UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13A-16 OR 15D-16 UNDER
THE SECURITIES EXCHANGE ACT OF 1934
For the month of January 2024
Commission File Number: 001-40010
Pharvaris N.V.
(Translation of registrant’s name into English)
Emmy Noetherweg 2
2333 BK Leiden
The Netherlands
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Note: Regulation S-T Rule 101(b)(1) only permits the submission in paper of a Form 6-K if submitted solely to provide an attached annual report to security holders.
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
Note: Regulation S-T Rule 101(b)(7) only permits the submission in paper of a Form 6-K if submitted to furnish a report or other document that the registrant foreign private issuer must furnish and make public under the laws of the jurisdiction in which the registrant is incorporated, domiciled or legally organized (the registrant’s “home country”), or under the rules of the home country exchange on which the registrant’s securities are traded, as long as the report or other document is not a press release, is not required to be and has not been distributed to the registrant’s security holders, and, if discussing a material event, has already been the subject of a Form 6-K submission or other Commission filing on EDGAR.
PHARVARIS N.V.
On January 22, 2024, Pharvaris N.V. (the “Company”) issued a press release. The press release is attached as Exhibit 99.1 hereto and is incorporated by reference herein. Also on January 22, 2024, the Company made available a corporate presentation on its website. A copy of the corporate presentation is attached hereto as Exhibit 99.2.
Exhibit 99.1 to this Report on Form 6-K shall be deemed to be incorporated by reference into the registration statements on Form F-3 (Registration Number 333-263198 and 333-273757) and Form S-8 (Registration Number 333-252897). Exhibit 99.2 to this Report on Form 6-K shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended or the Exchange Act.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
PHARVARIS N.V. |
|
|
Date: January 22, 2024 |
By: |
/s/ Berndt Modig |
|
Name: |
Berndt Modig |
|
Title: |
Chief Executive Officer |
EXHIBIT INDEX

Exhibit 99.1
Pharvaris Announces FDA Lifting of the Clinical Hold of Deucrictibant for the Prophylactic Treatment of HAE Attacks
Zug, Switzerland, January 22, 2024 – Pharvaris (Nasdaq: PHVS), a clinical-stage company developing novel, oral bradykinin B2 receptor antagonists to treat and prevent hereditary angioedema (HAE) attacks, today announced the U.S. Food and Drug Administration (FDA) has lifted the clinical hold on the Investigational New Drug (IND) application for deucrictibant for the prophylactic treatment of HAE attacks following review of data from a 26-week rodent toxicology study.
“The lift of the clinical hold in the U.S. enables us to progress the global development of deucrictibant for long-term prophylaxis, including resuming the open-label portion of CHAPTER-1, our Phase 2 proof-of-concept study of deucrictibant for the prevention of HAE attacks, in the U.S.,” said Berndt Modig, Chief Executive Officer of Pharvaris. “We are pleased to have worked collaboratively with the FDA to address the requests of the agency with the submission of additional nonclinical data, and we appreciate the agency’s comments and recommendations regarding study conduct. We will request an End-of-Phase 2 meeting with the FDA to align on key elements of CHAPTER-3, the anticipated global Phase 3 study of deucrictibant extended-release tablets (PHVS719) for the prophylactic treatment of HAE attacks.”
In August 2022, the FDA placed clinical studies of deucrictibant, including CHAPTER-1, on hold. Pharvaris notified ex-U.S. country-specific regulatory authorities of the clinical hold in the U.S., and the regulatory status of deucrictibant outside the U.S. was not affected. In June 2023, Pharvaris announced the FDA’s removal of the clinical hold of deucrictibant for the on-demand treatment of HAE in the U.S. following FDA review of data from a preplanned interim analysis of a 26-week rodent toxicology study. In December 2023, Pharvaris announced positive top-line clinical data from the Phase 2 CHAPTER-1 study of deucrictibant for the prophylactic treatment of HAE attacks.
About Deucrictibant
Deucrictibant is a potent, selective, and orally available antagonist of the bradykinin B2 receptor. By inhibiting bradykinin signaling through the bradykinin B2 receptor, deucrictibant has the potential to treat the clinical signs of an HAE attack and to prevent the occurrence of attacks. Based on its chemical properties, Pharvaris is developing two formulations of deucrictibant for oral administration; a capsule to enable rapid onset of activity for acute treatment, and an extended-release tablet to enable sustained absorption and efficacy in prophylactic treatment.
About Pharvaris
Building on its deep-seated roots in HAE, Pharvaris is a clinical-stage company developing novel, oral bradykinin B2 receptor antagonists to treat and prevent HAE attacks. By directly pursuing this clinically proven therapeutic target with novel small molecules, the Pharvaris team aspires to offer people with all sub-types of HAE efficacious, safe, and easy-to-administer alternatives to treat attacks, both on-demand and prophylactically. The company brings together the best talent in the industry with deep expertise in rare diseases and HAE. For more information, visit https://pharvaris.com/.
Forward-Looking Statements
This press release contains certain forward-looking statements that involve substantial risks and uncertainties. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements relating to our future plans, studies and trials, and any statements containing the words “believe,” “anticipate,” “expect,” “estimate,” “may,” “could,” “should,” “would,” “will,” “intend” and similar expressions. These forward-looking statements are based on management’s current expectations, are neither promises nor guarantees, and involve known and unknown risks, uncertainties and other important factors that may cause Pharvaris’ actual results, performance or achievements to be materially different from its expectations expressed or implied by the forward-looking statements. Such risks include but are not limited to the following: uncertainty in the outcome of our interactions with regulatory authorities, including the FDA; the expected timing, progress, or success of our clinical development programs, especially for deucrictibant immediate-release capsules (PHVS416) and deucrictibant extended-release tablets (PHVS719), which are in mid-stage global clinical trials; risks arising from epidemic diseases, such as the COVID-19 pandemic, which may adversely impact our business, nonclinical studies, and clinical trials; the outcome and timing of regulatory approvals; the value of our ordinary shares; the timing, costs and other limitations involved in obtaining regulatory approval for our product candidates, or any other product candidate that we may develop in the future; our ability to establish commercial capabilities or enter into agreements with third parties to market, sell, and distribute our product candidates; our ability to compete in the pharmaceutical industry, including with respect to existing therapies, emerging potentially competitive therapies and with competitive generic products; our ability to market, commercialize and achieve market acceptance for our product candidates; our ability to raise capital when needed and on acceptable terms; regulatory developments in the United States, the European Union and other jurisdictions; our ability to protect our intellectual property and know-how and operate our business without infringing the intellectual property rights or regulatory exclusivity of others; our ability to manage negative consequences from changes in applicable laws and regulations, including tax laws, our ability to successfully remediate the material weaknesses in our internal control over financial
reporting and to maintain an effective system of internal control over financial reporting; changes and uncertainty in general market, political and economic conditions, including as a result of inflation and the current conflict between Russia and Ukraine and the Hamas attack against Israel and the ensuing war; and the other factors described under the headings “Cautionary Statement Regarding Forward-Looking Statements” and “Item 3. Key Information—D. Risk Factors” in our Annual Report on Form 20-F and other periodic filings with the U.S. Securities and Exchange Commission. These and other important factors could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. While Pharvaris may elect to update such forward-looking statements at some point in the future, Pharvaris disclaims any obligation to do so, even if subsequent events cause its views to change. These forward-looking statements should not be relied upon as representing Pharvaris’ views as of any date subsequent to the date of this press release.
Contact
Maggie Beller
Executive Director, Head of External and Internal Communications
maggie.beller@pharvaris.com

Corporate Presentation January 2024 Pioneering science for patient choice Exhibit 99.2

Disclaimer This Presentation contains certain “forward‐looking statements” within the meaning of the federal securities laws that involve substantial risks and uncertainties. All statements contained in this Presentation that do not relate to matters of historical fact should be considered forward-looking statements including, without limitation, statements containing the words “believe,” “anticipate,” “expect,” “estimate,” “may,” “could,” “should,” “would,” “will,” “intend” and similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. Such forward-looking statements involve unknown risks, uncertainties and other factors which may cause our actual results, financial condition, performance or achievements, or industry results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Factors that might cause such a difference include, but are not limited to, uncertainty in the outcome of our interactions with regulatory authorities, including the FDA, the expected timing, progress, or success of our clinical development programs especially for PHVS416 and PHVS719 which are in mid-stage clinical trials, our ability to replicate the efficacy and safety demonstrated in the CHAPTER-1 Phase 2 study in ongoing and future nonclinical studies and clinical trials, risks associated with the COVID-19 pandemic which may adversely impact our business, nonclinical studies, and clinical trials, the outcome and timing of regulatory approvals, the value of our ordinary shares, the timing, costs and other limitations involved in obtaining regulatory approval for our product candidates, deucrictibant immediate-release capsules (PHVS416) and deucrictibant extended-release tablets (PHVS719), or any other product candidate that we may develop in the future, our ability to establish commercial capabilities or enter into agreements with third parties to market, sell, and distribute our product candidates, our ability to compete in the pharmaceutical industry, including with respect to existing therapies, emerging potentially competitive therapies and with competitive generic products, and with competitive generic products, our ability to market, commercialize and achieve market acceptance for our product candidates, our ability to raise capital when needed and on acceptable terms, regulatory developments in the United States, the European Union and other jurisdictions, our ability to protect our intellectual property and know-how and operate our business without infringing the intellectual property rights or regulatory exclusivity of others, our ability to manage negative consequences from changes in applicable laws and regulations, including tax laws, our ability to successfully remediate the material weaknesses in our internal control over financial reporting and to maintain an effective system of internal control over financial reporting, changes in general market, political and economic conditions, including as a result of the current conflict between Russia and Ukraine, the Israel-Hamas war, and the other factors described under the headings "Cautionary Statement Regarding Forward-Looking Statements" and "Item 3. Key Information--D. Risk Factors" in our Annual Report on Form 20-F and other periodic filings with the Securities and Exchange Commission. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. This presentation includes data for an investigational product not yet approved by regulatory authorities. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this Presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source.
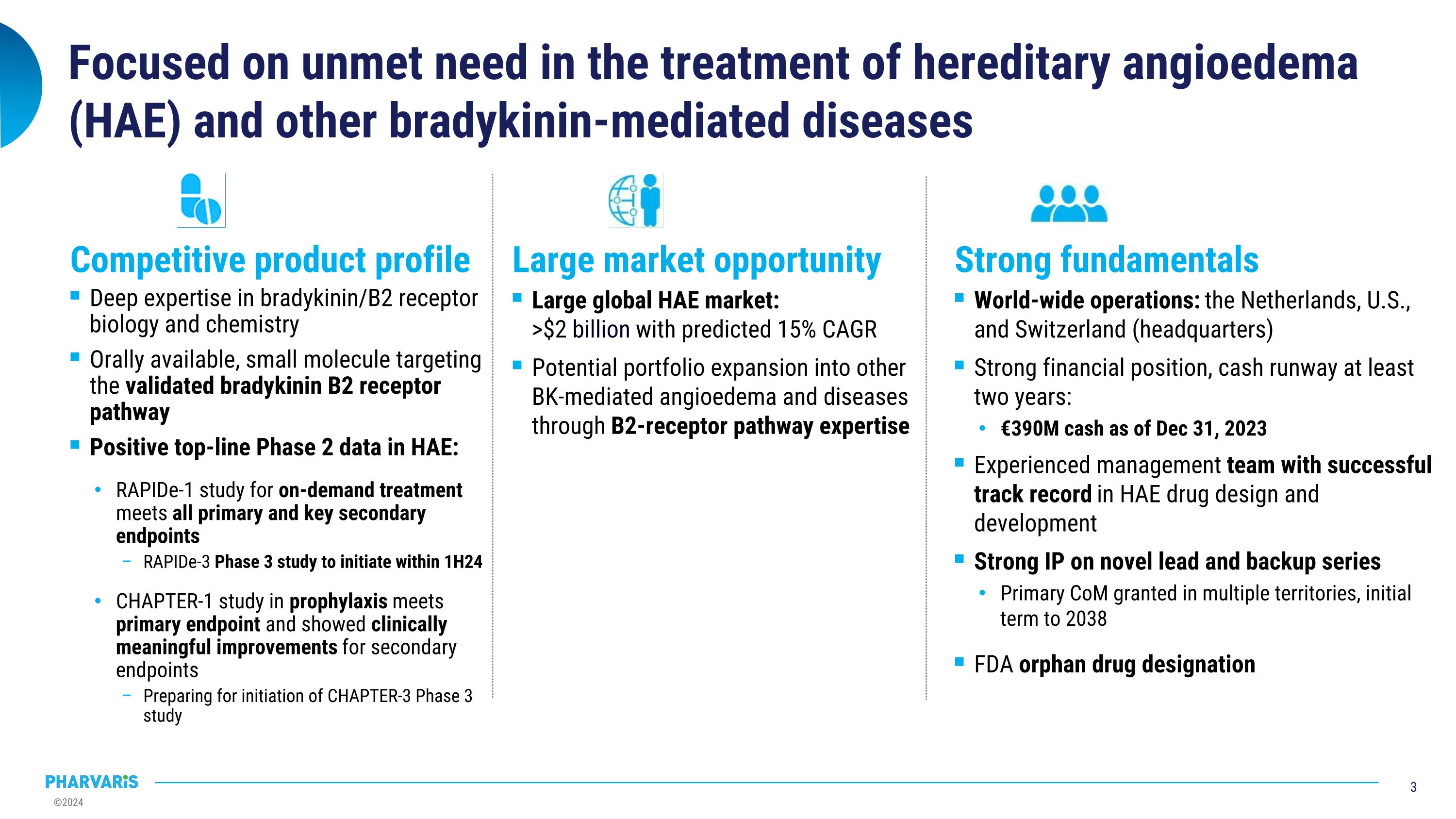
Focused on unmet need in the treatment of hereditary angioedema (HAE) and other bradykinin-mediated diseases Deep expertise in bradykinin/B2 receptor biology and chemistry Orally available, small molecule targeting the validated bradykinin B2 receptor pathway Positive top-line Phase 2 data in HAE: RAPIDe-1 study for on-demand treatment meets all primary and key secondary endpoints RAPIDe-3 Phase 3 study to initiate within 1H24 CHAPTER-1 study in prophylaxis meets primary endpoint and showed clinically meaningful improvements for secondary endpoints Preparing for initiation of CHAPTER-3 Phase 3 study Large global HAE market: �>$2 billion with predicted 15% CAGR Potential portfolio expansion into other BK-mediated angioedema and diseases through B2-receptor pathway expertise World-wide operations: the Netherlands, U.S., and Switzerland (headquarters) Strong financial position, cash runway at least two years: €390M cash as of Dec 31, 2023 Experienced management team with successful track record in HAE drug design and development Strong IP on novel lead and backup series Primary CoM granted in multiple territories, initial term to 2038 FDA orphan drug designation Competitive product profile Strong fundamentals Large market opportunity
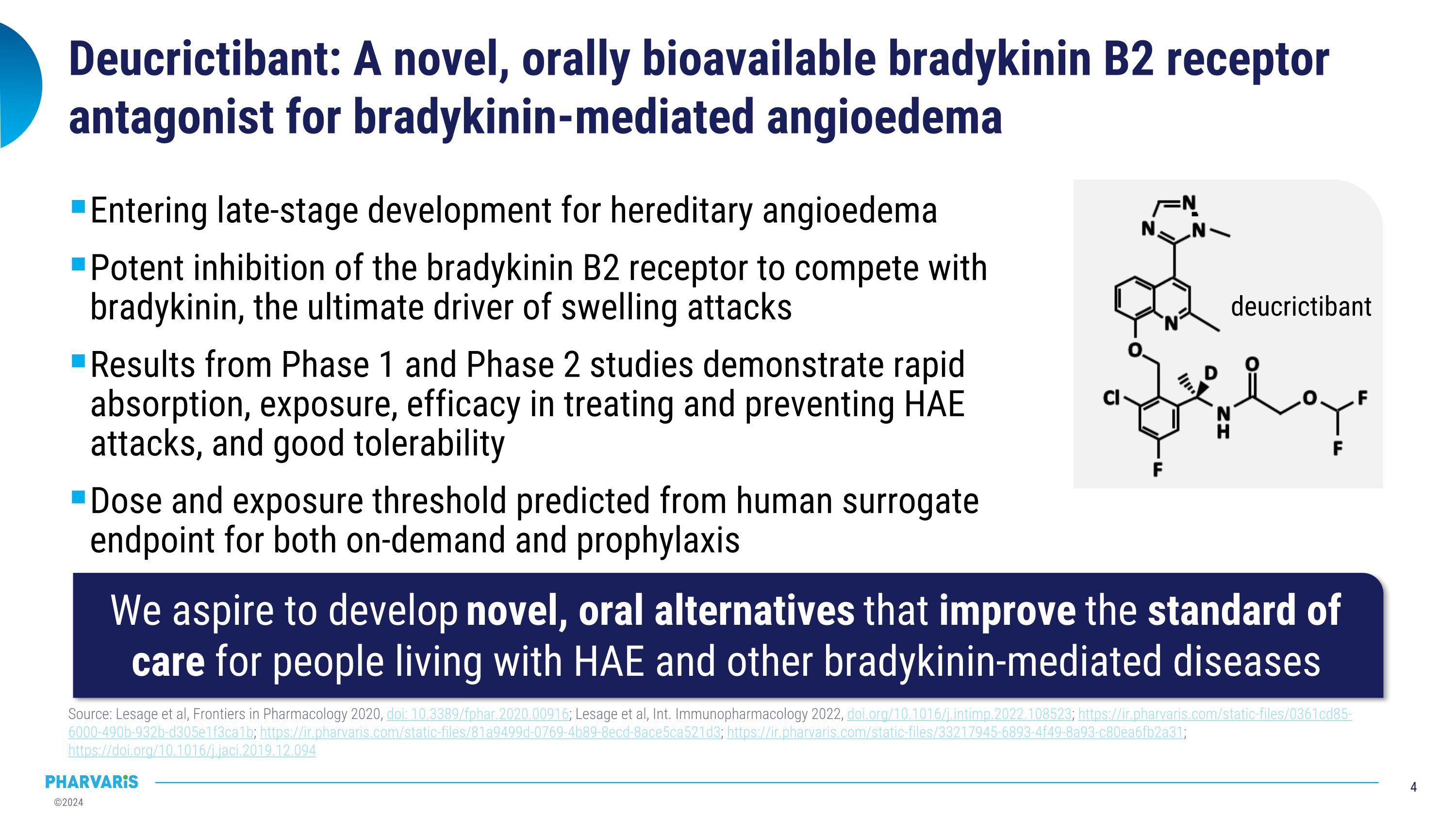
Deucrictibant: A novel, orally bioavailable bradykinin B2 receptor antagonist for bradykinin-mediated angioedema Entering late-stage development for hereditary angioedema Potent inhibition of the bradykinin B2 receptor to compete with bradykinin, the ultimate driver of swelling attacks Results from Phase 1 and Phase 2 studies demonstrate rapid absorption, exposure, efficacy in treating and preventing HAE attacks, and good tolerability Dose and exposure threshold predicted from human surrogate endpoint for both on-demand and prophylaxis Source: Lesage et al, Frontiers in Pharmacology 2020, doi: 10.3389/fphar.2020.00916; Lesage et al, Int. Immunopharmacology 2022, doi.org/10.1016/j.intimp.2022.108523; https://ir.pharvaris.com/static-files/0361cd85-6000-490b-932b-d305e1f3ca1b; https://ir.pharvaris.com/static-files/81a9499d-0769-4b89-8ecd-8ace5ca521d3; https://ir.pharvaris.com/static-files/33217945-6893-4f49-8a93-c80ea6fb2a31; https://doi.org/10.1016/j.jaci.2019.12.094 We aspire to develop novel, oral alternatives that improve the standard of care for people living with HAE and other bradykinin-mediated diseases deucrictibant
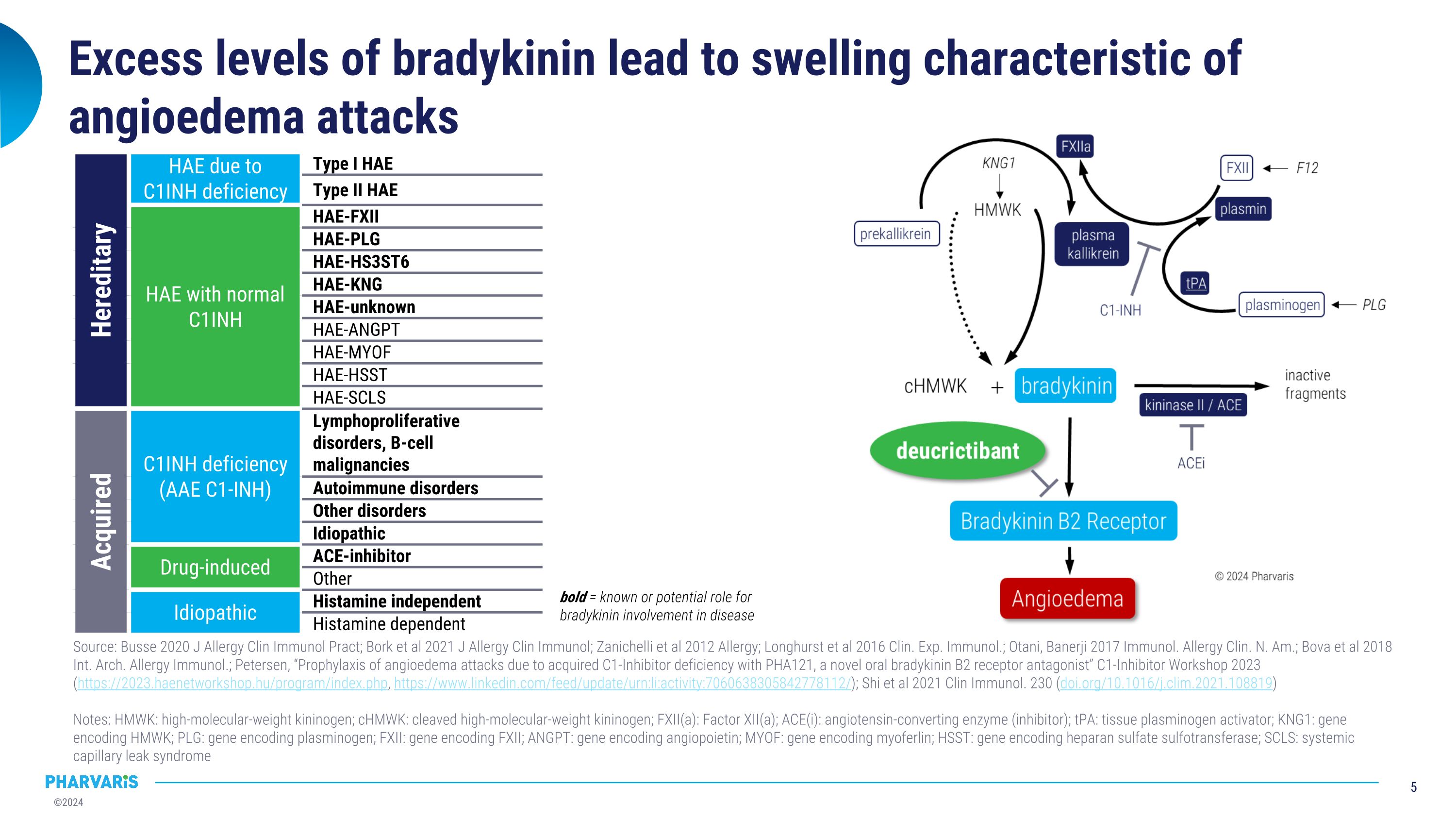
Excess levels of bradykinin lead to swelling characteristic of angioedema attacks Hereditary HAE due to C1INH deficiency Type I HAE Type II HAE HAE with normal C1INH HAE-FXII HAE-PLG HAE-HS3ST6 HAE-KNG HAE-unknown HAE-ANGPT HAE-MYOF HAE-HSST HAE-SCLS Acquired C1INH deficiency�(AAE C1-INH) Lymphoproliferative disorders, B-cell malignancies Autoimmune disorders Other disorders Idiopathic Drug-induced ACE-inhibitor Other Idiopathic Histamine independent Histamine dependent Source: Busse 2020 J Allergy Clin Immunol Pract; Bork et al 2021 J Allergy Clin Immunol; Zanichelli et al 2012 Allergy; Longhurst et al 2016 Clin. Exp. Immunol.; Otani, Banerji 2017 Immunol. Allergy Clin. N. Am.; Bova et al 2018 Int. Arch. Allergy Immunol.; Petersen, “Prophylaxis of angioedema attacks due to acquired C1-Inhibitor deficiency with PHA121, a novel oral bradykinin B2 receptor antagonist” C1-Inhibitor Workshop 2023 (https://2023.haenetworkshop.hu/program/index.php, https://www.linkedin.com/feed/update/urn:li:activity:7060638305842778112/); Shi et al 2021 Clin Immunol. 230 (doi.org/10.1016/j.clim.2021.108819) Notes: HMWK: high-molecular-weight kininogen; cHMWK: cleaved high-molecular-weight kininogen; FXII(a): Factor XII(a); ACE(i): angiotensin-converting enzyme (inhibitor); tPA: tissue plasminogen activator; KNG1: gene encoding HMWK; PLG: gene encoding plasminogen; FXII: gene encoding FXII; ANGPT: gene encoding angiopoietin; MYOF: gene encoding myoferlin; HSST: gene encoding heparan sulfate sulfotransferase; SCLS: systemic capillary leak syndrome bold = known or potential role for bradykinin involvement in disease
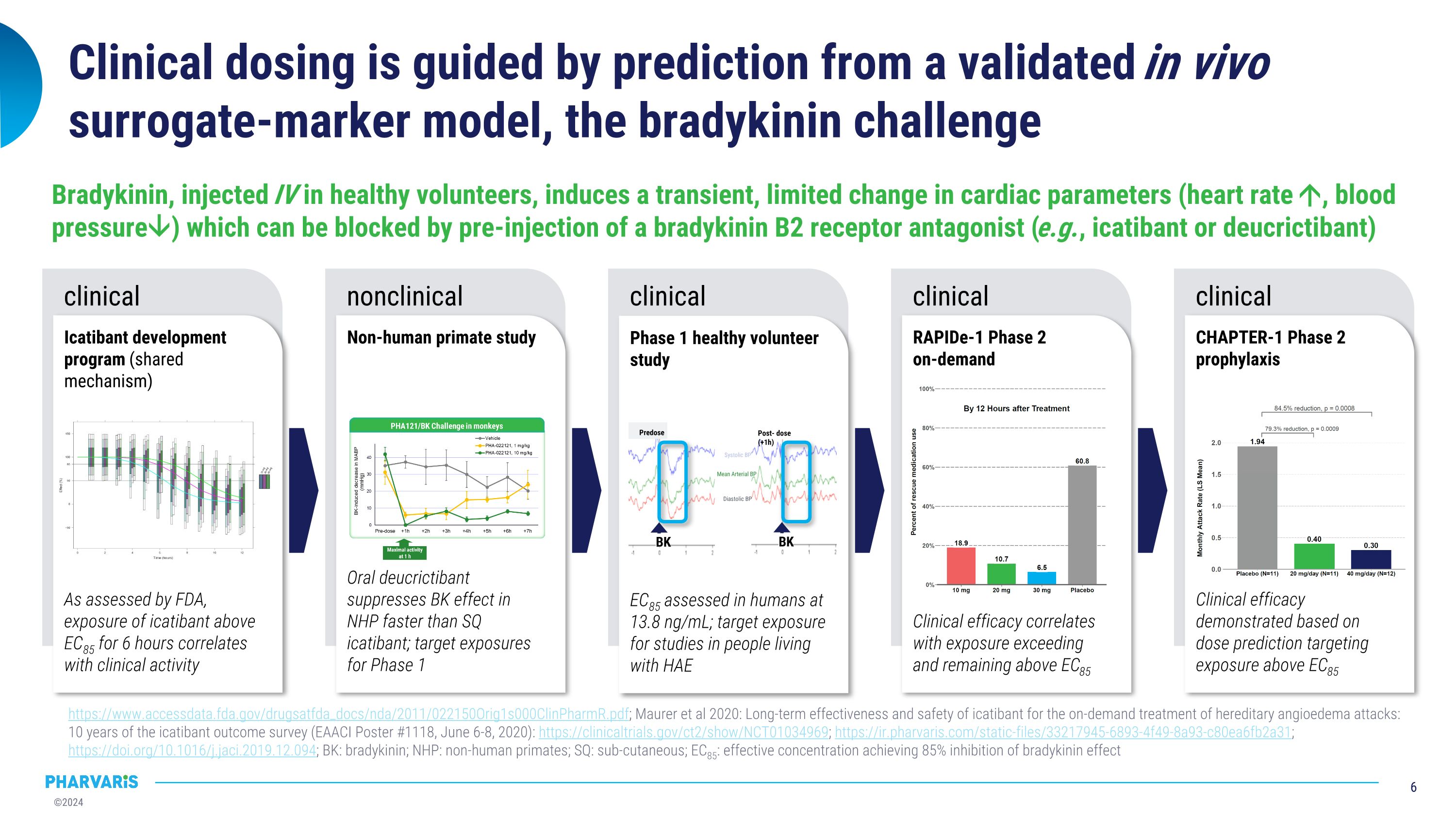
Clinical dosing is guided by prediction from a validated in vivo surrogate-marker model, the bradykinin challenge https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022150Orig1s000ClinPharmR.pdf; Maurer et al 2020: Long-term effectiveness and safety of icatibant for the on-demand treatment of hereditary angioedema attacks: 10 years of the icatibant outcome survey (EAACI Poster #1118, June 6-8, 2020): https://clinicaltrials.gov/ct2/show/NCT01034969; https://ir.pharvaris.com/static-files/33217945-6893-4f49-8a93-c80ea6fb2a31; https://doi.org/10.1016/j.jaci.2019.12.094; BK: bradykinin; NHP: non-human primates; SQ: sub-cutaneous; EC85: effective concentration achieving 85% inhibition of bradykinin effect clinical Icatibant development program (shared mechanism) As assessed by FDA, exposure of icatibant above EC85 for 6 hours correlates with clinical activity nonclinical Non-human primate study Oral deucrictibant suppresses BK effect in NHP faster than SQ icatibant; target exposures for Phase 1 clinical Phase 1 healthy volunteer study EC85 assessed in humans at 13.8 ng/mL; target exposure for studies in people living with HAE clinical RAPIDe-1 Phase 2 �on-demand Clinical efficacy correlates with exposure exceeding and remaining above EC85 clinical CHAPTER-1 Phase 2 prophylaxis Clinical efficacy demonstrated based on dose prediction targeting exposure above EC85 Bradykinin, injected IV in healthy volunteers, induces a transient, limited change in cardiac parameters (heart rate , blood pressure) which can be blocked by pre-injection of a bradykinin B2 receptor antagonist (e.g., icatibant or deucrictibant) Post- dose (+1h) BK BK Predose Systolic BP Diastolic BP Mean Arterial BP
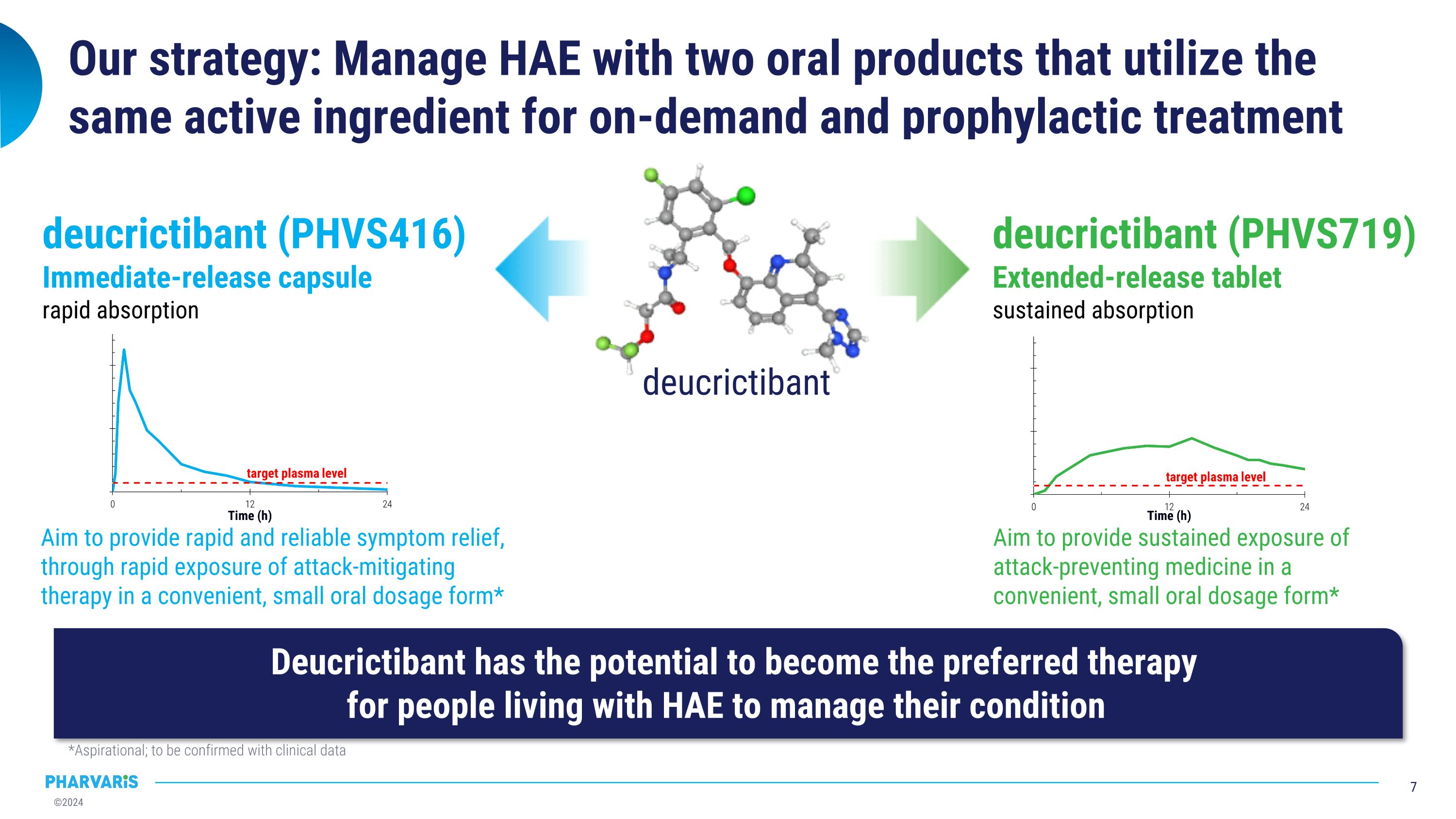
Our strategy: Manage HAE with two oral products that utilize the same active ingredient for on-demand and prophylactic treatment Deucrictibant has the potential to become the preferred therapy�for people living with HAE to manage their condition *Aspirational; to be confirmed with clinical data deucrictibant (PHVS416)�Immediate-release capsule�rapid absorption deucrictibant (PHVS719)�Extended-release tablet�sustained absorption Aim to provide sustained exposure of attack-preventing medicine in a convenient, small oral dosage form* Aim to provide rapid and reliable symptom relief, through rapid exposure of attack-mitigating therapy in a convenient, small oral dosage form* deucrictibant
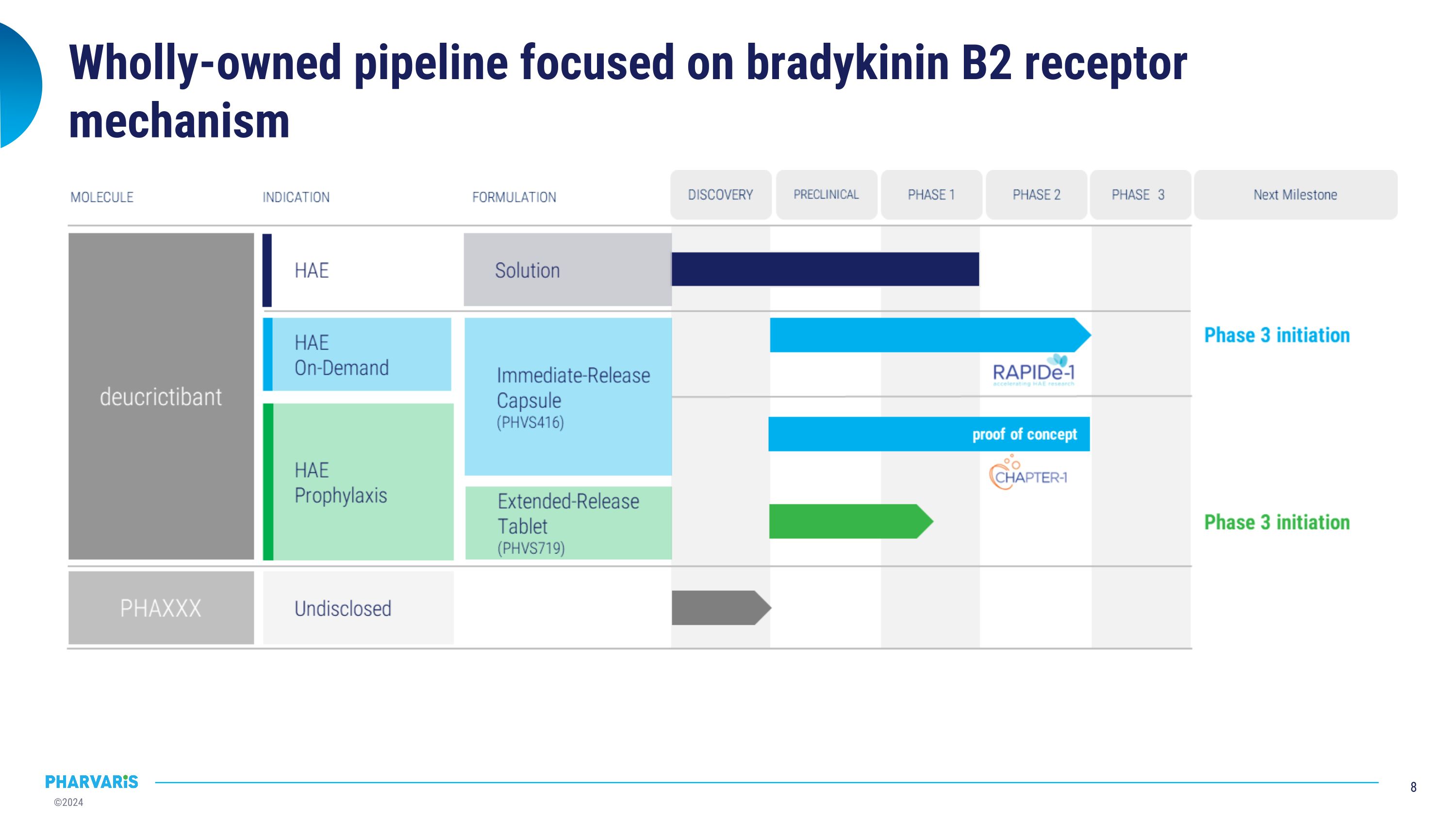
Wholly-owned pipeline focused on bradykinin B2 receptor mechanism
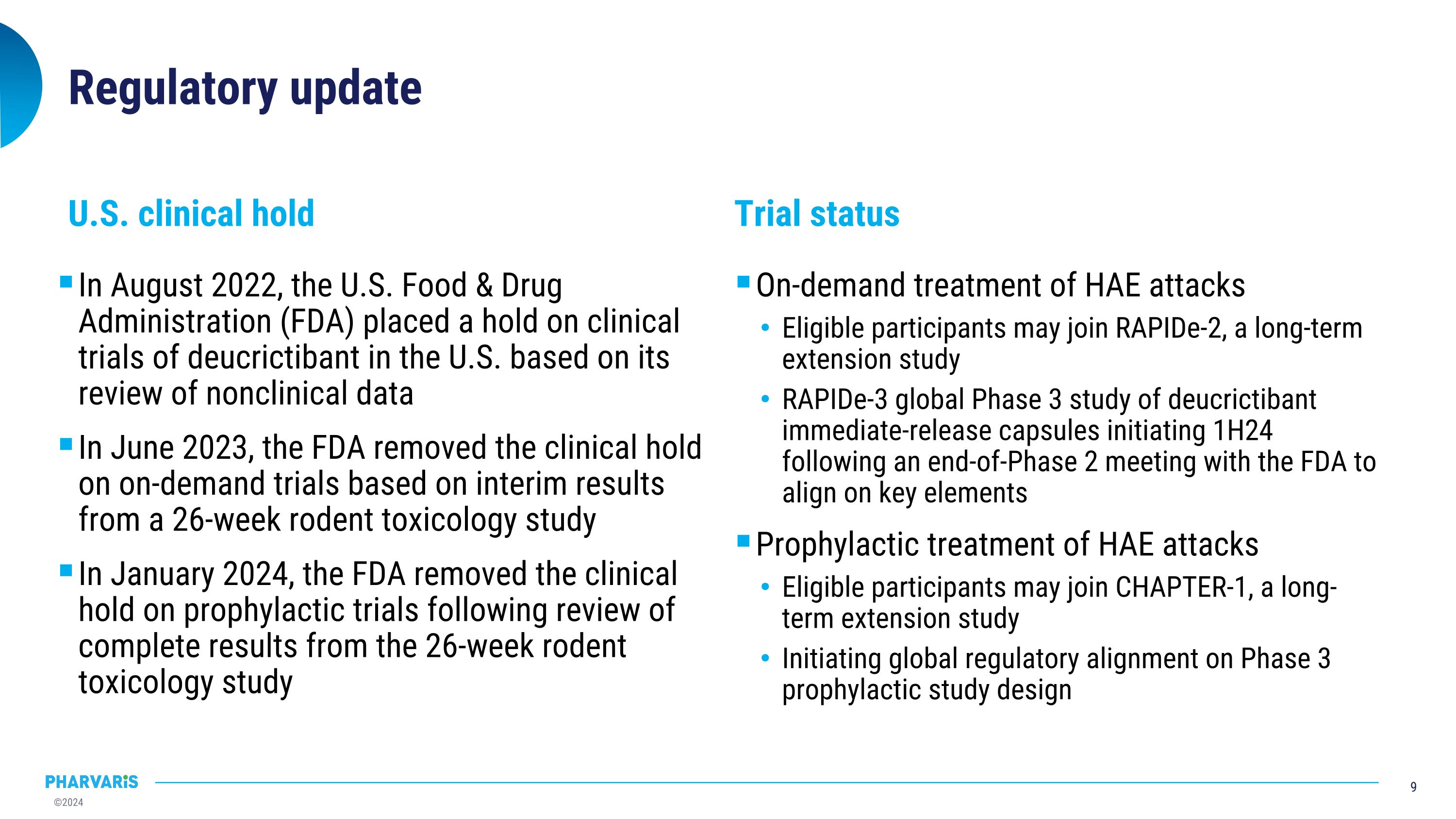
Regulatory update In August 2022, the U.S. Food & Drug Administration (FDA) placed a hold on clinical trials of deucrictibant in the U.S. based on its review of nonclinical data In June 2023, the FDA removed the clinical hold on on-demand trials based on interim results from a 26-week rodent toxicology study In January 2024, the FDA removed the clinical hold on prophylactic trials following review of complete results from the 26-week rodent toxicology study On-demand treatment of HAE attacks Eligible participants may join RAPIDe-2, a long-term extension study RAPIDe-3 global Phase 3 study of deucrictibant immediate-release capsules initiating 1H24 following an end-of-Phase 2 meeting with the FDA to align on key elements Prophylactic treatment of HAE attacks Eligible participants may join CHAPTER-1, a long-term extension study Initiating global regulatory alignment on Phase 3 prophylactic study design U.S. clinical hold Trial status
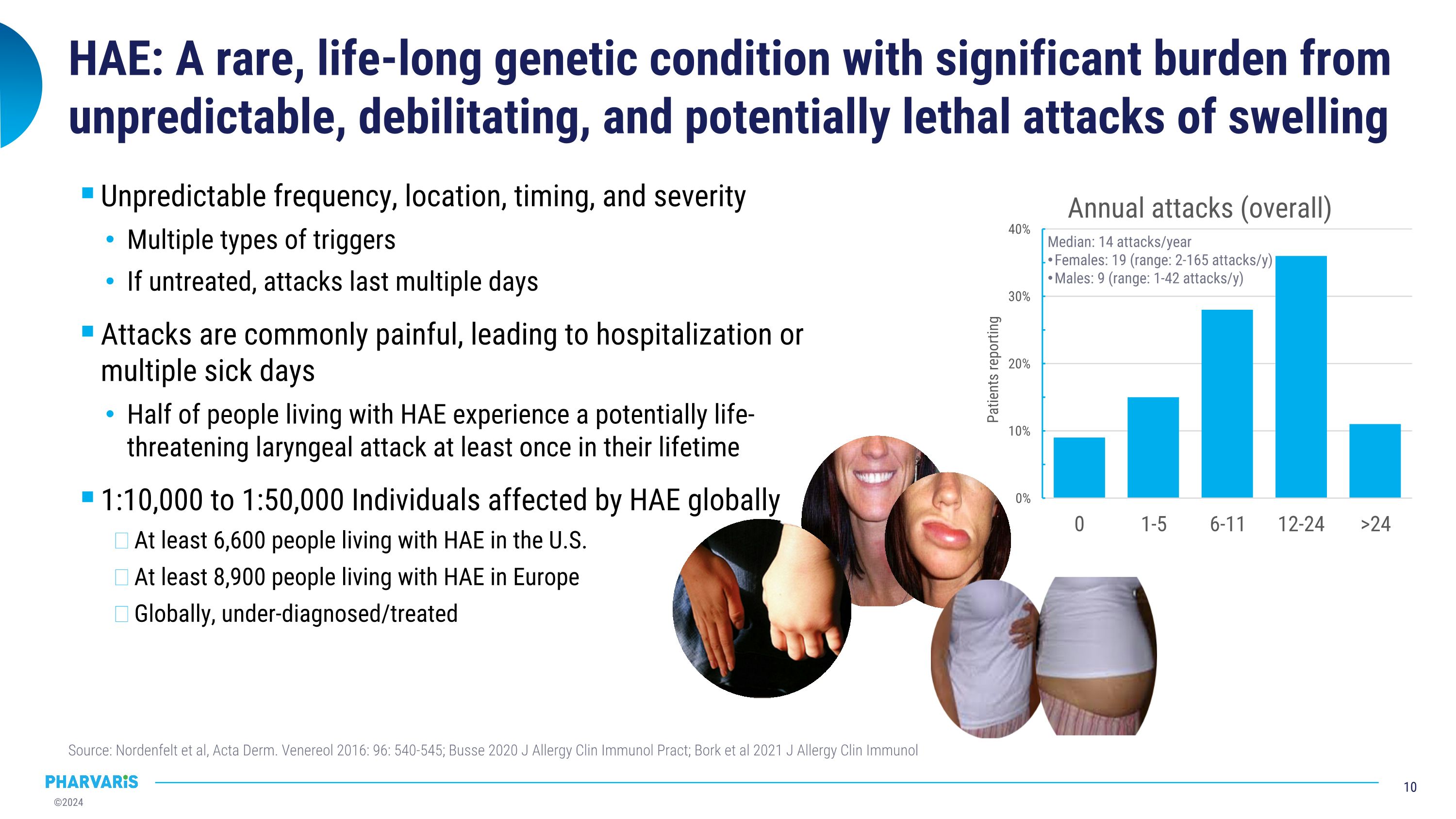
HAE: A rare, life-long genetic condition with significant burden from unpredictable, debilitating, and potentially lethal attacks of swelling Source: Nordenfelt et al, Acta Derm. Venereol 2016: 96: 540-545; Busse 2020 J Allergy Clin Immunol Pract; Bork et al 2021 J Allergy Clin Immunol Unpredictable frequency, location, timing, and severity Multiple types of triggers If untreated, attacks last multiple days Attacks are commonly painful, leading to hospitalization or multiple sick days Half of people living with HAE experience a potentially life-threatening laryngeal attack at least once in their lifetime 1:10,000 to 1:50,000 Individuals affected by HAE globally At least 6,600 people living with HAE in the U.S. At least 8,900 people living with HAE in Europe Globally, under-diagnosed/treated Median: 14 attacks/year Females: 19 (range: 2-165 attacks/y) Males: 9 (range: 1-42 attacks/y)
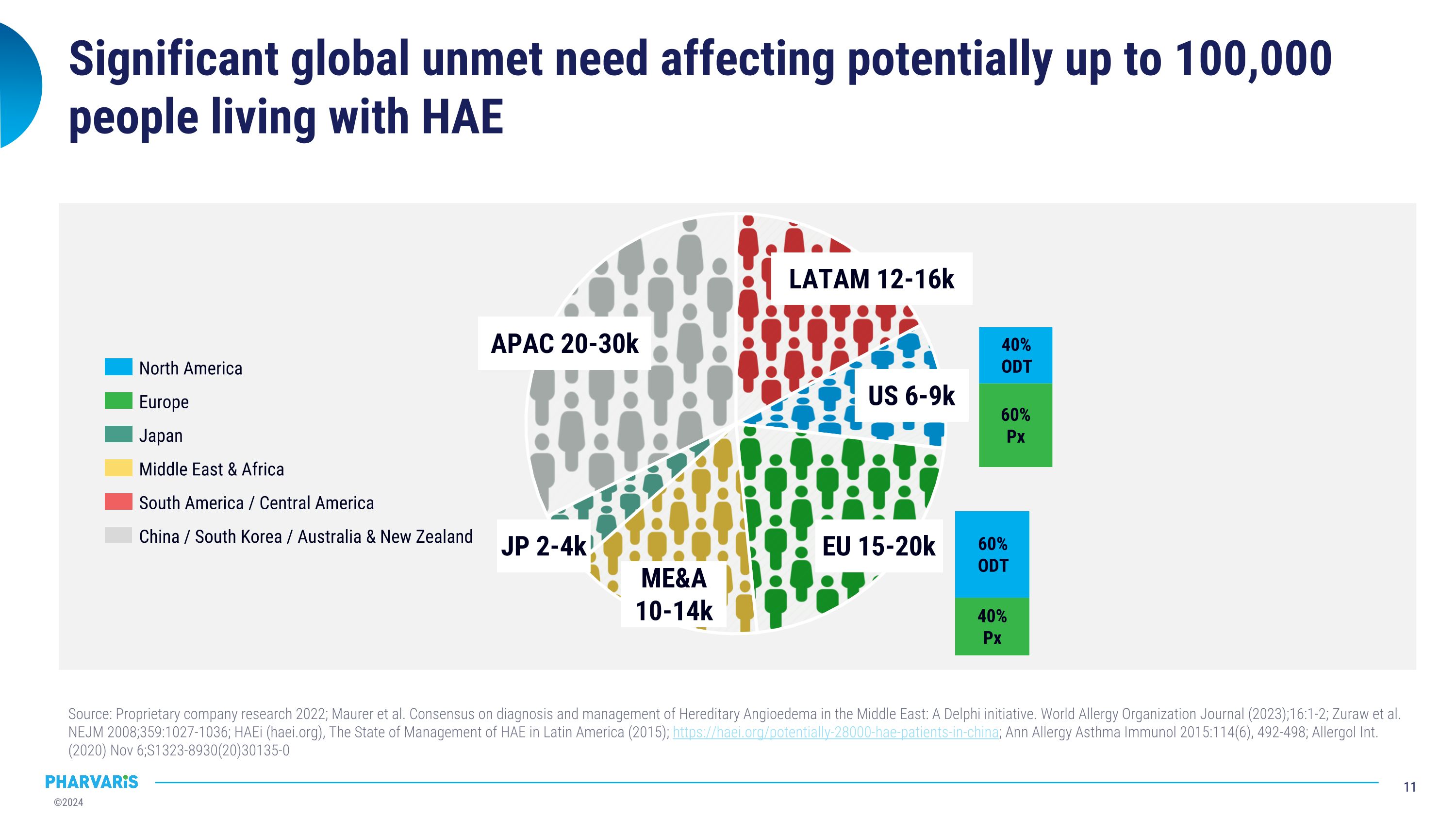
Significant global unmet need affecting potentially up to 100,000 people living with HAE Source: Proprietary company research 2022; Maurer et al. Consensus on diagnosis and management of Hereditary Angioedema in the Middle East: A Delphi initiative. World Allergy Organization Journal (2023);16:1-2; Zuraw et al. NEJM 2008;359:1027-1036; HAEi (haei.org), The State of Management of HAE in Latin America (2015); https://haei.org/potentially-28000-hae-patients-in-china; Ann Allergy Asthma Immunol 2015:114(6), 492-498; Allergol Int. (2020) Nov 6;S1323-8930(20)30135-0 North America Europe Japan South America / Central America Middle East & Africa China / South Korea / Australia & New Zealand EU 15-20k US 6-9k LATAM 12-16k JP 2-4k ME&A 10-14k APAC 20-30k

Source: Proprietary company research 2022 People living with HAE actively switch products, seeking improvement in efficacy, safety/tolerability, and convenience Efficacy is a prime concern … … but safety and tolerability drive exploration of alternatives … … while convenience has become a key driver for overall preference People living with HAE should not have to compromise
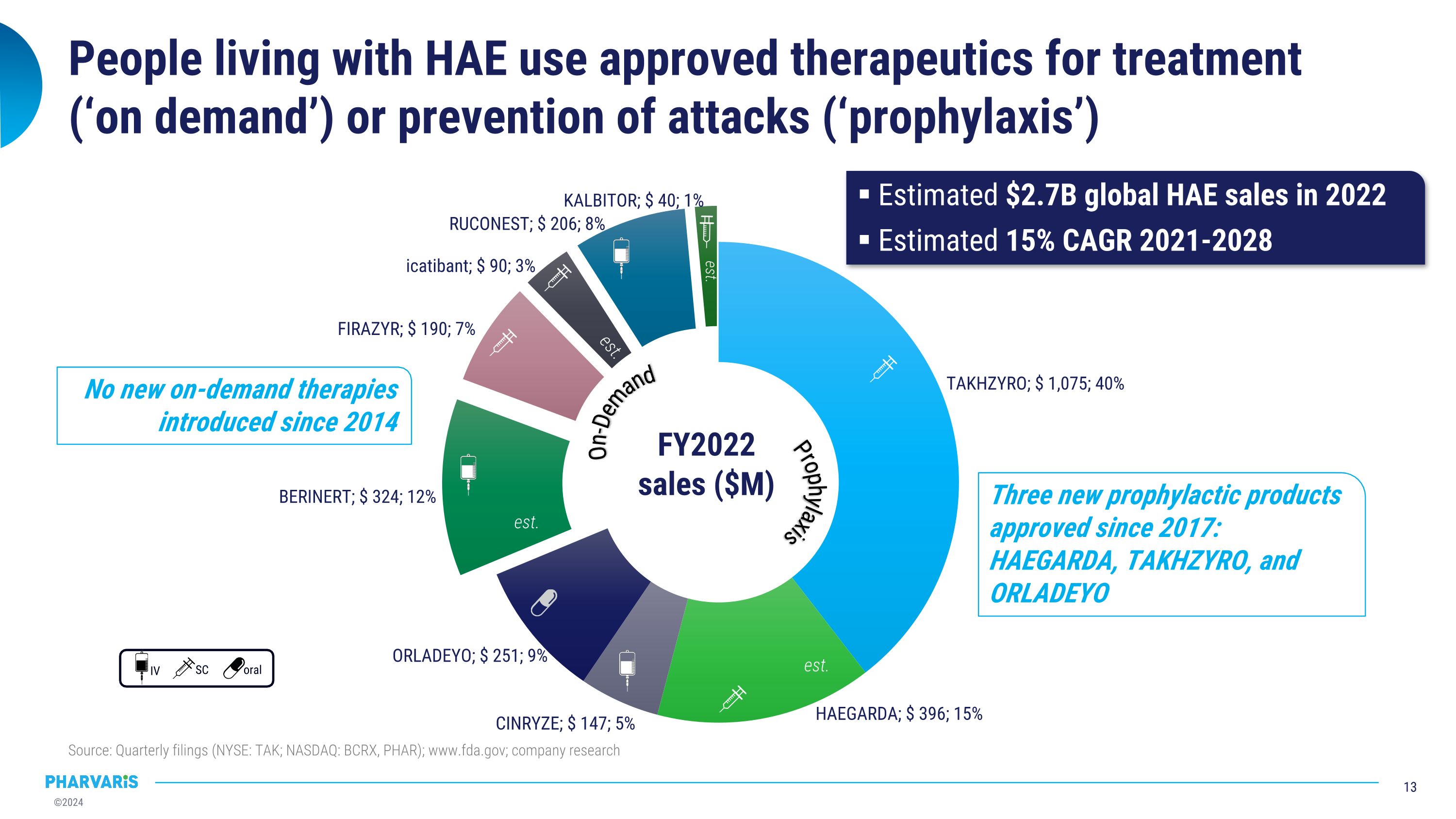
People living with HAE use approved therapeutics for treatment (‘on demand’) or prevention of attacks (‘prophylaxis’) Estimated $2.7B global HAE sales in 2022 Estimated 15% CAGR 2021-2028 SC oral IV No new on-demand therapies introduced since 2014 Three new prophylactic products approved since 2017: HAEGARDA, TAKHZYRO, and ORLADEYO Source: Quarterly filings (NYSE: TAK; NASDAQ: BCRX, PHAR); www.fda.gov; company research est. est. est. On-Demand Prophylaxis est.
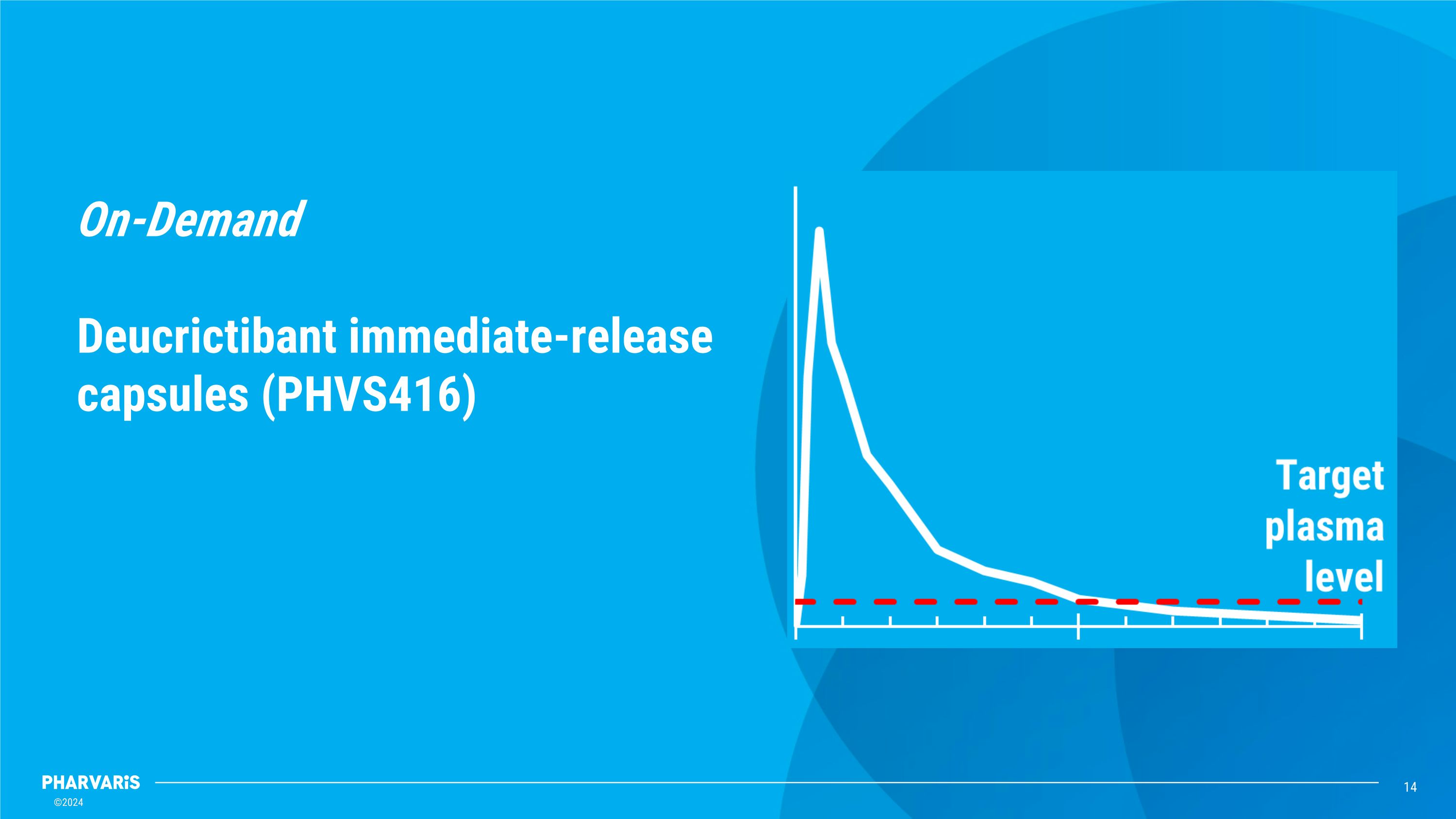
On-Demand��Deucrictibant immediate-release capsules (PHVS416)
Only injectable options: Significant unmet need in the on-demand treatment of HAE attacks Source: Proprietary Pharvaris research, 2022 (representative sample of patients, n = 103, and doctors, n = 100) Treatment today means painful injections … … and often one dose does not suffice … … while finding a place to administer the drug causes an extra burden As a result, people living with HAE often delay or even avoid therapy against clinical guideline recommendations
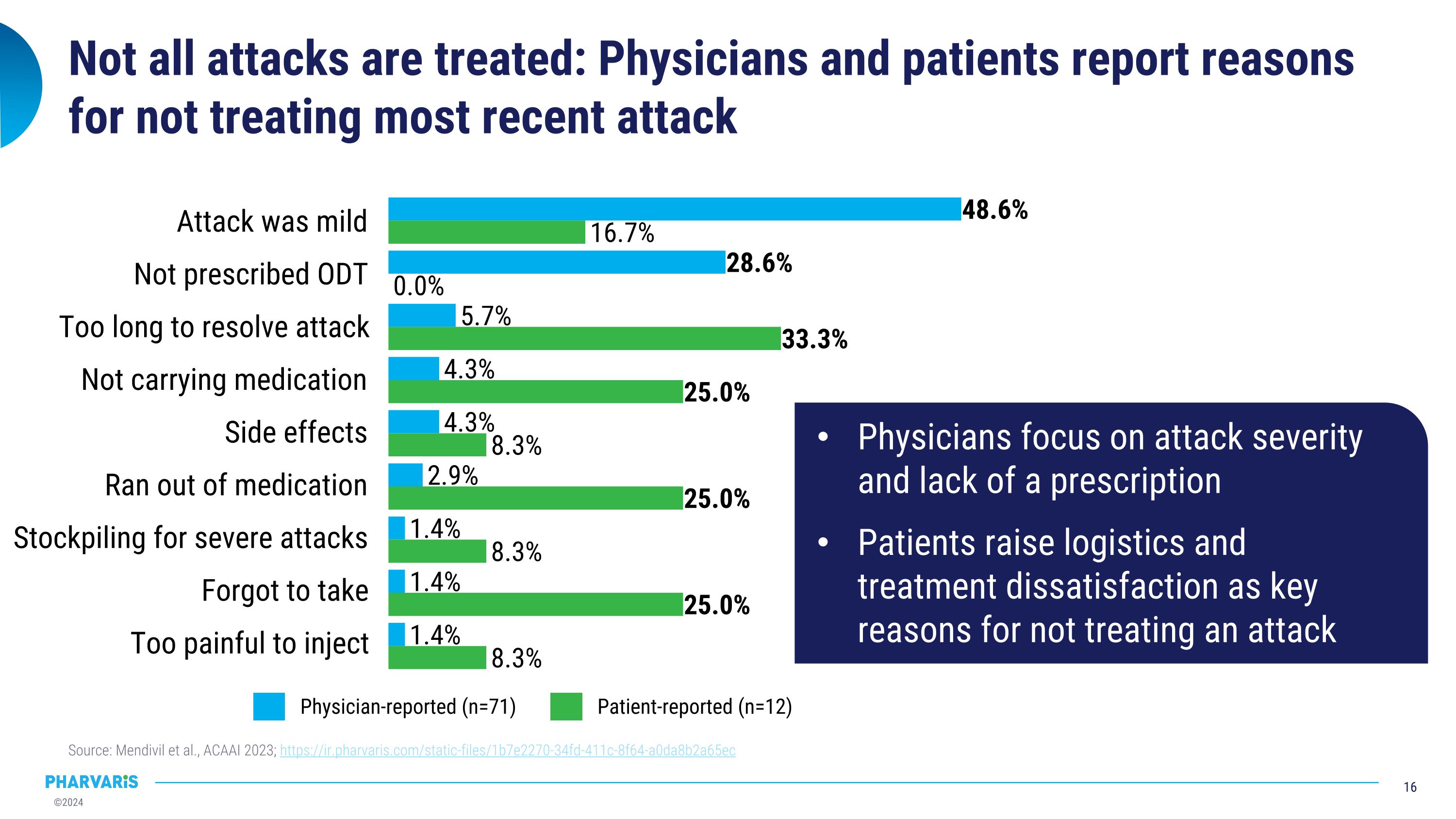
Not all attacks are treated: Physicians and patients report reasons for not treating most recent attack Source: Mendivil et al., ACAAI 2023; https://ir.pharvaris.com/static-files/1b7e2270-34fd-411c-8f64-a0da8b2a65ec Attack was mild Not prescribed ODT Too long to resolve attack Not carrying medication Side effects Ran out of medication Stockpiling for severe attacks Forgot to take Too painful to inject Physician-reported (n=71) Patient-reported (n=12) Physicians focus on attack severity and lack of a prescription Patients raise logistics and treatment dissatisfaction as key reasons for not treating an attack
People living with HAE are hoping for better on-demand therapies that offer rapid symptom relief with one single, oral dose Source: Proprietary Pharvaris research, 2022 (representative sample of patients, n = 103, and doctors, n = 100) Effectively targeting the bradykinin receptor with a small molecule has the potential to deliver on their hopes Patients want rapid onset of symptom relief … … with single dose durability … … in an oral pill
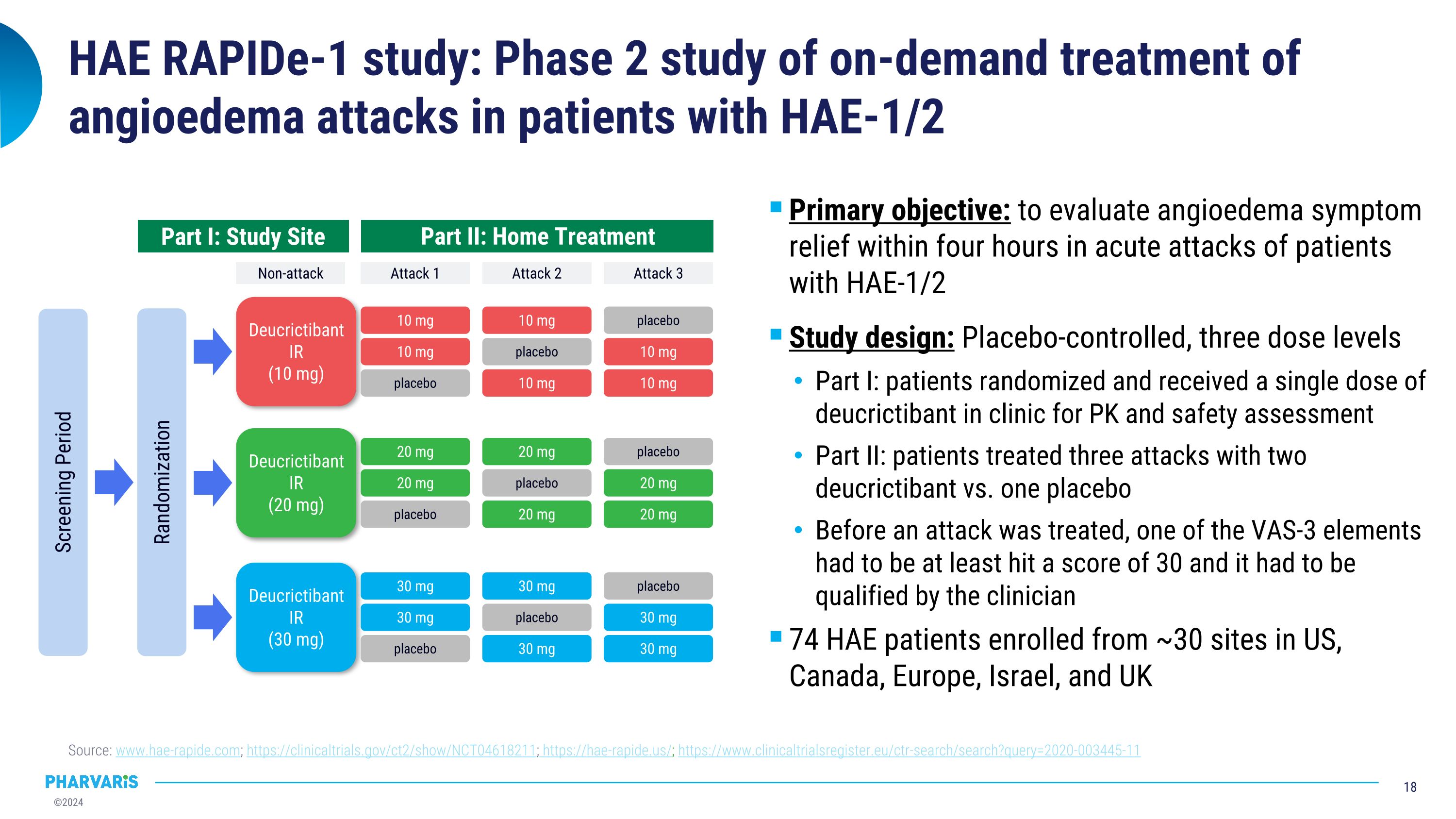
HAE RAPIDe-1 study: Phase 2 study of on-demand treatment of angioedema attacks in patients with HAE-1/2 Source: www.hae-rapide.com; https://clinicaltrials.gov/ct2/show/NCT04618211; https://hae-rapide.us/; https://www.clinicaltrialsregister.eu/ctr-search/search?query=2020-003445-11 Screening Period Randomization Non-attack Attack 1 Attack 2 Attack 3 Part I: Study Site Part II: Home Treatment Deucrictibant IR (10 mg) placebo 10 mg 10 mg placebo 10 mg 10 mg placebo 10 mg 10 mg Deucrictibant IR (20 mg) Deucrictibant IR (30 mg) placebo 20 mg 20 mg placebo 20 mg 20 mg placebo 20 mg 20 mg placebo 30 mg 30 mg placebo 30 mg 30 mg placebo 30 mg 30 mg Primary objective: to evaluate angioedema symptom relief within four hours in acute attacks of patients with HAE-1/2 Study design: Placebo-controlled, three dose levels Part I: patients randomized and received a single dose of deucrictibant in clinic for PK and safety assessment Part II: patients treated three attacks with two deucrictibant vs. one placebo Before an attack was treated, one of the VAS-3 elements had to be at least hit a score of 30 and it had to be qualified by the clinician 74 HAE patients enrolled from ~30 sites in US, Canada, Europe, Israel, and UK
Positive top-line Phase 2 data from RAPIDe-1 study of PHVS416 for the on-demand treatment of HAE attacks A total of 74 patients from 13 countries were enrolled to the study, 62 of them had 147 attacks that were treated with blinded study drug and included in efficacy evaluation The primary endpoint and all key secondary endpoints were met Deucrictibant IR showed rapid onset of action, symptom relief, and resolution of HAE attacks Deucrictibant IR substantially reduced the use of rescue medications Deucrictibant IR was well tolerated at all dose levels There were no treatment-related SAEs, no treatment-related AEs of severe severity, and no AEs leading to treatment discontinuation Consistent outcomes observed�across all endpoints and types of measurements
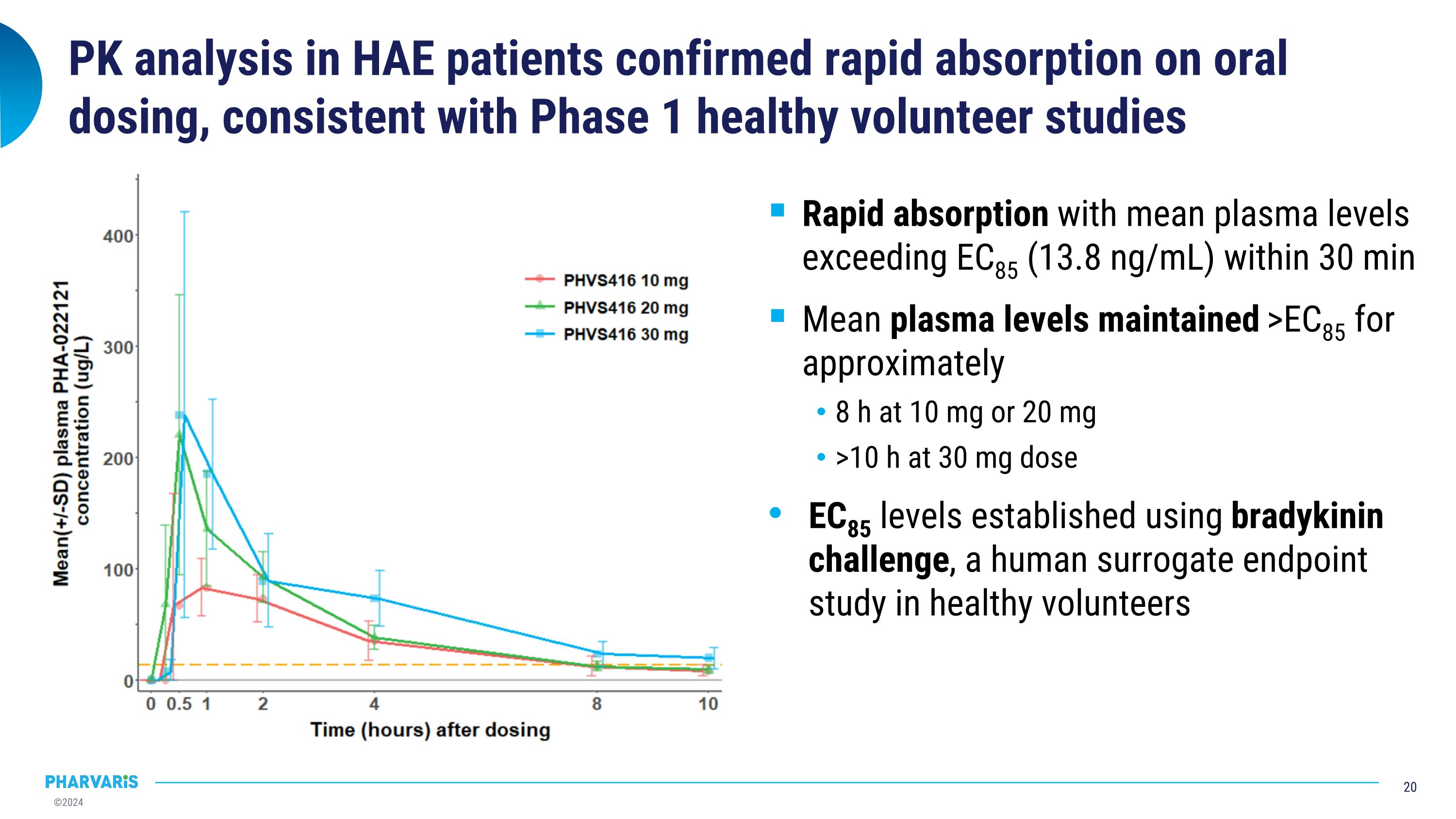
Rapid absorption with mean plasma levels exceeding EC85 (13.8 ng/mL) within 30 min Mean plasma levels maintained >EC85 for approximately 8 h at 10 mg or 20 mg >10 h at 30 mg dose EC85 levels established using bradykinin challenge, a human surrogate endpoint study in healthy volunteers PK analysis in HAE patients confirmed rapid absorption on oral dosing, consistent with Phase 1 healthy volunteer studies
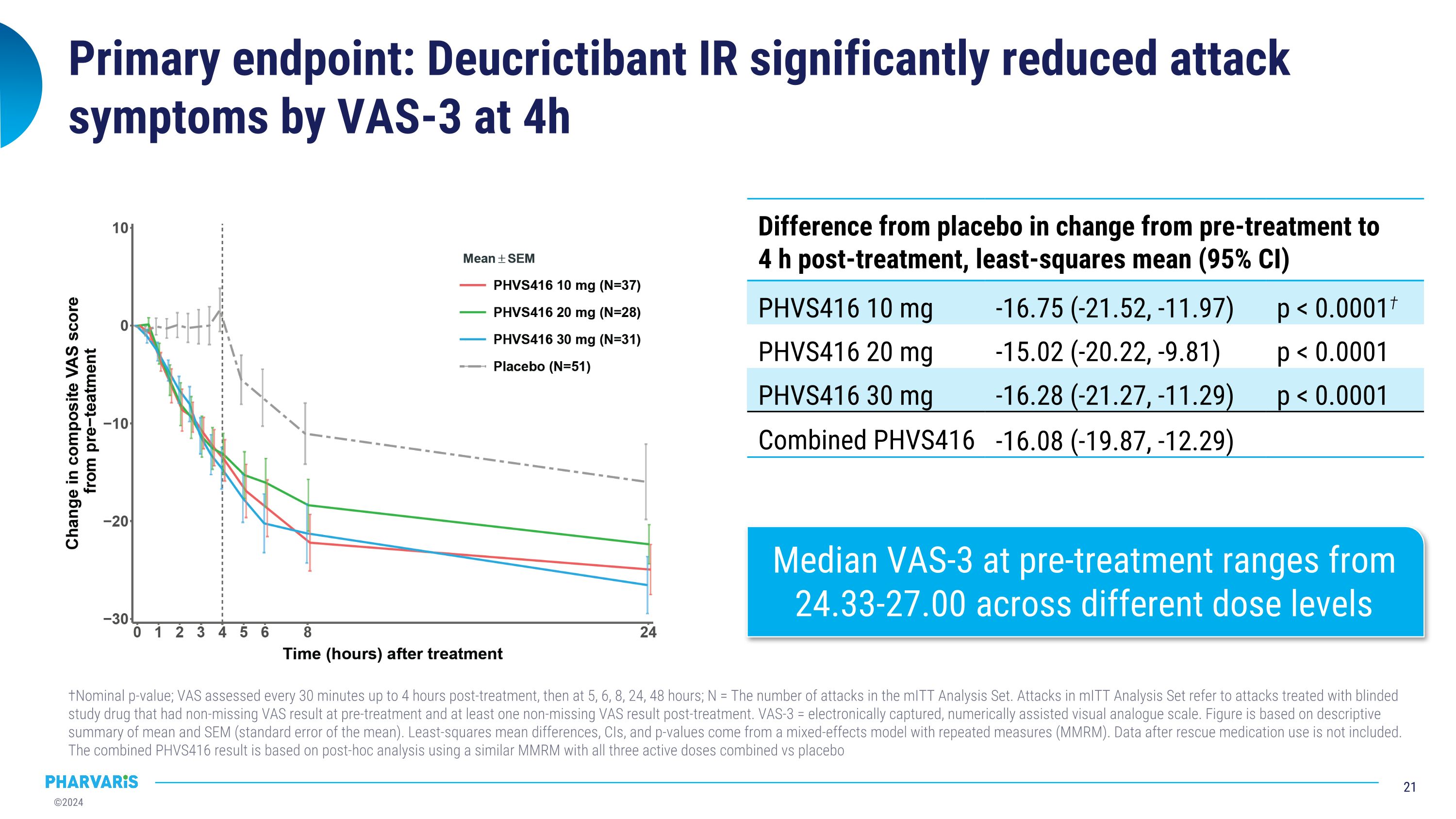
Primary endpoint: Deucrictibant IR significantly reduced attack symptoms by VAS-3 at 4h †Nominal p-value; VAS assessed every 30 minutes up to 4 hours post-treatment, then at 5, 6, 8, 24, 48 hours; N = The number of attacks in the mITT Analysis Set. Attacks in mITT Analysis Set refer to attacks treated with blinded study drug that had non-missing VAS result at pre-treatment and at least one non-missing VAS result post-treatment. VAS-3 = electronically captured, numerically assisted visual analogue scale. Figure is based on descriptive summary of mean and SEM (standard error of the mean). Least-squares mean differences, CIs, and p-values come from a mixed-effects model with repeated measures (MMRM). Data after rescue medication use is not included. The combined PHVS416 result is based on post-hoc analysis using a similar MMRM with all three active doses combined vs placebo Difference from placebo in change from pre-treatment to�4 h post-treatment, least-squares mean (95% CI) Median time in hours (95% CI) PHVS416 10 mg -16.75 (-21.52, -11.97) p < 0.0001† PHVS416 20 mg -15.02 (-20.22, -9.81) p < 0.0001 PHVS416 30 mg -16.28 (-21.27, -11.29) p < 0.0001 Combined PHVS416 -16.08 (-19.87, -12.29) Median VAS-3 at pre-treatment ranges from 24.33-27.00 across different dose levels
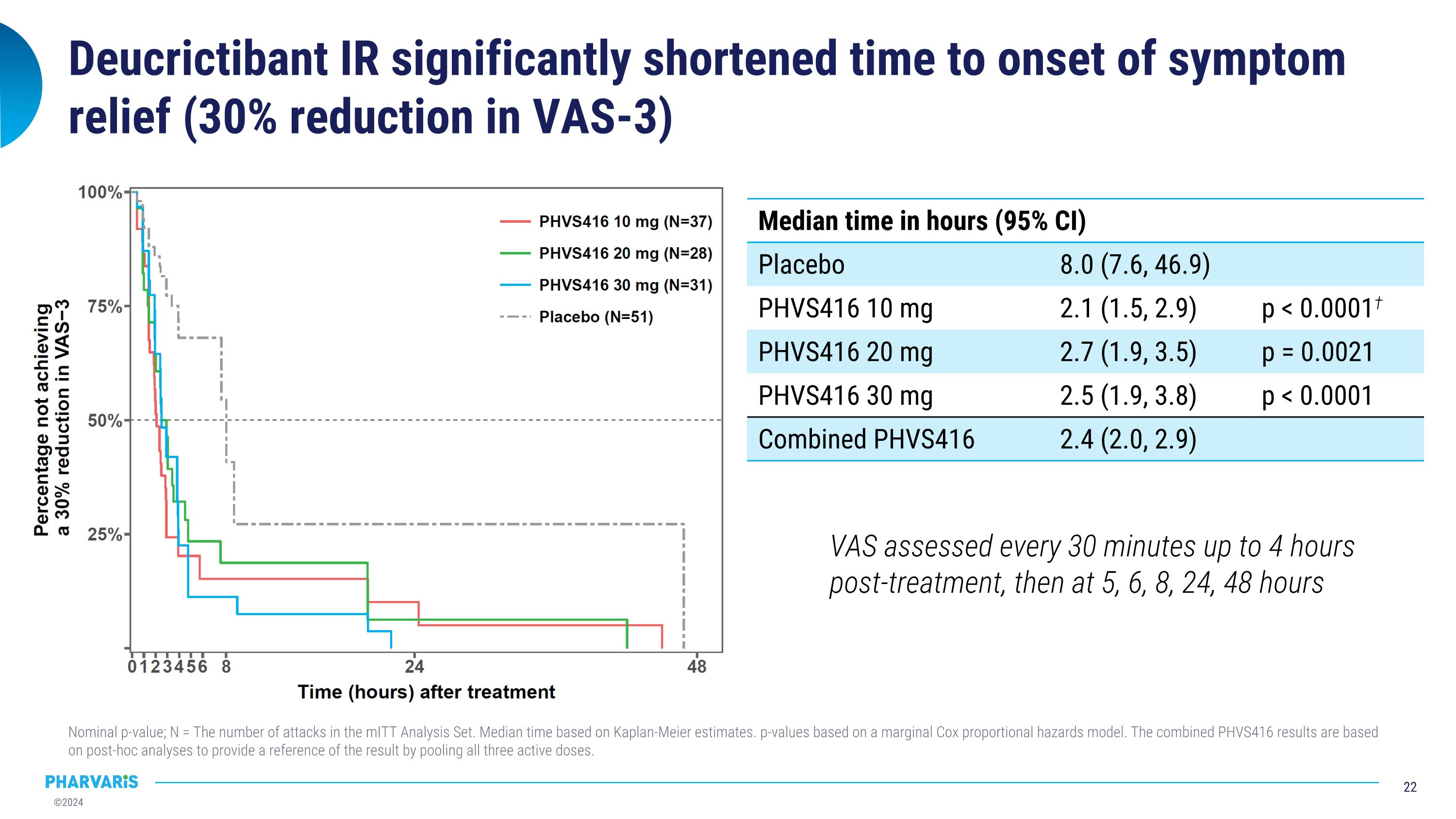
Deucrictibant IR significantly shortened time to onset of symptom relief (30% reduction in VAS-3) Nominal p-value; N = The number of attacks in the mITT Analysis Set. Median time based on Kaplan-Meier estimates. p-values based on a marginal Cox proportional hazards model. The combined PHVS416 results are based on post-hoc analyses to provide a reference of the result by pooling all three active doses. VAS assessed every 30 minutes up to 4 hours post-treatment, then at 5, 6, 8, 24, 48 hours Median time in hours (95% CI) Median time in hours (95% CI) Placebo 8.0 (7.6, 46.9) PHVS416 10 mg 2.1 (1.5, 2.9) p < 0.0001† PHVS416 20 mg 2.7 (1.9, 3.5) p = 0.0021 PHVS416 30 mg 2.5 (1.9, 3.8) p < 0.0001 Combined PHVS416 2.4 (2.0, 2.9)
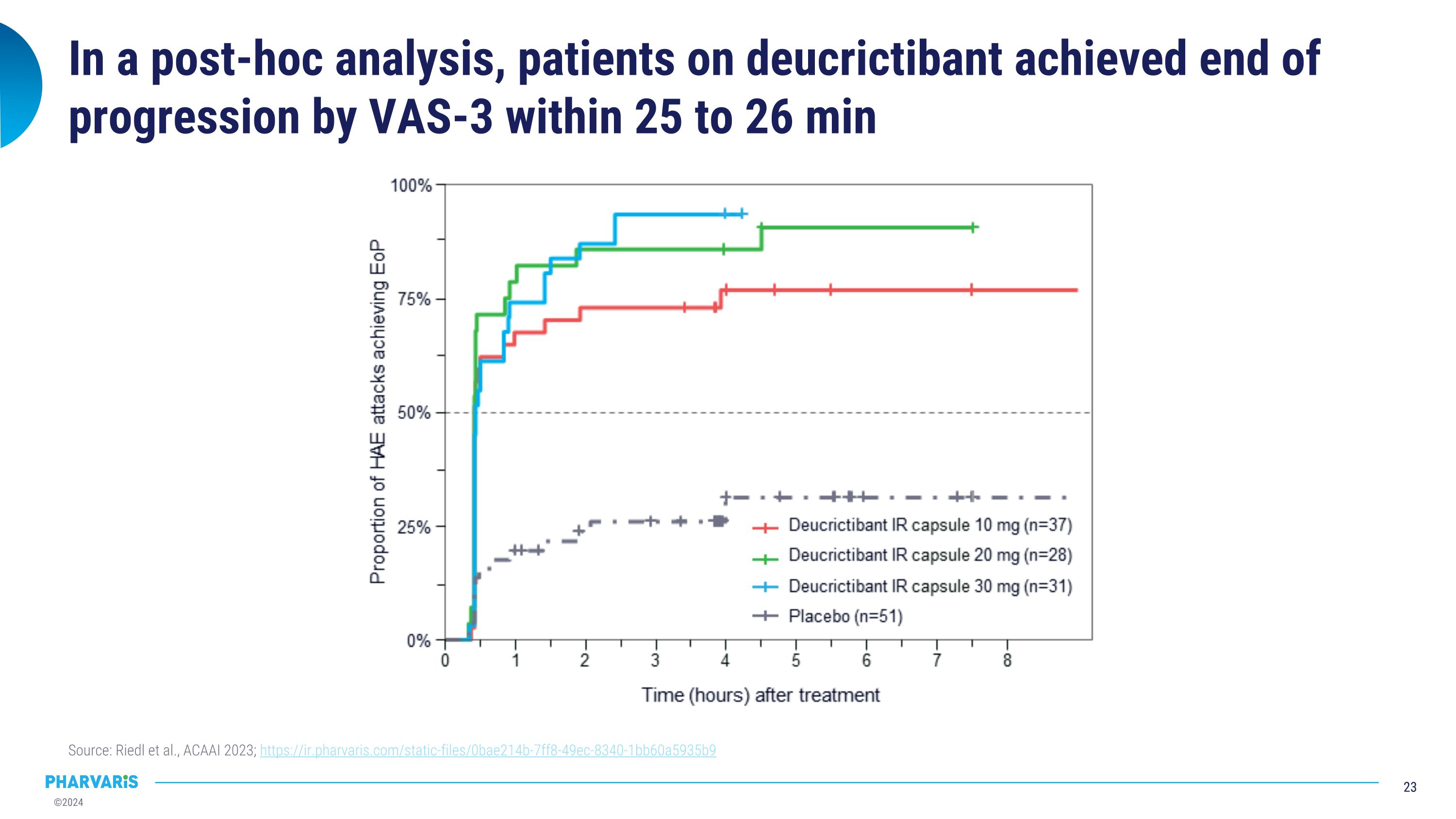
In a post-hoc analysis, patients on deucrictibant achieved end of progression by VAS-3 within 25 to 26 min Source: Riedl et al., ACAAI 2023; https://ir.pharvaris.com/static-files/0bae214b-7ff8-49ec-8340-1bb60a5935b9
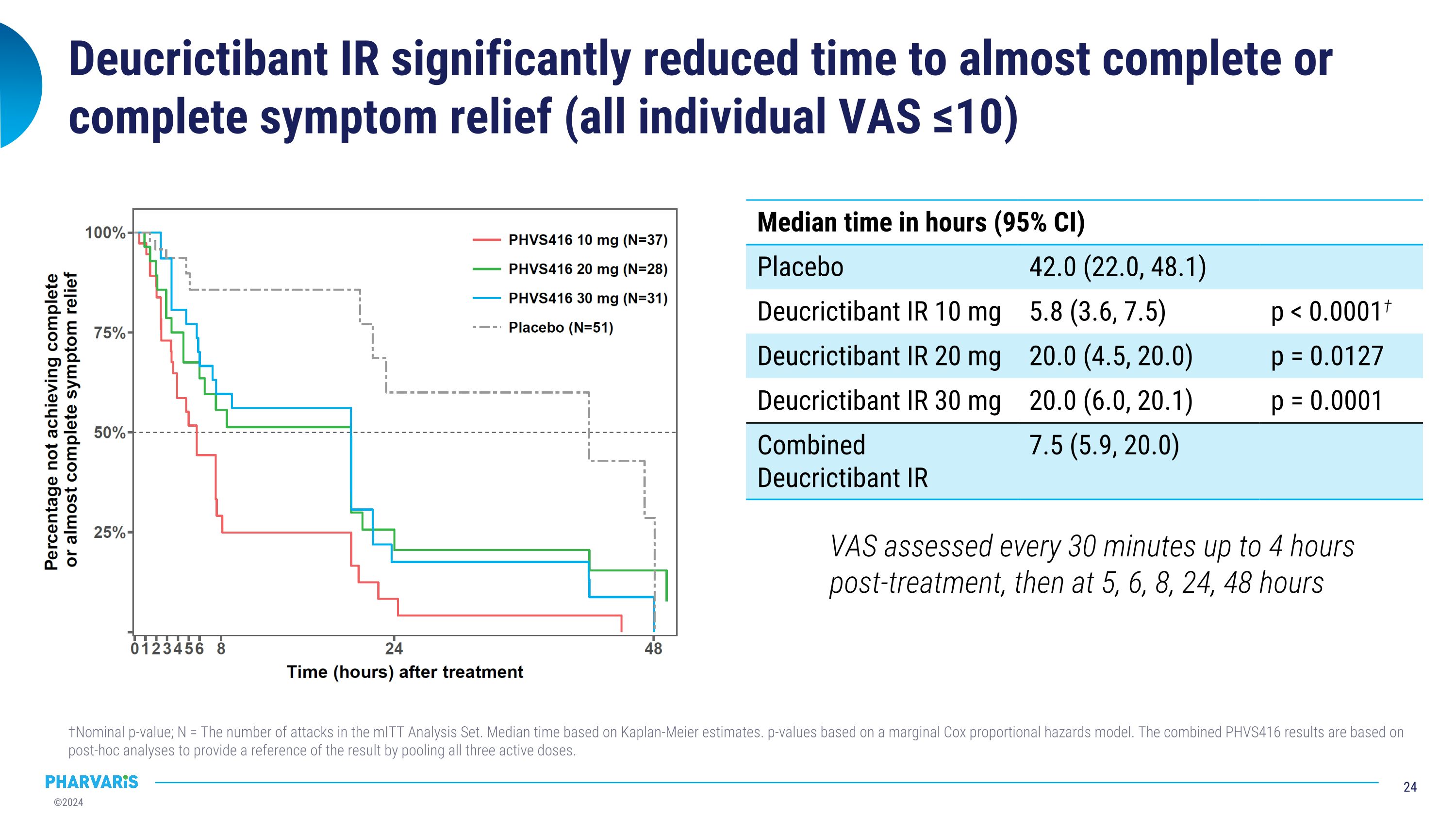
Deucrictibant IR significantly reduced time to almost complete or complete symptom relief (all individual VAS ≤10) †Nominal p-value; N = The number of attacks in the mITT Analysis Set. Median time based on Kaplan-Meier estimates. p-values based on a marginal Cox proportional hazards model. The combined PHVS416 results are based on post-hoc analyses to provide a reference of the result by pooling all three active doses. Median time in hours (95% CI) Median time in hours (95% CI) Placebo 42.0 (22.0, 48.1) Deucrictibant IR 10 mg 5.8 (3.6, 7.5) p < 0.0001† Deucrictibant IR 20 mg 20.0 (4.5, 20.0) p = 0.0127 Deucrictibant IR 30 mg 20.0 (6.0, 20.1) p = 0.0001 Combined Deucrictibant IR 7.5 (5.9, 20.0) VAS assessed every 30 minutes up to 4 hours post-treatment, then at 5, 6, 8, 24, 48 hours
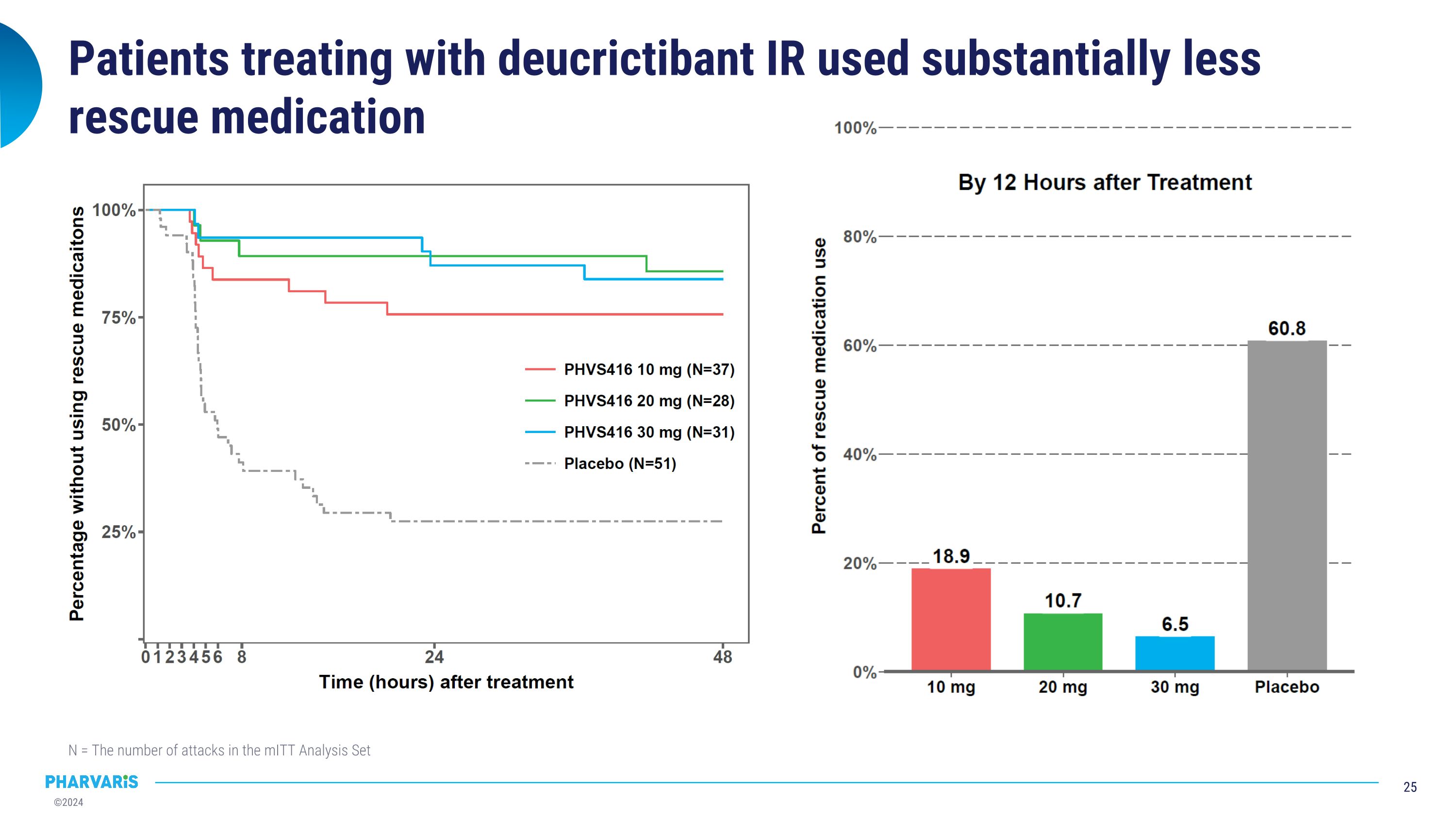
Patients treating with deucrictibant IR used substantially less rescue medication N = The number of attacks in the mITT Analysis Set
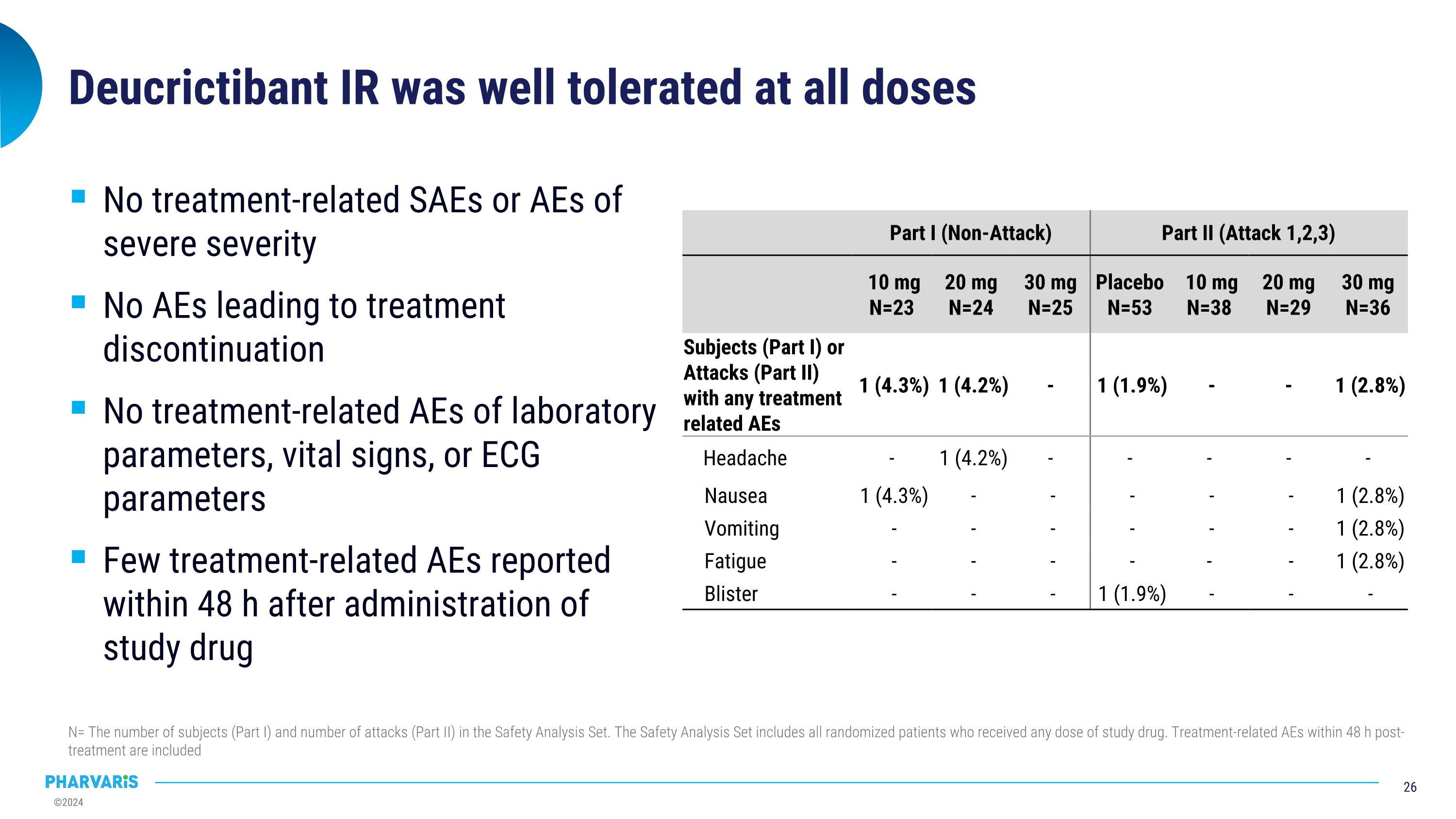
Deucrictibant IR was well tolerated at all doses N= The number of subjects (Part I) and number of attacks (Part II) in the Safety Analysis Set. The Safety Analysis Set includes all randomized patients who received any dose of study drug. Treatment-related AEs within 48 h post-treatment are included No treatment-related SAEs or AEs of severe severity No AEs leading to treatment discontinuation No treatment-related AEs of laboratory parameters, vital signs, or ECG parameters Few treatment-related AEs reported within 48 h after administration of study drug Part I (Non-Attack) Part II (Attack 1,2,3) Part II (Attack 1,2,3) 10 mg N=23 20 mg N=24 30 mg N=25 Placebo N=53 10 mg N=38 20 mg N=29 30 mg N=36 Subjects (Part I) or Attacks (Part II) with any treatment related AEs 1 (4.3%) 1 (4.2%) - 1 (1.9%) - - 1 (2.8%) Headache - 1 (4.2%) - - - - - Nausea 1 (4.3%) - - - - - 1 (2.8%) Vomiting - - - - - - 1 (2.8%) Fatigue - - - - - - 1 (2.8%) Blister - - - 1 (1.9%) - - -
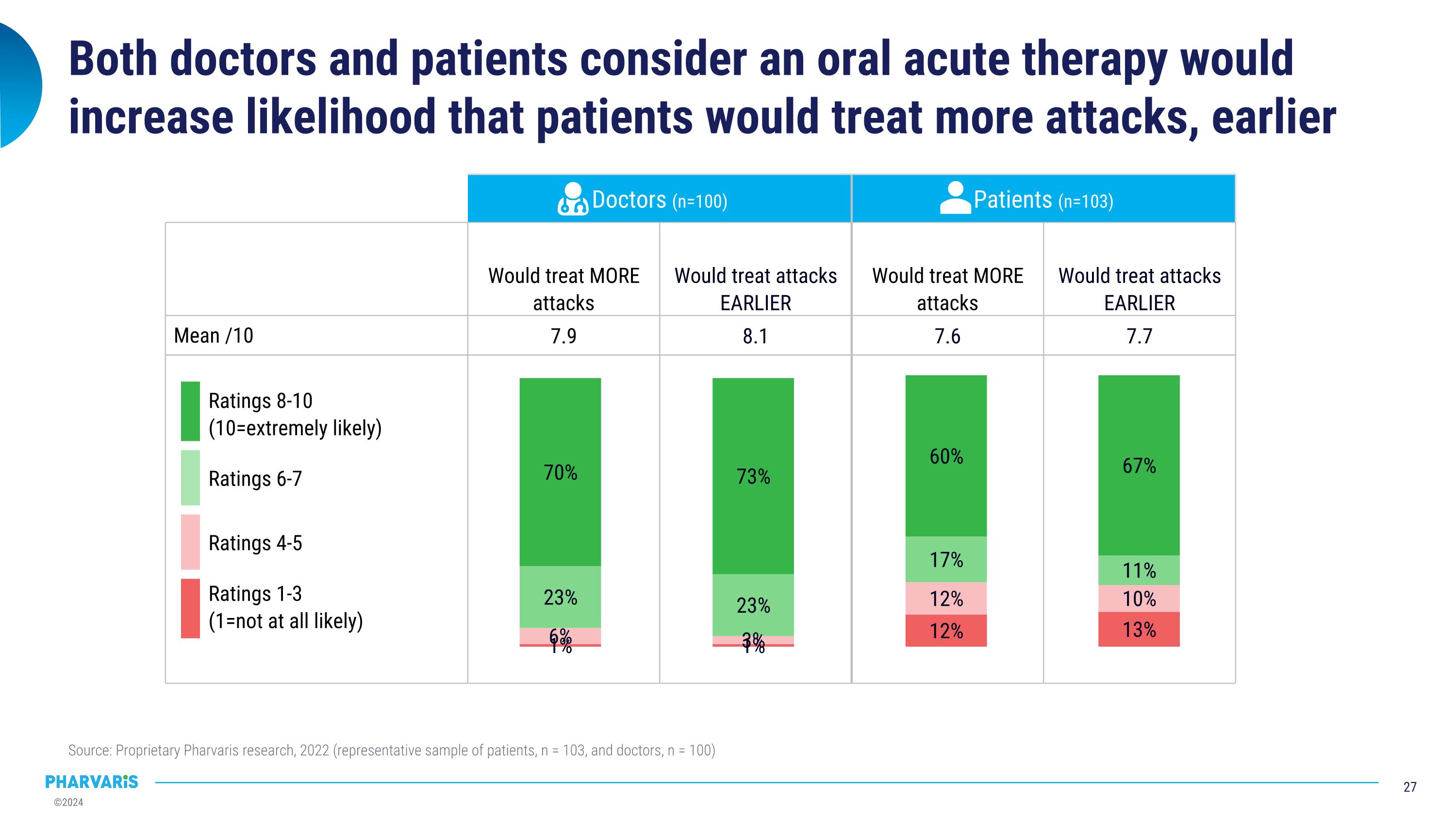
Both doctors and patients consider an oral acute therapy would increase likelihood that patients would treat more attacks, earlier Source: Proprietary Pharvaris research, 2022 (representative sample of patients, n = 103, and doctors, n = 100) Doctors (n=100) Patients (n=103) Anticipated impact of ORAL acute therapy on attacks treated Would treat MORE attacks Would treat attacks EARLIER Would treat MORE attacks Would treat attacks EARLIER Mean /10 7.9 8.1 7.6 7.7 Ratings 8-10 �(10=extremely likely) Ratings 6-7 Ratings 4-5 Ratings 1-3�(1=not at all likely)
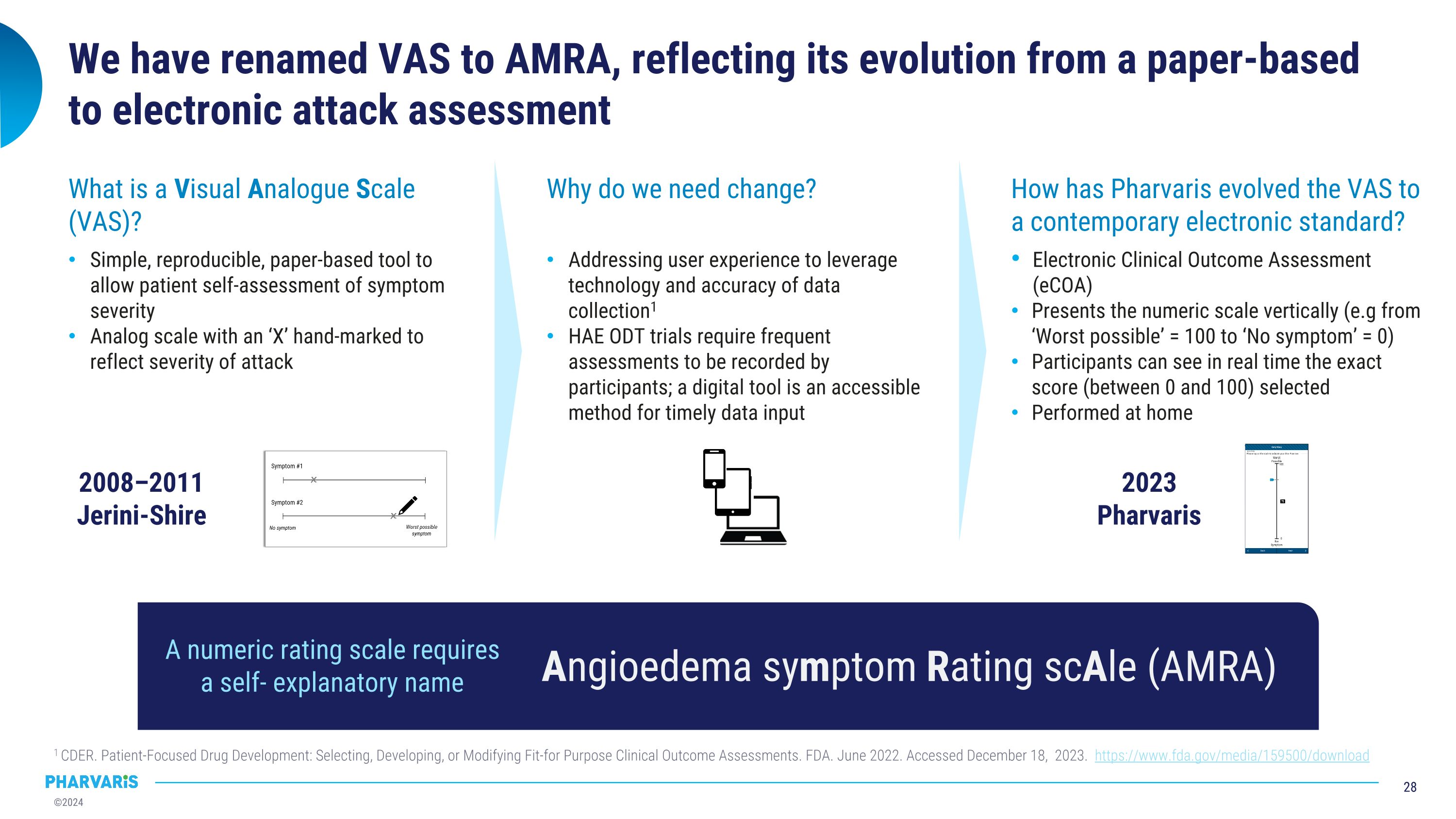
We have renamed VAS to AMRA, reflecting its evolution from a paper-based to electronic attack assessment 2008–2011 �Jerini-Shire Why do we need change? Addressing user experience to leverage technology and accuracy of data collection1 HAE ODT trials require frequent assessments to be recorded by participants; a digital tool is an accessible method for timely data input 2023 Pharvaris How has Pharvaris evolved the VAS to a contemporary electronic standard? Electronic Clinical Outcome Assessment (eCOA) Presents the numeric scale vertically (e.g from ‘Worst possible’ = 100 to ‘No symptom’ = 0) Participants can see in real time the exact score (between 0 and 100) selected Performed at home 1 CDER. Patient-Focused Drug Development: Selecting, Developing, or Modifying Fit-for Purpose Clinical Outcome Assessments. FDA. June 2022. Accessed December 18, 2023. https://www.fda.gov/media/159500/download What is a Visual Analogue Scale (VAS)? Simple, reproducible, paper-based tool to allow patient self-assessment of symptom severity Analog scale with an ‘X’ hand-marked to reflect severity of attack Angioedema symptom Rating scAle (AMRA) A numeric rating scale requires a self- explanatory name
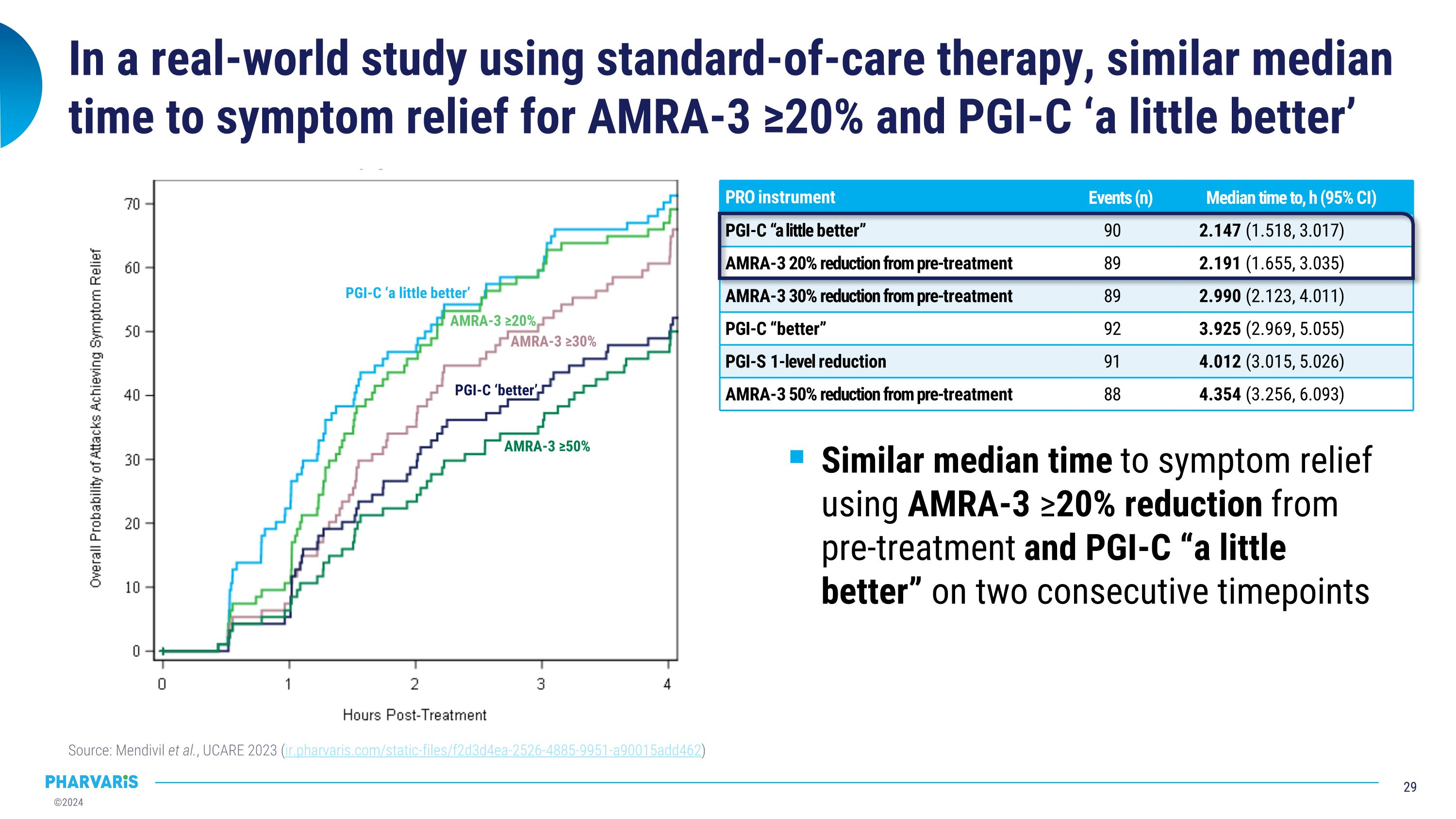
In a real-world study using standard-of-care therapy, similar median time to symptom relief for AMRA-3 ≥20% and PGI-C ‘a little better’ Source: Mendivil et al., UCARE 2023 (ir.pharvaris.com/static-files/f2d3d4ea-2526-4885-9951-a90015add462) PRO instrument Events (n) Median time to, h (95% CI) PGI-C “a little better” 90 2.147 (1.518, 3.017) AMRA-3 20% reduction from pre-treatment 89 2.191 (1.655, 3.035) AMRA-3 30% reduction from pre-treatment 89 2.990 (2.123, 4.011) PGI-C “better” 92 3.925 (2.969, 5.055) PGI-S 1-level reduction 91 4.012 (3.015, 5.026) AMRA-3 50% reduction from pre-treatment 88 4.354 (3.256, 6.093) Similar median time to symptom relief using AMRA-3 ≥20% reduction from pre-treatment and PGI-C “a little better” on two consecutive timepoints PGI-C ‘a little better’ AMRA-3 ≥20% AMRA-3 ≥30% PGI-C ‘better’ AMRA-3 ≥50%
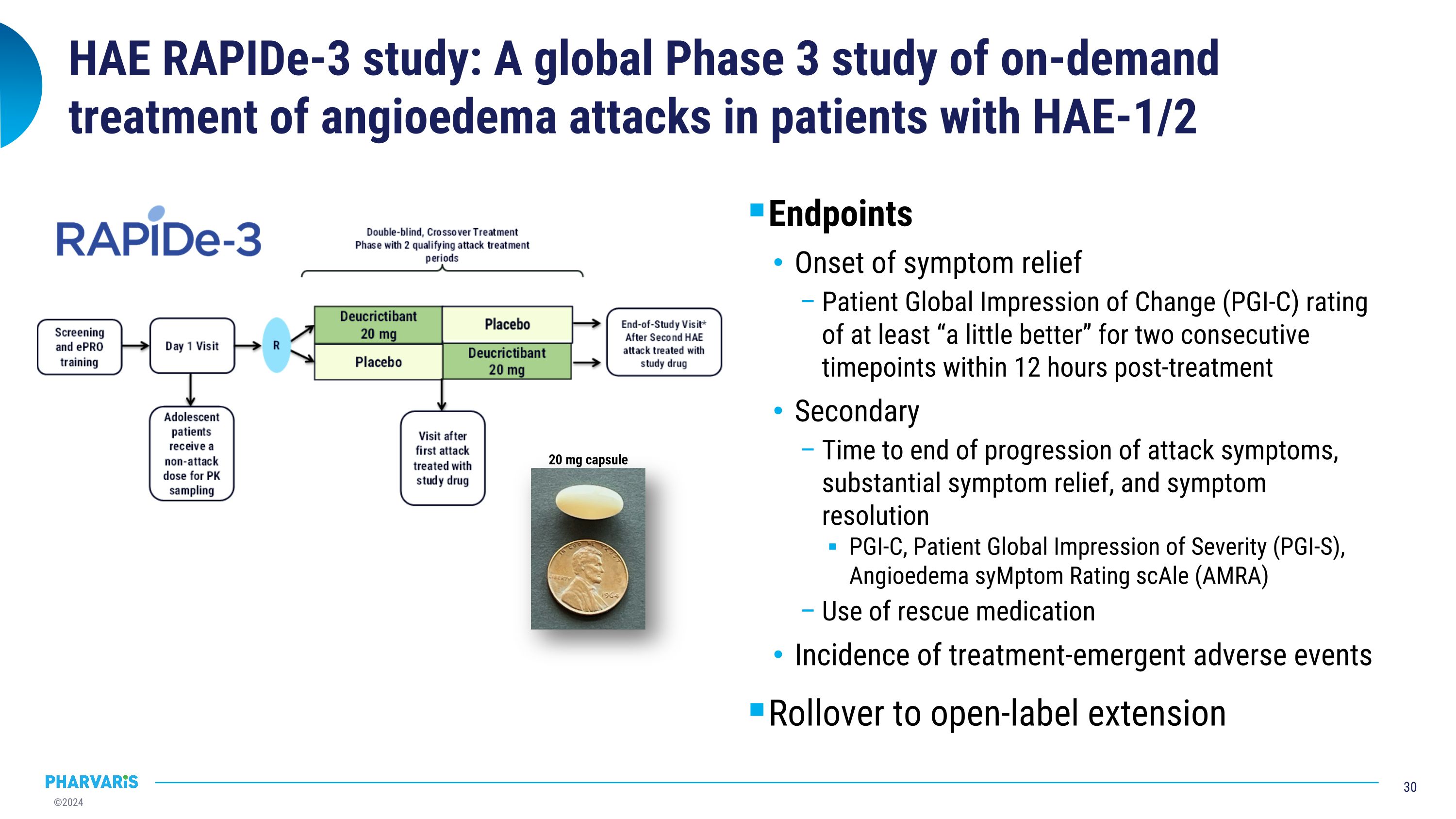
HAE RAPIDe-3 study: A global Phase 3 study of on-demand treatment of angioedema attacks in patients with HAE-1/2 Endpoints Onset of symptom relief Patient Global Impression of Change (PGI-C) rating of at least “a little better” for two consecutive timepoints within 12 hours post-treatment Secondary Time to end of progression of attack symptoms, substantial symptom relief, and symptom resolution PGI-C, Patient Global Impression of Severity (PGI-S), Angioedema syMptom Rating scAle (AMRA) Use of rescue medication Incidence of treatment-emergent adverse events Rollover to open-label extension 20 mg capsule
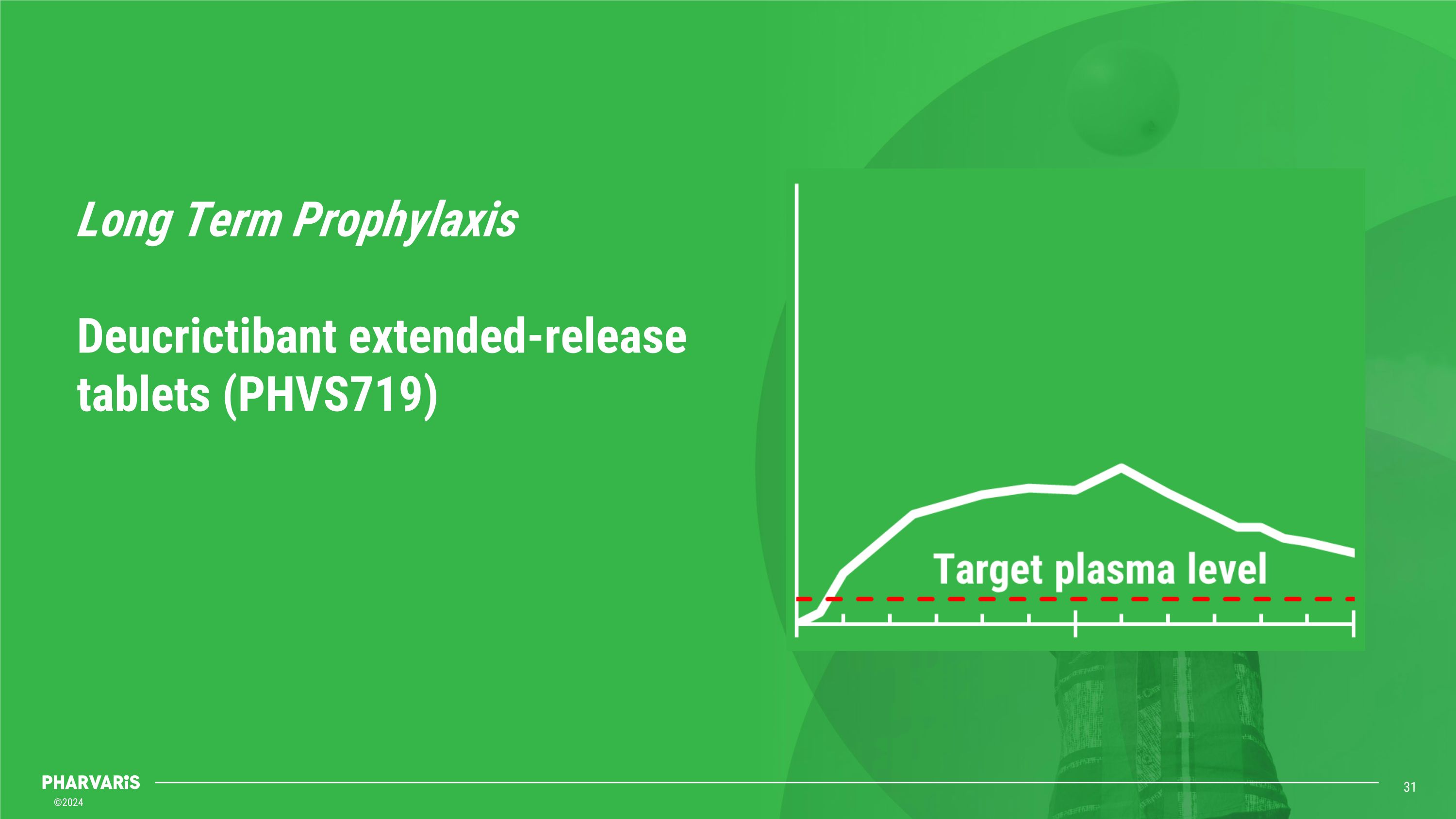
Long Term Prophylaxis��Deucrictibant extended-release tablets (PHVS719)
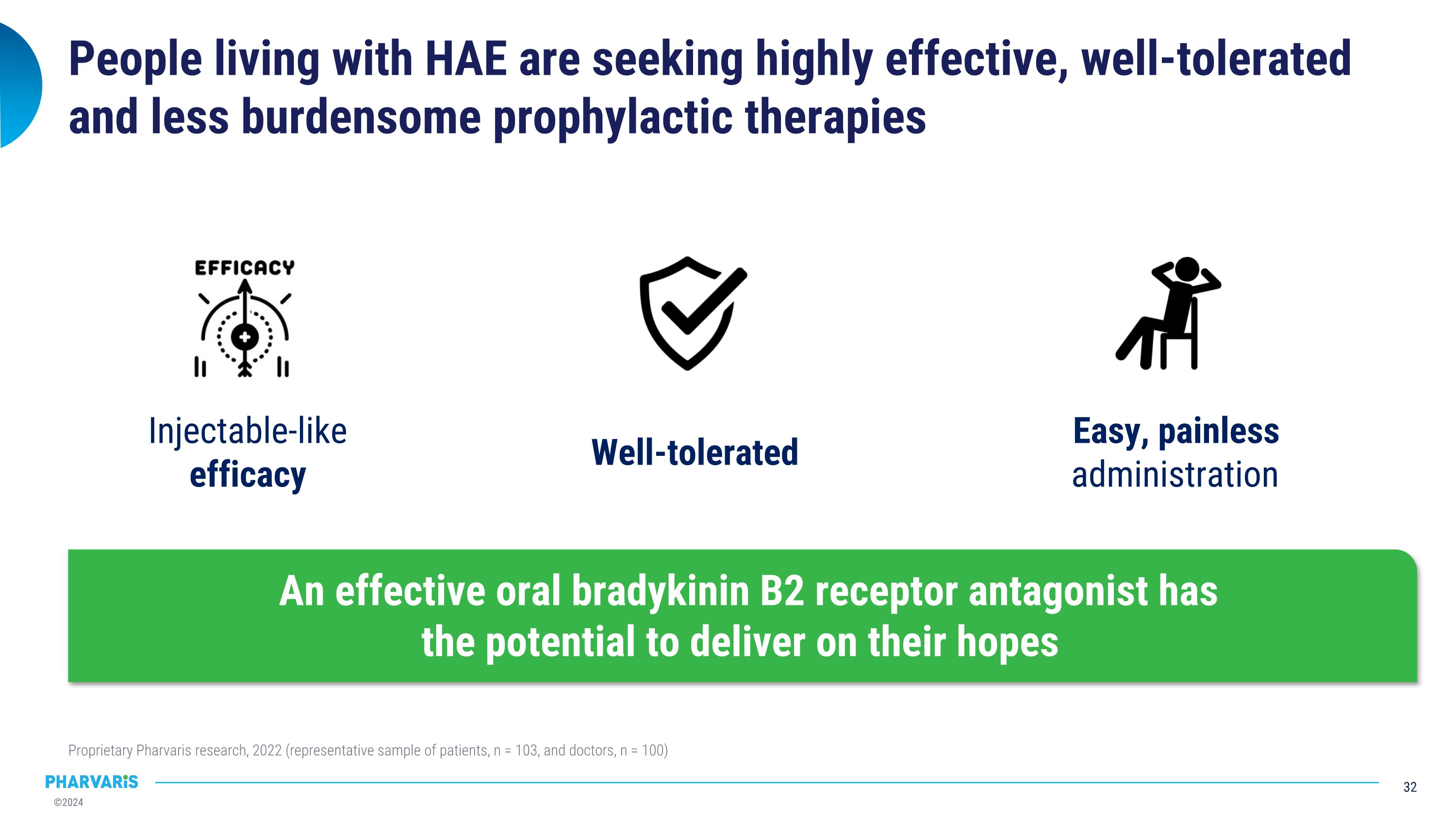
Injectable-like efficacy Easy, painless administration People living with HAE are seeking highly effective, well-tolerated and less burdensome prophylactic therapies Proprietary Pharvaris research, 2022 (representative sample of patients, n = 103, and doctors, n = 100) An effective oral bradykinin B2 receptor antagonist has�the potential to deliver on their hopes Well-tolerated
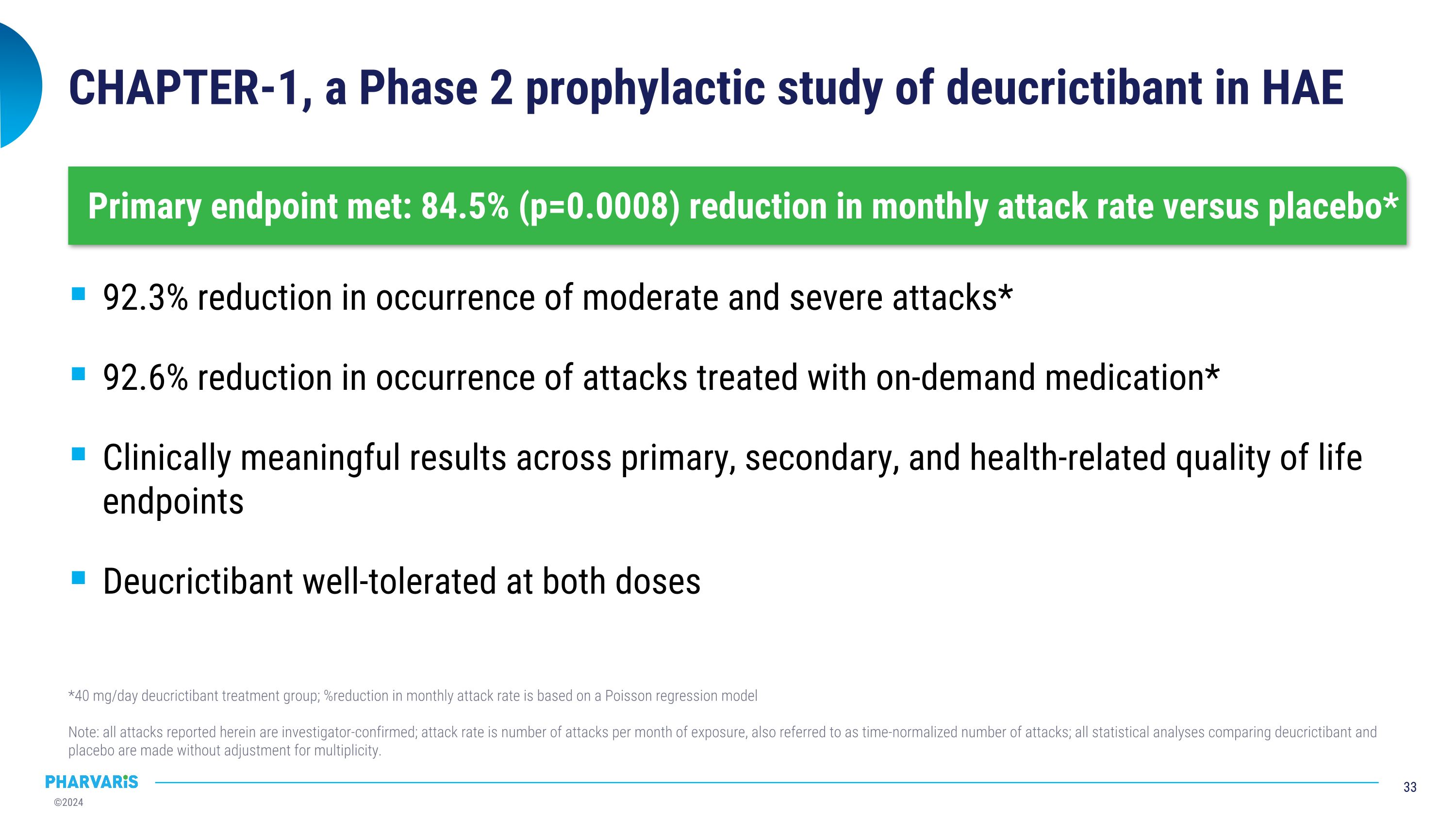
CHAPTER-1, a Phase 2 prophylactic study of deucrictibant in HAE 92.3% reduction in occurrence of moderate and severe attacks* 92.6% reduction in occurrence of attacks treated with on-demand medication* Clinically meaningful results across primary, secondary, and health-related quality of life endpoints Deucrictibant well-tolerated at both doses *40 mg/day deucrictibant treatment group; %reduction in monthly attack rate is based on a Poisson regression model Note: all attacks reported herein are investigator-confirmed; attack rate is number of attacks per month of exposure, also referred to as time-normalized number of attacks; all statistical analyses comparing deucrictibant and placebo are made without adjustment for multiplicity. Primary endpoint met: 84.5% (p=0.0008) reduction in monthly attack rate versus placebo*
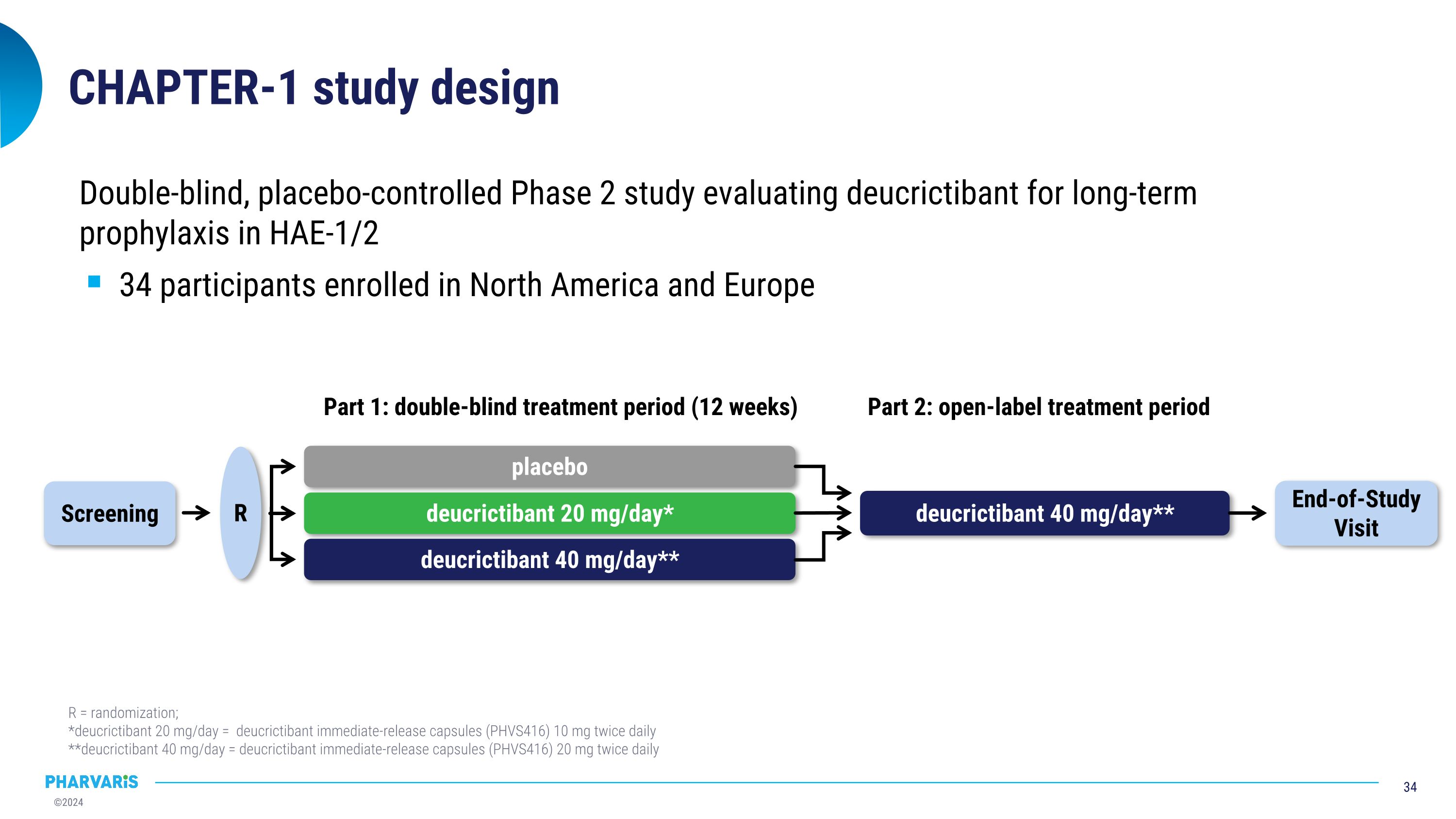
CHAPTER-1 study design Double-blind, placebo-controlled Phase 2 study evaluating deucrictibant for long-term prophylaxis in HAE-1/2 34 participants enrolled in North America and Europe R = randomization; *deucrictibant 20 mg/day = deucrictibant immediate-release capsules (PHVS416) 10 mg twice daily **deucrictibant 40 mg/day = deucrictibant immediate-release capsules (PHVS416) 20 mg twice daily placebo deucrictibant 20 mg/day* deucrictibant 40 mg/day** Part 1: double-blind treatment period (12 weeks) Screening R End-of-Study Visit deucrictibant 40 mg/day** Part 2: open-label treatment period
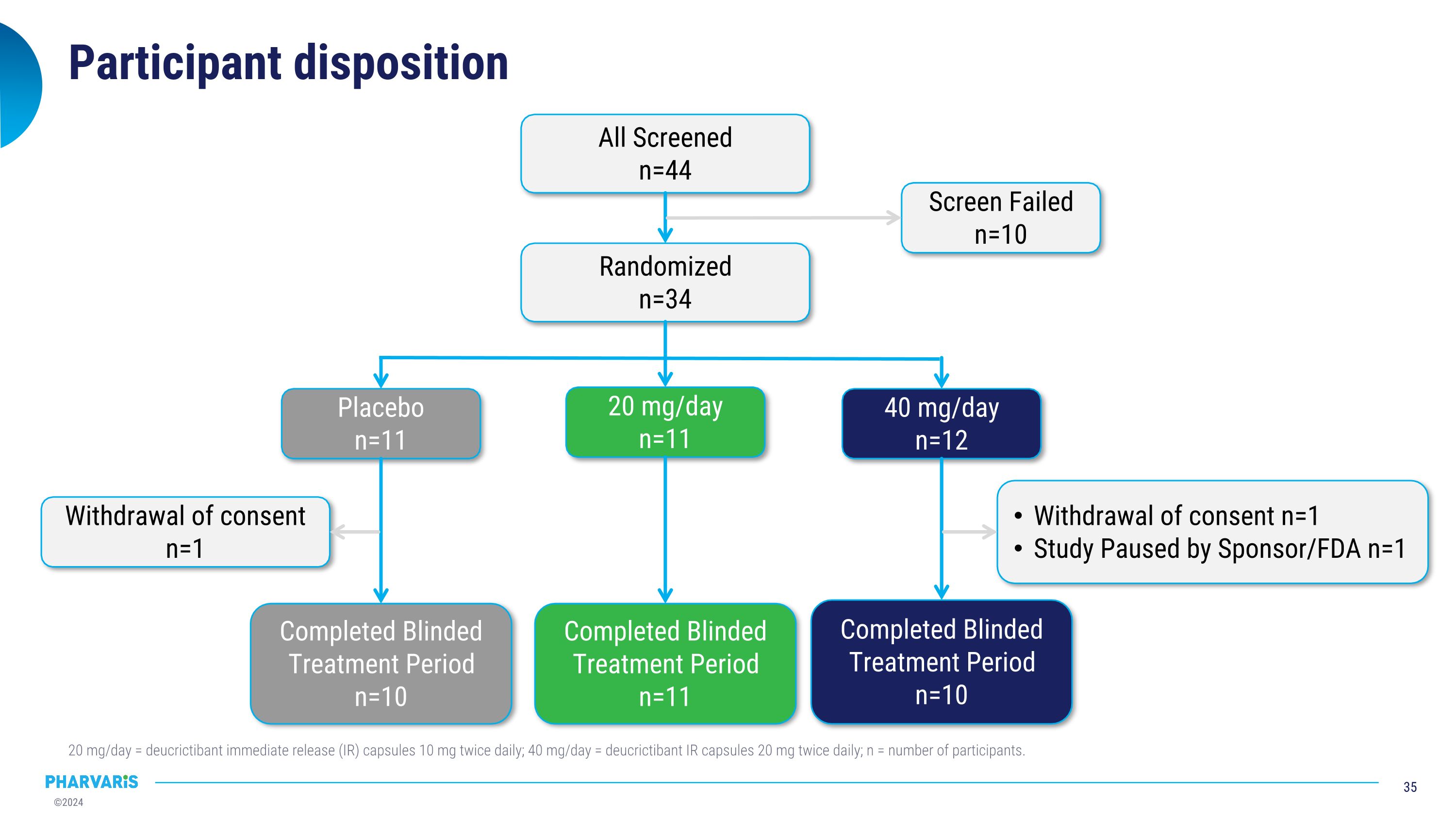
Participant disposition 20 mg/day = deucrictibant immediate release (IR) capsules 10 mg twice daily; 40 mg/day = deucrictibant IR capsules 20 mg twice daily; n = number of participants. All Screened n=44 Randomized n=34 Screen Failed n=10 Placebo n=11 20 mg/day n=11 40 mg/day n=12 Completed Blinded Treatment Period n=10 Withdrawal of consent n=1 Completed Blinded Treatment Period n=10 Withdrawal of consent n=1 Study Paused by Sponsor/FDA n=1 Completed Blinded Treatment Period n=11
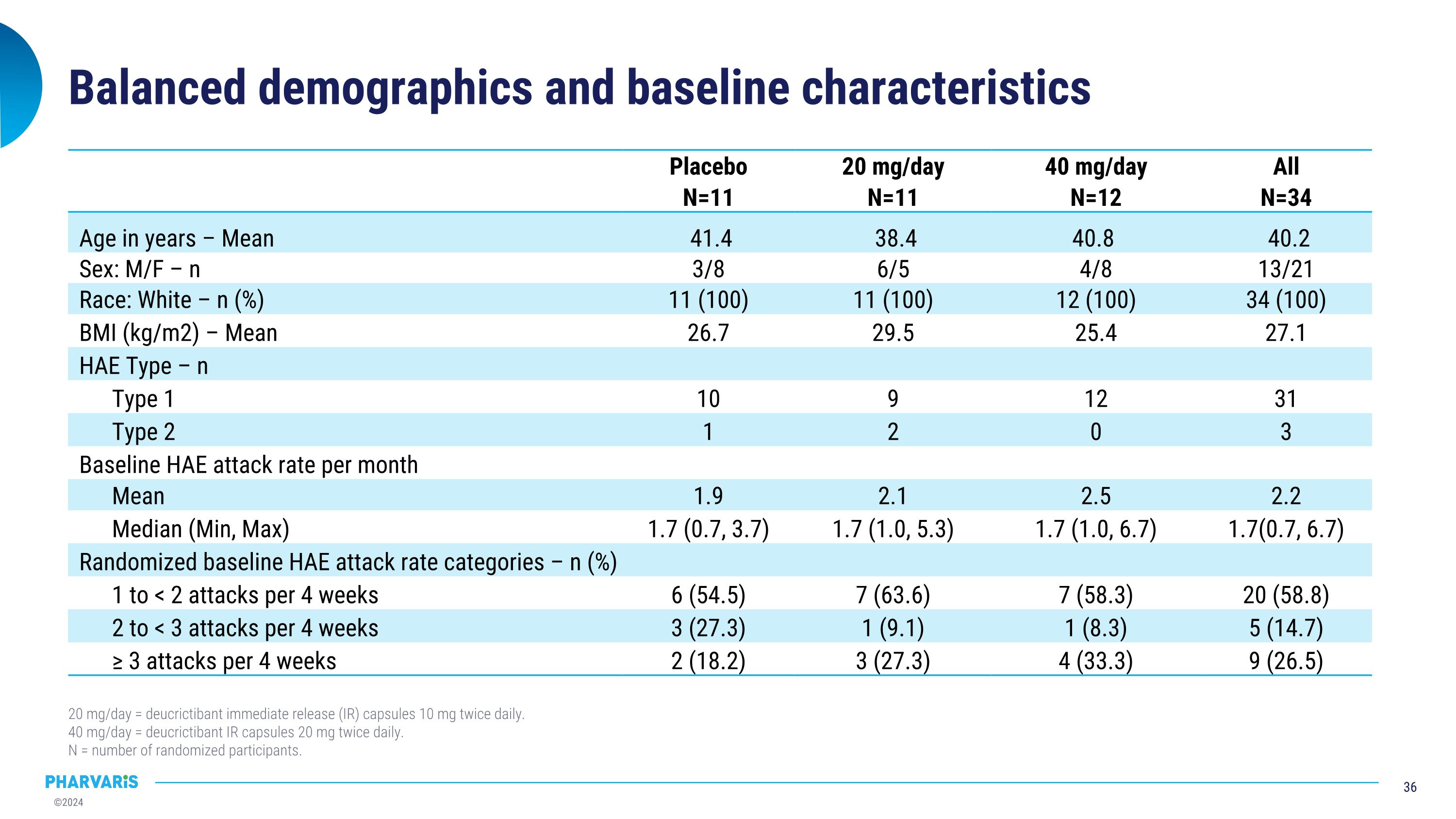
Balanced demographics and baseline characteristics 20 mg/day = deucrictibant immediate release (IR) capsules 10 mg twice daily. 40 mg/day = deucrictibant IR capsules 20 mg twice daily. N = number of randomized participants. Placebo N=11 20 mg/day N=11 40 mg/day N=12 All N=34 Age in years – Mean 41.4 38.4 40.8 40.2 Sex: M/F – n 3/8 6/5 4/8 13/21 Race: White – n (%) 11 (100) 11 (100) 12 (100) 34 (100) BMI (kg/m2) – Mean 26.7 29.5 25.4 27.1 HAE Type – n Type 1 10 9 12 31 Type 2 1 2 0 3 Baseline HAE attack rate per month Mean 1.9 2.1 2.5 2.2 Median (Min, Max) 1.7 (0.7, 3.7) 1.7 (1.0, 5.3) 1.7 (1.0, 6.7) 1.7(0.7, 6.7) Randomized baseline HAE attack rate categories – n (%) 1 to < 2 attacks per 4 weeks 6 (54.5) 7 (63.6) 7 (58.3) 20 (58.8) 2 to < 3 attacks per 4 weeks 3 (27.3) 1 (9.1) 1 (8.3) 5 (14.7) ≥ 3 attacks per 4 weeks 2 (18.2) 3 (27.3) 4 (33.3) 9 (26.5)
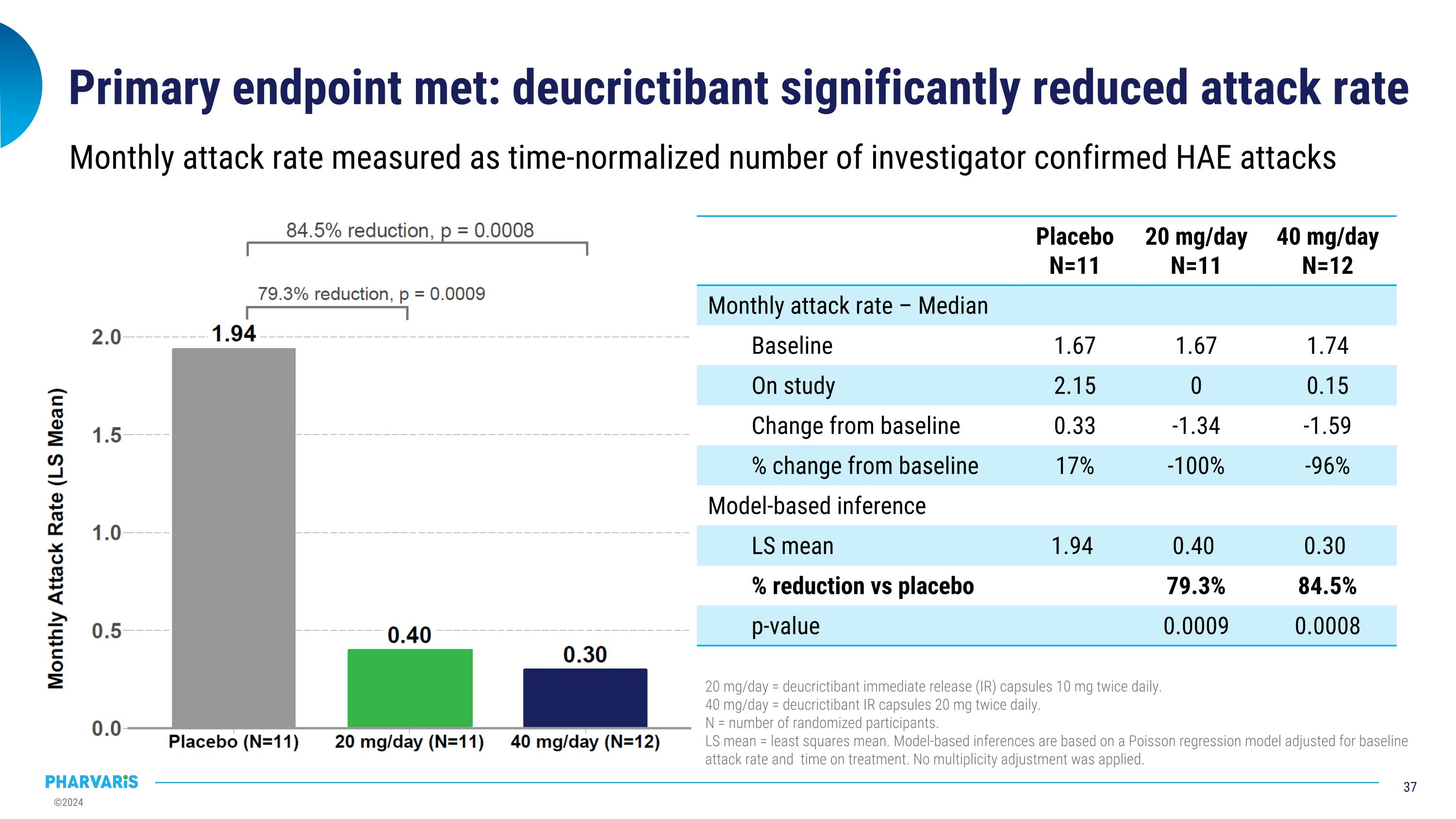
Primary endpoint met: deucrictibant significantly reduced attack rate Monthly attack rate measured as time-normalized number of investigator confirmed HAE attacks Placebo N=11 20 mg/day N=11 40 mg/day N=12 Monthly attack rate – Median Baseline 1.67 1.67 1.74 On study 2.15 0 0.15 Change from baseline 0.33 -1.34 -1.59 % change from baseline 17% -100% -96% Model-based inference LS mean 1.94 0.40 0.30 % reduction vs placebo 79.3% 84.5% p-value 0.0009 0.0008 20 mg/day = deucrictibant immediate release (IR) capsules 10 mg twice daily. 40 mg/day = deucrictibant IR capsules 20 mg twice daily. N = number of randomized participants. LS mean = least squares mean. Model-based inferences are based on a Poisson regression model adjusted for baseline attack rate and time on treatment. No multiplicity adjustment was applied.
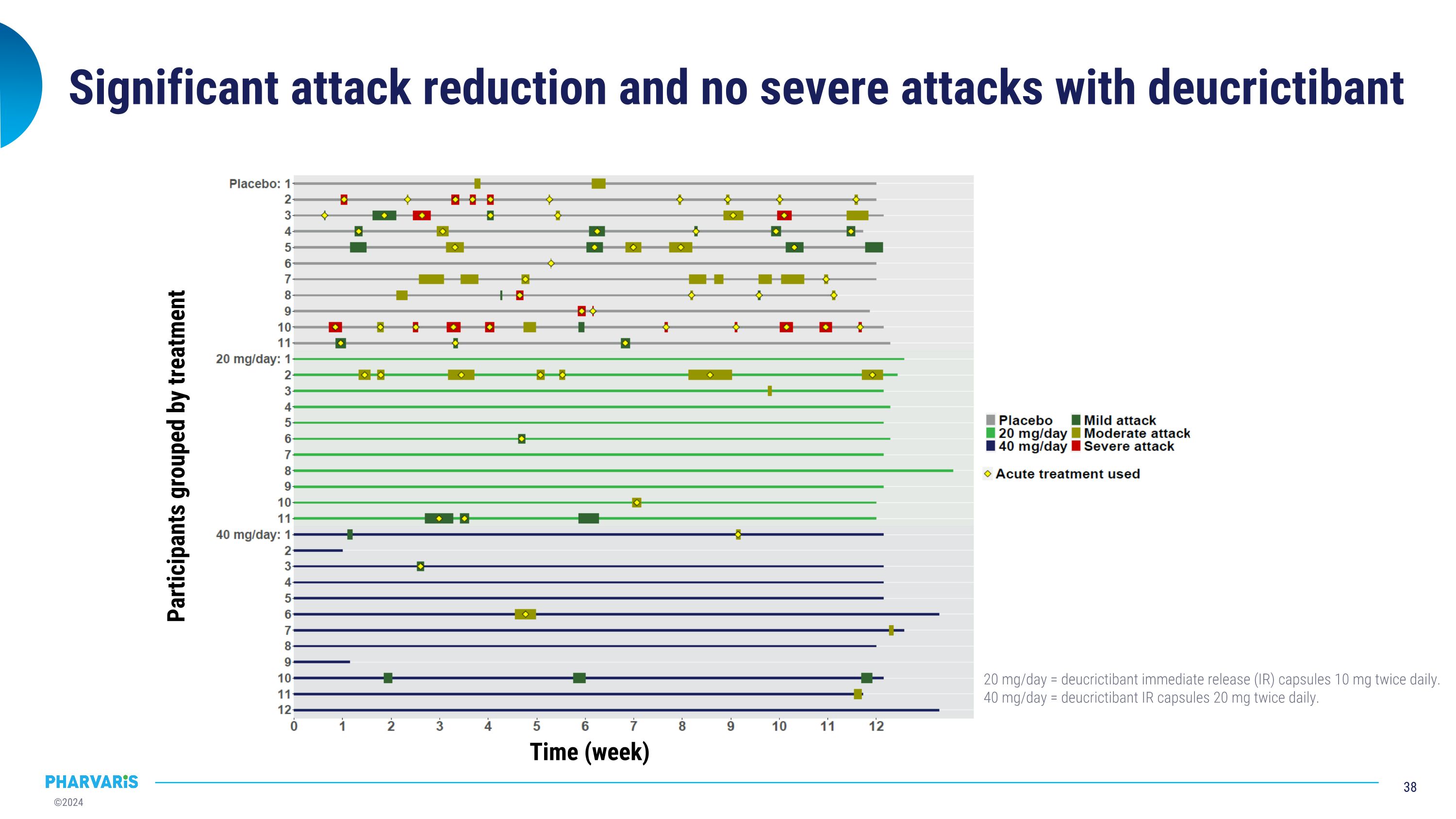
Significant attack reduction and no severe attacks with deucrictibant Participants grouped by treatment Time (week) 20 mg/day = deucrictibant immediate release (IR) capsules 10 mg twice daily. 40 mg/day = deucrictibant IR capsules 20 mg twice daily.
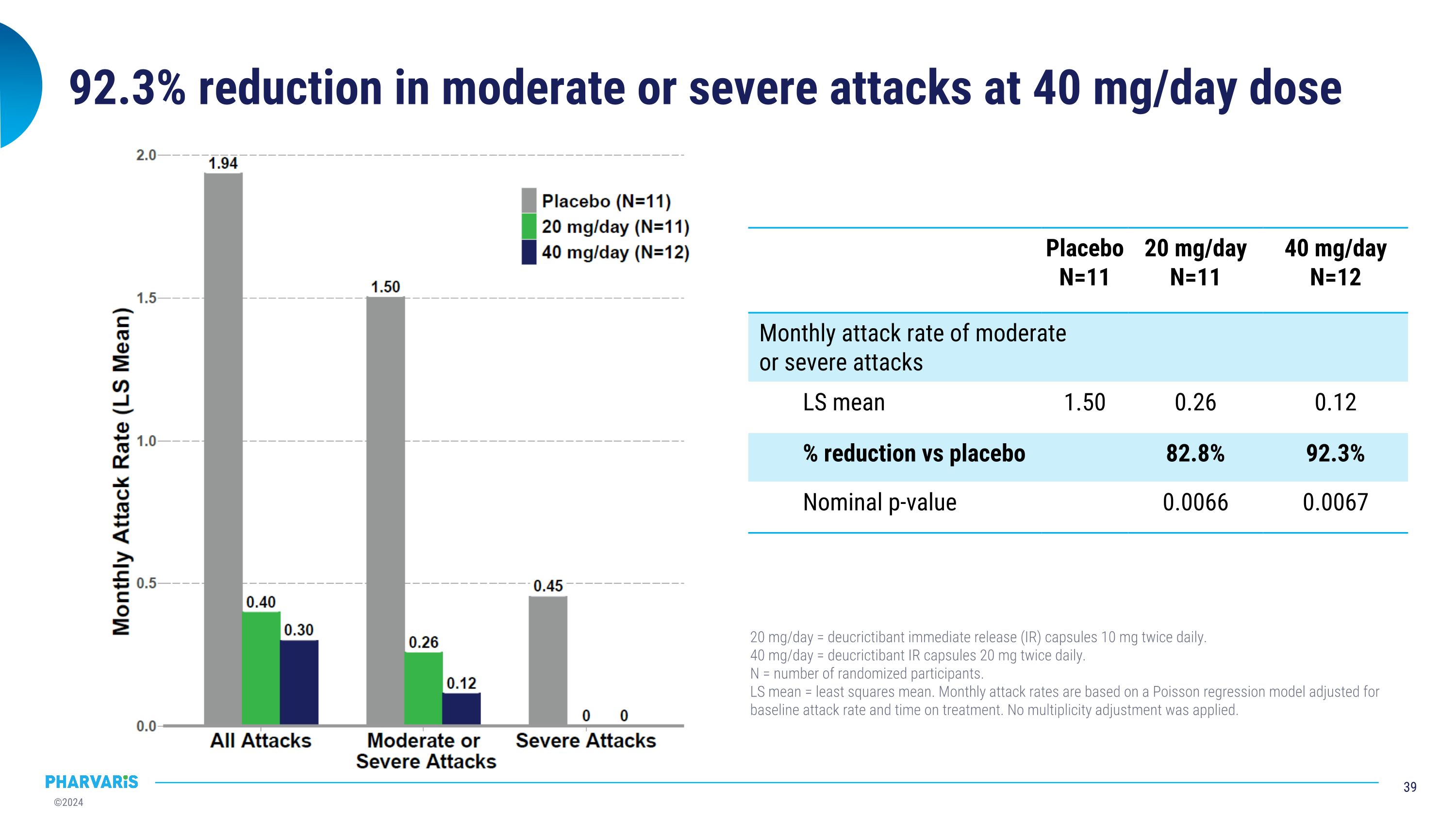
92.3% reduction in moderate or severe attacks at 40 mg/day dose Placebo N=11 20 mg/day N=11 40 mg/day N=12 Monthly attack rate of moderate or severe attacks LS mean 1.50 0.26 0.12 % reduction vs placebo 82.8% 92.3% Nominal p-value 0.0066 0.0067 20 mg/day = deucrictibant immediate release (IR) capsules 10 mg twice daily. 40 mg/day = deucrictibant IR capsules 20 mg twice daily. N = number of randomized participants. LS mean = least squares mean. Monthly attack rates are based on a Poisson regression model adjusted for baseline attack rate and time on treatment. No multiplicity adjustment was applied.
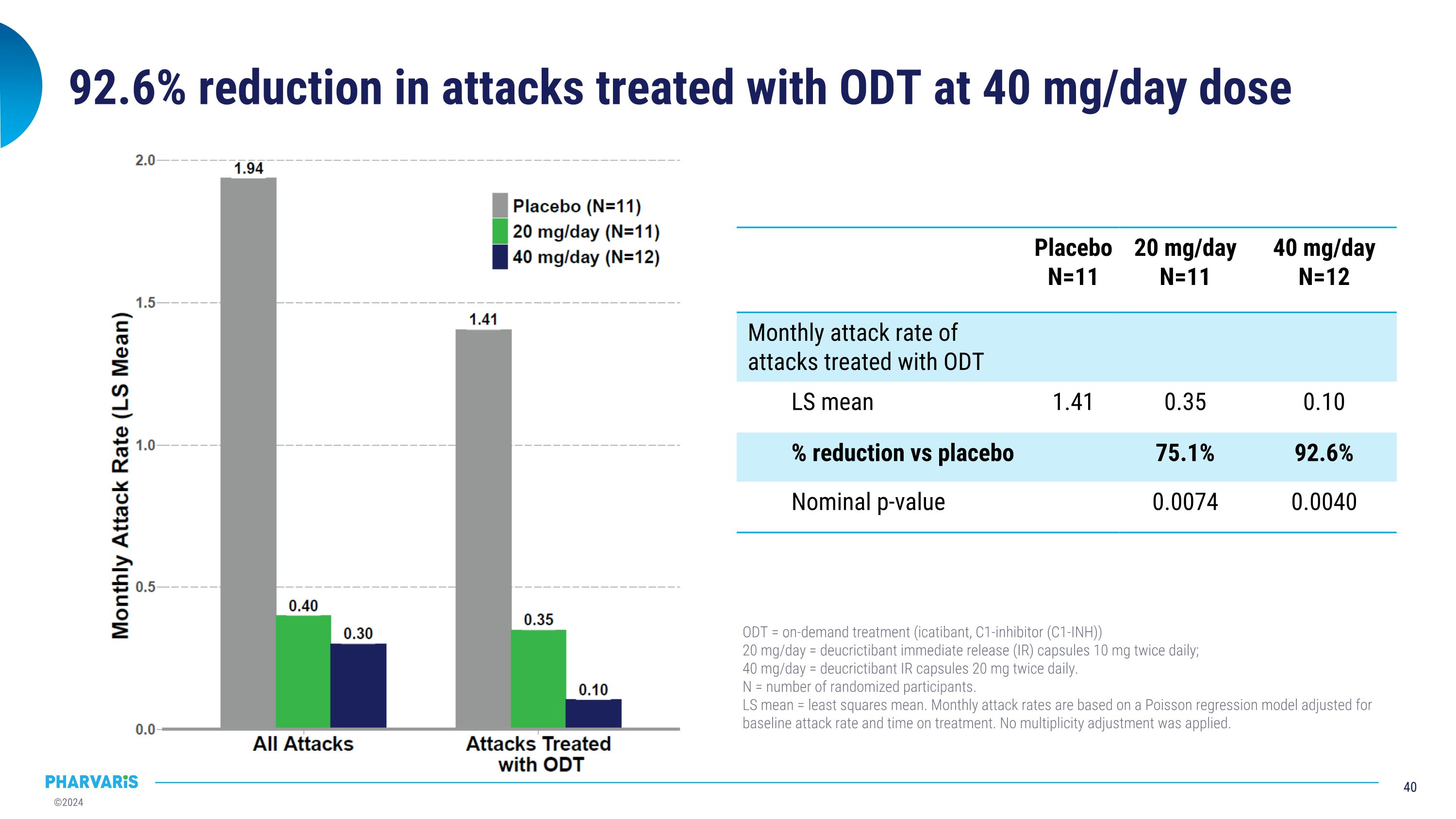
92.6% reduction in attacks treated with ODT at 40 mg/day dose Placebo N=11 20 mg/day N=11 40 mg/day N=12 Monthly attack rate of attacks treated with ODT LS mean 1.41 0.35 0.10 % reduction vs placebo 75.1% 92.6% Nominal p-value 0.0074 0.0040 ODT = on-demand treatment (icatibant, C1-inhibitor (C1-INH)) 20 mg/day = deucrictibant immediate release (IR) capsules 10 mg twice daily; 40 mg/day = deucrictibant IR capsules 20 mg twice daily. N = number of randomized participants. LS mean = least squares mean. Monthly attack rates are based on a Poisson regression model adjusted for baseline attack rate and time on treatment. No multiplicity adjustment was applied.
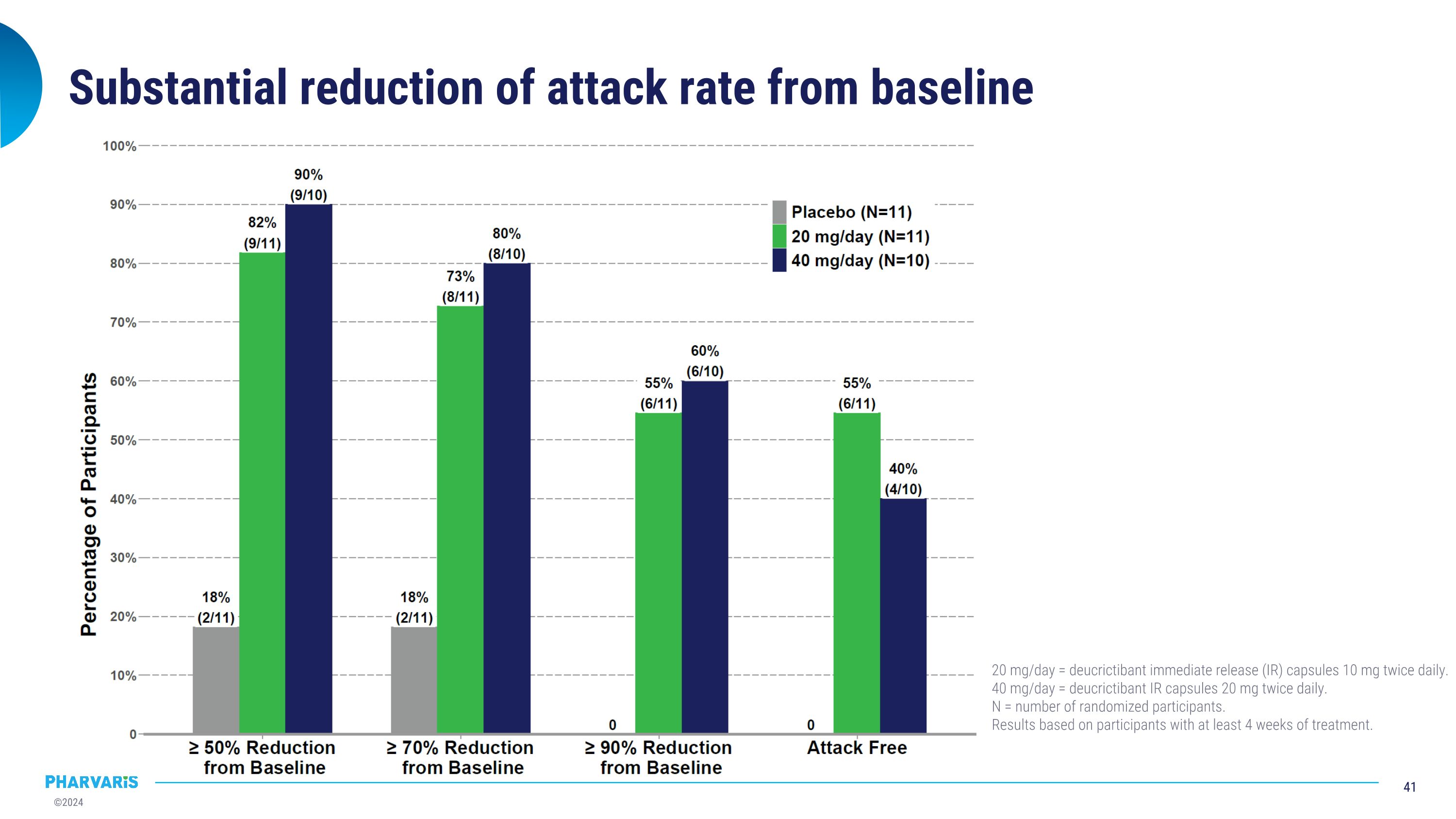
Substantial reduction of attack rate from baseline 20 mg/day = deucrictibant immediate release (IR) capsules 10 mg twice daily. 40 mg/day = deucrictibant IR capsules 20 mg twice daily. N = number of randomized participants. Results based on participants with at least 4 weeks of treatment.
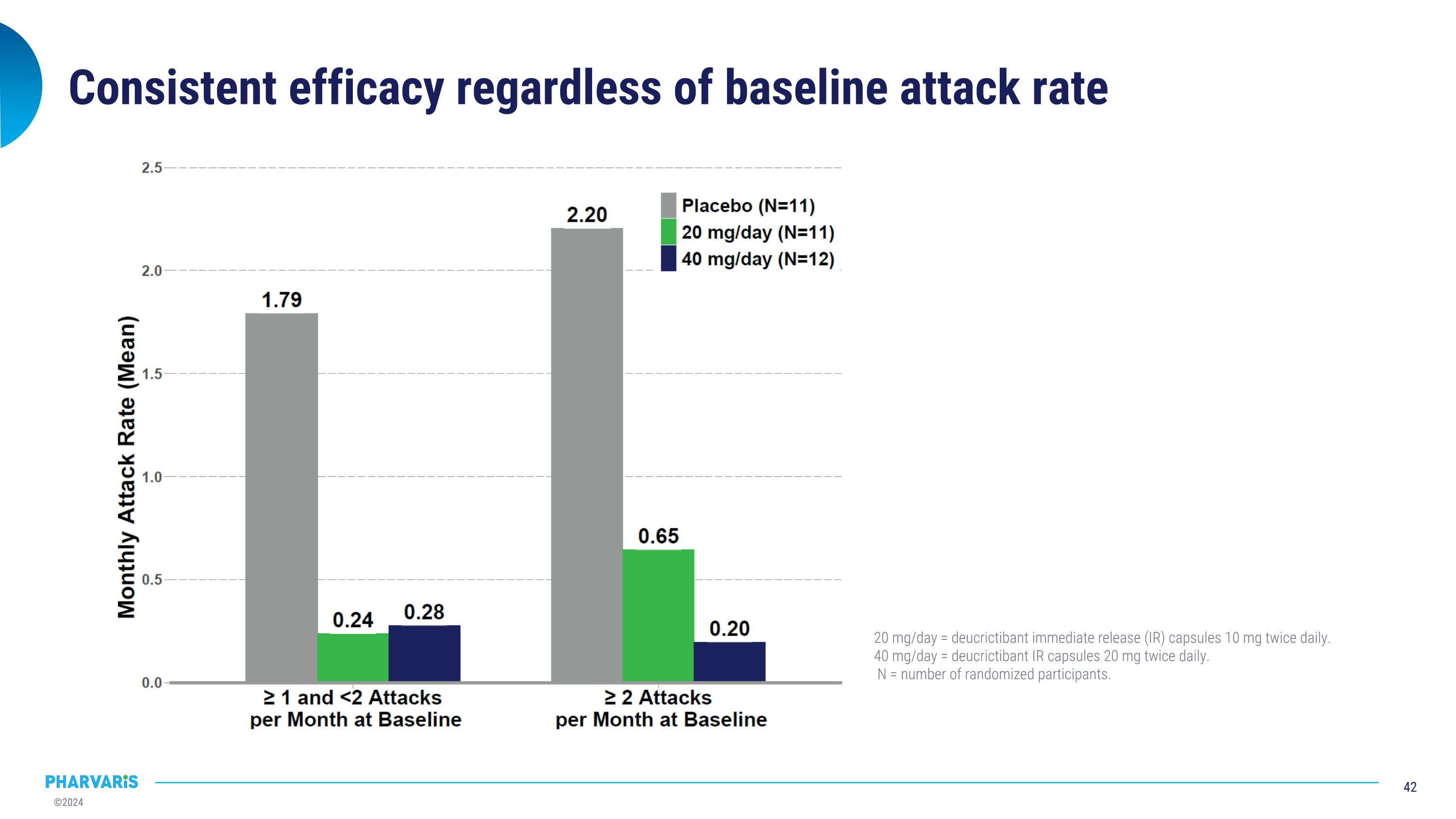
Consistent efficacy regardless of baseline attack rate 20 mg/day = deucrictibant immediate release (IR) capsules 10 mg twice daily. 40 mg/day = deucrictibant IR capsules 20 mg twice daily. N = number of randomized participants.
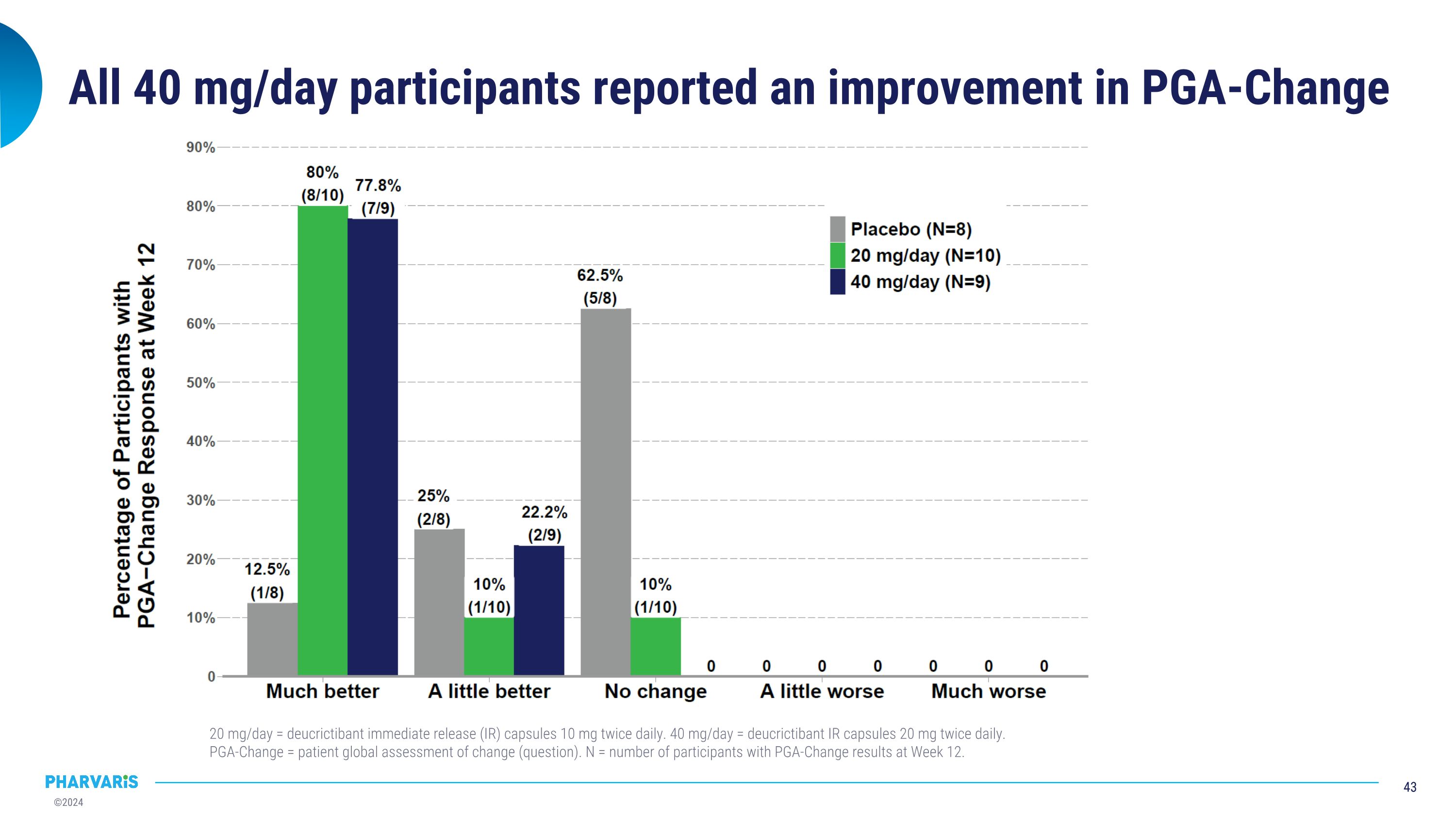
All 40 mg/day participants reported an improvement in PGA-Change 20 mg/day = deucrictibant immediate release (IR) capsules 10 mg twice daily. 40 mg/day = deucrictibant IR capsules 20 mg twice daily. PGA-Change = patient global assessment of change (question). N = number of participants with PGA-Change results at Week 12.
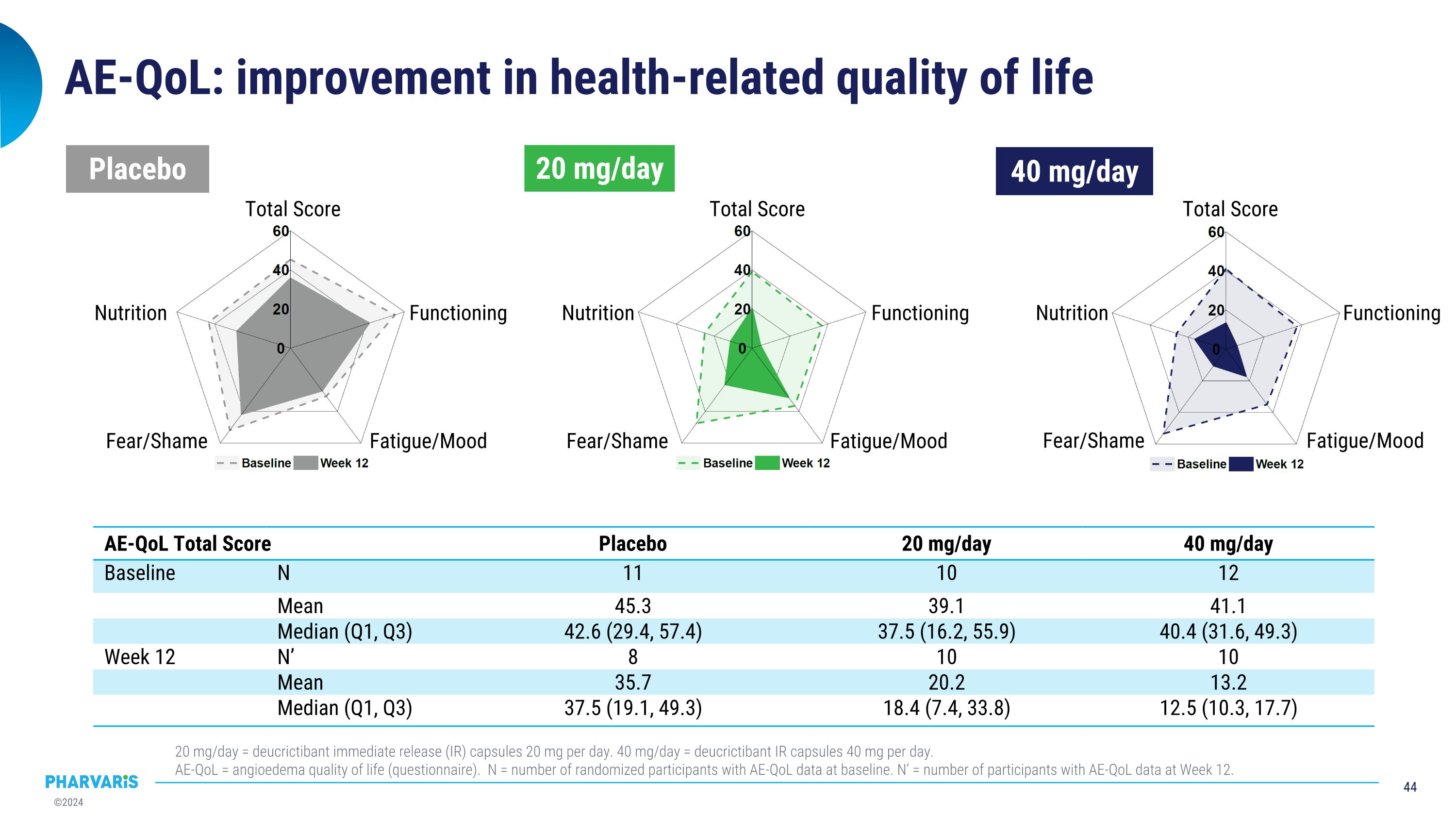
AE-QoL: improvement in health-related quality of life AE-QoL Total Score Placebo 20 mg/day 40 mg/day Baseline N 11 10 12 Mean 45.3 39.1 41.1 Median (Q1, Q3) 42.6 (29.4, 57.4) 37.5 (16.2, 55.9) 40.4 (31.6, 49.3) Week 12 N’ 8 10 10 Mean 35.7 20.2 13.2 Median (Q1, Q3) 37.5 (19.1, 49.3) 18.4 (7.4, 33.8) 12.5 (10.3, 17.7) 20 mg/day = deucrictibant immediate release (IR) capsules 20 mg per day. 40 mg/day = deucrictibant IR capsules 40 mg per day. AE-QoL = angioedema quality of life (questionnaire). N = number of randomized participants with AE-QoL data at baseline. N’ = number of participants with AE-QoL data at Week 12. Nutrition Total Score Functioning Fatigue/Mood Fear/Shame Placebo 20 mg/day 40 mg/day Nutrition Total Score Functioning Nutrition Total Score Functioning Fatigue/Mood Fear/Shame Fatigue/Mood Fear/Shame
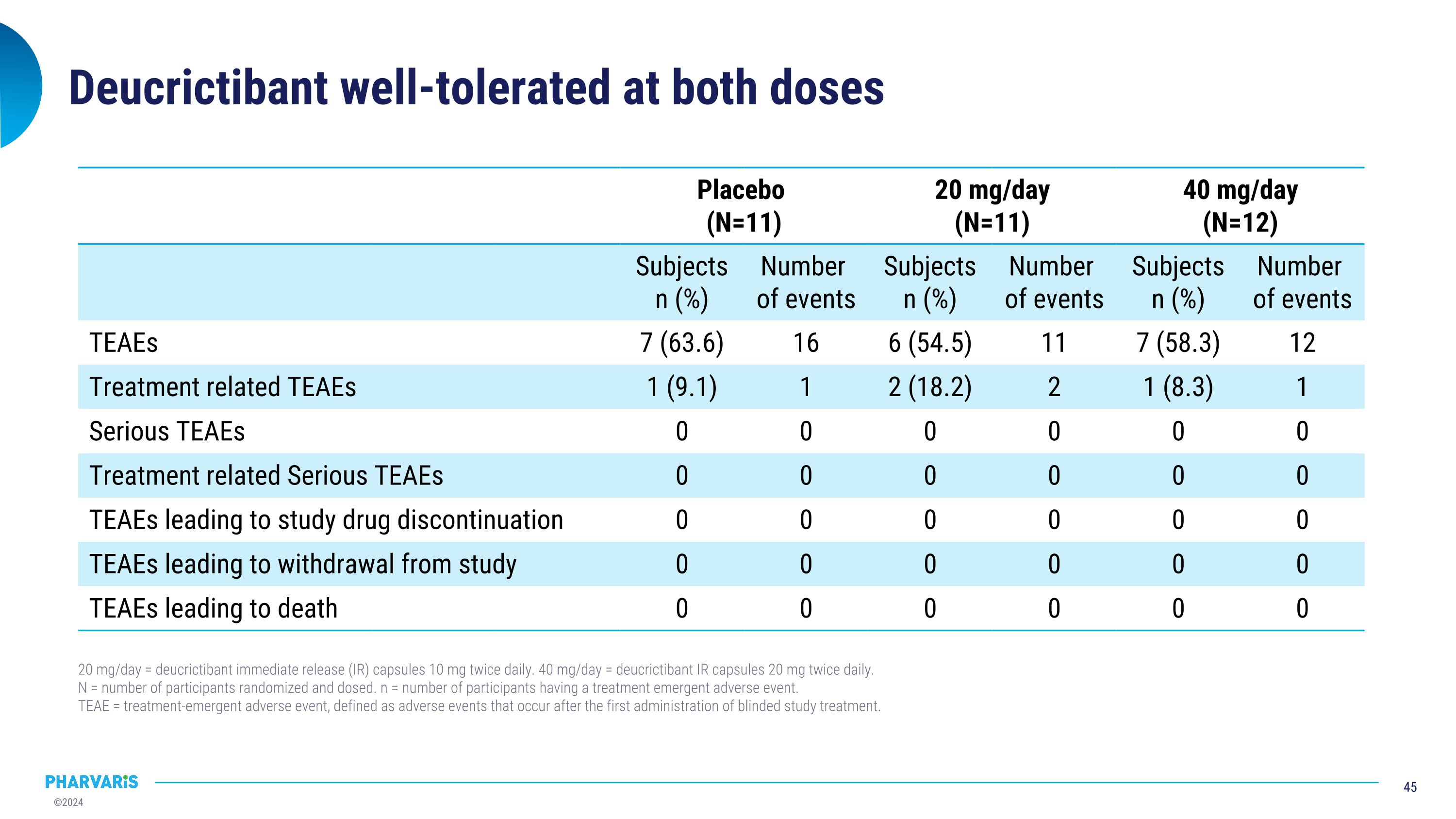
Deucrictibant well-tolerated at both doses Placebo (N=11) 20 mg/day (N=11) 40 mg/day (N=12) Subjects n (%) Number of events Subjects n (%) Number of events Subjects n (%) Number of events TEAEs TEAE 7 (63.6) 16 6 (54.5) 11 7 (58.3) 12 Treatment related TEAEs Treatment related TEAE 1 (9.1) 1 2 (18.2) 2 1 (8.3) 1 Serious TEAEs Serious TEAEs 0 0 0 0 0 0 Treatment related Serious TEAEs Treatment related TEAE 0 0 0 0 0 0 TEAEs leading to study drug discontinuation 0 0 0 0 0 0 TEAEs leading to withdrawal from study 0 0 0 0 0 0 TEAEs leading to death 0 0 0 0 0 0 20 mg/day = deucrictibant immediate release (IR) capsules 10 mg twice daily. 40 mg/day = deucrictibant IR capsules 20 mg twice daily. N = number of participants randomized and dosed. n = number of participants having a treatment emergent adverse event. TEAE = treatment-emergent adverse event, defined as adverse events that occur after the first administration of blinded study treatment.
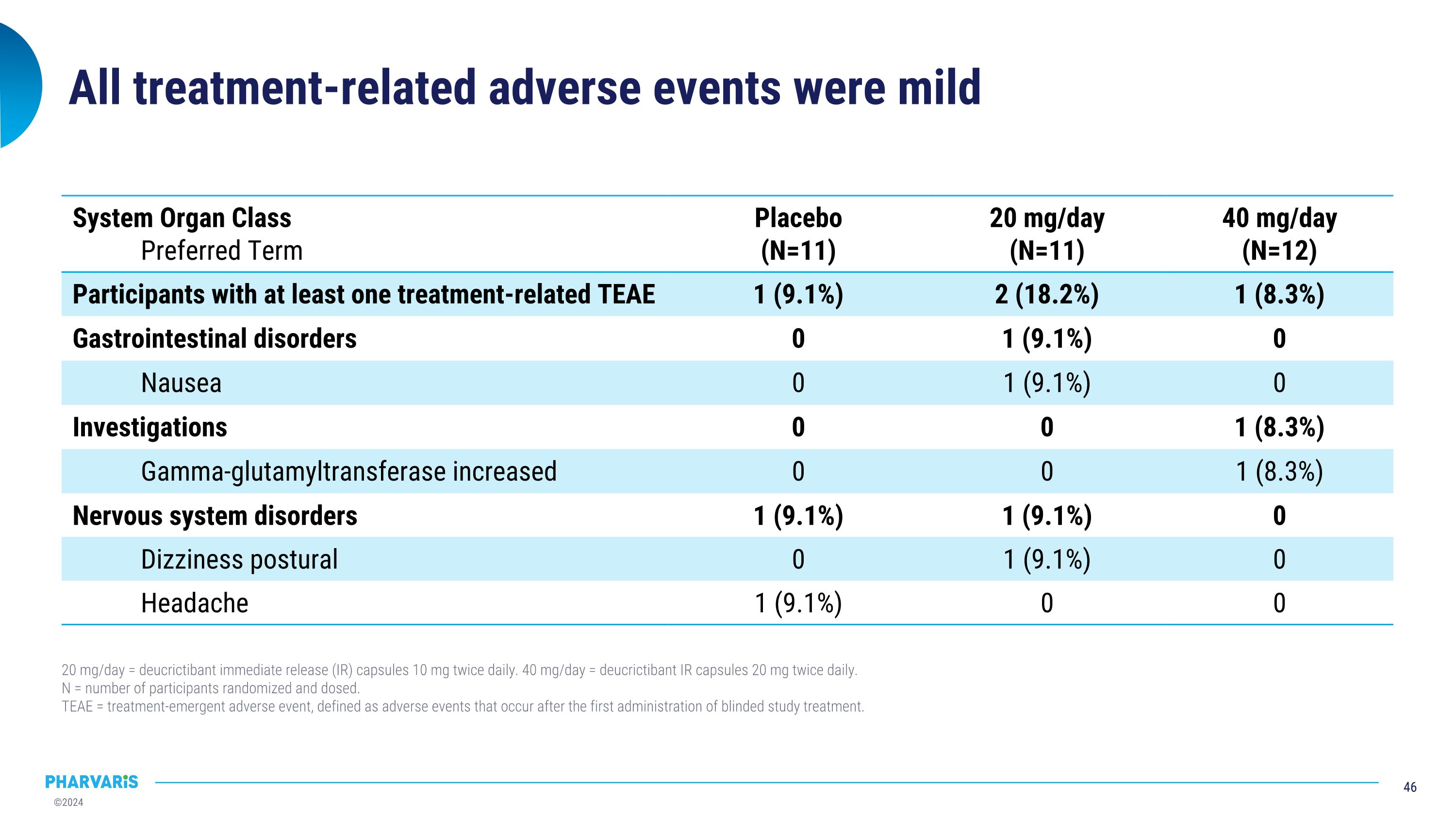
All treatment-related adverse events were mild System Organ Class Preferred Term Placebo (N=11) 20 mg/day (N=11) 40 mg/day (N=12) Participants with at least one treatment-related TEAE 1 (9.1%) 2 (18.2%) 1 (8.3%) Gastrointestinal disorders 0 1 (9.1%) 0 Nausea 0 1 (9.1%) 0 Investigations 0 0 1 (8.3%) Gamma-glutamyltransferase increased 0 0 1 (8.3%) Nervous system disorders 1 (9.1%) 1 (9.1%) 0 Dizziness postural 0 1 (9.1%) 0 Headache 1 (9.1%) 0 0 20 mg/day = deucrictibant immediate release (IR) capsules 10 mg twice daily. 40 mg/day = deucrictibant IR capsules 20 mg twice daily. N = number of participants randomized and dosed. TEAE = treatment-emergent adverse event, defined as adverse events that occur after the first administration of blinded study treatment.
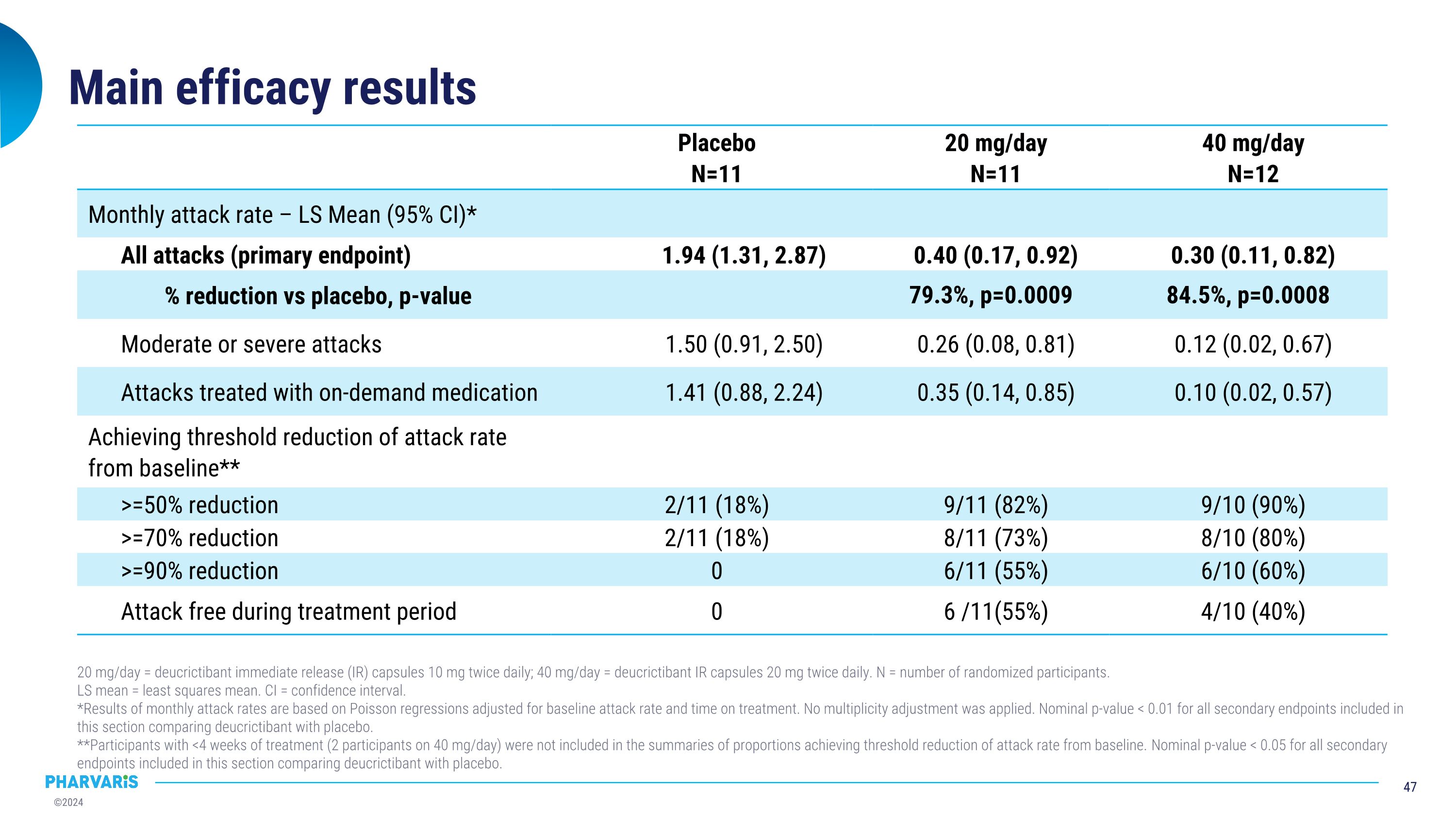
Main efficacy results 20 mg/day = deucrictibant immediate release (IR) capsules 10 mg twice daily; 40 mg/day = deucrictibant IR capsules 20 mg twice daily. N = number of randomized participants. LS mean = least squares mean. CI = confidence interval. *Results of monthly attack rates are based on Poisson regressions adjusted for baseline attack rate and time on treatment. No multiplicity adjustment was applied. Nominal p-value < 0.01 for all secondary endpoints included in this section comparing deucrictibant with placebo. **Participants with <4 weeks of treatment (2 participants on 40 mg/day) were not included in the summaries of proportions achieving threshold reduction of attack rate from baseline. Nominal p-value < 0.05 for all secondary endpoints included in this section comparing deucrictibant with placebo. Placebo N=11 20 mg/day N=11 40 mg/day N=12 Monthly attack rate – LS Mean (95% CI)* All attacks (primary endpoint) 1.94 (1.31, 2.87) 0.40 (0.17, 0.92) 0.30 (0.11, 0.82) % reduction vs placebo, p-value 79.3%, p=0.0009 84.5%, p=0.0008 Moderate or severe attacks 1.50 (0.91, 2.50) 0.26 (0.08, 0.81) 0.12 (0.02, 0.67) Attacks treated with on-demand medication 1.41 (0.88, 2.24) 0.35 (0.14, 0.85) 0.10 (0.02, 0.57) Achieving threshold reduction of attack rate from baseline** >=50% reduction 2/11 (18%) 9/11 (82%) 9/10 (90%) >=70% reduction 2/11 (18%) 8/11 (73%) 8/10 (80%) >=90% reduction 0 6/11 (55%) 6/10 (60%) Attack free during treatment period 0 6 /11(55%) 4/10 (40%)
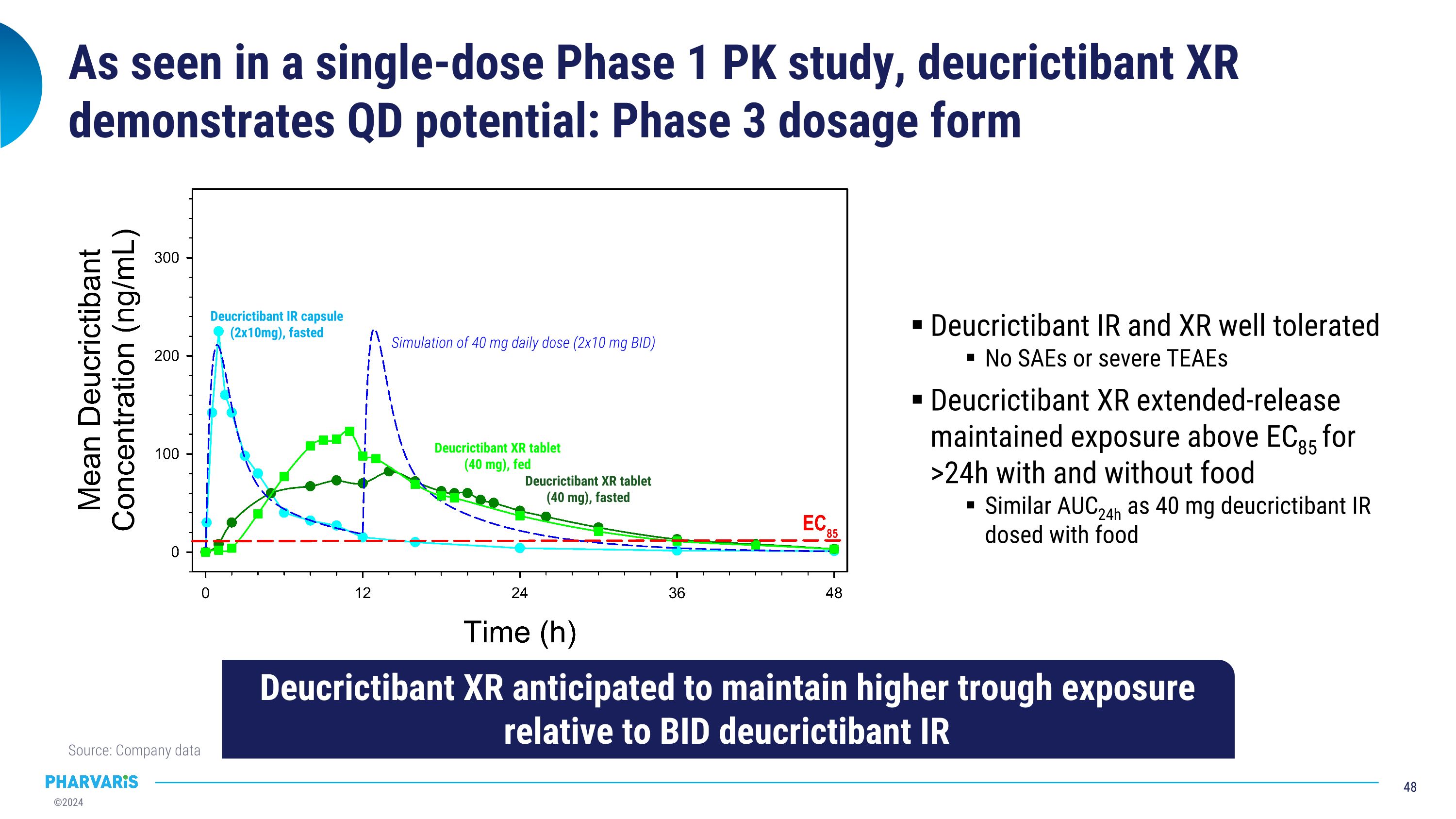
As seen in a single-dose Phase 1 PK study, deucrictibant XR demonstrates QD potential: Phase 3 dosage form Source: Company data Deucrictibant IR and XR well tolerated No SAEs or severe TEAEs Deucrictibant XR extended-release maintained exposure above EC85 for >24h with and without food Similar AUC24h as 40 mg deucrictibant IR dosed with food Simulation of 40 mg daily dose (2x10 mg BID) Deucrictibant XR anticipated to maintain higher trough exposure relative to BID deucrictibant IR Deucrictibant IR capsule�(2x10mg), fasted Deucrictibant XR tablet�(40 mg), fasted Deucrictibant XR tablet�(40 mg), fed
Our strategy: Manage HAE with two oral products that utilize the same active ingredient for on-demand and prophylactic treatment *Aspirational; to be confirmed with clinical data deucrictibant (PHVS416)�Immediate-release capsule�rapid absorption deucrictibant (PHVS719)�Extended-release tablet�sustained absorption Aim to provide sustained exposure of attack-preventing medicine in a convenient, small oral dosage form* Aim to provide rapid and reliable symptom relief, through rapid exposure of attack-mitigating therapy in a convenient, small oral dosage form* deucrictibant Based on the results in RAPIDe-1 and CHAPTER-1, deucrictibant has the potential to become the preferred option to treat and prevent HAE attacks
Focused on unmet need in the treatment of hereditary angioedema (HAE) and other bradykinin-mediated diseases Deep expertise in bradykinin/B2 receptor biology and chemistry Orally available, small molecule targeting the validated bradykinin B2 receptor pathway Positive top-line Phase 2 data in HAE: RAPIDe-1 study for on-demand treatment meets all primary and key secondary endpoints RAPIDe-3 Phase 3 study to initiate within 1H24 CHAPTER-1 study in prophylaxis meets primary endpoint and showed clinically meaningful improvements for secondary endpoints Preparing for initiation of CHAPTER-3 Phase 3 study Large global HAE market: �>$2 billion with predicted 15% CAGR Potential portfolio expansion into other BK-mediated angioedema and diseases through B2-receptor pathway expertise World-wide operations: the Netherlands, U.S., and Switzerland (headquarters) Strong financial position, cash runway at least two years: €390M cash as of Dec 31, 2023 Experienced management team with successful track record in HAE drug design and development Strong IP on novel lead and backup series Primary CoM granted in multiple territories, initial term to 2038 FDA orphan drug designation Competitive product profile Strong fundamentals Large market opportunity

www.pharvaris.com NASDAQ: PHVS Aspiring to free people from HAE or other bradykinin-mediated diseases

Additional RAPIDe-1 top-line clinical data Appendix
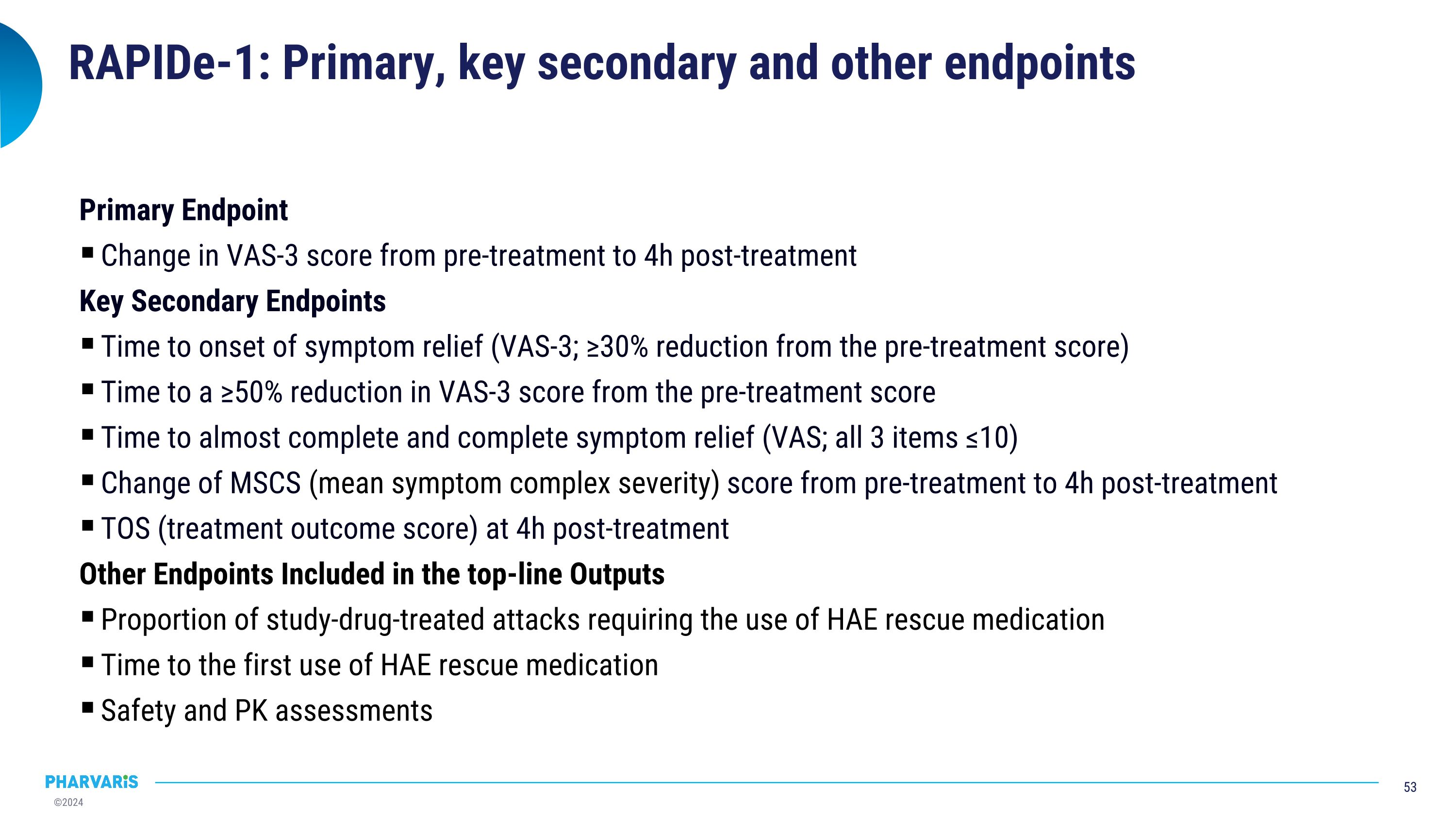
RAPIDe-1: Primary, key secondary and other endpoints Primary Endpoint Change in VAS-3 score from pre-treatment to 4h post-treatment Key Secondary Endpoints Time to onset of symptom relief (VAS-3; ≥30% reduction from the pre-treatment score) Time to a ≥50% reduction in VAS-3 score from the pre-treatment score Time to almost complete and complete symptom relief (VAS; all 3 items ≤10) Change of MSCS (mean symptom complex severity) score from pre-treatment to 4h post-treatment TOS (treatment outcome score) at 4h post-treatment Other Endpoints Included in the top-line Outputs Proportion of study-drug-treated attacks requiring the use of HAE rescue medication Time to the first use of HAE rescue medication Safety and PK assessments
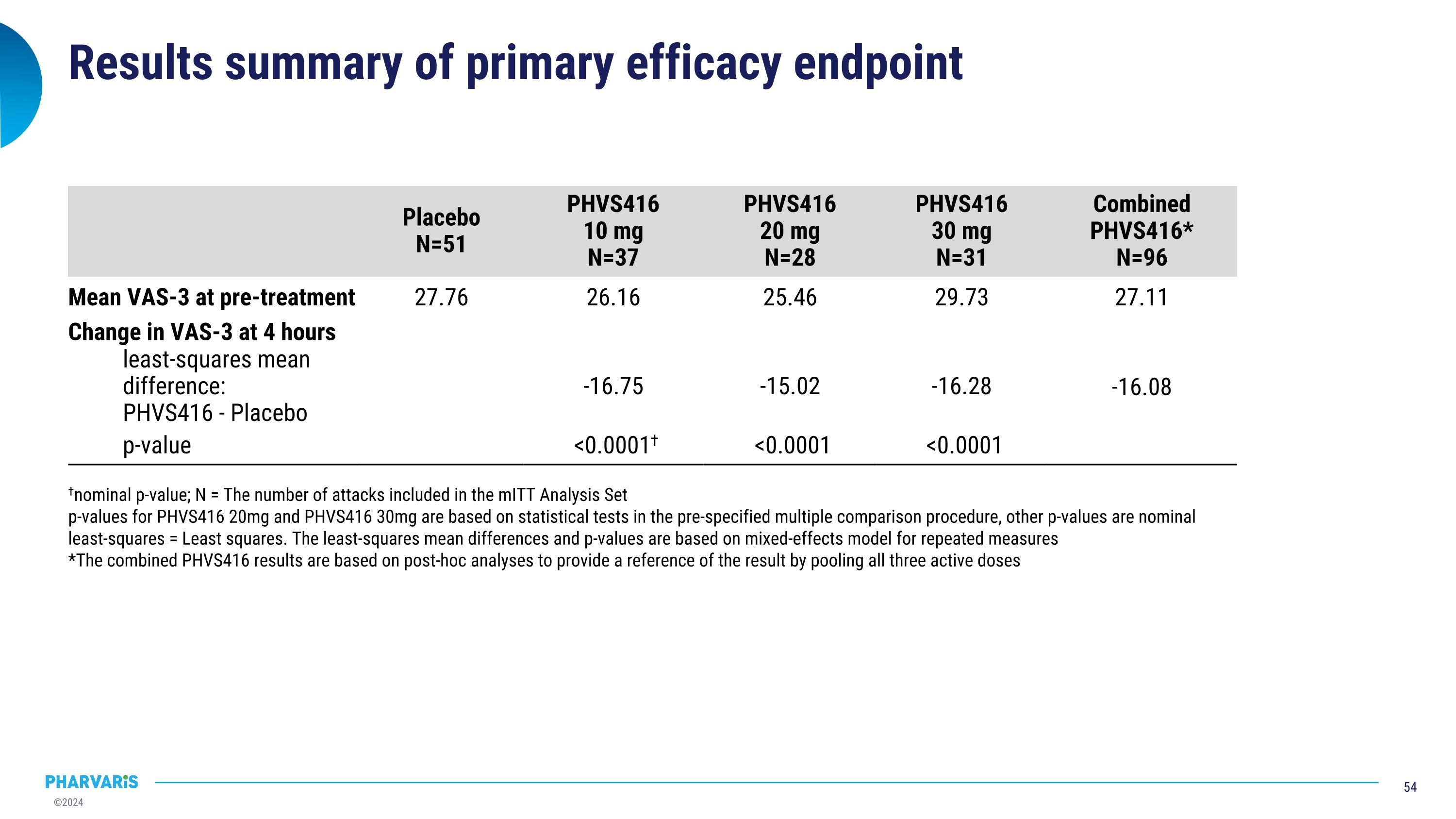
Results summary of primary efficacy endpoint Placebo N=51 PHVS416 10 mg N=37 PHVS416 20 mg N=28 PHVS416 30 mg N=31 Combined PHVS416* N=96 Mean VAS-3 at pre-treatment 27.76 26.16 25.46 29.73 27.11 Change in VAS-3 at 4 hours least-squares mean difference: PHVS416 - Placebo -16.75 -15.02 -16.28 -16.08 p-value <0.0001† <0.0001 <0.0001 †nominal p-value; N = The number of attacks included in the mITT Analysis Set p-values for PHVS416 20mg and PHVS416 30mg are based on statistical tests in the pre-specified multiple comparison procedure, other p-values are nominal least-squares = Least squares. The least-squares mean differences and p-values are based on mixed-effects model for repeated measures *The combined PHVS416 results are based on post-hoc analyses to provide a reference of the result by pooling all three active doses
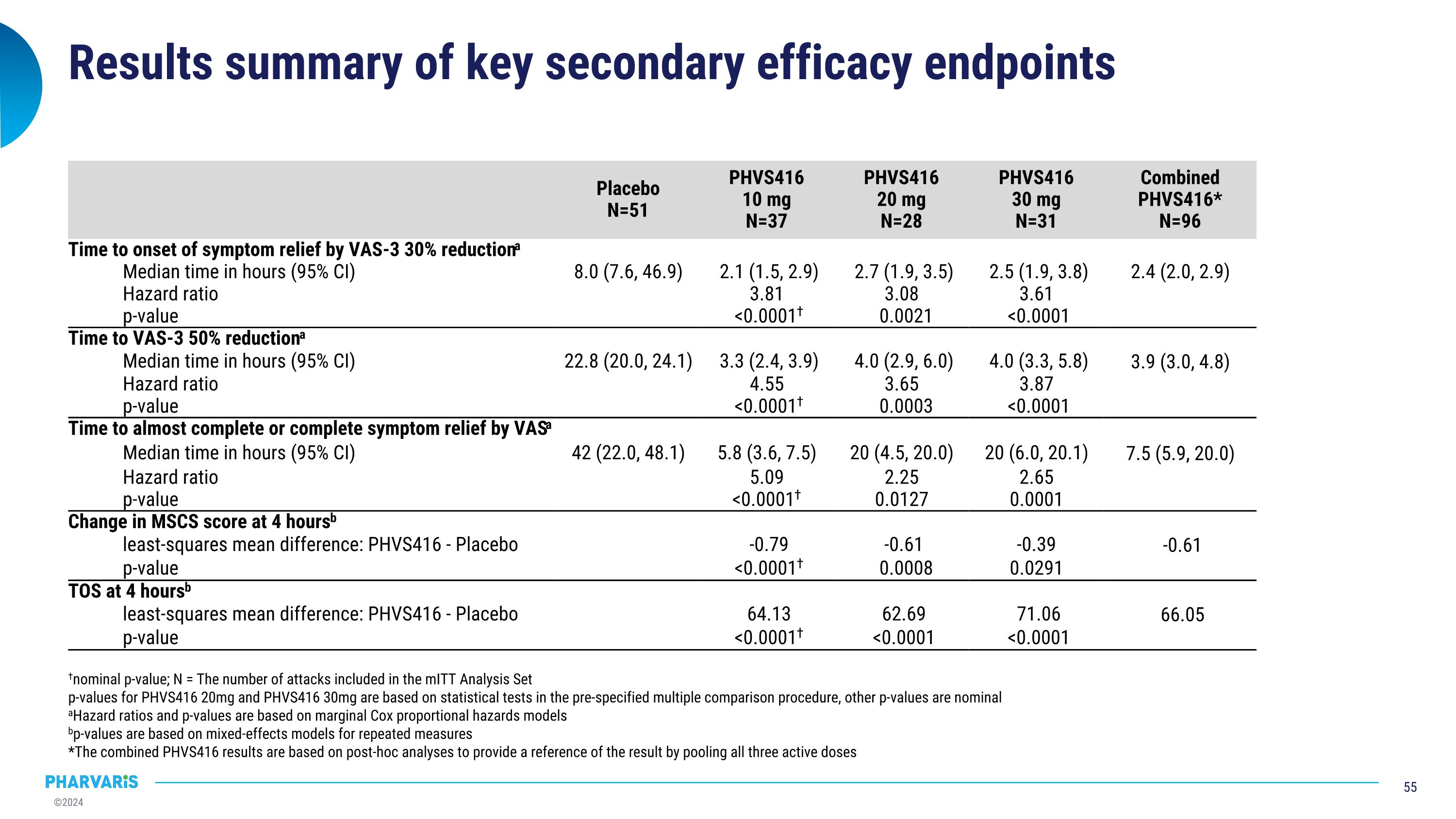
Results summary of key secondary efficacy endpoints Placebo N=51 PHVS416 10 mg N=37 PHVS416 20 mg N=28 PHVS416 30 mg N=31 Combined PHVS416* N=96 Time to onset of symptom relief by VAS-3 30% reductiona Median time in hours (95% CI) 8.0 (7.6, 46.9) 2.1 (1.5, 2.9) 2.7 (1.9, 3.5) 2.5 (1.9, 3.8) 2.4 (2.0, 2.9) Hazard ratio 3.81 3.08 3.61 p-value <0.0001† 0.0021 <0.0001 Time to VAS-3 50% reductiona Median time in hours (95% CI) 22.8 (20.0, 24.1) 3.3 (2.4, 3.9) 4.0 (2.9, 6.0) 4.0 (3.3, 5.8) 3.9 (3.0, 4.8) Hazard ratio 4.55 3.65 3.87 p-value <0.0001† 0.0003 <0.0001 Time to almost complete or complete symptom relief by VASa Median time in hours (95% CI) 42 (22.0, 48.1) 5.8 (3.6, 7.5) 20 (4.5, 20.0) 20 (6.0, 20.1) 7.5 (5.9, 20.0) Hazard ratio 5.09 2.25 2.65 p-value <0.0001† 0.0127 0.0001 Change in MSCS score at 4 hoursb least-squares mean difference: PHVS416 - Placebo -0.79 -0.61 -0.39 -0.61 p-value <0.0001† 0.0008 0.0291 TOS at 4 hoursb least-squares mean difference: PHVS416 - Placebo 64.13 62.69 71.06 66.05 p-value <0.0001† <0.0001 <0.0001 †nominal p-value; N = The number of attacks included in the mITT Analysis Set p-values for PHVS416 20mg and PHVS416 30mg are based on statistical tests in the pre-specified multiple comparison procedure, other p-values are nominal aHazard ratios and p-values are based on marginal Cox proportional hazards models bp-values are based on mixed-effects models for repeated measures *The combined PHVS416 results are based on post-hoc analyses to provide a reference of the result by pooling all three active doses
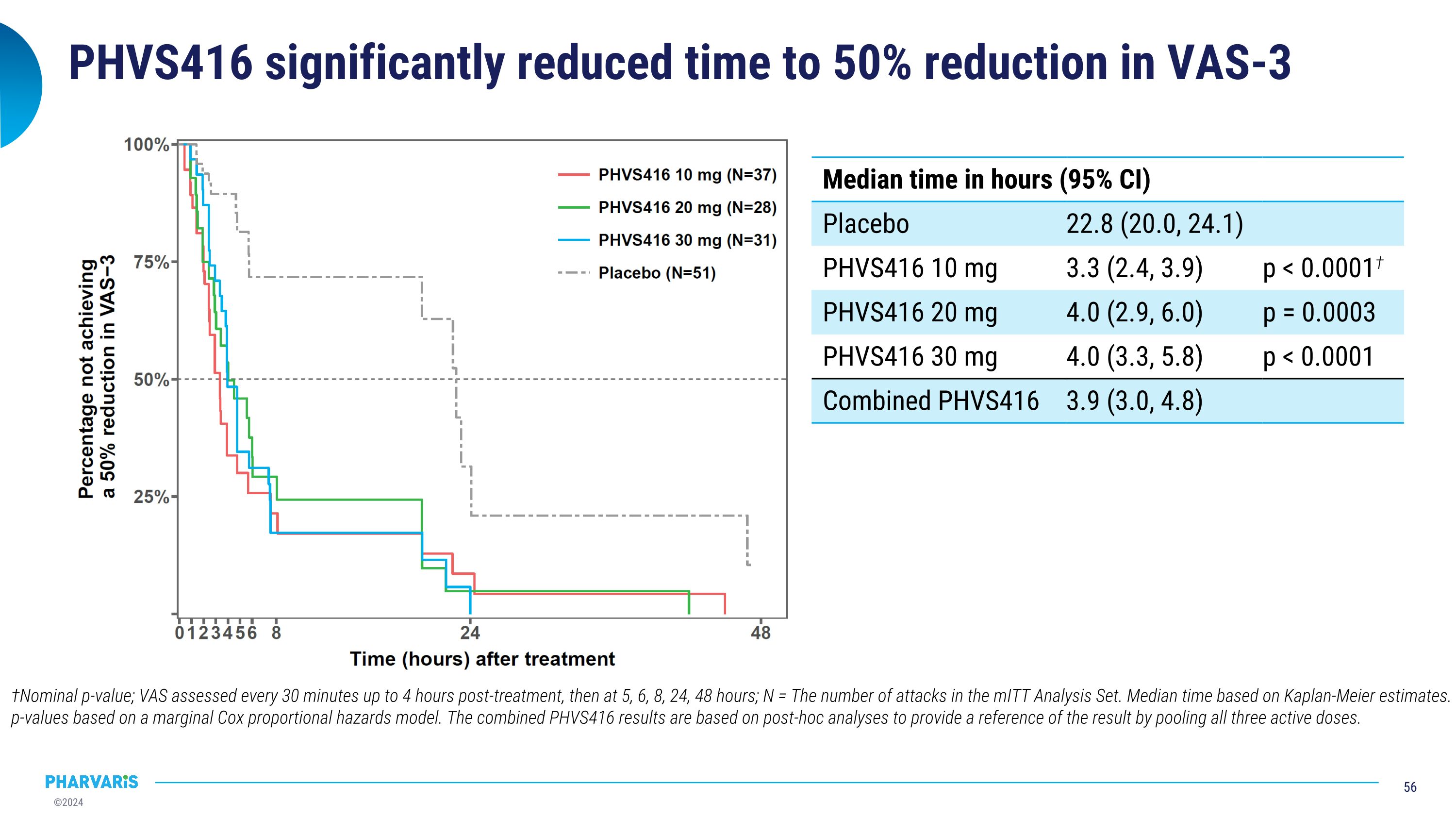
PHVS416 significantly reduced time to 50% reduction in VAS-3 †Nominal p-value; VAS assessed every 30 minutes up to 4 hours post-treatment, then at 5, 6, 8, 24, 48 hours; N = The number of attacks in the mITT Analysis Set. Median time based on Kaplan-Meier estimates. p-values based on a marginal Cox proportional hazards model. The combined PHVS416 results are based on post-hoc analyses to provide a reference of the result by pooling all three active doses. Median time in hours (95% CI) Median time in hours (95% CI) Placebo 22.8 (20.0, 24.1) PHVS416 10 mg 3.3 (2.4, 3.9) p < 0.0001† PHVS416 20 mg 4.0 (2.9, 6.0) p = 0.0003 PHVS416 30 mg 4.0 (3.3, 5.8) p < 0.0001 Combined PHVS416 3.9 (3.0, 4.8)
MSCS and TOS: definitions Validated patient-reported outcome measures to comprehensively capture symptom severity and change of HAE attacks MSCS (Mean Symptom Complex Severity) score is a point-in-time measure of symptom severity: Patients rated the severity of each affected symptom on a categorical scale (0 = normal, 1 = mild, 2 = moderate, 3 = severe) Calculated as average score from all affected anatomic sites of attack (symptom complexes or SC) pre-treatment Decrease in MSCS score reflects improvement in symptom severity TOS (Treatment Outcome Score) is a measure of symptom response to treatment: Patient assessment of response for each affected SC recorded on categorical scale (significant improvement [100], improvement [50], same [0], worsening [-50], significant worsening [-100]) Calculated as weighted average of the response at all SC using pre-treatment severity as the weight TOS value >0 reflects improvement in symptoms from pre-treatment
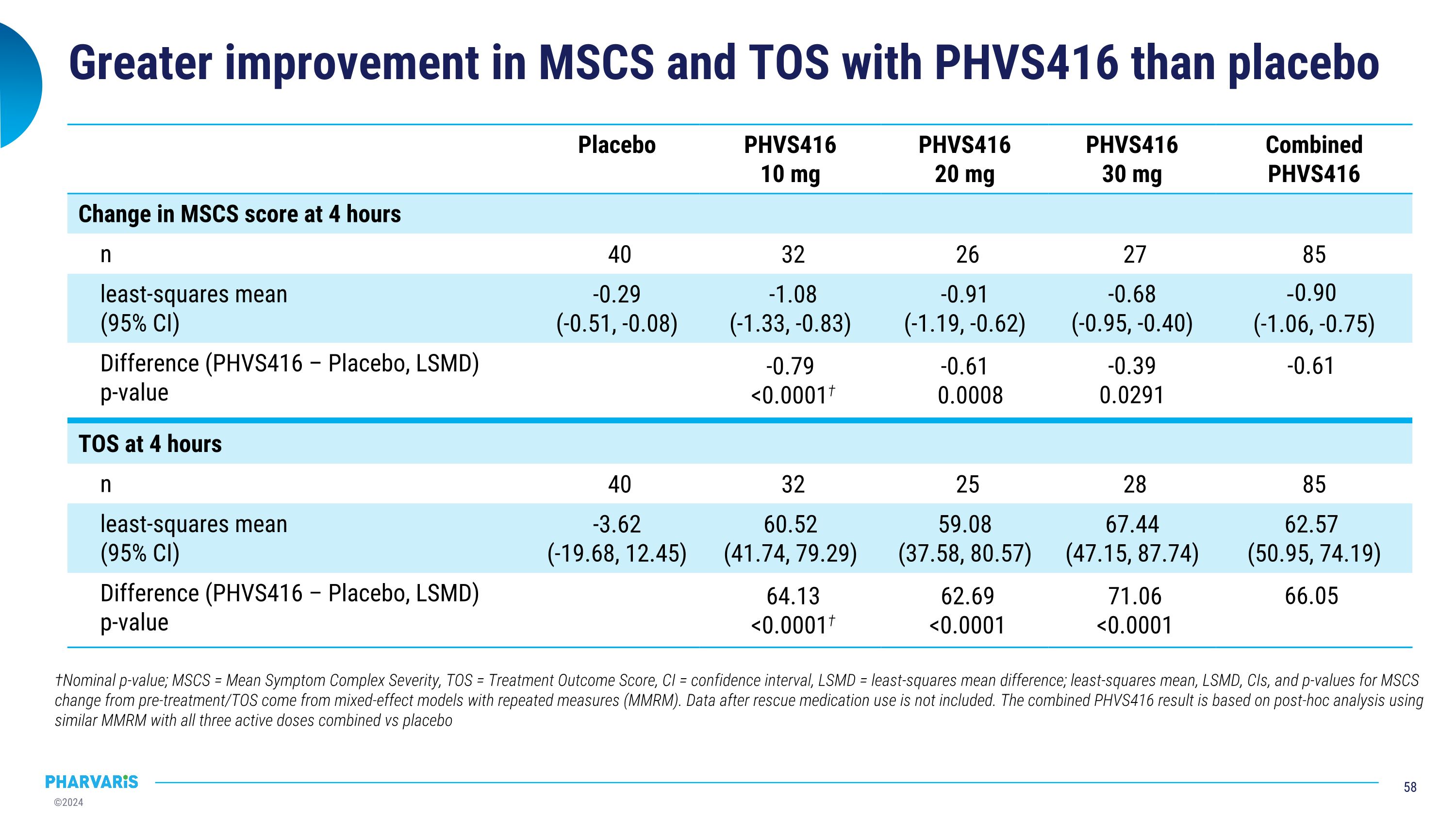
Greater improvement in MSCS and TOS with PHVS416 than placebo Placebo PHVS416 10 mg PHVS416 20 mg PHVS416 30 mg Combined PHVS416 Change in MSCS score at 4 hours n 40 32 26 27 85 least-squares mean (95% CI) -0.29 (-0.51, -0.08) -1.08 (-1.33, -0.83) -0.91 (-1.19, -0.62) -0.68 (-0.95, -0.40) -0.90 (-1.06, -0.75) Difference (PHVS416 – Placebo, LSMD) p-value -0.79 <0.0001† -0.61 0.0008 -0.39 0.0291 -0.61 TOS at 4 hours n 40 32 25 28 85 least-squares mean (95% CI) -3.62 (-19.68, 12.45) 60.52 (41.74, 79.29) 59.08 (37.58, 80.57) 67.44 (47.15, 87.74) 62.57 (50.95, 74.19) Difference (PHVS416 – Placebo, LSMD) p-value 64.13 <0.0001† 62.69 <0.0001 71.06 <0.0001 66.05 †Nominal p-value; MSCS = Mean Symptom Complex Severity, TOS = Treatment Outcome Score, CI = confidence interval, LSMD = least-squares mean difference; least-squares mean, LSMD, CIs, and p-values for MSCS change from pre-treatment/TOS come from mixed-effect models with repeated measures (MMRM). Data after rescue medication use is not included. The combined PHVS416 result is based on post-hoc analysis using similar MMRM with all three active doses combined vs placebo
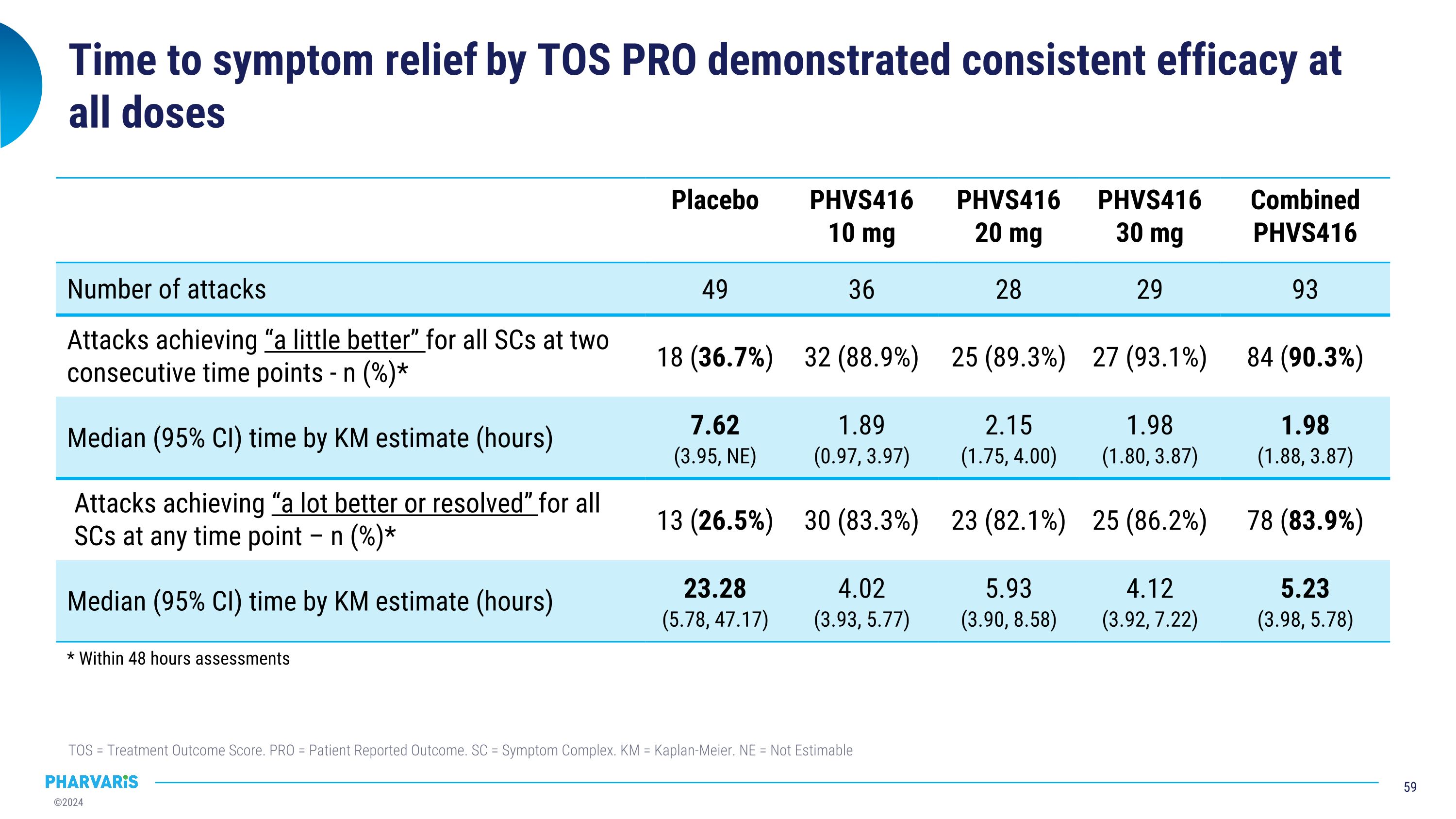
Placebo PHVS416 10 mg PHVS416 20 mg PHVS416 30 mg Combined PHVS416 Number of attacks 49 36 28 29 93 Attacks achieving “a little better” for all SCs at two consecutive time points - n (%)* 18 (36.7%) 32 (88.9%) 25 (89.3%) 27 (93.1%) 84 (90.3%) Median (95% CI) time by KM estimate (hours) 7.62 (3.95, NE) 1.89 (0.97, 3.97) 2.15 (1.75, 4.00) 1.98 (1.80, 3.87) 1.98 (1.88, 3.87) Attacks achieving “a lot better or resolved” for all SCs at any time point – n (%)* 13 (26.5%) 30 (83.3%) 23 (82.1%) 25 (86.2%) 78 (83.9%) Median (95% CI) time by KM estimate (hours) 23.28 (5.78, 47.17) 4.02 (3.93, 5.77) 5.93 (3.90, 8.58) 4.12 (3.92, 7.22) 5.23 (3.98, 5.78) Time to symptom relief by TOS PRO demonstrated consistent efficacy at all doses TOS = Treatment Outcome Score. PRO = Patient Reported Outcome. SC = Symptom Complex. KM = Kaplan-Meier. NE = Not Estimable * Within 48 hours assessments
Pharvaris NV (NASDAQ:PHVS)
Historical Stock Chart
From Mar 2024 to Apr 2024
Pharvaris NV (NASDAQ:PHVS)
Historical Stock Chart
From Apr 2023 to Apr 2024