false 0001604464 0001604464 2024-01-08 2024-01-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 8, 2024
Atara Biotherapeutics, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-36548 |
|
46-0920988 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer
Identification No.) |
|
|
| 2380 Conejo Spectrum Street Suite 200 |
|
|
| Thousand Oaks, California |
|
|
|
91320 |
| (Address of Principal Executive Offices) |
|
|
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (805) 623-4211
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share |
|
ATRA |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.05 |
Costs Associated with Exit or Disposal Activities. |
On January 8, 2024, Atara Biotherapeutics, Inc. (the “Company”) announced a reduction in its workforce that will impact approximately 25% of its current employees. The Company expects to substantially complete the workforce reduction by May 2024.
The Company expects to recognize approximately $4.5 million in total for severance and related benefits for employees laid off under the reduction in force. These charges are primarily one-time termination benefits and are primarily cash charges. The Company may also incur other charges or cash expenditures not currently contemplated due to events that may occur as a result of, or associated with, the workforce reduction.
Additional details will be provided in the Company’s Quarterly Report on Form 10-Q for the period ending March 31, 2024.
| Item 5.02 |
Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers. |
On January 8, 2024, the Company announced that the employment of Manher (AJ) Joshi, the Company’s Executive Vice President, Chief Medical Officer, would terminate effective as of February 2, 2024. Pursuant to the terms of the Executive Employment Agreement dated as of November 10, 2020 between Dr. Joshi and the Company, Dr. Joshi will be entitled to receive severance benefits of 12 months of base salary continuation and, subject to his timely election of coverage, payment by the Company of up to 12 months of continued health care benefits.
In connection with Dr. Joshi’s departure from the Company and subject to his execution of a general release in favor of the Company, the Company intends to enter into a consulting agreement with Dr. Joshi pursuant to which Dr. Joshi will provide consulting services to the Company through January 31, 2025. Dr. Joshi is expected to be paid a consulting fee of $3,000 monthly for up to a specified number of consulting hours per month and is expected to receive additional compensation of $200 per hour in excess of such specified number of consulting hours. Dr. Joshi’s outstanding restricted stock unit equity awards will continue to vest during the consulting term. In addition, Dr. Joshi will be entitled to receive $185,000 upon the approval by the United States Food and Drug Administration of a biologics license application for tabelecleucel.
| Item 7.01 |
Regulation FD Disclosure. |
The Company intends to conduct meetings with securities analysts, investors and others in connection with the 42nd Annual J.P. Morgan Healthcare Conference beginning on January 8, 2024. As part of these meetings, the Company intends to utilize the corporate slide presentation furnished with this Current Report on Form 8-K as Exhibit 99.1.
The information in this Item 7.01, including Exhibit 99.1 hereto, is being furnished, not filed, pursuant to Regulation FD. Accordingly, the information in Item 7.01 of this Current Report on Form 8-K and Exhibit 99.1 hereto will not be incorporated by reference into any registration statement filed by the Company under the Securities Act of 1933, as amended, unless specifically identified therein as being incorporated therein by reference. The furnishing of the information in this Current Report on Form 8-K is not intended to, and does not, constitute a determination or admission by the Company that the information in this Current Report on Form 8-K is material or complete, or that investors should consider this information before making an investment decision with respect to any security of the Company.
On January 8, 2024, the Company issued a press release titled “Atara Biotherapeutics to Present Recent Progress and Key Upcoming Milestones at the 42nd Annual J.P. Morgan Healthcare Conference.” A copy of the Company’s press release is attached as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated by reference herein.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
ATARA BIOTHERAPEUTICS, INC. |
|
|
|
|
| Date: January 8, 2024 |
|
|
|
By: |
|
/s/ Eric Hyllengren |
|
|
|
|
|
|
Eric Hyllengren
Chief Financial Officer
(Duly Authorized Officer and Principal Financial and Accounting Officer) |

Investor presentation 42nd Annual j.p.
morgan healthcare conference monday, January 8, 2024 Nasdaq: ATRA Exhibit 99.1

Forward-Looking Statements This
presentation and the accompanying oral presentation contain forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts
contained in this presentation, including statements regarding our future results of operations and financial position, future transactions, business strategy, product, product candidates, correspondence and discussions with regulatory authorities,
regulatory submissions, regulatory approvals, the initiation, timing, progress and results of preclinical studies and clinical trials and our research and development programs, the mechanistic link between EBV and multiple sclerosis and the ability
of ATA188 to specifically target such link, ability to sell, manufacture or otherwise commercialize our product and product candidates, research and development costs, timing and likelihood of success, plans and objectives of management for future
operations, any royalty payments, our ability to obtain and maintain intellectual property protection for our product and product candidates, and the sufficiency of Atara’s cash, cash equivalents, short-term investments to fund its planned
operations are forward-looking statements of Atara Biotherapeutics, Inc. (“Atara” or the “Company”). These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual
results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases you can identify these statements by forward-looking words
such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “would,” “project,”
“predict,” “plan,” “expect” or the negative or plural of these words or similar expressions. These forward-looking statements are subject to risks and uncertainties, including those discussed in
Atara’s filings with the Securities and Exchange Commission (SEC), including in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of the
Company’s most recently filed periodic reports on Form 10-K and Form 10-Q and subsequent filings and in the documents incorporated by reference therein. These risks and uncertainties include, without limitation, risks and uncertainties
associated with the costly and time-consuming pharmaceutical product development process and the uncertainty of clinical success; the COVID-19 pandemic, and the wars in Ukraine and the Middle East, which may significantly impact (i) our business,
research, clinical development plans and operations, including our operations in Southern California, Denver and at our clinical trial sites, as well as the business or operations of our third-party manufacturer, contract research organizations or
other third parties with whom we conduct business, (ii) our ability to access capital, and (iii) the value of our common stock; the impact of future and pending legislation and regulations; the use of our information technology and communication
systems and cybersecurity attacks; the sufficiency of our cash resources and need for additional capital, and other factors that may cause our or our industry's actual results, levels of activity, performance or achievements to be materially
different from those anticipated by the forward-looking statements. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control,
you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those
projected in the forward-looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed
circumstances or otherwise. Certain information contained in this presentation and statements made orally during this presentation relate to or are based on studies, publications, surveys and other data obtained from third-party sources and
Atara's own internal estimates and research. While Atara believes these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as
to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of Atara’s internal estimates or research and no
reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates and research. The content of this presentation is subject to copyright, which will be asserted by Atara and no part of
this presentation may be reproduced, stored in a retrieval system, or transmitted in any form or by any means without prior permission in writing from Atara.

First Company to Obtain Regulatory
Approval for an Allogeneic T-cell Immunotherapy EbvalloTM Approved by EMA in December 2022; BLA submission expected in Q2 2024 Expanded global tab-cel® partnership with Pierre Fabre closed in December 2023 Near-Term Milestones with
ATA3219, Allogeneic CD19 CAR T Cell Incorporating Clinically-Validated Technologies Lupus nephritis IND filing anticipated Q1 2024 IND cleared in relapsed/refractory B-cell NHL with initial clinical data anticipated H2 2024 Focused Operational
Activities and Associated Strategic Restructuring Extends Cash Runway into 2027 ATARA IS THE FIRST TO DELIVER ON THE TRANSFORMATIVE POTENTIAL OF ALLOGENEIC T-CELL THERAPY
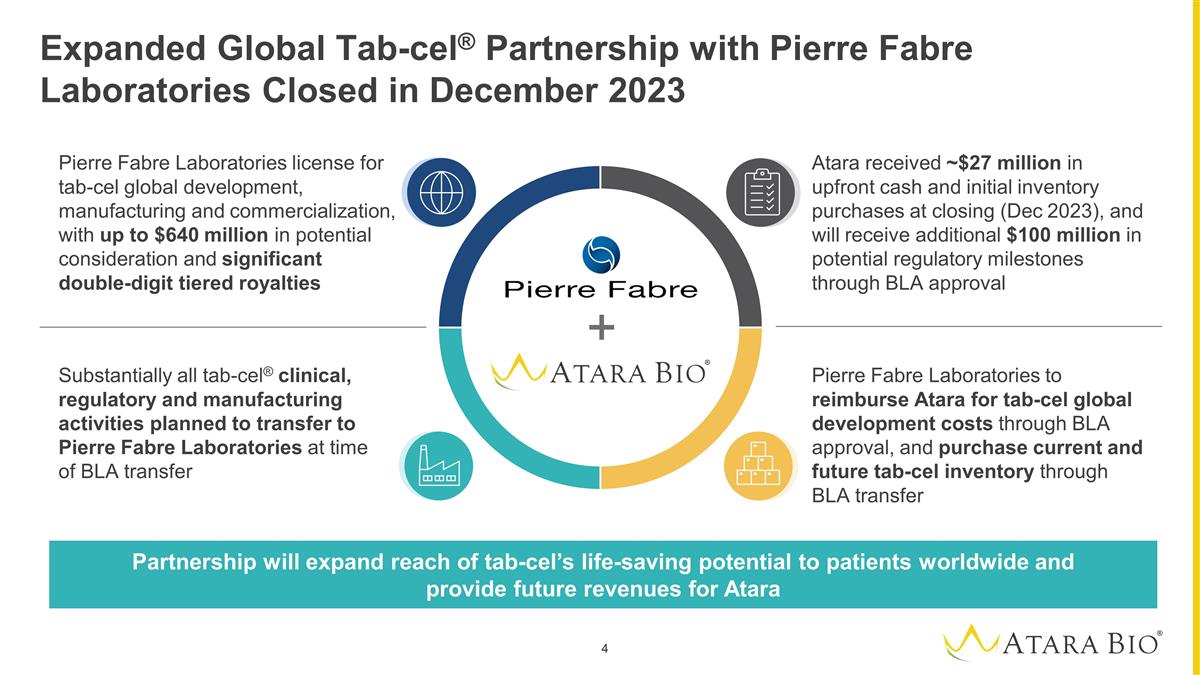
Expanded Global Tab-cel®
Partnership with Pierre Fabre Laboratories Closed in December 2023 Pierre Fabre Laboratories license for tab-cel global development, manufacturing and commercialization, with up to $640 million in potential consideration and significant
double-digit tiered royalties Pierre Fabre Laboratories to reimburse Atara for tab-cel global development costs through BLA approval, and purchase current and future tab-cel inventory through BLA transfer Substantially all tab-cel® clinical,
regulatory and manufacturing activities planned to transfer to Pierre Fabre Laboratories at time of BLA transfer Atara received ~$27 million in upfront cash and initial inventory purchases at closing (Dec 2023), and will receive additional $100
million in potential regulatory milestones through BLA approval Partnership will expand reach of tab-cel’s life-saving potential to patients worldwide and provide future revenues for Atara

Tab-cel BLA Submission on Track for Q2
2024 Based on Strong Clinical File Latest Phase 3 ALLELE data cut analysis reinforces confidence in tab-cel BLA filing package 49% ORR (p<0.0001) in patient population aligned with intended U.S. label Favorable and consistent safety profile
Other findings consistent with previous results, including DOR and estimated OS Separate pooled analysis including patients from ongoing tab-cel multicohort EBVision trial presented at ESMO-IO1 77.8% ORR in 18 EBV+ CNS PTLD patients, including first
line PTLD setting Long-term survival, and favorable and consistent safety profile 1Annals of Oncology (2023) 20 (suppl_1): 100520-100520. 10.1016/iotech/iotech100520 ORR – Objective Response Rate; DOR – Durability of Response; OS –
Overall Survival
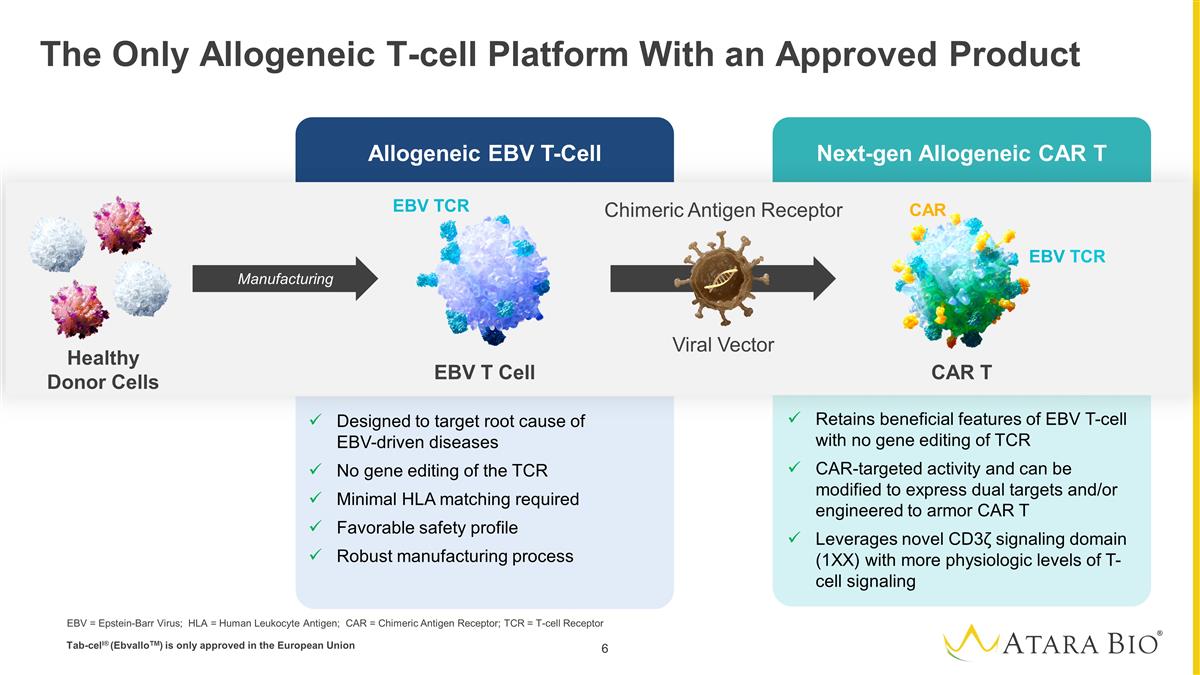
Allogeneic EBV T-Cell Next-gen
Allogeneic CAR T The Only Allogeneic T-cell Platform With an Approved Product Designed to target root cause of EBV-driven diseases No gene editing of the TCR Minimal HLA matching required Favorable safety profile Robust manufacturing process Retains
beneficial features of EBV T-cell with no gene editing of TCR CAR-targeted activity and can be modified to express dual targets and/or engineered to armor CAR T Leverages novel CD3ζ signaling domain (1XX) with more physiologic levels of T-cell
signaling EBV T Cell CAR T Chimeric Antigen Receptor EBV TCR EBV TCR CAR Manufacturing Viral Vector Healthy Donor Cells EBV = Epstein-Barr Virus; HLA = Human Leukocyte Antigen; CAR = Chimeric Antigen Receptor; TCR = T-cell Receptor
Tab-cell® (EbvalloTM) is only approved in the European Union
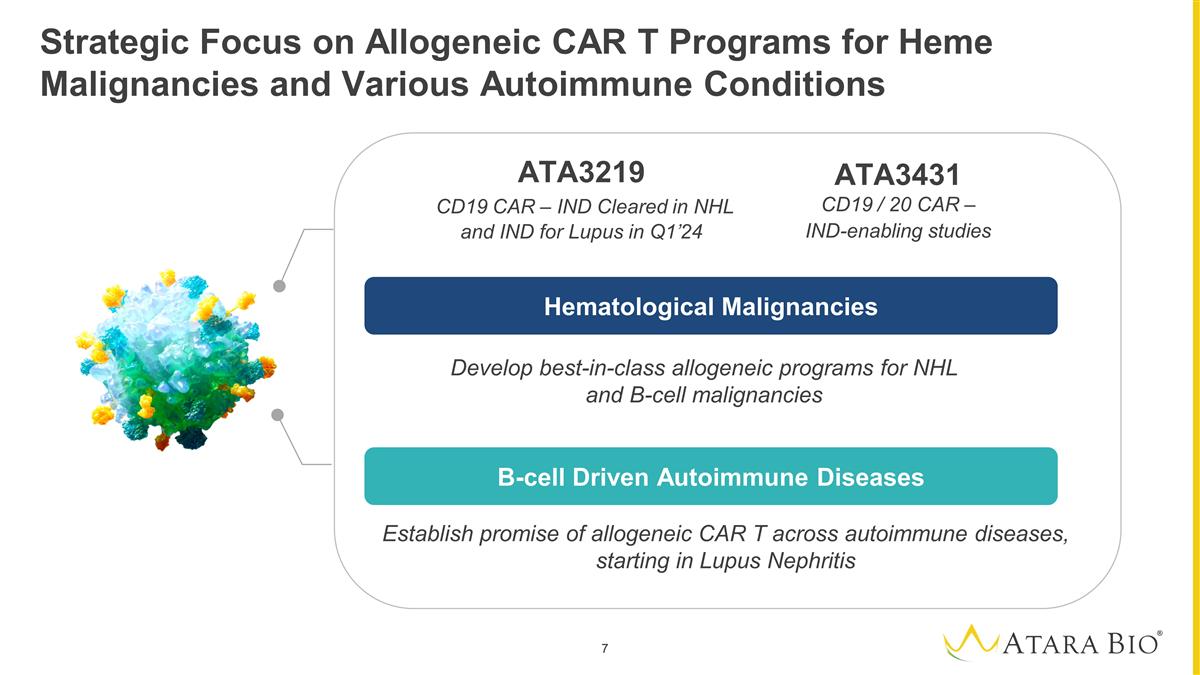
Strategic Focus on Allogeneic CAR T
Programs for Heme Malignancies and Various Autoimmune Conditions Develop best-in-class allogeneic programs for NHL and B-cell malignancies Establish promise of allogeneic CAR T across autoimmune diseases, starting in Lupus Nephritis ` `
Hematological Malignancies B-cell Driven Autoimmune Disease Hematological Malignancies B-cell Driven Autoimmune Diseases ATA3219 CD19 CAR – IND Cleared in NHL and IND for Lupus in Q1’24 ATA3431 CD19 / 20 CAR – IND-enabling
studies
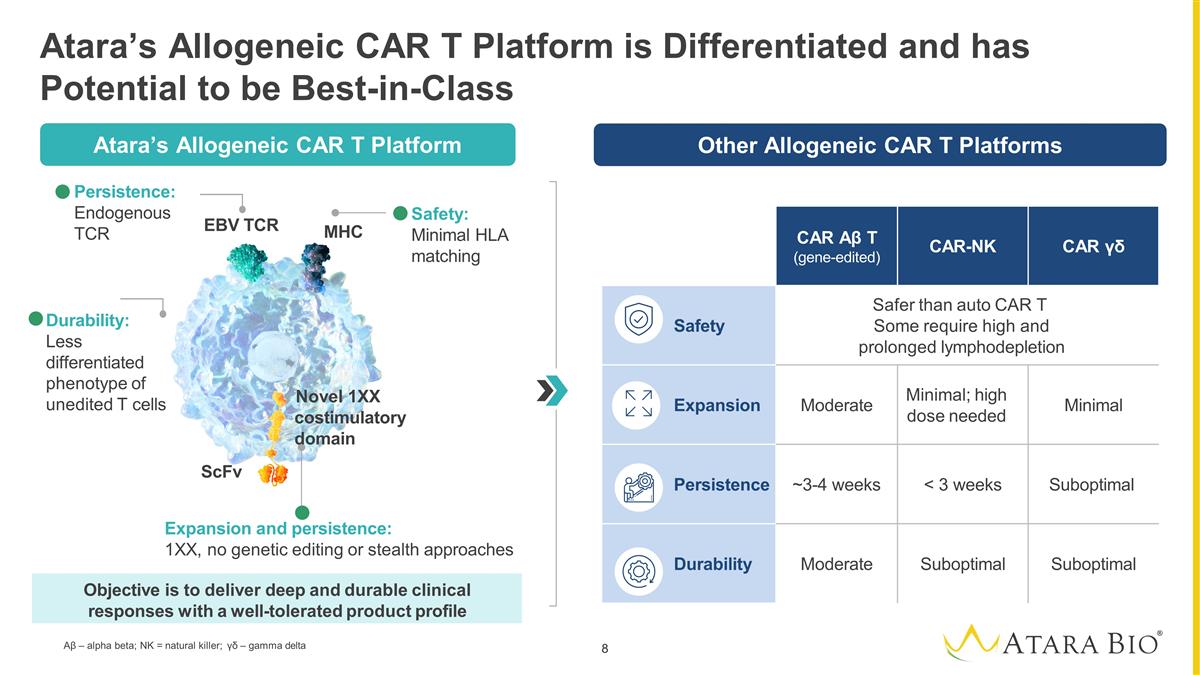
Other Allogeneic CAR Platforms
Atara’s Allogeneic CAR T Platform is Differentiated and has Potential to be Best-in-Class CAR Αβ T (gene-edited) CAR-NK CAR γδ Safety Safer than auto CAR T Some require high and prolonged lymphodepletion
Expansion Moderate Minimal; high dose needed Minimal Persistence ~3-4 weeks < 3 weeks Suboptimal Durability Moderate Suboptimal Suboptimal Other Allogeneic CAR T Platforms ScFv EBV TCR MHC Persistence: Endogenous TCR Expansion and persistence:
1XX, no genetic editing or stealth approaches Safety: Minimal HLA matching Objective is to deliver deep and durable clinical responses with a well-tolerated product profile Atara’s CAR Platform Durability: Less differentiated phenotype of
unedited T cells Novel 1XX costimulatory domain Atara’s Allogeneic CAR T Platform Αβ – alpha beta; NK = natural killer; γδ – gamma delta
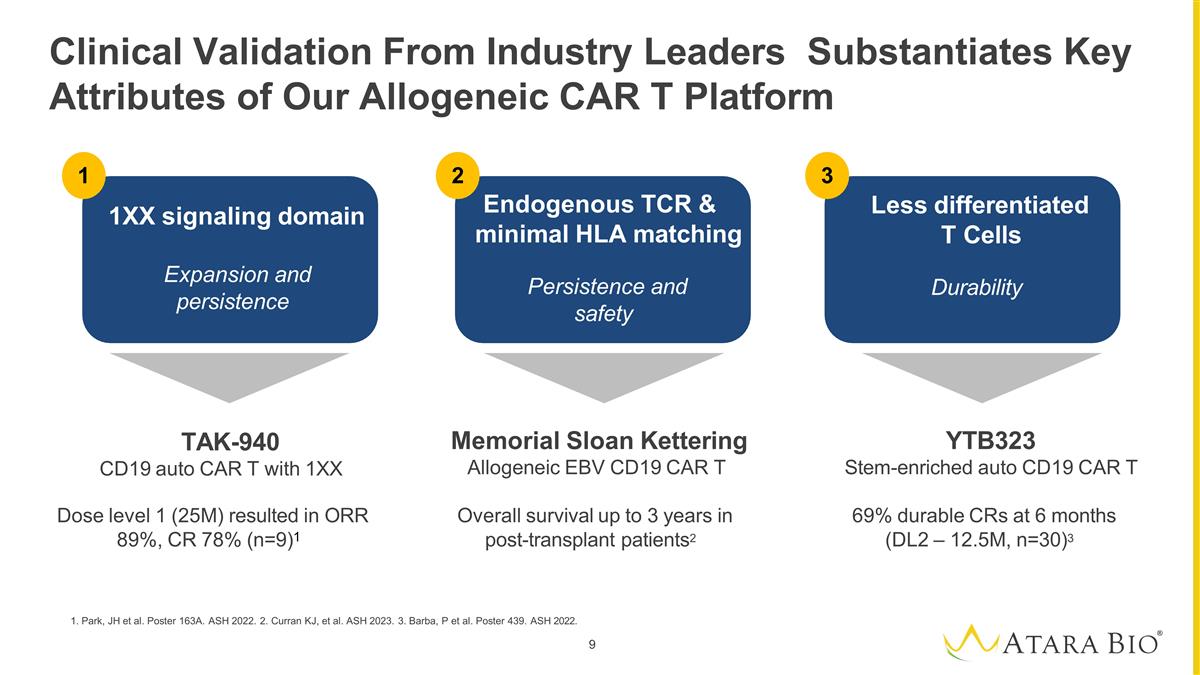
1. Park, JH et al. Poster 163A. ASH
2022. 2. Curran KJ, et al. ASH 2023. 3. Barba, P et al. Poster 439. ASH 2022. 9 Clinical Validation From Industry Leaders Substantiates Key Attributes of Our Allogeneic CAR T Platform TAK-940 CD19 auto CAR T with 1XX Dose level 1
(25M) resulted in ORR 89%, CR 78% (n=9)1 Memorial Sloan Kettering Allogeneic EBV CD19 CAR T Overall survival up to 3 years in post-transplant patients2 YTB323 Stem-enriched auto CD19 CAR T 69% durable CRs at 6 months (DL2 – 12.5M, n=30)3
1 2 3 Less differentiated T Cells Durability 1XX signaling domain Expansion and persistence Endogenous TCR & minimal HLA matching Persistence and safety
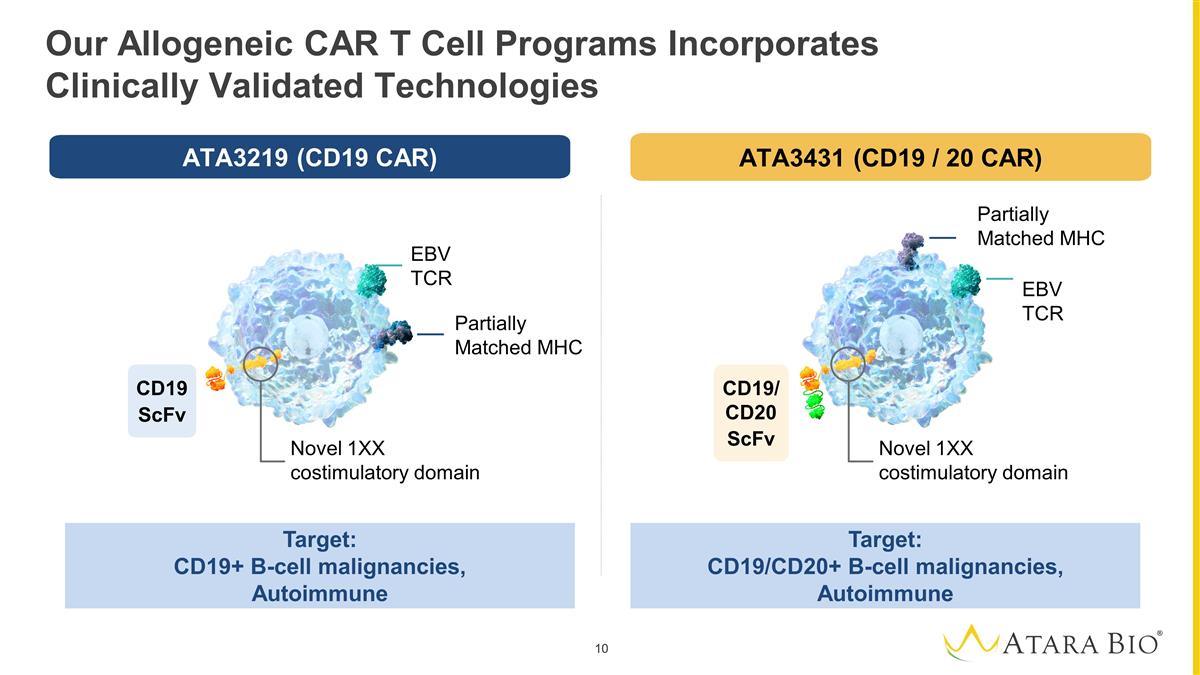
Our Allogeneic CAR T Cell Programs
Incorporates Clinically Validated Technologies Target: CD19+ B-cell malignancies, Autoimmune Target: CD19/CD20+ B-cell malignancies, Autoimmune EBV TCR Partially Matched MHC Novel 1XX costimulatory domain Novel 1XX costimulatory domain Partially
Matched MHC EBV TCR CD19 ScFv CD19/CD20 ScFv ATA3219 (CD19 CAR) ATA3431 (CD19 / 20 CAR)
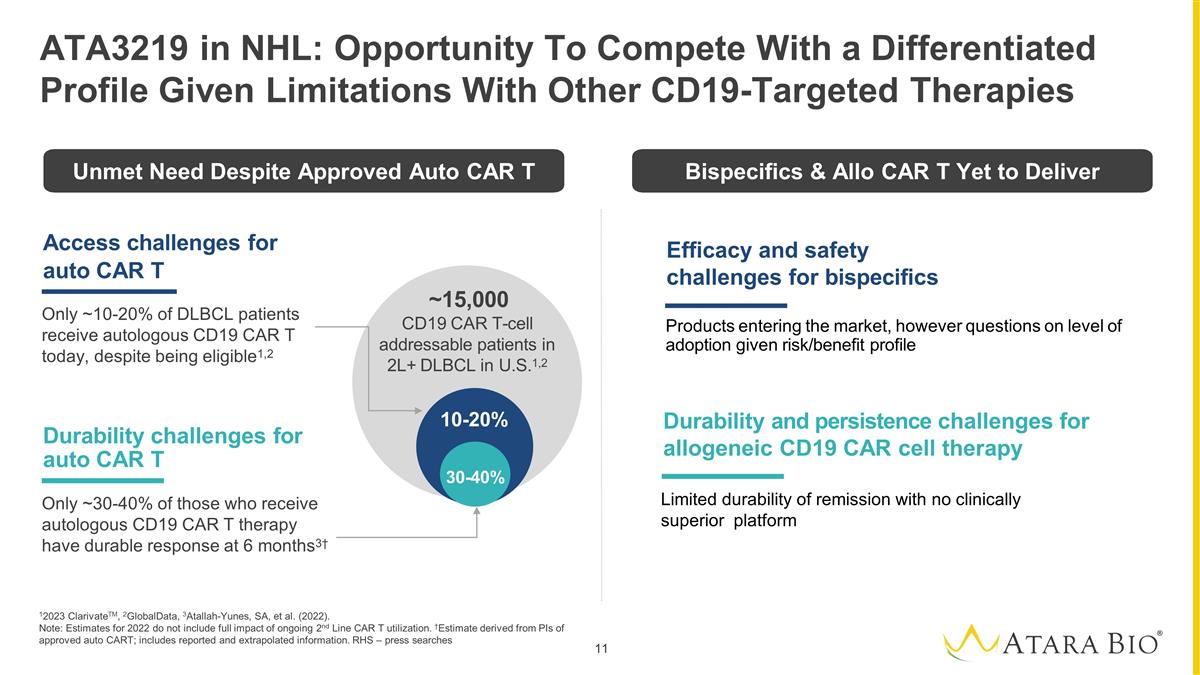
Access challenges for auto CAR T
Only ~10-20% of DLBCL patients receive autologous CD19 CAR T today, despite being eligible1,2 Durability challenges for auto CAR T Only ~30-40% of those who receive autologous CD19 CAR T therapy have durable response at 6 months3† 10-20%
30-40% ~15,000 CD19 CAR T-cell addressable patients in 2L+ DLBCL in U.S.1,2 12023 ClarivateTM, 2GlobalData, 3Atallah-Yunes, SA, et al. (2022). Note: Estimates for 2022 do not include full impact of ongoing 2nd Line CAR T utilization. †Estimate
derived from PIs of approved auto CART; includes reported and extrapolated information. RHS – press searches ATA3219 in NHL: Opportunity To Compete With a Differentiated Profile Given Limitations With Other CD19-Targeted Therapies Durability
and persistence challenges for allogeneic CD19 CAR cell therapy Efficacy and safety challenges for bispecifics Products entering the market, however questions on level of adoption given risk/benefit profile Unmet Need Despite Approved Auto CAR T
Bispecifics & Allo CAR T Yet to Deliver Limited durability of remission with no clinically superior platform
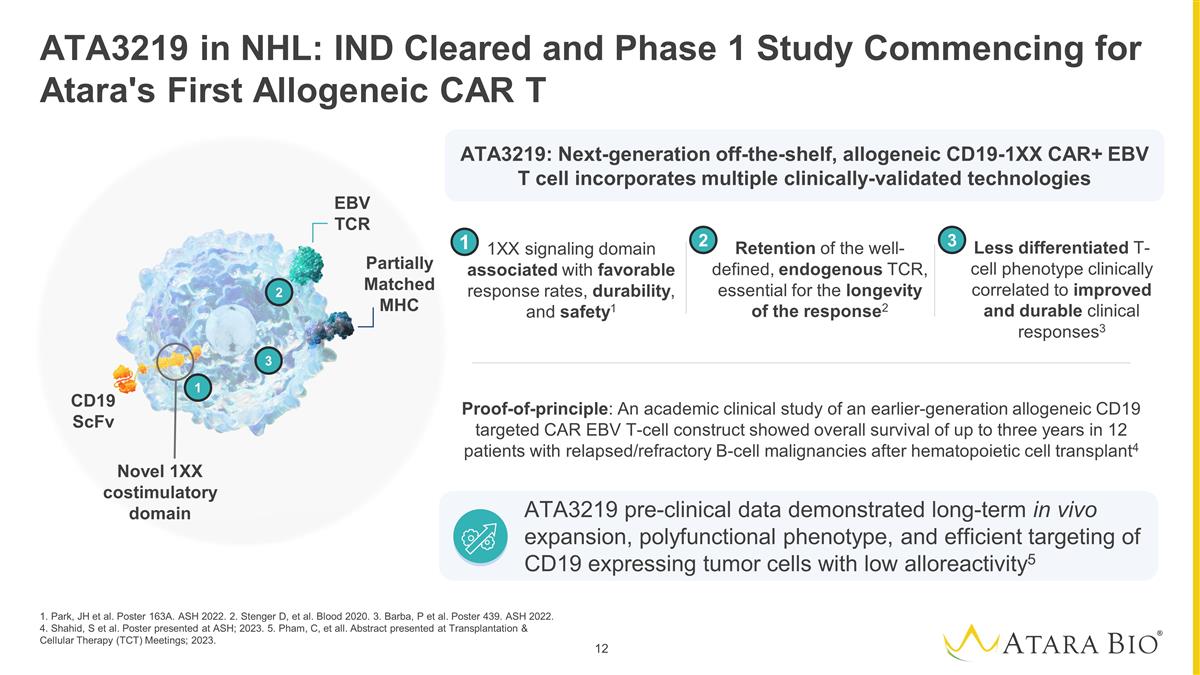
ATA3219 pre-clinical data
demonstrated long-term in vivo expansion, polyfunctional phenotype, and efficient targeting of CD19 expressing tumor cells with low alloreactivity5 ATA3219 in NHL: IND Cleared and Phase 1 Study Commencing for Atara's First Allogeneic CAR T
1. Park, JH et al. Poster 163A. ASH 2022. 2. Stenger D, et al. Blood 2020. 3. Barba, P et al. Poster 439. ASH 2022. 4. Shahid, S et al. Poster presented at ASH; 2023. 5. Pham, C, et all. Abstract presented at Transplantation & Cellular
Therapy (TCT) Meetings; 2023. Less differentiated T- cell phenotype clinically correlated to improved and durable clinical responses3 1XX signaling domain associated with favorable response rates, durability, and safety1 Retention of the
well-defined, endogenous TCR, essential for the longevity of the response2 Proof-of-principle: An academic clinical study of an earlier-generation allogeneic CD19 targeted CAR EBV T-cell construct showed overall survival of up to three years in 12
patients with relapsed/refractory B-cell malignancies after hematopoietic cell transplant4 2 1 3 Novel 1XX costimulatory domain EBV TCR CD19 ScFv Partially Matched MHC 2 1 3 ATA3219: Next-generation off-the-shelf, allogeneic CD19-1XX CAR+ EBV T cell
incorporates multiple clinically-validated technologies
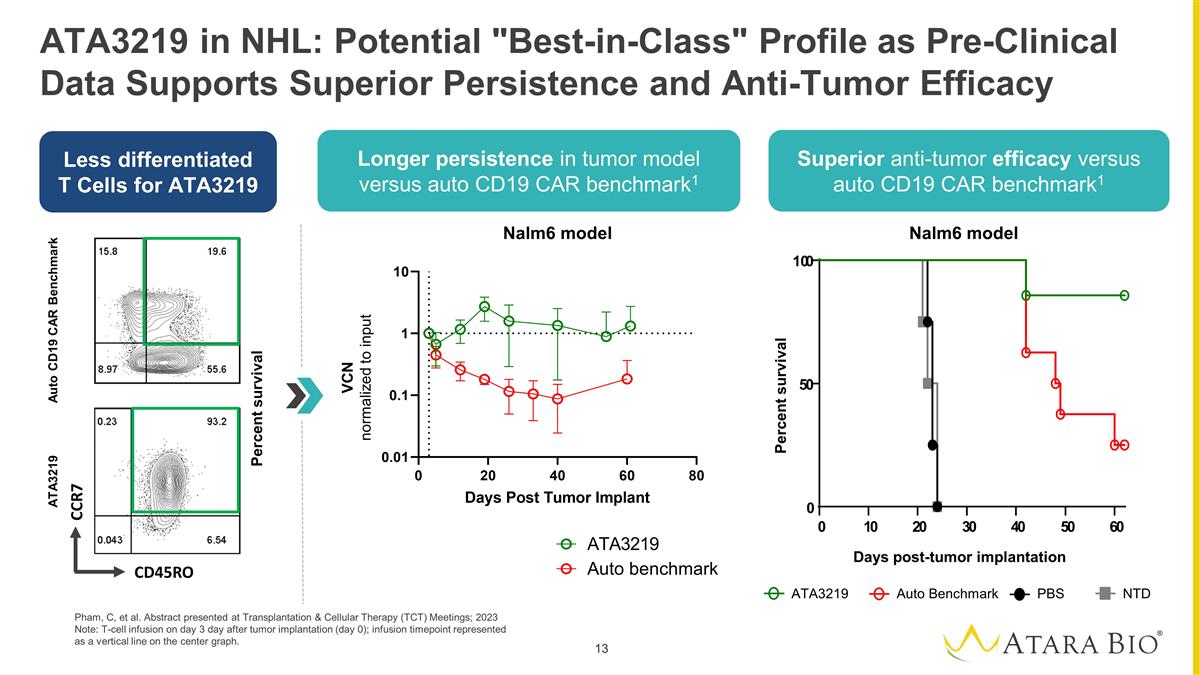
ATA3219 in NHL: Potential
"Best-in-Class" Profile as Pre-Clinical Data Supports Superior Persistence and Anti-Tumor Efficacy Pham, C, et al. Abstract presented at Transplantation & Cellular Therapy (TCT) Meetings; 2023 Note: T-cell infusion on day 3 day after tumor
implantation (day 0); infusion timepoint represented as a vertical line on the center graph. Less differentiated T Cells for ATA3219 Longer persistence in tumor model versus auto CD19 CAR benchmark1 Superior anti-tumor efficacy versus auto CD19 CAR
benchmark1 Nalm6 model ATA3219 Auto Benchmark PBS NTD Days post-tumor implantation Percent survival 0 1 0 2 0 3 0 4 0 5 0 6 0 0 5 0 10 0 VCN normalized to input Auto CD19 CAR Benchmark ATA3219 Percent survival CCR7 CD45RO Nalm6 model
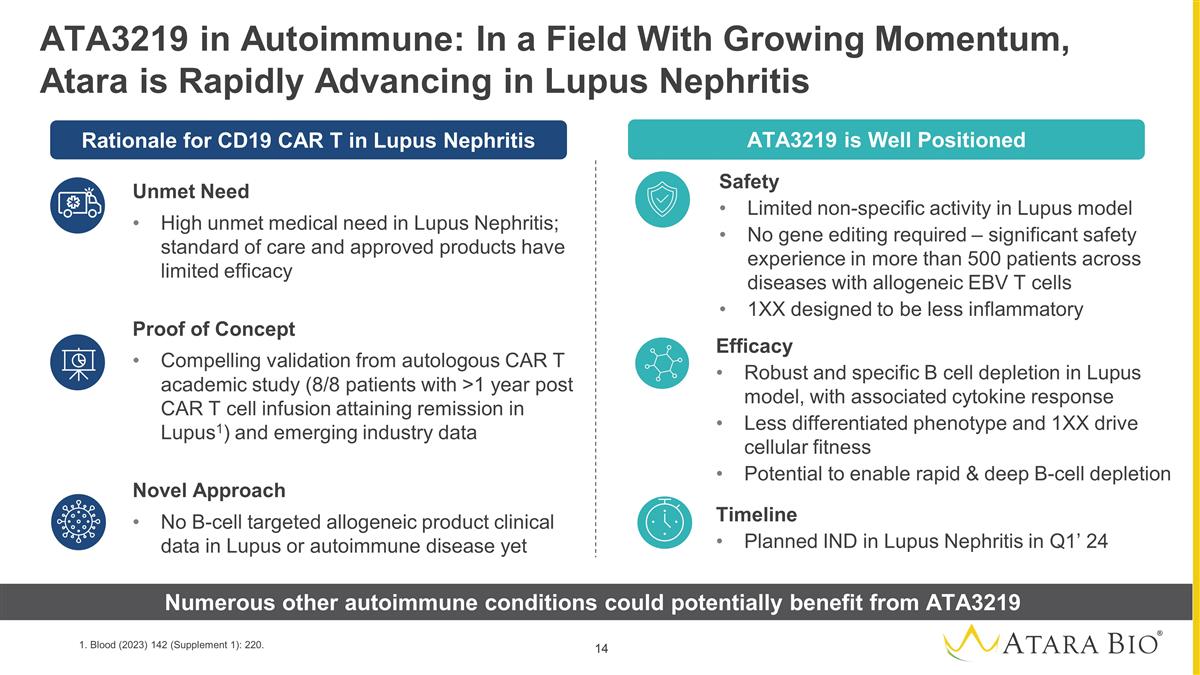
Timeline Planned IND in Lupus
Nephritis in Q1’ 24 ATA3219 in Autoimmune: In a Field With Growing Momentum, Atara is Rapidly Advancing in Lupus Nephritis Unmet Need High unmet medical need in Lupus Nephritis; standard of care and approved products have limited efficacy
Proof of Concept Compelling validation from autologous CAR T academic study (8/8 patients with >1 year post CAR T cell infusion attaining remission in Lupus1) and emerging industry data Novel Approach No B-cell targeted allogeneic product
clinical data in Lupus or autoimmune disease yet Rationale for CD19 CAR T in Lupus Nephritis ATA3219 is Well Positioned Efficacy Robust and specific B cell depletion in Lupus model, with associated cytokine response Less differentiated
phenotype and 1XX drive cellular fitness Potential to enable rapid & deep B-cell depletion Safety Limited non-specific activity in Lupus model No gene editing required – significant safety experience in more than 500 patients across
diseases with allogeneic EBV T cells 1XX designed to be less inflammatory Numerous other autoimmune conditions could potentially benefit from ATA3219 1. Blood (2023) 142 (Supplement 1): 220.
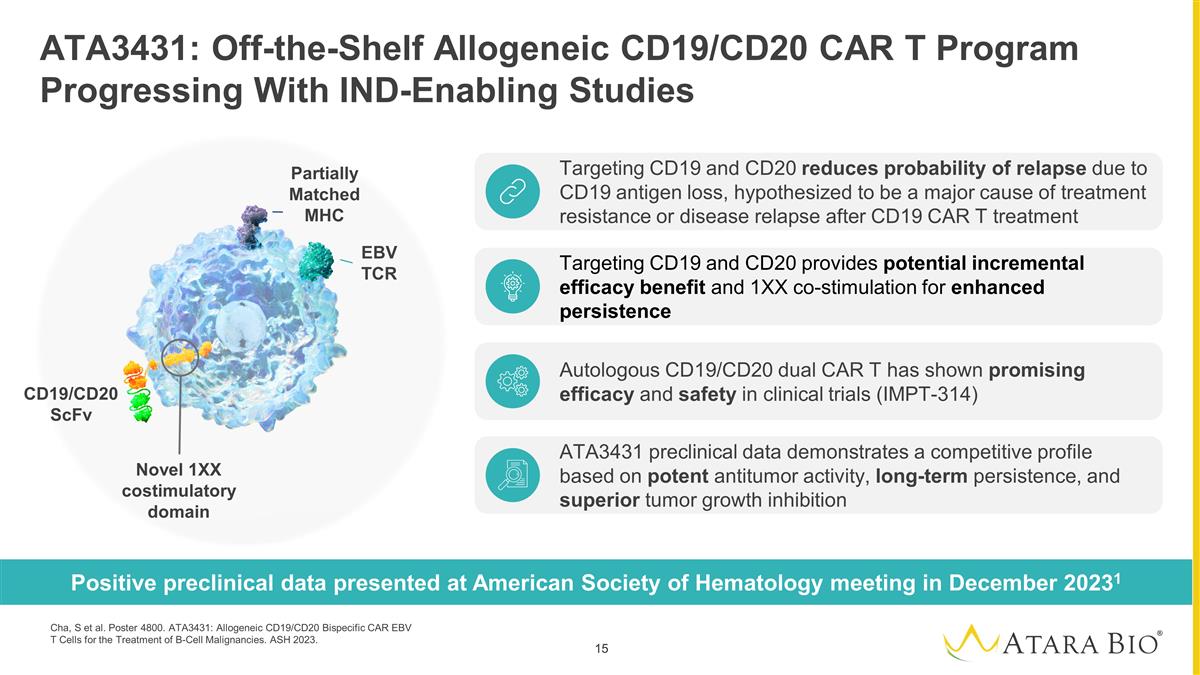
ATA3431: Off-the-Shelf Allogeneic
CD19/CD20 CAR T Program Progressing With IND-Enabling Studies Positive preclinical data presented at American Society of Hematology meeting in December 20231 Targeting CD19 and CD20 reduces probability of relapse due to CD19 antigen loss,
hypothesized to be a major cause of treatment resistance or disease relapse after CD19 CAR T treatment Targeting CD19 and CD20 provides potential incremental efficacy benefit and 1XX co-stimulation for enhanced persistence Autologous CD19/CD20
dual CAR T has shown promising efficacy and safety in clinical trials (IMPT-314) ATA3431 preclinical data demonstrates a competitive profile based on potent antitumor activity, long-term persistence, and superior tumor growth inhibition EBV TCR
Partially Matched MHC Novel 1XX costimulatory domain CD19/CD20 ScFv Cha, S et al. Poster 4800. ATA3431: Allogeneic CD19/CD20 Bispecific CAR EBV T Cells for the Treatment of B-Cell Malignancies. ASH 2023.
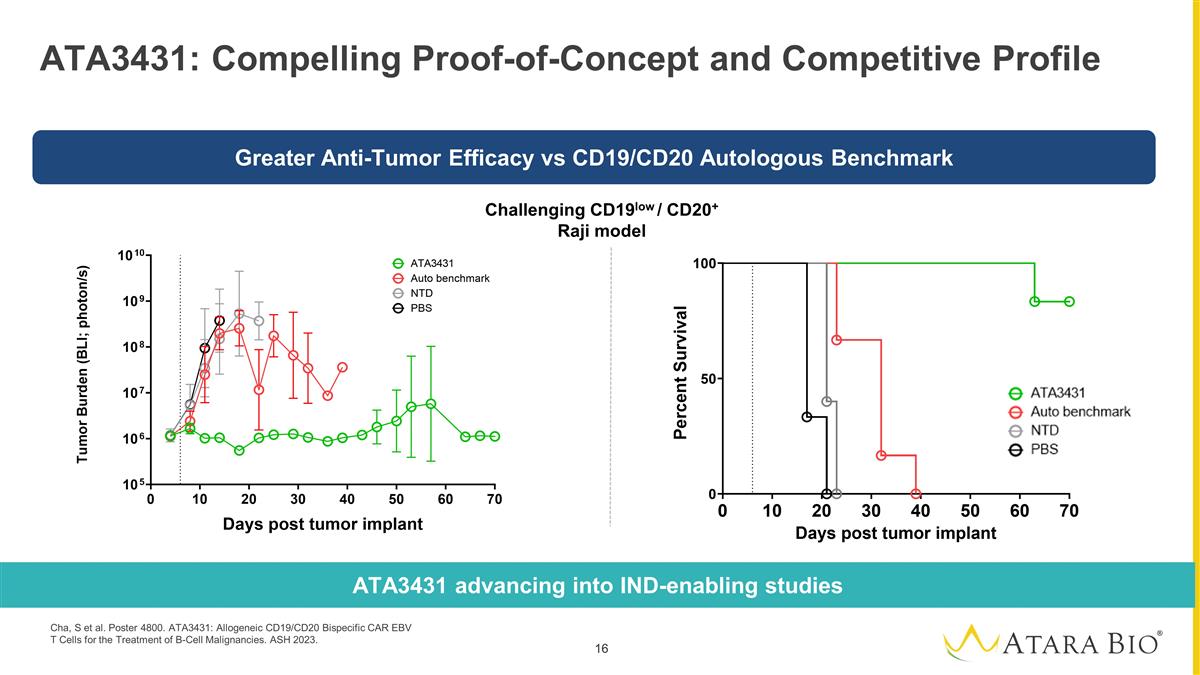
ATA3431: Compelling
Proof-of-Concept and Competitive Profile Challenging CD19low / CD20+ Raji model ATA3431 advancing into IND-enabling studies Greater Anti-Tumor Efficacy vs CD19/CD20 Autologous Benchmark Cha, S et al. Poster 4800. ATA3431: Allogeneic CD19/CD20
Bispecific CAR EBV T Cells for the Treatment of B-Cell Malignancies. ASH 2023.
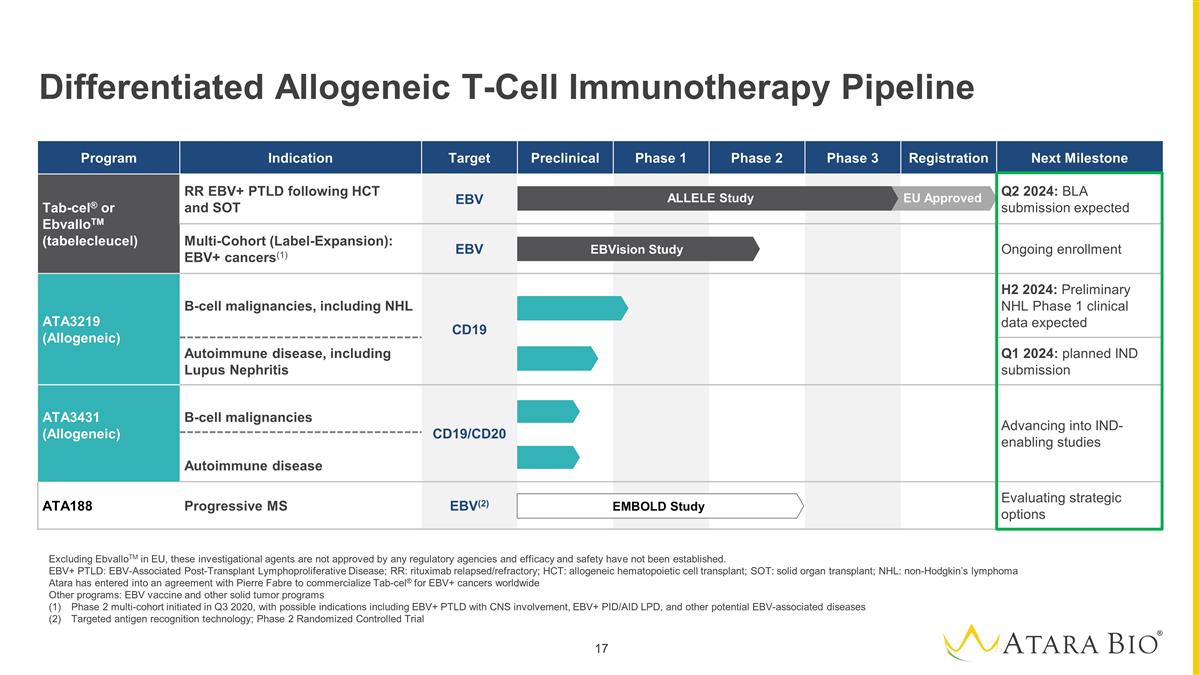
Differentiated Allogeneic T-Cell
Immunotherapy Pipeline Excluding EbvalloTM in EU, these investigational agents are not approved by any regulatory agencies and efficacy and safety have not been established. EBV+ PTLD: EBV-Associated Post-Transplant Lymphoproliferative Disease; RR:
rituximab relapsed/refractory; HCT: allogeneic hematopoietic cell transplant; SOT: solid organ transplant; NHL: non-Hodgkin’s lymphoma Atara has entered into an agreement with Pierre Fabre to commercialize Tab-cel® for EBV+ cancers
worldwide Other programs: EBV vaccine and other solid tumor programs Phase 2 multi-cohort initiated in Q3 2020, with possible indications including EBV+ PTLD with CNS involvement, EBV+ PID/AID LPD, and other potential EBV-associated diseases
Targeted antigen recognition technology; Phase 2 Randomized Controlled Trial Program Indication Target Preclinical Phase 1 Phase 2 Phase 3 Registration Next Milestone Tab-cel® or EbvalloTM (tabelecleucel) RR EBV+ PTLD following HCT and SOT EBV
Q2 2024: BLA submission expected Multi-Cohort (Label-Expansion): EBV+ cancers(1) EBV Ongoing enrollment ATA3219 (Allogeneic) B-cell malignancies, including NHL CD19 H2 2024: Preliminary NHL Phase 1 clinical data expected Autoimmune disease,
including Lupus Nephritis Q1 2024: planned IND submission ATA3431 (Allogeneic) B-cell malignancies CD19/CD20 Advancing into IND-enabling studies Autoimmune disease ATA188 Progressive MS EBV(2) Evaluating strategic options EMBOLD Study EBVision Study
EU Approved ALLELE Study

Thank You Nasdaq: ATRA
Exhibit 99.2
Atara Biotherapeutics to Present Recent Progress and Key Upcoming Milestones at the
42nd Annual J.P. Morgan Healthcare Conference
Closing of Transaction with
Pierre Fabre Laboratories to Expand Global Tab-cel® Partnership
Tab-cel
BLA on Track for Submission in Q2 2024 Following Positive New Data from Pivotal ALLELE Study
Expansion of Next-gen Allogeneic CAR-T Portfolio to Autoimmune Disease
ATA3219 IND in Lupus Nephritis Planned in Q1 2024
Focused Operational Activities and Associated Strategic Restructuring Extends Cash Runway into 2027
Thousand Oaks, Calif.—January 8, 2023--Atara Biotherapeutics, Inc. (Nasdaq: ATRA), a leader in T-cell
immunotherapy, leveraging its novel allogeneic Epstein-Barr virus (EBV) T-cell platform to develop transformative therapies for patients with cancer and autoimmune diseases, today announced Pascal Touchon,
President and Chief Executive Officer of Atara, will present the Company’s 2023 accomplishments across strategic priorities and key upcoming milestones at the 42nd Annual J.P. Morgan
Healthcare Conference on Thursday, January 11 at 9:45 a.m. PST / 12:45 p.m. EST.
“Our off-the-shelf, allogeneic CAR EBV T cell pipeline now spans both oncology and autoimmune indications and is designed to overcome current limitations of autologous CAR T and other allogeneic cell therapy
approaches. With preliminary clinical data expected later this year for ATA3219 in lymphoma and a planned IND in Lupus Nephritis in Q1, we enter 2024 with multiple opportunities for a potential best-in-class allogeneic product,” said Pascal Touchon, President and Chief Executive Officer of Atara. “Meanwhile, we are encouraged by our latest pivotal study data for tab-cel supporting our plan
to file a BLA in Q2 2024, while our global commercial partner Pierre Fabre is starting to prepare the U.S. launch.”
Tabelecleucel (tab-cel® or EBVALLOTM) for Post-Transplant Lymphoproliferative Disease (PTLD)
| |
• |
Atara is advancing toward filing a Biologics License Application (BLA) in Q2 2024, which will include the
latest pivotal ALLELE study data-cut that demonstrated a statistically significant 49% Objective Response Rate (ORR) (p<0.0001) and favorable safety profile consistent with previous analyses
|
| |
• |
This new data set augments the extensive database of pivotal and supportive data as part of the upcoming BLA
filing package, collectively consisting of approximately 450 patients treated with tab-cel across multiple life-threatening diseases |
| |
• |
The expanded global partnership with Pierre Fabre Laboratories for the U.S. and remaining global commercial
markets for tab-cel closed on December 20, 2023 |
| |
• |
Under the agreement, Atara received approximately USD 27 million in cash upfront at the closing of the
deal, with the potential to receive up to a total of USD 640 million in milestone payments, development funding, and significant double-digit tiered royalties on net sales |
Tab-cel for Potential Indication Expansion
| |
• |
Positive new clinical data from a combined analysis, including the first reported data from the multicohort
Phase 2 EBVision trial, were presented during an oral session at the ESMO Immuno-Oncology Annual Congress |
| |
• |
In the pooled analysis, an ORR of 77.8% was observed in 18 central nervous system (CNS) EBV+ PTLD patients
including 1 CNS EBV+ PTLD patient with no prior treatment, who achieved a complete response |
| |
• |
One- and two-year overall
survival rates were higher in responders (85.7% and 66.7%, respectively) versus non-responders (0% and 0%, respectively) |
| |
• |
Tab-cel was well tolerated, with no reports of serious treatment-related fatal or life-threatening
treatment-emergent adverse events (TEAEs), and no reports of serious treatment-related TEAEs of neurotoxicity, organ rejection, graft versus host disease, or tumor flare reaction of any grade |
| |
• |
Enrollment is continuing at sites in the potential label expansion multi-cohort Phase 2 EBVision trial
evaluating new patient populations, including 1L EBV+ PTLD and EBV+ immunodeficiency-associated lymphoproliferative diseases (IA-LPDs) |
CAR-T Programs (Hematological Malignancies and Autoimmune Conditions)
ATA3219
| |
• |
Atara is progressing development of ATA3219, an allogeneic, off-the-shelf CAR T targeting CD19, optimized for a memory phenotype and incorporating a next generation 1XX signaling domain |
| |
• |
Pre-clinical data support a potential best-in-class profile with longer persistence and superior anti-tumor efficacy compared to an autologous CD19 CAR T benchmark |
| |
• |
Site selection and activation is ongoing for the Phase 1 study in relapsed/refractory B-cell non-Hodgkin’s lymphoma (NHL) and progressing toward enrolling the first patient in Q1 2024 |
| |
• |
Preliminary clinical data in lymphoma anticipated H2 2024 |
| |
• |
Planned Q1 2024 IND submission in Lupus Nephritis following compelling clinical results from autologous CD19
CAR T academic clinical study showing 8/8 patients attaining remission1 |
| |
• |
Atara’s EBV CAR T cells may offer a differentiated therapeutic approach—off-the-shelf accessibility, no requirement for gene editing, and a less differentiated phenotype driving cellular fitness—with the potential for rapid and deep B-cell depletion |
| |
• |
ATA3219 autoimmune development is building upon the favorable safety profile of Atara’s allogeneic EBV T
cells in autoimmune disease |
ATA3431
| |
• |
|
Positive preclinical data presented at ASH for ATA3431, an allogeneic, dual-targeted CAR directed against CD20
and CD19 to mitigate CD19 antigen escape, built on Atara’s EBV T-cell platform with novel 1XX stimulation for enhanced persistence |
| |
• |
|
Data showed superior in vivo anti-tumor activity, survival, and functional persistence of ATA3431 compared
to an autologous CD20- CD19 CAR-T benchmark |
| |
• |
|
Atara is advancing ATA3431 into IND-enabling studies
|
Strategic Restructure and Financial Impact
| |
• |
|
Atara is undertaking a strategic restructuring and reducing its current workforce of 225 by approximately 25%
reflecting its evolving corporate strategy and pipeline focus to progress its potential best-in-class allogeneic CAR-T portfolio
for cancer and autoimmune diseases |
| |
• |
|
Atara will focus on executing its remaining responsibilities under the tab-cel collaboration with Pierre Fabre
Laboratories, including filing the BLA in Q2 2024, and advancing its differentiated allogeneic CAR-T (AlloCAR-T) ATA3219 and ATA3431 programs to key milestones in 2024 |
| |
• |
|
The strategic restructuring, combined with anticipated payments upon successful filing and approval of tab-cel
BLA from our expanded global partnership, and the Company’s existing cash, cash equivalents and short-term investments as of September 30, 2023, is expected to fund the Company’s planned operations into 2027 |
A live audio webcast of the presentation will be available by visiting the Investors & Media – News & Events section of
atarabio.com on Thursday, January 11, at 9:45 a.m. PST / 12:45 p.m. EST. An archived replay of the webcast will be available on the Company’s website for 30 days following the live presentation. A new corporate presentation will be
available on Monday, January 8 at 8:00 a.m. EST / 5:00 a.m. PST.
Next-Generation Allogeneic CAR-T
Approach
Atara is focused on applying Epstein-Barr virus (EBV) T-cell biology, featuring experience in over
500 patients treated, and novel chimeric antigen receptor (CAR) technologies to meet the current limitations of autologous and allogeneic CAR therapies head-on by advancing a potential best-in-class CAR-T pipeline
| 1 |
Blood (2023) 142 (Supplement 1): 220.
|
in oncology and autoimmune disease. Unlike gene-edited approaches aimed at inactivating T-cell receptor (TCR) function to reduce the risk for graft-vs-host disease, EBV T cells maintain expression of native TCRs that promote in vivo functional persistence while also demonstrating inherently low alloreactivity due to
their recognition of defined viral antigens and partial human leukocyte antigen (HLA) matching. A molecular toolkit of clinically-validated technologies—including the 1XX costimulatory domain designed for better cell fitness and less exhaustion
while maintaining stemness— offers a differentiated approach to addressing significant unmet need with the next generation CAR T.
About Atara
Biotherapeutics, Inc.
Atara is harnessing the natural power of the immune system to develop off-the-shelf cell therapies for difficult-to-treat cancers and autoimmune conditions that can be rapidly delivered to patients
within days. With cutting-edge science and differentiated approach, Atara is the first company in the world to receive regulatory approval of an allogeneic T-cell immunotherapy. Our advanced and versatile
Epstein-Barr virus (EBV) T-cell platform does not require T-cell receptor or HLA gene editing and forms the basis of a diverse portfolio of investigational therapies
that target EBV, the root cause of certain diseases, in addition to next-generation AlloCAR-Ts designed for best-in-class
opportunities across a broad range of hematological malignancies and B-cell driven autoimmune diseases. Atara is headquartered in Southern California. For more information,
visit atarabio.com and follow @Atarabio on X (formerly known as Twitter) and LinkedIn.
Forward-Looking Statements
This press release contains
or may imply “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. For example, forward-looking statements include statements
regarding the development, data, timing and progress, as applicable, of Atara’s (i) tab-cel program, including a potential BLA for tab-cel in the United States, and the amended and restated commercialization agreement with Pierre Fabre,
(ii) AlloCAR-T programs, including the Phase 1 study of ATA3219 in relapsed/refractory B-cell NHL, preclinical data for ATA3431, the potential characteristics and benefits of ATA3431, and potential IND
submissions for ATA3431 and for ATA3219 to treat Lupus Nephritis, (iii) restructuring, including the potential cost-savings and other financial impacts related thereto and (iv) cash runway. Because such statements deal with future events
and are based on Atara’s current expectations, they are subject to various risks and uncertainties and actual results, performance or achievements of Atara could differ materially from those described in or implied by the statements in this
press release. These forward-looking statements are subject to risks and uncertainties, including, without limitation, risks and uncertainties associated with the costly and time-consuming pharmaceutical product development process and the
uncertainty of clinical success; the COVID-19 pandemic and the wars in Ukraine and the Middle East, which may significantly impact (i) our business, research, clinical development plans and operations,
including our operations in Southern California and Denver and at our clinical trial sites, as well as the business or operations of our third-party manufacturer, contract research organizations or other third parties with whom we conduct business,
(ii) our ability to access capital, and (iii) the value of our common stock; the sufficiency of Atara’s cash resources and need for additional capital; and other risks and uncertainties affecting Atara’s and its development
programs, including those discussed in Atara’s filings with the Securities and Exchange Commission , including in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of
Operations” sections of the Company’s most recently filed periodic reports on Form 10-K and Form 10-Q and subsequent filings and in the documents incorporated
by reference therein. Except as otherwise required by law, Atara disclaims any intention or obligation to update or revise any forward-looking statements, which speak only as of the date hereof, whether as a result of new information, future events
or circumstances or otherwise.
Investor and Media Relations:
Alex Chapman
Vice President, Corporate Communications &
Investor Relations
(805) 456-4772
achapman@atarabio.com
Jason Awe, Ph.D.
Senior Director, Corporate Communications & Investor Relations
(805) 217-2287
jawe@atarabio.com
# # #
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Atara Biotherapeutics (NASDAQ:ATRA)
Historical Stock Chart
From Apr 2024 to May 2024
Atara Biotherapeutics (NASDAQ:ATRA)
Historical Stock Chart
From May 2023 to May 2024