UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10K
☒ANNUAL REPORT PURSUANT TO SECTION 13
OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended June 30, 2023
or
☐ TRANSITION REPORT PURSUANT TO SECTION
13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ________________
to ________________
Commission file number: 000-51060
CHINA HEALTH INDUSTRIES HOLDINGS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | | 86-0827216 |
| (State or other jurisdiction of | | (I.R.S. Employer |
| incorporation or organization) | | Identification No.) |
| 3199-1 Longxiang Road, Songbei District, Harbin City | | |
| Heilongjiang Province | | |
| People’s Republic of China | | 150028 |
| (Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including
area code: 86-451-88100688
Securities registered pursuant to Section 12(b)
of the Act:
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Not applicable |
|
Not applicable |
|
Not applicable |
Securities registered pursuant to Section 12(g)
of the Act:
Common Stock, $0.0001 par value
Title of Class
Indicate by check mark if the registrant is a
well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☐ Yes ☒ No
Indicate by check mark if the registrant is not
required to file reports pursuant to Section 13 or Section 15(d) of the Act. ☐ Yes ☒ No
Indicate by check mark whether the registrant
(1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months
(or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements
for the past 90 days. ☒ Yes ☐ No
Indicate by check mark whether the registrant
has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405
of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☒ Yes
☐ No
Indicate by check mark whether the registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company.
See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,”
and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | | Accelerated filer ☐ |
| Non-accelerated filer ☒ | | Smaller reporting company ☒ |
| | | Emerging Growth Company ☐ |
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant
has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial
reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or
issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant
included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether
any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the
registrant's executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant
is a shell company (as defined in Rule 12b-2 of the Act). ☐ Yes ☒ No
The aggregate market value of the voting and non-voting
common stock of the issuer held by non-affiliates as of December 31, 2022 was approximately $7,655,533 (45,032,549 shares of common stock
held by non-affiliates) based upon the closing price of the common stock on such date.
As of November 10, 2023, there were 65,539,737
shares of common stock, par value $0.0001 issued and outstanding.
Table of Contents
PART I
Item 1. Business.
China Health Industries Holdings, Inc. (“China
Health US”, “we”, “our”, or “us”) is not a Chinese operating Company, but a Delaware holding
company. Our operations are solely conducted through our subsidiaries in PRC. By investing in the securities of us, you are holding the
common stock of the Delaware holding company, under no circumstance will you have any shares of any of our operating PRC subsidiaries.
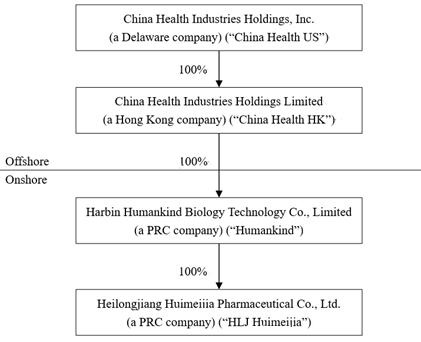
Our History and Corporate Structure
China Health Industries Holdings, Inc. (“China
Health US”) was incorporated in the State of Arizona on July 11, 1996 and was the successor of the business known as Arizona Mist,
Inc. which began in 1989. On May 9, 2005, it entered into a stock purchase agreement and share exchange (effecting a reverse merger) with
Edmonds 6, Inc. (“Edmonds 6”), a Delaware corporation, and changed its name to Universal Fog, Inc. Pursuant to this agreement,
Universal Fog, Inc. (which has been in continuous operation since 1996) became a wholly-owned subsidiary of Edmonds 6.
China Health Industries Holdings Limited (“China
Health HK”) was incorporated on July 20, 2007 in Hong Kong under the Companies Ordinance as a limited liability company. China Health
HK was formed for the purpose of seeking and consummating a merger or acquisition with a business entity organized as a private corporation,
partnership, or sole proprietorship as defined by FASB ACS Topic 915 (“Development Stage Entities”).
Harbin Humankind Biology Technology Co., Limited
(“Humankind”) was incorporated in Harbin City, Heilongjiang Province, the People’s Republic of China (the “PRC”)
on December 14, 2003, as a limited liability company under the Company Law of the PRC. Humankind is engaged in the manufacturing and sale
of health products.
On August 20, 2007, the sole shareholder of China
Health HK entered into a share purchase agreement (the “Share Purchase Agreement”) with the owners of Humankind. Pursuant
to the Share Purchase Agreement, China Health HK purchased 100% of the ownership in Humankind for a cash consideration of $60,408 (the
“Share Purchase”). Subsequent to the completion of the Share Purchase, Humankind became a wholly-owned subsidiary of China
Health HK. The Share Purchase was accounted for as a “reverse merger” since the owner of Humankind owned a majority of the
outstanding shares of China Health HK’s common stock immediately following the execution of the Share Purchase Agreement, it was
deemed to be the accounting acquirer in the reverse merger. Consequently, the assets and liabilities and the historical operations that
have been reflected in the financial statements for periods prior to the Share Purchase are those of Humankind and have been recorded
at the historical cost basis. After completion of the Share Purchase, China Health HK’s consolidated financial statements include
the assets and liabilities of both China Health HK and Humankind, the historical operations of Humankind, and the operations of China
Health HK and its subsidiaries from the closing date of the Share Purchase onward.
On October 14, 2008, Humankind set up a 99% owned
subsidiary, Harbin Huimeijia Medicine Company (“Huimeijia”), with its primary business being manufacturing and distributing
medicine. Mr. Xin Sun, the Company’s majority owner, owns 1% of Huimeijia. Huimeijia is consolidated in the consolidated financial
statements of China Health HK.
On December 31, 2008, China Health HK entered
into a reverse merger with Universal Fog, Inc., a U.S. publicly traded shell company (the “Transaction”). China Health HK
is the acquirer in the Transaction, and the Transaction was treated as a recapitalization of China Health US. After the Transaction and
a 20:1 reverse stock split, Mr. Xin Sun owned 61,203,088 shares of common stock, representing 98.3% of the 62,234,737 total outstanding
shares of common stock of China Health US. On April 7, 2009, Mr. Sun transferred 28,200,000 shares of common stock to 296 individuals,
leaving him with 33,003,088 shares of common stock of China Health US, or approximately 53.03% of the total outstanding shares of common
stock. Universal Fog, Inc. changed its name to China Health Industries Holdings, Inc. on February 19, 2009.
On November 22, 2013, Humankind completed the
acquisition of Heilongjiang Huimeijia Pharmaceutical Co., Ltd. (“HLJ Huimeijia”) for a total purchase price of $16,339,869
(RMB100,000,000). HLJ Huimeijia was founded on October 30, 2003, and is engaged in the manufacturing and distribution of tincture, ointments,
rubber paste (including hormones), topical solution, suppositories, liniment (including traditional Chinese medicine extractions), enemas
and oral liquids. HLJ Huimeijia’s predecessor is Heilongjiang Xue Du Pharmaceutical Co., Ltd., which has established its brand name
in the market through its supply of high-quality medical products. HLJ Huimeijia is categorized as a “high and new technology”
enterprise by the Science Technology Department in Heilongjiang Province. HLJ Huimeijia has 21 products which have been approved by, and
have received approval numbers issued by, the National Medical Products Administration (“NMPA”). In addition, HLJ Huimeijia
is the holder of one patent for utility models, five patents for external design and three trademarks in China, including the Chinese
brand name of “Xue Du” which has an established reputation among customers in northeastern China.
On December 24, 2014, Humankind entered into a
stock transfer agreement (the “Original Agreement”) with (i) Xiuzheng Pharmaceutical Group Co., Ltd. a company incorporated
under the laws of the PRC and located in Jilin province (“Xiuzheng Pharmacy” or the “Buyer”), (ii) Mr. Xin Sun,
the CEO of the Company, and (iii) Huimeijia, a subsidiary of Humankind that is 99% owned by Humankind and 1% owned by Mr. Xin Sun. Pursuant
to the Original Agreement, Humankind and Mr. Xin Sun (collectively, the “Equity Holders”), would sell their respective equity
interests in Huimeijia to Xiuzheng Pharmacy.
On February 9, 2015, the four parties entered
into a supplementary agreement (the “Supplementary Agreement”) to modify the terms of the Original Agreement, pursuant to
which the Equity Holders and Huimeijia (collectively, the “Asset Transferors”) would only sell 19 drug approval numbers (the
“Assets”) to Xiuzheng Pharmacy. The Equity Holders would have retained their equity interests in Huimeijia, but would have
pledged such equity interests to Xiuzheng Pharmacy until the Assets were transferred.
On October 12, 2016, the four parties agreed to
rescind the Supplementary Agreement and entered into a new supplementary agreement pursuant to which the parties agreed to execute the
transfer of the equity interests based on the Original Agreement, and the Equity Holders sold their respective equity interests in Huimeijia
to Xiuzheng Pharmacy for total cash consideration of RMB 8,000,000 (approximately $1,306,186 USD, the “Purchase Price”) to
the Equity Holders. As of October 12, 2016, Huimeijia had completed changes in its business registration, and Xiuzheng Pharmacy had obtained
a new business license issued by the local State Administration of Industry and Commerce in Harbin (“Harbin SAIC”) for Huimeijia,
in which Huimeijia’s ownership was recorded as held by Xiuzheng Pharmacy, and the legal representative (a person that is authorized
to take most corporate actions on behalf of a company under PRC corporate laws) of Huimeijia had been appointed by the Buyer.
On
June 27, 2023 (the “Signing Date”),
Harbin Humankind Biology Technology Co., Limited (“Humankind”),
a wholly owned subsidiary of China Health Industries Holdings, Inc. (“China
Health”), a corporation incorporated under the laws of the State
of Delaware, entered into certain equity transfer agreements (collectively, the “Agreements”)
with Mr. Xin Sun and Mr. Kai Sun (collectively, the “Sellers”).
Pursuant to the Agreements, the Sellers agreed to transfer to Humankind 99% and 1% of the equity interests of Heilongjiang HempCan Pharmaceuticals
Co., Ltd. (“HempCan”),
for a consideration of RMB292,050,000 (approximately $40,275,537) and RMB2,950,000 (approximately $406,824) respectively, totaling RMB295
million (approximately $40,682,360) (collectively, the “Purchase
Prices”). The Purchase Prices shall be fully paid to Sellers within
15 business days after the Signing Date, and the Sellers shall complete certain registration procedures, such as change of equity ownership
and change of business registration within 15 business days after full Purchase Prices are received. If Humankind is late in paying the
Purchase Prices, a 0.1% per day late penalty on the amount of Purchase Prices that is paid late shall be paid to the Sellers. Mr. Xin
Sun is China Health’s Chairman, sole director and sole executive
officer and Mr. Kai Sun is Mr. Xin Sun’s younger brother.
China Health US, China Health HK, Humankind and
HLJ Huimeijia are collectively referred herein to as the “Company.”
As of June 30, 2023, the Company’s corporate
structure was as follows:
Business Overview
Our principal business operations are conducted
through our wholly-owned subsidiaries, Humankind and HLJ Huimeijia.
The Company owns a GMP-certified plant and production
facilities and has the capacity to produce 21 different NMPA-approved medicines, 14 NMPA-approved health supplement products and 10 hemp
derivative products in soft capsule, hard capsule, tablet, granule, oral liquid forms. These products address the needs of some key sectors
in China, including the feminine, geriatric, and children’s markets.
HLJ Huimeijia was founded on October 30, 2003
and its latest GMP certificate is effective until April 24, 2023. On December 1, 2019, NMPA announced that it will no longer issue GMP
certificate for any pharmaceutical companies. However, NMPA will carry out conformity inspection to do compliance testing, as well as
flight check (unannounced inspection) for all pharmaceutical companies. HLJ Huimeijia engages in the manufacture and distribution of tincture,
ointments, rubber paste, including hormones, topical solution, suppositories, enemas, oral liquids, and liniment, including traditional
Chinese medicine extractions. HLJ Huimeijia’s predecessor was Heilongjiang Xue Du Pharmaceutical Co., Ltd., which established brand
recognition in the market through its supply of high-quality drug products. HLJ Huimeijia is a “high and new technology” enterprise
that provides the most comprehensive types of topical medical products in Heilongjiang Province, a northeastern province of China.
For the fiscal year 2023, we sell our products
to end customers through our own sales personnel as well as our sales agents, operating primarily in Heilongjiang Province, where most
of our revenues are generated. However, there is no such sales for the fiscal year 2022, which was primarily due to Humankind’s
enterprise transformation, and the lock down of factory due to COVID-19. Humankind is using existing materials to research and develop
new products. Humankind decided to transform the primary business to CBD extractive project, because the government intended to support
the company research in CBD aspect. However, by the end of fiscal year 2023, the support guidelines had not been published. Although the
research process is slow and unpredictable due to the zero-case policy and periodic quarantines caused by COVID-19 resurgence in the first
half of the fiscal year 2023, we expect the CBD extractive project to be completed by the end of calendar year of 2024 (“Transformation”).
We do not currently sell our products online, but we expect to do so in the future.
Cash Transfer
For the years ended June 30, 2023 and 2022, there
is no cash transfer among China Health US, China Health HK, or any of our PRC subsidiaries.
We have not paid dividends on our common stock
and do not anticipate to pay such dividends in the foreseeable future. We will rely on dividends from Humankind for our funds and PRC
regulations may limit the amount of funds distributed to us from Humankind, which will affect our ability to declare any dividends.
China Health US may encounter several limitations
related to cash transfer among its PRC subsidiaries, the holding company and its investors. Any funds we transfer to the PRC subsidiaries,
either as a shareholder loan or as an increase in registered capital, are subject to permission and approval by or registration with relevant
governmental authorities in China. According to the relevant PRC regulations on foreign invested enterprises in China, capital contributions
to our PRC subsidiaries are subject to the registration with the State Administration for Market Regulation or its local counterpart and
registration with a local bank authorized by SAFE. In addition, (i) any foreign loan procured by our PRC subsidiaries is required to be
registered with the SAFE or its local branches and (ii) any of our PRC subsidiaries may not procure loans which exceed the difference
between its total investment amount and registered capital or, as an alternative, only procure loans subject to the calculation approach
and limitation as provided by the People’s Bank of China. As a holding company with no operations, our ability to distribute dividends
largely depends on the distribution from our PRC subsidiaries. In addition, if China Health US is determined to be a PRC resident enterprise
for enterprise income tax purposes, we could be subject to PRC tax at a rate of 25% on our worldwide income, which could materially reduce
our net income, and we may be required to withhold a 10% withholding tax from dividends we pay to our shareholders that are non-resident
enterprises, including the holders of our ordinary shares, and non-resident enterprise shareholders (including our ordinary shareholders)
may be subject to PRC tax at a rate of 10% on gains realized on the sale or other disposition of ordinary shares, if such income is treated
as sourced from within China. An “indirect transfer” of PRC assets, including a transfer of equity interests in an unlisted
non-PRC holding company of a PRC resident enterprise, by non-PRC resident enterprises may be re-characterized and treated as a direct
transfer of the underlying PRC assets, if such arrangement does not have a reasonable commercial purpose and was established for the purpose
of avoiding payment of PRC enterprise income tax. As a result, gains derived from such indirect transfer may be subject to PRC enterprise
income tax, and the transferee or other person who is obligated to pay for the transfer is obligated to withhold the applicable taxes,
currently at a rate of 10% for the transfer of equity interests in a PRC resident enterprise.
Products
We are licensed to sell our products, including
our medical drugs, only in the PRC.
(i) Hemp Derivative Products
We have developed the following products that
are derived from hemp and obtained business license to manufacture and sell these products. We have begun to sell these products since
May 2018. Hemp Seed Beer, Hemp Oil, Hemp Protein Powder, Hemp Polypeptide and Collagen Peptide are sold through Humankind. Other products
are sold through HLJ Huimeijia. The revenue of the Hemp Seed Beer, Hemp Oil and Hemp Seed accounted for 100% and nil of the total revenues
for the fiscal year of 2023 and 2022, respectively.
| Serial No. |
|
Name |
| 1 |
|
Hemp Oil |
| 2 |
|
Hemp Protein Powder |
| 3 |
|
Hemp Polypeptide |
| 4 |
|
Collagen Peptide |
| 5 |
|
Natural Hemp Essence Repair Lotion |
| 6 |
|
Natural Hemp Revitalizing Essence |
| 7 |
|
Natural Hemp Anit-aging Brightening Eye Cream |
| 8 |
|
Natural Hemp Frozen Age Nourishing Cream |
| 9 |
|
Hemp Seed Beer |
| 10 |
|
Hemp Seed |
(ii) Health Products
Our “QunLe” brand Sailuozhi soft capsule,
a supplement made from frog oil, soybean isoflavone, procyanidine (made from grape seeds) and vitamin E, is for freckle removal and skin
moisture. “QunLe” brand Sailuozhi soft capsulechanged name to “QunLe” brand Frog Oil, Soybean Isoflavone and Vitamin
E Soft Capsul, register number is G20070356, which expired on April 6, 2026.
On May 12, 2010, we received a patent for this
product (Patent No. 200610010394.4) under the name “Run Chao” (which has since been changed to “QunLe”) with the
National Bureau of Intellectual Property.
Pursuant to a technology transfer agreement dated
October 12, 2007 (the “2007 Technology Transfer Agreement”), we purchased a health product known as “Kindlink”
brand propolis and black ant capsule made from propolis, black ant, acanthopanax and astragalus root from Beijing Jindelikang Bio-Technology
Co., Ltd (“Jindelikang”). The change of the ownership has been approved by the NMPA. This product is intended to boost one’s
immunity. The certification number issued by the NMPA on August 20, 2004, for the license to manufacture the product is GuoShiJianZi G20040906.
We have no continuing obligations under the 2007 Technology Transfer Agreement.
Pursuant to a technology transfer agreement dated
January 18, 2013 (the “2013 Technology Transfer Agreement”), we purchased 12 health products from Guangzhou Aoda Biology Beauty
Healthy Technology Co., Ltd, a non-affiliated party. These twelve products are the following:
| - | Dr.
Xiao Brand Honeysuckle Pearl Capsule (Guo Shi Jian Zi G20100656), which is designed to be effective in acne removal; |
| - | Dr.
Xiao Brand Multivitamin Tablet (Guo Shi Jian Zi G20080176), which is a multivitamin and mineral supplement; |
| - | Dr.
Xiao Brand Zhengdian Capsule (Guo Shi Jian Zi 20070261), which is designed to be effective in relieving eyestrain; |
| - | Dr.
Xiao Brand Shengui Capsule (Guo Shi Jian Zi G20080297), which is designed to be effective in increasing bone density; |
| - | Dr.
Xiao Brand Multivitamin Tablet (Woman) (Guo Shi Jian Zi G20070338), which is an iron and multivitamin supplement; |
| - | Dr.
Xiao Brand Shikong Soft Capsule (Guo Shi Jian Zi 20080096), which is designed to be effective in improving memory; |
| - | Dr.
Xiao Brand Huangjingdanggui Tablet (Guo Shi Jian Zi G20080201), which is designed to be effective in improving nutritional anemia and
chloasma; |
| - | Dr.
Xiao Brand Xingxing Soft Capsule (Guo Shi Jian Zi G20080080), which is designed to be effective in improving memory; |
| - | Dr.
Xiao Brand Vitamin A Fish Oil Soft Capsule (Guo Shi Jian Zi G20080406), which is designed to be effective in relieving eyestrain; |
| - | Dr.
Xiao Brand Colon Cleanser Granules (Guo Shi Jian Zi G20060061), which is designed to be effective in relaxing bowels and promoting the
discharge of lead; |
| - | Dr.
Xiao Brand Jianli Soft Capsule (Guo Shi Jian Zi G20050710), which is designed to be effective in increasing immunity and relieving physical
fatigue; and |
| - | LB
Brand Xinpin Capsule (Guo Shi Jian Zi G20050770), which is designed to be effective in dispelling chloasma. |
The major suppliers of raw materials for our products
who exceeded 10% of our total purchases in the fiscal years 2023 and 2022 are the following:
| | |
| |
Purchases | | |
| |
| | |
| |
(in U.S. | | |
% of | |
| | |
Name of Supplier | |
Dollars) | | |
Purchases | |
FY2023
| |
Harbin Delunbao Beer Co., Ltd
| |
| 68,803 | | |
| 96.71 | % |
| | |
| |
| | | |
| | |
| FY2022 | |
Nil | |
| | | |
| | |
The Company typically signs monthly purchase orders
with its major suppliers. All purchase orders with our other suppliers are on similar terms. We shall remit payment to a supplier’s
account no later than three business days after receiving raw materials. A supplier shall deliver raw materials no later than three business
days after receiving a purchase order. The cost of delivery is borne by the supplier.
(iii) Medical Drugs
HLJ Huimeijia has 21 products with approval numbers
issued by the NMPA as following:
| |
|
English Name |
|
Efficacy |
| 1 |
|
Enema Glycerini |
|
Lubricating laxative. Used for constipation. |
| |
|
|
|
|
| 2 |
|
UmguentumAcidi Borici Camphoratum |
|
Dermerethistica. Used for chilblain. |
| |
|
|
|
|
| 3 |
|
Ge Hong Beriberi Water |
|
Dehumidification insecticide. Used for tinea pedis and tinea manuum caused by damp toxin brewing and binding, and other skin diseases caused by enzyme. |
| |
|
|
|
|
| 4 |
|
Pelvic Inflammation Suppository |
|
Heat-clearing and detoxifying; activating blood to promote menstruation disperse swelling and relieve pain. Used for toxin and blood stasis stagnation in the uterus, distending pain in the lower abdomen, irregular menses, algomenorrhea and leukorrhagia, as well as pelvic inflammation and annexitis with the aforementioned symptoms. |
| |
|
|
|
|
| 5 |
|
Injury and Paralysis Tincture |
|
Warm channel and expelling cold, promoting blood circulation to arrest pain. Used to relieve pain caused by traumatic injury and sprain. |
| |
|
|
|
|
| 6 |
|
Indometacin and Furazolidone Suppositories |
|
Anti – inflammatory painkiller. Used to treat acute hemorrhoid, including internal hemorrhoids, external hemorrhoids, mixed hemorrhoids, anal fissure or archosyrinx and relieve pain; Used to ease pain after the operation of anal fissure, archosyrinx or hemorrhoids. |
| |
|
|
|
|
| 7 |
|
Injury and Rheumatism Relieving Paste |
|
Dispelling rheumatism and relieving pain. Used for headache, rheumatalgia, neuralgia, sprain and muscular soreness. |
| |
|
|
|
|
| 8 |
|
Refining GouPi Cream |
|
Relaxing tendon, invigorating the circulation of blood, dissipating cold and relieving pain. Used for arthralgia and myalgia, acute contusion, sprain, rheumatalgia, arthralgia, hypochondriac pain, muscular soreness, etc. |
| |
|
|
|
|
| 9 |
|
Muskiness Pain Relieving Paste |
|
Expelling wind and removing dampness, relaxing the tendons and unblocking collateral. Used for rheumatic arthralgia, low back cold pain, traumatic injury, etc. |
| |
|
English Name |
|
Efficacy |
| 10 |
|
Muskiness Bone Strengthener Paste |
|
Analgesia and anti-inflammatory. Used for rheumatalgia, arthralgia, backache, neuralgia, muscular soreness, sprain and contusion. |
| |
|
|
|
|
| 11 |
|
Matrine Suppositories |
|
Antibacterial and antiphlogistic drugs. Used for trichomonas and candida vaginitis, chronic cervicitis, pelvic inflammation, etc. |
| |
|
|
|
|
| 12 |
|
Ethacriding Lactate Solution |
|
Disinfectant and preservative drug. Used for disinfection of traumatic and disinfected wounds. |
| |
|
|
|
|
| 13 |
|
Triamcinolone Acetonide and Neomycin Paste |
|
Used for neurodermatitis circumscripta and chronic eczema. Also used for small-scale psoriasis. |
| |
|
|
|
|
| 14 |
|
Double – Coptis Suppository |
|
Course wind and resolving the exterior, heat-clearing and detoxifying. Used for influenza caused by affection of exogenous wind-heat, with symptoms of fever, cough and sore throat. Also used for upper respiratory tract infections and pneumonia, with symptoms of fever, cough and sore throat. |
| |
|
|
|
|
| 15 |
|
Methylrosanilinium Chloride Solution |
|
Disinfectant and preservative drug. |
| |
|
|
|
|
| 16 |
|
Iodine Tincture |
|
Disinfectant and preservative drug. |
| |
|
|
|
|
| 17 |
|
Mercurochrome Solution |
|
Disinfectant and preservative drug. |
| |
|
|
|
|
| 18 |
|
Hydrogen Peroxide Solution |
|
Disinfectant and preservative drug. |
| |
|
|
|
|
| 19 |
|
Halcinonide Cream |
|
Grucocorticoid. External use drug only to be used on the skin. Used for dermatoneuritis and psoriasis. |
| |
|
|
|
|
| 20 |
|
Compound Fluocinonide Tincture |
|
Grucocorticoid. Used for dermatoneuritis and psoriasis. |
| |
|
|
|
|
| 21 |
|
Policresulen Vaginal Suppository |
|
Anti-microbial and hemostasis drug. |
Distribution
Most of our products are sold to sales agents.
In the fiscal year of 2022, because of COVID-19 influence and the Transformation, we did not sell our products to sales agents. In the
fiscal year of 2023, our sales network covered Heilongjiang provinces or cities.
E-business
We are in the process of building the infrastructure
to conduct our business over the internet. A B2C e-business call and sales center has been established and will become an integral part
of our distribution channel in the future. We have employed graduates from Tsinghua University, Harbin Industry University and Harbin
Engineering University to develop the ERP (Enterprise Resource Planning), CRM (Customer Relationship Management) and Office Automation
software (“OA Software”) for our e-business. The OA Software has been used in our daily operation. The Company plans to sell
its products via internet in the fiscal year of 2024.
Our Customers
We sell most of our products to sales agents,
who are our customers. The sales agents sell the products to the end users.
Our customers who contributed more than 10% of
our consolidated revenues during the past two fiscal years are as follows:
| | |
| |
Sales | | |
Percent of | |
| Name | |
Products Sold | |
(in U.S. Dollars) | | |
Sales | |
| FY2023 | |
| |
| | |
| |
| Harbin Magic Nature Technology Co., Ltd | |
Hemp Seed Beer, Hemp Oil | |
| 70,824 | | |
| 63.47 | % |
| Ruiping Ji | |
Hemp Seed | |
| 36,516 | | |
| 32.73 | % |
| FY2022 | |
nil | |
| | | |
| | |
Manufacture
We manufacture our health food products on a plot
of land located in Jin Xing Industrial Park, Songbei District, Harbin. On June 7, 2004, the Company entered into a Land Use Purchase Contract
with the local government, pursuant to which the Company agreed to purchase the right to use a piece of land, approximately 8 acres (32,000
square meters), located in Harbin City, Heilongjiang Province for commercial purposes for a fifty-year period from June 7, 2004 through
June 6, 2054, for $637,261 (RMB5,248,000). The Company fully paid to the government the consideration for the land use right on June 13,
2004. The Department of Housing and Urban Development of Harbin City approved this transaction. The Company is in the process of applying
for the title certificate from the local government. The manufacturing facility on the land is 4,000 square meters and there are five
production lines which are sufficient for our purposes. We package our products in bottles, plastic containers and aluminum foil bags
there.
Since we acquired HLJ Huimeijia on November 22,
2013, we also manufacture our medicines and drugs using HLJ Huimeijia’s land, approximately 43,350 square meters, located in Hai-lin
Economic Development Zone, Mudanjiang City. The manufacturing facilities occupy approximately 5,710 square meters. We plan to build new
manufacturing facilities on the land. The expected construction cost is approximately $7,520,000 (RMB50,000,000).
Our Development Strategy
We will continue to focus on combining our products
with traditional Chinese medicine, creating new products such as our hemp-based products, and developing our B2C e-business and chain-stores.
We plan to implement health management projects in our future chain-stores throughout China and establish a database of our clients’
health data obtained from our B2C e-business and call center.
We plan to establish a one-stop shop for our customer’s
health needs. From conducting a genetic profile of our customer to determine his/her susceptibility to certain types of diseases and then
customizing health supplements and organic/green food to meet his/her needs, we plan to cater to our customer’s needs at all levels.
With the distribution network we hope to establish through our chain stores and B2C e-businesses, we plan to eventually branch out into
the sale and distribution of beauty products and medical appliances.
We plan to open chain stores of up to 100 stores
within the next 24 months; establish our oversea sales to North America, South Asia and European Union; acquire pharmaceuticals to enhance
marketing network and production capacity and increase investment in research and development of CBD drugs and hemp-based products.
The Future
Within the next ten years, our goals are to:
1. Increase product coverage in target markets
to achieve 20%-30% coverage
Our target market is the health industry market.
Presently, we believe that our product coverage is approximately 0.2%. We plan to open distribution stores in different provinces of China
to expand our coverage. We also plan to sell our products through B2C websites to our customers.
2. Develop to be among top 500 companies in the
medicine, health product, health industry in the PRC
Currently, we are not ranked in the top 500 medicine,
health product and health industry companies in the PRC. We believe that if our projected increase in revenue is achieved, we will achieve
our goal of becoming one of the top 500 medicine, health product, health industry companies in China.
3. Form a diversified management group
Currently, our management group comprises graduates
from the most prestigious universities in the PRC, such as Peking University and Renmin University of China. We plan to further diversify
our management group by hiring talent both in the PRC and abroad.
4. Enter into the international market and create
an internationally famous brand
Currently, our products are sold under the brand
names “Qunle”, “Kindlink”, “Huimeijia” and “Dr. Xiao” in the PRC. Our goal is eventually
to establish stable sales abroad in countries such as the United States of America, Russia, and Eastern Europe and South-east Asian countries.
Our Business Plan
The plans designed to meet our manufacturing,
marketing and profit targets include:
Manufacturing:
| (a) | improving
the manufacturing techniques and staff training; |
| (b) | guaranteeing
high quality material supply; |
| (c) | strengthening
the working procedure controls; |
| (d) | implementing
GMP to ensure a compliance standard in the food and medical industries; |
| (e) | ensuring
that all employees have adequate training in health regulations. |
Marketing:
Adopt an effective marketing strategy to:
| (a) | utilize
direct distribution of products to chain stores nationwide; |
| (b) | build
business alliances with well-known enterprises to create private label brands; |
| (c) | expand
the marketing of our products beyond the traditional methods. |
Product Distribution:
| (a) | enlarge
our sales and marketing force while developing new markets; |
| (b) | strengthen
the distribution channel by developing promotion strategies and participating in trade shows; |
| (c) | Develop
3-5 new products to market each year; |
| (d) | develop
new markets through innovation and research. |
Our approach to manufacturing, marketing, cost
control and products distribution, which is detailed above, is designed to minimize production costs and increase revenue at the same
time. We feel that our procedures will enable us to reach our sales goals with an optimal manufacturing cost. The result should yield
profits and a return to our investors.
Good Manufacturing Practice or “GMP”
is a term that is recognized worldwide for the control and management of manufacturing and quality control testing of foods and pharmaceutical
products. An important part of GMP is documentation of every aspect of the process, activities, and operations involved with drug and
medical device manufacture. Additionally, GMP requires that all manufacturing and testing equipment has been qualified as suitable for
use, and that all operational methodologies and procedures (such as manufacturing, cleaning, and analytical testing) utilized in the drug
manufacturing process have been validated (according to predetermined specifications), to demonstrate that they can perform their purported
function(s). On December 1, 2019 the newly revised Drug Administration Law (the “New Law”) came into effect. One of the major
amendments is the cancellation of GMP certification. The New Law eliminated the requirement that drug administration authorities shall
assess drug manufacture enterprises and drug trading enterprises, and issue assessment certificates. Instead, it requires that drug manufacturing
enterprises and drug trading enterprises establish and improve the quality management systems of manufacture and trade of drugs, and ensure
that the process of manufacturing and trading of drugs always meets all legal requirements. This means a stricter form of supervision
is implemented comparing to the prior GMP certificates system and our production lines are subject pilot inspection under the New Law.
The Market for Healthcare and Beauty Products
The health product industry is one of the mainstream
industries in the PRC, since it has a high level of recognition and importance. Recently there have been new policies for health products,
which control quality, manufacturing, manufacturing environments and techniques.
The Healthcare Product Market in the PRC
With thousands of years of history in health culture
and traditional Chinese medicine, the PRC currently utilizes advanced techniques and production capacity to initiate new health care trends,
from drugs and medicines to traditional health food and nutritional supplements, and from medical devices to health management and advice.
These trends demonstrate huge potential in the PRC’s health products market.
With the rise of the concept of “Great Health”,
the per capita expenditure of health products and the consumer group have been significantly improved.
Driven by the change of consumer treatment to
prevention, the promotion of health awareness, the refinement of health needs and the pursuit of high-quality health products, the market
scale of health products in China broke through RMB100 billion (USD16.2 billion) in 2014 and nearly RMB150 billion (USD22.2 billion) in
2017. Qianzhan Industry Research Institute in China predicted that the market scale of health products in China will reach RMB 243.5 billion
(USD37.7 billion) in 2025.
Comparing the consumption habits of Chinese and
American health products, we can see that there is still much room for development in China’s market. The penetration rate of health
products in the United States is 50%, while that in China is only 20%, and in terms of per capita consumption, China is only one-eighth
of that in the United States.
Legal and Operation Risks Associated with Being
Based in or Having the Majority of the Company’s Operations in China
China Health US’s PRC subsidiaries (as defined
below) face various legal and operational risks and uncertainties related to doing business in China. These risks could result in a material
adverse change in the Company’s business operations and cause the value of such securities to significantly decline or be worthless.
The PRC subsidiaries were formed under and are
governed by the laws of the PRC. The PRC legal system is based on written statutes. Prior court decisions may be cited for reference,
but have limited precedential value. As a significant part of our business is conducted in China, our operations are principally governed
by PRC laws and regulations. However, since the PRC legal system continues to evolve rapidly, the interpretations of many laws, regulations
and rules are not always uniform and enforcement of these laws, regulations and rules involves uncertainties, which may limit legal protections
available to us. In addition, some regulatory requirements issued by certain PRC government authorities may not be consistently applied
by other PRC government authorities (including local government authorities), thus making strict compliance with all regulatory requirements
impractical, or in some circumstances impossible. Considering PRC administrative and court authorities have discretion in interpreting
and implementing statutory and contractual terms, it may be more difficult to predict the outcome of administrative and court proceedings
and the level of legal protection we enjoy than in more developed legal systems. Furthermore, the PRC legal system is based in part on
government policies and internal rules, some of which are not published on a timely basis or at all and may have retroactive effect. As
a result, we may not be aware of our violation of these policies and rules until sometime after the violation. Such uncertainties, including
uncertainty over the scope and effect of our contractual, property (including intellectual property) and procedural rights, could materially
and adversely affect our business and impede our ability to continue our operations.
Furthermore, if China adopts more stringent standards
with respect to environmental protection or corporate social responsibilities, we may incur increased compliance costs or become subject
to additional restrictions in our operations. Intellectual property rights and confidentiality protections in China may also not be as
effective as in the United States or other countries. In addition, we cannot predict the effects of future developments in the PRC legal
system on our business operations, including the promulgation of new laws, or changes to existing laws or the interpretation or enforcement
thereof. These uncertainties could limit the legal protections available to us and our investors, including you. Moreover, any litigation
in China may be protracted and result in substantial costs and diversion of our resources and management attention.
China Health US’s corporate structure as
a Delaware holding company with operations primarily conducted by its subsidiaries in China involves unique risks to investors. Chinese
regulatory authorities could disallow this structure, which cause the incapability to continue operation without changing the corporate
structure or switching the business focus. This may in turn cause the value of the securities to significantly decline or even become
worthless. According to the Foreign Investment Law in China, the State Council shall promulgate or approve a list of special administrative
measures for market access of foreign investments, or the Negative List. The Foreign Investment Law grants national treatment to foreign-invested entities,
except for those foreign-invested entities that operate in industries specified as either “restricted” or “prohibited”
from foreign investment in the Negative List. The Foreign Investment Law provides that foreign-invested entities operating in “restricted”
or “prohibited” industries will require market entry clearance and other permissions or approvals from relevant PRC government
authorities. On December 27, 2021, the National Development and Reform Commission of China (“NDRC”) and the Ministry of Commerce
(“MOFCOM”) jointly issued the Special Administrative Measures for Foreign Investment Access (Negative List) (2021 Edition),
and the Special Administrative Measures for Foreign Investment Access in Pilot Free Trade Zones (Negative List) (2021 Edition), effective
January 1, 2022. As a company operating its business in pharmaceutical manufacture and distribution, which are not included in the 2021
Negative List, China Health US believes its business is not subject to any ownership restrictions. However, since the Negative List has
been adjusted and updated almost on an annual basis in the recent years, we cannot assure you that the aforementioned business segments
will continuously be beyond the “prohibited” category, which may significantly limit or completely hinder your ability to
offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless. The
PRC government will also establish a foreign investment information reporting system, according to which foreign investors or foreign-invested
enterprises shall submit investment information to the competent department for commerce concerned through the enterprise registration
system and the enterprise credit information publicity system, and a security review system under which the security review shall be conducted
for foreign investment affecting or likely affecting the state security.
The Chinese government has exercised and continues
to exercise substantial control over virtually every sector of the Chinese economy through regulation and state ownership. Furthermore,
recent statements made by the Chinese government have indicated an intent to increase the government’s oversight and control over
offerings of companies with significant operations in China that are to be conducted in foreign markets, as well as foreign investment
in China-based issuers like us. Our ability to operate in China may be harmed by changes in its laws and regulations. The central or local
governments of these jurisdictions may impose new, stricter regulations or interpretations of existing regulations that would require
additional expenditures and efforts on our part to ensure our compliance with such regulations or interpretations. Accordingly, government
actions in the future could have a significant effect on economic conditions in China or particular regions thereof, and could require
us to divest ourselves of any interest we then hold in Chinese properties. As such, our business segments may be subject to various government
and regulatory interference in the provinces in which they operate. We may incur increased costs necessary to comply with existing and
newly adopted laws and regulations or penalties for any failure to comply. The Chinese government may intervene or influence our operations
at any time with little advance notice, which could result in a material change in our operations and in the value of our ordinary shares.
Any actions by the Chinese government to exert more oversight and control over transaction that are conducted overseas and/or foreign
investment in China-based issuers could significantly limit or completely hinder our ability to offer or continue to offer securities
to investors and cause the value of such securities to significantly decline or be worthless.
On February 17, 2023, the CSRC promulgated the
Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Companies (the “Trial Measures”), which
became effective on March 31, 2023. The Trial Measures clarified and emphasized several aspects, which include but are not limited to:
(1) criteria and exemptions to determine whether an issuer will be required to go through the filing procedures under the Trial Measures;
(2) a negative list of types of issuers banned from listing or offering overseas, such as issuers whose affiliates have been recently
convicted of bribery and corruption; (3) issuers’ compliance with web security, data security, and other national security laws
and regulations; (4) issuers’ filing and reporting obligations, such as obligation to file with the CSRC after it submits an application
for initial public offering to overseas regulators, and obligation after offering or listing overseas to report to the CSRC material events
including change of control or voluntary or forced delisting of the issuer. Because the Company’s shares have been trading on the
over-the-counter market, the Trial Measures do not impose additional regulatory burden on us beyond the obligation to report to the CSRC
any future offerings of our securities, or material events such as a change of control. As the Trial Measures are newly issued, there
remains uncertainty as to how it will be interpreted or implemented. Therefore, there is uncertainty that if we are subject to such filing
requirements under the Trial Measures, we will be able to get clearance from the CSRC in a timely fashion.
Permissions and Approvals
Recently, the General Office of the Central Committee
of the Communist Party of China and the General Office of the State Council jointly issued the Opinions on Severe and Lawful Crackdown
on Illegal Securities Activities, which was available to the public on July 6, 2021. These opinions emphasized the need to strengthen
the administration over illegal securities activities and the supervision on overseas listings by China-based companies. These opinions
proposed to take effective measures, such as promoting the construction of relevant regulatory systems, to deal with the risks and incidents
facing China-based overseas-listed companies and the demand for cybersecurity and data privacy protection. Moreover, on January 4, 2022,
thirteen PRC regulatory agencies, namely, the Cyberspace Administration of China (“CAC”), the NDRC, the Ministry of Industry
and Information Technology, the Ministry of Public Security, the Ministry of State Security, the Ministry of Finance, MOFCOM, China State
Administration for Market Regulation (“SAMR”), the China Securities Regulatory Commission (“CSRC”), the People’s
Bank of China, the National Radio and Television Administration, National Administration of State Secrets Protection Network Data Security
(draft for public comments), and the National Cryptography Administration, jointly adopted and published the Measures for Cybersecurity
Review (2021), which became effective on February 15, 2022. The Measures for Cybersecurity Review (2021) required that, among others,
in addition to “operator of critical information infrastructure”, any “operator of network platform” holding personal
information of more than one million users which seeks to list in a foreign stock exchange should also be subject to cybersecurity review.
As of the date of this annual report, (i) the Company does not hold personal information of over one million users; (ii) the Company does
not involve data processing activities that affect or may affect national security, and (iii) the Company has not been informed by any
PRC governmental authority of any requirement that it file for a cybersecurity review; therefore, based on the foregoing, the Company
believes it is not required to pass cybersecurity review of CAC. However, we cannot assure you that the Company will remain fully compliant
with all new regulatory requirements of these opinions or any future implementation rules on a timely basis, or at all and any failure
to comply with applicable laws and obligations could have a material and adverse effect on our business, our trading, our financial condition,
and our results of operations.
As of the date of this annual report, the Company
has obtained all the approvals and permissions required to operate business in the relevant industry. A list of permissions and approvals
is below:
| No. |
|
Name
of Certificate/License |
|
Issued
Entity |
|
Issuing
Authority |
|
Issuance
Date |
|
Expiration
Date |
| 1 |
|
Food Manufacturing Certificate |
|
Harbin Humankind Biology Technology Co., Limited |
|
Heilongjiang Harbin New District Administrative Examination and Approval Bureau |
|
December 6, 2021 |
|
December 5, 2026 |
| 2 |
|
Food Distribution License |
|
Harbin Humankind Biology Technology Co., Limited |
|
Heilongjiang Harbin New District Administrative Examination and Approval Bureau |
|
February 14, 2019 |
|
February 13, 2024 |
However, if China Health US or its PRC subsidiaries
i) do not maintain such permissions or approvals; (ii) inadvertently conclude that such permissions or approvals are not required; or
(iii) applicable laws, regulations, or interpretations change and we are required to obtain such permissions or approvals, we may have
to spend great efforts and expenses to obtain such clearance, otherwise it may materially and adversely affect our business, operating
results, financial condition and the value of our ordinary shares, significantly limit or completely hinder our ability to offer or continue
to offer securities to investors, or cause such securities to significantly decline in value or become worthless.
Hemp and its Market and Industry in the PRC
Hemp was originated from the middle and lower
reaches of Yellow River with over eight-thousand years’ planting history. The hemp textile technology in our country has matured
as early as two thousand years in the Western Han Dynasty. “Plain Color Zen Clothing”, coming up from Han Tombs at Mawangdui
and other hemp textile products have become the milestone in the developing history of hemp textile technology.
Hemp, also known as Huoma, Xianma, Kuima, Hanma,
Dama. Species that containing less than 0.3% of tetrahydrocannabinol (THC) is considered as Hemp, and more than 0.3% is considered as
Marijuana and Hashish internationally. Hemp is a kind of economic plant with special effects and can be grown in large areas in all parts
of China. It has low requirements for soil and climate and can grow on relatively barren land. Planting hemp has become a way to get rich
and get out of poverty in some poor areas.
Hemp is full of treasures. The skin, stem, seed,
root, leaf and flower of hemp have utility value which can be widely applied in the fields of textile, paper-making, food, medicine, construction,
transportation, national defense and military industry and so on. As a kind of traditional economic plant, hemp fiber has the functions
of moisture absorption and perspiration, natural antimicrobial health care, good quality of adsorption, excellent quality of anti-UV and
unique wave adsorption and sound attenuation. The stem of hemp has high degree of lignification and can be used to produce high value
biologic additives, viscose fibers, top grade cigarette paper and wooden ceramics and so on. The leaf and flower of hemp can be used to
produce various kinds of health products and the seed of hemp can be used to manufacture top grade edible oil, essential oil and hemp
protein.
A hemp plant contains over 400 kinds of chemical
components and can be divided into cannabinoid and non-cannabinoid compounds. Cannabinol (CBN) in cannabinoid has the functions of anti-inflammatory,
analgesia, anti-convulsion and suppressing female hormone secretion. Cannabidiol (CBD) has the functions of anti-inflammatory, sterilization,
analgesia, antianxiety, antipsychotic, antioxidation, neural protection, reducing enterocinesia and improving learning and memory ability.
Hemp is the major high-yielding crop of making
traditional fiber products in China. It is a kind of high value-added economic crop with a wide range of uses and multi-purpose crop with
market prospects that provides fiber, hemp stem and seed. Hemp has many unique natural characteristics, especially its environmental benefits
and its natural versatility, which is a valuable kind of crop for ecological economy.
Textiles, clothing, military industry, construction
materials, food, medicines, health supplements, cosmetics and skin care products of the downstream of hemp industry in China are relatively
well-developed. The bottleneck that restricts the development of downstream industries is that the cultivation of hemp in the upstream
has long been the planting mode of scattered peasant households, and has not formed large-scale industrialized cultivation and local initial
processing capacity.
After years of development, Chinese consumers
have gradually rationalized their attitude and behavior in the consumption of health care products, paying more attention to the safety
and efficacy of health care products. At the same time, the government has gradually improved the laws and regulations of the health care
industry and tightened its supervision.
The rejuvenation of the modern industry of hemp
resulted from the major breakthrough in research and mass production of hemp. Firstly, cultivate new varieties of hemp with low activity
of anesthetic in agricultural scientific research. The main component of these varieties of anesthetics is THC and the content of THC
is controlled at 0.3% which approximates to non-toxicity. This has led to a renewed understanding and affirmation of the industrial use
of hemp, and made the cultivation of hemp mechanize, effectively contributing to the development of hemp industry.
In order to meet the needs of the development
of domestic and foreign market, China has successively reactivated the hemp industry projects in Yunnan, Heilongjiang, Shandong, Shanxi,
Anhui and Hubei provinces to set up factories and scientific research institutes to carry out research and development of various products.
The natural functional advantages of its related products are favored by consumers at home and abroad. The products are exported to countries
such as the United States, Canada, Australia, Japan, and Korea. Currently, related products of hemp have also become an export-oriented
pillar industry in China.
At present, on the basis of solving scientific
and technological problems of deep degumming of hemp, extraction of fine fiber, extraction of CBD, purification of hemp seed oil, extraction
of essential oil of hemp, extraction of hemp protein, biocomposites, fiber reinforced composite materials, carry out pilot plant test
and industrialized mass production of related products of hemp, and gradually develop the deep comprehensive utilization of hemp products,
demonstrating the strength of deep processing in the hemp industry.
Competition in the Healthcare Products Industry
We believe our competitors are:
Harbin DaZhong Pharmaceutical Co., Ltd. (Located
in Harbin, Heilongjiang Province);
Tsinghua Unisplendour Corporation Limited (Located
in Weihai City, Shandong Province);
Heilongjiang Tianlong Pharmaceuticals Co., Ltd
(Located in Heilongjiang Province);
HPGC Renmintongtai Pharmaceuticals Co., Ltd (Located
in Heilongjiang Province); and
Yunnan Hansu Biotechnology Co., Ltd. (Located
in Yunnan Province).
Our Competitive Advantages and Strategy
We believe that we have the following advantages
over our competitors:
| |
● |
We have more categories of products and a diversified production line; |
| |
● |
We have a strong and effective research and development team; |
| |
● |
We are a self-owned enterprise, and have the support of the local government; and |
| |
● |
We have a geographical advantage being located in Heilongjiang Province, the center of the healthcare industry in the PRC. |
Sales and Marketing
We plan to open more chain stores throughout the
PRC. Customers of our stores would be able to enjoy discounts on the price of our products and services. After establishing a sufficient
number of stores, we plan to develop a 24-hour delivery system for our B2C e-business.
Intellectual Property
We received a patent (200610010394.4) for our
“Qunle” brand Sailuozhi soft capsule from the National Bureau of Intellectual Property. We had initially applied for and used
the trade name of “RunChao” soft capsules, but the trade name was changed to “Qunle”, and the change was approved
by the National Bureau of Intellectual Property. The expiration date for “Qunle” is May 12, 2030.
Pursuant to a Technology Transfer Agreement dated
October 12, 2007 (“Kindlink Technology Transfer Agreement”), we purchased, for a total of RMB350,000, the technology, manufacturing,
and trademark rights to the health product known as “Kindlink” brand propolis and black ant capsule made from propolis, black
ant, acanthopanax, astragalus root from Jindelikang. The change of the ownership was approved by the NMPA. This product is consumed to
boost one’s immunity. The certification number issued by the NMPA on August 20, 2004, to permit the manufacture of the product is
GuoShiJianZi G20040906. We have no continuing obligations under the Kindlink Technology Transfer Agreement.
As of the date of this report, we have registered
the following 14 trademarks(1):
| Trademark |
|
Certificate No. |
|
Category |
|
Registrant |
|
Valid Term |
| “Qunle” |
|
3896026 |
|
No.5: Food preparations adapted for medical purposes; Albuminous milk; Dietetic beverages adapted for medical purposes; Milk sugar; Diabetic bread; Albuminous foodstuffs for medical purposes; Food for babies; Dietetic substances adapted for medical use; Nutritional additives for medical purposes |
|
Humankind |
|
7/7/2016 to 7/6/2026 |
| |
|
|
|
|
|
|
|
|
| “Wangzu” |
|
4857905 |
|
No.30: Molasses for food; Honey; pollen healthy grease; tortoise tuchahoe paste; breed columbine extract; helix alga; non-medical nutrition liquid; non-medical nutrition powder; non-medical nutrition capsule; sugar candy bird’s nest |
|
Humankind |
|
5/14/2018 to 5/13/2028 |
| |
|
|
|
|
|
|
|
|
| “Kindlink” |
|
3236981 |
|
No.5: Food preparations adapted for medical purposes; Dietetic substances adapted for medical use |
|
Humankind |
|
12/7/2013 to 12/06/2023 |
| |
|
|
|
|
|
|
|
|
| “Huimeijia” |
|
5280303 |
|
No.5: Medicine for human consumption; Medical nutrition capsule; Fibres (Edible plant) [non-nutritive]; Injection; Raw material drug; Troche; suppository; Food preparations adapted for medical purposes; Dietetic foods adapted for medical purposes; Dietetic substances adapted for medical use |
|
Humankind |
|
7/21/2019 to 7/20/2029 |
| |
|
|
|
|
|
|
|
|
| “Huide” |
|
5280304 |
|
No.5: Medicines for human consumption; Medical nutrition capsule; Fibres (Edible plant) [non-nutritive]; Injection; Raw material drug; Troche; suppository; Food preparations adapted for medical purposes; Dietetic foods adapted for medical purposes; Dietetic substances adapted for medical use |
|
Humankind |
|
7/21/2019 to 7/20/2029 |
| Trademark |
|
Certificate No. |
|
Category |
|
Registrant |
|
Valid Term |
| “KDLK” |
|
3230404 |
|
No.5: Food preparations adapted for medical purposes; Dietetic foods adapted for medical purposes; Dietetic substances adapted for medical use |
|
Humankind |
|
9/28/2023 to 9/27/2033 |
| |
|
|
|
|
|
|
|
|
| “dr.xiao” |
|
5176731 |
|
No.5: Disinfectant; Medicines for veterinary purposes; Insecticide; Sanitary napkin; Medicine health bag; Dental lacquer |
|
Humankind |
|
8/14/2019 to 8/13/2029 |
| |
|
|
|
|
|
|
|
|
| “dr.xiao” |
|
1610828 |
|
No.30: non-medical nutrition liquid; non-medical nutrition cream; non-medical nutrition powder; Honey; non-medical nutrition capsule; non-medical nutrition gum; Candy for food; Spirulina (non-medical nutrient); Candy; Pollen healthy grease |
|
Humankind |
|
7/28/2021 to 7/27/2031 |
| |
|
|
|
|
|
|
|
|
| “DaLeNing” |
|
5053772 |
|
No.5: Medicine for human; Chinese patent drugs; Suppository; Tincture; Water aqua; Paste; Liniment; Medical lotion; Patch; Chemical pharmaceuticals preparations |
|
HLJ Huimeijia |
|
5/7/2019 to 5/6/2029 |
| |
|
|
|
|
|
|
|
|
| “Xuedu” |
|
5053657 |
|
No.5: Medicine for human; Chinese patent drugs; Suppository; Tincture; Water aqua; Paste; Liniment; Medical lotion; Patch; Chemical pharmaceuticals preparations |
|
HLJ Huimeijia |
|
5/7/2019 to 5/6/2029 |
| |
|
|
|
|
|
|
|
|
| “Xuedu” with an image |
|
642099 |
|
No.5: Paste |
|
HLJ Huimeijia |
|
5/21/2023 to 5/20/2033 |
| |
|
|
|
|
|
|
|
|
| “Tai Yan Li” |
|
10014001 |
|
No.30: Honey, spirulina (non-medical), non-medical nutrient solution, non-medical nutrient lotion, non-medical nutrient powder, non-medical nutrient capsule, pastry, cereal, flour product. |
|
Humankind |
|
11/28/2022 to 11/27/2032 |
| |
|
|
|
|
|
|
|
|
| “Tai Yan Li” |
|
10013969 |
|
No.3: Cleanser lotion, soup, anti -bacterial hand soup, cleanser, cosmetics, conditioning gel, polish, essential oil. |
|
Humankind |
|
11/28/2022 to 11/27/2032 |
| |
|
|
|
|
|
|
|
|
| “Luo Qian’ |
|
8358643 |
|
No.3: essential oil, fragrance essential oil, nourishing essential oil, cosmetic mask, cosmetic tools, cosmetics, cosmetic cleanser, perfume, weight-losing cosmetics, banishing essence. |
|
Humankind |
|
6/14/2021 to 6/13/2031 |
| (1) | The
trademarks listed here have been spelt in Pinyin for the U.S. readers’ convenience. The original trademarks are in Chinese characters. |
We have the right to use the following patents
under the approval of National Bureau of Intellectual Property:
| |
|
|
|
|
|
|
|
|
|
Patent |
| Categories |
|
Name |
|
Inventor/Designer |
|
Patent No. |
|
Duration |
|
Owner |
| Invention Patent |
|
Runchao Soft Capsule and Its Manufacturing Method |
|
Xin Sun |
|
ZL200610010394.4 |
|
August 10, 2006-
August 9, 2026 |
|
Xin Sun* |
| * | Mr.
Sun verbally authorized the Company the right to use the patent. |
The following is a list of our patent applications:
The applicant of all the following patents is
Humankind, with their inventor being Mr. Xin Sun. In March 2017, we submitted the below patent applications which are currently in the
reviewing process and pending for approval.
| Serial No. |
|
Name |
|
Application |
|
Technology Field |
| 1 |
|
Cannabidiol (CBD) Cataplasmata |
|
Cannabidiol (CBD) is used for relieving muscle pain and its preparation method. |
|
The invention belongs to the research and application field of industrial hemp and relates to a kind of cataplasmata, in particular to Cannabidiol (CBD) which is used for relieving muscle pain and its preparation method. |
| |
|
|
|
|
|
|
| 2 |
|
Cannabidiol (CBD) Suspension |
|
Cannabidiol (CBD) Suspension is used for treating arthritis and its preparation method. |
|
The invention relates to the field of pharmaceutical preparations, in particular to a kind of Cannabidiol (CBD) Suspension which is used for treating arthritis and its preparation method. |
| |
|
|
|
|
|
|
| 3 |
|
Cannabidiol (CBD) Gel |
|
Cannabidiol (CBD) Gel has the effect of relieving nervous headache and its preparation method. |
|
The invention relates to gel preparations for medicine, in particular to a kind of Cannabidiol (CBD) Gel which has the effect of relieving nervous headache and its preparation method. |
| |
|
|
|
|
|
|
| 4 |
|
Cannabidiol (CBD) Paste |
|
Cannabidiol (CBD) Paste has the effect of relieving swelling and pain and its preparation method. |
|
The invention relates to the field of medicine, in particular to a kind of Cannabidiol (CBD) Paste which has the effect of relieving swelling and pain and its preparation method. |
| Serial No. |
|
Name |
|
Application |
|
Technology Field |
| 5 |
|
Cannabidiol (CBD) Soft Capsule |
|
Cannabidiol (CBD) Soft Capsule has the effect of improving diabetes and its preparation method. |
|
The invention relates to the field of soft capsule, in particular to a kind of Cannabidiol (CBD) Soft Capsule which has the effect of improving diabetes and its preparation method. |
| |
|
|
|
|
|
|
| 6 |
|
Cannabidiol (CBD) Suppository |
|
Cannabidiol (CBD) Suppository has the effect of heat sterilization and its preparation method. |
|
The invention relates to the field of medicine, in particular to a kind of Cannabidiol (CBD) Suppository which has the effect of heat sterilization and its preparation method. |
| |
|
|
|
|
|
|
| 7 |
|
Cannabidiol (CBD) Plaster |
|
Cannabidiol (CBD) Rubber Plaster has the effect of treating old bone disease and its preparation method. |
|
The invention relates to the field of medical plaster, in particular to a kind of Cannabidiol (CBD) Rubber Plaster which has the effect of treating old bone disease and its preparation method. |
| |
|
|
|
|
|
|
| 8 |
|
Cannabidiol (CBD) Rubber Plaster |
|
Cannabidiol (CBD) Rubber Plaster has the effect of dispelling wind and eliminating dampness and its preparation method. |
|
The invention relates to the field of medical plaster, in particular to a kind of Cannabidiol (CBD) Rubber Plaster which has the effect of dispelling wind and eliminating dampness and its preparation method. |
| |
|
|
|
|
|
|
| 9 |
|
Cannabidiol (CBD) Liquid Pharmaceutical Preparations |
|
Cannabidiol (CBD) Liquid Pharmaceutical Preparations has the anti-anxiety effect and its preparation method. |
|
The invention relates to the field of liquid pharmaceutical preparations, in particular to a kind of Cannabidiol (CBD) Liquid Pharmaceutical Preparations which has the anti-anxiety effect and its preparation method. |
| |
|
|
|
|
|
|
| 10 |
|
Moisturizing Cream with Hemp Seed Oil |
|
Moisturizing Cream with Hemp Seed Oil and its preparation method. |
|
The invention relates to the field of cosmetics, in particular to a kind of Moisturizing Cream which contains hemp seed oil and its preparation method. |
The laws governing our business are as follows:
| |
● |
Drug administration law of the PRC enacted August 27, 2019 |
| |
|
|
| |
● |
Healthcare registration and administration law, enacted January 7, 2005 |
| |
|
|
| |
● |
Measures for the Administration of Pharmaceutical Trade License, enacted January 4, 2004 |
| |
|
|
| |
● |
Measures for the Supervision Over and Administration of Pharmaceutical Production, enacted May 8, 2004 |
| |
|
|
| |
● |
Food Safety Law of the PRC, enacted June 1, 2009 |
| |
|
|
| |
● |
Regulation on the Implementation of the Food Safety Law of the PRC, enacted July 20, 2009 |
| |
● |
Regional regulation: Heilongjiang Regional Medicinal Materials Resource Protection Bylaw, enacted January 8, 2005 |
| |
|
|
| |
● |
Good Manufacturing Practice (GMP) Amendment, enacted January 17, 2011 |
| |
|
|
| |
● |
Hemp Industry 3-year Special Action Plan of Heilongjiang Province (2018-2020) and |
| |
|
|
| |
● |
Hemp Legislation of Heilongjiang Province in May 2017. |
In the PRC, a Good Manufacturing Practice Certification
(“GMP Certification”) is required for companies that produce medical drugs and health supplements. It is also required to
market our medical drugs and health supplements. According to the Administrative Rules of Drug Manufacturing and Certification issued
by the NMPA of the PRC on September 7, 2005, the NMPA is responsible for the review and issuance of GMP Certification. To obtain a GMP
Certification, a company shall submit its application; the NMPA will then conduct a technical review of the application materials; if
such company passes the technical review, the NMPA will inspect the manufacturing site. The NMPA also conducts follow-up inspections on
the manufacturing site. After the issuance of the GMP Certification, the NMPA may inspect the manufacturing site from time to time. However,
instead of issuing GMP, the NMPA now issues SC Permit to food manufacturers in China, allowing them to manufacture and sell health products.
The GMP Certifications of our wholly owned subsidiaries, Humankind, HLJ Huimeijia, are valid through February 14, 2019 and December 31,
2015, respectively. For the GMP Certificate of HLJ Huimeijia, the Company applied for a new certificate, which was obtained on April 25,
2018. Since obtaining the GMP Certification, we have been able to manufacture and market our products without further governmental approval.
As Humankind is in the business of manufacturing and selling health products, it is considered as a food manufacturer in China. Humankind
received its SC Permit on July 26, 2018, which will expire on September 12, 2021.The Company has applied for extension which is expect
to be approved at the end of October 2021. On December 1, 2019 the newly revised Drug Administration Law (the “New Law”) came
into effect. One of the major amendments is the cancellation of GMP certification. The New Law eliminated the requirement that drug administration
authorities shall assess drug manufacture enterprises and drug trading enterprises, and issue assessment certificates. Instead, it requires
that drug manufacturing enterprises and drug trading enterprises establish and improve the quality management systems of manufacture and
trade of drugs, and ensure that the process of manufacturing and trading of drugs always meets all legal requirements. This means a stricter
form of supervision is implemented comparing to the prior GMP certificates system and our production lines are subject pilot inspection
under the New Law.
PCAOB and Auditor’s Regulation
On April 21, 2020, SEC released a joint statement
highlighting the risks associated with investing in companies based in or have substantial operations in emerging markets including China.
The joint statement emphasized the risks associated with lack of access for the PCAOB to inspect auditors and audit work papers in China
and higher risks of fraud in emerging markets. On May 18, 2020, Nasdaq filed three proposals with the SEC to (i) apply minimum offering
size requirement for companies primarily operating in “Restrictive Market”, (ii) adopt a new requirement relating to the qualification
of management or board of director for Restrictive Market companies, and (iii) apply additional and more stringent criteria to an applicant
or listed company based on the qualifications of the Company’s auditors.
On May 20, 2020, the U.S. Senate passed the Holding
Foreign Companies Accountable Act (the “HFCAA”) requiring a foreign company to certify it is not owned or controlled by a
foreign government if the PCAOB is unable to audit specified reports because the Company uses a foreign auditor not subject to PCAOB inspection.
If the PCAOB is unable to inspect the Company’s auditors for three consecutive years, the issuer’s securities are prohibited
to trade on a national securities exchange or in the over the counter trading market in the U.S. On December 18, 2020, the HFCAA was signed
into law. The HFCAA has since then been subject to amendments by the U.S. Congress and interpretations and rulemaking by the SEC.
On June 22, 2021, the U.S. Senate passed the Accelerating
Holding Foreign Companies Accountable Act, which proposes to reduce the period of time for foreign companies to comply with PCAOB audits
from three to two consecutive years, thus reducing the time period before the securities of such foreign companies may be prohibited from
trading or delisted. On December 29, 2022, the Consolidated Appropriations Act, 2023 (the “CAA”), which the AHFCAA forms a
part, was signed into law, and it officially reduced the number of consecutive non-inspection years required for triggering the prohibitions
under the HFCAA from three years to two, thus, would reduce the time before an applicable issuer’s securities may be prohibited
from trading or delisted.
On December 16, 2021, PCAOB announced the PCAOB
HFCAA determinations relating to the PCAOB’s inability to inspect or investigate completely registered public accounting firms headquartered
in mainland China of the PRC or Hong Kong, a Special Administrative Region and dependency of the PRC, because of a position taken by one
or more authorities in the PRC or Hong Kong. The inability of the PCAOB to conduct inspections of auditors in China made it more difficult
to evaluate the effectiveness of these accounting firms’ audit procedures or quality control procedures as compared to auditors
outside of China that are subject to the PCAOB inspections, which could cause existing and potential investors in issuers operating in
China to lose confidence in such issuers’ procedures and reported financial information and the quality of financial statements.
Our auditor, Centurion ZD CPA & Co., the independent
registered public accounting firm that issues the audit report included herein, as an auditor of companies that are traded publicly in
the United States and a firm registered with the PCAOB, is subject to laws in the United States pursuant to which the PCAOB conducts regular
inspections to assess our auditor’s compliance with the applicable professional standards. Our auditor is headquartered in Hong
Kong, and was listed as an account firm subject to the Hong Kong determination in the PCAOB’s HFCAA Determination Report dated December
2021 (the “PCAOB 2021 Determinations”), as an accounting firm the PCAOB is unable to inspect or investigate completely.
On August 26, 2022, the PCAOB announced and signed
a Statement of Protocol (the “Protocol”) with the China Securities Regulatory Commission and the Ministry of Finance of the
People’s Republic of China (together, the “PRC Authorities”). The Protocol provides the PCAOB with: (1) sole discretion
to select the firms, audit engagements and potential violations it inspects and investigates, without any involvement of Chinese authorities;
(2) procedures for PCAOB inspectors and investigators to view complete audit work papers with all information included and for the PCAOB
to retain information as needed; (3) direct access to interview and take testimony from all personnel associated with the audits the PCAOB
inspects or investigates.
On October 21, 2022, the SEC issued its updated
“Conclusive list of issuers identified under the HFCAA” (the “List”) indicating that those companies
are now formally subject to the trading prohibition provisions if they remain on the List for two consecutive years, which we are included.
As of the date of this transition report, more than 170 public companies have been listed in as issuers identified under the AHFCAA, including
us. As we have been conclusively identified on October 21, 2022, if we continue to be so identified on the list until October 21, 2024,
the SEC will be required under the HFCAA to prohibit the trading of our securities on a national securities exchange and in the over-the-counter
market.
On December 15, 2022, the PCAOB announced in its
2022 HFCAA Determination Report (the “2022 Report”) its determination that the PCAOB was able to secure complete access to
inspect and investigate audit firms in the People’s Republic of China (PRC), and the PCAOB Board voted to vacate previous determinations
to the contrary. According to the 2022 Report, this determination was reached after the PCAOB had thoroughly tested compliance with every
aspect of the Protocol necessary to determine complete access, including on-site inspections and investigations in a manner fully consistent
with the PCAOB’s methodology and approach in the U.S. and globally. According to the 2022 Report, the PRC Authorities had fully
assisted and cooperated with the PCAOB in carrying out the inspections and investigations according to the Protocol, and have agreed to
continue to assist the PCAOB’s investigations and inspections in the future. Thus the PCAOB has vacated the PCAOB 2021 Determinations,
including the determination that the PCAOB is unable to inspect or investigate completely our auditor. The PCAOB may reassess its determinations
and issue new determinations consistent with the HFCAA at any time. We cannot assure you that the PRC Authorities will continue to fully
assist the PCAOB in the future. The PRC Authorities may, in the future, prevent or hinder the PCAOB from continuing to inspect or investigate,
either partially or completely, accounting firms headquartered in mainland China or Hong Kong, in which case we may get delisted from
the OTC Market if PCAOB determines that it is not able to fully inspect and investigate our auditors for two consecutive years if we do
not in time engage an alternative auditor fully subject to PCAOB inspection and investigation.
Further developments related to the HFCAA could
add uncertainties to our offering. Although PCAOB has now temporarily confirm the capability to inspect the auditors firms in PRC, and
thus the AHFCAA and the List will not affect our listing, we cannot assure you that will always be the case and what further actions the
SEC, the PCAOB or the stock exchanges will take to address these issues and what impact such actions will have on U.S. companies that
have significant operations in the PRC and have securities listed on a U.S. stock exchange (including a national securities exchange or
over-the-counter stock market). In addition, any additional actions, proceedings, or new rules resulting from these efforts to increase
U.S. regulatory access to audit information could create uncertainty for investors, the market price of our ordinary shares could be adversely
affected, and trading of our securities could become prohibited if we or our auditor are unable to meet the PCAOB inspection requirement.
Such a trading prohibitions would substantially impair your ability to sell or purchase our ordinary shares when you wish to do so, and
would have negative impacts on trading our ordinary shares, including decrease in trading price, increase in price volatility, decrease
in trading volume and liquidity, and loss of trading venues.
Enforceability
China Health US is a Delaware holding company
with its operation in PRC conducted by its PRC subsidiaries, with substantially all of the Company’s assets located there. In addition,
all Company’s senior executive officers, all reside within China for a significant portion of the time and most are PRC nationals.
As a result, it may be difficult for the shareholders to effect service of process upon China Health US or those persons inside China.
Shareholder claims that are common in the United
States, including securities law class actions and fraud claims, generally are difficult to pursue as a matter of law or practicality
in China. For example, in China, there are significant legal and other obstacles to obtaining information needed for shareholder investigations
or litigation outside China or otherwise with respect to foreign entities. Although the local authorities in China may establish a regulatory
cooperation mechanism with the securities regulatory authorities of another country or region to implement cross-border supervision and
administration, such regulatory cooperation with the securities regulatory authorities in the Unities States have not been efficient in
the absence of mutual and practical cooperation mechanism.
As China does not have treaties providing for
the reciprocal recognition and enforcement of judgments of courts with the United States and many other countries and regions, recognition
and enforcement in China of judgments of a court in any of these non-PRC jurisdictions in relation to any matter not subject to a binding
arbitration provision may be difficult or impossible. As a result, it may be difficult to enforce against us or them judgments obtained
in United States courts, including judgments predicated upon the civil liability provisions of the securities laws of the United States
or any state in the United States. It may also be difficult for a shareholder to enforce judgments obtained in U.S. courts based on the
civil liability provisions of the U.S. federal securities laws against us and these persons located in China.
Employees
As of June 30, 2023, we have 32 full-time employees
including 6 officers, 5 administrators, 12 workers, 4 technicians, and 5 accountants. Besides, we have 2 sales persons as part-time employees.
We believe that we are in compliance with local prevailing wage, contractor licensing and insurance regulations, and have good relations
with our employees.
Item 1A. Risk Factors.
We are a smaller reporting company and therefore
this item is not applicable to us.
Item 1B. Unresolved Staff Comments.
Not applicable.
Item 1C. Cybersecurity
Not applicable.
Item 2. Properties.
There is no private land ownership in PRC. Enterprises
and individuals can pay the government a fee to obtain a right to use a piece of land for commercial purpose or residential purpose for
an initial period of 50 years or 70 years, respectively. The land use right can be sold, purchased, and exchanged in the market. The land
use right of a successor owner will be reduced by the amount of time consumed by the predecessor owner.
We manufacture our products on a plot of land
located in Jin Xing Industrial Park, Songbei District, Harbin. On June 7, 2004, the Company entered into a Land Use Purchase Contract
with the local government, pursuant to which the Company agreed to purchase the right to use a piece of land, approximately 8 acres (32,000
square meters), located in Harbin County, Heilongjiang Province for commercial purposes for a fifty-year period from June 7, 2004 through
June 6, 2054, for $637,261 (RMB 5,248,000). The Company fully paid to the government the consideration for the land use right on June
13, 2004. The Department of Housing and Urban Development of Harbin City approved this transaction. The Company is in the process of applying
for the title certificate from the local government. Due to certain re-zoning conducted by the local government, the estimate time to
receive the title certificate is uncertain. The Company is striving to accelerate the process. The manufacturing facility on the land
is 4,000 square meters and there are five production lines which are sufficient for our operation. We package our products in bottles,
plastic containers and aluminum foil bags.
Since we acquired HLJ Huimeijia on November 22,
2013, we also manufacture our medicines and drugs using HLJ Huimeijia’s land, approximately 43,350 square meters, located in Hai-lin
Economic Development Zone, Mudanjiang City. The manufacturing facilities occupy approximately 5,710 square meters. We plan to build new
manufacturing facilities on the land. The expected construction cost is approximately $7,520,000 (RMB 50,000,000).
Item 3. Legal Proceedings.
We do not know of any material, active, pending
or threatened proceeding against us or our subsidiaries, nor are we, or any subsidiary, involved as a plaintiff or defendant in any material
proceeding or pending litigation.
Item 4. Mine Safety Disclosures.
This item is not applicable to us.
PART II
Item 5. Market for Registrant’s Common
Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock is quoted over-the-counter on
the OTC Markets QB Tier under the ticker “CHHE” and the market for the stock has been relatively inactive. The quotations
on the OTC Markets reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not necessarily represent actual
transactions.
As of November 10, 2023, we had approximately
502 shareholders of record of our common stock, such number of holders does not include street name holders who hold shares by brokerage
firms. The holders of common stock are entitled to one vote for each share held of record on all matters submitted to a vote of shareholders.
Holders of the common stock have no preemptive rights and no right to convert their common stock into any other securities. There are
no redemption or sinking fund provisions applicable to the common stock.
Dividends
We have not paid dividends on our common stock
and do not anticipate to pay such dividends in the foreseeable future. We will rely on dividends from Humankind for our funds and PRC
regulations may limit the amount of funds distributed to us from Humankind, which will affect our ability to declare any dividends.
Securities Authorized for Issuance Under
Equity Compensation Plans
On March 27, 2015 the Board of Directors (the
“Board”) adopted the Company’s 2015 Equity Incentive Plan (the “Plan”), which became effective as of such
date. The total number of authorized shares under the Plan is 6,000,000 shares of common stock.
The following table summarizes the number of shares
of our common stock authorized for issuance under our Plan as of June 30, 2023.
Equity Compensation Plan Information
| Plan category | |
Number
of
securities
to be issued
upon
exercise of
outstanding
options,
warrants
and rights
(a) | | |
Weighted-
average
exercise
price of
outstanding
options,
warrants
and rights
(b) | | |
Number
of
securities
remaining
available for
future
issuance
under equity
compensation
plans
(excluding
securities
reflected in
column(a))
(c) | |
| Equity compensation plans approved by security holders | |
| - | | |
| - | | |
| - | |
| Equity compensation plans not approved by security holders | |
| - | | |
| - | | |
| 2,700,000 | (1) |
| Total | |
| | | |
| | | |
| 2,700,000 | |
| (1) | The
Company granted an aggregate of 3.3 million shares of restricted shares to its CEO and an employee on March 30, 2015. The shares of the
Company are quoted on the OTC Markets. As a result, no security holders’ approval is required for the Company’s adoption
of an equity compensation plan. |
Registrar and Stock Transfer Agent
Our stock transfer agent is Direct Transfer LLC
at 1981 Murray Holladay Road, Suite 100, Salt Lake City, UT 84117. Their telephone number is (801) 272-9294, and their fax number is (801)
277-3147.
Shares Eligible for Future Sale
There is no active trading market for our common
stock. Future sales of substantial amounts of our common stock in the trading market could adversely affect market prices.
Penny Stock Regulations
Our shares of common stock are subject to the
“penny stock” rules of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and various rules
thereunder. In general terms, “penny stock” is defined as any equity security that has a market price less than $5.00 per
share, subject to certain exceptions. The rules provide that any equity security is considered to be a penny stock unless that security
is registered and traded on a national securities exchange meeting specified criteria set by the SEC, issued by a registered investment
company, and excluded from the definition on the basis of price (at least $5.00 per share), or based on the issuer’s net tangible
assets or revenues. In the last case, the issuer’s net tangible assets must exceed $3,000,000 if in continuous operation for at
least three years or $5,000,000 if in operation for less than three years or the issuer’s average revenues for each of the past
three years must exceed $6,000,000.
Trading in shares of penny stock is subject to
additional sales practice requirements for broker-dealers who sell penny stocks to persons other than established customers and accredited
investors. Accredited investors, in general, include individuals with assets in excess of $1,000,000 or annual income exceeding $200,000
(or $300,000 together with their spouse), and certain institutional investors. For transactions covered by these rules, broker-dealers
must make a special suitability determination for the purchase of the security and must have received the purchaser’s written consent
to the transaction prior to the purchase. Additionally, for any transaction involving a penny stock, the rules require the delivery, prior
to the first transaction, of a risk disclosure document relating to the penny stock. A broker-dealer also must disclose the commissions
payable to both the broker-dealer and the registered representative, and current quotations for the security. Finally, monthly statements
must be sent disclosing recent price information for the penny stocks. These rules may restrict the ability of broker-dealers to trade
or maintain a market in our common stock, to the extent it is penny stock, and may affect the ability of shareholders to sell their shares.
Recent Sale of Unregistered Securities
None.
Repurchase of Equity Securities
None.
Item 6. [Reserved]
Item 7. Management’s Discussion and Analysis
of Financial Condition and Results of Operations.
FORWARD LOOKING STATEMENTS
We make certain forward-looking statements in
this report. Statements concerning our future operations, prospects, strategies, financial condition, future economic performance (including
growth and earnings), demand for our services, and other statements of our plans, beliefs, or expectations, including the statements contained
under this caption as well as under captions elsewhere in this document, are forward-looking statements. In some cases, these statements
are identifiable through the use of words such as “anticipate”, “believe”, “estimate”, “expect”,
“intend”, “plan”, “project”, “target”, “can”, “could”, “may”,
“should”, “will”, “would”, and similar expressions. The forward-looking statements we make are not
guarantees of future performance and are subject to various assumptions, risks, and other factors that could cause actual results to differ
materially from those suggested by these forward-looking statements. These risks and uncertainties, together with the other risks described
from time to time in reports and documents that we file with the SEC should be considered in evaluating forward-looking statements. Because
such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by the forward-looking
statements. Indeed, it is likely that some of our assumptions will prove to be incorrect. Our actual results and financial position will
vary from those projected or implied in the forward-looking statements and the variances may be material. You are cautioned not to place
undue reliance on such forward-looking statements, which reflect our view only as of the date of this report.
Important factors that could cause actual results
to differ from those in the forward-looking statements include, without limitation, the following:
| |
● |
the effect of political conditions, economic conditions, market conditions, and geopolitical events; |
| |
|
|
| |
● |
legislative and regulatory changes that affect our business; |
| |
|
|
| |
● |
the availability of funds and working capital; and |
| |
|
|
| |
● |
the actions and initiatives of current and potential competitors. |
Except as required by applicable laws, regulations,
or rules, we do not undertake any responsibility to publicly release any revisions to these forward-looking statements to take into account
events or circumstances that occur after the date of this report. Additionally, we do not undertake any responsibility to update you on
the occurrence of any unanticipated events which may cause actual results to differ from those expressed or implied by any forward-looking
statements.
The following discussion and analysis should be
read in conjunction with our consolidated financial statements and the related notes thereto as filed with the SEC and other financial
information contained elsewhere in this report.
Except as otherwise indicated by the context,
references in this report to “we”, “us”, “our”, “the Registrant”, “our Company”,
or “the Company” are to China Health Industries Holdings, Inc., a Delaware corporation, China Health Industries Holdings Limited,
a limited liability company incorporated under the laws of Hong Kong, its wholly owned subsidiary in China, Harbin Humankind Biology Technology
Co. Limited (“Humankind”), and indirect wholly owned subsidiary, Heilongjiang Huimeijia Pharmaceutical Co., Ltd. (“HLJ
Huimeijia”). Unless the context otherwise requires, all references to (i) the “PRC” and “China” are to the
People’s Republic of China; (ii) “U.S. dollar,” “$” and “US$” are to United States dollars;
(iii) “RMB” are to Renminbi Yuan of China; (iv) “Securities Act” are to the Securities Act of 1933, as amended;
and (v) “Exchange Act” are to the Securities Exchange Act of 1934, as amended.
Business Overview
Our principal business operations are conducted
through our wholly-owned subsidiaries, Humankind and HLJ Huimeijia.
The Company owns a GMP-certified plant and production
facilities and has the capacity to produce 21 different NMPA-approved medicines, 14 NMPA-approved health supplement products and 10 hemp
derivative products in soft capsule, hard capsule, tablet, granule, oral liquid forms. These products address the needs of some key sectors
in China, including the feminine, geriatric, and children’s markets.
HLJ Huimeijia was founded on October 30, 2003
and its latest GMP certificate is effective until April 24, 2023. On December 1, 2019, NMPA announced that it will no longer issue GMP
certificate for any pharmaceutical companies. However, NMPA will carry out conformity inspection to do compliance testing, as well as
flight check (unannounced inspection) for all pharmaceutical companies. HLJ Huimeijia engages in the manufacture and distribution of tincture,
ointments, rubber paste, including hormones, topical solution, suppositories, enemas, oral liquids, and liniment, including traditional
Chinese medicine extractions. HLJ Huimeijia’s predecessor was Heilongjiang Xue Du Pharmaceutical Co., Ltd., which established brand
recognition in the market through its supply of high-quality drug products. HLJ Huimeijia is a “high and new technology” enterprise
that provides the most comprehensive types of topical medical products in Heilongjiang Province, a northeastern province of China.
We have developed the following products that
are derived from hemp and obtained business license to manufacture and sell these products. We have begun to sell these products since
May 2018. Hemp Seed Beer, Hemp Oil, Hemp Protein Powder, Hemp Polypeptide and Collagen Peptide are sold through Humankind. Other products
are sold through HLJ Huimeijia. The revenue of the Hemp Seed Beer, Hemp Oil and Hemp Seed accounted for 100% and nil of the total revenues
for the fiscal year of 2023 and 2022, respectively.
| Serial No. |
|
Name |
| 1 |
|
Hemp Oil |
| 2 |
|
Hemp Protein Powder |
| 3 |
|
Hemp Polypeptide |
| 4 |
|
Collagen Peptide |
| 5 |
|
Natural Hemp Essence Repair Lotion |
| 6 |
|
Natural Hemp Revitalizing Essence |
| 7 |
|
Natural Hemp Anit-aging Brightening Eye Cream |
| 8 |
|
Natural Hemp Frozen Age Nourishing Cream |
| 9 |
|
Hemp Seed Beer |
| 10 |
|
Hemp Seed |
We sell most of our products to sales agents, who
are our customers. The sales agents sell the products to the end users.
2023 Outlook
Overall, we anticipate our total revenues for
the year ended June 30, 2024 to reach to $5.5 million, with growth in all categories of our product sales, including the anticipated revenue
from Humankind for approximately $0.5 million, from HLJ Huimeijia for approximately $1.5 million and from HempCan for approximately $3.5
million. The gross profit margin for the year ended June 30, 2024 is expected to be approximately 40%, and we estimate our overall net
profit margin for the year ended June 30, 2024 to be approximately 15%. There is, however, no assurance that we will reach these projections.
Results of Operations
The following table summarizes the top lines
of the results of our operations for the years ended June 30, 2023 and 2022, respectively:
| | |
June 30,
2023 | | |
June 30,
2022 | | |
Variance | | |
% | |
| Revenues | |
$ | 111,579 | | |
$ | 267 | | |
| 111,312 | | |
| 41,689.9 | % |
| Humankind | |
| 111,579 | | |
| - | | |
| 111,579 | | |
| 100.0 | % |
| HLJ Huimeijia | |
| - | | |
| 267 | | |
| (267 | ) | |
| (100.0 | )% |
| Cost of Goods Sold | |
$ | 111,359 | | |
$ | 1,748 | | |
| 109,611 | | |
| 6,270.7 | % |
| Humankind | |
| 111,359 | | |
| - | | |
| 111,359 | | |
| 100.0 | % |
| HLJ Huimeijia | |
| - | | |
| 1,748 | | |
| (1,748 | ) | |
| (100.0 | )% |
| Gross Profit/(Loss) | |
$ | 220 | | |
$ | (1,481 | ) | |
| 1,701 | | |
| (114.9 | )% |
| Humankind | |
| 220 | | |
| - | | |
| 220 | | |
| 100.0 | % |
| HLJ Huimeijia | |
| - | | |
| (1,481 | ) | |
| 1,481 | | |
| (100.0 | )% |
Revenue
Total
revenues of the Company increased by $111,312 or 41,689.9%, for the year ended June 30, 2023 as compared to the year ended June 30, 2022.
The increase in revenues was primarily due to an increase of $111,579 or 100.0% in Humankind’s
revenues for the year ended June 30, 2023 as compared to the year ended June 30, 2022.The increase in Humankind’s
sales revenues was primarily derived from selling a new product, Hemp Seed Beer and selling raw material Hemp Seed.
Our
total cost of sales increased by $109,611 or 6,270.7% for the year ended June 30, 2023 as compared to the year ended June 30, 2022. The
increase in the overall cost of sales was attributable to an increase of $111,359 or 100.0% in Humankind’s
cost of sales in 2023 as compared to the year ended June 30, 2022. The increase in Humankind’s
sales revenues was primarily derived from cost on selling a new product, Hemp Seed Beer and selling raw material Hemp Seed.
Our gross profit increased by $1,701 from gross
loss $1,481 for the year ended June 30, 2022 to $220 for the year ended June 30, 2023. This change was consistent with the change of sales
and costs in Humankind. This growth mainly comes from the sales of the new product hemp seed beer and the raw material hemp seed.
Sales by Product Line
The following table summarizes a breakdown of
our sales by major product line for the years ended June 30, 2023 and 2022, respectively:
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
Quantity | | |
Sales | | |
| | |
Quantity | | |
Sales | | |
| |
| | |
(Unit) | | |
US$ | | |
% of | | |
(Unit) | | |
US$ | | |
% of | |
| Humankind | |
| | |
| | |
| | |
| | |
| | |
| |
| Hemp Oil | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Collagen Peptide | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Hemp Polypeptide | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Hemp Protein Powder | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Hemp Seed Beer | |
| 14,202 | | |
$ | 75,063 | | |
| 67.3 | % | |
| - | | |
$ | - | | |
| - | |
| Hemp Seed | |
| 35,000 | | |
| 36,516 | | |
| 32.7 | % | |
| - | | |
| - | | |
| - | |
| HLJ Huimeijia | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Natural Hemp Cosmetics | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Muskiness Bone Strengthener Paste | |
| - | | |
| - | | |
| - | | |
| 223 | | |
| 18 | | |
| 6.7 | % |
| ShangBiTongDing | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Indometacin and Furazolidone Suppositories | |
| - | | |
| - | | |
| - | | |
| 383 | | |
| 32 | | |
| 12.1 | % |
| Enema Glycerini | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Ge Hong Beriberi Water | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| UmguentumAcidi Borici Camphoratum | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Injury and Rheumatism Relieving Paste | |
| - | | |
| - | | |
| - | | |
| 1,852 | | |
| 152 | | |
| 56.9 | % |
| Refining GouPi Cream | |
| - | | |
| - | | |
| - | | |
| 788 | | |
| 65 | | |
| 24.3 | % |
| Natural Hemp supplies | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Total | |
| | | |
$ | 111,579 | | |
| 100.0 | % | |
| | | |
$ | 267 | | |
| 100.0 | % |
Operating Expenses
The following table summarizes our operating expenses
for the years ended June 30, 2023 and 2022, respectively:
| | |
June
30,
2023 | | |
June
30,
2022 | | |
Variance | | |
% | |
| Operating Expenses | |
| | |
| | |
| | |
| |
| Selling, general and administrative | |
$ | 1,285,313 | | |
$ | 934,927 | | |
| 350,386 | | |
| 37.5 | % |
| Depreciation and amortization | |
| 557,508 | | |
| 711,515 | | |
| (154,007 | ) | |
| (21.6 | )% |
| Total Operating Expenses | |
$ | 1,842,821 | | |
$ | 1,646,442 | | |
| 196,379 | | |
| 11.9 | % |
Total operating expenses for the year ended June
30, 2023 increased by $196,379 or 11.9%, as compared to the corresponding period in 2022. The increase in operating expenses was primarily
attributable to an increase of $320,070 or 34.2% in selling, general and administrative expenses. The increase in selling, general and
administrative expenses was mainly due to an increase in the amount of expired inventory destroyed in 2023 compared to the previous year.
Interest Income and Interest Expenses
Interest income was $113,615 for the year ended
June 30, 2023, as compared to $157,860 for the year ended June 30, 2022. This decrease of $44,245, or 28.0%, was primarily due to the
decreased average balance of bank deposits compared with the same period of 2022. This decline in bank balances was mainly due to the
effects of currency translation difference from RMB to USD during the periods and normal daily operating expenses payment.
Interest expense was $nil for the year ended June
30, 2023, and for the year ended June 30, 2022.
Other Income
Other
income was $1,320,791 expense for the year ended June 30, 2023, as compared to $1,139,746 for the year ended June 30, 2022. This increase
of $181,045 or 15.9%, was primarily due to revenue earned by the transfer of one of Huimeijia’s
production technique. This technique transfer would not affect future production of Huimeijia.
Income Taxes
Income taxes decreased by $2,934, or 100.0%, from
$2,934 for the year ended June 30, 2022 to $nil for the year ended June 30, 2023. This decrease of income tax expense incurred in the
fiscal year ended June 30, 2023 was primarily due to the decrease of net profits before income taxes.
Net Income (Loss) and Net Income per Share
Net loss after provision for income taxes increased
to $408,772, an increase of $54,822 for the year ended June 30, 2023, as compared to net loss of $353,950 for the year ended June 30,
2022. This increase of net loss was mainly because HLJ Huimeijia wrote off a large amount of inventory.
Net loss per share was $0.0062 for the year ended
June 30, 2023 and net loss per share was $0.0054 for the year ended June 30, 2022. This increase in net loss per share was primarily a
result of the aforementioned increase in net loss.
Liquidity and Capital Resources
We believe our current working capital position,
together with our expected future cash flows from operations, loans from our major shareholder, will be adequate to fund our operations
in the ordinary course of business, anticipated capital expenditures, debt payment requirements and other contractual obligations for
at least the next twelve months. However, this belief is based upon many assumptions and is subject to numerous risks, and there can be
no assurance that we will not require additional funding in the future.
The following table summarizes our cash and cash
equivalents position, our working capital, and our cash flow activity as of the fiscal years ended June 30, 2023 and 2022:
| | |
2023 | | |
2022 | |
| For the years ended June 30: | |
| | |
| |
| Cash and cash equivalent | |
$ | 47,246 | | |
$ | 44,789,999 | |
| | |
| | | |
| | |
| Working capital | |
$ | 34,997,532 | | |
$ | 37,652,693 | |
| | |
| | | |
| | |
| Inventories, net | |
$ | 40,608 | | |
$ | 521,229 | |
For the years ended June 30:
Cash provided by (used in):
| | |
2023 | | |
2022 | |
| Operating activities | |
$ | (42,272,498 | ) | |
$ | 2,115,726 | |
| | |
| | | |
| | |
| Investing activities | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Financing activities | |
$ | - | | |
$ | - | |
Due to investments in subsidiaries,Cash and cash
equivalents decreased by $44,742,753 from $44,789,999 as of June 30, 2022 to $47,246 as of June 30, 2023.
Our working capital as of June 30, 2023 was $34,997,532,
compared to working capital of $37,652,693 as of June 30, 2022. This decrease of $2,655,161 or 7.1% was mainly due to the decrease of
cash and cash equivalents in the amount of $44,742,753, which offset by the decrease of prepaid investment funds of $40,665,602.
Net cash used in operating activities
was $42,272,498 for the year ended June 30, 2023, compared to $2,115,726 for the year ended June 30, 2022. The decrease of $44,388,224
in the net cash provided by operating activities was primarily due to the purchase of subsidiary shares in June 2023.
Net cash used in investing activities was $nil
for both years ended June 30, 2023 and 2022.
Net cash provided by financing activities was
$nil for both years ended June 30, 2023 and 2022.
Other than as described in this report, we have
no present agreements or commitments with respect to any material acquisitions of businesses, products, product rights or technologies
or any other material capital expenditures. However, we will continue to evaluate acquisitions of, and/or investments in, products, technologies,
capital equipment or improvements or companies that complement our business and may make such acquisitions and/or investments in the future.
Accordingly, we may need to obtain additional sources of capital in the future to finance any such acquisitions and/or investments. We
may not be able to obtain such financing on commercially reasonable terms, if at all. Even if we are able to obtain additional financing,
it may contain undue restrictions on our operations, in the case of debt financing, or cause substantial dilution for our shareholders,
in the case of equity financing.
Related Party Debts
We had related party debts of $5,154,024 as of
June 30, 2023, as compared to $6,759,761 as of June 30, 2022, a decrease of $1,605,737 or 23.8%. The amount of related party debts mainly
consists of a loan from Mr. Xin Sun, the CEO of the Company. The loan is unsecured and non-interest bearing and has no fixed terms of
repayment. There was no written agreement for the loan. See Note 9.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements
that are currently material or reasonably likely to be material to our financial position or results of operations.
Critical Accounting Policies and Estimates
We regularly evaluate the accounting policies
and estimates that we use to make budgetary and financial statement assumptions. A complete summary of these policies is included in Note
2 to our consolidated financial statements, “Significant Accounting Policies”, and is incorporated herein by reference. In
general, management’s estimates are based on historical experience, on information from third party professionals, and on various other
assumptions that are believed to be reasonable under the facts and circumstances. Actual results could differ from those estimates made
by management.
Item 7A. Quantitative and Qualitative Disclosures
about Market Risk.
Not Applicable.
Item 8. Financial Statements and Supplementary
Data.
The financial statements required by this item
are set forth beginning on page F-1.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting
Firm
To the Shareholders and Board
of Directors of
China Health Industries Holdings, Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of China
Health Industries Holdings, Inc. and subsidiaries (the “Company”) as of June 30, 2023, and the related consolidated statements
of operations, comprehensive income (loss), changes in shareholders’ equity, and cash flows for the year then ended, and the related
notes and Schedule I (collectively referred to as the “financial statements”). In our opinion, the financial statements present
fairly, in all material respects, the financial position of the Company as of June 30, 2023, and the results of its operations and its
cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s
management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public
accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent
with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities
and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB.
Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free
of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit
of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control
over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control
over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material
misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures
included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included
evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation
of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Assentsure PAC
We have served as the Company’s auditors since 2023.
The Bencoolen, Singapore
November 13, 2023
 |
中正達會計師事務所
Centurion ZD CPA & Co.
Certified Public Accountants (Practising) |
| Unit 1304, 13/F, Two Harbourfront, 22 Tak Fung Street, Hunghom, Hong Kong. |
| 香港 紅磡 德豐街22號 海濱廣場二期 13樓1304室 |
| Tel 電話: (852) 2126 2388 Fax 傳真: (852) 2122 9078 |
| Email 電郵: info@czdcpa.com |
| |
| To: |
The board of directors and stockholders of |
| |
China Health Industries Holdings, Inc. (“the Company”) |
Report of Independent Registered Public Accounting Firm
Opinion on the Financial Statements
We have
audited the accompanying consolidated balance sheets of China Health Industries Holdings, Inc. and its subsidiaries (the “Company”)
as of June 30, 2022 and the related consolidated statements of operations and comprehensive income, changes in shareholders’ equity
and cash flows for the year then ended, and the related notes (collectively referred to as the “consolidated financial statements”).
In our opinion, the consolidated financial statements present fairly, in all material respects, the consolidated financial position of
the Company as of June 30, 2022 and the consolidated results of its operations and its cash flows for the year then ended, in conformity
with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated
financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s
consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight
Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S.
federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted
our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable
assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company
is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits,
we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion
on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits
included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error
or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding
the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used
and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements.
We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical
audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were
communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material
to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication
of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are
not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts
or disclosures to which they relate.
中正達會計師事務所
Centurion ZD CPA & Co.
Valuation
of Inventory
Inventories
consist of growing and processed plants and oils and are valued at the lower of cost or net realizable value. In evaluating whether inventories
are stated at lower of cost or net realizable value, management considers such factors as inventories in hand, estimated time to sell
such inventories and current market conditions. Write-offs for inventory obsolescence are recorded when, in the opinion of management,
the value of specific inventory items has been impaired.
Our audit procedures
to address the Company’s valuation of inventory included the following, among others. We updated to our understanding of the Company’s
inventory process, observations of year end quantities, and the testing of the cultivation, manufacturing and processing costs allocated
to the various stages of inventory during the year. We also tested the Company’s net realizable values across each inventory and
the assumptions used.
Impairment of long-lived
assets
The carrying
value of long-lived assets are reviewed when facts and circumstances suggest that the assets may be impaired or that the amortization
period may need to be changed. The Company considers internal and external factors relating to each asset, including cash flows, local
market developments, industry trends and other publicly available information. If these factors and the projected undiscounted cash flows
of the Company over the remaining amortization period indicate that the asset will not be recoverable, the carrying value will be adjusted
to the fair market value
Our audit
procedures relating to the Company’s assessment of impairment of long-lived assets included the following, among others. We evaluated
the methodologies and tested the significant assumptions and underlying data used by the Company. Such testing included assessing the
reasonableness of the undiscounted cash flow projections used by the Company in their assessment of impairment in comparison with historical
data and the Company’s assumptions used for the projections.
| /s/ Centurion ZD CPA & Co. |
|
| Centurion ZD CPA & Co. |
|
We have
served as the Company’s auditor since 2017
Hong Kong,
China
September
15, 2022
CHINA HEALTH INDUSTRIES HOLDINGS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
IN US DOLLARS
(Audited)
| | |
June
30,
2023 | | |
June
30,
2022 | |
| ASSETS | |
| | |
| |
| Current assets | |
| | |
| |
| Cash and cash equivalents | |
$ | 47,246 | | |
$ | 44,789,999 | |
| Accounts receivable, net | |
| - | | |
| 9,567 | |
| Inventory | |
| 40,608 | | |
| 521,229 | |
| Other receivables, net | |
| 1,921 | | |
| 31,778 | |
| Advances to suppliers | |
| 198,157 | | |
| 214,915 | |
| Prepaid investment funds | |
| 40,682,360 | | |
| - | |
| Prepayments | |
| - | | |
| 30,004 | |
| Total current assets | |
$ | 40,970,292 | | |
$ | 45,597,492 | |
| | |
| | | |
| | |
| Property, plants and equipment, net | |
| 2,727,170 | | |
| 3,158,021 | |
| Intangible assets, net | |
| 964,775 | | |
| 1,412,603 | |
| Construction in progress | |
| 420,092 | | |
| 579,402 | |
| Total assets | |
$ | 45,082,329 | | |
$ | 50,747,518 | |
| LIABILITIES AND EQUITY | |
| | | |
| | |
| Current liabilities | |
| | | |
| | |
| Accounts payable and accrued expenses | |
| 286,902 | | |
| 334,972 | |
| Other payables | |
| 94,293 | | |
| 82,323 | |
| Advances from customers | |
| 208,966 | | |
| 472,307 | |
| Related party debts | |
| 5,154,024 | | |
| 6,759,761 | |
| Wages payable | |
| 90,990 | | |
| 162,431 | |
| Taxes payable | |
| 137,585 | | |
| 133,005 | |
| Total current liabilities | |
$ | 5,972,760 | | |
$ | 7,944,799 | |
| | |
| | | |
| | |
| Equity | |
| | | |
| | |
Common stock, ($0.0001 par value per share, 300,000,000 shares authorized, 65,539,737 and
65,539,737 shares issued and outstanding as of June 30, 2023 and June 30, 2022, respectively) | |
| 6,554 | | |
| 6,554 | |
| Additional paid-in capital | |
| 521,987 | | |
| 521,987 | |
| Accumulated other comprehensive income | |
| (2,745,558 | ) | |
| 538,820 | |
| Statutory reserves | |
| 38,679 | | |
| 38,679 | |
| Retained earnings | |
| 41,287,907 | | |
| 41,696,679 | |
| Total stockholders’ equity | |
$ | 39,109,569 | | |
$ | 42,802,719 | |
| Total equity | |
$ | 39,109,569 | | |
$ | 42,802,719 | |
| | |
| | | |
| | |
| Total liabilities and equity | |
$ | 45,082,329 | | |
$ | 50,747,518 | |
The accompanying notes are an integral part of
these consolidated financial statements.
CHINA HEALTH INDUSTRIES HOLDINGS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE
INCOME
IN US DOLLARS
(Audited)
| | |
For the Year Ended | |
| | |
June
30,
2023 | | |
June
30,
2022 | |
| | |
| | |
| |
| REVENUE | |
$ | 111,579 | | |
$ | 267 | |
| COST OF GOODS SOLD | |
| 111,359 | | |
| 1,748 | |
| GROSS PROFIT/(LOSS) | |
| 220 | | |
| (1,481 | ) |
| | |
| | | |
| | |
| OPERATING EXPENSES | |
| | | |
| | |
| Selling, general and administrative expenses | |
| 1,285,313 | | |
| 934,927 | |
| Depreciation and amortization expenses | |
| 557,508 | | |
| 711,515 | |
| Total operating expenses | |
| 1,842,821 | | |
| 1,646,442 | |
| | |
| | | |
| | |
| LOSS FROM OPERATIONS | |
| (1,842,601 | ) | |
| (1,647,923 | ) |
| | |
| | | |
| | |
| OTHER INCOME/(EXPENSES) | |
| | | |
| | |
| Interest income | |
| 113,615 | | |
| 157,860 | |
| Other income, net | |
| 1,320,791 | | |
| 1,139,746 | |
| Bank charges | |
| (577 | ) | |
| (699 | ) |
| Total other income, net | |
| 1,433,829 | | |
| 1,296,907 | |
| | |
| | | |
| | |
| LOSS BEFORE INCOME TAXES | |
| (408,772 | ) | |
| (351,016 | ) |
| Provision for income taxes | |
| - | | |
| (2,934 | ) |
| NET LOSS | |
| (408,772 | ) | |
| (353,950 | ) |
The accompanying notes are an integral part of
these consolidated financial statements.
CHINA HEALTH INDUSTRIES HOLDINGS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE
INCOME
IN US DOLLARS
(Audited) (CONTINUED)
| | |
For the Year Ended | |
| | |
June
30,
2023 | | |
June
30,
2022 | |
| Foreign currency translation adjustment | |
| (3,284,378 | ) | |
| (1,617,972 | ) |
| COMPREHENSIVE LOSS | |
$ | (3,693,150 | ) | |
$ | (1,971,922 | ) |
| | |
| | | |
| | |
| Net (loss)/income per share: | |
| | | |
| | |
| | |
| | | |
| | |
Net loss per share Basic & diluted | |
$ | (0.0062 | ) | |
$ | (0.0054 | ) |
| | |
| | | |
| | |
| Weighted average shares outstanding: | |
| | | |
| | |
Basic & diluted | |
| 65,539,737 | | |
| 65,539,737 | |
The accompanying notes are an integral part of
these consolidated financial statements.
CHINA HEALTH INDUSTRIES HOLDINGS, INC.
AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
IN US DOLLARS
(Audited)
| | |
Common Shares | | |
Additional
Paid-in | | |
Retained | | |
Statutory | | |
Accumulated
Other
Comprehensive | | |
Total
Stockholders’ | | |
Non-
controlling | | |
Total | |
| | |
Shares | | |
Amount | | |
Capital | | |
Earnings | | |
Reserve | | |
Income (loss) | | |
Equity | | |
Interest | | |
Equity | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance, June 30, 2021 | |
| 65,539,737 | | |
$ | 6,554 | | |
$ | 521,987 | | |
| 42,050,629 | | |
| 38,679 | | |
| 2,156,792 | | |
| 44,774,641 | | |
| - | | |
| 44,774,641 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| (353,950 | ) | |
| - | | |
| - | | |
| (353,950 | ) | |
| - | | |
| (353,950 | ) |
| Other comprehensive loss - Translation adjustment | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (1,617,972 | ) | |
| (1,617,972 | ) | |
| - | | |
| (1,617,972 | ) |
| Balance, June 30, 2022 | |
| 65,539,737 | | |
| 6,554 | | |
| 521,987 | | |
| 41,696,679 | | |
| 38,679 | | |
| 538,820 | | |
| 42,802,719 | | |
| - | | |
| 42,802,719 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| (408, 772) | | |
| - | | |
| - | | |
| (408, 772) | | |
| - | | |
| (408, 772) | |
| Other comprehensive loss - Translation adjustment | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (3,284,378 | ) | |
| (3,284,378 | ) | |
| - | | |
| (3,284,378 | ) |
| Balance, June 30, 2023 | |
| 65,539,737 | | |
| 6,554 | | |
| 521,987 | | |
| 41,287,907 | | |
| 38,679 | | |
| (2,745,558 | ) | |
| 39,109,569 | | |
| - | | |
| 39,109,569 | |
The accompanying notes are an integral part of
these consolidated financial statements.
CHINA HEALTH INDUSTRIES HOLDINGS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
IN US DOLLARS
(Audited)
| | |
For the Year Ended | |
| | |
June
30,
2023 | | |
June
30,
2022 | |
| Cash Flows from Operating Activities | |
| | |
| |
| Net loss attributable to China Health Industries Holdings | |
$ | (408,772 | ) | |
$ | (353,950 | ) |
| | |
| | | |
| | |
| Adjustments to reconcile net income to net cash provided by operating activities: | |
| | | |
| | |
| Depreciation and amortization expenses | |
| 686,676 | | |
| 830,545 | |
| Provision for doubtful accounts | |
| 6,403 | | |
| (9,556 | ) |
| Deferred taxes gain | |
| - | | |
| 2,860 | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Inventory | |
| 459,731 | | |
| 215,391 | |
| Accounts receivable | |
| 2,813 | | |
| 2,288,077 | |
| Other receivables | |
| 12,737 | | |
| (114 | ) |
| Advance to suppliers and prepaid expenses | |
| 29,278 | | |
| (11,024 | ) |
| Prepaid investment funds | |
| (42,424,029 | ) | |
| - | |
| Accounts payables and accrued expenses | |
| (19,886 | ) | |
| (57,912 | ) |
| Advance from customers and other payables | |
| (171,488 | ) | |
| 358,179 | |
| Amounts due to related parties | |
| (374,064 | ) | |
| (1,057,059 | ) |
| Wages payable | |
| (61,577 | ) | |
| (48,844 | ) |
| Taxes payable | |
| (10,320 | ) | |
| (40,867 | ) |
| Net cash provided by operating activities | |
$ | (42,272,498 | ) | |
$ | 2,115,726 | |
| | |
| | | |
| | |
| Cash Flows from Investing Activities | |
| | | |
| | |
| Net cash (used in)/provided by investing activities | |
| - | | |
| - | |
| | |
| | | |
| | |
| Cash Flows from Financing Activities | |
| | | |
| | |
| Net cash (used in)/provided by financing activities | |
| - | | |
| - | |
| Effect of exchange rates change on cash and cash equivalents | |
| (2,470,255 | ) | |
| (1,672,471 | ) |
| | |
| | | |
| | |
| Net (decrease)/increase in cash and cash equivalents | |
| (44,742,753 | ) | |
| 443,255 | |
| Cash and cash equivalents, beginning of period | |
| 44,789,999 | | |
| 44,346,744 | |
| Cash and cash equivalents, end of period | |
$ | 47,246 | | |
$ | 44,789,999 | |
| Supplemental disclosure of cash flow information | |
| | | |
| | |
| Interest paid | |
$ | - | | |
$ | - | |
| Taxes paid | |
$ | - | | |
$ | - | |
| Non-cash activities: | |
| | | |
| | |
| Loan from related party for the construction of a facility | |
$ | - | | |
$ | - | |
The accompanying notes are an integral part of
these consolidated financial statements.
CHINA HEALTH INDUSTRIES HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1 - ORGANIZATION AND BUSINESS BACKGROUND
China Health Industries Holdings, Inc. (“China
Health US”) was incorporated in the State of Arizona on July 11, 1996 and was the successor of the business known as Arizona Mist,
Inc. which began in 1989. On May 9, 2005, it entered into a stock purchase agreement and Share Exchange (effecting a reverse merger) with
Edmonds 6, Inc. (“Edmonds 6”), a Delaware corporation, and changed its name to Universal Fog, Inc. Pursuant to this agreement,
Universal Fog, Inc. (which has been in continuous operation since 1996) became a wholly-owned subsidiary of Edmonds 6.
China Health Industries Holdings Limited (“China
Health HK”) was incorporated on July 20, 2007 in Hong Kong under the Companies Ordinance as a limited liability company. China Health
HK was formed for the purpose of seeking and consummating a merger or acquisition with a business entity organized as a private corporation,
partnership, or sole proprietorship as defined by FASB ACS Topic 915 (“Development Stage Entities”).
Harbin Humankind Biology Technology Co., Limited
(“Humankind”) was incorporated in Harbin City, Heilongjiang Province, the People’s Republic of China (the “PRC”)
on December 14, 2003, as a limited liability company under the Company Law of the PRC. Humankind is engaged in the manufacturing and sale
of health products.
On August 20, 2007, the sole shareholder of China
Health HK entered into a share purchase agreement (the “Share Purchase Agreement”) with the owners of Humankind. Pursuant
to the Share Purchase Agreement, China Health HK purchased 100% of the ownership in Humankind for a cash consideration of $60,408 (the
“Share Purchase”). Subsequent to the completion of the Share Purchase, Humankind became a wholly-owned subsidiary of China
Health HK. The Share Purchase was accounted for as a “reverse merger” since the owner of Humankind owned a majority of the
outstanding shares of China Health HK’s common stock immediately following the execution of the Share Purchase Agreement, it was
deemed to be the accounting acquirer in the reverse merger. Consequently, the assets and liabilities and the historical operations that
have been reflected in the financial statements for periods prior to the Share Purchase are those of Humankind and have been recorded
at the historical cost basis. After completion of the Share Purchase, China Health HK’s consolidated financial statements include
the assets and liabilities of both China Health HK and Humankind, the historical operations of Humankind, and the operations of China
Health HK and its subsidiaries from the closing date of the Share Purchase.
On October 14, 2008, Humankind set up a 99% owned
subsidiary, Harbin Huimeijia Medicine Company (“Huimeijia”), with its primary business being manufacturing and distributing
medicine. Mr. Xin Sun, the Company’s majority owner, owns 1% of Huimeijia. Huimeijia is consolidated in the consolidated financial
statements of China Health HK.
On December 31, 2008, China Health HK entered
into a reverse merger with Universal Fog, Inc., a U.S. publicly traded shell company (the “Transaction”). China Health HK
is the acquirer in the Transaction, and the Transaction has been treated as a recapitalization of China Health US. After the Transaction
and a 20:1 reverse stock split, Mr. Xin Sun owned 61,203,088 shares of common stock, representing 98.3% of the 62,234,737 total outstanding
shares of common stock of China Health US. On April 7, 2009, Mr. Sun transferred 28,200,000 shares of common stock to 296 individuals,
leaving him with 33,003,088 shares of common stock of China Health US, or approximately 53.03% of the total outstanding shares of common
stock. Universal Fog, Inc. changed its name to China Health Industries Holdings, Inc. on February 19, 2009.
On November 22, 2013, Humankind completed the
acquisition of Heilongjiang Huimeijia Pharmaceutical Co., Ltd. (“HLJ Huimeijia”) for a total purchase price of $16,339,869
(RMB100,000,000). HLJ Huimeijia was founded on October 30, 2003, and is engaged in the manufacturing and distribution of tincture, ointments,
rubber paste (including hormones), topical solution, suppositories, liniment (including traditional Chinese medicine extractions), enemas
and oral liquids. HLJ Huimeijia’s predecessor is Heilongjiang Xue Du Pharmaceutical Co., Ltd., which has established its brand name
in the market through its supply of high quality medical products. HLJ Huimeijia is categorized as a “high and new technology”
enterprise by the Science Technology Department in Heilongjiang Province. HLJ Huimeijia has 21 products which have been approved by, and
have received approval numbers issued by, the National Medical Products Administration (the “NMPA”). In addition, HLJ Huimeijia
is the holder of one patent for utility models, five patents for external design and three trademarks in China, including the Chinese
brand name of “Xue Du” which has an established reputation among customers in northeastern China.
On December 24, 2014, Humankind entered into a
stock transfer agreement (the “Original Agreement”) with Xiuzheng Pharmaceutical Group Co., Ltd. a company incorporated under
the laws of the PRC and located in Jilin province (“Xiuzheng Pharmacy” or the “Buyer”), Mr. Xin Sun, the CEO of
the Company, and Huimeijia, 99% owned by Humankind and 1% owned by Mr. Xin Sun. Pursuant to the Original Agreement, Humankind and Mr.
Xin Sun (the “Equity Holders”), would sell their respective equity interests in Huimeijia to Xiuzheng Pharmacy.
On February 9, 2015, the four parties entered
into a supplementary agreement (the “Supplementary Agreement”) to modify the terms of the Original Agreement, pursuant to
which the Equity Holders and Huimeijia (collectively the “Asset Transferors”) would sell only the 19 drug approval numbers
(including the tablet, capsule, powder, mixture, oral liquid, syrup and oral solution under the 19 approval numbers; licenses including
the original copies of Business License, Organization Code Certificate, Tax Registration Certificate, Drug Production Permit and GMP Certificate,
and other documents and original copies related to the production and operation of the 19 drugs) (the “Assets”) to Xiuzheng
Pharmacy. The Equity Holders would have retained their equity interests in Huimeijia, but would have pledged such equity interests to
Xiuzheng Pharmacy until the Assets were transferred, at which time the cash consideration would have been paid by the Buyer. Total cash
consideration would have been the same as under the Original Agreement, i.e., RMB 8,000,000 (approximately $1,306,186) to the Asset Transferors.
In the event that the Assets had failed to be transferred to the Buyer due to the fault of the Asset Transferors, the paid consideration
would have been returned to the Buyer with interest accrued. If the failure of the transfer of the Assets were a result of changes in
government policy or force majeure, the paid cash consideration would have been returned to the Buyer but without any interest.
On October 12, 2016, the four parties agreed to
rescind the Supplementary Agreement and entered into a new supplementary agreement (the “New Supplementary Agreement”), pursuant
to which the four parties agreed to execute the transfer of the equity interests based on the Original Agreement and the Equity Holders
agreed to sell their respective equity interests in Huimeijia to Xiuzheng Pharmacy. The transfer of 100% of the equity interests of Huimeijia
to the Buyer was for total cash consideration of RMB 8,000,000 (approximately $1,306,186) (the “Purchase Price”) to the Equity
Holders. 40% of the Purchase Price was due within 10 business days after the signing of the New Supplementary Agreement; 40% of the Purchase
Price was due within 10 business days after the completion of the changes in business registration described in the Original Agreement
and Xiuzheng Pharmacy obtaining documents evidencing its ownership on Huimeijia; 15% of the Purchase Price is due within 10 business days
after the transfer of all of the Assets is approved by Heilongjiang FDA; and 5% of the Purchase Price is due within 10 business days after
all of the Assets have been transferred to Xiuzheng Pharmacy or its designee and Humankind and Mr. Xin Sun have instructed Xiuzheng Pharmacy
complete three-batches production of all forms of the drugs included in the Assets. As of the date of this report, 80% of the Purchase
Price has been paid, the Company has completed changes in its business registration, and Xiuzheng Pharmacy has obtained a business license
issued by the local State Administration of Industry and Commerce in Harbin (“Harbin SAIC”) to Huimeijia, in which the ownership
of Huimeijia has been recorded as held by Xiuzheng Pharmacy, with Harbin SAIC and the legal representative (a person that is authorized
to take most of the corporate actions on behalf of a company under the corporate laws in China) of Huimeijia has been appointed by the
Buyer.
On
June 27, 2023 (the “Signing Date”),
Harbin Humankind Biology Technology Co., Limited (“Humankind”),
a wholly owned subsidiary of China Health Industries Holdings, Inc. (“China
Health”), a corporation incorporated under the laws of the State
of Delaware, entered into certain equity transfer agreements (collectively, the “Agreements”)
with Mr. Xin Sun and Mr. Kai Sun (collectively, the “Sellers”).
Pursuant to the Agreements, the Sellers agreed to transfer to Humankind 99% and 1% of the equity interests of Heilongjiang HempCan Pharmaceuticals
Co., Ltd. (“HempCan”),
for a consideration of RMB292,050,000 (approximately $40,275,537) and RMB2,950,000 (approximately $406,824) respectively, totaling RMB295
million (approximately $40,682,360) (collectively, the “Purchase
Prices”). The Purchase Prices shall be fully paid to Sellers within
15 business days after the Signing Date, and the Sellers shall complete certain registration procedures, such as change of equity ownership
and change of business registration within 15 business days after full Purchase Prices are received. If Humankind is late in paying the
Purchase Prices, a 0.1% per day late penalty on the amount of Purchase Prices that is paid late shall be paid to the Sellers. Mr. Xin
Sun is China Health’s Chairman, sole director and sole executive
officer and Mr. Kai Sun is Mr. Xin Sun’s younger brother.
China Health US, China Health HK, Humankind and
HLJ Huimeijia are collectively referred herein to as the “Company.”
As of June 30, 2023, the Company’s corporate
structure was as follows:
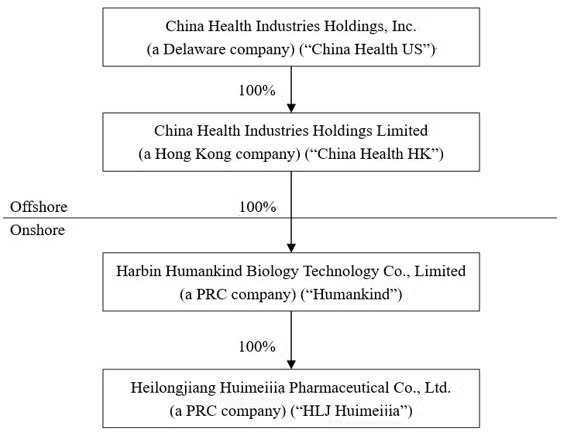
Note 2 - SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
This
summary of significant accounting policies of the Company is presented to assist in understanding the Company’s
consolidated financial statements. The financial statements and notes are representations of the Company’s
management, which is responsible for their integrity and objectivity. These accounting policies conform to generally accepted accounting
principles in the United States (“US GAAP”)
and have been consistently applied in the preparation of the consolidated financial statements.
Principles of Consolidation
The accompanying consolidated financial statements
include China Health US and its four subsidiary companies, including China Health HK, Humankind and HLJ Huimeijia. All significant intercompany
balances and transactions have been eliminated in consolidation and combination.
On
November 22, 2013, China Health US, through its wholly owned subsidiary Humankind, completed the acquisition of HLJ Huimeijia. HLJ Huimeijia
and Humankind are under the common control of Mr. Xin Sun, the CEO of the Company before and after the date of transfer. Humankind’s
accounting policy adopted the guidance in ASC 805-50-05-5 for the transfer of net assets between entities under common control to apply
a method similar to the pooling-of-interests’ method. Under this
method, the financial statements of Humankind shall report results of operations for the period in which the transfer occurs as though
the transfer of net assets had occurred at the beginning of the period. Results of operations for that period will thus comprise both
those of the previously separate entities combined from the beginning of the period to the date the transfer is completed and those of
the combined operations from that date to the end of the period. Similarly, Humankind shall present the statements of financial position
and other financial information as of the beginning of the period as though the assets and liabilities had been transferred at that date.
Financial statements and financial information of Humankind presented for prior years also shall be retrospectively adjusted to furnish
comparative information.
Segment Reporting
FASB
ASC Topic 280, “Segment Reporting,” established
standards for reporting information about operating segments on a basis consistent with the Company’s
internal organizational structure as well as information about geographical areas, business segments and major customers in financial
statements for details on the Company’s business segments. The Company
has three reportable operating segments: Humankind, HLJ Huimeijia and Others. The segments are grouped based on the types of products
provided.
Fair Value of Financial Instruments
The provisions of accounting guidance, FASB ASC
Topic 820 that applies to the Company requires all entities to disclose the fair value of financial instruments, both assets and liabilities
recognized and not recognized on the balance sheet, for which it is practicable to estimate fair value, and defines fair value of a financial
instrument as the amount at which the instrument could be exchanged in a current transaction between willing parties.
Fair Value Measurements
FASB
ASC Topic 820, “Fair Value Measurements and Disclosures,”
clarifies the definition of fair value for financial reporting, establishes a
framework for measuring fair value and requires additional disclosures about the use of fair value measurements.
Various
inputs are considered when determining the fair value of the Company’s
debt. The inputs or methodologies used for valuing securities are not necessarily an indication of the risk associated with investing
in these securities. These inputs are summarized in the three broad levels listed below.
| |
Level 1 |
– |
observable market inputs that are unadjusted quoted prices for identical assets or liabilities in active markets. |
| |
|
|
|
| |
Level 2 |
– |
other significant observable inputs (including quoted prices for similar securities, interest rates, credit risk, etc.). |
| |
|
|
|
| |
Level 3 |
– |
significant unobservable inputs (including the Company’s own assumptions in determining the fair value of investments). |
The carrying value of financial assets and liabilities
recorded at fair value is measured on a recurring or nonrecurring basis. Financial assets and liabilities measured on a non-recurring
basis are those that are adjusted to fair value when a significant event occurs. The Company had no financial assets or liabilities carried
and measured on a nonrecurring basis during the reporting periods. Financial assets and liabilities measured on a recurring basis are
those that are adjusted to fair value each time a financial statement is prepared. The Company had no financial assets or liabilities
carried and measured on a recurring basis during the reporting periods.
The availability of inputs observable in the market
varies from instrument to instrument and depends on a variety of factors including the type of instrument, whether the instrument is actively
traded, and other characteristics particular to the transaction. For many financial instruments, pricing inputs are readily observable
in the market, the valuation methodology used is widely accepted by market participants, and the valuation does not require significant
management discretion. For other financial instruments, pricing inputs are less observable in the market and may require management judgment.
Foreign Currency Translation and Transaction
Humankind,
Huimeijia and HLJ Huimeijia maintain their books and accounting records in PRC currency “Renminbi”
(“RMB”),
which has been determined as the functional currency. The functional currency of China Health HK is the Hong Kong Dollar (“HKD”).
Transactions denominated in currencies other than
the functional currencies are recorded at the exchange rates prevailing on the date of the transactions, as quoted by the Federal Reserve
Board. Foreign currency exchange gains and losses resulting from these transactions are included in operations.
Humankind,
Huimeijia, HLJ Huimeijia and China Health Hong Kong’s financial
statements are translated into the reporting currency, the United States Dollar (“USD”).
Assets and liabilities of the above entities are translated at the prevailing exchange rate at each reporting period end date. Contributed
capital accounts are translated using the historical rate of exchange when capital is injected. Income and expense accounts are translated
at the average rate of exchange during the reporting period. Translation adjustments resulting from the translation of these financial
statements are reflected as accumulated other comprehensive income in shareholders’ equity
and non-controlling interests.
For
the purpose of presenting these financial statements, the Company’s
assets and liabilities with functional currency of HKD are expressed in USD at the exchange rate on the balance sheet date, which was
7.8363 and 7.8472 as of June 30, 2023 and June 30, 2022, respectively; stockholder’s
equity accounts are translated at historical rates, and income and expense items are translated at the weighted average exchange rates
during the year, which was 7.8373 and 7.8049 for the years ended June 30, 2023 and 2022, respectively. For Renminbi currency, the Company’s
assets and liabilities are expressed in USD at the exchange rate on the balance sheet date, which was 7.2513 and 6.6981 as of June 30,
2023 and June 30, 2022, respectively; stockholder’s equity accounts
are translated at historical rates, and income and expense items are translated at the weighted average exchange rates during the year,
which was 6.9536 and 6.4554 for the years ended June 30, 2023 and 2022, respectively.
Statement of Cash Flows
In
accordance with Statement FASB ASC Topic 230, “Statement of Cash
Flows,” cash flow from the Company’s
operations is calculated based upon the local currencies and translated to the reporting currency using an average foreign exchange rate
for the reporting period. As a result, amounts related to assets and liabilities reported in the statement of cash flows will not necessarily
agree with changes in the corresponding balances on the balance sheet.
Use of Estimates and Assumptions
The
preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and judgments that affect the reported
amounts of assets and liabilities, disclosure of contingent assets and liabilities on the date of the financial statements and the reported
amounts of revenues and expenses during the reporting period. The Company bases its estimates and judgments on historical experience and
on various other assumptions and information that are believed to be reasonable under the circumstances. Estimates and assumptions of
future events and their effects cannot be perceived with certainty and, accordingly, these estimates may change as new events occur, as
more experience is acquired, as additional information is obtained and as the Company’s
operating environment changes. Significant estimates and assumptions by management include, among others; useful lives of long-lived assets
and intangible assets, valuation of inventory, accounts receivable and notes receivable, impairment analysis of long-lived assets, construction
in progress, intangible assets and deferred taxes. While the Company believes that the estimates and assumptions used in the preparation
of the financial statements are appropriate, actual results could differ from those estimates. Estimates and assumptions are periodically
reviewed and the effects of revisions are reflected in the financial statements in the period they are determined to be necessary.
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand,
deposits in banks with maturities of three months or less, and all highly liquid investments which are unrestricted as to withdrawal or
use, and which have original maturities of three months or less at the time of purchase.
As
of June 30, 2023, and 2022, the Company’s uninsured bank balance
was mainly maintained at financial institutions located in the PRC and Hong Kong, totaled $47,246 and $44,789,999, respectively. The Company
has no insured bank balance as of June 30, 2023 and 2022, respectively.
Accounts Receivable
Accounts
receivable are recorded at the invoiced amount and do not bear interest. The Company extends unsecured credit to its customers in the
ordinary course of business but mitigates the associated risks by performing credit checks and actively pursuing past due accounts. An
allowance for doubtful accounts is established and determined based on management’s
assessment of known requirements, aging of receivables, payment and bad debt history, the customer’s
current credit worthiness, changes in customer payment patterns and the economic environment. From November 1, 2013, the Company changed
its credit policy by offering ninety (90) day payment terms for sales agents, whereas the payment terms for sales agents before November
1, 2013 were thirty (30) day. As of June 30, 2023 and 2022, the balances of accounts receivable were $nil and $9,567, respectively. The
Company determines the allowance based on aging data, historical collection experience, customer specific facts and economic conditions.
Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery
is considered remote. The Company evaluated the nature of all accounts receivable then provided allowance for doubtful accounts. As of
June 30, 2023, and 2022, the balances of allowance for doubtful accounts were $51,188 and $48,769 respectively.
Advance to Suppliers
The Company periodically makes advances to certain
vendors for purchases of raw materials, or service providers for services relating to construction plans for our plant, equipment and
production lines for the GMP upgrading, and records these payments as advance to suppliers. The decrease in the amount of advance to suppliers
is due to fluctuation of exchange rate. As a result, as of June 30, 2023, and 2022, advance to suppliers amounted to $198,157 and $214,915,
respectively.
Inventory
Inventory consists of raw materials, work in progress
and finished goods of manufactured products.
Inventory
is stated at lower of cost or market and consists of materials, labor and overhead. HLJ Huimeijia uses the weighted average method for
inventory valuation. The other entities of the Company use the first-in, first-out (“FIFO”)
method for inventory valuation. Overhead costs included in finished goods include direct labor cost and other costs directly applicable
to the manufacturing process. The Company evaluates inventory for excess, slow moving, and obsolete inventory as well as inventory the
value of which is in excess of its net realizable value. This evaluation includes analysis of sales levels by product and projections
of future demand. If future demand or market conditions are less favorable than the Company’s
projections, a write-down of inventory may be required, and would be reflected in cost of goods sold in the period the revision is made.
The inventory allowance with an amount of $nil and $nil were provided for the years ended June 30, 2023, and 2022, respectively.
Impairment of Long-Lived Assets
The
Company’s long-lived assets and other assets are reviewed for impairment
in accordance with the guidance of the FASB ASC Topic 360-10, “Property,
Plant, and Equipment,” and FASB ASC Topic 205, “Presentation
of Financial Statements.” The Company tests for impairment losses
on long-lived assets used in operations whenever events or changes in circumstances indicate that the carrying amount of the asset may
not be recoverable. Recoverability of an asset to be held and used is measured by a comparison of the carrying amount of the asset to
the future undiscounted cash flows expected to be generated by the asset. If such asset is considered to be impaired, the impairment to
be recognized is measured by the amount by which the carrying amount of the asset exceeds its fair value. Impairment evaluations involve
management’s estimates on asset useful lives and future cash flows.
Actual useful lives and cash flows could be different from those estimated by management which could have a material effect on the Company’s
reporting results and financial position. Fair value is determined through various valuation techniques including discounted cash flow
models, quoted market values and third-party independent appraisals, as considered necessary. As of June 30, 2023, and 2022, the Company
has not experienced impairment losses on its long-lived assets. However, there can be no assurances that demand for the Company’s
products or services will continue, which could result in an impairment of long-lived assets in the future.
Property, Plant and Equipment
Property, plant and equipment are stated at cost
less accumulated depreciation and impairment losses. Maintenance, repairs and minor renewals are expensed as incurred, major renewals
and improvements that extend the lives or increase the capacity of plant assets are capitalized.
When assets are retired or disposed of, the cost
and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in the results of operations
in the reporting period of disposition.
Depreciation is calculated on a straight-line
basis over the estimated useful life of the assets. The depreciable lives applied are:
| Building, Warehouse and Improvements | |
20 to 30 years |
| Office Equipment | |
3 to 7 years |
| Vehicles | |
5 to15 years |
| Machinery and Equipment | |
7 to 15 years |
Intangible Assets
The
Company evaluates intangible assets in accordance with FASB ASC Topic 350, “Intangibles
— Goodwill and Other.” Intangible
assets deemed to have indefinite lives are not amortized, but are subject to annual impairment tests. If the assumptions and estimates
used to allocate the purchase price are not correct, or if business conditions change, purchase price adjustments or future asset impairment
charges could be required. The value of the Company’s intangible
assets could be impacted by future adverse changes such as: (i) any future declines in the Company’s
operating results, (ii) a decline in the valuation of technology, including the valuation of the Company’s
common stock, (iii) a significant slowdown in the worldwide economy, or (iv) any failure to meet the performance projections included
in the Company’s forecasts of future operating results. In accordance
with FASB ASC Topic 350, the Company tests intangible assets for impairment on an annual basis or more frequently if the Company believes
indicators of impairment exist. Impairment evaluations involve management estimates of asset useful lives and future cash flows. Significant
judgment by management is required in the forecasts of future operating results that are used in the evaluations. It is possible, however,
that the plans and estimates used may be incorrect. If the Company’s
actual results, or the plans and estimates used in future impairment analysis, are lower than the original estimates used to assess the
recoverability of these assets, we could incur additional impairment charges in a future period. Based on such evaluations, there were
no impairments recorded for intangible assets for the years ended June 30, 2023 and 2022, respectively.
Construction in Progress
Construction
in progress represents the costs incurred in connection with the construction of buildings or new additions to the Company’s
plant facilities. Costs classified as construction in progress include all costs of obtaining the asset and bringing it to the location
in the condition necessary for its intended use. No depreciation is provided for construction in progress until such time as the assets
are completed and are placed into service.
The
Company reviews the carrying value of construction in progress for impairment whenever events and circumstances indicate that the carrying
value of an asset may not be recoverable from the estimated future cash flows expected to result from its use and eventual disposition.
In cases where undiscounted expected future cash flows are less than the carrying value of the assets, an impairment loss is recognized
equal to an amount by which the carrying value exceeds the fair value of the assets. The factors considered by Management in performing
this assessment include current operating results, trends and prospects, the manner in which the property is used, and the effects of
obsolescence, demand, competition and other economic factors. Based on this assessment, there were no impairments recorded for construction
in progress, for the years ended June 30, 2023 and June 30, 2022, respectively. However, there can be no assurances that demand for the
Company’s products or services will continue, which could result
in an impairment of long-lived assets in the future.
Revenue Recognition
The
Company recognizes revenue at the amount to which it expects to be entitled when control of the products or services is transferred to
its customers based on any contract which has been identified. Control is generally transferred when the Company has a present right to
payment and title and the significant risks and rewards of ownership of products or services are transferred to its customers while performance
obligation are identified and completed. For most of the Company’s
products net sales, control transfers when products are shipped and transaction price are determined. The majority of the Company’s
revenue relates to the sale of inventory to customers, and revenue is recognized when control of the products or services is transferred
to its customers that reflects the performance obligations are properly allocated and satisfied with transaction price determined. Given
the nature of the Company’s business and the applicable rules guiding
revenue recognition, the Company’s revenue recognition practices
do not contain estimates that materially affect the results of operations. The Company records revenue at the discounted selling price
and allows its customers to return products for exchange or credit subject to certain limitations. A provision for such returns is recorded
based upon historical experience. There has been no provision recorded for returns based upon historical experience for the years ended
June 30, 2023 and 2022, respectively.
Cost of Goods Sold
Cost of goods sold consists primarily
of the costs of raw materials, freight charges, direct labor, depreciation of plants and machinery, warehousing and overhead costs associated
with the manufacturing process and commission expenses.
Income Taxes
The
Company adopts FASB ASC Topic 740, “Income Taxes,”
which requires the recognition of deferred tax assets and liabilities for the
expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred
income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and
their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which
the differences are expected to affect taxable income. A valuation allowance is established for deferred tax assets if it is more likely
than not that these items will either expire before the Company is able to realize the benefits or that future deductibility is uncertain.
Under
ASC 740, a tax position is recognized as a benefit only if it is “more
likely than not” that the tax position would be sustained in a tax
examination, with a tax examination being presumed to occur. The evaluation of a tax position is a two-step process. The first step is
to determine whether it is more-likely-than-not that a tax position will be sustained upon examination, including the resolution of any
related appeals or litigations based on the technical merits of that position. The second step is to measure a tax position that meets
the more-likely-than-not threshold to determine the amount of benefit to be recognized in the financial statements. A tax position is
measured at the largest amount of benefit that is greater than 50 percent likely of being realized upon ultimate settlement. Tax positions
that previously failed to meet the more-likely-than-not recognition threshold should be recognized in the first subsequent period in which
the threshold is met. Previously recognized tax positions that no longer meet the more-likely-than-not criteria should be de-recognized
in the first subsequent financial reporting period in which the threshold is no longer met. Penalties and interest incurred related to
underpayment of income tax are classified as income tax expense in the year incurred. GAAP also provides guidance on de-recognition, classification,
interest and penalties, accounting in interim periods, disclosures and transition.
As
a result of the implementation of FIN 48 (ASC 740-10), the Company undertook a comprehensive review of its portfolio of tax positions
in accordance with recognition standards established by FIN 48 (ASC 740-10). The Company recognized no material adjustments to liabilities
or shareholders’ equity as a result of the implementation. The adoption
of FIN 48 did not have a material impact on the Company’s financial
statements.
The
application of tax laws and regulations is subject to legal and factual interpretation, judgment and uncertainty. Tax laws and regulations
themselves are subject to change as a result of changes in fiscal policy, changes in legislation, the evolution of regulations and court
rulings. Therefore, the actual liability may be materially different from the Company’s
estimates, which could result in the need to record additional tax liabilities or potentially reverse previously recorded tax liabilities
or deferred tax asset valuation allowance.
Enterprise Income Tax
Under
the Provisional Regulations of PRC Concerning Income Tax on Enterprises promulgated by the PRC (the “EIT
Law”), income tax is payable by enterprises at a rate of 25% of
their taxable income.
Value Added Tax
The
Provisional Regulations of the PRC Concerning Value Added Tax promulgated by the State Council came into effect on January 1, 1994 and
revised on January 1, 2009. Under these regulations and the Implementing Rules of the Provisional Regulations of the PRC Concerning Value
Added Tax, value added tax (“VAT”)
is imposed on goods sold in, or imported into, the PRC and on processing, repair and replacement services provided within the PRC.
VAT payable in the PRC is charged on an aggregated
basis at a rate of 13% or 16% (depending on the type of goods involved) on the full price collected for the goods sold or, in the case
of taxable services provided, at a rate of 16% on the charges for the taxable services provided, but excluding, in respect of both goods
and services, any amount paid in respect of VAT included in the price or charges, and less any deductible VAT already paid by the taxpayer
on purchases of goods and services in the same financial year. As of June 30, 2023 and June 30, 2022, VAT payables were $37,119 and $29,835,
respectively.
Sales-Related Taxes
Pursuant to the tax law and regulations of the
PRC, the Company is obligated to pay 7% and 5% of the annual aggregate VAT paid by the Company as taxes for the purposes of maintaining
and building cities and educational facilities, which fees are included as sales-related taxes. Sales-related taxes are recorded when
sales revenue is recognized.
Concentrations of Business and Credit Risks
All
of the Company’s manufacturing is located in the PRC. There can
be no assurance that the Company will be able to successfully continue to manufacture its products and failure to do so would have a material
adverse effect on the Company’s financial position, results of operations
and cash flows. Moreover, the success of the Company’s operations
is subject to numerous contingencies, some of which are beyond management’s
control. These contingencies include general economic conditions, prices of raw materials, competition, governmental and political conditions,
and changes in regulations. Since the Company is dependent on trade in the PRC, the Company is subject to various additional political,
economic and other uncertainties. Among other risks, the Company’s
operations will be subject to the risks of restrictions on transfer of funds, domestic customs, changing taxation policies, foreign exchange
restrictions, and political and governmental regulations. The Company operates in China, which may give rise to significant foreign currency
risks from fluctuations and the degree of volatility of foreign exchange rates between U.S. dollars and the Chinese currency RMB. The
results of operations denominated in foreign currency are translated at the average rate of exchange during the reporting periods.
Earnings Per Share
Basic earnings per common share is computed by
dividing net earnings applicable to common shareholders by the weighted-average number of common shares outstanding during the period.
When applicable, diluted earnings per common share is determined using the weighted-average number of common shares outstanding during
the period, adjusted for the dilutive effect of common stock equivalents, consisting of shares that might be issued upon exercise of common
stock options and warrants. For the years ended June 30, 2023 and 2022, the Company had no potential dilutive common stock equivalents
outstanding.
Potential common shares issued are calculated
using the treasury stock method, which recognizes the use of proceeds that could be obtained upon the exercise of options and warrants
in computing diluted earnings per share. It assumes that any proceeds would be used to purchase common stock at the average market price
of the common stock during the period.
FASB
ASC Topic 260, “Earnings Per Share,” requires
a reconciliation of the numerator and denominator of the basic and diluted earnings per share (EPS) computations.
Recent Accounting Pronouncements
In
June 2016, the FASB issued ASU 2016-13, Financial Instruments-Credit Losses (Topic 326) – Measurement
of Credit Losses on Financial Instruments (ASU 2016-13). The main objective of the standard is to provide financial statement users with
more decision-useful information about the expected credit losses on financial instruments and other commitments to extend credit held
by a reporting entity at each reporting date. In issuing this standard, the FASB is responding to criticism that today’s
guidance delays recognition of credit losses. The standard will replace today’s
“incurred loss” approach
with an “expected loss” model.
The new model, referred to as the current expected credit loss (“CECL”)
model, will apply to: (1) financial assets subject to credit losses and measured at amortized cost, and (2) certain off-balance sheet
credit exposures. The standard is applicable to loans, accounts receivable, trade receivables, and other financial assets measured at
amortized cost, loan commitments and certain other off-balance sheet credit exposures, debt securities (including those held-to-maturity)
and other financial assets measured at fair value through other comprehensive income, and beneficial interests in securitized financial
assets. The CECL model does not apply to available-for-sale debt securities. For available-for-sale debt securities with unrealized losses,
entities will measure credit losses in a manner similar to what they do today, except that the credit losses will be recognized as allowances
rather than reductions in the amortized cost of the securities. Accordingly, the new methodology will be utilized when assessing the Company’s
financial instruments for impairment. As a result, entities will recognize improvements to estimated credit losses immediately in earnings
rather than as interest income over time, as they do today. The ASU also simplifies the accounting model for purchased credit-impaired
debt securities and loans. ASU 2016-13 also expands the disclosure requirements regarding an entity’s
assumptions, models, and methods for estimating the allowance for loan and lease losses. ASU 2016-13 is effective for years beginning
after December 15, 2019, including interim periods within those fiscal years under a modified retrospective approach. Early adoption is
permitted for the periods beginning after December 15, 2018. The Company adopted the guidance from July 1, 2020. The Company finalized
its analysis and determined that the adoption of this guidance has no material impact on the Company’s
consolidated financial statements and its internal controls over financial reporting.
In
August 2018, the FASB issued ASU 2018-13, Fair Value Measurement (Topic 820) – Disclosure
Framework – Changes to the Disclosure Requirements for Fair Value
Measurement (ASU 2018-13), which modifies the disclosure requirements on fair value measurements, including removing the requirement to
disclose (1) the amount of and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy, (2) the policy for timing
of transfers between levels and (3) the valuation processes for Level 3 fair value measurements. ASU 2018-13 also added new disclosures
including the requirement to disclose (a) the changes in unrealized gains and losses for the period included in other comprehensive income
for recurring Level 3 fair value measurements held at the end of the reporting period and (b) the range and weighted average of significant
unobservable inputs used to develop Level 3 fair value measurements. ASU 2018-13 is effective for fiscal years (and interim reporting
periods within those years) beginning after December 15, 2019 and early adoption is permitted. This standard will only impact the disclosures
pertaining to fair value measurements. The Company adopted the guidance from July 1, 2020. The Company finalized its analysis and determined
that the adoption of this guidance has no material impact on the Company’s
consolidated financial statements and its internal controls over financial reporting.
NOTE 3 - ASSETS SALE
On
December 24, 2014, Humankind entered into a stock transfer agreement (the “Agreement”)
with Xiuzheng Pharmaceutical Group Co., Ltd a company incorporated under the laws of the People’s
Republic of China and located in Jilin province (“Xiuzheng Pharmacy”
or the “Buyer”),
and Mr. Xin Sun, the CEO of the Company, and Huimeijia, pursuant to which, Humankind and Mr. Xin Sun (the “Equity
Holders”), shall sell their respective equity interests in Huimeijia
to Xiuzheng Pharmacy. The transfer of the 100% equity interests of Huimeijia to the Buyer was for a total cash consideration of RMB 8,000,000
(approximately $1,306,186) to the Equity Holders.
On
February 9, 2015, the four parties entered into a supplementary agreement (the “Supplementary
Agreement”) to modify the terms of the Agreement, pursuant to which,
the Equity Holders and Huimeijia (collectively the “Assets Transferors”)
shall only sell the 19 drug approval numbers (including the tablet, capsule, powder, mixture, oral liquid, syrup and oral solution under
the 19 approval numbers; licenses including the original copies of Business License, Organization Code Certificate, Tax Registration Certificate,
Drug Production Permit and GMP Certificate, and other documents and original copies related to the production and operation of the 19
drugs) (the “Assets”)
to Xiuzheng Pharmacy. The Equity Holders will retain the equity interests in Huimeijia, but will have the equity interests pledged to
Xiuzheng Pharmacy until the Assets are transferred, at which time all the cash consideration shall be paid by the Buyer. The total cash
consideration remains to be the same as under the Agreement, i.e., RMB 8,000,000 (approximately $1,306,186) to the Assets Transferors.
In the event that the Assets are failed to be transferred to the Buyer due to the fault of the Assets Transferors, the paid consideration
shall be returned to the Buyer with interests accrued. If the failure of the transfer of the Assets is a result of the government policy
changes or force majeure, the paid cash consideration shall be returned to the Buyer but without any interests.
As of June 30, 2016, the transfer of the Assets
had not been completed because the assets transfer crossed different provinces which resulted in a complicated interaction among the local
administrations of Heilongjiang Province, where Huimeijia is located and Jilin Province, where the transferee is located.
On
October 12, 2016, the four parties agreed to rescind the Supplementary Agreement and entered into a new supplementary agreement (the “New
Supplementary Agreement”), pursuant to which the four parties agreed
to execute the transfer of the equity interests based on the Original Agreement and the Equity Holders agreed to sell their respective
equity interests in Huimeijia to Xiuzheng Pharmacy. The transfer of 100% of the equity interests of Huimeijia to the Buyer was for a total
cash consideration of RMB 8,000,000 (approximately $1,306,186) (the “Purchase
Price”) to the Equity Holders. 40% of the Purchase Price was due
within 10 business days after the signing of the New Supplementary Agreement; 40% of the Purchase Price was due within 10 business days
after the completion of the changes in business registration described in the Original Agreement and Xiuzheng Pharmacy obtaining documents
evidencing its ownership on Huimeijia; 15% of the Purchase Price is due within 10 business days after the transfer of all of the Assets
is approved by Heilongjiang FDA; and 5% of the Purchase Price is due within 10 business days after all of the Assets have been transferred
to Xiuzheng Pharmacy or its designee and Humankind and Mr. Xin Sun have instructed Xiuzheng Pharmacy to complete three-batches production
of all forms of the drugs included in the Assets. As of the date of this report, 80% of the Purchase Price has been paid, the Company
has completed changes in its business registration, and Xiuzheng Pharmacy has obtained a business license issued by the local State Administration
of Industry and Commerce in Harbin (“Harbin SAIC”)
to Huimeijia, in which the ownership of Huimeijia has been recorded as held by Xiuzheng Pharmacy, with Harbin SAIC and the legal representative
(a person that is authorized to take most of the corporate actions on behalf of a company under the corporate laws in China) of Huimeijia
has been appointed by the Buyer.
On
June 27, 2023 (the “Signing Date”),
Harbin Humankind Biology Technology Co., Limited (“Humankind”),
a wholly owned subsidiary of China Health Industries Holdings, Inc. (“China
Health”), a corporation incorporated under the laws of the State
of Delaware, entered into certain equity transfer agreements (collectively, the “Agreements”)
with Mr. Xin Sun and Mr. Kai Sun (collectively, the “Sellers”).
Pursuant to the Agreements, the Sellers agreed to transfer to Humankind 99% and 1% of the equity interests of Heilongjiang HempCan Pharmaceuticals
Co., Ltd. (“HempCan”),
for a consideration of RMB292,050,000 (approximately $40,275,537) and RMB2,950,000 (approximately $406,824) respectively, totaling RMB295
million (approximately $40,682,360) (collectively, the “Purchase
Prices”). The Purchase Prices shall be fully paid to Sellers within
15 business days after the Signing Date, and the Sellers shall complete certain registration procedures, such as change of equity ownership
and change of business registration within 15 business days after full Purchase Prices are received. If Humankind is late in paying the
Purchase Prices, a 0.1% per day late penalty on the amount of Purchase Prices that is paid late shall be paid to the Sellers. Mr. Xin
Sun is China Health’s Chairman, sole director and sole executive
officer and Mr. Kai Sun is Mr. Xin Sun’s younger brother.
NOTE 4 - ACCOUNTS RECEIVABLE
The
Company’s accounts receivable amounted to $nil and $9,567 net of
allowance for doubtful accounts amounting to $51,188 and $48,769 as of June 30, 2023 and 2022, respectively.
NOTE 5 - INVENTORIES
Inventory consists of following:
| | |
June
30,
2023 | | |
June
30,
2022 | |
| Raw Materials | |
$ | 1,769 | | |
$ | 213,055 | |
| Supplies and Packing Materials | |
| - | | |
| 90,303 | |
| Work-in-Progress | |
| - | | |
| 82,412 | |
| Finished Goods | |
| 575 | | |
| 135,459 | |
| Self-made semi finished goods | |
| 38,264 | | |
| - | |
| Total | |
$ | 40,608 | | |
$ | 521,229 | |
The decrease in raw materials is mainly due to
the destruction of expired materials and the sale of hemp seeds.The inventory allowance with an amount of $nil and $nil were provided
for the years ended June 30, 2023 and 2022, respectively. The decrease in raw materials is mainly due to the destruction of expired raw
materials.
NOTE 6 - CONSTRUCTION IN PROGRESS
Construction in progress consisted of the following:
| | |
June
30,
2023 | | |
June
30,
2022 | |
| Plant - HLJ Huimeijia | |
$ | 420,092 | | |
$ | 579,402 | |
| Total | |
$ | 420,092 | | |
$ | 579,402 | |
On
April 6, 2012, HLJ Huimeijia entered into an agreement with a contractor for construction of the HLJ Huimeijia plant. The estimated total
cost of construction was approximately $1.86 million (RMB12,800,000). As
of June 30, 2023, 71.36% of construction has been completed, $1,259,608 (RMB9,133,796) has been recorded as costs of construction in progress
and construction in progress at an amount of $724,409 (RMB5,252,904) has been completed and converted into property, plant and equipment.
NOTE 7 - PROPERTY, PLANTS AND EQUIPMENT
Property, plants and equipment consisted of the
following:
| | |
June
30,
2023 | | |
June
30,
2022 | |
| Building, Warehouses and Improvements | |
$ | 3,768,300 | | |
$ | 3,946,492 | |
| Machinery and Equipment | |
| 1,693,740 | | |
| 1,832,446 | |
| Office Equipment | |
| 72,382 | | |
| 78,360 | |
| Vehicles | |
| 75,117 | | |
| 217,808 | |
| Others | |
| 889,225 | | |
| 962,667 | |
| Less Accumulated Depreciation | |
| (3,771,594 | ) | |
| (3,879,752 | ) |
| Total | |
$ | 2,727,170 | | |
$ | 3,158,021 | |
Depreciation expense was
$332,057 and $344,919 for the years ended June 30, 2023 and 2022, respectively. Depreciation expense charged to operations was $202,889
and $344,919 for the years ended June 30, 2023 and 2022, respectively. Depreciation expense charged to cost of goods sold was $nil and
$nil for the years ended June 30, 2023 and 2022, respectively.
NOTE 8 - INTANGIBLE ASSETS
The following is a summary of intangible assets:
| | |
June
30,
2023 | | |
June
30,
2022 | |
| Land Use Rights – Humankind | |
$ | 874,048 | | |
$ | 946,237 | |
| Health Supplement Product Patents – Humankind | |
| 4,137,188 | | |
| 4,478,882 | |
| Pharmaceutical Patents - HLJ Huimeijia | |
| 360,511 | | |
| 390,285 | |
| Land Use Rights - HLJ Huimeijia | |
| 597,836 | | |
| 647,211 | |
| Less: Accumulated Amortization | |
| (5,004,808 | ) | |
| (5,050,012 | ) |
| Intangible Assets, net, Held for Continuing Operations | |
$ | 964,775 | | |
$ | 1,412,603 | |
There is no private land ownership in PRC Enterprises
and individuals can pay the government a fee to obtain the right to use a piece of land for commercial purposes or residential purposes
for an initial period of 50 years or 70 years, respectively. The land use right can be sold, purchased, and exchanged in the market. The
successor owner of the land use right will have the right to use the land for the time remaining on the initial period. The patent has
amortized life of 10 years.
Amortization expense charged to operations was
$354,619 and $485,625 for the years ended June 30, 2023 and 2022, respectively.
NOTE 9 - RELATED PARTY DEBTS
Related party debts, which represent temporary
short-term loans from Mr. Xin Sun and Mr. Kai Sun consisted of the following:
| | |
June
30,
2023 | | |
June
30,
2022 | |
| Mr. Xin Sun | |
$ | 5,121,776 | | |
$ | 6,724,850 | |
| Mr. Kai Sun | |
| 32,248 | | |
| 34,911 | |
| Related Party Debts, Held for Continuing Operations | |
$ | 5,154,024 | | |
$ | 6,759,761 | |
These loans are unsecured and non-interest bearing
and have no fixed terms of repayment; therefore, they are deemed payable on demand. Mr. Kai Sun, a director of Humankind, is a PRC citizen
and a family member of Mr. Xin Sun, the CEO of the Company.
NOTE 10 - INCOME TAXES
(a) Corporate income taxes
China Health US was incorporated in the State
of Arizona on July 11, 1996. After the Company had acquired the business of China Health HK through the acquisition of all the share capital
of China Health HK under a share exchange agreement dated December 31, 2008, it became a holding company and did not conduct any substantial
operations or business of its own in the State of Delaware and in the U.S.
The Company also does not provide for U.S. taxes
or foreign withholding taxes on undistributed earnings from its non-U.S. subsidiaries, either owned directly or indirectly, because it
was elected to indefinitely reinvest such earnings outside the U.S to support non-U.S. liquidity needs to fund operations and growth of
its foreign subsidiaries and acquisitions.
United States
China
Health US had no taxable income for U.S. corporate income tax purposes for the years ended June 30, 2023 and 2022, respectively. As of
June 30, 2023 and 2022, China Health US had $1,470,989 and $1,793,042 in net operating loss carry forwards available to offset future
taxable income, respectively. The federal corporate net operating loss carryover is expired in 20 taxable years following the taxable
year of the loss. If not utilized, the federal net operating loss for the fiscal years 2023 and 2022 in an amount of $322,053 and $223,833,
respectively, will begin to expire in the years 2042 and 2041, respectively. Management believes that it is more likely than not that
the benefits from these accumulated net operating losses will not be realized in the future due to the Company’s
operating history and the continued losses of its U.S. operation. Accordingly, the Company has provided a full valuation allowance on
the deferred tax assets under its U.S. entity.
Hong Kong
China
Health Industries Holdings Limited (“China Health HK”)
was incorporated in Hong Kong on July 20, 2007 and is subject to Hong Kong profits taxation on its business activities conducted in Hong
Kong and income sourced in Hong Kong. As of June 30, 2023, and 2022, China Health Hong Kong had $10,646 and $11,030 in net operating loss
carry forwards available to offset future taxable income, respectively. Net operating losses of Hong Kong can generally be carried forward
indefinitely. The Company believes that it is more likely than not that these accumulated net operating losses will not be utilized in
the future due to the fact that the Company does not have and probably will not have any business operations in HK. Therefore, the Company
had provided full valuation allowance for the deferred tax assets arising from the losses in Hong Kong during the years ended June 30,
2023, and 2022, amounting $384 and $441, respectively. Accordingly, there is no net deferred tax assets under this entity.
People’s
Republic of China
Under the EIT Law, the standard EIT rate is 25%.
The PRC subsidiaries of the Company are subject to PRC income taxes on an entity basis on income arising in or derived from the tax jurisdiction
in which they operate.
The provision for income taxes consisted of the following
for the years ended June 30, 2023 and 2022:
As of June 30, 2023, and 2022, taxes payable consists
of:
| | |
June
30,
2023 | | |
June
30,
2022 | |
| Income tax payable | |
$ | 22,020 | | |
$ | 23,839 | |
| Value-added tax payable | |
| 37,119 | | |
| 29,835 | |
| Other taxes payable | |
| 78,446 | | |
| 79,331 | |
| Total | |
$ | 137,585 | | |
$ | 133,005 | |
A
reconciliation between the Company’s actual provision for income
taxes and the provision at the statutory rate is as follows:
| | |
June
30,
2023 | | |
June
30,
2022 | |
| Pre-tax book income | |
$ | (408,772 | ) | |
$ | (351,016 | ) |
| Federal statutory rate | |
| 21 | % | |
| 21 | % |
| Income tax computed at U.S. federal statutory rate | |
| (85,842 | ) | |
| (73,714 | ) |
| Non-deductible staff welfare | |
| - | | |
| - | |
| Foreign rate differential | |
| 175,725 | | |
| (7,874 | ) |
| Change in valuation allowance | |
| (89,883 | ) | |
| 84,522 | |
| Total provision for income taxes | |
$ | - | | |
$ | 2,934 | |
The
Company’s effective tax rate was nil and 0.8% for the years ended
June 30, 2023 and 2022, respectively.
The provision for income taxes on income consists
of the following for the years ended June 30, 2023 and 2022:
Provision for income taxes consisted of:
| | |
For the Years Ended | |
| | |
June 30, | |
| | |
2023 | | |
2022 | |
| Current provision: | |
| | |
| |
| Domestic | |
$ | - | | |
$ | - | |
| Foreign | |
| - | | |
| - | |
| Total current provision | |
| - | | |
| - | |
| Deferred provision: | |
| - | | |
| - | |
| Domestic | |
| - | | |
| - | |
| Foreign | |
| - | | |
| 2,934 | |
| Total deferred provision | |
| - | | |
| 2,934 | |
| Total provision for income taxes | |
$ | - | | |
$ | 2,934 | |
Significant components of deferred tax assets
were as follows:
| | |
June
30,
2023 | | |
June
30,
2022 | |
| Deferred tax assets | |
| | |
| |
| Net operating loss carry forward | |
$ | 1,007,658 | | |
$ | 1,096,936 | |
| Allowance for doubtful accounts | |
| (12,797 | ) | |
| (12,192 | ) |
| Valuation allowance | |
| (994,861 | ) | |
| (1,084,744 | ) |
| Deferred tax assets, net | |
$ | - | | |
$ | - | |
(b) Uncertain tax positions
There were no unrecognized tax benefits as of
June 30, 2023 and 2022, respectively. Management does not anticipate any potential future adjustments in the next twelve months which
would result in a material change to its tax positions. There was no interests and penalties arising from its tax payments for the years
ended June 30, 2023.
NOTE 11 - EARNINGS PER SHARE
Basic earnings per common share is computed by
dividing net earnings applicable to common shareholders by the weighted-average number of common shares outstanding during the period.
When applicable, diluted earnings per common share is determined using the weighted-average number of common shares outstanding during
the period, adjusted for the dilutive effect of common stock equivalents, consisting of shares that might be issued upon exercise of common
stock options and warrants.
Potential common shares issued are calculated
using the treasury stock method, which recognizes the use of proceeds that could be obtained upon the exercise of options and warrants
in computing diluted earnings per share. It assumes that any proceeds would be used to purchase common stock at the average market price
of the common stock during the period.
FASB ASC Topic 260, Earnings Per Share, requires
a reconciliation of the numerator and denominator of the basic and diluted earnings per share (EPS) computations.
For the years ended June 30, 2023, and 2022, the
Company does not have potential dilutive shares. The following table sets forth the computation of basic and diluted net income per share:
| | |
For the Years Ended | |
| | |
June 30, | | |
June 30, | |
| | |
2023 | | |
2022 | |
| Net loss | |
$ | (408,772 | ) | |
$ | (353,950 | ) |
| | |
| | | |
| | |
| Net income/(loss) per share: | |
| | | |
| | |
Net loss per share Basic & diluted | |
$ | (0.0062 | ) | |
$ | (0.0054 | ) |
| | |
| | | |
| | |
| Weighted average shares outstanding: | |
| | | |
| | |
Basic & diluted | |
| 65,539,737 | | |
| 65,539,737 | |
NOTE 12 - COMMITMENTS AND CONTINGENCIES
The
Company’s assets are located in the PRC and revenues are derived
from operations in the PRC.
In
terms of industry regulations and policies, the economy of the PRC has been transitioning from a planned economy to market oriented economy.
Although in recent years the Chinese government has implemented measures emphasizing the utilization of market forces for economic reforms,
the reduction of state ownership of productive assets and the establishment of sound corporate governance in business enterprises, a substantial
portion of productive assets in the PRC is still owned by the Chinese government. For example, there is no private land ownership in PRC
and all land is leased by government to business entities or individuals through the government’s
granting of Land Use Rights. The granting process is typically based on government policies at the time of granting and can be lengthy
and complex. This process may adversely affect the Company’s future
manufacturing expansions. The Chinese government also exercises significant control over the PRC’s
economic growth through the allocation of resources and providing preferential treatment to particular industries or companies. Uncertainties
may arise with changing of governmental policies and measures.
The
Company faces a number of risks and challenges not typically associated with companies in North America and Western Europe, since its
assets exist solely in the PRC, and its revenues are derived from its operations therein. The PRC is a developing country with an early
stage market economic system, overshadowed by the state. Its political and economic systems are very different from the more developed
countries and are in a state of change. The PRC also faces many social, economic and political challenges that may produce major shocks,
instabilities and even crises, in both its domestic arena and in its relationships with other countries, including the United States.
Such shocks, instabilities and crises may in turn significantly and negatively affect the Company’s
performance.
The Company had no rental commitment as of June
30, 2023.
NOTE 13 - MAJOR SUPPLIERS AND CUSTOMERS
For the year ended June 30, 2023, the Company
had five raw material suppliers,of which the largest proportion was the supplier of health products, with a purchase amount of $68,803,
accounting for 97%. For the year ended June 30, 2022, the Company had no suppliers due to its being temporarily out of production.
For
the year ended June 30, 2023, the Company had three customers that in aggregate accounted for 100% of the Company’s
total sales, with each customer accounting for 63%, 33%, and 4%, respectively. For the year ended June 30, 2022, the Company had no customers
due to the enterprise Transformation and the COVID-19 resurgence.
NOTE 14 - SEGMENT REPORTING
The
Company was organized into three main business segments based on the types of products being provided to customers: HLJ Huimeijia, Humankind
and others. Each of the three operating segments referenced above has separate and distinct general ledgers. The chief operating decision
maker (“CODM”)
receives financial information, including revenue, gross margin, operating income, and net income produced from the various general ledger
systems to make decisions about allocating resources and assessing performance; however, the principal measure of segment profitability
or loss used by the CODM is net income or loss by segment.
The following tables present summary information
by segment for the years ended June 30, 2023 and 2022, respectively:
| | |
For
the Year Ended
June 30, 2023
| | |
For
the Year Ended
June 30, 2022
| |
| | |
HLJ | | |
| | |
| | |
Consolidated | | |
HLJ | | |
| | |
| | |
Consolidated | |
| | |
Huimeijia | | |
Humankind | | |
Others | | |
operations | | |
Huimeijia | | |
Humankind | | |
Others | | |
operations | |
| Revenues | |
$ | - | | |
$ | 111,597 | | |
$ | - | | |
$ | 111,597 | | |
$ | 267 | | |
$ | - | | |
$ | - | | |
$ | 267 | |
| Cost of revenues | |
| - | | |
| 111,359 | | |
| - | | |
| 111,359 | | |
| 1,748 | | |
| - | | |
| - | | |
| 1,748 | |
| Gross profit (loss) | |
| - | | |
| 200 | | |
| - | | |
| 200 | | |
| (1,481 | ) | |
| - | | |
| - | | |
| (1,481 | ) |
| Interest income | |
| 386 | | |
| 113,201 | | |
| 28 | | |
| 113,615 | | |
| 322 | | |
| 157,536 | | |
| 2 | | |
| 157,860 | |
| Interest expense | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Depreciation and amortization | |
| 74,972 | | |
| 482,536 | | |
| - | | |
| 557,508 | | |
| 60,962 | | |
| 650,553 | | |
| - | | |
| 711,515 | |
| Income tax | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 2,934 | | |
| - | | |
| 2,934 | |
| Net income (loss) | |
| 525,522 | | |
| (611,857 | ) | |
| (322,437 | ) | |
| (408,772 | ) | |
| 509,990 | | |
| (637,666 | ) | |
| (226,274 | ) | |
| (353,950 | ) |
| Total capital expenditures | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Total assets | |
$ | 2,178,524 | | |
$ | 42,874,722 | | |
$ | 29,083 | | |
$ | 45,082,329 | | |
$ | 2,994,826 | | |
$ | 47,724,803 | | |
$ | 27,889 | | |
$ | 50,747,518 | |
NOTE 15 - SUBSEQUENT EVENTS
The Company has evaluated subsequent events from
the balance sheet date through the date the financial statements were issued and determined that there are no additional items required
to disclose.
Item 9. Changes in and Disagreements with Accountants
on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
We maintain a set of disclosure controls and procedures
designed to ensure that information required to be disclosed by us in our reports filed under the Securities Exchange Act, is recorded,
processed, summarized and reported within the time periods specified by the SEC’s rules and forms. Disclosure controls are also
designed with the objective of ensuring that this information is accumulated and communicated to our management, including our Chief Executive
Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Based upon their evaluation as of the end of the
periods covered by this report, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures
were not effective to satisfy the objectives, due to the material weakness in our internal control over financial reporting discussed
below.
Management’s Report on Internal Control
over Financial Reporting
Our management is responsible for establishing
and maintaining adequate internal control over financial reporting. Our internal control system was designed to provide reasonable assurance
to our management and our sole board member regarding the preparation and fair presentation of published financial statements.
Our management assessed the effectiveness of our
internal control over financial reporting as of June 30, 2023. In making this assessment, it used the criteria set forth by the Committee
of Sponsoring Organizations of the Treadway Commission (COSO) in 2013 in Internal Control—Integrated Framework. Our management
has implemented and tested our internal control over financial reporting based on these criteria and did not identify any significant
deficiencies and material weaknesses as of June 30, 2023. However, based on the fact that we do not have any full-time accounting personnel
who have U.S. GAAP experience, our management has considered this as a material weakness and determined that as of June 30, 2023, the
internal control over financial reporting was not effective.
In an effort to remedy this material weakness in
the future, we intend to do the following:
| |
● |
Develop a comprehensive training and development plan, for our finance, accounting and internal audit personnel, including our Chief Financial Officer, Financial Manager, and others, in the principles and rules of U.S. GAAP, SEC reporting requirements and the application thereof. |
| |
|
|
| |
● |
Design and implement a program to provide ongoing company-wide training regarding the Company’s internal controls, with particular emphasis on our finance and accounting staff. |
| |
|
|
| |
● |
Implement an internal review process over financial reporting to review all recent accounting pronouncements and to verify that the accounting treatment identified in such report have been fully implemented and confirmed by our internal control department. In the future, we will continue to improve our ongoing review and supervision of our internal control over financial reporting. |
| |
|
|
| |
● |
Hire an individual that possesses the requisite U.S. GAAP experience and education. |
Despite the material weakness reported above,
our management believes that our consolidated financial statements included in this report fairly present in all material respects our
financial condition, results of operations and cash flows for the periods presented.
This report does not include an attestation report
of our registered accounting firm regarding internal control over financial reporting. The management’s report was not subject to
attestation by our registered public accounting firm because we are a smaller reporting company.
Changes in Internal Control over Financial
Reporting
No changes in our internal control over financial
reporting have come to management’s attention during our last fiscal year that have materially affected, or are likely to materially
affect, our internal control over financial reporting.
Limitations on Controls
Management does not expect that our disclosure
controls and procedures or our internal control over financial reporting will prevent or detect all error and fraud. Any control system,
no matter how well designed and operated, is based upon certain assumptions and can provide only reasonable, not absolute, assurance that
its objectives will be met. Further, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud
will not occur or that all control issues and instances of fraud, if any, within the Company have been detected.
Item 9B. Other Information.
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions
that Prevent Inspections.
None.
Part III
Item 10. Directors, Executive Officers and
Corporate Governance.
The following table sets forth information regarding
our sole board member and executive officer. Our sole director holds office until the election and qualification of his successor.
| Name |
|
Age |
|
Position |
| Xin Sun |
|
57 |
|
Chairman (sole director), Chief Executive Officer, Chief Financial Officer and Treasurer |
Biography
Mr. Xin Sun, age 57, has been serving as our Chairman
of Board of Directors, Chief Executive Officer and Chief Financial Officer since 2009. From 1988 to 1991, he was the Production Manager
at Ha Yao Group Sanchine Medicine Joint-Stock Company Ltd. From 1991 to 1994, he was the District Director for the Northeast District
of China for Pfizer Pharmaceuticals Limited. Thereafter, he spent one year as the Director of Marketing for Ha Yao Group Sanchine Medicine
Joint-Stock Company Ltd. From 1996 to 2002, he was the Chief Executive Officer of a company he founded, Heilongjiang Bijie Chemical Industry
Co., Ltd., which is no longer in existence. From 2003 to the present, he has been the President and Chief Executive Officer of Humankind.
Mr. Sun is also President and General Manager of Huimeijia. Mr. Xin Sun attended Jia Mu Si Medical College with a major in Pharmacy from
1984 to 1988. He obtained his Masters of Business Administration from Renmin University of China in 2004. Mr. Sun attended the Doctor
of Business Administration program in Business Institute of Pennsylvania since September 2018 and received a doctor’s degree in
2019.
As a result of his professional experience, Mr.
Xin Sun is well known in the pharmaceutical field in Harbin, PRC. While he was studying at Renmin University, Mr. Xin Sun developed many
contacts in the pharmaceutical field, many of which later became district agents and other employees in Humankind’s distribution
system.
Our sole director, Mr. Xin Sun, does not hold
any directorships in other reporting companies and does not qualify as an “independent director” under the Rules of NASDAQ,
Marketplace Rule 4200(a)(15).
To our knowledge, during the last ten years, none
of our directors and executive officers (including those of our subsidiaries) have:
| |
(a) |
had a bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time. |
| |
(b) |
been convicted in a criminal proceeding or been subject to a pending criminal proceeding, excluding traffic violations and other minor offenses. |
| |
(c) |
been subject to any order, judgment or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities. |
| |
(d) |
been found by a court of competent jurisdiction (in a civil action), the SEC, or the Commodities Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended or vacated. |
Director Qualifications
Directors are responsible for overseeing the Company’s
business consistent with their fiduciary duty to the shareholders. This significant responsibility requires highly-skilled individuals
with various qualities, attributes and professional experience. Our sole director believes that there are general requirements for service
on the Board that are applicable to all directors and that there are other skills and experience that should be represented on the Board
as a whole but not necessarily by each director. The existing board member considers the qualifications of director and director candidates
individually and in the broader context of the Board’s overall composition and the Company’s current and future needs.
Qualifications for All Directors
In its assessment of each potential candidate,
including those recommended by the shareholders, the Board will consider the nominee’s judgment, integrity, experience, independence,
understanding of the Company’s business or other related industries and such other factors it determines are pertinent in light
of the current needs of the Board.
The Board also takes into account the ability
of a director to devote the time and effort necessary to fulfill his or her responsibilities to the Company.
The Board requires that each director be a recognized
person of high integrity with a proven record of success in his or her field. Each director must demonstrate innovative thinking, familiarity
with and respect for corporate governance requirements and practices, an appreciation of multiple cultures and a commitment to sustainability
and to dealing responsibly with social issues. In addition to the qualifications required of all directors, the Board conducts interviews
of potential director candidates to assess intangible qualities including the individual’s ability to ask difficult questions and,
simultaneously, to work collegially. The Board does not have a specific diversity policy, but considers diversity of race, ethnicity,
gender, age, cultural background and professional experiences in evaluating candidates for Board membership. Diversity is important because
a variety of points of view contribute to a more effective decision-making process.
Qualifications, Attributes, Skills and Experience
to be Represented on the Board as a Whole
The Board has identified particular qualifications,
attributes, skills and experiences that should be represented on the Board as a whole, in light of the Company’s current needs and
its business priorities. The Board believes that it should include some directors with a high level of financial literacy and some directors
who possess relevant business experiences as a chief executive officer, president or similar position at a company. Marketing is the core
focus of our business and the Company seeks to develop and deploy innovative and effective marketing and technology. Therefore, the Board
believes that marketing and technology experience should be represented on the Board.
Presently, Mr. Xin Sun is the sole director of
the Company. Mr. Xin Sun possesses many of the skills and experiences needed for our business. He has experience in the pharmaceutical
industry, having previously been the District Director for the Northeast District of China for Pfizer Pharmaceuticals Limited from 1991
to 1994. He has also been the Director of Marketing for Ha Yao Group Sanchine Medicine Joint-Stock Company Ltd., where he acquired strong
marketing experience. From 1996 to 2002, Mr. Xin Sun was the chief executive officer of a company he founded, Heilongjiang Bijie Chemical
Industry Co., Ltd., and from 2003 to the present, he has been the president and chief executive officer of Humankind.
The Board plans to eventually increase its membership
to include directors with skills and experiences complementary to Mr. Xin Sun’s background.
Board Leadership Structure and Role in Risk
Oversight
Mr. Xin Sun is the Company’s Chairman and
Chief Executive Officer. The Board’s role in the risk oversight of the Company includes, among other things:
| |
- |
appointing, retaining and overseeing the work of the independent auditors, including resolving disagreements between the management and the independent auditors relating to financial reporting; |
| |
- |
approving all auditing and non-auditing services permitted to be performed by the independent auditors; |
| |
- |
reviewing annually the independence and quality control procedures of the independent auditors; |
| |
- |
reviewing and approving all proposed related party transactions; |
| |
- |
discussing the annual audited financial statements with the management; and |
| |
- |
meeting separately with the independent auditors to discuss critical accounting policies, management letters, recommendations on internal controls, the auditor’s engagement letter and independence letter and other material written communications between the independent auditors and the management. |
Compliance with Section 16(a) of Exchange
Act
Section 16(a) of the Exchange Act requires our
executive officers and directors and persons who own more than ten percent of a registered class of our equity securities to file with
the SEC initial statements of beneficial ownership, reports of changes in ownership and annual reports concerning their ownership of our
common stock and other equity securities, on Form 3, 4 and 5 respectively. Executive officers, directors and greater than ten percent
shareholders are required by the SEC regulations to furnish the Company with copies of all Section 16(a) reports they file.
Based solely on our review of the copies of such
reports, we believe that, with respect to the fiscal year ended June 30, 2023, there is no triggering event for the filing of any report
under Section 16(a) by our sole officer and director, or by any of the persons known to us to own more than ten percent of our common
stock.
Meetings of Our Board of Directors
The Board held no meetings; however, the Board
had resolved matters in written consent one time during the fiscal year ended June 30, 2023.
Board Committees
Audit Committee. We intend to establish
an audit committee of the Board which will consist of soon-to-be-nominated independent directors. The audit committee’s duties will
be to recommend to the Board the engagement of independent auditors to audit our financial statements and to review our accounting and
auditing principles. The audit committee will review the scope, timing and fees for the annual audit and the results of audit examinations
performed by the internal auditors and independent public accountants, including their recommendations to improve the system of accounting
and internal controls. The audit committee will at all times be composed exclusively of directors who are, in the opinion of the Board,
free from any relationship which would interfere with the exercise of independent judgment as a committee member and who possess an understanding
of financial statements and generally accepted accounting principles.
Audit Committee Financial
Expert. The Board currently acts as our audit committee. Since we are still a developing company, the Board is still in the process
of finding an “audit committee financial expert” as defined in Regulation S-K and directors that are “independent”
as that term is used in Section 10A of the Exchange Act.
Compensation Committee. We intend to establish
a compensation committee of the Board. The compensation committee will review and approve our salary and benefits policies, including
compensation of executive officers.
Code of Ethics
We currently do not have a Code of Ethics because
we presently only have one director and one officer. We plan to adopt a Code of Ethics when the size of the Board and management increases.
Director Compensation
We did not compensate our director for the fiscal
year ended June 30, 2023. Going forward, however, we intend to implement a market-based director compensation program.
Limitations on Liability
Under Delaware law, a corporation may indemnify
its officers, directors, employees and agents under certain circumstances, including indemnification of such persons against liability
under the Securities Act. Those circumstances include that an officer, director, employee or agent may be indemnified if the person acted
in good faith and in a manner that he or she reasonably believed to be in, or not opposed to, the best interests of the corporation and,
with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was unlawful.
Article Seven of our Articles of Incorporation
provides that no director shall be personally liable to the Company or the shareholders for monetary damages for any breach of fiduciary
duty by such person in his or her capacity as a director. Notwithstanding the foregoing sentence, a director shall be liable to the extent
provided by applicable law, (i) for breach of his or her duty of loyalty to the Company or the shareholders, (ii) for acts or omissions
not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the Delaware
General Corporation law, or (iv) for any transaction from which he or she derived an improper personal benefit. No amendment to or repeal
of this provision shall apply to or have any effect on the liability or alleged liability of any director of the Company for or with respect
to any acts or omissions of such director occurring prior to such amendment.
We are also permitted to apply for insurance on
behalf of any director, officer, employee or other agent for liability arising out of his or her actions, whether or not the Delaware
General Corporation Law would permit indemnification.
Indemnification against Public Policy
Insofar as indemnification for liabilities arising
under the Securities Act may be permitted to directors, officers or persons controlling an issuer pursuant to the foregoing provisions,
the opinion of the SEC is that such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
In the event that a claim for indemnification against such liabilities (other than by a director, officer or controlling person in the
successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the
securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit
to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities
Act and will be governed by the final adjudication of such issue.
The effect of indemnification may be to limit
the rights of the Company and the shareholders (through shareholders’ derivative suits on behalf of the Company) to recover monetary
damages and expenses against a director for breach of fiduciary duty.
Directors and Officers of Humankind
The following table sets forth certain information
as of June 30, 2023 concerning the directors and executive officers of Humankind:
| Directors and Executive Officers |
|
Position/Title |
|
Age |
| Xin Sun |
|
Chairman, Chief Financial Officer, Treasurer |
|
57 |
| Baosen Ma |
|
President, Secretary, Director |
|
55 |
| Kai Sun |
|
Director |
|
52 |
The following is a summary of the biographical
information of those directors and officers of Humankind whose biographical information does not appear above:
Xin Sun, Chairman, Chief Financial Officer,
Treasurer
Mr. Sun’s biography is discussed above under
Item 10. Directors, Executive Officers and Corporate Governance and incorporated herein by reference.
Baosen Ma, President, Secretary and Director
Mr. Baosen Ma graduated from China University
of Political Science and Law with a major in Financial Accounting. From 1987 to 1992, he was an accountant with Harbin Keluola Solar Power
Co., Ltd. Thereafter, from 1992 to 1996, he was Vice General Manager and Sales Manager for Shanghai Dahua Solar Battery Co., Ltd. From
1996 to 2004, he was the East China Manager for the Ha Yao Group Sanchine Medicine Joint-Stock Ltd. In January 2004, Mr. Ma was appointed
President, Secretary and Director of Humankind.
Kai Sun, Director
Mr. Kai Sun graduated from Mu Dan Jiang University
with a major in Economics. From 1993 to 1995, he was Vice Director of Mu Dan Jiang Engine Factory. Thereafter, from 1995 to 1998, he was
a Director at Mu Dan Jiang Engine Factory. From 1998 to 2004, he was an Administration Director for Mu Dan Jiang Lysine Co., Ltd. Since
2004, he has been the Administration Director at Humankind. Mr. Kai Sun is the younger brother of Mr. Xin Sun, the Company’s Chairman,
Chief Financial Officer, Treasurer and sole director. In January 2004, Mr. Kai Sun was appointed a director of Humankind.
Item 11. Executive Compensation.
The following Summary Compensation Table sets
forth, for the years indicated, all cash compensation paid, distributed or accrued for services, including salary and bonus amounts, rendered
in all capacities by our Chief Executive Officer and all other executive officers who received or are entitled to receive remuneration
in excess of $100,000 during the stated periods. Mr. Xin Sun, our Chief Executive Officer and sole director, receives no additional compensation
for the services he provides in his capacity as director.
SUMMARY COMPENSATION TABLE
(all figures in US Dollars)
| | |
| | |
| | |
| | |
| | |
| | |
Non-Equity | | |
Non-qualified | | |
| | |
| |
| | |
| | |
| | |
| | |
| | |
| | |
Incentive | | |
Deferred | | |
All | | |
| |
| | |
| | |
| | |
| | |
Stock | | |
Option | | |
Plan | | |
Compensation | | |
Other | | |
| |
| | |
| | |
Salary | | |
Bonus | | |
Awards | | |
Awards | | |
Compensation | | |
Earnings | | |
Compensation | | |
Total | |
| Name and Principal Position | |
Year | | |
($) | | |
($) | | |
($) | | |
($) | | |
($) | | |
($) | | |
($) | | |
($) | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Xin Sun, Chief Executive Officer, | |
2023 | | |
| 12,523 | | |
| - | | |
| - | | |
| N/A | | |
| N/A | | |
| N/A | | |
| N/A | | |
| 12,523 | |
| Chief Financial Officer | |
2022 | | |
| 9,016 | | |
| - | | |
| - | | |
| N/A | | |
| N/A | | |
| N/A | | |
| N/A | | |
| 9,016 | |
Employment Agreements
We have no employment agreement with our sole
principal executive officer, Mr. Xin Sun.
Equity Compensation Plan Information
The following table sets forth information regarding
grants of awards and outstanding equity awards to Named Executive Officer during the year ended June 30, 2023:
Grants of Plan-Based Awards
| | |
| | | |
| Estimated
future payouts
under non-equity incentive
plan awards
| | |
| Estimated
future payouts
under equity incentive plan
awards
| | |
| All other
stock
awards:
Number of
shares of
stock or | | |
| All other option
awards:
Number of
securities
underlying | | |
| Exercise
or base price of
option | | |
| Grant date fair
value of
stock
and | |
| Name | |
| Grant date | | |
| Threshold($) | | |
| Target($) | | |
| Maximum($) | | |
| Threshold(#) | | |
| Target(#) | | |
| Maximum(#) | | |
| units (#) | | |
| options (#) | | |
| awards ($/Sh) | | |
| option awards | |
| (a) | |
| (b) | | |
| (c) | | |
| (d) | | |
| (e) | | |
| (f) | | |
| (g) | | |
| (h) | | |
| (i) | | |
| (j) | | |
| (k) | | |
| (l) | |
| Xin Sun, Chief Executive Officer, Chief Financial Officer | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
$ | - | |
Outstanding Equity Awards at Fiscal Year-End
| | |
| Option awards | | |
| Stock awards | |
| Name | |
| Number of securities underlying unexercised options(#) exercisable | | |
| Number of securities underlying unexercised options(#) unexercisable | | |
| Equity incentive plan awards: number of securities underlying unexercised unearned options(#) | | |
| Option exercise price($) | | |
| Option expiration date | | |
| Number of
shares or
units of
stock that have not vested(#) | | |
| Market value of shares or units of stock that have not vested(#) | | |
| Equity incentive plan awards: number of unearned shares, units or other rights that have not vested(#) | | |
| Equity incentive plan awards: market or payout value of unearned shares, units or other rights that have not vested($) | |
| (a) | |
| (b) | | |
| (c) | | |
| (d) | | |
| (e) | | |
| (f) | | |
| (g) | | |
| (h) | | |
| (i) | | |
| (j) | |
| Xin Sun, Chief Executive Officer, Chief Financial Officer | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
Option Exercises and Stock Vested
| | |
| Option awards | | |
| Stock awards | |
| Name | |
| Number of
shares acquired on
exercise (#) | | |
| Value
realized on
exercise ($) | | |
| Number of
shares acquired on
vesting (#) | | |
| Value realized on
vesting ($) | |
| (a) | |
| (b) | | |
| (c) | | |
| (d) | | |
| (e) | |
| Xin Sun, Chief Executive Officer, Chief Financial Officer | |
| - | | |
| - | | |
| - | | |
| - | |
Director’s and Officer’s Liability
Insurance
We currently do not have insurance insuring directors
and officers against liability; however, we are in the process of investigating the availability of such insurance.
Item 12. Security Ownership of Certain Beneficial
Owners and Management and Related Stockholder Matters.
The following table sets forth certain information
with respect to the beneficial ownership of our voting securities by (i) any person or group owning more than five percent of any class
of voting securities, (ii) our director, (iii) our chief executive officer and president and (iv) all executive officers and directors
as a group as of November 10, 2023.
The address of the beneficial owner listed below
is Harbin Humankind Biology Technology Co. Limited, 3199-1 Longxiang Road, Songbei District, Harbin, Heilongjiang Province, PRC.
| | |
| |
Amount and
Nature of | | |
| |
| Title of Class | |
Name | |
Beneficial
Owner | | |
Percent of
Class (1) | |
| Common Stock | |
Xin Sun, Chairman, Chief Executive Officer,
Chief Financial Officer and Treasurer | |
| 20,507,188 | | |
| 31.3 | % |
| | |
| |
| | | |
| | |
| Common Stock | |
All officers and directors as a group (1 person) | |
| 20,507,188 | | |
| 31.3 | % |
| (1) |
Based on 65,539,737 total issued and outstanding shares of the Company as of November 10, 2023. Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. Shares of common stock subject to options or warrants currently exercisable or convertible, or exercisable or convertible within 60 days of November 10, 2023 are deemed outstanding for computing the percentage of the person holding such option or warrant but are not deemed outstanding for computing the percentage of any other person. |
Mr. Xin Sun has the sole power to vote and dispose
all shares of common stock listed opposite his name. Mr. Sun did not and does not own any options or convertible securities.
Item 13. Certain Relationships and Related
Transactions, and Director Independence.
The Company received temporary short-term loans
from its majority owner and our sole director and principal executive officer, Mr. Xin Sun, a PRC citizen. These loans are unsecured and
non-interest bearing, and have no fixed terms of repayment; therefore, they are deemed payable on demand. Cash flows classified as due
to majority owner are classified as cash flows from financing activities.
The amounts due to related parties are used to
support the normal business operation of the Company, the related cash flows are classified as operating activities. The total amounts
due to Mr. Sun were $5,121,776 and $6,724,850 as of June 30, 2023 and June 30, 2022, respectively.
There were no interested imputed on the loans
for the fiscal years 2023 and 2022, respectively.
Item 14. Principal Accountant Fees and Services.
We were billed by Assentsure
PAC (“Assentsure”), our independent public accountants and CENTURION ZD
CPA & CO. (“Centurion”), our former independent public accountants, for the following professional services they performed
for us during the fiscal year ended June 30, 2023 and 2022, as set forth in the table below:
| | |
| |
| | |
Audit- | | |
| | |
| |
| | |
| |
Audit | | |
Related | | |
Tax | | |
Other | |
| | |
Auditors | |
Fees | | |
Fees | | |
Fees | | |
Fees | |
| 2023 | |
Assentsure | |
$ | 150,000 | | |
| - | | |
| - | | |
| - | |
| | |
Centurion | |
$ | 48,000 | | |
| | | |
| | | |
| | |
| | |
| |
| | | |
| | | |
| | | |
| | |
| 2022 | |
Centurion | |
$ | 140,000 | | |
| - | | |
| - | | |
| - | |
Audit Fees — This category includes the
audit of our annual financial statements and services that are normally provided by the independent auditors in connection with engagements
for those fiscal years.
Audit-Related Fees — This category consists
of assurance and related services by the independent auditors that are reasonably related to the performance of the audit or review of
our financial statements and are not reported above under “Audit Fees”.
Tax Fees — This category consists of professional
services rendered by the Company’s independent registered public accounting firm for tax compliance and tax advice. The services
for the fees disclosed under this category include tax return preparation and technical tax advice.
All Other Fees — This category consists of
fees for other miscellaneous items.
Pre-Approval Policies and Procedures
All of the services rendered to us by our independent
registered public accountants were pre-approved by the Board.
PART IV
Item 15. Exhibits and Financial Statement
Schedules
| Exhibit |
|
|
|
Filed |
|
|
| Index |
|
Description of Document |
|
Herewith |
|
Incorporated by Reference To: |
| 3.1 |
|
Articles of Incorporation. |
|
|
|
Exhibit 3.1 of the Company’s Registration Statement on Form 10-SB filed with the SEC on December 1, 2004. |
| |
|
|
|
|
|
|
| 3.2 |
|
By-laws. |
|
|
|
Exhibits 3.2 of the Company’s Registration Statement on Form 10-SB filed with the SEC on December 1, 2004. |
| |
|
|
|
|
|
|
| 3.3 |
|
Certificate of Amendment, dated May 11, 2005. |
|
|
|
Exhibits 3.3 of the Company’s Annual Report on Form 10-K/A filed with the SEC on November 16, 2010. |
| |
|
|
|
|
|
|
| 3.4 |
|
Certificate of Amendment, dated November 12, 2008. |
|
|
|
Exhibit 3.4 of the Company’s Annual Report on Form 10-K/A filed with the SEC on November 16, 2010. |
| |
|
|
|
|
|
|
| 3.5 |
|
Certificate of Amendment, dated February 11, 2009. |
|
|
|
Exhibit 3.5 of the Company’s Annual Report on Form 10-K/A filed with the SEC on November 16, 2010. |
| |
|
|
|
|
|
|
| 4.1 |
|
Specimen of Common Stock Certificate |
|
|
|
Exhibit 4.1 of the Company’s Annual Report on Form 10-K filed with the SEC on September 29, 2014. |
| |
|
|
|
|
|
|
| 4.2 |
|
Description of Securities Registered Pursuant to Section 12 of the Exchange Act. |
|
|
|
Exhibit 4.2 of the Company’s Annual Report on Form 10-K filed with the SEC on September 15, 2022. |
| |
|
|
|
|
|
|
| 10.1 |
|
English Translation of Land Use Agreement, dated June 7, 2004, between Harbin Humankind Biology Technology Co. Limited and Harbin City, Daochu District, Songbei Township, Jinxin Village. |
|
|
|
Exhibit 10.3 of the Company’s Annual Report on Form 10-K/A filed with the SEC on November 16, 2010. |
| |
|
|
|
|
|
|
| 10.2 |
|
English Translation of Cooperative Agreement, dated September 17, 2008, between Harbin Humankind Biology Technology Co. Limited and the Commercial Bureau of Qing’an County. |
|
|
|
Exhibit 10.5 of the Company’s Annual Report on Form 10-K/A filed with the SEC on November 16, 2010. |
| |
|
|
|
|
|
|
| 10.3 |
|
English Translation of Land Purchase Agreement, dated July 7, 2009, between Harbin Humankind Biology Technology Co. Limited and Harbin Songbei District Construction and Development Management Committee. |
|
|
|
Exhibit 10.6 of the Company’s Annual Report on Form 10-K/A filed with the SEC on November 16, 2010. |
| |
|
|
|
|
|
|
| 10.4 |
|
English Translation of Technology Transfer Agreement, dated October 12, 2007, between Harbin Humankind Biology Technology Co. Limited and Beijing Jindelikang Bio- Technology Co., Ltd. |
|
|
|
Exhibit 10.7 of the Company’s Annual Report on Form 10-K/A filed with the SEC on November 16, 2010. |
| Exhibit |
|
|
|
Filed |
|
|
| Index |
|
Description of Document |
|
Herewith |
|
Incorporated by Reference To: |
| 10.5 |
|
English Translation of Health Food Technology Transfer Agreement, dated January 18, 2013 by and between Harbin Humankind Biology Technology Co., Limited and Guangzhou Aoda Biology Beauty Healthy Technology Co., Ltd. |
|
|
|
Exhibit 10.22 of the Company’s Annual Report on Form 10-K filed with the SEC on October 15, 2013. |
| |
|
|
|
|
|
|
| 10.6 |
|
English Translation of Stock Transfer Agreement, dated April 10, 2013, by and between Stockholder of Heilongjiang Huimeijia Pharmaceuticals Co., Ltd, Liyuan Sun and Harbin Humankind Biology Technology Co., Limited. and its addendum dated June 18, 2013. |
|
|
|
Exhibit 10.23 of the Company’s Annual Report on Form 10-K filed with the SEC on October 15, 2013. |
| |
|
|
|
|
|
|
| 10.7 |
|
English Translation of Stock Transfer Agreement, dated April 10, 2013, by and between Stockholder of Heilongjiang Huimeijia Pharmaceuticals Co., Ltd, Wenbin Zhang and Harbin Humankind Biology Technology Co., Limited. and its addendum dated June 18, 2013. |
|
|
|
Exhibit 10.24 of the Company’s Annual Report on Form 10-K filed with the SEC on October 15, 2013. |
| |
|
|
|
|
|
|
| 10.8 |
|
English Translation of Stock Transfer Agreement dated December 24, 2014, by and among Harbin Humankind Biology Technology Co., Limited., Xin Sun, Harbin Huimeijia Medicine Company, and Xiuzheng Pharmaceutical Group Co., Ltd. |
|
|
|
Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the SEC on December 31, 2014. |
| |
|
|
|
|
|
|
| 10.9 |
|
English Translation of the Supplementary Agreement dated February 9, 2015, by and among Harbin Humankind Biology Technology Co., Limited., Xin Sun, Harbin Huimeijia Medicine Company, and Xiuzheng Pharmaceutical Group Co., Ltd. |
|
|
|
Exhibit 10.2 of the Company’s Quarterly Report on Form 10-Q filed with the SEC on February 13, 2015. |
| |
|
|
|
|
|
|
| 10.10 |
|
China Health Industries Holdings, Inc. 2015 Equity Incentive Plan, effective as of March 27, 2015 |
|
|
|
Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the SEC on April 2, 2015. |
| |
|
|
|
|
|
|
| 10.11 |
|
Form of the Restricted Stock Award Agreement |
|
|
|
Exhibit 10.2 of the Company’s Current Report on Form 8-K filed with the SEC on April 2, 2015. |
| 101.INS |
|
Inline XBRL Instance Document |
| |
|
|
| 101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document. |
|
|
|
|
| |
|
|
| 101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
|
|
|
|
| |
|
|
| 101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document. |
|
|
|
|
| |
|
|
| 101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document. |
|
|
|
|
| |
|
|
| 101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
|
|
|
|
| |
|
|
| 104 |
|
Cover Page Interactive Data File (formatted as Inline XBRL
and contained in Exhibit 101). |
|
|
|
|
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d)
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| |
CHINA HEALTH INDUSTRIES HOLDINGS, INC. |
| |
|
| |
By: |
/s/ Xin Sun |
| Date: |
November 13, 2023 |
Name: |
Xin Sun |
| |
Title: |
Chief Executive Officer
(principal
executive officer) |
Pursuant to the requirements of the Securities
Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and
on the dates indicated.
| By: |
/s/ Xin Sun |
|
| Name: |
Xin Sun |
|
| Title: |
Chief Executive Officer |
|
| |
(principal executive officer), |
|
| |
Chief Financial Officer |
|
| |
(principal financial officer and |
|
| |
principal accounting officer) and sole director |
|
Date: November 13, 2023
43
10-K
88100688
86-451
0.0054
0.0062
65539737
65539737
408772
408772
408772
0.0054
0.0062
65539737
65539737
false
FY
0001309057
0001309057
2022-07-01
2023-06-30
0001309057
2022-12-31
0001309057
2023-11-10
0001309057
2023-06-30
0001309057
2022-06-30
0001309057
us-gaap:RelatedPartyMember
2023-06-30
0001309057
us-gaap:RelatedPartyMember
2022-06-30
0001309057
2021-07-01
2022-06-30
0001309057
us-gaap:CommonStockMember
2021-06-30
0001309057
us-gaap:AdditionalPaidInCapitalMember
2021-06-30
0001309057
us-gaap:RetainedEarningsMember
2021-06-30
0001309057
chhe:StatutoryReservesMember
2021-06-30
0001309057
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-06-30
0001309057
us-gaap:ParentMember
2021-06-30
0001309057
us-gaap:NoncontrollingInterestMember
2021-06-30
0001309057
2021-06-30
0001309057
us-gaap:CommonStockMember
2021-07-01
2022-06-30
0001309057
us-gaap:AdditionalPaidInCapitalMember
2021-07-01
2022-06-30
0001309057
us-gaap:RetainedEarningsMember
2021-07-01
2022-06-30
0001309057
chhe:StatutoryReservesMember
2021-07-01
2022-06-30
0001309057
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-07-01
2022-06-30
0001309057
us-gaap:ParentMember
2021-07-01
2022-06-30
0001309057
us-gaap:NoncontrollingInterestMember
2021-07-01
2022-06-30
0001309057
us-gaap:CommonStockMember
2022-06-30
0001309057
us-gaap:AdditionalPaidInCapitalMember
2022-06-30
0001309057
us-gaap:RetainedEarningsMember
2022-06-30
0001309057
chhe:StatutoryReservesMember
2022-06-30
0001309057
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-06-30
0001309057
us-gaap:ParentMember
2022-06-30
0001309057
us-gaap:NoncontrollingInterestMember
2022-06-30
0001309057
us-gaap:CommonStockMember
2022-07-01
2023-06-30
0001309057
us-gaap:AdditionalPaidInCapitalMember
2022-07-01
2023-06-30
0001309057
us-gaap:RetainedEarningsMember
2022-07-01
2023-06-30
0001309057
chhe:StatutoryReservesMember
2022-07-01
2023-06-30
0001309057
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-07-01
2023-06-30
0001309057
us-gaap:ParentMember
2022-07-01
2023-06-30
0001309057
us-gaap:NoncontrollingInterestMember
2022-07-01
2023-06-30
0001309057
us-gaap:CommonStockMember
2023-06-30
0001309057
us-gaap:AdditionalPaidInCapitalMember
2023-06-30
0001309057
us-gaap:RetainedEarningsMember
2023-06-30
0001309057
chhe:StatutoryReservesMember
2023-06-30
0001309057
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-06-30
0001309057
us-gaap:ParentMember
2023-06-30
0001309057
us-gaap:NoncontrollingInterestMember
2023-06-30
0001309057
chhe:SharePurchaseAgreementMember
chhe:HarbinHumankindBiologyTechnologyCoLimitedMember
2007-08-20
0001309057
chhe:HarbinHumankindBiologyTechnologyCoLimitedMember
2007-08-02
2007-08-20
0001309057
chhe:BusinessCombinationMember
chhe:HarbinHuimeijiaMedicineCompanyMember
2008-10-14
0001309057
chhe:MrXinSunMember
2008-10-14
0001309057
chhe:ChinaHealthIndustriesHoldingsLimitedMember
2022-07-01
2023-06-30
0001309057
chhe:MrXinSunMember
chhe:ChinaHealthIndustriesHoldingsLimitedMember
2009-04-07
0001309057
chhe:HeilongjiangHuimeijiaPharmaceuticalCoLtdMember
2013-11-01
2013-11-22
0001309057
chhe:BusinessCombinationMember
chhe:HarbinHumankindBiologyTechnologyCoLimitedMember
2014-12-24
0001309057
chhe:MrXinSunMember
chhe:BusinessCombinationMember
2014-12-24
0001309057
chhe:SupplementaryAgreementMember
2015-02-02
2015-02-09
0001309057
chhe:NewSupplementaryAgreementMember
chhe:ChinaHealthIndustriesHoldingsLimitedMember
2016-10-12
0001309057
chhe:NewSupplementaryAgreementMember
2016-10-01
2016-10-12
0001309057
chhe:NewSupplementaryAgreementMember
2022-07-01
2023-06-30
0001309057
chhe:SupplementaryAgreementMember
2022-07-01
2023-06-30
0001309057
chhe:ChinaHealthIndustriesHoldingsLimitedMember
srt:MaximumMember
2023-06-27
0001309057
chhe:ChinaHealthIndustriesHoldingsLimitedMember
srt:MinimumMember
2023-06-27
0001309057
2023-06-27
2023-06-27
0001309057
chhe:ChinaHealthIndustriesHoldingsLimitedMember
2023-06-27
2023-06-27
0001309057
chhe:ExchangeRateMember
2023-06-30
0001309057
chhe:ExchangeRateMember
2022-06-30
0001309057
chhe:ExchangeRateOneMember
2023-06-30
0001309057
chhe:ExchangeRateOneMember
2022-06-30
0001309057
chhe:ExchangeRateTwoMember
2023-06-30
0001309057
chhe:ExchangeRateTwoMember
2022-06-30
0001309057
srt:MinimumMember
2022-07-01
2023-06-30
0001309057
srt:MaximumMember
2022-07-01
2023-06-30
0001309057
srt:MinimumMember
us-gaap:BuildingAndBuildingImprovementsMember
2023-06-30
0001309057
srt:MaximumMember
us-gaap:BuildingAndBuildingImprovementsMember
2023-06-30
0001309057
srt:MinimumMember
us-gaap:OfficeEquipmentMember
2023-06-30
0001309057
srt:MaximumMember
us-gaap:OfficeEquipmentMember
2023-06-30
0001309057
srt:MinimumMember
us-gaap:VehiclesMember
2023-06-30
0001309057
srt:MaximumMember
us-gaap:VehiclesMember
2023-06-30
0001309057
srt:MinimumMember
us-gaap:MachineryAndEquipmentMember
2023-06-30
0001309057
srt:MaximumMember
us-gaap:MachineryAndEquipmentMember
2023-06-30
0001309057
chhe:EquityInterestHuimeijiaMember
2014-12-24
0001309057
2014-12-24
2014-12-24
0001309057
2015-02-09
2015-02-09
0001309057
chhe:SupplementaryAgreementMember
2015-02-09
2015-02-09
0001309057
chhe:NewSupplementaryAgreementMember
chhe:EquityInterestHuimeijiaMember
2016-10-12
0001309057
chhe:NewSupplementaryAgreementMember
2016-10-12
2016-10-12
0001309057
us-gaap:AccountsReceivableMember
2023-06-30
0001309057
us-gaap:AccountsReceivableMember
2022-06-30
0001309057
2012-04-06
0001309057
chhe:PlantMember
2023-06-30
0001309057
chhe:PlantMember
2022-06-30
0001309057
us-gaap:BuildingAndBuildingImprovementsMember
2023-06-30
0001309057
us-gaap:BuildingAndBuildingImprovementsMember
2022-06-30
0001309057
us-gaap:MachineryAndEquipmentMember
2023-06-30
0001309057
us-gaap:MachineryAndEquipmentMember
2022-06-30
0001309057
us-gaap:OfficeEquipmentMember
2023-06-30
0001309057
us-gaap:OfficeEquipmentMember
2022-06-30
0001309057
us-gaap:VehiclesMember
2023-06-30
0001309057
us-gaap:VehiclesMember
2022-06-30
0001309057
us-gaap:OtherCapitalizedPropertyPlantAndEquipmentMember
2023-06-30
0001309057
us-gaap:OtherCapitalizedPropertyPlantAndEquipmentMember
2022-06-30
0001309057
chhe:CommercialPurposeMember
2023-06-30
0001309057
chhe:ResidentialPurposeMember
2023-06-30
0001309057
chhe:LandUseRightMember
chhe:HumankindMember
2023-06-30
0001309057
chhe:LandUseRightMember
chhe:HumankindMember
2022-06-30
0001309057
chhe:HealthSupplementProductPatentsMember
chhe:HumankindMember
2023-06-30
0001309057
chhe:HealthSupplementProductPatentsMember
chhe:HumankindMember
2022-06-30
0001309057
chhe:PharmaceuticalPatentsMember
chhe:HLJHuimeijiaMember
2023-06-30
0001309057
chhe:PharmaceuticalPatentsMember
chhe:HLJHuimeijiaMember
2022-06-30
0001309057
chhe:LandUseRightMember
chhe:HLJHuimeijiaMember
2023-06-30
0001309057
chhe:LandUseRightMember
chhe:HLJHuimeijiaMember
2022-06-30
0001309057
chhe:MrXinSunMember
2023-06-30
0001309057
chhe:MrXinSunMember
2022-06-30
0001309057
chhe:MrKaiSunMember
2023-06-30
0001309057
chhe:MrKaiSunMember
2022-06-30
0001309057
country:US
2023-06-30
0001309057
country:US
2022-06-30
0001309057
country:US
2022-07-01
2023-06-30
0001309057
country:HK
2023-06-30
0001309057
country:HK
2022-06-30
0001309057
chhe:PeoplesRepublicOfChinaMember
2022-07-01
2023-06-30
0001309057
chhe:PeoplesRepublicOfChinaMember
2021-07-01
2022-06-30
0001309057
us-gaap:SalesMember
chhe:SupplierOneMember
chhe:SupplierMember
2022-07-01
2023-06-30
0001309057
us-gaap:OtherCustomerMember
us-gaap:SalesMember
us-gaap:CustomerConcentrationRiskMember
2022-07-01
2023-06-30
0001309057
chhe:CustomerOneMember
us-gaap:SalesMember
us-gaap:CustomerConcentrationRiskMember
2022-07-01
2023-06-30
0001309057
chhe:CustomerTwoMember
us-gaap:SalesMember
us-gaap:CustomerConcentrationRiskMember
2022-07-01
2023-06-30
0001309057
chhe:CustomerThreeMember
us-gaap:SalesMember
us-gaap:CustomerConcentrationRiskMember
2022-07-01
2023-06-30
0001309057
chhe:HLJHuimeijiaMember
2022-07-01
2023-06-30
0001309057
chhe:HumankindMember
2022-07-01
2023-06-30
0001309057
chhe:OthersMember
2022-07-01
2023-06-30
0001309057
chhe:ConsolIdatedOperationsMember
2022-07-01
2023-06-30
0001309057
chhe:HLJHuimeijiaMember
2021-07-01
2022-06-30
0001309057
chhe:HumankindMember
2021-07-01
2022-06-30
0001309057
chhe:OthersMember
2021-07-01
2022-06-30
0001309057
chhe:ConsolIdatedOperationsMember
2021-07-01
2022-06-30
0001309057
chhe:HLJHuimeijiaMember
2023-06-30
0001309057
chhe:HumankindMember
2023-06-30
0001309057
chhe:OthersMember
2023-06-30
0001309057
chhe:ConsolIdatedOperationsMember
2023-06-30
0001309057
chhe:HLJHuimeijiaMember
2022-06-30
0001309057
chhe:HumankindMember
2022-06-30
0001309057
chhe:OthersMember
2022-06-30
0001309057
chhe:ConsolIdatedOperationsMember
2022-06-30
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
iso4217:CNY
As of June 30, 2023, China
Health Industries Holdings, Inc. (the “Company,” “we,” “us,” and “our” or “CHHE”)
had one class of securities registered under Section 12 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”),
which is our common stock, par value $0.0001 per share (the “common stock”).
We are incorporated in the
State of Delaware. The rights of our shareholders are generally covered by Delaware law and our certificate of incorporation and by-laws.
The terms of our common stock are therefore subject to Delaware law, including the Delaware General Corporation Law. The following is
a summary of the material terms of our common stock. This summary does not purport to be exhaustive and is qualified in its entirety by
reference to our certificate of incorporation, bylaws, to the applicable provisions of Delaware law.
As of September 15, 2023,
there were 65,539,737 shares of our common stock issued and outstanding, held by approximately 502 shareholders of record.
Our common stock is currently
traded on the OTC Markets QB Tier under the symbol “CHHE.”
Direct Transfer LLC, 1981
Murray Holladay Road, Suite 100, Salt Lake City, UT 84117, is the registrar and transfer agent of our common stock.
We are subject to Section
203 of the Delaware General Corporation Law, which prohibits a publicly-held Delaware corporation from engaging in a “business combination,”
except under certain circumstances, with an “interested shareholder” for a period of three years following the date such person
became an “interested shareholder” unless:
For purposes of Section
203, the term “interested shareholder” generally is defined as a person who, together with affiliates and associates, owns,
or, within the three years prior to the determination of interested shareholder status, owned, 15% or more of a corporation’s outstanding
voting stock. The term “business combination” includes mergers, asset or stock sales and other similar transactions resulting
in a financial benefit to an interested shareholder. Section 203 makes it more difficult for an “interested shareholder”
to effect various business combinations with a corporation for a three-year period. The existence of this provision would be expected
to have an anti-takeover effect with respect to transactions not approved in advance by our board of directors, including discouraging
attempts that might result in a premium over the market price for the shares of common stock held by shareholders. Presently, we have
not opted out of this provision.
We consent to the incorporation by reference in the Registration Statements
of China Health Industries Holdings, Inc. on Form S-8 (File No.333-214487) of our report dated November 13, 2023, with respect to our
audit of the consolidated financial statements of China Health Industries Holdings, Inc. for the year ended June 30, 2023, which report
is included in this Annual Report on Form 10-K for the year ended June 30, 2023
We also consent to the reference to us under the
heading “Experts” in such Registration Statement.
In connection with the Annual Report of China
Health Industries Holdings, Inc. (the “Company”) on Form 10-K for the year ended June 30, 2023, as filed with the Securities
and Exchange Commission on the date hereof (the “Report”), I, Xin Sun, Chief Executive Officer and Chief Financial Officer
of the Company, certify, pursuant to 18 U.S.C. section 1350 of the Sarbanes-Oxley Act of 2002, that:
A signed original of this written statement required
by Section 906 has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission
or its staff upon request.
The foregoing certification is being furnished
solely to accompany the Report pursuant to 18 U.S.C. Section 1350 and is not being filed for purposes of Section 18 of the Securities
Exchange Act of 1934, as amended, and is not to be incorporated by reference into any filing of the Company, whether made before or after
the date hereof, regardless of any general incorporation language in such filing.