false
0001433607
0001433607
2023-11-01
2023-11-01
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
DC 20549
FORM
8-K
CURRENT
REPORT PURSUANT
TO
SECTION 13 OR 15(d) OF THE
SECURITIES
EXCHANGE ACT OF 1934
Date
of report (Date of earliest event reported): November 1, 2023
InspireMD,
Inc.
(Exact
Name of Registrant as Specified in Its Charter)
Delaware
(State
or Other Jurisdiction of Incorporation)
| 001-35731 |
|
26-2123838 |
(Commission
File
Number) |
|
(IRS
Employer
Identification
No.) |
4
Menorat Hamaor St.
Tel
Aviv, Israel |
|
6744832 |
| (Address
of Principal Executive Offices) |
|
(Zip
Code) |
(888)
776-6804
(Registrant’s
Telephone Number, Including Area Code)
Check
the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under
any of the following provisions:
| ☐ |
Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
| ☐ |
Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities
registered pursuant to Section 12(b) of the Act:
| Title
of each class |
|
Trading
Symbol(s) |
|
Name
of each exchange on which registered |
| Common
Stock, par value $0.0001 per share |
|
NSPR |
|
The
Nasdaq Capital Market LLC |
Indicate
by check mark whether the registrant is an emerging growth company as defined in as defined in Rule 405 of the Securities Act of 1933
(§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company ☐
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
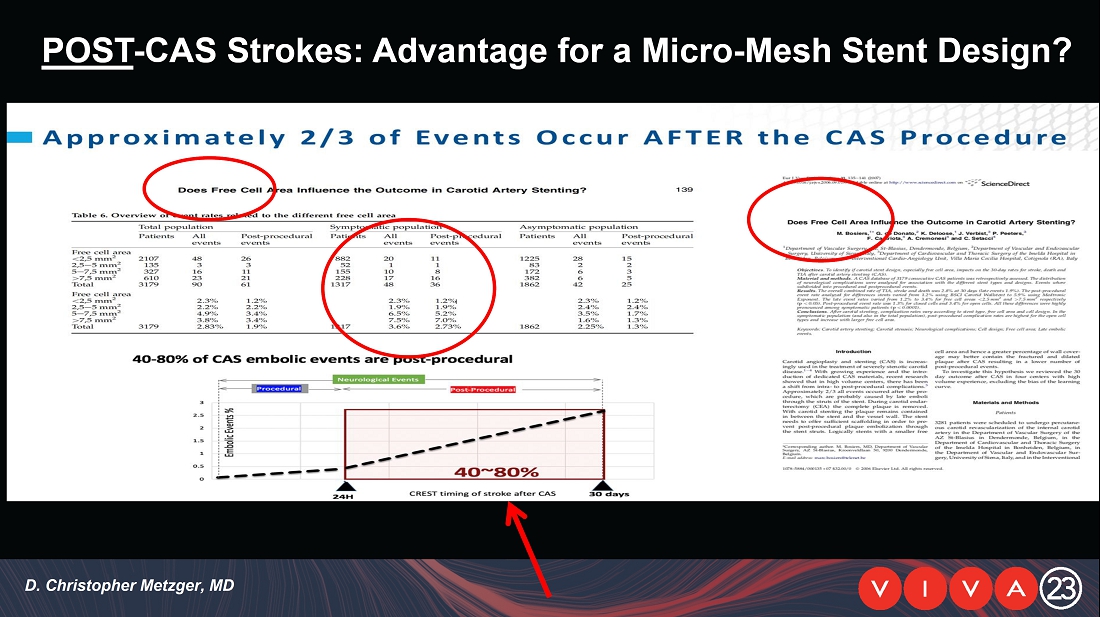
Item
7.01 Regulation FD Disclosure.
On
November 1, 2023, InspireMD, Inc. (the “Company”) issued a press release titled “InspireMD Presents Positive 30-Day
Follow-Up Results from the C-GUARDIANS U.S. Investigational Device Exemption (IDE) Clinical Trial at VIVA23”. A copy of the press
release is attached hereto as Exhibit 99.1 and is incorporated herein by reference. A copy of the
presentation that the Company presented at the Vascular InterVentional Advances meeting (“VIVA23”) is
attached hereto as Exhibit 99.2 and incorporated by reference in this Item 7.01.
In
accordance with General Instruction B.2 of Form 8-K, the information in this Current Report on Form 8-K that is furnished pursuant to
this Item 7.01, including Exhibit 99.1 and Exhibit 99.2, shall
not be deemed to be “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange
Act”), or otherwise subject to the liabilities of that section, and shall not be incorporated by reference into any registration
statement or other document filed under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set
forth by specific reference in such filing.
Item
8.01 Other Events.
On
November 1, 2023, the Company presented 30-day results from the C-GUARDIANS U.S. Investigational Device Exemption (IDE) clinical trial
at VIVA23.
From
July 2021 to June 2023, 316 patients were prospectively enrolled in a single-arm carotid artery stenting study performed at 24 sites
in the United States and the European Union. The primary endpoint in the clinical trial was a composite of: (1) incidence of major adverse
events including death (all-cause mortality), any stroke, or myocardial infarction (“DSMI”) through 30-days post index procedure,
or (2) ipsilateral stroke from day 31 to day 365 post-procedure. Stenting with the C-Guard carotid stent system in patients with carotid
artery stenosis and at high risk for carotid endarterectomy had a DSMI rate of 0.95%, measured from
the date of the procedure through 30 days follow-up post-procedure.
The
Company anticipates reporting primary endpoint results from C-GUARDIANS U.S. Investigational Device Exemption (IDE) clinical trial in
the second half of 2024 that may support a premarket approval application with the U.S. Food and
Drug Administration.
Item
9.01 Financial Statements and Exhibits.
(d)
Exhibits
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned hereunto duly authorized.
| |
INSPIREMD,
INC. |
| |
|
|
| Date:
November 1, 2023 |
By: |
/s/
Craig Shore |
| |
Name: |
Craig
Shore |
| |
Title: |
Chief
Financial Officer |
Exhibit
99.1

InspireMD
Presents Positive 30-Day Follow-Up Results from the C-GUARDIANS
U.S.
Investigational Device Exemption (IDE) Clinical Trial at VIVA23
Data
demonstrate a low major adverse event rate of 0.95% through 30 days post-procedure.
Tel
Aviv, Israel, and Miami, Florida — November 1, 2023 – InspireMD, Inc. (Nasdaq: NSPR), developer of the CGuard™
Embolic Prevention Stent System (EPS) for the prevention of stroke, today presented 30-day results from the C-GUARDIANS U.S. Investigational
Device Exemption (IDE) clinical trial at the Vascular InterVentional Advances (VIVA) meeting, which is being held October 30 through
November 2 in Las Vegas, NV. The presentation, which was accepted as a late-breaking abstract, was delivered by Dr. Chris Metzger, System
Vascular Chief at OhioHealth in Columbus, OH and principal investigator of the C-GUARDIANS trial.
C-GUARDIANS
is a pivotal trial designed to evaluate the safety and efficacy of the CGuard™ Carotid Stent System when used to treat symptomatic
and asymptomatic carotid artery stenosis in patients undergoing carotid artery stenting (CAS) and at a high risk for carotid endarterectomy
(CEA).
Presentation
Highlights:
| |
● |
From
July 2021 to June 2023, 316 patients were prospectively enrolled in this single-arm carotid artery stenting study performed at 24
sites in the US and the EU. |
| |
|
|
| |
● |
The
primary endpoint is a composite of: (1) incidence of major adverse events including death (all-cause mortality), any stroke, or myocardial
infarction (DSMI) through 30-days post index procedure, or (2) ipsilateral stroke from day 31 to day 365 post-procedure. |
| |
|
|
| |
● |
Stenting
with the C-Guard carotid stent system in patients with carotid artery stenosis and at high risk for carotid endarterectomy had a
DSMI rate of 0.95%, from procedure through 30 days follow-up. |
Marvin
Slosman, chief executive officer of InspireMD, stated, “The follow-up data from C-GUARDIANS once again supports the safety
of the C-Guard EPS stent, with its novel MicroNet™ technology, as reflected in the low rate of major adverse events observed through
30 days. We believe the neuroprotective qualities of C-Guard set it apart from competing stents on the market and should help accelerate
the ongoing shift in carotid revascularizations from ‘surgery first’ to an endovascular ‘stent first’ approach.
We look forward to reporting 12-month results as we continue to advance CGuard EPS toward potential FDA approval in the first half of
2025.”
InspireMD
anticipates reporting primary endpoint results from C-GUARDIANS in the second half of 2024 that may support a Premarket Approval (PMA)
application.
About
C-GUARDIANS
The
C-GUARDIANS clinical trial is evaluating the safety and efficacy of the CGuard™ Carotid Stent System for the treatment of carotid
artery stenosis. The study, which completed enrollment in June 2023, enrolled 316 patients across 24 trial sites in the U.S. and Europe.

The
trial includes both symptomatic and asymptomatic patients undergoing carotid artery stenting (CAS). The primary endpoint includes the
composite of the incidence of the following major adverse events: death (all-cause mortality), any stroke, or myocardial infarction (DSMI)
through 30-days post-index procedure or ipsilateral stroke from 31-365-day follow-up, based on Clinical Events Committee (CEC) adjudication.
The trial will be considered a success if the upper bound of the two-sided 95% confidence interval calculated from the observed primary
endpoint rate is less than the performance goal of 11.6%.
The
company anticipates primary endpoint results from the study in H2 2024.
About
VIVA
VIVA
(Vascular InterVentional Advances) is a global educational event for specialists caring for patients with vascular disease. VIVA brings
together attendees and faculty specializing in vascular surgery, interventional cardiology, interventional radiology, vascular medicine,
neurointervention/neurosurgery, and cardiothoracic surgery, offering a uniquely comprehensive educational experience with access to some
of the best minds in endovascular care.
About
InspireMD, Inc.
InspireMD
seeks to utilize its proprietary MicroNet® technology to make its products the industry standard for carotid stenting by providing
outstanding acute results and durable, stroke-free long-term outcomes. InspireMD’s common stock is quoted on the Nasdaq under the
ticker symbol NSPR.
We
routinely post information that may be important to investors on our website. For more information, please visit www.inspiremd.com.

Forward-looking
Statements
This
press release contains “forward-looking statements.” Forward-looking statements include, but are not limited to, statements
regarding InspireMD or its management team’s expectations, hopes, beliefs, intentions or strategies regarding the future. Such
statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,”
“anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,”
“hopes,” “potential”, “scheduled” or similar words. Examples of such statements include, but are
not limited to, statements relating to the C-GUARDIANS U.S. IDE clinical trial, including 30-day results from such trial, as well as
the timing and outcome of any subsequent results, PMA or potential launch. Forward-looking statements are not guarantees of future performance,
are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the company’s
control, and cannot be predicted or quantified and consequently, actual results may differ materially from those expressed or implied
by such forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated with
our history of recurring losses and negative cash flows from operating activities, significant future commitments and the uncertainty
regarding the adequacy of our liquidity to pursue our complete business objectives, and substantial doubt regarding our ability to continue
as a going concern; our need to raise additional capital to meet our business requirements in the future and such capital raising may
be costly or difficult to obtain and could dilute out stockholders’ ownership interests; market acceptance of our products; an
inability to secure and maintain regulatory approvals for the sale of our products; negative clinical trial results or lengthy product
delays in key markets; our ability to maintain compliance with the Nasdaq listing standards; our ability to generate revenues from our
products and obtain and maintain regulatory approvals for our products; our ability to adequately protect our intellectual property;
our dependence on a single manufacturing facility and our ability to comply with stringent manufacturing quality standards and to increase
production as necessary; the risk that the data collected from our current and planned clinical trials may not be sufficient to demonstrate
that our technology is an attractive alternative to other procedures and products; intense competition in our industry, with competitors
having substantially greater financial, technological, research and development, regulatory and clinical, manufacturing, marketing and
sales, distribution and personnel resources than we do; entry of new competitors and products and potential technological obsolescence
of our products; inability to carry out research, development and commercialization plans; loss of a key customer or supplier; technical
problems with our research and products and potential product liability claims; product malfunctions; price increases for supplies and
components; insufficient or inadequate reimbursement by governmental and other third-party payers for our products; our efforts to successfully
obtain and maintain intellectual property protection covering our products, which may not be successful; adverse federal, state and local
government regulation, in the United States, Europe or Israel and other foreign jurisdictions; the fact that we conduct business in multiple
foreign jurisdictions, exposing us to foreign currency exchange rate fluctuations, logistical and communications challenges, burdens
and costs of compliance with foreign laws and political and economic instability in each jurisdiction; the escalation of hostilities
in Israel, which could impair our ability to manufacture our products; and current or future unfavorable economic and market conditions
and adverse developments with respect to financial institutions and associated liquidity risk. More detailed information about the Company
and the risk factors that may affect the realization of forward-looking statements is set forth in the Company’s filings with the
Securities and Exchange Commission (SEC), including the Company’s Annual Report on Form 10-K and its Quarterly Reports on Form
10-Q. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at http://www.sec.gov.
The Company assumes no obligation to publicly update or revise its forward-looking statements as a result of new information, future
events or otherwise.
Investor
Contacts:
Craig
Shore
Chief
Financial Officer
InspireMD,
Inc.
888-776-6804
craigs@inspiremd.com
Chuck
Padala, Managing Director
LifeSci
Advisors
646-627-8390
chuck@lifesciadvisors.com
investor-relations@inspiremd.com
Exhibit 99.2




















v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
InspireMD (NASDAQ:NSPR)
Historical Stock Chart
From Mar 2024 to Apr 2024
InspireMD (NASDAQ:NSPR)
Historical Stock Chart
From Apr 2023 to Apr 2024