0001016504
INTEGRATED BIOPHARMA INC
false
--06-30
FY
2023
2,477,939
2,061
1,839
0
72
772
503
1,289
1,338
0.002
0.002
50,000,000
50,000,000
29,984,510
29,984,510
29,949,610
29,949,610
34,900
34,900
0
3
2016 2017 2018 2019
2
533
842
85
1
5
4
10
5
3
5
10
2.5
1
1
3
5
10
2
00010165042022-07-012023-06-30
iso4217:USD
00010165042022-12-31
xbrli:shares
00010165042023-09-15
thunderdome:item
00010165042021-07-012022-06-30
iso4217:USDxbrli:shares
00010165042023-06-30
00010165042022-06-30
0001016504inbp:VitaminRealtyLLCMember2023-06-30
0001016504inbp:VitaminRealtyLLCMember2022-06-30
0001016504us-gaap:CommonStockMember2021-06-30
0001016504us-gaap:AdditionalPaidInCapitalMember2021-06-30
0001016504us-gaap:RetainedEarningsMember2021-06-30
0001016504us-gaap:TreasuryStockCommonMember2021-06-30
00010165042021-06-30
0001016504us-gaap:CommonStockMember2021-07-012022-06-30
0001016504us-gaap:AdditionalPaidInCapitalMember2021-07-012022-06-30
0001016504us-gaap:RetainedEarningsMember2021-07-012022-06-30
0001016504us-gaap:TreasuryStockCommonMember2021-07-012022-06-30
0001016504us-gaap:CommonStockMember2022-06-30
0001016504us-gaap:AdditionalPaidInCapitalMember2022-06-30
0001016504us-gaap:RetainedEarningsMember2022-06-30
0001016504us-gaap:TreasuryStockCommonMember2022-06-30
0001016504us-gaap:CommonStockMember2022-07-012023-06-30
0001016504us-gaap:AdditionalPaidInCapitalMember2022-07-012023-06-30
0001016504us-gaap:RetainedEarningsMember2022-07-012023-06-30
0001016504us-gaap:TreasuryStockCommonMember2022-07-012023-06-30
0001016504us-gaap:CommonStockMember2023-06-30
0001016504us-gaap:AdditionalPaidInCapitalMember2023-06-30
0001016504us-gaap:RetainedEarningsMember2023-06-30
0001016504us-gaap:TreasuryStockCommonMember2023-06-30
0001016504inbp:BrandedProductsMember2022-07-012023-06-30
0001016504inbp:BrandedProductsMember2021-07-012022-06-30
0001016504us-gaap:ShippingAndHandlingMember2021-07-012023-06-30
0001016504us-gaap:ShippingAndHandlingMember2021-07-012022-06-30
utr:Y
0001016504us-gaap:StateAndLocalJurisdictionMemberus-gaap:NewJerseyDivisionOfTaxationMemberinbp:MDCMember2021-07-012022-06-30
0001016504us-gaap:StateAndLocalJurisdictionMemberus-gaap:NewJerseyDivisionOfTaxationMemberinbp:CIIMember2021-07-012022-06-30
0001016504us-gaap:BuildingAndBuildingImprovementsMember2022-06-30
0001016504us-gaap:MachineryAndEquipmentMember2022-06-30
0001016504us-gaap:AirTransportationEquipmentMember2022-06-30
00010165042020-07-012021-06-30
xbrli:pure
00010165042008-08-31
0001016504us-gaap:LandAndBuildingMember2023-06-30
0001016504us-gaap:LandAndBuildingMember2022-06-30
0001016504us-gaap:LeaseholdImprovementsMember2023-06-30
0001016504us-gaap:LeaseholdImprovementsMember2022-06-30
0001016504us-gaap:MachineryAndEquipmentMember2023-06-30
0001016504us-gaap:TransportationEquipmentMember2023-06-30
0001016504us-gaap:TransportationEquipmentMember2022-06-30
0001016504inbp:FullyDepreciatedPropertyMember2022-07-012023-06-30
0001016504inbp:FullyDepreciatedPropertyMember2021-07-012022-06-30
0001016504inbp:PropertyNotFullyDepreciatedMember2022-07-012023-06-30
0001016504inbp:PropertyNotFullyDepreciatedMember2021-07-012022-06-30
0001016504us-gaap:EquipmentMember2022-07-012023-06-30
0001016504us-gaap:EquipmentMember2021-07-012022-06-30
0001016504inbp:RevolvingAdvancesMember2023-06-30
0001016504inbp:RevolvingAdvancesMember2022-06-30
0001016504inbp:AmendedLoanAgreementMember2019-05-15
0001016504us-gaap:RevolvingCreditFacilityMemberinbp:AmendedLoanAgreementMember2019-05-15
0001016504inbp:AmendedLoanAgreementMemberinbp:TermLoanMember2019-05-15
0001016504us-gaap:RevolvingCreditFacilityMemberinbp:AmendedLoanAgreementMember2023-06-30
0001016504us-gaap:RevolvingCreditFacilityMemberinbp:AmendedLoanAgreementMember2022-06-30
0001016504us-gaap:RevolvingCreditFacilityMemberinbp:AmendedLoanAgreementMemberus-gaap:EurodollarMember2019-05-152019-05-15
0001016504inbp:AmendedLoanAgreementMemberinbp:TermLoanMember2022-06-30
0001016504inbp:AmendedLoanAgreementMemberinbp:TermLoanMemberus-gaap:EurodollarMember2019-05-152019-05-15
0001016504us-gaap:RevolvingCreditFacilityMemberinbp:AmendedLoanAgreementMember2023-05-11
0001016504inbp:AmendedLoanAgreementMemberus-gaap:SecuredOvernightFinancingRateSofrOvernightIndexSwapRateMember2023-01-012023-03-31
0001016504inbp:AmendedLoanAgreementMember2019-05-152019-05-15
0001016504inbp:AmendedLoanAgreementMemberinbp:TermLoanMember2019-05-152019-05-15
0001016504us-gaap:RevolvingCreditFacilityMemberinbp:AmendedLoanAgreementMember2019-05-152019-05-15
0001016504inbp:AmendedLoanAgreementMember2021-06-30
0001016504inbp:iBioStockMember2020-07-012021-06-30
0001016504us-gaap:SeniorNotesMember2022-07-012023-06-30
0001016504us-gaap:SeniorNotesMember2021-07-012022-06-30
0001016504inbp:DebtRelatedToInterestExpenseMember2023-06-30
0001016504inbp:DebtRelatedToInterestExpenseMember2022-06-30
0001016504us-gaap:DomesticCountryMember2023-06-30
0001016504us-gaap:StateAndLocalJurisdictionMember2023-06-30
0001016504us-gaap:CapitalLossCarryforwardMember2023-07-10
0001016504us-gaap:CapitalLossCarryforwardMemberinbp:TaxYear2025Member2021-06-30
0001016504us-gaap:CapitalLossCarryforwardMemberinbp:TaxYear2026Member2021-06-30
0001016504us-gaap:CapitalLossCarryforwardMemberinbp:TaxYear2028Member2021-06-30
0001016504inbp:DeferredTaxAssetsRelatedToNetOperatingLossMember2022-07-012023-06-30
0001016504us-gaap:StateAndLocalJurisdictionMemberus-gaap:NewJerseyDivisionOfTaxationMemberinbp:CIIMember2019-07-012020-06-30
0001016504us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMemberinbp:TwoCustomersMember2022-07-012023-06-30
0001016504us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMemberinbp:TwoCustomersMember2021-07-012022-06-30
0001016504us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMember2021-07-012022-06-30
0001016504us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMemberinbp:MajorCustomer1Memberinbp:ContractManufacturingMember2022-07-012023-06-30
0001016504us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMemberinbp:MajorCustomer2Memberinbp:ContractManufacturingMember2022-07-012023-06-30
0001016504us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMemberinbp:MajorCustomer1Memberinbp:ContractManufacturingMember2021-07-012022-06-30
0001016504us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMemberinbp:MajorCustomer2Memberinbp:ContractManufacturingMember2021-07-012022-06-30
0001016504us-gaap:AccountsReceivableMemberus-gaap:CustomerConcentrationRiskMemberinbp:TwoCustomersMember2022-07-012023-06-30
0001016504us-gaap:AccountsReceivableMemberus-gaap:CustomerConcentrationRiskMemberinbp:TwoCustomersMember2021-07-012022-06-30
0001016504us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMemberinbp:MajorCustomer1Memberinbp:OtherNutraceuticalBusinessMember2022-07-012023-06-30
0001016504us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMemberinbp:MajorCustomer2Memberinbp:OtherNutraceuticalBusinessMember2022-07-012023-06-30
0001016504us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMemberinbp:MajorCustomer3Memberinbp:OtherNutraceuticalBusinessMember2022-07-012023-06-30
0001016504us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMemberinbp:MajorCustomer1Memberinbp:OtherNutraceuticalBusinessMember2021-07-012022-06-30
0001016504us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMemberinbp:MajorCustomer2Memberinbp:OtherNutraceuticalBusinessMember2021-07-012022-06-30
0001016504us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMemberinbp:MajorCustomer3Memberinbp:OtherNutraceuticalBusinessMember2021-07-012022-06-30
0001016504us-gaap:SalesRevenueSegmentMemberus-gaap:CustomerConcentrationRiskMemberinbp:MajorCustomer1Memberinbp:BrandedNutraceuticalMember2022-07-012023-06-30
0001016504us-gaap:NumberOfEmployeesGeographicAreaMemberus-gaap:UnionizedEmployeesConcentrationRiskMember2022-07-012023-06-30
0001016504srt:MinimumMember2022-07-012023-06-30
0001016504srt:MaximumMember2022-07-012023-06-30
0001016504inbp:VitaminRealtyLLCMember2022-07-012023-06-30
0001016504inbp:UnrelatedPartyMember2022-07-012023-06-30
0001016504inbp:VitaminRealtyLLCMember2021-07-012022-06-30
0001016504inbp:UnrelatedPartyMember2021-07-012022-06-30
0001016504inbp:ChairmanChiefExecutiveOfficeAndMajorStockholderMember2021-07-012022-06-30
0001016504inbp:ManhattanDrugCompanyMember2012-01-04
0001016504inbp:ManhattanDrugCompanyMember2012-01-05
0001016504inbp:ManhattanDrugCompanyMember2012-01-052012-01-05
0001016504inbp:ManhattanDrugCompanyMember2022-07-15
0001016504inbp:ManhattanDrugCompanyMember2022-07-012022-07-01
0001016504inbp:AgrolabsMember2014-05-19
0001016504inbp:AgrolabsMember2014-05-192014-05-19
0001016504us-gaap:AccountsPayableMemberinbp:VitaminRealtyLLCMember2023-06-30
0001016504us-gaap:AccountsPayableMemberinbp:VitaminRealtyLLCMember2022-06-30
0001016504inbp:WarehouseLeaseMember2023-06-30
0001016504inbp:OfficeEquipmentLeasesMember2023-06-30
0001016504inbp:OfficeEquipmentLeasesMember2022-06-30
0001016504inbp:UnrelatedPartyMember2023-06-30
0001016504inbp:UnrelatedPartyMember2022-06-30
0001016504inbp:UnrelatedPartyMember2022-06-15
0001016504inbp:UnrelatedPartyMember2022-06-152022-06-15
0001016504inbp:OperatingLeaseRenewedForOfficeSpaceMember2023-03-31
0001016504inbp:ThirdAmendmentWithVitaminRealtyMember2023-03-31
utr:acre
0001016504inbp:FifthYearOfFinanceLeaseMember2023-03-31
0001016504us-gaap:TransportationEquipmentMemberus-gaap:SubsequentEventMember2023-08-04
0001016504us-gaap:AccountsPayableMember2023-06-30
0001016504inbp:StockOption2001PlanMember2022-06-30
0001016504inbp:StockOption2001PlanMember2021-07-012022-06-30
0001016504inbp:StockOption1997PlanMember2022-06-30
0001016504us-gaap:EmployeeStockOptionMembersrt:MaximumMember2021-07-012022-06-30
0001016504us-gaap:EmployeeStockOptionMembersrt:MinimumMember2021-07-012022-06-30
0001016504inbp:StockOption2022PlanMember2023-06-30
0001016504inbp:StockOption2022PlanMember2022-07-012023-06-30
0001016504us-gaap:EmployeeStockOptionMember2022-07-012023-06-30
0001016504us-gaap:EmployeeStockOptionMembersrt:MinimumMember2022-07-012023-06-30
0001016504us-gaap:EmployeeStockOptionMembersrt:MaximumMember2022-06-30
0001016504us-gaap:EmployeeStockOptionMemberus-gaap:SubsequentEventMembersrt:DirectorMember2023-07-31
0001016504us-gaap:EmployeeStockOptionMemberus-gaap:SubsequentEventMemberinbp:EachDirectorMember2023-07-31
0001016504us-gaap:EmployeeStockOptionMemberus-gaap:SubsequentEventMembersrt:DirectorMember2023-07-012023-07-31
0001016504us-gaap:EmployeeStockOptionMemberus-gaap:SubsequentEventMembersrt:DirectorMemberus-gaap:ShareBasedCompensationAwardTrancheOneMember2023-07-012023-07-31
0001016504srt:DirectorMember2022-07-012023-06-30
0001016504srt:MinimumMembersrt:DirectorMember2022-07-012023-06-30
0001016504srt:MaximumMembersrt:DirectorMember2022-07-012023-06-30
0001016504us-gaap:EmployeeStockOptionMembersrt:MinimumMember2020-11-012020-11-30
0001016504us-gaap:EmployeeStockOptionMembersrt:MaximumMember2020-11-012020-11-30
0001016504us-gaap:EmployeeStockOptionMember2021-07-012022-06-30
0001016504inbp:RangeOneMember2022-07-012023-06-30
0001016504inbp:RangeOneMember2023-06-30
0001016504inbp:RangeTwoMember2022-07-012023-06-30
0001016504inbp:RangeTwoMember2023-06-30
0001016504inbp:RangeThreeMember2022-07-012023-06-30
0001016504inbp:RangeThreeMember2023-06-30
0001016504inbp:RangeFourMember2022-07-012023-06-30
0001016504inbp:RangeFourMember2023-06-30
0001016504inbp:RangeFiveMember2022-07-012023-06-30
0001016504inbp:RangeFiveMember2023-06-30
0001016504inbp:RangeSixMember2022-07-012023-06-30
0001016504inbp:RangeSixMember2023-06-30
0001016504inbp:RangeSevenMember2022-07-012023-06-30
0001016504inbp:RangeSevenMember2023-06-30
0001016504inbp:RangeEightMember2022-07-012023-06-30
0001016504inbp:RangeEightMember2023-06-30
0001016504inbp:RangeNineMember2022-07-012023-06-30
0001016504inbp:RangeNineMember2023-06-30
0001016504inbp:EuropeAndCanadaMember2022-07-012023-06-30
0001016504inbp:EuropeAndCanadaMember2021-07-012022-06-30
0001016504inbp:ContractManufacturingMembercountry:US2022-07-012023-06-30
0001016504inbp:ContractManufacturingMemberus-gaap:NonUsMember2022-07-012023-06-30
0001016504inbp:ContractManufacturingMember2022-07-012023-06-30
0001016504inbp:ContractManufacturingMember2023-06-30
0001016504inbp:ContractManufacturingMembercountry:US2021-07-012022-06-30
0001016504inbp:ContractManufacturingMemberus-gaap:NonUsMember2021-07-012022-06-30
0001016504inbp:ContractManufacturingMember2021-07-012022-06-30
0001016504inbp:ContractManufacturingMember2022-06-30
0001016504inbp:OtherNutraceuticalBusinessMembercountry:US2022-07-012023-06-30
0001016504inbp:OtherNutraceuticalBusinessMemberus-gaap:NonUsMember2022-07-012023-06-30
0001016504inbp:OtherNutraceuticalBusinessMember2022-07-012023-06-30
0001016504inbp:OtherNutraceuticalBusinessMember2023-06-30
0001016504inbp:OtherNutraceuticalBusinessMembercountry:US2021-07-012022-06-30
0001016504inbp:OtherNutraceuticalBusinessMemberus-gaap:NonUsMember2021-07-012022-06-30
0001016504inbp:OtherNutraceuticalBusinessMember2021-07-012022-06-30
0001016504inbp:OtherNutraceuticalBusinessMember2022-06-30
0001016504country:US2022-07-012023-06-30
0001016504us-gaap:NonUsMember2022-07-012023-06-30
0001016504country:US2021-07-012022-06-30
0001016504us-gaap:NonUsMember2021-07-012022-06-30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
____________
FORM 10-K
__ __________
| ☒ Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 | |
| For the fiscal year ended June 30, 2023 | |
| ☐ Transition Report Pursuant to Section 13 Or 15(d) of the Securities Exchange Act Of 1934 | |
| For the transition period from to | |
Commission File Number 001-31668
INTEGRATED BIOPHARMA, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 22-2407475 |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
| 225 Long Ave., Hillside, New Jersey | 07205 |
| (Address of principal executive offices) | (Zip code) |
Registrant’s telephone number: (888) 319-6962
Securities registered under Section 12(b) of the Exchange Act:
| Title of Each Class | Trading Symbol | Name of Each Exchange on Which Registered |
| None | N/A | None |
Securities registered under Section 12(g) of the Exchange Act: Common Stock, $.002 par value per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, a non-accelerated filer, an accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “accelerated filer,” “large accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated Filer ☐ | Accelerated Filer ☐ | Non-accelerated Filer ☒ | Emerging Growth Company ☐ | Smaller reporting company ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant based on the last sale price of the registrant’s Common Stock on December 31, 2022 was $3,258,720.
The number of shares outstanding of each of the registrant’s classes of common equity, as of the latest practicable date:
| Class | Outstanding at September 15, 2023 |
| Common Stock, $.002 par value | 29,949,610 Shares |
DOCUMENTS INCORPORATED BY REFERENCE
The information required by part III will be incorporated by reference from certain portions of a definitive Proxy Statement which is expected to be filed by the Registrant within 120 days after the close of its fiscal year.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
FORM 10-K ANNUAL REPORT
INDEX
| Part I |
|
Page |
| |
|
|
| Item 1. |
Description of Business |
4 |
| Item 1A. |
Risk Factors |
9 |
| Item 1B. |
Unresolved Staff Comments |
14 |
| Item 2. |
Properties |
14 |
| Item 3. |
Legal Proceedings |
14 |
| Item 4. |
Mine Safety Disclosure |
14 |
| |
|
|
| Part II |
|
|
| |
|
|
| Item 5. |
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
15 |
| Item 6. |
[Reserved] |
16 |
| Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
16 |
| Item 7A. |
Quantitative and Qualitative Disclosures About Market Risk |
24 |
| Item 8. |
Financial Statements and Supplementary Data |
24 |
| Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
24 |
| Item 9A. |
Controls and Procedures |
24 |
| Item 9B. |
Other Information |
25 |
| Item 9C. |
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
25 |
| |
|
|
| Part III |
|
|
| |
|
|
| Item 10. |
Directors, Executive Officers and Corporate Governance |
25 |
| Item 11. |
Executive Compensation |
25 |
| Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
25 |
| Item 13. |
Certain Relationships and Related Transactions and Director Independence |
26 |
| Item 14. |
Principal Accountant Fees and Services |
26 |
| |
|
|
| Part IV |
|
|
| |
|
|
| Item 15. |
Exhibits and Financial Statement Schedules |
27 |
| Item 16. |
Form10-K Summary |
28 |
| |
|
|
| Signatures |
|
52 |
| |
|
|
| |
|
|
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
Certain statements in this Annual Report on Form 10-K may constitute forward-looking statements as defined in Section 27A of the Securities Act of 1933, as amended, (the “Securities Act”), Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), the Private Securities Litigation Reform Act of 1995 (the “PSLRA”) or in releases made by the Securities and Exchange Commission (“SEC”), all as may be amended from time to time. Such forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause the actual results, performance or achievements of Integrated BioPharma, Inc. and its subsidiaries (collectively, the “Company”) or industry results, to differ materially from any future results, performance or achievements expressed or implied by such forward-looking statements. Such factors include, among others, changes in general economic and business conditions; loss of market share through competition; introduction of competing products by other companies; the timing of regulatory approval and the introduction of new products by the Company; changes in industry capacity; pressure on prices from competition or from purchasers of the Company's products; regulatory changes in the pharmaceutical manufacturing industry and nutraceutical industry; regulatory obstacles to the introduction of new technologies or products that are important to the Company; availability of qualified personnel; the loss of any significant customers or suppliers; the impact of the war in Ukraine; the tightened labor markets and inflation; and other factors both referenced and not referenced in this Annual Report. Statements that are not historical fact are forward-looking statements. Forward looking-statements can be identified by, among other things, the use of forward-looking language, such as the words “plan”, “believe”, “expect”, “anticipate”, “intend”, “estimate”, “project”, “may”, “will”, “would”, “could”, “should”, “seeks”, or “scheduled to”, or other similar words, or the negative of these terms or other variations of these terms or comparable language, or by discussion of strategy or intentions. These cautionary statements are being made pursuant to the Securities Act, the Exchange Act and the PSLRA with the intention of obtaining the benefits of the “safe harbor” provisions of such laws. The Company cautions investors that any forward-looking statements made by the Company are not guarantees or indicative of future performance. Important assumptions and other important factors that could cause actual results to differ materially from those forward-looking statements with respect to the Company include, but are not limited to, the risks and uncertainties affecting their businesses described in Item 1A of this Annual Report on Form 10-K and in other filings by the Company with the SEC.
Although the Company believes that its plans, intentions and expectations reflected in or suggested by such forward-looking statements are reasonable, actual results could differ materially from a projection or assumption in any of its forward-looking statements. The Company’s future financial condition and results of operations, as well as any forward-looking statements, are subject to change and inherent risks and uncertainties. The forward-looking statements contained in this Annual Report on Form 10-K are made only as of the date hereof and the Company does not have or undertake any obligation to update or revise any forward-looking statements whether as a result of new information, subsequent events or otherwise, unless otherwise required by law.
PART I
Item 1. Description of Business
General
Integrated BioPharma, Inc., a Delaware corporation (together with its subsidiaries, the “Company”), is engaged primarily in manufacturing, distributing, marketing and sales of vitamins, nutritional supplements and herbal products. The Company’s customers are located primarily in the United States and Luxembourg. The Company was originally incorporated in the state of Delaware on August 31, 1995 under the name Chem International, Inc., on December 5, 2000, changed its name to Integrated Health Technologies, Inc. and, on January 29, 2003, changed its name to Integrated BioPharma, Inc. The Company restated its certificate of incorporation in Delaware in June 2006. The Company continues to do business as Chem International, Inc. with certain of its customers and certain vendors.
The Company’s business segments include: (a) Contract Manufacturing operated by Manhattan Drug Company, Inc. (“MDC”), which manufactures vitamins and nutritional supplements for sale to distributors, multilevel marketers and specialized health-care providers and (b) Other Nutraceutical Businesses which includes the operations of (i) AgroLabs, Inc. (“AgroLabs”), which distributed healthful nutritional products for sale through major mass market, grocery and drug and vitamin retailers under the following brands: Peaceful Sleep, and Wheatgrass and other products introduced into the market using the AgroLabs name (these are referred to as our branded products); (ii) The Vitamin Factory (the “Vitamin Factory”), which sells private label MDC products, as well as our AgroLabs products, through the Internet, (iii) IHT Health Products, Inc. (“IHT”) a distributor of fine natural botanicals, including multi minerals produced under a license agreement, (iv) MDC Warehousing and Distribution, Inc., a service provider for warehousing and fulfilment services and (v) Chem International, Inc., a distributor of certain raw materials for DSM Nutritional Products LLC. The Vitamin Factory had no products available for sale and AgroLabs had no sales of its branded products in each of the fiscal years ended June 30, 2023 and 2022.
Significant Revenues from Major Customers
In the fiscal years ended June 30, 2023 and 2022, a significant portion of our consolidated net sales, approximately 89% and 90%, respectively, were concentrated among two customers, Life Extension Quality Supplements and Vitamins, Inc. (“Life Extension”) and Herbalife Nutrition LTD (“Herbalife”), both customers in our Contract Manufacturing Segment. Life Extension and Herbalife represented approximately 70% and 24% and 67% and 26%, respectively, of our Contract Manufacturing Segment’s net sales in the fiscal years ended June 30, 2023 and 2022, respectively. Thermosource Tooling and Manufacturing, HotPack Global Inc. and ThermoFisher Scientific (customers of our Other Nutraceutical Businesses Segment), while not significant customers of our consolidated net sales, represented approximately 56%, 16% and 9% and 40%, 1% and 19%, respectively, of the Other Nutraceutical Businesses net sales in the fiscal years ended June 30, 2023 and 2022, respectively. The loss of any of these customers could have a significant adverse impact on our financial condition and results of operations.
Raw Materials
The principal raw materials used in the manufacturing process in the Company’s business are natural and synthetic vitamins, minerals, herbs, related nutritional supplements, vegetable and gelatin capsules, coating materials, organic and natural fruit extracts, fruit juices and the necessary components for packaging the finished products. The raw materials are available from numerous sources within the United States and abroad. The vegetable and gelatin capsules, coating materials and packaging materials are similarly widely available. The Company generally purchases its raw materials, on a purchase order basis, without long-term commitments in each of its operating segments. We have one principal supplier for our Other Nutraceutical Businesses segment, DSM Nutritional Products LLC and several suppliers in our Contract Manufacturing Segment.
Development and Supply Agreement
Effective July 15, 2009, the Company entered into development and supply agreements with Herbalife International of America, Inc. and Herbalife International of Luxembourg S.à.R.L, subsidiaries of Herbalife, pursuant to which the Company develops, manufactures and supplies certain nutritional products to Herbalife. This agreement is in the process of being extended through December 31, 2025. This agreement does not, however, obligate the Company to supply any particular amount of goods to Herbalife, nor does it obligate Herbalife to commit to a minimum order, if any. In its ordinary course of business, the Company enters into similar agreements with other customers in connection with its contract manufacturing business.
Seasonality
The nutraceutical business tends to be seasonal. We have found that in our first fiscal quarter ending on September 30th of each year, orders for our branded proprietary nutraceutical products usually slow (absent the addition of new customers or a new product launch with a significant first time order), as buyers in various markets may have purchased sufficient inventory to carry them through the summer months. Conversely, in our second fiscal quarter, ending on December 31st of each year, orders for our products increase as the demand for our branded nutraceutical products, as well as sales orders from our customers in our contract manufacturing segment, seem to increase in late December to early January as consumers become health conscious as they enter the new year.
The Company believes that there are other non-seasonal factors that also may influence the variability of quarterly results including, but not limited to, general economic and industry conditions that affect consumer spending, changing consumer demands and current news on nutritional supplements. Accordingly, a comparison of the Company’s results of operations from consecutive periods is not necessarily meaningful, and the Company’s results of operations for any period are not necessarily indicative of future periods.
Government Regulations
The manufacturing, processing, formulation, packaging, labeling and advertising of our products are subject to regulation by a number of federal agencies, including the Food and Drug Administration (“FDA”), the Federal Trade Commission (“FTC”), the United States Postal Service, the Consumer Product Safety Commission and the United States Department of Agriculture. Our activities are also regulated by various state and local agencies in states where our products are sold. The FDA is primarily responsible for the regulation of the manufacturing, labeling and sale of our products. The operation of our vitamin manufacturing facility is subject to regulation by the FDA as a dietary supplement manufacturing facility. The United States Postal Service and the FTC regulate advertising claims with respect to the Company’s products. In addition, we manufacture and market certain of our products in compliance with the guidelines promulgated by the United States Pharmacopoeia Convention, Inc. (“USP”) and other voluntary standards organizations.
The Dietary Supplement Health and Education Act of 1994 (“DSHEA”) was enacted on October 25, 1994. The Dietary Supplement Act amends the U.S. Federal Food, Drug and Cosmetic Act (“FFD&CA”) by defining dietary supplements, which include vitamins, minerals, nutritional supplements and herbs, and by providing a regulatory framework to ensure safe, quality dietary supplements and the dissemination of accurate information about such products. The FDA is generally prohibited from regulating the active ingredients in dietary supplements as food additives, or as drugs unless product claims trigger drug status. The DSHEA requires the FDA to regulate dietary supplements so as to guarantee consumer access to beneficial dietary supplements, allowing only truthful and proven claims. Generally, dietary ingredients that were on the market before October 15, 1994 may be sold without FDA pre-approval and without notifying the FDA. However, new dietary ingredients (those not used in dietary supplements marketed before October 15, 1994) require pre-market submission to the FDA of evidence of a history of their safe use, or other evidence establishing that they are reasonably expected to be safe. There can be no assurance that the FDA will accept the evidence of safety for any new dietary ingredient we may decide to use. The FDA’s refusal to accept such evidence could result in regulation of such dietary ingredients as food additives, requiring the FDA pre-approval based on newly conducted, costly safety testing.
DSHEA provides for specific nutritional labeling requirements for dietary supplements effective January 1, 1997. The Dietary Supplement Act permits substantiated, truthful and non-misleading statements of nutritional support to be made in labeling, such as statements describing general well-being from consumption of a dietary ingredient or the role of a nutrient or dietary ingredient in affecting or maintaining the structure or function of the body. The FDA requires the Company to notify the FDA of such statements. There can be no assurance that the FDA will not consider particular labeling statements used by us to be drug claims rather than acceptable statements of nutritional support, necessitating approval of a costly new drug application, or re-labeling to delete such statements. It is also possible that the FDA could allege false statements were submitted to it if structure/function claim notifications were either non-existent or so lacking in scientific support as to be plainly false.
As authorized by DSHEA, the FDA adopted Good Manufacturing Practices (“GMP”) specifically for dietary supplements (21 CFR Part 111). These GMP regulations, which became effective in June 2008, are more detailed than the GMPs that previously applied to dietary supplements and require, among other things, dietary supplements to be prepared, packaged and held in compliance with specific rules, and require quality controls similar to those required by GMP regulations for drugs. We believe our manufacturing and distribution practices comply with these rules.
Dietary supplements are also subject to the Nutrition, Labeling and Education Act (“NLEA”), which regulates health claims, ingredient labeling and nutrient content claims characterizing the level of a nutrient in a product. NLEA prohibits the use of any health claim for dietary supplements unless the health claim is supported by significant agreement within the scientific community and is pre-approved by the FDA.
In certain markets, including the United States, claims made with respect to dietary supplements may change the regulatory status of our products. For example, in the United States, the FDA could possibly take the position that claims made for some of our products classify those products as new drugs requiring pre-approval by the FDA. The FDA could also place those products within the scope of its over-the-counter (“OTC”) drug regulations and require us to comply with a published FDA OTC monograph. OTC monographs dictate permissible ingredients, appropriate labeling language and require the marketer or supplier of the products to register and file annual drug listing information with the FDA. We do not, at present, sell OTC drug products. If the FDA were to assert that our product claims cause them to be considered new drugs or to fall within the scope of OTC regulations, we would be required to either; file a new drug application, comply with the applicable monographs, or change the claims made in connection with those products.
The FTC regulates the marketing practices and advertising of all our products. In recent years, the FTC instituted enforcement actions against several dietary supplement companies for false and misleading marketing practices and advertising of certain products. These enforcement actions have resulted in consent decrees and monetary payments by the companies involved. Under FTC standards, the dissemination of any false advertising constitutes an unfair or deceptive act or practice actionable under Section 45 of the Fair Trade Commission Act and a false advertisement actionable under Section 52 of that Act. A false advertisement is one that is “misleading in a material respect.” In determining whether an advertisement or labeling information is misleading in a material respect, the FTC determines not only whether overt and implied representations are false but also whether the advertisement fails to reveal material facts. Under the FTC’s standards, any health benefit representation made in advertising must be backed by “competent and reliable scientific evidence” by which the FTC means: “tests, analyses, research studies, or other evidence based upon the expertise of professionals in the relevant area, that have been conducted and evaluated in an objective manner by persons qualified to do so, using procedures generally accepted by the profession to yield accurate and reliable results.”
The FTC has increased its review of the use of the type of testimonials that may be used to market our products. The FTC requires competent and reliable evidence substantiating claims and testimonials at the time that such claims of health benefit are first made. The failure to have this evidence when product claims are first made violates the Federal Trade Commission Act. Although the FTC has never threatened an enforcement action against the Company for the advertising of its products, there can be no assurance that the FTC will not question the advertising for our products in the future.
We believe we are currently in compliance with all applicable government regulations. We cannot predict what new legislation or regulations governing our operations will be enacted by legislative bodies or promulgated by agencies that regulate its activities. The FDA is expected to increase its enforcement activity against dietary supplements that it considers to be in violation of FFD&CA. In particular, the FDA is increasing its enforcement of DSHEA provisions. Those activities will be enhanced by the appropriation for increased FDA budgets for dietary supplement regulation enforcement.
We believe we may become subject to additional laws or regulations administered by the FDA or other federal, state, or foreign regulatory authorities. We also believe the laws or regulations which are considered favorable may be repealed, or more stringent interpretations of current laws or regulations may be implemented. Any or all of such requirements could be a burden to us. Future regulations could require us to:
● change the way we conduct business;
● use expanded or different labeling;
● recall, reformulate or discontinue certain products;
● keep additional records;
● increase the available documentation of the properties of its products; and/or
● increase the scientific proof of product ingredients, safety, and/or usefulness.
Competition
The business of manufacturing, distributing and marketing vitamins and nutritional supplements is highly competitive. Many of our competitors are substantially larger and have greater financial resources with which to manufacture and market their products. In particular, the retail segment is highly competitive. Many direct marketers not only focus on selling their own branded products, but offer national brands at discounts as well. Many competitors have established brand names recognizable to consumers. In addition, major pharmaceutical companies offer nationally advertised multivitamin products.
Many of our competitors in the retailing segment have the financial resources to advertise freely, to promote sales and to produce sophisticated catalogs and websites. In many cases, such competitors are able to offer price incentives for retail purchasers and to offer participation in frequent buyers programs. Some retail competitors also manufacture their own products whereby they have the ability and financial incentive to sell their own product.
We intend to compete by stressing the quality of our manufactured products, providing prompt service, competitive pricing of products in our marketing segment and by focusing on niche products in international retail markets.
Research and Development Activities
We do not conduct any significant research and development activities.
Environmental Compliance
We are subject to regulation under Federal, state and local environmental laws. While we believe we are in material compliance with applicable environmental laws, continued compliance may require substantial capital expenditures. We have not incurred any major costs for any environmental compliance during the years ended June 30, 2023 and 2022.
Employees
As of September 15, 2023, we had approximately 141 full time employees, including 106 who are members of the local unit of the Teamsters Union and are covered by a collective bargaining agreement which expires on August 31, 2027. The remaining 35 employees, not covered by a collective bargaining agreement, consist of approximately 15 administrative and professional personnel, 12 laboratory and quality assurance personnel, 3 sales and marketing personnel and 5 production and shipping personnel. We consider our relations with our employees to be good.
In December 2022, we entered into an agreement with a Professional Employer Organization (“PEO”) and terminated our agreement with the previous PEO. The PEO agreements established a three-way relationship between our non-union employees, the PEO and us. We and the PEO are co-employers of our non-union employees. The PEO has taken responsibility for our Human Resources administration and compliance, which allows us to continue to exercise control over our business while accessing quality employee benefits. We have been using PEOs since January 2007.
Available Information
We file annual, quarterly and current reports, proxy statements and other information with the SEC. These filings are available to the public via the Internet at the SEC's website located at http://www.sec.gov.
Our website is located at ir.ibiopharma.com. You may request a copy of our filings with the SEC (excluding exhibits) at no cost by writing or telephoning us at the following address or telephone number:
Integrated BioPharma, Inc.
225 Long Avenue, Bldg. 15
Hillside, New Jersey 07205
Attn: Investor Relations
Tel: 888-319-6962
Item 1A. Risk Factors
Please carefully consider the following risk factors which could materially adversely affect our business, financial condition, operating results and cash flows. The risk factors described below are not the only ones we face. Risks and uncertainties not known to us currently, or that we currently deem immaterial, also may materially adversely affect our business, financial condition, operating results and cash flows.
Our revenue could decline significantly if we lose one or more of our most significant customers, which could have a significant adverse impact on us.
A significant portion of our revenues are concentrated among four customers, Life Extension, Herbalife (customers in our Contract Manufacturing Segment), and Thermosource Tooling and Manufacturing and Hotpack Global, Inc. (customers of our Other Nutraceutical Businesses Segment). In the fiscal years ended June 30, 2023 and 2022, approximately 89% and 90% of our consolidated net sales, respectively, were derived from the two major customers in our Contract Manufacturing Segment. The loss of any of these customers could have a significant adverse impact on our financial condition and results of operations.
Supply chain disruptions resulting from geo-political events could have an impact on our financial results and ability to timely ship products to our customers.
These issues first arose as result of the COVID-19 pandemic and other geo-political events. Transportation, in general, continues to be an issue in the delay of receiving raw materials and our ability to meet promised delivery dates to our customers in the Contract Manufacturing Segment.
While we haven’t, to date, seen a significant negative impact in our margins resulting from the geo-political events, we are experiencing a slight negative impact on our margins due to inflation and tightened labor markets.
During the first quarter of calendar 2022, the war in Ukraine affected our customer’s business operations in Ukraine and Russia, resulting in the cancelation of some future orders. The war resulted in the imposition of sanctions by the United States, the United Kingdom and the European Union that affect the cross-border operations of businesses operating in Russia. In addition, many multinational companies ceased or suspended their operations in Russia. Therefore, the ability to continue operations in Russia by our customers is uncertain. Also, there may be a shortage of Sunflower Oil products in the near future and this may cause delays in production of certain raw materials and may require reformulation of products.
We have indebtedness, which may decrease our flexibility, increase our borrowing costs and adversely affect our liquidity.
We currently have $11.6 million in senior secured financing (the “Senior Credit Facility”) available under the Loan Agreement, dated as of June 27, 2012 and as amended on May 15, 2019 (the "Amended Loan Agreement"), by and among the Company, MDC, AgroLabs, IHT Health Products, Inc., IHT Properties Corp. (“IHT Properties”), and Vitamin Factory (collectively, the “Borrowers”) and PNC Bank, National Association ("PNC") and $2,623 in operating lease obligations. As of June 30, 2023, we have no amounts outstanding with PNC under the Senior Credit Facility.
Our level of indebtedness can have important consequences. For example, it may require a substantial portion of our cash flow from operations for the payment of principal of, and interest on, our indebtedness and reduce our ability to use our cash flow to fund working capital, capital expenditures and general corporate requirements or to pay dividends; and limit our flexibility to adjust to changing business and market conditions and make us more vulnerable to a downturn in general economic conditions as compared to our competitors.
There are various financial covenants and other restrictions in the Senior Credit Facility. If we fail to comply with any of these requirements, any related indebtedness (and other unrelated indebtedness) could become due and payable prior to its stated maturity. A default under the Senior Credit Facility may also significantly affect our ability to obtain additional or alternative financing. For example, PNC's ongoing obligation to extend credit under the Amended Loan Agreement is dependent upon our compliance with these covenants and restrictions.
To the extent we draw down additional funds under the Senior Credit Facility, our ability to make scheduled payments or to refinance our obligations with respect to indebtedness will depend on our operating and financial performance, which, in turn, is subject to prevailing economic conditions and to financial, business and other factors beyond our control. Our inability to refinance our indebtedness when necessary or to do so upon attractive terms would materially and adversely affect our liquidity and our ongoing results of operations.
Complying with new and existing government regulation, both in the U.S. and abroad, could increase our costs significantly and adversely affect our financial results.
The processing, formulation, manufacturing, packaging, labeling, advertising, distribution and sale of our products are subject to regulation by several U.S. federal agencies, including the FDA, the FTC, the Consumer Product Safety Commission, the Department of Agriculture and the EPA, as well as various state, local and international laws and agencies of the localities in which our products are sold. Government regulations may prevent or delay the introduction, or require the reformulation, of our products. Some agencies, such as the FDA or state agencies, could require us to remove a particular product from the market, delay or prevent the import of raw materials for the manufacture of our products, or otherwise disrupt the marketing of our products. Any such government actions would result in additional costs to us, including lost revenues from any additional products that we are required to remove from the market, which additional costs could be material. Any such government actions also could lead to liability, substantial costs and reduced growth prospects. Moreover, there can be no assurance that new laws or regulations imposing more stringent regulatory requirements on the dietary supplement industry will not be enacted or issued. In addition, complying with adverse event reporting requirements imposes additional costs on us, which costs could become significant in the event more demanding reporting requirements are put into place.
Additional or more stringent regulations of dietary supplements and other products have been considered from time to time. These developments could require reformulation of certain products to meet new standards, recalls or discontinuance of certain products that cannot be reformulated, additional record-keeping requirements, increased documentation of the properties of certain products, additional or different labeling, additional scientific substantiation, adverse event reporting or other new requirements. These developments also could increase our costs significantly. For example, the FDA issued rules which became effective in 2008 that imposed substantial new regulatory requirements for dietary supplements, including GMPs. Congress also passed legislation requiring adverse event reporting and related record keeping which imposed additional costs on us. See Item 1. "Description of Business—Government Regulations" for additional information.
We may be exposed to or the target of legal proceedings initiated by regulators or third parties either in the United States or abroad which could increase our costs and adversely affect our reputation, revenues and operating income.
In the United States and abroad, non-compliance with relevant legislation can result in regulators bringing administrative or, in some cases, criminal proceedings. As manufacturers of nutraceutical products, our products are regulated by various governments and it is common for regulators to prosecute retailers and manufacturers for non-compliance with legislation governing foodstuffs and medicines. Failures by us or our subsidiaries to comply with applicable legislation could occur from time to time and prosecution for any such violations could have a material adverse effect on our business, results of operations, financial condition and cash flows. Additionally, we are subject, from time to time, to claims by third parties under various legal theories. The defense of such claims, or any adverse outcome relating to any such claims, could have a material adverse effect on our liquidity, financial condition and cash flows.
We depend on our senior management, the loss of whom would have an adverse effect on us.
We presently are dependent upon the executive abilities of our Co-Chief Executive Officers, Christina Kay and Riva Sheppard, our Chief Financial Officer, Dina L. Masi and the Vice President of Operations for Manhattan Drug Company, Inc. Mireille Antinozzi. Our business and operations to date chiefly have been implemented under the direction of these individuals, who presently are, and in the future will be, responsible for the implementation of our anticipated plans and programs. The loss or unavailability of the services of one or more of our principal executives would have an adverse effect on us. We may encounter difficulty in our ability to recruit and ultimately hire any replacement or additional executive officers having similar background, experience and qualifications as those of our current executive officers.
We could be the target of a cybersecurity breach which could have an adverse effect on us.
A cybersecurity breach could result in the loss or theft of investor data or funds, the inability to access electronic systems, loss or theft of proprietary information or corporate data, disruption of our operations, physical damage to a computer or network system, or costs associated with system repairs. Such incidents could cause the Company to incur regulatory penalties, reputational damage, remediation costs, litigation costs, additional compliance costs, or financial loss. Intentional cybersecurity breaches include: unauthorized access to systems, networks, or devices (such as through “hacking” activity); infection from computer viruses or other malicious software code; and attacks that shut down, disable, slow, or otherwise disrupt operations, business processes, or website access or functionality. In addition, unintentional incidents can occur, such as the inadvertent release of confidential information (possibly resulting in the violation of applicable privacy laws.
There is no assurance that we will remain quoted or listed on an active trading market.
As of January 22, 2022, the Company’s common stock was upgraded to the OTCQX® Best Market of the OTC Markets Group, Inc. and commenced quotation under the symbol “INBP.” In order to maintain quotation on the OTCQX, the Company will be required to comply with certain minimum qualitative and quantitative requirements, including with respect to corporate governance. There can be no assurances that the Company will be able to comply with these qualitative and quantitative requirements for continued quotation of its common stock on the OTCQX. If the Company doesn’t maintain compliance with the OTCQX rules, the Company’s common stock may resume listing on the OTCQB and holders of the Company’s common stock may find it more difficult to sell their shares.
From September 23, 2009 until January 21, 2022, our common stock was quoted on the OTQB. From February 27, 2009 through September 22, 2009, our common stock was quoted on the Pink Sheets. Prior to February 27, 2009, our common stock was listed on the NASDAQ Global Market, and there can be no assurance that we will, in the future, be able to meet all the requirements for reinstatement on that or any other national securities exchange. The delisting of our common stock from the NASDAQ Global Market has adversely affected, and may in the future continue to adversely affect, the liquidity and trading of our common stock.
We have entered into several transactions with entities controlled by some of our officers and directors, which could pose a conflict of interest.
We have several agreements and arrangements, described in our previous SEC filings and to be described in our proxy statement for our 2023 annual meeting of stockholders, including the lease of real property from Vitamin Realty Associates, L.L.C. (“Vitamin Realty”), the sale of our financial debt securities, and issuance of our common stock, which involved transactions with entities owned, in whole or in part, by the Estate of the former Executive Chairman and our Co-Chief Executive Officers and other of our significant shareholders and/or directors, who collectively own a majority of our shares of common stock. Although we believe that these transactions were advantageous to us and were on terms no less favorable to us than could have been obtained from unaffiliated third parties, transactions with related parties can potentially pose a conflict of interest.
Our Executive Officers and Directors have majority voting power and may take actions that may not be in the best interest of other stockholders, but in their own interest.
Collectively, our Executive Officers, Directors and two other significant stockholders, beneficially own approximately 72% of our outstanding shares of common stock as of September 15, 2023. If these stockholders act together, they would be able to exert significant control over our management and affairs since significant corporate transactions require stockholder approval. This concentration of ownership may have the effect of delaying or preventing a change in control and might adversely affect the market price of our common stock. This concentration of ownership may not be in the best interests of all our stockholders.
We have a staggered Board of Directors, which could impede an attempt to acquire the Company or remove our management.
Our Board of Directors is divided into three classes, each of which serves for a staggered term of three years. This division of our Board of Directors could have the effect of impeding an attempt to take over our company or change or remove management, since only one class will be elected annually. Thus, only approximately one-third of the existing Board of Directors could be replaced at any election of directors.
Our product liability insurance may be insufficient to cover possible claims against us.
Our company, like other manufacturers, wholesalers and distributors of vitamin and nutritional supplement products, faces an inherent risk of exposure to product liability claims if, among other things, the use or ingestion of our products, results in sickness or injury. We currently maintain a product liability insurance policy that provides a total of $5.0 million of coverage per occurrence and $5.0 million of coverage in the aggregate. However, there can be no assurance that existing or future insurance coverage will be sufficient to cover any possible product liability risks or that such insurance will continue to be available to us on economically feasible terms.
Our nutraceutical products are manufactured using various raw materials consisting of vitamins, minerals, herbs, fruit extracts and other ingredients that we regard as safe when taken as recommended by us and that various scientific studies have suggested may provide health benefits. We could be adversely affected if any of our products or any similar products distributed by other companies should prove or be asserted to be harmful to consumers or should scientific studies provide unfavorable findings regarding the effectiveness of our products.
We may not be able to obtain raw materials used in certain of our manufactured products.
The principal raw materials used in the manufacturing process in the Company’s nutraceutical business are natural and synthetic vitamins, minerals, herbs, related nutritional supplements, vegetable and gelatin capsules, coating materials, fruit extracts, fruit juices and the necessary components for packaging the finished products. The raw materials are available from numerous sources within the United States and abroad. The vegetable and gelatin capsules, coating materials and packaging materials are similarly widely available. We generally purchase our raw materials, on a purchase order basis, without long-term commitments.
We have one principal supplier for our Other Nutraceutical Businesses Segment, DSM Nutritional Products LLC and several suppliers in our Contract Manufacturing Segment. If we are unable to maintain our relationships with our suppliers, we may not be able to find alternate sourcing of our raw materials or at the same pricing that we receive from our current suppliers and/or quickly enough to make timely shipments to our customers. This could decrease our sales and/or increase our cost of sales.
Current economic conditions may cause a decline in business and consumer spending which could adversely affect our business and financial performance.
Our operating results are impacted by the health of the North American economies. Our business and financial performance, including collection of our accounts receivable, recoverability of assets including investments, may be adversely affected by current and future economic conditions, such as a reduction in the availability of credit, financial market volatility, recession, etc. Additionally, we may experience difficulties in scaling our operations to react to economic pressures in the United States.
We may incur significant professional service fees and other control costs that impact our financial condition.
As a publicly traded corporation, we incur certain costs to comply with regulatory requirements. If regulatory requirements were to become more stringent or if controls thought to be effective later fail, we may be forced to make additional expenditures, the amounts of which could be material. Some of our competitors are privately owned so their accounting and control costs can be a competitive disadvantage for us. Should our sales decline or if we are unsuccessful at increasing prices to cover higher expenditures for internal controls, audits, consultants and legal, our costs associated with regulatory compliance will rise as a percentage of sales.
Other issues and uncertainties may include:
● New accounting pronouncements or changes in accounting policies; and
● Legislation or other governmental action that detrimentally impacts our expenses or reduces sales by adversely affecting our customers.
If we discover material weaknesses and other deficiencies in our internal control and accounting procedures, our stock price could decline significantly and raising capital could be more difficult.
If we fail to comply with the rules under the Sarbanes-Oxley Act, related to disclosure controls and procedures, or if we discover material weaknesses and other deficiencies in our internal control and accounting procedures, our stock price could decline significantly and raising capital could be more difficult. Moreover, effective internal controls are necessary for us to produce reliable financial reports and are important in helping prevent financial fraud. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock could drop significantly. While we have not identified any material weakness in our internal control over financial reporting, we cannot be certain that material weaknesses or significant deficiencies in our internal controls will not be discovered in the future.
We have not paid dividends on our common stock in the past and do not expect to pay dividends on our common stock for the foreseeable future. Any return on investment may be limited to the value of our common stock.
We have never paid any cash dividends on our common stock. We expect that any income received from operations will be devoted to our future operations and growth. We do not expect to pay cash dividends on our common stock in the near future. Payment of dividends would depend upon our profitability at the time, cash available for those dividends, and other factors that our board of directors may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on an investor’s investment will only occur if our stock price appreciates. Investors in our common stock should not rely on an investment in our company if they require dividend income.
A sale of a substantial number of shares of our common stock may cause the price of our common stock to decline and may impair our ability to raise capital in the future.
Our common stock is quoted on the OTCQX® Best Market and could be considered “thinly-traded,” meaning that the number of investors interested in purchasing our common stock at or near bid prices at any given time may be relatively small or non-existent. Finance transactions resulting in a large amount of newly issued shares that become readily tradable, or other events that cause current stockholders to sell shares, could place downward pressure on the trading price of our common stock. In addition, the lack of a robust resale market may require a stockholder who desires to sell a large number of shares of common stock to sell the shares in increments over time to mitigate any adverse impact of the sales on the market price of our stock.
If our stockholders sell, or the market perceives that our stockholders may sell for various reasons, including the ending of restriction on resale, substantial amounts of our common stock in the public market, including shares issued upon the exercise of outstanding options or warrants, the market price of our common stock could fall. Sales of a substantial number of shares of our common stock may make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
Item 1B. Unresolved Staff Comments
Not applicable.
Item 2. Properties
Warehouse and office facilities are leased from Vitamin Realty, which is 100% owned by the estate of our former Executive Chairman of the Board, President and major stockholder of the Company and our Co-Chief Executive Officers who are also directors of the Company. On January 5, 2012, MDC, a wholly-owned subsidiary of the Company, entered into a second amendment of the lease (the “Second Lease Amendment”) with Vitamin Realty for its office and warehouse space in Hillside, New Jersey increasing its rentable square footage from an aggregate of 74,898 square feet to 76,161 square feet and extending the expiration date to January 31, 2026. On July 15, 2022, MDC entered into a third amendment of the lease (the “Third Lease Amendment”) with Vitamin Realty, increasing its rentable square footage to 116,175. This Third Lease Amendment provides for minimum annual rental payments of $842, plus increases in real estate taxes and the building operating expenses allocation percentage and was effective as of July 1, 2022.
We also own a 40,000 square foot manufacturing facility in Hillside, New Jersey. The space is utilized for MDC’s tablet and capsule manufacturing operations.
In December 2022, MDC Warehousing and Distribution, Inc. entered into a lease agreement for 12,500 square feet of warehouse space in Elizabeth, New Jersey. This lease provides for minimum annual rent payments starting at $134 in year one, with a gradual increase to $150 by year 5. The lease expiration date is November 30, 2027.
On October 22, 2014, AgroLabs entered into a lease agreement for an office suite located in Miami, Florida. On December 18, 2022, AgroLabs renewed this lease with minimum annual payments of approximately $10. This renewed lease will expire in February 2024.
Item 3. Legal Proceedings
From time to time, we may become involved in various lawsuits and legal proceedings that arise in the ordinary course of business. However, litigation is subject to inherent uncertainties and an adverse result in these or other matters may arise from time to time that may harm our business. We are currently not aware of any such legal proceedings or claims that we believe will have a material adverse effect on our business, financial condition or operating results.
Item 4. Mine Safety Disclosure
Not Applicable
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Effective January 22, 2021, our common stock was upgraded to the OTCQX® Best Market of the OTC Markets Group, Inc. and commenced quotation under the symbol “INBP” on the OTCQX® Best Market. Prior to January 22, 2021, our common stock was quoted on the OTCQB under the symbol INBP.
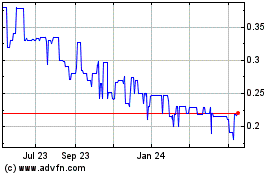
Set forth below are the high and low bid quotation of the Company’s common stock as quoted on the OTCQX® Best Market, for each of the fiscal quarters in the fiscal years ended June 30, 2023 and 2022. Such quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
| COMMON STOCK |
|
HIGH |
|
|
LOW |
|
| |
|
|
|
|
|
|
|
|
| FISCAL YEAR ENDED June 30, 2022 |
|
|
|
|
|
|
|
|
| First Quarter |
|
$ |
1.150 |
|
|
$ |
0.900 |
|
| Second Quarter |
|
$ |
1.090 |
|
|
$ |
0.880 |
|
| Third Quarter |
|
$ |
1.020 |
|
|
$ |
0.750 |
|
| Fourth Quarter |
|
$ |
0.800 |
|
|
$ |
0.410 |
|
| |
|
|
|
|
|
|
|
|
| FISCAL YEAR ENDED June 30, 2023 |
|
|
|
|
|
|
|
|
| First Quarter |
|
$ |
0.5500 |
|
|
$ |
0.4300 |
|
| Second Quarter |
|
$ |
0.4400 |
|
|
$ |
0.3502 |
|
| Third Quarter |
|
$ |
0.3800 |
|
|
$ |
0.2605 |
|
| Fourth Quarter |
|
$ |
0.3720 |
|
|
$ |
0.2200 |
|
| July 1, 2023 to September 8, 2023 |
|
$ |
0.3325 |
|
|
$ |
0.2600 |
|
Holders
As of September 15, 2023, there were approximately 71 holders of record of the Company’s common stock. This number does not include beneficial owners holding shares through nominee names.
Dividends
We have not declared or paid a dividend with respect to our common stock during the fiscal years ended June 30, 2023 and 2022, nor do we anticipate paying dividends in the foreseeable future.
Equity Compensation Plans
The following table provides information, as of June 30, 2023, about the Company's equity compensation plans:
| |
|
Equity Compensation Plan Information |
|
| |
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
|
|
Weighted-average exercise price of outstanding options, warrants and rights |
|
|
Number of securities remaining available for future issuance under equity compensation plans (excluding securities |
|
| |
|
(a) |
|
|
(b) |
|
|
reflected in column (a)) |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Equity compensation plans approved by security holders |
|
|
4,376,284 |
|
|
$ |
0.35 |
|
|
|
6,874,718 |
|
| Equity compensation plans not approved by security holders |
|
|
- |
|
|
|
- |
|
|
|
- |
|
| Totals |
|
|
4,376,284 |
|
|
$ |
0.35 |
|
|
|
6,874,718 |
|
Recent Sales of Unregistered Securities
None.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
During the quarter ended June 30, 2023, neither we nor any “affiliated purchaser,” as that term is defined in Rule 10b-18(a)(3) under the Exchange Act, purchased any of our common stock or other securities.
Item 6. [Reserved]
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations (dollars in thousands).
Certain statements set forth under this caption constitute “forward-looking statements.” See “Cautionary Statement Regarding Forward-Looking Statements” on page 3 of this Annual Report on Form 10-K for additional factors relating to such statements.
The Company is engaged primarily in the business of manufacturing, distributing, marketing and sale of vitamins, nutritional supplements and herbal products. The Company’s customers are located primarily throughout the United States and Luxembourg.
Our financial results are substantially dependent on net sales. Net sales are partly dependent on the mix of contract manufactured products and other nutraceutical sales, which are difficult to forecast. Factors that could cause demand to be different from our expectations include: customer acceptance of our pricing and our competitors’ pricing; changes in customer order patterns; changes in the level of customer inventory; and changes in business and economic conditions, including conditions in the credit market that could affect consumer confidence and result in lower than expected demand for our manufactured products and to a lesser extent, our other nutraceutical business products and services.
We believe that we have established and developed business relationships, facilities, personnel, product offerings, and competitive and financial resources in place for business success; however, future revenue, costs, gross margins, and profits are all influenced by a number of factors, including those discussed above, all of which are inherently difficult to forecast. Except as otherwise noted, all dollar amounts below are “in thousands”.
In the fiscal year ended June 30, 2023, our net sales from operations decreased by $5,574 to approximately $50,672 from approximately $56,246 in the fiscal year ended June 30, 2022. Our net sales in the Contract Manufacturing Segment decreased by $5,814 and net sales in our Other Nutraceuticals Segment increased by $240. Net sales decreased in our Contract Manufacturing Segment primarily due to decreased sales volumes to Life Extension and Herbalife, our two significant customers, in the amount of $2,571 and $2,708, respectively. In the fiscal year ended June 30, 2023, our gross profit decreased by approximately $2,491 to $4,061 from approximately $6,552 for the fiscal year ended June 30, 2022. Our profit margins decreased by 3.7% in the fiscal year ended June 30, 2023, from 11.7% in the fiscal year ended June 30, 2022 to 8.0% in the fiscal year ended June 30, 2023, primarily as a result of the decreased sales volume in the fiscal year ended June 30, 2023 compared to the fiscal year ended June 30, 2022. We had consolidated selling and administrative expenses of approximately $3,941 and $3,807 in the fiscal years ended June 30, 2023 and 2022, respectively. The increase in the consolidated selling and administrative expenses of $134, or approximately 3.5%, was primarily from the increase professional and consulting fees of $71 and office rent of $55. We had additional legal fees of $23, auditing fees of $20 and all other consulting of $28 in the fiscal year ended June 30, 2023 as compared to the fiscal year ended June 30, 2022. The increase in office rent was from the allocation of office space from the Third Lease Amendment with Vitamin Realty. In the fiscal years ended June 30, 2023 and 2022, we had operating income of approximately $120 and $2,745, respectively.
Our revenue from our two significant customers in our Contract Manufacturing Segment is dependent on their demand within their respective distribution channels for the products we manufacture for them. As in any competitive market, our ability to match or beat other contract manufacturers pricing for the same items may also alter our outlook and the ability to maintain or increase revenues. We will continue to focus on our core businesses and push forward in maintaining our cost structure in line with our sales and expanding our customer base.
We are currently experiencing negative impacts on our margins due to inflation and tightened labor markets. We may not be able to timely increase our selling prices to our customers resulting from price increases from our suppliers due to various economic factors, including inflation, labor and shipping costs and our own increases in shipping, labor and other operating costs. Our results of operations may also be affected by economic conditions, including inflationary pressures, that can impact consumer disposable income levels and spending habits, thereby reducing the orders we may receive from our significant customers.
We continue to experience minimal supply chain disruptions relating to fuel refinery and transportation issues as it pertains to shipping. These issues first arose as result of the COVID-19 pandemic and other geo-political events.
During the first quarter of calendar 2022, the war in Ukraine affected our customer’s business operations in Ukraine and Russia, resulting in the cancelation of some future orders. The war resulted in the imposition of sanctions by the United States, the United Kingdom, and the European Union, that affect the cross-border operations of businesses operating in Russia. In addition, many multinational companies ceased or suspended their operations in Russia. Therefore, the ability to continue operations in Russia by our customers remains uncertain.
Critical Accounting Policies and Estimates
Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. The most significant estimates include:
● sales returns and allowances;
● allowance for doubtful accounts;
● inventory valuation;
● valuation and recoverability of long-lived assets;
● income taxes and valuation allowances on deferred income taxes; and
● accruals for, and the probability of, the outcome of current litigation, if any.
On a continual basis, management reviews its estimates utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such reviews, and if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ from those estimates.
Allowances for Doubtful Accounts and Sales Returns
Our management makes judgments as to its ability to collect outstanding receivables and provides allowances for the portion of receivables for which collection becomes doubtful. Provisions are made based upon a specific review of all significant outstanding amounts. We continuously monitor payments from our customers and maintain allowances for estimated losses for doubtful accounts in the period they become known.
If the historical data we use to calculate the allowance provided for doubtful accounts does not reflect the future ability to collect outstanding receivables, additional provisions for doubtful accounts may be needed and the future results of operations could be materially affected. In recording any additional allowances, a respective charge against income is reflected in the general and administrative expenses; and would reduce the operating results in the period in which the increase is recorded.
Our return policy in our contract manufacturing business is to only accept returns for defective products. If defective products are returned, our agreement with our customers is to cure the defect and re-ship the product. Based on this policy, when the product is shipped we make an estimate of any potential returns or allowances. With respect to our branded proprietary nutraceutical products, our return policy is also to accept returns for defective products and re-ship replacement items for the damaged product. In most instances, the damaged goods are a small portion of the overall order and we instruct our customer to dispose of the damaged product and we issue them a credit for the dollar amount of the damaged goods plus any cost of disposal. We also estimate and make allowances at the time of shipment.
Inventory Valuation
Inventories are stated at the lower of cost or net realizable value, which reflects management’s estimates of net realizable value. Cost is determined using the first-in, first-out method. As a result of our inventory being manufactured primarily on a purchase order basis, the quantity of both raw materials and finished goods inventory provides for minimal risk of potential overstock or obsolescence.
Mail and Internet order inventory is expiration date sensitive. Accordingly, we review this inventory, consider sales levels (by SKU), term to expiration date, potential for retesting to extend expiration date, and evaluate potential for obsolescence or overstock.
Long Lived Assets
We record impairment losses on long lived assets when events and circumstances indicate that such assets might be impaired and the estimated fair value of any such asset is less than its recorded amount. The Company reviews the value of its long-lived assets for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable or that the useful lives of these assets are no longer appropriate. Conditions that would necessitate an impairment assessment include material adverse changes in operations, significant adverse differences in actual results in comparison with initial valuation forecasts prepared at the time of acquisition, a decision to abandon certain acquired products, services, or marketplaces, or other significant adverse changes that would indicate the carrying amount of the recorded asset might not be recoverable. Tests for impairment or recoverability are performed at least annually and require significant management judgment and the use of estimates which the Company believes are reasonable and appropriate at the time of the impairment test. The Company also re-evaluates the periods of amortization to determine whether circumstances warrant revised estimates of current useful lives. No impairment losses were identified in the fiscal years ended June 30, 2023 or 2022.
Income Taxes
The Company records deferred tax assets and liabilities for the estimated future tax effects of temporary differences between tax bases of assets and liabilities and amounts reported in the accompanying consolidated balance sheets, as well as operating losses and tax credit carry-forwards. The Company measures deferred tax assets and liabilities using enacted tax rates expected to be applied to taxable income in the years in which those temporary differences are expected to be recovered or settled. The Company reduces deferred tax assets by a valuation allowance if, based on available evidence, it is more likely than not that these benefits will not be realized.
The Company uses a recognition threshold and measurement attribute for financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities.
General Litigation
From time to time, the Company is a defendant or plaintiff in various legal actions which arise in the normal course of business. As such, the Company is required to assess the likelihood of any adverse outcomes to these matters as well as potential ranges of probable losses. A determination of the amount of the provision required for these commitments and contingencies, if any, which would be charged to earnings, is made after careful analysis of each matter. The provision may change in the future due to new developments or changes in circumstances. Changes in the provision could increase or decrease the Company’s earnings in the period the changes are made. In the opinion of management, after consultation with legal counsel, the ultimate resolution of these matters cannot be determined at this time as to the whether there could be material adverse effect on our financial condition or results of operations.
Revenue Recognition
The Company recognizes product sales revenue, the prices of which are fixed and determinable, when title and risk of loss have transferred to the customer, when estimated provisions for product returns, rebates, charge-backs and other sales allowances are reasonably determinable, and when collectability is reasonably assured. Accruals for these items are presented in the consolidated financial statements as reductions to sales. The Company’s net sales represent gross sales invoiced to customers, less certain related charges for discounts, returns, rebates, charge-backs and other allowances. Cost of sales includes the cost of raw materials and all labor and overhead associated with the manufacturing and packaging of the products. Gross margins are affected by, among other things, changes in the relative sales mix among our products and valuation and/or charge off of slow moving, expired or obsolete inventories. To perform revenue recognition for arrangements within the scope of ASC 606, the Company performs the following five steps:
| |
● |
identification of the promised goods or services in the contract; |
| |
● |
determination of whether the promised goods or serves are performance obligations including whether they are distinct in the context of the contract; |
| |
● |
measurement of the transaction price, including the constraint on variable consideration; |
| |
● |
allocation of the transaction price to the performance obligations based on estimated selling prices; and |
| |
● |
recognition of revenue when (or as) the Company satisfies each performance obligation. A performance obligation is a promise to transfer a distinct good or service to the customer and is the unit of account in ASC 606. |
Results of Operations (in thousands, except share and per share amounts)
The following table sets forth the income statement data of the Company as a percentage of net sales for the periods indicated:
| |
|
For the Fiscal Year Ended June 30, |
|
| |
|
2023 |
|
|
2022 |
|
| |
|
|
|
|
|
|
|
|
| Sales, net |
|
|
100.0 |
% |
|
|
100.0 |
% |
| |
|
|
|
|
|
|
|
|
| Costs and expenses: |
|
|
|
|
|
|
|
|
| Cost of sales |
|
|
92.0 |
% |
|
|
88.3 |
% |
| Selling and administrative |
|
|
7.8 |
% |
|
|
5.8 |
% |
| Total costs and expenses |
|
|
99.8 |
% |
|
|
95.1 |
% |
| Income from operations |
|
|
0.2 |
% |
|
|
4.9 |
% |
| |
|
|
|
|
|
|
|
|
| Other income (expense), net: |
|
|
|
|
|
|
|
|
| Interest expense |
|
|
(0.2 |
%) |
|
|
(0.3 |
%) |
| Unrealized gain (loss) on investment in iBio, Inc. |
|
|
0.1 |
% |
|
|
(0.1 |
%) |
| Realized loss on sale of iBio, Inc. common stock |
|
|
(0.1 |
%) |
|
|
- |
% |
| Other income (expense), net |
|
|
0.1 |
% |
|
|
0.1 |
% |
| Total other income (expense), net |
|
|
(0.1 |
%) |
|
|
(0.3 |
%) |
| Income before income taxes |
|
|
0.2 |
% |
|
|
4.6 |
% |
| |
|
|
|
|
|
|
|
|
| Federal and state income tax (expense) benefit, net |
|
|
(0.3 |
%) |
|
|
2.2 |
% |
| |
|
|
|
|
|
|
|
|
| Net (loss) income |
|
|
(0.1 |
%) |
|
|
6.8 |
% |
Year ended June 30, 2023 Compared to the Year ended June 30, 2022
Sales, net. Net sales for the fiscal year ended June 30, 2023 and 2022 were $50,672 and $56,246, respectively, a decrease of $5,574 or 9.9%. The decrease is comprised of the following:
| |
|
Fiscal Year Ended |
|
|
Dollar Increase |
|
|
Percentage |
|
| |
|
June 30, |
|
|
(Decrease) |
|
|
Change |
|
| |
|
2023 |
|
|
2022 |
|
|
2023 vs 2022 |
|
|
2023 vs 2022 |
|
| |
|
(dollars in thousands) |
|
| Contract Manufacturing: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| US Customers |
|
$ |
40,230 |
|
|
$ |
44,450 |
|
|
$ |
(4,220 |
) |
|
|
(9.5 |
%) |
| International Customers |
|
|
8,047 |
|
|
|
9,641 |
|
|
|
(1,594 |
) |
|
|
(16.5 |
)% |
| Net sales, Contract Manufacturing |
|
|
48,277 |
|
|
|
54,091 |
|
|
|
(5,814 |
) |
|
|
(10.7 |
%) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other Nutraceuticals: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| US Customers |
|
|
2,387 |
|
|
|
2,067 |
|
|
|
320 |
|
|
|
15.5 |
% |
| International Customers |
|
|
8 |
|
|
|
88 |
|
|
|
(80 |
) |
|
|
(90.9 |
%) |
| Net sales, Other Nutraceuticals |
|
|
2,395 |
|
|
|
2,155 |
|
|
|
240 |
|
|
|
11.1 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total net sales |
|
$ |
50,672 |
|
|
$ |
56,246 |
|
|
$ |
(5,574 |
) |
|
|
(9.9 |
%) |
In the fiscal years ended June 30, 2023 and 2022, a significant portion of our consolidated net sales, approximately 89% and 90%, respectively, were concentrated among two customers, Life Extension and Herbalife, customers in our Contract Manufacturing Segment. Life Extension and Herbalife represented approximately 70% and 24% and 67% and 26%, respectively, of our Contract Manufacturing Segment’s net sales in the fiscal years ended June 30, 2023 and 2022, respectively. Three other customers, Thermosource Tooling and Manufacturing, Hotpack Global, Inc. and ThermoFisher Scientific, (customers of our Other Nutraceutical Businesses Segment), while not significant customers of our consolidated net sales, represented 56%, 16% and 9% and 40%, 1% and 19%, respectively, of the Other Nutraceutical Businesses net sales in the fiscal years ended June 30, 2023 and 2022, respectively. The loss of any of these customers could have a significant adverse impact on our financial condition and results of operations.
The decrease in net sales of approximately $5,574 was primarily the result of decreased net sales in our Contract Manufacturing Segment by approximately $5,814 which was primarily due to decreased sales volumes to each of our major customers, Life Extension and Herbalife, in the amounts of $2,571 and $2,708, respectively, in the fiscal year ended June 30, 2023, compared to the comparable prior year. Net sales increased by $240 in the Other Nutraceuticals Segment primarily as a result of increased sales of $849 in MDC Warehousing and Distribution, Inc., offset by decreases in Chem and IHT of $491 and $118, respectively.
Cost of sales. Cost of sales decreased by $3,083 to $46,611 for the fiscal year ended June 30, 2023, as compared to $49,694 for the fiscal year ended June 30, 2022, a decrease of approximately 6.2%. Cost of sales as a percentage of sales was approximately 92% and 88% for the fiscal years ended June 30, 2023 and 2022, respectively. The decrease in the cost of goods sold amount is consistent and expected with the decrease in net sales. The increase in the cost of goods sold as a percentage of net sales and the resulting decrease in gross profit, was primarily the result of the decreased net sales and the increased costs of raw materials and freight charges that were not timely passed on to our customers.
Selling and Administrative Expenses. There was an increase in selling and administrative expenses of $134 or approximately 3.5% in the fiscal year ended June 30, 2023 as compared to the fiscal year ended June 30, 2022. As a percentage of sales, net, selling and administrative expenses were approximately 7.8% and 6.8% for the fiscal year ended June 30, 2023 and 2022, respectively. The increase was primarily from the increase professional and consulting fees of $71 and office rent of $55. We had additional legal fees of $23, auditing fees of $20 and all other consulting of $28 in the fiscal year ended June 30, 2023 as compared to the fiscal year ended June 30, 2022. The increase in office rent was from the allocation of office space from the Third Lease Amendment with Vitamin Realty. All other selling and administrative expenses had a net increase of $8, no other individual expense component changed by more the $40.
Other income (expense), net. Other income (expense), net was approximately $(20) for the fiscal year ended June 30, 2023 compared to $(148) for the fiscal year ended June 30, 2022, and is composed of:
| |
|
Fiscal Year Ended |
|
| |
|
June 30, |
|
| |
|
2023 |
|
|
2022 |
|
| |
|
(dollars in thousands) |
|
| Other income (expense): |
|
|
|
|
|
|
|
|
| Interest expense |
|
$ |
(52 |
) |
|
$ |
(128 |
) |
| Realized loss on sale of investment |
|
|
(35 |
) |
|
|
- |
|
| Unrealized gain (loss) on investment |
|
|
27 |
|
|
|
(55 |
) |
| Other income (expense), net |
|
|
40 |
|
|
|
35 |
|
| Total Other income (expense), net |
|
$ |
(20 |
) |
|
$ |
(148 |
) |
Our interest expense in the fiscal year ended June 30, 2023 decreased by $76 from the fiscal year ended June 30, 2022, primarily resulting from lower average daily balances outstanding under the Senior Credit Facility with PNC in the fiscal year ended June 30, 2023.
In the fiscal years ended June 30, 2023, we sold we sold our remaining iBio Stock, for a loss of $35 with no such sales in the fiscal year ended June 30, 2022. Also, in the fiscal years ended June 30, 2023 and 2022, we had an unrealized gain of approximately $27 and an unrealized loss of $55, respectively, on the remaining iBio Stock.
In the fiscal years ended June 30, 2023 and 2022, we had other income of $0 and $13, respectively from providing back office and operational support for unrelated entities that sell consumer products through retail and internet-based outlets. The balance of other income (expense), net was primarily from interest income of $39 and $1 from gain on the disposition of property and equipment in the fiscal year ended June 30, 2023 compared to $22 from gains on the disposition of property and equipment in the fiscal year ended June 30, 2022.
Federal and state income tax, net. In the fiscal years ended June 30, 2022, we released the valuation reserves for the deferred tax assets related to our federal net operating losses in the amount of $2,118. Management determined that the amount of the release was more likely than not to be realized by the Company. In the fiscal year ended June 30, 2023 and 2022, we had current federal income taxes of $110 and $600 and deferred income tax benefits of $55 and $2,106, respectively and current state tax expense of approximately $161 and $289, respectively and deferred state tax expense of $18 in the fiscal year ended June 30, 2023 and a deferred state tax benefit in the fiscal year ended June 30, 2022.
We continue to maintain a reserve on a portion of our deferred tax assets as it has been determined that it is “more likely than not” that the Company’s deferred tax assets, other than federal net operating losses, may not be fully realized.
The decrease in the state tax expense from 2022 to 2023 was the result of decreased taxable income for the combined group.
Net (loss) income. Our net (loss) income for the fiscal year ended June 30, 2023 and 2022 was approximately $(34) and $3,838, respectively. The decrease of approximately $3,872 was primarily the result of decreased operating income of $2,625, and net decrease in the income tax (expense) benefit, net in amount $1,375.
Liquidity and Capital Resources
The following table sets forth, for the periods indicated, the Company’s net cash flows provided by or used in operating, investing and financing activities:
| |
|
For the fiscal year ended June 30, |
|
| |
|
2023 |
|
|
2022 |
|
| |
|
(dollars in thousands) |
|
| Net cash provided by operating activities |
|
$ |
1,121 |
|
|
$ |
4,091 |
|
| Net cash used in investing activities |
|
$ |
(111 |
) |
|
$ |
(465 |
) |
| Net cash used in financing activities |
|
$ |
(136 |
) |
|
$ |
(3,505 |
) |
| Cash at end of year |
|
$ |
1,316 |
|
|
$ |
331 |
|
At June 30, 2023 and 2022, the Company had working capital of $11,544 and $11,363, respectively. Our current liabilities decreased by approximately $435 and our current assets decreased by $254 from June 30, 2022 to June 30, 2023. The decrease in current liabilities is primarily from the decrease of $101 in advances under revolving credit and a net decrease in accounts payable, accrued expenses and other current liabilities of $722, offset by an increase in operating lease obligations of $388. Our current assets decrease was primarily from a decrease in inventory and accounts receivable of $794 and $377, respectively, offset by the increase in cash of $985.
Operating Activities
Net cash provided by operating activities of $1,232 in the fiscal year ended June 30, 2023 includes a net loss of approximately $34. After excluding the effects of non-cash expenses and income, depreciation and amortization, compensation expense for employee stock options, accretion of financial instruments, changes in deferred tax assets and unrealized gains on investments, the adjusted cash used in operations before the effect of the changes in working capital components was an increase of approximately $1,483. Cash in the amount of approximately $251 from our working capital assets and liabilities was used in our operating activities and was primarily the result of decreases in operating lease obligations of approximately $804 and accounts payable and accrued expenses and other liabilities of $429, offset, by increases in accounts receivable of $156 and inventories of $794.
Net cash provided by operating activities of $4,091 in the fiscal year ended June 30, 2022 includes net income of approximately $3,838. After excluding the effects of non-cash expenses and income, depreciation and amortization, compensation expense for employee stock options, accretion of financial instruments, changes in deferred tax assets and unrealized gains on investments, the adjusted cash used in operations before the effect of the changes in working capital components was an increase of approximately $3,526. Cash in the amount of approximately $564 from our working capital assets and liabilities was provided from our operating activities and was primarily the result of decreases in accounts receivable of approximately $927 and inventories of $640, offset, by decreases in operating lease obligations of $501 and accounts payable and accrued expenses and other liabilities of $417, and an increase in prepaid expenses and other assets of approximately $84.
Investing Activities
Cash used in investing activities was used for the purchase of machinery and equipment for approximately $116 and $486 in the fiscal years ended June 30, 2023 and 2022, respectively, offset in the fiscal year ended June 30, 2023 and 2022 by proceeds (i) received from the sale of fixed assets of $1 and $21, and (ii) from the sale of iBio, Stock of $4 and $0, respectively, a net use of cash of approximately $111 and $465, respectively.
Financing Activities
Cash used in financing activities was approximately $136 for the fiscal year ended June 30, 2023, and was primarily from repayments of net advances under our revolving credit facility of $101 and payments under our financed lease obligation of $35.
Cash used in financing activities was approximately $3,505 for the fiscal year ended June 30, 2022 and consists of net payments under our revolving credit facility of $2,073 and repayments of principal under our term notes in the amount of $1,466 (See Note 5 to the consolidated financial statements included in this Annual Report on Form 10-K), offset by $34 received from the exercise of employee stock options.
As of June 30, 2023, we had cash of approximately $1,316, funds available under our revolving credit facility of approximately $6,269 and working capital of $11,544. We had income from operations of approximately $120 in the fiscal year ended June 30, 2023 and a net loss of approximately $34. The net loss includes a net federal tax provision of $55 that is a non-cash item due to our Federal net operating loss carryforwards and deferred tax assets offsetting the amounts owed. After taking into consideration our interim results and current projections, management believes that operations, together with the revolving credit facility and equipment financing will support our working capital requirements at least through the twelve-month period ending September 15, 2024.
Our current total annual commitments at June 30, 2023, for long term non-cancelable leases, of approximately $1,030 consists of obligations under operating leases for office and warehouse facilities and operating and finance lease obligations for the use of machinery and office equipment.
Capital Expenditures
The Company's capital expenditures in the fiscal years ended June 30, 2023 and 2022 were approximately $116 and $486, respectively. The Company has budgeted approximately $750 for capital expenditures for the fiscal year ending June 30, 2024. The total amount is expected to be funded from cash provided from the Company’s operations and from lease financing.
Off-Balance Sheet Arrangements
The Company has no off-balance sheet arrangements.
Impact of Inflation
The Company may not be able to timely increase its selling prices to its customer resulting from price increases from its suppliers due to various economic factors, including inflation, labor and shipping costs and its own increases in shipping, labor and other operating costs. The Company’s results of operations may also be affected by economic conditions, including inflationary pressures, that can impact consumer disposable income levels and spending habits, thereby reducing the orders it may receive from the Company’s significant customers.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
The Company is a smaller reporting company, as defined by Rule 12b-2 of the Exchange Act, and is not required to provide the information required under this item.
Item 8. Financial Statements and Supplementary Data
For a list of financial statements filed as part of this Annual Report on Form 10-K, see the index to consolidated financial statements on page 29.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not Applicable
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed by the Company in the reports it files or submits under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified by the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to provide reasonable assurance that information required to be disclosed by the Company in the reports it files or submits under the Exchange Act is accumulated and communicated to management, including the Co-Chief Executive Officers and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Under the supervision and with the participation of management, including the Co-Chief Executive Officers and Chief Financial Officer, the Company has evaluated the effectiveness of its disclosure controls and procedures (as such term is defined in Rule 13a-15(e) and 15d-15(e) under the Exchange Act) as of June 30, 2023, and, based upon this evaluation, the Co-Chief Executive Officers and Chief Financial Officer have concluded that these controls and procedures are effective in providing reasonable assurance of compliance.
Management’s Annual Report On Internal Control Over Financial Reporting
The Company’s management is responsible for establishing and maintaining an adequate system of internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Internal control over financial reporting is a process designed by, or under the supervision of, the Company’s principal executive and financial officers and effected by the Company’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
• Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company;
• Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and
• Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with policies and procedures may deteriorate.
The Company’s management, including the Co-Chief Executive Officers and Chief Financial Officer, has conducted an evaluation of the effectiveness of its internal control over financial reporting as of June 30, 2023 based on the criteria set forth in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013) (the “COSO Framework”). Based on that evaluation, management concluded that our internal control over financial reporting was effective as of June 30, 2023.
Changes in Internal Control over Financial Reporting
Under the supervision and with the participation of management, including the Co-Chief Executive Officers and Chief Financial Officer, the Company has evaluated changes in internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the quarter ended June 30, 2023 and have concluded that no change has materially affected, or is reasonably likely to materially affect, internal control over financial reporting.
Item 9B. Other Information
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
N/A
PART III
Item 10. Directors, Executive Officers and Corporate Governance of the Registrant.
Incorporated by reference from the Company’s Proxy Statement for Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year ended June 30, 2023.
Item 11. Executive Compensation
Incorporated by reference from the Company’s Proxy Statement for Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year ended June 30, 2023.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Incorporated by reference from the Company’s Proxy Statement for Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year ended June 30, 2023.
Item 13. Certain Relationships and Related Transactions and Director Independence
Incorporated by reference from the Company’s Proxy Statement for Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year ended June 30, 2023.
Item 14. Principal Accountant Fees and Services
Incorporated by reference from the Company’s Proxy Statement for Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year ended June 30, 2023.
PART IV
Item 15. Exhibits and Financial Statement Schedules
| |
(1) |
A list of the financial statements filed as part of this Annual Report on Form 10-K is set forth in the index to consolidated financial statements on Page 29 and is incorporated herein by reference. |
(2) An index of exhibits incorporated by reference or filed with this Annual Report on Form 10-K is provided below.
| Number |
Description |
| |
|
| 3.1 |
Certificate of Incorporation of Integrated BioPharma, Inc., as amended (7) |
| 3.2 |
Certificate of Amendment to the Restated Certificate of Incorporation of Integrated BioPharma, Inc. (7) |
| 3.3 |
By-Laws of Registrant (5) |
| 4.1 |
Certificate of Designation of Series and Determination of Rights and Preferences of Series A Convertible Preferred Stock of Integrated BioPharma, Inc. dated June 25, 2003 (1) |
| 4.2 |
Certificate of Designation of Series C and Determination of Rights and Preferences of Series C Convertible Preferred Stock of Integrated BioPharma, Inc. dated February 21, 2008 (6) |
| 10.1 |
Lease Agreement between the Company and Vitamin Realty Associates, dated January 10, 1997 (2) |
| 10.1.1 |
Second Amendment of Lease, dated as of January 5, 2012, between Vitamin Realty Associates, L.L.C. and InB:Manhattan Drug Company, Inc. (9) |
| 10.1.2 |
Third Amendment of Lease, dated as of July 15, 2022, between Vitamin Realty Associates, L.L.C. and Manhattan Drug Company, Inc. (14) |
| 10.2 |
Lease Agreement, dated as of January 5, 2012, between Vitamin Realty Associates, L.L.C. and AgroLabs, Inc. (9) |
| 10.2.1 |
Amendment of Lease Agreement, dated as of May 19, 2014, between Vitamin Realty Associates, L.L.C. and AgroLabs, Inc. (11) |
| 10.2.2 |
Termination of Lease Agreement, dated as of July 15, 2022, between Vitamin Realty Associates, L.L.C. and AgroLabs, Inc. (14) |
| 10.3 |
Integrated Health Technologies, Inc. 2001 Stock Option Plan, as amended (8) |
| 10.4 |
Separation and Distribution Agreement dated November 14, 2007, with our subsidiary INB:Biotechnologies (4) |
| 10.5 |
Revolving Credit, Term Loan and Security Agreement, dated as of June 27, 2012, by and among Integrated BioPharma, Inc., InB:Manhattan Drug Company, Inc., Agrolabs, Inc., IHT Health Products, Inc., IHT Properties Corp. and Vitamin Factory, Inc. and PNC Bank, National Association. (10) |
| 10.5.1 |
First Amendment to Revolving Credit, Term Loan and Security Agreement dated as of February 19, 2016 by and among Integrated BioPharma, Inc., InB: Manhattan Drug Company, Inc., AgroLabs, Inc., IHT Health Products, Inc., IHT Properties Corp. and Vitamin Factory, Inc. and PNC Bank, National Association. (12) |
| 10.5.2 |
Second Amendment to Revolving Credit, Term Loan and Security Agreement dated as of May 15, 2019 by and among Integrated BioPharma, Inc., InB: Manhattan Drug Company, Inc., AgroLabs, Inc., IHT Health Products, Inc., IHT Properties Corp. and Vitamin Factory, Inc. and PNC Bank, National Association. (13) |
| 10.5.3 |
Fourth Amendment to Revolving Credit, Term Loan and Security Agreement dated as of March 16, 2023 by and among Integrated BioPharma, Inc., Manhattan Drug Company, Inc. (successor-by-merger to InB:Manhattan Drug Company, Inc.), Agrolabs, Inc., IHT Health Products, Inc. IHT Properties, Inc. and Vitamin Factory, Inc. and PNC Bank, National Association. (15) |
| 10.6 |
Term Note, dated as of June 27, 2012, by and among Integrated BioPharma, Inc., InB:Manhattan Drug Company, Inc., Agrolabs, Inc., IHT Health Products, Inc., IHT Properties Corp. and Vitamin Factory, Inc. and PNC Bank, National Association, in the original principal amount of $3,727,000. (10) |
| 10.6.1 |
Amended and Restated Term Note dated as of February 19, 2016 by and among Integrated BioPharma, Inc., InB: Manhattan Drug Company, Inc., AgroLabs, Inc., IHT Health Products, Inc., IHT Properties Corp. and Vitamin Factory, Inc. and PNC Bank, National Association in the original principal amount of $3,422,160.00. (12) |
| 10.6.2 |
Second Amended and Restated Term Note dated as of May 15, 2019 by and among Integrated BioPharma, Inc., InB: Manhattan Drug Company, Inc., AgroLabs, Inc., IHT Health Products, Inc., IHT Properties, Inc. and Vitamin Factory, Inc. and PNC Bank, National Association in the original principal amount of $ $3,585,175. (13) |
| 10.7 |
Revolving Credit Note, dated as of June 27, 2012, by and among Integrated BioPharma, Inc., InB:Manhattan Drug Company, Inc., Agrolabs, Inc., IHT Health Products, Inc., IHT Properties Corp. and Vitamin Factory, Inc. and PNC Bank, National Association, in the original principal amount of $8,000,000. (10) |
| 10.7.1 |
First Amendment to Revolving Credit, Term Loan and Security Agreement dated as of February 19, 2016 by and among Integrated BioPharma, Inc., InB: Manhattan Drug Company, Inc., AgroLabs, Inc., IHT Health Products, Inc., IHT Properties Corp. and Vitamin Factory, Inc. and PNC Bank, National Association. (12) |
| 10.7.2 |
Second Amendment to Revolving Credit, Term Loan and Security Agreement dated as of May 15, 2019 by and among Integrated BioPharma, Inc., InB: Manhattan Drug Company, Inc., AgroLabs, Inc., IHT Health Products, Inc., IHT Properties Corp. and Vitamin Factory, Inc. and PNC Bank, National Association. (13) |
| 10.8 |
Stock Pledge Agreement, dated as of June 27, 2012, between Integrated BioPharma, Inc. and PNC Bank, National Association. (10) |
| 10.9 |
Mortgage and Security Agreement, dated as of June 27, 2012, by IHT Properties Corp. in favor of PNC Bank, National Association. (10) |
| 10.10 |
Environmental Indemnity Agreement, dated as of June 27, 2012, by and among Integrated BioPharma, Inc., InB:Manhattan Drug Company, Inc., Agrolabs, Inc., IHT Health Products, Inc., IHT Properties Corp. and Vitamin Factory, Inc. and PNC Bank, National Association (10) |
| 10.11 |
Lease Agreement between Elizabeth Industrial Park, LLC c/o Palin Enterprises, and MDC Warehousing and Distribution, Inc. dated November 22, 2022. (16) |
| 14 |
Code of Business Ethics (3) |
| 21 |
Subsidiaries of the Registrant (17) |
| 23.1 |
Consent of Independent Registered Public Accounting Firm (17) |
| 23.2 |
Consent of Independent Registered Public Accounting Firm (17) |
| 31.1 |
Certification of Periodic Report by Chief Executive Officer Pursuant to Rule 13a-14 and 15d-14 of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (17) |
| 31.2 |
Certification of Periodic Report by Chief Financial Officer Pursuant to Rule 13a-14 and 15d-14 of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (17) |
| 32.1 |
Certification by Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (17) |
| 32.2 |
Certification by Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (17) |
| |
|
| 101 |
The following financial information from Integrated BioPharma, Inc.’s Annual Report on Form 10-K for the fiscal year ended June 30, 2023, formatted in XBRL (extensible Business Reporting Language): (i) Consolidated Statements of Operations for the fiscal years ended June 30, 2023 and 2022, (ii) Consolidated Balance Sheets as of June 30, 2023 and 2022, (iii) Consolidated Statements of Changes in Stockholders’ Equity for the fiscal years ended June 30, 2023 and 2022 , (iv) Consolidated Statements of Cash Flows for the fiscal years ended June 30, 2023 and 2022, and (v) the Notes to Consolidated Statements. (17) |
|
|
Cover Page Interactive Date File (formatted as Inline XBRL and contained in Exhibit 101)
|
__________________________
| (1) |
Incorporated herein by reference to the Company’s Annual Report on Form 10-KSB for the fiscal year ended June 30, 2003, filed with the SEC on September 29, 2003. |
| (2) |
Incorporated herein by reference to the Company’s Annual Report on Form 10-KSB for the fiscal year ended June 30, 1997, filed with the SEC on September 29, 1997. |
| (3) |
Incorporated herein by reference to the Company’s Current Report on Form 8-K filed with the SEC on May 12, 2022. |
| (4) |
Incorporated herein by reference to the Company's Current Report on Form 8-K filed with the SEC on November 19, 2007. |
| (5) |
Incorporated herein by reference to the Company's Current Report on Form 8-K filed with the SEC on February 14, 2008. |
| (6) |
Incorporated herein by reference to the Company's Current Report on Form 8-K filed with the SEC on February 22, 2008. |
| (7) |
Incorporated herein by reference to the Company's Current Report on Form 8-K filed with the SEC on May 12, 2008 and to the Company’s Annual Report on Form 10-KSB for the fiscal year ended June 30, 2003 filed with the SEC on September 29, 2003. |
| (8) |
Incorporated herein by reference to the Company's Definitive Proxy Statement on Form DEF 14A, as revised, filed with the SEC on October 28, 2009. |
| (9) |
Incorporated herein by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2012 filed with the SEC on May 21, 2012. |
| (10) |
Incorporated herein by reference to the Company's Current Report on Form 8-K filed with the SEC on June 29, 2012. |
| (11) |
Incorporated herein by reference to the Company’s Annual Report on Form 10-K for the fiscal year ended June 30, 2014, filed with the SEC on September 8, 2014. |
| (12) |
Incorporated herein by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended December 31, 2015 filed with the SEC on February 19, 2016. |
| (13) |
Incorporated herein by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2019 filed with the SEC on May 15, 2019. |
| (14) |
Incorporated herein by reference to the Company’s Annual Report on Form 10-K for the fiscal year ended June 30, 2022 filed with the SEC on September 13, 2022. |
| (15) |
Incorporated herein by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2023 filed with the SEC on May 11, 2023. |
| (16) |
Incorporated herein by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended December 31, 2022 filed with the SEC on February 10, 2023. |
| (17) |
Filed herewith. |
Item 16. Form 10-K Summary
None.
Item 8: Financial Statements
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Report of Independent Registered Public Accounting Marcum LLP (PCAOB ID Number 688) and Friedman LLP (PCAOB ID Number 711) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 30 | |
| | | |
| Consolidated Statements of Operations for the fiscal years ended June 30, 2023 and 2022 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 32 | |
| | | |
| Consolidated Balance Sheets as of June 30, 2023 and 2022 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 33 | |
| | | |
| Consolidated Statements of Stockholders’ Equity for the fiscal years ended June 30, 2023 and 2022 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 34 | |
| | | |
| Consolidated Statements of Cash Flows for the fiscal years ended June 30, 2023 and 2022 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 35 | |
| | | |
| Notes to Consolidated Financial Statements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 36 | |
. . . . . . . . .
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Integrated BioPharma, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Integrated BioPharma, Inc. (the “Company”) as of June 30, 2023 and the related consolidated statements of operations, stockholders’ equity and cash flows for the year ended June 30, 2023, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2023, and the results of its operations and its cash flows for the year ended June 30, 2023, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/ Marcum LLP
Marcum LLP
We have served as the Company’s auditor since 2009 (such date takes into account the acquisition of certain assets of Friedman LLP effective September 1, 2022)
East Hanover, New Jersey
September 15, 2023
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of Directors of
Integrated BioPharma, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Integrated BioPharma, Inc. (the “Company”) as of June 30, 2022, the related consolidated statements of operations, and stockholders’ equity and cash flows for the year ended June 30, 2022, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2022, and the results of its operations and its cash flows for the year ended June 30, 2022, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Friedman LLP
We have served as the Company’s auditor from 2009 through 2022.
East Hanover, New Jersey
September 13, 2022
| INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES |
|
| CONSOLIDATED STATEMENTS OF OPERATIONS |
|
| FOR THE FISCAL YEARS ENDED JUNE 30, |
|
| (in thousands, except share and per share amounts) |
|
| |
|
|
|
|
|
|
| |
2023 |
|
2022 |
|
| |
|
|
|
|
|
|
| Sales, net |
$ |
50,672 |
|
$ |
56,246 |
|
| |
|
|
|
|
|
|
| Cost of sales |
|
46,611 |
|
|
49,694 |
|
| |
|
|
|
|
|
|
| Gross profit |
|
4,061 |
|
|
6,552 |
|
| |
|
|
|
|
|
|
| Selling and administrative expenses |
|
3,941 |
|
|
3,807 |
|
| |
|
|
|
|
|
|
| Operating income |
|
120 |
|
|
2,745 |
|
| |
|
|
|
|
|
|
| Other income (expense), net: |
|
|
|
|
|
|
| Interest expense |
|
(52 |
) |
|
(128 |
) |
| Unrealized gain (loss) on investment in iBio Stock |
|
27 |
|
|
(55 |
) |
| Realized loss on sale of investment in iBio Stock |
|
(35 |
) |
|
- |
|
| Other income |
|
40 |
|
|
35 |
|
| Total other income (expense), net |
|
(20 |
) |
|
(148 |
) |
| |
|
|
|
|
|
|
| Income before income taxes |
|
100 |
|
|
2,597 |
|
| |
|
|
|
|
|
|
| Income tax (expense) benefit, net |
|
(134 |
) |
|
1,241 |
|
| |
|
|
|
|
|
|
| Net (loss) income |
$ |
(34 |
) |
$ |
3,838 |
|
| |
|
|
|
|
|
|
| Basic net (loss) income per common share |
$ |
(0.00 |
) |
$ |
0.13 |
|
| |
|
|
|
|
|
|
| Diluted net (loss) income per common share |
$ |
(0.00 |
) |
$ |
0.12 |
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| Weighted average common shares outstanding - basic |
|
29,949,610 |
|
|
29,843,386 |
|
| Add: Equivalent shares outstanding - stock options |
|
- |
|
|
2,477,939 |
|
| Weighted average common shares outstanding - diluted |
|
29,949,610 |
|
|
32,321,326 |
|
See accompanying notes to consolidated financial statements.
| INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES | |
| CONSOLIDATED BALANCE SHEETS | |
| AS OF JUNE 30, | |
| (in thousands, except share and per share amounts) | |
| | | | | | | | | |
| | | 2023 | | | 2022 | |
| | | | | | | | | |
| Assets | | | | | | | | |
| Current Assets: | | | | | | | | |
| Cash | | $ | 1,316 | | | $ | 331 | |
| Accounts receivable, net | | | 4,511 | | | | 4,888 | |
| Inventories | | | 10,261 | | | | 11,055 | |
| Other current assets | | | 284 | | | | 352 | |
| Total current assets | | | 16,372 | | | | 16,626 | |
| | | | | | | | | |
| Property and equipment, net | | | 1,653 | | | | 1,910 | |
| Operating lease right-of-use assets (includes $2,061 and $1,839 with a related party) | | | 2,623 | | | | 1,867 | |
| Deferred tax assets, net | | | 4,726 | | | | 4,798 | |
| Security deposits and other assets | | | 57 | | | | 49 | |
| Total Assets | | $ | 25,431 | | | $ | 25,250 | |
| | | | | | | | | |
| Liabilities and Stockholders' Equity: | | | | | | | | |
| Current Liabilities: | | | | | | | | |
| Advances under revolving credit facility | | $ | - | | | $ | 101 | |
| Accounts payable (includes $0 and $72 due to a related party) | | | 2,266 | | | | 3,209 | |
| Accrued expenses and other current liabilities | | | 1,632 | | | | 1,411 | |
| Current portion of long term debt, net | | | 42 | | | | 32 | |
| Current portion of operating lease liabilities (includes $772 and $503 due to a related party) | | | 888 | | | | 510 | |
| Total current liabilities | | | 4,828 | | | | 5,263 | |
| | | | | | | | | |
| Long term debt, net | | | 7 | | | | 53 | |
| Operating lease liabilities (includes $1,289 and $1,338 due to a related party) | | | 1,735 | | | | 1,359 | |
| Total Liabilities | | | 6,570 | | | | 6,675 | |
| | | | | | | | | |
| Commitments and Contingencies (Note 10) | | | | | | | | |
| | | | | | | | | |
| Stockholders' Equity | | | | | | | | |
| Common Stock, $0.002 par value; 50,000,000 shares authorized; | | | | | | | | |
| 29,984,510 and 29,949,610 shares issued and outstanding, respectively | | | 60 | | | | 60 | |
| Additional paid-in-capital | | | 51,239 | | | | 50,919 | |
| Accumulated deficit | | | (32,339 | ) | | | (32,305 | ) |
| Less: Treasury stock, at cost, 34,900 shares | | | (99 | ) | | | (99 | ) |
| Total Stockholders' Equity | | | 18,861 | | | | 18,575 | |
| Total Liabilities and Stockholders' Equity | | $ | 25,431 | | | $ | 25,250 | |
See accompanying notes to consolidated financial statements.
| INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES |
|
| CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY |
|
| FOR THE FISCAL YEARS ENDED JUNE 30, |
|
| (in thousands, except shares) |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Common Stock |
|
|
Additional |
|
|
Accumulated |
|
|
Treasury Stock |
|
|
Total Stockholders' |
|
| |
|
Shares |
|
|
Par Value |
|
|
Paid-in-Capital |
|
|
Deficit |
|
|
Shares |
|
|
Cost |
|
|
Equity |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, July 1, 2021 |
|
|
29,838,177 |
|
|
$ |
60 |
|
|
$ |
50,516 |
|
|
$ |
(36,143 |
) |
|
|
34,900 |
|
|
$ |
(99 |
) |
|
$ |
14,334 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compensation expense for employee stock options |
|
|
- |
|
|
|
- |
|
|
|
369 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
369 |
|
| Shares issued upon exercise of stock options |
|
|
146,333 |
|
|
|
- |
|
|
|
34 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
34 |
|
| Net income |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
3,838 |
|
|
|
- |
|
|
|
- |
|
|
|
3,838 |
|
| Balance, June 30, 2022 |
|
|
29,984,510 |
|
|
|
60 |
|
|
|
50,919 |
|
|
|
(32,305 |
) |
|
|
34,900 |
|
|
|
(99 |
) |
|
|
18,575 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compensation expense for employee stock options |
|
|
- |
|
|
|
- |
|
|
|
320 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
320 |
|
| Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(34 |
) |
|
|
- |
|
|
|
- |
|
|
|
(34 |
) |
| Balance, June 30, 2023 |
|
|
29,984,510 |
|
|
$ |
60 |
|
|
$ |
51,239 |
|
|
$ |
(32,339 |
) |
|
|
34,900 |
|
|
$ |
(99 |
) |
|
$ |
18,861 |
|
See accompanying notes to consolidated financial statements.
| INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES |
|
| CONSOLIDATED STATEMENTS OF CASH FLOWS |
|
| FOR THE FISCAL YEARS ENDED JUNE 30, |
|
| (in thousands) |
|
| |
|
|
|
|
|
|
|
|
| |
|
2023 |
|
|
2022 |
|
| Cash flows from operating activities: |
|
|
|
|
|
|
|
|
| Net (loss) income |
|
$ |
(34 |
) |
|
$ |
3,838 |
|
| Adjustments to reconcile net (loss) income to net cash provided by operating activities: |
|
|
|
|
|
|
|
|
| Amortization of operating lease right-of-use assets |
|
|
803 |
|
|
|
498 |
|
| Depreciation and amortization |
|
|
351 |
|
|
|
336 |
|
| Deferred income taxes (benefit) |
|
|
72 |
|
|
|
(1,530 |
) |
| Accretion of financing instruments, amortization of prepaid financing costs and other |
|
|
12 |
|
|
|
21 |
|
| Compensation expense on employee stock options |
|
|
320 |
|
|
|
369 |
|
| Unrealized (gain) loss on investment in iBio Stock |
|
|
(27 |
) |
|
|
55 |
|
| Realized loss on sale of iBio Stock |
|
|
35 |
|
|
|
- |
|
| Bad debt expense |
|
|
3 |
|
|
|
13 |
|
| Gain on disposal of fixed assets |
|
|
(1 |
) |
|
|
(22 |
) |
| Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
| Decrease (increase) in: |
|
|
|
|
|
|
|
|
| Accounts receivable |
|
|
374 |
|
|
|
875 |
|
| Inventories |
|
|
794 |
|
|
|
640 |
|
| Prepaid expenses and other assets |
|
|
34 |
|
|
|
(84 |
) |
| (Decrease) increase in: |
|
|
|
|
|
|
|
|
| Operating lease obligations |
|
|
(804 |
) |
|
|
(501 |
) |
| Accounts payable |
|
|
(921 |
) |
|
|
(218 |
) |
| Accrued expenses and other current liabilities |
|
|
221 |
|
|
|
(199 |
) |
| Net cash provided by operating activities |
|
|
1,232 |
|
|
|
4,091 |
|
| |
|
|
|
|
|
|
|
|
| Cash flows from investing activities: |
|
|
|
|
|
|
|
|
| Purchase of property and equipment |
|
|
(116 |
) |
|
|
(486 |
) |
| Proceeds from sale of iBio Stock |
|
|
4 |
|
|
|
- |
|
| Proceeds from sale of fixed assets |
|
|
1 |
|
|
|
21 |
|
| Net cash used in investing activities |
|
|
(111 |
) |
|
|
(465 |
) |
| |
|
|
|
|
|
|
|
|
| Cash flows from financing activities: |
|
|
|
|
|
|
|
|
| Repayments of advances under revolving credit facility, net |
|
|
(101 |
) |
|
|
(2,073 |
) |
| Proceeds from exercises of stock options |
|
|
- |
|
|
|
34 |
|
| Repayments under term notes payable |
|
|
- |
|
|
|
(1,466 |
) |
| Repayments under financed lease obligations |
|
|
(35 |
) |
|
|
- |
|
| Net cash used in financing activities |
|
|
(136 |
) |
|
|
(3,505 |
) |
| |
|
|
|
|
|
|
|
|
| Net increase in cash |
|
|
985 |
|
|
|
121 |
|
| Cash at beginning of fiscal year |
|
|
331 |
|
|
|
210 |
|
| Cash at end of fiscal year |
|
$ |
1,316 |
|
|
$ |
331 |
|
| |
|
|
|
|
|
|
|
|
| Supplemental disclosures of cash flow information: |
|
|
|
|
|
|
|
|
| Cash paid during the periods for: |
|
|
|
|
|
|
|
|
| Interest |
|
$ |
41 |
|
|
$ |
113 |
|
| Income taxes |
|
$ |
- |
|
|
$ |
471 |
|
| Supplemental disclosures of non-cash transactions: |
|
|
|
|
|
|
|
|
| Amount owed on purchase of fixed assets |
|
$ |
- |
|
|
$ |
22 |
|
| Financing on financed lease obligation |
|
$ |
- |
|
|
$ |
85 |
|
| Acquisition of right-of-use asset, net and operating lease obligations, net |
|
$ |
1,560 |
|
|
$ |
- |
|
| Trade in value on like-kind exchanges |
|
$ |
- |
|
|
$ |
7 |
|
See accompanying notes to consolidated financial statements.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
Note 1. Business
Integrated BioPharma, Inc., a Delaware corporation (together with its subsidiaries, the “Company”), is engaged primarily in manufacturing, distributing, marketing and sales of vitamins, nutritional supplements and herbal products. The Company’s customers are located primarily in the United States and Luxembourg. The Company was originally incorporated in the state of Delaware on August 31, 1995 under the name Chem International, Inc., on December 5, 2000, changed its name to Integrated Health Technologies, Inc. and, on January 29, 2003, changed its name to Integrated BioPharma, Inc. The Company restated its certificate of incorporation in Delaware in June 2006. The Company continues to do business as Chem International, Inc. with certain of its customers and certain vendors.
The Company’s business segments include: (a) Contract Manufacturing operated by Manhattan Drug Company, Inc. (“MDC”), which manufactures vitamins and nutritional supplements for sale to distributors, multilevel marketers and specialized health-care providers and (b) Other Nutraceutical Businesses which includes the operations of (i) AgroLabs, Inc. (“AgroLabs”), which distributed healthful nutritional products for sale through major mass market, grocery and drug and vitamin retailers, under the following brands: Peaceful Sleep, Wheatgrass and other products introduced into the market using the AgroLabs name (these are referred to as our branded products), (ii) The Vitamin Factory (the “Vitamin Factory”), which did sell private label MDC products, as well as our AgroLabs products, through the Internet, (iii) IHT Health Products, Inc. (“IHT”) a distributor of fine natural botanicals, including multi minerals produced under a license agreement, (iv) MDC Warehousing and Distribution, Inc., a service provider for warehousing and fulfillment services and (v) Chem International, Inc. (“Chem”), a distributor of certain raw materials for DSM Nutritional Products LLC. The Vitamin Factory had no products available for sale and AgroLabs had no sales of its branded products in the fiscal years ended June 30, 2023 and 2022.
Note 2. Summary of Significant Accounting Policies
Principles of Consolidation. The accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. Intercompany transactions and accounts have been eliminated in consolidation.
Reclassifications. Certain prior year amounts have been reclassified to conform to the current year presentation.
Use of Estimates. The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. The most significant estimates include:
● sales returns and allowances;
● allowance for doubtful accounts;
● inventory valuation;
● valuation and recoverability of long-lived assets;
● income taxes and valuation allowance on deferred income taxes, and;
● accruals for, and the probability of, the outcome of current litigation, if any.
On a continual basis, management reviews its estimates utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such reviews, and if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ from those estimates.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
Revenue Recognition. The Company recognizes product sales revenue, the prices of which are fixed and determinable, when title and risk of loss have transferred to the customer, when estimated provisions for product returns, rebates, charge-backs and other sales allowances are reasonably determinable, and when collectability is reasonably assured. Accruals for these items are presented in the consolidated financial statements as reductions to sales. The Company’s net sales represent gross sales invoiced to customers, less certain related charges for discounts, returns, rebates, charge-backs and other allowances. Cost of sales includes the cost of raw materials and all labor and overhead associated with the manufacturing and packaging of the products. Gross margins are affected by, among other things, changes in the relative sales mix among our products and valuation and/or charge off of slow moving, expired or obsolete inventories. To perform revenue recognition for arrangements within the scope of ASC 606, the Company performs the following five steps:
| | ● | identification of the promised goods or services in the contract; |
| | ● | determination of whether the promised goods or serves are performance obligations including whether they are distinct in the context of the contract; |
| | ● | measurement of the transaction price, including the constraint on variable consideration; |
| | ● | allocation of the transaction price to the performance obligations based on estimated selling prices; and |
| | ● | recognition of revenue when (or as) the Company satisfies each performance obligation. A performance obligation is a promise to transfer a distinct good or service to the customer and is the unit of account in ASC 606. |
Shipping and Handling Costs. Shipping and handling costs were approximately $1,006 and $349 for the fiscal years ended June 30, 2023 and 2022, respectively, and are included in cost of sales in the accompanying Consolidated Statements of Operations.
Stock-Based Compensation. The Company has two stock-based compensation plans that have outstanding options issued in accordance with such plans. The Company periodically grants stock options to employees and directors in accordance with the provisions of its stock option plans, with the exercise price of the stock options being set at the closing market price of the common stock on the date of grant. Stock based compensation expense is recognized based on the estimated fair value, utilizing a Black-Scholes option pricing model, of the instrument on the date of grant over the requisite vesting period, which is generally three years.
Income Taxes. The Company accounts for income taxes using the asset and liability method. Accordingly, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in the tax rate is recognized in income or expense in the period that the change is effective. Tax benefits are recognized when it is probable that the deduction will be sustained. A valuation allowance is established when it is more likely than not that all or a portion of a deferred tax asset will not be realized.
The Company files a U.S. federal income tax return as well as returns for various states. The Company’s income taxes have not been examined by any tax authorities for the periods subject to review by such taxing authorities, except for the State of New Jersey tax filings for MDC and CII which were reviewed by the State of New Jersey for the then open tax periods of 2014 through 2017 and 2016 to 2019, respectively. Uncertain tax positions, if any, taken on our tax returns are accounted for as liabilities for unrecognized tax benefits. The Company recognizes interest and penalties, if any, related to unrecognized tax benefits in general and administrative expenses in the Consolidated Statements of Operations. There were no liabilities recorded for uncertain tax positions at June 30, 2023 or 2022.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
Leases. We determine if an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, other current liabilities, and operating lease liabilities on our consolidated balance sheets. The ROU assets related to Finance leases are included in property and equipment on our consolidated statement of financial condition.
Operating lease ROU assets and operating lease liabilities are recognized based on the present value of the future minimum lease payments over the lease term at commencement date. As most of our leases do not provide an implicit rate, we use our incremental borrowing rate based on the information available at commencement date in determining the present value of future payments. The operating lease ROU asset also includes any lease payments made and excludes lease incentives and initial direct costs incurred. Our lease terms may include options to extend or terminate the lease when it is reasonably certain that we will exercise that option. Lease expense for minimum lease payments is recognized on a straight-line basis over the lease term.
We have lease agreements with lease and non-lease components, which are generally accounted for separately. For certain equipment leases, such as vehicles, we account for the lease and non-lease components as a single lease component.
Earnings Per Share. Basic earnings per common share amounts are based on weighted average number of common shares outstanding. Diluted earnings per share amounts are based on the weighted average number of common shares outstanding, plus the incremental shares that would have been outstanding upon the assumed exercise of all potentially dilutive stock options, subject to anti-dilution limitations using the treasury stock method.
Fair Value of Financial Instruments. Generally accepted accounting principles require disclosing the fair value of financial instruments to the extent practicable for financial instruments which are recognized or unrecognized in the balance sheet. The fair value of the financial instruments disclosed herein is not necessarily representative of the amount that could be realized or settled, nor does the fair value amount consider the tax consequences of realization or settlement.
In assessing the fair value of financial instruments, the Company uses a variety of methods and assumptions, which are based on estimates of market conditions and risks existing at the time. For certain instruments, including cash and cash equivalents, accounts receivable, accounts payable, and accrued expenses, it was estimated that the carrying amount approximated fair value because of the short maturities of these instruments. All debt is based on current rates at which the Company could borrow funds with similar remaining maturities and approximates fair value.
Accounts Receivable and Allowance for Doubtful Accounts. In the normal course of business, the Company extends credit to customers. Accounts receivable, less the allowance for doubtful accounts, reflect the net realizable value of receivables, and approximate fair value. The Company believes there is no concentration of credit risk with any single customer whose failure or nonperformance would materially affect the Company’s results other than as discussed in Note 9(c) – Significant Risks and Uncertainties – Major Customers. On a regular basis, the Company evaluates its accounts receivables and establishes an allowance for doubtful accounts based on a combination of specific customer circumstances, credit conditions, and historical write-offs and collections. The allowance for doubtful accounts as of June 30, 2023 and 2022 was $37 and $85, respectively. Accounts receivable are charged off against the allowance after management determines that the potential for recovery is remote.
Inventories. Inventories are stated at the lower of cost or net realizable value. Cost is determined using the first-in, first-out method. Allowances for obsolete and overstock inventories are estimated based on “expiration dating” of inventory and projection of sales.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
Property and Equipment. Property and equipment are recorded at cost and are depreciated using the straight line method over the following estimated useful lives:
| Building | 15 Years |
| Leasehold Improvements | Shorter of estimated useful life or term of lease |
| Machinery and Equipment | 7 Years |
| Transportation Equipment | 5 Years |
Impairment of Long-Lived Assets. Long-lived assets are reviewed for impairment when circumstances indicate that the carrying value of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of the assets to the future net cash flows estimated by the Company to be generated by such assets. If such assets are considered to be impaired, the impairment to be recognized is the amount by which the carrying amount of the assets exceeds the fair value of the assets. Assets to be disposed of by sale are recorded as held for sale at the lower of carrying value or estimated net realizable value. Tests for impairment or recoverability are performed at least annually and require significant management judgment and the use of estimates which the Company believes are reasonable and appropriate at the time of the impairment test. Future unanticipated events affecting cash flows and changes in market conditions could affect such estimates and result in the need for an impairment charge. The Company also re-evaluates the periods of amortization to determine whether circumstances warrant revised estimates of current useful lives. No impairment losses were identified or recorded in the fiscal years ended June 30, 2023 and 2022 on the Company’s long-lived assets.
Investment in iBio, Inc. Prior to the adoption of ASU 2016-01 on July 1, 2018, the Company accounted for its investment in iBio, Inc. (“iBio”) common stock on the cost basis as it retained approximately 6% of its interest in iBio (the “iBio Stock”) at the time of the spin-off of this subsidiary in August 2008. The Company reviewed its investment in iBio for impairment and recorded a loss when there was deemed to be a permanent impairment of the investment. To date, there were cumulative impairment charges of approximately $2,562. ASU 2016-01, requires equity investments to be measured at fair value and recognize changes in fair value in net income. During the year ended June 30, 2023 and 2022, the Company recognized realized losses of $35 and $0, respectively and unrealized gain (loss) of $27 and $(55) for the fiscal years ended June 30, 2023 and 2022, respectively. As of June 30, 2023, the Company no longer owns any iBio Stock and as of June 3, 2022, the market value of the iBio Stock was approximately $12, based on the trade price at the close of trading on June 30, 2022. The investment in iBio is included in other current assets in the consolidated balance sheets as of June 30, 2022 at the respective market value.
Accounting Pronouncements Adopted
In June 2016, the FASB issued ASU 2016-13 “Financial Instruments-Credit Losses-Measurement of Credit Losses on Financial Instruments”. This guidance replaces the current incurred loss impairment methodology with a methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information to inform credit loss estimates. The guidance applies to loans, accounts receivable, trade receivables and other financial assets measured at amortized cost, loan commitments, debt securities and beneficial interests in securitized financial assets, but the effect on the Company is projected to be limited to accounts receivable. The guidance was effective for the fiscal year beginning on July 1, 2023, including interim periods within that year. The adoption of this standard did not have a material impact on the Company’s Consolidated Financial Statements.
In May 2019, the FASB issued ASU 2021-05 “Financial Instruments-Credit Losses (Topic 326)” which provides transition relief for companies adopting ASU 2016-13. This guidance amends ASU 2016-13 to allow companies to elect, upon adoption of ASU 2016-13, the fair value option on financial instruments that were previously recorded at amortized cost under certain circumstances. Companies are required to make this election on and instrument by instrument basis. The guidance will be effective for the fiscal year beginning on July 1, 2023, including interim periods within that year. The adoption of this standard did not have a material impact on the Company’s Consolidated Financial Statements.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
In October 2021, the FASB issued ASU 2021-10, Codification Improvements. The guidance contains improvements to the Codification by ensuring that all guidance that requires or provides an option or an entity to provide information in the notes to the financial statements is codified in the Disclosure Section of the Codification. The guidance also contains Codifications that are varied in nature and may affect the application of the guidance in cases in which the original guidance may have been unclear. For public business entities, the amendments in the ASU are effective for annual periods beginning after December 15, 2022, and interim periods within annual periods beginning after December 15, 2023. The adoption of ASU 2021-10 did not have a material impact on its consolidated financial statements.
Note 3. Inventories
Inventories are stated at the lower of cost or net realizable value using the first-in, first-out method and consist of the following:
| |
|
June 30, |
|
| |
|
2023 |
|
|
2022 |
|
| |
|
|
|
|
|
|
|
|
| Raw materials |
|
$ |
6,859 |
|
|
$ |
7,586 |
|
| Work-in-process |
|
|
2,148 |
|
|
|
1,759 |
|
| Finished goods |
|
|
1,254 |
|
|
|
1,440 |
|
| Total |
|
$ |
10,261 |
|
|
$ |
11,055 |
|
Note 4. Property and Equipment, net
Property and equipment consists of the following:
| | | June 30, | |
| | | 2023 | | | 2022 | |
| | | | | | | | | |
| Land and building | | $ | 1,250 | | | $ | 1,250 | |
| Leasehold improvements | | | 1,371 | | | | 1,371 | |
| Machinery and equipment | | | 6,801 | | | | 6,727 | |
| Transportation equipment | | | - | | | | 6 | |
| | | | 9,422 | | | | 9,354 | |
| Less: Accumulated depreciation and amortization | | | (7,769 | ) | | | (7,444 | ) |
| Total | | $ | 1,653 | | | $ | 1,910 | |
Depreciation and amortization expense was $351 and $336 for the fiscal years ended June 30, 2023 and 2022, respectively. Additionally, the Company disposed of fully depreciated property of $26 and $309 in the fiscal years ended June 30, 2023 and 2022, respectively. The Company also recognized a net gain on disposals of $0 and $2 on property, including property in the aggregate amount of $0 and $16 that were not fully depreciated and a gain on the sale of equipment of $1 and $20 in the fiscal years ended June 30, 2023 and 2022, respectively.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
Note 5. Senior Credit Facility
As of June 30, 2023 and 2022, the Company had the following debt outstanding:
| | | Principal Amount | | | Interest Rate | | Maturity Date |
| | | June 30, | | | | | | |
| | | 2023 | | | 2022 | | | | | | |
| Revolving advances under Senior Credit Facility with PNC Bank, National Association | | $ | - | | | $ | 101 | | | | 8.25 | % | 5/15/2024 |
| Total outstanding debt | | $ | - | | | $ | 101 | | | | | | |
SENIOR CREDIT FACILITY
On March 16, 2023, the Company, MDC, AgroLabs, IHT, IHT Properties Corp. (“IHT Properties”) and Vitamin Factory (collectively, the “Borrowers”) amended the Revolving Credit, Term Loan and Security Agreement (the “Amended Loan Agreement”) with PNC Bank, National Association as agent and lender (“PNC”) and the other lenders party thereto entered into on June 27, 2012, as amended on February 19, 2016 and May 15, 2019.
The Amended Loan Agreement provides for a total of $11,585 in senior secured financing (the “Senior Credit Facility”) as follows: (i) discretionary advances (“Revolving Advances”) based on eligible accounts receivable and eligible inventory in the maximum amount of $8,000 (the “Revolving Credit Facility”), and (ii) a term loan in the amount of $3,585 (the “Term Loan”). The Senior Credit Facility is secured by all assets of the Borrowers, including, without limitation, machinery and equipment, real estate owned by IHT Properties, and common stock of iBio owned by the Company. Revolving Advances bear interest at PNC’s Base Rate (8.25% and 4.75% as of June 30, 2023 and 2022, respectively) or the Eurodollar Rate, at Borrowers’ option, plus 2.50%. The Term Loan bore interest at PNC’s Base Rate (5.00% as of June 30, 2022) or the Eurodollar Rate at Borrowers’ option, plus 3.00%. As of September 15, 2023, the Revolving Advance interest rate is 8.50%.
As of March 16, 2023, the Amended Loan Agreement provides that any loans, advances and/or other extensions of credit denominated in U.S. Dollars prior to March 16, 2023 that bear interest or are permitted to bear interest, and have fees, commissions or other amounts based on the London Interbank Offered Rate administered by the ICE Benchmark Administration (which may be referred to as the “Eurodollar Rate” ( “LIBOR”) shall thereafter bear interest based on the Term SOFR Rate plus the SOFR Adjustment . The Term SOFR Rate, for any day, shall be equal to the secured overnight financing rate as administered by the Federal Reserve Bank of New York (or a successor administrator of the secured overnight financing rate). The SOFR Adjustment is defined as 10 basis points (0.10%).
Upon and after the occurrence of any event of default under the Amended Loan Agreement, and during the continuation thereof, interest shall be payable at the interest rate then applicable plus 2%. The Senior Credit Facility matures on May 15, 2024 (the “Senior Maturity Date”).
The principal balance of the Revolving Advances is payable on the Senior Maturity Date, subject to acceleration, based upon a material adverse event clause, as defined, subjective accelerations for borrowing base reserves, as defined or upon the occurrence of any event of default under the Amended Loan Agreement or earlier termination of the Amended Loan Agreement pursuant to the terms thereof. The Term Loan shall be repaid in eighty-four (84) consecutive monthly installments of principal, the first eighty-three (83) of which shall be in the amount of $43, commencing on the first business day of June 2019, and continuing on the first business day of each month thereafter, with a final payment of any unpaid balance of principal and interest payable on the Senior Maturity Date. The foregoing is subject to customary mandatory prepayment provisions and acceleration upon the occurrence of any event of default under the Amended Loan Agreement or earlier termination of the Amended Loan Agreement pursuant to the terms thereof. The Company satisfied all the principal payments required under the Term Note on January 3, 2022.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
The Revolving Advances are subject to the terms and conditions set forth in the Amended Loan Agreement and are made in aggregate amounts at any time equal to the lesser of (x) $8,000 or (y) an amount equal to the sum of: (i) up to 85%, subject to the provisions in the Amended Loan Agreement, of eligible accounts receivables (“Receivables Advance Rate”), plus (ii) up to the lesser of (A) 75%, subject to the provisions in the Amended Loan Agreement, of the value of the eligible inventory (“Inventory Advance Rate” and together with the Receivables Advance Rate, collectively, the “Advance Rates”), (B) 85% of the appraised net orderly liquidation value of eligible inventory (as evidenced by the most recent inventory appraisal reasonably satisfactory to PNC in its sole discretion exercised in good faith) and (C) the inventory sublimit in the aggregate at any one time (“Inventory Advance Rate” and together with the Receivables Advance Rate, collectively, the “Advance Rates”), minus (iii) the aggregate Maximum Undrawn Amount, as defined in the Amended Loan Agreement, of all outstanding letters of credit, minus (iv) such reserves as PNC may reasonably deem proper and necessary from time to time.
The Amended Loan Agreement contains customary mandatory prepayment provisions, including, without limitation the requirement to use any sales proceeds from the sale of iBio Stock to repay the Term Loan and to prepay the outstanding amount of the Term Note in an amount equal to twenty-five percent (25%) of Excess Cash Flow, as defined in the Amended Load Agreement, for each fiscal year commencing with the fiscal year ended June 30, 2016, payable upon delivery of the financial statements to PNC referred to in and required by the Amended Loan Agreement for such fiscal year but in any event not later than one hundred twenty (120) days after the end of each such fiscal year, which amount shall be applied ratably to the outstanding principal installments of the Term Loan in the inverse order of the maturities thereof. The Amended Loan Agreement also contains customary representations and warranties, covenants and events of default, including, without limitation, (i) a fixed charge coverage ratio maintenance requirement and (ii) an event of default tied to any change of control as defined in the Amended Loan Agreement. As of June 30, 2023, the Company was in compliance with the fixed charge coverage ratio maintenance requirement, with the required annual payments of 25% of the Excess Cash Flow for each fiscal year commencing with the fiscal year ended June 30, 2016 and used the proceeds of $96 from the sale of iBio Stock in the fiscal year ended June 30, 2021, to repay the Term Loan. Additionally, with the required annual payment of 25% of Excess Cash Flow for the fiscal year ended June 30, 2021, together with the required monthly installments of $43, the Company satisfied all the remaining principal payments required under the Term Note on January 3, 2022.
In connection with the Senior Credit Facility, the following loan documents were executed: (i) a Stock Pledge Agreement with PNC, pursuant to which the Company pledged to PNC the iBio Stock; (ii) a Mortgage and Security Agreement with PNC with IHT Properties; and (iii) an Environmental Indemnity Agreement with PNC.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
Note 6. Interest Expense
The components of interest expense for the fiscal years ended June 30, 2023 and 2022 are presented below:
| | | For the Fiscal Year Ended June 30, | |
| | | 2023 | | | 2022 | |
| | | | | | | | | |
| Interest on Senior Debt | | $ | 40 | | | $ | 103 | |
| Amortization of prepaid financing costs | | | 12 | | | | 21 | |
| Other interest expense | | | - | | | | 4 | |
| Interest Expense | | $ | 52 | | | $ | 128 | |
The weighted average interest rate paid was 5.67% and 3.33% in the fiscal years ended June 30, 2023 and 2022, respectively. As of June 30, 2023 and 2022, the Company had accrued unpaid interest of approximately $0 and $3, respectively.
Note 7. Income Taxes
The components of the provision for income taxes consists of the following:
| | | For the fiscal year | |
| | | ended June 30, | |
| | | 2023 | | | 2022 | |
| | | | | | | | | |
| Current - Federal tax expense | | $ | (110 | ) | | $ | (600 | ) |
| Current - State tax expense | | | (61 | ) | | | (289 | ) |
| Deferred - Federal benefit | | | 55 | | | | 2,106 | |
| Deferred - State tax (expense) benefit | | | (18 | ) | | | 24 | |
| Income tax (expense) benefit, net | | $ | (134 | ) | | $ | 1,241 | |
A reconciliation of the statutory tax rate to the effective tax rate is as follows:
| | | For the fiscal year | |
| | | ended June 30, | |
| | | 2023 | | | 2022 | |
| Statutory federal income tax rate | | | (21.0 | )% | | | (21.0 | )% |
| State income tax rate | | | (55.1 | )% | | | (11.5 | )% |
| Stock compensation | | | (166.0 | )% | | | (4.6 | )% |
| Change in valuation allowance | | | 201.2 | % | | | 85.6 | % |
| Other temporary differences | | | (62.1 | )% | | | (0.6 | )% |
| Loss on sale of iBio Stock | | | (18.4 | )% | | | - | |
| Non-deductible expenses | | | (12.6 | )% | | | (0.1 | )% |
| Effective income tax rate | | | (134 | )% | | | 47.8 | % |
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
Deferred income taxes reflect the tax effects of temporary differences between the carrying amounts of assets and liabilities for financial accounting purposes and the amounts used for income tax reporting. Significant components of the Company’s net deferred tax assets are as follows:
| | | June 30, | |
| | | 2023 | | | 2022 | |
| Deferred Tax Assets | | | | | | | | |
| Net operating loss | | $ | 5,105 | | | $ | 5,166 | |
| Capital loss carryover | | | 678 | | | | 485 | |
| Depreciation | | | (139 | ) | | | (157 | ) |
| Inventory | | | 180 | | | | 149 | |
| Other | | | 337 | | | | 296 | |
| Valuation allowance | | | (1,435 | ) | | | (1,141 | ) |
| Total deferred tax asset, net | | $ | 4,726 | | | $ | 4,798 | |
The Company has net operating losses (“NOL”) of approximately $21,700 for federal purposes which expire beginning in 2028, which were federal NOLs generated prior to 2018. NOLs generated post 2018 have an indefinite life, subject to 80% of taxable income, there were no NOLs generated post 2018. State NOL’s of approximately $3,997 expire beginning in 2031 through 2039. The Company also has capital loss carryforwards of $2,343 of which $271, $1,152 and $920 will expire in 2025, 2026 and 2028, respectively. The Company files a consolidated U.S. federal income tax return; however, the various state tax returns were filed on a stand-alone basis for the Company and its subsidiaries until the fiscal year ended June 30, 2021, which state income tax return are now filed on a combined basis. MDC has fully utilized its state NOL’s resulting in taxable income on a state level basis for the combined group.
Realization of the NOL carryforwards and other deferred tax temporary differences is contingent on future taxable earnings. The Company’s deferred tax asset was reviewed for expected utilization using a “more likely than not” approach by assessing the available positive and negative evidence surrounding its recoverability. Accordingly, a valuation allowance has been recorded against the Company’s deferred tax asset, as it was determined based upon past taxable losses and inconsistent taxable income in the past few years, that it was “more likely than not” that the Company’s deferred tax assets would not be realized. As of June 30, 2022, management determined that certain of the Company’s deferred tax assets were “more likely than not” to be realizable and the Company recognized deferred tax benefits related to the release of the valuation allowance on those assets of approximately $1,795.
The Company will continue to assess and evaluate strategies that will enable the deferred tax asset, or portion thereof, to be utilized, and will reduce the valuation allowance appropriately at such time when it is determined that the “more likely than not” criteria is satisfied.
There were no significant uncertain tax positions taken, or expected to be taken, in a tax return that would be determined to be an unrecognized tax benefit taken or expected to be taken in a tax return that should have been recorded on the Company’s consolidated financial statements for the year ended June 30, 2023. Additionally, there were no interest or penalties outstanding as of or for each of the fiscal years ended June 30, 2023 and 2022.
The latest three years of Federal and four years of state tax returns filed for the fiscal years ended through June 30, 2023 are currently open except for the State of New Jersey tax filings for CII which have been reviewed for the tax period of 2019. The tax returns for the year ended June 30, 2023 will be filed by March 15, 2024.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
Note 8. Profit-Sharing Plan
The Company maintains a profit-sharing plan, which qualifies under Section 401(k) of the Internal Revenue Code, covering all nonunion employees meeting age and service requirements. Contributions are determined by matching a percentage of employee contributions. For the fiscal years ended June 30, 2023 and 2022, the Company contributed approximately $81 and $86, respectively, into the plan for the benefit of the eligible employees participating in the plan.
Note 9. Significant Risks and Uncertainties
(a) Concentrations of Credit Risk-Cash. The Company maintains balances at several financial institutions. Deposits at each institution are insured by the Federal Deposit Insurance Corporation up to $250. As of June 30, 2023, the Company had $1,949 in uninsured deposits at these financial institutions.
(b) Concentrations of Credit Risk-Receivables. The Company routinely assesses the financial strength of its customers and, based upon factors surrounding the credit risk of its customers, establishes an allowance for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowances is limited. The Company does not require collateral in relation to its trade accounts receivable credit risk.
(c) Major Customers. In the fiscal years ended June 30, 2023 and 2022, approximately 89% and 90% of consolidated net sales, respectively, were derived from two customers. These two customers are in the Company’s Contract Manufacturing Segment and represent approximately 70% and 24% and 67% and 26%, respectively, of this segment’s net sales in the fiscal years ended June 30, 2023 and 2022, respectively. Accounts receivable from these two major customers represented approximately 84% and 70% of total net accounts receivable as of June 30, 2023 and 2022, respectively. Three other customers in the Other Nutraceutical Segment, while not significant customers of the Company’s consolidated net sales, represented approximately 56%, 16% and 9% and 40%, 1% and 19%, respectively, of net sales of the Other Nutraceutical Segment in the fiscal years ended June 30, 2023 and 2022, respectively. The loss of any of these customers could have an adverse effect on the Company’s operations. Major customers are those customers who account for more than 10% of net sales.
(d) Business Risks. The Company insures its business and assets against insurable risks, to the extent that it deems appropriate, based upon an analysis of the relative risks and costs. The Company believes that the risk of loss from non-insurable events would not have a material adverse effect on the Company’s operations as a whole.
The raw materials used by the Company are primarily commodities and agricultural-based products. Raw materials used by the Company in the manufacture of its nutraceutical products are purchased from independent suppliers. Raw materials are available from numerous sources and the Company believes that it will continue to obtain adequate supplies.
As of June 30, 2023, approximately 74% the Company’s employees are covered by a union contract and are employed in its New Jersey facilities. The contract was renewed effective September 1, 2022 and will expire on August 31, 2026.
While the Company hasn’t, to date, seen a significant negative impact in its margins resulting from the coronavirus outbreak, it is experiencing a negative impact on its margins due to inflation and tightened labor markets. The Company may not be able to timely increase its selling prices to its customer resulting from price increases from its suppliers due to various economic factors, including inflation, labor and shipping costs and its own increases in shipping, labor and other operating costs. The Company’s results of operations may also be affected by economic conditions, including inflationary pressures, that can impact consumer disposable income levels and spending habits, thereby reducing the orders it may receive from the Company’s significant customers.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
The Company continues to experience minimal supply chain disruptions relating to fuel refinery and transportation issues as it pertains to shipping. These issues first arose as result of the COVID-19 pandemic and other geo-political events. The significant outbreak of this contagious disease in the human population has resulted in a widespread health crisis that could adversely affect the economies and financial markets of many countries, resulting in an economic downturn that could affect demand for the Company’s products and impact the Company’s operating results.
During the first quarter of calendar 2022, the war in Ukraine affected the Company’s customer’s business operations in Ukraine and Russia, resulting in the cancelation of some future orders. The war resulted in the imposition of sanctions by the United States, the United Kingdom, and the European Union, that affect the cross-border operations of businesses operating in Russia. In addition, many multinational companies ceased or suspended their operations in Russia. Therefore, the ability to continue operations in Russia by the Company’s customers is uncertain. Also, there may be a shortage of Sunflower Oil products in the near future and this may cause delays in production of certain raw materials and may require reformulation of products.
Note 10. Commitments and Contingencies
(a) Leases. The Company has operating and finance leases for its corporate and sales offices, warehousing and packaging facilities and certain machinery and equipment, including office equipment. The Company’s leases have remaining terms of less than 1 year to less than 7 years.
The components of lease expense for the fiscal year ended June 30, 2023 and 2022 were as follows:
| | | 2023 | | | 2022 | |
| | | Related Party - Vitamin Realty | | | Other Leases | | | Totals | | | Related Party - Vitamin Realty | | | Other Leases | | | Totals | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Operating Lease Costs | | $ | 842 | | | $ | 88 | | | $ | 650 | | | $ | 566 | | | $ | 84 | | | $ | 650 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Finance Operating Lease Costs: | | | | | | | | | | | | | | | | | | | | | | | | |
| Amortization of right-of use assets | | $ | - | | | $ | 12 | | | $ | 12 | | | $ | - | | | $ | 3 | | | $ | 3 | |
| Total Finance Lease Costs | | $ | - | | | $ | 12 | | | $ | 12 | | | $ | - | | | $ | 3 | | | $ | 3 | |
Rent and lease amortization costs are included in cost of sales and selling and administrative expenses in the accompanying Condensed Consolidated Statements of Operations.
Operating Lease Liabilities
Related Party Operating Lease Liabilities. Warehouse and office facilities are leased from Vitamin Realty Associates, LLC (“Vitamin Realty”), which is 100% owned by the estate of the Company’s former chairman, and a major stockholder and certain of his family members, who are the Co-Chief Executive Officers and directors of the Company. On January 5, 2012, MDC entered into a second amendment of lease (the “Second Lease Amendment”) with Vitamin Realty for its office and warehouse space in New Jersey increasing its rentable square footage from an aggregate of 74,898 square feet to 76,161 square feet and extending the expiration date to January 31, 2026. This Second Lease Amendment provided for minimum annual rental payments of $533, plus increases in real estate taxes and building operating expenses. On July 15, 2022, MDC entered into a third amendment of the lease (the “Third Lease Amendment”) with Vitamin Realty, increasing its rentable square footage to 116,175. This Third Lease Amendment provided for minimum annual rental payments of $842, plus increases in real estate taxes and the building operating expenses allocation percentage and is effective as of July 1, 2022.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
On May 19, 2014, AgroLabs entered into an amendment to the lease agreement entered into on January 5, 2012, with Vitamin Realty for an additional 2,700 square feet of warehouse space in New Jersey, the term of which was to expire on January 31, 2020 to extend the expiration date to June 1, 2024. This additional lease provided for minimum lease payments of $27 with annual increases plus the proportionate share of operating expenses. The AgroLabs Lease was mutually terminated on July 15, 2022 with an effective date of July 1, 2022.
Rent expense, lease amortization costs and imputed interest costs on these related party leases were $1,269 and $896 for the fiscal years ended June 30, 2023 and 2022, respectively, and are included in cost of sales and selling and administrative expenses in the accompanying Consolidated Statements of Operations. As of June 30, 2023 and 2022, the Company had outstanding current obligations to Vitamin Realty of $0 and $72, respectively, included in accounts payable in the accompanying Consolidated Balance Sheets. Additionally, as of June 30, 2023 and 2022, the Company has operating lease obligations of $2,061 and $1,841, respectively, with Vitamin Realty as noted in the accompany Consolidated Balance Sheet.
Other Operating Lease Liabilities. The Company has entered into certain non-cancelable operating lease agreements expiring up through May 2023, related to machinery and equipment and office equipment.
As of June 30, 2023, the Company’s ROU assets, lease obligations and remaining cash commitment on these leases is as follows:
| | | Right-of-use Assets | | | Current Portion Operating Lease Obligations | | | Operating Lease Obligations | | | Remaining Cash Commitment | |
| | | | | | | | | | | | | | | | | |
| Vitamin Realty Leases | | $ | 2,061 | | | $ | 772 | | | $ | 1,289 | | | $ | 1,972 | |
| Warehouse Lease | | | 541 | | | | 108 | | | | 433 | | | | 631 | |
| Office equipment leases | | | 21 | | | | 8 | | | | 13 | | | | 23 | |
| | | $ | 2,623 | | | $ | 888 | | | $ | 1,735 | | | $ | 2,830 | |
As of June 30, 2022, the Company’s ROU assets, lease obligations and remaining cash commitment on these leases is as follows:
| | | Right-of-use Assets | | | Current Portion Operating Lease Obligations | | | Operating Lease Obligations | | | Remaining Cash Commitment | |
| | | | | | | | | | | | | | | | | |
| Vitamin Realty Leases | | $ | 1,839 | | | $ | 503 | | | $ | 1,338 | | | $ | 1,972 | |
| Office equipment leases | | | 28 | | | | 7 | | | | 21 | | | | 32 | |
| | | $ | 1,867 | | | $ | 510 | | | $ | 1,359 | | | $ | 2,004 | |
As of June 30, 2023 and 2022, the Company’s weighted average discount rate is 4.41% and 3.87%, for the fiscal years then ended, respectively, and the remaining term on lease liabilities is approximately 2.9 years and 3.5 years, respectively.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
Financed Lease Obligation.
On June 15, 2022, the Company entered into a financed lease obligation with LEAF Capital Funding, LLC in the amount of $85, which lease is secured by certain machinery and equipment and matures on August 15, 2024. The lease payment in the amount of approximately $4 is payable monthly, beginning September 15, 2023 and has a stated interest rate of 0.0%.
As of each June, 2023 and 2022, the Company’s weighted average discount rate for the outstanding finance lease obligation is 0% and the remaining term on finance lease obligation is approximately 1.2 years and 2.2 years, respectively. The ROU asset related to the finance lease obligation is included in Property and equipment, net in the accompanying Consolidated Balance Sheet.
Supplemental cash flows information related to leases for the fiscal year ended June 30, 2023 is as follows:
| | | Related Party - Vitamin Realty | | | Other Leases | | | Totals | |
| | | | | | | | | | | | | |
| Cash paid for amounts included in the measurement of lease liabilities: | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Operating cash flows from operating leases | | $ | 842 | | | $ | 147 | | | $ | 989 | |
| Operating cash flows from finance lease obligations | | | - | | | | - | | | | - | |
| Financing cash flows from finance lease obligations | | | - | | | | 35 | | | | 35 | |
Supplemental cash flows information related to leases for the fiscal year ended June 30, 2022 is as follows:
| | | Related Party - Vitamin Realty | | | Other Leases | | | Totals | |
| | | | | | | | | | | | | |
| Cash paid for amounts included in the measurement of lease liabilities: | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Operating cash flows from operating leases | | $ | 566 | | | $ | 84 | | | $ | 650 | |
| Operating cash flows from finance lease obligations | | | - | | | | - | | | | - | |
| Financing cash flows from finance lease obligations | | | - | | | | - | | | | - | |
In the fiscal year ended June 30, 2023, in addition to the Third Amendment with Vitamin Realty the Company renewed, for one year, an operating lease for office space with an annual commitment of $10 and entered into a five year lease for additional warehouse space of 12,500 square feet with an annual commitment of $134 in the first year increasing to $150 in the fifth year of the lease (the “Warehouse Lease”). The Warehouse Lease includes additional rent of not less than $1 per month for the Company’s pro rata portion of the lessor’s operating expenses and commenced on December 1, 2022. Additionally, on August 4, 2023, the Company entered into a four year operating lease for transportation equipment with an annual commitment of $21.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
Maturities of operating lease liabilities as of June 30, 2023 were as follows:
| Year ending June 30, | | Operating Lease Commitment | | | Related Party Operating Lease Commitment | | | Finance Lease Obligation | | | Total | |
| | | | | | | | | | | | | | | | | |
| 2024 | | $ | 146 | | | $ | 842 | | | $ | 42 | | | $ | 1,030 | |
| 2025 | | | 149 | | | | 842 | | | | 7 | | | | 998 | |
| 2026 | | | 148 | | | | 492 | | | | - | | | | 640 | |
| 2027 | | | 148 | | | | - | | | | - | | | | 148 | |
| 2028 | | | 63 | | | | - | | | | - | | | | 63 | |
| Total minimum lease payments | | | 654 | | | | 2,176 | | | | 49 | | | | 2,879 | |
| Imputed interest | | | (92 | ) | | | (115 | ) | | | - | | | | (207 | ) |
| Total | | $ | 562 | | | $ | 2,061 | | | $ | 49 | | | $ | 2,672 | |
(b) Legal Proceedings.
The Company is subject, from time to time, to claims by third parties under various legal theories. The defense of such claims, or any adverse outcome relating to any such claims, could have a material adverse effect on the Company’s liquidity, financial condition and cash flows.
Note 11. Related Party Transactions
See Note 10(a) - Leases for related party lease transactions.
Note 12. Equity Transactions and Stock-Based Compensation
Stock Option Plans. The Company has adopted a stock option plan for the granting of options or restricted shares to employees, officers, directors and consultants of the Company that originally provided for the issuance of up to 7,000,000 shares of common stock, at the discretion of the Board of Directors. Subsequent to the adoption, the Board of Directors and stockholders approved additional common stock shares aggregating 6,000,000 to be available for grant, for a total of 13,000,000 shares of common stock reserved for issuance under the Company’s 2001 Stock Option Plan, as amended (the “Plan”). The Company also has a 1997 Stock Option Plan with 5,000,000 shares of common stock reserved for issuance. Stock option grants may not be priced less than the fair market value of the Company’s common stock at the date of grant. Options granted are generally for ten-year periods, except that incentive stock options granted to a 10% stockholder (as defined) are limited to five-year terms. As of June 30, 2023, the Company has 6,823,569 shares of common stock remaining under the Plans.
In the fiscal year ended June 30, 2023, the Board of Directors authorized the issuance of up to 538,500 stock options to Company officers and employees. The Company issued 527,000 stock options with an exercise price ranging from $0.41 to $0.45, vesting over three years, with expiration terms of either five or ten years from the date of grant. For the fiscal years ended June 30, 2023 and 2022, the Company incurred stock-based compensation expense of $320 and $369, respectively. The Company expects to record additional stock-based compensation of $480 over the remaining estimated vesting period of approximately two and half years.
In July 2023, the Board of Directors authorized the issuance of 200,000 stock options to the non-executive directors of (50,000 each) with an exercise price of $0.33, vesting over one year, 25% at the end of each quarter ending September 30, 2023, December 21, 2023, March 31, 2024 and June 30, 2024.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
The Company used the following assumptions to calculate the fair value of the stock option grants using the Black-Scholes option pricing model on the measurement date during the year ended June 30, 2023:
| Risk Free Interest Rate | | | 3.85% to 3.00 | % |
| Volatility | | | 103.3% to 116.9 | % |
| Term | | | 4.5 to 7.5 years | |
| Dividend Rate | | | 0.00 | % |
| Closing Price of Common Stock | | $ | 0.41 | |
The Company calculates expected volatility for a stock-based grant based on historic daily stock price observations of its common stock during the period immediately preceding the grant that is equal in length to the expected term of the grant. The expected term of the options is estimated based on the Company’s historical exercise rate and forfeiture rates are estimated based on employment termination experience. The risk free interest rate is based on U.S. Treasury yields for securities in effect at the time of grants with terms approximating the term of the grants. The assumptions used in the Black-Scholes option valuation model are highly subjective, and can materially affect the resulting valuations.
In the fiscal year ended June 30, 2022, the Board of Directors authorized the issuance of 764,700 stock options to Company officers, employees and directors with an exercise price of $0.51, $0.95 or $1.045, vesting over one or three years, with terms of either five or ten years.
In the fiscal years ended June 30, 2023 and 2022, stock options in the aggregate amount of 4,376,284 and 537,600, were not included in the computation of weighted average diluted common shares outstanding as the effect of doing so would have been anti-dilutive.
The intrinsic value of options outstanding and exercisable at June 30, 2023 and 2022 was $461 and $1,002, respectively.
A summary of the Company’s stock option activity, and related information for the years ended June 30, follows:
| | | | | | | Weighted | |
| | | | | | | Average | |
| | | | | | | Exercise | |
| | | Options | | | Price | |
| | | | | | | | | |
| Outstanding as of July 1, 2021 | | | 3,916,666 | | | $ | 0.26 | |
| Granted | | | 764,700 | | | | 0.85 | |
| Exercised | | | (146,333 | ) | | | 0.23 | |
| Terminated | | | (44,700 | ) | | | 0.53 | |
| Expired | | | (46,400 | ) | | | 0.47 | |
| Outstanding as of June 30, 2022 | | | 4,443,933 | | | | 0.36 | |
| Granted | | | 527,000 | | | | 0.42 | |
| Exercised | | | - | | | | - | |
| Terminated | | | - | | | | - | |
| Expired | | | (594,649 | ) | | | 0.46 | |
| Outstanding as of June 30, 2023 | | | 4,376,284 | | | $ | 0.35 | |
| | | | | | | | | |
| Exercisable at June 30, 2022 | | | 3,524,500 | | | $ | 0.26 | |
| Exercisable at June 30, 2023 | | | 3,626,551 | | | $ | 0.30 | |
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share amounts)
The following table summarizes the range of exercise prices and weighted-average exercise prices for stock options outstanding and exercisable as of June 30, 2023 under the Company’s stock option plans:
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | Weighted | |
| | | | | | | | | | Weighted | | Weighted Average | | | | Average | |
| | | | | | Average | | Remaining | | | | Exercise | |
| Range of Exercise Price | | Outstanding | | Exercise Price | | Contractual Life (years) | Exercisable | | Price | |
| | | | | | | | | | | | | | | | | | | |
| $ | 0.09 | - | $ | 0.10 | | | 1,221,000 | | $ | 0.09 | | 1.9 | | 1,296,000 | | $ | 0.09 | |
| $ | 0.21 | - | $ | 0.21 | | | 1,194,000 | | | 0.21 | | 5.9 | | 1,209,000 | | | 0.21 | |
| $ | 0.23 | - | $ | 0.25 | | | 250,000 | | | 0.23 | | 3.4 | | 250,000 | | | 0.23 | |
| $ | 0.41 | - | $ | 0.41 | | | 426,500 | | | 0.41 | | 9.4 | | - | | | 0.00 | |
| $ | 0.51 | - | $ | 0.51 | | | 200,000 | | | 0.51 | | 9.0 | | 200,000 | | | 0.51 | |
| $ | 0.65 | - | $ | 0.65 | | | 570,667 | | | 0.65 | | 7.4 | | 431,000 | | | 0.65 | |
| $ | 0.72 | - | $ | 0.72 | | | 66,667 | | | 0.72 | | 0.7 | | 66,667 | | | 0.72 | |
| $ | 0.95 | - | $ | 0.95 | | | 425,783 | | | 0.95 | | 8.4 | | 242,217 | | | 0.95 | |
| $ | 1.05 | - | $ | 1.05 | | | 21,667 | | | 1.05 | | 0.7 | | 21,667 | | | 1.05 | |
| $ | 0.09 | - | $ | 1.05 | | | 4,376,284 | | $ | 0.35 | | 7.0 | | 3,626,551 | | $ | 0.30 | |
Note 13. Segment Information
The basis for presenting segment results generally is consistent with overall Company reporting. The Company reports information about its operating segments in accordance with GAAP which establishes standards for reporting information about a company’s operating segments.
The Company has divided its operations into two reportable segments; Contract Manufacturing and Other Nutraceutical Businesses. The international sales, concentrated primarily in Europe, for the fiscal years ended June 30, 2023 and 2022 were $8,055 and $9,729, respectively.
Financial information relating to the fiscal years ended June 30, 2023 and 2022 operations by business segment are as follows:
| | | | Sales, Net | | | Segment | | | | | | | | | | | | | |
| | | | U.S. | | | International | | | | | | | Gross | | | | | | | Capital | | | Total | |
| | | | Customers | | | Customers | | | Total | | | Profit | | | Depreciation | | | Expenditures | | | Assets | |
| Contract Manufacturing | 2023 | | $ | 40,230 | | | $ | 8,047 | | | $ | 48,277 | | | $ | 3,630 | | | $ | 348 | | | $ | 109 | | | $ | 19,507 | |
| | 2022 | | | 44,450 | | | | 9,641 | | | | 54,091 | | | | 5,917 | | | | 332 | | | | 486 | | | | 19,061 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Other Nutraceutical Businesses | 2023 | | | 2,387 | | | | 8 | | | | 2,395 | | | | 431 | | | | 3 | | | | 7 | | | | 5,924 | |
| | 2022 | | | 2,067 | | | | 88 | | | | 2,155 | | | | 635 | | | | 4 | | | | - | | | | 6,189 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total Company | 2023 | | | 42,617 | | | | 8,055 | | | | 50,672 | | | | 4,061 | | | | 351 | | | | 116 | | | | 25,431 | |
| | 2022 | | | 46,517 | | | | 9,729 | | | | 56,246 | | | | 6,552 | | | | 336 | | | | 486 | | | | 25,250 | |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Company has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| |
INTEGRATED BIOPHARMA, INC. |
| |
|
| |
|
| Date: September 15, 2023 |
By: /s/ Christina Kay |
| |
Christina Kay |
| |
Co-Chief Executive Officer |
| |
|
| Date: September 15, 2023 |
By: /s/ Dina L. Masi |
| |
Dina L. Masi |
| |
Chief Financial Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons in the capacities and on the dates indicated.
| Name |
|
Title |
|
Date |
| |
|
|
|
|
| /s/ Christina Kay |
|
Co-Chief Executive Officer and Director |
|
September 15, 2023 |
| Christina Kay |
|
(Principal Executive Officer) |
|
|
| |
|
|
|
|
| /s/ Riva Sheppard |
|
Co-Chief Executive Officer and Director |
|
September 15, 2023 |
| Riva Sheppard |
|
(Principal Executive Officer) |
|
|
| |
|
|
|
|
| /s/ Dina L. Masi |
|
Chief Financial Officer |
|
September 15, 2023 |
| Dina L. Masi |
|
(Principal Financial Officer and |
|
|
| |
|
Principal Accounting Officer) |
|
|
| |
|
|
|
|
| /s/ Robert Canarick |
|
Director |
|
September 15, 2023 |
| Robert Canarick |
|
|
|
|
| |
|
|
|
|
| /s/ Damon DeSantis |
|
Director |
|
September 15, 2023 |
| Damon DeSantis |
|
|
|
|
| |
|
|
|
|
| /s/ William H. Milmoe |
|
Director |
|
September 15, 2023 |
| William H. Milmoe |
|
|
|
|
| |
|
|
|
|
| /s/ Eric Friedman |
|
Director |
|
September 15, 2023 |
| Eric Friedman |
|
|
|
|
| |
|
|
|
|
EXHIBIT 21
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
SUBSIDIARIES OF THE REGISTRANT
|
Subsidiary Name
|
State of Incorporation
|
|
|
|
|
Manhattan Drug Company, Inc.
|
New Jersey
|
|
AgroLabs, Inc.
|
New Jersey
|
|
IHT Health Products, Inc.
|
Delaware
|
|
Vitamin Factory, Inc.
|
Delaware
|
|
IHT Properties, Inc.
|
Delaware
|
|
MDC Warehousing and Distribution, Inc. (f/k/a The Organic Beverage Company)
|
New Jersey
|
|
InB:Paxis Pharmaceuticals, Inc. (f/k/a Paxis Pharmaceuticals, Inc.) - inactive
|
Delaware
|
Exhibit 23.1
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM’S CONSENT
We consent to the incorporation by reference in the Registration Statements of Integrated BioPharma, Inc. on Form S-8 (File Nos. 333-229069, 333-37509, 333-87456 and 333-87458) and Form S-3 (File Nos. 333-144155 and 333-149855) of our report dated September 15, 2023, with respect to our audits of the consolidated financial statements of Integrated BioPharma, Inc. as of June 30, 2023 and for the year ended June 30, 2023, which report is included in this Annual Report on Form 10-K of Integrated BioPharma, Inc. for the year ended June 30, 2023.
/s/ Marcum LLP
Marcum LLP
East Hanover, NJ
September 15, 2023
EXHIBIT 23.2
CONSENT OF INDEPENDENT REGISTERED ACCOUNTANTING FIRM
We hereby consent to the incorporation by reference in the Registration Statements of Integrated BioPharma, Inc. on Form S-8 (File Nos. 333-229069, 333-37509, 333-87456 and 333-87458) and Form S-3 (File Nos. 333-144155 and 333-149855) of our report dated September 13, 2022, with respect to our audit of the consolidated financial statements as of June 30, 2022, and for the year ended June 30, 2022, which was included in the Company’s Annual Report on Form 10-K filed on September 15, 2023. We were dismissed as auditors on September 21, 2022 and, accordingly, we have not performed any audit or review procedures with respect to any financial statements incorporated by reference for the periods after the date of our dismissal.
|
/s/ Friedman LLP
|
| |
|
East Hanover, NJ
|
| |
|
September 15, 2023
|
Exhibit 31.1
CERTIFICATION
Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
We, Christina Kay and Riva Sheppard, Co-Chief Executive Officers, certify that:
|
|
1.
|
We have reviewed this annual report on Form 10-K of Integrated BioPharma, Inc.;
|
|
|
2.
|
Based on our knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
|
|
|
3.
|
Based on our knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
|
|
|
4.
|
The registrant's other certifying officer(s) and we are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
|
|
|
a)
|
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; and
|
|
|
b)
|
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
|
|
|
c)
|
Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
|
|
|
d)
|
Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
|
|
|
5.
|
The registrant's other certifying officer(s) and we have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors:
|
|
|
a)
|
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and
|
|
|
b)
|
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting.
|
|
Date: September 15, 2023
|
By: /s/ Christina Kay
|
|
|
Name: Christina Kay
|
|
|
Title: Co-Chief Executive Officer
|
| |
|
|
Date: September 15, 2023
|
By: /s/ Riva Sheppard
|
|
|
Name: Riva Sheppard
|
|
|
Title: Co-Chief Executive Officer
|
| |
|
| |
|
| |
|
Exhibit 31.2
CERTIFICATION
Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Dina L. Masi, Senior Vice President & Chief Financial Officer, certify that:
|
|
1.
|
I have reviewed this annual report on Form 10-K of Integrated BioPharma, Inc.;
|
|
|
2.
|
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
|
|
|
3.
|
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
|
|
|
4.
|
The registrant's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
|
|
|
a)
|
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; and
|
|
|
b)
|
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
|
|
|
c)
|
Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
|
|
|
d)
|
Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
|
|
|
5.
|
The registrant's other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors:
|
|
|
a)
|
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and
|
|
|
b)
|
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting.
|
|
Date: September 15, 2023
|
By: /s/ Dina L. Masi
|
|
|
Name: Dina L. Masi
|
|
|
Title: Senior Vice President & Chief Financial Officer
|
Exhibit 32.1
CERTIFICATION
As adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
In connection with the Annual Report on Form 10-K for the fiscal year ended June 30, 2023 of Integrated BioPharma, Inc. (the “Company”) as filed with the Securities and Exchange Commission on the date hereof (the “Report”), Christina Kay and Riva Sheppard, Co-Chief Executive Officers of Integrated BioPharma, Inc. (the "Company"), certifies, pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, 18 U.S.C. Section 1350, that to their knowledge:
|
(1)
|
the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (15 U.S.C. 78m or 78o(d)); and
|
|
(2)
|
the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
This certification accompanies the Report pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 and shall not, except to the extent required by the Sarbanes-Oxley Act of 2002, be deemed filed by the Company for purposes of Section 18 of the Securities and Exchange Act of 1934, as amended.
A signed original of this written statement has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.
|
Date: September 15, 2023
|
By: /s/ Christina Kay
|
|
|
Christina Kay
|
|
|
Co-Chief Executive Officer
|
| |
|
|
Date: September 15, 2023
|
By: /s/ Riva Sheppard
|
|
|
Riva Sheppard
|
|
|
Co-Chief Executive Officer
|
| |
|
| |
|
| |
|
Exhibit 32.2
CERTIFICATION OF PERIODIC REPORT
As adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
In connection with the Annual Report on Form 10-K for the fiscal year ended June 30, 2023 of Integrated BioPharma, Inc. (the “Company”) as filed with the Securities and Exchange Commission on the date hereof (the “Report”), Dina L. Masi, the Senior Vice President and Chief Financial Officer of Integrated BioPharma, Inc. (the "Company"), certifies, pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, 18 U.S.C. Section 1350, that to her knowledge:
|
(1)
|
the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (15 U.S.C. 78m or 78o(d)); and
|
|
(2)
|
the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
This certification accompanies the Report pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 and shall not, except to the extent required by the Sarbanes-Oxley Act of 2002, be deemed filed by the Company for purposes of Section 18 of the Securities and Exchange Act of 1934, as amended.
A signed original of this written statement has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.
|
Date: Date: September 15, 2023
|
By: /s/ Dina L. Masi
|
|
|
Dina L. Masi
|
|
|
Senior Vice President and Chief Financial Officer
|
v3.23.2
Document And Entity Information - USD ($)
|
12 Months Ended |
|
|
Jun. 30, 2023 |
Sep. 15, 2023 |
Dec. 31, 2022 |
| Document Information [Line Items] |
|
|
|
| Entity Central Index Key |
0001016504
|
|
|
| Entity Registrant Name |
INTEGRATED BIOPHARMA INC
|
|
|
| Amendment Flag |
false
|
|
|
| Current Fiscal Year End Date |
--06-30
|
|
|
| Document Fiscal Period Focus |
FY
|
|
|
| Document Fiscal Year Focus |
2023
|
|
|
| Document Type |
10-K
|
|
|
| Document Annual Report |
true
|
|
|
| Document Period End Date |
Jun. 30, 2023
|
|
|
| Document Transition Report |
false
|
|
|
| Entity File Number |
001-31668
|
|
|
| Entity Incorporation, State or Country Code |
DE
|
|
|
| Entity Tax Identification Number |
22-2407475
|
|
|
| Entity Address, Address Line One |
225 Long Ave.
|
|
|
| Entity Address, City or Town |
Hillside
|
|
|
| Entity Address, State or Province |
NJ
|
|
|
| Entity Address, Postal Zip Code |
07205
|
|
|
| City Area Code |
888
|
|
|
| Local Phone Number |
319-6962
|
|
|
| Title of 12(g) Security |
Common Stock, $.002 par value per share
|
|
|
| Entity Well-known Seasoned Issuer |
No
|
|
|
| Entity Voluntary Filers |
No
|
|
|
| Entity Current Reporting Status |
Yes
|
|
|
| Entity Interactive Data Current |
Yes
|
|
|
| Entity Filer Category |
Non-accelerated Filer
|
|
|
| Entity Emerging Growth Company |
false
|
|
|
| Entity Small Business |
true
|
|
|
| ICFR Auditor Attestation Flag |
false
|
|
|
| Document Financial Statement Error Correction [Flag] |
false
|
|
|
| Entity Shell Company |
false
|
|
|
| Entity Public Float |
|
|
$ 3,258,720
|
| Entity Common Stock, Shares Outstanding |
|
29,949,610
|
|
| Auditor Firm ID |
688
|
|
|
| Auditor Name |
Marcum LLP
|
|
|
| Auditor Location |
East Hanover, New Jersey
|
|
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPCAOB issued Audit Firm Identifier Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorFirmId |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:nonemptySequenceNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorLocation |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an annual report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentAnnualReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates whether any of the financial statement period in the filing include a restatement due to error correction. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection w
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 4: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentFinStmtErrorCorrectionFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter.
| Name: |
dei_EntityPublicFloat |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
| Name: |
dei_EntityVoluntaryFilers |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Is used on Form Type: 10-K, 10-Q, 8-K, 20-F, 6-K, 10-K/A, 10-Q/A, 20-F/A, 6-K/A, N-CSR, N-Q, N-1A. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 405
| Name: |
dei_EntityWellKnownSeasonedIssuer |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_IcfrAuditorAttestationFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(g) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection g
| Name: |
dei_Security12gTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Consolidated Statements of Operations - USD ($)
$ in Thousands |
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Sales, net |
$ 50,672
|
$ 56,246
|
| Cost of sales |
46,611
|
49,694
|
| Gross profit |
4,061
|
6,552
|
| Selling and administrative expenses |
3,941
|
3,807
|
| Operating income |
120
|
2,745
|
| Other income (expense), net: |
|
|
| Interest expense |
(52)
|
(128)
|
| Unrealized gain (loss) on investment in iBio Stock |
27
|
(55)
|
| Realized loss on sale of investment in iBio Stock |
(35)
|
0
|
| Other income |
40
|
35
|
| Total other income (expense), net |
(20)
|
(148)
|
| Income before income taxes |
100
|
2,597
|
| Income tax (expense) benefit, net |
(134)
|
1,241
|
| Net (loss) income |
$ (34)
|
$ 3,838
|
| Basic net (loss) income per common share (in dollars per share) |
$ (0.00)
|
$ 0.13
|
| Diluted net (loss) income per common share (in dollars per share) |
$ (0.00)
|
$ 0.12
|
| Weighted average common shares outstanding - basic (in shares) |
29,949,610
|
29,843,386
|
| Add: Equivalent shares outstanding - stock options (in shares) |
0
|
2,477,939
|
| Weighted average common shares outstanding - diluted (in shares) |
29,949,610
|
32,321,326
|
| X |
- DefinitionThe aggregate costs related to goods produced and sold and services rendered by an entity during the reporting period. This excludes costs incurred during the reporting period related to financial services rendered and other revenue generating activities. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479941/924-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.2(a),(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_CostOfGoodsAndServicesSold |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate revenue less cost of goods and services sold or operating expenses directly attributable to the revenue generation activity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.1,2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GrossProfit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAdditional shares included in the calculation of diluted EPS as a result of the potentially dilutive effect of share based payment arrangements using the treasury stock method. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480454/718-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-22
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 23
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-23
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-28A
| Name: |
us-gaap_IncrementalCommonSharesAttributableToShareBasedPaymentArrangements |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of the cost of borrowed funds accounted for as interest expense. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (210.5-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.7)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NonoperatingIncomeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of income (expense) related to nonoperating activities, classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherNonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of realized gain (loss) on investment. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
| Name: |
us-gaap_RealizedInvestmentGainsLosses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, including tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value-added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479941/924-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerIncludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total costs related to selling a firm's product and services, as well as all other general and administrative expenses. Direct selling expenses (for example, credit, warranty, and advertising) are expenses that can be directly linked to the sale of specific products. Indirect selling expenses are expenses that cannot be directly linked to the sale of specific products, for example telephone expenses, Internet, and postal charges. General and administrative expenses include salaries of non-sales personnel, rent, utilities, communication, etc. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_SellingGeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of unrealized gain (loss) on investment. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_UnrealizedGainLossOnInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Consolidated Balance Sheets - USD ($)
$ in Thousands |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Current Assets: |
|
|
| Cash |
$ 1,316
|
$ 331
|
| Accounts receivable, net |
4,511
|
4,888
|
| Inventories |
10,261
|
11,055
|
| Other current assets |
284
|
352
|
| Total current assets |
16,372
|
16,626
|
| Property and equipment, net |
1,653
|
1,910
|
| Operating lease right-of-use assets (includes $2,061 and $1,839 with a related party) |
2,623
|
1,867
|
| Deferred tax assets, net |
4,726
|
4,798
|
| Security deposits and other assets |
57
|
49
|
| Total Assets |
25,431
|
25,250
|
| Current Liabilities: |
|
|
| Advances under revolving credit facility |
0
|
101
|
| Accounts payable (includes $0 and $72 due to a related party) |
2,266
|
3,209
|
| Accrued expenses and other current liabilities |
1,632
|
1,411
|
| Current portion of long term debt, net |
42
|
32
|
| Current portion of operating lease liabilities |
888
|
510
|
| Total current liabilities |
4,828
|
5,263
|
| Long term debt, net |
7
|
53
|
| Operating lease liabilities |
1,735
|
1,359
|
| Total Liabilities |
6,570
|
6,675
|
| Commitments and Contingencies (Note 10) |
|
|
| Stockholders' Equity |
|
|
| Common Stock, $0.002 par value; 50,000,000 shares authorized; 29,984,510 and 29,949,610 shares issued and outstanding, respectively |
60
|
60
|
| Additional paid-in-capital |
51,239
|
50,919
|
| Accumulated deficit |
(32,339)
|
(32,305)
|
| Less: Treasury stock, at cost, 34,900 shares |
(99)
|
(99)
|
| Total Stockholders' Equity |
18,861
|
18,575
|
| Total Liabilities and Stockholders' Equity |
$ 25,431
|
$ 25,250
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allowance for credit loss, of right to consideration from customer for product sold and service rendered in normal course of business, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-9
| Name: |
us-gaap_AccountsReceivableNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expenses incurred but not yet paid nor invoiced, and liabilities classified as other.
| Name: |
us-gaap_AccruedLiabilitiesAndOtherLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.17)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.25)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value of amounts transferred to third parties for security purposes that are expected to be returned or applied towards payment after one year or beyond the operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DepositsAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after valuation and LIFO reserves of inventory expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-5
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.21)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe carrying value as of the balance sheet date of the current portion of long-term obligations drawn from a line of credit, which is a bank's commitment to make loans up to a specific amount. Examples of items that might be included in the application of this element may consist of letters of credit, standby letters of credit, and revolving credit arrangements, under which borrowings can be made up to a maximum amount as of any point in time conditional on satisfaction of specified terms before, as of and after the date of drawdowns on the line. Includes short-term obligations that would normally be classified as current liabilities but for which (a) postbalance sheet date issuance of a long term obligation to refinance the short term obligation on a long term basis, or (b) the enterprise has entered into a financing agreement that clearly permits the enterprise to refinance the short-term obligation on a long term basis and the following conditions are met (1) the agreement does not expire within 1 year and is not cancelable by the lender except for violation of an objectively determinable provision, (2) no violation exists at the BS date, and (3) the lender has entered into the financing agreement is expected to be financially capable of honoring the agreement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(13))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_LinesOfCreditCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt classified as current. Excludes lease obligation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermDebtCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt classified as noncurrent. Excludes lease obligation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermDebtNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of current assets classified as other. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount allocated to previously issued common shares repurchased by the issuing entity and held in treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481520/505-30-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481549/505-30-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.30)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_TreasuryStockCommonValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.2
Consolidated Balance Sheets (Parentheticals) - USD ($)
$ in Thousands |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Operating lease right-of-use assets |
$ 2,623
|
$ 1,867
|
| Accounts payable (includes $0 and $72 due to a related party) |
2,266
|
3,209
|
| Current portion of operating lease liabilities |
888
|
510
|
| Operating lease liabilities |
$ 1,735
|
$ 1,359
|
| Common stock, par value (in dollars per share) |
$ 0.002
|
$ 0.002
|
| Common stock, authorized (in shares) |
50,000,000
|
50,000,000
|
| Common stock, issued (in shares) |
29,984,510
|
29,984,510
|
| Common stock, outstanding (in shares) |
29,949,610
|
29,949,610
|
| Treasury Stock, Common, Shares |
34,900
|
34,900
|
| Vitamin Realty LLC [Member] |
|
|
| Operating lease right-of-use assets |
$ 2,061
|
$ 1,839
|
| Accounts payable (includes $0 and $72 due to a related party) |
0
|
72
|
| Current portion of operating lease liabilities |
772
|
503
|
| Operating lease liabilities |
$ 1,289
|
$ 1,338
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of previously issued common shares repurchased by the issuing entity and held in treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481549/505-30-45-1
| Name: |
us-gaap_TreasuryStockCommonShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.23.2
Consolidated Statements of Stockholders' Equity - USD ($)
$ in Thousands |
Common Stock [Member] |
Additional Paid-in Capital [Member] |
Retained Earnings [Member] |
Treasury Stock, Common [Member] |
Total |
| Balance (in shares) at Jun. 30, 2021 |
29,838,177
|
|
|
34,900
|
|
| Balance at Jun. 30, 2021 |
$ 60
|
$ 50,516
|
$ (36,143)
|
$ (99)
|
$ 14,334
|
| Compensation expense for employee stock options |
$ 0
|
369
|
0
|
$ 0
|
$ 369
|
| Exercised (in shares) |
146,333
|
|
|
0
|
146,333
|
| Shares issued upon exercise of stock options |
$ 0
|
34
|
0
|
$ 0
|
$ 34
|
| Net (loss) income |
0
|
0
|
3,838
|
0
|
3,838
|
| Net income (loss) |
$ 0
|
0
|
3,838
|
$ 0
|
3,838
|
| Balance (in shares) at Jun. 30, 2022 |
29,984,510
|
|
|
34,900
|
|
| Balance at Jun. 30, 2022 |
$ 60
|
50,919
|
(32,305)
|
$ (99)
|
18,575
|
| Compensation expense for employee stock options |
0
|
320
|
0
|
0
|
$ 320
|
| Exercised (in shares) |
|
|
|
|
0
|
| Net (loss) income |
0
|
0
|
(34)
|
0
|
$ (34)
|
| Net income (loss) |
$ 0
|
0
|
(34)
|
$ 0
|
(34)
|
| Balance (in shares) at Jun. 30, 2023 |
29,984,510
|
|
|
34,900
|
|
| Balance at Jun. 30, 2023 |
$ 60
|
$ 51,239
|
$ (32,339)
|
$ (99)
|
$ 18,861
|
| X |
- DefinitionAmount of increase to additional paid-in capital (APIC) for recognition of cost for option under share-based payment arrangement.
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalShareBasedCompensationStockOptionsRequisiteServicePeriodRecognition |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued as a result of the exercise of stock options. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29-31)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
Consolidated Statements of Cash Flows - USD ($)
$ in Thousands |
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Cash flows from operating activities: |
|
|
| Net (loss) income |
$ (34)
|
$ 3,838
|
| Adjustments to reconcile net (loss) income to net cash provided by operating activities: |
|
|
| Amortization of operating lease right-of-use assets |
803
|
498
|
| Depreciation and amortization |
351
|
336
|
| Deferred income taxes (benefit) |
72
|
(1,530)
|
| Accretion of financing instruments, amortization of prepaid financing costs and other |
12
|
21
|
| Compensation expense on employee stock options |
320
|
369
|
| Unrealized (gain) loss on investment in iBio Stock |
(27)
|
55
|
| Realized loss on sale of iBio Stock |
35
|
0
|
| Bad debt expense |
3
|
13
|
| Gain on disposal of fixed assets |
(1)
|
(22)
|
| Changes in operating assets and liabilities: |
|
|
| Accounts receivable |
374
|
875
|
| Inventories |
794
|
640
|
| Prepaid expenses and other assets |
34
|
(84)
|
| Operating lease obligations |
(804)
|
(501)
|
| Accounts payable |
(921)
|
(218)
|
| Accrued expenses and other current liabilities |
221
|
(199)
|
| Net cash provided by operating activities |
1,232
|
4,091
|
| Cash flows from investing activities: |
|
|
| Purchase of property and equipment |
(116)
|
(486)
|
| Proceeds from sale of iBio Stock |
4
|
0
|
| Proceeds from sale of fixed assets |
1
|
21
|
| Net cash used in investing activities |
(111)
|
(465)
|
| Cash flows from financing activities: |
|
|
| Repayments of advances under revolving credit facility, net |
(101)
|
(2,073)
|
| Proceeds from exercises of stock options |
0
|
34
|
| Repayments under term notes payable |
0
|
(1,466)
|
| Repayments under financed lease obligations |
(35)
|
0
|
| Net cash used in financing activities |
(136)
|
(3,505)
|
| Net increase in cash |
985
|
121
|
| Cash at beginning of fiscal year |
331
|
210
|
| Cash at end of fiscal year |
1,316
|
331
|
| Supplemental disclosures of cash flow information: |
|
|
| Interest |
41
|
113
|
| Income taxes |
0
|
471
|
| Supplemental disclosures of non-cash transactions: |
|
|
| Amount owed on purchase of fixed assets |
0
|
22
|
| Financing on financed lease obligation |
0
|
85
|
| Acquisition of right-of-use asset, net and operating lease obligations, net |
1,560
|
0
|
| Trade in value on like-kind exchanges |
$ 0
|
$ 7
|
| X |
- DefinitionThe increase (decrease) during the reporting period in operating lease liabilities.
| Name: |
inbp_IncreaseDecreaseInOperatingLeaseLiabilities |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionRepresents trade in value on like-kind exchanges.
| Name: |
inbp_TradeInValueOnLikekindExchanges |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFuture cash outflow to pay for purchases of fixed assets that have occurred. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-5
| Name: |
us-gaap_CapitalExpendituresIncurredButNotYetPaid |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in lease obligation from new lease. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
| Name: |
us-gaap_CapitalLeaseObligationsIncurred |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including, but not limited to, disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsIncludingDisposalGroupAndDiscontinuedOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_CashFlowNoncashInvestingAndFinancingActivitiesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate expense recognized in the current period that allocates the cost of tangible assets, intangible assets, or depleting assets to periods that benefit from use of the assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
| Name: |
us-gaap_DepreciationDepletionAndAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow for principal payment on finance lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-5
| Name: |
us-gaap_FinanceLeasePrincipalPayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of gain (loss) on sale or disposal of property, plant and equipment assets, including oil and gas property and timber property. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_GainLossOnSaleOfPropertyPlantEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in amount due within one year (or one business cycle) from customers for the credit sale of goods and services. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the period in the carrying value of derivative instruments reported as liabilities that are due to be disposed of within one year (or the normal operating cycle, if longer). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInDerivativeLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate value of all inventory held by the reporting entity, associated with underlying transactions that are classified as operating activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInInventories |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in other expenses incurred but not yet paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInOtherAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in current assets classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInOtherCurrentAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash paid for interest, excluding capitalized interest, classified as operating activity. Includes, but is not limited to, payment to settle zero-coupon bond for accreted interest of debt discount and debt instrument with insignificant coupon interest rate in relation to effective interest rate of borrowing attributable to accreted interest of debt discount. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-17
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-2
| Name: |
us-gaap_InterestPaidNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of periodic reduction over lease term of carrying amount of right-of-use asset from operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_OperatingLeaseRightOfUseAssetAmortizationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquirePropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the sale, maturity and collection of all investments such as debt, security and so forth during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-12
| Name: |
us-gaap_ProceedsFromSaleMaturityAndCollectionsOfInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the sale of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 12
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-12
| Name: |
us-gaap_ProceedsFromSaleOfPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from exercise of option under share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2A
-Subparagraph (a)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2A
| Name: |
us-gaap_ProceedsFromStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (reversal of expense) for expected credit loss on accounts receivable. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479319/326-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_ProvisionForDoubtfulAccounts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of realized gain (loss) on investment. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
| Name: |
us-gaap_RealizedInvestmentGainsLosses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow for payment of an obligation from a lender, including but not limited to, letter of credit, standby letter of credit and revolving credit arrangements. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(f))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_RepaymentsOfLinesOfCredit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for a borrowing supported by a written promise to pay an obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_RepaymentsOfNotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in right-of-use asset obtained in exchange for operating lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_RightOfUseAssetObtainedInExchangeForOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of unrealized gain (loss) on investment. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_UnrealizedGainLossOnInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.2
Note 1 - Business
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Organization, Consolidation and Presentation of Financial Statements Disclosure [Text Block] |
Note 1. Business
Integrated BioPharma, Inc., a Delaware corporation (together with its subsidiaries, the “Company”), is engaged primarily in manufacturing, distributing, marketing and sales of vitamins, nutritional supplements and herbal products. The Company’s customers are located primarily in the United States and Luxembourg. The Company was originally incorporated in the state of Delaware on August 31, 1995 under the name Chem International, Inc., on December 5, 2000, changed its name to Integrated Health Technologies, Inc. and, on January 29, 2003, changed its name to Integrated BioPharma, Inc. The Company restated its certificate of incorporation in Delaware in June 2006. The Company continues to do business as Chem International, Inc. with certain of its customers and certain vendors.
The Company’s business segments include: (a) Contract Manufacturing operated by Manhattan Drug Company, Inc. (“MDC”), which manufactures vitamins and nutritional supplements for sale to distributors, multilevel marketers and specialized health-care providers and (b) Other Nutraceutical Businesses which includes the operations of (i) AgroLabs, Inc. (“AgroLabs”), which distributed healthful nutritional products for sale through major mass market, grocery and drug and vitamin retailers, under the following brands: Peaceful Sleep, Wheatgrass and other products introduced into the market using the AgroLabs name (these are referred to as our branded products), (ii) The Vitamin Factory (the “Vitamin Factory”), which did sell private label MDC products, as well as our AgroLabs products, through the Internet, (iii) IHT Health Products, Inc. (“IHT”) a distributor of fine natural botanicals, including multi minerals produced under a license agreement, (iv) MDC Warehousing and Distribution, Inc., a service provider for warehousing and fulfillment services and (v) Chem International, Inc. (“Chem”), a distributor of certain raw materials for DSM Nutritional Products LLC. The Vitamin Factory had no products available for sale and AgroLabs had no sales of its branded products in the fiscal years ended June 30, 2023 and 2022.
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for organization, consolidation and basis of presentation of financial statements disclosure. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//810/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205/tableOfContent
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 2 - Summary of Significant Accounting Policies
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Significant Accounting Policies [Text Block] |
Note 2. Summary of Significant Accounting Policies
Principles of Consolidation. The accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. Intercompany transactions and accounts have been eliminated in consolidation.
Reclassifications. Certain prior year amounts have been reclassified to conform to the current year presentation.
Use of Estimates. The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. The most significant estimates include: ● sales returns and allowances; ● allowance for doubtful accounts; ● inventory valuation; ● valuation and recoverability of long-lived assets; ● income taxes and valuation allowance on deferred income taxes, and; ● accruals for, and the probability of, the outcome of current litigation, if any. On a continual basis, management reviews its estimates utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such reviews, and if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ from those estimates.
Revenue Recognition. The Company recognizes product sales revenue, the prices of which are fixed and determinable, when title and risk of loss have transferred to the customer, when estimated provisions for product returns, rebates, charge-backs and other sales allowances are reasonably determinable, and when collectability is reasonably assured. Accruals for these items are presented in the consolidated financial statements as reductions to sales. The Company’s net sales represent gross sales invoiced to customers, less certain related charges for discounts, returns, rebates, charge-backs and other allowances. Cost of sales includes the cost of raw materials and all labor and overhead associated with the manufacturing and packaging of the products. Gross margins are affected by, among other things, changes in the relative sales mix among our products and valuation and/or charge off of slow moving, expired or obsolete inventories. To perform revenue recognition for arrangements within the scope of ASC 606, the Company performs the following five steps:
| | ● | identification of the promised goods or services in the contract; |
| | ● | determination of whether the promised goods or serves are performance obligations including whether they are distinct in the context of the contract; |
| | ● | measurement of the transaction price, including the constraint on variable consideration; |
| | ● | allocation of the transaction price to the performance obligations based on estimated selling prices; and |
| | ● | recognition of revenue when (or as) the Company satisfies each performance obligation. A performance obligation is a promise to transfer a distinct good or service to the customer and is the unit of account in ASC 606. |
Shipping and Handling Costs. Shipping and handling costs were approximately $1,006 and $349 for the fiscal years ended June 30, 2023 and 2022, respectively, and are included in cost of sales in the accompanying Consolidated Statements of Operations.
Stock-Based Compensation. The Company has two stock-based compensation plans that have outstanding options issued in accordance with such plans. The Company periodically grants stock options to employees and directors in accordance with the provisions of its stock option plans, with the exercise price of the stock options being set at the closing market price of the common stock on the date of grant. Stock based compensation expense is recognized based on the estimated fair value, utilizing a Black-Scholes option pricing model, of the instrument on the date of grant over the requisite vesting period, which is generally three years.
Income Taxes. The Company accounts for income taxes using the asset and liability method. Accordingly, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in the tax rate is recognized in income or expense in the period that the change is effective. Tax benefits are recognized when it is probable that the deduction will be sustained. A valuation allowance is established when it is more likely than not that all or a portion of a deferred tax asset will not be realized. The Company files a U.S. federal income tax return as well as returns for various states. The Company’s income taxes have not been examined by any tax authorities for the periods subject to review by such taxing authorities, except for the State of New Jersey tax filings for MDC and CII which were reviewed by the State of New Jersey for the then open tax periods of 2014 through 2017 and 2016 to 2019, respectively. Uncertain tax positions, if any, taken on our tax returns are accounted for as liabilities for unrecognized tax benefits. The Company recognizes interest and penalties, if any, related to unrecognized tax benefits in general and administrative expenses in the Consolidated Statements of Operations. There were no liabilities recorded for uncertain tax positions at June 30, 2023 or 2022.
Leases. We determine if an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, other current liabilities, and operating lease liabilities on our consolidated balance sheets. The ROU assets related to Finance leases are included in property and equipment on our consolidated statement of financial condition. Operating lease ROU assets and operating lease liabilities are recognized based on the present value of the future minimum lease payments over the lease term at commencement date. As most of our leases do not provide an implicit rate, we use our incremental borrowing rate based on the information available at commencement date in determining the present value of future payments. The operating lease ROU asset also includes any lease payments made and excludes lease incentives and initial direct costs incurred. Our lease terms may include options to extend or terminate the lease when it is reasonably certain that we will exercise that option. Lease expense for minimum lease payments is recognized on a straight-line basis over the lease term. We have lease agreements with lease and non-lease components, which are generally accounted for separately. For certain equipment leases, such as vehicles, we account for the lease and non-lease components as a single lease component.
Earnings Per Share. Basic earnings per common share amounts are based on weighted average number of common shares outstanding. Diluted earnings per share amounts are based on the weighted average number of common shares outstanding, plus the incremental shares that would have been outstanding upon the assumed exercise of all potentially dilutive stock options, subject to anti-dilution limitations using the treasury stock method.
Fair Value of Financial Instruments. Generally accepted accounting principles require disclosing the fair value of financial instruments to the extent practicable for financial instruments which are recognized or unrecognized in the balance sheet. The fair value of the financial instruments disclosed herein is not necessarily representative of the amount that could be realized or settled, nor does the fair value amount consider the tax consequences of realization or settlement. In assessing the fair value of financial instruments, the Company uses a variety of methods and assumptions, which are based on estimates of market conditions and risks existing at the time. For certain instruments, including cash and cash equivalents, accounts receivable, accounts payable, and accrued expenses, it was estimated that the carrying amount approximated fair value because of the short maturities of these instruments. All debt is based on current rates at which the Company could borrow funds with similar remaining maturities and approximates fair value.
Accounts Receivable and Allowance for Doubtful Accounts. In the normal course of business, the Company extends credit to customers. Accounts receivable, less the allowance for doubtful accounts, reflect the net realizable value of receivables, and approximate fair value. The Company believes there is no concentration of credit risk with any single customer whose failure or nonperformance would materially affect the Company’s results other than as discussed in Note 9(c) – Significant Risks and Uncertainties – Major Customers. On a regular basis, the Company evaluates its accounts receivables and establishes an allowance for doubtful accounts based on a combination of specific customer circumstances, credit conditions, and historical write-offs and collections. The allowance for doubtful accounts as of June 30, 2023 and 2022 was $37 and $85, respectively. Accounts receivable are charged off against the allowance after management determines that the potential for recovery is remote.
Inventories. Inventories are stated at the lower of cost or net realizable value. Cost is determined using the first-in, first-out method. Allowances for obsolete and overstock inventories are estimated based on “expiration dating” of inventory and projection of sales.
Property and Equipment. Property and equipment are recorded at cost and are depreciated using the straight line method over the following estimated useful lives:
| Building | 15 Years |
| Leasehold Improvements | Shorter of estimated useful life or term of lease |
| Machinery and Equipment | 7 Years |
| Transportation Equipment | 5 Years |
Impairment of Long-Lived Assets. Long-lived assets are reviewed for impairment when circumstances indicate that the carrying value of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of the assets to the future net cash flows estimated by the Company to be generated by such assets. If such assets are considered to be impaired, the impairment to be recognized is the amount by which the carrying amount of the assets exceeds the fair value of the assets. Assets to be disposed of by sale are recorded as held for sale at the lower of carrying value or estimated net realizable value. Tests for impairment or recoverability are performed at least annually and require significant management judgment and the use of estimates which the Company believes are reasonable and appropriate at the time of the impairment test. Future unanticipated events affecting cash flows and changes in market conditions could affect such estimates and result in the need for an impairment charge. The Company also re-evaluates the periods of amortization to determine whether circumstances warrant revised estimates of current useful lives. No impairment losses were identified or recorded in the fiscal years ended June 30, 2023 and 2022 on the Company’s long-lived assets.
Investment in iBio, Inc. Prior to the adoption of ASU 2016-01 on July 1, 2018, the Company accounted for its investment in iBio, Inc. (“iBio”) common stock on the cost basis as it retained approximately 6% of its interest in iBio (the “iBio Stock”) at the time of the spin-off of this subsidiary in August 2008. The Company reviewed its investment in iBio for impairment and recorded a loss when there was deemed to be a permanent impairment of the investment. To date, there were cumulative impairment charges of approximately $2,562. ASU 2016-01, requires equity investments to be measured at fair value and recognize changes in fair value in net income. During the year ended June 30, 2023 and 2022, the Company recognized realized losses of $35 and $0, respectively and unrealized gain (loss) of $27 and $(55) for the fiscal years ended June 30, 2023 and 2022, respectively. As of June 30, 2023, the Company no longer owns any iBio Stock and as of June 3, 2022, the market value of the iBio Stock was approximately $12, based on the trade price at the close of trading on June 30, 2022. The investment in iBio is included in other current assets in the consolidated balance sheets as of June 30, 2022 at the respective market value.
Accounting Pronouncements Adopted In June 2016, the FASB issued ASU 2016-13 “Financial Instruments-Credit Losses-Measurement of Credit Losses on Financial Instruments”. This guidance replaces the current incurred loss impairment methodology with a methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information to inform credit loss estimates. The guidance applies to loans, accounts receivable, trade receivables and other financial assets measured at amortized cost, loan commitments, debt securities and beneficial interests in securitized financial assets, but the effect on the Company is projected to be limited to accounts receivable. The guidance was effective for the fiscal year beginning on July 1, 2023, including interim periods within that year. The adoption of this standard did not have a material impact on the Company’s Consolidated Financial Statements. In May 2019, the FASB issued ASU 2021-05 “Financial Instruments-Credit Losses (Topic 326)” which provides transition relief for companies adopting ASU 2016-13. This guidance amends ASU 2016-13 to allow companies to elect, upon adoption of ASU 2016-13, the fair value option on financial instruments that were previously recorded at amortized cost under certain circumstances. Companies are required to make this election on and instrument by instrument basis. The guidance will be effective for the fiscal year beginning on July 1, 2023, including interim periods within that year. The adoption of this standard did not have a material impact on the Company’s Consolidated Financial Statements.
In October 2021, the FASB issued ASU 2021-10, Codification Improvements. The guidance contains improvements to the Codification by ensuring that all guidance that requires or provides an option or an entity to provide information in the notes to the financial statements is codified in the Disclosure Section of the Codification. The guidance also contains Codifications that are varied in nature and may affect the application of the guidance in cases in which the original guidance may have been unclear. For public business entities, the amendments in the ASU are effective for annual periods beginning after December 15, 2022, and interim periods within annual periods beginning after December 15, 2023. The adoption of ASU 2021-10 did not have a material impact on its consolidated financial statements.
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 3 - Inventories
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Inventory Disclosure [Text Block] |
Note 3. Inventories
Inventories are stated at the lower of cost or net realizable value using the first-in, first-out method and consist of the following:
| |
|
June 30, |
|
| |
|
2023 |
|
|
2022 |
|
| |
|
|
|
|
|
|
|
|
| Raw materials |
|
$ |
6,859 |
|
|
$ |
7,586 |
|
| Work-in-process |
|
|
2,148 |
|
|
|
1,759 |
|
| Finished goods |
|
|
1,254 |
|
|
|
1,440 |
|
| Total |
|
$ |
10,261 |
|
|
$ |
11,055 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for inventory. Includes, but is not limited to, the basis of stating inventory, the method of determining inventory cost, the classes of inventory, and the nature of the cost elements included in inventory. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//330/tableOfContent
| Name: |
us-gaap_InventoryDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 4 - Property and Equipment, Net
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Property, Plant and Equipment Disclosure [Text Block] |
Note 4. Property and Equipment, net
Property and equipment consists of the following:
| | | June 30, | |
| | | 2023 | | | 2022 | |
| | | | | | | | | |
| Land and building | | $ | 1,250 | | | $ | 1,250 | |
| Leasehold improvements | | | 1,371 | | | | 1,371 | |
| Machinery and equipment | | | 6,801 | | | | 6,727 | |
| Transportation equipment | | | - | | | | 6 | |
| | | | 9,422 | | | | 9,354 | |
| Less: Accumulated depreciation and amortization | | | (7,769 | ) | | | (7,444 | ) |
| Total | | $ | 1,653 | | | $ | 1,910 | |
Depreciation and amortization expense was $351 and $336 for the fiscal years ended June 30, 2023 and 2022, respectively. Additionally, the Company disposed of fully depreciated property of $26 and $309 in the fiscal years ended June 30, 2023 and 2022, respectively. The Company also recognized a net gain on disposals of $0 and $2 on property, including property in the aggregate amount of $0 and $16 that were not fully depreciated and a gain on the sale of equipment of $1 and $20 in the fiscal years ended June 30, 2023 and 2022, respectively.
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//360/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-7
| Name: |
us-gaap_PropertyPlantAndEquipmentDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 5 - Senior Credit Facility and Other Long Term Debt
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Debt Disclosure [Text Block] |
Note 5. Senior Credit Facility
As of June 30, 2023 and 2022, the Company had the following debt outstanding:
| | | Principal Amount | | | Interest Rate | | Maturity Date |
| | | June 30, | | | | | | |
| | | 2023 | | | 2022 | | | | | | |
| Revolving advances under Senior Credit Facility with PNC Bank, National Association | | $ | - | | | $ | 101 | | | | 8.25 | % | 5/15/2024 |
| Total outstanding debt | | $ | - | | | $ | 101 | | | | | | |
SENIOR CREDIT FACILITY
On March 16, 2023, the Company, MDC, AgroLabs, IHT, IHT Properties Corp. (“IHT Properties”) and Vitamin Factory (collectively, the “Borrowers”) amended the Revolving Credit, Term Loan and Security Agreement (the “Amended Loan Agreement”) with PNC Bank, National Association as agent and lender (“PNC”) and the other lenders party thereto entered into on June 27, 2012, as amended on February 19, 2016 and May 15, 2019.
The Amended Loan Agreement provides for a total of $11,585 in senior secured financing (the “Senior Credit Facility”) as follows: (i) discretionary advances (“Revolving Advances”) based on eligible accounts receivable and eligible inventory in the maximum amount of $8,000 (the “Revolving Credit Facility”), and (ii) a term loan in the amount of $3,585 (the “Term Loan”). The Senior Credit Facility is secured by all assets of the Borrowers, including, without limitation, machinery and equipment, real estate owned by IHT Properties, and common stock of iBio owned by the Company. Revolving Advances bear interest at PNC’s Base Rate (8.25% and 4.75% as of June 30, 2023 and 2022, respectively) or the Eurodollar Rate, at Borrowers’ option, plus 2.50%. The Term Loan bore interest at PNC’s Base Rate (5.00% as of June 30, 2022) or the Eurodollar Rate at Borrowers’ option, plus 3.00%. As of September 15, 2023, the Revolving Advance interest rate is 8.50%.
As of March 16, 2023, the Amended Loan Agreement provides that any loans, advances and/or other extensions of credit denominated in U.S. Dollars prior to March 16, 2023 that bear interest or are permitted to bear interest, and have fees, commissions or other amounts based on the London Interbank Offered Rate administered by the ICE Benchmark Administration (which may be referred to as the “Eurodollar Rate” ( “LIBOR”) shall thereafter bear interest based on the Term SOFR Rate plus the SOFR Adjustment . The Term SOFR Rate, for any day, shall be equal to the secured overnight financing rate as administered by the Federal Reserve Bank of New York (or a successor administrator of the secured overnight financing rate). The SOFR Adjustment is defined as 10 basis points (0.10%).
Upon and after the occurrence of any event of default under the Amended Loan Agreement, and during the continuation thereof, interest shall be payable at the interest rate then applicable plus 2%. The Senior Credit Facility matures on May 15, 2024 (the “Senior Maturity Date”).
The principal balance of the Revolving Advances is payable on the Senior Maturity Date, subject to acceleration, based upon a material adverse event clause, as defined, subjective accelerations for borrowing base reserves, as defined or upon the occurrence of any event of default under the Amended Loan Agreement or earlier termination of the Amended Loan Agreement pursuant to the terms thereof. The Term Loan shall be repaid in eighty-four (84) consecutive monthly installments of principal, the first eighty-three (83) of which shall be in the amount of $43, commencing on the first business day of June 2019, and continuing on the first business day of each month thereafter, with a final payment of any unpaid balance of principal and interest payable on the Senior Maturity Date. The foregoing is subject to customary mandatory prepayment provisions and acceleration upon the occurrence of any event of default under the Amended Loan Agreement or earlier termination of the Amended Loan Agreement pursuant to the terms thereof. The Company satisfied all the principal payments required under the Term Note on January 3, 2022.
The Revolving Advances are subject to the terms and conditions set forth in the Amended Loan Agreement and are made in aggregate amounts at any time equal to the lesser of (x) $8,000 or (y) an amount equal to the sum of: (i) up to 85%, subject to the provisions in the Amended Loan Agreement, of eligible accounts receivables (“Receivables Advance Rate”), plus (ii) up to the lesser of (A) 75%, subject to the provisions in the Amended Loan Agreement, of the value of the eligible inventory (“Inventory Advance Rate” and together with the Receivables Advance Rate, collectively, the “Advance Rates”), (B) 85% of the appraised net orderly liquidation value of eligible inventory (as evidenced by the most recent inventory appraisal reasonably satisfactory to PNC in its sole discretion exercised in good faith) and (C) the inventory sublimit in the aggregate at any one time (“Inventory Advance Rate” and together with the Receivables Advance Rate, collectively, the “Advance Rates”), minus (iii) the aggregate Maximum Undrawn Amount, as defined in the Amended Loan Agreement, of all outstanding letters of credit, minus (iv) such reserves as PNC may reasonably deem proper and necessary from time to time.
The Amended Loan Agreement contains customary mandatory prepayment provisions, including, without limitation the requirement to use any sales proceeds from the sale of iBio Stock to repay the Term Loan and to prepay the outstanding amount of the Term Note in an amount equal to twenty-five percent (25%) of Excess Cash Flow, as defined in the Amended Load Agreement, for each fiscal year commencing with the fiscal year ended June 30, 2016, payable upon delivery of the financial statements to PNC referred to in and required by the Amended Loan Agreement for such fiscal year but in any event not later than one hundred twenty (120) days after the end of each such fiscal year, which amount shall be applied ratably to the outstanding principal installments of the Term Loan in the inverse order of the maturities thereof. The Amended Loan Agreement also contains customary representations and warranties, covenants and events of default, including, without limitation, (i) a fixed charge coverage ratio maintenance requirement and (ii) an event of default tied to any change of control as defined in the Amended Loan Agreement. As of June 30, 2023, the Company was in compliance with the fixed charge coverage ratio maintenance requirement, with the required annual payments of 25% of the Excess Cash Flow for each fiscal year commencing with the fiscal year ended June 30, 2016 and used the proceeds of $96 from the sale of iBio Stock in the fiscal year ended June 30, 2021, to repay the Term Loan. Additionally, with the required annual payment of 25% of Excess Cash Flow for the fiscal year ended June 30, 2021, together with the required monthly installments of $43, the Company satisfied all the remaining principal payments required under the Term Note on January 3, 2022.
In connection with the Senior Credit Facility, the following loan documents were executed: (i) a Stock Pledge Agreement with PNC, pursuant to which the Company pledged to PNC the iBio Stock; (ii) a Mortgage and Security Agreement with PNC with IHT Properties; and (iii) an Environmental Indemnity Agreement with PNC.
|
| X |
- DefinitionThe entire disclosure for information about short-term and long-term debt arrangements, which includes amounts of borrowings under each line of credit, note payable, commercial paper issue, bonds indenture, debenture issue, own-share lending arrangements and any other contractual agreement to repay funds, and about the underlying arrangements, rationale for a classification as long-term, including repayment terms, interest rates, collateral provided, restrictions on use of assets and activities, whether or not in compliance with debt covenants, and other matters important to users of the financial statements, such as the effects of refinancing and noncompliance with debt covenants. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 470
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//470/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1C
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1C
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1C
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1E
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1I
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1I
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1I
| Name: |
us-gaap_DebtDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 6 - Interest Expense
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Interest Expense [Text Block] |
Note 6. Interest Expense
The components of interest expense for the fiscal years ended June 30, 2023 and 2022 are presented below:
| | | For the Fiscal Year Ended June 30, | |
| | | 2023 | | | 2022 | |
| | | | | | | | | |
| Interest on Senior Debt | | $ | 40 | | | $ | 103 | |
| Amortization of prepaid financing costs | | | 12 | | | | 21 | |
| Other interest expense | | | - | | | | 4 | |
| Interest Expense | | $ | 52 | | | $ | 128 | |
The weighted average interest rate paid was 5.67% and 3.33% in the fiscal years ended June 30, 2023 and 2022, respectively. As of June 30, 2023 and 2022, the Company had accrued unpaid interest of approximately $0 and $3, respectively.
|
| X |
- DefinitionThe entire disclosure of interest expense.
| Name: |
inbp_InterestExpenseTextBlock |
| Namespace Prefix: |
inbp_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 7 - Income Taxes
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Income Tax Disclosure [Text Block] |
Note 7. Income Taxes
The components of the provision for income taxes consists of the following:
| | | For the fiscal year | |
| | | ended June 30, | |
| | | 2023 | | | 2022 | |
| | | | | | | | | |
| Current - Federal tax expense | | $ | (110 | ) | | $ | (600 | ) |
| Current - State tax expense | | | (61 | ) | | | (289 | ) |
| Deferred - Federal benefit | | | 55 | | | | 2,106 | |
| Deferred - State tax (expense) benefit | | | (18 | ) | | | 24 | |
| Income tax (expense) benefit, net | | $ | (134 | ) | | $ | 1,241 | |
A reconciliation of the statutory tax rate to the effective tax rate is as follows:
| | | For the fiscal year | |
| | | ended June 30, | |
| | | 2023 | | | 2022 | |
| Statutory federal income tax rate | | | (21.0 | )% | | | (21.0 | )% |
| State income tax rate | | | (55.1 | )% | | | (11.5 | )% |
| Stock compensation | | | (166.0 | )% | | | (4.6 | )% |
| Change in valuation allowance | | | 201.2 | % | | | 85.6 | % |
| Other temporary differences | | | (62.1 | )% | | | (0.6 | )% |
| Loss on sale of iBio Stock | | | (18.4 | )% | | | - | |
| Non-deductible expenses | | | (12.6 | )% | | | (0.1 | )% |
| Effective income tax rate | | | (134 | )% | | | 47.8 | % |
Deferred income taxes reflect the tax effects of temporary differences between the carrying amounts of assets and liabilities for financial accounting purposes and the amounts used for income tax reporting. Significant components of the Company’s net deferred tax assets are as follows:
| | | June 30, | |
| | | 2023 | | | 2022 | |
| Deferred Tax Assets | | | | | | | | |
| Net operating loss | | $ | 5,105 | | | $ | 5,166 | |
| Capital loss carryover | | | 678 | | | | 485 | |
| Depreciation | | | (139 | ) | | | (157 | ) |
| Inventory | | | 180 | | | | 149 | |
| Other | | | 337 | | | | 296 | |
| Valuation allowance | | | (1,435 | ) | | | (1,141 | ) |
| Total deferred tax asset, net | | $ | 4,726 | | | $ | 4,798 | |
The Company has net operating losses (“NOL”) of approximately $21,700 for federal purposes which expire beginning in 2028, which were federal NOLs generated prior to 2018. NOLs generated post 2018 have an indefinite life, subject to 80% of taxable income, there were no NOLs generated post 2018. State NOL’s of approximately $3,997 expire beginning in 2031 through 2039. The Company also has capital loss carryforwards of $2,343 of which $271, $1,152 and $920 will expire in 2025, 2026 and 2028, respectively. The Company files a consolidated U.S. federal income tax return; however, the various state tax returns were filed on a stand-alone basis for the Company and its subsidiaries until the fiscal year ended June 30, 2021, which state income tax return are now filed on a combined basis. MDC has fully utilized its state NOL’s resulting in taxable income on a state level basis for the combined group.
Realization of the NOL carryforwards and other deferred tax temporary differences is contingent on future taxable earnings. The Company’s deferred tax asset was reviewed for expected utilization using a “more likely than not” approach by assessing the available positive and negative evidence surrounding its recoverability. Accordingly, a valuation allowance has been recorded against the Company’s deferred tax asset, as it was determined based upon past taxable losses and inconsistent taxable income in the past few years, that it was “more likely than not” that the Company’s deferred tax assets would not be realized. As of June 30, 2022, management determined that certain of the Company’s deferred tax assets were “more likely than not” to be realizable and the Company recognized deferred tax benefits related to the release of the valuation allowance on those assets of approximately $1,795.
The Company will continue to assess and evaluate strategies that will enable the deferred tax asset, or portion thereof, to be utilized, and will reduce the valuation allowance appropriately at such time when it is determined that the “more likely than not” criteria is satisfied.
There were no significant uncertain tax positions taken, or expected to be taken, in a tax return that would be determined to be an unrecognized tax benefit taken or expected to be taken in a tax return that should have been recorded on the Company’s consolidated financial statements for the year ended June 30, 2023. Additionally, there were no interest or penalties outstanding as of or for each of the fiscal years ended June 30, 2023 and 2022.
The latest three years of Federal and four years of state tax returns filed for the fiscal years ended through June 30, 2023 are currently open except for the State of New Jersey tax filings for CII which have been reviewed for the tax period of 2019. The tax returns for the year ended June 30, 2023 will be filed by March 15, 2024.
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for income taxes. Disclosures may include net deferred tax liability or asset recognized in an enterprise's statement of financial position, net change during the year in the total valuation allowance, approximate tax effect of each type of temporary difference and carryforward that gives rise to a significant portion of deferred tax liabilities and deferred tax assets, utilization of a tax carryback, and tax uncertainties information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//740/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-14
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-21
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 270
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482526/740-270-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB TOPIC 6.I.5.Q1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 11.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482603/740-30-50-2
| Name: |
us-gaap_IncomeTaxDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 8 - Profit-sharing Plan
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Retirement Benefits [Text Block] |
Note 8. Profit-Sharing Plan
The Company maintains a profit-sharing plan, which qualifies under Section 401(k) of the Internal Revenue Code, covering all nonunion employees meeting age and service requirements. Contributions are determined by matching a percentage of employee contributions. For the fiscal years ended June 30, 2023 and 2022, the Company contributed approximately $81 and $86, respectively, into the plan for the benefit of the eligible employees participating in the plan.
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for retirement benefits. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 70
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480794/715-70-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480482/715-20-55-17
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480506/715-20-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480506/715-20-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (q)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480506/715-20-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (l)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480506/715-20-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//715/tableOfContent
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480506/715-20-50-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (o)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480506/715-20-50-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (p)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480506/715-20-50-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (r)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480506/715-20-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (r)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480506/715-20-50-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480126/715-20-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 60
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480266/715-60-50-3
| Name: |
us-gaap_PensionAndOtherPostretirementBenefitsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 9 - Significant Risks and Uncertainties
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Concentration Risk Disclosure [Text Block] |
Note 9. Significant Risks and Uncertainties
(a) Concentrations of Credit Risk-Cash. The Company maintains balances at several financial institutions. Deposits at each institution are insured by the Federal Deposit Insurance Corporation up to $250. As of June 30, 2023, the Company had $1,949 in uninsured deposits at these financial institutions.
(b) Concentrations of Credit Risk-Receivables. The Company routinely assesses the financial strength of its customers and, based upon factors surrounding the credit risk of its customers, establishes an allowance for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowances is limited. The Company does not require collateral in relation to its trade accounts receivable credit risk.
(c) Major Customers. In the fiscal years ended June 30, 2023 and 2022, approximately 89% and 90% of consolidated net sales, respectively, were derived from two customers. These two customers are in the Company’s Contract Manufacturing Segment and represent approximately 70% and 24% and 67% and 26%, respectively, of this segment’s net sales in the fiscal years ended June 30, 2023 and 2022, respectively. Accounts receivable from these two major customers represented approximately 84% and 70% of total net accounts receivable as of June 30, 2023 and 2022, respectively. Three other customers in the Other Nutraceutical Segment, while not significant customers of the Company’s consolidated net sales, represented approximately 56%, 16% and 9% and 40%, 1% and 19%, respectively, of net sales of the Other Nutraceutical Segment in the fiscal years ended June 30, 2023 and 2022, respectively. The loss of any of these customers could have an adverse effect on the Company’s operations. Major customers are those customers who account for more than 10% of net sales.
(d) Business Risks. The Company insures its business and assets against insurable risks, to the extent that it deems appropriate, based upon an analysis of the relative risks and costs. The Company believes that the risk of loss from non-insurable events would not have a material adverse effect on the Company’s operations as a whole.
The raw materials used by the Company are primarily commodities and agricultural-based products. Raw materials used by the Company in the manufacture of its nutraceutical products are purchased from independent suppliers. Raw materials are available from numerous sources and the Company believes that it will continue to obtain adequate supplies.
As of June 30, 2023, approximately 74% the Company’s employees are covered by a union contract and are employed in its New Jersey facilities. The contract was renewed effective September 1, 2022 and will expire on August 31, 2026.
While the Company hasn’t, to date, seen a significant negative impact in its margins resulting from the coronavirus outbreak, it is experiencing a negative impact on its margins due to inflation and tightened labor markets. The Company may not be able to timely increase its selling prices to its customer resulting from price increases from its suppliers due to various economic factors, including inflation, labor and shipping costs and its own increases in shipping, labor and other operating costs. The Company’s results of operations may also be affected by economic conditions, including inflationary pressures, that can impact consumer disposable income levels and spending habits, thereby reducing the orders it may receive from the Company’s significant customers.
The Company continues to experience minimal supply chain disruptions relating to fuel refinery and transportation issues as it pertains to shipping. These issues first arose as result of the COVID-19 pandemic and other geo-political events. The significant outbreak of this contagious disease in the human population has resulted in a widespread health crisis that could adversely affect the economies and financial markets of many countries, resulting in an economic downturn that could affect demand for the Company’s products and impact the Company’s operating results.
During the first quarter of calendar 2022, the war in Ukraine affected the Company’s customer’s business operations in Ukraine and Russia, resulting in the cancelation of some future orders. The war resulted in the imposition of sanctions by the United States, the United Kingdom, and the European Union, that affect the cross-border operations of businesses operating in Russia. In addition, many multinational companies ceased or suspended their operations in Russia. Therefore, the ability to continue operations in Russia by the Company’s customers is uncertain. Also, there may be a shortage of Sunflower Oil products in the near future and this may cause delays in production of certain raw materials and may require reformulation of products.
|
| X |
- DefinitionThe entire disclosure for any concentrations existing at the date of the financial statements that make an entity vulnerable to a reasonably possible, near-term, severe impact. This disclosure informs financial statement users about the general nature of the risk associated with the concentration, and may indicate the percentage of concentration risk as of the balance sheet date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//275/tableOfContent
| Name: |
us-gaap_ConcentrationRiskDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 10 - Commitments and Contingencies
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Commitments and Contingencies Disclosure [Text Block] |
Note 10. Commitments and Contingencies
(a) Leases. The Company has operating and finance leases for its corporate and sales offices, warehousing and packaging facilities and certain machinery and equipment, including office equipment. The Company’s leases have remaining terms of less than 1 year to less than 7 years.
The components of lease expense for the fiscal year ended June 30, 2023 and 2022 were as follows:
| | | 2023 | | | 2022 | |
| | | Related Party - Vitamin Realty | | | Other Leases | | | Totals | | | Related Party - Vitamin Realty | | | Other Leases | | | Totals | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Operating Lease Costs | | $ | 842 | | | $ | 88 | | | $ | 650 | | | $ | 566 | | | $ | 84 | | | $ | 650 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Finance Operating Lease Costs: | | | | | | | | | | | | | | | | | | | | | | | | |
| Amortization of right-of use assets | | $ | - | | | $ | 12 | | | $ | 12 | | | $ | - | | | $ | 3 | | | $ | 3 | |
| Total Finance Lease Costs | | $ | - | | | $ | 12 | | | $ | 12 | | | $ | - | | | $ | 3 | | | $ | 3 | |
Rent and lease amortization costs are included in cost of sales and selling and administrative expenses in the accompanying Condensed Consolidated Statements of Operations.
Operating Lease Liabilities
Related Party Operating Lease Liabilities. Warehouse and office facilities are leased from Vitamin Realty Associates, LLC (“Vitamin Realty”), which is 100% owned by the estate of the Company’s former chairman, and a major stockholder and certain of his family members, who are the Co-Chief Executive Officers and directors of the Company. On January 5, 2012, MDC entered into a second amendment of lease (the “Second Lease Amendment”) with Vitamin Realty for its office and warehouse space in New Jersey increasing its rentable square footage from an aggregate of 74,898 square feet to 76,161 square feet and extending the expiration date to January 31, 2026. This Second Lease Amendment provided for minimum annual rental payments of $533, plus increases in real estate taxes and building operating expenses. On July 15, 2022, MDC entered into a third amendment of the lease (the “Third Lease Amendment”) with Vitamin Realty, increasing its rentable square footage to 116,175. This Third Lease Amendment provided for minimum annual rental payments of $842, plus increases in real estate taxes and the building operating expenses allocation percentage and is effective as of July 1, 2022.
On May 19, 2014, AgroLabs entered into an amendment to the lease agreement entered into on January 5, 2012, with Vitamin Realty for an additional 2,700 square feet of warehouse space in New Jersey, the term of which was to expire on January 31, 2020 to extend the expiration date to June 1, 2024. This additional lease provided for minimum lease payments of $27 with annual increases plus the proportionate share of operating expenses. The AgroLabs Lease was mutually terminated on July 15, 2022 with an effective date of July 1, 2022.
Rent expense, lease amortization costs and imputed interest costs on these related party leases were $1,269 and $896 for the fiscal years ended June 30, 2023 and 2022, respectively, and are included in cost of sales and selling and administrative expenses in the accompanying Consolidated Statements of Operations. As of June 30, 2023 and 2022, the Company had outstanding current obligations to Vitamin Realty of $0 and $72, respectively, included in accounts payable in the accompanying Consolidated Balance Sheets. Additionally, as of June 30, 2023 and 2022, the Company has operating lease obligations of $2,061 and $1,841, respectively, with Vitamin Realty as noted in the accompany Consolidated Balance Sheet.
Other Operating Lease Liabilities. The Company has entered into certain non-cancelable operating lease agreements expiring up through May 2023, related to machinery and equipment and office equipment.
As of June 30, 2023, the Company’s ROU assets, lease obligations and remaining cash commitment on these leases is as follows:
| | | Right-of-use Assets | | | Current Portion Operating Lease Obligations | | | Operating Lease Obligations | | | Remaining Cash Commitment | |
| | | | | | | | | | | | | | | | | |
| Vitamin Realty Leases | | $ | 2,061 | | | $ | 772 | | | $ | 1,289 | | | $ | 1,972 | |
| Warehouse Lease | | | 541 | | | | 108 | | | | 433 | | | | 631 | |
| Office equipment leases | | | 21 | | | | 8 | | | | 13 | | | | 23 | |
| | | $ | 2,623 | | | $ | 888 | | | $ | 1,735 | | | $ | 2,830 | |
As of June 30, 2022, the Company’s ROU assets, lease obligations and remaining cash commitment on these leases is as follows:
| | | Right-of-use Assets | | | Current Portion Operating Lease Obligations | | | Operating Lease Obligations | | | Remaining Cash Commitment | |
| | | | | | | | | | | | | | | | | |
| Vitamin Realty Leases | | $ | 1,839 | | | $ | 503 | | | $ | 1,338 | | | $ | 1,972 | |
| Office equipment leases | | | 28 | | | | 7 | | | | 21 | | | | 32 | |
| | | $ | 1,867 | | | $ | 510 | | | $ | 1,359 | | | $ | 2,004 | |
As of June 30, 2023 and 2022, the Company’s weighted average discount rate is 4.41% and 3.87%, for the fiscal years then ended, respectively, and the remaining term on lease liabilities is approximately 2.9 years and 3.5 years, respectively.
Financed Lease Obligation.
On June 15, 2022, the Company entered into a financed lease obligation with LEAF Capital Funding, LLC in the amount of $85, which lease is secured by certain machinery and equipment and matures on August 15, 2024. The lease payment in the amount of approximately $4 is payable monthly, beginning September 15, 2023 and has a stated interest rate of 0.0%.
As of each June, 2023 and 2022, the Company’s weighted average discount rate for the outstanding finance lease obligation is 0% and the remaining term on finance lease obligation is approximately 1.2 years and 2.2 years, respectively. The ROU asset related to the finance lease obligation is included in Property and equipment, net in the accompanying Consolidated Balance Sheet.
Supplemental cash flows information related to leases for the fiscal year ended June 30, 2023 is as follows:
| | | Related Party - Vitamin Realty | | | Other Leases | | | Totals | |
| | | | | | | | | | | | | |
| Cash paid for amounts included in the measurement of lease liabilities: | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Operating cash flows from operating leases | | $ | 842 | | | $ | 147 | | | $ | 989 | |
| Operating cash flows from finance lease obligations | | | - | | | | - | | | | - | |
| Financing cash flows from finance lease obligations | | | - | | | | 35 | | | | 35 | |
Supplemental cash flows information related to leases for the fiscal year ended June 30, 2022 is as follows:
| | | Related Party - Vitamin Realty | | | Other Leases | | | Totals | |
| | | | | | | | | | | | | |
| Cash paid for amounts included in the measurement of lease liabilities: | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Operating cash flows from operating leases | | $ | 566 | | | $ | 84 | | | $ | 650 | |
| Operating cash flows from finance lease obligations | | | - | | | | - | | | | - | |
| Financing cash flows from finance lease obligations | | | - | | | | - | | | | - | |
In the fiscal year ended June 30, 2023, in addition to the Third Amendment with Vitamin Realty the Company renewed, for one year, an operating lease for office space with an annual commitment of $10 and entered into a five year lease for additional warehouse space of 12,500 square feet with an annual commitment of $134 in the first year increasing to $150 in the fifth year of the lease (the “Warehouse Lease”). The Warehouse Lease includes additional rent of not less than $1 per month for the Company’s pro rata portion of the lessor’s operating expenses and commenced on December 1, 2022. Additionally, on August 4, 2023, the Company entered into a four year operating lease for transportation equipment with an annual commitment of $21.
Maturities of operating lease liabilities as of June 30, 2023 were as follows:
| Year ending June 30, | | Operating Lease Commitment | | | Related Party Operating Lease Commitment | | | Finance Lease Obligation | | | Total | |
| | | | | | | | | | | | | | | | | |
| 2024 | | $ | 146 | | | $ | 842 | | | $ | 42 | | | $ | 1,030 | |
| 2025 | | | 149 | | | | 842 | | | | 7 | | | | 998 | |
| 2026 | | | 148 | | | | 492 | | | | - | | | | 640 | |
| 2027 | | | 148 | | | | - | | | | - | | | | 148 | |
| 2028 | | | 63 | | | | - | | | | - | | | | 63 | |
| Total minimum lease payments | | | 654 | | | | 2,176 | | | | 49 | | | | 2,879 | |
| Imputed interest | | | (92 | ) | | | (115 | ) | | | - | | | | (207 | ) |
| Total | | $ | 562 | | | $ | 2,061 | | | $ | 49 | | | $ | 2,672 | |
(b) Legal Proceedings.
The Company is subject, from time to time, to claims by third parties under various legal theories. The defense of such claims, or any adverse outcome relating to any such claims, could have a material adverse effect on the Company’s liquidity, financial condition and cash flows.
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//450/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480327/954-440-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for related party transactions. Examples of related party transactions include transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners; and (d) affiliates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(g)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//850/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
| Name: |
us-gaap_RelatedPartyTransactionsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 12 - Equity Transactions and Stock-based Compensation
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Equity [Text Block] |
Note 12. Equity Transactions and Stock-Based Compensation
Stock Option Plans. The Company has adopted a stock option plan for the granting of options or restricted shares to employees, officers, directors and consultants of the Company that originally provided for the issuance of up to 7,000,000 shares of common stock, at the discretion of the Board of Directors. Subsequent to the adoption, the Board of Directors and stockholders approved additional common stock shares aggregating 6,000,000 to be available for grant, for a total of 13,000,000 shares of common stock reserved for issuance under the Company’s 2001 Stock Option Plan, as amended (the “Plan”). The Company also has a 1997 Stock Option Plan with 5,000,000 shares of common stock reserved for issuance. Stock option grants may not be priced less than the fair market value of the Company’s common stock at the date of grant. Options granted are generally for ten-year periods, except that incentive stock options granted to a 10% stockholder (as defined) are limited to five-year terms. As of June 30, 2023, the Company has 6,823,569 shares of common stock remaining under the Plans.
In the fiscal year ended June 30, 2023, the Board of Directors authorized the issuance of up to 538,500 stock options to Company officers and employees. The Company issued 527,000 stock options with an exercise price ranging from $0.41 to $0.45, vesting over three years, with expiration terms of either five or ten years from the date of grant. For the fiscal years ended June 30, 2023 and 2022, the Company incurred stock-based compensation expense of $320 and $369, respectively. The Company expects to record additional stock-based compensation of $480 over the remaining estimated vesting period of approximately two and half years.
In July 2023, the Board of Directors authorized the issuance of 200,000 stock options to the non-executive directors of (50,000 each) with an exercise price of $0.33, vesting over one year, 25% at the end of each quarter ending September 30, 2023, December 21, 2023, March 31, 2024 and June 30, 2024.
The Company used the following assumptions to calculate the fair value of the stock option grants using the Black-Scholes option pricing model on the measurement date during the year ended June 30, 2023:
| Risk Free Interest Rate | | | 3.85% to 3.00 | % |
| Volatility | | | 103.3% to 116.9 | % |
| Term | | | 4.5 to 7.5 years | |
| Dividend Rate | | | 0.00 | % |
| Closing Price of Common Stock | | $ | 0.41 | |
The Company calculates expected volatility for a stock-based grant based on historic daily stock price observations of its common stock during the period immediately preceding the grant that is equal in length to the expected term of the grant. The expected term of the options is estimated based on the Company’s historical exercise rate and forfeiture rates are estimated based on employment termination experience. The risk free interest rate is based on U.S. Treasury yields for securities in effect at the time of grants with terms approximating the term of the grants. The assumptions used in the Black-Scholes option valuation model are highly subjective, and can materially affect the resulting valuations.
In the fiscal year ended June 30, 2022, the Board of Directors authorized the issuance of 764,700 stock options to Company officers, employees and directors with an exercise price of $0.51, $0.95 or $1.045, vesting over one or three years, with terms of either five or ten years.
In the fiscal years ended June 30, 2023 and 2022, stock options in the aggregate amount of 4,376,284 and 537,600, were not included in the computation of weighted average diluted common shares outstanding as the effect of doing so would have been anti-dilutive.
The intrinsic value of options outstanding and exercisable at June 30, 2023 and 2022 was $461 and $1,002, respectively.
A summary of the Company’s stock option activity, and related information for the years ended June 30, follows:
| | | | | | | Weighted | |
| | | | | | | Average | |
| | | | | | | Exercise | |
| | | Options | | | Price | |
| | | | | | | | | |
| Outstanding as of July 1, 2021 | | | 3,916,666 | | | $ | 0.26 | |
| Granted | | | 764,700 | | | | 0.85 | |
| Exercised | | | (146,333 | ) | | | 0.23 | |
| Terminated | | | (44,700 | ) | | | 0.53 | |
| Expired | | | (46,400 | ) | | | 0.47 | |
| Outstanding as of June 30, 2022 | | | 4,443,933 | | | | 0.36 | |
| Granted | | | 527,000 | | | | 0.42 | |
| Exercised | | | - | | | | - | |
| Terminated | | | - | | | | - | |
| Expired | | | (594,649 | ) | | | 0.46 | |
| Outstanding as of June 30, 2023 | | | 4,376,284 | | | $ | 0.35 | |
| | | | | | | | | |
| Exercisable at June 30, 2022 | | | 3,524,500 | | | $ | 0.26 | |
| Exercisable at June 30, 2023 | | | 3,626,551 | | | $ | 0.30 | |
The following table summarizes the range of exercise prices and weighted-average exercise prices for stock options outstanding and exercisable as of June 30, 2023 under the Company’s stock option plans:
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | Weighted | |
| | | | | | | | | | Weighted | | Weighted Average | | | | Average | |
| | | | | | Average | | Remaining | | | | Exercise | |
| Range of Exercise Price | | Outstanding | | Exercise Price | | Contractual Life (years) | Exercisable | | Price | |
| | | | | | | | | | | | | | | | | | | |
| $ | 0.09 | - | $ | 0.10 | | | 1,221,000 | | $ | 0.09 | | 1.9 | | 1,296,000 | | $ | 0.09 | |
| $ | 0.21 | - | $ | 0.21 | | | 1,194,000 | | | 0.21 | | 5.9 | | 1,209,000 | | | 0.21 | |
| $ | 0.23 | - | $ | 0.25 | | | 250,000 | | | 0.23 | | 3.4 | | 250,000 | | | 0.23 | |
| $ | 0.41 | - | $ | 0.41 | | | 426,500 | | | 0.41 | | 9.4 | | - | | | 0.00 | |
| $ | 0.51 | - | $ | 0.51 | | | 200,000 | | | 0.51 | | 9.0 | | 200,000 | | | 0.51 | |
| $ | 0.65 | - | $ | 0.65 | | | 570,667 | | | 0.65 | | 7.4 | | 431,000 | | | 0.65 | |
| $ | 0.72 | - | $ | 0.72 | | | 66,667 | | | 0.72 | | 0.7 | | 66,667 | | | 0.72 | |
| $ | 0.95 | - | $ | 0.95 | | | 425,783 | | | 0.95 | | 8.4 | | 242,217 | | | 0.95 | |
| $ | 1.05 | - | $ | 1.05 | | | 21,667 | | | 1.05 | | 0.7 | | 21,667 | | | 1.05 | |
| $ | 0.09 | - | $ | 1.05 | | | 4,376,284 | | $ | 0.35 | | 7.0 | | 3,626,551 | | $ | 0.30 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 13 - Segment Information
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes to Financial Statements |
|
| Segment Reporting Disclosure [Text Block] |
Note 13. Segment Information
The basis for presenting segment results generally is consistent with overall Company reporting. The Company reports information about its operating segments in accordance with GAAP which establishes standards for reporting information about a company’s operating segments.
The Company has divided its operations into two reportable segments; Contract Manufacturing and Other Nutraceutical Businesses. The international sales, concentrated primarily in Europe, for the fiscal years ended June 30, 2023 and 2022 were $8,055 and $9,729, respectively.
Financial information relating to the fiscal years ended June 30, 2023 and 2022 operations by business segment are as follows:
| | | | Sales, Net | | | Segment | | | | | | | | | | | | | |
| | | | U.S. | | | International | | | | | | | Gross | | | | | | | Capital | | | Total | |
| | | | Customers | | | Customers | | | Total | | | Profit | | | Depreciation | | | Expenditures | | | Assets | |
| Contract Manufacturing | 2023 | | $ | 40,230 | | | $ | 8,047 | | | $ | 48,277 | | | $ | 3,630 | | | $ | 348 | | | $ | 109 | | | $ | 19,507 | |
| | 2022 | | | 44,450 | | | | 9,641 | | | | 54,091 | | | | 5,917 | | | | 332 | | | | 486 | | | | 19,061 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Other Nutraceutical Businesses | 2023 | | | 2,387 | | | | 8 | | | | 2,395 | | | | 431 | | | | 3 | | | | 7 | | | | 5,924 | |
| | 2022 | | | 2,067 | | | | 88 | | | | 2,155 | | | | 635 | | | | 4 | | | | - | | | | 6,189 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total Company | 2023 | | | 42,617 | | | | 8,055 | | | | 50,672 | | | | 4,061 | | | | 351 | | | | 116 | | | | 25,431 | |
| | 2022 | | | 46,517 | | | | 9,729 | | | | 56,246 | | | | 6,552 | | | | 336 | | | | 486 | | | | 25,250 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisclosureTextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for reporting segments including data and tables. Reportable segments include those that meet any of the following quantitative thresholds a) it's reported revenue, including sales to external customers and intersegment sales or transfers is 10 percent or more of the combined revenue, internal and external, of all operating segments b) the absolute amount of its reported profit or loss is 10 percent or more of the greater, in absolute amount of 1) the combined reported profit of all operating segments that did not report a loss or 2) the combined reported loss of all operating segments that did report a loss c) its assets are 10 percent or more of the combined assets of all operating segments. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//280/tableOfContent
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 26
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-26
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 34
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-34
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-21
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-21
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_SegmentReportingDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Significant Accounting Policies (Policies)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Consolidation, Policy [Policy Text Block] |
Principles of Consolidation. The accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. Intercompany transactions and accounts have been eliminated in consolidation.
|
| Reclassification, Comparability Adjustment [Policy Text Block] |
Reclassifications. Certain prior year amounts have been reclassified to conform to the current year presentation.
|
| Use of Estimates, Policy [Policy Text Block] |
Use of Estimates. The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. The most significant estimates include: ● sales returns and allowances; ● allowance for doubtful accounts; ● inventory valuation; ● valuation and recoverability of long-lived assets; ● income taxes and valuation allowance on deferred income taxes, and; ● accruals for, and the probability of, the outcome of current litigation, if any. On a continual basis, management reviews its estimates utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such reviews, and if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ from those estimates.
|
| Revenue from Contract with Customer [Policy Text Block] |
Revenue Recognition. The Company recognizes product sales revenue, the prices of which are fixed and determinable, when title and risk of loss have transferred to the customer, when estimated provisions for product returns, rebates, charge-backs and other sales allowances are reasonably determinable, and when collectability is reasonably assured. Accruals for these items are presented in the consolidated financial statements as reductions to sales. The Company’s net sales represent gross sales invoiced to customers, less certain related charges for discounts, returns, rebates, charge-backs and other allowances. Cost of sales includes the cost of raw materials and all labor and overhead associated with the manufacturing and packaging of the products. Gross margins are affected by, among other things, changes in the relative sales mix among our products and valuation and/or charge off of slow moving, expired or obsolete inventories. To perform revenue recognition for arrangements within the scope of ASC 606, the Company performs the following five steps:
| | ● | identification of the promised goods or services in the contract; |
| | ● | determination of whether the promised goods or serves are performance obligations including whether they are distinct in the context of the contract; |
| | ● | measurement of the transaction price, including the constraint on variable consideration; |
| | ● | allocation of the transaction price to the performance obligations based on estimated selling prices; and |
| | ● | recognition of revenue when (or as) the Company satisfies each performance obligation. A performance obligation is a promise to transfer a distinct good or service to the customer and is the unit of account in ASC 606. |
|
| Cost of Goods, Shipping and Handling Costs [Policy Text Block] |
Shipping and Handling Costs. Shipping and handling costs were approximately $1,006 and $349 for the fiscal years ended June 30, 2023 and 2022, respectively, and are included in cost of sales in the accompanying Consolidated Statements of Operations.
|
| Share-Based Payment Arrangement [Policy Text Block] |
Stock-Based Compensation. The Company has two stock-based compensation plans that have outstanding options issued in accordance with such plans. The Company periodically grants stock options to employees and directors in accordance with the provisions of its stock option plans, with the exercise price of the stock options being set at the closing market price of the common stock on the date of grant. Stock based compensation expense is recognized based on the estimated fair value, utilizing a Black-Scholes option pricing model, of the instrument on the date of grant over the requisite vesting period, which is generally three years.
|
| Income Tax, Policy [Policy Text Block] |
Income Taxes. The Company accounts for income taxes using the asset and liability method. Accordingly, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in the tax rate is recognized in income or expense in the period that the change is effective. Tax benefits are recognized when it is probable that the deduction will be sustained. A valuation allowance is established when it is more likely than not that all or a portion of a deferred tax asset will not be realized. The Company files a U.S. federal income tax return as well as returns for various states. The Company’s income taxes have not been examined by any tax authorities for the periods subject to review by such taxing authorities, except for the State of New Jersey tax filings for MDC and CII which were reviewed by the State of New Jersey for the then open tax periods of 2014 through 2017 and 2016 to 2019, respectively. Uncertain tax positions, if any, taken on our tax returns are accounted for as liabilities for unrecognized tax benefits. The Company recognizes interest and penalties, if any, related to unrecognized tax benefits in general and administrative expenses in the Consolidated Statements of Operations. There were no liabilities recorded for uncertain tax positions at June 30, 2023 or 2022.
|
| Lessee, Leases [Policy Text Block] |
Leases. We determine if an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, other current liabilities, and operating lease liabilities on our consolidated balance sheets. The ROU assets related to Finance leases are included in property and equipment on our consolidated statement of financial condition. Operating lease ROU assets and operating lease liabilities are recognized based on the present value of the future minimum lease payments over the lease term at commencement date. As most of our leases do not provide an implicit rate, we use our incremental borrowing rate based on the information available at commencement date in determining the present value of future payments. The operating lease ROU asset also includes any lease payments made and excludes lease incentives and initial direct costs incurred. Our lease terms may include options to extend or terminate the lease when it is reasonably certain that we will exercise that option. Lease expense for minimum lease payments is recognized on a straight-line basis over the lease term. We have lease agreements with lease and non-lease components, which are generally accounted for separately. For certain equipment leases, such as vehicles, we account for the lease and non-lease components as a single lease component.
|
| Earnings Per Share, Policy [Policy Text Block] |
Earnings Per Share. Basic earnings per common share amounts are based on weighted average number of common shares outstanding. Diluted earnings per share amounts are based on the weighted average number of common shares outstanding, plus the incremental shares that would have been outstanding upon the assumed exercise of all potentially dilutive stock options, subject to anti-dilution limitations using the treasury stock method.
|
| Fair Value of Financial Instruments, Policy [Policy Text Block] |
Fair Value of Financial Instruments. Generally accepted accounting principles require disclosing the fair value of financial instruments to the extent practicable for financial instruments which are recognized or unrecognized in the balance sheet. The fair value of the financial instruments disclosed herein is not necessarily representative of the amount that could be realized or settled, nor does the fair value amount consider the tax consequences of realization or settlement. In assessing the fair value of financial instruments, the Company uses a variety of methods and assumptions, which are based on estimates of market conditions and risks existing at the time. For certain instruments, including cash and cash equivalents, accounts receivable, accounts payable, and accrued expenses, it was estimated that the carrying amount approximated fair value because of the short maturities of these instruments. All debt is based on current rates at which the Company could borrow funds with similar remaining maturities and approximates fair value.
|
| Loans and Leases Receivable, Allowance for Loan Losses Policy [Policy Text Block] |
Accounts Receivable and Allowance for Doubtful Accounts. In the normal course of business, the Company extends credit to customers. Accounts receivable, less the allowance for doubtful accounts, reflect the net realizable value of receivables, and approximate fair value. The Company believes there is no concentration of credit risk with any single customer whose failure or nonperformance would materially affect the Company’s results other than as discussed in Note 9(c) – Significant Risks and Uncertainties – Major Customers. On a regular basis, the Company evaluates its accounts receivables and establishes an allowance for doubtful accounts based on a combination of specific customer circumstances, credit conditions, and historical write-offs and collections. The allowance for doubtful accounts as of June 30, 2023 and 2022 was $37 and $85, respectively. Accounts receivable are charged off against the allowance after management determines that the potential for recovery is remote.
|
| Inventory, Policy [Policy Text Block] |
Inventories. Inventories are stated at the lower of cost or net realizable value. Cost is determined using the first-in, first-out method. Allowances for obsolete and overstock inventories are estimated based on “expiration dating” of inventory and projection of sales.
|
| Property, Plant and Equipment, Policy [Policy Text Block] |
Property and Equipment. Property and equipment are recorded at cost and are depreciated using the straight line method over the following estimated useful lives:
| Building | 15 Years |
| Leasehold Improvements | Shorter of estimated useful life or term of lease |
| Machinery and Equipment | 7 Years |
| Transportation Equipment | 5 Years |
|
| Goodwill and Intangible Assets, Policy [Policy Text Block] |
Impairment of Long-Lived Assets. Long-lived assets are reviewed for impairment when circumstances indicate that the carrying value of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of the assets to the future net cash flows estimated by the Company to be generated by such assets. If such assets are considered to be impaired, the impairment to be recognized is the amount by which the carrying amount of the assets exceeds the fair value of the assets. Assets to be disposed of by sale are recorded as held for sale at the lower of carrying value or estimated net realizable value. Tests for impairment or recoverability are performed at least annually and require significant management judgment and the use of estimates which the Company believes are reasonable and appropriate at the time of the impairment test. Future unanticipated events affecting cash flows and changes in market conditions could affect such estimates and result in the need for an impairment charge. The Company also re-evaluates the periods of amortization to determine whether circumstances warrant revised estimates of current useful lives. No impairment losses were identified or recorded in the fiscal years ended June 30, 2023 and 2022 on the Company’s long-lived assets.
|
| Investment, Policy [Policy Text Block] |
Investment in iBio, Inc. Prior to the adoption of ASU 2016-01 on July 1, 2018, the Company accounted for its investment in iBio, Inc. (“iBio”) common stock on the cost basis as it retained approximately 6% of its interest in iBio (the “iBio Stock”) at the time of the spin-off of this subsidiary in August 2008. The Company reviewed its investment in iBio for impairment and recorded a loss when there was deemed to be a permanent impairment of the investment. To date, there were cumulative impairment charges of approximately $2,562. ASU 2016-01, requires equity investments to be measured at fair value and recognize changes in fair value in net income. During the year ended June 30, 2023 and 2022, the Company recognized realized losses of $35 and $0, respectively and unrealized gain (loss) of $27 and $(55) for the fiscal years ended June 30, 2023 and 2022, respectively. As of June 30, 2023, the Company no longer owns any iBio Stock and as of June 3, 2022, the market value of the iBio Stock was approximately $12, based on the trade price at the close of trading on June 30, 2022. The investment in iBio is included in other current assets in the consolidated balance sheets as of June 30, 2022 at the respective market value.
|
| New Accounting Pronouncements, Policy [Policy Text Block] |
Accounting Pronouncements Adopted In June 2016, the FASB issued ASU 2016-13 “Financial Instruments-Credit Losses-Measurement of Credit Losses on Financial Instruments”. This guidance replaces the current incurred loss impairment methodology with a methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information to inform credit loss estimates. The guidance applies to loans, accounts receivable, trade receivables and other financial assets measured at amortized cost, loan commitments, debt securities and beneficial interests in securitized financial assets, but the effect on the Company is projected to be limited to accounts receivable. The guidance was effective for the fiscal year beginning on July 1, 2023, including interim periods within that year. The adoption of this standard did not have a material impact on the Company’s Consolidated Financial Statements. In May 2019, the FASB issued ASU 2021-05 “Financial Instruments-Credit Losses (Topic 326)” which provides transition relief for companies adopting ASU 2016-13. This guidance amends ASU 2016-13 to allow companies to elect, upon adoption of ASU 2016-13, the fair value option on financial instruments that were previously recorded at amortized cost under certain circumstances. Companies are required to make this election on and instrument by instrument basis. The guidance will be effective for the fiscal year beginning on July 1, 2023, including interim periods within that year. The adoption of this standard did not have a material impact on the Company’s Consolidated Financial Statements.
In October 2021, the FASB issued ASU 2021-10, Codification Improvements. The guidance contains improvements to the Codification by ensuring that all guidance that requires or provides an option or an entity to provide information in the notes to the financial statements is codified in the Disclosure Section of the Codification. The guidance also contains Codifications that are varied in nature and may affect the application of the guidance in cases in which the original guidance may have been unclear. For public business entities, the amendments in the ASU are effective for annual periods beginning after December 15, 2022, and interim periods within annual periods beginning after December 15, 2023. The adoption of ASU 2021-10 did not have a material impact on its consolidated financial statements.
|
| X |
- DefinitionDisclosure of accounting policy for the shipping and handling costs of product sold.
| Name: |
inbp_CostOfGoodsShippingAndHandlingCostsPolicyTextBlock |
| Namespace Prefix: |
inbp_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy regarding (1) the principles it follows in consolidating or combining the separate financial statements, including the principles followed in determining the inclusion or exclusion of subsidiaries or other entities in the consolidated or combined financial statements and (2) its treatment of interests (for example, common stock, a partnership interest or other means of exerting influence) in other entities, for example consolidation or use of the equity or cost methods of accounting. The accounting policy may also address the accounting treatment for intercompany accounts and transactions, noncontrolling interest, and the income statement treatment in consolidation for issuances of stock by a subsidiary. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1
| Name: |
us-gaap_ConsolidationPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for determining the fair value of financial instruments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 60
-Paragraph 1
-SubTopic 10
-Topic 820
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482053/820-10-60-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 825
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-1
| Name: |
us-gaap_FairValueOfFinancialInstrumentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for goodwill and intangible assets. This accounting policy also may address how an entity assesses and measures impairment of goodwill and intangible assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 30
-Topic 350
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-1
| Name: |
us-gaap_GoodwillAndIntangibleAssetsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(h)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-25
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-28
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-19
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-20
| Name: |
us-gaap_IncomeTaxPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of inventory accounting policy for inventory classes, including, but not limited to, basis for determining inventory amounts, methods by which amounts are added and removed from inventory classes, loss recognition on impairment of inventories, and situations in which inventories are stated above cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483489/210-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 912
-SubTopic 330
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482105/912-330-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//330/tableOfContent
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483080/330-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483080/330-10-50-4
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 6
-Subparagraph (a)
-SubTopic 10
-Topic 270
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482989/270-10-45-6
| Name: |
us-gaap_InventoryPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for investment in financial asset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 12
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-12
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 19
-Subparagraph (2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-19
| Name: |
us-gaap_InvestmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for leasing arrangement entered into by lessee. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-1
| Name: |
us-gaap_LesseeLeasesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for estimating the allowance for losses on loans and lease receivables. The disclosure may include (a) how the entity determines each element of the allowance, (b) which loans are evaluated individually and which loans are evaluated as a group, (c) how the entity determines both the allocated and unallocated portions of the allowance, (d) how the entity determines the loss factors applied to graded loans in order to develop a general allowance, and (e) what self-correcting mechanism the entity uses to reduce differences between estimated and actual losses. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 310
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-9
| Name: |
us-gaap_LoansAndLeasesReceivableAllowanceForLoanLossesPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for reclassification affecting comparability of financial statement. Excludes amendment to accounting standards, other change in accounting principle, and correction of error. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 205
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483504/205-10-50-1
| Name: |
us-gaap_PriorPeriodReclassificationAdjustmentDescription |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for revenue from contract with customer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-17
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-19
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-18
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-18
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-20
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-20
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-20
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-20
Reference 9: http://www.xbrl.org/2003/role/exampleRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (e)
-SubTopic 10
-Topic 235
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 606
-Publisher FASB
-URI https://asc.fasb.org//606/tableOfContent
| Name: |
us-gaap_RevenueFromContractWithCustomerPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for award under share-based payment arrangement. Includes, but is not limited to, methodology and assumption used in measuring cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.C.Q3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.D.1.Q5)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.D.3.Q2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.D.2.Q6)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//718/tableOfContent
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationOptionAndIncentivePlansPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 3 - Inventories (Tables)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes Tables |
|
| Schedule of Inventory, Current [Table Text Block] |
| |
|
June 30, |
|
| |
|
2023 |
|
|
2022 |
|
| |
|
|
|
|
|
|
|
|
| Raw materials |
|
$ |
6,859 |
|
|
$ |
7,586 |
|
| Work-in-process |
|
|
2,148 |
|
|
|
1,759 |
|
| Finished goods |
|
|
1,254 |
|
|
|
1,440 |
|
| Total |
|
$ |
10,261 |
|
|
$ |
11,055 |
|
|
| X |
- DefinitionTabular disclosure of the carrying amount as of the balance sheet date of merchandise, goods, commodities, or supplies held for future sale or to be used in manufacturing, servicing or production process. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483489/210-10-50-1
| Name: |
us-gaap_ScheduleOfInventoryCurrentTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 4 - Property and Equipment, Net (Tables)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes Tables |
|
| Property, Plant and Equipment [Table Text Block] |
| | | June 30, | |
| | | 2023 | | | 2022 | |
| | | | | | | | | |
| Land and building | | $ | 1,250 | | | $ | 1,250 | |
| Leasehold improvements | | | 1,371 | | | | 1,371 | |
| Machinery and equipment | | | 6,801 | | | | 6,727 | |
| Transportation equipment | | | - | | | | 6 | |
| | | | 9,422 | | | | 9,354 | |
| Less: Accumulated depreciation and amortization | | | (7,769 | ) | | | (7,444 | ) |
| Total | | $ | 1,653 | | | $ | 1,910 | |
|
| X |
- DefinitionTabular disclosure of physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation and depletion expense and method used, including composite depreciation, and accumulated deprecation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 5 - Senior Credit Facility and Other Long Term Debt (Tables)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes Tables |
|
| Schedule of Debt [Table Text Block] |
| | | Principal Amount | | | Interest Rate | | Maturity Date |
| | | June 30, | | | | | | |
| | | 2023 | | | 2022 | | | | | | |
| Revolving advances under Senior Credit Facility with PNC Bank, National Association | | $ | - | | | $ | 101 | | | | 8.25 | % | 5/15/2024 |
| Total outstanding debt | | $ | - | | | $ | 101 | | | | | | |
|
| X |
- DefinitionTabular disclosure of information pertaining to short-term and long-debt instruments or arrangements, including but not limited to identification of terms, features, collateral requirements and other information necessary to a fair presentation.
| Name: |
us-gaap_ScheduleOfDebtTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- DefinitionThe tabular disclosure for interest expense.
| Name: |
inbp_InterestExpenseDisclosureTableTableTextBlock |
| Namespace Prefix: |
inbp_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 7 - Income Taxes (Tables)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes Tables |
|
| Schedule of Components of Income Tax Expense (Benefit) [Table Text Block] |
| | | For the fiscal year | |
| | | ended June 30, | |
| | | 2023 | | | 2022 | |
| | | | | | | | | |
| Current - Federal tax expense | | $ | (110 | ) | | $ | (600 | ) |
| Current - State tax expense | | | (61 | ) | | | (289 | ) |
| Deferred - Federal benefit | | | 55 | | | | 2,106 | |
| Deferred - State tax (expense) benefit | | | (18 | ) | | | 24 | |
| Income tax (expense) benefit, net | | $ | (134 | ) | | $ | 1,241 | |
|
| Schedule of Effective Income Tax Rate Reconciliation [Table Text Block] |
| | | For the fiscal year | |
| | | ended June 30, | |
| | | 2023 | | | 2022 | |
| Statutory federal income tax rate | | | (21.0 | )% | | | (21.0 | )% |
| State income tax rate | | | (55.1 | )% | | | (11.5 | )% |
| Stock compensation | | | (166.0 | )% | | | (4.6 | )% |
| Change in valuation allowance | | | 201.2 | % | | | 85.6 | % |
| Other temporary differences | | | (62.1 | )% | | | (0.6 | )% |
| Loss on sale of iBio Stock | | | (18.4 | )% | | | - | |
| Non-deductible expenses | | | (12.6 | )% | | | (0.1 | )% |
| Effective income tax rate | | | (134 | )% | | | 47.8 | % |
|
| Schedule of Deferred Tax Assets and Liabilities [Table Text Block] |
| | | June 30, | |
| | | 2023 | | | 2022 | |
| Deferred Tax Assets | | | | | | | | |
| Net operating loss | | $ | 5,105 | | | $ | 5,166 | |
| Capital loss carryover | | | 678 | | | | 485 | |
| Depreciation | | | (139 | ) | | | (157 | ) |
| Inventory | | | 180 | | | | 149 | |
| Other | | | 337 | | | | 296 | |
| Valuation allowance | | | (1,435 | ) | | | (1,141 | ) |
| Total deferred tax asset, net | | $ | 4,726 | | | $ | 4,798 | |
|
| X |
- DefinitionTabular disclosure of the components of income tax expense attributable to continuing operations for each year presented including, but not limited to: current tax expense (benefit), deferred tax expense (benefit), investment tax credits, government grants, the benefits of operating loss carryforwards, tax expense that results from allocating certain tax benefits either directly to contributed capital or to reduce goodwill or other noncurrent intangible assets of an acquired entity, adjustments of a deferred tax liability or asset for enacted changes in tax laws or rates or a change in the tax status of the entity, and adjustments of the beginning-of-the-year balances of a valuation allowance because of a change in circumstances that causes a change in judgment about the realizability of the related deferred tax asset in future years. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 9
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
| Name: |
us-gaap_ScheduleOfComponentsOfIncomeTaxExpenseBenefitTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the components of net deferred tax asset or liability recognized in an entity's statement of financial position, including the following: the total of all deferred tax liabilities, the total of all deferred tax assets, the total valuation allowance recognized for deferred tax assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ScheduleOfDeferredTaxAssetsAndLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the reconciliation using percentage or dollar amounts of the reported amount of income tax expense attributable to continuing operations for the year to the amount of income tax expense that would result from applying domestic federal statutory tax rates to pretax income from continuing operations. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 12
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-12
| Name: |
us-gaap_ScheduleOfEffectiveIncomeTaxRateReconciliationTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 10 - Commitments and Contingencies (Tables)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes Tables |
|
| Lease, Cost [Table Text Block] |
| | | 2023 | | | 2022 | |
| | | Related Party - Vitamin Realty | | | Other Leases | | | Totals | | | Related Party - Vitamin Realty | | | Other Leases | | | Totals | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Operating Lease Costs | | $ | 842 | | | $ | 88 | | | $ | 650 | | | $ | 566 | | | $ | 84 | | | $ | 650 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Finance Operating Lease Costs: | | | | | | | | | | | | | | | | | | | | | | | | |
| Amortization of right-of use assets | | $ | - | | | $ | 12 | | | $ | 12 | | | $ | - | | | $ | 3 | | | $ | 3 | |
| Total Finance Lease Costs | | $ | - | | | $ | 12 | | | $ | 12 | | | $ | - | | | $ | 3 | | | $ | 3 | |
|
| Leases and Remaining Commitments [Table Text Block] |
| | | Right-of-use Assets | | | Current Portion Operating Lease Obligations | | | Operating Lease Obligations | | | Remaining Cash Commitment | |
| | | | | | | | | | | | | | | | | |
| Vitamin Realty Leases | | $ | 2,061 | | | $ | 772 | | | $ | 1,289 | | | $ | 1,972 | |
| Warehouse Lease | | | 541 | | | | 108 | | | | 433 | | | | 631 | |
| Office equipment leases | | | 21 | | | | 8 | | | | 13 | | | | 23 | |
| | | $ | 2,623 | | | $ | 888 | | | $ | 1,735 | | | $ | 2,830 | |
| | | Right-of-use Assets | | | Current Portion Operating Lease Obligations | | | Operating Lease Obligations | | | Remaining Cash Commitment | |
| | | | | | | | | | | | | | | | | |
| Vitamin Realty Leases | | $ | 1,839 | | | $ | 503 | | | $ | 1,338 | | | $ | 1,972 | |
| Office equipment leases | | | 28 | | | | 7 | | | | 21 | | | | 32 | |
| | | $ | 1,867 | | | $ | 510 | | | $ | 1,359 | | | $ | 2,004 | |
|
| Supplemental Cash Flow Information, Leases [Table Text Block] |
| | | Related Party - Vitamin Realty | | | Other Leases | | | Totals | |
| | | | | | | | | | | | | |
| Cash paid for amounts included in the measurement of lease liabilities: | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Operating cash flows from operating leases | | $ | 842 | | | $ | 147 | | | $ | 989 | |
| Operating cash flows from finance lease obligations | | | - | | | | - | | | | - | |
| Financing cash flows from finance lease obligations | | | - | | | | 35 | | | | 35 | |
| | | Related Party - Vitamin Realty | | | Other Leases | | | Totals | |
| | | | | | | | | | | | | |
| Cash paid for amounts included in the measurement of lease liabilities: | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Operating cash flows from operating leases | | $ | 566 | | | $ | 84 | | | $ | 650 | |
| Operating cash flows from finance lease obligations | | | - | | | | - | | | | - | |
| Financing cash flows from finance lease obligations | | | - | | | | - | | | | - | |
|
| Lessee, Operating Lease and Finance Lease, Liability, Maturity [Table Text Block] |
| Year ending June 30, | | Operating Lease Commitment | | | Related Party Operating Lease Commitment | | | Finance Lease Obligation | | | Total | |
| | | | | | | | | | | | | | | | | |
| 2024 | | $ | 146 | | | $ | 842 | | | $ | 42 | | | $ | 1,030 | |
| 2025 | | | 149 | | | | 842 | | | | 7 | | | | 998 | |
| 2026 | | | 148 | | | | 492 | | | | - | | | | 640 | |
| 2027 | | | 148 | | | | - | | | | - | | | | 148 | |
| 2028 | | | 63 | | | | - | | | | - | | | | 63 | |
| Total minimum lease payments | | | 654 | | | | 2,176 | | | | 49 | | | | 2,879 | |
| Imputed interest | | | (92 | ) | | | (115 | ) | | | - | | | | (207 | ) |
| Total | | $ | 562 | | | $ | 2,061 | | | $ | 49 | | | $ | 2,672 | |
|
| X |
- DefinitionA tabular disclosure of leases and remaining lease commitments.
| Name: |
inbp_LeasesAndRemainingCommitmentsTableTextBlock |
| Namespace Prefix: |
inbp_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of undiscounted cash flows of lessee's operating lease and finance lease liability.
| Name: |
inbp_LesseeOperatingLeaseAndFinanceLeaseLiabilityMaturityTableTextBlock |
| Namespace Prefix: |
inbp_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA tabular disclosure of supplemental cash flow information related to leases.
| Name: |
inbp_SupplementalCashFlowInformationLeasesTableTextBlock |
| Namespace Prefix: |
inbp_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of lessee's lease cost. Includes, but is not limited to, interest expense for finance lease, amortization of right-of-use asset for finance lease, operating lease cost, short-term lease cost, variable lease cost and sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCostTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 12 - Equity Transactions and Stock-based Compensation (Tables)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes Tables |
|
| Schedule of Share-Based Payment Award, Stock Options, Valuation Assumptions [Table Text Block] |
| Risk Free Interest Rate | | | 3.85% to 3.00 | % |
| Volatility | | | 103.3% to 116.9 | % |
| Term | | | 4.5 to 7.5 years | |
| Dividend Rate | | | 0.00 | % |
| Closing Price of Common Stock | | $ | 0.41 | |
|
| Share-Based Payment Arrangement, Option, Activity [Table Text Block] |
| | | | | | | Weighted | |
| | | | | | | Average | |
| | | | | | | Exercise | |
| | | Options | | | Price | |
| | | | | | | | | |
| Outstanding as of July 1, 2021 | | | 3,916,666 | | | $ | 0.26 | |
| Granted | | | 764,700 | | | | 0.85 | |
| Exercised | | | (146,333 | ) | | | 0.23 | |
| Terminated | | | (44,700 | ) | | | 0.53 | |
| Expired | | | (46,400 | ) | | | 0.47 | |
| Outstanding as of June 30, 2022 | | | 4,443,933 | | | | 0.36 | |
| Granted | | | 527,000 | | | | 0.42 | |
| Exercised | | | - | | | | - | |
| Terminated | | | - | | | | - | |
| Expired | | | (594,649 | ) | | | 0.46 | |
| Outstanding as of June 30, 2023 | | | 4,376,284 | | | $ | 0.35 | |
| | | | | | | | | |
| Exercisable at June 30, 2022 | | | 3,524,500 | | | $ | 0.26 | |
| Exercisable at June 30, 2023 | | | 3,626,551 | | | $ | 0.30 | |
|
| Share-Based Payment Arrangement, Option, Exercise Price Range [Table Text Block] |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | Weighted | |
| | | | | | | | | | Weighted | | Weighted Average | | | | Average | |
| | | | | | Average | | Remaining | | | | Exercise | |
| Range of Exercise Price | | Outstanding | | Exercise Price | | Contractual Life (years) | Exercisable | | Price | |
| | | | | | | | | | | | | | | | | | | |
| $ | 0.09 | - | $ | 0.10 | | | 1,221,000 | | $ | 0.09 | | 1.9 | | 1,296,000 | | $ | 0.09 | |
| $ | 0.21 | - | $ | 0.21 | | | 1,194,000 | | | 0.21 | | 5.9 | | 1,209,000 | | | 0.21 | |
| $ | 0.23 | - | $ | 0.25 | | | 250,000 | | | 0.23 | | 3.4 | | 250,000 | | | 0.23 | |
| $ | 0.41 | - | $ | 0.41 | | | 426,500 | | | 0.41 | | 9.4 | | - | | | 0.00 | |
| $ | 0.51 | - | $ | 0.51 | | | 200,000 | | | 0.51 | | 9.0 | | 200,000 | | | 0.51 | |
| $ | 0.65 | - | $ | 0.65 | | | 570,667 | | | 0.65 | | 7.4 | | 431,000 | | | 0.65 | |
| $ | 0.72 | - | $ | 0.72 | | | 66,667 | | | 0.72 | | 0.7 | | 66,667 | | | 0.72 | |
| $ | 0.95 | - | $ | 0.95 | | | 425,783 | | | 0.95 | | 8.4 | | 242,217 | | | 0.95 | |
| $ | 1.05 | - | $ | 1.05 | | | 21,667 | | | 1.05 | | 0.7 | | 21,667 | | | 1.05 | |
| $ | 0.09 | - | $ | 1.05 | | | 4,376,284 | | $ | 0.35 | | 7.0 | | 3,626,551 | | $ | 0.30 | |
|
| X |
- DefinitionTabular disclosure of option exercise prices, by grouped ranges, including the upper and lower limits of the price range, the number of shares under option, weighted average exercise price and remaining contractual option terms. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the significant assumptions used during the year to estimate the fair value of stock options, including, but not limited to: (a) expected term of share options and similar instruments, (b) expected volatility of the entity's shares, (c) expected dividends, (d) risk-free rate(s), and (e) discount for post-vesting restrictions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (f)(2)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 13 - Segment Information (Tables)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Notes Tables |
|
| Schedule of Segment Reporting Information, by Segment [Table Text Block] |
| | | | Sales, Net | | | Segment | | | | | | | | | | | | | |
| | | | U.S. | | | International | | | | | | | Gross | | | | | | | Capital | | | Total | |
| | | | Customers | | | Customers | | | Total | | | Profit | | | Depreciation | | | Expenditures | | | Assets | |
| Contract Manufacturing | 2023 | | $ | 40,230 | | | $ | 8,047 | | | $ | 48,277 | | | $ | 3,630 | | | $ | 348 | | | $ | 109 | | | $ | 19,507 | |
| | 2022 | | | 44,450 | | | | 9,641 | | | | 54,091 | | | | 5,917 | | | | 332 | | | | 486 | | | | 19,061 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Other Nutraceutical Businesses | 2023 | | | 2,387 | | | | 8 | | | | 2,395 | | | | 431 | | | | 3 | | | | 7 | | | | 5,924 | |
| | 2022 | | | 2,067 | | | | 88 | | | | 2,155 | | | | 635 | | | | 4 | | | | - | | | | 6,189 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total Company | 2023 | | | 42,617 | | | | 8,055 | | | | 50,672 | | | | 4,061 | | | | 351 | | | | 116 | | | | 25,431 | |
| | 2022 | | | 46,517 | | | | 9,729 | | | | 56,246 | | | | 6,552 | | | | 336 | | | | 486 | | | | 25,250 | |
|
| X |
- DefinitionTabular disclosure of the profit or loss and total assets for each reportable segment. An entity discloses certain information on each reportable segment if the amounts (a) are included in the measure of segment profit or loss reviewed by the chief operating decision maker or (b) are otherwise regularly provided to the chief operating decision maker, even if not included in that measure of segment profit or loss. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-25
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 30
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
| Name: |
us-gaap_ScheduleOfSegmentReportingInformationBySegmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- DefinitionAmount, including tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value-added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479941/924-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerIncludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=inbp_BrandedProductsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 2 - Summary of Significant Accounting Policies (Details Textual) - USD ($)
$ in Thousands |
12 Months Ended |
24 Months Ended |
|
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 30, 2021 |
Jun. 30, 2020 |
Jun. 30, 2023 |
Aug. 31, 2008 |
| Cost of Goods and Services Sold |
$ 46,611
|
$ 49,694
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Award Vesting Period (Year) |
|
3 years
|
|
|
|
|
| Liability for Uncertainty in Income Taxes, Current |
|
|
$ 0
|
|
|
|
| Accounts Receivable, Allowance for Credit Loss |
37
|
$ 85
|
|
|
$ 37
|
|
| Impairment of Intangible Assets, Finite-Lived |
|
|
$ 0
|
|
|
|
| Cost Method Investment, Ownership Percentage |
|
|
|
|
|
6.00%
|
| Asset Impairment Charges, Total |
|
2,562
|
|
|
|
|
| Realized Investment Gains (Losses) |
(35)
|
0
|
|
|
|
|
| Unrealized Gain (Loss) on Investments |
$ 27
|
(55)
|
|
|
|
|
| Investment Owned, at Fair Value |
|
$ 12
|
|
|
|
|
| Building and Building Improvements [Member] |
|
|
|
|
|
|
| Property, Plant and Equipment, Useful Life (Year) |
|
15 years
|
|
|
|
|
| Machinery and Equipment [Member] |
|
|
|
|
|
|
| Property, Plant and Equipment, Useful Life (Year) |
|
7 years
|
|
|
|
|
| Air Transportation Equipment [Member] |
|
|
|
|
|
|
| Property, Plant and Equipment, Useful Life (Year) |
|
5 years
|
|
|
|
|
| State and Local Jurisdiction [Member] | New Jersey Division of Taxation [Member] | MDC [Member] |
|
|
|
|
|
|
| Income Tax Examination, Year under Examination |
|
2014
|
|
|
|
|
| State and Local Jurisdiction [Member] | New Jersey Division of Taxation [Member] | CII [Member] |
|
|
|
|
|
|
| Income Tax Examination, Year under Examination |
|
2016
|
|
2016 2017 2018 2019
|
|
|
| Shipping and Handling [Member] |
|
|
|
|
|
|
| Cost of Goods and Services Sold |
|
$ 349
|
|
|
$ 1,006
|
|
| X |
- DefinitionThe percentage of ownership of common stock or equity participation in the investee accounted for under the cost method of accounting.
| Name: |
inbp_CostMethodInvestmentOwnershipPercentage |
| Namespace Prefix: |
inbp_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of allowance for credit loss on accounts receivable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479344/326-20-45-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-4
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479319/326-20-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479319/326-20-50-13
| Name: |
us-gaap_AllowanceForDoubtfulAccountsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of write-down of assets recognized in the income statement. Includes, but is not limited to, losses from tangible assets, intangible assets and goodwill. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482130/360-10-45-4
| Name: |
us-gaap_AssetImpairmentCharges |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs related to goods produced and sold and services rendered by an entity during the reporting period. This excludes costs incurred during the reporting period related to financial services rendered and other revenue generating activities. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479941/924-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.2(a),(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_CostOfGoodsAndServicesSold |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of impairment loss recognized in the period resulting from the write-down of the carrying amount of a finite-lived intangible asset to fair value. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-3
| Name: |
us-gaap_ImpairmentOfIntangibleAssetsFinitelived |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionFair value of investment in security owned. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 55
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480493/946-210-55-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-2
Reference 10: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(2)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(2)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column F))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(b)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column F)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column F)(Footnote 7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
| Name: |
us-gaap_InvestmentOwnedAtFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount recognized for uncertainty in income taxes classified as current. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilityForUncertainTaxPositionsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionUseful life of long lived, physical assets used in the normal conduct of business and not intended for resale, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Examples include, but not limited to, land, buildings, machinery and equipment, office equipment, furniture and fixtures, and computer equipment.
| Name: |
us-gaap_PropertyPlantAndEquipmentUsefulLife |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of realized gain (loss) on investment. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
| Name: |
us-gaap_RealizedInvestmentGainsLosses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod over which grantee's right to exercise award under share-based payment arrangement is no longer contingent on satisfaction of service or performance condition, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, combination of market, performance or service condition. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingPeriod1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of unrealized gain (loss) on investment. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_UnrealizedGainLossOnInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_BuildingAndBuildingImprovementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_MachineryAndEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_AirTransportationEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=inbp_MDCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=inbp_CIIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=us-gaap_ShippingAndHandlingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 3 - Inventories - Inventories (Details) - USD ($)
$ in Thousands |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Raw materials |
$ 6,859
|
$ 7,586
|
| Work-in-process |
2,148
|
1,759
|
| Finished goods |
1,254
|
1,440
|
| Total |
$ 10,261
|
$ 11,055
|
| X |
- DefinitionCarrying amount, net of valuation reserves and adjustments, as of the balance sheet date of merchandise or goods held by the company that are readily available for sale. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.BB)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480581/330-10-S99-2
| Name: |
us-gaap_InventoryFinishedGoodsNetOfReserves |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after valuation and LIFO reserves of inventory expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying amount, net of valuation reserves and adjustments, as of the balance sheet date of unprocessed items to be consumed in the manufacturing or production process. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.BB)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480581/330-10-S99-2
| Name: |
us-gaap_InventoryRawMaterialsNetOfReserves |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying amount, net of reserves and adjustments, as of the balance sheet date of merchandise or goods which are partially completed. This inventory is generally comprised of raw materials, labor and factory overhead costs, which require further materials, labor and overhead to be converted into finished goods, and which generally require the use of estimates to determine percentage complete and pricing. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.BB)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480581/330-10-S99-2
| Name: |
us-gaap_InventoryWorkInProcessNetOfReserves |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.2
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of gain (loss) on sale or disposal of property, plant and equipment assets, including oil and gas property and timber property. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_GainLossOnSaleOfPropertyPlantEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of divestiture of long-lived, physical assets used in the normal conduct of business and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, furniture and fixtures, and computer equipment.
| Name: |
us-gaap_PropertyPlantAndEquipmentDisposals |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=inbp_FullyDepreciatedPropertyMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=inbp_PropertyNotFullyDepreciatedMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_EquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 4 - Property and Equipment, Net - Property and Equipment, Net (Details) - USD ($)
$ in Thousands |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Property and equipment, gross |
$ 9,422
|
$ 9,354
|
| Less: Accumulated depreciation and amortization |
(7,769)
|
(7,444)
|
| Total |
1,653
|
1,910
|
| Land and Building [Member] |
|
|
| Property and equipment, gross |
1,250
|
1,250
|
| Leasehold Improvements [Member] |
|
|
| Property and equipment, gross |
1,371
|
1,371
|
| Machinery and Equipment [Member] |
|
|
| Property and equipment, gross |
6,801
|
6,727
|
| Transportation Equipment [Member] |
|
|
| Property and equipment, gross |
$ 0
|
$ 6
|
| X |
- DefinitionAmount of accumulated depreciation, depletion and amortization for physical assets used in the normal conduct of business to produce goods and services. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_AccumulatedDepreciationDepletionAndAmortizationPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(13))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_LandAndBuildingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_LeaseholdImprovementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_MachineryAndEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_TransportationEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 5 - Senior Credit Facility and Other Long Term Debt (Details Textual)
$ in Thousands |
|
3 Months Ended |
12 Months Ended |
|
|
May 15, 2019
USD ($)
|
Mar. 31, 2023 |
Jun. 30, 2023
USD ($)
|
Jun. 30, 2022
USD ($)
|
Jun. 30, 2021
USD ($)
|
May 11, 2023 |
| Proceeds from Sale, Maturity and Collection of Investments, Total |
|
|
$ 4
|
$ 0
|
|
|
| iBio Stock [Member] |
|
|
|
|
|
|
| Proceeds from Sale, Maturity and Collection of Investments, Total |
|
|
|
|
$ 96
|
|
| Amended Loan Agreement [Member] |
|
|
|
|
|
|
| Senior Notes, Total |
$ 11,585
|
|
|
|
|
|
| Debt Instrument, Debt Default, Interest Rate Basic Spread |
2.00%
|
|
|
|
|
|
| Line of Credit Facility Covenant Prepayment Provisions Percentage of Excess Cash flow |
|
|
|
|
25.00%
|
|
| Amended Loan Agreement [Member] | Secured Overnight Financing Rate (SOFR) Overnight Index Swap Rate [Member] |
|
|
|
|
|
|
| Debt Instrument, Basis Spread on Variable Rate |
|
0.10%
|
|
|
|
|
| Amended Loan Agreement [Member] | Term Loan [Member] |
|
|
|
|
|
|
| Debt Instrument, Face Amount |
$ 3,585
|
|
|
|
|
|
| Line of Credit Facility, Interest Rate at Period End |
|
|
|
5.00%
|
|
|
| Number of Consecutive Monthly Installments |
84
|
|
|
|
|
|
| Number of Consecutive Monthly Installments, Fixed Amount |
83
|
|
|
|
|
|
| Debt Instrument, Periodic Payment, Total |
$ 43
|
|
|
|
|
|
| Line of Credit Facility Covenant Prepayment Provisions Percentage of Excess Cash flow |
25.00%
|
|
|
|
|
|
| Amended Loan Agreement [Member] | Term Loan [Member] | Eurodollar [Member] |
|
|
|
|
|
|
| Debt Instrument, Basis Spread on Variable Rate |
3.00%
|
|
|
|
|
|
| Amended Loan Agreement [Member] | Revolving Credit Facility [Member] |
|
|
|
|
|
|
| Line of Credit Facility, Maximum Borrowing Capacity |
$ 8,000
|
|
|
|
|
|
| Line of Credit Facility, Interest Rate at Period End |
|
|
8.25%
|
4.75%
|
|
8.50%
|
| Line of Credit Facility Covenant Maximum Aggregate Revolving Advance |
$ 8,000
|
|
|
|
|
|
| Line of Credit Facility Covenant Aggregate Revolving Advance Receivables Advance Rate |
85.00%
|
|
|
|
|
|
| Line of Credit Facility Covenant Aggregate Revolving Advance Inventory Advance Rate |
75.00%
|
|
|
|
|
|
| Line of Credit Facility, Covenan,t Aggregate Revolving Advance, Appraised Liquidation Value, Inventory Advance Rate |
85.00%
|
|
|
|
|
|
| Amended Loan Agreement [Member] | Revolving Credit Facility [Member] | Eurodollar [Member] |
|
|
|
|
|
|
| Debt Instrument, Basis Spread on Variable Rate |
2.50%
|
|
|
|
|
|
| X |
- DefinitionPercentage points added to the interest rate in case of debt instrument defaulted.
| Name: |
inbp_DebtInstrumentDebtDefaultInterestRateBasicSpread |
| Namespace Prefix: |
inbp_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe percentage of appraised net orderly liquidation value of eligible inventory that used to calculate the aggregate amount of revolving advances that can be made to the company.
| Name: |
inbp_LineOfCreditFacilityCovenantAggregateRevolvingAdvanceAppraisedLiquidationValueInventoryAdvanceRate |
| Namespace Prefix: |
inbp_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe inventory advance rate used to calculate the aggregate amount of revolving advances that can be made to the company.
| Name: |
inbp_LineOfCreditFacilityCovenantAggregateRevolvingAdvanceInventoryAdvancerate |
| Namespace Prefix: |
inbp_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum percentage of eligible accounts receivable used to calculate the aggregate amount of revolving advances that can be made to the company.
| Name: |
inbp_LineOfCreditFacilityCovenantAggregateRevolvingAdvanceReceivablesAdvancerate |
| Namespace Prefix: |
inbp_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum amount of aggregate revolving advances that can be made under the credit facility.
| Name: |
inbp_LineOfCreditFacilityCovenantMaximumAggregateRevolvingadvance |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe prepayment requirement as a percentage of excess cashflow for each fiscal year.
| Name: |
inbp_LineOfCreditFacilityCovenantPrepaymentProvisionsPercentageOfExcessCashflow |
| Namespace Prefix: |
inbp_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the number of consecutive monthly payments for a term loan.
| Name: |
inbp_NumberOfConsecutiveMonthlyInstallments |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the number of consecutive monthly installments that have a fixed amount.
| Name: |
inbp_NumberOfConsecutiveMonthlyInstallmentsFixedAmount |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPercentage points added to the reference rate to compute the variable rate on the debt instrument.
| Name: |
us-gaap_DebtInstrumentBasisSpreadOnVariableRate1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFace (par) amount of debt instrument at time of issuance. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482900/835-30-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69B
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69C
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-2
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482949/835-30-55-8
| Name: |
us-gaap_DebtInstrumentFaceAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of the required periodic payments including both interest and principal payments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 470
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480848/942-470-50-3
| Name: |
us-gaap_DebtInstrumentPeriodicPayment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe effective interest rate at the end of the reporting period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(b),22(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LineOfCreditFacilityInterestRateAtPeriodEnd |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionMaximum borrowing capacity under the credit facility without consideration of any current restrictions on the amount that could be borrowed or the amounts currently outstanding under the facility. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(b),22(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LineOfCreditFacilityMaximumBorrowingCapacity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow associated with the sale, maturity and collection of all investments such as debt, security and so forth during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-12
| Name: |
us-gaap_ProceedsFromSaleMaturityAndCollectionsOfInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionIncluding the current and noncurrent portions, carrying value as of the balance sheet date of Notes with the highest claim on the assets of the issuer in case of bankruptcy or liquidation (with maturities initially due after one year or beyond the operating cycle if longer). Senior note holders are paid off in full before any payments are made to junior note holders. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.16)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_SeniorNotes |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=inbp_iBioStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=inbp_AmendedLoanAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_VariableRateAxis=us-gaap_SecuredOvernightFinancingRateSofrOvernightIndexSwapRateMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_LongtermDebtTypeAxis=inbp_TermLoanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_VariableRateAxis=us-gaap_EurodollarMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_CreditFacilityAxis=us-gaap_RevolvingCreditFacilityMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionEffective interest rate for the funds borrowed under the debt agreement considering interest compounding and original issue discount or premium. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482900/835-30-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-6
| Name: |
us-gaap_DebtInstrumentInterestRateEffectivePercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt. Excludes lease obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69B
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69C
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1D
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-4
| Name: |
us-gaap_LongTermDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionIncluding the current and noncurrent portions, aggregate carrying amount of all types of notes payable, as of the balance sheet date, with initial maturities beyond one year or beyond the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_NotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_LineOfCreditFacilityAxis=inbp_RevolvingAdvancesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionWeighted average interest rate of debt outstanding.
| Name: |
us-gaap_DebtWeightedAverageInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of interest payable on debt, including, but not limited to, trade payables. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(15)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.15(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_InterestPayableCurrentAndNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
| X |
- DefinitionAmount of the cost of borrowed funds accounted for as interest expense. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (210.5-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate amount of interest paid or due on all long-term debt. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Regulation S-K (SK)
-Number 229
-Section 1402
-Paragraph (a)
-Publisher SEC
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Regulation S-K (SK)
-Number 229
-Section 1402
-Paragraph (b)
-Subparagraph (1)
-Publisher SEC
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.8)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_InterestExpenseLongTermDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest expense classified as other.
| Name: |
us-gaap_InterestExpenseOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionInterest expense incurred during the reporting period on subordinated notes and debentures. Includes amortization of expenses incurred in the issuance of subordinated notes and debentures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.8)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_InterestExpenseSubordinatedNotesAndDebentures |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_LongtermDebtTypeAxis=us-gaap_SeniorNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 7 - Income Taxes (Details Textual) - USD ($)
$ in Thousands |
12 Months Ended |
|
|
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 30, 2020 |
Jul. 10, 2023 |
Jun. 30, 2021 |
| Valuation Allowance, Deferred Tax Asset, Increase (Decrease), Amount |
$ (18)
|
$ 24
|
|
|
|
| Liability for Uncertainty in Income Taxes, Current |
|
|
|
|
$ 0
|
| Income Tax Examination, Penalties and Interest Accrued, Total |
|
|
|
|
0
|
| Deferred Tax Assets Related to Net Operating Loss [Member] |
|
|
|
|
|
| Valuation Allowance, Deferred Tax Asset, Increase (Decrease), Amount |
1,795
|
|
|
|
|
| Capital Loss Carryforward [Member] |
|
|
|
|
|
| Tax Credit Carryforward, Amount |
|
|
|
$ 2,343
|
|
| Capital Loss Carryforward [Member] | Tax Year 2025 [Member] |
|
|
|
|
|
| Tax Credit Carryforward, Amount |
|
|
|
|
271
|
| Capital Loss Carryforward [Member] | Tax Year 2026 [Member] |
|
|
|
|
|
| Tax Credit Carryforward, Amount |
|
|
|
|
1,152
|
| Capital Loss Carryforward [Member] | Tax Year 2028 [Member] |
|
|
|
|
|
| Tax Credit Carryforward, Amount |
|
|
|
|
$ 920
|
| Domestic Tax Authority [Member] |
|
|
|
|
|
| Operating Loss Carryforwards |
21,700
|
|
|
|
|
| State and Local Jurisdiction [Member] |
|
|
|
|
|
| Operating Loss Carryforwards |
$ 3,997
|
|
|
|
|
| State and Local Jurisdiction [Member] | New Jersey Division of Taxation [Member] | CII [Member] |
|
|
|
|
|
| Income Tax Examination, Year under Examination |
|
2016
|
2016 2017 2018 2019
|
|
|
| X |
- DefinitionAmount recognized for uncertainty in income taxes classified as current. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilityForUncertainTaxPositionsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of operating loss carryforward, before tax effects, available to reduce future taxable income under enacted tax laws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_OperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of the tax credit carryforward, before tax effects, available to reduce future taxable income under enacted tax laws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_TaxCreditCarryforwardAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in the valuation allowance for a specified deferred tax asset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ValuationAllowanceDeferredTaxAssetChangeInAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=us-gaap_CapitalLossCarryforwardMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=inbp_TaxYear2025Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=inbp_TaxYear2026Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=inbp_TaxYear2028Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=inbp_CIIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionAmount of current federal tax expense (benefit) attributable to income (loss) from continuing operations. Includes, but is not limited to, current national tax expense (benefit) for non-US (United States of America) jurisdiction. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(1)(Note 1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-SubTopic 10
-Topic 740
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 6.I.7)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-1
| Name: |
us-gaap_CurrentFederalTaxExpenseBenefit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of current state and local tax expense (benefit) attributable to income (loss) from continuing operations. Includes, but is not limited to, current regional, territorial, and provincial tax expense (benefit) for non-US (United States of America) jurisdiction. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(1)(Note 1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-SubTopic 10
-Topic 740
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 6.I.7)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-1
| Name: |
us-gaap_CurrentStateAndLocalTaxExpenseBenefit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of deferred federal, state, and local tax expense (benefit) attributable to income (loss) from continuing operations. Includes, but is not limited to, deferred national, regional, territorial, and provincial tax expense (benefit) for non-US (United States of America) jurisdiction. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-10
| Name: |
us-gaap_DeferredFederalStateAndLocalTaxExpenseBenefit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in the valuation allowance for a specified deferred tax asset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ValuationAllowanceDeferredTaxAssetChangeInAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.2
v3.23.2
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible capital loss carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsCapitalLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from derivative instruments. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsDerivativeInstruments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from inventory. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsInventory |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allocation of valuation allowances and deferred tax liability, of deferred tax asset attributable to deductible differences and carryforwards, without jurisdictional netting. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsLiabilitiesNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible operating loss carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsOperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax assets for which it is more likely than not that a tax benefit will not be realized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsValuationAllowance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax liability attributable to taxable temporary differences from property, plant, and equipment. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxLiabilitiesPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
| X |
- DefinitionAmount of discretionary contributions made by an employer to a defined contribution plan.
| Name: |
us-gaap_DefinedContributionPlanEmployerDiscretionaryContributionAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
Note 9 - Significant Risks and Uncertainties (Details Textual)
$ in Thousands |
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022
USD ($)
|
| Cash, FDIC Insured Amount |
|
$ 250
|
| Cash, Uninsured Amount |
|
$ 1,949
|
| Revenue Benchmark [Member] | Customer Concentration Risk [Member] |
|
|
| Number of Major Customers |
|
2
|
| Revenue Benchmark [Member] | Customer Concentration Risk [Member] | Two Customers [Member] |
|
|
| Concentration Risk, Percentage |
89.00%
|
90.00%
|
| Revenue Benchmark [Member] | Customer Concentration Risk [Member] | Major Customer 1 [Member] | Contract Manufacturing [Member] |
|
|
| Concentration Risk, Percentage |
70.00%
|
67.00%
|
| Revenue Benchmark [Member] | Customer Concentration Risk [Member] | Major Customer 1 [Member] | Other Nutraceutical Business [Member] |
|
|
| Concentration Risk, Percentage |
56.00%
|
40.00%
|
| Revenue Benchmark [Member] | Customer Concentration Risk [Member] | Major Customer 2 [Member] | Contract Manufacturing [Member] |
|
|
| Concentration Risk, Percentage |
24.00%
|
26.00%
|
| Revenue Benchmark [Member] | Customer Concentration Risk [Member] | Major Customer 2 [Member] | Other Nutraceutical Business [Member] |
|
|
| Concentration Risk, Percentage |
16.00%
|
1.00%
|
| Revenue Benchmark [Member] | Customer Concentration Risk [Member] | Major Customer 3 [Member] | Other Nutraceutical Business [Member] |
|
|
| Concentration Risk, Percentage |
9.00%
|
19.00%
|
| Accounts Receivable [Member] | Customer Concentration Risk [Member] | Two Customers [Member] |
|
|
| Concentration Risk, Percentage |
84.00%
|
70.00%
|
| Revenue, Segment Benchmark [Member] | Customer Concentration Risk [Member] | Major Customer 1 [Member] | Branded Nutraceutical [Member] |
|
|
| Concentration Risk, Percentage |
10.00%
|
|
| Number of Employees, Geographic Area [Member] | Unionized Employees Concentration Risk [Member] |
|
|
| Concentration Risk, Percentage |
74.00%
|
|
| X |
- DefinitionRepresents the number of major customers accounting for 10% or more of the specified concentration risk benchmark, which includes, but not limited to, sales revenue, accounts receivable, etc.
| Name: |
inbp_NumberOfMajorCustomers |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of cash deposited in financial institutions as of the balance sheet date that is insured by the Federal Deposit Insurance Corporation.
| Name: |
us-gaap_CashFDICInsuredAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of cash as of the balance sheet date that is not insured by the Federal Deposit Insurance Corporation.
| Name: |
us-gaap_CashUninsuredAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionFor an entity that discloses a concentration risk in relation to quantitative amount, which serves as the "benchmark" (or denominator) in the equation, this concept represents the concentration percentage derived from the division. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 21
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-21
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-20
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-18
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-20
| Name: |
us-gaap_ConcentrationRiskPercentage1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_SalesRevenueNetMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByTypeAxis=us-gaap_CustomerConcentrationRiskMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_MajorCustomersAxis=inbp_TwoCustomersMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_MajorCustomersAxis=inbp_MajorCustomer1Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=inbp_ContractManufacturingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=inbp_OtherNutraceuticalBusinessMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_MajorCustomersAxis=inbp_MajorCustomer2Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_MajorCustomersAxis=inbp_MajorCustomer3Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_AccountsReceivableMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_SalesRevenueSegmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=inbp_BrandedNutraceuticalMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_NumberOfEmployeesGeographicAreaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByTypeAxis=us-gaap_UnionizedEmployeesConcentrationRiskMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 10 - Commitments and Contingencies (Details Textual)
|
|
|
|
|
12 Months Ended |
|
|
|
|
|
Jul. 01, 2022
USD ($)
|
Jun. 15, 2022
USD ($)
|
May 19, 2014
USD ($)
ft²
|
Jan. 05, 2012
USD ($)
ft²
|
Jun. 30, 2023
USD ($)
|
Jun. 30, 2022
USD ($)
|
Aug. 04, 2023
USD ($)
|
Mar. 31, 2023
USD ($)
a
|
Jul. 15, 2022
ft²
|
Jan. 04, 2012
ft²
|
| Subsequent Event [Member] | Transportation Equipment [Member] |
|
|
|
|
|
|
|
|
|
|
| Lessee, Operating Lease, Term of Contract (Year) |
|
|
|
|
|
|
4 years
|
|
|
|
| Lessee, Operating Lease, Liability, Annual Commitment |
|
|
|
|
|
|
$ 21
|
|
|
|
| Operating Lease Renewed for Office Space [Member] |
|
|
|
|
|
|
|
|
|
|
| Lessee, Operating Lease, Term of Contract (Year) |
|
|
|
|
|
|
|
1 year
|
|
|
| Lessee, Operating Lease, Liability, Annual Commitment |
|
|
|
|
|
|
|
$ 10,000
|
|
|
| Third Amendment With Vitamin Realty [Member] |
|
|
|
|
|
|
|
|
|
|
| Area of Real Estate Property (Square Foot) | a |
|
|
|
|
|
|
|
12,500
|
|
|
| Lessee, Operating Lease, Term of Contract (Year) |
|
|
|
|
|
|
|
5 years
|
|
|
| Lessee, Operating Lease, Liability, Annual Commitment |
|
|
|
|
|
|
|
$ 134,000
|
|
|
| Fifth Year of Finance Lease [Member] |
|
|
|
|
|
|
|
|
|
|
| Lessee, Operating Lease, Liability, Annual Commitment |
|
|
|
|
|
|
|
$ 150,000
|
|
|
| Unrelated Party [Member] |
|
|
|
|
|
|
|
|
|
|
| Operating Lease, Liability |
|
|
|
|
$ 562,000
|
|
|
|
|
|
| Lessee, Operating Lease, Discount Rate |
|
|
|
|
4.41%
|
3.87%
|
|
|
|
|
| Operating Lease, Weighted Average Remaining Lease Term (Year) |
|
|
|
|
2 years 10 months 24 days
|
3 years 6 months
|
|
|
|
|
| Finance Lease, Liability |
|
$ 85,000
|
|
|
|
|
|
|
|
|
| Debt Instrument, Periodic Payment, Total |
|
$ 4,000
|
|
|
|
|
|
|
|
|
| Debt Instrument, Interest Rate, Stated Percentage |
|
0.00%
|
|
|
|
|
|
|
|
|
| Lessee, Finance Lease, Discount Rate |
|
|
|
|
0.00%
|
|
|
|
|
|
| Finance Lease, Weighted Average Remaining Lease Term (Year) |
|
|
|
|
1 year 2 months 12 days
|
2 years 2 months 12 days
|
|
|
|
|
| Accounts Payable [Member] |
|
|
|
|
|
|
|
|
|
|
| Finance Lease, Liability |
|
|
|
|
$ 49,000
|
|
|
|
|
|
| Manhattan Drug Company [Member] |
|
|
|
|
|
|
|
|
|
|
| Area of Real Estate Property (Square Foot) | ft² |
|
|
|
76,161
|
|
|
|
|
116,175
|
74,898
|
| Payments for Rent |
$ 842,000
|
|
|
$ 533,000
|
|
|
|
|
|
|
| AgroLabs [Member] |
|
|
|
|
|
|
|
|
|
|
| Area of Real Estate Property (Square Foot) | ft² |
|
|
2,700
|
|
|
|
|
|
|
|
| Payments for Rent |
|
|
$ 27,000
|
|
|
|
|
|
|
|
| Chairman, Chief Executive Office and Major Stockholder [Member] |
|
|
|
|
|
|
|
|
|
|
| Percent of Ownership for Warehouse and Office Facilities Leased |
|
|
|
|
|
100.00%
|
|
|
|
|
| Vitamin Realty LLC [Member] |
|
|
|
|
|
|
|
|
|
|
| Operating Lease, Expense |
|
|
|
|
1,269,000
|
$ 896,000
|
|
|
|
|
| Operating Lease, Liability |
|
|
|
|
2,061,000
|
1,841,000
|
|
|
|
|
| Vitamin Realty LLC [Member] | Accounts Payable [Member] |
|
|
|
|
|
|
|
|
|
|
| Operating Lease, Liability |
|
|
|
|
$ 0
|
$ 72,000
|
|
|
|
|
| Minimum [Member] |
|
|
|
|
|
|
|
|
|
|
| Lessee, Lease, Term of Contract (Year) |
|
|
|
|
1 year
|
|
|
|
|
|
| Maximum [Member] |
|
|
|
|
|
|
|
|
|
|
| Lessee, Lease, Term of Contract (Year) |
|
|
|
|
7 years
|
|
|
|
|
|
| X |
- DefinitionTerm of lessee's operating and finance leases, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days.
| Name: |
inbp_LesseeLeaseTermOfContract |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe annual commitment related to the operating lease.
| Name: |
inbp_LesseeOperatingLeaseLiabilityAnnualCommitment |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the percentage of ownership for warehouse and office facilities leased.
| Name: |
inbp_PercentOfOwnershipForWarehouseAndOfficeFacilitiesLeased |
| Namespace Prefix: |
inbp_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionArea of a real estate property.
| Name: |
us-gaap_AreaOfRealEstateProperty |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:areaItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionContractual interest rate for funds borrowed, under the debt agreement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DebtInstrumentInterestRateStatedPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of the required periodic payments including both interest and principal payments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 470
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480848/942-470-50-3
| Name: |
us-gaap_DebtInstrumentPeriodicPayment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from finance lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_FinanceLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining lease term for finance lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_FinanceLeaseWeightedAverageRemainingLeaseTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDiscount rate used by lessee to determine present value of finance lease payments. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeFinanceLeaseDiscountRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDiscount rate used by lessee to determine present value of operating lease payments. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseDiscountRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTerm of lessee's operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseTermOfContract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of operating lease expense. Excludes sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-4
| Name: |
us-gaap_OperatingLeaseExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionCash payments to lessor's for use of assets under operating leases. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (g)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_PaymentsForRent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_LeaseContractualTermAxis=us-gaap_TransportationEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BalanceSheetLocationAxis=us-gaap_AccountsPayableMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=inbp_ManhattanDrugCompanyMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=inbp_AgrolabsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionAmount of expense on finance lease liability.
| Name: |
inbp_FinanceLeaseExpense |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of amortization expense attributable to right-of-use asset from finance lease. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_FinanceLeaseRightOfUseAssetAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.2
Note 10 - Commitments and Contingencies - Leases and Commitments (Details) - USD ($)
$ in Thousands |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Operating lease right-of-use assets |
$ 2,623
|
$ 1,867
|
| Current portion operating lease obligations |
888
|
510
|
| Operating lease obligations |
1,735
|
1,359
|
| Remaining cash commitment |
2,830
|
2,004
|
| Office Equipment Leases [Member] |
|
|
| Operating lease right-of-use assets |
21
|
28
|
| Current portion operating lease obligations |
8
|
7
|
| Operating lease obligations |
13
|
21
|
| Remaining cash commitment |
23
|
32
|
| Vitamin Realty LLC [Member] |
|
|
| Operating lease right-of-use assets |
2,061
|
1,839
|
| Current portion operating lease obligations |
772
|
503
|
| Operating lease obligations |
1,289
|
1,338
|
| Remaining cash commitment |
1,972
|
$ 1,972
|
| Warehouse Lease [Member] |
|
|
| Operating lease right-of-use assets |
541
|
|
| Current portion operating lease obligations |
108
|
|
| Operating lease obligations |
433
|
|
| Remaining cash commitment |
$ 631
|
|
| X |
- DefinitionAmount of remaining lease commitment.
| Name: |
inbp_LeaseCommitmentAmount |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=inbp_OfficeEquipmentLeasesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionThe cash outflow for the obligation including interest for a lease meeting the criteria for capitalization (with maturities exceeding one year or beyond the operating cycle of the entity, if longer).
| Name: |
inbp_RepaymentsOfLongTermCapitalLeaseObligationsIncludingInterest |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest paid on finance lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-5
| Name: |
us-gaap_FinanceLeaseInterestPaymentOnLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow from operating lease, excluding payments to bring another asset to condition and location necessary for its intended use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-5
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeasePayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.2
Note 10 - Commitments and Contingencies - Minimum Rental Commitment for Long-term Non-cancelable Leases (Details) - USD ($)
$ in Thousands |
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 15, 2022 |
| 2024 |
$ 42
|
|
|
| 2024 |
1,030
|
|
|
| 2025 |
7
|
|
|
| 2025 |
998
|
|
|
| 2026 |
0
|
|
|
| 2026 |
640
|
|
|
| 2027 |
0
|
|
|
| 2027 |
148
|
|
|
| 2028 |
0
|
|
|
| 2028 |
63
|
|
|
| Total minimum lease payments, Capital Lease Obligation |
49
|
|
|
| Total minimum lease payments |
2,879
|
|
|
| Imputed interest, Capital Lease Obligation |
0
|
|
|
| Imputed interest |
(207)
|
|
|
| Total |
2,672
|
|
|
| Accounts Payable [Member] |
|
|
|
| Total, Capital Lease Obligation |
49
|
|
|
| Vitamin Realty LLC [Member] |
|
|
|
| 2024 |
842
|
|
|
| 2025 |
842
|
|
|
| 2026 |
492
|
|
|
| 2027 |
0
|
|
|
| 2028 |
0
|
|
|
| Total minimum lease payments, Operating Lease |
2,176
|
|
|
| Imputed interest, Operating Lease |
(115)
|
|
|
| Total, Operating Lease |
2,061
|
$ 1,841
|
|
| Vitamin Realty LLC [Member] | Accounts Payable [Member] |
|
|
|
| Total, Operating Lease |
0
|
$ 72
|
|
| Unrelated Party [Member] |
|
|
|
| 2024 |
146
|
|
|
| 2025 |
149
|
|
|
| 2026 |
148
|
|
|
| 2027 |
148
|
|
|
| 2028 |
63
|
|
|
| Total minimum lease payments, Operating Lease |
654
|
|
|
| Imputed interest, Operating Lease |
(92)
|
|
|
| Total, Operating Lease |
$ 562
|
|
|
| Total, Capital Lease Obligation |
|
|
$ 85
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments for operating and finance leases.
| Name: |
inbp_LesseeOperatingAndFinanceLeaseLiabilityToBePaidTotal |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating and finance lease to be paid in the fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).
| Name: |
inbp_LesseeOperatingAndFinanceLeaseLiabilityToBePaidYearFive |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating and finance lease to be paid in the fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).
| Name: |
inbp_LesseeOperatingAndFinanceLeaseLiabilityToBePaidYearFour |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating and finance lease to be paid in first fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).
| Name: |
inbp_LesseeOperatingAndFinanceLeaseLiabilityToBePaidYearOne |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating and finance lease to be paid in the third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).
| Name: |
inbp_LesseeOperatingAndFinanceLeaseLiabilityToBePaidYearThree |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating and finance lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).
| Name: |
inbp_LesseeOperatingAndFinanceLeaseLiabilityToBePaidYearTwo |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for finance and operating leases.
| Name: |
inbp_LesseeOperatingAndFinanceLeaseLiabilityUndiscountedExcessAmount |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from finance and operating leases.
| Name: |
inbp_LesseeOperatingAndFinanceLeaseTotal |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from finance lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_FinanceLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments for finance lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_FinanceLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for finance lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_FinanceLeaseLiabilityPaymentsDueNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for finance lease to be paid in fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_FinanceLeaseLiabilityPaymentsDueYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for finance lease to be paid in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_FinanceLeaseLiabilityPaymentsDueYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for finance lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_FinanceLeaseLiabilityPaymentsDueYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for finance lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_FinanceLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for finance lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_FinanceLeaseLiabilityUndiscountedExcessAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityUndiscountedExcessAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_BalanceSheetLocationAxis=us-gaap_AccountsPayableMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 12 - Equity Transactions and Stock-based Compensation (Details Textual) - USD ($)
$ / shares in Units, $ in Thousands |
1 Months Ended |
12 Months Ended |
|
Jul. 31, 2023 |
Nov. 30, 2020 |
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 30, 2021 |
| Share-Based Compensation Arrangement by Share-Based Payment Award, Number of Shares Available for Grant (in shares) |
|
|
|
6,823,569
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
527,000
|
764,700
|
|
| Share-Based Payment Arrangement, Option, Exercise Price Range, Lower Range Limit (in dollars per share) |
|
|
$ 0.09
|
|
|
| Share-Based Payment Arrangement, Option, Exercise Price Range, Upper Range Limit (in dollars per share) |
|
|
1.05
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Award Vesting Period (Year) |
|
|
|
3 years
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Outstanding, Weighted Average Exercise Price (in dollars per share) |
|
|
0.35
|
$ 0.36
|
$ 0.26
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
$ 0.42
|
$ 0.85
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Exercisable, Intrinsic Value |
|
|
$ 461
|
$ 1,002
|
|
| Director [Member] |
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
764,700
|
|
|
| Share-Based Payment Arrangement, Option, Exercise Price Range, Lower Range Limit (in dollars per share) |
|
|
$ 0.51
|
|
|
| Maximum [Member] | Director [Member] |
|
|
|
|
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
1.045
|
|
|
| Minimum [Member] | Director [Member] |
|
|
|
|
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price (in dollars per share) |
|
|
$ 0.95
|
|
|
| Share-Based Payment Arrangement, Option [Member] |
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Award Vesting Period (Year) |
|
|
3 years
|
|
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share, Amount (in shares) |
|
|
4,376,284
|
537,600
|
|
| Share-Based Payment Arrangement, Option [Member] | Subsequent Event [Member] | Director [Member] |
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Number of Shares Authorized (in shares) |
200,000
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Award Vesting Period (Year) |
1 year
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Outstanding, Weighted Average Exercise Price (in dollars per share) |
$ 0.33
|
|
|
|
|
| Share-Based Payment Arrangement, Option [Member] | Subsequent Event [Member] | Director [Member] | Share-Based Payment Arrangement, Tranche One [Member] |
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Award Vesting Rights, Percentage |
25.00%
|
|
|
|
|
| Share-Based Payment Arrangement, Option [Member] | Subsequent Event [Member] | Each Director [Member] |
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Number of Shares Authorized (in shares) |
50,000
|
|
|
|
|
| Share-Based Payment Arrangement, Option [Member] | Maximum [Member] |
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Expiration Period (Year) |
|
10 years
|
|
10 years
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Award Vesting Period (Year) |
|
|
|
3 years
|
|
| Share-Based Payment Arrangement, Expense |
|
|
|
$ 369
|
|
| Share-Based Payment Arrangement, Nonvested Award, Cost Not yet Recognized, Amount, Total |
|
|
|
$ 480
|
|
| Share-Based Payment Arrangement, Nonvested Award, Cost Not yet Recognized, Period for Recognition (Year) |
|
|
|
2 years 6 months
|
|
| Share-Based Payment Arrangement, Option [Member] | Minimum [Member] |
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Expiration Period (Year) |
|
5 years
|
|
5 years
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Award Vesting Period (Year) |
|
|
|
1 year
|
|
| Share-Based Payment Arrangement, Expense |
|
|
$ 320
|
|
|
| Stock Option 2001 Plan [Member] |
|
|
|
|
|
| Share Based Compensation Arrangement by Share Based Payment Award Number of Shares Originally Authorized (in shares) |
|
|
|
7,000,000
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Number of Additional Shares Authorized (in shares) |
|
|
|
6,000,000
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Number of Shares Authorized (in shares) |
|
|
|
13,000,000
|
|
| Stock Option 1997 Plan [Member] |
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Number of Shares Authorized (in shares) |
|
|
|
5,000,000
|
|
| Stock Option 2022 Plan [Member] |
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Number of Shares Authorized (in shares) |
|
|
538,500
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross (in shares) |
|
|
527,000
|
|
|
| Share-Based Payment Arrangement, Option, Exercise Price Range, Lower Range Limit (in dollars per share) |
|
|
$ 0.41
|
|
|
| Share-Based Payment Arrangement, Option, Exercise Price Range, Upper Range Limit (in dollars per share) |
|
|
$ 0.45
|
|
|
| X |
- DefinitionThe maximum number of shares (or other type of equity) originally approved (usually by shareholders and board of directors) for awards under the equity-based compensation plan.
| Name: |
inbp_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesOriginallyAuthorized |
| Namespace Prefix: |
inbp_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cost not yet recognized for nonvested award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average period over which cost not yet recognized is expected to be recognized for award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedPeriodForRecognition1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod over which grantee's right to exercise award under share-based payment arrangement is no longer contingent on satisfaction of service or performance condition, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, combination of market, performance or service condition. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingPeriod1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of additional shares authorized for issuance under share-based payment arrangement.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfAdditionalSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares authorized for issuance under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe difference between the maximum number of shares (or other type of equity) authorized for issuance under the plan (including the effects of amendments and adjustments), and the sum of: 1) the number of shares (or other type of equity) already issued upon exercise of options or other equity-based awards under the plan; and 2) shares (or other type of equity) reserved for issuance on granting of outstanding awards, net of cancellations and forfeitures, if applicable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAvailableForGrant |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe floor of a customized range of exercise prices for purposes of disclosing shares potentially issuable under outstanding stock option awards on all stock option plans and other required information pertaining to awards in the customized range. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeLowerRangeLimit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe ceiling of a customized range of exercise prices for purposes of disclosing shares potentially issuable under outstanding stock option awards on all stock option plans and other required information pertaining to awards in the customized range. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeUpperRangeLimit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPercentage of vesting of award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardAwardVestingRightsPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod from grant date that an equity-based award expires, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of difference between fair value of the underlying shares reserved for issuance and exercise price of vested portions of options outstanding and currently exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_DirectorMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_VestingAxis=us-gaap_ShareBasedCompensationAwardTrancheOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=inbp_EachDirectorMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=inbp_StockOption2001PlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=inbp_StockOption1997PlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=inbp_StockOption2022PlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPrice of a single share of a number of saleable stocks of a company.
| Name: |
us-gaap_SharePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 12 - Equity Transactions and Stock-based Compensation - Summary of the Company's Stock Option Activity, and Related Information (Details) - $ / shares
|
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Balance (in shares) |
4,443,933
|
3,916,666
|
| Balance, weighted average exercise price (in dollars per share) |
$ 0.36
|
$ 0.26
|
| Granted (in shares) |
527,000
|
764,700
|
| Granted, weighted average exercise price (in dollars per share) |
$ 0.42
|
$ 0.85
|
| Exercised (in shares) |
0
|
(146,333)
|
| Exercised, weighted average exercise price (in dollars per share) |
$ 0
|
$ 0.23
|
| Terminated (in shares) |
0
|
(44,700)
|
| Terminated, weighted average exercise price (in dollars per share) |
$ 0
|
$ 0.53
|
| Expired (in shares) |
(594,649)
|
(46,400)
|
| Expired, weighted average exercise price (in dollars per share) |
$ 0.46
|
$ 0.47
|
| Balance (in shares) |
4,376,284
|
4,443,933
|
| Balance, weighted average exercise price (in dollars per share) |
$ 0.35
|
$ 0.36
|
| Exercised (in shares) |
0
|
146,333
|
| Terminated (in shares) |
0
|
44,700
|
| Balance (in shares) |
4,376,284
|
4,443,933
|
| Balance, weighted average exercise price (in dollars per share) |
$ 0.35
|
$ 0.36
|
| Exercisable (in shares) |
3,626,551
|
3,524,500
|
| Exercisable, weighted average exercise price (in dollars per share) |
$ 0.30
|
$ 0.26
|
| X |
- DefinitionThe number of shares into which fully or partially vested stock options outstanding as of the balance sheet date can be currently converted under the option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted-average price as of the balance sheet date at which grantees can acquire the shares reserved for issuance on vested portions of options outstanding and currently exercisable under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options or other stock instruments for which the right to exercise has lapsed under the terms of the plan agreements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExpirationsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which option holders acquired shares when converting their stock options into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options of the plan that expired. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExpirationsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options that were terminated. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 12 - Equity Transactions and Stock-based Compensation - Share-based Compensation, Shares Authorized under Stock Option Plans, by Exercise Price Range (Details) - $ / shares
|
12 Months Ended |
|
|
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 30, 2021 |
| Range of Exercise Price, Lower Limit (in dollars per share) |
$ 0.09
|
|
|
| Range of Exercise Price, Upper Limit (in dollars per share) |
$ 1.05
|
|
|
| Outstanding (in shares) |
4,376,284
|
4,443,933
|
3,916,666
|
| Weighted average exercise price (in dollars per share) |
$ 0.35
|
$ 0.36
|
$ 0.26
|
| Weighted Average Remaining Contractual Life (Year) |
7 years
|
|
|
| Exercisable (in shares) |
3,626,551
|
3,524,500
|
|
| Weighted Average Exercise Price, Exercisable (in dollars per share) |
$ 0.30
|
$ 0.26
|
|
| Range One [Member] |
|
|
|
| Range of Exercise Price, Lower Limit (in dollars per share) |
0.09
|
|
|
| Range of Exercise Price, Upper Limit (in dollars per share) |
$ 0.10
|
|
|
| Outstanding (in shares) |
1,221,000
|
|
|
| Weighted average exercise price (in dollars per share) |
$ 0.09
|
|
|
| Weighted Average Remaining Contractual Life (Year) |
1 year 10 months 24 days
|
|
|
| Exercisable (in shares) |
1,296,000
|
|
|
| Weighted Average Exercise Price, Exercisable (in dollars per share) |
$ 0.09
|
|
|
| Range Two [Member] |
|
|
|
| Range of Exercise Price, Lower Limit (in dollars per share) |
0.21
|
|
|
| Range of Exercise Price, Upper Limit (in dollars per share) |
$ 0.21
|
|
|
| Outstanding (in shares) |
1,194,000
|
|
|
| Weighted average exercise price (in dollars per share) |
$ 0.21
|
|
|
| Weighted Average Remaining Contractual Life (Year) |
5 years 10 months 24 days
|
|
|
| Exercisable (in shares) |
1,209,000
|
|
|
| Weighted Average Exercise Price, Exercisable (in dollars per share) |
$ 0.21
|
|
|
| Range Three [Member] |
|
|
|
| Range of Exercise Price, Lower Limit (in dollars per share) |
0.23
|
|
|
| Range of Exercise Price, Upper Limit (in dollars per share) |
$ 0.25
|
|
|
| Outstanding (in shares) |
250,000
|
|
|
| Weighted average exercise price (in dollars per share) |
$ 0.23
|
|
|
| Weighted Average Remaining Contractual Life (Year) |
3 years 4 months 24 days
|
|
|
| Exercisable (in shares) |
250,000
|
|
|
| Weighted Average Exercise Price, Exercisable (in dollars per share) |
$ 0.23
|
|
|
| Range Four [Member] |
|
|
|
| Range of Exercise Price, Lower Limit (in dollars per share) |
0.41
|
|
|
| Range of Exercise Price, Upper Limit (in dollars per share) |
$ 0.41
|
|
|
| Outstanding (in shares) |
426,500
|
|
|
| Weighted average exercise price (in dollars per share) |
$ 0.41
|
|
|
| Weighted Average Remaining Contractual Life (Year) |
9 years 4 months 24 days
|
|
|
| Exercisable (in shares) |
0
|
|
|
| Weighted Average Exercise Price, Exercisable (in dollars per share) |
$ 0.00
|
|
|
| Range Five [Member] |
|
|
|
| Range of Exercise Price, Lower Limit (in dollars per share) |
0.51
|
|
|
| Range of Exercise Price, Upper Limit (in dollars per share) |
$ 0.51
|
|
|
| Outstanding (in shares) |
200,000
|
|
|
| Weighted average exercise price (in dollars per share) |
$ 0.51
|
|
|
| Weighted Average Remaining Contractual Life (Year) |
9 years
|
|
|
| Exercisable (in shares) |
200,000
|
|
|
| Weighted Average Exercise Price, Exercisable (in dollars per share) |
$ 0.51
|
|
|
| Range Six [Member] |
|
|
|
| Range of Exercise Price, Lower Limit (in dollars per share) |
0.65
|
|
|
| Range of Exercise Price, Upper Limit (in dollars per share) |
$ 0.65
|
|
|
| Outstanding (in shares) |
570,667
|
|
|
| Weighted average exercise price (in dollars per share) |
$ 0.65
|
|
|
| Weighted Average Remaining Contractual Life (Year) |
7 years 4 months 24 days
|
|
|
| Exercisable (in shares) |
431,000
|
|
|
| Weighted Average Exercise Price, Exercisable (in dollars per share) |
$ 0.65
|
|
|
| Range Seven [Member] |
|
|
|
| Range of Exercise Price, Lower Limit (in dollars per share) |
0.72
|
|
|
| Range of Exercise Price, Upper Limit (in dollars per share) |
$ 0.72
|
|
|
| Outstanding (in shares) |
66,667
|
|
|
| Weighted average exercise price (in dollars per share) |
$ 0.72
|
|
|
| Weighted Average Remaining Contractual Life (Year) |
8 months 12 days
|
|
|
| Exercisable (in shares) |
66,667
|
|
|
| Weighted Average Exercise Price, Exercisable (in dollars per share) |
$ 0.72
|
|
|
| Range Eight [Member] |
|
|
|
| Range of Exercise Price, Lower Limit (in dollars per share) |
0.95
|
|
|
| Range of Exercise Price, Upper Limit (in dollars per share) |
$ 0.95
|
|
|
| Outstanding (in shares) |
425,783
|
|
|
| Weighted average exercise price (in dollars per share) |
$ 0.95
|
|
|
| Weighted Average Remaining Contractual Life (Year) |
8 years 4 months 24 days
|
|
|
| Exercisable (in shares) |
242,217
|
|
|
| Weighted Average Exercise Price, Exercisable (in dollars per share) |
$ 0.95
|
|
|
| Range Nine [Member] |
|
|
|
| Range of Exercise Price, Lower Limit (in dollars per share) |
1.05
|
|
|
| Range of Exercise Price, Upper Limit (in dollars per share) |
$ 1.05
|
|
|
| Outstanding (in shares) |
21,667
|
|
|
| Weighted average exercise price (in dollars per share) |
$ 1.05
|
|
|
| Weighted Average Remaining Contractual Life (Year) |
8 months 12 days
|
|
|
| Exercisable (in shares) |
21,667
|
|
|
| Weighted Average Exercise Price, Exercisable (in dollars per share) |
$ 1.05
|
|
|
| X |
- DefinitionThe number of shares into which fully or partially vested stock options outstanding as of the balance sheet date can be currently converted under the option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted-average price as of the balance sheet date at which grantees can acquire the shares reserved for issuance on vested portions of options outstanding and currently exercisable under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe floor of a customized range of exercise prices for purposes of disclosing shares potentially issuable under outstanding stock option awards on all stock option plans and other required information pertaining to awards in the customized range. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeLowerRangeLimit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe ceiling of a customized range of exercise prices for purposes of disclosing shares potentially issuable under outstanding stock option awards on all stock option plans and other required information pertaining to awards in the customized range. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeUpperRangeLimit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=inbp_RangeOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=inbp_RangeTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=inbp_RangeThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=inbp_RangeFourMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=inbp_RangeFiveMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=inbp_RangeSixMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=inbp_RangeSevenMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=inbp_RangeEightMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=inbp_RangeNineMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 13 - Segment Information (Details Textual)
|
12 Months Ended |
|
Jun. 30, 2023
$ / shares
|
Jun. 30, 2022
$ / shares
|
| Number of Reportable Segments |
2
|
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Expirations in Period, Weighted Average Exercise Price (in dollars per share) |
$ 0.46
|
$ 0.47
|
| Europe and Canada [Member] |
|
|
| Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Expirations in Period, Weighted Average Exercise Price (in dollars per share) |
$ 8,055
|
$ 9,729
|
| X |
- DefinitionNumber of segments reported by the entity. A reportable segment is a component of an entity for which there is an accounting requirement to report separate financial information on that component in the entity's financial statements. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-18
| Name: |
us-gaap_NumberOfReportableSegments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options of the plan that expired. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExpirationsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=inbp_EuropeAndCanadaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 13 - Segment Information - Operations by Business Segment (Details) - USD ($)
$ in Thousands |
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Sales, net |
$ 50,672
|
$ 56,246
|
| Gross Profit |
4,061
|
6,552
|
| Depreciation |
351
|
336
|
| Capital Expenditures |
116
|
486
|
| Assets |
25,431
|
25,250
|
| Contract Manufacturing [Member] |
|
|
| Sales, net |
48,277
|
54,091
|
| Gross Profit |
3,630
|
5,917
|
| Depreciation |
348
|
332
|
| Capital Expenditures |
109
|
486
|
| Assets |
19,507
|
19,061
|
| Other Nutraceutical Business [Member] |
|
|
| Sales, net |
2,395
|
2,155
|
| Gross Profit |
431
|
635
|
| Depreciation |
3
|
4
|
| Capital Expenditures |
7
|
0
|
| Assets |
5,924
|
6,189
|
| UNITED STATES |
|
|
| Sales, net |
42,617
|
46,517
|
| UNITED STATES | Contract Manufacturing [Member] |
|
|
| Sales, net |
40,230
|
44,450
|
| UNITED STATES | Other Nutraceutical Business [Member] |
|
|
| Sales, net |
2,387
|
2,067
|
| Non-US [Member] |
|
|
| Sales, net |
8,055
|
9,729
|
| Non-US [Member] | Contract Manufacturing [Member] |
|
|
| Sales, net |
8,047
|
9,641
|
| Non-US [Member] | Other Nutraceutical Business [Member] |
|
|
| Sales, net |
$ 8
|
$ 88
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate revenue less cost of goods and services sold or operating expenses directly attributable to the revenue generation activity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.1,2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GrossProfit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for purchases of and capital improvements on property, plant and equipment (capital expenditures), software, and other intangible assets. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 805
-SubTopic 50
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480060/805-50-25-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 805
-SubTopic 50
-Name Accounting Standards Codification
-Section 30
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480027/805-50-30-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 805
-SubTopic 50
-Name Accounting Standards Codification
-Section 30
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480027/805-50-30-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireProductiveAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, including tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value-added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479941/924-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerIncludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=inbp_ContractManufacturingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=inbp_OtherNutraceuticalBusinessMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=country_US |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=us-gaap_NonUsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Integrated BioPharma (QX) (USOTC:INBP)
Historical Stock Chart
From Mar 2024 to Apr 2024
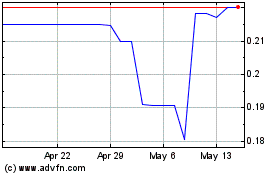
Integrated BioPharma (QX) (USOTC:INBP)
Historical Stock Chart
From Apr 2023 to Apr 2024