UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
Form
6-K
Report
of Foreign Private Issuer
Pursuant
to Rule 13a-16 or 15d-16
under
the Securities Exchange Act of 1934
For
the month of September 2023
Commission
file number: 001-39957
NLS
PHARMACEUTICS LTD.
(Translation
of registrant’s name into English)
The
Circle 6
8058
Zurich, Switzerland
(Address
of principal executive offices)
Indicate
by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form
20-F ☒ Form 40-F ☐
CONTENTS
On
September [__], 2023, NLS Pharmaceutics Ltd., or the Registrant, made available a presentation on its website. A copy of the presentation
is attached hereto as Exhibit 99.1.
EXHIBIT
INDEX
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned, thereunto duly authorized.
| |
NLS Pharmaceutics Ltd. |
| |
|
|
| Date:
September 13, 2023 |
By: |
/s/
Alexander Zwyer |
| |
|
Name: |
Alexander Zwyer |
| |
|
Title: |
Chief Executive Officer |
2
Exhibit 99.1

1 Re defining Central Nervous System Therapies NLS Pharmaceutics September 2023

2 Forward - Looking Statements This presentation contains express or implied forward - looking statements within the Private Securities Litigation Reform Act of 1995 and other US Federal securities laws. For example, we are using forward - looking statements when we discuss the results of our clinical trials, the expected timing of our future clinical trials, the receipt of the results from clinical trials and obtaining regulatory approval for Mazindol ER; our strategic priorities in 2023; our pipeline of product candidates for various indications; the anticipated development timeline for Mazindol ER; proposed trials that may occur in the future; and the potential benefits our product candidates could have on improving patient health care. These forward - looking statements and their implications are based on the current expectations of our management only and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward - looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward - looking statements: changes in technology and market requirements; we may encounter delays or obstacles in launching and/or successfully completing its clinical trials; our products may not be approved by regulatory agencies, our technology may not be validated as it progresses further and its methods may not be accepted by the scientific community; we may be unable to retain or attract key employees whose knowledge is essential to the development of its products; unforeseen scientific difficulties may develop with our process; our products may wind up being more expensive than it anticipates; results in the laboratory may not translate to equally good results in real clinical settings; results of preclinical studies may not correlate with the results of human clinical trials; our patents may not be sufficient; our products may harm recipients; changes in legislation may adversely impact us; inability to timely develop and introduce new technologies, products and applications; loss of market share and pressure on pricing resulting from competition, which could cause our actual results or performance to differ materially from those contemplated in such forward - looking statements. Except as otherwise required by law, we undertake no obligation to publicly release any revisions to these forward - looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. More detailed information about the risks and uncertainties affecting us is contained under the heading "Risk Factors" in our annual report on Form 20 - F for the year ended December 31, 2022, filed with the Securities and Exchange Commission (SEC), which is available on the SEC's website, www.sec.gov , and in subsequent filings made by us with the SEC. Our logo and some of our trademarks and tradenames are used or incorporated by reference in this presentation. This presentation also includes trademarks, tradenames and service marks that are the property of other organizations. Solely for convenience, trademarks, tradenames and service marks referred to in this presentation may appear without the ®, TM and SM symbols, but those references are not intended to indicate in any way that we will not assert to the fullest extent under applicable law our rights or the rights of the applicable licensor to these trademarks, tradenames and service marks. We obtained the statistical data, market data and other industry data and forecasts described by reference in this presentation from market research, publicly available information and industry publications. Industry publications generally state that they obtain their information from sources that they believe to be reliable, but they do not guarantee the accuracy and completeness of the information. Similarly, while we believe that the statistical data, industry data and forecasts and market research are reliable, we have not independently verified the data, and we do not make any representation as to the accuracy of the information.

General Overview

4 Business Highlights NLS develops drug treatments for rare and complex Central Nervous System (CNS) disorders • Mazindol ER has successfully completed the Phase 2 trials, including OLE, for narcolepsy treatment: projected to be $4.5B annual market by 2027* • AMAZE Phase 3 Program to start in 2H 2023 • Orphan Drug Designation (ODD) granted in the US and Europe for 2 indications • Strong IP protection on once - daily formulation until 2037 • Unique sleep franchise (incl. MNE) • Pipeline progressed and expanded with long - dated IP protections in major markets • Named Patient Program for patients suffering from narcolepsy & idiopathic hypersomnia launched in target markets across Europe and potentially ROW (rest - of - world) * ResearchAndMarkets.com (October 2022)

NLS Leadership Team Alex Zwyer, MBA Chief Executive Officer & Co - Founder A co - founder of the company with extensive operational, C - level pharmaceutical experience. A serial entrepreneur and strong leader with a proven track - record to get “deals done”. Served as Chief Operating Officer at Viforpharma AG, a global specialty pharmaceutical company. And a retired Swiss army Captain. George Apostol, MD, MS Chief Medical Officer Worked in pharma R&D organizations for more than 20 years. Broad drug development expertise across early, middle and late phases of development at the Global R&D organizations of Eli Lilly, Pfizer, Abbott, Novartis, Shire and Endo. Received distinguished R&D awards and achieved multiple regulatory approvals in US, EU and Japan. Elena Thyen - Pighin Chief Financial Officer Eric Konofal, MD, PhD Chief Scientific Officer & Co - Founder A drug - hunter and co - founder of the company with a deep knowledge and experience in clinical and scientific research. He is a senior medical consultant for the Pediatric Sleep Disorders Center at the Robert - Debre University of Paris (APHP) and served as Principal Clinical Investigator at the Clinical Pharmacology & Pharmacogenetic Department at Robert - Debre University of Paris. Ms. Thyen - Pighin holds extensive experience is leadership and management functions as both head of finance and human resources across a number of industries. Based in Switzerland, Ms. Thyen - Pighin has a successful track record, most notably in accounting for both private and publicly listed enterprises..

6 Marianne Lambertson, MSc, MSW Head of Corporate Communications and Investor Relations Harvey Kushner Sr. Biostatistical Consultant Tom Curatolo, MBA Head of US Commercial Operations Beat Widler, PhD Head of Quality Hervé Girsault, MBA Head of Business Development & Licensing Christina Allgeier US GAAP Controller Pelin Vatanacan Finance Operations Manager Jennifer Franco Head of Scientific Excellence Jeff Bernier Head of Clinical Operations Sharon Tan, MBA Head of Program Management & Strategy Bao Le, MS Head of Regulatory Experienced Team Assembled Andreas Fischer Head of Supply Chain Sam Salamone, MS, MBA, RAC Head of CMC Jim Herman Head of Toxicology Alex Zwyer, MBA Chief Executive Officer Co - Founder George Apostal, MD, MS Chief Medical Officer Eric Konofal, MD, PhD Chief Scientific Officer Co - Founder Elena Thyen - Pighin Chief Financial Officer

NLS Pipeline Overview Phase 3 Phase 2 IND enabling Preclinical Compound Indication Therapeutic Area Over 100 patents in over 140 countries including technology, mechanism of action and application for: • Sleep Disorders including Narcolepsy, Idiopathic Hypersomnia, and Kleine - Levin Syndrome • ADHD • Neurodegenerative diseases with Lewy Body Disease, Parkinson’s Disease and/or Alzheimer's Disease • CNS diseases treatment associated with sleep disorders Mazindol ER Narcolepsy NT1 Narcolepsy NT2 Idiopathic Hypersomnia Sleep Disorders NLS - 4 Idiopathic Hypersomnia NLS - 11 Kleine - Levin Syndrome NLS - 4 CFS/ME Long COVID CNS/Immunology NLS - 8 Alzheimer’s Disease Neurodegenerative Diseases NLS - 12 Lewy Body Disease NLS - 1 ADHD Opioid Use Disorder Psychiatry/ Addiction NLS - 3 ADHD Autism Spectrum Disorder Substance Use Disorder
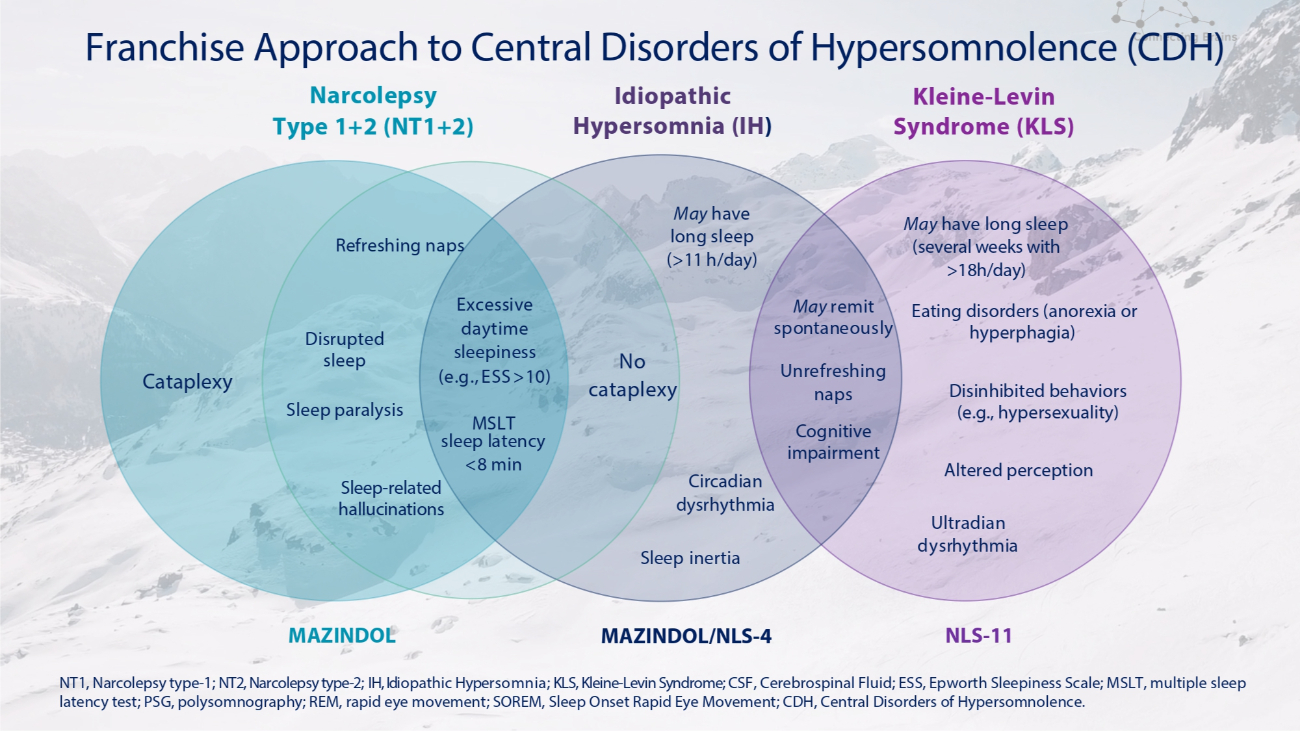
Narcolepsy Type 1+2 (NT1+2) Idiopathic Hypersomnia (IH ) Cataplexy Refreshing naps Disrupted sleep Sleep paralysis Sleep - related hallucinations No cataplexy Excessive daytime sleepiness (e . g . , ESS > 10 ) MSLT sleep latency <8 min Sleep inertia May have long sleep (> 11 h/day) May remit spontaneously Circadian dysrhythmia Altered perception Kleine - Levin Syndrome (KLS) Unrefreshing naps Cognitive impairment Ultradian dysrhythmia May have long sleep (several weeks with >18h/day) Eating disorders (anorexia or hyperphagia) Disinhibited behaviors (e.g., hypersexuality) MAZINDOL MAZINDOL/NLS - 4 NLS - 11 NT1, Narcolepsy type - 1; NT2, Narcolepsy type - 2; IH, Idiopathic Hypersomnia; KLS, Kleine - Levin Syndrome; CSF, Cerebrospinal Fluid; ESS, Epworth Sleepiness Scale; MSLT, multiple sleep latency test; PSG, polysomnography; REM, rapid eye movement; SOREM, Sleep Onset Rapid Eye Movement; CDH, Central Disorders of Hypersomnolence. Franchise Approach to Central Disorders of Hypersomnolence (CDH)

Narcolepsy at a Glance

10 1. www.ncbi.nlm.nih.gov/pmc/articles/PMC6492289/ 2. Polaris Market Research (April 2023) 3. 2018 National Know Narcolepsy Survey. https://knownarcolepsy.com/hcp/impact Narcolepsy Debilitating Disorder; Large and Underserved Market • 3 Million people suffer worldwide • 200,000 in the US alone • 60 - 70'000 diagnosed • 17,000 taking SXB (2022) Source: www.narcolepsynetwork.org • Classic narcolepsy is associated with very low or undetectable levels of the hormone, orexin, in the brain 1 • Global narcolepsy therapeutics market envisaged to reach USD 7.75 Billion By 2032 (2022: USD 3.14 Billion), at 9.5% CAGR Growth 2 • No commercially available products address the orexin deficit; most patients remain unsatisfied with their treatment 3
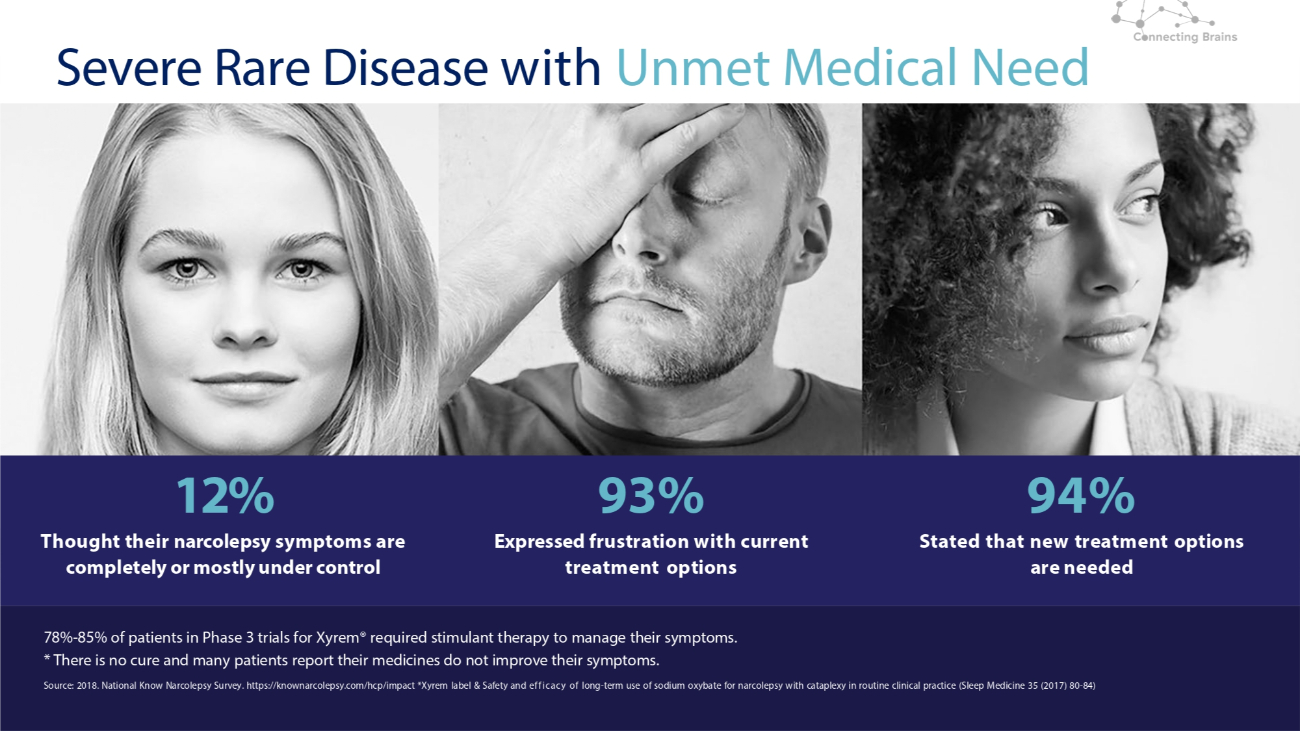
11 12% Thought their narcolepsy symptoms are completely or mostly under control 93% Expressed frustration with current treatment options 94% Stated that new treatment options are needed Severe Rare Disease with Unmet Medical Need 78% - 85% of patients in Phase 3 trials for Xyrem® required stimulant therapy to manage their symptoms. * There is no cure and many patients report their medicines do not improve their symptoms. Source: 2018. National Know Narcolepsy Survey. https://knownarcolepsy.com/hcp/impact *Xyrem label & Safety and efficacy of long - term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice (Sleep Medicine 35 (2017) 80 - 84)
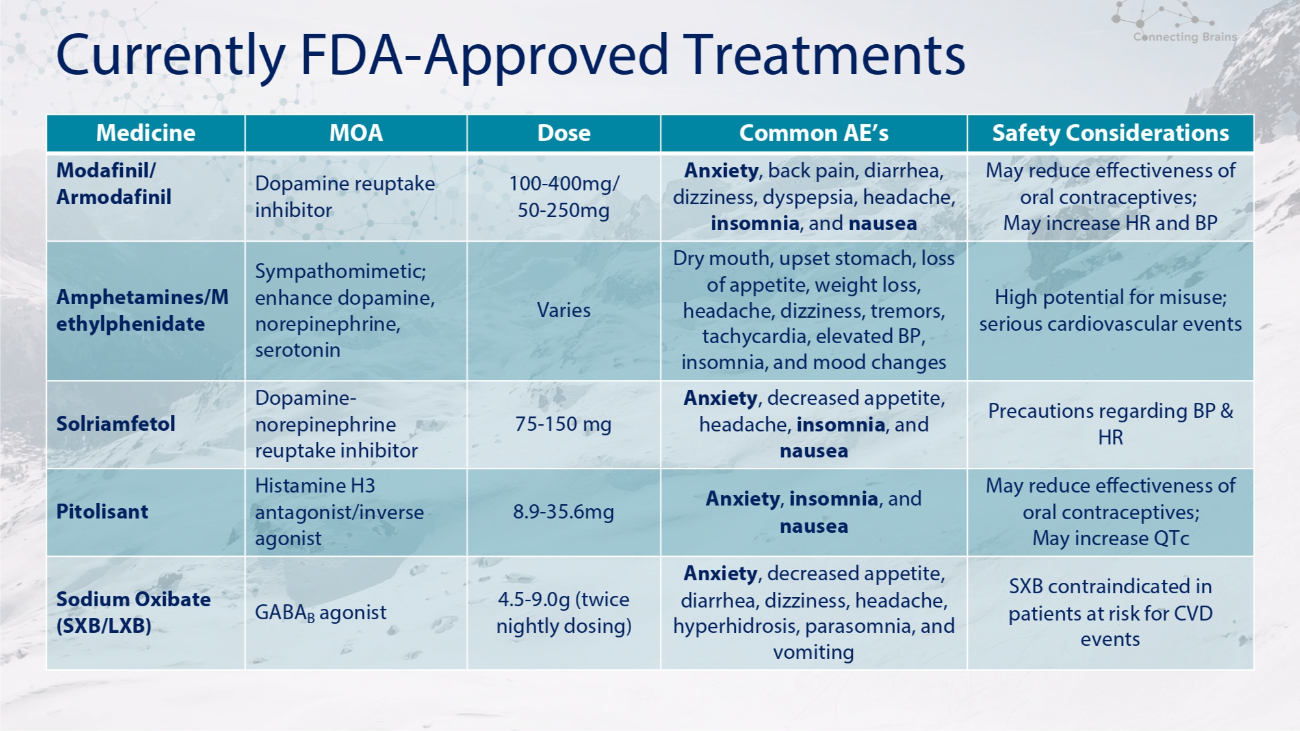
Currently FDA - Approved Treatments Safety Considerations Common AE’s Dose MOA Medicine May reduce effectiveness of oral contraceptives; May increase HR and BP Anxiety , back pain, diarrhea, dizziness, dyspepsia, headache, insomnia , and nausea 100 - 400mg/ 50 - 250mg Dopamine reuptake inhibitor Modafinil/ Armodafinil High potential for misuse; serious cardiovascular events Dry mouth, upset stomach, loss of appetite, weight loss, headache, dizziness, tremors, tachycardia, elevated BP, insomnia, and mood changes Varies Sympathomimetic; enhance dopamine, norepinephrine, serotonin Amphetamines/M ethylphenidate Precautions regarding BP & HR Anxiety , decreased appetite, headache, insomnia , and nausea 75 - 150 mg Dopamine - norepinephrine reuptake inhibitor Solriamfetol May reduce effectiveness of oral contraceptives; May increase QTc Anxiety , insomnia , and nausea 8.9 - 35.6mg Histamine H3 antagonist/inverse agonist Pitolisant SXB contraindicated in patients at risk for CVD events Anxiety , decreased appetite, diarrhea, dizziness, headache, hyperhidrosis, parasomnia, and vomiting 4.5 - 9.0g (twice nightly dosing) GABA B agonist Sodium Oxibate (SXB/LXB)
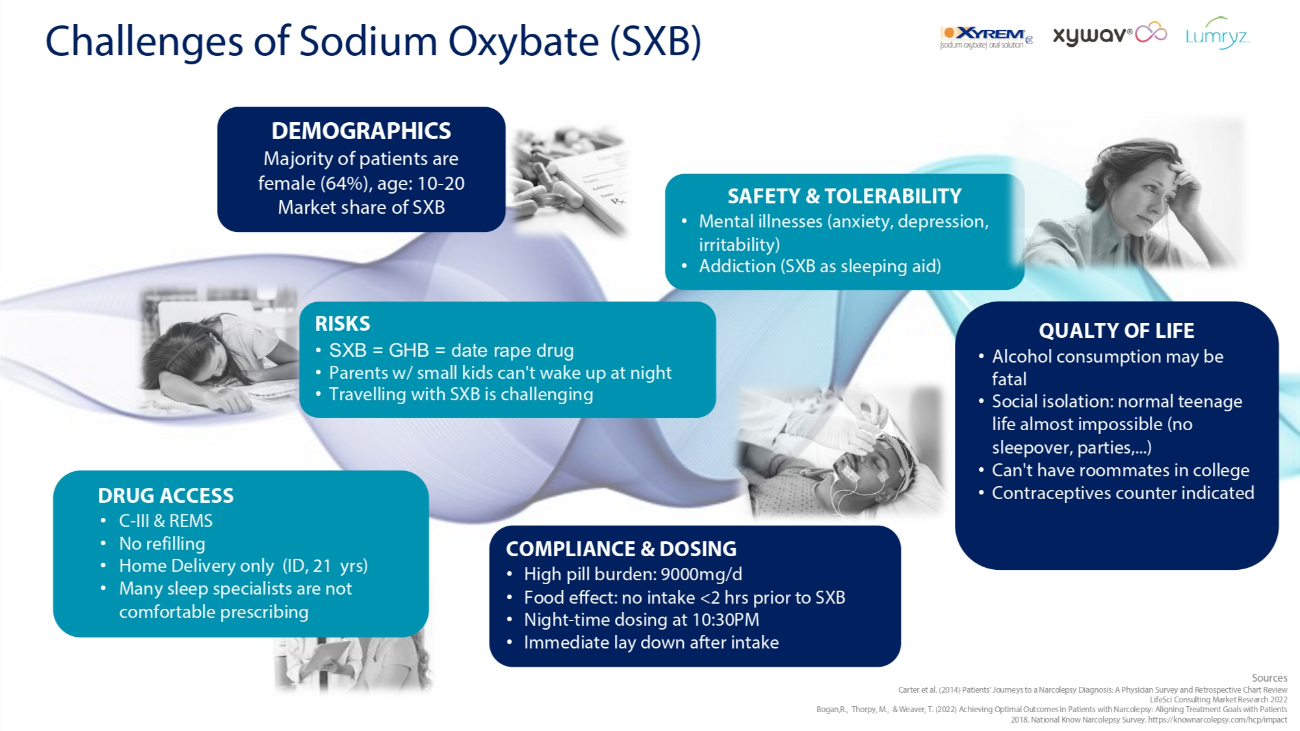
Challenges of Sodium Oxybate (SXB) COMPLIANCE & DOSING • High pill burden: 9000mg/d • Food effect: no intake <2 hrs prior to SXB • Night - time dosing at 10:30PM • Immediate lay down after intake Sources Carter et al. (2014) Patients' Journeys to a Narcolepsy Diagnosis: A Physician Survey and Retrospective Chart Review LifeSci Consulting Market Research 2022 Bogan,R., Thorpy, M., & Weaver, T. (2022) Achieving Optimal Outcomes in Patients with Narcolepsy: Aligning Treatment Goals with Patients 2018. National Know Narcolepsy Survey. https://knownarcolepsy.com/hcp/impact DEMOGRAPHICS Majority of patients are female (64%), age: 10 - 20 Market share of SXB RISKS • SXB = GHB = date rape drug • Parents w/ small kids can't wake up at night • Travelling with SXB is challenging QUALTY OF LIFE • Alcohol consumption may be fatal • Social isolation: normal teenage life almost impossible (no sleepover, parties,...) • Can't have roommates in college • Contraceptives counter indicated SAFETY & TOLERABILITY • Mental illnesses (anxiety, depression, irritability) • Addiction (SXB as sleeping aid) DRUG ACCESS • C - III & REMS • No refilling • Home Delivery only (ID, 21 yrs) • Many sleep specialists are not comfortable prescribing
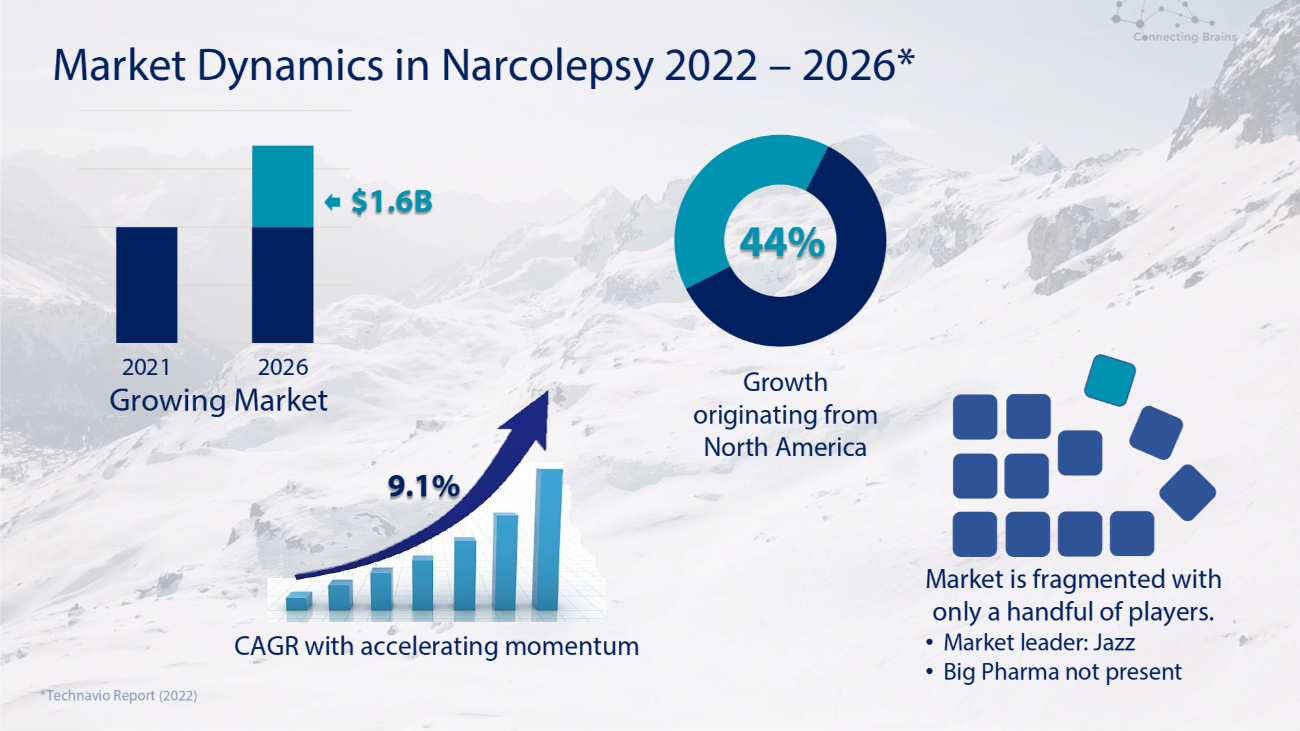
Market Dynamics in Narcolepsy 2022 – 2026* Growth originating from North America *Technavio Report (2022) Market is fragmented with only a handful of players. • Market leader: Jazz • Big Pharma not present 2021 2026 Growing Market CAGR with accelerating momentum 9.1% $1.6B 44%

Mazindol ER (3mg)

• Long history of the active ingredient (mazindol) used to treat narcolepsy (“Compassionate Use” Programs) • Novel dual mechanism of action with partial Orexin - 2 receptor activation & triple monoamine reuptake inhibition • Positive Phase 2 results: met most endpoints with high statistical significance and favorable safety / tolerability • Once - daily formulation • Quick Onset of Action • Low drug - drug interaction potential • Low abuse potential: Schedule IV • Potential as monotherapy treatment of narcolepsy Mazindol ER dual SNDRI/OX2R agonist
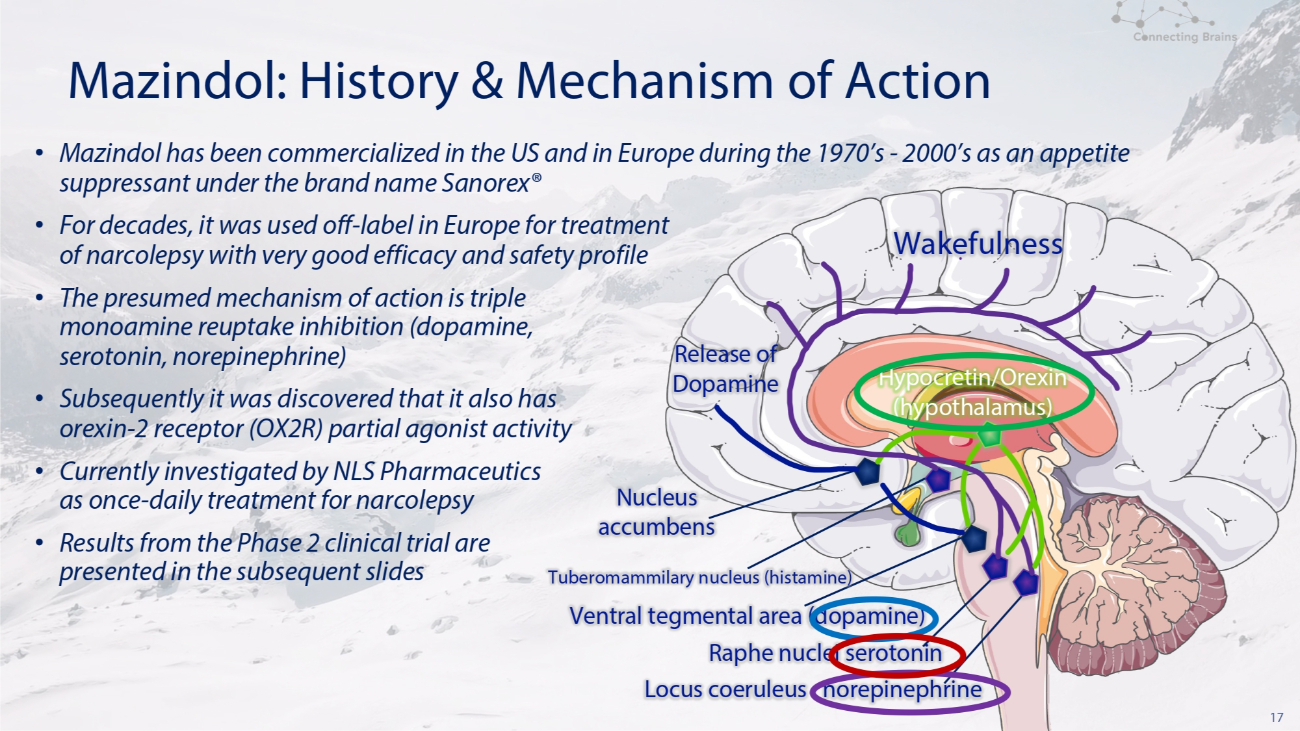
17 Mazindol: History & Mechanism of Action Wakefulness Release of Dopamine Nucleus accumbens Tuberomammilary nucleus (histamine) Ventral tegmental area (dopamine) Raphe nuclei serotonin Locus coeruleus norepinephrine • Mazindol has been commercialized in the US and in Europe during the 1970’s - 2000’s as an appetite suppressant under the brand name Sanorex® • For decades, it was used off - label in Europe for treatment of narcolepsy with very good efficacy and safety profile • The presumed mechanism of action is triple monoamine reuptake inhibition (dopamine, serotonin, norepinephrine) • Subsequently it was discovered that it also has orexin - 2 receptor (OX 2 R) partial agonist activity • Currently investigated by NLS Pharmaceutics as once - daily treatment for narcolepsy • Results from the Phase 2 clinical trial are presented in the subsequent slides Hypocretin/Orexin (hypothalamus)

Mazindol ER Clinical Development Program POLARIS

19 POLARIS: Mazindol ER Development Program in Narcolepsy The Mazindol ER development program POLARIS consisted of two US clinical trials • NLS - 1021 Phase 2, A four - week double - blind, placebo controlled, randomized, US multi - center study of Mazindol ER 3 mg once daily vs. placebo (1:1) • NLS - 1022 Open Label Extension, An Open Label Extension (OLE) Study available for individuals following completion of the four - week NLS 1021 study. This OLE study offers participants the opportunity to take oral Mazindol ER once daily in the morning for up to six months.
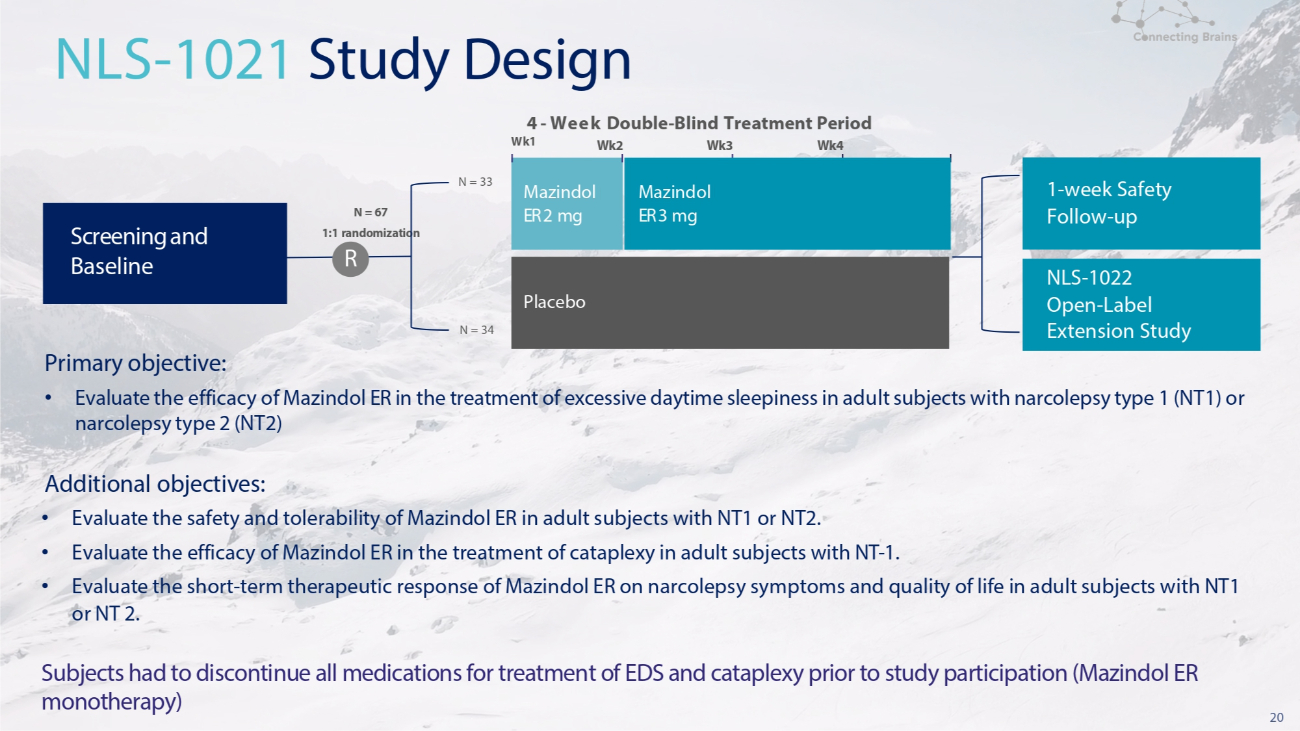
20 Primary objective: • Evaluate the efficacy of Mazindol ER in the treatment of excessive daytime sleepiness in adult subjects with narcolepsy type 1 (NT1) or narcolepsy type 2 (NT2) Additional objectives: • Evaluate the safety and tolerability of Mazindol ER in adult subjects with NT1 or NT2. • Evaluate the efficacy of Mazindol ER in the treatment of cataplexy in adult subjects with NT - 1. • Evaluate the short - term therapeutic response of Mazindol ER on narcolepsy symptoms and quality of life in adult subjects with NT1 or NT 2. Subjects had to discontinue all medications for treatment of EDS and cataplexy prior to study participation (Mazindol ER monotherapy) N = 33 Wk1 N = 34 4 - Week Double - Blind Treatment Period Wk2 Wk3 Wk4 NLS - 1021 Study Design Mazindol ER 2 mg Placebo Mazindol ER 3 mg Screening and Baseline 1 - week Safety Follow - up NLS - 1022 Open - Label Extension Study N = 67 1:1 randomization R

Phase 2 Primary Efficacy Results Excessive Daytime Sleepiness (EDS)
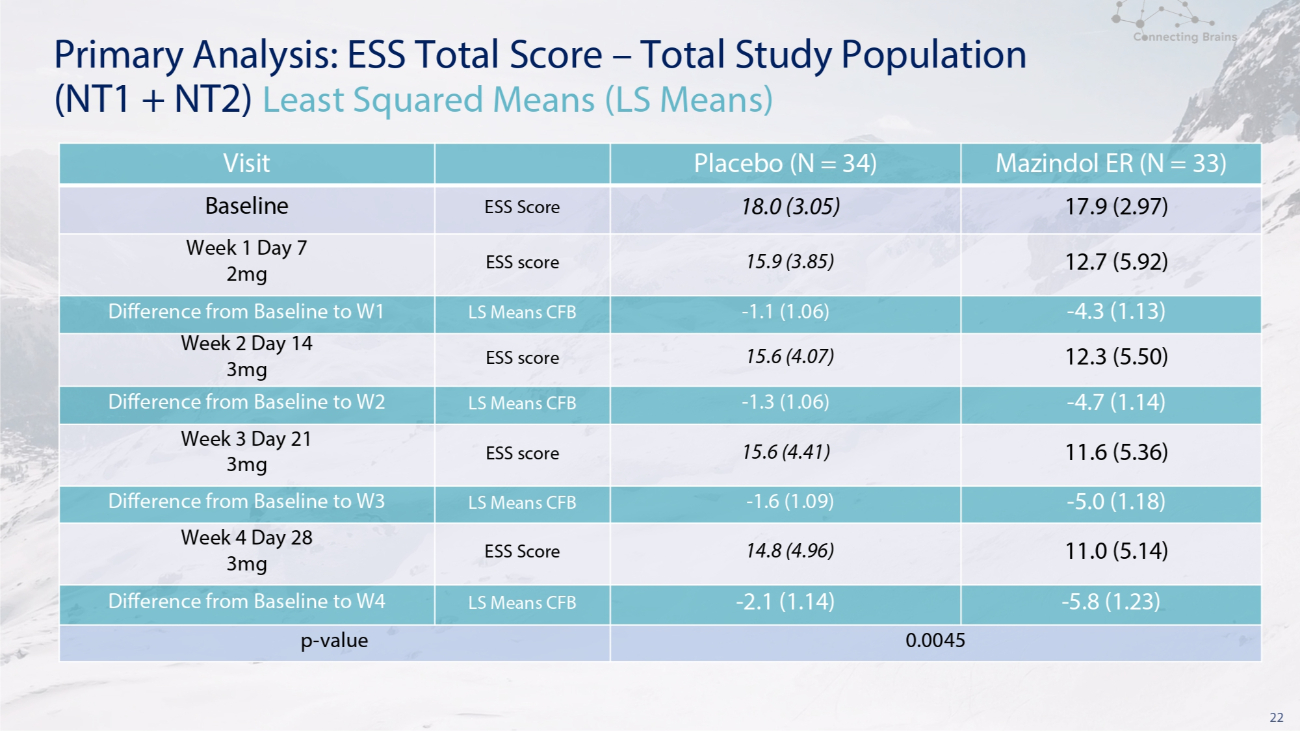
22 Primary Analysis: ESS Total Score – Total Study Population (NT1 + NT2) Least Squared Means (LS Means) Mazindol ER (N = 33) Placebo (N = 34) Visit 17.9 (2.97) 18.0 (3.05) ESS Score Baseline 12.7 (5.92) 15.9 (3.85) ESS score Week 1 Day 7 2mg - 4.3 (1.13) - 1.1 (1.06) LS Means CFB Difference from Baseline to W1 12.3 (5.50) 15.6 (4.07) ESS score Week 2 Day 14 3mg - 4.7 (1.14) - 1.3 (1.06) LS Means CFB Difference from Baseline to W2 11.6 (5.36) 15.6 (4.41) ESS score Week 3 Day 21 3mg - 5.0 (1.18) - 1.6 (1.09) LS Means CFB Difference from Baseline to W3 11.0 (5.14) 14.8 (4.96) ESS Score Week 4 Day 28 3mg - 5.8 (1.23) - 2.1 (1.14) LS Means CFB Difference from Baseline to W4 0.0045 p - value
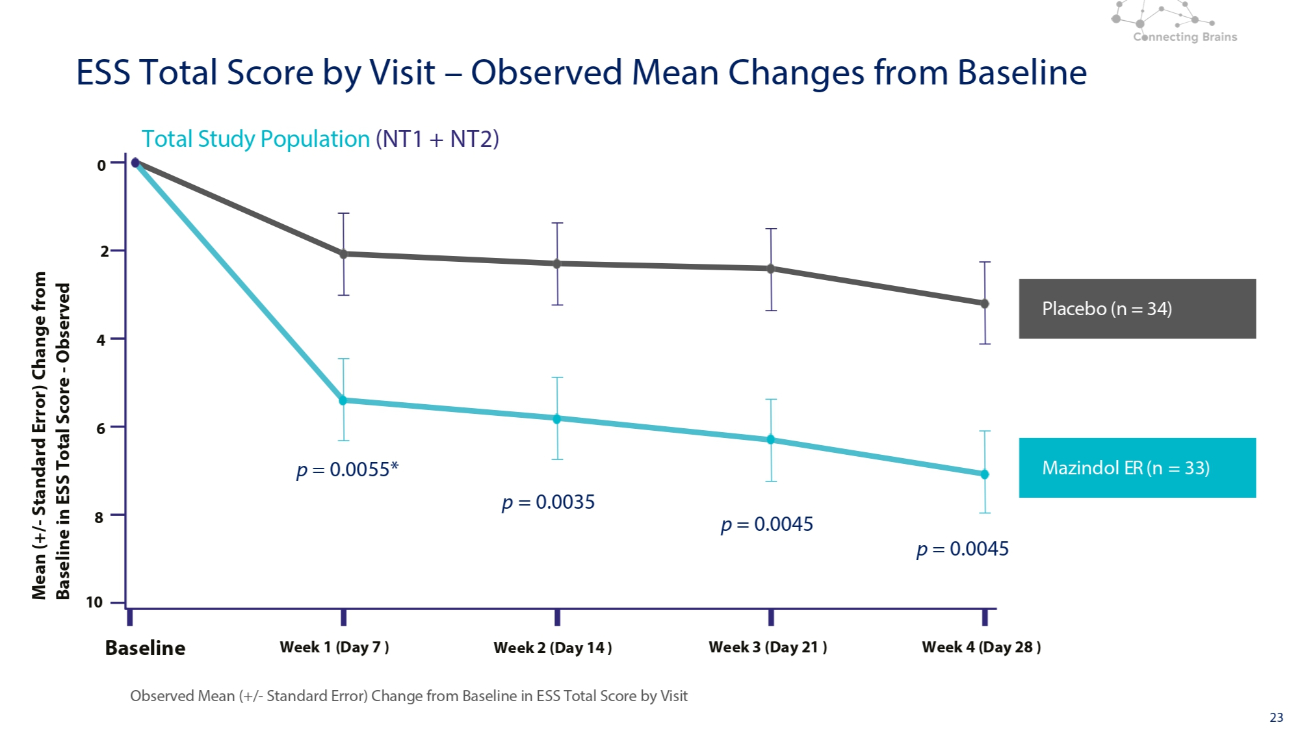
23 Placebo (n = 34) Mazindol ER (n = 33) Total Study Population (NT1 + NT2) Observed Mean (+/ - Standard Error) Change from Baseline in ESS Total Score by Visit 0 2 4 6 8 10 Baseline Week 1 (Day 7 ) Week 2 (Day 14 ) Week 3 (Day 21 ) Week 4 (Day 28 ) Mean (+/ - Standard Error) Change from Baseline in ESS Total Score - Observed ESS Total Score by Visit – Observed Mean Changes from Baseline p = 0.0055* p = 0.0045 p = 0.0045 p = 0.0035

Phase 2 Secondary Efficacy Results Cataplexy
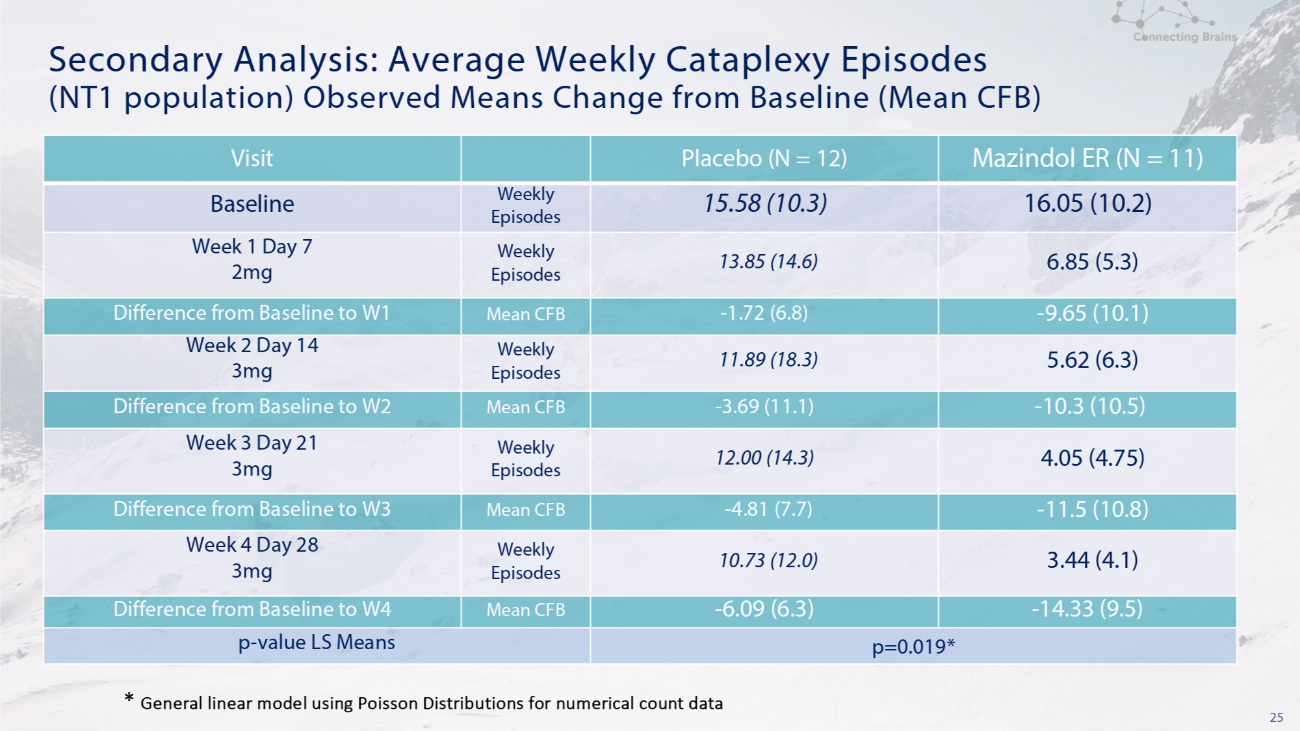
25 Secondary Analysis: Average Weekly Cataplexy Episodes (NT1 population) Observed Means Change from Baseline (Mean CFB) Mazindol ER (N = 11) Placebo (N = 12) Visit 16.05 (10.2) 15.58 (10.3) Weekly Episodes Baseline 6.85 (5.3) 13.85 (14.6) Weekly Episodes Week 1 Day 7 2mg - 9.65 (10.1) - 1.72 (6.8) Mean CFB Difference from Baseline to W1 5.62 (6.3) 11.89 (18.3) Weekly Episodes Week 2 Day 14 3mg - 10.3 (10.5) - 3.69 (11.1) Mean CFB Difference from Baseline to W2 4.05 (4.75) 12.00 (14.3) Weekly Episodes Week 3 Day 21 3mg - 11.5 (10.8) - 4.81 (7.7) Mean CFB Difference from Baseline to W3 3.44 (4.1) 10.73 (12.0) Weekly Episodes Week 4 Day 28 3mg - 14.33 (9.5) - 6.09 (6.3) Mean CFB Difference from Baseline to W4 p=0.019* p - value LS Means * General linear model using Poisson Distributions for numerical count data
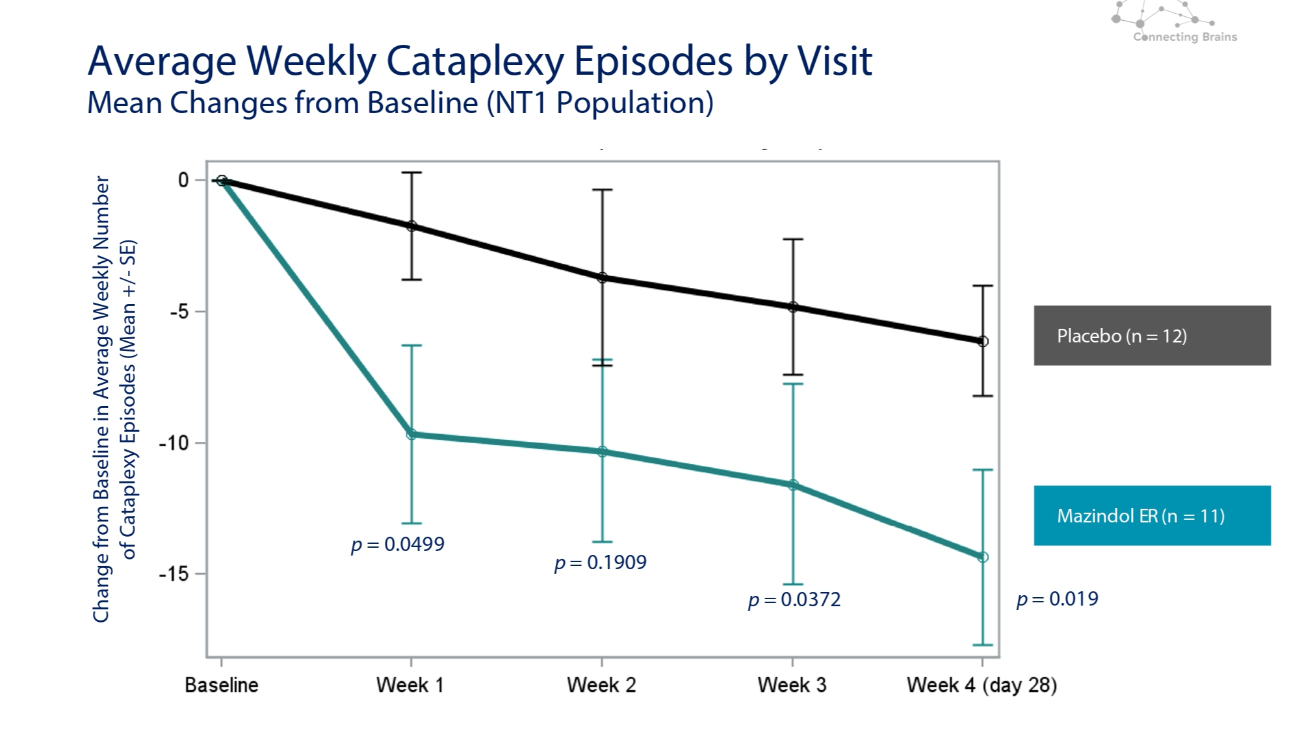
Average Weekly Cataplexy Episodes by Visit Mean Changes from Baseline (NT1 Population) Change from Baseline in Average Weekly Number of Cataplexy Episodes (Mean +/ - SE) Placebo (n = 12) Mazindol ER (n = 11) p = 0.0499 p = 0.1909 p = 0.0372 p = 0.019
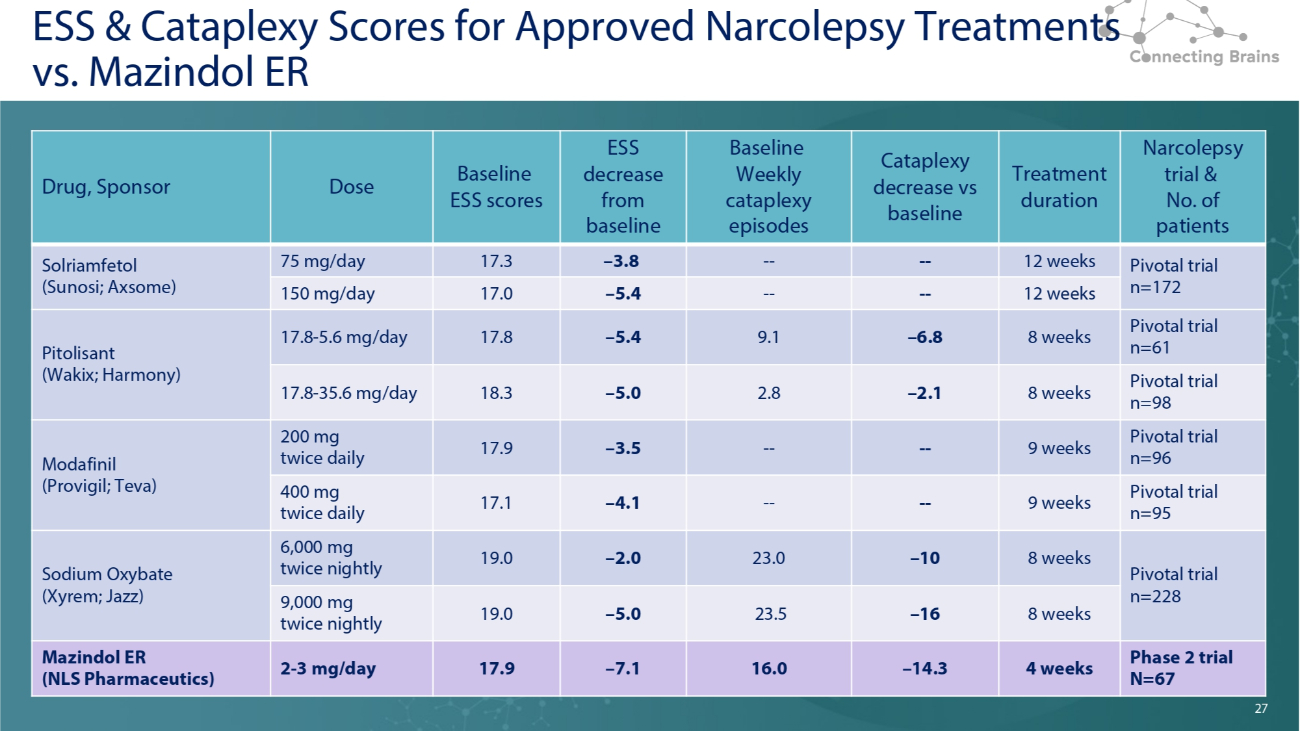
27 ESS & Cataplexy Scores for Approved Narcolepsy Treatments vs. Mazindol ER Narcolepsy trial & No. of patients Treatment duration Cataplexy decrease vs baseline Baseline Weekly cataplexy episodes ESS decrease from baseline Baseline ESS scores Dose Drug, Sponsor Pivotal trial n=172 12 weeks - - - - – 3.8 17.3 75 mg/day Solriamfetol (Sunosi; Axsome) 12 weeks - - - - – 5.4 17.0 150 mg/day Pivotal trial n=61 8 weeks – 6.8 9.1 – 5.4 17.8 17.8 - 5.6 mg/day Pitolisant (Wakix; Harmony) Pivotal trial n=98 8 weeks – 2.1 2.8 – 5.0 18.3 17.8 - 35.6 mg/day Pivotal trial n=96 9 weeks - - - - – 3.5 17.9 200 mg twice daily Modafinil (Provigil; Teva) Pivotal trial n=95 9 weeks - - - - – 4.1 17.1 400 mg twice daily Pivotal trial n=228 8 weeks – 10 23.0 – 2.0 19.0 6,000 mg twice nightly Sodium Oxybate (Xyrem; Jazz) 8 weeks – 16 23.5 – 5.0 19.0 9,000 mg twice nightly Phase 2 trial N=67 4 weeks – 14.3 16.0 – 7.1 17.9 2 - 3 mg/day Mazindol ER (NLS Pharmaceutics) 27

28 Summary Efficacy Results • Clinically meaningful improvement on both Sleepiness and Cataplexy at 4 weeks treatment vs placebo • Fast onset of action (within 1 week) even on 2 mg • Sustained EDS and cataplexy improvements at all time points despite small sample size • Strong placebo effect did not prevent Mazindol ER from meaningfully separating on most primary and secondary measures evaluated

Phase 2 Results Safety & Tolerability
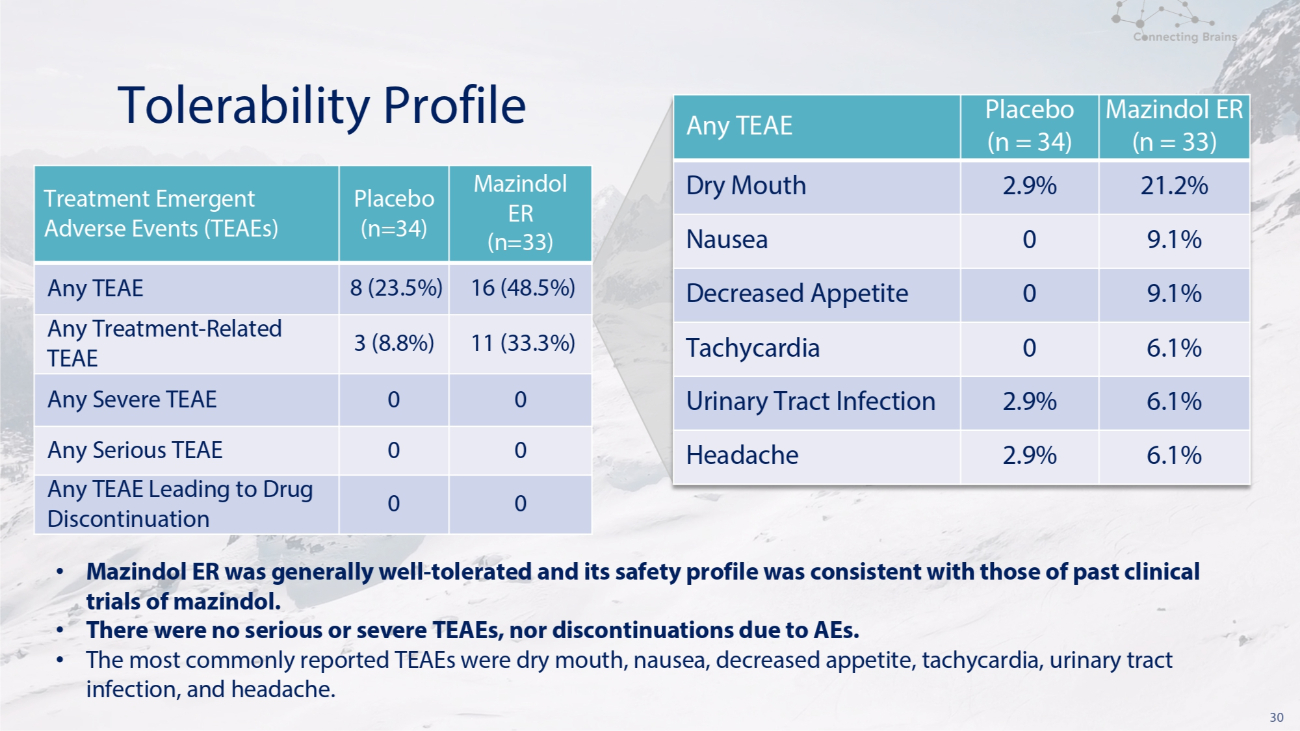
30 Tolerability Profile Mazindol ER (n=33) Placebo (n=34) Treatment Emergent Adverse Events (TEAEs) 16 (48.5%) 8 (23.5%) Any TEAE 11 (33.3%) 3 (8.8%) Any Treatment - Related TEAE 0 0 Any Severe TEAE 0 0 Any Serious TEAE 0 0 Any TEAE Leading to Drug Discontinuation Mazindol ER (n = 33) Placebo (n = 34) Any TEAE 21.2% 2.9% Dry Mouth 9.1% 0 Nausea 9.1% 0 Decreased Appetite 6.1% 0 Tachycardia 6.1% 2.9% Urinary Tract Infection 6.1% 2.9% Headache • Mazindol ER was generally well - tolerated and its safety profile was consistent with those of past clinical trials of mazindol. • There were no serious or severe TEAEs, nor discontinuations due to AEs. • The most commonly reported TEAEs were dry mouth, nausea, decreased appetite, tachycardia, urinary tract infection, and headache.

31 Summary Safety Results • No patients discontinued due to adverse reactions • All adverse events where either mild or moderate • None of the adverse events were severe • Most resolved spontaneously (no intervention was required) • No unexpected adverse events • Mazindol ER was well - tolerated, with even the most common adverse reactions (dry mouth, nausea, decreased appetite) being benign in nature and occurring in one in five patients or less • No meaningful differences on mazindol vs PBO on biochemistry, hematology, ECG or vital signs except for an increase in heart rate (~11bpm) and weight loss (~1.3kg)

Open Label Extension Study Results Efficacy
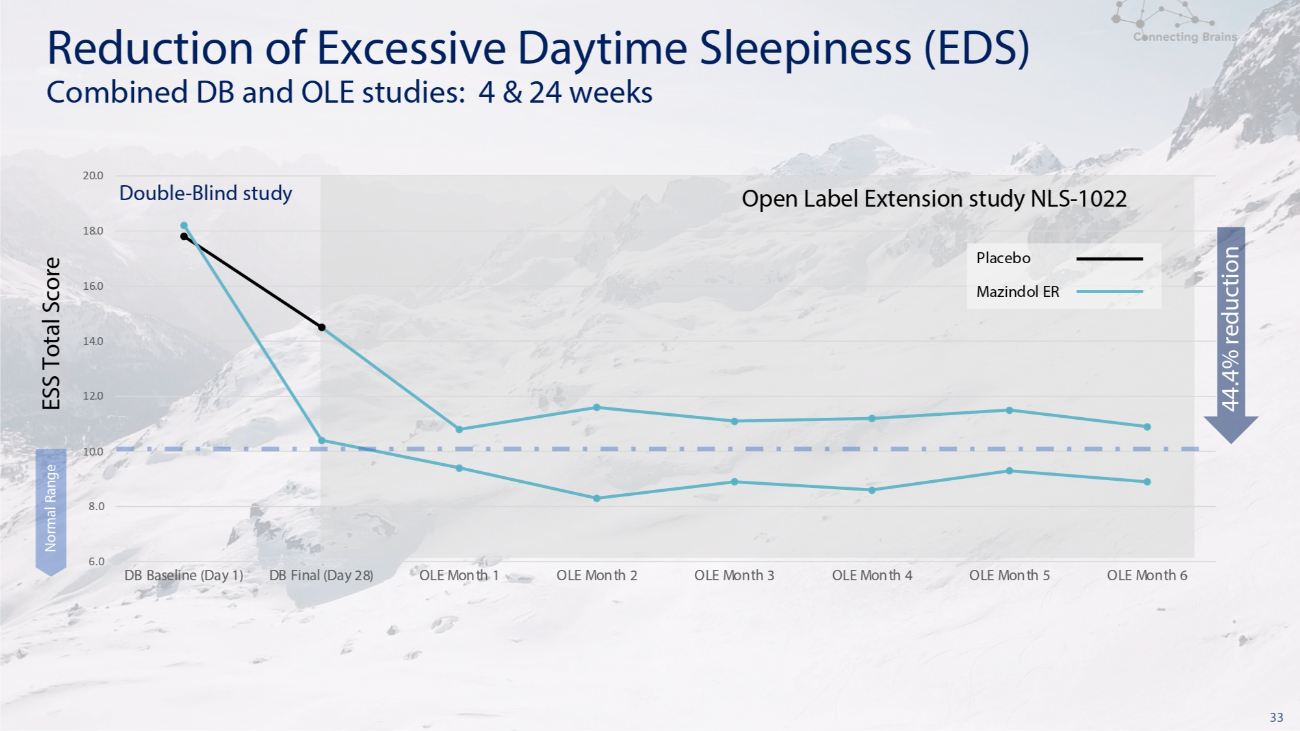
33 6.0 8.0 10.0 12.0 14.0 16.0 18.0 20.0 DB Baseline (Day 1) DB Final (Day 28) OLE Month 1 OLE Month 2 OLE Month 3 OLE Month 4 OLE Month 5 OLE Month 6 Normal Range Placebo Mazindol ER Double - Blind study Open Label Extension study NLS - 1022 Reduction of Excessive Daytime Sleepiness (EDS) Combined DB and OLE studies: 4 & 24 weeks 44.4% reduction ESS Total Score
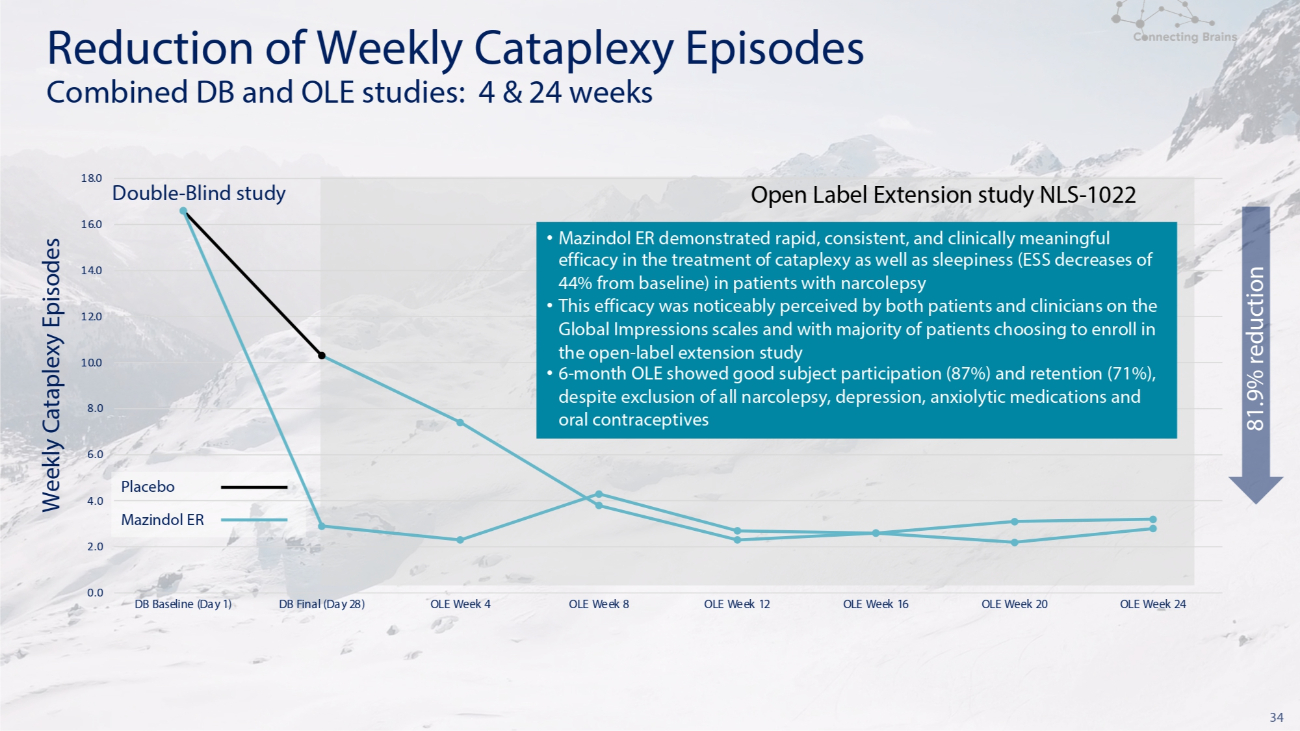
34 Double - Blind study Open Label Extension study NLS - 1022 Reduction of Weekly Cataplexy Episodes Combined DB and OLE studies: 4 & 24 weeks 6.0 Placebo 4.0 Mazindol ER 2.0 0.0 8.0 10.0 12.0 14.0 16.0 18.0 DB Baseline (Day 1) DB Final (Day 28) OLE Week 4 OLE Week 8 OLE Week 12 OLE Week 16 OLE Week 20 OLE Week 24 81.9% reduction Weekly Cataplexy Episodes • Mazindol ER demonstrated rapid, consistent, and clinically meaningful efficacy in the treatment of cataplexy as well as sleepiness (ESS decreases of 44% from baseline) in patients with narcolepsy • This efficacy was noticeably perceived by both patients and clinicians on the Global Impressions scales and with majority of patients choosing to enroll in the open - label extension study • 6 - month OLE showed good subject participation ( 87 % ) and retention ( 71 % ), despite exclusion of all narcolepsy, depression, anxiolytic medications and oral contraceptives
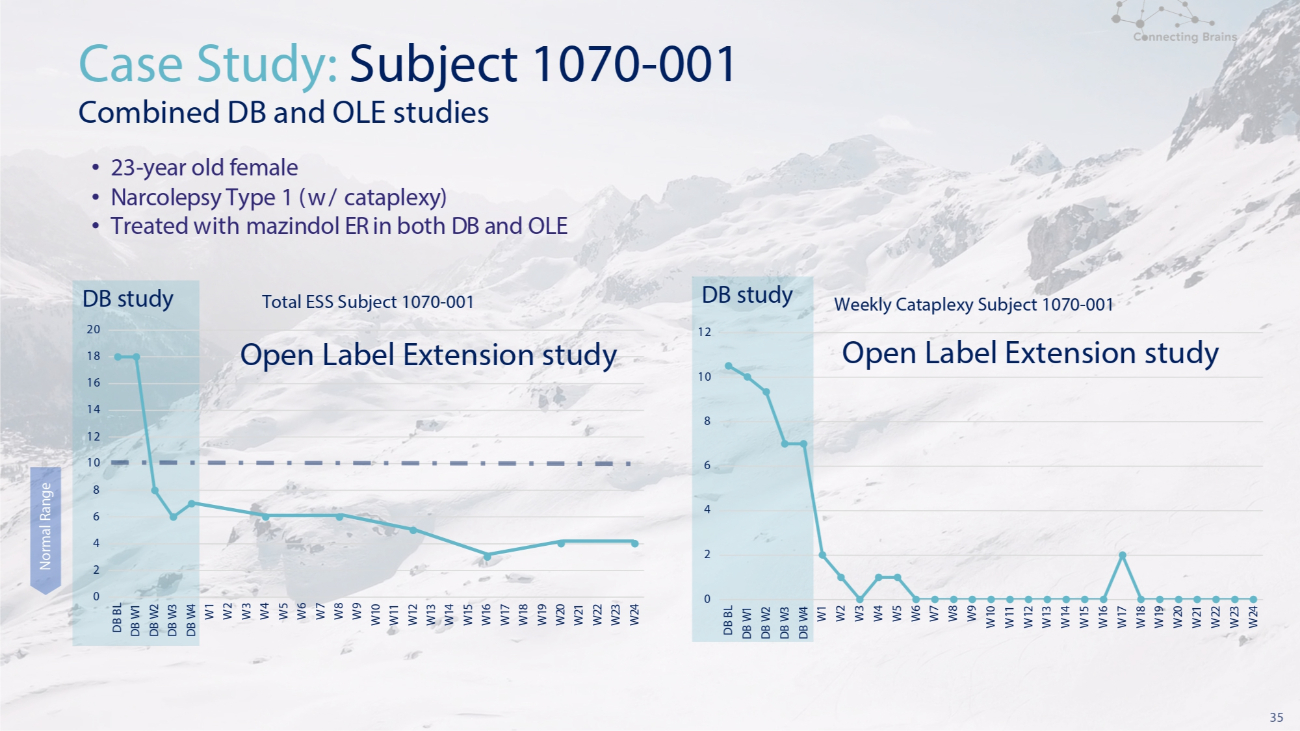
35 18 16 14 12 10 8 6 4 2 0 DB study 20 DB BL DB W 1 DB W 2 DB W 3 DB W 4 W 1 W 2 W 3 W 4 W 5 W 6 W 7 W 8 W 9 W 10 W 11 W 12 W 13 W 14 W 15 W 16 W 17 W 18 W 19 W 20 W 21 W 22 W 23 W 24 Total ESS Subject 1070 - 001 Open Label Extension study Case Study: Subject 1070 - 001 Combined DB and OLE studies • 23 - year old female • Narcolepsy Type 1 (w/ cataplexy) • Treated with mazindol ER in both DB and OLE W1 W 2 W 3 W 4 W 5 W 6 W 7 W 8 W 9 W 10 W 11 W 12 W 13 W 14 W 15 W 16 W 17 W 18 W 19 W 20 W 21 W 22 W 23 W 24 Weekly Cataplexy Subject 1070 - 001 DB study 12 Open Label Extension study 10 8 6 4 2 0 DB BL DB W 1 DB W 2 DB W 3 DB W 4 Normal Range

Open Label Extension Study Results Safety & Tolerability
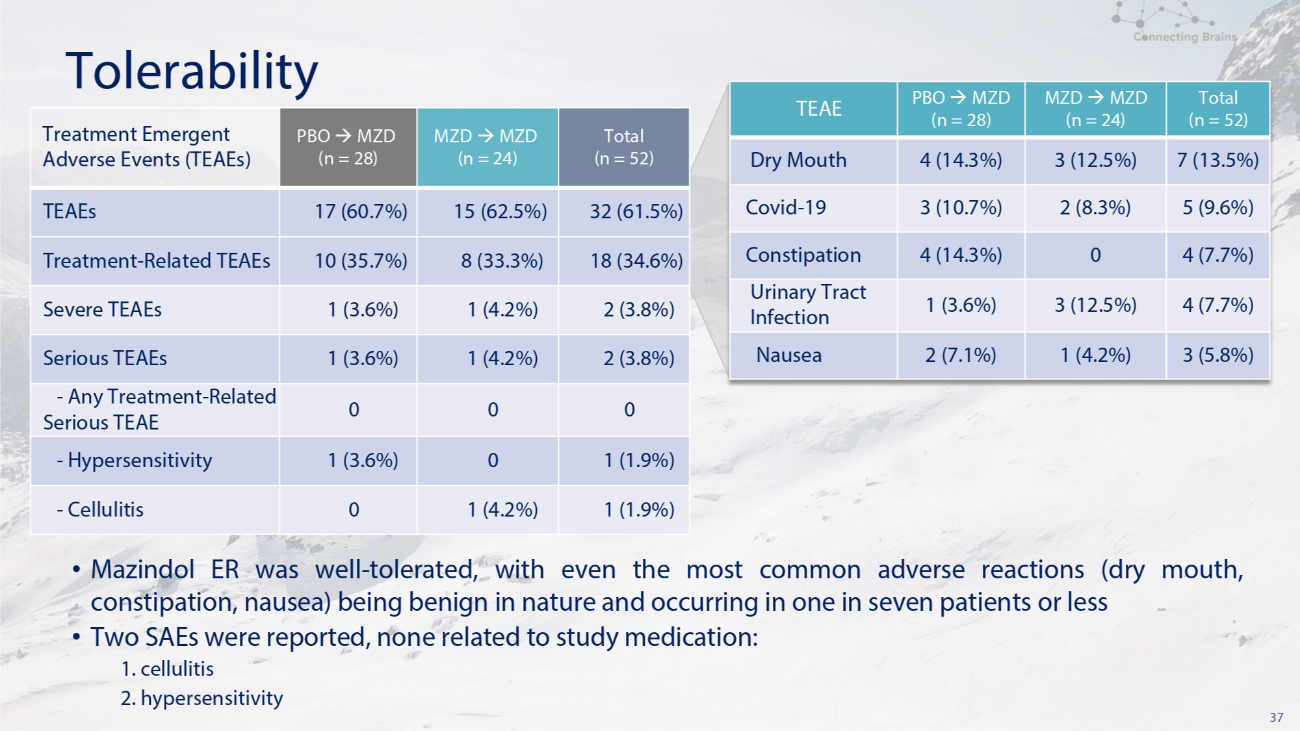
37 Tolerability Total (n = 52) MZD MZD (n = 24) PBO MZD (n = 28) TEAE 7 (13.5%) 3 (12.5%) 4 (14.3%) Dry Mouth 5 (9.6%) 2 (8.3%) 3 (10.7%) Covid - 19 4 (7.7%) 0 4 (14.3%) Constipation 4 (7.7%) 3 (12.5%) 1 (3.6%) Urinary Tract Infection 3 (5.8%) 1 (4.2%) 2 (7.1%) Nausea Total (n = 52) MZD MZD (n = 24) PBO MZD (n = 28) Treatment Emergent Adverse Events (TEAEs) 32 (61.5%) 15 (62.5%) 17 (60.7%) TEAEs 18 (34.6%) 8 (33.3%) 10 (35.7%) Treatment - Related TEAEs 2 (3.8%) 1 (4.2%) 1 (3.6%) Severe TEAEs 2 (3.8%) 1 (4.2%) 1 (3.6%) Serious TEAEs 0 0 0 - Any Treatment - Related Serious TEAE 1 (1.9%) 0 1 (3.6%) - Hypersensitivity 1 (1.9%) 1 (4.2%) 0 - Cellulitis • Mazindol ER was well - tolerated, with even the most common adverse reactions (dry mouth, constipation, nausea) being benign in nature and occurring in one in seven patients or less • Two SAEs were reported, none related to study medication: 1. cellulitis 2. hypersensitivity

38 POLARIS: OLE Conclusions • This 6 - month OLE narcolepsy study showed good subject participation (87%) and retention (71%), especially given the exclusion of any narcolepsy/depression/anxiolytic medications • High treatment compliance with few drug down - titrations reported (11.5%) • Solid efficacy data confirming the interim results: • ESS decreases of 44% from DB study baseline • Cataplexy decreases of 82% from DB study baseline • Mazindol was well tolerated, with 61.5% of patients reporting at least one AE and 34.6% reporting at least one treatment - related AE • Most frequent AEs were dry mouth, constipation, and nausea and occurred in less than one in seven patients • Two SAEs were reported, none related to study medication (cellulitis and hypersensitivity) • The previously - described CV effects of mazindol were confirmed in this study and the magnitude was similar to that seen with stimulants & SNRIs

39 Phase 3 Program NLS - 1031, NLS - 1032 and NLS - 1033
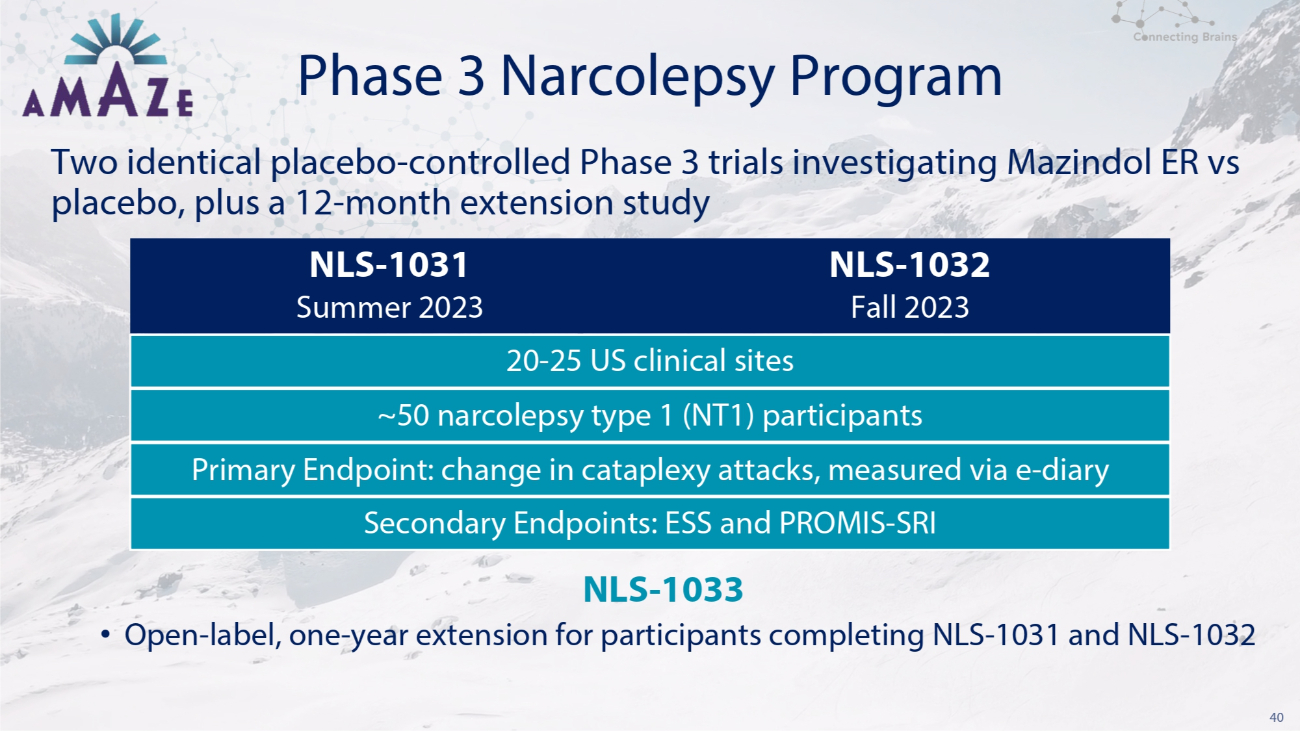
40 Phase 3 Narcolepsy Program Two identical placebo - controlled Phase 3 trials investigating Mazindol ER vs placebo, plus a 12 - month extension study NLS - 1033 • Open - label, one - year extension for participants completing NLS - 1031 and NLS - 1032 NLS - 1031 NLS - 1032 Summer 2023 Fall 2023 20 - 25 US clinical sites ~50 narcolepsy type 1 (NT1) participants Primary Endpoint: change in cataplexy attacks, measured via e - diary Secondary Endpoints: ESS and PROMIS - SRI
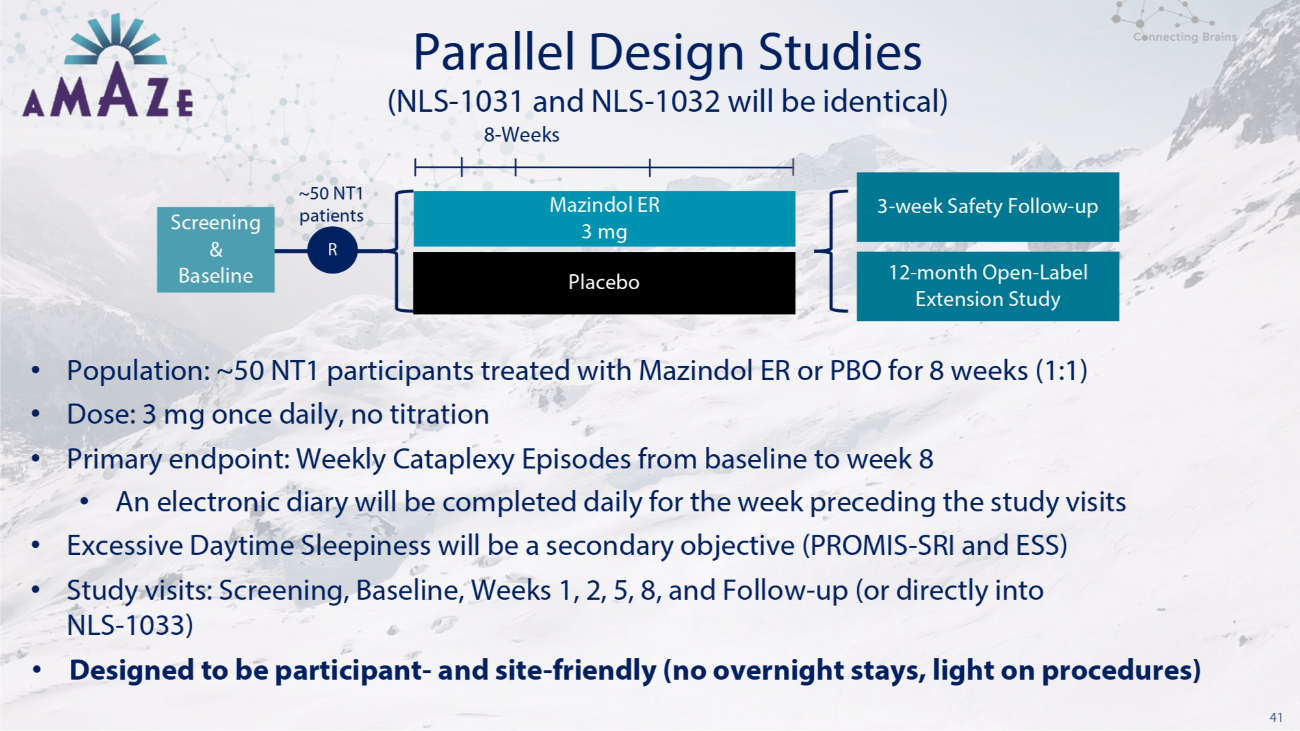
41 Parallel Design Studies • Population: ~50 NT1 participants treated with Mazindol ER or PBO for 8 weeks (1:1) • Dose: 3 mg once daily, no titration • Primary endpoint: Weekly Cataplexy Episodes from baseline to week 8 • An electronic diary will be completed daily for the week preceding the study visits • Excessive Daytime Sleepiness will be a secondary objective (PROMIS - SRI and ESS) • Study visits: Screening, Baseline, Weeks 1, 2, 5, 8, and Follow - up (or directly into NLS - 1033) • Designed to be participant - and site - friendly (no overnight stays, light on procedures) Placebo (NLS - 1031 and NLS - 1032 will be identical) 8 - Weeks Screening & Baseline ~50 NT1 patients R Mazindol ER 3 mg 3 - week Safety Follow - up 12 - month Open - Label Extension Study

42 12 - Month OLE Study (NLS - 1033) • Study Design: A 52 - week, open - label, multi - center study of Mazindol ER 3 mg once daily • Study population: ~100 participants (Completers from studies NLS - 1031 and NLS - 1032) • Rescue narcolepsy/psychiatric medications allowed in the study • Primary objective: Long - term safety and tolerability of Mazindol ER • Secondary objective: Long - term therapeutic response to Mazindol ER in the treatment of Cataplexy and EDS • Study visits: Baseline, Weeks 2, 4, 12, 26, 40, 52, Follow up NLS - 1032 52 - Week Open - Label Treatment Period Mazindol ER 3 mg 3 - week Safety Follow - up NLS - 1031 ~100 NT1 patients

Mazindol ER (3mg) Competitive Advantages
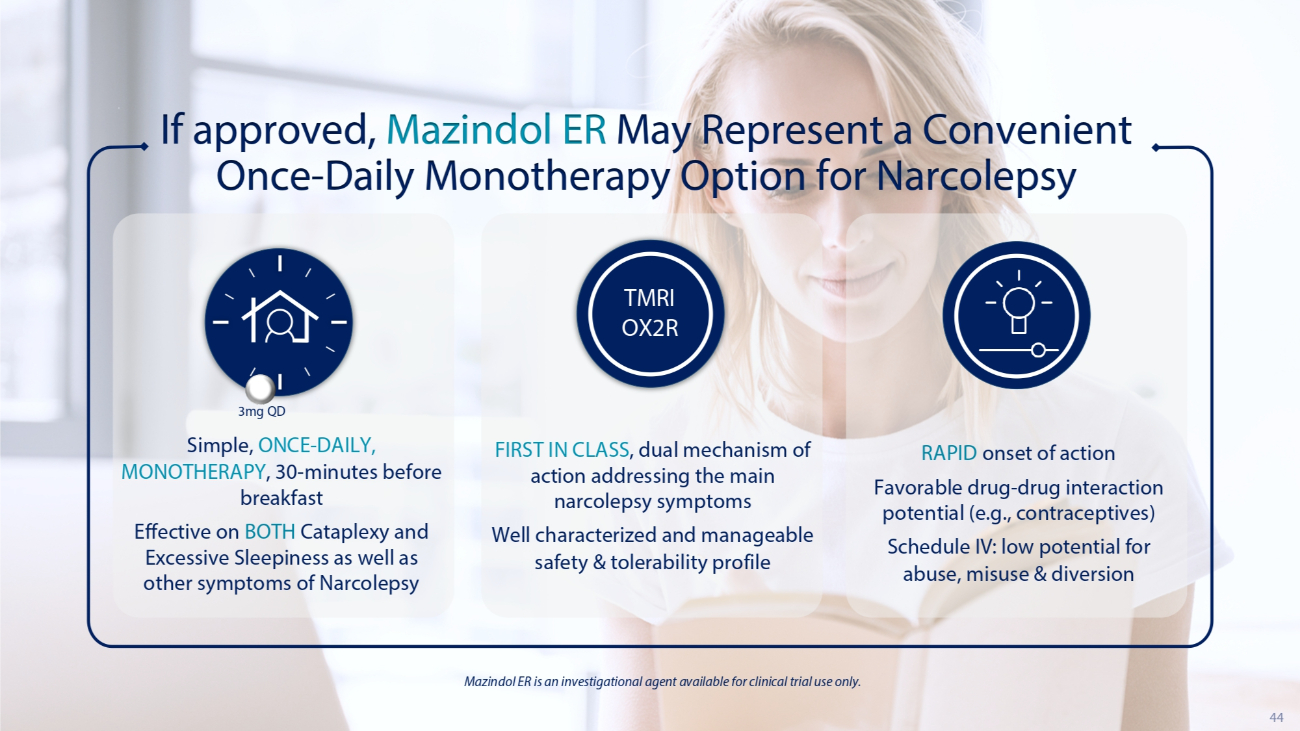
44 If approved, Mazindol ER May Represent a Convenient Once - Daily Monotherapy Option for Narcolepsy Simple, ONCE - DAILY, MONOTHERAPY , 30 - minutes before breakfast Effective on BOTH Cataplexy and Excessive Sleepiness as well as other symptoms of Narcolepsy RAPID onset of action Favorable drug - drug interaction potential (e.g., contraceptives) Schedule IV: low potential for abuse, misuse & diversion Mazindol ER is an investigational agent available for clinical trial use only. 3mg QD FIRST IN CLASS , dual mechanism of action addressing the main narcolepsy symptoms Well characterized and manageable safety & tolerability profile TMRI OX2R
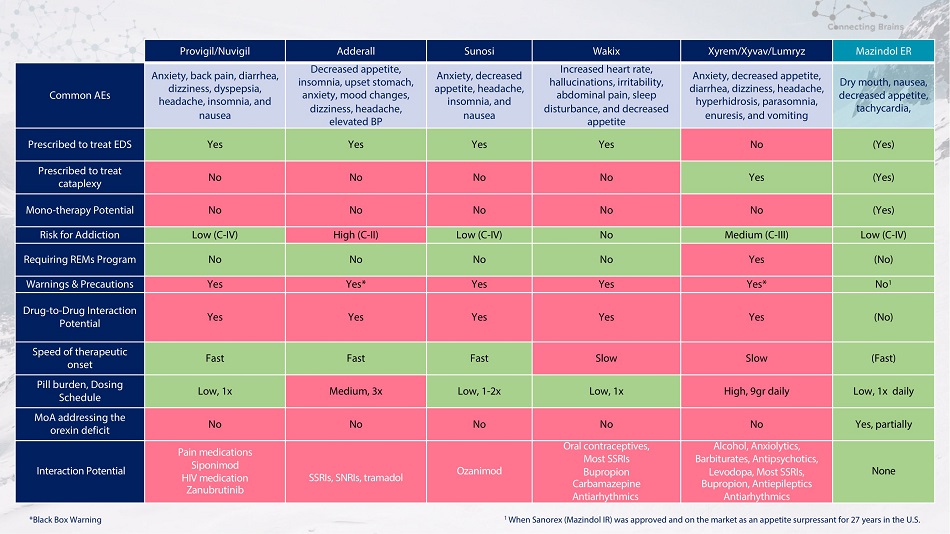
Provigil/Nuvigil Adderall Sunosi Wakix Xyrem/Xyvav/Lumryz Mazindol ER Common AEs Anxiety, back pain, diarrhea, dizziness, dyspepsia, headache, insomnia, and nausea Decreased appetite, insomnia, upset stomach, anxiety, mood changes, dizziness, headache, elevated BP Anxiety, decreased appetite, headache, insomnia, and nausea Increased heart rate, hallucinations, irritability, abdominal pain, sleep disturbance, and decreased appetite Anxiety, decreased appetite, diarrhea, dizziness, headache, hyperhidrosis, parasomnia, enuresis, and vomiting Dry mouth, nausea, decreased appetite, tachycardia, Prescribed to treat EDS Yes Yes Yes Yes No (Yes) Prescribed to treat cataplexy No No No No Yes (Yes) Mono - therapy Potential No No No No No (Yes) Risk for Addiction Low (C - IV) High (C - II) Low (C - IV) No Medium (C - III) Low (C - IV) Requiring REMs Program No No No No Yes (No) Warnings & Precautions Yes Yes* Yes Yes Yes* No 1 Drug - to - Drug Interaction Potential Yes Yes Yes Yes Yes (No) Speed of therapeutic onset Fast Fast Fast Slow Slow (Fast) Pill burden, Dosing Schedule Low, 1x Medium, 3x Low, 1 - 2x Low, 1x High, 9gr daily Low, 1x daily MoA addressing the orexin deficit No No No No No Yes, partially Interaction Potential Pain medications Siponimod HIV medication Zanubrutinib SSRIs, SNRIs, tramadol Ozanimod Oral contraceptives, Most SSRIs Bupropion Carbamazepine Antiarhythmics Alcohol, Anxiolytics, Barbiturates, Antipsychotics, Levodopa, Most SSRIs, Bupropion, Antiepileptics Antiarhythmics None 1 When Sanorex (Mazindol IR) was approved and on the market as an appetite surpressant for 27 years in the U.S. *Black Box Warning

Timeline
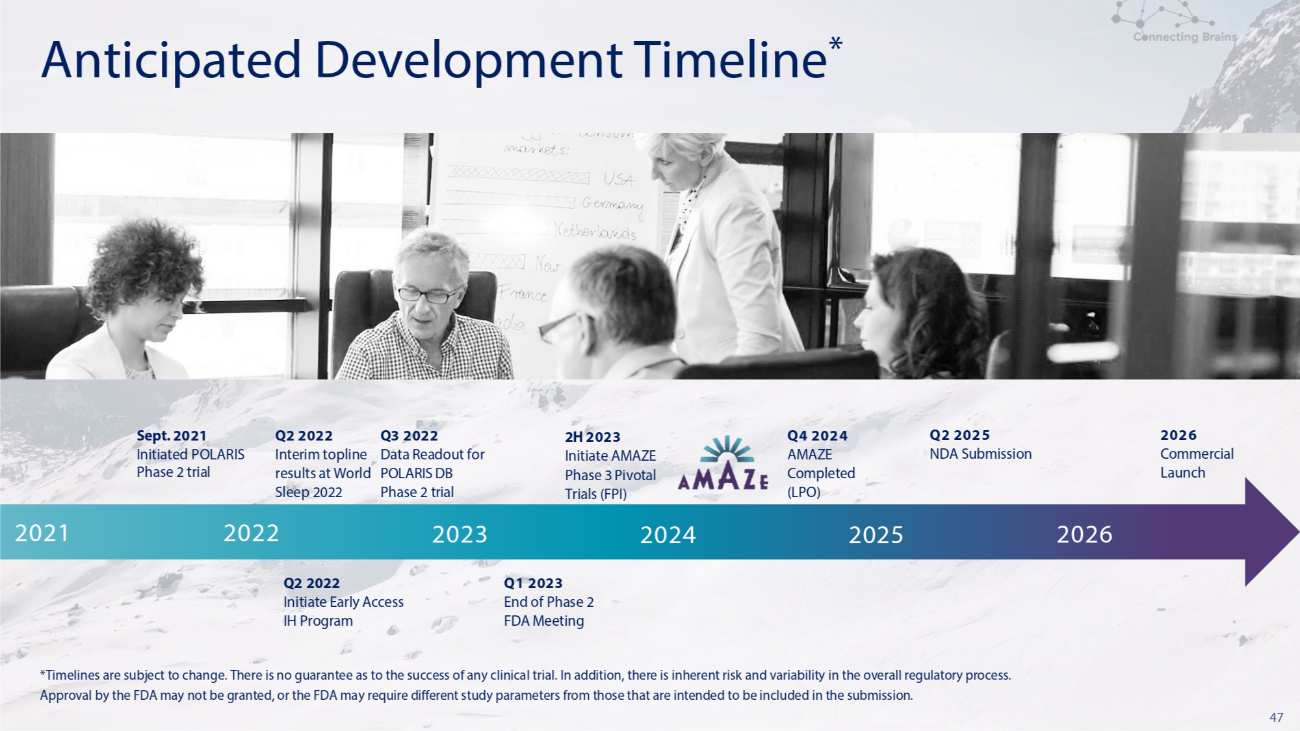
47 *Timelines are subject to change. There is no guarantee as to the success of any clinical trial. In addition, there is inherent risk and variability in the overall regulatory process. Approval by the FDA may not be granted, or the FDA may require different study parameters from those that are intended to be included in the submission. Anticipated Development Timeline * 2026 Q2 2025 Q4 2024 2H 2023 Q3 2022 Q2 2022 Sept. 2021 Commercial NDA Submission AMAZE Initiate AMAZE Data Readout for Interim topline Initiated POLARIS Launch Completed Phase 3 Pivotal POLARIS DB results at World Phase 2 trial (LPO) Trials (FPI) Phase 2 trial Sleep 2022 2024 2025 2026 2023 2021 2022 Q1 2023 End of Phase 2 FDA Meeting Q2 2022 Initiate Early Access IH Program
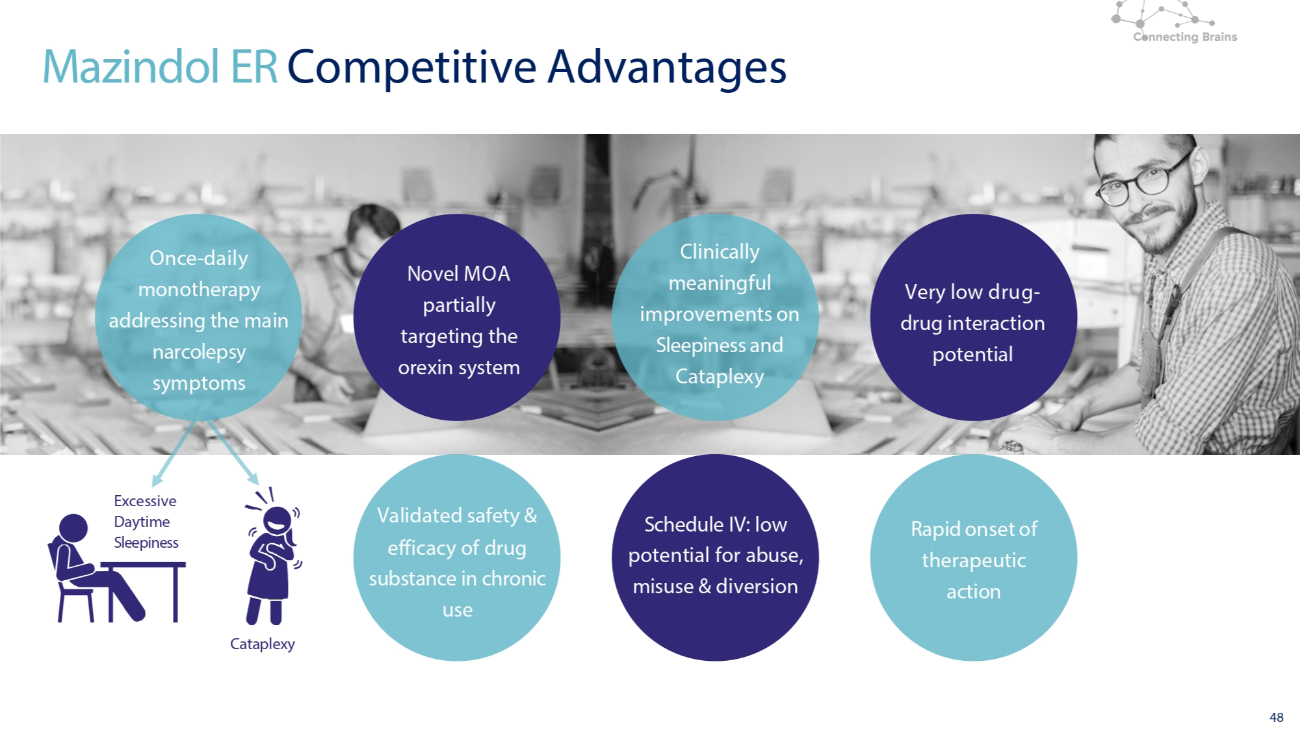
48 Novel MOA partially targeting the orexin system Validated safety & efficacy of drug substance in chronic use Clinically meaningful improvements on Sleepiness and Cataplexy Schedule IV: low potential for abuse, misuse & diversion Very low drug - drug interaction potential Rapid onset of therapeutic action Mazindol ER Competitive Advantages Cataplexy Excessive Daytime Sleepiness Once - daily monotherapy addressing the main narcolepsy symptoms

49 Redefining Central Nervous System Therapies
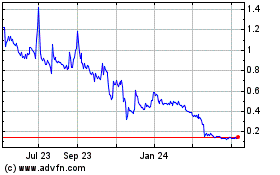
NLS Pharmaceutics (NASDAQ:NLSP)
Historical Stock Chart
From Apr 2024 to May 2024
NLS Pharmaceutics (NASDAQ:NLSP)
Historical Stock Chart
From May 2023 to May 2024