Miromatrix Applauds the FDA for Its Continued Commitment to the Field of Organ Transplant
July 05 2022 - 7:01AM

Miromatrix Medical Inc. (NASDAQ: MIRO), a life sciences company
pioneering a novel technology for bioengineering fully
transplantable organs to help save and improve patients' lives,
applauds the U.S. Food and Drug Administration (FDA) for hosting a
two-day public advisory committee meeting to discuss scientific and
regulatory topics relating to the field of organ transplant,
specifically xenotransplantation. Opening remarks from the FDA
highlighted the limited availability of human organs for transplant
and recognized the tremendous unmet need for new treatments for
patients with end-stage organ failure.
"We are appreciative of the FDA's significant
commitment of time and resources to discuss topics that may
ultimately help address the extensive shortage of organs available
for transplant. We are also excited by the possibility that our
proprietary decellularization and recellularization technology may
provide an additional treatment alternative for organ transplant
patients," said Jeff Ross Ph.D., Miromatrix's CEO. "Our proprietary
decellularization and recellularization technology platform was
designed in such a manner that we believe it is not classified as
xenotransplantation, and it may address some of the challenges that
have historically faced xenotransplantation technologies, including
gene editing technologies."
Scott Nyberg M.D., Ph.D., a Mayo Clinic transplant
surgeon and Miromatrix collaborator, added that "Miromatrix's
two-step method of decellularization and recellularization is
designed to remove the porcine cells from the organs obtained from
pigs and replace them with unmodified human cells to reduce the
risk of organ rejection amongst other potential benefits."
The FDA published its original guidance for the
xenotransplantation industry in 2003 and updated that guidance in
2016. The FDA noted that the updated guidance addresses many of the
topics discussed during the two-day meeting and that the science is
changing rapidly.
About MiromatrixMiromatrix Medical
Inc. is a life sciences company pioneering a novel technology for
bioengineering fully transplantable human organs to help save and
improve patients' lives. The Company has developed a proprietary
perfusion technology platform for bioengineering organs that it
believes will efficiently scale to address the shortage of
available human organs. The Company's initial development focus is
on human livers and kidneys. For more information,
visit miromatrix.com.
Investor ContactGreg
Chodaczek347-620-7010 ir@miromatrix.com
Media
Contact:press@miromatrix.com
MiroMatrix Medical (NASDAQ:MIRO)
Historical Stock Chart
From Mar 2024 to Apr 2024
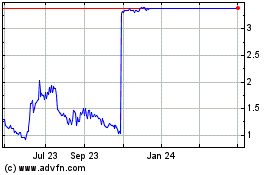
MiroMatrix Medical (NASDAQ:MIRO)
Historical Stock Chart
From Apr 2023 to Apr 2024