Filed
Pursuant to Rule 424(b)(5)
Registration
No. 333-262311
PROSPECTUS
SUPPLEMENT
(To
Prospectus dated February 1, 2022)
$50,000,000

Beyond
Air, Inc.
Common
Stock
We
have entered into a sales agreement with Truist Securities, Inc., or Truist, and Oppenheimer & Co. Inc., or Oppenheimer, each of
whom we refer to as an agent, and together as the agents, relating to shares of our common stock offered by this prospectus supplement.
In accordance with the terms of the sales agreement, we may offer and sell shares of our common stock having an aggregate offering price
of up to $50,000,000 from time to time through the agents.
Our
common stock is listed on the Nasdaq Capital Market under the symbol “XAIR.” On February 2, the last reported sales price
for our common stock was $7.24 per share.
Sales
of our common stock, if any, under this prospectus supplement may be made in sales deemed to be “at the market offerings”
as defined in Rule 415 promulgated under the Securities Act of 1933, as amended, or the Securities Act, including sales made directly
on or through the Nasdaq Capital Market, the existing trading market for our common stock, or any other existing trading market for our
common stock. The agents are not required to sell any specific number or dollar amount of securities, but will act as sales agents, using
commercially reasonable efforts consistent with their normal trading and sales practices, on mutually agreed terms among the agents and
us. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
The
compensation to the agents for sales of common stock sold pursuant to the sales agreement will be an amount up to 3.0% of the gross proceeds
of any shares of common stock sold under the sales agreement. See “Plan of Distribution” beginning on page S-13 for additional
information regarding the compensation to be paid to the agents. In connection with the sale of the common stock on our behalf, each
of the agents will be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation of the
agents will be deemed to be underwriting commissions or discounts. We have also agreed to provide indemnification and contribution to
the agents with respect to certain liabilities, including liabilities under the Securities Act or the Exchange Act of 1934, as amended,
or the Exchange Act.
Investing
in our securities involves a high degree of risk. You should review carefully the risks and uncertainties described under the heading
“Risk Factors” on page S-9 of this prospectus supplement and under similar headings in the other documents that are incorporated
by reference into this prospectus supplement.
NEITHER
THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED
IF THIS PROSPECTUS SUPPLEMENT AND THE ACCOMPANYING PROSPECTUS ARE TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL
OFFENSE.
|
Truist
Securities
|
|
Oppenheimer
& Co.
|
February
4, 2022
TABLE
OF CONTENTS
Prospectus
Supplement
Prospectus
ABOUT
THIS PROSPECTUS SUPPLEMENT
This
document is comprised of two parts. The first part is this prospectus supplement, which describes the specific terms of this offering
of our shares of common stock. The second part is the accompanying prospectus, including the documents incorporated by reference into
the accompanying prospectus, which provides more general information, some of which may not apply to this offering. The information included
or incorporated by reference in this prospectus supplement also adds to, updates and changes information contained or incorporated by
reference in the accompanying prospectus. If information included or incorporated by reference in this prospectus supplement is inconsistent
with the accompanying prospectus or the information incorporated by reference therein, then this prospectus supplement or the information
incorporated by reference in this prospectus supplement will apply and will supersede the information in the accompanying prospectus
and the documents incorporated by reference therein.
This
prospectus supplement is part of a registration statement on Form S-3 (File No. 333-262311) that we filed with the Securities and Exchange
Commission, or the SEC, using a “shelf” registration process. Under the shelf registration process, we may from time to time
offer and sell any combination of the securities described in the accompanying prospectus up to a total dollar amount of $200,000,000,
of which this offering is a part. Under this prospectus supplement, we may offer shares of our common stock having a total aggregate
offering price of up to $50,000,000 from time to time at prices and on terms to be determined by market conditions at the time of offering.
We
have not authorized anyone to provide you with information other than that contained or incorporated by reference in this prospectus
supplement, the accompanying prospectus and any free writing prospectus that we have authorized for use in connection with this offering.
We do not take any responsibility for, and can provide no assurance as to the reliability of, any other information that others may give
you. Our business, financial condition, results of operations and prospects may have changed since those dates. You should not assume
that the information contained or incorporated in this prospectus supplement and the accompanying prospectus is accurate as of any date
other than their respective dates, regardless of the time of delivery. You should read this prospectus supplement, the accompanying prospectus,
the documents incorporated by reference in this prospectus supplement and the accompanying prospectus, and any free writing prospectus
that we have authorized for use in connection with this offering when making your investment decision. We and the agents are not making
an offer to sell our common stock offered hereto in any jurisdiction where the offer or sale is not permitted.
This
prospectus supplement, the accompanying prospectus and the information incorporated herein and therein by reference includes trademarks,
service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included or incorporated
by reference into this prospectus supplement or the accompanying prospectus are the property of their respective owners.
Unless
the context indicates otherwise, in this prospectus supplement and the accompanying prospectus the terms, the “Company,”
“we,” “our” or “us” refer to Beyond Air, Inc. and its wholly-owned subsidiaries.
CAUTIONARY
STATEMENTS CONCERNING FORWARD-LOOKING STATEMENTS
This
prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein may contain forward looking statements
within the meaning of Section 27A of the Securities Act of 1933 (the “Securities Act”), and Section 21E of the Securities
Exchange Act of 1934 (the “Exchange Act”) that involve risks and uncertainties. All statements other than statements of historical
fact contained in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein, including
statements regarding future events, our future financial performance, business strategy, and plans and objectives of management for future
operations, are forward-looking statements. We have attempted to identify forward-looking statements by terminology including “anticipates,”
“believes,” “can,” “continue,” “could,” “estimates,” “expects,”
“intends,” “may,” “plans,” “potential,” “predicts,” “should,”
or “will” or the negative of these terms or other comparable terminology. Although we do not make forward looking statements
unless we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy. These statements are only predictions
and involve known and unknown risks, uncertainties and other factors, including the risks outlined under “Risk Factors” or
elsewhere in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein, which may cause
our or our industry’s actual results, levels of activity, performance or achievements expressed or implied by these forward-looking
statements. Moreover, we operate in a highly regulated, very competitive, and rapidly changing environment. New risks emerge from time
to time and it is not possible for us to predict all risk factors, nor can we address the impact of all factors on our business or the
extent to which any factor, or combination of factors, may cause our actual results to differ materially from those contained in any
forward-looking statements.
We
have based these forward-looking statements largely on our current expectations and projections about future events and financial trends
that we believe may affect our financial condition, results of operations, business strategy, short term and long-term business operations,
and financial needs. These forward-looking statements are subject to certain risks and uncertainties that could cause our actual results
to differ materially from those reflected in the forward looking statements. Factors that could cause or contribute to such differences
include, but are not limited to, those discussed in this this prospectus supplement, the accompanying prospectus and the documents incorporated
by reference herein, and in particular, the risks discussed below and under the heading “Risk Factors” and those discussed
in other documents we file with the SEC. We undertake no obligation to revise or publicly release the results of any revision to these
forward-looking statements, except as required by law. In light of these risks, uncertainties and assumptions, the forward-looking events
and circumstances discussed in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein
may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statement.
You
should not place undue reliance on any forward-looking statement, each of which applies only as of the date of this prospectus supplement.
Except as required by law, we undertake no obligation to update or revise publicly any of the forward-looking statements after the date
of this prospectus supplement to conform our statements to actual results or changed expectations.
PROSPECTUS
SUPPLEMENT SUMMARY
This
summary highlights selected information about our company, this offering and information appearing elsewhere in this prospectus supplement,
in the accompanying prospectus, and in the documents we incorporate by reference. This summary is not complete and does not contain all
the information that you should consider before investing in our securities. You should carefully read this entire prospectus supplement
and the accompanying prospectus, particularly the information included under the heading “Risk Factors” contained in this
prospectus supplement beginning on page S-9, and the risk factors, financial statements and notes incorporated by reference herein,
before making an investment decision. This prospectus supplement may add to, update or change information in the accompanying prospectus.
Company
Overview
We
are a clinical-stage medical device and biopharmaceutical company developing a nitric oxide (“NO”) generator and delivery
system (the “LungFit® system”) capable of generating NO from ambient air. The LungFit® platform
can generate NO up to 400 parts per million for delivery to a patient’s lungs directly or via a ventilator. LungFit®
can deliver NO either continuously or for a fixed amount of time at various flow rates and has the ability to either titrate dose on
demand or maintain a constant dose. We believe that LungFit® can be used to treat patients on ventilators that require
NO, as well as patients with chronic or acute severe lung infections via delivery through a breathing mask or similar apparatus. Furthermore,
we believe that there is a high unmet medical need for patients suffering from certain severe lung infections that the LungFit®
platform can potentially address. Our current
areas of focus with LungFit® are persistent pulmonary hypertension of the newborn (PPHN), community-acquired viral pneumonia
(CAVP) including COVID-19 (previously termed acute viral pneumonia, or AVP), bronchiolitis (BRO) and nontuberculous mycobacteria (NTM)
lung infection. The Company’s current product candidates will be subject to premarket reviews and approvals by the U.S. Food and
Drug Administration, (the “FDA”), CE marking conformity assessment by a notified body in the European Union (the “E.U.”),
as well as similar regulatory agencies’ reviews or approvals in other countries or regions.
If approved, our system will be marketed as a medical device in the U.S.
An
additional focus of the Company is solid tumors, through our majority-owned affiliate Beyond Cancer, Ltd. For the solid tumor indication
the LungFit® platform is not utilized due to need for ultra-high concentrations of gaseous nitric oxide (“UNO”).
A proprietary delivery system has been developed that can safely deliver UNO in excess of 10,000 ppm directly to a solid tumor. This
program is in preclinical development and will require approval from the FDA or similar regulatory agencies in other countries to enter
human studies. We anticipate beginning the enrollment of patients into the first human trial in the first quarter of calendar year 2022.
We
believe LungFit® will be the first approved system with patented technology that generates NO using room air, enabling
the delivery of unlimited, on-demand NO regardless of dose or flow. To generate the NO, a pump within the LungFit® flows
room air through a small chamber where power, equivalent to a 60-watt lightbulb, ionizes the oxygen and nitrogen molecules. The molecules
recombine as NO. We believe that the on-demand delivery, either to a ventilator circuit or directly to a patient’s lungs, is safe
due to the Company’s system design and the Company’s proprietary nitrogen dioxide (“NO2”) filter.
The NO2 filter removes toxic NO2 for 12 hours when used for PPHN and shorter periods for treating other conditions
that require NO concentrations of 150 ppm or more.
With
respect to PPHN, our novel LungFit® PH is designed to deliver a dosage of NO to the lungs that is consistent with current
guidelines for delivery of 20 ppm NO with a range of 0.5 ppm – 80 ppm (low-concentration NO) for ventilated patients. We believe
the ability of LungFit® PH to generate NO from ambient air provides us with many competitive advantages over the current
standard of NO delivery systems in the U.S., the E.U., Japan and other markets. For example, LungFit® PH does not require
the use of a high-pressure cylinder, does not require cumbersome purging procedures and places less burden on hospital staff in carrying
out safety procedures.
Our
novel LungFit® platform can also deliver a high concentration (>150 ppm) of NO directly to the lungs, which
we believe has the potential to eliminate microbial infections including bacteria, fungi and viruses, among others. We believe that current
FDA-approved NO vasodilation treatments would have limited success in treating microbial infections given the low concentrations of NO
being delivered (<100 ppm). Given that NO is produced naturally by the body as an innate immunity mechanism, at a concentration of
200 ppm, supplemental high dose NO should aid in the body’s fight against infection. Based on our preclinical and clinical studies,
we believe that 150 ppm is the minimum therapeutic dose to achieve the desired pulmonary antimicrobial effect of NO. To date, neither
the FDA nor equivalent regulatory agencies in other countries or regions have approved any NO formulation and/or delivery system for
>80 ppm NO.
LungFit®
PH for the treatment of Persistent Pulmonary Hypertension of the Newborn
In
November 2020 we submitted a PMA application to the FDA for the use of LungFit® PH in PPHN. There is a standard 180-day
review process that starts upon the FDA’s acknowledgement of the submission, though PMA reviews oftentimes take much longer, sometimes
over a year or more. Moreover, the ongoing COVID-19 pandemic and an increased volume of submissions have led to longer review times by
the FDA. We anticipate an FDA decision on the PMA in the first half of the calendar year 2022.
We
also expect to receive the CE Mark under the Medical Device Regulation (“MDR”) in the E.U. in the first half of calendar
year 2022. According to the most recent year-end report from Mallinckrodt Pharmaceuticals, sales of NO were $574.1 million in 2020 (up
from $571.4 million in 2019) for the United States, Canada, Japan, Mexico and Australia, with >90% in the United States. However,
due to increased competition following the launch of competing nitric oxide delivery systems in the United States, Mallinckrodt Pharmaceutical’s
sales of NO faced a downwards trajectory, reporting $134 million in the first quarter of 2021, $106 million in the second quarter of
2021 and $98 million in the third quarter of 2021. Outside of the U.S. there are multiple market participants which translates to considerably
lower sales than in the U.S. We believe the U.S. sales potential of LungFit® PH in PPHN to be greater than $300 million
and worldwide sales potential to be greater than $600 million. If regulatory approval is obtained, we anticipate a product launch in
the U.S. in the first half of calendar year 2022 and will continue to launch in the EU and globally in 2022 and beyond.
LungFit®
PRO for the treatment of viral lung infections in hospitalized patients
Community-Acquired
Viral Pneumonia (including COVID-19)
Viral
pneumonia in adults is most commonly caused by rhinovirus, respiratory syncytial virus (“RSV”) and influenza virus. However,
newly emerging viruses (including SARS-CoV-1, SARS-CoV-2, avian influenza A, and H1N1 viruses) have been identified as pathogens contributing
to the overall burden of adult viral pneumonia. COVID-19 is an infectious disease caused by SARS-CoV-2, that has resulted in a global
pandemic. Excluding the pandemic, there are approximately 350,000 annual viral pneumonia hospitalizations in the US, and 16 million annual
viral pneumonia hospitalizations globally. For the broader annual viral pneumonia, we believe U.S. sales potential to be greater than
$1.5 billion and worldwide market potential to be greater than $3 billion.
We
initiated a pilot study in late 2020 using our novel LungFit® PRO system at 150 ppm to treat patients with CAVP, including
COVID-19. The ongoing trial is a multi-center, open-label, randomized clinical trial in Israel, including patients infected with SARS-CoV-2.
Patients are randomized in a 1:1 ratio to receive either inhalations of 150 ppm NO given intermittently for 40 minutes four times per
day for up to seven days in addition to standard supportive treatment (“NO+SST”) or standard supportive treatment alone (“SST”).
Endpoints related to safety (primary endpoint), oxygen saturation and ICU admission, among others, will be assessed.
We
reported interim data from this ongoing trial at the American Thoracic Society or ATS International Conference 2021, which was held virtually
from May 14, 2021 through May 19, 2021. At the time of the data cut off, the intent-to-treat (“ITT”) analysis population
included 19 COVID-19 patients (9 NO + SST vs 10 SST). The data readout showed that 150 ppm NO treatment administered via LungFit®
PRO was safe and well tolerated and demonstrated encouraging efficacy signals. From a safety perspective, there were no treatment-related,
or possibly related, adverse events or severe adverse events. NO2 levels were below 4 ppm at all timepoints (trial safety
threshold is 5 ppm) and methemoglobin (“MetHb”) levels were below 4% at all times (trial safety threshold is 10%). With respect
to the requirement of oxygen support beyond hospital stay, 22.2% of subjects in the NO + SST group compared with 40% of control subjects
had this requirement. There was an observable trend of shortening the duration of hospital stay and duration on oxygen support for treated
patients. The pilot study in adult viral pneumonia, including COVID-19, remains active with trial sites open for enrollment. Additional
detailed study results may be submitted for presentation at an upcoming scientific meeting in April 2022.
Bronchiolitis
(BRO)
Bronchiolitis
is the leading cause of hospital admission in children less than 1 year of age. The incidence is estimated to be 150 million new cases
a year worldwide, with 2-3% (over 3 million) of them severe enough to require hospitalization. Worldwide, 95%3 of all cases
occur in developing countries. In the U.S., there are more than 120,000 annual bronchiolitis hospitalizations and approximately 3.2 million
annual child hospitalizations globally. Currently, there is no approved treatment for bronchiolitis. The treatment for acute viral lung
infections that cause bronchiolitis in infants is largely supportive care and is based primarily on prolonged hospitalization during
which the infant receives a constant flow of oxygen to treat hypoxemia, a reduced concentration of oxygen in the blood. In addition,
systemic steroids and inhalation with bronchodilators are sometimes utilized until recovery, but we believe that these treatments do
not successfully reduce hospital length of stay. We believe the U.S. market potential for bronchiolitis to be greater than $500 million
and worldwide market potential to be greater than $1.2 billion.
Our
BRO program is currently on hold due to the COVID-19 pandemic. The pivotal study for bronchiolitis was originally set to be performed
in the winter of 2020/21 but was delayed due to the pandemic. We have completed three successful pilot studies for bronchiolitis. A further
analysis of the three previously reported pilot studies was presented at the ATS International Conference 2021, which was held virtually
from May 14, 2021 through May 19, 2021. Analysis across the studies (n=198 infants, mean age 3.9 months) showed that 150 ppm –
160 ppm NO administered intermittently was generally safe and well tolerated with adverse event rates similar among treatment groups
with no reported treatment-related serious adverse events. The short course of treatments with intermittent high concentration inhaled
NO was effective in shortening hospital length of stay and accelerating time to fit for discharge – a composite endpoint of clinical
signs and symptoms to indicate readiness to be evaluated for hospital discharge. This treatment was also effective in accelerating time
to stable oxygen saturation – measured as SpO2 ≥ 92% in room air. Additionally, NO at a dose of 85 ppm NO showed no difference
compared to control for all efficacy endpoints, while 150 ppm NO showed statistical significance when compared to control.
We
believe that the entirety of data at 150 ppm - 160 ppm NO in both adult and infant patient populations supports further development of
LungFit® PRO in a pivotal study for patients hospitalized with viral pneumonia.
LungFit®
GO for the treatment of Nontuberculous mycobacteria (NTM)
NTM
lung infection is a rare and serious pulmonary disease associated with increased morbidity and mortality. Patients with NTM lung disease
may experience a multitude of symptoms such as fever, weight loss, cough, lack of appetite, night sweats, blood in the sputum and fatigue.
Patients with NTM lung disease, specifically Mycobacterium abscessus (M.abscessus) representing 20% - 25% of all NTM and
other forms of NTM that are refractory to antibiotic therapy, frequently require lengthy and repeated hospital stays to manage
their condition. There are no treatments specifically indicated for the treatment of M. abscessus lung disease in North America,
Europe or Japan.
There
are approximately 50,000 to 90,000 people with NTM infections in the U.S. In Asia, the number of patients suffering from NTM surpasses
what is seen in the U.S. There is one inhaled antibiotic approved for the treatment of refractory Mycobacterium avium complex (“MAC”).
Current guideline-based approaches to treat NTM lung disease involve multi-drug regimens of antibiotics that may cause severe, long lasting
side effects, and treatment can be as long as 18 months or more. Median survival for NTM MAC patients is approximately 13 years while
median survival for patients with other variations of NTM is typically 4.6 years. The prevalence of human disease attributable to NTM
has increased over the past two decades. In a study conducted between 2007 and 2016, researchers found that the prevalence of NTM in
the U.S. is increasing at approximately 7.5% per year. M. abscessus treatment costs are estimated to be more than double that of MAC.
In total, a 2015 publication by co-authors from several U.S. government departments stated that annual cases in 2014 cost the U.S. healthcare
system approximately $1.7 billion. For this indication, we believe U.S. sales potential to be greater than $1 billion and worldwide sales
potential to be greater than $2.5 billion.
In
December 2020 we began a 12-week, multi-center, open-label clinical trial in Australia and we plan to enroll approximately 20 adult patients
with chronic refractory NTM lung disease. We received a grant of up to $2.17 million from the CFF to fund this study and advance the
clinical development of inhaled NO to treat NTM pulmonary disease. The trial is enrolling both cystic fibrosis (“CF”) and
non-CF patients infected with MAC or M. abscessus. The study consists of a run-in period followed by two treatment phases. The
run-in period provides a baseline for the efficacy endpoints. The first treatment phase takes place over a two-week period and begins
in the hospital setting where patients will be titrated from 150 ppm NO up to 250 ppm NO over several days. During this phase patients
receive NO for 40 minutes, four times per day while MetHb levels are monitored. Patients are also trained to use LungFit®
GO and subsequently discharged to complete the remaining portion of the two-week treatment period at their home at the highest tolerated
NO concentration. For the second treatment phase, a 10-week maintenance phase, the administration is twice daily. The study is evaluating
safety, quality of life, physical function, and bacterial load among other parameters.
We
reported positive interim results in October 2021. At the time of data cutoff on September 6, 2021, eight subjects were successfully
titrated up to 250 ppm NO in the hospital setting, and none required dose reductions during the subsequent at-home portion of the study.
The mean age of subjects was 56.6 years (range: 22 – 73 years) with the majority female (87.5%), a distribution consistent with
real-world NTM disease, and occurring at a higher rate in older adult women than men. 250 ppm NO was well-tolerated in all subjects with
no study discontinuations or treatment-related serious adverse events observed. Methemoglobin and NO2 concentrations remained
within acceptable ranges in all subjects during NO treatment, and below the safety thresholds of 10% and 5 ppm, respectively. The study
continues to enroll patients, and we anticipate reporting the complete efficacy and safety results in the first half of calendar year
2022. If the trial is successful, we would anticipate commencing a pivotal study in the first half of calendar year 2024.
The
Company’s program in chronic obstructive pulmonary disease (“COPD”) is in the preclinical stage and we anticipate beginning
a pilot study in calendar year 2022 or 2023.
Ultra-High
Concentration NO in solid tumors through majority-owned affiliate Beyond Cancer, Ltd.
In
November 2021, we secured commitments of $23.9 million in a concurrent private placement of common shares, not to exceed $30 million,
providing the investors with up to 20% equity ownership in Beyond Cancer, a new and independently managed, private company. The funding
is expected to be used to accelerate ongoing preclinical work including the completion of IND-enabling studies, completion of a Phase
1 study, expansion of preclinical programs for combination studies, hiring of additional Beyond Cancer team members, and optimization
of the delivery system, as well as for general corporate purposes. The concurrent private placement is expected to close in the fourth
fiscal quarter.
Beyond
Cancer will benefit from Beyond Air’s NO expertise, IP portfolio, preclinical oncology team, and regulatory progress, and will
pay Beyond Air a single digit royalty on all future revenues. Beyond Cancer will be led by a seasoned leadership team with experience
in emerging healthcare companies and clinical oncology.
Ultra-high
concentration NO has shown anticancer properties in preclinical trials by eliciting an immune response from the host. We have released
this preclinical data at several medical/scientific conferences showing the promise of delivering NO at concentrations of 20,000 ppm
– 200,000 ppm directly to tumors. Results showed that local tumor ablation with NO conveyed anti-tumor immunity to the host. In
our most recent release of data, 8 of 11 mice treated with a single administration of 25,000 ppm NO over five minutes were resistant
to a subsequent tumor challenge and 11 of 11 mice treated with 50,000 ppm NO were resistant to a subsequent tumor challenge. Preclinical
work will continue with a goal of beginning the enrollment of patients in a first-in-human study in the first half of calendar year 2022.
For
more information about us, please refer to other documents that we have filed with the SEC and that are incorporated by reference into
this prospectus supplement, as listed under the heading “Incorporation of Documents by Reference.”
Litigation
Update
On
March 16, 2018, Empery Asset Master, Ltd. (“Empery Master”), Empery Tax Efficient, LP (“Empery I) and Empery Tax Efficient
II, LP (“Empery II), (collectively, “Empery”) filed a complaint in the Supreme Court of the State of New York (the
“Trial Court”), relating to the notice of adjustment of both the exercise price of and the number of warrant shares issuable
under warrants issued to Empery in January 2017. Empery alleges that, as a result of certain circumstances in connection with the February
2018 financing transaction, the 166,672 warrants issued to Empery in January 2017 provide for adjustments to both the exercise price
of the warrants and the number of warrant shares issuable upon such exercise. On August 20, 2020, the Trial Court denied the Company’s
summary judgment motion as to the first and third claim for relief, but dismissed the second claim for declaratory judgment as moot (the
“August 20 Decision”). The Appellate Division First Department denied the Company’s appeal of the August 20 Decision
on September 30, 2021. Following a three day bench trial, the Trial Court issued a decision on October 14, 2021, finding in favor of
Empery on the two remaining claims, granting reformation of the Warrant Agreement, and awarding Empery damages in the aggregate amount
of approximately $5.8 million (the “October 14 Decision”). On November 12, 2021, we filed a notice of appeal. Pending appeal,
we are required to use approximately $7.4 million of cash as collateral to secure a supersedeas bond for the full amount of damages and
interest in the case that we are unsuccessful in our appeal. On September 30, 2021, we recorded an estimate for a contingent loss of
$2.4 million related to the Empery litigation. We, in consultation with outside legal counsel, believe that we have several meritorious
defenses against the claims, and the decision of the Trial Court.
On
December 28, 2021, Hudson Bay Master Fund (“Hudson”) filed a complaint in the Trial Court relating to the notice of adjustment
of both the exercise price of and the number of warrant shares issuable under warrants issued to Hudson in January 2017. Hudson’s
complaint alleges breach of contract and that it is entitled to damages estimated at approximately $2.6 million. We, in consultation
with outside legal counsel, believe that we have several meritorious defenses against Hudson’s claims. We believe that Hudson’s
claims have no merit and we shall vigorously defend such lawsuit.
Corporate
Information
We
were incorporated on April 28, 2015 under Delaware law. On June 25, 2019, our name was changed to Beyond Air, Inc. from AIT Therapeutics,
Inc.
Our
principal executive offices are located at 900 Stewart Avenue, Suite 301, Garden City, New York 11530, and our telephone number is (516)
665-8200. Our website address is www.beyondair.net. The information contained on, or that can be accessed through, our website is not
part of this prospectus. We have included our website address in this prospectus supplement solely as an inactive textual reference.
THE
OFFERING
|
Common
Stock Offered By Us
|
|
Shares
of our common stock having an aggregate offering price of up to $50,000,000.
|
|
Manner
of Offering
|
|
“At
the market offering” that may be made from time to time through our agents, Truist and Oppenheimer. See “Plan of Distribution”
on page S-13 of this prospectus supplement.
|
|
Use
of Proceeds
|
|
We
currently intend to use the net proceeds from this offering primarily to further advance our pipeline of product candidates and for
working capital and general corporate purposes. See “Use of Proceeds” on page S-11 of this prospectus supplement.
|
|
Risk
Factors
|
|
Investing
in our common stock involves significant risks. See “Risk Factors” on page S-9 of this prospectus supplement, and under
similar headings in other documents incorporated by reference into this prospectus supplement and the accompanying prospectus.
|
|
Nasdaq
Capital Market Symbol
|
|
“XAIR”.
|
RISK
FACTORS
Investing
in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should carefully consider
the risk factors we describe in this prospectus supplement, any related free writing prospectus for a specific offering of securities,
as well as those incorporated by reference into this prospectus supplement or accompanying prospectus (including, without limitation,
in our Annual Report on Form 10-K for the year ended March 31, 2021). You should also carefully consider other information contained
and incorporated by reference in this prospectus supplement and the accompanying prospectus, including our financial statements and the
related notes thereto incorporated by reference herein. The risks and uncertainties described in this prospectus supplement and our other
filings with the SEC incorporated by reference herein are not the only ones we face. Additional risks and uncertainties not presently
known to us or that we currently consider immaterial may also adversely affect us. Our business, financial condition or results of operations
could be materially harmed by these risks. In such case, the value of our securities could decline and you may lose all or part of your
investment.
Risks
Relating to Our Business Operations
Recent
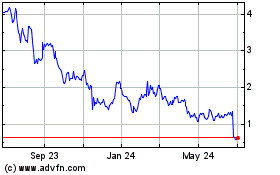
trading in our common stock has been volatile and may continue to be volatile in the future.
Our
common stock has recently experienced extreme volatility. In November 2021, our common stock closed as high as $15.61 per share and in
January 2022 as low as $6.06 per share. Our common stock may continue to be volatile and could materially fall for a number of reasons
including:
|
|
●
|
Announcements
by competitors that they have successfully produced an effective vaccine or other treatment option for COVID-19;
|
|
|
●
|
Public
announcement that the rapid spread of COVID-19 has receded;
|
|
|
●
|
Our
ability to obtain FDA approval of the PMA for our LungFit® system;
|
|
|
●
|
The
continued large declines in major stock market indexes which causes investors to sell our common stock;
|
|
|
●
|
The
termination of any other factors which may have created volatility and spike in volume; or
|
|
|
●
|
Other
possible reasons for volatility which we have disclosed in our reports filed with the SEC and incorporated by reference into this
prospectus supplement.
|
We
cannot assure you that our stock price and volume will remain at current levels in which case investors may sustain large losses.
We
face business disruption and related risks resulting from the COVID-19 pandemic, which could have a material adverse effect on our business
plan.
The
development of our product candidates could be further disrupted and materially adversely affected by a resurgence of the COVID-19 pandemic.
We experienced significant delays in the supply chain for LungFit® due to the redundancy in parts and suppliers with ventilator
manufacturing which has since been remedied. We continuously assess the impact that COVID-19 may have on our business plans and our ability
to conduct the preclinical studies and clinical trials as well as on our reliance on third-party manufacturing and our supply chain.
However, there can be no assurance that we will be able to avoid part or all of any impact from COVID-19 or its consequences if a resurgence
occurs.
The
ultimate resolution of two lawsuits against us, while not material to our operations, may be material to our financial statements.
On
September 30, 2021, we recorded an estimate for a contingent loss of $2.4 million related to the lawsuit filed against us in 2018 by
Empery relating to the notice of adjustment of the exercise price of and the number of warrant shares issuable under warrants issued
to Empery in January 2017. In December 2021 Hudson filed a lawsuit against us related to the notice of adjustment of the exercise price
of and the number of warrant shares issuable under warrants issued to Hudson in January 2017. The ultimate resolution of these two lawsuits
against us, and potential lawsuits that may be filed by other holders of warrants issued in January 2017 relating to the adjustment of
such warrants’ exercise price and number of underlying shares, if unfavorable, could result in losses in excess of our current
estimates which may be material to our financial statements.
Risks
Related to This Offering
Our
management will have broad discretion over the use of any net proceeds from this offering, you may not agree with how we use the proceeds,
and the proceeds may not be invested successfully.
Our
management will have broad discretion as to the use of any net proceeds from this offering and could use them for purposes other than
those contemplated at the time of this offering. Accordingly, you will be relying on the judgment of our management with regard to the
use of any proceeds from the sale of shares of common stock in this offering, and you will not have the opportunity, as part of your
investment decision, to assess whether the proceeds are being used appropriately. It is possible that the proceeds will be invested in
a way that does not yield a favorable, or any, return for you.
You
may experience dilution.
The
offering price per share in this offering may exceed the net tangible book value per share of our common stock outstanding prior to this
offering. Assuming that an aggregate of 6,906,077 shares of our common stock are sold at a price of $7.24 per share, the last reported
sale price of our common stock on the Nasdaq Capital Market on February 2, 2022, for aggregate gross proceeds of $50,000,000, and after
deducting commissions and estimated offering expenses payable by us, you would experience immediate dilution of $4.47 per share, representing
the difference between our as adjusted net tangible book value per share as of September 30, 2021, after giving effect to this offering,
and the assumed offering price. The exercise of outstanding stock options and warrants would result in further dilution of your investment.
See the section entitled “Dilution” below for a more detailed illustration of the dilution you would incur if you participate
in this offering. Because the sales of the shares offered hereby will be made directly into the market, the prices at which we sell these
shares will vary and these variations may be significant. Purchasers of the shares we sell, as well as our existing stockholders, will
experience significant dilution if we sell shares at prices significantly below the price at which they invested.
Investors
in this offering may experience future dilution as a result of future equity offerings.
In
order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into
or exchangeable for our common stock. Investors purchasing our shares or other securities in the future could have rights superior to
existing common stockholders, and the price per share at which we sell additional shares of our common stock or other securities convertible
into or exchangeable for our common stock in future transactions may be higher or lower than the price per share in this offering.
Sales
of a significant number of shares of our common stock in the public markets, or the perception that such sales could occur, could depress
the market price of our common stock.
Sales
of a substantial number of shares of our common stock in the public markets could depress the market price of our common stock and impair
our ability to raise capital through the sale of additional equity securities. We cannot predict the effect that future sales of our
common stock would have on the market price of our common stock.
We
do not intend to pay any cash dividends on our common stock in the foreseeable future and, therefore, any return on your investment in
our common stock must come from increases in the fair market value and trading price of our common stock.
We
do not intend to pay any cash dividends on our common stock in the foreseeable future and, therefore, any return on your investment in
our common stock must come from increases in the fair market value and trading price of our common stock.
USE
OF PROCEEDS
We
may issue and sell shares of our common stock having aggregate sales proceeds of up to $50,000,000 from time to time in this offering.
Because there is no minimum offering amount required as a condition to close this offering, the actual total public offering amount,
commissions and proceeds to us, if any, are not determinable at this time. There can be no assurance that we will sell any shares under
or fully utilize the sales agreement with the agents as a source of financing.
We
intend to use net proceeds from this offering for general corporate purposes, which may include working capital, capital expenditures,
research and development expenditures, clinical trial expenditures, commercial expenditures, acquisitions of new technologies, products
or businesses, and investments. The amounts and timing of these expenditures will depend on numerous factors, including the development
of our current business initiatives. Pending use of the net proceeds from this offering as described above, we may invest the net proceeds
in short-term interest-bearing investment grade instruments.
DILUTION
If
you purchase shares in this offering, you will experience dilution to the extent of the difference between the public offering price
of the shares and the net tangible book value per share of our common stock immediately after this offering.
Our
net tangible book value as of September 30, 2021 was approximately $40,565,000 or $1.61 per share of common stock. Net tangible book
value per share is determined by dividing our total tangible assets, less total liabilities, by the number of our shares of common stock
outstanding as of September 30, 2021. Dilution in net tangible book value per share represents the difference between the amount per
share paid by purchasers of shares in this offering and the net tangible book value per share of our common stock immediately after this
offering.
After
giving effect to the assumed sale of 7,429,421 shares of our common stock in this offering at an assumed offering price of $7.24 per
share, the last reported sale price of our common stock on the Nasdaq Capital Market on February 2, 2022 and after deducting commissions
and estimated offering expenses payable by us, our as adjusted net tangible book value as of September 30, 2021 would have been approximately
$89,065,000, or $2.77 per share. This represents an immediate increase in net tangible book value of $1.16 per share to existing stockholders
and immediate dilution of $4.47 per share to investors purchasing our common stock in this offering at the assumed offering price. The
following table illustrates this dilution on a per share basis:
|
Assumed
public offering price per share
|
|
$
|
7.24
|
|
|
Net
tangible book value per share as of September 30, 2021
|
|
$
|
1.61
|
|
|
Increase
per share attributable to this offering
|
|
$
|
1.16
|
|
|
As
adjusted net tangible book value per share as of September 30, 2021 after this offering
|
|
$
|
2.77
|
|
|
Dilution
per share to new investors participating in this offering
|
|
$
|
4.47
|
|
The
number of shares of our common stock that will be issued and outstanding immediately after this offering as shown above is based on 25,209,749
shares of common stock issued and outstanding as of September 30, 2021 and excludes, as of that date:
|
|
●
|
4,271,160
shares of our common stock issuable upon exercise of outstanding stock options;
|
|
|
●
|
3,109,627
shares of our common stock issuable upon exercise of outstanding warrants;
|
|
|
●
|
787,200
shares of our common stock underlying restricted stock units pursuant to our amended and restated 2013 Equity Incentive Plan (“2013
Plan”); and
|
|
|
●
|
520,511
shares of our common stock reserved for future issuance under our 2013 Plan and 2021 Employee Stock Purchase Plan (“2021 Plan”).
|
The
table above assumes for illustrative purposes that an aggregate of 7,429,421 shares of our common stock are sold during the term of the
sales agreement with the agents at a price of $7.24 per share, the last reported sale price of our common stock on the Nasdaq Capital
Market on February 2, 2022 for aggregate gross proceeds of $50,000,000. The shares sold in this offering, if any, will be sold from time
to time at various prices. An increase of $1.00 per share in the price at which the shares are sold from the assumed offering price of
$7.24 per share, assuming all of our common stock in the aggregate amount of $50,000,000 during the term of the sales agreement with
the agents is sold at that price, would decrease our adjusted net tangible book value per share after the offering to $1.24 share and
would increase the dilution in net tangible book value per share to new investors in this offering to $5.39 per share, after deducting
commissions and estimated offering expenses payable by us. A decrease of $1.00 per share in the price at which the shares are sold from
the assumed offering price of $7.24 per share, assuming all of our common stock in the aggregate amount of $50,000,000 is sold at that
price, would decrease our adjusted net tangible book value per share after the offering to $1.07per share and would decrease the dilution
in net tangible book value per share to new investors in this offering to $3.56 per share, after deducting commissions and estimated
offering expenses payable by us. This information is supplied for illustrative purposes only.
We
may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds
for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible
debt securities, the issuance of these securities may result in further dilution to our shareholders. To the extent that outstanding
options or warrants outstanding as of September 30, 2021 have been or may be exercised or other shares issued, investors purchasing our
common stock in this offering may experience further dilution.
PLAN
OF DISTRIBUTION
We
have entered into a sales agreement with Truist and Oppenheimer, under which we may issue and sell from time to time up to $50,000,000
of our common stock through Truist and Oppenheimer as our sales agents. Sales of our common stock, if any, will be made at market prices
by any method that is deemed to be an “at the market offering” as defined in Rule 415 under the Securities Act, including
sales made directly on the Nasdaq Capital Market or any other trading market for our common stock. If authorized by us in writing, the
agents may purchase shares of our common stock as principal.
The
agents will offer our common stock subject to the terms and conditions of the sales agreement on a daily basis or as otherwise agreed
upon by us and the agents. We will designate the maximum amount of common stock to be sold through the agents on a daily basis or otherwise
determine such maximum amount together with the agents. Subject to the terms and conditions of the sales agreement, the agents will use
their commercially reasonable efforts to sell on our behalf all of the shares of common stock requested to be sold by us. We may instruct
the agents not to sell common stock if the sales cannot be effected at or above the price designated by us in any such instruction. The
agents or we may suspend the offering of our common stock being made through the agents under the sales agreement upon proper notice
to the other parties. The agents and we each have the right, by giving written notice as specified in the sales agreement, to terminate
the sales agreement in each party’s sole discretion at any time.
The
aggregate compensation payable to Truist and Oppenheimer as sales agents equals up to 3.0% of the gross sales price of the shares sold
through them pursuant to the sales agreement. We have also agreed to reimburse the agents for up to $50,000 of the actual outside legal
expenses incurred by the agents. We estimate that the total expenses in connection with the transactions contemplated by the sales agreement
payable by us, excluding commissions payable to the agents under the sales agreement, will be approximately $125,000.
The
remaining sales proceeds, after deducting any expenses payable by us and any transaction fees imposed by any governmental, regulatory,
or self-regulatory organization in connection with the sales, will equal our net proceeds for the sale of such common stock.
The
agents will provide written confirmation to us following the close of trading on the Nasdaq Capital Market on each day in which common
stock is sold through them as sales agents under the sales agreement. Each confirmation will include the number of shares of common stock
sold through the agents as sales agent on that day, the volume weighted average price of the shares sold, the percentage of the daily
trading volume and the net proceeds to us.
Settlement
for sales of common stock will occur, unless the parties agree otherwise, on the second business day that is also a trading day following
the date on which any sales were made in return for payment of the net proceeds to us. There is no arrangement for funds to be received
in an escrow, trust or similar arrangement.
In
connection with the sales of our common stock on our behalf, each of the agents may be deemed to be an “underwriter” within
the meaning of the Securities Act, and the compensation paid to the agents may be deemed to be underwriting commissions or discounts.
We have agreed in the sales agreement to provide indemnification and contribution to the agents against certain liabilities, including
liabilities under the Securities Act. As sales agents, the agents will not engage in any transactions that stabilizes our common stock.
Our
common stock is listed on the Nasdaq Capital Market and trades under the symbol “XAIR”. The transfer agent of our common
stock is Action Stock Transfer Corporation.
The
agents and/or their affiliates have provided, and may in the future provide, various investment banking and other financial services
for us for which services they have received and, may in the future receive, customary fees.
LEGAL
MATTERS
Certain
legal matters with respect to the legality of the issuance of the securities offered by this prospectus supplement will be passed upon
for us by Sichenzia Ross Ference LLP, New York, New York. Goodwin Procter LLP, New York, New York will pass upon certain legal matters
in connection with the offering for Truist and Oppenheimer.
EXPERTS
The
consolidated financial statements as of March 31, 2021 and 2020 and for each of the years in the two year period ended March 31, 2021
incorporated by reference in this prospectus supplement from our Annual Report on Form 10-K for the year ended March 31, 2021 have been
so included in reliance on the report of Friedman LLP, an independent registered public accounting firm, given on the authority of said
firm as experts in accounting and auditing.
WHERE
YOU CAN FIND ADDITIONAL INFORMATION
We
file annual, quarter and periodic reports, proxy statements and other information with the SEC. You may access such reports and information
at the SEC’s website at www.sec.gov.
This
prospectus supplement and the accompanying prospectus are part of the registration statement on Form S-3 filed with the SEC under the
Securities Act for the common stock offered by this prospectus supplement. This prospectus supplement does not contain all of the information
set forth in the registration statement, certain parts of which have been omitted in accordance with the rules and regulations of the
SEC. For further information, reference is made to the registration statement and its exhibits. Whenever we make references in this prospectus
supplement or the accompanying prospectus to any of our contracts, agreements or other documents, the references are not necessarily
complete and you should refer to the exhibits attached to the registration statement for the copies of the actual contract, agreement
or other document.
INCORPORATION
OF DOCUMENTS BY REFERENCE
We
are “incorporating by reference” in this prospectus certain documents we file with the SEC, which means that we can disclose
important information to you by referring you to those documents. The information in the documents incorporated by reference is considered
to be part of this prospectus. Statements contained in documents that we file with the SEC and that are incorporated by reference in
this prospectus will automatically update and supersede information contained in this prospectus, including information in previously
filed documents or reports that have been incorporated by reference in this prospectus, to the extent the new information differs from
or is inconsistent with the old information. We incorporate by reference the following information or documents that we have filed with
the SEC (excluding those portions of any Form 8-K that are not deemed “filed” pursuant to the General Instructions of Form
8-K):
|
|
●
|
our
Annual Report on Form 10-K for the fiscal year ended March 31, 2021, filed with the SEC on June 10, 2021, as amended by our Annual
Report on Form 10-K/A filed with the SEC on July 23, 2021;
|
|
|
●
|
our
Proxy Statement filed with the SEC on January 21, 2022;
|
|
|
●
|
our
Quarterly Reports on Form 10-Q for the quarterly period ended June 30, 2021, filed with the SEC on August 10, 2021, and September
30, 2021, filed with the SEC on November 12, 2021;
|
|
|
●
|
our
Current Reports on Form 8-K, filed with the SEC on May 13, 2021, May 26, 2021, August 25, 2021, September 27, 2021, October 20, 2021,
November 5, 2021, November 15, 2021 (except Item 2.02 and the portions of Item 99.1 covered by Item 2.02) and December 10, 2021;
and
|
|
|
●
|
the
description of our common stock contained in our Registration Statement on Form 8-A, filed with the SEC on May 3, 2019, including
any amendments or reports filed for the purpose of updating such description.
|
Any
information in any of the foregoing documents will automatically be deemed to be modified or superseded to the extent that information
in this prospectus supplement or the accompanying prospectus or in a later filed document that is incorporated or deemed to be incorporated
herein by reference modifies or replaces such information.
We
also incorporate by reference any future filings (excluding information furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits
filed on such form that are related to such items) made with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act,
until we sell all of the securities offered by this prospectus supplement. Information in such future filings updates and supplements
the information provided in this prospectus supplement. Any statements in any such future filings will automatically be deemed to modify
and supersede any information in any document we previously filed with the SEC that is incorporated or deemed to be incorporated herein
by reference to the extent that statements in the later filed document modify or replace such earlier statements.
You
may request, orally or in writing, a copy of these documents, which will be provided to you at no cost (other than exhibits, unless such
exhibits are specifically incorporated by reference), by contacting Adam T. Newman, c/o Beyond Air, Inc., at 900 Stewart Avenue, Suite
301, Garden City, New York 11530. Our telephone number is (516) 665-8200. Information about us is also available at our website at http://www.beyondair.net.
The information in our website is not a part of this prospectus and is not incorporated by reference.
PROSPECTUS

$200,000,000
Common
Stock
Preferred
Stock
Warrants
Debt
Securities
Units
We
may offer to the public from time to time in one or more series or issuances:
|
|
●
|
shares
of our common stock;
|
|
|
|
|
|
|
●
|
shares
of our preferred stock;
|
|
|
|
|
|
|
●
|
warrants
to purchase shares of our common stock, preferred stock and/or debt securities;
|
|
|
|
|
|
|
●
|
debt
securities consisting of debentures, notes or other evidences of indebtedness;
|
|
|
|
|
|
|
●
|
units
consisting of a combination of the foregoing securities; or
|
|
|
|
|
|
|
●
|
any
combination of these securities.
|
The
aggregate initial offering price of all securities sold by us pursuant to this prospectus will not exceed $200,000,000.
This
prospectus provides a general description of the securities that we may offer. Each time that we offer securities under this prospectus,
we will provide the specific terms of the securities offered, including the public offering price, in a supplement to this prospectus.
Any prospectus supplement may add to, update or change information contained in this prospectus.
The
securities may be sold by us to or through underwriters or dealers, directly to purchasers or through agents designated from time to
time. For additional information on the methods of sale, you should refer to the section entitled “Plan of Distribution”
in this prospectus and the comparable section of any applicable prospectus supplement. If any underwriters are involved in the sale of
the securities with respect to which this prospectus is being delivered, the names of such underwriters and any applicable discounts
or commissions and over-allotment options will be set forth in the applicable prospectus supplement.
Our
common stock trades on the Nasdaq Capital Market under the ticker symbol “XAIR.” On January 21, 2022, the last reported
sale price per share of our common stock was $6.77. We have not yet determined whether the other securities that may be offered
by this prospectus will be listed on any exchange, interdealer quotation system or over-the-counter market. If we decide to seek the
listing of any such securities upon issuance, the prospectus supplement relating to those securities will disclose the exchange, quotation
system or market on which those securities will be listed.
INVESTING
IN OUR SECURITIES INVOLVES A HIGH DEGREE OF RISK. RISKS ASSOCIATED WITH AN INVESTMENT IN OUR SECURITIES WILL BE DESCRIBED IN THE APPLICABLE
PROSPECTUS SUPPLEMENT AND CERTAIN OF OUR FILINGS WITH THE SECURITIES AND EXCHANGE COMMISSION INCORPORATED BY REFERENCE INTO THIS PROSPECTUS,
AS DESCRIBED UNDER “RISK FACTORS” ON PAGE 4.
You
should read this prospectus and any applicable prospectus supplement together with additional information described under the heading
“Where You Can Find More Information” before you make your investment decision.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The
date of this prospectus is February 1, 2022.
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
This
prospectus is a part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or the SEC, using
a “shelf” registration process. Under this shelf registration process, we may offer to sell any of the securities, or any
combination of the securities, described in this prospectus, in each case in one or more offerings, up to a total dollar amount of $200,000,000.
This
prospectus provides you only with a general description of the securities that we may offer. Each time securities are sold under the
shelf registration statement, we will provide a prospectus supplement that will contain specific information about the terms of those
securities and the terms of that offering. The prospectus supplement may also add, update or change information contained in this prospectus.
If there is any inconsistency between the information in this prospectus and any prospectus supplement, you should rely on the information
in the prospectus supplement. You should read both this prospectus and any prospectus supplement, including all documents incorporated
by reference herein and therein, together with the additional information described under “Where You Can Find More Information”
below.
The
information contained in this prospectus is not complete and may be changed. You should rely only on the information provided in or incorporated
by reference in this prospectus or in any prospectus supplement, or documents to which we otherwise refer you. We have not authorized
anyone else to provide you with different information.
We
have not authorized any dealer, agent or other person to give any information or to make any representation other than those contained
or incorporated by reference in this prospectus and any accompanying prospectus supplement. You must not rely upon any information or
representation not contained or incorporated by reference in this prospectus or an accompanying prospectus supplement. This prospectus
and the accompanying prospectus supplement, if any, do not constitute an offer to sell or the solicitation of an offer to buy any securities
other than the registered securities to which they relate, nor do this prospectus and the accompanying prospectus supplement, if any,
constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful
to make such offer or solicitation in such jurisdiction. You should not assume that the information contained in this prospectus and
the accompanying prospectus supplement, if any, is accurate on any date subsequent to the date set forth on the front of such document
or that any information we have incorporated by reference is correct on any date subsequent to the date of the document incorporated
by reference, even though this prospectus and any accompanying prospectus supplement is delivered or securities are sold on a later date.
References
in this prospectus to the terms “the Company,” “Beyond Air,” “we,” “our” and “us”
or other similar terms mean Beyond Air, Inc. and our wholly owned subsidiaries, unless we state otherwise or the context indicates otherwise.
FORWARD-LOOKING
STATEMENTS
This
prospectus and the documents incorporated by reference herein contain, and any prospectus supplement and the documents incorporated therein,
contain forward-looking statements. We intend such forward-looking statements to be covered by the safe harbor provisions for forward-looking
statements contained in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. All statements
other than statements of historical facts contained in this prospectus, any prospectus supplement or the documents incorporated herein
and therein by reference, including statements regarding our future results of operations and financial position, business strategy,
prospective product candidates and products, product approvals, timing of our clinical development activities, research and development
costs, timing and likelihood of success and the plans and objectives of management for future operations and future results of anticipated
products are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors
that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements
express or implied by the forward-looking statements.
In
some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,”
“expect,” “plan,” “anticipate,” “expect,” “could,” “intend,”
“target,” “project,” “contemplate,” “believe,” “estimate,” “predict,”
“potential” or “continue” or the negative of these terms or other similar conditional expressions. The forward-looking
statements in this prospectus, any prospectus supplement or the documents incorporated herein and therein by reference, are only predictions.
We have based these forward-looking statements largely on our current expectations and projections about future events and financial
trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements speak
only as of the date of this prospectus and are subject to a number of important factors that could cause actual results to differ materially
from those in the forward-looking statements, including the factors described under Item 1A “Risk Factors” contained in our
most recently filed Annual Report on Form 10-K, as well as the following:
|
|
●
|
our
status as a development-stage company and our expectation to incur losses in the future;
|
|
|
|
|
|
|
●
|
our
future capital needs and our need to raise additional funds;
|
|
|
|
|
|
|
●
|
our
ability to obtain U.S. Food and Drug Administration (“FDA”) approval of the Premarket
Approval Application for the LungFit® system;
|
|
|
|
|
|
|
●
|
our
ability to build a pipeline of product candidates and develop and commercialize products;
|
|
|
|
|
|
|
●
|
our
ability to enroll patients in clinical trials, timely and successfully complete those trials
and receive necessary regulatory approvals;
|
|
|
|
|
|
|
●
|
our
ability to maintain our existing or future collaborations or licenses;
|
|
|
|
|
|
|
●
|
our
ability to protect and enforce our intellectual property rights;
|
|
|
|
|
|
|
●
|
federal,
state and foreign regulatory requirements, including the FDA regulation of our product candidates;
|
|
|
|
|
|
|
●
|
our
ability to obtain and retain key executives and attract and retain qualified personnel;
|
|
|
|
|
|
|
●
|
our
ability to successfully manage our growth; and
|
|
|
|
|
|
|
●
|
our
ability to address business disruption and related risks resulting from the COVID-19 pandemic,
which could have a material adverse effect on our business plan.
|
Moreover,
we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management
to predict all risk factors and uncertainties.
We
cannot guarantee that the results and other expectations expressed, anticipated or implied in any forward-looking statement will be realized.
The risks set forth under Item 1A of our Annual Report on Form 10-K for the fiscal year ended March 31, 2021, as revised or supplemented
by our Quarterly Reports on Form 10-Q and other documents we file with the SEC, describe major risks to our business, and you should
read and interpret any forward-looking statements together with these risks. A variety of factors, including these risks, could cause
our actual results and other expectations to differ materially from the anticipated results or other expectations expressed, anticipated
or implied in our forward-looking statements. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate,
actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements.
You should bear this in mind as you consider any forward-looking statements.
You
should read this prospectus, any prospectus supplement and the documents that we incorporate by reference herein and therein completely
and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking
statements by these cautionary statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking
statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
MARKET,
INDUSTRY AND OTHER DATA
This
prospectus and any applicable prospectus supplement and the documents incorporated by reference herein and therein contain estimates,
projections, market research and other information concerning our industry, our business, markets for LungFit® PH and
our other product candidates and the size of those markets, the prevalence of certain medical conditions, LungFit® PH
market access, prescription data and other physician, patient and payor data. Unless otherwise expressly stated, we obtain this information
from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical
and general publications, government data and similar sources as well as from our own internal estimates and research and from publications,
research, surveys and studies conducted by third parties on our behalf. Information that is based on estimates, projections, market research
or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events
and circumstances that are reflected in this information. As a result, you are cautioned not to give undue weight to such information.
SUMMARY
This
summary highlights selected information from this prospectus and does not contain all of the information that you need to consider in
making your investment decision. You should carefully read the entire prospectus, the applicable prospectus supplement and any related
free writing prospectus, including the risks of investing in our securities discussed under the heading “Risk Factors” contained
in the applicable prospectus supplement and any related free writing prospectus, and under similar headings in the other documents that
are incorporated by reference into this prospectus. You should also carefully read the information incorporated by reference into this
prospectus, including our financial statements, and the exhibits to the registration statement of which this prospectus is a part.
Company
Overview
We
are a clinical-stage medical device and biopharmaceutical company developing a nitric oxide (“NO”) generator and delivery
system (the “LungFit® system”) capable of generating NO from ambient air. The LungFit® platform
can generate NO up to 400 parts per million for delivery to a patient’s lungs directly or via a ventilator. LungFit® can
deliver NO either continuously or for a fixed amount of time at various flow rates and has the ability to either titrate dose on demand
or maintain a constant dose. We believe that LungFit® can be used to treat patients on ventilators that require NO, as
well as patients with chronic or acute severe lung infections via delivery through a breathing mask or similar apparatus. Furthermore,
we believe that there is a high unmet medical need for patients suffering from certain severe lung infections that the LungFit®
platform can potentially address. The Company’s current areas of focus with LungFit® are persistent pulmonary
hypertension of the newborn (PPHN), acute viral pneumonia (AVP) including COVID-19, bronchiolitis (BRO) and nontuberculous mycobacteria
(NTM) lung infection. The Company’s current product candidates will be subject to premarket reviews and approvals by the FDA, CE
marking conformity assessment by a notified body in the European Union, as well as similar regulatory agencies’ reviews or approvals
in other countries or regions. If approved, the Company’s system will be marketed as a medical device in the U.S.
For
more information about the Company, please refer to other documents that we have filed with the SEC and that are incorporated by reference
into this prospectus, as listed under the heading “Incorporation by Reference.”
Corporate
Information
We
were incorporated on April 28, 2015 under Delaware law. On June 25, 2019, our name was changed to Beyond Air, Inc. from AIT Therapeutics,
Inc.
Our
principal executive offices are located at 900 Stewart Avenue, Suite 301, Garden City, New York 11530, and our telephone number is (516)
665-8200. Our website address is www.beyondair.net. The information contained on, or that can be accessed through, our website is not
part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
Beyond
AirTM, the Beyond Air logo and other trademarks or service marks of Beyond Air, Inc. appearing in this prospectus are the
property of Beyond Air, Inc. This prospectus and any applicable prospectus supplement and the documents incorporated by reference herein
and therein may also include trademarks, tradenames and service marks that are the property of other organizations. Solely for convenience,
trademarks and tradenames referred to in this prospectus and any applicable prospectus supplement and the documents incorporated by reference
herein and therein appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that
we will not assert, to the fullest extent under applicable law, our rights, or that the applicable owner will not assert its rights,
to these trademarks and tradenames.
RISK
FACTORS
Investing
in our securities involves a high degree of risk. The prospectus supplement applicable to each offering of our securities will contain
a discussion of the risks applicable to an investment in our securities. Prior to making a decision about investing in our securities,
you should carefully consider the specific factors discussed under the heading “Risk Factors” in the applicable prospectus
supplement, together with all of the other information contained or incorporated by reference in the prospectus supplement or appearing
or incorporated by reference in this prospectus. You should also consider the risks, uncertainties and assumptions discussed under the
heading “Risk Factors” in our most recent Annual Report on Form 10-K and our Quarterly Reports on Form 10-Q and other documents
that we file with the SEC, which are incorporated herein by reference as described in this prospectus under the heading “Where
You Can Find More Information”. The risks and uncertainties we have described in such documents are not the only risks that we
face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our operations.
USE
OF PROCEEDS
Except
as otherwise provided in the applicable prospectus supplement relating to a specific offering, we intend to use the net proceeds from
the sale of securities by us under this prospectus for general corporate purposes, which may include working capital, capital expenditures,
research and development expenditures, clinical trial expenditures, commercial expenditures, acquisitions of new technologies, products
or businesses, and investments. Additional information on the use of net proceeds from the sale of securities by us under this prospectus
may be set forth in the prospectus supplement relating to the specific offering.
PLAN
OF DISTRIBUTION
We
may sell the securities, from time to time pursuant to public offerings, negotiated transactions, block trades, “At the Market
Offerings,” within the meaning of Rule 415(a)(4) of the Securities Act into an existing trading market, at prevailing market prices,
or a combination of these methods. We may sell the securities to or through underwriters or dealers, through agents or remarketing firms,
or directly to one or more purchasers. We may distribute securities from time to time in one or more transactions:
|
|
●
|
at
a fixed price or prices, which may be changed;
|
|
|
|
|
|
|
●
|
at
market prices prevailing at the time of sale;
|
|
|
|
|
|
|
●
|
at
prices related to such prevailing market prices; or
|
|
|
|
|
|
|
●
|
at
negotiated prices.
|
A
prospectus supplement or supplements (and any related free writing prospectus that we may authorize to be provided to you) will describe
the terms of the offering of the securities, including, to the extent applicable:
|
|
●
|
the
name or names of the underwriters, dealers or agents, if any;
|
|
|
|
|
|
|
●
|
if
the securities are to be offered through the selling efforts of brokers or dealers, the plan
of distribution and the terms of any agreement, arrangement, or understanding entered into
with broker(s) or dealer(s) prior to the effective date of the registration statement, and,
if known, the identity of any broker(s) or dealer(s) who will participate in the offering
and the amount to be offered through each;
|
|
|
|
|
|
|
●
|
the
purchase price of the securities or other consideration therefor, and the proceeds, if any,
we will receive from the sale;
|
|
|
|
|
|
|
●
|
if
any of the securities being registered are to be offered otherwise than for cash, the general
purposes of the distribution, the basis upon which the securities are to be offered, the
amount of compensation and other expenses of distribution, and by whom they are to be borne;
|
|
|
|
|
|
|
●
|
any
delayed delivery arrangements;
|
|
|
|
|
|
|
●
|
any
options under which underwriters may purchase additional securities from us;
|
|
|
|
|
|
|
●
|
any
agency fees or underwriting discounts and other items constituting agents’ or underwriters’
compensation;
|
|
|
|
|
|
|
●
|
any
public offering price;
|
|
|
|
|
|
|
●
|
any
discounts, commissions or concessions allowed or reallowed or paid to dealers;
|
|
|
|
|
|
|
●
|
the
identity and relationships of any finders, if applicable; and
|
|
|
|
|
|
|
●
|
any
securities exchange or market on which the securities may be listed.
|
Only
underwriters named in the prospectus supplement will be underwriters of the securities offered by the prospectus supplement.
If
underwriters are used in the sale, they will acquire the securities for their own account and may resell the securities from time to
time in one or more transactions at a fixed public offering price or at varying prices determined at the time of sale. The obligations
of the underwriters to purchase the securities will be subject to the conditions set forth in the applicable underwriting agreement.
We may offer the securities to the public through underwriting syndicates represented by managing underwriters or by underwriters without
a syndicate. Unless otherwise indicated in the prospectus supplement, subject to certain conditions, the underwriters will be obligated
to purchase all of the securities offered by the prospectus supplement, other than securities covered by any over-allotment or other
option. Any public offering price and any discounts or concessions allowed or reallowed or paid to dealers may change from time to time.
We may use underwriters, dealers or agents with whom we have a material relationship. We will describe in the prospectus supplement,
naming the underwriter, dealer or agent, the nature of any such relationship.
We
may use a remarketing firm to offer the securities in connection with a remarketing arrangement upon their purchase. Remarketing firms
will act as principals for their own account or as agents for us. These remarketing firms will offer or sell the securities pursuant
to the terms of the securities. A prospectus supplement will identify any remarketing firm and the terms of its agreement, if any, with
us and will describe the remarketing firm’s compensation. Remarketing firms may be deemed to be underwriters in connection the
securities they remarket.
If
we offer and sell securities through a dealer, we or an underwriter will sell the securities to the dealer, as principal. The dealer
may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale. The name of the
dealer and the terms of the transaction will be set forth in the applicable prospectus supplement.
We
may sell securities directly or through agents we designate from time to time. We will name any agent involved in the offering and sale
of securities and we will describe any commissions we will pay to the agent in the prospectus supplement. Unless the prospectus supplement
states otherwise, our agent will act on a best-efforts basis for the period of its appointment.
Dealers
and agents participating in the distribution of the securities may be deemed to be underwriters, and compensation received by them on
resale of the securities may be deemed to be underwriting discounts. If such dealers or agents were deemed to be underwriters, they may
be subject to statutory liabilities under the Securities Act.
We
may sell securities directly to one or more purchasers without using underwriters or agents. Underwriters, dealers and agents that participate
in the distribution of the securities may be underwriters as defined in the Securities Act, and any discounts or commissions they receive
from us and any profit on their resale of the securities may be treated as underwriting discounts and commissions under the Securities
Act.
We
may authorize agents or underwriters to solicit offers by certain types of institutional investors to purchase securities from us at
the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery
on a specified date in the future. We will describe the conditions to these contracts and the commissions we must pay for solicitation
of these contracts in the prospectus supplement.
We
may provide agents, underwriters and dealers with indemnification against civil liabilities, including liabilities under the Securities
Act, or contribution with respect to payments that the agents, underwriters or dealers may make with respect to these liabilities. Agents,
underwriters and dealers, or their respective affiliates, may engage in transactions with, or perform services for, us in the ordinary
course of business.
All
securities we may offer, other than common stock, will be new issues of securities with no established trading market. Any underwriter
may make a market in these securities, but will not be obligated to do so and may discontinue any market making at any time without notice.
We cannot guarantee the liquidity of the trading markets for any securities.
Any
underwriter may engage in over-allotment, stabilizing transactions, short-covering transactions and penalty bids in accordance with Regulation
M under the Exchange Act. Over-allotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions
permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum price. Syndicate-covering
or other short-covering transactions involve purchases of the securities, either through exercise of the over-allotment option or in
the open market after the distribution is completed, to cover short positions. Penalty bids permit the underwriters to reclaim a selling
concession from a dealer when the securities originally sold by the dealer are purchased in a stabilizing or covering transaction to
cover short positions. Those activities may cause the price of the securities to be higher than it would otherwise be. If commenced,
the underwriters may discontinue any of the activities at any time.
Any
underwriters that are qualified market makers on the Nasdaq Stock Market may engage in passive market making transactions in the common
stock on the Nasdaq Stock Market in accordance with Regulation M under the Securities Exchange Act of 1934, as amended, or the Exchange
Act, during the business day prior to the pricing of the offering, before the commencement of offers or sales of the common stock. Passive
market makers must comply with applicable volume and price limitations and must be identified as passive market makers. In general, a
passive market maker must display its bid at a price not in excess of the highest independent bid for such security; if all independent
bids are lowered below the passive market maker’s bid, however, the passive market maker’s bid must then be lowered when
certain purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which
might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
GENERAL
DESCRIPTION OF OUR SECURITIES
We
may offer and sell, at any time and from time to time:
|
|
●
|
shares
of our common stock;
|
|
|
|
|
|
|
●
|
shares
of our preferred stock;
|
|
|
|
|
|
|
●
|
warrants
to purchase shares of our common stock, preferred stock and/or debt securities;
|
|
|
|
|
|
|
●
|
debt
securities consisting of debentures, notes or other evidences of indebtedness;
|
|
|
|
|
|
|
●
|
units
consisting of a combination of the foregoing securities; or
|
|
|
|
|
|
|
●
|
any
combination of these securities.
|
The
terms of any securities we offer will be determined at the time of sale. We may issue debt securities that are exchangeable for and/or
convertible into common stock or any of the other securities that may be sold under this prospectus. When particular securities are offered
by us, a supplement to this prospectus will be filed with the SEC, which will describe the terms of the offering and sale of the offered
securities.
DESCRIPTION
OF OUR COMMON STOCK
The
following summary of the terms of our common stock is subject to and qualified in its entirety by reference to our certificate of incorporation
and bylaws, copies of which are on file with the SEC as exhibits to previous filings with the SEC. Please refer to “Where You Can
Find More Information” below for directions on obtaining these documents.
Our
certificate of incorporation authorizes us to issue up to 110,000,000 shares, 100,000,000 of which is designated as common stock with
a par value of $0.0001 per share. As of January 19, 2022, there were 29,798,950 shares of common stock outstanding, held by 106 stockholders
of record. This figure does not reflect the number of beneficial owners of shares of our common stock as a single stockholder of record
often holds shares in nominee name (also referred to as, in “street name”) on behalf of multiple beneficial owners.
Voting
Rights
Holders
of shares of our common stock are entitled to one vote for each share held of record on all matters to be voted on by stockholders, including
the election of directors. When a quorum is present at any meeting, a plurality of the votes properly cast for election to any office
shall elect to such office and a majority of the votes properly cast upon any question other than an election to an office shall decide
the question, except when a larger vote is required by law, by our certificate of incorporation or by our bylaws.
Our
certificate of incorporation and bylaws do not provide for cumulative voting rights. Because of this, the holders of a majority of the
shares of common stock entitled to vote in any election of directors can elect all of the directors standing for election, if they should
so choose.
Dividend
Rights
Subject
to the preferences that may be applicable to any then outstanding preferred stock, the holders of our outstanding shares of common stock
are entitled to receive dividends, if any, as may be declared from time to time by our board of directors out of legally available funds.
We have never paid a dividend and we do not anticipate paying a dividend in the foreseeable future.
Liquidation
Rights
In
the event of our liquidation, dissolution or winding up, holders of our common stock will be entitled to share ratably in the net assets
legally available for distribution to stockholders after the payment of all of our debts and other liabilities, subject to the satisfaction
of any liquidation preference granted to the holders of any outstanding shares of preferred stock.
Other
Rights and Preferences
The
terms of our common stock do not include any preemptive, conversion or subscription rights, nor any redemption or sinking fund provisions.
The common stock is not subject to future calls or assessments by us. The rights, preferences and privileges of the holders of our common
stock are subject to, and may be adversely affected by, the rights of shares of any series of our preferred stock that we may classify
and issue in the future.
Outstanding
Stock Options
As
of January 19, 2022, we had outstanding options to purchase 4,102,631 shares of our common stock at a weighted-average exercise
price of $5.15 per share, pursuant to our Third Amended and Restated 2013 Equity Incentive Plan (the “2013 Plan”).
As of January 19, 2022, there were 233,761 shares of our common stock reserved for future issuance under our 2013 Plan.
As
of January 19, 2022, we had outstanding options to purchase 75,000 shares of our common stock at a weighted-average exercise price of
$10.68 per share, which options were issued outside of our equity compensation plans as an inducement material to certain individuals
entering into employment with us in accordance with Nasdaq Listing Rule 5635(c)(4).
Outstanding
Stock Units
As
of January 19, 2022, we had 584,600 shares of our common stock underlying outstanding restricted stock units pursuant to our 2013 Plan.
2021
Employee Stock Purchase Plan
As
of January 19, 2022, there were 750,000 shares of our common stock reserved for future issuance under our 2021 Employee Stock Purchase
Plan.
Outstanding
Warrants
As
of January 19, 2022, we had outstanding:
|
|
●
|
warrants
held by investors in a March 2017 offering, to purchase up to an aggregate of 68,330 shares
of our common stock, at an exercise price of $3.66 per share.
|
|
|
●
|
warrants
held by the placement agent in a March 2017 offering, to purchase up to an aggregate of 7,541
shares of our common stock, at an exercise price of $3.66 per share.
|
|
|
●
|
warrants
held by NitricGen, Inc. in partial consideration for a licensing agreement to purchase up
to an aggregate of 80,000 shares of our common stock, at an exercise price of $6.90 per share.
|
|
|
●
|
warrants
held by a third party pursuant to a third-party license agreement, to purchase up to an aggregate
of 208,333 shares of our common stock, at an exercise price of $4.80 per share.
|
|
|
●
|
warrants
held by lenders in a March 2020 loan, to purchase up to an aggregate of 172,187 shares of
our common stock, at an exercise price of $7.26 per share.
|
The
warrants issued in the March 2017 offering have down round protection.
Description
of Certain Provisions of Delaware Law and our Certificate of Incorporation and Bylaws
Section
203 of the Delaware General Corporation Law
We
are subject to the provisions of Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a publicly held
Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year
period following the time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed
manner. Under Section 203, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies
one of the following conditions:
|
|
●
|
prior
to the date of the transaction, the board of directors of the corporation approved either
the business combination or the transaction which resulted in the stockholder becoming an
interested stockholder;
|
|
|
●
|
the
interested stockholder owned at least 85% of the voting stock of the corporation outstanding
upon consummation of the transaction, excluding for purposes of determining the number of
shares outstanding (1) shares owned by persons who are directors and also officers and (2)
shares owned by employee stock plans in which employee participants do not have the right
to determine confidentially whether shares held subject to the plan will be tendered in a
tender or exchange offer; or
|
|
|
|
|
|
|
●
|
on
or subsequent to the consummation of the transaction, the business combination is approved
by the board of directors and authorized at an annual or special meeting of stockholders,
and not by written consent, by the affirmative vote of at least 66-2/3% of the outstanding
voting stock which is not owned by the interested stockholder.
|
Section
203 defines a business combination to include:
|
|
●
|
any
merger or consolidation involving the corporation and the interested stockholder;
|
|
|
|
|
|
|
●
|
any
sale, transfer, lease, pledge or other disposition involving the interested stockholder of
10% or more of the assets of the corporation;
|
|
|
|
|
|
|
●
|
subject
to exceptions, any transaction that results in the issuance or transfer by the corporation
of any stock of the corporation to the interested stockholder;
|
|
|
|
|
|
|
●
|
subject
to exceptions, any transaction involving the corporation that has the effect of increasing
the proportionate share of the stock of any class or series of the corporation beneficially
owned by the interested stockholder; and
|
|
|
|
|
|
|
●
|
the
receipt by the interested stockholder of the benefit of any loans, advances, guarantees,
pledges or other financial benefits provided by or through the corporation.
|
In
general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting
stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Certificate
of Incorporation and Bylaws
Provisions
of our certificate of incorporation and bylaws may delay or discourage transactions involving an actual or potential change of control
or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares, or transactions
that our stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price
of our common stock. Among other things, our certificate of incorporation and our bylaws:
|
|
●
|
permit
our board of directors to issue up to 10,000,000 shares of preferred stock, with any rights,
preferences and privileges as it may designate, which issuance could result in the loss of
voting control by other stockholders;
|
|
|
|
|
|
|
●
|
subject
to the rights of the holders of any series of preferred stock, provide that all vacancies
on our board of directors, including as a result of newly created directorships, may, except
as otherwise required by law, be filled only by the affirmative vote of a majority of directors
then in office, even if less than a quorum;
|
|
|
|
|
|
|
●
|
provide
that stockholders seeking to present proposals before a meeting of stockholders or to nominate
candidates for election as directors at a meeting of stockholders must provide advance notice
in writing, and also specify requirements as to the form and content of a stockholder’s
notice;
|
|
|
|
|
|
|
●
|
do
not provide for cumulative voting rights, thereby allowing the holders of a majority of the
shares of common stock entitled to vote in any election of directors to elect all of the
directors standing for election;
|
|
|
|
|
|
|
●
|
provide
that special meetings of our stockholders may be called only by the (i) the chairperson of
the board; (ii) our chief executive officer; or (iii) a majority of the number of authorized
directors; and
|
|
|
|
|
|
|
●
|
provide
that the Court of Chancery of the State of Delaware is the sole and exclusive forum for:
(A) any derivative action or proceeding brought on behalf of us; (B) any action asserting
a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees
to us or our stockholders; (C) any action asserting a claim against us arising pursuant to
any provision of the Delaware General Corporation Law, our certificate of incorporation or
our bylaws; or (D) any action asserting a claim against us governed by the internal affairs
doctrine. Any person or entity purchasing or otherwise acquiring any interest in shares of
our capital stock shall be deemed to have notice of and to have consented to the foregoing
exclusive forum. Section 27 of the Exchange Act creates exclusive federal jurisdiction over
all suits brought to enforce any duty or liability created by the Exchange Act or the rules
and regulations thereunder. As a result, the exclusive forum provision will not apply to
suits brought to enforce any duty or liability created by the Exchange Act or any other claim
for which the federal courts have exclusive jurisdiction. In addition, Section 22 of the
Securities Act creates concurrent jurisdiction for federal and state courts over all suits
brought to enforce any duty or liability created by the Securities Act or the rules and regulations
thereunder. As a result, the exclusive forum provision will not apply to suits brought to
enforce any duty or liability created by the Securities Act or any other claim for which
the federal and state courts have concurrent jurisdiction.
|
The
Nasdaq Capital Market
Our
shares of common stock are listed for trading on the Nasdaq Capital Market under the symbol “XAIR.”
Transfer
Agent and Registrar
The
transfer agent and registrar for our common stock is Action Stock Transfer Corporation.
DESCRIPTION
OF OUR PREFERRED STOCK
We
currently have authorized 10,000,000 shares of preferred stock, par value $0.0001 per share, of which no shares have been designated.
Our
board of directors may, without further action by our stockholders, from time to time, direct the issuance of shares of preferred stock
in series and may, at the time of issuance, determine and fix the number of shares of such series and the designation of such series,
the voting powers, if any, of the shares of such series, the preferences and relative, participating, optional or other special rights,
if any, and the qualifications, limitations or restrictions thereof, including without limitation thereof, dividend rights, conversion
rights, redemption privileges and liquidation preferences, of the shares of such series. Satisfaction of any dividend preferences of
outstanding shares of our preferred stock would reduce the amount of funds available for the payment of dividends on shares of our common
stock. Holders of shares of our preferred stock may be entitled to receive a preference payment in the event of any liquidation, dissolution
or winding-up of our Company before any payment is made to the holders of shares of our common stock. In some circumstances, the issuance
of shares of preferred stock may render more difficult or tend to discourage a merger, tender offer or proxy contest, the assumption
of control by a holder of a large block of our securities or the removal of incumbent management. Upon the affirmative vote of our board
of directors, without stockholder approval, we may issue shares of preferred stock with voting and conversion rights which could adversely
affect the holders of shares of our common stock. It is not possible to state the actual effect of the issuance of any shares of preferred
stock on the rights of holders of common stock until the board of directors determines the specific rights attached to that preferred
stock. We have no current plan to issue any shares of preferred stock.
If
we offer a specific series of preferred stock under this prospectus, we will describe the terms of the preferred stock in the prospectus
supplement for such offering and will file a copy of the certificate establishing the terms of the preferred stock with the SEC. To the
extent required, this description will include:
|
|
●
|
the
title and stated value;
|
|
|
|
|
|
|
●
|
the
number of shares offered, the liquidation preference per share, and the purchase price;
|
|
|
|
|
|
|
●
|
the
dividend rate(s), period(s), and/or payment date(s), or method(s) of calculation for such
dividends;
|
|
|
|
|
|
|
●
|
whether
dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends
will accumulate;
|
|
|
|
|
|
|
●
|
the
procedures for any auction and remarketing, if any;
|
|
|
|
|
|
|
●
|
the
provisions for a sinking fund, if any;
|
|
|
|
|
|
|
●
|
the
provisions for redemption, if applicable;
|
|
|
|
|
|
|
●
|
any
listing of the preferred stock on any securities exchange or market;
|
|
|
|
|
|
|
●
|
whether
the preferred stock will be convertible into our common stock or our other securities and,
if applicable, the conversion price (or how it will be calculated), the conversion period
and any other terms of conversion (including any anti-dilution provisions, if any);
|
|
|
|
|
|
|
●
|
whether
the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange
price (or how it will be calculated), the exchange period and any other terms of exchange
(including any anti-dilution provisions, if any);
|
|
|
|
|
|
|
●
|
voting
rights, if any, of the preferred stock;
|
|
|
|
|
|
|
●
|
a
discussion of any material and/or special U.S. federal income tax considerations applicable
to the preferred stock;
|
|
|
|
|
|
|
●
|
the
relative ranking and preferences of the preferred stock as to dividend rights and rights
upon liquidation, dissolution, or winding up of our affairs;
|
|
|
|
|
|
|
●
|
any
material limitations on issuance of any class or series of preferred stock ranking senior
to or on a parity with the series of preferred stock as to dividend rights and rights upon
our liquidation, dissolution, or winding up; and
|
|
|
|
|
|
|
●
|
any
other affirmative, negative or other covenants or contractual rights which might be attendant
with the specific series of preferred stock.
|
The
preferred stock offering by this prospectus, when issued, will not have, or be subject to, any preemptive or similar rights.
Transfer
Agent and Registrar
The
transfer agent and registrar for any series of preferred stock will be set forth in each applicable prospectus supplement.
DESCRIPTION
OF OUR WARRANTS
We
may issue warrants to purchase shares of our common stock, preferred stock and/or debt securities in one or more series together with
other securities or separately, as described in each applicable prospectus supplement. Below is a description of certain general terms
and provisions of the warrants that we may offer. Particular terms of the warrants will be described in the applicable warrant agreements
and the applicable prospectus supplement for the warrants.
The
applicable prospectus supplement will contain, where applicable, the following terms of and other information relating to the warrants:
|
|
●
|
the
specific designation and aggregate number of, and the price at which we will issue, the warrants;
|
|
|
|
|
|
|
●
|
the
currency or currency units in which the offering price, if any, and the exercise price are
payable;
|
|
|
|
|
|
|
●
|
the
designation, amount and terms of the securities purchasable upon exercise of the warrants;
|
|
|
|
|
|
|
●
|
if
applicable, the exercise price for shares of our common stock and the number of shares of
common stock to be received upon exercise of the warrants;
|
|
|
|
|
|
|
●
|
if
applicable, the exercise price for shares of our preferred stock, the number of shares of
preferred stock to be received upon exercise of the warrants, and a description of that series
of our preferred stock;
|
|
|
|
|
|
|
●
|
if
applicable, the exercise price for our debt securities, the amount of our debt securities
to be received upon exercise of the warrants, and a description of that series of debt securities;
|
|
|
|
|
|
|
●
|
the
date on which the right to exercise the warrants will begin and the date on which that right
will expire or, if the warrants may not be continuously exercised throughout that period,
the specific date or dates on which the warrants may be exercised;
|
|
|
|
|
|
|
●
|
whether
the warrants will be issued in fully registered form or bearer form, in definitive or global
form or in any combination of these forms, although, in any case, the form of a warrant included
in a unit will correspond to the form of the unit and of any security included in that unit;
|
|
|
|
|
|
|
●
|
any
applicable material U.S. federal income tax or foreign tax consequences;
|
|
|
|
|
|
|
●
|
the
identity of the warrant agent for the warrants, if any, and of any other depositaries, execution
or paying agents, transfer agents, registrars or other agents;
|
|
|
|
|
|
|
●
|
the
proposed listing, if any, of the warrants or any securities purchasable upon exercise of
the warrants on any securities exchange or market;
|
|
|
|
|
|
|
●
|
if
applicable, the date from and after which the warrants and the common stock, preferred stock
and/or debt securities will be separately transferable;
|
|
|
|
|
|
|
●
|
if
applicable, the minimum or maximum amount of the warrants that may be exercised at any one
time;
|
|
|
|
|
|
|
●
|
information
with respect to book-entry procedures, if any
|
|
|
|
|
|
|
●
|
the
anti-dilution provisions of the warrants, if any;
|
|
|
|
|
|
|
●
|
any
redemption, put or call provisions;
|
|
|
|
|
|
|
●
|
whether
the warrants are to be sold separately or with other securities as parts of units; and
|
|
|
|
|
|
|
●
|
any
additional terms of the warrants, including terms, procedures and limitations relating to
the exchange and exercise of the warrants.
|
Transfer
Agent and Registrar
The
transfer agent and registrar for any warrants will be set forth in the applicable prospectus supplement.
Description
of Outstanding Warrants
As
of January 19, 2022, there were a total of 536,391 warrants to purchase shares of our common stock outstanding. See “Description
of Our Capital Stock - Description of Our Common Stock – Outstanding Warrants.”
DESCRIPTION
OF OUR DEBT SECURITIES
This
section describes the general terms and provisions of the debt securities that we may offer under this prospectus, any of which may be
issued as convertible or exchangeable debt securities. We will set forth the particular terms of the debt securities we offer in a prospectus
supplement. The extent, if any, to which the following general provisions apply to particular debt securities will be described in the
applicable prospectus supplement. The following description of general terms relating to the debt securities and the indenture under
which the debt securities will be issued are summaries only and therefore are not complete. You should read the indenture and the prospectus
supplement regarding any particular issuance of debt securities.
We
will issue the debt securities offered by this prospectus and any accompanying prospectus supplement under an indenture to be entered
into between us and the trustee identified in the applicable prospectus supplement. The terms of the debt securities will include those
stated in the indenture and those made part of the indenture by reference to the Trust Indenture Act of 1939, as in effect on the date
of the indenture. We have filed or will file a copy of the form of indenture as an exhibit to the registration statement in which this
prospectus is included. The indenture will be subject to and governed by the terms of the Trust Indenture Act of 1939.
We
may offer under this prospectus up to an aggregate principal amount of $200,000,000 in debt securities, or if debt securities are issued
at a discount, or in a foreign currency, foreign currency units or composite currency, the principal amount as may be sold for an aggregate
initial public offering price of up to $200,000,000. Unless otherwise specified in the applicable prospectus supplement, the debt securities
will represent direct, unsecured obligations of Beyond Air and will rank equally with all of our other unsecured indebtedness.
The
following statements relating to the debt securities and the indenture are summaries, qualified in their entirety by reference to the
detailed provisions of the indenture and the final form indenture as may be filed with a future prospectus supplement.
General
We
may issue the debt securities in one or more series with the same or various maturities, at par, at a premium, or at a discount. We will
describe the particular terms of each series of debt securities in a prospectus supplement relating to that series, which we will file
with the SEC.
The
prospectus supplement will set forth, to the extent required, the following terms of the debt securities in respect of which the prospectus
supplement is delivered:
|
|
●
|
the
title of the series;
|
|
|
|
|
|
|
●
|
the
aggregate principal amount;
|
|
|
|
|
|
|
●
|
the
issue price or prices, expressed as a percentage of the aggregate principal amount of the
debt securities;
|
|
|
|
|
|
|
●
|
any
limit on the aggregate principal amount;
|
|
|
|
|
|
|
●
|
the
date or dates on which principal is payable;
|
|
|
|
|
|
|
●
|
the
interest rate or rates (which may be fixed or variable) or, if applicable, the method used
to determine such rate or rates;
|
|
|
|
|
|
|
●
|
the
date or dates from which interest, if any, will be payable and any regular record date for
the interest payable;
|
|
|
|
|
|
|
●
|
the
place or places where principal and, if applicable, premium and interest, is payable;
|
|
|
|
|
|
|
●
|
the
terms and conditions upon which we may, or the holders may require us to, redeem or repurchase
the debt securities;
|
|
|
|
|
|
|
●
|
the
denominations in which such debt securities may be issuable, if other than denominations
of $1,000 or any integral multiple of that number;
|
|
|
|
|
|
|
●
|
whether
the debt securities are to be issuable in the form of certificated debt securities (as described
below) or global debt securities (as described below);
|
|
|
|
|
|
|
●
|
the
portion of principal amount that will be payable upon declaration of acceleration of the
maturity date if other than the principal amount of the debt securities;
|
|
|
|
|
|
|
●
|
the
currency of denomination;
|
|
|
|
|
|
|
●
|
the
designation of the currency, currencies or currency units in which payment of principal and,
if applicable, premium and interest, will be made;
|
|
|
|
|
|
|
●
|
if
payments of principal and, if applicable, premium or interest, on the debt securities are
to be made in one or more currencies or currency units other than the currency of denomination,
the manner in which the exchange rate with respect to such payments will be determined;
|
|
|
|
|
|
|
●
|
if
amounts of principal and, if applicable, premium and interest may be determined by reference
to an index based on a currency or currencies or by reference to a commodity, commodity index,
stock exchange index or financial index, then the manner in which such amounts will be determined;
|
|
|
|
|
|
|
●
|
the
provisions, if any, relating to any collateral provided for such debt securities;
|
|
|
|
|
|
|
●
|
any
addition to or change in the covenants and/or the acceleration provisions described in this
prospectus or in the indenture;
|
|
|
|
|
|
|
●
|
any
events of default, if not otherwise described below under “Events of Default”;
|
|
|
|
|
|
|
●
|
the
terms and conditions, if any, for conversion into or exchange for shares of our common stock
or preferred stock;
|
|
|
|
|
|
|
●
|
any
depositaries, interest rate calculation agents, exchange rate calculation agents or other
agents; and
|
|
|
|
|
|
|
●
|
the
terms and conditions, if any, upon which the debt securities shall be subordinated in right
of payment to other indebtedness of Beyond Air.
|
We
may issue discount debt securities that provide for an amount less than the stated principal amount to be due and payable upon acceleration
of the maturity of such debt securities in accordance with the terms of the indenture. We may also issue debt securities in bearer form,
with or without coupons. If we issue discount debt securities or debt securities in bearer form, we will describe material U.S. federal
income tax considerations and other material special considerations that apply to these debt securities in the applicable prospectus
supplement.
We
may issue debt securities denominated in or payable in a foreign currency or currencies or a foreign currency unit or units. If we do,
we will describe the restrictions, elections, and general tax considerations relating to the debt securities and the foreign currency
or currencies or foreign currency unit or units in the applicable prospectus supplement.
Exchange
and/or Conversion Rights
We
may issue debt securities which can be exchanged for or converted into shares of our common stock or preferred stock. If we do, we will
describe the terms of exchange or conversion in the prospectus supplement relating to these debt securities.
Transfer
and Exchange
We
may issue debt securities that will be represented by either:
|
|
●
|
“book-entry
securities,” which means that there will be one or more global securities registered
in the name of a depositary or a nominee of a depositary; or
|
|
|
|
|
|
|
●
|
“certificated
securities,” which means that they will be represented by a certificate issued in definitive
registered form.
|
We
will specify in the prospectus supplement applicable to a particular offering whether the debt securities offered will be book-entry
or certificated securities.
Certificated
Debt Securities
If
you hold certificated debt securities issued under an indenture, you may transfer or exchange such debt securities in accordance with
the terms of the indenture. You will not be charged a service charge for any transfer or exchange of certificated debt securities but
may be required to pay an amount sufficient to cover any tax or other governmental charge payable in connection with such transfer or
exchange.
Global
Securities
The
debt securities of a series may be issued in the form of one or more global securities that will be deposited with a depositary or its
nominees identified in the prospectus supplement relating to the debt securities. In such a case, one or more global securities will
be issued in a denomination or aggregate denominations equal to the portion of the aggregate principal amount of outstanding debt securities
of the series to be represented by such global security or securities.
Unless
and until it is exchanged in whole or in part for debt securities in definitive registered form, a global security may not be registered
for transfer or exchange except as a whole by the depositary for such global security to a nominee of the depositary and except in the
circumstances described in the prospectus supplement relating to the debt securities. The specific terms of the depositary arrangement
with respect to a series of debt securities will be described in the prospectus supplement relating to such series.
Protection
in the Event of Change of Control
Any
provision in an indenture that governs our debt securities covered by this prospectus that includes any covenant or other provision providing
for a put or increased interest or otherwise that would afford holders of our debt securities additional protection in the event of a
recapitalization transaction, a change of control of the Company, or a highly leveraged transaction will be described in the applicable
prospectus supplement.
Covenants
Unless
otherwise indicated in this prospectus or the applicable prospectus supplement, our debt securities may not have the benefit of any covenant
that limits or restricts our business or operations, the pledging of our assets or the incurrence by us of indebtedness. We will describe
in the applicable prospectus supplement any material covenants in respect of a series of debt securities.
Consolidation,
Merger and Sale of Assets
We
may agree in any indenture that governs the debt securities of any series covered by this prospectus that we will not consolidate with
or merge into any other person or convey, transfer, sell or lease our properties and assets substantially as an entirety to any person,
unless such person and such proposed transaction meets various criteria, which we will describe in detail in the applicable prospectus
supplement.
Defaults
and Notice
The
debt securities of any series will contain events of default to be specified in the applicable prospectus supplement, which may include,
without limitation:
|
|
●
|
failure
to pay the principal of, or premium or make-whole amount, if any, on any debt security of
such series when due and payable (whether at maturity, by call for redemption, through any
mandatory sinking fund, by redemption at the option of the holder, by declaration or acceleration
or otherwise);
|
|
|
|
|
|
|
●
|
failure
to make a payment of any interest on any debt security of such series when due;
|
|
|
|
|
|
|
●
|
failure
to perform or observe any other covenants or agreements in the indenture with respect to
the debt securities of such series;
|
|
|
|
|
|
|
●
|
certain
events relating to our bankruptcy, insolvency or reorganization; and
|
|
|
|
|
|
|
●
|
certain
cross defaults, if and as applicable.
|
If
an event of default with respect to debt securities of any series shall occur and be continuing, we may agree that the trustee or the
holders of at least 25% in aggregate principal amount of the then outstanding debt securities of such series may declare the principal
amount (or, if the debt securities of such series are issued at an original issue discount, such portion of the principal amount as may
be specified in the terms of the debt securities of such series) of all debt securities of such series or such other amount or amounts
as the debt securities or supplemental indenture with respect to such series may provide, to be due and payable immediately. Any provisions
pertaining to events of default and any remedies associated therewith will be described in the applicable prospectus supplement.
Any
indenture that governs our debt securities covered by this prospectus may require that the trustee under such indenture shall, within
90 days after the occurrence of a default, give to holders of debt securities of any series notice of all uncured defaults with respect
to such series known to it. However, in the case of a default that results from the failure to make any payment of the principal of,
premium or make-whole amount, if any, or interest on the debt securities of any series, or in the payment of any mandatory sinking fund
installment with respect to debt securities of such series, if any, the trustee may withhold such notice if it in good faith determines
that the withholding of such notice is in the interest of the holders of debt securities of such series. Any terms and provisions relating
to the foregoing types of provisions will be described in further detail in the applicable prospectus supplement.
Any
indenture that governs our debt securities covered by this prospectus will contain a provision entitling the trustee to be indemnified
by holders of debt securities before proceeding to exercise any trust or power under the indenture at the request of such holders. Any
such indenture may provide that the holders of at least a majority in aggregate principal amount of the then outstanding debt securities
of any series may direct the time, method and place of conducting any proceedings for any remedy available to the trustee, or of exercising
any trust or power conferred upon the trustee with respect to the debt securities of such series. However, the trustee under any such
indenture may decline to follow any such direction if, among other reasons, the trustee determines in good faith that the actions or
proceedings as directed may not lawfully be taken, would involve the trustee in personal liability or would be unduly prejudicial to
the holders of the debt securities of such series not joining in such direction.
Any
indenture that governs our debt securities covered by this prospectus may endow the holders of such debt securities to institute a proceeding
with respect to such indenture, subject to certain conditions, which will be specified in the applicable prospectus supplement and which
may include, that the holders of at least a majority in aggregate principal amount of the debt securities of such series then outstanding
make a written request upon the trustee to exercise its power under the indenture, indemnify the trustee and afford the trustee reasonable
opportunity to act. Even so, such holders may have an absolute right to receipt of the principal of, premium or make-whole amount, if
any, and interest when due, to require conversion or exchange of debt securities if such indenture provides for convertibility or exchangeability
at the option of the holder and to institute suit for the enforcement of such rights. Any terms and provisions relating to the foregoing
types of provisions will be described in further detail in the applicable prospectus supplement.
Modification
of the Indenture
We
and the trustee may modify any indenture that governs our debt securities of any series covered by this prospectus with or without the
consent of the holders of such debt securities, under certain circumstances to be described in a prospectus supplement.
Defeasance;
Satisfaction and Discharge
The
prospectus supplement will outline the conditions under which we may elect to have certain of our obligations under the indenture discharged
and under which the indenture obligations will be deemed to be satisfied.
Regarding
the Trustee
We
will identify the trustee and any relationship that we may have with such trustee, with respect to any series of debt securities, in
the prospectus supplement relating to the applicable debt securities. You should note that if the trustee becomes a creditor of Beyond
Air, the indenture and the Trust Indenture Act of 1939 limit the rights of the trustee to obtain payment of claims in certain cases,
or to realize on certain property received in respect of any such claim, as security or otherwise. The trustee and its affiliates may
engage in, and will be permitted to continue to engage in, other transactions with us and our affiliates. If, however, the trustee acquires
any “conflicting interest” within the meaning of the Trust Indenture Act of 1939, it must eliminate such conflict or resign.
Governing
Law
The
law governing the indenture and the debt securities will be identified in the prospectus supplement relating to the applicable indenture
and debt securities.
DESCRIPTION
OF OUR UNITS
The
following description, together with the additional information we include in any applicable prospectus supplement, summarizes the material
terms and provisions of the units that we may offer under this prospectus. Units may be offered independently or together with common
stock, preferred stock, debt securities and/or warrants offered by any prospectus supplement, and may be attached to or separate from
those securities. While the terms we have summarized below will generally apply to any future units that we may offer under this prospectus,
we will describe the particular terms of any series of units that we may offer in more detail in the applicable prospectus supplement.
The terms of any units offered under a prospectus supplement may differ from the terms described below.
We
will incorporate by reference into the registration statement of which this prospectus forms a part the form of unit agreement, including
a form of unit certificate, if any, that describes the terms of the series of units we are offering before the issuance of the related
series of units. The following summaries of material provisions of the units, and the unit agreements, are subject to, and qualified
in their entirety by reference to, all the provisions of the unit agreement applicable to a particular series of units. We urge you to
read the applicable prospectus supplements related to the units that we sell under this prospectus, as well as the complete unit agreements
that contain the terms of the units.
General
We
may issue units comprised of one or more shares of our common stock or preferred stock, debt securities and warrants in any combination.
Each unit will be issued so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of
a unit will have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may
provide that the securities included in the unit may not be held or transferred separately, at any time or at any time before a specified
date.
We
will describe in the applicable prospectus supplement the terms of the series of units, including:
|
|
●
|
the
designation and terms of the units and of the securities comprising the units, including
whether, and under what circumstances, those securities may be held or transferred separately;
|
|
|
|
|
|
|
●
|
the
rights and obligations of the unit agent, if any;
|
|
|
|
|
|
|
●
|
any
provisions of the governing unit agreement that differ from those described below; and
|
|
|
|
|
|
|
●
|
any
provisions for the issuance, payment, settlement, transfer or exchange of the units or of
the securities comprising the units.
|
The
provisions described in this section, as well as those described under “Description of Our Common Stock,” “Description
of our Preferred Stock,” “Description of Our Debt Securities” and “Description of Our Warrants,” will apply
to each unit and to any common stock, preferred stock, debt securities or warrants included in each unit, respectively.
Issuance
in Series
We
may issue units in such amounts and in numerous distinct series as we determine.
WHERE
YOU CAN FIND MORE INFORMATION
We
file annual, quarterly and current reports, proxy statements and other information with the SEC. The SEC maintains an Internet website
at www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers that file electronically
with the SEC. Our reports on Forms 10-K, 10-Q and 8-K, and amendments to those reports, are also available for download, free of charge,
as soon as reasonably practicable after these reports are filed with, or furnished to, the SEC, at our website at www.beyondair.net.
Information contained on or accessible through our website is not a part of this prospectus or any prospectus supplement, and the inclusion
of our website address in this prospectus is an inactive textual reference only.
INCORPORATION
BY REFERENCE
The
SEC allows us to “incorporate by reference” into this prospectus the information in other documents that we file with it.
This means that we can disclose important information to you by referring you to those documents. The information incorporated by reference
is considered to be a part of this prospectus, and information in documents that we file later with the SEC will automatically update
and supersede information contained in documents filed earlier with the SEC or contained in this prospectus. We incorporate by reference
in this prospectus (i) the documents listed below, (ii) all documents that we file with the SEC under Section 13(a), 13(c), 14 or 15(d)
of the Exchange Act after the date of the initial filing of the registration statement of which this prospectus is included and prior
to the effectiveness of such registration statement, and (iii) and any future filings that we may make with the SEC under Sections 13(a),
13(c), 14, or 15(d) of the Exchange Act prior to the termination of the offering under this prospectus; provided, however, that we are
not incorporating, in each case, any documents or information deemed to have been furnished and not filed, including any information
that we disclose under Items 2.02 or 7.01 of any Current Report on Form 8-K, in accordance with SEC rules:
|
|
●
|
our
Annual Report on Form 10-K for the fiscal year ended March 31, 2021, filed with the SEC on
June 10, 2021, as amended by our Annual Report on Form 10-K/A filed with the SEC on July
23, 2021;
|
|
|
|
|
|
|
●
|
our
Quarterly Reports on Form 10-Q for the quarterly period ended June 30, 2021, filed with the
SEC on August 10, 2021, and September 30, 2021, filed with the SEC on November 12, 2021;
|
|
|
|
|
|
|
●
|
our
Current Reports on Form 8-K, filed with the SEC on May 13, 2021, May 26, 2021, August 25, 2021, September 27, 2021, October 20, 2021, November 5, 2021, November 15, 2021 (except Item
2.02 and the portions of Item 99.1 covered by Item 2.02) and December 10, 2021; and
|
|
|
|
|
|
|
●
|
the
description of our common stock contained in our Registration Statement on Form 8-A, filed
with the SEC on May 3, 2019, including any amendments or reports filed for the purpose of
updating such description.
|
You
may request, orally or in writing, a copy of any or all of the documents incorporated herein by reference. These documents will be provided
to you at no cost, by contacting: Adam T. Newman, c/o Beyond Air, Inc., at 900 Stewart Avenue, Suite 301, Garden City, New York 11530.
In addition, copies of any or all of the documents incorporated herein by reference may be accessed at our website at www.beyondair.net.
The information on such website is not incorporated by reference and is not a part of this prospectus.
LEGAL
MATTERS
The
validity of the issuance of the securities offered hereby will be passed upon for us by Hogan Lovells US LLP. As appropriate, legal counsel
representing the underwriters, dealers or agents will be named in the accompanying prospectus supplement and may opine to certain legal
matters.
EXPERTS
The
consolidated financial statements as of March 31, 2021 and 2020 and for each of the years in the two year period ended March 31,
2021 incorporated by reference in this prospectus from our Annual Report on Form 10-K for the year ended March 31, 2021 have been so
included in reliance on the report of Friedman LLP, an independent registered public accounting firm, given on the authority of said
firm as experts in accounting and auditing.

$200,000,000
Common
Stock
Preferred
Stock
Warrants
Debt
Securities
Units
PROSPECTUS
,
2022
Beyond Air (NASDAQ:XAIR)
Historical Stock Chart
From Mar 2024 to Apr 2024
Beyond Air (NASDAQ:XAIR)
Historical Stock Chart
From Apr 2023 to Apr 2024