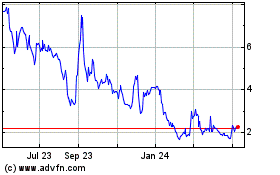
Our common stock and Series Z Warrants are
listed for trading on the NASDAQ Capital Market under the symbols “PAVM” and “PAVMZ,” respectively. On
October 16, 2018, the last reported sale prices of our common stock and Series Z Warrants were $1.14 and $0.39,
respectively.
ABOUT
THIS PROSPECTUS
This
prospectus is part of a registration statement on Form S-3 (the “Registration Statement”) that we have filed with
the Securities and Exchange Commission (the “SEC”). It is important for you to read and consider all of the information
contained in or incorporated by reference into this prospectus and any applicable prospectus supplement before making any decision
whether to invest in our common stock. This prospectus incorporates by reference important business and financial information
about us that is not included in or delivered with this document, as described in “
Where You Can Find More Information
”
beginning on page 24 in this prospectus. You should also read and consider the additional information contained in the documents
that we have incorporated into this prospectus by reference.
You
should rely only on the information contained in or incorporated by reference into this prospectus or any applicable prospectus
supplement. We have not authorized anyone to give or provide any information different from the information that is contained
in or incorporated by reference into this prospectus or any accompanying prospectus supplement and, if given, such information
must not be relied upon as having been made or authorized by us. The information contained in this prospectus is accurate only
as of the date on the front of this prospectus and information appearing in any applicable prospectus supplement is accurate only
as of the date of the applicable prospectus supplement. Additionally, any information we have incorporated by reference in this
prospectus or any applicable prospectus supplement is accurate only as of the date of the document incorporated by reference,
regardless of the time of delivery of this prospectus, any applicable prospectus supplement or any sale of our common stock. Our
business, financial condition, results of operations and prospects may have changed since that date.
This
prospectus or any accompanying prospectus supplement does not constitute an offer or solicitation by anyone in any state in which
such offer or solicitation is not authorized or in which the person making such offer or solicitation is not qualified to do so
or to anyone to whom it is unlawful to make such offer or solicitation.
Unless
otherwise indicated or unless the context otherwise requires, all references in this prospectus to the “
Company
”
and to “
we
,” “
us
,” and “
our
” mean PAVmed Inc. and its subsidiaries and
“
Lucid Diagnostics
” means Lucid Diagnostics Inc., a majority-owned subsidiary of ours.
TRADEMARKS
We
have proprietary rights to trademarks used in this prospectus, including PAVmed™, Lucid Diagnostics™, Caldus™,
CarpX™, DisappEAR™, EsoCheck™, NextCath™, NextFlo™, PortIO™ and “Innovating at the Speed
of Life”™. Solely for our convenience, trademarks and trade names referred to in this prospectus may appear without
the “®” or “™” symbols, but such references are not intended to indicate, in any way, that we
will not assert, to the fullest extent possible under applicable law, our rights or the rights to these trademarks and trade names.
We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply a relationship
with, or endorsement or sponsorship of us by, any other companies. Each trademark, trade name, or service mark of any other company
appearing in this prospectus is the property of its respective holder.
PROSPECTUS
SUMMARY
Company
Summary
We
are a highly-differentiated multi-product medical device company organized to advance a broad pipeline of innovative medical technologies
we believe address unmet clinical needs and possess attractive market opportunities to commercialization. Our goal is to enhance
and accelerate value creation by employing a business model focused on capital efficiency and speed to market. Since our inception
on June 26, 2014, our activities have focused on advancing the lead products in our pipeline towards regulatory approval and commercialization,
while protecting our intellectual property, and strengthening our corporate infrastructure and management team. As resources permit,
we will continue to explore internal and external innovations that fulfill our project selection criteria without limiting ourselves
to any target specialty or condition.
The
following is a brief overview of the products currently in our pipeline, including our lead products of CarpX™, PortIO™,
and DisappEAR™ together with our recent addition, EsoCheck™. These products are all in various phases of development
and have not yet received regulatory approval. Among other things:
|
●
|
We
have filed final nonprovisional patent applications for PortIO™ and CarpX™ and entered into a licensing agreement
with a group of academic centers securing the worldwide rights in perpetuity to a family of patents and patent applications
underlying our DisappEAR™ product.
|
|
|
|
|
●
|
On
May 12, 2018, Lucid Diagnostics, Inc., a majority-owned subsidiary of the Company, entered into a worldwide license agreement
with Case Western Reserve University for the intellectual property rights to the “EsoCheck™ Technology”,
for the detection of Barrett’s Esophagus, the primary precursor to esophageal cancer, as further discussed herein below.
|
|
|
|
|
●
|
We
have advanced, in partnership with our design and contract manufacturing partners, our CarpX™ product from concept to
working prototypes, completed successful benchtop and cadaver testing confirming the device consistently cuts the transverse
carpal ligament, as well as commercial design and development, and performed pre-submission verification and validation testing.
On November 27, 2017 we filed a 510(k) premarket notification submission with the Federal Food and Drug Administration (“
FDA
”)
for CarpX™ using a commercially available carpel tunnel release device as a predicate. On August 22, 2018, we were notified
by the lead FDA branch reviewing the submission that it had not reached a consensus with the consulting FDA branch within
the review period allotted under the FDA’s rules and regulations. Accordingly, the lead branch recommended to us that
we take the appropriate steps to extend the review process through a resubmission, which we subsequently completed. We have
engaged FDA counsel to assist with the resubmission process and any appeals. In addition, we are preparing to submit for CE
Mark clearance in Europe and have been approved for a first-in-man clinical series outside of the United States. We recently,
hired a Chief Commercial Officer to further develop and implement our commercialization strategy in the United States and
commercialization partnerships worldwide.
|
|
|
|
|
●
|
As
noted above, in May 2018, our majority-owned subsidiary, Lucid Diagnostics Inc. entered into a licensed agreement with Case
Western Reserve University (“
CWRU
”) for the worldwide rights to the EsoCheck™ technology (the “
EsoCheck™
License Agreement
”). The EsoCheck™ technology is comprised of a cell sample collection device (the “
EsoCheck™
Cell Sample Collection Device
”) and highly accurate proprietary DNA biomarkers (the “
EsoCheck™ DNA
Biomarkers
”) that are used to detect “Barrett’s Esophagus,” the primary precursor to esophageal
cancer. The incidence of esophageal adenocarcinoma (“
EAC
”), the most common cancer of the esophagus, the
pipe through which food passes to the stomach, has quadrupled over the past 30 years. Its prognosis, however, remains dismal,
with less than 20% of patients surviving five years.
|
|
|
In
a five-minute office-based test, the patient swallows the EsoCheck™ Cell Sample Collection Device, a vitamin-sized silicone-covered
capsule containing a small inflatable balloon attached to a thin catheter, which swabs the target area for cell collection
as the catheter is withdrawn. The collected cell sample is then tested against a panel of the proprietary EsoCheck™
DNA Biomarkers recently shown to be highly accurate in detecting Barrett’s Esophagus.
|
|
|
|
|
|
The
primary cause of EAC cancer is Gastroesophageal Reflux Disease (“
GERD
”), commonly known as chronic heartburn
or acid reflux. GERD, where stomach acid refluxes into the esophagus, affects 20-40% of Western adult populations, according
to published epidemiological data. The repeated exposure of the esophagus to acid can lead to pre-cancerous changes in its
lining, called Barrett’s Esophagus. Nearly all patients diagnosed with EAC cancer have evidence of previously undetected
Barrett’s Esophagus. If detected before EAC cancer develops, Barrett’s Esophagus can be successfully treated,
usually with non-surgical approaches. Heartburn symptoms, commonly seen in patients with acid reflux with or without Barrett’s,
can easily be treated by over-the counter medications, while endoscopy, the standard diagnostic test, is expensive, invasive
and requires sedation. As a result, wide screening for Barrett’s is not practical or cost-effective. We are pursuing
the development of the EsoCheck™ technology to provide the estimated 50 million at-risk patients a non-invasive, less
costly test to detect Barrett’s to treat it before it turns deadly.
|
|
|
|
|
|
The
proprietary EsoCheck™ DNA Biomarkers was developed by the laboratory of the EsoCheck™ technology co-inventor Sanford
D. Markowitz, MD, PhD, the Ingalls Professor of Cancer Genetics medical oncologist at University Hospitals Seidman Cancer
Center, NCI Outstanding Investigator Awardee, and head of the NIH-Case GI Cancers Program of Research Excellence and GI cancer
genetics program at the Case Comprehensive Cancer Center. In an article published in the periodical Science Translational
Medicine clinical data showed DNA methylation of the VIM and CCNA1 genes is diagnostic of Barrett’s Esophagus and the
EsoCheck™ technology, which combines the proprietary EsoCheck™ Cell Sample Collection Device with these biomarkers,
was over 90% accurate at identifying patients without Barrett’s Esophagus. Another of the EsoCheck™ technology’s
three physician co-inventors, Dr. Joseph E. Willis, MD, professor of pathology and pathology vice-chair for clinical affairs,
at University Hospitals Cleveland Medical Center, is leading an ongoing NIH-supported effort to create a CLIA-certified VIM/CCNA1
DNA methylation test suitable for commercialization.
|
|
|
|
|
|
Our
initial goal is a U.S. launch of the first commercial EsoCheck™ technology-based product early next year. We have begun
building EsoCheck™ Cell Sample Collection Devices for verification and validation testing with FDA 510(k) submission
targeted for the end of this year. We are also working closely with the reference laboratory performing the EsoCheck™
DNA Biomarker test to complete the CLIA certification and clinical validation testing steps required to achieve a designation
of the EsoCheck™ technology as a commercial Laboratory Developed Test, a process which is on target for early next year.
The ongoing multicenter National Institutes of Health-funded clinical study, which seeks to establish the definitive clinical
evidence for widespread EsoCheck™ technology screening of Barrett’s Esophagus, has expanded to eight enrolling
centers. Through our majority-owned subsidiary Lucid Diagnostics, we are working closely with the investigators to provide
all necessary support to accelerate enrollment and ensure the data is of the highest quality for future regulatory submission.
|
|
|
|
|
●
|
We
have advanced, in partnership with our design and contract manufacturing partners, our PortIO™ product from concept
to working prototypes, benchtop, animal, and cadaver testing, commercial design and development, verification and validation
testing, and an initial submission to the FDA for 510(k) market clearance for use in patients requiring 24-hour emergency
type vascular access. After further discussion with the FDA, we have decided to pursue a broader clearance for use in patients
with a need for vascular access up to seven days under section 513(f)2 of the Federal Food, Drug and Cosmetic Act, also referred
to as de novo classification. We have filed a de novo pre-submission package with the FDA, which was followed by an in-person
meeting on January 9, 2018 to discuss the risk assessment and proposed mitigation for the de novo application. Based on FDA
recommendations, we will initiate a seven-day animal study, having successfully completed a pilot animal study which showed
excellent function of the device over the seven-day implant period and on explant. In anticipation of having to follow-up
the animal study with a human clinical safety trial, we have accelerated our strategic partnership efforts to include the
pre-clearance phase.
|
|
●
|
We
have advanced, in partnership with our design and contract manufacturing partners and our academic partners at Tufts University
and Harvard Medical School, our DisappEAR™ product. Our efforts have focused on sourcing commercially ready aqueous
silk and optimizing manufacturing processes consistent with the necessary cost of goods for the commercial product. Preparations
are underway to begin an animal study to assess resorption rates of DisappEAR™, our resorbable, antimicrobial pediatric
ear tube, which we are now able to machine from solid silk rods.
|
|
|
|
|
●
|
We
have advanced the design and development of the NextFlo™ device, including a redesign which dramatically simplifies
the product, lowers the projected cost of goods and expands its application to routine inpatient infusion sets. We have completed
benchtop testing of a working prototype demonstrating constant flows across the range of pressures encountered in clinical
situations. We have recently elevated NextFlo to lead product status having established proof of concept and we are proceeding
with design work to ensure a cost-effective device with the potential to drive down health care costs. We believe this technology
will permit hospitals to return to gravity-driven infusions and eliminate expensive and troublesome electronic pumps for most
of the over 1 million hospital infusions performed in the U.S. each day.
|
|
|
|
|
●
|
Although
we have focused the majority of our resources on our lead products, we have additional products in our pipeline which are
currently in different stages of development. We have completed initial design work on the first product in the NextCath™
product line, completed head-to-head testing of retention forces, comparing our working prototype to several competing products,
which has validated our approach and advanced the commercial design and development process focusing on optimizing the self-anchoring
helical portion as well as cost of materials and manufacturing processes.
|
|
|
|
|
●
|
We
are evaluating which initial applications for our Caldus™ disposable tissue ablation technology to pursue from a clinical
and commercial point-of-view and will reinitiate development activity on this product once resources are available.
|
|
|
|
|
●
|
We
remain actively engaged with our full-service regulatory consulting partner who is working closely with our contract design,
engineering and manufacturing partners as our products advance towards regulatory submission, clearance, and commercialization.
|
|
|
|
|
●
|
We
are evaluating a number of product opportunities and intellectual property covering a spectrum of clinical conditions, which
have been presented to us by clinician innovators and academic medical centers, for consideration of a partnership to develop
and commercialize these products; we are also exploring opportunities to partner with larger medical device companies to commercialize
our lead products as they move towards regulatory clearance and commercialization.
|
|
|
|
|
●
|
We
are exploring other opportunities to grow our business and enhance shareholder value through the acquisition of pre-commercial
or commercial stage products and /or companies with potential strategic corporate and commercial synergies consistent with
our growth strategy.
|
We
were incorporated on June 26, 2014 in the State of Delaware under the name PAXmed Inc. In April 2015, we changed our name to PAVmed
Inc. Our business address is One Grand Central Place, Suite 4600, New York, New York 10165, and our telephone number is (212)
949-4319. Our corporate website is www.pavmed.com. The information contained on, or that can be assessed through, our website
is not incorporated by reference into this prospectus and you should not consider information on our website to be part of this
prospectus or in deciding whether to purchase our securities.
Background
of the Offering
Pre-IPO
and IPO Transactions
Immediately
prior to our initial public offering (“
IPO
”), we had 12,250,000 shares of our common stock and 9,560,296 warrants
to purchase shares of our common stock outstanding, all of which had been issued in private placements. On April 28, 2016, we
consummated our initial public offering of 1,060,000 units, each unit consisting of one share of common stock and one warrant.
The units were sold at an offering price of $5.00 per unit, generating gross proceeds of $5.3 million, and net cash proceeds of
$4.2 million, after deducting cash selling agent discounts and commissions and offering expenses. Upon consummation of our IPO,
the warrants outstanding immediately prior to the IPO automatically converted into warrants identical to those issued in our IPO.
In this prospectus, we refer to the warrants issued in private placements prior to our IPO and to those issued in our IPO as the
“
IPO Series W Warrants
” and the “
Pre-IPO Series W Warrants
,” respectively, and together
as the “
Series W Warrants
.” The Series W Warrants have an exercise price of $5.00 per share (subject to adjustment
for stock splits, stock dividends and similar events), are currently exercisable and expire on January 29, 2022.
On
April 5, 2018, we completed an exchange offer, pursuant to which we offered to all holders of the Series W Warrants the opportunity
to exchange each Series W Warrant they held for 0.5 Series Z warrants to purchase common stock (the “
Series Z Warrants
”).
Pursuant to the exchange offer, 10,151,682 Series W Warrants, including 849,163 IPO Series W Warrants and 9,302,519 Pre-IPO Series
W Warrants, were validly tendered and not withdrawn. We accepted for exchange all the securities so tendered and issued approximately
5,075,849 Series Z Warrants in exchange for such Series W Warrants, including 424,581 Series Z Warrants (the “
IPO Series
Z Warrants
”) in exchange for the IPO Series W Warrants and 4,651,268 Series Z Warrants (the “
Pre-IPO Series
Z Warrants
”) in exchange for Pre-IPO Series W Warrants. The Series Z Warrants have an exercise price of $1.60 per share
(subject to adjustment for stock splits, stock dividends and similar events), are currently exercisable and expire on April 30,
2024. After the exchange, 124,042 IPO Series W Warrants and 257,776 Pre-IPO Series W Warrants remained outstanding.
In
addition, as of the date of this prospectus, 86,795 IPO Series W Warrants had been exercised and 12,450 Pre-IPO Series Z Warrants
had been publicly transferred in accordance with the exemption from registration provided by Section 4(a)(1) of the Securities
Act of 1933, as amended (the “
Securities Act
”), and Rule 144 thereunder.
As
a result, this prospectus covers the resale of 257,776 shares of common stock underlying Pre-IPO Series W Warrants and 4,638,818
shares of common stock underlying Pre-IPO Series Z Warrants, as well as the initial issuance of such shares to the extent such
warrants are publicly transferred prior to their exercise. This prospectus also covers the initial issuance of 124,042 shares
of common stock underlying the IPO Series W Warrants, 424,581 shares of common stock underlying the IPO Series Z Warrants and
12,450 shares of common stock underlying the Pre-IPO Series Z Warrants that have been publicly transferred.
UPOs
On
April 28, 2016, in connection with the closing of our IPO, we issued 53,000 unit purchase options to the selling agents in our
IPO. The unit purchase options entitled the holders thereof to purchase 53,000 units, each unit consisting of one share of our
common stock and one Series W Warrant, for an exercise price of $5.50 per unit. On August 22, 2018, we entered into an exchange
agreement pursuant to which the outstanding unit purchase options were exchanged for a like number of new unit purchase options
(the “
UPOs
”). The new UPOs entitle the holders thereof to purchase 53,000 units, each unit consisting of one
share of our common stock and one Series Z Warrant (the “
UPO Series Z Warrants
”), for an exercise price of
$5.50 per unit. The UPOs also may be exercised, in whole or in part, on a cashless basis.
This
prospectus covers the resale of 53,000 shares of common stock issuable upon exercise of the UPOs, 53,000 UPO Series Z Warrants
and 53,000 shares of common stock underlying the UPO Series Z Warrants, as well as the initial issuance of the shares underlying
the UPO Series Z Warrants to the extent such warrants are publicly transferred prior to their exercise.
Preferred
Stock Financing
On
January 26, 2017, January 31, 2017 and March 8, 2017, we sold 422,838 shares of Series A Convertible Preferred Stock (the “
Series
A Preferred Stock
”) and 422,838 Series A warrants to purchase common stock (the “
Series A Warrants
”)
in a private placement. The Series A Preferred Stock and Series A Warrants were sold in units consisting of one share and one
warrant, at a purchase price of $6.00 per unit.
On
August 4, 2017, we sold 125,000 shares of Series A-1 Convertible Preferred Stock (the “
Series A-1 Preferred Stock
”)
and 125,000 Series A-1 warrants to purchase common stock (the “
Series A-1 Warrants
”) in a private placement.
The Series A-1 Preferred Stock and Series A-1 Warrants were sold in units consisting of one share and one warrant, at a purchase
price of $4.00 per unit.
On
November 17, 2017, we completed a private exchange offer, pursuant to which we offered to all the holders of our Series A Preferred
Stock and Series A Warrants the opportunity to exchange each share of Series A Preferred Stock for 1.5 shares of Series A-1 Preferred
Stock and each Series A Warrant for one Series A-1 Warrant. 154,837 shares of the Series A Preferred Stock and 154,837 Series
A Warrants were validly tendered and not withdrawn. We accepted for exchange all of the securities so tendered and issued an aggregate
of 232,259 shares of Series A-1 Preferred Stock and 154,837 Series A-1 Warrants. In addition, in November and December of 2017,
18,334 shares of Series A Preferred Stock were converted into 22,093 shares of our commons stock in accordance with their terms.
On
March 15, 2018, we completed another private exchange offer, pursuant to which we offered (i) to all holders of our Series A Preferred
Stock and Series A Warrants, the opportunity to exchange each share of Series A Preferred Stock for two shares of Series B Convertible
Preferred Stock (the “
Series B Preferred Stock
”) and each Series A Warrant for five Series Z Warrants; and
(ii) to all holders of our Series A-1 Preferred Stock and Series A-1 Warrants, the opportunity to exchange each share of Series
A-1 Preferred Stock exchanged for 1.33 shares of Series B Preferred Stock and each Series A-1 Warrant for five Series Z Warrants.
All of the Series A Preferred Stock and Series A Warrants and all of the Series A-1 Preferred Stock and Series A-1 Warrants were
validly tendered and not withdrawn. We accepted for exchange all of the securities so tendered and issued an aggregate of 975,568
shares of Series B Preferred Stock and an aggregate of 2,739,190 Series Z Warrants (the “
Preferred Stock Financing Series
Z Warrants
”). The Series B Preferred Stock has a stated value of $3.00 per share, has a conversion price of $3.00
per share (subject to adjustment for stock splits, stock dividends and similar events) and is currently convertible at the holder’s
election.
As
of the date of this prospectus, 33,325 of shares of Series B Preferred Stock have been converted into 33,325 shares of our common
stock. In addition, 106,045 shares of Series B Preferred Stock have been issued as payment-in-kind dividends. All of the shares
of common stock issued or issuable upon conversion of the outstanding Series B Preferred Stock (including all payment-in-kind
dividends) may be resold without volume or manner of sale restrictions in accordance with the exemption from registration provided
by Section 4(a)(1) of the Securities Act and Rule 144 thereunder.
As
a result, this prospectus covers the resale of 2,739,190 shares underlying Preferred Stock Financing Series Z Warrants, as well
as the initial issuance of such shares to the extent such warrants are publicly transferred prior to their exercise.
Scopia
Debt Financing
On
June 30, 2017, we entered into a Note and Securities Purchase Agreement (the “
NSPA
”) with Scopia Holdings,
LLC (“
Scopia
”). Effective July 3, 2017, we consummated the sale of securities pursuant to the NSPA, upon (i)
the issuance by us of a 15.0% senior secured promissory note with a principal amount of $5,000,000 (the “
Promissory Note
”)
to Scopia; (ii) the issuance by us of Series S warrants to purchase 2,660,000 shares of our common stock (the
“
Series S Warrants
”) to Scopia and its designees; and (iii) the delivery by Scopia to us of $4.8 million in
cash, representing the principal amount of the Promissory Note net of Scopia’s costs.
The
Series S Warrants have an exercise price of $0.01 per share (subject to adjustment for stock splits, stock dividends and similar
events), are currently exercisable and expire on June 30, 2032. The Series S Warrants may also be exercised, in whole or in part,
on a cashless basis. Any outstanding Series S Warrants on the expiration date shall be automatically exercised via cashless exercise.
As
of the date of this prospectus, the Series S Warrants have been exercised for cash as to 1,338,257 shares and on a cashless basis
as to 122,360 shares, resulting in the issuance of 1,460,337 shares of our common stock. Series S Warrants to purchase 1,199,383
shares of our common stock remain outstanding.
As
a result, this prospectus covers the resale of the 1,460,337 shares of common stock issued upon exercise of the Series S Warrants
and 1,199,383 shares underlying the remaining unexercised Series S Warrants.
The
Offering
|
Common
stock to be offered by the selling securityholders
|
10,401,504
shares of common stock
|
|
|
|
|
Series
Z Warrants to be offered by the selling securityholders
|
53,000
Series Z Warrants
|
|
|
|
|
Common
stock to be offered by us
|
8,249,857
shares of common stock
|
|
|
|
|
Common
stock outstanding after the offering
|
36,045,219
shares of common stock(1)
|
|
|
|
|
Series
Z Warrants outstanding after the offering
|
16,868,039
Series Z Warrants(2)
|
|
|
|
|
Use
of proceeds
|
We
will not receive any of the proceeds from the sale or other disposition of the securities by the selling securityholders.
However, we will receive up to approximately $13,481,987 in gross proceeds upon the cash exercise of the UPOs, Series S Warrants,
Series W Warrants and Series Z Warrants covering certain of the securities offered for resale, and we will receive up to approximately
$1,319,460 in gross proceeds upon the cash exercise of the publicly held Series W Warrants and Series Z Warrants. We will
use any such proceeds for working capital. See “
Use of Proceeds
” beginning on page 11 of this prospectus.
|
|
|
|
|
Nasdaq
Capital Market symbols
|
PAVM
(common stock)
PAVMZ (Series Z Warrants)
|
|
|
|
|
Risk
Factors
|
See
“
Risk Factors
” beginning on page 9 of this prospectus and the other information included in or incorporated
by reference into this prospectus for a discussion of the factors you should consider before making an investment decision.
|
|
(1)
|
Based
on 26,542,979 shares of common stock outstanding as of October 16, 2018. Assumes the exercise for cash of all the options
and warrants covering the shares offered for resale or initial issuance hereby. Excludes the following: (1) 1,048,288 shares
of common stock underlying our Series B Convertible Preferred Stock, (2) 9,000,000 shares of common stock underlying our Series
Z Warrants that are not covered by this registration statement, and (3) 3,277,140 shares of common stock underlying our outstanding
stock options.
|
|
(2)
|
Based
on 16,815,039 Series Z Warrants outstanding as of October 16, 2018. Assumes the exercise for cash of all the UPOs,
but no exercise of the Series Z Warrants.
|
NOTE
ON FORWARD-LOOKING STATEMENTS
The
statements contained in this prospectus and in the documents incorporated by reference in this prospectus that are not purely
historical are forward-looking statements. Forward-looking statements include, but are not limited to, statements regarding expectations,
hopes, beliefs, intentions or strategies regarding the future, such as:
|
●
|
our
expectations regarding our existing capital resources will be sufficient to enable us to successfully meet the capital requirements
for all of our current and future products;
|
|
|
|
|
●
|
our
estimates regarding expenses, future revenue, capital requirements and needs for additional financing; and
|
|
|
|
|
●
|
expectations
regarding the time during which we will be an Emerging Growth Company under the JOBS Act.
|
In
addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including
any underlying assumptions, are forward-looking statements. The words “anticipates,” “believes,” “continues,”
“could,” “estimates,” “expects,” “intends,” “may,” “might,”
“plans,” “possible,” “potential,” “predicts,” “projects,” “should,”
“would” and similar expressions may identify forward-looking statements, but the absence of these words does not mean
that a statement is not forward-looking.
The
forward-looking statements contained in this prospectus and in the documents incorporated by reference in this prospectus are
based on current expectations and beliefs concerning future developments and their potential effects on us. There can be no assurance
that future developments will be those that have been anticipated. These forward-looking statements involve a number of risks,
uncertainties (some of which are beyond our control) or other assumptions that may cause actual results or performance to be materially
different from those expressed or implied by these forward-looking statements. These risks and uncertainties include, but are
not limited to, those factors incorporated by reference or described in “
Risk Factors
,” as well as the following:
|
●
|
our
limited operating history;
|
|
|
|
|
●
|
our
ability to generate revenue;
|
|
|
|
|
●
|
the
ability of our products to achieve regulatory approval and market acceptance;
|
|
|
|
|
●
|
our
success in retaining or recruiting, or changes required in, our officers, key employees or directors;
|
|
|
|
|
●
|
our
ability to obtain additional financing when and if needed;
|
|
|
|
|
●
|
our
ability to protect our intellectual property rights;
|
|
|
|
|
●
|
our
ability to complete strategic acquisitions;
|
|
|
|
|
●
|
our
ability to manage growth and integrate acquired operations;
|
|
|
|
|
●
|
the
liquidity and trading of our securities; and
|
|
|
|
|
●
|
regulatory
or operational risks.
|
Should
one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual results may
vary in material respects from those projected in these forward-looking statements. We do not undertake any obligation to update
or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be
required under applicable securities laws.
RISK
FACTORS
Any
investment in our securities involves a high degree of risk. Potential investors are urged to read and consider the risks and
uncertainties relating to an investment in our company set forth below and those set forth in or incorporated by reference into
this prospectus, including those set forth in our most recent annual report on Form 10-K and those set forth in our quarterly
reports on Form 10-Q for the fiscal quarters commencing after the end of the fiscal year covered by such annual report. Additional
risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our business and results
of operations. If any of these risks actually occur, our business, financial condition or results of operations could be seriously
harmed. In that event, the market price for our common stock could decline and you may lose all or part of your investment.
We
have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
We
will have broad discretion in the application of the balance of the proceeds from this offering and could spend the proceeds in
ways that do not improve our results of operations or enhance the value of our common stock. If we fail to apply these funds effectively
it could result in financial losses that could have a material adverse effect on our business, cause the price of our common stock
to decline and delay the commercialization of any products we may develop. Pending their use, we may invest the net proceeds from
this offering in a manner that does not produce income or that loses value.
Only
a limited market exists for our common stock and Series Z Warrants, which could lead to price volatility.
Our
common stock and Series Z Warrants trade on the Nasdaq Capital Market. However, trading volumes for our common stock and
Series Z Warrants have been low from time to time. The limited trading market for our common stock and Series Z Warrants
may cause fluctuations in the market value of our common stock and Series Z Warrants to be exaggerated, leading to
price volatility in excess of that which would occur in a more active trading market for our common stock and Series Z Warrants.
Sales
of substantial amounts of our common stock by the selling securityholders, or the perception that these sales could occur, could
adversely affect the price of our common stock.
The
sale by the selling securityholders of a significant number of shares of common stock could have a material adverse effect on
the market price of our common stock. In addition, the perception in the public markets that the selling securityholders may sell
all or a portion of their shares as a result of the registration of such shares pursuant to the Registration Statement could also
in and of itself have a material adverse effect on the market price of our common stock.
The
exercise of the UPOs, Series S Warrants, Series W Warrants and Series Z Warrants described herein will dilute our equity, and
there may be future sales or other dilution of our equity, which may adversely affect the market price of our common stock.
The
exercise prices of the UPOs, Series S Warrants, Series W Warrants and Series Z Warrants are $5.50 per unit, $0.01 per share,
$5.00 per share and $1.60 per share, respectively. Such warrants likely will be exercised only at a time when it is economically
beneficially for the holder to do so. Accordingly, the exercise of these options and warrants by the selling securityholders likely
will dilute our other equity holders. In addition, we may issue additional shares of common stock and/or other securities that
are convertible into or exchangeable for, or that represent the right to receive, shares of common stock. The market price of
our shares could decline as a result of sales of our common stock or such other securities, or the perception that such sales
could occur.
Holders
of our Series Z Warrants will have no rights as a common stockholder until such holders exercise their Series Z Warrants and acquire
our common stock.
Until
holders of Series Z Warrants acquire shares of our common stock upon exercise of the Series Z Warrants, holders of Series Z Warrants
will have no rights with respect to the shares of our common stock underlying such Series Z Warrants. Upon exercise of the Series
Z Warrants, the holders thereof will be entitled to exercise the rights of a common stockholder only as to matters for which the
record date occurs after the exercise date.
The
market price of our common stock may never exceed the exercise price of the Series Z Warrants offered hereby.
The
Series Z Warrants offered hereby are currently exercisable and will expire on April 30, 2024, or earlier upon
certain redemption provisions. The market price of our common stock may never exceed the exercise price of the Series Z Warrants
prior to their date of expiration. Any Series Z Warrants not exercised by their date of expiration will expire worthless and we
will be under no further obligation to the warrant holder.
An
investor will only be able to exercise a Series Z Warrant if the issuance of shares of common stock upon such exercise has been
registered or qualified or is deemed exempt under the securities laws of the state of residence of the holder of the warrants.
No
Series Z Warrants will be exercisable for cash and we will not be obligated to issue shares of common stock unless the shares
of common stock issuable upon such exercise has been registered or qualified or deemed to be exempt under the securities laws
of the state of residence of the holder of the warrants. Our common stock is currently listed on Nasdaq, which provides an exemption
from registration in every state. However, we cannot assure you that our common stock will remain so listed. If the shares of
common stock issuable upon exercise of the Series Z Warrants are not qualified or exempt from qualification in the jurisdictions
in which the holders of the warrants reside, the Series Z Warrans may be deprived of any value, the market for the warrants may
be limited and they may expire worthless if they cannot be sold.
We
may amend the terms of the Series Z Warrants in a way that may be adverse to holders with the approval by the holders of a majority
of the then outstanding Series Z Warrants.
Our
Series Z Warrants are issued in registered form under a warrant agreement between Continental Stock Transfer & Trust Company,
as warrant agent, and us. The warrant agreement provides that the terms of the Series Z Warrants may be amended without the consent
of any holder to cure any ambiguity or correct any defective provision. The warrant agreement requires the approval by the holders
of a majority of the then outstanding Series Z Warrants (including those held by our affiliates, which presently account for 18%
of the outstanding Series Z Warrants) in order to make any change that adversely affects the interests of the registered holders.
We
may redeem the Series Z Warrants at a time that is not beneficial to investors.
We
may call our Series Z Warrants, other than those held by our founders, certain members of management and their respective affiliates,
for redemption at any time after the redemption criteria described elsewhere in this prospectus have been satisfied. If we call
such warrants for redemption, holders may be forced to accept a nominal redemption price or sell or exercise the Series Z Warrants
when they may not wish to do so.
Our
ability to require holders of our Series Z Warrants to exercise such warrants on a cashless basis will cause holders to receive
fewer shares of common stock upon their exercise of the Series Z Warrants than they would have received had they been able to
exercise their warrants for cash.
If
we call our Series Z Warrants for redemption after the redemption criteria described elsewhere in this prospectus have been satisfied,
we will have the option to require any holder that wishes to exercise its warrant (including any warrants held by our initial
stockholders or their permitted transferees) to do so on a “cashless basis.” If we choose to require holders to exercise
their Series Z Warrants on a cashless basis, the number of shares of common stock received by a holder upon exercise will be fewer
than it would have been had such holder exercised his warrant for cash. This will have the effect of reducing the potential “upside”
of the holder’s investment in our company.
Since
the Series Z Warrants are executory contracts, they may have no value in a bankruptcy or reorganization proceeding.
In
the event a bankruptcy or reorganization proceeding is commenced by or against us, a bankruptcy court may hold that any unexercised
Series Z Warrants are executory contracts that are subject to rejection by us with the approval of the bankruptcy court. As a
result, holders of the Series Z Warrants may, even if we have sufficient funds, not be entitled to receive any consideration for
their Series Z Warrants or may receive an amount less than they would be entitled to if they had exercised their Series Z Warrants
prior to the commencement of any such bankruptcy or reorganization proceeding.
USE
OF PROCEEDS
Certain
of the shares sold under this prospectus will be sold or otherwise disposed of for the account of the selling securityholders,
or their pledgees, assignees or successors-in-interest. We will not receive any of the proceeds from the sale or other disposition
of the shares by the selling securityholders. However, we will receive up to approximately $13,481,987 in gross proceeds upon
the cash exercise of the UPOs, Series S Warrants, Series W Warrants and Series Z Warrants covering certain of the shares offered
for resale, and we will receive up to approximately $1,319,460 in gross proceeds upon the cash exercise of the publicly held Series
W Warrants and Series Z Warrants. We will use any such proceeds for working capital.
DESCRIPTION
OF SECURITIES
Capital
Stock
We
are authorized to issue 75,000,000 shares of common stock, par value $0.001, and 20,000,000 shares of preferred stock, par value
$0.001. As of October 16, 2018, we had outstanding:
|
●
|
26,542,979
shares of our common stock.
|
|
|
|
|
●
|
1,048,288
shares of Series B Convertible Preferred Stock, each with a stated value of $3.00 and convertible at the holder’s option
into shares of common stock at a price of $3.00 per share, subject to adjustment.
|
|
|
|
|
●
|
381,818
Series W Warrants, each entitling the holder to purchase one share of common stock at $5.00 per share, subject to adjustment,
and expiring on January 29, 2022.
|
|
|
|
|
●
|
16,815,039
Series Z Warrants, each entitling the holder to purchase one share of common stock at $1.60 per share, subject to adjustment,
and expiring on April 30, 2024.
|
|
|
|
|
●
|
1,199,383
Series S Warrants, each entitling the holder to purchase one share of common stock at an exercise price of $0.01 per share,
subject to adjustment, and expiring on June 30, 2032.
|
|
|
|
|
●
|
53,000
UPOs, each entitling the holder to purchase one unit, comprised of one share of common stock and one Series Z Warrant, at
an exercise price of $5.00 per unit, subject to adjustment, and expiring on January 29, 2021.
|
|
|
|
|
●
|
S
tock
options entitling the holders thereof to purchase 3,277,140 shares of common stock at a weighted average exercise price
of $3.72 per share.
|
Holders
of common stock are entitled to one vote per share on matters on which our stockholders vote. There are no cumulative voting rights.
Subject to any preferential dividend rights of any outstanding shares of preferred stock, holders of common stock are entitled
to receive dividends, if declared by our board of directors, out of funds that we may legally use to pay dividends. If we liquidate
or dissolve, holders of common stock are entitled to share ratably in our assets once our debts and any liquidation preference
owed to any then-outstanding preferred stockholders are paid. Our certificate of incorporation does not provide the common stock
with any redemption, conversion or preemptive rights. All shares of common stock that are outstanding as of the date of this Prospectus
will be fully-paid and non-assessable.
Our
certificate of incorporation authorizes the issuance of blank check preferred stock. Accordingly, our board of directors is empowered,
without stockholder approval, to issue shares of preferred stock with dividend, liquidation, redemption, voting or other rights
which could adversely affect the voting power or other rights of the holders of shares of our common stock. In addition, shares
of preferred stock could be utilized as a method of discouraging, delaying or preventing a change in control of us.
As
of the date of this prospectus, we have authorized one series of preferred stock, the Series B Convertible Preferred Stock. The
Series B Preferred Stock has no voting rights. It provides for dividends at a rate of 8% per annum on the stated value of the
Series B Preferred Stock, with such dividends compounded quarterly, accumulate, and are payable in arrears upon being declared
by our Board of Directors. The Series B Preferred Stock dividends from April 1, 2018 through October 1, 2021 are payable-in-kind
in additional shares of Series B Preferred Stock. The dividends may be settled after October 1, 2021, at the option of the Company,
through any combination of the issuance of additional Series B Preferred Stock, shares of common stock, and /or cash payment.
Series
Z Warrants
We
have 16,815,039 Series Z Warrants outstanding. A Series Z Warrant may be exercised for one share of common stock at an exercise
price of $1.60 per share, with such exercise price not subject to further adjustment, except for the effect of stock dividends,
stock splits or similar events affecting the common stock, and will expire after the close of business on April 30, 2024. The
Series Z Warrants are immediately exercisable.
Commencing
on May 1, 2019, we may redeem the outstanding Series Z Warrants, at our option, in whole or in part, at a price of $0.01 per Series
Z Warrant at any time while the Series Z Warrants are exercisable, upon a minimum of 30 days’ prior written notice of redemption,
if, and only if, the volume weighted average closing price of the common stock equals or exceeds $9.00 (subject to adjustment)
for any 20 out of 30 consecutive trading days ending three business days before we issue our notice of redemption, and provided
the average daily trading volume in the common stock during such 30-day period is at least 20,000 shares per day; and if, and
only if, there is a current registration statement in effect with respect to the shares of common stock underlying such Series
Z Warrants. Under the terms of the warrant agreement governing the Series Z Warrants, all modifications or amendments to the Series
Z Warrants (other than amendments for the purpose of curing or correcting ambiguities and/or defective provisions), including
any amendment to increase the warrant price or shorten the exercise period of the Series Z Warrants, shall require the written
consent or vote of the registered holders of at least two-thirds of the then outstanding Series Z Warrants (including any Series
Z Warrants held by our officers and directors or their respective affiliates). Notwithstanding the foregoing, pursuant to the
terms of the warrant agreement governing the Series Z Warrants, we may lower the warrant price or extend the duration of the exercise
period without the consent of the registered holders.
Under
the terms of the warrant agreement governing the Series Z Warrants, we have agreed to use our best efforts to cause a registration
statement covering the shares of common stock underlying the Series Z Warrants to continue to be effective until the expiration
of the Series Z Warrants in accordance with the terms of the warrant agreement.
Anti-Takeover
Provisions
Provisions
of the Delaware General Corporation Law (the “
DGCL
”) and our certificate of incorporation and bylaws could
make it more difficult to acquire us by means of a tender offer, a proxy contest or otherwise, or to remove incumbent officers
and directors. These provisions, summarized below, are expected to discourage certain types of coercive takeover practices and
takeover bids that our board of directors may consider inadequate and to encourage persons seeking to acquire control of us to
first negotiate with our board of directors. We believe that the benefits of increased protection of our ability to negotiate
with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging
takeover or acquisition proposals because, among other things, negotiation of these proposals could result in improved terms for
our stockholders.
Delaware
Anti-Takeover Statute
. We are subject to Section 203 of the DGCL, an anti-takeover statute. In general, Section 203 of the
DGCL prohibits a publicly-held Delaware corporation from engaging in a “business combination” with an “interested
stockholder” for a period of three years following the time the person became an interested stockholder, unless the business
combination or the acquisition of shares that resulted in a stockholder becoming an interested stockholder is approved in a prescribed
manner. Generally, a “business combination” includes a merger, asset or stock sale, or other transaction resulting
in a financial benefit to the interested stockholder. Generally, an “interested stockholder” is a person who, together
with affiliates and associates, owns (or within three years prior to the determination of interested stockholder status did own)
15% or more of a corporation’s voting stock. The existence of this provision would be expected to have an anti-takeover
effect with respect to transactions not approved in advance by the board of directors, including discouraging attempts that might
result in a premium over the market price for the shares of common stock held by stockholders.
Amendments
to Our Certificate of Incorporation
. Under the DGCL, the affirmative vote of a majority of the outstanding shares entitled
to vote thereon and a majority of the outstanding stock of each class entitled to vote thereon is required to amend a corporation’s
certificate of incorporation. Under the DGCL, the holders of the outstanding shares of a class of our capital stock shall be entitled
to vote as a class upon a proposed amendment, whether or not entitled to vote thereon by the certificate of incorporation, if
the amendment would:
|
|
●
|
increase
or decrease the aggregate number of authorized shares of such class;
|
|
|
|
|
|
|
●
|
increase
or decrease the par value of the shares of such class; or
|
|
|
|
|
|
|
●
|
alter
or change the powers, preferences or special rights of the shares of such class so as to affect them adversely.
|
If
any proposed amendment would alter or change the powers, preferences or special rights of one or more series of any class of our
capital stock so as to affect them adversely, but shall not so affect the entire class, then only the shares of the series so
affected by the amendment shall be considered a separate class for the purposes of this provision.
Classified
Board
. Our board of directors is divided into three classes. The number of directors in each class is as nearly equal as possible.
Directors elected to succeed those directors whose terms expire shall be elected for a term of office to expire at the third succeeding
annual meeting of stockholders after their election. The existence of a classified board may extend the time required to make
any change in control of the board when compared to a corporation with an unclassified board. It may take two annual meetings
for our stockholders to effect a change in control of the board, because in general less than a majority of the members of the
board will be elected at a given annual meeting. Because our board is classified and our certificate of incorporation does not
otherwise provide, under Delaware law, our directors may only be removed for cause.
Vacancies
in the Board of Directors
. Our certificate of incorporation and bylaws provide that, subject to limitations, any vacancy occurring
in our board of directors for any reason may be filled by a majority of the remaining members of our board of directors then in
office, even if such majority is less than a quorum. Each director elected to fill a vacancy resulting from the death, resignation
or removal of a director shall hold office until the expiration of the term of the director whose death, resignation or removal
created the vacancy.
Special
Meetings of Stockholders
. Under our bylaws, special meetings of stockholders may be called by the directors, or the president
or the chairman, and shall be called by the secretary at the request in writing of stockholders owning a majority in amount of
the entire capital stock of the corporation issued and outstanding and entitled to vote.
No
Cumulative Voting
. The DGCL provides that stockholders are denied the right to cumulate votes in the election of directors
unless our certificate of incorporation provides otherwise. Our certificate of incorporation does not provide for cumulative voting.
Dividends
We
have not paid any cash dividends on our shares of common stock to date. The payment of cash dividends in the future will be dependent
upon our revenues and earnings, if any, capital requirements and general financial condition and will be within the discretion
of our board of directors. It is the present intention of our board of directors to retain all earnings, if any, for use in our
business operations and, accordingly, our board of directors does not anticipate declaring any dividends in the foreseeable future.
Transfer
Agent and Registrar
The
transfer agent and registrar for our common stock and Series Z Warrants is Continental Stock Transfer & Trust Company.
Listing
of our Securities
Our
common stock, Series W Warrants, and Series Z Warrants are traded on the Nasdaq Capital Market under the symbols “PAVM”,
“PAVMW” and “PAVMZ,” respectively.
SELLING
SECURITYHOLDERS
The
selling securityholders, or their pledgees, assignees or successors-in-interest, are offering for resale, from time to time, up
to an aggregate of 10,401,504 shares of our common stock and 53,000 Series Z Warrants. As more fully described in “
Prospectus
Summary – Background of the Offering
,” such shares consist of:
|
|
●
|
257,776
shares of common stock underlying Pre-IPO Series W Warrants and 4,638,818 shares of common stock underlying the Pre-IPO Series
Z Warrants.
|
|
|
|
|
|
|
●
|
53,000
shares of common stock and 53,000 UPO Series Z Warrants issuable upon exercise of the UPOs and 53,000 shares of common stock
underlying the UPO Series Z Warrants;
|
|
|
|
|
|
|
●
|
2,739,190
shares of common stock underlying the Preferred Stock Financing Series Z Warrants; and
|
|
|
|
|
|
|
●
|
1,460,337
shares of common stock issued and 1,199,383 shares of common stock issuable upon exercise of the Series S Warrants.
|
Because
the Series S Warrants, the UPOs and, in certain circumstances, the Series W Warrants and Series Z Warrants permit cashless
exercise, the number of shares that ultimately will be issuable upon any exercise thereof (and, in the case of the UPO, of the
underlying UPO Series Z Warrants) may be less than the number of shares being offered by this prospectus. The selling securityholders
may sell all, some or none of their shares in this offering. The selling securityholders also may sell, transfer or otherwise
dispose of some or all their shares in transactions exempt from, or not subject to the registration requirements of, the Securities
Act. See “
Plan of Distribution
.”
The
tables below have been prepared based solely on information supplied to us by the selling securityholders, or included in statements
on Schedule 13D or 13G or other public documents filed by the selling securityholders with the SEC, and assumes the sale of all
the shares offered hereby. Other than as described in the footnotes below, none of the selling securityholders have, within the
past three years, had any position, office or other material relationship with us or any of our predecessors or affiliates other
than as a holder of our securities, or are broker-dealers or affiliates of a broker-dealer. Information concerning the selling
securityholders may change from time to time and, if necessary and required, we will amend or supplement this prospectus accordingly.
Beneficial
ownership is determined in accordance with Section 13(d) of the Securities Exchange Act of 1934, as amended, or the “Exchange
Act,” and generally includes shares over which the selling securityholder has voting or dispositive power, including any
shares that the selling securityholder has the right to acquire within 60 days of October 16, 2018. The percentages of
ownership before the offering are calculated based on 26,542,979 shares outstanding as of October 16, 2018. The percentages
of ownership after the offering assume the exercise of all the UPOs, Series S Warrants, Series W Warrants and Series Z Warrants
the underlying shares of which are offered for resale hereby, and the sale by each selling securityholder of all of the shares
offered for resale hereby.
Pre-IPO
Selling Securityholders
|
|
|
Beneficial
Ownership
Before
|
|
|
Shares
|
|
|
Beneficial
Ownership
|
|
|
|
|
Offering
|
|
|
Offered
|
|
|
After
Offering
|
|
|
Selling
Securityholder
|
|
Shares
|
|
|
Hereby
|
|
|
Shares
|
|
|
Percent
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lishan Aklog, M.D.(1)
|
|
|
1,307,098
|
|
|
|
350,588
|
|
|
|
956,510
|
|
|
|
2.7
|
%
|
|
Jeffrey P. Bergholtz(2)
|
|
|
418,089
|
|
|
|
139,363
|
|
|
|
278,726
|
|
|
|
*
|
|
|
Paul Christie IRA(3)
|
|
|
175,597
|
|
|
|
41,809
|
|
|
|
133,788
|
|
|
|
*
|
|
|
Graubard Miller(4)
|
|
|
48,777
|
|
|
|
48,777
|
|
|
|
0
|
|
|
|
*
|
|
|
Matthew J. Glennon(5)
|
|
|
209,045
|
|
|
|
69,682
|
|
|
|
139,363
|
|
|
|
*
|
|
|
Ira S. Greenspan(6)
|
|
|
1,227,729
|
|
|
|
775,828
|
|
|
|
451,901
|
|
|
|
1.3
|
%
|
|
Robert M. Greenspan(7)
|
|
|
24,388
|
|
|
|
3,484
|
|
|
|
20,904
|
|
|
|
*
|
|
|
HCFP Inc.(8)
|
|
|
508,492
|
|
|
|
448,492
|
|
|
|
60,000
|
|
|
|
*
|
|
|
HCFP LLC(9)
|
|
|
250,000
|
|
|
|
250,000
|
|
|
|
0
|
|
|
|
*
|
|
|
Neil Kaufman(10)
|
|
|
22,872
|
|
|
|
6,968
|
|
|
|
15,904
|
|
|
|
*
|
|
|
Peter M. Kendall(11)
|
|
|
196,545
|
|
|
|
57,182
|
|
|
|
139,363
|
|
|
|
*
|
|
|
Josh Lamstein(12)
|
|
|
131,589
|
|
|
|
81,589
|
|
|
|
50,000
|
|
|
|
*
|
|
|
Lawrence M. Levinson(13)
|
|
|
290,760
|
|
|
|
87,629
|
|
|
|
203,131
|
|
|
|
*
|
|
|
Pavilion Venture Partners, LLC(1)
|
|
|
6,528,855
|
|
|
|
2,072,285
|
|
|
|
4,456,570
|
|
|
|
12.6
|
%
|
|
Richard J. Salute(14)
|
|
|
680,318
|
|
|
|
226,773
|
|
|
|
453,545
|
|
|
|
1.3
|
%
|
|
Richard X. Seet(15)
|
|
|
83,618
|
|
|
|
27,873
|
|
|
|
55,745
|
|
|
|
*
|
|
|
Chris P. Vieira(16)
|
|
|
452,930
|
|
|
|
34,841
|
|
|
|
418,089
|
|
|
|
1.2
|
%
|
|
James P. Ward(17)
|
|
|
418,089
|
|
|
|
139,363
|
|
|
|
278,726
|
|
|
|
*
|
|
|
Ella Damiano(18)
|
|
|
19,902
|
|
|
|
3,501
|
|
|
|
16,401
|
|
|
|
*
|
|
|
Michael Damiano(18)
|
|
|
19,902
|
|
|
|
3,501
|
|
|
|
16,401
|
|
|
|
*
|
|
|
Lawrence Howard(19)
|
|
|
11,540
|
|
|
|
1,347
|
|
|
|
10,193
|
|
|
|
*
|
|
|
Londonderry Capital LLC(20)
|
|
|
13,847
|
|
|
|
1,616
|
|
|
|
12,231
|
|
|
|
*
|
|
|
Sheryl Masella(21)
|
|
|
4,616
|
|
|
|
808
|
|
|
|
3,808
|
|
|
|
*
|
|
|
Amy Newmark(18)
|
|
|
19,902
|
|
|
|
3,501
|
|
|
|
16,401
|
|
|
|
*
|
|
|
Rosemary Rouhana(18)
|
|
|
19,902
|
|
|
|
3,501
|
|
|
|
16,401
|
|
|
|
*
|
|
|
Timothy Rouhana(18)
|
|
|
19,902
|
|
|
|
3,501
|
|
|
|
16,401
|
|
|
|
*
|
|
|
William J. Rouhana, Jr. (18)
|
|
|
19,902
|
|
|
|
3,501
|
|
|
|
16,401
|
|
|
|
*
|
|
|
Alan Salzbank(22)
|
|
|
4,616
|
|
|
|
539
|
|
|
|
4,077
|
|
|
|
*
|
|
|
Lauren Smith(23)
|
|
|
47,410
|
|
|
|
22,154
|
|
|
|
25,256
|
|
|
|
*
|
|
|
Stewart and Sons LLC(24)
|
|
|
45,002
|
|
|
|
5,251
|
|
|
|
39,751
|
|
|
|
*
|
|
|
Mark J. Wishner(19)
|
|
|
11,540
|
|
|
|
1,347
|
|
|
|
10,193
|
|
|
|
*
|
|
|
*
|
Less
than 1%.
|
|
|
|
|
(1)
|
Dr.
Aklog is our Chairman of the Board and Chief Executive Officer. Dr. Aklog’s beneficial ownership includes (i) 618,413
shares held by Dr. Akog, 2,303 shares held by his daughter, 2,280 shares held by his son and 20,000 shares held by HCFP/AG
LLC, an entity co-controlled by Dr. Aklog, (ii) 363,313 shares underlying Series Z Warrants (including 350,888 offered hereby)
held by Dr. Aklog, 1,018 shares underlying Series Z Warrants held by his daughter, 980 shares underlying Series Z Warrants
held by his son, and 10,000 shares underlying Series Z Warrants held by HCFP/AG LLC, and (iii) 288,791 shares underlying employee
stock options held by Dr. Aklog, which excludes 185,043 shares underlying employee stock options that are not exercisable
and will not become exercisable within 60 days. Pavilion Venture Partners LLC is controlled by Lishan Aklog, M.D., our Chairman
of the Board and Chief Executive Officer, as manager. Accordingly, Dr. Aklog may be deemed to beneficially own the shares
of common stock and warrants held by such entity. Pavilion Venture Partners, LLC’s beneficial ownership includes 4,456,570
shares and 2,078,285 shares underlying Series Z Warrants held by it.
|
|
|
|
|
(2)
|
Mr.
Bergholtz’s beneficial ownership includes 278,726 shares and 139,363 shares underlying Series Z Warrants (all of which
are offered hereby).
|
|
|
|
|
(3)
|
Paul
Christie may be deemed to have beneficial ownership of the shares of common stock held by the Paul Christie IRA. The Paul
Christie IRA’s beneficial ownership includes 133,788 shares and 41,809 shares underlying Series Z Warrants (all of which
are offered hereby).
|
|
|
|
|
(4)
|
Graubard
Miller is our outside general counsel. David Alan Miller, Managing Partner of Graubard Miller, exercises voting and dispositive
power over securities held by such entity. Graubard Miller’s beneficial ownership includes 48,777 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
(5)
|
Mr.
Glennon’s beneficial ownership includes 139,363 shares and 69,682 shares underlying Series Z Warrants (all of which
are offered hereby).
|
|
|
|
|
(6)
|
Mr.
Ira Greenspan is a former member of our board of directors. Mr. Ira Greenspan’s beneficial ownership includes (i) 421,691
shares and 776,038 shares underlying Series Z Warrants (including 775,828 of which are offered hereby) held by Mr. Ira Greenspan
and (ii) 20,000 shares and 10,000 shares underlying Series Z Warrants held by HCFP/AG LLC, an entity co-controlled by Mr.
Ira Greenspan.
|
|
|
|
|
(7)
|
Mr.
Robert Greenspan is Mr. Ira Greenspan’s son. Mr. Robert Greenspan’s beneficial ownership includes 20,904 shares
and 3,484 shares underlying Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(8)
|
HCFP
Inc.’s beneficial ownership includes 60,000 shares and 448,492 shares underlying Series Z Warrants (all of which are
offered hereby).
|
|
|
|
|
(9)
|
HCFP
LLC’s beneficial ownership includes 250,000 shares underlying Series W Warrants (all of which are offered hereby).
|
|
|
|
|
(10)
|
Mr.
Kaufman’s beneficial ownership includes 15,904 shares and 6,968 shares underlying Series W Warrants (all of which are
offered hereby).
|
|
|
|
|
(11)
|
Mr.
Kendall is affiliated with MKM Partners, a broker-dealer. Mr. Kendall’s beneficial ownership includes 139,363 shares
and 57,182 shares underlying Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(12)
|
Mr.
Lamstein is a former member of our board of directors. Mr. Lamstein’s beneficial ownership includes 50,000 shares and
81,589 shares underlying Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(13)
|
Mr.
Levinson’s beneficial ownership includes 203,131 shares and 87,629 shares underlying Series Z Warrants (all of which
are offered hereby).
|
|
|
|
|
(14)
|
Mr.
Salute’s beneficial ownership includes 453,545 shares and 226,773 shares underlying Series Z Warrants (all of which
are offered hereby).
|
|
|
|
|
(15)
|
Mr.
Seet’s beneficial ownership includes 55,745 shares and 27,873 shares underlying Series Z Warrants (all of which are
offered hereby).
|
|
|
|
|
(16)
|
Mr.
Vieira’s beneficial ownership includes 418,089 shares and 34,841 shares underlying Series Z Warrants (all of which are
offered hereby).
|
|
|
|
|
(17)
|
Mr.
Ward’s beneficial ownership includes 278,726 shares and 139,363 shares underlying Series Z Warrants (all of which are
offered hereby).
|
|
|
|
|
(18)
|
Each
of Ms. Damino, Mr. Damiano, Ms. Newmark, Ms. Rouhana, Mr. Timothy Rouhana and Mr. William Rouhana’s beneficial ownership
includes 10,051 shares and 9,851 shares underlying Series Z Warrants (3,501 of which are offered hereby).
|
|
|
|
|
(19)
|
Mr.
Wishner is a partner of Greenberg Traurig LLP, counsel to the company. Each of Ms. Howard and Mr. Wishner’s beneficial
ownership includes 7,693 shares and 3,847 shares underlying Series Z Warrants (1,347 of which are offered hereby).
|
|
|
|
|
(20)
|
Londonderry
Capital LLC’s beneficial ownership includes 9,231 shares and 4,616 shares underlying Series Z Warrants (1,616 of which
are offered hereby).
|
|
|
|
|
(21)
|
Ms.
Masella’s beneficial ownership includes 2,308 shares and 2,308 shares underlying Series W Warrants (808 of which are
offered hereby).
|
|
|
|
|
(22)
|
Mr.
Salzbank’s beneficial ownership includes 3,077 shares and 1,539 shares underlying Series Z Warrants (539 of which are
offered hereby).
|
|
|
|
|
(23)
|
Ms.
Smith’s beneficial ownership includes 12,308 shares, 26,154 shares underlying Series Z Warrants (22,154 of which are
offered hereby) and 8,948 shares underlying Series B Preferred Stock.
|
|
|
|
|
(24)
|
Stewart
and Sons LLC’s beneficial ownership includes 30,001 shares and 15,001 shares underlying Series Z Warrants (5,251 of
which are offered hereby).
|
UPO
Selling Securityholders
|
|
|
Shares
|
|
|
Warrants
|
|
|
Selling
|
|
Beneficial
Ownership Before
|
|
|
Shares
Offered
|
|
|
Beneficial
Ownership After Offering
|
|
|
Beneficial
Ownership Before
|
|
|
Offered
|
|
|
Beneficial
Ownership
After Offering
|
|
|
Securityholder(1)
|
|
Offering
|
|
|
Hereby
|
|
|
Shares
|
|
|
Percent
|
|
|
Offering
|
|
|
Hereby
|
|
|
Number
|
|
|
Percent
|
|
|
Jamil Aboumeri
|
|
|
12,366
|
|
|
|
12,366
|
|
|
|
0
|
|
|
|
*
|
|
|
|
6,183
|
|
|
|
6,183
|
|
|
|
0
|
|
|
|
*
|
|
|
The Benchmark Company
|
|
|
31,800
|
|
|
|
31,800
|
|
|
|
0
|
|
|
|
*
|
|
|
|
15,900
|
|
|
|
15,900
|
|
|
|
0
|
|
|
|
*
|
|
|
Hilary Bergman
|
|
|
1,230
|
|
|
|
1,230
|
|
|
|
0
|
|
|
|
*
|
|
|
|
615
|
|
|
|
615
|
|
|
|
0
|
|
|
|
*
|
|
|
John Borer
|
|
|
12,366
|
|
|
|
12,366
|
|
|
|
0
|
|
|
|
*
|
|
|
|
6,183
|
|
|
|
6,183
|
|
|
|
0
|
|
|
|
*
|
|
|
Braden Carlsson
|
|
|
12,368
|
|
|
|
12,368
|
|
|
|
0
|
|
|
|
*
|
|
|
|
6,184
|
|
|
|
6,184
|
|
|
|
0
|
|
|
|
*
|
|
|
Ksenia Kolesnikov
|
|
|
1,766
|
|
|
|
1,766
|
|
|
|
0
|
|
|
|
*
|
|
|
|
883
|
|
|
|
883
|
|
|
|
0
|
|
|
|
*
|
|
|
Seth Moskowitz
|
|
|
15,334
|
|
|
|
15,334
|
|
|
|
0
|
|
|
|
*
|
|
|
|
7,667
|
|
|
|
7,667
|
|
|
|
0
|
|
|
|
*
|
|
|
Steven Shaffer
|
|
|
14,000
|
|
|
|
14,000
|
|
|
|
0
|
|
|
|
*
|
|
|
|
7,000
|
|
|
|
7,000
|
|
|
|
0
|
|
|
|
*
|
|
|
Robert Shapiro
|
|
|
4,770
|
|
|
|
4,770
|
|
|
|
0
|
|
|
|
*
|
|
|
|
2,385
|
|
|
|
2,385
|
|
|
|
0
|
|
|
|
*
|
|
|
*
|
Less
than 1%.
|
|
|
|
|
(1)
|
Each
of the selling securityholders is a broker-dealer or an affiliate of a broker-dealer. The shares beneficially owned by each
selling securityholder consist entirely of an equal amount of shares underlying UPOs and shares issuable upon exercise of
the Series Z Warrants underlying UPOs. The Series Z Warrants beneficially owned by each selling securityholder consist entirely
of Series Z Warrants underlying UPOs.
|
Preferred
Stock Financing Selling Securityholders
|
|
|
Beneficial
Ownership Before
|
|
|
Shares
|
|
|
Beneficial
Ownership
|
|
|
|
|
Offering
|
|
|
Offered
|
|
|
After
Offering
|
|
|
Selling
Securityholder
|
|
Shares
|
|
|
Hereby
|
|
|
Shares
|
|
|
Percent
|
|
|
David Adams, M.D.(1)
|
|
|
130,607
|
|
|
|
93,750
|
|
|
|
36,857
|
|
|
|
*
|
|
|
Eric Bland(2)
|
|
|
123,023
|
|
|
|
85,000
|
|
|
|
38,023
|
|
|
|
*
|
|
|
Stephen W. Boyce(3)
|
|
|
120,343
|
|
|
|
83,335
|
|
|
|
37,008
|
|
|
|
*
|
|
|
John Campeau, Jr.(4)
|
|
|
180,509
|
|
|
|
125,000
|
|
|
|
55,509
|
|
|
|
*
|
|
|
Dubreville Family Trust dtd 7/1/97(5)
|
|
|
506,732
|
|
|
|
350,000
|
|
|
|
156,732
|
|
|
|
*
|
|
|
Paige R. Dubreville(6)
|
|
|
50,674
|
|
|
|
35,000
|
|
|
|
15,674
|
|
|
|
*
|
|
|
Preston M. Dubreville(6)
|
|
|
50,674
|
|
|
|
35,000
|
|
|
|
15,674
|
|
|
|
*
|
|
|
Daniel Engelman(7)
|
|
|
120,343
|
|
|
|
83,335
|
|
|
|
37,008
|
|
|
|
*
|
|
|
Bradley Foster, M.D.(8)
|
|
|
120,343
|
|
|
|
83,335
|
|
|
|
37,008
|
|
|
|
*
|
|
|
Marc W. Gerdish(9)
|
|
|
681,670
|
|
|
|
83,335
|
|
|
|
598,335
|
|
|
|
1.7
|
%
|
|
Stephen Greenberg(10)
|
|
|
60,175
|
|
|
|
41,670
|
|
|
|
18,505
|
|
|
|
*
|
|
|
Edward S. Gutman(11)
|
|
|
28,883
|
|
|
|
20,000
|
|
|
|
8,883
|
|
|
|
*
|
|
|
Allen D. Hamdan(12)
|
|
|
60,175
|
|
|
|
41,670
|
|
|
|
18,505
|
|
|
|
*
|
|
|
C. Geoffrey Hampson(13)
|
|
|
30,089
|
|
|
|
20,835
|
|
|
|
9,254
|
|
|
|
*
|
|
|
HJJD Associates, L.P.(14)
|
|
|
80,406
|
|
|
|
62,500
|
|
|
|
17,906
|
|
|
|
*
|
|
|
LaGrossa Family Trust dtd 1/29/08(15)
|
|
|
180,977
|
|
|
|
125,000
|
|
|
|
55,977
|
|
|
|
*
|
|
|
Elisabeth Levin(16)
|
|
|
201,011
|
|
|
|
156,250
|
|
|
|
44,761
|
|
|
|
*
|
|
|
Lincoln Park Capital Fund, LLC(17)
|
|
|
353,248
|
|
|
|
125,000
|
|
|
|
228,248
|
|
|
|
*
|
|
|
Jacqueline Lindenbaum(18)
|
|
|
50,000
|
|
|
|
50,000
|
|
|
|
0
|
|
|
|
*
|
|
|
Arthur L. Loeb(19)
|
|
|
160,809
|
|
|
|
125,000
|
|
|
|
35,809
|
|
|
|
*
|
|
|
Krishna Nathan(20)
|
|
|
60,157
|
|
|
|
41,665
|
|
|
|
18,492
|
|
|
|
*
|
|
|
David Richer(21)
|
|
|
18,052
|
|
|
|
12,500
|
|
|
|
5,552
|
|
|
|
*
|
|
|
Mark A. Romney(22)
|
|
|
138,674
|
|
|
|
83,335
|
|
|
|
55,339
|
|
|
|
*
|
|
|
Rooney & Associates Communications
LLC Pension Plan(23)
|
|
|
120,654
|
|
|
|
83,335
|
|
|
|
37,319
|
|
|
|
*
|
|
|
S&S Borgardt Family Trust utd 8/22/07(24)
|
|
|
65,153
|
|
|
|
45,000
|
|
|
|
20,153
|
|
|
|
*
|
|
|
Doron Hay Saar(25)
|
|
|
361,952
|
|
|
|
250,000
|
|
|
|
111,952
|
|
|
|
*
|
|
|
Stephen Sadove(26)
|
|
|
677,795
|
|
|
|
83,335
|
|
|
|
594,460
|
|
|
|
1.7
|
%
|
|
William Schreier(27)
|
|
|
18,052
|
|
|
|
12,500
|
|
|
|
5,552
|
|
|
|
*
|
|
|
Stephen L. Schwartz(28)
|
|
|
28,948
|
|
|
|
20,000
|
|
|
|
8,948
|
|
|
|
*
|
|
|
The Ronald Shear Revocable Trust(29)
|
|
|
108,549
|
|
|
|
75,000
|
|
|
|
33,549
|
|
|
|
*
|
|
|
Paul Spence(30)
|
|
|
57,513
|
|
|
|
41,670
|
|
|
|
15,843
|
|
|
|
*
|
|
|
Justin Steele(31)
|
|
|
120,320
|
|
|
|
83,335
|
|
|
|
36,985
|
|
|
|
*
|
|
|
Trust U/W Carl M. Loeb FBO Jean L. Troubh(32)
|
|
|
80,406
|
|
|
|
62,500
|
|
|
|
17,906
|
|
|
|
*
|
|
|
*
|
Less
than 1%.
|
|
|
|
|
(1)
|
Dr.
Adams’ beneficial ownership includes 10,000 shares, 26,857 shares underlying Series B Preferred Stock and 93,750 shares
underlying Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(2)
|
Mr.
Bland’s beneficial ownership includes 38,023 shares underlying Series B Preferred Stock and 85,000 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(3)
|
Mr.
Boyce’s beneficial ownership includes 37,008 shares underlying Series B Preferred Stock and 83,335 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(4)
|
Mr.
Campeau’s beneficial ownership includes 55,509 shares underlying Series B Preferred Stock and 125,000 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(5)
|
The
Dubreville Family Trust’s beneficial ownership includes 156,732 shares underlying Series B Preferred Stock and 350,000
shares underlying Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(6)
|
Each
of Ms. Dubreville and Mr. Dubreville’s beneficial ownership includes 15,674 shares
underlying Series B Preferred Stock and 35,000 shares underlying Series Z Warrants (all
of which are offered hereby).
|
|
|
|
|
|
(7)
|
Mr.
Engelman’s beneficial ownership includes 37,008 shares underlying Series B Preferred Stock and 83,335 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(8)
|
Dr.
Foster’s beneficial ownership includes 37,008 shares underlying Series B Preferred Stock and 83,335 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
(9)
|
Mr.
Gerdish’s beneficial ownership includes 561,302 shares, 37,008 shares underlying Series B Preferred Stock and 83,360
shares underlying Series Z Warrants (83,335 of which are offered hereby).
|
|
|
|
|
(10)
|
Mr.
Greenberg’s beneficial ownership includes 18,505 shares underlying Series B Preferred Stock and 41,670 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(11)
|
Mr.
Gutman’s beneficial ownership includes 8,883 shares underlying Series B Preferred Stock and 20,000 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(12)
|
Mr.
Hamdan’s beneficial ownership includes 18,505 shares underlying Series B Preferred Stock and 41,670 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(13)
|
Mr.
Hampson’s beneficial ownership includes 9,254 shares underlying Series B Preferred Stock and 20,835 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(14)
|
HJJD
Associates, L.P.’s beneficial ownership includes 17,906 shares underlying Series B Preferred Stock and 62,500 shares
underlying Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(15)
|
LaGrossa
Family Trust’s beneficial ownership includes 55,977 shares underlying Series B Preferred Stock and 125,000 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(16)
|
Ms.
Levin’s beneficial ownership includes 44,761 shares underlying Series B Preferred Stock and 156,250 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(17)
|
Lincoln
Park Capital Fund, LLC’s beneficial ownership includes 2,484 shares underlying Series B Preferred Stock and 350,764
shares underlying Series Z Warrants (125,000 of which are offered hereby).
|
|
|
|
|
(18)
|
Ms.
Lindenbaum’s beneficial ownership includes 50,000 shares underlying Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(19)
|
Mr.
Loeb’s beneficial ownership includes 35,809 shares underlying Series B Preferred Stock and 125,000 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(20)
|
Mr.
Nathan’s beneficial ownership includes 18,492 shares underlying Series B Preferred Stock and 41,665 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(21)
|
Mr.
Richer’s beneficial ownership includes 5,552 shares underlying Series B Preferred Stock and 12,500 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(22)
|
Mr.
Romney’s beneficial ownership includes 16,000 shares, 37,339 shares underlying Series B Preferred Stock and 85,335 shares
underlying Series Z Warrants (83,335 of which are offered hereby).
|
|
|
|
|
(23)
|
Rooney
& Associates Communications LLC Pension Plan’s beneficial ownership includes 37,319 shares underlying Series B Preferred
Stock and 83,335 shares underlying Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(24)
|
S&S
Borgardt Family Trust’s beneficial ownership includes 20,153 shares underlying Series B Preferred Stock and 45,000 shares
underlying Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(25)
|
Mr.
Hay Saar’s beneficial ownership includes 111,952 shares underlying Series B Preferred Stock and 250,000 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(26)
|
Mr.
Sadove’s beneficial ownership includes 557,452 shares, 37,008 shares underlying Series B Preferred Stock and 83,335
shares underlying Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(27)
|
Mr.
Schreier’s beneficial ownership includes 5,552 shares underlying Series B Preferred Stock and 12,500 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(28)
|
Mr.
Schwartz’s beneficial ownership includes 8,948 shares underlying Series B Preferred Stock and 20,000 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(29)
|
The
Ronald Shear Revocable Trust’s beneficial ownership includes 33,549 shares underlying Series B Preferred Stock and 75,000
shares underlying Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(30)
|
Mr.
Spence’s beneficial ownership includes 13,943 shares and 43,570 shares underlying Series Z Warrants (41,670 of which
are offered hereby).
|
|
|
|
|
(31)
|
Mr.
Steele’s beneficial ownership includes 36,985 shares underlying Series B Preferred Stock and 83,335 shares underlying
Series Z Warrants (all of which are offered hereby).
|
|
|
|
|
(32)
|
The
Trust’s beneficial ownership includes 17,906 shares underlying Series B Preferred
Stock and 62,500 shares underlying Series Z Warrants (all of which are offered hereby).
|
Debt
Financing Selling Securityholders
|
|
|
Beneficial
Ownership Before
|
|
|
Shares
|
|
|
Beneficial
Ownership
|
|
|
|
|
Offering
|
|
|
Offered
|
|
|
After
Offering
|
|
|
Selling
Securityholder
|
|
Shares
|
|
|
Hereby
|
|
|
Shares
|
|
|
Percent
|
|
|
The Boomer Fund, L.P.(1)
|
|
|
1,198,000
|
|
|
|
266,000
|
|
|
|
932,000
|
|
|
|
2.6
|
%
|
|
David Broser(2)
|
|
|
532,000
|
|
|
|
532,000
|
|
|
|
0
|
|
|
|
*
|
|
|
2003 Hochman Family LLC(3)
|
|
|
86,539
|
|
|
|
26,539
|
|
|
|
60,000
|
|
|
|
*
|
|
|
Hochman Family Partnership(4)
|
|
|
86,539
|
|
|
|
26,539
|
|
|
|
60,000
|
|
|
|
*
|
|
|
Carol Hochman(5)
|
|
|
53,270
|
|
|
|
13,270
|
|
|
|
40,000
|
|
|
|
*
|
|
|
Jason Hochman(6)
|
|
|
27,962
|
|
|
|
7,962
|
|
|
|
20,000
|
|
|
|
*
|
|
|
Nathaniel Hochman(7)
|
|
|
27,962
|
|
|
|
7,962
|
|
|
|
20,000
|
|
|
|
*
|
|
|
Richard & Carol Hochman(8)
|
|
|
139,808
|
|
|
|
39,808
|
|
|
|
100,000
|
|
|
|
*
|
|
|
Jeremy Mindich(2)
|
|
|
532,000
|
|
|
|
532,000
|
|
|
|
0
|
|
|
|
*
|
|
|
Matthew Sirovich(1)
|
|
|
3,197,127
|
|
|
|
1,207,640
|
|
|
|
3,188,870
|
|
|
|
8.7
|
%
|
|
*
|
Less
than 1%.
|
|
|
|
|
(1)
|
Mr.
Sirovich’s beneficial ownership includes (i) 8,257 shares issued upon the exercise of Series S Warrants (all of which
are offered hereby) held by him, (ii) 458,257 additional shares held by him, (iii) 458,257 shares underlying Series Z
Warrants held by him, (iv) 1,414,904 shares held by The Sirovich Family Charitable Foundation, an entity controlled by
Mr. Sirovich, and (v) 857,452 shares underlying Series Z Warrants held by The Sirovich Family Charitable Foundation.
Although Mr. Sirovich also is offering for resale by this prospectus 1,199,383 shares underlying unexercised Series S
Warrants, such shares are not included in his beneficial ownership as of the date hereof, because the Series S Warrants
cannot be exercised to the extent Mr. Sirovich would beneficially own greater than 4.75% of our outstanding common stock
following such exercise. The Boomer Fund, L.P. is controlled by Matthew Sirovich, as general partner. Accordingly, Mr.
Sirovich may be deemed to beneficially own the shares of common stock and warrants held by such entity. The Boomer Fund,
L.P.’s beneficial ownership includes 266,000 shares issued upon the exercise of Series S Warrants (all of which are
offered hereby), 466,000 additional shares and 466,000 shares underlying Series Z Warrants.
|
|
|
|
|
(2)
|
The
selling securityholders’ beneficial ownership consists entirely of shares issued upon the exercise of Series S Warrants
(all of which are offered hereby).
|
|
|
|
|
(3)
|
The
2003 Hochman Family LLC is controlled by Richard Hochman, as a member. Accordingly, Mr. Hochman may be deemed to beneficially
own the shares of common stock held by such entity. The 2003 Hochman Family LLC’s beneficial ownership includes 26,539
shares issued upon the exercise of Series S Warrants (all of which are offered hereby), 30,000 additional shares and 30,000
shares underlying Series Z Warrants.
|
|
|
|
|
(4)
|
The
Hochman Family Partnership is controlled by Richard Hochman, as general partner. Accordingly, Mr. Hochman may be deemed to
beneficially own the shares of common stock held by such entity. The Hochman Family Partnership’s beneficial ownership
includes 26,539 shares issued upon the exercise of Series S Warrants (all of which are offered hereby), 30,000 additional
shares and 30,000 shares underlying Series Z Warrants.
|
|
|
|
|
(5)
|
Ms.
Hochman’s beneficial ownership includes 13,270 shares issued upon the exercise of Series S Warrants (all of which are
offered hereby), 20,000 additional shares and 20,000 shares underlying Series Z Warrants.
|
|
|
|
|
(6)
|
Mr.
Jason Hochman’s beneficial ownership includes 7,962 shares issued upon the exercise of Series S Warrants (all of which
are offered hereby), 10,000 additional shares and 10,000 shares underlying Series Z Warrants.
|
|
|
|
|
(7)
|
Mr.
Nathaniel Hochman’s beneficial ownership includes 7,962 shares issued upon the exercise of Series S Warrants (all of
which are offered hereby), 10,000 additional shares and 10,000 shares underlying Series Z Warrants.
|
|
|
|
|
(8)
|
Richard
& Carol Hochman’s beneficial ownership includes 39,808 shares issued upon the exercise of Series S Warrants (all
of which are offered hereby), 50,000 additional shares and 50,000 shares underlying Series Z Warrants
|
PLAN
OF DISTRIBUTION
We
are registering 10,401,504 shares of common stock issued or issuable upon exercise of UPOs, Series S Warrants, Series W Warrants
and Series Z Warrants (including the Series Z Warrants underlying the UPOs) and 53,000 Series Z Warrants issuable upon exercise
of the UPOs to permit the resale of those securities from time to time after the date of this prospectus by the selling securityholders.
The term “selling securityholders,” as used herein, includes any pledgee, assignee or successor-in-interest to any
selling securityholder listed herein selling such shares or interests in such shares received after the date of this prospectus
from a selling securityholder as a pledge, gift, partnership distribution or other transfer.
The
selling securityholders may, from time to time, sell, transfer or otherwise dispose of any or all of their securities or interests
in such securities on any stock exchange, market or trading facility on which the securities are traded or in private transactions.
The securities may be offered by the selling securityholders at fixed prices, at prevailing market prices at the time of sale,
at prices related to the prevailing market price, at varying prices determined at the time of sale, or at negotiated prices.
The
selling securityholders may use any one or more of the following methods when disposing of the securities or interests therein:
|
|
●
|
on
any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale;
|
|
|
|
|
|
|
●
|
ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
|
|
|
|
|
●
|
in
the over the counter market;
|
|
|
|
|
|
|
●
|
block
trades in which the broker-dealer will attempt to sell the securities as agent, but may position and resell a portion of the
block as principal to facilitate the transaction;
|
|
|
|
|
|
|
●
|
purchases
by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
|
|
|
|
|
●
|
an
exchange distribution or other future transaction exchange-related transaction in accordance with the rules of the applicable
exchange or exchange-related transaction;
|
|
|
|
|
|
|
●
|
privately
negotiated transactions;
|
|
|
|
|
|
|
●
|
short
sales;
|
|
|
|
|
|
|
●
|
through
the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise;
|
|
|
|
|
|
|
●
|
broker-dealers
may agree with the selling securityholders to sell a specified number of such securities at a stipulated price per share;
|
|
|
|
|
|
|
●
|
a
combination of any such methods of sale; and
|
|
|
|
|
|
|
●
|
any
other method permitted pursuant to applicable law.
|
The
selling securityholders may, from time to time, pledge or grant a security interest in some or all of the securities owned by
them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell
the pledged securities, from time to time, under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3)
or other applicable provision of the Securities Act amending the list of selling securityholders to include the pledgee, transferee
or other successors in interest as selling securityholders under this prospectus. The selling securityholders also may transfer
the securities in other circumstances, in which case the pledgees, assignees or other successors-in-interest will be the selling
beneficial owners for purposes of this prospectus; provided, however, that prior to any such transfer the following information
(or such other information as may be required by the federal securities laws from time to time) with respect to each such selling
beneficial owner must be added to the prospectus by way of a prospectus supplement or post-effective amendment, as appropriate:
(1) the name of the beneficial owner; (2) any material relationship the beneficial owner has had within the past three years with
us or any of our predecessors or affiliates; (3) the amount of securities owned by the beneficial owner before the offering; (4)
the amount to be offered for the beneficial owner’s account; and (5) the amount and (if one percent or more) the percentage
of securities to be owned by the beneficial owner after the offering is complete.
In
connection with the sale of our securities or interests therein, the selling securityholders may enter into hedging transactions
with broker-dealers or other financial institutions, which may in turn engage in short sales of the securities in the course of
hedging the positions they assume. The selling securityholders may also sell securities short and deliver these securities to
close out their short positions, or loan or pledge securities to broker-dealers that in turn may sell these securities. The selling
securityholders may also enter into option or other transactions with broker-dealers or other financial institutions or the creation
of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of securities
offered by this prospectus, which securities such broker-dealer or other financial institution may resell pursuant to this prospectus
(as supplemented or amended to reflect such transaction).
The
aggregate proceeds to the selling securityholders from the sale of the securities offered by them will be the purchase price of
the securities less discounts or commissions, if any. Each of the selling securityholders reserves the right to accept and, together
with their agents from time to time, to reject, in whole or in part, any proposed purchase of securities to be made directly or
through agents. We will not receive any of the proceeds from the sale or other disposition of the securities by the selling securityholders.
However, we will receive up to approximately $13,481,987 in gross proceeds upon the cash exercise of the UPOs, Series S Warrants,
Series W Warrants and Series Z Warrants covering certain of the securities offered for resale, and we will receive up to approximately
$1,319,460 in gross proceeds upon the cash exercise of the publicly held Series W Warrants and Series Z Warrants.
The
selling securityholders also may resell all or a portion of the securities in open market transactions in reliance upon Rule 144
under the Securities Act of 1933, provided that they meet the criteria and conform to the requirements of that rule.
The
selling securityholders and any underwriters, broker-dealers or agents that participate in the sale of the securities or interests
therein may be “underwriters” within the meaning of Section 2(11) of the Securities Act. Any discounts, commissions,
concessions or profit they earn on any resale of the securities may be underwriting discounts and commissions under the Securities
Act. Selling securityholders who are “underwriters” within the meaning of Section 2(11) of the Securities Act will
be subject to the prospectus delivery requirements of the Securities Act.
To
the extent required, the securities to be sold, the names of the selling securityholders, the respective purchase prices and public
offering prices, the names of any agents, dealer or underwriter, any applicable commissions or discounts with respect to a particular
offer will be set forth in an accompanying prospectus supplement or, if appropriate, a post-effective amendment to the Registration
Statement.
The
maximum amount of compensation to be received by any FINRA member or independent broker-dealer for the sale of any securities
registered under this prospectus will not be greater than 8.0% of the gross proceeds from the sale of such securities.
In
order to comply with the securities laws of some states, if applicable, the securities may be sold in these jurisdictions only
through registered or licensed brokers or dealers. In addition, the securities may not be sold unless they have been registered
or qualified for sale under the applicable state securities laws, or an exemption from registration or qualification requirements
is available and is complied with, or registration or qualification is otherwise not required.
We
have advised the selling securityholders that the anti-manipulation rules of Regulation M under the Exchange Act may apply to
sales of securities in the market and to the activities of the selling securityholders and their affiliates. The selling securityholders
may indemnify any broker-dealer that participates in transactions involving the sale of the securities against certain liabilities,
including liabilities arising under the Securities Act.
We
are also registering for initial issuance 7,868,039 shares of common stock underlying Series Z Warrants and 381,818 shares of
common stock underlying Series W Warrants.
Pursuant to the terms of the UPOs, Series S Warrants, Series W Warrants and Series Z Warrants, the securities to be issued upon
exercise of such options and warrants will be distributed only to those warrant holders who duly provide notice of exercise and,
unless exercised on a cashless basis, provide payment of the exercise price, all in accordance with the applicable agreement governing
such options and warrants. The applicable option and warrant agreements are included or incorporated by reference as exhibits
to the Registration Statement.
LEGAL
MATTERS
The
legality of the common stock offered by this prospectus has been passed upon by Graubard Miller, New York, New York. Graubard
Miller and its partners own warrants to purchase shares of our common stock, which represent, in the aggregate, beneficial ownership
of less than 1% of our common stock.
EXPERTS
The
consolidated financial statements of PAVmed Inc. and Subsidiary as of and for the years ended December 31, 2017 and 2016, which
are incorporated in this prospectus by reference to the Annual Report on Form 10-K, as amended, for the year ended December 31,
2017, have been so incorporated in reliance on the report (which contains an explanatory paragraph relating to the Company’s
ability to continue as a going concern as described in Note 1 to the Consolidated Financial Statements) of Citrin Cooperman &
Company, LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and
accounting.
WHERE
YOU CAN FIND MORE INFORMATION
We
file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available
to the public over the Internet at the SEC’s web site at http://www.sec.gov. You may also read and copy any document we
file with the SEC at the SEC’s public reference room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC
at 1-800-SEC-0330 for further information about the public reference room.
The
SEC allows us to incorporate by reference the information we file with it, which means that we can disclose important information
to you by referring you to those documents. The information incorporated by reference is an important part of this prospectus,
and information that we file later with the SEC will automatically update and supersede this information. This prospectus incorporates
by reference the documents listed below, all filings we make under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after
the initial filing date of the Registration Statement and prior to effectiveness of the Registration Statement, and all filings
we make with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after effectiveness of the Registration Statement
and prior to the sale of all of the securities offered hereby:
|
|
●
|
our
annual report on Form 10-K for the fiscal year ended December 31, 2017 filed with the SEC on March 14, 2018 as amended by
Amendment No. 1 to our annual report on Form 10-K/A filed on April 30, 2018;
|
|
|
|
|
|
|
●
|
our
quarterly reports on Form 10-Q for the fiscal quarter ended March 31, 2018 filed with the SEC on May 21, 2018 and for the
fiscal quarter ended June 30, 2018 filed with the SEC on August 13, 2018;
|
|
|
|
|
|
|
●
|
our
current reports on Form 8-K filed with the SEC on January 19, 2018, January 23, 2018,
January 25, 2018, February 12, 2018, February 16, 2018, March 9, 2018, April 5, 2018,
April 16, 2018 (as amended on April 20, 2018), May 15, 2018, May 17, 2018, June 8, 2018,
June 12, 2018, June 27, 2018 and October 2, 2018;
|
|
|
|
|
|
|
●
|
our
proxy statement on Schedule 14A filed with the SEC on August 24, 2018; and
|
|
|
|
|
|
|
●
|
our
registration statement on Form 8-A filed on January 28, 2016, registering our common stock and Series W Warrants under Section
12(b) of the Exchange Act, and our registration statement on Form 8-A filed on April 5, 2018, registering our Series Z Warrants
under Section 12(b) of the Exchange Act.
|
Any
statement contained in a document filed before the date of this prospectus and incorporated by reference herein shall be deemed
to be modified or superseded for purposes of this prospectus to the extent that a statement contained herein modifies or supersedes
such statement. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute
a part of this prospectus. Any information that we file after the date of this prospectus with the SEC will automatically update
and supersede the information contained in this prospectus. Notwithstanding the foregoing, we are not incorporating any document
or portion thereof or information deemed to have been furnished and not filed in accordance with SEC rule.
We
will provide you with a copy of any or all of the information that has been incorporated by reference in this prospectus, without
charge, upon written or oral request directed to PAVmed Inc., One Grand Central Place, Suite 4600, New York, New York 10165, telephone
number (212) 949-4319.
PAVmed (NASDAQ:PAVM)
Historical Stock Chart
From Mar 2024 to Apr 2024
PAVmed (NASDAQ:PAVM)
Historical Stock Chart
From Apr 2023 to Apr 2024