Filed Pursuant to Rule 424(b)(3)
Registration No. 333-221783
Prospectus

APTOSE BIOSCIENCES INC.
6,041,567 Common Shares
This prospectus
relates to the sale, from time to time, of up to 6,041,567 of our common shares by the selling shareholder, Aspire Capital Fund, LLC, an Illinois limited liability company, or “
Aspire Capital
.” Aspire Capital is also referred
to in this prospectus as the selling shareholder. The prices at which the selling shareholder may sell the shares will be determined by the prevailing market price for the shares or in negotiated transactions. We will not receive proceeds from the
sale of the shares by the selling shareholder. However, we may receive proceeds of up to $15,500,000 from the sale of our common shares to the selling shareholder, pursuant to a common shares purchase agreement entered into with the selling
shareholder on October 27, 2017 (referred to as the “
Purchase Agreement
”) once the registration statement, of which this prospectus is a part, is declared effective. Under the terms of the Purchase Agreement, on
October 31, 2017, Aspire Capital made an initial purchase of 357,143 common shares at a price of $1.40 per share, representing gross proceeds of approximately $500,000. As the date of this prospectus supplement, 2,162,995 common shares remain
available to be issued to Aspire Capital under the terms of the Purchase Agreement.
Aspire Capital is an
“
underwriter
” within the meaning of Section 2(a)(11) of the United States Securities Act of 1933, as amended, or the “
Securities Act
.” Aspire Capital may sell the common shares described in this
prospectus in a number of different ways and at varying prices. See “Plan of Distribution” for more information about how Aspire Capital may sell the common shares being registered pursuant to this prospectus. We will pay the expenses
incurred in registering the common shares to which this prospectus relates, including legal and accounting fees. See “Plan of Distribution.”
Our common shares are listed on Toronto Stock Exchange, which we refer to as the “TSX” under the symbol “APS” and the
Nasdaq Capital Market, which we refer to as “Nasdaq”, under the symbol “APTO.” On April 12, 2018, the last reported sale price per share of our common shares was CDN$4.17 per share on the TSX and $3.34 per share on Nasdaq.
You should read this prospectus and any prospectus supplement, together with additional information described under the headings
“Documents Incorporated by Reference” and “Where You Can Find More Information,” carefully before you invest in any of our securities.
Investing in our common shares involves a high degree of risk. See “
Risk Factors
” beginning on page
10.
Neither the United States Securities and Exchange Commission nor any state securities commission has approved or disapproved
of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this
prospectus is April 13, 2018.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is a part of a registration statement that we have filed with the SEC. Aspire Capital may sell the securities described in
this prospectus from time to time, in one or more offerings. This prospectus provides you with a general description of the securities that Aspire Capital may offer.
Before investing in our securities, please carefully read both this prospectus together with the documents incorporated by reference into this
prospectus, as listed under “Documents Incorporated by Reference,” and the additional information described below under “Where You Can Find More Information.”
Owning securities may subject you to tax consequences in the United States and in Canada. This prospectus or any applicable prospectus
supplement may not describe these tax consequences fully. You should read the tax discussion in any prospectus supplement with respect to a particular offering and consult your own tax advisor with respect to your own particular circumstances.
You should rely only on the information contained in or incorporated by reference into this prospectus or a prospectus supplement. We
have not authorized anyone to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. The distribution or possession of this prospectus in or from certain jurisdictions
may be restricted by law. This prospectus is not an offer to sell the securities and is not soliciting an offer to buy the securities in any jurisdiction where the offer or sale is not permitted or where the person making the offer or sale is not
qualified to do so or to any person to whom it is not permitted to make such offer or sale. You should assume that the information contained in this prospectus and in any applicable prospectus supplement is accurate only as of the date on the front
cover of this prospectus or prospectus supplement, as applicable, and the information incorporated by reference into this prospectus or any prospectus supplement is accurate only as of the date of the document incorporated by reference. Our
business, financial condition, results of operations and prospects may have changed since that date.
In this prospectus, unless the
context otherwise requires, references to “Aptose,” the “Company,” “we,” “us,” or “our” refer to Aptose Biosciences Inc. and its wholly owned subsidiaries through which it conducts its business.
FINANCIAL STATEMENTS AND EXCHANGE RATE INFORMATION
The consolidated financial statements incorporated by reference into this prospectus and the documents incorporated by reference into this
prospectus, and the financial data derived from those consolidated financial statements included in this prospectus, are presented in U.S. dollars, unless otherwise specified, and have been prepared in accordance with International Financial
Reporting Standards as issued by the International Accounting Standards Board, which we refer to as “
IFRS
”. References in this prospectus to “dollars”, “US$” or “$” are to United States dollars.
Canadian dollars are indicated by the symbol “Cdn$”.
The following table lists, for each period presented, the high and low
exchange rates, the average of the exchange rates during the period indicated, and the exchange rates at the end of the period indicated, for one Canadian dollar, expressed in United States dollars. For the 2014, 2015, and 2016 years, the exchange
rate is based on the noon exchange rate published by the Bank of Canada. For the 2017 year, the exchange rate is based on the daily average exchange rate published by the Bank of Canada.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31,
|
|
|
|
|
2017
|
|
|
2016
|
|
|
2015
|
|
|
2014
|
|
|
High for the period
|
|
$
|
0.8245
|
|
|
$
|
0.7977
|
|
|
$
|
0.8511
|
|
|
$
|
0.9399
|
|
|
Low for the period
|
|
$
|
0.7276
|
|
|
$
|
0.6869
|
|
|
$
|
0.7161
|
|
|
$
|
0.8579
|
|
|
End of period
|
|
$
|
0.7971
|
|
|
$
|
0.7448
|
|
|
$
|
0.7225
|
|
|
$
|
0.8620
|
|
|
Average for the period
|
|
$
|
0.7708
|
|
|
$
|
0.7550
|
|
|
$
|
0.7821
|
|
|
$
|
0.9053
|
|
On April 12, 2018, the Bank of Canada’s daily average exchange rate was Cdn$1.00 = $0.7939.
1
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement under the Securities Act, with respect to the resale by the selling shareholder of our
common shares pursuant to this prospectus. This prospectus, which is a part of the registration statement, does not contain all of the information contained in the registration statement or the exhibits and schedules to the registration statement,
and you should refer to the complete registration statement. Statements made in this prospectus concerning the contents of any contract, agreement or other document filed as an exhibit to the registration statement are summaries of all of the
material terms of such contracts, agreements or documents, but do not repeat all of their terms. Reference is made to each such exhibit for a more complete description of the matters involved and the summary statements are qualified in their
entirety by reference to the complete document filed as an exhibit. The registration statement and its exhibits, and the reports and other information we have filed with the SEC under the Exchange Act, may be inspected and copied by the public at
the public reference facilities maintained by the SEC Our filings are also available from commercial document retrieval services.
We are
subject to the information reporting requirements of the United States Securities Exchange Act of 1934, which we refer to as the “
Exchange Act
”, and we therefore file reports and other information with the SEC through its
Electronic Document Gathering Retrieval System, which is commonly known by the acronym EDGAR and may be accessed at www.sec.gov. The reports and other information filed by us with the SEC may be read and copied at the SEC’s public reference
room at 100 F Street, N.E., Washington, D.C. 20549. Copies of the same documents can also be obtained from the public reference room of the SEC in Washington by paying a fee. Please call the SEC at
1-800-SEC-0330
for further information on the public reference room. In addition, we are subject to continuous disclosure obligations under Canadian securities laws.
Therefore, we file disclosure documents, reports, statements and other information with the securities commissions or similar regulatory authorities in Canada. We make our filings on the Canadian System for Electronic Document Analysis and
Retrieval, which is commonly known by the acronym SEDAR and which may be accessed at www.sedar.com. SEDAR is the Canadian equivalent of EDGAR. In addition, our documents may be viewed at our head office located at 5955 Airport Road Suite #228,
Mississauga, Ontario, Canada, L4V 1R9.
As a “foreign private issuer” under the Exchange Act, we are exempt from the rules under
the Exchange Act prescribing the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and
“short-swing
profit” recovery
provisions contained in Section 16 of the Exchange Act. In addition, we are not required to publish financial statements as promptly as United States companies.
ENFORCEMENT OF CIVIL LIABILITIES
The enforcement by investors of civil liabilities under U.S. federal securities laws may be affected adversely by the fact that we are
incorporated under the laws of Canada, that many of our directors are residents of countries other than the United States, that some of the experts named in this prospectus are residents of countries other than the United States, and that some of
our assets and the assets of said persons are located outside the United States.
In particular, it may be difficult to bring and enforce
suits against us or said persons under U.S. federal securities laws. It may be difficult for U.S. holders of our common shares to effect service of process on us or said persons within the United States or to enforce judgments obtained in the United
States based on the civil liability provisions of the U.S. federal securities laws against us or said persons. In addition, a shareholder should not assume that the courts of Canada (i) would enforce judgments of U.S. courts obtained in actions
against us, our officers or directors, or other said persons, predicated upon the civil liability provisions of the U.S. federal securities laws or other laws of the United States, or (ii) would enforce, in original actions, liabilities against
us, our officers or directors or other said persons predicated upon the U.S. federal securities laws or other laws of the United States.
2
DOCUMENTS INCORPORATED BY REFERENCE
The SEC allows us to “incorporate by reference” the information we have filed with the SEC. This means that we can disclose
important information by referring you to another document filed separately with the SEC. The information incorporated by reference is considered to be a part of this prospectus and information that we file later with the SEC will also be deemed to
be incorporated by reference into this prospectus and be part hereof from the date of filing of such documents and will automatically update and supersede previously filed information, including information contained in this document. The following
documents filed with or furnished to the SEC are specifically incorporated by reference into, and form a part of, this prospectus:
|
|
•
|
|
our Annual Report on Form
40-F
for the fiscal year ended December 31, 2017, filed on March 27, 2018;
|
|
|
•
|
|
the description of our common shares set forth in our registration statement on Form
8-A,
filed on October 21, 2014 (which incorporates by reference the description of our
common shares included in our Annual Report on Form
20-F
for the fiscal year ended May 31, 2014, filed on July 30, 2014); and
|
|
|
•
|
|
all other documents filed by us under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, after the date of this prospectus but before the end of the offering of the securities pursuant to this prospectus.
|
In addition, all subsequent Annual Reports on Form
20-F,
Form
40-F,
or Form
10-K,
and all subsequent filings on Form
10-Q
or Form
8-K,
that we file pursuant
to the Exchange Act prior to the termination of this offering, are hereby incorporated by reference into this prospectus. Also, we may incorporate by reference future reports on Form
6-K
that we furnish
subsequent to the date of this prospectus by stating in those Form
6-Ks
that they are being incorporated by reference into this prospectus.
Any statement contained in a document incorporated by reference into this prospectus shall be deemed to be modified or superseded for purposes
of this prospectus to the extent that a statement contained in this prospectus, in one of those other documents or in any other later filed document that is also incorporated by reference into this prospectus modifies or supersedes that statement.
Any such statement so modified shall not be deemed, except as so modified, to constitute a part of this prospectus. Any such statement so superseded shall be deemed not to constitute a part of this prospectus.
Any person receiving a copy of this prospectus, including any beneficial owner, may obtain without charge, upon written or oral request, a
copy of any of the documents incorporated by reference into this prospectus, except for the exhibits to those documents unless the exhibits are specifically incorporated by reference into those documents. Requests should be directed to our principal
executive offices, 5955 Airport Road, Suite #228, Mississauga, Ontario, Canada L4V 1R9, Attention: Chief Financial Officer (telephone: (647)
479-9829).
3
PROSPECTUS SUMMARY
This summary highlights certain information about us, this offering and selected information contained in the prospectus. This summary is
not complete and does not contain all of the information that you should consider before deciding whether to invest in our common shares. For a more complete understanding of our company and this offering, we encourage you to read and consider the
more detailed information in the prospectus and the documents incorporated by reference into this prospectus, including “Risk Factors” and the financial statements and related notes. Unless we specify otherwise, all references in this
prospectus to “Aptose,” “we,” “our,” “us” and “our company” refer to Aptose Biosciences, Inc. and the subsidiaries through which it conducts its business.
Company Overview
Aptose is a
science-driven biotechnology company advancing highly differentiated agents to treat unmet medical needs in life-threatening cancers, such as acute myeloid leukemia, or “AML”, high-risk myelodysplastic syndromes, or “MDS”, and
other hematologic malignancies. Based on insights into the genetic and epigenetic profiles of certain cancers and patient populations, we are building a pipeline of novel and targeted oncology therapies directed at dysregulated processes and
signaling pathways in cancer cells, and this strategy is intended to optimize efficacy and quality of life by minimizing the cytotoxic side effects associated with conventional therapies and minimize the emergence of drug resistance. Our product
pipeline includes cancer drug candidates that exert potent activity as stand-alone agents and that enhance the activities of other anticancer agents without causing overlapping toxicities.
We believe the future of cancer treatment and management lies in the prospective selection and treatment of patients having genotype-specific
malignancies that are genetically or epigenetically predisposed to response based on a drug’s unique mechanism of action. We are of the view that many drugs currently approved for the treatment and management of cancer are not selective for the
specific genetic alterations (targets) that cause the patient’s tumor and hence lead to significant toxicities due to
off-target
effects. Aptose’s strategy is to develop agents that target underlying
disease-promoting mutations or altered pathways within a patient population, and we intend to apply this strategy across several therapeutic indications in oncology, including hematologic malignancies and solid tumor indications, particularly those
cancers that offer orphan drug designation opportunities.
We have a clinical-stage program, a late preclinical stage program, and a third
program that is discovery-stage and positioned for partnering. Aptose’s
pan-FLT3
/ BTK program, CG’806, is currently in preclinical development and moving toward Investigational New Drug, or
“
IND
” submission, with anticipation of commencing a Phase 1 trial during 2018.
APTO-253
is our second program and at the Phase 1b clinical stage for the treatment of patients with
relapsed / refractory blood cancers, including AML and high-risk MDS, under an IND allowed by the United States Food and Drug Administration to evaluate
APTO-253
as a therapeutic agent dosed on a weekly
administration schedule for the treatment of certain hematologic malignancies. The
APTO-253
program has received orphan drug designation from the FDA for the treatment of AML, and is currently on clinical hold
while attempts are made to manufacture a newly formulated and stable clinical supply.
As noted above, we are committed to the development
of anticancer drugs that target aberrant oncologic signaling processes that underlie a particular life-threatening malignancy. This targeted approach is intended to impact the disease-causing events in cancer cells without affecting normal processes
within cells. Such an approach requires that we first identify critical underlying oncogenic mechanisms in cancer cells and then develop a therapeutic that selectively impacts such oncogenic mechanisms. As a multi-kinase
pan-FLT3
/
pan-BTK
inhibitor, CG’806 targets multiple critical pathways that overlap to lead to the proliferation of cancer cells, including the
B-cell
receptor signaling pathways (drive certain B cell malignancies) and FLT3 receptor pathways (drive AML) that converge at various points in the signaling cascade. Further, we created the
4
APTO-253
small molecule targeted drug that inhibits expression of the
c-Myc
oncogene and is under development as a
novel therapy for AML and the related MDS.
In June 2016, we announced an exclusive global option and license agreement focused on the
development of CG’806. CG’806 is a highly potent
first-in-class
pan-FLT3 /
pan-BTK
inhibitor. This small molecule therapeutic agent, exhibits a picomolar IC50 toward the
FMS-like
tyrosine kinase 3 with the Internal Tandem Duplication
(FLT3-ITD)
mutation and significant potency against other mutant forms of FLT3 and the wild type form of FLT3.
Because of the potency of CG’806 against the FLT3 enzyme, it may become an effective therapy for AML patients, including the subset of
patients having the
FLT3-ITD,
which occurs in approximately 30% of patients with AML and is associated with poor prognosis. Importantly, CG’806 targets the wild type and all mutant types of FLT3, as well
as other oncogenic kinases which are operative in AML. Consequently, CG’806 has potential to treat a broad range of AML patients, including the
difficult-to-treat
genotype-specific AML patient population having altered FLT3.
In addition to potent inhibition of wild type and mutant forms of the FLT3
enzyme, CG’806 is a highly potent, reversible,
non-covalent
inhibitor of the wild type and mutant forms of the BTK enzymes. Overexpression of BTK drives certain B cell malignancies, including chronic
lymphocytic leukemia, or “CLL”, mantle cell lymphoma, or “MCL”, diffuse large B cell lymphoma, or “DLBCL”, and others. Treatment of such B cell malignancies with covalent BTK inhibitors that target the cysteine residue
in the active site of BTK can lead to drug resistance via mutation of the cysteine amino acid residue to a serine residue
(BTK-C481S
mutant). CG’806 targets the
ATP-binding
pocket of BTK through a reversible,
non-covalent
mechanism, thereby allowing CG’806 to retain low nM potency against the
BTK-C481S
mutant enzyme. In addition, CG’806 inhibits other kinase driven oncogenic pathways (BTK, ERK, AURK, FLT3 and others) on which the malignant B cells depend. Thus, CG’806 may serve as a novel
therapeutic agent to treat B cell malignancy patients that are refractory, resistant or intolerant to covalent BTK inhibitors.
APTO-253,
our second therapeutic program, is a small molecule therapeutic agent that inhibits expression of the
c-Myc
oncogene without causing general myelosuppression of the
healthy bone marrow, and
APTO-253
is currently allowed for development to treat AML in a Phase Ib trial under an IND with the FDA. The
c-Myc
oncogene is overexpressed in
hematologic cancers, including AML.
C-Myc
is a transcription factor that regulates cell growth, proliferation, differentiation and apoptosis, and overexpression amplifies new sets of genes to promote
oncogenesis.
APTO-253
dramatically down-regulates expression of the
c-Myc
oncogene in AML cells and depletes those cells of the
c-Myc
oncoprotein, leading to apoptotic cell death in AML cells. Thus,
APTO-253
may serve as safe and effective
c-Myc
inhibitor
for AML that combines well with other agents and does not impact the normal bone marrow.
Corporate Information
Our principal office is located at 5955 Airport Road, Suite 228, Mississauga, Ontario, Canada, L4V 1R9 and our phone number is (647)
479-9828.
We maintain a website at www.aptose.com where general information about us is available. Investors can obtain copies of our filings with the Securities and Exchange Commission, or SEC, from this site free
of charge, as well as from the SEC website at www.sec.gov. The contents of our website are not incorporated by reference into this prospectus.
Recent
Developments
CG’806
We have
invested significant time, effort and capital to create a scalable synthetic route for the manufacture of CG’806 drug substance, to develop an oral formulation for clinical development, and to study the actions of
5
CG’806 in various preclinical biological pathway studies. We now have solved the synthetic route, have manufactured okg levels of API, and have initiated
in-life
preclinical pharmacokinetic, Dose Range Finding Studies and toxicology studies. Likewise, we also reported that we selected the oral formulation that we intend to take into
first-in-human
clinical trials.
Provided the studies continue on the anticipated timeline, we expect to initiate a
first-in-human
clinical trial during 2018, and greater granularity on the timing of the IND submission and clinical trial will be provided in the coming months. CG’806 is being developed with the intent
to deliver the agent as an oral therapeutic and to develop it in parallel for AML and for appropriate B cell malignancies (likely CLL).
On May 7, 2017, we presented preclinical data for our
pan-FLT3/pan-BTK
inhibitor CG’806 at the 2017 American Association for Cancer Research, or “AACR”, Conference for Hematologic Malignancies: Translating
Discoveries to Novel Therapies in Boston, MA. Two separate presentations highlighting CG’806 were presented. In one presentation, our scientists, with researchers from the Knight Cancer Institute at Oregon Health & Science University,
or “OHSU”, presented data relating to the potency of CG’806 against samples derived from patients with various hematologic malignancies. In a separate presentation, our scientists, with researchers from the MD Anderson Cancer Center,
presented data demonstrating CG’806’s potent activity against AML cells harboring wild type or specific mutant forms of FLT3.
On August 4, 2017 we received a notice from the United States Patent and Trademark Office (the “
USPTO
”) stating
that our U.S. Patent Application is allowed for issuance as a patent. The allowed application claims numerous compounds, including the CG’806 compound, pharmaceutical compositions comprising the CG’806 compound, and methods of
treating various diseases caused by abnormal or uncontrolled activation of protein kinases. The notice of allowance is not a grant of patent rights and although it is uncommon, the USPTO can withdraw the allowed application from issuance.
On December 11, 2017 at the American Society of Hematology Annual Meeting, we presented with the OHSU Knight Cancer Institute preclinical
data demonstrating that CG’806, a
pan-FLT3/pan-BTK
inhibitor, has broad and potent drug activity against AML, CLL and other hematologic disease subtypes. We also
announced the presentation of preclinical data from research led by The University of Texas MD Anderson Cancer Center demonstrating that CG’806 exerts a profound anti-leukemia effect in human and murine leukemia cell lines harboring
FLT-3
ITD mutations, mutations that are usually associated with very poor prognoses in leukemia patients. In addition, CG’806 induces apoptosis, or programmed cell death, in AML patient samples by multiple
mechanisms and is able to overcome resistance that is seen with other FLT3 inhibitors. The data were highlighted in poster presentations on December 10 and 11, 2017 at the American Society of Hematology Annual Meeting.
On December 26, 2017, we announced that the FDA has granted orphan drug designation to CG’806 for the treatment of patients with
AML. Orphan drug designation is granted by the FDA to encourage companies to develop therapies for the treatment of diseases that affect fewer than 200,000 individuals in the United States. Orphan drug status provides research and development tax
credits, an opportunity to obtain grant funding, exemption from FDA application fees and other benefits. If CG’806 is approved to treat AML, the orphan drug designation provides Aptose with seven years of marketing exclusivity.
On March 15, 2018, we announced two abstracts related to the mechanistic properties of CG’806 in AML cells and in B cell malignancy
cells have been accepted for poster presentations at the upcoming 2018 Annual Meeting of the American Association for Cancer Research (AACR).
On March 28, 2018 we announced that we entered into an
“at-the-market”
sales agreement with Cantor Fitzgerald & Co (“
Cantor
”). Under the terms of the sales agreement, we may issue
and sell through Cantor, acting as sole agent, up to thirty million common shares of the company through
at-the-market
distributions.
6
APTO-253
Clinical Hold and Current Status
In 2016, our Phase Ib trial for
APTO-253
was placed on clinical hold in order to solve a
chemistry-based formulation issue, and the chemistry of the API and the formulation had undergone minor modifications to deliver a stable and soluble drug product for return to the clinical setting. In December 2016, we announced that we had
successfully manufactured multiple
non-GMP
batches of a new drug product formulation for
APTO-253,
including a batch that had been stable and soluble for over six
months. However, the 40L batch that was the intended clinical supply encountered an unanticipated mishap during the filling process that compromised the stability of that batch of drug product. On January 23, 2017, we announced that the root
cause and corrective action studies would take longer than originally expected and that we would temporarily delay clinical activities with
APTO-253
in order to elucidate the cause of manufacturing setback,
with the intention of restoring the molecule to a state supporting clinical development and partnering. Formal root cause analyses studies have now been completed and we have identified the mishap that resulted in drug product stability failure, and
we have established a corrective and prevention action plan for the manufacture of future batches of drug product that avoid the earlier mishap. Given these findings, we manufactured a new clinical batch of drug product, and studies required to
demonstrate fitness of the drug product for clinical usage are currently ongoing. We will then present the findings to the FDA with the hope of having the clinical hold removed and returning
APTO-253
to the
clinical trial. Although the Company expects the clinical supply to perform favorably and the clinical hold to be lifted during the first half of 2018, there can be no assurance that the FDA will remove the clinical hold.
Finally, on March 15, 2018, we announced that one abstract related to the mechanistic properties of
APTO-253
was accepted for presentation at the 2018 Annual Meeting of the American Association for Cancer Research.
7
THE OFFERING
This summary highlights information presented in greater detail elsewhere in this prospectus. This summary is not complete and does not
contain all the information you should consider before investing in our common shares. You should carefully read this entire prospectus before investing in our common shares including the section entitled “
Risk
Factors
,” our consolidated financial statements and the documents incorporated herein.
|
Common shares offered by the selling shareholder:
|
Up to 6,041,567 common shares.
|
|
Common shares outstanding:
|
30,702,053 (as of March 27, 2018)
|
|
Use of proceeds:
|
The selling shareholder will receive all of the proceeds from the sale of the shares offered for sale by it under this prospectus. We will not receive proceeds from the sale of the shares by the selling shareholder. However, we may receive up to
$15,500,000 in proceeds from the sale of our common shares to the selling shareholder under the Purchase Agreement. Any proceeds from the selling shareholder that we receive under the Purchase Agreement are expected be used to fund our growth plans,
for working capital, and for other general corporate purposes, including capital expenditures related to our research and development activities.
|
|
Toronto Stock Exchange symbol:
|
“APS”
|
|
Nasdaq Capital Market symbol:
|
“APTO”
|
|
Risk factors:
|
An investment in our securities involves risk. Before you invest in our securities, you should carefully consider the risks involved. Accordingly, you should carefully consider the information contained in or incorporated by reference into this
prospectus, including the risks described below and in our Annual Report on Form
40-F
for the fiscal year ended December 31, 2017, which is incorporated by reference into this prospectus. The discussion
of risks related to our business contained in or incorporated by reference into this prospectus comprises material risks of which we are aware. If any of the events or developments described actually occurs, our business, financial condition or
results of operations would likely be adversely affected.
|
On October 27, 2017, we entered into the Purchase Agreement
with Aspire Capital, which provides that, upon the terms and subject to the conditions and limitations set forth therein, Aspire Capital is committed to purchase up to an aggregate of $15,500,000 of our common shares over the approximately
thirty-month term of the Purchase Agreement. In consideration for entering into the Purchase Agreement, concurrently with the execution of the Purchase Agreement, we issued to Aspire Capital 321,429 common shares as a commitment fee (referred to in
this prospectus as the “
Commitment Shares
”). Upon execution of the Purchase Agreement, the Company agreed to sell to Aspire Capital 357,143 common shares (referred to in this prospectus as the “
Initial Purchase
Shares
”), at $1.40 per share for gross proceeds of $500,000. Concurrently with entering into the Purchase Agreement, we also entered into a registration rights agreement with Aspire Capital (referred to in this prospectus as the
“
Registration Rights Agreement
”), in which we agreed to file one or more registration
8
statements, including the registration statement of which this prospectus is a part, as permissible and necessary to register under the Securities Act, the sale of the common shares that have
been and may be issued to Aspire Capital under the Purchase Agreement. From January 1, 2018 to the date of this registration statement, the Company issued 3,200,000 shares to Aspire Capital at prices from $2.17 to $3.29. As at March 27,
2018, there remain available 2,162,995 common shares to be issued to Aspire under the terms of the Purchase Agreement.
As of
March 27, 2018, there were 30,702,053 common shares outstanding (30,395,271 shares held by
non-affiliates)
which include 3,878,572 shares offered that have been issued to Aspire Capital pursuant to the
Purchase Agreement. If all of the 2,162,995 remaining available common shares offered here were issued and outstanding as of the date hereof, such shares would represent 6.58% of the total common shares outstanding or 6.64% of the
non-affiliate
shares of common shares outstanding as of the date hereof. The number of common shares ultimately offered for sale by Aspire Capital is dependent upon the number of shares purchased by Aspire Capital
under the Purchase Agreement.
Pursuant to the Purchase Agreement and the Registration Rights Agreement, we have registered 6,041,567
common shares under the Securities Act, which includes the Commitment Shares and the Initial Purchase Shares that have already been issued to Aspire Capital, 3,200,000 common shares that have been issued to Aspire Capital between January 1,
2018 and March 27, 2018 and 2,162,995 common shares which we may issue to Aspire Capital. All 6,041,567 common shares are being offered pursuant to this prospectus.
On December 29, 2017, the conditions necessary for purchases under the Purchase Agreement to commence were satisfied. On any trading day
on which the closing sale price of our common shares is not less than $0.25 per share, we have the right, in our sole discretion, to present Aspire Capital with a purchase notice (each, a “
Purchase Notice
”), directing Aspire Capital
(as principal) to purchase up to 200,000 common shares per trading day, up to $15,500,000 of our common shares in the aggregate at a per share price, or the “Purchase Price”, calculated by reference to the prevailing market price of our
common shares (as more specifically described below).
In addition, on any date on which we submit a Purchase Notice for 200,000 shares to
Aspire Capital, we also have the right, in our sole discretion, to present Aspire Capital with a volume-weighted average price purchase notice, or a “
VWAP Purchase Notice
”, directing Aspire Capital to purchase a number of common
shares equal to up to 30% of our aggregate common shares traded on Nasdaq on the next trading day, or the “
VWAP Purchase Date
”, subject to a maximum number of shares we may determine, which we refer to as the “
VWAP Purchase
Share Volume Maximum
”, and a minimum trading price, or the “
VWAP Minimum Price Threshold
” (as more specifically described below). The purchase price per Purchase Share pursuant to such VWAP Purchase Notice, or the
“
VWAP Purchase Price
”, is calculated by reference to the prevailing market price of our common shares (as more specifically described below).
The Purchase Agreement provides that we and Aspire Capital shall not effect any sales under the Purchase Agreement on any purchase date where
the closing sale price of our common shares is less than $0.25 per share, which we refer to as the “
Floor Price
”. This Floor Price and the respective prices and share numbers in the preceding paragraphs shall be appropriately
adjusted for any reorganization, recapitalization,
non-cash
dividend, stock split, reverse stock split or other similar transaction. There are no trading volume requirements or restrictions under the Purchase
Agreement, and we will control the timing and amount of any sales of our common shares to Aspire Capital. Aspire Capital has no right to require any sales by us, but is obligated to make purchases from us as we direct in accordance with the Purchase
Agreement. There are no limitations on use of proceeds, financial or business covenants, restrictions on future fundings, rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement. Aspire Capital may
not assign its rights or obligations under the Purchase Agreement. The Purchase Agreement may be terminated by us at any time, at our discretion, without any penalty or cost to us. The Purchase Agreement may be terminated by Aspire Capital in case
of default by us. The TSX has approved the transaction, but under no circumstance shall we issue, or make issuable, more than 6,041,567 common shares in aggregate, without shareholder approval.
9
RISK FACTORS
An investment in our securities involves risk. Before you invest in our securities, you should carefully consider the risks involved.
Accordingly, you should carefully consider the information contained in or incorporated by reference into this prospectus, including the risks described below and in our Annual Report on Form
40-F
for the
fiscal year ended December 31, 2017, which is incorporated by reference into this prospectus. The discussion of risks related to our business contained in or incorporated by reference into this prospectus comprises material risks of which we
are aware. If any of the events or developments described actually occurs, our business, financial condition or results of operations would likely be adversely affected.
Risks Related to this Offering
We
will need to raise substantial additional capital in the future to fund our operations and we may be unable to raise such funds when needed and on acceptable terms
Our ability to utilize the Purchase Agreement with Aspire Capital as a source of funding will depend on a number of factors, including the
prevailing market price of our common shares, the volume of trading in our common shares and continuing the listing of our common stock on the Nasdaq Capital Market or an equivalent stock exchange. The number of shares that we may sell to Aspire
Capital under the Purchase Agreement on any given day and during the term of the agreement is limited. See “The Aspire Capital Transaction” section of this prospectus for additional information. Additionally, we and Aspire Capital may not
effect any sales of our common shares under the Purchase Agreement during the continuance of an event of default or on any trading day that the closing sale price of our common shares is less than $0.25 per share, but under no circumstances shall we
issue, or make issuable, more than 6,041,567 common shares in aggregate without shareholder approval. Even if we are able to access the full committed amount of $15,500,000 under the Purchase Agreement, we will still need additional capital to fully
implement our business, operating and development plans.
We will have broad discretion in how we use the proceeds, and we may use
the proceeds in ways in which you and other shareholders may disagree.
We intend to use the net proceeds we receive from the
issuance of common shares to Aspire Capital pursuant to the Purchase Agreement for working capital and general corporate purposes. Our management will have broad discretion in the application of the proceeds from this offering and could spend the
proceeds in ways that do not necessarily improve our operating results or enhance the value of our common shares.
The sale of our
common shares to Aspire Capital may cause substantial dilution to our existing shareholders and the sale of common shares acquired by Aspire Capital could cause the price of our common shares to decline.
We have registered for sale the Commitment Shares and Initial Purchase Shares that we have issued and 5,362,995 shares that we may sell to
Aspire Capital (of which 2,162,995 are available to be sold to Aspire Capital) under the Purchase Agreement. It is anticipated that shares registered in this offering will be sold over a period of up to approximately thirty months from the date of
this prospectus. The number of shares ultimately offered for sale by Aspire Capital under this prospectus is dependent upon the number of shares we elect to sell to Aspire Capital under the Purchase Agreement. Depending on a variety of factors,
including market liquidity of our common shares, the sale of shares under the Purchase Agreement may cause the trading price of our common shares to decline.
Aspire Capital may ultimately purchase all, some or none of the common shares that, together with the Commitment Shares and Initial Purchase
Shares (for aggregate value, including the Commitment Shares and Initial Purchase Shares, of up to $15,500,000), is the subject of this prospectus. Aspire Capital may sell all, some or none of our shares that it holds or comes to hold under the
Purchase Agreement. Sales by Aspire Capital of shares acquired pursuant to the Purchase Agreement under the registration statement, of which this prospectus is a part, may result in dilution to the interests of other holders of our common shares.
The sale of a substantial
10
number of common shares by Aspire Capital in this offering, or anticipation of such sales, could cause the trading price of our common shares to decline or make it more difficult for us to sell
equity or equity-related securities in the future at a time and at a price that we might otherwise desire. However, we have the right under the Purchase Agreement to control the timing and amount of sales of our shares to Aspire Capital, and the
Purchase Agreement may be terminated by us at any time at our discretion without any penalty or cost to us.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, including the information that we incorporate by reference, contains forward-looking statements within the meaning of the
Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this prospectus, including statements regarding our future operating results and
financial position, business strategy, and plans and objectives of management for future operations, are forward-looking statements. In many cases, you can identify forward-looking statements by terms such as “may,” “should,”
“expects,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,”
“potential,” or “continue” or the negative of these terms or other similar expressions.
The forward-looking
statements contained in this prospectus reflect our views as of the date of this prospectus about future events and are subject to risks, uncertainties, assumptions, and changes in circumstances that may cause our actual results, performance, or
achievements to differ significantly from those expressed or implied in any forward-looking statement. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future events, results,
performance, or achievements. A number of important factors could cause actual results to differ materially from those indicated by the forward-looking statements, including, without limitation, those factors described in “Risk Factors.”
In addition, those “Risk Factors” may be updated from time to time by our filings under the Securities Exchange Act of 1934, as amended, or the Exchange Act. Except as required by law, after the date of this prospectus, we are under no
duty to update or revise any of the forward-looking statements contained or incorporated by reference herein, whether as a result of new information, future events or otherwise.
You should read this prospectus, including the information that we incorporate by reference, completely and with the understanding that our
actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
CAPITALIZATION AND INDEBTEDNESS
The following provides our capitalization and indebtedness as at December 31, 2017, in accordance with IFRS as issued by the
International Accounting Standards Board.
|
|
|
|
|
|
|
|
|
|
|
(in thousands other than securities numbers)
(1)
|
|
Actual
|
|
|
Proforma
As Adjusted
(2)
|
|
|
Common shares (unlimited authorized, 27,502,053 issued and outstanding, without par
value)
|
|
$
|
231,923
|
|
|
$
|
240,784
|
|
|
Stock options (exchangeable into 2,342,840 common shares)
|
|
$
|
6,456
|
|
|
$
|
6,456
|
|
|
Contributed surplus
|
|
$
|
22,909
|
|
|
$
|
22,909
|
|
|
Accumulated other comprehensive income
|
|
$
|
(4,298
|
)
|
|
$
|
(4,298
|
)
|
|
Deficit
|
|
$
|
(246,788
|
)
|
|
$
|
(246,788
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total capitalization
|
|
$
|
10,202
|
|
|
$
|
19,063
|
|
|
|
|
|
|
|
|
|
|
|
|
(1)
|
The table above is based on 27,502,053 common shares outstanding on December 31, 2017.
|
|
(2)
|
The Proforma As Adjusted column reflects 3,200,000 common shares that have been issued by us for the period from January 1, 2018 to March 28, 2018 at an average price of $2.77.
|
11
You should read this table in conjunction with our audited consolidated financial statements as
at and for the year ended December 31, 2017, which are incorporated by reference in this prospectus.
OFFER AND LISTING
Our common shares have been listed on Nasdaq since October 23, 2014 under the symbol “APTO.” Our common shares are also listed
on the TSX, under the symbol “APS.”
The following table sets out the price ranges of our common shares on the TSX for the
periods indicated below, as adjusted to reflect the 1 for 12 share consolidation of our common shares that occurred on October 1, 2014.
TSX
(CDN$)
|
|
|
|
|
|
|
|
|
|
|
Five most recent full fiscal years:
|
|
High
|
|
|
Low
|
|
|
Year ended December 31, 2017
|
|
$
|
3.00
|
|
|
$
|
1.05
|
|
|
Year ended December 31, 2016
|
|
$
|
5.13
|
|
|
$
|
1.12
|
|
|
Year ended December 31, 2015
|
|
$
|
8.73
|
|
|
$
|
3.06
|
|
|
7 month period ended December 31, 2014
|
|
$
|
9.14
|
|
|
$
|
4.80
|
|
|
|
|
|
|
Year ended December 31, 2017
|
|
$
|
3.00
|
|
|
$
|
1.05
|
|
|
Quarter ended December 31, 2017
|
|
$
|
3.00
|
|
|
$
|
1.64
|
|
|
Quarter ended September 30, 2017
|
|
$
|
2.20
|
|
|
$
|
1.63
|
|
|
Quarter ended June 30, 2017
|
|
$
|
2.20
|
|
|
$
|
1.05
|
|
|
Quarter ended March 31, 2017
|
|
$
|
1.93
|
|
|
$
|
1.23
|
|
|
|
|
|
|
Year ended December 31, 2016
|
|
$
|
5.13
|
|
|
$
|
1.12
|
|
|
Quarter ended December 31, 2016
|
|
$
|
4.02
|
|
|
$
|
1.12
|
|
|
Quarter ended September 30, 2016
|
|
$
|
3.65
|
|
|
$
|
2.51
|
|
|
Quarter ended June 30, 2016
|
|
$
|
5.13
|
|
|
$
|
2.80
|
|
|
Quarter ended March 31, 2016
|
|
$
|
4.21
|
|
|
$
|
2.74
|
|
|
|
|
|
|
Most recent six months:
|
|
|
|
|
|
|
|
|
|
March 31, 2018
|
|
$
|
5.18
|
|
|
$
|
3.37
|
|
|
February 28, 2018
|
|
$
|
3.71
|
|
|
$
|
3.16
|
|
|
January 31, 2018
|
|
$
|
4.80
|
|
|
$
|
2.69
|
|
|
December 31, 2017
|
|
$
|
3.00
|
|
|
$
|
2.17
|
|
|
November 30, 2017
|
|
$
|
2.92
|
|
|
$
|
1.95
|
|
|
October 31, 2017
|
|
$
|
2.07
|
|
|
$
|
1.64
|
|
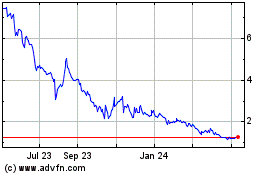
The following table sets out the price ranges and trading volumes of our common shares on Nasdaq for the
periods indicated below.
Nasdaq
(US$)
|
|
|
|
|
|
|
|
|
|
|
Five most recent full fiscal years:
|
|
High
|
|
|
Low
|
|
|
Year ended December 31, 2017
|
|
$
|
2.58
|
|
|
$
|
0.78
|
|
|
Year ended December 31, 2016
|
|
$
|
4.30
|
|
|
$
|
0.83
|
|
|
Year ended December 31, 2015
|
|
$
|
6.81
|
|
|
$
|
2.17
|
|
|
7 month period ended December 31, 2014
|
|
$
|
7.78
|
|
|
$
|
5.60
|
|
12
|
|
|
|
|
|
|
|
|
|
|
Five most recent full fiscal years:
|
|
High
|
|
|
Low
|
|
|
|
|
|
|
Year ended December 31, 2017
|
|
$
|
2.58
|
|
|
$
|
0.78
|
|
|
Quarter ended December 31, 2017
|
|
$
|
2.58
|
|
|
$
|
1.30
|
|
|
Quarter ended September 30, 2017
|
|
$
|
1.75
|
|
|
$
|
1.25
|
|
|
Quarter ended June 30, 2017
|
|
$
|
1.70
|
|
|
$
|
0.78
|
|
|
Quarter ended March 31, 2017
|
|
$
|
1.43
|
|
|
$
|
0.91
|
|
|
|
|
|
|
Year ended December 31, 2016
|
|
$
|
4.30
|
|
|
$
|
0.83
|
|
|
Quarter ended December 31, 2016
|
|
$
|
3.20
|
|
|
$
|
0.83
|
|
|
Quarter ended September 30, 2016
|
|
$
|
2.82
|
|
|
$
|
1.92
|
|
|
Quarter ended June 30, 2016
|
|
$
|
4.30
|
|
|
$
|
2.12
|
|
|
Quarter ended March 31, 2016
|
|
$
|
3.41
|
|
|
$
|
1.93
|
|
|
|
|
|
|
Most recent six months:
|
|
|
|
|
|
|
|
|
|
March 31, 2018
|
|
$
|
3.97
|
|
|
$
|
2.62
|
|
|
February 28, 2018
|
|
$
|
3.03
|
|
|
$
|
2.51
|
|
|
January 31, 2018
|
|
$
|
3.90
|
|
|
$
|
2.10
|
|
|
December 31, 2017
|
|
$
|
2.58
|
|
|
$
|
1.68
|
|
|
November 30, 2017
|
|
$
|
2.30
|
|
|
$
|
1.50
|
|
|
October 31, 2017
|
|
$
|
1.61
|
|
|
$
|
1.30
|
|
USE OF PROCEEDS
This prospectus relates to our common shares that may be offered and sold from time to time by Aspire Capital. We will receive no proceeds
from the sale of our common shares by Aspire Capital in this offering. However, we may receive gross proceeds of up to $15,500,000 from Aspire Capital under the Purchase Agreement. We estimate that the net proceeds to us from the sale of our common
shares to Aspire Capital pursuant to the Purchase Agreement will be up to $15,353,458 over an approximately
30-month
period, assuming that we sell the full amount of our common shares that we have the right,
but not the obligation, to sell to Aspire Capital under the Purchase Agreement and other estimated fees and expenses. See “
Plan of Distribution
” elsewhere in this prospectus for more information.
Unless otherwise indicated in the applicable prospectus supplement, information incorporated by reference or free writing prospectus, we
expect to use any proceeds that we receive under the Purchase Agreement to fund our growth plans, for working capital, and for other general corporate purposes, including capital expenditures related to our research and development activities.
THE ASPIRE CAPITAL TRANSACTION
General
On October 27, 2017,
we entered into the Purchase Agreement which provides that, upon the terms and subject to the conditions and limitations set forth therein, Aspire Capital is committed to purchase up to an aggregate of $15,500,000 of our common shares over the term
of the Purchase Agreement. In consideration for entering into the Purchase Agreement, concurrently with the execution of the Purchase Agreement, we issued to Aspire Capital the Commitment Shares. Upon execution of the Purchase Agreement, we agreed
to sell to Aspire Capital 357,143 initial Purchase Shares at a price of $1.40 per share, for gross proceeds of $500,000. Concurrently with entering into the Purchase Agreement, we also entered into the Registration Rights Agreement, in which we
agreed to file one or more registration statements as permissible and necessary to register under the Securities Act, the sale of the shares of our common shares that have been and may be issued to Aspire Capital under the Purchase Agreement. From
January 1, 2018 to the date of this registration statement,
13
the Company issued 3,200,000 shares to Aspire Capital at prices from $2.17 to $3.29. As at March 27, 2018, there remain available 2,162,995 common shares to be issued to Aspire under the
terms of the Purchase Agreement.
As of March 27, 2018, there were 30,702,053 common shares outstanding (30,395,271 shares held by
non-affiliates)
which include 3,878,572 shares offered that have been issued to Aspire Capital pursuant to the Purchase Agreement. If all of the 2,162,995 remaining available common shares offered here were issued
and outstanding as of the date hereof, such shares would represent 6.58% of the total common shares outstanding or 6.64% of the
non-affiliate
shares of common shares outstanding as of the date hereof. The
number of common shares ultimately offered for sale by Aspire Capital is dependent upon the number of shares purchased by Aspire Capital under the Purchase Agreement.
Pursuant to the Purchase Agreement and the Registration Rights Agreement, we have registered 6,041,567 common shares under the Securities Act,
which includes the Commitment Shares and the Initial Purchase Shares that have already been issued to Aspire Capital, 3,200,000 common shares that have been issued to Aspire between January 1 2018 and March 27, 2018 and 2,162,995 common
shares which we may issue to Aspire Capital. All 6,041,567 common shares are being offered pursuant to this prospectus.
On
December 29, 2017, the conditions necessary for purchases under the Purchase Agreement to commence were satisfied. On any trading day on which the closing sale price of our common shares is not less than $0.25 per share, we have the right,
in our sole discretion, to present Aspire Capital with a Purchase Notice, directing Aspire Capital (as principal) to purchase up to 200,000 common shares per business day, up to $15,500,000 of our common shares in the aggregate over the term of
the Purchase Agreement, at a Purchase Price calculated by reference to the prevailing market price of our common shares over the preceding
10-business
day period (as more specifically described below);
however, no sale pursuant to a Purchase Notice may exceed $500,000 per trading day.
In addition, on any date on which we submit a
Purchase Notice to Aspire Capital for 200,000 Purchase Shares, we also have the right, in our sole discretion, to present Aspire Capital with a VWAP Purchase Notice directing Aspire Capital to purchase a number of common shares equal to up to 30% of
the aggregate common shares of the Company traded on the Nasdaq Capital Market on the next trading day, subject to the VWAP Purchase Share Volume Maximum and the VWAP Minimum Price Threshold. The VWAP Purchase Price is calculated by reference to the
prevailing market price of our common shares (as more specifically described below).
The Purchase Agreement provides that we and Aspire
Capital shall not effect any sales under the Purchase Agreement on any purchase date where the closing sale price of our common shares is less than the Floor Price. There are no trading volume requirements or restrictions under the Purchase
Agreement, and we will control the timing and amount of any sales of our common shares to Aspire Capital. Aspire Capital has no right to require any sales by us, but is obligated to make purchases from us as we direct in accordance with the Purchase
Agreement. There are no limitations on use of proceeds, financial or business covenants, restrictions on future financings, rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement. Aspire Capital may
not assign its rights or obligations under the Purchase Agreement. The Purchase Agreement may be terminated by us at any time, at our discretion, without any penalty or cost to us.
Purchase of Common Shares Under the Common Shares Purchase Agreement
Under the Purchase Agreement, on any trading day selected by us on which the closing sale price of our common shares is not less than $0.25 per
share, we may direct Aspire Capital to purchase up to 200,000 common shares per trading day. The Purchase Price of such shares is equal to the lesser of:
|
|
•
|
|
the lowest sale price of our common shares on the purchase date; or
|
14
|
|
•
|
|
the arithmetic average of the three lowest closing sale prices for our common shares on the Nasdaq Capital Market (or successor principal market) during the ten consecutive trading days ending on the trading day
immediately preceding the purchase date.
|
In addition, on any date on which we submit a Purchase Notice to Aspire Capital
for purchase of 200,000 shares, we also have the right to direct Aspire Capital to purchase a number of common shares equal to up to 30% of the aggregate common shares of the Company traded on the Nasdaq Capital Market on the next trading day,
subject to the VWAP Purchase Share Volume Maximum and the VWAP Minimum Price Threshold, which is equal to the greater of (a) 80% of the closing price of our common shares on the business day immediately preceding the VWAP Purchase Date or
(b) such higher price as set forth by us in the VWAP Purchase Notice. The VWAP Purchase Price of such shares is the lower of:
|
|
•
|
|
the Closing Sale Price on the VWAP Purchase Date; or
|
|
|
•
|
|
97% of the volume-weighted average price for our common shares traded on the Nasdaq Capital Market:
|
|
|
•
|
|
on the VWAP Purchase Date, if the aggregate shares to be purchased on that date have not exceeded the VWAP Purchase Share Volume Maximum or
|
|
|
•
|
|
during that portion of the VWAP Purchase Date until such time as the sooner to occur of (i) the time at which the aggregate shares traded on the Nasdaq Capital Market exceed the VWAP Purchase Share Volume Maximum
or (ii) the time at which the sale price of our common shares falls below the VWAP Minimum Price Threshold.
|
The TSX
has approved the transaction and the Nasdaq Capital Market has completed its review of the listing of the common shares issuable in connection with the transaction, but under no circumstance shall we issue, or make issuable, more than 6,041,567
common shares in aggregate, without shareholder approval.
Maximum Number of Shares
The TSX has approved the transaction, but under no circumstance shall we issue, or make issuable, more than 6,041,567 common shares in
aggregate, without shareholder approval.
Minimum Share Price
Under the Purchase Agreement, we and Aspire Capital may not effect any sales of common shares under the Purchase Agreement on any trading day
that the closing sale price of our common shares is less than $0.25 per share.
Events of Default
Generally, Aspire Capital may terminate the Purchase Agreement upon the occurrence of any of the following, among other, events of default:
|
|
•
|
|
the effectiveness of any registration statement that is required to be maintained effective pursuant to the terms of the Registration Rights Agreement between us and Aspire Capital lapses for any reason (including,
without limitation, the issuance of a stop order) or is unavailable to Aspire Capital for sale of common shares, and such lapse or unavailability continues for a period of ten consecutive business days or for more than an aggregate of thirty
business days in any
365-day
period, which is not in connection with a post-effective amendment to any such registration statement; in connection with any post-effective amendment to such registration
statement that is required to be declared effective by the SEC such lapse or unavailability may continue for a period of no more than 40 consecutive business days;
|
15
|
|
•
|
|
the suspension from trading or failure of our common shares to be listed on our principal market for a period of three consecutive business days;
|
|
|
•
|
|
the delisting of our common shares from our principal market (currently the Nasdaq Capital Market), provided our common shares are not immediately thereafter trading on the New York Stock Exchange, the NYSE MKT, the
Nasdaq Capital Market, the Nasdaq Global Select Market, the Nasdaq Global Market, the OTB Bulletin Board or the OTCQB marketplace or OTCQX marketplace of the OTC Markets Group;
|
|
|
•
|
|
our transfer agent’s failure to issue to Aspire Capital common shares which Aspire Capital is entitled to receive under the Purchase Agreement within five business days after an applicable purchase date;
|
|
|
•
|
|
any breach by us of the representations or warranties or covenants contained in the Purchase Agreement or any related agreements which could have a material adverse effect on us, subject to a cure period of five
business days;
|
|
|
•
|
|
if we become insolvent or are generally unable to pay our debts as they become due; or
|
|
|
•
|
|
any participation or threatened participation in insolvency or bankruptcy proceedings by or against us.
|
Our Termination Rights
The
Purchase Agreement may be terminated by us at any time, at our discretion, without any penalty or cost to us.
No Short-Selling or Hedging by Aspire
Capital
Aspire Capital has agreed that neither it nor any of its agents, representatives and affiliates shall engage in any direct
or indirect short-selling or hedging of our common shares during any time prior to the termination of the Purchase Agreement.
Effect of Performance
of the Purchase Agreement on Our Shareholders
The Purchase Agreement does not limit the ability of Aspire Capital to sell any or
all of the 6,041,567 common shares registered in this offering. It is anticipated that common shares registered in this offering will be sold over a period of up to approximately thirty months from the date of this prospectus. The sale by Aspire
Capital of a significant amount of shares registered in this offering at any given time could cause the market price of our common shares to decline and/or to be highly volatile. Aspire Capital may ultimately purchase all, some or none of the
5,362,995 common shares not yet issued but registered in this offering. After it has acquired such common shares, it may sell all, some or none of such shares. Therefore, sales to Aspire Capital by us pursuant to the Purchase Agreement also may
result in substantial dilution to the interests of other holders of our common shares. However, we have the right to control the timing and amount of any sales of our shares to Aspire Capital and the Purchase Agreement may be terminated by us at any
time at our discretion without any penalty or cost to us.
Percentage of Outstanding Shares After Giving Effect to the Purchased Shares Issued to
Aspire Capital
In connection with entering into the Purchase Agreement, we authorized the sale to Aspire Capital of up to
$15,500,000 of our common shares. However, we estimate that we will sell no more than 5,362,995 shares to Aspire Capital under the Purchase Agreement (exclusive of the Commitment Shares and Initial Purchase Shares), all of which are included in this
offering. Subject to any required approval by our board of directors, we have the right but not the obligation to issue more than the 6,041,567 shares included in this prospectus to Aspire Capital under the Purchase Agreement. In the event we elect
to issue more than 6,041,567 shares under the Purchase Agreement, we will be required to file a new registration statement and have it declared effective by the SEC. The number of shares ultimately offered for sale by Aspire Capital in this offering
is dependent upon the number
16
of shares purchased by Aspire Capital under the Purchase Agreement. The following table sets forth the number and percentage of outstanding shares to be held by Aspire Capital after giving effect
to the sale of common shares issued to Aspire Capital at varying purchase prices:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assumed Average
Purchase Price
|
|
|
Proceeds from the Sale
of Shares to Aspire Capital
Under the Purchase
Agreement Registered
in
this Offering
|
|
|
Number of Shares to be Issued
in this Offering at the Assumed
Average Purchase Price
(1)
|
|
|
Percentage of Outstanding
Shares After Giving Effect to
the Purchased Shares Issued
to
Aspire Capital
(2)
|
|
|
$
|
0.25
|
|
|
$
|
1,340,749.00
|
|
|
|
5,362,995
|
|
|
|
*
|
|
|
$
|
0.50
|
|
|
$
|
2,681,498.00
|
|
|
|
5,362,995
|
|
|
|
16.5
|
%
|
|
$
|
1.00
|
|
|
$
|
5,362,995.00
|
|
|
|
5,362,995
|
|
|
|
16.5
|
%
|
|
$
|
1.50
|
|
|
$
|
8,044,493.00
|
|
|
|
5,362,995
|
|
|
|
16.5
|
%
|
|
$
|
2.50
|
|
|
$
|
13,407,488.00
|
|
|
|
5,362,995
|
|
|
|
16.5
|
%
|
|
$
|
5.00
|
|
|
$
|
15,000,000.00
|
|
|
|
3,000,000
|
|
|
|
10.0
|
%
|
|
$
|
10.00
|
|
|
$
|
15,000,000.00
|
|
|
|
1,500,000
|
|
|
|
5.3
|
%
|
|
(1)
|
Excludes 321,429 Commitment Shares and 357,143 Initial Purchase Shares issued under the Purchase Agreement between us and Aspire Capital.
|
|
(2)
|
The denominator is based on 27,049,724 shares outstanding as of November 22, 2017, which includes the 678,572 shares previously issued to Aspire Capital and the number of shares set forth in the adjacent column
which we would have sold to Aspire Capital. The numerator is based on the number of shares which we may issue to Aspire Capital under the Purchase Agreement (that are the subject of this offering) at the corresponding assumed purchase price set
forth in the adjacent column.
|
SELLING SHAREHOLDER
The selling shareholder may from time to time offer and sell any or all of the common shares set forth below pursuant to this prospectus. When
we refer to the “selling shareholder” in this prospectus, we mean the entity listed in the table below, and its respective pledgees, donees, permitted transferees, assignees, successors and others who later come to hold any of the selling
shareholder’s interests in common shares other than through a public sale.
The following table sets forth, as of the date of this
prospectus, the name of the selling shareholder for whom we have registered shares for sale to the public, the number of common shares beneficially owned by the selling shareholder prior to this offering, the total number of common shares that the
selling shareholder may offer pursuant to this prospectus and the number of common shares that the selling shareholder will beneficially own after this offering. Except as noted below, the selling shareholder does not have, or within the past three
years has not had, any material relationship with us or any of our predecessors or affiliates and the selling shareholder is not or was not affiliated with registered broker-dealers.
Based on the information provided to us by the selling shareholder, assuming that the selling shareholder sells all of the common shares
beneficially owned by it that have been registered by us and does not acquire any additional shares during the offering, the selling shareholder will not own any shares other than those appearing in the column entitled “Beneficial Ownership
After This Offering.” We cannot advise you as to whether the selling shareholder will in fact sell any or all of such common shares. In addition, the selling shareholder may have sold, transferred or otherwise disposed of, or may sell, transfer
or otherwise dispose of, at any time and
17
from time to time, the common shares in transactions exempt from the registration requirements of the Securities Act after the date on which it provided the information set forth in the table
below.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares of
Common
Stock
Owned
Prior to this
Offering
|
|
|
Shares of
Common
Stock Being
Offered
|
|
|
Beneficial Ownership
After this Offering
(1)
|
|
|
Name
|
|
|
|
Number of
Shares
|
|
|
%
(2)(3)(
*
)
|
|
|
Aspire Capital Fund, LLC
(4)
155 North Wacker Drive, Suite 1600
Chicago, Illinois 60606
|
|
|
978,572
|
(5)
|
|
|
6,041,567
|
|
|
|
0
|
|
|
|
0
|
%
|
* Represents
less than 1% of outstanding shares
|
(1)
|
Assumes the sale of common shares registered pursuant to this prospectus, although the selling shareholder is under no obligation known to us to sell any common shares at this time.
|
|
(2)
|
Based on 27,049,724 common shares outstanding on November 22, 2017.
|
|
(3)
|
Under the terms of the Purchase Agreement, Aspire Capital will limit its share ownership in Aptose to 9.99%.
|
|
(4)
|
Aspire Capital Partners LLC (“
Aspire Partners
”) is the Managing Member of Aspire Capital Fund LLC (“
Aspire Fund
”). SGM Holdings Corp (“
SGM
”) is the
Managing Member of Aspire Partners. Mr. Steven G. Martin (“Mr. Martin”) is the president and sole shareholder of SGM, as well as a principal of Aspire Partners. Mr. Erik J. Brown (“Mr. Brown”) is the
president and sole shareholder of Red Cedar Capital Corp (“
Red Cedar
”), which is a principal of Aspire Partners. Mr. Christos Komissopoulos (“
Mr.
Komissopoulos
”)
is president and sole shareholder of Chrisko Investors Inc. (“
Chrisko
”), which is a principal of Aspire Partners. Each of Aspire Partners, SGM, Red Cedar, Chrisko, Mr. Martin, Mr. Brown, and Mr. Komissopoulos
may be deemed to be a beneficial owner of common shares held by Aspire Fund. Each of Aspire Partners, SGM, Red Cedar, Chrisko, Mr. Martin, Mr. Brown, and Mr. Komissopoulos disclaims beneficial ownership of the common shares held by
Aspire Fund.
|
|
(5)
|
As of the date hereof, 678,572 of our common shares have been acquired by Aspire Capital under the Purchase Agreement, consisting of the Commitment Shares and Initial Purchase Shares, and 300,000 of our common shares
were acquired by Aspire Capital on the open market. We may elect in our sole discretion to sell to Aspire Capital up to an additional 5,362,995 shares under the Purchase Agreement but Aspire Capital does not presently beneficially own those shares
as determined in accordance with the rules of the SEC.
|
DESCRIPTION OF SHARE CAPITAL
We are authorized to issue an unlimited number of common shares, no par value. As of March 27, 2018, we had 30,702,053 common shares
issued and outstanding.
Our common shares are issued in registered, not bearer, form. The holders of our common shares are entitled to
one vote per share at meetings of shareholders, to receive such dividends as declared by us and to receive our remaining property and assets upon dissolution or winding up. Our common shares are not subject to any future call or assessment and there
are no
pre-emptive,
conversion or redemption rights attached to such common shares.
The transfer
agent for our common shares in Canada is Computershare Investor Services Inc. at its principal office in Toronto, Canada.
Common Shares
On October 27, 2017, we entered into the Common Stock Purchase Agreement with Aspire Capital Fund, LLC, or Aspire Capital. Pursuant to the
terms of this agreement, Aspire Capital purchased 357,143 shares of our
18
common stock at $1.40 per share and we issued 321,429 shares of our common stock to Aspire Capital in consideration for entering into the agreement. From January 1, 2018 to March 27,
2018, the Company issued 3,200,000 shares to Aspire Capital at prices from $2.17 to $3.29. As at March 27, 2018, there remain available 2,162,995 common shares to be issued to Aspire under the terms of the Purchase Agreement.
Warrants
Our board of directors
authorized or ratified the issuances of the warrants set forth in the tables below and the issuance of one common share upon the due exercise of each warrant in accordance with its terms and the receipt by us of the designated exercise price payable
in respect of the share prior to the time of expiry on the designated expiry date.
As at December 31, 2017 and March 27, 2018,
we had no outstanding warrants:
Stock Options
Our board of directors authorized or ratified the issuances of the options set forth in the tables below and the issuance of one common share
upon the due exercise of each option in accordance with its terms and the receipt by us of the designated exercise price payable in respect of the share prior to the time of expiry on the designated expiry date.
As at December 31, 2017, we had the following outstanding stock options:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Option numbers
are in (000’s)
|
|
|
|
|
|
Options outstanding
|
|
|
Range of exercise prices
|
|
Options
|
|
|
Weighted
average
remaining
contractual
life (years)
|
|
|
Weighted
average
exercise
price
|
|
|
$1.03-$1.15
|
|
|
241
|
|
|
|
9.4
|
|
|
$
|
1.05
|
|
|
$1.16-$1.86
|
|
|
527
|
|
|
|
9.2
|
|
|
|
1.26
|
|
|
$1.87-$4.37
|
|
|
444
|
|
|
|
7.8
|
|
|
|
3.11
|
|
|
$4.38-$5.08
|
|
|
614
|
|
|
|
6.4
|
|
|
|
4.61
|
|
|
$5.09-$34.37
|
|
|
518
|
|
|
|
6.9
|
|
|
|
5.79
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,344
|
|
|
|
7.7
|
|
|
$
|
3.46
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As at March 28, 2018, we had the following outstanding stock options:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Option numbers
are in (000’s)
|
|
|
|
|
|
Options outstanding
|
|
|
Range of exercise prices
|
|
Options
|
|
|
Weighted
average
remaining
contractual
life (years)
|
|
|
Weighted
average
exercise
price
|
|
|
$1.03-$1.15
|
|
|
241
|
|
|
|
9.2
|
|
|
$
|
1.04
|
|
|
$1.16-$1.86
|
|
|
527
|
|
|
|
9.0
|
|
|
|
1.23
|
|
|
$1.87-$4.37
|
|
|
2,503
|
|
|
|
9.4
|
|
|
|
2.96
|
|
|
$4.38-$5.08
|
|
|
614
|
|
|
|
6.2
|
|
|
|
4.49
|
|
|
$5.09-$33.51
|
|
|
518
|
|
|
|
6.6
|
|
|
|
5.64
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,403
|
|
|
|
8.6
|
|
|
$
|
3.18
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
19
Issuances of Common Shares
Share capital issued — for the year ended December 31, 2017
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares
|
|
|
|
|
Number
(in thousands)
|
|
|
Amount
(in thousands)
|
|
|
Balance, December 31, 2016
|
|
|
15,722
|
|
|
$
|
218,034
|
|
|
Common shares under the ATM (b)(ii)
|
|
|
10,952
|
|
|
|
13,394
|
|
|
Common shares issued under share purchase agreement (b(i))
|
|
|
678
|
|
|
|
324
|
|
|
Common shares issued under redemption of restricted share units
|
|
|
150
|
|
|
|
171
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2017
|
|
|
27,502
|
|
|
$
|
231,923
|
|
|
|
|
|
|
|
|
|
|
|
Share purchase agreement
On October 27, 2017, we entered into the Aspire Purchase Agreement, which provides that, upon the terms and subject to the conditions and
limitations set forth therein, Aspire Capital is committed to purchase up to an aggregate of $15,500,000 of common shares over approximately 30 months. Pursuant to the terms of this agreement, on October 31, 2017, Aspire Capital purchased
357,143 common shares for gross proceeds of $500 thousand ($324 thousand net of cash share issue costs). We also issued 321,429 common shares to Aspire Capital in consideration for entering into the Aspire Purchase Agreement.
At-The-Market
(“ATM”) Facility
On April 2, 2015, Aptose entered into an
at-the-market
(“ATM”) equity facility with Cowen and Company, LLC, acting as sole agent. Under the terms of the ATM, Aptose was permitted to sell common shares having an aggregate offering value of US$20,000,000 on NASDAQ. The ATM expired on
December 29, 2017 and as at that date the Company had issued a cumulative $20,000,000 of common shares pursuant to this facility.
During the year ended December 31, 2017, the Company issued 10,952,093 common shares under the ATM at an average price of $1.27 per share
for gross proceeds of $13.9 million ($13.4 million net of share issue costs). Costs associated with the proceeds included a 3% cash commission as well as legal and accounting fees
Restricted Share Units
On
March 28, 2017 the Company granted 150,000 restricted share units with a vesting term of three months, and accordingly, on June 28, 2017 all of these restricted share units vested and were redeemed for 150,000 common shares. During the
year ending December 31, 2017, the Company recorded share-based payment expense of $171 thousand (2016 – nil, 2015- nil) related to the issued RSUs.
Share capital issued — for the year ended December 31, 2016
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares
|
|
|
|
|
Number
(in thousands)
|
|
|
Amount
(in thousands)
|
|
|
Balance, December 31, 2015
|
|
|
12,048
|
|
|
$
|
212,308
|
|
|
Common shares under the ATM (b)(ii)
|
|
|
3,674
|
|
|
|
5,726
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2016
|
|
|
15,722
|
|
|
$
|
218,034
|
|
|
|
|
|
|
|
|
|
|
|
20
During the year ended December 31, 2016, the Company issued 3,673,933 common shares under
the ATM at an average price of $1.65 per share for gross proceeds of $6.05 million ($5.7 million net of share issue costs). Costs associated with the proceeds included a 3% cash commission as well as legal and accounting fees.
Share capital issued — for the year ended December 31, 2015
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares
|
|
|
|
|
Number
(in thousands)
|
|
|
Amount
(in thousands)
|
|
|
Balance, December 31, 2014
|
|
|
11,700
|
|
|
$
|
210,454
|
|
|
Warrant exercises
|
|
|
81
|
|
|
|
429
|
|
|
Option exercises
|
|
|
143
|
|
|
|
1,075
|
|
|
Common shares issued under ATM (b)(ii)
|
|
|
2
|
|
|
|
8
|
|
|
Promissory note conversion
|
|
|
122
|
|
|
|
342
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2015
|
|
|
12,048
|
|
|
$
|
212,308
|
|
|
|
|
|
|
|
|
|
|
|
During the year ended December 31, 2015, the Company issued 1,504 common shares under the ATM at an
average price of $5.20 per share for net proceeds of approximately $8 thousand.
During the year ended December 31, 2015, the
Company issued 81,466 common shares upon the exercise of warrants at an average exercise price of $3.42 for gross proceeds of approximately $279 thousand. In addition to the cash proceeds received, the original fair value related to these
warrants of $150 thousand was transferred from warrants to share capital. This resulted in a total amount of $429 thousand credited to share capital.
During the year ended December 31, 2015, the Company issued 143,084 common shares upon the exercise of options at an average exercise
price of $3.63 for gross proceeds of approximately $519 thousand. In addition to the cash proceeds received, the original fair value related to these warrants of $556 thousand was transferred from stock options to share capital. This
resulted in a total amount of $1,075 thousand credited to share capital.
During the year ended December 31, 2015, the Company
issued 121,528 common shares upon the conversion of convertible promissory notes issued in September 2013. There were no cash proceeds received by the company upon conversion.
MEMORANDUM AND ARTICLES OF ASSOCIATION
We are incorporated pursuant to the laws of Canada (Corporation Number: 6650309). Our articles of incorporation (“
Articles
”)
and
by-laws
provide no restrictions as to the nature of our business operations. Under Canadian law, a director must inform us, at a meeting of the Board, of any interest in a material contract or material
transaction, proposed material contract or proposed material transaction with us. Directors may not vote in respect of any such contracts or transactions made with us or in any such contract or transaction in which a director is interested, and such
directors shall not be counted for purposes of determining a quorum. However, these provisions do not apply to a contract or transaction, (i) relating primarily to their remuneration as a director, officer, employee or agent of the Company
or an affiliate, (ii) for their indemnity or insurance as permitted under the
Canada Business Corporations Act
, or (iii) with an affiliate.
We are authorized to issue an unlimited number of common shares. Our shareholders have no rights to share in our profits, are subject to no
redemption or sinking fund provisions, have no liability for further capital calls and are not subject to any discrimination due to number of Common Shares owned. By not more than 50 days nor less than seven days in advance of a dividend, the
Board may establish a record date for the determination of the persons entitled to such dividend.
21
The rights of holders of our common shares can be changed at any time in a shareholder meeting
where the modifications are approved by 66 2/3% of the common shares represented by proxy or in person at a meeting at which a quorum exists.
All holders of our common shares are entitled to vote at annual or special meetings of shareholders, provided that they were shareholders as
of the record date. The record date for shareholder meetings may precede the meeting date by no more than 60 days and not less than 21 days, provided that notice by way of advertisement is given to shareholders at least seven days before
such record date. Notice of the time and place of meetings of shareholders may not be less than 21days nor greater than 50 days prior to the date of the meeting. There are no:
|
|
•
|
|
limitations on share ownership;
|
|
|
•
|
|
provisions of the Articles or
by-laws
that would have the effect of delaying, deferring or preventing a change of control of our company;
|
|
|
•
|
|
by-law
provisions that govern the ownership threshold above which shareholder ownership must be disclosed; and
|
|
|
•
|
|
conditions imposed by the Articles or
by-laws
governing changes in capital, but Canadian corporate law requires any changes to the terms of share capital be approved by 66 2/3% of
the common shares represented by proxy or in person at a shareholders’ meeting convened for that purpose at which a quorum exists.
|
MATERIAL CONTRACTS
Other than the agreements described below, we have not, in the two years preceding the date hereof, entered into any material agreements other
than contracts in the ordinary course of business.
|
|
1.
|
Executive Employment Agreement between the Company and Dr. William G. Rice, dated October 25, 2013 and the Amendment dated August 19, 2014.
|
|
|
2.
|
Sales Agreement between the Company and Cowen and Company, LLC, dated April 2, 2015. The Sales Agreement expired at the end of the 2017 fiscal year.
|
|
|
3.
|
License agreement with CrystalGenomics, Inc, dated June 1, 2016.
|
|
|
4.
|
Common Shares Purchase Agreement, dated October 27, 2017, between the Company and Aspire Capital.
|
|
|
5.
|
Registration Rights Agreement, dated October 27, 2017, between the Company and Aspire Capital.
|
|
|
6.
|
License Agreement, dated March 7, 2018 with OHM Oncology.
|
EXCHANGE CONTROLS
There is no law or governmental decree or regulation in Canada that restricts the export or import of capital, or affects the remittance of
dividends, interest or other payments to
non-resident
holders of our voting common shares, other than withholding tax requirements.
There is no limitation imposed by Canadian law or by our Articles or other charter documents on the right of a
non-resident
to hold or vote voting common shares, other than as provided by the
Investment Canada Act
, the
North American Free Trade Agreement Implementation Act
(Canada) and the
World Trade
Organization Agreement Implementation Act
.
22
The
Investment Canada Act
requires notification and, in certain cases, advance review and
approval by the government of Canada of the acquisition by a
non-Canadian
of control of a Canadian business, all as defined in the
Investment Canada Act.
Generally, the threshold for review will be
higher in monetary terms for a member of the World Trade Organization or North American Free Trade Agreement.
DIVIDENDS AND PAYING AGENTS
The transfer agent and registrar for our common shares is Computershare Investor Services Inc. at its principal office in the City of Toronto.
SUBSIDIARY INFORMATION
The Company has three subsidiaries: NuChem, a company incorporated under the laws of Ontario, Canada, Aptose USA, a company incorporated under
the laws of Delaware, USA and Aptose Suisse GmbH a company incorporated under the laws of Zug, Switzerland. The Company owns 80% of the issued and outstanding voting share capital of NuChem and 100% of the issued and outstanding voting share capital
of Aptose USA and Aptose Suisse.
EXPENSES RELATING TO THIS OFFERING
The following table sets forth the estimated costs and expenses payable by us in connection with the sale of the securities being registered.
All amounts except the SEC registration fee are estimates.
|
|
|
|
|
|
|
SEC registration fee
|
|
$
|
1,542
|
|
|
Legal fees and expenses
|
|
|
100,000
|
|
|
Accounting fees and expenses
|
|
|
30,000
|
|
|
Miscellaneous expenses
|
|
|
15,000
|
|
|
Total
|
|
$
|
146,542
|
|
PLAN OF DISTRIBUTION
The common shares offered by this prospectus are being offered by the selling shareholder, Aspire Capital. The common shares may be sold or
distributed from time to time by the selling shareholder directly to one or more purchasers or through brokers, dealers, or underwriters who may act solely as agents at market prices prevailing at the time of sale, at prices related to the
prevailing market prices, at negotiated prices, or at fixed prices, which may be changed. The sale of the common shares offered by this prospectus could be effected in one or more of the following methods:
|
|
•
|
|
ordinary brokers’ transactions;
|
|
|
•
|
|
transactions involving cross or block trades;
|
|
|
•
|
|
through brokers, dealers, or underwriters who may act solely as agents
|
|
|
•
|
|
“
at the market
” into an existing market for the common shares;
|
|
|
•
|
|
in other ways not involving market makers or established business markets, including direct sales to purchasers or sales effected through agents;
|
|
|
•
|
|
in privately negotiated transactions; or
|
|
|
•
|
|
any combination of the foregoing.
|
23
In order to comply with the securities laws of certain states, if applicable, the shares may be
sold only through registered or licensed brokers or dealers. In addition, in certain states, the shares may not be sold unless they have been registered or qualified for sale in the state or an exemption from the registration or qualification
requirement is available and complied with.
The selling shareholder may transfer the common shares by other means not described in this
prospectus.
Aspire Capital has informed us that it intends to use an unaffiliated broker-dealer to effectuate all sales, if any, of the
common shares that it may purchase from us pursuant to the Purchase Agreement. Such sales will be made at prices and at terms then prevailing or at prices related to the then current market price. Each such unaffiliated broker-dealer will be an
underwriter within the meaning of Section 2(a)(11) of the Securities Act. Aspire Capital has informed us that each such broker-dealer will receive commissions from Aspire Capital that will not exceed customary brokerage commissions.
Brokers, dealers, underwriters or agents participating in the distribution of the common shares as agents may receive compensation in the form
of commissions, discounts, or concessions from the selling shareholder and/or purchasers of the common shares for whom the broker-dealers may act as agent. The compensation paid to a particular broker-dealer may be less than or in excess of
customary commissions. Neither we nor Aspire Capital can presently estimate the amount of compensation that any agent will receive.
Aspire Capital is an “underwriter” within the meaning of the Securities Act.
Neither we nor Aspire Capital can presently estimate the amount of compensation that any agent will receive. We know of no existing
arrangements between Aspire Capital, any other shareholder, broker, dealer, underwriter, or agent relating to the sale or distribution of the shares offered by this prospectus. At the time a particular offer of shares is made, a prospectus
supplement, if required, will be distributed that will set forth the names of any agents, underwriters, or dealers and any compensation from the selling shareholder, and any other required information.
We will pay all of the expenses incident to the registration, offering, and sale of the shares to the public other than commissions or
discounts of underwriters, broker-dealers, or agents. We have agreed to indemnify Aspire Capital and certain other persons against certain liabilities in connection with the offering of common shares offered hereby, including liabilities arising
under the Securities Act or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities. Aspire Capital has agreed to indemnify us against liabilities under the Securities Act that may arise from
certain written information furnished to us by Aspire Capital specifically for use in this prospectus or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers, and controlling
persons, we have been advised that in the opinion of the SEC this indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Aspire Capital and its affiliates have agreed not to engage in any direct or indirect short selling or hedging of our common shares during the
term of the Purchase Agreement.
We have advised Aspire Capital that while it is engaged in a distribution of the shares included in this
prospectus it is required to comply with Regulation M promulgated under the Exchange Act. With certain exceptions, Regulation M precludes the selling shareholder, any affiliated purchasers, and any broker-dealer or other person who participates in
the distribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase any security which is the subject of the distribution until the entire distribution is complete. Regulation M also prohibits any bids or
purchases made in order to stabilize the price of a security in connection with the distribution of that security. All of the foregoing may affect the marketability of the shares offered hereby this prospectus.
24
We may suspend the sale of shares by Aspire Capital pursuant to this prospectus for certain
periods of time for certain reasons, including if the prospectus is required to be supplemented or amended to include additional material information.
This offering will terminate on the date that all shares offered by this prospectus have been sold by Aspire Capital.
CERTAIN UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following discussion is limited to certain material U.S. federal income tax considerations relating to the purchase, ownership and
disposition of the common shares by U.S. Holders (as defined below). This discussion applies to U.S. Holders that hold common shares as capital assets. This summary is for general information purposes only and does not purport to be a complete
analysis or listing of all potential U.S. federal income tax considerations that may apply to a U.S. Holder arising from and relating to the acquisition, ownership, and disposition of common shares. Accordingly, this summary is not intended to be,
and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder.
No legal opinion from U.S. legal
counsel or ruling from the Internal Revenue Service (the “
IRS
”) has been requested, or will be obtained, regarding the U.S. federal income tax consequences of the acquisition, ownership, and disposition of common shares. This
summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions taken in this summary. In addition, because the authorities on which this summary is based are subject to
various interpretations, the IRS and the U.S. courts could disagree with one or more of the conclusions described in this summary.
This
discussion is based on the U.S. Internal Revenue Code of 1986, as amended (the “
Code
”), U.S. Treasury regulations promulgated thereunder and administrative and judicial interpretations thereof, all as in effect on the date
hereof and all of which are subject to change, possibly with retroactive effect. This summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation.
This discussion does not address all of the U.S. federal income tax considerations that may be relevant to specific U.S. Holders in light of
their particular circumstances or to U.S. Holders subject to special treatment under U.S. federal income tax law (such as certain financial institutions, insurance companies, broker-dealers and traders in securities or other persons that generally
mark their securities to market for U.S. federal income tax purposes,
tax-exempt
entities, retirement plans, regulated investment companies, real estate investment trusts, certain former citizens or residents
of the United States, persons who hold common shares as part of a “straddle,” “hedge,” “conversion transaction,” “synthetic security” or integrated investment, persons that have a “functional
currency” other than the U.S. dollar, persons that own (or are deemed to own) 10% or more (by voting power or value) of our common shares, corporations that accumulate earnings to avoid U.S. federal income tax, persons required to accelerate
the recognition of any item of gross income with respect to common shares as a result of such income being recognized on an applicable financial statement, partnerships and other pass-through entities, and investors in such pass-through entities).
This discussion does not address any U.S. state or local or
non-U.S.
tax considerations or any U.S. federal estate, gift or alternative minimum tax considerations. In addition, except as specifically set forth
below, this summary does not discuss applicable tax reporting requirements.
As used in this discussion, the term “
U.S.
Holder
” means a beneficial owner of the common shares that is, for U.S. federal income tax purposes, (1) an individual who is a citizen or resident of the United States, (2) a corporation (or entity treated as a corporation
for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof, or the District of Columbia, (3) an estate the income of which is subject to U.S. federal income tax regardless of its
source or (4) a trust (x) with respect to which a court within the United States is able to exercise primary supervision over its administration and one or more United States persons have the authority to control all of its substantial
decisions or (y) that has elected under applicable U.S. Treasury regulations to be treated as a domestic trust for U.S. federal income tax purposes.
25
If an entity treated as a partnership for U.S. federal income tax purposes holds the common
shares, the U.S. federal income tax considerations relating to an investment in the common shares will depend in part upon the status and activities of such entity and the particular partner. Any such entity should consult its own tax advisor
regarding the U.S. federal income tax considerations applicable to it and its partners of the purchase, ownership and disposition of the common shares.
Persons holding common shares should consult their own tax advisors as to the particular tax considerations applicable to them relating to
the purchase, ownership and disposition of common shares, including the applicability of U.S. federal, state and local tax laws and
non-U.S.
tax laws.
Distributions
Subject to the discussion
below under “
Passive Foreign Investment Company Considerations
,” a U.S. Holder that receives a distribution with respect to the common shares generally will be required to include the gross amount of such distribution (before
reduction for any Canadian withholding taxes) in gross income as a dividend when actually or constructively received to the extent of the U.S. Holder’s pro rata share of our current and/or accumulated earnings and profits (as determined under
U.S. federal income tax principles). To the extent a distribution received by a U.S. Holder is not a dividend because it exceeds the U.S. Holder’s pro rata share of our current and accumulated earnings and profits, it will be treated first as a
tax-free
return of capital and reduce (but not below zero) the adjusted tax basis of the U.S. Holder’s common shares. To the extent the distribution exceeds the adjusted tax basis of the U.S.
Holder’s common shares, the remainder will be taxed as capital gain. Because we may not calculate our earnings and profits under U.S. federal income tax principles, U.S. Holders should expect all distributions to be reported to them as
dividends.
The U.S. dollar value of any distribution on the common shares made in Canadian dollars generally should be calculated by
reference to the exchange rate between the U.S. dollar and the Canadian dollar in effect on the date of receipt (or deemed receipt) of such distribution by the U.S. Holder regardless of whether the Canadian dollars so received are in fact converted
into U.S. dollars at that time. If the Canadian dollars received are converted into U.S. dollars on the date of receipt (or deemed receipt), a U.S. Holder generally should not recognize currency gain or loss on such conversion. If the Canadian
dollars received are not converted into U.S. dollars on the date of receipt (or deemed receipt), a U.S. Holder generally will have a basis in such Canadian dollars equal to the U.S. dollar value of such Canadian dollars on the date of receipt (or
deemed receipt). Any gain or loss on a subsequent conversion or other disposition of such Canadian dollars by such U.S. Holder generally will be treated as ordinary income or loss and generally will be income or loss from sources within the United
States for U.S. foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method of tax accounting. Each U.S. Holder should consult its own U.S. tax advisors regarding the U.S. federal income tax consequences of
receiving, owning, and disposing of foreign currency.
Distributions on the common shares that are treated as dividends generally will
constitute income from sources outside the United States for foreign tax credit purposes and generally will constitute passive category income. Such dividends will not be eligible for the “dividends received” deduction generally allowed to
corporate shareholders with respect to dividends received from U.S. corporations. Dividends paid by a “qualified foreign corporation” are eligible for taxation at a reduced capital gains rate rather than the marginal tax rates generally
applicable to ordinary income provided that a holding period requirement (more than 60 days of ownership, without protection from the risk of loss, during the
121-day
period beginning 60 days before the
ex-dividend
date) and certain other requirements are met. However, if we are a PFIC for the taxable year in which the dividend is paid or the preceding taxable year (see discussion below under “
Passive
Foreign Investment Company Considerations
”), we will not be treated as a qualified foreign corporation, and therefore the reduced capital gains tax rate described above will not apply. Each U.S. Holder is advised to consult its own tax
advisors regarding the availability of the reduced tax rate on dividends.
If a U.S. Holder is subject to Canadian withholding tax on
dividends paid on the holder’s common shares, the U.S. Holder may be eligible, subject to a number of complex limitations, to claim a credit against its U.S.
26
federal income tax for the Canadian withholding tax imposed on the dividends. A U.S. Holder may claim a deduction for the Canadian withholding tax in lieu of a credit, but only for a year in
which the U.S. Holder elects to do so for all creditable foreign income taxes. The rules governing the foreign tax credit are complex. Each U.S. Holder is advised to consult its tax advisor regarding the availability of the foreign tax credit under
its particular circumstances.
Sale, Exchange or Other Disposition of Common Shares
Subject to the discussion below under “
Passive Foreign Investment Company Considerations
,” a U.S. Holder generally will
recognize capital gain or loss for U.S. federal income tax purposes upon the sale, exchange or other disposition of common shares. The amount of gain recognized will equal the excess of the amount realized (i.e., the amount of cash plus the
fair market value of any property received) over the U.S. Holder’s adjusted tax basis in the common shares sold or exchanged. The amount of loss recognized will equal the excess of the U.S. Holder’s adjusted tax basis in the common shares
sold or exchanged over the amount realized. Such capital gain or loss generally will be long-term capital gain or loss if, on the date of sale, exchange or other disposition, the common shares were held by the U.S. Holder for more than one year. Net
long-term capital gain derived by a
non-corporate
U.S. Holder currently is subject to tax at reduced rates. The deductibility of a capital loss is subject to limitations. Any gain or loss recognized from the
sale, exchange or other disposition of common shares will generally be gain or loss from sources within the United States for U.S. foreign tax credit purposes, except as otherwise provided in an applicable income tax treaty and if an election is
properly made under the Code.
Passive Foreign Investment Company Considerations
In general, a corporation organized outside the United States will be treated as a PFIC in any taxable year in which either (1) at least
75% of its gross income is “passive income” or (2) at least 50% of the average quarterly value of its assets is attributable to assets that produce passive income or are held for the production of passive income. Passive income for
this purpose generally includes, among other things, dividends, interest, royalties, rents, and gains from commodities transactions and from the sale or exchange of property that gives rise to passive income. In determining whether a foreign
corporation is a PFIC, a proportionate share of the items of gross income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) are taken into account.
We believe we were a PFIC for our taxable year ended December 31, 2017 based on the nature of our business, the projected composition of
our gross income and the projected composition and estimated fair market values of our assets, we expect to be a PFIC for our taxable year ending December 31, 2018 and may be a PFIC in subsequent tax years. No opinion of legal counsel or ruling
from the IRS concerning our status as a PFIC has been obtained or is currently planned to be requested. However, the determination of our PFIC status is made annually after the close of each taxable year and it is difficult to predict before such
determination whether we will be a PFIC for any given taxable year. Even if we determine that we are not a PFIC after the close of a taxable year, there can be no assurance that the IRS will agree with our conclusion. No assurance can be provided
regarded our PFIC status, and neither we nor our United States counsel expresses any opinion with respect to our PFIC status for the taxable year ended December 31, 2017 or for any other taxable year.
If we are a PFIC at any time when a U.S. Holder owns common shares, such U.S. Holder will generally be subject to federal tax under the excess
distribution regime on (1) distributions paid during a taxable year that are greater than 125% of the average annual distributions paid in the three preceding taxable years, or, if shorter, the U.S. Holder’s holding period for the
common shares, and (2) any gain recognized on a sale, exchange or other disposition (which would include a pledge) of common shares. Under the excess distribution regime, the U.S. Holder’s tax liability will be determined by allocating
such distribution or gain ratably to each day in the U.S. Holder’s holding period for the common shares. The amount allocated to the current taxable year (i.e., the year in which the distribution occurs or the gain is recognized) and any
year prior to the first taxable year in which we
27
were a PFIC in the holding period will be taxed as ordinary income earned in the current taxable year. The amount allocated to other taxable years will be taxed at the highest marginal rate in
effect (for individuals or corporations as applicable) for ordinary income in each such taxable year, and an interest charge, generally that applicable to the underpayment of tax, will be added to the tax. Once we are a PFIC with respect to a
particular U.S. Holder, we generally will remain a PFIC with respect to the U.S. Holder, unless we cease to meet the gross income and asset tests described above and the U.S. Holder makes a “deemed sale” election with respect to all of the
U.S. Holder’s common shares. If such election is made, the U.S. Holder will be deemed to have sold the common shares held at their fair market value on the last day of the last taxable year in which we qualified as a PFIC, and any gain from
such deemed sale would be taxed under the excess distribution regime described above. After the deemed sale election, the U.S. Holder’s common shares would not be treated as common shares of a PFIC unless we subsequently became a PFIC.
If we are a PFIC for any taxable year during which a U.S. Holder holds the common shares and one of our
non-United
States subsidiaries is also a PFIC (i.e., a lower-tier PFIC), the U.S. Holder will be treated as owning a proportionate amount (by value) of the common shares of the lower-tier PFIC and will be
subject to the rules described above on certain distributions by the lower-tier PFIC and a disposition (or deemed disposition) of common shares of the lower-tier PFIC, even though the U.S. Holder would not receive the distributions or the proceeds
from the disposition of the common shares of the lower-tier PFIC. Each U.S. Holder is advised to consult its tax advisors regarding the application of the PFIC rules to any of our subsidiaries.
The tax considerations that would apply if we were a PFIC would be different from those described above if a U.S. Holder were able to make a
valid “qualified electing fund,” or “QEF election.” We do not intend to provide U.S. Holders with the information required to permit them to make a QEF election and, accordingly, prospective investors should assume that a QEF
election will not be available.
A U.S. Holder may avoid taxation under the excess distribution regime if the holder makes a valid
“mark-to-market”
election. An electing U.S. Holder generally would take into account as ordinary income each year, the excess of the fair market value of the common
shares held at the end of the taxable year over the adjusted tax basis of such common shares. The U.S. Holder would also take into account, as an ordinary loss each year, the excess of the adjusted tax basis of such common shares over their fair
market value at the end of the taxable year, but only to the extent of the excess of amounts previously included in income over ordinary losses deducted as a result of the
mark-to-market
election. The U.S. Holder’s tax basis in the common shares would be adjusted to reflect any income or loss recognized as a result of the
mark-to-market
election. Any gain from a sale, exchange or other disposition of the common shares in any taxable year in which we are a PFIC, (i.e., when we meet the gross
income test or asset test described above) would be treated as ordinary income and any loss from a sale, exchange or other disposition would be treated first as an ordinary loss (to the extent of any net
mark-to-market
gains previously included in income) and thereafter as a capital loss. If we cease to be a PFIC, any gain or loss recognized by a U.S. Holder on the sale or exchange of the common shares would
be classified as a capital gain or loss.
A
mark-to-market
election is available to a U.S. Holder only for “marketable stock.” Generally, stock will be considered marketable stock if it is “regularly traded” on a “qualified exchange” within the meaning of applicable U.S.
Treasury regulations. A class of stock is regularly traded during any calendar year during which such class of stock is traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. The common shares should be
marketable stock as long as they are listed on the TSX and are regularly traded. A
mark-to-market
election will not apply to the common shares for any taxable year
during which we are not a PFIC, but will remain in effect with respect to any subsequent taxable year in which we again become a PFIC. Such election will not apply to any subsidiary that we own. Accordingly, a U.S. Holder may continue to be subject
to the PFIC rules with respect to any lower-tier PFICs notwithstanding the U.S. Holder’s
mark-to-market
election.
Each U.S. person who is a shareholder of a PFIC generally must file an annual report with the IRS containing certain information, and the
failure to file such report could result in the imposition of penalties on
28
such U.S. person and in the extension of the statute of limitations with respect to federal income tax returns filed by such U.S. person.
The U.S. federal income tax rules relating to PFICs are very complex. U.S. Holders are urged to consult their own tax advisors with respect
to the purchase, ownership and disposition of common shares, the consequences to them of an investment in a PFIC, any elections available with respect to the common shares and the IRS information reporting obligations with respect to the purchase,
ownership and disposition of common shares in the event we are considered a PFIC.
Additional Tax on Passive Income
Certain U.S. Holders that are individuals, estates or trusts (other than trusts that are exempt from tax) will be subject to a 3.8% tax on all
or a portion of their “net investment income,” which includes dividends on the common shares, and net gains from the disposition of the common shares. Further, excess distributions treated as dividends, gains treated as excess
distributions, and
mark-to-market
inclusions and deductions are all included in the calculation of net investment income.
Treasury regulations provide, subject to the election described in the following paragraph, that solely for purposes of this additional tax,
that distributions of previously taxed income will be treated as dividends and included in net investment income subject to the additional 3.8% tax. Additionally, to determine the amount of any capital gain from the sale or other taxable disposition
of common shares that will be subject to the additional tax on net investment income, a U.S. Holder who has made a QEF election will be required to recalculate its basis in the common shares excluding QEF election basis adjustments.
Alternatively, a U.S. Holder may make an election which will be effective with respect to all interests in controlled foreign corporations and
QEF election held in that year or acquired in future years. Under this election, a U.S. Holder pays the additional 3.8% tax on QEF election income inclusions and on gains calculated after giving effect to related tax basis adjustments. U.S. Holders
that are individuals, estates or trusts should consult their own tax advisors regarding the applicability of this tax to any of their income or gains in respect of the common shares.
Information Reporting with Respect to Foreign Financial Assets
U.S. individuals that own “specified foreign financial assets” with an aggregate fair market value exceeding certain threshold
amounts generally are required to file an information report on IRS Form 8938 with respect to such assets with their tax returns. Significant penalties may apply to persons who fail to comply with these rules. Specified foreign financial assets
include not only financial accounts maintained in foreign financial institutions, but also, unless held in accounts maintained by a financial institution, any stock or security issued by a
non-U.S.
person.
Upon the issuance of future U.S. Treasury regulations, these information reporting requirements may apply to certain U.S. entities that own specified foreign financial assets. The failure to report information required under the current regulations
could result in substantial penalties and in the extension of the statute of limitations with respect to federal income tax returns filed by a U.S. Holder. U.S. Holders should consult their own tax advisors regarding the possible implications of
these U.S. Treasury regulations for an investment in our common shares.
Special Reporting Requirements for Transfers to Foreign Corporations
A U.S. Holder that acquires common shares generally will be required to file Form 926 with the IRS if (1) immediately after the
acquisition such U.S. Holder, directly or indirectly, owns at least 10% of the common shares, or (2) the amount of cash transferred in exchange for common shares during the
12-month
period ending on the
date of the acquisition exceeds US$100,000. Significant penalties may apply for failing to satisfy these filing requirements. U.S. Holders are urged to contact their tax advisors regarding these filing requirements.
29
Information Reporting and Backup Withholding
Dividends on and proceeds from the sale or other disposition of common shares may be reported to the IRS unless the U.S. Holder establishes a
basis for exemption. Backup withholding may apply to amounts subject to reporting if (1) the holder fails to provide an accurate taxpayer identification number or otherwise establish a basis for exemption, or (2) is described in certain
other categories of persons.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules
generally will be allowed as a refund or a credit against a U.S. Holder’s U.S. federal income tax liability if the required information is furnished by the U.S. Holder on a timely basis to the IRS.
THE DISCUSSION ABOVE IS A GENERAL SUMMARY. IT DOES NOT COVER ALL TAX MATTERS THAT MAY BE OF IMPORTANCE TO A US HOLDER. EACH US HOLDER IS
URGED TO CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO IT OF AN INVESTMENT IN COMMON SHARES IN LIGHT OF THE INVESTOR’S OWN CIRCUMSTANCES.
CERTAIN CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
The following is, as of the date hereof, a summary of certain Canadian federal income tax considerations under the
Income Tax Act
(Canada) (the “
Tax Act
”) generally applicable to a person who holds, as beneficial owner, common shares of the Company and who, for purposes of the Tax Act and at all relevant times, is neither resident in Canada nor deemed
to be resident in Canada for purposes of the Tax Act and any applicable income tax treaty or convention, and who does not use or hold (and is not deemed to use or hold) common shares in carrying on a business in Canada, deals at arm’s length
with and is not affiliated with the Company and holds common shares as capital property (a “
Holder
”). Generally, common shares will be considered to be capital property to a Holder thereof provided that the Holder does not
hold common shares in the course of carrying on a business of buying and selling securities and such Holder has not acquired them in one or more transactions considered to be an adventure or concern in the nature of trade.
This summary does not apply to a Holder (i) that is a “financial institution” for purposes of the
mark-to-market
rules contained in the Tax Act; (ii) that is a “specified financial institution” as defined in the Tax Act; (iii) an interest in which is a
“tax shelter investment” as defined in the Tax Act; or (iv) that has elected to report its tax results in a functional currency other than Canadian currency. Special rules, which are not discussed in this summary, may apply to a
Holder that is an “authorized foreign bank” within the meaning of the Tax Act or an insurer carrying on business in Canada and elsewhere. Such Holders should consult their own tax advisors.
This summary is based upon the provisions of the Tax Act (including the regulations (“
Regulations
”) thereunder) in
force as of the date hereof and our understanding of the current administrative policies and assessing practices of the Canada Revenue Agency (the “
CRA
”) published in writing by the CRA prior to the date hereof. This summary
takes into account all specific proposals to amend the Tax Act (and the Regulations) publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the “
Tax Proposals
”) and assumes that the
Tax Proposals will be enacted in the form proposed, although no assurance can be given that the Tax Proposals will be enacted in their current form or at all. This summary does not otherwise take into account any changes in law or in the
administrative policies or assessing practices of the CRA, whether by legislative, governmental or judicial decision or action. This summary is not exhaustive of all possible Canadian federal income tax considerations, and does not take into account
other federal or any provincial, territorial or foreign income tax legislation or considerations, which may differ materially from those described in this summary.
This summary is of a general nature only and is not, and is not intended to be, and should not be construed to be, legal or tax advice to any
particular Holder, and no representations concerning the tax consequences to any particular Holder are made.
Holders should consult their own tax advisors regarding the income tax considerations applicable to them having regard to their
particular circumstances.
30
Dividends
Dividends paid or credited (or deemed to be paid or credited) to a Holder by the Company are subject to Canadian withholding tax at the rate of
25% unless reduced by the terms of an applicable tax treaty. For example, under the Canada-United States Income Tax Convention (1980) (the “
US Treaty
”), as amended, the dividend withholding tax rate is generally reduced to
15% in respect of a dividend paid or credited to a Holder beneficially entitled to the dividend who is resident in the U.S. for purposes of the US Treaty and whose entitlement to the benefits of the US Treaty is not limited by the limitation of
benefits provisions of the US Treaty.
Holders are urged to consult their own tax advisors to determine their entitlement to relief under the US Treaty or any other applicable tax treaty as well as their ability to claim foreign tax credits with
respect to any Canadian withholding tax, based on their particular circumstances.
Disposition of Common Shares
A Holder generally will not be subject to tax under the Tax Act in respect of a capital gain realized on the disposition or deemed disposition
of a common share, unless the common share constitutes or is deemed to constitute “taxable Canadian property” to the Holder thereof for purposes of the Tax Act, and the gain is not exempt from tax pursuant to the terms of an applicable tax
treaty.
In general, provided the common shares are listed on a “designated stock exchange” (which currently includes the TSX
and the Nasdaq Capital Market) at the date of the disposition, such common shares will only constitute “taxable Canadian property” of a Holder if, at any time within the
60-month
period preceding the
disposition, the following two conditions are met concurrently: (i) such Holder, persons with whom the Holder did not deal at arm’s length, partnerships in which the Holder, or a person with whom the Holder did not deal at arm’s
length, holds a membership interest directly or indirectly through one or more partnerships, or any combination thereof, owned 25% or more of the issued shares of any class or series of the Company’s share capital, and (ii) more than 50%
of the fair market value of the common shares was derived directly or indirectly from one or any combination of (A) real or immovable property situated in Canada, (B) “Canadian resource properties” (as defined in the Tax Act), (C)
“timber resource properties” (as defined in the Tax Act), and (D) options in respect of, or interests in, or for civil law rights in, property described in any of subparagraphs (ii)(A) to (C), whether or not the property exists.
However, and despite the foregoing, in certain circumstances the common shares may be deemed to be “taxable Canadian property” under the Tax Act.
Holders whose common shares may be “taxable Canadian property” should consult their own tax advisors.
INTERESTS OF NAMED EXPERTS AND COUNSEL
None.
EXPERTS
The financial statements incorporated into this prospectus by reference to the Annual Report on Form
40-F
for the year ended December 31, 2017, have been so incorporated in reliance on the reports of KPMG LLP, an independent registered public accounting firm, given on the authority of said firm as
experts in auditing and accounting. The offices of KPMG LLP are located at 100 New Park Place, Suite 1400, Vaughan, Ontario, L4K 0J3, Canada.
LEGAL MATTERS
The validity of the common shares and certain other matters of Canadian law will be passed upon for us by McCarthy Tetrault LLP, Toronto,
Ontario, our Canadian counsel. Certain matters of United States law will be passed upon for us by Dorsey & Whitney LLP, Vancouver, B.C. and Denver, Colorado, our United States counsel.
31
MATERIAL CHANGES
Except as otherwise described in our Annual Report on Form 40-F for the fiscal period ended December 31, 2017, in our Reports on Form 6-K
filed or furnished under the Exchange Act and incorporated by reference herein and as disclosed in this prospectus, no material changes have occurred since December 31, 2017.
32

6,041,567
Common Shares
Prospectus
April 13, 2018
Aptose Biosciences (NASDAQ:APTO)
Historical Stock Chart
From Mar 2024 to Apr 2024
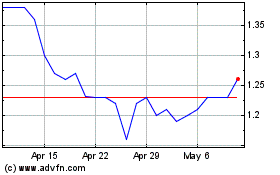
Aptose Biosciences (NASDAQ:APTO)
Historical Stock Chart
From Apr 2023 to Apr 2024