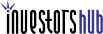Xenoport (XNPT) focuses on developing and commercializing a portfolio of internally discovered product candidates for the treatment of neurological disorders.
XenoPort is working on advancing XP23829 into phase III studies for treatment of relapsing forms of multiple sclerosis. The company will also submit an Investigational New Drug (IND) application to the U.S. Food and Drug Administration (:FDA) for XP23829 for the treatment of moderate-to-severe chronic plaque psoriasis. A phase II study is slated to start by mid-2014.
XenoPort has several products in different stages of development. The company currently has one approved product on the market called Horizant (gabapentin enacarbil), which is used to treat Restless Leg Syndrome. The product is marketed in both the United States and Japan. Astellas Pharma is partnered with XenoPort and has commercial rights for the product in Japan.
The company is also developing XP21279 for Parkinson’s Disease and has completed Phase II clinical trials. XP21279 is a Xenoport’s patented oral product candidate that utilizes naturally-occurring, high-capacity nutrient transporters in the gastrointestinal tract to generate active, efficient absorption into the body. Once absorbed, XP21279 is rapidly converted into levodopa, a drug that acts to replace dopamine in the brain.
XP23829 is currently being studied in a Phase 1 clinical trial in healthy subjects and may have potential as a treatment for relapsing-remitting multiple sclerosis (RRMS) and/or psoriasis. However, these trials are in very early stages, but we think the market opportunity is so large, that XenoPort could attract new institutional ownership.
For a company with a $300M market cap, we feel it’s rather under speculated, and could attract more market wide attention in the coming months, as the company will be releasing data points.
Additionally, XenoPort is also expecting to release data from a post-marketing study for Horizant patients in the coming months, which should drive the stock in the near term. The company is also presenting at the 34th Annual Health Care Conference at 10:40 a.m EST on March 5, 2014.
Top Institutional Holders
| Holder | Shares | % Out | Value* | Reported |
|---|---|---|---|---|
| Orbimed Advisors LLC. | 4,590,800 | 7.69 | 26,397,100 | Dec 31, 2013 |
| Deerfield Management | 3,719,040 | 6.23 | 21,384,480 | Dec 31, 2013 |
| Capital World Investors | 3,620,000 | 6.06 | 20,815,000 | Dec 31, 2013 |
| BlackRock Fund Advisors | 1,739,961 | 2.91 | 10,004,775 | Dec 31, 2013 |
| BlackRock Institutional Trust Company, N.A. | 1,652,806 | 2.77 | 9,503,634 | Dec 31, 2013 |
| FMR, LLC | 1,425,707 | 2.39 | 8,197,815 | Dec 31, 2013 |
| Vanguard Group, Inc. (The) | 1,215,613 | 2.04 | 6,989,774 | Dec 31, 2013 |
| Wellington Management Company, LLP | 6,670,334 | 11.17 | 38,354,420 | Dec 31, 2013 |
| Millennium Management LLC | 1,170,767 | 1.96 | 6,731,910 | Dec 31, 2013 |
| Clinton Group, Inc. | 1,110,221 | 1.86 | 6,383,770 | Dec 31, 2013 |
XenoPort has strong institution ownership, with Orbimed Advisors owning a substantial block. Additionally, the company recently engaged in a public offering of 12,000,000 shares of its common stock at a price to the public of $6.00 per share, without warrants.
While certainly a speculative bet, we feel XenoPort could be on to something with its proprietary platform, and if proven sucessfull, could end up being a highly valuated company in time, provided management stays on a correct course.
Chart:
The chart above clearly shows strong accumulation and money flow, along with the MACD just crossing into positive. The last time the stock had a strong MACD cross, it hit $7.20. We feel if the market maintains and does not roll over, that $7.50 is within range here.