0001442999
false
0001442999
2021-04-01
2021-06-30
0001442999
2021-10-11
2021-10-11
0001442999
2021-06-30
0001442999
2021-03-31
0001442999
us-gaap:CommonStockMember
2021-01-06
0001442999
us-gaap:AdditionalPaidInCapitalMember
2021-01-06
0001442999
us-gaap:ComprehensiveIncomeMember
2021-01-06
0001442999
us-gaap:RetainedEarningsMember
2021-01-06
0001442999
2021-01-06
0001442999
us-gaap:CommonStockMember
2021-01-07
2021-03-31
0001442999
us-gaap:AdditionalPaidInCapitalMember
2021-01-07
2021-03-31
0001442999
us-gaap:ComprehensiveIncomeMember
2021-01-07
2021-03-31
0001442999
us-gaap:RetainedEarningsMember
2021-01-07
2021-03-31
0001442999
2021-01-07
2021-03-31
0001442999
us-gaap:CommonStockMember
2021-03-31
0001442999
us-gaap:AdditionalPaidInCapitalMember
2021-03-31
0001442999
us-gaap:ComprehensiveIncomeMember
2021-03-31
0001442999
us-gaap:RetainedEarningsMember
2021-03-31
0001442999
us-gaap:CommonStockMember
2021-04-01
2021-06-30
0001442999
us-gaap:AdditionalPaidInCapitalMember
2021-04-01
2021-06-30
0001442999
us-gaap:ComprehensiveIncomeMember
2021-04-01
2021-06-30
0001442999
us-gaap:RetainedEarningsMember
2021-04-01
2021-06-30
0001442999
us-gaap:CommonStockMember
2021-06-30
0001442999
us-gaap:AdditionalPaidInCapitalMember
2021-06-30
0001442999
us-gaap:ComprehensiveIncomeMember
2021-06-30
0001442999
us-gaap:RetainedEarningsMember
2021-06-30
0001442999
ABTI:IssuanceOneMember
2010-04-02
2010-05-03
0001442999
ABTI:IssuanceTwoMember
2021-01-01
2021-01-29
0001442999
2021-05-28
0001442999
ABTI:AuditFeesMember
2021-06-30
0001442999
ABTI:AuditFeesMember
2021-03-31
0001442999
ABTI:AccountingMember
2021-06-30
0001442999
ABTI:AccountingMember
2021-03-31
0001442999
ABTI:ResearchAndDevelopmentMember
2021-06-30
0001442999
ABTI:ResearchAndDevelopmentMember
2021-03-31
0001442999
ABTI:LegalAndTransferAgentMember
2021-06-30
0001442999
ABTI:LegalAndTransferAgentMember
2021-03-31
0001442999
2009-04-16
2021-06-30
0001442999
ABTI:TransitionalPeriodMember
2021-03-31
0001442999
ABTI:TransitionalPeriodMember
2020-09-30
0001442999
ABTI:TransitionalPeriodMember
2019-09-30
0001442999
ABTI:TransitionalPeriodMember
2020-10-01
2021-03-31
0001442999
ABTI:TransitionalPeriodMember
2019-10-01
2020-03-31
0001442999
ABTI:TransitionalPeriodMember
2019-10-01
2020-09-30
0001442999
ABTI:TransitionalPeriodMember
2018-10-01
2019-09-30
0001442999
us-gaap:CommonStockMember
ABTI:TransitionalPeriodMember
2019-09-30
0001442999
us-gaap:AdditionalPaidInCapitalMember
ABTI:TransitionalPeriodMember
2019-09-30
0001442999
ABTI:SharesHeldInTrustMember
ABTI:TransitionalPeriodMember
2019-09-30
0001442999
us-gaap:ComprehensiveIncomeMember
ABTI:TransitionalPeriodMember
2019-09-30
0001442999
us-gaap:CommonStockMember
ABTI:TransitionalPeriodMember
2019-10-01
2020-09-30
0001442999
us-gaap:AdditionalPaidInCapitalMember
ABTI:TransitionalPeriodMember
2019-10-01
2020-09-30
0001442999
ABTI:SharesHeldInTrustMember
ABTI:TransitionalPeriodMember
2019-10-01
2020-09-30
0001442999
us-gaap:ComprehensiveIncomeMember
ABTI:TransitionalPeriodMember
2019-10-01
2020-09-30
0001442999
us-gaap:CommonStockMember
ABTI:TransitionalPeriodMember
2020-09-30
0001442999
us-gaap:AdditionalPaidInCapitalMember
ABTI:TransitionalPeriodMember
2020-09-30
0001442999
ABTI:SharesHeldInTrustMember
ABTI:TransitionalPeriodMember
2020-09-30
0001442999
us-gaap:ComprehensiveIncomeMember
ABTI:TransitionalPeriodMember
2020-09-30
0001442999
us-gaap:CommonStockMember
ABTI:TransitionalPeriodMember
2020-10-01
2021-03-31
0001442999
us-gaap:AdditionalPaidInCapitalMember
ABTI:TransitionalPeriodMember
2020-10-01
2021-03-31
0001442999
ABTI:SharesHeldInTrustMember
ABTI:TransitionalPeriodMember
2020-10-01
2021-03-31
0001442999
us-gaap:ComprehensiveIncomeMember
ABTI:TransitionalPeriodMember
2020-10-01
2021-03-31
0001442999
us-gaap:CommonStockMember
ABTI:TransitionalPeriodMember
2021-03-31
0001442999
us-gaap:AdditionalPaidInCapitalMember
ABTI:TransitionalPeriodMember
2021-03-31
0001442999
ABTI:SharesHeldInTrustMember
ABTI:TransitionalPeriodMember
2021-03-31
0001442999
us-gaap:ComprehensiveIncomeMember
ABTI:TransitionalPeriodMember
2021-03-31
0001442999
ABTI:TransitionalPeriodMember
2018-09-30
0001442999
ABTI:TransitionalPeriodMember
2020-03-31
0001442999
2020-10-01
2021-03-31
0001442999
ABTI:AuditFeesMember
ABTI:TransitionalPeriodMember
2021-03-31
0001442999
ABTI:AuditFeesMember
ABTI:TransitionalPeriodMember
2020-09-30
0001442999
ABTI:AccountingMember
ABTI:TransitionalPeriodMember
2021-03-31
0001442999
ABTI:AccountingMember
ABTI:TransitionalPeriodMember
2020-09-30
0001442999
ABTI:LegalAndTransferAgentMember
ABTI:TransitionalPeriodMember
2021-03-31
0001442999
ABTI:LegalAndTransferAgentMember
ABTI:TransitionalPeriodMember
2020-09-30
0001442999
ABTI:TransitionalPeriodMember
ABTI:OfficerIssuanceMember
2018-10-01
2019-09-30
0001442999
ABTI:IssuanceTwoMember
ABTI:TransitionalPeriodMember
2021-01-01
2021-01-29
0001442999
2020-09-30
0001442999
ABTI:TransitionalPeriodMember
2009-01-16
2021-03-31
0001442999
ABTI:TransitionalPeriodMember
2021-05-01
2021-05-24
0001442999
ABTI:ABTIPharmaMember
2021-03-31
0001442999
ABTI:ABTIPharmaMember
2020-03-31
0001442999
ABTI:OrdinarySharesMember
2021-03-31
0001442999
ABTI:OrdinarySharesMember
2020-03-31
0001442999
ABTI:ABTIPharmaMember
2020-04-01
2021-03-31
0001442999
ABTI:ABTIPharmaMember
2019-04-01
2020-03-31
0001442999
us-gaap:CommonStockMember
ABTI:ABTIPharmaMember
2019-03-31
0001442999
us-gaap:AdditionalPaidInCapitalMember
ABTI:ABTIPharmaMember
2019-03-31
0001442999
us-gaap:RetainedEarningsMember
ABTI:ABTIPharmaMember
2019-03-31
0001442999
us-gaap:ComprehensiveIncomeMember
ABTI:ABTIPharmaMember
2019-03-31
0001442999
ABTI:ABTIPharmaMember
2019-03-31
0001442999
us-gaap:CommonStockMember
ABTI:ABTIPharmaMember
2019-04-01
2020-03-31
0001442999
us-gaap:AdditionalPaidInCapitalMember
ABTI:ABTIPharmaMember
2019-04-01
2020-03-31
0001442999
us-gaap:RetainedEarningsMember
ABTI:ABTIPharmaMember
2019-04-01
2020-03-31
0001442999
us-gaap:ComprehensiveIncomeMember
ABTI:ABTIPharmaMember
2019-04-01
2020-03-31
0001442999
us-gaap:CommonStockMember
ABTI:ABTIPharmaMember
2020-03-31
0001442999
us-gaap:AdditionalPaidInCapitalMember
ABTI:ABTIPharmaMember
2020-03-31
0001442999
us-gaap:RetainedEarningsMember
ABTI:ABTIPharmaMember
2020-03-31
0001442999
us-gaap:ComprehensiveIncomeMember
ABTI:ABTIPharmaMember
2020-03-31
0001442999
us-gaap:CommonStockMember
ABTI:ABTIPharmaMember
2020-04-01
2021-03-31
0001442999
us-gaap:AdditionalPaidInCapitalMember
ABTI:ABTIPharmaMember
2020-04-01
2021-03-31
0001442999
us-gaap:RetainedEarningsMember
ABTI:ABTIPharmaMember
2020-04-01
2021-03-31
0001442999
us-gaap:ComprehensiveIncomeMember
ABTI:ABTIPharmaMember
2020-04-01
2021-03-31
0001442999
us-gaap:CommonStockMember
ABTI:ABTIPharmaMember
2021-03-31
0001442999
us-gaap:AdditionalPaidInCapitalMember
ABTI:ABTIPharmaMember
2021-03-31
0001442999
us-gaap:RetainedEarningsMember
ABTI:ABTIPharmaMember
2021-03-31
0001442999
us-gaap:ComprehensiveIncomeMember
ABTI:ABTIPharmaMember
2021-03-31
0001442999
2020-04-01
2021-03-31
0001442999
2020-03-31
0001442999
ABTI:AlterolaBiotechMember
2021-03-31
0001442999
ABTI:ABTIPharmaLimitedMember
2021-03-31
0001442999
ABTI:ProformaAdjustmentsMember
2021-03-31
0001442999
ABTI:ProformaAsAdjustedMember
2021-03-31
0001442999
ABTI:ProFormaFinancialsMember
2021-03-31
0001442999
ABTI:AlterolaBiotechMember
2020-04-01
2021-03-31
0001442999
ABTI:ABTIPharmaLimitedMember
2020-04-01
2021-03-31
0001442999
ABTI:ProformaAdjustmentsMember
2020-04-01
2021-03-31
0001442999
ABTI:ProformaAsAdjustedMember
2020-04-01
2021-03-31
0001442999
2021-05-01
2021-05-24
0001442999
ABTI:ProFormaFinancialsMember
2020-04-01
2021-03-31
0001442999
2021-05-01
2021-05-28
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
iso4217:GBP
iso4217:GBP
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Alterola Biotech, Inc.
(Exact name of registrant as specified in its charter)
|
Nevada
|
|
2834
|
|
82-1317032
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(Primary Standard Industrial
Classification Code Number)
|
|
(I.R.S. Employer
Identification No.)
|
47 Hamilton Square Birkenhead Merseyside
CH41 5AR United Kingdom
(800) 706-0806
(Address, including zip code, and telephone number,
including area code, of registrant’s principal executive office)
Spring Valley Solutions, LLC
4955 S. Durango Rd. Ste. 165
Las Vegas, NV 89113
(702) 982-5686
(Name, address, including zip code, and telephone
number, including area code, of agent for service)
Copies to:
Scott Doney
The Doney Law Firm
4955 S. Durango Rd. Ste. 165
Las Vegas, NV 89113
(702) 982-5686
Approximate date of commencement
of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being
registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check
the following box. [X]
If this Form is filed to register
additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities
Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective
amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement
number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective
amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement
number of the earlier effective registration statement for the same offering. [ ]
Indicate by check mark whether
the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging
growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting
company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer
|
☐
|
Accelerated filer
|
☐
|
|
Non-accelerated Filer
|
☒
|
Smaller reporting company
|
☒
|
|
|
Emerging Growth Company
|
☐
|
If an emerging growth company,
indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial
accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
|
Title of Each Class of
Securities to be Registered
|
|
Number of
Shares of
Common
Stock to be
Registered(1)
|
|
|
Proposed
Maximum
Offering
Price Per
Share
|
|
|
Proposed
Maximum
Aggregate
Offering
Price(1)
|
|
|
Amount of
Registration
Fee(2)
|
|
|
Common stock, par value $0.001 per share
|
|
|
55,000,000
|
|
|
$
|
2.00
|
|
|
$
|
110,000,000
|
|
|
$
|
10,197
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1)
|
Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended.
|
|
(2)
|
Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price.
|
The Registrant hereby amends
this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further
amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a)
of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and
Exchange Commission, acting pursuant to Section 8(a), may determine.
The information
in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed
with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer
to buy these securities in any state or other jurisdiction where the offer or sale is not permitted.
|
PRELIMINARY PROSPECTUS
|
SUBJECT
TO COMPLETION, DATED OCTOBER 18, 2021
|

Alterola Biotech, Inc.
55,000,000 Shares of Common Stock
Our common stock is quoted on
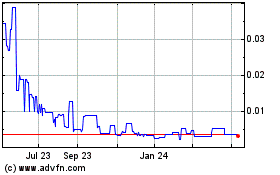
the OTC Markets under the symbol “ABTI.” The last reported sale price of our common stock on October 18, 2021 was $0.343 per
share.
This prospectus relates to the
offer and sale of up to 55,000,000 shares of common stock, par value $0.001, of Alterola Biotech Inc., a Nevada corporation, by EMC2 Capital,
LLC, or EMC2 or the Selling Stockholder.
The shares of common stock being
offered by the Selling Stockholder have been or may be issued pursuant to the purchase agreement dated August 11, 2021, that we entered
into with EMC2. See “The EMC2 Transaction” on page 7 for a description of that agreement and “Selling Stockholder”
on page 38 for additional information regarding EMC2. The prices at which EMC2 may sell the shares will be determined by the prevailing
market price for the shares or in negotiated transactions.
We are not selling any securities
under this prospectus and will not receive any of the proceeds from the sale of shares by the Selling Stockholder.
The Selling Stockholder may sell
the shares of common stock described in this prospectus in a number of different ways and at varying prices. See “Plan of Distribution”
on page 34 for more information about how the Selling Stockholder may sell the shares of common stock being registered pursuant to this
prospectus. The Selling Stockholder is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act of 1933,
as amended.
We will pay the expenses incurred
in registering the shares, including legal and accounting fees. See “Plan of Distribution”.
Investing in our common
stock involves a high degree of risk. See “Risk Factors” on page 4 in this prospectus to read about the factors you
should consider before buying shares of our common stock.
We may amend or supplement this
prospectus from time to time by filing amendments or supplements as required. You should read the entire prospectus and any amendments
or supplements carefully before you make your investment decision.
Neither the Securities and Exchange
Commission nor any other state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy
of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is
October 18, 2021
TABLE OF CONTENTS
You should rely only on the
information contained in this prospectus and in any free writing prospectus that we may provide to you in connection with this offering.
We have not authorized anyone to provide you with information different from, or in addition to, that contained in this prospectus or
any such free writing prospectus. If anyone provides you with different or inconsistent information, you should not rely on it. We can
provide no assurance as to the reliability of any other information that others may give you. We are not making an offer to sell or seeking
offers to buy these securities in any jurisdiction where or to any person to whom the offer or sale is not permitted. The information
in this prospectus is accurate only as of the date on the front cover of this prospectus, and the information in any free writing prospectus
that we may provide you in connection with this offering is accurate only as of the date of such free writing prospectus. Our business,
financial condition, results of operations and prospects may have changed since those dates. Neither we, nor any of our officers, directors,
or agents, makes any representation to you about the legality of an investment in our common stock. You should not interpret the contents
of this prospectus or any free writing prospectus to be legal, business, investment or tax advice. You should consult with your own advisors
for that type of advice and consult with them about the legal, tax, business, financial and other issues that you should consider before
investing in our common stock.
PROSPECTUS SUMMARY
This summary highlights information
about this offering and the information included in this prospectus. This summary does not contain all of the information that you should
consider before investing in our securities. You should carefully read this entire prospectus, especially the sections titled “Risk
Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated
financial statements included herein, including the notes thereto, before making an investment decision. References in this prospectus
to “we,” “us,” “our,” “the company” and the “Company” refer to Alterola Biotech,
Inc. and, where appropriate, its subsidiaries, unless expressly indicated or the context otherwise requires.
Business Overview
Our goal is to provide better
medicines for patients across the globe. We believe in harnessing the therapeutic potential of cannabinoids and cannabinoid- like compounds,
which can bring valuable treatments to seriously ill patients. Rather than just focussing on one method of identifying, researching and
developing such medicines, we are interested in developing new medicines from all sources including botanical, traditional chemical synthesis
and biosynthetic methodologies.
On May 28, 2021, we acquired ABTI
Pharma Limited, a company registered in England and Wales (“ABTI Pharma”), with the purchase of all of its capital stock in
exchange for 600,000,000 shares of our common stock pro rata to the ABTI Pharma shareholders.
As a result of the acquisition,
we are a pharmacuetical company working with cannabinoid and cannabinoid like molecules. We have three areas of focus:
1) Development of regulated pharmaceuticals
(human and animal health) and regulated food products. This has been achieved via the strategic acquisition of Phytotherapeutix Ltd.
2) Production of low cost of goods
Active Pharmaceutical Ingredient (API) and food-grade ingredients (supported by the strategic acquisition of Ferven Ltd), and
3) Formulation, and drug delivery,
providing improved bioavailability, solubility and stability (supported by the exclusive licensing of IP and technology from Nano4M Ltd).
Phytotherapeutix Ltd, a subsidiary
of ABTI Pharma, has generated a number of molecules with patents pending, some of which have demonstrable pharmacological activity, similar
to that of CBD. This means that some of these molecules are anticipated to have a similar market potential to CBD across a range of therapeutic
areas.
Ferven Ltd, another subsidiary
of ABTI Pharma, is looking to produce cannabinoids by fermentation. The exclusively licensed organism has the potential to be genetically
modified to produce multiple cannabinoids at a very low cost of goods. It is anticipated that the selected genetically modified organisms
will grow very quickly, which in turn, reduces the cost of production.
Nano4M Ltd is a company which
has exclusively licensed its nano-formulation patents and know-how to ABTI Pharma Ltd.
Additionally, we may consider
entering into Joint Venture Partnerships, Acquisition of Companies with complimentary portfolios or Licencing Agreements to enhance the
product portfolio. These are strategies the Company may implement and any such opportunities will be assessed on a case by case basis
and on their merit at the time.
ABTI Pharma management has extensive
proven experience, know-how and connections in the cannabinoid medicines sector, and is looking to utilize this knowledge and experience
for the development of such medicines from existing cannabinoids and cannabinoid-like molecules.
Our address is 47 Hamilton Square
Birkenhead Merseyside CH41 5AR United Kingdom. Our telephone number is +44 151 601 9477. Our website is www.alterolabio.com.
We do not incorporate the information
on or accessible through our websites into this Registration Statement, and you should not consider any information on, or that can be
accessed through, our websites a part of this Registration Statement.
The EMC2 Transaction
On August 11, 2021, we entered
into a purchase agreement with EMC2, which we refer to in this prospectus as the Purchase Agreement, pursuant to which EMC2 has agreed
to purchase from us up to an aggregate of $125,000,000 of our common stock (subject to certain limitations) from time to time over the
term of the Purchase Agreement. Also, on August 11, 2021, we entered into a registration rights agreement with EMC2, which we refer to
in this prospectus as the Registration Rights Agreement, pursuant to which we are required to file with the SEC a registration statement
that includes this prospectus to register for resale under the Securities Act of 1933, as amended, or the Securities Act, the shares of
common stock that have been or may be issued to EMC2 under the Purchase Agreement. Pursuant to the terms of the Purchase Agreement, at
the time we signed the Purchase Agreement and the Registration Rights Agreement, we issued 7,500,000 shares of our common stock and 15,000,000
warrants to EMC2 as consideration for its commitment to purchase shares of our common stock under the Purchase Agreement, which we refer
to in this prospectus as the Commitment Shares and Commitment Warrants.
We do not have the right to commence
any sales of our common stock to EMC2 under the Purchase Agreement until certain conditions set forth in the Purchase Agreement, all of
which are outside of EMC2’s control, have been satisfied, including that the SEC has declared effective the registration statement
that includes this prospectus. Thereafter, we may, from time to time and at our sole discretion, direct EMC2 to purchase shares of our
common stock in amounts up to 100,000 shares on any single business day, subject to a maximum of $1,000,000 per purchase, plus other “VWAP
Purchases” under certain circumstances. There are no trading volume requirements or restrictions under the Purchase Agreement, and
we will control the timing and amount of any sales of our common stock to EMC2. The purchase price of the shares that may be sold to EMC2
under the Purchase Agreement will be based on the market price of our common stock preceding the time of sale as computed under the Purchase
Agreement. The purchase price per share will be equitably adjusted for any reorganization, recapitalization, non-cash dividend, stock
split, or other similar transaction occurring during the business days used to compute such price. We may at any time in our sole discretion
terminate the Purchase Agreement without fee, penalty or cost upon one business day notice. There are no restrictions on future financings,
rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement or Registration Rights Agreement,
other than a prohibition on entering into a “Variable Rate Transaction,” as defined in the Purchase Agreement. EMC2 may not
assign or transfer its rights and obligations under the Purchase Agreement.
As of August 11, 2021, there were
754,280,000 shares of our common stock outstanding, of which 115,130,000 shares were held by non-affiliates. Although the Purchase Agreement
provides that we may sell up to $125,000,000 of our common stock to EMC2, only 55,000,000 shares of our common stock are being offered
under this prospectus, which represents: (i) 7,500,000 shares that we issued to EMC2 as a commitment fee for making the commitment under
the Purchase Agreement; (ii) 15,000,000 shares underlying the Commitment Warrants; and (iii) an additional 32,500,000 shares which may
be issued to EMC2 in the future under the Purchase Agreement, if and when we sell shares to EMC2 under the Purchase Agreement. Depending
on the market price of our common stock at the time we elect to issue and sell shares to EMC2 under the Purchase Agreement, we may need
to register for resale under the Securities Act additional shares of our common stock in order to receive aggregate gross proceeds equal
to the $125,000,000 total commitment available to us under the Purchase Agreement. If all of the 55,000,000 shares offered by EMC2 under
this prospectus were issued and outstanding as of the date hereof, such shares would represent 6.7% of the total number of shares of our
common stock outstanding and 32.33% of the total number of outstanding shares held by non-affiliates, in each case as of the date hereof.
If we elect to issue and sell more than the 55,000,000 shares offered under this prospectus to EMC2, which we have the right, but not
the obligation, to do, we must first register for resale under the Securities Act any such additional shares, which could cause additional
substantial dilution to our stockholders. The number of shares ultimately offered for resale by EMC2 is dependent upon the number of shares
we sell to EMC2 under the Purchase Agreement.
The Purchase Agreement also prohibits
us from directing EMC2 to purchase any shares of common stock if those shares, when aggregated with all other shares of our common stock
then beneficially owned by EMC2 and its affiliates, would result in EMC2 and its affiliates having beneficial ownership, at any single
point in time, of more than 4.99% of the then total outstanding shares of our common stock, as calculated pursuant to Section 13(d) of
the Securities Exchange Act of 1934, as amended, or the Exchange Act, and Rule 13d-3 thereunder, which limitation we refer to as the Beneficial
Ownership Cap.
Issuances of our common stock
in this offering will not affect the rights or privileges of our existing stockholders, except that the economic and voting interests
of each of our existing stockholders will be diluted as a result of any such issuance. Although the number of shares of common stock that
our existing stockholders own will not decrease, the shares owned by our existing stockholders will represent a smaller percentage of
our total outstanding shares after any such issuance to EMC2.
The Offering
|
Common stock outstanding prior to this offering
|
|
754,280,000 shares of common stock
|
|
|
|
|
|
Common stock offered by the Selling Stockholder
|
|
55,000,000 shares of common stock consisting of: 7,500,000 Commitment Shares issued to EMC2; 15,000,000 shares underlying the Commitement Warrants issued to EMC2; and 32,500,000 shares we may sell to EMC2 under the Purchase Agreement from time to time after the date of this prospectus.
|
|
|
|
|
|
Common stock to be outstanding immediately after this offering(1)
|
|
809,280,000 shares of common stock. If issued presently, the 55,000,000 shares of common stock registered for resale by EMC2 would represent approximately 6.7% of our issued and outstanding shares of common stock. Additionally, the 55,000,000 shares of common stock registered for resale herein would represent approximately 33% of our public float.
|
|
|
|
|
|
Offering price per share
|
|
EMC2 (the selling stockholder identified in this prospectus) may sell all or a portion of the shares being offered pursuant to this prospectus at fixed prices and prevailing market prices at the time of sale, at varying prices or at negotiated prices.
|
|
|
|
|
|
Use of proceeds
|
|
We will not receive any proceeds from the sale of the shares of our common stock by EMC2 (the selling stockholder identified in this prospectus). However, we will receive proceeds from our initial sale of shares to EMC2, pursuant to the Purchase Agreement. The proceeds from the initial sale of shares will be used for the purpose of working capital and that the Board of Directors, in good faith deem to be in the best interest of the Company. See “Use of Proceeds”
|
|
|
|
|
|
Duration of this offering
|
|
The offering shall terminate on the earlier of (i) the date when the sale of all 55,000,000 shares is completed, or (ii) August 11, 2024.
|
|
|
|
|
|
Risk factors
|
|
Investing in our common stock involves a high
degree of risk, and the purchasers of our common stock may lose all or part of their investment. Before deciding to invest in our
securities, please carefully read the section entitled “Risk Factors” beginning on page 4 and the other information in
this prospectus.
|
|
|
|
|
|
OTC Markets trading symbol
|
|
Our common stock is quoted on the OTC Markets under the symbol “ABTI.”
|
RISK FACTORS
An investment in our securities
involves a high degree of risk. In addition to the other information contained in this prospectus, prospective investors should carefully
consider the following risks before investing in our securities. If any of the following risks actually occur, as well as other risks
not currently known to us or that we currently consider immaterial, our business, operating results and financial condition could be materially
adversely affected. As a result, the trading price of our common stock could decline, and you may lose all or part of your investment
in our common stock. The risks discussed below also include forward-looking statements, and our actual results may differ substantially
from those discussed in these forward-looking statements. See “Cautionary Note Regarding Forward-Looking Statements” in this
prospectus. In assessing the risks below, you should also refer to the other information contained in this prospectus, including the financial
statements and the related notes, before deciding to purchase any of our securities.
Risk Related to Covid 19
Our business and future
operations may be adversely affected by epidemics and pandemics, such as the recent COVID-19 outbreak.
We may face risks related
to health epidemics and pandemics or other outbreaks of communicable diseases, which could result in a widespread health crisis that could
adversely affect general commercial activity and the economies and financial markets of the world as a whole. For example, the outbreak
of COVID-19, which began in China, has been declared by the World Health Organization to be a “pandemic,” has spread across
the globe, including the United States of America. A health epidemic or pandemic or other outbreak of communicable diseases, such as the
current COVID-19 pandemic, poses the risk that we, or potential business partners may be disrupted or prevented from conducting business
activities for certain periods of time, the durations of which are uncertain, and may otherwise experience significant impairments of
business activities, including due to, among other things, operational shutdowns or suspensions that may be requested or mandated by national
or local governmental authorities or self-imposed by us, our users or other business partners. For example, due to COVID-19, we have been
unable to travel across the relevant jurisdictions pertaining to our business and foresee this as an ongoing issue. While it is not possible
at this time to estimate the full impact that COVID-19 could have on our business, potential users or other potential business partners,
the continued spread of COVID-19, the measures taken by the local and federal government, actions taken to protect employees, and the
impact of the pandemic on various business activities could adversely affect our results of operations and financial condition.
Risks Relating to Our Financial Condition
There are doubts about our ability to continue
as a going concern.
We have
generated no revenue, and have an accumulated deficit of $1,649,936 through June 30, 2021. These factors raise substantial doubt about
our ability to continue as a going concern.
There
can be no assurance that sufficient funds required during the next year or thereafter will be generated from operations or that funds
will be available from external sources, such as debt or equity financings or other potential sources. The lack of additional capital
resulting from the inability to generate cash flow from operations, or to raise capital from external sources would force us to substantially
curtail or cease operations and would, therefore, have a material adverse effect on its business. Furthermore, there can be no assurance
that any such required funds, if available, will be available on attractive terms or that they will not have a significant dilutive effect
on our existing stockholders.
We seek
to overcome the circumstances that impact our ability to remain a going concern through a combination of the growth of revenues, with
interim cash flow deficiencies being addressed through additional equity and debt financing. We anticipate raising additional funds through
public or private financing, strategic relationships or other arrangements in the near future to support its business operations; however,
we may not have commitments from third parties for a sufficient amount of additional capital. We cannot be certain that any such financing
will be available on acceptable terms, or at all, and our failure to raise capital when needed could limit our ability to continue operations.
Our ability to obtain additional funding will determine its ability to continue as a going concern. Failure to secure additional financing
in a timely manner and on favorable terms would have a material adverse effect on our financial performance, results of operations and
stock price and require us to curtail or cease operations, sell off our assets, seek protection from our creditors through bankruptcy
proceedings, or otherwise. Furthermore, additional equity financing may be dilutive to the holders of our common stock, and debt financing,
if available, may involve restrictive covenants, and strategic relationships, if necessary, to raise additional funds, and may require
that we relinquish valuable rights.
Because we have a limited
operating history, you may not be able to accurately evaluate our operations.
We have
had limited operations to date. Therefore, we have a limited operating history upon which to evaluate the merits of investing in our company.
Potential investors should be aware of the difficulties normally encountered by new companies and the high rate of failure of such enterprises.
The likelihood of success must be considered in light of the problems, expenses, difficulties, complications and delays encountered in
connection with the operations that we plan to undertake. These potential problems include, but are not limited to, unanticipated problems
relating to the ability to generate sufficient cash flow to operate our business, and additional costs and expenses that may exceed current
estimates. We expect to continue to incur significant losses into the foreseeable future. We recognize that if the effectiveness of our
business plan is not forthcoming, we will not be able to continue business operations. There is no history upon which to base any assumption
as to the likelihood that we will prove successful, and it is doubtful that we will generate any operating revenues or ever achieve profitable
operations. If we are unsuccessful in addressing these risks, our business will most likely fail.
We are dependent on
outside financing for continuation of our operations.
Because
we have generated no revenues and currently operate at a loss, we are completely dependent on the continued availability of financing
in order to continue our business operations. There can be no assurance that financing sufficient to enable us to continue our operations
will be available to us in the future.
We will
need additional funds to complete further development of our business plan to achieve a sustainable level where ongoing operations can
be funded out of revenues. We anticipate that we must raise $25,000,000 for our operations for the next 12 months, and $81,000,000 for
our initial clinical development program for each of the molecules and therapeutic indications. We will require further funding to fully
implement our business plan to its fullest potential and achieve our growth plans. There is no assurance that any additional financing
will be available or if available, on terms that will be acceptable to us.
Our failure
to obtain future financing or to produce levels of revenue to meet our financial needs could result in our inability to continue as a
going concern and, as a result, our investors could lose their entire investment.
Our operating results
may fluctuate, which could have a negative impact on our ability to grow our client base, establish sustainable revenues and succeed overall.
Our results
of operations may fluctuate as a result of a number of factors, some of which are beyond our control including but not limited to:
|
|
§
|
general economic conditions in the geographies and industries where we sell our services and conduct operations; legislative policies where we sell our services and conduct operations;
|
|
|
§
|
the budgetary constraints of our customers; seasonality;
|
|
|
§
|
success of our strategic growth initiatives;
|
|
|
§
|
costs associated with the launching or integration of new or acquired businesses; timing of new product introductions by us, our suppliers and our competitors; product and service mix, availability, utilization and pricing;
|
|
|
§
|
the mix, by state and country, of our revenues, personnel and assets; movements in interest rates or tax rates;
|
|
|
§
|
changes in, and application of, accounting rules; changes in the regulations applicable to us; and litigation matters.
|
As a
result of these factors, we may not succeed in our business and we could go out of business.
As a growing company,
we have yet to achieve a profit and may not achieve a profit in the near future, if at all.
We have
not yet produced any revenues or profit and may not in the near future, if at all. We cannot be certain that we will be able to realize
sufficient revenue to achieve profitability. Further, many of our competitors have a significantly larger industry presence and revenue
stream but have yet to achieve profitability. Our ability to continue as a going concern is dependent upon raising capital from financing
transactions, increasing revenue and keeping operating expenses below our revenue levels in order to achieve positive cash flows, none
of which can be assured.
Risks Related with Management and Control Persons
We are dependent on the continued services of
our Chairman and Chief Operating Officer and if we fail to keep them or fail to attract and retain qualified senior executive and key
technical personnel, our business will not be able to expand.
We are dependent on the continued
availability of Dominic Schiller and Hunter Land, and the availability of new employees to implement our business plans. The market for
skilled employees is highly competitive, especially for employees in our industry. Although we expect that our planned compensation programs
will be intended to attract and retain the employees required for us to be successful, there can be no assurance that we will be able
to retain the services of all our key employees or a sufficient number to execute our plans, nor can there be any assurance we will be
able to continue to attract new employees as required.
Our lack of adequate D&O insurance may also
make it difficult for us to retain and attract talented and skilled directors and officers.
In the future we may be subject
to additional litigation, including potential class action and stockholder derivative actions. Risks associated with legal liability are
difficult to assess and quantify, and their existence and magnitude can remain unknown for significant periods of time. To date, we have
not obtained directors and officers liability (“D&O”) insurance. Without adequate D&O insurance, the amounts we would
pay to indemnify our officers and directors should they be subject to legal action based on their service to the Company could have a
material adverse effect on our financial condition, results of operations and liquidity. Furthermore, our lack of adequate D&O insurance
may make it difficult for us to retain and attract talented and skilled directors and officers, which could adversely affect our business.
Our personnel may voluntarily terminate their
relationship with us at any time, and competition for qualified personnel is intense. The process of locating additional personnel with
the combination of skills and attributes required to carry out our strategy could be lengthy, costly and disruptive.
If we lose the services of key
personnel or fail to replace the services of key personnel who depart, we could experience a severe negative effect on our financial results
and stock price. The loss of the services of any key personnel, marketing or other personnel or our failure to attract, integrate, motivate
and retain additional key employees could have a material adverse effect on our business, operating and financial results and stock price
Our officers and directors have substantial
control over us and our policies and will be able to influence corporate matters.
Our officers and directors presently
beneficially own 60% of our common stock. They are able to exercise significant influence over all matters requiring approval by our stockholders,
including the election of directors, the approval of significant corporate transactions, and any change of control of our company. They
could prevent transactions, which would be in the best interests of the other shareholders. Our officers and directors’ interests
may not necessarily be in the best interests of the shareholders in general.
The elimination of monetary liability against
our directors, officers and employees under our Articles of Incorporation and the existence of indemnification rights to our directors,
officers and employees may result in substantial expenditures by our Company and may discourage lawsuits against our directors, officers
and employees.
Our Articles of Incorporation
contain provisions that eliminate the liability of our directors for monetary damages to our Company and shareholders. Our bylaws also
require us to indemnify our officers and directors. We may also have contractual indemnification obligations under our agreements with
our directors, officers and employees. The foregoing indemnification obligations could result in our company incurring substantial expenditures
to cover the cost of settlement or damage awards against directors, officers and employees that we may be unable to recoup. These provisions
and resulting costs may also discourage our company from bringing a lawsuit against directors, officers and employees for breaches of
their fiduciary duties, and may similarly discourage the filing of derivative litigation by our shareholders against our directors, officers
and employees even though such actions, if successful, might otherwise benefit our Company and shareholders.
Our officers and directors have limited experience
managing a public company.
Our officers and directors have
limited managing a public company. Consequently, we may not be able to raise any funds or run our public company successfully. Our executive’s
officer’s and director’s lack of experience of managing a public company could cause you to lose some or all of your investment.
Risks Relating to our Common Stock and Offering
We will likely conduct further offerings of
our equity securities in the future, in which case your proportionate interest may become diluted.
We will likely be required to
conduct equity offerings in the future to finance our current projects or to finance subsequent projects that we decide to undertake.
If our common stock shares are issued in return for additional funds, the price per share could be lower than that paid by our current
shareholders. We anticipate continuing to rely on equity sales of our common stock shares in order to fund our business operations. If
we issue additional common stock shares or securities convertible into shares of our common stock, your percentage interest in us could
become diluted.
Our common stock price may be volatile and could
fluctuate widely in price, which could result in substantial losses for investors.
Our common stock is quoted on
the OTCPink under the symbol, “ABTI.” The market price of our common stock is likely to be highly volatile and could fluctuate
widely in price in response to various factors, many of which are beyond our control, including:
|
|
§
|
government regulation of our products and services;
|
|
|
§
|
the establishment of partnerships with sports development companies;
|
|
|
§
|
intellectual property disputes;
|
|
|
§
|
additions or departures of key personnel;
|
|
|
§
|
sales of our common stock
|
|
|
§
|
our ability to integrate operations, technology, products and services;
|
|
|
§
|
our ability to execute our business plan;
|
|
|
§
|
operating results below expectations;
|
|
|
§
|
loss of any strategic relationship;
|
|
|
§
|
economic and other external factors; and
|
|
|
§
|
period-to-period fluctuations in our financial results.
|
Because we have no revenues to
date, you should consider any one of these factors to be material. Our stock price may fluctuate widely as a result of any of the above.
In addition, the securities markets
have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular
companies. These market fluctuations may also materially and adversely affect the market price of our common stock.
Our existing stockholders may experience significant
dilution from the sale of our common stock pursuant to the EMC2 Purchase Agreement.
The sale of our common stock to
EMC2 in accordance with the Purchase Agreement may have a dilutive impact on our shareholders. As a result, the market price of our common
stock could decline. In addition, the lower our stock price is at the time we exercise our put options, the more shares of our common
stock we will have to issue to EMC2 in order to exercise a put under the Purchase Agreement. If our stock price decreases, then our existing
shareholders would experience greater dilution for any given dollar amount raised through the offering.
The perceived risk of dilution
may cause our stockholders to sell their shares, which may cause a decline in the price of our common stock. Moreover, the perceived risk
of dilution and the resulting downward pressure on our stock price could encourage investors to engage in short sales of our common stock.
By increasing the number of shares offered for sale, material amounts of short selling could further contribute to progressive price declines
in our common stock.
The issuance of shares pursuant to the EMC2
Purchase Agreement may have significant dilutive effect.
Depending on the number of shares
we issue pursuant to the EMC2 Purchase Agreement, it could have a significant dilutive effect upon our existing shareholders. Although
the number of shares that we may issue pursuant to the Purchase Agreement will vary based on our stock price (the higher our stock price,
the less shares we have to issue), there may be a potential dilutive effect to our shareholders, based on different potential future stock
prices, if the full amount of the Purchase Agreement is realized. Dilution is based upon common stock put to EMC2 and the stock price
discounted to 91% of the lowest sales price on the purchase date.
EMC2 will pay less than the then-prevailing
market price of our common stock which could cause the price of our common stock to decline.
Our common stock to be issued
under the EMC2 Purchase Agreement will be purchased at 91% of the lowest sales price on the purchase date. EMC2 has a financial incentive
to sell our shares immediately upon receiving them to realize the profit between the discounted price and the market price. If EMC2 sells
our shares, the price of our common stock may decrease. If our stock price decreases, EMC2 may have further incentive to sell such shares.
Accordingly, the discounted sales price in the Purchase Agreement may cause the price of our common stock to decline.
We may not have access to the full amount under
the Purchase Agreement.
At an assumed purchase price under the Purchase Agreement of $ 0.312
(equal to 91% of the lowest sales price on October 18, 2021, we will be able to receive up to $ 17,160,000 in gross proceeds, assuming
the sale of the entire 55,000,000 purchase notice shares being registered hereunder pursuant to the Purchase Agreement. At an assumed
purchase price of $0.312 under the Purchase Agreement, we would be required to register 345,641,026 additional shares of our common stock
to obtain the balance of $ 107,840,000 of the total $125,000,000 under the Purchase Agreement. Due to the floating offering price, we
are not able to determine the exact number of shares that we will issue under the Purchase Agreement.
Our ability to draw down funds
and sell shares under the Purchase Agreement with EMC2 requires that the registration statement of which this prospectus forms a part
to be declared effective and continue to be effective. The registration statement of which this prospectus forms a part registers the
resale of 55,000,000 shares issuable under the Purchase Agreement with EMC2, and our ability to sell any remaining shares issuable under
the investment with EMC2 is subject to our ability to prepare and file one or more additional registration statements registering the
resale of these shares. These registration statements may be subject to review and comment by the staff of the Securities and Exchange
Commission and will require the consent of our independent registered public accounting firm. Therefore, the timing of effectiveness of
these registration statements cannot be assured. The effectiveness of these registration statements is a condition precedent to our ability
to sell all of the shares of our common stock to EMC2 under the Purchase Agreement. Even if we are successful in causing one or more registration
statements registering the resale of some or all of the shares issuable under the purchase agreement with EMC2 to be declared effective
by the Securities and Exchange Commission in a timely manner, we may not be able to sell the shares unless certain other conditions are
met. For example, we might have to increase the number of our authorized shares in order to issue the shares to EMC2. Increasing the number
of our authorized shares will require board and stockholder approval. Accordingly, because our ability to draw down any amounts under
the Purchase Agreement with EMC2 is subject to a number of conditions, there is no guarantee that we will be able to draw down any portion
or all of the proceeds of $125,000,000 under the investment with EMC2.
We have never declared or paid any cash dividends
or distributions on our capital stock. And we do not anticipate paying any cash dividends on our common stock in the foreseeable future.
We have never declared or paid
any cash dividends or distributions on our capital stock. We currently intend to retain our future earnings, if any, to support operations
and to finance expansion and therefore we do not anticipate paying any cash dividends on our common stock in the foreseeable future.
The declaration, payment and amount
of any future dividends will be made at the discretion of the board of directors, and will depend upon, among other things, the results
of our operations, cash flows and financial condition, operating and capital requirements, and other factors as the board of directors
considers relevant. There is no assurance that future dividends will be paid, and, if dividends are paid, there is no assurance with respect
to the amount of any such dividend.
We may become involved in securities class action
litigation that could divert management’s attention and harm our business.
The stock market in general, and
the shares of early-stage companies in particular, have experienced extreme price and volume fluctuations. These fluctuations have often
been unrelated or disproportionate to the operating performance of the companies involved. If these fluctuations occur in the future,
the market price of our shares could fall regardless of our operating performance. In the past, following periods of volatility in the
market price of a particular company’s securities, securities class action litigation has often been brought against that company.
If the market price or volume of our shares suffers extreme fluctuations, then we may become involved in this type of litigation, which
would be expensive and divert management’s attention and resources from managing our business.
As a public company, we may also
from time to time make forward-looking statements about future operating results and provide some financial guidance to the public markets.
Projections may not be made timely or set at expected performance levels and could materially affect the price of our shares. Any failure
to meet published forward-looking statements that adversely affect the stock price could result in losses to investors, stockholder lawsuits
or other litigation, sanctions or restrictions issued by the SEC.
Our common stock is currently deemed a “penny
stock,” which makes it more difficult for our investors to sell their shares.
Our common stock is currently
deemed a “penny stock,” which makes it more difficult for our investors to sell their shares. The SEC has adopted rule 3a51-1
which establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market
price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction
involving a penny stock, unless exempt, Rule 15g-9 requires:
• that
a broker or dealer approve a person’s account for transactions in penny stocks, and
• the
broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny
stock to be purchased.
In order to approve a person’s
account for transactions in penny stocks, the broker or dealer must:
• obtain
financial information and investment experience objectives of the person, and
• make
a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge
and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
The broker or dealer must also
deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which,
in highlight form:
• sets
forth the basis on which the broker or dealer made the suitability determination and
• that
the broker or dealer received a signed, written agreement from the investor prior to the transaction.
Generally, brokers may be less
willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors
to dispose of our common stock and cause a decline in the market value of our stock.
Risks Relating to Our Company and Industry
Our future
success will largely depend on the success of our drug candidates, which development will require significant
capital resources and years of clinical development effort.
We currently have no drug products
on the market, and none of our drug development projects / pipeline drug candidates has reached
preclinical study or clinical trial status. Our business depends almost entirely on the successful clinical
development, regulatory approval and commercialization of our pipeline drug candidates. Investors need to be aware
that substantial additional investments including preclinical and clinical development and regulatory approval efforts will
be required before we are permitted to market and commercialize our pipeline drug
candidates, if ever. It may be several years before we can commence clinical trials, if ever.
Any clinical trial will be subject to extensive and rigorous review and regulation
by numerous government authorities in the United States, the European Union, and other jurisdictions where
we intend, if approved, to market our pipeline drug candidates. Before obtaining regulatory approvals for any of our
pipeline drug candidates, we must demonstrate through preclinical testing and clinical trials that the pipeline drug candidate
is safe and effective for its specific application. This process can take many years and may include post- marketing
studies and surveillance, which would require the expenditure of substantial resources. Of the large number of drugs in development
for approval in the United States, European Union (and the rest of the world), only a small percentage
will successfully complete the FDA regulatory approval process or be granted authorization to be marketed in
the European Commission or the other competent authorities in the European Union (“EU”) Member States, or the rest
of the world. Accordingly, even if we obtain the sufficient financing to fund our planned research, development and
clinical programs, we cannot assure you that any of our pipeline drug candidates will be successfully developed or commercialized.
We
may be unable to formulate or scale-up any or all of our pipeline drug candidates. There is no guarantee that any of the pipeline
drug candidates will be or are able to be manufactured or produced in a manner to meet the FDA’s criteria for
product stability, content uniformity and all other criteria necessary for product approval in the United States and other
markets. Any of our pipeline drug candidates may fail to achieve their specified endpoints in clinical trials.
Furthermore,
pipeline drug candidates may not be approved even if they achieve their specified endpoints in clinical trials.
The FDA may disagree with our trial design and our interpretation of data from clinical trials, or may change the requirements
for approval even after it has reviewed and commented on the design for our clinical trials. The FDA may also approve a drug
for fewer or more limited indications than we request, or may grant approval contingent on the performance of
costly post-approval clinical trials (i.e., Phase IV trials). In addition, the FDA may not approve the labeling
claims that we believe are necessary or desirable for the successful commercialization of our pipeline drug candidates.
If we are unable to expand our
pipeline and obtain regulatory approval for our pipeline drug candidates within the timelines
we anticipate, we will not be able to execute our business strategy effectively and our ability to substantially grow
our revenues will be limited, which would have a material adverse impact on our long-term business, results of operations,
financial condition, and prospects.
Our drug
development projects, if approved, may be unable to achieve the expected market acceptance and, consequently,
limit our ability to generate revenue
Even when drug development is
successful and regulatory approval has been obtained, our ability to generate significant revenue
depends on the acceptance of our (then) approved medicines by physicians, prescribers and patients. We cannot assure you that
any of our pipeline drug candidates will achieve the expected market acceptance and revenue, if and when we obtain the regulatory approvals.
The market acceptance of any drug depends on a number of factors, including the indication statement and warnings approved by regulatory
authorities for the drug label, continued demonstration of efficacy and safety in commercial
use, physicians’ / prescribers willingness to prescribe the drug, reimbursement from third-party payers such as government
health care systems and insurance companies, the price of the drug, the nature of any post-approval risk
management plans mandated by regulatory authorities, competition, and marketing and distribution support. Any factors preventing
or limiting the market acceptance of our drugs could have a material adverse effect on our business, results of operations and financial
condition.
Results
of preclinical studies and earlier clinical trials are not necessarily predictive indicators of future results.
Any
positive results from future preclinical testing of our pipeline drug candidates and potential future clinical trials may
not necessarily be predictive of the results from Phase 1, Phase 2 or Phase 3 clinical trials. In addition, our interpretation of results
derived from clinical data or our conclusions based on our preclinical data may prove inaccurate.
Frequently, pharmaceutical and biotechnology companies have suffered significant setbacks
in clinical trials after achieving positive results in preclinical testing and early phase clinical trials, and we cannot
be certain that we will not face similar setbacks. These setbacks may be caused by the fact
that preclinical and clinical data can be susceptible to varying interpretations and analyses.
Furthermore, certain pipeline drug candidates may perform satisfactorily in preclinical
studies and clinical trials, but nonetheless fail to obtain FDA approval, a marketing authorization granted by the European
Commission, or appropriate approvals by the appropriate medicines regulatory authorities in other countries.
If we fail to produce positive results in our clinical trials for our pipeline drug candidates, the development timeline
and regulatory approval and commercialization prospects for them and as a result our business and financial prospects, would
be materially adversely affected.
The regulatory
approval processes with the FDA, the EMA and other comparable foreign regulatory authorities is lengthy and
inherently unpredictable.
We
are not permitted to market our drug candidates as medicines in the United
States or the European Union or other countries until we receive approval of a
New Drug Application (“NDA”) from the FDA or a Marketing Authorization Application (“MAA”)
from the European Commission, respectively, or in any foreign countries until we receive the approval from the regulatory
authorities of such countries. Prior to submitting an NDA to the FDA or an MAA to the EMA for approval
of our drug candidates we will need to have completed our preclinical studies and clinical trials. Successfully completing any
clinical program and obtaining approval of an NDA or MAA is a complex, lengthy, expensive and uncertain process, and the FDA or EMA (or
other country medicines regulatory body) may delay, limit or deny approval of pipeline drug candidates for many reasons, including, among
others, because:
|
|
§
|
an inability to demonstrate that our pipeline drug
candidates are safe and effective
in treating patients to the satisfaction of the FDA or EMA (or any other country’s medicine regulatory body);
|
|
|
§
|
results of clinical trials that may not meet the
level of statistical
or clinical significance required by the FDA or EMA (or any other country’s medicine regulatory body);
|
|
|
§
|
disagreements with the FDA or EMA (or any other
country’s medicine regulatory body)
with respect to the number, design, size, conduct or implementation of clinical trials;
|
|
|
§
|
requirements by the FDA and EMA (or any other country’s
medicine regulatory body)
to conduct additional clinical trials;
|
|
|
§
|
disapproval by the FDA or EMA or other applicable
foreign regulatory authorities of certain formulations, labeling
or specifications of pipeline drug candidates;
|
|
|
§
|
findings by the FDA or EMA (or any other country’s
medicine regulatory
body) that the data from preclinical studies and clinical trials are insufficient;
|
|
|
§
|
the FDA or EMA (or any other country’s medicine regulatory body) may
disagree with the interpretation of data from preclinical studies and clinical trials; and
|
|
|
§
|
the FDA, European Commission or other applicable foreign regulatory agencies
may change their approval policies or adopt new regulations.
|
Any
of these factors, many of which are beyond our control, could increase development time
and / or costs or jeopardize our ability to obtain regulatory approval for
our drug candidates.
We may apply
for orphan drug status granted by the FDA and / or EMA for some
of our drug candidates for the treatment of rare diseases.
Regulatory
authorities in some jurisdictions, including the United States and the European Union, may designate drugs for
relatively small patient populations as orphan drugs. The FDA may grant orphan drug designation to drugs intended to treat
a rare disease or condition that affects fewer than 200,000 individuals annually in the United States. In the European
Union, the EMA’s Committee for Orphan Medicinal Products grants orphan drug designation to promote the development
of drugs that are intended for the diagnosis, prevention or treatment of life-threatening or chronically debilitating conditions
affecting not more than 5 in 10,000 persons in the European Union. Additionally, such designation is granted for drugs intended for the
diagnosis, prevention or treatment of a life-threatening, seriously debilitating or
serious and chronic condition and when, without incentives, it is unlikely that sales of the drug in the European Union would be sufficient
to justify the necessary investment in developing the drug.
In the USA, orphan drug designation
entitles a party to financial incentives, such as opportunities for grant funding towards clinical
trial costs, tax credits for certain research and user fee waivers under certain circumstances. In addition, if a drug receives
the first FDA approval for the drug and indication for which it has orphan drug designation, the drug is entitled to seven years of market
exclusivity, which means the FDA may not approve any other application for the same drug for the same indication for a period of seven
years, except in limited circumstances, such as a showing of clinical superiority over the
drug with orphan drug exclusivity. Orphan drug exclusivity does not prevent the FDA from approving a different drug for the
same disease or condition, or the same drug for a different disease or condition.
In
the European Union, orphan drug designation also entitles a party to financial incentives such as reduction of fees or fee
waivers and ten years of market exclusivity following drug approval. This period may be reduced to six years if the orphan
drug designation criteria are no longer met, including where it is shown that the drug is sufficiently profitable so that
market exclusivity is no longer justified.
Whilst the company may wish to
apply for ODDs for some or all of its pipeline drug candidates, there is no guarantee that FDA or EMA (or any other international regulatory
body) will grant an ODD for any of the company’s pipeline drug candidates.
Our
drug candidates may become subject to controlled substance laws and regulations in the U.S.
While cannabis and some cannabinoids
are controlled substances under the CSA in the United States, we plan to initially focus our drug development projects using cannabinoids
and other molecules that are produced from a variety of sources: (1) produced via chemical
synthesis and / or (2) produced biosynthetically and / or (3) produced via botanical means.
A number of cannabinoid-containing
medicines, such as Marinol® or
Syndros® (containing
dronabinol), or Epidiolex (containing botanically-derived cannabidiol) or Cesamet® (containing
nabilone) have been approved by the FDA for variousindications.
In the USA, while plant-derived
cannabinoids – during development - are categorized as Schedule I substances under the CSA, the scheduling changes once
a medicine has been approved by the FDA.
Marinol®,
a capsule formulation which contains synthetic tetrahydrocannabinol, or THC when
formulated is a Schedule III medicine.
Syndros® (which
also contains synthetic THC, dronabinol) is a liquid formulation as is classified as Schedule II.
Epidiolex® was
initially a Schedule V medicine when it was introduced in 2018, but was descheduled by the DEA in 2020.
It
is our intention to produce pipeline drug candidates via synthetic, and / or biosynthetic and
/ or botanical means, which may produce complex extracts or purified drug
substance as API.
Depending upon the content of
our selected API(s), and their subsequent controlled drug status in the USA, and if the company conducts preclinical
studies or clinical trials in the United States, we will become subject
to the CSA laws and regulation in addition to FDA regulations. If the Company decides to proceed
with APIs which are controlled drugs, it will evaluate where it is best to conduct its research and preclinical and
clinical trials. This may or may not be the USA.
Nevertheless, our finished drug
products may contain controlled substances as defined in the CSA. Pipeline drug candidates which contain controlled substances are subject
to a high degree of regulation under the CSA, which establishes, among other things, certain
registration, manufacturing quotas, security, recordkeeping, reporting, import, export
and other requirements administered by the DEA. The DEA classifies controlled substances into five schedules: Schedule I,
II, III, IV or V substances. Schedule I substances, by definition, have a high potential for abuse, have no currently
“accepted medical use” in the United States, lack accepted safety for use under medical supervision, and may not
be prescribed, marketed or sold in the United States. Pharmaceutical products approved for use in the United States may
be listed as Schedule II, III, IV or V, with Schedule II substances considered to present the highest potential for abuse
or dependence and Schedule V substances the lowest relative risk of abuse among such substances. Schedule I and
II drugs are subject to the strictest controls under the CSA, including manufacturing and procurement quotas, security
requirements and criteria for importation. In addition, dispensing of Schedule II drugs is further restricted. For example,
they may not be refilled without a new prescription.
While
cannabis and certain of its derivatives and certain cannabinoids are Schedule I controlled substances, drugs approved
for medical use in the United States that contain cannabis, cannabis extracts or certain cannabinoids must be placed
in Schedules II - V, since approval by the FDA satisfies the “accepted medical use” requirement. If, and when any
of our pipeline drug candidates receive FDA approval, for those that are considered
controlled substances under the CSA, the DEA will make a scheduling determination
and place it in a schedule other than Schedule I for it to be prescribed for patients in the United States. If approved by the FDA, depending
upon the products potential for abuse amongst other factors, we expect the finished dosage forms of any of our pipeline drug
candidates to be listed by the DEA as a Schedule II-V controlled substance. Consequently, their manufacture,
importation, exportation, domestic distribution, storage, sale and legitimate use will be subject to a significant degree
of regulation by the DEA (in the USA) and the corresponding competent authorities around
the world.
The scheduling process may take
one or more years beyond FDA approval in the USA, thereby significantly delaying
the launch of our drugs / medicines. However, the DEA must issue a temporary order scheduling the drug within 90 days after the FDA approves
the drug and the DEA receives a scientific and medical evaluation and scheduling recommendation
from the Department of Health and Human Services. Furthermore, if the FDA, DEA
or any foreign regulatory authority determines that any of our drugs may have potential for abuse, it may require
us to generate more clinical data than that which is currently anticipated, which could increase the cost and/or delay the
launch of our drugs / medicines or APIs (or food or cosmetic ingredients outside of the USA).
Clinical
trials of cannabinoid-based drug candidates are novel with very limited or non-existing history; we face a significant
risk that the trials will not result in commercially viable drugs and treatments.
At present, there is only a very
limited documented clinical trial history from which we can derive any scientific conclusions
for our drug pipeline candidates, or prove that our present assumptions for the current and planned research are scientifically compelling. The
API content of the Investigational Medicinal Products (IMPs) can vary from one IMP to another – hence it is not necessarily possible
to extrapolate results from studies with one product and predict efficacy of safety with another product containing a similar API a different
source. Whilst the principal cannabinoid component may be similar, the APIs may differ in terms of minor cannabinoid content, impurity
profiles or degradant profiles. While we are encouraged by the results of clinical trials
by others (where they exist), there can be no assurance that any preclinical study or clinical
trial will result in producing results which will lead to commercially viable drugs or treatments.
Clinical
trials are expensive, time consuming and difficult to design and implement. We, as well as the regulatory authorities
may suspend, delay or terminate our clinical trials at any time, may require us, for various reasons, to conduct
additional clinical trials, or may require a particular clinical trial to continue for a longer duration than originally planned,
including, among others:
|
|
§
|
lack of effectiveness of any API, formulation or delivery system during
clinical trials;
|
|
|
§
|
discovery of serious or unexpected toxicities or side effects experienced
by trial participants or other safety issues;
|
|
|
§
|
slower than expected rates of subject recruitment and enrollment rates in
clinical trials;
|
|
|
§
|
delays or inability in manufacturing or obtaining sufficient quantities
of GMP-grade materials for use in clinical trials due to regulatory and manufacturing constraints;
|
|
|
§
|
delays in obtaining regulatory authorization to commence a trial, including
Institutional Review Board (“IRB”) approvals or DEA approvals, licenses required for obtaining and using cannabis , cannabis-derived
cannabinoid or cannabinoid-like substances for research, either before or after a trial is commenced;
|
|
|
§
|
unfavorable results from ongoing pre-clinical studies and clinical trials;
|
|
|
§
|
patients or investigators failing to comply with clinical trial protocols;
|
|
|
§
|
patients failing to return for post-treatment follow-up at the expected
rate;
|
|
|
§
|
sites participating in an ongoing clinical trial withdraw, requiring us
to engage new sites;
|
|
|
§
|
third-party clinical investigators decline to participate in our clinical
trials, do not perform the clinical trials on the anticipated schedule, or act in ways inconsistent with the established investigator
agreement, clinical trial protocol, good clinical practices, and other IRB requirements;
|
|
|
§
|
third-party entities do not perform data collection and analysis in a timely
or accurate manner or at all; or
|
|
|
§
|
regulatory inspections of our clinical trials require us to undertake corrective
action or suspend or terminate our clinical trials.
|
Any
of the foregoing could have a material adverse effect on our business, results of operations and financial condition.
The
FDA has not approved any complex botanically-derived cannabinoid drug as a safe and effective drug for any indication.
To date, the FDA has not approved
any complex botanical cannabinoid medicine as safe and effective for any indication. It has
however approved a cannabinoid medicine containing a highly purified cannabinoid (CBD) medicine (Epidiolex®)
for a limited number of indications. However, the FDA is
aware that there is considerable interest in the use of
complex botanical medicines (e.g. Sativex® -
which is not approved in the USA, but is approved in some other countries) or
purified cannabinoids (e.g. Epidiolex®) or synthesized cannabinoid medicines (e.g. Marinol) to attempt to treat
a number of medical conditions.
Before conducting testing in humans
of a drug that has not been approved by the FDA, we will need to submit an investigational
new drug (“IND”) application to the FDA (or a Clinical Trial Authorisation (CTA) to the EMA). Failure to comply with applicable U.S.
requirements may subject a company to a variety of administrative or judicial sanctions,
such as the FDA’s refusal to approve pending NDAs, warning letters, product recalls,
product seizures, total or partial suspension of production or distribution, injunctions,
fines, civil penalties and criminal prosecution. Failure to comply with similarly applicable regulatory requirements
in other countries may also subject a company to a variety of administrative or judicial sanctions within their country.
We
face a potentially highly competitive market.
Demand
for cannabinoid-containing or cannabis-based medicines will likely be dependent on a number of social, political
and economic factors that are beyond our control. While we believe that there will be a demand for such drugs, and that the
demand will grow, there is no assurance that such demand will happen, that we will benefit from any demand or that our business, in fact,
will ever generate revenues from our drug development programs or become profitable.
The emerging markets for cannabinoid-containing
or cannabis-derived medicines and medical research and development is and will likely remain
competitive. The development and commercialization of drugs / medicines is highly competitive. We compete with a variety of
multinational pharmaceutical companies and specialized biotechnology companies, as well as products and processes being developed by universities
and other research institutions. Many of our competitors have developed, are developing, or
will develop drugs and processes which may be competitive with our drug candidates.
Competitive therapeutic treatments include those that have already been approved
by medicines regulators and accepted by the medical community and any new treatments that may enter the market. For some of
our drug development programs / areas of therapeutic interest, other treatment options are currently
available, under development, and may become commercially available in the future. If any of our pipeline drug
candidates is approved for the diseases and conditions we are currently pursuing, they may compete with a range of medicines
/ therapeutic treatments that are either in development, will be developed in the future or currently marketed.
We
are aware of many companies that are engaged in cannabinoid-derived drug development activities. In addition, other
U.S.-based and foreign-based companies are in early stage discovery and preclinical
development utilizing the cannabinoids CBD and/or THC.
Established
companies may have a competitive advantage over us due to their size and experiences, financial resources, and
institutional networks. Many of our competitors may have significantly greater financial, technical and human resources
than we do. Due to these factors, our competitors may have an advantage in marketing their approved drugs and may obtain regulatory
approval of their drug candidates before we are able to, which may limit our ability to develop or commercialize our drug candidates.
Our competitors may also develop drugs / medicines that are safer, more effective, more widely
used and less expensive than ours. These advantages could materially impact our ability to develop and, if approved, commercialize
our pipeline drug candidates successfully. Furthermore, some of these competitors may make
acquisitions or establish collaborative relationships among themselves or with third parties to increase their ability to
rapidly gain market share.
Our
pipeline drug candidates may compete with other cannabinoid or cannabis-based drugs, in addition to competing with state-licensed
medical and recreational marijuana, in markets where the recreational and/or medical use of marijuana
is legal. There is continuing support in the USA for further state legalization of marijuana. In markets where recreational
and/or medical marijuana is not legal, our pipeline drug candidates, once approved by regulators, may compete
with marijuana or marijuana-based products purchased in the illegal drug market. This may or may not affect the
commercial price that we may be able to achieve for our cannabinoid-containing or other
non-cannabinoid-containing regulatory-approved medicines, should they be approved by the FDA.
Moreover,
as generic versions of drug products enter the market, the price for such medicines may be expected to decline rapidly and
substantially. Even if we are the first to obtain FDA approval of one of our pipeline drug candidates,
the future potential approval of generics could adversely affect the price we are able to charge and the profitability of
our product(s) will likely decline.
Mergers
and acquisitions in the pharmaceutical and biotechnology industries may result in more resources being concentrated
among a smaller number of our competitors. Smaller and other early-stage companies may also prove to be
significant competitors, particularly through collaborative arrangements with large and established companies.
These
companies may compete with us in recruiting and retaining qualified scientific, management and commercial personnel,
utilizing contract manufacturing facilities or contract research organizations (CROs),
or establishing clinical trial sites and subject registration for clinical trials, as well as in acquiring technologies complementary to
our research projects.
Our
failure to comply with existing and potential future laws and regulations relating to drug development could
harm our plan of operations.
Our business is, and will be,
subject to wide-ranging existing federal and state laws and regulations and other governmental
bodies in each of the countries we may develop and/or market our pipeline drug candidates. We must comply with all regulatory
requirements if we expect to be successful.
If
any of our cannabinoid-containing or cannabis-based pipeline drug candidates are controlled substances and are approved in
the United States, they will be subject to ongoing regulatory requirements including federal and state requirements.
As a result, we and our collaborators and/or joint venture partners must continue to expend time, money and
effort in all areas of regulatory compliance, including, if applicable, manufacturing, production, quality control and assurance, preclinical
research and development and, of upmost importance, clinical trials. We will also be required to report certain adverse reactions
and production problems, if any and applicable, to the FDA, and to comply with
advertising and promotion requirements for our cannabinoid-containing drug candidates.
Any
failure to comply with ongoing regulatory or controlled drug requirements may significantly and adversely affect our
ability to conduct clinical trials which are prerequisites to our ability to commercialize our cannabinoid-based drugs and
related treatments. If regulatory sanctions are applied or if regulatory approval, once obtained, is for any reason suspended
or withdrawn, the value of our business and our operating results could be materially adversely affected.
Our
failure to be able to out-licence some or all of our pipeline drug candidates could
harm our plan of operations.
The cost of drug development is
high and the attrition rate of new drug pipeline candidates is also high during the drug development process. In order to help fund the
development of some of our pipeline drug candidates, the company may wish / need to out-licence some of its assets to other (big) pharmaceutical
or biotechnology companies. The aim of such out-licensing would be generate funds for the company which may take the form of up-front
payments and / or milestone payments and / or royalties. Such decisions will be taken on a case-by-case basis, as the opportunity arises
or is required.
There is no guarantee that the
company will generate pipeline drug candidates which are suitable for out-licensing. In addition, even if the company does produce pipeline
drug candidates that are suitable for out-licensing there is no guarantee that the company will be successful in being able to identify
potential licencees and successfully negotiate such out-licensing agreements, on agreeable terms if and when required. Any failure to
secure such out-licensing agreements may materially affect our ability to finance or develop and / or commercialize one or more of our
pipeline drug candidates. Any such failure may materially adversely affect our business.
Our
failure to be able to enter Research and Development (R & D) Collaboration
Agreements or Joint Venture (JV) Agreements for some or all of our pipeline drug candidates could harm our plan of operations
As mentioned above, the cost of
drug development is high. In order to help fund the development of some of our pipeline drug candidates, the company may wish to enter
into Research and Development Collaboration Agreements or Joint Venture Agreements with
other (big) pharmaceutical or biotechnology companies to help research and develop some of its assets and for those companies pay for
some or all of the associated R & D costs. The aim of such Collaboration or JV agreements would be to offset some of the company’s
R & D costs. Depending upon the outcome of such R & D or JV Agreements, it may lead to the opportunity to outlicence one or more
of the assets investigated under the Collaboration Agreement to the same other (big) pharmaceutical or biotechnology company who may be
our R &D Collaboration / JV partner. If successful, this may generate funds for the company which may take the form of up-front payments
and / or milestone payments and / or royalties. Such decisions will be taken on a case-by-case basis, as the opportunity arises or is
required.
There is no guarantee that the
company will generate pipeline drug candidates which are suitable for R & D Collaborations or JV Agreements. In addition, even if
the company does produce pipeline drug candidates that are suitable for such collaborations or JVs, there is no guarantee that the company
will be successful in being able to identify potential R & D collaboration partners or JV partners and successfully negotiate such
collaboration or JV agreements, on agreeable terms if and when required. Depending upon the financial status of the company, any failure
to secure such collaboration or JV agreements agreements may materially affect our ability to finance or develop and / or commercialize
one or more of our pipeline drug candidates. Any such failure may materially adversely affect our business.
The introduction of new businesses,
products, services, and technologies, our activities in certain jurisdictions, or other actions we take may subject us to additional laws
and regulations. The costs of compliance with these laws and regulations are high and are likely to increase in the future. Any failure
on our part to comply with laws and regulations can result in negative publicity and diversion of management time and effort and may subject
us to significant liabilities and other penalties.
We could be subject to litigation, allegations
or other legal claims.
Our assets or our business activities
may be subject to disputes that may result in litigation or other legal claims. We may be subject to allegations through press, social
media, the courts or other mediums that may or may not be founded. We may be required to respond to or defend against these claims and/or
allegations, which will divert resources away from our principal business. There can be no assurance that our defense of such claims and/or
allegations would be successful, and we may be required to make material settlements. This could have a material adverse effect on our
business prospects, results of operations, cash flows, financial condition and corporate reputation.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus includes statements
that express our opinions, expectations, beliefs, plans, objectives, assumptions or projections regarding future events or future results
and therefore are, or may be deemed to be, “forward-looking statements.” All statements other than statements of historical
facts contained in this prospectus may be forward-looking statements. These forward-looking statements can generally be identified by
the use of forward-looking terminology, including the terms “believes,” “estimates,” “continues,”
“anticipates,” “expects,” “seeks,” “projects,” “intends,” “plans,”
“may,” “will,” “would” or “should” or, in each case, their negative or other variations
or comparable terminology. They appear in a number of places throughout this prospectus, and include statements regarding our intentions,
beliefs or current expectations concerning, among other things, our results of operations, financial condition, liquidity, prospects,
growth, strategies, future acquisitions and the industry in which we operate.
By their nature, forward-looking
statements involve risks and uncertainties because they relate to events and depend on circumstances that may or may not occur in the
future. We believe that these risks and uncertainties include, but are not limited to, those described in the “Risk Factors”
section of this prospectus. Those factors should not be construed as exhaustive and should be read with the other cautionary statements
in this prospectus.
Although we base these forward-looking
statements on assumptions that we believe are reasonable when made, we caution you that forward-looking statements are not guarantees
of future performance and that our actual results of operations, financial condition and liquidity, and industry developments may differ
materially from statements made in or suggested by the forward-looking statements contained in this prospectus. The matters summarized
under “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition
and Results of Operations,” “Business” and elsewhere in this prospectus could cause our actual results to differ significantly
from those contained in our forward-looking statements. In addition, even if our results of operations, financial condition and liquidity,
and industry developments are consistent with the forward-looking statements contained in this prospectus, those results or developments
may not be indicative of results or developments in subsequent periods.
In light of these risks and uncertainties,
we caution you not to place undue reliance on these forward-looking statements. Any forward-looking statement that we make in this prospectus
speaks only as of the date of such statement, and we undertake no obligation to update any forward-looking statement or to publicly announce
the results of any revision to any of those statements to reflect future events or developments, except as required by applicable law.
Comparisons of results for current and any prior periods are not intended to express any future trends or indications of future performance,
unless specifically expressed as such, and should only be viewed as historical data.
USE OF PROCEEDS
We will not receive any proceeds
from the sale of the shares of our Common Stock by EMC2 (the selling stockholder identified in this prospectus). However, we will receive
proceeds from our sale of shares to EMC2, pursuant to the Purchase Agreement. The proceeds from the initial sale of shares will be used
for the purpose of working capital or for other purposes that the Board of Directors, in good faith deem to be in the best interest of
the Company.
DETERMINATION OF OFFERING PRICE
We have not set an offering price
for the shares registered hereunder, as the only shares being registered are those sold pursuant to the EMC2 Purchase Agreement. EMC2
may sell all or a portion of the shares being offered pursuant to this prospectus at fixed prices and prevailing market prices at the
time of sale, at varying prices or at negotiated prices.
PRICE RANGE OF THE REGISTRANT’S COMMON EQUITY
Market Information
Our common stock is quoted under
the symbol “ABTI” on the OTC Pink operated by OTC Markets Group, Inc.
There is currently no active trading
market for our securities. There is no assurance that a regular trading market will develop, or if developed, that it will be sustained.
Therefore, a shareholder may be unable to resell his securities in our company.
Penny Stock
The Securities Exchange Commission
(“SEC”) has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks
are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges
or quoted on the NASDAQ system, provided that current price and volume information with respect to transactions in such securities is
provided by the exchange or system. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock, to deliver
a standardized risk disclosure document prepared by the Commission, that: (a) contains a description of the nature and level of risk in
the market for penny stocks in both public offerings and secondary trading;(b) contains a description of the broker's or dealer's duties
to the customer and of the rights and remedies available to the customer with respect to a violation to such duties or other requirements
of Securities' laws; (c) contains a brief, clear, narrative description of a dealer market, including bid and ask prices for penny stocks
and the significance of the spread between the bid and ask price;(d) contains a toll-free telephone number for inquiries on disciplinary
actions;(e) defines significant terms in the disclosure document or in the conduct of trading in penny stocks; and;(f) contains such other
information and is in such form, including language, type, size and format, as the Commission shall require by rule or regulation.
The broker-dealer also
must provide, prior to effecting any transaction in a penny stock, the customer with; (a) bid and offer quotations for the penny stock;(b)
the compensation of the broker-dealer and its salesperson in the transaction;(c) the number of shares to which such bid and ask prices
apply, or other comparable information relating to the depth and liquidity of the market for such stock; and (d) a monthly account statements
showing the market value of each penny stock held in the customer's account.
In addition, the penny stock rules
require that prior to a transaction in a penny stock not otherwise exempt from those rules; the broker-dealer must make a special written
determination that the penny stock is a suitable investment for the purchaser and receive the purchaser's written acknowledgment of the
receipt of a risk disclosure statement, a written agreement to transactions involving penny stocks, and a signed and dated copy of a written
suitability statement.
These disclosure requirements
may have the effect of reducing the trading activity in the secondary market for our stock if it becomes subject to these penny stock
rules. Therefore, because our common stock is subject to the penny stock rules, stockholders may have difficulty selling those securities.
Holders of Our Common Stock
Currently, we have approximately
125 holders of record of our common stock.
Stock Option Grants
To
date, we have not granted any stock options.
Dividends
There are no restrictions in our
articles of incorporation or bylaws that prevent us from declaring dividends. The Nevada Revised Statutes, however, do prohibit us from
declaring dividends where after giving effect to the distribution of the dividend:
|
1.
|
we would not be able to pay our debts as they become due in the usual course of business, or;
|
|
2.
|
our total assets would be less than the sum
of our total liabilities plus the amount that would be needed to
satisfy the rights of shareholders
who have preferential rights superior to those receiving the distribution.
|
We have not declared any dividends
and we do not plan to declare any dividends in the foreseeable future.
Recent Sales of Unregistered Securities
On
June 28, 2018, the company issued one million (1,000,000) common shares for consulting services with a deemed value
of $90,000. As the services are to be provided over a period from April 1, 2018 to January 31, 2019, the company has recorded
$63,000 as deferred stock based compensation.
During
the year ended September 30, 2019, the Company issued 1,000,000 shares of common stock to an officer for services rendered with a deemed
value of services provided of $90,000.
During
the year ended September 30, 2020, the Company issued 13,000,000 shares of common stock to individuals for services rendered with a deemed
value of services provided of $130,000.
During
the period ended March 31, 2021, the Company issued 3,200,000 shares of common stock for services rendered with a deemed value of services
provided of $32,000.
We
issued 600,000,000 shares of common stock to the shareholders of ABTI Pharma Limited in connection with a Stock
Transfer Agreement dated January 19, 2021. As part of the transaction, the 200,000,000 shares to Amsterdam Café
Holdings Ltd. have been cancelled and Bulls Run Investments Limited was issued 19,100,000 shares of common stock. Also, 2,000,000
shares of common stock were issued for services rendered, and with the above transactions, amounts to acquisition with a deemed value
of $ 621,100.
These
securities were issued pursuant to Section 4(2) of the Securities Act and/or Rule 506 promulgated thereunder. The holders
represented their intention to acquire the securities for investment only and not with a view towards distribution.
The investors were given adequate information about us to make an informed investment decision. We did not
engage in any general solicitation or advertising. We directed our transfer agent to issue the stock certificates with the
appropriate restrictive legend affixed to the restricted stock.
Securities Authorized for Issuance under Equity
Compensation Plans
We did not issue any securities
under any equity compensation plan as of March 31, 2021.
DIVIDEND POLICY
Holders of our common stock are
entitled to receive dividends as may be declared from time to time by our board of directors. We have not paid any cash dividends since
inception on our common stock and do not anticipate paying any in the foreseeable future. Although we intend to retain our earnings, if
any, to finance the exploration and growth of our business, our board of directors will have the discretion to declare and pay dividends
in the future. Payment of dividends in the future will depend upon our earnings, capital requirements, and other factors, which our board
of directors may deem relevant.
DILUTION
Not applicable. The shares registered
under this registration statement are not being offered for purchase by the Company. The shares are being registered on behalf of EMC2
(the selling stockholder identified in this prospectus) pursuant to the EMC2 Purchase Agreement.
OUR BUSINESS
Overview
Our goal is to provide better
medicines for patients across the globe. We believe in harnessing the therapeutic potential of cannabinoids and cannabinoid- like compounds,
which can bring valuable treatments to seriously ill patients. Rather than just focussing on one method of identifying, researching and
developing such medicines, we are interested in developing new medicines from all sources including botanical, traditional chemical synthesis
and biosynthetic methodologies.
On May 28, 2021, we acquired ABTI
Pharma Limited, a company registered in England and Wales (“ABTI Pharma”), with the purchase of all of its capital stock in
exchange for 600,000,000 shares of our common stock pro rata to the ABTI Pharma shareholders.
As a result of the acquisition,
we are a pharmacuetical company working with cannabinoid and cannabinoid like molecules. We have three areas of focus:
1) Development of regulated pharmaceuticals
(human and animal health) and regulated food products. This has been achieved via the strategic acquisition of Phytotherapeutix Ltd.
2) Production of low cost of goods
Active Pharmaceutical Ingredient (API) and food-grade ingredients (supported by the strategic acquisition of Ferven Ltd), and
3) Formulation, and drug delivery,
providing improved bioavailability, solubility and stability (supported by the exclusive licensing of IP and technology from Nano4M Ltd).
Phytotherapeutix Ltd, a subsidiary
of ABTI Pharma, has generated a number of molecules with patents pending, some of which have demonstrable pharmacological activity, similar
to that of CBD. This means that some of these molecules are anticipated to have a similar market potential to CBD across a range of therapeutic
areas.
Ferven Ltd, another subsidiary
of ABTI Pharma, is looking to produce cannabinoids by fermentation. The exclusively licensed organism has the potential to be genetically
modified to produce multiple cannabinoids at a very low cost of goods. It is anticipated that the selected genetically modified organisms
will grow very quickly, which in turn, reduces the cost of production.
Nano4M Ltd is a company which
has exclusively licensed its nano-formulation patents and know-how to ABTI Pharma Ltd.
Additionally, we may consider
entering into Joint Venture Partnerships, Acquisition of Companies with complimentary portfolios or Licencing Agreements to enhance the
product portfolio. These are strategies the Company may implement and any such opportunities will be assessed on a case by case basis
and on their merit at the time.
ABTI Pharma management has extensive
proven experience, know-how and connections in the cannabinoid medicines sector, and is looking to utilize this knowledge and experience
for the development of such medicines from existing cannabinoids and cannabinoid-like molecules.
Our address is 47 Hamilton Square
Birkenhead Merseyside CH41 5AR United Kingdom. Our telephone number is +44 151 601 9477. Our website is www.alterolabiotech.com.
We do not incorporate the information
on or accessible through our websites into this Registration Statement, and you should not consider any information on, or that can be
accessed through, our websites a part of this Registration Statement.
Competition
Pharmaceutical Sector
The
cannabinoid-based and cannabinoid-like pharmaceutical medicine research and development
sector and is and will likely remain competitive. In general, the biotechnology and pharmaceutical industries are characterized
by rapidly advancing technologies, intense competition, and a strong emphasis on proprietary drugs / medicines.
We expect that Alterola will be
required to compete with a variety of multinational pharmaceutical companies and specialized
biotechnology companies, as well as drugs and processes being developed at universities and other research institutions. Our competitors
may develop or may already have developed drugs comparable or competitive with our pipeline
drug candidates. Competitive therapeutic treatments for diseases, disorders and medical conditions that are included
in our pipeline development projects have already been approved by the pharmaceutical regulatory bodies around the world (e.g.
FDA, EMA etc.) and used / prescribed by the medical community and any new treatments that may enter the market would face fierce competition.
We
are aware of a number of companies that are engaged in cannabinoid-based drug development. In addition, several other
U.S.-based companies are in early stage discovery and preclinical development utilizing synthetic and/or plant- derived cannabinoids
such as CBD and/or THC.
Non-Pharmaceutical Sector
Due to Federal regulation,
it is not currently possible to develop THC or CBD-containing products for non- pharmaceutical
use (e.g. as food ingredients or dietary supplements) in the USA. However, it is possible to develop cannabinoid- containing
ingredients and products in the food sector in Europe through the Novel Food Approvals route.
Again
this sector is and will likely remain competitive in territories where it is legal to develop and sell such products. Further
it is also possible to develop cannabinoid-containing ingredients in the cosmetics sector.
For
both pharmaceutical and non-pharmaceutical markets, established companies may have a competitive advantage due to their size and experiences,
positive cash flows and institutional networks. Many of our pharma and non-pharma competitors
may have significantly greater financial, technical and human resources than we do. Due to these factors, our competitors
may have a range of competitive advantages and may obtain regulatory approval of their active pharmaceutical
ingredient (API), or medicines; or food ingredients or food products or cosmetic ingredients before we are
able to develop or commercialize our pharma or non pharma active ingredients or products. Our competitors may also develop
ingredients or products that are safer, more effective, more widely used and less expensive than ours.
Furthermore, some of these competitors
may make acquisitions or establish collaborative relationships among themselves or with third
parties to increase their ability to rapidly gain market share and/or increase their ingredient or product lines.
Mergers
and acquisitions in the pharmaceutical and biotechnology and non-pharmaceutical industries may result in even more resources
being concentrated among a smaller number of competitors. Smaller and other early-stage companies,
such as ours, may also prove to be significant competitors, particularly through collaborative
arrangements with large and established companies. We aim to compete with large and small companies in recruiting and retaining qualified
scientific, management and commercial personnel, and using our management knowhow and
expertise in the sector to develop ingredients and products in a compliant manner, as well as in acquiring technologies complementary
to our development programs.
Intellectual Property:
Through
the acquisition of ABTI Pharma, Alterola has acquired ABTI Pharma’s IP portfolio, which includes:
|
|
1)
|
IP including patent applications pertaining
to novel compounds for development of pharmaceutical drug candidates and
their therapeutic use;
|
|
|
2)
|
IP (including organisms, protocols and knowhow)
pertaining to low cost of goods production of Active Pharmaceutical
Ingredient (API) and food-grade ingredients; and
|
|
|
3)
|
IP including granted patents pertaining
to particle engineering technology, formulation, and drug delivery technologies,
which will provide improved drug performance.
|
In
addition, ABTI Pharma have in principle agreements to
bring in additional complimentary technologies with incumbent IP.
Regulatory Matters Pharmaceuticals USA
As a development stage company
that intends to have its pipeline drug candidates approved in the U.S., we are subject to extensive regulation by regulatory agencies.
The U.S. Food, Drug, and Cosmetic Act and its implementing regulations set forth, among other things, requirements for the research, testing,
development, manufacture, quality control, safety, effectiveness, approval, labeling, storage, record keeping, reporting, distribution,
import, export, advertising and promotion of our drugs (medicines). Generally, our activities in other countries will be subject to regulations
that are similar in nature and scope as those in the United States, although there can be important differences. Additionally, some significant
aspects of regulation in the European Union are addressed in a centralized way through the European Medicines Agency (“EMA”)
and the European Commission, but country- specific regulation remains essential in many respects. The process of obtaining regulatory
marketing approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require
the expenditure of substantial time and financial resources and we may not be successful.
Given that the active ingredients
present in our APIs, food ingredients and cosmetic ingredients are in some cases considered to be controlled substances in certain jurisdictions
/ territories, there are additional regulations which are applicable to the research, development, import, receipt, possession, storage,
preparation, extraction, synthesis, biosynthesis,
manufacture, processing, analysis, release, formulation, dispensing, packaging and labelling,
import/export, transport, commercialization, advertising and supply / distribution of Controlled Substances. This means that Alterola
needs to be compliant with competent authorities such as the DEA (USA), The Home Office (UK) and the corresponding authorities in each
country.
We intend to conduct some of our
research and development relating to our drug candidates in the United States, at which time, our research and development, future manufacturing,
distribution and sale of our drugs will become subject to the United States Federal Controlled Substances Act of 1970 and regulations
promulgated thereunder.
While cannabis is a Schedule I
controlled substance, drugs approved for medical use in the United States that contain cannabis or cannabis extracts must be placed in
Schedules II-V, since approval by the FDA satisfies the “accepted medical use” requirement. If any of our pipeline drug candidates
will receive approval by the FDA, it must be listed by the DEA as an appropriately scheduled controlled substance to be allowed for commercialization.
Consequently, the manufacture,
importation, exportation, domestic distribution, storage, sale and legitimate use of our future drugs will be subject to a significant
degree of regulation by the DEA. In addition, individual states in the United States have also established controlled substance laws and
regulations. Though state-controlled substances laws often mirror federal law, because the states are separate jurisdictions, they may
separately schedule our drugs.
Europe
It is the company’s intention
have its pipeline drug candidates approved in countries in addition to the USA and hence we are subject to extensive regulation by other
international regulatory agencies, and the applicable local laws and regulations.
Similarly to the U.S. Food, Drug, and Cosmetic Act
in the USA and its implementing regulations, there are similar laws and regulations in Europe for the research, testing, development,
manufacture, quality control, safety, effectiveness, approval, labeling, storage, record keeping, reporting, distribution, import, export,
advertising and promotion of our drugs (medicines). Again, our activities in Europe will be subject to regulations that are similar in
nature and scope as those in the United States, although there can be important differences.
Our pipeline candidates
may be developed or approved through the Centralized Procedure or Decentralized Procedure through the or through the Mutual Recognition
Procedure (MRP) through the European Medicines Agency (“EMA”) and the European Commission; however it should be noted that
country-specific regulation remains essential in many respects. The process of obtaining regulatory marketing approvals and the subsequent
compliance with the appropriate national, federal, state, local and foreign statutes and regulations require the expenditure of substantial
time and financial resources and we may not be successful.
Again, given that the active ingredients
present in our APIs, food ingredients and cosmetic ingredients are in some countries are considered to be controlled substances in certain
European jurisdictions / territories, there are additional regulations which are applicable to the research, development, import, receipt,
possession, storage, preparation, extraction, synthesis, biosynthesis, manufacture, processing, analysis, release, formulation, dispensing,
packaging and labelling, import/export, transport, commercialisation, advertising and supply / distribution of Controlled Substances.
This means that Alterola needs to be compliant with each competent authority in each European country as applicable.
Japan
It is the company’s intention
have its pipeline drug candidates in due course approved in Japan and hence we are subject to extensive regulation by the pharmaceutical
regulatory authority of Japan: the Pharmaceutical and Food Safety Bureau (PFSB) of the Japanese Ministry of Health, Labor and Welfare
(MHLW), and the Japanese applicable local laws and regulations.
Japan has its own laws and regulations
for the research, testing, development, manufacture, quality control, safety, effectiveness, approval, labeling, storage, record keeping,
reporting, distribution, import, export, advertising and promotion of our drugs (medicines).
Again, given that the active ingredients
present in our APIs, food ingredients and cosmetic ingredients are in some countries are considered to be controlled substances in Japan,
there are additional regulations which are applicable to the research, development, import, receipt, possession, storage, preparation,
extraction, synthesis, biosynthesis, manufacture, processing, analysis, release, formulation, dispensing, packaging and labelling, import/export,
transport, commercialization, advertising and supply / distribution of Controlled Substances. This means that Alterola needs to be compliant
with the Japanese competent authority requirements.
The process of obtaining regulatory
marketing approvals and the subsequent compliance with the appropriate national and local statutes and regulations of Japan require the
expenditure of substantial time and financial resources and we may not be successful.
Rest of the World
It is the company’s intention
have its pipeline drug candidates in due course approved in other countries around the world (Rest of World) and hence we are subject
to extensive regulation by the various national pharmaceutical regulatory authorities which govern the various countries, and the applicable
local laws and regulations.
Different countries have
different laws and regulations for the research, testing, development, manufacture, quality control, safety, effectiveness, approval,
labeling, storage, record keeping, reporting, distribution, import, export, advertising and promotion of our drugs (medicines).
Again, given that the active ingredients
present in our APIs, food ingredients and cosmetic ingredients are in some countries are considered to be controlled substances in some
countries, there are additional regulations which are applicable to the research, development, import, receipt, possession, storage, preparation,
extraction, synthesis, biosynthesis, manufacture, processing, analysis, release, formulation, dispensing, packaging and labelling, import/export,
transport, commercialization, advertising and supply / distribution of Controlled Substances. This means that Alterola needs to be compliant
with each competent authority in each country as applicable.
The process of obtaining regulatory
marketing approvals and the subsequent compliance with the appropriate national, federal, state, local and foreign statutes and regulations
of other countries (ex-US, Europe and Japan) require the expenditure of substantial time and financial resources and we may not be successful.
The Regulatory Process for the approval
of New Medicines
The Company operates in a highly
controlled new drugs / medicines regulatory environment. Strict regulations establish requirements relating to demonstration of quality,
safety and efficacy of a medicine. Regulations also cover preclinical and clinical research and development, manufacturing and reporting
procedures, both pre- and post- approval. Failure to comply with regulations can result in stringent sanctions, including product recalls,
withdrawal of approvals, seizure of products and criminal prosecution. Further, many countries have stringent regulations relating to
the possession and use of cannabis or cannabinoid or cannabis-based medicines.
Before obtaining regulatory approvals
for the commercial sale of our future drug candidates, we must demonstrate that the proposed medicine demonstrates quality, safety and
efficacy. From a quality perspective this is done through demonstrating appropriate chemistry and manufacturing controls (CMC), and from
a safety and efficacy perspective, this is done through demonstrating that our drug candidates are safe and effective in preclinical studies
and clinical trials. Historically, the results from preclinical studies and early clinical trials often have not accurately predicted
results of later clinical trials. In addition, many pharmaceuticals have shown promising results in clinical trials but subsequently failed
to establish sufficient safety and efficacy results to obtain necessary regulatory approvals.
We expect to incur substantial
expense for, and devote a significant amount of time to, the development of quality ingredients and products as well as preclinical studies
and clinical trials. Many factors can delay the commencement and rate of completion of clinical trials, including the inability to recruit
patients at the expected rate, the inability to follow patients adequately after treatment, the failure to manufacture sufficient quantities
of materials used for clinical trials, and the emergence of unforeseen safety issues and governmental and regulatory delays. If a drug
candidate fails to demonstrate safety and efficacy in clinical trials, this failure may delay development of other drug candidates and
hinder our ability to develop and / or conduct related preclinical studies and clinical trials. Additionally, if we have pipeline candidate
failures, we may also be expected to experience challenges, delays or even the inability to obtain additional financing at acceptable
terms and conditions to develop these or other drug candidates.
Governmental authorities in all
major markets require that a new drug be approved or exempted from approval before it is marketed, and have established high standards
for technical appraisal, which can result in an expensive and lengthy approval process. The time to obtain approval of a new medicine
or indication varies by country and some drugs are never approved. The lengthy process of conducting new product or formulation development,
preclinical studies and clinical trials, seeking approval and the subsequent compliance with applicable statutes and regulations, if approval
is obtained, are very costly and require the expenditure of substantial resources.
United States
In the United States, the Public
Health Service Act and the Federal Food, Drug, and Cosmetic Act, as amended, and the regulations promulgated thereunder, and other federal
and state statutes and regulations govern, among other things, the safety and effectiveness standards for our drugs and the raw materials
and components used in the production of, testing, manufacture, labeling, storage, record keeping, approval, distribution, advertising
and promotion of drug candidates on a product-by-product basis.
Preclinical tests include in vitro
and in vivo evaluation of the drug candidate, including animal studies to assess potential safety and efficacy. Certain preclinical tests
must be conducted in compliance with good laboratory practice regulations. Violations of these regulations can, in some cases, lead to
invalidation of the studies, requiring them to be replicated. In addition, non-clinical studies (Chemistry and Manufacturing Controls,
CMC) are undertaken to evaluate a new drug’s chemistry, and to determine, amongst other things, the active ingredients’ and
finished product formulation’s stability and batch-to-batch reproducibility.
After laboratory analysis and
preclinical testing, a Sponsor files an Investigational New Drug Application, or IND, to begin clinical development (clinical trials in
humans). Typically, a manufacturer conducts a three-phase human clinical development program which itself is subject to numerous laws
and regulatory requirements, including adequate monitoring, reporting, record keeping and informed consent. In Phase I, small clinical
trials are conducted to determine the safety and tolerability of drug candidates. In Phase II, clinical trials are conducted to assess
safety and gain preliminary evidence of the efficacy of drug candidates, and to determine appropriate dose ranges in patients with the
target indication. In Phase III, clinical trials are conducted in appropriate patient populations to provide sufficient data for the statistically
valid evidence of safety and efficacy. The time and expense that will be required for us to perform this clinical development can vary
and is substantial. We cannot be certain that we will successfully complete Phase I, Phase II or Phase III clinical trials within any
specific period, if at all. Furthermore, the FDA, the IRB are responsible for approving and monitoring the clinical trials at a given
site, the Data Safety Monitoring Board, where one is used, or we may suspend the clinical trials at any time on various grounds, including
a finding that subjects or patients are exposed to unacceptable health risk. Given that a number of our clinical trials are likely to
be performed using drug candidates containing controlled substances, there is the added requirement for compliance with DEA regulations
(or equivalent competent authority in ex-US countries where the preclinical studies and clinical trials may be conducted). DEA requirements
for State and Federal DEA Registration for receipt, storage and dispensing of controlled substances vary from state to state and the DEA
Registration process can be lengthy and requirement multiple site visits by DEA personnel. This is further complicated if the controlled
substance needs temperature regulation as well as controlled access / storage. Failure to gain or delay to gaining the necessary DEA registrations
at one or more non-clinical (CMC), laboratory or manufacturing or packaging or labelling sites. preclinical study sites, analytical laboratories
or clinical trial sites may delay the delivery of materials to key stakeholders. For example, delay of delivery of investigational product
to a clinical trial site, may ultimately delay the initiation, conduct or completion of clinical trials critical for the approval of the
product. These failures or delays may delay also the development of other drug candidates and hinder our ability to develop and / or conduct
related preclinical studies and clinical trials. Additionally, if we have failures or delays in DEA registrations in pivotal or critical
programs, we may also be expected to experience challenges, delays or even the inability to obtain additional financing at acceptable
terms and conditions to develop these or other drug candidates.
If the clinical data from these
clinical trials (Phases I, II and III) are deemed to support the safety and effectiveness of the drug candidate for its intended use,
and the preclinical and quality data are also acceptable, then we may proceed to seek to file with the FDA, a New Drug Application, or
NDA, with the US FDA seeking approval to market a new drug for one or more specified intended uses. We have not completed our non-clinical
(CMC) studies or preclinical studies or clinical trials for any candidate drug for any intended use and therefore, we cannot ascertain
whether the clinical data will support and justify filing an NDA. Nevertheless, if and when we are able to ascertain that the clinical
data supports and justifies filing an NDA, we intend to make such appropriate filing.
The purpose of the NDA is to provide
the FDA with sufficient information so that it can assess whether the candidate drug has a positive benefit / risk profile and whether
it should approve the drug candidate for marketing for specific intended uses.
The fact that the FDA has previously
granted a candidate drug an IND, or designated a drug as an orphan drug for a specific intended use, or granted it Breakthrough status,
or fast track status or an expedited review does not mean that the drug has been approved for marketing. Only after an NDA has been approved
by the FDA is marketing allowed. A request for orphan drug status (orphan drug designation) must be filed before the NDA is filed. The
orphan drug designation, though, provides certain benefits, including a seven-year period of market exclusivity subject to certain exceptions.
The NDA normally includes, but
is not limited to, sections describing the quality safety and efficacy of the medicine. The quality section describes the chemistry, manufacturing,
and controls, the preclinical (non-clinical) section describes the non-clinical pharmacology, safety pharmacology, drug metabolism and
pharmacokinetics (DMPK) and toxicology, human pharmacokinetics and bioavailability, , and the clinical section describes the efficacy
and safety results of the clinical trials, and the proposed labeling which contains, among other things, the intended uses of the candidate
drug. Importantly for drug candidates containing controlled substances, studies investigating the medicine’s potential for abuse
are also undertaken and reported.
We cannot take any action to market
any new drug or biologic drug in the United States until our appropriate marketing application has been approved by the FDA. The FDA has
substantial discretion over the approval process and may disagree with our interpretation of the data submitted. The process may be significantly
extended by requests for additional information or clarification regarding information already provided. As part of this review, the FDA
may refer the application to an appropriate advisory committee, typically a panel of clinicians. Satisfaction of these and other regulatory
requirements typically takes several years, and the actual time required may vary substantially based upon the type, complexity and novelty
of the drug. Government regulation may delay or prevent marketing of potential drugs for a considerable period and impose costly procedures
on our activities. We cannot be certain that the FDA or other regulatory agencies will approve any of our drugs on a timely basis, if
at all. Success in preclinical or early stage clinical trials does not assure success in later-stage clinical trials. Even if a drug receives
regulatory approval, the approval may be significantly limited to specific indications or uses and these limitations may adversely affect
the commercial viability of the drug / medicine. Delays in obtaining, or failures to obtain regulatory approvals, would have a material
adverse effect on our business.
Even after we obtain FDA approval,
we may be required to conduct further studies which may be additional preclinical studies or clinical trials (e.g. Phase IV trials) and
provide additional data on safety and effectiveness. We are also required to gain separate approval for the use of an approved drug as
a treatment for indications other than those initially approved. In addition, side effects or adverse events that are reported during
clinical trials can delay, impede or prevent marketing approval. Similarly, adverse events that are reported after marketing approval
can result in additional limitations being placed on the drug’s use and, potentially, withdrawal of the drug from the market. Any
adverse event, either before or after marketing approval, can result in product liability claims against the company.
As an alternate path
for FDA approval of new indications or new formulations of previously-approved drugs, a company may file a Section 505(b)(2) NDA, instead
of a “stand-alone” or “full” NDA. Section 505(b)(2) of the Food, Drug, and Cosmetic Act was enacted as part of
the Drug Price Competition and Patent Term Restoration Act of 1984, otherwise known as the Hatch-Waxman Amendments. Section 505(b)(2)
permits the submission of an NDA where at least some of the
information required
for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference.
Some examples of drugs that may be allowed to follow a 505(b)(2) path to approval are drugs that have a new dosage form, strength, route
of administration, formulation or indication. The Hatch-Waxman Amendments permit the applicant to rely upon certain published nonclinical
or clinical studies conducted for an approved drug or the FDA’s conclusions from prior review of such studies. The FDA may require
companies to perform additional studies or measurements to support any changes from the approved drug. The FDA may then approve the new
drug for all or some of the labeled indications for which the referenced listed drug has been approved, as well as for any new indication
supported by the NDA. While references to nonclinical and clinical data not generated by the applicant or for which the applicant does
not have a right of reference are allowed, all development, process, stability, qualification and validation data related to the manufacturing
and quality of the new drug must be included in an NDA submitted under Section 505(b)(2).
To the extent that the
Section 505(b)(2) applicant is relying on the FDA’s conclusions regarding studies conducted for an already approved drug, the applicant
is required to certify to the FDA concerning any patents listed for the approved drug in the FDA’s “Orange Book” publication.
Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired;
(iii) the listed patent has not expired but will expire on a particular date and approval is sought after patent expiration; or (iv) the
listed patent is invalid or will not be infringed by the new drug. The Section 505(b)(2) application also will not be approved until any
non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the reference
drug has expired. Thus, the Section 505(b)(2) applicant may invest a significant amount of time and expense in the development of its
drugs only to be subject to significant delay and patent litigation before its drugs may be commercialized.
In addition to regulating and
auditing human clinical trials, the FDA regulates and inspects equipment, facilities, laboratories and processes used in the manufacturing
and testing of such drugs prior to providing approval to market a drug.
Orphan Drug Designation in the U.S.
Under the Orphan Drug Act, the
FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition, which is a disease or condition that affects
fewer than 200,000 individuals in the United States. If the disease or condition affects more than 200,000 individuals in the United States,
orphan drug designation may nevertheless be available if there is no reasonable expectation that the cost of developing and making the
drug would be recovered from sales in the United States. In the United States, a drug that has received orphan drug designation is eligible
for financial incentives, such as opportunities for grant funding towards clinical trial costs, tax credits for certain research and user
fee waivers under certain circumstances. The Orphan Drug Act provides that, if a designated drug is approved for the rare disease or condition
for which it was designated, the approved drug will be granted seven years of orphan drug exclusivity, which means the FDA generally will
not approve any other application for a drug containing the same active moiety for the same indication for a period of seven years, except
in limited circumstances, such as a showing of clinical superiority over the drug with orphan drug exclusivity. Orphan drug exclusivity
does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or
condition.
Orphan drug designation must be
requested before submission of an application for marketing approval. Products that qualify for orphan designation may also qualify for
other FDA programs that are intended to expedite the development and approval process and, as a practical matter, clinical trials for
orphan products may be smaller, simply because of the smaller patient population. Nonetheless, the same approval standards apply to orphan-
designated products as for other drugs. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory
review and approval process.
Europe
The drug development process in
Europe is essentially the same as that required to develop drugs in an acceptable manner, in that a drug must meet the requirements for
quality safety and efficacy. The international regulators (including the FDA) have a system which allows them to mutually recognize the
standards of drug development. This is called the ICH standard (international Conference on Harmonization). This avoids the need for pharmaceutical
companies to repeat their costly drug development programs for different jurisidctions / international territories. There are nuances
between the requirements of the USA, Europe and Japan – but the standards to which development programs must be conducted are essentially
the same.
There are essentially three mechanisms
for obtaining a marketing authorization (MA) in Europe
|
|
1)
|
the Centralized Procedure
|
|
|
2)
|
the De-Centralized Procedure
|
|
|
3)
|
the Mutual Recognition Procedure
|
Centralized Procedure (CP)
The advantage of the centralized
procedure is that it requires a single application which, if successful, results in a single marketing authorization with the same product
information available in all EU languages and valid in all EU member states / countries,
as well as Iceland, Liechtenstein, and Norway. The scientific assessment of the marketing authorization application is carried out by
the Committee on Human Medicinal Products (CHMP). The scientific review process consists of alternating periods of active evaluation and
periods during which the clock is stopped in order to give the applicant time to resolve any issues identified during the evaluation.
In total, the duration of the process is up to 210 ‘active’ days before an opinion is issued by the CHMP. Once an opinion
has been given, it is forwarded to the European Commission which then has 67 days to issue a legally binding decision on the marketing
authorization.
Once a marketing authorization
has been granted, the applicant can start to market the medicine in any EU Member State of its choice. However, in practice before a medicine
is marketed, it will be subject to pricing negotiations and a review of its cost-effectiveness. This is carried out at national level
by Member States to determine reimbursement criteria. Initially, the centralized procedure was mandatory only for biotechnology medicines,
as was the case with the previous concertation procedure. Over time, however, the mandatory scope of the centralized procedure has been
gradually expanded and by 2005, it included orphan medicines (medicines for rare diseases) as well as human medicines that contain a new
active substance (not previously authorized in the Union before 20 November 2005) and that are intended for the treatment of AIDS, cancer,
neurodegenerative disorders, diabetes, auto-immune and other immune dysfunctions, and viral diseases. In 2009, the centralized procedure
also became mandatory for advanced therapy medicines. The centralized procedure is also optional for other medicines that contain a new
active substance not authorized in the Union before 20 November 2005, and for products which are considered to be a significant therapeutic,
scientific, or technical innovation, or for which an EU-wide authorization is considered to be in the interests of public health.
The Decentralized Procedure (DCP)
In the decentralized procedure,
the applicant chooses one country as the reference Member State when making its application for marketing authorization. The chosen reference
Member State then prepares a draft assessment report that is submitted to the other Member States where approval is sought for their simultaneous
consideration and approval. In allowing the other Member States access to this assessment at an early stage, any issues and concerns can
be dealt with quickly without delay, which sometimes is known to occur with the mutual recognition procedure (MRP, see below). Compared
with the MRP, the decentralized procedure has the advantage that the marketing authorization in all chosen Member States is received simultaneously,
enabling simultaneous marketing of the medicine and reducing the administrative and regulatory burden.
The Mutual Recognition Procedure (MRP)
The mutual recognition procedure
has been in place since 1995 and evolved from the multi-state licensing procedure. The applicant must initially receive national approval
in one EU Member State, referred to as the “Reference Member State” (RMS) and then seek approval for the medicine in other,
so-called ‘Concerned Member States’ in a second step based on the assessment done in the RMS. This process has significant
differences from the former multi-state licensing procedure, notably the requirement that disagreements between Member States must now
be resolved at EU level. Disagreements are handled by the Co-ordination Group for Mutual Recognition and Decentralized Procedures –
Human (CMDh), a body representing Member States, which is responsible for any questions in two or more Member States relating to the Marketing
Authorization (MA) of a medicinal product approved through the mutual recognition or the decentralized procedure. If there is a disagreement
between Member States on grounds of a potential serious risk to public health, the CMDh considers the matter in order to reach an agreement
within 60 days. If resolution is not possible by the CMDh, the procedure is referred to the CHMP in a procedure called a referral. The
CHMP will then carry out a scientific assessment of the relevant medicine on behalf of the EU. In contrast to the previous (multi-state)
procedure, the outcome of the CHMP is binding on the Member States involved once it has been adopted by the European Commission. The timelines
for assessment by CHMP is 60 days. Since the introduction of the decentralized procedure, the mutual recognition procedure is used for
extending existing marketing authorizations to other countries.
There are other nuances to Marketing
Authorization approval of medicines in Europe compared with the FDA. For example, a Pediatric Investigation Plan (PIP) is a development
plan aimed at ensuring that the necessary data are obtained through studies in children, to support the authorization of a medicine for
children. All applications for marketing authorization for new medicines have to include the results of studies as described in an agreed
PIP, unless the medicine is exempt because of a deferral or waiver.
Orphan Drug Designation in Europe
In the European Union, it is also
possible to obtain an orphan drug designation for a pipeline drug candidate. This also entitles a company to financial incentives such
as a reduction of fees or fee waivers and ten years of market exclusivity following drug approval. This period may be reduced to six years
if the orphan drug designation criteria are no longer met, including where it is shown that the drug is sufficiently profitable not to
justify maintenance of market exclusivity. The definition of what qualifies as a rare disease in Europe is slightly different to the USA
definition.
To qualify for orphan
designation in Europe, a medicine must meet a number of criteria:
|
|
§
|
it must be intended for the treatment, prevention or diagnosis of a disease
that is life-threatening or chronically debilitating;
|
|
|
§
|
the prevalence of the condition in the EU must not be more than 5 in 10,000
or it must be unlikely that marketing of the medicine would generate sufficient returns to justify the investment needed for its development;
|
|
|
§
|
no satisfactory method of diagnosis, prevention or treatment of the condition
concerned can be authorized, or, if such a method exists, the medicine must be of significant benefit to those affected by the condition.
|
As with the USA, European Orphan
drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
In the same way that there is
no guarantee than any medicines developed by Alterola will be approved in the USA, there is similarly no guarantee that any of Alterola’s
medicines will be approved in Europe.
Non-Pharmaceuticals
Food, Drinks & Dietary Supplements
USA
According to the FDA, it is currently
illegal to market THC or CBD by adding it to a food or labeling it as a dietary supplement. Based on available evidence, FDA has concluded
that THC and CBD products are excluded from the dietary supplement definition under section 201(ff)(3)(B) of the FD&C Act [21 U.S.C.
§ 321(ff)(3)(B)]. Under that provision, if a substance (such as THC or CBD) is an active ingredient in a drug product that has been
approved under section 505 of the FD&C Act [21 U.S.C. § 355], or has been authorized for investigation as a new drug for which
substantial clinical investigations have been instituted and for which the existence of such investigations has been made public, then
products containing that substance are excluded from the definition of a dietary supplement. FDA considers a substance to be "authorized
for investigation as a new drug" if it is the subject of an Investigational New Drug application (IND) that has gone into effect.
Under FDA’s regulations (21 CFR 312.2), unless a clinical investigation meets the limited criteria in that regulation, an IND is
required for all clinical investigations of products that are subject to section 505 of the FD&C Act.
There is an exception to section
201(ff)(3)(B) if the substance was "marketed as" a dietary supplement or as a conventional food before the drug was approved
or before the new drug investigations were authorized, as applicable. However, based on available evidence, FDA has concluded that this
is not the case for THC or CBD. FDA is not aware of any evidence that would call into question its current conclusions that THC and CBD
products are excluded from the dietary supplement definition under section 201(ff)(3)(B) of the FD&C Act. FDA continues to review
information that is submitted to FDA on this issue, but to date this has not caused FDA to change their conclusions.
Given the legal / regulatory situation
at present in the USA, at this time, Alterola will not be looking to commercialize cannabinoid-containing ingredients or products in the
food, drinks or dietary supplements sector in the USA.
Europe - Novel Food Application (Europe)
Under EU regulations,
any food that was not consumed “significantly” prior to May 1997 is considered to be a “Novel Food”. The category
covers new foods, food from new sources, new substances used in food as well as new ways and technologies for producing food. There is
a specific procedure for gaining a Novel Food Approval in Europe.
The novel food status of CBD extracts
was confirmed in January 2019. This means that applicants need to apply for authorisation of CBD extracts and isolates using the procedure
for full applications (rather than a traditional food) outlined in the European Food Standards Agency (EFSA) guidance.
In general, the process is as
follows: (1) The applicant submits a Novel Food application; (2) the application is reviewed and if compliant validated by the European
Commission to see if it falls within the scope of Novel Food Regulation (EU) 2015 / 2283 (EC validity check); (3) the European Food Standards
Agency (EFSA) undertakes a suitability check to see if the application fulfils the requirements of article 10(2) of (EU) 2015 / 2283;
(4) EFSA reviews and performs a risk assessment and gives an opinion within 9 months of receipt of a valid application (5) the EC drafts
an implementing act authorizing the placement on the market of a Novel Food and updating the EU list, within 7 months of the EFSA opinion.
This process can take approximately 18 months from receipt of a valid application, although it can take longer in some cases.
Given the legal and regulated
process in Europe, Alterola intends to submit Novel Food applications for cannabinoid-containing ingredients and / or products in the
food, drinks or dietary supplements sector in Europe, where it is legal to do so. It may be several years before we can obtain approval
and commence commercialization of such ingredients, if ever.
Rest of The World (RoW)
Given the varying legal
and regulated processes for regulatory approval of for cannabinoid-containing ingredients and / or products in the food, drinks or dietary
supplements sector in countries outside of the USA and Europe, Alterola will consider gaining such approval in countries / territories
where it is legal to do so. These will be considered on a case-by-case basis as appropriate. It may be several years before we can obtain
approval and commence commercialization of such ingredients, if ever.
Cosmetics
USA
A cosmetic is defined in the Food,
Drug and Cosmetics Act 201(i) as "(1) articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise
applied to the human body or any part thereof for cleansing, beautifying, promoting attractiveness, or altering the appearance, and (2)
articles intended for use as a component of any such articles; except that such term shall not include soap."
Under the FD&C Act, cosmetic
products and ingredients are not subject to premarket approval by FDA, except for most color additives. Certain cosmetic ingredients are
prohibited or restricted by regulation, but currently that is not the case for any cannabis or cannabis-derived ingredients. Ingredients
not specifically addressed by regulation must nonetheless comply with all applicable requirements, and no ingredient – including
a cannabis or cannabis-derived ingredient – cannot be used in a cosmetic if it causes the product to be adulterated or misbranded
in any way. A cosmetic generally is adulterated if it bears or contains any poisonous or deleterious substance which may render it injurious
to users under the conditions of use prescribed in the labeling, or under such conditions of use as are customary or usual (section 601(a)
of the FD&C Act [21 U.S.C. § 361(a)]).
Alterola may choose to
supply active ingredient(s) to cosmetic companies within the USA where it is legal to do so. However, although the company is focussed
upon producing low cost of goods ingredients, there is no guarantee that the company will be able to produce cosmetic ingredients at the
purity required of at a cost of goods which will enable the company to compete within other suppliers of cosmetic ingredients to cosmetic
companies. Alterola has no intention in producing its own cosmetic products. It may be several years before we can obtain approval and
commence commercialization of such ingredients, if ever.
Europe
The use of CBD in cosmetics is
harmonised within the European Cosmetic Regulation 1223/2009, under entry 306 ‘Narcotics, natural and synthetic’ of Annex
II, and has been for some time. The regulation prohibits use of cannabis and cannabis extracts in cosmetics, as they are controlled substances
in Schedule I of the 1961 Single Convention on Narcotic Drugs. However, CBD specifically is not referenced in this convention. At the
beginning of 2019, the European Commission (EC) added two entries to its database of cosmetics ingredients for CBD to differentiate between:
CBD “derived from extract or tincture or resin of cannabis” and CBD “synthetically produced”. Both entries contain
the same text: “Cannabidiol (CBD) as such, irrespective of its source, is not listed in the Schedules of the 1961 Single Convention
on Narcotic Drugs. However, it shall be prohibited from use in cosmetic products (II/306) if it is prepared as an extract or tincture
or resin of Cannabis in accordance with the Single Convention. Please note that national legislations on controlled substances may also
apply.” Essentially, use of naturally-derived CBD from cannabis plants is prohibited in the EU but use of hemp-derived or synthetically-produced
CBD is allowed. However, the Single Convention’s banned ingredients list does not include cannabis seeds or leaves without tops,
meaning use of CBD derived from these parts of the cannabis plant is not currently prohibited.
It is Alterola’s intention
to supply active ingredient(s) to cosmetic companies within the EU where it is legal to do so. However, although the company is focussed
upon producing low cost of goods ingredients, there is no guarantee that the company will be able to produce cosmetic ingredients at the
purity required of at a cost of goods which will enable the company to compete within other suppliers of cosmetic ingredients to cosmetic
companies. Alterola has no intention in producing its own cosmetic products. It may be several years before we can obtain approval and
commence commercialization of such ingredients, if ever.
Rest of the World
Given the varying legal and regulated
processes for regulatory approval of for cannabinoid-containing ingredients and / or products in the cosmetic sector in countries outside
of the USA and Europe, Alterola will consider gaining such approval in countries / territories where it is legal to do so. These will
be considered on a case-by-case basis as appropriate.
Employees
At present, we have no other employees
other than our officers and directors. They oversee all responsibilities in corporate administration, business development and research.
If finances permit, however, we intend to expand our current management to retain skilled directors, officers and employees with experience
relevant to our business focus.
Property
We do
not own any real property. We maintain our corporate offices at 47 Hamilton Square Birkenhead Merseyside CH41 5AR United Kingdom. One
of the company directors has a beneficial ownership in the property, which is leased on “arms length” terms.
Legal Proceedings
From
time to time, we may become party to various lawsuits, claims and other legal proceedings that arise in the ordinary course of our business.
We are not currently a party, as plaintiff or defendant, to any legal proceedings that we believe to be material or which, individually
or in the aggregate, would be expected to have a material effect on our business, financial condition or results of operation if determined
adversely to us.
Smaller Reporting Company
The Company
is a “smaller reporting company” as defined in Rule 12b-2 under the Exchange Act. There are certain exemptions available to
us as a smaller reporting company, including: (1) not being required to comply with the auditor attestation requirements of Section 404(b)
of the Sarbanes Oxley Act; (2) scaled executive compensation disclosures; and (3) the requirement to provide only two years of audited
financial statements, instead of three years. As long as we maintain our status as a “smaller reporting company”, these exemptions
will continue to be available to us.
SELLING STOCKHOLDER
This prospectus relates to the
possible resale by the selling stockholder, EMC2, of shares of common stock that have been or may be issued to EMC2 pursuant to the Purchase
Agreement. We are filing the registration statement of which this prospectus forms a part pursuant to the provisions of the Registration
Rights Agreement, which we entered into with EMC2 on August 11, 2021, concurrently with our execution of the Purchase Agreement, in which
we agreed to provide certain registration rights with respect to sales by EMC2 of the shares of our common stock that have been or may
be issued to EMC2 under the Purchase Agreement.
EMC2, as the selling stockholder,
may, from time to time, offer and sell pursuant to this prospectus any or all of the shares that we have issued or may sell to EMC2 under
the Purchase Agreement. The selling stockholder may sell some, all or none of its shares. We do not know how long the selling stockholder
will hold the shares before selling them, and we currently have no agreements, arrangements or understandings with the selling stockholder
regarding the sale of any of the shares.
The following table presents information
regarding the selling stockholder and the shares that it may offer and sell from time to time under this prospectus. The table is prepared
based on information supplied to us by the selling stockholder and reflects its holdings as of October 18, 2021. Neither EMC2 nor any of
its affiliates has held a position or office, or had any other material relationship, with us or any of our predecessors or affiliates.
Beneficial ownership is determined in accordance with Section 13(d) of the Exchange Act and Rule 13d-3 thereunder.
|
Selling Stockholder
|
|
Shares Beneficially Owned Before this Offering
|
|
Percentage of Outstanding Shares Beneficially Owned Before this Offering
|
|
Shares to be Sold in this Offering Assuming The Company issues the Maximum Number of Shares Under the Purchase Agreement
|
|
Percentage of Outstanding Shares Beneficially Owned After this Offering
|
|
EMC2 Capital, LLC (1)
|
|
22,500,000 (2)
|
|
2.9% (3)
|
|
55,000,000 (4)
|
|
0
|
|
|
(1)
|
Barrett Evans, the Managing Member of EMC2 Capital, LLC, is deemed to be beneficial owners of all of the shares of common stock owned by EMC2 Capital Fund, LLC. Mr. Evans has sole voting and investment power over the shares being offered under the prospectus filed with the SEC in connection with the transactions contemplated under the Purchase Agreement. EMC2 Capital, LLC is not a licensed broker dealer or an affiliate of a licensed broker dealer.
|
|
|
(2)
|
Represents (i) 7,500,000 Commitment Shares of our common stock issued to EMC2 upon our execution of the Purchase Agreement as a fee for its commitment to purchase shares of our common stock under the Purchase Agreement, all of which shares are covered by the registration statement that includes this prospectus; and (ii) an aggregate of 15,000,000 shares of our common stock, representing shares that may be issued to EMC2 as of the date of this prospectus upon exercise of warrants to purchase our common stock, at certain fixed prices (that may be subject to adjustment as provided in such warrants), which warrants were acquired by EMC2 in connection with the Purchase Agreement. EMC2 may not exercise these warrants if such shares, when aggregated with all other shares of our common stock then beneficially owned by EMC2 and its affiliates, would result in EMC2 and its affiliates having beneficial ownership of more than 4.99% of the then total outstanding shares of our common stock, as calculated in accordance with the terms of such warrants. In accordance with rule 13d-3(d) under the Exchange Act, we have excluded from the number of shares beneficially owned prior to the offering all of the shares of common stock that EMC2 may be required to purchase pursuant to the Purchase Agreement because the issuance of such shares is solely at our discretion and is subject to certain conditions, the satisfaction of all of which are outside of EMC2’s control, including the registration statement of which this prospectus is a part becoming and remaining effective. See the description under the heading “The EMC2 Transaction” for more information about the Purchase Agreement.
|
|
|
(3)
|
Based on 754,280,000 outstanding shares of our common stock as of October 18, 2021, which excludes the 7,500,000 Commitment Shares we have already issued to EMC2 pursuant to the Purchase Agreement.
|
|
|
(4)
|
Although the Purchase Agreement provides that we may sell up to $125,000,000 of our common stock to EMC2, only 55,000,000 shares of our common stock are being offered under this prospectus, which represents: (i) 7,500,000 Commitment Shares issued to EMC2 upon our execution of the Purchase Agreement as a fee for its commitment to purchase shares of our common stock under the Purchase Agreement; (ii) 15,000,000 Commitment Warrants issued to EMC2 upon our execution of the Purchase Agreement; and (iii) an aggregate of 32,500,000 shares of our common stock that may be sold by us to EMC2 at our discretion from time to time over a 36-month period commencing after the satisfaction of certain conditions set forth in the Purchase Agreement, including that the SEC has declared effective the registration statement that includes this prospectus. Depending on the price per share at which we sell our common stock to EMC2 pursuant to the Purchase Agreement, we may need to sell to EMC2 under the Purchase Agreement more shares of our common stock than are offered under this prospectus in order to receive aggregate gross proceeds equal to the $125,000,000 total commitment available to us under the Purchase Agreement. If we choose to do so, we must first register for resale under the Securities Act such additional shares. The number of shares ultimately offered for resale by EMC2 is dependent upon the number of shares we sell to EMC2 under the Purchase Agreement.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The
following discussion and analysis of our results of operations and financial condition should be read in conjunction with our consolidated
financial statements and the notes to those consolidated financial statements that are included elsewhere in this prospectus. Our discussion
includes forward-looking statements based upon current expectations that involve risks and uncertainties, such as our plans, objectives,
expectations and intentions. Actual results and the timing of events could differ materially from those anticipated in these forward-looking
statements as a result of a number of factors. See “Cautionary Note Regarding Forward-Looking Statements” at the beginning
of this prospectus.
Results of Operations for the Three Months
Ended June 30, 2021 and 2020
We have
generated no revenues since inception and we do not anticipate earning revenues until such time that we are able to market and sell our
products.
We incurred
operating expenses of $150,560 for the three months ended June 30, 2021, consisting mainly of research and development of $136,291.
We expect
that our operation expenses will increase significantly for the balance of the fiscal year ended March 31, 2022. This would be the result
of increased research and development expenses associated with our product candidates, the regulatory process of approval of those products,
as well as the expenses associated with our reporting obligations with the Securities and Exchange Commission.
We recorded
a net loss of $150,560 for the three months ended June 30, 2021.
As a
newly formed pharmaceutical company, the company has limited operations to date, and expects to have reoccurring losses, as is typical
with companies in the pharmaceutical industry, for the foreseeable future. As explained above, the company intends to raise capital and
ramp up its efforts to bring its product candidates to market. This will require significant capital, product development to continue
and complete and momentum on those product candidates through the regulatory process. There are no assurances that we will be able to
generate revenues and achieve profitable operations.
Results of Operations for Six Months Ended
March 31, 2021 and 2020
We have generated no
revenues since inception and we do not anticipate earning revenues until such time that we are able to market and sell our products.
We incurred operating
expenses of $309,500 for the six months ended March 31, 2021, compared with $139,001 for the six months ended March 31, 2020.
Our operating expenses
for the six months ended March 31, 2021, mainly consisted of $243,000 in professional fees, $30,000 in director fees, and $26,500 in accounting
and audit fees. Our operating expenses for the six months ended March 31, 2020, mainly consisted of $60,000 in director fees, $60,000
in consulting fees, and $10,500 in audit fees.
We recorded other expense
of zero for the six months ended March 31, 2021, as compared with other income of $79,000 for the six months ended March 31, 2020. Our
other income for March 31, 2020, consisted of third-party consideration to the company for effecting a change in stock symbol.
We recorded a net loss
of $98,500 for the six months ended March 31, 2021, compared with a net loss of $60,001 for the six months ended March 31, 2020.
Results of Operations for the Year Ended
September 30, 2020 and 2019
We generated no revenue
for the period from July 21, 2008 (Date of Inception) until September 30, 2020. We do not anticipate earning revenues until such time
that we are able to market and sell our products.
We had operating expenses
of $329,511 for the year ended September 30, 2020, as compared with operating expenses of $258,453 for the year ended September 30, 2019.
Our operating expenses for the year ended September 30, 2020, consisted of director fees of $120,000, stock-based compensation of $130,000,
consulting fees of $60,000 and accounting and audit fees of $11,000. Our operating expenses for the year ended September 30, 2019, consisted
of director fees of $120,000, stock-based compensation of $126,000, and accounting and audit fees of $11,000.
We anticipate our operating
expenses will increase as we implement our business plan.
We had other income $79,000
for the year ended September 30, 2020, which consisted of third-party consideration to the company for effecting a change in stock symbol,
as compared with other expenses of $249 for the year ended September 30, 2019, which consisted of interest expense.
We recorded a net loss
of $250,511 for the year ended September 30, 2020, as compared with a net loss of $258,702 for the year ended September 30, 2019.
Liquidity and Capital
Resources
As of
June 30, 2021, we had $15,227 in current assets and currently liabilities of $910,723. We had a working capital deficit of $895,496 as
of June 30, 2021.
We had
insignificant operating, investing or financing cash flows to report for the three months ended June 30, 2021, and 2020.
Based
upon our current financial condition, we do not have sufficient cash to operate our business at the current level for the next 12 months.
We intend to fund operations through increased sales and debt and/or equity financing arrangements, which may be insufficient to fund
expenditures or other cash requirements. We plan to seek additional financing in a private equity offering to secure funding for operations.
There can be no assurance that we will be successful in raising additional funding. If we are not able to secure additional funding, the
implementation of our business plan will be impaired. There can be no assurance that such additional financing will be available to us
on acceptable terms or at all.
Subsequent
to the reporting period, we received $100,000 in funding in July and we entered into an equity line financing for up to $125,000,000.
The company is hopeful that this financing may assist the company to raise the funds needed to implement its business plan. The financing,
however, is conditional on filing a registration statement with the Securities and Exchange Commission and other factors set forth in
the definitive agreements. If we are unable to use the equity line, or we are limited in the amounts of funds we are able to draw from
such line, we may not realize the funds necessary to implement our business plan exclusively from this equity line financing.
Off Balance Sheet Arrangements
As of
June 30, 2021, we had no off-balance sheet arrangements.
Going Concern
Our financial
statements were prepared assuming we will continue as a going concern which contemplates the realization of assets and satisfaction of
liabilities in the normal course of business. We have negative working capital of $895,496 at June 30, 2021 and have incurred losses since
inception to the same time of $1,649,936. We expect to incur further losses in the development of our business and have been dependent
on funding operations from inception. These conditions raise substantial doubt about our ability to continue as a going concern. Management’s
plans include continuing to finance operations through the private or public placement of debt and/or equity securities and the reduction
of expenditures. However, no assurance can be given at this time as to whether we will be able to achieve these objectives. The financial
statements do not include any adjustment relating to the recoverability and classification of recorded asset amounts or the amounts and
classification of liabilities that might be necessary should we be unable to continue as a going concern.
MANAGEMENT
The following information sets
forth the names, ages, and positions of our current directors and executive officers.
|
Name
|
|
Age
|
|
Positions and Offices Held
|
|
Timothy Rogers
|
|
|
58
|
|
|
Chairman, CFO and Director
|
|
Seamus McAuley
|
|
|
45
|
|
|
Chief Executive Officer, Secretary and Director
|
|
Colin Stott
|
|
|
55
|
|
|
Chief Operating Officer and Director
|
|
Hunter Land
|
|
|
37
|
|
|
Vice President of Translational Research and Director
|
|
Dominic Schiller
|
|
|
57
|
|
|
Director
|
|
Daniel Reshef
|
|
|
69
|
|
|
Director
|
|
Lalit Kumar Verma
|
|
|
40
|
|
|
Director
|
|
Ning Qu
|
|
|
52
|
|
|
Director
|
Set forth below is a
brief description of the background and business experience of each of our current executive officers and directors.
Timothy Paul Rogers – CFO - Director - Chairman
Age 57
Timothy. Rogers is an international
business leader with 35 years’ experience in global sales and marketing, specifically launching products from an intellectual property
platform. Mr. Rogers is multi-lingual, and has been involved with start-ups in Singapore, South East Asia, Africa, Australia, the United
States, Canada and Europe in the pharmaceutical, agriculture, essential oil, biocide, oil and gas and cosmetic sectors. He has gained
success from a number of industry disturbing products and services, leading in particular, to being part of the team taking control of
Alterola. Trained as an accountant, he is known for his finance connections, his complex business interests across the globe and specifically
in Africa, ranging from mining, agriculture and controlled substances and linking them all to a focused coffee based social equity program
for economic empowerment of African agricultural workers. He is known for his closeness to a number of African politicians and business
leaders, and his co-operation with these 21st century African entrepreneurs is with the aim to establish a new foreign
investment policy in Africa to use the vast resource of that continent to benefit the most disadvantaged in society. Tim has lived and
worked in UK, Ireland, France, Australia, U.S.A and Thailand for the past 40 years and has conducted business in over 40 countries across
the world physically visiting each one personally.
He currently serves as a Director
of Novagean International Limited. a medical device and therapeutic diagnostic manufacturer and clinical research company based in China
and Galway Ireland.
In recent years, Mr. Rogers has
focused his time building a multi-sector agro-pharma drug development business in Africa which includes controlled substances.
Mr. Rogers earned diplomas including
Business Studies from Birkenhead Technical College, and Animation at the Fisher School of English in Paris, France
Seamus McAuley – Director
– Chief Executive Officer- Age 45
Seamus McAuley is a proven Senior
Commercial Executive with extensive experience in bringing products to market in the pharmaceutical, biotech, diagnostic and device sectors.
He is the founder and CEO of Opes Medical Holdings Ltd., a consultancy offering strategic executive services for the development of new
and innovative medical technologies and in- vitro diagnostics, accessing funding sources and commercial launch of products. Related services
include corporate due diligence, market projection assessment, down-stream value strategies, implementing customized distribution strategies
and deal negotiations. Opes has interests in multiple technologies and innovations which hold great commercial promise and has led investment
rounds and grant applications for product development through vehicles including Horizon 2020 and the Disruptive Technology Innovation
Fund.
Before founding OPES, Mr. McAuley
held several senior level sales and commercialization positions, most recently as European Corporate Development Manager for Diploma PLC,
an international group of businesses supplying specialized technical products and services to the Life Sciences sector, where he was responsible
for identifying, targeting, assessing and closing company acquisitions in strategically identified geographic zones and market sectors.
Prior to that, he was Sales and Commercial Director (UK & Ireland) for Technopath Distribution Ltd, an international manufacturer
and distributor of clinical diagnostic products, where he more than doubled sales.
Mr. McAuley began his life sciences
career as a nurse practitioner in ICU, surgical and trauma wards, before transitioning to the corporate side with GlaxoSmithKline. He
quickly gained recognition for his sales capabilities - consistently ranking in the top 2% of GSK sales executives during his tenure -
and for developing and executing record setting campaigns for a number of high-profile products, including the UK rollout of the Papilloma
virus vaccine, neurological therapies for Parkinson's, smoking cessation, diabetes, depression, urology and erectile dysfunction products.
Mr. McAuley earned Diplomas in Counselling and Nursing from the University of Ulster.
Dominic Schiller – Director – IP Counsel
Age 57
Mr. Schiller is a Chartered and
European Patent Attorney with over 30 years of experience, largely in the pharmaceutical, botanical and nutraceutical industries. He is
the founder and CEO of Equipped 4 Holdings Limited, the parent company of Equipped 4 (IP) Limited, an Intellectual Property law practice,
specializing in building patent portfolios for biotech companies, most notably GW Pharmaceuticals and Compass Pathways.
A pioneer in innovative pharmaceutical
sectors, Mr. Schiller successfully secured some of the earliest and most prominent cannabinoid related patents for GW Pharma, helping
them establish an IP portfolio comprising claims directed to plants, plant extracts, extraction technology, pharmaceutical formulations,
drug delivery and the therapeutic uses of cannabinoids, as well as plant variety rights. He was also the patent attorney behind Compass
Pathways, a mental health care company, where he drafted and prosecuted to grant, patents relating to a psilocybin polymorph, formulations
and their medical use to treat drug resistant depression. For Phynova, a natural products company, he has secured patents for Chinese
herbal products, products with Food Approvals and products with MHRA approvals under the Traditional Herbal Medicinal Product (THMP) directive.
Mr. Schiller serves as a director
and/or advisor to other life sciences companies, including The Life Sciences Division (an investment bank) and Atai Life Sciences (a leading
mental health company), and plays an active management role for a number of companies which he helped found. He is also an inventor on
two key GW Pharmaceutical patents relating to “The use of cannabinoids in the treatment of epilepsy” and “The use of
cannabinoids in the treatment of mental disorders.”
Mr. Schiller holds a
combined honors degree in Biochemistry and Genetics from Leeds University and earned his MBA from Liverpool University.
Colin Stott – Chief Operating Officer
and Director - Age 55
From November 2020 –
present, Mr. Stott has been Founder and Chief Executive Officer of Phytotherapeutix Holdings Ltd. From April 2019 – present, Mr.
Stott has been Founder and Chief Executive Officer of Phytotherapeutix Ltd. From July 1, 2019 – December 1, 2020, he served as Chief
Operating Officer for Alinova Biosciences Ltd. From June 1, 2017 – May 31, 2019, he served as Scientific Affairs Director, International
Division for GW Pharmaceuticals plc. From January 2001 – May 31, 2017, he was R & D Operations Director for GW Pharmaceuticals
plc.
Hunter Land – Age 37
Hunter Land has over
20 years of R&D expertise across 15 different indications, as well as 10 years of cannabinoid-focused research. As an expert in the
field of cannabinoid science, he has developed a pipeline of discovery work on over 20 novel cannabinoids and terpenes. Previously, Hunter
acted as the Sr. Scientific Director, Director of Cannabinoid Research, and scientific spokesperson at Canopy Growth Corporation. Most
notably, Hunter co-established R&D for GW Pharmaceuticals within the US, where he authored multiple protocols in refractory epilepsy
(Dravet Syndrome and Lennox-Gastaut Syndrome), Multiple Sclerosis, pain, and led the clinical development of Epidiolex® (FDA approved
prescription CBD). Hunter acts as the Sr. Scientific Advisor for the National Hockey League Alumni Association in conjunction with NEEKA
Brain Health, a board member of Veterinary Cannabis Society, and lectures at the University of Wisconsin. He has been a featured speaker
at over 50 scientific conferences, a named inventor on 6 patent applications, and has over 20 publications.
Dr. Daniel Reshef - Director – Age 69
Dr. Daniel Reshef is
an Executive Director with substantial clinical experience and demonstrated history of strategic work in the pharmaceuticals industry.
Skilled in Immuno-Oncology, Oncology, Biomarkers, Epidemiology, Vaccines, Ophthalmology, and Clinical Pharmacology, he is Board certified
in Ophthalmology. Dan has extensive experience in clinical, industry, and public health settings, technical skills, project management
and data quality.
Dr. Reshef worked at Roche, Genentech
and served as Therapeutic Area Lead – Immuno- Oncology at a leading pharmaceutical company. Dan has also been successfully involved
in numerous entrepreneurial ventures in the past 20 years. He has been active in diverse areas such as the hotel industry, technology
start- ups, Customer Relations Management (CRM), innovative novel energy sources, blockchain, cryptocurrencies and Forex. Dr. Reshef earned
his MPH & PhD in Epidemiology from Johns Hopkins University.
Lalit Kumar - Age 40
Lalit Kumar is a Director. He
was formerly the CEO of Sakthi Automotive Group. He brings several years of executive international experience working in India, Japan,
China, Korea and the US. His expertise is in supply chain management and global purchasing at OEMs like GM, Honda and Bombardier. He obtained
his MA from Delhi College of Engineering and his MBA from the Institute of Management Technology in Ghaziabad, India. He brings a strong
operational, and manufacturing expertise to support the future growth of Alterola Biotech Inc. and he is working on initiatives to expand
into the company into Europe, India, and China.
Prof. Dr. Ning Qu – Age 52
Ning Qu was born in China in 1968.
He finished his Medical School in China Medical University in 1991 (Cum Laude). He received his medical specialist training in Cardiothoracic
Surgery in Shanghai Chest Hospital and University Medical Center Groningen (UMCG). He is a registered clinical practitioner both in the
Netherlands and China. His strong clinical interest in cardiac surgery is Organ Transplantation (Lung) and open heart surgical intervention
on Atrial Fibrillation. He got his PhD from Groningen University in Lung Transplantation Immunology, and is currently holding two professor
(visiting) positions in Cardiac Surgery and Translational Medicine. He is also one of the four founding professors of Medical Academy
in 2018 of Tianjin University, China.
Term of Office
Our directors are appointed for
a one-year term to hold office until the next annual general meeting of our shareholders or until removed from office in accordance with
our bylaws. Our officers are appointed by our board of directors and hold office until removed by the board.
Family Relationships
There are no family relationships
between or among the directors, executive officers or persons nominated or chosen by us to become directors or executive officers.
Advisory Board
We currently do not have an advisory
board, but we intend to establish one at a later date.
Involvement in Certain Legal Proceedings
During the past 10 years, none
of our current directors, nominees for directors or current executive officers has been involved in any legal proceeding identified in
Item 401(f) of Regulation S-K, including:
1. Any
petition under the Federal bankruptcy laws or any state insolvency law filed by or against, or a receiver, fiscal agent or similar officer
was appointed by a court for the business or property of such person, or any partnership in which he or she was a general partner at or
within two years before the time of such filing, or any corporation or business association of which he or she was an executive officer
at or within two years before the time of such filing;
2. Any
conviction in a criminal proceeding or being named a subject of a pending criminal proceeding (excluding traffic violations and other
minor offenses);
3. Being
subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently
or temporarily enjoining him or her from, or otherwise limiting, the following activities:
|
|
i.
|
Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity;
|
|
|
ii.
|
Engaging in any type of business practice; or
|
|
|
|
|
|
|
iii.
|
Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of Federal or State securities laws or Federal commodities laws;
|
4. Being
subject to any order, judgment or decree, not subsequently reversed, suspended or vacated, of any Federal or State authority barring,
suspending or otherwise limiting for more than 60 days the right of such person to engage in any type of business regulated by the Commodity
Futures Trading Commission, securities, investment, insurance or banking activities, or to be associated with persons engaged in any such
activity;
5. Being found by a court of competent
jurisdiction in a civil action or by the SEC to have violated any Federal or State securities law, and the judgment in such civil action
or finding by the Commission has not been subsequently reversed, suspended, or vacated;
6. Being
found by a court of competent jurisdiction in a civil action
or by the Commodity Futures Trading Commission to have violated any Federal commodities law, and the judgment in such civil action or
finding by the Commodity Futures Trading Commission has not been subsequently reversed, suspended or vacated;
7. Being
subject to, or a party to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed,
suspended or vacated, relating to an alleged violation of:
|
|
i.
|
Any Federal or State securities or commodities law or regulation; or
|
ii. Any
law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction,
order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition
order; or
|
|
iii.
|
Any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
|
8. Being
subject to, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self- regulatory organization
(as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of
the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary
authority over its members or persons associated with a member.
Committees of the Board
Our
company currently does not have nominating or compensation committees performing similar functions nor does our company have a written nominating
or compensation committee charter. Our directors believe that it is not necessary to have such committees, at this time, because the functions
of such committees can be adequately performed by the board of directors.
We do have an Audit and Compliance
Committee, with Ning Qu as Chairperson. We also have Brendan McAleer and Duncan Boxwell on the Committee. The Committee will be responsible
for our accounting and financial reporting processes and the audit of our financial statements.
Our company does not have any
defined policy or procedural requirements for shareholders to submit recommendations or nominations for directors. The board of directors
believes that, given the stage of our development, a specific nominating policy would be premature and
of little assistance until our business operations develop to a more advanced level. Our company does not currently have any specific
or minimum criteria for the election of nominees to the board of directors and we
do not have any specific process or procedure for evaluating such nominees. The board of directors
will assess all candidates, whether submitted by management or shareholders, and make recommendations for election or appointment.
A shareholder who wishes to communicate
with our board of directors may do so by directing a written request addressed to our CEO and
director Seamus McAuley, at the address appearing on the first page of this annual report.
Code of Ethics
We have not adopted a Code of
Ethics that applies our principal executive officer, principal financial officer, principal accounting officer or controller, or persons
performing similar functions.
EXECUTIVE AND DIRECTOR COMPENSATION
The table below summarizes all compensation awarded
to, earned by, or paid to our executive officer for all services rendered in all capacities to us for the periods ended March 31, 2021,
and 2020.
|
SUMMARY COMPENSATION TABLE
|
|
|
Name and principal position
|
|
|
Year
|
|
|
|
Salary
($)
|
|
|
|
Bonus
($)
|
|
|
Stock
Awards
($)
|
|
Option
Awards
($)
|
|
Non-Equity
Incentive Plan
Compensation
($)
|
|
Nonqualified
Deferred
Compensation
Earnings
($)
|
|
All Other
Compensation
($)
|
|
|
Total
($)
|
|
|
Timothy Rogers
Chairman
|
|
|
2021
2020
|
|
|
|
0
0
|
|
|
|
0
0
|
|
|
0
0
|
|
0
0
|
|
0
0
|
|
0
0
|
|
0
0
|
|
|
0
0
|
|
|
Seamus McAuley
Chief Executive Officer
|
|
|
2021
2020
|
|
|
|
0
0
|
|
|
|
0
0
|
|
|
0
0
|
|
0
0
|
|
0
0
|
|
0
0
|
|
0
0
|
|
|
0
0
|
|
|
Rene Lauritsen,
Former officer
|
|
|
2021
2020
|
|
|
|
0
0
|
|
|
|
0
0
|
|
|
0
0
|
|
0
0
|
|
0
0
|
|
0
0
|
|
0
0
|
|
|
0
0
|
|
|
Peter Maddocks
Former officer
|
|
|
2021
2020
|
|
|
|
0
0
|
|
|
|
0
0
|
|
|
0
0
|
|
0
0
|
|
0
0
|
|
0
0
|
|
0
0
|
|
|
0
0
|
|
|
Dheeraj Jain
Former officer
|
|
|
2021
2020
|
|
|
|
0
0
|
|
|
|
0
0
|
|
|
0
0
|
|
0
0
|
|
0
0
|
|
0
0
|
|
0
0
|
|
|
0
0
|
|
|
Lalit Kumar
Former officer
|
|
|
2021
2020
|
|
|
|
0
0
|
|
|
|
0
0
|
|
|
0
0
|
|
0
0
|
|
0
0
|
|
0
0
|
|
0
0
|
|
|
0
0
|
|
Narrative to Summery Compensation Table
We did not compensate our executive officers for the
years ended March 31, 2021, or 2020. With the closing of the stock transfer agreement, we expect to enter into employment agreements with
executive officers for their services.
On March 28, 2021, we entered into an employment agreement
with Larson Elmore. The three-year agreement provides an annual salary to Mr. Elmore of $160,000 and he shall be entitled to receive a
onetime bonus equaling (10%) of salary with the financial close of financing for each plant location obtained by the company. Mr. Elmore
will be entitled to an additional equity interest in the Company in the amount of (4,000,000) four million restricted shares subject to
financing and vesting. Mr. Elmore is entitled to paid sick and vacation and may participate in any benefit programs we make available.
On September 28, 2021, our board and shahreolder removed
Mr. Elmore and the employment agreement terminated.
Outstanding Equity Awards at Fiscal Year-End
We had no outstanding equity awards at fiscal year-end.
Director Compensation
We did not pay our directors for their services to
us in for the year ended March 31, 2021.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other than described below or
the transactions described under the heading “Executive Compensation” (or with respect to which such information is omitted
in accordance with SEC regulations), there have not been, and there is not currently proposed, any transaction or series of similar transactions
to which we were or will be a participant in which the amount involved exceeded or will exceed the lesser of $120,000 or one percent of
the average of our total assets at year-end for the last two completed fiscal years, and in which any director, executive officer, holder
of 5% or more of any class of our capital stock or any member of the immediate family of any of the foregoing persons had or will have
a direct or indirect material interest.
On January 19, 2021, we entered into an Stock Transfer
Agreement (the “Agreement”) with ABTI Pharma Limited, a company registered in England and Wales (“ABTI Pharma”),
pursuant to which the Company will acquire all of the outstanding shares of capital stock of ABTI Pharma from its shareholders in exchange
for 600,000,000 shares of the Company pro rata to the ABTI Pharma shareholders.
On May 24, 2021, we and the shareholders of ABTI Pharma
memorialized a new closing date in an amendment to the Agreement (the “Amendment”). We have already issued the 600,000,000
shares in anticipation of the closing and the transaction closed on May 26, 2021, upon the filing of our December 31, 2020 quarterly report
on Form 10-Q with the Securities and Exchange Commission.
Timothy Rogers, Colin Stott and Dominic Schiller received
the majority of the 600,000,000 shares in the transaction.
During the period ended March 31, 2021, the
Company accrued director’s fees payable of $330,000 to Peter Maddocks.
During the period ended March 31, 2021, Bulls
Run Limited (Leslie Greyling) made advances to the company to fund operating expenses in the amount of $50,000. These advances are non
– interest bearing and have no specified terms of repayment.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS
AND
MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth, as of October 18, 2021,
certain information as to shares of our common stock owned by (i) each person known by us to beneficially own more than 5% of our outstanding
common stock, (ii) each of our directors, and (iii) all of our executive officers and directors as a group:
|
Name and Address of Beneficial Owners of Common Stock
|
|
Title of Class
|
|
Amount and Nature of Beneficial Ownership 1
|
|
% of Common Stock 2
|
|
Timothy Rogers(3)
|
|
Common Stock
|
|
180,000,000 shares
|
|
|
23.9%
|
|
Seamus McAuley(4)
|
|
Common Stock
|
|
30,000,000 shares
|
|
|
4.0%
|
|
Dominic Schiller(5)
|
|
Common Stock
|
|
180,000,000 shares
|
|
|
23.9%
|
|
Daniel Reshef
|
|
-
|
|
4,400,000 shares
|
|
|
Less than 1%
|
|
Lahit Kumar Verma(6)
|
|
Common Stock
|
|
27,750,000 shares
|
|
|
3.6%
|
|
Ning Qu(7)
|
|
Common Stock
|
|
30,000,000 shares
|
|
|
4.0%
|
|
Colin Stott (8)
|
|
Common Stock
|
|
180,000,000
|
|
|
23.9%
|
|
Hunter Land
|
|
Common Stock
|
|
-
|
|
|
-
|
|
Officers and Directors as a Group (8 persons)
|
|
Common Stock
|
|
632,150,000
|
|
|
83.8%
|
|
5% SHAREHOLDERS
|
|
|
|
|
|
|
|
|
NONE
|
|
|
|
|
|
|
|
|
1.
|
As used in this table, "beneficial ownership" means the sole or shared power to vote, or to direct the voting of, a security, or the sole or shared investment power with respect to a security (i.e., the power to dispose of, or to direct the disposition of, a security). In addition, for purposes of this table, a person is deemed, as of any date, to have "beneficial ownership" of any security that such person has the right to acquire within 60 days after such date.
|
|
2.
|
The percentage shown is based on denominator of 754,280,000 shares of common stock issued and outstanding for the company as of October 18, 2021, not including the 7,500,000 Commitment Shares.
|
|
|
3.
|
All shares are held in TPR Holdings Limited, in which Mr. Rogers has voting and investment control over the shares.
|
|
|
4.
|
All shares are held in Opesmedical Holdings Ltd., in which Mr. McAuley has voting and investment control over the shares.
|
|
|
5.
|
All shares are held in Equipped 4 Holdings, in which Mr. Schiller has voting and investment control over the shares.
|
|
|
6.
|
All shares are held in Future Trends, Ltd., in which Mr. Verma has voting and investment control over the shares.
|
|
|
7.
|
All shares are held in Partner Investments B.V. in which Mr. Qu has voting and investment control over the shares.
|
|
|
8.
|
All shares are held in Phytotherapeutix Holdings Ltd in which Mr. Stott has voting and investment control over the shares.
|
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON
ACCOUNTING AND FINANCIAL DISCLOSURE
On May 20, 2021, AJ Robbins CPA,
LLC (the “Former Accountant”) resigned as the Company’s independent registered public accounting firm and, on May 20,
2021, the Company engaged Gries & Associates, LLC (the “New Accountant”) as the Company’s independent registered
public accounting firm. The engagement of the New Accountant was approved by the Company’s Board of Directors.
DESCRIPTION OF CAPITAL STOCK
General
Our authorized capital stock consists of 2,000,000,000 shares of common
stock and 10,000,000 shares of preferred stock, par value $0.001 per share. As of October 18, 2021, there were 754,280,000 shares of our
common stock issued and outstanding (not including the Commitment Shares) and 0 shares of our preferred stock issued and outstanding.
Common Stock
Our common stock is entitled to
one vote per share on all matters submitted to a vote of the stockholders, including the election of directors. Except as otherwise required
by law or provided in any resolution adopted by our board of directors with respect to any series of preferred stock, the holders of our
common stock will possess all voting power. Generally, all matters to be voted on by stockholders must be approved by a majority (or,
in the case of election of directors, by a plurality) of the votes entitled to be cast by all shares of our common stock that are present
in person or represented by proxy, subject to any voting rights granted to holders of any preferred stock. Holders of our common stock
representing fifty percent (50%) of our capital stock issued, outstanding and entitled to vote, represented in person or by proxy, are
necessary to constitute a quorum at any meeting of our stockholders. A vote by the holders of a majority of our outstanding shares is
required to effectuate certain fundamental corporate changes such as liquidation, merger or an amendment to our Articles of Incorporation.
Our Articles of Incorporation do not provide for cumulative voting in the election of directors.
Subject to any preferential rights
of any outstanding series of preferred stock created by our board of directors from time to time, the holders of shares of our common
stock will be entitled to such cash dividends as may be declared from time to time by our board of directors from funds available therefore.
Subject to any preferential rights
of any outstanding series of preferred stock created from time to time by our board of directors, upon liquidation, dissolution or winding
up, the holders of shares of our common stock will be entitled to receive pro rata all assets available for distribution to such holders.
In the event of any merger or
consolidation with or into another company in connection with which shares of our common stock are converted into or exchangeable for
shares of stock, other securities or property (including cash), all holders of our common stock will be entitled to receive the same kind
and amount of shares of stock and other securities and property (including cash). Holders of our common stock have no pre-emptive rights,
no conversion rights and there are no redemption provisions applicable to our common stock.
Preferred Stock
Our board of directors may become
authorized to authorize preferred shares of stock and to divide the authorized shares of our preferred stock into one or more series,
each of which must be so designated as to distinguish the shares of each series of preferred stock from the shares of all other series
and classes. Our board of directors is authorized, within any limitations prescribed by law and our articles of incorporation, to fix
and determine the designations, rights, qualifications, preferences, limitations and terms of the shares of any series of preferred stock
including, but not limited to, the following:
|
|
(1)
|
The number of shares constituting that series and the distinctive designation of that series, which may be by distinguishing number, letter or title;
|
|
|
(2)
|
The dividend rate on the shares of that series, whether dividends will be cumulative, and if so, from which date(s), and the relative rights of priority, if any, of payment of dividends on shares of that series;
|
|
|
(3)
|
Whether that series will have voting rights, in addition to the voting rights provided by law, and, if so, the terms of such voting rights;
|
|
|
(4)
|
Whether that series will have conversion privileges, and, if so, the terms and conditions of such conversion, including provision for adjustment of the conversion rate in such events as the Board of Directors determines;
|
|
|
(5)
|
Whether or not the shares of that series will be redeemable, and, if so, the terms and conditions of such redemption, including the date or date upon or after which they are redeemable, and the amount per share payable in case of redemption, which amount may vary under different conditions and at different redemption dates;
|
|
|
(6)
|
Whether that series will have a sinking fund for the redemption or purchase of shares of that series, and, if so, the terms and amount of such sinking fund;
|
|
|
(7)
|
The rights of the shares of that series in the event of voluntary or involuntary liquidation, dissolution or winding up of the corporation, and the relative rights of priority, if any, of payment of shares of that series; and
|
|
|
(8)
|
Any other relative rights, preferences and limitations of that series.
|
On December 7, 2020, we filed
a Certificate of Designation with the Nevada Secretary of State to designate a class of Series A Preferred Stock. The Series A Preferred
Stock features are summarized below:
1) Consists
of 8,000,000 shares;
2) Super
voting rights of 10 votes of common stock per share;
3) Liquidation
preference of $1.00 per share; and
4) Conversion
rights into common on a 1:10 basis with adjustments.
There are no outstanding shares
of Series A Preferred Stock as of the date of this prospectus
Provisions in Our Articles of Incorporation and
By-Laws That Would Delay, Defer or Prevent a Change in Control
Our articles of incorporation
authorize our board of directors to issue a class of preferred stock commonly known as a “blank check” preferred stock. Specifically,
the preferred stock may be issued from time to time by the board of directors as shares of one (1) or more classes or series. Our board
of directors, subject to the provisions of our Articles of Incorporation and limitations imposed by law, is authorized to adopt resolutions;
to issue the shares; to fix the number of shares; to change the number of shares constituting any series; and to provide for or change
the following: the voting powers; designations; preferences; and relative, participating, optional or other special rights, qualifications,
limitations or restrictions, including the following: dividend rights, including whether dividends are cumulative; dividend rates; terms
of redemption, including sinking fund provisions; redemption prices; conversion rights and liquidation preferences of the shares constituting
any class or series of the preferred stock.
In each such case, we will not
need any further action or vote by our shareholders. One of the effects of undesignated preferred stock may be to enable the board of
directors to render more difficult or to discourage an attempt to obtain control of us by means of a tender offer, proxy contest, merger
or otherwise, and thereby to protect the continuity of our management. The issuance of shares of preferred stock pursuant to the board
of director’s authority described above may adversely affect the rights of holders of common stock. For example, preferred stock
issued by us may rank prior to the common stock as to dividend rights, liquidation preference or both, may have full or limited voting
rights and may be convertible into shares of common stock. Accordingly, the issuance of shares of preferred stock may discourage bids
for the common stock at a premium or may otherwise adversely affect the market price of the common stock.
Share Purchase Warrants
We have no outstanding warrants to purchase our securities, aside from
the Commitment Warrants.
Options
We have no outstanding options
to purchase our securities, aside from the Commitment Warrants.
Convertible Securities
We have not issued and do not
have outstanding any securities convertible into shares of our common stock or any rights convertible or exchangeable into shares of our
common stock.
Certain Anti-Takeover Provisions
Nevada Revised Statutes sections
78.378 to 78.379 provide state regulation over the acquisition of a controlling interest in certain Nevada corporations unless the articles
of incorporation or bylaws of the corporation provide that the provisions of these sections do not apply. Our articles of incorporation
and bylaws do not state that these provisions do not apply. The statute creates a number of restrictions on the ability of a person or
entity to acquire control of a Nevada company by setting down certain rules of conduct and voting restrictions in any acquisition attempt,
among other things. The statute is limited to corporations that are organized in the state of Nevada and that have 200 or more stockholders,
at least 100 of whom are stockholders of record and residents of the State of Nevada; and does business in the State of Nevada directly
or through an affiliated corporation. Because of these conditions, the statute currently does not apply to our company.
PLAN OF DISTRIBUTION
The common stock offered by this
prospectus is being offered by the selling stockholder, EMC2. The common stock may be sold or distributed from time to time by the selling
stockholder directly to one or more purchasers or through brokers, dealers, or underwriters who may act solely as agents at market prices
prevailing at the time of sale, at prices related to the prevailing market prices, at negotiated prices, or at fixed prices, which may
be changed. The sale of the common stock offered by this prospectus could be effected in one or more of the following methods:
|
|
·
|
ordinary brokers’ transactions;
|
|
|
·
|
transactions involving cross or block trades;
|
|
|
·
|
through brokers, dealers, or underwriters who may act solely as agents;
|
|
|
·
|
“at the market” into an existing market for the common stock;
|
|
|
·
|
in other ways not involving market makers or established business markets, including direct sales to purchasers or sales effected through agents;
|
|
|
·
|
in privately negotiated transactions; or
|
|
|
·
|
any combination of the foregoing.
|
In order to comply with the securities
laws of certain states, if applicable, the shares may be sold only through registered or licensed brokers or dealers. In addition, in
certain states, the shares may not be sold unless they have been registered or qualified for sale in the state or an exemption from the
state’s registration or qualification requirement is available and complied with.
EMC2 is an “underwriter”
within the meaning of Section 2(a)(11) of the Securities Act.
EMC2 has informed us that it intends
to use an unaffiliated broker-dealer to effectuate all sales, if any, of the common stock that it may purchase from us pursuant to the
Purchase Agreement. Such sales will be made at prices and at terms then prevailing or at prices related to the then current market price.
Each such unaffiliated broker-dealer will be an underwriter within the meaning of Section 2(a)(11) of the Securities Act. EMC2 has informed
us that each such broker-dealer will receive commissions from EMC2 that will not exceed customary brokerage commissions.
Brokers, dealers, underwriters
or agents participating in the distribution of the shares as agents may receive compensation in the form of commissions, discounts, or
concessions from the selling stockholder and/or purchasers of the common stock for whom the broker-dealers may act as agent. The compensation
paid to a particular broker-dealer may be less than or in excess of customary commissions. Neither we nor EMC2 can presently estimate
the amount of compensation that any agent will receive.
We know of no existing arrangements
between EMC2 or any other stockholder, broker, dealer, underwriter or agent relating to the sale or distribution of the shares offered
by this prospectus. At the time a particular offer of shares is made, a prospectus supplement, if required, will be distributed that will
set forth the names of any agents, underwriters or dealers and any compensation from the selling stockholder, and any other required information.
We will pay the expenses incident
to the registration, offering, and sale of the shares to EMC2. We have agreed to indemnify EMC2 and certain other persons against certain
liabilities in connection with the offering of shares of common stock offered hereby, including liabilities arising under the Securities
Act or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities. EMC2 has agreed to
indemnify us against liabilities under the Securities Act that may arise from certain written information furnished to us by EMC2 specifically
for use in this prospectus or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities.
EMC2 has represented to us that
at no time prior to the Purchase Agreement has EMC2 or its agents, representatives or affiliates engaged in or effected, in any manner
whatsoever, directly or indirectly, any short sale (as such term is defined in Rule 200 of Regulation SHO of the Exchange Act) of our
common stock or any hedging transaction, which establishes a net short position with respect to our common stock. EMC2 agreed that during
the term of the Purchase Agreement, it, its agents, representatives or affiliates will not enter into or effect, directly or indirectly,
any of the foregoing transactions.
We have advised EMC2 that it is
required to comply with Regulation M promulgated under the Exchange Act. With certain exceptions, Regulation M precludes the selling stockholder,
any affiliated purchasers, and any broker-dealer or other person who participates in the distribution from bidding for or purchasing,
or attempting to induce any person to bid for or purchase any security which is the subject of the distribution until the entire distribution
is complete. Regulation M also prohibits any bids or purchases made in order to stabilize the price of a security in connection with the
distribution of that security. All of the foregoing may affect the marketability of the securities offered by this prospectus.
This offering will terminate on
the date that all shares offered by this prospectus have been sold by EMC2.
Our common stock is quoted on
The OTC Markets under the symbol “ABTI”.
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Sections 78.7502 and 78.751 of
the Nevada Revised Statutes authorize a court to award, or a corporation’s board of directors to grant, indemnity to directors and
officers in terms sufficiently broad to permit indemnification, including reimbursement of expenses incurred, under certain circumstances
for liabilities arising under the Securities Act. In addition, our bylaws provide that we have the authority to indemnify our directors
and officers and may indemnify our employees and agents (other than officers and directors) against liabilities to the fullest extent
permitted by Nevada law. We are also empowered under our bylaws to purchase insurance on behalf of any person whom we are required or
permitted to indemnify.
Insofar as indemnification for
liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling us pursuant to the
foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy
as expressed in the Act and is, therefore, unenforceable.
LEGAL MATTERS
The
validity of the securities offered hereby will be passed upon for us by The Doney Law Firm, Las Vegas, Nevada.
EXPERTS
AJ Robbins CPA, LLC and Gries
& Associates, LLC have audited our financial statements included in this prospectus and registration statement to the extent and for
the periods set forth in their audit reports. AJ Robbins CPA, LLC and Gries & Associates, LLC have presented their reports with respect
to our audited financial statements. The reports of AJ Robbins CPA, LLC and Gries & Associates, LLC is included in reliance upon their
authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We are subject to the information
requirements of the Exchange Act and, in accordance therewith, file annual, quarterly and special reports, proxy statements and other
information with the SEC. These documents also may be accessed through the SEC’s electronic data gathering, analysis and retrieval
system, or EDGAR, via electronic means, including the SEC’s home page on the Internet (www.sec.gov). At some point in the near future
we intend to make our reports, amendments thereto, and other information available, free of charge, on our website. At this time, we do
not provide a link on its website to such filings, and there is no estimate for when such a link on our website will be available.
We have filed with the SEC a
registration statement on Form S-1 under the Securities Act, with respect to the securities being offered hereby. This prospectus, which
constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the
exhibits and schedules filed with the registration statement. For further information about us and the securities offered hereby, we refer
you to the registration statement and the exhibits filed with the registration statement. Statements contained in this prospectus regarding
the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete,
and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit
to the registration statement.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
Unaudited Financial Statements:
|
|
F-1
|
Consolidated Balance Sheets as of June 30, 2021 and March 31, 2021;
|
|
F-3
|
Consolidated Statements of Operations for three months ended June 30, 2021 and 2020;
|
|
F-4
|
Consolidated Statements of Cash Flows for three months ended June 30, 2021 and 2020;
|
|
F-5
|
Consolidated Statement of Stockholders’ Equity as of June 30, 2021; and
|
|
F-6
|
Notes to Consolidated Financial Statements.
|
|
Audited Financial Statements:
|
|
F-10
|
Reports of Independent Registered Public Accounting Firms
|
|
F-11
|
Balance Sheets as of March 31, 2021, September 30, 2020 and September 30, 2019;
|
|
F-12
|
Statements of Operations for the six months ended March 31, 2021 and 2020 and the years ended September 30, 2020 and 2019;
|
|
F-13
|
Statement of Stockholders’ Deficit for the six months ended March 31, 2021 and 2020 and the years ended September 30, 2020 and 2019
|
|
F-14
|
Statements of Cash Flows for the six months ended March 31, 2021 and 2020 and the years ended September 30, 2020 and 2019;
|
|
F-15
|
Notes to Financial Statements
|
|
|
|
|
F-21
|
Report of Independent Registered Public Accounting Firm
|
|
F-22
|
Consolidated Balance Sheet of ABTI Pharma Limited as of March 31, 2021 and March 31, 2020
|
|
F-23
|
Consolidated Statements of Operations of ABTI Pharma Limited for the years ended March 31, 2021 and March 31, 2020
|
|
F-24
|
Consolidated Statement of Shareholders’ Deficit of ABTI Pharma Limited for March 31, 2021 and March 31, 2020
|
|
F-25
|
Consolidated Statements of Cash Flows of ABTI Pharma Limited for the years ended March 31, 2021 and March 31, 2020
|
|
F-26
|
Notes to Consolidated Financial Statements
|
|
|
|
|
F-30
|
Pro Forma Financial Information (unaudited)
|
ALTEROLA
BIOTECH, INC.
UNAUDITED
CONSOLIDATED BALANCE SHEETS
AS
OF JUNE 30, 2021 AND MARCH 31, 2021
|
|
|
June
30, 2021
|
|
March
31, 2021
|
|
ASSETS
|
|
|
|
|
|
|
|
|
Current Assets
|
|
|
|
|
|
|
|
|
Bank
|
|
$
|
387
|
|
|
$
|
519
|
|
Funds
in attorney trust account
|
|
|
12,773
|
|
|
|
|
|
Prepaid
|
|
|
2,067
|
|
|
|
—
|
|
Total
Current Assets
|
|
|
15,227
|
|
|
|
519
|
|
|
|
|
|
|
|
|
|
|
TOTAL
ASSETS
|
|
$
|
15,227
|
|
|
$
|
519
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES
AND STOCKHOLDERS’ DEFICIT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Liabilities
|
|
|
|
|
|
|
|
|
Accounts
payable
|
|
$
|
337,281
|
|
|
$
|
98,379
|
|
Accrued
expenses
|
|
|
164,346
|
|
|
|
20,244
|
|
Accrued
expenses- related party
|
|
|
330,000
|
|
|
|
562,665
|
|
Advances
from related party
|
|
|
79,096
|
|
|
|
78,350
|
|
Total
Current Liabilities
|
|
|
910,723
|
|
|
|
759,638
|
|
|
|
|
|
|
|
|
|
|
Total
Liabilities
|
|
|
910,723
|
|
|
|
759,638
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Equity
(Deficit)
|
|
|
|
|
|
|
|
|
Preferred
Stock, $.001 par value, 10,000,000 shares authorized, -0- shares issued and outstanding
|
|
|
—
|
|
|
|
—
|
|
Common
Stock, $.001 par value, 2,000,000,000 shares authorized, 754,280,000 and 754,280,000 shares issued and outstanding, respectively
|
|
|
754,280
|
|
|
|
754,280
|
|
Foreign
currency translation adjustment
|
|
|
160
|
|
|
|
(14,023)
|
|
Additional
paid-in capital
|
|
|
—
|
|
|
|
0
|
|
Accumulated
deficit
|
|
|
(1,649,936
|
)
|
|
|
(1,499,376)
|
|
Total
Stockholders’ Equity (Deficit)
|
|
|
(895,496
|
)
|
|
|
(759,119)
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT
|
|
$
|
15,227
|
|
|
$
|
519
|
See
accompanying notes to financial statements.
ALTEROLA BIOTECH, INC.
UNAUDITED
CONSOLIDATED STATEMENT OF OPERATIONS
FOR
THE THREE MONTHS ENDED JUNE 30, 2021
|
|
|
|
|
|
|
|
Three Months Ended June 30, 2021
|
|
|
|
|
|
|
REVENUES
|
|
$
|
—
|
|
|
|
|
|
|
OPERATING EXPENSES
|
|
|
|
|
Accounting and audit fees
|
|
|
8,392
|
|
Research and development
|
|
|
136,291
|
|
Legal fees
|
|
|
719
|
|
Directors fees
|
|
|
—
|
|
General and administrative expenses
|
|
|
5,158
|
|
TOTAL OPERATING EXPENSES
|
|
|
150,560
|
|
|
|
|
|
|
LOSS FROM OPERATIONS
|
|
|
(150,560)
|
|
|
|
|
|
|
OTHER INCOME (EXPENSE)
|
|
|
|
|
Miscellaneous sale
|
|
|
—
|
|
TOTAL OTHER INCOME (EXPENSE)
|
|
|
—
|
|
|
|
|
|
|
PROVISION FOR INCOME TAXES
|
|
|
—
|
|
|
|
|
|
|
NET LOSS
|
|
$
|
(150,560)
|
|
|
|
|
|
|
NET LOSS PER SHARE: BASIC AND DILUTED
|
|
$
|
(0.00)
|
|
|
|
|
|
|
WEIGHTED AVERAGE NUMBER OF SHARES OUTSTANDING: BASIC AND DILUTED
|
|
|
754,280,000
|
See
accompanying notes to financial statements.
ALTEROLA
BIOTECH, INC.
UNAUDITED
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR
THE PERIOD FROM JANUARY 7, 2021 (INCEPTION) TO MARCH 31 2021 AND JUNE 30, 2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
stock
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares
|
|
|
|
Amount
|
|
|
|
Additional
paid in capital
|
|
|
|
Accumulated
other comprehensive income ( loss)
|
|
|
|
Deficit
|
|
|
|
Total
|
|
Balance, January 7, 2021(inception)
|
|
|
100
|
|
|
$
|
136
|
|
|
|
—
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
136
|
|
Related party interest forgiven
|
|
|
—
|
|
|
|
—
|
|
|
|
1,544
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,544
|
|
Recapitalization on reverse merger
|
|
|
754,279,900
|
|
|
|
754,144
|
|
|
|
(1,544
|
)
|
|
|
—
|
|
|
|
(1,156,343
|
)
|
|
|
(403,743)
|
|
Change in foreign currency
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(14,023
|
)
|
|
|
|
|
|
|
(14,023)
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(343,033
|
)
|
|
|
(343,033)
|
|
Balance, March 31, 2021
|
|
|
754,280,000
|
|
|
$
|
754,280
|
|
|
|
—
|
|
|
$
|
(14,023
|
)
|
|
$
|
(1,499,376
|
)
|
|
$
|
(759,119)
|
|
Change in foreign currency
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
14,183
|
|
|
|
—
|
|
|
|
14,183
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(150,560
|
)
|
|
|
(150,560)
|
|
Balance, June 30, 2021
|
|
|
754,280,000
|
|
|
$
|
754,280
|
|
|
|
0
|
|
|
$
|
160
|
|
|
$
|
(1,649,936
|
)
|
|
$
|
(895,496)
|
See
accompanying notes to financial statements.
ALTEROLA
BIOTECH, INC.
UNAUDITED
CONSOLIDATED STATEMENT OF CASH FLOWS
FOR
THREE MONTHS ENDED JUNE 30, 2021
|
|
|
|
|
|
|
|
Three
Months Ended June 30, 2021
|
|
CASH FLOWS
FROM OPERATING ACTIVITIES
|
|
|
|
|
Net
loss for the period
|
|
$
|
(150,560)
|
|
Changes
in assets and liabilities:
|
|
|
|
|
Increase
(decrease) in prepaid assets
|
|
|
(2,067)
|
|
Increase
(decrease) in accrued expenses -related party
|
|
|
(232,832)
|
|
Increase
(decrease) in accrued expenses
|
|
|
144,590
|
|
Increase
(decrease) in accounts payable
|
|
|
240,151
|
|
Net Cash Used by Operating
Activities
|
|
|
(718)
|
|
|
|
|
|
|
CASH
FLOWS FROM INVESTING ACTIVITIES
|
|
|
|
|
Net Cash Used by Investing
Activities
|
|
|
0
|
|
|
|
|
|
|
CASH FLOWS
FROM FINANCING ACTIVITIES
|
|
|
|
|
Due
from related parties
|
|
|
746
|
|
Net Cash Provided by Financing
Activities
|
|
|
746
|
|
|
|
|
|
|
Effect of exchange rate adjustments
on cash
|
|
|
(160)
|
|
|
|
|
|
|
Net Increase (Decrease) in
Cash and Cash Equivalents
|
|
|
(132)
|
|
|
|
|
|
|
Cash
and cash equivalents, beginning of period
|
|
|
519
|
|
Cash
and cash equivalents, end of period
|
|
$
|
387
|
|
|
|
|
|
|
SUPPLEMENTAL
CASH FLOW INFORMATION
|
|
|
|
|
Interest
paid
|
|
$
|
—
|
|
Income
taxes paid
|
|
$
|
—
|
|
|
|
|
|
|
NON-CASH
INVESTING AND FINANCING INFORMATION
|
|
|
|
See
accompanying notes to financial statements.
ALTEROLA
BIOTECH, INC.
NOTES
TO THE UNAUDITED CONSOIDATED FINANCIAL STATEMENTS
JUNE
30, 2021
NOTE
1 – NATURE OF BUSINESS
After
formation, the Company was in the business of mineral exploration. On May 3, 2010, the Company sold its mineral exploration business
and entered into an Intellectual Property Assignment Agreement (“IP Agreement”) with Soren Nielsen pursuant to which Mr.
Nielsen transferred his right, title and interest in all intellectual property relating to certain chewing gum compositions having appetite
suppressant activity (the “IP”) to the Company for the issuance of 55,000,000 shares of the Company’s common stock.
Following
the acquisition of the IP the Company changed its business direction to pursue the development of chewing gums for the delivery of Nutraceutical/functional
ingredients for applications such as appetite suppressant, cholesterol suppressant, vitamin delivery, antioxidant delivery and motion
sickness suppressant.
The
business plan of the company will no longer be focused on a chewing gum delivery system but it will re-focus its activities to the development
of cannabinoid, cannabinoid-like, and non-cannabinoid pharmaceutical active pharmaceutical ingredients (APIs), pharmaceutical medicines
made from cannabinoid, cannabinoid-like, and non-cannabinoid APIs and European novel food approval of cannabinoid-based, cannabinoid-like
and non-cannabinoid ingredients and products .In addition, the company plans to develop such bulk ingredients for supply into the cosmetic
sector.
On
January 19, 2021, the Company entered into an Stock Purchase Agreement (the “Agreement”) with ABTI Pharma Limited, a company
registered in England and Wales (“ABTI Pharma”), pursuant to which the Company agreed to acquire all of the outstanding shares
of capital stock of ABTI Pharma from its shareholders in exchange for 600,000,000 shares of the Company pro rata to the ABTI Pharma shareholders.
The shares were issued on January 29, 2021 in anticipation of the closing and the parties to the transaction agreed in a March 24, 2021
amendment to close upon the ABTI Pharma Limited Shares being transferred to the Company, which was to occur upon the filing by the Company
of its outstanding December 31, 2020 quarterly report on Form 10-Q, which was filed on May 28, 2021 with the Securities and Exchange
Commission. The transaction closed on May 28, 2021.
The
transaction is being accounted for as a reverse acquisition and recapitalization. ABTI Pharma is the acquirer for accounting purposes
and the Company is the issuer. The historical financial statements presented are the financial statements of ABTI. The Agreement was
treated as a recapitalization and not as a business combination; at the date of the acquisition, the net liabilities of the legal acquirer,
Alterola, were $389,721.
NOTE
2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis
of Presentation
The
accompanying consolidated financial statements were prepared in accordance with accounting principles generally accepted in the United
State of America (GAAP accounting) and include the accounts of Alterola and its wholly owned subsidiaries ABTI Pharma, Phytotherapeutix
Ltd, Ferven Ltd., and Nano4M Ltd. All material intercompany transactions and balances have been eliminated.
ALTEROLA
BIOTECH, INC.
NOTES
TO THE UNAUDITED CONSOLIDATEDEDFINANCIAL STATEMENTS
JUNE
30, 2021
NOTE
2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
The
Company had a September 30 fiscal year end. Subsequent to the Agreement with ABTI Pharma, the Company has changed its year end from September
30 to March 31.
Use
of Estimates
The
preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported
amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date the financial statements and the reported
amount of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash
and Equivalents
For
purposes of the statement of cash flows, the Company considers highly liquid financial instruments purchased with a maturity of three
months or less to be cash equivalents.
Funds
in attorney trust account
The
company does not have its own bank account. Amounts due from attorney represents fund held on behalf of the Company in trust by its legal
counsel.
Fair
Value of Financial Instruments
Alterola’s
financial instruments consist of cash and equivalents, accrued expenses, accrued interest and notes payable. The carrying amount of these
financial instruments approximates fair value (“FV”) due either to length of maturity or interest rates that approximate
prevailing market rates unless otherwise disclosed in these financial statements.
FV
is defined as the price that would be received upon sale of an asset or paid upon transfer of a liability in an orderly transaction between
market participants at the measurement date and in the principal or most advantageous market for that asset or liability. The FV should
be calculated based on assumptions that market participants would use in pricing the asset or liability, not on assumptions specific
to the entity. In addition, the FV of liabilities should include consideration of non-performance risk including our own credit risk.
In
addition to defining FV, the disclosure requirements around FV establish a FV hierarchy for valuation inputs which is expanded. The hierarchy
prioritizes the inputs into three levels based on the extent to which inputs used in measuring FV are observable in the market. Each
FV measurement is reported in one of the three levels which is determined by the lowest level input that is significant to the FV measurement
in its entirety. These levels are:
Level
1 – inputs are based upon unadjusted quoted prices for identical instruments traded in active markets.
Level
2 – inputs are based upon significant observable inputs other than quoted prices included in Level 1, such as quoted prices for
identical or similar instruments in markets that are not active, and model-based valuation techniques for which all significant assumptions
are observable in the market or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
ALTEROLA
BIOTECH, INC.
NOTES
TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
JUNE
30, 2021
NOTE
2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Fair
Value of Financial Instruments (continued)
Level
3 – inputs are generally unobservable and typically reflect management’s estimates of assumptions that market participants
would use in pricing the asset or liability. The FV are therefore determined using model-based techniques that include option pricing
models, discounted cash flow models, and similar techniques.
The
carrying value of the Company’s financial assets and liabilities which consist of cash, accounts payable and accrued liabilities,
and notes payable are valued using level 1 inputs. The Company believes that the recorded values approximate their FV due to the short
maturity of such instruments. Unless otherwise noted, it is management’s opinion that the Company is not exposed to significant
interest, exchange or credit risks arising from these financial instruments.
Income
taxes are computed using the asset and liability method. Under the asset and liability method, deferred income tax assets and liabilities
are determined based on the differences between the financial reporting and tax bases of assets and liabilities and are measured using
the currently enacted tax rates and laws. A valuation allowance is provided for the amount of deferred tax assets that, based on available
evidence, are not expected to be realized.
Foreign
Currency Translation
The
financial statements are presented in US Dollars. Transactions with foreign subsidiaries where US dollars are not the functional currency
will be recorded in accordance with Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”)
Topic 830 Foreign Currency Transaction. According to Topic 830, all assets and liabilities are translated at the exchange rate
on the balance sheet date, stockholders’ equity is translated at historical rates and statement of operations items are translated
at the weighted average exchange rate for the period. The resulting translation adjustments are reported under other comprehensive income
(loss) in accordance with ASC Topic 220, Comprehensive Income . Gains and losses resulting from the translations of foreign currency
transactions and balances are reflected in the statement of operations and comprehensive income (loss )
Revenue
Recognition
On
January 1, 2018, the Company adopted ASC Topic 606, Revenue from Contracts with Customers ("ASC 606"), using the modified
retrospective method applied to those contracts which were not completed as of January 1, 2018. Results for reporting periods
beginning after January 1, 2018 are presented under ASC 606, while prior period amounts are not adjusted and continue to be reported
in accordance with our historic accounting under ASC 605. As of and for the year ended June 30, 2021, the financial statements were
not materially impacted as a result of the application of Topic 606 compared to Topic 605.
Loss
Per Common Share
Basic
loss per share is calculated using the weighted-average number of common shares outstanding during each reporting period. Diluted loss
per share includes potentially dilutive securities such as outstanding options and warrants, using various methods such as the treasury
stock or modified treasury stock method in the determination of dilutive shares outstanding during each reporting period. The Company
does not have any potentially dilutive instruments.
ALTEROLA
BIOTECH, INC.
NOTES
TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
JUNE
30, 2021
NOTE
2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Stock-Based
Compensation
Stock-based
compensation is accounted for at FV in accordance with ASC Topic 718. To date, the Company has not adopted a stock option plan and has
not granted any stock options
Risks
and Uncertainties
On
January 30, 2020, the World Health Organization declared the coronavirus outbreak a “Public Health Emergency of International Concern”
and on March 10, 2020, declared it to be a pandemic. Actions taken around the world to help mitigate the spread of the coronavirus
include restrictions on travel, and quarantines in certain areas, and forced closures for certain types of public places and business.
The Coronavirus and actions taken to mitigate it have had and are expected to have an adverse impact on the economies and financial markets
of many countries, including the geographical area in which the Company plans to operate.”
Recent
Accounting Pronouncements
Alterola
does not expect the adoption of recently issued accounting pronouncements to have a significant impact on the Company’s results
of operations, financial position or cash flow.
NOTE
3 – ACCRUED EXPENSES
Accrued
expenses consisted of the following at June 30, 2021 and March 31, 2021:
|
|
|
June 30, 2021
|
|
March 31, 2021
|
|
Audit fees
|
|
$
|
17,687
|
|
|
$
|
—
|
|
|
Accounting
|
|
|
5,600
|
|
|
|
5,600
|
|
|
Research and development
|
|
|
126,415
|
|
|
|
—
|
|
|
Legal fees and transfer agent
|
|
|
14,644
|
|
|
|
15,644
|
|
|
Total Accrued Expenses
|
|
$
|
164,346
|
|
|
$
|
20,244
|
|
NOTE
4 – CAPITAL STOCK
The
Company has 2,000,000,000 shares of $.001 par value common stock authorized and 10,000,000 shares of $.001 par value preferred stock
authorized.
The
Company has 754,280,000 and 754,280,000 shares of common stock issued and outstanding as of June 30, 2021 and March 31, 2021, respectively.
There are no shares of preferred stock issued and outstanding as of June 30, 2021 and March 31, 2021.
ALTEROLA
BIOTECH, INC.
NOTES
TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
JUNE
30, 2021
NOTE
5 – RELATED PARTY TRANSACTIONS
Alterola
neither owns nor leases any real or personal property. An officer has provided office space without charge. There is no obligation for
the officer to continue this arrangement. Such costs are immaterial to the financial statements and accordingly are not reflected herein.
The officers and directors are involved in other business activities and most likely will become involved in other business activities
in the future.
During
the period ended June 30, 2021, a shareholder made advances to the company to fund operating expenses in the amount of $79,096. These
advances are non – interest bearing and have no specified terms of repayment.
During
the period ended June 30, 2021, the Company accrued director’s fees payable of $330,000.
NOTE
6 – LIQUIDITY & GOING CONCERN
Alterola
has negative working capital of $895,496, has incurred losses since inception of $1,649,936, and has not received revenues from sales
of products or services. These factors create substantial doubt about the Company’s ability to continue as a going concern. The
financial statements do not include any adjustment that might be necessary if the Company is unable to continue as a going concern.
The
ability of Alterola to continue as a going concern is dependent on the Company generating cash from the sale of its common stock and/or
obtaining debt financing and attaining future profitable operations. Management’s plans include selling its equity securities and
obtaining debt financing to fund its capital requirement and ongoing operations; however, there can be no assurance the Company will
be successful in these efforts.
NOTE
7 – SUBSEQUENT EVENTS
In
accordance with ASC Topic 855-10, the Company analyzed its operations subsequent to June 30, 2021 to the date these financial statements
were issued, and determined it does not have any material subsequent events to disclose in these financial statements.
Gries
& Associates, LLC
Certified Public Accountants
400 South Colorado
Blvd, Ste 870
Denver, Colorado 80246

REPORT OF INDEPENDENT REGISTERED
PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders Alterola Biotech,
Inc.
Opinion on the Financial Statements
We have audited the accompanying balance
sheets of Alterola Biotech, Inc. (the Company) as of March 31, 2021 and the related statement of operations, stockholders’ deficit
and cash flows for the period then ended and the related notes (collectively referred to as the financial statements). In our opinion,
the financial statements present fairly, in all material respects, the financial position of the Company as of March 31, 2021, and the
results of its operations and its cash flows for each of the period then ended in conformity
with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our
audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required
to be independent with respect to the Company in accordance with the
U.S. federal securities laws and the applicable
rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance
with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether
the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were
we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding
of internal control over financial reporting, but not for the purpose of expressing an opinion on
the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures
to assess the risks of material misstatement of the financial statements, whether due to error or fraud and performing procedures that
respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial
statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as
evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion
.
Going Concern Uncertainty
The accompanying financial statements have been prepared assuming
that the Company will continue as a going concern. As discussed in note 7 to the financial statements, the Company has negative working
capital of $389,521, has incurred losses since inception of $1,531,288, and has not received any revenues These factors create an uncertainty
as to the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described
in note 7. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Emphasis of Matters-Risks and Uncertainties
The Company is not able to predict the ultimate
impact that COVID -19 will have on its business. However, if the current economic conditions continue, the pandemic could have an adverse
impact on the economies and financial markets of many countries, including the geographical area in which the Company plans to operate.
/s/ Gries & Associates, LLC
We have served as the Company’s auditor
since 2021. Denver, Colorado
June 4, 2021

blaze@griesandassociates.com
400 South Colorado Blvd, Suite 870,
Denver, Colorado 80246 (O)720-464-2875 (M)773-255-5631 (F)720-222-5846

|
AJ Robbins CPA, LLC
Certified Public Accountants
|
REPORT OF INDEPENDENT REGISTERED PUBLIC
ACCOUNTING FIRM
To the Board of Directors and Stockholders
Alterola Biotech, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets
of Alterola Biotech, Inc. (the Company) as of September 30, 2020 and 2019 and the related statements of operations, stockholders’
deficit and cash flows for each of the
years then ended and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements
present fairly, in all material respects, the financial position of the Company as of September 30, 2020 and 2019, and the results of
its operations and its cash flows for each of the years
then ended in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our
audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required
to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations
of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards
of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements
are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform,
an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal
control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal
control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess
the risks of material misstatement of the financial statements, whether due to error or fraud and performing procedures that respond to
those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements.
Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating
the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion
.
Going Concern Uncertainty
The accompanying financial statements have been prepared assuming that the
Company will continue as a going concern. As discussed in note 7 to the financial statements, the Company has negative
working capital of $323,221, has incurred losses since inception of $1,221,788, and has not received any revenues These factors
create an uncertainty as to the Company’s ability to continue as a going concern. Management’s plans in regard to these matters
are also described in note 7. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Emphasis of Matters-Risks and Uncertainties
The Company is not able to predict the ultimate
impact that COVID -19 will have on its business. However, if the current economic conditions continue, the pandemic could have an
adverse impact on the economies and financial markets of many countries, including the geographical area in which the Company plans
to operate.
|
|
|
/s/AJ Robbins CPA LLC
|
|
|
|
|
|
We have served as the Company’s auditor since 2019.
|
|
|
|
|
Denver, Colorado
March 23, 2021
|
|
|
|
aj@ajrobbins.com
400 South Colorado Blvd, Suite 870, Denver, Colorado 80246
(B)303-537-5898 (M)720-339-5566 (F)303-586-6261
ALTEROLA
BIOTECH, INC.
BALANCE
SHEETS
AS
OF MARCH 31, 2021, SEPTEMBER 30, 2020 AND SEPTEMBER 30, 2019
|
|
|
March
31, 2021
|
|
September
30, 2020
|
|
September
30, 2019
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
Funds
in attorney trust account
|
|
$
|
12,773
|
|
|
$
|
15,273
|
|
|
$
|
14,742
|
|
Total Current Assets
|
|
|
12,773
|
|
|
|
15,273
|
|
|
|
14,742
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL
ASSETS
|
|
$
|
12,773
|
|
|
$
|
15,273
|
|
|
$
|
14,742
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES
AND STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
Accrued
expenses
|
|
$
|
20,244
|
|
|
$
|
36,244
|
|
|
$
|
35,202
|
|
Accrued
directors’ fees
|
|
|
330,000
|
|
|
|
300,000
|
|
|
|
180,000
|
|
Advances
from related party
|
|
|
52,250
|
|
|
|
2,250
|
|
|
|
2,250
|
|
Total
Current Liabilities
|
|
|
402,494
|
|
|
|
338,494
|
|
|
|
217,452
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
Liabilities
|
|
|
402,494
|
|
|
|
338,494
|
|
|
|
217,452
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Deficit
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred
Stock, $.001 par value, 10,000,000 shares authorized, -0- shares issued and outstanding
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
Common
Stock, $.001 par
value, 2,000,000,000 shares
authorized, 754,280,000 and 129,980,000
shares issued and outstanding, respectively
|
|
|
754,280
|
|
|
|
129,980
|
|
|
|
116,980
|
|
Additional
paid-in capital
|
|
|
987,287
|
|
|
|
768,587
|
|
|
|
651,587
|
|
Common
stock held in trust
|
|
|
(600,000
|
)
|
|
|
—
|
|
|
|
—
|
|
Accumulated
deficit
|
|
|
(1,531,288
|
)
|
|
|
(1,221,788
|
)
|
|
|
(971,277)
|
|
Total
Stockholders’ Deficit
|
|
|
(389,721
|
)
|
|
|
(323,221
|
)
|
|
|
(202,710)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
$
|
12,773
|
|
|
$
|
15,273
|
|
|
$
|
14,742
|
See
accompanying notes to financial statements.
ALTEROLA
BIOTECH, INC.
STATEMENTS
OF OPERATIONS
FOR
THE SIX MONTHS ENDED MARCH 31, 2021 AND 2020 AND THE YEARS ENDED SEPTEMBER 30, 2020 AND 2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six
Months Ended March 31, 2021
|
|
Six
Months Ended March 31, 2020
|
|
Year
Ended September 30, 2020,
|
|
Year
Ended September 30, 2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
REVENUES
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPERATING
EXPENSES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounting
and audit fees
|
|
|
26,500
|
|
|
|
10,500
|
|
|
|
11,000
|
|
|
|
10,000
|
|
Professional
fees
|
|
|
243,000
|
|
|
|
—
|
|
|
|
130,000
|
|
|
|
126,000
|
|
Consulting
fees
|
|
|
—
|
|
|
|
60,000
|
|
|
|
60,000
|
|
|
|
—
|
|
Legal
fees
|
|
|
5,000
|
|
|
|
4,034
|
|
|
|
4,034
|
|
|
|
930
|
|
Directors
fees
|
|
|
30,000
|
|
|
|
60,000
|
|
|
|
120,000
|
|
|
|
120,000
|
|
General
and administrative expenses
|
|
|
5,000
|
|
|
|
4,467
|
|
|
|
4,477
|
|
|
|
523
|
|
TOTAL
OPERATING EXPENSES
|
|
|
309,500
|
|
|
|
139,001
|
|
|
|
329,511
|
|
|
|
258,453
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LOSS FROM
OPERATIONS
|
|
|
(309,500
|
)
|
|
|
(139,001
|
)
|
|
|
(329,511
|
)
|
|
|
(258,453)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME
(EXPENSE)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Miscellaneous
sale
|
|
|
—
|
|
|
|
79,000
|
|
|
|
79,000
|
|
|
|
—
|
|
Interest
expense
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(249)
|
|
TOTAL
OTHER INCOME (EXPENSE)
|
|
|
—
|
|
|
|
79,000
|
|
|
|
79,000
|
|
|
|
(249)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PROVISION
FOR INCOME TAXES
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET
LOSS
|
|
$
|
(309,500
|
)
|
|
$
|
(60,001
|
)
|
|
$
|
(250,511
|
)
|
|
$
|
(258,702)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET
LOSS PER SHARE: BASIC AND DILUTED
|
|
$
|
(0.00
|
)
|
|
$
|
(0.00)
|
|
|
$
|
(0.00
|
)
|
|
$
|
(0.00)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
WEIGHTED
AVERAGE NUMBER OF SHARES OUTSTANDING: BASIC AND DILUTED
|
|
|
442,363,333
|
|
|
|
116,980,000
|
|
|
|
118,063,333
|
|
|
|
116,563,333
|
See
accompanying notes to financial statements.
ALTEROLA
BIOTECH, INC.
STATEMENT
OF STOCKHOLDERS’ DEFICIT
FOR
THE PERIOD ENDED MARCH 31, 2021 AND THE YEAR ENDED SEPTEMBER 30, 2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
stock
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares
|
|
|
|
Amount
|
|
|
|
Additional
paid-in Capital
|
|
|
|
Shares
held in trust
|
|
|
|
Accumulated
Deficit
|
|
|
|
Total
|
|
Balance, September
30, 2019
|
|
|
116,980,000
|
|
|
$
|
116,980
|
|
|
$
|
651,587
|
|
|
|
—
|
|
|
$
|
(971,277
|
)
|
|
$
|
(202,710)
|
|
Common
stock issued for services
|
|
|
13,000,000
|
|
|
|
13,000
|
|
|
|
117,000
|
|
|
|
—
|
|
|
|
|
|
|
|
130,000
|
|
Net
loss for the year ended September 30, 2020
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(250,511
|
)
|
|
|
(250,511)
|
|
Balance,
September 30, 2020
|
|
|
129,980,000
|
|
|
$
|
129,980
|
|
|
$
|
768,587
|
|
|
|
—
|
|
|
$
|
(1,221,788
|
)
|
|
$
|
(323,221)
|
|
Common
stock issued for services
|
|
|
24,300,000
|
|
|
|
24,300
|
|
|
|
218,700
|
|
|
|
|
|
|
|
|
|
|
|
243,000
|
|
Common
stock issued for acquisition
|
|
|
600,000,000
|
|
|
|
600,000
|
|
|
|
—
|
|
|
|
(600,000
|
)
|
|
|
—
|
|
|
|
—
|
|
Net
loss for the period ended March 31, 2021
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(309,500
|
)
|
|
|
(309,500)
|
|
Balance,
March 31, 2021
|
|
|
754,280,000
|
|
|
$
|
754,280
|
|
|
$
|
987,287
|
|
|
|
(600,000
|
)
|
|
$
|
(1,531,288
|
)
|
|
$
|
(389,721)
|
See
accompanying notes to financial statements.
ALTEROLA
BIOTECH, INC.
STATEMENTS
OF CASH FLOWS
FOR
THE SIX MONTHS ENDED MARCH 31, 2021 AND 2020, AND YEARS ENDED SEPTEMBER 30, 2020 AND 2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six
months ended March 31, 2021
|
|
Six
months ended March 31, 2020
|
|
Twelve
months ended September 30, 2020
|
|
Twelve
months ended September 30, 2019
|
|
CASH
FLOWS FROM OPERATING ACTIVITIES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss for the period
|
|
$
|
(309,500
|
)
|
|
$
|
(60,001
|
)
|
|
$
|
(250,511
|
)
|
|
$
|
(258,702)
|
|
Non-cash
items related to operations
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock
based compensation
|
|
|
243,000
|
|
|
|
0
|
|
|
|
130,000
|
|
|
|
126,000
|
|
Changes
in assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Increase
(decrease) in accrued expenses
|
|
|
14,000
|
|
|
|
60,533
|
|
|
|
121,042
|
|
|
|
130,953
|
|
Increase
(decrease) in accrued interest
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
249
|
|
Increase
(decrease) in advance from related party
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
1,500
|
|
Increase
(decrease) in due from attorney
|
|
|
2,500
|
|
|
|
(532
|
)
|
|
|
(531
|
)
|
|
|
0
|
|
Net
Cash Used by Operating Activities
|
|
|
(50,000
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH
FLOWS FROM INVESTING ACTIVITIES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquisition
of intellectual property
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
Website
development
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
Net
Cash Used by Investing Activities
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH
FLOWS FROM FINANCING ACTIVITIES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Due
from related parties
|
|
|
50,000
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
Proceeds
from notes payable
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
Net
Cash Provided by Financing Activities
|
|
|
50,000
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Increase
(Decrease) in Cash and Cash Equivalents
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
and cash equivalents, beginning of period
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
Cash
and cash equivalents, end of period
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL
CASH FLOW INFORMATION
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest
paid
|
|
$
|
0
|
|
|
$
|
0
|
|
|
|
0
|
|
|
|
0
|
|
Income
taxes paid
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NON-CASH
INVESTING AND FINANCING INFORMATION
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
stock to be issued for acquisition
|
|
$
|
600,000
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
Common
stock issued for services
|
|
$
|
243,000
|
|
|
$
|
0
|
|
|
$
|
130,000
|
|
|
$
|
0
|
See
accompanying notes to financial statements.
ALTEROLA
BIOTECH, INC.
NOTES
TO THE FINANCIAL STATEMENTS
NOTE
1 – NATURE OF BUSINESS
After
formation, the Company was in the business of mineral exploration. On May 3, 2010, the Company sold its mineral exploration business
and entered into an Intellectual Property Assignment Agreement (“IP Agreement”) with Soren Nielsen pursuant to which Mr.
Nielsen transferred his right, title and interest in all intellectual property relating to certain chewing gum compositions having appetite
suppressant activity (the “IP”) to the Company for the issuance of 55,000,000 shares of the Company’s common stock.
Following
the acquisition of the IP the Company changed its business direction to pursue the development of chewing gums for the delivery of Nutraceutical/functional
ingredients for applications such as appetite suppressant, cholesterol suppressant, vitamin delivery, antioxidant delivery and motion
sickness suppressant.
The
business plan of the company will no longer be focused on a chewing gum delivery system but it will re-focus its activities to the development
of cannabinoid, cannabinoid-like, and non-cannabinoid pharmaceutical active pharmaceutical ingredients (APIs), pharmaceutical medicines
made from cannabinoid, cannabinoid-like, and non-cannabinoid APIs and European novel food approval of cannabinoid-based, cannabinoid-like
and non-cannabinoid ingredients and products .In addition, the company plans to develop such bulk ingredients for supply into the cosmetic
sector.
On
January 19, 2021, the Company entered into an Stock Transfer Agreement (the “Agreement”) with ABTI Pharma Limited, a company
registered in England and Wales (“ABTI Pharma”), pursuant to which the Company will acquire all of the outstanding shares
of capital stock of ABTI Pharma from its shareholders in exchange for 600,000,000 shares of the Company pro rata to the ABTI Pharma shareholders.
The shares have been issued in anticipation of the closing and the transaction will close upon the ABTI Pharma Limited Shares being transferred
to the Company which will occur upon the filing by the Company of its outstanding annual report and form 10-K for 2019, and its quarterly
reports for 2020, which were filed on May 28, 2021. The shares were issued January 29, 2021 in anticipation of a closing. The transaction
will be accounted for as a reverse acquisition upon closing. As of March 31, 2021, the shares have been recorded at $600,000 and has
been reflected in the statement of equity as common stock held in trust as the shares have not been released to ABTI Pharma.
NOTE
2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Accounting
Basis
The
Company uses the accrual basis of accounting and accounting principles generally accepted in the United States of America (“GAAP”
accounting). The Company has a September 30 fiscal year end. Subsequent to the Agreement with ABTI Pharma Limited, the Company
has changed its year end from September 30 to March 31.
Use
of Estimates
The
preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported
amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date the financial statements and the reported
amount of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash
and Equivalents
For
purposes of the statement of cash flows, the Company considers highly liquid financial instruments purchased with a maturity of three
months or less to be cash equivalents.
ALTEROLA
BIOTECH, INC.
NOTES
TO THE FINANCIAL STATEMENTS
NOTE
2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Funds
in attorney trust account
The
company does not have its own bank account. Amounts due from attorney represents fund held on behalf of the Company in trust by its legal
counsel.
Fair
Value of Financial Instruments
Alterola’s
financial instruments consist of cash and equivalents, accrued expenses, accrued interest and notes payable. The carrying amount of these
financial instruments approximates fair value (“FV”) due either to length of maturity or interest rates that approximate
prevailing market rates unless otherwise disclosed in these financial statements.
FV
is defined as the price that would be received upon sale of an asset or paid upon transfer of a liability in an orderly transaction between
market participants at the measurement date and in the principal or most advantageous market for that asset or liability. The FV should
be calculated based on assumptions that market participants would use in pricing the asset or liability, not on assumptions specific
to the entity. In addition, the FV of liabilities should include consideration of non-performance risk including our own credit risk.
In
addition to defining FV, the disclosure requirements around FV establish a FV hierarchy for valuation inputs which is expanded. The hierarchy
prioritizes the inputs into three levels based on the extent to which inputs used in measuring FV are observable in the market. Each
FV measurement is reported in one of the three levels which is determined by the lowest level input that is significant to the FV measurement
in its entirety. These levels are:
Level
1 – inputs are based upon unadjusted quoted prices for identical instruments traded in active markets.
Level
2 – inputs are based upon significant observable inputs other than quoted prices included in Level 1, such as quoted prices for
identical or similar instruments in markets that are not active, and model-based valuation techniques for which all significant assumptions
are observable in the market or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level
3 – inputs are generally unobservable and typically reflect management’s estimates of assumptions that market participants
would use in pricing the asset or liability. The FV are therefore determined using model-based techniques that include option pricing
models, discounted cash flow models, and similar techniques.
The
carrying value of the Company’s financial assets and liabilities which consist of cash, accounts payable and accrued liabilities,
and notes payable are valued using level 1 inputs. The Company believes that the recorded values approximate their FV due to the short
maturity of such instruments. Unless otherwise noted, it is management’s opinion that the Company is not exposed to significant
interest, exchange or credit risks arising from these financial instruments.
ALTEROLA
BIOTECH, INC.
NOTES
TO THE FINANCIAL STATEMENTS
NOTE
2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Income
Taxes
Income
taxes are computed using the asset and liability method. Under the asset and liability method, deferred income tax assets and liabilities
are determined based on the differences between the financial reporting and tax bases of assets and liabilities and are measured using
the currently enacted tax rates and laws. A valuation allowance is provided for the amount of deferred tax assets that, based on available
evidence, are not expected to be realized.
Foreign
Currency Translation
The
financial statements are presented in US Dollars. Transactions with foreign subsidiaries where US dollars are not the functional currency
will be recorded in accordance with Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”)
Topic 830 Foreign Currency Transaction. According to Topic 830, all assets and liabilities are translated at the exchange rate
on the balance sheet date, stockholders’ equity is translated at historical rates and statement of operations items are translated
at the weighted average exchange rate for the period. The resulting translation adjustments are reported under other comprehensive income
(loss) in accordance with ASC Topic 220, Comprehensive Income . Gains and losses resulting from the translations of foreign currency
transactions and balances are reflected in the statement of operations and comprehensive income (loss )
Revenue
Recognition
On
January 1, 2018, the Company adopted ASC Topic 606, Revenue from Contracts with Customers ("ASC 606"), using the modified
retrospective method applied to those contracts which were not completed as of January 1, 2018. Results for reporting periods
beginning after January 1, 2018 are presented under ASC 606, while prior period amounts are not adjusted and continue to be reported
in accordance with our historic accounting under ASC 605. As of and for the year ended March 31, 2021, the financial statements were
not materially impacted as a result of the application of Topic 606 compared to Topic 605.
Loss
Per Common Share
Basic
loss per share is calculated using the weighted-average number of common shares outstanding during each reporting period. Diluted loss
per share includes potentially dilutive securities such as outstanding options and warrants, using various methods such as the treasury
stock or modified treasury stock method in the determination of dilutive shares outstanding during each reporting period. The Company
does not have any potentially dilutive instruments.
Stock-Based
Compensation
Stock-based
compensation is accounted for at FV in accordance with ASC Topic 718. To date, the Company has not adopted a stock option plan and has
not granted any stock options
Risks
and Uncertainties
On
January 30, 2020, the World Health Organization declared the coronavirus outbreak a “Public Health Emergency of International Concern”
and on March 10, 2020, declared it to be a pandemic. Actions taken around the world to help mitigate the spread of the coronavirus
include restrictions on travel, and quarantines in certain areas, and forced closures for certain types of public places and business.
The Coronavirus and actions taken to mitigate it have had and are expected to have an adverse impact on the economies and financial markets
of many countries, including the geographical area in which the Company plans to operate.”
Recent
Accounting Pronouncements
Alterola
does not expect the adoption of recently issued accounting pronouncements to have a significant impact on the Company’s results
of operations, financial position or cash flow.
ALTEROLA
BIOTECH, INC.
NOTES
TO THE FINANCIAL STATEMENTS
NOTE
3 – ACCRUED EXPENSES
Accrued
expenses consisted of the following at March 31, 2021 and September 30, 2020:
|
|
|
March 31, 2021
|
|
September 30, 2020
|
|
Audit fees
|
|
$
|
—
|
|
|
$
|
10,000
|
|
Accounting
|
|
|
5,600
|
|
|
|
6,600
|
|
Legal fees and transfer agent
|
|
|
14,644
|
|
|
|
19,644
|
|
Total Accrued Expenses
|
|
$
|
20,244
|
|
|
$
|
36,244
|
NOTE
4 – CAPITAL STOCK
The
Company has 2,000,000,000 shares of $.001 par value common stock authorized and 10,000,000 shares of $.001 par value preferred stock
authorized.
During
the year ended September 30, 2019, the Company issued 1,000,000 shares of common stock to an officer for services rendered with a deemed
value of services provided of $90,000.
During the year ended September 30, 2020, the
Company issued 13,000,000 shares of common stock to individuals for services rendered with a deemed value of services provided
of $130,000.
During
the period ended March 31, 2021, the Company issued 24,300,000 shares of common stock for services rendered with a deemed value of services
provided of $243,000.
On
January 29, 2021, the Company issued 600,000,000 shares of common stock for an acquisition with a deemed value of $600,000. The shares
have not been transferred and are held in trust as of March 31, 2021.
The
Company has 754,280,000 and 129,980,000 shares of common stock issued and outstanding as of March 31, 2021 and September 30, 2020 respectively.
There are no shares of preferred stock issued and outstanding as of March 31, 2021 and September 30, 2020.
ALTEROLA
BIOTECH, INC.
NOTES
TO THE FINANCIAL STATEMENTS
NOTE
5 – INCOME TAX
Due
to uncertainties surrounding the Company’s ability to generate future taxable income to realize these assets, a full valuation
allowance has been established to offset the net deferred tax asset. The income tax effects of the Tax Cuts and Jobs Act have been completed
in accordance with FASB ASC 740.
The
provision for income tax consists of the following components at March 31, 2021 and September 30, 2020:
|
|
|
2021
|
|
2020
|
|
Current:
|
|
|
|
|
|
|
|
|
|
Federal income taxes (benefit)
|
|
|
(33,490
|
)
|
|
$
|
(82,472
|
)
|
|
State income taxes
|
|
|
—
|
|
|
|
—
|
|
|
Deferred Benefit from net operating loss
|
|
|
33,490
|
|
|
|
82,472
|
|
|
|
|
$
|
(0
|
)
|
|
$
|
(0
|
)
|
The
following reconciles income taxes reported in the financial statements to taxes that would be obtained by applying regular tax rates
to income before taxes:
|
|
|
2021
|
|
2020
|
|
Expected tax expense (benefit) using regular rates
|
|
$
|
33,490
|
|
|
$
|
82,472
|
|
|
State minimum tax valuation allowance
|
|
|
(33,490
|
)
|
|
|
(82,472
|
)
|
|
Tax Provision
|
|
$
|
—
|
|
|
$
|
—
|
|
The
Company has loss carry forwards totaling $1,372,534 that may be offset against future federal income taxes. If not used, the carry forwards
will expire between 2028 and 2040. The change in control may limit the amount of loss carryforward that may be utilized.
At
March 31, 2021 and September 30, 2020, the significant components of the deferred tax assets are summarized below:
|
|
|
March 31, 2021
|
|
September 30, 2020
|
|
Deferred income tax asset
|
|
|
|
|
|
|
|
|
Net operation loss carryforwards
|
|
|
466,662
|
|
|
|
433,172
|
|
Total deferred income tax asset
|
|
|
466,662
|
|
|
|
433,172
|
|
Less: valuation allowance
|
|
|
(466,662
|
)
|
|
|
(433,172)
|
|
Total deferred income tax asset
|
|
$
|
—
|
|
|
$
|
—
|
The
federal income tax returns of the Company for 2021 and 2020 are subject to examination by the IRS, generally for three years after they
were filed.
ALTEROLA
BIOTECH, INC.
NOTES
TO THE FINANCIAL STATEMENTS
NOTE
6 – RELATED PARTY TRANSACTIONS
Alterola
neither owns nor leases any real or personal property. An officer has provided office space without charge. There is no obligation for
the officer to continue this arrangement. Such costs are immaterial to the financial statements and accordingly are not reflected herein.
The officers and directors are involved in other business activities and most likely will become involved in other business activities
in the future.
During
the period ended March 31, 2021, a shareholder made advances to the company to fund operating expenses in the amount of $50,000. These
advances are non – interest bearing and have no specified terms of repayment.
During
the period ended March 31, 2021, the Company accrued director’s fees payable of $330,000.
NOTE
7 – LIQUIDITY & GOING CONCERN
Alterola
has negative working capital of $389,521, has incurred losses since inception of $1,531,288, and has not received revenues from sales
of products or services. These factors create substantial doubt about the Company’s ability to continue as a going concern. The
financial statements do not include any adjustment that might be necessary if the Company is unable to continue as a going concern.
The
ability of Alterola to continue as a going concern is dependent on the Company generating cash from the sale of its common stock and/or
obtaining debt financing and attaining future profitable operations. Management’s plans include selling its equity securities and
obtaining debt financing to fund its capital requirement and ongoing operations; however, there can be no assurance the Company will
be successful in these efforts.
NOTE
8 – OTHER INCOME
The
Company recognized other income of $79,000 during the year ended September 30, 2020. The income consists of payments received from third
parties for effecting a change in stock symbol.
NOTE
9 – SUBSEQUENT EVENTS
In
accordance with ASC Topic 855-10, the Company analyzed its operations subsequent to March 31, 2021
to the date these financial statements were issued.
On
May 24, 2021, the Company and the shareholders of ABTI Pharma memorialized a new closing date in an amendment to the Agreement. The Company
issued the 600,000,000 shares in anticipation of the closing and the transaction will close upon the ABTI Pharma shares being transferred
to the Company, which will occur upon the filing of the Company’s December 31, 2020 quarterly form on Form 10-Q With the Securities
and Exchange Commission (”SEC”). The December 31, 2020 Form 10-Q was filed with the SEC on May 28, 2021.
Pursuant
to the Agreement, the Company will provide funding to ABTI Pharma to pay for operating expenses including salaries, office expenses and
additional expenses or projects in the amount of US$500,000 within fifteen (15) days from closing the Agreement and shall fund an additional
US $200,000 every 30 days thereafter until a total funding of US $1,100,000 has been delivered.
Report of Independent Registered Public
Accounting Firm
To the shareholders and the board of directors of
ABTI Pharma Limited
Opinion on the Financial Statements
We have audited the accompanying consolidated
balance sheets of ABTI Pharma Limited (the "Company") as of March 31, 2021 and 2020, the related consolidated statement of operations,
stockholders' equity, and cash flows for the years ended March 31, 2021 and 2020, and the related notes (collectively referred to as the
"financial statements"). In our opinion, the consolidated financial statements present fairly, in all material respects, the
financial position of the Company as of March 31, 2021 and 2020, and the results of its operations and its cash flows for the years ended
through March 31, 2021 and 2020, in conformity with accounting principles generally accepted in the United States.
Substantial Doubt about the Company’s
Ability to Continue as a Going Concern
The accompanying financial statements have
been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company’s
significant operating losses raise substantial doubt about its ability to continue as a going concern. The financial statements do not
include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility
of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audit. We
are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are
required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and
regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with
the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the
financial statements are free of material misstatement, whether due to error or fraud.
Our audit included performing procedures
to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that
respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial
statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as
evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ BF Borgers CPA PC
BF Borgers CPA PC
Served as Auditor since 2021
Lakewood, CO
June 16, 2021
ABTI Pharma Limited
Consolidated Balance Sheets
|
|
|
March 31,
2021
|
|
March 31,
2020
|
|
ASSETS
|
|
|
|
|
|
Current Assets
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
519
|
|
|
$
|
2
|
|
Total Current Assets
|
|
|
519
|
|
|
|
2
|
|
|
|
|
|
|
|
|
|
|
Total Assets
|
|
$
|
519
|
|
|
$
|
2
|
|
|
|
|
|
|
|
|
|
|
Liabilities and Shareholders' Equity (Deficit)
|
|
|
|
|
|
|
|
|
Current Liabilities
|
|
|
|
|
|
|
|
|
Accounts payable and accrued liabilities
|
|
$
|
97,130
|
|
|
$
|
—
|
|
Accounts payable – related party
|
|
|
232,665
|
|
|
|
|
|
Loan payable - related party
|
|
|
26,100
|
|
|
|
—
|
|
Total Current Liabilities
|
|
|
355,895
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
Total Liabilities
|
|
|
355,895
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
Shareholders' Deficit
|
|
|
|
|
|
|
|
|
Ordinary shares: GBP
£1.00 ($1.36) par value 100 shares issued and outstanding
|
|
|
136
|
|
|
|
3
|
|
Additional paid in capital
|
|
|
1,544
|
|
|
|
—
|
|
Accumulated deficit
|
|
|
(343,033
|
)
|
|
|
—
|
|
Accumulated other comprehensive loss
|
|
|
(14,023
|
)
|
|
|
(1)
|
|
Total Shareholders' Equity (Deficit)
|
|
|
(355,376
|
)
|
|
|
2
|
|
Total Liabilities and Shareholders' Equity (Deficit)
|
|
$
|
519
|
|
|
$
|
2
|
The accompanying notes are an integral part of these
audited financial statements.
ABTI Pharma Limited
Consolidated Statements of Comprehensive Loss
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended March 31,
|
|
|
|
2021
|
|
2020
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
Operating Expenses:
|
|
|
|
|
|
|
|
|
General and administrative
|
|
|
28,838
|
|
|
|
—
|
|
Professional fees
|
|
|
92,219
|
|
|
|
—
|
|
Research and development – related party
|
|
|
220,512
|
|
|
|
—
|
|
Total operating expenses
|
|
|
341,569
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
(341,569
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
Other income (expense)
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
(1,464
|
)
|
|
|
—
|
|
Total other expense
|
|
|
(1,464
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
Net loss before taxes
|
|
|
(343,033
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
Income tax benefit
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
Net Loss
|
|
$
|
(343,033
|
)
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income (loss)
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment
|
|
|
(14,022
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
Comprehensive Loss
|
|
$
|
(357,055
|
)
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
Net loss per ordinary
share, basic and diluted
|
|
$
|
(3,430
|
)
|
|
$
|
—
|
|
Basic and diluted weighted average ordinary shares outstanding
|
|
|
100
|
|
|
|
2
|
The accompanying notes are an integral part of these
audited financial statements.
ABTI Pharma Limited
Consolidated Statement of Shareholders’ Deficit
For the Years Ended March 31, 2021 and 2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of Shares
|
|
Share Capital
|
|
Additional Paid in Capital
|
|
Accumulated Deficit
|
|
Accumulated Other Comprehensive Loss
|
|
Total Shareholders' Equity (Deficit )
|
|
Balance - March 31, 2019
|
|
|
2
|
|
|
$
|
3
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
(1
|
)
|
|
$
|
2
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
Balance - March 31, 2020
|
|
|
2
|
|
|
|
3
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1
|
)
|
|
|
2
|
|
Issuance of ordinary shares
|
|
|
98
|
|
|
|
133
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
133
|
|
Related party interest forgiven
|
|
|
—
|
|
|
|
—
|
|
|
|
1,544
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,544
|
|
Foreign currency translation adjustment
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(14,022
|
)
|
|
|
(14,022)
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(343,033
|
)
|
|
|
—
|
|
|
|
(343,033)
|
|
Balance - March 31, 2021
|
|
|
100
|
|
|
$
|
136
|
|
|
$
|
1,544
|
|
|
$
|
(343,033
|
)
|
|
$
|
(14,023
|
)
|
|
$
|
(355,376)
|
The accompanying notes are an integral part of these
audited financial statements.
ABTI Pharma Limited
Consolidated Statements of Cash Flows
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended March 31,
|
|
|
|
2021
|
|
2020
|
|
|
|
|
|
|
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(343,033
|
)
|
|
$
|
—
|
|
Changes in current assets and liabilities:
|
|
|
|
|
|
|
|
|
Accounts payable – related party
|
|
|
220,512
|
|
|
|
—
|
|
Accounts payable and accrued liabilities
|
|
|
93,520
|
|
|
|
—
|
|
Net cash used in operating activities
|
|
|
(29,000
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
|
|
|
|
|
|
|
|
Proceeds from issuance of ordinary shares
|
|
|
128
|
|
|
|
—
|
|
Proceeds from related party loans
|
|
|
24,736
|
|
|
|
—
|
|
Net cash provided by financing activities
|
|
|
24,864
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate in cash
|
|
|
4,653
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
Net change in cash for the period
|
|
|
517
|
|
|
|
—
|
|
Cash at beginning of period
|
|
|
2
|
|
|
|
2
|
|
Cash at end of period
|
|
$
|
519
|
|
|
$
|
2
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL CASH FLOW INFORMATION:
|
|
|
|
|
|
|
|
|
Cash paid for income taxes
|
|
$
|
—
|
|
|
$
|
—
|
|
Cash paid for interest
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
NON-CASH INVESTING AND FINANCING ACTIVITIES
|
|
|
|
|
|
|
|
|
Forgiveness of related party accrued interest
|
|
$
|
1,464
|
|
|
$
|
—
|
The accompanying notes are an integral part of these
audited financial statements.
ABTI Pharma Limited
Notes to the Consolidated Financial Statements
March 31, 2021 and 2020
NOTE 1 - ORGANIZATION AND DESCRIPTION OF BUSINESS
ABTI Pharma Limited (the “Company”)
was incorporated in the United Kingdom on January 7, 2021. The Company is a UK based pharmaceutical company developing cannabinoid,
cannabinoid-like, and non-cannabinoid pharmaceutical active pharmaceutical ingredients (APIs), pharmaceutical medicines made from cannabinoid,
cannabinoid-like, and non-cannabinoid APIs and targeting European novel food approval of cannabinoid-based, cannabinoid-like and non-cannabinoid
ingredients and products .In addition, the company is seeking to develop such bulk ingredients for supply into the cosmetic sector. To
date, the Company’s activities have been limited to its formation and the raising of equity capital. The Company’s fiscal
year end is March 31.
Subsidiaries
On January 27, 2021, the
Company acquired 100% of Ferven Limited and Phytotherapeutix Ltd. The Companies were under common control before the acquisition and are
consolidated in accordance to ASC-805-50, in which the assets and liabilities of Ferven Limited and Phytotherapeutix Ltd. have been presented
at their carrying values at the date of common control.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The Financial Statements and related disclosures have
been prepared pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”). The Financial Statements
have been prepared using the accrual basis of accounting in accordance with Generally Accepted Accounting Principles (“GAAP”)
of the United States.
Principles of Consolidation
The consolidated financial statements include the
accounts of the Company and its wholly owned subsidiaries, Ferven Limited (United Kingdom) and Phytotherapeutix Ltd (United Kingdom).
All significant intercompany transactions and balances have been eliminated in consolidation.
Use of estimates
The preparation of financial statements in conformity
with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure
of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the
reporting period. Actual results could differ from those estimates.
Cash and cash equivalents
Cash and cash equivalents consist of cash and short-term
investments with original maturities of less than 90 days. Cash equivalents are placed with high credit quality financial institutions
and are primarily in money market funds. The carrying value of those investments approximates fair value. The Company had $519 and $2
in cash as of March 31, 2021 and 2020, respectively.
Foreign Currency Translations
The Company’s functional currency is in British
pound sterling (“GBP”). All transactions are translated into U.S. dollars in accordance with ASC 830-30, “Translation
of Financial Statements,” as follows:
|
|
1)
|
Monetary assets and liabilities at the rate of exchange in effect at the
balance sheet date.
|
|
|
2)
|
Equity at historical rates.
|
|
|
3)
|
Revenue and expense items at the average rate of exchange prevailing during
the period.
|
Adjustments arising from such translations are deferred
until realization and are included as a separate component of stockholders’ equity as a component of comprehensive income or loss.
Therefore, translation adjustments are not included in determining net income (loss) but reported as other comprehensive income (loss).
Gains and losses from foreign currency transactions are included in earnings in the period of settlement.
Financial Instruments
FASB ASC 820, Fair Value Measurements and
Disclosures (“ASC 820”) establishes a framework for all fair value measurements and expands disclosures related to fair
value measurement and developments. ASC 820 defines fair value as the price that would be received to sell an asset or paid to transfer
a liability in an orderly transaction between market participants at the measurement date.
The carrying amounts of cash, accounts payable and
accrued liabilities, and due to related parties approximate fair value because of the short-term nature of these items.
Concentrations of Credit Risks
The Company’s financial instruments that are
exposed to concentrations of credit risk primarily consist of its cash and related party payables it will likely incur in the near future.
The Company places its cash and cash equivalents with financial institutions of high credit worthiness. At times, its cash with a particular
financial institution may exceed any applicable government insurance limits. The Company’s management plans to assess the financial
strength and credit worthiness of any parties to which it extends funds, and as such, it believes that any associated credit risk exposures
are limited.
Income Taxes
The Company accounts for income taxes using the asset
and liability method in accordance with ASC 740, “Accounting for Income Taxes”. The asset and liability method provides that
deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the financial
reporting and tax bases of assets and liabilities and for operating loss and tax credit carry forwards. Deferred tax assets and liabilities
are measured using the currently enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company
records a valuation allowance to reduce deferred tax assets to the amount that is believed more likely than not to be realized.
As of March 31, 2021, and 2020, the Company did not
have any amounts recorded pertaining to uncertain tax positions.
Loss per Share
The Company has adopted ASC 260, “Earnings Per
Share,” (“EPS”) which requires presentation of basic and diluted EPS on the face of the income statement for all entities
with complex capital structures, and requires a reconciliation of the numerator and denominator of the basic EPS computation to the numerator
and denominator of the diluted EPS computation. In the accompanying financial statements, basic loss per share is computed by dividing
net loss by the weighted average number of shares of ordinary stock outstanding during the period.
The Company has no potentially dilutive securities
currently issued and outstanding.
Recent Accounting Pronouncements
Management has considered all recent accounting pronouncements
issued since the last audit of its financial statements. The Company’s management believes that these recent pronouncements will
not have a material effect on the Company’s financial statements.
NOTE 3 - GOING CONCERN
The accompanying financial statements have been prepared
on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business.
The Company has not generated any revenues since inception. The Company has a net loss of $343,033, for the period ended March
31, 2021, working capital deficiency of $355,376 and an accumulated deficit of $343,033 at March 31, 2021. These factors among others
raise substantial doubt about the ability of the Company to continue as a going concern for a reasonable period of time. The accompanying
financial statements do not include any adjustments that might result from the outcome of this uncertainty.
The continuing operations of the Company are dependent
upon its ability to continue to raise adequate financing, shareholder loans and to commence profitable operations in the future and
repay its liabilities arising from normal business operations as they become due. The Company intends to fund operations through equity
financing arrangements, which may be insufficient to fund its capital expenditures, working capital and other cash requirements for future
periods.
NOTE 4 - INCOME TAXES
The Company follows ASC 740. Deferred income taxes
reflect the net effect of (a) temporary difference between carrying amounts of assets and liabilities for financial purposes and the amounts
used for income tax reporting purposes, and (b) net operating loss carry-forwards. No net provision for refundable income tax has been
made in the accompanying statement of loss because no recoverable taxes were paid previously. Similarly, no deferred tax asset attributable
to the net operating loss carry-forward has been recognized, as it is not deemed likely to be realized.
The provisions for income tax, consist of the following:
|
|
|
March 31,
2021
|
|
Net Operating Loss carryforward
|
|
$
|
65,176
|
|
Valuation allowance
|
|
|
(65,176)
|
|
Net deferred tax asset
|
|
$
|
—
|
The tax effects of temporary differences that give
rise to the Company’s net deferred tax assets are as follows:
|
|
|
March 31,
2021
|
|
March 31,
2020
|
|
Net Operating Profit
|
|
$
|
(343,033
|
)
|
|
$
|
—
|
|
Effective tax rate
|
|
|
19%
|
|
|
|
—
|
|
Income Tax expense
|
|
|
(65,176
|
)
|
|
|
—
|
|
Less: valuation allowance
|
|
|
65,176
|
|
|
|
—
|
|
Income Tax Expense
|
|
$
|
—
|
|
|
$
|
—
|
A valuation allowance has been established for our
tax assets as their use is dependent on the generation of sufficient future taxable income, which cannot be predicted at this time. As
of March 31, 2021, we had no material unrecognized tax benefits and no adjustments to liabilities or operations were required. No interest
and penalties have been recognized by us to date. Our net operating loss carryforwards are subject to review and possible adjustment
by HM Revenue & Customs. Tax returns for the years ended 2019 through 2021 are subject to review.
NOTE 5 - RELATED PARTIES TRANSACTIONS
In support of the Company’s efforts and cash
requirements, it may rely on advances from related parties until such time that the company can support its operations or attains adequate
financing through sales of its equity or traditional debt financing. There is no formal written commitment for continued support by shareholders
or directors. Amounts represent advances or amounts paid in satisfaction of liabilities. The advances were considered temporary in nature
and were not formalized by a promissory note.
During the year ended March 31, 2021, the subsidiary’s
director advanced to the Company an amount of $24,736 by the way of loan and the Company accrued $1,544 interest at 13% per annum. As
of March 31,2021, the subsidiary’s director forgave the accrued interest payable of $1,544 and it was recorded to additional paid-in-capital.
During the year ended March 31, 2021, research and
development services of $232,665 were rendered to the Company by a related party. As of March 31, 2021, the unpaid invoice for research
and development services was $232,665.
NOTE 6 - EQUITY
The Company has ordinary shares with a par value of
GBP 1.00 per share.
During the year ended March 31, 2021, 100 ordinary
shares were issued for GBP 100 ($133) in cash.
As of March 31, 2021 and 2020, there were 100
and 2 ordinary shares issued and outstanding, respectively.
NOTE 7 - SUBSEQUENT EVENTS
Management has evaluated subsequent events through
the date these financial statements were available to be issued. Based on our evaluation no material events have occurred that require
disclosure.
ALTEROLA BIOTECH INC.
Unaudited
Pro Forma Condensed Combined Balance Sheet
As of March
31, 2021
|
|
|
Alterola
Biotech Inc.
|
|
ABTI
Pharma Limtied
|
|
Proforma
Adjustments
|
|
Notes
|
|
Proforma
As Adjusted
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
12,773
|
|
|
$
|
519
|
|
|
$
|
—
|
|
|
|
|
|
|
$
|
13,292
|
|
Total
Current assets
|
|
|
12,773
|
|
|
|
519
|
|
|
|
—
|
|
|
|
|
|
|
|
13,292
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
Assets
|
|
$
|
12,773
|
|
|
$
|
519
|
|
|
$
|
—
|
|
|
|
|
|
|
$
|
13,292
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities
and Shareholders' Deficit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current
Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts
payable and accrued liabilities
|
|
$
|
20,244
|
|
|
$
|
97,130
|
|
|
$
|
—
|
|
|
|
|
|
|
$
|
117,374
|
|
Accounts
payable - related party
|
|
|
330,000
|
|
|
|
232,665
|
|
|
|
—
|
|
|
|
|
|
|
|
562,665
|
|
Loans
payable - related parties
|
|
|
52,250
|
|
|
|
26,100
|
|
|
|
—
|
|
|
|
|
|
|
|
78,350
|
|
Total
Current Liabilities
|
|
|
402,494
|
|
|
|
355,895
|
|
|
|
—
|
|
|
|
|
|
|
|
758,389
|
|
Total
Liabilities
|
|
|
402,494
|
|
|
|
355,895
|
|
|
|
—
|
|
|
|
|
|
|
|
758,389
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’
Deficit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred
Stock, $.001 par value, 10,000,000 shares authorized, -0- shares issued and outstanding
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
—
|
|
Common
stock: 2,000,000,000 authorized; $.001 par value 754,280,000 shares issued and outstanding
|
|
|
754,280
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
754,280
|
|
Ordinary
shares: GBP £1.00 ($1.36) par value 100 shares issued and outstanding
|
|
|
—
|
|
|
|
136
|
|
|
|
(136
|
)
|
|
|
4(a)
|
|
|
|
—
|
|
Additional
paid in capital
|
|
|
987,287
|
|
|
|
1,544
|
|
|
|
(599,864
|
)
|
|
|
|
|
|
|
388,967
|
|
Common
stock held in trust
|
|
|
(600,000
|
)
|
|
|
—
|
|
|
|
600,000
|
|
|
|
|
|
|
|
—
|
|
Accumulated
deficit
|
|
|
(1,531,288
|
)
|
|
|
(343,033
|
)
|
|
|
—
|
|
|
|
|
|
|
|
(1,874,321
|
|
Accumulated
other comprehensive loss
|
|
|
—
|
|
|
|
(14,023
|
)
|
|
|
—
|
|
|
|
|
|
|
|
(14,023)
|
|
Total
Stockholder’s Deficit
|
|
|
(389,721
|
)
|
|
|
(355,376
|
)
|
|
|
—
|
|
|
|
|
|
|
|
(745,097)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
Liabilities and Stockholders' Deficit
|
|
$
|
12,773
|
|
|
$
|
519
|
|
|
$
|
—
|
|
|
|
|
|
|
$
|
13,292
|
See
accompanying notes to the Unaudited Pro Forma Condensed Combined Financial Statements.
ALTEROLA
BIOTECH INC.
Unaudited
Pro Forma Condensed Combined Statement of Operations
Year
Ended March 31, 2021
|
|
|
Alterola
Biotech Inc.
|
|
ABTI Pharma
Limited
|
|
Proforma
Adjustments
|
|
Notes
|
|
Proforma
As Adjusted
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
$
|
—
|
|
Operating Expenses
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
—
|
|
|
|
220,512
|
|
|
|
—
|
|
|
|
|
|
220,512
|
|
Accounting and audit fees
|
|
|
23,500
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
23,500
|
|
Professional fees
|
|
|
373,000
|
|
|
|
92,219
|
|
|
|
—
|
|
|
|
|
|
465,219
|
|
Legal fees
|
|
|
5,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
5,000
|
|
Directors fees
|
|
|
90,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
90,000
|
|
General and administrative expenses
|
|
|
5,010
|
|
|
|
28,838
|
|
|
|
—
|
|
|
|
|
|
33,848
|
|
Total operating expenses
|
|
|
496,510
|
|
|
|
341,569
|
|
|
|
—
|
|
|
|
|
|
838,079
|
|
Operating loss
|
|
|
(496,510
|
)
|
|
|
(341,569
|
)
|
|
|
—
|
|
|
|
|
|
(838,079)
|
|
Other Income (Expense)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
—
|
|
|
|
(1,464
|
)
|
|
|
—
|
|
|
|
|
|
(1,464)
|
|
Total other expense
|
|
|
—
|
|
|
|
(1,464
|
)
|
|
|
—
|
|
|
|
|
|
(1,464)
|
|
Income taxes
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
—
|
|
Net loss
|
|
$
|
(496,510
|
)
|
|
$
|
(343,033
|
)
|
|
$
|
—
|
|
|
|
|
$
|
(839,543)
|
|
Other comprehensive loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment
|
|
|
—
|
|
|
|
(14,022
|
)
|
|
|
—
|
|
|
|
|
|
(14,022)
|
|
Total comprehensive loss
|
|
$
|
(496,510
|
)
|
|
$
|
(357,055
|
)
|
|
$
|
—
|
|
|
|
|
$
|
(853,565)
|
|
Basic and dilutive loss per common share
|
|
$
|
(0.00
|
)
|
|
$
|
(0.00
|
)
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of common shares outstanding
|
|
|
442,363,333
|
|
|
|
442,363,333
|
|
|
|
|
|
|
|
|
|
|
See
accompanying notes to the Unaudited Pro Forma Condensed Combined Financial Statements.
ALTEROLA
BIOTECH INC.
Notes
to the Unaudited Pro Forma Condensed Combined Financial Statements
On
January 19, 2021, Alterola Biotech Inc. (the “Company”, “Alterola”) entered into a Stock Purchase Agreement (the
“Agreement”) with ABTI Pharma Limited, a company registered in England and Wales (“ABTI Pharma”, “ABTI”),
pursuant to which the Company will acquire all of the outstanding shares of capital stock of ABTI Pharma from its shareholders in exchange
for 600,000,000 shares of the Company issued pro rata to the ABTI Pharma shareholders.
On
May 24, 2021, the Company and the shareholders of ABTI Pharma memorialized a new closing date in an amendment to the Agreement (the “Amendment”).
The Company has already issued the 600,000,000 shares in anticipation of the closing and the transaction will close upon the ABTI Pharma
shares being transferred to the Company, which will occur upon the filing of the Company’s December 31, 2020 quarterly report on
Form 10-Q with the Securities and Exchange Commission (“SEC”).
On
May 28, 2021, having completed all conditions under the Agreement, the Company closed the transaction.
NOTE
1. BASIS OF PRO FORMA PRESENTATION
The
unaudited pro forma condensed combined financial statements are based on the Company’s and ABTI’s historical consolidated
financial statements as adjusted to give effect to the acquisition of ABTI and the shares issued as part of the acquisition. The unaudited
pro forma combined statements of operations for the year ended March 31, 2021 give effect to the ABTI acquisition as if it had occurred
on April 1, 2020. The unaudited proforma combined balance sheet as of March 31, 2021 gives effect to the ABTI acquisition as if it had
occurred on March 31, 2021.
Historical
financial information has been adjusted in the pro forma balance sheet to pro forma events that are: (1) directly attributable to the
Acquisition; (2) factually supportable; and (3) expected to have a continuing impact on the Company’s results of operations. The
pro forma adjustments presented in the pro forma combined balance sheet and statement of operations are described in Note 4— Pro
Forma Adjustments.
The
unaudited pro forma condensed combined financial information is for illustrative purposes only. These companies may have performed differently
had they actually been combined for the periods presented. You should not rely on the pro forma combined financial information as being
indicative of the historical results that would have been achieved had the companies always been combined or the future results that
the combined companies will experience after the acquisition.
NOTE
2. ACCOUNTING PERIODS PRESENTED
Certain
pro forma adjustments were made to conform ABTI accounting policies to the Company’s accounting policies as noted below.
The
unaudited pro forma condensed combined balance sheet as of March 31, 2021 is presented as if the acquisition had occurred on March 31,
2021 and combines the historical balance sheet of the Company at March 31, 2021 and the historical balance sheet of the ABTI at March
31, 2021.
The
unaudited pro forma condensed combined statement of operations for the year ended March 31, 2021 has been prepared by combining the Company’s
historical consolidated statement of operations for the year ended March 31, 2021, with the historical statement of operations of ABTI
for the year ended March 31, 2021.
NOTE
3. PRELIMINARY PURCHASE PRICE ALLOCATION
On
May 28, 2021, the Company acquired ABTI for total consideration of 600,000,000 shares of Company’s common stock. The unaudited
pro forma condensed combined financial statements include various assumptions, including those related to the preliminary purchase price
allocation of the assets acquired and liabilities assumed of SwissLink based on management’s best estimates of fair value. The
final purchase price allocation may vary based on final appraisals, valuations and analysis of fair value of the acquired assets and
assumed liabilities. Accordingly, pro forma adjustments are preliminary and have been made solely for illustrative purposes.
NOTE
4. PRO FORMA ADJUSTMENTS
The
pro forma adjustments are based on our preliminary estimates and assumptions that are subject to change. The following adjustments have
been reflected in the unaudited pro forma condensed combined financial information:
|
|
a)
|
To
eliminate 600,000,000 shares of common stock held in trust and equity of ABTI.
|

Alterola Biotech, Inc.
55,000,000 Shares of Common Stock
Prospectus
October 18, 2021
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth
the costs and expenses paid or payable by us in connection with the issuance and distribution of the securities being registered. All
amounts shown are estimates, except for the SEC registration fee.
|
|
|
Amount Paid
or to be Paid
|
|
|
SEC registration fee
|
|
$
|
10,197
|
|
|
Legal fees and expenses
|
|
|
10,000
|
|
|
Accounting fees and expenses
|
|
|
10,000
|
|
|
Miscellaneous expenses
|
|
|
0
|
|
|
Total
|
|
$
|
30,197
|
|
Item 14. Indemnification of Directors and Officers
Under our bylaws, every person
who was or is a party to, or is threatened to be made a party to, or is involved in any action, suit, or proceeding, whether civil, criminal,
administrative, or investigative, by reason of the fact that he is or was our director or officer, or is or was serving at our request
as a director or officer of another corporation, or as its representative in a partnership, joint venture, trust, or other enterprise,
shall be indemnified and held harmless to the fullest extent legally permissible under the laws of the State of Nevada from time to time
against all expenses, liability, and loss (including attorneys’ fees judgments, fines, and amounts paid or to be paid in settlement)
reasonably incurred or suffered by him or her in connection therewith. Such right of indemnification shall be a contract right, which
may be enforced in any manner desired by such person. The expenses of officers and directors incurred in defending a civil or criminal
action, suit, or proceeding must be paid by us as they are incurred and in advance of the final disposition of the action, suit, or proceeding,
upon receipt of an undertaking by or on behalf of the director or officer to repay the amount if it is ultimately determined by a court
of competent jurisdiction that he is not entitled to be indemnified by us. Such right of indemnification shall not be exclusive of any
other right which such directors, officers, or representatives may have or hereafter acquire, and, without limiting the generality of
such statement, they shall be entitled to their respective rights of indemnification under any bylaw, agreement, vote of shareholders,
provision of law, or otherwise.
Without limiting the application
of the foregoing, our board of directors may adopt bylaws from time to time with respect to indemnification, to provide at all times the
fullest indemnification permitted by the laws of the State of Nevada, and may cause us to purchase and maintain insurance on behalf of
any person who is or was our director or officer, or is or was serving at our request as a director or officer of another corporation,
or as its representative in a partnership, joint venture, trust, or other enterprise against any liability asserted against such person
and incurred in any such capacity or arising out of such status, whether or not we would have the power to indemnify such person. The
indemnification provided shall continue as to a person who has ceased to be a director, officer, employee, or agent, and shall inure to
the benefit of the heirs, executors and administrators of such person.
Insofar as indemnification for
liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing
provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities
Act and is therefore unenforceable.
We have not entered into any agreements
with our directors and executive officers that require us to indemnify these persons against expenses, judgments, fines, settlements and
other amounts actually and reasonably incurred (including expenses of a derivative action) in connection with any proceeding, whether
actual or threatened, to which any such person may be made a party by reason of the fact that the person is or was our director or officer
or any of our affiliated enterprises. We have an insurance policy covering our officers and directors with respect to certain liabilities,
including liabilities arising under the Securities Act, or otherwise.
Item 15. Recent Sales of Unregistered Securities
On
June 28, 2018, the company issued one million (1,000,000) common shares for consulting services with a deemed value
of $90,000. As the services are to be provided over a period from April 1, 2018 to January 31, 2019, the company has recorded
$63,000 as deferred stock-based compensation.
During
the year ended September 30, 2019, the Company issued 1,000,000 shares of common stock to an officer for services rendered with a deemed
value of services provided of $90,000.
During
the year ended September 30, 2020, the Company issued 13,000,000 shares of common stock to individuals for services rendered with a deemed
value of services provided of $130,000.
During
the period ended March 31, 2021, the Company issued 3,200,000 shares of common stock for services rendered with a deemed value of services
provided of $32,000.
We
issued 600,000,000 shares of common stock to the shareholders of ABTI Pharma Limited in connection with a Stock
Transfer Agreement dated January 19, 2021. As part of the transaction, the 200,000,000 shares to Amsterdam Café
Holdings Ltd. have been cancelled and Bulls Run Investments Limited was issued 19,100,000 shares of common stock. Also, 2,000,000
shares of common stock were issued for services rendered, and with the above transactions, amounts to acquisition with a deemed value
of $ 621,100.
These
securities were issued pursuant to Section 4(2) of the Securities Act and/or Rule 506 promulgated thereunder. The holders
represented their intention to acquire the securities for investment only and not with a view towards distribution.
The investors were given adequate information about us to make an informed investment decision. We did not
engage in any general solicitation or advertising. We directed our transfer agent to issue the stock certificates with the
appropriate restrictive legend affixed to the restricted stock.
Item 16. Exhibits and Financial Statement Schedules
(a) Exhibits
The following documents are filed as exhibits to this
registration statement.
|
Number
|
|
Exhibit Description
|
|
2.1
|
|
Stock Transfer Agreement, dated January 19, 2021 (filed as Exhibit 2.1 to the Current Report on Form 8-K filed with the SEC on March 16, 2021 and incorporated herein by reference).
|
|
2.2
|
|
Amendment to Stock Transfer Agreement, dated May 24, 2021 (filed as Exhibit 2.1 to the Current Report on Form 8-K filed with the SEC on May 25, 2021 and incorporated herein by reference).
|
|
3.1
|
|
Articles of Incorporation, dated July 16, 2008 (filed as Exhibit 3.1 to the Form S-1 filed with the SEC on December 12, 2008 and incorporated herein by reference).
|
|
3.2
|
|
Certificate of Amendment, dated October 26, 20210(filed as Exhibit 3.1 to the Current Report on Form 8-K filed with the SEC on October 28, 2020 and incorporated herein by reference).
|
|
3.3
|
|
Certificate of Designation (filed as Exhibit 3.4 to the Annual Report on Form 10-KT filed with the SEC on June 9, 2021 and incorporated herein by reference).
|
|
3.4
|
|
Amended and Restated Bylaws (filed as Exhibit 3.2 to the Current Report on Form 8-K filed with the SEC on October 28, 2020 and incorporated herein by reference).
|
|
4.1
|
|
Common Stock Purchase Warrant, dated August 11, 2021 (filed as Exhibit 4.1 to the Quarterly Report on Form 10-Q filed with the SEC on August 19, 2021 and incorporated herein by reference).
|
|
4.2
|
|
Convertible Promissory Note, dated June 8, 2021 (filed as Exhibit 4.1 to the Annual Report on Form 10-KT filed with the SEC on June 9, 2021 and incorporated herein by reference).
|
|
5.1
|
|
Legal opinion of the Doney Law Firm with consent to use*
|
|
10.1
|
|
Common Stock Purchase Agreement, dated August 11, 2021 (filed as Exhibit 10.1 to the Quarterly Report on Form 10-Q filed with the SEC on August 19, 2021 and incorporated herein by reference).
|
|
10.2
|
|
Registration Rights Agreement, dated August 11, 2021 (filed as Exhibit 10.2 to the Quarterly Report on Form 10-Q filed with the SEC on August 19, 2021 and incorporated herein by reference).
|
|
10.3
|
|
Employment Agreement, dated March 28, 2021 (filed as Exhibit 10.1 to the Annual Report on Form 10-KT filed with the SEC on June 9, 2021 and incorporated herein by reference).
|
|
23.1
|
|
Consent of AJ Robbins CPA, LLC*
|
|
23.2
|
|
Consent of Gries & Associates, LLC*
|
|
99.1
|
|
Audit and Complaince Committee Charter (filed as Exhibit 99.1 to the Current Report on Form 8-K filed with the SEC on October 5, 2021
|
* Filed Herewith
Item 17. Undertakings
The undersigned registrant hereby
undertakes:
(a) (1) To file, during any period in which
offers or sales are being made, a post-effective amendment to this registration statement;
(i) To include any prospectus required
by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in
the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment
thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement.
Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered
would not exceed that which was registered) and any deviation from the low or high and of the estimated maximum offering range may be
reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and
price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration
Fee” table in the effective registration statement.
(iii) To include any
material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change
to such information in the registration statement.
(A) Paragraphs (a)(1)(i)
and (a)(1)(ii) of this section do not apply if the registration statement is on Form S-8, and the information required to be included
in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant
pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934, as amended (“Securities Exchange Act of 1934”)
(15 U.S.C. 78m or 78o(d)) that are incorporated by reference in the registration statement; and
(B) Paragraphs (a)(1)(i),
(a)(1)(ii) and (a)(1)(iii) of this section do not apply if the registration statement is on Form S-3 or Form F-3 and the information required
to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by
the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the
registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
(2) That, for the
purpose of determining any liability under the Securities Act of 1933, as amended (“Securities Act”) each such post-effective
amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities
at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from
registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the
offering.
(4) That, for the
purpose of determining any liability under the Securities Act, each Prospectus filed pursuant to Rule 424(b) as part of a registration
statement relating to an offering, other than registration statements relying on Rule 430B or other than Prospectuses filed in reliance
on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness.
Provided, however, that no statement made in a registration statement or Prospectus that is part of the registration statement
or made in a document incorporated or deemed incorporated by reference into the registration statement or Prospectus that is part of the
registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement
that was made in the registration statement or Prospectus that was part of the registration statement or made in any such document immediately
prior to such date of first use.
(5) That, for the
purpose of determining liability under the Securities Act to any purchaser:
(i) If the registrant
is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other
than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part
of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement
made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed
incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser
with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement
or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(6) That, for the
purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities:
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration
statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to
such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will
be considered to offer or sell such securities to such purchaser:
(i) Any preliminary
prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing
prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of
any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities
provided by or on behalf of the undersigned registrant; and
(iv) Any other communication
that is an offer in the offering made by the undersigned registrant to the purchaser.
(h) Insofar as indemnification
for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant
pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange
Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a
claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director,
officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director,
officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel
the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification
by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(i) The undersigned
registrant hereby undertakes that:
(1) For purposes of
determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this
registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1)
or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose
of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall
be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time
shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
Pursuant to the requirements
of the Securities Act of 1933, as amended, the registrant has duly caused this Registration Statement on Form S-1 to be signed on its
behalf by the undersigned, thereunto duly authorized, in the City of Liverpool, UK on October 18, 2021.
Alterola Biotech, Inc.
By: /s/ Timothy Rogers
Timothy Rogers
Chairman , Principal Executive Officer, Principal Financial Officer, Principal
Accounting Officer and Director
By: /s/ Seamus McAuley
Seamus McAuley
Chief Executive Officer Officer, Company Secretary and Director
By: /s/ Colin Stott
Colin Stott
Chief Operting Officer and Director
Pursuant to the requirements of the Securities Act of 1933, this
report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
By: /s/ Timothy Rogers
Timothy Rogers
Chairman, Principal Executive Officer, Principal Financial Officer, Principal
Accounting Officer and Director,
October 18, 2021
By: /s/ Seamus McAuley
Seamus McAuley
Chief Executive Officer Officer, Company Secretary and Director
October 18, 2021
By: /s/ Colin Stott
Colin Stott
Chief Operting Officer and Director
October 18, 2021
By: /s/ Dominic Schiller
Dominic Schiller Director
October 18, 2021
By: /s/ Daniel Reshef
Daniel Reshef Director
October 18, 2021
By: /s/ Lalit Kumar Verma
Lalit Kumar Verma Director
October 18, 2021
By: /s/ Ning Qu
Ning Qu Director
October 18, 2021
Alterola Biotech (PK) (USOTC:ABTI)
Historical Stock Chart
From Nov 2024 to Dec 2024
Alterola Biotech (PK) (USOTC:ABTI)
Historical Stock Chart
From Dec 2023 to Dec 2024