Sientra Gets FDA Approval for Low Plus Profile Projection Breast Implant
July 06 2022 - 9:44AM
Dow Jones News
By Chris Wack
Sientra Inc. said the U.S. Food and Drug Administration approved
the Low Plus Profile Projection Breast Implant for breast
augmentation in women at least 22 years old, and for women of all
ages undergoing breast reconstruction.
The company said the product will be commercially available for
board-certified and board-eligible plastic surgeons in the U.S. in
late July.
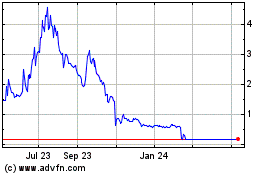
Sientra shares were up 13% to 99 cents in premarket trading.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
July 06, 2022 09:29 ET (13:29 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
Sientra (NASDAQ:SIEN)
Historical Stock Chart
From Apr 2024 to May 2024
Sientra (NASDAQ:SIEN)
Historical Stock Chart
From May 2023 to May 2024