Cara Therapeutics, Vifor Pharma Say FDA Approves Korsuva Injection
August 23 2021 - 4:56PM
Dow Jones News
By Stephen Nakrosis
Cara Therapeutics and Vifor Pharma on Monday said the U.S. Food
and Drug Administration approved the Korsuva injection to treat
pruritus in hemodialysis patients.
The companies said the Korsuva, or difelikefalin, injection was
approved to treat moderate-to-severe pruritus in hemodialysis
patients.
The companies said the Korsuva injection is the first and only
therapy approved by the FDA to treat pruritus associated with
chronic kidney disease in adult patients undergoing
hemodialysis.
The companies said the promotional launch of Korsuva injection
in the U.S. is expected in the first quarter of next year.
Write to Stephen Nakrosis at stephen.nakrosis@wsj.com
(END) Dow Jones Newswires
August 23, 2021 16:46 ET (20:46 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
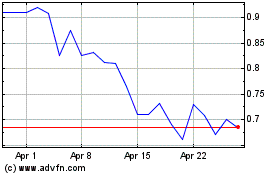
Cara Therapeutics (NASDAQ:CARA)
Historical Stock Chart
From Mar 2024 to Apr 2024
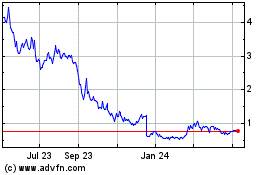
Cara Therapeutics (NASDAQ:CARA)
Historical Stock Chart
From Apr 2023 to Apr 2024