false0001720580NONE00017205802024-01-162024-01-16
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): January 16, 2024 |
Adicet Bio, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-38359 |
81-3305277 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
200 Berkeley Street, 19th Floor |
|
Boston, Massachusetts |
|
02116 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (650) 503-9095 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, par value $0.0001 per share |
|
ACET |
|
The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On January 16, 2024, Adicet Bio, Inc. (the “Company”) posted to the “Presentations & Events” section of the Company’s website at investor.adicetbio.com an updated corporate presentation (the “Corporate Presentation”). A copy of the Corporate Presentation is furnished herewith as Exhibit 99.1.
The information in this Item 7.01, including Exhibit 99.1 attached hereto, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 9.01 Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
ADICET BIO, INC. |
|
|
|
|
Date: |
January 16, 2024 |
By: |
/s/ Nick Harvey |
|
|
Name: Title: |
Nick Harvey
Chief Financial Officer |

Leaders in Developing Allogeneic CAR γδ1 Cell Therapies to Fight Autoimmune Diseases and Cancer January 16, 2024 γδ= Gamma delta; CAR= Chimeric antigen receptor

Forward-Looking Statements This presentation contains "forward-looking statements" of Adicet Bio, Inc. (Adicet or the Company) within the meaning of the Private Securities Litigation Reform Act of 1995 relating to business and operations of Adicet. These forward-looking statements include, but are not limited to, express or implied statements regarding: preclinical and clinical development of Adicet's product candidates, including future plans or expectations for ADI-001 and ADI-270 and the potential safety, durability, tolerability and efficacy of these product candidates; the expected progress, timing and success of the Phase 1 clinical trial of ADI-001, including continued enrollment and expectations around a clinical update in the second half of 2024; the Company's plan to initiate a Phase 1 clinical trial of ADI-001 in lupus nephritis, including the potential for a clinical update in the second half of 2024; the Company's expansion into other autoimmune indications in the future, including IND submissions, acceptances and the initiation of clinical trials; the Company's expectations regarding the submission of an IND for ADI-270 in renal cell carcinoma in the second quarter of 2024 and timing for future clinical updates; and expectations regarding its uses of capital, expenses and financial results, including the expected cash runway. Any forward-looking statements in this presentation are based on management’s current expectations and beliefs of future events, and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements, including without limitation, the effect of global economic conditions and public health emergencies on Adicet’s business and financial results, including with respect to disruptions to its preclinical and clinical studies, business operations, and ability to raise additional capital; Adicet’s ability to execute on its strategy, including obtaining the requisite regulatory approvals on the expected timeline if at all; that positive results, including interim results, from a preclinical or clinical study may not necessarily be predictive of the results of future or ongoing studies; clinical studies may fail to demonstrate adequate safety and efficacy of Adicet's product candidates, which would prevent, delay, or limit the scope of regulatory approval and commercialization and regulatory approval processes of the FDA and comparable foreign regulatory authorities are lengthy, time-consuming, and inherently unpredictable. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause Adicet’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in Adicet’s most recent annual report on Form 10-K and subsequent filings with the U.S. Securities and Exchange Commission, All information in this presentation is as of the date its release, and Adicet undertakes no duty to update this information unless required by law. Industry and Market Information Information regarding market share, market position and industry data pertaining to Adicet’s business contained in this presentation consists of estimates based on data and reports compiled by industry professional organizations and analysts and Adicet’s knowledge of their industry. Although Adicet believes the industry and market data to be reliable, this information could prove to be inaccurate. You should carefully consider the inherent risks and uncertainties associated with the market and other industry data contained in this presentation. Forward-looking information obtained from third-party sources is subject to the same qualifications and the additional uncertainties as the other forward-looking statements in this presentation.
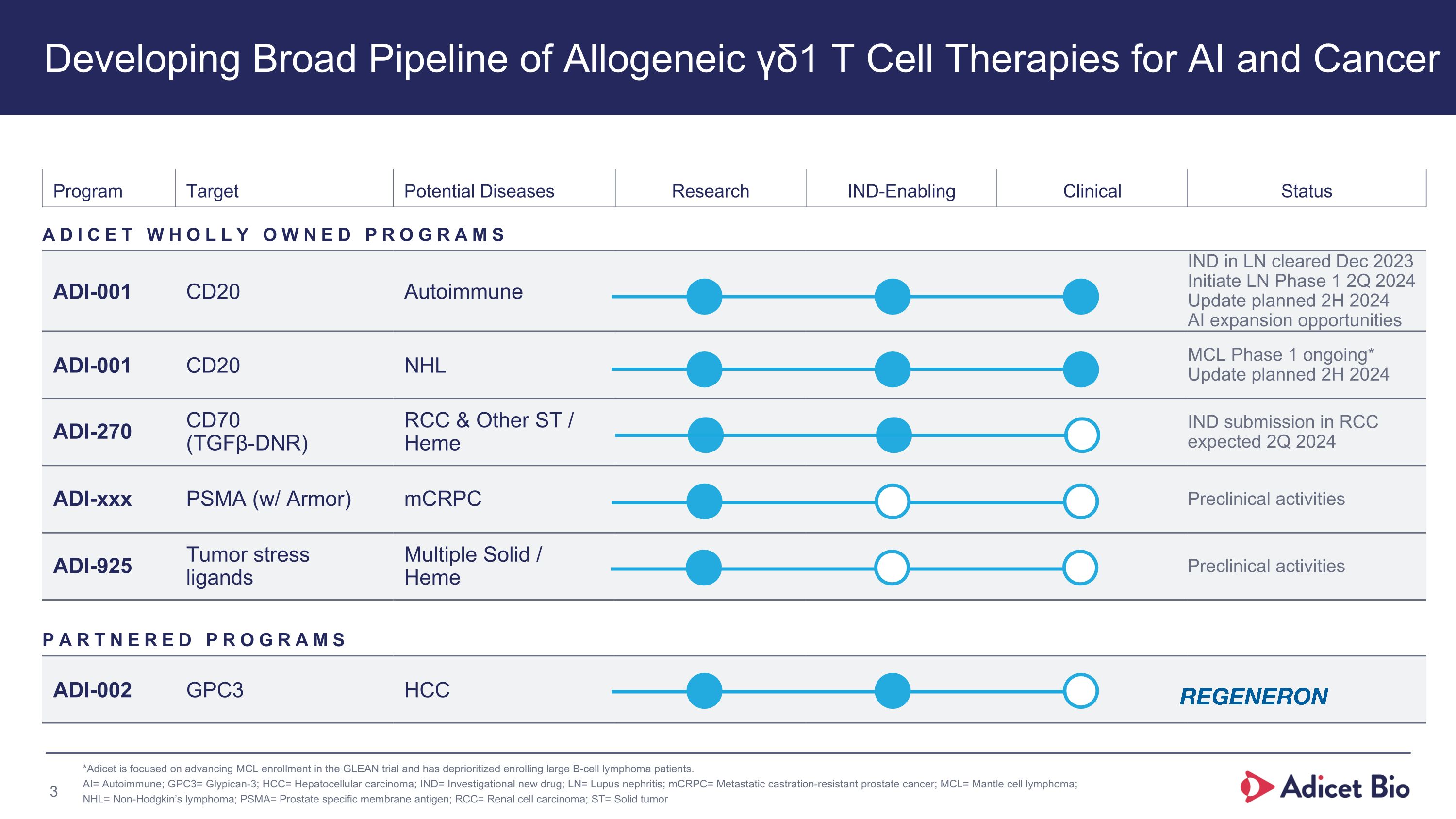
Program Target Potential Diseases Research IND-Enabling Clinical Status ADICET WHOLLY OWNED PROGRAMS ADI-001 CD20 Autoimmune IND in LN cleared Dec 2023 Initiate LN Phase 1 2Q 2024 Update planned 2H 2024 AI expansion opportunities ADI-001 CD20 NHL MCL Phase 1 ongoing* Update planned 2H 2024 ADI-270 CD70 �(TGFβ-DNR) RCC & Other ST / Heme IND submission in RCC expected 2Q 2024 ADI-xxx PSMA (w/ Armor) mCRPC Preclinical activities ADI-925 Tumor stress �ligands Multiple Solid / �Heme Preclinical activities PARTNERED PROGRAMS ADI-002 GPC3 HCC *Adicet is focused on advancing MCL enrollment in the GLEAN trial and has deprioritized enrolling large B-cell lymphoma patients. AI= Autoimmune; GPC3= Glypican-3; HCC= Hepatocellular carcinoma; IND= Investigational new drug; LN= Lupus nephritis; mCRPC= Metastatic castration-resistant prostate cancer; MCL= Mantle cell lymphoma; NHL= Non-Hodgkin’s lymphoma; PSMA= Prostate specific membrane antigen; RCC= Renal cell carcinoma; ST= Solid tumor Developing Broad Pipeline of Allogeneic γδ1 T Cell Therapies for AI and Cancer
Adicet Bio Leadership Team Francesco Galimi, M.D., Ph.D. Chief Medical Officer Chen Schor �President and CEO Nick Harvey Chief Financial Officer Don Healey, Ph.D. Chief Technology Officer Blake Aftab, Ph.D. Chief Scientific Officer Amy Locke Head of Human Resources Nancy Boman, M.D., Ph.D. Chief Regulatory Officer
ADI-001�Autoimmune Diseases
Exposure Consistent with Approved Autologous CAR T (Cmax, Day 28 Persistence and AUC) B-Cell Depletion Consistent with Autologous CD19 CAR T in SLE, SSC and IIM Preferentially Trafficking to Organs/ Tissues No Significant Risk of CRS,�ICANS, or T cell Malignancies Compared to Autologous CAR T Readily Available,�“Off-the-Shelf” Potential to Dose in Community Setting Adicet γδ1 CAR T Cell Therapy For Autoimmune Indications AUC= Area Under the Curve; Cmax= Peak plasma concentration; CRS= Cytokine release syndrome; ICANS= Immune effector cell-associated neurotoxicity syndrome; IIM= idiopathic inflammatory myopathy; SLE= systemic lupus erythematosus; SSC= systemic sclerosis ADI-001 Data in NHL Provides Strong Foundation for Future Development in AI
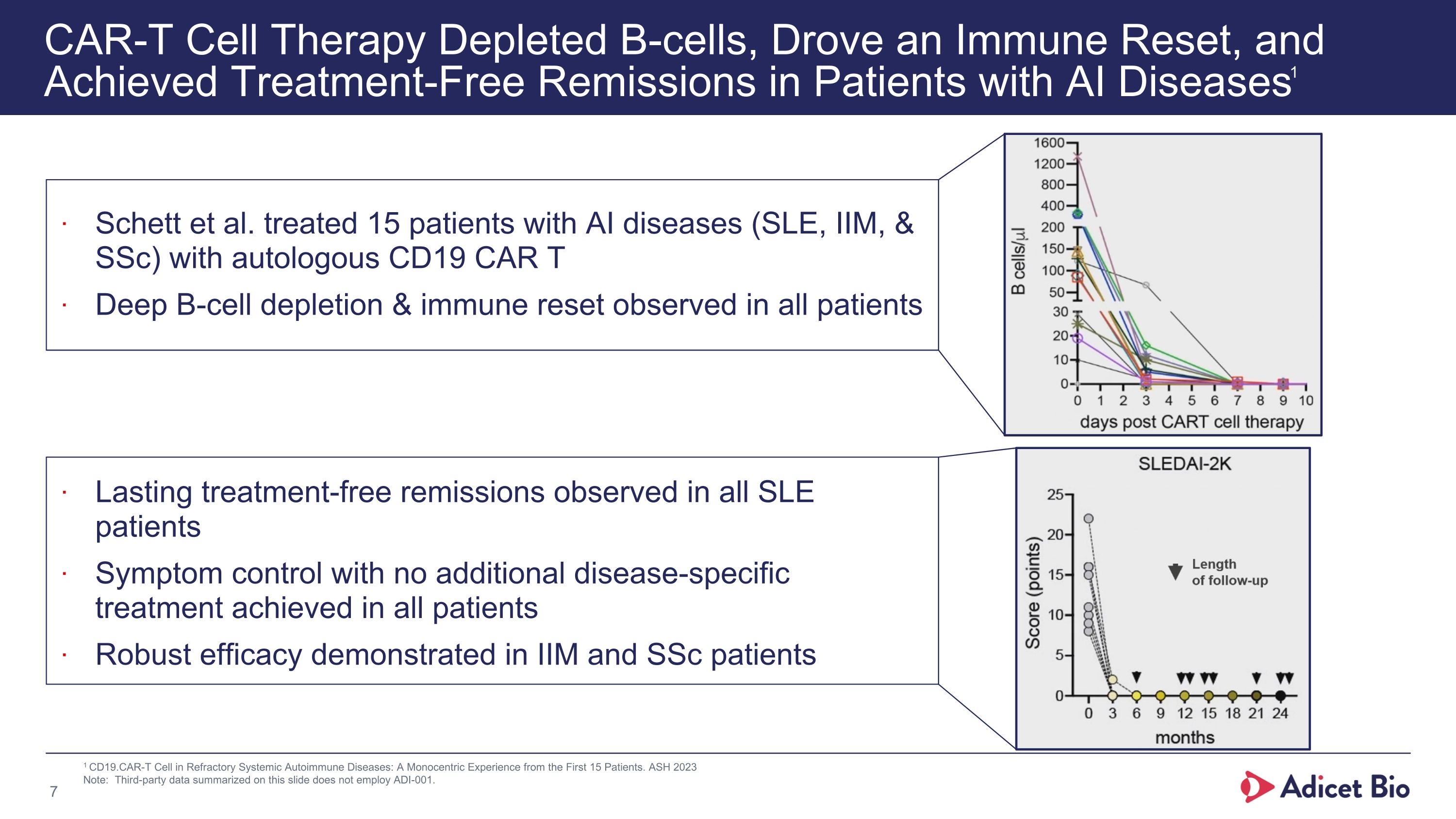
CAR-T Cell Therapy Depleted B-cells, Drove an Immune Reset, and Achieved Treatment-Free Remissions in Patients with AI Diseases1 1 CD19.CAR-T Cell in Refractory Systemic Autoimmune Diseases: A Monocentric Experience from the First 15 Patients. ASH 2023 Note: Third-party data summarized on this slide does not employ ADI-001. Schett et al. treated 15 patients with AI diseases (SLE, IIM, & SSc) with autologous CD19 CAR T Deep B-cell depletion & immune reset observed in all patients Lasting treatment-free remissions observed in all SLE patients Symptom control with no additional disease-specific treatment achieved in all patients Robust efficacy demonstrated in IIM and SSc patients
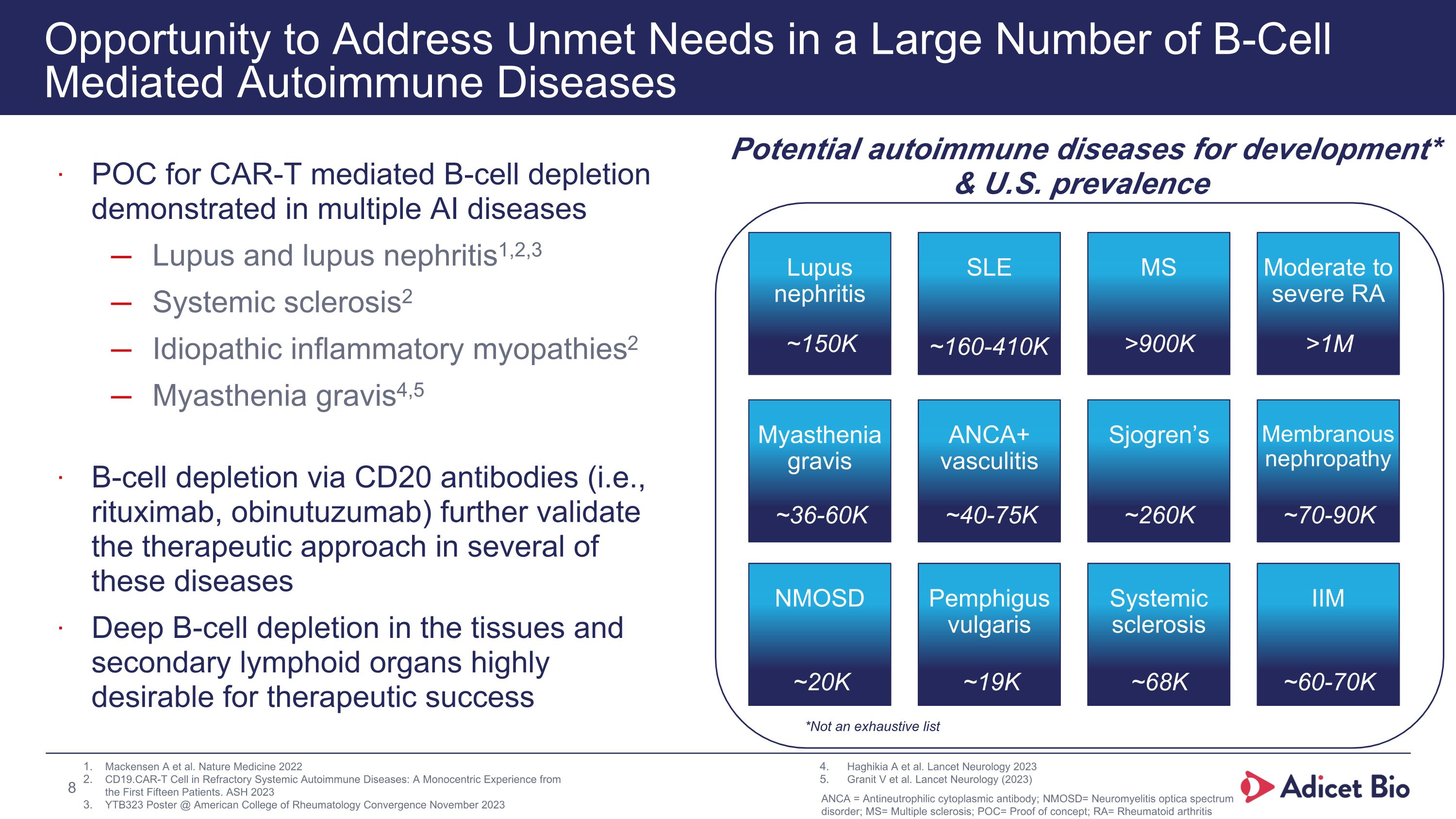
Opportunity to Address Unmet Needs in a Large Number of B-Cell Mediated Autoimmune Diseases POC for CAR-T mediated B-cell depletion demonstrated in multiple AI diseases Lupus and lupus nephritis1,2,3 Systemic sclerosis2 Idiopathic inflammatory myopathies2 Myasthenia gravis4,5 B-cell depletion via CD20 antibodies (i.e., rituximab, obinutuzumab) further validate the therapeutic approach in several of these diseases Deep B-cell depletion in the tissues and secondary lymphoid organs highly desirable for therapeutic success Potential autoimmune diseases for development* & U.S. prevalence * Not exhaustive Lupus nephritis SLE MS Moderate to severe RA Myasthenia gravis ANCA+ vasculitis Sjogren’s Membranous nephropathy NMOSD Pemphigus vulgaris Systemic sclerosis IIM Mackensen A et al. Nature Medicine 2022 CD19.CAR-T Cell in Refractory Systemic Autoimmune Diseases: A Monocentric Experience from the First Fifteen Patients. ASH 2023 YTB323 Poster @ American College of Rheumatology Convergence November 2023 Haghikia A et al. Lancet Neurology 2023 Granit V et al. Lancet Neurology (2023) ~150K ~160-410K >900K >1M ~36-60K ~40-75K ~260K ~70-90K ~20K ~19K ~68K ~60-70K *Not an exhaustive list 8 ANCA = Antineutrophilic cytoplasmic antibody; NMOSD= Neuromyelitis optica spectrum disorder; MS= Multiple sclerosis; POC= Proof of concept; RA= Rheumatoid arthritis
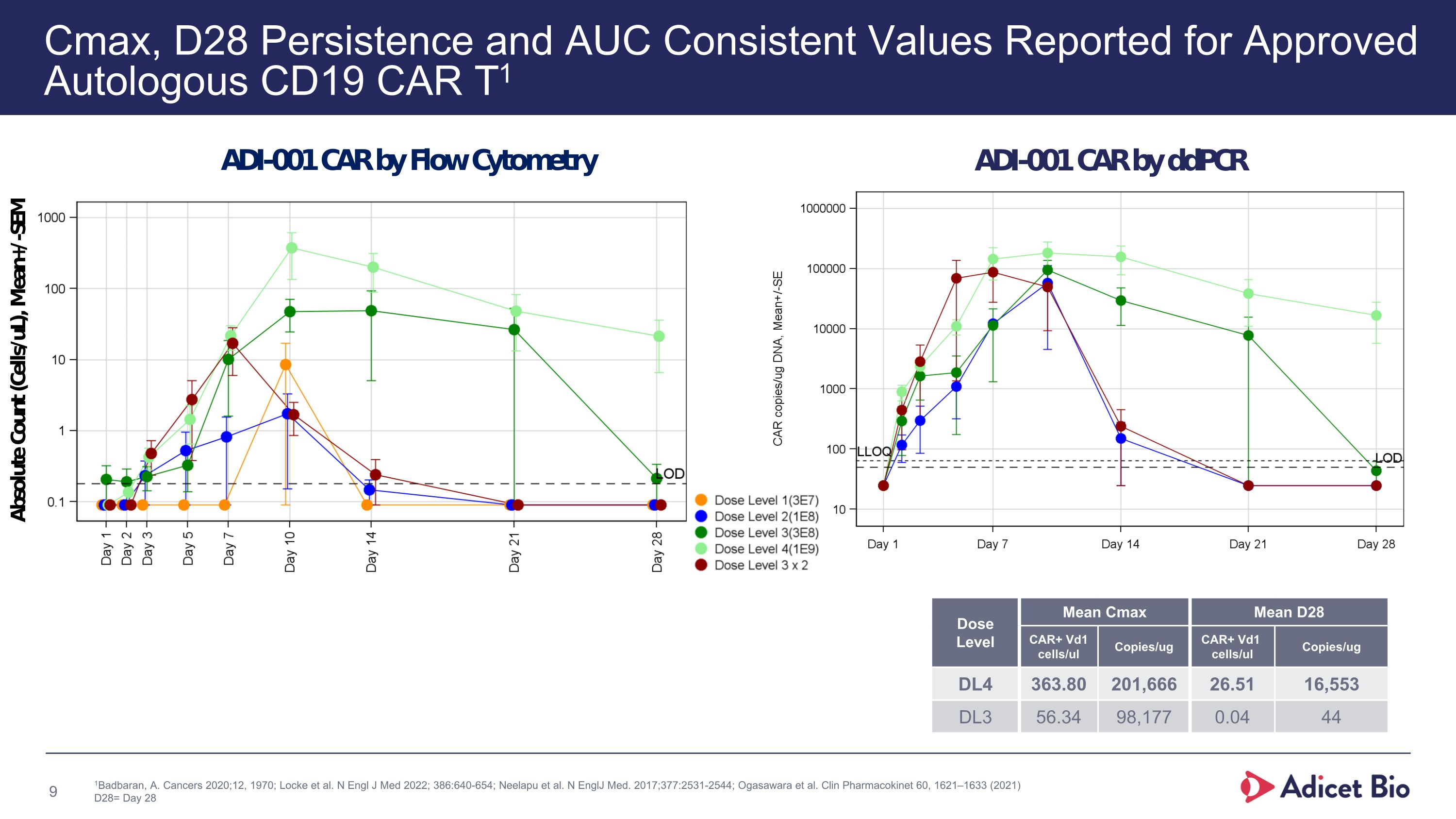
Cmax, D28 Persistence and AUC Consistent Values Reported for Approved Autologous CD19 CAR T1 1Badbaran, A. Cancers 2020;12, 1970; Locke et al. N Engl J Med 2022; 386:640-654; Neelapu et al. N EnglJ Med. 2017;377:2531-2544; Ogasawara et al. Clin Pharmacokinet 60, 1621–1633 (2021) D28= Day 28 xxx Dose Level Mean Cmax Mean D28 CAR+ Vd1 cells/ul Copies/ug CAR+ Vd1 cells/ul Copies/ug DL4 363.80 201,666 26.51 16,553 DL3 56.34 98,177 0.04 44
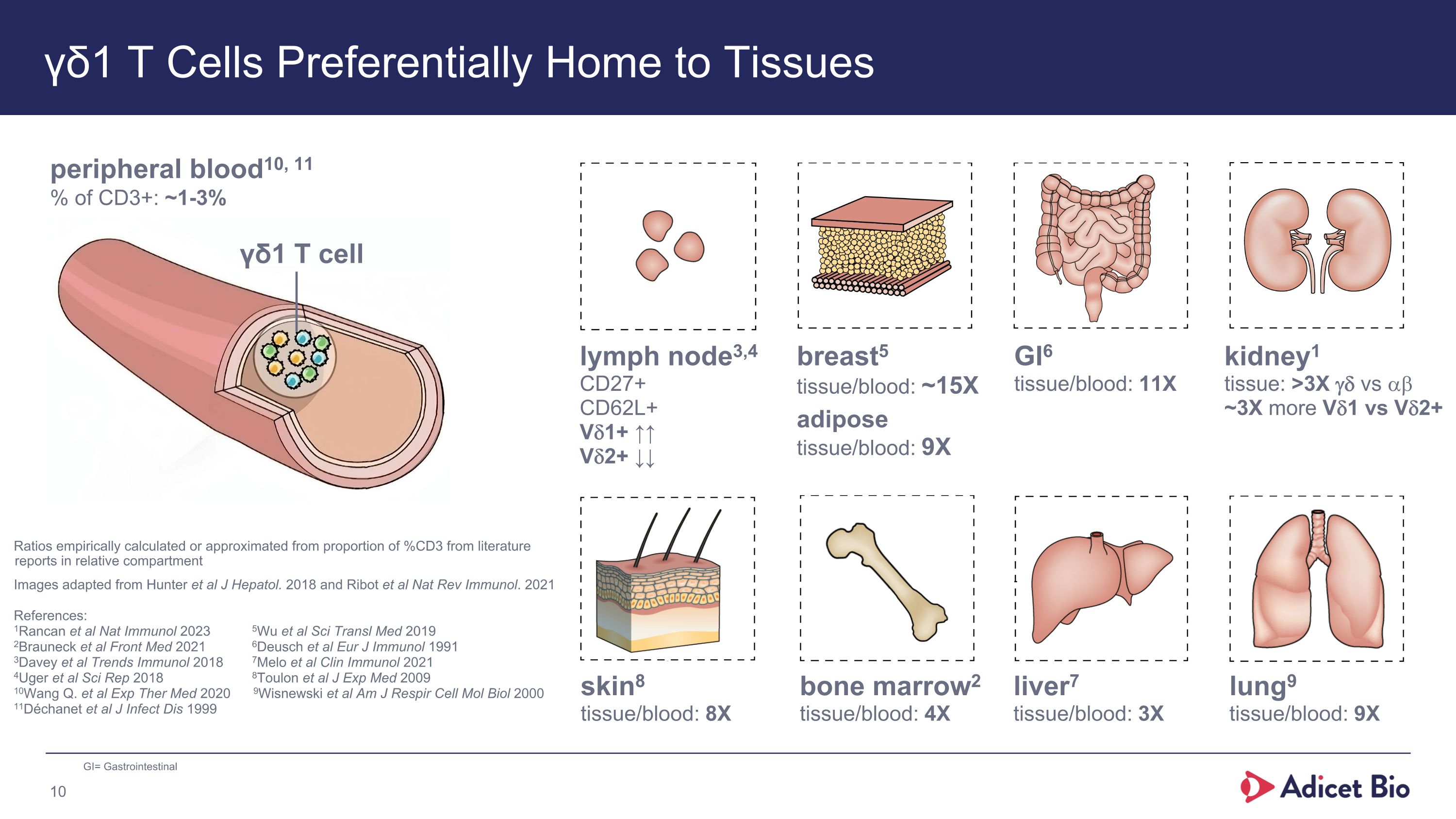
γδ1 T Cells Preferentially Home to Tissues peripheral blood10, 11 % of CD3+: ~1-3% γδ1 T cell lung9 tissue/blood: 9X liver7 tissue/blood: 3X skin8 tissue/blood: 8X GI6 tissue/blood: 11X breast5�tissue/blood: ~15X adipose�tissue/blood: 9X lymph node3,4 CD27+ CD62L+ Vd1+ ↑↑ Vd2+ ↓↓ bone marrow2 tissue/blood: 4X Ratios empirically calculated or approximated from proportion of %CD3 from literature reports in relative compartment Images adapted from Hunter et al J Hepatol. 2018 and Ribot et al Nat Rev Immunol. 2021 References: 1Rancan et al Nat Immunol 2023 5Wu et al Sci Transl Med 2019 2Brauneck et al Front Med 2021 6Deusch et al Eur J Immunol 1991 3Davey et al Trends Immunol 2018 7Melo et al Clin Immunol 2021 4Uger et al Sci Rep 2018 8Toulon et al J Exp Med 2009 10Wang Q. et al Exp Ther Med 2020 9Wisnewski et al Am J Respir Cell Mol Biol 2000 11Déchanet et al J Infect Dis 1999 GI= Gastrointestinal kidney1 tissue: >3X gd vs ab ~3X more Vd1 vs Vd2+
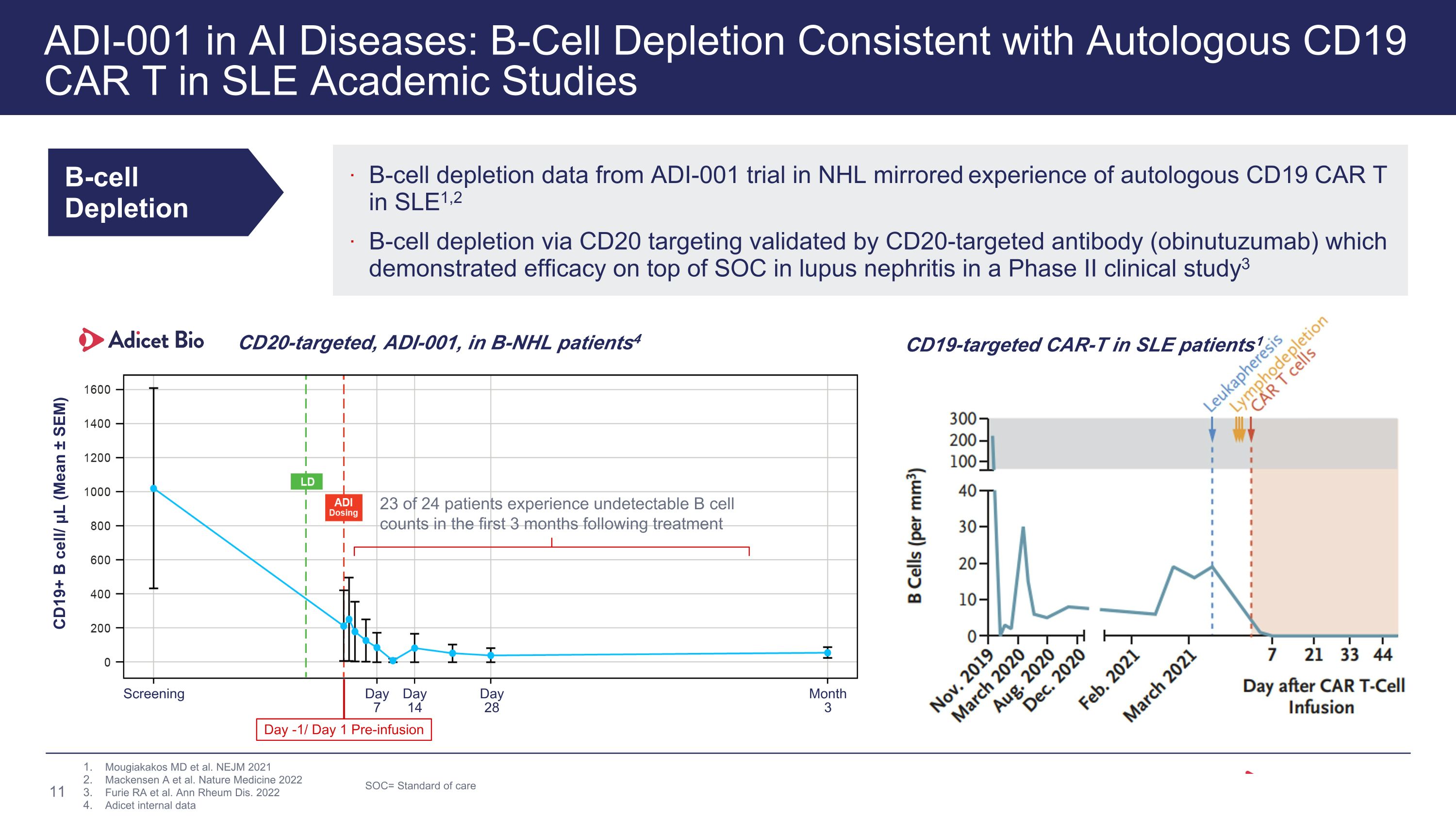
ADI-001 in AI Diseases: B-Cell Depletion Consistent with Autologous CD19 CAR T in SLE Academic Studies B-cell Depletion B-cell depletion data from ADI-001 trial in NHL mirrored experience of autologous CD19 CAR T in SLE1,2 B-cell depletion via CD20 targeting validated by CD20-targeted antibody (obinutuzumab) which demonstrated efficacy on top of SOC in lupus nephritis in a Phase II clinical study3 Mougiakakos MD et al. NEJM 2021 Mackensen A et al. Nature Medicine 2022 Furie RA et al. Ann Rheum Dis. 2022 Adicet internal data CD19-targeted CAR-T in SLE patients1 SOC= Standard of care CD20-targeted, ADI-001, in B-NHL patients4 CD19+ B cell/ μL (Mean ± SEM) Screening Month�3 Day�28 Day�14 Day�7 Day -1/ Day 1 Pre-infusion 23 of 24 patients experience undetectable B cell counts in the first 3 months following treatment
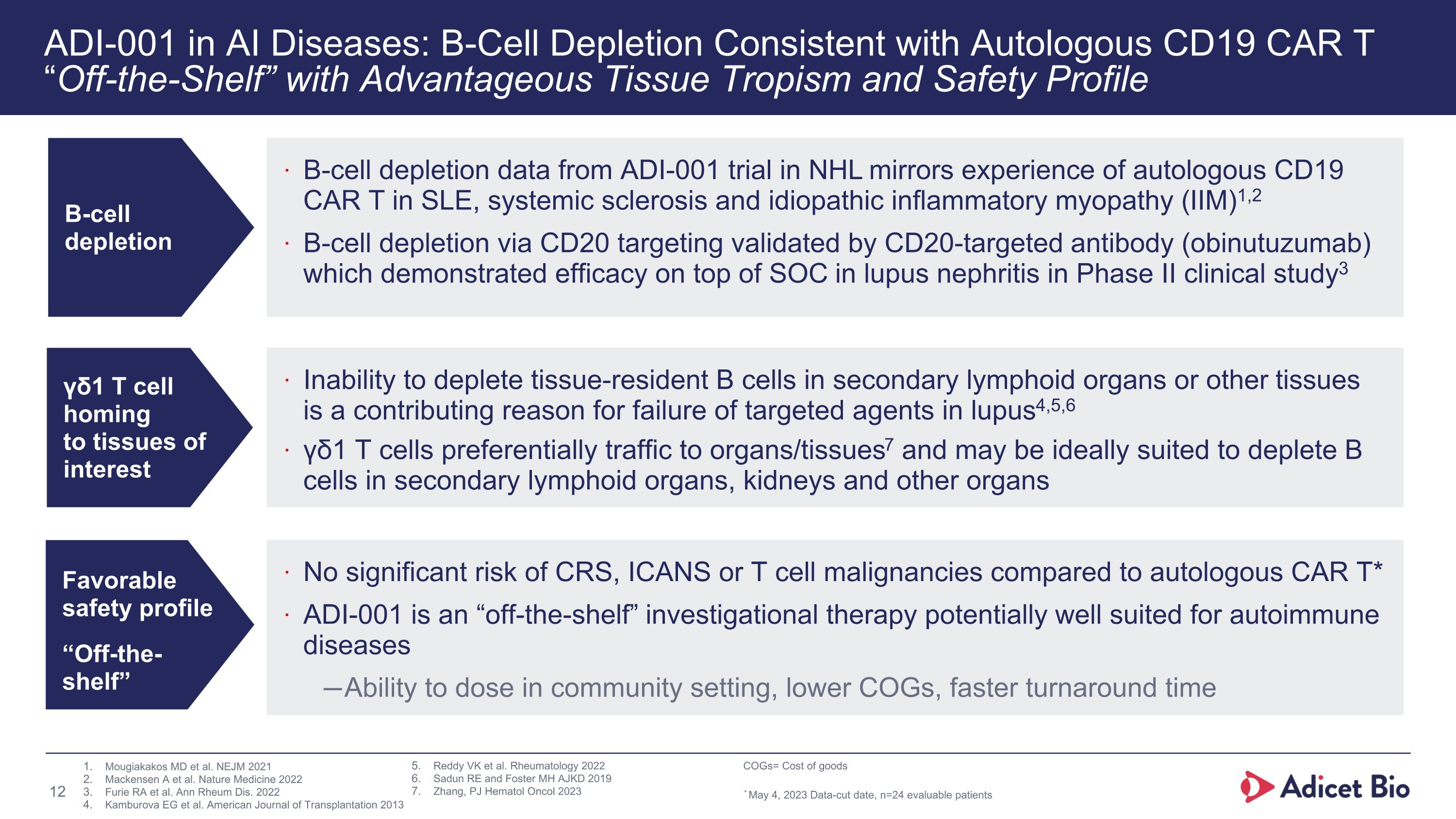
ADI-001 in AI Diseases: B-Cell Depletion Consistent with Autologous CD19 CAR T �“Off-the-Shelf” with Advantageous Tissue Tropism and Safety Profile B-cell depletion γδ1 T cell �homing �to tissues of �interest B-cell depletion data from ADI-001 trial in NHL mirrors experience of autologous CD19 CAR T in SLE, systemic sclerosis and idiopathic inflammatory myopathy (IIM)1,2 B-cell depletion via CD20 targeting validated by CD20-targeted antibody (obinutuzumab) which demonstrated efficacy on top of SOC in lupus nephritis in Phase II clinical study3 Inability to deplete tissue-resident B cells in secondary lymphoid organs or other tissues is a contributing reason for failure of targeted agents in lupus4,5,6 γδ1 T cells preferentially traffic to organs/tissues7 and may be ideally suited to deplete B cells in secondary lymphoid organs, kidneys and other organs Favorable safety profile “Off-the-shelf” No significant risk of CRS, ICANS or T cell malignancies compared to autologous CAR T* ADI-001 is an “off-the-shelf” investigational therapy potentially well suited for autoimmune diseases Ability to dose in community setting, lower COGs, faster turnaround time Mougiakakos MD et al. NEJM 2021 Mackensen A et al. Nature Medicine 2022 Furie RA et al. Ann Rheum Dis. 2022 Kamburova EG et al. American Journal of Transplantation 2013 Reddy VK et al. Rheumatology 2022 Sadun RE and Foster MH AJKD 2019 Zhang, PJ Hematol Oncol 2023 COGs= Cost of goods * May 4, 2023 Data-cut date, n=24 evaluable patients
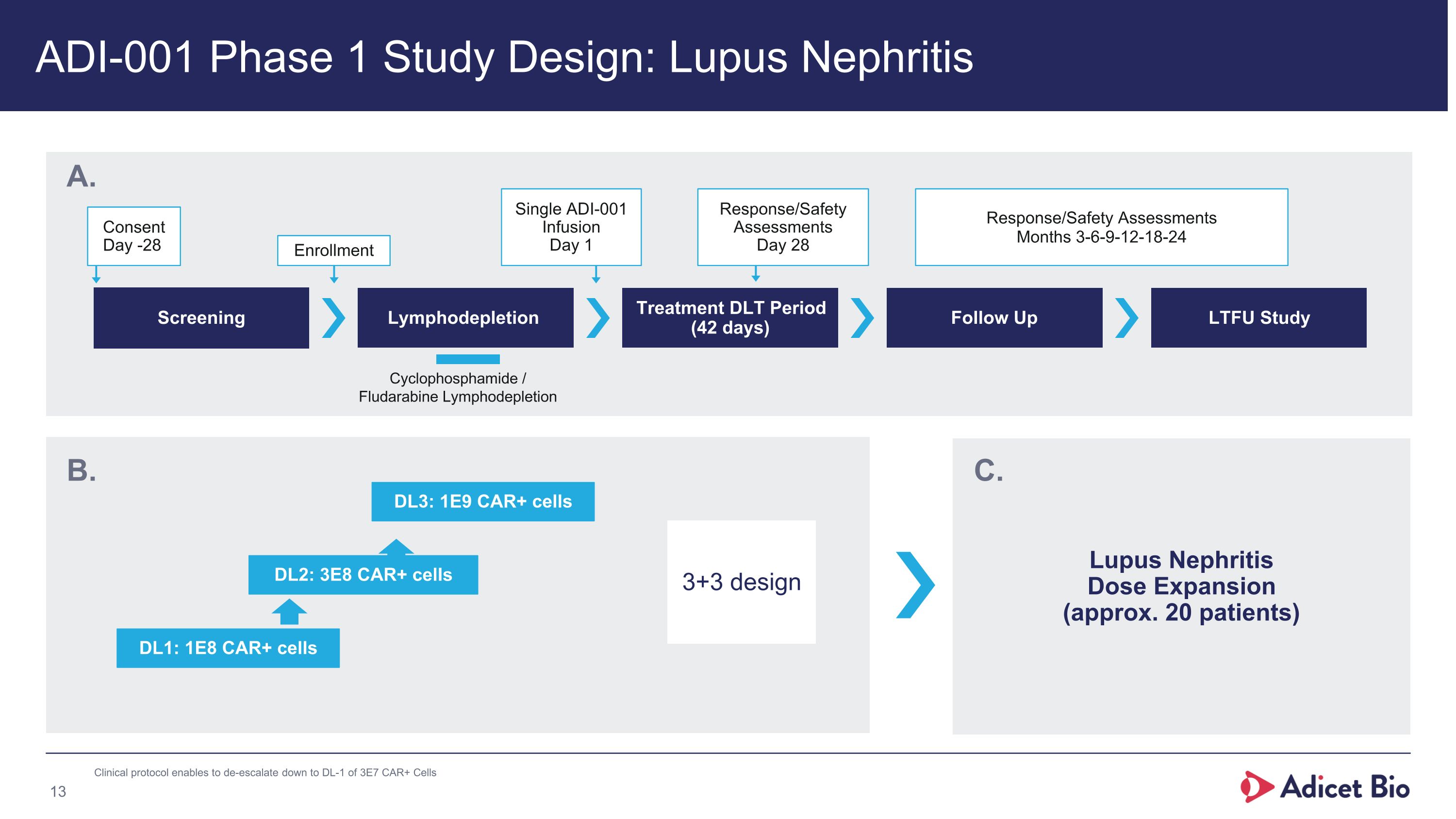
ADI-001 Phase 1 Study Design: Lupus Nephritis Screening Lymphodepletion Treatment DLT Period (42 days) Follow Up LTFU Study Consent Day -28 Enrollment Single ADI-001 Infusion Day 1 Response/Safety Assessments Day 28 Response/Safety Assessments Months 3-6-9-12-18-24 Cyclophosphamide / �Fludarabine Lymphodepletion DL1: 1E8 CAR+ cells DL2: 3E8 CAR+ cells 3+3 design A. B. DL3: 1E9 CAR+ cells Lupus Nephritis�Dose Expansion�(approx. 20 patients) C. Clinical protocol enables to de-escalate down to DL-1 of 3E7 CAR+ Cells
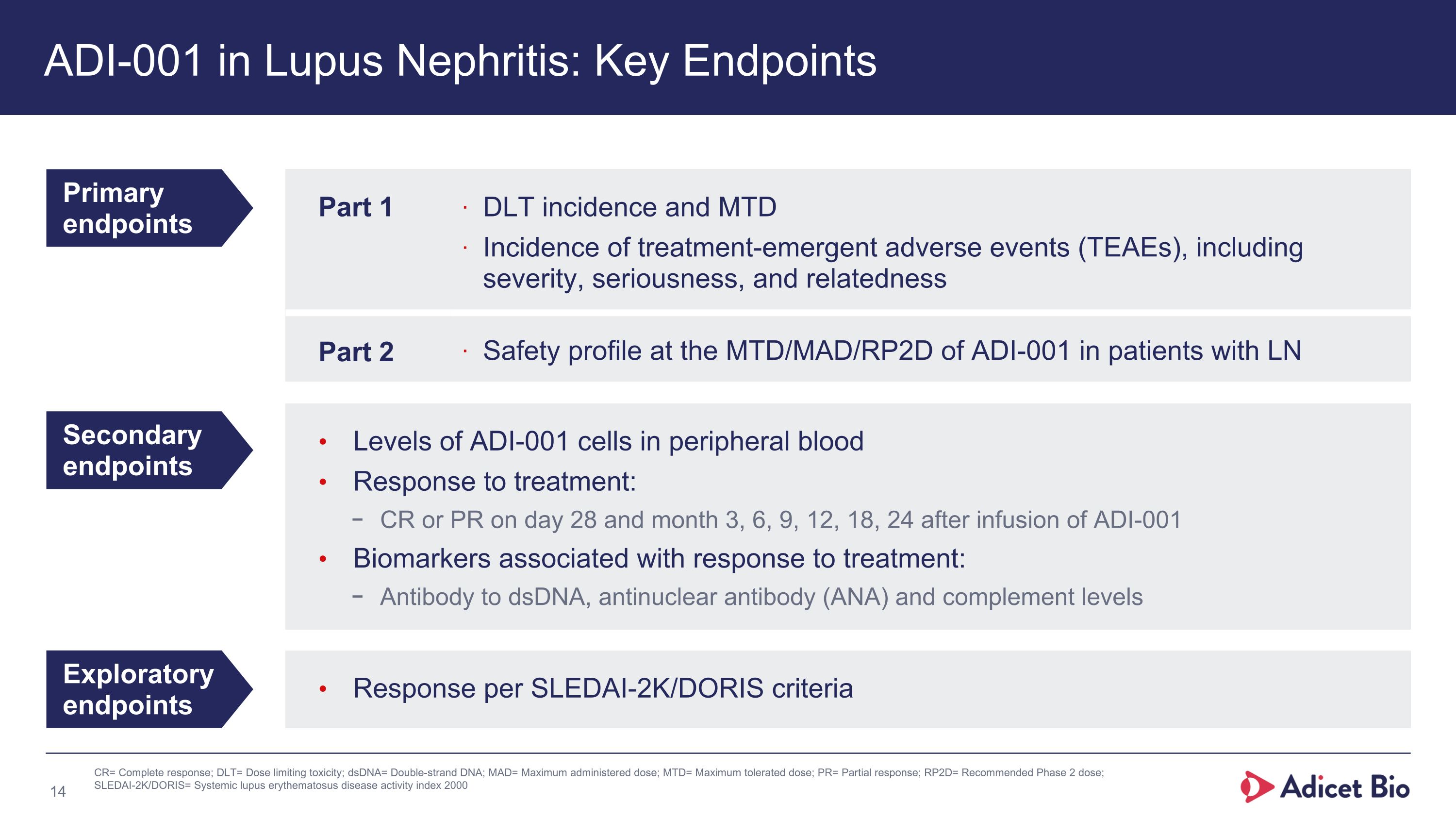
Levels of ADI-001 cells in peripheral blood Response to treatment: CR or PR on day 28 and month 3, 6, 9, 12, 18, 24 after infusion of ADI-001 Biomarkers associated with response to treatment: Antibody to dsDNA, antinuclear antibody (ANA) and complement levels Response per SLEDAI-2K/DORIS criteria ADI-001 in Lupus Nephritis: Key Endpoints Primary endpoints Secondary endpoints Exploratory endpoints Part 1 DLT incidence and MTD Incidence of treatment-emergent adverse events (TEAEs), including severity, seriousness, and relatedness Part 2 Safety profile at the MTD/MAD/RP2D of ADI-001 in patients with LN CR= Complete response; DLT= Dose limiting toxicity; dsDNA= Double-strand DNA; MAD= Maximum administered dose; MTD= Maximum tolerated dose; PR= Partial response; RP2D= Recommended Phase 2 dose; SLEDAI-2K/DORIS= Systemic lupus erythematosus disease activity index 2000
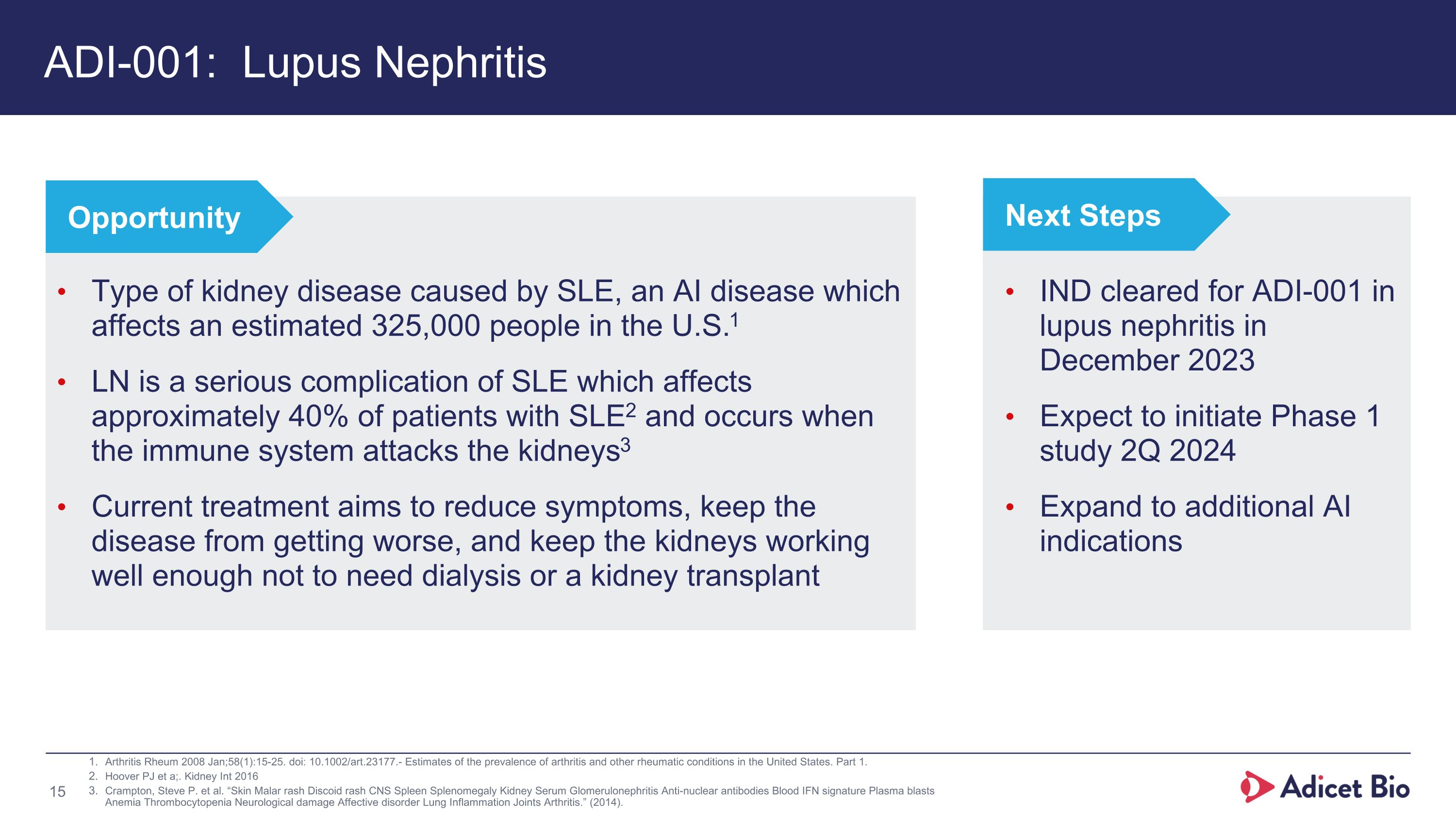
ADI-001: Lupus Nephritis Arthritis Rheum 2008 Jan;58(1):15-25. doi: 10.1002/art.23177.- Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part 1. Hoover PJ et a;. Kidney Int 2016 Crampton, Steve P. et al. “Skin Malar rash Discoid rash CNS Spleen Splenomegaly Kidney Serum Glomerulonephritis Anti-nuclear antibodies Blood IFN signature Plasma blasts Anemia Thrombocytopenia Neurological damage Affective disorder Lung Inflammation Joints Arthritis.” (2014). Type of kidney disease caused by SLE, an AI disease which affects an estimated 325,000 people in the U.S.1 LN is a serious complication of SLE which affects approximately 40% of patients with SLE2 and occurs when the immune system attacks the kidneys3 Current treatment aims to reduce symptoms, keep the disease from getting worse, and keep the kidneys working well enough not to need dialysis or a kidney transplant Opportunity IND cleared for ADI-001 in lupus nephritis in December 2023 Expect to initiate Phase 1 study 2Q 2024 Expand to additional AI indications Next Steps
ADI-001�Mantle Cell Lymphoma
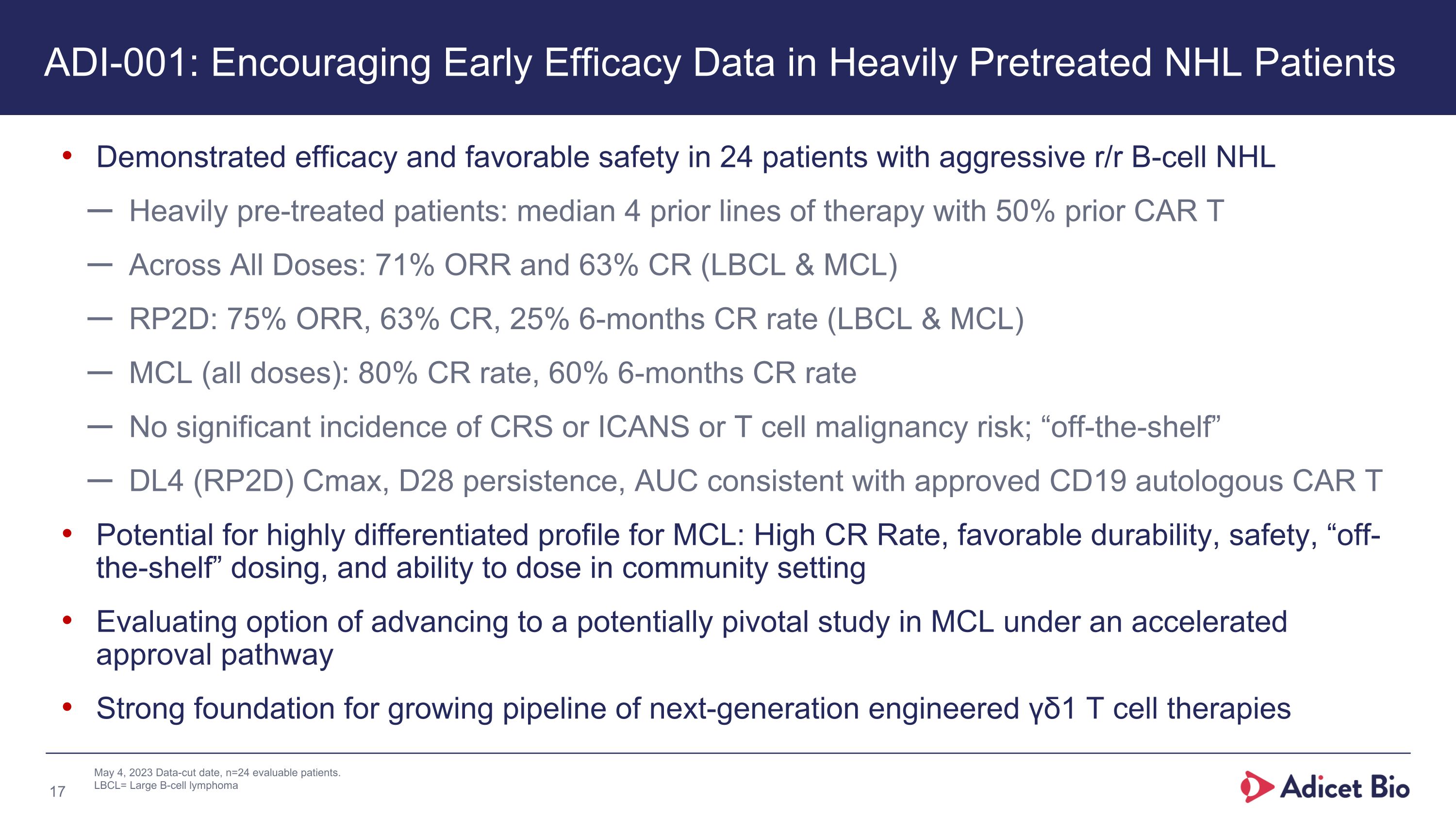
May 4, 2023 Data-cut date, n=24 evaluable patients. LBCL= Large B-cell lymphoma Demonstrated efficacy and favorable safety in 24 patients with aggressive r/r B-cell NHL Heavily pre-treated patients: median 4 prior lines of therapy with 50% prior CAR T Across All Doses: 71% ORR and 63% CR (LBCL & MCL) RP2D: 75% ORR, 63% CR, 25% 6-months CR rate (LBCL & MCL) MCL (all doses): 80% CR rate, 60% 6-months CR rate No significant incidence of CRS or ICANS or T cell malignancy risk; “off-the-shelf” DL4 (RP2D) Cmax, D28 persistence, AUC consistent with approved CD19 autologous CAR T Potential for highly differentiated profile for MCL: High CR Rate, favorable durability, safety, “off-the-shelf” dosing, and ability to dose in community setting Evaluating option of advancing to a potentially pivotal study in MCL under an accelerated approval pathway Strong foundation for growing pipeline of next-generation engineered γδ1 T cell therapies ADI-001: Encouraging Early Efficacy Data in Heavily Pretreated NHL Patients
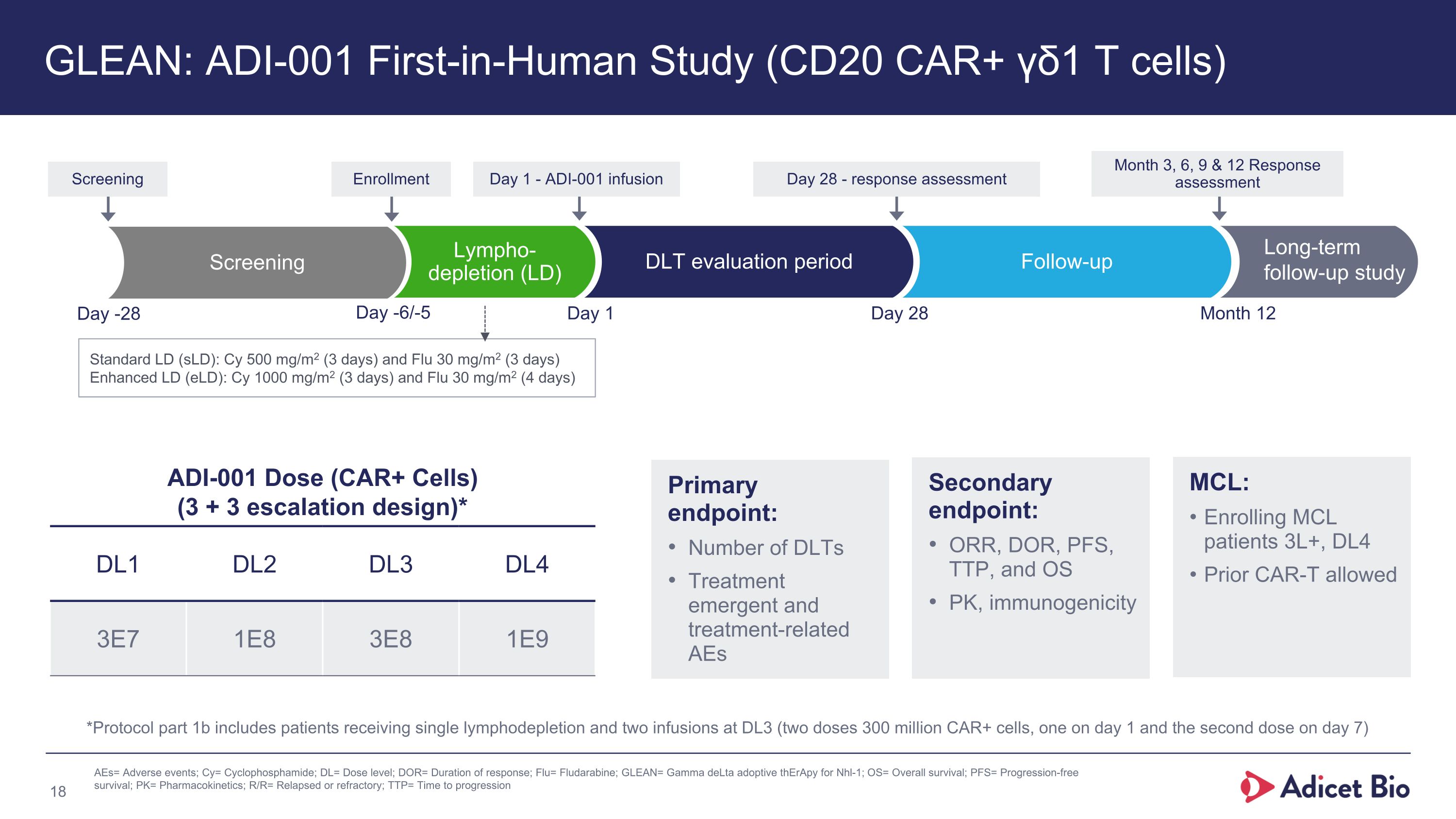
GLEAN: ADI-001 First-in-Human Study (CD20 CAR+ γδ1 T cells) Follow-up Day -6/-5 Day 1 Day 28 Month 12 Long-term�follow-up study Lympho-�depletion (LD) Day 28 - response assessment Month 3, 6, 9 & 12 Response assessment Day 1 - ADI-001 infusion ADI-001 Dose (CAR+ Cells) �(3 + 3 escalation design)* DL1 DL2 DL3 DL4 3E7 1E8 3E8 1E9 Standard LD (sLD): Cy 500 mg/m2 (3 days) and Flu 30 mg/m2 (3 days) Enhanced LD (eLD): Cy 1000 mg/m2 (3 days) and Flu 30 mg/m2 (4 days) MCL: Enrolling MCL patients 3L+, DL4 Prior CAR-T allowed Primary �endpoint: Number of DLTs Treatment emergent and treatment-related AEs Secondary endpoint: ORR, DOR, PFS, TTP, and OS PK, immunogenicity Enrollment DLT evaluation period Screening Screening Day -28 AEs= Adverse events; Cy= Cyclophosphamide; DL= Dose level; DOR= Duration of response; Flu= Fludarabine; GLEAN= Gamma deLta adoptive thErApy for Nhl-1; OS= Overall survival; PFS= Progression-free survival; PK= Pharmacokinetics; R/R= Relapsed or refractory; TTP= Time to progression *Protocol part 1b includes patients receiving single lymphodepletion and two infusions at DL3 (two doses 300 million CAR+ cells, one on day 1 and the second dose on day 7)
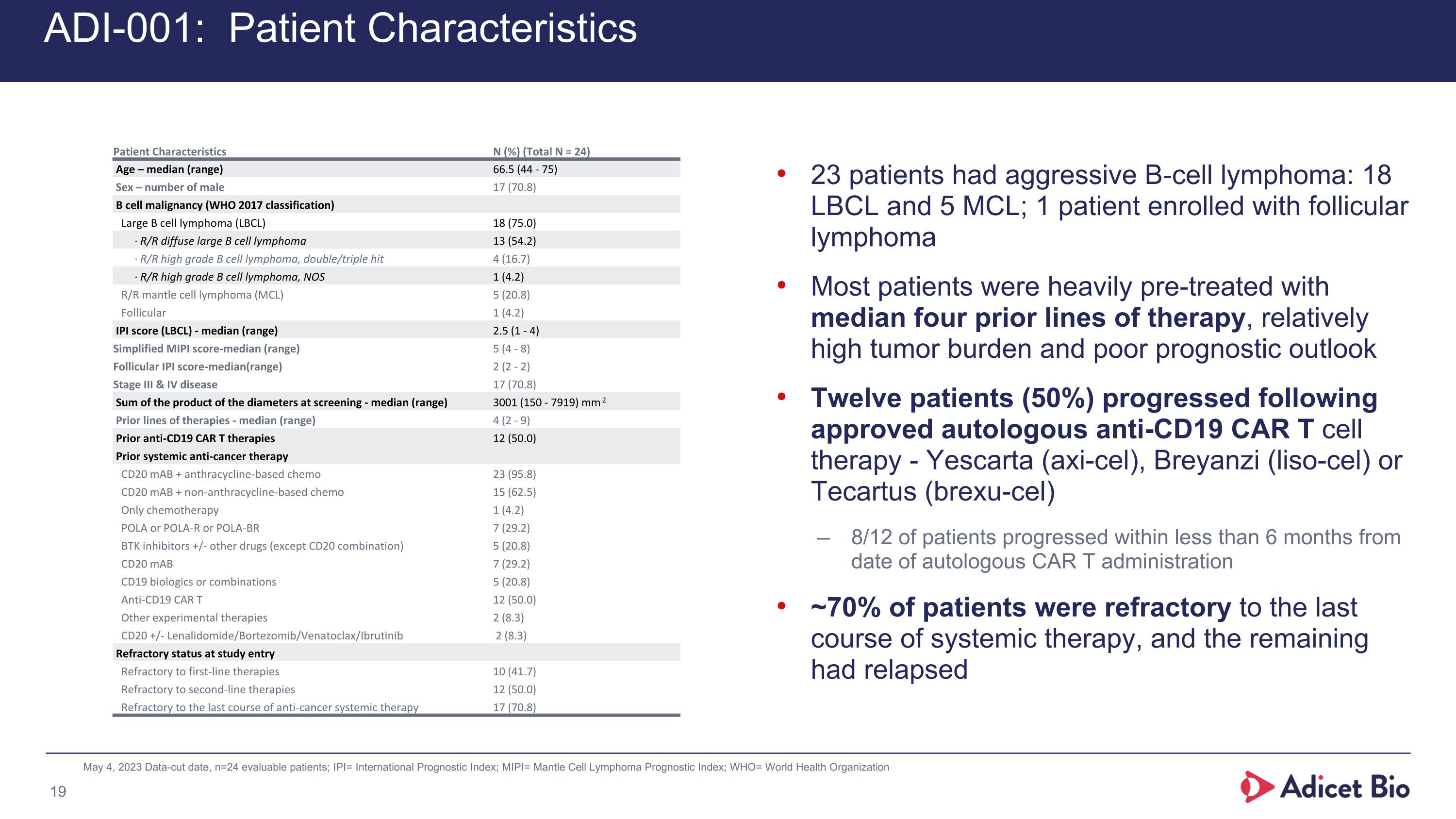
ADI-001: Patient Characteristics 23 patients had aggressive B-cell lymphoma: 18 LBCL and 5 MCL; 1 patient enrolled with follicular lymphoma Most patients were heavily pre-treated with median four prior lines of therapy, relatively high tumor burden and poor prognostic outlook Twelve patients (50%) progressed following approved autologous anti-CD19 CAR T cell therapy - Yescarta (axi-cel), Breyanzi (liso-cel) or Tecartus (brexu-cel) 8/12 of patients progressed within less than 6 months from date of autologous CAR T administration ~70% of patients were refractory to the last course of systemic therapy, and the remaining had relapsed May 4, 2023 Data-cut date, n=24 evaluable patients; IPI= International Prognostic Index; MIPI= Mantle Cell Lymphoma Prognostic Index; WHO= World Health Organization Patient Characteristics N (%) (Total N = 24) Age – median (range) 66.5 (44 - 75) Sex – number of male 17 (70.8) B cell malignancy (WHO 2017 classification) Large B cell lymphoma (LBCL) 18 (75.0) ∙ R/R diffuse large B cell lymphoma 13 (54.2) ∙ R/R high grade B cell lymphoma, double/triple hit 4 (16.7) ∙ R/R high grade B cell lymphoma, NOS 1 (4.2) R/R mantle cell lymphoma (MCL) 5 (20.8) Follicular 1 (4.2) IPI score (LBCL) - median (range) 2.5 (1 - 4) Simplified MIPI score-median (range) 5 (4 - 8) Follicular IPI score-median(range) 2 (2 - 2) Stage III & IV disease 17 (70.8) Sum of the product of the diameters at screening - median (range) 3001 (150 - 7919) mm2 Prior lines of therapies - median (range) 4 (2 - 9) Prior anti-CD19 CAR T therapies 12 (50.0) Prior systemic anti-cancer therapy CD20 mAB + anthracycline-based chemo 23 (95.8) CD20 mAB + non-anthracycline-based chemo 15 (62.5) Only chemotherapy 1 (4.2) POLA or POLA-R or POLA-BR 7 (29.2) BTK inhibitors +/- other drugs (except CD20 combination) 5 (20.8) CD20 mAB 7 (29.2) CD19 biologics or combinations 5 (20.8) Anti-CD19 CAR T 12 (50.0) Other experimental therapies 2 (8.3) CD20 +/- Lenalidomide/Bortezomib/Venatoclax/Ibrutinib 2 (8.3) Refractory status at study entry Refractory to first-line therapies 10 (41.7) Refractory to second-line therapies 12 (50.0) Refractory to the last course of anti-cancer systemic therapy 17 (70.8)
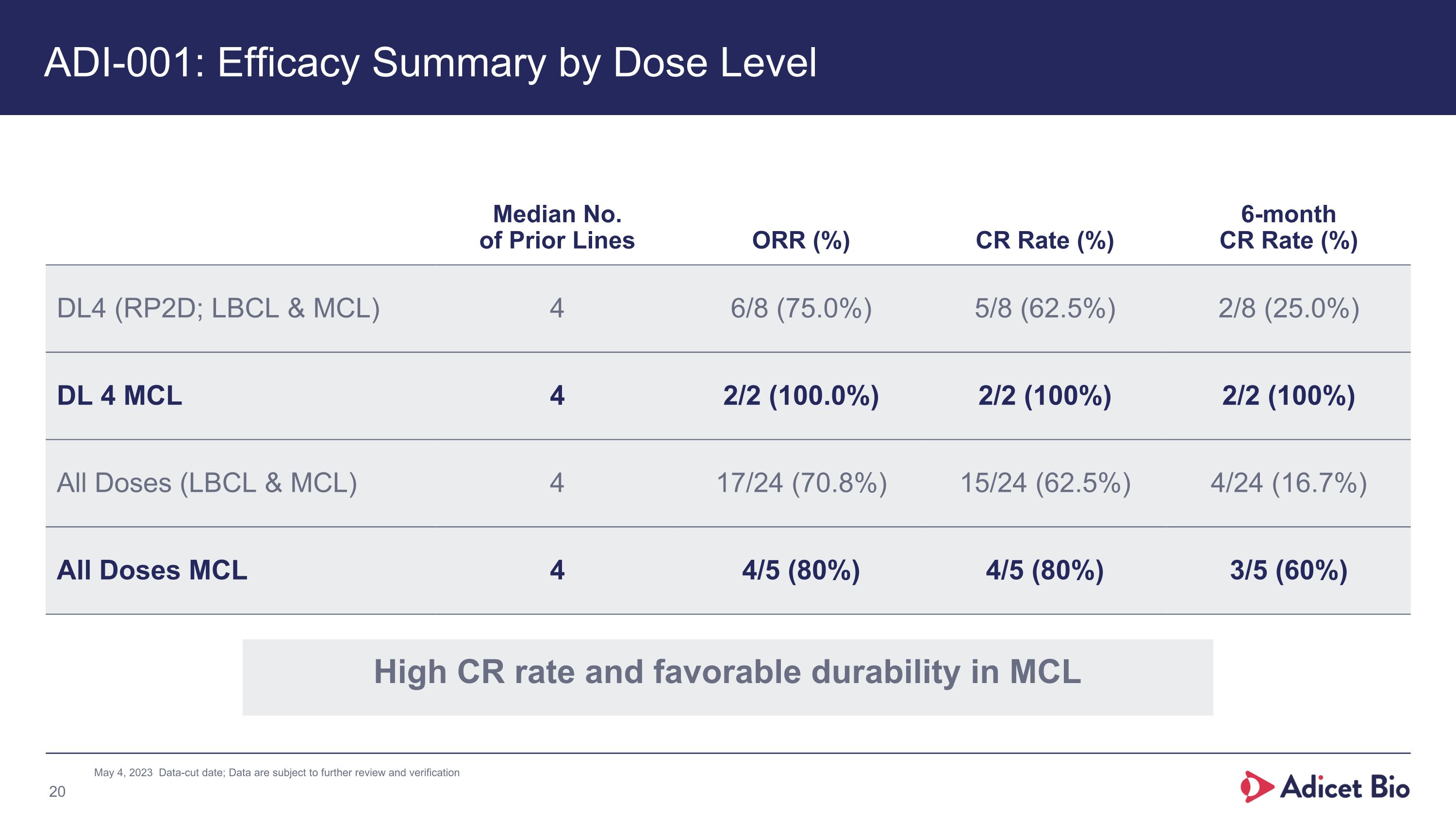
ADI-001: Efficacy Summary by Dose Level Median No.�of Prior Lines ORR (%) CR Rate (%) 6-month CR Rate (%) DL4 (RP2D; LBCL & MCL) 4 6/8 (75.0%) 5/8 (62.5%) 2/8 (25.0%) DL 4 MCL 4 2/2 (100.0%) 2/2 (100%) 2/2 (100%) All Doses (LBCL & MCL) 4 17/24 (70.8%) 15/24 (62.5%) 4/24 (16.7%) All Doses MCL 4 4/5 (80%) 4/5 (80%) 3/5 (60%) May 4, 2023 Data-cut date; Data are subject to further review and verification High CR rate and favorable durability in MCL
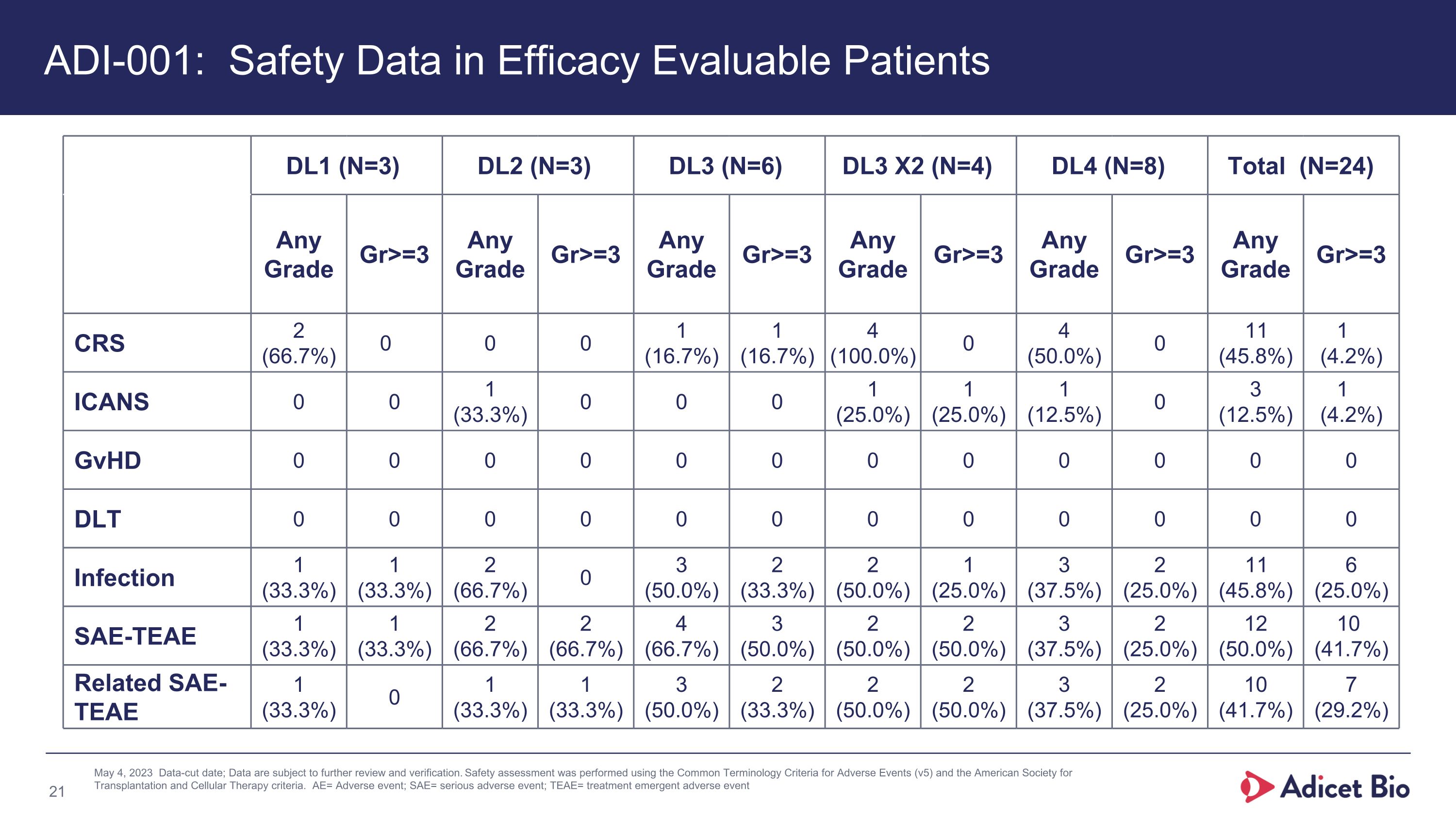
DL1 (N=3) DL2 (N=3) DL3 (N=6) DL3 X2 (N=4) DL4 (N=8) Total (N=24) Any Grade Gr>=3 Any Grade Gr>=3 Any Grade Gr>=3 Any Grade Gr>=3 Any Grade Gr>=3 Any Grade Gr>=3 CRS 2 (66.7%) 0 0 0 1 (16.7%) 1 (16.7%) 4 (100.0%) 0 4 (50.0%) 0 11 (45.8%) 1 (4.2%) ICANS 0 0 1 (33.3%) 0 0 0 1 (25.0%) 1 (25.0%) 1 (12.5%) 0 3 (12.5%) 1 (4.2%) GvHD 0 0 0 0 0 0 0 0 0 0 0 0 DLT 0 0 0 0 0 0 0 0 0 0 0 0 Infection 1 (33.3%) 1 (33.3%) 2 (66.7%) 0 3 (50.0%) 2 (33.3%) 2 (50.0%) 1 (25.0%) 3 (37.5%) 2 (25.0%) 11 (45.8%) 6 (25.0%) SAE-TEAE 1 (33.3%) 1 (33.3%) 2 (66.7%) 2 (66.7%) 4 (66.7%) 3 (50.0%) 2 (50.0%) 2 (50.0%) 3 (37.5%) 2 (25.0%) 12 (50.0%) 10 (41.7%) Related SAE-TEAE 1 (33.3%) 0 1 (33.3%) 1 (33.3%) 3 (50.0%) 2 (33.3%) 2 (50.0%) 2 (50.0%) 3 (37.5%) 2 (25.0%) 10 (41.7%) 7 (29.2%) May 4, 2023 Data-cut date; Data are subject to further review and verification. Safety assessment was performed using the Common Terminology Criteria for Adverse Events (v5) and the American Society for Transplantation and Cellular Therapy criteria. AE= Adverse event; SAE= serious adverse event; TEAE= treatment emergent adverse event % added ADI-001: Safety Data in Efficacy Evaluable Patients
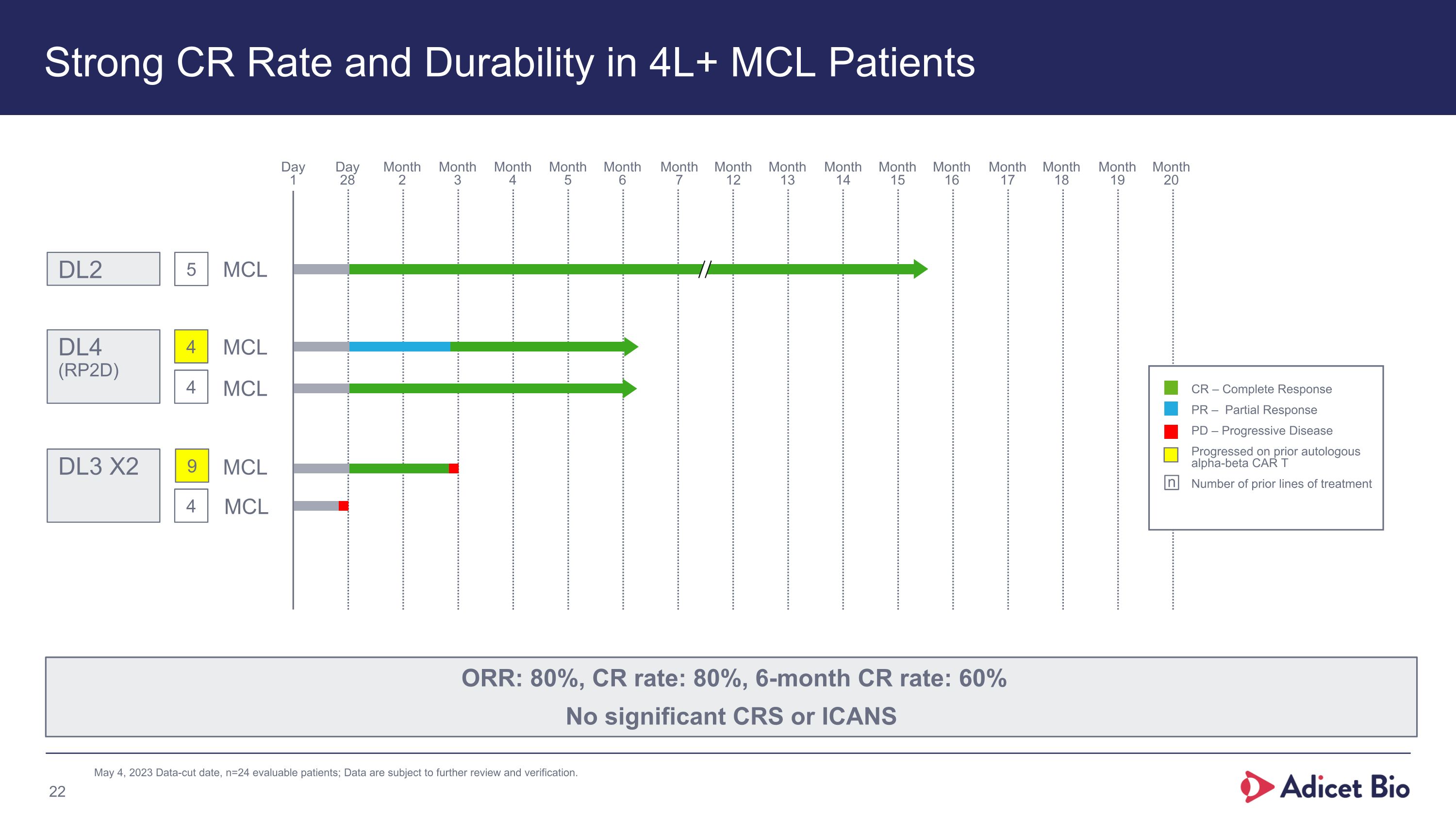
Strong CR Rate and Durability in 4L+ MCL Patients May 4, 2023 Data-cut date, n=24 evaluable patients; Data are subject to further review and verification. ORR: 80%, CR rate: 80%, 6-month CR rate: 60% No significant CRS or ICANS Day�1 Day�28 Month�6 Month�7 Month�5 Month�4 Month�3 Month�2 Month�12 Month�13 Month�14 Month�15 Month�16 Month�17 Month�18 Month�19 Month�20 CR – Complete Response PR – Partial Response PD – Progressive Disease Progressed on prior autologous alpha-beta CAR T Number of prior lines of treatment n 5 DL2 9 4 DL3 X2 4 4 DL4 (RP2D) MCL MCL MCL MCL MCL
Data Provides Strong Foundation for Future Development in MCL High CR rate Favorable durability in late-line patients Superior cell killing potency compared to autologous CAR T1 Cmax, Day 28 persistence and AUC consistent with approved CD19 autologous CAR T Favorable safety profile with no significant risk of CRS, ICANS, or T-cell malignancy Potential to dose in community setting 1. Nishimoto et al. ISCT 2022 May 4, 2023 Data-cut date, n=24 evaluable patients
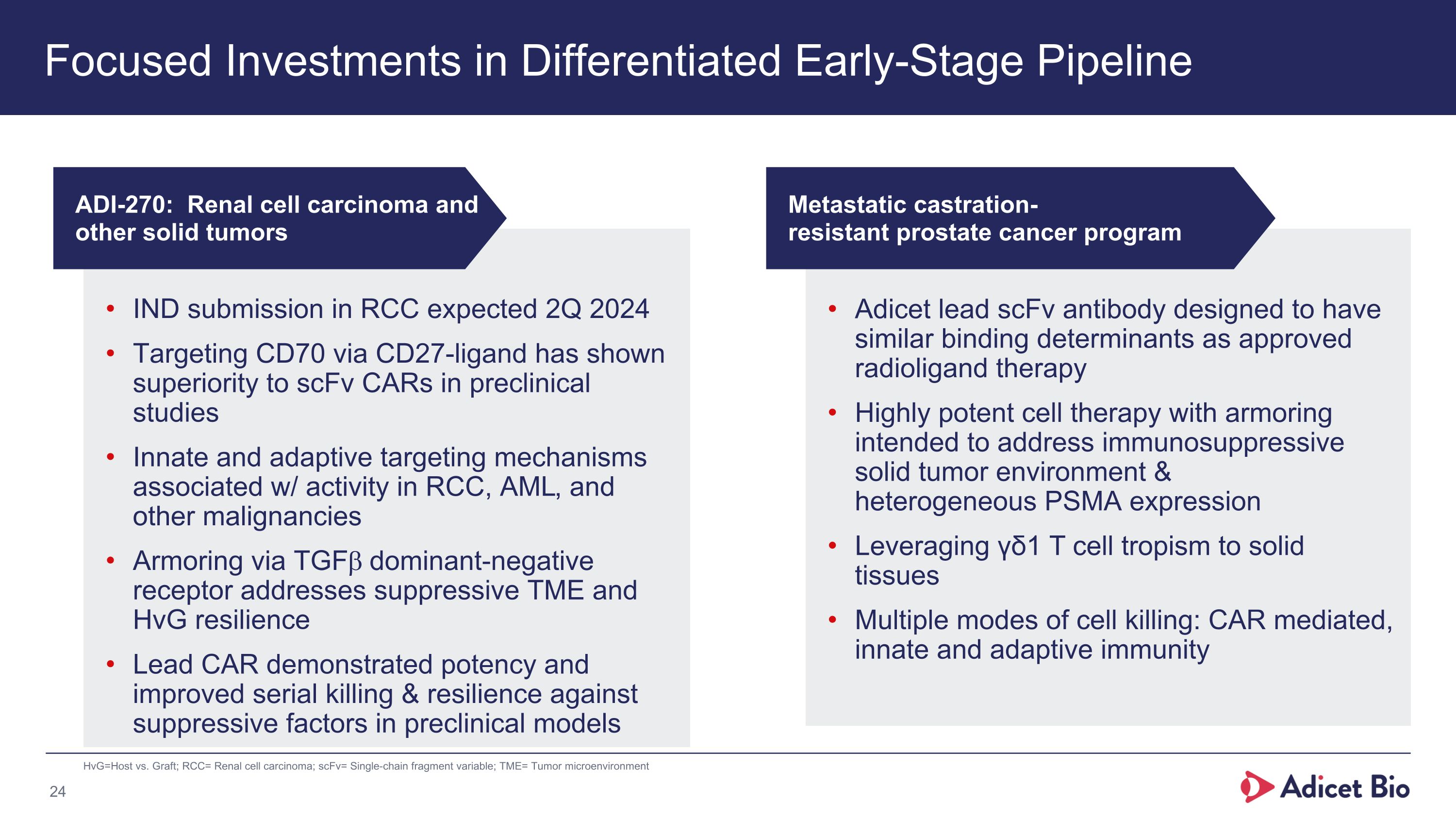
Focused Investments in Differentiated Early-Stage Pipeline HvG=Host vs. Graft; RCC= Renal cell carcinoma; scFv= Single-chain fragment variable; TME= Tumor microenvironment IND submission in RCC expected 2Q 2024 Targeting CD70 via CD27-ligand has shown superiority to scFv CARs in preclinical studies Innate and adaptive targeting mechanisms associated w/ activity in RCC, AML, and other malignancies Armoring via TGFb dominant-negative receptor addresses suppressive TME and HvG resilience Lead CAR demonstrated potency and improved serial killing & resilience against suppressive factors in preclinical models Adicet lead scFv antibody designed to have similar binding determinants as approved radioligand therapy Highly potent cell therapy with armoring intended to address immunosuppressive solid tumor environment & heterogeneous PSMA expression Leveraging γδ1 T cell tropism to solid tissues Multiple modes of cell killing: CAR mediated, innate and adaptive immunity ADI-270: Renal cell carcinoma and other solid tumors Metastatic castration-�resistant prostate cancer program
ADI-270�Renal Cell Carcinoma
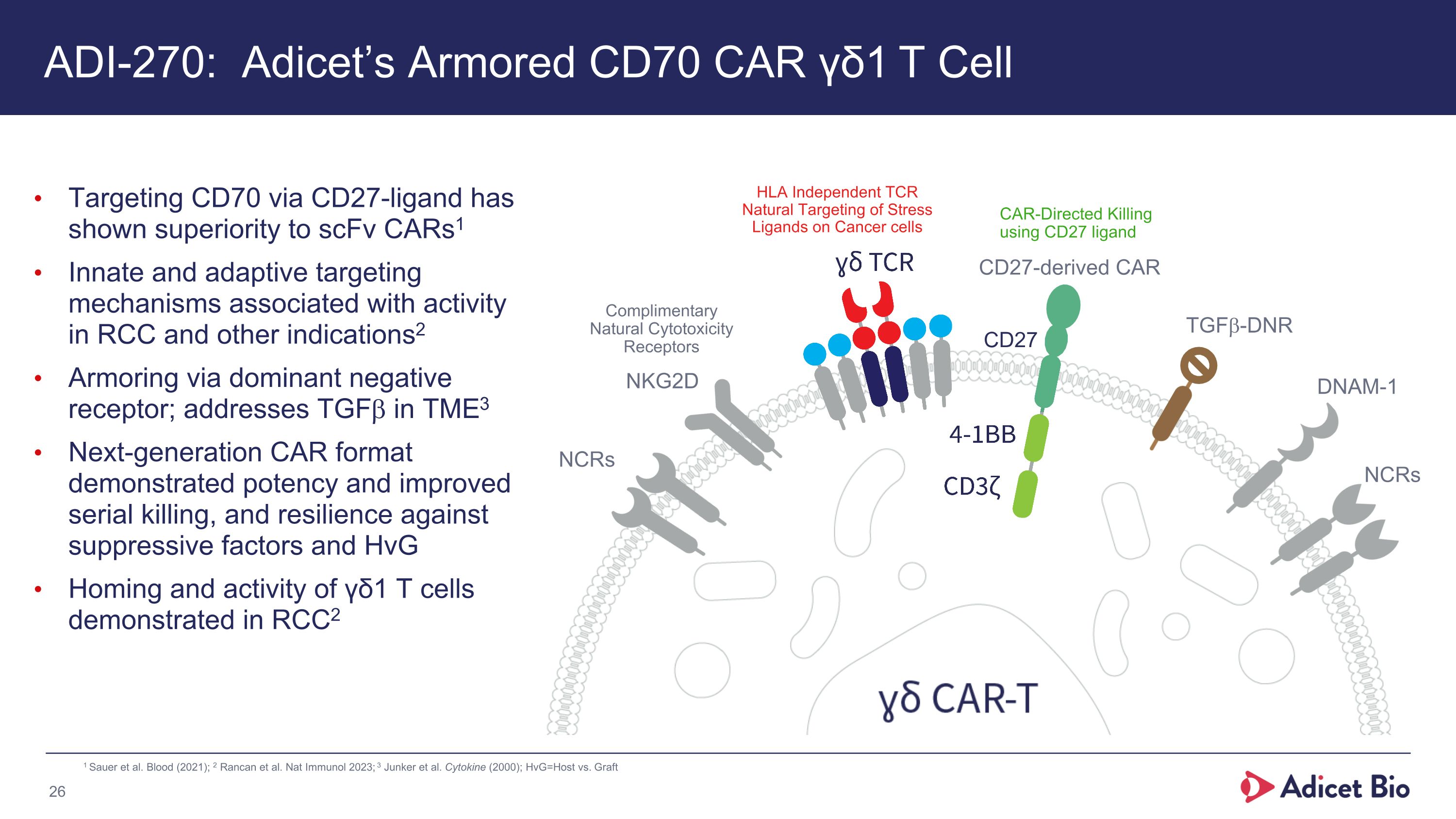
ADI-270: Adicet’s Armored CD70 CAR γδ1 T Cell Targeting CD70 via CD27-ligand has shown superiority to scFv CARs1 Innate and adaptive targeting mechanisms associated with activity in RCC and other indications2 Armoring via dominant negative receptor; addresses TGFb in TME3 Next-generation CAR format demonstrated potency and improved serial killing, and resilience against suppressive factors and HvG Homing and activity of γδ1 T cells demonstrated in RCC2 Complimentary Natural Cytotoxicity Receptors HLA Independent TCR Natural Targeting of Stress Ligands on Cancer cells CAR-Directed Killing using CD27 ligand NCRs NKG2D DNAM-1 NCRs TGFb-DNR CD27-derived CAR CD27 1 Sauer et al. Blood (2021); 2 Rancan et al. Nat Immunol 2023; 3 Junker et al. Cytokine (2000); HvG=Host vs. Graft
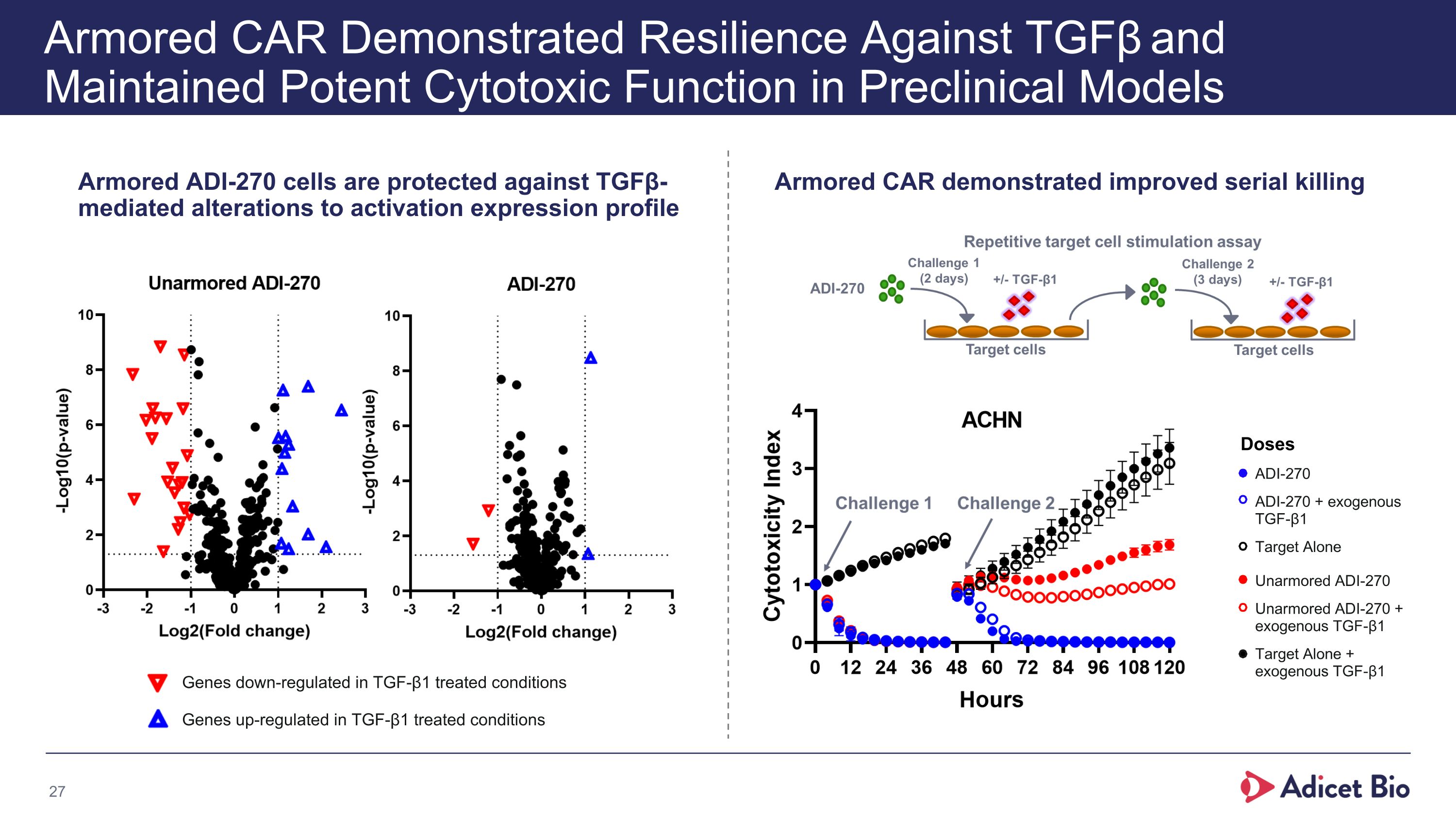
Armored CAR Demonstrated Resilience Against TGFβ and Maintained Potent Cytotoxic Function in Preclinical Models Armored ADI-270 cells are protected against TGFβ-mediated alterations to activation expression profile Armored CAR demonstrated improved serial killing Doses Genes down-regulated in TGF-β1 treated conditions Genes up-regulated in TGF-β1 treated conditions ADI-270 ADI-270 + exogenous TGF-β1 Target Alone Unarmored ADI-270 Unarmored ADI-270 + exogenous TGF-β1 Target Alone + exogenous TGF-β1
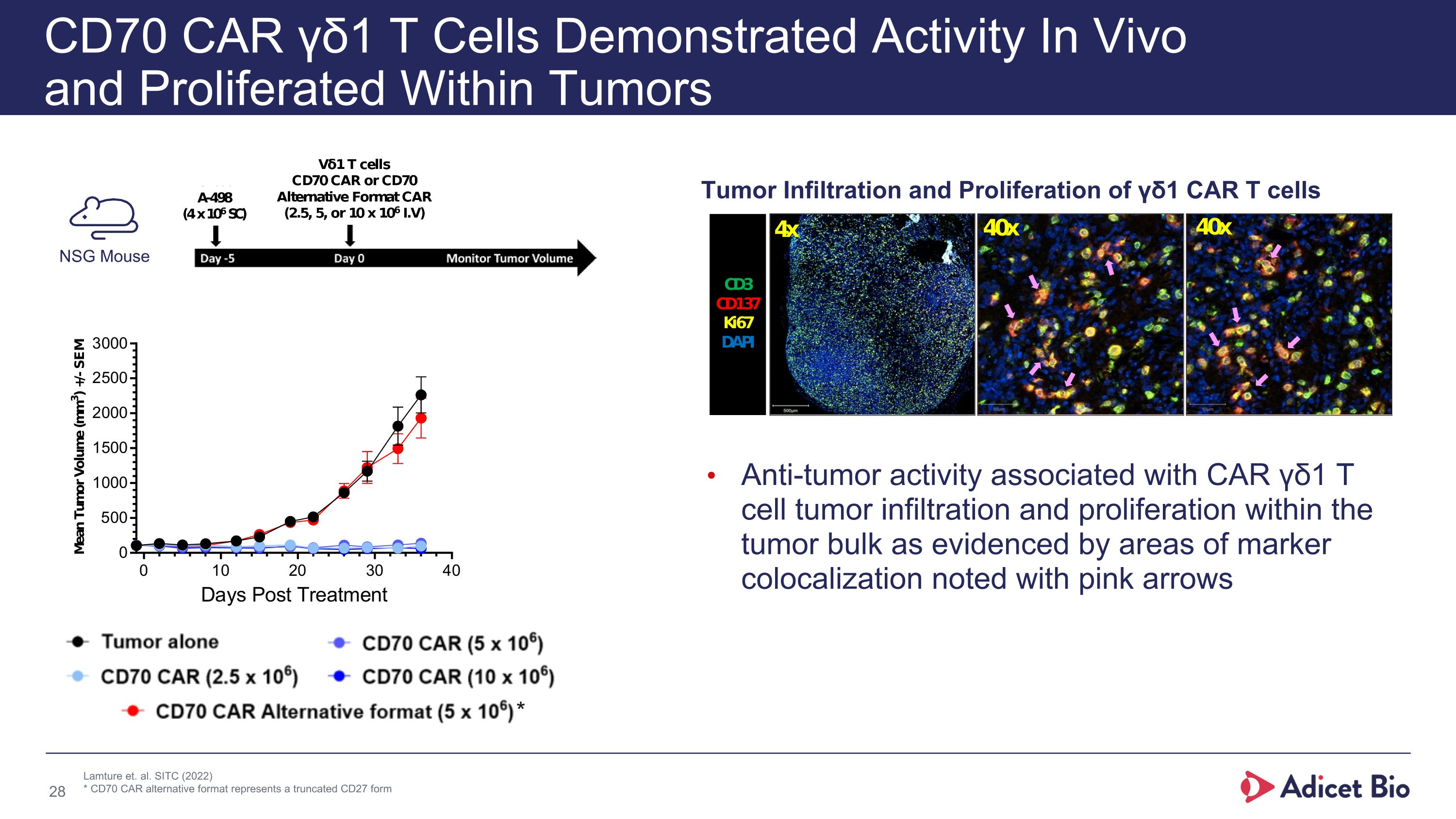
CD70 CAR γδ1 T Cells Demonstrated Activity In Vivo �and Proliferated Within Tumors Anti-tumor activity associated with CAR γδ1 T cell tumor infiltration and proliferation within the tumor bulk as evidenced by areas of marker colocalization noted with pink arrows Tumor Infiltration and Proliferation of γδ1 CAR T cells NSG Mouse Lamture et. al. SITC (2022) * CD70 CAR alternative format represents a truncated CD27 form *
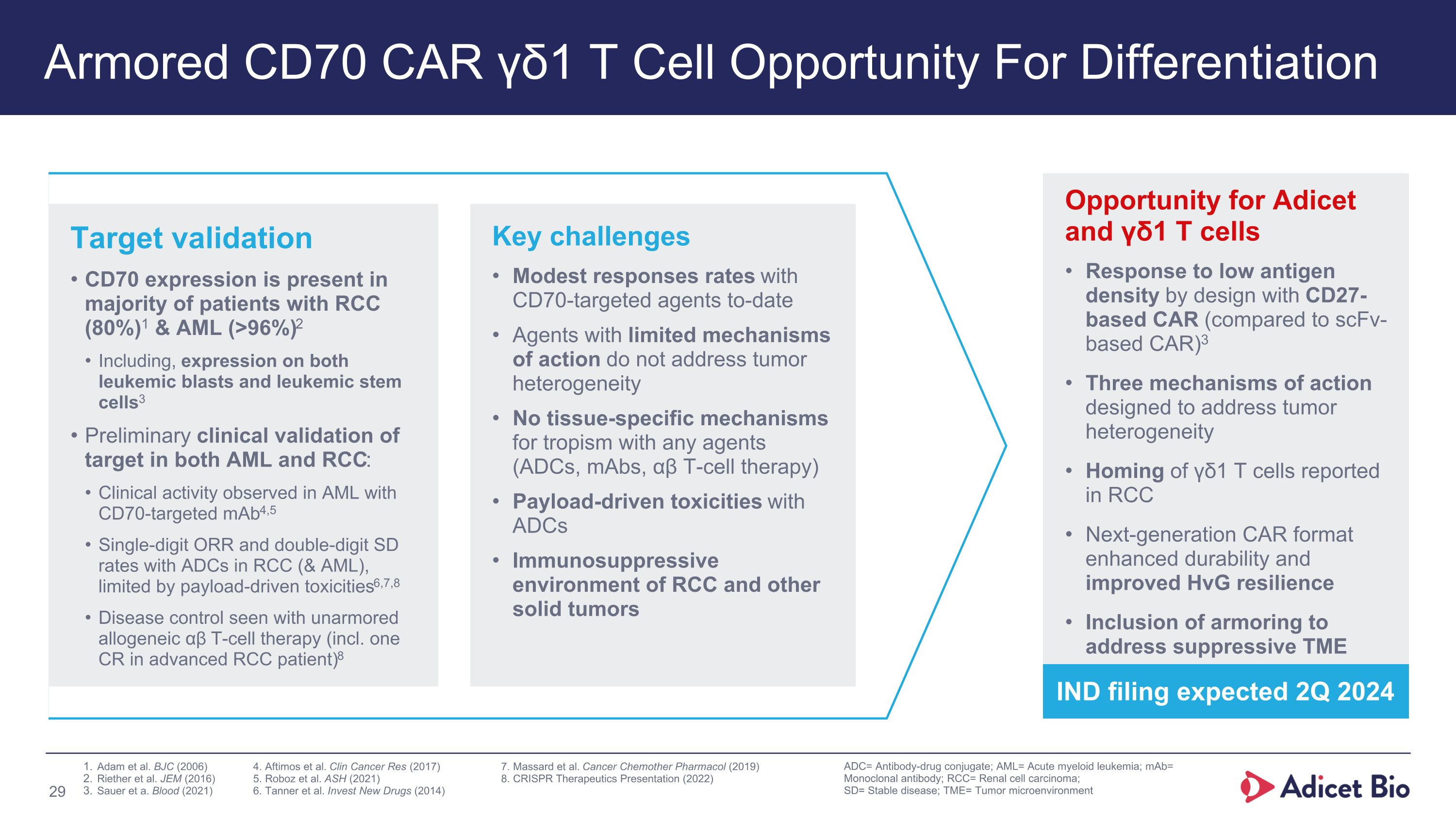
Armored CD70 CAR γδ1 T Cell Opportunity For Differentiation ADC= Antibody-drug conjugate; AML= Acute myeloid leukemia; mAb= Monoclonal antibody; RCC= Renal cell carcinoma; SD= Stable disease; TME= Tumor microenvironment Opportunity for Adicet and γδ1 T cells Response to low antigen density by design with CD27-based CAR (compared to scFv-based CAR)3 Three mechanisms of action designed to address tumor heterogeneity Homing of γδ1 T cells reported in RCC Next-generation CAR format enhanced durability and improved HvG resilience Inclusion of armoring to address suppressive TME Key challenges Modest responses rates with CD70-targeted agents to-date Agents with limited mechanisms of action do not address tumor heterogeneity No tissue-specific mechanisms for tropism with any agents (ADCs, mAbs, αβ T-cell therapy) Payload-driven toxicities with ADCs Immunosuppressive environment of RCC and other solid tumors Target validation CD70 expression is present in majority of patients with RCC (80%)1 & AML (>96%)2 Including, expression on both leukemic blasts and leukemic stem cells3 Preliminary clinical validation of target in both AML and RCC: Clinical activity observed in AML with CD70-targeted mAb4,5 Single-digit ORR and double-digit SD rates with ADCs in RCC (& AML), limited by payload-driven toxicities6,7,8 Disease control seen with unarmored allogeneic αβ T-cell therapy (incl. one CR in advanced RCC patient)8 Adam et al. BJC (2006) 4. Aftimos et al. Clin Cancer Res (2017) 7. Massard et al. Cancer Chemother Pharmacol (2019) Riether et al. JEM (2016) 5. Roboz et al. ASH (2021) 8. CRISPR Therapeutics Presentation (2022) Sauer et a. Blood (2021) 6. Tanner et al. Invest New Drugs (2014) IND filing expected 2Q 2024

PSMA Program
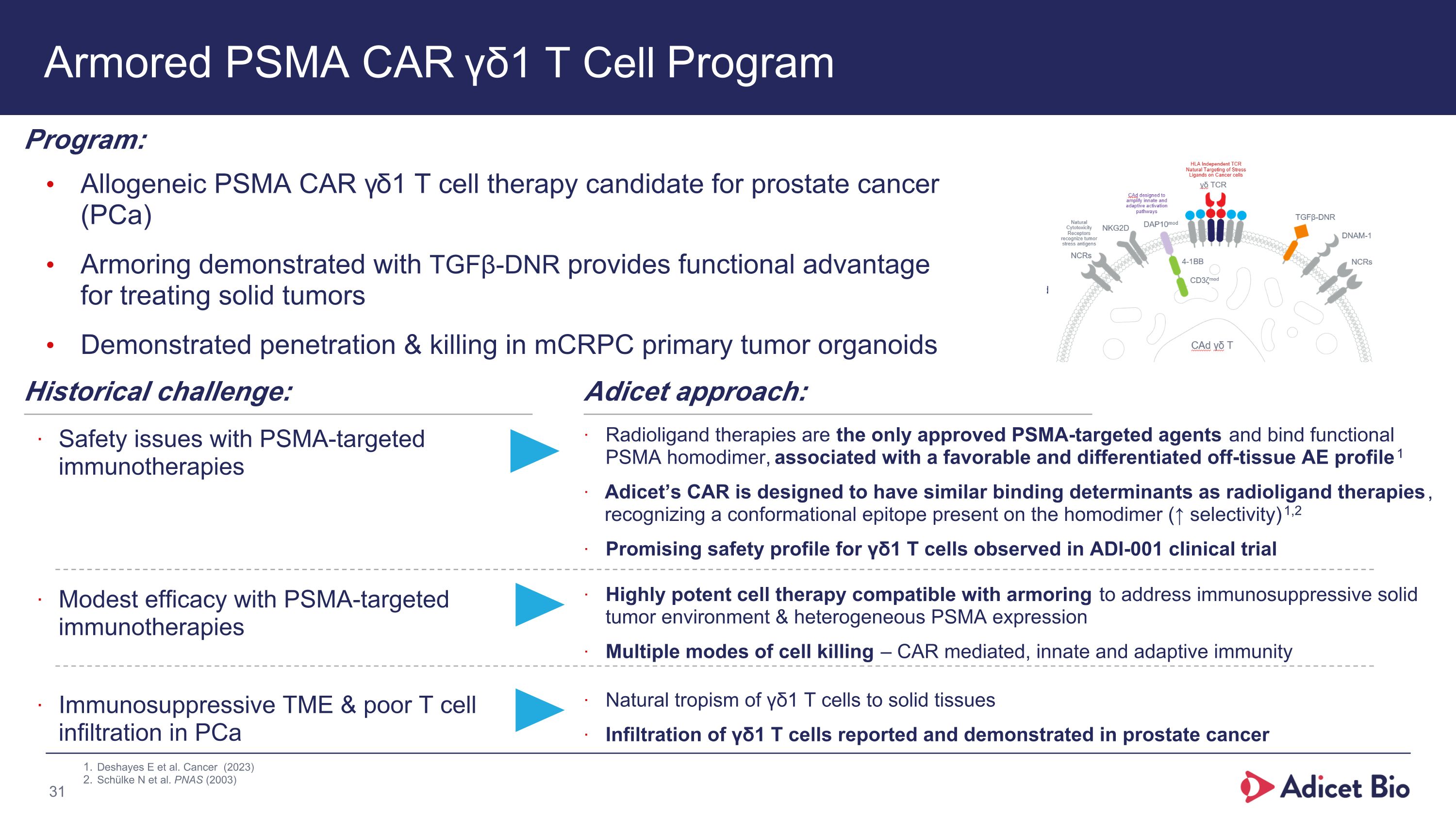
Armored PSMA CAR γδ1 T Cell Program Allogeneic PSMA CAR γδ1 T cell therapy candidate for prostate cancer (PCa) Armoring demonstrated with TGFβ-DNR provides functional advantage for treating solid tumors Demonstrated penetration & killing in mCRPC primary tumor organoids Program: Historical challenge: Safety issues with PSMA-targeted immunotherapies Adicet approach: Radioligand therapies are the only approved PSMA-targeted agents and bind functional PSMA homodimer, associated with a favorable and differentiated off-tissue AE profile1 Adicet’s CAR is designed to have similar binding determinants as radioligand therapies, recognizing a conformational epitope present on the homodimer (↑ selectivity)1,2 Promising safety profile for γδ1 T cells observed in ADI-001 clinical trial Modest efficacy with PSMA-targeted immunotherapies Immunosuppressive TME & poor T cell infiltration in PCa Highly potent cell therapy compatible with armoring to address immunosuppressive solid tumor environment & heterogeneous PSMA expression Multiple modes of cell killing – CAR mediated, innate and adaptive immunity Natural tropism of γδ1 T cells to solid tissues Infiltration of γδ1 T cells reported and demonstrated in prostate cancer Deshayes E et al. Cancer (2023) Schülke N et al. PNAS (2003)
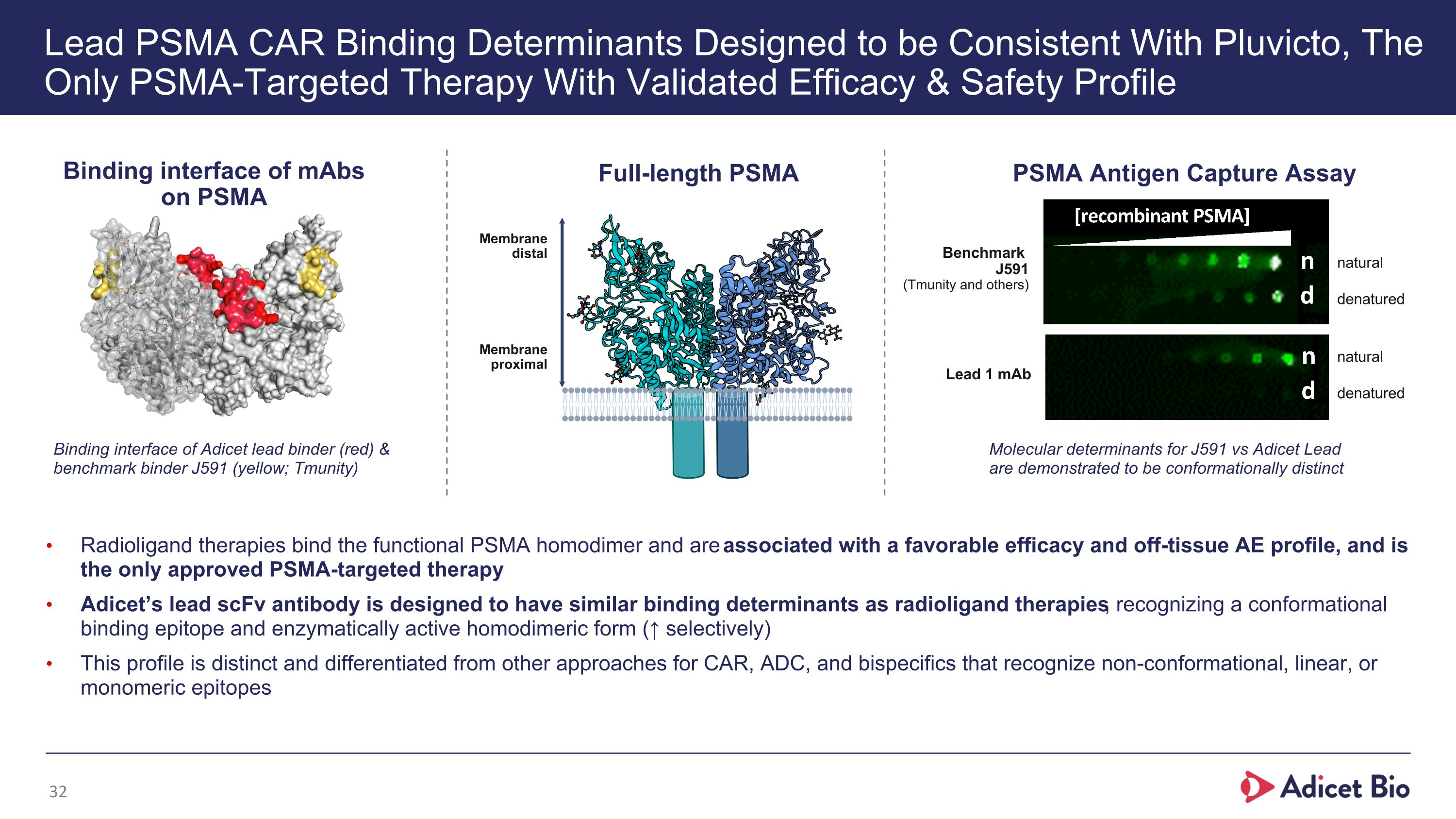
Lead PSMA CAR Binding Determinants Designed to be Consistent With Pluvicto, The Only PSMA-Targeted Therapy With Validated Efficacy & Safety Profile Radioligand therapies bind the functional PSMA homodimer and are associated with a favorable efficacy and off-tissue AE profile, and is the only approved PSMA-targeted therapy Adicet’s lead scFv antibody is designed to have similar binding determinants as radioligand therapies, recognizing a conformational binding epitope and enzymatically active homodimeric form (↑ selectively) This profile is distinct and differentiated from other approaches for CAR, ADC, and bispecifics that recognize non-conformational, linear, or monomeric epitopes Molecular determinants for J591 vs Adicet Lead are demonstrated to be conformationally distinct natural denatured natural denatured PSMA Antigen Capture Assay Binding interface of mAbs on PSMA Full-length PSMA Binding interface of Adicet lead binder (red) & benchmark binder J591 (yellow; Tmunity) Benchmark �J591�(Tmunity and others) Membrane distal Membrane proximal Lead 1 mAb
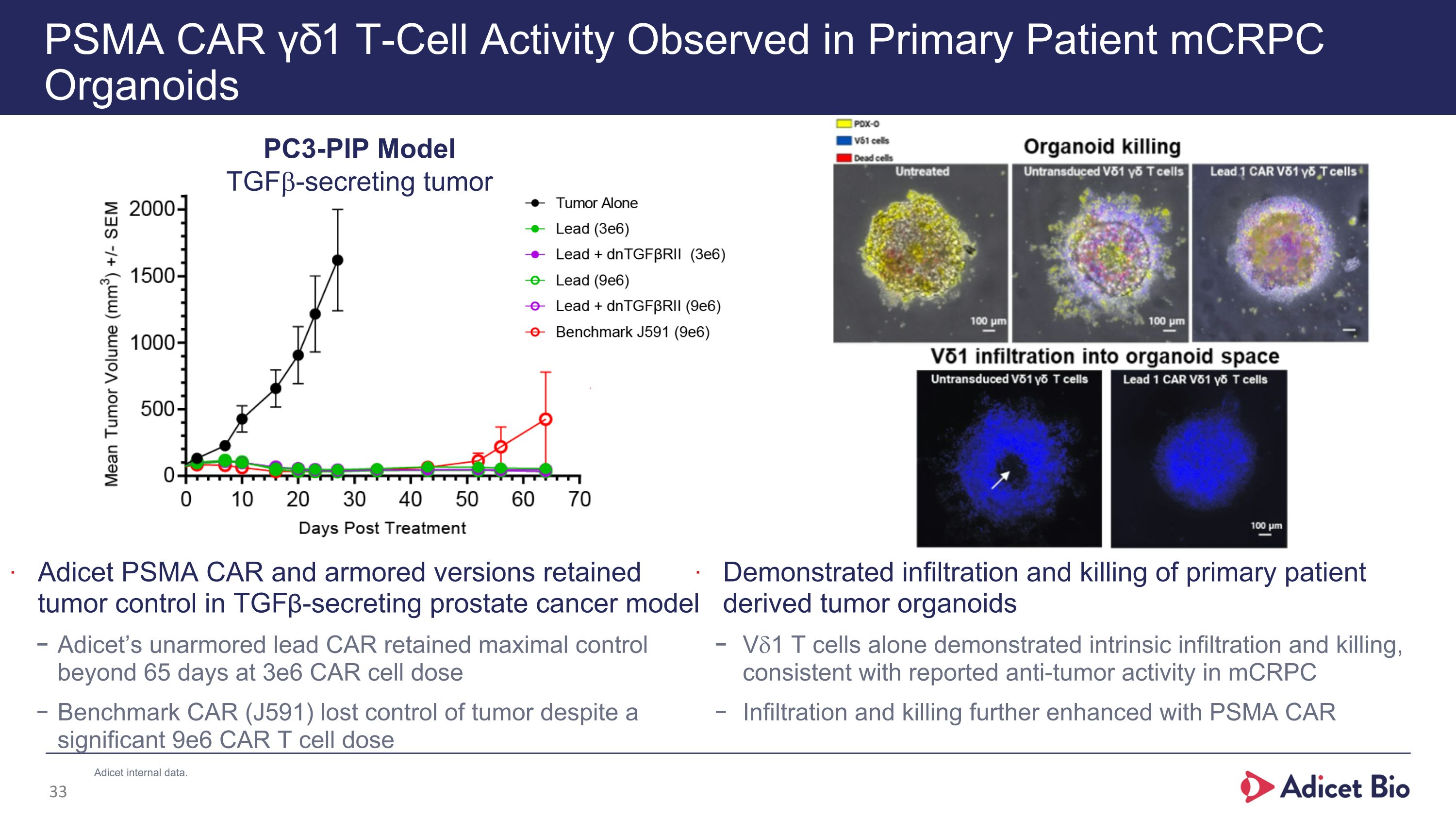
PSMA CAR γδ1 T-Cell Activity Observed in Primary Patient mCRPC Organoids Adicet PSMA CAR and armored versions retained tumor control in TGFβ-secreting prostate cancer model Adicet’s unarmored lead CAR retained maximal control beyond 65 days at 3e6 CAR cell dose Benchmark CAR (J591) lost control of tumor despite a significant 9e6 CAR T cell dose PC3-PIP Model TGFb-secreting tumor Demonstrated infiltration and killing of primary patient derived tumor organoids Vd1 T cells alone demonstrated intrinsic infiltration and killing, consistent with reported anti-tumor activity in mCRPC Infiltration and killing further enhanced with PSMA CAR Adicet internal data.
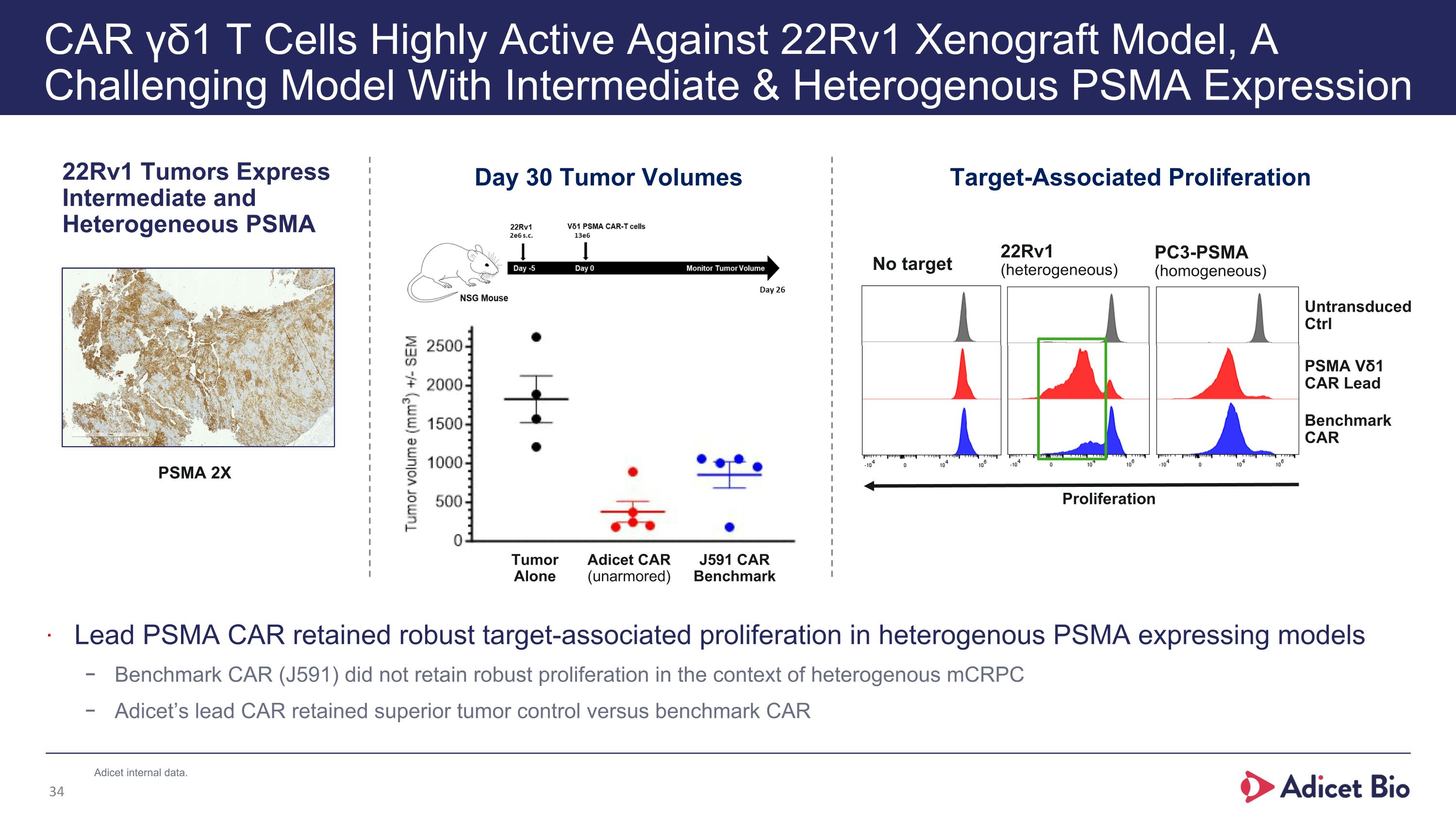
CAR γδ1 T Cells Highly Active Against 22Rv1 Xenograft Model, A Challenging Model With Intermediate & Heterogenous PSMA Expression 22Rv1 Tumors Express Intermediate and Heterogeneous PSMA Target-Associated Proliferation 22Rv1 (heterogeneous) PC3-PSMA (homogeneous) No target Untransduced Ctrl PSMA Vδ1 CAR Lead Benchmark CAR Proliferation Lead PSMA CAR retained robust target-associated proliferation in heterogenous PSMA expressing models Benchmark CAR (J591) did not retain robust proliferation in the context of heterogenous mCRPC Adicet’s lead CAR retained superior tumor control versus benchmark CAR Day 30 Tumor Volumes Tumor Alone Adicet CAR (unarmored) J591 CAR Benchmark PSMA 2X Adicet internal data.
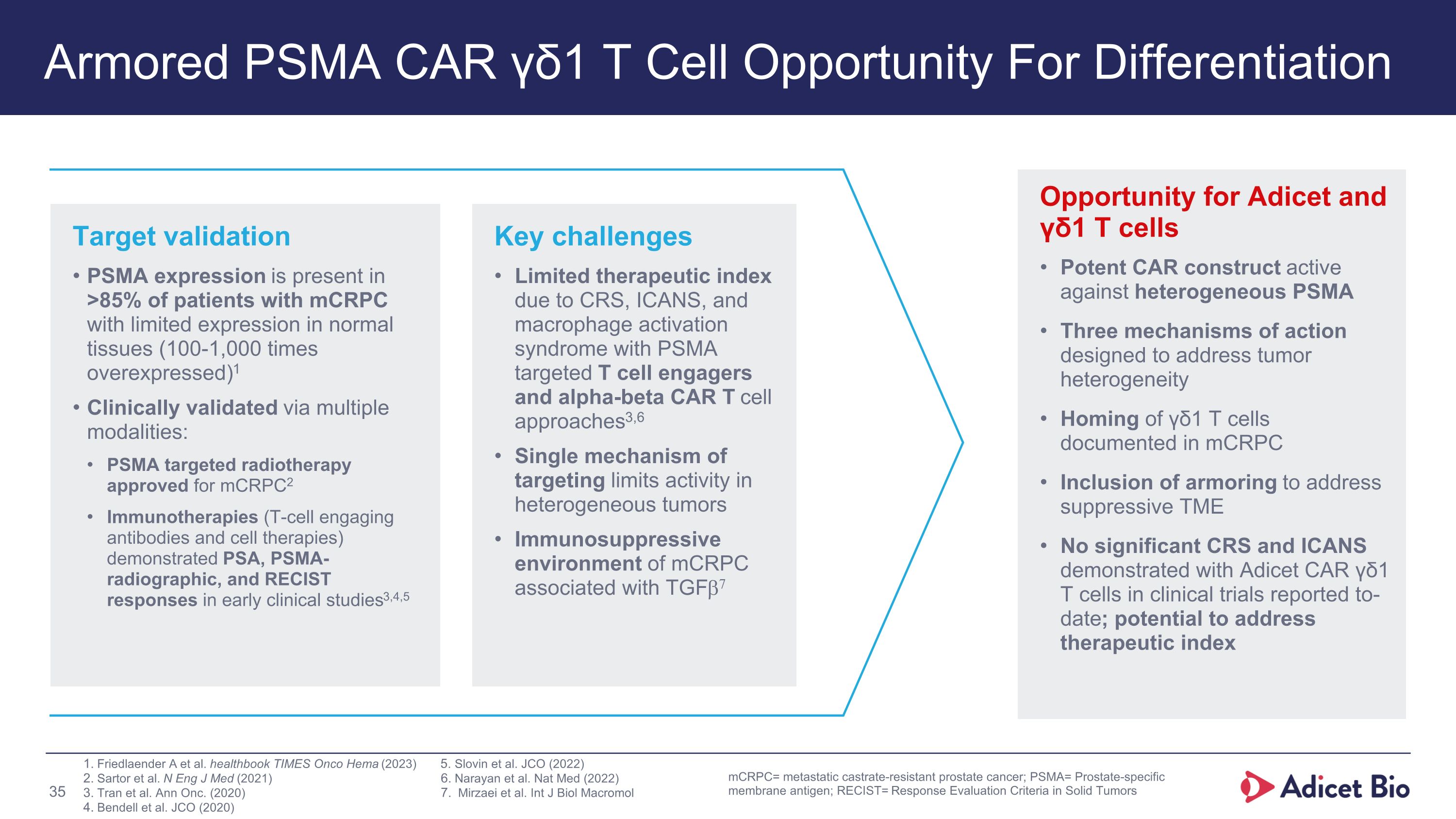
Armored PSMA CAR γδ1 T Cell Opportunity For Differentiation Opportunity for Adicet and γδ1 T cells Potent CAR construct active against heterogeneous PSMA Three mechanisms of action designed to address tumor heterogeneity Homing of γδ1 T cells documented in mCRPC Inclusion of armoring to address suppressive TME No significant CRS and ICANS demonstrated with Adicet CAR γδ1 T cells in clinical trials reported to-date; potential to address therapeutic index Key challenges Limited therapeutic index due to CRS, ICANS, and macrophage activation syndrome with PSMA targeted T cell engagers and alpha-beta CAR T cell approaches3,6 Single mechanism of targeting limits activity in heterogeneous tumors Immunosuppressive environment of mCRPC associated with TGFb7 Target validation PSMA expression is present in >85% of patients with mCRPC with limited expression in normal tissues (100-1,000 times overexpressed)1 Clinically validated via multiple modalities: PSMA targeted radiotherapy approved for mCRPC2 Immunotherapies (T-cell engaging antibodies and cell therapies) demonstrated PSA, PSMA-radiographic, and RECIST responses in early clinical studies3,4,5 Slovin et al. JCO (2022) Narayan et al. Nat Med (2022) Mirzaei et al. Int J Biol Macromol mCRPC= metastatic castrate-resistant prostate cancer; PSMA= Prostate-specific membrane antigen; RECIST= Response Evaluation Criteria in Solid Tumors Friedlaender A et al. healthbook TIMES Onco Hema (2023) Sartor et al. N Eng J Med (2021) Tran et al. Ann Onc. (2020) Bendell et al. JCO (2020)
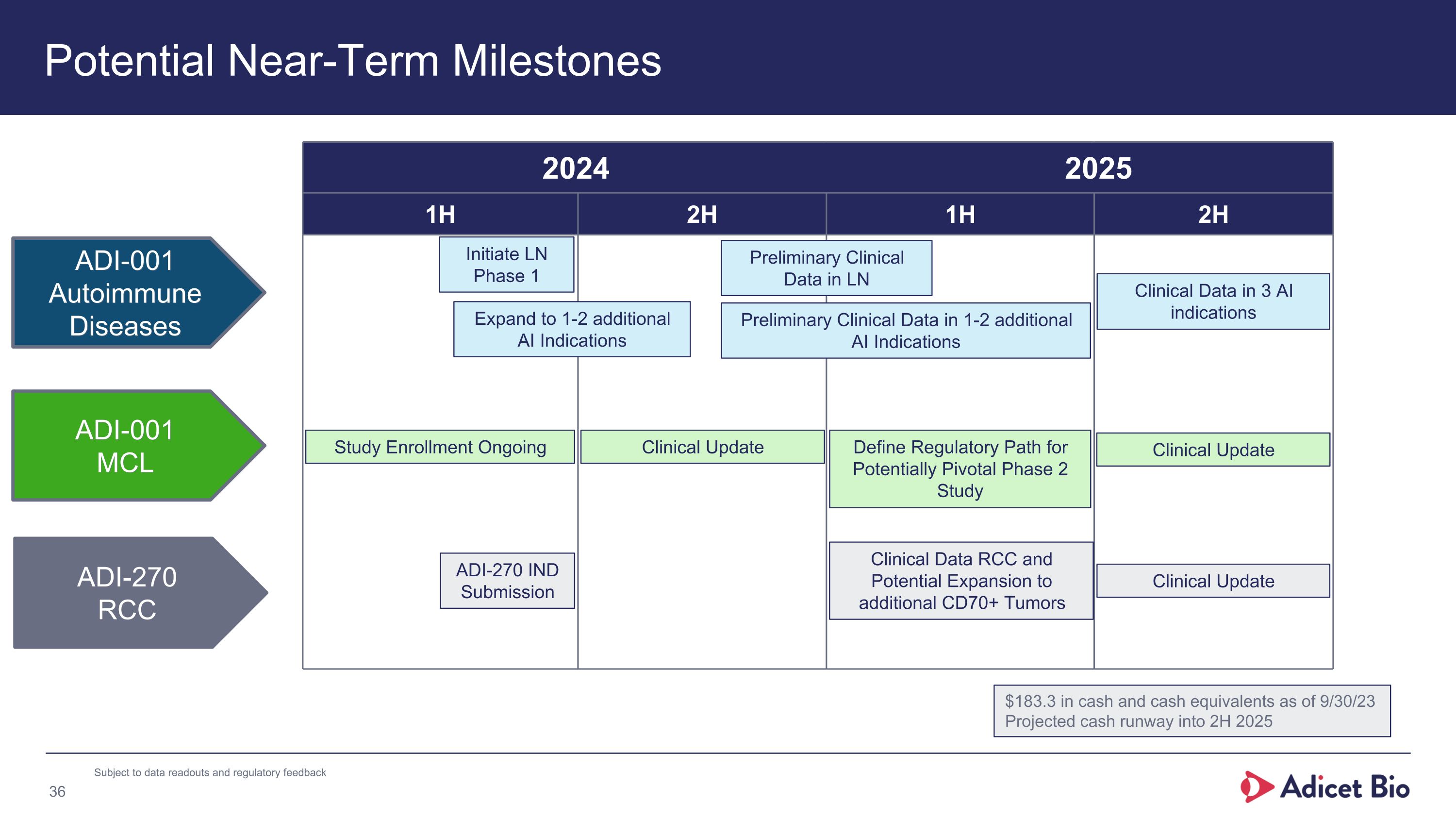
Potential Near-Term Milestones 2024 2025 1H 2H 1H 2H ADI-001 MCL ADI-001 Autoimmune Diseases ADI-270 RCC Clinical Update ADI-270 IND Submission Study Enrollment Ongoing Initiate LN Phase 1 Expand to 1-2 additional AI Indications Preliminary Clinical Data in LN Preliminary Clinical Data in 1-2 additional AI Indications Clinical Data RCC and Potential Expansion to additional CD70+ Tumors Clinical Update Clinical Data in 3 AI indications $183.3 in cash and cash equivalents as of 9/30/23 Projected cash runway into 2H 2025 Define Regulatory Path for Potentially Pivotal Phase 2 Study Clinical Update Subject to data readouts and regulatory feedback

Leaders in Developing Allogeneic CAR γδ1 Cell Therapies to Fight Autoimmune Diseases and Cancer
v3.23.4
Document And Entity Information
|
Jan. 16, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 16, 2024
|
| Entity Registrant Name |
Adicet Bio, Inc.
|
| Entity Central Index Key |
0001720580
|
| Entity Emerging Growth Company |
false
|
| Entity File Number |
001-38359
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
81-3305277
|
| Entity Address, Address Line One |
200 Berkeley Street, 19th Floor
|
| Entity Address, City or Town |
Boston
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02116
|
| City Area Code |
(650)
|
| Local Phone Number |
503-9095
|
| Entity Information, Former Legal or Registered Name |
Not applicable
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.0001 per share
|
| Trading Symbol |
ACET
|
| Security Exchange Name |
NONE
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
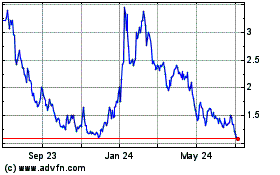
Adicet Bio (NASDAQ:ACET)
Historical Stock Chart
From Jun 2024 to Jul 2024
Adicet Bio (NASDAQ:ACET)
Historical Stock Chart
From Jul 2023 to Jul 2024