NASDAQ false 0001422143 0001422143 2024-01-30 2024-01-30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 30, 2024
KURA ONCOLOGY, INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-37620 |
|
61-1547851 |
| (State or Other Jurisdiction of Incorporation) |
|
(Commission
File Number) |
|
(IRS Employer Identification No.) |
|
|
| 12730 High Bluff Drive, Suite 400, San Diego, CA |
|
92130 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (858) 500-8800
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instructions A.2. below):
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange
on which registered |
| Common Stock, par value $0.0001 per share |
|
KURA |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02 |
Results of Operations and Financial Condition. |
On January 30, 2024, Kura Oncology, Inc. (the “Company”) announced that its preliminary unaudited cash, cash equivalents and short-term investments as of December 31, 2023 were approximately $424 million and that the net proceeds from its recent private placement that closed on January 26, 2024 were approximately $146 million.
The preliminary unaudited cash position discussed above is subject to the completion of financial closing procedures and other developments that may arise between now and the time the financial results for the fourth quarter of 2023 are finalized, as well as the completion of the audit of the 2023 financial statements. Therefore, actual results may differ materially from these estimates. In addition, the above estimates do not present all information necessary for an understanding of the Company’s financial condition as of December 31, 2023.
The information contained in this Current Report on Form 8-K under Item 2.02 is being furnished and shall not be deemed to be “filed” for the purposes of Section 18 of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section and will not be incorporated by reference into any filing by the Company under the Securities Act of 1933, as amended, or the Exchange Act, unless specifically identified as being incorporated therein by reference.
On January 30, 2024, the Company reported preliminary clinical data from the first 20 patients in KOMET-007, a Phase 1 dose-escalation trial of the Company’s potent and selective menin inhibitor, ziftomenib, in combination with standards of care, including cytarabine/daunorubicin (“7+3”) and venetoclax/azacitidine (“ven/aza”), in patients with NPM1-mutant (“NPM1-m”) and KMT2A-rearranged (“KMT2A-r”) acute myeloid leukemia (“AML”).
The first 20 patients were enrolled in KOMET-007 between July 2023 and November 2023, including five newly diagnosed patients with adverse risk1 NPM1-m or KMT2A-r AML and 15 patients with refractory/relapsed (“R/R”) NPM1-m or KMT2A-r AML.
Continuous daily dosing of ziftomenib at 200 mg QD has been well tolerated and the safety profile is consistent with features of underlying disease and backbone therapies. No differentiation syndrome events of any grade were reported, and no dose-limiting toxicities, evidence of QTc prolongation, drug-drug interactions or additive myelosuppression were observed.
As of the data cutoff on January 11, 2024, all newly diagnosed patients treated with ziftomenib and 7+3 achieved a complete remission (“CR”) with full count recovery, for a CR rate of 100% (5/5), including four patients with NPM1-m AML and one patient with KMT2A-r AML.
The overall response rate (“ORR”) among R/R patients treated with ziftomenib and ven/aza was 53% (8/15). Among all patients treated with ziftomenib and ven/aza, 40% (6/15) received prior treatment with a menin inhibitor. The CR/CRh2 rate in patients who were menin inhibitor naïve was 56% (5/9), including 60% (3/5) in patients with NPM1-m AML and 50% (2/4) in patients with KMT2A-r AML. The ORR in patients who received prior venetoclax was 40% (4/10), including 60% (3/5) in patients with NPM1-m AML.
As of the data cutoff, 80% (16/20) of patients remain on trial, including 100% (11/11) of all NPM1-m patients.
The 200 mg dose of ziftomenib has been cleared in the R/R ven/aza cohorts and enrollment at the 400 mg dose is ongoing. Upon determination of a recommended Phase 2 dose, the Company plans to initiate a Phase 1b dose validation/expansion in combination with ven/aza in newly diagnosed patients with NPM1-m (without adverse risk) or KMT2A-r AML.
| 1 |
Age > 60 years and/or treatment-related AML and/or adverse risk cytogenetics per European LeukemiaNet |
| 2 |
CR with partial hematologic recovery |
The Company expects to complete enrollment of all 85 patients in KOMET-001, the Company’s Phase 2 registration-directed trial of ziftomenib in patients with R/R NPM1-m AML, by the middle of this year.
The Company’s current cash, cash equivalents and investments, including the proceeds from the Company’s recently announced private placement, are expected to fund operations into 2027.
On January 30, 2024, the Company will host a virtual investor event and present certain materials related to the Company (the “Presentation”). A copy of the Presentation is attached hereto as Exhibit 99.1 and incorporated herein by reference.
Forward-Looking Statements
Statements contained in this Current Report on Form 8-K regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements, including, but not limited to, statements regarding, among other things, the efficacy, safety and therapeutic potential of ziftomenib, potential benefits of combining ziftomenib with appropriate standards of care, plans regarding future clinical trials in the ziftomenib program, plans and expected timing for enrollment in the Phase 2 registration-directed trial of ziftomenib, and the Company’s cash runway.
Any forward-looking statements in this Current Report on Form 8-K are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include: the risk that compounds that appeared promising in early research or clinical trials do not demonstrate safety and/or efficacy in later preclinical studies or clinical trials, the risk that the Company may not obtain approval to market its product candidates, uncertainties associated with performing clinical trials, regulatory filings, applications and other interactions with regulatory bodies, risks associated with reliance on third parties to successfully conduct clinical trials, risks associated with the Company’s cash needs, the risks associated with reliance on outside financing to meet capital requirements, and other risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such drugs, as well as those risks and uncertainties set forth more fully under the caption “Risk Factors” in the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2023 filed with the Securities and Exchange Commission (“SEC”) on November 2, 2023, as well as discussions of potential risks, uncertainties and other important factors in the Company’s other filings and reports with the SEC. All forward-looking statements contained in this Current Report on Form 8-K speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
KURA ONCOLOGY, INC. |
|
|
|
|
| Date: January 30, 2024 |
|
|
|
By: |
|
/s/ Teresa Bair |
|
|
|
|
|
|
Teresa Bair |
|
|
|
|
|
|
Chief Legal Officer |

Exhibit 99.1 DEVELOPING PRECISION MEDICINES FOR THE TREATMENT OF CANCER
Preliminary Data from KOMET-007 – January 30, 2024

WELCOME AND INTRODUCTION Troy Wilson, Ph.D., J.D. – President
& Chief Executive Officer, Kura Oncology

Forward-Looking Statements This presentation contains forward-looking
statements. Such statements include, but are not limited to, statements regarding our research, preclinical and clinical development activities, plans and projected timelines for ziftomenib, tipifarnib and KO-2806, plans regarding regulatory
filings, our expectations regarding the relative benefits of our product candidates versus competitive therapies, our expectations regarding the therapeutic and commercial potential of our product candidates, our expectations regarding our cash
runway, and our expectations regarding our intended use of the net proceeds from the private placement that closed on January 26, 2024. The words “believe,” “may,” “should,” “will,”
“estimate,” “promise,” “plan”, “continue,” “anticipate,” “intend,” “expect,” “potential” and similar expressions (including the negative thereof), are
intended to identify forward-looking statements. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Risks that contribute to the
uncertain nature of the forward-looking statements include: our preclinical studies and clinical trials may not be successful; the U.S. Food and Drug Administration (FDA) may not agree with our interpretation of the data from clinical trials of our
product candidates; we may decide, or the FDA may require us, to conduct additional clinical trials or to modify our ongoing clinical trials; we may experience delays in the commencement, enrollment, completion or analysis of clinical testing for
our product candidates, or significant issues regarding the adequacy of our clinical trial designs or the execution of our clinical trials may arise, which could result in increased costs and delays, or limit our ability to obtain regulatory
approval; the commencement, enrollment and completion of clinical trials and the reporting of data therefrom; the COVID-19 pandemic may disrupt our business and that of the third parties on which we depend, including delaying or otherwise disrupting
our clinical trials and preclinical studies, manufacturing and supply chain, or impairing employee productivity; our product candidates may not receive regulatory approval or be successfully commercialized; unexpected adverse side effects or
inadequate therapeutic efficacy of our product candidates could delay or prevent regulatory approval or commercialization; we may not be able to obtain additional financing; risks associated with market conditions and the satisfaction of closing
conditions related to the private placement; risks associated with our cash needs; and risks and uncertainties associated with our business and finances in general. Additional risks and uncertainties may emerge from time to time, and it is not
possible for Kura’s management to predict all risk factors and uncertainties. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Other risks and uncertainties affecting us are
described more fully in our filings with the Securities and Exchange Commission. We undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. This presentation
also contains statistical and clinical data obtained from and prepared by third parties. The recipient is cautioned not to give undue weight to such disclosures. Neither the Company nor any other person makes any representation as to the accuracy or
completeness of such data or undertakes any obligation to update such data after the date of this presentation. 3
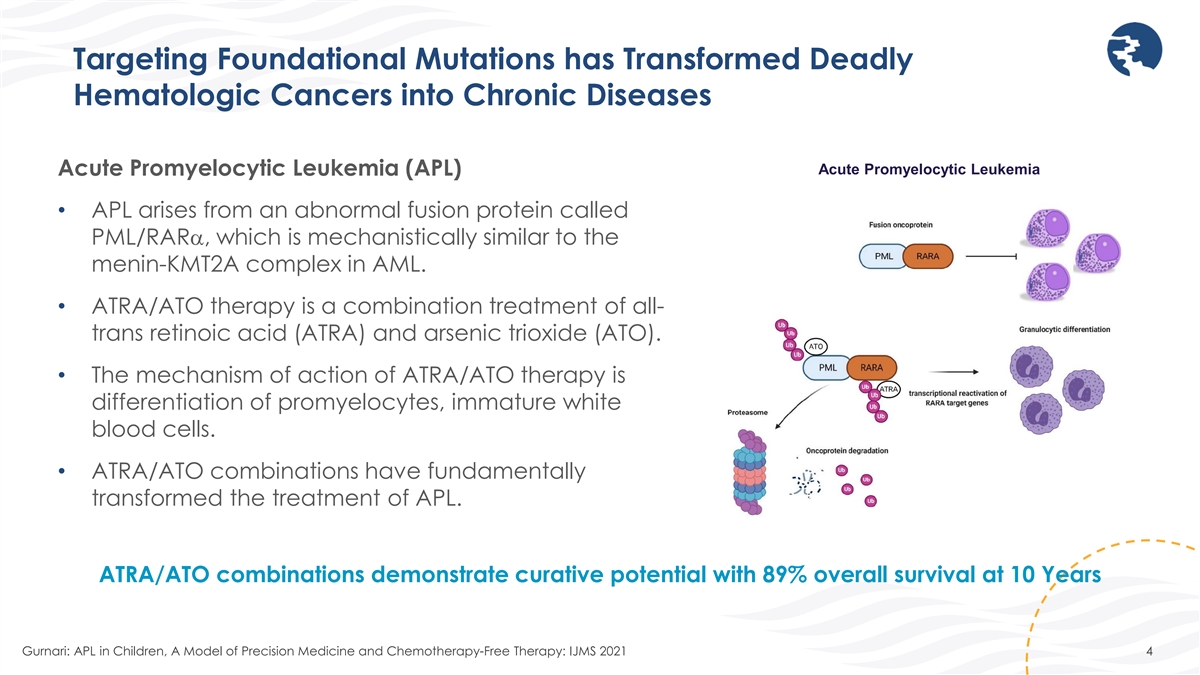
Targeting Foundational Mutations has Transformed Deadly Hematologic
Cancers into Chronic Diseases Acute Promyelocytic Leukemia Acute Promyelocytic Leukemia (APL) • APL arises from an abnormal fusion protein called PML/RARα, which is mechanistically similar to the menin-KMT2A complex in AML. •
ATRA/ATO therapy is a combination treatment of all- trans retinoic acid (ATRA) and arsenic trioxide (ATO). • The mechanism of action of ATRA/ATO therapy is differentiation of promyelocytes, immature white blood cells. • ATRA/ATO
combinations have fundamentally transformed the treatment of APL. ATRA/ATO combinations demonstrate curative potential with 89% overall survival at 10 Years Gurnari: APL in Children, A Model of Precision Medicine and Chemotherapy-Free Therapy: IJMS
2021 4
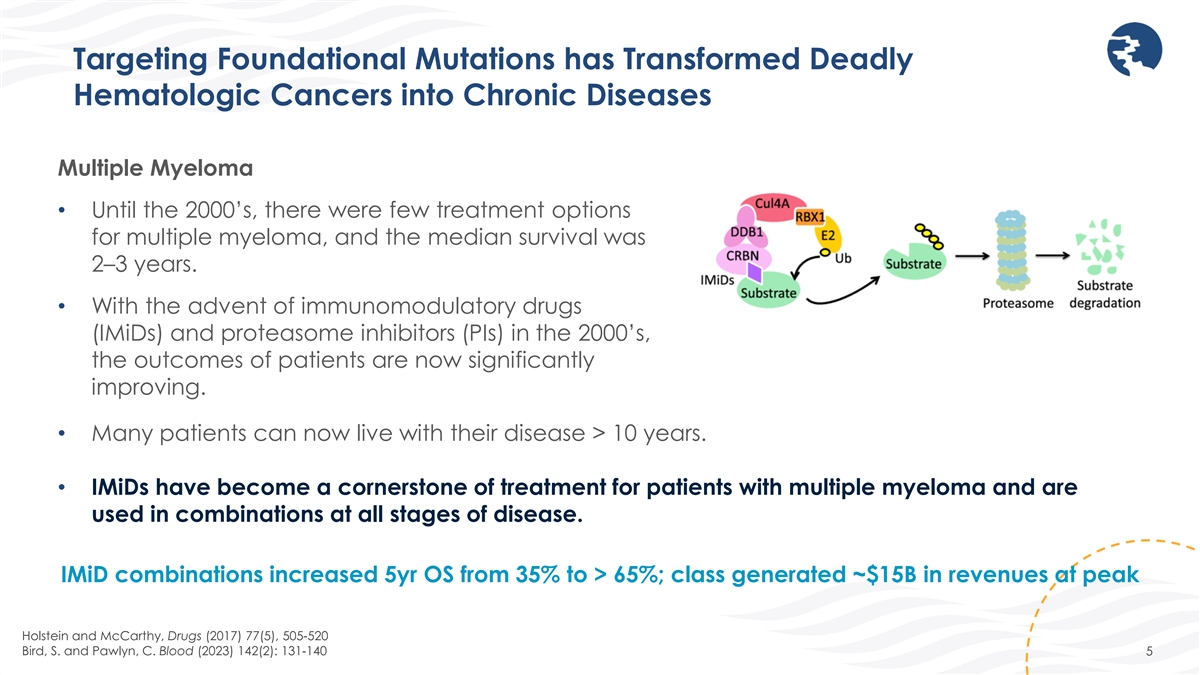
Targeting Foundational Mutations has Transformed Deadly Hematologic
Cancers into Chronic Diseases Multiple Myeloma • Until the 2000’s, there were few treatment options for multiple myeloma, and the median survival was 2–3 years. • With the advent of immunomodulatory drugs (IMiDs) and
proteasome inhibitors (PIs) in the 2000’s, the outcomes of patients are now significantly improving. • Many patients can now live with their disease > 10 years. • IMiDs have become a cornerstone of treatment for patients with
multiple myeloma and are used in combinations at all stages of disease. IMiD combinations increased 5yr OS from 35% to > 65%; class generated ~$15B in revenues at peak Holstein and McCarthy, Drugs (2017) 77(5), 505-520 Bird, S. and Pawlyn, C.
Blood (2023) 142(2): 131-140 5

ZIFTOMENIB OPPORTUNITY AND INTRODUCTION TO KOMET-007 INVESTIGATORS
Stephen Dale, M.D. – Chief Medical Officer, Kura Oncology
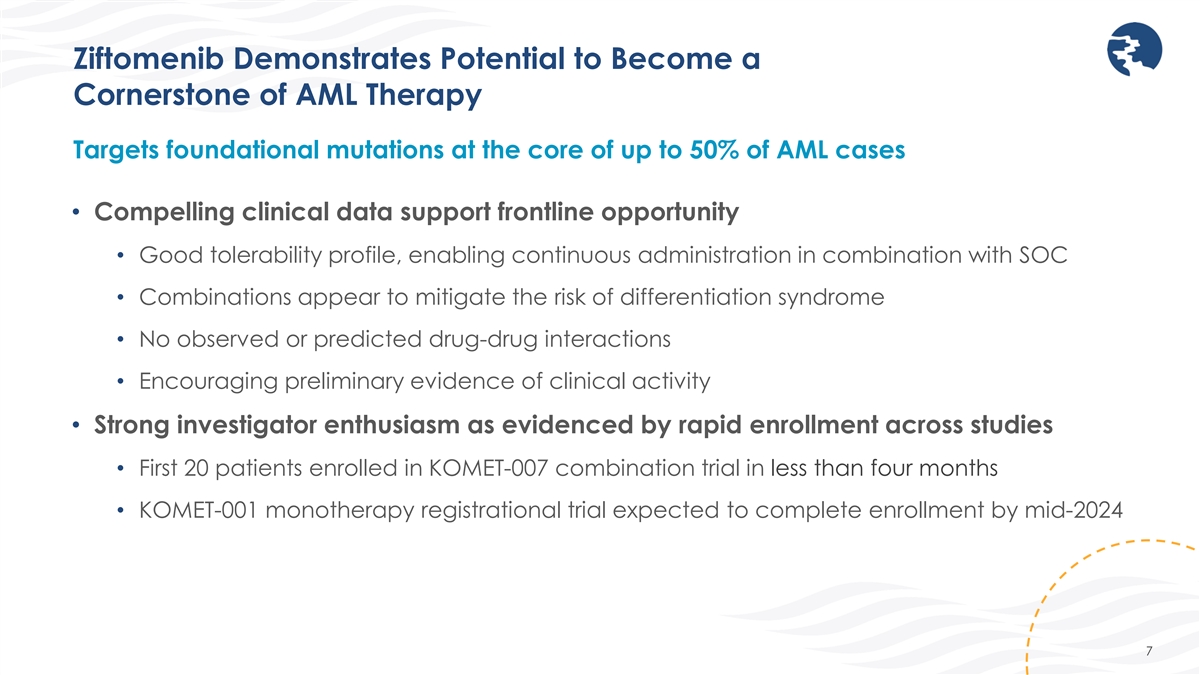
Ziftomenib Demonstrates Potential to Become a Cornerstone of AML Therapy
Targets foundational mutations at the core of up to 50% of AML cases • Compelling clinical data support frontline opportunity • Good tolerability profile, enabling continuous administration in combination with SOC • Combinations
appear to mitigate the risk of differentiation syndrome • No observed or predicted drug-drug interactions • Encouraging preliminary evidence of clinical activity • Strong investigator enthusiasm as evidenced by rapid enrollment
across studies • First 20 patients enrolled in KOMET-007 combination trial in less than four months • KOMET-001 monotherapy registrational trial expected to complete enrollment by mid-2024 7

KOMET-007 Investigators Amir Fathi, M.D. Amer Zeidan, MBBS •
Program Director, Center for Leukemia, • Interim Chief, Division of Hematologic Massachusetts General Hospital Cancer Center Malignancies, Director of Hematology Early Therapeutics Research, Yale Cancer Center • Associate Professor of
Medicine, Harvard Medical School • Associate Professor of Medicine (Hematology), Yale University 8

ZIFTOMENIB AS MONOTHERAPY / OPPORTUNITY IN COMBINATION Amir Fathi, M.D.
– Massachusetts General Hospital

KOMET-001 Phase 1/2 Study of Ziftomenib in Relapsed/Refractory AML
Phase 1a Phase 1b Phase 2 Phase 1b Dose Escalation Expansion Registration-Enabling Validation Cohorts (Ongoing) Completed Completed Completed Ongoing Expansion of 600 mg QD Cohort 1: 200 mg QD 50 mg 100 mg 1000 mg 600 mg QD QD QD QD Cohort 2: 600 mg
QD NPM1-m, KMT2A-r, Other NPM1-m NPM1-m NPM1-m or KMT2A-r OBJECTIVES • Safety and tolerability Continue enrollment of Phase • Primary endpoint: • Safety and tolerability • CR/CRh • Pharmacokinetics 1b validation
cohort(s) • Pharmacokinetics • Early evidence of antitumor consistent with FDA’s Project • Clinical activity • Secondary endpoints: activity Optimus • Duration of CR/CRh • Safety and tolerability •
Transfusion independence • Pharmacokinetics • CR/CRh MRD negativity • Adverse events • Clinical activity CR, complete remission; CRh, complete remission with partial hematological recovery; FDA, United States Food and Drug
Administration; MRD, measurable residual disease; R/R, relapsed/refractory; RP2D, recommended phase 2 dose. 10 ~ ~
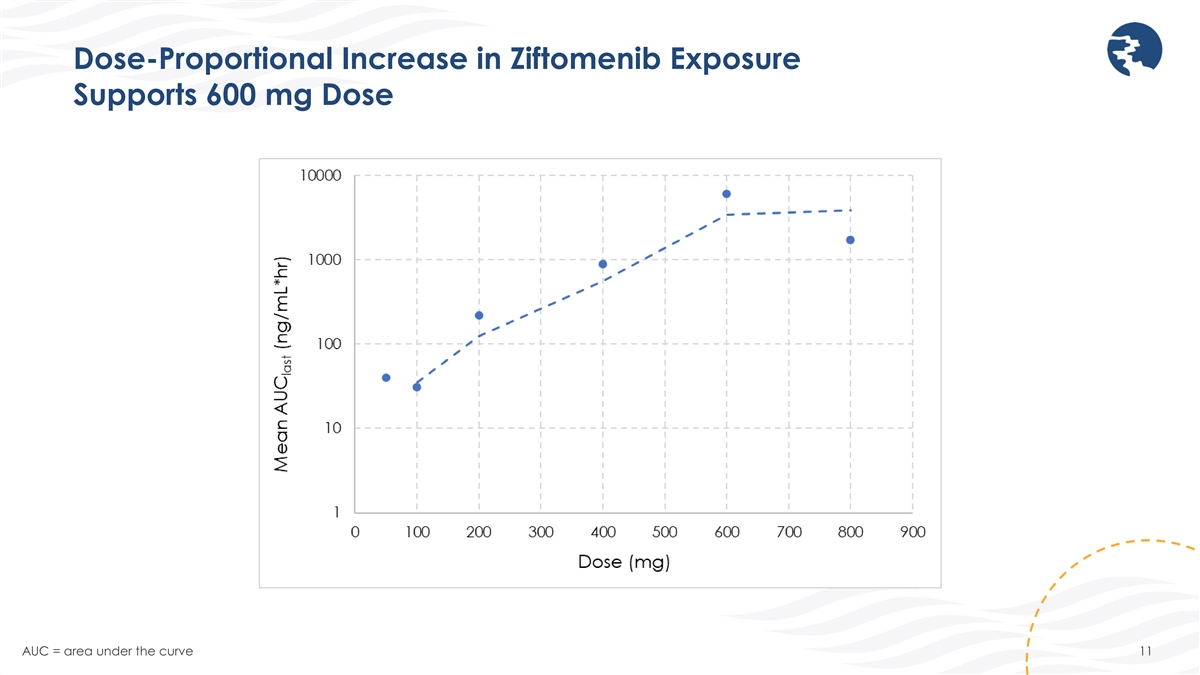
Dose-Proportional Increase in Ziftomenib Exposure Supports 600 mg Dose
AUC = area under the curve 11
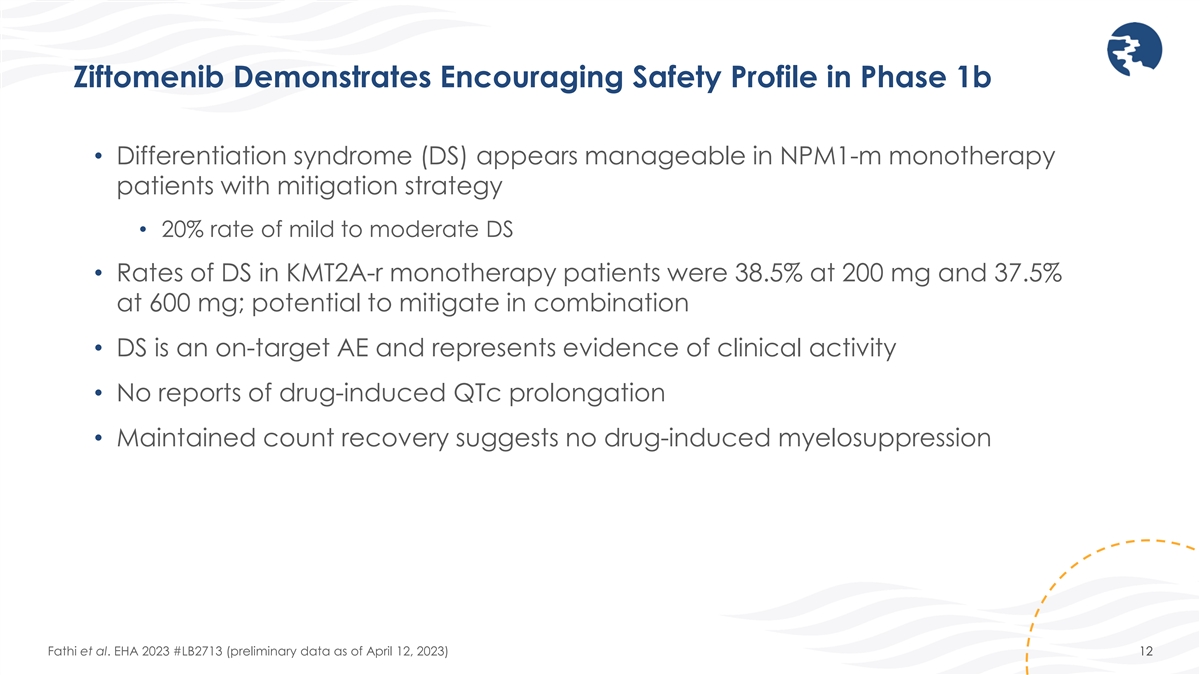
Ziftomenib Demonstrates Encouraging Safety Profile in Phase 1b •
Differentiation syndrome (DS) appears manageable in NPM1-m monotherapy patients with mitigation strategy • 20% rate of mild to moderate DS • Rates of DS in KMT2A-r monotherapy patients were 38.5% at 200 mg and 37.5% at 600 mg; potential
to mitigate in combination • DS is an on-target AE and represents evidence of clinical activity • No reports of drug-induced QTc prolongation • Maintained count recovery suggests no drug-induced myelosuppression Fathi et al. EHA
2023 #LB2713 (preliminary data as of April 12, 2023) 12

Ziftomenib has Highly Differentiated Monotherapy Activity Best Overall
Response 600 mg Differentiated CR Rates vs. SOC in NPM1-m Phase 1a + 1b (n=20) 40% of NPM1 Heavily Pretreated Patients patients CR 7 (35.0) achieved a MEDIAN CR/CRh 7 (35.0) MUTATION CR % mDOR CR during PRIORS course of CRc 8 (40.0) NPM1m 8.2 mo*
35% study Ziftomenib 1 MRD negativity 4(50.0) FLT3m - 3 33% 600mg QD IDH 1/2 - 50% ORR 9 (45.0) Gilteritinib FLT3m 14.2% 14.8 mo 1 KMT2A-r Phase 1a + 1b (n=18) Enasidenib IDH2 19% 8.2 mo 2 CR/CRh 2 (11.1) Ivosidenib IDH1 25% 10.1 mo 2 CRc 3 (16.7)
*Median DoR for CRc without censoring at HSCT MRD negativity 3 (100.0) Source: USPI’s ORR 3 (16.7) (preliminary data as of April 10, 2023) Ø High activity, durable responses and favorable profile suggest potential for ziftomenib to become
a backbone therapy across the continuum of AML care 1 MRD was assessed for 6/8 CRc patients; 4 of those 6 patients (67%) tested were MRD negative 13 CRc includes CR, CRh, CRi, CRp; ORR includes CR, CRh, CRi, CRp, MLFS
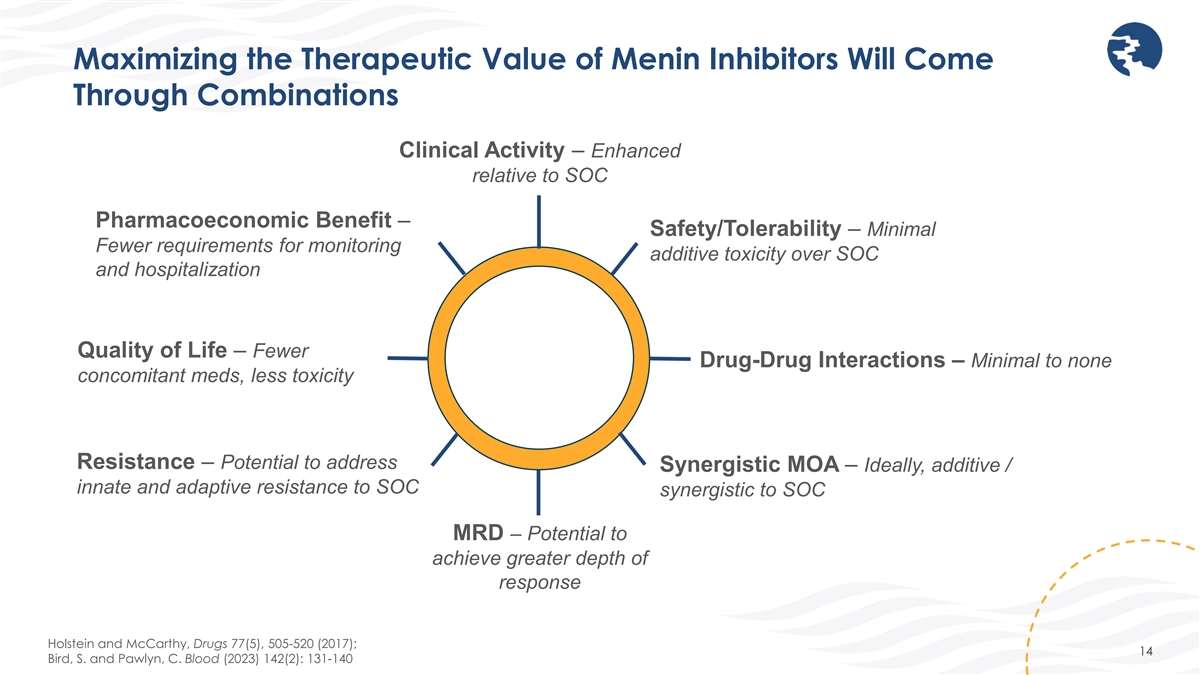
Maximizing the Therapeutic Value of Menin Inhibitors Will Come Through
Combinations Clinical Activity – Enhanced relative to SOC Pharmacoeconomic Benefit – Safety/Tolerability – Minimal Fewer requirements for monitoring additive toxicity over SOC and hospitalization Quality of Life – Fewer
Drug-Drug Interactions – Minimal to none concomitant meds, less toxicity Resistance – Potential to address Synergistic MOA – Ideally, additive / innate and adaptive resistance to SOC synergistic to SOC MRD – Potential to
achieve greater depth of response Holstein and McCarthy, Drugs 77(5), 505-520 (2017); 14 Bird, S. and Pawlyn, C. Blood (2023) 142(2): 131-140

PRELIMINARY COMBINATION DATA FROM KOMET-007 TRIAL Amer Zeidan, MBBS
– Yale Cancer Center Disclosure: Honoraria or consultation fees provided by Kura Oncology

KOMET-007: Phase 1 Combination Trial of Ziftomenib in Patients with
Newly Diagnosed or R/R AML Ziftomenib/cytarabine/daunorubicin (7+3) combination • Ziftomenib dosing will begin on Cycle 1 Day 8 and be administered continuously thereafter • Cytarabine will be administered on C1 Day 1-7; administration
of an additional cycle based on C1 bone marrow biopsy results • Daunorubicin will be administered on C1 Day 1- 3; administration of an additional cycle based on C1 bone marrow biopsy results • Dose escalation conducted in patients with
adverse risk* *Age > 60 years and/or treatment-related AML and/or adverse risk cytogenetics per ELN 16 DL = ziftomenib dose level; zifto = ziftomenib; 7+3 = cytarabine/daunorubicin; RP2D = recommended Phase 2 dose; 1L = first-line; IC = intensive
chemotherapy
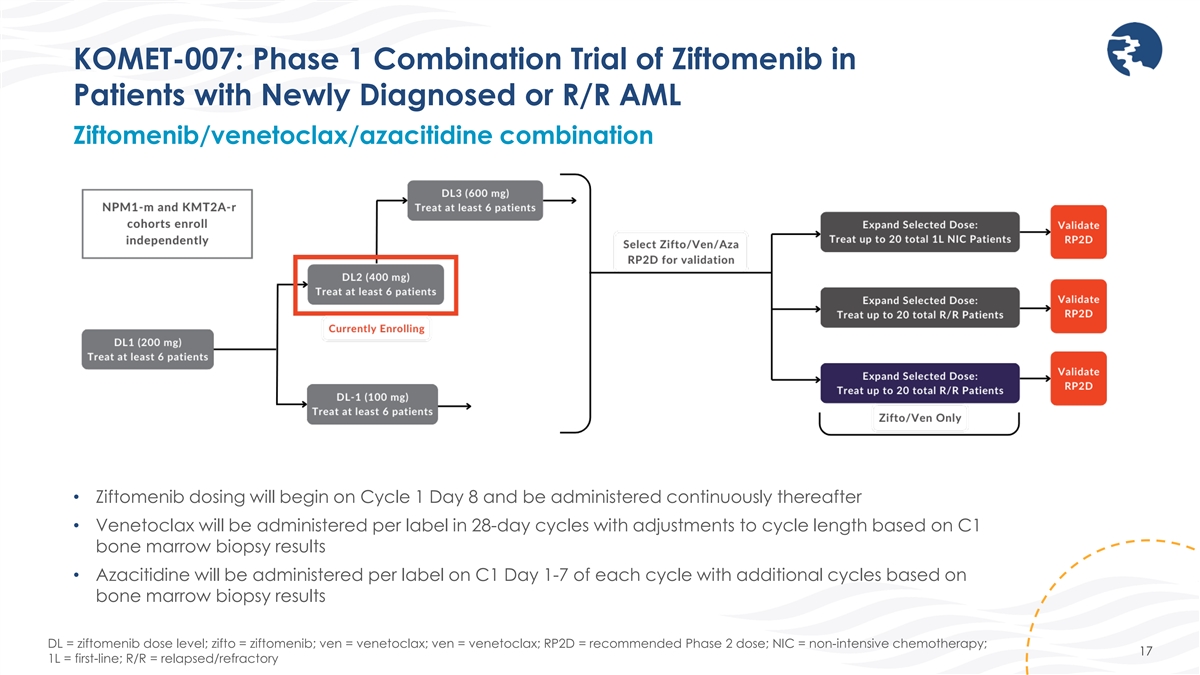
KOMET-007: Phase 1 Combination Trial of Ziftomenib in Patients with
Newly Diagnosed or R/R AML Ziftomenib/venetoclax/azacitidine combination • Ziftomenib dosing will begin on Cycle 1 Day 8 and be administered continuously thereafter • Venetoclax will be administered per label in 28-day cycles with
adjustments to cycle length based on C1 bone marrow biopsy results • Azacitidine will be administered per label on C1 Day 1-7 of each cycle with additional cycles based on bone marrow biopsy results DL = ziftomenib dose level; zifto =
ziftomenib; ven = venetoclax; ven = venetoclax; RP2D = recommended Phase 2 dose; NIC = non-intensive chemotherapy; 17 1L = first-line; R/R = relapsed/refractory
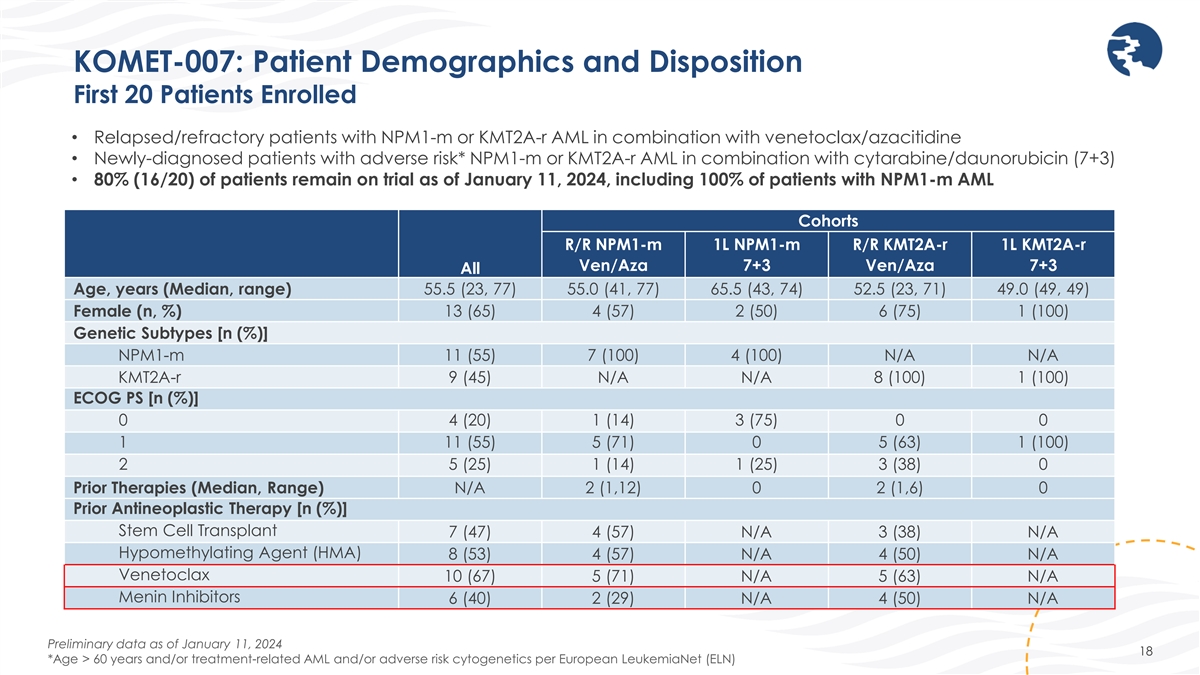
KOMET-007: Patient Demographics and Disposition First 20 Patients
Enrolled • Relapsed/refractory patients with NPM1-m or KMT2A-r AML in combination with venetoclax/azacitidine • Newly-diagnosed patients with adverse risk* NPM1-m or KMT2A-r AML in combination with cytarabine/daunorubicin (7+3) •
80% (16/20) of patients remain on trial as of January 11, 2024, including 100% of patients with NPM1-m AML Cohorts R/R NPM1-m 1L NPM1-m R/R KMT2A-r 1L KMT2A-r Ven/Aza 7+3 Ven/Aza 7+3 All Age, years (Median, range) 55.5 (23, 77) 55.0 (41, 77) 65.5
(43, 74) 52.5 (23, 71) 49.0 (49, 49) 13 (65) 4 (57) 2 (50) 6 (75) 1 (100) Female (n, %) Genetic Subtypes [n (%)] NPM1-m 11 (55) 7 (100) 4 (100) N/A N/A KMT2A-r 9 (45) N/A N/A 8 (100) 1 (100) ECOG PS [n (%)] 0 4 (20) 1 (14) 3 (75) 0 0 1 11 (55) 5
(71) 0 5 (63) 1 (100) 2 5 (25) 1 (14) 1 (25) 3 (38) 0 Prior Therapies (Median, Range) N/A 2 (1,12) 0 2 (1,6) 0 Prior Antineoplastic Therapy [n (%)] Stem Cell Transplant 7 (47) 4 (57) N/A 3 (38) N/A Hypomethylating Agent (HMA) 8 (53) 4 (57) N/A 4
(50) N/A Venetoclax 10 (67) 5 (71) N/A 5 (63) N/A Menin Inhibitors 6 (40) 2 (29) N/A 4 (50) N/A Preliminary data as of January 11, 2024 18 *Age > 60 years and/or treatment-related AML and/or adverse risk cytogenetics per European LeukemiaNet
(ELN)

KOMET-007: Promising Safety and Tolerability Profile in Combination
Combinations mitigate risk of differentiation syndrome (DS) Grade ≥ 3 TEAEs (≥ 10%) n (%) Grade ≥ 3 Ziftomenib-Related AEs (All) n (%) Patients with Grade ≥ 3 TEAEs 18 (90) Patients with Grade ≥ 3 Ziftomenib-Related AEs
6 (30) Platelet count decreased 6 (30) Platelet count decreased 3 (15) Febrile neutropenia 5 (25) Anemia 1 (5) White blood cell count decreased 4 (20) Febrile neutropenia 1 (5) Pneumonia 3 (15) Leukopenia 1 (5) Hypoxia 2 (10) Neutrophil count 1 (5)
Neutrophil count decreased 2 (10) Thrombocytopenia 1 (5) Sepsis 2 (10) Thrombocytopenia 2 (10) • No DS events reported • No dose-limiting toxicities (DLTs) observed to date, including delayed hematologic count recovery • No QTc
prolongation observed • TEAEs consistent with underlying disease and backbone therapies Preliminary data as of January 11, 2024 19 TEAEs = Treatment-emergent adverse events
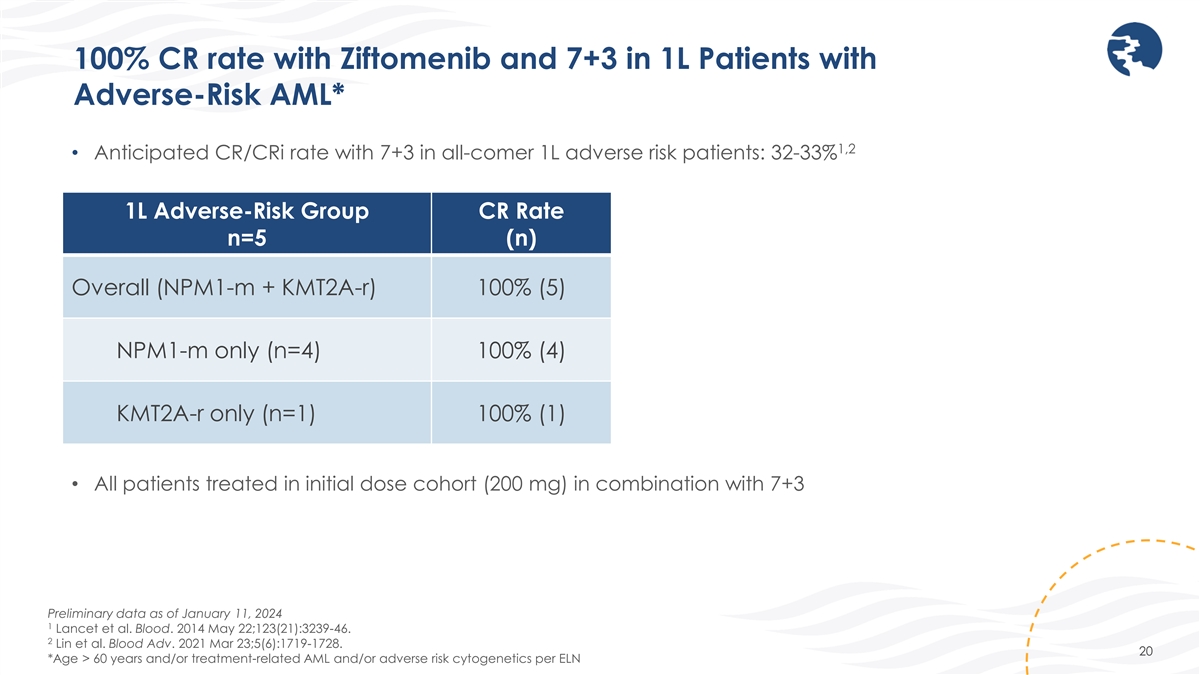
100% CR rate with Ziftomenib and 7+3 in 1L Patients with Adverse-Risk
AML* 1,2 • Anticipated CR/CRi rate with 7+3 in all-comer 1L adverse risk patients: 32-33% 1L Adverse-Risk Group CR Rate n=5 (n) Overall (NPM1-m + KMT2A-r) 100% (5) NPM1-m only (n=4) 100% (4) KMT2A-r only (n=1) 100% (1) • All patients
treated in initial dose cohort (200 mg) in combination with 7+3 Preliminary data as of January 11, 2024 1 Lancet et al. Blood. 2014 May 22;123(21):3239-46. 2 Lin et al. Blood Adv. 2021 Mar 23;5(6):1719-1728. 20 *Age > 60 years and/or
treatment-related AML and/or adverse risk cytogenetics per ELN

Ziftomenib + Ven/Aza with Pronounced Activity in Menin Inhibitor
Naïve Patients 1 • ~35-45% CR/CRi is expected in ven-naïve relapsed/refractory patients 2 • Anticipated response rate in KMT2A-r relapsed/refractory AML <10% ORR • 53% ORR in mITT population (n=15, including six menin
experienced patients) • 40% (6/15) of patients treated with ven/aza received prior treatment with a menin inhibitor Menin Inhibitor Naïve Group ORR CR/CRi Rate CR/CRh Rate n=9 (n) (n) (n) Overall (NPM1-m + KMT2A-r) 78% (7) 67% (6) 56% (5)
NPM1-m (n=5) 100% (5) 80% (4) 60% (3) KMT2A-r (n=4) 50% (2) 50% (2) 50% (2) • All patients treated in initial dose cohort (200 mg) in combination with Ven/Aza • Enrollment in 400 mg dose cohort ongoing Preliminary data as of January 11,
2024 1 Stahl, M. et al., Blood Advances 5(5), 1552-1564 (2021) 2 Issa, Syndax ASH Investor Event (Dec. 2023) 21 ORR includes CR, CRh, CRi, MLFS
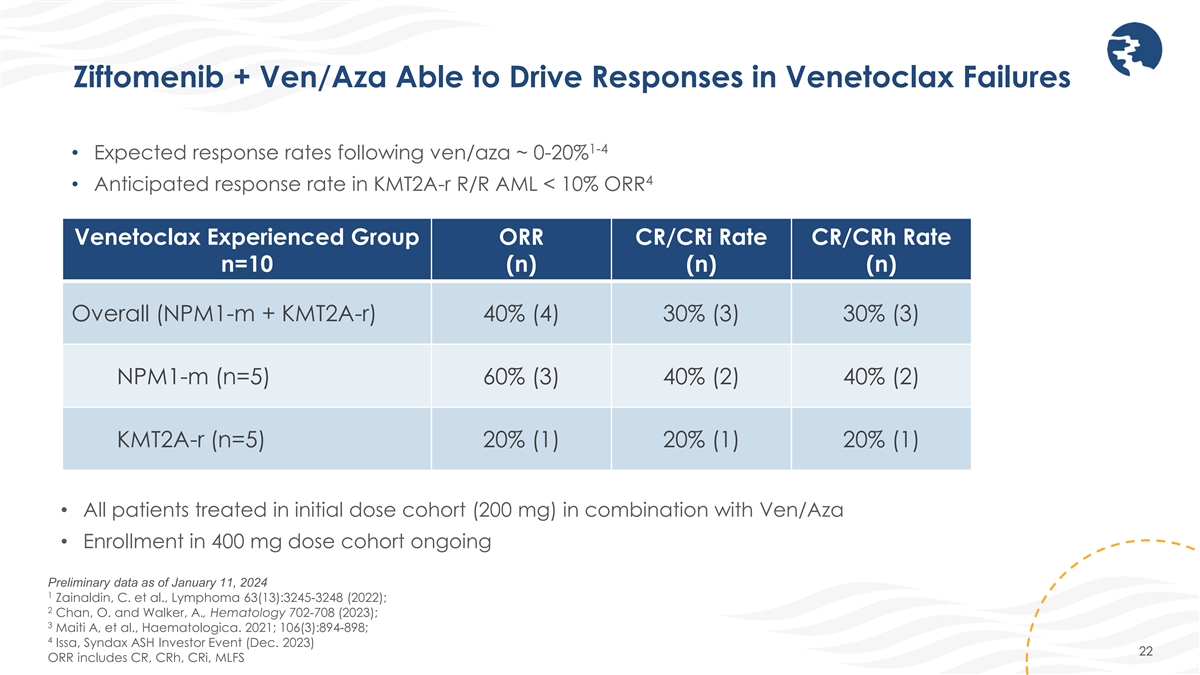
Ziftomenib + Ven/Aza Able to Drive Responses in Venetoclax Failures 1-4
• Expected response rates following ven/aza ~ 0-20% 4 • Anticipated response rate in KMT2A-r R/R AML < 10% ORR Venetoclax Experienced Group ORR CR/CRi Rate CR/CRh Rate n=10 (n) (n) (n) Overall (NPM1-m + KMT2A-r) 40% (4) 30% (3) 30%
(3) NPM1-m (n=5) 60% (3) 40% (2) 40% (2) KMT2A-r (n=5) 20% (1) 20% (1) 20% (1) • All patients treated in initial dose cohort (200 mg) in combination with Ven/Aza • Enrollment in 400 mg dose cohort ongoing Preliminary data as of January
11, 2024 1 Zainaldin, C. et al., Lymphoma 63(13):3245-3248 (2022); 2 Chan, O. and Walker, A., Hematology 702-708 (2023); 3 Maiti A, et al., Haematologica. 2021; 106(3):894-898; 4 Issa, Syndax ASH Investor Event (Dec. 2023) 22 ORR includes CR, CRh,
CRi, MLFS
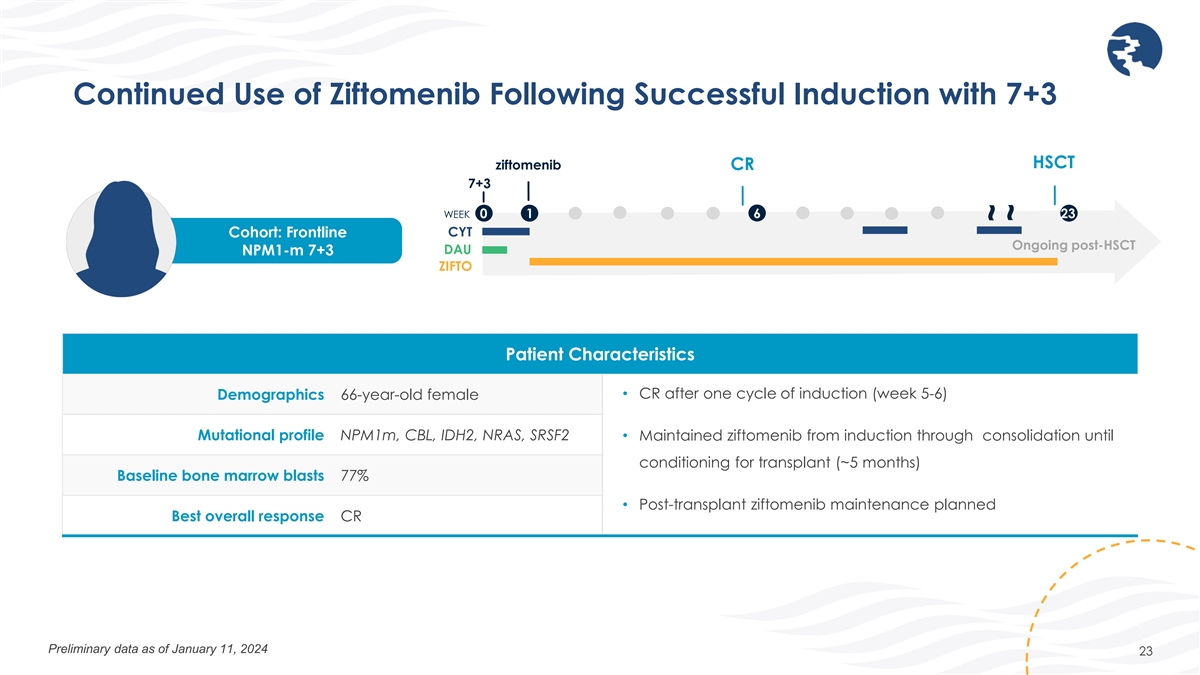
Continued Use of Ziftomenib Following Successful Induction with 7+3
HSCT ziftomenib CR 7+3 WEEK 0 1 6 23 CYT Cohort: Frontline Ongoing post-HSCT DAU NPM1-m 7+3 ZIFTO Patient Characteristics • CR after one cycle of induction (week 5-6) Demographics 66-year-old female Mutational profile NPM1m, CBL, IDH2, NRAS,
SRSF2 • Maintained ziftomenib from induction through consolidation until conditioning for transplant (~5 months) Baseline bone marrow blasts 77% • Post-transplant ziftomenib maintenance planned Best overall response CR Preliminary data
as of January 11, 2024 23 ~ ~
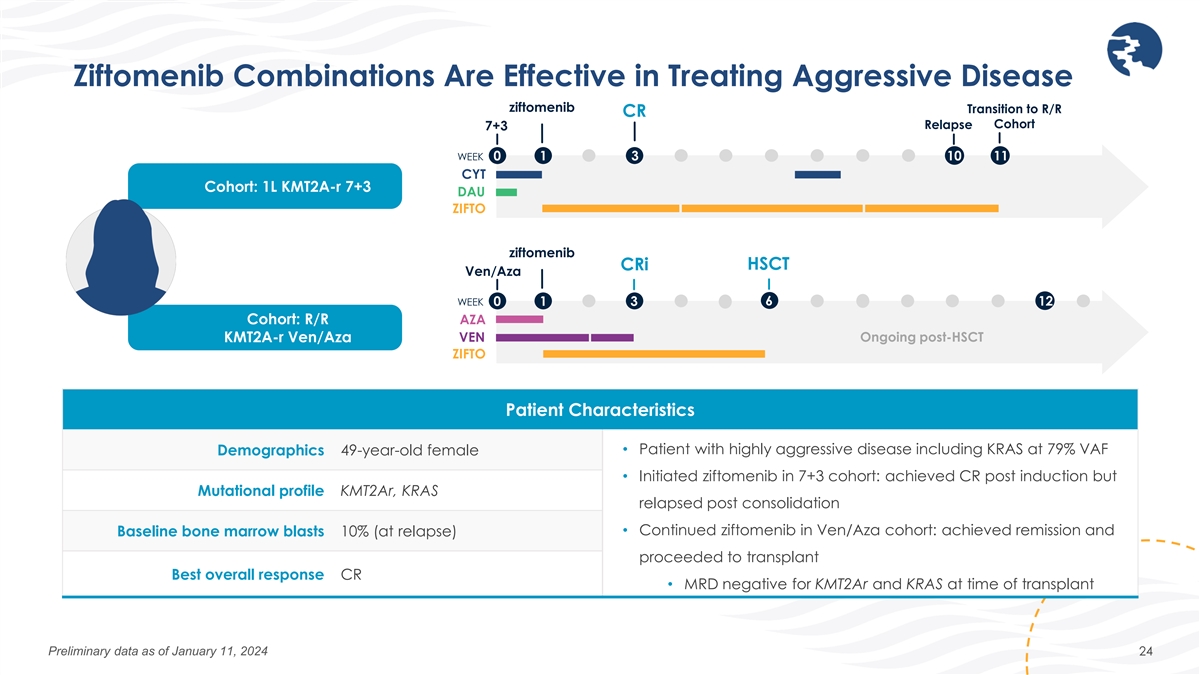
Ziftomenib Combinations Are Effective in Treating Aggressive Disease
ziftomenib Transition to R/R CR Relapse Cohort 7+3 WEEK 0 1 3 10 11 CYT Cohort: 1L KMT2A-r 7+3 DAU ZIFTO ziftomenib CRi HSCT Ven/Aza 6 WEEK 0 1 3 12 Cohort: R/R AZA KMT2A-r Ven/Aza VEN Ongoing post-HSCT ZIFTO Patient Characteristics Demographics
49-year-old female • Patient with highly aggressive disease including KRAS at 79% VAF • Initiated ziftomenib in 7+3 cohort: achieved CR post induction but Mutational profile KMT2Ar, KRAS relapsed post consolidation • Continued
ziftomenib in Ven/Aza cohort: achieved remission and Baseline bone marrow blasts 10% (at relapse) proceeded to transplant Best overall response CR • MRD negative for KMT2Ar and KRAS at time of transplant Preliminary data as of January 11, 2024
24
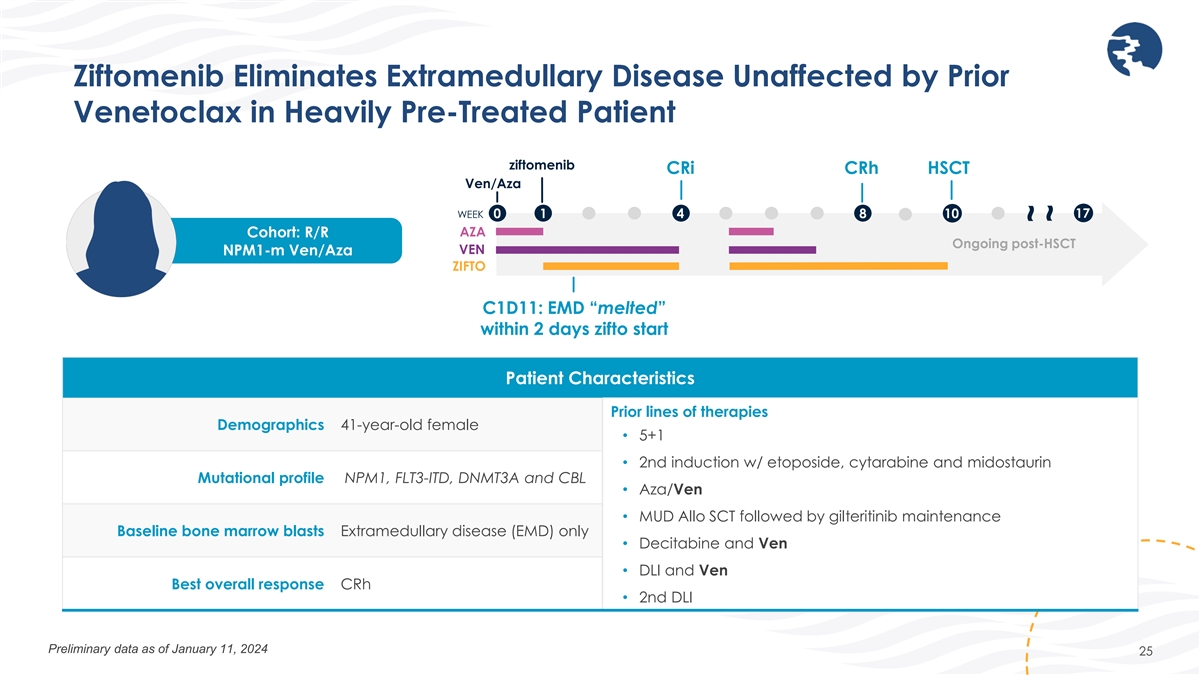
Ziftomenib Eliminates Extramedullary Disease Unaffected by Prior
Venetoclax in Heavily Pre-Treated Patient ziftomenib CRi CRh HSCT Ven/Aza 17 WEEK 0 1 4 8 10 AZA Cohort: R/R Ongoing post-HSCT VEN NPM1-m Ven/Aza ZIFTO C1D11: EMD “melted” within 2 days zifto start Patient Characteristics Prior lines of
therapies Demographics 41-year-old female • 5+1 • 2nd induction w/ etoposide, cytarabine and midostaurin Mutational profile NPM1, FLT3-ITD, DNMT3A and CBL • Aza/Ven • MUD Allo SCT followed by gilteritinib maintenance Baseline
bone marrow blasts Extramedullary disease (EMD) only • Decitabine and Ven • DLI and Ven Best overall response CRh • 2nd DLI Preliminary data as of January 11, 2024 25 ~ ~

CLINICAL DEVELOPMENT PLAN Mollie Leoni, M.D. – Executive Vice
President, Clinical Development

Ziftomenib Clinical Development Path DEVELOPMENT APPROACH PLANNED STUDY
STARTUP PHASE 1 REGISTRATION DIRECTED TRIAL NPM1-mutant acute myeloid leukemia (AML) MONOTHERAPY Non- NPM1-m/KMT2A-r AML (Relapsed/refractory) KMT2A-rearranged ALL COMBINATION WITH NPM1-mutant AML VENETOCLAX + AZACITIDINE KMT2A-rearranged AML
(Relapsed/refractory, frontline) COMBINATION WITH NPM1-mutant AML CYTARABINE + DAUNORUBICIN (7+3) KMT2A-rearranged AML (Relapsed/refractory, frontline) COMBINATIONS WITH GILTERITINIB, NPM1-mutant AML FLAG-IDA, LDAC KMT2A-rearranged AML
(Relapsed/refractory) NPM1-mutant AML Investigator / Company- POST-TRANSPLANT MAINTENANCE sponsored studies KMT2A-rearranged AML Pediatric AML & ALL COMBINATION WITH FLA Investigator-sponsored studies Pediatric ALL COMBINATION WITH BV-DAM
27

MARKET OPPORTUNITY AND UPCOMING MILESTONES Troy Wilson, Ph.D., J.D.
– President & Chief Executive Officer, Kura Oncology
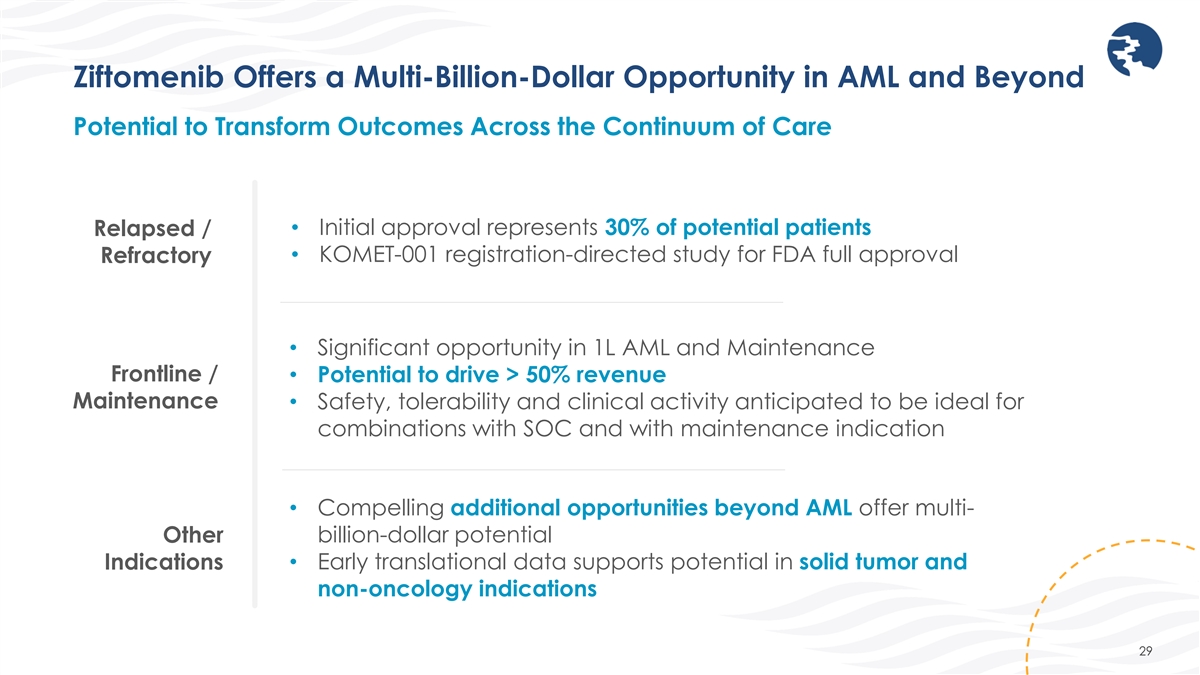
Ziftomenib Offers a Multi-Billion-Dollar Opportunity in AML and Beyond
Potential to Transform Outcomes Across the Continuum of Care • Initial approval represents 30% of potential patients Relapsed / • KOMET-001 registration-directed study for FDA full approval Refractory • Significant opportunity in
1L AML and Maintenance Frontline / • Potential to drive > 50% revenue Maintenance • Safety, tolerability and clinical activity anticipated to be ideal for combinations with SOC and with maintenance indication • Compelling
additional opportunities beyond AML offer multi- Other billion-dollar potential Indications • Early translational data supports potential in solid tumor and non-oncology indications 29

Upcoming Milestones for Ziftomenib in Acute Leukemia ESTIMATED TIME
MILESTONE OF ACHIEVEMENT Dose first patients in KOMET-008 trial in combination with FLT3 inhibitor gilteritinib, Q1 2024 LDAC and FLAG-IDA Initiate post-transplant maintenance program Q1 2024 Expand ziftomenib development to acute lymphoblastic
leukemia (ALL) Q1 2024 Complete enrollment of 85 patients in KOMET-001 registration-directed trial Mid-2024 Determine recommended Phase 2 dose in combination with ven/aza Mid-2024 Initiate dose validation/expansion in combination with ven/aza in 1L
AML Mid-2024 Provide next KOMET-007 combination update 2024 • $570 million in pro forma cash* provides runway into 2027, enabling aggressive research, development and pre-commercial activities to maximize value of ziftomenib and other pipeline
assets * Includes $424M in cash, cash equivalents and short-term investments as of 12/31/23 and estimated proceeds net of offering expenses of $146M from private placement closed on January 26, 2024 30
DEVELOPING PRECISION MEDICINES FOR THE TREATMENT OF CANCER Preliminary
Data from KOMET-007 – January 30, 2024
v3.24.0.1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Kura Oncology (NASDAQ:KURA)
Historical Stock Chart
From Mar 2024 to Apr 2024
Kura Oncology (NASDAQ:KURA)
Historical Stock Chart
From Apr 2023 to Apr 2024