0000898437
false
0000898437
2023-09-21
2023-09-21
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities
Exchange Act of 1934
Date of Report (Date of earliest event reported):
September 21, 2023
Anika Therapeutics, Inc.
(Exact Name of Registrant as Specified in its
Charter)
| Delaware |
|
001-14027 |
|
04-3145961 |
|
(State or Other
Jurisdiction of Incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
| 32 Wiggins Avenue |
|
| Bedford, MA |
01730 |
| (Address of Principal Executive Offices) |
(Zip Code) |
Registrant’s telephone number, including
area code: (781) 457-9000
Not Applicable
(Former Name or Former Address, If Changed Since
Last Report)
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading symbol |
|
Name of each exchange on which registered |
| Common Stock, par value $0.01 per share |
|
ANIK |
|
Nasdaq Global Select Market |
Indicate
by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2
of the Securities Exchange Act of 1934: Emerging growth company ☐
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01. | Regulation FD Disclosure. |
Anika Therapeutics,
Inc. (the “Company”) from time to time participates in various industry conferences and clinical, scientific, community and
investors meetings, during which representatives of the Company may refer to information regarding the Company’s business. A copy
of the slide deck (the “Investor Presentation”) containing certain corporate updates, including information on the Company’s
products and pipeline, which the Company may refer to at upcoming meetings, is attached hereto as Exhibit 99.1 and is incorporated into
this Item 7.01 of this Current Report on Form 8-K. The Investor Presentation is
available on the Investor Relations section of the Company’s website at https://ir.anika.com/.
The information contained in Item 7.01 of this Current Report on Form
8-K, including Exhibit 99.1 attached hereto, is being furnished and shall not be deemed “filed” for the purposes of Section
18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that
section. It shall not be deemed to be incorporated by reference into any of the Company’s filings under the Exchange Act or the
Securities Act of 1933, as amended, whether made before or after the date hereof and regardless of any general incorporation language
in such filings, except to the extent expressly set forth by specific reference in such filing.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURE
Pursuant to the requirements of the Securities
Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, hereunto duly authorized.
| |
ANIKA THERAPEUTICS, INC. |
| |
|
| |
|
| |
By: |
/s/ MICHAEL LEVITZ |
| |
|
Executive Vice President, Chief Financial Officer, and Treasurer |
Dated: September 21, 2023
3
Exhibit 99.1

Anika. Restore Active Living. ΠSIDOTI SMALL CAP INVESTOR CONFERENCE INVESTOR OVERVIEW SEPTEMBER 21, 2023

Safe Harbor Statements 2 Cautionary Note on Forward - looking Statements The statements made in, and during the course of, this presentation that are not statements of historical fact, including those related to the Company’s commercial capabilities, initiatives and production, its product pipeline and associated timelines, its upcoming corporate milestones, and its growth strategy and projections, are forward looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward - looking statements in “believe,” “will,” “would,” “expect,” “anticipate,” “intend,” “estimate,” “plan,” “likely,” and other expressions, which are predictions of, or indicate future events and trends, and which do not constitute historical matters, identify forward - looking statements, including, without limitation, relating to the impact of macroeconomic volatility, including global pandemics and the related effects of those on our ongoing business operations, clinical studies and future expectations with respect to our annual and multi - year business objectives and financial performance, those statements related to the Company’s product pipeline and the launch and anticipated adoption and growth of new products, the regulatory status, including plans for expanded indications, of the Company’s current and future products, the market potential of the Company’s products, and management’s discussion of the Company’s growth and strategic plans. The Company's actual results could differ materially from any anticipated future results, performance or achievements described in the forward - looking statements as a result of a number of factors (some of which may be outside of the Company’s control), both known and unknown, including, without limitation, future strategic decisions made by the Company, the results of its research and development efforts and the timing of regulatory approvals. Additional factors and risks are described in the Company's periodic reports filed with the Securities and Exchange Commission, and they are available on the SEC's website at www.sec.gov . Forward - looking statements are made based on information available to the Company on the date of this presentation, and the Company assumes no obligation to update the information contained herein or otherwise presented. Cautionary Note on Non - GAAP Financial Measures This presentation refers to certain non - GAAP financial measures. These non - GAAP financial measures should not be considered replacements for, and should be read together with, the most comparable GAAP financial measures. A reconciliation of these non - GAAP financial measures to the most directly comparable GAAP financial measures, calculated and presented in accordance with GAAP, is available under the “Quarterly Results” tab in the Investor Relations section of the Company’s website at www.anika.com . Note: this document contains proprietary information of Anika Therapeutics, Inc. Unauthorized use, duplication, dissemination or disclosure to third parties is strictly prohibited. © 2023 Anika Therapeutics, Inc. All rights reserved. ANIKA, CINGAL, HYALOFAST, INTEGRITY, MONOVISC, ORTHOVISC, REVOMOTION, TACTOSET, X - SPLINE, and X - TWIST are trademarks or registered trademarks of Anika Therapeutics, Inc. or its subsidiaries. This document may also contain trademarks and service marks that are the property of other companies, including certain trademarks licensed to us. The use of third - party trademarks does not constitute an endorsement or imply a relationship or other affiliation.

6+ NEW PRODUCT LAUNCHES in Fastest Growing Segments of Joint Preservation SIGNIFICANT & ONGOING CASH FLOW GENERATION STRONG HYALURONIC ACID (HA) FRANCHISE Leading osteoarthritis (OA) pain management franchise with Monovisc® and Orthovisc® and Cingal® OUS Newly launched products are expected to generate accelerating double - digit topline growth Anika: A Well - Positioned Company With A Compelling Future Strong base business generates resilient cash flow that is funding new product launches in large, growing markets X - Twist TM Fixation System Integrity TM Patch System Tactoset ® Expansion Hyalofast ® (US) Cartilage Repair Cingal ® (US) Next - Gen OA RevoMotion TM Reverse Shoulder 3
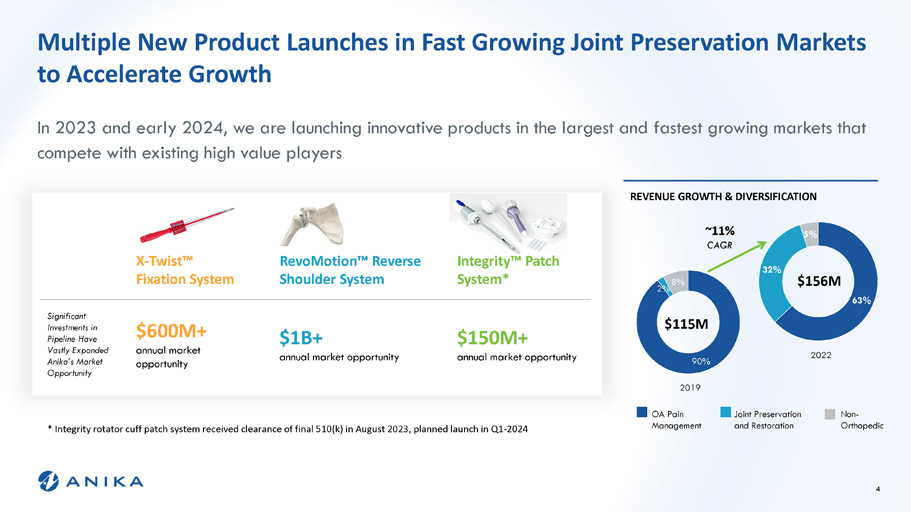
Multiple New Product Launches in Fast Growing Joint Preservation Markets to Accelerate Growth 63% 32% 5% 90% 2% 8% REVENUE GROWTH & DIVERSIFICATION $115M $156M 2019 2022 In 2023 and early 2024, we are launching innovative products in the largest and fastest growing markets that compete with existing high value players ~11% CAGR OA Pain Management Integrity Ρ Patch System* RevoMotion Ρ Reverse Shoulder System X - Twist Ρ Fixation System $150M+ annual market opportunity $1B+ annual market opportunity $600M+ annual market opportunity Significant Investments in Pipeline Have Vastly Expanded Anika’s Market Opportunity Joint Preservation and Restoration Non - Orthopedic * Integrity rotator cuff patch system received clearance of final 510(k) in August 2023, planned launch in Q1 - 2024 4

Versatile Suture Eyelet Strong Fixation Open Design Supports Healing Fast and Easy to Insert (X - Spline Ρ ) Successful Full Market Launch in 2023 of X - Twist Ρ Fixation System X - Twist Fixation System is a feature - rich suture anchor platform for soft tissue repairs in the shoulder and other extremities Easy - to - use and highly effective competitor to the market leading offerings Growth to be amplified in 2024 with recently 510(k) - cleared biocomposite to accompany the PEEK version introduced in 2023 The X - Twist is a new addition to the Anika sports medicine portfolio that brings their anchor options to the highest standard in orthopedics in the market. The instrumentation is easy to use and intuitive, and fixation is robust. The X - Twist can easily be used for nearly any soft tissue repair within the shoulder and throughout the body . ” Dr . Christopher E . Baker, M . D . Florida Orthopedics Institute, Tampa, FL X - Twist Biocomposite Cleared Sept. 19, 2023 5

Bone Preserving Design Smallest Diameter Threaded Baseplate Personalized Options Anatomic Mid - Lay Humeral Enhanced Facility Efficiency Two Instrument Tray Design Limited Market Launch of RevoMotion Ρ Reverse Shoulder Arthroplasty System Moves to Full Market Launch in September 2023 So far, early into my experience with using RevoMotion reverse total shoulder arthroplasty, my patients have had universally exceptional outcomes and reviews. I am already booking patients for their contralateral shoulder RevoMotion as their first has performed so well." Dr. Christopher Baker, M.D. Florida Orthopeadic Institute, Tampa, FL Accelerated full market launch to September at OSET annual meeting Positive feedback from limited market launch 6

Strong Hybrid*, multifilament structure provides superior implant handling and strength vs. collagen - based products Versatile Differentiated lateral - to - medial fixation and technique via tacks and darts, provides confidence across continuum of tears Proven Higher regenerative capacity vs. first generation collagen patches 1 . Composed of 80% HYAFF ® material with 15 years of safe clinical use Easy to Use Pliable, soft matrix supports arthroscopic insertion * 80% Hyaff / 20% PET v/v Cleared Integrity Ρ Regenerative Rotator Cuff Patch System Launches Q1 - 2024 Differentiated, regenerative, arthroscopic, HA - based; Superior to collagen patches Leveraged our hyaluronic acid (HA) and joint preservation expertise to design a better, easier to use delivery system for our second - generation HA - based product competing against market leader Regeneten (Smith & Nephew), as well as Embody and BioRez products, all of whom use collagen Integrity is generating substantial initial surgeon interest, even before market launch, as existing collagen technology is not easy to work with and lacks proper fixation Integrity’s instrumentation and technique are a game changer. Securing the implant laterally helps ensure proper coverage across the repair site and the rolling deployment tool provides consistent and repeatable implant placement.” Dr. Timothy P. Codd, M.D. University of Maryland Medical System 1 Clinical data on file 7

Integrity Ρ Patch System Highly Differentiated Compared to Collagen Patches Greater mechanical integrity for easier insertion Greater regenerative capacity of HA - based patch vs. collagen through 26 weeks 1 Histology showing greater regenerative capacity with Integrity vs. collagen INTEGRITY 8 COMPETITIVE COLLAGEN 26 weeks 12 weeks 6 weeks • Bars at 6, 12, & 26 weeks show difference in regenerative capability • Both devices ~1mm thick at t=0 • Collagen competition - collagen layer doesn’t increase in thickness • Integrity becomes a cellular collagen construct ~3x thicker than competition, significantly augmenting rotator cuff 26 weeks 12 weeks 6 weeks 1 Clinical data on file

Advancing Hyalofast® and Cingal® Towards FDA Approval Expecting final PMA module filing with FDA in 2025 for Hyalofast US Launch in 2026 • Single - stage, off - the - shelf, resorbable, HA - based scaffold for cartilage repair • Provides entry into $1 billion U.S. knee cartilage repair market • Recently completed enrollment of pivotal phase III study in U.S. • Designated as breakthrough device by FDA allowing prioritized interaction and review • Already selling in more than 30 countries OUS • Highly competitive with Vericel’s market - leading MACI based on clinical data 1 Engaging with FDA regarding Next Steps for Cingal • Cingal is poised to be a game - changing OA pain product in the U.S., as demonstrated by its consistent double - digit international growth with a >$1 billion U.S. market opportunity • Highly differentiated next generation OA pain product with significant clinical and real - world advantages vs. existing therapies as demonstrated by continued growth in over 35 countries OUS • Evaluating significant interest from potential partners in Asia, as well as the US • Engaging with FDA for next steps toward potential approval • Highly competitive with Pacira Biosciences’ Zilretta based on clinical data 1 1 Clinical data on file 9

Driving Commercial Focus While Actively Managing Costs to Enhance Margins and Profitability Actively managing OpEx spend, while preparing to support significant growth in coming years • Significant progress in both launching breakthrough products and addressing Medical Device Regulations (MDR) enables savings and improved operating leverage • Continue to deploy cost - conscious hybrid sales model but enhancing to drive focus - Executing on a targeted approach utilizing key direct sales resources to penetrate accounts not currently a priority for existing distributors - Addition of direct sales resources will not increase overall OpEx spend Confident in margin expansion as Anika pivots to accelerated top and bottom - line growth • Targeting double digit Joint Preservation and Restoration (JPR) growth with RevoMotion, Tactoset, and X - Twist in the market and Integrity launching in early 2024 • Committed to driving operating leverage as growth accelerates and costs remain stable 10

Key Value Drivers RevoMotion fully launched in September 2023 on very positive surgeon feedback from limited release Executing market launch of Integrity in Q1 - 2024 expanding opportunities in fast growing rotator cuff augmentation market Driving double - digit joint preservation growth with new launches underway (RevoMotion, X - Twist, Tactoset fully launched; Integrity launch in Q1 - 2024) Implementation of focused direct sales effort to augment existing hybrid commercial structure Beginning modular PMA filing for Hyalofast in 2024, with final module in 2025 Focused efforts to securing US and OUS Cingal partnerships Continue to engage FDA on solidifying pathway to approval in the U.S. Focused cost control with stable OpEx Driving operating leverage as growth accelerates and costs remain stable 11 11

Appendix
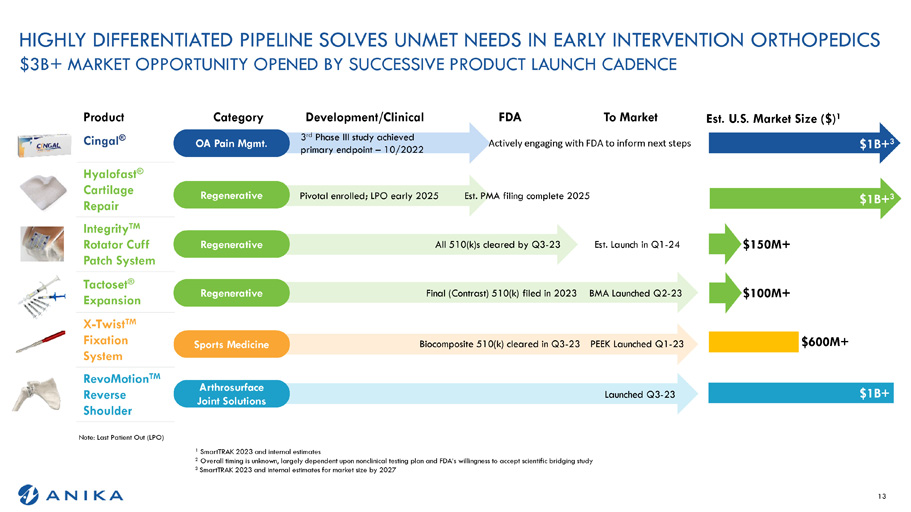
HIGHLY DIFFERENTIATED PIPELINE SOLVES UNMET NEEDS IN EARLY INTERVENTION ORTHOPEDICS $3B+ MARKET OPPORTUNITY OPENED BY SUCCESSIVE PRODUCT LAUNCH CADENCE Note: Last Patient Out (LPO) 1 SmartTRAK 2023 and internal estimates 2 Overall timing is unknown, largely dependent upon nonclinical testing plan and FDA’s willingness to accept scientific bridging study 3 SmartTRAK 2023 and internal estimates for market size by 2027 2 - 23 - 23 1 - 23 1 - 24 Est. U.S. Market Size ($) 1 $1B+ 3 $600M+ $100M+ $150M+ $1B+ To Market FDA Development/Clinical Category Product with FDA to inform nex Actively engaging 3 rd Phase III study achieved primary endpoint – 10/2022 OA Pain Mgmt. Cingal ® 2025 Est. PMA filing complete Pivotal enrolled; LPO early 2025 Regenerative Hyalofast ® Cartilage Repair Est. Launch in Q 0(k)s cleared by Q3 - 23 All 51 Regenerative Integrity TM Rotator Cuff Patch System 023 BMA Launched Q ntrast) 510(k) filed in 2 Final (Co Regenerative Tactoset ® Expansion - 23 PEEK Launched Q ite 510(k) cleared in Q3 Biocompos Sports Medicine X - Twist TM Fixation System Launched Q3 Arthrosurface Joint Solutions RevoMotion TM Reverse Shoulder 13 $1B+ 3 t steps
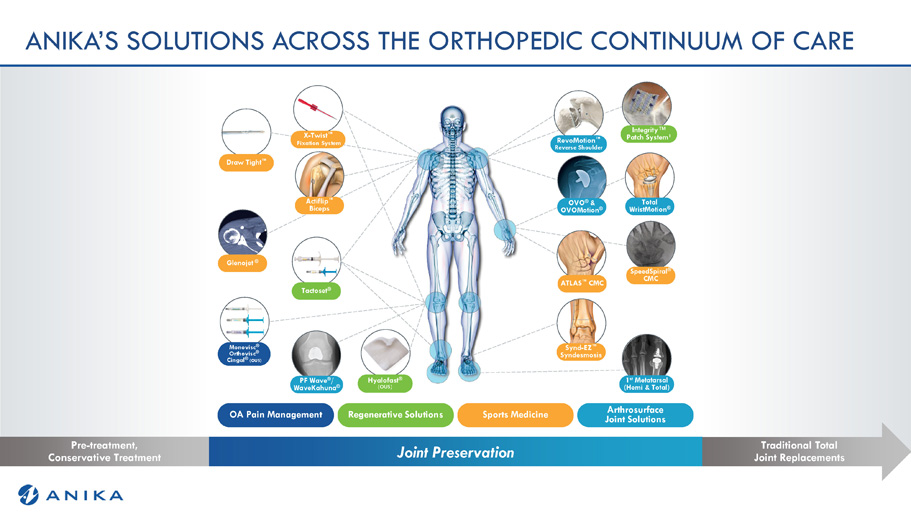
Joint Preservation Pre - treatment, Conservative Treatment Traditional Total Joint Replacements OVO ® & OVOMotion ® Tactoset ® SpeedSpiral ® CMC PF Wave ® / WaveKahuna ® Hyalofast ® (OUS) Total WristMotion ® Monovisc ® Orthovisc ® Cingal ® (OUS) Synd - EZ Œ Syndesmosis 1 st Metatarsal (Hemi & Total) Glenojet ® Draw Tight Œ X - Twist Œ Fixation System Actiflip Œ Biceps RevoMotion Œ Reverse Shoulder OA Pain Management Arthrosurface Joint Solutions Sports Medicine Regenerative Solutions ATLAS Œ CMC ANIKA’S SOLUTIONS ACROSS THE ORTHOPEDIC CONTINUUM OF CARE Integrity Œ Patch System 1

EXPANDING MARKET OPPORTUNITY IN JOINT PRESERVATION 1 Combination of iData, SmartTRAK, and internal estimates; 2 SmartTRAK and Anika internal estimates. CAGR 2021 - 2026 3 Received 510(k) clearance from FDA in Q3 - 2023; planned launch in Q1 - 2024 Extremities >$4B HA/Visco >$1B Regen >$1B Soft Tissue >$2B ANIKA PORTFOLIO OA Pain Management (HA/Visco) Monovisc ® Orthovisc ® Cingal ® (International) ► Cingal (U.S.) Regenerative Solutions Tactoset ® Hyalofast ® (International) ► Integrity TM Rotator Cuff Patch System 3 ► Hyalofast (U.S.) Sports Medicine (Soft Tissue) X. Twist TM Fixation System Suture Anchors ► Biocomposite Anchors 3 ► Implants, Instruments and Kits Arthrosurface Joint Solutions (Extremities) OVOMotion ® Shoulder RevoMotion TM Reverse Shoulder WristMotion ® Arthroplasty Upper and Lower Extremity Implants ► Shoulder, Foot and Ankle Implants ► Denotes Product Development Roadmap MARKET GROWTH RATES 2 FROM $1B IN 2019 TO $8B+ TODAY IN GLOBAL MARKET OPPORTUNITY 1 ~7% CAGR ~1% CAGR ~5 % CAGR ~8 % CAGR 15

SEASONED LEADERSHIP TEAM DRIVING GROWTH STRATEGY DECADES OF EXPERIENCE LEADING ORTHOPEDIC, REGENERATIVE MEDICINE, AND MEDICAL DEVICE COMPANIES Mike Levitz EVP, CFO & Treasurer 2020 David Colleran EVP, General Counsel & Corporate Secretary 2020 James Chase SVP, International Sales & Marketing 2018 Dawn Wilson VP, Research & Development 2020 Lisa Funiciello VP, Human Resources 2022 Mira Leiwant VP, Regulatory, Quality & Clinical Affairs 2019 Ben Joseph VP, Commercial & Corporate Development 2020 Cheryl R. Blanchard, Ph.D. President & CEO Joined: 2020 Anne Nunes SVP, Chief Operations Officer 2021 Robert Delp VP, U.S. Sales 2022 16
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Anika Therapeutics (NASDAQ:ANIK)
Historical Stock Chart
From Mar 2024 to Apr 2024
Anika Therapeutics (NASDAQ:ANIK)
Historical Stock Chart
From Apr 2023 to Apr 2024