Gamida Cell to Begin Biologics License Application Submission for Omidubicel
January 19 2022 - 7:43AM
Dow Jones News
By Chris Wack
Gamida Cell Ltd. said that after getting a positive Type B
meeting correspondence from the U.S. Food and Drug Administration
on Tuesday, it plans to initiate a rolling Biologics License
Application submission for omidubicel, a treatment for patients
with blood cancers in need of stem cell transplant.
The company previously disclosed that the FDA requested a
revised analysis of the manufacturing data generated at Gamida
Cell's wholly owned commercial manufacturing facility to
demonstrate the analytical comparability to the Lonza clinical
manufacturing site that produced omidubicel for the Phase 3
study.
Gamida Cell and the FDA have now reached alignment that
analytical comparability has been established between the
commercial manufacturing facility and the product that was
manufactured for the Phase 3 study.
Based on this demonstration of comparability, along with the
positive clinical results of the Phase 3 study, the FDA has agreed
that the initiation of a rolling BLA submission is appropriate.
Additional clinical data won't be required to initiate the BLA
submission.
Gamida Cell said it plans to complete the full BLA submission in
the first half of this year.
Gamida Cell shares were up 12% to $2.49 in premarket
trading.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
January 19, 2022 07:28 ET (12:28 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
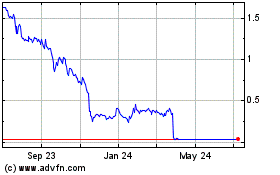
Gamida Cell (NASDAQ:GMDA)
Historical Stock Chart
From Aug 2024 to Sep 2024
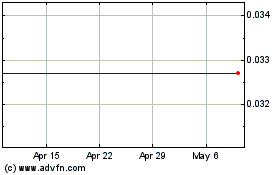
Gamida Cell (NASDAQ:GMDA)
Historical Stock Chart
From Sep 2023 to Sep 2024