Registration No. 333-256053
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
S-1/A
REGISTRATION
STATEMENT UNDER THE SECURITIES ACT OF 1933
Todos Medical Ltd.
(Exact name of registrant as
specified in its charter)
Israel
(State or other jurisdiction
of incorporation or organization)
2835
(Primary Standard Industrial
Classification Code Number)
N/A
(I.R.S. Employer Identification
Number)
121 Derech Menachem Begin, 30th Floor, Tel Aviv,
6701203 Israel, +972 (52) 642-0126
(Address, including zip code,
and telephone number,
including area code, of registrant’s
principal executive offices)
Puglisi & Associates
850 Library Avenue, Suite 204
Newark, Delaware 19711
302-738-6680
(Name,
address, including zip code, and telephone number,
including area code, of agent for service)
Copies of all Correspondence to:
|
Carl M. Sherer
Rimon PC
100 Park Ave, 16th floor
New York, NY 10017
Telephone No. (800) 930-7271
Facsimile No.: (617)997-0098
|
|
Jeffrey J. Fessler
Sheppard, Mullin, Richter &
Hampton LLP
30 Rockefeller Plaza
New York, NY 10112
Telephone No. (212) 634-3067
Facsimile No.: (917) 438-6133
|
As soon as practicable after the effective date
of this Registration Statement.
(Approximate date of commencement
of proposed sale to the public)
If any of the securities being registered on
this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities
Act of 1933 check the following box: ☒
If this Form is filed to register additional
securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities
Act registration statement number of the earlier effective registration statement for the same offering.
If this Form is a post-effective amendment
filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement
number of the earlier effective registration statement for the same offering.
If this Form is a post-effective amendment
filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement
number of the earlier effective registration statement for the same offering.
Indicate by check mark whether the registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company.
See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,”
and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ☐
|
|
Accelerated filer ☐
|
|
Non-accelerated filer ☒
|
|
Smaller reporting company ☒
|
|
|
|
Emerging growth company ☐
|
If an emerging growth company,
indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised
financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
Calculation of Registration Fee
|
Title
of each class of securities to be registered
|
|
Amount
to
be Registered (1)
|
|
|
Proposed
Maximum Offering Price Per Share (2)
|
|
|
Proposed
Maximum Aggregate
Offering
Price
|
|
|
Amount
of
Registration
Fee
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ordinary
shares, par value NIS 0.01, issuable upon exercise of Warrants
|
|
|
137,980,949
|
|
|
$
|
.064
|
|
|
$
|
8,830,781
|
|
|
$
|
818.61
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ordinary
Shares, par value NIS 0.01, issuable upon conversion of Convertible Notes
|
|
|
123,086,088
|
|
|
$
|
.064
|
|
|
|
7,877,510
|
|
|
$
|
730.25
|
|
|
TOTAL
|
|
|
261,067,037
|
|
|
|
|
|
|
$
|
16,708,291
|
|
|
$
|
1,548.86
|
|
|
(1)
|
Pursuant to Rule 416 under the Securities Act of 1933, as amended (the “Securities Act”), the ordinary shares issuable upon the exercise of warrants, and ordinary shares issuable upon conversion of Convertible Notes being registered hereunder include such indeterminate number of ordinary shares as may be issuable as a result of share splits, share dividends or similar transactions.
|
|
|
|
|
(2)
|
Estimated solely for the purpose of calculating the registration fee
pursuant to Rule 457 (c) under the Securities Act of 1933, as amended. Calculated based upon the closing bid price for the Company’s
shares on the OTCQB Venture Market on January 13, 2022.
|
The Registrant hereby amends this Registration
Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment
which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of
the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant
to said Section 8(a), may determine.
The
information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration
statement filed with the Securities and Exchange Commission becomes effective. This preliminary prospectus is not an offer to sell nor
does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
|
Preliminary
Prospectus
|
Subject
to Completion. Dated: January 18, 2022
|

TODOS
MEDICAL LTD.
261,067,037
Ordinary Shares
This
prospectus relates to the resale by the selling shareholders named herein, from time to time, of up to (i) 123,086,088 ordinary
shares, (the “Convertible Note Shares”), issuable upon the exercise of outstanding convertible notes (the “Convertible
Notes”), (ii) 78,332,201 ordinary shares, (the “Purchaser Warrant Shares”), issuable upon exercise of outstanding
warrants (the “2021 Warrants”), and (iii) 59,648,748 ordinary shares (the “Prior Warrant Shares”) issuable
upon the exercise of outstanding warrants (the “2020 Warrants”). The Convertible Notes and the 2021 Warrants were initially
issued by us in a private placement (the “Private Placement”), pursuant to nine securities purchase agreements, dated
as of July 6, 2021, July 7, 2021, August 9, 2021, September 15, 2021, October 18, 2021, November 2, 2021, November 24, 2021, December
14, 2021, and December 21, 2021 between us and certain selling shareholders. The 2020 Warrants were initially issued by us in a series
of private placements to fifteen purchasers between November 1, 2019, and July 8, 2020. The Purchaser Warrant Shares, the
Prior Warrant Shares and the Convertible Note Shares are referred to herein as the “Ordinary Shares” or the “Securities.”
We are not registering the resale of the Convertible Notes, the 2021 Warrants or the 2020 Warrants.
The
conversion price for the Convertible Notes is subject to adjustment downwards under certain circumstances. All share numbers in this
prospectus assume that such conversion price will not be adjusted. The maximum adjustment of the conversion price under the Convertible
Notes is 20%.
We
will not receive any proceeds from the sale of Securities by the selling shareholders. We will, however, receive the proceeds of any
Warrants exercised for cash in the future, which will total up to approximately $13,506,515, based on the Warrants’ respective
exercise prices. See “Use of Proceeds” in this prospectus.
The
selling shareholders may offer and sell the Securities from time to time at varying prices and in a number of different ways as each
selling shareholder may determine through public or private transactions or through other means described under “Plan of Distribution.”
Each selling shareholder may also sell shares under Rule 144 under the Securities Act of 1933, as amended, to the extent available pursuant
to the restrictions thereunder, rather than under this prospectus.
The
selling shareholders will bear all commissions, discounts and concessions, if any, attributable to the sale or disposition of the Securities.
Other than in connection with our indemnification obligations with respect to the selling shareholders, we will bear only the costs,
expenses and fees in connection with the registration of the Securities. We will not be paying any underwriting commissions or discounts
in offerings under this prospectus. For more information, see “Plan of Distribution.”
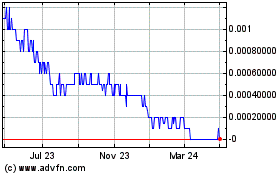
Our
ordinary shares are traded on the OTCQB under the symbol “TOMDF.” The last reported sales price of our ordinary shares on
the OTCQB on January 13, 2022 was $.064 per ordinary share.
INVESTING
IN OUR SECURITIES INVOLVES A HIGH DEGREE OF RISK. SEE “RISK FACTORS” IN THIS PROSPECTUS.
None
of the Securities and Exchange Commission, the Israel Securities Authority or any state securities commission has approved or disapproved
of the securities being offered by this prospectus, or determined if this prospectus is truthful or complete. Any representation to the
contrary is a criminal offense.
The
date of this prospectus is January 18, 2022
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
This
prospectus relates to the resale from time to time by selling shareholders of up to (i) 123,086,088 ordinary shares, (the “Convertible
Note Shares”), issuable upon the exercise of outstanding convertible notes (the “Convertible Notes”), (ii) 78,332,201
ordinary shares, (the “Purchaser Warrant Shares”), issuable upon exercise of outstanding warrants (the “2021 Warrants”),
and (iii) 59,648,748 ordinary shares (the “Prior Warrant Shares”) issuable upon the exercise of outstanding warrants
(the “2020 Warrants”). Before buying any of the ordinary shares that the selling shareholders are offering, we urge you to
read this prospectus carefully. These documents contain important information that you should consider when making your investment decision.
We
have not authorized anyone to provide you with information that is different from that contained in this
prospectus, any amendment or supplement to this prospectus, or in any free writing prospectus we may authorize to be
delivered or made available to you. We do not take responsibility for, and can provide no assurance as to the reliability of,
any other information that others may give you. The selling shareholders are offering to sell our ordinary shares and seeking
offers to purchase our ordinary shares only in jurisdictions where offers and sales are permitted. The information contained in
this prospectus is accurate only as of the date on the front of this prospectus, regardless of the time of delivery of this
prospectus or any sale of our ordinary shares. Our business, financial condition, results of operations and prospects may have
changed since the date on the front cover of this prospectus.
For
investors outside the United States: We have not done anything that would permit offerings under this prospectus, or possession or distribution
of this prospectus, in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the
United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the
offering of the ordinary shares and the distribution of this prospectus outside of the United States.
Unless
the context clearly indicates otherwise, references in this prospectus to “we,” “our,” “ours,” “us,”
“the Company” and “Todos” refer to Todos Medical Ltd. and its subsidiaries.
CAUTIONARY
STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
In
addition to historical information, this prospectus contains forward-looking statements within the meaning of the U.S. Private Securities
Litigation Reform Act of 1995, Section 27A of the U.S. Securities Act of 1933 (the “Securities Act”), and Section 21E of
the U.S. Securities Exchange Act of 1934 (the “Exchange Act”). Such forward-looking statements may include projections regarding
the Company’s future performance and other statements that are not statements of historical fact and, in some cases, may be identified
by words like “anticipate,” “assume,” “believe,” “continue,” “could,” “estimate,”
“expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,”
“future,” “will,” “should,” “would,” “seek” and similar terms or phrases.
These
forward-looking statements are based on our management’s current expectations, which are subject to uncertainty, risks and changes
in circumstances that are difficult to predict, and many of which are outside of our control. Important factors that could cause our
actual resu0lts to differ materially from those indicated in the forward-looking statements include, among others, the factors discussed
under the heading “Risk Factors.”
You
should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected
in the forward-looking statements are reasonable, we cannot guarantee that future results, levels of activity, performance and events
and circumstances reflected in the forward-looking statements will be achieved or will occur.
Any
forward-looking statements made in this prospectus speak only as of the date of the particular statement. Factors or events that could
cause the Company’s actual results to differ from the statements contained herein or therein may emerge from time to time, and
it is not possible for the Company to predict all of them. Except as required by law, the Company undertakes no obligation to publicly
update any forward-looking statements, whether as a result of new information, future developments or otherwise.
PROSPECTUS
SUMMARY
This
summary highlights information contained elsewhere in this prospectus. It may not contain all of the information that you should consider
before investing in our securities. You should read this entire prospectus carefully, including the “Risk Factors”, “Management’s
Discussion and Analysis of Financial Condition and Results of Operations” sections, and the financial statements and related notes
included herein. This prospectus includes forward-looking statements that involve risks and uncertainties. See “Forward-Looking
Statements.”
Overview
|
|
●
|
Todos
Medical Ltd. (“Todos Medical,” “Todos,” the “Company,” “we,” “our,”
“us”), is a comprehensive medical diagnostics and related solutions company focused on distributing comprehensive
solutions for COVID-19 screening and diagnosis, and immune resisting supplements, and on developing blood tests for the early
detection of cancer and Alzheimer’s disease.
|
|
|
|
|
|
|
●
|
Todos
has entered into distribution agreements with companies to distribute certain novel coronavirus (COVID-19) test kits. The agreements
cover multiple international suppliers of PCR testing kits and related materials and supplies, as well as antibody testing kits from
multiple manufacturers after completing validation of said testing kits and supplies in its partner CLIA/CAP certified laboratory
in the United States. Todos has combined the PCR testing kits with automated lab equipment to create lab workflows capable of performing
up to 40,000 PCR tests per day. Todos has entered into supply agreements with CLIA/CAP certified laboratories in the United States
to deploy these PCR workflows. Todos has formed strategic partnerships with Meridian Health and other strategic partners to deploy
COVID-19 antigen and antibody testing in the United States. Additionally, the Company is developing a lab-based COVID-19 3CL protease
test to determine whether a COVID-19 positive patient remains contagious after quarantine is complete and is further developing point-of-care-based
embodiments of the lab test for use in screening programs worldwide.
|
|
|
|
|
|
|
●
|
In
December 2020, Todos announced the commercial launch of its proprietary 3CL protease inhibitor dietary supplement Tollovid™ at
The Alchemist’s Kitchen in the SoHo district in Manhattan, New York. Tollovid, a mix of botanical extracts, is being targeted
to support healthy immune function against circulating coronaviruses. Tollovid’s mechanism of action is to inhibit the
activity of the 3CL protease, a key protease required for the intracellular replication of coronaviruses. Tollovid was granted a
Certificate of Free Sale by the US Food & Drug Administration in August 2020, allowing its commercial sale anywhere in the
United States. Tollovid has begun online sales through Amazon, Shopify and other online marketplaces.
|
|
|
|
|
|
|
●
|
Additionally,
the Company’s patented Todos Biochemical Infrared Analyses (TBIA) is a cancer-screening technology using peripheral blood analysis
that deploys deep examination into cancer’s influence on the immune system, looking for biochemical changes in blood mononuclear
cells and plasma. Todos’ two internally developed cancer-screening tests, TMB-1 and TMB-2 have received a CE mark in Europe.
In April 2021, Todos completed the acquisition of U.S.-based medical diagnostics company Provista Diagnostics, Inc. to gain rights
to its Alpharetta, Georgia-based CLIA/CAP certified lab and Provista’s proprietary commercial-stage Videssa® breast cancer
blood test.
|
|
|
|
|
|
|
●
|
Todos
is also developing blood tests for the early detection of neurodegenerative disorders, such as Alzheimer’s disease.
|
|
|
|
|
|
|
●
|
In
July 2020, Todos completed the acquisition of Breakthrough Diagnostics, Inc., the owner of the LymPro Test intellectual property,
from Amarantus Bioscience Holdings, Inc.
|
|
|
|
|
|
|
●
|
In
July 2021, Todos completed the acquisition of Provista Diagnostics Inc. (“Provista”), a CLIA laboratory performing COVID
testing for the general population as well as other scientific functions.
|
|
|
|
|
|
|
●
|
At
our annual general meeting of shareholders held on July 26, 2021, our shareholders voted to approve a reverse share split of the
Company’s Ordinary Shares within a range of 2:1 to 500:1, to be effective at the ratio and on a date to be determined by the
Board of Directors of the Company (the “Reverse Split”). Although our shareholders approved the Reverse Split, all per
share amounts and calculations in this prospectus and the accompanying consolidated financial statements do not reflect the effects
of the Reverse Split, as the Board of Directors has not determined the final ratio or the effective date of the Reverse Split.
|
Recent
Developments
On
January 22, 2021, we entered into a Securities Purchase Agreement (the “SPA”) with Yozma Global Genomic Fund (the “Purchaser”)
pursuant to which on January 29, 2021, the Company issued a promissory convertible note (the “Note”) to the Purchaser in
the principal amount of $4,857,142.86 for proceeds of $3,400,000 (the “Transaction”). The Note has a maturity date of one
year from the date of issuance and pays interest at a rate of 4% per annum. The Note is convertible into Ordinary Shares of the Company
(the “Conversion Shares”) at a conversion price of $0.0599 (the “Conversion Price). In addition, the Purchaser received
a warrant (the “Warrant”) to purchase up to 16,956,929 Ordinary Shares (the “Warrant Shares”) of the Company
with an exercise price equal to $0.107415 per share. The Warrant is exercisable for 5 years from the date of issuance. In the event that
the Company effectuates a reverse split of its ordinary shares for a ratio in excess of 20:1, the resulting adjusted Warrant Shares and
Exercise Price are limited to a 20:1 ratio.
This
registration statement registers for resale the Warrant Shares. Subsequent to the effective date of such Registration
Statement, if the closing sale price of the Ordinary Shares averages less than the then Conversion Price over a period of ten (10) consecutive
trading days, the Conversion Price shall reset to such average price. If the 10 day volume weighted average price of the Ordinary Shares
continues to be less than the Conversion Price, then the Conversion Price should reset to such 10-day average price. The maximum discount
from the initial Conversion Price under the Note is 20%.
The
foregoing descriptions of the SPA, the Note and the Warrant do not purport to be complete and are qualified in their entirety by reference
to the full text of the SPA, Note and Warrant, forms of which are attached as Exhibit 10.1, 10.2 and 10.3, respectively, to the Company’s
Current Report on Form 8-K dated January 26, 2021.
The
Company and Leviston Resources LLC, a Delaware limited liability company (the “Purchaser”) are parties to that certain Securities
Purchase Agreement, dated as of July 9, 2020 (the “Purchase Agreement”), pursuant to which the Purchaser purchased an aggregate
principal amount of $850,000 of convertible notes (the “July 2020 Convertible Notes”) from the Company. On March 3, 2021,
the Company and the Purchaser entered into a Closing Agreement (the “Closing Agreement”) pursuant to which the Purchaser
exercised its right to invest an additional $847,570 into the Company of July 2020 Convertible Notes (the “Tranche 2 Securities”).
This
registration statement registers for resale the Ordinary Shares underlying the Tranche 2 Securities.
The
foregoing description of the Closing Agreement does not purport to be complete and is qualified in its entirety by reference to the full
text of the Closing Agreement, a form of which is attached as Exhibit 10.1 to the Company’s Current Report on Form 8-K dated March
10, 2021.
During
the first quarter of 2021, the Company’s contractual agreement to supply Covid-19 testing kits to NOAH Laboratories, Inc., a significant
customer expired. At the customer’s request, the Company continued to supply Covid-19 testing kits until such time as the customer
requested that the Company stop doing so. The customer has not yet paid for some of the Covid-19 testing kits so supplied and has not
yet renewed its agreement with the Company. On November 15, 2021, Todos USA sent a demand letter (the “Demand Letter”) to
the significant customer with which our contractual agreement to supply Covid-19 testing kits expired. The Demand Letter seeks (a) payment
for testing kits that Todos USA supplied for which it was not paid, in the amount of $3,465,000, (b) the return of Todos USA’s
equipment, title to which remains with Todos USA unless and until the significant customer meets a minimum purchase requirement, and
(c) payment of damages as a result of the significant customer’s unlawful retention of Todos USA’s equipment, in an amount
anticipated to be $2 million. The Company and Todos USA are negotiating a settlement with NOAH Laboratories, Inc., which the Company
believes will result in most of the Company’s demands being met.
On
April 8, 2021, the Company entered into a Securities Purchase Agreement (the “SPA”) with Kips Bay Select LP (the “Purchaser”)
pursuant to which the Company agreed to issue a promissory convertible note (the “Note”) to the Purchaser in the principal
amount of $4,285,714.29 for proceeds of $3,000,000 (the “Transaction”). The closing occurred on April 12, 2021 (the “Closing
Date”). The Note has a maturity date of one year from the date of issuance and pays interest at a rate of 4% per annum. The Note
is convertible into Ordinary Shares (the “Conversion Shares”) at a conversion price of $0.0599 (the “Conversion Price).
In addition, the Purchaser received a warrant (the “Warrant”) to purchase up to 16,000,000 Ordinary Shares (the “Warrant
Shares”) of the Company with an exercise price equal to $0.107415 per share. The Warrant is exercisable for 5 years from the date
of issuance. In the event that the Company effectuates a reverse split of its Ordinary shares for a ratio in excess of 20:1, the resulting
adjusted Warrant Shares and Exercise Price are limited to a 20:1 ratio. The Company used the net proceeds from this Note to initiate
the Phase 2 for Tollovir™ clinical trial in COVID-19 patients, complete the acquisition of Provista Diagnostics, Inc. and for general
corporate purposes.
This
registration statement registers for resale the Warrant Shares. Subsequent to the effective date of such registration
statement, if the closing sale price of our Ordinary Shares averages less than the then Conversion Price over a period of ten (10) consecutive
trading days, the Conversion Price shall reset to such average price. If the 10 day volume weighted average price of our Ordinary Shares
continues to be less than the Conversion Price then the Conversion Price should reset to such 10-day average price with a maximum of
a 20% discount from the initial Conversion Price.
The
Purchaser has the option to purchase an additional Note in the principal amount of $5,285,714.20 for proceeds of $3,700,000 and an additional
Warrant to purchase 16,000,000 Ordinary Shares within 90 days of the effective date of the registration statement on Form S-1 described
in the previous paragraph.
The
foregoing descriptions of the SPA, the Note and the Warrant do not purport to be complete and are qualified in their entirety by
reference to the full text of the SPA, Note and Warrant, forms of which are attached as Exhibit 10.1, 10.2 and 10.3, respectively,
to the Company’s Current Report on Form 8-K dated April 14, 2021.
On
April 8, 2021, the Company received a notice of allowance (‘Letter of Intent to Grant a Patent’) from the European Patent
Office covering the use of the Company’s proprietary Total Biochemical Infrared Analysis (‘TBIA’) method that uses
blood (plasma and/or peripheral blood mononuclear cells ‘PBMCs’) to distinguish between patients with benign tumors vs. malignant
tumors vs. no tumors (healthy controls).
The
patent application specifically covers methods for capturing consistent data from infrared spectroscopy readers, as well as the application
of various artificial intelligence algorithm development methods to the data. The ability of TBIA to make a diagnosis of cancer has first
been applied to the detection of breast and colon cancers, where Todos has received CE Marks in Europe paving the way for commercialization
initially focused on TMB-2 (dense breast / inconclusive mammogram secondary screening) and TMB-1 (general breast cancer screening) cancer
detection tests.
On
April 19, 2021, the Company entered into an Agreement to Purchase Provista Diagnostics, Inc. (“Agreement to Purchase”) with
Strategic Investment Holdings, LLC (“SIH”), Ascenda BioSciences LLC (“Ascenda”) and Provista Diagnostics, Inc.
(“Provista”). Ascenda was the sole owner of the outstanding securities of Provista and SIH is the sole owner of all the outstanding
securities of Ascenda.
Pursuant
to the Agreement to Purchase, the Company acquired Provista from Ascenda and SIH for an aggregate purchase price of $7.5 million consisting
of an initial cash payment of $1.25 million, the issuance of $1.5 million in Ordinary Shares priced at $0.0512 per share, the issuance
to SIH of a $3.5 million convertible promissory note dated April 19, 2021 (the “Note”) and the payment on for before July
1, 2021 of $1.25 million in cash (the “July Payment”), which payment the Company had the right to, and did, extend to July
15, 2021. The Provista shares acquired by the Company remained in an escrow account until the July Payment was made.
The
Note has a maturity date of April 8, 2025, and is convertible beginning on October 20, 2021, into Ordinary Shares of the Company at a
conversion price equal to the lesser of $0.05 or the volume weighted average price of the last 20 trading days for the Ordinary Shares
prior to the date of conversion. In the event SIH delivers a Notice of Conversion to the Company at a per share price less than $0.05
($0.05), the Company has the right to immediately notify SIH of its intention to pay the conversion amount in cash within three (3) business
days of receipt of the Notice of Conversion (i.e., before SIH would take possession of shares converted under the Notice of Conversion).
If, at any time between October 20, 2021 and April 20, 2022, the average of the lowest bid and closing sale price at 4:00:00 p.m., New
York time (or such other time as such market publicly announces is the official close of trading) is below ($0.05), the Company has the
option to buy out all or any portion of the Note (the “Buyback Option”). In the event the Company exercises the Buyback Option
for an amount equal to or greater than one million, one hundred seventy thousand dollars ($1,170,000) (the “Buyback Amount”),
SIH may not submit any conversions below five cents ($0.05) for ninety (90) days from receipt of the Buyback Amount (“90 Day Period”).
In
the event that the Company uplists its Ordinary Shares to a national securities exchange, the Note shall automatically be exchanged into
Series B preferred stock with a conversion price equal to the lesser of (a) $0.05, (b) the opening price on the day of the uplisting
provides there is no transaction associated with the uplisting or (c) the deal price of an uplisting transaction.
As
of the date of this prospectus, SIH has not submitted a Conversion Notice.
The
foregoing descriptions of the Agreement to Purchase, the SPA, the Note and the Security Agreement do not purport to be complete and are
qualified in their entirety by reference to the full text of the Agreement to Purchase, SPA, Note and the Security Agreement, forms of
which are attached as Exhibit 10.1, 10.2 and 10.3, respectively, to the Company’s Current Report on Form 8-K dated April 23, 2021.
On
July 7, 2021, the Company entered into a Securities Purchase Agreement (the “SPA”) with Kips Bay Select LP (the “Purchaser”)
pursuant to which the Company agreed to issue a promissory convertible note (the “Note”) to the Purchaser in the principal
amount of $1,535,714 for proceeds of $1,075,000 (the “Transaction”). The closing occurred on July 7, 2021 (the “Closing
Date”). The Note has a maturity date of one year from the date of issuance and pays interest at a rate of 4% per annum. The Note
is convertible into Ordinary Shares of the Company (the “Conversion Shares”) at a conversion price of $0.0599 (the “Conversion
Price). In addition, the Purchaser received a warrant (the “Warrant”) to purchase up to 3,440,000 Ordinary Shares (the “Warrant
Shares”) of the Company with an exercise price equal to $0.107415 per share. The Warrant is exercisable for 5 years from the date
of issuance. From the Closing Date until 180 days thereafter, the Company shall be restricted from issuing or entering into any agreement
to issue any Ordinary Shares, except under certain circumstances, including an uplisting. This provision shall no longer be in effect
if the closing sale price of the Ordinary shares exceeds $0.10. The Company intends to use the net proceeds for general corporate purposes.
This
registration statement registers for resale the Warrant Shares. Subsequent to the effective date of such registration
statement, if the closing sale price of the Ordinary Shares averages less than the then Conversion Price over a period of ten (10) consecutive
trading days, the Conversion Price shall reset to such average price. If the 10-day volume weighted average price of the Ordinary Shares
continues to be less than the Conversion Price then the Conversion Price should reset to such 10-day average price with a maximum of
a 20% discount from the initial Conversion Price.
The
foregoing descriptions of the SPA, the Note and the Warrant do not purport to be complete and are qualified in their entirety by reference
to the full text of the SPA, Note and Warrant, forms of which are attached as Exhibit 10.1, 10.2 and 10.3, respectively, to the Company’s
Current Report on Form 8-K filed July 8, 2021.
On
September 23, 2021, the Company completed the conditions precedent required to enter into a Securities Purchase Agreement (the “SPA”)
with Mercer Street Global Opportunity Fund, LLC (the “Purchaser”) pursuant to which the Company issued a promissory convertible
note (the “Note”) to the Purchaser in the principal amount of $2,285,142.86 for proceeds of $2,000,000 (the “Transaction”).
The Note has a maturity date of one year from the date of issuance and pays interest at a rate of 4% per annum. The Note is convertible
into Ordinary Shares of the Company (the “Conversion Shares”) at a conversion price of $0.0599 (the “Conversion Price).
In addition, the Purchaser received a warrant (the “Warrant”) to purchase up to 11,924,636 Ordinary Shares (the “Warrant
Shares”) of the Company with an exercise price equal to $0.107415 per share. The Warrant is exercisable for 5 years from the date
of issuance. The Company intends to use the net proceeds from this Note to initiate Phase 2/3 trials for Tollovir™ COVID-19 patients,
initiate digital marketing for its dietary supplement Tollovid®, increase sales & marketing for Provista Diagnostics, and for
general corporate purposes.
Univest
Securities, LLC acted as placement agent for the offering.
This
registration statement registers for resale the Conversion Shares and the Warrant Shares. Subsequent to the effective date of the registration
statement, if the closing sale price of the Ordinary shares averages less than the then Conversion Price over a period of ten (10) consecutive
trading days, the Conversion Price shall reset to such average price. If the 10 day volume weighted average price of the Ordinary
shares continues to be less than the Conversion Price then the Conversion Price should reset to such 10-day average price with a maximum
of a 20% discount from the initial Conversion Price.
The
foregoing descriptions of the SPA, the Note and the Warrant do not purport to be complete and are qualified in their entirety by reference
to the full text of the SPA, Note and Warrant, forms of which are attached as Exhibit 10.1, 10.2 and 10.3, respectively, to the Company’s
Current Report on Form 8-K filed September 24, 2021.
On
July 22, 2021, the US Food & Drug Administration (FDA) granted a new Certificate of Free Sale for Tollovid Daily™, the newest
member of the Company’s Tollovid™ dietary supplement product line.
The
Certificate of Free Sale is for a twice-daily dosing regimen and, critically, a 3CL protease inhibitor claim. Each 60-pill bottle of
Tollovid Daily can help support and maintain healthy immune function for 30 days. The Company intends to establish a monthly subscription
model as part of its marketing launch campaign for Tollovid Daily immune system support. Tollovid™ and Tollovid Daily are both
3CL protease inhibitor products developed under a joint venture with NLC Pharma.
On
November 15, 2021, Todos USA sent a demand letter (the “Demand Letter”) to a significant customer with which our contractual
agreement to supply Covid-19 testing kits expired. The Demand Letter seeks (a) payment for testing kits that Todos USA supplied for which
it was not paid, in the amount of $3,465,000, (b) the return of Todos USA’s equipment, title to which remains with Todos USA unless
and until the significant customer meets a minimum purchase requirement, and (c) payment of damages as a result of the significant customer’s
unlawful retention of Todos USA’s equipment, in an amount anticipated to be $2 million. Todos USA has yet to receive a response
to the Demand Letter.
On
November 18, 2021, Todos entered into a license agreement (the “License Agreement”) with T-Cell Protect Hellas S.A. (“T-Cell
Protect”) pursuant to which the Company will license the European manufacturing and distribution rights to a product based on the
Company’s Tollovid® and Tollovid Daily™ 3CL protease inhibitor and immune support dietary supplements to T-Cell Protect
(the “Product”). The License Agreement became effective on November 22, 2021 when the Company received a purchase order for
50,000 bottles of Tollovid Daily, per the terms of the License Agreement. The Product is expected to be marketed under the T-Cell Protect
brand in Europe. In addition, the Company will grant to T-Cell Protect an exclusive license to manufacture, sell and distribute the Product
in Greece. The License Agreement provides for royalty in the low double digits to the Company and a minimum of 500,000 bottles in sales
over the 18 months after the date of the License Agreement. In the event T-Cell Protect establishes its own manufacturing of the Product
in the European market, the 500,000 bottle minimum referred to in the previous sentence will become a minimum sales requirement. In the
License Agreement, T-Cell Protect acknowledged that the Company intends to assign the License Agreement to a subsidiary being formed
by the Company to focus on the development of 3CL protease biology called 3CL Sciences, Inc. (“3CL Sciences”).
On
November 22, 2021, the Company entered into a Securities Purchase Agreement (the “SPA”) with T-Cell pursuant to which the
Company issued a promissory convertible note (the “Note”) to T-Cell Protect in the principal amount of €1,000,000 (the
“Transaction”). The Note has a maturity date of one year from the date of issuance and pays interest at a rate of 10% per
annum. The Note is convertible into ordinary shares (the “Conversion Shares”) at a conversion price of $0.0599 (the “Conversion
Price). At any time prior to the Company uplisting its ordinary shares to a national securities exchange, T-Cell Protect may exchange
the Note into either (a) a direct equity investment in 3CL Sciences at the same terms as a financing round of at least $5,000,000 (the
“Sub”) or (b) into a note in the Sub, bearing 10% interest that converts into direct equity in the Sub at the same terms
as a financing round of at least $5,000,000. The proceeds from this Transaction are intended to be used for the clinical development
of Tollovir, the Company’s therapeutic candidate for hospitalized COVID-19 patients.
On
November 24, 2021, the Company entered into a binding letter of intent (the “LOI”) with NLC Pharma Ltd. (“NLC”)
pursuant to which 3CL Sciences will purchase all therapeutic, diagnostic, dietary supplement and pharmaceutical assets from NLC that
relate to 3CL protease biology in exchange for a 40% equity interest in 3CL Sciences that NLC will own, single digit royalties and Company
ordinary shares. Promptly following execution of the LOI, the Company will deliver $325,000 to pay outstanding invoices related to the
interim analysis of the ongoing clinical trial of Tollovir. The Company shall be responsible for providing or assisting in the raising
of a total of $10 million into 3CL Sciences over a period of 7 months from execution of the LOI. The Company and NLC agree to identify
a seasoned biopharmaceutical CEO to run 3CL Sciences going forward. The board of directors of 3CL Sciences will be made up of five (5)
individuals: two (2) appointed by the Company, two (2) appointed by NLC and one (1) to be mutually agreed upon by the Company and NLC.
In addition, NLC will be granted one (1) seat on the Company’s Board of Directors.
The
Company has agreed to file a registration statement with the Securities and Exchange Commission registering for resale the Conversion
Shares (the “Registration Statement).
The
foregoing descriptions of the License Agreement, SPA, the Note and LOI do not purport to be complete and are qualified in their entirety
by reference to the full text of the License Agreement, SPA, Note and LOI, forms of which are attached as Exhibit 10.1, 10.2, 10.3 and
10.4, respectively, to the Company’s Current Report on Form 8-K dated November 29, 2021.
Contractual
Obligations
The
following table summarizes our contractual obligations as of September 30, 2021:
|
|
|
Payments due by period
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(US$)
|
|
|
Less than 1
|
|
|
|
|
|
|
|
|
More than
|
|
|
|
|
Total
|
|
|
year
|
|
|
1-3 years
|
|
|
3-5 years
|
|
|
5 years
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible bridge loans, net
|
|
|
15,560,000
|
|
|
|
15,560,000
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Other loans, net
|
|
|
2,756,000
|
|
|
|
2,756,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Royalties to BGU (1)
|
|
|
501,000
|
|
|
|
296,000
|
|
|
|
95,000
|
|
|
|
39,000
|
|
|
|
71,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total (2)
|
|
|
18,817,000
|
|
|
|
18,612,000
|
|
|
|
95,000
|
|
|
|
39,000
|
|
|
|
71,000
|
|
(1)
This balance was measured based on the future cash payments discounted using an interest rate of 21%, which represents, according to
management’s estimate, the applicable rate of risk for us.
(2)
This does not include the repayment of approximately $272,000 of grants we received from the Israel Innovation Authority (“IIA”)
and interest thereon, which shall be repaid as royalties upon the commercialization of our products.
Summary
of Risk Factors
Our
business is subject to significant risks and uncertainties that make an investment in us speculative and risky. Below we summarize what
we believe are the principal risk factors, but these risks are not the only ones we face, and you should carefully review and consider
the full discussion of our risk factors in the section titled “Risk Factors”, together with the other information in this
prospectus. If any of the following risks actually occurs (or if any of those listed elsewhere in this prospectus occur), our business,
reputation, financial condition, results of operations, revenue, and future prospects could be seriously harmed. Additional risks and
uncertainties that we are unaware of, or that we currently believe are not material, may also become important factors that adversely
affect our business.
We
have a history of losses, may incur future losses and may not achieve profitability.
We
have a need for substantial additional financing and will have to significantly delay, curtail or cease operations if we are unable to
secure such financing.
The
report of our former independent registered public accounting firm expresses substantial doubt about our ability to continue as a going
concern.
There
can be no assurance of market acceptance for our COVID-19 antibody test.
We
rely on a third party to manufacture the COVID-19 antibody tests for us, and if such third party refuses or is unable to supply us with
the COVID-19 test kits, our business will be materially harmed.
We
may not succeed in completing the development of our cancer detection products, commercializing our products or generating significant
revenues.
We
are currently in the process of improving our technology and adapting to the high throughput methodology.
We
will require additional funding in order to commercialize our cancer detection kits and to develop and commercialize any future products.
We
may not successfully maintain our existing license agreement with BGU and Soroka, and we are currently not in compliance with the repayment
terms of the license agreement, which could adversely affect our ability to develop and commercialize our product candidates.
If
we are unable to protect our intellectual property rights, our competitive position could be harmed.
Because
the medical device industry is litigious, we are susceptible to intellectual property suits that could cause us to incur substantial
costs or pay substantial damages or prohibit us from selling our cancer detection kits.
If
we or our future distributors do not obtain and maintain the necessary regulatory clearances or approvals in a specific country or region,
we will not be able to market and sell our cancer detection kits or future products in that country or region.
If
we are unable to successfully complete clinical trials with respect to our cancer detection kits, we may be unable to receive regulatory
approvals or clearances for our cancer detection kits and/or our ability to achieve market acceptance of our cancer detection kits will
be harmed.
Conditions
in Israel could materially and adversely affect our business
Your
rights and responsibilities as a shareholder will be governed by Israeli law which differs in some material respects from the rights
and responsibilities of shareholders of U.S. companies.
It
may be difficult to enforce a judgment of a U.S. court against us, or against our officers and directors in Israel, or to assert U.S.
securities laws claims in Israel or to serve process on our officers and directors in Israel.
The
sale or issuance of our Ordinary Shares to Lincoln Park Capital may cause dilution and the sale of the Ordinary Shares acquired by Lincoln
Park, or the perception that such sales may occur, could cause the price of our Ordinary Shares to fall.
We
may not have access to the full amount available under the Purchase Agreement with Lincoln Park.
An
active trading market for our Ordinary Shares may not develop and our shareholders may not be able to resell their Ordinary Shares.
You
should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected
in the forward-looking statements are reasonable, we cannot guarantee that future results, levels of activity, performance and events
and circumstances reflected in the forward-looking statements will be achieved or will occur.
Any
forward-looking statements made in this prospectus speak only as of the date of the particular statement. Factors or events that could
cause the Company’s actual results to differ from the statements contained herein or therein may emerge from time to time, and
it is not possible for the Company to predict all of them. Except as required by law, the Company undertakes no obligation to publicly
update any forward-looking statements, whether as a result of new information, future developments or otherwise.
Corporate
Information
We
were incorporated in the State of Israel in April 2010, and are subject to the Israel Companies Law, 5760-1999 (the “Companies
Law”). Since March 7, 2017, our Ordinary Shares have been quoted on the OTCQB under the symbol TOMDF.
In
January 2016, we incorporated our wholly owned subsidiary, Todos (Singapore) Pte. Ltd. In March 2016, Todos (Singapore) Pte. Ltd. changed
its name to Todos Medical Singapore Pte. Ltd., or Todos Singapore. Todos Singapore has not yet commenced its business operations.
In
January 2020, we incorporated Todos Medical USA, a Nevada corporation (“Todos US”), for the purpose of conducting
business as a medical importer and distributor focused on the distribution the Company’s testing products and services to
customers in North America and Latin America. In addition, in March 2020, Todos US formed a subsidiary, Corona Diagnostics, LLC, in
the State of Nevada, for the purpose of marketing COVID-19 related products in the United States. In July 2020, we completed the
acquisition of Breakthrough Diagnostics, Inc, which is now a wholly owned subsidiary of Todos. In July 2021, we completed the
acquisition of Provista Diagnostics, Inc., which is now a wholly owned subsidiary of Todos.
Effective
January 1, 2021, we began filing periodic reports with the SEC as a U.S. domestic issuer, after we determined that, as of June 30, 2020,
we no longer qualified as a foreign private issuer under SEC rules. As a U.S. domestic issuer, we must now, for the first time, make
our SEC filings under the rules applicable to U.S. domestic issuers, and must include certain disclosures that were not previously required.
The
current corporate organizational structure of the Company and how we have operated substantially for the past year appears below.

Our
principal executive office is located at 121 Derech Menachem Begin, 30th Floor, Tel Aviv, 6701203 Israel, and our telephone number in
Israel is +972-52-642-0126. Our web address is www.todosmedical.com. The information contained on our website or available through our
website is not incorporated by reference into and should not be considered a part of this prospectus, and the reference to our website
in this prospectus is an inactive textual reference only. Puglisi & Associates is our agent in the United States, and its address
is 850 Library Avenue, Suite 204 Newark, Delaware 19711.
All
per share amounts and calculations in this prospectus and the accompanying financial statements do not reflect the effects of
the Reverse Split discussed elsewhere in this prospectus.
Our
former independent registered public accounting firm indicated in its report on our financial statements for the year ended December
31, 2020, as included elsewhere in this prospectus, that conditions raise substantial doubts about our ability to continue as
a “going concern.” In addition, our financial status creates substantial doubt whether we will continue as a going concern.
Shares
offered for sale in this prospectus
The
shares offered in this prospectus relate to the resale by selling shareholders of an aggregate of (i) 123,086,088 ordinary shares
issuable upon the conversion of eight convertible notes (the “2021 Convertible Notes”) which were issued in private
placements to six institutional investors, (ii) 78,332,201 ordinary shares, (the “Purchaser Warrant Shares”),
issuable upon exercise of outstanding warrants (the “2021 Warrants”), and (iii) 59,648,748 ordinary shares (the “Prior
Warrant Shares”) issuable upon the exercise of outstanding warrants (the “2020 Warrants”). We sold the 2021 Convertible
Notes and the 2021 Warrants pursuant to nine securities purchase agreements between us and the respective investors party thereto,
dated July 6, 2021, July 7, 2021, August 9, 2021, September 15, 2021, October 18, 2021, November 2, 2021, November 24, 2021, December
14, 2021, and December 21, 2021, respectively (the “2021 Purchase Agreements”). The 2020 Warrants were initially issued
by us in a series of private placements to fifteen purchasers between November 1, 2019 and July 8, 2020.
The
2021 Convertible Notes include:
|
|
●
|
nine
convertible notes issued
to seven different investors in the original principal amounts ranging from $142,857
to $2,857,143 respectively, which may be
converted to purchase up to 123,086,088 ordinary shares at a conversion price of $0.0599
per share, which were issued to certain of the Selling Shareholders pursuant to the 2021
Purchase Agreements. The conversion price for the Convertible Notes is subject to adjustment
downwards under certain circumstances. All share numbers in this prospectus assume that such
conversion price will not be adjusted. The maximum adjustment of the conversion price under
the Convertible Notes is 20%. One convertible note did not include warrants.
|
The
2021 Warrants include:
|
|
●
|
fourteen
warrants issued to eight
different investors to purchase between 266,667 and 16,956,929 ordinary
shares respectively, at an exercise price of $0.10741, which were issued to certain
of the Selling Shareholders pursuant to the 2021 Purchase Agreements.
|
The
2020 Warrants include:
|
|
●
|
twenty-one
warrants issued to fifteen different investors to purchase up to an aggregate
of 59,648,748 ordinary shares (ranging from 446,428 to 8,000,000 ordinary shares)
at exercise prices ranging from $0.04 to $0.10 per ordinary share, which were issued
to certain of the Selling Shareholders in a series of private placements between November
2019 and July 2020.
|
All
of the 2021 Convertible Notes, 2021 Warrants and 2020 Warrants were issued pursuant to the exemption from the registration requirements
in Section 4(a)(2) of the Securities Act of 1933, as amended, and/or Regulation D thereunder. We are filing the registration statement
on Form S-1, of which this prospectus is a part, to enable the holders of the 2021 Convertible Notes to resell the underlying ordinary
shares upon converting the convertible notes to ordinary shares, and the holders of the 2021 Warrants and the 2020 Warrants to resell
the underlying ordinary shares after exercising the warrants for cash.
The
Offering
|
Securities
offered by the selling shareholders
|
|
123,086,088
ordinary shares issuable
upon the conversion of the 2021 Convertible Notes, 78,332,201 ordinary shares issuable
upon exercise of the outstanding 2021 Warrants, and 59,648,748 ordinary shares issuable
upon exercise of the outstanding 2020 Warrants.
|
|
|
|
|
|
Ordinary
shares outstanding before this offering
|
|
977,144,432
ordinary shares, based on the number of shares
outstanding as of January 13, 2022
|
|
|
|
|
|
Ordinary
shares to be outstanding after this offering
|
|
1,505,466,119
ordinary shares (assuming
(a) the conversion of all of the Convertible Notes, (b) the exercise of all
the outstanding 2021 Warrants, (c) the exercise of all of the outstanding 2020 Warrants,
and the resale of all underlying ordinary shares by the selling shareholders in offerings
under this prospectus, (d) the conversion by Yozma Global Genomic Fund 1 of $9,571,429
in principal amount of convertible notes into 166,181,732 ordinary shares, and (e) the conversion
by Kips Bay Select LP of an additional $5,821,428 in principal amount of convertible notes
into 101,072,918 ordinary shares). The conversion price for the Convertible Notes is subject
to adjustment downwards under certain circumstances. All share numbers in this prospectus
assume that such conversion price will not be adjusted. The maximum adjustment of the conversion
price under the Convertible Notes is 20%.
|
|
|
|
|
|
Use
of proceeds
|
|
We
will not receive any proceeds from the sale of ordinary shares issuable upon conversion of the 2021 Convertible Notes by the selling
shareholders. We will, however, receive the proceeds of any Warrants exercised for cash in the future. Such net proceeds will be
up to approximately $13,506,515, based on the 2020 Warrants’ and the 2021 Warrants’ respective exercise prices.
See “Use of Proceeds” in this prospectus.
|
|
|
|
|
|
Dividend
policy
|
|
We
have never declared or paid any cash dividends on our ordinary shares. We do not anticipate paying any cash dividends in the foreseeable
future.
|
|
|
|
|
|
Risk
factors
|
|
You
should carefully consider the risk factors described in the section of this prospectus entitled “Risk Factors,” together
with all of the other information included in this prospectus, before deciding to purchase our ordinary shares.
|
SUMMARY
CONSOLIDATED FINANCIAL INFORMATION
The
following tables present the Company’s Condensed Consolidated Financial Statements as of September 30, 2021.
The
summarized financial information presented below is derived from and should be read in conjunction with our audited consolidated financial
statements including the notes to those financial statements, which are included elsewhere in this prospectus along with the section
entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our historical results
are not necessarily indicative of our future results.
TODOS
MEDICAL LTD.
CONDENSED
CONSOLIDATED BALANCE SHEETS
(U.S.
dollars in thousands except share and per share amounts)
The
accompanying notes are an integral part of these condensed consolidated financial statements.
TODOS
MEDICAL LTD.
CONDENSED
STATEMENTS OF OPERATIONS
(U.S.
dollars in thousands except share and per share amounts)
The
accompanying notes are an integral part of these condensed consolidated financial statements.
TODOS
MEDICAL LTD.
CONDENSED
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ DEFICIT
(U.S.
dollars in thousands except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ordinary shares
|
|
|
Additional paid-in
|
|
|
Accumulated
|
|
|
Total Shareholders’
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
capital
|
|
|
deficit
|
|
|
deficit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2019
|
|
|
103,573,795
|
|
|
$
|
280
|
|
|
$
|
10,979
|
|
|
$
|
(17,508
|
)
|
|
$
|
(6,249
|
)
|
|
Changes during the three months period ended March 31, 2020:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares for call option to acquire potential acquiree
|
|
|
17,091,096
|
|
|
|
49
|
|
|
|
951
|
|
|
|
-
|
|
|
|
1,000
|
|
|
Issuance of ordinary shares as settlement of previous commitments
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares as settlement of previous commitments, shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Partial conversion of convertible bridge loans into ordinary shares
|
|
|
27,336,061
|
|
|
|
78
|
|
|
|
1,508
|
|
|
|
-
|
|
|
|
1,586
|
|
|
Issuance of ordinary shares upon modification of terms relating to convertible
straight loan transaction
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares upon modification of terms relating to convertible
straight loan transaction, shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Classification of derivative warrants liability into equity as result of partial
conversion of convertible bridge loans into ordinary shares
|
|
|
-
|
|
|
|
-
|
|
|
|
333
|
|
|
|
-
|
|
|
|
333
|
|
|
Issuance of ordinary shares as partial settlement of financial liability
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares as partial settlement of financial liability, shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares and stock warrants upon modification of terms relating
to convertible bridge loans transactions
|
|
|
350,000
|
|
|
|
1
|
|
|
|
376
|
|
|
|
-
|
|
|
|
377
|
|
|
Commitment to issue units consisting of ordinary shares and stock warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
30
|
|
|
|
-
|
|
|
|
30
|
|
|
Issuance of stock warrants as part of convertible bridge loan received
|
|
|
-
|
|
|
|
-
|
|
|
|
466
|
|
|
|
-
|
|
|
|
466
|
|
|
Issuance of ordinary shares in exchange for equity line received
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares in exchange for equity line received, shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares as collateral for loan repayment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares as collateral for loan repayment, shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares or commitment for issuance of fixed number of ordinary
shares to service providers
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares as commitment shares in exchange for equity
line granted
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares as commitment shares in exchange for equity
line granted, shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares through equity line
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares through equity line, shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares as consideration to obtain control over affiliated
company
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares as consideration to obtain control over affiliated
company, shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares as commitment shares in exchange for receivables
financing facility
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares as commitment shares in exchange for receivables
financing facility, shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of units consisting of ordinary shares (or fixed number of shares to
be issued) and warrants
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of units consisting of ordinary shares (or fixed number of shares to
be issued) and warrants, shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amount related to fixed number of ordinary shares to be issued as contingent
consideration
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share based compensation for employees & directors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitment to issue shares in acquisition of subsidiary
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation to service providers
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of shares in acquisition of subsidiary
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of shares in acquisition of subsidiary, shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares to service providers
|
|
|
5,718,588
|
|
|
|
17
|
|
|
|
815
|
|
|
|
-
|
|
|
|
832
|
|
|
Net loss for the period
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(4,638
|
)
|
|
|
(4,638
|
)
|
|
Balance as of March 31, 2020 (unaudited)
|
|
|
154,069,540
|
|
|
|
425
|
|
|
|
15,458
|
|
|
|
(22,146
|
)
|
|
|
(6,263
|
)
|
|
Changes during the three months period ended June 30, 2020:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares for call option to acquire potential acquiree
|
|
|
13,008,976
|
|
|
|
37
|
|
|
|
963
|
|
|
|
-
|
|
|
|
1,000
|
|
|
Partial conversion of convertible bridge loans into ordinary shares
|
|
|
13,015,711
|
|
|
|
36
|
|
|
|
866
|
|
|
|
-
|
|
|
|
902
|
|
|
Classification of derivative warrants liability into equity as result of partial
conversion of convertible bridge loans into ordinary shares
|
|
|
-
|
|
|
|
-
|
|
|
|
193
|
|
|
|
-
|
|
|
|
193
|
|
|
Issuance of stock warrants as part of convertible bridge loan received
|
|
|
-
|
|
|
|
-
|
|
|
|
126
|
|
|
|
-
|
|
|
|
126
|
|
|
Issuance of ordinary shares as partial settlement of financial liability
|
|
|
13,750,000
|
|
|
|
39
|
|
|
|
910
|
|
|
|
-
|
|
|
|
949
|
|
|
Issuance of ordinary shares and stock warrants upon modification of terms relating
to convertible bridge loans transactions
|
|
|
720,000
|
|
|
|
2
|
|
|
|
39
|
|
|
|
-
|
|
|
|
41
|
|
|
Issuance of ordinary shares to service providers
|
|
|
7,309,915
|
|
|
|
21
|
|
|
|
966
|
|
|
|
-
|
|
|
|
987
|
|
|
Net loss for the period
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(2,585
|
)
|
|
|
(2,585
|
)
|
|
Balance as of June 30, 2020 (unaudited)
|
|
|
201,874,142
|
|
|
$
|
560
|
|
|
$
|
19,521
|
|
|
$
|
(24,731
|
)
|
|
$
|
(4,650
|
)
|
|
Changes during the three months period ended September 30, 2020:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Partial conversion of convertible bridge loans into ordinary shares
|
|
|
14,017,973
|
|
|
|
41
|
|
|
|
1,413
|
|
|
|
-
|
|
|
|
1,454
|
|
|
Issuance of stock warrants as part of convertible bridge loan received
|
|
|
-
|
|
|
|
-
|
|
|
|
582
|
|
|
|
-
|
|
|
|
582
|
|
|
Issuance of ordinary shares as commitment shares in exchange for equity line
granted
|
|
|
5,812,500
|
|
|
|
17
|
|
|
|
465
|
|
|
|
-
|
|
|
|
482
|
|
|
Issuance of ordinary shares through equity line
|
|
|
14,437,500
|
|
|
|
43
|
|
|
|
1,253
|
|
|
|
-
|
|
|
|
1,296
|
|
|
Issuance of ordinary shares as consideration to obtain control over affiliated
company
|
|
|
67,599,796
|
|
|
|
193
|
|
|
|
5,891
|
|
|
|
-
|
|
|
|
6,084
|
|
|
Issuance of ordinary shares for call option to acquire potential acquire
|
|
|
18,608,113
|
|
|
|
54
|
|
|
|
946
|
|
|
|
-
|
|
|
|
1,000
|
|
|
Issuance of ordinary shares as commitment shares in exchange for receivables
financing facility
|
|
|
3,500,000
|
|
|
|
10
|
|
|
|
305
|
|
|
|
-
|
|
|
|
315
|
|
|
Issuance of ordinary shares and stock warrants upon modification of terms relating
to convertible bridge loans transactions
|
|
|
9,333,333
|
|
|
|
27
|
|
|
|
1,191
|
|
|
|
-
|
|
|
|
1,218
|
|
|
Issuance of units consisting of ordinary shares (or fixed number of shares to
be issued) and warrants
|
|
|
1,000,000
|
|
|
|
3
|
|
|
|
(3
|
)
|
|
|
-
|
|
|
|
-
|
|
|
Classification of derivative warrants liability into equity as result of partial
conversion of convertible bridge loans into ordinary shares
|
|
|
-
|
|
|
|
-
|
|
|
|
70
|
|
|
|
-
|
|
|
|
70
|
|
|
Amount related to fixed number of ordinary shares to be issued as contingent
consideration
|
|
|
-
|
|
|
|
-
|
|
|
|
1,300
|
|
|
|
-
|
|
|
|
1,300
|
|
|
Share based compensation for employees & directors
|
|
|
-
|
|
|
|
-
|
|
|
|
460
|
|
|
|
-
|
|
|
|
460
|
|
|
Share based compensation for service providers
|
|
|
-
|
|
|
|
-
|
|
|
|
57
|
|
|
|
-
|
|
|
|
57
|
|
|
Net loss for the period
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(18,035
|
)
|
|
|
(18,035
|
)
|
|
Balance as of September 30, 2020 (unaudited)
|
|
|
336,183,357
|
|
|
$
|
948
|
|
|
$
|
33,451
|
|
|
$
|
(42,766
|
)
|
|
$
|
(8,367
|
)
|
The
accompanying notes are an integral part of these condensed consolidated financial statements.
TODOS
MEDICAL LTD.
CONDENSED
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ DEFICIT
(U.S.
dollars in thousands except share and per share amounts)
|
|
|
Ordinary shares
|
|
|
Additional paid-in
|
|
|
Accumulated
|
|
|
Total Shareholders’
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
capital
|
|
|
deficit
|
|
|
deficit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2020
|
|
|
376,335,802
|
|
|
$
|
1,059
|
|
|
$
|
35,211
|
|
|
$
|
(47,281
|
)
|
|
$
|
(11,011
|
)
|
|
Changes during the three months period ended March 31, 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of ordinary shares as settlement of previous commitments
|
|
|
2,500,000
|
|
|
|
8
|
|
|
|
(8
|
)
|
|
|
-
|
|
|
|
-
|
|
|
Partial conversion of convertible bridge loans into ordinary shares
|
|
|
134,358,817
|
|
|
|
409
|
|
|
|
6,461
|
|
|
|
-
|
|
|
|
6,870
|
|
|
Issuance of ordinary shares upon modification of terms relating to convertible
straight loan transaction
|
|
|
2,000,000
|
|
|
|
6
|
|
|
|
82
|
|
|
|
-
|
|
|
|
88
|
|
|
Issuance of stock warrants as part of convertible bridge loan received
|
|
|
-
|
|
|
|
-
|
|
|
|
792
|
|
|
|
-
|
|
|
|
792
|
|
|
Issuance of ordinary shares in exchange for equity line received
|
|
|
5,229,809
|
|
|
|
16
|
|
|
|
239
|
|
|
|
-
|
|
|
|
255
|
|
|
Issuance of ordinary shares as collateral for loan repayment
|
|
|
20,000,000
|
|
|
|
61
|
|
|
|
809
|
|
|
|
-
|
|
|
|
870
|
|
|
Issuance of ordinary shares or commitment for issuance of fixed number of ordinary
shares to service providers
|
|
|
|
|
|
36
|
|
|
|
30
|
|
|
|
-
|
|
|
|
66
|
|
|
Stock-based compensation to employees and directors
|
|
|
-
|
|
|
|
-
|
|
|
|
169
|
|
|
|
-
|
|
|
|
169
|
|
|
Net loss for the period
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(17,557
|
)
|
|
|
(17,557
|
)
|
|
Balance as of March 31, 2021 (unaudited)
|
|
|
552,345,481
|
|
|
|
1,595
|
|
|
|
43,785
|
|
|
|
(64,838
|
)
|
|
|
(19,458
|
)
|
|
Changes during the three months period ended June 30, 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Partial conversion of convertible bridge loans into ordinary shares
|
|
|
55,415,011
|
|
|
|
170
|
|
|
|
1,606
|
|
|
|
-
|
|
|
|
1,776
|
|
|
Issuance of stock warrants as part of convertible bridge loan received
|
|
|
-
|
|
|
|
-
|
|
|
|
3,430
|
|
|
|
-
|
|
|
|
3,430
|
|
|
Stock-based compensation to service providers
|
|
|
-
|
|
|
|
-
|
|
|
|
21
|
|
|
|
-
|
|
|
|
21
|
|
|
Commitment to issue shares in acquisition of subsidiary
|
|
|
-
|
|
|
|
-
|
|
|
|
1,699
|
|
|
|
-
|
|
|
|
1,699
|
|
|
Stock-based compensation to employees and directors
|
|
|
-
|
|
|
|
-
|
|
|
|
143
|
|
|
|
-
|
|
|
|
143
|
|
|
Net income for the period
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
3,390
|
|
|
|
3,390
|
|
|
Balance as of June 30, 2021 (unaudited)
|
|
|
607,760,492
|
|
|
$
|
1,765
|
|
|
$
|
50,684
|
|
|
$
|
(61,448
|
)
|
|
$
|
(8,999
|
)
|
|
Changes during the three months period ended September 30, 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Partial conversion of convertible bridge loans into ordinary shares
|
|
|
238,190,489
|
|
|
|
739
|
|
|
|
7,179
|
|
|
|
-
|
|
|
|
7,918
|
|
|
Issuance of stock warrants as part of convertible bridge loan received
|
|
|
-
|
|
|
|
-
|
|
|
|
728
|
|
|
|
-
|
|
|
|
728
|
|
|
Stock-based compensation to service providers
|
|
|
|
|
|
9
|
|
|
|
76
|
|
|
|
-
|
|
|
|
85
|
|
|
Issuance of shares in acquisition of subsidiary
|
|
|
25,862,069
|
|
|
|
80
|
|
|
|
(80
|
)
|
|
|
-
|
|
|
|
-
|
|
|
Stock-based compensation to employees and directors
|
|
|
-
|
|
|
|
-
|
|
|
|
148
|
|
|
|
-
|
|
|
|
148
|
|
|
Net loss for the period
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(10,379
|
)
|
|
|
(10,379
|
)
|
|
Net income (loss)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(10,379
|
)
|
|
|
(10,379
|
)
|
|
Balance as of September 30, 2021 (unaudited)
|
|
|
874,813,050
|
|
|
$
|
2,593
|
|
|
$
|
58,735
|
|
|
$
|
(71,827
|
)
|
|
$
|
(10,499
|
)
|
The
accompanying notes are an integral part of these condensed consolidated financial statements.
TODOS
MEDICAL LTD.
CONDENSED
CONSOLIDATED STATEMENTS OF CASH FLOWS
(U.S.
dollars in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine
months period ended
September
30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
|
|
Unaudited
|
|
|
Unaudited
|
|
|
Cash flows from operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(24,547
|
)
|
|
$
|
(25,258
|
)
|
|
Adjustments required to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Depreciation
|
|
|
556
|
|
|
|
38
|
|
|
Liability for minimum royalties
|
|
|
53
|
|
|
|
29
|
|
|
Stock-based compensation
|
|
|
633
|
|
|
|
2,334
|
|
|
Expiration of call options to acquire potential acquiree
|
|
|
-
|
|
|
|
3,000
|
|
|
Impairment of intangible IPR&D, net of taxes
|
|
|
-
|
|
|
|
8,157
|
|
|
Impairment of investment in affiliated company
|
|
|
-
|
|
|
|
2,823
|
|
|
Revaluation of investment in affiliated company to fair value
|
|
|
-
|
|
|
|
(1,623
|
)
|
|
Share in losses of affiliated company
|
|
|
1,499
|
|
|
|
-
|
|
|
Modification of terms relating to straight loan transaction
|
|
|
88
|
|
|
|
-
|
|
|
Modification of terms relating to convertible bridge loans transactions
|
|
|
-
|
|
|
|
(3,495
|
)
|
|
Exchange differences relating to loans from shareholders
|
|
|
-
|
|
|
|
40
|
|
|
Issuance of shares as a settlement in excess of the carrying amount of financial
liabilities
|
|
|
-
|
|
|
|
499
|
|
|
Issuance of ordinary shares and stock warrants upon modification of terms relating
to convertible bridge loans transactions
|
|
|
-
|
|
|
|
415
|
|
|
Amortization of discounts and accrued interest on convertible bridge loans
|
|
|
18,080
|
|
|
|
8,393
|
|
|
Amortization of discounts and accrued interest on straight loans
|
|
|
2,290
|
|
|
|
-
|
|
|
Change in fair value of derivative warrants liability and fair value of warrants
expired
|
|
|
(299
|
)
|
|
|
-
|
|
|
Change in fair value of liability related to conversion feature of convertible
bridge loans
|
|
|
(3,777
|
)
|
|
|
-
|
|
|
Increase in trade receivables
|
|
|
(1,629
|
)
|
|
|
(206
|
)
|
|
Increase in inventories
|
|
|
(806
|
)
|
|
|
(440
|
)
|
|
Decrease (increase) in other current assets
|
|
|
704
|
|
|
|
48
|
|
|
Increase (decrease) in accounts payables
|
|
|
(961
|
)
|
|
|
203
|
|
|
Decrease in deferred revenues
|
|
|
(857
|
)
|
|
|
-
|
|
|
Increase (decrease) in other current liabilities
|
|
|
(326
|
)
|
|
|
1,376
|
|
|
Net cash used in operating activities
|
|
|
(9,299
|
)
|
|
|
(3,667
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of property and equipment
|
|
|
(965
|
)
|
|
|
(346
|
)
|
|
Restricted cash
|
|
|
-
|
|
|
|
5
|
|
|
Purchase of intangible IPR&D
|
|
|
-
|
|
|
|
(450
|
)
|
|
Cash used in purchased of subsidiary consolidated for the first time
|
|
|
(1,176
|
)
|
|
|
-
|
|
|
Investment in other companies
|
|
|
(1,024
|
)
|
|
|
(560
|
)
|
|
Net cash used in investing activities
|
|
|
(3,165
|
)
|
|
|
(1,351
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from straight loans, net
|
|
|
2,496
|
|
|
|
812
|
|
|
Repayment of Receivables financing facility
|
|
|
(1,249
|
)
|
|
|
-
|
|
|
Repayment of straight loans
|
|
|
(1,329
|
)
|
|
|
-
|
|
|
Repayment of convertible bridge loans
|
|
|
(2,165
|
)
|
|
|
-
|
|
|
Proceeds from issuance of units consisting of convertible bridge loans, stock
warrants and shares, net
|
|
|
13,687
|
|
|
|
3,087
|
|
|
Proceeds from issuance of units consisting of ordinary shares and stock warrants
|
|
|
-
|
|
|
|
30
|
|
|
Proceeds from issuance of ordinary shares through equity
line
|
|
|
255
|
|
|
|
1,296
|
|
|
Net cash provided by financing activities
|
|
|
11,695
|
|
|
|
5,225
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in cash, cash equivalents
|
|
|
(769
|
)
|
|
|
207
|
|
|
Cash, cash equivalents at beginning of period
|
|
|
935
|
|
|
|
12
|
|
|
Cash, cash equivalents at end of period
|
|
$
|
166
|
|
|
$
|
219
|
|
The
accompanying notes are an integral part of these condensed consolidated financial statements.
TODOS
MEDICAL LTD.
CONDENSED
CONSOLIDATED STATEMENTS OF CASH FLOWS (Cont.)
(U.S.
dollars in thousands)
|
|
|
Nine months period ended September
30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
|
|
Unaudited
|
|
|
Unaudited
|
|
|
Supplemental disclosure of non-cash activities:
|
|
|
|
|
|
|
|
Issuance of warrants as part of bridge loan transactions
|
|
|
4,157
|
|
|
|
948
|
|
|
Partial conversion of convertible bridge loans and liability related to conversion
feature of convertible bridge loans into ordinary shares
|
|
|
(16,493
|
)
|
|
|
(3,943
|
)
|
|
Issuance of stock warrants as part of convertible bridge loan received
|
|
|
(870
|
)
|
|
|
|
|
|
Issuance of shares upon acquisition of an IPR&D
|
|
|
-
|
|
|
|
6,084
|
|
|
Issuance of shares for receiving an equity line
|
|
|
-
|
|
|
|
(315
|
)
|
|
Issuance of shares or commitment to issue fixed number of shares for receiving
convertible bridge loans
|
|
|
-
|
|
|
|
482
|
|
|
Issuance of ordinary shares upon modification of terms relating to convertible
straight loan transaction
|
|
|
792
|
|
|
|
|
|
|
Issuance of shares as settlement of financial liabilities
|
|
|
-
|
|
|
|
(450
|
)
|
|
Investment in affiliated company by issuance shares and commitment for issued
shares as contingent consideration and commitment for funding
|
|
|
-
|
|
|
|
(2,615
|
)
|
|
Classification of warrants from liability into equity upon partial conversion
of convertible bridge loans into ordinary shares
|
|
|
-
|
|
|
|
(595
|
)
|
|
Conversion of loan from shareholder into ordinary shares
|
|
|
-
|
|
|
|
40
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash used in purchased of subsidiary consolidated for the first time:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Working capital (excluding cash and cash equivalents)
|
|
|
(18
|
)
|
|
|
|
|
|
Fixed assets
|
|
|
183
|
|
|
|
|
|
|
Long term assets
|
|
|
3
|
|
|
|
|
|
|
Net assets acquired
|
|
|
168
|
|
|
|
|
|
|
Goodwill acquired
|
|
|
7,761
|
|
|
|
|
|
|
Intangible assets acquired
|
|
|
1,500
|
|
|
|
|
|
|
Second cash installment payable
|
|
|
(1,250
|
)
|
|
|
|
|
|
Consideration in convertible promissory note
|
|
|
(4,989
|
)
|
|
|
|
|
|
Consideration in Shares
|
|
|
(1,699
|
)
|
|
|
|
|
|
Deferred tax liability
|
|
|
(315
|
)
|
|
|
|
|
|
Net cash used in purchase of subsidiary consolidated for the first time
|
|
|
1,176
|
|
|
|
|
|
RISK
FACTORS
An
investment in our securities involves risks. Our business, financial condition or results of operations could be materially and adversely
affected by any of these risks. If any of these risks occur, the value of our Ordinary Shares and our other securities may decline.
Before investing in our Ordinary Shares, you should consider carefully any risk factors set forth in this prospectus, any prospectus
supplement hereto or any free writing prospectus that we have authorized for use in connection with this offering and the risk factors
described below.
An
investment in our Ordinary Shares involves a high degree of risk. You should carefully consider the following factors and other information
in this prospectus before deciding to invest in us. If any of the following risks actually occur, our business, financial condition,
results of operations and prospects for growth would likely suffer. As a result, you could lose all or part of your investment. Additional
risks and uncertainties not presently known to us or that we currently deem immaterial also may materially and adversely affect our business,
financial condition and results of operations.
Risks
Related to Our Business
We
have a history of losses, may incur future losses and may not achieve profitability.
Until
March 2020, we were solely a clinical-stage medical diagnostics company with a limited operating history. We have incurred net losses
in each fiscal year since we commenced operations in 2010. We incurred net losses of $11,904,000 and $11,814,515 in the fiscal years
ended December 31, 2020, and 2019, respectively, and we may incur a net loss in 2021. As of December 31, 2020, our accumulated deficit
was $47,142,000. Our losses could continue for the foreseeable future, as we continue our investment in research and development and
clinical trials to complete the development of our technology and to attain regulatory approvals, begin the commercialization efforts
for our cancer detection kits, increase our marketing and selling expenses, and incur additional costs as a result of being a publicly
reporting company in the United States. The extent of our future operating losses and the timing of becoming profitable are highly uncertain,
and we may never achieve or sustain profitability.
We
have a need for substantial additional financing and will have to significantly delay, curtail or cease operations if we are unable to
secure such financing.
The
Company requires substantial additional financing to fund its operations. As of September 30, 2021, we had cash and cash equivalents
of $166,000. We will need to raise additional funds prior to commercializing our cancer detection products. Additional financing may
not be available to us on a timely basis on terms acceptable to us, or at all. In addition, any additional financing may be dilutive
to our shareholders or may require us to grant a lender a security interest in our assets.
The
report of our independent registered public accounting firm expresses substantial doubt about our ability to continue as a going concern.
Our
former independent registered public accounting firm indicated in its report on our financial statements for the year ended December
31, 2020, included elsewhere in this prospectus, that conditions exist that raise substantial doubt about our ability to continue as
a going concern. A going concern paragraph included in our independent registered public accounting firm’s report on our financial
statements, could color investor perceptions and impair our ability to finance our operations through the sale of equity, incurring debt,
or other financing alternatives. Our ability to continue as a going concern will depend upon many factors beyond our control including
the availability and terms of future funding and the demand for COVID-19 tests. If we are unable to achieve our goals and raise the necessary
funds to finance our operations, our business would be jeopardized, and we may not be able to continue. If we ceased operations, it is
likely that all of our investors would lose their investment.
Risks
Related to our COVID-19 Antibody Test
In
connection with the marketing and sale of our COVID-19 antibody test, we are relying on FDA policies and guidance provisions that have
recently changed and may continue to change. If we misinterpret this guidance or the guidance changes unexpectedly and/or materially,
potential sales of our COVID-19 antibody test could be impacted.
The
FDA issued non-binding guidance for manufacturers relating to the pathway to enable FDA notification following confirmed validation for
devices related to testing for COVID-19 under the Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency. Following
the issuance of the guidance published on March 16, 2020, revised guidance specific to COVID-19 ‘antibody tests’ was issued.
Newer guidance was published on May 4, 2020 further describing the requirements for serology tests to continue to be marketed under an
Emergency Use Authorization. If our interpretation of the newly revised guidance is incorrect or specifics around the guidance change,
sales of our COVID-19 antibody test could be materially impacted.
There
can be no assurance of market acceptance for our COVID-19 antibody test.
The
commercial success of our COVID-19 antibody test will depend upon its acceptance as medically useful and cost-effective by physicians
and other members of the medical community, patients and third-party payers. Broad market acceptance can be achieved only with substantial
education about the benefits and limitations of such tests, as well as resolution of concerns about their appropriate use. Our reputation
and the public image of our COVID-19 antibody test kits may be impaired if they fail to perform as expected or are perceived as difficult
to use. Despite quality control testing, defects or errors could occur with the tests. Thus, there can be no assurance our COVID-19 antibody
test will gain market acceptance on a timely basis, if at all, and purchasers of such tests could choose to purchase competitors’
tests instead. Failure to achieve market acceptance and/or the impact of strong competition will have a material adverse effect on our
business, financial condition and results of operations.
We
rely on a third party to manufacture the COVID-19 antibody tests for us, and if such third party refuses or is unable to supply us with
the COVID-19 test kits, our business will be materially harmed.
We
rely on a third party to manufacture the COVID-19 antibody tests. If any issues arise with respect to the manufacturer’s ability
to manufacture and deliver to us the COVID-19 tests, our business could be materially harmed. In addition, the manufacturer may be unable
to provide us with an adequate supply of COVID-19 antibody tests for various reasons, including, among others, if it becomes insolvent,
if a United States regulatory authority or other governments block the import or sale of the COVID-19 tests or if it fails to maintain
its rights to manufacture the COVID-19 test. If we are unable to keep up with demand for the COVID-19 antibody test kits, our revenue
growth could be impaired, market acceptance for the test could be adversely affected, and our customers might instead purchase our competitors’
diagnostic tests.
We
have relied and expect to continue to rely on third parties to conduct studies of the COVID-19 diagnostic tests that will be required
by the FDA or other regulatory authorities, and those third parties may not perform satisfactorily.
Although
we are selling our COVID-19 antibody test kits by virtue of recent FDA guidance allowing for reduced product clinical and analytical
studies, we have relied on third parties, such as independent testing laboratories and hospitals, to conduct such studies. Our reliance
on these third parties will reduce our control over these activities. These third-party contractors may not complete activities on schedule
or conduct studies in accordance with regulatory requirements or our study design. We cannot control whether they devote sufficient time,
skill and resources to our studies. Our reliance on third parties that we do not control will not relieve us of any applicable requirement
to prepare, and ensure compliance with, various procedures required under good clinical practices. If these third parties do not successfully
carry out their contractual duties or regulatory obligations or meet expected deadlines, if the third parties need to be replaced or
if the quality or accuracy of the data they obtain is compromised due to their failure to adhere to our clinical protocols or regulatory
requirements or for other reasons, our studies may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory
approval for additional diagnostic tests.
We
may not succeed in completing the development of our cancer detection products, commercializing our products or generating significant
revenues.
From
the commencement of our operations until March 2020, we have focused on the research and development and limited clinical trials of our
cancer detection kits. Our ability to generate revenues and achieve profitability in the long run depends on our ability to successfully
complete the development of our products, obtain market approval and generate significant revenues. The future success of our business
cannot be determined at this time, and we do not anticipate generating revenues from cancer detection product sales for the foreseeable
future. At the same time, we recognize that the recent development of several vaccines for COVID-19 may make our COVID-19 tests less
valuable. In addition, we face a number of challenges with respect to our future commercialization efforts of our products that do not
relate to COVID-19, including, among others, that:
|
|
●
|
we
may not have adequate financial or other resources to complete the development of our products;
|
|
|
|
|
|
|
●
|
we
may not be able to manufacture our products in commercial quantities, at an adequate quality or at an acceptable cost;
|
|
|
|
|
|
|
●
|
we
may not be able to meet the timing schedule for (a) completing successful clinical trials in the U.S.; and (b) receiving U.S. Food
and Drug Administration, or FDA, approval within our goal of approximately two to four years;
|
|
|
|
|
|
|
●
|
we
may not be able to maintain our CE mark due to the regulatory changes;
|
|
|
|
|
|
|
●
|
we
may never receive FDA approval, for our intended development plan;
|
|
|
|
|
|
|
●
|
we
may not be able to establish adequate sales, marketing and distribution channels;
|
|
|
|
|
|
|
●
|
healthcare
professionals and patients may not accept our cancer detection kits;
|
|
|
|
|
|
|
●
|
technological
breakthroughs in cancer detection, treatment and prevention may reduce the demand for our products;
|
|
|
|
|
|
|
●
|
changes
in the market for cancer detection, new alliances between existing market participants and the entrance of new market participants
may interfere with our market penetration efforts;
|
|
|
|
|
|
|
●
|
third-party
payors may not agree to reimburse patients for any or all of the purchase price of our products, which may adversely affect patients’
willingness to purchase our cancer detection kits;
|
|
|
|
|
|
|
●
|
uncertainty
as to market demand may result in inefficient pricing of our cancer detection kits;
|
|
|
|
|
|
|
●
|
we
may face third-party claims of intellectual property infringement;
|
|
|
|
|
|
|
●
|
we
may fail to obtain or maintain regulatory approvals for our cancer detection kits in our target markets or may face adverse regulatory
or legal actions relating to our cancer detection kits even if regulatory approval is obtained; and
|
|
|
|
|
|
|
●
|
we
are dependent upon the results of ongoing clinical studies relating to our cancer detection kits and the products of our competitors.
We may fail in obtaining positive results.
|
If
we are unable to meet any one or more of these challenges successfully, our ability to effectively commercialize our cancer detection
kits could be limited, which in turn could have a material adverse effect on our business, financial condition and results of operations.
We
are currently in the process of improving our technology and adapting to the high throughput methodology.
We
believe our existing protocols and measurement instruments are sufficient to support the initial commercial launch in Israel and Europe.
However, we plan to change our protocol and measurement instrument as well as our sample handling in order to adapt it to new high throughput
methodology once we have successfully commercialized and have begun research activities on this second-generation protocols and measurement
instruments. The changes we plan to implement in the second-generation protocol and measurement instrument are significant. The new protocol
aims to be more robust, reproducible, fast and easy to handle, however, this transformation from the older manual protocol to the new
protocol incurs several risks. To our management’s knowledge, the new protocol will not impact the previously obtained European
Conformity, or CE, mark of approval of the TBIA test. The results may not be as promising as the former version and although some procedures
may be more reproducible, these procedures will unfortunately damage some molecules, which were part of the diagnostic features in the
previous protocol.
The
previous tests we performed were preliminary or limited un-blinded studies.
We
consider the tests conducted by us, as of the current date, under our method, to be preliminary or limited, as they include a relatively
small number of test subjects. Thus, there is a risk in having less sufficient sensitivity and/or specificity in the trials we plan on
conducting with larger populations, in comparison to the preliminary data we have gathered thus far. Increasing the population can increase
the variance in the medical condition of the control patients as well as the cancer patients, thus affecting our test performances with
regard to cancer detection.
If
healthcare professionals do not recommend our product to their patients, our cancer detection kits may not achieve market acceptance
and we may not become profitable.
Cancer
detection candidates are generally referred to a specified device by their healthcare professional and detection technologies are purchased
by prescription. If healthcare professionals, including physicians, do not recommend or prescribe our product to their patients, our
cancer detection kits may not achieve market acceptance and we may not become profitable. In addition, physicians have historically been
slow to change their medical diagnostic and treatment practices because of perceived liability risks arising from the use of new products.
Delayed adoption of our cancer detection kits by healthcare professionals could lead to a delayed adoption by patients and third-party
payors. Healthcare professionals may not recommend or prescribe our cancer detection kits until certain conditions have been satisfied,
including, among others:
|
|
●
|
sufficient
long-term clinical evidence to convince them to supplement their existing detection methods and device recommendations;
|
|
|
|
|
|
|
●
|
recommendations
from other prominent physicians, educators and/or associations that our cancer detection kits are safe and effective;
|
|
|
|
|
|
|
●
|
obtainment
of favorable data from clinical studies for our cancer detection kits; and
|
|
|
|
|
|
|
●
|
availability
of reimbursement or insurance coverage from third-party payors.
|
We
cannot predict when, if ever, healthcare professionals and patients may adopt the use of our cancer detection kits. Even if favorable
data is obtained from clinical studies for our cancer detection kits, there can be no assurance that physicians would endorse it or that
future clinical studies will continue to produce favorable data regarding our cancer detection kits. In addition, prolonged market exposure
may also be a pre-requisite to reimbursement or insurance coverage from third-party payors. If our cancer detection kits do not achieve
an adequate level of acceptance by patients, healthcare professionals and third-party payors, we may not generate significant product
revenues and we may not become profitable.
Our
reliance on limited source suppliers could harm our ability to meet demand for our product in a timely manner or within budget.
We
currently depend on a limited number of source suppliers for some of the components necessary for the production of our product. Our
current suppliers have been able to supply the required quantities of such components to date. However, if the supply of these components
is disrupted or terminated or if our current suppliers are unable to supply required quantities of components, we may not be able to
find alternative sources for these key components in a timely manner. Although we are planning to maintain strategic inventory of key
components, the inventory may not be sufficient to satisfy the demand for our products if such supply is interrupted or otherwise affected
by catastrophic events such as a fire at our storage facility. As a result, we may be unable to meet the demand for our testing kits,
which could harm our ability to generate revenues, lead to customer dissatisfaction and damage our reputation. If we are required to
change the manufacturer of any of these key components, there may be a significant delay in locating a suitable alternative manufacturer.
The delays associated with the identification of a new manufacturer could delay our ability to manufacture our testing kits in a timely
manner or within budget. Furthermore, in the event that the manufacturer of a key component of our testing kits ceases operations or
otherwise ceases to do business with us, we may not have access to the information necessary to enable another supplier to manufacture
the component. The occurrence of any of these events could harm our ability to meet demand for our testing kits in a timely manner or
within budget.
We
have sent a demand letter to a significant customer, which has not yet paid for some of the Covid-19 testing kits that we supplied to
it.
During
the first quarter of 2021, the Company’s contractual agreement to supply Covid-19 testing kits to NOAH Laboratories, Inc., a significant
customer, expired. At the customer’s request, the Company continued to supply Covid-19 testing kits until such time as the customer
requested that the Company stop doing so. The customer has not yet paid for some of the Covid-19 testing kits so supplied, and has not
renewed its agreement with the Company. On November 15, 2021, Todos USA sent a demand letter (the “Demand Letter”) to a significant
customer with which our contractual agreement to supply Covid-19 testing kits expired. The Demand Letter seeks (a) payment for testing
kits that Todos USA supplied for which it was not paid, in the amount of $3,465,000, (b) the return of Todos USA’s equipment, title
to which remains with Todos USA unless and until the significant customer meets a minimum purchase requirement, and (c) payment of damages
as a result of the significant customer’s unlawful retention of Todos USA’s equipment, in an amount anticipated to be $2
million. The Company and Todos USA are negotiating a settlement with NOAH Laboratories, Inc., which the Company believes will
result in most of the Company’s demands being met. The Company believes that ultimately the customer will pay for
the Covid-19 testing kits so supplied. Should the customer not make payment in full of all amounts owed to the Company, it could have
a material adverse effect on the Company’s revenues from the sale of Covid-19 testing kits.
The
use of any of our products could result in product liability or similar claims that could have an adverse effect on our business, financial
condition, results of operations and our reputation.
Our
business exposes us to an inherent risk of potential product liability or similar claims related to the manufacturing, marketing and
sale of medical devices. The medical device industry has historically been litigious, and we face financial exposure to product liability
or similar claims if the use of our kits were to cause or contribute to injury or death, including, without limitation, harm to the body
caused by the procedure or inaccurate diagnoses from the procedure that could affect treatment options. There is also the possibility
that defects in the design or manufacture of any of these products might necessitate a product recall. Although we plan to maintain product
liability insurance, the coverage limits of these policies may not be adequate to cover future claims. In the future, we may be unable
to maintain product liability insurance on acceptable terms or at reasonable costs and such insurance may not provide us with adequate
coverage against potential liabilities. A product liability claim, regardless of merit or ultimate outcome, or any product recall could
result in substantial costs to us, damage to our reputation, customer dissatisfaction and frustration, and a substantial diversion of
management attention. A successful claim brought against us in excess of, or outside of, our insurance coverage could have a material
adverse effect on our business, financial condition, results of operations and our reputation.
We
will require additional funding in order to commercialize our cancer detection kits and to develop and commercialize any future products.
Assuming
we are successful in raising additional capital, we will continue our efforts to commercialize our cancer detection kits.
In
order to market and sell our products in Israel, we require the approval of the Ministry of Health. To the best of our knowledge, approval
of our products by the Ministry of Health requires us to comply with CE mark approval and International Organization for Standardization
(ISO) 13485 (both of which we have already obtained). We were previously approved to sell our products in Israel, and we have restarted
the regulatory approval process in Israel and expect the regulatory approval process in Israel to take until the end of the second quarter
of 2022, although there may be delays due to the backlog from the Coronavirus pandemic.
Furthermore,
if adequate additional financing on acceptable terms is not available, we may not be able to develop our cancer detection kits at the
rate or to the stage we desire, and we may have to delay or abandon the commercialization of our cancer detection kits. Alternatively,
we may be required to prematurely license to third parties the rights to further develop or to commercialize our cancer detection kits
on terms that are not favorable to us. Any of these factors could materially adversely affect our business, financial condition and results
of operations.
We
are entering a potentially highly competitive market.
Early
detection is vital to the treatment of cancer, which is also the focus area of our products. The diagnostic, pharmaceutical and biopharmaceutical
industries are characterized by intense competition and rapid, significant technological changes. Many companies, research institutions
and universities are conducting research and development in a number of areas similar to those that we focus on that could lead to the
development of new products which could possibly compete with our own. Most of the companies against which we will compete have substantially
greater financial, technical, manufacturing, marketing, distribution and other resources. A number of these companies may have or may
develop technologies for developing products for detecting various cancers that could prove to be the same or even superior to ours.
We expect technological developments in the diagnostic, pharmaceutical, biopharmaceutical and related fields to occur at a rapid rate,
and we believe competition will intensify as advances in these fields are made.
Our
future success depends in part on our ability to retain our executive officers and to attract, retain and motivate other qualified personnel.
We
are highly dependent on the principal members of our management, research and development team and scientific staff. In order to implement
our business strategy, we will need to attract and retain key personnel with expertise in the areas of research and development, clinical
testing, government regulation, manufacturing, finance, marketing and sales. The inability to recruit and retain qualified personnel,
or the loss of the services of our executive officers, without proper replacement, may impede the progress of our development and commercialization
objectives.
There
are future financial risks associated with funding our business operations with loans.
It
is highly likely that we will find it necessary to borrow funds from banks or other financial institutions. In particular, despite the
fact that we have repurchased a significant portion of our convertible debt, we may still need to borrow money in order to repurchase
additional convertible debt that we have issued to finance our operations, the conversion of which may have a depressant effect on our
stock price. No assurances can be given that, at the time we desire to borrow funds, banks or other financial institutions will be willing
to loan funds to us or that, if willing, they will do so on terms acceptable to our management. If we cannot borrow sufficient funds
for our operations, that might have a material adverse effect on our business, financial condition or operating results.
We
may become involved in legal proceedings.
From
time to time, we may become involved in various lawsuits and legal proceedings, including securities class action litigation. In the
past, biotechnology and pharmaceutical companies have experienced significant share price volatility, particularly when associated with
binary events such as clinical trials and product approvals. If we face such litigation, it could result in substantial costs and a diversion
of management’s attention and resources, which could harm our business and result in a decline in the market price of our Ordinary
Shares.
We
may face tax exposure as a result of the Provista transaction.
On
April 19, 2021, the Company entered into an Agreement to Purchase Provista Diagnostics, Inc. (“Agreement to Purchase”) with
Strategic Investment Holdings, LLC (“SIH”), Ascenda BioSciences LLC (“Ascenda”) and Provista Diagnostics, Inc.
(“Provista”). Ascenda was the sole owner of the outstanding securities of Provista and SIH is the sole owner of all the outstanding
securities of Ascenda.
Pursuant
to the Agreement to Purchase, the Company acquired Provista from Ascenda and SIH for an aggregate purchase price of $7.5 million consisting
of an initial cash payment of $1.25 million, the issuance of $1.5 million in Ordinary Shares of the Company priced at $0.0512 per share,
the issuance of a $3.5 million convertible promissory note dated April 19, 2021 (the “Note”) and the payment on for before
July 1, 2021 of $1.25 million in cash (the “July Payment”), which payment the Company had the right to, and did, extend to
July 15, 2021. The Provista shares acquired by the Company remained in an escrow account until the July Payment was made. The Note has
a maturity date of April 8, 2025, and is convertible beginning on October 20, 2021, into Ordinary Shares of the Company at a conversion
price equal to the lesser of $0.05 or the volume weighted average price of the last 20 trading days for the Ordinary Shares prior to
the date of conversion. In the event SIH delivers a Notice of Conversion to the Company at a per share price less than $0.05 ($0.05),
the Company has the right to immediately notify SIH of its intention to pay the conversion amount in cash within three (3) business days
of receipt of the Notice of Conversion (i.e., before SIH would take possession of shares converted under the Notice of Conversion). If,
at any time between October 20, 2021 and April 20, 2022, the average of the lowest bid and closing sale price at 4:00:00 p.m., New York
time (or such other time as such market publicly announces is the official close of trading) is below ($0.05), the Company has the option
to buy out all or any portion of the Note (the “Buyback Option”). In the event the Company exercises the Buyback Option for
an amount equal to or greater than one million, one hundred seventy thousand dollars ($1,170,000) (the “Buyback Amount”),
SIH may not submit any conversions below five cents ($0.05) for ninety (90) days from receipt of the Buyback Amount (“90 Day Period”).
In
the event that the Company uplists its Ordinary Shares to a national securities exchange, the Note shall automatically be exchanged into
Series B preferred stock with a conversion price equal to the lesser of (a) $0.05, (b) the opening price on the day of the uplisting
provides there is no transaction associated with the uplisting or (c) the deal price of an uplisting transaction.
As
of the date of this prospectus, SIH has not submitted a Conversion Notice.
To
the extent that the value of the assets transferred to us in the transaction are not comparable to the value of our Ordinary Shares issued
to SIH pursuant to the Option Agreement, we may face tax exposure in both Israel and the United States.
We
may face tax liability as a result of the Amarantus transaction.
On
February 27, 2019, we entered into a joint venture agreement with Amarantus Bioscience Holdings, Inc. (“Amarantus”)
pursuant to which we issued Ordinary Shares representing 19.99% of the then-issued and outstanding Ordinary Shares of our Company to
Amarantus, in exchange for Amarantus transferring to us 19.99% of Breakthrough, which was then a wholly-owned subsidiary of
Amarantus, and for Amarantus assigning its amended and restated license agreement with the University of Leipzig for an exclusive
license to develop and commercialize the LymPro Test®, an immune-based neurodiagnostic blood test for the detection of
Alzheimer’s disease (the “License”). On April 14, 2019, we notified Amarantus of the Company’s decision to
exercise its option to acquire the remaining 80.01% of Breakthrough held by Amarantus in exchange for the issuance to Amarantus of
Ordinary Shares of the Company representing an additional thirty percent (30%) of the Company’s then-issued and outstanding
share capital, such that the Company would own 100% of Breakthrough, and Amarantus would own 49.99% of the Company. At the annual
meeting of shareholders of the Company held on April 29, 2019, the Company’s shareholders voted on a resolution approving the
Company’s exercise of this option. On July 28, 2020, the Company entered into Amendment No. 1 to the Binding Joint Venture
Agreement with Amarantus pursuant to which the parties agreed that the Company would issue 49.9% of its Ordinary Shares as of
December 31, 2019 to Amarantus in exchange for the 80.01% equity interest it does not own of Breakthrough Diagnostics, Inc. In
addition, Amarantus will receive a 10% royalty on LymPro intellectual property. On July 28, 2020, the Company exercised this option
and issued an additional 67,599,796 Ordinary Shares to Amarantus. To the extent that the value of the assets transferred to us in
the transaction is not comparable to the value of our Ordinary Shares issued to Amarantus in this transaction, we may face a tax
exposure in both Israel and the United States.
Our
U.S. Holders may suffer adverse tax consequences if we were to be characterized as a passive foreign investment company, or PFIC.
Generally,
if for any taxable year 75% or more of our gross income is passive income, or at least 50% of our assets are held for the production
of, or produce, passive income, we would be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax
purposes. There can be no assurance that we will not be classified as a PFIC in any year. If we were to be characterized as a PFIC for
U.S. federal income tax purposes in any taxable year during which a U.S. Holder, as defined in “Taxation— U.S. Tax
Considerations”, owns Ordinary Shares, such U.S. Holder could face adverse U.S. federal income tax consequences, including
having gains realized on the sale of our Ordinary Shares classified as ordinary income, rather than as capital gains, a loss of the preferential
rate applicable to dividends received on our Ordinary Shares by individuals who are U.S. Holders and having interest charges apply to
distributions by us and the proceeds of share sales. Certain elections exist that may alleviate some of the adverse consequences of PFIC
status and would result in an alternative treatment (such as mark-to-market treatment) of our Ordinary Shares; however, we do not intend
to provide the information necessary for U.S. Holders to make “qualified electing fund elections”, or QEF elections, if we
are classified as a PFIC, and, accordingly, such elections would not be available to U.S. Holders. See “Taxation —
U.S. Tax Considerations”.
Our
business may be adversely affected by the ongoing Coronavirus pandemic.
The
outbreak of the novel Coronavirus (COVID-19) has evolved into a global pandemic. The Coronavirus has spread to many regions of the world.
The extent to which the Coronavirus impacts our business and operating results will depend on future developments that are highly uncertain
and cannot be accurately predicted, including new information that may emerge concerning the Coronavirus and the actions to contain the
Coronavirus or treat its impact, among others.
As
a result of the continuing spread of the Coronavirus, certain aspects of our cancer testing business operations may be delayed. Specifically,
as a result of shelter-in-place orders and other mandated local travel restrictions, among other things, the research and development
activities of certain of our partners may be affected, resulting in delays to our clinical trials, and we can provide no assurance as
to when such trials will resume at this time.
Furthermore,
site initiation, participant recruitment and enrollment, participant dosing, distribution of clinical trial materials, study
monitoring, and data analysis may be paused or delayed due to changes in hospital or university policies, federal, state or local or
foreign regulations, prioritization of hospital resources toward pandemic efforts, or other reasons related to the pandemic. If the
Coronavirus continues to spread, some participants and clinical investigators may not be able to comply with clinical trial
protocols. For example, quarantines or other travel limitations (whether voluntary or required) may impede participant movement,
affect sponsor access to study sites, or interrupt healthcare services, and we may be unable to conduct our clinical trials.
Further, if the spread of the Coronavirus pandemic continues and our operations are adversely impacted, we risk a delay, default
and/or non-performance under existing agreements which may increase our costs. These cost increases may not be fully recoverable or
adequately covered by insurance.
Infections
and deaths related to the pandemic may disrupt the United States’ or other countries’ healthcare and healthcare regulatory
systems. Such disruptions could divert healthcare resources away from, or materially delay FDA or foreign regulatory agency review and/or
approval with respect to, our clinical trials. It is unknown how long these disruptions could continue, were they to occur. Any elongation
or de-prioritization of our clinical trials or delay in regulatory review resulting from such disruptions could materially affect the
development and study of our product candidates.
We
currently utilize third parties to, among other things, manufacture raw materials. If any third-party party in the supply chain for materials
used in the production of our product candidates is adversely impacted by restrictions resulting from the Coronavirus outbreak, our supply
chain may be disrupted, limiting our ability to manufacture our product candidates for our clinical trials and research and development
operations.
On
the other hand, we have produced revenue as a result of the Coronavirus pandemic through our testing products and our Tollovid nutritional
supplement. If the Coronavirus pandemic were to end completely, these revenues will disappear. Notwithstanding the development of several
vaccines against the Coronavirus, it appears that for now, at least, the Coronavirus pandemic is not ending in many countries, and we
expect to continue to produce revenue from our Coronavirus testing and from Tollovid (as well as from Tollovir if it is commercialized)
for at least the next 12 months.
The
spread of the Coronavirus, which has caused a broad impact globally, including restrictions on travel and quarantine policies put into
place by businesses and governments, may have a material economic effect on our business. While the potential economic impact brought
by and the duration of the pandemic may be difficult to assess or predict, it has already caused, and is likely to result in further,
significant disruption of global financial markets, which may reduce our ability to access capital either at all or on favorable terms.
In addition, a recession, depression or other sustained adverse market event resulting from the spread of the Coronavirus could materially
and adversely affect our business and the value of our Ordinary Shares.
The
ultimate impact of the current pandemic, or any other health epidemic, is highly uncertain and subject to change. We do not yet know
the full extent of potential delays or impacts on our business, our clinical trials, our research programs, healthcare systems or the
global economy as a whole. However, these effects could have a material impact on our operations, and we will continue to monitor the
situation closely.
Risks
Related to Our Intellectual Property
We
may not successfully maintain our existing license agreement with BGU and Soroka, and we are currently not in compliance with the repayment
terms of the license agreement, which could adversely affect our ability to develop and commercialize our product candidates.
We
rely on our existing License Agreement with BGU and Soroka with respect to the development of our core cancer technology, TBIA. Our failure
to maintain our existing license could adversely affect our ability to develop and commercialize our product candidates and could adversely
affect our business prospects, financial condition or ability to develop and commercialize our product candidates.
We
may not be able to further establish or maintain such licensing and collaboration arrangements necessary to develop and commercialize
our product candidates. Even if we are able to maintain or establish licensing or collaboration arrangements, these arrangements may
not be on favorable terms and may contain provisions that will restrict our ability to develop, test and market our product candidates.
Any failure to maintain or establish licensing or collaboration arrangements on favorable terms could adversely affect our business prospects,
financial condition or ability to develop and commercialize our product candidates.
Our
license agreement contains provisions that could give rise to disputes regarding the rights and obligations of the parties. These and
other possible disagreements could lead to termination of the agreement or delays in collaborative research, development, supply, or
commercialization of certain product candidates, or could require or result in litigation or arbitration. Moreover, disagreements could
arise with our collaborators over rights to intellectual property or our rights to share in any of the future revenues of products developed
by our collaborators. These kinds of disagreements could result in costly and time-consuming litigation. Any such conflicts with our
collaborators could reduce our ability to obtain future collaboration agreements and could have a negative impact on our relationship
with existing collaborators.
If
we are unable to protect our intellectual property rights, our competitive position could be harmed.
Our
success and ability to compete depends in large part upon our ability to protect our intellectual property. We face several risks and
uncertainties in connection with our intellectual property rights, including, among others:
|
|
●
|
pending
and future patent applications may not result in the issuance of patents or, if issued, may not be issued in a form that will be
advantageous to us;
|
|
|
|
|
|
|
●
|
our
issued patents may be challenged, invalidated or legally circumvented by third parties;
|
|
|
|
|
|
|
●
|
our
patents may not be upheld as valid and enforceable or prevent the development of competitive products;
|
|
|
|
|
|
|
●
|
the
eligibility of certain inventions related to diagnostic medicine, more specifically diagnostic methods and processes, for patent
protection in the United States has been limited recently which may affect our ability to enforce our issued patents in the United
States or may make it difficult to obtain broad patent protection going forward in the United States;
|
|
|
|
|
|
|
●
|
for
a variety of reasons, we may decide not to file for patent protection on various improvements or additional features; and
|
|
|
|
|
|
|
●
|
intellectual
property protection and/or enforcement may be unavailable or limited in some countries where laws or law enforcement practices may
not protect our proprietary rights to the same extent as the laws of the United States, the European Union, or Israel.
|
Consequently,
our competitors could develop, manufacture and sell products that directly compete with our products, which could decrease our sales
and diminish our ability to compete. In addition, competitors could attempt to develop their own competitive technologies that fall outside
of our intellectual property rights. If our intellectual property does not adequately protect us from our competitors’ products
and methods, our competitive position could be materially adversely affected.
Because
the medical device industry is litigious, we are susceptible to intellectual property suits that could cause us to incur substantial
costs or pay substantial damages or prohibit us from selling our cancer detection kits.
There
is a substantial amount of litigation over patent and other intellectual property rights in the medical device industry. Whether or not
a product infringes a patent involves complex legal and factual considerations, the determination of which is often uncertain. Our management
is presently unaware of any other parties’ valid patents and proprietary rights which our evolving product designs would infringe.
Searches typically performed to identify potentially infringed patents of third parties are often not conclusive and, because patent
applications can take many years to issue, there may be applications now pending, which may later result in issued patents which our
current or future products may infringe. In addition, our competitors or other parties may assert that our cancer detection kits, and
the methods employed may be covered by patents held by them. If any of our products infringes a valid patent, we could be prevented from
manufacturing or selling such product unless we are able to obtain a license or able to redesign the product in such a manner as to avoid
infringement. A license may not always be available or may require us to pay substantial royalties. We also may not be successful in
any attempt to redesign our product to avoid infringement. Infringement and other intellectual property claims, with or without merit,
can be expensive and time-consuming to litigate and could divert our management’s attention from operating our business.
The
steps we have taken to protect our intellectual property may not be adequate, which could have a material adverse effect on our ability
to compete in the market.
In
addition to filing patent applications, we rely on confidentiality, non-compete, non-disclosure and assignment of inventions provisions,
as appropriate, in our agreements with our employees, consultants, and service providers, to protect and otherwise seek to control access
to, and distribution of, our proprietary information. These measures may not be adequate to protect our intellectual property from unauthorized
disclosure, third-party infringement or misappropriation, for the following reasons:
|
|
●
|
the
agreements may be breached, may not provide the scope of protection we believe they provide or may be determined to be unenforceable;
|
|
|
|
|
|
|
●
|
we
may have inadequate remedies for any breach;
|
|
|
|
|
|
|
●
|
proprietary
information could be disclosed to our competitors; or
|
|
|
|
|
|
|
●
|
others
may independently develop substantially equivalent or superior proprietary information and techniques or otherwise gain access to
our trade secrets or disclose such technologies.
|
Specifically,
with respect to non-compete agreements, we may be unable to enforce these agreements, in whole or in part, and it may be difficult for
us to restrict our competitors from gaining the expertise that our former employees gained while working for us. If our intellectual
property is disclosed or misappropriated, it could harm our ability to protect our rights and could have a material adverse effect on
our business, financial condition and results of operations.
We
may need to initiate lawsuits to protect or enforce our patents and other intellectual property rights, which could be expensive and,
if we lose, could cause us to lose some of our intellectual property rights, which would harm our ability to compete in the market.
We
rely on patents to protect a portion of our intellectual property and our competitive position. Patent law relating to the scope of claims
in the technology fields in which we operate is still evolving and, consequently, patent positions in the medical device industry are
generally uncertain. In order to protect or enforce our patent rights, we may initiate patent and related litigation against third parties,
such as infringement suits or interference proceedings. Any lawsuits that we initiate could be expensive, take significant time and divert
our management’s attention from other business concerns and the outcome of litigation to enforce our intellectual property rights
in patents, copyrights, trade secrets or trademarks is highly unpredictable. Litigation also puts our patents at risk of being invalidated
or interpreted narrowly and our patent applications at risk of not issuing. In addition, we may provoke third parties to assert claims
against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, including attorney fees,
if any, may not be commercially valuable. The occurrence of any of these events could have a material adverse effect on our business,
financial condition and results of operations.
Risks
Related to Regulations
If
we or our future distributors do not obtain and maintain the necessary regulatory clearances or approvals in a specific country or region,
we will not be able to market and sell our cancer detection kits or future products in that country or region.
We
intend to market our cancer detection kits in a number of international markets. To be able to market and sell our cancer detection kits
in a specific country or region, we or our distributors must comply with the regulations of that country or region. While the regulations
of some countries do not impose barriers to marketing and selling part or all of our products or only require notification, others require
that we or our distributors obtain the approval of a specified regulatory authority. These regulations, including the requirements for
approvals and the time required for regulatory review, vary from country to country. Obtaining regulatory approvals is expensive and
time-consuming, and we cannot be certain that we or our distributors will receive regulatory approvals for our cancer detection kits
or any future products in each country or region in which we plan to market such products. If we modify our cancer detection kits or
any future products, we or our distributors may need to apply for new regulatory approvals or regulatory authorities may need to review
the planned changes before we are permitted to sell them.
We
obtained approval to use the European CE mark on December 9, 2013 with the receipt of a Certificate of Conformance from our regulatory
authorized representative in Europe. The European regulatory demands regarding IVD have recently been revised and major changes need
to be made in order to keep our CE Mark. These changes will need to be made by 2022. We may not meet the quality and safety standards
required to maintain any authorizations we receive in the future or maintain the CE Certificate of Conformance that we have already received.
If we or our distributors are unable to maintain our authorizations or CE Certificate of Conformance in a particular country or region,
we will no longer be able to sell our cancer detection kits or any future products in that country or region, and our ability to generate
revenues will be materially and adversely affected.
If
we are unable to successfully complete clinical trials with respect to our cancer detection kits, we may be unable to receive regulatory
approvals or clearances for our cancer detection kits and/or our ability to achieve market acceptance of our cancer detection kits will
be harmed.
The
development of cancer diagnostics typically includes pre-clinical studies. Certain other devices require the submission of data generated
from clinical trials, which can be a long, expensive and uncertain process, subject to delays and failure at any stage. The data obtained
from the studies and trials may be inadequate to support regulatory clearances or approvals, or to obtain third country approval equivalent
to the European CE mark of approval, or to allow market acceptance of the products being studied. Our cancer detection kits are currently
undergoing clinical development.
We
conducted clinical studies in cooperation with leading hospitals in Israel. A study with Soroka (along with BGU) formed the basis of
our methodology. We then conducted studies, with both Rabin Medical Center, or Rabin, and Kaplan Medical Center, or Kaplan, which focused
on breast and colorectal cancers.
As
for the FDA, our products’ intended use or other specifications that are under development today may not be accepted by the FDA.
Under such circumstances, we may be required to change the intended use or specifications of our products, and be required to perform
additional trials and provide new supportive material to the FDA.
We
are an IVD company, developing proprietary technology which will analyze a blood sample to detect the presence of various cancers. Since
we are not developing a drug, we believe that we will not need to submit an investigational new drug application to the FDA prior to
conducting clinical trials in the U.S. We believe that we will only need institutional review board, or IRB, approval prior to conducting
clinical trials in the U.S.
We
expect that obtaining FDA approval for the marketing and selling of our products in the U.S. will take anywhere between two to four years
and will cost us approximately $10 million to $15 million. As we do not have this amount of money, we would need to raise additional
funds to perform clinical trials in the U.S. in order to receive FDA approval. If we are unable to raise such funds, we will not be able
proceed with our efforts to obtain FDA approval. Inability to obtain FDA approval would significantly harm our viability as a company.
We
estimate that we will need a “small pilot” clinical trial (less than 100 patients) to enable us to approach the FDA with
the results and begin a dialogue with the FDA to seek the FDA’s recommendation (not their approval) as to trial size and the protocols
for future U.S. clinical trials. We plan to submit a formal application to the FDA for approval of the TBIA method after we have completed
our clinical trials in the U.S.
Upon
the closing of the Provista transaction, we acquired a Clinical Laboratory Improvement Amendments laboratory, or CLIA laboratory, and
retain our product as a Laboratory Developed Test, or LDT, which are assays developed in the laboratory for internal use, in parallel
to the FDA evaluation.
Further,
any regulatory authority whose approval we will require in order to market and sell our products in any territory may require us to submit
data on a greater number of patients than we originally anticipated and/or for a longer follow-up period, or may change the data collection
requirements or data analysis applicable to our clinical trials.
The
commencement or completion of any of our clinical studies or trials may be delayed or halted, or be inadequate to support regulatory
clearance, approval or product acceptance, or to obtain local regulatory approvals in any country that we wish to sell our products,
for numerous reasons, including, among others:
|
|
●
|
patients
do not enroll in the clinical trial at the rate we expect;
|
|
|
|
|
|
|
●
|
patients
do not comply with trial protocols;
|
|
|
|
|
|
|
●
|
patient
follow-up is not at the rate we expect;
|
|
|
|
|
|
|
●
|
patients
experience adverse side effects;
|
|
|
|
|
|
|
●
|
patients
die during a clinical trial, even though their death may be unrelated to our product;
|
|
|
|
|
|
|
●
|
regulatory
authorities do not approve a clinical trial protocol or a clinical trial, or place a clinical trial on hold;
|
|
|
|
|
|
|
●
|
IRBs,
Ethics Committees and third-party clinical investigators may delay or reject our trial protocol and Informed Consent Form;
|
|
|
|
|
|
|
●
|
third-party
clinical investigators decline to participate in a study or trial or do not perform a study or trial on our anticipated schedule
or consistent with the investigator agreements, study or trial protocol, good clinical practices or FDA, IRBs, Ethics Committees,
or other applicable requirements;
|
|
|
|
|
|
|
●
|
third-party
organizations such as the Contract Research Organization, do not perform data collection, monitoring and analysis in a timely or
accurate manner or consistent with the study or trial protocol or investigational or statistical plans;
|
|
|
●
|
regulatory
inspections of our studies, trials or manufacturing facilities may require us to, among other things, undertake corrective action
or suspend or terminate our studies or clinical trials;
|
|
|
|
|
|
|
●
|
changes
in governmental regulations or administrative actions;
|
|
|
|
|
|
|
●
|
the
interim or final results of the study or clinical trial are inconclusive or unfavorable as to safety or efficacy; and
|
|
|
|
|
|
|
●
|
a
regulatory agency or our notified body concludes that our trial design is or was inadequate to demonstrate different parameters of
the assay.
|
The
results of pre-clinical and clinical studies do not necessarily predict future clinical trial results, and predecessor clinical trial
results may not be repeated in subsequent clinical trials. Additionally, any regulatory authority whose approval we will require in order
to market and sell our products in any territory may disagree with our interpretation of the data from our pre-clinical studies and clinical
trials, or may find the clinical trial design, conduct or results inadequate to demonstrate safety or efficacy, and may require us to
pursue additional pre-clinical studies or clinical trials, which could further delay the clearance or approval of the sale of our products.
The data we collect from our non-clinical testing, our pre-clinical studies and other clinical trials may not be sufficient to support
regulatory approval.
If
the third parties on which we rely to conduct our clinical trials and clinical development do not perform as contractually required or
expected, we may not be able to obtain regulatory clearance or approval for, or commercialize, our cancer detection kits or future products.
We
do not have the ability to independently conduct our clinical trials for our cancer detection kits and we must rely on third parties,
such as contract research organizations, medical institutions, clinical investigators and contract laboratories to conduct such trials.
If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if
these third parties need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere
to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials
may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory clearance for, or successfully commercialize,
our cancer detection kits or future products on a timely basis, if at all, and our business, operating results and prospects may be adversely
affected. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside
of their control.
The
results of our clinical trials may not support our product claims or may result in the discovery of adverse side effects.
Even
if our clinical trials are completed as planned, we cannot be certain that their results will support our product claims or that any
regulatory authority whose approval we will require in order to market and sell our products in any territory will agree with our conclusions
regarding them. Success in pre-clinical studies and early clinical trials does not ensure that later clinical trials will be successful,
and we cannot be sure that clinical trials will replicate the results of prior trials and pre-clinical studies. The clinical trial process
may fail to demonstrate that our cancer detection kits, or any future products, are safe and effective for the proposed indicated uses,
which could cause us to abandon a product and may delay development of others. Any delay or termination of our clinical trials will delay
the filing of our product submissions and, ultimately, our ability to commercialize our cancer detection kits, or any future products,
and generate revenues. It is also possible that patients enrolled in clinical trials will experience adverse side effects that are not
currently part of the product candidate’s profile.
Our
cancer detection kits may in the future be subject to product recalls that could harm our reputation, business and financial results.
The
FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of
material deficiencies or defects in design or manufacture. In the case of the FDA, the authority to require a recall must be based on
an FDA finding that there is a reasonable probability that the device would cause serious injury or death. In addition, foreign governmental
bodies have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacture.
Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. A government-mandated
or voluntary recall by us or one of our distributors could occur as a result of component failures, manufacturing errors, design or labeling
defects or other deficiencies and issues. Once marketed, recalls of any of our products, including our cancer detection kits, would divert
managerial and financial resources and have an adverse effect on our business, financial condition and results of operations. The FDA
requires that certain classifications of recalls be reported to the FDA within 10 working days after the recall is initiated. Companies
are required to maintain certain records of recalls, even if they are not reportable to the FDA. We may initiate voluntary recalls involving
our products in the future that we determine do not require us to notify the FDA. If the FDA disagrees with our determinations, they
could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively
affect our sales. In addition, the FDA could take enforcement action against us based on our failure to report the recalls when they
were conducted.
If
we are unable to achieve reimbursement and coverage from third-party payors for laboratory tests using our cancer detection kits, or
if reimbursement is insufficient to create an economic benefit for purchasing or using our cancer detection kits when compared to alternative
tests, demand for our products may not grow at the rate we expect.
The
demand for our cancer detection kits will depend significantly on the eligibility of the tests performed using our cancer detection kits
for reimbursement through government-sponsored healthcare payment systems and private third-party payors. Reimbursement practices vary
significantly from country to country and within some countries, by region, and we must obtain reimbursement approvals on a country-by-country
and/or region-by-region basis. In general, the process of obtaining reimbursement and coverage approvals has been longer outside of the
United States. We may not be able to obtain reimbursement approvals in a timely manner or at all and existing reimbursement and coverage
policies may be revised from time to time by third-party payors. If physicians, hospitals and other healthcare providers are unable to
obtain sufficient coverage and reimbursement from third-party payors for tests using our cancer detection kits, if reimbursement is,
or is perceived by our customers to be, insufficient to create an economic incentive for purchasing or using our cancer detection kits,
or if such reimbursement does not adequately compensate physicians and health care providers compared to the other tests they offer,
demand for our products may not grow at the rate we expect.
Federal
and state privacy laws, and equivalent laws of third countries, may increase our costs of operation and expose us to civil and criminal
sanctions.
The
Health Insurance Portability and Accountability Act of 1996, as amended, and the regulations that have been issued under it, or collectively
HIPAA, and similar laws outside the United States, contain substantial restrictions and requirements with respect to the use and disclosure
of individuals’ protected health information. The HIPAA privacy rules prohibit “covered entities,” such as healthcare
providers and health plans, from using or disclosing an individual’s protected health information, unless the use or disclosure
is authorized by the individual or is specifically required or permitted under the privacy rules. Under the HIPAA security rules, covered
entities must establish administrative, physical and technical safeguards to protect the confidentiality, integrity and availability
of electronic protected health information maintained or transmitted by them or by others on their behalf. While we do not believe that
we will be a covered entity under HIPAA, we believe many of our customers will be covered entities subject to HIPAA. Such customers may
require us to enter into business associate agreements, which will obligate us to safeguard certain health information we obtain in the
course of our relationship with them, restrict the manner in which we use and disclose such information and impose liability on us for
failure to meet our contractual obligations.
In
addition, under The Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, certain of HIPAA’s privacy
and security requirements are now also directly applicable to “business associates” of covered entities and subject them
to direct governmental enforcement for failure to comply with these requirements. We may be deemed as a “business associate”
of some of our customers. As a result, we may be subject to civil and criminal penalties for failure to comply with applicable privacy
and security rule requirements. Moreover, HITECH created a new requirement obligating “business associates” to report any
breach of unsecured, individually identifiable health information to their covered entity customers and imposes penalties for failing
to do so.
In
addition to HIPAA, most U.S. states have enacted patient confidentiality laws that protect against the disclosure of confidential medical
information, and many U.S. states have adopted or are considering adopting further legislation in this area, including privacy safeguards,
security standards, and data security breach notification requirements. These U.S. state laws, which may be even more stringent than
the HIPAA requirements, are not preempted by the federal requirements, and we are therefore required to comply with them to the extent
they are applicable to our operations.
These
and other possible changes to HIPAA or other U.S. federal or state laws or regulations, or comparable laws and regulations in countries
where we conduct business, could affect our business and the costs of compliance could be significant. Failure by us to comply with any
of the standards regarding patient privacy, identity theft prevention and detection, and data security may subject us to penalties, including
civil monetary penalties and in some circumstances, criminal penalties. In addition, such failure may damage our reputation and adversely
affect our ability to retain customers and attract new customers.
The
protection of personal data, particularly patient data, is subject to strict laws and regulations in many countries. The collection and
use of personal health data in the EU are governed by the provisions of the General Data Protection Regulation (GDPR). GDPR carries provisions
that require businesses to protect the personal data and privacy of EU citizens for transactions that occur within EU member states.
The GDPR also regulates the exportation of personal data outside the EU. The GDPR will levy harsh fines against those who violate its
privacy and security standards, with penalties reaching into the tens of millions of euros. The GDPR defines several roles that are responsible
for ensuring compliance: data controller (defines how personal data is processed and the purposes for which it is processed), data processor
(liable for breaches or non-compliance) and the data protection officer (liable to process, store and monitor large amounts of EU and
Non-EU citizen data). Under the GDPR, health data is considered a special category of personal data, demanding even further steps for
its protection than other, regular types of personal data. To lawfully process special category data, both a legal basis and a separate
condition for processing must be identified. The GDPR also requires that upon processing special category data, you must keep records
and include documenting the categories of the data you process. The GDPR doesn’t explicitly state how long an organization is allowed
to hold on to personal data, however healthcare organizations should ensure that the information relating to health data is not kept
for longer than needed. For that reason, retention periods must be clearly established and communicated to data subjects, such as patients.
Additionally, the GDPR requires that before processing data that is likely to be high risk to the rights and freedoms of data subjects,
a Data Protection Impact Assessment is to be conducted in order to identify the potential risks that could be faced.
The
GDPR also imposes strict rules on the transfer of personal data out of the EU. Failure to comply with the requirements of the GDPR may
result in fines and other administrative penalties and harm our business. We may incur extensive costs in ensuring compliance with these
laws and regulations, particularly if we are considered to be a data controller within the meaning of the GDPR.
Once
we commercialize our product, if ever, security breaches, loss of data and other disruptions could compromise sensitive information related
to our business or prevent us from accessing critical information and expose us to liability, which could adversely affect our business
and our reputation.
Once
we commercialize our product, in the ordinary course of our business, it is highly likely that we and our third-party providers will
collect and store sensitive data, including legally protected health information and personally identifiable information about patients,
our healthcare provider customers and payors. We also may store sensitive intellectual property and other proprietary business information,
including that of our customers and payors. We plan to manage and maintain our data utilizing a combination of on-site systems and cloud-based
data center systems. This data will encompass a wide variety of business-critical information, including research and development information,
commercial information and business and financial information.
We
face four primary risks relative to protecting this critical information: loss of access risk, inappropriate disclosure risk, inappropriate
modification risk and the risk of our being unable to identify and audit our controls over the first three risks.
We
will be highly dependent on information technology networks and systems, including the Internet, to securely process, transmit and store
this critical information. Security breaches of this infrastructure, including physical or electronic break-ins, computer viruses, attacks
by hackers and similar breaches, can create system disruptions, shutdowns or unauthorized disclosure or modification of confidential
information. The secure processing, storage, maintenance and transmission of this critical information will be vital to our operations
and business strategy, and we plan to devote significant resources to protecting such information. Although we will take measures to
protect sensitive information from unauthorized access or disclosure, our information technology and infrastructure, and that of our
third-party providers, may be vulnerable to attacks by hackers or viruses or breached due to employee error, malfeasance or other disruptions.
A
security breach or privacy violation that leads to disclosure or modification of or prevents access to consumer information (including
personally identifiable information or protected health information) could harm our reputation, compel us to comply with disparate state
breach notification laws, require us to verify the correctness of database contents and otherwise subject us to liability under laws
that protect personal data, resulting in increased costs or loss of revenue. If we are unable to prevent such security breaches or privacy
violations or implement satisfactory remedial measures, our operations could be disrupted, and we may suffer loss of reputation, financial
loss and other regulatory penalties because of lost or misappropriated information, including sensitive consumer data. In addition, these
breaches and other inappropriate access can be difficult to detect, and any delay in identifying them may lead to increased harm of the
type described above.
Any
such breach or interruption could compromise our networks or those of our third-party providers, and the information stored there could
be inaccessible or could be accessed by unauthorized parties, publicly disclosed, lost or stolen. Any such interruption in access, improper
access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy
of personal information, such as HIPAA, and regulatory penalties. Unauthorized access, loss or dissemination could also disrupt our operations,
including our ability to perform tests, provide test results, bill payers or patients, process claims and appeals, provide customer assistance
services, conduct research and development activities, collect, process and prepare company financial information, provide information
about our current and future products and other patient and clinician education and outreach efforts through our website, and manage
the administrative aspects of our business and damage our reputation, any of which could adversely affect our business. Any such breach
could also result in the compromise of our trade secrets and other proprietary information, which could adversely affect our competitive
position.
In
addition, the interpretation and application of consumer, health-related, privacy and data protection laws in the U.S., the EU and elsewhere
are often uncertain, contradictory and in flux. It is possible that these laws may be interpreted and applied in a manner that is inconsistent
with our practices. If so, this could result in government-imposed fines or orders requiring that we change our practices, which could
adversely affect our business. Complying with these various laws could cause us to incur substantial costs or require us to change our
business practices and compliance procedures in a manner adverse to our business.
If
we fail to comply with the U.S. federal Anti-Kickback Statute and similar state and third country laws, we could be subject to criminal
and civil penalties and exclusion from federally funded healthcare programs including the Medicare and Medicaid programs and equivalent
third country programs, which would have a material adverse effect on our business and results of operations.
A
provision of the Social Security Act, commonly referred to as the federal Anti-Kickback Statute, prohibits the knowing and willful offer,
payment, solicitation or receipt of any form of remuneration, directly or indirectly, in cash or in kind, to induce or reward the referring,
ordering, leasing, purchasing or arranging for, or recommending the ordering, purchasing or leasing of, items or services payable, in
whole or in part, by Medicare, Medicaid or any other federal healthcare program. Although there are a number of statutory exemptions
and regulatory safe harbors to the federal Anti-Kickback Statute protecting certain common business arrangements and activities from
prosecution or regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that do not fit squarely within
an exemption or safe harbor may be subject to scrutiny. The federal Anti-Kickback Statute is very broad in scope and many of its provisions
have not been uniformly or definitively interpreted by existing case law or regulations. In addition, most of the states have adopted
laws similar to the federal Anti-Kickback Statute, and some of these laws are even broader than the federal Anti- Kickback Statute in
that their prohibitions may apply to items or services reimbursed under Medicaid and other state programs or, in several states, apply
regardless of the source of payment. Violations of the federal Anti-Kickback Statute may result in substantial criminal, civil or administrative
penalties, damages, fines and exclusion from participation in federal healthcare programs.
All
of our future financial relationships with U.S. healthcare providers, purchasers, formulary managers, and others who provide products
or services to federal healthcare program beneficiaries will potentially be governed by the federal Anti-Kickback Statute and similar
state laws. We believe our operations will be in compliance with the federal Anti-Kickback Statute and similar state laws. However, we
cannot be certain that we will not be subject to investigations or litigation alleging violations of these laws, which could be time-consuming
and costly to us and could divert management’s attention from operating our business, which in turn could have a material adverse
effect on our business. In addition, if our arrangements were found to violate the federal Anti-Kickback Statute or similar state laws,
the consequences of such violations would likely have a material adverse effect on our business, results of operations and financial
condition.
There
are other federal and state laws that may affect our ability to operate, including the federal civil False Claims Act, which prohibits,
among other things, individuals or entities from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment
of government funds or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to
pay money to the government or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to
pay money to the federal government. Moreover, we may be subject to other federal false claim laws, including, among others, federal
criminal healthcare fraud and false statement statutes that extend to non-government health benefit programs. Moreover, there are analogous
state laws. Violations of these laws can result in substantial criminal, civil or administrative penalties, damages, fines and exclusion
from participation in federal healthcare programs.
Similar
restrictions are imposed by the national legislation of many third countries in which our medical devices will be marketed. Moreover,
the provisions of the Foreign Corrupt Practices Act of 1997 and other similar anti-bribery laws in other jurisdictions generally prohibit
companies and their intermediaries from providing money or anything of value to officials of foreign governments, foreign political parties,
or international organizations with the intent to obtain or retain business or seek a business advantage. Recently, there has been a
substantial increase in anti-bribery law enforcement activity by U.S. regulators, with more aggressive and frequent investigations and
enforcement by both the SEC and the Department of Justice. A determination that our operations or activities violated U.S. or foreign
laws or regulations could result in imposition of substantial fines, interruption of business, loss of supplier, vendor or other third-party
relationships, termination of necessary licenses and permits, and other legal or equitable sanctions. In addition, lawsuits brought by
private litigants may also follow as a consequence.
Risks
Related to Our Operations in Israel
Conditions
in Israel could materially and adversely affect our business.
Many
of Todos’ employees and consultants, including its Chief Financial Officer and Chief Business Officer, are residents of Israel.
Accordingly, political, economic, and military conditions in Israel and the surrounding region may directly affect Todos’ business
and operations. In recent years, Israel has been engaged in sporadic armed conflicts with Hamas, an Islamist terrorist group that controls
the Gaza Strip, with Hezbollah, an Islamist terrorist group that controls large portions of southern Lebanon, and with Iranian-backed
military forces in Syria. In addition, Iran has threatened to attack Israel and may be developing nuclear weapons. Some of these hostilities
were accompanied by missiles being fired from the Gaza Strip against civilian targets in various parts of Israel, including areas in
which Todos’ employees are located, and negatively affected business conditions in Israel. Any hostilities involving Israel or
the interruption or curtailment of trade between Israel and its trading partners could adversely affect our operations and results of
operations.
Todos’
commercial insurance does not cover losses that may occur as a result of events associated with war and terrorism. Although the Israeli
government currently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, Todos cannot
assure you that this government coverage will be maintained or that it will sufficiently cover Todos’ potential damages. Any losses
or damages incurred by Todos could have a material adverse effect on its business. Any armed conflicts or political instability in the
region would likely negatively affect business conditions and could harm our results of operations.
Further,
in the past, the State of Israel and Israeli companies have been subjected to economic boycotts. Several countries still restrict business
with the State of Israel and with Israeli companies. These restrictive laws and policies may have an adverse impact on Todos’ results
of operations, financial condition or the expansion of its business. A campaign of boycotts, divestment, and sanctions has been undertaken
against Israel, which could also adversely affect Todos’ business. Actual or perceived political instability in Israel or any negative
changes in the political environment, may individually or in the aggregate adversely affect the Israeli economy and, in turn, Todos’
business, financial condition, results of operations, and prospects.
In
addition, many Israeli citizens are obligated to perform several weeks of annual military reserve duty each year until they reach the
age of 40 (or older, for reservists who are military officers or who have certain occupations) and, in the event of a military conflict,
may be called to active duty. In response to increases in terrorist activity, there have been periods of significant call-ups of military
reservists. It is possible that there will be military reserve duty call-ups in the future. Todos’ operations could be disrupted
by such call-ups, which may include the call-up of members of its management. Such disruption could materially adversely affect its business,
prospects, financial condition, and results of operations.
Your
rights and responsibilities as a shareholder will be governed by Israeli law which differs in some material respects from the rights
and responsibilities of shareholders of U.S. companies.
The
rights and responsibilities of the holders of our Ordinary Shares are governed by our articles of association, as amended (the “Amended
Articles”), and by Israeli law. These rights and responsibilities differ in some material respects from the rights and responsibilities
of shareholders in U.S.-based corporations. For instance, a shareholder of an Israeli company has a duty to act in good faith and in
a customary manner in exercising its rights and performing its obligations towards the company and other shareholders, and to refrain
from abusing its power in the company, including, among other things, in voting at a general meeting of shareholders on matters such
as amendments to a company’s articles of association, increases in a company’s authorized share capital, mergers and acquisitions
and related party transactions requiring shareholder approval. In addition, a shareholder who is aware that it possesses the power to
determine the outcome of a shareholder vote or to appoint or prevent the appointment of a director or executive officer in the company
has a duty of fairness toward the company. There is limited case law available to assist us in understanding the nature of this duty
or the implications of these provisions. These provisions may be interpreted to impose additional obligations and liabilities on holders
of our Ordinary Shares that are not typically imposed on shareholders of U.S. corporations.
It
may be difficult to enforce a judgment of a U.S. court against us, or against our officers and directors in Israel, or to assert U.S.
securities laws claims in Israel or to serve process on our officers and directors in Israel.
We
were incorporated in Israel. A majority of our executive officers and directors reside outside of the United States, and some of our
assets and most of the assets of these persons are located outside the United States. Therefore, a judgment obtained against us, or any
of these persons, including a judgment based on the civil liability provisions of the U.S. federal securities laws, may not be collectible
in the U.S. and may not necessarily be enforced by an Israeli court. It also may be difficult to affect service of process on these persons
in the United States or to assert U.S. securities law claims in original actions instituted in Israel. Additionally, it may be difficult
for an investor, or any other person or entity, to initiate an action with respect to U.S. securities laws in Israel. Israeli courts
may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is not the most appropriate forum
in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not
U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proven as a fact
by expert witnesses, which can be a time consuming and costly process. Certain matters of procedure will also be governed by Israeli
law. There is little binding case law in Israel that addresses the matters described above. As a result of the difficulty associated
with enforcing a judgment against us in Israel, you may not be able to collect any damages awarded by either a U.S. or foreign court.
Provisions
of Israeli law and our Amended Articles may delay, prevent or otherwise impede a merger with, or an acquisition of, us, even when the
terms of such a transaction are favorable to us and our shareholders.
Israeli
corporate law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special approvals
for transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant to such types
of transactions. For example, a tender offer for all of a company’s issued and outstanding shares can only be completed if the
acquirer receives positive responses from the holders of at least 95% of the issued share capital and the approval of a majority of the
offerees that do not have a personal interest in the tender offer, unless at least 98% of the company’s outstanding shares are
tendered. Furthermore, the shareholders, including those who indicated their acceptance of the tender offer (unless the acquirer stipulated
in its tender offer that a shareholder that accepts the offer may not seek appraisal rights), may, at any time within six months following
the completion of the tender offer, petition an Israeli court to alter the consideration for the acquisition. In addition, a merger may
not be consummated unless at least 50 days have passed from the date on which a proposal for approval of the merger was filed by each
party with the Israeli Registrar of Companies and at least 30 days have passed from the date on which the merger was approved by the
shareholders of each party. See “Provisions Restricting Change in Control in our Company” for additional information.
Furthermore,
Israeli tax considerations may make potential transactions unappealing to us or to our shareholders whose country of residence does not
have a tax treaty with Israel exempting such shareholders from Israeli tax. For example, Israeli tax law does not recognize tax-free
share exchanges to the same extent as U.S. tax law. With respect to mergers, Israeli tax law allows for tax deferral in certain circumstances
but makes the deferral contingent on the fulfillment of a number of conditions, including, in some cases, a holding period of two years
from the date of the transaction during which sales and dispositions of shares of the participating companies are subject to certain
restrictions. Moreover, with respect to certain share swap transactions, the tax deferral is limited in time, and when such time expires,
the tax becomes payable even if no disposition of the shares has occurred.
We
received Israeli government grants for certain of our research and development activities. The terms of those grants may require us to
pay royalties and to satisfy specified conditions in order to manufacture products and transfer technologies outside of Israel. We may
be required to pay penalties in addition to repayment of the grants.
From
inception through December 31, 2020, we have been awarded an aggregate of $272,237 in the form of grants from Israel Innovation Authority,
or the IIA, formerly known as Israel’s Office of the Chief Scientist of the Ministry of Economy. The requirements and restrictions
for such grants are found in the Israeli Encouragement of Research and Development Law, 5744-1984 and the regulations thereunder (the
“Research Law”). Under the Research Law, royalties of 3% to 5% on the revenues derived from sales of products or services
developed in whole or in part using these IIA grants are payable to the Israeli government. We developed our technologies, at least in
part, with funds from these grants, and accordingly we would be obligated to pay these royalties on sales of any of our product candidates
that achieve regulatory approval. The maximum aggregate royalties paid generally cannot exceed 100% of the grants made to us, plus annual
interest equal to the 12-month LIBOR applicable to dollar deposits, as published on the first business day of each calendar year. As
of December 31, 2020, we had not paid any royalties to the IIA. In 2020, we did not receive a grant from the IIA. When a company develops
know-how, technology or products using IIA grants, the terms of these grants and the Research Law restrict the transfer of such know-how,
and the transfer of manufacturing or manufacturing rights of such products, technologies or know-how outside of Israel, without the prior
approval of the IIA. Therefore, the discretionary approval of an IIA committee would be required for any transfer to third parties inside
or outside of Israel of know-how or manufacturing or manufacturing rights related to those aspects of such technologies. We may not receive
those approvals. Furthermore, the IIA may impose certain conditions on any arrangement under which it permits us to transfer technology
or development out of Israel, including an increased royalty rate.
The
transfer of IIA-supported technology or know-how outside of Israel may involve the payment of significant amounts, depending upon the
value of the transferred technology or know-how, our research and development expenses, the amount of IIA support, the time of completion
of the IIA-supported research project and other factors. These restrictions and requirements for payment may impair our ability to sell
or otherwise transfer our technology assets outside of Israel or to outsource or transfer development or manufacturing activities with
respect to any product or technology outside of Israel. Furthermore, the consideration available to our shareholders in a transaction
involving the transfer outside of Israel of technology or know-how developed with IIA funding (such as a merger or similar transaction)
may be reduced by any amounts that we are required to pay to the IIA.
These
restrictions will continue to apply even after we have repaid the full amount of royalties on the grants. For the years ended December
31, 2020 and 2019, we did not apply for or receive any grants from the IIA.
Risks
Related to Our Ordinary Shares
The
sale or issuance of our Ordinary Shares to Lincoln Park
may cause dilution and the sale of the Ordinary Shares acquired by Lincoln Park, or the perception that such sales may occur,
could cause the price of our Ordinary Shares to fall.
On
August 4, 2020, we entered into a Purchase Agreement with Lincoln Park Capital, pursuant to which Lincoln Park has committed to purchase
up to $10,000,000 of our Ordinary Shares. Upon the execution of the Purchase Agreement, we issued 5,812,500 Commitment Shares to Lincoln
Park, and Lincoln Park purchased 3,437,500 Initial Purchase Shares. The remaining 40,750,000 Ordinary Shares that may be issued under
the Purchase Agreement may be sold by us to Lincoln Park at our discretion from time to time over a 36-month period commencing after
the satisfaction of certain conditions set forth in the Purchase Agreement, including the SEC declaring effective a registration statement
that includes the shares included in the Purchase Agreement, which registration statement was declared effective on August 18, 2020.
The purchase price for the Ordinary Shares that we may sell to Lincoln Park under the Purchase Agreement will fluctuate based on the
price of our Ordinary Shares. Depending on market liquidity at the time, sales of such shares may cause the trading price of our Ordinary
Shares to fall.
We
generally have the right to control the timing and amount of any future sales of our shares to Lincoln Park. Sales of our Ordinary Shares,
if any, to Lincoln Park will depend upon market conditions and other factors to be determined by us. We may ultimately decide to sell
to Lincoln Park all, some or none of the additional Ordinary Shares that may be available for us to sell pursuant to the Purchase Agreement.
If and when we do sell shares to Lincoln Park, after Lincoln Park has acquired the shares, Lincoln Park may resell all, some or none
of those shares at any time or from time to time in its discretion. Therefore, sales to Lincoln Park by us could result in substantial
dilution to the interests of other holders of our Ordinary Shares. Additionally, the sale of a substantial number of our Ordinary Shares
to Lincoln Park, or the anticipation of such sales, could make it more difficult for us to sell equity or equity-related securities in
the future at a time and at a price that we might otherwise wish to effect sales.
We
may not have access to the full amount available under the Purchase Agreement with Lincoln Park.
Under
our Purchase Agreement with Lincoln Park, we may, at our discretion from time to time over a 24-month period commencing after the satisfaction
of certain conditions set forth in the Purchase Agreement, on any single business day on which the closing price of our Ordinary Shares
is above $.02 per share (subject to adjustment for any reorganization, recapitalization, non-cash dividend, share split, or other similar
transaction as provided in the Purchase Agreement), direct Lincoln Park to purchase our Ordinary Shares in amounts up to 500,000 shares,
which amounts may be increased to up to 1,000,000 shares depending on the market price of our Ordinary Shares at the time of sale and
subject to a maximum commitment by Lincoln Park of $500,000 per single regular purchase.
Depending on the market prices
of our Ordinary Shares at the time we elect to issue and sell shares to Lincoln Park under the Purchase Agreement, we may need to register
for resale under the Securities Act additional Ordinary Shares in order to receive aggregate gross proceeds equal to the $10,000,000
total commitment available to us under the Purchase Agreement. Assuming a purchase price of $0.064 per share (the closing sale price
of the Ordinary Shares on January 13, 2022), total gross proceeds to us would only be $2,608,000. As of January 13, 2022, we had issued
an aggregate of 37,977,388 Ordinary Shares to Lincoln Park, at prices ranging from $0.038 to $0.115 per shares for total gross proceeds
of approximately $2,593,725.
The
extent we rely on Lincoln Park as a source of funding will depend on a number of factors, including the prevailing market price of our
Ordinary Shares and the extent to which we are able to secure working capital from other sources. If obtaining sufficient funding from
Lincoln Park were to prove unavailable or prohibitively dilutive, we will need to secure another source of funding in order to satisfy
our working capital needs. If we elect to issue and sell more than 50,000,000 shares, which we have the right, but not the obligation,
to do, we must first register for resale under the Securities Act any such additional shares, which could cause additional substantial
dilution to our shareholders. Even if we sell all $10,000,000 under the Purchase Agreement to Lincoln Park, we may still need additional
capital to fully implement our business, operating and development plans. Should the financing we require to sustain our working capital
needs be unavailable or prohibitively expensive when we require it, the consequences could be a material adverse effect on our business,
operating results, financial condition and prospects.
Future
issuance of our Ordinary Shares could dilute the interests of existing shareholders.
We
may issue additional Ordinary Shares in the future. The issuance of a substantial number of Ordinary Shares could have the effect of
substantially diluting the interests of our shareholders. In addition, the sale of a substantial amount of Ordinary Shares in the public
market, in the initial issuance, in a situation in which we acquire a company, and the acquired company receives Ordinary Shares as consideration
and the acquired company subsequently sells its Ordinary Shares, or by investors who acquired such Ordinary Shares in a private placement,
could have an adverse effect on the market price of our Ordinary Shares.
Sales
of a substantial number of our Ordinary Shares in the public market by our existing shareholders could cause our share price to fall.
Sales
of a substantial number of shares of our Ordinary Shares in the public market, or the perception that these sales might occur, could
depress the market price of our Ordinary Shares and could impair our ability to raise capital through the sale of additional equity securities.
We are unable to predict the effect that sales may have on the prevailing market price of our Ordinary Shares. Sales of shares by these
shareholders could have a material adverse effect on the trading price of our Ordinary Shares. We intend to register the offering, issuance,
and sale of all Ordinary Shares that we may issue under our equity compensation plans. Once we register these shares, they can be freely
sold in the public market upon issuance, subject to volume limitations applicable to affiliates and any lock-up agreements. Also, we
are required to register the shares that would result from (a) the conversion of certain convertible notes we issued in 2021, and (b)
the exercise of certain warrants we issued alongside convertible notes in 2020 and 2021. The sales of a significant number of those shares
could have a depressant effect on our share price.
Until December 31, 2021, we were an Emerging
Growth Company, which may have reduced the amount of information available to investors.
Until December 31, 2021,
we were an Emerging Growth Company. The Jumpstart Our Business Start-ups Act, or the JOBS Act, allowed us to postpone the date by which
we must comply with some of the laws and regulations intended to protect investors and to reduce the amount of information we provided
in our reports filed with the SEC, which could have adversely undermined investor confidence in our company and adversely affected the
market price of our Ordinary Shares. Because we have not yet filed a periodic report since our Emerging Growth Company status expired,
we have not yet complied with the above-mentioned laws and regulations.
For as long as we remained
an “emerging growth company” as defined in the JOBS Act, we took advantage of certain exemptions from various requirements
that are applicable to public companies that are not emerging growth companies including:
|
|
●
|
the
provisions of the Sarbanes-Oxley Act requiring that our independent registered public accounting firm provide an attestation report
on the effectiveness of our internal control over financial reporting;
|
|
|
|
|
|
|
●
|
Section
107 of the JOBS Act, which provides that an “emerging growth company” can take advantage of the extended transition period
provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised
accounting standards. This means that an “emerging growth company” can delay the adoption of certain accounting standards
until those standards would otherwise apply to private companies. We may elect to delay such adoption of new or revised accounting
standards. As a result of this adoption, our financial statements may not be comparable to companies that comply with the public
company effective date; and
|
|
|
|
|
|
|
●
|
any
rules that may be adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement
to the auditor’s report on the financial statements.
|
We took advantage of these
exemptions until we were no longer an “emerging growth company.” We were an emerging growth company until the last day of
the fiscal year ending on December 31, 2021.
We
have never paid cash dividends on our Ordinary Shares, and we do not anticipate paying any dividends in the foreseeable future. Consequently,
any gains from an investment in our Ordinary Shares will likely depend on whether the price of our Ordinary Shares increases, which may
not occur.
We
have not paid cash dividends on any of our share capital to date and we currently intend to retain our future earnings, if any, to fund
the development and growth of our business. In addition, the Companies Law imposes restrictions on our ability to declare and pay dividends.
As a result, capital appreciation, if any, of our Ordinary Shares will be your sole source of gain for the foreseeable future. Consequently,
in the foreseeable future, you will likely only experience a gain from your investment in our Ordinary Shares if the price of our Ordinary
Shares increases beyond the price in which you originally acquired the Ordinary Shares.
The
potential future application of the SEC’s “penny stock” rules to our Ordinary Shares could limit trading activity in
the market, and our shareholders may find it more difficult to sell their shares.
If
our Ordinary Shares trade at less than $5.00 per share we will be subject to the SEC’s penny stock rules. Rule 15g-9 under the
Exchange Act establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that
has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions.
For any transaction involving a penny stock, unless exempt, the rules require: (a) that a broker or dealer approve a person’s account
for transactions in penny stocks; and (b) the broker or dealer receive from the investor a written agreement to the transaction, setting
forth the identity and quantity of the penny stock to be purchased.
In
order to approve a person’s account for transactions in penny stocks, the broker or dealer must: (a) obtain financial information
and investment experience objectives of the person and (b) make a reasonable determination that the transactions in penny stocks are
suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the
risks of transactions in penny stocks.
The
broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to
the penny stock market, which, in highlight form: (a) sets forth the basis on which the broker or dealer made the suitability determination;
and (b) confirms that the broker or dealer received a signed, written agreement from the investor prior to the transaction. Generally,
brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more
difficult for investors to dispose of our Ordinary Shares and cause a decline in the market value of our Ordinary Shares.
Disclosure
also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions
payable to both the broker or dealer and the registered representative, current quotations for the securities and the rights and remedies
available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent
price information for the penny stock held in the account and information on the limited market in penny stocks.
The
market price of our Ordinary Shares may be volatile.
The
market price of our Ordinary Shares may be highly volatile. Some of the factors that may materially affect the market price of our Ordinary
Shares are beyond our control, such as changes in financial estimates by industry and securities analysts, conditions or trends in the
industry in which we operate or sales of our Ordinary Shares. These factors may materially adversely affect the market price of our Ordinary
Shares, regardless of our performance. In addition, the public stock markets have experienced extreme price and trading volume volatility.
This volatility has significantly affected the market prices of securities of many companies for reasons frequently unrelated to the
operating performance of the specific companies. These broad market fluctuations may adversely affect the market price of our Ordinary
Shares.
Our
executive officers, directors and principal shareholders will maintain the ability to exert significant control over matters submitted
to our shareholders for approval.
Our
executive officers, directors and principal shareholders who own more than 5% of our outstanding Ordinary Shares beneficially own shares
representing approximately 17.63% of our share capital. As a result, if these shareholders were to act together, they would be able to
control all matters submitted to our shareholders for approval, as well as our management and affairs. For example, these persons, if
they act together, could control the election of directors and approval of any merger, consolidation or sale of all or substantially
all of our assets. This concentration of voting power could delay or prevent an acquisition of our company on terms that other shareholders
may desire or may result in management of our company being appointed despite our other shareholders’ disapproval.
If
securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they
adversely change their recommendations or publish negative reports regarding our business or our shares, our share price and trading
volume could decline.
The
trading market for our Ordinary Shares will be influenced by the research and reports that industry or securities analysts may publish
about us, our business, our market or our competitors. We do not have any control over these analysts, and we cannot provide any assurance
that analysts will cover us or provide favorable coverage. If any of the analysts who may cover us adversely change their recommendation
regarding our shares, or provide more favorable relative recommendations about our competitors, our share price would likely decline.
If any analyst who may cover us were to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility
in the financial markets, which in turn could cause our share price or trading volume to decline.
USE
OF PROCEEDS
This
prospectus relates to our ordinary shares that may be offered and sold from time to time by the selling shareholders. We will receive
no proceeds from the sale of ordinary shares by the selling shareholders in this offering. However, we may receive the proceeds of any
exercise by certain of the selling shareholders of warrants owned by them.
We
intend to use the net proceeds for research and development of our cancer detection kits, and for general corporate purposes, including
working capital needs. We may also use such proceeds for potential acquisitions in complementary businesses, although we do not currently
have any agreement or understanding with respect to an acquisition in which we plan to invest such proceeds.
We
will not be paying any underwriting discounts or commissions in offerings under this prospectus. The selling shareholders will bear discounts
or commissions, if any, attributable to their sale or disposition of the ordinary shares. Other than in connection with our indemnification
obligations with respect to the selling shareholders, we will bear all costs, expenses and fees in connection with the registration of
the shares (which do not include the fees and expenses of any selling shareholder counsel).
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS
OF OPERATIONS
The
following discussion and analysis should be read in conjunction with our financial statements and related notes, which have been publicly
filed with the Securities Exchange Commission and are included with this prospectus. This discussion and other parts of this offering
memorandum contain forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results
and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of several
factors, including those set forth under “Risk Factors” and elsewhere in this offering memorandum. We report financial information
under US GAAP and our financial statements were prepared in accordance with generally accepted accounting principles in the United States.
Overview
Todos
Medical is an in-vitro-diagnostic (“IVD”) firm engaging in the development and commercialization of a series of patient-friendly
blood tests that enable screening of a variety of cancers. Detecting cancer at an early stage may lead to more effective treatment and
possible better survival rate. Our goal is to establish our name in the cancer detection industry worldwide. Our cancer tests are still
in the development phase, have not yet been approved by the FDA and are not yet being marketed.
Screening
tests for cancer, specifically breast, colon and lung cancers, have compliance shortcomings due to their scientific limitations, invasive
procedures, and expensive nature of more accurate diagnostic methods. More importantly, many of the most effective diagnostic methods
remain too expensive for adoption as screening tests for all those at risk are used too rarely and not quickly enough to allow for the
most effective treatment. Blood based tests are the future of cancer screening.
Our
Provista Diagnostics Laboratory serves as a hub for our diagnostic development programs, including our flagship Videssa blood test, as
well as support for our automation solutions customers.
Diagnostic
testing helps health care providers screen for or monitor specific diseases or conditions. It also helps assess patient health to make
clinical decisions for patient care. Our Provista Diagnostics Laboratory is approved under the Clinical Laboratory Improvement Amendments
(CLIA). The Clinical Laboratory Improvement Amendments (CLIA) regulate laboratory testing and require clinical laboratories to be certified
by the Center for Medicare and Medicaid Services (CMS) before they can accept human samples for diagnostic testing. Laboratories can
obtain multiple types of CLIA certificates, based on the kinds of diagnostic tests they conduct.
We
have also focused our COVID-19 diagnostic testing efforts at Provista to prioritize delivering diagnostic services, including PCR and
neutralizing antibody testing, becoming a direct provider to healthcare professionals.
This
expansion into testing services allows us to diversify our business into higher margin revenue in the COVID-19 space, as well as help
us to expand our business development opportunities with the labs we work with by providing reference lab testing services as we increase
Provista’s automated testing capabilities. The Company intends to build Provista into a highly automated lab capable of running
multiple platforms in parallel in order to offer clients comprehensive testing solutions that meet their needs, especially in cancer,
infectious disease, immune monitoring and Alzheimer’s disease.
The
Company is also a developer and distributor of immune support products and antivirals that target the inhibition of 3CL protease for
the treatment of Covid-19. Todos has acquired exclusive distribution rights to the dietary supplement Tollovid™. Tollovid is a
powerful proprietary blend of plant extracts that help support healthy immune function for today’s challenges.
Todos
is also developing a more concentrated version of Tollovid for COVID-19 infected patients, using a proprietary blend of botanical extracts
with an active chemical ingredient that limits replication of coronaviruses. Todos is currently supporting randomized, placebo-controlled
clinical trials managed by joint venture partner NLC Pharma in Israel. Tollovir is the result of over 15 years of development and an
investment of over $18 million to date.
Videssa
Breast
Current
methods of breast cancer detection have known limitations, particularly in women with abnormal or difficult-to-interpret imaging findings.
While clinical examination and imaging technologies are critical elements for detecting breast cancer, the high rate of false positive
and false negative results from these approaches can significantly impact patient care. In an effort to improve the accuracy of early
breast cancer detection, complementary blood-based approaches are being developed to help address the current limitations of breast imaging.
By utilizing new detection strategies, healthcare providers will be able to improve the accuracy of breast cancer detection and minimize
the consequences of false positive and false negative results. To help address the diagnostic challenges in breast cancer, we developed
Videssa Breast—the first blood test of its kind to detect the presence or absence of breast cancer in women with abnormal or difficult-to-interpret
imaging findings. When combined with imaging, Videssa Breast improves diagnostic accuracy and provides greater confidence and clarity
when clinical assessment is challenging.
Videssa
Breast was developed to provide physicians with actionable information regarding breast cancer risk in women following an inconclusive
mammogram result (BI-RADS III or IV), which primarily occurs in women with dense breasts. The data provided from the test, which has
demonstrated specificity of ~99% in both women over and under 50 years of age, arms physicians with a powerful tool to help guide decisions
of whether to continue to monitor a low-risk patient intermittently, or whether to advance an at-risk patient immediately into a more
expensive and invasive diagnostic assessment that likely includes a breast biopsy. With Videssa as the proprietary centerpiece of our
cancer diagnostic strategy, we will be looking to offer highly advanced, comprehensive cancer testing solutions to OB-GYNs, general practitioners
and other stakeholders in the medical community who will ultimately be managing patients likely to be strong candidates for Videssa.
Videssa
Breast combines multiple Serum Protein Biomarkers (SPBs) and Tumor-Associated Autoantibodies (TAAbs), along with patient clinical data,
to generate a unique protein signature for breast cancer. As these protein biomarkers are released into the bloodstream, they act as
biological cues for the presence of a malignancy, providing a snapshot of what’s going on inside of a woman’s body to complement
the anatomical features visible on imaging. Unlike genetic testing which determines the future risk of developing breast cancer, Videssa
Breast is designed to detect real-time disease status. By identifying early biochemical warning signals of breast cancer in the bloodstream,
such as “protein biomarkers,” Videssa Breast provides information not detectable through imaging technologies, allowing for
a more comprehensive assessment.
LymPro
Test™:
The
Lymphocyte Proliferation (LymPro) Test™ measures markers of immune cells present in the blood as a surrogate for loss of nerve
cell function and the toxic accumulation of beta-amyloid plaques in the brain, which is a hallmark of Alzheimer’s disease. Based
on differences observed in the response of cells from patients with Alzheimer’s disease as compared with age-matched controls and
patients with other dementias, it appears that the test has high potential as an adjunctive diagnostic for Alzheimer’s disease.
LymPro exploits the fact that abnormalities in replication (or the cell cycle) seem to extend to immune cells in the blood. The test
specifically measures the alterations in cell cycle activity in blood lymphocytes (a type of immune cell) as a biomarker of neuronal
damage, for the early identification and screening of Alzheimer’s. Areas for deployment include initial Investigational Use Only
(“IUO”) testing followed by full diagnostic testing for patients with mild cognitive impairment (“MCI”) and dementia
for differential diagnosis. Todos owns the exclusive worldwide rights to this Alzheimer’s blood test as a result of its acquisition
of Breakthrough from Amarantus as follows. On February 27, 2019, we entered into a joint venture agreement with Amarantus, pursuant to
which we issued Ordinary Share representing 19.99% of our then outstanding Ordinary Shares to Amarantus, in exchange for Amarantus transferring
to us 19.99% of Breakthrough, then a wholly-owned subsidiary of Amarantus, and for Amarantus assigning the license for the LymPro test
to Breakthrough. As part of the transaction, we agreed to provide working capital to Breakthrough to support Breakthrough’s operations.
As part of the Breakthrough joint venture, we were granted an exclusive option to acquire the remaining 80.01% of Breakthrough from Amarantus.
At our 2019 annual meeting of shareholders, our shareholders approved a resolution authorizing us to exercise our option to acquire the
remaining 80.01% of Breakthrough from Amarantus in exchange for an additional 30% of our then issued and outstanding Ordinary Shares.
We closed the acquisition of Breakthrough in July 2020.
There
are a few blood-based approaches to Alzheimer’s, most of which focus on identifying canonical Alzheimer’s markers –
Amyloid or Tau. The rationale for these tests is that they serve as a proxy for brain concentration Amyloid and Tau-based imaging. Given
the failure of these two mechanisms to demonstrate improvement across hundreds of clinical trials, we believe that looking upstream from
Amyloid and Tau is where both true diagnostic and therapeutic avenues exist. LymPro captures both Amyloid and Tau-based information by
proxy. Given the expectation that the Alzheimer’s therapeutic market could reach $13.57 billion by 20271, we believe
LymPro could also help drive mid-term value for Todos as progress is made. Taken together with our core patented Todos Biochemical Infrared
Analyses (“TBIA”), which uses a platform based upon a highly sensitive mid infrared equipment called fourier transform infrared
spectrometers (“FTIR”), we believe Todos is positioned to become a worldwide leader in the field of immune-based diagnostics.
COVID-19
Proprietary Lab and Distribution Business:
We
provide advanced technologies addressing bottlenecks, whether they be scientific, technical or logistical, to enable laboratories to
rapidly expand testing capacity while reducing operational costs. To forward this business, we entered into distribution agreements with
multiple companies (such as 3D Biomedicine Science and Technology Col. Ltd., Meridian Health Services Network, Inc., and PCL Inc.) to
gain rights to rapid IgM/IgG COVID-19 antibody test kits, RNA extraction machines, RNA extraction reagents, qPCR reagents, digital PCR
reagents and automated liquid handler machines, in order to offer a comprehensive suite of solutions to laboratories worldwide. In the
second quarter of 2020, we began marketing a turnkey automation services solution to laboratories seeking to expand their COVID-19 testing
capabilities and started generating revenue from the distribution of products to support laboratory COVID testing through the automated
machinery we provided.
Our
Provista Diagnostics Laboratory serves as a hub for our diagnostic development programs, including our flagship Videssa blood test, as
well as support for our automation solutions customers. We have focused our COVID-19 diagnostic testing efforts at Provista to prioritize
delivering diagnostic services, including PCR and neutralizing antibody testing, becoming a direct provider to healthcare professionals.
We have partnered with Fosun Pharma to offer the first neutralizing antibody test, cPass™ SARS-CoV-2 Neutralizing Antibody Detection
Kit, which has received Emergency Use Authorization (“EUA”) from the US FDA for the detection of SARS-CoV-2 receptor binding
domain (“RBD” or “neutralizing”) antibodies. We believe this test can serve as a key marker for physicians, businesses
and schools to access Covid-19 immunity risk among their populations. This expansion into testing services allows us to diversify our
business into higher margin revenue in the COVID-19 space, as well as help us to expand our business development opportunities with the
labs we work with by providing reference lab testing services as we increase Provista’s automated testing capabilities. The Company
intends to build Provista into a highly automated lab capable of running multiple platforms in parallel in order to offer clients comprehensive
testing solutions that meet their needs, especially in cancer, infectious disease, immune monitoring and Alzheimer’s disease.
With
the Delta variant posing a significant risk for breakthrough infections, we see neutralizing antibody testing becoming critical for informed
decision making to assess who may be best suited for booster shots, as well as at what point someone previously infected with COVID begins
to show waning immunity and may decide to receive vaccination as a result. We see a large market opportunity developing for the cPass™
SARS-CoV-2 Neutralizing Antibody Detection Kit that we believe will begin to encroach on the COVID-19 PCR testing market. The cPass test
will enable individuals to take charge of their health by making data-driven decisions to protect themselves beyond vaccination, such
as masking or avoiding certain higher-risk activities when armed with this crucial information. A key differentiator for this novel cPass
test is that it detects neutralizing antibodies in patient samples without the use of live virus and with very fast turnaround times,
as compared to the conventional method of measuring neutralizing antibodies in patient samples, which requires the use of live cells.
We believe immune monitoring will be the primary driver of COVID-19 testing growth going forward. As time advances, and more individuals
are several months from their initial vaccine dose, it will become increasingly important for individuals and healthcare providers to
assess and monitor neutralizing antibody levels in order to make data-driven decisions with respect to booster shots and behavioral changes.
We are currently in the process of automating the cPass test at our laboratory, Provista Diagnostics, to add high-capacity neutralizing
antibody testing to its test menu. Provista plans to offer cPass as a testing service to other CLIA labs on a reference basis, as well
as directly to the public through healthcare professionals.
1https://www.medgadget.com/2021/01/alzheimers-therapeutics-market-to-reach-usd-13-57-billion-by-2027-size-share-industry-
analysis-and-global-forecast-to-2027.html
We
intend to focus on ways of leveraging our existing testing business and our client base to deliver actionable high value testing that
will improve outcomes while lowering cost of care. We believe that our establishment of a strong commercial infrastructure is the key
to unlocking the value of our intellectual property portfolio for our Company and its shareholders.
TBIA
Platform
Todos
Medical’s TBIA platform represents a cost effective, scalable, and patient-friendly screening method for cancer screening. Todos
has developed the “Total Biochemical Infrared Analysis” (TBIA) method, a proprietary method for screening of solid tumors
using peripheral blood spectroscopy analysis. The process involves observing the immune system’s response to tumor presence rather
than looking for the tumor cells themselves or specific markers. TBIA analyzes the entire biochemical signatures spectrum (including
proteins, lipids, nucleic acids and carbohydrates) of affected immune cells from peripheral blood, using infrared spectroscopy.
|
|
●
|
Advances
in mid infrared spectroscopy using fourier transform infrared spectrometers (FTIR) open new
diagnostic frontiers
|
|
|
|
|
|
|
●
|
Immune
system changes detected in plasma and mononuclear cells via FTIR
|
|
|
|
|
|
|
●
|
Immune
response acts as body’s sensor for cancer
|
|
|
|
|
|
|
●
|
FTIR
allows observation of distinct immune response to breast, colorectal, lung and other cancer
types
|
The
test offered under our TBIA method could help reduce false positives and improve detection rate by reporting to the physician the probability
of the presence/absence of cancer prior to more expensive tests. A large, underserved population of unscreened and inadequately screened
patients represents a significant opportunity for a patient friendly screening test. Furthermore, traditional tests are more likely to
expose patients to radiation and other risks inherent in those tests, our products offer may become a viable solution for these patients,
as it is a simple blood test.
Each
of the existing screening diagnosis methods have at least one of the following significant drawbacks:
|
-
|
Expensive
|
|
-
|
Low
sensitivity or specificity
|
|
-
|
Uncomfortable
to use
|
|
-
|
Not
accessible to the general public
|
|
-
|
Require
specialists for results interpretation
|
|
-
|
Possible
medical side effects from radiation and invasive tests
|
Many
patients who need to be checked regularly for cancer, avoid undertaking periodic examinations due to one or more of the above disadvantages.
The objective of our Company is to provide a more reliable alternative to the current methods of testing and to thereby overcome patients’
fear of regular cancer checkups, leveraging our proprietary technology in TBIA.
Despite
the various indications of the positive potential of our products, in order to establish our product in the market we still need to conduct
larger and more focused blind trials and we need to invest in a large-scale validation blind trial on the same cancer to confirm these
preliminary results.
Our
test is for cancer screening and cannot be regarded as a final diagnosis. However, it only requires a simple blood test causing minor
risk and pain (as below diagram demonstrates). Following the results of the test for positive or negative for specific cancer, the physician
will refer the patient for additional screenings such as colonoscopy for further examination of cancer presence.
Immune
Support Products and 3CL Protease Inhibiting Antivirals
The
Company entered into a joint venture with Israeli-based biotech company, NLC Pharma, to advance a theragnostic program targeting the
3CL protease, a key enzyme required for coronaviruses to replicate and infect other cells. We have funded the development of a novel
enzymatic 3CL protease diagnostic test that determines whether a coronavirus is actively replicating vs. inactively being cleared from
the body by the immune system, as well as 3CL protease inhibitors that aim to slow the replication of the virus in order to be able to
further support the body’s ability to be able to overcome a potential coronavirus exposure or infection.
Tollovir:
Furthermore,
the partnership is in the development phase of our own antiviral, Tollovir™, a potent 3CL protease inhibitor for the treatment
of hospitalized COVID-19 patients, which is currently undergoing a Phase 2 clinical trial in Israel with plans to expand the clinical
development program to India.
In
light of the emerging delta variant circulating widely worldwide, there is now a clear need for novel COVID-19 anti-viral therapies to
protect the unvaccinated and those for whom authorized vaccines do not confer immunity, which includes a large portion of the elderly
and those taking immune suppressants, against COVID-19 infection. In the United States, the Biden administration recently underscored
this need by pledging to invest $3.2 billion into research for COVID-19 anti-viral therapies, similar to the US Government’s investments
into COVID-19 vaccines in 2020 at the beginning of the pandemic. We believe this government recognition of the need for antivirals will
provide a significant tailwind for the development of our Tollovir™ anti-viral that is currently undergoing a Phase 2 clinical
trial in Israel with plans progressing rapidly to expand the clinical development program to India. As part of the ongoing scientific
effort to further elucidate the mechanisms that have enabled Tollovir to achieve its very positive early clinical results, NLC Pharma
identified an anti-inflammatory mechanism of action of Tollovir to complement its 3CL protease inhibiting mechanism. This dual mechanism
of action helps explain the significant reduction in symptoms and the biomarker C Reactive Protein (CRP) that was documented in the earliest
clinical COVID-19 data sets produced in Israel, which could not be explained by a reduction in viral load alone likely caused by Tollovir’s
3CL protease inhibiting mechanism. As a result of these quite complementary 3CL protease inhibiting and anti-inflammatory mechanisms,
given that we are in a race with Pfizer to get to market with the first 3CL protease inhibiting COVID-19 antiviral for which therapeutic
claims could be made, we believe it is critical to rapidly expand our clinical development programs to gather additional data in multiple
clinical settings to demonstrate Tollovir’s ability to help patients suffering from COVID-19. As part of that effort, our Israel-based
Principal Investigators have introduced us to physician clinical collaborators in India who work with a highly-respected local Clinical
Research Organization (CRO) with extensive experience in running COVID-19 clinical trials. This CRO has near-immediate access to 6 clinical
sites that have previously enrolled patients into clinical trials for hospitalized COVID-19 patients and 5 clinical sites that have previously
enrolled patients into clinical trials for non-hospitalized COVID-19 patients. We believe this CRO relationship will allow for the rapid
expansion of enrollment for Tollovir’s clinical data acquisition, and allow us to quickly prepare for a Phase 3 international clinical
development program to support regulatory approval under Emergency Use Authorization.
Tollovid:
The
Company’s 3CL protease inhibitor botanical product, Tollovid, is a dietary supplement that helps to support and maintain healthy
immune function. This technology will potentially have a significant impact for the development of virus targeting therapeutic development
strategies, as well as clearance for return to life activities post-infection.
We
are very pleased that the Company’s dietary supplement Tollovid, which provides immune support as a protease inhibitor, received
FDA authorization for a new 5-day dosing regimen in April 2021. We believe this authorization underscores the emerging need in the marketplace
for immune support supplements supported by strong scientific and safety data, as well as provides international regulatory authorities
with a high degree of comfort of Tollovid’s safety profile. Going forward, the Company sees two critical areas of expansion in
the advancement of Tollovid:
|
|
1)
|
International
distribution partnerships in jurisdictions where high value dietary supplements are distributed
by reputable pharmacies and other high-end wellness stores that can engage with consumers
directly on the value and underlying science of their products; and
|
|
|
|
|
|
|
2)
|
US
marketing campaign to dramatically expand awareness of Tollovid for consumers and distribution
partners who are looking for products to further support immune function.
|
We
believe that as we continue to grow our automation services business, we are creating a natural distribution base for the Videssa test,
as well as for the eventual commercialization of our proprietary TBIA platform tests and diagnostics developed with NLC Pharma. We intend
to seek out additional opportunities to leverage our expanding base of laboratory partners in the coming years.
Operating
Results
Revenues
During
the nine and three months ended September 30, 2021, we have generated revenues of $7,773,000 and $1,010,000, respectively, through our
U.S. subsidiaries, Corona Diagnostics, LLC and Provista Diagnostic, Inc .
Operating
Expenses
Our
current operating expenses consist of four components - cost of revenues, research and development expenses, marketing expenses and general
and administrative expenses.
Cost
of revenues
Our
cost of revenues consists primarily of materials, depreciation and other related cost of revenues expenses.
The
following table discloses the breakdown of cost of revenues:
|
|
|
Nine
Months Ended
September
30,
|
|
|
Three
Months Ended
September
30,
|
|
|
U.S.
dollars
|
|
2021
|
|
|
2020
|
|
|
2021
|
|
|
2020
|
|
|
Salaries
and related expenses
|
|
$
|
355,000
|
|
|
$
|
-
|
|
|
$
|
290,000
|
|
|
$
|
-
|
|
|
Materials
and other costs
|
|
|
4,354,000
|
|
|
|
894,000
|
|
|
|
581,000
|
|
|
|
883,000
|
|
|
Depreciation
|
|
|
482,000
|
|
|
|
-
|
|
|
|
172,000
|
|
|
|
-
|
|
|
Total
|
|
$
|
5,191,000
|
|
|
$
|
894,000
|
|
|
$
|
1,043,000
|
|
|
$
|
883,000
|
|
Research
and Development Expenses
Our
research and development expenses consist primarily of salaries and related personnel expenses, subcontracted work and consulting, liabilities
for royalties and other related research and development expenses.
The
following table discloses the breakdown of research and development expenses:
|
|
|
Nine
Months Ended
September 30,
|
|
|
Three
Months Ended
September 30,
|
|
|
U.S.
dollars
|
|
2021
|
|
|
2020
|
|
|
2021
|
|
|
2020
|
|
|
Stock-based
compensation
|
|
$
|
-
|
|
|
$
|
60,000
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Professional
fees
|
|
|
165,000
|
|
|
|
800,000
|
|
|
|
41,000
|
|
|
|
365,000
|
|
|
IPR&D
acquired as part of asset acquisition
|
|
|
-
|
|
|
|
8,157,000
|
|
|
|
-
|
|
|
|
8,157,000
|
|
|
Laboratory
and materials
|
|
|
646,000
|
|
|
|
572,000
|
|
|
|
144,000
|
|
|
|
511,000
|
|
|
Depreciation
|
|
|
22,000
|
|
|
|
38,000
|
|
|
|
7,000
|
|
|
|
25,000
|
|
|
Insurance
and other expenses
|
|
|
2,000
|
|
|
|
28,000
|
|
|
|
-
|
|
|
|
28,000
|
|
|
Total
|
|
$
|
835,000
|
|
|
$
|
9,655,000
|
|
|
$
|
192,000
|
|
|
$
|
9,086,000
|
|
We
expect that our research and development expenses will materially increase as we plan to rapidly recruit more employees in order to accelerate
our research and development efforts.
Sales
and Marketing Expenses
Sales
and marketing expenses consist primarily of salaries and share-based compensation expense.
The
following table discloses the breakdown of sales and marketing expenses:
|
|
|
Nine
Months Ended
September 30,
|
|
|
Three
Months Ended
September 30,
|
|
|
U.S.
dollars
|
|
2021
|
|
|
2020
|
|
|
2021
|
|
|
2020
|
|
|
Salaries
and related expenses
|
|
$
|
496,000
|
|
|
$
|
-
|
|
|
$
|
243,000
|
|
|
$
|
-
|
|
|
Share Based Compensation
|
|
|
45,000
|
|
|
|
1,463,000
|
|
|
|
-
|
|
|
|
33,000
|
|
|
Professional
Fees
|
|
|
1,846,000
|
|
|
|
724,000
|
|
|
|
186,000
|
|
|
|
724,000
|
|
|
Total
|
|
$
|
2,387,000
|
|
|
$
|
2,187,000
|
|
|
$
|
429,000
|
|
|
$
|
757,000
|
|
General
and Administrative
General
and administrative expenses consist primarily of salaries, share-based compensation expense, professional service fees (for accounting,
legal, bookkeeping, intellectual property and facilities), directors fees and insurance and other general and administrative expenses.
The
following table discloses the breakdown of general and administrative expenses:
|
|
|
Nine
Months Ended
September 30,
|
|
|
Three
Months Ended
September 30,
|
|
|
U.S.
dollars
|
|
2021
|
|
|
2020
|
|
|
2021
|
|
|
2020
|
|
|
Salaries
and related expenses
|
|
$
|
127,000
|
|
|
$
|
123,000
|
|
|
$
|
43,000
|
|
|
$
|
43,000
|
|
|
Share-based
compensation
|
|
|
513,000
|
|
|
|
712,000
|
|
|
|
148,000
|
|
|
|
398,000
|
|
|
Professional
fees
|
|
|
3,533,000
|
|
|
|
826,000
|
|
|
|
1,418,000
|
|
|
|
342,000
|
|
|
Insurance
and other expenses
|
|
|
875,000
|
|
|
|
68,000
|
|
|
|
234,000
|
|
|
|
21,000
|
|
|
Total
|
|
$
|
5,048,000
|
|
|
$
|
1,729,000
|
|
|
$
|
1,843,000
|
|
|
$
|
804,000
|
|
Comparison
of the Three and Nine Months Ended September 30, 2021 and September 30, 2020:
Results
of Operations
Revenues.
Our revenues for the three months ended September 30, 2021, were $1,010,000, compared to $1,284,000 during the three months ended September
30, 2020.
Our
revenues for the nine months ended September 30, 2021 were $7,773,000, compared to $1,316,000 during the nine months ended September
30, 2020.
The
increase in our revenues is a result of the sales of our COVID-19 testing products through our U.S. subsidiaries, Corona Diagnostics,
LLC and Provista Diagnostic Inc,. Revenues for the nine and three months ended September 30, 2021 include $4,290,000 of revenues during
the first quarter of 2021 to our significant customer with which Company’s contractual agreement to supply Covid-19 testing kits
to a significant customer expired.
Cost
of revenues. Our cost of revenues for the three months ended September 30, 2021, were $1,043,000, compared to $883,000 during the three
months ended September 30, 2020, and $5,191,000 during the nine months ended September 30, 2021, compared to $894,000 during the nine
months ended September 30, 2020. The increase in our cost of revenues is related to the sales of our COVID-19 testing products.
Research
and Development Expenses. Our research and development expenses for the three months ended September 30, 2021, were $192,000 compared
to $9,086,000 for the three months ended September 30, 2020, representing a net decrease of $8,894,000, or 98%, and $835,000 for the
nine months ended September 30, 2021, compared to $9,655,000 during the nine months ended September 30, 2020, a decrease of $8,820,000,
or 91%. The decrease in the nine months ended September 30, 2021 is primarily due to IPR&D expenses associated with the acquisition
of our subsidiary in the nine months ended September 30, 2020 offset by a decrease in Laboratory and materials and other research and
development costs in connection with providing Covid testing services mainly through our wholly-owned subsidiary Corona Diagnostics,
LLC and stock-based compensation used for continued development of our products.
Sales
and Marketing Expenses. Our sales and marketing expenses increased from $429,000 in the three months ended September 30, 2020, to $757,000
in the three months ended September 30, 2021, providing an increase of $328,000 or 43%, and increased from $2,187,000 in the nine months
ended September 30, 2020 to $2,387,000 in the nine months ended September 30, 2021, providing an increase of $200,000 or 9%. This increase
was principally due to increases in marketing and public relations efforts and costs associated with the sales of our Covid products
offset by a decrease in stock-based compensation.
General
and Administrative Expenses. Our general and administrative expenses for the three months ended September 30, 2021, were $1,843,000,
compared to $804,000 for the three months ended September 30, 2020, providing an increase of $1,039,000 or 129%, and $5,048,000 for the
nine months ended September 30, 2021, compared to $1,729,000 for the nine months ended September 30, 2020, providing an increase of $3,319,000
or 192%. The increase is primarily due to the increase in professional services which consists mainly of legal fees, directors fees and
other professional services.
Finance
Expenses, Net. Our net finance expenses for the three months ended September 30, 2021 was $6,875,000 compared to net finance expenses
of $7,055,000 for the three months ended September 30, 2020, providing a decrease of $180,000 or 3%, and $17,360,000 for the nine months
ended September 30, 2021, compared to net finance expenses of $11,375,000 for the nine months ended September 30, 2020, providing an
increase of $5,985,000 or 53%. The increase is primarily due to change in fair value of warrants liability, loss from extinguishment
of loans from shareholders and amortization of discounts and accrued interest on convertible bridge loans. It should be noted that during
the third quarter of 2021, most of the Company’s convertible bridge loans were repaid.
Share
in losses of affiliated company is accounted for under the equity method. Our share in losses of affiliated company accounted for under
the equity method amounted to $1,007,000 in the three months and $734,000 in the nine months ended September 30, 2021.
Net
Loss. Our net loss for the three months ended September 30, 2021 was $10,379,000, compared to net loss of $18,035,000 for the three months
ended September 30, 2020, providing a decrease of $7,656,000 or 42%. Our net loss for the nine months ended September 30, 2021 was $24,547,000,
compared with a net loss of $25,258,000 for the nine months ended September 30, 2020, a decrease in net loss of $711,000 or 3%. The decrease
is primarily due to the changes as mentioned above.
We
prepare our financial statements in accordance with US GAAP. At the time of the preparation of the financial statements, our management
is required to use estimates, evaluations, and assumptions which affect the application of the accounting policy and the amounts reported
for assets, obligations, revenues and expenses. Any estimates and assumptions are continually reviewed. The changes to the accounting
estimates are credited during the period in which the change to the estimate is made.
Subject
to certain conditions set forth in the JOBS Act, as an “emerging growth company,” we elected to rely on other exemptions,
including without limitation, (i) providing an auditor’s attestation report on our internal control over financial reporting pursuant
to Section 404 of the Sarbanes-Oxley Act and (ii) complying with any requirement that may be adopted by the PCAOB regarding mandatory
audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial
statements (auditor discussion and analysis). These exemptions applied until the last day of the 2021 fiscal year (the
fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement under
the Securities Act).
Going
Concern Uncertainty
Until
2020, we devoted substantially all of our efforts to research and development and raising capital. In 2020, we raised significant capital,
but we also generated revenues for the first time as a result of our activities related to Covid-19. There is no certainty as to the
continuance of our revenues related to Covid-19. The development and commercialization of our other products, which are necessary for
our long term financial health, are expected to require substantial further expenditures. We remain dependent upon external sources for
financing our operations. Since inception, we have incurred substantial accumulated losses, negative working capital, and negative operating
cash flow, and have a significant shareholders’ deficit. These factors raise substantial doubt about our ability to continue as
a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. We plan
to finance our operations through the sale of equity and, to the extent available, short term and long-term loans. There can be no assurance
that we will succeed in obtaining the necessary financing to continue our operations.
Liquidity
and Capital Resources
Overview
To
date, we have funded our operations primarily through revenue and with convertible bridge loans, grants from the IIA, and issuing Ordinary
Shares and stock warrants (including warrants’ exercise).
The
table below presents our cash flows:
STATEMENTS
OF CASH FLOWS
U.S.
dollars in thousands
|
|
|
Nine
months period ended
September
30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
Cash
flows from operating activities:
|
|
|
|
|
|
|
|
|
|
Net
loss
|
|
$
|
(24,547
|
)
|
|
$
|
(25,257
|
)
|
|
Adjustments
required to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Depreciation
|
|
|
556
|
|
|
|
38
|
|
|
Liability
for minimum royalties
|
|
|
53
|
|
|
|
29
|
|
|
Stock-based
compensation
|
|
|
633
|
|
|
|
2,334
|
|
|
Expiration
of call options to acquire potential acquiree
|
|
|
-
|
|
|
|
3,000
|
|
|
Impairment
of intangible IPR&D, net of taxes
|
|
|
-
|
|
|
|
8,157
|
|
|
Impairment
of investment in affiliated company
|
|
|
-
|
|
|
|
2,823
|
|
|
Revaluation
of investment in affiliated company to fair value
|
|
|
-
|
|
|
|
(1,623
|
)
|
|
Share
in losses of affiliated company
|
|
|
1,498
|
|
|
|
-
|
|
|
Modification
of terms relating to straight loan transaction
|
|
|
88
|
|
|
|
-
|
|
|
Modification
of terms relating to convertible bridge loans transactions
|
|
|
-
|
|
|
|
(3,495
|
)
|
|
Exchange
differences relating to loans from shareholders
|
|
|
-
|
|
|
|
40
|
|
|
Issuance
of shares as a settlement in excess of the carrying amount of financial liabilities
|
|
|
-
|
|
|
|
499
|
|
|
Issuance
of ordinary shares and stock warrants upon modification of terms relating to convertible bridge loans transactions
|
|
|
-
|
|
|
|
415
|
|
|
Amortization
of discounts and accrued interest on convertible bridge loans
|
|
|
18,080
|
|
|
|
8,393
|
|
|
Amortization
of discounts and accrued interest on straight loans
|
|
|
2,290
|
|
|
|
-
|
|
|
Change
in fair value of derivative warrants liability and fair value of warrants expired
|
|
|
(299
|
)
|
|
|
-
|
|
|
Change
in fair value of liability related to conversion feature of convertible bridge loans
|
|
|
(3,777
|
)
|
|
|
-
|
|
|
Increase
in trade receivables
|
|
|
(1,629
|
)
|
|
|
(206
|
)
|
|
Increase
in inventories
|
|
|
(1,806
|
)
|
|
|
(440
|
)
|
|
Decrease
(increase) in other current assets
|
|
|
705
|
|
|
|
47
|
|
|
Increase
(decrease) in accounts payables
|
|
|
(961
|
)
|
|
|
203
|
|
|
Decrease
in deferred revenues
|
|
|
(857
|
)
|
|
|
-
|
|
|
Increase
(decrease) in other current liabilities
|
|
|
(326
|
)
|
|
|
1,376
|
|
|
Net
cash used in operating activities
|
|
|
(9,299
|
)
|
|
|
(3,667
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash
flows from investing activities:
|
|
|
|
|
|
|
|
|
|
Purchase
of property and equipment
|
|
|
(965
|
)
|
|
|
(346
|
)
|
|
Restricted
cash
|
|
|
-
|
|
|
|
5
|
|
|
Purchase
of intangible IPR&D
|
|
|
-
|
|
|
|
(450
|
)
|
|
Cash
used in purchased of subsidiary consolidated for the first time
|
|
|
(1,176
|
)
|
|
|
-
|
|
|
Investment
in other companies
|
|
|
(1,024
|
)
|
|
|
(560
|
)
|
|
Net
cash used in investing activities
|
|
|
(3,165
|
)
|
|
|
(1,351
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash
flows from financing activities:
|
|
|
|
|
|
|
|
|
|
Proceeds
from straight loans, net
|
|
|
2,496
|
|
|
|
812
|
|
|
Repayment
of Receivables financing facility
|
|
|
(1,249
|
)
|
|
|
-
|
|
|
Repayment
of straight loans
|
|
|
(1,329
|
)
|
|
|
-
|
|
|
Repayment
of convertible bridge loans
|
|
|
(2,165
|
)
|
|
|
-
|
|
|
Proceeds
from issuance of units consisting of convertible bridge loans, stock warrants and shares, net
|
|
|
13,687
|
|
|
|
3,087
|
|
|
Proceeds
from issuance of units consisting of ordinary shares and stock warrants
|
|
|
-
|
|
|
|
30
|
|
|
Proceeds
from issuance of ordinary shares through equity line
|
|
|
255
|
|
|
|
1,296
|
|
|
Net
cash provided by financing activities
|
|
|
11,695
|
|
|
|
5,225
|
|
|
|
|
|
|
|
|
|
|
|
|
Change
in cash, cash equivalents
|
|
|
(769
|
)
|
|
|
207
|
|
|
Cash,
cash equivalents at beginning of period
|
|
|
935
|
|
|
|
12
|
|
|
Cash,
cash equivalents at end of period
|
|
$
|
166
|
|
|
$
|
219
|
|
Operating
Activities
Net
cash used in operating activities for the nine months ended September 30, 2021 was $9,329,000 compared to $3,667,000 in the nine months
ended September 30, 2020. The increase in the cash flow used in operating activities in 2021 compared to 2020 is primarily due to our
operating loss less stock-based compensation, change in fair value of convertible bridge loans, amortization of discounts and accrued
interest on convertible bridge loans and changes in other current assets, plus change in fair value of derivative warrants liability
and fair value of warrants expired, change in fair value of liability related to conversion feature of convertible bridge loans, increase
in inventory, increase in trade receivables and decrease in deferred revenues.
Investing
Activities
Net
cash used in investing activities for the for the nine months ended September 30, 2021 was $3,135,000, compared to $1,351,000 in the
nine months ended September 30, 2020. The primary reason for the increase in investing activities was due to the purchase of laboratory
equipment by our U.S. subsidiary, Corona Diagnostics, LLC, the investment in Provista Diagnostics Inc, and investments in other laboratories.
Financing
Activities
Net
cash provided by financing activities for the nine months ended September 30, 2021 was $11,695,000, compared to net cash provided by
financing activities for the nine months ended September 30, 2020 of $5,225,000. This increase is primarily due to cash received from
issuance of units consisting of convertible bridge loans, stock warrants and shares, net and from the proceeds from straight loans offset
by repayment of receivables financing facility, repayment of straight loans and repayment of convertible bridge loans.
Current
Outlook
We
cannot assure that our cancer detection kits will be commercialized, work as indicated, or that they will receive regulatory approval
and that we will earn revenues sufficient to support our operations or that we will ever be profitable. Furthermore, since we have no
committed source of financing, we cannot assure that we will be able to raise money as and when we need it to continue our operations.
If we cannot raise funds as and when we need them, we may be required to curtail, or even to cease, our operations.
We
have limited experience with in-vitro-diagnostics. As such, these budget estimates may not be accurate. In addition, the actual work
to be performed is not known at this time, other than a broad outline, as is normal with any scientific work. As further work is performed,
additional work may become necessary or changes in plans or workload may occur. Such changes may have an adverse impact on our estimated
budget. Such changes may also have an adverse impact on our projected timeline of drug development.
We
are currently distributing COVID-19 testing kits as a means of funding our operations.
If
we are unable to raise additional funds, we will need to do one or more of the following:
|
|
●
|
delay,
scale-back or eliminate some or all of our research and product development programs;
|
|
|
|
|
|
|
●
|
provide
licenses to third parties to develop and commercialize products or technologies that we would otherwise seek to develop and commercialize
ourselves;
|
|
|
|
|
|
|
●
|
seek
strategic alliances or business combinations;
|
|
|
●
|
attempt
to sell our Company;
|
|
|
|
|
|
|
●
|
cease
operations; or
|
|
|
|
|
|
|
●
|
declare
bankruptcy.
|
Any
debt financing secured by us in the future could involve restrictive covenants relating to our capital raising activities and other financial
and operational matters, which may make it more difficult for us to obtain additional capital and to pursue business opportunities, including
potential acquisitions. We may not be able to secure additional debt or equity financing in a timely manner, or at all, which could require
us to scale back our business plan and operations.
The
above conditions raise substantial doubt about our ability to continue as a going concern. The financial statements included elsewhere
herein were prepared under the assumption that we would continue our operations as a going concern. Our financial statements do not include
any adjustments that may result from the outcome of this uncertainty. Without additional funds from debt or equity financing, sales of
our intellectual property or technologies, or from a business combination or a similar transaction, we will soon exhaust our resources
and will be unable to continue operations. If we cannot continue as a viable entity, our shareholders may lose some or all of their investment
in us.
Our
management intends to attempt to secure additional required funding primarily through additional equity or debt financings. We may also
seek to secure required funding through sales or out-licensing of intellectual property assets, seeking partnerships with other pharmaceutical
companies or third parties to co-develop and fund research and development efforts, or similar transactions. However, there can be no
assurance that we will be able to obtain required funding. If we are unsuccessful in securing funding from any of these sources, we will
defer, reduce or eliminate certain planned expenditures in our research protocols. If we do not have sufficient funds to continue operations,
we could be required to seek bankruptcy protection or other alternatives that could result in our shareholders losing some or all of
their investment in us.
Recent
Developments
On
January 22, 2021, we entered into a Securities Purchase Agreement (the “SPA”) with Yozma Global Genomic Fund (the “Purchaser”)
pursuant to which on January 29, 2021, the Company issued a promissory convertible note (the “Note”) to the Purchaser in
the principal amount of $4,857,142.86 for proceeds of $3,400,000 (the “Transaction”). The Note has a maturity date of one
year from the date of issuance and pays interest at a rate of 4% per annum. The Note is convertible into Ordinary Shares of the Company
(the “Conversion Shares”) at a conversion price of $0.0599 (the “Conversion Price). In addition, the Purchaser received
a warrant (the “Warrant”) to purchase up to 16,956,929 Ordinary Shares (the “Warrant Shares”) of the Company
with an exercise price equal to $0.107415 per share. The Warrant is exercisable for 5 years from the date of issuance. In the event that
the Company effectuates a reverse split of its Ordinary shares for a ratio in excess of 20:1, the resulting adjusted Warrant Shares and
Exercise Price are limited to a 20:1 ratio.
This
registration statement includes the Warrant Shares. Subsequent to the effective date of such registration statement,
if the closing sale price of the Ordinary Shares averages less than the then Conversion Price over a period of ten (10) consecutive trading
days, the Conversion Price shall reset to such average price. If the 10 day volume weighted average price of the Ordinary Shares continues
to be less than the Conversion Price, then the Conversion Price should reset to such 10-day average price. The maximum discount from
the initial Conversion Price under the Note is 20%.
The
foregoing descriptions of the SPA, the Note and the Warrant do not purport to be complete and are qualified in their entirety by the
full text of the SPA, Note and Warrant, forms of which are attached as Exhibit 10.1, 10.2 and 10.3, respectively, to the Company’s
Current Report on Form 8-K dated January 26, 2021.
The
Company and Leviston Resources LLC, a Delaware limited liability company (the “Purchaser”) are parties to that certain Securities
Purchase Agreement, dated as of July 9, 2020 (the “Purchase Agreement”), pursuant to which the Purchaser purchased an aggregate
principal amount of $850,000 of convertible notes (the “July 2020 Convertible Notes”) from the Company. On March 3, 2021,
the Company and the Purchaser entered into a Closing Agreement (the “Closing Agreement”) pursuant to which the Purchaser
exercised its right to invest an additional $847,570 into the Company of July 2020 Convertible Notes (the “Tranche 2 Securities”).
This
registration statement includes the Ordinary Shares underlying the Tranche 2 Securities, but such Registration Statement has not yet
become effective.
The
foregoing description of the Closing Agreement does not purport to be complete and is qualified in its entirety by the full text of the
Closing Agreement, a form of which is attached as Exhibit 10.1 to the Company’s Current Report on Form 8-K dated March 10, 2021.
During
the first quarter of 2021, the Company’s contractual agreement to supply Covid-19 testing kits to NOAH Laboratories, Inc., a significant
customer expired. At the customer’s request, the Company continued to supply Covid-19 testing kits until such time as the customer
requested that the Company stop doing so. The customer has not yet paid for some of the Covid-19 testing kits so supplied, and has not
yet renewed its agreement with the Company. On November 15, 2021, Todos USA sent a demand letter (the “Demand Letter”) to
the significant customer with which our contractual agreement to supply Covid-19 testing kits expired. The Demand Letter seeks (a) payment
for testing kits that Todos USA supplied for which it was not paid, in the amount of $3,465,000, (b) the return of Todos USA’s
equipment, title to which remains with Todos USA unless and until the significant customer meets a minimum purchase requirement, and
(c) payment of damages as a result of the significant customer’s unlawful retention of Todos USA’s equipment, in an amount
anticipated to be $2 million. The Company and Todos USA are negotiating a settlement with NOAH Laboratories, Inc., which the Company believes will
result in most of the Company’s demands being met.
On
April 8, 2021, the Company entered into a Securities Purchase Agreement (the “SPA”) with Kips Bay Select LP (the “Purchaser”)
pursuant to which the Company agreed to issue a promissory convertible note (the “Note”) to the Purchaser in the principal
amount of $4,285,714.29 for proceeds of $3,000,000 (the “Transaction”). The closing occurred on April 12, 2021 (the “Closing
Date”). The Note has a maturity date of one year from the date of issuance and pays interest at a rate of 4% per annum. The Note
is convertible into Ordinary Shares (the “Conversion Shares”) at a conversion price of $0.0599 (the “Conversion Price).
In addition, the Purchaser received a warrant (the “Warrant”) to purchase up to 16,000,000 Ordinary Shares (the “Warrant
Shares”) of the Company with an exercise price equal to $0.107415 per share. The Warrant is exercisable for 5 years from the date
of issuance. In the event that the Company effectuates a reverse split of its Ordinary shares for a ratio in excess of 20:1, the resulting
adjusted Warrant Shares and Exercise Price are limited to a 20:1 ratio. The Company used the net proceeds from this Note to initiate
the Phase 2 for Tollovir™ clinical trial in COVID-19 patients, complete the acquisition of Provista Diagnostics, Inc. and for general
corporate purposes.
This
registration statement includes the Warrant Shares. Subsequent to the effective date of such registration statement,
if the closing sale price of the Ordinary Shares averages less than the then Conversion Price over a period of ten (10) consecutive trading
days, the Conversion Price shall reset to such average price. If the 10 day volume weighted average price of the Ordinary Shares continues
to be less than the Conversion Price then the Conversion Price should reset to such 10-day average price with a maximum of a 20% discount
from the initial Conversion Price.
The
Purchaser has the option to purchase an additional Note in the principal amount of $5,285,714.20 for proceeds of $3,700,000 and an additional
Warrant to purchase 16,000,000 Ordinary Shares within 90 days of the effective date of the registration statement on Form S-1 described
in the previous paragraph.
The
foregoing descriptions of the SPA, the Note and the Warrant do not purport to be complete and are qualified in their entirety by the
full text of the SPA, Note and Warrant, forms of which are attached as Exhibit 10.1, 10.2 and 10.3, respectively, to the Company’s
Current Report on Form 8-K dated April 14, 2021.
On
April 8, 2021, the Company received a notice of allowance (‘Letter of Intent to Grant a Patent’) from the European Patent
Office covering the use of the Company’s proprietary Total Biochemical Infrared Analysis (‘TBIA’) method that uses
blood (plasma and/or peripheral blood mononuclear cells ‘PBMCs’) to distinguish between patients with benign tumors vs. malignant
tumors vs. no tumors (healthy controls).
The
patent application specifically covers methods for capturing consistent data from infrared spectroscopy readers, as well as the application
of various artificial intelligence algorithm development methods to the data. The ability of TBIA to make a diagnosis of cancer has first
been applied to the detection of breast and colon cancers, where Todos has received CE Marks in Europe paving the way for commercialization
initially focused on TMB-2 (dense breast / inconclusive mammogram secondary screening) and TMB-1 (general breast cancer screening) cancer
detection tests.
On
April 19, 2021, the Company entered into an Agreement to Purchase Provista Diagnostics, Inc. (“Agreement to Purchase”) with
Strategic Investment Holdings, LLC (“SIH”), Ascenda BioSciences LLC (“Ascenda”) and Provista Diagnostics, Inc.
(“Provista”). Ascenda was the sole owner of the outstanding securities of Provista and SIH is the sole owner of all the outstanding
securities of Ascenda.
Pursuant
to the Agreement to Purchase, the Company acquired Provista from Ascenda and SIH for an aggregate purchase price of $7.5 million consisting
of an initial cash payment of $1.25 million, the issuance of $1.5 million in Ordinary Shares priced at $0.0512 per share, the issuance
of a $3.5 million convertible promissory note dated April 19, 2021 (the “Note”) and the payment on for before July 1, 2021
of $1.25 million in cash (the “July Payment”), which payment the Company had the right to, and did, extend to July 15, 2021.
The Provista shares acquired by the Company remained in an escrow account until the July Payment was made. The Note has a maturity date
of April 8, 2025, and is convertible beginning on October 20, 2021, into Ordinary Shares of the Company at a conversion price equal to
the lesser of $0.05 or the volume weighted average price of the last 20 trading days for the Ordinary Shares prior to the date of conversion.
In the event that the Company uplists its Ordinary Shares to a national securities exchange, the Note shall automatically be exchanged
into preferred stock with a conversion price equal to the lesser of (a) $0.05, (b) the opening price on the day of the uplisting provided
there is no transaction associated with the uplisting or (c) the deal price of an uplisting transaction.
The
foregoing descriptions of the Agreement to Purchase, the SPA, the Note and the Security Agreement do not purport to be complete and are
qualified in their entirety by the full text of the Agreement to Purchase, SPA, Note and the Security Agreement, forms of which are attached
as Exhibit 10.1, 10.2 and 10.3, respectively, to the Company’s Current Report on Form 8-K dated April 23, 2021.
On
July 7, 2021, the Company entered into a Securities Purchase Agreement (the “SPA”) with Kips Bay Select LP (the “Purchaser”)
pursuant to which the Company agreed to issue a promissory convertible note (the “Note”) to the Purchaser in the principal
amount of $1,535,714 for proceeds of $1,075,000 (the “Transaction”). The closing occurred on July 7, 2021 (the “Closing
Date”). The Note has a maturity date of one year from the date of issuance and pays interest at a rate of 4% per annum. The Note
is convertible into Ordinary Shares of the Company (the “Conversion Shares”) at a conversion price of $0.0599 (the “Conversion
Price). In addition, the Purchaser received a warrant (the “Warrant”) to purchase up to 3,440,000 Ordinary Shares (the “Warrant
Shares”) of the Company with an exercise price equal to $0.107415 per share. The Warrant is exercisable for 5 years from the date
of issuance. From the Closing Date until 180 days thereafter, the Company shall be restricted from issuing or entering into any agreement
to issue any Ordinary Shares, except under certain circumstances, including an uplisting. This provision shall no longer be in effect
if the closing sale price of the Ordinary shares exceeds $0.10. The Company intends to use the net proceeds for general corporate purposes.
This
registration statement includes the Warrant Shares. Subsequent to the effective date of such registration statement,
if the closing sale price of the Ordinary Shares averages less than the then Conversion Price over a period of ten (10) consecutive trading
days, the Conversion Price shall reset to such average price. If the 10-day volume weighted average price of the Ordinary Shares continues
to be less than the Conversion Price then the Conversion Price should reset to such 10-day average price with a maximum of a 20% discount
from the initial Conversion Price.
The
foregoing descriptions of the SPA, the Note and the Warrant do not purport to be complete and are qualified in their entirety by the
full text of the SPA, Note and Warrant, forms of which are attached as Exhibit 10.1, 10.2 and 10.3, respectively, to the Company’s
Current Report on Form 8-K filed July 8, 2021.
On
September 23, 2021, the Company completed the conditions precedent required to enter into a Securities Purchase Agreement (the “SPA”)
with Mercer Street Global Opportunity Fund, LLC (the “Purchaser”) pursuant to which the Company issued a promissory convertible
note (the “Note”) to the Purchaser in the principal amount of $2,285,142.86 for proceeds of $2,000,000 (the “Transaction”).
The Note has a maturity date of one year from the date of issuance and pays interest at a rate of 4% per annum. The Note is convertible
into Ordinary Shares of the Company (the “Conversion Shares”) at a conversion price of $0.0599 (the “Conversion Price).
In addition, the Purchaser received a warrant (the “Warrant”) to purchase up to 11,924,636 Ordinary Shares (the “Warrant
Shares”) of the Company with an exercise price equal to $0.107415 per share. The Warrant is exercisable for 5 years from the date
of issuance. The Company intends to use the net proceeds from this Note to initiate Phase 2/3 trials for Tollovir™ COVID-19 patients,
initiate digital marketing for its dietary supplement Tollovid®, increase sales & marketing for Provista Diagnostics, and for
general corporate purposes.
Univest
Securities, LLC acted as placement agent for the offering.
This
registration statement includes the Conversion Shares and the Warrant Shares. Subsequent to the effective date of the registration statement,
if the closing sale price of the Ordinary shares averages less than the then Conversion Price over a period of ten (10) consecutive trading
days, the Conversion Price shall reset to such average price. If the 10 day volume weighted average price of the Ordinary shares
continues to be less than the Conversion Price then the Conversion Price should reset to such 10-day average price with a maximum of
a 20% discount from the initial Conversion Price.
The
foregoing descriptions of the SPA, the Note and the Warrant do not purport to be complete and are qualified in their entirety by the
full text of the SPA, Note and Warrant, forms of which are attached as Exhibit 10.1, 10.2 and 10.3, respectively, to the Company’s
Current Report on Form 8-K filed September 24, 2021.
On
July 22, 2021, the US Food & Drug Administration (FDA) granted a new Certificate of Free Sale for Tollovid Daily™, the newest
member of the Company’s Tollovid™ dietary supplement product line.
The
Certificate of Free Sale is for a twice-daily dosing regimen and, critically, a 3CL protease inhibitor claim. Each 60-pill bottle of
Tollovid Daily can help support and maintain healthy immune function for 30 days. The Company intends to establish a monthly subscription
model as part of its marketing launch campaign for Tollovid Daily immune system support. Tollovid™ and Tollovid Daily are both
3CL protease inhibitor products developed under a joint venture with NLC Pharma.
On
November 18, 2021, Todos entered into a license agreement (the “License Agreement”) with T-Cell Protect Hellas S.A. (“T-Cell
Protect”) pursuant to which the Company will license the European manufacturing and distribution rights to a product based on the
Company’s Tollovid® and Tollovid Daily™ 3CL protease inhibitor and immune support dietary supplements to T-Cell Protect
(the “Product”). The License Agreement became effective on November 22, 2021 when the Company received a purchase order for
50,000 bottles of Tollovid Daily, per the terms of the License Agreement. The Product is expected to be marketed under the T-Cell Protect
brand in Europe. In addition, the Company will grant to T-Cell Protect an exclusive license to manufacture, sell and distribute the Product
in Greece. The License Agreement provides for royalty in the low double digits to the Company and a minimum of 500,000 bottles in sales
over the 18 months after the date of the License Agreement. In the event T-Cell Protect establishes its own manufacturing of the Product
in the European market, the 500,000 bottle minimum referred to in the previous sentence will become a minimum sales requirement. In the
License Agreement, T-Cell Protect acknowledged that the Company intends to assign the License Agreement to a subsidiary being formed
by the Company to focus on the development of 3CL protease biology called 3CL Sciences, Inc. (“3CL Sciences”).
On
November 22, 2021, the Company entered into a Securities Purchase Agreement (the “SPA”) with T-Cell pursuant to which the
Company issued a promissory convertible note (the “Note”) to T-Cell Protect in the principal amount of €1,000,000 (the
“Transaction”). The Note has a maturity date of one year from the date of issuance and pays interest at a rate of 10% per
annum. The Note is convertible into ordinary shares (the “Conversion Shares”) at a conversion price of $0.0599 (the “Conversion
Price). At any time prior to the Company uplisting its ordinary shares to a national securities exchange, T-Cell Protect may exchange
the Note into either (a) a direct equity investment in 3CL Sciences at the same terms as a financing round of at least $5,000,000 (the
“Sub”) or (b) into a note in the Sub, bearing 10% interest that converts into direct equity in the Sub at the same terms
as a financing round of at least $5,000,000. The proceeds from this Transaction are intended to be used for the clinical development
of Tollovir, the Company’s therapeutic candidate for hospitalized COVID-19 patients.
On
November 24, 2021, the Company entered into a binding letter of intent (the “LOI”) with NLC Pharma Ltd. (“NLC”)
pursuant to which 3CL Sciences will purchase all therapeutic, diagnostic, dietary supplement and pharmaceutical assets from NLC that
relate to 3CL protease biology in exchange for a 40% equity interest in 3CL Sciences that NLC will own, single digit royalties and Company
ordinary shares. Promptly following execution of the LOI, the Company will deliver $325,000 to pay outstanding invoices related to the
interim analysis of the ongoing clinical trial of Tollovir. The Company shall be responsible for providing or assisting in the raising
of a total of $10 million into 3CL Sciences over a period of 7 months from execution of the LOI. The Company and NLC agree to identify
a seasoned biopharmaceutical CEO to run 3CL Sciences going forward. The board of directors of 3CL Sciences will be made up of five (5)
individuals: two (2) appointed by the Company, two (2) appointed by NLC and one (1) to be mutually agreed upon by the Company and NLC.
In addition, NLC will be granted one (1) seat on the Company’s Board of Directors.
The
Company has agreed to file a registration statement with the Securities and Exchange Commission registering for resale the Conversion
Shares (the “Registration Statement).
The
foregoing descriptions of the License Agreement, SPA, the Note and LOI do not purport to be complete and are qualified in their entirety
by reference to the full text of the License Agreement, SPA, Note and LOI, forms of which are attached as Exhibit 10.1 , 10.2, 10.3 and
10.4, respectively, to the Company’s Current Report on Form 8-K dated November 29, 2021.
Contractual
Obligations
The
following table summarizes our contractual obligations as of September 30, 2021:
|
|
|
Payments
due by period
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(US$)
|
|
|
Less
than 1
|
|
|
|
|
|
|
|
|
More
than
|
|
|
|
|
Total
|
|
|
year
|
|
|
1-3
years
|
|
|
3-5
years
|
|
|
5
years
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible
bridge loans, net
|
|
|
15,560,000
|
|
|
|
15,560,000
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Other
loans, net
|
|
|
2,756,000
|
|
|
|
2,756,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Royalties
to BGU (1)
|
|
|
501,000
|
|
|
|
296,000
|
|
|
|
95,000
|
|
|
|
39,000
|
|
|
|
71,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total (2)
|
|
|
18,817,000
|
|
|
|
18,612,000
|
|
|
|
95,000
|
|
|
|
39,000
|
|
|
|
71,000
|
|
(1)
This balance was measured based on the future cash payments discounted using an interest rate of 21%, which represents, according to
management’s estimate, the applicable rate of risk for us.
(2)
This does not include the repayment of approximately $272,000 of grants we received from the Israel Innovation Authority and interest
thereon, which shall be repaid as royalties upon the commercialization of our products.
BUSINESS
Overview
Todos
Medical is an in-vitro-diagnostic (“IVD”) firm engaging in the development and commercialization of a series of patient-friendly
blood tests that enable screening of a variety of cancers. Detecting cancer at an early stage may lead to more effective treatment and
possible better survival rate. Our goal is to establish our name in the cancer detection industry worldwide. Our cancer tests are still
in the development phase, have not yet been approved by the FDA and are not yet being marketed.
Screening
tests for cancer, specifically breast, colon and lung cancers, have compliance shortcomings due to their scientific limitations, invasive
procedures, and expensive nature of more accurate diagnostic methods. More importantly, many of the most effective diagnostic methods
remain too expensive for adoption as screening tests for all those at risk are used too rarely and not quickly enough to allow for the
most effective treatment. Blood based tests are the future of cancer screening.
Our
Provista Diagnostics Laboratory serves as a hub for our diagnostic development programs, including our flagship Videssa blood test, as
well as support for our automation solutions customers.
Diagnostic
testing helps health care providers screen for or monitor specific diseases or conditions. It also helps assess patient health to make
clinical decisions for patient care. Our Provista Diagnostics Laboratory is approved under the Clinical Laboratory Improvement Amendments
(CLIA) in the U.S. The Clinical Laboratory Improvement Amendments (CLIA) regulate laboratory testing and require clinical laboratories
to be certified by the Center for Medicare and Medicaid Services (CMS) before they can accept human samples for diagnostic testing. Laboratories
can obtain multiple types of CLIA certificates, based on the kinds of diagnostic tests they conduct.
Three
federal agencies are responsible for CLIA: The Food and Drug Administration (FDA), Center for Medicaid Services (CMS), and the Centers
for Disease Control and Prevention (CDC). Each agency has a unique role in assuring quality laboratory testing.
FDA
|
|
●
|
Categorizes
tests based on complexity
|
|
|
●
|
Reviews
requests for Waiver by Application
|
|
|
●
|
Develops
rules/guidance for CLIA complexity categorization
|
CMS
|
|
●
|
Issues
laboratory certificates
|
|
|
●
|
Conducts
inspections and enforces regulatory compliance
|
|
|
●
|
Approves
private accreditation organizations for performing inspections, and approves state exemptions
|
|
|
●
|
Monitors
laboratory performance on Proficiency Testing (PT) and approves PT programs
|
|
|
●
|
Publishes
CLIA rules and regulations
|
CDC
|
|
●
|
Provides
analysis, research, and technical assistance
|
|
|
●
|
Develops
technical standards and laboratory practice guidelines, including standards and guidelines
for cytology
|
|
|
●
|
Conducts
laboratory quality improvement studies
|
|
|
●
|
Monitors
proficiency testing practices
|
|
|
●
|
Develops
and distributes professional information and educational resources
|
|
|
●
|
Manages
the Clinical Laboratory Improvement Advisory Committee (CLIAC)
|
The
FDA categorizes diagnostic tests by their complexity—from the least to the most complex: waived tests, moderate complexity tests,
and high complexity tests.
CLIA
categorization is determined after the FDA has cleared or approved a marketing submission, or upon request for legally marketed devices,
as described in the FDA guidance Administrative Procedures for CLIA Categorization. Tests that are waived by regulation under 42 CFR
493.15(c), or cleared or approved for home use, are categorized as waived. Otherwise, the FDA determines the test’s complexity
by reviewing the package insert test instructions, and using a criteria “scorecard” to categorize a test as moderate or high
complexity (42 CFR 493.17). Each test is graded for level of complexity by assigning scores of 1, 2, or 3 for each of the seven criteria
on the scorecard.
A
score of 1 indicates the lowest level of complexity, and a score of 3 indicates the highest level. The scores for the 7 criteria are
added together and tests with a score of 12 or less are categorized as moderate complexity, while those with a score above 12 are categorized
as high complexity. The FDA will notify the sponsor of their CLIA categorization —usually within two weeks of the marketing clearance
or approval.
Following
categorization, a manufacturer of a test categorized as moderate complexity may request categorization of the test as waived through
a CLIA Waiver by Application (CW) submission to the FDA.
We
have also focused our COVID-19 diagnostic testing efforts at Provista to prioritize delivering diagnostic services, including PCR and
neutralizing antibody testing, becoming a direct provider to healthcare professionals.
This
expansion into testing services allows us to diversify our business into higher margin revenue in the COVID-19 space, as well as help
us to expand our business development opportunities with the labs we work with by providing reference lab testing services as we increase
Provista’s automated testing capabilities. The Company intends to build Provista into a highly automated lab capable of running
multiple platforms in parallel in order to offer clients comprehensive testing solutions that meet their needs, especially in cancer,
infectious disease, immune monitoring and Alzheimer’s disease.
The
Company is also a developer and distributor of immune support products and antivirals that target the inhibition of 3CL protease for
the treatment of Covid-19. Todos has acquired exclusive distribution rights to the dietary supplement Tollovid™. Tollovid is a
powerful proprietary blend of plant extracts that help support healthy immune function for today’s challenges.
Todos
is also developing a more concentrated version of Tollovid for COVID-19 infected patients, using a proprietary blend of botanical extracts
with an active chemical ingredient that limits replication of coronaviruses. Todos is currently supporting randomized, placebo-controlled
clinical trials managed by joint venture partner NLC Pharma in Israel. Tollovir is the result of over 15 years of development and an
investment of over $18 million to date.
The
following table summarizes the current status of each of our technologies:

Diagnostics
Videssa
Breast:
Current
methods of breast cancer detection have known limitations, particularly in women with abnormal or difficult-to-interpret imaging findings.
While clinical examination and imaging technologies are critical elements for detecting breast cancer, the high rate of false positive
and false negative results from these approaches can significantly impact patient care. In an effort to improve the accuracy of early
breast cancer detection, complementary blood-based approaches are being developed to help address the current limitations of breast imaging.
By utilizing new detection strategies, healthcare providers will be able to improve the accuracy of breast cancer detection and minimize
the consequences of false positive and false negative results. To help address the diagnostic challenges in breast cancer, we developed
Videssa Breast—the first blood test of its kind to detect the presence or absence of breast cancer in women with abnormal or difficult-to-interpret
imaging findings. When combined with imaging, Videssa Breast improves diagnostic accuracy and provides greater confidence and clarity
when clinical assessment is challenging.
Videssa
Breast was developed to provide physicians with actionable information regarding breast cancer risk in women following an inconclusive
mammogram result (BI-RADS III or IV), which primarily occurs in women with dense breasts. The data provided from the test, which has
demonstrated specificity of ~99% in both women over and under 50 years of age, arms physicians with a powerful tool to help guide decisions
of whether to continue to monitor a low-risk patient intermittently, or whether to advance an at-risk patient immediately into a more
expensive and invasive diagnostic assessment that likely includes a breast biopsy. With Videssa as the proprietary centerpiece of our
cancer diagnostic strategy, we will be looking to offer highly advanced, comprehensive cancer testing solutions to OB-GYNs, general practitioners
and other stakeholders in the medical community who will ultimately be managing patients likely to be strong candidates for Videssa.

Videssa
Breast combines multiple Serum Protein Biomarkers (SPBs) and Tumor-Associated Autoantibodies (TAAbs), along with patient clinical data,
to generate a unique protein signature for breast cancer. As these protein biomarkers are released into the bloodstream, they act as
biological cues for the presence of a malignancy, providing a snapshot of what’s going on inside of a woman’s body to complement
the anatomical features visible on imaging. Unlike genetic testing which determines the future risk of developing breast cancer, Videssa
Breast is designed to detect real-time disease status. By identifying early biochemical warning signals of breast cancer in the bloodstream,
such as “protein biomarkers,” Videssa Breast provides information not detectable through imaging technologies, allowing for
a more comprehensive assessment.
LymPro
Test™:
The
Lymphocyte Proliferation (LymPro) Test™ measures markers of immune cells present in the blood as a surrogate for loss of nerve
cell function and the toxic accumulation of beta-amyloid plaques in the brain, which is a hallmark of Alzheimer’s disease. Based
on differences observed in the response of cells from patients with Alzheimer’s disease as compared with age-matched controls and
patients with other dementias, it appears that the test has high potential as an adjunctive diagnostic for Alzheimer’s disease.
LymPro exploits the fact that abnormalities in replication (or the cell cycle) seem to extend to immune cells in the blood. The test
specifically measures the alterations in cell cycle activity in blood lymphocytes (a type of immune cell) as a biomarker of neuronal
damage, for the early identification and screening of Alzheimer’s. Areas for deployment include initial Investigational Use Only
(“IUO”) testing followed by full diagnostic testing for patients with mild cognitive impairment (“MCI”) and dementia
for differential diagnosis. Todos owns the exclusive worldwide rights to this Alzheimer’s blood test as a result of its acquisition
of Breakthrough from Amarantus as follows. On February 27, 2019, we entered into a joint venture agreement with Amarantus, pursuant to
which we issued Ordinary Share representing 19.99% of our then outstanding Ordinary Shares to Amarantus, in exchange for Amarantus transferring
to us 19.99% of Breakthrough, then a wholly-owned subsidiary of Amarantus, and for Amarantus assigning the license for the LymPro test
to Breakthrough. As part of the transaction, we agreed to provide working capital to Breakthrough to support Breakthrough’s operations.
As part of the Breakthrough joint venture, we were granted an exclusive option to acquire the remaining 80.01% of Breakthrough from Amarantus.
At our 2019 annual meeting of shareholders, our shareholders approved a resolution authorizing us to exercise our option to acquire the
remaining 80.01% of Breakthrough from Amarantus in exchange for an additional 30% of our then issued and outstanding Ordinary Shares.
We closed the acquisition of Breakthrough in July 2020.
There
are a few blood-based approaches to Alzheimer’s, most of which focus on identifying canonical Alzheimer’s markers –
Amyloid or Tau. The rationale for these tests is that they serve as a proxy for brain concentration Amyloid and Tau-based imaging. Given
the failure of these two mechanisms to demonstrate improvement across hundreds of clinical trials, we believe that looking upstream from
Amyloid and Tau is where both true diagnostic and therapeutic avenues exist. LymPro captures both Amyloid and Tau-based information by
proxy. Given the expectation that the Alzheimers therapeutic market could reach $13.57 billion by 2027 2 ,
we believe LymPro could also help drive mid-term value for Todos as progress is made. Taken together with our core patented Todos Biochemical
Infrared Analyses (“TBIA”), which uses a platform based upon a highly sensitive mid infrared equipment called fourier transform
infrared spectrometers (“FTIR”), we believe Todos is positioned to become a worldwide leader in the field of immune-based
diagnostics.

2https://www.medgadget.com/2021/01/alzheimers-therapeutics-market-to-reach-usd-13-57-billion-by-2027-size-
share-industry-analysis-and-global-forecast-to-2027.html

TBIA
Platform:
Further,
Todos Medical’s TBIA platform represents a cost effective, scalable, and patient-friendly screening method for cancer screening.
Todos has developed the “Total Biochemical Infrared Analysis” (TBIA) method, a proprietary method for screening of solid
tumors using peripheral blood spectroscopy analysis. The process involves observing the immune system’s response to tumor presence
rather than looking for the tumor cells themselves or specific markers. TBIA analyzes the entire biochemical signatures spectrum (including
proteins, lipids, nucleic acids and carbohydrates) of affected immune cells from peripheral blood, using infrared spectroscopy.
|
|
●
|
Advances
in mid infrared spectroscopy using fourier transform infrared spectrometers (FTIR) open new
diagnostic frontiers
|
|
|
●
|
Immune
system changes detected in plasma and mononuclear cells via FTIR
|
|
|
●
|
Immune
response acts as body’s sensor for cancer
|
|
|
●
|
FTIR
allows observation of distinct immune response to breast, colorectal, lung and other cancer
types
|
The
test offered under our TBIA method could help reduce false positives and improve detection rate by reporting to the physician the probability
of the presence/absence of cancer prior to more expensive tests. A large, underserved population of unscreened and inadequately screened
patients represents a significant opportunity for a patient friendly screening test. Furthermore, traditional tests are more likely to
expose patients to radiation and other risks inherent in those tests, our products offer may become a viable solution for these patients,
as it is a simple blood test.
Each
of the existing screening diagnosis methods have at least one of the following significant drawbacks:
|
-
|
Expensive
|
|
-
|
Low
sensitivity or specificity
|
|
-
|
Uncomfortable
to use
|
|
-
|
Not
accessible to the general public
|
|
-
|
Require
specialists for results interpretation
|
|
-
|
Possible
medical side effects from radiation and invasive tests
|
Many
patients who need to be checked regularly for cancer, avoid undertaking periodic examinations due to one or more of the above disadvantages.
The objective of our Company is to provide a more reliable alternative to the current methods of testing and to thereby overcome patients’
fear of regular cancer checkups, leveraging our proprietary technology in TBIA.
Despite
the various indications of the positive potential of our products, in order to establish our product in the market we still need to conduct
larger and more focused blind trials and we need to invest in a large-scale validation blind trial on the same cancer to confirm these
preliminary results.
Our
test is for cancer screening and cannot be regarded as a final diagnosis. However, it only requires a simple blood test causing minor
risk and pain (as below diagram demonstrates). Following the results of the test for positive or negative for specific cancer, the physician
will refer the patient for additional screenings such as colonoscopy for further examination of cancer presence.


Our
Proprietary Technology
We
developed the TBIA method, a proprietary method for screening of solid tumors using peripheral blood analysis by infrared spectroscopy.
The method is multidisciplinary and incorporates hematology, biochemistry, physics and signal processing. TBIA detection method is based
on the cancers influence on the immune system which triggers cellular and biochemical changes in the peripheral blood mononuclear cells
(“PBMC”) and plasma. These biochemical changes are detected by a highly sensitive mid infrared equipment called the fourier
transform infrared spectrometers (“FTIR”). The FTIR results undergo rigorous testing of sophisticated signal processing in
order to detect if the entire biochemical signature under detection has the typical biochemical indications for cancer existence.
The
principle behind our proprietary technology, TBIA, is to observe the immune system response to tumor presence anywhere in the body rather
than looking for the tumor cells themselves. We analyze multiple elements of the biochemical signature (including proteins, lipids, nucleic
acids and carbohydrates) of the affected immune cells from the peripheral blood using infrared spectroscopy, instead of focusing on a
single specific protein as a biomarker.
Our
research, using spectral analysis, thus far indicates that the “infrared signatures” of several types of cancer are significantly
distinct from the “infrared signatures” of healthy patients. These differences can be related to several biological effects
which exist during malignancy.
Our
cancer screening kit includes a special glass slide upon which the PBMC and the plasma are placed. Some tests might also include a salt
solution that is needed for the blood separation process. There is a different test for each cancer type.
The
Todos Medical Innovation Solution:
|
|
-
|
The
immune system and plasma undergo biochemical changes due to cancer existence.
|
|
|
|
|
|
|
-
|
Mid
infrared spectroscopy can detect these biochemical changes (“spectral signature”).
|
|
|
-
|
The
result is generated automatically by a sophisticated mathematical algorithm which enhances
the efficiency for handling massive amounts of tests per year.
|
Clinical
Trials
Since
our inception, we have entered into agreements with a number of medical centers for the clinical studies of our screening kits in Israel,
including Wolfson Medical Center, Rabin Medical Center, Ichilov Medical Center, and Soroka Medical Center. We are currently conducting
a multi-center clinical study on breast cancer at Kaplan Medical Center and Soroka Medical Center.
Distribution
Channels
The
CE mark we have enables us to sell in Europe. We currently have one distributor in the Ukraine who acts as our regional distributor.
We are currently evaluating the possibility of a second distributor in Germany. The Ukraine. distributor, ABS Medical, is a small private
clinic which specifically added the TBIA blood separation capabilities to their practice to enable the testing, selling, and distributing
of our cancer screening kits. Meanwhile, we have yet to find a suitable distributor in Germany. Our goal is to collaborate with well-established
partners that could provide us with access to different markets around the world, but there can be no assurance that we will be able
to find any partners in the foreseeable future.
Because
we have not yet applied, and do not intend to apply for US Food and Drug Administration (“FDA”) approval in the next 12 months,
and do not have an established market demand for our products, we have not yet secured any reimbursement policy with any major third
party provider that could mass distribute our products.
Raw
Materials and Suppliers
We
have several vendors that provide us raw material from various geographic locations. While we are currently relying on these suppliers,
we plan to locate other suppliers upon strict inspection. We plan to utilize capital raised from this Offering to establish relationships
with other suppliers and check for compatibilities among supplies from these different vendors. We plan to have a minimum of two vendors
for each component in our system and it is our intention to eventually produce the raw material internally. However, because we are in
a highly specialized industry, there can be no assurance that we will be able to achieve that.
Our
current material suppliers, none of whom individually represents more than 10% of our supplies, are listed below. There is no assurance
that they will be able to continue supply our raw materials or that we will find replacement and vendors on a timely basis on favorable
terms, we may not be able to enter into agreements with them on terms and conditions favorable to us.
List
of the raw material suppliers for kits
|
SUPPLIERS
|
|
MATERIAL
|
|
SIGMA
|
|
HISTOPAQUE
|
|
guylop
|
|
DISICATORES
|
|
Crystaltechno
ltd., Alkor Technologies
|
|
ZnSe
uncoated
|
|
LA
MEDIMARKET
|
|
Saline
solution (0.9% NaCl)
|
|
ALEX
RED
|
|
Slide
Safe Box
|
|
SAGAM
|
|
TM
FABRIC
|
Suppliers
for our cancer screening kits
|
Manufacturer
Name
|
|
Supplier
Name
|
|
Service
Description / Component Description
|
|
BD
|
|
Bactlab
diagnostic ltd.
|
|
BD
Vacutainer K2EDTA 3ml 13*75P LAVEND 1K/c
|
|
Sigma
Aldrich
|
|
Sigma
Aldrich Israel ltd.
|
|
Histopaque-1077
|
|
Aguettant,
Lyon, France.
|
|
Medi-Market
|
|
Sodium
Chloride Solution, 0.9%, Embryo
|
|
Sigma
Aldrich
|
|
Sigma
Aldrich Israel ltd.
|
|
Ethanol
Absolut, Spectranal
|
Customers
Our
goal is to have a diversified pool of customers worldwide, including the United States. However, we plan to only focus on the Western
EU nations and Israel in the near future since we have the CE mark, and it will require more time and effort to achieve FDA approval.
Competitive
Advantage
Not
all tumors have applicable methods for screening. Current prevailing cancer screening tests utilize the standard procedures which are
typically uncomfortable or potentially hazardous, such as colonoscopy for colorectal cancer and mammography for breast cancer. In addition,
these tests generally have medium to low sensitivities/specificity, along with adverse risks. Furthermore, many of the existing screening
methods depend on the technicians or the physician’s capabilities, knowledge and interpretation. They also carry a significantly higher
cost to provide.
In
light of these drawbacks, we believe the primary advantages of the TBIA platform are the high accuracy (sensitivity and specificity)
and low-cost structure due to the biological information being captured using spectroscopy versus biological antibody capture methods
that require the manufacture of multiple antibodies to capture a biological signature. Within the early detection/screening universe
for breast cancer, Todos is the only spectroscopy-based assay that is mechanism agnostic, solely looking for differences in immune profiling
between disease and healthy normal based upon algorithms developed using artificial intelligence. We believe the TBIA test overcomes
many obstacles and limits imposed on the screening process by today’s traditional tests, thus enabling a larger population to be
checked for a cancer with a test that is simple, low-cost, and less harmful.
Early
detection is vital to the treatment of cancer, which is also the focus area of our products. The diagnostic, pharmaceutical and biopharmaceutical
industry is characterized by intense competition and rapid, significant technological changes. Many companies, research institutions
and universities are conducting research and development in a number of areas similar to those that we focus on that could lead to the
development of new products which could compete with our products. Most of the companies against which we compete have substantially
greater financial, technical, manufacturing, marketing, distribution and other resources. A number of these companies may have or may
develop technologies for developing products for treating various diseases that could prove to be the same or even superior to ours.
We expect technological developments in the diagnostic, pharmaceutical and biopharmaceutical and related fields to occur at a rapid rate,
and we believe competition will intensify as advances in these fields are made.
We
believe, however, that our products have their own competitive advantages and have the potential to become popular products for medical
professionals and patients. Our competitors lack our unique technology and, consequently, we believe we have advantages which we plan
to capitalize upon in order to rapidly gain market share.
Intellectual
Property
In
order to protect our proprietary technologies, we rely on combinations of patent, and trade secret protection, as well as confidentiality
agreements with employees, consultants, and third parties.
Our
success depends upon our ability to protect our technologies through patents, trademarks, know-how and confidentiality agreements. However,
there can be no assurance that the above-mentioned patent application will be approved by the appropriate regulatory agencies.
The
following table summarizes the status and uses of our patents and patent applications:
|
Patent
Number
|
Title
|
Description
|
Status
|
Exp.
Date
|
Next
Action
|
Due
Date
|
Payment
|
|
Category
I: These applications relate to analysis of an IR spectrum of a PBMC sample. Claims are generally directed to indicating the presence
of a solid tumor based on analysis of an IR spectrum of a PBMC sample.
|
US
Patent 9,606,057
(Patent Application No. 13/701,262)
|
BIOCHEMICAL
ANALYSIS OF PBMC
|
Claims
for a method indicating the presence of a solid tumor in breast tissue based on analysis of an IR spectrum of a PBMC sample.
|
Granted
March 28, 2017
|
Jun
1, 2031
|
Renewal
|
Sep
28, 2024
|
|
US
Patent Application No. 16/789,978
(Continuation of patent application No. 15/443,674)
|
INFRARED
(IR) SPECTROSCOPY SYSTEM
|
|
Pending
/ Filed
February 13, 2020
|
---
|
Awaiting
Official Notification
|
Jan
15, 2022
|
|
European
Patent No. 2577298
(Application No. 11789348.7)
|
DIAGNOSIS
OF CANCER
|
Claims
for a method indicating the presence of a solid tumor based on analysis of an IR spectrum of a PBMC sample.
|
Granted
February 26, 2020
|
---
|
---
|
---
|
|
German
Patent No. 60 2011 065 248.6
Validation of European Patent No. 2577298 (Application No. 11789348.7)
|
DIAGNOSIS
OF CANCER
|
|
Granted
February 26, 2020
|
Jun
1, 2031
|
Renewal
|
Jun
1, 2022
|
|
French
Patent No. 2577298
Validation of European Patent No. 2577298 (Application No. 11789348.7)
|
DIAGNOSIS
OF CANCER
|
|
Granted
February 26, 2020
|
Jun
1, 2031
|
Renewal
|
Jun
1, 2022
|
|
British
Patent No. 2577298
Validation of European Patent No. 2577298 (Application No. 11789348.7)
|
DIAGNOSIS
OF CANCER
|
|
Granted
February 26, 2020
|
Jun
1, 2031
|
Renewal
|
Jun
1, 2022
|
|
|
Israeli
Patent No. 223237
|
DIAGNOSIS
OF CANCER
|
Claims
for a method and for a computer program product, for indicating the presence of a solid tumor based on analysis of an IR spectrum
of a PBMC sample or white blood cells.
|
Granted
September 30, 2020
|
Jun
1, 2031
|
Renewal
|
Jun
1, 2025
|
|
|
Category
II: These applications relate to analysis of an IR spectrum of a blood plasma sample. Claims are generally directed to indicating
the presence of a solid tumor based on analysis of an IR spectrum of a blood plasma sample.
|
US
Patent No. 9,719,937
(Patent Application No. 14/116,506)
|
DIAGNOSIS
OF CANCER
|
Claims
for a method (process), a system, and for a computer program product. The claims are generally directed to indicating
the presence of a solid tumor in a gastrointestinal tract based on analysis of an IR spectrum of a blood plasma sample.
|
Granted
August 1, 2017
|
May
10, 2032
|
Renewal
|
Feb
1, 2025
|
|
US
Patent No. 10,139,349
(Patent Application No. 15/645,168)
(Continuation of patent application No. 14/116,506)
|
DIAGNOSIS
OF CANCER
|
Claims
for a method (process), a system, and for a computer program product. The claims are generally directed indicating the presence of
a solid tumor in breast tissue based on analysis of an IR spectrum of a blood plasma sample.
|
Granted
November 27, 2018
|
May
10, 2032
|
Renewal
|
May
27, 2022
|
|
US
Patent No. 10,379,056
(Patent Application 16/173,838)
(Continuation of patent application No. 15/645,168)
|
DIAGNOSIS
OF CANCER
|
Claims
for a method (process), a system, and a computer program product. The claims are generally directed to indicating the presence of
a solid tumor in lung tissue based on analysis of an IR spectrum of a blood plasma sample.
|
Granted
August 13, 2019
|
May
10, 2032
|
Renewal
|
Feb
13, 2023
|
|
US
Patent Application No. 16/530,473
(Continuation of patent application No. 16/173,838)
|
DIAGNOSIS
OF CANCER
|
|
Pending
/ Filed
August 2, 2019
|
---
|
Response
to Final Office Action
|
Nov
5, 2021
|
|
|
European
Patent Application No. 12782256.7
|
DIAGNOSIS
OF CANCER
|
|
Pending
/ Filed
December 10, 2013
|
---
|
Renewal
|
May
10, 2022
|
|
|
IL
Patent No. 229109
|
DIAGNOSIS
OF CANCER
|
Claims
for a method (process), a system, and for a computer program product, directed to indicating the presence of a solid tumor based
on analysis of an IR spectrum of a blood plasma sample.
|
Granted
September 1, 2018
|
May
10, 2032
|
Renewal
|
May
10, 2022
|
|
|
Category
III: Application relates to analysis of an IR spectrum of a blood plasma sample and PBMC samples.
|
US
Patent No. 9,804,145
(Patent Application No. 14/894,128)
|
DIFFERENTIAL
DIAGNOSIS OF BENIGN TUMORS
|
Method
claims generally directed to (i) analysis of an infrared (“IR”) spectrum of a peripheral blood mononuclear cells (“PBMC”)
to indicate the presence of a benign tumor in breast tissue and in the gastrointestinal tract, and (ii) analysis of an infrared (“IR”)
spectrum of a blood plasma sample to indicate the presence of a benign tumor.
|
Granted
October 31, 2017
|
Nov
14, 2033
|
Renewal
|
Apr
30, 2025
|
|
US
Patent No. 10,732,165
(Patent Application No. 15/785,801)
(Continuation of Patent Application No. 14/894,128)
|
DIFFERENTIAL
DIAGNOSIS OF BENIGN TUMORS
|
Method
claims generally directed to (i) analysis of an infrared (“IR”) spectrum of a peripheral blood mononuclear cells (“PBMC”)
to indicate the presence of a benign tumor in ovarian tissue and in the gastrointestinal tract, and (ii) analysis of an infrared
(“IR”) spectrum of a blood plasma sample to indicate the presence of a benign tumor.
|
Granted
August 4, 2020
|
Nov
14, 2033
|
Renewal
|
Feb
4, 2024
|
|
US
Patent Application No. 16/942,714
(Continuation of Patent Application No. 15/785,801)
|
DIFFERENTIAL
DIAGNOSIS OF BENIGN TUMORS
|
|
Pending
/ Filed
July 29, 2020
|
---
|
Awaiting
Official Notification
|
Jan
29, 2022
|
|
European
Patent No. 3004870
(Application No. 13885931.9)
|
DIFFERENTIAL
DIAGNOSIS OF BENIGN TUMORS
|
Method
claims generally directed to indicating the presence of a benign tumor in breast tissue based on analysis of an IR spectrum of a
PBMC sample.
|
Granted
January 2, 2019
|
---
|
---
|
---
|
|
German
Patent No. 60 2013 049 402.9
Validation of European Patent No. 3004870 (Application No. 13885931.9)
|
DIFFERENTIAL
DIAGNOSIS OF BENIGN TUMORS
|
|
Granted
January 2, 2019
|
Nov
14, 2033
|
Renewal
|
Nov
14, 2021
|
|
French
Patent No. 3004870
Validation of European Patent No. 3004870 (Application No. 13885931.9)
|
DIFFERENTIAL
DIAGNOSIS OF BENIGN TUMORS
|
|
Granted
January 2, 2019
|
Nov
14, 2033
|
Renewal
|
Nov
14, 2021
|
British
Patent No. 3004870
Validation of European Patent No. 3004870 (Application No. 13885931.9)
|
DIFFERENTIAL
DIAGNOSIS OF BENIGN TUMORS
|
|
Granted
January 2, 2019
|
Nov
14, 2033
|
Renewal
|
Nov
14, 2021
|
Dutch
Patent No. 3004870
Validation of European Patent No. 3004870 (Application No. 13885931.9)
|
DIFFERENTIAL
DIAGNOSIS OF BENIGN TUMORS
|
|
Granted
January 2, 2019
|
Nov
14, 2033
|
Renewal
|
Nov
14, 2021
|
European
Patent No. 3489659
(Application No. 18214760.3)
|
DIFFERENTIAL
DIAGNOSIS OF BENIGN TUMORS
|
Method
claims generally directed to indicating the presence of a benign tumor in GI tract based on analysis of an IR spectrum of a PBMC
sample.
|
Granted
March 3, 2021
|
---
|
---
|
---
|
German
Patent No. DE 60 2013 076 116.7
Validation of European Patent No. 3489659 (Application No. 18214760.3)
|
DIFFERENTIAL
DIAGNOSIS OF BENIGN TUMORS
|
|
Granted
March 3, 2021
|
Nov
14, 2033
|
Renewal
|
Nov
14, 2021
|
|
Spanish
Patent No. 3489659
Validation of European Patent No. 3489659 (Application No. 18214760.3)
|
DIFFERENTIAL
DIAGNOSIS OF BENIGN TUMORS
|
|
Granted
March 3, 2021
|
Nov
14, 2033
|
Renewal
|
Nov
14, 2021
|
|
French
Patent No. 3489659
Validation of European Patent No. 3489659 (Application No. 18214760.3)
|
DIFFERENTIAL
DIAGNOSIS OF BENIGN TUMORS
|
|
Granted
March 3, 2021
|
Nov
14, 2033
|
Renewal
|
Nov
14, 2021
|
|
British
Patent No. 3489659
Validation of European Patent No. 3489659 (Application No. 18214760.3)
|
DIFFERENTIAL
DIAGNOSIS OF BENIGN TUMORS
|
|
Granted
March 3, 2021
|
Nov
14, 2033
|
Renewal
|
Nov
14, 2021
|
|
Italian
Patent No. IT 502021000048371
Validation of European Patent No. 3489659 (Application No. 18214760.3)
|
DIFFERENTIAL
DIAGNOSIS OF BENIGN TUMORS
|
|
Granted
March 3, 2021
|
Nov
14, 2033
|
Renewal
|
Nov
14, 2021
|
|
Licensing
Agreement
On
April 8, 2010, we entered into a certain research and license agreement with BG Negev Technology and Application Ltd. (“BGU”)
and Mor Research and Application Ltd. (together with BGU, the “Licensor”). The Licensor, pursuant to the agreement, granted
us an exclusive, worldwide, license to commercialize certain intellectual property covered by the agreement (i.e., research, develop,
manufacture, market, distribute, and sell any product containing the licensable IP under the agreement). The Licensed IP under the agreement
included (a) provisional patent application number 61/318,395 filed on March 29th 2010 (“Existing IP”); (b) all patent applications
that may thereafter be filed by or on behalf of the Licensor, in any jurisdiction, which either are based on or claim priority from any
Existing IP; (c) any issued patent(s) and pending patent application(s) relating to leukemia identification and screening and filed pursuant
to this Agreement to protect the Existing IP, whether filed as an original application, continuation, continuation-in-part or divisional
application; (d) all patents which may be granted pursuant to any of the foregoing patent applications; (e) all IP using the FTIR for
cancer diagnosis and follow up, which has not been developed by Todos (“Licensed IP”). The license is perpetual unless the
agreement is terminated in accordance with its terms. The agreement requires that Todos pay a percentage of its sales from products produced
using the Licensed IP. To date, Todos has had no such sales.
Breast
Cancer Market and Competitive Landscape
The
breast cancer market report is segmented on the basis of type, treatment, end user and by geographical levels. The global breast cancer
diagnostic and drug market reached $20.9 billion in 2019 and is expected to increase to $28.2 billion by 2024, at a compound annual growth
rate (CAGR) of 6.2%3. Major factors in driving the growth of the breast cancer market include rising incidents of breast
cancer, increasing female geriatric population and growing governmental initiatives for new therapies. Furthermore, growing obesity,
lack of physical exercise, overexposure to radiation and drinking alcohol are common causes for the proliferation of the disease.
Based
upon treatment, the breast cancer market is classified into:
|
|
●
|
surgery
& radiation therapy,
|
Based
upon end users, the breast cancer market is classified into hospital pharmacies, private pharmacies and others.
In
2021, an estimated 281,550 new cases of invasive breast cancer are expected to be diagnosed in women in the U.S., along with 49,290 new
cases of non-invasive (in situ) breast cancer. About 2,650 new cases of invasive breast cancer are expected to be diagnosed in men in
2021. A man’s lifetime risk of breast cancer is about 1 in 833. 4
3
Source https://www.bccresearch.com/market-research/healthcare/breast-cancer-diagnostic-and-drug-
technologies-global-markets-report.html
4
Source https://www.breastcancer.org/symptoms/understand_bc/statistics
According
to the WHO, the global cancer burden is estimated to have risen to 18.1 million new cases and 9.6 million deaths in 2018. On the upside,
due to the advancement in cancer research, more patients with cancer have been successfully treated. However, high capital investment,
expensive medications, long approval time for the drugs and lack of awareness in several countries still hamper the development of the
breast cancer testing market.
North
America dominates the breast cancer market because of increased awareness about advanced treatment for cancer, presence of leading players,
established healthcare infrastructure and availability of branded drugs, changing lifestyle and increasing prevalence of breast cancer.
Favorable government initiatives and an increase in the number of research collaborations will hopefully accelerate the regional market
growth going forward.
The
Asia-Pacific region is expected to emerge as the fastest-growing regional market due to the increase in healthcare expenditure, growing
geriatric population rate, growing prevalence of breast cancer, rising awareness about early diagnosis, developing healthcare infrastructure,
and availability of effective treatment in emerging countries, such as China and India. In the Asia-Pacific region, rising disposable
income, adoption of western lifestyle, living more sedentary lives and consuming junk foods with higher energy and fat are major contributors
to this developing market. According to the Organization for Economic Cooperation and Development (OECD), the share of the population
aged over 65 years is expected to double to reach and on average 27.6% in 2050 in high income Asia-Pacific countries. 5
Cancer
diagnostic tests are very expensive, putting a financial burden on patients and families. Currently available tests show false positive
results in some cases. Thus, supportive confirmatory tests are required, which adds an extra layer of out-of-pocket expenses. Imaging
modalities are widely used for imaging tumorous growth in the body. However, injection of radioactive agents to locate the cancerous
growth accurately increases the risk of hazards caused by radiation exposure to the patient, as well as radiologists and imaging center
staff. Considering the side effects and risk associated with radiation exposure, imaging centers are required to adhere to stringent
practice guidelines to ensure safety of patients and staff. Thus, skilled professionals are mandatory in such centers, which adds additional
cost burden to imaging centers. Furthermore, government insurance frameworks are not strong in developing countries, resulting in the
middle-class patient population taking the biggest hit, since most. are not covered under any insurance (private or government). Also,
many private insurance players do not cover cost associated with cancer diagnosis in these countries. As a result, high cost of diagnosis
is challenging the adoption of cancer screening tools, especially in developing countries. However, the scenario is expected to change
in the near future due to booming medical tourism in some countries, where healthcare treatments and diagnostic solutions are becoming
increasingly more affordable.
Competitors:
Check4Cancer
(Cambridge, England)
Check4Cancer
is a provider of cancer detection and genetic services for self-funding patients, insurers & corporate customers. They work with
employers, their advisers and their insurers to help employees become the first line of defense against the rising incidence of the most
common cancers. They can be funded by the employer or the employee, reflecting the objectives and budget of both.
Biosignatures
(England)
Biosignatures
is an AI Cloud software company. They support advanced blood sample analysis for use in a health ecosystem. Biosignatures contribute
a core, Cloud delivered, software analytics technology to the ecosystem that can provide health insights for multiple conditions from
a single drop of blood. Their approach takes a powerful research technique and, through systematically improved workflows and algorithms,
adapts it for consistent use in large populations. The technology provides not only information about multiple proteins, but also distributions
of modifications (including N and O glycosylations). These modifications have long been known to have significant involvement in infection
and disease processes. They have completed an ‘in clinic’ proof of concept in prostate cancer and are currently extending
into other cancers and chronic disease areas.
5
Source https://www.oecd.org/newsroom/asia-pacific-should-reduce-inequalities-in-access-to-care-for-the-most-
marginalised-groups.htm
MSDX,
Inc. (Tucson, AZ)
MSDx,
Inc. is commercializing both Research Use Only (RUO) and In Vitro Diagnostic (IVD) blood test products for neurological disorders. Multiple
Sclerosis is the first focus. Ultimately, this technology can be used to monitor patients with conditions such as brain and spinal cord
injury, Parkinson’s disease, and chronic fatigue syndrome.
COVID-19
Proprietary Lab and Distribution Business:
We
provide advanced technologies addressing bottlenecks, whether they be scientific, technical or logistical, to enable laboratories to
rapidly expand testing capacity while reducing operational costs. To forward this business, we entered into distribution agreements with
multiple companies (such as 3D Biomedicine Science and Technology Col. Ltd., Meridian Health Services Network, Inc., and PCL Inc.) to
gain rights to rapid IgM/IgG COVID-19 antibody test kits, RNA extraction machines, RNA extraction reagents, qPCR reagents, digital PCR
reagents and automated liquid handler machines, in order to offer a comprehensive suite of solutions to laboratories worldwide. In the
second quarter of 2020, we began marketing a turnkey automation services solution to laboratories seeking to expand their COVID-19 testing
capabilities and started generating revenue from the distribution of products to support laboratory COVID testing through the automated
machinery we provided.
Our
Provista Diagnostics Laboratory serves as a hub for our diagnostic development programs, including our flagship Videssa blood test, as
well as support for our automation solutions customers. We have focused our COVID-19 diagnostic testing efforts at Provista to prioritize
delivering diagnostic services, including PCR and neutralizing antibody testing, becoming a direct provider to healthcare professionals.
We have partnered with Fosun Pharma to offer the first neutralizing antibody test, cPass™ SARS-CoV-2 Neutralizing Antibody Detection
Kit, which has received Emergency Use Authorization (“EUA”) from the US FDA for the detection of SARS-CoV-2 receptor binding
domain (“RBD” or “neutralizing”) antibodies. We believe this test can serve as a key marker for physicians, businesses
and schools to access Covid-19 immunity risk among their populations. This expansion into testing services allows us to diversify our
business into higher margin revenue in the COVID-19 space, as well as help us to expand our business development opportunities with the
labs we work with by providing reference lab testing services as we increase Provista’s automated testing capabilities. The Company
intends to build Provista into a highly automated lab capable of running multiple platforms in parallel in order to offer clients comprehensive
testing solutions that meet their needs, especially in cancer, infectious disease, immune monitoring and Alzheimer’s disease.
With
the Delta variant posing a significant risk for breakthrough infections, we see neutralizing antibody testing becoming critical for informed
decision making to assess who may be best suited for booster shots, as well as at what point someone previously infected with COVID begins
to show waning immunity and may decide to receive vaccination as a result. We see a large market opportunity developing for the cPass™
SARS-CoV-2 Neutralizing Antibody Detection Kit that we believe will begin to encroach on the COVID-19 PCR testing market. The cPass test
will enable individuals to take charge of their health by making data-driven decisions to protect themselves beyond vaccination, such
as masking or avoiding certain higher-risk activities when armed with this crucial information. A key differentiator for this novel cPass
test is that it detects neutralizing antibodies in patient samples without the use of live virus and with very fast turnaround times,
as compared to the conventional method of measuring neutralizing antibodies in patient samples, which requires the use of live cells.
We believe immune monitoring will be the primary driver of COVID-19 testing growth going forward. As time advances, and more individuals
are several months from their initial vaccine dose, it will become increasingly important for individuals and healthcare providers to
assess and monitor neutralizing antibody levels in order to make data-driven decisions with respect to booster shots and behavioral changes.
We are currently in the process of automating the cPass test at our laboratory, Provista Diagnostics, to add high-capacity neutralizing
antibody testing to its test menu. Provista plans to offer cPass as a testing service to other CLIA labs on a reference basis, as well
as directly to the public through healthcare professionals.
We
intend to focus on ways of leveraging our existing testing business and our client base to deliver actionable high value testing that
will improve outcomes while lowering cost of care. We believe that our establishment of a strong commercial infrastructure is the key
to unlocking the value of our intellectual property portfolio for our Company and its shareholders.
Immune
Support Products and 3CL Protease Inhibiting Antivirals
The
Company entered into a joint venture with Israeli-based biotech company, NLC Pharma, to advance a theragnostic program targeting the
3CL protease, a key enzyme required for coronaviruses to replicate and infect other cells. We have funded the development of a novel
enzymatic 3CL protease diagnostic test that determines whether a coronavirus is actively replicating vs. inactively being cleared from
the body by the immune system, as well as 3CL protease inhibitors that aim to slow the replication of the virus in order to be able to
further support the body’s ability to be able to overcome a potential coronavirus exposure or infection.
Tollovir:
Furthermore,
the partnership is in the development phase of our own antiviral, Tollovir™, a potent 3CL protease inhibitor for the treatment
of hospitalized COVID-19 patients, which is currently undergoing a Phase 2 clinical trial in Israel with plans to expand the clinical
development program to India.
In
light of the emerging delta variant circulating widely worldwide, there is now a clear need for novel COVID-19 anti-viral therapies to
protect the unvaccinated and those for whom authorized vaccines do not confer immunity, which includes a large portion of the elderly
and those taking immune suppressants, against COVID-19 infection. In the United States, the Biden administration recently underscored
this need by pledging to invest $3.2 billion into research for COVID-19 anti-viral therapies, similar to the US Government’s investments
into COVID-19 vaccines in 2020 at the beginning of the pandemic. We believe this government recognition of the need for antivirals will
provide a significant tailwind for the development of our Tollovir™ anti-viral that is currently undergoing a Phase 2 clinical
trial in Israel with plans progressing rapidly to expand the clinical development program to India. As part of the ongoing scientific
effort to further elucidate the mechanisms that have enabled Tollovir to achieve its very positive early clinical results, NLC Pharma
identified an anti-inflammatory mechanism of action of Tollovir to complement its 3CL protease inhibiting mechanism. This dual mechanism
of action helps explain the significant reduction in symptoms and the biomarker C Reactive Protein (CRP) that was documented in the earliest
clinical COVID-19 data sets produced in Israel, which could not be explained by a reduction in viral load alone likely caused by Tollovir’s
3CL protease inhibiting mechanism. As a result of these quite complementary 3CL protease inhibiting and anti-inflammatory mechanisms,
given that we are in a race with Pfizer to get to market with the first 3CL protease inhibiting COVID-19 antiviral for which therapeutic
claims could be made, we believe it is critical to rapidly expand our clinical development programs to gather additional data in multiple
clinical settings to demonstrate Tollovir’s ability to help patients suffering from COVID-19. As part of that effort, our Israel-based
Principal Investigators have introduced us to physician clinical collaborators in India who work with a highly-respected local Clinical
Research Organization (CRO) with extensive experience in running COVID-19 clinical trials. This CRO has near-immediate access to 6 clinical
sites that have previously enrolled patients into clinical trials for hospitalized COVID-19 patients and 5 clinical sites that have previously
enrolled patients into clinical trials for non-hospitalized COVID-19 patients. We believe this CRO relationship will allow for the rapid
expansion of enrollment for Tollovir’s clinical data acquisition, and allow us to quickly prepare for a Phase 3 international clinical
development program to support regulatory approval under Emergency Use Authorization.

Tollovid:
The
Company’s 3CL protease inhibitor botanical product, Tollovid, is a dietary supplement that helps to support and maintain healthy
immune function. This technology will potentially have a significant impact for the development of virus targeting therapeutic development
strategies, as well as clearance for return to life activities post-infection.
We
are very pleased that the Company’s dietary supplement Tollovid, which provides immune support as a protease inhibitor, received
FDA authorization for a new 5-day dosing regimen in April 2021. We believe this authorization underscores the emerging need in the marketplace
for immune support supplements supported by strong scientific and safety data, as well as provides international regulatory authorities
with a high degree of comfort of Tollovid’s safety profile. Going forward, the Company sees two critical areas of expansion in
the advancement of Tollovid:
|
|
1)
|
International
distribution partnerships in jurisdictions where high value dietary supplements are distributed
by reputable pharmacies and other high-end wellness stores that can engage with consumers
directly on the value and underlying science of their products; and
|
|
|
|
|
|
|
2)
|
US
marketing campaign to dramatically expand awareness of Tollovid for consumers and distribution
partners who are looking for products to further support immune function.
|
We
believe that as we continue to grow our automation services business, we are creating a natural distribution base for the Videssa test,
as well as for the eventual commercialization of our proprietary TBIA platform tests and diagnostics developed with NLC Pharma. We intend
to seek out additional opportunities to leverage our expanding base of laboratory partners in the coming years.
Covid-19
Diagnostics Market Scope
The
infectious disease diagnostics market is segmented into reagents, kits, and consumables; instruments; and software & services. The
reagents, kits, and consumables segment, critical components in Covid testing, accounted for the highest growth rate in the infectious
disease diagnostics market in 2020. The requirement of reagents, kits and consumables in large numbers compared to instruments is the
main factor contributing to this segment’s high growth rate. This segment’s market growth can also be attributed to repeat purchases
of reagents, kits, and consumables compared to instruments. The World Health Organization (WHO) has urged nations to ramp up their diagnostics
capacities, which subsequently will cause a boost in the uptake of reagents and kits at a larger scale. Several regulatory bodies have
issued mass testing; however, laboratories are facing a shortage of reagents required for large batches of testing.
The
Centers of Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) are addressing the challenges surfacing due
to the recent shortage of reagents in the U.S. To combat any hindrance caused by shortages of pivotal testing reagents and kits in the
U.S., the FDA has granted an Emergency Use Authorization (EUA) to various COVID-19 detection tests. This strategy is aimed at relaxing
various ordinary standards enabling the market players to seek easy and rapid approval for their kits as well as reagents. This is expected
to increase the supply of kits and reagents, contributing to the segment growth.
The
portable handheld instruments are gaining immense popularity for their use in controlling coronavirus cases, hence the segment is anticipated
to witness lucrative growth over the forecast period. These portable instruments accelerate clinical decision-making in decentralized
settings. Thus, increasing usage of rapid antigen tests is anticipated to boost the revenue of instrument segments. While supply issues
have been a well-publicized factor for rt-PCR testing, supply issues can or cannot become an issue in point-of-care testing.
Sample
Type Insights
Nasopharyngeal
swabs are the preferred choice for sample collection as recommended by the CDC. In case of nasal swab shortage, oropharyngeal swabs are
also acceptable. The use of both swabs in a single tube maximizes the test sensitivity and limits the usage of testing resources, which
drives their adoption rate in COVID-19 sample collection.
In
addition, the FDA has recently allowed using a wide range of swabs, especially polyester swabs, for diagnosis of COVID-19. These synthetic
swabs are easier to manufacture and address the challenges pertaining to a shortage of swabs. In response, the FDA announced in April
that U.S. Cotton has planned to manufacture polyester-based swabs in large quantities that can also be put to use in sample collection
for coronavirus testing. These initiatives are expected to further accelerate the adoption rate of swabs.
Blood
sample-based tests are largely employed in antibody detection tests along with rapid detection tests. This sample type provides an assessment
of both short- and long-term trajectories of antibody response, abundance, and diversity in the affected patients, which is difficult
in viral RNA detection tests. Thus, the blood sample type is expected to gain traction across research and molecular studies.
Test
Type Insights
A
majority of the COVID-19 detection tests rely on Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) for quantification of RNA in
the samples of coronavirus patients. Large-scale employment of PCR across the CDC, commercial laboratories, hospitals, and public health
laboratories have made significant contributions to the revenue generated by this technology in the global market.
Continuous
approval of RT-PCR-based molecular diagnostic tests for early detection of the infection is anticipated to drive the segment. In March
2020, Roche’s Cobas SARS-CoV-2 test was the first test to receive EUA. Subsequently, the TaqPath COVID-19 Combo Kit of Thermo Fisher
received EUA during the same month. Other available tests are Lyra SARS-CoV-2 Assay by Quidel, SARS-CoV-2 Qualitative Real-Time RT-PCR
by Quest, COVID-19 RT-PCR Test by LabCorp, and others.
With
55% revenue share, the non-POC (centralized) tests segment is anticipated to dominate the market in 2020, due to the low sensitivity
of rapid tests that has boosted the adoption of non-POC tests by the end-users. As a result, government bodies have advised the healthcare
settings to conduct RT-PCR test for symptomatic individuals to confirm the diagnosis, if the rapid antigen test results are negative.
Furthermore, conventional laboratory testing remains the primary testing mode due to its ability to conduct a high volume of tests at
a given point in time. These factors are key driving forces for this segment. On the other hand, companies are validating and developing
rapid POC immunodiagnostic tests to facilitate decentralized testing. For instance, in August 2020, Abbott announced EUA authorization
for its COVID-19 antigen test that delivers results in 15 minutes. The company planned the commencement of product shipping in early
October with 50 million kits per month. Prior to this approval, BD and Quidel already secured regulatory approval for their antigen tests
making mass testing more feasible.
End-use
Insights
The
laboratories are constantly increasing their testing capacities to address the rising demand for COVID-19 testing. Moreover, private
laboratories are also inclined toward coronavirus testing. As reported in April 2020, Orchard Laboratories was the first private laboratory
in Michigan to offer rapid COVID-19 testing by using RT-PCR technology. Initially, it conducted 400 tests per day.
Moreover,
laboratories have leveraged high-throughput technologies to process a large volume of coronavirus tests accurately and rapidly. This
is propelled by robust reimbursement policies implemented effectively in the developed economies. For instance, the Centers for Medicare
& Medicaid Services (CMS) has doubled the Medicare reimbursement rate for high-molecular tests pertaining to COVID-19 detection.
Government
authorities and companies are undertaking efforts to develop tests that can be specifically deployed in diagnostics centers, clinics,
or physician’s offices. The FDA has recently approved decentralized testing platforms to diversify testing capabilities across
clinics. For instance, Abbott’s ID NOW platform is highly implemented in urgent-care clinics and doctors’ offices in the
U.S. The small size makes it portable and thus, its use would not be limited to only hospital settings.
Key
players are constantly accelerating the production and supply of diagnostic tests to keep pace with the increasing need for disease containment.
For instance, in March 2020, Roche initiated shipment of the first allotment of the Cobas SARS-CoV-2 test for COVID-19 diagnosis. Approximately
400,000 tests were shipped to a targeted network of reference laboratories as well as hospitals across the U.S. Roche also planned for
a shipment of 400,000 additional tests to the laboratory testing facilities every week. Companies such as Quest Diagnostics and Labcorp
are focusing on the aggressive expansion of capacity and are also working towards the return-to-work testing market through employer-focused
COVID testing offerings. In May 2020, Quest Diagnostics announced its collaboration with Ortho Clinical Diagnostics aimed at the expansion
of COVID-19 antibody testing across more than 20 laboratories of Quest throughout the U.S. The company applied VITROS Immunodiagnostic
Products Anti-SARS-CoV-2 IgG Test of Ortho to ramp up the coronavirus testing in patients. Some of the prominent players in the COVID-19
diagnostics market include:
|
|
▪
|
F.
Hoffman-La Roche Ltd.
|
|
|
▪
|
Thermo
Fisher Scientific, Inc.
|
|
|
▪
|
altona
Diagnostics GmbH
|
|
|
▪
|
Mylab
Discovery Solutions Pvt Ltd.
|
|
|
▪
|
Laboratory
Corporation of America Holdings
|
Government
Regulation
The
FDA and other regulatory authorities at federal, state and local levels, as well as in foreign countries, extensively regulate, among
other things, the research, development, testing, manufacture, quality control, import, export, safety, effectiveness, labeling, packaging,
storage, distribution, record keeping, approval, advertising, promotion, marketing, post-approval monitoring and post-approval reporting
of diagnostic tests such as those we are developing, as well as medicines such as those we are developing for the treatment of Covid-19.
Seasonality
To
date, our business is not subject to any detectable seasonality.
Web
Domain
Our
official website is currently under the domain of www.todosmedical.com.
Employees
As
of the date of this prospectus, we have eight full-time employees. We believe that we have good relations with our employees.
DESCRIPTION
OF OUR SECURITIES
The
following description of our share capital does not purport to be complete, and is qualified in its entirety by reference to our amended
and restated articles of association (the “Amended Articles”), a copy of which is filed as Exhibit 99.1 to the Company’s
current report on Form 6-K (File No. 333-209744) filed with the Commission on March 30, 2017. This summary is subject to the Companies
Law and to the complete text of our Amended Articles.
General
As of January 13, 2022, our
authorized share capital consists of 5,000,000,000 Ordinary Shares, par value NIS 0.01 per share, of which 977,144,432 shares are issued
and outstanding, 50,000 Preferred A Shares par value NIS 0.01 each, none of which are issued and outstanding, and 5,000 Preferred B Shares
par value NIS 0.01 each, none of which are issued and outstanding. All of our outstanding Ordinary Shares are validly issued, fully paid
and non-assessable. Our Ordinary Shares are not redeemable and do not have any preemptive rights.
Registration
Number and Purposes of the Company
Our
registration number with the Israeli Registrar of Companies is 51-443712-8. Our purpose as set forth in our Amended Articles is to engage
in any lawful activity. Our Amended Articles state that the liability of our shareholders is limited, subject to the provisions of the
Companies Law.
Transfer
of Shares
Our
fully paid Ordinary Shares are issued in registered form and may be freely transferred under our Amended Articles, unless the transfer
is restricted or prohibited by another instrument, applicable law or the rules of a stock exchange on which the shares are listed for
trade. The ownership or voting of our Ordinary Shares by non-residents of Israel is not restricted in any way by our Amended Articles
or the laws of the State of Israel, except for ownership by nationals of some countries that are, or have been, in a state of war with
Israel.
Election
of Directors
The
Ordinary Shares do not have cumulative voting rights for the election of directors. Rather, under the Amended Articles the Company’s
directors (other than external directors) are elected at a shareholders meeting by a simple majority of Ordinary Shares for a term of
service ending upon the next annual general meeting. Under the Companies Law, external directors are elected by a simple majority of
Ordinary Shares, which majority includes at least a majority of the shares held by non-controlling shareholders who do not have a personal
interest in the matter (excluding a personal interest unrelated to the relationship with a controlling shareholder) voted at the meeting,
or the total number of shares held by such non-controlling shareholders who do not have a personal interest voted against the election
of the external director does not exceed two percent of the aggregate voting rights in the Company. External directors are elected for
a term of service ending upon the third annual general meeting.
As
a result, the holders of Ordinary Shares that represent more than 50% of the voting power represented at a shareholder meeting have the
power to elect any or all of the Company’s directors whose positions are being filled at that meeting, subject to the additional
approval requirements for external directors.
Dividend
and Liquidation Rights
We
may declare a dividend to be paid to the holders of our Ordinary Shares in proportion to their respective shareholdings. Under the Companies
Law, dividend distributions are determined by the board of directors and do not require the approval of the shareholders of a company
unless the company’s articles of association provide otherwise. Our Amended Articles do not require shareholder approval of a dividend
distribution and provide that dividend distributions may be determined by our board of directors.
Pursuant
to the Companies Law, we may declare and pay dividends only if, upon the determination of our board of directors, there is no reasonable
concern that the distribution will prevent us from being able to meet the terms of our existing and foreseeable obligations as they become
due. Under the Companies Law, the distribution amount is further limited to the greater of retained earnings or earnings generated over
the two most recent years legally available for distribution according to our then last reviewed or audited financial statements, provided
that the date of the financial statements is not more than six months prior to the date of distribution. In the event that we do not
have retained earnings or earnings generated over the two most recent years legally available for distribution, we must seek the approval
of the court in order to distribute a dividend. The court may approve our request if it is convinced that there is no reasonable concern
that the payment of a dividend will prevent us from satisfying our existing and foreseeable obligations as they become due.
In
the event of our liquidation, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of our Ordinary
Shares in proportion to the nominal value of their shareholdings. This right, as well as the right to receive dividends, may be affected
by the grant of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that may be
authorized in the future.
Exchange
Controls
There
are currently no Israeli currency control restrictions on remittances of dividends on our Ordinary Shares, proceeds from the sale of
the shares or interest or other payments to non-residents of Israel, except for shareholders who are residents, citizens or subjects
of countries that are, or have been, in a state of war with Israel.
Shareholder
Meetings
Under
Israeli law, we are required to hold an annual general meeting of our shareholders once every calendar year that must be held no later
than 15 months after the date of the previous annual general meeting. All meetings other than the annual general meeting of shareholders
are referred to in our Amended Articles as special general meetings. Our board of directors may call special general meetings whenever
it sees fit, at such time and place, within or outside of Israel, as it may determine. In addition, the Companies Law provides that our
board of directors is required to convene a special general meeting upon the written request of (i) any two of our directors or one-quarter
of the serving members of our board of directors; or (ii) one or more shareholders holding, in the aggregate, either (a) 5% or more of
our outstanding shares and 1% of our outstanding voting power or (b) 5% or more of our outstanding voting power.
Furthermore,
the Companies Law requires that resolutions regarding the following matters be approved by our shareholders at a general meeting:
|
|
●
|
amendments
to our articles of association;
|
|
|
|
|
|
|
●
|
appointment,
terms of service and termination of service of our auditors;
|
|
|
|
|
|
|
●
|
appointment
of external directors;
|
|
|
|
|
|
|
●
|
approval
of certain related party transactions;
|
|
|
|
|
|
|
●
|
increases
or reductions of our authorized share capital;
|
|
|
|
|
|
|
●
|
mergers;
and
|
|
|
|
|
|
|
●
|
the
exercise of our board of director’s powers by a general meeting, if our board of directors is unable to exercise its powers
and the exercise of any of its powers is essential for our proper management.
|
Subject
to the provisions of the Companies Law and regulations promulgated thereunder, shareholders entitled to participate and vote at general
meetings are the shareholders of record on a date to be decided by the board of directors, which, as a company listed on an exchange
outside Israel, may be between four and 40 days prior to the date of the meeting.
The
Companies Law requires that a notice of any annual general meeting or special general meeting be provided to shareholders at least 21
days prior to the meeting and if the agenda of the meeting includes, among other things, the appointment or removal of directors, the
approval of transactions with office holders or interested or related parties, an approval of a merger or the approval of the compensation
policy, notice must be provided at least 35 days prior to the meeting.
Under
the Companies Law, our shareholders are not permitted to take action via written consent in lieu of a meeting.
Voting
Rights
Quorum
Requirements
Pursuant
to our Amended Articles, holders of our Ordinary Shares have one vote for each ordinary share held on all matters submitted to a vote
before the shareholders at a general meeting. The quorum required for general meetings of our shareholders is at least two shareholders
present in person, by proxy or written ballot, who hold or represent between them at least 25% of the total outstanding voting rights
(or if a higher percentage is required by law, such higher percentage), within half an hour of the time fixed for the commencement of
the meeting. A meeting adjourned for lack of a quorum is adjourned either to the same day in the following week at the same time and
place or to such day, time and place as specified in the notice of the meeting or to such day, time and place as the chairman of the
general meeting shall determine. At the reconvened meeting, at least two shareholders present in person or by proxy shall constitute
a lawful quorum, unless the meeting of shareholders was convened at the demand of shareholders, in which case, the quorum shall be the
presence of one or more shareholders holding at least 5% of our issued share capital and at least one percent of the voting power of
our shares, or one or more shareholders with at least 5% of the voting power of our shares.
Vote
Requirements
Our
Amended Articles provide that all resolutions of our shareholders require a simple majority vote, unless otherwise required by the Companies
Law or by our Amended Articles. Under the Companies Law, certain actions require a special majority, including: (i) appointment of external
directors; (ii) approval of an extraordinary transaction with a controlling shareholder or in which the controlling shareholder has a
personal interest and the terms of employment or other engagement of the controlling shareholder or a relative of the controlling shareholder
(even if not extraordinary); (iii) approval of a compensation policy; and (iv) approval of executive officer compensation inconsistent
with our office holder compensation policy or the compensation of our chief executive officer (subject to limited exceptions).
In
addition, under the Companies Law the authorization of the chairman of the board to assume the role or responsibilities of the chief
executive officer, or the authorization of the chief executive officer or his or her relative thereof to assume the role or responsibilities
of the chairman of the board, for periods of no longer than three years each, is subject to receipt of the approval of a majority of
the shares voting on the matter, provided that either (i) included in such majority are at least two-thirds of the shares of shareholders
who are non-controlling shareholders and shareholders who do not have a personal interest in the resolution that are voted at the meeting
on the matter (excluding any abstentions); or (ii) the total number of shares of shareholders specified in clause (i) who voted against
the resolution does not exceed 2% of the voting rights in the company.
Another
exception to the simple majority vote requirement is a resolution for the voluntary winding up, or an approval of a scheme of arrangement
or reorganization, of the company pursuant to Section 350 of the Companies Law, which requires the approval of holders of 75% of the
voting rights represented at the meeting and voting on the resolution.
Access
to Corporate Records
Under
the Companies Law, shareholders are provided access to: (i) minutes of the general meetings of our shareholders; (ii) our shareholders
register and principal shareholders register, articles of association and financial statements; and (iii) any document that we are required
by law to file publicly with the Israeli Companies Registrar or the Israel Securities Authority. In addition, shareholders may request
to be provided with any document in the company’s possession related to an action or transaction requiring shareholder approval
under the related party transaction provisions of the Companies Law. We may deny this request if we believe it has not been made in good
faith or if such denial is necessary to protect our interest or protect a trade secret or patent.
Modification
of Class Rights
Under
the Companies Law and our Amended Articles, the rights attached to any class of shares, such as voting, liquidation and dividend rights,
may be modified or cancelled by adoption of a shareholders’ resolution amending the Articles, together with the approval of the
simple majority of voting rights of a separate meeting consisting of the shareholders of that class; and any creation or modification
of such rights (or other amendment to the Articles) which would prejudice or compromise the rights held by a particular class of shareholders
is subject to the approval of a separate meeting consisting of the shareholders of that class.
Registration
Rights
On
April 19, 2021, the Company entered into an Agreement to Purchase Provista Diagnostics, Inc. (“Agreement to Purchase”) with
Strategic Investment Holdings, LLC (“SIH”), Ascenda BioSciences LLC (“Ascenda”) and Provista Diagnostics, Inc.
(“Provista”). Ascenda was the sole owner of the outstanding securities of Provista and SIH is the sole owner of all the outstanding
securities of Ascenda.
Pursuant
to the Agreement to Purchase, the Company acquired Provista from Ascenda and SIH for an aggregate purchase price of $7.5 million consisting
of an initial cash payment of $1.25 million, the issuance of $1.5 million in Ordinary Shares priced at $0.0512 per share, the issuance
to SIH of a $3.5 million convertible promissory note dated April 19, 2021 (the “Note”) and the payment on for before July
1, 2021 of $1.25 million in cash (the “July Payment”), which payment the Company had the right to, and did, extend to July
15, 2021. The Provista shares acquired by the Company remained in an escrow account until the July Payment was made.
The
Note has a maturity date of April 8, 2025, and is convertible beginning on October 20, 2021, into Ordinary Shares of the Company at a
conversion price equal to the lesser of $0.05 or the volume weighted average price of the last 20 trading days for the Ordinary Shares
prior to the date of conversion. In the event SIH delivers a Notice of Conversion to the Company at a per share price less than $0.05
($0.05), the Company has the right to immediately notify SIH of its intention to pay the conversion amount in cash within three (3) business
days of receipt of the Notice of Conversion (i.e., before SIH would take possession of shares converted under the Notice of Conversion).
If, at any time between October 20, 2021 and April 20, 2022, the average of the lowest bid and closing sale price at 4:00 p.m., New York
time (or such other time as such market publicly announces is the official close of trading) is below ($0.05), the Company has the option
to buy out all or any portion of the Note (the “Buyback Option”). In the event the Company exercises the Buyback Option for
an amount equal to or greater than one million, one hundred seventy thousand dollars ($1,170,000) (the “Buyback Amount”),
SIH may not submit any conversions below five cents ($0.05) for ninety (90) days from receipt of the Buyback Amount (“90 Day Period”).
In
the event that the Company uplists its Ordinary Shares to a national securities exchange, the Note shall automatically be exchanged into
Series B preferred stock with a conversion price equal to the lesser of (a) $0.05, (b) the opening price on the day of the uplisting
provided there is no transaction associated with the uplisting or (c) the deal price of an uplisting transaction.
As
of the date of this prospectus, SIH has not submitted a Conversion Notice.
Acquisitions
under Israeli Law
Full
Tender Offer
A
person wishing to acquire shares of an Israeli public company, and who would as a result hold over 90% of the target company’s
issued and outstanding share capital, is required by the Companies Law to make a tender offer to all of the company’s shareholders
for the purchase of all of the issued and outstanding shares of the company. A person wishing to acquire shares of an Israeli public
company, and who would as a result hold over 90% of the issued and outstanding share capital of a certain class of shares of the company,
is required to make a tender offer to all of the shareholders who hold shares of the relevant class for the purchase of all of the issued
and outstanding shares of that class. If the shareholders who do not accept the offer hold less than 5% of the issued and outstanding
share capital of the company or of the applicable class, and more than half of the shareholders who do not have a personal interest in
the offer accept the offer, all of the shares that the acquirer offered to purchase will be transferred to the acquirer by operation
of law. However, a tender offer will also be accepted if the shareholders who do not accept the offer hold less than 2% of the issued
and outstanding share capital of the company or of the applicable class of shares.
Upon
a successful completion of such a full tender offer, any shareholder that was an offeree in such tender offer, whether such shareholder
accepted the tender offer or not, may, within six months from the date of acceptance of the tender offer, petition an Israeli court to
determine whether the tender offer was for less than fair value and that the fair value should be paid as determined by the court. However,
under certain conditions, the offeror may include in the terms of the tender offer that an offeree who accepted the offer will not be
entitled to petition the Israeli court as described above.
If
(a) the shareholders who did not respond or accept the tender offer hold at least 5% of the issued and outstanding share capital of the
company, or of the applicable class, or the shareholders who accept the offer constitute less than a majority of the offerees that do
not have a personal interest in the acceptance of the tender offer, or (b) the shareholders who did not accept the tender offer hold
2% or more of the issued and outstanding share capital of the company (or of the applicable class), the acquirer may not acquire shares
of the company that will increase its holdings to more than 90% of the company’s issued and outstanding share capital or of the
applicable class from shareholders who accepted the tender offer.
Special
Tender Offer
The
Companies Law provides that an acquisition of shares of an Israeli public company must be made by means of a special tender offer if
as a result of the acquisition the purchaser would become a holder of 25% or more of the voting rights in the company, if there is no
other shareholder that holds 25% or more of the voting rights in the company, subject to exceptions. Similarly, the Companies Law provides
that an acquisition of shares in an Israeli public company must be made by means of a special tender offer if as a result of the acquisition
the purchaser would become a holder of more than 45% of the voting rights in the company, if there is no other shareholder of the company
who holds more than 45% of the voting rights in the company, subject to certain exceptions. No tender offer is required if the acquisition
of shares: (i) occurs in the context of a private placement, that was approved by the company’s shareholders and whose purpose
is to give the acquirer at least 25% of the voting rights in the company if there is no person who holds 25% or more of the voting rights
in the company, or as a private placement whose purpose is to give the acquirer 45% of the voting rights in the company, if there is
no person who holds 45% of the voting rights in the company; (ii) was from a holder of 25% or more of the voting rights in the company
following which the purchaser will hold 25% or more of the voting rights in the company; or (iii) was from a holder of more than 45%
of the voting rights in the company following which the purchaser will hold more than 45% of the voting rights in the company.
A
special tender offer must be extended to all shareholders of a company, but the offeror is not required to purchase shares representing
more than 5% of the voting power attached to the company’s outstanding shares, regardless of how many shares are tendered by shareholders.
A special tender offer may be consummated only if (i) at least 5% of the voting power attached to the company’s outstanding shares
will be acquired by the offeror; and (ii) the number of shares tendered in the offer exceeds the number of shares whose holders objected
to the offer (excluding the purchaser, its controlling shareholders, holders of 25% or more of the voting rights in the company or any
person having a personal interest in the acceptance of the tender offer, or anyone on their behalf, including any such person’s
relatives and entities under their control). If a special tender offer is accepted, then the purchaser or any person or entity controlling
it, at the time of the offer, and any person or entity under common control with the purchaser or such controlling person or entity may
not make a subsequent tender offer for the purchase of shares of the target company and may not enter into a merger with the target company
for a period of one year from the date of the offer, unless the purchaser or such person or entity undertook to effect such an offer
or merger in the initial special tender offer.
Merger
The
Companies Law permits merger transactions if approved by each party’s board of directors and, unless certain requirements described
under the Companies Law are met, by a majority vote of each party’s shares, and, in the case of the target company, a majority
vote of each class of its shares, voted on the proposed merger at a shareholders meeting. The board of directors of a merging company
may not approve the merger if it determines that there exists a reasonable concern that, as a result of the merger, the surviving company
will be unable to satisfy the obligations of the merging entities.
For
purposes of the shareholder vote of a merging company whose shares are held by the other merging company or a person or entity holding
25% or more of any of the means of control of the other merging entity, unless a court rules otherwise, the merger will not be deemed
approved if a majority of the votes of shares voting on the matter at the shareholders meeting (excluding abstentions) that are held
by parties other than the other party to the merger, or by any other person or entity who holds 25% or more of the voting rights or the
right to appoint 25% or more of the directors of the other party, or any one on their behalf including their relatives or corporations
controlled by any of them, vote against the merger. If, however, the merger involves a merger with a company’s own controlling
shareholder or if the controlling shareholder has a personal interest in the merger, then the merger is instead subject to the same Special
Majority approval that governs all extraordinary transactions with controlling shareholders (as described under “Directors,
Executive Officers, Promotors and Control Persons – Approval of Related Party Transactions under Israeli Law – Disclosure
of Personal Interests of Controlling Shareholders and Approval of Certain Transactions”).
If
the transaction would have been approved by the shareholders of a merging company but for the separate approval of each class or the
exclusion of the votes of certain shareholders as provided above, a court may still approve the merger upon the request of holders of
at least 25% of the voting rights of a company, if the court holds that the merger is fair and reasonable, taking into account the valuation
of the merging companies and the consideration offered to the shareholders.
Upon
the request of a creditor of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there
exists a reasonable concern that, as a result of the merger, the surviving company will be unable to satisfy the obligations of the merging
entities, and may further give instructions to secure the rights of creditors.
In
addition, a merger may not be consummated unless at least 50 days have passed from the date on which a proposal for approval of the merger
was filed by each party with the Israeli Registrar of Companies and at least 30 days have passed from the date on which the merger was
approved by the shareholders of each party.
Anti-Takeover
Measures under Israeli Law
The
Companies Law allows us to create and issue shares having rights different from those attached to our Ordinary Shares, including shares
providing certain preferred rights with respect to voting, distributions or other matters and shares having pre-emptive rights. We currently
have two classes of preferred shares. While our current classes of preferred shares do not have the ability to do so, in the future,
if we do authorize, create and issue an additional class of preferred shares, such class of shares, depending on the specific rights
that may be attached to it, may have the ability to frustrate or prevent a takeover or otherwise prevent our shareholders from realizing
a potential premium over the market value of their Ordinary Shares. The authorization and designation of an additional class of preferred
shares will require an amendment to our Amended Articles, which requires the prior approval of the holders of a majority of the voting
power attached to our issued and outstanding shares at a general meeting. The convening of the meeting, the shareholders entitled to
participate, and the majority vote required to be obtained at such a meeting will be subject to the requirements set forth in the Companies
Law and our Amended Articles as described above in “– Voting Rights.”
Borrowing
Powers
Pursuant
to the Companies Law and our Amended Articles, our board of directors may exercise all powers and take all actions that are not required
under law or under our Amended Articles to be exercised or taken by our shareholders, including the power to borrow money for company
purposes.
Changes
in Capital
Our
Amended Articles enable us to increase or reduce our share capital. Any such changes are subject to the provisions of the Companies Law
and must be approved by a resolution duly passed by our shareholders at a general meeting by voting on such change in the capital. In
addition, transactions that have the effect of reducing capital, such as the declaration and payment of dividends in the absence of sufficient
retained earnings or profits, require the approval of both our board of directors and an Israeli court.
Transfer
Agent and Registrar
Our
transfer agent for our Ordinary Shares is Word Wide Stock Transfer, LLC, 1 University Plaza Drive #505, Hackensack, NJ, phone number:
(201) 820-2008, and fax number: (201) 820-2010.
Warrants
Included in the Offering
Public
Warrants. This offering includes the Convertible Note Shares, the Purchaser Warrant Shares and the Prior Warrant Shares. The holders
of the Purchaser Warrants and the Prior Warrants must pay the exercise price in cash upon exercise of the warrants, unless they are utilizing
the cashless exercise provision of the warrants, which is only available in certain circumstances, such as if the issuance and sale of
the underlying shares is not registered with the SEC pursuant to an effective registration statement. We intend to keep the registration
statement of which this prospectus forms a part effective when the warrants are exercised. Except as otherwise provided in the warrants
or by virtue of such holder’s ownership of Ordinary Shares, the holder of a warrant does not have the rights or privileges of a
holder of our Ordinary Shares, including any voting rights, until the holder exercises the warrant.
The
number of warrants outstanding, and the exercise price of those securities, will be adjusted proportionately in the event of a reverse
or forward stock split of our Ordinary Shares, a recapitalization or reclassification of our Ordinary Shares, payment of dividends or
distributions in shares to our shareholders, or similar transactions. In the event that the Company effects a rights offering to its
ordinary shareholders or a pro rata distribution of its assets among its shareholders, then the holder of the warrants will have the
right to participate in such distribution and rights offering to the extent of their pro rata share of the Company’s outstanding
Ordinary Shares assuming they owned the number of Ordinary Shares issuable upon the exercise of their warrants. In the event of a “Fundamental
Transaction” by the Company, such as a merger or consolidation of it with another company, the sale or other disposition of all
or substantially all of the Company’s assets in one or a series of related transactions, a purchase offer, tender offer or exchange
offer, or any reclassification, reorganization or recapitalization of the Company’s Ordinary Shares, then the warrant holder will
have the right to receive, for each ordinary share issuable upon the exercise of the warrant, at the option of the holder, the number
of Ordinary Shares of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional
consideration payable as a result of the Fundamental Transaction, that would have been issued or conveyed to the warrant holder had the
holder exercised the warrant immediately preceding the closing of the Fundamental Transaction. In lieu of receiving such Ordinary Shares
and additional consideration in the Fundamental Transaction, the warrant holder may elect to have the Company or the successor entity
purchase the warrant holder’s warrant for its fair market value measured by the Black Scholes method.
A
holder will not have the right to exercise any portion of the warrant if the holder (together with its affiliates) would beneficially
own in excess of 4.99% (or, upon election of the holder, 9.99%) of the number of our Ordinary Shares outstanding immediately after giving
effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants. However, any holder
may increase or decrease such percentage, provided that any increase will not be effective until the 61st day after such election.
The
Company will promptly notify the warrant holders in writing of any adjustment to the exercise price or to the number of the outstanding
warrants, declaration of a dividend or other distribution, a special non-recurring cash dividend on or a redemption of the Ordinary Shares,
the authorization of a rights offering, the approval of the shareholders required for any proposed reclassification of the Ordinary Shares,
a consolidation or merger by the Company, sale of all or substantially all of the assets of the Company, any compulsory share exchange,
or the authorization of any voluntary or involuntary dissolution, liquidation, or winding up of the Company.
The
warrants contain a contractual provision stating that all questions concerning the construction, validity, enforcement and interpretation
of the warrants are governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard
to the principles of conflicts of law.
We
have not applied, and do not intend to apply, for listing of the warrants on any securities exchange or other trading system.
MARKET
PRICE AND DIVIDEND POLICY
Our
Ordinary Shares have been quoted on the OTCQB under the symbol “TOMDF” since March 7, 2017. The OTCQB is a regulated quotation
service that displays real-time quotes, last-sale prices, and volume information in over-the-counter equity securities. The OTCQB is
a quotation medium for subscribing members, not an issuer listing service, and should not be confused with The NASDAQ Stock Market.
On January 13, 2022, the last
reported sale price of our Ordinary Shares on the OTCQB was $ 0.064 per ordinary share.
Holders
We had 143 holders of record
for our Ordinary Shares as of January 13, 2022.
We
have not declared or paid any dividend since inception on our Ordinary Shares. We do not anticipate that we will declare or pay dividends
in the foreseeable future on our Ordinary Shares. Instead, we anticipate that all of our earnings will be used for the operation and
growth of our business. Any future determination to declare cash dividends would be subject to the discretion of our board of directors
and would depend upon various factors, including our results of operations, financial condition and liquidity requirements, restrictions
that may be imposed by applicable law and our contracts and other factors deemed relevant by our board of directors.
PROPERTIES
The
Company does not own any real property. Our principal executive office and mailing address is 121 Derech Ben Gurion, Tel Aviv, Israel,
which we have leased since March 2021 pursuant to a month-to-month agreement. The Company additionally has an office at 40 Wall Street,
Suite 2702, New York, New York 10005, which is subject to an annual lease that is currently due to expire at the end of March 2022 and
which is shared with two of our United States subsidiaries, Todos Medical USA and Corona Diagnostics LLC. Our Provista Diagnostics laboratory
is located in a leased facility located at 2001 Westside Parkway, Alpharetta, GA 30004. The lease is currently due to expire June
30, 2023. We believe that all of our leases provide for rental payments at a market rate.
LEGAL PROCEEDINGS
The Company and its subsidiaries are involved
in certain legal proceedings and certain business relationships that arise from time to time in the ordinary course of their business
and in connection with certain agreements with third parties (such as with respect to certain royalty agreements). Except for income
tax contingencies, the Company applies the provisions of ASC Topic 450, Contingencies. Thus, the Company records accruals for contingencies
to the extent that the management concludes that the occurrence is probable and that the related liabilities are estimable. Legal expenses
associated with contingencies are expensed as incurred.
In
January 2022, Toledo Advisors, LLC filed suit against the Company and Corona Diagnostics in a Nevada state court, alleging breach of
contract. The Company and Corona Diagnostics have not yet filed an answer in this case, but intend to vigorously contest it.
DIRECTORS, OFFICERS AND CORPORATE GOVERNANCE
Directors
The
following table sets forth information regarding the directors and officers
of Todos as of September 30, 2021:
|
Name
|
|
Age
|
|
Director
Since
|
|
Term
Ends
|
|
Gerald
Commissiong, Chief Executive Officer and Director
|
|
39
|
|
2020
|
|
2022
|
|
Daniel
Hirsch, Chief Financial Officer and Director
|
|
53
|
|
2020
|
|
2022
|
|
Dr.
Herman Weiss, Chairman of the Board
|
|
51
|
|
2017
|
|
2022
|
|
*Dr.
Lauren Chung, Director
|
|
49
|
|
2020
|
|
2024
|
|
*Moshe
Schlisser, Director
|
|
33
|
|
2016
|
|
2024
|
|
**Moshe
Abramovitz, Director
|
|
39
|
|
2016
|
|
2022
|
* Independent Director and External Director under Israeli Law
** Independent Director
|
Gerald
Commissiong
Chief
Executive Officer and Director
|
|
Mr.
Commissiong has served as our Chief Executive Officer and director
since January 5, 2020. In addition, Mr. Commissiong serves as Chief Executive Officer, President and a member of the Board of Directors
of Amarantus Bioscience Holdings, Inc. (“Amarantus”), of which he is a co-founder. Prior to becoming Chief Executive
Officer of Amarantus in October 2011, Mr. Commissiong was the Chief Operating Officer of Amarantus. Mr. Commissiong graduated from
Stanford University in Management Science and Engineering with a focus on Financial Decisions. The Board believes that Mr. Commissiong’s
executive leadership and healthcare expertise qualifies Mr. Commissiong to serve as a director.
|
|
|
|
|
|
Daniel
Hirsch
Chief
Financial Officer and Director
|
|
Mr.
Hirsch has served as our Chief Financial Officer and director since January 5, 2020. Mr.
Hirsch has been managing Partner of First Line Capital, LLC since 2002. Prior to 2002, Mr.
Hirsch served as Senior Consultant at Integrated Healthcare based in Greenwich, Connecticut
providing turn around services for large medical practices. From 1992 to1998, Mr. Hirsch
was Director of Primary Care for Hackensack University Medical Center in Hackensack, New
Jersey. Mr. Hirsch holds a bachelor’s degree in Economics from Yeshiva University and
a master’s degree in Public Health from the New School of Social Research. The Board
believes that Mr. Hirsch’s financial expertise in the healthcare industry qualifies
Mr. Hirsch to serve as a director.
|
|
|
|
|
|
Dr.
Herman Weiss
Chairman
of the Board of Directors
|
|
Dr.
Herman Weiss has served as a director of the Company since June 22, 2017 and Chairman of
the Board of Directors since January 5, 2020. Dr. Weiss served as Chief Executive Officer
of the Company from July 30, 2018 to January 5, 2020. In addition, from 2016-2018,
Dr. Weiss served as the Vice President of Medical Affairs and Clinical Development at Juniper
Pharmaceuticals Inc. in Boston, MA. Since 2016, Dr. Weiss has also served as
a Professor at Yeshiva University. Dr. Weiss has served as a consultant to
multiple medical device and pharmaceutical companies, including American Medical Systems,
and to venture capital firms in New York City, and also founded and served as the Chief
Medical Officer of FibroControl, a biotech medical device company in Herzliya, Israel. Dr.
Weiss owns multiple patents and is the author of numerous publications in the area of women’s
health and gynecology. Dr. Weiss holds an M.B.A. from the George Washington University, Washington
DC, an M.D. from the Ohio State University College of Medicine, and a B.A. in Philosophy
(summa cum laude) from the Ramapo College of New Jersey. The Board believes that Dr. Weiss’
experience in the medical device and pharmaceutical industries qualifies him to serve as
Chairman of the Board.
|
|
Lauren
Chung,
External Director
|
|
Dr.
Lauren Chung has served as director of the Company since April 2020. In 2012, Dr. Chung founded, and since then, she has served as
Chief Executive Officer of MINLEIGH LLC, identifying, evaluating and partnering with companies for investments and strategic, operational,
and commercial opportunities. Dr. Chung has over 20 years of healthcare investment management, investment banking, and advisory experience.
Dr. Chung was a managing director in Healthcare Research at WestPark Capital and an analyst at Maxim Group from 2016-2019. From
2006 - 2016, Dr. Chung was a co-founder of Tokum Capital Management, a global healthcare fund, which merged with Perella Weinberg
Partners. From 2000 - 2006 Dr. Chung managed healthcare investment portfolios at RBR Capital, Kingdon Capital, and Pequot
Capital. From 1994-2000 , Dr. Chung was a recognized research scientist conducting cutting edge research in neurodegenerative
and genetic disorders at Massachusetts General Hospital/Harvard Medical School and Boston Children’s Hospital. Dr. Chung has
published many leading peer-reviewed scientific journals. As a current and former director of public and private companies, Dr. Chung
brings a valuable perspective for the Company’s strategy and operations as well as extensive scientific insights. Dr. Chung
holds a Ph.D. in Neuropathology from Columbia University-College of Physicians & Surgeons, and a BA with honors in Biochemistry
and Economics from Wellesley College. The Board believes that Dr. Chung’s experience with companies in the healthcare industry
qualifies her to serve as a director.
|
|
Moshe
Schlisser
External
Director
|
|
Mr.
Moshe Schlisser has served as a director of the Company since February 2016. Mr. Schlisser currently also serves as a director at
SmartGreen Ltd, Tantel Group Ltd and III Pte Ltd. Since 2018, Mr. Schlisser has been serving as General Partner at Shefa Capital
Ltd, a Growth Venture Fund with a focus on mid to later stage deep technology investments. Until 2018, Mr. Schlisser was the managing
partner at Iberica Investments International, a private equity firm. Mr. Schlisser has held managerial positions in various investment
firms and has experience with investments, structured finance and mergers and acquisitions. In 2010, Mr. Schlisser co-founded and
currently serves as a director of a soup kitchen in Jerusalem that serves a hot prepared dinner every night to over 50 homeless and
underprivileged individuals and delivers weekend food packages to over 250 underprivileged families. The Board of Directors believes
that Mr. Schlisser’s experience in the capital markets qualifies him to serve as a director.
|
|
|
|
|
|
Moshe
Abramovitz
Director
|
|
Mr.
Moshe Abramovitz has served as a director of the Company since February 27, 2016. Mr. Abramovitz has held managerial positions in
various organizations (Israeli companies and charities) including serving as the Deputy Chief Executive Officer of A.S. Mehadrin
Ltd. Since 2011, Mr. Abramovitz has been a project manager in the construction industry in Israel. Mr. Abramovitz holds a
B.A. in business administration, specializing in information systems, from Ono Academic College and an MBA in business administration
specializing in business strategy from Ono Academic College. Mr. Abramowitz received training and a certificate to serve as a mediator
from Bar Ilan University. The Board believes that Mr. Abramowitz’s managerial positions qualify him to serve as a director.
|
Family
Relationships
There
are no family relationships among any of our executive officers or directors.
Section
16(a) Beneficial Ownership Reporting Compliance
Not
applicable.
Involvement
in Certain Legal Proceedings
During
the past ten years, none of the persons serving as executive officers and/or directors of the Company has been the subject matter of
any of the following legal proceedings that are required to be disclosed pursuant to Item 401(f) of Regulation S-K including: (a) any
bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time
of the bankruptcy or within two years prior to that time; (b) any criminal convictions; (c) any order, judgment, or decree permanently
or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities;
(d) any finding by a court, the SEC or the CFTC to have violated a federal or state securities or commodities law, any law or regulation
respecting financial institutions or insurance companies, or any law or regulation prohibiting mail or wire fraud; or (e) any sanction
or order of any self-regulatory organization or registered entity or equivalent exchange, association or entity. Further, no such legal
proceedings are believed to be contemplated by governmental authorities against any director or executive officer.
Corporate
Governance
In
considering its corporate governance requirements and best practices, including, but not limited to, director independence, at the conclusion
of this offering, we will be required to comply with Nasdaq’s listing and maintenance rules, in addition to complying with the
Companies Law due to our being an Israeli corporation. Because we are not a foreign private issuer, we may not elect to follow home country
practice under the NASDAQ Rules. As a result, among other things, at the next Annual Meeting of Shareholders following the closing of
this offering, we will recommend to our shareholders to amend our Articles of Association to raise the requirement for a quorum at our
shareholders’ meetings to 33%.
Certain provisions of Israeli law regarding “External Directors”
As an Israeli company, we
are subject to the Israeli Companies Law, which provides that at least two of the Company’s directors must be external directors,
whose terms of office, and procedures for appointment or removal, must conform with the provisions of the Companies Law, Section 239
et seq. (the “External Directors”).
External Directors must meet
certain statutory qualifications, as detailed in the Israeli Companies Law, Section 239 et seq. (the “Statutory Qualifications”).
The Statutory Qualifications include certain provisions designed to ensure the External Directors’ independent judgment, as well
as criteria for the External Directors’ professional skills, as follows (the “Professional Qualifications”): (i) at
least one of the External Directors must have “accounting and financial expertise”, and (ii) each External Director must
have either “accounting and financial expertise” or “professional skills”; all as such terms are defined by the
regulations promulgated under the Companies Law.
Each External Director must
file a written declaration affirming that she or he meets the Statutory Qualifications, including documentation of her or his Professional
Qualifications, before her or his nomination can be properly considered by the Shareholders’ Meeting. Each of Dr. Lauren Chung
and Mr. Moshe Schlisser submitted their respective written declarations to the Board, as required under the Companies Law before our
July 26, 2021 Annual Meeting (the “July 2021 Meeting”). After considering their respective declarations, our Board of Directors
determined that Dr. Chung and Mr. Schlisser possess the required Professional Qualifications to be appointed as External Directors and,
specifically, that Dr. Chung has “accounting and financial expertise,” as that term is defined by the regulations promulgated
under the Companies Law.
Compensation package and expenses of External Directors
The compensation and allowable
expenses of External Directors are regulated by the Companies Law and regulations promulgated pursuant thereto (the “Compensation
Regulations”). Generally speaking, the Compensation Regulations set a fixed range including minimum, maximum, and suggested median
tariffs for External Directors’ compensation, on the basis of a fixed cash salary, in addition to fixed cash amount per meeting.
The relevant fixed ranges are based on the Company’s shareholders’ equity and, for Companies whose securities are listed
on a non-Israeli exchange, the increased burden of the demands and obligations imposed by the laws and regulations to which companies
listed on that exchange are subject. However, the fixed amounts set forth in the Compensation Regulations may not adequately account
for the unique position in which every company is situated. Therefore, Regulations 8A and 8B of the Compensation Regulations allow the
Company to adopt a compensation package, including cash as well as equity, which goes beyond the parameters otherwise imposed by the
fixed range of the Compensation Regulations. Based on the legal advice it has received, the Board has determined that the Compensation
Schedule is in keeping with the requirements of the Compensation Regulations.
External Directorship exemptions for companies listed on a “Qualified
Exchange”
Regulations promulgated pursuant
to Section 364(a) of the Companies Law allow for an exemption from some provisions of the Companies Law for companies (such as Todos)
whose securities are listed on a non-Israeli exchange only (the “Foreign Listing Leniencies”). Regulation 5D of the Foreign
Listing Leniencies provides certain exemptions from the Companies Law requirements regarding the corporate governance role of External
Directors, for Israeli companies listed on certain non-Israeli exchanges (the “Qualified Exchanges”); provided that: (i)
the Israeli company is fully compliant with all laws and regulations of the Applicable Jurisdiction regarding the appointment of independent
directors, and the composition of audit and/or compensation committees, as they apply to companies incorporated in the Applicable Jurisdiction,
such regulations being deemed to include any rules or guidelines generally practiced at the relevant Qualified Exchange (the “Qualified
Exchange Regulations”), where “Applicable Jurisdiction” means the jurisdiction in which the company’s securities
are either (x) offered to the public, or (y) listed for trade on the Qualified Exchange; (ii) there is no one person or syndicate exercising
“Control” over the Company, as that term is defined under the Israeli Securities Law, 1968 (see definition of “Control”
in the “required vote” section of this Proposal); (iii) the composition of the company’s Board includes at least one
member of each of the male and female genders; and (iv) the company has elected to comply with the Qualified Exchange Regulations in
lieu of the provisions of the Companies Law relating to the appointment of independent directors, and the composition of audit and/or
compensation committees.
As a matter of Israeli law,
the Minister of Justice may from time to time, after consulting with the Israel Securities Authority and after receiving the approbation
of the Law and Constitution Committee of the Knesset, revise the Foreign Listing Leniencies. In the future, such revisions may include
the addition, removal and/or revision of leniencies, as well as the addition of other exchanges to (or removal of exchanges from) the
list of Qualified Exchanges. At time of this writing, the Qualified Exchanges as per the Foreign Listing Leniencies are the NYSE, AMEX,
NASDAQ Global Select, NASDAQ Global, and NASDAQ Capital. Should the Company’s shares become listed on a Qualified Exchange in the
future, provided that the conditions for exemption as described above apply, the Board may consider the feasibility of procuring the
services of Dr. Chung and Mr. Schlisser as “independent directors” under and subject to the applicable Qualified Exchange
Regulations, in place of their current service as External Directors under the Companies Law.
At the July 2021 Meeting,
the Company’s shareholders adopted an amendment to its Articles of Association which would ensure the validity of such leniencies
in the future, should the Board decide to comply with the Qualified Exchange Regulations in lieu of the relevant Companies Law provisions,
as described above.
Role of the Board and the Audit Committee
in Risk Oversight
While management is charged
with the day-to-day management of risks that the Company faces, the Board, and the Audit Committee of the Board, have been responsible
for oversight of risk management. The full Board, and the Audit Committee since it was formed, have responsibility for general oversight
of risks facing the Company. Specifically, the Audit Committee reviews and assesses the adequacy of the Company’s risk management
policies and procedures with regard to identification of the Company’s principal risks, both financial and non-financial, and review
updates on these risks from the Chief Financial Officer and the Chief Executive Officer. The Audit Committee also reviews and assesses
the adequacy of the implementation of appropriate systems to mitigate and manage the principal risks.
Review and Approval of Transactions with
Related Parties
The Board adopted a policy
to comply with Item 404 of Regulation S-K of the Exchange Act as well as the Nasdaq Rules requiring that disinterested directors approve
transactions with related parties which are not market-based transactions.
Generally, the Board will
approve transactions only to the extent the disinterested directors believe that they are in the best interests of the Company and on
terms that are fair and reasonable (in the judgment of the disinterested directors) to the Company. Our policy is available on our Company
website at www.todosmedical.com.
The Companies Law also contains
requirements for the approval of transaction with related parties by disinterested directors. Those requirements are discussed at Description
of Our Securities – Voting Rights – Vote Requirements and
at Certain Relationships and Related Party Transactions, and Director Independence.
Audit Committee
The Audit Committee was established
to oversee the Company’s corporate accounting and financial reporting processes and audits of its financial statements. The members
of our Audit Committee are Moshe Schlisser, Lauren Chung and Moshe Abramovitz. Moshe Schlisser serves as chairman of the Audit Committee.
The Board determined that all members of our Audit Committee were independent under SEC Rule 10A-3(b)(1) and Nasdaq Rule 5605(a)(2).
The Board has determined that all current members of the Audit Committee are “financially literate” as interpreted by the
Board in its business judgment. Lauren Chung has been qualified as an audit committee financial expert, as defined in the applicable
rules of the SEC.
The Audit Committee meets
periodically with our independent accountants and management to review the scope and results of the annual audit and to review our financial
statements and related reporting matters prior to the submission of the financial statements to the Board. In addition, the Audit Committee
meets with the independent auditors at least on a quarterly basis to review and discuss the annual audit or quarterly review of our financial
statements.
We have established an Audit
Committee Charter that deals with the establishment of the Audit Committee and sets out its duties and responsibilities. Our Audit Committee
Charter complies with Section 3(a)(58)(A) of the Exchange Act and Nasdaq Rule 5605. The Audit Committee is required to review and reassess
the adequacy of the Audit Committee Charter on an annual basis. The Audit Committee Charter is available on our Company website at www.todosmedical.com.
Compensation Committee
The members of our Compensation
Committee are Moshe Schlisser, Lauren Chung and Moshe Abramovitz. Lauren Chung serves as chairwoman of the Compensation Committee. The
Compensation Committee is responsible for making recommendations to the Board regarding the compensation of executive officers, to review
and administer our Company’s equity compensation plans, to review, discuss, and evaluate at least annually the relationship between
risk management policies and practices and compensation, as well as oversee the Company’s engagement with shareholders and proxy
advisors.
The Compensation Committee
consists of only independent directors in accordance with Nasdaq Rule 5605(a)(2) and all non-employee directors for purposes of Rule
16b-3 of the Exchange Act. The compensation of our CEO, Mr. Commissiong, must be determined by the Compensation Committee and the CEO
may not be present during voting or deliberations for his compensation.
Although Nasdaq Rule 5605(d)(3)
provides that the Compensation Committee may (in its discretion, not Board discretion) retain compensation consultants, independent legal
counsel, and other advisors, the independent directors acting as the Compensation Committee have not decided to do so. Our Compensation
Committee Charter is available at our website:www.todosmedical.com.
Code of Business Conduct and Ethics
Our Board has adopted a Code
of Business Conduct and Ethics Policy (the “Code of Conduct”) applicable to all of the Company’s and its subsidiaries’
employees, including the Company’s Chief Executive Officer and Chief Financial Officer. The Code of Conduct contains written standards
that are designed to deter wrongdoing and to promote honest and ethical conduct, including the ethical handling of actual or apparent
conflicts of interest; full, fair, accurate, timely and understandable public disclosures and communications, including financial reporting;
compliance with applicable laws, rules and regulations; prompt internal reporting of violations of the code; and accountability for adherence
to the code. A copy of our Code of Business Conduct and Ethics Policy of the Company is posted at our website at www.todosmedical.com.
Insider Trading Policy and Policy on Trading
Blackout Periods, Benefit Plans and Section 16 Reporting
Our Insider Trading Policy
and policy on Trading Blackout Periods, Benefit Plans and Section 16 Reporting applies to all of our officers, directors, and employees
and provides strict guidelines as to restrictions on trading activity in the Company’s stock. These policies are posted at our
website: www.todosmedical.com
Director Independence
The Board has determined that
Moshe Schlisser, Lauren Chung and Moshe Abramovitz are “independent directors” under the rules and regulations of the SEC
and Nasdaq Rule 5062(a)(2). In making this determination, our Board considered the current and prior relationships that each non-employee
director has with the Company and all other facts and circumstances our Board deemed relevant in determining their independence, including
such individual’s beneficial ownership of our Ordinary Shares.
EXECUTIVE
AND DIRECTOR COMPENSATION
Compensation-Related
Requirements of the Companies Law
As
approved at the July 2021 Meeting, and as required by the Companies Law, we have adopted a Compensation Policy
regarding the terms of office and employment of our “office holders” (as defined under the Companies Law, which includes
directors, the CEO, other executive officers and any other managers directly subordinate to the CEO), including cash compensation, equity-based
awards, releases from liability, indemnification and insurance, severance and other benefits (the “Terms of Office and Employment”).
Each of our directors and executive officers is an “office holder” within the meaning of the Companies Law. The Compensation
Policy is reviewed from time to time by the Compensation Committee and Board to ensure its alignment with our compensation philosophy
and to consider its appropriateness for Todos and is required to be brought at least once every three years to our shareholders for approval.
Pursuant
to the Companies Law, arrangements between Todos and its office holders must generally be consistent with the Compensation
Policy. However, under certain circumstances, we may approve an arrangement that is not consistent with the Compensation Policy,
if the arrangement is approved by a majority of our shareholders, provided that (i) the majority includes a majority of the votes
cast by shareholders who are present and voting (abstentions are disregarded) who (A) are not controlling shareholders and (B)
do not have a personal interest in the matter, or (ii) the votes cast against the arrangement by shareholders who are not controlling
shareholders and who do not have a personal interest in the matter who were present and voted constitute two percent or less of
the voting power of the Company (a “special majority”). Under certain circumstances, if the Compensation Policy is
not approved by the shareholders, the Compensation Committee and the Board may nonetheless approve such policy.
In
addition, pursuant to the Companies Law, the Terms of Office and Employment generally require the approval of the Compensation
Committee and the Board. The Terms of Office and Employment as applicable to directors further require the approval of the shareholders
by a simple majority. The Terms of Office and Employment with respect to a CEO generally require the approval of the shareholders by
the special majority referenced in the immediately preceding paragraph, in addition to the approval of the Compensation Committee and
the Board. Pursuant to regulations promulgated under the Companies Law, shareholder approval is not required with respect to
Terms of Office and Employment granted to a director or a CEO for the period following his or her appointment until the next general
meeting of shareholders, provided these terms are (i) approved by the Compensation Committee and the Board, (ii) consistent with the
Compensation Policy and (iii) on similar or less favorable terms than those of the person’s predecessor. In addition, under certain
circumstances, shareholder approval is not required with respect to the Terms of Office and Employment of a candidate for CEO if the
Compensation Committee determines that the engagement will be frustrated if the approval is pursued, provided that the terms are consistent
with the Compensation Policy. Compensation packages for our office holders were approved at the Company’s Annual General Meeting
on July 26, 2021.
Under
certain circumstances, if the Terms of Office and Employment of office holders who are not directors are not approved by the shareholders,
where such approval is required, the Compensation Committee and the Board may nonetheless approve such terms. In addition, non-material
amendments of the Terms of Office and Employment of office holders who are not directors may be approved by the Compensation Committee
only and non-material amendments of the Terms of Office and Employment of office holders who are not directors and excluding the
CEO may be approved by the CEO only, provided such approvals are permitted under the Compensation Policy and consistent therewith.
Accordingly, for as long as not otherwise determined by the Compensation Committee and the Board, our President and CEO is currently
authorized to approve benefits and perquisites for any other executive officer with respect to any calendar year, provided that
it does not exceed the value of such executive officer’s one month base salary.
2021
Equity Incentive Plan
At our annual meeting on July
26, 2021, we adopted our 2021 Equity Incentive Plan.
The following paragraphs provide
a summary of the principal features of the Equity Incentive Plan and its operation. The Plan is set forth in Annex C to Exhibit
99.1 to our Proxy Statement as filed on Form 8-K on June 28, 2021.
Background and Purpose of the Plan
The Plan is intended to attract,
motivate, and retain employees, consultants, directors, and other service providers who provide significant services to us.
Administration of the Plan
Our Board of Directors or a committee
appointed by our Board of Directors (the “Committee”) administers the Plan. Subject to the terms of the Plan, the Committee
will have the power to recommend to our Board of Directors, and our Board of Directors will have the power and authority to select the
employees and consultants who will receive stock option awards and determine the terms and conditions of stock option awards (for example,
the exercise price and vesting schedule). The Committee will have full power and authority to interpret the provisions of the Plan and
outstanding options granted thereunder.
If a stock option expires or
is cancelled without having been fully exercised or vested, the shares covered by such option will be returned to the available pool
of the Company’s ordinary shares (the “Shares”) reserved for issuance under the 2021 Plan. Also, if we experience a
share dividend, recapitalization or other change in our capital structure, the number, class, and kind of shares available for issuance
under the Plan, and the outstanding stock option awards, may be adjusted as appropriate to reflect the share dividend or other change.
Eligibility to Receive Awards
Our Board of Directors, upon
the recommendation of the Committee, selects the employees and consultants who will be granted discretionary stock option awards under
the Plan. The actual number of individuals who will receive a stock option award under the Plan cannot be determined in advance because
our Board of Directors has the discretion to select the participants.
Our Board of Directors may, in
its discretion, elect to grant stock options awards under the Plan in order to fulfill some or all of the equity compensation to be received
by the Company’s Directors and/or Officers, as described in the Compensation Schedule (pending approval of the Compensation Schedule
at this Meeting) or otherwise.
Stock Options
A stock option is the right to
acquire Shares at a fixed exercise price for a fixed period of time. Under the Plan, our Board of Directors may grant options that qualify
for favorable tax treatment under Israeli or United States tax law. Our Board of Directors will determine the number of Shares covered
by each option.
The exercise price of the Shares
subject to each option is set by the Board of Directors in its discretion in accordance with applicable law.
An option granted under the Plan
cannot generally be exercised until it becomes vested. Our Board of Directors establishes the vesting schedule of each option at the
time of grant. Options become exercisable at the times and on the terms established by our Board of Directors. Options granted under
the Plan expire at the times established by our Board of Directors.
The exercise price of each option
granted under the Plan must be paid in full at the time of exercise. The Plan also contains provisions for the cashless exercise of options.
The participant must pay any taxes the Company is required to withhold at the time of exercise.
Change of Control
In the event of a merger, acquisition,
or reorganization by or with one or more entities, in which the Company is not the surviving entity, or a sale of all or substantially
all of the assets of the Company, the successor corporation will either assume or provide a substitute stock option award for each outstanding
stock option. In the event the successor corporation refuses to assume or provide a substitute stock option award, the Committee will
provide at least 15 days’ notice that the option will immediately vest and become exercisable as to all of the Shares subject to
such award and that such award will terminate upon the expiration of such notice period.
Stock Option Awards to Be Granted to Certain Individuals and Groups
The number of stock option awards
that an employee or consultant may receive under the Plan is in the discretion of the Committee and therefore cannot be determined in
advance.
Limited Transferability of Stock Options
Stock options granted under the
Plan generally may not be sold, transferred, pledged, assigned, or otherwise alienated or hypothecated, other than by will or by the
applicable laws of descent and distribution.
Amendment and Termination of the Plan
The Board generally may amend
or terminate the Plan at any time and for any reason.
Summary
Compensation Table
The
following table provides certain summary information concerning compensation awarded to, earned by or paid to our Principal Executive
Officer and our two other highest paid executive officer whose total annual salary and bonus exceeded $100,000 (collectively,
the “named executive officers”) for fiscal year 2020.
|
Name and principal position
|
|
Year
|
|
|
Salary ($)
|
|
|
Bonus
($)
|
|
|
Nonequity incentive plan compensation ($)
|
|
|
Nonqualified deferred compensation earnings
($)
|
|
|
All other compensation ($)
|
|
|
Total
($)
|
|
|
Gerald Commissiong, PEO
|
|
2020
|
|
|
$
|
156,250
|
|
|
|
-
|
|
|
|
-
|
|
|
$
|
219,851
|
|
|
|
-
|
|
|
$
|
376,101
|
|
|
Daniel Hirsch, CFO
|
|
2020
|
|
|
$
|
93,750
|
|
|
|
-
|
|
|
|
-
|
|
|
$
|
111,128
|
|
|
|
-
|
|
|
$
|
204,878
|
|
Director
Compensation
The
following table provides certain summary information concerning compensation awarded to, earned by or paid to our Directors for
fiscal year 2020.
|
Name
|
|
Fees earned or paid in cash ($)
|
|
|
Non-equity incentive plan compensation ($)
|
|
|
Nonqualified deferred compensation earnings ($)
|
|
|
All other compensation ($)
|
|
|
Total
($)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dr. Herman Weiss
|
|
$
|
27,083
|
|
|
|
-
|
|
|
$
|
63,699
|
|
|
|
|
|
|
$
|
90,782
|
|
|
Gerald Commissiong
|
|
$
|
27,083
|
|
|
|
-
|
|
|
$
|
56,993
|
|
|
|
|
|
|
$
|
84,076
|
|
|
Daniel Hirsh
|
|
$
|
27,083
|
|
|
|
-
|
|
|
$
|
56,993
|
|
|
|
|
|
|
$
|
84,076
|
|
|
Lauren Chung
|
|
$
|
27,083
|
|
|
|
-
|
|
|
$
|
56,993
|
|
|
$
|
6,250
|
|
|
$
|
90,326
|
|
|
Moshe Abramovitz
|
|
$
|
27,083
|
|
|
|
-
|
|
|
$
|
56,993
|
|
|
$
|
4,167
|
|
|
$
|
88,243
|
|
|
Moshe Schlisser
|
|
$
|
33,854
|
|
|
|
-
|
|
|
$
|
56,993
|
|
|
$
|
8,333
|
|
|
$
|
99,180
|
|
Outstanding
Equity Awards at Fiscal Year End
The
following table provides certain summary information concerning equity awards made to our executive officers as of December 31,
2020.
|
|
|
Option
awards
|
|
Name
|
|
Number
of securities underlying unexercised options (#) exercisable
|
|
|
Number
of securities underlying unexercised options (#) unexercisable
|
|
|
Equity
incentive plan awards: Number of securities underlying unexercised unearned options (#)
|
|
|
Option
exercise price ($)
|
|
|
Option
expiration date
|
|
Gerald
Commissiong
|
|
|
305,870
|
|
|
|
1,733,263
|
|
|
|
|
|
|
|
0.095
|
|
|
July
29, 2025
|
|
Daniel
Hirsch
|
|
|
75,893
|
|
|
|
430,058
|
|
|
|
|
|
|
|
0.095
|
|
|
July
29, 2025
|
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Security
Ownership
The
following table describes, as of September 30, 2021, the beneficial ownership of Todos Ordinary Shares by:
|
|
●
|
each
person we believe beneficially holds more than 5% of the outstanding Ordinary Shares based solely on our review of SEC filings;
|
|
|
●
|
each
of our named executive officers;
|
|
|
●
|
each
of our directors; and
|
|
|
●
|
all
of our directors and executive officers as a group.
|
|
|
|
No. of Shares Beneficially Owned
|
|
|
Percentage Owned
|
|
|
|
|
|
|
|
|
|
|
Directors and executive officers:
|
|
|
|
|
|
|
|
|
|
Dr. Herman Weiss
|
|
|
300,000
|
|
|
|
*
|
|
|
Gerald Commissiong(1)
|
|
|
79,077,125
|
|
|
|
8.6
|
%
|
|
Lauren Chung
|
|
|
0
|
|
|
|
*
|
|
|
Moshe Abramovitz
|
|
|
0
|
|
|
|
*
|
|
|
Moshe Schlisser
|
|
|
0
|
|
|
|
*
|
|
|
Daniel Hirsch
|
|
|
54,000
|
|
|
|
*
|
|
|
All directors and executive officers as a group (7 persons)
|
|
|
82,854,975
|
|
|
|
9.03
|
%
|
|
Persons or Groups Holding More than 5% of Ordinary
Shares
|
|
|
|
|
|
|
|
|
|
Amarantus Bioscience Holdings, Inc.
|
|
|
78,025,645
|
|
|
|
8.51
|
%
|
|
The Strategic Group
|
|
|
44,744,827
|
|
|
|
4.88
|
%
|
The
address of each shareholder is c/o Todos Medical Limited, 121 Derech Menachem Begin, 30th Floor, Tel Aviv, 6701203 Israel.
|
*
|
Indicates
beneficial ownership of less than 1% of the total ordinary shares outstanding.
|
(1)
Includes 78,025,645 shares owned by Amarantus Bioscience Holdings, Inc (“Amarantus”). Gerald Commissiong is the Executive
Chairman and controlling shareholder of Amarantus and in such capacity holds voting and dispositive power over the securities held by
such entity.
CERTAIN
RELATIONSHIPS AND RELATED PARTY TRANSACTIONS, AND DIRECTOR INDEPENDENCE
On
February 27, 2019, we entered into a joint venture agreement with Amarantus, pursuant to which we issued Ordinary Share representing
19.99% of our then outstanding Ordinary Shares to Amarantus, in exchange for Amarantus transferring to us 19.99% of Breakthrough, a wholly-owned
subsidiary of Amarantus, and for Amarantus assigning the exclusive license to develop and commercialize the LymPro Test®, an immune-based
neurodiagnostic blood test for the detection of Alzheimer’s disease (the “License”) to Breakthrough. As part of
the transaction, we agreed to provide working capital to Breakthrough to support Breakthrough’s operations. As part of the Breakthrough
joint venture, we were granted an exclusive option, which was limited to an exercise period of 60 days from its date, to acquire the
remaining 80.01% of Breakthrough from Amarantus. At our 2019 annual meeting of shareholders, our shareholders approved a resolution authorizing
us to exercise our option to acquire the remaining 80.01% of Breakthrough from Amarantus in exchange for an additional 30% of our then
issued and outstanding Ordinary Shares. Our Chief Executive Officer, Gerald Commissiong, is also the Executive Chairman and controlling
shareholder of Amarantus.
The
Amarantus transaction was valued at approximately $8,623,000 and Amarantus’ holdings in Todos have a value of approximately $5,617,846
based upon the Company’s closing market price on January 13, 2022.
The
related party transaction described above was reviewed and approved in accordance with the provisions of the Companies
Law, as described below.
Approval
of Related Party Transactions
The
Companies Law requires that an “office holder” (as defined in the Companies Law) of a company promptly
disclose any personal interest that he or she may have and all related material information known to him or her, in connection
with any existing or proposed transaction of the company.
Pursuant
to the Companies Law, any transaction with an office holder or in which the office holder has a personal interest (other
than with respect to such office holder’s Terms of Office and Employment) must be brought before the Audit Committee, in
order to determine whether such transaction is an “extraordinary transaction” (defined as a transaction not in the
ordinary course of business, not on market terms or likely to have a material impact on the company’s profitability, assets
or liabilities).
Pursuant
to the Companies Law, the Amended Articles and Todos policy, in the event that the Audit Committee determines that the
transaction is not an extraordinary transaction, the transaction will require only Audit Committee approval; if, however, it is
determined to be an extraordinary transaction, Board approval is also required and, in some circumstances, shareholder approval
may also be required. Such a transaction may only be approved by the Board if it is determined to be in the best interests of
Todos.
A
person with a personal interest in the matter generally may not be present at meetings of the Board or certain committees where
the matter is being considered and, if a member of the Board or a committee, may generally not vote on the matter.
Transactions
with Controlling Shareholders
Under
Israeli law, extraordinary transactions with a controlling shareholder, or in which the controlling shareholder has a personal
interest, and any engagement with a controlling shareholder, or a controlling shareholder’s relative, with respect to the
provision of services to the company or their Terms of Office and Employment as an office holder or as another employee, generally
require the approval of the Audit Committee (or with respect to Terms of Office and Employment, the Compensation Committee), the
Board of Directors and the shareholders. If required, shareholder approval must include (i) at least a majority of the shareholders
who do not have a personal interest in the transaction and are present and voting at the meeting (abstentions are disregarded),
or, alternatively, that (ii) the total shareholdings of the disinterested shareholders who vote against the transaction do not
represent more than two percent of the voting rights in the company. Transactions for a period of more than three years generally
need to be brought for approval in accordance with the above procedures every three years.
A
shareholder who holds 25% or more of the voting rights in a company is considered a controlling shareholder for these purposes
if no other shareholder holds more than 50% of the voting rights. If two or more shareholders are interested parties in the same
transaction, their shareholdings are combined for the purposes of calculating percentages. We are currently not aware of the existence
of any controlling shareholder or any shareholder arrangements which would constitute “control” of Todos under Israeli
law.
Director
Independence
We
define an independent director as a member of the board of directors who (1) does not have a material relationship with the
Company, (2) is not part of the Company’s executive team, and (3) is not involved with the day-to-day operations of the
Company.
The
Board of Directors has determined that all of the directors that currently serve on the Board of Directors are, and all of the
directors that served on the Board of Directors during 2020 were, independent, except for Gerald Commissiong (who is also our Chief
Executive Officer) and Daniel Hirsch (who is also our Chief Financial Officer). At our annual meeting on July 26, 2021, Dr.
Lauren Chung and Mr. Moshe Schlisser were elected ‘public directors’ (or ‘external directors’) under the
Israeli Companies Law.
SELLING
SHAREHOLDERS
This prospectus relates to the
possible resale by the resale of up to (i) 137,980,949 ordinary shares that may be issuable upon the exercise of warrants held
by warrantholders identified in this prospectus, and (ii) 123,086,088 ordinary shares as may be issued from time to time to the holders
of convertible notes held by the convertible noteholders identified in this prospectus, which convertible notes have an aggregate principal
amount of $8,625,000. The conversion price for the Convertible Notes is subject to adjustment downwards under certain circumstances.
All share numbers in this prospectus assume that such conversion price will not be adjusted. The maximum adjustment of the conversion
price under the Convertible Notes is 20%. We are filing the registration statement of which this prospectus forms a part pursuant to
registration rights that were granted to the selling shareholders pursuant to their respective agreements with us.
The
selling shareholders, respectively, may, from time to time, offer and sell pursuant to this prospectus any or all of the ordinary
shares that such selling shareholder may receive by exercise of a warrant or conversion of a convertible note. Each “selling
shareholder” may sell some, all or none of its ordinary shares. We do not know how long the selling shareholders will hold
the ordinary shares before selling them, and we currently have no agreements, arrangements or understandings with the selling
shareholders regarding the sale of any of the ordinary shares.
The
following table presents information regarding each selling shareholder and the ordinary shares that it may offer and sell from time
to time under this prospectus. The table is prepared based on information supplied to us by the selling shareholders and reflects their
holdings as of January 13, 2022. Except as noted below, none of the selling shareholders has held a position or office, or had
any other material relationship, with us or any of our predecessors or affiliates. Beneficial ownership is determined in accordance with
Section 13(d) of the Exchange Act and Rule 13d-3 thereunder. The percentage of ordinary shares beneficially owned prior to the offering
is based on 977,144,432 ordinary shares actually outstanding as of January 13, 2022, modified as described in footnote 2 to
the table below.
|
|
|
Ordinary
Shares
Beneficially
Owned
Prior
to Offering
|
|
|
Number
of
Ordinary
Shares
Being
|
|
|
Ordinary
Shares
Beneficially
Owned
After
Offering
|
|
|
Name
|
|
Number
(1)
|
|
|
%(2)
|
|
|
Offered
|
|
|
Number
(3)
|
|
|
%(4)
|
|
|
Yozma
Global Genomic Fund 1 (5)
|
|
|
199,596,856
|
|
|
|
13.26
|
%
|
|
|
33,415,125
|
|
|
|
166,181,731
|
|
|
|
11.04
|
|
|
Kips
Bay Select LP (6)
|
|
|
148,756,454
|
|
|
|
9.88
|
|
|
|
47,683,236
|
|
|
|
101,073,218
|
|
|
|
6.71
|
|
|
SB
Nihul Ltd.(7)
|
|
|
5,958,115
|
|
|
|
0.40
|
|
|
|
5,958,115
|
|
|
|
0
|
|
|
|
0.0
|
|
|
Quick
Capital, LLC (8)
|
|
|
5,958,115
|
|
|
|
0.40
|
|
|
|
5,958,115
|
|
|
|
0
|
|
|
|
0.0
|
|
|
GreenTree
Financial
|
|
|
23,479,727
|
|
|
|
1.56
|
|
|
|
21,979,727
|
|
|
|
2,250,000
|
|
|
|
0.15
|
|
|
Avner
Krohn
|
|
|
5,080,643
|
|
|
|
0.34
|
|
|
|
1,339,284
|
|
|
|
2,991,359
|
|
|
|
0.20
|
|
|
BelHar
Investments
|
|
|
30,432,734
|
|
|
|
2.02
|
|
|
|
8,928,562
|
|
|
|
21,504,172
|
|
|
|
1.43
|
|
|
Ethel
Zieleniec
|
|
|
8,928,562
|
|
|
|
0.59
|
|
|
|
8,928,562
|
|
|
|
0
|
|
|
|
0.0
|
|
|
DPH
Investments
|
|
|
3,571,424
|
|
|
|
0.24
|
|
|
|
3,571,424
|
|
|
|
0
|
|
|
|
0.0
|
|
|
Shmuel
Rotbard
|
|
|
1,116,070
|
|
|
|
0.07
|
|
|
|
1,116,070
|
|
|
|
0
|
|
|
|
0.0
|
|
|
Tehresa
Yee Ling Tan
|
|
|
5,753,538
|
|
|
|
0.38
|
|
|
|
2,232,140
|
|
|
|
3,521,398
|
|
|
|
0.23
|
|
|
Infusion
51a
|
|
|
2,000,000
|
|
|
|
0.13
|
|
|
|
2,000,000
|
|
|
|
0
|
|
|
|
0.0
|
|
|
Daniel
Reich
|
|
|
9,088,597
|
|
|
|
0.60
|
|
|
|
3,000,000
|
|
|
|
6,088,597
|
|
|
|
0.40
|
|
|
Shmuel
Bar On
|
|
|
500,000
|
|
|
|
0.03
|
|
|
|
500,000
|
|
|
|
0
|
|
|
|
0.0
|
|
|
Leviston
Capital
|
|
|
5,513,513
|
|
|
|
0.37
|
|
|
|
5,513,513
|
|
|
|
0
|
|
|
|
0.0
|
|
|
Leonite
Capital
|
|
|
34,961,990
|
|
|
|
2.32
|
|
|
|
34,961,990
|
|
|
|
0
|
|
|
|
0.0
|
|
|
Ben
Tsion Khasid
|
|
|
1,834,757
|
|
|
|
0.12
|
|
|
|
446,428
|
|
|
|
1,388,329
|
|
|
|
0.09
|
|
|
Mercer
St. Global Opportunity Fund, LLC (9)
|
|
|
61,531,123
|
|
|
|
4.09
|
|
|
|
61,531,123
|
|
|
|
0
|
|
|
|
0.0
|
|
|
David
Wasserman
|
|
|
1,935,932
|
|
|
|
0.13
|
|
|
|
892,856
|
|
|
|
1,043,076
|
|
|
|
0.07
|
|
|
Ascendant
Partners Ltd. (10)
|
|
|
6,460,768
|
|
|
|
0.43
|
|
|
|
4,964,569
|
|
|
|
0
|
|
|
|
0.0
|
|
|
|
(1)
|
Includes
all shares that are being offered for sale under this prospectus that would result from the
exercise of warrants held by the selling shareholders, and all shares that are being offered
for sale under this prospectus that would result from the conversion of convertible notes
held by selling shareholders. The conversion price for the Convertible Notes is subject
to adjustment downwards under certain circumstances. All share numbers in this prospectus
assume that such conversion price will not be adjusted. The maximum adjustment of the conversion
price under the Convertible Notes is 20%.
|
|
|
|
|
|
|
(2)
|
Calculated
by dividing the total number of ordinary shares beneficially owned by each selling shareholder as reflected in column 1 by the number
of ordinary shares outstanding as of January 13, 2022, assuming that (a) all shares that are being offered for sale
under this prospectus that would result from the exercise of warrants held by selling shareholders have been exercised and (b)
all shares that are being offered for sale under this prospectus that would result from the conversion of convertible notes held
by selling shareholders have been converted, and (c) all shares that would result from the conversion of convertible notes held
by Yozma Global Genomic Fund 1 and Kips Bay Select LP (that are not part of this prospectus) have been converted. The conversion
price for the Convertible Notes is subject to adjustment downwards under certain circumstances. All share numbers in this prospectus
assume that such conversion price will not be adjusted. The maximum adjustment of the conversion price under the Convertible Notes
is 20%.
|
|
|
|
|
|
|
(3)
|
Assumes
all shares offered by the selling shareholder are sold.
|
|
|
|
|
|
|
(4)
|
Calculated
by dividing the total number of ordinary shares beneficially owned by each selling shareholder after all shares offered by the
selling shareholder are sold, by the number of ordinary shares outstanding as of January 13, 2022, assuming that all
shares that are being offered for sale under this prospectus that would result from the exercise of warrants held by selling
shareholders have been exercised and all shares that are being offered for sale under this prospectus that would result from the
conversion of convertible notes held by selling shareholders have been converted, and all shares that would result from the
conversion of convertible notes held by Yozma Global Genomic Fund 1 and Kips Bay Select LP (that are not part of this prospectus)
have been converted. The conversion price for the Convertible Notes is subject to adjustment downwards under certain circumstances.
All share numbers in this prospectus assume that such conversion price will not be adjusted. The maximum adjustment of the
conversion price under the Convertible Notes is 20%.
|
|
|
|
|
|
|
(5)
|
Mr.
Wonjac Lee is the Chief Executive Officer of Yozma Global Genomic
Fund 1 and in such capacity holds voting and dispositive power over the securities held by such entity
|
|
|
|
|
|
|
(6)
|
Mr.
John Miller is the General Partner of Kips Bay Select LP and in
such capacity holds voting and dispositive power over the securities held by such entity
|
|
|
|
|
|
|
(7)
|
Mr.
Baruch Saar is the Chief Executive Officer of SB Nihul Ltd. and in such capacity holds voting and dispositive power over the securities
held by such entity.
|
|
|
|
|
|
|
(8)
|
Mr.
Eilon Natan is the Manager of Quick Capital, LLC and in such capacity holds voting and dispositive power over the securities held
by such entity
|
|
|
|
|
|
|
(9)
|
Mr.
Jonathan Juchno is the Manager of Mercer Street Global Opportunity Fund, LLC and in such capacity holds voting power over the securities
held by such entity
|
|
|
|
|
|
|
(10)
|
Richard Galterio is the General Partner of Ascendant
Partners Ltd. and in such capacity holds voting power over the securities held by such entity.
|
PLAN
OF DISTRIBUTION
The
ordinary shares offered by this prospectus are being offered by the selling shareholders. The ordinary shares may be sold or distributed
from time to time by the selling shareholders directly to one or more purchasers or through brokers, dealers, or underwriters
who may act solely as agents at market prices prevailing at the time of sale, at prices related to the prevailing market prices,
at negotiated prices, or at fixed prices, which may be changed. The sale of the ordinary shares offered by this prospectus could
be effected in one or more of the following methods:
ordinary
brokers’ transactions;
transactions
involving cross or block trades;
through
brokers, dealers, or underwriters who may act solely as agents;
“at
the market” into an existing market for the ordinary shares;
in
other ways not involving market makers or established business markets, including direct sales to purchasers or sales effected
through agents;
in
privately negotiated transactions; or
any
combination of the foregoing.
In
order to comply with the securities laws of certain states, if applicable, the ordinary shares may be sold only through registered
or licensed brokers or dealers. In addition, in certain states, the ordinary shares may not be sold unless they have been registered
or qualified for sale in the state or an exemption from the state’s registration or qualification requirement is available
and complied with.
Each
selling shareholder is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act.
Each
selling shareholder has informed us that it intends to use an unaffiliated broker-dealer to effectuate all sales, if any, of the
ordinary shares that it has acquired and may in the future acquire from us pursuant to the Purchase Agreement. Such sales will
be made at prices and at terms then prevailing or at prices related to the then current market price. Each such unaffiliated broker-dealer
will be an underwriter within the meaning of Section 2(a)(11) of the Securities Act. Each selling shareholder has informed us
that each such broker-dealer will receive commissions from such selling shareholder that will not exceed customary brokerage commissions.
Brokers,
dealers, underwriters or agents participating in the distribution of the ordinary shares offered by this prospectus may receive
compensation in the form of commissions, discounts, or concessions from the purchasers, for whom the broker-dealers may act as
agent, of the ordinary shares sold by the selling shareholders through this prospectus. The compensation paid to any such particular
broker-dealer by any such purchasers of ordinary shares sold by the selling shareholders may be less than or in excess of customary
commissions. Neither we nor any selling shareholder can presently estimate the amount of compensation that any agent will receive
from any purchasers of ordinary shares sold by such selling shareholder.
We
know of no existing arrangements between any selling shareholder or any other shareholder, broker, dealer, underwriter or agent
relating to the sale or distribution of the ordinary shares offered by this prospectus.
We
may from time to time file with the SEC one or more supplements to this prospectus or amendments to the registration statement
of which this prospectus forms a part to amend, supplement or update information contained in this prospectus, including, if and
when required under the Securities Act, to disclose certain information relating to a particular sale of ordinary shares offered
by this prospectus by the selling shareholder, including the names of any brokers, dealers, underwriters or agents participating
in the distribution of such ordinary shares by the selling shareholder, any compensation paid by the selling shareholders to any
such brokers, dealers, underwriters or agents, and any other required information.
We
will pay the expenses incident to the registration under the Securities Act of the offer and sale of the ordinary shares covered
by this prospectus by the selling shareholders. We have agreed to indemnify the selling shareholders and certain other persons
against certain liabilities in connection with the offering of ordinary shares offered hereby, including liabilities arising under
the Securities Act or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities.
Each selling shareholder has agreed to indemnify us against liabilities under the Securities Act that may arise from certain written
information furnished to us by such selling shareholder specifically for use in this prospectus or, if such indemnity is unavailable,
to contribute amounts required to be paid in respect of such liabilities.
Insofar
as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers, and controlling
persons, we have been advised that in the opinion of the SEC this indemnification is against public policy as expressed in the
Securities Act and is therefore, unenforceable.
Each
Selling Shareholder has represented to us that at no time prior to the Purchase Agreement has such selling shareholder or its
agents, representatives or affiliates engaged in or effected, in any manner whatsoever, directly or indirectly, any short sale
(as such term is defined in Rule 200 of Regulation SHO of the Exchange Act) of our ordinary shares or any hedging transaction,
which establishes a net short position with respect to our ordinary shares. Each selling shareholder agreed that for so long as
the registration statement of which this prospectus is part remains effective, and such selling shareholder has unsold shares
registered hereunder, it, its agents, representatives or affiliates will not enter into or effect, directly or indirectly, any
of the foregoing transactions.
We
have advised each selling shareholder that it is required to comply with Regulation M promulgated under the Exchange Act. With
certain exceptions, Regulation M precludes the selling shareholder, any affiliated purchasers, and any broker-dealer or other
person who participates in the distribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase
any security which is the subject of the distribution until the entire distribution is complete. Regulation M also prohibits any
bids or purchases made in order to stabilize the price of a security in connection with the distribution of that security. All
of the foregoing may affect the marketability of the securities offered by this prospectus.
This
offering will terminate on the date that all ordinary shares offered by this prospectus have been sold by the selling shareholders.
Our ordinary shares are currently
quoted on the U.S. OTCQB marketplace of OTC Link, or OTCQB, under the symbol “TOMDF”. On January 13, 2022, the closing
price of our ordinary shares, as reported on the OTCQB, was $.064 per share.
Blue
Sky Restrictions on Resale
If
a selling shareholder desires to sell our ordinary shares under this prospectus in the United States, then such selling shareholder
will also need to comply with state securities laws, also known as “Blue Sky laws,” with regard to secondary sales.
All states offer a variety of exemptions from registration for secondary sales. Many states, for example, have an exemption for
secondary trading of securities registered under Section 12(g) of the Exchange Act or for securities of issuers that publish continuous
disclosure of financial and non-financial information in a recognized securities manual, such as Standard & Poor’s.
Any
person who purchases our ordinary shares from a selling shareholder under this prospectus who then desires to sell such shares
also will have to comply with Blue Sky laws regarding secondary sales.
MATERIAL
TAX CONSIDERATIONS AND GOVERNMENT PROGRAMS
The
following description is not intended to constitute a complete analysis of all tax consequences relating to the acquisition, ownership
and disposition of our securities. You should consult your own tax advisor concerning the tax consequences in your particular
situation, as well as any tax consequences that may arise under the laws of any taxing jurisdiction.
Israeli Tax Considerations
The following is a brief
summary of certain material Israeli tax laws applicable to us, and certain Israeli government programs that benefit
us. This section also contains a discussion of certain material Israeli tax consequences concerning the ownership and disposition
of our securities offered hereby. This summary does not discuss all the aspects of Israeli tax law that may be relevant to
a particular investor in light of his or her personal investment circumstances or to some types of investors subject to special treatment
under Israeli law. Examples of such investors include residents of Israel or traders in securities who are subject
to special tax regimes not covered in this discussion. To the extent that the discussion is based on tax legislation that
has not yet been subject to judicial or administrative interpretation, we cannot assure you that the appropriate tax authorities
or the courts will accept the views expressed in this discussion. The discussion below is not intended, and should not be
construed, as legal or professional tax advice and is not exhaustive of all possible tax considerations. The discussion
is subject to change, including due to amendments under Israeli law or changes to the applicable judicial or administrative interpretations
of Israeli law, which change could affect the tax consequences described below, possibly with a retroactive effect.
THEREFORE, YOU ARE
URGED TO CONSULT YOUR OWN TAX ADVISORS AS TO THE ISRAELI OR OTHER TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND DISPOSITION
OF OUR SECURITIES OFFERED HEREBY, INCLUDING, IN PARTICULAR, THE EFFECT OF ANY FOREIGN, STATE OR LOCAL TAXES.
General
Corporate Tax Structure in Israel
Israeli companies are generally
subject to corporate tax at a flat rate. In December 2016, the Israeli Parliament approved the Economic Efficiency Law (Legislative
Amendments for Applying the Economic Policy for the 2017 and 2018 Budget Years), or EEL which reduced the corporate income tax
rate from 25% to 24% effective from January 1, 2017, and to 23% effective from January 1, 2018 and thereafter. However, the
effective tax rate payable by a company that derives income from an Approved Enterprise, a Preferred Enterprise, a Benefited
Enterprise or a Technological Enterprise (as discussed below) may be considerably less. Capital gains derived by an Israeli
company are generally subject to corporate tax rate.
Law
for the Encouragement of Industry (Taxes), 5729-1969
The Law for the Encouragement
of Industry (Taxes), 5729-1969, generally referred to as the Industry Encouragement Law, or IEL, provides several
tax benefits for “Industrial Companies.” We may qualify as an Industrial Company within the meaning of the IEL.
The IEL defines an “Industrial
Company” as an Israeli resident-company, of which 90% or more of its income in any tax year, other than income from certain
government loans, capital gains, interest and dividends, is derived from an “Industrial Enterprise” owned by it and
located in Israel or in the “Area,” in accordance with the definition under Section 3A of the Israeli Income
Tax Ordinance (New Version) 1961, or the Ordinance. An “Industrial Enterprise” is defined as an enterprise whose principal
activity in a given tax year is industrial production.
The
following are the main tax benefits available to Industrial Companies:
|
|
●
|
Amortization
of the cost of purchased patent, rights to use a patent, and know-how, which are used for the development or advancement of the Industrial
Enterprise, over an eight-year period, commencing on the year in which such rights were first exercised;
|
|
|
|
|
|
|
●
|
Under
limited conditions, an election to file consolidated tax returns with controlled Israeli Industrial Companies;
and
|
|
|
|
|
|
|
●
|
Expenses
related to a public offering are deductible in equal amounts over three years commencing on the year of the offering.
|
Eligibility
for benefits under the Industry Encouragement Law is not contingent
upon approval of any governmental authority.
Tax
Benefits and Grants for Research and Development
Israeli
tax law allows, under certain conditions, a tax deduction for expenditures related to scientific research and development projects, including
capital expenditures, for the year in which they are incurred. Expenditures are deemed related to scientific research and development
projects, if:
|
|
●
|
The
expenditures are approved by the relevant Israeli government ministry, determined by the field of research;
|
|
|
|
|
|
|
●
|
The
research and development must be for the promotion of the company; and
|
|
|
|
|
|
|
●
|
The
research and development is carried out by or on behalf of the company seeking such tax deduction.
|
The
amount of such deductible expenses is reduced by the sum of any funds received through government grants for the finance of such scientific
research and development projects. No deduction under these research and development deduction rules is allowed if such deduction is
related to an expense invested in an asset depreciable under the general depreciation rules of the Ordinance. Expenditures that are unqualified
under the conditions above are deductible in equal amounts over three years.
From
time to time we may apply to the Israel Innovation Authority, or IIA for approval to allow a tax deduction for all or most of research
and development expenses during the year incurred. There can be no assurance that such application will be accepted. If we will not be
able to deduct research and development expenses during the year of the payment, we may be able to deduct research and development expenses
in equal amounts over a period of three years commencing in the year of the payment of such expenses.
Law
for the Encouragement of Capital Investments, 5719-1959
The
Law for the Encouragement of Capital Investments, 5719-1959, or the Investment Law, provides certain incentives for capital
investments in production facilities (or other eligible assets). Generally, an investment program that is implemented in accordance
with the provisions of the Investment Law, referred to as an Approved Enterprise, a Beneficiary Enterprise, a Preferred Enterprise, a
Preferred Technological Enterprise, or a Special Preferred Technological Enterprise, is entitled to benefits as discussed below. These
benefits may include cash grants from the Israeli government and tax benefits, based upon, among other things, the geographic location
in Israel of the facility in which the investment is made. In order to qualify for these incentives, the Company is required to comply
with the requirements of the Investment Law.
The Investment Law was significantly
amended effective as of April 1, 2005, or the 2005 Amendment, as of January 1, 2011, or the 2011 Amendment, and as of January
1, 2017, or the 2017 Amendment. Pursuant to the 2005 Amendment, tax benefits granted in accordance with the provisions of the
Investment Law prior to its revision by the 2005 Amendment remain in force but any benefits granted subsequently are subject to the provisions
of the amended Investment Law. Similarly, the 2011 Amendment introduced new benefits to replace those granted in accordance with the
provisions of the Investment Law in effect prior to the 2011 Amendment. However, companies entitled to benefits under the Investment
Law as in effect prior to January 1, 2011 were entitled to choose to continue to enjoy such benefits, provided that certain conditions
are met, or elect instead, irrevocably, to forego such benefits and have the benefits of the 2011 Amendment apply. The 2017 Amendment
introduces new benefits for Technological Enterprises, alongside the existing tax benefits.
Tax
Benefits Under the 2011 Amendment
The
2011 Amendment canceled the availability of the benefits granted to Industrial Companies under the Investment Law prior to 2011
and, instead, introduced new benefits for income generated by a “Preferred Company” through its “Preferred Enterprise”
(as such terms are defined in the Investment Law) as of January 1, 2011. The definition of a Preferred Company includes a company incorporated
in Israel that is not fully owned by a governmental entity, and that has, among other things, Preferred Enterprise status and is controlled
and managed from Israel.
Pursuant to the 2011 Amendment,
a Preferred Company is entitled to a reduced corporate tax rate of 15% with respect to its income derived by its Preferred Enterprise
in 2011 and 2012, unless the Preferred Enterprise is located in a specified development zone, in which case the rate will be 10%.
Under the 2011 Amendment, such corporate tax rate was reduced from 15% and 10%, respectively, to 12.5% and 7%, respectively, in 2013,
16% and 9% respectively, in 2014, 2015 and 2016, and 16% and 7.5%, respectively, in 2017 and thereafter. Income derived by a Preferred
Company from a “Special Preferred Enterprise” (as such term is defined in the Investment Law) would be entitled, subject
to certain conditions and during a benefits period of 10 years, to further reduced tax rates of 8%, or 5% if the Special Preferred
Enterprise is located in a certain development zone.
Dividends distributed from
income which is attributed to a “Preferred Enterprise” will be subject to tax at the following rates: (i) Israeli resident
corporations –0% (although, if such dividends are subsequently distributed to individuals or a non-Israeli company the
below rates detailed in sub sections (ii) and (iii) shall apply) (ii) Israeli resident individuals–20% (iii) non-Israeli residents
(individuals and corporations)–20%, subject to a reduced tax rate under the provisions of any applicable tax treaty. The withholding
tax rate applicable to distribution of dividend from such income to non-Israeli residents is 25% (or 30% if distributed to a “substantial
shareholder” at the time of the sale or at any time during the preceding twelve months period, as defined below), which may be
reduced under an applicable tax treaty by applying in advance for a withholding certificate from the Israel Tax Authority, or ITA. A
“substantial shareholder” is generally a person who alone or together with such person’s relative or another person
who collaborates with such person on a permanent basis, holds, directly or indirectly, at least 10% of any of the “Means of Control”
of the corporation. “Means of control” generally include the right to vote, receive profits, nominate a director or an executive
officer, receive assets upon liquidation, or order someone who holds any of the aforesaid rights how to act, regardless of the source
of such right.
The 2011 Amendment also
provided transitional provisions to address companies already enjoying existing tax benefits under the Investment Law. These transitional
provisions provide, among other things, that unless an irrevocable request is made to apply the provisions of the Investment Law as amended
in 2011 with respect to income to be derived as of January 1, 2011, a Beneficiary Enterprise can elect to continue to benefit from the
benefits provided to it before the 2011 Amendment came into effect, provided that certain conditions are met.
New
Tax Benefits Under the 2017 Amendment That Became Effective on January 1, 2017
The
2017 Amendment was enacted as part of the EEL that was published on December 29, 2016 and is effective as of January 1, 2017.
The 2017 Amendment provides new tax benefits for two types of “Technological Enterprises,” as described below, and
is in addition to the other existing tax beneficial programs under the Investment Law.
The
2017 Amendment provides that a Preferred Company satisfying certain conditions will qualify as having a “Preferred
Technological Enterprise” and will thereby enjoy a reduced corporate tax rate of 12% on income that qualifies as “Preferred
Technological Income,” as defined in the Investment Law. The corporate tax rate is further reduced to 7.5% with
respect to a Preferred Technological Enterprise located in development zone “A.” In addition, a Preferred
Technological Company will enjoy a reduced corporate tax rate of 12% on capital gain derived from the sale of certain “Benefitted
Intangible Assets” (as defined in the Investment Law) to a related foreign company if the Benefitted Intangible Assets were acquired
from a foreign company on or after January 1, 2017 for at least NIS 200 million, and the sale receives prior approval from the
IIA.
The
2017 Amendment further provides that a Preferred Company satisfying certain conditions (including group consolidated revenues
of at least NIS 10 billion) will qualify as a “Special Preferred Technological Enterprise” and will thereby enjoy
a reduced corporate tax rate of 6% on “Preferred Technological Income” regardless of the company’s geographic
location within Israel. In addition, a Special Preferred Technological Enterprise will enjoy a reduced corporate tax rate of 6%
on capital gain derived from the sale of certain “Benefitted Intangible Assets” to a related foreign company if the Benefitted
Intangible Assets were either developed by the Special Preferred Enterprise or acquired from a foreign company on or after January
1, 2017, and the sale received prior approval from the IIA. A Special Preferred Technological Enterprise that acquires
Benefitted Intangible Assets from a foreign company for more than NIS 500 million will be eligible for these benefits for at least ten
years, subject to certain approvals as specified in the Investment Law.
Dividends
distributed by a Preferred Technological Enterprise or a Special Preferred Technological Enterprise, paid out of Preferred Technological
Income, are generally subject to tax at the rate of 20% or such lower rate as may be provided in an applicable tax treaty.
The withholding tax rate applicable to distribution of dividend from such income to non-Israeli residents is 25% (or 30% if distributed
to a “substantial shareholder” at the time of the sale or at any time during the preceding twelve months period), which may
be reduced under an applicable tax treaty by applying in advance for a withholding certificate from the ITA. In addition, if such dividends
are distributed to a foreign company that holds solely or together with other foreign companies 90% or more in the Israeli company
and other conditions are met, the withholding tax rate will be 4% (subject to the receipt in advance of a valid certificate from
the ITA allowing for a reduced tax rate). However, if such dividends are paid to an Israeli company, no tax is required to be withheld.
Taxation
of Our Securityholders
Capital gains Tax on Sales of our Ordinary
Shares and Warrants
Israeli law generally imposes
a capital gains tax on the sale of any capital assets by Israeli residents, as defined for Israeli tax purposes, and on the sale of capital
assets located in Israel, including shares of Israeli companies, by both Israeli residents and non-Israeli residents, unless a specific
exemption is available or unless a tax treaty between Israel and the shareholder’s country of residence provides otherwise. The
Ordinance distinguishes between real gain and inflationary surplus. The inflationary surplus is a portion of the total capital gain equivalent
to the increase of the relevant asset’s purchase price attributable to an increase in the Israeli consumer price index, or a foreign
currency exchange rate, between the date of purchase and the date of sale. Inflationary surplus is currently not subject to tax in Israel.
The real gain is the excess of the total capital gain over the inflationary surplus.
Capital Gains Taxes Applicable to Non-Israeli
Resident Securityholders
A non-Israeli resident who
derives capital gains from the sale of shares or warrants in an Israeli resident company that were purchased after the company was listed
for trading on a stock exchange outside of Israel, will
be exempt from Israeli tax
as long as (among other conditions) the securities were not held through a permanent establishment that the non-resident maintains in
Israel. However, non-Israeli corporations will not be entitled to the foregoing exemption if Israeli residents: (i) have a controlling
interest of more than 25% in such non-Israeli corporation or (ii) are the beneficiaries of, or are entitled to, 25% or more of the revenues
or profits of such non-Israeli corporation, whether directly or indirectly. In addition, such exemption is not applicable to a person
whose gains from selling or otherwise disposing of the securities are deemed to be business income.
Additionally, a sale of
securities by a non-Israeli resident may be exempt from Israeli capital gains tax under the provisions of an applicable tax treaty. For
example, under the Convention Between the Government of the United States of America and the Government of the State of Israel with respect
to Taxes on Income, as amended, or the U.S. Israel Tax Treaty, the sale, exchange or other disposition of shares by a shareholder who
is a United States resident (for purposes of the treaty) holding the shares as a capital asset and is entitled to claim the benefits
afforded to such a resident by the U.S. Israel Tax Treaty (a “U.S. Resident”) is generally exempt from Israeli capital gains
tax unless: (i) the capital gain arising from such sale, exchange or disposition is attributed to real estate located in Israel; (ii)
the capital gain arising from such sale, exchange or disposition is attributed to royalties; (iii) the capital gain arising from the
such sale, exchange or disposition is attributed to a permanent establishment in Israel, under certain terms; (iv) such U.S. Resident
holds, directly or indirectly, shares representing 10% or more of the voting capital during any part of the 12 month period preceding
the disposition, subject to certain conditions; or (v) such U.S. Resident is an individual and was present in Israel for 183 days or
more during the relevant taxable year. In any such case, the sale, exchange or disposition of such shares by the U.S. Resident would
be subject to Israeli tax (unless exempt under the Israeli domestic law as described above). Under the U.S. Israel Tax Treaty, the gain
may be treated as foreign source income for United States foreign tax credit purposes, upon an election by the U.S. Resident, and such
U.S. Resident may be permitted to claim a credit for such taxes against the United States federal income tax imposed on such sale, subject
to the limitations under the United States federal income tax laws applicable to foreign tax credits. The U.S. Israel Tax Treaty does
not provide such credit against any United States state or local taxes.
Regardless of whether securityholders
may be liable for Israeli tax on the sale of our securities, the payment of the consideration may be subject to the withholding of Israeli
tax at source. Securityholders may be required to demonstrate that they are exempt from tax on their capital gains in order to avoid
withholding at source at the time of sale (i.e., provide a non-Israeli resident certificate and other documentation).
Capital Gains Taxes Applicable to Israeli
Resident Securityholders
An Israeli resident corporation
who derives capital gains from the sale of shares or warrants in an Israeli resident company that were purchased after the company was
listed for trading on a stock exchange outside of Israel will generally be subject to tax on the real capital gains generated on such
sale at the corporate tax rate (currently of 23%). An Israeli resident individual will generally be subject to capital gain tax at the
rate of 25%. However, if the individual securityholder is claiming deduction of interest expenditures or he is a “substantial shareholder”
at the time of the sale or at any time during the preceding twelve months period, such gain will be taxed at the rate of 30%. Individual
holders dealing in securities in Israel for whom the income from the sale of securities is considered “business income” as
defined in section 2(1) of the Ordinance are taxed at the marginal tax rates applicable to business income (up to 47% in 2021 plus 3%
Surtax). Certain Israeli institutions who are exempt from tax under section 9(2) or section 129(C)(a)(1) of the Ordinance (such as exempt
trust funds and pension funds) may be exempt from capital gains tax from the sale of the securities.
Exercise of Warrants and Certain Adjustments
to the Warrants
Purchasers will generally
not recognize gain or loss for Israeli tax purposes on the exercise of a Warrant and related receipt of an ordinary share (unless, for
instance, cash is received in lieu of the issuance of a fractional ordinary share). Nevertheless, the Israeli income tax treatment and
the tax consequences of a cashless exercise of Warrants into ordinary shares is unclear. Furthermore, the exercise terms of the Warrants
may be adjusted in certain circumstances. An adjustment to the number of ordinary shares that will be issued on the exercise of the Warrants
or an adjustment to the exercise price of a Warrant may be treated as a taxable event under Israeli tax law even if such holder does
not receive any cash or other property in connection with the adjustment. Purchasers should consult their tax advisors regarding the
proper treatment of any exercise of and/or adjustments to the Warrants.
Taxation of Israeli Shareholders on Receipt
of Dividends
An Israeli resident individual
is generally subject to Israeli income tax on the receipt of dividends paid on our ordinary shares at the rate of 25%. With respect to
a person who is a “substantial shareholder” at the time of receiving the dividend or on any time during the preceding twelve
months, the applicable tax rate is 30%. Such dividends are generally subject to Israeli withholding tax at a rate of 25% if the shares
are registered with a nominee company (whether the recipient is a substantial shareholder or not). If the recipient of the dividend is
an Israeli resident corporation such dividend income will be exempt from tax provided the income from which such dividend is distributed
was derived or accrued within Israel and was received directly or indirectly from another corporation that is liable to Israeli corporate
tax. An exempt trust fund, pension fund or other entity that is exempt from tax under section 9(2) or section 129C(a)(1) of the Ordinance
is exempt from tax on a dividend.
Dividend distribution by
a Preferred Technology Enterprise or a Special Preferred Technology Enterprise is subject to beneficial withholding tax rates. For a
further discussion, see “Taxation and Government Programs—Israeli Tax Considerations—Law for the Encouragement of Capital
Investments, 5719-1959—New Tax Benefits Under the 2017 Amendment that Became Effective on January 1, 2017.”
Taxation of Non-Israeli Shareholders on
Receipt of Dividends
Non-Israeli residents (either
individuals or corporations) are generally subject to Israeli income tax on the receipt of dividends paid on our ordinary shares at the
rate of 25%, which tax will be withheld at source, unless relief is provided in a treaty between Israel and the shareholder’s country
of residence. With respect to a person who is a “substantial shareholder” at the time of receiving the dividend or on any
time during the preceding twelve months, the applicable tax rate is 30%. Such dividends are generally subject to Israeli withholding
tax at a rate of 25% if the shares are registered with a nominee company (whether the recipient is a substantial shareholder or not)
unless a reduced rate is provided under an applicable tax treaty (subject to the receipt in advance of a valid certificate from the ITA
allowing for a reduced tax rate). For example, under the U.S. Israel Tax Treaty, the maximum rate of tax withheld at source in Israel
on dividends paid to a holder of our ordinary shares who is a U.S. Resident is 25%. However, generally, the maximum rate of withholding
tax on dividends, not generated by a Preferred Technological Enterprise, Preferred Enterprise, Approved Enterprise or Beneficial Enterprise,
that are paid to a United States corporation holding 10% or more of the outstanding voting capital throughout the tax year in which the
dividend is distributed as well as during the previous tax year, is 12.5%, provided that not more than 25% of the gross income for such
preceding year consists of certain types of dividends and interest.
Notwithstanding the foregoing,
dividends distributed from income attributed to an Approved Enterprise, Preferred Technological Enterprise, Benefited Enterprise or Preferred
Enterprise are not entitled to such reduction under the tax treaty but are subject to a withholding tax rate of 15% for a shareholder
that is a U.S. corporation, provided that the conditions related to the outstanding voting rights and the gross income for the previous
year (as set forth in the previous sentences) are met. If the dividend is attributable partly to income derived from an Approved Enterprise,
Preferred Technological Enterprise, Benefited Enterprise or Preferred Enterprise, and partly to other sources of income, the withholding
rate will be a blended rate reflecting the relative portions of the two types of income. We cannot assure you that we will designate
the profits that we may distribute in a way that will reduce shareholders’ tax liability. Application for the reduced tax rate
requires appropriate documentation presented and specific instruction received from the ITA. To the extent tax is withheld at source
at the maximum rates (see above), a qualified tax treaty recipient will have to comply with some administrative procedures with the ITA
in order to receive a refund of any excess tax withheld.
A foreign resident who had
income from a dividend that was accrued from Israeli source, from which the full tax was deducted, will be exempt from filing a tax return in Israel, unless he is liable to additional Surtax (see below) in accordance with section 121B
of the Ordinance.
Dividend distribution by a Preferred Technology Enterprise or a Special Preferred Technology Enterprise is subject to
beneficial withholding tax rates. For a further discussion, see “Taxation and Government Programs —Israeli Tax Considerations—Law
for the Encouragement of Capital Investments, 5719-1959—New Tax benefits Under the 2017 Amendment that Became Effective on January
1, 2017.”
Surtax
Subject to the provisions
of an applicable tax treaty, individuals who are subject to tax in Israel are also subject to an additional tax at a rate of 3% on annual
income (including, but not limited to, dividends, interest and capital gain) exceeding NIS 647,640 for 2021, which amount is linked to
the annual change in the Israeli consumer price index.
Estate
and Gift Tax
Israeli
law presently does not impose estate or gift taxes.
Foreign Exchange Regulations
Non-residents of Israel
who hold our Ordinary Shares are able to receive any dividends, and any amounts payable upon the dissolution, liquidation and winding
up of our affairs, repayable in non-Israeli currency at the rate of exchange prevailing at the time of conversion. However, Israeli income
tax is generally required to have been paid or withheld on these amounts. In addition, the statutory framework for the potential imposition
of currency exchange control has not been eliminated and may be restored at any time by administrative action.
Material
U.S. Federal Income Tax Consequences to U.S. Holders
The following discussion describes
the material U.S. federal income tax consequences relating to the ownership and disposition of our Ordinary Shares by U.S. Holders
(as defined below). This discussion applies to U.S. Holders that purchase Ordinary Shares pursuant to this offering and hold such
Ordinary Shares as capital assets within the meaning of Section 1221 of the U.S. Internal Revenue Code of 1986, as amended (the
“Code”). This discussion is based on the Code, U.S. Treasury regulations promulgated thereunder and administrative and judicial
interpretations thereof, all as in effect on the date hereof and all of which are subject to change, possibly with retroactive effect.
This discussion does not address all of the U.S. federal income tax consequences that may be relevant to specific U.S. Holders in light
of their particular circumstances or to U.S. Holders subject to special treatment under U.S. federal income tax law (such as certain
financial institutions, insurance companies, broker-dealers and traders in securities or other persons that generally mark their securities
to market for U.S. federal income tax purposes, tax-exempt entities, retirement plans, regulated investment companies, real estate investment
trusts, certain former citizens or residents of the United States, persons who hold Ordinary Shares as part of a “straddle,”
“hedge,” “conversion transaction,” “synthetic security” or integrated investment, persons who received
their Ordinary Shares as compensatory payments, persons that have a “functional currency” other than the U.S. dollar,
persons that own directly, indirectly or through attribution 10% or more of our shares by vote or value, persons who are subject to Section
451(b) of the Code, corporations that accumulate earnings to avoid U.S. federal income tax, partnerships and other pass-through entities
and arrangements that are classified as partnerships for U.S. federal income tax purposes, and investors in such pass-through entities).
This discussion does not address any U.S. state or local or non-U.S. tax consequences or any U.S. federal estate, gift or alternative
minimum tax consequences.
As
used in this discussion, the term “U.S. Holder” means a beneficial owner of Ordinary Shares that is, for U.S. federal
income tax purposes, (1) an individual who is a citizen or resident of the United States, (2) a corporation (or entity treated as a corporation
for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof, or the District
of Columbia, (3) an estate the income of which is subject to U.S. federal income tax regardless of its source or (4) a trust (x) with
respect to which a court within the United States is able to exercise primary supervision over its administration and one or more United
States persons have the authority to control all of its substantial decisions or (y) that has elected under applicable U.S. Treasury
regulations to be treated as a domestic trust for U.S. federal income tax purposes.
If an entity or arrangement
treated as a partnership for U.S. federal income tax purposes holds Ordinary Shares, the U.S. federal income tax consequences
relating to an investment in the Ordinary Shares will depend in part upon the status and activities of such entity or arrangement
and the particular partner. Any such entity or arrangement should consult its own tax advisor regarding the U.S. federal income tax consequences
applicable to it and its partners of the purchase, ownership and disposition of Ordinary Shares.
Persons considering an investment
in Ordinary Shares should consult their own tax advisors as to the particular tax consequences applicable to them relating to
the purchase, ownership and disposition of Ordinary Shares, including the applicability of U.S. federal, state and local tax laws
and non-U.S. tax laws.
Passive
Foreign Investment Company Consequences
In
general, a corporation organized outside the United States will be treated as a passive foreign investment company, or PFIC, for
any taxable year in which either (1) at least 75% of its gross income is “passive income”, the PFIC income test, or
(2) on average at least 50% of its assets, determined on a quarterly basis, are assets that produce passive income or are held
for the production of passive income, the PFIC asset test. Passive income for this purpose generally includes, among other things,
dividends, interest, royalties, rents, and gains from the sale or exchange of property that gives rise to passive income. Assets
that produce or are held for the production of passive income generally include cash, even if held as working capital or raised
in a public offering, marketable securities, and other assets that may produce passive income. Generally, in determining whether
a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly
or indirectly, at least a 25% interest (by value) is taken into account.
Our
status as a PFIC will depend on the nature and composition of our income and the nature, composition and value of our assets (which,
assuming we are not a “controlled foreign corporation,” or a CFC, under Section 957(a) of the Internal Revenue Code of 1986,
as amended, or the Code, for the year being tested, may be determined based on the fair market value of each asset, with the value of
goodwill and going concern value being determined in large part by reference to the market value of our Ordinary Shares, which
may be volatile). Based upon the value of our assets, including any goodwill and the nature and composition of our income and assets,
we do not believe that we were classified as a PFIC for the taxable year ended December 31, 2018 and we do not believe that we will be
classified as a PFIC for the taxable year ending December 31, 2019 or in the immediately foreseeable future. Even if we determine that
we are not a PFIC for a taxable year, there can be no assurance that the IRS will agree with our conclusion and that the IRS would not
successfully challenge our position. Our status as a PFIC is a fact-intensive determination made on an annual basis after the end of
each taxable year. Accordingly, our U.S. counsel expresses no opinion with respect to our PFIC status for our taxable year ended December
31, 2018, and also expresses no opinion with regard to our expectations regarding our PFIC status in the future.
If we are a PFIC in any taxable
year during which a U.S. Holder owns Ordinary Shares, the U.S. Holder could be liable for additional taxes and interest charges
under the “PFIC excess distribution regime” upon (1) a distribution paid during a taxable year that is greater than 125%
of the average annual distributions paid in the three preceding taxable years, or, if shorter, the U.S. Holder’s holding period
for the Ordinary Shares, and (2) any gain recognized on a sale, exchange or other disposition, including a pledge, of the Ordinary
Shares, whether or not we continue to be a PFIC. Under the PFIC excess distribution regime, the tax on such distribution or gain
would be determined by allocating the distribution or gain ratably over the U.S. Holder’s holding period for Ordinary Shares.
The amount allocated to the current taxable year (i.e., the year in which the distribution occurs or the gain is recognized) and any
year prior to the first taxable year in which we are a PFIC will be taxed as ordinary income earned in the current taxable year. The
amount allocated to other taxable years will be taxed at the highest marginal rates in effect for individuals or corporations, as applicable,
to ordinary income for each such taxable year, and an interest charge, generally applicable to underpayments of tax, will be added to
the tax.
If we are a PFIC for any year
during which a U.S. Holder holds Ordinary Shares, we must generally continue to be treated as a PFIC by that holder for all succeeding
years during which the U.S. Holder holds the Ordinary Shares, unless we cease to meet the requirements for PFIC status and the
U.S. Holder makes a “deemed sale” election with respect to the Ordinary Shares. If the election is made, the U.S.
Holder will be deemed to sell the Ordinary Shares it holds at their fair market value on the last day of the last taxable year
in which we qualified as a PFIC, and any gain recognized from such deemed sale would be taxed under the PFIC excess distribution regime.
After the deemed sale election, the U.S. Holder’s Ordinary Shares would not be treated as shares of a PFIC unless we subsequently
become a PFIC.
If we are a PFIC for any taxable
year during which a U.S. Holder holds Ordinary Shares and one of our non-U.S. corporate subsidiaries is also a PFIC (i.e., a lower-tier
PFIC), such U.S. Holder would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC and would be
taxed under the PFIC excess distribution regime on distributions by the lower-tier PFIC and on gain from the disposition of shares of
the lower-tier PFIC even though such U.S. Holder would not receive the proceeds of those distributions or dispositions. Each U.S. Holder
is advised to consult its tax advisors regarding the application of the PFIC rules to our non-U.S. subsidiaries.
If we are a PFIC, a U.S. Holder
will not be subject to tax under the PFIC excess distribution regime on distributions or gain recognized on Ordinary Shares if
such U.S. Holder makes a valid “mark-to-market” election for our Ordinary Shares. A mark-to-market election is available
to a U.S. Holder only for “marketable stock.” Our Ordinary Shares will be marketable stock as long as they remain
listed on The Nasdaq Capital Market and are regularly traded, other than in de minimis quantities, on at least 15 days during
each calendar quarter. If a mark-to-market election is in effect, a U.S. Holder generally would take into account, as ordinary income
for each taxable year of the U.S. holder, the excess of the fair market value of Ordinary Shares held at the end of such taxable
year over the adjusted tax basis of such Ordinary Shares. The U.S. Holder would also take into account, as an ordinary loss each
year, the excess of the adjusted tax basis of such Ordinary Shares over their fair market value at the end of the taxable year,
but only to the extent of the excess of amounts previously included in income over ordinary losses deducted as a result of the mark-to-
market election. The U.S. Holder’s tax basis in Ordinary Shares would be adjusted to reflect any income or loss recognized
as a result of the mark- to-market election. Any gain from a sale, exchange or other disposition of Ordinary Shares in any taxable
year in which we are a PFIC would be treated as ordinary income and any loss from such sale, exchange or other disposition would be treated
first as ordinary loss (to the extent of any net mark-to-market gains previously included in income) and thereafter as capital loss.
A mark-to-market election will
not apply to Ordinary Shares for any taxable year during which we are not a PFIC, but will remain in effect with respect to any
subsequent taxable year in which we become a PFIC. Such election will not apply to any non-U.S. subsidiaries that we may organize or
acquire in the future. Accordingly, a U.S. Holder may continue to be subject to tax under the PFIC excess distribution regime with respect
to any lower-tier PFICs that we may organize or acquire in the future notwithstanding the U.S. Holder’s mark-to-market election
for the Ordinary Shares.
The tax consequences that would
apply if we are a PFIC would also be different from those described above if a U.S. Holder were able to make a valid qualified electing
fund, or QEF, election. At this time, we do not expect to provide U.S. Holders with the information necessary for a U.S. Holder to make
a QEF election. Prospective investors should assume that a QEF election will not be available.
Each
U.S. person that is an investor of a PFIC is generally required to file an annual information return on IRS Form 8621 containing
such information as the U.S. Treasury Department may require. The failure to file IRS Form 8621 could result in the imposition
of penalties and the extension of the statute of limitations with respect to U.S. federal income tax.
The U.S. federal income
tax rules relating to PFICs are very complex. Prospective U.S. investors are strongly urged to consult their own tax advisors with respect
to the impact of PFIC status on the purchase, ownership and disposition of Ordinary Shares, the consequences to them of an investment
in a PFIC, any elections available with respect to the Ordinary Shares and the IRS information reporting obligations with
respect to the purchase, ownership and disposition of Ordinary Shares of a PFIC.
Distributions
As described in the section
entitled “– Dividend Policy,” we do not anticipate declaring or paying dividends to holders of our Ordinary
Shares in the foreseeable future. However, if we make a distribution contrary to the expectation, subject to the discussion above
under “— Passive Foreign Investment Company Consequences,” a U.S. Holder that receives a distribution with respect
to Ordinary Shares generally will be required to include the gross amount of such distribution in gross income as a dividend when
actually or constructively received to the extent of the U.S. Holder’s pro rata share of our current and/or accumulated earnings
and profits (as determined under U.S. federal income tax principles). To the extent a distribution received by a U.S. Holder is not a
dividend because it exceeds the U.S. Holder’s pro rata share of our current and accumulated earnings and profits, it will be treated
first as a tax-free return of capital and reduce (but not below zero) the adjusted tax basis of the U.S. Holder’s Ordinary Shares.
To the extent the distribution exceeds the adjusted tax basis of the U.S. Holder’s Ordinary Shares, the remainder will be
taxed as capital gain. Because we may not account for our earnings and profits in accordance with U.S. federal income tax principles,
U.S. Holders should expect all distributions to be reported to them as dividends.
Distributions on Ordinary
Shares that are treated as dividends generally will constitute income from sources outside the United States for foreign tax credit
purposes and generally will constitute passive category income. Subject to certain complex conditions and limitations, Israeli taxes
withheld on any distributions on Ordinary Shares may be eligible for credit against a U.S. Holder’s federal income tax liability.
The rules relating to the determination of the U.S. foreign tax credit are complex, and U.S. Holders should consult their tax advisors
regarding the availability of a foreign tax credit in their particular circumstances and the possibility of claiming an itemized deduction
(in lieu of the foreign tax credit) for any foreign taxes paid or withheld.
Dividends paid by a “qualified
foreign corporation” are eligible for taxation to non-corporate U.S. Holders at a reduced capital gains rate rather than the marginal
tax rates generally applicable to ordinary income provided that certain requirements are met. Each U.S. Holder is advised to consult
its tax advisors regarding the availability of the reduced tax rate on dividends with regard to its particular circumstances. Each U.S.
Holder is advised to consult its tax advisors regarding the availability of the reduced tax rate on dividends with regard to its particular
circumstances. Distributions on Ordinary Shares that are treated as dividends generally will not be eligible for the “dividends
received” deduction generally allowed to corporate shareholders with respect to dividends received from U.S. corporations.
A non-United States corporation
(other than a corporation that is classified as a PFIC for the taxable year in which the dividend is paid or the preceding taxable year)
generally will be considered to be a qualified foreign corporation (a) if it is eligible for the benefits of a comprehensive tax treaty
with the United States which the Secretary of Treasury of the United States determines is satisfactory for purposes of this provision
and which includes an exchange of information provision, or (b) with respect to any dividend it pays on shares that are readily tradable
on an established securities market in the United States. Our Ordinary Shares will generally be considered to be readily tradable
on an established securities market in the United States if they are listed on The Nasdaq Capital Market, as we intend our Ordinary
Shares will be. We believe that we qualify as a resident of Israel for purposes of, and are eligible for the benefits of, the U.S.-Israel
Double Tax Treaty, although there can be no assurance in this regard. Further, the IRS has determined that the U.S.-Israel Double Tax
Treaty is satisfactory for purposes of the qualified dividend rules and that it includes an exchange of information provision. Therefore,
subject to the discussion above under “— Passive Foreign Investment Company Consequences,” if the U.S.-Israel
Double Tax Treaty is applicable, or if our Ordinary Shares are readily tradable on an established securities market in the United
States, such dividends will generally be “qualified dividend income” in the hands of individual U.S. Holders, provided that
certain conditions are met, including holding period and the absence of certain risk reduction transaction requirements. Each U.S. Holder
is advised to consult its tax advisors regarding the availability of the reduced tax rate on dividends with regard to its particular
circumstances.
Sale, Exchange or Other Disposition of Ordinary
Shares
Subject to the discussion above
under “— Passive Foreign Investment Company Consequences,” a U.S. Holder generally will recognize capital gain
or loss for U.S. federal income tax purposes upon the sale, exchange or other disposition of Ordinary Shares in an amount equal
to the difference, if any, between the amount realized (i.e., the amount of cash plus the fair market value of any property received)
on the sale, exchange or other disposition and such U.S. Holder’s adjusted tax basis in the Ordinary Shares. Such capital
gain or loss generally will be long-term capital gain taxable at a reduced rate for non-corporate U.S. Holders or long-term capital loss
if, on the date of sale, exchange or other disposition, the Ordinary Shares were held by the U.S. Holder for more than one year.
Any capital gain of a non-corporate U.S. Holder that is not long-term capital gain is taxed at ordinary income rates. The deductibility
of capital losses is subject to limitations. Any gain or loss recognized from the sale or other disposition of Ordinary Shares
will generally be gain or loss from sources within the United States for U.S. foreign tax credit purposes.
Medicare Tax
Certain U.S. Holders that are
individuals, estates or trusts and whose income exceeds certain thresholds generally are subject to a 3.8% tax on all or a portion of
their net investment income, which may include their gross dividend income and net gains from the disposition of Ordinary Shares.
If you are a United States person that is an individual, estate or trust, you are encouraged to consult your tax advisors regarding the
applicability of this Medicare tax to your income and gains in respect of your investment in Ordinary Shares.
Information Reporting and Backup Withholding
U.S. Holders may be required
to file certain U.S. information reporting returns with the IRS with respect to an investment in Ordinary Shares, including, among
others, IRS Form 8938 (Statement of Specified Foreign Financial Assets). As described above under “Passive Foreign Investment
Company Consequences”, each U.S. Holder who is a shareholder of a PFIC must file an annual report containing certain information.
U.S. Holders paying more than US$100,000 for Ordinary Shares may be required to file IRS Form 926 (Return by a U.S. Transferor
of Property to a Foreign Corporation) reporting this payment. Substantial penalties may be imposed upon a U.S. Holder that fails to comply
with the required information reporting.
Dividends on and proceeds from
the sale or other disposition of Ordinary Shares may be reported to the IRS unless the U.S. Holder establishes a basis for exemption.
Backup withholding may apply to amounts subject to reporting if the holder (1) fails to provide an accurate United States taxpayer identification
number or otherwise establish a basis for exemption (usually on IRS Form W-9), or (2) is described in certain other categories of persons.
However, U.S. Holders that are corporations generally are excluded from these information reporting and backup withholding tax rules.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules generally will be allowed as a refund
or a credit against a U.S. Holder’s U.S. federal income tax liability if the required information is furnished by the U.S. Holder
on a timely basis to the IRS.
U.S.
Holders should consult their own tax advisors regarding the backup withholding tax and information reporting rules.
EACH
PROSPECTIVE INVESTOR IS URGED TO CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO IT OF AN INVESTMENT IN ORDINARY SHARES IN
LIGHT OF THE INVESTOR’S OWN CIRCUMSTANCES.
LEGAL
MATTERS
No expert or counsel named
in this prospectus as having prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities
being registered or upon other legal matters in connection with the registration or offering of the Ordinary Shares was employed on a
contingency basis or had, or is to receive, in connection with the offering, a substantial interest, directly or indirectly, in the registrant
or its subsidiary. Nor was any such person connected with the Registrant or any of its parents, subsidiaries as a promoter, managing
or principal underwriter, voting trustee, Director, officer or employee.
The
validity of our ordinary shares registered hereby and certain other matters of Israeli law will be passed upon for us by Rimon
PC and certain matters of U.S. federal law will be passed upon for us by Sheppard, Mullin, Richter & Hampton LLP, New York,
New York.
EXPERTS
The
consolidated financial statements as of December 31, 2020 and 2019 and for each of the years in the two-year period ended December 31,
2020 included in this prospectus and elsewhere in the registration statement have been so included in reliance upon the report
of Fahn Kanne & Co., our former independent registered public accountants, upon the authority of said firm as experts
in accounting and auditing.
The
unaudited condensed consolidated financial statements as of September 30, 2021 and September 30, 2020 have been prepared by Yarel + Partners,
CPA, independent registered public accountants, upon the authority of said firm as experts in accounting and auditing.
EXPENSES
OF THIS OFFERING
The
estimated expenses payable by us in connection with the offering described in this prospectus will be as set forth in
the table below. With the exception of the U.S. Securities and Exchange Commission registration fee, all amounts are estimates. All such
expenses will be borne by the Registrant.
|
Item
|
|
Amount
to be Paid
|
|
|
SEC registration fee
|
|
$
|
1,783
|
|
|
Legal fees and expenses
|
|
|
50,000
|
|
|
Accounting fees and expenses
|
|
|
45,000
|
|
|
Miscellaneous expenses
|
|
|
2,000
|
|
|
Total
|
|
$
|
98,783
|
|
WHERE
YOU CAN FIND MORE INFORMATION
We
have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the securities offered by
this prospectus. This prospectus, which is part of our registration statement on Form S-1, omits certain non-material information,
exhibits, schedules and undertakings set forth in the registration statement. For further information about us and the securities
offered by this prospectus, please refer to the registration statement. You may access copies at the SEC’s website (www.sec.gov).
We
are subject to the information reporting requirements of the Exchange Act applicable to U.S. domestic issuers and, as such, file
annual, quarterly and current reports, proxy statements and other information with the SEC. The SEC’s website (www.sec.gov)
also contains reports, proxy statements and other information regarding issuers, such as us, that file electronically with the
SEC. We also maintain a website (www.todosmedical.com), from which you can access such reports and other information free of charge
as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. Information contained
on, or that can be accessed through, our website does not constitute a part of this prospectus.
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
Indemnification
of Directors, Officers, Employees and Agents
Under
the Companies Law, a company may not exculpate an office holder from liability for a breach of the duty of loyalty. A company
may exculpate an office holder in advance from liability to the company, in whole or in part, for damages caused to the company
as a result of a breach of the duty of care but only if a provision authorizing such exculpation is included in its articles of
association. Our amended and restated articles of association include such a provision. An Israeli company may not exculpate a
director from liability arising out of a breach of the duty of care with respect to a dividend or distribution to shareholders.
Under
the Companies Law and the Securities Law, 5728—1968, or the Securities Law, a company may indemnify an office holder in
respect of the following liabilities, payments and expenses incurred for acts performed as an office holder, either pursuant to
an undertaking made in advance of an event or following an event, provided a provision authorizing such indemnification is contained
in its articles of association:
|
|
●
|
a
monetary liability incurred by or imposed on him or her in favor of another person pursuant to a judgment, including a settlement
or arbitrator’s award approved by a court. However, if an undertaking to indemnify an office holder with respect to
such liability is provided in advance, then such undertaking must be limited to certain events which, in the opinion of the
board of directors, can be foreseen based on the company’s activities when the undertaking to indemnify is given, and
to an amount or according to criteria determined by the board of directors as reasonable under the circumstances, and such
undertaking shall detail the foreseen events and described above amount or criteria;
|
|
|
|
|
|
|
●
|
reasonable
litigation expenses, including reasonable attorneys’ fees, incurred by the office holder as (1) a result of an investigation
or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided
that (i) no indictment was filed against such office holder as a result of such investigation or proceeding; and (ii) no financial
liability was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation or proceeding
or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal
intent; or (2) in connection with a monetary sanction; a monetary liability imposed on him or her in favor of an injured party
at an Administrative Procedure (as defined below) pursuant to Section 52(54)(a)(1)(a) of the Securities Law;
|
|
|
|
|
|
|
●
|
expenses
incurred by an office holder in connection with an Administrative Procedure under the Securities Law, including reasonable
litigation expenses and reasonable attorneys’ fees; and
|
|
|
|
|
|
|
●
|
reasonable
litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings instituted
against him or her by the company, on its behalf or by a third party or in connection with criminal proceedings in which the
office holder was acquitted or as a result of a conviction for an offense that does not require proof of criminal intent.
|
“Administrative
Procedure” is defined as a procedure pursuant to chapters H3 (Monetary Sanction by the Israeli Securities Authority), H4
(Administrative Enforcement Procedures of the Administrative Enforcement Committee) or I1 (Arrangement to prevent Procedures or
Interruption of procedures subject to conditions) to the Securities Law.
Under
the Companies Law and the Securities Law, a company may insure an office holder against the following liabilities incurred for
acts performed by him or her as an office holder if and to the extent provided in the company’s articles of association:
|
|
●
|
a
breach of duty of care to the company or to a third party, to the extent such a breach arises out of the negligent conduct
of the office holder;
|
|
|
|
|
|
|
●
|
a
breach of duty of loyalty to the company, provided that the office holder acted in good faith and had a reasonable basis to
believe that the act would not harm the company;
|
|
|
|
|
|
|
●
|
a
monetary liability imposed on the office holder in favor of a third party;
|
|
|
|
|
|
|
●
|
a
monetary liability imposed on the office holder in favor of an injured party at an Administrative Procedure pursuant to Section
52(54)(a)(1)(a) of the Securities Law; and
|
|
|
|
|
|
|
●
|
expenses
incurred by an office holder in connection with an Administrative Procedure, including reasonable litigation expenses and
reasonable attorneys’ fees.
|
|
|
Under
the Companies Law, a company may not indemnify, exculpate or insure an office holder against any of the following:
|
|
|
|
|
|
|
●
|
a
breach of duty of loyalty, except for indemnification and insurance for a breach of the duty of loyalty to the company to
the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm the
company;
|
|
|
|
|
|
|
●
|
a
breach of the duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct
of the office holder;
|
|
|
|
|
|
|
●
|
an
act or omission committed with intent to derive illegal personal benefit; or
|
|
|
|
|
|
|
●
|
a
fine or forfeit levied against the office holder.
|
Under
the Companies Law, exculpation, indemnification and insurance of office holders must be approved by the compensation committee
and the board of directors and, with respect to certain office holders or under certain circumstances, also by the shareholders.
Our
amended and restated articles of association permit us to, exculpate, indemnify and insure our office holders as permitted under
the Companies Law. Our office holders are currently covered by a directors and officers’ liability insurance policy. As
of the date of this registration statement, no claims for directors’ and officers’ liability insurance have been filed
under this policy, we are not aware of any pending or threatened litigation or proceeding involving any of our directors or officers
in which indemnification is sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification
by any director or officer.
Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons
controlling the registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the Securities
and Exchange Commission such indemnification is against public policy as expressed in the Act and is therefore unenforceable.
Recent
Sales of Unregistered Securities
Set
forth below are the sales of all securities of the registrant sold by the registrant within the past three years which were not
registered under the Securities Act. All such sales were made under either Section 4(a)(2) of the Securities Act or in reliance
upon the exemption contained in Regulation S under the Securities Act:
On
November 18, 2018, the Company signed a share purchase agreement with an investor for an investment of $100,000 in exchange for the issuance
of 800,000 Ordinary Shares and 600,000 warrants with a three year exercise period at an exercise price of the lower of $0.125
or the lowest price during the 5 trading days before the exercise notice.
On February 27, 2019, we entered
into a joint venture agreement with Amarantus Bioscience Holdings, Inc. (“Amarantus”) pursuant to which we issued Ordinary
Shares representing 19.99% of the then-issued and outstanding Ordinary Shares of our Company to Amarantus, in exchange for Amarantus
transferring to us 19.99% of Breakthrough, which was then a wholly-owned subsidiary of Amarantus, and for Amarantus assigning its amended
and restated license agreement with the University of Leipzig for an exclusive license to develop and commercialize the LymPro Test®,
an immune-based neurodiagnostic blood test for the detection of Alzheimer’s disease (the “License”). On April 14, 2019,
we notified Amarantus of the Company’s decision to exercise its option to acquire the remaining 80.01% of Breakthrough held by
Amarantus in exchange for the issuance to Amarantus of Ordinary Shares of the Company representing an additional thirty percent (30%)
of the Company’s then-issued and outstanding share capital, such that the Company would own 100% of Breakthrough, and Amarantus
would own 49.99% of the Company. At the annual meeting of shareholders of the Company held on April 29, 2019, the Company’s shareholders
voted on a resolution approving the Company’s exercise of this option. On July 28, 2020, the Company entered into Amendment No.
1 to the Binding Joint Venture Agreement with Amarantus pursuant to which the parties agreed that the Company would issue 49.9% of
its Ordinary Shares as of December 31, 2019 to Amarantus in exchange for the 80.01% equity interest it does not own of Breakthrough
Diagnostics, Inc. In addition, Amarantus will receive a 10% royalty on LymPro intellectual property. On July 28, 2020, the Company
exercised this option and issued an additional 67,599,796 Ordinary Shares to Amarantus.
In 2019, the Company raised $1,215,450
from the sale of convertible notes, which had an outstanding principal balance of $1,350,500. On February 27, 2019, we entered into a
convertible bridge loan agreement, and issued notes and warrants relating thereto, to obtain loans from several private lenders, including
DPH Investment Ltd., a holder of 11.5% of our shares (as of such date), to finance the Company’s activities through the consummation
of a proposed public offering and our planned uplisting to the NASDAQ Capital Market. As of April 15, 2019, we had obtained $1,010,500
in bridge loan financing, and had commitments for an additional $340,000 subject to certain milestones. The loans, which have an original
issue discount of ten percent (10%), bear interest at a flat rate of ten percent (10%) and have a maturity date six months after receipt
of the loan funds. The loans are convertible into Ordinary Shares of the Company after the maturity date at a conversion price
equal to 70% of the average closing bid price of the Company’s Ordinary Shares in the five days prior to the conversion.
In the event the Company defaults under the loan agreement, the conversion price will be reduced to 60% of the average closing bid price
of the Company’s Ordinary Shares in the 15 days prior to the conversion. In addition, each lender received a warrant to
purchase an amount of Ordinary Shares equal to 25% of the amount loaned by such lender, with the warrant exercise price to be
equal to the offering price in the proposed public offering, or, in the event its loan is converted into shares, the warrant exercise
price will be equal to the applicable closing bid price of the Company’s shares at the time of the conversion of the loan. The
warrants may be exercised only during the period beginning on the date that is six months after the date that the warrant exercise price
and the number of warrant shares are determined and ending on the date that is three years thereafter.
On March 10, 2019, we entered
into an amendment to the bridge loan agreement. The amendment provides for a 10% penalty if we repay the loan prior to the maturity date.
In addition, we agreed to grant each lender a warrant to purchase an additional amount of Ordinary Shares equal to 25% of the
amount loaned by such lender, under the same terms as the original warrant, but with a warrant exercise price equal to 150% of the closing
bid price of our shares on the day prior to the closing of the bridge loan transaction.
During the first quarter of 2021,
we redeemed the convertible notes that we issued in 2019 as described above. The warrants issued alongside those notes remain outstanding.
On February 10, 2020, the Company
entered into a Business Development Agreement (the “BDA”) with Orion Capital Advisors, LLC (“BDC”) whereby BDC
was to provide business development services to the Company which included inter alia (a) review and advice concerning
the technical design of existing and planned products or services; (b) business development assistance including terms of possible transactions
and suggestions during negotiations; (c) sales assistance through the development of business models and sales strategy; (d) advice regarding
financing, review of proposed term sheets, capitalization planning and, where appropriate, participation in negotiations; (e) strategic
consulting regarding product planning, market development, marketing and public relations; (f) consulting on corporate structure, employee
stock option structure, warrant arrangements and intellectual property planning; (g) introductions to potential strategic partners and
other alliance candidates; (h) introductions to prospective customers for the Company’s products or services.
Upon signing the BDA, the Company
issued 2,500,000 unregistered Ordinary Shares to BDC.
This Agreement expired on August
10, 2020.
Between January and March 2020,
the Company entered into six different loan agreements with Bel Har Investments Ltd. (two agreements), Ethel Zieleniec, DPH Investments
Ltd., Avner Krohn and Shmuel Rothbard. The amounts borrowed under each agreement ranged from $25,000 to $142,857. Each loan was evidenced
by a senior or a senior secured convertible promissory note bearing interest at a rate of 10% per annum, and included warrants exercisable
by the note holder for five years in amounts ranging from 1,116,070 to 4,464,281 Ordinary Shares with an exercise price of 10
cents per share. In each case, either the loan agreement or the note included registration rights. The total amount borrowed pursuant
to these six agreements was $437,857.
On July 28, 2020, the Company
held a final closing of a financing round of $2,015,000 in convertible notes in the aggregate. The Company entered into multiple securities
purchase agreements with institutional and high net worth investors who included Rotbard, Bar On, Reich, Leviston and Leonite (each as
defined below, and collectively, the “Todos Investors”) pursuant to which the Company agreed to issue to the Todos Investors
secured promissory convertible notes in an aggregate principal amount of $2,686,666 (the “Convertible Note”). The Convertible
Notes bore interest at 2% per annum. The Convertible Notes were convertible into Ordinary Shares of the Company (“Conversion
Shares”) for 40 days following the date of closing at 150% of the closing bid price of the Company’s Ordinary Shares
on such closing date. After the 40 days, the conversion price equaled the lower of (i) 60% of the lowest VWAP trading price of the Ordinary
Shares during the eleven trading days immediately prior to the date of conversion, (ii) 150% of the closing bid price of the Company’s
Ordinary Shares on such closing date and (iii) 150% of the closing bid price on the date of effectiveness of the Company’s
registration statement covering the converted shares. A total of $2,000,000 was disbursed to the Company as a result of the issuance
of the Convertible Notes. In addition, the Company issued to Leviston and Leonite 4,000,000 and 3,333,333 shares, respectively as a commitment
fee (the “Commitment Shares”), and issued to Leviston an additional 2,000,000 shares as a diligence fee (the “Diligence
Shares”). The Company also issued warrants to the Todos Investors to purchase up to an aggregate 23,500,000 shares (the “Warrant
Shares”) at an exercise price of $0.10 per share exercisable at any time until expiration dates ranging from July 9, 2025 to July
28, 2025. Pursuant to a Registration Rights Agreement, the Company agreed to file within 17 days after the closing date, a registration
statement on Form F-1 registering for resale the Conversion Shares, Commitment Shares, Diligence Shares and the Warrant Shares. The Company
agreed to use its reasonable best efforts to cause the registration statement to be effective within 90 days after the closing date.
On June 15, 2020 (the “Issuance
Date”), the Company issued a convertible note in the original principal amount of $375,000 (the “Rotbard Note”) to Mr.
Shmuel Rotbard (“Rotbard”), a resident of Israel, in a transaction that is exempt from registration under Regulation S under
the Securities Act. We received $315,000 under the Rotbard Note, which reflected an original issue discount equal to $60,000. The Rotbard
Note bears interest at a rate of 2% per annum. Both principal and interest were payable in one installment on June 15, 2021.
Rotbard was entitled, at his
option, at any time, to convert all or any amount of the principal face amount of the Rotbard Note and the accumulated interest then
outstanding into the Company’s Ordinary Shares at a price equal to 80% of the lower of (i) the lowest closing bid price
on the trading day prior to the Issuance Date or (ii) the lowest trading price of the Ordinary Shares as reported by the trading
market on which the Company’s shares are traded, for the 20 prior trading days including the day upon which a conversion notice
is received (the “Conversion Price”).
Upon the occurrence of a Sale
Event as defined in the Rotbard Note, the Company was required to, upon request of Rotbard, redeem the Rotbard Note in cash for
in an amount equal to 150% of the principal amount, plus accrued but unpaid interest through the date of redemption, or at the election
of Rotbard, Rotbard may convert the unpaid principal amount of the Rotbard Note and the unpaid interest into Ordinary Shares of
the Company at the Conversion Price immediately prior to such Sale Event.
Upon the occurrence of an Event
of Default (as defined in the Rotbard Note), the Rotbard Note would accrue interest at the lower of (i) 24% per annum or (ii) the highest
rate of interest permitted by law. In addition, the Company will be subject to the penalty fee as described in paragraph 8 of the Rotbard
Note.
On June 23, 2020, we entered
into a Securities Purchase Agreement with Daniel Reich (“Reich”) pursuant to which Reich purchased from Todos (a) a convertible
note in the original principal amount of $400,000 including an original issue discount of $100,000, and a warrant to purchase up to 3,000,000
Ordinary Shares of the Company at an exercise price of ten cents per share for a period of five years.
On June 29, 2020, we entered
into a Securities Purchase Agreement with Alexsander Shmuel Bar On (“Bar On”) pursuant to which Bar On purchased from Todos
(a) a convertible note in the original principal amount of $62,500 including an original issue discount of $12,500 and interest payable
in Ordinary Shares of Todos, and (b) a warrant to purchase up to 500,000 Ordinary Shares of the Company at an exercise
price of ten cents per share for a period of up to five years.
On July 9, 2020, we entered into
a Securities Purchase Agreement with Leviston Resources, LLC (“Leviston”) pursuant to which Leviston purchased from Todos
(a) 4,000,000 Ordinary Shares, (b) a convertible note in the original principal amount of up to $2,000,000, less an original issue
discount of up to $500,000 (with said note being the subject of future advances), and (c) warrants to purchase up to 5,000,000 Ordinary
Shares for a period of five years having an exercise price equal to the lower of (i) $0.10 and (ii) the lowest exercise price of
issued and outstanding warrants, subject to adjustment as described therein. Todos also issued an additional 2,000,000 shares to Leviston
as a diligence fee. Todos also entered into a customary registration rights agreement with Leviston, and into a Security Agreement with
Leviston, pursuant to which Leviston was granted a security interest in all of Todos’ property.
On July 17, 2020, the Company
entered into a Securities Purchase Agreement with Leonite Capital LLC (“Leonite”) pursuant to which Leonite purchased from
Todos, (a) a convertible note in the original principal amount of up to $666,667, less an original issue discount of up to $166,667 (with
said note being subject to future advances), and (b) warrants to purchase up to 5,000,000 Ordinary Shares for a period of five
years with an exercise price equal to the lower of (i) $0.10 and (ii) the lowest exercise price of issued and outstanding warrants, subject
to adjustment therein. Todos also agreed to issue an additional 3,333,333 original shares to Leonite as a commitment fee under the above-mentioned
Securities Purchase Agreement.
During the first quarter of
2021, we redeemed the convertible notes that we issued in 2020 as described above. The warrants issued alongside those notes remain outstanding.
On August 4, 2020, the Company
issued 3,500,000 Ordinary Shares to Toledo Advisors LLC in exchange for Toledo agreeing to amendments to the terms of their purchase
order financing agreement with Todos, including, among other things, a lowered fee and a loss of exclusivity on with respect to purchase
order financing.
On August 11, 2020, we filed
a registration statement on Form F-1 with respect up to 50,000,000 Ordinary Shares to be issued pursuant to a purchase agreement
with Lincoln Park Capital LLC.
On January 22, 2021, the Company
entered into a Securities Purchase Agreement (the “Yozma SPA”) with Yozma Global Genomic Fund (“Yozma”) pursuant
to which the Company agreed to issue a promissory convertible note (the “Yozma Note”) to Yozma in the principal amount of
$4,857,142.86 for proceeds of $3,400,000 (the “Transaction”). The closing took place on January 29, 2021. The Yozma Note
has a maturity date of one year from the date of issuance and pays interest at a rate of 4% per annum. The Note is convertible into Ordinary
Shares (the “Yozma Conversion Shares”) at a conversion price of $0.0599 (the “Yozma Conversion Price). In addition,
Yozma received a warrant (the “Yozma Warrant”) to purchase up to 16,956,929 Ordinary Shares (the “Yozma Warrant
Shares”) of the Company with an exercise price equal to $0.107415 per share. The Yozma Warrant is exercisable for 5 years from
the date of issuance.
On April 9, 2021, the Company
entered into a Securities Purchase Agreement with a Family Office Investor (the “Family Office”) to which the Company agreed
to issue a promissory convertible note (the “Note”, or the “Yozma Crossover Round”) to the Purchaser in the principal
amount of $5,821,428.29 for proceeds of $3,000,000 (the “Transaction”). The closing occurred on April 12, 2021. The
Note has a maturity date of one year from the date of issuance and pays interest at a rate of 4% per annum. The Note is convertible into
Ordinary Shares (the “Family Office Conversion Shares”) at a conversion price of $0.0599 (the “Conversion Price).
In addition, the Purchaser received a warrant (the “Warrant”) to purchase up to 16,000,000 Ordinary Shares (the “Family
Office Warrant Shares”) of the Company with an exercise price equal to $0.107415 per share. The Warrant is exercisable for 5 years
from the date of issuance.
On April 27, 2021, the Company
entered into a Securities Purchase Agreement (the “SPA”) with Yozma Global Genomic Fund (the “Purchaser”) pursuant
to which the Company has agreed to issue a promissory convertible note (the “Note”) to the Purchaser in the principal amount
of $4,714,285.71 for proceeds of $3,300,000 (the “Transaction”). The Note has a maturity date of one year from the date
of issuance and pays interest at a rate of 4% per annum. The Note is convertible into Ordinary Shares (the “Conversion Shares”)
at a conversion price of $0.0599 (the “Conversion Price). In addition, the Purchaser received a warrant (the “Warrant”)
to purchase up to 16,458,196 Ordinary Shares (the “Warrant Shares”) of the Company with an exercise price equal to $0.107415
per share. The Warrant is exercisable for 5 years from the date of issuance.
On July 7, 2021, the Company
entered into a Securities Purchase Agreement (the “SPA”) with an institutional investor (the “Purchaser”) pursuant
to which the Company has agreed to issue a promissory convertible note (the “Note”) to the Purchaser in the principal amount
of $1,535,714 for proceeds of $1,075,000 (the “Transaction”). The closing occurred on July 7, 2021 (the “Closing Date”).
The Note has a maturity date of one year from the date of issuance and pays interest at a rate of 4% per annum. The Note is convertible
into Ordinary Shares (the “Conversion Shares”) at a conversion price of $0.0599 (the “Conversion Price). In addition,
the Purchaser received a warrant (the “Warrant”) to purchase up to 3,440,000 Ordinary Shares (the “Warrant Shares”)
of the Company with an exercise price equal to $0.107415 per share. The Warrant is exercisable for 5 years from the date of issuance.
From the Closing Date until 180 days thereafter, the Company shall be restricted from issuing or entering into any agreement to issue
any Ordinary Shares, except under certain circumstances. This provision shall no longer be in effect if the closing sale price of the
Ordinary Shares exceeds $0.10. The Company intends to use the net proceeds for general corporate purposes.
On September 23, 2021, the
Company completed the conditions precedent required to enter into a Securities Purchase Agreement (the “SPA”) with an institutional
investor (the “Purchaser”) pursuant to which the Company issued a promissory convertible note (the “Note”) to
the Purchaser in the principal amount of $2,285,142.86 for proceeds of $2,000,000 (the “Transaction”). The Note has a maturity
date of one year from the date of issuance and pays interest at a rate of 4% per annum. The Note is convertible into Ordinary Shares
(the “Conversion Shares”) at a conversion price of $0.0599 (the “Conversion Price). In addition, the Purchaser received
a warrant (the “Warrant”) to purchase up to 11,924,636 shares of Ordinary shares (the “Warrant Shares”) of the
Company with an exercise price equal to $0.107415 per share. The Warrant is exercisable for 5 years from the date of issuance. The Company
intends to use the net proceeds from this Note to initiate Phase 2/3 trials for Tollovir™ COVID-19 patients, initiate digital marketing
for its dietary supplement Tollovid®, increase sales & marketing for Provista Diagnostics, and for general corporate purposes.
Univest Securities, LLC acted
as placement agent for the offering.
On April 19, 2021, the Company
entered into an Agreement to Purchase Provista Diagnostics, Inc. (“Agreement to Purchase”) with Strategic Investment Holdings,
LLC (“SIH”), Ascenda BioSciences LLC (“Ascenda”) and Provista Diagnostics, Inc. (“Provista”). Ascenda
was the sole owner of the outstanding securities of Provista and SIH is the sole owner of all the outstanding securities of Ascenda.
Pursuant to the Agreement
to Purchase, the Company acquired Provista from Ascenda and SIH for an aggregate purchase price of $7.5 million consisting of an initial
cash payment of $1.25 million, the issuance of $1.5 million in Ordinary Shares of the Company priced at $0.0512 per share, the issuance
of a $3.5 million convertible promissory note dated April 19, 2021 (the “Note”) and the payment on for before July 1, 2021
of $1.25 million in cash (the “July Payment”). The Provista shares acquired by the Company remained in an escrow account
until the July Payment is made. The Note has a maturity date of April 8, 2025 and is convertible beginning on October 20, 2021 into Ordinary
Shares of the Company at a conversion price equal to the lesser of $0.05 or the volume weighted average price of the last 20 trading
days for the Ordinary Shares prior to the date of conversion. In the event that the Company uplists its Ordinary Shares to a national
securities exchange, the Note shall automatically be exchanged into preferred stock with a conversion price equal to the lesser of (a)
$0.05, (b) the opening price on the day of the uplisting provides there is no transaction associated with the uplisting or (c) the deal
price of an uplisting transaction. The Company’s obligation to deliver the July Payment by July 1, 2021 is secured by the Provista
shares through a Security Agreement dated as of April 19, 2021 by and between SIH, Ascenda and Provista. The Company had the option of
extending the payment of the July Payment to July 15, 2021 by paying SIH and Ascenda $250,000 on or before July 1, 2021 (the “Extension
Payment”). In the event the Company paid the July Payment by July 15, 2021, the Extension Payment would be credited towards the
July Payment. The Company paid the Extension Payment and paid the July payment in a timely manner.
On September 23, 2021, the
Company completed the conditions precedent required to enter into a Securities Purchase Agreement (the “SPA”) with Mercer
Street Global Opportunity Fund, LLC (the “Purchaser”) pursuant to which the Company issued a promissory convertible note
(the “Note”) to the Purchaser in the principal amount of $2,285,142.86 for proceeds of $2,000,000 (the “Transaction”).
The Note has a maturity date of one year from the date of issuance and pays interest at a rate of 4% per annum. The Note is convertible
into Ordinary Shares of the Company (the “Conversion Shares”) at a conversion price of $0.0599 (the “Conversion Price).
In addition, the Purchaser received a warrant (the “Warrant”) to purchase up to 11,924,636 Ordinary Shares (the “Warrant
Shares”) of the Company with an exercise price equal to $0.107415 per share. The Warrant is exercisable for 5 years from the date
of issuance. The Company intends to use the net proceeds from this Note to initiate Phase 2/3 trials for Tollovir™ COVID-19 patients,
initiate digital marketing for its dietary supplement Tollovid®, increase sales & marketing for Provista Diagnostics, and for
general corporate purposes.
Univest Securities, LLC
acted as placement agent for the offering.
This registration statement
registers for resale the Conversion Shares and the Warrant Shares (the “Registration Statement). Subsequent to the effective date
of the Registration Statement, if the closing sale price of the Ordinary shares averages less than the then Conversion Price over a period
of ten (10) consecutive trading days, the Conversion Price shall reset to such average price. If the 10 day volume weighted average
price of the Ordinary shares continues to be less than the Conversion Price then the Conversion Price should reset to such 10-day average
price with a maximum of a 20% discount from the initial Conversion Price.
The foregoing descriptions
of the SPA, the Note and the Warrant do not purport to be complete and are qualified in their entirety by the full text of the SPA, Note
and Warrant, forms of which are attached as Exhibit 10.1, 10.2 and 10.3, respectively, to the Company’s Current Report on Form
8-K filed September 24, 2021.
On October 21, 2021, Todos
Medical Ltd. (the “Company”) entered into a Securities Purchase Agreement (the “SPA”) with Kips Bay Select LP
(the “Purchaser”) pursuant to which the Company has agreed to issue a promissory convertible note (the “Note”)
to the Purchaser in the principal amount of $1,428,571.43 for proceeds of $1,000,000 (the “Transaction”). The closing occurred
on October 22, 2021 (the “Closing Date”). The Note has a maturity date of one year from the date of issuance and pays
interest at a rate of 4% per annum. The Note is convertible into shares of Common Stock (the “Conversion Shares”) at a conversion
price of $0.0599 (the “Conversion Price). In addition, the Purchaser received a warrant (the “Warrant”) to purchase
up to 3,440,000 shares of Common Stock (the “Warrant Shares”) of the Company with an exercise price equal to $0.107415 per
share. The Warrant is exercisable for 5 years from the date of issuance. The Company intends to use the net proceeds from this Note
to continue funding the ongoing Phase 2 clinical trial of Tollovir® in hospitalized COVID-19 patients, beginning the initial marketing
campaign for the cPass neutralizing antibody test launch at Provista Diagnostics and general corporate purposes.
On November 2, 2021, the Company
entered into a Securities Purchase Agreement (the “SPA”) with Leonite Fund I, LP (the “Purchaser”) pursuant to
which the Company issued a promissory convertible note (the “Note”) to the Purchaser in the principal amount of $1,503,571
for proceeds of $1,050,000 (the “Transaction”). The closing occurred on November 2, 2021 (the “Closing Date”).
The Note has a maturity date of one year from the date of issuance and pays interest at a rate of 4% per annum. The Note is convertible
into Ordinary Shares (the “Conversion Shares”) at a conversion price of $0.0599 (the “Conversion Price). In addition,
the Purchaser received a warrant (the “Warrant”) to purchase up to 5,613,334 Ordinary Shares (the “Warrant Shares”)
of the Company with an exercise price equal to $0.107415 per share. The Warrant is exercisable for 5 years from the date of issuance.
From the Closing Date until 180 days thereafter, the Company shall be restricted from issuing or entering into any agreement to issue
any Ordinary Shares, except under certain circumstances. This provision shall no longer be in effect if the closing sale price of the
Ordinary Shares exceeds $0.10. The Company intends to use the net proceeds for general corporate purposes
On November 22, 2021, the
Company entered into a Securities Purchase Agreement (the “SPA”) with T-Cell Protect Hellas S.A. (“T-Cell”) pursuant
to which the Company issued a promissory convertible note (the “Note”) to T-Cell Protect in the principal amount of €1,000,000
(the “Transaction”). The Note has a maturity date of one year from the date of issuance and pays interest at a rate of 10%
per annum. The Note is convertible into shares of Common Stock (the “Conversion Shares”) at a conversion price of $0.0599
(the “Conversion Price). At any time prior to the Company uplisting its ordinary shares to a national securities exchange, T-Cell
Protect may exchange the Note into either (a) a direct equity investment in 3CL Sciences at the same terms as a financing round of at
least $5,000,000 (the “Sub”) or (b) into a note in the Sub, bearing 10% interest that converts into direct equity in the
Sub at the same terms as a financing round of at least $5,000,000. The proceeds from this Transaction are intended to be used for the
clinical development of Tollovir, the Company’s therapeutic candidate for hospitalized COVID-19 patients.
We
claimed exemption from registration under the Securities Act for these issuances described above under Section 4(a)(2) or Regulation
S promulgated under the Securities Act, as well as, with respect to grants of share options, under Rule 701 of the Securities
Act as transactions pursuant to written compensatory plans or pursuant to a written contract relating to compensation. No form
of general solicitation or general advertising was conducted in connection with any of these sales, and no underwriters were employed.
Exhibits
and Financial Statement Schedules
(a)
Exhibits:
The
following exhibits are filed as part of this registration statement:
|
3.1
|
|
Amended and Restated Articles of Association of Todos Medical Ltd. (filed as Exhibit 99.1 to the Company’s current report on Form 6-K (File No. 333-209744) filed on March 30, 2017.
|
|
|
|
|
|
4.1
|
|
Todos
Medical Ltd. 2015 Israeli Share Option Plan (filed as Exhibit 10.7 to the Company’s registration statement on Form F-1 (File
No. 333-209744) filed on February 26, 2016.
|
|
|
|
|
|
5.1
|
|
Opinion
of Rimon, PC.
|
|
|
|
|
|
10.1
|
|
Summary
English Translation of Lease Agreement for Corporate Offices in Rehovot, Israel (filed as Exhibit 10.4 to the Company’s registration
statement on Form F-1 (File No. 333-209744) filed on February 26, 2016).
|
|
|
|
|
|
10.2
|
|
Employment
Agreement, dated March 16, 2017, between Todos Medical Singapore Pte Ltd. and Dr. Wee Yue Chew and warrant agreement, dated March
16, 2017, between Todos Medical Ltd. and Dr. Wee Yue Chew (filed as Exhibit 4.12 to Form 20-F (File No. 333-209744) filed on May
1, 2017).
|
|
|
|
|
|
10.3
|
|
Convertible Bridge Loan Agreement, dated February 27, 2019, filed as Exhibit 4.1 to the Company’s Form 6-K filed on February 28, 2019
|
|
|
|
|
|
10.4
|
|
Amendment to Convertible Bridge Loan Agreement, dated February 27, 2019, filed as Exhibit 4.1 to the Company’s Form 6-K filed on March 12, 2019
|
|
|
|
|
|
10.5
|
|
Share Purchase and Assignment of License Agreement among Todos Medical Ltd., Amarantus Bioscience Holdings, Inc., and Breakthrough Diagnostics, Inc., dated February 27, 2019, filed as Exhibit 4.4 to the Company’s Form 6-K filed on February 28, 2019
|
|
|
|
|
|
10.6
|
|
Assignment
and Loan Conversion Agreement among the Company, Adeline Holdings Ltd., Yitzhak Ostrovitzky, and Sorry Doll Ltd. and S.B.
Nihul Merkakein Ltd., dated November 28, 2018, filed as Exhibit 4.9 to the Company’s Form 20-F filed on March 28, 2019.
|
|
|
|
|
|
10.7
|
|
Marketing
and Reseller Agreement, between the Company and Care G.B. Plus Ltd., dated December 20, 2018 filed as Exhibit 4.10 to the Company’s
Form 20-F filed on March 28, 2019.
|
|
|
|
|
|
10.8
|
|
Exclusive
option agreement among the Company, Strategic Investment Holdings, LLC, Ascenda BioSciences LLC and Provista Diagnostics, Inc. dated
January 6, 2020. filed as Exhibit 10.8 to the Company’s Registration Statement on Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.9
|
|
2%
Convertible Redeemable Note made by the Company in favor of Shmuel Rotbard in the original principal amount of $375,000 dated June
15, 2020. filed as Exhibit 10.9 to the Company’s Registration Statement on Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.10
|
|
Securities
Purchase Agreement with Daniel Reich, dated June 23, 2020. filed as Exhibit 10.10 to the Company’s Registration Statement on
Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.11
|
|
Securities
Purchase Agreement with Alexsander Shmuel Bar On, dated June 29, 2020. filed as Exhibit 10.11 to the Company’s Registration
Statement on Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.12
|
|
Securities
Purchase Agreement, dated July 9, 2020, with Leviston Resources, LLC. filed as Exhibit 10.12 to the Company’s Registration
Statement on Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.13
|
|
Form
of convertible note dated July 28, 2020, between the Company and the Todos Investors. filed as Exhibit 10.13 to the Company’s
Registration Statement on Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.14
|
|
Purchase Agreement dated as of August 4, 2020 by and between Todos Medical Ltd. and Lincoln Park Capital Fund, LLC. filed as Exhibit 10.1 to the Company’s Form 6-K filed on August 6, 2020.
|
|
10.15
|
|
Registration Rights Agreement dated as of August 4, 2020 by and between Todos Medical Ltd. and Lincoln Park Capital Fund, LLC, filed as Exhibit 10.2 to the Company’s Form 6-K filed on August 6, 2020.
|
|
|
|
|
|
10.16
|
|
Research
and License Agreement with B.G. Negev Technologies and Applications Ltd. and Mor Research Applications Ltd., dated April 26, 2010,
as amended June 25, 2012 (filed as Exhibit 10.1 to the Company’s registration statement on Form F-1 (File No. 333- 209744)
filed on February 26, 2016).
|
|
|
|
|
|
10.17
|
|
Addendum
No. 2 to Research and License Agreement Dated March 19, 2017, as amended on June 25, 2012 with B.G. Negev Technologies and Applications
Ltd. and Mor Research Applications Ltd. (filed as Exhibit 4.2 to Form 20-F (File No. 333- 209744) filed on May 1, 2017).
|
|
|
|
|
|
10.18
|
|
Employment
Agreement between the Company and Dr. Herman Weiss, dated March 25, 2019, filed as Exhibit 10.11 to the Company’s Registration
Statement on Form F-1 filed on April 22, 2019.
|
|
|
|
|
|
10.19
|
|
Loan
Agreement dated March 24, 2020 by and between Todos Medical Ltd. and Ethel Zieleniec, filed as Exhibit 10.19 on the Company’s
Registration Statement on Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.20
|
|
Loan
Agreement dated February 25, 2020 by and between Todos Medical Ltd. and Ethel Zieleniec, filed as Exhibit 10.20 on the Company’s
Registration Statement on Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.21
|
|
Loan
Agreement dated January 23, 2020, by and between Todos Medical Ltd. and Bel Har Investments Ltd., filed as Exhibit 10.21 on the Company’s
Registration Statement on Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.22
|
|
Loan
Agreement dated March 23, 2020 by and between Todos Medical Ltd. and Bel Har Investments Ltd., filed as Exhibit 10.22 on the Company’s
Registration Statement on Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.23
|
|
Loan
Agreement dated March 24, 2020 by and between Todos Medical Ltd. and DPH Investments Ltd., filed as Exhibit 10.23 on the Company’s
Registration Statement on Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.24
|
|
Loan
Agreement dated March 22, 2020 by and between Todos Medical Ltd. and Avner Krohn, filed as Exhibit 10.24 on the Company’s Registration
Statement on Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.25
|
|
Loan
Agreement dated March 15, 2020 by and between Todos Medical Ltd. and Shmuel Rotbard, filed as Exhibit 10.25 on the Company’s
Registration Statement on Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.26
|
|
Form
of Loan Agreement dated March 24, 2020 by and between Todos Medical Ltd. and DPH Investments Ltd., filed as Exhibit 10.26 on the
Company’s Registration Statement on Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.27
|
|
Form
of Loan Agreement dated March 24, 2020 by and between Todos Medical Ltd. and Tehresa Yee Ling Tan, filed as Exhibit 10.27 on the
Company’s Registration Statement on Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.28
|
|
Loan
Agreement dated January 27, 2020 by and between Todos Medical Ltd. and Greentree Financial Group Inc., filed as Exhibit 10.28 on
the Company’s Registration Statement on Form F-1/A, filed on August 17, 2020.
|
|
|
|
|
|
10.29
|
|
Loan
Conversion Agreement dated May 10, 2020, by and among Todos Medical Ltd., Shmuel Mellman, Meir Ben Zur and Shay Zaga, filed as Exhibit
10.29 on the Company’s Annual Report on Form 10-K, filed on April 21, 2021.
|
|
|
|
|
|
10.30
|
|
Receivables
Financing Agreement effective as of June 19, 2020 by and among Toledo Advisors L.L.C., Corona Diagnostics LLC, Todos Medical USA,
a Nevada corporation and Todos Medical Ltd. filed as Exhibit 10.30 on the Company’s Annual Report on Form 10-K, filed on April
21, 2021.
|
|
10.31
|
|
Amendment
to Receivables Financing Agreement effective as of November 19, 2020 by and among Toledo Advisors L.L.C., Corona Diagnostics LLC,
Todos Medical USA, a Nevada corporation and Todos Medical Ltd. filed as Exhibit 10.31 on the Company’s Annual Report on Form
10-K, filed on April 21, 2021.
|
|
|
|
|
|
10.32
|
|
Secured
Convertible Equipment Loan Agreement, dated November 4, 2020, between Todos Medical Ltd. and Friends of Yeshiva Orot Hateshuva Inc.
filed as Exhibit 10.32 on the Company’s Annual Report on Form 10-K, filed on April 21, 2021.
|
|
|
|
|
|
10.33
|
|
Secured
Convertible Equipment Loan Agreement, dated December 31, 2020, between Todos Medical Ltd. and Harper Advance LLC filed as Exhibit
10.33 on the Company’s Annual Report on Form 10-K, filed on April 21, 2021.
|
|
|
|
|
|
10.34
|
|
Non-Exclusive
Distribution Agreement, dated March 17, 2020 between Todos Medical Ltd. and 3D Biomedicine Science and Technology Col. Ltd. filed
as Exhibit 10.34 on the Company’s Annual Report on Form 10-K, filed on April 21, 2021.
|
|
|
|
|
|
10.35
|
|
Medical
Device Distribution Agreement, dated June 4, 2020 between Todos Medical Ltd. and 3D Biomedicine Science and Technology Col. Ltd.
filed as Exhibit 10.35 on the Company’s Annual Report on Form 10-K, filed on April 21, 2021.
|
|
|
|
|
|
10.36
|
|
Distribution
Agreement dated June 18, 2020 between Todos Medical Ltd. and Meridian Health Services Network, Inc. filed as Exhibit 10.36 on the
Company’s Annual Report on Form 10-K, filed on April 21, 2021.
|
|
|
|
|
|
10.37
|
|
Distribution
Agreement, dated July 23, 2020, between Todos Medical Ltd. and PCL Inc. filed as Exhibit 10.37 on the Company’s Annual Report
on Form 10-K, filed on April 21, 2021.
|
|
|
|
|
|
10.38
|
|
Amendment
No. 1, dated July 28, 2020, to the Binding Joint Venture Agreement between Todos Medical Ltd. and Amarantus Bioscience Holdings,
Inc. filed as Exhibit 10.38 on the Company’s Annual Report on Form 10-K, filed on April 21, 2021.
|
|
|
|
|
|
10.39
|
|
Securities
Purchase Agreement dated as of January 22, 2021, between Todos Medical Ltd and Yozma Global Genomic Fund 1, filed as Exhibit 10.1
on the Company’s Form 8-K filed January 26, 2021.
|
|
|
|
|
|
10.40
|
|
Form
of Promissory Convertible Note issued by Todos Medical Ltd to Yozma Global Genomic Fund 1, filed as Exhibit 10.2 on the Company’s
Form 8-K filed January 26, 2021.
|
|
|
|
|
|
10.41
|
|
Form
of Ordinary Share Purchase Warrant issued by Todos Medical Ltd. to Yozma Korea Group Ltd., filed as Exhibit 10.3 on the Company’s
Form 8-K filed on January 26, 2021.
|
|
|
|
|
|
10.42
|
|
Form
of Securities Purchase Agreement, dated April 8, 2021, between Todos Medical Ltd. and the Purchaser, filed as Exhibit 10.1 on the
Company’s Form 8-K filed April 14, 2021.
|
|
|
|
|
|
10.43
|
|
Form
of Promissory Convertible Note issued by Todos Medical Ltd. to the Purchaser, filed as Exhibit 10.2 on the Company’s Form 8-K
filed April 14, 2021.
|
|
|
|
|
|
10.44
|
|
Form
of Ordinary Share Purchase Warrant issued by Todos Medical Ltd. to the Purchaser, filed as Exhibit 10.3 on the Company’s Form
8-K filed on April 14, 2021.
|
|
|
|
|
|
10.45
|
|
Agreement
to Purchase Provista Diagnostics, Inc. dated April 19, 2021, filed as Exhibit 10.1 on the Company’s Form 8-K filed on April
23, 2021.
|
|
|
|
|
|
10.46
|
|
Securities
Purchase Agreement dated April 19, 2021, filed as Exhibit 10.2 on the Company’s Form 8-K filed on April 23, 2021.
|
|
10.47
|
|
Convertible
Promissory Note dated April 19, 2021, filed as Exhibit 10.3 on the Company’s Form 8-K filed on April 23, 2021.
|
|
|
|
|
|
10.48
|
|
Security
Agreement dated April 19, 2021, filed as Exhibit 10.4 on the Company’s Form 8-K filed on April 23, 2021.
|
|
|
|
|
|
10.49
|
|
Proxy
Statement filed as Exhibit 99.1 on the Company’s Form 8-K filed on June 28, 2021.
|
|
|
|
|
|
10.50
|
|
Securities
Purchase Agreement dated as of April 27, 2021, filed as Exhibit 10.1 on the Company’s Report on Form 8-K filed on April 30,2021.
|
|
|
|
|
|
10.51
|
|
Promissory
Convertible Note dated April 2021, filed as Exhibit 10.2 on the Company’s Report on Form 8-K filed on April 30, 2021.
|
|
|
|
|
|
10.52
|
|
Ordinary
Share Purchase Warrant dated April 2021, filed as Exhibit 10.3 on the Company’s Report on Form 8-K filed on April 30, 2021.
|
|
|
|
|
|
10.53
|
|
Securities
Purchase Agreement dated as of July 7, 2021, filed as Exhibit 10.1 on the Company’s Report on Form 8-K filed on July 8, 2021.
|
|
|
|
|
|
10.54
|
|
Promissory
Convertible Note dated July 7, 2021, filed as Exhibit 10.2 on the Company’s Report on Form 8-K filed on July 8, 2021.
|
|
|
|
|
|
10.55
|
|
Ordinary
Shares Purchase Warrant dated July 7, 2021, filed as Exhibit 10.3 on the Company’s Report on Form 8-K filed on July 8, 2021.
|
|
|
|
|
|
10.56
|
|
Securities
Purchase Agreement dated as of September 15, 2021, filed as Exhibit 10.1 on the Company’s Report on Form 8-K filed on September
24, 2021.
|
|
|
|
|
|
10.57
|
|
Promissory
Convertible Note dated September 15, 2021, filed as Exhibit 10.2 on the Company’s Report on Form 8-K filed on September 24,
2021.
|
|
|
|
|
|
10.58
|
|
Ordinary
Shares Purchase Warrant dated September 15, 2021, filed as Exhibit 10.3 on the Company’s Report on Form 8-K filed on September
24, 2021.
|
|
|
|
|
|
21.1
|
|
List
of Subsidiaries (filed as Exhibit 21.1 to the Company’s annual report on Form 10-K, filed with the SEC on April 21,
2021).
|
|
|
|
|
|
23.1
|
|
Consent
of Fahn Kanne & Co. Grant Thornton Israel.
|
|
|
|
|
|
23.2
|
|
Consent
of Rimon PC (incorporated in Exhibit 5.1).
|
|
|
|
|
|
24.1
|
|
Power
of Attorney (included on signature page to registration statement).
|
(b)
Financial statement schedules
No
financial statement schedules are provided because the information called for is not applicable or is shown in the financial statements
or notes.
Undertakings.
(1)
The undersigned registrant hereby undertakes:
(a)
to file, during any period in which offers or sales are being made, a post-effective amendment to this Registration Statement:
|
(i)
|
to include any prospectus required by Section 10(a)(3) of the Securities Act;
|
|
|
|
|
(ii)
|
to
reflect in the prospectus any facts or events arising after the effective date of this Registration Statement (or the most
recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information
set forth in this Registration Statement. Notwithstanding the foregoing, any increase or decrease in volume of securities
offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from
the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities
and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than
a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table
in the effective registration statement; and
|
|
|
|
|
(iii)
|
to
include any material information with respect to the plan of distribution not previously disclosed in the Registration Statement
or any material change to such information in this Registration Statement;
|
provided,
however, that paragraphs (i), (ii) and (iii) above do not apply if the information required to be included in a post-effective
amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to
Section 13 or Section 15(d) of the Exchange Act that are incorporated by reference in this Registration Statement.
(b)
that, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed
to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time
shall be deemed to be the initial bona fide offering thereof.
(c)
to remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold
at the termination of the offering.
(d)
that, for the purpose of determining liability of a registrant under the Securities Act to any purchaser in the initial distribution
of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant
pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if
the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant
will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
|
(i)
|
any
preliminary prospectus or prospectus of the undersigned registrant to the offering required to be filed pursuant to Rule 424;
|
|
(ii)
|
any
free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred
to by an undersigned registrant;
|
|
(iii)
|
the
portion of any other free writing prospectus relating to the offering containing material information about the undersigned
registrant or its securities provided by or on behalf of the undersigned registrant; and
|
|
(iv)
|
any
other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
|
|
(2)
|
The
undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing
of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Exchange Act (and, where applicable,
each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Exchange Act) that is incorporated
by reference in this Registration Statement shall be deemed to be a new registration statement relating to the securities
offered herein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
|
|
(3)
|
Insofar
as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling
persons of the registrant pursuant to the provisions referred to in Item 15, or otherwise, the registrant has been advised
that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in
the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities
(other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the
registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling
person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter
has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification
by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
|
|
(4)
|
The
undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, the information
omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained
in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall
be deemed to be part of this Registration Statement as of the time it was declared effective.
|
|
(5)
|
The
undersigned registrant hereby undertakes that, for the purpose of determining any liability under the Securities Act, each
post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to
the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide
offering thereof.
|
Signatures
In
accordance with the requirements of the Securities Act of 1933, the Registrant certifies that it has reasonable grounds to believe
that it meets all of the requirements for filing this Form S-1 and has authorized this registration statement to be signed on its
behalf by the undersigned, thereunto duly authorized, in New York, New York and Tel Aviv, Israel on January 18, 2022.
|
|
TODOS
MEDICAL LTD.
|
|
|
(Registrant)
|
|
|
|
|
|
|
By:
|
/s/
Gerald Commissiong
|
|
|
|
Gerald
Commissiong
|
|
|
|
Chief
Executive Officer and Director
|
|
|
|
(Principal
Executive Officer)
|
|
|
|
|
|
|
By:
|
/s/
Daniel Hirsch
|
|
|
|
Chief
Financial Officer
|
|
|
|
(Principal
Financial and Accounting Officer)
|
POWER
OF ATTORNEY
We,
the undersigned, hereby severally constitute and appoint Gerald Commissiong, in his individual capacity, our true and lawful attorney-in-fact
and agent, with full power of substitution and resubstitution, for him and his name, place and stead, in any and all capacities,
to execute any and all amendments (including post-effective amendments) to this registration statement, to sign any registration
statement filed pursuant to Rule 462(b) of the Securities Act of 1933, as amended, and to cause the same to be filed with all
exhibits thereto, and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact
and agent, full power and authority to do and perform each and every act and thing requisite and desirable to be done in and about
the premises as fully and to all intents and purposes as we might or could do in person, hereby ratifying and confirming all facts
and things that said attorney-in-fact and agent, or his substitute may lawfully do or cause to be done by virtue thereof.
In
accordance with the requirements of the Securities Act of 1933, this registration statement was signed by the following persons
in the capacities and on the dates stated:
|
SIGNATURE
|
|
TITLE
|
|
DATE
|
|
|
|
|
|
|
|
/s/
Gerald Commissiong
|
|
Chief
Executive Officer (Principal
|
|
January 18, 2022
|
|
Gerald
Commissiong
|
|
Executive
Officer) and Director
|
|
|
|
|
|
|
|
|
|
/s/
Daniel Hirsch
|
|
Chief
Financial Officer (Principal
|
|
January 18, 2022
|
|
Daniel
Hirsch
|
|
Financial
and Accounting Officer) and Director
|
|
|
|
|
|
|
|
|
|
/s/
Moshe Schlisser
|
|
Director
|
|
January 18, 2022
|
|
Moshe
Schlisser
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Moshe Abramovitz
|
|
Director
|
|
January 18, 2022
|
|
Moshe
Abramovitz
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Lauren Chung
|
|
Director
|
|
January 18, 2022
|
|
Lauren
Chung
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Herman Weiss
|
|
Director
|
|
January 18, 2022
|
|
Dr.
Herman Weiss
|
|
|
|
|
SIGNATURE
OF AUTHORIZED REPRESENTATIVE IN THE UNITED STATES
Pursuant
to the Securities Act of 1933, as amended, the undersigned, the duly authorized representative in the United States of Todos Medical
Ltd., has signed this registration statement on January 18, 2022.
|
Authorized
U.S. Representative
|
|
|
|
|
|
/s/
Donald J. Puglisi
|
|
|
Managing
Director
|
|
|
Puglisi
& Associates
|
|
Todos Med (CE) (USOTC:TOMDF)
Historical Stock Chart
From Mar 2024 to Apr 2024
Todos Med (CE) (USOTC:TOMDF)
Historical Stock Chart
From Apr 2023 to Apr 2024