Form 8-K - Current report
October 16 2023 - 8:05AM
Edgar (US Regulatory)
false 0001515673 0001515673 2023-10-14 2023-10-14
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 14, 2023
Ultragenyx Pharmaceutical Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-36276 |
|
27-2546083 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer
Identification No.) |
|
|
|
|
|
| 60 Leveroni Court |
|
|
|
|
| Novato, California |
|
|
|
94949 |
| (Address of Principal Executive Offices) |
|
|
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (415) 483-8800
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, $0.001 par value |
|
RARE |
|
Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02 |
Results of Operations and Financial Condition |
The information contained in Item 7.01 under “Preliminary Third Quarter Revenue (unaudited)” and “Preliminary Third Quarter Ending Cash Position (unaudited)” is incorporated by reference herein.
The information set forth in this Item 2.02 shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, except as shall be expressly set forth by specific reference in such filing.
| Item 7.01 |
Regulation FD Disclosure. |
Data from Phase 2 portion of Phase 2/3 Orbit study of UX143
On October 14, 2023, Ultragenyx Pharmaceutical Inc. (the “Company”) issued a press release announcing interim data from the Phase 2 portion of the Phase 2/3 Orbit study of UX143. The press release is attached hereto as Exhibit 99.1.
Analyst Day Update
On October 16, 2023, the Company issued a press release providing updates on its development pipeline, including setrusumab (UX143) for osteogenesis imperfecta (“OI”), GTX-102 for Angelman syndrome (“AS”), UX701 in Wilson disease and the rest of the Company’s gene therapy portfolio to be presented at an Analyst Day held in New York City and by webcast. The press release is attached hereto as Exhibit 99.2.
Preliminary Third Quarter Revenue (unaudited)
The Company’s preliminary unaudited total revenue for the third quarter of fiscal 2023 is $96 million to $100 million, preliminary unaudited Crysvita revenue for the third quarter is $74 million to $76 million and preliminary unaudited Dojolvi revenue for the third quarter is $16 million to $17 million. Third quarter Crysvita revenue in the United States was impacted by a decrease in channel inventory related to Kyowa Kirin Co., Ltd.’s (“KKC”) change from Ultragenyx labeled product to KKC’s labeled product as part of the transition of North America commercialization responsibilities for Crysvita from the Company to KKC. This one-time change occurred in the third quarter, and the Company expects Crysvita channel inventories to increase to more normal levels at the end of the year.
Preliminary Third Quarter Ending Cash Position (unaudited)
Cash, cash equivalents, and marketable debt securities were approximately $525 million as of September 30, 2023.
These amounts are preliminary, have not been audited and are subject to change pending completion of the Company’s unaudited financial statements for the quarter ended September 30, 2023. Additional information and disclosures would be required for a more complete understanding of the Company’s financial position and results of operations as of September 30, 2023. The Company’s independent registered public accounting firm has not audited, reviewed or performed any procedures with respect to these preliminary results and, accordingly, does not express an opinion or any other form of assurance about them.
The information set forth in this Item 7.01 and in the press releases attached hereto as Exhibit 99.1 and Exhibit 99.2 shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, except as shall be expressly set forth by specific reference in any such filing.
Data from Phase 2 portion of Phase 2/3 Orbit study of UX143
On October 14, 2023, the Company and Mereo BioPharma Group plc announced interim data from the Phase 2 portion of the Phase 2/3 Orbit study demonstrating that treatment with setrusumab (UX143) significantly reduced incidence of fractures in patients with osteogenesis imperfecta (“OI”) with at least six months of follow-up and continues to demonstrate ongoing and meaningful improvements in lumbar spine bone mineral density (“BMD”). The data were presented in a late-breaker presentation at the American Society for Bone and Mineral Research (“ASBMR”) 2023 Annual Meeting.
As of the cut-off date and following at least six months of treatment with setrusumab, the annualized fracture rate across all 24 patients in the Phase 2 portion of the study was reduced by 67%. In the two years prior to treatment with setrusumab all patients experienced at least one fracture. The median annualized fracture rate of 0.72 in the two years prior to treatment was reduced to 0.00 (n=24, p=0.042) during the mean treatment duration period of nine months. Following initiation of treatment with setrusumab, 20 patients experienced no radiographic-confirmed fractures, and four patients experienced seven radiographic-confirmed fractures in five separate events. These fractures exclude fractures of the fingers, toes, skull, and face consistent with the Phase 3 study design.
The reduction in annualized fracture rates was associated with a clinically meaningful increase in BMD. At the six-month timepoint, treatment with setrusumab resulted in a mean increase in lumbar spine BMD from baseline of 13% at 20 mg/kg (n=11) and 16% at 40 mg/kg (n=8), which represents the same substantial mean improvement in Z-score of +0.85 for both dose groups at six months compared to a combined mean baseline Z-score of –1.68. The small apparent difference in BMD change from baseline is likely related to differences in patients assigned to the two treated groups. There was no statistically significant difference in BMD percent change or Z-score change from baseline between the 20 and 40 mg/kg dosing cohorts.
As of the data cut-off, there were no treatment-related serious adverse events observed in the study. Reported adverse events were generally consistent with those observed in the ASTEROID study with infusion-related events and headache determined to be the most common adverse events related to the study drug. There have been no reported hypersensitivity reactions related to setrusumab. There were no notable safety-related differences observed between dosing groups or age groups.
The Phase 3 portion of the study is currently enrolling approximately 195 patients at 50 sites across 12 countries.
Analyst Day Updates
On October 16, 2023, the Company announced the following updates on its development pipeline, including setrusumab (UX143) for OI, GTX-102 for AS, UX701 in Wilson disease and the rest of the Company’s gene therapy portfolio to be presented at an Analyst Day held in New York City and by webcast:
| |
• |
|
UX143 (setrusumab) monoclonal antibody for OI: Interim Phase 2 data from the Phase 2/3 Orbit study show statistically significant decrease in annualized fracture rates following at least six months of treatment. |
| |
• |
|
Data presented at the ASBMR 2023 Annual Meeting show that treatment with setrusumab reduced the annualized fracture rate by 67% and this reduction was associated with continuing large and meaningful improvements in BMD. |
| |
• |
|
Setrusumab was generally well tolerated with no drug related serious adverse events (“SAEs”) reported and no reports of drug-related hypersensitivity. |
| |
• |
|
The Company plans to provide updated Phase 2 data next year. |
| |
• |
|
GTX-102 antisense oligonucleotide for AS: Data from the extension cohorts in the Phase 1/2 study show clinically meaningful improvements in multiple domains. |
| |
• |
|
Quantitative data show improvements across multiple clinical domains compared to natural history data, where available, and clinical changes were associated with quantitative changes in EEG. |
| |
• |
|
Long term data showed patients who stopped and restarted treatment reacquired previously gained developmental skills when they were re-dosed with the current regimen. |
| |
• |
|
There have been no additional treatment-related SAEs, including lower extremity weakness, since November 2022. |
| |
• |
|
Data from the dose expansion cohorts on at least 20 patients who have been on therapy for at least six months is anticipated in the first half of 2024. |
| |
• |
|
UX701 AAV gene therapy for Wilson disease: Four of five patients in the lowest-dose cohort of the Phase 1/2/3 Cypress2+ study show improvements in tapering standard of care. |
| |
• |
|
Four out of five patients in the low-dose Cohort 1 have had reductions in urinary copper and are tapering off of chelators and/or zinc therapy, including two of three earlier treated patients in the Cohort that are now completely off standard therapy. |
| |
• |
|
UX701 has been generally well tolerated with no treatment-related SAEs. |
| |
• |
|
The seamless study is expected to complete dosing of all three dose cohorts in Stage 1 at the end of 2023 and these data are expected in the first half of 2024. |
| |
• |
|
The Company also provided updates on other late-stage gene therapy candidates: |
| |
• |
|
DTX401 AAV gene therapy for Glycogen Storage Disease Type Ia (GSDIa): The Phase 3 GlucoGene study was fully enrolled in the first quarter of 2023 and the Company plans to provide preliminary data in the first half of 2024. |
| |
• |
|
UX111 for Sanfilippo syndrome (MPS IIIA): The pivotal Transpher A study has been fully enrolled and the Company plans to meet with the FDA in the fourth quarter of 2023. |
| |
• |
|
DTX301 AAV gene therapy for Ornithine Transcarbamylase (OTC) Deficiency: The Phase 3 Enh3ance study is expected to complete enrollment in the first half of 2024. |
A presentation regarding the updates to GTX-102 is attached hereto as Exhibit 99.3.
Cautionary Note Regarding Forward-Looking Statements
This Current Report on Form 8-K contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of words such as, but not limited to, “anticipates,” “continue,” “will,” or other similar terms or expressions that concern the Company’s expectations, plans and intentions. Forward-looking statements include, without limitation, statements regarding its future operating results and financial performance, business plans and objectives for UX143, the clinical benefit, tolerability and safety of UX143, future clinical and regulatory developments for UX143, the clinical benefit, tolerability and safety of GTX-102, future clinical and regulatory developments for GTX-102, the clinical benefit, tolerability and safety of UX701, future clinical and regulatory developments for UX701, timing for enrollment, dosing and data for Ultragenyx’s investigational therapies and gene therapy candidates, regulatory meetings and Crysvita channel inventories. Such forward-looking statements involve substantial risks and uncertainties that could cause the Company’s clinical development programs, collaboration with third parties, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, the uncertainty of clinical drug development and unpredictability and lengthy process for obtaining regulatory approvals, risks related to serious or undesirable side effects of the Company’s product candidates, the Company’s ability to achieve its projected development goals in its expected timeframes, risks related to reliance on third party partners to conduct certain activities on the Company’s behalf, the Company’s limited experience in generating revenue from product sales, risks related to product liability lawsuits, smaller than anticipated market opportunities for the Company’s products and product candidates, manufacturing risks, competition from other therapies or products, and other matters that could affect the sufficiency of existing cash, cash equivalents and short-term investments to fund operations, the Company’s future operating results and financial performance, the timing of clinical trial activities and reporting results from same, and the availability or commercial potential of the Company’s products and drug candidates. The Company undertakes no obligation to update or revise any forward-looking statements. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to the business of the Company in general, see the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission (SEC) on August 4, 2023, and its subsequent periodic reports filed with the SEC.
| Item 9.01 |
Financial Statements and Exhibits |
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
| Date: October 16, 2023 |
|
Ultragenyx Pharmaceutical Inc. |
|
|
|
|
|
|
|
|
By: |
|
/s/ Emil D. Kakkis |
|
|
|
|
|
|
Emil D. Kakkis, M.D., Ph.D. |
|
|
|
|
|
|
President and Chief Executive Officer |
Exhibit 99.1
Ultragenyx and Mereo BioPharma Announce Interim Phase 2 Data from Phase 2/3 Orbit Study
Demonstrating Setrusumab (UX143) Significantly Reduced Fracture Rates in Patients with Osteogenesis Imperfecta (OI)
Phase 2 data
presented at ASBMR 2023 show treatment with setrusumab resulted in 67% reduction in annualized fracture rate associated with continuous and meaningful improvements in bone mineral density (BMD)
Ultragenyx hosting Analyst Day on Monday, October 16 at 8:30 a.m. ET
NOVATO, Calif., VANCOUVER, British Columbia and LONDON, UK — Oct. 14, 2023 — Ultragenyx Pharmaceutical Inc. (NASDAQ: RARE) and Mereo
BioPharma Group plc (NASDAQ: MREO) today announced interim data from the Phase 2 portion of the Phase 2/3 Orbit study demonstrating that treatment with setrusumab (UX143) significantly reduced incidence of fractures in patients with OI with
at least 6 months of follow-up and continues to demonstrate ongoing and meaningful improvements in lumbar spine bone mineral density (BMD). The data were presented in a late-breaker presentation at the
American Society for Bone and Mineral Research 2023 Annual Meeting (ASBMR).
As of the cut-off date and following
at least 6 months of treatment with setrusumab, the annualized fracture rate across all 24 patients in the Phase 2 portion of the study was reduced by 67%. In the 2 years prior to treatment with setrusumab all patients experienced at least 1
fracture. The median annualized fracture rate of 0.72 in the 2 years prior to treatment was reduced to 0.00 (n=24, p=0.042) during the mean treatment duration period of 9 months. Following initiation of treatment with setrusumab, 20 patients
experienced no radiographic-confirmed fractures, and 4 patients experienced 7 radiographic-confirmed fractures in 5 separate events. These fractures exclude fractures of the fingers, toes, skull, and face consistent with the Phase 3 study design.
“I have not yet encountered a patient with a fragility fracture while on setrusumab, and this may result from setrusumab’s effects on the
skeleton, improving the rate of new bone formation and bone quality,” said Gary Gottesman, M.D., Professor of Pediatrics and Medicine, Washington University School of Medicine. “Some of the kids feel well enough they are participating in
activities that they might normally avoid and have suffered some relatively minor fractures.”
The reduction in annualized fracture rates was associated with a clinically meaningful increase in BMD. At
the 6-month timepoint, treatment with setrusumab resulted in a mean increase in lumbar spine BMD from baseline of 13% at 20 mg/kg (n=11) and 16% at 40 mg/kg (n=8), which represents the same substantial mean
improvement in Z-score of +0.85 for both dose groups at 6 months compared to a combined mean baseline Z-score of –1.68. The small apparent difference in BMD change
from baseline is likely related to differences in patients assigned to the two treated groups. There was no statistically significant difference in BMD percent change or Z-score change from baseline between
the 20 and 40 mg/kg dosing cohorts.
“These data provide compelling evidence that improved bone mineral density, resulting from this unique mechanism
of action, reduced the risk of fractures and that treatment with setrusumab could allow patients with OI to lead much more active lives with fewer fractures,” said Eric Crombez, M.D., chief medical officer at Ultragenyx. “I want to
acknowledge the OI community and especially thank the people living with OI and their caregivers who have aided the setrusumab development program so that we may potentially offer the first approved treatment option for this severe and disabling
disease.”
As of the data cut-off, there were no treatment-related serious adverse events observed in the
study. Reported adverse events were generally consistent with those observed in the ASTEROID study with infusion-related events and headache determined to be the most common adverse events related to the study drug. There have been no
reported hypersensitivity reactions related to setrusumab. There were no notable safety-related differences observed between dosing groups or age groups.
The Phase 3 portion of the study is currently enrolling approximately 195 patients at 50 sites across 12 countries.
U.S. residents can learn more by visiting ultraclinicaltrials.com.
Analyst Day and Webcast Information
Ultragenyx will host
an Analyst Day at 8:30 a.m. ET on Monday, October 16, 2023 to discuss these data and to provide an update on the company’s development pipeline. A live video webcast of the program will be available at
https://www.webcaster4.com/Webcast/Page/359/49192.
An archived version of the remarks will also be available through the Ultragenyx website.
The Setrusumab Phase 3 Program
The global, seamless
Phase 2/3 Orbit study is evaluating the effect of setrusumab on clinical fracture rate in patients aged 5 to <26 years. In the Phase 2 portion, 24 patients were randomized 1:1 to receive setrusumab at one of two doses to determine the optimal
dosing strategy for Phase 3. The pivotal Phase 3 portion of the study will include approximately 195
patients at 50 sites across 12 countries, randomized 2:1 to receive setrusumab or placebo, with a primary efficacy endpoint of annualized clinical fracture rate, excluding fingers, toes, skull,
and face. All patients will transition to an extension period and receive open-label setrusumab after the Phase 3 primary analysis is complete.
The
global Phase 3 Cosmic study is an open-label, randomized, active-controlled study in patients aged 2 to <7 years evaluating setrusumab compared to intravenous bisphosphonates (IV-BP) therapy on reduction in
total fracture rate, including morphometric vertebral fractures. The Cosmic study will enroll approximately 65 patients at more than 20 sites across 8 countries.
About Osteogenesis Imperfecta (OI)
Osteogenesis
Imperfecta (OI) includes a group of genetic disorders impacting bone metabolism. Approximately 85% to 90% of OI cases are caused by mutations in the COL1A1 or COL1A2 genes, leading to either reduced or abnormal
collagen and changes in bone metabolism. The collagen mutations in OI can result in increased bone brittleness, which contributes to a high rate of fractures. Patients with OI also exhibit inadequate production of new bone, which leads to decreased
bone mass, bone fragility and weakness. OI can also lead to bone deformities, abnormal spine curvature, pain, decreased mobility, and short stature. No treatments are approved for OI, which affects approximately 60,000 people in the developed world.
About Setrusumab (UX143)
Setrusumab is a fully
human monoclonal antibody that inhibits sclerostin, a negative regulator of bone formation. Blocking sclerostin is expected to increase new bone formation, bone mineral density and bone strength in OI. In mouse models of OI, the use of
anti-sclerostin antibodies was shown to increase bone formation, improve bone mass to normal levels, and increase bone strength against fracture force testing to normal levels.
In 2019 Mereo BioPharma completed the Phase 2b dose-finding study (ASTEROID) for setrusumab in 112 adults with OI. The ASTEROID study
demonstrated treatment with setrusumab resulted in a clear, dose-dependent and statistically significant effect on bone formation and bone density at multiple anatomical sites among adult participants with OI.
Ultragenyx and Mereo BioPharma are collaborating on the development of setrusumab globally based on the collaboration and license agreement between the
parties. The companies have developed a comprehensive late-stage program to continue development of setrusumab in pediatric and young adult patients across OI sub-types I, III and IV.
About Ultragenyx
Ultragenyx is a biopharmaceutical company committed to bringing novel products to patients for the treatment of serious rare and ultra-rare genetic diseases.
The company has built a diverse portfolio of approved therapies and product candidates aimed at addressing diseases with high unmet medical need and clear biology for treatment, for which there are typically no approved therapies treating the
underlying disease.
The company is led by a management team experienced in the development and commercialization of rare disease therapeutics.
Ultragenyx’s strategy is predicated upon time- and cost-efficient drug development, with the goal of delivering safe and effective therapies to patients with the utmost urgency. For more information on Ultragenyx, please visit
ultragenyx.com.
About Mereo BioPharma
Mereo
BioPharma is a biopharmaceutical company focused on the development of innovative therapeutics for rare diseases. The Company has two rare disease product candidates, setrusumab for the treatment of Osteogenesis Imperfecta (OI) and alvelestat
primarily for the treatment of severe alpha-1-antitrypsin deficiency-associated lung disease (AATD-LD). The Company’s
partner, Ultragenyx Pharmaceutical, Inc., has initiated a pivotal Phase 2/3 pediatric study in young adults (5 to <26 years old) for setrusumab in OI and a Phase 3 study in pediatric patients (2 to <7 years old) in the first half of 2023. The
partnership with Ultragenyx includes potential milestone payments of up to $245 million (following the recent $9 million milestone) and royalties to Mereo on commercial sales in Ultragenyx territories. Mereo has retained EU and UK
commercial rights and will pay Ultragenyx royalties on commercial sales in those territories. Setrusumab has received orphan designation for osteogenesis imperfecta from the EMA and FDA, PRIME designation from the EMA and has pediatric disease
designation from the FDA. Alvelestat has received U.S. Orphan Drug Designation for the treatment of AATD, Fast Track designation from the FDA, and positive data were reported from a Phase 2 proof-of-concept study in North America, Europe and the UK. In addition to the rare disease programs, Mereo has two oncology product candidates in clinical development. Etigilimab (anti-TIGIT) has completed
enrollment in a Phase 1b/2 basket study evaluating its safety and efficacy in combination with an anti-PD-1 in a range of tumor types including three rare tumors and
three gynecological carcinomas—cervical, ovarian, and endometrial and is in an ongoing Phase 1b/2 investigator led study at the MD Anderson Cancer Center in clear cell ovarian cancer; navicixizumab, for the treatment of late line ovarian
cancer, has completed a Phase 1 study and has been partnered with OncXerna Therapeutics, Inc. in a global licensing agreement that includes payments of up to $300 million in milestones and royalties.
For more information on Mereo BioPharma, please visit www.mereobiopharma.com.
Ultragenyx Forward-Looking Statements and Use of Digital Media
Except for the historical information contained herein, the matters set forth in this press release, including statements related to Ultragenyx’s
expectations and projections regarding its future operating results and financial performance, business plans and objectives for UX143, expectations regarding the tolerability and safety of UX143, and future clinical and regulatory developments for
UX143 are forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve substantial risks and uncertainties that could cause
our clinical development programs, collaboration with third parties, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among
others, the uncertainty of clinical drug development and unpredictability and lengthy process for obtaining regulatory approvals, the ability of the company and Mereo BioPharma to successfully develop UX143, the company’s ability to achieve its
projected development goals in its expected timeframes, risks related to adverse side effects, risks related to reliance on third party partners to conduct certain activities on the company’s behalf, the potential for any license or
collaboration agreement, including the company’s collaboration agreement with Mereo to be terminated, smaller than anticipated market opportunities for the company’s products and product candidates, manufacturing risks, competition from
other therapies or products, and other matters that could affect sufficiency of existing cash, cash equivalents and short-term investments to fund operations, the company’s future operating results and financial performance, the timing of
clinical trial activities and reporting results from same, and the availability or commercial potential of Ultragenyx’s products and drug candidates. Ultragenyx undertakes no obligation to update or revise any forward-looking statements. For a
further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to the business of Ultragenyx in general, see Ultragenyx’s
Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC) on August 4, 2023, and its subsequent periodic reports filed with the SEC.
In addition to its SEC filings, press releases and public conference calls, Ultragenyx uses its investor relations website and social media outlets to
publish important information about the company, including information that may be deemed material to investors, and to comply with its disclosure obligations under Regulation FD. Financial and other information about Ultragenyx is routinely posted
and is accessible on Ultragenyx’s Investor Relations website (https://ir.ultragenyx.com/) and LinkedIn website
(https://www.linkedin.com/company/ultragenyx-pharmaceutical-inc-/).
Mereo BioPharma Forward-Looking Statements
This press
release contains “forward-looking statements.” All statements other than statements of historical fact contained in this press release are forward-looking statements within the meaning of Section 27A of the United States Securities
Act of 1933, as amended (the “Securities Act”), and Section 21E of the United States Securities Exchange Act of 1934, as amended (the “Exchange Act”). Forward-looking statements usually relate to future events and
anticipated revenues, earnings, cash flows or other aspects of Mereo BioPharma’s operations or operating
results. Forward-looking statements are often identified by the words “believe,” “expect,” “anticipate,” “plan,” “intend,” “foresee,”
“should,” “would,” “could,” “may,” “estimate,” “outlook” and similar expressions, including the negative thereof. The absence of these words, however, does not mean that the statements are
not forward-looking. These forward-looking statements are based on Mereo BioPharma’s current expectations, beliefs and assumptions concerning future developments and business conditions and their potential effect on Mereo. While management
believes that these forward-looking statements are reasonable as and when made, there can be no assurance that future developments affecting Mereo BioPharma will be those that it anticipates.
All of Mereo BioPharma’s forward-looking statements involve known and unknown risks and uncertainties some of which are significant or beyond its
control and assumptions that could cause actual results to differ materially from Mereo BioPharma’s historical experience and its present expectations or projections.
Such risks and uncertainties include, among others, the uncertainties inherent in the clinical development process; Mereo BioPharma’s reliance on
third parties to conduct and provide funding for its clinical trials; Mereo’s dependence on enrollment of patients in its clinical trials; and Mereo’s dependence on its key executives. You should carefully consider the foregoing factors
and the other risks and uncertainties that affect Mereo BioPharma’s business, including those described in the “Risk Factors” section of its latest Annual Report on Form 20-F, reports on Form 6-K and other documents furnished or filed from time to time by Mereo BioPharma with the Securities and Exchange Commission. Mereo BioPharma wishes to caution you not to place undue reliance on any forward-looking
statements, which speak only as of the date hereof. Mereo BioPharma undertakes no obligation to publicly update or revise any of our forward-looking statements after the date they are made, whether as a result of new information, future events or
otherwise, except to the extent required by law.
Contacts
Ultragenyx Pharmaceutical Inc.
Investors
Joshua Higa
415-475-6370
ir@ultragenyx.com
Media
Jeff Blake
415-612-7784
media@ultragenyx.com
Mereo BioPharma Group plc
Denise Scots-Knight, Chief Executive Officer
Christine Fox,
Chief Financial Officer
+44 (0)333 023 7300
Burns
McClellan (Investor Relations Advisor to Mereo)
Lee Roth +01 646-930-4406
investors@mereobiopharma.com
Exhibit 99.2

Ultragenyx Announces Program and Pipeline Updates at Analyst Day Including Interim Data from Ongoing
Studies in Osteogenesis Imperfecta (OI), Angelman Syndrome (AS) and Wilson Disease
Treatment with setrusumab (UX143) for at least 6
months resulted in 67% reduction in annualized fracture rate in patients with OI in Phase 2/3 Orbit study
Quantitative data from
the Phase 1/2 study of GTX-102 for AS show clinically meaningful improvements in multiple domains as compared to natural history
4 of 5 patients in lowest-dose cohort of Phase 1/2/3 study of UX701 in Wilson disease are tapering off of chelators and/or zinc therapy,
including 2 that are now completely off standard therapy
Ultragenyx Analyst Day live webcast available today at 8:30 a.m. ET
NOVATO, Calif. — Oct. 16, 2023 — Ultragenyx Pharmaceutical Inc. (NASDAQ: RARE) today will provide updates on its development pipeline,
including setrusumab (UX143) for osteogenesis imperfecta (OI), GTX-102 for Angelman syndrome (AS), UX701 in Wilson disease and the rest of the company’s gene therapy portfolio at an Analyst Day held in
New York City and by webcast.
“The data we are presenting today show that these investigational therapies are having meaningful clinical effects on
difficult diseases with limited or no approved treatments and are potentially transformative for people living with these diseases if proven safe and effective in Phase 3 studies,” said Emil D. Kakkis, M.D., Ph.D., chief executive officer and
president of Ultragenyx. “We also have one of the largest, late-stage gene therapy pipelines in rare disease with full end-to-end capabilities in-house that uniquely position us to deliver high quality robust commercial manufacturing at scale to support our large pipeline.”
Analyst Day Updates
UX143 (setrusumab) monoclonal
antibody for Osteogenesis Imperfecta (OI): Interim Phase 2 data from the Phase 2/3 Orbit study show statistically significant decrease in annualized fracture rates following at least 6 months of treatment
| |
• |
|
Data presented at the American Society for Bone and Mineral Research 2023 Annual Meeting (ASBMR) show that
treatment with setrusumab reduced the annualized fracture rate by 67% and this reduction was associated with continuing large and meaningful improvements in bone mineral density (BMD). |
| |
• |
|
Setrusumab was generally well tolerated with no drug related serious adverse events (SAEs) reported and no
reports of drug-related hypersensitivity. |
| |
• |
|
The company plans to provide updated Phase 2 data next year. |
GTX-102 antisense oligonucleotide for Angelman syndrome: Data from the extension cohorts in the Phase 1/2 study
show clinically meaningful improvements in multiple domains
| |
• |
|
Quantitative data show improvements across multiple clinical domains compared to natural history data, where
available, and clinical changes were associated with quantitative changes in EEG. |
| |
• |
|
Long term data showed patients who stopped and restarted treatment reacquired previously gained developmental
skills when they were re-dosed with the current regimen. |
| |
• |
|
There have been no additional treatment-related SAEs, including lower extremity weakness, since November 2022.
|
| |
• |
|
Data from the dose expansion cohorts on at least 20 patients who have been on therapy for at least 6 months is
anticipated in the first half of 2024. |
UX701 AAV gene therapy for Wilson disease: Four of five patients in the lowest-dose cohort
of the Phase 1/2/3 Cypress 2+ study show improvements in tapering standard of care
| |
• |
|
Four out of 5 patients in the low-dose Cohort 1 have had reductions in
urinary copper and are tapering off of chelators and/or zinc therapy, including 2 of 3 earlier treated patients in the Cohort that are now completely off standard therapy. |
| |
• |
|
UX701 has been generally well tolerated with no treatment-related SAEs. |
| |
• |
|
The seamless study is expected to complete dosing of all 3 dose cohorts in Stage 1 at the end of 2023 and these
data are expected in the first half of 2024. |
Company also provided update on other late-stage gene therapy candidates
| |
• |
|
DTX401 AAV gene therapy for Glycogen Storage Disease Type Ia (GSDIa): The Phase 3 GlucoGene study
was fully enrolled in the first quarter of 2023 and the company plans to provide preliminary data in the first half of 2024. |
| |
• |
|
UX111 for Sanfilippo syndrome (MPS IIIA): The pivotal Transpher A study has been fully enrolled and
the company plans to meet with the FDA in the fourth quarter of 2023. |
| |
• |
|
DTX301 AAV gene therapy for Ornithine Transcarbamylase (OTC) Deficiency: The Phase 3 Enh3ance study is expected to complete enrollment in the first half of 2024. |
Analyst Day and Webcast Information
Ultragenyx will host
an Analyst Day at 8:30 a.m. ET on Monday, October 16, 2023 to discuss these data and to provide an update on the company’s development pipeline. A live video webcast of the program will be available at
https://www.webcaster4.com/Webcast/Page/359/49192. An archived version of the remarks will also be available through the Ultragenyx website.
About Ultragenyx
Ultragenyx is a biopharmaceutical company committed to bringing novel products to patients for the treatment of serious rare and ultra-rare genetic diseases.
The company has built a diverse portfolio of approved therapies and product candidates aimed at addressing diseases with high unmet medical need and clear biology for treatment, for which there are typically no approved therapies treating the
underlying disease.
The company is led by a management team experienced in the development and commercialization of rare disease therapeutics.
Ultragenyx’s strategy is predicated upon time- and cost-efficient drug development, with the goal of delivering safe and effective therapies to patients with the utmost urgency. For more information on Ultragenyx, please visit
ultragenyx.com.
Ultragenyx Forward-Looking Statements and Use of Digital Media
Except for the historical information contained herein, the matters set forth in this press release, including statements related to Ultragenyx’s
expectations and projections regarding its future operating results and financial performance, business plans and objectives for UX143, expectations regarding the clinical benefit, tolerability and safety of UX143, future clinical and regulatory
developments for UX143, the clinical benefit, tolerability and safety of GTX-102, future clinical and regulatory developments for GTX-102, the clinical benefit,
tolerability and safety of UX701, future clinical and regulatory developments for UX701, timing for enrollment, dosing and data for Ultragenyx’s investigational therapies and gene therapy candidates and regulatory meetings are forward-looking
statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve substantial risks and uncertainties that could cause our clinical development
programs, collaboration with third parties, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, the uncertainty of
clinical drug development and unpredictability and lengthy process for obtaining regulatory approvals, the ability of the company and Mereo BioPharma to successfully develop UX143, the company’s ability to achieve its projected development
goals in its expected timeframes, risks related to adverse side effects, risks related to reliance on third party partners to conduct certain activities on the company’s behalf, the potential for any license or collaboration agreement,
including the company’s collaboration agreement with Mereo to be terminated, smaller than anticipated market opportunities for the company’s products and product candidates, manufacturing risks, competition from other therapies or
products, and other matters that could affect sufficiency of existing cash, cash equivalents and short-term investments to fund operations, the company’s future operating results and financial performance, the timing of clinical trial
activities and reporting results from same, and the availability or commercial potential of Ultragenyx’s products and drug candidates. Ultragenyx undertakes no obligation to update or revise any
forward-looking statements. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as
risks relating to the business of Ultragenyx in general, see Ultragenyx’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC) on August 4, 2023, and its subsequent
periodic reports filed with the SEC.
In addition to its SEC filings, press releases and public conference calls, Ultragenyx uses its investor
relations website and social media outlets to publish important information about the company, including information that may be deemed material to investors, and to comply with its disclosure obligations under Regulation FD. Financial and other
information about Ultragenyx is routinely posted and is accessible on Ultragenyx’s Investor Relations website (https://ir.ultragenyx.com/) and LinkedIn website
(https://www.linkedin.com/company/ultragenyx-pharmaceutical-inc-/).
Contacts
Ultragenyx Pharmaceutical Inc.
Investors
Joshua Higa
415-475-6370
ir@ultragenyx.com
Media
Carolyn Wang
415-225-5050
media@ultragenyx.com

GTX-102 for Angelman Syndrome Phase
1/2 Clinical Study Update © 2023 Ultragenyx Pharmaceutical Inc. | Confidential and Proprietary Exhibit 99.3
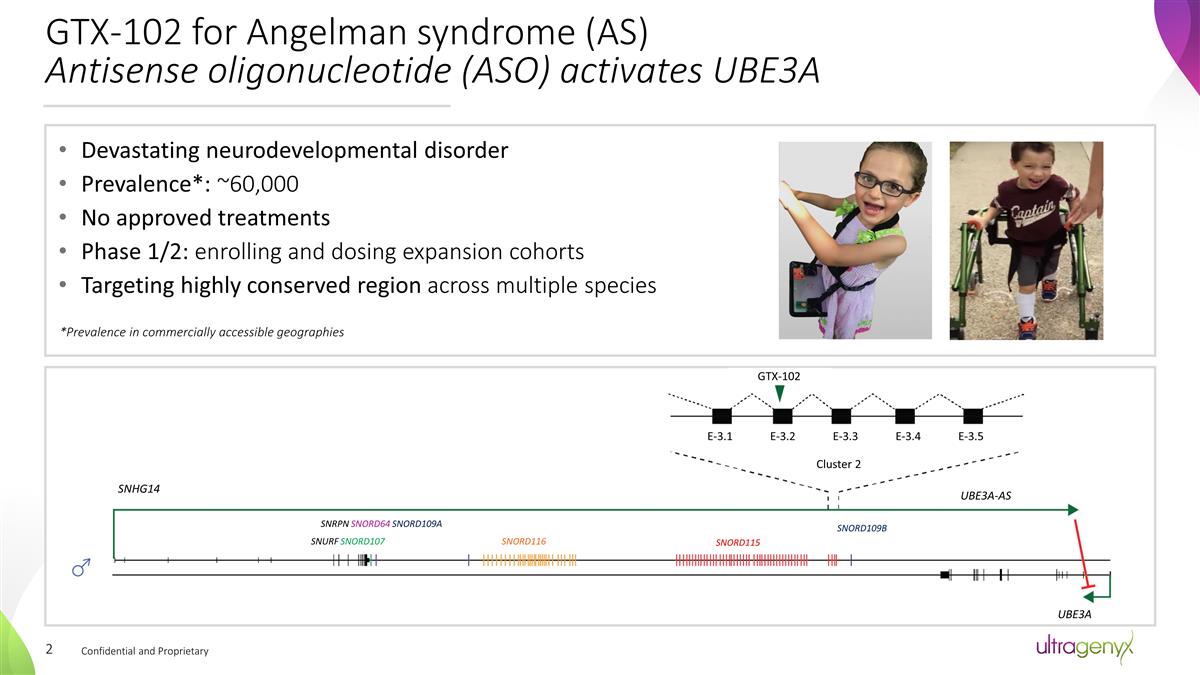
GTX-102 for Angelman syndrome
(AS) Antisense oligonucleotide (ASO) activates UBE3A Confidential and Proprietary Devastating neurodevelopmental disorder Prevalence*: ~60,000 No approved treatments Phase 1/2: enrolling and dosing expansion cohorts Targeting highly
conserved region across multiple species *Prevalence in commercially accessible geographies SNHG14 SNRPN SNORD64 SNORD109A SNURF SNORD107 SNORD116 SNORD115 SNORD109B UBE3A-AS UBE3A Cluster 2 GTX-102 E-3.1 E-3.2 E-3.3 E-3.4 E-3.5
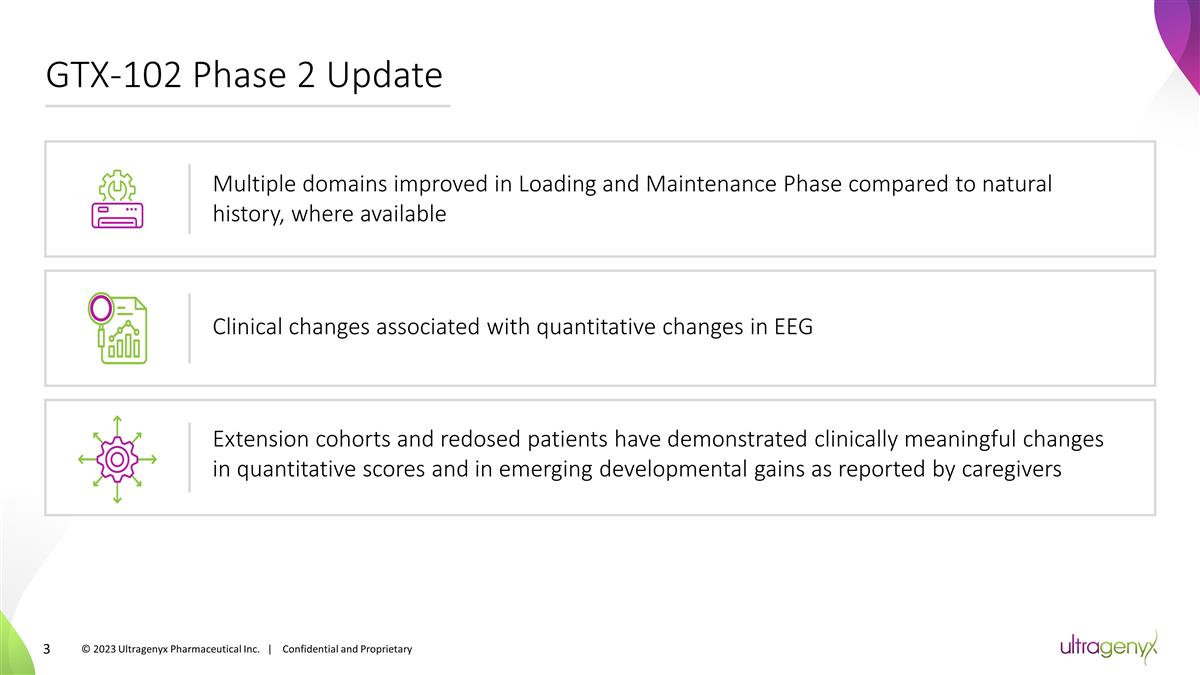
GTX-102 Phase 2 Update © 2023
Ultragenyx Pharmaceutical Inc. | Confidential and Proprietary Multiple domains improved in Loading and Maintenance Phase compared to natural history, where available Clinical changes associated with quantitative changes in EEG Extension cohorts and
redosed patients have demonstrated clinically meaningful changes in quantitative scores and in emerging developmental gains as reported by caregivers
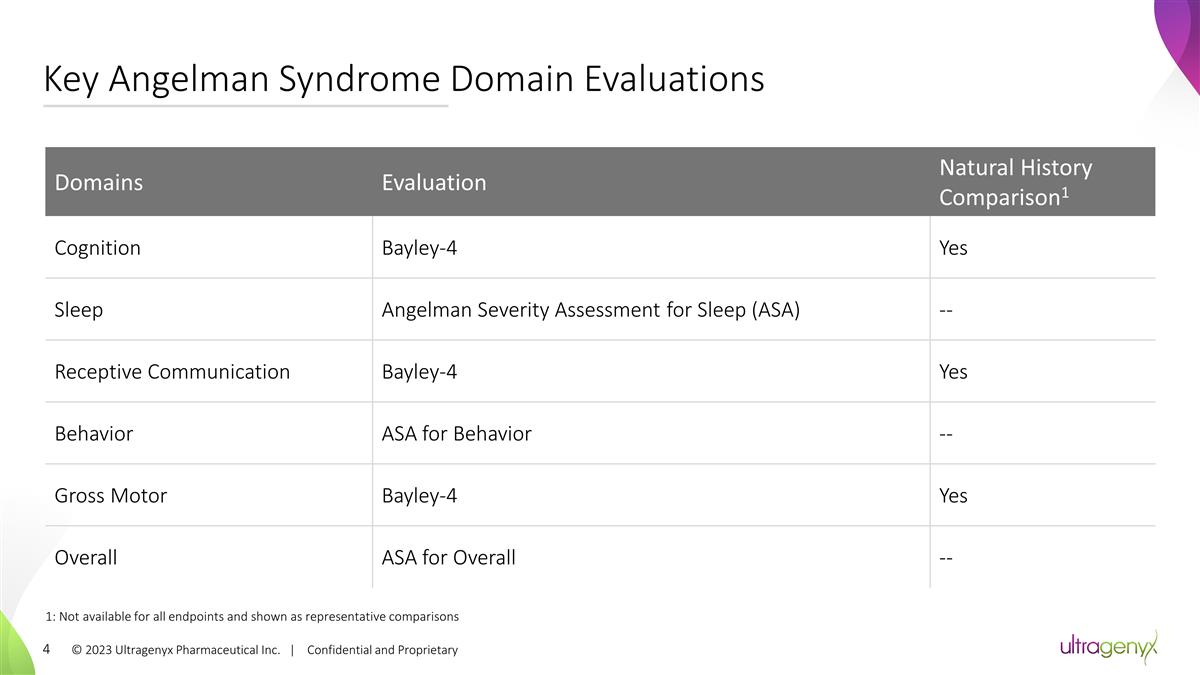
© 2023 Ultragenyx Pharmaceutical
Inc. | Confidential and Proprietary Key Angelman Syndrome Domain Evaluations Domains Evaluation Natural History Comparison1 Cognition Bayley-4 Yes Sleep Angelman Severity Assessment for Sleep (ASA) -- Receptive Communication Bayley-4 Yes Behavior
ASA for Behavior -- Gross Motor Bayley-4 Yes Overall ASA for Overall -- 1: Not available for all endpoints and shown as representative comparisons
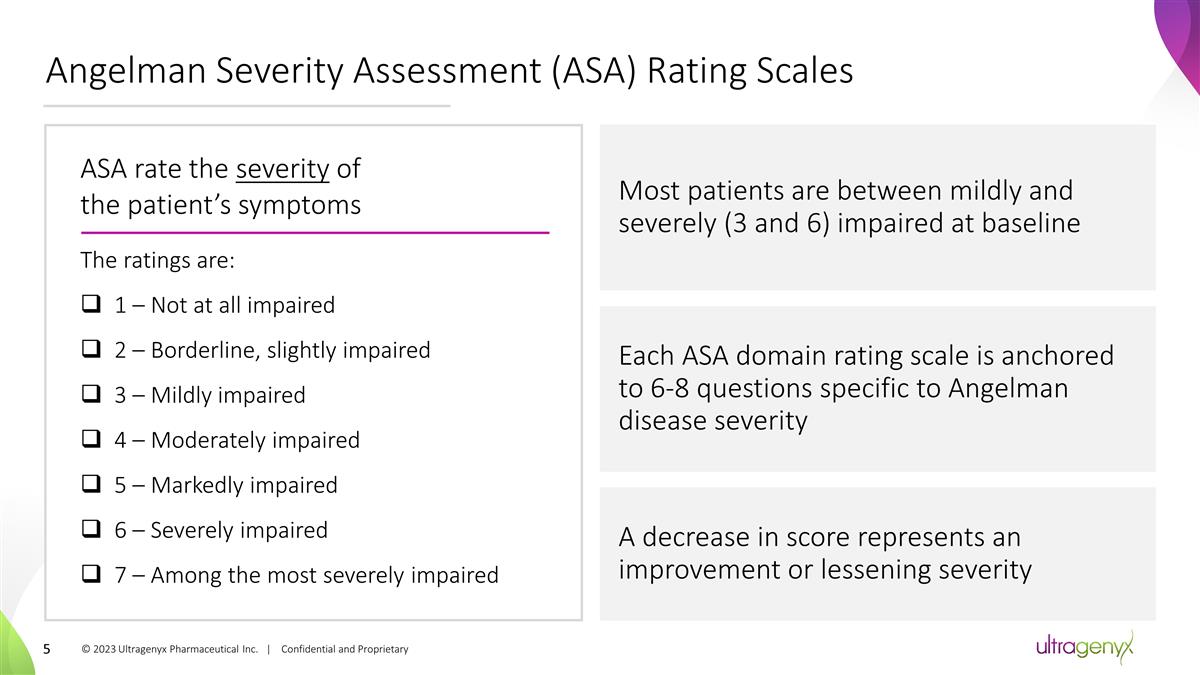
Angelman Severity Assessment (ASA)
Rating Scales © 2023 Ultragenyx Pharmaceutical Inc. | Confidential and Proprietary ASA rate the severity of the patient’s symptoms The ratings are: 1 – Not at all impaired 2 – Borderline, slightly impaired 3 – Mildly
impaired 4 – Moderately impaired 5 – Markedly impaired 6 – Severely impaired 7 – Among the most severely impaired Most patients are between mildly and severely (3 and 6) impaired at baseline Each ASA domain rating scale is
anchored to 6-8 questions specific to Angelman disease severity A decrease in score represents an improvement or lessening severity
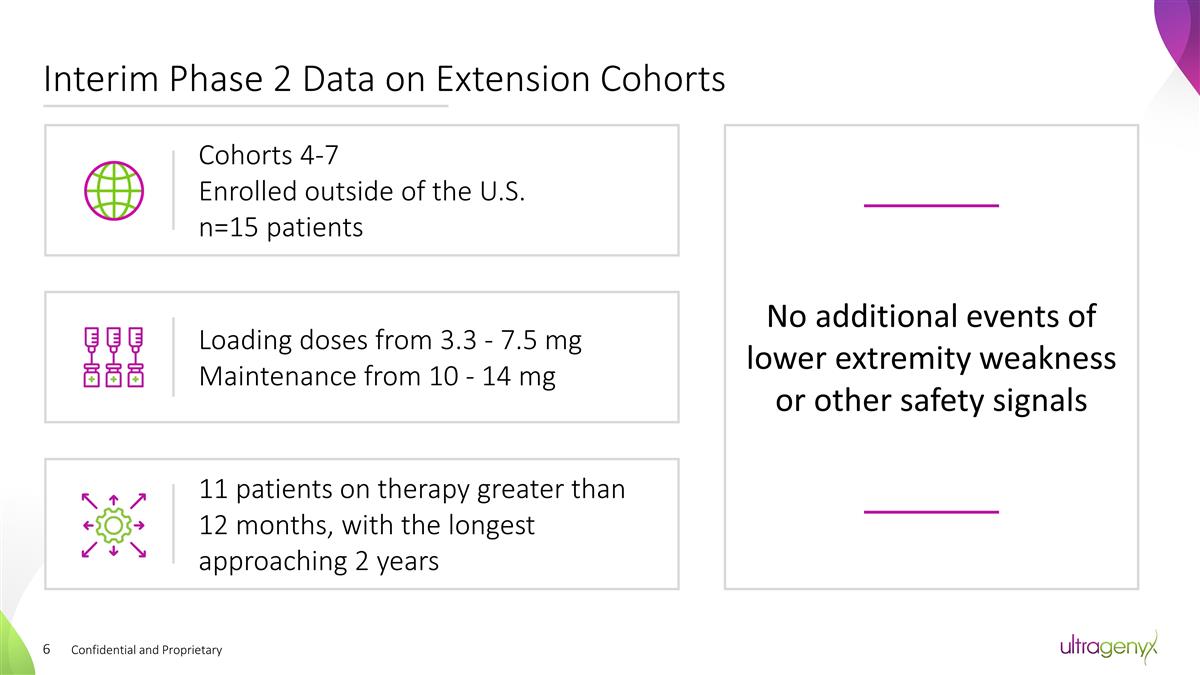
Confidential and Proprietary Interim
Phase 2 Data on Extension Cohorts No additional events of lower extremity weakness or other safety signals Cohorts 4-7 Enrolled outside of the U.S. n=15 patients Loading doses from 3.3 - 7.5 mg Maintenance from 10 - 14 mg 11 patients on
therapy greater than 12 months, with the longest approaching 2 years
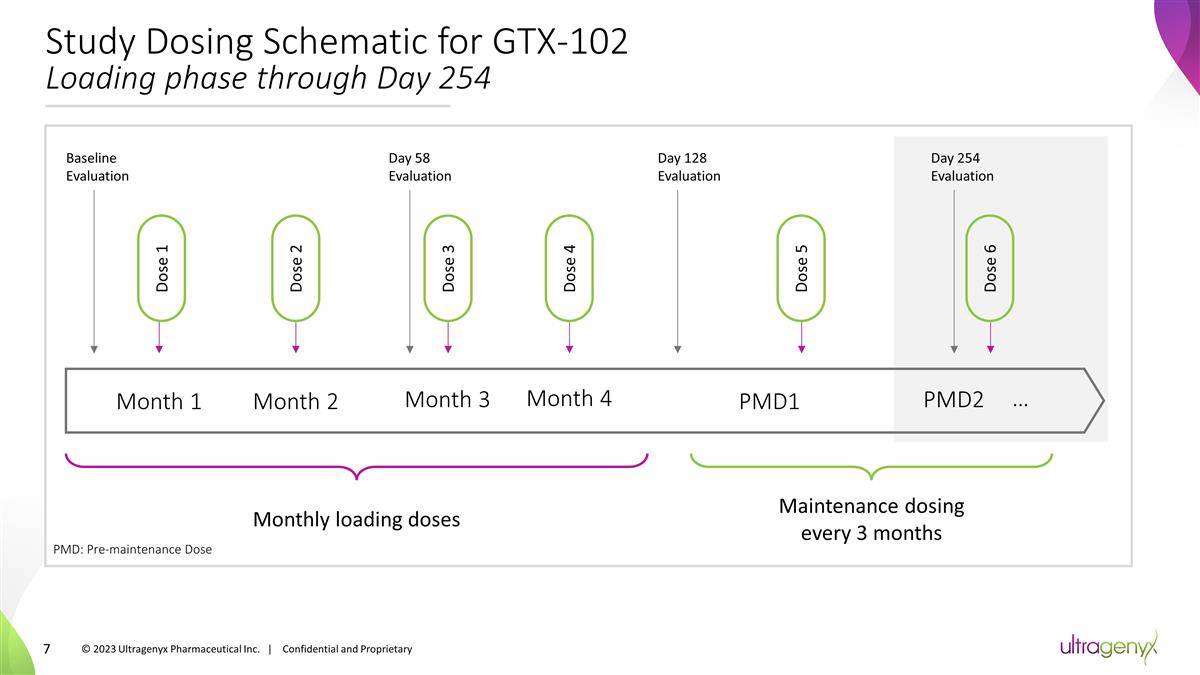
Study Dosing Schematic for GTX-102
Loading phase through Day 254 Month 1 … PMD2 PMD1 Month 4 Month 2 Month 3 Baseline Evaluation Day 58 Evaluation Day 128 Evaluation Day 254 Evaluation Dose 1 Dose 2 Dose 3 Dose 4 Dose 5 Dose 6 Monthly loading doses Maintenance dosing every 3
months © 2023 Ultragenyx Pharmaceutical Inc. | Confidential and Proprietary PMD: Pre-maintenance Dose
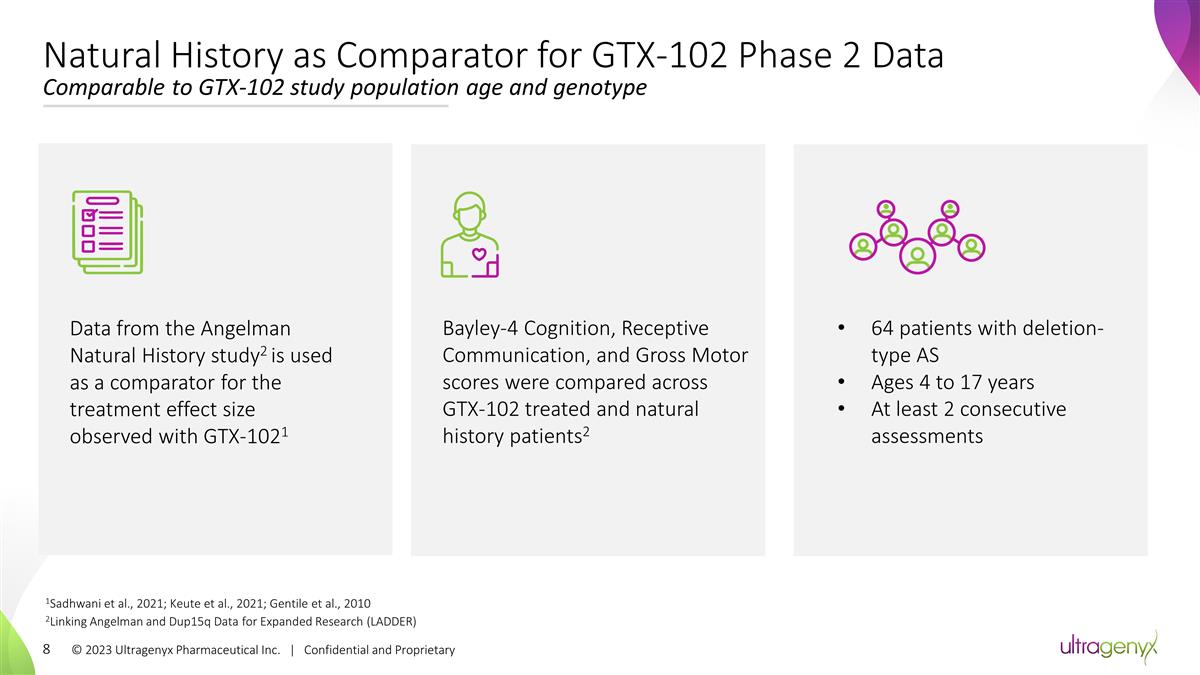
© 2023 Ultragenyx Pharmaceutical
Inc. | Confidential and Proprietary Natural History as Comparator for GTX-102 Phase 2 Data Comparable to GTX-102 study population age and genotype 1Sadhwani et al., 2021; Keute et al., 2021; Gentile et al., 2010 2Linking Angelman and Dup15q Data for
Expanded Research (LADDER) Data from the Angelman Natural History study2 is used as a comparator for the treatment effect size observed with GTX-1021 Bayley-4 Cognition, Receptive Communication, and Gross Motor scores were compared across GTX-102
treated and natural history patients2 64 patients with deletion-type AS Ages 4 to 17 years At least 2 consecutive assessments
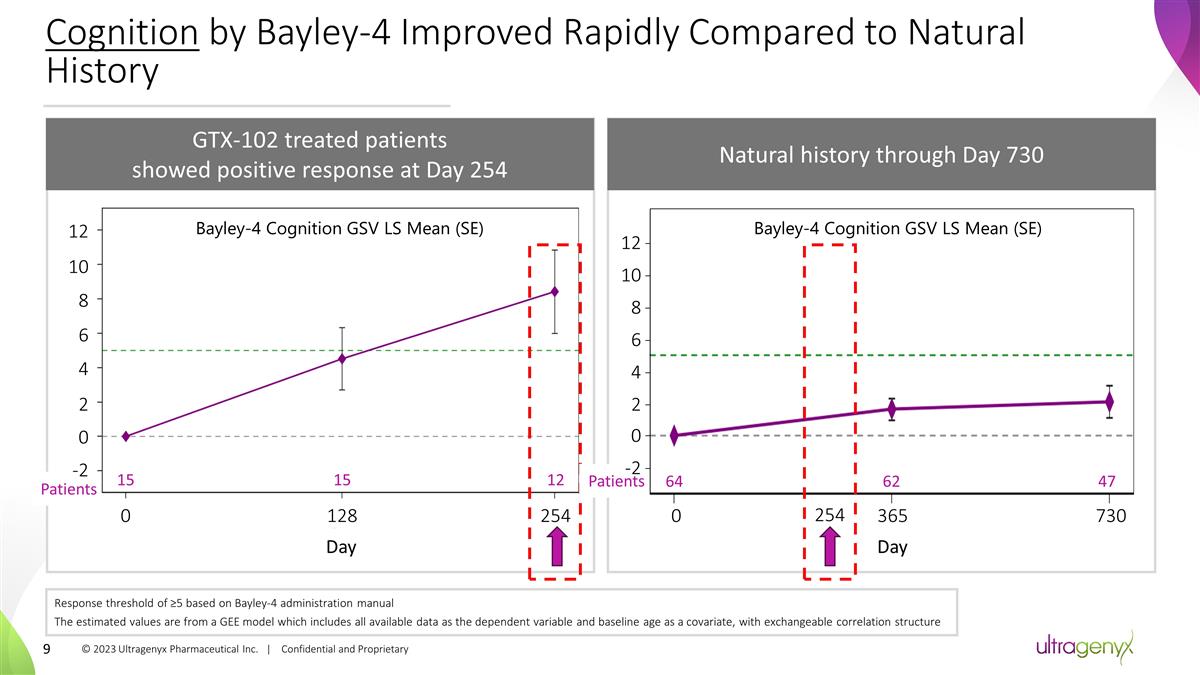
Cognition by Bayley-4 Improved Rapidly
Compared to Natural History GTX-102 treated patients showed positive response at Day 254 Natural history through Day 730 15 15 12 0 128 254 Day Patients -2 0 2 4 6 8 10 12 Bayley-4 Cognition GSV LS Mean (SE) -5 0 365 730 254 Patients 64 62 47
Bayley-4 Cognition GSV LS Mean (SE) Day Response threshold of ≥5 based on Bayley-4 administration manual The estimated values are from a GEE model which includes all available data as the dependent variable and baseline age as a covariate,
with exchangeable correlation structure -2 0 2 4 6 8 10 12 © 2023 Ultragenyx Pharmaceutical Inc. | Confidential and Proprietary
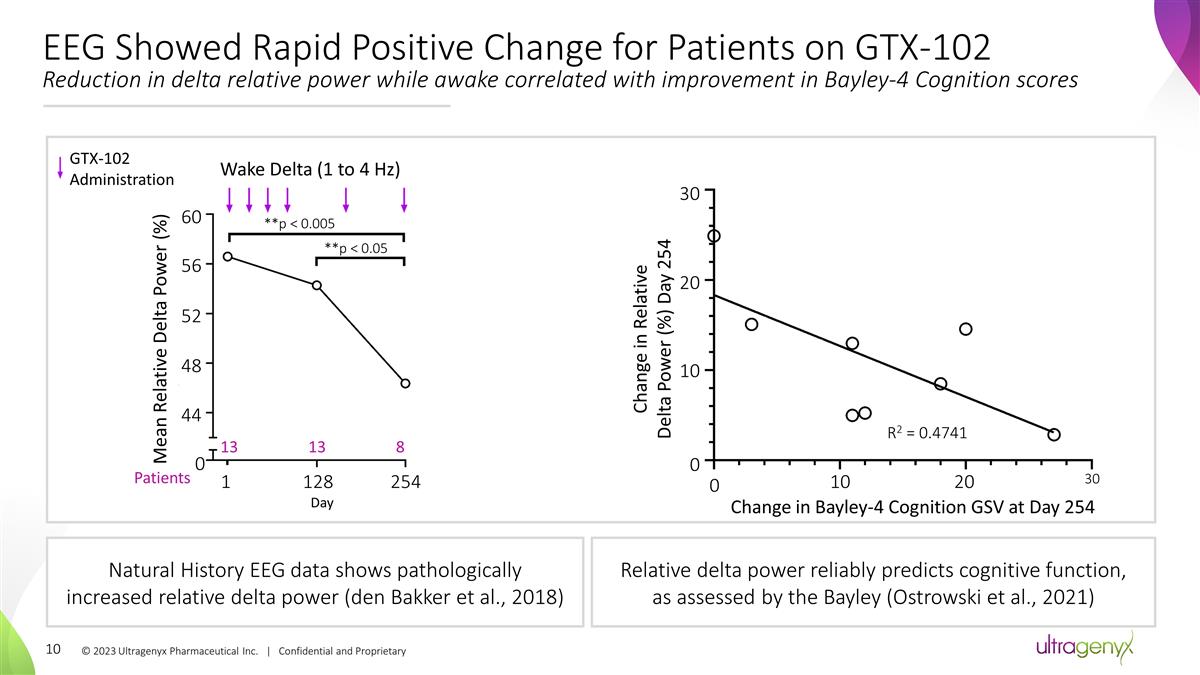
Natural History EEG data shows
pathologically increased relative delta power (den Bakker et al., 2018) Relative delta power reliably predicts cognitive function, as assessed by the Bayley (Ostrowski et al., 2021) EEG Showed Rapid Positive Change for Patients on GTX-102 Reduction
in delta relative power while awake correlated with improvement in Bayley-4 Cognition scores © 2023 Ultragenyx Pharmaceutical Inc. | Confidential and Proprietary Wake Delta (1 to 4 Hz) **p < 0.005 **p < 0.05 60 56 52 48 44 0 1 128 254 Day
Mean Relative Delta Power (%) Change in Relative Delta Power (%) Day 254 30 20 10 0 0 Change in Bayley-4 Cognition GSV at Day 254 10 20 30 R2 = 0.4741 GTX-102 Administration Patients 13 13 8
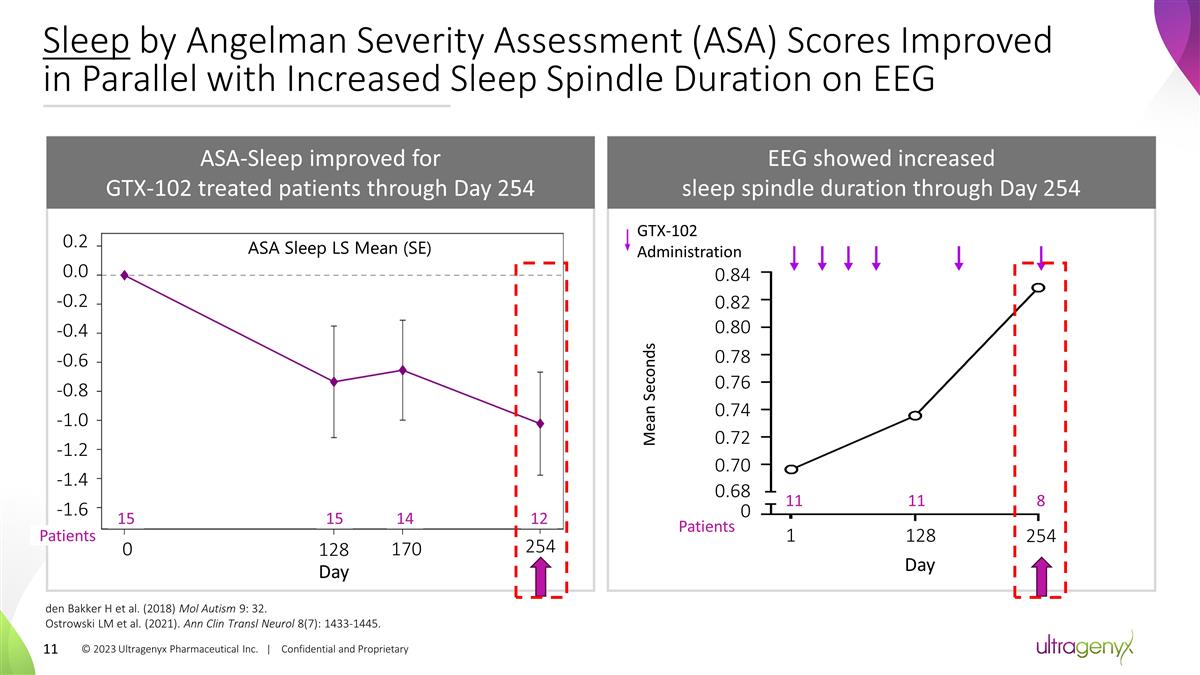
Sleep by Angelman Severity
Assessment (ASA) Scores Improved in Parallel with Increased Sleep Spindle Duration on EEG den Bakker H et al. (2018) Mol Autism 9: 32. Ostrowski LM et al. (2021). Ann Clin Transl Neurol 8(7): 1433-1445. ASA-Sleep improved for GTX-102 treated
patients through Day 254 EEG showed increased sleep spindle duration through Day 254 15 0 15 128 14 170 12 254 Day Patients 0.84 0.82 0 Mean Seconds 0.80 0.78 0.76 0.74 0.72 0.70 0.68 1 128 254 Day GTX-102 Administration -1.6 -1.4 -1.2 -1.0 -0.8
-0.6 -0.4 -0.2 0.0 0.2 11 11 8 Patients ASA Sleep LS Mean (SE) © 2023 Ultragenyx Pharmaceutical Inc. | Confidential and Proprietary

GTX-102 Maintenance Phase
Confidential and Proprietary
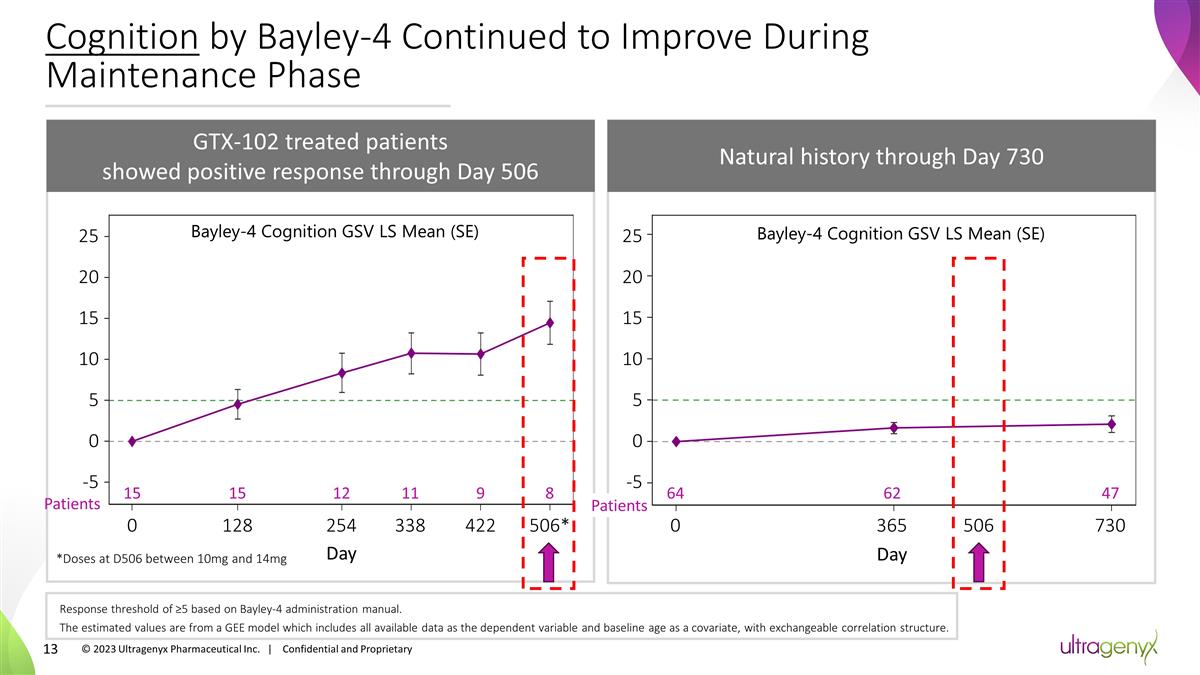
Cognition by Bayley-4 Continued to
Improve During Maintenance Phase GTX-102 treated patients showed positive response through Day 506 Patients 15 0 15 128 12 254 11 338 9 422 8 506* Day 25 20 15 10 5 0 -5 *Doses at D506 between 10mg and 14mg Bayley-4 Cognition GSV LS Mean (SE)
Natural history through Day 730 Bayley-4 Cognition GSV LS Mean (SE) 0 365 730 Day 64 62 47 Patients 506 Response threshold of ≥5 based on Bayley-4 administration manual. The estimated values are from a GEE model which includes all available
data as the dependent variable and baseline age as a covariate, with exchangeable correlation structure. 25 20 15 10 5 0 -5 © 2023 Ultragenyx Pharmaceutical Inc. | Confidential and Proprietary
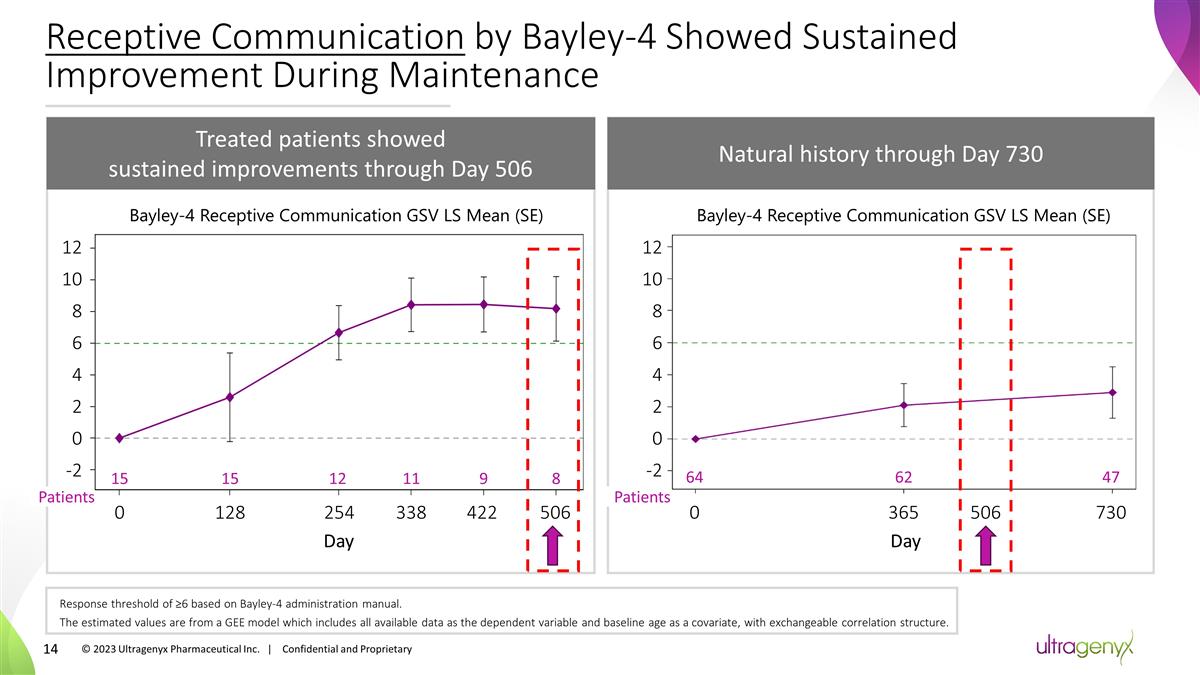
Receptive Communication by Bayley-4
Showed Sustained Improvement During Maintenance Treated patients showed sustained improvements through Day 506 Natural history through Day 730 15 12 8 0 128 422 15 11 9 338 506 254 -2 0 2 4 6 8 10 12 Patients 0 365 730 Day 506 Bayley-4 Receptive
Communication GSV LS Mean (SE) Bayley-4 Receptive Communication GSV LS Mean (SE) Day Response threshold of ≥6 based on Bayley-4 administration manual. The estimated values are from a GEE model which includes all available data as the dependent
variable and baseline age as a covariate, with exchangeable correlation structure. 64 62 47 Patients -2 0 2 4 6 8 10 12 © 2023 Ultragenyx Pharmaceutical Inc. | Confidential and Proprietary
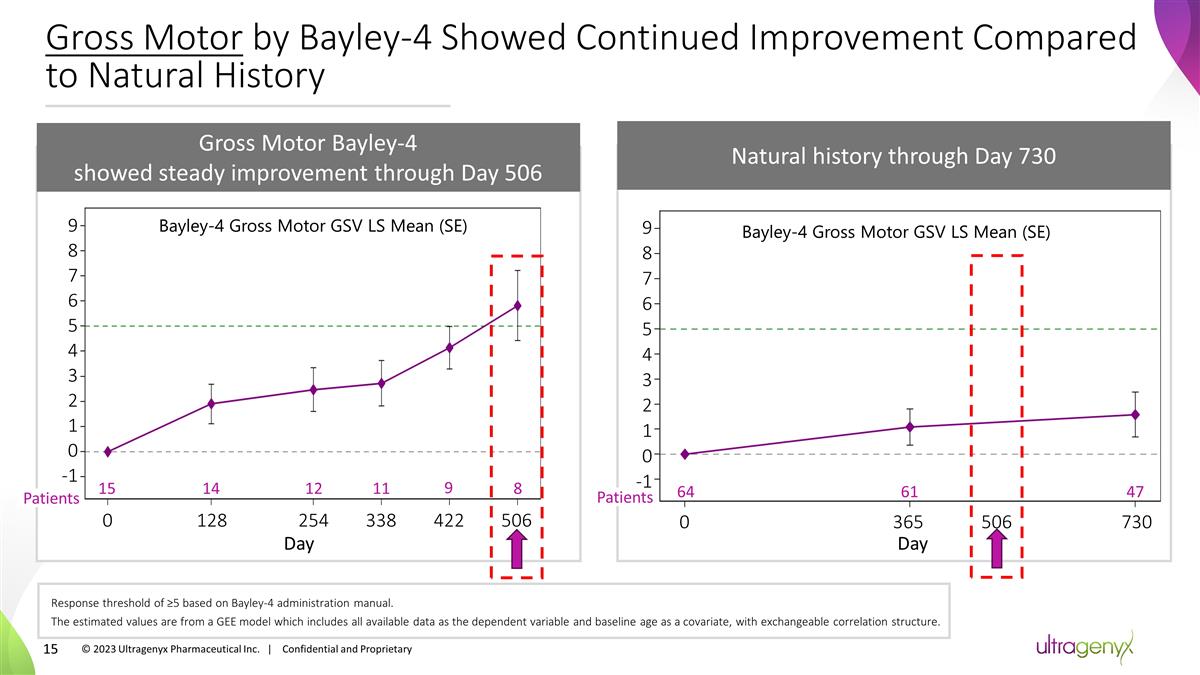
Gross Motor by Bayley-4 Showed
Continued Improvement Compared to Natural History Gross Motor Bayley-4 showed steady improvement through Day 506 Response threshold of ≥5 based on Bayley-4 administration manual. The estimated values are from a GEE model which includes all
available data as the dependent variable and baseline age as a covariate, with exchangeable correlation structure. Natural history through Day 730 64 0 Patients 61 365 47 730 506 Day Bayley-4 Gross Motor GSV LS Mean (SE) Bayley-4 Gross Motor GSV LS
Mean (SE) -1 0 1 2 3 4 6 9 5 7 8 2 3 4 6 9 5 7 8 Patients 15 14 12 11 9 8 128 254 338 422 506 Day 0 -1 0 1 © 2023 Ultragenyx Pharmaceutical Inc. | Confidential and Proprietary
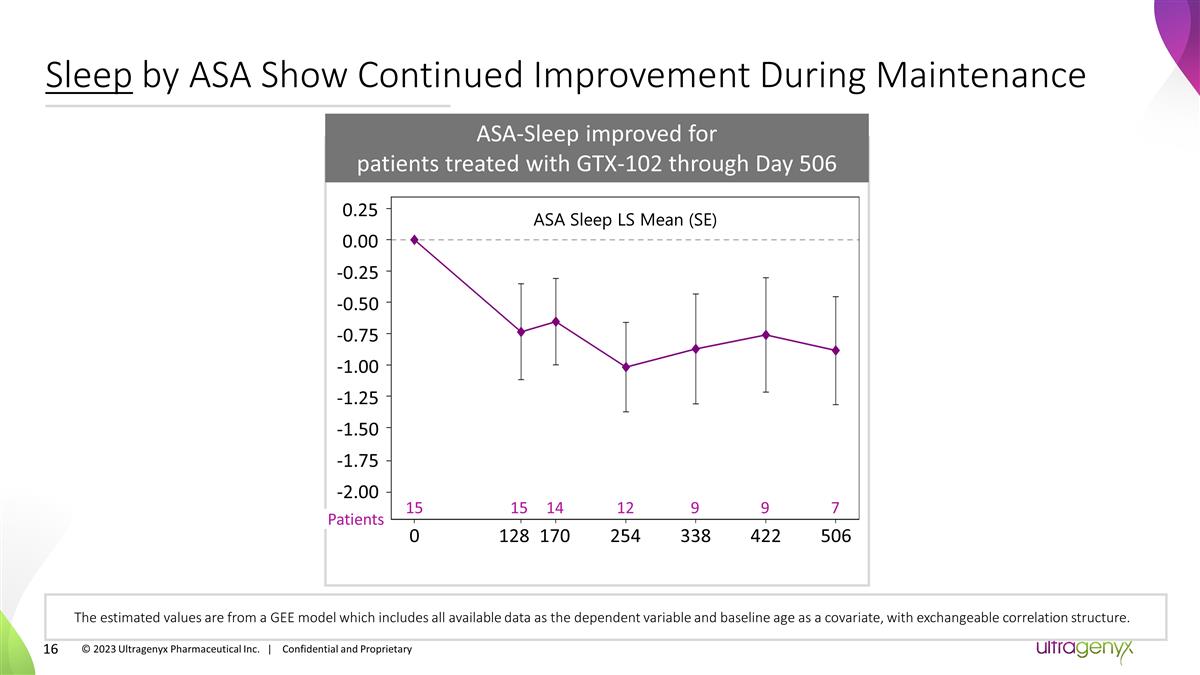
Sleep by ASA Show Continued
Improvement During Maintenance The estimated values are from a GEE model which includes all available data as the dependent variable and baseline age as a covariate, with exchangeable correlation structure. Day Patients ASA-Sleep improved for
patients treated with GTX-102 through Day 506 0.25 0.00 -0.25 -0.50 -0.75 -1.00 -1.25 -1.50 -1.75 ASA Sleep LS Mean (SE) 15 12 7 0 128 422 15 9 9 254 338 506 14 170 -2.00 © 2023 Ultragenyx Pharmaceutical Inc. | Confidential and
Proprietary
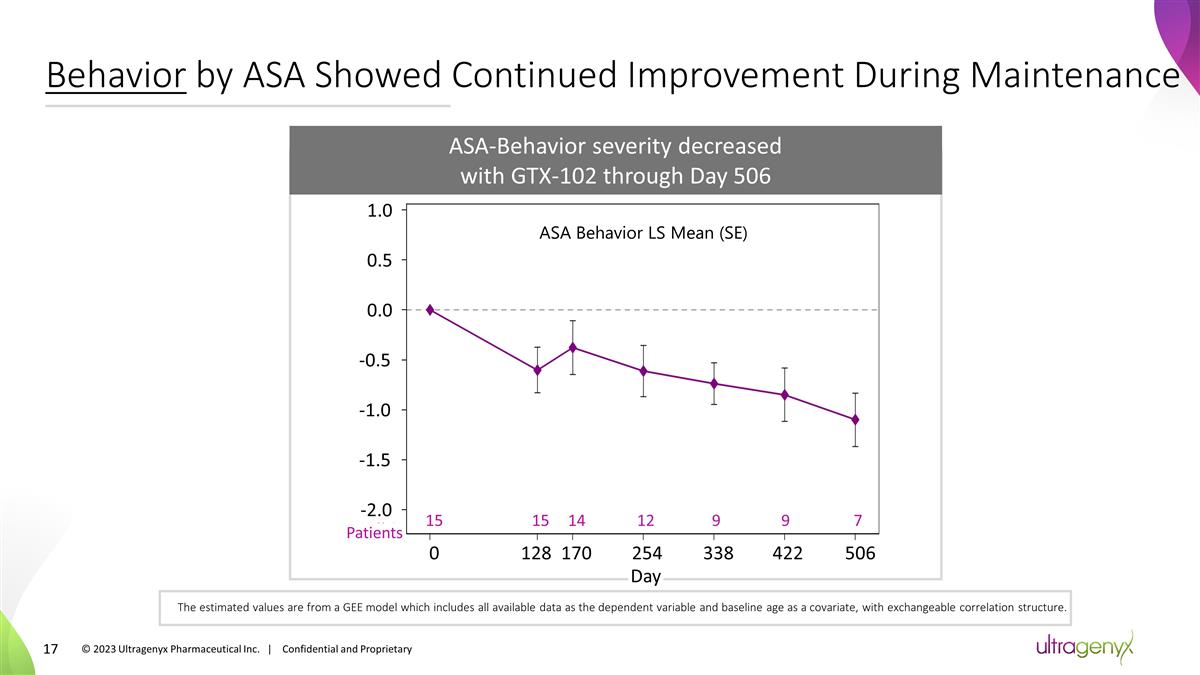
Behavior by ASA Showed Continued
Improvement During Maintenance 15 12 7 0 128 422 Day 15 9 9 254 338 506 Patients 14 170 The estimated values are from a GEE model which includes all available data as the dependent variable and baseline age as a covariate, with exchangeable
correlation structure. ASA-Behavior severity decreased with GTX-102 through Day 506 1.0 0.5 0.0 -0.5 -1.0 -1.5 -2.0 ASA Behavior LS Mean (SE) © 2023 Ultragenyx Pharmaceutical Inc. | Confidential and Proprietary
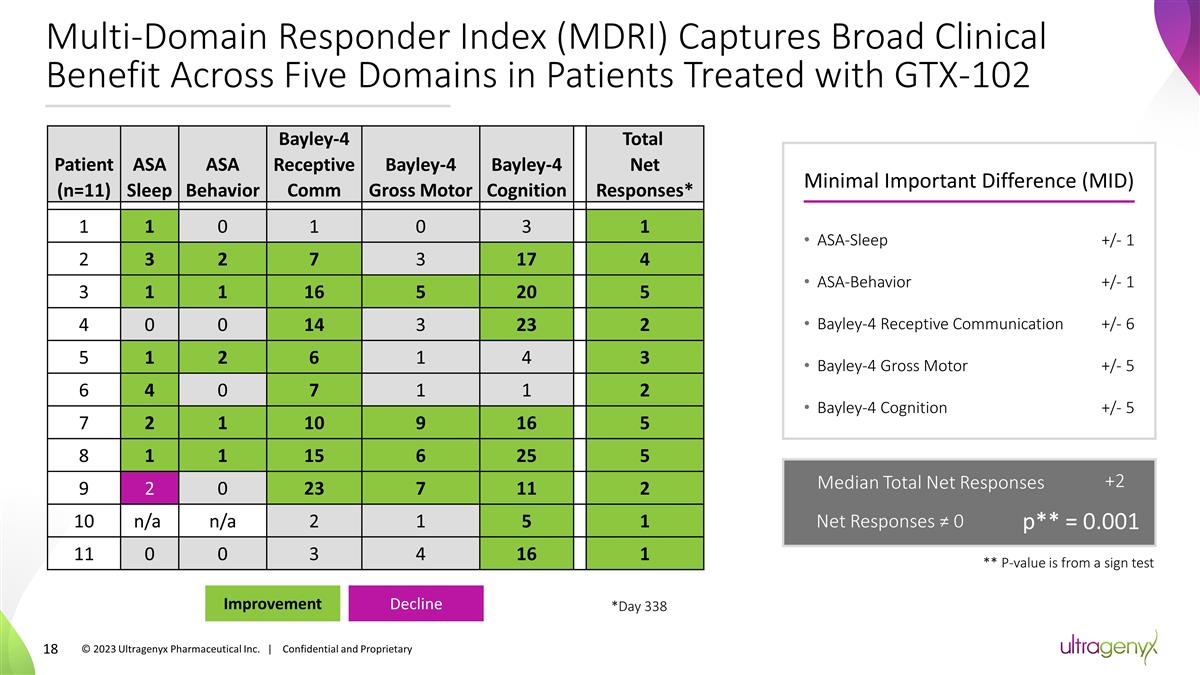
Multi-Domain Responder Index (MDRI)
Captures Broad Clinical Benefit Across Five Domains in Patients Treated with GTX-102 Patient (n=11) ASA Sleep ASA Behavior Bayley-4 Receptive Comm Bayley-4 Gross Motor Bayley-4 Cognition Total Net Responses* 1 1 0 1 0 3 1 2 3 2 7
3 17 4 3 1 1 16 5 20 5 4 0 0 14 3 23 2 5 1 2 6 1 4 3 6 4 0 7 1 1 2 7 2 1 10 9 16 5 8 1 1 15 6 25 5 9 2 0 23 7 11 2 10 n/a n/a 2 1 5 1 11 0 0 3 4 16 1 Decline Improvement Minimal Important Difference (MID) ASA-Sleep +/- 1 ASA-Behavior +/- 1
Bayley-4 Receptive Communication +/- 6 Bayley-4 Gross Motor +/- 5 Bayley-4 Cognition +/- 5 ** P-value is from a sign test © 2023 Ultragenyx Pharmaceutical Inc. | Confidential and Proprietary Median Total Net Responses +2 Net
Responses ≠ 0 p** = 0.001 *Day 338
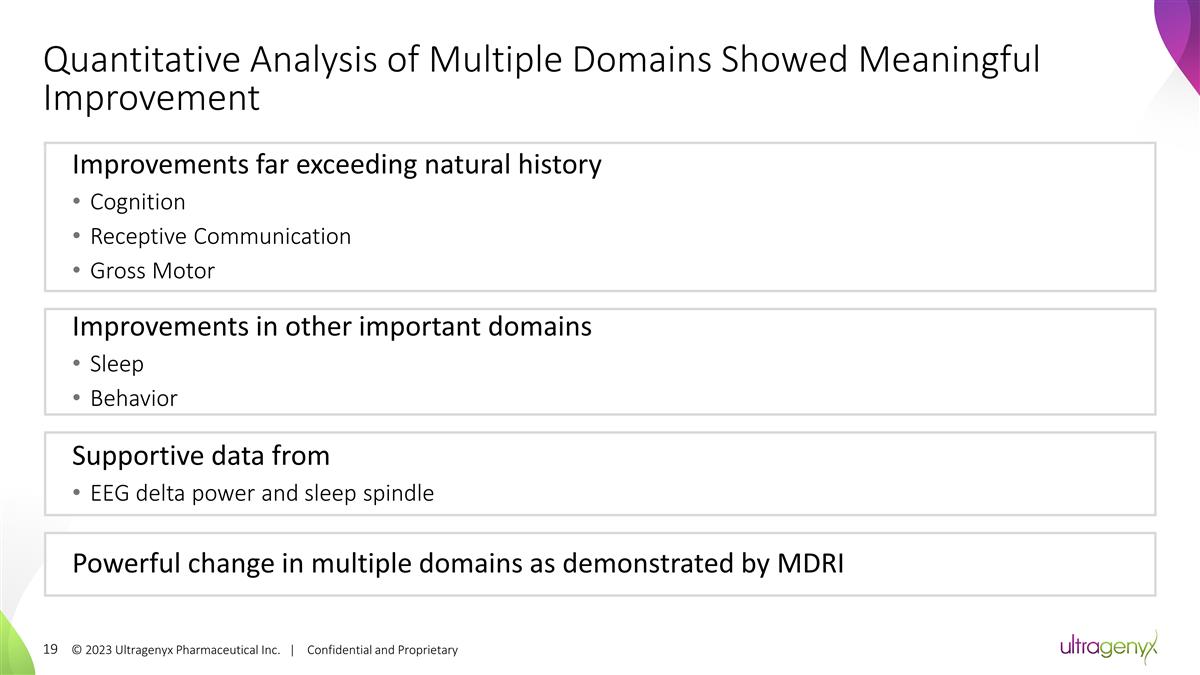
© 2023 Ultragenyx
Pharmaceutical Inc. | Confidential and Proprietary Quantitative Analysis of Multiple Domains Showed Meaningful Improvement Improvements far exceeding natural history Cognition Receptive Communication Gross Motor Supportive data from EEG delta power
and sleep spindle Improvements in other important domains Sleep Behavior Powerful change in multiple domains as demonstrated by MDRI
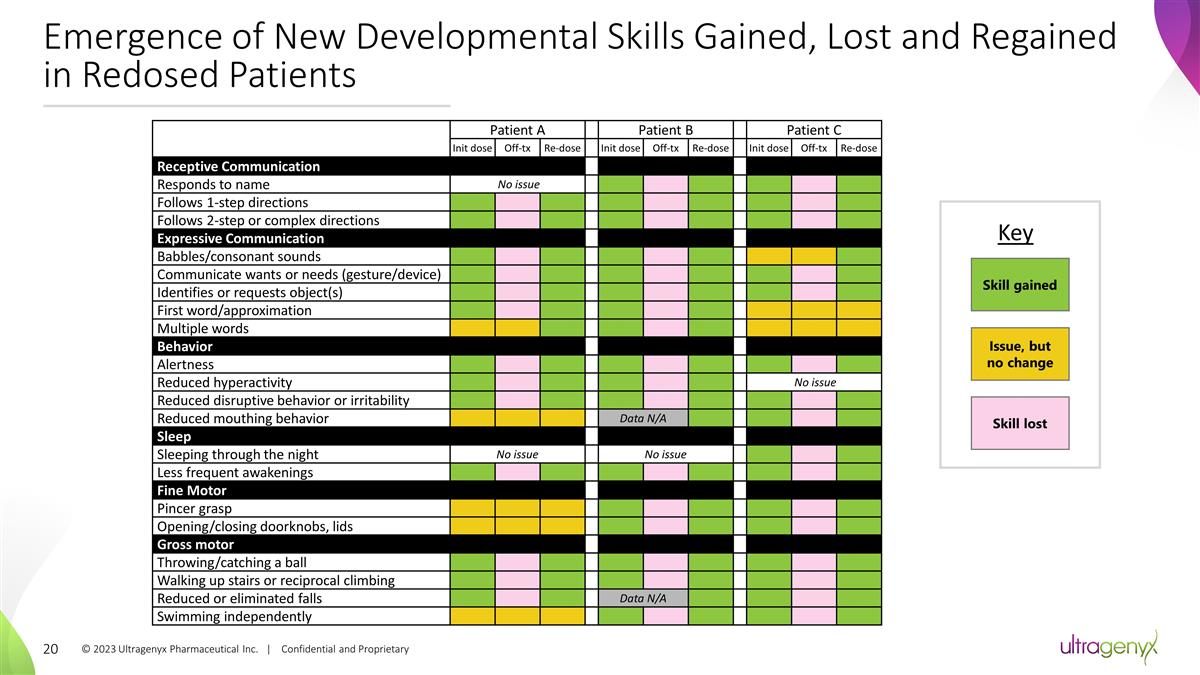
Emergence of New Developmental
Skills Gained, Lost and Regained in Redosed Patients Patient A Patient B Patient C 103-004 Init dose Off-tx Re-dose Init dose Off-tx Re-dose Init dose Off-tx Re-dose Receptive Communication Responds to name No issue
Follows 1-step directions Follows 2-step or complex directions Expressive Communication Babbles/consonant sounds Communicate wants or needs (gesture/device)
Identifies or requests object(s) First word/approximation Multiple words Behavior Alertness Reduced hyperactivity
No issue Reduced disruptive behavior or irritability Reduced mouthing behavior Data N/A Sleep Sleeping through the night No issue No issue Less frequent awakenings
Fine Motor Pincer grasp Opening/closing doorknobs, lids Gross motor Throwing/catching a ball Walking up stairs or reciprocal
climbing Reduced or eliminated falls Data N/A Swimming independently Skill gained Key Skill lost Issue, but no change © 2023 Ultragenyx Pharmaceutical Inc. | Confidential and
Proprietary
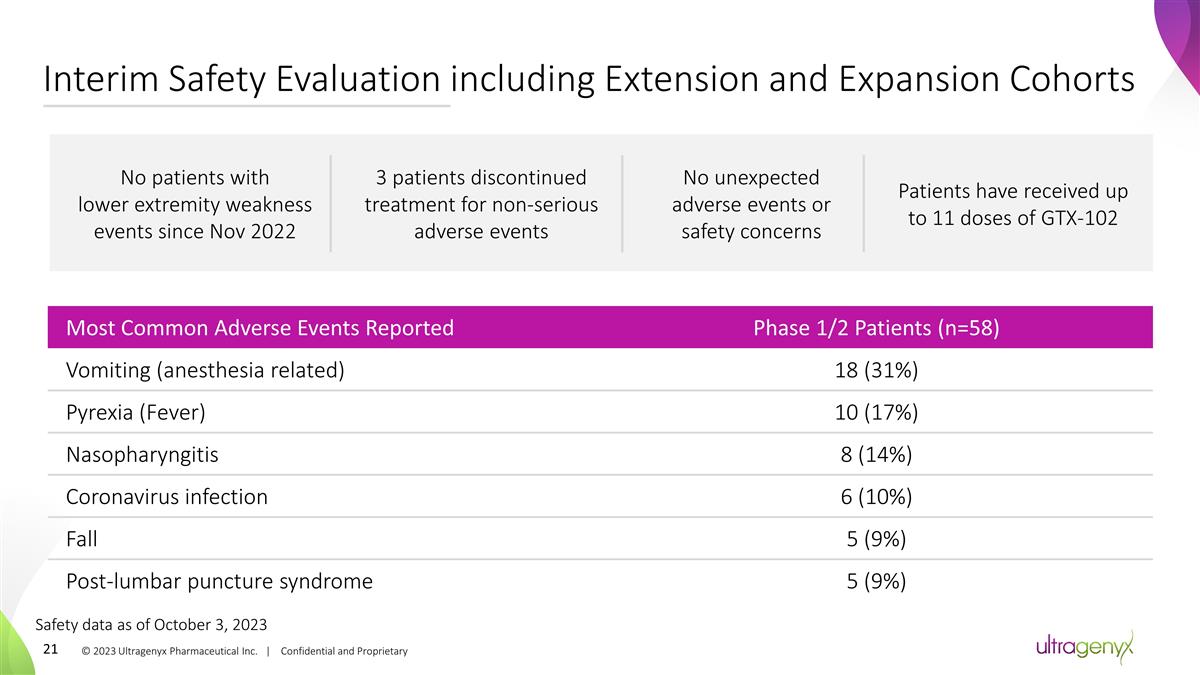
Interim Safety Evaluation including
Extension and Expansion Cohorts Most Common Adverse Events Reported Phase 1/2 Patients (n=58) Vomiting (anesthesia related) 18 (31%) Pyrexia (Fever) 10 (17%) Nasopharyngitis 8 (14%) Coronavirus infection 6 (10%) Fall 5 (9%) Post-lumbar puncture
syndrome 5 (9%) No patients with lower extremity weakness events since Nov 2022 No unexpected adverse events or safety concerns 3 patients discontinued treatment for non-serious adverse events © 2023 Ultragenyx Pharmaceutical Inc. |
Confidential and Proprietary Safety data as of October 3, 2023 Patients have received up to 11 doses of GTX-102
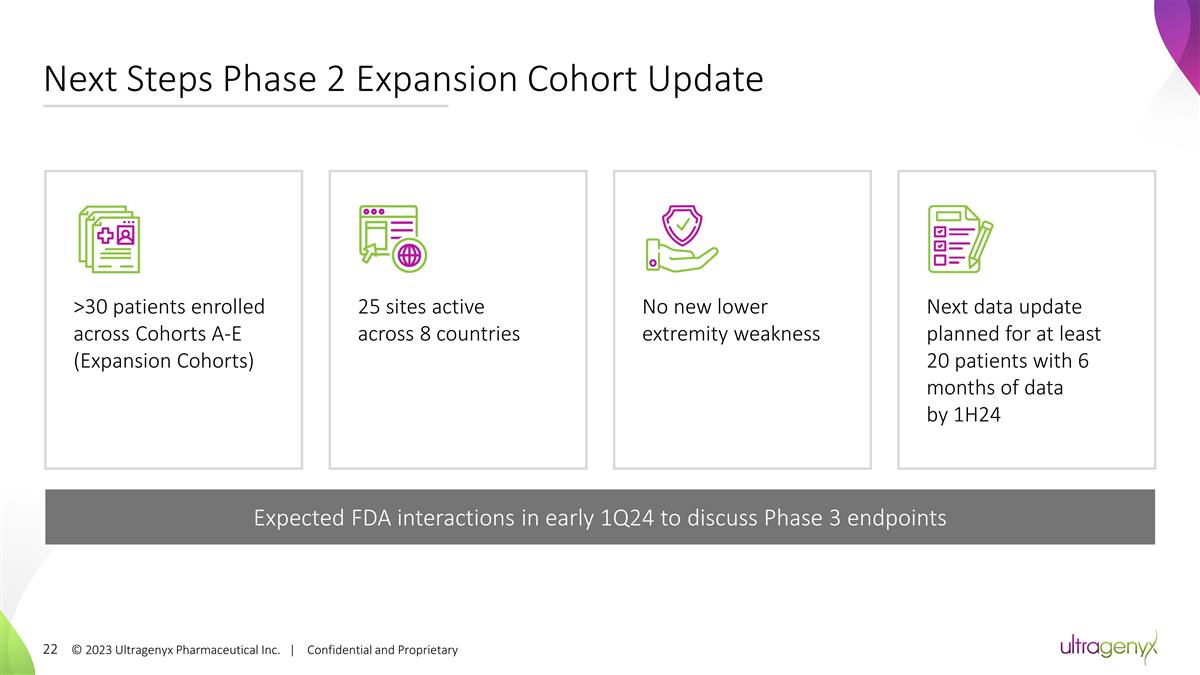
Next Steps Phase 2 Expansion Cohort
Update © 2023 Ultragenyx Pharmaceutical Inc. | Confidential and Proprietary >30 patients enrolled across Cohorts A-E (Expansion Cohorts) 25 sites active across 8 countries Next data update planned for at least 20 patients with 6 months of
data by 1H24 Expected FDA interactions in early 1Q24 to discuss Phase 3 endpoints No new lower extremity weakness
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Ultragenyx Pharmaceutical (NASDAQ:RARE)
Historical Stock Chart
From Apr 2024 to May 2024
Ultragenyx Pharmaceutical (NASDAQ:RARE)
Historical Stock Chart
From May 2023 to May 2024