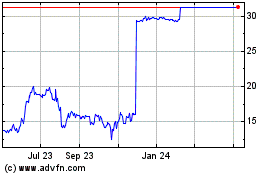
ImmunoGen Shares Rise 96% on Positive Data From Elahere Trial
May 03 2023 - 9:01AM
Dow Jones News
By Chris Wack
ImmunoGen Inc. shares were up 96% to $10.20 in premarket trading
after the company said it saw positive top-line data from a Phase 3
trial evaluating the safety and efficacy of Elahere mirvetuximab
soravtansine-gynx.
The company said the trial compared patients who received
Elahere, with chemotherapy in patients with folate receptor
alpha-positive platinum-resistant ovarian cancer who have received
one to three prior lines of therapy.
Based on this data, the company said it plans to submit a
Marketing Authorization Application in Europe and a supplemental
Biologics License Application in the U.S. for the conversion to a
regular approval of Elahere.
ImmunoGen said the safety profile of Elahere consists
predominantly of low-grade ocular and gastrointestinal events.
In November 2022, the U.S. Food and Drug Administration granted
accelerated approval for Elahere for the treatment of adult
patients with receptor alpha-positive platinum-resistant ovarian
cancer, fallopian tube, or primary peritoneal cancer who have
received one to three prior systemic treatment regimens.
ImmunoGen plans to submit an MAA to the European Medicines
Agency and an sBLA to the FDA in the second half of this year.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
May 03, 2023 08:46 ET (12:46 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
ImmunoGen (NASDAQ:IMGN)
Historical Stock Chart
From Jun 2024 to Jul 2024
ImmunoGen (NASDAQ:IMGN)
Historical Stock Chart
From Jul 2023 to Jul 2024