Abbott Shipping 150,000 Coronavirus Tests
March 18 2020 - 6:44PM
Dow Jones News
By Josh Beckerman
Abbott Laboratories said the U.S. Food and Drug Administration
has issued Emergency Use Authorization for the company's molecular
test for Covid-19.
The company is immediately shipping 150,000 Abbott RealTime
SARS-CoV-2 EUA tests to existing customers in the U.S. The tests
are used on Abbott's m2000 RealTime System.
Abbott is ramping up production, with the goal of providing up
to 1 million tests per week.
Write to Josh Beckerman at josh.beckerman@wsj.com
(END) Dow Jones Newswires
March 18, 2020 18:29 ET (22:29 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
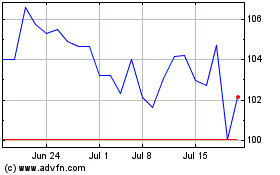
Abbott Laboratories (NYSE:ABT)
Historical Stock Chart
From Mar 2024 to Apr 2024
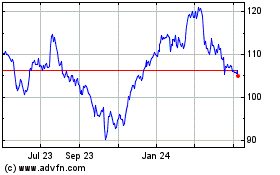
Abbott Laboratories (NYSE:ABT)
Historical Stock Chart
From Apr 2023 to Apr 2024