Form 10-Q - Quarterly report [Sections 13 or 15(d)]
August 03 2023 - 7:11AM
Edgar (US Regulatory)
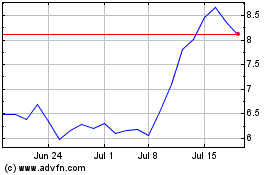
Fulcrum Therapeutics (NASDAQ:FULC)
Historical Stock Chart
From Apr 2024 to May 2024
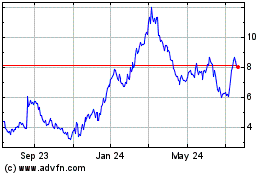
Fulcrum Therapeutics (NASDAQ:FULC)
Historical Stock Chart
From May 2023 to May 2024