CorMedix Shares Drop 14% After Type A Meeting With FDA for DefenCath
April 26 2023 - 10:47AM
Dow Jones News
By Chris Wack
CorMedix Inc. shares were down 14% at $4 each after the company
said that the Food and Drug Administration may conduct a
pre-approval inspection at its manufacturing facility as part of a
New Drug Application for DefenCath.
The biopharmaceutical company said that following its Type A
meeting with the FDA, it intends to resubmit its new drug
application for DefenCath by the middle of May.
At the Type A meeting, the FDA informed CorMedix that it is in
receipt of the close-out report for inspectional observations
received from its existing contract manufacturing organization, and
NDA resubmission can be done at the company's discretion.
CorMedix shares are up 4% in the past 12 months.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
April 26, 2023 10:32 ET (14:32 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
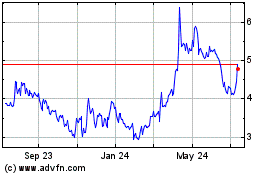
CorMedix (NASDAQ:CRMD)
Historical Stock Chart
From Apr 2024 to May 2024
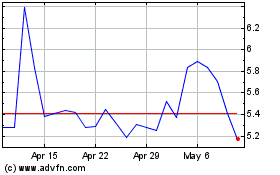
CorMedix (NASDAQ:CRMD)
Historical Stock Chart
From May 2023 to May 2024