false
0001029125
0001029125
2024-02-01
2024-02-01
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
February 1, 2024
|
|
Date of Report (Date of Earliest Event Reported)
|
|
Panbela Therapeutics, Inc.
|
|
(Exact Name of Registrant as Specified in its Charter)
|
|
Delaware
|
|
001-39468
|
|
88-2805017
|
|
(State of Incorporation)
|
|
(Commission File Number)
|
|
(I.R.S. Employer Identification No.)
|
|
712 Vista Blvd #305
Waconia, Minnesota
|
|
55387
|
|
(Address of Principal Executive Offices)
|
|
(Zip Code)
|
|
(952) 479-1196
|
|
(Registrant’s Telephone Number, Including Area Code)
|
| |
|
(Former Name or Former Address, if Changed Since Last Report.)
|
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
☐
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
|
|
☐
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
|
|
☐
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
|
|
☐
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
|
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
|
Trading Symbol
|
|
Name of each exchange on which registered
|
|
Common Stock, $0.001 par value
|
|
PBLA
|
|
The Nasdaq Stock Market LLC
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2 of the Securities Exchange Act of 1934.
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
|
Item 7.01
|
Regulation FD Disclosure.
|
On February 1, 2024, Panbela Therapeutics, Inc. (the “Company”) issued a press release announcing the closing of its previously announced offering of common stock and warrants to purchase common stock, the text of which is furnished as Exhibit 99.1 and incorporated by reference herein.
The information contained in this Item 7.01, including Exhibit 99.1, shall not be deemed to be “filed” with the Commission for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed to be incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
Following the completion of the offering, and the issuance of shares of common stock upon the exercise of a portion of the pre-funded warrants sold in the offering, the Company had a total of 3,481,298 shares of its common stock issued and outstanding at the close of business on January 31, 2024.
|
Item 9.01
|
Financial Statements and Exhibits.
|
|
Exhibit
No.
|
|
Description
|
| |
|
|
|
99.1
|
|
|
| |
|
|
|
104
|
|
Cover Page Interactive Data File (the cover page XBRL tags are embedded in the Inline XBRL document).
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
Panbela Therapeutics, Inc.
|
|
Date: February 1, 2024
|
By:
|
/s/ Susan Horvath
|
| |
|
Susan Horvath
|
| |
|
Chief Financial Officer
|
Exhibit 99.1

Panbela Announces Closing of Approximately $9.0 Million Public Offering
MINNEAPOLIS, January 31, 2024 (GLOBE NEWSWIRE) -- Panbela Therapeutics, Inc. (Nasdaq: PBLA), a clinical stage company developing disruptive therapeutics for the treatment of patients with urgent unmet medical needs, today announced the closing of its previously announced public offering of (i) 4,375,000 shares of its common stock or pre-funded warrants in lieu thereof and (ii) two classes of warrants to purchase up to an aggregate of 8,750,000 shares of its common stock (the “Public Warrants”) at a purchase price of $2.06 per share and associated Public Warrants and $2.059 per pre-funded warrant and associated Public Warrants. The pre-funded warrants have an exercise price of $0.001 per share. The Public Warrants have an exercise price of $2.06 per share, are exercisable upon issuance, and will expire five years following the date of issuance. The Public Warrants do not have any alternative cashless exercise or other provisions to adjust their exercise price beyond customary proportionate adjustments for recapitalizations and similar events.
Roth Capital Partners acted as sole placement agent of the offering.
Gross proceeds, before deducting placement agent fees and commissions and offering expenses, were approximately $9.0 million. The Company intends to use the net proceeds from the proposed offering for the continued clinical development of its product candidates, working capital, business development and other general corporate purposes, which may include repayment of debt.
The securities described above were offered pursuant to a registration statement on Form S-1 (File No. 333-276367), as amended, that was declared effective by the U.S. Securities and Exchange Commission (“SEC”), on January 26, 2024. The offering was made solely by means of a prospectus. Copies of the accompanying prospectus relating to and describing the terms of the offering may be obtained at the SEC’s website at www.sec.gov or by contacting Roth Capital Partners, LLC, 888 San Clemente Drive, Suite 400, Newport Beach, CA 92660 or by email at rothecm@roth.com.
This press release does not and shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or other jurisdiction. Any offer, if at all, will be made only by means of a prospectus, including a prospectus supplement, forming a part of the effective registration statement.
About Panbela
Panbela Therapeutics, Inc. is a clinical-stage biopharmaceutical company developing disruptive therapeutics for patients with urgent unmet medical needs. Panbela’s lead assets are Ivospemin (SBP-101) and Flynpovi. Further information can be found at www.panbela.com. Panbela’s common stock is listed on The Nasdaq Stock Market LLC under the symbol “PBLA”.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains “forward-looking statements,” including within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as: “anticipate,” “believe,” “can,” “design,” “expect,” “focus,” “intend,” “looking forward,” “may,” “plan,” “positioned,” “potential,” and “will.” All statements other than statements of historical fact are statements that should be deemed forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations, and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially and adversely from the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: (i) our ability to obtain additional funding to execute our business and clinical development plans; (ii) progress and success of our clinical development program; (iii) the impact of the current COVID-19 pandemic on our ability to conduct our clinical trials; (iv) our ability to demonstrate the safety and effectiveness of our product candidates: ivospemin (SBP-101) and eflornithine (CPP-1X); (v) our reliance on a third party for the execution of the registration trial for our product candidate Flynpovi ; (vi) our ability to obtain regulatory approvals for our product candidates, SBP-101 and CPP-1X in the United States, the European Union or other international markets; (vii) the market acceptance and level of future sales of our product candidates, SBP-101 and CPP-1X; (viii) the cost and delays in product development that may result from changes in regulatory oversight applicable to our product candidates, SBP-101 and CPP-1X; (ix) the rate of progress in establishing reimbursement arrangements with third-party payors; (x) the effect of competing technological and market developments; (xi) the costs involved in filing and prosecuting patent applications and enforcing or defending patent claims; (xii) our ability to maintain the listing of our common stock on a national securities exchange; and (xiv) such other factors as discussed in Part I, Item 1A under the caption “Risk Factors” in our most recent Annual Report on Form 10-K, any additional risks presented in our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K. Any forward-looking statement made by us in this press release is based on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to publicly update any forward-looking statement or reasons why actual results would differ from those anticipated in any such forward-looking statement, whether written or oral, whether as a result of new information, future developments or otherwise.
Contact Information:
Investors:
James Carbonara
Hayden IR
(646) 755-7412
james@haydenir.com
Media:
Tammy Groene
Panbela Therapeutics, Inc.
(952) 479-1196
IR@panbela.com
v3.24.0.1
Document And Entity Information
|
Feb. 01, 2024 |
| Document Information [Line Items] |
|
| Entity, Registrant Name |
Panbela Therapeutics, Inc.
|
| Document, Type |
8-K
|
| Document, Period End Date |
Feb. 01, 2024
|
| Entity, Incorporation, State or Country Code |
DE
|
| Entity, File Number |
001-39468
|
| Entity, Tax Identification Number |
88-2805017
|
| Entity, Address, Address Line One |
712 Vista Blvd #305
|
| Entity, Address, City or Town |
Waconia
|
| Entity, Address, State or Province |
MN
|
| Entity, Address, Postal Zip Code |
55387
|
| City Area Code |
952
|
| Local Phone Number |
479-1196
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock
|
| Trading Symbol |
PBLA
|
| Security Exchange Name |
NASDAQ
|
| Entity, Emerging Growth Company |
false
|
| Amendment Flag |
false
|
| Entity, Central Index Key |
0001029125
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Panbela Therapeutics (NASDAQ:PBLA)
Historical Stock Chart
From Apr 2024 to May 2024
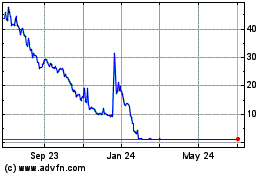
Panbela Therapeutics (NASDAQ:PBLA)
Historical Stock Chart
From May 2023 to May 2024