Amylyx Shares Rally Premarket on FDA AdCom Meeting Plans
July 05 2022 - 9:53AM
Dow Jones News
By Colin Kellaher
Shares of Amylyx Pharmaceuticals Inc. jumped more than 15% in
premarket trading Tuesday after the biopharmaceutical company said
the U.S. Food and Drug Administration plans to reconvene an
advisory committee meeting on its amyotrophic lateral sclerosis
drug AMX0035 on Sept. 7.
Amylyx said the meeting will focus on the additional analyses of
study data that the Cambridge, Mass., company submitted earlier
this year on the proposed treatment for amyotrophic lateral
sclerosis, a progressive neurodegenerative disease also known as
Lou Gehrig's disease that robs patients of their ability to move
and speak.
The FDA had previously extended its target action date on
AMX0035 to Sept. 29 from June 29 to allow more time to review the
additional analyses.
The FDA's advisory panel of neuroscience experts in March had
voted 6-to-4 that a study hadn't provided sufficient evidence that
AMX0035 works, raising fears that the FDA would reject the drug.
However, the agency's extension of the target action date raised
renewed hopes for the drug, which in June won approval from
Canadian health authorities.
Amylyx shares, which closed Friday at $19.47, were recently up
16.2% to $22.63 in premarket trading.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
July 05, 2022 09:38 ET (13:38 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
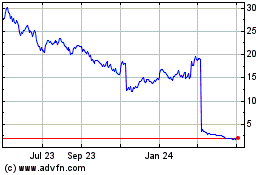
Amylyx Pharmaceuticals (NASDAQ:AMLX)
Historical Stock Chart
From Mar 2024 to Apr 2024
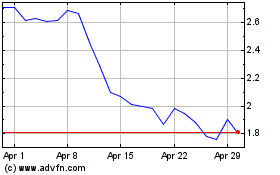
Amylyx Pharmaceuticals (NASDAQ:AMLX)
Historical Stock Chart
From Apr 2023 to Apr 2024