VistaGen Therapeutics Receives Notices of Allowance in Australia and Japan for AV-101 Patents Covering Treatment of Depressio...
November 12 2018 - 8:30AM

VistaGen Therapeutics (NASDAQ: VTGN), a clinical-stage
biopharmaceutical company developing new generation medicines for
central nervous system (CNS) diseases and disorders with high unmet
need, today announced receiving Notices of Allowance from IP
Australia and the Japan Patent Office (JPO) related to methods of
treating depression with AV-101, VistaGen’s oral NMDA
(N-methyl-D-aspartate) receptor glycine B antagonist in Phase 2
development for adjunctive treatment of major depressive disorder
(MDD).
“These patents, when issued, will extend our commercial
protection of AV-101 into Australia and Japan, two additional major
pharmaceutical markets,” stated Shawn Singh, Chief Executive
Officer of VistaGen. “As we continue to move forward with our
clinical development of AV-101 for adjunctive treatment of MDD,
having important AV-101 intellectual property outside of the U.S.
and Europe is essential for potential strategic partnering
opportunities in selected regional markets and to support our
mission to bring new treatment alternatives for CNS conditions with
unmet need to individuals around the world."
About VistaGen VistaGen Therapeutics, Inc. is a
clinical-stage biopharmaceutical company developing new generation
medicines for multiple CNS diseases and disorders with high unmet
need. For more information, please
visit www.vistagen.com and connect with VistaGen
on Twitter, LinkedIn and Facebook.
About AV-101 AV-101 is an investigational,
orally bioavailable, small molecule NMDA (N-methyl-D-aspartate)
receptor glycine B antagonist with the potential to be a treatment
for multiple CNS indications with high unmet need. AV-101 is
currently in Phase 2 clinical development in the United States for
adjunctive treatment of MDD. The FDA has granted Fast
Track designation for development of AV-101 as both a
potential adjunctive treatment of MDD and as a non-opioid treatment
for neuropathic pain.
Forward-Looking Statements This release
contains various statements concerning VistaGen's future
expectations, plans and prospects, including without limitation,
our expectations regarding development and commercialization of our
drug candidates, including AV-101 for adjunctive treatment of MDD
and as a non-opioid treatment for neuropathic pain, as well as our
intellectual property and commercial protection of AV-101, all of
which constitute forward-looking statements for the purposes of the
safe harbor provisions under the Private Securities Litigation
Reform Act of 1995. These forward-looking statements are neither
promises nor guarantees of future performance and are subject to a
variety of risks and uncertainties, many of which are beyond our
control, and may cause actual results to differ materially from
those contemplated in these forward-looking statements. Among these
risks is the possibility that (i) we may encounter unexpected
adverse events in patients during our clinical development of any
product candidate that cause us to discontinue further development,
(ii) we may not be able to successfully demonstrate the safety and
efficacy of our product candidates at each stage of clinical
development, (iii) success in preclinical studies or in early-stage
clinical trials may not be repeated or observed in ongoing or
future studies, and ongoing or future preclinical and clinical
results may not support further development of, or be sufficient to
gain regulatory approval to market our drug candidates, (iv)
decisions or actions of regulatory agencies may negatively affect
the progress of, and our ability to proceed with, further clinical
studies or to obtain marketing approval for our drug candidates,
(v) we may not be able to obtain or maintain adequate intellectual
property protection and other forms of marketing and data
exclusivity for our product candidates, (vi) we may not have access
to or be able to secure substantial additional capital to support
our operations, including our ongoing clinical development
activities; and (vii) we may encounter technical and other
unexpected hurdles in the manufacturing and development of any of
our product candidates. Certain other risks are more fully
discussed in the section entitled "Risk Factors" in our most recent
annual report on Form 10-K, and subsequent quarterly reports on
Form 10-Q, as well as discussions of potential risks,
uncertainties, and other important factors in our other filings
with the Securities and Exchange Commission (SEC). Our SEC filings
are available on the SEC's website at www.sec.gov. In
addition, any forward-looking statements represent our views only
as of the issuance of this release and should not be relied upon as
representing our views as of any subsequent date. We explicitly
disclaim any obligation to update any forward-looking
statements.
Company Contact Mark A. McPartland VistaGen
Therapeutics Inc. Phone: +1 (650) 577-3600
Email: IR@vistagen.com
Investor Contact Valter Pinto / Allison Soss
KCSA Strategic Communications Phone: +1 (212) 896-1254/+1 (212)
896-1267 Email: VistaGen@KCSA.com
Media Contact Caitlin Kasunich / Lisa Lipson
KCSA Strategic Communications Phone: +1 (212) 896-1241/+1 (508)
843-6428 Email: VistaGen@KCSA.com
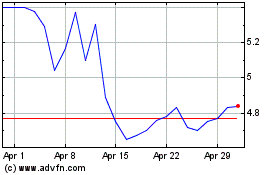
VistaGen Therapeutics (NASDAQ:VTGN)
Historical Stock Chart
From Mar 2024 to Apr 2024
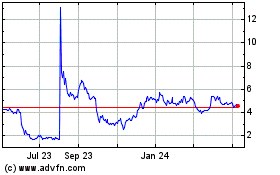
VistaGen Therapeutics (NASDAQ:VTGN)
Historical Stock Chart
From Apr 2023 to Apr 2024