0001165320
GB SCIENCES INC
false
Q1
2022
57,322
43,096
272,122
296,504
141,123
154,590
0.0001
0.0001
600,000,000
600,000,000
317,429,078
317,429,078
315,340,411
315,340,411
1
3
3
3
3
3
3
3
3
0.05
3
3
43,096
12,287
296,504
608,580
154,590
0
0.0001
0.0001
600,000,000
600,000,000
315,340,411
315,340,411
275,541,602
275,541,602
3
3
3
3
3
3
3
3
0.05
10
3
3
10
10
33.33
33.33
33.33
3
5
5
3
3
00011653202021-04-012021-06-30
thunderdome:item
iso4217:USD
00011653202021-06-30
00011653202021-03-31
iso4217:USDxbrli:shares
xbrli:shares
00011653202020-04-012020-06-30
00011653202020-03-31
00011653202020-06-30
0001165320us-gaap:CommonStockMember2021-03-31
0001165320us-gaap:AdditionalPaidInCapitalMember2021-03-31
0001165320us-gaap:RetainedEarningsMember2021-03-31
0001165320us-gaap:CommonStockMember2021-04-012021-06-30
0001165320us-gaap:AdditionalPaidInCapitalMember2021-04-012021-06-30
0001165320us-gaap:RetainedEarningsMember2021-04-012021-06-30
0001165320us-gaap:CommonStockMember2021-06-30
0001165320us-gaap:AdditionalPaidInCapitalMember2021-06-30
0001165320us-gaap:RetainedEarningsMember2021-06-30
0001165320us-gaap:CommonStockMember2020-03-31
0001165320us-gaap:AdditionalPaidInCapitalMember2020-03-31
0001165320us-gaap:RetainedEarningsMember2020-03-31
0001165320us-gaap:CommonStockMember2020-04-012020-06-30
0001165320us-gaap:AdditionalPaidInCapitalMember2020-04-012020-06-30
0001165320us-gaap:RetainedEarningsMember2020-04-012020-06-30
0001165320us-gaap:CommonStockMember2020-06-30
0001165320us-gaap:AdditionalPaidInCapitalMember2020-06-30
0001165320us-gaap:RetainedEarningsMember2020-06-30
xbrli:pure
00011653202020-04-012021-03-31
0001165320us-gaap:SegmentDiscontinuedOperationsMember2021-06-30
0001165320us-gaap:SegmentDiscontinuedOperationsMember2021-03-31
0001165320us-gaap:SegmentDiscontinuedOperationsMember2021-04-012021-06-30
0001165320us-gaap:SegmentDiscontinuedOperationsMember2020-04-012020-06-30
0001165320us-gaap:SegmentContinuingOperationsMember2021-06-30
0001165320gblx:SegmentContinuingAndDiscontinuedOperationsMember2021-06-30
0001165320us-gaap:SegmentContinuingOperationsMember2021-03-31
0001165320gblx:SegmentContinuingAndDiscontinuedOperationsMember2021-03-31
0001165320us-gaap:SegmentContinuingOperationsMember2021-04-012021-06-30
0001165320gblx:SegmentContinuingAndDiscontinuedOperationsMember2021-04-012021-06-30
0001165320us-gaap:SegmentContinuingOperationsMember2020-04-012020-06-30
0001165320gblx:SegmentContinuingAndDiscontinuedOperationsMember2020-04-012020-06-30
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMember2021-06-30
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMember2021-03-31
0001165320srt:MaximumMember2021-06-30
0001165320us-gaap:LineOfCreditMember2019-11-27
0001165320us-gaap:LineOfCreditMember2019-11-272021-03-31
0001165320us-gaap:LineOfCreditMember2021-06-30
0001165320gblx:ProductionLicenseMembergblx:NevadaMedicalMarijuanaProductionLicenseAgreementMember2017-10-23
0001165320gblx:CultivationLicenseMembergblx:NevadaMedicalMarijuanaProductionLicenseAgreementMember2017-10-23
0001165320gblx:NevadaMedicalMarijuanaProductionLicenseAgreementMember2017-10-232017-10-23
0001165320gblx:The0PercentNotePayableDatedOctober232017Membergblx:PromissoryNoteMember2017-10-23
utr:Y
0001165320gblx:The0PercentNotePayableDatedOctober232017Membergblx:PromissoryNoteMember2017-10-232017-10-23
0001165320gblx:The0PercentNotePayableDatedOctober232017Membergblx:PromissoryNoteMember2017-10-242021-06-30
0001165320gblx:The0PercentNotePayableDatedOctober232017Membergblx:PromissoryNoteMember2021-06-30
0001165320gblx:The0PercentNotePayableDatedOctober232017Membergblx:MembershipInterestPurchaseAgreementForSaleOfInterestInGBSciencesNopahLLCMembergblx:PromissoryNoteMember2020-08-10
0001165320us-gaap:RevolvingCreditFacilityMembergblx:TheJuly24NoteMember2020-07-24
0001165320gblx:TheJuly24NoteMembergblx:TecoMember2020-07-24
0001165320gblx:TheJuly24NoteMembergblx:TecoMember2020-12-292020-12-29
0001165320gblx:TheJuly24NoteMembergblx:TecoMember2020-12-29
0001165320gblx:TheJuly24NoteMembergblx:TecoMember2020-04-012021-03-31
0001165320gblx:TheJuly24NoteMembergblx:TecoMember2021-04-012021-06-30
0001165320gblx:TheJuly24NoteMembergblx:TecoMember2021-06-30
0001165320gblx:The0PercentNotePayableDatedOctober232017Memberus-gaap:ConvertibleNotesPayableMember2021-06-30
0001165320gblx:The8PercentLineOfCreditDatedNovember272019Memberus-gaap:LineOfCreditMember2021-06-30
0001165320gblx:The8PercentLineOfCreditDatedJuly242020Memberus-gaap:LineOfCreditMember2021-06-30
0001165320gblx:The6PercentNotePayableDueNovember302018Memberus-gaap:ConvertibleNotesPayableMember2021-06-30
0001165320gblx:TheAmended8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2021-06-30
0001165320gblx:The6PercentNotesPayableDueJanuary182022Memberus-gaap:ConvertibleNotesPayableMember2021-06-30
0001165320gblx:PromissoryNoteMember2021-06-30
0001165320gblx:The6PercentNotePayableDatedSeptember302023Memberus-gaap:ConvertibleDebtMember2021-06-30
0001165320gblx:The6PercentNotePayableDatedDecember312023Memberus-gaap:ConvertibleDebtMember2021-06-30
0001165320gblx:PromissoryNoteMember2021-06-30
0001165320gblx:March2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleNotesPayableMember2017-03-31
0001165320gblx:March2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleNotesPayableMemberus-gaap:CommonStockMember2017-03-012017-03-31
0001165320gblx:March2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleNotesPayableMemberus-gaap:CommonStockMember2017-03-31
0001165320gblx:WarrantsIssuedInMarch2017ConvertibleNoteOfferingMember2017-03-012017-03-31
0001165320gblx:WarrantsIssuedInMarch2017ConvertibleNoteOfferingMember2017-03-31
0001165320gblx:March2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleNotesPayableMember2017-05-31
0001165320gblx:March2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleNotesPayableMember2017-03-012017-05-31
0001165320gblx:March2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleNotesPayableMemberus-gaap:CommonStockMember2017-03-012017-05-31
0001165320gblx:WarrantsIssuedInMarch2017ConvertibleNoteOfferingMember2017-03-012017-05-31
0001165320gblx:WarrantsIssuedInMarch2017ConvertibleNoteOfferingMember2017-05-31
0001165320gblx:July2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleNotesPayableMember2017-07-31
0001165320gblx:July2017ConvertibleNoteOfferingMembergblx:PromissoryNoteMember2017-07-31
0001165320gblx:July2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleNotesPayableMemberus-gaap:CommonStockMember2017-07-012017-07-31
0001165320gblx:July2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleNotesPayableMemberus-gaap:CommonStockMember2017-07-31
0001165320gblx:WarrantsIssuedInMarch2017AndJuly2017ConvertibleNoteOfferingsMember2017-07-012017-07-31
0001165320gblx:WarrantsRelatedToJuly2017ConvertibleNoteOfferingMember2017-07-31
0001165320gblx:July2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleNotesPayableMember2017-07-012017-07-31
0001165320gblx:July2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleNotesPayableMember2017-12-31
0001165320gblx:July2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleNotesPayableMember2017-07-012017-12-31
0001165320gblx:July2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleNotesPayableMemberus-gaap:CommonStockMember2017-07-012017-12-31
0001165320gblx:WarrantsRelatedToJuly2017ConvertibleNoteOfferingMember2017-07-012017-12-31
0001165320gblx:WarrantsRelatedToJuly2017ConvertibleNoteOfferingMember2017-12-31
0001165320gblx:MarchAndJuly2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleNotesPayableMember2021-03-31
0001165320gblx:WarrantsIssuedInMarch2017AndJuly2017ConvertibleNoteOfferingsMember2020-04-012021-03-31
0001165320gblx:WarrantsIssuedInSeptember302023ConvertibleNoteOfferingMember2021-03-31
0001165320gblx:MarchAndJuly2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleDebtMember2020-04-012021-03-31
0001165320gblx:MarchAndJuly2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleDebtMember2021-03-31
0001165320gblx:MarchAndJuly2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleDebtMember2021-04-012021-06-30
0001165320gblx:MarchAndJuly2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleDebtMember2021-06-30
0001165320us-gaap:ConvertibleDebtMember2021-06-30
0001165320us-gaap:ConvertibleDebtMember2021-04-012021-06-30
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-02-28
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMemberus-gaap:CommonStockMember2019-02-282019-02-28
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMemberus-gaap:CommonStockMember2019-02-28
0001165320us-gaap:CollateralPledgedMembergblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:GBSciencesNevadaLLCMembergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-02-28
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-02-282019-02-28
0001165320gblx:CSWVenturesLPMembergblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-05-282019-05-28
0001165320gblx:CSWVenturesLPMembergblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-05-28
0001165320gblx:CSWVenturesLPMembergblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMemberus-gaap:CommonStockMember2019-05-282019-05-28
0001165320gblx:CSWVenturesLPMembergblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMemberus-gaap:CommonStockMember2019-05-28
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-07-12
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-07-122019-07-12
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMemberus-gaap:CommonStockMember2019-07-12
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-08-012019-08-01
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMemberus-gaap:CommonStockMember2019-08-01
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMemberus-gaap:CommonStockMember2019-08-012019-08-01
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-08-01
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMemberus-gaap:CommonStockMember2019-10-23
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-10-23
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-10-232019-10-23
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-04-012020-03-31
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-11-27
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMemberus-gaap:CommonStockMember2019-11-27
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-11-272019-11-27
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-12-162019-12-16
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMemberus-gaap:CommonStockMember2019-12-16
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMemberus-gaap:CommonStockMember2019-12-162019-12-16
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-12-16
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2021-01-012021-03-31
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMemberus-gaap:CommonStockMember2021-03-31
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMemberus-gaap:CommonStockMember2021-01-012021-03-31
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2021-06-30
0001165320gblx:TecoMember2021-06-30
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Memberus-gaap:ConvertibleNotesPayableMember2019-04-23
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Memberus-gaap:ConvertibleNotesPayableMember2019-04-232019-04-23
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Memberus-gaap:ConvertibleNotesPayableMember2019-04-012020-03-31
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Memberus-gaap:ConvertibleNotesPayableMember2019-10-302019-10-30
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Memberus-gaap:ConvertibleNotesPayableMember2019-10-30
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Memberus-gaap:ConvertibleNotesPayableMember2019-11-182019-11-18
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Memberus-gaap:ConvertibleNotesPayableMember2019-11-18
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Memberus-gaap:ConvertibleNotesPayableMember2020-04-22
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Memberus-gaap:ConvertibleNotesPayableMember2020-04-222020-04-22
0001165320gblx:RepaymentOfIliadNoteMemberus-gaap:JudicialRulingMember2020-07-142020-07-14
0001165320gblx:RepaymentOfIliadNoteMemberus-gaap:JudicialRulingMember2020-07-14
0001165320gblx:RepaymentOfIliadNoteMemberus-gaap:JudicialRulingMember2020-11-20
0001165320gblx:RepaymentOfIliadNoteMemberus-gaap:JudicialRulingMember2020-12-09
0001165320gblx:RepaymentOfIliadNoteMemberus-gaap:JudicialRulingMember2020-12-162020-12-16
0001165320gblx:The6PercentConvertibleNotePayableDatedDecember312023Member2020-12-18
0001165320gblx:ThreeInvestorsMembergblx:The6PercentConvertibleNotePayableDatedDecember312023Member2021-03-31
0001165320gblx:ThreeInvestorsMembergblx:The6ConvertibleNotePayableMatureInDecember2021Member2021-03-31
0001165320gblx:ThreeInvestorsMembergblx:The6ConvertibleNotePayableMatureInDecember2023Member2021-03-31
0001165320gblx:The6PercentConvertibleNotePayableIssuedWithInMoneyConversionFeaturesMember2020-04-012021-03-31
0001165320gblx:The6PercentConvertibleNotePayableIssuedWithInMoneyConversionFeaturesMember2021-03-31
0001165320gblx:The6ConvertibleNotePayableMatureInDecember2023Member2021-06-30
0001165320gblx:The6ConvertibleNotePayableMatureInDecember2023Member2021-04-012021-06-30
0001165320gblx:WarrantsIssuedToInvestorsInPrivatePlacementsMembersrt:MinimumMember2020-04-01
0001165320gblx:WarrantsIssuedToInvestorsInPrivatePlacementsMembersrt:MaximumMember2020-04-01
0001165320gblx:WarrantsIssuedToInvestorsInPrivatePlacementsMemberus-gaap:SubsequentEventMember2021-07-18
0001165320gblx:WarrantsIssuedToInvestorsInPrivatePlacementsMember2021-04-012021-06-30
0001165320gblx:PaymentOfServicesProvidedByContractorMemberus-gaap:PendingLitigationMember2020-04-222020-04-22
0001165320gblx:PaymentOfServicesProvidedByContractorMemberus-gaap:SettledLitigationMember2020-09-172020-09-17
0001165320gblx:OfficersAndDirectorsMember2021-06-30
0001165320gblx:TecoMember2019-11-152019-11-15
0001165320gblx:TecoMember2020-03-242020-03-24
0001165320gblx:TecoMember2020-03-24
utr:M
0001165320us-gaap:SecuredDebtMembergblx:TheJuly24NoteMembergblx:AjeManagementLLCMember2020-07-24
0001165320gblx:July24NoteMembergblx:TecoMember2020-07-24
0001165320us-gaap:SecuredDebtMembergblx:TheJuly24NoteMembergblx:AjeManagementLLCMember2020-07-252021-06-30
0001165320gblx:July24NoteMembergblx:TecoMember2020-12-292020-12-29
0001165320gblx:July24NoteMembergblx:TecoMember2020-12-29
0001165320gblx:TheJuly24NoteMember2020-12-292020-12-29
0001165320gblx:July24NoteMember2020-12-29
0001165320gblx:July24NoteMembergblx:TecoMember2020-12-29
0001165320gblx:July24NoteMembergblx:TecoMember2020-07-242020-07-24
0001165320gblx:TecoMember2021-04-012021-06-30
0001165320gblx:TecoMember2021-05-112021-05-11
0001165320gblx:GBSciencesNopahLLCMember2019-11-272019-11-27
0001165320gblx:GBSciencesNopahLLCMember2020-08-102020-08-10
0001165320gblx:The0PercentNotePayableDatedOctober232017Membergblx:PromissoryNoteMembergblx:GBSciencesNopahLLCMember2020-08-10
0001165320gblx:The0PercentNotePayableDatedOctober232017Membergblx:PromissoryNoteMember2020-08-10
0001165320gblx:The6ConvertiblePromissoryNoteDueJuly12022Memberus-gaap:SubsequentEventMember2021-07-02
00011653202019-04-012020-03-31
0001165320us-gaap:CommonStockMember2019-03-31
0001165320us-gaap:AdditionalPaidInCapitalMember2019-03-31
0001165320us-gaap:RetainedEarningsMember2019-03-31
0001165320us-gaap:NoncontrollingInterestMember2019-03-31
00011653202019-03-31
0001165320us-gaap:CommonStockMember2019-04-012020-03-31
0001165320us-gaap:AdditionalPaidInCapitalMember2019-04-012020-03-31
0001165320us-gaap:RetainedEarningsMember2019-04-012020-03-31
0001165320us-gaap:NoncontrollingInterestMember2019-04-012020-03-31
0001165320us-gaap:AccountingStandardsUpdate201602Membersrt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:CommonStockMember2019-03-31
0001165320us-gaap:AccountingStandardsUpdate201602Membersrt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:AdditionalPaidInCapitalMember2019-03-31
0001165320us-gaap:AccountingStandardsUpdate201602Membersrt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:RetainedEarningsMember2019-03-31
0001165320us-gaap:AccountingStandardsUpdate201602Membersrt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:NoncontrollingInterestMember2019-03-31
0001165320us-gaap:AccountingStandardsUpdate201602Membersrt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2019-03-31
0001165320us-gaap:NoncontrollingInterestMember2020-03-31
0001165320us-gaap:CommonStockMember2020-04-012021-03-31
0001165320us-gaap:AdditionalPaidInCapitalMember2020-04-012021-03-31
0001165320us-gaap:RetainedEarningsMember2020-04-012021-03-31
0001165320us-gaap:NoncontrollingInterestMember2020-04-012021-03-31
0001165320us-gaap:NoncontrollingInterestMember2021-03-31
0001165320us-gaap:ConvertibleNotesPayableMember2020-04-012021-03-31
0001165320us-gaap:ConvertibleNotesPayableMember2019-04-012020-03-31
00011653202018-03-31
00011653202018-04-08
00011653202019-08-15
0001165320gblx:WellcanaGroupLLCMembergblx:GBSciencesLouisianaLLCSaleOfEquityMember2019-11-152019-11-15
0001165320gblx:WellcanaNoteMember2020-12-162020-12-16
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Memberus-gaap:JudicialRulingMemberus-gaap:ConvertibleNotesPayableMember2020-07-142020-07-14
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Membergblx:JudgmentSettlementAgreementMemberus-gaap:ConvertibleNotesPayableMember2020-11-20
0001165320gblx:WellcanaPlusLLCMembergblx:The8PercentConvertiblePromissoryNoteDatedApril232019Membergblx:JudgmentSettlementAgreementMemberus-gaap:ConvertibleNotesPayableMember2020-11-20
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Membergblx:WellcanaPlusLLCMembergblx:JudgmentSettlementAgreementMemberus-gaap:ConvertibleNotesPayableMember2020-11-202020-12-08
0001165320gblx:TecoMember2019-11-152019-11-15
0001165320gblx:TecoMember2019-11-15
0001165320gblx:GBSciencesNopahLLCMember2019-11-272019-11-27
0001165320gblx:GBSciencesNopahLLCMember2020-08-10
0001165320gblx:GBSciencesNopahLLCMembergblx:The0PercentNotePayableDatedOctober232017Member2020-08-10
0001165320us-gaap:SegmentDiscontinuedOperationsMember2020-03-31
0001165320gblx:TecoMember2019-04-012020-03-31
0001165320gblx:ProductionLicenseMember2019-04-012020-03-31
0001165320gblx:ProductionLicenseMember2019-03-31
0001165320gblx:ProductionLicenseMember2020-03-31
0001165320srt:MinimumMember2020-04-012021-03-31
0001165320srt:MaximumMember2020-04-012021-03-31
0001165320gblx:GBSciencesLouisianaLLCMember2021-03-31
0001165320us-gaap:SegmentContinuingOperationsMember2020-03-31
0001165320gblx:SegmentContinuingAndDiscontinuedOperationsMember2020-03-31
0001165320us-gaap:SegmentContinuingOperationsMember2020-04-012021-03-31
0001165320us-gaap:SegmentDiscontinuedOperationsMember2020-04-012021-03-31
0001165320gblx:SegmentContinuingAndDiscontinuedOperationsMember2020-04-012021-03-31
0001165320us-gaap:SegmentContinuingOperationsMember2019-04-012020-03-31
0001165320us-gaap:SegmentDiscontinuedOperationsMember2019-04-012020-03-31
0001165320gblx:SegmentContinuingAndDiscontinuedOperationsMember2019-04-012020-03-31
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMembergblx:NevadaSubsidiariesMember2020-04-012021-03-31
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMembergblx:GBSciencesLouisianaLLCMember2020-04-012021-03-31
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMember2020-04-012021-03-31
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMembergblx:NevadaSubsidiariesMember2019-04-012020-03-31
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMembergblx:GBSciencesLouisianaLLCMember2019-04-012020-03-31
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMember2019-04-012020-03-31
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMember2020-03-31
0001165320us-gaap:AccountingStandardsUpdate201602Member2019-04-01
0001165320srt:MinimumMember2021-03-31
0001165320srt:MaximumMember2021-03-31
0001165320us-gaap:GeneralAndAdministrativeExpenseMemberus-gaap:SegmentContinuingOperationsMember2020-04-012021-03-31
0001165320us-gaap:GeneralAndAdministrativeExpenseMemberus-gaap:SegmentContinuingOperationsMember2019-04-012020-03-31
0001165320gblx:The0PercentNotePayableDatedOctober232017Membergblx:PromissoryNoteMember2017-10-242021-03-31
0001165320gblx:The0PercentNotePayableDatedOctober232017Membergblx:PromissoryNoteMember2021-03-31
0001165320gblx:The0PercentNotePayableDatedOctober232017Membergblx:PromissoryNoteMember2020-04-012021-03-31
0001165320us-gaap:LineOfCreditMember2019-11-282021-03-31
0001165320us-gaap:LineOfCreditMember2021-03-31
0001165320gblx:The8NotePayableDatedMay72020Memberus-gaap:ConvertibleDebtMember2020-05-072020-05-07
0001165320gblx:The8NotePayableDatedMay72020Memberus-gaap:ConvertibleDebtMember2020-05-07
0001165320gblx:RepricedPreexistingWarrantsWithConvertibleDebtIssuanceMember2020-05-07
0001165320gblx:RepricedPreexistingWarrantsWithConvertibleDebtIssuanceMember2020-05-06
0001165320gblx:RepricedPreexistingWarrantsWithConvertibleDebtIssuanceMember2020-05-072020-05-07
0001165320gblx:The8NotePayableDatedMay72020Memberus-gaap:ConvertibleDebtMember2020-04-012021-03-31
0001165320gblx:The8NotePayableDatedMay72020Memberus-gaap:ConvertibleDebtMember2020-10-052020-10-05
0001165320gblx:TheJuly24NoteMember2020-07-24
0001165320gblx:TheJuly24NoteMembergblx:TecoMember2021-03-31
0001165320gblx:The0PercentNotePayableDatedOctober232017Memberus-gaap:ConvertibleNotesPayableMember2021-03-31
0001165320gblx:The8PercentLineOfCreditDatedNovember272019Memberus-gaap:LineOfCreditMember2021-03-31
0001165320gblx:The8PercentLineOfCreditDatedJuly242020Memberus-gaap:LineOfCreditMember2021-03-31
0001165320gblx:The6PercentNotePayableDueNovember302018Memberus-gaap:ConvertibleNotesPayableMember2021-03-31
0001165320gblx:TheAmended8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2021-03-31
0001165320gblx:The6PercentNotesPayableDueJanuary182022Memberus-gaap:ConvertibleNotesPayableMember2021-03-31
0001165320gblx:PromissoryNoteMember2021-03-31
0001165320gblx:The6PercentNotePayableDatedSeptember302023Memberus-gaap:ConvertibleDebtMember2021-03-31
0001165320gblx:The6PercentNotePayableDatedDecember312023Memberus-gaap:ConvertibleDebtMember2021-03-31
0001165320gblx:PromissoryNoteMember2021-03-31
0001165320gblx:The0PercentNotePayableDatedOctober232017Memberus-gaap:ConvertibleNotesPayableMember2020-03-31
0001165320gblx:The8PercentLineOfCreditDatedNovember272019Memberus-gaap:LineOfCreditMember2020-03-31
0001165320gblx:The6PercentNotePayableDueNovember302018Memberus-gaap:ConvertibleNotesPayableMember2020-03-31
0001165320gblx:TheAmended8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2020-03-31
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Memberus-gaap:ConvertibleNotesPayableMember2020-03-31
0001165320gblx:PromissoryNoteMember2020-03-31
0001165320us-gaap:ConvertibleDebtMember2021-03-31
0001165320us-gaap:ConvertibleDebtMember2020-04-012021-03-31
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2021-03-31
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2020-04-012021-03-31
0001165320gblx:TecoMember2021-03-31
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Memberus-gaap:ConvertibleNotesPayableMember2020-04-012021-03-31
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Memberus-gaap:ConvertibleNotesPayableMember2020-11-212021-03-31
0001165320gblx:JudgmentSettlementAgreementMemberus-gaap:JudicialRulingMember2020-04-012021-03-31
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Memberus-gaap:JudicialRulingMemberus-gaap:ConvertibleNotesPayableMember2020-04-012021-03-31
0001165320gblx:The8PercentConvertiblePromissoryNoteDatedApril232019Memberus-gaap:JudicialRulingMemberus-gaap:ConvertibleNotesPayableMember2021-03-31
0001165320gblx:The6PercentConvertibleNotePayableDatedDecember312023Member2021-03-31
0001165320gblx:The6PercentConvertibleNotePayableDatedDecember312023Member2020-04-012021-03-31
0001165320us-gaap:FurnitureAndFixturesMember2021-03-31
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMemberus-gaap:FurnitureAndFixturesMember2021-03-31
0001165320us-gaap:ComputerEquipmentMember2021-03-31
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMemberus-gaap:ComputerEquipmentMember2021-03-31
0001165320us-gaap:MachineryAndEquipmentMember2021-03-31
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMemberus-gaap:MachineryAndEquipmentMember2021-03-31
0001165320us-gaap:LeaseholdsAndLeaseholdImprovementsMember2021-03-31
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMemberus-gaap:LeaseholdsAndLeaseholdImprovementsMember2021-03-31
0001165320us-gaap:ConstructionInProgressMember2021-03-31
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMemberus-gaap:ConstructionInProgressMember2021-03-31
0001165320gblx:FinanceLeaseRightOfUseAssetMember2021-03-31
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMembergblx:FinanceLeaseRightOfUseAssetMember2021-03-31
0001165320gblx:WarrantsIssuedToInvestorsInPrivatePlacementsMember2020-04-012021-03-31
0001165320gblx:ScientistAndResearcherMember2020-04-012021-03-31
0001165320us-gaap:EmployeeStockOptionMembergblx:ScientistAndResearcherMember2020-04-012021-03-31
0001165320gblx:ConvertibleWarrantMember2020-04-012021-03-31
0001165320gblx:MarchAndJuly2017ConvertibleNoteOfferingMemberus-gaap:ConvertibleNotesPayableMember2020-04-012021-03-31
0001165320gblx:ConvertibleWarrantMember2021-03-31
0001165320gblx:FormerDirectorMember2020-11-16
0001165320gblx:FormerDirectorMember2020-11-162020-11-16
0001165320gblx:EmployeesAndDirectorWarrantsMembergblx:EmployeesAndDirectorsMember2020-12-07
0001165320gblx:EmployeesAndDirectorWarrantsMembergblx:EmployeesAndDirectorsMember2020-12-072020-12-07
0001165320gblx:EmployeesAndDirectorsMember2020-12-152020-12-15
0001165320us-gaap:EmployeeStockOptionMembergblx:EmployeesAndDirectorsMember2020-12-152020-12-15
0001165320us-gaap:EmployeeStockOptionMembergblx:EmployeesAndDirectorsMemberus-gaap:ShareBasedCompensationAwardTrancheOneMember2020-12-152020-12-15
0001165320us-gaap:EmployeeStockOptionMembergblx:EmployeesAndDirectorsMemberus-gaap:ShareBasedCompensationAwardTrancheTwoMember2020-12-152020-12-15
0001165320us-gaap:EmployeeStockOptionMembergblx:EmployeesAndDirectorsMemberus-gaap:ShareBasedCompensationAwardTrancheThreeMember2020-12-152020-12-15
0001165320gblx:EmployeesAndDirectorsMember2020-04-012021-03-31
0001165320gblx:EmployeesAndDirectorsMember2021-03-31
0001165320gblx:CurrentEmployeesMember2020-12-15
0001165320gblx:CurrentEmployeesMember2020-12-152020-12-15
0001165320us-gaap:EmployeeStockOptionMember2020-12-152020-12-15
0001165320gblx:CompensationWarrantsIssuedToBrokersMember2021-01-02
0001165320gblx:CompensationWarrantsIssuedToBrokersMembersrt:MinimumMember2021-01-01
0001165320gblx:CompensationWarrantsIssuedToBrokersMembersrt:MaximumMember2021-01-01
0001165320gblx:CompensationWarrantsIssuedToBrokersMember2021-01-01
0001165320gblx:CompensationWarrantsIssuedToBrokersMember2021-01-022021-01-02
0001165320gblx:ReplacementWarrantsMember2021-02-08
0001165320gblx:WarrantsMember2021-03-31
0001165320gblx:WarrantsMembersrt:MinimumMember2021-03-31
0001165320gblx:WarrantsMembersrt:MaximumMember2021-03-31
0001165320us-gaap:ShareBasedPaymentArrangementEmployeeMember2021-03-31
0001165320us-gaap:ShareBasedPaymentArrangementNonemployeeMember2021-03-31
0001165320gblx:The8PercentSeniorSecuredConvertiblePromissoryNoteDatedFebruary282019Membergblx:SeniorSecuredConvertiblePromissoryNoteMember2019-12-31
0001165320gblx:WarrantsIssuedToInvestorsInPrivatePlacementsMember2019-07-11
0001165320gblx:WarrantsIssuedToInvestorsInPrivatePlacementsMembersrt:MinimumMember2019-07-11
0001165320gblx:WarrantsIssuedToInvestorsInPrivatePlacementsMembersrt:MaximumMember2019-07-11
0001165320gblx:WarrantsIssuedToInvestorsInPrivatePlacementsMember2019-04-012019-07-11
0001165320gblx:WarrantsIssuedToInvestorsInPrivatePlacementsMember2019-07-122019-07-12
0001165320gblx:WarrantsIssuedToInvestorsInPrivatePlacementsMember2019-07-12
0001165320gblx:WarrantsIssuedToInvestorsInPrivatePlacementsMembersrt:MinimumMember2019-12-31
0001165320gblx:WarrantsIssuedToInvestorsInPrivatePlacementsMembersrt:MaximumMember2019-12-31
0001165320gblx:WarrantsIssuedToInvestorsInPrivatePlacementsMember2019-12-012019-12-31
0001165320srt:MinimumMember2019-04-012020-03-31
0001165320srt:MaximumMember2019-04-012020-03-31
0001165320us-gaap:RestrictedStockMembergblx:GBSciencesInc2007AmendedStockOptionPlanMember2008-02-06
0001165320us-gaap:EmployeeStockOptionMembergblx:The2014EquityCompensationPlanMember2015-06-30
0001165320gblx:GbSciencesInc2018StockPlanMember2018-10-25
0001165320us-gaap:EmployeeStockOptionMember2020-04-012021-03-31
0001165320us-gaap:EmployeeStockOptionMember2019-04-012020-03-31
0001165320us-gaap:RestrictedStockMember2020-04-012021-03-31
0001165320us-gaap:ShareBasedPaymentArrangementEmployeeMember2019-03-31
0001165320us-gaap:ShareBasedPaymentArrangementEmployeeMember2018-04-012019-03-31
0001165320us-gaap:ShareBasedPaymentArrangementEmployeeMember2019-04-012020-03-31
0001165320us-gaap:ShareBasedPaymentArrangementEmployeeMember2020-03-31
0001165320us-gaap:ShareBasedPaymentArrangementEmployeeMember2020-04-012021-03-31
0001165320us-gaap:ShareBasedPaymentArrangementNonemployeeMember2019-03-31
0001165320us-gaap:ShareBasedPaymentArrangementNonemployeeMember2018-04-012019-03-31
0001165320us-gaap:ShareBasedPaymentArrangementNonemployeeMember2019-04-012020-03-31
0001165320us-gaap:ShareBasedPaymentArrangementNonemployeeMember2020-03-31
0001165320us-gaap:ShareBasedPaymentArrangementNonemployeeMember2020-04-012021-03-31
0001165320gblx:AgreementWithLouisianaStateUniversityAgCenterMember2017-09-182017-09-18
0001165320gblx:AgreementWithLouisianaStateUniversityAgCenterMember2017-09-18
0001165320gblx:AgreementWithLouisianaStateUniversityAgCenterMember2020-04-012021-03-31
0001165320gblx:AgreementWithLouisianaStateUniversityAgCenterMember2017-09-182021-03-31
0001165320gblx:AgreementWithLouisianaStateUniversityAgCenterMember2020-08-242020-08-24
0001165320gblx:UnpaidSeveranceCompensationMembergblx:KseniaGriswoldMember2020-11-162020-11-16
0001165320gblx:UnpaidSeveranceCompensationMembergblx:KseniaGriswoldMember2021-01-022021-01-02
0001165320gblx:UnpaidSeveranceCompensationMembergblx:KseniaGriswoldMember2021-01-02
0001165320gblx:UnpaidSeveranceCompensationMembergblx:LeslieBocskorMember2020-11-162020-11-16
0001165320gblx:LeslieBocskorMember2020-11-16
0001165320gblx:LeslieBocskorMember2020-11-162020-11-16
0001165320gblx:LeslieBocskorMember2020-04-012021-03-31
0001165320gblx:LeslieBocskorMember2019-04-012020-03-31
0001165320gblx:JohnDavisMember2021-03-31
0001165320gblx:WellcanaGroupLLCMembergblx:GBSciencesLouisianaLLCMember2019-11-152019-11-15
0001165320gblx:WellcanaGroupLLCMembergblx:GBSciencesLouisianaLLCMember2019-11-15
0001165320gblx:WellcanaNoteMember2021-03-31
0001165320gblx:WellcanaNoteMember2020-04-012021-03-31
0001165320gblx:WellcanaNoteMember2019-11-15
00011653202019-11-15
0001165320gblx:GBSciencesLouisianaLLCMember2019-11-152019-11-15
0001165320gblx:GBSciencesLouisianaLLCMember2019-11-15
00011653202019-11-152019-11-15
0001165320gblx:WellcanaGroupLLCMembergblx:GBSciencesLouisianaLLCMember2020-08-242020-10-15
0001165320gblx:WellcanaGroupLLCMembergblx:GBSciencesLouisianaLLCMember2020-08-23
0001165320gblx:WellcanaGroupLLCMembergblx:GBSciencesLouisianaLLCMember2020-08-24
0001165320gblx:WellcanaNoteMember2020-06-012020-10-15
0001165320gblx:WellcanaNoteMember2020-03-31
0001165320gblx:WellcanaGroupLLCMembergblx:GBSciencesLouisianaLLCMember2020-08-012020-08-31
0001165320gblx:WellcanaGroupLLCMembergblx:GBSciencesLouisianaLLCMember2020-09-012020-09-30
0001165320gblx:WellcanaGroupLLCMembergblx:GBSciencesLouisianaLLCMember2020-12-31
0001165320gblx:WellcanaGroupLLCMembergblx:GBSciencesLouisianaLLCMember2020-10-15
0001165320gblx:WellcanaGroupLLCMembergblx:GBSciencesLouisianaLLCMember2020-12-08
0001165320gblx:WellcanaGroupLLCMembergblx:GBSciencesLouisianaLLCMember2020-10-292020-10-29
0001165320gblx:WellcanaGroupLLCMembergblx:GBSciencesLouisianaLLCMember2020-12-082020-12-08
0001165320gblx:WellcanaGroupLLCMembergblx:GBSciencesLouisianaLLCMember2020-12-162020-12-16
0001165320us-gaap:LineOfCreditMembergblx:TecoNoteMember2019-11-15
0001165320us-gaap:SecuredDebtMembergblx:TheJuly24NoteMembergblx:AjeManagementLLCMember2020-07-252021-03-31
0001165320gblx:TheJuly24NoteMember2020-12-29
0001165320gblx:TecoMember2021-03-31
0001165320gblx:TecoMember2020-04-012021-03-31
0001165320gblx:The0PercentNotePayableDatedOctober232017Membergblx:PromissoryNoteMembergblx:GBSciencesNopahLLCMember2019-11-27
0001165320gblx:The0PercentNotePayableDatedOctober232017Membergblx:PromissoryNoteMember2019-11-27
0001165320us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMember2020-04-012021-03-31
0001165320us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMembergblx:CustomerOneMember2020-04-012021-03-31
0001165320us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMembergblx:CustomerTwoMember2020-04-012021-03-31
0001165320us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMembergblx:CustomerThreeMember2020-04-012021-03-31
0001165320us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMember2019-04-012020-03-31
0001165320us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMembergblx:CustomerOneMember2019-04-012020-03-31
0001165320us-gaap:SalesRevenueNetMemberus-gaap:CustomerConcentrationRiskMembergblx:CustomerTwoMember2019-04-012020-03-31
0001165320stpr:NV2019-04-012020-03-31
0001165320us-gaap:SalesRevenueNetMemberus-gaap:GeographicConcentrationRiskMemberstpr:NV2019-04-012020-03-31
0001165320stpr:LA2019-04-012020-03-31
0001165320us-gaap:SalesRevenueNetMemberus-gaap:GeographicConcentrationRiskMemberstpr:LA2019-04-012020-03-31
0001165320gblx:TecoMemberus-gaap:SubsequentEventMember2021-05-112021-05-11
0001165320us-gaap:SubsequentEventMember2021-06-142021-06-14
0001165320us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMember2021-04-012021-06-30
0001165320gblx:NevadaSubsidiariesMember2021-04-012021-06-30
0001165320gblx:GBSciencesLouisianaLLCSaleOfEquityMember2020-04-012021-03-31
0001165320gblx:NevadaSubsidiariesMember2020-04-012021-03-31
This prospectus relates to the resale, by the Selling Security Holders identified in this prospectus, of up to an aggregate of 119,899,091 shares of our common stock, par value $0.0001 per share (“Common Stock”), issuable upon the conversion of convertible debt issued in private placements and issuable upon the exercise of warrants and options issued in private placements and to employees and consultants in exchange for services rendered to the Company.
The Selling Security Holders may sell all or a portion of the shares of Common Stock from time to time in market transactions through any market on which our shares of Common Stock are then traded, in negotiated transactions or otherwise, and at prices and on terms that will be determined by the then prevailing market price or at negotiated prices directly or through a broker or brokers, who may act as agent or as principal or by a combination of such methods of sale. See “Plan of Distribution.”
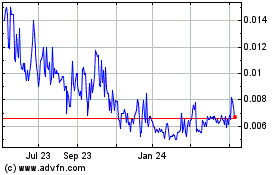
Our Common Stock is listed on The OTC Markets under the symbol “GBLX.” On September 7, 2021, the last reported sale price of our Common Stock was $0.037 per share.
The date of this prospectus is ___, 2021.
RISK FACTORS
This offering involves a high degree of risk. You should carefully consider the risks and uncertainties described below in addition to the other information contained in this prospectus before deciding whether to invest in shares of our common stock. If any of the following risks actually occur, our business, financial condition or operating results could be harmed. In that case, the trading price of our common stock could decline and you may lose part or all of your investment. In the opinion of management, the risks discussed below represent the material risks known to the Company. Additional risks and uncertainties not currently known to us or that we currently deem immaterial may also impair our business operations and adversely affect the market price of our common stock.
RISKS RELATING TO OUR BUSINESS AND INDUSTRY
We have a limited operating history, which may make it difficult for investors to predict future performance based on current operations.
We have a limited operating history upon which investors may base an evaluation of our potential future performance. In particular, we have not proven that we can supply growing equipment in a manner that enables us to be profitable and meet customer requirements, develop intellectual property to enhance our product lines, obtain the necessary permits to develop medical grade cannabis, develop and maintain relationships with key manufacturers and strategic partners to extract value from our intellectual property, raise sufficient capital in the public and/or private markets, or respond effectively to competitive pressures. As a result, there can be no assurance that we will be able to develop or maintain consistent revenue sources, or that our operations will be profitable and/or generate positive cash flows.
Any forecasts we make about our operations may prove to be inaccurate. We must, among other things, determine appropriate risks, rewards, and level of investment in our product lines, respond to economic and market variables outside of our control, respond to competitive developments and continue to attract, retain and motivate qualified employees. There can be no assurance that we will be successful in meeting these challenges and addressing such risks and the failure to do so could have a materially adverse effect on our business, results of operations and financial condition. Our prospects must be considered in light of the risks, expenses, and difficulties frequently encountered by companies in the early stage of development. As a result of these risks, challenges and uncertainties, the value of your investment could be significantly reduced or completely lost.
Our independent auditors’ report for the fiscal years ended March 31, 2021 and 2020 have expressed doubts about our ability to continue as a going concern;
Due to the uncertainty of our ability to meet our current operating and capital expenses, in our audited annual financial statements as of and for the years ended March 31, 2021 and 2020 our independent auditors included a note to our financial statements regarding concerns about our ability to continue as a going concern. The Company has incurred recurring losses and has generated limited revenue since inception. These factors and the need for additional financing in order for the Company to meet its business plan, raise substantial doubt about the ability to continue as a going concern. The presence of the going concern note to our financial statements may have an adverse impact on the relationships we are developing and plan to develop with third parties as we continue the commercialization of our products and could make it challenging and difficult for us to raise additional financing, all of which could have a material adverse impact on our business and prospects and result in a significant or complete loss of your investment.
We have incurred significant losses in prior periods, and losses in the future could cause the quoted price of our Common Stock to decline or have a material adverse effect on our financial condition, our ability to pay our debts as they become due and on our cash flows.
We have incurred significant losses in prior periods. For the years ended March 31, 2021 and 2020, we incurred net losses of $3,725,027 and $12,373,579 respectively, and we had an accumulated deficit of $103,886,232 and $97,387,205 respectively. Any losses in the future could cause the quoted price of our common stock to decline or have a material adverse effect on our financial condition, our ability to pay our debts as they become due, and on our cash flows.
We will need additional capital to sustain our operations and will need to seek further financing, which we may not be able to obtain on acceptable terms or at all. If we fail to raise additional capital, as needed, our ability to implement our business plan could be compromised.
We have limited capital resources and operations. To date, our operations have been funded primarily from the proceeds of debt and equity financings. We expect to require substantial additional capital in the near future to implement our strategies, develop our intellectual property base, and establish our targeted levels of commercial production. There is no assurance that we will be able to raise the amount of capital needed for future growth plans.
Even if financing is available, it may not be on terms that are acceptable. If unable to raise the necessary capital at the times required, the Company may have to materially change the business plan, including delaying implementation of aspects of the business plan or curtailing or abandoning the business plan. Even if we obtain financing for our near-term operations, we expect that we will require additional capital thereafter, especially if we are to develop our Science division and start to conduct, individually or with joint venture partners, pre-clinical and clinical trials for potential pharmaceutical, or nutraceutical products derived from cannabis. Our capital needs will depend on numerous factors including: (i) our profitability; (ii) the release of competitive products by our competition; (iii) the level of our investment requirements for research and development; and (iv) the amount of our capital expenditures, including acquisitions. We cannot assure you that we will be able to obtain capital in the future to meet our needs.
If we raise additional funds through the issuance of equity or convertible debt securities, the percentage ownership held by our existing stockholders will be reduced and our stockholders may experience significant dilution. In addition, new securities may contain rights, preferences or privileges that are senior to those of our common stock. If we raise additional capital by incurring debt, this will result in increased interest expense. If we raise additional funds through the issuance of securities, market fluctuations in the price of our shares of common stock could limit our ability to obtain equity financing.
We cannot give you any assurance that any additional financing will be available to us, or if available, will be on terms favorable to us. If we are unable to raise capital when needed, our business, financial condition, and results of operations would be materially adversely affected, and we could be forced to reduce or discontinue our operations.
Drug research and development programs typically involves huge expenditures, long periods to obtain FDA approvals and the potential that such prospective pharmaceutical products will not prove to be safe and effective.
The production of FDA-approved pharmaceutical products and related drug is typically a highly expensive a long and drawn out process, typically involving hundreds of millions of dollars and a decade or more to achieve. Although we believe that some, if not all, of our planned cannabinoid based pharmaceutical protocols can qualify for “orphan drug” status and be accelerated through the FDA approval process, there can be no assurance that this will be the case.
In addition, we do not now have, and do not expect in the foreseeable future to have, the capital resources to fund our drug discovery programs, nor do we have the infrastructure to conduct such program alone. For that reason, we intend to engage in joint ventures with third parties, including hospitals, clinics, foundations and other qualified sources. Although we are in preliminary discussions with various potential partners, to date, we have not entered into any definitive drug development joint venture or partnership agreement. Our failure or inability to enter into one or more drug development agreements will materially and adversely affect our ability to develop our Science division. Even if we are able to obtain such joint drug development agreements there can be no assurance that it will be on terms and conditions that will be favorable to us.
There is the further risk that the anticipated costs of producing an FDA approved drug will not escalate to the point that will cause us and any of our prospective development partners to abandon such efforts.
Even if we do develop an FDA-approved pharmaceutical product, there is the risk that it will not be saleable to a major pharmaceutical company (either before or after completion of the FDA approval process), or that other competing drugs will not be produced providing the same medical benefits.
Accordingly, there is a significant risk that we will never be able to generate a return on our investment, and we could lose our entire investment in GBS Global Biopharma, Inc. Either of such events, would have a material adverse effect on our business prospects and equity value.
There has been limited study on the effects of cannabinoids and future clinical research studies may lead to conclusions that dispute or conflict with our understanding and belief regarding the medical benefits, viability, safety, efficacy, dosing and social acceptance of cannabinoid-based active ingredients.
Research regarding the medical benefits, viability, safety, efficacy and dosing of cannabinoids (such as CBD) remains in relatively early stages. There have been few clinical trials on the benefits of cannabinoids conducted by us or by others, but the number of trials is growing.
Future research and clinical trials may draw opposing conclusions to statements contained in the articles, reports and studies we have relied on or could reach different or negative conclusions regarding the medical benefits, viability, safety, efficacy, dosing or other facts and perceptions related to cannabinoid-containing prescription medicines. However, our proprietary formulations will have been through the rigorous premarket approval process of the US FDA prior to marketing.
Federal law prohibits the use of cannabis for the purposes in which the Company expects to engage.
Under the federal Controlled Substances Act (“CSA”), cannabis is deemed to be a Schedule One narcotic that has no medical benefit. Therefore, a range of activities including cultivation and the personal use of cannabis is prohibited and is a criminal offense. Unless and until Congress amends the CSA with respect to medical cannabis, as to the timing or scope of any which amendments there can be no assurance, there is a risk that federal authorities may enforce current federal law. The risk of strict enforcement of the CSA in light of Congressional activity, judicial holdings, and stated federal policy remains uncertain.
The current policy and regulations of the Federal government and its agencies, including the U.S. Drug Enforcement Agency and the FDA, are that cannabis has no medical benefit and a range of activities including cultivation and use of cannabis for personal use is prohibited on the basis of Federal law. Although thirty-three states and District of Columbia have passed legislation permitting the cultivation and dispensing of medical cannabis, these laws are, in many jurisdictions, subject to strict regulation and limitations and are still being developed. Active enforcement of the current federal regulatory position on cannabis on a regional or national basis may directly and adversely affect the ability of the Company to develop its business plan even though it is allowed by state regulation in the various states in which the Company intends to operate. Although research and development in the growing and processing of cannabis products for medicinal purposes and in seeking to obtain state permits for the cultivation and sale of cannabis products are not in violation of Federal law, our business plan to conduct our Solutions and Products divisions, even if conducted within the parameters of any state licenses or permits we are able to obtain, will violate federal laws, as currently in effect. Accordingly, although the Company was successful in obtaining a cultivation and production license in Nevada or other states and operates pursuant to such licenses, if federal law does not change, we believe the Company will at that time be in violation of federal law. If existing federal laws are enforced by the United States Department of Justice or the FDA, it is likely that our proposed business will be significantly and materially adversely affected.
Because the Company's sales are subject to IRC 280E, we may owe federal income taxes even though we are incurring losses.
Under the federal Controlled Substances Act (“CSA”), cannabis is deemed to be a Schedule One narcotic that has no medical benefit. The production and distribution of Schedule One narcotics is subject to Internal Revenue Code Section 280E, which prohibits the Company from deducting any ordinary and necessary business expenses from taxable gross profit related to the sale of cannabis products. Without the deduction of business expenses, it is possible that the Company will owe income taxes while generating losses. If we are unable to pay those taxes we may be subject to penalties and IRS enforcement action.
FDA regulation of marijuana and the possible registration of facilities where medical marijuana is grown could negatively affect the cannabis industry which would directly affect our financial condition.
Should the federal government legalize marijuana for medical use, it is possible that the U.S. Food and Drug Administration (FDA) would seek to regulate it under the Food, Drug and Cosmetics Act of 1938. Additionally, the FDA may issue rules and regulations including cGMPs (current good manufacturing practices) related to the growth, cultivation, harvesting and processing of medical marijuana. Clinical trials may be needed to verify efficacy and safety. It is also possible that the FDA would require that facilities where medical marijuana is grown be registered with the FDA and comply with certain federally prescribed regulations. In the event that some or all of these regulations are imposed, we do not know what the impact would be on the medical marijuana industry, what costs, requirements and possible prohibitions may be enforced.
If no additional states allow the medicinal use of cannabis, or if one or more states that currently allow it reverse their position, we may not be able to continue our growth, or the market for our products and services may decline.
Currently, thirty-three states and the District of Columbia allow the use of medicinal cannabis. While we believe that the number of states that allow the use of medicinal cannabis will grow, there can be no assurance that it will, and if it does not, there can be no assurance that the thirty-three existing states and/or the District of Columbia won’t reverse their position and disallow it. If either of these things happens, then not only will the growth of our business be materially impacted, we may experience declining revenue as the market for our products and services declines.
Because the business activities of some of our customers are illegal under Federal law, we may be deemed to be aiding and abetting illegal activities through the services that we provide to those customers. As a result, we may be subject to actions by law enforcement authorities which would materially and adversely affect our business.
We provide services to customers that are engaged in businesses involving the possession, use, cultivation, and transfer of cannabis. As a result, law enforcement authorities may seek to bring an action or actions against us, including, but not limited, to a claim of aiding and abetting another’s criminal activities. Such an action would have a material effect on our business and operations.
In the states where medicinal cannabis is permitted, local laws and regulations could adversely affect our clients, including causing some of them to close, which would materially and adversely affect our business.
Even in areas where the medicinal use of cannabis is legal under state law, there are also local laws and regulations that affect our clients. These local laws and regulations may cause some of our customers to close and having a material effect on our business and operations. In addition, the enforcement of identical rules or regulations as it pertains to medicinal cannabis may vary from municipality to municipality, or city to city.
Variations in state and local regulation and enforcement in states that have legalized medical cannabis that may restrict cannabis-related activities, including activities related to medical cannabis may negatively impact our revenues and profits.
Individual state laws do not always conform to the federal standard or to other states laws. A number of states have decriminalized cannabis to varying degrees, other states have created exemptions specifically for medical cannabis, and several have both decriminalization and medical laws. Variations exist among states that have legalized, decriminalized, or created medical cannabis exemptions. For example, Colorado has limits on the number of cannabis plants that can be homegrown. In most states, the cultivation of cannabis for personal use continues to be prohibited except for those states that allow small-scale cultivation by the individual in possession of medical cannabis needing care or that person’s caregiver. Active enforcement of state laws that prohibit personal cultivation of cannabis may indirectly and adversely affect our business and our revenue and profits.
It is possible that federal or state legislation could be enacted in the future that would prohibit us from selling our products or any resulting cannabis products, and if such legislation were enacted, it could prevent us from generating revenue, leading to a loss in your investment.
We are not aware of any federal or state regulation that regulates the sale of indoor cultivation equipment to medical or recreational cannabis growers. The extent to which the regulation of drug paraphernalia under the CSA is applicable to our business and the sale of our products is found in the definition of “drug paraphernalia.” Drug paraphernalia means any equipment, product, or material of any kind that is primarily intended or designed for use in manufacturing, compounding, converting, concealing, producing processing, preparing, injecting, ingesting, inhaling, or otherwise introducing into the human body a controlled substance, possession of which is unlawful.
If federal and/or state legislation is enacted which prohibits the sale of our growing equipment to medical cannabis growers, our revenues would decline, leading to a loss of a material portion of your investment.
Prospective customers may be deterred from doing business with a company with a significant nationwide online presence because of fears of federal or state enforcement of laws prohibiting possession and sale of medical or recreational cannabis.
Internet websites are visible by people everywhere, not just in jurisdictions where the medical or recreational use of cannabis is considered legal. Our website is visible in jurisdictions where medicinal and/or recreational use of cannabis is not permitted and, as a result, we may be found to be violating the laws of those jurisdictions. We could lose potential customers as they could fear federal prosecution. In most states in which the production and sale of cannabis have been legalized, there are additional laws or licenses required and some states altogether prohibit home cultivation, all of which could make the loss of potential customers more likely.
We may not obtain the necessary permits and authorizations to operate the cannabis business.
We may not be able to obtain or maintain the necessary licenses, permits, authorizations, or accreditations, or may only be able to do so at great cost, to operate its medical cannabis business. In addition, we may not be able to comply fully with the wide variety of laws and regulations applicable to the medical cannabis industry. Failure to comply with or to obtain the necessary licenses, permits, authorizations, or accreditations could result in restrictions on our ability to operate the medical cannabis business, which could have a material adverse effect on our business.
Any failure on our part to comply with applicable regulations could prevent us from being able to carry on our business.
Nevada Department of Taxation inspectors routinely assess the Teco Facility for compliance with applicable regulatory requirements. Any failure by us to comply with the applicable regulatory requirements could require extensive changes to our operations; result in regulatory or agency proceedings or investigations, increased compliance costs, damage awards, civil or criminal fines or penalties or restrictions on our operations; and harm our reputation or give rise to material liabilities or a revocation of our licenses and other permits. There can be no assurance that any pending or future regulatory or agency proceedings, investigations or audits will not result in substantial costs, a diversion of management’s attention and resources or other adverse consequences to us and our business.
If we incur substantial liability from litigation, complaints, or enforcement actions, our financial condition could suffer.
Our participation in the medical cannabis industry may lead to litigation, formal or informal complaints, enforcement actions, and inquiries by various federal, state, or local governmental authorities against these subsidiaries. Litigation, complaints, and enforcement actions involving these subsidiaries could consume considerable amounts of financial and other corporate resources, which could have a negative impact on our sales, revenue, profitability, and growth prospects.
We are subject to risks inherent in an agricultural business, including the risk of crop failure.
We grow cannabis, which is an agricultural process. As such, our business is subject to the risks inherent in the agricultural business, including risks of crop failure presented by weather, insects, plant diseases and similar agricultural risks.
We have difficulty accessing the service of banks, which may make it difficult for us to operate.
Since the use of cannabis is illegal under Federal law, there is an argument that banks should not accept for deposit funds from businesses involved with the cannabis industry. Consequently, such businesses often have difficulty finding a bank willing to accept their business.
On February 14, 2014, the U.S. government issued rules allowing banks to legally provide financial services to state licensed marijuana businesses. A memorandum issued by the Justice Department to federal prosecutors re-iterated guidance previously given, this time to the financial industry that banks can do business with legal marijuana businesses and “may not” be prosecuted. The Treasury Department's Financial Crimes Enforcement Network (FinCEN) issued guidelines to banks that “it is possible to provide financial services" to state-licensed marijuana businesses and still be in compliance with federal anti-money laundering laws.
Notwithstanding the above federal guidelines and in addition to potential federal sanctions, regulators in the states in which we are able to conduct business may make it difficult for local banks to do business with companies considered to be engaged in cultivating and dispensing cannabis. Failure to establish a permanent banking relationship could have a material and adverse effect on our future business operations.
We face intense competition and many of our competitors have greater resources that may enable them to compete more effectively.
The industry in which we operate is subject to intense and increasing competition. Some of our competitors have greater capital resources, facilities and diversity of product lines, which may enable them to compete more effectively in this market. Our competitors may devote their resources to developing and marketing products that will directly compete with our product lines. Due to this competition, there is no assurance that we will not encounter difficulties in obtaining revenues and market share or in the positioning of our products. There are no assurances that competition in our respective industries will not lead to reduced prices for our products. If we are unable to successfully compete with existing companies and new entrants to the market this will have a negative impact on our business and financial condition.
If we fail to protect or develop our intellectual property, our business could be adversely affected.
Our viability will depend, in part, on our ability to develop and maintain the proprietary aspects of our technology to distinguish our products from our competitors’ products. We will rely on patents, copyrights, trademarks, trade secrets, and confidentiality provisions to establish and protect our intellectual property.
Any infringement or misappropriation of our intellectual property could damage its value and limit our ability to compete. We may have to engage in litigation to protect the rights to our intellectual property, which could result in significant litigation costs and require a significant amount of our time. In addition, our ability to enforce and protect our intellectual property rights may be limited in certain countries outside the United States, which could make it easier for competitors to capture market position in such countries by utilizing technologies that are similar to those developed or licensed by us.
Competitors may also harm our sales by designing products that mirror the capabilities of our products or technology without infringing on our intellectual property rights. If we do not obtain sufficient protection for our intellectual property, or if we are unable to effectively enforce our intellectual property rights, our competitiveness could be impaired, which would limit our growth and future revenue.
We may also find it necessary to bring infringement or other actions against third parties to seek to protect our intellectual property rights. Litigation of this nature, even if successful, is often expensive and time-consuming to prosecute and there can be no assurance that we will have the financial or other resources to enforce our rights or be able to enforce our rights or prevent other parties from developing similar technology or designing around our intellectual property.
Although we believe that our intellectual property does not and will not infringe upon the patents or violate the proprietary rights of others, it is possible such infringement or violation has occurred or may occur, which could have a material adverse effect on our business.
We are not aware of any infringement by us of any person’s or entity’s intellectual property rights. In the event that products we sell are deemed to infringe upon the patents or proprietary rights of others, we could be required to modify our products or obtain a license for the manufacture and/or sale of such products or cease selling such products. In such event, there can be no assurance that we would be able to do so in a timely manner, upon acceptable terms and conditions, or at all, and the failure to do any of the foregoing could have a material adverse effect upon our business.
There can be no assurance that we will have the financial or other resources necessary to enforce or defend a patent infringement or proprietary rights violation action. If our products or proposed products are deemed to infringe or likely to infringe upon the patents or proprietary rights of others, we could be subject to injunctive relief and, under certain circumstances, become liable for damages, which could also have a material adverse effect on our business and our financial condition.
Our trade secrets may be difficult to protect.
Our success depends upon the skills, knowledge, and experience of our scientific and technical personnel, our consultants and advisors, as well as our licensors and contractors. Because we operate in several highly competitive industries, we rely in part on trade secrets to protect our proprietary technology and processes. However, trade secrets are difficult to protect. We enter into confidentiality or non-disclosure agreements with our corporate partners, employees, consultants, outside scientific collaborators, developers, and other advisors. These agreements generally require that the receiving party keep confidential and not disclose confidential information developed by the receiving party or made known to the receiving party by us during the course of the receiving party’s relationship with us. These agreements also generally provide that inventions conceived by the receiving party in the course of rendering services to us will be our exclusive property, and we enter into assignment agreements to perfect our rights.
These confidentiality, inventions and assignment agreements may be breached and may not effectively assign intellectual property rights to us. Our trade secrets also could be independently discovered by competitors, in which case we would not be able to prevent the use of such trade secrets by our competitors. The enforcement of a claim alleging that a party illegally obtained and was using our trade secrets could be difficult, expensive and time consuming and the outcome would be unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets. The failure to obtain or maintain meaningful trade secret protection could adversely affect our competitive position.
Our future success depends on our key executive officers and our ability to attract, retain, and motivate qualified personnel.
Our future success largely depends upon the continued services of our executive officers and management team. If one or more of our executive officers are unable or unwilling to continue in their present positions, we may not be able to replace them readily, if at all. Additionally, we may incur additional expenses to recruit and retain new executive officers. If any of our executive officers joins a competitor or forms a competing company, we may lose some of our potential customers. Finally, we do not maintain “key person” life insurance on any of our executive officers. Because of these factors, the loss of the services of any of these key persons could adversely affect our business, financial condition, and results of operations, and thereby an investment in our stock.
Our continuing ability to attract and retain highly qualified personnel will also be critical to our success because we will need to hire and retain additional personnel as our business grows. There can be no assurance that we will be able to attract or retain highly qualified personnel. We face significant competition for skilled personnel in our industry. This competition may make it more difficult and expensive to attract, hire, and retain qualified managers and employees. Because of these factors, we may not be able to effectively manage or grow our business, which could adversely affect our financial condition or business. As a result, the value of your investment could be significantly reduced or completely lost.
We may not be able to effectively manage our growth or improve our operational, financial, and management information systems, which would impair our results of operations.
In the near term, we intend to expand the scope of our operations activities significantly. If we are successful in executing our business plan, we will experience growth in our business that could place a significant strain on our business operations, finances, management and other resources. The factors that may place strain on our resources include, but are not limited to, the following:
|
|
●
|
The need for continued development of our financial and information management systems;
|
|
|
●
|
The need to manage strategic relationships and agreements with manufacturers, customers and partners; and
|
|
|
●
|
Difficulties in hiring and retaining skilled management, technical, and other personnel necessary to support and manage our business.
|
Additionally, our strategy could produce a period of rapid growth that may impose a significant burden on our administrative and operational resources. Our ability to effectively manage growth will require us to substantially expand the capabilities of our administrative and operational resources and to attract, train, manage, and retain qualified management and other personnel. There can be no assurance that we will be successful in recruiting and retaining new employees or retaining existing employees.
We cannot provide assurances that our management will be able to manage this growth effectively. Our failure to successfully manage growth could result in our sales not increasing commensurately with capital investments or otherwise materially adversely affecting our business, financial condition, or results of operations.
If we are unable to continually innovate and increase efficiencies, our ability to attract new customers may be adversely affected.
In the area of innovation, we must be able to develop new technologies and products that appeal to our customers. This depends, in part, on the technological and creative skills of our personnel and on our ability to protect our intellectual property rights. We may not be successful in the development, introduction, marketing, and sourcing of new technologies or innovations, that satisfy customer needs, achieve market acceptance, or generate satisfactory financial returns.
Litigation may adversely affect our business, financial condition, and results of operations.
From time to time in the normal course of our business operations, we may become subject to litigation that may result in liability material to our financial statements as a whole or may negatively affect our operating results if changes to our business operations are required. The cost to defend such litigation may be significant and may require a diversion of our resources. There also may be adverse publicity associated with litigation that could negatively affect customer perception of our business, regardless of whether the allegations are valid or whether we are ultimately found liable. Insurance may not be available at all or in sufficient amounts to cover any liabilities with respect to these or other matters. A judgment or other liability in excess of our insurance coverage for any claims could adversely affect our business and the results of our operations.
If we fail to implement and maintain proper and effective internal controls and disclosure controls and procedures pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, our ability to produce accurate and timely financial statements and public reports could be impaired, which could adversely affect our operating results, our ability to operate our business, and investors’ views of us.
As of March 31, 2021, management assessed the effectiveness of our internal controls over financial reporting. Management concluded, as of the fiscal year ended March 31, 2021, that our internal controls and procedures were not effective to detect the inappropriate application of U.S. GAAP rules. Management concluded that our internal controls were adversely affected by deficiencies in the design or operation of our internal controls, which management considered to be material weakness; specifically, no member of our board of directors qualifies as an “audit committee financial expert” as defined in Item 407(d)(5) of Regulation S-K promulgated under the Securities Act.
The failure to implement and maintain proper and effective internal controls and disclosure controls could result in material weaknesses in our financial reporting such as errors in our financial statements and in the accompanying footnote disclosures that could require restatements. Investors may lose confidence in our reported financial information and disclosure, which could negatively impact our stock price.
We do not expect that our internal controls over financial reporting will prevent all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. Over time, controls may become inadequate because changes in conditions or deterioration in the degree of compliance with policies or procedures may occur. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
Our insurance coverage may be inadequate to cover all significant risk exposures; because we are in the cannabis industry, we have a difficult time obtaining the various insurances that are desired to operate our business, which may expose us to additional risk and financial liabilities.
We will be exposed to liabilities that are unique to the products we provide. While we intend to maintain insurance for certain risks, the amount of our insurance coverage may not be adequate to cover all claims or liabilities, and we may be forced to bear substantial costs resulting from risks and uncertainties of our business. It is also not possible to obtain insurance to protect against all operational risks and liabilities. The failure to obtain adequate insurance coverage on terms favorable to us, or at all, could have a material adverse effect on our business, financial condition and results of operations. We do not have any business interruption insurance. Any business disruption or natural disaster could result in substantial costs and diversion of resources. We do not have directors' and officers' liability insurance in place and could incur substantial costs to indemnify our directors and officers against any claims that may arise.
Currently we have insurance coverage in place for business personal properties located at 3550 W. Teco Avenue, Las Vegas, Nevada 89118, workers’ compensation insurance, and general liability insurance.
Insurance that is otherwise readily available is more difficult for us to find, and more expensive, because we engaged in the medicinal cannabis industry. There are no guarantees that we will be able to find such insurances in the future, or that the cost will be affordable to us. If we are forced to go without such insurances, it may prevent us from entering into certain business sectors, may inhibit our growth, and may expose us to additional risk and financial liabilities.
RISKS RELATED TO AN INVESTMENT IN OUR SECURITIES
We expect to experience volatility in the price of our common stock, which could negatively affect stockholders’ investments.
The trading price of our common stock may be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. The stock market in general has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of companies with securities traded in those markets. Broad market and industry factors may seriously affect the market price of companies’ stock, including ours, regardless of actual operating performance. All of these factors could adversely affect your ability to sell your shares of common stock or, if you are able to sell your shares, to sell your shares at a price that you determine to be fair or favorable.
Our common stock is categorized as “penny stock,” which may make it more difficult for investors to sell their shares of common stock due to suitability requirements.
Our common stock is categorized as “penny stock”. The Securities and Exchange Commission (the “SEC”) has adopted Rule 15g-9 which generally defines “penny stock” to be any equity security that has a market price (as defined) less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exceptions. The price of our common stock is significantly less than $5.00 per share and is therefore considered “penny stock.” This designation imposes additional sales practice requirements on broker-dealers who sell to persons other than established customers and accredited investors. The penny stock rules require a broker-dealer buying our securities to disclose certain information concerning the transaction, obtain a written agreement from the purchaser and determine that the purchaser is reasonably suitable to purchase the securities given the increased risks generally inherent in penny stocks. These rules may restrict the ability and/or willingness of brokers or dealers to buy or sell our common stock, either directly or on behalf of their clients, may discourage potential stockholders from purchasing our common stock, or may adversely affect the ability of stockholders to sell their shares.
Financial Industry Regulatory Authority (“FINRA”) sales practice requirements may also limit a stockholder’s ability to buy and sell our common stock, which could depress the price of our common stock.
In addition to the “penny stock” rules described above, FINRA has adopted rules that require a broker-dealer to have reasonable grounds for believing that the investment is suitable for that customer before recommending an investment to a customer. Prior to recommending speculative low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative, low-priced securities will not be suitable for at least some customers. Thus, the FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our shares of common stock, have an adverse effect on the market for our shares of common stock, and thereby depress our price per share of common stock.
The elimination of monetary liability against our directors, officers, and employees under Nevada law and the existence of indemnification rights for or obligations to our directors, officers, and employees may result in substantial expenditures by us and may discourage lawsuits against our directors, officers, and employees.
Our Articles of Incorporation contain a provision permitting us to eliminate the personal liability of our directors to us and our stockholders for damages for the breach of a fiduciary duty as a director or officer to the extent provided by Nevada law. We may also have contractual indemnification obligations under any future employment agreements with our officers. The foregoing indemnification obligations could result in us incurring substantial expenditures to cover the cost of settlement or damage awards against directors and officers, which we may be unable to recoup. These provisions and the resulting costs may also discourage us from bringing a lawsuit against directors and officers for breaches of their fiduciary duties and may similarly discourage the filing of derivative litigation by our stockholders against our directors and officers even though such actions, if successful, might otherwise benefit us and our stockholders. We do not have directors' and officers' liability insurance in place and could incur substantial costs to indemnify our directors and officers against any claims that may arise.
We may issue additional shares of common stock in the future, which could cause significant dilution to all stockholders.
Our Articles of of Incorporation authorize the issuance of up to 600,000,000 shares with a par value of $0.0001 per share. As of August 13, 2021, we had 317,429,078 shares of common stock outstanding. However, we require additional capital and will likely issue additional shares of Common Stock in the future in connection with one or more financings or an acquisition. Such issuances may not require the approval of our stockholders. In addition, certain of our outstanding rights to purchase additional shares of common stock or securities convertible into our common stock are subject to full-ratchet anti-dilution protection, which could result in the right to purchase significantly more shares of common stock being issued or a reduction in the purchase price for any such shares or both. Any issuance of additional shares of our common stock, or equity securities convertible into our common stock, including but not limited to, warrants, and options, will dilute the percentage ownership interest of all stockholders, may dilute the book value per share of our common stock, and may negatively impact the market price of our common stock.
Because we do not intend to pay any cash dividends on our common stock, our stockholders will not be able to receive a return on their shares unless they sell them.
We intend to retain any future earnings to finance the development and expansion of our business. We do not anticipate paying any cash dividends on our common stock in the foreseeable future. Declaring and paying future dividends, if any, will be determined by our Board, based upon earnings, financial condition, capital resources, capital requirements, restrictions in our Articles of Incorporation, contractual restrictions, and such other factors as our Board deems relevant. Unless we pay dividends, our stockholders will not be able to receive a return on their shares unless they sell them. There is no assurance that stockholders will be able to sell shares when desired.
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion highlights the Company’s results of operations and the principal factors that have affected our financial condition, as well as our liquidity and capital resources for the periods described and provides information that management believes is relevant for an assessment and understanding of the statements of financial condition and results of operations presented herein. The following discussion and analysis is based on the Company’s financial statements contained in this prospectus, which we have prepared in accordance with United States generally accepted accounting principles. You should read this discussion and analysis together with such financial statements and the related notes thereto.
Overview
GB Sciences, Inc. is a phytomedical research and biopharmaceutical drug development company whose goal is to create patented formulations of plant-inspired, complex therapeutic mixtures for the prescription drug market that target a variety of medical conditions. The Company is engaged in the research and development of plant-based medicines and plans to produce plant-inspired, complex therapeutic mixtures based on its portfolio of intellectual property.
Through its wholly owned Canadian subsidiary, GBS Global Biopharma, Inc. (“GBSGB”), the Company is engaged in the research and development of plant-based medicines, primarily cannabinoid medicines, with virtual operations in North America and Europe. GBSGB’s assets include a portfolio of intellectual property containing both proprietary cannabinoid-containing formulations and our AI-enabled drug discovery platform, as well as critical research contracts and key supplier arrangements. GBSGB’s intellectual property covers a range of medical conditions and several programs are in the pre-clinical animal stage of development including Parkinson’s disease, neuropathic pain, and cardiovascular therapeutic programs. GBSGB runs a lean drug development program and takes effort to minimize expenses, including personnel, overhead, and fixed capital expenses through strategic partnerships with Universities and Contract Research Organizations (“CROs”). GBSGB’s intellectual property portfolio includes five USPTO issued patents, nine USPTO nonprovisional patent applications pending in the US, and one provisional patent application in the US. In addition to the USPTO patents and patent applications, the company has filed 35 patent applications internationally to protect its proprietary technology. We recently filed a provisional USPTO patent application to further protect aspects of our proprietary drug discovery engine, “Phytomedical Analytics for Research Optimization at Scale," or PhAROS™.
Plan of Operation
Drug Discovery and Development of Novel Cannabis-Based Therapies
Through its wholly owned Canadian subsidiary, GBS Global Biopharma, Inc. ("GBSGB"), the Company has conducted ground-breaking research embracing the rational design of plant-based medicines led by Dr. Andrea Small-Howard, the Company’s Chief Science Officer and Director, and Dr. Helen Turner, Vice President of Innovation and Dean of the Natural Sciences and Mathematics Department at Chaminade University. Small-Howard and Turner posited that complex mixtures of plant-based ingredients would provide more targeted and effective treatments for specific disease conditions than either single ingredient or whole plant formulations. They developed a rapid screening and assaying system which tested thousands of combinations of cannabinoids and terpenes in vitro against cell-based models of disease. This process identified precise mixtures of cannabinoids and terpenes, many of which contained no THC, to treat categories of disease conditions, including neurological disorders, inflammation, heart disease, metabolic syndrome, chronic and neuropathic pain.
GBSGB’s drug discovery engine involves both high throughput screening of cell models of disease and a data analytics/machine learning tool to expedite drug discovery. Initially, GBSGB explored the potential medical uses of specific mixtures derived from cannabis-based raw materials, but these tools are also effective for investigating the medical applications of complex therapeutic mixtures from any plant-derived starting material. In 2014, GBSGB developed its first rapid screening and assaying system which tested thousands of combinations of cannabinoids and terpenes against cell-based models of diseases. This process has been refined over the years and now has identified precise mixtures of cannabinoids and terpenes, many of which contained no THC, to treat categories of disease conditions, including neurological disorders, inflammation, heart disease, metabolic syndrome, chronic and neuropathic pain. GBSGB has filed for patent protection on these plant-inspired, complex therapeutic mixtures, and they are testing them in disease-specific animal models in preparation for human trials.
GBSGB’s drug discovery process combines: 1) HTS: high throughput screening of tens of thousands of combinations of compounds derived from plants in well-established cellular models of diseases, and 2) PhAROS™: Phytomedical Analytics for Research Optimization at Scale for the prediction of complex therapeutic mixtures from plant-based materials. This combined approach to drug discovery increases research efficiency and accuracy reducing the time from ideation to patenting from 7 years to 1.5 years. Screening of plant-based mixtures for drug discovery involves the testing of specific combinations of plant chemicals from many naturally occurring plants and the use of live models for these diseases that have been well established by other researchers. First, the Company finds plant materials that show some therapeutic activity, and then refines these natural mixtures to optimize their effectiveness in cellular assays by removing compounds that do not act synergistically with the others in the mixtures. The Company also use its PhAROS™ Platform to prioritize and eliminate some potential combinations, which reduces the time in the discovery period. PhAROS™ can also be used to identify and predict the efficacy of plant-derived, complex therapeutic mixtures for specific diseases in silico, which are then tested in the cell models.
The U.S. Patent and Trademark Office allows complex mixtures to be claimed as Active Pharmaceutical Ingredients ("APIs"). GBSGB has three issued patents and a series of pending patents containing cannabis-derived complex mixtures that act as therapeutic agents for specific disease categories, as described below. GBSGB’s pending patents are protected whether the individual compounds are derived from the cannabis plant, another plant, synthetically produced, or derived from a combination of sources for the individual chemical compounds in these mixtures.
Drug Development Progress
GBS Global Biopharma, Inc. has made significant strides in the past year with respect to both its drug discovery research and product development programs. Our lead pharmaceutical programs in both Parkinson’s disease and chronic neuropathic pain are now in preclinical animal studies with Dr. Lee Ellis of the National Research Council ("NRC") Canada in Halifax, Nova Scotia. Our complex therapeutic mixtures for the treatment of Cytokine Release Syndrome in COVID-19 and other severe hyperinflammatory conditions are now being tested in preclinical studies with Dr. Norbert Kaminski at Michigan State University. In addition, the two patents which protect GBSGB’s formulations in our lead development programs have been issued by the US Patent and Trademark Office ("USPTO"). On December 8, 2020, our third US patent was issued on complex therapeutic mixtures for the treatment of the hyper inflammatory condition, Mast Cell Activation Syndrome ("MCAS"). Achieving these significant milestones is driving interest in these novel therapeutic programs.
For its lead program in PD therapeutics, GBSGB announced that it has obtained the statistically significant reduction of Parkinson’s-disease like symptoms using its proprietary complex mixtures in an animal model of Parkinson’s disease ("PD"). Several of GBSGB’s PD formulations significantly reduced the symptoms, while the most effective formula reduced the symptoms back to the baseline activity of normal animals. In addition, the toxicity studies for these PD formulas came back without any significant negative findings. These important preclinical results will be included in GBS’ Investigational New Drug ("IND") application with the US FDA to enter human clinical trials as soon as possible. New therapies to address Parkinson’s disease symptoms are needed to help those afflicted with this debilitating disease. The combined direct and indirect costs associated with Parkinson’s disease are estimated at $52 billion in the U.S. alone.
For Parkinson’s disease, the initial clinical prototypes of GBSGB’s Cannabinoid-Containing Complex Mixtures ("CCCM™") are being formulated by Catalent Pharma using Catalent’s Zydis® Orally Disintegrating Tablet ("ODT") technology. This ODT format was selected for the PD formulas because it dissolves on the tongues of patients without the need to swallow for ease of use in patients with PD, who often have difficulties with swallowing. GBSGB selected Catalent as its development partner for the PD therapies due to Catalent’s prior experience in working on US FDA-approved, cannabinoid-containing drugs, their Schedule I drug manufacturing facilities, their familiarity with US FDA and international regulatory and manufacturing requirements, their expertise in tackling formulation challenges, and their ability to achieve the stability and dosing necessary for these novel complex mixtures. In addition to its Zydis® technology, Catalent has early drug development services and additional oral drug delivery solutions available for the efficient delivery of GBSGB's proprietary APIs.
For its lead chronic neuropathic pain program, GBSGB is testing its Cannabinoid-Containing Complex Mixtures and Myrcene-Containing Complex Mixtures ("MCCM") both as encapsulated, time-released nanoparticles, as well as in non-encapsulated forms of these therapeutic mixtures in an animal model at the NRC in Halifax, Nova Scotia. In preparation for human clinical trials, our standard MCCM and the time-released MCCM are currently being compared in an animal model that demonstrates their potential effectiveness at treating chronic pain. The early results from this preclinical research project look very promising.
The three patents which protect formulations in the Company’s lead therapeutic programs have been issued by the USPTO. The issuance of U.S. Patent No. 10,653,640 entitled "Cannabinoid-Containing Complex Mixtures for the Treatment of Neurodegenerative Diseases" on May 19, 2020 protects methods of using GBSGB’s proprietary cannabinoid-containing complex mixtures (CCCM™) for treating Parkinson’s Disease. This was an important milestone in the development of these vitally-important therapies and validates GBSGB’s drug discovery platform. In the US alone, the combined direct and indirect costs associated with Parkinson’s disease are estimated at $52 billion, and new therapies to address Parkinson’s disease symptoms are greatly needed. This was also the first time that a US patent has been awarded for a cannabis-based complex mixture defined using this type of drug discovery method. The first US patent for PD therapies validated our drug discovery platform and strengthened our intellectual property portfolio of unique CCCM’s™, each targeting one of up to 60 specific clinical applications.
The issuance of GBSGB’s second US patent for active pharmaceutical ingredients that are complex mixtures identified by our biotech platform further confirms that GBSGB’s pharmaceutical compositions can be patent-protected for use as biopharmaceutical and nutraceutical products. The US Patent entitled “Myrcene-Containing Complex Mixtures Targeting TRPV1” protects methods of using GBSGB’s proprietary Myrcene-Containing Complex Mixtures for the treatment of pain disorders related to arthritis, shingles, irritable bowel syndrome, sickle cell disease, and endometriosis. In the US alone, chronic pain represents an estimated health burden of between $560 and $650 billion dollars, and an estimated 20.4% of U.S. adults suffer from chronic pain that significantly decreases their quality of life. Despite the widespread rates of addiction and death, opioids remain the standard of care treatment for most people with chronic pain. The Company believes that it is important to create safer, less addictive alternatives to opioids for the treatment of chronic pain disorders, like GBSGB’s myrcene-containing complex mixtures.
Favorable Research Updates from our university collaborators reveal the promise in our discovery programs with Michigan State University (HIV-Associated Neurodegenerative Disorder and COVID-19 therapies), Chaminade University (Chronic Neuropathic Pain, Metabolic Syndrome, Cannabis Metabolomics with the University of Athens), the University of Athens, Greece (Cannabis Metabolomics), the University of Seville, Spain (Time-Released Nanoparticles), and the National Research Council of Canada (Parkinson’s Disease, Chronic Neuropathic Pain).
Partnering Strategy
GBSGB runs a lean drug development program and minimizes expenses, including personnel, overhead, and fixed capital expenses (such as lab and diagnostic equipment), through strategic partnerships with Universities and Contract Research Organizations (“CROs”). Through these research and development agreements, GBSGB has created a virtual pipeline for the further development of novel medicines extracted from the cannabis plant. The partners bring both expertise and infrastructure at a reasonable cost to the life sciences program. In most instances, GBSGB has also negotiated with these partners to keep 100% of the ownership of the IP within GBSGB for original patent filings.
GBSGB currently has on-going research agreements with the following institutions covering the indicated areas of research:
Chaminade University: Broad-based research program to support the drug discovery platform that has yielded many of GBSGB’s original patents to date in the areas of neurodegenerative diseases, heart disease, inflammatory diseases, neuropathic and chronic pain. They have also performed the bioassay portion of the Cannabis Metabolomics study performed with the University of Athens, Greece and GBSGB.
University of Athens: Broad-based metabolomics analysis of over 100 cannabis genotypes including both hemp and THC-producing cannabis varieties, in combination with GBSGB’s bioassay data linking genotypes and potential disease-remediations. This project has the potential to define active ingredients from plant-derived mixtures beyond the standard cannabinoids and terpenoids. The discovery potential is huge, and novel agents have recently been discovered.
Michigan State University: Discovery work using a cutting-edge, multi-cellular model of the human immune system and a multi-cell model of the brain to explore CCCM™s for use in the prevention of HIV-Associated Neurocognitive Disorders (HAND). Although combination antiretroviral therapy keeps symptoms for most HIV-patients well controlled, between 40% and 70% of these well-controlled HIV patients end up with HAND symptoms that range from movement disorders to dementia-like symptoms. The results from this work were included in a new patent application that will be filed in Q3 of 2021. In addition, MSU has performed experiments using their novel model of the human-immune system that have allowed GBSGB to prepare cannabis-based formulas for the potential treatment of virally-induced hyperinflammation/cytokine storm syndrome that has led to the majority of COVID-19 deaths. The new patent application for our novel, cannabinoid-containing complex mixtures (CCCM™) for the treatment of hyperinflammation and cytokine release syndrome in COVID-19 patients was filed August 18, 2020.
The University of Seville: Bringing their novel expertise to the development and functional testing of time-released and disease-targeted nanoparticles of cannabis-based complex mixtures for oral administration. These specialized nanoparticles are being used for the precise and time-released delivery of several of our therapies, including GBSGB’s MCCM™ and CCCM™’s used in the preclinical animal testing performed at the NRC Canada. The University of Seville has completed functional testing on nanoparticles containing myrcene, nerolidol, and beta-caryophyllene for our Myrcene-Containing Complex Mixtures. In these cell-based assays, the effectiveness and kinetics of the nanoparticle-forms of these terpenes were compared with the “naked” terpenes both individually and in mixtures. In all cases, the effectiveness of the nanoparticles were superior to the naked terpenes, however, the mixtures were dramatically more effective than the individuals. These results from Seville are very promising as these nanoparticles have entered the animal testing phase at the NRC in Halifax.
The National Research Center (NRC) of Canada, Halifax, Nova Scotia: Two animal-phase studies are being performed by Dr. Lee Ellis’ group at the NRC. An animal safety and efficacy study was initiated in Q4 of 2018 for GBSGB’s Parkinson’s disease therapies, and the NRC has demonstrated that the company’s PD formulations were able to reduce behavioral changes associated with the loss of dopamine-producing neurons, which underlies the pathology of Parkinson’s disease in the animal model. Based on achieving the statistically significant reduction in Parkinson’s disease symptomology, GBSGB has signed an amendment to include a final phase of testing, which will study the mechanism of action for these promising formulations. In Q1 of 2019, GBSGB started a safety and efficacy study in animals for GBSGB’s Chronic Neuropathic Pain (CNP) formulas. The midterm results for these preclinical pain studies are promising.
The University of Cadiz: Testing the safety and efficacy of the above-mentioned time-released nanoparticles in rodent models of chronic pain. Proof of concept complete for one formulation.
University of Hawaii: Validating the efficacy of a complex cannabis-based mixture for the treatment of cardiac hypertrophy and cardiac disease in a rodent model. Proof of concept work is complete.
Path to Market: Drug Development Stages and Proposed Clinical Trials
GBSGB has cannabis-based therapeutic products in the following stages of drug development: Discovery, Pre-Clinical, and entering the Clinical Phase. It has also licensed therapeutic products that the Company intends to develop through partners, labeled Partner Programs.
The completion of pre-clinical studies, clinical trials, and obtaining FDA-approvals for pharmaceutical products is traditionally a long and expensive process. However, GBSGB asserts that its cannabis-based drug discovery engine, lean development program, novel regulatory strategy, experienced development partners, and aggressive licensing of these products at early clinical stages can mitigate some of the risks. The Company uses a combination of in silico discovery methods and automated screening of cellular models of disease to decrease the time in Discovery prior to filing novel patent applications for disease-specific therapeutics. GBSGB’s original patent applications cover new chemical entities (“NCE”) based on complex combinations of plant-derived compounds. Its Exploratory IND/Phase 0 Program gets the Company to First-in-Man sooner than traditional programs, which reduces translational risks, and includes preliminary efficacy measures for responsible development decisions. In contrast, a traditional phased-development path would not provide any efficacy measures until Phase II. After the completion of our Phase 0 study, which compares the efficacies of multiple related cannabis-based formulations, the Company plans to advance the lead drug candidate using an adaptive trial design that is more efficient than the traditional phased-development pathway. GBSGB has entered into research contracts, partnerships, and/or joint ventures with several respected, independent contract research organizations, medical schools, universities, and other scientific researchers to increase developmental efficiencies. If and when one or more of GBSGB’s drugs, therapies or treatments are approved by the FDA, GBSGB will seek to market them under licensing arrangements with major biotechnology or pharmaceutical companies.
There can be no assurance that we will ever be able to enter into any joint ventures or other arrangements with third parties to finance our drug development program or that if we are able to do so, that any of our projected therapies will ever be approved by the FDA. Even if we obtain FDA approval for a therapy, there can be no assurance that it could be successfully marketed or would not be superseded by another cannabis-based therapy produced by one or more of our competitors. It also may be anticipated that even if we enter into a joint venture development with a financially stable pharmaceutical or institutional partner, we will still be required to raise significant additional capital in the future to achieve the strategic goals of GBSGB. There can be no assurance that we will be able to obtain such additional capital on reasonable terms, if at all. If GBSGB fails to achieve its goal of producing one or more cannabis-based pharmaceuticals or therapies, it would have a material adverse effect on our future financial condition and business prospects.
Other Operations
In addition to our key biopharmaceutical research and development activities described in detail above, the Company has operated in the medical and adult-use cannabis markets under State-issued cultivation and production licenses. Our wholly owned subsidiary GB Sciences Nevada, LLC (“GBSN”) leases a warehouse facility at 3550 W. Teco Avenue, Las Vegas Nevada (the "Teco Facility") and operates a cannabis cultivation facility under Nevada licenses for the medical and adult-use markets. Our wholly owned subsidiary GB Sciences Las Vegas, LLC ("GBLV") holds Nevada certificates for medical and adult-use cannabis production and produces extracts and concentrates for the wholesale market.
On March 24, 2020, we entered into the Membership Interest Purchase Agreement ("Teco MIPA") which formalized the sale of the Teco Subsidiaries. Pursuant to the Teco MIPA, the Company will sell 100% of its membership interests in GBSN and GBLV for $4.0 million cash upon close and will receive a $4.0 million 8% promissory note to be paid in monthly installments over 36 months.
The Company also holds a Nevada license for cultivation of medical marijuana located in Sandy Valley, Nevada (the “Nopah License”). The license is owned by the Company’s wholly owned subsidiary, GB Sciences Nopah, LLC ("Nopah"). Operations have not begun under the Nopah License. On August 10, 2020, the Company entered into the Membership Interest Purchase Agreement ("Nopah MIPA") and Promissory Note Modification Agreement with the purchaser of GB Sciences Nopah, LLC. As consideration for the transfer of the license and membership interest in GB Sciences Nopah, LLC, the Company will receive $300,000 and the purchaser will pay all expenses related to the upkeep and maintenance of the Nopah License. The transfer of the Nopah License is subject to the same restrictions on license transfers currently in effect in the State of Nevada.
The sales of the Teco Facility and Nopah are expected to close upon the successful transfer of the Nevada cultivation and production licenses. The transfer of cannabis licenses in the State of Nevada was subject to an indefinite moratorium from October 2019 through August of 2020, and the processing of license transfers has been further delayed by the COVID-19 pandemic. In a meeting held on July 21, 2020, the Nevada Cannabis Compliance Board lifted the moratorium, however, the board has indicated that there were over 90 requests pending and it will take up to several months to process the entire backlog of pending license transfers. Based on this information, we cannot provide any assurances as to the timing of the close of the sale.
Results of Operations
The following table sets forth certain of our Statements of Operations data from continuing operations for the Company's most recently completed fiscal quarter:
|
|
|
For the Three Months Ended
|
|
|
|
|
June 30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative expenses
|
|
$
|
493,405
|
|
|
$
|
515,253
|
|
|
LOSS FROM OPERATIONS
|
|
|
(493,405
|
)
|
|
|
(515,253
|
)
|
|
OTHER EXPENSE
|
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
(65,254
|
)
|
|
|
(662,370
|
)
|
|
Debt default penalty
|
|
|
-
|
|
|
|
(286,059
|
)
|
|
Other expense
|
|
|
-
|
|
|
|
(11,182
|
)
|
|
LOSS BEFORE INCOME TAXES
|
|
|
(558,659
|
)
|
|
|
(1,474,864
|
)
|
|
Income tax expense
|
|
|
-
|
|
|
|
-
|
|
|
LOSS FROM CONTINUING OPERATIONS
|
|
$
|
(558,659
|
)
|
|
$
|
(1,474,864
|
)
|
Comparison of the Three Months Ended June 30, 2021 and 2020
General and Administrative Expenses
General and Administrative Expenses decreased by $(21,848) to $493,405 for the three months ended June 30, 2021, compared to $515,253 for the three months ended June 30, 2020. The Company is continuing its efforts to maintain administrative costs at a minimum and to make the best use of its limited resources in advancing research & development of the Company's intellectual property portfolio.
Interest Expense
Interest expense decreased by $(597,116) to $65,254 for the three months ended June 30, 2021, compared to $662,370 in the prior year quarter. The decrease is primarily attributable to less interest-bearing debt outstanding during the current quarter as the result of the payoff of the note payable to Iliad Research and Trading, L.P. in December 2020. In addition, notes with balances totaling $2.1 million at June 30, 2021 are no longer accruing interest beginning December 1, 2020, as the result of the Omnibus Amendment to the agreements surrounding the sale of the Company's Nevada Subsidiaries.
Debt Default Penalty
The Company recorded a default penalty of $286,059 in the prior year quarter, related to the Company's failure to timely repay the principal and interest owed under the note payable to Iliad Research and Trading, L.P. on April 1, 2020. The penalty was 10% of the principal and accrued interest balances outstanding at the time of default.
The following table sets forth certain of our Statement of Operations data from continuing operations for the Company's most recently completed fiscal year:
|
|
|
For the Years Ended
|
|
|
|
|
March 31,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
|
|
|
|
|
|
|
|
|
|
SALES REVENUE
|
|
$
|
-
|
|
|
$
|
-
|
|
|
COST OF GOODS SOLD
|
|
|
-
|
|
|
|
-
|
|
|
GROSS PROFIT (LOSS)
|
|
|
-
|
|
|
|
-
|
|
|
GENERAL AND ADMINISTRATIVE EXPENSES
|
|
|
2,001,617
|
|
|
|
5,741,514
|
|
|
LOSS FROM OPERATIONS
|
|
|
(2,001,617
|
)
|
|
|
(5,741,514
|
)
|
|
OTHER INCOME (EXPENSE)
|
|
|
|
|
|
|
|
|
|
Gain/(loss) on extinguishment
|
|
|
467,872
|
|
|
|
(216,954
|
)
|
|
Gain on settlement of accounts payable
|
|
|
422,414
|
|
|
|
-
|
|
|
Gain on deconsolidation
|
|
|
-
|
|
|
|
4,393,242
|
|
|
Interest expense
|
|
|
(1,285,460
|
)
|
|
|
(1,109,031
|
)
|
|
Loss on modification of line of credit
|
|
|
(650,000
|
)
|
|
|
-
|
|
|
Loss on modification of note receivable
|
|
|
-
|
|
|
|
(1,895,434
|
)
|
|
Debt default penalty
|
|
|
(286,059
|
)
|
|
|
-
|
|
|
Other expense
|
|
|
-
|
|
|
|
(179,368
|
)
|
|
Total other income/(expense)
|
|
|
(1,331,233
|
)
|
|
|
992,455
|
|
|
NET LOSS BEFORE INCOME TAX EXPENSE
|
|
|
(3,332,850
|
)
|
|
|
(4,749,059
|
)
|
|
INCOME TAX EXPENSE
|
|
|
-
|
|
|
|
-
|
|
|
LOSS FROM CONTINUING OPERATIONS
|
|
|
(3,332,850
|
)
|
|
|
(4,749,059
|
)
|
|
LOSS FROM DISCONTINUED OPERATIONS
|
|
|
(392,177
|
)
|
|
|
(8,362,626
|
)
|
|
NET LOSS
|
|
|
(3,725,027
|
)
|
|
|
(13,111,685
|
)
|
|
NET LOSS ATTRIBUTABLE TO NON-CONTROLLING INTEREST
|
|
|
-
|
|
|
|
(738,106
|
)
|
|
NET LOSS ATTRIBUTABLE TO GB SCIENCES, INC.
|
|
$
|
(3,725,027
|
)
|
|
$
|
(12,373,579
|
)
|
General and Administrative Expenses. General and administrative expense decreased $(3,739,897) to $2,001,617 for the year ended March 31, 2021 as compared to $5,741,514 for the same period last year. The decrease is attributable to a company-wide initiative to reduce general and administrative costs, including a substantial reduction in the number of employees involved in administrative functions and related salaries & wages expense.
Gain/(Loss) on extinguishment. The gain on extinguishment of $467,872 for the year ended March 31, 2021 relates to the Judgment Settlement Agreement with Iliad Research & Trading, L.P. In order to settle the lawsuit brought by Iliad, the Company paid $3,006,015 in full satisfaction of the principal and accrued interest balance of $3,473,886. Prior year losses on extinguishment of $216,954 relate to modifications of the note payable to CSW Ventures, LP, which were accounted for as extinguishments.
Gain on settlement of accounts payable. During the year ended March 31, 2021, the Company settled accounts payable at a discount in exchange for immediate lump sum payments and recorded income from cancellation of accounts payable totaling $422,414, compared to $0 in the prior year.
Gain on deconsolidation. The Company recorded a gain on deconsolidation of $4,393,242 related to the sale of its 50% membership interest in GB Sciences Louisiana, LLC during the year ended March 31, 2020, compared to $0 in the current year.
Interest Expense. Interest for the year ended March 31, 2021 was $1,285,460, compared to $1,109,031 for the year ended March 31, 2020. The increase is primarily due to interest income of $509,265 related to the Wellcana note receivable included in the prior year amount as a net reduction, and offset by a decrease in interest expense resulting from the amortization of note discounts. Primarily as the result of the Company's largest outstanding notes payable becoming fully amortized during the year, interest expense from amortization of debt discounts decreased from $1,150,995 in the prior year to $776,122 in the current year.
Loss on modification of line of credit. As a result of the Omnibus Amendment dated December 29, 2020, the Company accrued a modification expense of $650,000. The amount represents an increase to the note balance to a total of $1,025,000, which will reduce the note receivable issued to the Company at the closing of the sale of the Teco Facility.
Loss on modification of note receivable. As the result of the Company's August 24, 2020 letter agreement with Wellcana, the Company determined that the amount of the note receivable from Wellcana that was collectible as of March 31, 2020 was $5,224,423 and recorded a loss on modification of note receivable in the amount of $1,895,434.
LIQUIDITY AND CAPITAL RESOURCES
Current Liquidity
The Company will need additional capital to implement its strategies. There is no assurance that it will be able to raise the amount of capital needed for future growth plans. Even if financing is available, it may not be on terms that are acceptable. If unable to raise the necessary capital at the times required, the Company may have to materially change the business plan, including delaying implementation of aspects of the business plan or curtailing or abandoning the business plan. The Company represents a speculative investment and investors may lose all of their investment. In order to be able to achieve the strategic goals, the Company needs to further expand its business and financing activities. Based on the Company's cash position, it is necessary to raise additional capital by the end of the next quarter in order to continue to fund current operations. These factors raise substantial doubt about the ability to continue as a going concern. The Company is pursuing several alternatives to address this situation, including the raising of additional funding through equity or debt financing. In order to finance existing operations and pay current liabilities over the next twelve months, the Company will need to raise additional capital. No assurance can be given that the Company will be able to operate profitably on a consistent basis, or at all, in the future.
The principal sources of liquidity to date have been cash generated from sales of debt and equity securities and loans.
In continuing operations at June 30, 2021, cash was $582,971, other current assets excluding cash were $296,081, and our working capital deficit was $5,841,001. Current liabilities in continuing operations were $6,720,053 and consisted principally of $1,463,806 in accounts payable, $1,552,148 in accrued liabilities, $3,619,186 in notes and convertible notes payable, and $84,913 in indebtedness to related parties. At June 30, 2021, current assets from discontinued operations were $2,248,757, current liabilities from discontinued operations were $1,987,787, and working capital from discontinued operations was $260,970.
At March 31, 2021, continuing operations included a cash balance of $793,040, other current assets excluding cash were $256,251, and our working capital deficit was $5,494,572. Current liabilities in continuing operations were $6,543,863, which consisted principally of $1,412,459 in accounts payable, $1,451,687 in accrued liabilities, $3,594,804 in notes and convertible notes payable, and $84,913 of indebtedness to related parties. At March 31, 2021, current assets from discontinued operations were $2,494,564, current liabilities from discontinued operations were $2,054,585, and working capital from discontinued operations was $439,979.
Sources and Uses of Cash
Operating Activities
Net cash used in operating activities was $636,583, including $2,954 used by discontinued operations for the three months ended June 30, 2021, compared to $323,845 net of $235,779 provided by operating activities of discontinued operations for the three months ended June 30, 2020. We anticipate that cash flows from operations will be insufficient to fund business operations for the next twelve-month period. Accordingly, we will have to generate additional liquidity or cash flow to fund our current and anticipated operations. This will likely require the sale of additional common stock or other securities. There is no assurance that we will be able to realize any significant proceeds from such sales, if at all.
Investing Activities
During the three months ended June 30, 2021, $157,435 was provided by investing activities, including $7,435 provided by discontinued operations. Cash provided by investing activities of continuing operations consisted of $200,000 received by the Company as an advancement of the purchase price of the Teco facility, and was offset by $50,000 paid to acquire intangible assets. During the three months ended June 30, 2020, no cash was used or provided by investing activities.
Financing Activities
During the three months ended June 30, 2021, cash flows provided by financing activities totaled $29,182, net of $33,478 used in discontinued operations. Cash flows provided by financing activities of continuing operations related to $62,660 in gross proceeds from warrant exercises. Cash provided by financing activities for the three months ended June 30, 2020 was $273,309, net of $12,772 used in discontinued operations. Cash provided by financing activities for the three months ended June 30, 2020 related primarily to $151,202 in proceeds from warrant exercises and $150,000 in proceeds from the sale of a note payable, offset by $15,121 of brokerage fees for warrant exercises.
Going Concern
The Company’s financial statements have been prepared assuming the Company will continue as a going concern. The Company has sustained net losses since inception, which have caused an accumulated deficit of $104,681,665 at June 30, 2021. The Company had a working capital deficit of $5,580,031 at June 30, 2021, net of working capital of $260,970 classified as discontinued operations, compared to $5,054,593 at March 31, 2021, net of working capital of $439,979 classified as discontinued operations. In addition, the Company has consumed cash in its operating activities of $636,583 for the three months ended June 30, 2021, including $2,954 used by discontinued operations, compared to $323,845 used in operating activities, net of $235,779 provided by discontinued operations for the three months ended June 30, 2020. These factors, among others, raise substantial doubt about the Company’s ability to continue as a going concern.
Management has been able, thus far, to finance the losses through a public offering, private placements and obtaining operating funds from stockholders. The Company is continuing to seek sources of financing. There are no assurances that the Company will be successful in achieving its goals.
Furthermore, Management believes the COVID-19 pandemic may have a significant impact on the Company's business. The pandemic presents a risk to the global economy, and it is possible that it could have an impact on the operations of the Company in the near term that could materially impact the Company’s financials and ability to continue as a going concern. Management has not been able to measure the potential financial impact on the Company and continues to monitor the impact of the pandemic closely, although the extent to which the COVID-19 outbreak will impact our operations, financing ability or future financial results is uncertain.
In view of these conditions, the Company’s ability to continue as a going concern is dependent upon its ability to obtain additional financing or capital sources, to meet its financing requirements, and ultimately to achieve profitable operations. Management believes that its current and future plans provide an opportunity to continue as a going concern. The accompanying financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classification of liabilities that may be necessary in the event the Company is unable to continue as a going concern.
Variables and Trends
We have limited operating history with respect to the current business plan. In the event we are able to obtain the necessary financing to move forward with the business plan, we expect business expenses to increase significantly as we go operational. Accordingly, the comparison of the financial data for the periods presented may not be a meaningful indicator of future performance and must be considered in light these circumstances.
Off Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on the financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Critical Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. The Company regularly evaluates estimates and assumptions related to allowances for doubtful accounts, inventory valuation, valuation of initial right-of-use assets and corresponding lease liabilities, valuation of beneficial conversion features in convertible debt, valuation of the assets and liabilities of discontinued operations, stock-based compensation expense, purchased intangible asset valuations, deferred income tax asset valuation allowances, uncertain tax positions, litigation and other loss contingencies. These estimates and assumptions are based on current facts, historical experience and various other factors that the Company believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the recording of costs and expenses that are not readily apparent from other sources. The actual results the Company experiences may differ materially and adversely from these estimates.
Discontinued Operations
Discontinued operations comprise those activities that were disposed of during the period or which were classified as held for sale at the end of the period and represent a separate major line of business or geographical area that can be clearly distinguished for operational and financial reporting purposes. The Company has included its subsidiaries GB Sciences Louisiana, LLC, GB Sciences Nevada, LLC, GB Sciences Las Vegas, LLC, and GB Sciences Nopah, LLC in discontinued operations due to the sale of the Company's Louisiana cultivation and extraction facility and the pending sale of the Company's Nevada cultivation and extraction facilities.
Inventory
We value our inventory at the lower of the actual cost of our inventory, as determined using the first-in, first-out method, or its current estimated market value. We periodically review our physical inventory for excess, obsolete, and potentially impaired items and reserve accordingly. Our reserve estimate for excess and obsolete inventory is based on expected future use. Indirect costs, which primarily relate to the lease and operation costs of the Teco Facility, are allocated based on square footage of the facility used in the production of inventory.
Indefinite-Lived Intangible Assets
Our indefinite-lived intangible assets primarily represent the value of our patents pending and includes the costs paid to draft and file patent applications. Upon issuance of the patents, the indefinite-lived intangible assets will have finite lives. Intangible assets also include the acquisition cost of a cannabis production license with an indefinite life. We amortize our finite-lived intangible assets over their estimated useful lives using the straight-line method, and we periodically evaluate the remaining useful lives of our finite-lived intangible assets to determine whether events or circumstances warrant a revision to the remaining period of amortization. During the year ended March 31, 2020, the Company entered into the Membership Interest Purchase Agreement ("Teco MIPA") to sell 100% of the membership interests in the Teco Facility. As a result of this agreement, the Company determined that the long-lived assets of the Teco Facility including a production license acquired through purchase might be impaired due to the current expectation that the asset group will more likely than not be disposed of by sale significantly before the end of its previously estimated useful life. The Company recorded an impairment loss of $449,801 related to the license for the year ended March 31, 2020, and reduced the carrying value of the related intangible asset from $1,021,067 to $571,264. The license asset and the impairment loss are included in discontinued operations in the accompanying financial statements.
Long-Lived Assets
Property and equipment comprise a significant portion of our total assets. We evaluate the carrying value of property and equipment if impairment indicators are present or if other circumstances indicate that impairment may exist under authoritative guidance. The annual testing date is March 31. When management believes impairment indicators may exist, projections of the undiscounted future cash flows associated with the use of and eventual disposition of property and equipment are prepared. If the projections indicate that the carrying value of the property and equipment are not recoverable, we reduce the carrying values to fair value. These impairment tests are heavily influenced by assumptions and estimates that are subject to change as additional information becomes available.
During the year ended March 31, 2020, the Company entered into the Membership Interest Purchase Agreement ("Teco MIPA") to sell 100% of the membership interests in the Teco Facility. As a result of this agreement, the Company determined that the long-lived assets of the Teco Facility might be impaired due to the current expectation that the asset group will more likely than not be disposed of by sale significantly before the end of its previously estimated useful life. The Company estimated future undiscounted cash flows related to the Teco Facility to be $8.0 million, which was less than the carrying amount of the Teco Facility asset group of $11.9 million. Using a discounted cash flow approach, the Company estimated the fair value of the asset group to be approximately $7.3 million, resulting in a write-down of $4,645,054 related to the Teco Facility asset group. Fair value was based on expected future cash flows using level 3 inputs under ASC 820. The cash flows are the proceeds expected to be generated from the sale of the assets under the Teco MIPA, discounted to present value at a rate of 17%. The impairment loss and the related long-lived assets are included in discontinued operations in the accompanying financial statements.
Beneficial Conversion Feature of Convertible Notes Payable
The Company accounts for convertible notes payable in accordance with the guidelines established by the Financial Accounting Standards Board’s (“FASB”) Accounting Standards Codification (“ASC”) Topic 470-20, Debt with Conversion and Other Options and Emerging Issues Task Force (“EITF”) 00-27, “Application of Issue No. 98-5 to Certain Convertible Instruments”. A beneficial conversion feature (“BCF”) exists on the date a convertible note is issued when the fair value of the underlying common stock to which the note is convertible into is in excess of the remaining unallocated proceeds of the note after first considering the allocation of a portion of the note proceeds to the fair value of any attached equity instruments, if any related equity instruments were granted with the debt. In accordance with this guidance, the BCF of a convertible note is measured by allocating a portion of the note's proceeds to the warrants, if applicable, and as a reduction of the carrying amount of the convertible note equal to the intrinsic value of the conversion feature, both of which are credited to additional paid-in-capital. The Company calculates the fair value of warrants issued with the convertible notes using the Black-Scholes valuation model and uses the same assumptions for valuing any employee options in accordance with ASC Topic 718 Compensation – Stock Compensation. The only difference is that the contractual life of the warrants is used.
The value of the proceeds received from a convertible note is then allocated between the conversion features and warrants on a relative fair value basis. The allocated fair value is recorded in the financial statements as a debt discount (premium) from the face amount of the note and such discount is amortized over the expected term of the convertible note (or to the conversion date of the note, if sooner) and is charged to interest expense.
Equity-Based Compensation
The Company accounts for equity instruments issued to employees in accordance with the provisions of ASC 718 Stock Compensation (ASC 718) and Equity-Based Payments to Non-employees pursuant to ASC 505-50 (ASC 505-50). The computation of the expense associated with stock-based compensation requires the use of a valuation model. The FASB-issued accounting guidance requires significant judgment and the use of estimates, particularly surrounding Black-Scholes assumptions such as stock price volatility, expected option lives, and expected option forfeiture rates, to value equity-based compensation. We currently use a Black-Scholes option pricing model to calculate the fair value of our stock options. We primarily use historical data to determine the assumptions to be used in the Black-Scholes model and have no reason to believe that future data is likely to differ materially from historical data. However, changes in the assumptions to reflect future stock price volatility and future stock award exercise experience could result in a change in the assumptions used to value awards in the future and may result in a material change to the fair value calculation of stock-based awards. This accounting guidance requires the recognition of the fair value of stock compensation in net income. Although every effort is made to ensure the accuracy of our estimates and assumptions, significant unanticipated changes in those estimates, interpretations and assumptions may result in recording stock option expense that may materially impact our financial statements for each respective reporting period.
Income Taxes
The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been included in financial statements or tax returns. Deferred tax items are reflected at the enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Due to the uncertainty regarding the success of future operations, management has valued the deferred tax asset allowance at 100% of the related deferred tax assets.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1 under the Securities Act with the Securities and Exchange Commission with respect to the sale or resale of an aggregate of 119,899,091 shares of common stock. This prospectus was filed as a part of that registration statement but does not contain all of the information contained in the registration statement and exhibits. Reference is thus made to the omitted information. Statements made in this prospectus are summaries of the material terms of contracts, agreements and documents and are not necessarily complete; however, all information we considered material has been disclosed. Reference is made to each exhibit for a more complete description of the matters involved and these statements are qualified in their entirety by the reference. You may inspect the registration statement, exhibits and schedules filed with the Securities and Exchange Commission at the Securities and Exchange Commission's principle office in Washington, D.C. Copies of all or any part of the registration statement may be obtained from the Public Reference Section of the Securities and Exchange Commission, 100 F. Street, N.E., Washington, D.C. 20549. The Securities and Exchange Commission also maintains a web site (http://www.sec.gov) that contains this filed registration statement, reports, proxy statements and information regarding us that we have filed electronically with the Commission. For more information pertaining to our company and the sale or resale of an aggregate of 119,899,091 shares of common stock, reference is made to the registration statement.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” information into this prospectus. This means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information that we incorporate by reference is considered to be part of this prospectus. Because we are incorporating by reference our future filings with the SEC, this prospectus is continually updated and those future filings may modify or supersede some or all of the information included or incorporated in this prospectus. This means that you must look at all of the SEC filings that we incorporate by reference to determine if any of the statements in this prospectus or in any document previously incorporated by reference have been modified or superseded.
This prospectus incorporates by reference the documents listed below that have been previously filed with the SEC:
We also incorporate by reference all future documents (except as to any portion of any report or document that is not deemed filed under such provisions) we file with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, subsequent to the date of this prospectus and prior to the termination of the offering.
You should rely only on the information incorporated by reference or provided in this prospectus. We have not authorized anyone else to provide you with different information. Any statement contained in a document incorporated by reference into this prospectus will be deemed to be modified or superseded for the purposes of this prospectus to the extent that a later statement contained in this prospectus or in any other document incorporated by reference into this prospectus modifies or supersedes the earlier statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus. You should not assume that the information in this prospectus is accurate as of any date other than the date of this prospectus or the date of the documents incorporated by reference in this prospectus.
We will provide without charge to each person, including any beneficial owner, to whom this prospectus is delivered, upon written or oral request, a copy of any or all documents that are incorporated by reference into this prospectus, but not delivered with the prospectus, other than exhibits to such documents unless such exhibits are specifically incorporated by reference into the documents that this prospectus incorporates. You should direct oral or written requests by one of the following methods. Attention: Investor Relations, GB Sciences, Inc., 3550 W. Teco Avenue, Las Vegas, NV 89118. You may also access these documents, free of charge on the SEC’s website at www.sec.gov or on the “Finance” page of our website at https://gbsciences.com. The information found on our website, or that may be accessed by links on our website, is not part of this prospectus. We have included our website address solely as an inactive textual reference. Investors should not rely on any such information in deciding whether to purchase our securities.
FINANCIAL STATEMENTS
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
CONDENSED CONSOLIDATED BALANCE SHEETS AS OF JUNE 30, 2021 AND MARCH 31, 2021 (Unaudited)
|
56
|
|
|
|
|
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS - THREE MONTHS ENDED JUNE 30, 2021 AND 2020 (Unaudited)
|
57
|
|
|
|
|
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS – THREE MONTHS ENDED JUNE 30, 2021 AND 2020 (Unaudited)
|
58
|
|
|
|
|
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' DEFICIT – THREE MONTHS ENDED JUNE 30, 2021 AND 2020 (Unaudited)
|
60
|
|
|
|
|
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited)
|
61
|
|
|
|
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
|
81
|
|
|
|
|
CONSOLIDATED BALANCE SHEETS AS OF MARCH 31, 2021 AND MARCH 31, 2020
|
83
|
|
|
|
|
CONSOLIDATED STATEMENTS OF OPERATIONS - YEARS ENDED MARCH 31, 2021 AND 2020
|
84
|
|
|
|
|
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY/(DEFICIT) - YEARS ENDED MARCH 31, 2021 AND 2020
|
85
|
|
|
|
|
CONSOLIDATED STATEMENTS OF CASH FLOWS - YEARS ENDED MARCH 31, 2021 AND 2020
|
86
|
|
|
|
|
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
|
88
|
GB SCIENCES, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED BALANCE SHEETS
|
|
|
As of June 30,
|
|
|
As of March 31,
|
|
|
|
|
2021
|
|
|
2021
|
|
|
CURRENT ASSETS:
|
|
(unaudited)
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
582,971
|
|
|
$
|
793,040
|
|
|
Prepaid expenses and other current assets
|
|
|
296,081
|
|
|
|
256,251
|
|
|
Current assets from discontinued operations
|
|
|
2,248,757
|
|
|
|
2,494,564
|
|
|
TOTAL CURRENT ASSETS
|
|
|
3,127,809
|
|
|
|
3,543,855
|
|
|
Property and equipment, net
|
|
|
21,895
|
|
|
|
25,022
|
|
|
Intangible assets, net of accumulated amortization of $57,322 and $43,096 at June 30, 2021 and March 31, 2021, respectively
|
|
|
1,797,920
|
|
|
|
1,706,762
|
|
|
Long term assets from discontinued operations
|
|
|
5,383,005
|
|
|
|
5,530,415
|
|
|
TOTAL ASSETS
|
|
$
|
10,330,629
|
|
|
$
|
10,806,054
|
|
|
CURRENT LIABILITIES:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
1,463,806
|
|
|
$
|
1,412,459
|
|
|
Accrued interest
|
|
|
521,145
|
|
|
|
493,741
|
|
|
Accrued liabilities
|
|
|
1,031,003
|
|
|
|
957,946
|
|
|
Notes and convertible notes payable and line of credit, net of unamortized discount of $272,122 and $296,504 at June 30, 2021 and March 31, 2021, respectively
|
|
|
3,619,186
|
|
|
|
3,594,804
|
|
|
Indebtedness to related parties
|
|
|
84,913
|
|
|
|
84,913
|
|
|
Current liabilities from discontinued operations
|
|
|
1,987,787
|
|
|
|
2,054,585
|
|
|
TOTAL CURRENT LIABILITIES
|
|
|
8,707,840
|
|
|
|
8,598,448
|
|
|
Convertible notes payable, net of unamortized discount of $141,123 and $154,590 at June 30, 2021 and March 31, 2021, respectively
|
|
|
305,877
|
|
|
|
292,410
|
|
|
Long term liabilities from discontinued operations
|
|
|
3,347,363
|
|
|
|
3,389,124
|
|
|
TOTAL LIABILITIES
|
|
|
12,361,080
|
|
|
|
12,279,982
|
|
|
Commitments and contingencies (Note 7)
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS' DEFICIT:
|
|
|
|
|
|
|
|
|
|
Common Stock, $0.0001 par value, 600,000,000 shares authorized, 317,429,078 and 315,340,411 outstanding at June 30, 2021 and March 31, 2021, respectively
|
|
|
31,743
|
|
|
|
31,534
|
|
|
Additional paid-in capital
|
|
|
102,619,471
|
|
|
|
102,380,770
|
|
|
Accumulated deficit
|
|
|
(104,681,665
|
)
|
|
|
(103,886,232
|
)
|
|
TOTAL STOCKHOLDERS' DEFICIT
|
|
|
(2,030,451
|
)
|
|
|
(1,473,928
|
)
|
|
TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT
|
|
$
|
10,330,629
|
|
|
$
|
10,806,054
|
|
The accompanying unaudited notes are an integral part of these unaudited condensed consolidated financial statements.
GB SCIENCES, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(unaudited)
|
|
|
For the Three Months Ended June 30,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2021
|
|
|
2020
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales revenue
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Cost of goods sold
|
|
|
-
|
|
|
|
-
|
|
|
Gross profit
|
|
|
-
|
|
|
|
-
|
|
|
General and administrative expenses
|
|
|
493,405
|
|
|
|
515,253
|
|
|
LOSS FROM OPERATIONS
|
|
|
(493,405
|
)
|
|
|
(515,253
|
)
|
|
OTHER EXPENSE
|
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
(65,254
|
)
|
|
|
(662,370
|
)
|
|
Debt default penalty
|
|
|
-
|
|
|
|
(286,059
|
)
|
|
Other expense
|
|
|
-
|
|
|
|
(11,182
|
)
|
|
Total other expense
|
|
|
(65,254
|
)
|
|
|
(959,611
|
)
|
|
LOSS BEFORE INCOME TAXES
|
|
|
(558,659
|
)
|
|
|
(1,474,864
|
)
|
|
Income tax expense
|
|
|
-
|
|
|
|
-
|
|
|
LOSS FROM CONTINUING OPERATIONS
|
|
|
(558,659
|
)
|
|
|
(1,474,864
|
)
|
|
Loss from discontinued operations
|
|
|
(73,758
|
)
|
|
|
(371,836
|
)
|
|
NET LOSS
|
|
$
|
(632,417
|
)
|
|
$
|
(1,846,700
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to common stockholders of GB Sciences, Inc.
|
|
|
|
|
|
|
|
|
|
Continuing operations
|
|
$
|
(558,659
|
)
|
|
$
|
(1,474,864
|
)
|
|
Discontinued operations
|
|
|
(73,758
|
)
|
|
|
(371,836
|
)
|
|
Net loss
|
|
$
|
(632,417
|
)
|
|
$
|
(1,846,700
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per common share – basic and diluted
|
|
|
|
|
|
|
|
|
|
Continuing operations
|
|
$
|
(0.00
|
)
|
|
$
|
(0.01
|
)
|
|
Discontinued operations
|
|
$
|
(0.00
|
)
|
|
$
|
(0.00
|
)
|
|
Net loss
|
|
$
|
(0.00
|
)
|
|
$
|
(0.01
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding - basic and diluted
|
|
|
315,675,539
|
|
|
|
277,968,516
|
|
The accompanying unaudited notes are an integral part of these unaudited condensed consolidated financial statements.
GB SCIENCES, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(unaudited)
|
|
|
Three Months Ended June 30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
OPERATING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(632,417
|
)
|
|
$
|
(1,846,700
|
)
|
|
Loss from discontinued operations
|
|
|
(73,758
|
)
|
|
|
(371,836
|
)
|
|
Net loss from continuing operations
|
|
|
(558,659
|
)
|
|
|
(1,474,864
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
17,354
|
|
|
|
5,562
|
|
|
Stock-based compensation
|
|
|
19,500
|
|
|
|
-
|
|
|
Amortization of debt discount and beneficial conversion feature
|
|
|
37,849
|
|
|
|
510,885
|
|
|
Debt default penalty
|
|
|
-
|
|
|
|
286,059
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Prepaid expenses and other current assets
|
|
|
(39,830
|
)
|
|
|
-
|
|
|
Accounts payable
|
|
|
(10,304
|
)
|
|
|
34,739
|
|
|
Accrued expenses
|
|
|
(126,943
|
)
|
|
|
(141,102
|
)
|
|
Accrued interest
|
|
|
27,404
|
|
|
|
158,806
|
|
|
Indebtedness to related parties
|
|
|
-
|
|
|
|
60,291
|
|
|
Net cash used in operating activities of continuing operations
|
|
|
(633,629
|
)
|
|
|
(559,624
|
)
|
|
Net cash provided by/(used in) operating activities of discontinued operations
|
|
|
(2,954
|
)
|
|
|
235,779
|
|
|
Net cash used in operating activities
|
|
|
(636,583
|
)
|
|
|
(323,845
|
)
|
|
INVESTING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Advancement of proceeds from sale of Nevada subsidiaries
|
|
|
200,000
|
|
|
|
-
|
|
|
Acquisition of intangible assets
|
|
|
(50,000
|
)
|
|
|
-
|
|
|
Net cash provided by investing activities of continuing operations
|
|
|
150,000
|
|
|
|
-
|
|
|
Net cash provided by investing activities of discontinued operations
|
|
|
7,435
|
|
|
|
-
|
|
|
Net cash provided by investing activities
|
|
|
157,435
|
|
|
|
-
|
|
|
FINANCING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Gross proceeds from warrant exercises
|
|
|
62,660
|
|
|
|
151,202
|
|
|
Proceeds of note payable
|
|
|
-
|
|
|
|
150,000
|
|
|
Brokerage fees for warrant exercises
|
|
|
-
|
|
|
|
(15,121
|
)
|
|
Net cash provided by financing activities of continuing operations
|
|
|
62,660
|
|
|
|
286,081
|
|
|
Net cash used in financing activities of discontinued operations
|
|
|
(33,478
|
)
|
|
|
(12,772
|
)
|
|
Net cash provided by financing activities
|
|
|
29,182
|
|
|
|
273,309
|
|
|
Net change in cash and cash equivalents
|
|
|
(449,966
|
)
|
|
|
(50,536
|
)
|
|
CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD
|
|
|
1,145,633
|
|
|
|
151,766
|
|
|
CASH AND CASH EQUIVALENTS AT END OF PERIOD
|
|
|
695,667
|
|
|
|
101,230
|
|
|
Less: cash and cash equivalents classified as discontinued operations
|
|
|
(112,696
|
)
|
|
|
(50,135
|
)
|
|
CASH AND CASH EQUIVALENTS AT END OF PERIOD FROM CONTINUING OPERATIONS
|
|
$
|
582,971
|
|
|
$
|
51,095
|
|
The accompanying unaudited notes are an integral part of these unaudited condensed consolidated financial statements.
GB SCIENCES, INC. AND SUBSIDIARIES
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION
For the Three Months Ended June 30, 2021 and 2020
(unaudited)
|
|
|
Three Months Ended June 30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
Cash paid for interest
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Cash paid for income tax
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-cash investing and financing transactions:
|
|
|
|
|
|
|
|
|
|
Depreciation capitalized in inventory (discontinued operations)
|
|
$
|
134,511
|
|
|
$
|
164,654
|
|
|
Patent drafting and filing costs capitalized in intangible assets
|
|
$
|
55,385
|
|
|
$
|
78,226
|
|
|
Brokerage fees from warrant exercises in accounts payable
|
|
$
|
6,266
|
|
|
$
|
-
|
|
|
Induced dividend from warrant exercises
|
|
$
|
163,016
|
|
|
$
|
17,236
|
|
|
Discount on note payable attributable to warrant modification
|
|
$
|
-
|
|
|
$
|
150,000
|
|
The accompanying unaudited notes are an integral part of these unaudited condensed consolidated financial statements.
GB SCIENCES, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' DEFICIT
For the Three Months Ended June 30, 2021 and 2020
(unaudited)
|
|
|
Shares
|
|
|
Amount
|
|
|
Additional
Paid-In
Capital
|
|
|
Accumulated
Deficit
|
|
|
Total
|
|
|
Balance at March 31, 2021
|
|
|
315,340,411
|
|
|
$
|
31,534
|
|
|
$
|
102,380,770
|
|
|
$
|
(103,886,232
|
)
|
|
$
|
(1,473,928
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of warrants for stock, net of issuance costs
|
|
|
2,088,667
|
|
|
|
209
|
|
|
|
56,185
|
|
|
|
-
|
|
|
|
56,394
|
|
|
Share based compensation expense
|
|
|
-
|
|
|
|
-
|
|
|
|
19,500
|
|
|
|
-
|
|
|
|
19,500
|
|
|
Inducement dividend from warrant exercises
|
|
|
-
|
|
|
|
-
|
|
|
|
163,016
|
|
|
|
(163,016
|
)
|
|
|
-
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(632,417
|
)
|
|
|
(632,417
|
)
|
|
Balance at June 30, 2021
|
|
|
317,429,078
|
|
|
$
|
31,743
|
|
|
$
|
102,619,471
|
|
|
$
|
(104,681,665
|
)
|
|
$
|
(2,030,451
|
)
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Additional
Paid-In
Capital
|
|
|
Accumulated
Deficit
|
|
|
Total
|
|
|
Balance at March 31, 2020
|
|
|
275,541,602
|
|
|
$
|
27,554
|
|
|
$
|
97,271,157
|
|
|
$
|
(97,387,205
|
)
|
|
$
|
(88,494
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of warrants for stock, net of issuance costs
|
|
|
4,991,084
|
|
|
|
500
|
|
|
|
135,581
|
|
|
|
-
|
|
|
|
136,081
|
|
|
Discount on convertible note payable from warrant modification
|
|
|
-
|
|
|
|
-
|
|
|
|
150,000
|
|
|
|
-
|
|
|
|
150,000
|
|
|
Inducement dividend from warrant exercises
|
|
|
-
|
|
|
|
-
|
|
|
|
17,263
|
|
|
|
(17,263
|
)
|
|
|
-
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,846,700
|
)
|
|
|
(1,846,700
|
)
|
|
Balance at June 30, 2020
|
|
|
280,532,686
|
|
|
$
|
28,054
|
|
|
$
|
97,574,001
|
|
|
$
|
(99,251,168
|
)
|
|
$
|
(1,649,113
|
)
|
The accompanying unaudited notes are an integral part of these unaudited condensed consolidated financial statements.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
Note 1 – Background and Significant Accounting Policies
GB Sciences, Inc. (“the Company”, “GB Sciences”, “we”, “us”, or “our”) is a phytomedical research and biopharmaceutical drug development company whose goal is to create patented formulations of plant-inspired, complex therapeutic mixtures for the prescription drug market that target a variety of medical conditions. The Company is engaged in the research and development of plant-based medicines and plans to produce plant-inspired, complex therapeutic mixtures based on its portfolio of intellectual property.
Through its wholly owned Canadian subsidiary, GBS Global Biopharma, Inc. (“GBSGB”), the Company is engaged in the research and development of plant-based medicines, primarily cannabinoid medicines, with virtual operations in North America and Europe. GBSGB’s assets include a portfolio of intellectual property containing both proprietary cannabinoid-containing formulations and our AI-enabled drug discovery platform, as well as critical research contracts and key supplier arrangements. GBSGB’s intellectual property covers a range of medical conditions and several programs are in the pre-clinical animal stage of development including Parkinson’s disease, neuropathic pain, and cardiovascular therapeutic programs. GBSGB runs a lean drug development program and takes effort to minimize expenses, including personnel, overhead, and fixed capital expenses through strategic partnerships with Universities and Contract Research Organizations (“CROs”). GBSGB’s intellectual property portfolio includes five USPTO issued patents, nine USPTO nonprovisional patent applications pending in the US, and one provisional patent application in the US. In addition to the USPTO patents and patent applications, the company has filed 35 patent applications internationally to protect its proprietary technology. We recently filed a provisional USPTO patent application to further protect aspects of our proprietary drug discovery engine, “Phytomedical Analytics for Research Optimization at Scale," or PhAROS™.
Basis of Presentation
The accompanying unaudited interim condensed consolidated financial statements of GB Sciences, Inc. (the “Company,” “We” or “Us”) have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”) for interim financial information and with the instructions to Form 10-Q and Article 8 of Regulations S-X. Accordingly, they do not include all of the information and footnotes required by U.S. GAAP for complete financial statements. In the opinion of management, all adjustments (consisting of normal recurring accruals) considered necessary for a fair presentation have been included. Operating results for the periods presented are not necessarily indicative of the results that may be expected for the year ending March 31, 2022. The balance sheet at March 31, 2021 has been derived from the audited financial statements at that date but does not include all of the information and footnotes required by U.S. GAAP for complete financial statements. The unaudited interim condensed consolidated financial statements should be read in conjunction with the consolidated financial statements and footnotes thereto included in the Company’s annual report on Form 10-K for the year ended March 31, 2021.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
Principles of Consolidation
We prepare our consolidated financial statements in accordance with generally accepted accounting principles (GAAP) for the United States of America. Our consolidated financial statements include all operating divisions and majority-owned subsidiaries, reported as a single operating segment, for which we maintain controlling interests. Intercompany accounts and transactions have been eliminated in consolidation. All subsidiaries were wholly owned by the Company for the periods presented.
Use of Estimates
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. The Company regularly evaluates estimates and assumptions related to allowances for doubtful accounts, inventory valuation and standard cost allocations, valuation of initial right-of-use assets and corresponding lease liabilities, valuation of beneficial conversion features in convertible debt, valuation of the assets and liabilities of discontinued operations, stock-based compensation expense, purchased intangible asset valuations, deferred income tax asset valuation allowances, uncertain tax positions, litigation, other loss contingencies, and impairment of long lived assets. These estimates and assumptions are based on current facts, historical experience and various other factors that the Company believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the recording of costs and expenses that are not readily apparent from other sources. The actual results the Company experiences may differ materially and adversely from these estimates.
Reclassifications
Certain reclassifications have been made to the comparative period amounts in order to conform to the current period presentation. In particular, the assets, liabilities, profit and loss, and cash flows of GB Sciences Nevada LLC, GB Sciences Las Vegas, LLC, and GB Sciences Nopah, LLC, have been separated from the comparative period amounts to conform to the current period presentation as discontinued operations as the result of the pending sale of the Company's Nevada operations. The reclassifications had no effect on the reported financial position, results of operations or cash flows of the Company.
Discontinued Operations
See Note 3.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
Long-Lived Assets
We evaluate the carrying value of property and equipment if impairment indicators are present or if other circumstances indicate that impairment may exist under authoritative guidance. The annual testing date is March 31. When management believes impairment indicators may exist, projections of the undiscounted future cash flows associated with the use of and eventual disposition of property and equipment are prepared. If the projections indicate that the carrying value of the property and equipment are not recoverable, we reduce the carrying values to fair value. These impairment tests are heavily influenced by assumptions and estimates that are subject to change as additional information becomes available. No indicators of impairment were identified by the Company as of June 30, 2021.
Inventory
We value our inventory at the lower of the actual cost of our inventory, as determined using the first-in, first-out method, or its current estimated market value. We periodically review our physical inventory for excess, obsolete, and potentially impaired items and reserve accordingly. Our reserve estimate for excess and obsolete inventory is based on expected future use. Indirect costs, which primarily relate to the lease and operation costs of the Teco Facility, which are included in discontinued operations, are allocated based on square footage of the facility used in the production of inventory.
Beneficial Conversion Feature of Convertible Notes Payable
The Company accounts for convertible notes payable in accordance with the guidelines established by the Financial Accounting Standards Board’s (“FASB”) Accounting Standards Codification (“ASC”) Topic 470-20, Debt with Conversion and Other Options and Emerging Issues Task Force (“EITF”) 00-27, “Application of Issue No. 98-5 to Certain Convertible Instruments”. A beneficial conversion feature (“BCF”) exists on the date a convertible note is issued when the fair value of the underlying common stock to which the note is convertible into is in excess of the remaining unallocated proceeds of the note after first considering the allocation of a portion of the note proceeds to the fair value of any attached equity instruments, if any related equity instruments were granted with the debt. In accordance with this guidance, the BCF of a convertible note is measured by allocating a portion of the note's proceeds to the warrants, if applicable, and as a reduction of the carrying amount of the convertible note equal to the intrinsic value of the conversion feature, both of which are credited to additional paid-in-capital. The Company calculates the fair value of warrants issued with the convertible notes using the Black-Scholes valuation model and uses the same assumptions for valuing any employee options in accordance with ASC Topic 718 Compensation – Stock Compensation. The only difference is that the contractual life of the warrants is used.
The value of the proceeds received from a convertible note is then allocated between the conversion features and warrants on a relative fair value basis. The allocated fair value is recorded in the financial statements as a debt discount (premium) from the face amount of the note and such discount is amortized over the expected term of the convertible note (or to the conversion date of the note, if sooner) and is charged to interest expense.
Revenue Recognition
The FASB issued Accounting Standards Codification (“ASC”) 606 as guidance on the recognition of revenue from contracts with customers. Revenue recognition depicts the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The guidance also requires disclosures regarding the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. The guidance permits two methods of adoption: retrospectively to each prior reporting period presented, or retrospectively with the cumulative effect of initially applying the guidance recognized at the date of initial application (the cumulative catch-up transition method). The Company adopted the guidance on April 1, 2018 and applied the cumulative catch-up transition method.
The Company’s only material revenue source is part of discontinued operations and derives from sales of cannabis and cannabis products, distinct physical goods. Under ASC 606, the Company is required to separately identify each performance obligation resulting from its contracts from customers, which may be a good or a service. A contract may contain one or more performance obligations. All of the Company’s contracts with customers, past and present, contain only a single performance obligation, the delivery of distinct physical goods. Because fulfillment of the company’s performance obligation to the customer under ASC 606 results in the same timing of revenue recognition as under the previous guidance (i.e. revenue is recognized upon delivery of physical goods), the Company did not record any material adjustment to report the cumulative effect of initial application of the guidance.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
Income Taxes
The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been included in financial statements or tax returns. Deferred tax items are reflected at the enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Due to the uncertainty regarding the success of future operations, management has valued the deferred tax asset allowance at 100% of the related deferred tax assets.
Because the Company operates in the State-licensed cannabis industry through the Nevada Subsidiaries, it is subject to the limitations of Internal Revenue Code Section 280E (“280E”) for U.S. income tax purposes. Under 280E, the Company is allowed to deduct expenses that are directly related to the production of its products, i.e. cost of goods sold, but is allowed no further deductions for ordinary and necessary business expenses from its gross profit. The Company believes that the deductions disallowed include the deduction of NOLs. The unused NOLs will continue to carry forward and may be used by the Company to offset future taxable income that is not subject to the limitations of 280E.
Loss per Share
The Company’s basic loss per share has been calculated using the weighted average number of common shares outstanding during the period. The Company had 160,314,865 and 164,049,941 potentially dilutive common shares at June 30, 2021 and March 31, 2021, respectively. However, such common stock equivalents were not included in the computation of diluted net loss per share, as their inclusion would have been anti-dilutive.
Recent Accounting Pronouncements
Standards Not Yet Adopted
In May 2021, the FASB issued ASU No. 2021-04, Issuer's Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options. This guidance clarifies and reduces diversity in an issuer’s accounting for modifications or exchanges of freestanding equity-classified written call options due to a lack of explicit guidance in the FASB Codification. The ASU 2021-04 is effective for The Company's fiscal year beginning April 1, 2022. Early adoption is permitted. The Company is evaluating the impact of adopting ASU 2021-04 and does not expect the adoption of this ASU to materially impact its consolidated financial statements.
On June 16, 2016, the FASB issued ASU No. 2016-13, Measurement of Credit Losses on Financial Instruments. The standard requires the use of an “expected loss” model on certain types of financial instruments. The standard also amends the impairment model for available-for-sale debt securities and requires estimated credit losses to be recorded as allowances instead of reductions to amortized cost of the securities. The amendments in this ASU are effective for the Company's fiscal year beginning April 1, 2023. The Company is currently evaluating the impact of ASU 2016-13 on its financial statements.
In June 2020, the FASB issued ASU No. 2020-06, Accounting for Convertible Instruments and Contracts in an Entity's Own Equity. The guidance simplifies the current guidance for convertible instruments and the derivatives scope exception for contracts in an entity’s own equity. Additionally, the amendments affect the diluted EPS calculation for instruments that may be settled in cash or shares and for convertible instruments. This ASU will be effective for the Company's fiscal year beginning April 1, 2024. Early adoption is permitted. The amendments in this update must be applied on either full retrospective basis or modified retrospective basis through a cumulative-effect adjustment to retained earnings/(deficit) in the period of adoption. The Company is currently evaluating the impact of ASU 2020-06 on its consolidated financial statements and related disclosures, as well as the timing of adoption.
All other newly issued accounting pronouncements have been deemed either immaterial or not applicable.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
Note 2 – Going Concern
The Company’s financial statements have been prepared assuming the Company will continue as a going concern. The Company has sustained net losses since inception, which have caused an accumulated deficit of $104,681,665 at June 30, 2021. The Company had a working capital deficit of $5,580,031 at June 30, 2021, net of working capital of $260,970 classified as discontinued operations, compared to $5,054,593 at March 31, 2021, net of working capital of $439,979 classified as discontinued operations. In addition, the Company has consumed cash in its operating activities of $636,583 for the three months ended June 30, 2021, including $2,954 used by discontinued operations, compared to $323,845 used in operating activities, net of $235,779 provided by discontinued operations for the three months ended June 30, 2020. These factors, among others, raise substantial doubt about the Company’s ability to continue as a going concern.
Management has been able, thus far, to finance the losses through a public offering, private placements and obtaining operating funds from stockholders. The Company is continuing to seek sources of financing. There are no assurances that the Company will be successful in achieving its goals.
Furthermore, Management believes the COVID-19 pandemic may have a significant impact on the Company's business. The pandemic presents a risk to the global economy, and it is possible that it could have an impact on the operations of the Company in the near term that could materially impact the Company’s financials and ability to continue as a going concern. As the result of the pandemic, gross revenue of the Company's discontinued operations was significantly reduced for the three months ended June 30, 2020, due to temporary closures of retail stores who purchase products from the Teco Facility. Management has not been able to measure the potential future impact on the Company's financial statements and continues to monitor the impact of the pandemic closely, although the extent to which the COVID-19 outbreak will impact our operations, financing ability or future financial results is uncertain.
In view of these conditions, the Company’s ability to continue as a going concern is dependent upon its ability to obtain additional financing or capital sources, to meet its financing requirements, and ultimately to achieve profitable operations. Management believes that its current and future plans provide an opportunity to continue as a going concern. The accompanying financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classification of liabilities that may be necessary in the event the Company is unable to continue as a going concern.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
Note 3 – Discontinued Operations
Discontinued operations comprise those activities that were disposed of during the period or which were classified as held for sale at the end of the period and represent a separate major line of business or geographical area that can be clearly distinguished for operational and financial reporting purposes. The Company has included its subsidiaries GB Sciences Nevada, LLC, GB Sciences Las Vegas, LLC, and GB Sciences Nopah, LLC in discontinued operations due to the pending sale of the Company's Nevada cultivation and extraction facilities (Note 9).
The assets and liabilities associated with discontinued operations included in our condensed consolidated balance sheets as of June 30, 2021 and March 31, 2021 were as follows:
|
|
|
June 30, 2021
|
|
|
March 31, 2021
|
|
|
|
|
Continuing
|
|
|
Discontinued
|
|
|
Total
|
|
|
Continuing
|
|
|
Discontinued
|
|
|
Total
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
582,971
|
|
|
$
|
112,696
|
|
|
$
|
695,667
|
|
|
$
|
793,040
|
|
|
$
|
352,593
|
|
|
$
|
1,145,633
|
|
|
Accounts receivable, net
|
|
|
-
|
|
|
|
643,594
|
|
|
|
643,594
|
|
|
|
-
|
|
|
|
400,175
|
|
|
|
400,175
|
|
|
Inventory, net
|
|
|
-
|
|
|
|
1,462,009
|
|
|
|
1,462,009
|
|
|
|
-
|
|
|
|
1,689,304
|
|
|
|
1,689,304
|
|
|
Prepaid and other current assets
|
|
|
296,081
|
|
|
|
30,458
|
|
|
|
326,539
|
|
|
|
256,251
|
|
|
|
52,492
|
|
|
|
308,743
|
|
|
TOTAL CURRENT ASSETS
|
|
|
879,052
|
|
|
|
2,248,757
|
|
|
|
3,127,809
|
|
|
|
1,049,291
|
|
|
|
2,494,564
|
|
|
|
3,543,855
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment, net
|
|
|
21,895
|
|
|
|
4,736,272
|
|
|
|
4,758,167
|
|
|
|
25,022
|
|
|
|
4,876,247
|
|
|
|
4,901,269
|
|
|
Intangible assets, net
|
|
|
1,797,920
|
|
|
|
571,264
|
|
|
|
2,369,184
|
|
|
|
1,706,762
|
|
|
|
571,264
|
|
|
|
2,278,026
|
|
|
Deposits and other noncurrent assets
|
|
|
-
|
|
|
|
75,469
|
|
|
|
75,469
|
|
|
|
-
|
|
|
|
82,904
|
|
|
|
82,904
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL ASSETS
|
|
$
|
2,698,867
|
|
|
$
|
7,631,762
|
|
|
$
|
10,330,629
|
|
|
$
|
2,781,075
|
|
|
$
|
8,024,979
|
|
|
$
|
10,806,054
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
1,463,806
|
|
|
$
|
385,320
|
|
|
$
|
1,849,126
|
|
|
$
|
1,412,459
|
|
|
$
|
509,477
|
|
|
$
|
1,921,936
|
|
|
Accrued interest
|
|
|
521,145
|
|
|
|
49,211
|
|
|
|
570,356
|
|
|
|
493,741
|
|
|
|
49,211
|
|
|
|
542,952
|
|
|
Accrued expenses
|
|
|
1,031,003
|
|
|
|
122,714
|
|
|
|
1,153,717
|
|
|
|
957,946
|
|
|
|
105,421
|
|
|
|
1,063,367
|
|
|
Notes payable, net
|
|
|
3,619,186
|
|
|
|
485,000
|
|
|
|
4,104,186
|
|
|
|
3,594,804
|
|
|
|
485,000
|
|
|
|
4,079,804
|
|
|
Indebtedness to related parties
|
|
|
84,913
|
|
|
|
-
|
|
|
|
84,913
|
|
|
|
84,913
|
|
|
|
-
|
|
|
|
84,913
|
|
|
Income tax payable
|
|
|
-
|
|
|
|
793,292
|
|
|
|
793,292
|
|
|
|
-
|
|
|
|
761,509
|
|
|
|
761,509
|
|
|
Finance lease obligations, current
|
|
|
-
|
|
|
|
152,250
|
|
|
|
152,250
|
|
|
|
-
|
|
|
|
143,967
|
|
|
|
143,967
|
|
|
TOTAL CURRENT LIABILITIES
|
|
|
6,720,053
|
|
|
|
1,987,787
|
|
|
|
8,707,840
|
|
|
|
6,543,863
|
|
|
|
2,054,585
|
|
|
|
8,598,448
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible notes payable
|
|
|
305,877
|
|
|
|
-
|
|
|
|
305,877
|
|
|
|
292,410
|
|
|
|
-
|
|
|
|
292,410
|
|
|
Finance lease obligations, long term
|
|
|
-
|
|
|
|
3,347,363
|
|
|
|
3,347,363
|
|
|
|
-
|
|
|
|
3,389,124
|
|
|
|
3,389,124
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES
|
|
$
|
7,025,930
|
|
|
$
|
5,335,150
|
|
|
$
|
12,361,080
|
|
|
$
|
6,836,273
|
|
|
$
|
5,443,709
|
|
|
$
|
12,279,982
|
|
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
Discontinued Operations - Revenues and Expenses
The revenues and expenses associated with discontinued operations included in our condensed consolidated statements of operations for the three months ended June 30, 2021 and 2020 were as follows:
|
|
|
For the Three Months Ended June 30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
|
|
Continuing
|
|
|
Discontinued
|
|
|
Total
|
|
|
Continuing
|
|
|
Discontinued
|
|
|
Total
|
|
|
Sales revenue
|
|
$
|
-
|
|
|
$
|
1,342,586
|
|
|
$
|
1,342,586
|
|
|
$
|
-
|
|
|
$
|
551,197
|
|
|
$
|
551,197
|
|
|
Cost of goods sold
|
|
|
-
|
|
|
|
(1,219,041
|
)
|
|
|
(1,219,041
|
)
|
|
|
-
|
|
|
|
(491,795
|
)
|
|
|
(491,795
|
)
|
|
Gross profit/(loss)
|
|
|
-
|
|
|
|
123,545
|
|
|
|
123,545
|
|
|
|
-
|
|
|
|
59,402
|
|
|
|
59,402
|
|
|
General and administrative expenses
|
|
|
493,405
|
|
|
|
84,078
|
|
|
|
577,483
|
|
|
|
515,253
|
|
|
|
289,455
|
|
|
|
804,708
|
|
|
INCOME/(LOSS) FROM OPERATIONS
|
|
|
(493,405
|
)
|
|
|
39,467
|
|
|
|
(453,938
|
)
|
|
|
(515,253
|
)
|
|
|
(230,053
|
)
|
|
|
(745,306
|
)
|
|
OTHER EXPENSE
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
(65,254
|
)
|
|
|
(102,331
|
)
|
|
|
(167,585
|
)
|
|
|
(662,370
|
)
|
|
|
(132,943
|
)
|
|
|
(795,313
|
)
|
|
Debt default penalty
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(286,059
|
)
|
|
|
-
|
|
|
|
(286,059
|
)
|
|
Other income/(expense)
|
|
|
-
|
|
|
|
20,889
|
|
|
|
20,889
|
|
|
|
(11,182
|
)
|
|
|
-
|
|
|
|
(11,182
|
)
|
|
Total other expense
|
|
|
(65,254
|
)
|
|
|
(81,442
|
)
|
|
|
(146,696
|
)
|
|
|
(959,611
|
)
|
|
|
(132,943
|
)
|
|
|
(1,092,554
|
)
|
|
LOSS BEFORE INCOME TAXES
|
|
|
(558,659
|
)
|
|
|
(41,975
|
)
|
|
|
(600,634
|
)
|
|
|
(1,474,864
|
)
|
|
|
(362,996
|
)
|
|
|
(1,837,860
|
)
|
|
Income tax expense
|
|
|
-
|
|
|
|
(31,783
|
)
|
|
|
(31,783
|
)
|
|
|
-
|
|
|
|
(8,840
|
)
|
|
|
(8,840
|
)
|
|
NET LOSS
|
|
$
|
(558,659
|
)
|
|
$
|
(73,758
|
)
|
|
$
|
(632,417
|
)
|
|
$
|
(1,474,864
|
)
|
|
$
|
(371,836
|
)
|
|
$
|
(1,846,700
|
)
|
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
Discontinued Operations - Inventory
Raw materials consist of supplies, materials, and consumables used in the cultivation and extraction processes. Work-in-progress includes live plants and cannabis in the drying, curing, and trimming processes. Finished goods includes completed cannabis flower, trim, and extracts in bulk and packaged forms. Inventory is included in current assets from discontinued operations in the Company's unaudited condensed consolidated balance sheets at June 30, 2021 and March 31, 2021.
|
|
|
June 30, 2021
|
|
|
March 31, 2021
|
|
|
|
|
|
|
|
|
|
|
|
|
Raw materials
|
|
$
|
112,857
|
|
|
$
|
86,076
|
|
|
Work in progress
|
|
|
814,166
|
|
|
|
743,844
|
|
|
Finished goods
|
|
|
601,807
|
|
|
|
866,195
|
|
|
Subtotal
|
|
|
1,528,830
|
|
|
|
1,696,115
|
|
|
Allowance to reduce inventory to net realizable value
|
|
|
(66,821
|
)
|
|
|
(6,811
|
)
|
|
Total inventory, net
|
|
$
|
1,462,009
|
|
|
$
|
1,689,304
|
|
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
Discontinued Operations - Leases
The Company evaluates all finance and operating leases, and they are measured on the balance sheet with a lease liability and right-of-use asset (“ROU”) at inception. ROU assets represent the Company’s right to use an underlying asset for the lease term, and lease liabilities represent the obligation to make scheduled lease payments. Lease terms include options to extend when it is reasonably certain that the option will be exercised. Leases with a term of 12 months or less are not recorded on the consolidated balance sheet. The present value of lease payments is calculated using the incremental borrowing rate at lease commencement, which takes into consideration recent debt issuances as well as other applicable market data available.
The Company's only remaining lease commitment is a finance lease for the Teco Facility, which is classified as discontinued operations in the Company's unaudited condensed consolidated financial statements. This lease has a remaining non-cancelable term that ends December 31, 2025 with an option to extend through December 31, 2030. The rate used to discount this lease was 11.5%.
Finance leases are included in property and equipment (long term assets from discontinued operations), finance lease obligations, short term (current liabilities from discontinued operations), and finance lease obligations, long term (long term liabilities from discontinued operations), on the unaudited condensed consolidated balance sheets.
During the three months ended June 30, 2021, finance lease costs included in discontinued operations were $140,258, of which $101,583 represents interest expense and $38,675 represents amortization of the right-of-use asset.
The future minimum lease payments of lease liabilities as of June 30, 2021, from discontinued operations are as follows:
|
Year Ending
|
|
|
|
|
|
March 31,
|
|
Finance Leases
|
|
|
|
|
|
|
|
|
2022 (9 months)
|
|
$
|
409,235
|
|
|
2023
|
|
|
560,625
|
|
|
2024
|
|
|
577,444
|
|
|
2025
|
|
|
594,767
|
|
|
2026
|
|
|
612,610
|
|
|
Thereafter
|
|
|
3,168,492
|
|
|
Total minimum lease payments
|
|
|
5,923,173
|
|
|
Less: Amount representing interest
|
|
|
(2,423,560
|
)
|
|
Present value of minimum lease payments
|
|
|
3,499,613
|
|
|
Less: Current maturities of capital lease obligations
|
|
|
(152,250
|
)
|
|
Long-term capital lease obligations
|
|
$
|
3,347,363
|
|
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
Discontinued Operations - 8% Line of Credit dated November 27, 2019
In connection with the Binding Letter of Intent dated November 27, 2019 (Note 9), the Teco Subsidiaries entered into a promissory note and line of credit for up to $470,000 from the purchaser of the membership interests in the Teco Subsidiaries. The purpose of the line of credit was to supply working capital for the Teco Subsidiaries, and the note matures upon the close of the sale of the Teco Subsidiaries. The principal and accrued interest balances outstanding at the time of closing will be considered paid in full upon closing and will not reduce the purchase price received by GB Sciences. As of March 31, 2021, the Teco Subsidiaries have received $485,000 in advances under the line of credit, reflecting an informal agreement with the lender to increase the line of credit by $15,000. On December 29, 2020, the Company entered into the Omnibus Amendment with the purchaser of the Teco Facility, which provides that no further interest will be accrued on this note after November 30, 2020. The balance of the line of credit was $485,000 at June 30, 2021 and accrued interest was $49,211. The note and related interest expense are included in current liabilities from discontinued operations and loss from discontinued operations.
Note 4 – Notes Payable and Line of Credit
0% Note Payable dated October 23, 2017
On October 23, 2017, the Company amended the existing Nevada Medical Marijuana Production License Agreement (“Amended Production License Agreement”). Per the terms of the Amended Production License Agreement, GB Sciences purchased the remaining percentage of the production license resulting in the 100% ownership of the license. GB Sciences also received 100% ownership of the cultivation license included in the original Nevada Medical Marijuana Production License Agreement. In exchange, GB Sciences made one-time payment of $500,000 and issued a 0% Promissory Note in the amount of $700,000 payable in equal monthly payments over a three-year period commencing on January 1, 2018. The present value of the note was $521,067 on the date of its issuance based on an imputed interest rate of 20.3% and the Company recorded a discount on notes payable of $178,933 related to the difference between the face value and present value of the note.
To date, the Company has made principal payments totaling $330,555 and the principal balance of the note was $369,445 at June 30, 2021. The remaining unamortized discount as of June 30, 2021, was $0.
On August 10, 2020, the Company entered into the Membership Interest Purchase Agreement ("Nopah MIPA") for the sale of its interest in GB Sciences Nopah, LLC (Note 9). The Nopah MIPA will close upon successful transfer of the Nevada Medical Marijuana Cultivation Facility Registration Certificate. Upon close, the principal balance of the note will be reduced to $190,272. The maturity date of the note was extended to July 31, 2021, with no payments of principal or interest due until maturity. In addition, the note will no longer bear interest at the penalty rate of 15% unless there is a new event of default. The Company is negotiating terms of an extension to the note with the note holder.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
8% Line of Credit dated July 24, 2020
On July 24, 2020, the Company entered into the Loan Agreement, 8% Secured Promissory Note, and Security Agreement (together, the "July 24 Note") with AJE Management, LLC, which established a revolving loan of up to $500,000 that the Company may draw on from time to time. The loan is collateralized by the Teco Facility, subject to the pre-existing lien held by CSW Ventures, L.P. in connection with the 8% Senior Secured Convertible Promissory Note dated February 28, 2019. Any advances will be made at the sole discretion of the lender following a written request made by the Company. Contemporaneously with the Loan Agreement, the Company and AJE Management entered into the Amendment to the Membership Interest Purchase Agreement with AJE Management. The amendment provides that any balances outstanding under the July 24 Note at the time of the close of the sale of the Teco Facility will be forgiven in exchange for a reduction to the $4,000,000 note receivable that the Company will receive as consideration for the sale of the Teco Facility. The reduction to the note receivable will be equal to 3 times the balance outstanding under the July 24 Note on the date of the close of the sale of the Teco Facility. The balance outstanding under the note plus accrued interest may be repaid at any time prior to the close of the sale of the Teco facility (Note 9).
On December 29, 2020, the Company entered into the Omnibus Amendment with the purchaser of the Teco Facility. The Omnibus Amendment reduces the amount of the note receivable that the Company will receive from the sale of the Teco Facility by $975,000 (three times $325,000 in advances made under the July 24 Note) to $3,025,000. Any advances made to the Company under the July 24 Note in excess of $325,000 will reduce the amount of cash received upon close of the sale of Teco one-for-one, i.e., such advances will be considered advance payments of the $4,000,000 cash purchase price. The note will not accrue any additional interest subsequent to November 30, 2020. The Company also agreed that it will not repay the balances outstanding under the July 24 Note prior to the closing of the Teco sale. As a result of the Omnibus Amendment, the Company accrued a modification expense of $650,000 during the year ended March 31, 2021 (two times $325,000 in addition to $325,000 in advances already recorded under the July 24 Note). As of June 30, 2021, the Company has received $50,000 in additional advances above $325,000 during the fiscal year ended March 31, 2021, bringing the total balance to $1,025,000 at June 30, 2021. Accrued interest was $58,495 at June 30, 2021.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
Summary of Notes and Convertible Notes Payable
As of June 30, 2021, the following notes payable were recorded in the Company’s consolidated balance sheet:
|
|
|
As of June 30, 2021
|
|
|
Short-Term Notes Payable
|
|
Face Value
|
|
|
Discount
|
|
|
Carrying
Value
|
|
|
0% Note Payable dated October 23, 2017 (Note 4)
|
|
$
|
369,445
|
|
|
$
|
-
|
|
|
$
|
369,445
|
|
|
8% Line of Credit dated November 27, 2019 (Note 3)
|
|
|
485,000
|
|
|
|
-
|
|
|
|
485,000
|
|
|
8% Line of Credit dated July 24, 2020 (Note 4)
|
|
|
1,025,000
|
|
|
|
-
|
|
|
|
1,025,000
|
|
|
6% Convertible promissory notes payable (Note 5)
|
|
|
1,060,000
|
|
|
|
-
|
|
|
|
1,060,000
|
|
|
8% Convertible Secured Promissory Note dated February 28, 2019, as amended (Note 5)
|
|
|
1,111,863
|
|
|
|
-
|
|
|
|
1,111,863
|
|
|
6% Convertible notes payable due January 18, 2022 (Note 5)
|
|
|
325,000
|
|
|
|
(272,122
|
)
|
|
|
52,878
|
|
|
Total short-term notes and convertible notes payable
|
|
|
4,376,308
|
|
|
|
(272,122
|
)
|
|
|
4,104,186
|
|
|
Less: Notes payable classified as discontinued operations
|
|
|
(485,000
|
)
|
|
|
-
|
|
|
|
(485,000
|
)
|
|
Total short-term notes payable classified as continuing operations
|
|
$
|
3,891,308
|
|
|
$
|
(272,122
|
)
|
|
$
|
3,619,186
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6% Convertible promissory notes payable due September 30, 2023 (Note 5)
|
|
|
197,000
|
|
|
|
(37,204
|
)
|
|
|
159,796
|
|
|
6% Convertible note payable due December 31, 2023 (Note 5)
|
|
|
250,000
|
|
|
|
(103,919
|
)
|
|
|
146,081
|
|
|
Total long-term notes payable classified as continuing operations
|
|
$
|
447,000
|
|
|
$
|
(141,123
|
)
|
|
$
|
305,877
|
|
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
Note 5 – Convertible Notes
March 2017 and July 2017 Convertible Note Offerings
In March 2017, the Company entered into a Placement Agent’s Agreement with a third-party brokerage firm to offer units consisting of a $1,000 6% promissory note convertible into 4,000 shares of the Company’s common stock at $0.25 per share and 4,000 warrants to purchase shares of the Company’s’ common stock at an exercise price of $0.60 per share for the period of three years. Between March 2017 and May 2017, the Company issued short-term Promissory Notes (“Notes”) to various holders with combined face value of $2,000,000. The Notes are payable within three years of issuance and are convertible into 8,000,000 shares of the Company’s common stock. The Company also issued 8,000,000 common stock warrants to the Noteholders. The warrants are exercisable at any time and from time to time before maturity at the option of the holder. Each warrant gives the Noteholder the right to purchase one share of common stock of the Company at an exercise price of $0.60 per share for a period of three years. The Company recorded an aggregate discount on convertible notes of $1,933,693, which included $904,690 related to the relative fair value of beneficial conversion features and $1,029,003 for the relative fair value of the warrants issued with each note. The fair value of warrants was derived using the Black-Scholes valuation model.
In July 2017, the Company entered into a Placement Agent’s Agreement with a third-party brokerage firm to offer units consisting of a $1,000 6% promissory note convertible into 4,000 shares of the Company’s common stock at $0.25 per share and 4,000 warrants to purchase shares of the Company’s’ common stock at an exercise price of $0.65 per share for the period of three years. Between July 2017 and December 2017, the Company issued short-term Promissory Notes (“Notes”) to various holders with combined face value of $7,201,000. The Notes are payable within three years of issuance and are convertible into 28,804,000 shares of the Company’s common stock. The Company also issued 28,804,000 common stock warrants to the Note holders. The warrants are exercisable at any time and from time to time before maturity at the option of the holder. Each warrant gives the Noteholder the right to purchase one share of common stock of the Company at an exercise price of $0.60 per share for a period of three years. The Company recorded an aggregate discount on convertible notes of $7,092,796, which included $3,142,605 related to the relative fair value of beneficial conversion features and $3,950,191 for the relative fair value of the warrants issued with each note. The fair value of warrants was derived using the Black-Scholes valuation model.
All notes from the March and July 2017 offerings have passed their maturity dates. During the year ended March 31, 2021, the Company agreed to extensions with the holders of a total of $197,000 of the $1,257,000 that remains outstanding. For the $197,000 of extended notes, the Company agreed to reduce the conversion price to $0.10 per share and issued a total of 788,000 additional warrants to the holders of the notes with a term of three years and an exercise price of $0.10 per share. In exchange, the maturity date of the notes was extended to September 30, 2023. Using the Black-Scholes model, the Company valued the warrants at $13,396 and the change in the fair value of the conversion feature at $33,490. Because the change in the fair value of the conversion feature exceeded 10% of the carrying amount of the notes, the Company accounted for the modification of the notes as an extinguishment and recorded a discount on the new convertible notes of $46,886 related to the fair value of the new warrants and the change in the fair value of the conversion feature. The Company recorded interest expense of $6,303 on the new notes during the three months ended June 30, 2021, of which $3,356 represented amortization of the note discounts. Accrued interest on the $197,000 extended notes is $47,279 at June 30, 2021, which includes $38,438 accrued prior to the extinguishments.
Three convertible notes totaling $1,060,000 held by the same investor are past maturity and are currently in default. The Company is negotiating the terms of an extension with the note holder. The notes do not provide for a default penalty or penalty interest rate. Interest expense during the three months ended June 30, 2021, was $15,856 and there is no remaining unamortized discount. Accrued interest on the $1,060,000 notes was $244,129 at June 30, 2021.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
8% Senior Secured Convertible Promissory Note dated February 28, 2019
On February 28, 2019, the Company issued a $1,500,000 8% Senior Secured Convertible Promissory Note and entered into the Note Purchase Agreement and Security Agreement with CSW Ventures, L.P. (together, “CSW Note”). The note matured on August 28, 2020, and was convertible at any time until maturity into 8,823,529 shares of the Company’s common stock at $0.17 per share. Collateral pledged as security for the note includes all of the Company’s 100% membership interests in GB Sciences, Nevada, LLC and GB Sciences Las Vegas, LLC, which together represent substantially all of the Company’s cannabis cultivation and production operations and assets located at the Teco facility in Las Vegas, Nevada. The intrinsic value of the beneficial conversion feature resulting from the market price of the Company’s common stock in excess of the conversion price was $176,471 on the date of issuance, and the Company recorded a discount on the CSW Note in that amount.
On May 28, 2019, the Company received notice from CSW Ventures, L.P. of the conversion of a total of $170,000 of the principal balance of the 8% Senior Secured Promissory Note dated February 28, 2019. Accordingly, the Company issued 1,000,000 shares of its common stock based on a $0.17 per share conversion price. In connection with the conversions, $17,225 in unamortized discount was recorded as interest expense and the Company reduced the carrying amount of convertible notes payable by $152,775. After conversion, the remaining balance outstanding was $1,330,000.
On July 12, 2019, the Company entered into the Amendment to Note Documents and the Amended and Restated 8% Senior Secured Promissory Note (together, “Amended CSW Note”). The Amended CSW Note increased the note balance by $100,000 to reflect an additional $100,000 advanced to the Company on July 12, 2019, and by $41,863 to add accrued interest to date to the principal balance, and decreased the conversion price to $0.11 per share, with the remaining terms substantially unchanged from the original CSW Note.
The Company evaluated the modification under the guidance in ASC 470-50 and determined that the amendment represents an extinguishment because the change in the fair value of the conversion feature exceeded 10% of the carrying value of the CSW Note on the amendment date. The carrying value of the amended note on the date of extinguishment was $1,338,057, net of a beneficial conversion feature discount of $133,806, and we recorded a loss on extinguishment of $124,158.
On August 1, 2019, the Company received notice from CSW Ventures, L.P. of the conversion of a total of $110,000 of the principal balance of the Amended CSW Note at $0.11 per share. Accordingly, the Company issued 1,000,000 shares of its common stock. In connection with the conversions, $9,579 in unamortized discount was recorded as interest expense and the Company has reduced the carrying amount of convertible notes payable by $100,421. After conversion, the remaining balance outstanding was $1,361,863.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
On October 23, 2019, the Company entered into the Amendment to Promissory Note. The October 23, 2019 amendment decreased the conversion price to $0.08 per share, with the remaining terms substantially unchanged from the Amended CSW Note.
We evaluated the modification under the guidance in ASC 470-50 and determined that the amendment represents an extinguishment because the change in the fair value of the conversion feature exceeded 10% of the carrying value of the Amended CSW Note immediately prior to the 2nd Amended CSW Note. The carrying value of the Amended CSW Note on the date of extinguishment was $1,269,067, net of a beneficial conversion feature discount of $92,796, and we recorded a loss on extinguishment of $92,796 during the year ended March 31, 2020.
On November 27, 2019, the Company entered into the Second Amendment to Note Documents and the Second Amended and Restated 8% Senior Secured Promissory Note (together, “2nd Amended CSW Note”). The 2nd Amended CSW Note decreased the conversion price to $0.04 per share and increased the note balance by $30,000 to reflect an advance received on that date, with the remaining terms substantially unchanged from the Amended CSW Note.
We evaluated the modification under the guidance in ASC 470-50 and determined that the 2nd Amended CSW Note represents an extinguishment because the change in the fair value of the conversion feature exceeded 10% of the carrying value of the Amended CSW Note immediately prior to the 2nd Amended CSW Note; however, no loss on extinguishment was recorded because the net consideration paid for the 2nd Amended CSW Note was equal to the extinguished carrying value of the Amended CSW Note. The carrying value of the Amended CSW Note on the date of extinguishment was $1,361,863.
On December 16, 2019, the Company received notice from CSW Ventures, L.P. of the conversion of a total of $120,000 of the principal balance of the Amended CSW Note at $0.04 per share and we issued 3,000,000 shares of common stock. In connection with the conversions, $57,551 in unamortized discount was recorded as interest expense, and the Company has reduced the carrying amount of convertible notes payable by $62,449. After conversion, the remaining balance outstanding was $1,271,863 and the carrying amount of the note was $687,021, net of $584,842 in unamortized discount from the beneficial conversion feature.
On December 29, 2020, the Company entered into the Omnibus Amendment, and the note holder agreed to cease interest accrual on the CSW Note after November 30, 2020.
During the quarter ended March 31, 2021, the Company received notice of the conversion of $160,000 total principal balance at $0.04 per share and issued 4,000,000 shares of common stock to the note holder. After the conversions that occurred during the year ended March 31, 2021, the remaining principal balance and carrying amount of the note is $1,111,863 as of June 30, 2021. Accrued interest was $144,994. The total outstanding balance of principal and accrued interest totaling $1,256,857 will reduce the $4,000,000 cash payment received by the Company upon the close of the sale of the Teco Facility, and no further interest expense will be accrued on the note.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
8% Convertible Promissory Note dated April 23, 2019
On April 23, 2019, the Company entered into the Note Purchase Agreement with Iliad Research and Trading, L.P. ("Iliad") and issued an 8% Convertible Promissory Note with a face value of $2,765,000. The Note was issued with original issue discount of $265,000 and is convertible into shares of the Company’s common stock at a price of $0.17 per share at the option of the note holder at any time until the Note is repaid. The Note matured on April 22, 2020. A total discount of $440,000 was recorded on the note, which includes $265,000 of original issue discount and $175,000 in fees paid to brokers.
During the year ended March 31, 2020, the Company honored the conversion of a total of a total of $125,000 of accrued interest on the Iliad Note at reduced conversion rates. On October 30, 2019, the Company received notice of the conversion of $75,000 at $0.06 per share and issued 1,250,000 shares of its common stock. The fair value of the shares issued exceeded the fair value of the shares issuable under the original terms of the Note by $64,706, and the Company recorded an induced conversion expense. On November 18, 2019, the Company received notice of the conversion of $50,000 of the note balance at $0.0375 per share and issued 1,333,333 shares of its common stock.
On April 22, 2020, the Company failed to make payment of the principal and accrued interest due under the Iliad Note, resulting in a default. Upon the occurrence of the default, the principal and accrued interest balances outstanding increased by 10%. As the result of the default, Company recorded an expense of $9,559 related to a 10% increase in the accrued interest balance, which is recorded in interest expense, and $276,500 related to the 10% increase in the principal balance, which is recorded in debt default penalty and other expense.
On May 20, 2020, Iliad filed a lawsuit against the Company in the Third Judicial District Court of Salt Lake County in the State of Utah demanding repayment of the note. The lawsuit further sought to compel the Company to participate in arbitration pursuant to the arbitration provisions contained within the Note Purchase Agreement and to prohibit the Company to raise funds through the issuance of its common stock unless the note is paid in full simultaneously with such issuance. On July 14, 2020, the Court entered judgment in favor of Iliad in the amount of $3,264,594 plus reasonable attorney's fees and costs and accrued post-judgment interest at the default rate of 15% per annum.
On November 20, 2020, the Company, Iliad, and Wellcana Plus, LLC entered into the Judgment Settlement Agreement, whereby Iliad agreed to discharge all amounts owed to it by the Company upon receipt of payment totaling $3,006,015 directly from the proceeds of the Wellcana Note Receivable on or before December 8, 2020. On December 8, 2020, Wellcana failed to make payment to the Company. On December 9, 2020, the Company entered into a letter agreement with Iliad extending the Judgment Settlement agreement in exchange for payment of $25,000 plus $25,000 per week until the payment totaling $3,006,015 is received by Iliad, with such payments not reducing the amount owed under the Judgment Settlement Agreement. On December 16, 2020, Wellcana made payment of the full amount owed to the Company, of which $3,006,015 was paid directly to Iliad in full satisfaction of the Judgment Settlement Agreement. On December 18, 2020, Iliad filed a Satisfaction of Judgment in the Third Judicial District Court of Salt Lake County in the State of Utah, and the lawsuit was dismissed. The Company has no further obligations to Iliad.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
December 2020 $575,000 6% Convertible Note Offering
On December 18, 2020, the Company began an offering of 6.0% convertible notes for the purpose of funding a pre-clinical study of the Company's patent-pending Cannabinoid-Containing Complex Mixtures for the treatment of Cytokine Release Syndromes, including Acute Respiratory Distress Syndrome, in COVID-19 patients. The Company pledged the related intellectual property as security for the notes. The notes are convertible at a rate of $0.05 per share at the lender's request. During the year ended March 31, 2021, the Company issued $575,000 in convertible notes under the offering to three investors. $325,000 of the notes mature in December 2021, and $250,000 mature in December 2023. Payment of accrued interest and principal is due at maturity. The Company received cash of $500,250, net of issuance costs, and recorded a discount on convertible notes of $74,750. Notes totaling $425,000 were issued with in-the-money conversion features, and the Company recorded beneficial conversion feature discounts totaling $347,000 on the related notes.
At June 30, 2021, notes with a carrying amount of $52,878 were included in short term notes and convertible notes payable, net of unamortized discounts of $272,122. Notes with a carrying amount of $146,081 were included in long term notes and convertible notes payable, net of unamortized discounts of $103,919. Interest expense related to the notes was $24,458 for the three months ended June 30, 2021, which includes $15,857 from amortization of the note discounts.
Note 6 – Capital Transactions
Sale of Common Stock and Exercise of Warrants
On April 1, 2020, the Company entered into the Advisory Agreement with its brokers and effected a temporary decrease in the exercise price of the Company's outstanding warrants to $0.03-$.05 per share. On July 18, 2021, the Company entered into an amendment to the Advisory Agreement extending the temporary decrease through September 30, 2021 and agreed that each exercising warrant holder will receive an equal number of replacement warrants to purchase one share of the Company's common stock at $0.10 for three years. During the three months ended June 30, 2021,the Company received notice of the exercise of 2,088,667 warrants at $0.03 per share and received proceeds of $56,394, net of accrued brokerage fees of $6,266. As the result of the exercises, the Company recorded an inducement dividend of $163,016, which includes $62,660 related to the intrinsic value of the exercised warrants at the dates of exercise and $100,356 related to the Black-Scholes fair value of the 2,088,667 replacement warrants issued to the exercising investors.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
Note 7 – Commitments and Contingencies
On April 22, 2020, the Company failed to repay any of the outstanding balance of the Convertible Promissory Note Payable to Iliad Research and Trading, L.P., resulting in a default. On May 20, 2020, Iliad filed a lawsuit against the Company in the Third Judicial District Court of Salt Lake County in the State of Utah demanding repayment of the note. On July 14, 2020, the Court entered judgment in favor of Iliad in the amount of $3,264,594. The Company's obligation to Iliad was satisfied in full on December 16, 2020 upon payment of $3,006,015 pursuant to the Judgment Settlement Agreement (Note 5).
On April 22, 2020, the Company was served notice of a lawsuit filed in the Eighth Judicial District Court in Clark County, Nevada, filed by a contractor who had been hired to perform architectural and design services. The lawsuit demanded payment of $73,050 for the services provided. On September 17, 2020, the Company entered into a Mutual Compromise, Settlement, and Release Agreement with the contractor and made payment of $25,000 in full satisfaction of the alleged debt and reduced the cost of the related fixed asset by $48,050.
From time to time, the Company may become involved in certain legal proceedings and claims which arise in the ordinary course of business. In management’s opinion, based on consultations with outside counsel, the results of any of these ordinary course matters, individually and in the aggregate, are not expected to have a material effect on our results of operations, financial condition, or cash flows. As more information becomes available, if management should determine that an unfavorable outcome is probable on such a claim and that the amount of such probable loss that it will incur on that claim is reasonably estimable, the Company would record a reserve for the claim in question. If and when the Company records such a reserve, it could be material and could adversely impact its results of operations, financial condition, and cash flows.
Note 8 – Related Party Transactions
As of June 30, 2021 the Company was indebted to executive officers for unpaid compensation totaling $84,913.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
Note 9 – Sale of Membership Interests in Nevada Subsidiaries
On November 15, 2019, we entered into a Binding Letter of Intent ("Teco LOI") to sell 75% of the Company's membership interest interests in GB Sciences Nevada, LLC, and GB Sciences Las Vegas, LLC ("Teco Subsidiaries"). On March 24, 2020, we entered into the Membership Interest Purchase Agreement ("Teco MIPA") with AJE Management, LLC, which formalized the sale of the Teco Subsidiaries and modified the terms of the sale. Pursuant to the Teco MIPA, the Company will sell 100% of its membership interests in GBSN and GBLV for $4,000,000 cash upon close and will receive a $4,000,000 8% promissory note to be paid in monthly installments over 36 months with payments beginning 30 months after the close of the sale.
On July 24, 2020, the Company entered into the Loan Agreement, 8% Secured Promissory Note, and Security Agreement (together, the "July 24 Note") with AJE Management, LLC. Contemporaneously with the Loan Agreement, the Company and AJE Management entered into the Amendment to the Membership Interest Purchase Agreement with AJE Management. The amendment provides that any balances outstanding under the July 24 Note at the time of the close of the sale of the Teco Facility will be forgiven in exchange for a reduction to the $4,000,000 note receivable that the Company will receive as consideration for the sale of the Teco Facility. The Company has received $375,000 in advances under the note (Note 4).
On December 29, 2020, the Company entered into the Omnibus Amendment with the purchaser of the Teco Facility. The Omnibus Amendment reduces the amount of the note receivable that the Company will receive from the sale of the Teco Facility by $975,000 to $3,025,000, and any advances made to the Company above $325,000 will reduce the amount of cash received upon close of the sale of Teco one-for-one, rather than reducing the note receivable by three times the amount of the balance outstanding. The Company also agreed that it will not repay the balances outstanding under the July 24 Note prior to the closing of the Teco sale. As a result of the Omnibus Amendment, the Company accrued an expense of $650,000 to increase the balance outstanding under the July 24 Note to three times $325,000, to a total of $975,000 which will reduce the $4,000,000 note receivable that the Company will receive upon close of the sale of the Teco Facility, and an additional $50,000 that will be treated as a reduction of the $4,000,000 cash purchase price paid at close.
The Omnibus Amendment also amends the Management Services Agreement to provide that no further management fees will accrue after November 30, 2020. As of June 30, 2021, the Company has paid $150,000 and accrued $700,000 in management fees, which will reduce the $4,000,000 in cash proceeds received upon the close of the sale. The form of the note receivable that the Company will receive on close was amended to accelerate payments such that the Company will receive payment in full within three years, rather than over 36 months with payments beginning 30 months after the close of the sale.
On May 11, 2021, the Company entered into a Second Omnibus Amendment, which extends the Management Services Agreement through December 31, 2021, and received $200,000 from the purchaser as a partial advancement of the $4,000,000 cash purchase price. The advancement is recorded in accrued liabilities in the Company's condensed consolidated balance sheet as of June 30, 2021.
The sale is expected to close upon the successful transfer of the Nevada cultivation and production licenses. The transfer of cannabis licenses in the State of Nevada was subject to an indefinite moratorium beginning in October 2019. In a meeting held on July 21, 2020, the Nevada Cannabis Compliance Board lifted the moratorium, however, the board has indicated that there were over 90 requests pending, and it will take up to several months to process the entire backlog of pending license transfers. The lifting of the moratorium and processing of cannabis license transfers have been delayed by the COVID-19 pandemic and could be further delayed if the pandemic continues.
The Company also holds a Nevada license for cultivation of medical marijuana located in Sandy Valley, Nevada (the “Nopah License”). The license is owned by the Company’s wholly owned subsidiary, GB Sciences Nopah, LLC ("Nopah"). Operations have not begun under the Nopah License. On November 27, 2019, the Company entered into a Binding Letter of Intent to sell its 100% interest in GB Sciences Nopah, LLC. On August 10, 2020, the Company entered into the Membership Interest Purchase Agreement ("Nopah MIPA") and Promissory Note Modification Agreement with the purchaser of GB Sciences Nopah, LLC. As consideration for the transfer of the license and membership interest in GB Sciences Nopah, LLC, the Company will receive $300,000 and the purchaser will pay all expenses related to the upkeep and maintenance of the Nopah License until closing. The $300,000 purchase price will be applied as a reduction to the balance of the 0% Note payable dated October 23, 2017 (Note 4), which is held by an affiliate of the purchaser of the Nopah license. The transfer of the Nopah License is subject to the same restrictions on license transfers discussed above.
Because the moratorium on license transfers has been lifted, the Company determined that the Teco Facility and Nopah Facility qualify for presentation as discontinued operations, and the income, assets, and cash flows of the Teco Subsidiaries and GB Sciences Nopah, LLC have been reclassified as discontinued operations for all periods presented in the Company's consolidated financial statements.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021
(unaudited)
Note 10 – Subsequent Events
Capital Transactions
On July 2, 2021, the Company received $50,000 from an investor and issued a 6% convertible promissory note due July 1, 2022 under the convertible note offering that began in December 2020. The note is convertible at $0.05 per share into the Company's common stock.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Audit Committee of
GB Sciences, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of GB Sciences, Inc. (the Company) as of March 31, 2021 and 2020, and the related consolidated statements of operations, stockholders’ deficit and cash flows for each of the years in the two-year period ended March 31, 2021 and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of March 31, 2021 and 2020, and the results of its operations and its cash flows for each of the years in the two-year period ended March 31, 2021, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph- Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company has suffered recurring losses for the year ended March 31, 2021. The Company had a net loss of $3,725,027, accumulated deficit of $103,886,232, net cash used in operating activities of $2,185,220 and had negative working capital of $5,054,593. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 3. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.2
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Inventory Costs
As summarized in Note 2 “Inventory” to the consolidated financial statements, the Company’s inventory consists of three categories, Raw materials, which consists of supplies, materials, and consumables used in the cultivation and extraction processes; work-in-progress which includes live plants and cannabis in the drying, curing, and trimming processes and finished goods includes completed cannabis flower, trim, and extracts in bulk and packaged forms. The inventory, net of reserve was $1,689,304 as of March 31, 2021. Management records the cost of inventory on the consolidated balance sheet based on the costs incurred throughout the year in each category less the amounts transferred to cost of goods sold for sales in the year. Labor costs and overhead costs comprise the majority of overall inventory cost.
We identified the allocation of labor and overhead costs to inventory as a critical audit matter because of the significant estimates management used in the allocation of labor and overhead costs to inventory. Auditing management’s allocations of internal costs to the inventory was complex and involved a high degree of subjectivity.
The primary procedures we performed to address this critical audit matter included (a) Obtained an understanding of management’s process for allocating labor and overhead costs, (b) Tested the accuracy and completeness of allocated labor, including testing, on a sample basis, total labor costs incurred, (c) Tested the accuracy and completeness of overhead costs allocated, including testing, on a sample basis, overhead costs incurred (d) Evaluated the reasonableness of management’s significant assumptions used in such allocation, (e) compared the Company’s inventory ratios and per unit production costs to industry data, and (f) Recomputed the unit cost and total inventory costs.
/s/ Assurance Dimensions
|
|
|
We have served as the Company’s auditor since 2020.
|
|
|
|
Margate, Florida
July 2, 2021
|
GB SCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
|
|
|
As of March 31,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
CURRENT ASSETS:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
793,040
|
|
|
$
|
2,406
|
|
|
Prepaid expenses and other current assets
|
|
|
256,251
|
|
|
|
18,776
|
|
|
Note receivable
|
|
|
-
|
|
|
|
5,224,423
|
|
|
Current assets from discontinued operations
|
|
|
2,494,564
|
|
|
|
1,755,275
|
|
|
TOTAL CURRENT ASSETS
|
|
|
3,543,855
|
|
|
|
7,000,880
|
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment, net
|
|
|
25,022
|
|
|
|
37,821
|
|
|
Intangible assets, net of accumulated amortization of $43,096 and $12,287 at March 31, 2021 and 2020, respectively
|
|
|
1,706,762
|
|
|
|
1,128,702
|
|
|
Long term assets from discontinued operations
|
|
|
5,530,415
|
|
|
|
6,185,465
|
|
|
TOTAL ASSETS
|
|
$
|
10,806,054
|
|
|
$
|
14,352,868
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
1,412,459
|
|
|
$
|
1,913,049
|
|
|
Accrued interest
|
|
|
493,741
|
|
|
|
366,865
|
|
|
Accrued liabilities
|
|
|
957,946
|
|
|
|
813,618
|
|
|
Notes and convertible notes payable, net of unamortized discount of $296,504 and $608,580 at March 31, 2021 and 2020, respectively
|
|
|
3,594,804
|
|
|
|
5,054,728
|
|
|
Indebtedness to related parties
|
|
|
84,913
|
|
|
|
586,512
|
|
|
Note payable to related party
|
|
|
-
|
|
|
|
151,923
|
|
|
Current liabilities from discontinued operations
|
|
|
2,054,585
|
|
|
|
1,999,062
|
|
|
TOTAL CURRENT LIABILITIES
|
|
|
8,598,448
|
|
|
|
10,885,757
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible notes payable, net of unamortized discount of $154,590 and $0 at March 31, 2021 and 2020, respectively
|
|
|
292,410
|
|
|
|
-
|
|
|
Long term liabilities from discontinued operations
|
|
|
3,389,124
|
|
|
|
3,555,605
|
|
|
TOTAL LIABILITIES
|
|
|
12,279,982
|
|
|
|
14,441,362
|
|
|
Commitments and contingencies (Note 11)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS' EQUITY/(DEFICIT):
|
|
|
|
|
|
|
|
|
|
Common Stock, $0.0001 par value, 600,000,000 shares authorized, 315,340,411 and 275,541,602 outstanding at March 31, 2021 and 2020, respectively
|
|
|
31,534
|
|
|
|
27,554
|
|
|
Additional paid-in capital
|
|
|
102,380,770
|
|
|
|
97,271,157
|
|
|
Accumulated deficit
|
|
|
(103,886,232
|
)
|
|
|
(97,387,205
|
)
|
|
TOTAL STOCKHOLDERS' DEFICIT
|
|
|
(1,473,928
|
)
|
|
|
(88,494
|
)
|
|
TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT
|
|
$
|
10,806,054
|
|
|
$
|
14,352,868
|
|
The accompanying notes are an integral part of these consolidated financial statements
GB SCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
|
|
|
For the Years Ended
|
|
|
|
|
March 31,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales revenue
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Cost of goods sold
|
|
|
-
|
|
|
|
-
|
|
|
Gross profit (loss)
|
|
|
-
|
|
|
|
-
|
|
|
General and administrative expenses
|
|
|
2,001,617
|
|
|
|
5,741,514
|
|
|
LOSS FROM OPERATIONS
|
|
|
(2,001,617
|
)
|
|
|
(5,741,514
|
)
|
|
OTHER INCOME (EXPENSE)
|
|
|
|
|
|
|
|
|
|
Gain/(loss) on extinguishment
|
|
|
467,872
|
|
|
|
(216,954
|
)
|
|
Gain on settlement of accounts payable
|
|
|
422,414
|
|
|
|
-
|
|
|
Gain on deconsolidation
|
|
|
-
|
|
|
|
4,393,242
|
|
|
Interest expense
|
|
|
(1,285,460
|
)
|
|
|
(1,109,031
|
)
|
|
Loss on modification of line of credit
|
|
|
(650,000
|
)
|
|
|
-
|
|
|
Loss on modification of note receivable
|
|
|
-
|
|
|
|
(1,895,434
|
)
|
|
Debt default penalty
|
|
|
(286,059
|
)
|
|
|
-
|
|
|
Other expense
|
|
|
-
|
|
|
|
(179,368
|
)
|
|
Total other income/(expense)
|
|
|
(1,331,233
|
)
|
|
|
992,455
|
|
|
LOSS BEFORE INCOME TAXES
|
|
|
(3,332,850
|
)
|
|
|
(4,749,059
|
)
|
|
Income tax expense (Note 8)
|
|
|
-
|
|
|
|
-
|
|
|
LOSS FROM CONTINUING OPERATIONS
|
|
|
(3,332,850
|
)
|
|
|
(4,749,059
|
)
|
|
Net loss from discontinued operations (Note 4)
|
|
|
(392,177
|
)
|
|
|
(8,362,626
|
)
|
|
NET LOSS
|
|
|
(3,725,027
|
)
|
|
|
(13,111,685
|
)
|
|
Net loss attributable to non-controlling interest
|
|
|
-
|
|
|
|
(738,106
|
)
|
|
NET LOSS ATTRIBUTABLE TO GB SCIENCES, INC.
|
|
$
|
(3,725,027
|
)
|
|
$
|
(12,373,579
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to common stockholders of GB Sciences, Inc.
|
|
|
|
|
|
|
|
|
|
Continuing operations
|
|
$
|
(3,332,850
|
)
|
|
$
|
(4,749,059
|
)
|
|
Discontinued operations
|
|
|
(392,177
|
)
|
|
|
(7,624,520
|
)
|
|
Net loss
|
|
$
|
(3,725,027
|
)
|
|
$
|
(12,373,579
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per common share – basic and diluted
|
|
|
|
|
|
|
|
|
|
Continuing operations
|
|
$
|
(0.01
|
)
|
|
$
|
(0.02
|
)
|
|
Discontinued operations
|
|
$
|
(0.00
|
)
|
|
$
|
(0.03
|
)
|
|
Net loss
|
|
$
|
(0.01
|
)
|
|
$
|
(0.05
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding - basic and diluted
|
|
|
285,190,729
|
|
|
|
258,450,641
|
|
The accompanying notes are an integral part of these consolidated financial statements
GB SCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY/(DEFICIT)
|
|
|
|
|
|
|
|
|
|
|
Additional
Paid-
|
|
|
Accumulated
|
|
|
Non-
Controlling
|
|
|
|
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
In Capital
|
|
|
Deficit
|
|
|
Interest
|
|
|
Total
|
|
|
Balance at March 31, 2019
|
|
|
240,627,102
|
|
|
$
|
24,063
|
|
|
$
|
93,020,015
|
|
|
$
|
(84,743,836
|
)
|
|
$
|
8,855,757
|
|
|
$
|
17,155,999
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of stock for debt conversion
|
|
|
7,583,333
|
|
|
|
758
|
|
|
|
524,242
|
|
|
|
-
|
|
|
|
-
|
|
|
|
525,000
|
|
|
Exercise of warrants for stock, net of issuance costs
|
|
|
17,563,000
|
|
|
|
1,756
|
|
|
|
1,155,971
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,157,727
|
|
|
Issuance of stock for services
|
|
|
2,100,000
|
|
|
|
210
|
|
|
|
213,790
|
|
|
|
-
|
|
|
|
-
|
|
|
|
214,000
|
|
|
Share based compensation expense
|
|
|
-
|
|
|
|
-
|
|
|
|
287,260
|
|
|
|
-
|
|
|
|
-
|
|
|
|
287,260
|
|
|
Issuance of stock for cash, net of issuance costs
|
|
|
7,668,167
|
|
|
|
767
|
|
|
|
717,929
|
|
|
|
-
|
|
|
|
-
|
|
|
|
718,696
|
|
|
Beneficial conversion feature on notes payable
|
|
|
-
|
|
|
|
-
|
|
|
|
829,737
|
|
|
|
-
|
|
|
|
-
|
|
|
|
829,737
|
|
|
Contributions from non-controlling interest
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
590,000
|
|
|
|
590,000
|
|
|
Compensation warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
132,914
|
|
|
|
-
|
|
|
|
-
|
|
|
|
132,914
|
|
|
Inducement dividend from warrant exercises
|
|
|
-
|
|
|
|
-
|
|
|
|
262,240
|
|
|
|
(262,240
|
)
|
|
|
-
|
|
|
|
-
|
|
|
Induced conversions of accrued interest on notes payable
|
|
|
-
|
|
|
|
-
|
|
|
|
127,059
|
|
|
|
-
|
|
|
|
-
|
|
|
|
127,059
|
|
|
Cumulative effect of the new lease standard
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(7,550
|
)
|
|
|
-
|
|
|
|
(7,550
|
)
|
|
Deconsolidation of GB Sciences Louisiana, LLC
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(8,707,651
|
)
|
|
|
(8,707,651
|
)
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(12,373,579
|
)
|
|
|
-
|
|
|
|
(12,373,579
|
)
|
|
Loss attributable to non-controlling interest
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(738,106
|
)
|
|
|
(738,106
|
)
|
|
Balance at March 31, 2020
|
|
|
275,541,602
|
|
|
|
27,554
|
|
|
|
97,271,157
|
|
|
|
(97,387,205
|
)
|
|
|
-
|
|
|
|
(88,494
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of stock for debt conversion
|
|
|
4,000,000
|
|
|
|
400
|
|
|
|
159,600
|
|
|
|
-
|
|
|
|
-
|
|
|
|
160,000
|
|
|
Exercise of warrants for stock, net of issuance costs
|
|
|
35,798,809
|
|
|
|
3,580
|
|
|
|
964,443
|
|
|
|
-
|
|
|
|
-
|
|
|
|
968,023
|
|
|
Share based compensation expense
|
|
|
-
|
|
|
|
-
|
|
|
|
436,349
|
|
|
|
-
|
|
|
|
-
|
|
|
|
436,349
|
|
|
Beneficial conversion feature on notes payable
|
|
|
-
|
|
|
|
-
|
|
|
|
543,886
|
|
|
|
-
|
|
|
|
-
|
|
|
|
543,886
|
|
|
Compensation warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
231,335
|
|
|
|
-
|
|
|
|
-
|
|
|
|
231,335
|
|
|
Inducement dividend from warrant exercises
|
|
|
-
|
|
|
|
-
|
|
|
|
2,774,000
|
|
|
|
(2,774,000
|
)
|
|
|
-
|
|
|
|
-
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
(3,725,027
|
)
|
|
|
-
|
|
|
|
(3,725,027
|
)
|
|
Balance at March 31, 2021
|
|
|
315,340,411
|
|
|
$
|
31,534
|
|
|
$
|
102,380,770
|
|
|
$
|
(103,886,232
|
)
|
|
$
|
-
|
|
|
$
|
(1,473,928
|
)
|
The accompanying notes are an integral part of these consolidated financial statements
GB SCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
|
Year Ended March 31,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
OPERATING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(3,725,027
|
)
|
|
$
|
(13,111,685
|
)
|
|
Loss from discontinued operations
|
|
|
(392,177
|
)
|
|
|
(8,362,626
|
)
|
|
Net loss from continuing operations
|
|
|
(3,332,850
|
)
|
|
|
(4,749,059
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
47,353
|
|
|
|
125,502
|
|
|
Stock-based compensation
|
|
|
436,349
|
|
|
|
287,260
|
|
|
Stock issued for services
|
|
|
-
|
|
|
|
214,000
|
|
|
Compensation warrants
|
|
|
231,335
|
|
|
|
132,914
|
|
|
Amortization of debt discount and beneficial conversion feature
|
|
|
776,122
|
|
|
|
1,150,995
|
|
|
Debt default penalty
|
|
|
286,059
|
|
|
|
-
|
|
|
Interest expense on conversion of notes payable
|
|
|
-
|
|
|
|
84,354
|
|
|
Loss on modification of line of credit
|
|
|
650,000
|
|
|
|
-
|
|
|
Loss/(gain) on extinguishment
|
|
|
(467,872
|
)
|
|
|
216,954
|
|
|
Gain on settlement of accounts payable
|
|
|
(422,414
|
)
|
|
|
-
|
|
|
Loss on disposal of assets and termination of operating lease
|
|
|
-
|
|
|
|
147,953
|
|
|
Loss on induced conversion of note payable
|
|
|
-
|
|
|
|
127,059
|
|
|
Loss on note receivable modification
|
|
|
-
|
|
|
|
1,895,434
|
|
|
Gain on deconsolidation
|
|
|
-
|
|
|
|
(4,393,242
|
)
|
|
Interest income receivable and amortization of discount on note receivable
|
|
|
-
|
|
|
|
(509,265
|
)
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
-
|
|
|
|
150,137
|
|
|
Prepaid expenses and other current assets
|
|
|
(237,475
|
)
|
|
|
20,932
|
|
|
Decrease in deposits and other noncurrent assets
|
|
|
-
|
|
|
|
110,485
|
|
|
Inventory
|
|
|
-
|
|
|
|
83,750
|
|
|
Accounts payable
|
|
|
(248,115
|
)
|
|
|
739,415
|
|
|
Accrued expenses
|
|
|
166,828
|
|
|
|
697,429
|
|
|
Accrued interest
|
|
|
549,703
|
|
|
|
464,279
|
|
|
Indebtedness to related parties
|
|
|
(501,599
|
)
|
|
|
738,435
|
|
|
Net cash used in operating activities of continuing operations
|
|
|
(2,066,576
|
)
|
|
|
(2,264,279
|
)
|
|
Net cash used in operating activities of discontinued operations
|
|
|
(118,644
|
)
|
|
|
(2,215,434
|
)
|
|
Net cash used in operating activities
|
|
|
(2,185,220
|
)
|
|
|
(4,479,713
|
)
|
|
INVESTING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Proceeds of note receivable
|
|
|
5,051,923
|
|
|
|
-
|
|
|
Acquisition of intangible assets
|
|
|
(292,675
|
)
|
|
|
(91,862
|
)
|
|
Net cash provided by/(used in) investing activities of continuing operations
|
|
|
4,759,248
|
|
|
|
(91,862
|
)
|
|
Net cash used in investing activities of discontinued operations
|
|
|
(103,729
|
)
|
|
|
(446,922
|
)
|
|
Net cash provided by/(used in) investing activities
|
|
|
4,655,519
|
|
|
|
(538,784
|
)
|
|
FINANCING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock
|
|
|
-
|
|
|
|
790,225
|
|
|
Proceeds from warrant exercises
|
|
|
1,075,396
|
|
|
|
1,274,790
|
|
|
Proceeds from convertible notes payable
|
|
|
725,000
|
|
|
|
2,630,000
|
|
|
Proceeds from line of credit
|
|
|
375,000
|
|
|
|
-
|
|
|
Principal payment on notes payable and operating lease obligation
|
|
|
(3,156,014
|
)
|
|
|
(84,869
|
)
|
|
Principal payment on related party note
|
|
|
(151,923
|
)
|
|
|
-
|
|
|
Brokerage fees from warrant exercises and stock issuances
|
|
|
(107,373
|
)
|
|
|
(188,593
|
)
|
|
Fees for issuance of convertible notes
|
|
|
(74,750
|
)
|
|
|
(175,000
|
)
|
|
Net cash provided by/(used in) financing activities of continuing operations
|
|
|
(1,314,664
|
)
|
|
|
4,246,553
|
|
|
Net cash provided by/(used in) financing activities of discontinued operations
|
|
|
(161,768
|
)
|
|
|
741,655
|
|
|
Net cash provided by/(used in) financing activities
|
|
|
(1,476,432
|
)
|
|
|
4,988,208
|
|
|
NET CHANGE IN CASH AND CASH EQUIVALENTS
|
|
|
993,867
|
|
|
|
(30,289
|
)
|
|
CASH AND CASH EQUIVALENTS AT BEGINNING OF YEAR
|
|
|
151,766
|
|
|
|
182,055
|
|
|
CASH AND CASH EQUIVALENTS AT END OF YEAR
|
|
|
1,145,633
|
|
|
|
151,766
|
|
|
Less: cash and cash equivalents classified as discontinued operations
|
|
|
(352,593
|
)
|
|
|
(149,360
|
)
|
|
CASH AND CASH EQUIVALENTS AT END OF YEAR FROM CONTINUING OPERATIONS
|
|
$
|
793,040
|
|
|
$
|
2,406
|
|
The accompanying notes are an integral part of these consolidated financial statements
GB SCIENCES, INC. AND SUBSIDIARIES
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION
|
|
|
Year Ended March 31,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid for interest
|
|
$
|
241,014
|
|
|
$
|
451,040
|
|
|
Cash paid for income tax
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Noncash investing and financing transactions:
|
|
|
|
|
|
|
|
|
|
Accrued liabilities forgiven in connection with Wellcana Note settlement
|
|
$
|
172,500
|
|
|
$
|
-
|
|
|
Depreciation capitalized in inventory (discontinued operations)
|
|
$
|
532,785
|
|
|
$
|
811,508
|
|
|
Accrued interest capitalized in convertible note principal
|
|
$
|
223,094
|
|
|
$
|
-
|
|
|
Property capitalized under operating leases
|
|
$
|
-
|
|
|
$
|
182,624
|
|
|
Patent acquisition costs capitalized in intangible assets
|
|
$
|
319,939
|
|
|
$
|
247,646
|
|
|
Stock options issued for preparing patent applications
|
|
$
|
168,000
|
|
|
$
|
-
|
|
|
Stock issued upon conversion of notes payable
|
|
$
|
160,000
|
|
|
$
|
525,000
|
|
|
Inducement dividend from warrant exercises
|
|
$
|
2,774,000
|
|
|
$
|
262,240
|
|
|
Beneficial conversion feature on notes payable
|
|
$
|
543,886
|
|
|
$
|
829,737
|
|
|
Cumulative effect of the new lease standard
|
|
$
|
-
|
|
|
$
|
7,550
|
|
The accompanying notes are an integral part of these consolidated financial statements
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Note 1 - Background and Nature of Operations
Business
GB Sciences, Inc. (“the Company”, “GB Sciences”, “we”, “us”, or “our”) is a phytomedical research and biopharmaceutical drug development company whose goal is to create patented formulations of plant-inspired, complex therapeutic mixtures for the prescription drug market that target a variety of medical conditions. The Company is engaged in the research and development of plant-based medicines and plans to produce plant-inspired, complex therapeutic mixtures based on its portfolio of intellectual property.
Through its wholly owned Canadian subsidiary, GBS Global Biopharma, Inc. (“GBSGB”), the Company is engaged in the research and development of plant-based medicines, primarily cannabinoid medicines, with virtual operations in North America and Europe. GBSGB’s assets include a portfolio of intellectual property containing both proprietary cannabinoid-containing formulations and our AI-enabled drug discovery platform, as well as critical research contracts and key supplier arrangements. GBSGB’s intellectual property covers a range of medical conditions and several programs are in the pre-clinical animal stage of development including Parkinson’s disease, neuropathic pain, and cardiovascular therapeutic programs. GBSGB runs a lean drug development program and takes effort to minimize expenses, including personnel, overhead, and fixed capital expenses through strategic partnerships with Universities and Contract Research Organizations (“CROs”). GBSGB’s intellectual property portfolio includes five USPTO issued patents, nine USPTO nonprovisional patent applications pending in the US, and one provisional patent application in the US. In addition to the USPTO patents and patent applications, the company has filed 35 patent applications internationally to protect its proprietary technology. We recently filed a provisional USPTO patent application to further protect aspects of our proprietary drug discovery engine, “Phytomedical Analytics for Research Optimization at Scale," or PhAROS™.
We were incorporated in the State of Delaware on April 4, 2001, under the name “Flagstick Venture, Inc.” On March 28, 2008, stockholders owning a majority of our outstanding common stock approved changing our then name “Signature Exploration and Production Corp.” as our business model had changed.
On April 4, 2014, we changed our name from Signature Exploration and Production Corporation to Growblox Sciences, Inc. Effective December 12, 2016, the Company amended its Certificate of Corporation pursuant to shareholder approval, and the Company’s name was changed from Growblox Sciences, Inc. to GB Sciences, Inc.
Effective April 8, 2018, Shareholders of the Company approved the change in corporate domicile from the State of Delaware to the State of Nevada and increase in the number of authorized capital shares from 250,000,000 to 400,000,000. Effective August 15, 2019, Shareholders of the Company approved an increase in authorized capital shares from 400,000,000 to 600,000,000.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Recent Developments
Sale of Membership Interest in GB Sciences Louisiana, LLC
On November 15, 2019, the Company entered into a Membership Interest Purchase Agreement (the “Agreement”) with Wellcana Plus, LLC, a Louisiana limited liability company ("Wellcana"), whereby Wellcana would acquire the Company’s 50.01% membership interest (the “Membership Interest”) in GB Sciences Louisiana LLC, a Louisiana limited liability company. Since entering into the agreement, certain modifications of the Agreement were made. It was ultimately agreed that Wellcana would pay the Company $4,900,000 in cash for the Membership Interest. On December 16, 2020, Wellcana made the final payment totaling $4,900,000 which completed the disposition of the Membership Interest (Note 13).
Convertible Note Payable to Iliad Research and Trading, L.P.
On April 23, 2019, the Company issued an 8% Convertible Promissory Note (the “Note”) in the face amount of $2,765,000 to Iliad Research and Trading, L.P. (“Iliad”). On April 22, 2020, the Company defaulted on its obligation to pay the Note by that date. Based upon the default, Iliad filed a lawsuit against the Company in the Third Judicial District Court of Salt Lake County, State of Utah (the “Court”). On July 14, 2020, the Court issued a judgment in favor of Iliad in the amount of $3,264,594 (the “Judgment”).
On November 20, 2020, the Company, Iliad, and Wellcana entered into the Judgment Settlement Agreement (the Agreement), in which the Company agreed to pay Iliad $3,006,015 on or before December 8, 2020, in full satisfaction of the Judgment. In addition to the Company and Iliad, the Agreement was signed by Wellcana Plus LLC (“Wellcana”). By signing the Agreement, Wellcana agreed to pay $3,006,015 of what it owed the Company, directly to Iliad to satisfy the Company’s obligation to Iliad. Of the $4,150,000 paid by Wellcana, $3,006,015 was sent directly by Wellcana to Iliad in satisfaction of the Company’s obligation pursuant to the Settlement Agreement (Note 6).
Intellectual Property Portfolio
On October 14th, 2020, GB Sciences filed a provisional patent application to protect its machine learning algorithm for the prediction of novel active ingredients from traditional, plant-based medical preparations. The new provisional patent application is entitled “In Silico Meta-Pharmacopeia Assembly from Non-Western Medical Systems Using Advanced Data Analytic Techniques to Identify and Design Phytotherapeutic Strategies”. GBSGB’s proprietary data analytics tool uses in silico convergence analysis to deconvolve modes of action and predict desirable components of plant-based formulations established in traditional medical practice based on computational consensus analysis across cultures and medical systems.
On September 23rd, GB Sciences received a Notice of Allowance from the United States Patent and Trademark Office (USPTO) for claims protecting their Cannabinoid Containing Complex Mixtures (CCCMs) for the Treatment of Mast Cell Activation Syndrome (MCAS). The patent is owned by GBSGB. MCAS is a severe immunological condition in which mast cells inappropriately and excessively release inflammatory mediators, resulting in a range of severe chronic hyperinflammatory symptoms and life-threatening anaphylaxis attacks. There is no single recommended treatment for MCAS patients. Instead, patients, with their doctor’s guidance, attempt to manage MCAS symptoms primarily by avoiding ‘triggers’ and using rescue medicines for their severe hyperinflammatory attacks. Therefore, MCAS patients need new therapeutic options to control their mast cell related symptoms, and the Company’s CCCM™ were designed to simultaneously control multiple inflammatory pathways within mast cells as a comprehensive treatment option. The application, entitled “Cannabinoid-Containing Complex Mixtures for the Treatment of Mast Cell-Associated or Basophil-Mediated Inflammatory Disorders” was originally filed on January 31, 2018 and describes CCCMs that can be used for the treatment of Crohn's disease, Inflammatory Bowel Disease (IBD), Irritable Bowel Syndrome (IBS), rheumatoid arthritis, osteoarthritis, allergic asthma, Chronic Obstructive Pulmonary Disease (COPD), psoriasis, eczema, urticarias, dermatitis, mastocytosis, or anaphylactic sting. Claims for these additional indications will be examined by the USPTO in the future. On December 8, 2020, the patent was issued as United States Patent 10,857,107.
On April 7th, 2020, GB Sciences received a Notice of Allowance from the United States Patent and Trademark Office (USPTO) for claims protecting Cannabinoid Containing Complex Mixtures ("CCCMs") for the Treatment of Parkinson’s disease (PD), which is owned by GBSGB. On May 19, 2020, the patent was issued as United States Patent 10,653,640.
On May 12th, 2020, GB Sciences received a Notice of Allowance from the United States Patent and Trademark Office (USPTO) for claims protecting Myrcene Containing Complex Mixtures ("MCCMs") for the Treatment of Neuropathic Pain. Intellectual property rights to this application and the MCCM contained within it are owned by GBSGB. The Company's MCCMs are protected for use in the treatment of pain related to arthritis, shingles, irritable bowel syndrome, sickle cell disease, and endometriosis. The patent was issued on July 14, 2020, as United States Patent 10,709,670.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Planned Divestiture of Nevada Cannabis Operations
On November 15, 2019, we entered into a Binding Letter of Intent (the "LOI") to sell the Company's membership interest interests in GBSN and GBLV (together, the "Teco Subsidiaries"). In connection with the LOI, we entered into a Management Agreement with the purchaser whereby the facilities will be managed by an affiliate of the purchaser until the close of the sale. On March 24, 2020, we entered into the Membership Interest Purchase Agreement ("Teco MIPA") which formalized the sale of the Teco Subsidiaries and modified the terms of the sale. Pursuant to the Teco MIPA, the Company will sell 100% of its membership interests in GBSN and GBLV for $4,000,000 cash upon close and $4,000,000 in the form of an 8% promissory note (Note 14).
On November 27, 2019, we entered into a Binding Letter of Intent to sell the Company's 100% interest in GB Sciences Nopah, LLC. On August 10, 2020, the Company entered into the Membership Interest Purchase Agreement ("Nopah MIPA") and Promissory Note Modification Agreement with the purchaser of GB Sciences Nopah, LLC. The Company will receive $300,000 upon closing, and the purchaser will pay all expenses related to the upkeep and maintenance of the Nopah License from the date of the agreement. The $300,000 purchase price will be paid as a reduction to the balance of the 0% Note payable dated October 23, 2017, which is held by an affiliate of the purchaser of the Nopah license (Note 14).
The sales of the Teco and Nopah Subsidiaries are expected to close upon the successful transfer of the Nevada cannabis cultivation and production licenses held by those subsidiaries. The transfer of cannabis licenses in the State of Nevada was subject to an indefinite moratorium beginning in October 2019. In a meeting held on July 21, 2020, the Nevada Cannabis Compliance Board lifted the moratorium, however, the board has indicated that there were initially 90 requests pending, and it will likely take several months to process the entire backlog of pending license transfers. Based on this information, we cannot provide any assurances as to the timing of the close of the sale. In addition, the lifting of the moratorium and the processing of cannabis license transfers have been delayed by the COVID-19 pandemic and could be further delayed if the pandemic continues.
Note 2 - Going Concern
The Company’s consolidated financial statements have been prepared assuming the Company will continue as a going concern. The Company has sustained net losses since inception, which have caused an accumulated deficit of $103,886,232 at March 31, 2021. The Company had a working capital deficit of $5,054,593, net of working capital of $439,979 from discontinued operations as of March 31, 2021, compared to a working capital deficit of $3,884,877, including a working capital deficit of $243,787 from discontinued operations at March 31, 2020. In addition, the Company has consumed cash in its operating activities of $2,185,220 including $118,644 used in discontinued operations for the year ended March 31, 2021, compared to $4,479,713 including $2,215,434 used in discontinued operations for the year ended March 31, 2020. These factors, among others, raise substantial doubt about the Company’s ability to continue as a going concern.
Management has been able, thus far, to finance the losses through a public offering, private placements of debt and equity, and obtaining operating funds from stockholders. The Company is continuing to seek sources of financing. There are no assurances that the Company will be successful in achieving its goals.
Furthermore, Management believes the COVID-19 pandemic may have a significant impact on the Company's business. The pandemic presents a risk to the global economy, and it is possible that it could have an impact on the operations of the Company in the near term that could materially impact the Company’s financials and ability to continue as a going concern. Management has not been able to measure the potential financial impact on the Company and continues to monitor the impact of the pandemic closely, although the extent to which the COVID-19 outbreak will impact our operations, financing ability or future financial results is uncertain.
In view of these conditions, the Company’s ability to continue as a going concern is dependent upon its ability to obtain additional financing or capital sources, to meet its financing requirements, and ultimately to achieve profitable operations. Management believes that its current and future plans provide an opportunity to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classification of liabilities that may be necessary in the event the Company is unable to continue as a going concern.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Note 3 - Basis of Presentation and Summary of Significant Accounting Policies
Principles of Consolidation
We prepare our consolidated financial statements in accordance with generally accepted accounting principles (GAAP) for the United States of America. Our consolidated financial statements include all operating divisions and majority-owned subsidiaries, reported as a single operating segment, for which we maintain controlling interests.
The subsidiaries of the Company are:
Continuing Operations:
GBS Global Biopharma, Inc.
ECRX, Inc.
The PhAROS Institute, LLC
GB Sciences Texas, LLC
Discontinued Operations:
GB Sciences Nevada, LLC
GB Sciences Las Vegas, LLC
GB Sciences Nopah, LLC
Intercompany accounts and transactions have been eliminated in consolidation. The ownership interest of non-controlling participants in subsidiaries that are not wholly owned is included as a separate component of equity. The non-controlling participants’ share of the net loss is included as “Net loss attributable to non-controlling interest” on the consolidated statements of operations.
Use of Estimates
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. The Company regularly evaluates estimates and assumptions related to allowances for doubtful accounts, inventory valuation and standard cost allocations, valuation of initial right-of-use assets and corresponding lease liabilities, valuation of beneficial conversion features in convertible debt, valuation of the assets and liabilities of discontinued operations, stock-based compensation expense, purchased intangible asset valuations, deferred income tax asset valuation allowances, uncertain tax positions, litigation, other loss contingencies, and impairment of long lived assets. These estimates and assumptions are based on current facts, historical experience and various other factors that the Company believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the recording of costs and expenses that are not readily apparent from other sources. The actual results the Company experiences may differ materially and adversely from these estimates.
Reclassifications
Certain reclassifications have been made to the comparative period amounts in order to conform to the current period presentation. In particular, the assets, liabilities, income, and cash flows of GB Sciences Nevada LLC, GB Sciences Las Vegas, LLC, and GB Sciences Nopah, LLC, have been separated from the comparative period amounts to conform to the current period presentation as discontinued operations as the result of the pending sale of the Company's Nevada operations. The reclassifications had no effect on the reported financial position, results of operations or cash flows of the Company.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Discontinued Operations
See Note 4.
Fair Value of Financial Instruments
The Company adopted ASC 820, Fair Value Measurements and Disclosures (ASC 820). ASC 820 defines fair value, establishes a three-level valuation hierarchy for disclosures of fair value measurement and enhances disclosure requirements for fair value measures. The three levels are defined as follows:
|
|
-
|
Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
|
|
|
-
|
Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument.
|
|
|
-
|
Level 3 inputs to valuation methodology are unobservable and significant to the fair measurement.
|
The carrying value of cash, accounts receivable, accounts payable and accrued expenses are estimated by management to approximate fair value, primarily due to the short-term nature of the instruments.
Cash and Cash Equivalents
The Company considers all short-term investments with an original maturity of three months or less when purchased to be cash equivalents. The Company had no short-term investments classified as cash equivalents at March 31, 2021 and 2020.
Accounts Receivable
Accounts receivable are carried at their estimated collectible amounts. Trade accounts receivable are periodically evaluated for collectability based on aging and subsequent collections.
Inventory
We value our inventory at the lower of the actual cost of our inventory, as determined using the first-in, first-out method, or its current estimated market value. We periodically review our physical inventory for excess, obsolete, and potentially impaired items and reserve accordingly. Our reserve estimate for excess and obsolete inventory is based on expected future use. Indirect costs, which primarily relate to the lease and operation costs of the Teco Facility, are allocated based on square footage of the facility used in the production of inventory.
Indefinite-Lived Intangible Assets
Our indefinite-lived intangible assets primarily represent the value of our patents pending and includes the costs paid to draft and file patent applications. Upon issuance of the patents, the indefinite-lived intangible assets will have finite lives. Intangible assets also include the acquisition cost of a cannabis production license with an indefinite life. We amortize our finite-lived intangible assets over their estimated useful lives using the straight-line method, and we periodically evaluate the remaining useful lives of our finite-lived intangible assets to determine whether events or circumstances warrant a revision to the remaining period of amortization. During the year ended March 31, 2020, the Company entered into the Membership Interest Purchase Agreement ("Teco MIPA") to sell 100% of the membership interests in the Teco Facility. As a result of this agreement, the Company determined that the long-lived assets of the Teco Facility including a production license acquired through purchase might be impaired due to the current expectation that the asset group will more likely than not be disposed of by sale significantly before the end of its previously estimated useful life. The Company recorded an impairment loss of $449,801 related to the license for the year ended March 31, 2020, and reduced the carrying value of the related intangible asset from $1,021,067 to $571,264. The license asset and the impairment loss are included in discontinued operations in the accompanying financial statements.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Operating Lease Right-of-Use Asset and Liability
The Company determines if an arrangement is a lease at inception and has lease agreements for office facilities, equipment, and other space and assets with non-cancelable lease terms. Certain real estate and property leases, and various other operating leases are measured on the balance sheet with a lease liability and right-of-use asset ("ROU").
ROU assets represent the Company's right to use an underlying asset for the lease term, and lease liabilities represent the obligation to make scheduled lease payments. ROU assets and liabilities are recognized on the lease commencement date based on the present value of lease payments over the lease term. The present value of lease payments is calculated using the incremental borrowing rate at lease commencement, which takes into consideration recent debt issuances as well as other applicable market data available.
Lease payments include fixed payments, variable payments based on an index or rate, reasonably certain purchase options, termination penalties, and others as required by the New Lease Standard. Lease payments do not include variable lease payments other than those that depend on an index or rate, any guarantee by the lessee of the lessor’s debt, or any amount allocated to non-lease components.
Lease terms include options to extend when it is reasonably certain that the option will be exercised. Leases with a term of twelve months or less are not recorded on the balance sheet. Additionally, lease and non-lease components are accounted for as a single lease component for real estate agreements.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets: 3-8 years for machinery and equipment, and leasehold improvements are amortized over the shorter of the estimated useful lives or the underlying lease term. Property under finance leases and related obligations are initially recorded at an amount equal to the present value of future minimum lease payments computed on the basis of the Company’s incremental borrowing rate, and depreciation is recorded on a straight-line basis and is included within depreciation and amortization expense. Repairs and maintenance expenditures which do not extend the useful lives of related assets are expensed as incurred.
Long-Lived Assets
Property and equipment comprise a significant portion of our total assets from discontinued operations. We evaluate the carrying value of property and equipment if impairment indicators are present or if other circumstances indicate that impairment may exist under authoritative guidance. The annual testing date is March 31. When management believes impairment indicators may exist, projections of the undiscounted future cash flows associated with the use of and eventual disposition of property and equipment are prepared. If the projections indicate that the carrying value of the property and equipment are not recoverable, we reduce the carrying values to fair value. These impairment tests are heavily influenced by assumptions and estimates that are subject to change as additional information becomes available.
During the year ended March 31, 2020, the Company entered into the Membership Interest Purchase Agreement ("Teco MIPA") to sell 100% of the membership interests in the Teco Facility (Note 14). As a result of this agreement, the Company determined that the long-lived assets of the Teco Facility might be impaired due to the current expectation that the asset group will more likely than not be disposed of by sale significantly before the end of its previously estimated useful life. The Company estimated future undiscounted cash flows related to the Teco Facility to be $8.0 million, which was less than the carrying amount of the Teco Facility asset group of $11.9 million. Using a discounted cash flow approach, the Company estimated the fair value of the asset group to be approximately $7.3 million, resulting in a write-down of $4,645,054 related to the Teco Facility asset group. Fair value was based on expected future cash flows using level 3 inputs under ASC 820. The cash flows are the proceeds expected to be generated from the sale of the assets under the Teco MIPA, discounted to present value at a rate of 17%. The cash flow projection includes the $4.0 million in cash flows that the Company anticipates receiving from the Note Receivable that it will receive from the sale of the Teco facility and the $4.0M payment that will be received at the close of the sale. The impairment loss and the related long-lived assets are included in discontinued operations in the accompanying financial statements.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Beneficial Conversion Feature of Convertible Notes Payable
The Company accounts for convertible notes payable in accordance with the guidelines established by the Financial Accounting Standards Board’s (“FASB”) Accounting Standards Codification (“ASC”) Topic 470-20, Debt with Conversion and Other Options and Emerging Issues Task Force (“EITF”) 00-27, “Application of Issue No. 98-5 to Certain Convertible Instruments”. A beneficial conversion feature (“BCF”) exists on the date a convertible note is issued when the fair value of the underlying common stock to which the note is convertible into is in excess of the remaining unallocated proceeds of the note after first considering the allocation of a portion of the note proceeds to the fair value of any attached equity instruments, if any related equity instruments were granted with the debt. In accordance with this guidance, the BCF of a convertible note is measured by allocating a portion of the note's proceeds to the warrants, if applicable, and as a reduction of the carrying amount of the convertible note equal to the intrinsic value of the conversion feature, both of which are credited to additional paid-in-capital. The Company calculates the fair value of warrants issued with the convertible notes using the Black-Scholes valuation model and uses the same assumptions for valuing any employee options in accordance with ASC Topic 718 Compensation – Stock Compensation. The only difference is that the contractual life of the warrants is used.
The value of the proceeds received from a convertible note is then allocated between the conversion features and warrants on a relative fair value basis. The allocated fair value is recorded in the financial statements as a debt discount (premium) from the face amount of the note and such discount is amortized over the expected term of the convertible note (or to the conversion date of the note, if sooner) and is charged to interest expense.
Revenue Recognition
The FASB issued Accounting Standards Codification (“ASC”) 606 as guidance on the recognition of revenue from contracts with customers. Revenue recognition depicts the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The guidance also requires disclosures regarding the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. The guidance permits two methods of adoption: retrospectively to each prior reporting period presented, or retrospectively with the cumulative effect of initially applying the guidance recognized at the date of initial application (the cumulative catch-up transition method). The Company adopted the guidance on April 1, 2018 and applied the cumulative catch-up transition method.
The Company’s only material revenue source is part of discontinued operations and derives from sales of cannabis and cannabis products, distinct physical goods. Under ASC 606, the Company is required to separately identify each performance obligation resulting from its contracts from customers, which may be a good or a service. A contract may contain one or more performance obligations. All of the Company’s contracts with customers, past and present, contain only a single performance obligation, the delivery of distinct physical goods. Because fulfillment of the company’s performance obligation to the customer under ASC 606 results in the same timing of revenue recognition as under the previous guidance (i.e. revenue is recognized upon delivery of physical goods), the Company did not record any material adjustment to report the cumulative effect of initial application of the guidance.
Research and Development Costs
Research and development costs are expensed as incurred. During the years ended March 31, 2021 and 2020, the Company recorded $352,274 and $1,543,397, respectively, in research and development expense, which is included in general and administrative expense in the Company's consolidated financial statements.
Equity-Based Compensation
The Company accounts for equity instruments issued to employees and non-employees in accordance with the provisions of ASC 718 Stock Compensation (ASC 718). The computation of the expense associated with stock-based compensation requires the use of a valuation model. The FASB-issued accounting guidance requires significant judgment and the use of estimates, particularly surrounding Black-Scholes assumptions such as stock price volatility, expected option lives, and expected option forfeiture rates, to value equity-based compensation. We currently use a Black-Scholes option pricing model to calculate the fair value of our stock options. We primarily use historical data to determine the assumptions to be used in the Black-Scholes model and have no reason to believe that future data is likely to differ materially from historical data. However, changes in the assumptions to reflect future stock price volatility and future stock award exercise experience could result in a change in the assumptions used to value awards in the future and may result in a material change to the fair value calculation of stock-based awards. This accounting guidance requires the recognition of the fair value of stock compensation in net income. Although every effort is made to ensure the accuracy of our estimates and assumptions, significant unanticipated changes in those estimates, interpretations and assumptions may result in recording stock option expense that may materially impact our financial statements for each respective reporting period.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Income Taxes
The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been included in financial statements or tax returns. Deferred tax items are reflected at the enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Due to the uncertainty regarding the success of future operations, management has valued the deferred tax asset allowance at 100% of the related deferred tax assets.
Because the Company operates in the State-licensed cannabis industry, it is subject to the limitations of Internal Revenue Code Section 280E (“280E”) for U.S. income tax purposes. Under 280E, the Company is allowed to deduct expenses that are directly related to the production of its products, i.e. cost of goods sold, but is allowed no further deductions for ordinary and necessary business expenses from its gross profit. The Company believes that the deductions disallowed include the deduction of NOLs. The unused NOLs will continue to carry forward and may be used by the Company to offset future taxable income that is not subject to the limitations of 280E.
Loss per Share
The Company’s basic loss per share has been calculated using the weighted average number of common shares outstanding during the period. The Company had 164,049,941 and 158,404,020 potentially dilutive common shares at March 31, 2021 and 2020, respectively. However, such common stock equivalents were not included in the computation of diluted net loss per share as their inclusion would have been anti-dilutive.
Recent Accounting Pronouncements
Recently Adopted Standards
In August 2018, the Financial Accounting Standards Board ("FASB") issued ASU No. 2018-13, Disclosure Framework-Changes to the Disclosure Requirements for Fair Value Measurement. This ASU eliminates, adds and modifies certain disclosure requirements for fair value measurements as part of its disclosure framework project. The standard is effective for all entities for financial statements issued for fiscal years beginning after December 15, 2019, and interim periods within those fiscal years. The Company adopted the standard on April 1, 2020, and it did not have a material impact on the Company’s financial statements.
Standards Not Yet Adopted
In May 2021, the FASB issued ASU No. 2021-04, Issuer's Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options. This guidance clarifies and reduces diversity in an issuer’s accounting for modifications or exchanges of freestanding equity-classified written call options due to a lack of explicit guidance in the FASB Codification. The ASU 2021-04 is effective for The Company's fiscal year beginning April 1, 2022. Early adoption is permitted. The Company is currently evaluating the impact of adopting ASU 2021-04 on its consolidated financial statements.
On June 16, 2016, the FASB issued ASU No. 2016-13, Measurement of Credit Losses on Financial Instruments. The standard requires the use of an “expected loss” model on certain types of financial instruments. The standard also amends the impairment model for available-for-sale debt securities and requires estimated credit losses to be recorded as allowances instead of reductions to amortized cost of the securities. The amendments in this ASU are effective for the Company's fiscal year beginning April 1, 2023. The Company is currently evaluating the impact of ASU 2016-13 on its financial statements.
In June 2020, the FASB issued ASU No. 2020-06, Accounting for Convertible Instruments and Contracts in an Entity's Own Equity. The guidance simplifies the current guidance for convertible instruments and the derivatives scope exception for contracts in an entity’s own equity. Additionally, the amendments affect the diluted EPS calculation for instruments that may be settled in cash or shares and for convertible instruments. This ASU will be effective for the Company's fiscal year beginning April 1, 2024. Early adoption is permitted. The amendments in this update must be applied on either full retrospective basis or modified retrospective basis through a cumulative-effect adjustment to retained earnings/(deficit) in the period of adoption. The Company is currently evaluating the impact of ASU 2020-06 on its consolidated financial statements and related disclosures, as well as the timing of adoption.
All other newly issued accounting pronouncements have been deemed either immaterial or not applicable.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Note 4 - Discontinued Operations
Discontinued Operations
Discontinued operations comprise those activities that were disposed of during the period, or which were classified as held for sale at the end of the period and represent a separate major line of business or geographical area that can be clearly distinguished for operational and financial reporting purposes. The Company has included its subsidiaries GB Sciences Louisiana, LLC, GB Sciences Nevada, LLC, GB Sciences Las Vegas, LLC, and GB Sciences Nopah, LLC in discontinued operations due to the sale of the Company's Louisiana cultivation and extraction facility (Note 13) and the pending sale of the Company's Nevada cultivation and extraction facilities (Note 14).
There were no assets and liabilities from discontinued operations attributable to GB Sciences Louisiana, LLC at March 31, 2021 and 2020. The assets and liabilities associated with discontinued operations included in our consolidated balance sheets as of March 31, 2021 and 2020 were as follows:
|
|
|
March 31, 2021
|
|
|
March 31, 2020
|
|
|
|
|
Continuing
|
|
|
Discontinued
Nevada
Subsidiaries
|
|
|
Total
|
|
|
Continuing
|
|
|
Discontinued
Nevada
Subsidiaries
|
|
|
Total
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
793,040
|
|
|
$
|
352,593
|
|
|
$
|
1,145,633
|
|
|
$
|
2,406
|
|
|
$
|
149,360
|
|
|
$
|
151,766
|
|
|
Accounts receivable, net
|
|
|
-
|
|
|
|
400,175
|
|
|
|
400,175
|
|
|
|
-
|
|
|
|
117,967
|
|
|
|
117,967
|
|
|
Inventory, net
|
|
|
-
|
|
|
|
1,689,304
|
|
|
|
1,689,304
|
|
|
|
-
|
|
|
|
1,445,839
|
|
|
|
1,445,839
|
|
|
Prepaid and other current assets
|
|
|
256,251
|
|
|
|
52,492
|
|
|
|
308,743
|
|
|
|
18,776
|
|
|
|
42,109
|
|
|
|
60,885
|
|
|
Note receivable
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
5,224,423
|
|
|
|
-
|
|
|
|
5,224,423
|
|
|
TOTAL CURRENT ASSETS
|
|
|
1,049,291
|
|
|
|
2,494,564
|
|
|
|
3,543,855
|
|
|
|
5,245,605
|
|
|
|
1,755,275
|
|
|
|
7,000,880
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment, net
|
|
|
25,022
|
|
|
|
4,876,247
|
|
|
|
4,901,269
|
|
|
|
37,821
|
|
|
|
5,496,012
|
|
|
|
5,533,833
|
|
|
Intangible assets, net
|
|
|
1,706,762
|
|
|
|
571,264
|
|
|
|
2,278,026
|
|
|
|
1,128,702
|
|
|
|
571,264
|
|
|
|
1,699,966
|
|
|
Deposits and other noncurrent assets
|
|
|
-
|
|
|
|
82,904
|
|
|
|
82,904
|
|
|
|
-
|
|
|
|
91,504
|
|
|
|
91,504
|
|
|
Operating lease right-of-use assets, net
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
26,685
|
|
|
|
26,685
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL ASSETS
|
|
$
|
2,781,075
|
|
|
$
|
8,024,979
|
|
|
$
|
10,806,054
|
|
|
$
|
6,412,128
|
|
|
$
|
7,940,740
|
|
|
$
|
14,352,868
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
1,412,459
|
|
|
$
|
509,477
|
|
|
$
|
1,921,936
|
|
|
$
|
1,913,049
|
|
|
$
|
646,865
|
|
|
$
|
2,559,914
|
|
|
Accrued interest
|
|
|
493,741
|
|
|
|
49,211
|
|
|
|
542,952
|
|
|
|
366,865
|
|
|
|
30,787
|
|
|
|
397,652
|
|
|
Accrued expenses
|
|
|
957,946
|
|
|
|
105,421
|
|
|
|
1,063,367
|
|
|
|
813,618
|
|
|
|
74,394
|
|
|
|
888,012
|
|
|
Notes and convertible notes payable, net
|
|
|
3,594,804
|
|
|
|
485,000
|
|
|
|
4,079,804
|
|
|
|
5,054,728
|
|
|
|
480,000
|
|
|
|
5,534,728
|
|
|
Indebtedness to related parties
|
|
|
84,913
|
|
|
|
-
|
|
|
|
84,913
|
|
|
|
586,512
|
|
|
|
-
|
|
|
|
586,512
|
|
|
Note payable to related party
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
151,923
|
|
|
|
-
|
|
|
|
151,923
|
|
|
Income tax payable
|
|
|
-
|
|
|
|
761,509
|
|
|
|
761,509
|
|
|
|
-
|
|
|
|
592,982
|
|
|
|
592,982
|
|
|
Finance lease obligations, current
|
|
|
-
|
|
|
|
143,967
|
|
|
|
143,967
|
|
|
|
-
|
|
|
|
166,769
|
|
|
|
166,769
|
|
|
Operating lease obligations, current
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
7,265
|
|
|
|
7,265
|
|
|
TOTAL CURRENT LIABILITIES
|
|
|
6,543,863
|
|
|
|
2,054,585
|
|
|
|
8,598,448
|
|
|
|
8,886,695
|
|
|
|
1,999,062
|
|
|
|
10,885,757
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible notes payable, net
|
|
|
292,410
|
|
|
|
-
|
|
|
|
292,410
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Operating lease obligations, long term
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
22,515
|
|
|
|
22,515
|
|
|
Finance lease obligations, long term
|
|
|
-
|
|
|
|
3,389,124
|
|
|
|
3,389,124
|
|
|
|
-
|
|
|
|
3,533,090
|
|
|
|
3,533,090
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES
|
|
$
|
6,836,273
|
|
|
$
|
5,443,709
|
|
|
$
|
12,279,982
|
|
|
$
|
8,886,695
|
|
|
$
|
5,554,667
|
|
|
$
|
14,441,362
|
|
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
The revenues and expenses associated with discontinued operations included in our consolidated statements of operations for the years ended March 31, 2021 and 2020, were as follows:
|
|
|
For the Year Ended March 31,
|
|
|
For the Year Ended March 31,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
|
|
Continuing
|
|
|
Discontinued
|
|
|
Total
|
|
|
Continuing
|
|
|
Discontinued
|
|
|
Total
|
|
|
Sales revenue
|
|
$
|
-
|
|
|
$
|
4,110,456
|
|
|
$
|
4,110,456
|
|
|
$
|
-
|
|
|
$
|
3,689,697
|
|
|
$
|
3,689,697
|
|
|
Cost of goods sold
|
|
|
-
|
|
|
|
(3,506,722
|
)
|
|
|
(3,506,722
|
)
|
|
|
-
|
|
|
|
(4,576,627
|
)
|
|
|
(4,576,627
|
)
|
|
Gross profit (loss)
|
|
|
-
|
|
|
|
603,734
|
|
|
|
603,734
|
|
|
|
-
|
|
|
|
(886,930
|
)
|
|
|
(886,930
|
)
|
|
General and administrative expenses
|
|
|
2,001,617
|
|
|
|
276,986
|
|
|
|
2,278,603
|
|
|
|
5,741,514
|
|
|
|
2,034,612
|
|
|
|
7,776,126
|
|
|
Loss on impairment of long-lived assets
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
4,645,054
|
|
|
|
4,645,054
|
|
|
LOSS FROM OPERATIONS
|
|
|
(2,001,617
|
)
|
|
|
326,748
|
|
|
|
(1,674,869
|
)
|
|
|
(5,741,514
|
)
|
|
|
(7,566,596
|
)
|
|
|
(13,308,110
|
)
|
|
OTHER INCOME/(EXPENSE)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gain/(loss) on extinguishment
|
|
|
467,872
|
|
|
|
-
|
|
|
|
467,872
|
|
|
|
(216,954
|
)
|
|
|
-
|
|
|
|
(216,954
|
)
|
|
Gain on settlement of accounts payable
|
|
|
422,414
|
|
|
|
54,958
|
|
|
|
477,372
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Gain on deconsolidation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
4,393,242
|
|
|
|
-
|
|
|
|
4,393,242
|
|
|
Interest expense
|
|
|
(1,285,460
|
)
|
|
|
(486,481
|
)
|
|
|
(1,771,941
|
)
|
|
|
(1,109,031
|
)
|
|
|
(694,313
|
)
|
|
|
(1,803,344
|
)
|
|
Loss on modification of line of credit
|
|
|
(650,000
|
)
|
|
|
-
|
|
|
|
(650,000
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Loss on modification of note receivable
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,895,434
|
)
|
|
|
-
|
|
|
|
(1,895,434
|
)
|
|
Debt default penalty
|
|
|
(286,059
|
)
|
|
|
-
|
|
|
|
(286,059
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Other expense
|
|
|
-
|
|
|
|
(118,875
|
)
|
|
|
(118,875
|
)
|
|
|
(179,368
|
)
|
|
|
(14,880
|
)
|
|
|
(194,248
|
)
|
|
TOTAL OTHER INCOME/(EXPENSE)
|
|
|
(1,331,233
|
)
|
|
|
(550,398
|
)
|
|
|
(1,881,631
|
)
|
|
|
992,455
|
|
|
|
(709,193
|
)
|
|
|
283,262
|
|
|
NET LOSS BEFORE INCOME TAXES
|
|
|
(3,332,850
|
)
|
|
|
(223,650
|
)
|
|
|
(3,556,500
|
)
|
|
|
(4,749,059
|
)
|
|
|
(8,275,789
|
)
|
|
|
(13,024,848
|
)
|
|
Income tax expense
|
|
|
-
|
|
|
|
(168,527
|
)
|
|
|
(168,527
|
)
|
|
|
-
|
|
|
|
(86,837
|
)
|
|
|
(86,837
|
)
|
|
NET LOSS
|
|
$
|
(3,332,850
|
)
|
|
$
|
(392,177
|
)
|
|
$
|
(3,725,027
|
)
|
|
$
|
(4,749,059
|
)
|
|
$
|
(8,362,626
|
)
|
|
$
|
(13,111,685
|
)
|
The components of revenues and expenses associated with discontinued operations included in our consolidated statements of operations for the years ended March 31, 2021 and 2020 were as follows:
|
|
|
For the Year Ended March 31,
|
|
|
For the Year Ended March 31,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
|
|
Nevada
|
|
|
Louisiana
|
|
|
Total
|
|
|
Nevada
|
|
|
Louisiana
|
|
|
Total
|
|
|
Sales revenue
|
|
$
|
4,110,456
|
|
|
$
|
-
|
|
|
$
|
4,110,456
|
|
|
$
|
3,120,620
|
|
|
$
|
569,077
|
|
|
$
|
3,689,697
|
|
|
Cost of goods sold
|
|
|
(3,506,722
|
)
|
|
|
-
|
|
|
|
(3,506,722
|
)
|
|
|
(4,002,083
|
)
|
|
|
(574,544
|
)
|
|
|
(4,576,627
|
)
|
|
Gross profit (loss)
|
|
|
603,734
|
|
|
|
-
|
|
|
|
603,734
|
|
|
|
(881,463
|
)
|
|
|
(5,467
|
)
|
|
|
(886,930
|
)
|
|
General and administrative expenses
|
|
|
276,986
|
|
|
|
-
|
|
|
|
276,986
|
|
|
|
741,999
|
|
|
|
1,292,613
|
|
|
|
2,034,612
|
|
|
Loss on impairment of long-lived assets
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
4,645,054
|
|
|
|
-
|
|
|
|
4,645,054
|
|
|
LOSS FROM OPERATIONS
|
|
|
326,748
|
|
|
|
-
|
|
|
|
326,748
|
|
|
|
(6,268,516
|
)
|
|
|
(1,298,080
|
)
|
|
|
(7,566,596
|
)
|
|
OTHER INCOME/(EXPENSE)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gain on settlement of accounts payable
|
|
|
54,958
|
|
|
|
-
|
|
|
|
54,958
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Interest expense
|
|
|
(486,481
|
)
|
|
|
-
|
|
|
|
(486,481
|
)
|
|
|
(516,173
|
)
|
|
|
(178,140
|
)
|
|
|
(694,313
|
)
|
|
Other expense
|
|
|
(118,875
|
)
|
|
|
-
|
|
|
|
(118,875
|
)
|
|
|
(14,880
|
)
|
|
|
-
|
|
|
|
(14,880
|
)
|
|
TOTAL OTHER INCOME/(EXPENSE)
|
|
|
(550,398
|
)
|
|
|
-
|
|
|
|
(550,398
|
)
|
|
|
(531,053
|
)
|
|
|
(178,140
|
)
|
|
|
(709,193
|
)
|
|
NET LOSS BEFORE INCOME TAXES
|
|
|
(223,650
|
)
|
|
|
-
|
|
|
|
(223,650
|
)
|
|
|
(6,799,569
|
)
|
|
|
(1,476,220
|
)
|
|
|
(8,275,789
|
)
|
|
Income tax expense
|
|
|
(168,527
|
)
|
|
|
-
|
|
|
|
(168,527
|
)
|
|
|
(86,837
|
)
|
|
|
-
|
|
|
|
(86,837
|
)
|
|
NET LOSS
|
|
$
|
(392,177
|
)
|
|
$
|
-
|
|
|
$
|
(392,177
|
)
|
|
$
|
(6,886,406
|
)
|
|
$
|
(1,476,220
|
)
|
|
$
|
(8,362,626
|
)
|
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Accounts Receivable
Accounts receivable are carried at their estimated collectible amounts. Trade accounts receivable are periodically evaluated for collectability based on aging and subsequent collections. During the year ended March 31, 2021, the Company recorded $24,768 in bad debt recoveries as the result of a $94,912 decrease in the allowance for doubtful accounts and $70,144 of accounts written off as uncollectible. Accounts receivable are included in current assets from discontinued operations in the Company's consolidated balance sheets at March 31, 2021 and 2020.
Inventory
We value our inventory at the lower of the actual cost of our inventory, as determined using the first-in, first-out method, or its current estimated market value. We periodically review our physical inventory for excess, obsolete, and potentially impaired items and reserve accordingly. Our reserve estimate for excess and obsolete inventory is based on expected future use. Indirect costs, which primarily relate to the lease and operation costs of the Teco Facility, are allocated based on square footage of the facility used in the production of inventory.
Raw materials consist of supplies, materials, and consumables used in the cultivation and extraction processes. Work-in-progress includes live plants and cannabis in the drying, curing, and trimming processes. Finished goods includes completed cannabis flower, trim, and extracts in bulk and packaged forms. Inventory is included in current assets from discontinued operations in the Company's consolidated balance sheets at March 31, 2021 and 2020.
|
|
|
March 31,
2021
|
|
|
March 31,
2020
|
|
|
|
|
|
|
|
|
|
|
|
|
Raw materials
|
|
$
|
86,076
|
|
|
$
|
91,465
|
|
|
Work in progress
|
|
|
743,844
|
|
|
|
1,166,511
|
|
|
Finished goods
|
|
|
866,195
|
|
|
|
466,319
|
|
|
Subtotal
|
|
|
1,696,115
|
|
|
|
1,724,295
|
|
|
Allowance to reduce inventory to NRV
|
|
|
(6,811
|
)
|
|
|
(278,456
|
)
|
|
Total inventory, net
|
|
$
|
1,689,304
|
|
|
$
|
1,445,839
|
|
Deposits and Noncurrent Assets
Deposits and noncurrent assets from discontinued operations were $82,904 and $91,504 at March 31, 2021 and 2020, respectively. The decrease in deposits and prepayments is due to refunds of security deposits. Deposits and noncurrent assets are included in long term assets from discontinued operations in the Company's consolidated balance sheets at March 31, 2021 and 2020.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Leases
In February 2016, the Financial Accounting Standards Board ("FASB") issued ASU 2016-02, Leases (Topic 842), (the "New Lease Standard"). This standard requires leases, other than short-term, to be recognized on the balance sheet as a lease liability and a corresponding right-of-use asset.
Lease payments include fixed payments, variable payments based on an index or rate, reasonably certain purchase options, termination penalties, and others as required by the standard. Lease payments do not include variable lease payments other than those that depend on an index or rate, any guarantee by the lessee of the lessor’s debt, or any amount allocated to non-lease components. The Company adopted the standard as of April 1, 2019. The Company also elected the package of practical expedients, which among other things, does not require reassessment of lease classification.
The Company adopted the New Lease Standard using the modified retrospective transition approach as of the effective date as permitted by the amendments in ASU 2018-11, "Targeted Improvements - Leases (Topic 842)." Under this method, the cumulative effect adjustment to the opening balance of retained earnings is recognized at the adoption date. As a result, the Company was not required to adjust its comparative period financial information for effects of the standard or make the new required lease disclosures for periods before the date of adoption on April 1, 2019.
The Company determines if an arrangement is a lease at inception and has had lease agreements for warehouses, office facilities, and equipment.
As a result of the adoption of ASC 842 on April 1, 2019, certain real estate and equipment operating leases were recorded on the balance sheet with a lease liability and right-of-use asset ("ROU"). Application of this standard resulted in the recognition of ROU assets of $182,624, net of accumulated amortization, and a corresponding lease liability of $190,173 at the date of adoption. Accounting for finance leases is substantially unchanged.
All of the Company's lease commitments previously recorded as operating leases have terminated as of March 31, 2021. The Company's only remaining lease commitment as of March 31, 2021, is a finance lease for the Teco Facility, which is classified as discontinued operations in the Company's financial statements for the years ended March 31, 2021 and 2020. This lease has a remaining non-cancelable term that ends December 31, 2025 with an option to extend through December 31, 2030.
Operating leases are included in discontinued operations as operating lease right-of-use assets, operating lease obligations, current, and operating lease obligations, long term on the Company's balance sheets. Finance leases are included in property and equipment, finance lease obligations, current, and finance lease obligations, long term, on the Company's balance sheets. ROU assets represent the Company's right to use an underlying asset for the lease term, and lease liabilities represent the obligation to make scheduled lease payments. ROU assets and liabilities are recognized on the lease commencement date based on the present value of lease payments over the lease term. The present value of lease payments is calculated using the incremental borrowing rate at lease commencement, which takes into consideration recent debt issuances as well as other applicable market data available. The rates used to discount finance leases previously recorded as capital leases range from 10.2% to 11.5%. Operating leases were discounted at a rate of 17.0%.
Lease terms include options to extend when it is reasonably certain that the option will be exercised. Leases with a term of 12 months or less are not recorded on the consolidated balance sheet.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
The lease costs recorded in the Company's financial statements for the years ended March 31, 2021 and 2020 are set forth in the table below:
|
|
|
|
March 31,
|
|
|
|
Classification on the Statements of Operations
|
|
2021
|
|
|
2020
|
|
|
Discontinued operations:
|
|
|
|
|
|
|
|
|
|
|
Finance leases - amortization of ROU assets
|
Loss from discontinued operations
|
|
$
|
154,699
|
|
|
$
|
252,973
|
|
|
Finance leases - interest on lease liabilities
|
Loss from discontinued operations
|
|
|
414,993
|
|
|
|
426,374
|
|
|
Operating leases
|
Loss from discontinued operations
|
|
|
3,243
|
|
|
|
13,648
|
|
|
Total lease cost, discontinued operations
|
|
|
572,935
|
|
|
|
692,995
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating leases, continuing operations
|
General and administrative expense
|
|
|
-
|
|
|
|
61,658
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total lease cost
|
|
$
|
572,935
|
|
|
$
|
754,653
|
|
The future minimum lease payments of lease liabilities, including the costs of the lease extension, classified as discontinued operations at March 31, 2021, are as follows:
|
Year Ending
|
|
|
|
|
|
March 31,
|
|
Finance Leases
|
|
|
|
|
|
|
|
|
2022
|
|
$
|
544,296
|
|
|
2023
|
|
|
560,625
|
|
|
2024
|
|
|
577,444
|
|
|
2025
|
|
|
594,767
|
|
|
2026
|
|
|
612,610
|
|
|
Thereafter
|
|
|
3,168,492
|
|
|
Total minimum lease payments
|
|
|
6,058,234
|
|
|
Less: Amount representing interest
|
|
|
(2,525,143
|
)
|
|
Present value of minimum lease payments
|
|
|
3,533,091
|
|
|
Less: Current maturities of capital lease obligations
|
|
|
(143,967
|
)
|
|
Long-term capital lease obligations
|
|
$
|
3,389,124
|
|
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Note 5 – Notes Payable and Line of Credit
0% Note Payable dated October 23, 2017
On October 23, 2017, the Company amended the existing Nevada Medical Marijuana Production License Agreement (“Amended Production License Agreement”). Per the terms of the Amended Production License Agreement, GB Sciences purchased the remaining percentage of the production license resulting in the 100% ownership of the license. GB Sciences also received 100% ownership of the cultivation license included in the original Nevada Medical Marijuana Production License Agreement. In exchange, GB Sciences made one-time payment of $500,000 and issued a 0% Promissory Note in the amount of $700,000 payable in equal monthly payments over a three-year period commencing on January 1, 2018. The present value of the note was $521,067 on the date of its issuance based on an imputed interest rate of 20.3% and the Company recorded a discount on notes payable of $178,933 related to the difference between the face value and present value of the note.
To date, the Company has made principal payments totaling $330,555 and the principal balance of the note was $369,445 at March 31, 2021. During the year ended March 31, 2021, the Company recorded interest expense of $13,929 related to amortization of the note discount. The remaining unamortized discount as of March 31, 2021, was $0.
On August 10, 2020, the Company entered into the Membership Interest Purchase Agreement ("Nopah MIPA") for the sale of its interest in GB Sciences Nopah, LLC (Note 14). The Nopah MIPA will close upon successful transfer of the Nevada Medical Marijuana Cultivation Facility Registration Certificate. Upon close, the principal balance of the note will be reduced to $190,272. The maturity date of the note was be extended to July 31, 2021, with no payments of principal or interest due until maturity. In addition, the note will no longer bear interest at the penalty rate of 15% unless there is a new event of default.
8% Line of Credit dated November 27, 2019
In connection with the Binding Letter of Intent dated November 27, 2019 (Note 14), the Teco Subsidiaries entered into a promissory note and line of credit for up to $470,000 from the purchaser of the membership interests in the Teco Subsidiaries. The purpose of the line of credit is to supply working capital for the Teco Subsidiaries, and the note matures upon the close of the sale of the Teco Subsidiaries. The principal and accrued interest balances outstanding at the time of closing will be considered paid in full upon closing and will not reduce the purchase price received by GB Sciences. As of March 31, 2021, the Teco Subsidiaries have received $485,000 in advances under the line of credit, reflecting an informal agreement with the lender to increase the line of credit by $15,000. The Company accrued interest of $38,767 on the line of credit for the year ended March 31, 2021, and the balance of the line of credit was $485,000 at March 31, 2021. The note and related interest expense are included in current liabilities from discontinued operations and loss from discontinued operations.
8% Note Payable dated May 7, 2020
On May 7, 2020, the Company received $135,000 cash from an investor, net of $15,000 in brokerage fees, and issued a $150,000 promissory note. The note bears interest at a rate of 8.0% per annum. The note was to be repaid upon the first proceeds received from the $8,000,000 promissory note related to the sale of the Company's membership interest in GB Sciences Louisiana, LLC, or from the proceeds of the sale of the Teco Facility. As inducement to enter into the note transaction, the Company repriced 8,002,500 preexisting warrants held by the investor to an exercise price of $0.04. The repriced warrants were valued at $272,085 on the date of the transaction using the Black-Scholes Model, which exceeded the value of the warrants prior to the price reduction of $49,525 by $222,560. As the result of the increase in the estimated fair value of the warrants, the Company recorded a full discount on notes payable of $150,000. During the year ended March 31, 2021, the Company recorded interest expense of $154,964 related to the note consisting of accrued interest of $4,964 and $150,000 related to amortization of the note discount. The Company paid $154,964 on October 5, 2020, in full satisfaction of the note.
8% Line of Credit dated July 24, 2020
On July 24, 2020, the Company entered into the Loan Agreement, 8% Secured Promissory Note, and Security Agreement (together, the "July 24 Note") with AJE Management, LLC, which established a revolving loan of up to $500,000 that the Company may draw on from time to time. The loan is collateralized by the Teco Facility, subject to the pre-existing lien held by CSW Ventures, L.P. in connection with the 8% Senior Secured Convertible Promissory Note dated February 28, 2019. Any advances will be made at the sole discretion of the lender following a written request made by the Company. Contemporaneously with the Loan Agreement, the Company and AJE Management entered into the Amendment to the Membership Interest Purchase Agreement with AJE Management. The amendment provides that any balances outstanding under the July 24 Note at the time of the close of the sale of the Teco Facility will be forgiven in exchange for a reduction to the $4,000,000 note receivable that the Company will receive as consideration for the sale of the Teco Facility. The reduction to the note receivable will be equal to 3 times the balance outstanding under the July 24 Note on the date of the close of the sale of the Teco Facility. The balance outstanding under the note plus accrued interest may be repaid at any time prior to the close of the sale of the Teco facility (Note 14).
101
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
On December 29, 2020, the Company entered into the Omnibus Amendment with the purchaser of the Teco Facility. The Omnibus Amendment reduces the amount of the note receivable that the Company will receive from the sale of the Teco Facility by $975,000 (three times $325,000 in advances made under the July 24 Note) to $3,025,000. Any advances made to the Company under the July 24 Note in excess of $325,000 will reduce the amount of cash received upon close of the sale of Teco one-for-one, i.e., such advances will be considered advance payments of the $4,000,000 cash purchase price. The Company also agreed that it will not repay the balances outstanding under the July 24 Note prior to the closing of the Teco sale. As a result of the Omnibus Amendment, the Company accrued a modification expense of $650,000 (two times $325,000 in addition to $325,000 in advances already recorded under the July 24 Note). The Company has received $50,000 in additional advances above $325,000 bringing the total balance to $1,025,000 at March 31, 2021. Interest expense was $12,510 for the year ended March 31, 2021.
Summary of Notes Payable
As of March 31, 2021, the following notes payable were recorded in the Company’s consolidated balance sheet:
|
|
|
As of March 31, 2021
|
|
|
|
|
Face Value
|
|
|
Discount
|
|
|
Carrying
Value
|
|
|
0% Note Payable dated October 23, 2017 (Note 5)
|
|
$
|
369,445
|
|
|
$
|
-
|
|
|
$
|
369,445
|
|
|
8% Line of Credit dated November 27, 2019 (Note 5)
|
|
|
485,000
|
|
|
|
-
|
|
|
|
485,000
|
|
|
8% Line of Credit dated July 24, 2020 (Note 5)
|
|
|
1,025,000
|
|
|
|
-
|
|
|
|
1,025,000
|
|
|
6% Convertible promissory notes payable (Note 6)
|
|
|
1,060,000
|
|
|
|
-
|
|
|
|
1,060,000
|
|
|
8% Convertible Secured Promissory Note dated February 28, 2019, as amended (Note 6)
|
|
|
1,111,863
|
|
|
|
-
|
|
|
|
1,111,863
|
|
|
6% Convertible notes payable due January 18, 2022 (Note 6)
|
|
|
325,000
|
|
|
|
(296,504
|
)
|
|
|
28,496
|
|
|
Total short-term notes and convertible notes payable
|
|
|
4,376,308
|
|
|
|
(296,504
|
)
|
|
|
4,079,804
|
|
|
Less: Notes payable classified as discontinued operations
|
|
|
(485,000
|
)
|
|
|
-
|
|
|
|
(485,000
|
)
|
|
Total short term notes and convertible notes payable classified as continuing operations
|
|
$
|
3,891,308
|
|
|
$
|
(296,504
|
)
|
|
$
|
3,594,804
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6% Convertible promissory notes payable due September 30, 2023 (Note 6)
|
|
$
|
197,000
|
|
|
$
|
(40,561
|
)
|
|
$
|
156,439
|
|
|
6% Convertible note payable due December 31, 2023 (Note 6)
|
|
|
250,000
|
|
|
|
(114,029
|
)
|
|
|
135,971
|
|
|
Total long term convertible notes payable classified as continuing operations
|
|
$
|
447,000
|
|
|
$
|
(154,590
|
)
|
|
$
|
292,410
|
|
As of March 31, 2020, the following notes payable were recorded in the Company’s consolidated balance sheet:
|
|
|
As of March 31, 2020
|
|
|
|
|
Face Value
|
|
|
Discount
|
|
|
Carrying
Value
|
|
|
0% Note Payable dated October 23, 2017 (Note 5)
|
|
$
|
369,445
|
|
|
$
|
(13,929
|
)
|
|
$
|
355,516
|
|
|
8% Line of Credit dated November 27, 2019 (Note 5)
|
|
|
480,000
|
|
|
|
-
|
|
|
|
480,000
|
|
|
6% Convertible promissory notes payable (Note 6)
|
|
|
1,257,000
|
|
|
|
(155,340
|
)
|
|
|
1,101,660
|
|
|
8% Convertible Secured Promissory Note dated February 28, 2019, as amended (Note 6)
|
|
|
1,271,863
|
|
|
|
(409,481
|
)
|
|
|
862,382
|
|
|
8% Convertible Promissory Note dated April 23, 2019 (Note 6)
|
|
|
2,765,000
|
|
|
|
(29,830
|
)
|
|
|
2,735,170
|
|
|
Total short term notes and convertible notes payable
|
|
|
6,143,308
|
|
|
|
(608,580
|
)
|
|
|
5,534,728
|
|
|
Less: Notes payable classified as discontinued operations
|
|
|
(480,000
|
)
|
|
|
-
|
|
|
|
(480,000
|
)
|
|
Total short term notes and convertible notes payable classified as continuing operations
|
|
$
|
5,663,308
|
|
|
$
|
(608,580
|
)
|
|
$
|
5,054,728
|
|
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Note 6 – Convertible Notes
March 2017 and July 2017 Convertible Note Offerings
In March 2017, the Company entered into a Placement Agent’s Agreement with a third-party brokerage firm to offer units consisting of a $1,000 6% promissory note convertible into 4,000 shares of the Company’s common stock at $0.25 per share and 4,000 warrants to purchase shares of the Company’s’ common stock at an exercise price of $0.60 per share for the period of three years. Between March 2017 and May 2017, the Company issued short-term Promissory Notes (“Notes”) to various holders with combined face value of $2,000,000. The Notes are payable within three years of issuance and are convertible into 8,000,000 shares of the Company’s common stock. The Company also issued 8,000,000 common stock warrants to the Noteholders. The warrants are exercisable at any time and from time to time before maturity at the option of the holder. Each warrant gives the Noteholder the right to purchase one share of common stock of the Company at an exercise price of $0.60 per share for a period of three years. The Company recorded an aggregate discount on convertible notes of $1,933,693, which included $904,690 related to the relative fair value of beneficial conversion features and $1,029,003 for the relative fair value of the warrants issued with each note. The fair value of warrants was derived using the Black-Scholes valuation model.
In July 2017, the Company entered into a Placement Agent’s Agreement with a third-party brokerage firm to offer units consisting of a $1,000 6% promissory note convertible into 4,000 shares of the Company’s common stock at $0.25 per share and 4,000 warrants to purchase shares of the Company’s’ common stock at an exercise price of $0.65 per share for the period of three years. Between July 2017 and December 2017, the Company issued short-term Promissory Notes (“Notes”) to various holders with combined face value of $7,201,000. The Notes are payable within three years of issuance and are convertible into 28,804,000 shares of the Company’s common stock. The Company also issued 28,804,000 common stock warrants to the Note holders. The warrants are exercisable at any time and from time to time before maturity at the option of the holder. Each warrant gives the Noteholder the right to purchase one share of common stock of the Company at an exercise price of $0.60 per share for a period of three years. The Company recorded an aggregate discount on convertible notes of $7,092,796, which included $3,142,605 related to the relative fair value of beneficial conversion features and $3,950,191 for the relative fair value of the warrants issued with each note. The fair value of warrants was derived using the Black-Scholes valuation model.
All notes from the March and July 2017 offerings have passed their maturity dates. During the year ended March 31, 2021, the Company agreed to extensions with the holders of a total of $197,000 of the $1,257,000 that remains outstanding. For the $197,000 of extended notes, the Company agreed to reduce the conversion price to $0.10 per share and issued a total of 788,000 additional warrants to the holders of the notes with a term of three years and an exercise price of $0.10 per share. In exchange, the maturity date of the notes was extended to September 30, 2023. Using the Black-Scholes model, the Company valued the warrants at $13,396 and the change in the fair value of the conversion feature at $33,490. Because the change in the fair value of the conversion feature exceeded 10% of the carrying amount of the notes, the Company accounted for the modification of the notes as an extinguishment and recorded a discount on the new convertible notes of $46,886 related to the fair value of the new warrants issued and the change in the fair value of the conversion feature. The Company recorded interest expense of $28,306 on the new notes during the year ended March 31, 2021, of which $22,412 represented amortization of the note discounts. Accrued interest on the $197,000 extended notes is $44,332 at March 31, 2021, which includes $38,438 accrued prior to the extinguishments.
Three convertible notes totaling $1,060,000 held by the same investor are past maturity and are currently in default. The Company is negotiating the terms of an extension with the note holder. The notes do not provide for a default penalty or penalty interest rate. Interest expense during the year ended March 31, 2021, was $208,779, of which $139,253 represents amortization of the note discount. Accrued interest on the $1,060,000 notes was $228,373 at March 31, 2021.
8% Senior Secured Convertible Promissory Note dated February 28, 2019
On February 28, 2019, the Company issued a $1,500,000 8% Senior Secured Convertible Promissory Note and entered into the Note Purchase Agreement and Security Agreement with CSW Ventures, L.P. (together, “CSW Note”). The note matured on August 28, 2020, and was convertible at any time until maturity into 8,823,529 shares of the Company’s common stock at $0.17 per share. Collateral pledged as security for the note includes all of the Company’s 100% membership interests in GB Sciences, Nevada, LLC and GB Sciences Las Vegas, LLC, which together represent substantially all of the Company’s cannabis cultivation and production operations and assets located at the Teco facility in Las Vegas, Nevada. The intrinsic value of the beneficial conversion feature resulting from the market price of the Company’s common stock in excess of the conversion price was $176,471 on the date of issuance, and the Company recorded a discount on the CSW Note in that amount.
103
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
On May 28, 2019, the Company received notice from CSW Ventures, L.P. of the conversion of a total of $170,000 of the principal balance of the 8% Senior Secured Promissory Note dated February 28, 2019. Accordingly, the Company issued 1,000,000 shares of its common stock based on a $0.17 per share conversion price. In connection with the conversions, $17,225 in unamortized discount was recorded as interest expense and the Company reduced the carrying amount of convertible notes payable by $152,775. After conversion, the remaining balance outstanding was $1,330,000.
On July 12, 2019, the Company entered into the Amendment to Note Documents and the Amended and Restated 8% Senior Secured Promissory Note (together, “Amended CSW Note”). The Amended CSW Note increased the note balance by $100,000 to reflect an additional $100,000 advanced to the Company on July 12, 2019, and by $41,863 to add accrued interest to date to the principal balance, and decreased the conversion price to $0.11 per share, with the remaining terms substantially unchanged from the original CSW Note.
The Company evaluated the modification under the guidance in ASC 470-50 and determined that the amendment represents an extinguishment because the change in the fair value of the conversion feature exceeded 10% of the carrying value of the CSW Note on the amendment date. The carrying value of the amended note on the date of extinguishment was $1,338,057, net of a beneficial conversion feature discount of $133,806, and we recorded a loss on extinguishment of $124,158.
On August 1, 2019, the Company received notice from CSW Ventures, L.P. of the conversion of a total of $110,000 of the principal balance of the Amended CSW Note at $0.11 per share. Accordingly, the Company issued 1,000,000 shares of its common stock. In connection with the conversions, $9,579 in unamortized discount was recorded as interest expense and the Company has reduced the carrying amount of convertible notes payable by $100,421. After conversion, the remaining balance outstanding was $1,361,863.
On October 23, 2019, the Company entered into the Amendment to Promissory Note. The October 23, 2019 amendment decreased the conversion price to $0.08 per share, with the remaining terms substantially unchanged from the Amended CSW Note.
We evaluated the modification under the guidance in ASC 470-50 and determined that the amendment represents an extinguishment because the change in the fair value of the conversion feature exceeded 10% of the carrying value of the Amended CSW Note immediately prior to the 2nd Amended CSW Note. The carrying value of the Amended CSW Note on the date of extinguishment was $1,269,067, net of a beneficial conversion feature discount of $92,796, and we recorded a loss on extinguishment of $92,796 during the year ended March 31, 2020.
On November 27, 2019, the Company entered into the Second Amendment to Note Documents and the Second Amended and Restated 8% Senior Secured Promissory Note (together, “2nd Amended CSW Note”). The 2nd Amended CSW Note decreased the conversion price to $0.04 per share and increased the note balance by $30,000 to reflect an advance received on that date, with the remaining terms substantially unchanged from the Amended CSW Note.
We evaluated the modification under the guidance in ASC 470-50 and determined that the 2nd Amended CSW Note represents an extinguishment because the change in the fair value of the conversion feature exceeded 10% of the carrying value of the Amended CSW Note immediately prior to the 2nd Amended CSW Note; however, no loss on extinguishment was recorded because the net consideration paid for the 2nd Amended CSW Note was equal to the extinguished carrying value of the Amended CSW Note. The carrying value of the Amended CSW Note on the date of extinguishment was $1,361,863.
On December 16, 2019, the Company received notice from CSW Ventures, L.P. of the conversion of a total of $120,000 of the principal balance of the Amended CSW Note at $0.04 per share and we issued 3,000,000 shares of common stock. In connection with the conversions, $57,551 in unamortized discount was recorded as interest expense, and the Company has reduced the carrying amount of convertible notes payable by $62,449. After conversion, the remaining balance outstanding was $1,271,863 and the carrying amount of the note was $687,021, net of $584,842 in unamortized discount from the beneficial conversion feature.
On December 29, 2020, the Company entered into the Omnibus Amendment (Note 14), and the note holder agreed to cease interest accrual on the CSW Note after November 30, 2020.
During the quarter ended March 31, 2021, the Company received notice of the conversion of $160,000 total principal balance at $0.04 per share and issued 4,000,000 shares of common stock to the note holder. After the conversions, the remaining principal balance and carrying amount of the note is $1,111,863 as of March 31, 2021.
During the year ended March 31, 2021, we recorded interest expense of $477,500 related to the CSW Note and its amendments consisting of $68,019 in stated interest and $409,481 related to amortization of the note discount. The total outstanding balance of principal and accrued interest totaling $1,256,857 will reduce the $4,000,000 cash payment received by the Company upon the close of the sale of the Teco Facility, and no further interest expense will be accrued on the note.
104
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
8% Convertible Promissory Note dated April 23, 2019
On April 23, 2019, the Company entered into the Note Purchase Agreement with Iliad Research and Trading, L.P. ("Iliad") and issued an 8% Convertible Promissory Note with a face value of $2,765,000. The Note was issued with original issue discount of $265,000 and is convertible into shares of the Company’s common stock at a price of $0.17 per share at the option of the note holder at any time until the Note is repaid. The Note matured on April 22, 2020. A total discount of $440,000 was recorded on the note, which includes $265,000 of original issue discount and $175,000 in fees paid to brokers.
During the year ended March 31, 2020, the Company honored the conversion of a total of a total of $125,000 of accrued interest on the Iliad Note at reduced conversion rates. On October 30, 2019, the Company received notice of the conversion of $75,000 at $0.06 per share and issued 1,250,000 shares of its common stock. The fair value of the shares issued exceeded the fair value of the shares issuable under the original terms of the Note by $64,706, and the Company recorded an induced conversion expense. On November 18, 2019, the Company received notice of the conversion of $50,000 of the note balance at $0.0375 per share and issued 1,333,333 shares of its common stock.
On April 22, 2020, the Company failed to make payment of the principal and accrued interest due under the Iliad Note, resulting in a default. Upon the occurrence of the default, the principal and accrued interest balances outstanding increased by 10%. As the result of the default, Company recorded an expense of $9,559 related to a 10% increase in the accrued interest balance, which is recorded in interest expense, and $276,500 related to the 10% increase in the principal balance, which is recorded in debt default penalty and other expense.
On May 20, 2020, Iliad filed a lawsuit against the Company in the Third Judicial District Court of Salt Lake County in the State of Utah demanding repayment of the note. The lawsuit further sought to compel the Company to participate in arbitration pursuant to the arbitration provisions contained within the Note Purchase Agreement and to prohibit the Company to raise funds through the issuance of its common stock unless the note is paid in full simultaneously with such issuance. On July 14, 2020, the Court entered judgment in favor of Iliad in the amount of $3,264,594 plus reasonable attorney's fees and costs and accrued post-judgment interest at the default rate of 15% per annum.
On November 20, 2020, the Company, Iliad, and Wellcana Plus, LLC entered into the Judgment Settlement Agreement, whereby Iliad agreed to discharge all amounts owed to it by the Company upon receipt of payment totaling $3,006,015 directly from the proceeds of the Wellcana Note Receivable (Note 14) on or before December 8, 2020. On December 8, 2020, Wellcana failed to make payment to the Company. On December 9, 2020, the Company entered into a letter agreement with Iliad extending the Judgment Settlement agreement in exchange for payment of $25,000 plus $25,000 per week until the payment totaling $3,006,015 is received by Iliad, with such payments not reducing the amount owed under the Judgment Settlement Agreement. On December 16, 2020, Wellcana made payment of the full amount owed to the Company, of which $3,006,015 was paid directly to Iliad in full satisfaction of the Judgment Settlement Agreement. On December 18, 2020, Iliad filed a Satisfaction of Judgment in the Third Judicial District Court of Salt Lake County in the State of Utah, and the lawsuit was dismissed. The Company has no further obligations to Iliad.
During the year ended March 31, 2021, interest expense related to the Iliad Note was $379,956, of which $29,831 relates to amortization of the note discount, $140,833 relates to accrued interest prior to the judgment, and $209,292 was accrued post-judgment interest. The Company also recorded $25,000 in other expense as the result of the letter agreement to extend the Judgment Settlement Agreement. As of the date of final payment, the outstanding judgment balance of $3,264,594 plus accrued post-judgment interest of $209,292 totaled $3,473,886, and the Company recorded a gain on extinguishment of $467,872.
December 2020 $575,000 6% Convertible Notes
On December 18, 2020, the Company began an offering of 6.0% convertible notes for the purpose of funding a pre-clinical study of the Company's patent-pending Cannabinoid-Containing Complex Mixtures for the treatment of Cytokine Release Syndromes, including Acute Respiratory Distress Syndrome, in COVID-19 patients. The Company pledged the related intellectual property as security for the notes. The notes are convertible at a rate of $0.05 per share at the lender's request. During the year ended March 31, 2021, the Company issued $575,000 in convertible notes under the offering to three investors. $325,000 of the notes mature in December 2021, and $250,000 mature in December 2023. Payment of accrued interest and principal is due at maturity. The Company received cash of $500,250, net of issuance costs, and recorded a discount on convertible notes of 74,750. Notes totaling $425,000 were issued with in-the-money conversion features, and the Company recorded beneficial conversion feature discounts totaling $347,000 on the related notes.
At March 31, 2021, notes with a carrying amount of $28,496 were included in short term notes and convertible notes payable, net of unamortized discounts of $296,504. Notes with a carrying amount of $135,971 were included in long term notes and convertible notes payable, net of unamortized discounts of $114,029. Interest expense related to the notes was $16,354 for the year ended March 31, 2021, which includes $11,217 from amortization of the note discounts.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Note 7 - Property and Equipment
Property and equipment is recorded at cost and depreciated using the straight-line method over the estimated useful life of the asset or, in the case of leasehold improvements amortized over the lesser of the useful life of the asset or the underlying lease term. We recorded depreciation expense of $34,555 and $541,462 on a consolidated basis for the years ended year ended March 31, 2021 and 2020, respectively, net of depreciation capitalized in inventory of $532,785 and $811,508. Discontinued operations included $21,855 and $424,501 for the years ended March 31, 2021 and 2020, respectively. Property and equipment is comprised of the following:
|
|
|
March 31, 2021
|
|
|
|
|
Continuing
Operations
|
|
|
Discontinued
Operations
|
|
|
Total
|
|
|
Furniture and fixtures
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Computer and software
|
|
|
151,748
|
|
|
|
-
|
|
|
|
151,748
|
|
|
Machinery and equipment
|
|
|
619,631
|
|
|
|
289,035
|
|
|
|
908,666
|
|
|
Leaseholds
|
|
|
-
|
|
|
|
3,455,600
|
|
|
|
3,455,600
|
|
|
Construction in progress
|
|
|
-
|
|
|
|
21,069
|
|
|
|
21,069
|
|
|
Finance lease right-of-use asset
|
|
|
-
|
|
|
|
1,663,013
|
|
|
|
1,663,013
|
|
|
|
|
|
771,379
|
|
|
|
5,428,717
|
|
|
|
6,200,097
|
|
|
Less accumulated depreciation and amortization
|
|
|
(746,357
|
)
|
|
|
(552,471
|
)
|
|
|
(1,298,828
|
)
|
|
Property and Equipment, Net
|
|
$
|
25,022
|
|
|
$
|
4,876,247
|
|
|
$
|
4,901,269
|
|
During the year ended March 31, 2020, the Company entered into the Membership Interest Purchase Agreement ("Teco MIPA") to sell 100% of the membership interests in the Teco Facility (Note 14). As a result of this agreement, the Company determined that the long-lived assets of the Teco Facility might be impaired due to the current expectation that the asset group will more likely than not be disposed of by sale significantly before the end of its previously estimated useful life. The Company estimated future undiscounted cash flows related to the Teco Facility to be $8.0 million, which was less than the carrying amount of the Teco Facility asset group of $11.9 million. Using a discounted cash flow approach, the Company estimated the fair value of the asset group to be approximately $7.3 million, resulting in a write-down of $4,645,054 related to the Teco Facility asset group. Fair value was based on expected future cash flows using level 3 inputs under ASC 820. The cash flows are the proceeds expected to be generated from the sale of the assets under the Teco MIPA, discounted to present value at a rate of 17%.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Note 8 – Income Taxes
The Company files income tax returns in the U.S. federal jurisdiction. The Company operates in the state of Nevada, which does not levy an income tax. The Company has analyzed filing positions for all open tax years in the federal jurisdiction where it is required to file income tax returns. The Company identified its federal tax return as its “major” tax jurisdiction, as defined under generally accepted accounting principles.
The Company’s effective tax rate was -3.6% and 0.0% for the years ended March 31, 2021 and 2020, respectively.
Income tax expense was $168,527 for the year ended March 31, 2021, which includes $48,076 in penalties and interest related to a $486,145 tax liability from the March 31, 2018 tax year. Income tax payable at March 31, 2021 was $761,509 including a $486,145 income tax liability related to the March 31, 2018 tax year and accrued penalties and interest of $154,914. Income tax expense was $86,837 for the year ended March 31, 2020. This amount represents penalties and interest on the March 31, 2018 tax liability. Income tax payable was $592,982 as of March 31, 2020. Income tax expense and income tax payable are included in discontinued operations in the Company's financial statements for the years ended March 31, 2021 and 2020.
Because the Company operates in the State-licensed cannabis industry, it is subject to the limitations of Internal Revenue Code Section 280E (“280E”) for U.S. income tax purposes. Under 280E, the Company is allowed to deduct expenses that are directly related to the production of its products, i.e., cost of goods sold, but is allowed no further deductions for ordinary and necessary business expenses from its gross profit. The Company believes that the deductions disallowed include the deduction of NOLs. The unused NOLs will continue to carry forward and may be used by the Company to offset future taxable income that is not subject to the limitations of 280E.
At March 31, 2021 and 2020 respectively, the Company had net operating loss carryforwards (“NOLs”) for income tax purposes of $51,776,062 and $50,596,940. $34,481,122 of the Company's NOL carryforwards are expected to expire at various times from 2025 through 2039. $17,294,940 of the NOL carryforwards generated in tax years ending March 31, 2019 to present have no expiration date. These NOLs have the potential to be used to offset future ordinary taxable income and reduce future cash tax liabilities. Utilization of the Company’s net operating losses may be subject to substantial annual limitation if the Company experiences a 50% change in ownership, as provided by the Internal Revenue Code. Such an ownership change would substantially increase the possibility of net operating losses expiring before complete utilization.
The provision for income taxes included in discontinued operations is different than would result from applying the U.S. statutory rate to profit before taxes for the reasons set forth in the following reconciliation:
|
|
|
2021
|
|
|
2020
|
|
|
Tax benefit computed at U.S. statutory rates
|
|
$
|
(697,040
|
)
|
|
$
|
(2,178,478
|
)
|
|
Increases (decreases) in taxes resulting from:
|
|
|
|
|
|
|
|
|
|
IRC Section 280E
|
|
|
173,045
|
|
|
|
202,877
|
|
|
Other permanent items
|
|
|
14,407
|
|
|
|
22,948
|
|
|
Change in valuation allowance
|
|
|
26,720
|
|
|
|
1,952,653
|
|
|
Adjustments to valuation of deferred tax assets
|
|
|
603,319
|
|
|
|
-
|
|
|
Total provision for income taxes
|
|
|
120,451
|
|
|
|
-
|
|
|
Penalties and interest on prior year tax liabilities
|
|
|
48,076
|
|
|
|
86,837
|
|
|
Total income tax expense
|
|
$
|
168,527
|
|
|
$
|
86,837
|
|
The tax effects of the primary temporary differences giving rise to the Company’s deferred tax assets and liabilities are as follows for the year ended March 31, 2021 and 2020:
|
|
|
2021
|
|
|
2020
|
|
|
Deferred tax assets:
|
|
|
|
|
|
|
|
|
|
Stock based compensation
|
|
$
|
3,131,344
|
|
|
$
|
2,943,816
|
|
|
Net operating loss carryforward
|
|
|
10,460,788
|
|
|
|
10,625,357
|
|
|
Impairment of long-lived assets
|
|
|
975,461
|
|
|
|
975,461
|
|
|
Depreciation and Amortization expense
|
|
|
(458,938
|
)
|
|
|
(324,707
|
)
|
|
Other temporary items
|
|
|
220,795
|
|
|
|
136,243
|
|
|
Total deferred tax assets
|
|
|
14,329,450
|
|
|
|
14,356,170
|
|
|
Less valuation allowance
|
|
|
(14,329,450
|
)
|
|
|
(14,356,170
|
)
|
|
Net deferred tax asset
|
|
$
|
-
|
|
|
$
|
-
|
|
107
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Deferred tax assets are evaluated by considering historical levels of income, estimates of future taxable income and the impact of tax planning strategies. The Company continues to evaluate its deferred tax asset valuation allowance on a quarterly basis. The Company concluded that, as of March 31, 2021, it is more likely than not that the Company will not have sufficient taxable income within the applicable net operating loss carry-forward period to realize any portion of its deferred tax assets.
The Company believes that the tax positions taken in its tax returns would be sustained upon examination by taxing authorities. The Company files income tax returns in the U.S. federal jurisdiction and other required state jurisdictions. The Company's periodic tax returns filed in 2018 and thereafter are subject to examination by taxing authorities under the normal statutes of limitations in the applicable jurisdictions.
Note 9 – Capital Transactions
Year Ended March 31, 2021
On April 1, 2020, the Company entered into the Advisory Agreement with its brokers and effected a temporary decrease in the exercise price of the Company's outstanding warrants to $0.03-$.05 per share. As a result of the price reduction, the Company received notice of the exercise of 35,798,809 warrants during the year ended March 31, 2021, and received proceeds of $968,023, net of brokerage fees of $(107,373). The Company recorded inducement dividends totaling $1,591,080 as the difference between the reduced exercise price of the warrants and the stock price on the date of exercise.
During the year ended March 31, 2021, the Company granted 3,500,000 immediately vesting options to purchase one share of the Company's Common Stock at the price of $0.05 per share for a period of ten years, as compensation to a scientist and researcher for drafting and filing U.S. and international patents. The options were valued at $168,000 using the Black-Scholes model.
During the year ended March 31, 2021, the Company issued a total of 788,000 warrants to convertible note holders with a term of three years and an exercise price of $0.10 per share in exchange for a three-year extension of notes having an aggregate principal balance of $197,000 (Note 6). Using the Black-Scholes model, the Company valued the warrants at $13,396.
On November 16, 2020, the Company entered into a Severance Agreement with Leslie Bocskor, a former member of the Company's board of directors, and re-priced 450,000 options held by the director to that day's closing share price of $0.03. The term of the options was extended to November 16, 2025, from June 1, 2023. Using the Black-Scholes Model, the Company valued the options at $4,950 immediately prior to the modification and at $11,250 immediately after the modification, and the Company recorded share-based compensation expense of $6,300.
On December 7, 2020, the board of directors approved the issuance of warrants to purchase a total of 3,500,000 shares of the Company's common stock at $0.04 per share for a term of ten years to current employees and directors. The Company valued the warrants at $133,000 using the Black-Scholes Model and recorded share-based compensation expense of $133,000 related to the warrants.
On December 15, 2020, the board of directors approved the issuance of options to purchase a total of 3,250,000 shares of the Company's common stock at $0.05 per share for a term of ten years to current employees and directors. The options vest one-third upon grant, one-third after one year of service, and one-third after two years of service. The Company valued the options at $156,000 using the Black-Scholes Model and recorded share-based compensation expense of $62,000 related to the options for the year ended March 31, 2021. Remaining unrecognized compensation cost related to the options was $78,000 at March 31, 2021.
On December 15, 2020, the board of directors approved the re-pricing of 6,050,000 options held by current employees to an exercise price of $0.05, the closing stock price on that date. All of the options subject to the modification were fully vested. Using the Black-Scholes Model, the Company valued the options at $199,600 immediately prior to the modification and at $250,650 immediately after the modification, and the Company recorded share-based compensation expense of $51,050.
108
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
On January 2, 2021, the board of directors approved the re-pricing and extension of 9,424,613 outstanding compensation warrants issued to the Company's brokers. The exercise price of the warrants was reduced to $0.01, and the warrants were extended to the expiration date of January 2, 2024. Prior to the extension and re-pricing, the warrants had expiration dates ranging from December 11, 2020 through October 1, 2023, and had exercise prices ranging from $0.25 to $1.00. The company valued the modified warrants at $367,196 using the Black-Scholes model. The Black-Scholes value of the warrants immediately prior to the modification was $135,861, and the Company recorded compensation expense of $231,335.
During the quarter ended March 31, 2021, the Company received notice of the conversion of $160,000 total principal balance of the note payable to CSW Ventures, L.P. at $0.04 per share and issued 4,000,000 shares of common stock to the note holder (Note 6).
On February 8, 2021, the board of directors approved the issuance of 42,705,809 replacement warrants to investors who had exercised warrants at prices that were near or at-the-money beginning in December of 2019 in order to provide working capital to the Company. The replacement warrants expire three years from the date of the initial warrant exercise and have a strike price of $0.10 per share. The Company valued the warrants at $1,182,920 using the Black-Scholes model and recorded the value of the warrants as an inducement dividend.
At March 31, 2021, there were 85,843,036 warrants at exercise prices ranging from $0.01 to $0.90 per share outstanding, exclusive of warrants held by employees. At the same date, 17,733,334 employee options and warrants with a weighted average exercise price of $0.11 were outstanding, and 5,883,000 nonemployee options with a weighted average exercise price of $0.14 remain outstanding.
Year Ended March 31, 2020
Stock Issued for Debt Conversions
During the year ended March 31, 2020, the Company issued a total of 7,583,333 shares of common stock for the conversion of notes payable:
On May 28, 2019, the Company received notice from CSW Ventures, L.P. of the conversion of a total of $170,000 of the principal balance of the 8% Senior Secured Promissory Note dated February 28, 2019 (See Note 6). Accordingly, the Company issued 1,000,000 shares of its common stock based on a $0.17 per share conversion price. In connection with the conversions, $17,225 in unamortized discount was recorded as interest expense and the Company has reduced the carrying amount of convertible notes payable by $152,775.
On August 1, 2019, the Company received notice from CSW Ventures, L.P. of the conversion of a total of $110,000 of the principal balance of the Amended CSW Note at $0.11 per share. Accordingly, the Company issued 1,000,000 shares of its common stock. In connection with the conversions, $9,579 in unamortized discount was recorded as interest expense and the Company has reduced the carrying amount of convertible notes payable by $100,421. After conversion, the remaining balance outstanding was $1,361,863.
On December 16, 2019, the Company received notice from CSW Ventures, L.P. of the conversion of a total of $120,000 of the principal balance of the Amended CSW Note at $0.04 per share and we issued 3,000,000 shares of common stock. In connection with the conversions, $57,551 in unamortized discount was recorded as interest expense and the Company has reduced the carrying amount of convertible notes payable by $62,449. After conversion, the remaining balance outstanding was $1,271,863 and the carrrying amount of the note was $687,021, net of $584,842 in unamortized discount from the beneficial conversion feature.
During the year ended March 31, 2020, the Company has honored the conversion of a total of a total of $125,000 of debt owed under the Iliad Note at reduced conversion rates. On October 30, 2019, the Company received notice of the conversion of $75,000 at $0.06 per share and issued 1,250,000 shares of its common stock. The fair value of the shares issued exceeded the fair value of the shares issuable under the original terms of the Note by $64,706, and the Company recorded an expense in that amount. On November 18, 2019, the Company received notice of the conversion of $50,000 of the note balance at $0.0375 per share and issued 1,333,333 shares of its common stock. The fair value of the shares issued exceeded the fair value of the shares issuable under the original terms of the Note by $62,353, and the Company recorded an expense in that amount. In total, the Company recorded $127,059 in noncash expense for the two conversions of the Iliad note at below contractual conversion rates for the year ended March 31, 2020.
109
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Exercise of Warrants for Stock
During the year ended March 31, 2020, the Company issued 17,563,000 shares of common stock for exercises of warrants:
In order to encourage the exercise of approximately 70.5 million warrants issued to investors in private placements of convertible notes and common stock having exercise prices ranging between $0.65 and $0.30, the Company effected a temporary decrease in the exercise price of the warrants to $0.10 per share until July 11, 2019. On July 12, 2019, the Company extended the repricing of the warrants through August 30, 2019, and on July 31, 2019, the Company extended the repricing of the warrants to December 31, 2019. As a result of the price reduction, the Company received notice of the exercise of 9,449,750 warrants and received proceeds of $850,478, net of brokerage fees of $94,498. In connection with the induced exercise of the warrants, the Company recorded an inducement dividend of $230,025.
In order to encourage the further exercise of the warrants, the Company effected a temporary decrease in the exercise price of the warrants to $0.03-$.05 per share beginning in December 2019. As a result of the price reduction, the Company received notice of the exercise of an additional 8,113,250 warrants and received proceeds of $307,249, net of brokerage fees of $22,566. In connection with the induced exercise of the warrants, the Company recorded an inducement dividend of $32,215.
Issuance of Stock for Services
During the year ended March 31, 2020, the Company issued 2,100,000 shares of common stock for consulting services and recorded related expense of $214,000 based on the fair value of the stock on the date of the related consulting agreements.
Warrants Outstanding
Presented below is a summary of the Company’s warrant activity, exclusive of warrants held by employees (see Note 10), for the years ended March 31, 2021 and 2020:
|
|
|
Warrants Outstanding
|
|
|
|
|
Number of Shares
|
|
|
Exercise Price
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at March 31, 2019
|
|
|
99,790,989
|
|
|
|
|
|
|
Warrants issued
|
|
|
7,622,780
|
|
|
|
$0.30
|
|
|
Warrants exercised
|
|
|
(17,563,000
|
)
|
|
0.03550.1010
|
|
|
Warrants expired/cancelled
|
|
|
(5,312,608
|
)
|
|
0.5002.0000
|
|
|
Outstanding at March 31, 2020
|
|
|
84,538,161
|
|
|
|
|
|
|
Warrants issued
|
|
|
43,493,809
|
|
|
|
$0.10
|
|
|
Warrants exercised
|
|
|
(35,798,809
|
)
|
|
0.03000.03535
|
|
|
Warrants expired/cancelled
|
|
|
(6,390,125
|
)
|
|
0.6000.9090
|
|
|
Outstanding at March 31, 2021
|
|
|
85,843,036
|
|
|
|
|
|
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Note 10 – Employee Benefit Plan
Share-Based Employee Compensation
On February 6, 2008, the board of directors adopted the GB Sciences, Inc. 2007 Amended Stock Option Plan (“2007 Plan”). Under the 2007 Plan, 4,500,000 shares of the Company’s restricted common stock may be issuable upon the exercise of options issued to employees, advisors and consultants. The Company revised the plan, and the board of directors adopted the new 2014 Equity Compensation Plan. On June 30, 2015, GB Sciences filed a Form S-8 Registration Statement with the SEC to register 8,500,000 shares of common stock issuable under stock options to grant to employees and consultants. At the Company’s special meeting of the shareholders held on April 6, 2018, the adoption by the board of directors of the 2014 Equity Compensation Plan was ratified by a majority of shareholders present at the meeting, either in person or by proxy and the Company adopted the GB Sciences, Inc 2018 Stock Plan. On October 25, 2018, GB Sciences filed a Form S-8 Registration Statement with the SEC to register 10,000,000 shares of common stock issuable under the 2018 Plan. There were 2,022,443 shares available for issuance under the stock plans at March 31, 2021.
Compensation Expense
For the years ended March 31, 2021 and 2020, the Company recorded share-based compensation expense of $436,349 and $287,260, respectively, which includes $211,000 and $103,472, respectively, related to employee stock options and warrants. There was no expense for restricted stock. Unrecognized compensation cost for non-vested awards was $78,000 as of March 31, 2021.
Fair Value
The closing price of the Company's stock on the date of grant is used as the fair value for issuances of restricted stock. The fair value of stock options granted is estimated as of the grant date using the Black-Scholes option pricing model.
The following range of assumptions in the Black-Scholes option pricing model was used to determine fair value:
|
|
|
Year Ended
|
|
|
|
|
March 31,
2021
|
|
|
March 31,
2020
|
|
|
Weighted-average volatility
|
|
|
127
|
%
|
|
|
N/A
|
|
|
Expected term (in years)
|
|
|
10
|
|
|
|
N/A
|
|
|
Risk-free interest rate
|
|
|
0.93
|
%
|
|
|
N/A
|
|
Expected volatilities used for award valuation are based on historical volatility of the Company's common stock. The risk-free interest rate for periods equal to the expected term of an award is based on Federal Reserve yields for U.S. Treasury securities.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Stock Options
A summary of employee option activity, including warrants issued to employees, as of March 31, 2021 and 2020, and changes during the years then ended, is presented below:
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Average
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Remaining
|
|
|
Aggregate
|
|
|
|
|
|
|
|
|
Exercise
|
|
|
Contractual
|
|
|
Intrinsic
|
|
|
Employee options and warrants
|
|
Options
|
|
|
Price $
|
|
|
Life (years)
|
|
|
Value ($)
|
|
|
Outstanding at March 31, 2019
|
|
|
12,583,334
|
|
|
$
|
0.28
|
|
|
|
7.18
|
|
|
$
|
43,000
|
|
|
Granted
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forfeited
|
|
|
(1,600,000
|
)
|
|
$
|
0.27
|
|
|
|
|
|
|
|
|
|
|
Outstanding at March 31, 2020
|
|
|
10,983,334
|
|
|
$
|
0.28
|
|
|
|
6.02
|
|
|
$
|
-
|
|
|
Granted
|
|
|
6,750,000
|
|
|
$
|
0.045
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forfeited
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at March 31, 2021
|
|
|
17,733,334
|
|
|
$
|
0.11
|
|
|
|
6.80
|
|
|
$
|
172,000
|
|
|
Fully vested and expected to vest at March 31, 2021
|
|
|
17,733,334
|
|
|
$
|
0.11
|
|
|
|
|
|
|
|
|
|
|
Exercisable at March 31, 2021
|
|
|
15,566,668
|
|
|
$
|
0.12
|
|
|
|
|
|
|
|
|
|
The table below sets forth nonemployee option activity for the years ended March 31, 2021 and 2020:
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Average
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Remaining
|
|
|
Aggregate
|
|
|
|
|
|
|
|
|
Exercise
|
|
|
Contractual
|
|
|
Intrinsic
|
|
|
Nonemployee options
|
|
Options
|
|
|
Price $
|
|
|
Life (years)
|
|
|
Value ($)
|
|
|
Outstanding at March 31, 2019
|
|
|
2,383,000
|
|
|
$
|
0.27
|
|
|
|
7.76
|
|
|
$
|
492,250
|
|
|
Granted
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forfeited
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at March 31, 2020
|
|
|
2,383,000
|
|
|
$
|
0.27
|
|
|
|
6.75
|
|
|
$
|
-
|
|
|
Granted
|
|
|
3,500,000
|
|
|
$
|
0.05
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forfeited
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at March 31, 2021
|
|
|
5,883,000
|
|
|
$
|
0.14
|
|
|
|
8.11
|
|
|
$
|
35,000
|
|
|
Fully vested and expected to vest at March 31, 2021
|
|
|
5,883,000
|
|
|
$
|
0.14
|
|
|
|
|
|
|
|
|
|
|
Exercisable at March 31, 2021
|
|
|
5,883,000
|
|
|
$
|
0.14
|
|
|
|
|
|
|
|
|
|
Restricted stock awards
No restricted stock awards were granted during the years ended March 31, 2021 and 2020.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Note 11 – Commitments and Contingencies
On September 18, 2017, GB Sciences finalized its agreement with Louisiana State University (“LSU”) AgCenter to be the sole operator of LSU’s medical marijuana program. The LSU Board of Supervisors entered into a five-year agreement that has an option to renew for two additional five-year terms with GB Sciences. The contract includes the Company’s commitment to make annual research investments of $500,000 to the LSU AgCenter. The Company initially retained its 50% interest in the research relationship with LSU after the sale of its membership interest in GB Sciences Louisiana, LLC, and accordingly remained obligated for $250,000 of the $500,000 annual research investment for three years, or a total commitment of $750,000. On August 4, 2020, the Company received its first payment under the Wellcana Note Receivable, net of the $250,000 research contribution due for the twelve-month period ended September 30, 2020, and our commitment for that twelve month period was paid by the purchaser with the funds withheld from the note payment. On August 24, 2020, the Company entered into a letter agreement with Wellcana to discount the note receivable in exchange for accelerated payment. Pursuant to the letter of intent, the purchaser assumed the annual $250,000 research contribution commitment to LSU and the Company retains no rights in the intellectual property developed under the research relationship (Note 14).
An individual filed a Charge of Discrimination with the Nevada Equal Rights Commission ("NERC") against the Company on April 2, 2019, alleging sexual harassment and retaliatory discharge. The matter was amicably resolved, and the charges against the Company were dismissed on May 11, 2021.
On April 22, 2020, the Company failed to repay any of the outstanding balance of the Convertible Promissory Note Payable to Iliad Research and Trading, L.P., resulting in a default. On May 20, 2020, Iliad filed a lawsuit against the Company in the Third Judicial District Court of Salt Lake County in the State of Utah demanding repayment of the note. On July 14, 2020, the Court entered judgment in favor of Iliad in the amount of $3,264,594. The Company's obligation to Iliad was satisfied in full on December 16, 2020, upon payment of $3,006,015 pursuant to the Judgment Settlement Agreement (Note 6).
On April 22, 2020, the Company was served notice of a lawsuit filed in the Eighth Judicial District Court in Clark County, Nevada, filed by a contractor who had been hired to perform architectural and design services. The lawsuit demanded payment of $73,050 for the services provided. On September 17, 2020, the Company entered into a Mutual Compromise, Settlement, and Release Agreement with the contractor and made payment of $25,000 in full satisfaction of the alleged debt and reduced the cost of the related fixed asset by $48,050.
From time to time, the Company may become involved in certain legal proceedings and claims which arise in the ordinary course of business. In management’s opinion, based on consultations with outside counsel, the results of any of these ordinary course matters, individually and in the aggregate, are not expected to have a material effect on our results of operations, financial condition, or cash flows. As more information becomes available, if management should determine that an unfavorable outcome is probable on such a claim and that the amount of such probable loss that it will incur on that claim is reasonably estimable, the Company would record a reserve for the claim in question. If and when the Company records such a reserve, it could be material and could adversely impact its results of operations, financial condition, and cash flows.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Note 12 - Related Party Transactions
As of March 31, 2021, the Company is indebted to executive officers for unpaid compensation totaling $84,913.
On September 1, 2019, the Company and its former CFO and COO, Ksenia Griswold, terminated their relationship and entered into the Severance Agreement. Pursuant to the Severance Agreement, Ms. Griswold would receive severance payments of $20,000 per month for 9 months following the date of separation and would continue receive health insurance coverage for the same period. On January 2, 2021, the Company entered into the Settlement Agreement with Ms. Griswold, and made payment of $57,000 in full satisfaction of $114,159 in Severance compensation owed to Ms. Griswold. As the result of the settlement, the Company recorded a gain of $57,159, which is included in gain on settlement of accounts payable in the Company's consolidated financial statements.
On November 16, 2020, the Company entered into a Severance Agreement with Leslie Bocskor, a former member of the board of directors. Pursuant to the Severance Agreement, the Company made payments totaling $84,745 to Mr. Bocskor subsequent to his departure consisting of $78,245 in compensation accrued during Mr. Bocskor's service on the board of directors and $6,500 related to health insurance, for which the Company had agreed to reimburse until the compensation was paid in full. Prior to the termination of his board service, Mr. Bocskor was paid $44,192 in director's compensation during the year ended March 31, 2021. During the year ended March 31, 2020, the Company made payments totaling $40,192 to Mr. Bocksor.
In connection with the sale of membership interest in GB Sciences Louisiana, LLC, the Company issued a note payable in the amount of $151,923 to John Davis, the Company's former General Counsel and President of GB Sciences Louisiana, LLC, for unpaid compensation and bonuses. The note matured upon receipt of the first payment from the Wellcana Note Receivable. The principal balance of the note and accrued interest were repaid in full on August 4, 2020, when the related funds were withheld from the first payment to the Company under the Wellcana Note Receivable (Note 14).
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Note 13 – Sale of 50% Membership Interest in GB Sciences Louisiana, LLC
On November 15, 2019, the Company entered into the Membership Interest Purchase Agreement ("MIPA") with Wellcana Plus, LLC ("Wellcana"). In consideration for the sale of its 50.01% controlling membership interest in GB Sciences Louisiana, LLC (“GBSLA"), the Company received an $8,000,000 Promissory Note ("Wellcana Note") with the potential to receive up to an additional $8,000,000 in earn-out payments.
The Wellcana note bore interest at a rate of 5% per annum and payments were due beginning June 1, 2020 and ending December 1, 2021. The Company recorded a $1,389,408 discount on the note receivable based on an imputed interest rate of 17.0%, and the carrying amount of the note was $6,610,592 as of November 15, 2019:
|
November 15, 2019 Note Receivable
|
|
Note
Payments
|
|
|
June 1, 2020
|
|
$
|
500,000
|
|
|
September 1, 2020
|
|
|
750,000
|
|
|
December 1, 2020
|
|
|
1,000,000
|
|
|
March 1, 2020
|
|
|
1,250,000
|
|
|
June 1, 2021
|
|
|
1,500,000
|
|
|
September 1, 2021
|
|
|
1,500,000
|
|
|
December 1, 2021
|
|
|
1,500,000
|
|
|
Total proceeds
|
|
|
8,000,000
|
|
|
Discount on note receivable
|
|
|
(1,389,408
|
)
|
|
Net present value
|
|
$
|
6,610,592
|
|
Upon close of the sale on November 15, 2019, the Company recorded a gain on deconsolidation of $4,393,242 related to the sale of its membership interest in GBSLA, calculated as follows:
|
|
|
As of
|
|
|
Gain on Deconsolidation
|
|
November 15,
2019
|
|
|
Present value of promissory note
|
|
$
|
6,610,592
|
|
|
Carrying amount of non-controlling interest
|
|
|
8,707,651
|
|
|
Total
|
|
|
15,318,243
|
|
|
|
|
|
|
|
|
Carrying amount of assets
|
|
|
14,715,798
|
|
|
Carrying amount of liabilities
|
|
|
(3,790,797
|
)
|
|
Net assets deconsolidated
|
|
|
10,925,001
|
|
|
Gain on deconsolidation
|
|
$
|
4,393,242
|
|
115
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
On August 24, 2020, the Company entered into a letter of intent with Wellcana to discount the note receivable in exchange for accelerated payment. Pursuant to the letter of intent, the Company would receive payments totaling $5,224,423, including the repayment of a note payable to related party of $151,923, the forgiveness by Wellcana of $172,500 in liabilities and the payment of $4,900,000 in cash, on or before October 15, 2020, less any cash payments made by Wellcana up to the date of the final payment. Upon receipt of the payment, all liabilities owed to the Company by Wellcana, including the $8,000,000 note receivable and any potential earn-out payments were to be considered satisfied in full. Wellcana would assume the annual $250,000 research contribution commitment to LSU (Note 11), and the Company would retain no rights in the intellectual property developed under the research relationship. In addition, the Company agreed to reduce the $750,000 note payment due on September 1, 2020, to $500,000.
As a result of the August 24, 2020 letter of intent, the Company determined that the amount of the note that was collectible as of March 31, 2020 was $5,224,423 and recorded a loss on modification of note receivable calculated as follows:
|
August 24, 2020 Modification
|
|
March 31, 2020
|
|
|
Total cash payments to be made by October 15, 2020
|
|
$
|
4,900,000
|
|
|
Liabilities to be forgiven upon receipt of October 15, 2020 payment
|
|
|
324,423
|
|
|
Total receivable (as modified)
|
|
|
5,224,423
|
|
|
|
|
|
|
|
|
Carrying value of note receivable as of March 31, 2020
|
|
|
6,969,720
|
|
|
Accrued interest as of March 31, 2020
|
|
|
150,137
|
|
|
Total amount receivable (prior to modification)
|
|
|
7,119,857
|
|
|
|
|
|
|
|
|
Loss on modification of note receivable
|
|
$
|
(1,895,434
|
)
|
The Company received payments from Wellcana totaling $550,000 in August and September of 2020, which in combination with the repayment of a note payable to related party of $151,923 and the $172,500 liabilities assumed by Wellcana reduced the final payment owed under the letter agreement to $4,350,000.
On October 15, 2020, the Company was notified that Wellcana would be unable to make the payment of $4,350,000 by October 15, 2020, and the parties entered into a letter agreement, which extended the due date of the final $4,350,000 payment to December 8, 2020. The letter agreement also required Wellcana to provide proof of $4,350,000 in funds and an escrow deposit of $250,000, which was to be released to the Company in the event that Wellcana were unable to make the $4,350,000 payment on or before December 8, 2020. On October 24, 2020, the parties entered into the Escrow Agreement and Wellcana made the $250,000 escrow payment on October 29, 2020.
On December 8, 2020, Wellcana failed to make the payment and the $250,000 escrow deposit was disbursed to the Company. The Company retained $50,000 of the escrow disbursement as compensation for Wellcana's failure to meet the agreed-upon deadline of December 8, 2020, which is recorded in other income, and did not offset that amount against the $4,350,000 balance owed by Wellcana. On December 16, 2020, Wellcana made payment of the remaining balance of $4,150,000, satisfying its obligations to the Company in full.
The Company's statements of operations and cash flows for the year ended March 31, 2020 include activity through the close of the sale on November 15, 2019, classified as discontinued operations.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Note 14 - Sale of 100% Membership Interest in Teco Facility and Nopah License
On November 15, 2019, we entered into a Binding Letter of Intent ("Teco LOI") to sell 75% of the Company's membership interest interests in GB Sciences Nevada, LLC, and GB Sciences Las Vegas, LLC ("Teco Subsidiaries"), for $3,000,000 cash upon close and up to an additional $3,000,000 in earn-out payments after close. In connection with the Teco LOI, we entered into a Management Agreement with the purchaser whereby the facilities will be managed by an affiliate of the purchaser until the close of the sale. As part of the transaction, the Teco Subsidiaries also entered into a Line of Credit of up to $470,000 with the purchaser to provide working capital for the Teco Subsidiaries (Note 5). The Line of Credit will be considered satisfied in full upon close of the sale of the Teco Subsidiaries.
On March 24, 2020, we entered into the Membership Interest Purchase Agreement ("Teco MIPA") with AJE Management, LLC, which formalized the sale of the Teco Subsidiaries and modified the terms of the sale. Pursuant to the Teco MIPA, the Company will sell 100% of its membership interests in GBSN and GBLV for $4,000,000 cash upon close and will receive a $4,000,000 8% promissory note to be paid in monthly installments over 36 months with payments beginning 30 months after the close of the sale.
On July 24, 2020, the Company entered into the Loan Agreement, 8% Secured Promissory Note, and Security Agreement (together, the "July 24 Note") with AJE Management, LLC. Contemporaneously with the Loan Agreement, the Company and AJE Management entered into the Amendment to the Membership Interest Purchase Agreement with AJE Management. The amendment provides that any balances outstanding under the July 24 Note at the time of the close of the sale of the Teco Facility will be forgiven in exchange for a reduction to the $4,000,000 note receivable that the Company will receive as consideration for the sale of the Teco Facility. The reduction to the note receivable was equal to 3 times the balance outstanding under the July 24 Note on the date of the close of the sale of the Teco Facility. As of March 31, 2021, the Company has received advances totaling $375,000 under the July 24 Note (Note 5).
On December 29, 2020, the Company entered into the Omnibus Amendment with the purchaser of the Teco Facility. The Omnibus Amendment reduces the amount of the note receivable that the Company will receive from the sale of the Teco Facility by $975,000 to $3,025,000, and any advances made to the Company above $325,000 will reduce the amount of cash received upon close of the sale of Teco one-for-one, rather than reducing the note receivable by three times the amount of the balance outstanding. The Company also agreed that it will not repay the balances outstanding under the July 24 Note prior to the closing of the Teco sale. As a result of the Omnibus Amendment, the Company accrued an expense of $650,000 to increase the balance outstanding under the July 24 Note to three times $325,000, to total $1,025,000, which will offset the $4,000,000 note receivable that the Company will receive upon close of the sale of the Teco Facility.
The Omnibus Amendment also amends the Management Services Agreement to provide that no further management fees will accrue after November 30, 2020. As of March 31, 2021, the Company has accrued $850,000 which will reduce the $4,000,000 in cash proceeds received upon the close of the sale. The form of the note receivable that the Company will receive on close was amended to accelerate payments such that the Company will receive payment in full within three years, rather than over 36 months with payments beginning 30 months after the close of the sale.
The sale is expected to close upon the successful transfer of the Nevada cultivation and production licenses. The transfer of cannabis licenses in the State of Nevada was subject to an indefinite moratorium beginning in October 2019. In a meeting held on July 21, 2020, the Nevada Cannabis Compliance Board lifted the moratorium, however, the board has indicated that there are over 90 requests pending, and it will take up to several months to process the entire backlog of pending license transfers. The lifting of the moratorium and processing of cannabis license transfers have been delayed by the COVID-19 pandemic and could be further delayed if the pandemic continues.
The Company also holds a Nevada license for cultivation of medical marijuana located in Sandy Valley, Nevada (the “Nopah License”). The license is owned by the Company’s wholly owned subsidiary, GB Sciences Nopah, LLC ("Nopah"). Operations have not begun under the Nopah License. On November 27, 2019, the Company entered into a Binding Letter of Intent to sell its 100% interest in GB Sciences Nopah, LLC. On August 10, 2020, the Company entered into the Membership Interest Purchase Agreement ("Nopah MIPA") and Promissory Note Modification Agreement with the purchaser of GB Sciences Nopah, LLC. As consideration for the transfer of the license and membership interest in GB Sciences Nopah, LLC, the Company will receive $300,000 and the purchaser will pay all expenses related to the upkeep and maintenance of the Nopah Licens until closing. The $300,000 purchase price will be applied as a reduction to the balance of the 0% Note payable dated October 23, 2017 (Note 5), which is held by an affiliate of the purchaser of the Nopah license. The transfer of the Nopah License is subject to the same restrictions on license transfers discussed above.
Because the moratorium on license transfers has been lifted, the Company determined that the Teco Facility and Nopah Facility qualify for presentation as discontinued operations, and the income, assets, and cash flows of the Teco Subsidiaries and GB Sciences Nopah, LLC have been reclassified as discontinued operations for all periods presented in the Company's consolidated financial statements.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
Note 15 – Concentrations
For the year ended March 31, 2021, there were three customers that accounted for 18.4%, 16.7%, and 13.3% of total revenue classified as discontinued operations. As of March 31, 2021, two customers accounted for 26% and 13% of accounts receivable classified as discontinued operations. We held cash in excess of FDIC limits of $513,901 as of March 31, 2021. All of the Company's sales classified as discontinued operations for the year ended March 31, 2021 were located in the State of Nevada. Of the Company's total sales classified as discontinued operations of $3,120,620 during the year ended March 31, 2020, $3,120,620, or 85%, were in the State of Nevada and $569,077, or 15%, were part of discontinued operations in the State of Louisiana.
Note 16 – Subsequent Events
On May 11, 2021, the Company entered into the Second Omnibus Amendment with the purchaser of the Teco Subsidiaries. Pursuant to the amendment, the Company received a $200,000 advance of the cash purchase price and the Teco Management Agreement was extended to December 31, 2021.
On June 14, 2021, the Company received notice of the exercise of warrants to purchase 1,672,000 shares of common stock at $0.03 per share and received $50,160 cash proceeds.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
The following table sets forth the expenses expected to be incurred in connection with the issuance and distribution of common stock registered hereby, all of which expenses, except for the Securities and Exchange Commission registration fee, are estimated.
|
Securities and Exchange Commission registration fee
|
|
$
|
523.00
|
|
|
Miscellaneous expenses
|
|
|
500.00
|
|
|
Legal
|
|
|
3,000.00
|
|
|
Accounting fees and expenses
|
|
|
3,000.00
|
|
|
Total
|
|
$
|
7,023.00
|
|
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS
We are a Nevada corporation and generally governed by the Nevada Private Corporations Code, Title 78 of the Nevada Revised Statutes, or NRS.
Section 78.138 of the NRS provides that, unless the corporation’s articles of incorporation provide otherwise, a director or officer will not be individually liable unless it is proven that (i) the director’s or officer’s acts or omissions constituted a breach of his or her fiduciary duties, and (ii) such breach involved intentional misconduct, fraud or a knowing violation of the law.
Section 78.7502 of the NRS permits a Nevada corporation to indemnify its directors and officers against expenses, judgments, fines, and amounts paid in settlement actually and reasonably incurred in connection with a threatened, pending, or completed action, suit, or proceeding, except an action by or on behalf of the corporation, if the officer or director (i) is not liable pursuant to NRS 78.138, or (ii) acted in good faith and in a manner the officer or director reasonably believed to be in or not opposed to the best interests of the corporation and, if a criminal action or proceeding, had no reasonable cause to believe the conduct of the officer or director was unlawful. Section 78.7502 of the NRS also requires a corporation to indemnify its officers and directors if they have been successful on the merits or otherwise in defense of any claim, issue, or matter resulting from their service as a director or officer.
Section 78.751 of the NRS permits a Nevada corporation to indemnify its officers and directors against expenses incurred by them in defending a civil or criminal action, suit, or proceeding as they are incurred and in advance of final disposition thereof, upon determination by the stockholders, the disinterested board members, or by independent legal counsel. Section 78.751 of NRS requires a corporation to advance expenses as incurred upon receipt of an undertaking by or on behalf of the officer or director to repay the amount if it is ultimately determined by a court of competent jurisdiction that such officer or director is not entitled to be indemnified by the corporation if so provided in the corporation’s articles of incorporation, bylaws, or other agreement. Section 78.751 of the NRS further permits the corporation to grant its directors and officers additional rights of indemnification under its articles of incorporation, bylaws or other agreement.
Section 78.752 of the NRS provides that a Nevada corporation may purchase and maintain insurance or make other financial arrangements on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another company, partnership, joint venture, trust or other enterprise, for any liability asserted against him and liability and expenses incurred by him in his capacity as a director, officer, employee or agent, or arising out of his status as such, whether or not the corporation has the authority to indemnify him against such liability and expenses.
Our Articles of Incorporation are silent on the indemnification and insurance provisions of Chapter 78 of the NRS. However, our Bylaws provide as follows:
“INDEMNIFICATION AND INSURANCE.
6.1 Right to Indemnification. Each person who was or is a party or is threatened to be
made a party to or is involved in any action, suit or proceeding, whether civil, criminal, administrative or investigative (a "proceeding"), by reason of the fact that he, or a person of whom he is the legal representative, is or was a director or officer of the corporation or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or of a partnership, joint venture, trust or other enterprise, including service with respect to employee benefit plans, whether the basis of such proceeding is alleged action or inaction in an official capacity or in any other capacity while serving as director, officer, employee or agent, shall be indemnified and held harmless by the corporation to the fullest extent permitted by the General Corporation Law of Delaware, as amended from time to time, against all costs, charges, expenses, liabilities and losses (including attorneys' fees, judgments, fines, ERISA excise taxes or penalties and amounts paid or to be paid in settlement) reasonably incurred or suffered by such person in connection therewith, and that indemnification shall continue as to a person who has ceased to be a director, officer, employee or agent and shall inure to the benefit of his heirs, executors and administrators; provided, however, that, except as provided in section 6.2, the corporation shall indemnify any such person seeking indemnification in connection with a proceeding (or part thereof) initiated by that person, only if that proceeding (or part thereof) was authorized by the Board. The right to indemnification conferred in these by-laws shall be a contract right and shall include the right to be paid by the corporation the expenses incurred in defending any such proceeding in advance of its final disposition; provided, however, that, if the General Corporation Law of Delaware, as amended from time to time, requires, the payment of such expenses incurred by a director or officer in his capacity as a director or officer (and not in any other capacity in which service was or is rendered by that person while a director or officer, including, without limitation, service to an employee benefit plan) in advance of the final disposition of a proceeding shall be made only upon delivery to the corporation of an undertaking, by or on behalf of such director or officer, to repay all amounts so advanced, if it shall ultimately be determined that such director or officer is not entitled to be indemnified under these by-laws or otherwise. The corporation may, by action of its Board, provide indemnification to employees and agents of the corporation with the same scope and effect as the foregoing indemnification of directors and officers.
6.2 Right of Claimant to Bring Suit. If a claim under section 6.1 is not paid in full by the corporation within 30 days after a written claim has been received by the corporation, the claimant may at any time thereafter bring suit against the corporation to recover the unpaid amount of the claim and, if successful in whole or in part, the claimant also shall be entitled to be paid the expense of prosecuting that claim. It shall be a defense to any such action (other than an action brought to enforce a claim for expenses incurred in defending any proceeding in advance of its final disposition, where the required undertaking, if any, is required and has been tendered to the corporation) that the claimant has failed to meet a standard of conduct that makes it permissible under Delaware law for the corporation to indemnify the claimant for the amount claimed. Neither the failure of the corporation (including its Board, its independent legal counsel or its stockholders) to have made a determination prior to the commencement of such action that indemnification of the claimant is permissible in the circumstances because he has met that standard of conduct, nor an actual determination by the corporation (including its Board, its independent counsel or its stockholders) that the claimant has not met that standard of conduct, shall be a defense to the action or create a presumption that the claimant has failed to meet that standard of conduct.
6.3 Non-Exclusivity of Rights. The right to indemnification and the payment of expenses incurred in defending a proceeding in advance of its final disposition conferred in this section 6 shall not be exclusive of any other right any person may have or hereafter acquire under any statute, provision of the certificate of incorporation, by-law, agreement, vote of stockholders or disinterested directors or otherwise.
6.4 Insurance. The corporation may maintain insurance, at its expense, to protect itself and any director, officer, employee or agent of the corporation or another corporation, partnership, joint venture, trust or other enterprise against any such expense, liability or loss, whether or not the corporation would have the power to indemnify such person against that expense, liability or loss under Delaware law.
6.5 Expenses as a Witness. To the extent any director, officer, employee or agent of the corporation is by reason of such position, or a position with another entity at the request of the corporation, a witness in any action, suit or proceeding, he shall be indemnified against all costs and expenses actually and reasonably incurred by him or on his behalf in connection therewith.
6.6 Indemnity Agreements. The corporation may enter into agreement with any director, officer, employee or agent of the corporation providing for indemnification to the fullest extent permitted by Delaware law.”
By way of explanation, the Company was originally incorporated in the State of Delaware and our bylaws were prepared as a Delaware corporation. The Company was subsequently redomiciled in Nevada with no adjustment to the Bylaws being made to reflect Nevada law. It is likely that the Company has the authority to indemnify and insure its officers and directors against liability to the fullest extent permitted under Chapter 78 of the NRS.
At the present time, there is no pending litigation or proceeding involving a director, officer, employee or other agent of ours in which indemnification would be required or permitted. We are not aware of any threatened litigation or proceeding which may result in a claim for such indemnification.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES
During the past three years, the Company sold securities as follows:
|
|
●
|
During the nine months ended December 31, 2018, the Company received notice from convertible note holders of the conversion of notes having a total of $4,470,000 face value and $170,971 in accrued interest. Accordingly, the Company has issued 18,563,885 shares of its common stock based on a $0.25 per share conversion price.
|
|
|
●
|
During the nine months ended December 31, 2018, the Company issued 3,885,412 shares in exchange for past and future consulting services and recorded a related expense of $0.8 million and recorded $0.3 million in prepaid expenses.
|
|
|
●
|
During the nine months ended December 31, 2018, in order to encourage the exercise of the 8,000,000 warrants issued to investors in the private offering of convertible notes dated March 2017 and the 28,804,000 warrants issued to investors in the private offering of convertible notes dated July 2017, the Company effected a temporary decrease in the exercise price of the warrants from $0.60 and $0.65, respectively, to $0.30 and $0.325 per share. As a result of the price reduction, the Company issued 12,332,750 shares of its common stock and received net proceeds of approximately $3.9 million.
|
|
|
●
|
On December 4, 2018, the Company entered into a Placement Agent’s Agreement to offer a total of 15,000,000 units at the price of $0.20 per unit up to a total of $3 million. Each unit consisted of one share of the Company’s common stock and one warrant to purchase one share of the Company’s common stock at the price of $0.60 for a period of five years. On January 15, 2019, the Placement Agent’s Agreement was amended to decrease the unit price from $0.20 per unit to $0.15 per unit and decrease the exercise price of the warrants included in each unit from $0.60 to $0.30, applied retroactively to funds raised prior to the date of the amendment, with no other changes to the agreement. Between December 4, 2018 and March 31, 2019, the Company received a total of $2,072,125 in proceeds from the private placement, net of $309,620 in brokerage fees paid to Network 1 Financial Securities, Inc., and issued 15,878,302 shares of its common stock and 15,878,302 warrants to purchase one share of its common stock at $0.30 per share.
|
|
|
●
|
During the nine months ended December 31, 2018, the Company issued 277,778 shares of its common stock to an investor for the cash purchase of shares at $0.36 per share.
|
|
|
●
|
In connection with a termination agreement with a note holder, the Company issued 500,000 shares on December 21, 2018, upon deferment of payment on the $0.5 million promissory note. The company recorded $95,000 in other expense related to those shares.
|
|
|
●
|
During 2019, the Company issued 71,878 shares of its common stock to a consultant for consulting services.
|
|
|
●
|
On May 28, 2019, the Company received notice from a noteholder of the conversion of a total of $170,000 of the principal balance of the note. Accordingly, the Company issued 1,000,000 shares of its common stock based on a $0.17 per share conversion price.
|
|
|
●
|
On August 1, 2019, the Company received notice from a noteholder of the conversion of a total of $110,000 of the principal balance of the note at $0.11 per share. Accordingly, the Company issued 1,000,000 shares of its common stock.
|
|
|
●
|
On October 30, 2019, the Company received notice of the conversion of a portion of a note in the conversion request amount of $75,000 at $0.06 per share and issued 1,250,000 shares of its common stock. On November 18, 2019, the Company received notice of the conversion of $50,000 of the same note balance at $0.0375 per share and issued 1,333,333 shares of its common stock.
|
|
|
●
|
On December 16, 2019, the Company received notice from a noteholder of the conversion of a total of $120,000 of the principal balance of the note at $0.04 per share and we issued 3,000,000 shares of common stock.
|
|
|
●
|
In order to encourage the exercise of approximately 70.5 million warrants issued to investors in private placements of convertible notes and common stock having exercise prices ranging between $0.65 and $0.30, the Company effected a temporary decrease in the exercise price of the warrants to $0.10 per share until July 11, 2019. On July 12, 2019, the Company extended the repricing of the warrants through August 30, 2019, and on July 31, 2019, the Company extended the repricing of the warrants to December 31, 2019. As a result of the price reduction, the Company received notice of the exercise of 9,449,750 warrants and received proceeds of $850,478, net of brokerage fees of $94,498 paid to Network 1 Financial Securities, Inc.
|
|
|
●
|
In order to encourage the further exercise of the warrants, the Company effected a temporary decrease in the exercise price of the warrants to $0.03-$.05 per share beginning in December 2019. As a result of the price reduction, the Company received notice of the exercise of an additional 8,113,250 warrants and received proceeds of $307,249, net of brokerage fees of $22,566 paid to Network 1 Financial Securities, Inc.
|
|
|
●
|
During the year ended March 31, 2020, the Company issued 2,100,000 shares of common stock for consulting services and recorded related expense of $214,000 based on the fair value of the stock on the date of the related consulting agreements.
|
|
|
●
|
During 2020, the Company issued 3,668,167 shares of its common stock and 3,668,167 warrants to purchase one share of its common stock at $0.30 per share in exchange for $478,696 in proceeds net of $71,529 in brokerage fees paid to Network 1 Financial Securities, Inc. The sale was in connection with the December 2018 Placement Agent’s Agreement. The Company also issued 1,954,613 compensation warrants to Network 1 Financial Securities, Inc. in connection with the private placement. The warrants are exercisable at $0.30 for a period of five years. The warrants were valued at $132,914 at the grant date using the Black-Scholes Model.
|
|
|
●
|
On October 10, 2019, the Company issued 4,000,000 shares of common stock and 2,000,000 warrants to purchase one share of common stock at $0.08 per share for a period of three years to an investor for $240,000 cash. The warrants were valued at $110,000 on the date of issuance using the Black-Scholes model.
|
|
|
●
|
During the year ended March 31, 2019, the Company received notice from convertible note holders of the conversion of a total of $4,470,000 in face value and $170,971 in accrued interest on the related convertible notes. Accordingly, the Company issued 18,563,885 shares of its common stock based on a $0.25 per share conversion price.
|
|
|
●
|
During the year ended March 31, 2019, in order to encourage the exercise of the 8,000,000 warrants issued to investors in the private offering of convertible notes dated March 2017 and the 28,804,000 warrants issued to investors in the private offering of convertible notes dated July 2017, the Company effected a temporary decrease in the exercise price of the warrants from $0.60 and $0.65, respectively, to $0.30 and $0.325 per share. As a result of the price reduction, the Company issued 12,332,750 shares of its common stock and received net proceeds of approximately $3.9 million.
|
|
|
●
|
During the year ended March 31, 2019, the Company issued 4,032,407 shares in exchange for past and future consulting services and recorded a related expense of $0.9 million and prepaid expense of $0.3 million. The shares and services were valued at the closing price of the Company’s common stock on the dates granted under the related consulting agreements.
|
|
|
●
|
During the year ended March 31, 2019, the Company issued 277,778 shares of its common stock to an investor for the cash purchase of shares at $0.36 per share.
|
|
|
●
|
On December 1, 2018, the Company entered into an agreement with a consultant for business advisory and consulting services. In consideration for the services, the Company issued warrants to purchase 8 million shares of the Company’s common stock at $0.1125 per share. The Company valued the warrants at $969,197 using the Black-Scholes valuation model.
|
|
|
●
|
In connection with the agreement with a business consultant, the Company issued 2 million restricted shares of the Company’s common stock on December 6, 2018. The Company also issued to the consultant warrants to purchase 2 million shares of the Company’s common stock at $0.1125 per share. The company recorded $81,333 in expense related to the warrants in its Consolidated Statement of Operations for the year ended March 31, 2019.
|
|
|
●
|
During the year ended March 31, 2021, the Company granted 3,500,000 immediately vesting options to purchase one share of the Company's Common Stock at the price of $0.05 per share for a period of ten years, as compensation to a scientist and researcher for drafting and filing U.S. and international patents. The options were valued at $168,000 using the Black-Scholes model.
|
|
|
●
|
During the year ended March 31, 2021, the Company issued a total of 788,000 warrants to convertible note holders with a term of three years and an exercise price of $0.10 per share in exchange for a three-year extension of notes having an aggregate principal balance of $197,000. Using the Black-Scholes model, the Company valued the warrants at $13,396.
|
|
|
●
|
On April 1, 2020, the Company entered into the Advisory Agreement with its brokers and effected a temporary decrease in the exercise price of the Company's outstanding warrants to $0.03-$.05 per share. As a result of the price reduction, the Company received notice of the exercise of 35,798,809 warrants during the year ended March 31, 2021, and received proceeds of $968,023, net of brokerage fees of $107,373 paid to Network 1 Financial Securities, Inc.
|
|
|
●
|
During the quarter ended March 31, 2021, the Company received notice of the conversion of $160,000 total principal balance of a note at $0.04 per share and issued 4,000,000 shares of common stock to the note holder.
|
The above mentioned securities were issued in reliance on the exemption from registration provided by Section 4(2) of the Securities Act of 1933 (the "Securities Act") and/or Rule 506 of Regulation D under the Securities Act, as amended.
ITEM 16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
No.
|
|
Description
|
|
3.1
|
|
Articles of Incorporation (Incorporated by reference to an exhibit to Form SB-2 No. 333-82580 filed with the Commission on February 12, 2002)
|
|
3.2
|
|
Amendment to Articles of Incorporation (Incorporated by reference to Exhibit 3.2 to Form S-1/A No. 333-82580 filed with the Commission on October 6, 2014 and Exhibit 3.2 to Form 10-K No. 333-82580 filed with the Commission on June 27, 2014)
|
|
3.3
|
|
Articles of Incorporation (Incorporated by reference to Exhibit 3.3 to the Annual Report on Form 10-K filed with the Commission on August 28, 2020)
|
|
3.4
|
|
Amendment to Articles of Incorporation (Incorporated by reference to Exhibit 3.3 to the Annual Report on Form 10-K filed with the Commission on August 28, 2020)
|
|
3.5
|
|
Bylaws (Incorporated by reference to an exhibit to Form SB-2 No. 333-82580 filed with the Commission on February 12, 2002
|
|
4.6
|
|
Description of Registrant's Securities (Incorporated by reference to Exhibit 4.6 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
5.1
|
|
Opinion of Gary R. Henrie, Attorney at Law regarding the legality of the common stock being registered.
|
|
10.1
|
|
2005 Restricted Stock Plan (Incorporated by reference to Annex A to Schedule 14A No. 333-82580 filed with the Commission on June 14, 2005)
|
|
10.2
|
|
2007 Restricted Stock Plan (Incorporated by reference to Exhibit 4.2 to Form S-8/POS No. 333-141467 filed with the Commission on February 8, 2008)
|
|
10.3
|
|
Amended Employment Agreement between Registrant and Andrea Small-Howard dated June 19, 2014 (Incorporated by reference to Exhibit 10.5 to Form 10-K No. 333-82580 filed with the Commission on June 27, 2014)
|
|
10.4
|
|
Employment Agreement between Registrant and John Poss dated August 10, 2015 (Incorporated by reference to Exhibit 10.1 to Form 10-Q No. 000-55462 filed with the Commission on November 18, 2015)
|
|
10.5
|
|
Operating Agreement of GB Sciences Nevada LLC (Incorporated by reference to Exhibit 10.4 to Form S-1/A No. 333-82580 filed with the Commission on October 6, 2014)
|
|
10.6
|
|
2014 Equity Incentive Plan (Incorporated by reference to Exhibit 10.6 to Form S-1/A No. 333-198967 filed with the Commission on December 23, 2014)
|
|
10.7
|
|
Amended Employment Agreement between Registrant and John Poss dated June 1, 2016 (Incorporated by reference to Exhibit 10.23 to Form 10-K No. 333-82580 filed with the Commission on July 14, 2016)
|
|
10.8
|
|
Amended Employment Agreement between Registrant and Andrea Small-Howard dated June 1, 2016 (Incorporated by reference to Exhibit 10.24 to Form 10-K No. 333-82580 filed with the Commission on July 14, 2016)
|
|
10.9
|
|
Audit Committee Charter (Incorporated by reference to Exhibit 10.25 to Form 10-K No. 333-82580 filed with the Commission on July 14, 2016)
|
|
10.10
|
|
Compensation Committee Charter (Incorporated by reference to Exhibit 10.26 to Form 10-K No. 333-82580 filed with the Commission on July 14, 2016)
|
|
10.11
|
|
Note Purchase Agreement between Registrant and CSW Ventures, LP dated February 28, 2019 (Incorporated by reference to Exhibit 10.29 to March 31, 2019 Form 10-K filed with the Commission on July 15, 2019)
|
|
10.12
|
|
8% Convertible Promissory Note between Registrant and CSW Ventures, LP dated February 28, 2019 (Incorporated by reference to Exhibit 10.29 to March 31, 2019 Form 10-K filed with the Commission on July 15, 2019)
|
|
10.13
|
|
Security Agreement between Registrant and CSW Ventures, LP dated February 28, 2019 (Incorporated by reference to Exhibit 10.29 to March 31, 2019 Form 10-K filed with the Commission on July 15, 2019)
|
|
10.14
|
|
Note Purchase Agreement between Registrant and Iliad Research and Trading, LP dated April 23, 2019 (Incorporated by reference to Exhibit 10.29 to March 31, 2019 Form 10-K filed with the Commission on July 15, 2019)
|
|
10.15
|
|
8% Convertible Promissory Note between Registrant and Iliad Research and Trading, LP dated April 23, 2019 (Incorporated by reference to Exhibit 10.29 to March 31, 2019 Form 10-K filed with the Commission on July 15, 2019)
|
|
10.16
|
|
Membership Interest Purchase Agreement between Registrant, Wellcana Plus, LLC, and GB Sciences Louisiana, LLC, dated November 15, 2019 (Incorporated by reference to Exhibit 10.1 to December 31, 2019 Form 10-Q filed with the Commission on February 18, 2020)
|
|
10.17
|
|
Membership Interest Purchase Agreement by and among Registrant, GB Sciences Nevada, LLC, GB Sciences Las Vegas, LLC, and AJE Management, LLC dated March 25, 2020 (Incorporated by reference to Exhibit 10.29 to the Annual Report on Form 10-K filed with the Commission on August 28, 2020)
|
|
10.18
|
|
Management Services Agreement by and among Registrant, GB Sciences Nevada, LLC, GB Sciences Las Vegas, LLC, and AJE Management, LLC dated December 6, 2019 (Incorporated by reference to Exhibit 10.30 to the Annual Report on Form 10-K filed with the Commission on August 28, 2020)
|
|
10.19
|
|
Amendment to Note Documents between Registrant and CSW Ventures, LP dated July 12, 2019 (Incorporated by reference to Exhibit 10.31 to the Annual Report on Form 10-K filed with the Commission on August 28, 2020)
|
|
10.20
|
|
Amended and Restated 8% Senior Secured Convertible Promissory Note payable to CSW Ventures, LP dated July 12, 2019 (Incorporated by reference to Exhibit 10.32 to the Annual Report on Form 10-K filed with the Commission on August 28, 2020)
|
|
10.21
|
|
Amendment to Promissory Note between Registrant and CSW Ventures, LP dated July 12, 2019 (Incorporated by reference to Exhibit 10.33 to the Annual Report on Form 10-K filed with the Commission on August 28, 2020)
|
|
10.22
|
|
Second Amendment to Note Documents between Registrant and CSW Ventures, LP dated November 27, 2019 (Incorporated by reference to Exhibit 10.34 to the Annual Report on Form 10-K filed with the Commission on August 28, 2020)
|
|
10.23
|
|
Second Amended and Restated 8% Senior Secured Convertible Promissory Note payable to CSW Ventures, LP dated November 27, 2019 (Incorporated by reference to Exhibit 10.35 to the Annual Report on Form 10-K filed with the Commission on August 28, 2020)
|
|
10.24
|
|
Loan Agreement between Registrant and AJE Management, LLC dated December 3, 2020 (Incorporated by reference to Exhibit 10.36 to the Annual Report on Form 10-K filed with the Commission on August 28, 2020)
|
|
10.25
|
|
8% Promissory Note payable to AJE Management, LLC (Incorporated by reference to Exhibit 10.37 to the Annual Report on Form 10-K filed with the Commission on August 28, 2020)
|
|
10.26
|
|
Promissory Note payable to John Davis dated November 15, 2019 (Incorporated by reference to Exhibit 10.38 to the Annual Report on Form 10-K filed with the Commission on August 28, 2020)
|
|
10.27
|
|
Judgment Settlement Agreement between Registrant, Iliad Research and Trading, L.P., and Wellcana Plus, LLC, dated November 20, 2020 (Incorporated by reference to Exhibit 10.1 to December 31, 2020 Form 10-Q filed with the Commission on February 16, 2021)
|
|
10.28
|
|
Amendment to Membership Interest Purchase Agreement between Registrant, AJE Management, LLC, GB Sciences Nevada, LLC, and GB Sciences Las Vegas, LLC dated May 11, 2020 (Incorporated by reference to Exhibit 10.28 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
10.29
|
|
Second Amendment to Membership Interest Purchase Agreement between Registrant, AJE Management, LLC, GB Sciences Nevada, LLC, and GB Sciences Las Vegas, LLC dated July 24, 2020 (Incorporated by reference to Exhibit 10.29 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
10.30
|
|
Third Amendment to Membership Interest Purchase Agreement between Registrant, AJE Management, LLC, GB Sciences Nevada, LLC, and GB Sciences Las Vegas, LLC dated May 11, 2020 (Incorporated by reference to Exhibit 10.30 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
10.31
|
|
8% Promissory Note Payable to AJE Management, LLC, dated July 24, 2020 (Incorporated by reference to Exhibit 10.31 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
10.32
|
|
Security Agreement to AJE Management, LLC, dated July 24, 2020 (Incorporated by reference to Exhibit 10.32 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
10.33
|
|
Omnibus Amendment between Registrant, Wellcana Plus, LLC, and GB Sciences Louisiana, LLC dated December 15, 2020 (Incorporated by reference to Exhibit 10.33 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
10.34
|
|
Amendment to Membership Interest Purchase Agreement between Registrant, Wellcana Plus, LLC, and GB Sciences Louisiana, LLC, dated December 15, 2020 (Incorporated by reference to Exhibit 10.34 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
10.35
|
|
Judgment Settlement Agreement between Registrant and Iliad Research and Trading, L.P. dated November 20, 2020(Incorporated by reference to Exhibit 10.35 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
10.36
|
|
Membership Interest Purchase Agreement between Registrant, 483 Management, LLC, and GB Sciences Nopah, LLC dated August 10, 2020 (Incorporated by reference to Exhibit 10.36 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
10.37
|
|
Management Services Agreement between Registrant, 483 Management, LLC, and GB Sciences Nopah, LLC dated August 10, 2020 (Incorporated by reference to Exhibit 10.37 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
10.38
|
|
Promissory Note Modification Agreement between Registrant and 483 Management, LLC dated August 10, 2020 (Incorporated by reference to Exhibit 10.38 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
10.39
|
|
Indemnification Agreement between Registrant and Edmond A. DeFrank dated November 16, 2020(Incorporated by reference to Exhibit 10.39 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
10.40
|
|
Indemnification Agreement between Registrant and Leslie Bocskor dated November 16, 2020 (Incorporated by reference to Exhibit 10.40 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
10.41
|
|
Indemnification Agreement between Registrant and John Poss dated November 16, 2020 (Incorporated by reference to Exhibit 10.41 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
10.42
|
|
Indemnification Agreement between Registrant and Andrea Small-Howard dated November 16, 2020 (Incorporated by reference to Exhibit 10.42 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
|
10.43
|
|
Indemnification Agreement between Registrant and Zach Swarts dated November 16, 2020 (Incorporated by reference to Exhibit 10.43 to the Annual Report on Form 10-K filed with the Commission on July 6, 2021)
|
(b) Financial Statement Schedules
See the Index to Financial Statements included on page 55 for a list of the financial statements included in this registration statement.
ITEM 17. UNDERTAKINGS
The undersigned registrant hereby undertakes:
(a) The undersigned Registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post- effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement;
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
Provided, however, that paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the SEC by the registrant pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement.
(2) That for the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and this offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned Registrant undertakes that in a primary offering of securities of the undersigned Registrant pursuant to this registration statement, regardless of the method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned Registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned Registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned Registrant or used or referred to by the undersigned Registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned Registrant or its securities provided by or on behalf of the undersigned Registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned Registrant to the purchaser.
(5) That for purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4), or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(b) The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the provisions described in Item 6 hereof, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Las Vegas, State of Nevada, on September 17, 2021.
|
|
|
GB SCIENCES, INC.
|
|
|
By:
|
/s/ John Poss
|
|
|
|
John Poss
|
|
|
|
Chief Executive Officer
|
|
|
|
|
POWER OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints John Poss his true and lawful attorney-in-fact and agent with full power of substitution and re-substitution, for him or her and in his or her name, place, and stead, in any and all capacities, to sign any and all (1) amendments (including post-effective amendments) and additions to this Registration Statement and (2) Registration Statements, and any and all amendments thereto (including post-effective amendments), relating to the offering contemplated pursuant to Rule 462(b) under the Securities Act of 1933, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or his or her substitute or substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed below by the following persons in the capacities and on the dates indicated.
/s/ John Poss
John Poss, CEO and Director
September 17, 2021
/s/ Andrea Small-Howard
Andrea Small-Howard, President and Director
September 17, 2021
/s/ Ed DeFrank
Ed DeFrank, Director
September 17, 2021
/s/ Zach Swarts
Zach Swarts, CFO and Chief Accounting Officer
September 17, 2021
GB Sciences (PK) (USOTC:GBLX)
Historical Stock Chart
From Mar 2024 to Apr 2024
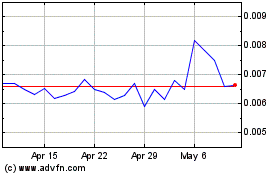
GB Sciences (PK) (USOTC:GBLX)
Historical Stock Chart
From Apr 2023 to Apr 2024