false 0001609809 0001609809 2024-01-09 2024-01-09 0001609809 dei:FormerAddressMember 2024-01-09 2024-01-09
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 9, 2024
SERES THERAPEUTICS, INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-37465 |
|
27-4326290 |
(State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
101 Cambridgepark Drive Cambridge, MA |
|
02140 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (617) 945-9626
200 Sidney Street - 4th Floor, Cambridge, MA 02139
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
| Common stock, par value $0.001 per share |
|
MCRB |
|
The Nasdaq Stock Market LLC (Nasdaq Global Select Market) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01. Regulation FD Disclosure.
On January 9, 2024, Seres Therapeutics, Inc. (the “Company”) posted an updated corporate presentation in the “Investors and News” portion of its website at www.serestherapeutics.com. A copy of the slide presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K and incorporated herein by reference.
The information in Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.1 attached hereto, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1.
| Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits.
The following Exhibit 99.1 relates to Item 7.01 and shall be deemed to be furnished, and not filed:
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
| Date: January 9, 2024 |
|
SERES THERAPEUTICS, INC. |
|
|
|
|
|
|
|
|
By: |
|
/s/ Thomas J. DesRosier |
|
|
|
|
Name: |
|
Thomas J. DesRosier |
|
|
|
|
Title: |
|
Chief Legal Officer and Executive Vice President |

January 10, 2024 Seres
Therapeutics Investor Presentation Exhibit 99.1

Some of the statements in this
presentation constitute “forward looking statements” under the Private Securities Litigation Reform Act of 1995, including, but not limited to the anticipated supply and degree of market acceptance of VOWST; the potential for microbiome
therapeutics to protect against infection; the timing and outcome of clinical development; our development opportunities and plans; the ultimate safety and efficacy data for our products; access to additional debt tranches; the availability of
cash to fund operations, and other statements which are not historical fact. Such statements are subject to important factors, risks and uncertainties, such as those discussed under the caption "Risk Factors" in the Company’s Quarterly Report
on Form 10-Q filed on November 2, 2023, and its other filings with the Securities and Exchange Commission ("SEC"), that may cause actual results to differ materially from those expressed or implied by such forward looking statements. Any
forward-looking statements included herein represent our views as of today only. We may update these statements, but we disclaim any obligation to do so. Forward Looking Statements | Seres Therapeutics, Inc. © 2024

January Updates Drove significant
broad demand with 2,015 new patient starts since commercial launch in June SER-155 SER-155 received US FDA Fast Track designation; Phase 1b clinical data expected Q3 2024 | Seres Therapeutics, Inc. © 2024
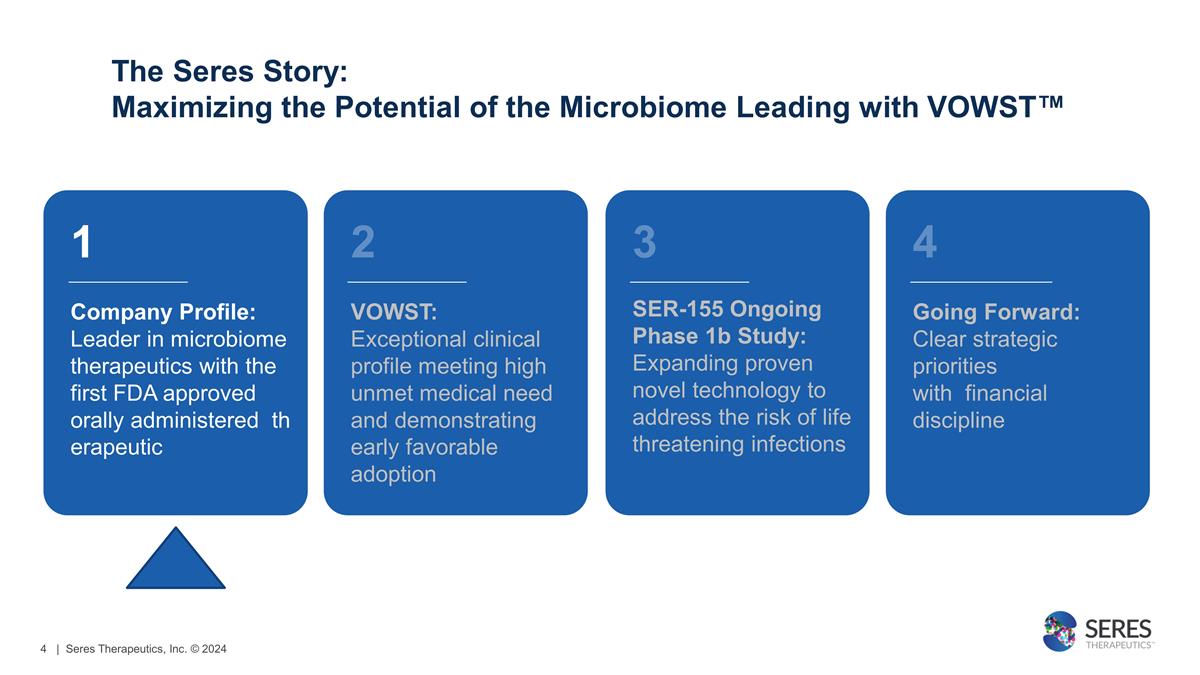
The Seres Story: Maximizing the
Potential of the Microbiome Leading with VOWST™ 1 Company Profile: Leader in microbiome therapeutics with the first FDA approved orally administered therapeutic VOWST: Exceptional clinical profile meeting high
unmet medical need and demonstrating early favorable adoption 3 SER-155 Ongoing Phase 1b Study: Expanding proven novel technology to address the risk of life threatening infections 4 Going Forward: Clear strategic priorities with
financial discipline 2 | Seres Therapeutics, Inc. © 2024
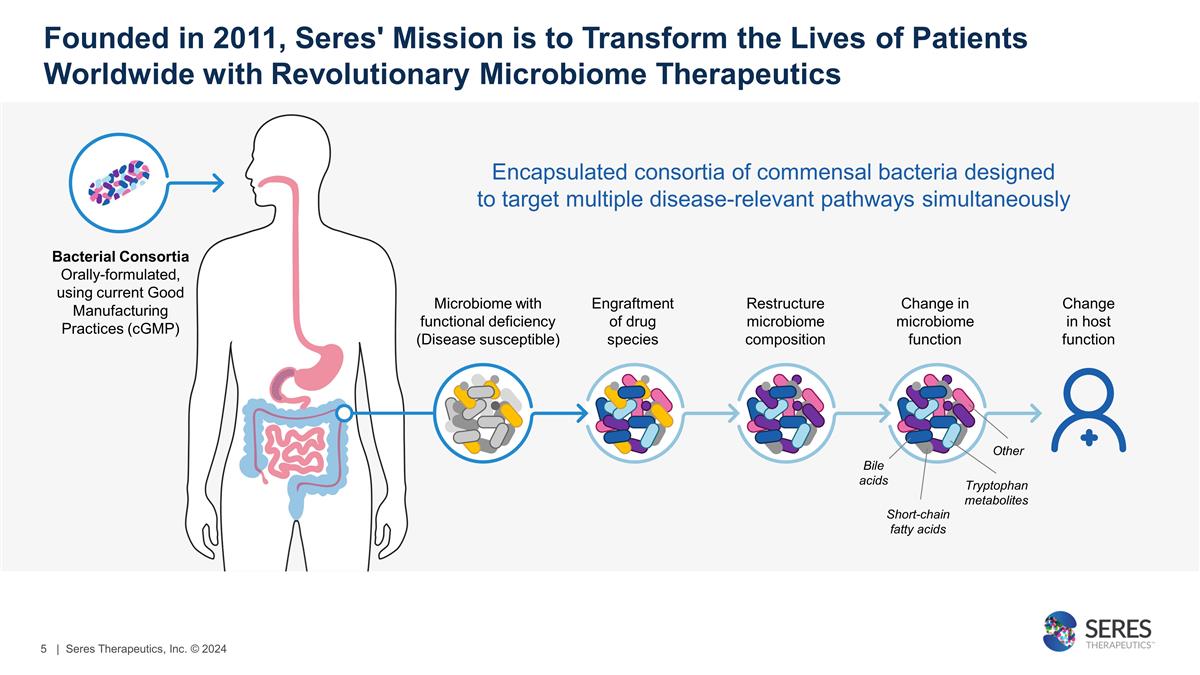
Founded in 2011, Seres' Mission is to
Transform the Lives of Patients Worldwide with Revolutionary Microbiome Therapeutics Encapsulated consortia of commensal bacteria designed to target multiple disease-relevant pathways simultaneously Bacterial Consortia Orally-formulated, using
current Good Manufacturing Practices (cGMP) Microbiome with functional deficiency (Disease susceptible) Engraftment of drug species Restructure microbiome composition Change in microbiome function Change in host function Bile acids Short-chain fatty
acids Tryptophan metabolites Other | Seres Therapeutics, Inc. © 2024

In April 2023,
VOWST™ Became the First FDA Approved Orally Administered Microbiota-Based Therapeutic VOWSTTM is indicated to prevent the recurrence of C. difficile infection (CDI) in individuals 18 years of age or older following antibacterial treatment
for recurrent CDI (rCDI). | Seres Therapeutics, Inc. © 2024
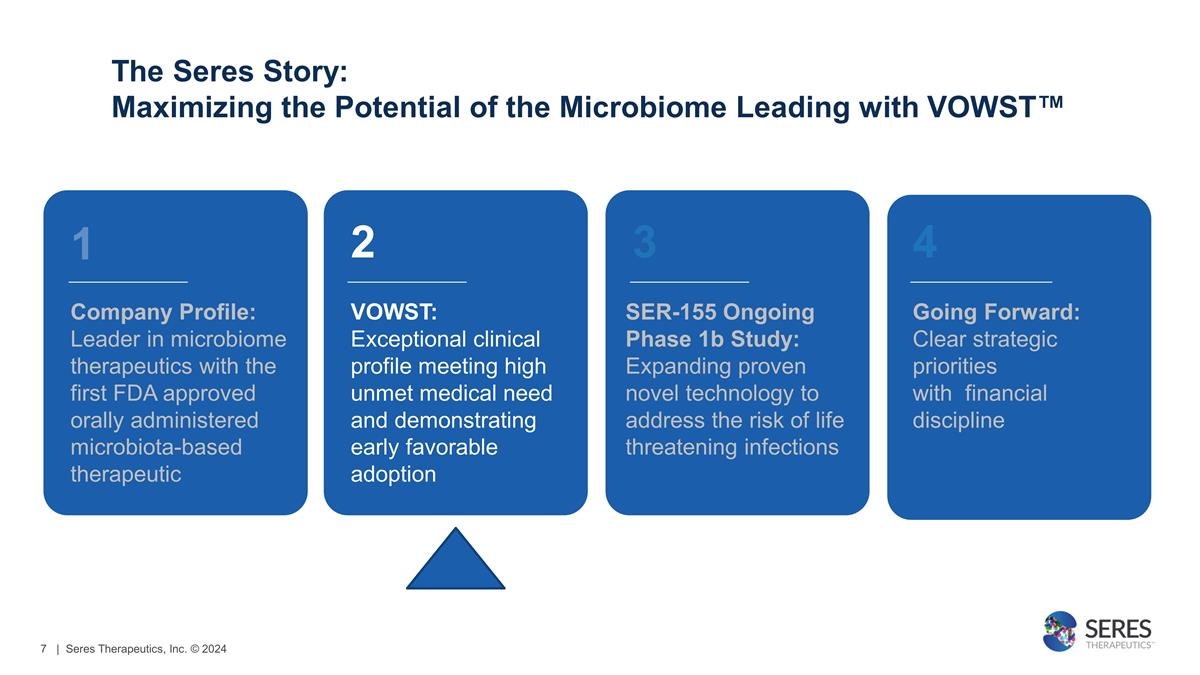
Company Profile: Leader in
microbiome therapeutics with the first FDA approved orally administered microbiota-based therapeutic 2 VOWST: Exceptional clinical profile meeting high unmet medical need and demonstrating early favorable adoption 3 SER-155 Ongoing Phase
1b Study: Expanding proven novel technology to address the risk of life threatening infections 4 Going Forward: Clear strategic priorities with financial discipline 1 The Seres Story: Maximizing the Potential of the Microbiome Leading
with VOWST™ | Seres Therapeutics, Inc. © 2024
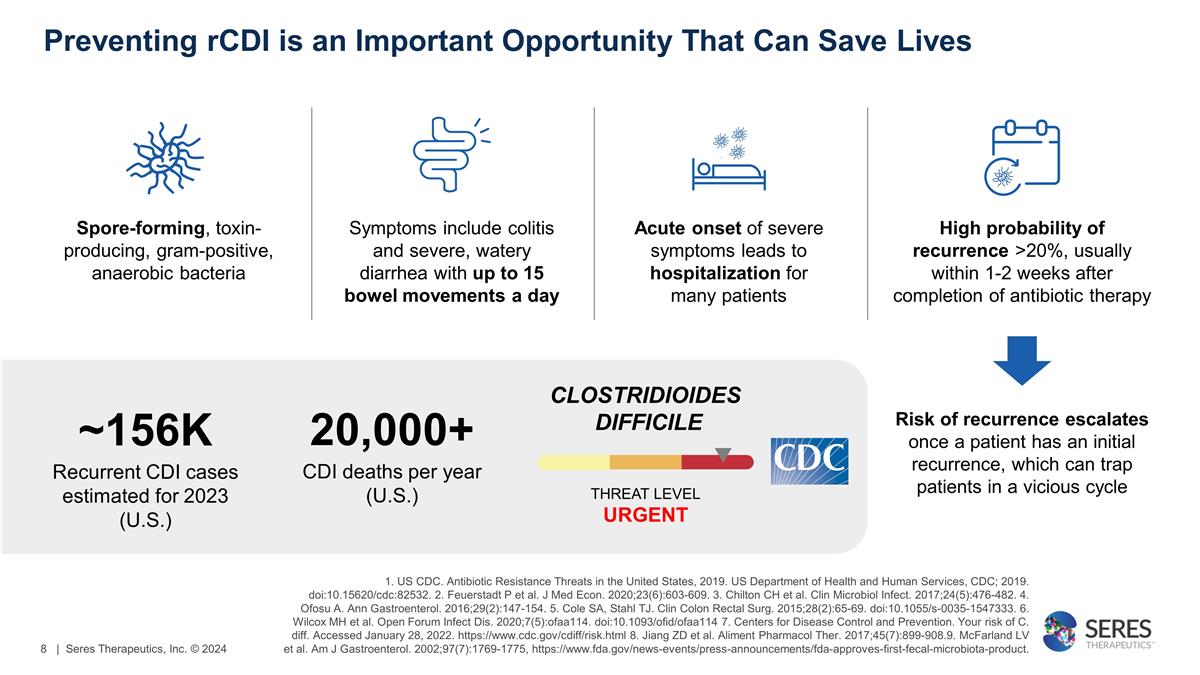
Preventing rCDI is an Important
Opportunity That Can Save Lives High probability of recurrence >20%, usually within 1-2 weeks after completion of antibiotic therapy Symptoms include colitis and severe, watery diarrhea with up to 15 bowel movements a day Spore-forming,
toxin-producing, gram-positive, anaerobic bacteria Acute onset of severe symptoms leads to hospitalization for many patients Risk of recurrence escalates once a patient has an initial recurrence, which can trap patients in a vicious cycle 20,000+
CDI deaths per year (U.S.) CLOSTRIDIOIDES DIFFICILE THREAT LEVEL URGENT ~156K Recurrent CDI cases estimated for 2023 (U.S.) 1. US CDC. Antibiotic Resistance Threats in the United States, 2019. US Department of Health and Human Services, CDC; 2019.
doi:10.15620/cdc:82532. 2. Feuerstadt P et al. J Med Econ. 2020;23(6):603-609. 3. Chilton CH et al. Clin Microbiol Infect. 2017;24(5):476-482. 4. Ofosu A. Ann Gastroenterol. 2016;29(2):147-154. 5. Cole SA, Stahl TJ. Clin Colon Rectal Surg.
2015;28(2):65-69. doi:10.1055/s-0035-1547333. 6. Wilcox MH et al. Open Forum Infect Dis. 2020;7(5):ofaa114. doi:10.1093/ofid/ofaa114 7. Centers for Disease Control and Prevention. Your risk of C. diff. Accessed January 28, 2022.
https://www.cdc.gov/cdiff/risk.html 8. Jiang ZD et al. Aliment Pharmacol Ther. 2017;45(7):899-908.9. McFarland LV et al. Am J Gastroenterol. 2002;97(7):1769-1775,
https://www.fda.gov/news-events/press-announcements/fda-approves-first-fecal-microbiota-product. | Seres Therapeutics, Inc. © 2024
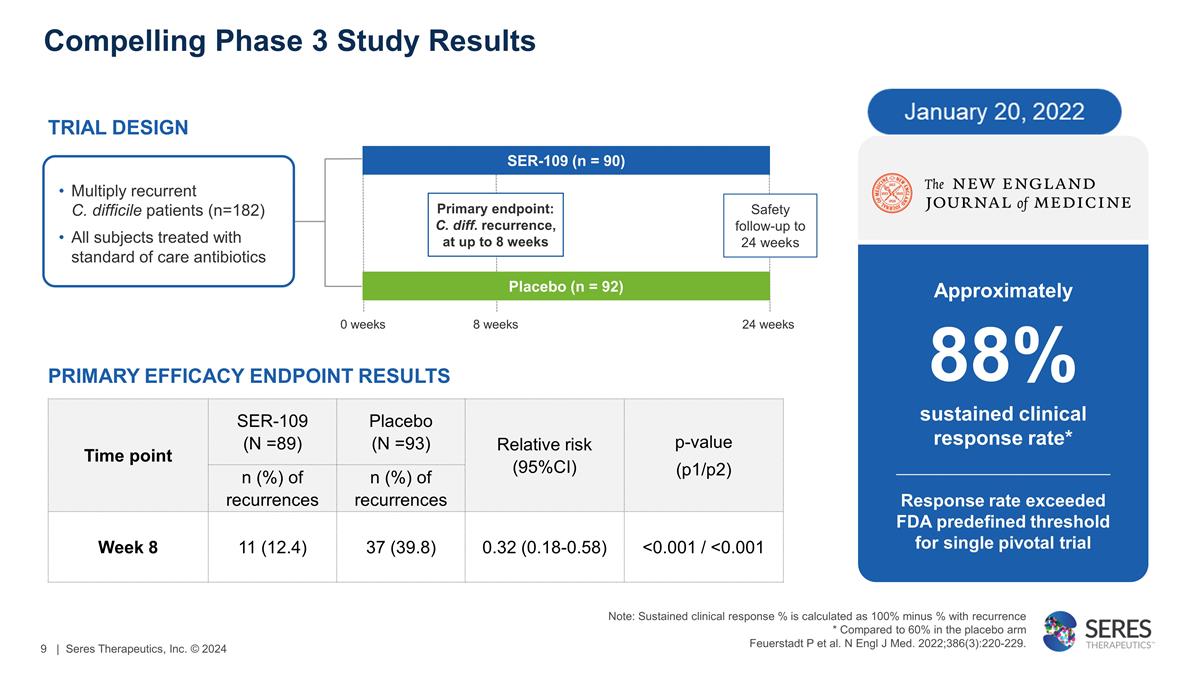
Compelling Phase 3 Study Results Time
point SER-109 (N =89) Placebo (N =93) Relative risk (95%CI) p-value (p1/p2) n (%) of recurrences n (%) of recurrences Week 8 11 (12.4) 37 (39.8) 0.32 (0.18-0.58) <0.001 / <0.001 TRIAL DESIGN Multiply recurrent C. difficile patients (n=182) All
subjects treated with standard of care antibiotics 0 weeks 8 weeks 24 weeks PRIMARY EFFICACY ENDPOINT RESULTS Approximately 88% sustained clinical response rate* Response rate exceeded FDA predefined threshold for single pivotal trial Placebo (n =
92) Primary endpoint: C. diff. recurrence, at up to 8 weeks Safety follow-up to 24 weeks SER-109 (n = 90) Note: Sustained clinical response % is calculated as 100% minus % with recurrence * Compared to 60% in the placebo arm Feuerstadt P et al. N
Engl J Med. 2022;386(3):220-229. | Seres Therapeutics, Inc. © 2024

VOWST Offers a Highly Attractive
Product Profile Well-tolerated in Phase 3 clinical studies. The most common adverse reactions (reported in ≥5% of participants) were abdominal distension (31.1%), fatigue (22.2%), constipation (14.4%), chills (11.1%), and diarrhea (10.0%) Oral
dosing - 4 capsules once daily for 3 consecutive days following antibiotic treatment and laxative No refrigeration requirements Approximately 88% recurrence-free at 8 weeks* Broad product label covering adults with rCDI, including at first
recurrence ✔ ✔ ✔ ✔ ✔ Highlights of Prescribing Information Indication statement VOWST is indicated to prevent the recurrence of Clostridioides difficile infection (CDI) in individuals 18 years of age and older
following antibiotic treatment for recurrent CDI (rCDI) Limitations of use VOWST is not indicated for the treatment of CDI Full prescribing information available at vowst.com | Seres Therapeutics, Inc. © 2024
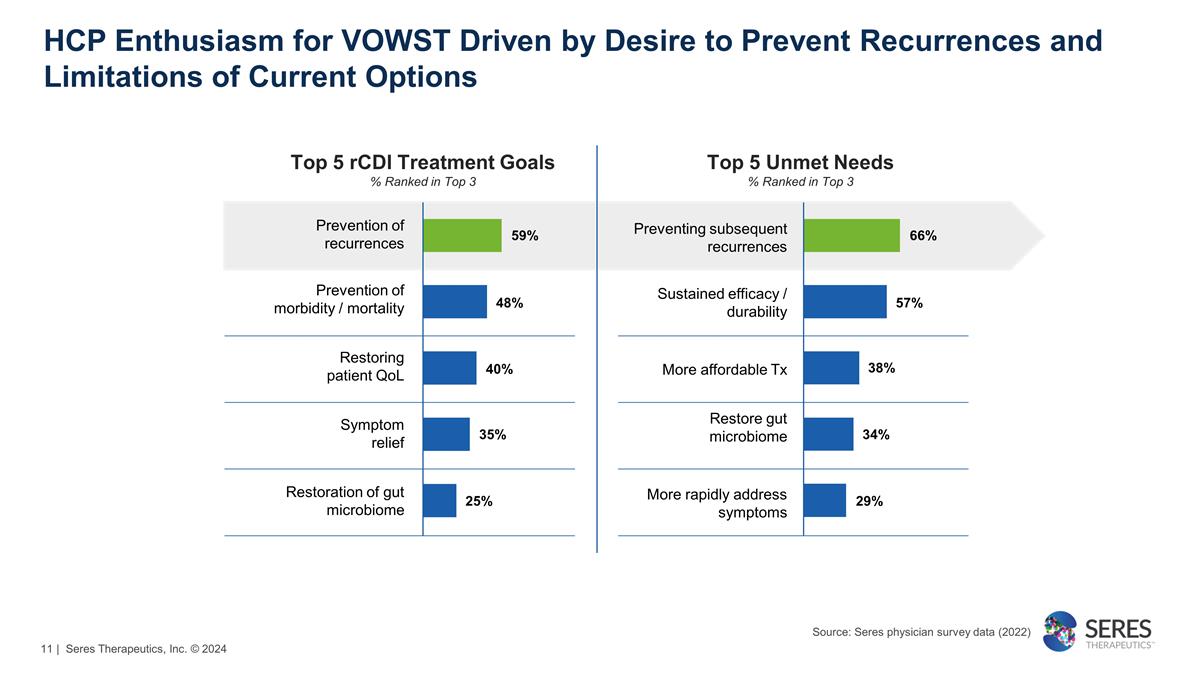
HCP Enthusiasm for VOWST Driven by
Desire to Prevent Recurrences and Limitations of Current Options Top 5 rCDI Treatment Goals % Ranked in Top 3 Prevention of recurrences Prevention of morbidity / mortality Restoring patient QoL Symptom relief Restoration of gut microbiome Preventing
subsequent recurrences Sustained efficacy / durability More affordable Tx Restore gut microbiome More rapidly address symptoms Top 5 Unmet Needs % Ranked in Top 3 59% 48% 40% 35% 25% 66% 57% 38% 34% 29% Source: Seres physician survey data (2022) |
Seres Therapeutics, Inc. © 2024
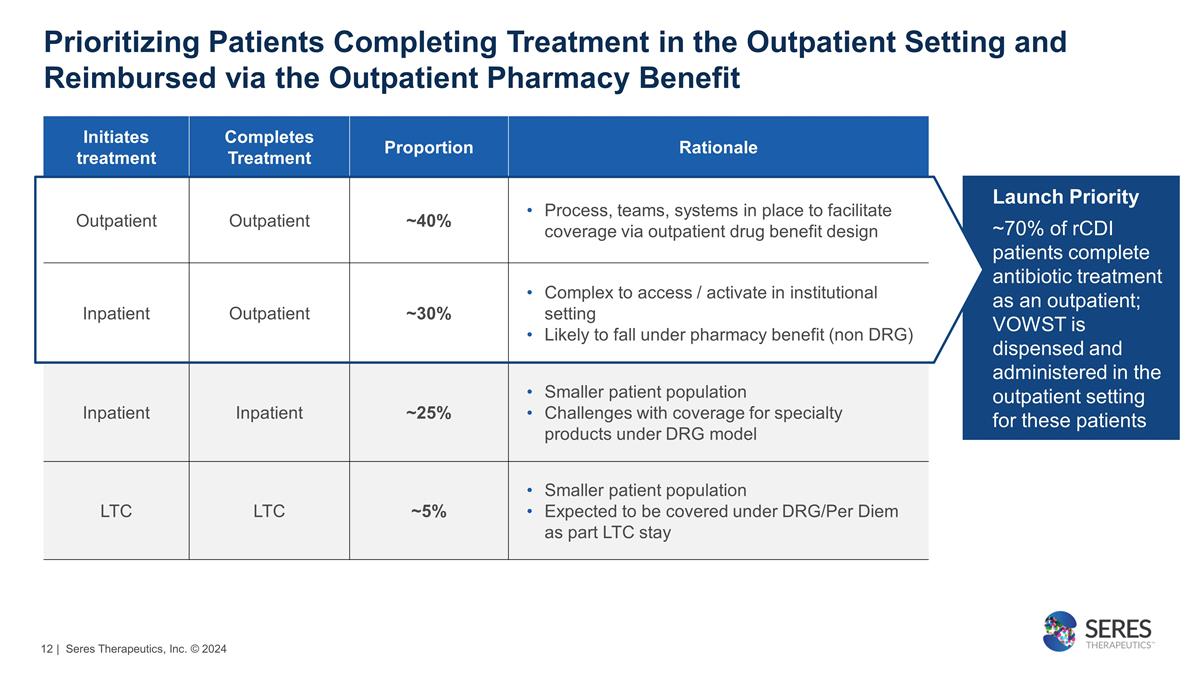
Launch Priority ~70% of rCDI
patients complete antibiotic treatment as an outpatient; VOWST is dispensed and administered in the outpatient setting for these patients Prioritizing Patients Completing Treatment in the Outpatient Setting and Reimbursed via the Outpatient Pharmacy
Benefit Initiates treatment Completes Treatment Proportion Rationale Outpatient Outpatient ~40% Process, teams, systems in place to facilitate coverage via outpatient drug benefit design Inpatient Outpatient ~30% Complex to access / activate in
institutional setting Likely to fall under pharmacy benefit (non DRG) Inpatient Inpatient ~25% Smaller patient population Challenges with coverage for specialty products under DRG model LTC LTC ~5% Smaller patient population Expected to be covered
under DRG/Per Diem as part LTC stay | Seres Therapeutics, Inc. © 2024
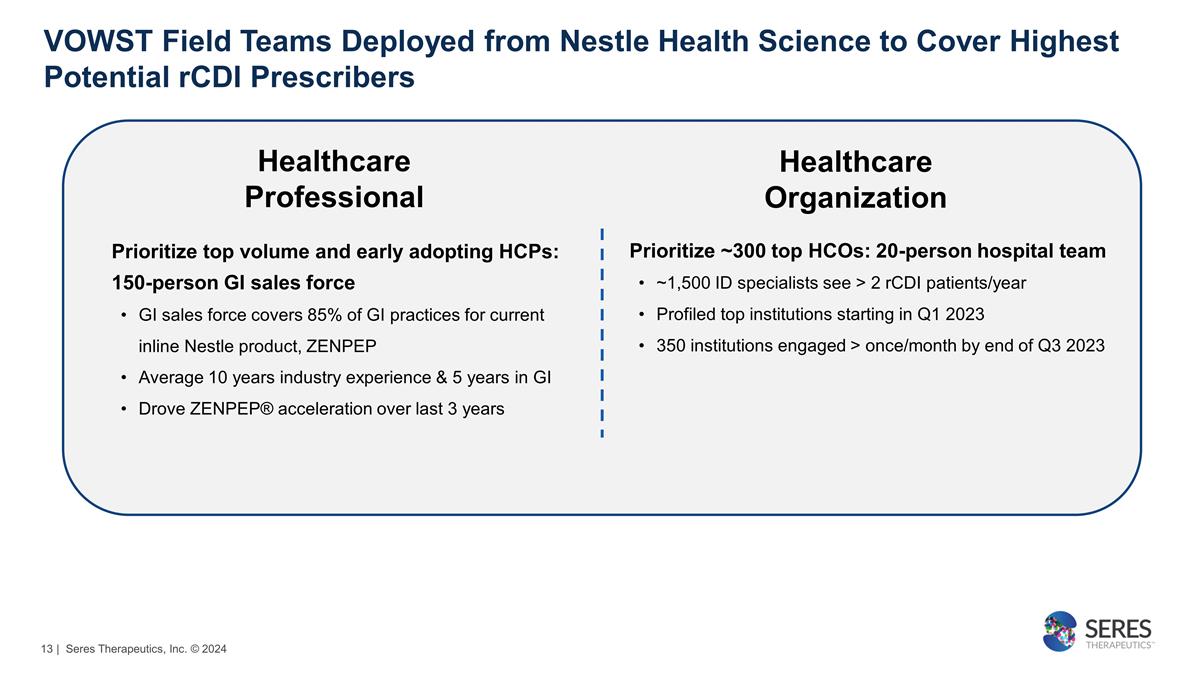
Prioritize top volume and early
adopting HCPs: 150-person GI sales force GI sales force covers 85% of GI practices for current inline Nestle product, ZENPEP Average 10 years industry experience & 5 years in GI Drove ZENPEP® acceleration over last 3 years Prioritize ~300
top HCOs: 20-person hospital team ~1,500 ID specialists see > 2 rCDI patients/year Profiled top institutions starting in Q1 2023 350 institutions engaged > once/month by end of Q3 2023 VOWST Field Teams Deployed from Nestle Health Science
to Cover Highest Potential rCDI Prescribers Healthcare Professional Healthcare Organization | Seres Therapeutics, Inc. © 2024
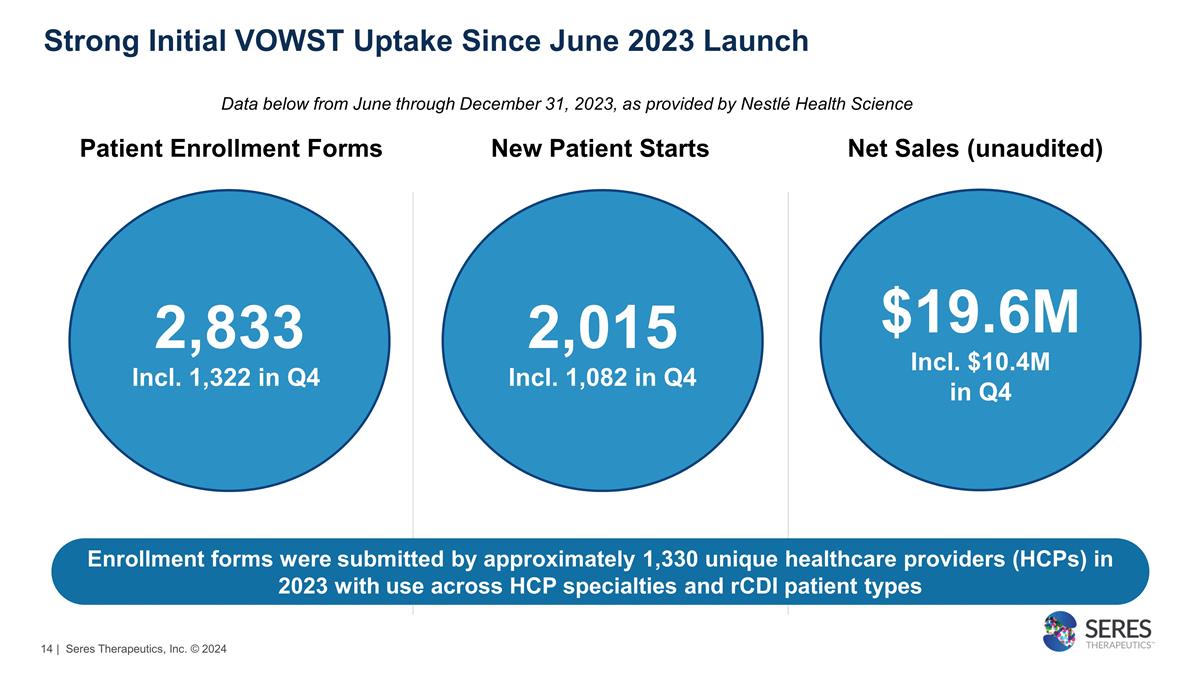
Strong Initial VOWST Uptake
Since June 2023 Launch Data below from June through December 31, 2023, as provided by Nestlé Health Science Patient Enrollment Forms New Patient Starts Net Sales (unaudited) 2,833 Incl. 1,322 in Q4 2,015 Incl. 1,082 in Q4 $19.6M Incl.
$10.4M in Q4 Enrollment forms were submitted by approximately 1,330 unique healthcare providers (HCPs) in 2023 with use across HCP specialties and rCDI patient types | Seres Therapeutics, Inc. © 2024
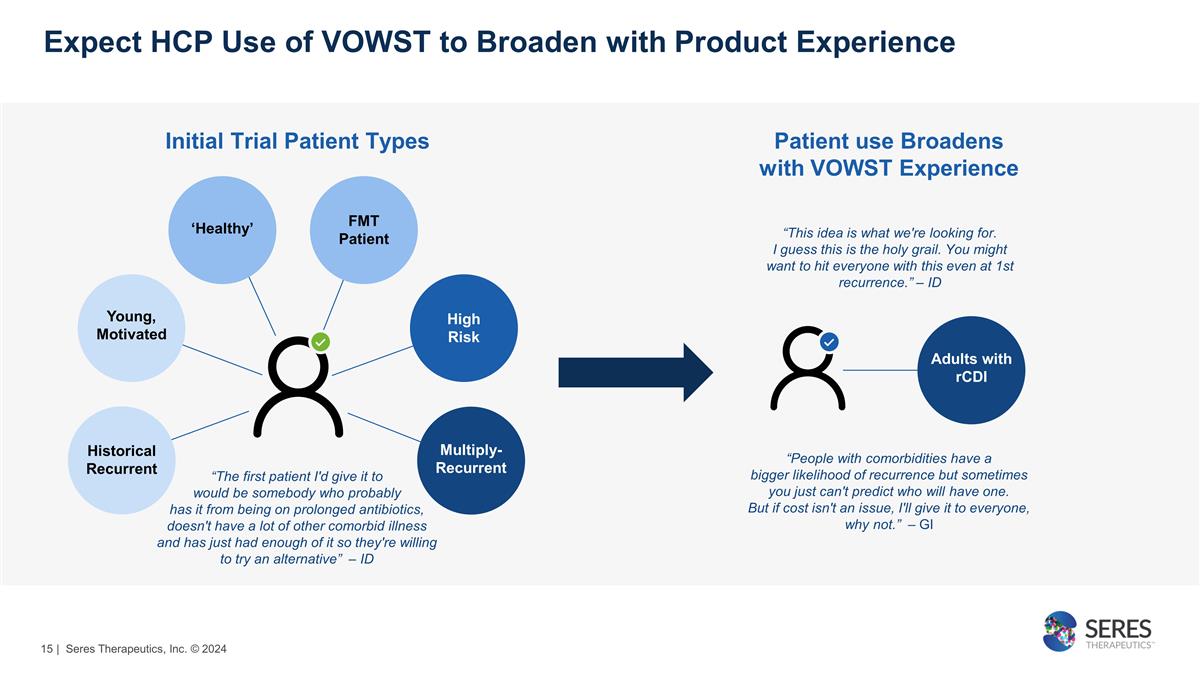
Expect HCP Use of VOWST to Broaden
with Product Experience Initial Trial Patient Types “People with comorbidities have a bigger likelihood of recurrence but sometimes you just can't predict who will have one. But if cost isn't an issue, I'll give it to everyone, why not.”
– GI “The first patient I'd give it to would be somebody who probably has it from being on prolonged antibiotics, doesn't have a lot of other comorbid illness and has just had enough of it so they're willing to try an alternative”
– ID Multiply-Recurrent Historical Recurrent Young, Motivated ‘Healthy’ FMT Patient High Risk Patient use Broadens with VOWST Experience Adults with rCDI “This idea is what we're looking for. I guess this is the holy grail.
You might want to hit everyone with this even at 1st recurrence.” – ID | Seres Therapeutics, Inc. © 2024
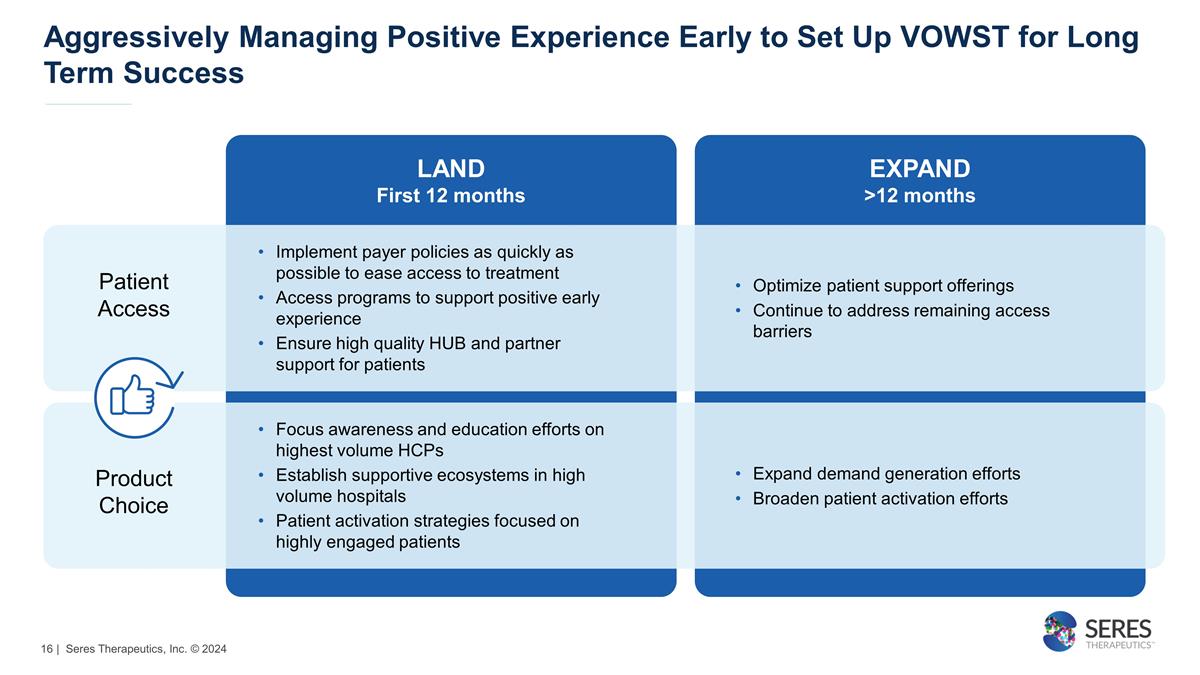
Aggressively Managing Positive
Experience Early to Set Up VOWST for Long Term Success Patient Access Product Choice LAND First 12 months EXPAND >12 months Implement payer policies as quickly as possible to ease access to treatment Access programs to support positive early
experience Ensure high quality HUB and partner support for patients Optimize patient support offerings Continue to address remaining access barriers Focus awareness and education efforts on highest volume HCPs Establish supportive ecosystems
in high volume hospitals Patient activation strategies focused on highly engaged patients Expand demand generation efforts Broaden patient activation efforts | Seres Therapeutics, Inc. © 2024
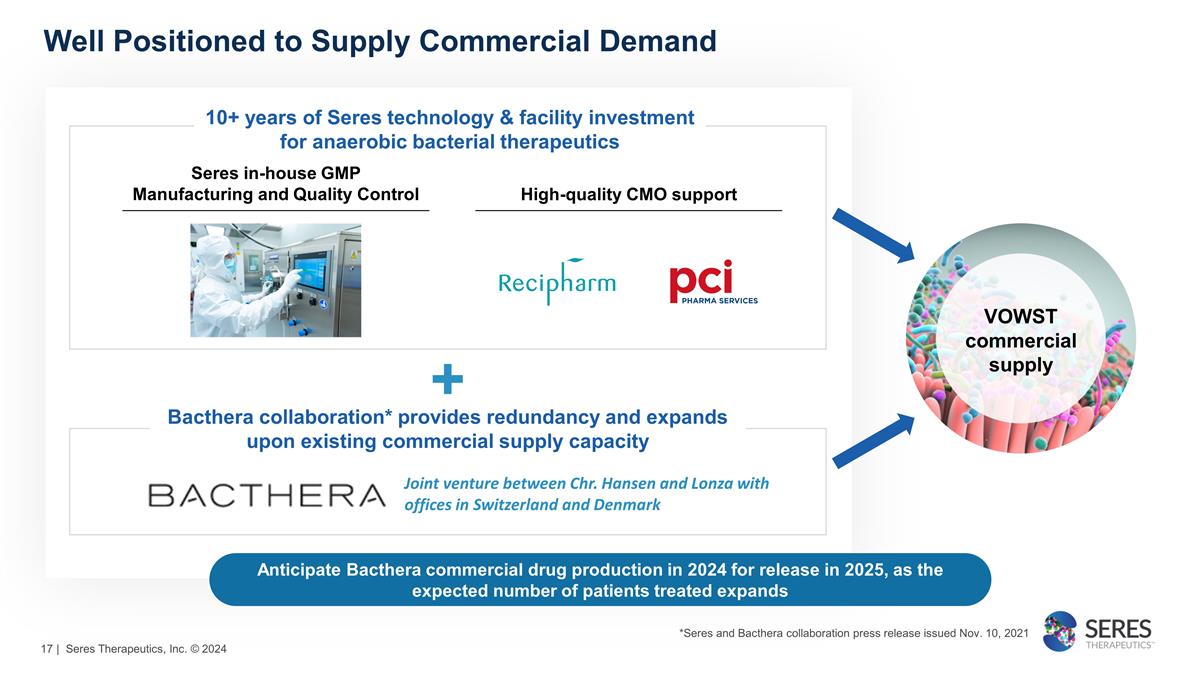
Well Positioned to
Supply Commercial Demand VOWST commercial supply *Seres and Bacthera collaboration press release issued Nov. 10, 2021 + 10+ years of Seres technology & facility investment for anaerobic bacterial therapeutics Joint venture between Chr.
Hansen and Lonza with offices in Switzerland and Denmark Bacthera collaboration* provides redundancy and expands upon existing commercial supply capacity Anticipate Bacthera commercial drug production in 2024 for release in 2025, as the expected
number of patients treated expands Seres in-house GMP Manufacturing and Quality Control High-quality CMO support | Seres Therapeutics, Inc. © 2024
Company Profile: Leader in
microbiome therapeutics with the first FDA approved orally administered microbiota-based therapeutic VOWST: Exceptional clinical profile meeting high unmet medical need and demonstrating early favorable adoption SER-155 Ongoing Phase 1b
Study: Expanding proven novel technology to address the risk of life threatening infections Going Forward: Clear strategic priorities with financial discipline 3 4 2 1 The Seres Story: Maximizing the Potential of the Microbiome Leading
with VOWST™ | Seres Therapeutics, Inc. © 2024
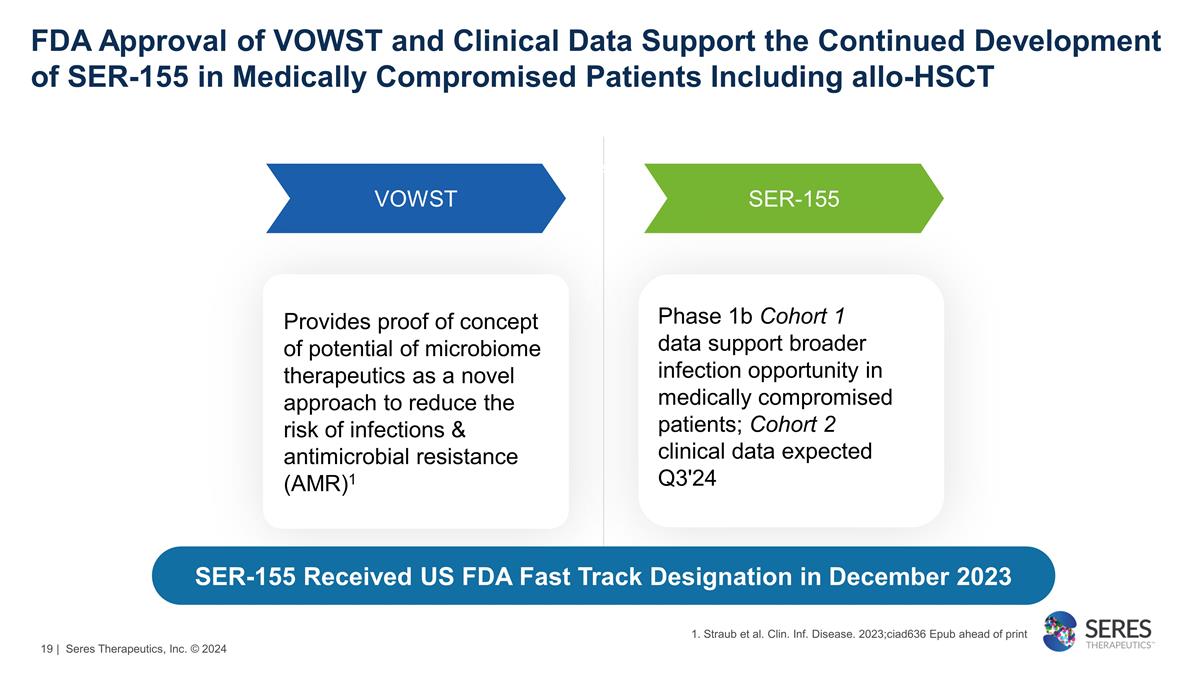
FDA Approval of VOWST and Clinical
Data Support the Continued Development of SER-155 in Medically Compromised Patients Including allo-HSCT VOWST SER-155 Phase 1b Cohort 1 data support broader infection opportunity in medically compromised
patients; Cohort 2 clinical data expected Q3'24 Provides proof of concept of potential of microbiome therapeutics as a novel approach to reduce the risk of infections & antimicrobial resistance (AMR)1 We are here
SER-155 Received US FDA Fast Track Designation in December 2023 1. Straub et al. Clin. Inf. Disease. 2023;ciad636 Epub ahead of print | Seres Therapeutics, Inc. © 2024
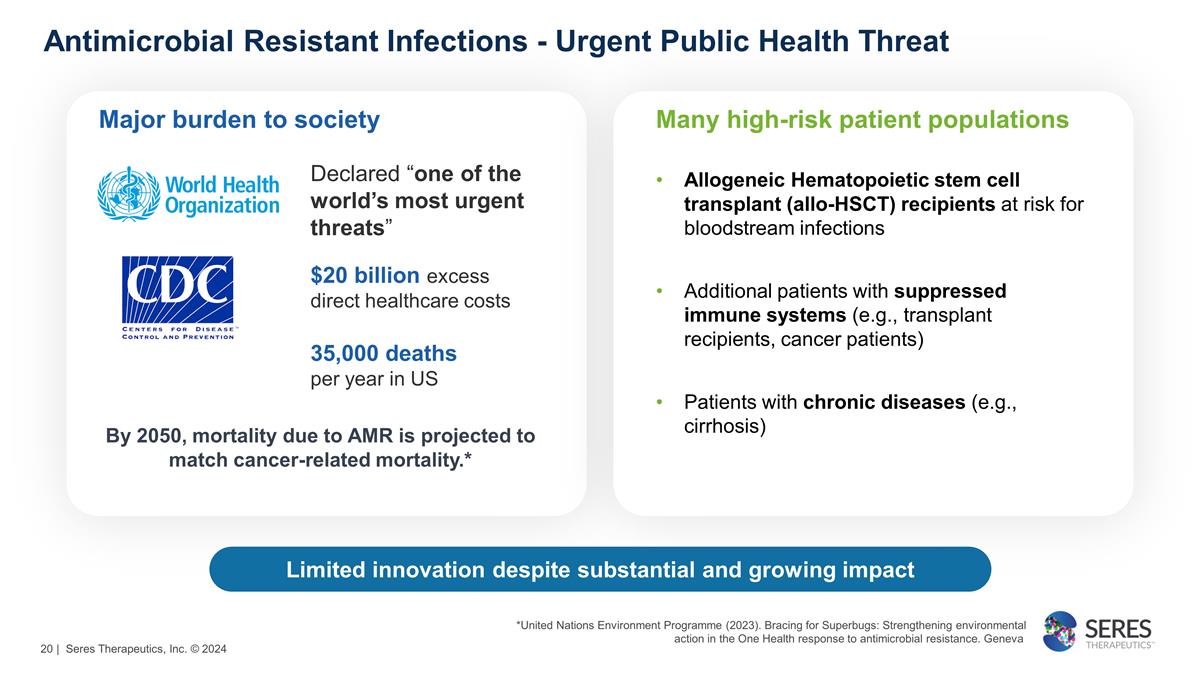
Antimicrobial Resistant Infections
- Urgent Public Health Threat Limited innovation despite substantial and growing impact Declared “one of the world’s most urgent threats” $20 billion excess direct healthcare costs 35,000 deaths per year in US Major burden to
society Many high-risk patient populations Allogeneic Hematopoietic stem cell transplant (allo-HSCT) recipients at risk for bloodstream infections Additional patients with suppressed immune systems (e.g., transplant recipients, cancer patients)
Patients with chronic diseases (e.g., cirrhosis) By 2050, mortality due to AMR is projected to match cancer-related mortality.* *United Nations Environment Programme (2023). Bracing for Superbugs: Strengthening environmental action in the One
Health response to antimicrobial resistance. Geneva | Seres Therapeutics, Inc. © 2024
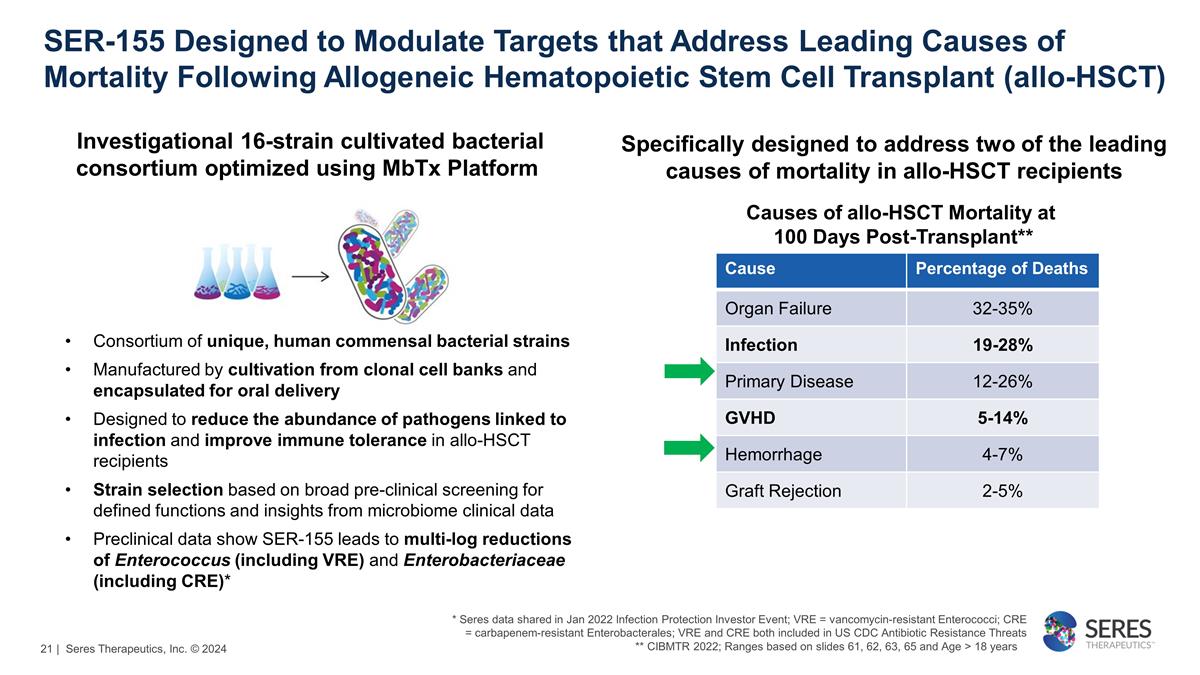
SER-155 Designed to Modulate
Targets that Address Leading Causes of Mortality Following Allogeneic Hematopoietic Stem Cell Transplant (allo-HSCT) Consortium of unique, human commensal bacterial strains Manufactured by cultivation from clonal cell banks and
encapsulated for oral delivery Designed to reduce the abundance of pathogens linked to infection and improve immune tolerance in allo-HSCT recipients Strain selection based on broad pre-clinical screening for defined functions and insights
from microbiome clinical data Preclinical data show SER-155 leads to multi-log reductions of Enterococcus (including VRE) and Enterobacteriaceae (including CRE)* Investigational 16-strain cultivated bacterial consortium optimized
using MbTx Platform * Seres data shared in Jan 2022 Infection Protection Investor Event; VRE = vancomycin-resistant Enterococci; CRE = carbapenem-resistant Enterobacterales; VRE and CRE both included in US CDC Antibiotic Resistance Threats **
CIBMTR 2022; Ranges based on slides 61, 62, 63, 65 and Age > 18 years Specifically designed to address two of the leading causes of mortality in allo-HSCT recipients Causes of allo-HSCT Mortality at 100 Days
Post-Transplant** Cause Percentage of Deaths Organ Failure 32-35% Infection 19-28% Primary Disease 12-26% GVHD 5-14% Hemorrhage 4-7% Graft Rejection 2-5% | Seres Therapeutics, Inc. © 2024
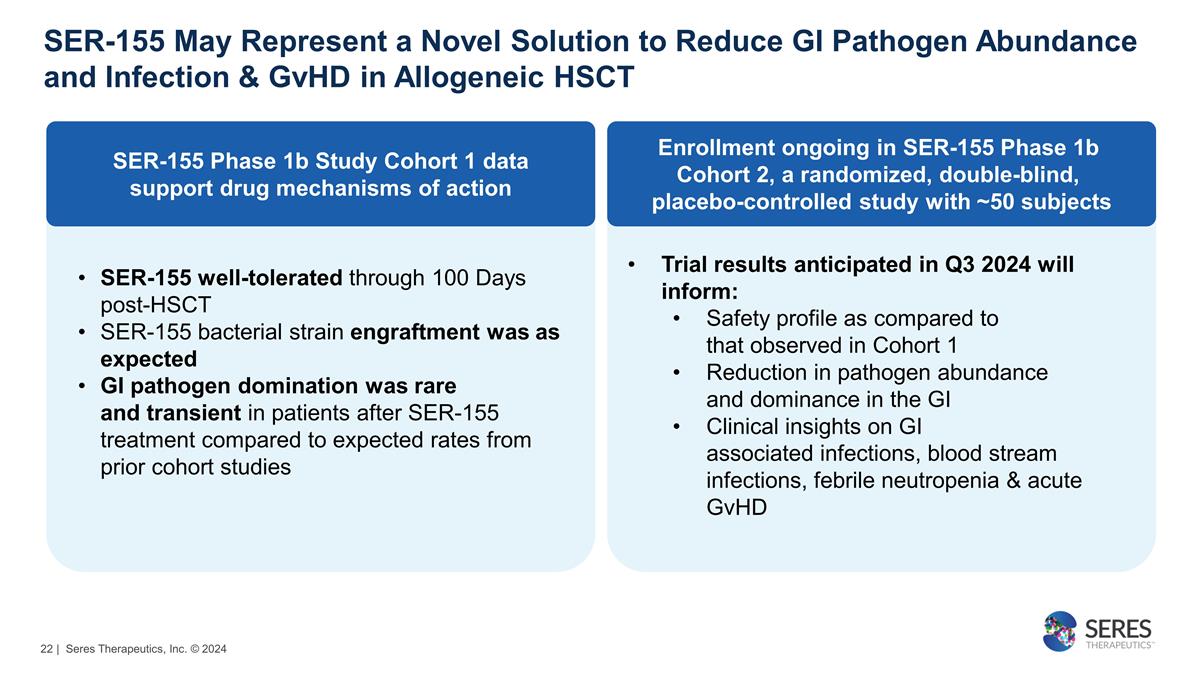
SER-155 May Represent a Novel
Solution to Reduce GI Pathogen Abundance and Infection & GvHD in Allogeneic HSCT | Seres Therapeutics, Inc. © 2024 SER-155 well-tolerated through 100 Days post-HSCT SER-155 bacterial strain engraftment was as
expected GI pathogen domination was rare and transient in patients after SER-155 treatment compared to expected rates from prior cohort studies Trial results anticipated in Q3 2024 will inform: Safety profile as compared to
that observed in Cohort 1 Reduction in pathogen abundance and dominance in the GI Clinical insights on GI associated infections, blood stream infections, febrile neutropenia & acute GvHD SER-155 Phase 1b Study Cohort 1
data support drug mechanisms of action Enrollment ongoing in SER-155 Phase 1b Cohort 2, a randomized, double-blind, placebo-controlled study with ~50 subjects
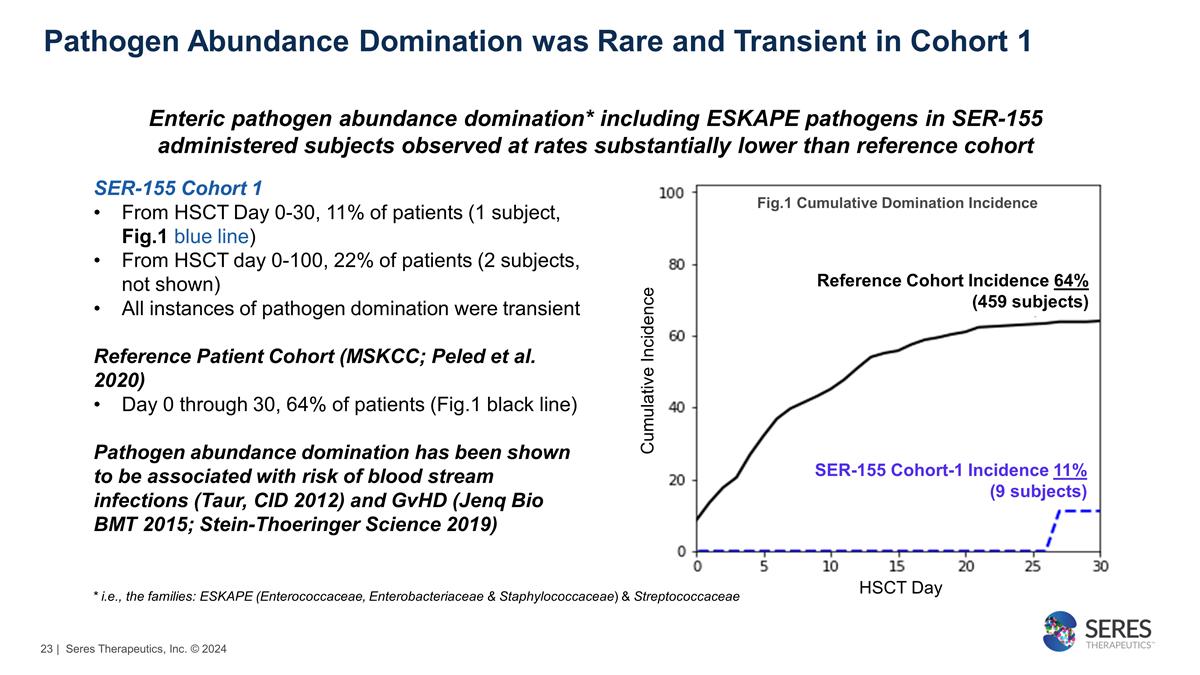
Pathogen Abundance Domination was
Rare and Transient in Cohort 1 SER-155 Cohort 1 From HSCT Day 0-30, 11% of patients (1 subject, Fig.1 blue line) From HSCT day 0-100, 22% of patients (2 subjects, not shown) All instances of pathogen domination were transient Reference
Patient Cohort (MSKCC; Peled et al. 2020) Day 0 through 30, 64% of patients (Fig.1 black line) Pathogen abundance domination has been shown to be associated with risk of blood stream infections (Taur, CID 2012) and GvHD (Jenq Bio BMT 2015;
Stein-Thoeringer Science 2019) Cumulative Incidence HSCT Day Fig.1 Cumulative Domination Incidence Reference Cohort Incidence 64% (459 subjects) SER-155 Cohort-1 Incidence 11% (9 subjects) * i.e., the families: ESKAPE (Enterococcaceae,
Enterobacteriaceae & Staphylococcaceae) & Streptococcaceae Enteric pathogen abundance domination* including ESKAPE pathogens in SER-155 administered subjects observed at rates substantially lower than reference
cohort | Seres Therapeutics, Inc. © 2024
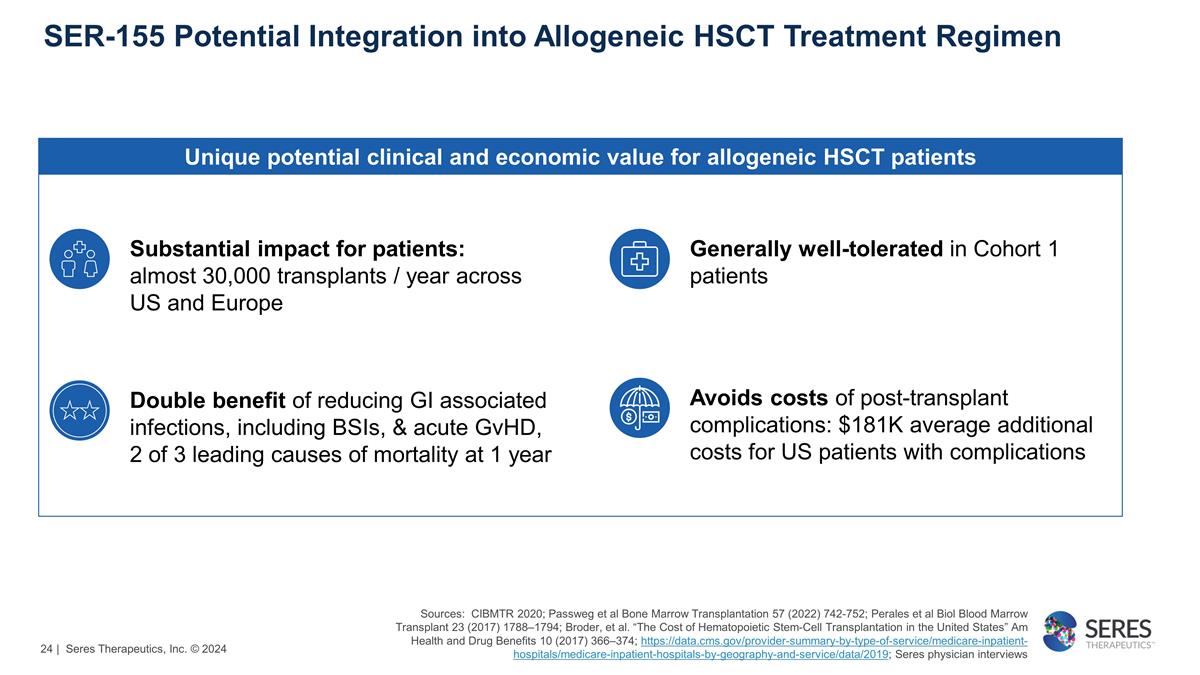
SER-155 Potential Integration into
Allogeneic HSCT Treatment Regimen Unique potential clinical and economic value for allogeneic HSCT patients Double benefit of reducing GI associated infections, including BSIs, & acute GvHD, 2 of 3 leading causes of mortality at 1 year Avoids
costs of post-transplant complications: $181K average additional costs for US patients with complications Sources: CIBMTR 2020; Passweg et al Bone Marrow Transplantation 57 (2022) 742-752; Perales et al Biol Blood Marrow Transplant 23 (2017)
1788–1794; Broder, et al. “The Cost of Hematopoietic Stem-Cell Transplantation in the United States” Am Health and Drug Benefits 10 (2017) 366–374;
https://data.cms.gov/provider-summary-by-type-of-service/medicare-inpatient-hospitals/medicare-inpatient-hospitals-by-geography-and-service/data/2019; Seres physician interviews Generally well-tolerated in Cohort 1 patients
Substantial impact for patients: almost 30,000 transplants / year across US and Europe | Seres Therapeutics, Inc. © 2024
Company Profile: Leader in
microbiome therapeutics with the first FDA approved orally administered microbiota-based therapeutic VOWST: Exceptional clinical profile meeting high unmet medical need and demonstrating early favorable adoption SER-155 Ongoing Phase 1b
Study: Expanding proven novel technology to address the risk of life threatening infections Going Forward: Clear strategic priorities with financial discipline 4 1 3 2 The Seres Story: Maximizing the Potential of the Microbiome Leading
with VOWST™ | Seres Therapeutics, Inc. © 2024
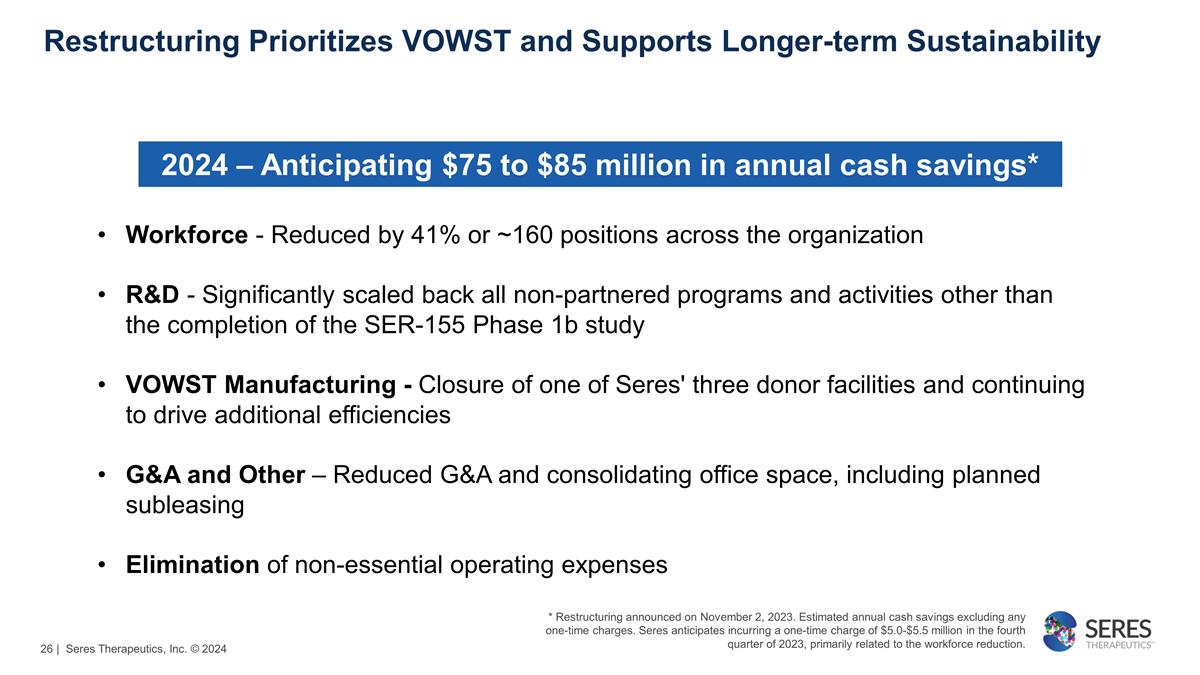
Restructuring Prioritizes VOWST and
Supports Longer-term Sustainability Before Strategic Restructuring 2024 – Anticipating $75 to $85 million in annual cash savings* Workforce - Reduced by 41% or ~160 positions across the organization R&D - Significantly scaled
back all non-partnered programs and activities other than the completion of the SER-155 Phase 1b study VOWST Manufacturing - Closure of one of Seres' three donor facilities and continuing to drive additional efficiencies G&A and
Other – Reduced G&A and consolidating office space, including planned subleasing Elimination of non-essential operating expenses * Restructuring announced on November 2, 2023. Estimated annual cash savings excluding any one-time
charges. Seres anticipates incurring a one-time charge of $5.0-$5.5 million in the fourth quarter of 2023, primarily related to the workforce reduction. | Seres Therapeutics, Inc. © 2024 26
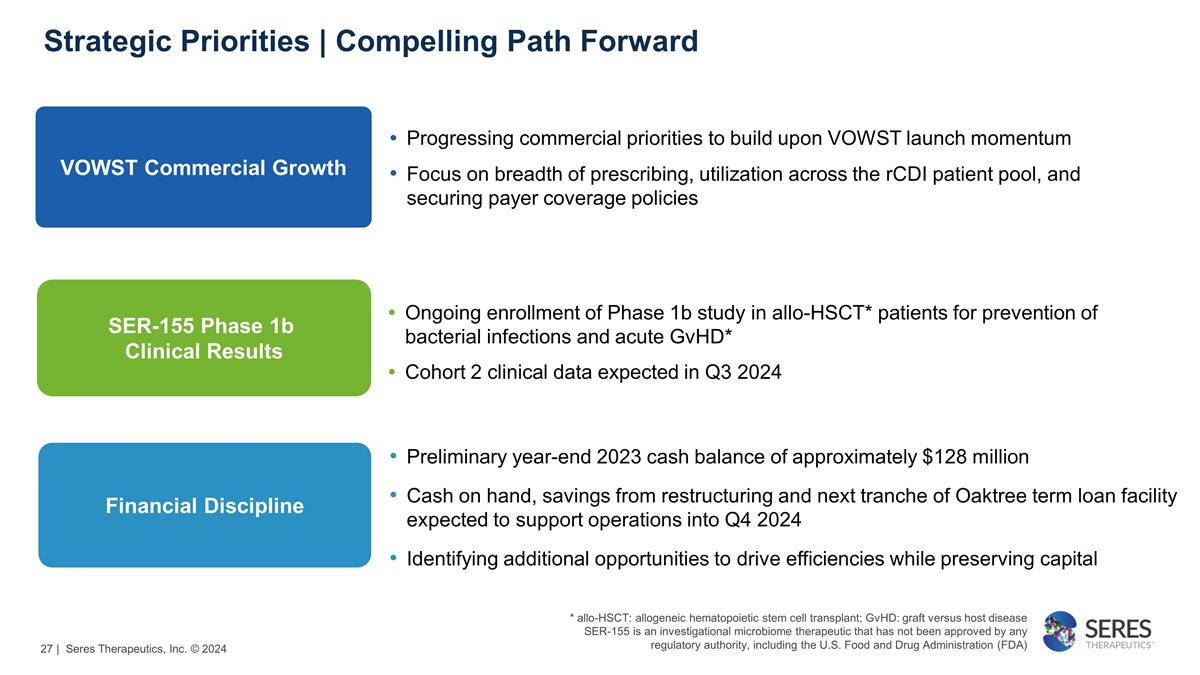
Strategic Priorities | Compelling
Path Forward VOWST Commercial Growth SER-155 Phase 1b Clinical Results Financial Discipline Progressing commercial priorities to build upon VOWST launch momentum Focus on breadth of prescribing, utilization across the rCDI patient
pool, and securing payer coverage policies Ongoing enrollment of Phase 1b study in allo-HSCT* patients for prevention of bacterial infections and acute GvHD* Cohort 2 clinical data expected in Q3 2024 Preliminary year-end 2023 cash balance of
approximately $128 million Cash on hand, savings from restructuring and next tranche of Oaktree term loan facility expected to support operations into Q4 2024 Identifying additional opportunities to drive efficiencies while
preserving capital * allo-HSCT: allogeneic hematopoietic stem cell transplant; GvHD: graft versus host disease SER-155 is an investigational microbiome therapeutic that has not been approved by any regulatory authority, including the U.S. Food and
Drug Administration (FDA) | Seres Therapeutics, Inc. © 2024
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Seres Therapeutics (NASDAQ:MCRB)
Historical Stock Chart
From Apr 2024 to May 2024
Seres Therapeutics (NASDAQ:MCRB)
Historical Stock Chart
From May 2023 to May 2024