Adagene Gets FDA Clearance for Phase 1b/2 Trial in Solid Tumors
March 16 2022 - 7:59AM
Dow Jones News
By Michael Dabaie
Adagene Inc. said it received Food and Drug Administration
clearance to proceed with a trial of ADG126 in combination with
pembrolizumab in solid tumors.
The global Phase 1b/2 trial will evaluate patients with
advanced/metastatic solid tumors at multiple sites in the U.S. and
Asia Pacific, the company, which is focused on antibody-based
therapies, said.
The company said it expects the ADG126-P001 trial to dose the
first patients soon. The trial will evaluate safety and
tolerability, and determine the recommended Phase 2 dose for ADG126
in combination with pembrolizumab, it said.
The trial will begin with dose escalation, followed by dose
expansion at the recommended dose for early efficacy evaluation. A
combination cohort of ADG126 with anti-PD-1 therapy toripalimab is
also being initiated in Australia, the company said.
Write to Michael Dabaie at michael.dabaie@wsj.com
(END) Dow Jones Newswires
March 16, 2022 07:44 ET (11:44 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
Adagene (NASDAQ:ADAG)
Historical Stock Chart
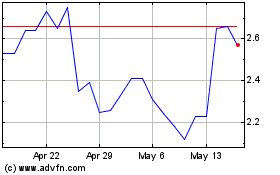
From Jun 2024 to Jul 2024
Adagene (NASDAQ:ADAG)
Historical Stock Chart
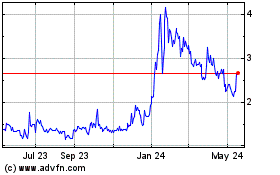
From Jul 2023 to Jul 2024