Annovis Bio Shares Rise 6% After FDA Approves Buntanetap Study
October 06 2022 - 10:34AM
Dow Jones News
By Chris Wack
Annovis Bio Inc. shares were up 6% to $14.18 early Thursday
after the drug-platform company said the U.S. Food and Drug
Administration authorized the Phase 2/3 clinical study of
buntanetap in moderate Alzheimer's Disease.
Following the submission of the Phase 2a clinical safety data
and the chronic toxicology data in animals, the company requested
approval to further pursue the development of buntanetap in AD.
The FDA approved the company's development plan, study protocol
and authorized the initiation of the Phase 2/3 clinical study of
buntanetap in AD.
Annovis stock is up 40% in the last month.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
October 06, 2022 10:19 ET (14:19 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
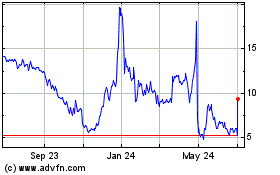
Annovis Bio (NYSE:ANVS)
Historical Stock Chart
From Mar 2024 to Apr 2024
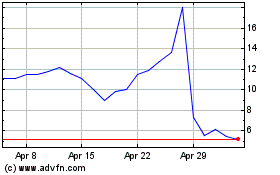
Annovis Bio (NYSE:ANVS)
Historical Stock Chart
From Apr 2023 to Apr 2024