Table of Contents
Registration No. 333-267039
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
____________________
AMENDMENT NO. 4
to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Cannabis Bioscience
International Holdings, Inc.
(formerly named China Infrastructure Construction
Corp.)
(Exact name of Registrant
as specified in its charter)
____________________
| Colorado |
8999; 8099 |
84-4901229 |
| (State or other jurisdiction of |
Primary Standard |
(I.R.S. Employer Identification No.) |
| Incorporation or organization) |
Industrial Classification |
|
| |
Code Numbers |
|
____________________
6201 Bonhomme Road, Suite 466S,
Houston, TX 77036
Telephone: (832) 606-7500
(Address, including zip
code and telephone number,
including area code, of Registrant’s principal executive offices)
____________________
Dante Picazo
Chief Executive Officer
Cannabis Bioscience International Holdings,
Inc.
6201 Bonhomme Road, Suite 466S
Houston, TX 91789
Telephone: (832) 606-7500
(Name, address, including zip code, and telephone
number,
including area code, of agent for service)
____________________
With a copy to:
Barry J. Miller, Esq.
Barry J. Miller PLLC
7146 Pebble Park Drive
West Bloomfield, MI 48322
Telephone: (248) 232-8039
Fax: (248) 246-9524
____________________
Approximate date of commencement of proposed sale to the public:
As soon as practicable following the effective
date of this registration statement is declared effective by the Registrant and from time to time thereafter, as determined by the Selling
Stockholders.
If any of the securities being registered on
this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following
box: ☒
If this form is filed to register additional
securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration
statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed
pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of
the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed
pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of
the earlier effective registration statement for the same offering. ☐
___________________
Indicate by check mark whether the Registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company or an emerging growth company.
See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company”
and “emerging growth company” in Rule 12b-2 of the Exchange Act. (check one)
| Large accelerated filer |
☐ |
Accelerated filer |
☐ |
| Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
| |
|
Emerging growth company |
☒ |
If an emerging growth company, indicate by check
mark if the Registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
___________________
The Registrant hereby amends this registration
statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which
specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities
Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting
pursuant to said Section 8(a), may determine.
The information in this prospectus
is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange
Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities
in any jurisdiction where the offer or sale is not permitted.
Subject to Completion, dated
July 10, 2023.
PROSPECTUS
Cannabis
Bioscience International Holdings, Inc.
10,283,940,599 SHARES OF COMMON STOCK
This
Prospectus relates to the offer and sale of up to 10,283,940,599 shares of the common stock,
without par value (“Common Stock”), of Cannabis Bioscience International Holdings,
Inc., a Colorado corporation (the “Shares”), of which 6,250,000,000 shares are
offered by the Company and 4,033,940,599 shares are offered by the Selling Stockholders.
The Company will receive the proceeds of sales of the shares that it sells, but none of the
proceeds of the sales of the shares that are sold by the Selling Stockholders. The Company
is offering the shares to be sold by it at an aggregate offering price of $5,000,000.
An investment
in Common Stock is speculative and involves a high degree of risk. Therefore, before purchasing Common Stock, investors should carefully
consider the risk factors and other uncertainties described in this Prospectus. See “Risk Factors.”
We are
an “emerging growth company” as defined under the U.S. federal securities laws and, as such, may elect to comply with reduced
public company reporting requirements for this Prospectus and future filings. See “Prospectus Summary – Implications
of Being an Emerging Growth Company.”
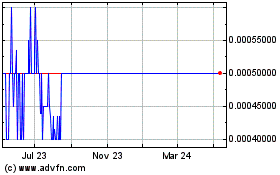
The Common Stock is quoted on the OTC Pink tier
of the alternate trading system operated by OTC Markets Group Inc. (“OTC”).
The Company and the Selling Stockholders will
offer their shares at $0.0008 per share (the “Fixed Offering Price”). See “Plan of Distribution”
for further information. The Selling Stockholders may sell any, all or none of their shares and the Company does not know when, in what
amounts or in what manner they may sell their shares.
Any investment in the shares offered herein
involves a high degree of risk. You should only purchase shares if you can afford a loss of your investment. Our independent
registered public accounting firm has issued an audit opinion for the Company’s audited consolidated financial statements for the
year ended May 31, 2022, that includes a statement expressing substantial doubt as to our ability to continue as a going concern.
On December 6, 2022, the Company changed its
corporate name from China Infrastructure Construction Corp. to Cannabis Bioscience International Holdings, Inc. and has applied to the
Financial Industry Regulatory Authority (“FINRA”) to implement the name change and to obtain a new trading symbol reflecting
the name change. It is not certain whether FINRA will process the application. See “Risk Factors – Risks Related to the Common
Stock and This Offering – If FINRA does not process the Company’s application to change its name on FINRA’s records,
the Company and its shareholders would be adversely affected.”
Dante Picazo, the Company’s chief executive
officer and one of its directors, has voting control of the Company, with power to elect and remove its directors, and will continue
to have such control after the offering.
The Selling Stockholders and any broker-dealers
or agents involved in selling the Shares may be deemed to be underwriters within the meaning of the Securities Act of 1933, as amended
(the “Securities Act”), in connection with such sales. In such event, any commissions received by such broker-dealers or
agents and any profit on the resale of the Shares purchased by them may be deemed to be underwriting commissions or discounts under the
Securities Act.
The Selling
Stockholders and any other person participating in the sale of the Shares will be subject to the provisions of the Securities Exchange
Act of 1934 (the “Exchange Act”) and the rules and regulations promulgated thereunder. These rules include, without limitation,
Regulation M, which may limit the timing of purchases and sales of any of the Shares by the Selling Stockholders and any other person.
In addition, Regulation M may restrict the ability of any person engaged in the distribution of the Shares to engage in market-making
activities with respect to the particular shares being distributed, which may affect the marketability of the Shares and the ability
of any person or entity to engage in market-making activities with respect to the Shares.
Once sold
under the registration statement of which this Prospectus forms a part, the Shares will be freely tradeable in the hands of persons other
than our affiliates.
We have
paid and will pay all expenses incurred in registering the shares, whether offered by the Company or the Selling Stockholders, including
legal and accounting fees. See “Plan of Distribution.” For information regarding expenses of registration,
see “Use of Proceeds.”
The Jumpstart
Our Business Startups Act, or the JOBS Act, was enacted in April 2012 to encourage capital formation in the United States and reduce
the regulatory burden on new-public companies that qualify as “emerging growth companies.” We are an “emerging growth
company” within the meaning of the JOBS Act. As an “emerging growth company,” we intend to take advantage of certain
exemptions from various public reporting requirements, including the requirement that our internal control over financial reporting be
audited by our independent registered public accounting firm pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley
Act, certain requirements related to the disclosure of executive compensation in this Prospectus and our periodic reports and proxy statements,
and the requirement that we hold a non-binding advisory vote on executive compensation and any golden parachute payments. We may take
advantage of these exemptions until we are no longer an “emerging growth company.”
NEITHER THE SECURITIES AND EXCHANGE COMMISSION
NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE.
ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this Prospectus is July __, 2023.
TABLE OF CONTENTS
Through and including _____________, 2023
(the 25th day after the date of this Prospectus), all dealers effecting transactions in these securities, whether or not participating
in this offering, may be required to deliver a prospectus, in addition to a dealer’s obligation to deliver a prospectus when acting
as an underwriter and with respect to an unsold allotment or subscription.
This Prospectus forms a part of a registration
statement on Form S-1 that we filed with the SEC. Under this registration statement, the Selling Stockholders may, from time to time,
sell their shares, as described in this Prospectus. We will not receive any proceeds from the sale of the Shares by any such Selling
Stockholders. See “Use of Proceeds.”
Neither we nor the Selling Stockholders have
authorized anyone to provide any information or make any representations other than those contained in this Prospectus or any free writing
prospectuses we have prepared. Neither we nor the Selling Stockholders take responsibility for and cannot assure as to the reliability
of any information that others may give you, other than the information contained in this Prospectus. This Prospectus is an offer
to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful. The information contained
in this Prospectus is current only as of its date, regardless of the time of delivery of this Prospectus or any sale of Common Stock.
For investors outside the United States: Neither
we nor the Selling Stockholders have taken any action that would permit this offering or possession or distribution of this Prospectus
in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who
come into possession of this Prospectus must inform themselves about and observe any restrictions relating to the offering of the shares
of Common Stock and the distribution of this Prospectus outside the United States.
PROSPECTUS SUMMARY
This summary highlights information contained
elsewhere in this Prospectus. Because this is only a summary, it does not contain all information that may be important to you. You should
read the entire Prospectus and should consider, among other things, the matters set forth under “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and the notes
thereto appearing elsewhere in this Prospectus before making an investment decision. This Prospectus contains forward-looking statements
and information relating to the Company. See “Cautionary Notes.”
The Company is based in Houston, Texas, and was
established in 2003. For more detailed information respecting its corporate history, see “Description of Business
– History.” The address of our principal executive office is 6201 Bonhomme Road, Suite 466S, Houston, TX 77036, and our
telephone number is (832) 606-7500. Its website is www.chnc-hdh.com. The information contained thereon is not intended to be incorporated
into this Prospectus or the registration statement of which it is a part.
We provide educational and other services to
the cannabis industry (the “Pharmacology University Business”) (see “Description of Business –
Business – Pharmacology University Business”), clinical trial services to Sponsors and CROs (the “Alpha
Research Business”) (see “Description of Business – Business – Alpha Research
Business”) and diagnostic services related to sleep disorders through the Sleep Center (the “Sleep Center
”) (see “Description of Business – Business – Sleep Center Business”).
“Sponsor” means a person who takes responsibility for and initiates a clinical investigation of a drug or medical
device, including an individual or pharmaceutical company, governmental agency, academic institution, private organization, or other
organization. “CRO” means a person that assumes, as an independent contractor with a Sponsor, one or more of the
obligations of a Sponsor, such as the design of a protocol, selection or monitoring of investigations, evaluation of reports, and
preparation of materials to be submitted to the U.S. Food and Drug Administration (the “FDA”).
Implications of Being an Emerging Growth Company
We are an “emerging growth company”
as defined in the Jumpstart Our Business Startups Act (known as the “JOBS Act”). Under the JOBS Act, we may utilize reduced
reporting requirements that are otherwise applicable to public companies, including delaying auditor attestation of internal control
over financial reporting, providing only two years of audited financial statements and related Management’s Discussion and Analysis
of Financial Condition and Results of Operations in this Prospectus and the reports that we will file with the U.S. Securities and Exchange
Commission (the “SEC”), including reduced executive compensation disclosures.
We are permitted to remain an emerging growth
company for up to five years from the date of the first sale in this offering. However, if certain events occur before the end of that
period, including our becoming a “large accelerated filer,” our annual gross revenue’s exceeding $1.07 billion or our
issuance of more than $1.0 billion of nonconvertible debt in any three-year period, we will cease to be an emerging growth company.
We have elected to take advantage of certain
of the reduced disclosure obligations in this Prospectus and the registration statement of which it is a part and may elect to take advantage
of other reduced reporting requirements in future filings. In particular, in this Prospectus, we have provided only two years of audited
financial statements and have not included all of the information relating to executive compensation that would be required if we were
not an emerging growth company. As a result, the information that we provide to our stockholders may be different from that which might
be received from public reporting companies that are not emerging growth companies. We have irrevocably elected to avail ourselves of
the extended transition period for complying with new or revised accounting standards and therefore, we will be subject to the same new
or revised accounting standards as private companies.
Recent Developments
The COVID-19 pandemic has harmed the Company
Early in 2020, the COVID-19 pandemic resulted
in decreased business activity and restrictions on the conduct of businesses, including mandatory lockdowns. Because of these restrictions,
all our classrooms and public venues were closed and other Pharmacology University Business activities that required face-to-face contact,
such as its consulting services and franchising and marketing efforts, were sharply reduced or terminated. Among other things, the Pharmacology
University Business closed its seminars in Ecuador and the Dominican Republic; ceased holding classes at the University of Tadeo in Bogota,
Cartagena and Santa Marta, Colombia; and ceased all travel. The business conducted by the Alpha Research Business has also been adversely
affected because several of the clinical studies in which it was participating were deferred, shortened or canceled. These restrictions
have been reduced or eliminated in many jurisdictions, but if the pandemic resurges, they could be reimposed. We have not been able to
resume classroom teaching and seminars, consulting services, franchising and marketing efforts and the Alpha Research Business has continued
to be adversely impacted.
As a result of the pandemic, we experienced substantial
reductions in our revenues and our losses increased in our educational and clinical trial businesses. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Impact of the Covid-19 Pandemic.” To protect
our business from disruption caused by the COVID-19 pandemic and to enable our students to continue to be educated, we created online
courses. We currently have over 100 online videos in English, Spanish, Portuguese, Italian and Arabic. We also commenced the use of Zoom
meetings to hold virtual classes to teach students and be able to respond to their questions in real time. We believe that these measures
have helped us to manage our business prudently during the pandemic; nevertheless, much of our business depends on personal contacts,
and we have not been able to reduce the adverse effects of the pandemic’s reducing or eliminating personal contact.
On December 6, 2022, we changed our corporate
name from China Infrastructure Construction Corp. to Cannabis Bioscience International Holdings, Inc. We have applied to FINRA to implement
the name change and to obtain a new trading symbol reflecting the name change. It is not certain whether FINRA will process the application.
See “Risk Factors – Risks Related to the Common Stock and This Offering – If FINRA does not process the Company’s
application to change its name on FINRA’s records, the Company and its shareholders would be adversely affected.”
Risk Factors Summary
Our business is subject to many risks and uncertainties
of which you should be aware before deciding whether to invest in Common Stock, in addition to general business risks. These risks are
more fully described in the section titled “Risk Factors” immediately following this Prospectus Summary. These risks include,
among others, the following:
| |
· |
The COVID-19 pandemic and the impact of actions to mitigate the COVID-19 pandemic have materially and adversely impacted and will continue materially and adversely to impact our business, results of operations and financial condition. In particular, our revenues have decreased and our losses have increased, in each case materially, since the onset of the pandemic. |
| |
|
|
| |
· |
The Company expects to encounter significant challenges in recovering from the adverse effects of the Covid-19 pandemic and can give no assurances respecting its success in meeting them. |
| |
|
|
| |
· |
The Company has incurred net losses each year since its inception and
may not be able to achieve profitability. It has incurred net losses of $885,171, $159,308, and $541,152 for the fiscal years ended
May 31, 2022, May 31, 2021, and May 31, 2020, respectively, and $756,573 for the nine months ended February 28, 2023. Its accumulated
deficit for the fiscal years ended May 31, 2022, May 31, 2021, and May 31, 2020, were $3,650,156 and $2,764,985, respectively, and
was $4,406,658 for the nine months ended February 28, 2023. |
| |
|
|
| |
· |
The Alpha Research Business is conducted in a highly competitive industry and may not be able to compete successfully with its current or future competitors. |
| |
|
|
| |
· |
Both the Pharmacology University Business and the Alpha Research Business are subject to a wide variety of complex, evolving, and, with respect to the Pharmacology University Business, sometimes inconsistent and ambiguous laws and regulations that may adversely impact their operations and could cause the Company to incur significant liabilities including fines and criminal penalties, which could have a material adverse effect on its business, results of operations, and financial condition. |
| |
|
|
| |
· |
The Alpha Research Business is conducted in a highly competitive industry and may not be able to compete successfully with its current or future competitors. |
| |
|
|
| |
· |
Following the Offering, there will be a large number of shares of Common Stock that may be sold in the public markets, which may substantially and adversely affect their market price. For further information, see “Risk Factors – Risks Related to the Common Stock and the Offering – There will be a larger number of shares of Common Stock that will be eligible to be sold in the public markets” and “Shares Eligible for Future Sale.” |
| |
|
|
| |
· |
The Company may not be able to sell all of the Shares at the Fixed Offering Price. See “Risk Factors – Risks Related to the Common Stock and the Offering – We may change the Fixed Offering Price. |
THE
OFFERING
| Amount of Offering by us: |
|
$5,000,000 |
| |
|
|
| Offering Price per Share: |
|
The shares offered by the Company will be sold at the Fixed Offering Price of $0.0008
per share for the duration of the offering (the “Fixed Offering Price”). The Selling Stockholders may offer their shares in different ways and at varying prices. See “Plan of Distribution.” |
| |
|
|
| Shares of Common Stock offered by the Company: |
|
6,250,000,000 shares |
| |
|
|
Shares
of Common Stock offered by the Selling Stockholders:
|
|
4,033,405,599 shares |
| |
|
|
Shares
of Common Stock outstanding prior to the Offering:
|
|
10,294,249,347 shares |
| |
|
|
Shares
of Common Stock outstanding after the Offering:
|
|
16,544,249,347shares |
| |
|
|
| |
|
The number of shares of Common Stock to be outstanding after the Offering is based on 10,294,249,347
shares of Common Stock outstanding as of July 10, 2023. |
| |
|
|
| Voting rights: |
|
Each share of Common Stock and Series A Preferred is entitled to one vote per share. The Series B
Preferred has 60% of the voting power in the Company and all of the outstanding shares are held by the Company’s chief
executive officer, who is also a director. By virtue of his holdings of Series B Preferred, he has the power to control the outcome
of all matters submitted to stockholders for approval, including the election of directors and the approval of any change-of-control
transaction. See “Description of Capital Stock.” |
| |
|
|
| Use of Proceeds: |
|
The proceeds that we receive from sales of the shares offered by the Company will be used for the purposes
set forth under “Use of Proceeds.”. We will not receive any proceeds from the sale of the Shares offered
by the Selling Stockholders. |
| |
|
|
| Trading symbol: |
|
CHNC |
| |
|
|
| Risk Factors: |
|
An investment in Common Stock is highly speculative and involves a high degree of risk for the reasons
set forth in “Risk Factors” and elsewhere in this Prospectus. |
| |
|
|
| Fees and Expenses: |
|
We will pay all expenses incident to the registration of the shares offered by this Prospectus, except
for sales commissions and other expenses of the Selling Stockholders. |
RISK FACTORS
An investment in
Common Stock involves a high degree of risk. Prospective investors should carefully consider the risks described below and all of the
other information contained in this Prospectus, including the Company’s consolidated financial statements and the related notes
and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” before deciding whether
to invest in Common Stock. If any of the events described below occur, the Company’s business, business prospects, cash flow, results
of operations or financial condition could be materially and adversely harmed. In these events, the trading price of the Common Stock
could decline, and investors might lose all or part of their investments. Investors should read the section entitled “Forward-Looking Statements” for a discussion of what types of statements are forward-looking, as well as the significance of such statements
in the context of this Prospectus.
The following is a discussion of the risk
factors that the Company believes are currently material. These risks and uncertainties are not the only ones facing the Company,
and in addition to general business risks, there may be other matters of which the Company is not aware or that it currently considers
immaterial. All of these could adversely affect the Company’s business, business prospects, cash flow, results of operations
or financial condition.
Business-Related Risks
The COVID-19 pandemic
and the efforts to mitigate its impact may have an adverse effect on the Company’s business, liquidity, results of operations,
financial condition and price of its securities.
The Covid-19 pandemic has materially and adversely
impacted the Company and its results of operations, particularly as a result of limitations on the ability of the Pharmacology University
Business to conduct classes and other face-to-face activities due to lockdowns. Public health authorities and governments at local, national
and international levels have from time to time announced various measures of varying intensity to respond to this pandemic. Some measures
that have directly or indirectly impacted the Company’s business include voluntary or mandatory quarantines and business closures,
restrictions on travel and limiting gatherings of people in public places.
For detailed information respecting the impact
of the pandemic on the Company’s financial results, see “Management’s Discussion and Analysis of Financial
Condition and Results of Operations – Impact of the Covid-19 Pandemic.”
Although many of the measures introduced to combat
the COVID-19 pandemic have been relaxed and in some cases terminated, the Company does not know when it will be able to resume its normal
operations, particularly in the classroom, franchising and consulting activities of the Pharmacology University Business. However, we
expect that returning to normal operations will require time, will involve substantial costs and will involve uncertainties, including
(i) whether the pandemic will continue to abate, (ii) what measures governments will take if the pandemic intensifies and (iii) the ability
of our customers and suppliers to recover from the effects of the pandemic.
To the extent the pandemic has and may continue
to affect the Company’s business and financial results adversely, it may also have the effect of heightening many of the other
risks to which the Company is subject, whether or not described under “Risk Factors.” If the pandemic does
not continue to abate or it intensifies, the Company’s ability to execute its business plan on a timely basis or at all may be
materially impeded.
We have a limited operating history, making
it difficult to forecast our revenue and evaluate our business and prospects.
We have a limited operating history and as a
result, our ability to forecast our future results of operations and plan for growth is limited and subject to many uncertainties. We
have encountered and expect to continue to encounter risks and uncertainties frequently experienced by growing companies in rapidly evolving
industries, such as the risks and uncertainties described herein. Accordingly, we may be unable to prepare accurate internal financial
forecasts or replace anticipated revenue that we do not receive as a result of delays arising from these factors, and our results of
operations in future reporting periods may be below the expectations of investors. If we do not address these risks successfully, our
results of operations could differ materially from our estimates and forecasts or the expectations of investors, causing our business
to suffer and the market price of the Common Stock to decline.
We
have a history of net losses, we anticipate increasing operating expenses in the future, and we may not be able to achieve and, if achieved,
maintain profitability.
We have incurred significant net losses each
year since our inception (see “Management’s Discussion and Analysis of Financial Condition and Results of Operations”).
We expect to continue to incur net losses for the foreseeable future and we may not achieve or maintain profitability in the future.
It is difficult for us to predict our future results of operations or the limits of our market opportunity. We expect our operating expenses
to significantly increase over the next several years as we hire additional personnel, particularly in sales and marketing, and expand
our operations, both domestically and internationally. We may also selectively pursue acquisitions. In addition, because we will become
subject to the reporting and other requirements of the Exchange Act as a result of the effectiveness of the registration statement of
which this Prospectus is a part, we will incur additional significant legal, accounting, and other expenses that we did not incur previously.
If our revenue does not increase to offset the expected increases in our operating expenses, we will not become profitable. Our growth
could be impeded for many reasons, including, but not limited to, those set forth under “Risk Factors.” Our failure to sustain
consistent profitability could cause the market price of the Common Stock to decline.
The Company requires substantial additional
capital. If the Company cannot raise capital, it may have to curtail its operations or it could fail.
The Company requires substantial additional capital through public
or private debt or equity financings to continue operating, as well as to fund its operating losses, increase its sales and marketing
capacity, take advantage of opportunities for internal expansion or acquisitions, hire, train and retain employees, develop and complete
existing services and new services and products and respond to economic and competitive pressures. The Company needs $5,000,000 to execute
its business plan and meet its other corporate expenses, some or all of which may be provided from the sale of the Shares. If it cannot
raise such capital, it may have to alter its business plan or curtail its operations, or it could fail. The financial condition of the
Company presents a material risk to investors and may make it difficult to attract additional capital or adversely affect the terms on
which the Company can obtain it. For further information, see “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – General Statement of Business – Going Concern”
and “– Liquidity and Capital Resources.”
The Company has received no commitment for financing
from investors or banks and no assurance can be given that any such commitment will be forthcoming or, if so, in what amount and on what
terms.
The preceding risk factors raise substantial
doubt about our ability to continue as a going concern and our independent registered public accounting firm has included an explanatory
paragraph relating to our ability to continue as a going concern in its report on our audited consolidated financial statements contained
in this Prospectus.
The consolidated financial statements contained
in the Prospectus were prepared on the assumption that we will continue as a going concern. Accordingly, the accompanying financial statements
do not include any adjustments that might be necessary should we be unable to continue as a going concern. We do not have adequate funds
available, and the Offering may not provide sufficient proceeds to fund our anticipated expenses without obtaining significant additional
financing. This raises substantial doubt about our ability to continue as a going concern. The perception that we may not be able to
continue as a going concern may materially limit our ability to raise additional funds through the issuance of new debt or equity securities
or otherwise and no assurance can be given that sufficient funding will be available when needed to allow us to continue as a going concern.
This perception may also make it more difficult to operate our business due to concerns about our ability to meet our contractual obligations.
Our ability to continue as a going concern is contingent upon, among other factors, our ability to sell shares of Common Stock, including
those that we are offering by this Prospectus, and obtaining additional capital. We cannot provide any assurance that we will be able
to raise additional capital. If we cannot secure additional capital, we may be required to curtail our operations and take measures to
reduce costs to conserve cash in amounts sufficient to sustain operations and meet our obligations. These measures could cause significant
delays in the realization of our business plan. It is not presently possible for us to predict the potential success of our business
plan. We cannot predict the revenue or the income potential of our proposed businesses and operations. If we cannot operate as a viable
entity, you may lose some or all of your investment.
In
addition, the report of our independent registered public accounting firm with respect to our consolidated financial statements appearing
elsewhere in this Prospectus contains an explanatory paragraph stating that the Company had negative working capital at May 31, 2021,
had incurred recurring losses and recurring negative cash flow from operating activities, and had an accumulated deficit, raising substantial
doubt about its ability to continue as a going concern. For information about Management’s evaluation of and plans regarding these
matters, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources” and Note 3 to its audited consolidated financial statements.
Delays in payments by Sponsors and CROs
have affected and may continue to affect the Company’s cash flows.
The Company has from time to time been affected
by delays in payments by Sponsors and CROs. More specifically, at May 31, 2022, and May 31, 2021, the Company had accounts payable from
Sponsors and CROs of $5,614 and $1,295, respectively, of which $470 and $1295, respectively, were not paid timely; all of these accounts
payable have been collected. At February 28, 2023, and February 28, 2022, the Company had accounts payable from Sponsors and CROs of
$11,379 and $5,174, respectively, of which $5,485 and $4,209, respectively, were not paid timely; $2,735 of these accounts payable remains
unpaid, while all of the accounts payable at February 28, 2022, have been collected. At May 31, 2022, the balance of accounts receivable
was 2.6% of the total revenue of $214,980 generated for that fiscal year; at February 28, 2023, the balance of accounts receivable, was
9.0% of the total revenue of $51,251 generated for the three months then ended; and at February 28, 2023, the balance of accounts receivable
was 1.7% of the total revenue of $270,413 generated for the nine months then ended.
Although the Company has been able to cope
with these delays in the past, it may not be able to do so in the future. If payments were delayed, the proceeds of the Offering were
insufficient and other available resources were insufficient to pay its obligations, the Company would have to sell shares of Common
Stock, probably at below-market prices and/or borrow money at rates of interest that could be high. If the Company were unable to sell
shares or borrow money, it might have to alter its business plan or curtail its operations, or it could fail.
For further information about delays in payments, see Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources.
We May be Affected by Inflation.
Inflation rates have increased and may
continue to rise. Companies from which we purchase goods and services may raise their prices and we may be unable to pass these
increases on to our customers. Although we have not yet been affected, inflation could adversely affect our business,
including our competitive position, market share, revenues and operating income.
We May be Affected by Increasing
Interest Rates.
Interest rates have recently risen, but we
have not been materially affected. However, we cannot assure that rising interest rates will not affect us in the future, thereby reducing
our ability to borrow or increasing our expenses if we were to borrow, either of which would adversely affect our business plans and
growth, increase the cost of our borrowings and reduce our earnings.
Because the Pharmacology University Business
deals with persons that operate in the cannabis industry, it faces unique, unpredictable and evolving risks.
The Company does not grow or sell cannabis
or manufacture or sell cannabis-related paraphernalia and believes that it is not directly subject to the risks that may arise from violation
of the federal, state and foreign laws relating thereto. However, some of the Company’s customers and potential customers engage
in one or more of these activities, and are subject to these risks, the eventuation of which may adversely affect demand for the services
and products offered by the Pharmacology University Business and the Company’s ability to collect receivables from its customers.
Specific risks faced by these customers and potential customers include the following:
Cannabis is illegal
under federal law, the laws of certain states and certain foreign countries.
Cannabis is illegal under
federal law, as is growing, cultivating, selling or possessing it for any purpose or assisting or conspiring with those who do so. Additionally,
it is unlawful to knowingly open, lease, rent, use, or maintain any place, whether permanently or temporarily, for the purpose of manufacturing,
distributing, or using cannabis. The laws of some states and foreign countries in which the Company operates prohibit one or more of
these activities. Even in states in which the use of cannabis has been legalized, its use, growth, cultivation, sale and possession remain
violations of federal law, because federal law preempts state laws. Strict enforcement of these federal, state or foreign laws would
likely result in the inability of some or all of the Company’s U.S. customers and potential customers to operate, which could adversely
affect demand for the services offered by the Pharmacology University Business and the Company’s ability to collect receivables.
In particular, some of
the Company’s customers may be directly affected by these laws and, as a result the Company may be indirectly affected by the cannabis-related
laws of other jurisdictions in which it operates. These jurisdictions and summaries of the relevant laws are as follows:
| |
· |
Texas. Possession of (i) up to two ounces of cannabis
is a Class B misdemeanor, punishable by up to 180 days in jail and a fine of up to $2,000, (ii) from two up to four ounces is a Class
A misdemeanor, punishable by imprisonment of up to one year and a fine of up to $4,000, (iii) from four ounces up to five pounds
is a felony, punishable by imprisonment of up to two years and a fine of up to $10,000; (iv) from five pounds up to 50 pounds is
a felony, punishable by imprisonment of up to 10 years and a fine of up to $10,000; (v) from 50 pounds up to 2,000 pounds is a felony,
punishable by imprisonment of up to 20 years and a fine of up to $10,000; and more than 2,000 pounds is a felony, punishable by imprisonment
of up to 99 years and a fine of up to $50,000. The Texas Compassionate Use Act (TBCUA), passed in 2015, permits the use of low-THC
cannabis oil, containing no more than 0.5% THC and at least 10% CBD, for individuals diagnosed with intractable epilepsy, but this
legislation limits access to a small number of patients and requires physicians to register with the Texas Department of Public Safety
to prescribe the oil. |
| |
|
|
| |
· |
Colombia. In 2015, the Colombian Constitutional Court
ruled that personal possession and consumption of drugs, including cannabis, could not be criminal offenses. In 2016, legalized medical
cannabis and established a framework for its cultivation, production and export. In 2017, Colombia decriminalized the possession
of up to 20 grams of cannabis for personal use. Possession of more than 20 grams and any activity regarded as commercial cultivation,
sale, or export is punishable by imprisonment. |
| |
|
|
| |
· |
Mexico. The production, sale, and use of cannabis
for recreational and medical purposes is lawful. Possession of more than 28 grams up to 200 grams is subject to a fine of $500 and
possession of more than 200 grams is subject to imprisonment of up to six years. |
| |
|
|
| |
· |
Jordan. Recreational and medical use of cannabis is illegal.
Possession of cannabis is punishable by imprisonment, except that first-time offenders are placed in rehabilitation. The growth and
sale of cannabis are punishable by imprisonment or, in the case of the latter, death. |
| |
|
|
| |
· |
Ecuador. Cannabis is illegal in Ecuador for both medical
and recreational use. Possession, cultivation, sale, and trafficking are strictly prohibited, and persons violating these laws are
subject to imprisonment, except that the possession of up to 10 grams for personal use is legal, as is medical use. Cultivation and
sale are illegal. |
| |
|
|
| |
· |
Venezuela. The cultivation, sale, and possession of
cannabis for recreational purposes are illegal in Venezuela. However, the possession for personal use of up to 20 grams of cannabis
and 5 grams of genetically modified cannabis have been decriminalized. |
| |
|
|
| |
· |
Argentina. The cultivation, sale, and possession of
cannabis for recreational purposes are illegal and are punishable by imprisonment ranging from 4 to 15 years; personal recreational
use is lawful, as is medical use. |
| |
|
|
| |
· |
Brazil. Cannabis is illegal, except for possession
and cultivation of personal amounts and for private use and use of cannabis medications is allowed for terminally ill patients or
those who have exhausted other treatment options. |
| |
|
|
| |
· |
Canada. Canada permits the possession of cannabis
and the growth of cannabis in limited amounts. Medical cannabis is lawful for persons who have received authorization from their
healthcare providers. Penalties for possession over the legal limit, illegal distribution or sale, illegal production of cannabis,
illegal exportation and other cannabis-related offenses can be as high as imprisonment for 14 years. |
The Company does not
believe that it and its personnel are subject to direct risk under these laws, inasmuch as its operations in these jurisdictions involve
teaching, writing, editing, translating and other activities that do not involve the production, sale, use, cultivation or export of
cannabis. However, customers of the Company to whom it extends credit or who are required to make periodic payments, such as persons
to whom the Company provides consulting services or the Company’s franchises, could be directly affected by these laws, with the
result that their ability to pay amounts that they owe to the Company timely or all could be impaired or negated.
The Company may face
risks in the above foreign jurisdictions and others in which it may in the future have operations because of its lack of familiarity
and burdens of complying with foreign laws, legal standards and regulatory requirements relating to cannabis. See “Risk Factors
– Because our success depends in part on our ability to expand our operations outside the United States, our business will be susceptible
to risks associated with international operations” for information as to general risks that that the Company may encounter in conducting
foreign operations.
Uncertainties exist
respecting enforcement.
The enforcement of federal
laws relating to cannabis has varied and may continue to vary in intensity. Some administrations have indicated that they intend to enforce
such laws vigorously, while others have deprioritized enforcement to varying degrees, based, for example, on whether the laws of a state
in which an offense occurred have legalized cannabis or whether the offense relates to the recreational of medical use of cannabis. The
Company believes that the Department of Justice (the “DOJ”) under the Biden administration is not prioritizing enforcement
of the CSA, but the extent to which the DOJ will seek to enforce the CSA under a future administration and against whom enforcement will
be sought is unclear.
Since 2014, it has been
the policy of the Department of the Treasury to deprioritize enforcement of the Bank Secrecy Act against financial institutions and marijuana-related
businesses which utilize them. If the Department of the Treasury were to change this policy, it would be more difficult for our clients
and potential clients to access the U.S. banking systems and conduct financial transactions, which could in turn adversely affect our
operations.
Since 2014, in annual bills,
Congress has prohibited federal funds from being used to prevent states from implementing their own medical marijuana laws, but has not
codified federal protections for medical marijuana patients and producers. Despite this prohibition, the DOJ maintains that it can prosecute
violations of the federal marijuana ban. No assurance can be given that Congress will continue to pass such bills. If it does not do so,
the risk of federal enforcement that overrides such state laws could increase.
The Company cannot predict
the vigor with which state and foreign laws relating to cannabis are or may be enforced, but to the degree that they are enforced, its
customers and potential customers could be adversely affected, which in turn, could adversely affect demand for the Company’s services
and its ability to collect receivables.
On December 2, 2022,
the Medical Marijuana and Cannabidiol Research Expansion Act, which established a new, separate registration process to facilitate research
on marijuana, became law.
Recently, Congress has
considered several bills relating to cannabis:
| · | The
Marijuana Opportunity Reinvestment and Expungement Act (known as the “MORE Act”)
would among other things decriminalize cannabis by removing it from the list of scheduled
substances under the Controlled Substances Act, was passed by the House of representatives
on May 1, 2022, but has not been taken up by the Senate. |
| | | |
| · | The
Cannabis Administration and Opportunity Act, which would decriminalize cannabis at the federal
level and expunge federal cannabis-related criminal records, was introduced in the Senate,
but has not been acted on. |
| | | |
| · | The
Secure and Fair Enforcement (SAFE) Banking Act, which generally prohibits a federal banking
regulator from penalizing a depository institution for providing banking services to a legitimate
cannabis-related business, was passed by the House of Representatives on April 19, 2021,
but was not acted upon by the Senate. |
The Company believes
that these bills or similar legislation will be reintroduced in the Congress during 2023 but cannot predict whether any of them will
become law.
The Company cannot foresee
developments relating to the above matters and cannot predict how and the extent to which we could be affected by them; however, these
effects could be sudden and adverse.
The Company could
become subject to racketeering laws.
While the Company does not
grow, handle, process or sell cannabis or products derived from it, its receipt of money from clients that do so exposes it to risks
related to the Racketeer Influenced Corrupt Organizations Act (“RICO”). RICO is a federal statute providing civil and criminal
penalties for acts performed as part of an ongoing criminal organization. Under RICO, it is unlawful for any person who has received
income derived from a pattern of racketeering activity (which includes most felonious violations of the federal laws relating to cannabis)
to use or invest any of that income in the acquisition of any interest, or the establishment or operation of, any enterprise which is
engaged in interstate commerce. RICO also authorizes private parties whose properties or businesses are harmed by such patterns of racketeering
activity to initiate civil actions. A violation of RICO could result in fines, penalties, administrative sanctions, convictions or settlements
arising from civil or criminal proceedings, seizure of assets, disgorgement of profits, cessation of business activities or divestiture.
Banking regulations
could limit access to banking services and expose the Company to risk.
Receipt of payments from
clients engaged in the cannabis business could subject the Company to the consequences of federal laws and regulations relating to money
laundering, financial record keeping and proceeds of crime, including the Bank Secrecy Act, as amended by the “Patriot Act.”
Since the Company may receive money from persons whose activities are illegal, many banks and other financial institutions could be concerned
that their receipt of these funds from the Company could violate federal statutes such as those relating to money laundering, unlicensed
money remittances and the Bank Secrecy Act. As a result, banks may refuse to provide services to the Company. Such refusal could make
it difficult for the Company to operate. Additionally, some courts have denied cannabis-related businesses bankruptcy protection, thus,
making it difficult for lenders to recoup their investments, which may make it more difficult for the Company to raise capital through
loans. While the Company has not encountered difficulty in obtaining banking services, no assurance can be given that it will be able
to do so.
Since 2014, the DOJ has
de-prioritized enforcement of the Bank Secrecy Act against financial institutions and cannabis-related businesses which utilize them.
If such enforcement were to increase, it might become more difficult for the Company and its clients and potential clients to access
the U.S. banking systems and conduct financial transactions, which could adversely affect the Company’s operations.
Dividends and distributions
could be prevented if receipt of payments from clients is deemed to be proceeds of crime.
While the Company has no
intention to declare or pay dividends in the foreseeable future, if any of its revenues were found to have resulted from violations of
money laundering laws or otherwise the proceeds of crime, the Company might determine to or be required to suspend the declaration declaring
or payment of dividends.
Further legislative
developments beneficial to the Company’s operations are not assured.
The Pharmacology University
Business involves providing services to persons who may be directly or indirectly engaged in the cultivation, distribution, manufacture,
storage, transportation or sale of cannabis and cannabis products. Its success depends on the continued development of the cannabis industry.
Such development depends upon continued legislative and regulatory legalization of cannabis at the state level and either legalization
at the federal level or a continued “hands-off” approach by federal enforcement agencies. However, regulatory developments
beneficial to the industry cannot be assured. While there may be ample public support for legislative action, other factors, such as the
willingness of legislative bodies to act, election results, scientific findings or intangible events, could slow or halt progressive legislation
relating to cannabis and or reduce the current tolerance for the use of cannabis, which could adversely affect the demand for the Company’s
services.
The House of Representatives,
in its most recent term, passed bills that would decriminalize cannabis, remove it from the list of scheduled substances under the Controlled
Substances Act, eliminate criminal penalties for individuals who manufacture, distribute, or possess cannabis, and prohibit a federal
banking regulator from penalizing a depository institution for providing banking services to legitimate cannabis- or hemp-related businesses
or ordering a depository institution to terminate a customer account unless (i) the agency has a valid reason for doing so, and (ii) that
reason is not based solely on reputation risk. Neither of these bills became law because the Senate did not pass them. None of these bills
was adopted by Congress. No assurance can be given that any similar bill will be adopted by the present or any future Congress.
We may be subject
to risks relating to bankruptcy laws.
Some courts have denied
marijuana-related businesses bankruptcy protection, thus, making it very difficult for lenders to recoup their investments, which may
limit the willingness of banks to lend to our clients and us. The lack of banking and financial services presents unique and significant
challenges to businesses in the cannabis industry. We could experience difficulties obtaining and maintaining regular banking and financial
services because of the activities of our clients.
Changes in legislation
or clients’ violations of law could adversely affect the Company.
The voters or legislatures
of states in which cannabis has been legalized could repeal or amend these laws, which could adversely affect the demand for the Company’s
services. In addition, changes to and interpretations of laws and regulations could detrimentally affect its clients and, in turn, result
in a material adverse effect on its operations. Violations of these laws, or allegations of such violations, could disrupt our clients’
business, thereby adversely affecting the Company.
Changes
in government regulation could affect the Alpha Research Business.
Governmental
agencies worldwide, including in the United States, strictly regulate the drug development process. The Alpha Research Business is subject
to regulation and its activities involve providing services helping pharmaceutical and biotechnology companies and CROs that are subject
to regulation. Changes in regulations, especially those that affect clinical trials, could adversely affect demand for our services.
Also, if government efforts to contain drug costs or changes in the practices of health insurers impact pharmaceutical and biotechnology
companies’ profits from new drugs, they may spend less, or reduce their growth in spending on research and development, thereby
reducing the market for clinical trials.
Failure
to comply with existing regulations or contractual obligations could result in a loss of revenue or earnings or increased costs.
Failure
on the part of the Alpha Research Business to comply with applicable regulations, whether imposed directly or required to be complied
with by contract, could have adverse effects. If this were to happen, we could be contractually required to repeat the trial at no further
cost to our customer, but at substantial cost to us, or the contract could be terminated; in either case, we could be exposed to a lawsuit
seeking substantial monetary damages.
We may
bear financial losses because most of our clinical trial contracts are fixed price and may be delayed or terminated or reduced in scope
for reasons beyond our control.
Many of
our clinical trials contracts provide for services on a fixed-price or capped fee-for-service basis and they may be terminated or reduced
in scope either immediately or upon notice. Cancellations may occur for a variety of reasons, including the inefficacy of a drug or device;
its failure to meet safety requirements; unexpected or undesired results; insufficient patient enrollment; insufficient investigator
recruitment; a client’s decision to terminate the development of a product or to end a particular study; and our failure to perform
our duties under the contract properly.
The loss,
reduction in scope or delay of a contract or the loss, delay or conclusion of multiple contracts could materially adversely affect our
business, although our contracts often entitle us to receive the costs of winding down terminated projects, as well as all fees earned
by us up to the time of termination.
We may
suffer losses if we underprice our contracts or incur overrun costs.
Since
Alpha Research Institute’s contracts are often structured based on a fixed price or a fee for service with a cap, we would bear
the loss if we were to misestimate costs. Underpricing or cost overruns could have a material adverse effect on our business, results
of operations, financial condition, and cash flows.
The
potential loss or delay of a contract or multiple contracts could adversely affect our results.
Most of our contracts for clinical trials can
be terminated by our customers upon 30 to 90 days’ notice or immediately in certain circumstances. Our clients may delay, terminate
or reduce the scope of our contracts for a variety of reasons beyond our control, including but not limited to decisions to forego or
terminate a particular clinical trial; lack of available financing, budgetary limits or changing priorities; actions by regulatory authorities;
production problems resulting in shortages of the drug being tested; failure of products being tested to satisfy safety requirements
or efficacy criteria; unexpected or undesired clinical results for products; insufficient patient enrollment in a clinical trial; insufficient
investigator recruitment; shift of business to a competitor or internal resources; product withdrawal following market launch; shut down
of manufacturing facilities; or our failure to comply with the provisions of a contract.
In the event of termination, our contracts often
provide for fees for winding down the project, but these fees may not be sufficient for us to realize the full amount of revenues or
profits anticipated thereunder.
If the Alpha Research Business fails to
perform services in accordance with contractual requirements, regulatory standards and ethical considerations, we could be subject to
significant costs or liability and our reputation could be harmed.
We contract
with Sponsors and CROs in performing clinical trials to assist them in bringing new drugs to market. Clinical trials are complex and
subject to contractual requirements, regulatory standards and ethical considerations. If we fail to perform in accordance with these
requirements, regulatory agencies may take action against us or customers may terminate contracts. Customers may also bring claims against
us for breach of our contractual obligations and patients in the clinical trials and patients taking drugs approved on the basis of those
clinical trials may bring personal injury claims against us for negligence. Any such action could have a material adverse effect on our
results of operations, financial condition and reputation. The occurrence of any of the foregoing could impact our ability to provide
the same level of service to our clients, require us to modify our services or increase our costs, which could materially and adversely
affect our operating results and financial condition.
We are subject to federal and state health
privacy laws and regulations. If we cannot comply or have not fully complied with such laws and regulations, we could face government
enforcement actions, civil penalties, criminal sanctions, or damages, which could harm our reputation and adversely affect our business.
The Health Insurance Portability and Accountability
Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act and their respective implementing regulations
(“collectively, HIPAA”), establishes federal privacy and security standards for the protection of individually identifiable
health information that apply to health plans, healthcare clearinghouses, and healthcare providers that submit certain covered transactions,
or “covered entities.” A subset of these standards also applies to “business associates,” which are persons or
entities that perform certain services for, or on behalf of, a covered entity that involve creating, receiving, maintaining, or transmitting
protected health information.
Some of our customers may be HIPAA-covered entities
and service providers, and in that context, we may function as a business associate under HIPAA. Among other things, this status means
that, for certain activities, we must comply with applicable administrative, technical, and physical safeguards as required by HIPAA,
including stringent data security obligations. Failure to comply with HIPAA can result in significant civil monetary penalties and, in
certain circumstances, criminal penalties with fines or imprisonment.
The HIPAA-covered entities and service providers
that we serve as business associates may require us to enter into HIPAA-compliant business associate agreements with them. If
we were unable to comply with our obligations as a HIPAA business associate, we could face contractual liability under the applicable
business associate agreement.
In addition, many state laws govern the privacy
and security of health information in certain circumstances, many of which differ from HIPAA. There may also be costs associated with
responding to government investigations regarding alleged violations of these and other laws and regulations, even if there are ultimately
no findings of violations or no penalties imposed. These costs could consume our resources and impact our business. Publicity from alleged
violations could harm our reputation.
If we are unable to meet the requirements of
HIPAA, our business associate agreements or state health privacy laws, we could face contractual liability or civil and criminal liability
under HIPAA, all of which could have an adverse impact on our business and generate negative publicity, which, in turn, could have an
adverse effect on our ability to attract new customers and adversely affect our business condition and prospects.
We may be adversely affected by client
concentration.
We derive the majority of our revenues from a
few customers. If any of them decreases or terminates its relationship with us, our business, results of operations or financial condition
could be materially adversely affected. For further information, see “Description of Business – Concentration of Revenues.”
Our business could incur liability if a
drug causes harm to a patient. While we are generally indemnified and insured against such risks, we may still suffer financial losses.
We could suffer liability for harm allegedly
caused by a drug or device for which we conduct a clinical trial, either as a result of a lawsuit against the Sponsor or CRO to which
we are joined or an action launched by a regulatory body. While we are generally indemnified for such harm under our agreements with
Sponsors and CROs, we could nonetheless incur financial losses, regulatory penalties or both. Further, the indemnification obligations
of Sponsors and CROS are enforceable by us only if specific facts, which may be difficult to prove or may be subject to dispute, exist.
Any claim could result in potential liability for us if the claim is outside the scope of such indemnification, the Sponsor or CRO does
not comply with its indemnification obligations or our liability exceeds applicable indemnification limits or available insurance coverage.
Further, we do not carry insurance to cover damages for which we are liable. Such a claim could have an adverse impact on our financial
condition and results of operations. Furthermore, the associated negative publicity could have an adverse effect on our business and
reputation.
If we fail to maintain an effective system
of disclosure controls and internal control over financial reporting, our ability to produce timely and accurate financial statements
or comply with applicable regulations could be impaired.
The Sarbanes-Oxley Act of 2002 (“SOX”)
requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting.
However, our independent registered public accounting has advised management that we have the following material weaknesses in internal
control: lack of in-house personnel with insufficient technical knowledge to identify and address certain accounting matters; insufficient
accounting personnel to perform duties over financial transaction processing, key account reconciliations and reporting; insufficient
written policies and procedures over accounting transaction processing such that routine transactions are recorded on an accrual basis
in a timely manner; and insufficient in-house knowledge on monitoring accounting standards for the impact of complex accounting standards.
Accordingly, we need to develop and refine
our disclosure controls and other procedures to ensure that information required to be disclosed by us in the reports that we will file
under the Exchange Act is accumulated and communicated to our principal executive and financial officers. We are also continuing to improve
our internal control over financial reporting. To maintain and improve effective disclosure controls and procedures and internal control
over financial reporting, we will need to expend significant resources, including accounting-related costs and significant management
oversight.
Our current controls and any new controls that
we develop may become inadequate because of changes in conditions in our business. Further, weaknesses in our disclosure controls and
internal control over financial reporting may be discovered. Any failure to develop or maintain effective controls or any difficulties
encountered in their implementation or improvement could adversely affect our results of operations or cause us to fail to meet our reporting
obligations and result in a restatement of our consolidated financial statements. Failure to implement and maintain effective internal
control over financial reporting could also adversely affect the results of periodic management evaluations and annual independent registered
public accounting firm attestation reports regarding the effectiveness of our internal control over financial reporting that we will
eventually be required to include in our periodic reports filed with the SEC. Ineffective disclosure controls and procedures and internal
control over financial reporting could also cause investors to lose confidence in our reported financial and other information, which
could have a negative effect on the trading price of Common Stock. We are not currently required to comply with the SEC rules that implement
Section 404 of SOX and are therefore not required to make a formal assessment of the effectiveness of our internal control over financial
reporting for that purpose. After the registration statement of which this Prospectus forms a part is made effective, we will be required
to provide an annual management report on the effectiveness of our internal control over financial reporting commencing with our second
annual report on Form 10-K.
Our independent registered public accounting
firm will not be required to attest formally to the effectiveness of our internal control over financial reporting until after we cease
to be an “emerging growth company” as defined in the JOBS Act. At that time, our independent registered public accounting
firm may issue an adverse report if it is not satisfied with the level at which our internal control over financial reporting is documented,
designed or operating. Any failure to maintain effective disclosure controls and internal control over financial reporting could harm
our business, results of operations, and financial condition and could cause a decline in the price of Common Stock.
The Company is an “emerging growth
company,” as defined in the Securities Act (an “EGC”), and a “smaller reporting company,” as defined in
Rule 405 promulgated under the Securities Act (an “SRC”) and intends to take advantage of certain exemptions from disclosure
requirements available to it. Doing so could make the Common Stock less attractive to investors and make it more difficult to compare
the performance of the Company with that of other public companies.
As long as the Company is an EGC, it intends to
utilize certain exemptions from reporting requirements that apply to public companies that are not EGCs. Among the reporting requirements
from which the Company is so exempted are the auditor attestation requirements of SOX, certain disclosures relating to executive compensation,
holding a nonbinding advisory vote on executive compensation and stockholder approval of “golden parachute” payments. The
Company is permitted to be an emerging growth company for up to five years or until the earliest of (i) the last day of the first
fiscal year in which its annual gross revenues exceed $1 billion, (ii) the date that it becomes a “large accelerated filer”
as defined in Rule 12b-2 under the Exchange Act, which would occur if the market value of Common Stock that is held by non-affiliates
exceeds $700 million as of the last business day of its most recently completed second fiscal quarter, or (iii) the date on which
it has issued more than $1 billion in nonconvertible debt during the preceding three-year period.
As an SRC, the Company intends to utilize certain
reduced disclosure requirements, including publishing two years of audited financial statements instead of three years, as required for
companies that are not SRCs. The Company will remain an SRC until the last day of the fiscal year in which it had (i) a public float
that exceeded $250 million or (ii) annual revenues of more than $100 million and a public float that exceeded $700 million. To the extent
the Company takes advantage of such reduced disclosure obligations, it may make comparison of its financial statements with those
of other public companies difficult or impossible.
After the Company ceases to be an EGC, it is
expected to incur additional management time and cost to comply with the more stringent reporting requirements applicable to companies
that are accelerated filers or large accelerated filers, including complying with the auditor attestation requirements of Section 404
of SOX.
Changes in existing financial accounting
standards or practices may harm our results of operations.
Changes in existing accounting rules or practices,
including generally accepted accounting principles in the United States (“GAAP”), new accounting pronouncements or varying
interpretations of current accounting pronouncements or practices could harm our results of operations or the manner in which we conduct
our business. Further, such changes could potentially affect our reporting of transactions completed before such changes are effective.
GAAP is subject to interpretation by the Financial Accounting Standards Board, FASB, the SEC and various bodies formed to promulgate and
interpret appropriate accounting principles. A change in these principles or interpretations could have a significant effect on our reported
financial results and affect the reporting of transactions completed before the announcement of a change. Any difficulties in implementing
these pronouncements could cause us to fail to meet our financial reporting obligations, which could result in regulatory discipline and
harm investors’ confidence in us.
If our estimates or judgments relating
to our critical accounting policies prove to be incorrect, our results of operations could be adversely affected.
Preparing financial statements in conformity
with GAAP requires management to make estimates and assumptions that affect the amounts reported in our consolidated financial statements
and related notes. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under
the circumstances, as provided in “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
The results of these estimates form the basis for making assumptions and judgments affecting our consolidated financial statements, including
those related to revenue recognition, stock-based compensation, the fair value of Common Stock, valuation of strategic investments, periods
of benefit for deferred costs, and uncertain tax positions. Our results of operations may be adversely affected if our assumptions change
or if actual circumstances differ from those in our assumptions, which could cause our results of operations to fall below the expectations
of securities analysts and investors, resulting in a decline in the trading price of Common Stock.
The Company’s business depends substantially
on the continuing efforts of its executive officers, and its business may be severely disrupted if it were to lose the services rendered
by any of them.
The Company’s future success depends substantially
on the continued services of its executive officers. The Company does not maintain key-man life insurance on its executive officers.
If any of these executive officers were unable or unwilling to continue in their present positions, the Company might not be able to
replace them readily, if at all. The loss of any of these officers could cause the Company’s business to be disrupted, and it could
incur additional expenses to recruit and retain new officers.
This risk is increased because the Company has
no employment contracts with its officers and is paying them sporadically and in varying amounts as the Company’s financial condition
permits. Further, their salaries are not commensurate with their contributions and abilities. While none of these officers has indicated
when or whether he would terminate his employment if he continues to be paid on the basis set forth above, the Company believes that
they may not work for it indefinitely without appropriate and regularly paid compensation. If the Company were to lose any of its officers,
its ability to operate would be materially impaired.
The Company’s business depends substantially
on recruiting additional members of management and key personnel and its business could be severely disrupted if it were unable to hire
such personnel or lose their services.
The Company needs to attract, hire and retain
additional managers and key employees to implement its business plan. If it were unable to do so or if, after being hired, any
of the members of the Company’s management were lost, it would have to spend a considerable amount of time and resources searching,
recruiting, and integrating their replacements, which would substantially divert management’s attention from and severely disrupt
its business. The Company may face difficulties in attracting and retaining additional management and, if it were to lose any of them,
in attracting and retaining their replacements because it cannot presently pay competitive compensation and its future is uncertain.
Litigation could adversely affect the Company’s
business, financial condition and results of operations.
From time to time, the Company may become subject
to litigation that may result in liability materially adverse to its financial condition or may negatively affect its operating results
if changes to its business operation are required. The cost of defending such litigation could be significant and require the diversion
of its resources. Adverse publicity associated with litigation could negatively affect perceptions of the Company, regardless of whether
the allegations are valid or whether the Company is ultimately found not to be liable. As a result, litigation could adversely affect
the Company’s business, financial condition and results of operations.
Acquisitions, other strategic alliances
and investments could result in operating difficulties, dilution, and other harmful consequences that may adversely impact the Company’s
business and results of operations.
The Company may acquire other businesses and
these transactions could be material to its financial condition and results of operations. The areas where it may encounter risks in
connection with acquisitions include, but are not limited to, the failure to successfully further develop the acquired business, the
implementation or remediation of controls, procedures and policies at the acquired business, the transition of operations, users and
customers onto our existing platforms, and the challenges associated with integrating the acquired business and its employees into the
Company’s organization, as well as retaining employees of the acquired businesses. Failure to address these risks or other problems
encountered in connection with acquisitions successfully could cause the Company to fail to realize the anticipated benefits of such
acquisitions, investments or alliances, incur unanticipated liabilities, and harm its business generally.
Such acquisitions could also result in dilutive
issuances of the Company’s equity securities, the incurrence of debt, contingent liabilities or amortization expenses, impairment
of goodwill and purchased long-lived assets, or restructuring charges, any of which could adversely affect its financial condition, results
of operations and cash flows. Also, the anticipated benefits and synergies of acquisitions may not materialize.
Because our success depends in part on
our ability to expand our operations outside the United States, our business will be susceptible to risks associated with international
operations.
We currently maintain operations and have personnel
outside the United States in Mexico, Jordan, Ecuador, Columbia, Venezuela, Argentina and Brazil. We plan to expand our international
operations into Argentina, Chile, Peru, Panama and other countries where our activities are lawful. In the nine months ended February
28, 2023, and the fiscal years ended May 31, 2022, and May 31, 2021, our non-U.S. revenue was approximately 5.3%, 4.2% and
5% of our total revenue, respectively. We expect to continue to expand our international operations, but these efforts may not be successful.
In addition, conducting international operations subjects us to new risks, some of which we have not generally faced in the United States
or other countries where we currently operate. These risks include, among other things: lack of familiarity and burdens of complying
with foreign laws, legal standards, regulatory requirements and other barriers, and the risk of penalties to the Company, its management
and employees if its practices are deemed to be out of compliance; unexpected changes in regulatory requirements, taxes, trade laws,
tariffs, export quotas, customs duties, or other trade restrictions; longer accounts receivable payment cycles and difficulties in collecting
accounts receivable; increased financial accounting and reporting burdens and complexities; difficulties in managing and staffing international
operations including the proper classification of independent contractors and other contingent workers, differing employer/employee relationships,
and local employment laws; increased costs involved with recruiting and retaining an expanded employee population outside the United
States through cash- and equity-based incentive programs and unexpected legal costs and regulatory restrictions in issuing our shares
to employees outside the United States; global political and regulatory changes that may lead to restrictions on immigration and travel
for our employees outside the United States; potentially adverse tax consequences, including the complexities of foreign value added
tax (or other tax) systems, and restrictions on the repatriation of earnings; and permanent establishment risks and complexities in connection
with international payroll, tax, and social security requirements for international employees.
Additionally, operating in international markets
requires significant management attention and financial resources. There is no certainty that the investments and additional resources
required to establish operations in other countries will produce the desired revenue or profitability.
Compliance with laws and regulations applicable
to our global operations also substantially increases our cost of doing business in foreign jurisdictions. We have limited experience
in operating outside the United States, which increases the risk that any operations that we may undertake will not be successful. If
we invest substantial time and resources to expand our international operations and are unable to do so successfully and timely, our business,
results of operations, and financial condition will suffer. We may be unable to keep current with changes in government requirements as
they change from time to time. Failure to comply with these regulations could harm our business. In many countries, it is common for others
to engage in business practices that are prohibited by United States law and regulation or by our policies and procedures.
A portion
of our operations is conducted in foreign jurisdictions and is subject to the economic, political, legal and business environments of
the countries where we do business. Risks associated with such international operations could negatively affect our business, financial
condition, results of operations and cash flows.
We have operations outside the United
States and plan to expand them. International operations inherently subject us to a number of risks and uncertainties, including those
arising from compliance with governmental controls, trade restrictions, restrictions on direct investments, quotas, embargoes, import
and export restrictions, tariffs, duties, and regulatory and licensing requirements by domestic or foreign entities, including restrictions
administered by the Office of Foreign Assets Control of the U.S. Department of the Treasury; difficulties in building, staffing and managing
foreign operations (including a geographically dispersed workforce) and maintaining compliance with foreign labor laws; burdens to comply
with, and different levels of protection offered by, multiple and potentially conflicting foreign laws and regulations, including those
relating to environmental, health and safety requirements and intellectual property; changes in laws, regulations, government controls
or enforcement practices with respect to our business and the businesses of our customers; political and social instability, including
crime, civil disturbance, terrorist activities, armed conflicts and natural and other disasters; ongoing instability or changes in a
country’s or region’s regulatory, economic or political conditions; local business and cultural factors that differ from
our standards and practices, including business practices prohibited by the Foreign Corrupt Practices Act and other anti-corruption laws
and regulations; longer payment cycles and increased exposure to counterparty risk; and differing needs of foreign customers.
The international nature of our business subjects
us to potential risks that various taxing authorities may challenge the pricing of our cross-border arrangements and subject us to additional
tax, adversely impacting our effective tax rate and tax liability.
In addition, international transactions may involve
increased financial and legal risks due to differing legal systems and customs. Compliance with these requirements may prohibit the import
or export of certain products and technologies or require us to obtain licenses before importing or exporting certain products or technology.
Our failure to comply with any of these laws, regulations or requirements could result in civil or criminal legal proceedings, monetary
or non-monetary penalties, or both, disruptions to our business, limitations on our ability to import and export products and services,
and damage to our reputation.
While the impact of these factors is difficult
to predict, any of them could have a material adverse effect on our business, financial condition, results of operations and cash flows.
Changes in any of these laws, regulations or requirements, or the political environment in a particular country, may affect our ability
to engage in business transactions in certain markets, including investment, procurement and repatriation of earnings.
We are subject to anti-corruption, anti-bribery,
and similar laws, and non-compliance with them could subject us to criminal penalties or significant fines and harm our business and
reputation.
We are subject to anti-corruption and anti-bribery
and similar laws, such as the Foreign Corrupt Practices Act of 1977 (the “FCPA”), and other anti-corruption, anti-bribery
and anti-money laundering laws in the United States and in the countries in which we conduct activities. These laws prohibit companies
and their employees and agents from promising, authorizing, making, or offering improper payments or other benefits to government officials
and others in the private sector. As we increase our international operations, our risks under these laws may increase. Anti-corruption
and anti-bribery laws have been enforced vigorously in recent years and interpreted broadly. Noncompliance with these laws could subject
us to investigations, sanctions, settlements, prosecution, other enforcement actions, disgorgement of profits, significant fines, damages,
other civil and criminal penalties or injunctions, adverse media coverage, and other consequences. Any investigations, actions or sanctions
could harm our business, results of operations, and financial condition. Under some of these laws, we may be held liable for the corrupt
or other illegal activities of intermediaries, and our employees, representatives, contractors, partners, and agents, even if we do not
explicitly authorize such activities. We intend to implement an anti-corruption compliance program but cannot assure that all of these
persons will not take actions in violation of our policies and applicable law, for which we may be ultimately held responsible. Any violation
of the FCPA, other applicable anti-corruption laws or anti-money laundering laws could result in whistleblower complaints, negative
media coverage, investigations, loss of privileges and severe criminal or civil sanctions, any of which could have a materially adverse
effect on our reputation, business, results of operations, and prospects.
Our business is exposed to domestic and
foreign currency fluctuations that could have a material adverse effect on our business, financial condition, results of operations and
cash flows.
In the nine months ended February 28, 2023,
and the fiscal years ended May 31, 2022, and May 31, 2021, our non-U.S. revenue was approximately 5.3%, 4.2% and 5% of our
total revenue, respectively. With the abatement of the Covid-19 pandemic permitting the reopening of classrooms in Latin America, this
percentage may increase. Changes in non-U.S. currencies relative to the U.S. dollar impact our revenues, profits, assets and liabilities.
In addition, the weakening or strengthening of the U.S. dollar may result in significant favorable or unfavorable translation effects
when the operating results of our non-U.S. business activity are translated into U.S. dollars and could cause our results of operations
to differ from our expectations and the expectations of our investors. For our international sales denominated in U.S. dollars, an increase
in the value of the U.S. dollar relative to foreign currencies could make our products and services less competitive in international
markets. Alternatively, a weakening of the currencies in which sales are generated relative to those in which costs are denominated would
decrease operating profits and cash flow. Changes in currency exchange rates may also affect the relative prices at which we provide
services in foreign markets. In addition, the impact of currency devaluations in countries experiencing high inflation rates or significant
currency exchange fluctuations could negatively impact our operating results. While we may use financial instruments to mitigate the
impact of fluctuations in currency exchange rates on our cash flows, unhedged exposures would continue to be subject to currency fluctuations.
If we fail to manage our growth effectively,
we may be unable to execute our business plan or maintain high service levels and customer satisfaction.
We hope to attain rapid growth. Doing so will
place significant demands on our management and operational and financial resources. We have established international operations, including
Mexico, Peru, Ecuador, Columbia and the Dominican Republic. We plan to expand into Argentina, Chile, Brazil, Panama and other countries
where its activities are lawful. In addition, our organizational structure will become more complex as we grow, as will our operational,
financial and management controls and reporting systems and procedures. To manage growth in our operations, we will need to continue
to grow and improve our operational, financial and management controls and reporting systems and procedures. We will require significant
capital expenditures and the allocation of valuable management resources to grow and change in these areas. Our growth will place a significant
strain on our management and may distract management from other important functions. If we cannot manage our growth effectively, our
reputation, as well as our business, results of operations and financial condition, could be harmed.
We may not be able to compete effectively.
While we believe that the market served by the
Pharmacology University Business has few participants, if one or more competitors were to enter this market, we might not be able to
compete effectively for many reasons, including a competitor’s greater financial resources, better services, a more effective sales
organization or a superior website. The Sleep Center, in contrast, provides services in a market that is highly fragmented and
has many competitors, and in which the ability to compete successfully depends on quality of service, the ability to form and maintain
professional relationships and satisfy demanding customers.
Many of our existing competitors have, and some
of our potential competitors could have, substantial competitive advantages such as greater recognition and longer operating histories,
larger sales and marketing budgets and resources, and, especially in the case of the Alpha Research Business and the Sleep Center
Business, established relationships with customers, greater resources to make acquisitions, lower labor costs and substantially greater
financial and other resources. Competitors with greater financial and operating resources may be able to respond more quickly and effectively
than we can to new or changing opportunities, developments or customer requirements. Conditions relating to the Alpha Research Business
and the Sleep Center Business could also change rapidly and significantly, potentially adversely, as a result of changes in the
laws relating to cannabis, especially at the federal level.
If we do not compete effectively with established
companies as well as new market entrants, our business, results of operations, and financial condition could be harmed. Competitive pressures
could result in price reductions; fewer customers; reduced revenue, gross profit and gross margins; increased net losses; and loss of
market share.
Risks Related to the Common Stock and This
Offering
There are risks, including stock market
volatility, inherent in owning Common Stock.
The market price and volume of the Common Stock
have been, and may continue to be, subject to significant fluctuations and trading in Common Stock has often been sporadic. These fluctuations
may arise from general stock market conditions, the impact of risk factors described herein on our results of operations and financial
position, or a change in opinion in the market regarding our business prospects or other factors, many of which may be outside our control.
We believe that this has and may continue to materially and adversely affect our ability to fund our business through sales of equity
securities and could adversely affect the retentive power of our 2022 Equity Incentive Plan. The lack of an active market for Common
Stock may impair investors’ ability to sell their shares when they wish to sell them or at prices that they consider reasonable,
may reduce the fair market value of their shares and may impair the Company’s ability to raise capital to continue to fund operations
by selling shares and may impair its ability to acquire additional intellectual property assets by using our shares as consideration.
We may change the Fixed Offering Price.
If we cannot sell the Shares at the Fixed
Offering Price, we may amend the Registration Statement of which this Prospectus is a part to reduce it one or more times. In any such
event, investors who had purchased Shares before the reduction would suffer an immediate and perhaps permanent loss in the market value
of their Shares.
There will be a large number of shares
of Common Stock that will be eligible to be sold in the public markets.
In addition to the 10,283,940,599 shares of
Common Stock that are offered by this Prospectus, (i) approximately 935,000,000 shares of Common Stock held by persons who are not affiliates
of the Company will be permitted to be sold after January 13, 2024, under Rule 144 promulgated by the SEC under the Securities Act (“Rule
144”) without notice to the SEC in unlimited amounts and without restriction as to the manner of sale and (ii) 3,512,111,700 shares
of Common Stock held by a person who is an affiliate of the Company will be permitted to be sold under Rule 144 after January 13, 2024,
in limited amounts, subject to notice to the SEC and subject to restriction as to the manner of sale and (iii) up to 600,000,000 shares
of Common Stock that may be issued under the Company's 2022 Equity Incentive Plan may be sold in the public markets, subject to limitations
in the case of shares issued under that plan to affiliates of the Company, upon the filing of a registration statement on Form S-8 with
respect thereto or without registration under Rule 144 after being held by for the period required by that rule. The sale of these shares
or the perception that they may be sold may substantially and adversely affect the market price of the Common Stock, with the result
that persons who acquire shares of Common Stock in the Offering may be able to resell them only at substantial losses. For further information
concerning shares that are eligible for future resale, see “Shares Eligible for Future Resale.”
If FINRA does not process the Company’s
application to change its name on FINRA’s records, the Company and its shareholders would be adversely affected.
On December 6, 2022, the Company filed an amendment
to its articles of incorporation changing its corporate name to its present name with the Secretary of State of the State of Colorado.
In order for the Common Stock to trade under the new name, FINRA must process the Company’s application to change its name on FINRA’s
records. FINRA has requested that the Company furnish additional information before FINRA will commence such processing. Some of this
information is unavailable and the Company is asking FINRA to proceed with processing because such information is not relevant to review
of the application. If FINRA does review the application, it may deny processing for a number of reasons, the most significant of which
is that the Company failed to file reports with the SEC from 2012 to 2015, if FINRA determines that such denial is “necessary for
the protection of investors, the public interest and to maintain fair and orderly markets.” The Company does not know whether FINRA
will review the application or, if it does, whether it will deny processing because of the Company’s failure to file reports or
for some other reason. If FINRA does not review the application or if having determined to review it, FINRA denies processing, the Company
intends to contest such failure to review or denial by appeal within FINRA and to the SEC and/or in court, but may not succeed. Unless
and until FINRA processes the application, the Common Stock will continue to trade under the Company’s former name and the Company
may determine to resume its former name. The Company believes that in these events, its ability to sell the shares offered by this Prospectus
will be impaired, as will the ability of investors who purchase such shares to resell them. If FINRA will not process the name change,
the Company believes that it will be difficult for it to process dividends and other matters subject to FINRA’s processing.
The Company may fail to comply with
its reporting obligations.
The Company is required to file reports with
the SEC, including annual and quarterly reports. These reports are required to contain information about the Company’s business
and operations, as well as financial statements (which will be audited by the Company’s public accounting firm in the case of annual
reports and reviewed by such firm in the case of quarterly reports) and provide other information upon which investors will rely in making
investment decisions about the Company. The Company, under prior management, failed to file these reports. The Company’s current
management intends to cause it to comply with its reporting obligations, but investors should consider such failure in making a decision
as to whether to purchase the shares offered by this Prospectus. The Company may also become delinquent if its funding were to become
insufficient to pay the legal and accounting costs of preparing these reports. If the Company were again to become delinquent, investors
would be deprived of material information about the Company, which could adversely affect the market price for the Common Stock, such
that holders of Common Stock could lose some or all of their investment and could encounter limited or no markets for the sale of their
securities.
If the Company issues additional equity
or equity-linked securities, investors may incur immediate and substantial dilution in the book value of their shares.
If
the Company issues additional shares of Common Stock (including under stock options or warrants)
or securities convertible into or exchangeable or exercisable for shares of Common Stock,
its stockholders, including investors who purchase shares of Common Stock in the Offering,
may experience additional dilution. Any such issuances may result in downward pressure on
the price of the Common Stock. No assurance can be given that investors will be able to sell
shares sold pursuant to this Prospectus at a price per share that is equal to or greater
than the prices that they pay. The Company has sold substantial amounts of shares at below-market
prices. For example, since February 28, 2023, when the Company had 8,846,919,983 shares of
Common Stock outstanding, it has sold 824,572,248 shares of Common Stock at below-market
prices for proceeds totaling $250,000. To the extent that the Company does not sell all of
the shares of Common Stock offered by this Prospectus, it may continue this practice. If
it does so, shareholders will suffer substantial dilution.
The Company does not intend to pay dividends
for the foreseeable future and investors must rely on increases in the market price of the Common Stock for returns on their investments.
For the foreseeable future, the Company intends
to retain its earnings, if any, to finance the development and expansion of our business, and the Company does not anticipate paying
any cash dividends on the Common Stock. Accordingly, investors must be prepared to rely on sales of their Common Stock after price appreciation
to earn an investment return, but no assurance can be given that the price of the Common Stock will appreciate or if it does, that it
will remain at or rise above the level to which it has appreciated. Any determination to pay dividends in the future will be made at
the discretion of the Company’s board of directors (the “Board”) and will depend on our results of operations, financial
condition, capital needs, contractual restrictions, restrictions imposed by applicable law and other factors the Board
deems relevant.
Because the Common Stock is subject to
the penny stock rules, it may be more difficult to sell.
The SEC has adopted rules that regulate broker-dealer
practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00
(subject to exceptions that do not apply to the Common Stock). The penny stock rules require a broker-dealer, at least two business days
prior to a transaction in a penny stock not otherwise exempt from those rules, to deliver to the customer a standardized risk disclosure
document containing specified information and to obtain from the customer a signed and date an acknowledgment of receipt of that
document. In addition, these rules require that, prior to effecting any transaction in a penny stock not otherwise exempt from those rules,
a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive:
(i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions
involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These requirements may have the effect of
reducing the trading activity in Common Stock, and therefore stockholders may have difficulty selling their shares.
One person has voting control of the company
and may authorize or prevent corporate actions to the detriment of other stockholders.
One person, who is an officer and director of
the Company, through his ownership of Series B Preferred, has voting control of the Company. Accordingly, he has the power to determine
the outcome of all matters requiring the approval of the stockholders, including the election of directors and the approval of mergers
and other significant corporate transactions. His interests could conflict with the interests of other stockholders.
CAUTIONARY NOTES
Regarding Forward-Looking Statements
This Prospectus contains forward-looking statements
about us and our industry that involve substantial risks and uncertainties. All statements other than statements of historical facts
contained in this Prospectus, including statements regarding our strategy, future financial condition, future operations, projected costs,
prospects, plans, objectives of management, and expected market growth, are forward-looking statements. In some cases, you can identify
forward-looking statements because they contain words such as “may,” “will,” “shall,” “should,”
“expects,” “plans,” “anticipates,” “could,” “intends,” “target,”
“projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential,”
“goal,” “objective,” “seeks,” or “continue” or the negative of these words or other similar
terms or expressions that concern our expectations, strategy, plans, or intentions. Forward-looking statements contained in this Prospectus
include, but are not limited to, statements about the effects of the COVID-19 pandemic on our business and the U.S. and global economies
generally; our expectations regarding our financial performance; our expectations regarding future operating performance; our ability
to attract and retain customers; our ability to compete in our industries; our ability to meet our liquidity needs; our ability to effectively
manage our exposure to fluctuations in foreign currency exchange rates; the increased expenses associated with being a public company;
the size of our addressable markets, market share, and market trends, including our ability to grow our business in the countries we
have identified as near- term priorities; anticipated trends, developments, and challenges in our industry, business, and the highly
competitive markets in which we operate; our ability to anticipate market needs or develop new or enhanced offerings and services to
meet those needs; our ability to manage expansion into international markets and new industries; our ability to comply with laws and
regulations, including laws affecting the cannabis and pharmaceutical industries, that currently apply or may become applicable to our
business both in the United States and internationally; our ability to effectively manage our growth and expand our infrastructure and
maintain our corporate culture; our ability to identify, recruit, and retain skilled personnel, including key members of senior management;
our ability to successfully defend litigation brought against us; our ability to successfully identify, manage, and integrate any existing
and potential acquisitions; our ability to maintain, protect, and enhance our intellectual property; and our intended use of the net
proceeds from this offering.
You should not rely upon forward-looking statements
as predictions of future events. We have based the forward-looking statements in this Prospectus primarily on our current expectations,
estimates, forecasts, and projections about future events and trends that we believe may affect our business, results of operations, financial
condition, and prospects. Although we believe that we have a reasonable basis for each such forward-looking statement, we cannot guarantee
that the future results, activity levels, performance, or events and circumstances reflected in the forward-looking statements will be
achieved. The outcome of the events described or discussed in these forward-looking statements is subject to risks, uncertainties, and
other factors described in the section titled “Risk Factors” and elsewhere in this Prospectus. Moreover, we operate in a very
competitive and rapidly changing environment. New risks and uncertainties emerge from time to time, and we cannot predict all of
them that could have an impact on the forward-looking statements contained in this Prospectus. The results, events, and circumstances
reflected in the forward-looking statements may not be achieved or occur, and actual results, events or circumstances could differ materially
from those described in the forward-looking statements.
The forward-looking statements in this Prospectus
relate only to events or circumstances as of the date on which they are made. We undertake no obligation to update any forward-looking
statement in this Prospectus to reflect events or circumstances after the date of this Prospectus or to reflect new information or the
occurrence of unanticipated events, except as required by law. We may not achieve the plans, intentions, or expectations disclosed in
our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Our forward-looking statements
do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures, or investments we may make.
In addition, statements that “we believe”
and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available
to us as of the date of this Prospectus, and while we believe such information forms a reasonable basis for such statements, such information
may be limited or incomplete, and such statements should not be read to indicate that we have conducted an exhaustive inquiry into, or
review of, all potentially available relevant information. These statements are inherently uncertain, and you should not unduly rely
upon them.
You should read this Prospectus and the documents
referred to in it completely and with the understanding that our actual future results may be materially different from what we expect.
All forward-looking statements in this Prospectus are qualified by these cautionary statements.
Third-Party Information
This Prospectus includes information and estimates
based on reports and other publications, sources from industry analysts, market research firms and other independent sources that were
generally available to the public and not commissioned by us, in addition to management’s good-faith estimates and analyses.
We believe that such reports and publications are reliable but have not independently verified them or their underlying data sources,
methodologies or assumptions. They contain information and estimates that are based on estimates, forecasts, projections, market research,
or similar methodologies and are inherently subject to uncertainties. Actual events or circumstances may differ materially from events
and circumstances reflected in these reports.
Descriptions of Contracts
This Prospectus may contain descriptions of contracts
and instruments to which the Company or its officers and directors are parties or by which it is affected. These contracts and instruments
are exhibits to the Registration Statement of which this Prospectus is a part and are identified in Item 16, Exhibits, Financial Statement
Schedules. Where any such contract or instrument is described in this Prospectus, you are referred to the related exhibit, which
may be found on the SEC’s website, and the description thereof is qualified by such reference.
USE OF PROCEEDS AND BUSINESS
PLAN
This Prospectus relates in part to shares of
Common Stock that may be offered and sold from time to time by the Company and in part to shares being offered and sold by the Selling
Shareholders. We will receive proceeds from sales of the shares that we are offering and none of the proceeds of sales of shares offered
by the Selling Stockholders. See “Plan of Distribution.”
We will realize gross proceeds from the Offering
of $1,250,000 if 25% of the shares offered by us are sold, $2,500,000 if 50% of such shares are sold, $3,750,000 if 75% of such shares
are sold and $5,000,000 if 100% of such shares are sold.
Use of Proceeds
The following table shows how we expect to
use the net proceeds from our sales of the shares in executing our business plan, which is discussed below. Further details as to such
use appear in “Business Plan.” The table does not represent the order of priority in which such proceeds may be applied.
Estimated
Use of Proceeds for 25%, 50%, 75%, and 100% of Offering
| | |
25% of
Offering | | |
50% of
Offering | | |
75% of
Offering | | |
100%
of Offering | |
| | |
Dollar
Amount | | |
|
% of Gross Proceeds |
|
|
|
Dollar Amount | | |
|
% of Gross Proceeds | | |
Dollar
Amount | | |
% of
Gross Proceeds | | |
Dollar
Amount | | |
% of
Gross Proceeds | |
| Alpha Research Institute | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Increase employees from
8 to 35 | |
$ | 131,250 | | |
| 10.5% | | |
$ | 262,500 | | |
| 10.5% | | |
$ | 393,750 | | |
| 10.5% | | |
$ | 525,000 | | |
| 10.5% | |
| Obtain new contacts with health professionals,
sponsors and CROs | |
$ | 7,500 | | |
| 0.6% | | |
$ | 15,000 | | |
| 0.6% | | |
$ | 22,500 | | |
| 0.6% | | |
$ | 30,000 | | |
| 0.6% | |
| Contract with at least ten new principal
investigators specializing in various areas of medicine | |
$ | 25,000 | | |
| 2.0% | | |
$ | 50,000 | | |
| 2.0% | | |
$ | 75,000 | | |
| 2.0% | | |
$ | 100,000 | | |
| 2.0% | |
| Conduct at least
six seminars with the expectation of generating relationships | |
$ | 5,000 | | |
| 0.4% | | |
$ | 10,000 | | |
| 0.4% | | |
$ | 15,000 | | |
| 0.4% | | |
$ | 20,000 | | |
| 0.4% | |
| Total Alpha Research Institute | |
$ | 168,750 | | |
| 13.5% | | |
$ | 337,500 | | |
| 13.5% | | |
$ | 506,250 | | |
| 13.5% | | |
$ | 675,000 | | |
| 13.5% | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
Pharmacology
University | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Increase the number of annual paid
subscriptions to Cannabis World Journals to at least 5,000 | |
$ | 12,500 | | |
| 1.0% | | |
$ | 25,000 | | |
| 1.0% | | |
$ | 37,500 | | |
| 1.0% | | |
$ | 50,000 | | |
| 1.0% | |
| Sell educational materials to third
parties | |
$ | 25,000 | | |
| 2.0% | | |
$ | 50,000 | | |
| 2.0% | | |
$ | 75,000 | | |
| 2.0% | | |
$ | 100,000 | | |
| 2.0% | |
| Resume and increase classroom and
seminar teaching | |
$ | 62,500 | | |
| 5.0% | | |
$ | 125,000 | | |
| 5.0% | | |
$ | 187,500 | | |
| 5.0% | | |
$ | 250,000 | | |
| 5.0% | |
| Increase our
portfolio of cannabis-related educational material | |
$ | 50,000 | | |
| 4.0% | | |
$ | 100,000 | | |
| 4.0% | | |
$ | 150,000 | | |
| 4.0% | | |
$ | 200,000 | | |
| 4.0% | |
| Total Pharmacology University | |
$ | 150,000 | | |
| 12.0% | | |
$ | 300,000 | | |
| 12.0% | | |
$ | 450,000 | | |
| 12.0% | | |
$ | 600,000 | | |
| 12.0% | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Alpha Fertility and Sleep
Center | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Expand to be
capable of performing sleep tests for 20 patients per month and open a second sleep center | |
$ | 200,000 | | |
| 16.0% | | |
$ | 400,000 | | |
| 16.0% | | |
$ | 600,000 | | |
| 16.0% | | |
$ | 800,000 | | |
| 16.0% | |
| Add additional
staff for in-house sleep studies | |
$ | 87,500 | | |
| 7.0% | | |
$ | 175,000 | | |
| 7.0% | | |
$ | 262,500 | | |
| 7.0% | | |
$ | 350,000 | | |
| 7.0% | |
| Total Alpha Fertility and Sleep Center | |
$ | 287,500 | | |
| 23.0% | | |
$ | 575,000 | | |
| 23.0% | | |
$ | 862,500 | | |
| 23.0% | | |
$ | 1,150,000 | | |
| 23.0% | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Corporate | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Operating costs | |
$ | 243,750 | | |
| 19.5% | | |
$ | 487,500 | | |
| 19.5% | | |
$ | 731,250 | | |
| 19.5% | | |
$ | 975,000 | | |
| 19.5% | |
| Overhead | |
$ | 150,000 | | |
| 12.0% | | |
$ | 300,000 | | |
| 12.0% | | |
$ | 450,000 | | |
| 12.0% | | |
$ | 600,000 | | |
| 12.0% | |
| Legal and accounting | |
$ | 50,000 | | |
| 4.0% | | |
$ | 100,000 | | |
| 4.0% | | |
$ | 150,000 | | |
| 4.0% | | |
$ | 200,000 | | |
| 4.0% | |
| Operating capital | |
$ | 200,000 | | |
| 16.0% | | |
$ | 400,000 | | |
| 16.0% | | |
$ | 600,000 | | |
| 16.0% | | |
$ | 800,000 | | |
| 16.0% | |
| Total Corporate | |
$ | 643,750 | | |
| 51.5% | | |
$ | 1,287,500 | | |
| 51.5% | | |
$ | 1,931,250 | | |
| 51.5% | | |
$ | 2,575,000 | | |
| 51.5% | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total Use of Proceeds | |
$ | 1,250,000 | | |
| 100% | | |
$ | 2,500,000 | | |
| 100% | | |
$ | 3,750,000 | | |
| 100% | | |
$ | 5,000,000 | | |
| 100% | |
The foregoing represents our best estimate as
to how the proceeds of the shares offered by the Company will be expended. We reserve the right to redirect any portion of the funds either
among the items referred to above or to such other projects as our management considers to be in our best interest.
Business Plan
Background
Since the year ended on
May 31, 2020, the Company has striven to grow and improve in the following ways:
Alpha Research Institute
Alpha Research Institute
has devoted time and attention to bettering interinstitutional relationships with the pharmaceutical industry; improving operational
values; creating and re-establishing alliances with clinical study contractors; understanding the needs of its staff and its patients,
improving documentation and internal and external communications, offering transportation to patients and increasing participation in
clinical trials by addressing potential patients’ Covid-related concerns.
Alpha Fertility and Sleep
Center
The mission of the Sleep
Center, which opened in June 2022, is to provide superior care in sleep medicine and, in doing so, to address the concerns of each
patient and his referring physician’s concerns effectively and satisfactorily.
Pharmacology University
As a result of the Covid-19
pandemic, which made classroom education impossible, Pharmacology University has focused on the production of educational materials for
sale on online platforms (including those operated by Amazon, Zinio, Apple, Walmart/Kobo, Barnes & Noble and Google Books), while
maintaining its relationships with academic venues. During the pandemic, in an effort to continue to provide cannabis-related education,
Pharmacology University recorded over 100 online classes that were available on our platform as well as third-party platforms.
With the abatement of
the COVID-19 pandemic, it has resumed classroom education by holding a class in Austin, Texas, on April 22, 2023, and mixed-mode (personal
and virtual) classes in Cartagena, Colombia, beginning on April 14, 2023. The Company believes that over time, but subject to the availability
of capital, it will be successful in resuming classroom education.
We have published 50
cannabis-related eBooks in five languages, have produced videos to offer online and have recorded over 13,000 minutes of audio in five
languages. We have also engaged artificial intelligence services to generate translations of these materials in up to 100 additional
languages; while this activity has resulted in increased expenses while producing minimal revenue and no profit, we believe that it will
become profitable and become a significant segment of our business.
Several of our online publications
have been unified into a single magazine, Cannabis World Journals, which began publication in five languages, beginning in the
third and fourth quarters of the year ending May 31, 2022.
Operating Goals
The Company has established
the following principal goals, for the attainment of which it expects to expend approximately $2,425,000 for the period ending May 31,
2024:
Alpha Research Institute
The Company’s principal
goals for Alpha Research Institute are:
| |
· |
To increase its revenue from clinical trials from $706,008 in the year ended May 31, 2021, to $1,500,000. To achieve this goal, the number of Alpha Research Institute’s employees will be increased from its present six to approximately 35, comprising approximately 25 employees in the Houston office and approximately ten patient recruiters, for which the Company will need to spend approximately $525,000. |
| |
|
|
| |
· |
To obtain new contacts with health professionals, sponsors and CROs to obtain new and diverse clinical trials at an approximate cost of $30,000. |
| |
|
|
| |
· |
To contract with at
least ten new principal investigators, specializing in various areas of medicine, including cancer, PTSD, rare diseases and glaucoma,
and organize six training programs on clinical research for health professionals at an approximate cost of $100,000. |
| |
|
|
| |
· |
To conduct at least six seminars in Houston with the expectation of generating relationships with personnel in the U.S. pharmaceutical industry in charge of finding new clinical trials at the cost of approximately $20,000. |
The total cost of these
goals is approximately $675,000.
Pharmacology University
The Company’s principal
goals for Pharmacology University are:
| |
· |
To resume and increase classroom and seminar teaching, which involved approximately four classrooms
in two countries, generating revenues of approximately $38,440, to 20 classrooms in 5 countries, generating revenues of $1,000,000,
at an approximate cost of $250,000. |
| |
|
|
| |
· |
To increase the number of annual paid subscriptions to Cannabis World Journals to at least 5,000, which
will require better positioning and improving traffic on the internet and social media by using search engine optimization (SEO) and
search engine marketing (SEM) strategists at an approximate cost of $50,000. |
| |
|
|
| |
· |
To increase our portfolio of cannabis-related educational material from 50 eBooks in five languages,
154 online videos in five languages, and over 13,000 minutes of audio in 5 languages to 150 eBooks in five languages, 300 online videos
in more than 100 languages, and over 40,000 minutes of audio in five languages, generating revenues of $1,500,000 at an approximate
cost of $200,000. |
| |
|
|
| |
· |
To sell the above educational materials to third parties, who would resell them worldwide on their
platforms, we will need to increase our sales staff and supervisors. We believe that this activity could generate revenues of $500,000
at an approximate cost of $100,000. |
The total cost of these
goals is approximately $600,000.
Alpha Fertility and Sleep
Center
The Company’s principal
goals for the Sleep Center are:
| |
· |
To expand the existing facility to be capable of performing sleep tests for 20 patients per month,
with a view to opening a second facility having a larger capacity and a complete laboratory, generating revenues of $2,500,000 at an
approximate cost of $800,000. |
| |
|
|
| |
· |
Add additional staff for in-house sleep studies at an approximate cost of $350,000. |
The total cost of these
goals is approximately $1,150,000.
The Company intends to devote
its manpower and capital resources to execute its business plan, which it believes will enable it to become profitable. No assurance can
be given, however, that the Company can obtain any of the goals set forth above, in whole or in part.
Even if the Company sells all of the shares of Common Stock
offered by it under this Prospectus, it will need to obtain additional financing to attain the above goals. For further information
regarding the Company’s capital needs and its ability to meet them, see “Management’s Discussion and
Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources.”
DIVIDEND POLICY
We intend to retain any future earnings and do
not anticipate declaring or paying cash dividends in the foreseeable future. If we raise capital through borrowing, the terms of the
related instruments may restrict our ability to pay dividends or make distributions. Future determination to declare cash dividends will
be made at the discretion of our Board, subject to applicable laws, and will depend on many factors, including our financial condition,
results of operations, capital requirements, contractual restrictions, general business conditions and such other factors as the Board
may deem relevant.
CAPITALIZATION
The following table sets forth our capitalization
as of February 28, 2023, and as adjusted at that date to give effect to the issuance of all of the shares offered by this Prospectus
at the Fixed Offering Price.
| | |
As of February 28, 2023 | |
| | |
Actual | | |
As Adjusted | |
| Long-term debt: | |
$ | 234,210 | | |
$ | 234,210 | |
| Stockholders’ equity: | |
| | | |
| | |
| Common Stock | |
| – | | |
| – | |
| Series A Preferred | |
| – | | |
| – | |
| Series B Preferred | |
| – | | |
| – | |
| Additional paid-in capital | |
| 3,925,071 | | |
| 8,676,771 | |
| Accumulated deficit | |
| (4,406,729 | ) | |
| (4,082,072 | ) |
| Total capitalization (stockholders’ deficit) | |
$ | (247,448 | ) | |
$ | 4,828,909 | |
DILUTION
If you invest in the shares offered by this
Prospectus, your ownership interest will be diluted to the extent of the difference between the offering price per share of Common Stock
and the pro forma as adjusted net tangible book value per share of Common Stock immediately after this offering. Dilution results from
the fact that the per share offering price of the Common Stock is substantially higher than the book value per share attributable to
our existing stockholders. Pro forma net tangible book value per share represents the amount of stockholders’ equity (deficit),
excluding intangible assets, divided by the number of shares of Common Stock outstanding at that date, without giving effect to the conversion
of the Series A Convertible Preferred Stock, which is convertible into shares of Common Stock, but cannot be so converted, now or for
the foreseeable future, on an economically rational basis.
Our
historical net tangible book value deficit as of February 28, 2023, was $514,923, or ($0.00005) per
share of Common Stock. This deficit is our total tangible assets minus our total liabilities.
Historical net tangible book value (deficit) per share is historical net tangible book value
(deficit) divided by the number of shares of Common Stock outstanding as of February 28,
2023.
After giving effect to our sale in the offering
of the shares of Common Stock offered by the Company at an assumed initial public offering price of $0.0008 per share, the midpoint of
the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated
offering expenses payable by us, our pro forma as adjusted net tangible book value as of February 28, 2023, would have been approximately
$4,335,077, or $0.00028 per share of Common Stock. This represents an immediate dilution of $0.00052
per share, or 65.61% per share, to investors purchasing shares in the Offering.
The following table illustrates such dilution.
| Assumed initial public offering price per share | |
$ | 0.0008 | |
| Net tangible book value per share as of February 28, 2023 | |
| (0.00005 | ) |
| | |
| | |
| Increase in net tangible book value per share attributable
to investors in the Offering | |
| 0.00078 | |
| Net tangible book value per share after
the Offering | |
$ | 0.00028 | |
| | |
| | |
| Dilution in net tangible book value
per share to investors in the Offering | |
$ | 0.00052 | |
The following table summarizes, on a pro forma
as adjusted basis described above as of February 28, 2023, the total cash consideration paid and the average price per share paid
by our existing stockholders and by our new investors purchasing shares in this offering at the Offering Price of $0.0008 per share,
before deducting the estimated offering expenses payable by us:
| | |
Shares
Purchased | | |
Total
Consideration | | |
Average
Price | |
| | |
Number | | |
Percent | | |
Amount | | |
Percent | | |
Per Share | |
| Existing stockholders1 | |
| 9,459,677,919 | | |
| 60.22 | | |
$ | (514,923 | ) | |
| (11.88 | ) | |
$ | (0.00005 | ) |
| New investors1 | |
| 6,250,000,000 | | |
| 39.78 | | |
| 4,850,000 | | |
| 111.88 | | |
| 0.00078 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total | |
| 15,709,677,919 | | |
| 100 | | |
| 4,335,077 | | |
| 100 | | |
| 0.00028 | |
___________
1 After estimated offering
expenses of $150,000.
The foregoing tables and calculations (other
than the historical net tangible book value calculation) are based on 16,544,249,347 shares of Common Stock outstanding after the Offering
and exclude (i) 834,571,428 shares of Common Stock that have been issued since February 28, 2023, at below market prices, for the effect
of which on dilution, see the next paragraph, (ii) 600,000,000 shares of Common Stock that may be issued under the Incentive Plan and
(iii) shares of Common Stock that may be issued upon the conversion of the Series A Convertible Preferred Stock, which is convertible
into shares of Common Stock, but cannot be so converted, now or for the foreseeable future, on an economically rational basis. To the
extent that shares of Common Stock are issued under the Incentive Plan or we issue additional shares of Common Stock in the future, there
will be further dilution to investors participating in the Offering. If we raise additional capital through the sale of equity or convertible
debt securities, the issuance of these securities could result in further dilution to our stockholders.
The Company received $250,000 for the 834,571,428
shares of Common Stock described in the previous paragraph. The following table summarizes, on a pro forma as adjusted basis described
above as of February 28, 2023, the total cash consideration paid and the average price per share that would be paid by our existing stockholders
and by our new investors purchasing shares in the Offering at the Offering Price of $0.0008 per share, before deducting the estimated
offering expenses payable by us, if these shares had been issued and paid for and the Offering had occurred on that date:
| | |
Shares
Purchased | | |
Total
Consideration | | |
Average
Price | |
| | |
Number | | |
Percent | | |
Amount | | |
Percent | | |
Per Share | |
| Existing stockholders1 | |
| 10,294,249,347 | | |
| 62.22 | | |
$ | (514,923 | ) | |
| (11.88 | ) | |
$ | (0.00005 | ) |
| New investors1 | |
| 6,250,000,000 | | |
| 37.78 | | |
| 4,850,000 | | |
| 111.88 | | |
| 0.00078 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total | |
| 16,544,249,347 | | |
| 100.00 | | |
| 4,335,077 | | |
| 100 | | |
| 0.00028 | |
___________
1 After estimated offering
expenses of $150,000.
MANAGEMENT’S DISCUSSION
AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The financial information discussed below
is derived from the Company’s unaudited consolidated financial statements for the nine months ended February 28, 2023, and its
audited consolidated financial statements for the year ended May 31, 2022, which were prepared and presented in accordance with generally
accepted accounting principles (“GAAP”). This financial information is only a summary and should be read in conjunction with
the audited financial statements and related notes contained herein, which more fully present the Company’s financial condition
and results of operations at that date. The results outlined in these consolidated financial statements are not necessarily indicative
of the Company’s future performance. This section and other parts of this Offering Circular contain forward-looking statements
that involve risks and uncertainties. Actual results may differ significantly from the results discussed in forward-looking statements.
Information about the Company
The Company, headquartered in Houston, Texas,
offers services in clinical trials through Alpha Research Institute; cannabis-related education in classrooms, seminars and online through
Pharmacology University; and sleep disorder problems through the Sleep Center. For detailed information about the Company and its operations,
see “Description of Business.”
The Company’s fiscal year begins on
June 1 in each year and ends on May 31 in the following year.
Going Concern
As indicated in Note 3 of the notes to the
audited consolidated financial statements for the year ended May 31, 2022, there is substantial doubt as to the ability of the Company
to continue as a going concern. The Company has generated material operating losses since inception and its ability to continue as a
going concern depends on the successful execution of its operating plan, which includes the resumption of services that were interrupted
by the Covid-19 pandemic, increasing sales of existing services and introducing new services, as well as raising either debt or equity
financing.
The Company needs substantial additional capital
to fund its business, including the completion of its business plan and repayment of its debts. No assurance can be given that any additional
capital can be obtained or, if obtained, will be adequate to meet its needs, and the Company may need to take measures to remain a going
concern. If adequate capital cannot be obtained on a timely basis and satisfactory terms, the Company’s operations could be materially
negatively impacted, or it could be forced to terminate its operations.
Impact of the Covid-19 Pandemic
The COVID-19 pandemic has adversely impacted
the Company and its financial results in different ways, depending on the particular business operation. Principally as a result of the
pandemic.
Pharmacology University Business. The
Company encountered quarantines, restrictions on gatherings and other governmental regulations that precluded classroom education, as
well as restrictions on travel that reduced consulting activities. The Company reduced the impact of the pandemic by developing online
educational programs and transitioning its workforce to a remote working environment without reducing its workforce. Revenue from this
operation was increased from $18,323 (unaudited) in the year ended May 31, 2019, to $44,799 and $38,440 in the years ended May 31, 2020,
and May 31, 2021, respectively; revenue for the year ended May 31, 2022, was $13,985; revenue for the nine months ended February 28,
2023, was $39,920.
Clinical Trials. Quarantines,
restrictions on gatherings and other governmental regulations, amplified by potential patients’ fears of contracting Covid-19 at
the Company’s clinics, negatively affected clinical trials. In addition, these clinics were subject to closure if cases of the
virus were detected. Revenue from this operation changed from $165,666 (unaudited) in the year ended May 31, 2019, to $84,979 and $706,008
in the years ended May 31, 2020, and May 31, 2021, respectively; revenue for the year ended May 31, 2022, was $196,637; revenue for the
nine months ended February 28, 2023, was $230,493. The Company believes that it may have been negatively impacted by the association
of the pandemic with the People’s Republic of China because “China” appeared in its former corporate name. Although
the Company has no operations in or any relationship with China, the Company believes that potential investors may have been deterred
from considering the Company because of concerns related to that country. For this reason, and because the Company’s corporate
name does not reflect its activities, it intends to change its name to Cannabis Bioscience International Holdings, Inc.
Overview
The Company provides educational systems focused
on medical cannabis in the United States and Latin America, as well as worldwide through online education; services in therapeutic areas
of clinical trials; and services relating to sleep disorders through its Sleep Center in Houston, Texas. The Company’s operating
units and their activities are:
| · | Alpha
Research Institute – Clinical trials and medical research. |
| | | |
| · | Pharmacology
University: – Education, consulting, digital publishing, marketing, and franchising
related to medical cannabis. |
| | | |
| · | Sleep
Center – services related to sleep disorders. |
For further information concerning the Company
and its business, see “Description of Business.”
Results of Operations
Comparison of the Nine Months Ended
February 28, 2023, and February 28, 2022
The following table sets forth information
from the consolidated statements of operations for the nine months ended February 28, 2023, and February 28, 2022:
| | |
Nine Months Ended February 28, | |
| | |
2023 | | |
2022 | |
| Revenues | |
$ | 270,413 | | |
$ | 141,981 | |
| Cost of revenues | |
| 77,443 | | |
| 31,515 | |
| Gross profit | |
| 192,970 | | |
| 110,466 | |
| | |
| | | |
| | |
| Operating expenses | |
| 901,443 | | |
| 728,659 | |
| Operating loss | |
| (708,473 | ) | |
| (618,193 | ) |
| | |
| | | |
| | |
| Non-operating income (expense): | |
| | | |
| | |
| Other income (expense) | |
| (48,101 | ) | |
| (11,957 | ) |
| Net loss | |
$ | (756,573 | ) | |
$ | (630,150 | ) |
Revenues
Revenues were $270,413 and $148,981 for the
nine months ended February 28, 2023, and February 28, 2022, respectively, primarily due to an increase of $96,496 in revenues from clinical
trials, which were $133,547 in the earlier period and $230,493 in the later. The Company attributes this increase to the lifting of Covid-19
restrictions and its increase in marketing efforts to find clinical trial sponsors. Primarily due to the increase in sales on-line courses
and start-up of universities, revenues from cannabis-related educational classes and seminars increased by 31,487, from $8,433 for the
nine months ended February 28, 2022, to $39,920 for the nine months ended February 28, 2023. Cost of revenues was $77,443 and $31,515
for the nine months ended February 28, 2023, and February 28, 2022, respectively, primarily due to an increase of $45,928 in costs for
payments to doctors and laboratories in connection with clinical trials.
Operating Expenses
The following table sets forth information
about the Company’s operating expenses from the consolidated statements of operations for the nine months ended February 28, 2023,
and February 28, 2022:
| | |
Nine Months Ended February 28, | |
| | |
2023 | | |
2022 |
|
| General and administrative | |
$ | 102,005 | | |
$ | 98,880 |
|
| Contract labor | |
| 528,610 | | |
| 397,216 |
|
| Professional fees | |
| 173,767 | | |
| 108,911 |
|
| Officer compensation | |
| 36,235 | | |
| 54,797 |
|
| Rent and lease | |
| 55,915 | | |
| 60,353 |
|
| Travel | |
| 4,911 | | |
| 8,502 |
|
| Total operating expenses | |
$ | 901,443 | | |
$ | 728,659 |
|
The increase in contract labor was due to
adding staff to write, translate, and produce audiobooks, e-books, and online videos as well as added staff for clinical trials. Professional
fees increased due to the auditing costs incurred principally in connection with the preparation of the Company’s audited financial
statements for the year ended May 31, 2021, and legal expenses incurred in connection with the preparation of the registration statement
of which this Prospectus is a part, as well in the preparation of reports that the Company filed with OTC Markets Group Inc.
Operating Loss
For the reasons set forth above, operating
loss increased from $618,193 for the nine months ended February 28, 2022, to $708,473 for the nine months ended February 28, 2023.
Other Income (Expense)
In the nine months ended February 28, 2023,
and February 28, 2022, the Company recorded other income of $41,666 and $33,708, respectively, from forgiveness of its PPP Loans. The
Company accrued interest of $89,767 and $45,665 in the nine months ended February 28, 2023, and February 28, 2022, respectively.
Net Loss
Net loss for the nine months ended February
28, 2023, was $756,573, compared with net loss of $630,150 for the nine months ended February 28, 2022, for the reasons set forth above
in relation to loss from operations and the effect of other income received in the nine months ended February 28, 2023, and February
28, 2022.
Comparison of the Year Ended May
31, 2022, and the Year Ended May 31, 2021
The following table sets forth information
from the consolidated statements of operations for the years ended May 31, 2022, and May 31, 2021.
| | |
Year Ended May 31, | |
| | |
2022 | | |
2021 | |
| Revenues | |
$ | 214,980 | | |
$ | 761,737 | |
| Cost of revenues | |
| 46,763 | | |
| 108,311 | |
| Gross profit | |
| 168,217 | | |
| 653,426 | |
| | |
| | | |
| | |
| Operating expenses | |
| 1,056,275 | | |
| 769,732 | |
| Operating loss | |
| (888,058 | ) | |
| (116,306 | ) |
| | |
| | | |
| | |
| Non-operating income (expense): | |
| | | |
| | |
| Interest | |
| (51,036 | ) | |
| (43,002 | ) |
| Other income | |
| 53,923 | | |
| – | |
| Net income (loss) | |
$ | (885,171 | ) | |
$ | (159,308 | ) |
Revenues
Revenues were $214,980 and $761,737 for the
years ended May 31, 2022, and May 31, 2021, respectively, primarily due to a decrease of $509,371 in revenues from clinical trial contracts,
which were $706,008 in the earlier period and $196,637 in the later. The Company attributes this reduction to Covid-19, in that patients
for clinical studies were reluctant to visit clinics or doctor’s offices, resulting in the early termination or cancellation of
studies. Revenues from cannabis-related educational classes and seminars decreased by $24,455, from $38,440 for the year ended May 31,
2021, to $13,985 for the year ended May 31, 2022, primarily due to the effects of the Covid-19 pandemic. Franchise fees were $0 for both
years.
Operating Expenses
Operating expenses for the years ended May
31, 2022, and May 31, 2021, consisted of the following:
| | |
Years Ended May 31, | |
| | |
2022 | | |
2021 | |
| General and administrative | |
$ | 134,351 | | |
$ | 90,4721 | |
| Contract labor | |
| 544,760 | | |
| 263,138 | |
| Professional fees | |
| 222,535 | | |
| 101,336 | |
| Officer compensation | |
| 70,983 | | |
| 211,312 | |
| Rent | |
| 75,226 | | |
| 72,244 | |
| Travel | |
| 8,240 | | |
| 31,320 | |
| Total operating expenses | |
$ | 1,056,275 | | |
$ | 769,732 | |
Increased contract labor was due to adding
staff to write, translate, and produce audiobooks, e-books, and online videos. Professional fees decreased due to the reduction of $36,316
in study fees due to Covid-19 causing a decrease in clinical trials, even though increases in auditing costs incurred principally in
connection with the preparation of the Company’s audited financial statements for the year ended May 31, 2021, and legal expenses
incurred in connection with the preparation of the registration statement of which this Prospectus is a part, as well in the preparation
of reports that the Company filed with OTC Markets Group Inc. Officer compensation decreased because an officer left the Company and
was not replaced. Travel decreased because of Covid-19 restrictions.
Operating Loss
For the reasons set forth above, operating
loss increased from $116,306 in the year ended May 31, 2021, to $888,058 in the year ended May 31, 2022.
Interest
Interest was $51,306 in the year ended May
31, 2022, and $43,302 in the year ended May 31, 2021.
Other Income
In the year ended May 31, 2022, the Company
recorded other income of $53,923 from the forgiveness of PPP loans.
Net Loss
Net loss for the year ended May 31, 2022,
was $885,171, compared with a net loss of $159,308 for the year ended May 31, 2021, for the reasons set forth above in relation to income
(loss) from operations and the effect of other income received in the year ended May 31, 2022.
Liquidity and Capital Resources
At February 28, 2023, the Company had $25,655
in cash and cash equivalents and accounts receivable of $4,484, negative working capital of $280,713 and no commitments for capital expenditures.
The Company had cash in the amount of $_______ on the date of this Prospectus.
During the nine months ended February 28,
2023, and February 28, 2022, the Company had cash flow used in operations of $710,515 and $584,728, respectively, and cash flow provided
by financing activities of $704,189 and $548,192, respectively. During the years ended May 31, 2022, and May 31, 2021, the Company had
cash flow used in operations of $855,704 and $507,705, respectively, and cash flow provided by financing activities of $846,364 and $421,372,
respectively. The Company had accumulated deficits of $3,650,156 and $2,764,985 at May 31, 2022, and May 31, 2021, respectively, and
an accumulated deficit of $4,406,729 at February 28, 2023.
Delays in payments by Sponsors and CROs
have affected and may continue to affect the Company’s cash flows, although accounts receivable were $4,484
and $8,442, at February 28, 2023, and February 28, 2022, respectively. At May 31, 2022, the
balance of accounts receivable was 2.6% of the total revenue of $214,980 generated for that fiscal year; at February 28, 2023, the
balance of accounts receivable, was 9.0% of the total revenue of $51,251 generated for the three months then ended; and at February
28, 2023, the balance of accounts receivable was 1.7% of the total revenue of $270,413 generated for the nine months then ended. The
Company has a good history of collecting from its customers and in the judgment of management, no allowance for bad debt has been
necessary. However, such delays have required the Company to take measures to pay its obligations that it might not otherwise taken,
including sales of Common Stock at below-market prices and borrowing at high rates of interest, the former of which has had a
dilutive effect on some holders of Common Stock and the latter of which has increased the Company’s expenses.
Since June 1, 2021, the Company has raised
capital as follows:
| · | In
the year ended May 31, 2021, the Company received $56,881 of PPP loans under the Coronavirus
Aid, Relief, and Economic Security Act (the “CARES Act”). The Company has been
notified that all of these loans and the interest accrued thereon have been forgiven in full,
subject to review by the SBA. The amounts forgiven have been recorded as non-operating income
in the consolidated statement of operations for the year ended May 31, 2022. |
| | | |
| · | In
the year ended May 31, 2021, the Company received SBA loans of $106,200. |
| | | |
| · | In
the years ended May 31, 2022, and the year ended May 31, 2021, the Company received $261,000
and $813,290, respectively, from sales of its Common Stock to private investors and in the
nine months ended February 28, 2023, the Company received $635,966 from such sales. Since
February 28, 2023, it has received $250,000 from such sales. |
| | | |
| · | In
the years ended May 31, 2022, and the year ended May 31, 2022, the Company received loans,
in addition to those described above, of $48,074 and $0, respectively, and in the nine months
ended February 28, 2023, the Company received no such loans. |
The Company believes that it will require
$2,425,000 to attain the goals described under “Business Plan” and estimates that other capital needs, including operating
costs of $975,000, legal/accounting costs of $200,000, overhead of $600,000 and a reserve for contingencies of $800,000 for the next
two years, will approximate $2,575,000, totaling $5,000,000.
To the extent that capital needs cannot be
met by revenue from operations, profits and the proceeds of the Offering, the Company will need to raise additional capital through the
sale of debt or equity securities to public and private investors. There is no assurance that such funding will be available on acceptable
terms or at all or that the Company will attain profitability. If the Company cannot raise sufficient funds when required or on acceptable
terms, it may have to reduce its operations significantly or discontinue them entirely. To the extent that funds are raised by issuing
equity securities or securities that are convertible into the Company’s equity securities, its stockholders may experience significant
dilution.
As indicated in this Prospectus, the Company
was materially and adversely impacted by the Covid-19 pandemic. With the lifting of the restrictions imposed in response to the pandemic,
the Company is resuming normal operations in its Pharmacology University and Alpha Research Businesses and has opened its Sleep Center
Business. The Company believes that these businesses will produce revenues of $80,000, $600,000 and $120,000, respectively, for the year
ending May 31, 2023, totaling $800,000. The Company believes that cost or revenues and operating expenses will total approximately $1,100,000
for that year, resulting in an operating loss of approximately$300,000, compared with operating losses of $885,171 and $159,308 for the
years ended May 31, 2022, and May 31, 2021. If the Company is successful in carrying out its business plan, it expects to become profitable
in the year ending May 31, 2024, and beyond.
Off-Balance Sheet Arrangements
The Company has no off-balance sheet arrangements.
DESCRIPTION OF BUSINESS
History
The Company was formed in the State of Colorado
on February 28, 2003, as a limited liability company under the name Fidelity Aircraft Partners LLC. On December 16, 2009, it converted
to a corporation under the name Fidelity Aviation Corporation, and on August 24, 2009, it changed its name to China Infrastructure Construction
Corp. On February 28, 2018, the Company changed its name to Hippocrates Direct Healthcare, Inc. and on July 4, 2018, it resumed its present
name. On December 6, 2022, it changed its corporate name to Cannabis Bioscience International Holdings, Inc.
From its inception to 2009, the Company
sold used aircraft parts and airframe components salvaged from non-flying jet aircraft. Beginning on October 8, 2009, the Company
terminated that business and entered into concrete production in the People’s Republic of China and Hong Kong through
subsidiaries, which were sold to an unrelated party in 2016. No information about the Company’s operations or management was
available from early 2012 to early 2015, but the current management believes that the Company was dormant during that period and
that its directors and officers abandoned their positions. In February 2015, an independent investor obtained control of the
Company. On July 25, 2016, the Company disposed of its subsidiaries and on January 6, 2017, transferred control of the Company to
another independent investor. On February 5, 2018, control of the Company was acquired by a former member of its management. On
December 20, 2019, the present management acquired control of the Company as a result of the acquisition of Pharmacology University,
Inc. (see below). The Company began to file reports with OTC in 2018 under its Alternate Reporting Standard and has been a
“Pink Sheet” company since then. Market prices for the Common Stock have been volatile and its trading volume has
fluctuated extremely. See “Risks Related to the Common Stock and This Offering – There are risks, including stock market
volatility, inherent in owning Common Stock.” In the past, the Company failed to comply with its reporting requirements under
the Securities Exchange Act of 1934. See “Risk Factors – Risk Factors Related to the Common Stock and to This Offering
– The Company has failed to comply with its reporting obligations.”
Acquisition of Hippocrates
On December 17, 2017, the Company acquired
Hippocrates Direct Healthcare, LLC, a Texas limited liability company (“Hippocrates”). Before this acquisition, the Company
had no operations and no or nominal assets (a “Rule 144 Shell Company”). As a result of this acquisition, the Company ceased
to be a Rule 144 Shell Company. The business of Hippocrates (the “Hippocrates Business”), which was terminated on October
31, 2020, was concierge healthcare.
Acquisition of Pharmacology University Inc.
Pharmaceutical University Inc.(“PUI”) was
incorporated in the State of Delaware on January 5, 2017, under the corporate name Canna-Pharmacology University Inc.; on March 15,
2017, its certificate of incorporation was amended to change its corporate name to Pharmacology University Inc. On December 20,
2019, PUI was merged with and into the Company, such that the shareholders of PUI received 4,875,000,000 shares of Common Stock and
2,000,000 shares of the Company’s Series A Convertible Preferred Stock (“Series A Preferred”) as merger
consideration. The Company conducts the business acquired by this merger (the “Pharmacology University Business”) under
the trade name Pharmacology University. The Pharmacology University Business is generally cannabis-related research and education.
For a more detailed description of the Pharmacology University Business, see “Description of Business –
Pharmacology University Business.” For a description of the interest of certain members of the management of the Company
in this merger, “Certain Relationships and Related Party Transactions – Merger with Pharmacology
University, Inc.”
Acquisition of Precision Research Institute
On March 31, 2019, the Company entered into the
Alpha Research Business by acquiring all of the outstanding units in Precision Research Institute, LLC, a Texas limited liability company
(“PRI”), which was formed on May 18, 2016, from the Company’s then president. On August 20, 2020, PRI was merged with
and into the Company. The Company conducts the Alpha Research Business under the trade name Alpha Research Institute. For a detailed
description of the Alpha Research Business, see “Description of Business – Alpha Research Business.”
Businesses
The Company has three operations, namely,
the Pharmacology University Business, the Alpha Research Business and the Hippocrates Business. Its vision is to provide superior
services, while adhering to its core values of integrity, respect, compassion, inclusiveness, social responsibility, excellence and innovation.
Pharmacology University Business
The Cannabis Industry
The cannabis industry is
fast-growing, increasingly complex, and rapidly changing. The Company believes that the growing cannabis industry in numerous U.S. states
and other countries represents a significant market opportunity for the Pharmacology University Business, as persons involved in the
industry need the educational and other services that it furnishes, as more fully described below.
The U.S. cannabis industry
is undergoing rapid growth and change, particularly with the recent opening of opportunities for federally sanctioned research on cannabis
in partnership with the Drug Enforcement Administration (the “DEA”), as well as the federal legalization of hemp and corresponding
state and federal hemp research programs.
The cannabis market generally
is large and growing. In 2023, according to Brightfield’s 2023 US Cannabis Market Forecast, the USA cannabis market had approximately
$27 million in sales in 2022 and is forecast to reach $50.7 by 2028. According to a report by New Frontier Data, the U.S. legal cannabis
market is predicted to reach $41.5 billion in sales by 2025.
In the medical market,
the demand for cannabis for research is likely to increase significantly over the next few years and decades, due to the increasing number
of states legalizing cannabis and the strong public support for cannabis legalization. By 2025, 5.4 million Americans, or 2.4% of U.S.
adults, are predicted to be registered patients in medical cannabis states, according to a report by New Frontier Data (“New Frontier”).
New Frontier also projects that the medical cannabis market will nearly double to over $16 billion in that time, taking into account
more geographies within the U.S. legalizing cannabis, which will lead to market expansion, the normalization of cannabis which will increase
the number of consumers, and medical cannabis patients turning to cannabis as an alternative to prescription drugs. The global medical
cannabis market is projected to reach $87.4 billion by 2027, according to Global Market Insights (“GMI”). The DEA’s
aggregate production quotas for cannabis were 3,200 kg in 2022 for dried flower (an estimated $35 million market) and 1,000 kg for cannabis
extract (an estimated $100 million market). These aggregate production quotas are expected to continue increasing to meet increasing
demand for cannabis research in the U.S. In addition to government funding, some institutions are already receiving private investment
in cannabis research. For example, Harvard and MIT recently received a $9 million donation to fund research into cannabis’ influence
on brain health and behavior. Additionally, Skylight Health Group (formerly named “CB2 Insights”) has noted that average
prescriptions for qualifying conditions such as chronic pain, PTSD, sleep disorders, epilepsy and anxiety saw a decline of 11% in favor
of medical cannabis replacement leading the company to estimate that more than $4 billion in sales that currently go to pharmaceutical
products could be redirected towards medical cannabis. Further research on cannabis legalization and its impact on public health is needed
and is likely to take place over the coming years, as the DEA has recognized the increased need for cannabis-related research.
In 2019, large pharmaceutical
companies in the U.S. spent $83 billion on drug research and development. The private research market, like the federal DEA research program,
has an interest in investigating the uses and risks of cannabis and hemp derivatives, not only in states that have legalized medical cannabis,
but also in anticipation of potential full legalization.
The high prevalence of cancer
is expected to be one of the factors driving the demand for legal cannabis. For instance, according to the World Health Organization
(WHO), cancer is the second leading cause of death worldwide and was responsible for about 8.8 million deaths in 2015. In addition, the
growing disease burden of chronic pain and significant side effects associated with opioid usage is expected to drive the demand for
medical cannabis, which has proved to be a potent product for chronic pain management. The Company believes that these and other applications
will lead to increased demand.
Expanding Legalization of Cannabis
The 2018 Farm Bill in the
U.S. created an opportunity for hemp-derived cannabidiol (CBD) products in retail and pharmaceutical channels. Also, many countries,
including Canada, China, Italy, Australia, and South Korea, have legalized hemp for growth and export. In the United States, CBD is widely
available from retailers, including online, drug and convenience stores, natural products, beauty, grocery, and pet stores. According
to the Grand View Research Industrial Hemp Market Analysis, the global CBD market was valued at $4.6 billion in 2018 and is expected
to grow at a CAGR of 22.2% from 2019 to 2025. Additionally, the global industrial hemp market size was estimated at $4.71 billion in
2019 and is expected to show a revenue-based compound annual growth rate of 15.8%.
A recent CBS News poll found
that 88% of Americans support the legal use of medical cannabis when recommended by a doctor. Sales in the cannabis industry are projected
by Cannabis Business Daily to be $15.5 billion and $20.3 billion in 2020 and 2021, respectively and sales could be as high as $37 billion
in 2024. The size of the industry was only $3.4 billion industry in 2015. Sharp sales increases in recently launched medical cannabis
programs – as well as continued gains in adult-use markets – are expected to fuel much of the industry’s growth over
the coming years.
Thirty-eight U.S. states,
the District of Columbia, Puerto Rico and Guam have legalized some form of whole-plant cannabis cultivation, sales and use for certain
medical purposes. Eighteen of those states and the District of Columbia and Northern Mariana have also legalized cannabis for adults for
non-medical purposes (sometimes referred to as adult use). Under federal law, however, those activities are illegal. Cannabis, other than
hemp (defined by the U.S. government as Cannabis sativa L. with a THC concentration of not more than 0.3% on a dry weight basis), is a
Schedule I controlled substance under the CSA. Even in states or territories that have legalized cannabis to some extent, the cultivation,
possession, and sale of cannabis, whether in-state or interstate, violate the CSA and are punishable by imprisonment, substantial fines
and forfeiture. Moreover, individuals and entities may violate federal law if they aid and abet another in violating the CSA or conspire
with another to violate the law. Violation of the CSA is a predicate for violation of other criminal laws, including money laundering
laws and RICO. The U.S. Supreme Court has ruled that the federal government has the authority to regulate and criminalize the sale, possession
and use of cannabis, even for individual medical purposes, regardless of whether it is legal under state law.
While the U.S. government
has not enforced these laws against companies complying with state cannabis laws, it retains the authority to do so, and therefore, the
likelihood of any future adverse enforcement against companies complying with state cannabis laws remains uncertain. See “Risk Factors--Because
the Pharmacology University Business deals with persons that operate in the cannabis industry, it faces unique, unpredictable and evolving
risks.” U.S. Attorneys can prosecute violations of the CSA, including cannabis activities that comply with state law; however, U.S.
Attorneys have not targeted state-law-compliant entities in recent years. The Company believes that the policy of not prosecuting such
entities is likely to continue under current U.S. Attorney General Merrick Garland.
Since 2014, versions of
the U.S. omnibus spending bill have included provisions prohibiting the DOJ, which includes the DEA, from using appropriated funds to
prevent states from implementing their medical-use cannabis laws. In 2016, the U.S. Court of Appeals for the Ninth Circuit held that this
provision prohibits the DOJ from spending funds to prosecute individuals who engage in conduct permitted by state medical-use cannabis
laws and who strictly comply with such laws and other courts that have considered the issue have ruled similarly. However, the court noted
that if these provisions were not continued, prosecutors could prosecute conduct that occurred even while the provision was previously
in force. This decision does not apply to adult-use businesses.
Despite the ongoing federal
illegality of cannabis, the DEA has authorized certain institutions to conduct research using cannabis. Between January 2017 and January
2019, the DEA’s projections for federally approved cannabis research projects increased dramatically: the number of federally registered
cannabis researchers increased from 384 to 542. In 2019, the DEA announced that it would further facilitate and expand scientific and
medical research for cannabis in the United States, including registering additional entities to produce cannabis for researchers and
increasing the amount and variety of cannabis available for research in order “facilitate research, advance scientific understanding
about the effects of marijuana, and potentially aid in the development of safe and effective drug products that may be approved for marketing
by the Food and Drug Administration.” Further, this announcement acknowledged the possibility that medical cannabis or related
products may, in the future, require FDA approval and come under the FDA’s FDCA jurisdiction.
On December 18,
2020, the DEA finalized regulations pertaining to applications by entities seeking to become registered with the DEA to grow
cannabis as bulk manufacturers for authorized purposes. Under these and other applicable regulations, applicants are responsible for
demonstrating they have met various requirements, including requirements to possess appropriate state authority, document that their
customers are licensed to perform research, and employ adequate safeguards to prevent diversion.
On May 14, 2021, the DEA
announced that memorandums of agreement were provided to an unspecified and unnamed number of companies to collaborate with the DEA “to
facilitate the production, storage, packaging, and distribution of marijuana under the new regulations as well as other applicable legal
standards and relevant laws.” To the extent these memorandums of agreement are finalized, DEA anticipates issuing DEA registrations
to these manufacturers. Each applicant will then be authorized to cultivate cannabis – up to an allotted quota – in support
of the more than 575 DEA-licensed researchers nationwide. As of 2022, six companies have been granted DEA registrations to bulk-manufacture
cannabis.
If the DEA continues the
above policies and activities (as to which no assurance can be given), the Company believes that demand for medical cannabis, and in turn,
the Company’s products and services, will increase.
According to the Biden
campaign website: “A Biden Administration will support the legalization of cannabis for medical purposes and reschedule cannabis
as a CSA Schedule II drug so researchers can study its positive and negative impacts. This will include allowing the VA to research the
use of medical cannabis to treat veteran-specific health needs.”
The Company believes
that the anticipated growth of the cannabis industry, propelled in significant part by the increasing legalization of cannabis, offers
the Company opportunities to expand. The industry requires skilled and educated cannabis professionals to operate.
Overview of the Pharmacology
University Business
Through the Pharmacology
University Business, the Company provides knowledge and promotes professionalism in the rapidly growing worldwide cannabis industry through
education in and research about the medical properties and healing virtues of this substance. The Company does not cultivate, sell or
distribute cannabis or cannabis-infused products and has no plans to do so. Pharmacology University is not an institution of higher education,
is not chartered, regulated or accredited by any governmental or private agency and does not offer training that qualifies recipients
to become pharmacists or pharmacologists.
The Pharmacology University
Business and its prospects depend on the growth of the cannabis industry and the need for experienced, educated professional persons to
lead and grow that industry ethically and responsibly in the United States and other countries where the Company’s activities are
legal. While the Company embraces the legal cannabis industry generally, its primary focus is on educating cannabis industry workers and
leaders and scientific research and development of hemp and cannabis for medicinal and commercial applications. One of the Company’s
most important assets is the close relationship of its personnel to and cooperation with law enforcement agencies in the locations where
it does business. Police agencies in several countries have appeared as guest speakers at the Company’s cannabis seminars.
In the United
States, the Company has conducted instructional seminars and cannabis classes in the states of Texas, Arkansas, Florida, Illinois,
Missouri, Oklahoma and Georgia, as well as Puerto Rico, and is planning to do likewise in the remaining states. Currently, the
Company is holding seminars and classes only in the state of Texas. The Company has conducted instructional seminars and cannabis
classes in Mexico, Peru, Ecuador, Columbia and the Dominican Republic, but is presently conducting them only in Colombia. With the
Covid-19 pandemic having abated, it plans to resume these activities Mexico, Peru, Ecuador and the Dominican Republic, and to expand
into Argentina, Chile, Brazil, Panama and other Latin American countries where its activities are lawful.
Before the pandemic,
the Company offered, and with the abatement of the pandemic, it intends to offer, opportunities for learning, discovery and engagement
to students, doctors, scientists, entrepreneurs and others in a real-world setting. The Company offers a full range of educational
programs at all levels and pursues a broad agenda of research, innovative and creative activities and builds partnerships with other
educational institutions, community organizations, government agencies and the private sector in many jurisdictions, including Jorge
Tadeo Lozano University in Bogota, Cartagena, and Santa Marta, Colombia; Clayton State University, Atlanta, Georgia; Autonomous University
of Santo Domingo, Dominican Republic; EUFLORIA Medical Cannabis Dispensary, Tulsa, Oklahoma; the Polytechnic University of Puerto Rico
in San Juan, Dispensarios 420, Puerto Rico; Cannapolis Scientific Farm in Colombia; Hemp Ecuador in Ecuador.
Educational Services
The Company has offered,
and after the abatement of the pandemic, intends to offer, multilevel educational services to entrepreneurs, medical and legal professionals,
cultivators, dispensary technicians, manufacturers, patients and others who desire to participate in the cannabis industry or who are
otherwise interested in cannabis. These services include:
| · | Continuing
medical education courses for physicians |
| | | |
| · | Continuing
legal education courses for attorneys |
| | | |
| · | Certification
courses for physicians |
| | | |
| · | Certification
for industry workers |
| | | |
| · | General
education seminars |
| | | |
| · | On-site
training |
These courses cover all
aspects of the medical cannabis industry. For the general public, they focus on the history of cannabis, its medicinal value, dispensary
concepts, legal issues and ethics, production, growing and extracts, security, operations and economics. For doctors, our courses and
seminars cover subjects such as medicinal uses of cannabis, the biochemistry of cannabis, functions of the endocannabinoid system, pharmacology,
cannabis use and abuse, and administration and dosage of cannabis medications. The cultivation course focuses on germination, cultivation
practices, cloning, growth stages and harvesting, drying and curing, and the manufacturing course covers the chemical composition of
cannabis plants, extraction of oils, laboratory practices, the manufacture of cannabis products and marketing. Overall, we have certified
and graduated several thousand students in our courses in the United States, Puerto Rico and Colombia.
Courses are taught and seminars
are led by degreed professionals, university professors, and industry experts with at least two years of commercial experience in the
particular subject. For example, the cultivation course might be taught by a professor of horticulture, an individual with an M.S. degree
in agriculture, or a master grower with three years’ experience growing crops of at least 500 plants. Before the Covid-19 pandemic,
classes were usually held at local colleges and universities in classrooms with projectors, screens and microphones. Among these colleges
and universities were the University of Texas, Houston; Texas Women’s University; University of Oklahoma; Oklahoma State University;
Clayton State University, Atlanta; Polytechnic University, San Juan; Texas A&M University; and Jorge Tadeo Lozano University in Bogota,
Cartagena, and Santa Marta, Colombia.
Students learn about Pharmacology
University through its website, social media, ticket venues, and local cannabis groups. Upon completing a course of study, students receive
certifications of completion, which are not certifications of their ability to work in a particular field, but recognition of their completion
of a non-accredited class. A 130-hour course lasting a semester was available at the University of Tadeo in Colombia, and the students
who completed it received a certificate entitled “Diplomado en Cannabis.” In addition, CME and CLE credits were available
for doctors and lawyers taking the classes. The Company has received CLE approvals for courses that it has offered in Arkansas (Office
of Professional Programs), Oklahoma (Oklahoma MCLE Commission) and Texas (State Bar of Texas) and received CME approvals for courses
that it has offered in Arkansas (University of Arkansas for Medical Sciences Office of Continuing Education), Texas and Florida (Ponce
Medical School Foundation). We were the first company approved by the Department of Health of the Commonwealth of Puerto Rico as
a provider of all training certifications, including medical education, agriculture and manufacturing education, dispensary education,
and others in the medicinal cannabis industry.
After the advent of the
Covid-19 pandemic, all of our classrooms and public venues were forced to close. We also canceled all travel plans to further our
expansion. To meet this pandemic, we created online courses. We currently have more than 100 videos available online in English, Spanish,
Portuguese, Italian and Arabic and we plan to add other languages. Additionally, we have used Zoom to hold virtual classes to teach students
and be able to respond to their questions in real time during the courses. However, revenue received from online courses has not replaced
the revenue that we believe we would have generated if our classrooms and public venues had remained open. The Company intends to resume
its former courses and add new courses as the Covid-19 pandemic abates.
With the restrictions
imposed in response to the pandemic being lifted and increasing activity in the cannabis industry, we are resuming classroom and seminar
activities. We expect to hold two classes and one seminar during the year ending May 31, 2023, producing revenue of $18,000. We are continuing
to expand our online business by promoting products across 30 platforms and we are continuing to promote Cannabis World Journals. We
expect to produce total revenue of $25,000 from our Pharmacology University Business during the year ending May 31, 2023.
Digital Products
As a result of the Covid-19
pandemic, which made classroom education impossible, Pharmacology University has focused on the production of educational materials for
sale on online platforms (including those operated by Amazon, Zinio, Apple, Walmart/Kobo, Barnes & Noble and Google Books), which
maintaining its relationships with academic venues where it expects to resume classroom teaching when the pandemic abates (including
ICESI, TADEO and UTB). It also focuses on entering into subscription and commercial agreements with universities and e-commerce platforms.
We have published 50
cannabis-related eBooks in five languages, have produced videos to offer online and have recorded over 13,000 minutes of audio in 5 languages.
We have also engaged artificial intelligence services to generate translations of these materials in up to 100 additional languages;
while this activity has resulted in increased expenses, while producing minimal revenue and no profit, we believe that it will become
profitable and be a significant component of our business.
We have aimed to publish
our educational content on different marketplaces that host products in languages commonly used worldwide. We work with platforms from
Brazil, Spain, England, Mexico, Canada, the United States, Germany, and more. We currently have four types of products published on different
platforms:
| · | E-Books:
We publish fifty titles in Spanish, English, Portuguese, Italian, and Arabic on Amazon,
Kobo and Google Books. In addition, Smashwords distributes our content on Barnes & Noble,
Apple, Baker & Taylor's Axis 360, OverDrive, Scribd, cloudLibrary, Gardners Extended
Retail, Odilo and Gardners Library. |
| | | |
| · | Audiobooks:
Findawayvoices distributes our content on 3Leaf Group, Axiell, Baker & Taylor, Bibliotheca,
Bidi, EBSCO, Follett, hoopla, LOL, dilo, Overdrive, Perma-Bound, Ulverscroft and Wheelers,
as well as on 24symbols, Anyplay, Apple, Audiobooks.com, AudiobooksNow, AudiobooksNZ, BajaL,
BingeBooks, Bokus Play, Bookmate, Chirp, Cliq, Downpour, eStories, Google Play, Hummingbird,
Instaread, Leamos, Libro.FM, Milkbox, Nextory, NOOK, Scribd, and Ubook. |
| | | |
| · | Video
courses: We publish 161 titles on Amazon (6 courses), Sympla (17 courses), Teachlr
(62 courses), Edusity (13 courses), Simplivlearning (16 courses), Alugha (40 courses), Aprendum (4 courses), and Unihance (105 courses). |
| | | |
| · | Cannabis
Worlds. We publish Cannabis Worlds, our digital magazine, on Google books (25
issues in five languages), Zinio (25 issues in Spanish and English), Pocketmags (25 issues in English) and Magzter (25 issues in five
languages). |
The Company believes that the amount and scope
of its digital products exceed those offered by any of its competitors in cannabis-related education.
Franchising
We offered, until the Covid-19
pandemic, educational programs to franchisees worldwide. A franchisee purchased the right to provide our courses in its particular city.
In addition to an initial franchise fee, a franchisee paid a 10% franchise fee and a 2% advertising fee on all gross sales. We assisted
in creating and registering a franchisee’s business identity; developing and activating its websites; creating its social media
platforms; providing it with marketing plans; assisting in finding venues for their classes; explaining how to find qualified instructors;
providing PowerPoint presentations as well as books for students and instructors; and providing one week of one-on-one training relating
to the operation of the franchise. In addition, we provided one month of marketing assistance. Before the pandemic, we had four franchisees
that produced revenue of $0 and $17,361 in the years ended May 31, 2021, and May 31, 2020, respectively. As a result of the pandemic,
we received no revenue from these franchisees in the year ended May 31, 2022, and the nine months ended February 28, 2023. Although
the Covid-19 pandemic has abated, we have not resumed franchise operations because we have not completed the revision of our franchise
model, which we expect will include more online classes.
Consulting
We have offered, and after
the abatement of the pandemic, intend to offer, consulting services that consist of assisting persons or companies that wish to obtain
a license to enter the legal cannabis marketplace. The cost of these services is based on the nature of each assignment. These services
may include:
| · | creating
and presenting advertising material for campaigns in traditional and digital media, including
publicity strategy, campaign creation, design of flyers, advertising social networks, newspapers
and magazines and creation of audiovisual content. |
| | | |
| · | consulting services to entrepreneurs
who are considering entering the cannabis industry, manufacturers and growers, including
preparation of business plans, guidance in business structure, guidance in seeking investment,
preparation of license and other applications and development of operating procedures. |
We have provided consulting
services in many states and Puerto Rico and have assisted in obtaining over 40 licenses for our clients for dispensaries, cultivation,
manufacturing and a full analytical laboratory.
A staff of 29 independent
contractors in Venezuela, Argentina, Colombia, Brazil, Mexico, Jordan, Canada and Texas, hold classes; research, edit and materials;
translate materials; prepare audio-visual materials; and engage in web development.
Alpha Research Business
Through the Alpha Research
Business, based in Houston, Texas, the Company offers specialized services in all therapeutic areas of clinical trials and has conducted
over 20 clinical trials. These trials have included drugs relating to diseases in the areas of asthma, allergies, renal disorders, neurology
disorders, cardiac and vascular disorders, nutrition/metabolism, obstetrics/gynecology, dermatology, oncology, ophthalmology, orthopedics,
gastroenterology, psychiatric disorders, infectious diseases, pulmonary and respiratory diseases, urology and Covid-19, as well as devices
for orthopedic and cardiovascular problems. Our clients have included Sponsors such as Pfizer Inc., Merck & Co, Inc., Shionogi &
Co., Ltd., Medtronic plc, Novartis, GlaxoSmithKline plc, Gilead Sciences, Inc. and Johnson & Johnson, and CROs, such as PPD, Inc.,
Icon plc, Parexel, PRA Health Sciences, Inc., Covance, IQVIA Holdings Inc. and Medpace Holdings, Inc.
Clinical trials are a
clinical research method designed to evaluate and test new drugs or devices. They are typically conducted in four phases, each of which
has a different purpose and helps scientists answer different questions.
| · | Phase
I. Researchers test an experimental drug or treatment in a small group of people for
the first time. The researchers evaluate the treatment’s safety, determine a safe dosage
range, and identify side effects. |
| | | |
| · | Phase II. The experimental
drug or treatment is given to a larger group of people to ascertain whether it is effective
and to evaluate its safety further. |
| | | |
| · | Phase III. The experimental
study drug or treatment is administered to large groups of people. Researchers confirm its
effectiveness, monitor side effects, compare it to commonly used treatments, and collect
information that will allow the experimental drug or treatment to be used safely. |
| | | |
| · | Phase IV. Post-marketing
studies, which are conducted after a treatment is approved for use by the FDA, provide additional
information, including information relating to treatment, risks, benefits and best use. |
Clinical trials are conducted
by Sponsors or CROs. The Company will contract with a Sponsor or CRO to provide services in connection with a clinical trial after it
has provided information respecting its ability to provide them and after a visit by the Sponsor or CRO to our facilities to confirm
our ability to conduct the trial and to establish communications procedures. After further measures, which include establishing a budget
and providing additional information about the Company and a second visit to our facilities, we will enter into a contract with the Sponsor
or CRO, which will issue a “Site Activation Letter.” When we receive this letter, we begin enrolling volunteer test subjects.
The Company’s facilities
are equipped with examination and blood drawing rooms, storage for investigational medication and study-related equipment. The Company
employs only clinical research coordinators (“CRCs”) with at least five years of experience. CRCs are involved in supervising
drug trials and medical research, which involves recruiting patients for medical and drug trials and screening them to ensure that they
meet the guidelines of the trial, as well as following good clinical practice, overseeing the progress of the clinical trial and ensuring
that it is properly conducted, recorded, and reported.
The recruitment of subjects
from minority, rural and economically disadvantaged groups is important to clinical trials because the benefits and risks of new drugs
with respect to them may differ from other groups due to genetic, environmental and other factors. To enhance such recruitment, the Company
has worked with community organizations, churches, social services and public agencies and has provided transportation services.
The Alpha Research Business
is staffed by six personnel responsible for regulatory and Investigational Review Board (“IRB”) processes and a staff of two
auditors. An IRB is an independent body required by federal regulation, comprising medical, scientific, and nonscientific members, the
responsibility of which is to ensure the protection of the rights, safety, and well-being of human subjects involved in a clinical trial.
An IRB reviews and approves clinical trials, protocols, amendments, methods and materials to be used in obtaining and documenting
informed consents from the trial subjects.
We have more than 20 principal
investigators, who are physicians who prepare and perform or oversee clinical trials, usually in conjunction with their medical practices.
These investigators are independent contractors. In addition, we have four professional personnel who analyze data and report the results
of trials to Sponsors and CROs, all of whom are independent contractors; they are encouraged to keep up to date on good clinical practices
and regulations relating to clinical research and a part-time accountant.
Alpha Research obtains customers
in three ways:
| · | Recruitment
websites. On these websites, we search for trials that are within our competence and
contact the related Sponsors or CROs, providing relevant information about ourselves, who
will respond if they are interested in our services. The Sponsor or CRO will consider entering
into a contract for the study only after it has met with our personnel and has visited our
facilities and if the Sponsor or CRO is satisfied that we can conduct the trial and comply
with the terms of its contract, which, as indicated above, are complex. Even then, the Sponsor
or CRO may award the contract to a firm that it considers better qualified. |
| | | |
| · | Sponsor
or CRO websites. The process is similar to that described above for recruitment websites. |
| | | |
| · | Personal
contact. |
We are currently conducting clinical trials in
non-cirrhotic non-alcoholic steatohepatitis, chronic obstructive pulmonary disease, a multivalent pneumococcal vaccine, iron deficiency
anemia and the collection of biospecimen collections and samples across all ages and various therapeutic areas, and multiple medical
conditions. We are actively seeking contracts, have bid on four and believe that we will be successful in obtaining some of them. We
expect to produce revenue of approximately $600,000 from our clinical trials business for the year ending May 31, 2023.
Sleep Center Business
In July 2022, the Company opened its Sleep
Center, which serves Houston-area patients who are interested in improving their sleep quality and enhancing their physical and mental
well-being. The Sleep Center utilizes state-of-the-art equipment. Its goal is to assess, diagnose, and treat sleep problems and provide
patients with convenient and flexible care.
The Sleep Center is intended as a one-stop source
for patients’ sleep disorder needs. It is overseen by Dr. Esteban Berberian, a primary care physician and board-certified internal
medicine physician. Working with Dr. Berberian are registered sleep specialists, many of whom are Registered Polysomnographic Technologists
(“RGSPTs”). RGSPTs are healthcare professionals certified by the American Board of Sleep Medicine (the “ABSM”)
who clinically assess patients with sleep disorders. The ABSM is a nonprofit organization that certifies physicians, PhDs, specialists
and technologists in sleep medicine. Dr. Berberian is an independent contractor, who conduct his activities with the sleep center
in connection with his medical practice.
Millions of Americans suffer from sleep disorders,
resulting in poor quality and a limited quantity of sleep that significantly interferes with their overall functioning. These disorders
include insomnia, obstructive sleep apnea, excessive daytime sleepiness and cataplexy (narcolepsy), restless leg syndrome (RLS), REM
sleep behavior disorder and snoring. Sleep disorders can cause sexual problems, such as loss of libido and erectile dysfunction. There
is a high correlation between sleep disorders and irregular menstrual cycle or premenstrual symptoms and infertility in women and low
testosterone in men. The Sleep Center does not diagnose or treat sexual or fertility problems arising from sleep disorders.
Sleep-related disorders are a nationwide problem, according to
the American Academy of Sleep Medicine:
| |
· |
about 30 percent of adults have symptoms of insomnia and about 10 percent of adults have insomnia that
is severe enough to cause daytime consequences. |
| |
|
|
| |
· |
about 26 percent of adults between the ages of 30 and 70 years have sleep apnea. |
| |
|
|
| |
· |
about 2 percent of adults suffer from RLS. |
| |
|
|
| |
· |
about 1 percent of people have narcolepsy and REM sleep behavior disorder. |
The Sleep Center acquires patients through referrals and
marketing efforts.
The development of the Sleep Center is
in its early stages. It is leasing space and equipment in two locations as needed. Its staff is being hired on a part-time basis until
our patient load is sufficient to support full-time staffing. The current staff comprises a medical director, who has general oversight
of the Sleep Center, a polysomnographic technologist, who performs diagnostic procedures (“sleep studies”), a Sleep Board
registered physician and a Registered Sleep Tech Scorer, who evaluates, interprets and scores sleep studies, and an office manager who
it shares with PRI. Our staff works for us only when we have patients. We believe that our staff, space and equipment are adequate for
the current operations of the Sleep Center.
Our goal is to expand our existing facility to
be capable of performing sleep tests for 20 patients per month, with a view, as patient load increases, to opening a second facility having
a larger capacity and a complete laboratory. Staffing would be increased to include a director of business operations and a receptionist.
When a patient is referred to the Sleep Center
for testing, it may perform an at-home sleep test, which monitors a patient’s breathing, oxygen levels, and breathing effort.
If the at-home test indicates further testing, or if the Sleep Center determines that at-home testing would not be useful, it
will perform a sleep study (“polysomnography”), which is a comprehensive test used to diagnose sleep disorders by monitoring
sleep stages and cycles to identify if or when they are disrupted and why. This test records a patient’s brain waves, blood oxygen
level, heart rate, breathing rate and eye and leg movements.
Polysomnography may be performed at a sleep disorder
unit in a hospital or a sleep center. In a typical test, a patient arrives in the evening and stays overnight. The test is performed in
a dark and quiet room with a bed and a bathroom and is equipped with a low-light video camera so that the polysomnography technologist
can observe the room when lights are extinguished. After a patient prepares for sleep, a technologist attaches sensors to the patient’s
scalp, temples, chest and legs and an oximeter to his finger or earlobe; these devices are connected to a computer, which records data
indicating his sleep patterns during the night. While the patient sleeps, a technologist observes the video and monitors his brainwaves,
eye movements, heart rate, breathing pattern, blood oxygen level, body position, chest, abdominal and limb movement, and snoring. If sleep
apnea is being treated, the technician may have the patient try a positive airway pressure machine or device that delivers a constant
stream of air that keeps his airway passages open while sleeping.
The information gathered during polysomnography
is evaluated first by a polysomnography technologist, who uses the data to chart sleep stages and cycles. The measurements recorded during
the polysomnography provide information about a patient’s sleep patterns. For example:
| |
· |
Brain wave activity and eye movements during sleep can help to identify disruptions in the stages that
may occur due to sleep disorders such as narcolepsy and REM sleep behavior disorder. |
| |
|
|
| |
· |
Abnormal variations in heart and breathing rate in blood oxygen may suggest sleep apnea. |
| |
|
|
| |
· |
Correct settings for positive airway pressure machines or oxygen if prescribed. |
| |
|
|
| |
· |
Frequent leg movements that disrupt sleep may indicate periodic limb movement disorder. |
| |
|
|
| |
· |
Other unusual movements or behaviors during sleep may indicate REM sleep behavior disorder or another
sleep disorder. |
A report is generated, reviewed for accuracy,
scored and given to the doctor who will diagnose the sleep disorder. After diagnosis, the following therapies are offered:
| |
· |
Continuous positive airway pressure (CPAP). If a patient has moderate to severe sleep apnea, he may
benefit from using a machine that delivers air pressure through a mask while asleep. |
| |
|
|
| |
· |
Other airway pressure devices. An airway pressure device that automatically adjusts while a patient
sleeps (auto-CPAP) is available. Units that supply bilevel positive airway pressure (BPAP) also are available. These provide more pressure
when a patient inhales and less when he exhales. |
| |
|
|
| |
· |
Oral appliances. Oral appliances are designed to open a patient’s throat by bringing your jaw
forward, which can sometimes relieve snoring and mild obstructive sleep apnea. |
| |
|
|
| |
· |
Supplemental oxygen. Using supplemental oxygen while a patient is asleep may help if he has central
sleep apnea. |
| |
|
|
| |
· |
Adaptive servo-ventilation (ASV). This recently approved airflow device learns a patient’s
normal breathing pattern and stores the information. |
The Sleep Center is now treating approximately
six patients. We expect that its patient load will increase in the coming months and that it will produce revenue of $1,000 for
the year ending May 31, 2023.
Employees
The Company has two employees, namely its
executive officers, who serve on a full-time basis. It meets its other manpower needs through approximately 34 independent
contractors; the number of these personnel changes from time to time in accordance with the Company’s staffing needs. For a
description of the services provided by these independent contractors, see the descriptions of the three segments of the
Company’s business.
Hippocrates Business
The Hippocrates Business provided concierge healthcare
services. From its inception, it did not provide sufficient revenue and was discontinued on October 31, 2020.
Concentration of Revenues
The Company has depended on a few customers of
the Alpha Research Business for substantial portions of its revenue. For the year ended May 31, 2021, the Company had revenues of $761,737,
of which 72% was received from one customer. For the year ended May 31, 2022, the Company had revenues of $214,980, of which 36.7%, 14.8%,
10.1% and 7.6% were received from four customers. For the nine months ended February 28, 2023, the Company had revenues of $270,413,
of which 49.6%, 29.4% and 5.0% were received from three customers. As the Company implements its business plan, the Company expects,
but cannot assure, that such dependence may be reduced.
Description of Property
The Company leases premises of approximately
4,500 square feet located at 6201 Bonhomme Road, Suites 460S and 466S, Houston, Texas. The lease provides for a base rent of $3,381.96
per month, increasing to (i) $3,529 per month on July 1, 2020, (ii) $3,676 per month on July 1, 2021, and (iii) $3,823 per month
on July 1, 2022, subject to CPI increase. The lease expires on June 30, 2023. The leased space is shared by PUI, Alpha Research Institute
and the Sleep Center. On March 23, 2023, the Company amended the lease to extend its term to June 30, 2024, at a base rent of $4,779
per month.
Two of the Company’s officers lease
1,400 square feet in Houston, Texas, at 1625 Main St, Houston, Texas, under a lease the term of which commenced on March 15, 2023, and
will expire on September 14, 2023, at a rent of $3,168 per month; these officers have made a portion of these premises available to the
Company for use as office space, for which the Company pays them $2,817 per month.
Legal Proceedings
The Company has not been and is not a party to
any litigation and is not aware of any threatened litigation.
Off-Balance Sheet Arrangements
We have no off-balance-sheet arrangements.
MANAGEMENT
The following table presents information with
respect to our officers and directors:
| Name |
|
Age |
|
Position |
| Dante Picazo |
|
67 |
|
Chief Executive Officer and Director |
| Henry Levinski |
|
71 |
|
Vice President and Director |
| Jose Torres |
|
64 |
|
Secretary and Director |
Each of our directors serves until his
death, resignation or removal or until his successor is elected and qualified. Each of our officers is elected by the Board to a
term of one year and serves until his successor is duly elected and qualified or until he dies, resigns or is removed. Members of
the Board receive no compensation for their services as such.
Biographical Information Regarding Officers and Directors
Dante Picazo
Mr. Picazo has been the chief executive officer
and a director of the Company since the merger of PUI into the company on December 19, 2019, and was the co-founder of PUI, serving as
one of its directors and as its chief executive officer and president from its incorporation in 2009 to that merger.
He has 45 years of experience in operating and
growing from concept to profitability, originating marketing and branding efforts, leading to initial public offerings for three companies.
He graduated from Cornell University School of
Hotel Administration in Ithaca, N.Y., and is fluent in three languages.
Mr. Picazo’s control of the Company through
his ownership of its capital stock, together with his knowledge of the Pharmacology University Business and his extensive experience
in international business and finance, led to the conclusion that he should serve as a member of the board.
Henry Levinski
Mr. Levinski has served as treasurer and a director
of the Company since the merger of PUI into the company on December 19, 2019, and was the co-founder of PUI, serving as one of its directors
and its chief executive officer from its incorporation in 2009. He has over 40 years of experience in operations, marketing, purchasing
and training.
Mr. Levinski’s knowledge of the Pharmacology
University Business, together with his prior experience, led to the conclusion that he should serve as a board member.
Jose Torres
Dr. Torres has served as a director and national
medical director of the Company since the merger of PUI into the company on December 19, 2019. He served in like positions with PUI until
the merger. He is board-certified in General and internal medicine and is an Anti-aging medicine Specialist with 35 years of medical
practice experience.
He received his medical degree from the Autonomous
University of Guerrero in Chilpancingo, Guerrero, Mexico, and completed a residency in internal medicine residency at Caguas Regional
Hospital in Puerto Rico. He is certified in urgent care and by World Link Medical. He is a Member of the American College of Physicians,
the Puerto Rico College of Physicians and the American Academy of Cannabinoid Medicine. He is an expert in the medical uses of cannabis
and is involved in research respecting its use in treating several medical conditions, including sleep disorders, pain management, treatment
of nausea and vomiting associated with cancer and chemotherapy, asthma and other bronchial ailments, and decreased libido.
Mr. Torres’ experience with the medicinal
use of cannabis and with sleep disorders led to the conclusion that he should serve as a member of the board.
EXECUTIVE COMPENSATION
Compensation of Officers
The following table sets forth information concerning
all compensation awarded to, earned by, or paid to our principal executive officer and our only other executive officer, who were serving
as such on May 31, 2023, for the fiscal years ended May 31, 2023, and May 31, 2022.
SUMMARY COMPENSATION TABLE
| Name and principal
position |
|
Year |
|
Salary
($) |
|
Bonus
($) |
|
Stock
Awards
($) |
|
Option
Awards
($) |
|
Non-equity
incentive plan compensation
($) |
|
Change in pension
value and nonqualified deferred compensation earnings
($) |
|
All Other
Compensation
($) |
|
Total
($) |
| (a) |
|
(b) |
|
(c) |
|
(d) |
|
(e) |
|
(f) |
|
(g) |
|
(h) |
|
(i) |
|
(j) |
| Dante Picazo |
|
2023 |
|
24,500 |
|
– |
|
– |
|
– |
|
– |
|
– |
|
– |
|
24,500 |
| CEO |
|
2022 |
|
24,000 |
|
– |
|
– |
|
– |
|
– |
|
– |
|
– |
|
24,000 |
Henry Levinski
|
|
2023 |
|
21,375 |
|
– |
|
– |
|
– |
|
– |
|
– |
|
– |
|
21,375 |
| VP |
|
2022 |
|
24,000 |
|
– |
|
– |
|
– |
|
– |
|
– |
|
– |
|
24,000 |
Compensation Discussion and Analysis
The Company has determined the amount paid as
salary to the persons named in the above table based on the Company’s ability to pay. The Company believes that these salaries
are lower than those that these persons could earn in equivalent positions in other companies and that these persons have elected to
receive these salaries and remain with the Company because of their equity positions in the Company, their belief in the prospects of
the Company and intangible reasons of which the Company may not be aware. In the case of Mr. Levinski, the Company has provided additional
compensation in the form of shares of Common Stock. The Company believes that it needs to be able to provide competitive compensation
to these persons, as well as persons that it hires in the future, but will not be able to do so until it can generate materially increased
revenue. Until then, the Company is subject to the risk that one or more of these persons will seek employment elsewhere. The Company
has adopted its 2022 Equity Incentive Plan (see “Incentive Plan”) and may explore the adoption of plans that will enable
it to reward and retain the loyalty of these and other employees through awards of share-based compensation, such as stock options, restricted
stock and restricted stock units.
Incentive Plan
General Information
On July 20, 2022, the Board
adopted, and the shareholders approved, the 2022 Equity Incentive Plan (the “Incentive Plan”), which provides for the grant
of stock options, stock appreciation rights, restricted stock, unrestricted stock, restricted stock units, and performance awards to directors,
officers, employees and consultants (“Grantees”). The Incentive Plan is administered by the Board, which has the authority,
among other things, to select eligible persons to receive awards and determine the terms of awards.
The Company will recognize
as share-based compensation expense all share-based payments to Grantees over the requisite service period (generally the vesting period)
in its consolidated statements of income based on the fair values of the awards that are ultimately expected to vest. As a result, for
most awards, recognized share-based compensation expense will be reduced for estimated forfeitures prior to vesting, primarily based initially
on the judgment of management and thereafter, estimated forfeitures will be reassessed in subsequent periods based on facts and circumstances.
As no awards were made under the Incentive Plan during the periods covered by the consolidated financial statements included in this Prospectus,
no expense for share-based compensation was recorded therein.
The Company adopted the
Incentive Plan because it believes that long-term incentives for Grantees will be a significant factor in generating returns for its
shareholders based upon the Incentive Plan’s ability to focus on long-term performance. By providing grantees with opportunities
to acquire a meaningful equity stake in the Company, it can better align their interests with those of its shareholders and create value
for them.
The Company expects to make
periodic awards to its executive officers, employees and consultants, as well as awards in connection with promotions or new hires, the
occurrence of significant events or to promote retention of employees.
Awards will generally be
subject to time- or performance-based vesting over periods determined by the Board. Performance-based goals will be determined by the
Board. We believe that performance-based awards will encourage Grantees to achieve key strategic objectives and maximize value creation
for our shareholders.
No awards have been made
as of the date of this Prospectus.
Provisions of the
Incentive Plan
The following is a description
of the material terms of the Incentive Plan, which is not a complete description and is qualified in its entirety by reference to the
Incentive Plan, which is filed as an exhibit to the registration statement of which this Prospectus is a part.
Authorized shares.
Subject to adjustment in certain events, the maximum number of shares of Common Stock that may be issued in satisfaction of awards
is 600,000,000. As of the date of this Prospectus, no awards had been granted.
Eligibility. The
Board may select participants from among employees and directors of and consultants to the Company.
Types of awards; vesting.
The Incentive Plan provides for various awards, including incentive stock options (“ISOs”), nonstatutory stock options,
stock appreciation rights, restricted and unrestricted stock and stock units, performance awards and cash. The Board has the authority
to determine the vesting schedule applicable to each award and to accelerate the vesting or exercisability of any award.
Termination of awards.
Unless otherwise provided
in an award agreement, upon termination of employment or service, a participant’s options and SARS will terminate and the participant
will have no further right, title or interest therein, the shares of Common Stock subject thereto or any consideration in respect thereof.
If employment or service terminates otherwise than for cause, the Participant may exercise his Option or SAR to the extent vested,
but only within the following period or, if applicable, such other period provided in the Award Agreement.
Except as otherwise provided
in the Award Agreement or other written agreement, if a Participant’s continuous service terminates for any reason, (i) the Company
may receive through a forfeiture condition or a repurchase right any or all of the shares of Common Stock held by the participant under
his restricted stock award that have not vested as of the date of such termination as set forth in such agreement and (ii) any portion
of his RSU award that has not vested shall terminate upon such termination and he shall have no further right, title or interest in the
RSU award, the shares of Common Stock issuable pursuant thereto the RSU Award or any consideration in respect thereof the RSU.
Except as provided in an
award agreement, in the event of a dissolution or liquidation of the Company, outstanding awards (other than those consisting of vested
and outstanding shares of Common Stock not subject to a forfeiture condition or the Company’s right of repurchase) shall terminate
prior to the completion of such dissolution or liquidation, and the shares of Common Stock subject to the Company’s repurchase
rights or subject to a forfeiture condition may be repurchased or reacquired by the Company, provided that the Board may cause some or
all expired or terminated Awards to become fully vested, exercisable or no longer subject to repurchase or forfeiture before the dissolution
or liquidation is completed but contingent on its completion.
Transferability.
Options and SARs may not
be transferred to financial institutions for value and the Board may impose such additional limitations on the transferability of an
option or SAR as it determines. In the absence of any such determination, the following restrictions shall apply (provided that, except
as explicitly provided in the Incentive Plan, an option or a SAR may not be transferred for consideration and, if an option is an ISO,
it may be deemed to be a nonstatutory stock option as a result of such transfer):
An option or SAR shall not
be transferable, except by will or by the laws of descent and distribution, and shall be exercisable during the lifetime of a participant
only by him (provided that, in certain cases, the Board may permit the transfer of an Option or SAR in a manner that is not prohibited
by applicable tax and securities laws upon the Participant’s request, including to a trust if the Participant is considered to
be the sole beneficial owner of such trust (as determined under Section 671 of the U.S. Internal Revenue Code of 1986, as amended (the
“Code”), and applicable state law) while such Option or SAR is held in such trust, provided that the Participant and the
trustee enter into a transfer and other agreements required by the Company.
Subject to the execution
of transfer documentation in a format acceptable to the Company and subject to the approval of the Board or a duly authorized officer,
an Option or SAR may be transferred pursuant to a domestic relations order.
Corporate transactions.
In the event of certain corporate transactions (including merger, consolidation, reorganization, recapitalization, reincorporation,
stock dividend, dividend in property other than cash, large nonrecurring cash dividend, stock split, reverse stock split, liquidating
dividend, combination of shares, exchange of shares, change in corporate structure), the Board shall appropriately and proportionately
adjust (a) the class or classes and the maximum number of shares of Common Stock subject to the Plan, (b) the class or classes and the
maximum number of shares that may be issued pursuant to the exercise of ISOs and (c) the class or classes and the number of securities
and exercise price, strike price or purchase price of Common Stock subject to outstanding Awards.
Acceleration.
The Board may accelerate the time at which an award may first be exercised or the time during which an award or any part thereof will
vest.
Change in control.
In the event of a change in control of the Company (as defined in the Incentive Plan), the Board shall have discretion (i) settle
awards for an amount of cash or securities equal to their value, where in the case of options and SARs, the value of such Awards, if
any, shall be equal to their in-the-money spread value (if any), as determined in the sole discretion of the Board, (ii) arrange for
the surviving corporation or acquiring corporation (or its parent company) to assume or continue the award or to substitute a substantially
similar award, (iii) arrange for the assignment of any reacquisition or repurchase rights held by the Company in respect of Common Stock
issued pursuant to the award to the surviving corporation or acquiring corporation (or its parent company), (iv) modify the terms of
awards to add events, conditions or circumstances (including termination of employment within any specified period after a change in
control) upon which the vesting of such awards or lapse of restrictions thereon shall accelerate or deem any performance conditions satisfied
at target, maximum or actual performance through closing or provide for the performance conditions to continue after closing, (v) arrange
for the lapse, in whole or in part, of any reacquisition or repurchase rights held by the Company with respect to awards, (vi) cancel
or arrange for the cancellation of awards, to the extent not vested or not exercised prior to the effective time of the change in control,
in exchange for such cash consideration, if any, as the Board may consider appropriate, or(vii) provide that, for at least 20 days
prior to the change in control, any Options or SARs that would not otherwise become exercisable prior thereto shall be exercisable as
to all shares of Common Stock subject thereto, contingent upon and subject to the occurrence of the change in control, and that any options
or SARs not exercised prior to the consummation of the change in control shall terminate and be of no further force and effect as of
the consummation thereof.
Amendment and termination.
The Board may amend the Incentive Plan or outstanding awards, except that it may not materially impair the rights and obligations
under any award except with the written consent of the affected participant.
Retirement, Resignation or Termination Plans
We have or sponsor no plan, whether written or
verbal, that would provide compensation or benefits of any type to an executive upon retirement or any plan that would provide payment
for retirement, resignation, or termination as a result of a change in control of our company or as a result of a change in the responsibilities
of an executive following a change in control of our company.
Pension Benefits
The Company has no plan under which retirement
payments and benefits, or payments and benefits that will be provided primarily following retirement, may be or have been or may be paid.
Nonqualified Defined Contribution and Other
Nonqualified Deferred Compensation Plans
The Company has no defined contribution or other
plan that provides for the deferral of compensation.
Potential Payments upon Termination or Change-in-Control
The Company is not a party to any contract, agreement,
plan or arrangement, whether written or unwritten, that provides for payment to any of its executive officers at, following or in connection
with any termination, including without limitation resignation, severance, retirement or constructive termination, or a change in control
of the Company or a change in any of their responsibilities.
Compensation of Directors
Our directors receive no compensation in their
capacities as such.
CERTAIN RELATIONSHIPS AND
RELATED PARTY TRANSACTIONS
Issuance of Shares
On December 19, 2019, Pharmacology University,
Inc., a Delaware corporation (“PUI”), with and into the Company pursuant to an Agreement and Plan of Merger, dated as of November
7, 2019, under which PUI was merged with and into the Company (the “PU Merger Agreement”). Pursuant to this agreement, the
Company issued 4,595,467,025 shares of Common Stock to the former holders of the common stock of PUI and also issued 2,000,000 shares
of its Series A Preferred to Dante Picazo, who became the Company’s chief executive officer president upon the closing of the PU
Merger Agreement. As a result of these issuances, Mr. Picazo acquired sole control of the Company.
On January 5, 2020, the Company issued 50,000,000
shares of Common Stock to Henry Levinski, a director and vice president of the Company in consideration of his employment. These shares
had a market value of $5,000 on the date of their issuance.
On January 5, 2020, the Company issued 40,000,000
shares of Common Stock to Jose Torres, a director and secretary of the Company, in consideration of $40,000. Based on the closing price
for the Common Stock on January 3, 2020, which was the most recent date on which it was traded, these shares had a market value of $8,000.
The Company and Henry Levinski entered into
an agreement, dated as of November 1, 2022, under which he agreed, in consideration of the issuance to him of 50,000,000 shares of Common
Stock, to provide services in connection with causing the registration statement of which this Prospectus is a part to be declared effective
by the SEC and, after it is declared effective, in assisting the Company in the marketing of the shares of the Common Stock registered
thereunder for a period of the earlier of (i) two years after the registration statement is made effective or (ii) the date on which
all of the shares offered by the Company under the registration statement have been sold. He also agreed that he would not resign as
an officer or director of the Company during such period. A quorum of the board of directors, which did not include Mr. Levinski, approved
this transaction. These shares had a market value of $60,000 on the date of the agreement.
Lease
Dante Picazo and Henry Levinski, two of the Company’s
officers, leased 1,400 square feet in Houston, Texas, at 1625 Main St, Houston, Texas, under a lease, the term of which commenced on
February 29, 2020, and expired on September 30, 2022, at a rent of $3,449 per month. These officers have made a portion of these premises
available to the Company for office space, for which the Company pays them $2,817 per month. On September 15, 2022, these officers re-leased
these premises under a lease that expired on March 14, 2023, at a rent of $3,038 per month, and they continued to make a portion of these
premises available to the Company for use as office space, for which the Company paid them $2,817 per month. On March 2, 2023, these
officers entered into a new lease for the same premises, which expires on September 14, 2023, at a rent of $3,168 per month, and they
are continuing to make a portion of these premises available to the Company for use as office space, for which the Company is paying
them $2,817 per month. The Company believes that these rentals are the fair market value of the space rented.
Exchange of Shares
On August 15, 2022, pursuant to resolutions
of the Board, Mr. Picazo exchanged 595,467,205 shares of Common Stock for 1,000 shares of the Company’s Series B Preferred Stock
(“Series B Preferred”). By his ownership of these shares, Mr. Picazo has voting control of the Company. Since Mr. Picazo
had an interest in this exchange, he did not vote on the adoption of this resolution. A quorum of the board of directors, which did not
include Mr. Picazo, approved this transaction.
Loans
The Company has received loans from Messrs. Picazo
and Levinski from time to time since the year ended May 31, 2022. All of these loans are non-interest-bearing and have no set maturity
date. The Company expects to repay these loans when funds become available. During the years ended May 31, 2021, and May 31, 2022, and
during the nine months ended February 28, 2023, the Company received and repaid loans from and to them as follows:
| | |
Dante Picazo | | |
Henry Levinski | |
| Balance at June 1, 2020 | |
$ | 3,925 | | |
$ | 300 | |
| Year ended May 31, 2021 | |
| | | |
| | |
| Amounts loaned | |
| 2,930 | | |
| 54,148 | |
| Amounts repaid: | |
| (5,400 | ) | |
| (43,640 | ) |
| Balance at May 31, 2021 | |
$ | 1,455 | | |
$ | 10,808 | |
| Year ended May 31, 2022 | |
| | | |
| | |
| Amounts loaned | |
| 850 | | |
| 58,000 | |
| Amounts repaid: | |
| (2,350 | ) | |
$ | (52,925 | ) |
| Balance at May 31, 2022 | |
$ | (45 | ) | |
| 15,883 | |
| Nine months ended February 28, 2023 | |
| | | |
| | |
| Amounts loaned | |
| 14,425 | | |
| 73,426 | |
| Amount repaid | |
| (1,850 | ) | |
| (12,355 | ) |
| Balance at February 28, 2023 | |
$ | 12,530 | | |
$ | 76,954 | |
Since February 28, 2023, Mr. Picazo has loaned
the Company $2,500 and has been repaid $1,188 and Mr. Levinski has loaned the Company $34,400 and has been repaid $18,801. As of June
30, 2023, the balances that the Company owes to Messrs. Picazo and Levinski were $13,842 and $92,553, respectively.
PRINCIPAL AND SELLING STOCKHOLDERS
The table below sets forth the beneficial
ownership of Common Stock as of the date of this Prospectus. At that date, 10,294,249,347 shares of Common Stock were issued and outstanding.
The following table provides information with
respect to the beneficial ownership of Common Stock by the following (i) each of our named executive officers, (ii) each of our directors,
(ii) all directors and executive officers as a group, (iii) each person known to beneficially own more than 5% of Common Stock (excluding
the Selling Stockholders) and (iv) the Selling Stockholders.
The amounts and percentages of shares beneficially
owned are reported as required by the SEC’s rules respecting the determination of beneficial ownership of securities. Under these
rules, a person is deemed to be a “beneficial owner” of a security if he has or shares voting power or investment power, which
includes the power to dispose of or to direct the disposition of such security; and is also deemed to be a beneficial owner of any securities
of which he has a right to acquire beneficial ownership within 60 days after the determination date. Securities that can be so acquired
are deemed to be outstanding for purposes of determining such person’s ownership percentage, but not for purposes of determining
any other person’s ownership percentage. Under these rules, more than one person may be deemed to be a beneficial owner of
the same securities and a person may be deemed to be a beneficial owner of securities in which he has no economic interest.
| |
|
Shares Beneficially
Owned Prior to the Offering |
|
|
|
|
|
Shares Beneficially
Owned After the Offering1 |
|
| Name and Address of Beneficial
Owner2 |
|
Title of Class or Series |
|
Number |
|
Percent of
Outstanding Shares3 |
|
|
Shares Being Offered |
|
|
Number |
|
|
Percent of
Outstanding Shares4 |
|
| Named Executive Officers and Directors: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Dante Picazo |
|
Common Stock |
|
4,002,611,700 |
4, 5 |
|
38.8 |
|
|
|
490,500,000 |
|
|
|
3,512,111,700 |
5, 6 |
|
|
21.2 |
|
| |
|
Series A Preferred |
|
2,000,000 |
|
|
80.00 |
|
|
|
– |
|
|
|
2,000,000 |
|
|
|
80.00 |
|
| |
|
Series B Preferred |
|
1,000 |
|
|
100.00 |
|
|
|
– |
|
|
|
1,000 |
|
|
|
100.00 |
|
| Henry Levinski |
|
Common Stock |
|
100,000,000 |
|
|
<1 |
|
|
|
100,000,000 |
|
|
|
– |
|
|
|
– |
|
| Jose A. Torres |
|
Common Stock |
|
40,000,000 |
|
|
<1 |
|
|
|
40,000,000 |
|
|
|
– |
|
|
|
– |
|
| All directors and executive officers as a group (3 persons): |
|
Common Stock |
|
4,142,611,700 |
|
|
40.2 |
|
|
|
630,500,000 |
|
|
|
3,512,111,700 |
5, 6 |
|
|
21.2 |
|
| |
|
Series A Preferred |
|
2,000,000 |
|
|
100.0 |
|
|
|
– |
|
|
|
2,500,000 |
|
|
|
100.00 |
|
| |
|
Series B Preferred |
|
1,000 |
|
|
100.0 |
|
|
|
– |
|
|
|
1,000 |
|
|
|
100.00 |
|
| The Selling Stockholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| John Jones |
|
Common Stock |
|
1,103,888,888 |
|
|
10.9 |
|
|
|
1,103,888,888 |
|
|
|
– |
|
|
|
– |
|
| Ibeth Corrales |
|
Common Stock |
|
625,000,000 |
|
|
6.1 |
|
|
|
625,000,000 |
|
|
|
– |
|
|
|
– |
|
| Julian J. Gonzalez |
|
Common Stock |
|
321,428,572 |
|
|
3.1 |
|
|
|
65,285,714 |
|
|
|
256,142,858 |
|
|
|
1.5 |
|
| John Neville |
|
Common Stock |
|
300,000,000 |
|
|
3.0 |
|
|
|
300,000,000 |
|
|
|
– |
|
|
|
– |
|
| Mark Herbert |
|
Common Stock |
|
203,000,000 |
|
|
2.0 |
|
|
|
203,000,000 |
|
|
|
– |
|
|
|
– |
|
| Harry Feinberg |
|
Common Stock |
|
164,705,881 |
|
|
1.6 |
|
|
|
164,705,881 |
|
|
|
– |
|
|
|
– |
|
| Tony Brown |
|
Common Stock |
|
75,892,857 |
|
|
<1 |
|
|
|
15,178,572 |
|
|
|
60,714,285 |
|
|
|
<1 |
|
| Richard Meikle and Laurie Meikle |
|
Common Stock |
|
62,500,000 |
|
|
<1 |
|
|
|
62,500,000 |
|
|
|
– |
|
|
|
– |
|
| Asad H. Shalami and Razia T. Quareshi-Shalami |
|
Common Stock |
|
62,500,000 |
|
|
<1 |
|
|
|
62,500,000 |
|
|
|
– |
|
|
|
– |
|
| Jeffery Lien |
|
Common Stock |
|
55,000,000 |
|
|
<1 |
|
|
|
55,000,000 |
|
|
|
– |
|
|
|
– |
|
| Esteban Berberian |
|
Common Stock |
|
51,470,588 |
|
|
<1 |
|
|
|
10,294,118 |
|
|
|
41,176,470 |
|
|
|
<1 |
|
| Stephen A. Khoury |
|
Common Stock |
|
50,000,000 |
|
|
<1 |
|
|
|
20,000,000 |
|
|
|
30,000,000 |
|
|
|
<1 |
|
| Casaro, S.A. |
|
Common Stock |
|
50,000,000 |
|
|
<1 |
|
|
|
20,000,000 |
|
|
|
30,000,000 |
|
|
|
<1 |
|
| Paola Cedano |
|
Common Stock |
|
50,000,000 |
|
|
<1 |
|
|
|
10,000,000 |
|
|
|
40,000,000 |
|
|
|
<1 |
|
| Clifford Miller |
|
Common Stock |
|
41,025,641 |
|
|
<1 |
|
|
|
20,512,820 |
|
|
|
20,512,821 |
|
|
|
<1 |
|
| Katerin Osuna Robles |
|
Common Stock |
|
39,285,714 |
|
|
<1 |
|
|
|
39,285,714 |
|
|
|
– |
|
|
|
– |
|
| Alfonso Campos |
|
Common Stock |
|
35,000,000 |
|
|
<1 |
|
|
|
33,000,000 |
|
|
|
2,000,000 |
|
|
|
<1 |
|
| Leroy Wilits |
|
Common Stock |
|
31,904,762 |
|
|
<1 |
|
|
|
6,380,953 |
|
|
|
25,523,809 |
|
|
|
<1 |
|
| Maria Madelena Pinedo |
|
Common Stock |
|
28,571,428 |
|
|
<1 |
|
|
|
28,571,428 |
|
|
|
– |
|
|
|
– |
|
| Dwight Gaddis |
|
Common Stock |
|
25,000,000 |
|
|
|
|
|
|
25,000,000 |
|
|
|
– |
|
|
|
– |
|
| Richard Royall |
|
Common Stock |
|
25,000,000 |
|
|
|
|
|
|
25,000,000 |
|
|
|
– |
|
|
|
– |
|
| Fabian Ortega |
|
Common Stock |
|
25,000,000 |
|
|
<1 |
|
|
|
25,000,000 |
|
|
|
– |
|
|
|
– |
|
| Miriam Maciel Rivera |
|
Common Stock |
|
22,500,000 |
|
|
<1 |
|
|
|
22,500,000 |
|
|
|
– |
|
|
|
– |
|
| Nicola Abate |
|
Common Stock |
|
22,222,222 |
|
|
<1 |
|
|
|
22,222,222 |
|
|
|
– |
|
|
|
– |
|
| Shane Leupold |
|
Common Stock |
|
21,250,000 |
|
|
<1 |
|
|
|
21,250,000 |
|
|
|
– |
|
|
|
– |
|
| Jorge Verar |
|
Common Stock |
|
20,000,000 |
|
|
<1 |
|
|
|
5,000,000 |
|
|
|
15,000,000 |
|
|
|
<1 |
|
| Guadeloupe G. Ramirez |
|
Common Stock |
|
20,000,000 |
|
|
<1 |
|
|
|
20,000,000 |
|
|
|
– |
|
|
|
– |
|
| Brandon Milatovic |
|
Common Stock |
|
20,000,000 |
|
|
<1 |
|
|
|
20,000,000 |
|
|
|
– |
|
|
|
– |
|
| Rosa Casares |
|
Common Stock |
|
16,975,703 |
|
|
<1 |
|
|
|
3,395,141 |
|
|
|
13,580,562 |
|
|
|
<1 |
|
| Frank and Maria Hernandez |
|
Common Stock |
|
16,071,428 |
|
|
<1 |
|
|
|
3,214,285 |
|
|
|
12,857,143 |
|
|
|
<1 |
|
| Jonathan Eisner |
|
Common Stock |
|
16,000,000 |
|
|
<1 |
|
|
|
16,000,000 |
|
|
|
– |
|
|
|
– |
|
| Adriane Kearney |
|
Common Stock |
|
15,000,000 |
|
|
<1 |
|
|
|
3,000,000 |
|
|
|
12,000,000 |
|
|
|
<1 |
|
| Ludvina Martinez |
|
Common Stock |
|
14,705,882 |
|
|
<1 |
|
|
|
2,941,177 |
|
|
|
11,764,705 |
|
|
|
<1 |
|
| Andres Mesa |
|
Common Stock |
|
14,285,715 |
|
|
<1 |
|
|
|
2,857,143 |
|
|
|
11,428,572 |
|
|
|
<1 |
|
| Laura and Jesus Grimaldo |
|
Common Stock |
|
13,161,764 |
|
|
<1 |
|
|
|
2,632,353 |
|
|
|
10,529,411 |
|
|
|
<1 |
|
| Yoselet Isamark Ortiz |
|
Common Stock |
|
12,500,000 |
|
|
<1 |
|
|
|
12,500,000 |
|
|
|
– |
|
|
|
– |
|
| Lucy Caldera |
|
Common Stock |
|
12,500,000 |
|
|
<1 |
|
|
|
12,500,000 |
|
|
|
– |
|
|
|
– |
|
| David Ward |
|
Common Stock |
|
10,080,645 |
|
|
<1 |
|
|
|
2,016,129 |
|
|
|
8,064,516 |
|
|
|
<1 |
|
| Dianely Heredia |
|
Common Stock |
|
10,000,000 |
|
|
<1 |
|
|
|
10,000,000 |
|
|
|
– |
|
|
|
– |
|
| Will Morey | |
Common Stock | |
10,000,000 | |
| <1 | | |
| 10,000,000 | | |
| – | | |
| – | |
| Robert A. Fleming | |
Common Stock | |
10,000,000 | |
| <1 | | |
| 2,000,000 | | |
| 8,000,000 | | |
| <1 | |
| Dolores Diaz | |
Common Stock | |
8,928,857 | |
| <1 | | |
| 1,785,772 | | |
| 7,143,085 | | |
| <1 | |
| Shawna M. Heisler | |
Common Stock | |
7,000,000 | |
| <1 | | |
| 1,400,000 | | |
| 5,600,000 | | |
| <1 | |
| Stephen Joshua Bertrand | |
Common Stock | |
7,000,000 | |
| <1 | | |
| 1,400,000 | | |
| 5,600,000 | | |
| <1 | |
| Lourdes Perez Ruiz and Cesar A Oliver Canabal | |
Common Stock | |
7,000,000 | |
| <1 | | |
| 7,000,000 | | |
| – | | |
| – | |
| Ana and Raul Hernandez | |
Common Stock | |
6,944,444 | |
| <1 | | |
| 1,388,889 | | |
| 5,555,555 | | |
| <1 | |
| Cannapolis Scientific Farm SAS | |
Common Stock | |
6,429,000 | |
| <1 | | |
| 1,285,800 | | |
| 5,143,200 | | |
| <1 | |
| Juan de Dios Martinez | |
Common Stock | |
6,250,000 | |
| <1 | | |
| 1,250,000 | | |
| 5,000,000 | | |
| <1 | |
| Rosa Galindo | |
Common Stock | |
6,250,000 | |
| <1 | | |
| 1,250,000 | | |
| 5,000,000 | | |
| <1 | |
| Paola Perales | |
Common Stock | |
6,000,000 | |
| <1 | | |
| 1,200,000 | | |
| 4,800,000 | | |
| <1 | |
| David Esparza | |
Common Stock | |
5,882,353 | |
| <1 | | |
| 1,176,471 | | |
| 4,705,882 | | |
| <1 | |
| George and Sky Noel | |
Common Stock | |
5,018,939 | |
| <1 | | |
| 1,003,788 | | |
| 4,015,151 | | |
| <1 | |
| Alex J. Cruz Valez | |
Common Stock | |
5,000,000 | |
| <1 | | |
| 1,000,000 | | |
| 4,000,000 | | |
| <1 | |
| Eduardo Ibarra | |
Common Stock | |
5,000,000 | |
| <1 | | |
| 5,000,000 | | |
| – | | |
| <1 | |
| Bob Wood | |
Common Stock | |
5,000,000 | |
| <1 | | |
| 1,000,000 | | |
| 4,000,000 | | |
| <1 | |
| Brian Cuban | |
Common Stock | |
5,000,000 | |
| <1 | | |
| 1,000,000 | | |
| 4,000,000 | | |
| <1 | |
| Maria Magdelena Pinedo | |
Common Stock | |
5,000,000 | |
| <1 | | |
| 1,000,000 | | |
| 4,000,000 | | |
| <1 | |
| Victor Montanez | |
Common Stock | |
5,000,000 | |
| <1 | | |
| 1,000,000 | | |
| 4,000,000 | | |
| <1 | |
| Marianna Jazmin Sardi Nobles | |
Common Stock | |
5,000,000 | |
| <1 | | |
| 5,000,000 | | |
| – | | |
| – | |
| Teresa Lafond | |
Common Stock | |
4,000,000 | |
| <1 | | |
| 4,000,000 | | |
| – | | |
| – | |
| Ericka and Marcos Nava | |
Common Stock | |
3,750,000 | |
| <1 | | |
| 750,000 | | |
| 3,000,000 | | |
| <1 | |
| Wyntrea Cunningham | |
Common Stock | |
3,571,428 | |
| <1 | | |
| 714,286 | | |
| 2,857,142 | | |
| <1 | |
| Shana Rodriguez | |
Common Stock | |
3,125,000 | |
| <1 | | |
| 685,000 | | |
| 2,440,000 | | |
| <1 | |
| Dante Rodriguez | |
Common Stock | |
3,000,000 | |
| <1 | | |
| 600,000 | | |
| 2,400,000 | | |
| <1 | |
| Eugenio E. Ibarra Pereira | |
Common Stock | |
3,000,000 | |
| <1 | | |
| 600,000 | | |
| 2,400,000 | | |
| <1 | |
| Leidy Marulanda Escudero | |
Common Stock | |
3,000,000 | |
| <1 | | |
| 600,000 | | |
| 2,400,000 | | |
| <1 | |
| Arturo Gomez | |
Common Stock | |
2,500,000 | |
| <1 | | |
| 500,000 | | |
| 2,000,000 | | |
| <1 | |
| Teresa Serrano-Lamm | |
Common Stock | |
2,500,000 | |
| <1 | | |
| 500,000 | | |
| 2,000,000 | | |
| <1 | |
| Cardamom Export Company SAS | |
Common Stock | |
2,143,000 | |
| <1 | | |
| 428,600 | | |
| 1,714,400 | | |
| <1 | |
| Billy and Krista Foxworth | |
Common Stock | |
2,000,000 | |
| <1 | | |
| 400,000 | | |
| 1,600,000 | | |
| <1 | |
| Cecil Bishop, Jr. | |
Common Stock | |
2,000,000 | |
| <1 | | |
| 400,000 | | |
| 1,600,000 | | |
| <1 | |
| Martina A Cortez | |
Common Stock | |
2,000,000 | |
| <1 | | |
| 400,000 | | |
| 1,600,000 | | |
| <1 | |
| Presly Schoenman | |
Common Stock | |
2,000,000 | |
| <1 | | |
| 400,000 | | |
| 1,600,000 | | |
| <1 | |
| Tom Tusing | |
Common Stock | |
2,000,000 | |
| <1 | | |
| 400,000 | | |
| 1,600,000 | | |
| <1 | |
| Fernando and Ramon Najera | |
Common Stock | |
2,000,000 | |
| <1 | | |
| 2,000,000 | | |
| – | | |
| – | |
| Lizeth Vega | |
Common Stock | |
1,893,939 | |
| <1 | | |
| 378,788 | | |
| 1,515,151 | | |
| <1 | |
| Steven and Sonia Flores | |
Common Stock | |
1,562,500 | |
| <1 | | |
| 312,500 | | |
| 1,250,000 | | |
| <1 | |
| Eric Dangler, Timothy Borgmann and David Farmos | |
Common Stock | |
1,500,000 | |
| <1 | | |
| 300,000 | | |
| 1,200,000 | | |
| <1 | |
| Leavery Y. Davidson | |
Common Stock | |
1,500,000 | |
| <1 | | |
| 300,000 | | |
| 1,200,000 | | |
| <1 | |
| Akil Thomas | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Annabel Velasquez | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Jeanette Cantu and Ricardo Beltran | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Maryanne Velasquez | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Ricardo Delacruz | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Robert Gomez | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Shanner Fugett | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Tommy Hampton | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Victor and Rene Gonzalez | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Carrie Ray | |
Common Stock | |
1,177,000 | |
| <1 | | |
| 235,400 | | |
| 941,600 | | |
| <1 | |
| Denise Rodriguez Steidel | |
Common Stock | |
1,000,000 | |
| <1 | | |
| 200,000 | | |
| 800,000 | | |
| <1 | |
| Jill Rocha | |
Common Stock | |
1,000,000 | |
| <1 | | |
| 200,000 | | |
| 800,000 | | |
| <1 | |
| Kristina Gallegos Martinez | |
Common Stock | |
1,000,000 | |
| <1 | | |
| 200,000 | | |
| 800,000 | | |
| <1 | |
| Monique Lucy Castillo Velosa | |
Common Stock | |
1,000,000 | |
| <1 | | |
| 200,000 | | |
| 800,000 | | |
| <1 | |
| Rosangel del Valle Andrades Fuentes | |
Common Stock | |
1,000,000 | |
| <1 | | |
| 1,000,000 | | |
| – | | |
| – | |
| Barbara Collazo Cortes | |
Common Stock | |
500,000 | |
| <1 | | |
| 500,000 | | |
| – | | |
| – | |
| Daniela Montana Arevalo | |
Common Stock | |
500,000 | |
| <1 | | |
| 500,000 | | |
| – | | |
| – | |
| Erika Daniel | |
Common Stock | |
500,000 | |
| <1 | | |
| 100,000 | | |
| 400,000 | | |
| <1 | |
| Travis Slater | |
Common Stock | |
500,000 | |
| <1 | | |
| 100,000 | | |
| 400,000 | | |
| <1 | |
| Brenda Gonzalez | |
Common Stock | |
250,000 | |
| <1 | | |
| 250,000 | | |
| – | | |
| – | |
| Sara and Maria Jaramillo Castillo | |
Common Stock | |
250,000 | |
| <1 | | |
| 50,000 | | |
| 200,000 | | |
| <1 | |
| Alexis Marie Molina | |
Common Stock | |
150,000 | |
| <1 | | |
| 30,000 | | |
| 120,000 | | |
| <1 | |
___________
(1) Assumes the
sale of all of the shares of Common Stock being offered by the Company, which would result in 16,284,677,919 shares of outstanding Common
Stock.
(2) The address
for each person is c/o Cannabis Bioscience International Holdings, Inc., 6201 Bonhomme Road, Suite 466S, Houston, TX 91789.
(3) Applicable
percentage of ownership is based on 10,294,249,347 shares of Common Stock outstanding on the date of this Prospectus, plus the 2,500,000
shares of Common Stock into which the outstanding shares of Series A Preferred Stock are convertible, totaling 10,296,749,347n Stock.
(4) Applicable
percentage of ownership is based on 16,544,249,347 shares of Common Stock to be outstanding assuming that all of the shares offered by
the Company pursuant to this Prospectus are sold, plus the 2,500,000 shares of Common Stock into which the outstanding shares of Series
A Preferred Stock are convertible, totaling 16,546,749,347 shares of Common Stock.
(5) Assumes the
conversion of all of the shares of Series A Preferred.
(6) Includes 117,000
shares of Common Stock beneficially owned together with Henry Levinski.
Corporate Governance
Director Independence
OTC defines “independent director”
as a person other than an executive officer or employee of a company or any other person having a relationship which, in the opinion
of the Board, would interfere with the exercise of independent judgment in carrying out their responsibilities as a director. The persons
who are not considered independent for purposes of this definition are (i) a director who is, or at any time during the past three
years was, employed by the company; (ii) a director who accepted or has a family member who accepted any compensation from the company
in excess of $120,000 during any fiscal year within the three years preceding the determination of independence, other than compensation
for board or board committee service; compensation paid to a family member who is an employee (other than an executive officer) of the
company or benefits under a tax-qualified retirement plan, or non-discretionary compensation or (iii) a director who is the family member
of a person who is, or at any time during the past three years was, employed by the Company as an executive officer.
Inasmuch as all of the directors of the Company
are employed by the Company as its officers, none of them is an independent director.
A director is not considered independent if he
is also an executive officer or employee of the corporation.
Compensation Committee
The Company does not have a standing compensation
committee or a committee performing similar functions because the Board believes that, in light of the Company’s early stage of
development and the fact that its compensation structure is not complex, such a committee is not presently warranted. Accordingly, the
whole Board participates in considering executive compensation and will do so if, in the future, directors are compensated for their services
as such.
MARKET PRICE FOR OUR COMMON
EQUITY
AND RELATED SHAREHOLDER MATTERS
The Common Stock is quoted on the OTC Pink tier
of the alternate trading system operated by OTC under the symbol CHNC. Market quotations for shares of Common Stock shown on OTC’s
quotation system reflect inter-dealer prices without retail mark-up, mark-down or commission and may not necessarily represent actual
transactions.
On the date of this Prospectus, the closing price
for the Common Stock quoted by OTC was $_____.
As of June 30, 2023, there were 431 record holders
of the shares of the Common Stock, of which 2,335,975,553 shares were freely tradable.
The exemption from registration afforded by Rule
144 will not be available until January 13, 2024, at the earliest.
DESCRIPTION OF CAPITAL STOCK
Our authorized capital stock comprises 20,000,000,000
shares of Common Stock, without par value, of which 9,022,937,656 shares are outstanding, and 10,000,000 shares of preferred stock,
without par value, issuable in series, of which 2,500,000 shares have been designated Series A Convertible Preferred Stock (“Series
A Preferred”) and 1,000 shares have been designated Series B Convertible Preferred Stock (“Series B Preferred”), all
of which are outstanding. The rights of the holders of each class and series are as follows:
Common Stock
Holders of Common Stock are entitled to cast
one vote for each share of Common Stock on all matters submitted to a vote of the stockholders; to receive, on a pro-rata basis, dividends
and distributions, if any, that the Board may declare out of legally available funds, subject to preferences that are applicable to the
Series A Preferred and Series B Preferred, and, if any, to series of preferred stock that may be designated in the future; and upon liquidation,
dissolution or winding up, to share equally and ratably in any assets remaining after the payment of all debts and other liabilities,
subject to the prior rights of the holders of the Series A Preferred.
We do not expect to declare or pay dividends
on Common Stock for the foreseeable future. See “Dividend Policy.”
The holders of Common Stock do not have any preemptive,
cumulative voting, subscription, conversion, redemption or sinking fund rights. The Common Stock is not subject to calls or assessments.
The rights and privileges of holders of the Common Stock are subject to those of the Series A Preferred, which are described below, and
to any other series of preferred stock that we may issue in the future.
The Common Stock is quoted on the OTC Pink tier
of the alternate trading system operated by OTC under the symbol “CHNC.”
Preferred Stock
The Company has authorized 10,000,000 shares
of preferred stock, of which 2,500,000 shares have been designated Series A Convertible Preferred Stock and 1,000 shares have been designated
Series B Preferred Stock (“Series B Preferred”). The rights and preferences of the Series A Preferred Stock are the Series
B Preferred Stock are as follows:
Series A Preferred
The Series A Preferred Stock
is senior to the Common Stock and subordinate to all other series of preferred stock.
Each share of Series A Preferred
is entitled to receive out of the funds of the Company legally available therefor, on the date on which such dividend or other distribution
is paid or made to the holders of Common Stock, a dividend or distribution equal to the dividend or distribution that would be paid on
the number of shares of Common Stock into which such share of Series A Preferred Stock is convertible immediately prior to the record
date for such dividend.
In the event of any liquidation,
dissolution or winding up of the Company, the holders of the outstanding shares of Series A Preferred shall be entitled to be paid out
of the assets of the Corporation available for distribution to its shareholders, whether from capital, surplus funds or earnings, and
before any payment is made in respect of the shares of Common Stock, an amount equal to the greater of (i) the Market Price (as defined
in the restated articles of incorporation) of the Series A Preferred Stock on the date of the liquidation, or (ii) ten cents ($0.10) per
share of Series A Preferred, plus accrued but unpaid dividends.
The Series A Preferred may
be redeemed, as a whole or in part, at any time or from time to time, as determined by the Board in its discretion. Upon redemption,
each share of Series A Preferred shall receive as the full redemption payment the number of shares of Common Stock into which it is then
convertible. The Board shall select the shares of Series A Preferred to be redeemed in its sole and unfettered discretion and need not
do so on a pro-rata basis. The Series A Preferred is not redeemable at the option of the holders.
Each share of Series A Preferred
is entitled to one vote for each share of Common Stock into which it is convertible, and except as otherwise required by law, vote as
a group with the holders of Common Stock.
Each share of Series A Preferred
may be converted, at the option of the holder, into the number of shares of Common Stock equal to the quotient obtained by dividing the
current Series A Preference Price by the Series A Conversion Price, which is the greater of (i) $0.10 or (ii) 75% of the Market
Price of the Common Stock on the Conversion Date.
Series B Preferred
The Series B Preferred is
senior to the Common Stock and the Series A Preferred.
In the event of liquidation,
the shares of Series B Preferred shall not be entitled to receive any distribution of cash or other property whatsoever.
The Series B Preferred is
not redeemable at the option of the holder or the Company.
The holders of the Series
B Preferred vote as a group with the holders of all other classes and series of the Corporation’s capital stock and have 60% of
the voting power of the Company on all matters, except that the holders of the Series B Preferred vote as a separate voting group on
all matters affecting their rights as such or as otherwise specified by law. No series of preferred stock having voting rights equal
or superior to the voting rights of the Series B Preferred may designated without the unanimous vote of all of the holders thereof.
The holders of Series B
Preferred have no conversion rights.
Anti-Takeover Effects of the Series B Preferred
The provisions of the restated articles of incorporation
designating the Series B Preferred vest 60% of the voting power of the Company in the holders thereof. These provisions prevent the holders
of Common Stock from taking any action without the approval of the holders of the Series B Preferred. These provisions may have an anti-takeover
effect and may delay, deter or prevent a tender offer, takeover attempt or other transaction that might be in a stockholder’s best
interest, including an attempt that might result in the receipt of a premium over the market price for shares of Common Stock.
Indemnification
The Company’s amended and restated articles
of incorporation require it to indemnify, to the full extent permitted by law, any person who is or was a director or officer of the Company
and may indemnify any other person against any claim, liability or expense arising against or incurred by such person made a party to
a proceeding because he is or was a director, officer, agent, fiduciary or employee of the Company or because he is or was serving another
entity as a director, officer, partner, trustee, employee, fiduciary or agent at the Company’s request.
Elimination of Personal Liability
The Company’s amended and restated articles
of incorporation provide that the personal liability of the Company’s directors to the Company or its stockholders is limited to
the full extent permitted by the CBCA.
Annual Stockholders Meeting
Our amended and restated by-laws provide that
annual stockholder meetings will be held at a date, time and place selected by resolution adopted by a majority of our entire Board or,
if duly authorized by the affirmative vote of a majority of our entire Board, by a committee thereof, or by the chairman of our Board
(if delegated such authority by resolution adopted by a majority of our entire Board). We are permitted to conduct stockholder meetings
by remote communications.
The affirmative vote of holders of a majority
of the outstanding shares of our capital stock present, in person or by proxy, at any annual or special meeting of stockholders and entitled
to vote will decide all matters voted on by stockholders at such meeting, provided that such shares constitute a quorum, unless the question
is one upon which, by express provision of law, under our amended and restated certificate of incorporation, or under our amended and
restated by-laws, a different vote is required, in which case such provision will control.
SHARES ELIGIBLE FOR FUTURE
SALE
Future sales of substantial amounts of Common
Stock, including shares issued upon the exercise of outstanding options or warrants, in the public market after the Offering, or the
perception that such sales may occur, could cause the market price for the Common Stock to fall or impair our ability to raise capital
through sales of our equity securities.
Upon the termination of the Offering,
assuming that all of the shares offered by the Company are sold, there will be 15,275,437,656 shares of Common Stock outstanding.
In
addition to the 10,283,940,599 shares of Common Stock that are offered by this Prospectus,
(i) approximately 935,000,000 shares of Common Stock held by persons who are not affiliates
of the Company will be permitted to be sold after January 13, 2024, under Rule 144 promulgated
by the SEC under the Securities Act (“Rule 144”) without notice to the SEC in
unlimited amounts and without restriction as to the manner of sale and (ii) 3,512,111,700
shares of Common Stock held by a person who is an affiliate of the Company will be permitted
to be sold under Rule 144 after January 13, 2024, in limited amounts, subject to notice to
the SEC and subject to restriction as to the manner of sale and (iii) up to 600,000,000 shares
of Common Stock that may be issued under the Company's 2022 Equity Incentive Plan may be
sold in the public markets, subject to limitations in the case of shares issued under that
plan to affiliates of the Company, upon the filing of a registration statement on Form S-8
with respect thereto or without registration under Rule 144 after being held by for the period
required by that rule. The sale of these shares or the perception that they may be sold may
substantially and adversely affect the market price of the Common Stock, with the result
that persons who acquire shares of Common Stock in the Offering may be able to resell them
only at substantial losses.
Rule 144
In general, under Rule 144, beginning on January
13, 2024, any person who is not our affiliate and has held their shares for at least six months, including the holding period of any
prior owner, except for our affiliates, may sell shares without restriction, subject to the availability of current public information
about us. In addition, under Rule 144, any person who is not and has not been our affiliate at any time during the preceding three months
and has held his shares for at least one year, including the holding period of any prior owner, except for our affiliates, would be entitled
to sell an unlimited number of shares immediately in the event that no current public information about us is available. Beginning on
January 13, 2024, a person who is our affiliate or who was our affiliate at any time during the preceding three months and who has beneficially
owned restricted securities for at least six months, including the holding period of any prior owner other than one of our affiliates,
will be entitled to sell a number of shares within any three-month period that does not exceed the greater of: (i) 1% of the number
of shares of Common Stock outstanding, which will be approximately 152,754,376 shares immediately after the termination of the Offering,
assuming that all of the shares offered by the Company are sold, and (ii) the average weekly trading volume of Common Stock during
the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. Such sales will be subject to certain
manner-of-sale provisions, notice requirements and the availability of current public information about us.
Incentive Plan Registration Statement
We intend to file with the SEC a registration
statement on Form S-8 under the Securities Act covering the shares of common stock that are issuable to existing and future awards under
the Incentive Plan. Shares covered by such registration statement will be available for sale in the open market following its effective
date, subject to limitations applicable to shares of Common Stock held by our affiliates.
Registration rights
Persons to whom we sell shares of Common Stock
or securities convertible into Common Stock pursuant to exemptions from registration under the Securities Act may acquire these shares
or securities under agreements pursuant to which they may demand that we register the sale of the purchased shares under the Securities
Act or, if we file a registration statement under the Securities Act other than a registration statement on Form S-8 covering securities
issuable under the Incentive Plan or on Form S-4, may have the right to include their shares in such registration. Following such registered
sales, these shares will be freely tradable without restriction under the Securities Act, unless they are held by our affiliates.
MATERIAL U.S. FEDERAL INCOME
TAX CONSEQUENCES TO NON-U.S. HOLDERS OF COMMON STOCK
The following is a summary of the material U.S.
federal income tax consequences to non-U.S. holders (as defined below) of the acquisition, ownership, and disposition of Common Stock
issued pursuant to this offering. This discussion is not a complete analysis of all potential U.S. federal income tax consequences relating
thereto, does not address the potential application of the Medicare contribution tax on net investment income, the alternative minimum
tax, or the special tax accounting rules under Section 451(b) of the Code, and does not address any estate or gift tax consequences or
any tax consequences arising under any state, local, or foreign tax laws, or any other U.S. federal tax laws. This discussion is based
on the Code and applicable Treasury Regulations promulgated thereunder, judicial decisions, and published rulings and administrative
pronouncements of the Internal Revenue Service, or IRS, all as in effect as of the date hereof. These authorities are subject to differing
interpretations and may change, possibly retroactively, resulting in U.S. federal income tax consequences different from those discussed
below. We have not requested a ruling from the IRS with respect to the statements made and the conclusions reached in the following summary,
and there can be no assurance that the IRS or a court will agree with such statements and conclusions.
This discussion is limited to non-U.S. holders
who purchase Common Stock pursuant to this offering and who hold Common Stock as a “capital asset” within the meaning of
Section 1221 of the Code (generally, property held for investment). This discussion does not address all of the U.S. federal income tax
consequences that may be relevant to a particular holder in light of such holder’s particular circumstances. This discussion also
does not consider any specific facts or circumstances that may be relevant to holders subject to special rules under the U.S. federal
income tax laws, including:
| · |
|
certain former citizens or long-term residents of the United States;
|
| |
|
|
| · |
|
“controlled foreign corporations”; |
| |
|
|
| · |
|
“passive foreign investment companies”; |
| |
|
|
| · |
|
corporations that accumulate earnings to avoid U.S. federal income
tax; |
| |
|
|
| · |
|
banks, financial institutions, investment funds, insurance companies,
brokers, dealers, or traders in securities; |
| |
|
|
| · |
|
tax-exempt organizations and governmental organizations; |
| |
|
|
| · |
|
tax-qualified retirement plans; |
| |
|
|
| · |
|
“qualified foreign pension funds” as defined in Section 897(l)(2)
of the Code and entities, all of the interests of which are held by qualified foreign pension funds; |
| |
|
|
| · |
|
persons that own, or have owned, actually or constructively, more than
5% of Common Stock at any time; |
| |
|
|
| · |
|
persons who have elected to mark securities to market. |
If an entity or arrangement that is classified
as a partnership for U.S. federal income tax purposes holds Common Stock, the U.S. federal income tax treatment of the partnership and
the partners thereof generally depend on the status of the partner and the activities
of the partnership. Partnerships holding Common Stock and the partners in such partnerships are urged to consult their tax advisors about
the particular U.S. federal income tax consequences to them of holding and disposing of Common Stock.
THIS DISCUSSION IS FOR INFORMATIONAL PURPOSES
ONLY AND IS NOT TAX ADVICE. PROSPECTIVE INVESTORS SHOULD CONSULT THEIR TAX ADVISORS REGARDING THE PARTICULAR U.S. FEDERAL INCOME TAX
CONSEQUENCES TO THEM OF ACQUIRING, OWNING, AND DISPOSING OF COMMON STOCK, AS WELL AS ANY TAX CONSEQUENCES ARISING UNDER ANY STATE, LOCAL,
OR FOREIGN TAX LAWS AND ANY OTHER U.S. FEDERAL TAX LAWS.
Definition of non-U.S. holder
For purposes of this discussion, the term “non-U.S.
holder” means any beneficial owner of Common Stock that is not a “U.S. person” or a partnership (including any entity
or arrangement treated as a partnership) for U.S. federal income tax purposes. A U.S. person is any person that, for U.S. federal income
tax purposes, is or is treated as any of the following:
| · |
|
an individual who is a citizen or resident of the United States; |
| |
|
|
| · |
|
a corporation (or entity treated as a corporation for U.S. federal
income tax purposes) created or organized under the laws of the United States, any state thereof, or the District of Columbia; |
| |
|
|
| · |
|
an estate, the income of which is subject to U.S. federal income tax
regardless of its source; or |
| |
|
|
| · |
|
a trust (1) whose administration is subject to the primary supervision
of a U.S. court and which has one or more U.S. persons who have the authority to control all substantial decisions of the trust or
(2) that has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
Distributions on Common Stock
We have not paid dividends on Common Stock and
do not anticipate paying dividends on Common Stock for the foreseeable future. However, if we make cash or other property distributions
on Common Stock, such distributions will constitute dividends for U.S. federal income tax purposes to the extent paid from our current
or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S.
federal income tax purposes will constitute a return of capital and will first be applied against and reduce a holder’s tax basis
in Common Stock, but not below zero. Any excess will be treated as gain realized on the sale or other disposition of Common Stock and
will be treated as described under the section titled “— Gain on disposition of Common Stock” below.
Subject to the discussions below regarding effectively
connected income, backup withholding and Sections 1471 through 1474 of the Code (commonly referred to as FATCA), dividends paid to a
non-U.S. holder generally will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends or such
lower rate specified by an applicable income tax treaty. To receive the benefit of a reduced treaty rate, a non-U.S. holder must furnish
a valid IRS Form W-8BEN or IRS Form W-8BEN-E (or applicable successor form) and satisfy applicable certification and other requirements.
This certification must be provided before the payment of dividends and must be updated periodically. If the non-U.S. holder holds the
stock through a financial institution or other agent acting on the non-U.S. holder’s behalf, the non-U.S. holder will be required
to provide appropriate documentation to the agent, which then will be required to provide certification, either directly or through other
intermediaries.
If a non-U.S. holder holds Common Stock in connection
with the conduct of a trade or business in the United States, and dividends paid on Common Stock are effectively connected with such
holder’s U.S. trade or business (and are attributable to such holder’s permanent establishment in the United States if required
by an applicable tax treaty), the non-U.S. holder will be exempt from U.S. federal withholding tax. To claim the exemption, the non-U.S.
holder must generally furnish a valid IRS Form W-8ECI (or applicable successor form) to us or our paying agent. However, any such effectively
connected dividends paid on Common Stock generally will be subject to U.S. federal income tax on a net income basis at the regular U.S.
federal income tax rates in the same manner as if such holder were a resident of the United States. A non-U.S. holder that is a foreign
corporation also may be subject to an additional branch profits tax equal to 30% (or such lower rate specified by an applicable income
tax treaty) of its effectively connected earnings and profits for the taxable year, as adjusted for certain items. Non-U.S. holders should
consult their tax advisors regarding any applicable income tax treaties that may provide for different rules.
Non-U.S. holders that do not provide the required
certification on a timely basis, but that qualify for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely
filing an appropriate claim for refund with the IRS.
Gain on disposition of Common Stock
Subject to the discussions below regarding backup
withholding and FATCA, a non-U.S. holder generally will not be subject to U.S. federal income tax on any gain realized on the sale or
other disposition of Common Stock unless:
| · |
|
the gain is effectively connected with the non-U.S. holder’s
conduct of a trade or business in the United States and, if required by an applicable income tax treaty, is attributable to a permanent
establishment maintained by the non-U.S. holder in the United States; |
| |
|
|
| · |
|
the non-U.S. holder is a nonresident alien individual present in the
United States for 183 days or more during the taxable year of the disposition, and certain other requirements are met; or |
| |
|
|
| · |
|
Common Stock constitutes a “United States real property interest,”
or USRPI, by reason of our status as a United States real property holding corporation, or USRPHC, for U.S. federal income tax purposes
at any time within the shorter of the five-year period preceding the disposition or the non-U.S. holder’s holding period for
Common Stock. |
The determination of whether we are a USRPHC
depends on the fair market value of our USRPIs relative to the fair market value of worldwide real property interests and our other assets
used or held for use in a trade or business. We believe that we are not and do not anticipate becoming a USRPHC for U.S. federal
income tax purposes, although there can be no assurance that we will not become a USRPHC. Even if we are or were to become a USRPHC,
gain arising from the sale or other taxable disposition of Common Stock by a non-U.S. holder will not be subject to U.S. federal income
tax if Common Stock is “regularly traded” (as defined by applicable Treasury Regulations) on an established securities market,
and such non-U.S. holder owned, actually and constructively, 5% or less of Common Stock throughout the shorter of the five-year period
ending on the date of the sale or other taxable disposition or the non-U.S. holder’s holding period. Prospective investors are
encouraged to consult their own tax advisors regarding the possible consequences to them if we are, or were to become, a USRPHC.
Gain described in the first bullet point above
generally will be subject to U.S. federal income tax on a net income basis at the regular U.S. federal income tax rates in the same manner
as if such holder were a resident of the United States. A non-U.S. holder that is a foreign corporation also may be subject to an additional
branch profits tax equal to 30% (or such lower rate specified by an applicable income tax treaty) of its effectively connected earnings
and profits for the taxable year, as adjusted for certain items. Gain described in the second bullet point above will be subject to U.S.
federal income tax at a flat 30% rate (or such lower rate specified by an applicable income tax treaty), but may be offset by certain
U.S.-source capital losses (even though the individual is not considered a resident of the United States), provided that the non-U.S.
holder has timely filed U.S. federal income tax returns with respect to such losses. Non-U.S. holders should consult their tax advisors
regarding any applicable income tax treaties that may provide for different rules.
Information reporting and backup withholding
Annual reports are required to be filed with
the IRS and provided to each non-U.S. holder indicating the amount of distributions on Common Stock paid to such holder and the amount
of any tax withheld with respect to those distributions. These information reporting requirements apply regardless of whether such distributions
constitute dividends and even if no withholding was required. This information also may be made available under a specific treaty or
agreement with the tax authorities in the country in which the non-U.S. holder resides or is established. Backup withholding, currently
at a 24% rate, generally will not apply to payments to a non-U.S. holder of dividends on or the gross proceeds of a disposition of Common
Stock provided the non-U.S. holder furnishes the required certification for its non-U.S. status, such as by providing a valid IRS Form
W-8BEN, IRS Form W-8BEN-E, or IRS Form W-8ECI, or certain other requirements are met. Backup withholding may apply if the payor has actual
knowledge, or reason to know, that the holder is a U.S. person who is not an exempt recipient.
Backup withholding is not an additional tax.
If any amount is withheld under the backup withholding rules, the non-U.S. holder should consult with a U.S. tax advisor regarding the
possibility of and procedure for obtaining a refund or a credit against the non-U.S. holder’s U.S. federal income tax liability,
if any.
FATCA
FATCA imposes a U.S. federal withholding tax
of 30% on certain payments made to a “foreign financial institution” (as specially defined under these rules) unless such
institution enters into an agreement with the U.S. government to withhold on certain payments and to collect and provide to the U.S.
tax authorities substantial information regarding certain U.S. account holders of such institution (which includes certain equity and
debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners) or an exemption applies.
FATCA also generally will impose a U.S. federal withholding tax of 30% on certain payments made to a non-financial foreign entity unless
such entity provides the withholding agent a certification identifying certain direct and indirect U.S. owners of the entity or an exemption
applies. An intergovernmental agreement between the United States and an applicable foreign country may modify these requirements. Under
certain circumstances, a non-U.S. holder might be eligible for refunds or credits of such taxes. FATCA currently applies to dividends
paid on Common Stock. Under applicable Treasury Regulations and administrative guidance, withholding under FATCA would have applied to
payments of gross proceeds from the sale or other disposition of stock, but under proposed regulations (the preamble to which specifies
that taxpayers are permitted to rely on such proposed regulations pending finalization), no withholding would apply with respect to payments
of gross proceeds.
Prospective investors are encouraged to consult
with their own tax advisors regarding the possible implications of this legislation on their investment in Common Stock.
PLAN OF DISTRIBUTION
By the Company
The Company is offering up to 6,250,000,000
shares of Common Stock at the Fixed Offering Price, unless modified by a post-effective amendment to the registration statement of which
this Prospectus is a part.
Each time we offer and sell such shares, we
will, if required, make available a Prospectus that will describe the method of distribution and set forth the terms of the offering.
We may terminate the offering before all shares
are sold. There is no minimum number of shares that must be sold before we may use the proceeds. Proceeds will not be returned to investors
if we sell less than all of the shares offered by this Prospectus. The proceeds from the sales of the shares will not be placed in an
escrow account.
The offering by the Company will be conducted
by the executive officers of the Company. Under Rule 3a 4-1 of the Exchange Act, an issuer may conduct a direct offering of its securities
without registration as a broker-dealer using officers who perform substantial duties for or on behalf of the issuer otherwise than in
connection with securities transactions and who were not brokers or dealers or associated persons of brokers or dealers within the preceding
12 months and who have not participated in selling an offering of securities for any issuer more than once every 12 months, with certain
exceptions. Furthermore, such persons may not be subject to statutory disqualification under Section 3(a)(39) of the Securities Exchange
Act and may not be compensated in connection with securities offerings by payment of commission or other remuneration based either directly
or indirectly on transactions in securities and at the time of offering our shares may not be associated persons of a broker or dealer.
Mr. Picazo and our other executive officers meet these requirements.
During the Offering, the Company may offer
unregistered shares of Common Stock to investors in private placements at prices per share that may be higher or lower than the
public offering price, but only to the extent that it is permitted to do so by the federal securities laws, including Rule 152
promulgated under the Securities Act.
By the Selling Stockholders
The Selling Stockholders identified in this Prospectus
may offer, from time to time, up to 3,860,369,171 shares of Common Stock up to the respective amounts set forth in this Prospectus.
We will not receive any of the proceeds of such sales. There can be no assurance that the Selling Stockholders will offer or sell any
or all of such Common Stock.
Messrs. Picazo, Levinski and Torres, who are
officers and directors of the Company, and Mr. John Jones, who owns more than ten percent of the outstanding shares of Common Stock,
may be regarded as underwriters. In addition, Mr. Picazo has indicated that he may reinvest all or a portion of the proceeds of sales
of his shares, in the form of equity or debt, on terms to be approved by the Board in the manner provided by Colorado law respecting
transactions in which officers and directors of the Company have an interest and may be regarded as an underwriter in respect of such
reinvestments.
The Selling Stockholders and their successors,
including their transferees, may sell all or a portion of their shares directly to purchasers or through broker-dealers
or agents, who may receive compensation in the form of discounts, concessions or commissions from the Selling Stockholders or the purchasers
of the shares. These discounts, concessions or commissions as to any particular underwriter, broker-dealer or agent may be in excess of
those customary in the types of transactions involved.
These shares may be sold in one or more transactions
on any national securities exchange or alternate trading system on which the shares may be listed or quoted at the time of sale or in
the over-the-counter market or transactions otherwise than on these exchanges or systems in one or more transactions. The shares will
be sold at the Fixed Offering Price. These sales may be effected in transactions, which may involve crosses or block transactions.
Additionally, the Selling Stockholders may enter into derivative transactions with third parties or sell securities not covered by this
Prospectus to third parties in privately negotiated transactions. The Selling Stockholders may use any one or more of the following methods
when selling shares:
| · | on
any national securities exchange or alternated trading system on which the shares may be
listed or quoted at the time of sale, including NASDAQ; |
| | | |
| · | in
transactions otherwise than on these exchanges or services or in the over-the-counter market; |
| · | through
the writing or settlement of options, whether the options are listed on an options exchange
or otherwise; |
| · | ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| · | block
trades in which the broker-dealer will attempt to sell the shares as agent but may position
and resell a portion of the block as principal to facilitate the transaction; |
| · | purchases
by a broker-dealer as principal and resale by the broker-dealer for its account; |
| · | an
exchange distribution in accordance with the rules of the applicable exchange; |
| · | a
debt-for-equity exchange; |
| · | privately
negotiated transactions; |
| · | settlement
of short sales entered into after the effective date of the registration statement of which
this Prospectus forms a part; |
| · | broker-dealers
may agree with the Selling Stockholders to sell a specified number of such shares at a stipulated
price per share; |
| · | a
combination of any such methods of sale; and |
| · | any
other method permitted by applicable law. |
The anti-manipulation rules of Regulation M under
the Exchange Act may apply to sales of the shares of Common Stock offered pursuant to this Prospectus and the activities of the
Selling Stockholders. In addition, we will make copies of this Prospectus available to the Selling Stockholders to satisfy the prospectus
delivery requirements of the Securities Act. To the extent applicable, Regulation M may also restrict the ability of any person engaged
in the distribution of the Common Stock to engage in market-making activities with respect to the Common Stock. All of the foregoing
may affect the marketability of the Common Stock and the ability of any person or entity to engage in market-making activities with respect
to the Common Stock.
In addition, any securities that qualify for sale
pursuant to Rule 144, Regulation S under the Securities Act or Section 4(1) under the Securities Act may be sold under such rules rather
than pursuant to this Prospectus.
The Selling Stockholders may sell short
the shares and deliver Common Stock to close out short positions, or loan or pledge the shares to broker-dealers that in turn may
sell these shares. The Selling Stockholders may also enter into option or other transactions with broker-dealers or other financial
institutions or the creation of one or more derivative securities that require the delivery to such broker-dealer or other financial
institution of shares offered by this Prospectus, which shares such broker-dealer or other financial institution may resell pursuant
to this Prospectus. The Selling Stockholders also may transfer and donate the shares in other circumstances, in which case the
transferees, donees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this
Prospectus.
The aggregate proceeds to the Selling Stockholders
from the sale of the shares of Common Stock will be the sale price for the shares, less discounts and commissions, if any.
In offering the shares of Common Stock covered
by this Prospectus, the Selling Stockholders and any broker-dealers who execute sales for the Selling Stockholders may be deemed to be
“underwriters” within the meaning of Section 2(a)(11) of the Securities Act in connection with such sales. Any profits realized
by the Selling Stockholders and the compensation of any broker-dealer may be deemed to be underwriting discounts and commissions. Selling
Stockholders who are “underwriters” within the meaning of Section 2(a)(11) of the Securities Act will be subject to the prospectus
delivery requirements of the Securities Act and may be subject to certain statutory and regulatory liabilities, including liabilities
imposed pursuant to Sections 11, 12 and 17 of the Securities Act and Rule 10b-5 under the Exchange Act.
To comply with the securities laws of certain
states, if applicable, the shares of Common Stock must be sold in such jurisdictions only through registered or licensed brokers or dealers.
In addition, in certain states the shares may not be sold unless the shares are registered or qualified for sale in the applicable state
or an exemption from the registration or qualification requirement is available and is complied with.
LEGAL MATTERS
The validity of the shares of Common Stock offered
by this Prospectus has been passed upon by Barry J. Miller of West Bloomfield, Michigan. Mr. Miller is the indirect beneficial holder
of 50,000,000 shares of Common Stock.
EXPERTS
The consolidated financial statements of the
Company for the years ended on May 31, 2022, and May 31, 2021, have been included in this Prospectus and in the registration statement
of which it forms a part in reliance upon the report of PWR CPA, LLP, the Company’s independent registered public accounting
firm, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement
on Form S-1, including exhibits and schedules, under the Securities Act, with respect to the shares of Common Stock being offered by
this Prospectus. This Prospectus, which constitutes part of the registration statement, does not contain all of the information in the
registration statement and its exhibits. For further information with respect to us and the Common Stock offered by this Prospectus,
we refer you to the registration statement and its exhibits. Statements contained in this Prospectus as to the contents of any contract
or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other
document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You can read our SEC filings, including the registration
statement, free of charge, over the Internet at the SEC’s website at www.sec.gov.
We will be subject to the information reporting
requirements of the Exchange Act and we will file reports and other information with the SEC. You may access these materials free of
charge on the SEC’s website as soon as they are filed with the SEC.
Information on or accessible through our website
is not a part of this Prospectus, and the inclusion of our website address in this Prospectus is an inactive textual reference only.
INDEX
TO CONSOLIDATED FINANCIAL STATEMENTS
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS,
INC.
(formerly named China Infrastructure Construction
Corp.)
CONSOLIDATED BALANCE SHEETS
| | |
February 28, 2023 | | |
May 31, 2022 | |
| | |
(Unaudited) | | |
(Audited) | |
| ASSETS |
|
|
|
|
|
|
|
|
| CURRENT ASSETS | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 25,655 | | |
$ | 31,982 | |
| Accounts receivable | |
| 4,484 | | |
| 5,614 | |
| Related party receivables | |
| 12,000 | | |
| 12,000 | |
| TOTAL CURRENT ASSETS | |
| 42,139 | | |
| 49,596 | |
| Right-of-use asset | |
| 33,265 | | |
| 60,298 | |
| TOTAL ASSETS | |
$ | 75,404 | | |
$ | 109,894 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | |
| | | |
| | |
| | |
| | | |
| | |
| CURRENT LIABILITIES | |
| | | |
| | |
| Accounts payables and accrued expenses | |
$ | 86,879 | | |
$ | 68,210 | |
| Related party payables | |
| 89,484 | | |
| 15,838 | |
| Short-term loans | |
| 116,794 | | |
| 48,074 | |
| SBA loan - current | |
| 14,592 | | |
| 5,561 | |
| PPP loan | |
| – | | |
| 41,666 | |
| Lease liabilities | |
| 15,103 | | |
| 44,054 | |
| TOTAL CURRENT LIABILITIES | |
| 322,852 | | |
| 223,403 | |
| LONG-TERM LIABILITIES | |
| | | |
| | |
| SBA loan | |
| 234,210 | | |
| 243,738 | |
| Lease liabilities | |
| – | | |
| 3,804 | |
| TOTAL LONG-TERM LIABILITIES | |
| 234,210 | | |
| 247,542 | |
| TOTAL LIABILITIES | |
| 557,062 | | |
| 470,945 | |
| STOCKHOLDERS’ DEFICIT | |
| | | |
| | |
| Series A Convertible Preferred Stock: 2,500,000 shares designated and outstanding at February 28, 2023, and May 31, 2022 (without par value at February 28, 2023, and par value $0.001 per share at May 31, 2022) | |
| – | | |
| 2,500 | |
| Series B Convertible Preferred Stock, without par value: 1,000 shares designated and outstanding at February 28, 2023; none designated at May 31, 2022 | |
| – | | |
| – | |
| Common stock, without par value: 20,000,000,000 shares authorized; 9,459,677,919. and 8,612,998,299 shares issued and outstanding as of February 28, 2023, and May 31, 2022, respectively. | |
| – | | |
| – | |
| Additional paid-in capital | |
| 3,925,071 | | |
| 3,286,605 | |
| Accumulated deficit | |
| (4,406,729 | ) | |
| (3,650,156 | ) |
| TOTAL STOCKHOLDERS’ DEFICIT | |
| (481,658 | ) | |
| (361,051 | ) |
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | |
$ | 75,404 | | |
$ | 109,894 | |
The accompanying notes are an integral part of
these unaudited consolidated financial statements.
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS,
INC.
(formerly named China Infrastructure Construction
Corp.)
CONSOLIDATED STATEMENTS OF
OPERATIONS
(Unaudited)
| | |
Nine Months Ended February 28, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Revenue | |
$ | 270,413 | | |
$ | 141,981 | |
| Cost of revenues | |
| 77,443 | | |
| 31,515 | |
| Gross profit | |
| 192,970 | | |
| 110,466 | |
| | |
| | | |
| | |
| Cost and expenses | |
| | | |
| | |
| General and administrative | |
| 102,005 | | |
| 98,880 | |
| Contract labor | |
| 528,610 | | |
| 397,216 | |
| Professional fees | |
| 173,767 | | |
| 108,911 | |
| Officer compensation | |
| 36,235 | | |
| 54,797 | |
| Rent and lease | |
| 55,915 | | |
| 60,353 | |
| Travel | |
| 4,911 | | |
| 8,502 | |
| Total operating expenses | |
| 901,443 | | |
| 728,659 | |
| | |
| | | |
| | |
| Operating loss | |
| (708,473 | ) | |
| (618,193 | ) |
| | |
| | | |
| | |
| Other income (expense) | |
| | | |
| | |
| Forgiveness of debt | |
| 41,666 | | |
| 33,708 | |
| Interest | |
| (89,767 | ) | |
| (45,665 | ) |
| Total other income | |
| (48,101 | ) | |
| (11,957 | ) |
| | |
| | | |
| | |
| Net loss | |
$ | (756,573 | ) | |
$ | (630,150 | ) |
| | |
| | | |
| | |
| Average common stock outstanding | |
| 8,724,596,387 | | |
| 7,882,105,374 | |
| Average loss per share | |
$ | (0.00009 | ) | |
$ | (0.00008 | ) |
The accompanying notes are
an integral part of these unaudited consolidated financial statements.
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS,
INC.
(formerly named China Infrastructure Construction
Corp.)
CONSOLIDATED STATEMENTS
OF CASH FLOWS
(Unaudited)
| | |
Nine Months Ended February 28, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| OPERATING ACTIVITIES: | |
| | | |
| | |
| Net loss | |
$ | (757,573 | ) | |
$ | (630,150 | ) |
| Adjustments to reconcile net income: | |
| | | |
| | |
| Amortization of right-of-use asset and liability | |
| (5,722 | ) | |
| (1,629 | ) |
| Forgiveness of PPP loan | |
| (41,666 | ) | |
| (31,750 | ) |
| Changes in assets and liabilities: | |
| | | |
| | |
| Accounts receivable | |
| 1,130 | | |
| 7,147 | |
| Accounts payable and accrued expenses | |
| 18,669 | | |
| 55,626 | |
| Deferred revenue | |
| – | | |
| 31,860 | |
| Prepaid expenses | |
| – | | |
| 1,538 | |
| Related party payable | |
| 73,646 | | |
| – | |
| NET CASH USED IN OPERATIONS | |
| (710,516 | ) | |
| (584,728 | ) |
| | |
| | | |
| | |
| FINANCING ACTIVITIES: | |
| | | |
| | |
| Proceeds from sales of common stock | |
| 635,966 | | |
| 532,500 | |
| Proceeds of short-term loans | |
| 68,270 | | |
| 5,465 | |
| Proceeds from PPP loans | |
| – | | |
| 5,000 | |
| Repayment of SBA loan | |
| (497 | ) | |
| – | |
| Proceeds from related party loan | |
| – | | |
| 5,227 | |
| NET CASH PROVIDED BY FINANCING ACTIVITIES | |
| 704,189 | | |
| 548,192 | |
| | |
| | | |
| | |
| NET DECREASE IN CASH | |
| (6,327 | ) | |
| (36,536 | ) |
| | |
| | | |
| | |
| CASH AT BEGINNING OF PERIOD | |
| 31,982 | | |
| 41,322 | |
| | |
| | | |
| | |
| CASH AT END OF PERIOD | |
$ | 25,655 | | |
$ | 4,786 | |
| | |
| | | |
| | |
| Supplemental disclosure of cash flow information | |
| | | |
| | |
| Cash paid for interest | |
$ | 89,767 | | |
$ | 17,981 | |
| Cash paid for taxes | |
$ | – | | |
$ | – | |
The accompanying notes are
an integral part of these unaudited consolidated financial statements.
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS,
INC.
(formerly named China Infrastructure Construction
Corp.)
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIT
(Unaudited)
| | |
Series A Convertible Preferred Stock | | |
Series B Preferred
Convertible Stock | | |
Common Stock | | |
Additional Paid | | |
Accumulated | | |
| |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
cCapital | | |
Deficit | | |
Total | |
| Balances May 31, 2021 | |
| 2,500,000 | | |
$ | 2,500 | | |
| – | | |
$ | – | | |
| 7,814,238,100 | | |
$ | 2,461,315 | | |
$ | (2,764,983 | ) | |
$ | (301,168 | ) |
| Sales of common stock for cash | |
| – | | |
| – | | |
| – | | |
| – | | |
| 36,134,739 | | |
| 82,500 | | |
| – | | |
| 82,500 | |
| Net loss for the quarter | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (171,287 | ) | |
| (171,287 | ) |
| Balance August 31, 2021 | |
| 2,500,000 | | |
$ | 2,500 | | |
| – | | |
$ | – | | |
| 7,850,372,839 | | |
$ | 2,543,815 | | |
$ | (2,936,272 | ) | |
$ | (389,957 | ) |
| Sales of common stock for cash | |
| – | | |
| – | | |
| – | | |
| – | | |
| 51,893,939 | | |
| 95,000 | | |
| – | | |
| 95,000 | |
| Net loss for the quarter | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (192,506 | ) | |
| (192,506 | ) |
| Prior fiscal year adjustments | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | |
| Balance November 30, 2021 | |
| 2,500,000 | | |
$ | 2,500 | | |
| – | | |
$ | – | | |
| 7,902,266,778 | | |
$ | 2,638,815 | | |
$ | (3,128,778 | ) | |
$ | (487,463 | ) |
| Sales of common stock for cash | |
| – | | |
| – | | |
| – | | |
| – | | |
| 363,333,333 | | |
| 355,000 | | |
| – | | |
| 355,000 | |
| Net loss for the quarter | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (266,357 | ) | |
| (266,357 | ) |
| Balance February 28, 2022 | |
| 2,500,000 | | |
$ | 2,500 | | |
| – | | |
$ | – | | |
| 8,265,600,111 | | |
$ | 2,993,815 | | |
$ | (3,395,135 | ) | |
$ | (398,820 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance May 31, 2022 | |
| 2,500,000 | | |
$ | 2,500 | | |
| – | | |
$ | – | | |
| 8,612,998,299 | | |
$ | 3,286,605 | | |
$ | (3,650,156 | ) | |
$ | (361,051 | ) |
| Sales of common stock for cash | |
| – | | |
| – | | |
| – | | |
| – | | |
| 125,000,000 | | |
| 75,000 | | |
| – | | |
| 75,000 | |
| Change in par value of common stock | |
| – | | |
| (2,500 | ) | |
| – | | |
| – | | |
| – | | |
| 2,500 | | |
| – | | |
| – | |
| Exchange of Series B Preferred Stock for common stock | |
| – | | |
| – | | |
| 1,000 | | |
| – | | |
| (595,467,205 | ) | |
| – | | |
| – | | |
| – | |
| Net loss for the quarter | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (212,030 | ) | |
| (212,030 | ) |
| Balance August 31, 2022 | |
| 2,500,000 | | |
$ | – | | |
| 1,000 | | |
$ | – | | |
| 8,142,531,094 | | |
$ | 3,364,105 | | |
$ | (3,862,186 | ) | |
$ | (498,081 | ) |
| Sales of common stock for cash | |
| – | | |
| – | | |
| – | | |
| – | | |
| 704,388,889 | | |
| 312,666 | | |
| – | | |
| 312,666 | |
| Net loss for the Quarter | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (219,886 | ) | |
| (219,886 | ) |
| Balance November 30, 2022 | |
| 2,500,000 | | |
$ | – | | |
| 1,000 | | |
$ | – | | |
| 8,846,919,983 | | |
$ | 3,676,771 | | |
$ | (4,082,072 | ) | |
$ | (405,301 | ) |
| Sales of common stock for cash | |
| – | | |
| – | | |
| – | | |
| – | | |
| 612,757,936 | | |
| 248,300 | | |
| – | | |
| 248,300 | |
| Net loss for the quarter | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (324,657 | ) | |
| (324,657 | ) |
| Balance February 28, 2023 | |
| 2,500,000 | | |
$ | – | | |
| 1,000 | | |
$ | – | | |
| 9,459,677,919 | | |
$ | 3,925,071 | | |
$ | (4,406,729 | ) | |
$ | (481,658 | ) |
The accompanying notes are
an integral part of these unaudited consolidated financial statements.
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS,
INC.
(formerly named China Infrastructure Construction
Corp.)
Notes to Unaudited Consolidated
Financial Statements
February 28, 2023
Note 1 – Organization and Business
Organization and Operations
Cannabis Bioscience International Holdings, Inc.,
a Colorado corporation (the “Company”), was formed on February 28, 2003, as a limited liability company under the name Fidelity
Aircraft Partners LLC. On December 16, 2009, it converted to a corporation under the name Fidelity Aviation Corporation, and on August
24, 2009, it changed its name to China Infrastructure Construction Corp. On February 28, 2018, the Company changed its name to Hippocrates
Direct Healthcare, Inc.; on July 4, 2018, it resumed the name China Infrastructure Construction Corp. On December 6, 2022, it changed
its name to its present name. The Company provides educational systems focused on medical cannabis in cities throughout the United States
and six countries in Latin America. The Company provides services in therapeutic areas of clinical trials and services relating to sleep
disorders through its sleep center in Houston, Texas. The Company offered concierge medicine at an affordable price through a membership-based
model through its wholly owned subsidiary, Hippocrates Direct Healthcare, LLC, a Texas limited liability company, formed on September
11, 2017; this business was discontinued during the quarter ended August 31, 2020. The Company has one subsidiary, Alpha Fertility and
Sleep Center, LLC, a Texas limited liability company, through which it conducts its sleep center business.
Note 2 – Summary of Significant Accounting Policies
Accounting Principles
The accompanying unaudited consolidated financial
statements have been prepared using the accrual basis of accounting in accordance with accounting principles generally accepted in the
United States of America (“GAAP”) for interim financial statements. Accordingly, they do not contain all information and footnotes
required by GAAP for annual financial statements. In the opinion of the Company’s management, the accompanying unaudited consolidated
financial statements contain all the adjustments necessary (consisting only of normal recurring accruals) to present the consolidated
financial position of the Company at February 28, 2023, and the results of operations and cash flows for the periods presented. The results
of operations for the nine months ended February 28, 2023, are not necessarily indicative of the operating results for the full fiscal
year or any future period. These unaudited consolidated financial statements should be read in conjunction with the consolidated financial
statements and related notes thereto included in the Company’s audited financial statements for the fiscal year ended May 31, 2022.
Use of Estimates
The preparation of financial statements in conformity
with U.S. GAAP requires management to make significant estimates and assumptions that affect the reported amounts of assets and liabilities
and disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenue and expenses
during the reporting periods. Making estimates requires management to exercise significant judgment. Certain of these estimates could
be affected by external conditions, including those unique to the Company’s businesses and general economic conditions. These external
conditions could have an effect on the Company’s estimates that could cause actual results to differ materially from its estimates.
Actual results could differ from those estimates. The Company re-evaluates all of its accounting estimates at least quarterly based on
these conditions and records adjustments when necessary.
Significant estimates relied upon in preparing
these statements include revenue recognition, accounts receivable reserves, accrued expenses, share-based compensation and the recoverability
of the Company’s net deferred tax assets and any related valuation allowance.
Principles of Consolidation
The unaudited consolidated financial statements
include the accounts of the Company and its wholly-owned subsidiaries. All intercompany accounts and transactions have been eliminated
in consolidation.
Reclassification
Certain amounts in the prior consolidated financial
statements have been reclassified to conform to the presentation of the current period financial statements. These reclassifications had
no impact on the results of operations, changes in equity, or cash flows.
Cash and Cash Equivalents
Cash equivalents are short-term, highly liquid
investments that are readily convertible to cash with original maturities of three months or less at the date acquired. The Company had
$188 and $300 of investment securities that were deemed cash equivalents at February 28, 2023, and May 31, 2022, respectively.
Accounts Receivable
Included in accounts receivable on the balance
sheets are amounts primarily related to customers. The Company estimates losses on receivables based on known troubled accounts and historical
experience of losses incurred. Receivables are considered impaired and written off when it is probable that all contractual payments due
will not be collected in accordance with the terms of the related agreement. Based on experience and the judgment of management, there
was no allowance for doubtful accounts at February 28, 2023, and May 31, 2022.
Revenue Recognition
The Company
follows Accounting Standards Codification 606 (“ASC 606”), Revenue from Contracts with Customers, which
requires it to recognize revenues when it transfers goods or services to customers in an amount that reflects the consideration it expects
to receive for them.
Under ASC
606, the Company recognizes revenue when a customer obtains control of promised goods or services or goods are shipped to a customer in
an amount that reflects the consideration it expects to receive in exchange for them. The Company recognizes revenues following the five-step
model prescribed under ASC 606: (i) it identifies a contract with a customer, (ii) it identifies the performance obligations in the contract,
(iii) it determines the transaction price, (iv) It allocates the transaction price to the performance obligations in the contract and
(v) it recognizes revenues when (or as) it satisfies its performance obligation.
The Company
generates revenue from multiple streams: namely, clinical trials, consulting fees, seminars, and merchandise sales. Revenues from product
sales are recognized when a customer obtains control of the Company’s product, which occurs at a point in time or over time, typically
upon shipment to the customer or when services are fulfilled, and the customers benefit from such services. Revenue is deferred, and a
liability is established to the extent the Company receives payments from customers before goods are shipped or services are rendered.
The Company
expenses incremental costs of obtaining a contract as and when incurred if the expected amortization period of the asset that it would
have been recognized is one year or less or the amount is immaterial.
A performance
obligation is a contractual promise to transfer a distinct product or service to a customer and is the unit of account in the new revenue
standard. The contract transaction price is allocated to each distinct performance obligation and recognized as revenue when, or as, the
performance obligation is satisfied. Each contract has a single performance obligation as the promise to transfer the individual goods
or services is not separately identifiable from other promises in the contracts and, therefore, not distinct. Revenue from contracts that
satisfy the criteria for overtime recognition is recognized as the work progresses. The majority of our revenue is derived from services
provided to customers and is executed typically over a period that is typically between 1 to 12 months, based on evaluation of when these
services are rendered. Our contracts will continue to be recognized over time because of the continuous transfer of control to the customer
as services are rendered to customers. Payments made by customers in advance of services being rendered are recorded as deferred revenue.
Our significant payment terms for our customer
contracts vary based on the revenue stream. Franchising business clients are required to advance a percentage of the franchise fee upon
acceptance of the contract. These advances when received are accounted for as contract liabilities on the consolidated balance sheet and
are subsequently recognized in revenue when they are earned. Contracts for our clinical trials typically provided for progress payments
based on the number of patients seen, with final payments generally due within 30 days upon completion of work or the termination of the
contract. Revenue is recognized when all performance obligations under the terms of a contract are satisfied. The Company requires advance
payments from our consulting customers and these payments are recorded as contract liabilities on the consolidated balance sheet until
service is performed and revenue is recognized. These advance payments are not treated as financing component based on the guidance in
ASC 606-10-32-16 and -17, whereby the timing of when services are provided are at the discretion of the customers or a substantial amount
of the consideration promised by the customer is variable and not in the control of the customer or the Company. There
is no significant financing component to any of our contracts.
Contract Modifications
Contracts
for the Company’s clinical trial business are subject to modification. These modifications may create new, or change existing, enforceable
rights and obligations of the parties thereto. Modifications are generally effected pursuant to an amendment or addendum to the original
contract. A contract modification is accounted for as a new contract if it reflects an increase in scope that is regarded as distinct
from the original contract and is priced in line with the standalone price for the related services. If a contract modification is not
considered a new contract, the modification is combined with the original contract and the impact on revenue recognition will depend on
whether the remaining services are distinct from the original contract. If they are distinct from those in the original contract, all
remaining performance obligations will be accounted for on a prospective basis with unrecognized consideration allocated to the remaining
performance obligations. If the remaining goods or services are not distinct, the modification will be treated as if it were a part of
the existing contract and the effect that the contract modification has on the transaction price and on the measure of progress toward
satisfaction of the performance obligations are recognized as an adjustment to revenue (either as an increase in or a reduction of revenue)
at the date of the contract modification on a cumulative catch-up basis.
Remaining Performance Obligations
The Company follows ASC 606, which requires the
allocation of the transaction price to the remaining performance obligations of a contract and applies a practical expedient allowing
it not to disclose the amount of the transaction price allocated to the remaining performance obligations for contracts with an original
expected duration of one year or less. As of February 28, 2023, and May 31, 2022, the Company had no remaining performance obligations.
Share-Based Payments
ASC 718,
“Compensation – Stock Compensation,” prescribes accounting and reporting standards for all share-based
payment transactions. In June 2018, FASB issued ASU No. 2018-07, Compensation – Stock Compensation (Topic 718): Improvements
to Nonemployee Share-Based Payment Accounting, which aligns accounting for share-based payments issued to nonemployees to that of
employees under the existing guidance of Topic 718, with certain exceptions. This update supersedes previous guidance for share-based
payments to nonemployees under Subtopic 505-50, Equity – Equity-Based Payments to Nonemployees. This guidance became effective
for the Company on January 1, 2019. Based on its completed analysis, the Company has determined that adopting this guidance will not have
a material impact on its financial statements. The Company follows FASB guidance related to equity-based payments, which requires that
equity-based compensation be accounted for using a fair value method and recognized as expense in the accompanying statements of operations.
Equity-based compensation expense will be recognized as compensation expense.
Leases
The Company has adopted ASU 2016-02, Leases
(Topic 842), along with related clarifications and improvements, under which lessees are required to recognize a lease liability,
which represents the discounted obligation to make future minimum lease payments and a corresponding right-of-use asset on the balance
sheet for most leases. The guidance retains the historical accounting for lessors and does not make significant changes to the recognition,
measurement, and presentation of expenses and cash flows by a lessee. Enhanced disclosures are also required to give financial statement
users the ability to assess the amount, timing and uncertainty of cash flows arising from leases.
Cash Flows
The Company follows ASU 2016-18, “Statement
of Cash Flows (Topic 230),” requiring that the statement of cash flows explain the change in the total cash, cash equivalents,
and amounts generally described as restricted cash or restricted cash equivalents. The provisions of this guidance are to be applied using
a retrospective approach, which requires application of the guidance for all periods presented.
Fair Value Measurements
The Company has adopted ASC Topic 820, Fair
Value Measurements, which defines fair value as used in numerous accounting pronouncements, establishes a framework for measuring
fair value and expands disclosure of fair-value measurements.
The estimated fair value of certain financial
instruments, including cash and cash equivalents, accounts receivable, accounts payable and accrued expenses, is carried at historical
cost basis, which approximates their fair values because of the short-term nature of these instruments. The carrying amounts of the Company’s
short- and long-term credit obligations approximate fair value because the effective yields on these obligations, which include contractual
interest rates taken together with other features, such as concurrent issuances of warrants and/or embedded conversion options, are comparable
to rates of returns for instruments of similar credit risk.
ASC Topic 820 defines fair value as the exchange
price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market
for the asset or liability in an orderly transaction between market participants on the measurement date. ASC Topic 820 also establishes
a fair-value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs
when measuring fair value. ASC Topic 820 describes three levels of inputs that may be used to measure fair value:
Level 1: Quoted prices in active markets for identical assets
or liabilities.
Level 2: Quoted prices for similar assets and liabilities
in active markets or inputs that are observable.
Level 3: Inputs that are unobservable (for example, cash
flow modeling inputs based on assumptions).
Income Taxes
The Company accounts for income taxes in accordance
with Accounting Standards Codification No. 740, “Income Taxes” (“ASC 740”). This codification prescribes
the use of the asset and liability method whereby deferred tax asset and liability account balances are determined based on differences
between financial reporting and tax bases of assets and liabilities and for carryforward tax losses. Deferred taxes are measured using
the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company provides a valuation allowance,
if necessary, to reduce deferred tax assets to their estimated realizable value if it is more likely than not that some portion or all
of the deferred tax asset will not be realized.
Deferred tax liabilities and assets are classified
as current or noncurrent based on the classification of the related asset or liability for financial reporting or according to the expected
reversal dates of the specific temporary differences, if not related to an asset or liability for financial reporting.
The Company accounts for uncertain tax positions
in accordance with the provisions of ASC 740, which provides guidance as to the determination of whether tax benefits claimed or expected
to be claimed on a tax return should be recorded in its unaudited financial statements, under which a company may recognize the tax benefit
from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing
authorities, based on the technical merits of the position.
The tax benefits recognized in unaudited financial
statements from such a position are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon
ultimate settlement. Accordingly, the Company would report a liability for unrecognized tax benefits resulting from uncertain tax positions
taken or expected to be taken in a tax return. The Company elects to recognize any interest and penalties, if any, related to unrecognized
tax benefits in tax expense.
Loss per Share
The Company computes basic earnings per share
amounts in accordance with Accounting Standards Codification Topic 260, “Earnings per Share.” Basic earnings per share
is calculated by dividing net income (loss) available to holders of Common Stock by the weighted average number of shares of Common Stock
outstanding during the reporting period. Diluted loss per share is computed by dividing net loss by the weighted average number of shares
of Common Stock, common stock equivalents and potentially dilutive securities outstanding during the period. As of February 28, 2023,
and February 28, 2022, the Company had no dilutive securities.
Recently Issued Accounting Standards
The Company does not believe there are any other
recently issued, but not yet effective, accounting standards that would have a significant impact on the Company’s financial position
or results of operations.
Note 3 – Going Concern
The accompanying unaudited consolidated
financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America
(“U.S. GAAP”), which contemplate the Company’s continuation as a going concern in accordance with ASC 240-40-50.
The Company’s history of recurring losses, negative working capital and negative cash flows from operating activities raises
substantial doubt about its ability to continue as a going concern. The Company has not generated any profits since inception and
its current cash balances will not meet its working capital needs. For the nine months ended February 28, 2023, the Company had a
net loss from operations of $708,473, net cash used in operations of $710,516, a working capital deficit of $280,713 and an
accumulated deficit of $4,406,729. The Company’s independent registered public accounting firm has issued an audit opinion for
the Company’s audited consolidated financial statements for the year ended May 31, 2022, that includes a statement expressing
substantial doubt as to our ability to continue as a going concern.
The ability of the Company to continue as a going
concern depends on the successful execution of its operating plan, which includes expanding its operations and raising either debt or
equity financing. There is no assurance that the Company will be able to expand its operations or obtain such financing on satisfactory
terms or at all. If the Company is unsuccessful in these endeavors, it may be required to curtail or cease its operations.
The accompanying unaudited consolidated financial
statements do not include any adjustments related to the recoverability or classification of asset carrying amounts or the amounts and
classification of liabilities that may result should the Company be unable to continue as a going concern.
Note 4 – Debt
PPP Loans
During 2021 and 2020, the Company received one
loan of $31,750, two loans of $20,833 each and three loans of $5,000 each under the Payroll Protection Program (“PPP”). The
PPP was established in 2020 as part of the Coronavirus Aid, Relief and Economic Security Act (“CARES Act”) to provide loans
to qualifying businesses for amounts up to 2.5 times their average monthly payroll expenses. At May 31, 2022, the Company’s outstanding
PPP loans of $41,666 was recorded as a current liability; these loans were forgiven during the nine months ended February 28, 2023.
EIDL Loan
In May 2020, the Company received $143,100 from
the Small Business Administration as an Economic Injury Disaster Loan (“EIDL”) to help fund its operations during the Covid-19
pandemic. The loan bears interest at the rate of 3.75% per annum and is payable in monthly installments of $698 over a 30-year period,
with deferral of payments for the first 12 months. An additional $10,000 borrowed under EIDL, which was provided for payroll, was forgiven
and recorded as Other Income.
In June 2020, the Company received proceeds of
$106,300 from the Small Business Administration through a second EIDL loan to help fund its operations during the Covid-19 pandemic. The
loan bears interest at the rate of 3.75% per annum and is payable in monthly installments of $518 over a 30-year period. An additional
$4,000 under EIDL, which was provided for payroll, was forgiven and recorded as Other Income.
The Company’s EIDL loans were recorded in
the balance sheet as follows:
| | |
February 28, 2023 | | |
May 31, 2022 | |
| SBA (EIDL) current portion | |
$ | 14,592 | | |
$ | 5,561 | |
| SBA (EIDL) noncurrent portion | |
| 234,210 | | |
| 243,738 | |
| | |
$ | 247,240 | | |
$ | 249,299 | |
Line of Credit
On November 16, 2020, the Company received proceeds
of $15,000 under a line of credit provided by an unrelated party with a limit of $15,000. Borrowings under the line of credit bear interest
at the rate of 4.17% per month. There were balances of $12,962 and $0 outstanding at February 28, 2023, and May 31, 2022.
Short-Term Loans
The Company has entered into agreements under
which it sold receivables to third parties. In accordance with ASC 470, these transactions are treated as loans encumbering the receivables
of the Company in the event of default and are accounted for as a debt, such that payments are allocated to principal and interest expense
as they are made. These transactions are as follows:
| · | On December 10, 2020, the Company entered into
a financing agreement with an unrelated party for a loan of $45,000 at an annual interest rate of 42%, to be repaid at the weekly rate
of $1,997. This loan was repaid in May 2021. |
| | | |
| · | On January 14, 2021, the Company entered into
a financing agreement with an unrelated party for a loan of $22,500 at an annual interest rate of 46%, to be repaid at the rate of $1,027
per week for 32 weeks. The loan was repaid in May 2021. |
| | | |
| · | In
May 2022, the Company entered into a financing agreement with an unrelated party for a loan
of $50,000 at an annual interest rate of 20.9%, to be repaid at the rate of $1,218 per week
for one year. On October 5, 2022, this loan was refinanced such that the Company received
an additional $29,360; the refinanced loan bears interest at the annual rate of 25.6% and
is to be repaid at the rate of $1208 per week for 18 months. At February 28, 2023, and May
31, 2022, the outstanding balances, including interest, were $84,486 and $60,814, respectively. |
| | | |
| · | On August 8, 2022, the Company entered into a
financing agreement with an unrelated party for a loan of $45,000 at an annual interest rate of 26.4%, to be repaid at the rate of $3,057
per week for 20 weeks, On October 17, 2022, this loan was refinanced to include an additional $10,000, such that it bears interest at
an annual interest rate of 26.4% and was to be repaid at the rate of $3,057 per week for four weeks. |
| | | |
| · | On December 20, 2022, the Company borrowed increased the loan to $76,000
and modified the financing agreement such that the loan bears interest at an annual interest rate of 26.4% and is to be repaid at the
rate of $6114 per week for 17 weeks. The outstanding balance as of February 28, 2023, including interest, was $45,201. |
On June 29, 2022, the Company borrowed $12,500
from an unrelated party at an annual interest rate of 14%. This loan is payable at the weekly rate of $589 for 24 weeks. On October 13,
2022, an additional loan of $6,304 was obtained with a weekly payment of $297 for 24 weeks. At February 28, 2023, the outstanding balance
of this loan, including interest, was $ 11,833.
On August 3, 2022, the Company borrowed $15,000
from an unrelated party at an annual interest rate of 42.5%, repayable at the rate of $1,188 per month for 18 months. At February 28,
2023, the outstanding balance of this loan, including interest, was $12,962.
Note 5 – Right-of-Use Assets and Lease
Liabilities
The Company leases real property from unrelated
parties under leases that are classified as operating leases. The right-of-use assets for operating leases are included in right-of-use
assets on the balance sheets, with the corresponding lease liability in liabilities. Lease expense is recognized on a straight-line basis
over the lease term. Renewals and terminations are included in the calculation of right-of-use assets and lease liabilities when they
are considered reasonably certain to be exercised. When the implicit rate is unknown, the incremental borrowing rate, based on the commencement
date, is used in determining the present value of lease payments.
The following
amounts related to leases were recorded in the balance sheets:
| | |
February 28, 2023 | | |
May 31, 2022 | |
| Right of use asset | |
$ | 155,387 | | |
$ | 63,213 | |
| Less: accumulated amortization | |
| (122,122 | ) | |
| (2,915 | ) |
| Right of use asset, net | |
$ | 33,265 | | |
$ | 60,298 | |
| | |
| | | |
| | |
| Lease liabilities – current | |
$ | 15,103 | | |
$ | 44,054 | |
| Lease liabilities – noncurrent | |
| – | | |
| 3,804 | |
| | |
$ | 15,103 | | |
$ | 47,858 | |
The Company reimburses for an office space operating
lease under a month-to-month arrangement, payable at the discretion of management. The Company’s total operating lease expense was
$55,915 and $60,353 during the nine months ended February 28, 2023, and February 28, 2022, respectively. See Note 10 for additional lease
information.
Note 6 -- Revenue
Most of the Company’s revenue is generated
by the performance of services to customers and recognized at a point in time based on the evaluation of when the customer obtains control
of the products. Revenue is recognized when all performance obligations under the terms of a contract are satisfied, net of certain taxes
and gain/loss resulting from changes in foreign currency. Revenue is recorded when customer acceptance is received and all performance
obligations have been satisfied. Sales of goods typically do not include multiple products and/or service elements.
The table below summarizes the Company’s
disaggregated revenue information:
| | |
Nine Months Ended
February 28, | |
| | |
2023 | | |
2022 | |
| Clinical trials | |
$ | 230,492 | | |
$ | 133,547 | |
| Consulting fees | |
| – | | |
| – | |
| Video Course | |
| 2,497 | | |
| 151 | |
| Seminar fees | |
| 16,433 | | |
| 7,972 | |
| Royalty | |
| 42 | | |
| 311 | |
| Merchandise | |
| 20,949 | | |
| – | |
| Total revenue | |
$ | 270,413 | | |
$ | 141,981 | |
Cost of Revenue
Cost of revenue consists of third-party costs
associated with the patient stipend, sleep study fees and audio/video fees. For the nine months ending February 28, 2023, and February
28, 2022, cost of revenue totaled $77,443 and $31,515, respectively.
Note 7 – Stockholders’ Deficit
The Company is authorized to issue 20,010,000,000
of capital stock, of which 20,000,000,000 shares are Common Stock, without par value, and 10,000,000 are preferred stock.
Preferred Stock
The Company has designated 2,500,000 shares of
preferred stock as Series A Convertible Preferred Stock (the “Series A Stock”). Until July 20, 2022, each share had a par
value of $0.001; on that date, the Company amended its articles of incorporation to provide that each such share has no par value. Under
this amendment, (i) Series A Stock is entitled to receive dividends on the shares of Common Stock into which such shares are convertible,
(ii) has the voting power of the number of shares of Common Stock into which such shares are convertible, (iii) is redeemable at the option
of the Company for a redemption price equal to the number of shares of Common Stock into which the redeemed shares are convertible and
(iv) are senior to the Common Stock and junior to the Series B Convertible Preferred Stock described below.
On July 20, 2022, the Company designated a series
of preferred stock, named Series B Preferred Convertible Preferred Stock, comprising 1,000 shares (“Series B Preferred”).
The shares of this series have no par value, are not entitled to dividends, have no liquidation rights, are not redeemable, are not convertible,
have 60% of the Company’s voting power and rank senior to the Common Stock and Series A Convertible Preferred Stock. The 1,000 preferred
shares were issued in exchange for Common Stock to an existing common shareholder. The Company has deemed the value of the preferred and
common shares to be the same, resulting in no change to additional paid capital.
Common Stock
Issuances and Surrenders
During the year May 31, 2022, the Company sold
798,760,199 shares of Common Stock for $825,290 and during the year ended May 31, 2021, and during the year ended May 31, 2020, the Company
sold 117,797,617 shares of Common Stock for $183,868.
During the year ended May 31, 2022, the Company
issued 20,000,000 shares of Common Stock for services rendered; these shares had a market value of $12,000 on the date of their issuance.
On June 26, 2022, the Company issued 125,000,000
shares of Common Stock to an unrelated party for $75,000.
On August 10, 2022, the Company issued 1,000
shares of Series B Preferred to one of its officers in exchange for his surrender of 595,467,205 shares of Common Stock.
On September 7, 2022, the Company issued 62,500,000
shares of Common Stock and 16,888,889 shares of Common Stock to two unrelated parties in consideration of $50,000 and $12,667, respectively.
On September 15, 2022, the officers that leased
the Officers’ Leased Property entered into a new lease for the same premises, which expires on March 14, 2023, at a rent of $3,038
per month, and these officers continued to make a portion of these premises available to the Company for use as office space, for which
the Company is paying them $2,817 per month.
On September 30, 2022, the Company issued 11,250,000
shares of Common Stock to an unrelated party in consideration of services rendered; these shares had a market value of $11,812 on the
date of their issuance.
On October 17, 2022, the Company issued 625,000,000
shares of Common Stock to an unrelated party in consideration of $250,000.
On December 1, 2022, the Company issued 4,000,000
shares of Common Stock to an unrelated party in consideration of $2,000.
On December 6, 2022, the Company changed its corporate
name to Cannabis Bioscience International Holdings, Inc.
On December 9, 2022, the Company issued 20,000,000
shares of Common Stock to an unrelated party in consideration of $10,000.
On December 12, 2022, the Company issued 22,222,222
shares of Common Stock to an unrelated party in consideration of $10,000.
On January 9, 2023, the Company issued 116,000,000
shares of Common Stock to two unrelated parties in consideration of $29,800.
On January 18, 2023, the Company issued 300,000,000
shares of Common Stock to an unrelated party in consideration of $100,000.
On January 23, 2023, the Company issued 125,000,000
shares of Common Stock to an unrelated party in consideration of $50,000.
On February 28, 2023, the Company issued 14,285,714
shares of Common Stock to an unrelated party in consideration of $5,000.
During the nine months ended February 28, 2023,
the Company sold 1,442,146,825 shares of Common Stock for $635,966 and during the nine months ended February 28, 2022, the Company sold
88,028,678 shares of Common Stock for $532,500.
As of February 28, 2023, and May 31, 2022, there
were respectively 9,459,677,919 and 8,612,998,299 shares of Common Stock issued and outstanding.
Note 8 – Share-Based Compensation
During the nine months ended February 28, 2023,
and February 28, 2022, the Company issued no shares of Common Stock to its employees as additional compensation.
On July 20, 2022, the Company adopted its 2022
Equity Incentive Plan, which provides for the grant of incentive and non-statutory stock options, stock appreciation rights, restricted
stock, unrestricted stock, restricted stock units and performance awards to directors, officers, employees and consultants, as determined
by the Board, as plan administrator. The Company will recognize as share-based compensation expense all share-based payments to employees
over the requisite service period (generally the vesting period) in its consolidated statements of operations based on the fair values
of the awards that are issued.
Note 9 – Income Taxes
The Company provides for income taxes under ASC
740. Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recorded based on the differences between
the financial statement and tax basis of assets and liabilities and the tax rates in effect when these differences are expected to reverse.
A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize tax
assets through future operations.
On December 22, 2017, the 2017 Tax Cuts and Jobs
Act (the “Tax Act”) was enacted into law, making significant changes to the Code. These changes included a federal corporate
tax rate decrease from 35% to 21% for tax years beginning after December 31, 2017, the transition of U.S. international taxation
from a worldwide tax system to a territorial system and a one-time transition tax on the mandatory deemed repatriation of foreign earnings.
The Company is required to recognize the effect of the tax law changes in the period of enactment, such as re-measuring its U.S. deferred
tax assets and liabilities as well as reassessing the net realizability of our deferred tax assets and liabilities. The Tax Act did not
give rise to any material impact on the balance sheets and statements of operations due to the Company’s historical worldwide loss
position and the full valuation allowance on its net U.S. deferred tax assets.
Note 10 – Commitments and Contingencies
The Company leases premises of approximately 4,500
square feet located at 6201 Bonhomme Road, Suites 460S and 466S, Houston, Texas. The lease currently provides for the base rent of $3,382
per month, increasing to (i) $3,529 per month on July 1, 2020, (ii) $3,676.04 per month on July 1, 2021, and (iii) $3,823 per
month on July 1, 2022, subject to CPI increase. On March 23, 2023, the Company amended the lease to extend its term to June 30, 2024,
at a base rent of $4,779 per month. For information regarding the recording of the right-of-use asset and the lease liability in the balance
sheets in respect of this lease, see Note 5.
Two of the Company’s officers leased 1,400
square feet in Houston, Texas (the “Officers’ Leased Property”), under a lease, the term of which commenced on February
29, 2020, and expired on March 14, 2022, at a rent of $3,449 per month. These officers made a portion of these premises available to the
Company for use as office space on a month-to-month basis, for which the Company paid them $2,817 per month. On March 15, 2022, these
officers entered into a new lease for the same premises, which expires on September 14, 2022, at a rent of $3,008 per month, and these
officers continued to make a portion of these premises available to the Company for use as office space, for which the Company is paying
them $2,817 per month on a month-to-month basis. On September 15, 2022, the officers that leased the Officers’ Leased Property entered
into a new lease for these premises, which expired on March 14, 2023, at a rent of $3,038 per month, and these officers continued to make
a portion of these premises available to the Company for use as office space, for which the Company paid them $2,817 per month. On March
2, 2023, these officers entered into a new lease for the same premises, which expires on September 14, 2023, at a rent of $3,168 per month;
they are continuing to make a portion of these premises available to the Company for use as office space, for which the Company is paying
them $2,817 per month.
Note 11 – Related Party Transactions
See Note 7 – Issuance and Surrenders for
information respecting the Company’s purchase of Common Stock from one of its officers and Note 10 for information respecting the
lease of real property to the Company by two of its officers. During the year ended May 31, 2021, the Company advanced $15,000 to one
of its stockholders, of which $12,000 remains outstanding. The Company also had related party liabilities outstanding to certain shareholders
totaling $89,484 and $15,838 as of February 28, 2023, and May 31, 2022, respectively.
Note 12 – Off-Balance-Sheet Arrangements
The Company has no off-balance sheet arrangements.
Note 13 – Concentration of Risk
The Company has revenue, net of taxes and foreign
currency gain/loss of $270,413 and $141,981 for the nine months ended February 28, 2023, and February 28, 2022, respectively. The Company
had two customers that provided 79% of revenue for the nine months ended February 28, 2023, and four customers that provided 75% of revenue
for the nine months ended February 28, 2021.
Note 14 – Subsequent Events
During the years ended May 31, 2022, and May 31,
2021, and during the nine months ended February 28, 2023, the COVID-19 pandemic had a material adverse effect on the Company’s educational
business because governmental measures that we imposed to control it resulted in the closing of classrooms and other educational venues,
and also hindered the Company’s franchising and consulting activities. As the pandemic has abated, some of these restrictions have
been removed and the Company is beginning to resume normal operations. If the pandemic does not continue to abate, because of infections
resulting from emerging virus variants or for other reasons, restrictions could be reimposed or increased. The ultimate impact of the
pandemic will depend on future developments, which are highly uncertain and cannot be predicted.
On March 7, 2023, the Company issued 12,500,000
shares of Common Stock to an unrelated party in consideration of $5,000.
On March 9, 2023, the Company issued 150,000,000
shares of Common Stock to an unrelated party in consideration of $50,000.
On March 24, 2023, the Company issued 62,500,000
shares of Common Stock to an unrelated party in consideration of $25,000.
On March 30, 2023, the Company issued 150,000,000
shares of Common Stock to an unrelated party in consideration of $50,000.
On March 30, 2023, the Company borrowed $47,000
from an unrelated party. The loan is repayable in 11 weekly installments of $6,000 and is secured by $68,373 of future receivables.
On April 20, 2023, the Company borrowed $25,000
from an unrelated party. The loan is repayable in 11 weekly installments of $3,345 and is secured by $37,475 of future receipts.
On April 25, 2023, the Company issued 200,000,000
shares of Common Stock to an unrelated party in consideration of $50,000.
On May 31, 2023, the Company issued 25,000,000
shares of Common Stock to an unrelated party in consideration of $10,000.
On June 7, 2023, the Company issued 25,000,000
shares of Common Stock to an unrelated party in consideration of $5,000.
As of the date of these unaudited consolidated
financial statements, there were 10,084,677,919 shares of Common Stock outstanding.
On June 20, 2023, the Company issued 25,000,000
shares of Common Stock to an unrelated party in consideration of $10,000.
On June 22, 2023, the Company issued 25,000,000
shares of Common Stock to an unrelated party in consideration of $10,000.
On June 29, 2023, the Company issued 10,000,000
shares of Common Stock to an unrelated party in consideration of $5,000.
On June 29, 2023, the Company issued 28,571,428
shares of Common Stock to an unrelated party in consideration of $10,000.
On June 30, 2023, the Company issued 10,000,000
shares of Common Stock to an unrelated party in consideration of $10,000.
On July 1, 2023, the Company issued 25,000,000
shares of Common Stock to an unrelated party in consideration of $10,000.
Since March 1, 2023, two of the Company’s
officers have made loans to the Company and have been repaid portions thereof. As of the date when these unaudited financial statements
were issued, these loans totaled $16,911, net of repayments.
Management has evaluated all other subsequent
events when these unaudited financial statements were issued and has determined that none of them requires disclosure herein.

REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of China Infrastructure Construction
Corp.
Opinion on the Financial Statements
We have audited the accompanying consolidated
balance sheets of China Infrastructure Construction Corp. (the Company) as of May 31, 2022, and 2021, and the related consolidated statements
of operations, cash flows and stockholders’ equity (deficit) for each of the years in the two-year period ended May 31, 2022, and
the related notes (collectively referred to as the financial statements). In our opinion, the consolidated financial statements present
fairly, in all material respects, the financial position of the Company as of May 31, 2022, and 2021, and the results of its operations
and its cash flows for each of the two years in the two-year ended May 31, 2022, in conformity with accounting principles generally accepted
in the United States of America.
Basis for Opinion
These financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our
audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States)(PCAOB) and are required
to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations
of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the
standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged
to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding
of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s
internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess
the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond
to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements.
Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating
the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
As discussed in Note 3 to the financial statements,
the Company’s recurring losses from operations, working capital deficit, negative cash flows from operating activities, and its
need for additional financing in order to fund its projected loss in 2022 raise substantial doubt about its ability to continue as a going
concern. These 2022 and 2021 financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Going Concern
The accompanying financial statements have been
prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company had
negative working capital at May 31, 2022, has incurred recurring losses and recurring negative cash flow from operating activities, and
has an accumulated deficit which raises substantial doubt about its ability to continue as a going concern. Management’s plans concerning
these matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome
of this uncertainty.
Critical Audit Matters
Critical audit matters arising from the current
period audit of the financial statements that were communicated or required to be communicated to the audit committee and that (1) relate
to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex
judgments. We determined that there were no critical audit matters.
/s/ PWR CPA, LLP
Houston, Texas
PCAOB #6686
We have served as the Company’s auditor
since 2021.
November 28, 2022
CHINA INFRASTRUCTURE
CONSTRUCTION CORP.
CONSOLIDATED
BALANCE SHEETS
| | |
Year Ended May 31, | |
| | |
2022 | | |
2021 | |
| ASSETS | |
| CURRENT ASSETS: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 31,982 | | |
$ | 41,322 | |
| Accounts receivable | |
| 5,614 | | |
| 1,295 | |
| Related party receivables | |
| 12,000 | | |
| 12,000 | |
| TOTAL CURRENT ASSETS | |
| 49,596 | | |
| 54,617 | |
| Right-of-use asset | |
| 60,298 | | |
| 94,172 | |
| TOTAL ASSETS | |
$ | 109,894 | | |
$ | 148,789 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | |
| CURRENT LIABILITIES: | |
| | | |
| | |
| Accounts payables and accrued expenses | |
$ | 68,210 | | |
$ | 16,346 | |
| Related party payables | |
| 15,838 | | |
| 10,808 | |
| Short term loan | |
| 48,074 | | |
| – | |
| SBA loan | |
| 5,561 | | |
| 27,731 | |
| PPP loans | |
| 41,666 | | |
| 88,631 | |
| Lease liabilities – current | |
| 44,054 | | |
| 43,963 | |
| TOTAL CURRENT LIABILITIES | |
| 223,403 | | |
| 187,479 | |
| LONG-TERM LIABILITIES: | |
| | | |
| | |
| SBA loan | |
| 243,738 | | |
| 221,569 | |
| Lease liabilities | |
| 3,804 | | |
| 40,911 | |
| TOTAL LONG-TERM LIABILITIES | |
| 470,945 | | |
| 449,959 | |
| TOTAL LIABILITIES | |
| | | |
| | |
| | |
| | | |
| | |
| STOCKHOLDERS’ DEFICIT | |
| | | |
| | |
| Series A Convertible Preferred Stock, par value $0.001 per share: | |
| | | |
| | |
| 10,000,000 shares authorized; 2,500,000 shares issued and outstanding at May 31, 2022, and May 31, 2021 | |
| 2,500 | | |
| 2,500 | |
| Common Stock, without par value, 20,000,000,000 shares authorized 8,737,998,299 and 7,814,238,100 shares issued and outstanding at May 31, 2022, and May 31, 2021 | |
| – | | |
| – | |
| Additional paid-in capital | |
| 3,286,605 | | |
| 2,461,315 | |
| Accumulated deficit | |
| (3,650,156 | ) | |
| (2,764,985 | ) |
| TOTAL STOCKHOLDERS’ DEFICIT | |
| (361,051 | ) | |
| (301,170 | ) |
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | |
$ | 109,894 | | |
$ | 148,789 | |
The accompanying notes are
an integral part of these consolidated financial statements.
CHINA INFRASTRUCTURE
CONSTRUCTION CORP.
CONSOLIDATED
STATEMENTS OF OPERATION
| | |
Year Ended May 31, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| Revenues | |
$ | 214,980 | | |
$ | 761,737 | |
| Cost of Revenues | |
| 46,763 | | |
| 108,311 | |
| Gross profit | |
| 168,217 | | |
| 653,426 | |
| | |
| | | |
| | |
| Cost and expenses | |
| | | |
| | |
| General and administrative | |
| 134,351 | | |
| 90,472 | |
| Contract labor | |
| 544,760 | | |
| 263,138 | |
| Professional fees | |
| 222,535 | | |
| 101,336 | |
| Officer compensation | |
| 70,983 | | |
| 211,312 | |
| Rent and lease | |
| 75,226 | | |
| 72,244 | |
| Travel | |
| 8,420 | | |
| 31,230 | |
| Total operating expenses | |
| 1,056,275 | | |
| 769,732 | |
| | |
| | | |
| | |
| Operating loss | |
| (888,058 | ) | |
| (116,306 | ) |
| | |
| | | |
| | |
| Interest | |
| (51,036 | ) | |
| (43,002 | ) |
| Other income | |
| 53,923 | | |
| – | |
| | |
| | | |
| | |
| Net loss before taxes | |
| (885,171 | ) | |
| (159,308 | ) |
| | |
| | | |
| | |
| Income tax provision | |
| – | | |
| – | |
| | |
| | | |
| | |
| Net loss | |
$ | (885,171 | ) | |
$ | (159,308 | ) |
| | |
| | | |
| | |
| Average common stock outstanding | |
| 8,090,501,599 | | |
| 8,246,111,316 | |
| Average loss per share | |
| (0.00011 | ) | |
| (0.00002 | ) |
The accompanying notes are
an integral part of these consolidated financial statements.
CHINA INFRASTRUCTURE
CONSTRUCTION CORP.
CONSOLIDATED
STATEMENTS OF CASH FLOWS
| | |
Year Ended May 31, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| OPERATING ACTIVITIES: | |
| | | |
| | |
| Net loss | |
$ | (885,171 | ) | |
$ | (159,308 | ) |
| Adjustments to reconcile net income | |
| | | |
| | |
| Amortization of right-of-use asset and liability | |
| 33,874 | | |
| 3,644 | |
| Forgiveness of PPP loan | |
| (31,965 | ) | |
| – | |
Share-based compensation | |
| 12,000 | | |
| – | |
| Changes in assets and liabilities: | |
| | | |
| | |
| Accounts receivable | |
| (4,319 | ) | |
| (795 | ) |
| Accounts payable and accrued expenses | |
| 51,864 | | |
| (77,360 | ) |
| Deferred revenues | |
| – | | |
| (268,469 | ) |
| Related party accounts receivable | |
| 5,030 | | |
| – | |
| Related party payables | |
| – | | |
| (5,417 | ) |
| Lease liability | |
| (37,017 | ) | |
| – | |
| NET CASH USED IN OPERATIONS | |
| (855,704 | ) | |
| (507,705 | ) |
| FINANCING ACTIVITIES: | |
| | | |
| | |
| Proceeds from sales of common stock | |
| 813,290 | | |
| 261,000 | |
| Repurchase of common stock | |
| – | | |
| (1,000 | ) |
| Payments of short-term loan | |
| (15,000 | ) | |
| (1,709 | ) |
| Proceeds from short-term loan | |
| 48,074 | | |
| – | |
| Proceeds from PPP loans | |
| – | | |
| 56,881 | |
| Proceeds from SBA loan | |
| – | | |
| 106,200 | |
| | |
| | | |
| | |
| | |
| | | |
| | |
| NET CASH PROVIDED BY FINANCING ACTIVITIES | |
| 846,364 | | |
| 421,372 | |
| NET DECREASE IN CASH | |
| (9,340 | ) | |
| (86,333 | ) |
| CASH AT BEGINNING OF PERIOD | |
| 41,322 | | |
| 127,655 | |
| CASH AT END OF PERIOD | |
$ | 31,982 | | |
$ | 41,322 | |
| | |
| | | |
| | |
| Supplemental disclosure of cash flow information: | |
| | | |
| | |
| Cash paid for interest | |
$ | 3,104 | | |
$ | 30,236 | |
The accompanying notes are
an integral part of these consolidated financial statements.
CHINA INFRASTRUCTURE
CONSTRUCTION CORP.
CONSOLIDATED
STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
| | |
Series A Convertible Preferred Stock | | |
Common Stock | | |
Additional Paid-In | | |
Accumulated | | |
| |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Total | |
| Balances - May 31, 2020 | |
| 2,500,000 | | |
$ | 2,500 | | |
| 8,715,256,416 | | |
$ | – | | |
$ | 2,189,365 | | |
$ | (2,605,677 | ) | |
$ | (413,812 | ) |
| Sales of common stock | |
| – | | |
| – | | |
| 98,981,684 | | |
| – | | |
| 272,950 | | |
| – | | |
| 272,950 | |
| Repurchase of common stock | |
| – | | |
| – | | |
| (1,000,000,000 | ) | |
| – | | |
| (1,000 | ) | |
| – | | |
| (1,000 | ) |
| Net loss | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (159,308 | ) | |
| (159,308 | ) |
| Balances - May 31, 2021 | |
| 2,500,000 | | |
$ | 2,500 | | |
| 7,814,238,100 | | |
$ | – | | |
$ | 2,461,315 | | |
$ | (2,764,985 | ) | |
$ | (301,170 | ) |
| Sales of common stock | |
| – | | |
| – | | |
| 798,760,199 | | |
| – | | |
| 825,290 | | |
| – | | |
| 825,290 | |
| Net loss | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (885,171 | ) | |
| (885,171 | ) |
| Balances - May 31, 2022 | |
| 2,500,000 | | |
$ | 2,500 | | |
| 8,612,998,299 | | |
$ | – | | |
$ | 3,286,605 | | |
$ | (3,650,156 | ) | |
$ | (361,051 | ) |
The accompanying notes are
an integral part of these consolidated financial statements.
CHINA INFRASTRUCTURE
CONSTRUCTION CORP.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
May 31, 2022
Note 1 – Organization and Business
Organization and Operations
China Infrastructure Construction Corp., a Colorado
corporation (the “Company”), was formed on February 28, 2003, as a limited liability company under the name Fidelity Aircraft
Partners LLC. On December 16, 2009, it converted to a corporation under the name Fidelity Aviation Corporation, and on August 24, 2009,
it changed its name to China Infrastructure Construction Corp. On February 28, 2018, the Company changed its name to Hippocrates Direct
Healthcare, Inc.; on July 4, 2018, it resumed its present name. The Company provides educational systems focused on medical cannabis in
cities throughout the United States and six countries in Latin America. The Company provides services in therapeutic areas of clinical
trials and services relating to sleep disorders through its sleep center in Houston, Texas. The Company offered concierge medicine at
an affordable price through a membership-based model through its wholly owned subsidiary, Hippocrates Direct Healthcare, LLC, a Texas
limited liability company, formed on September 11, 2017; this business was discontinued during the quarter ended August 31, 2020. The
Company has one subsidiary, Alpha Fertility and Sleep Center, LLC, through which it conducts its Sleep Center business.
Note 2 – Summary of Significant Accounting
Policies
Accounting Principles
The financial statements and notes thereto have
been prepared by management using the accrual basis of accounting in accordance with accounting principles generally accepted in the United
States of America (“U.S. GAAP”).
Use of Estimates
The preparation of financial statements in
conformity with U.S. GAAP requires management to make significant estimates and assumptions that affect the reported amounts of
assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements and the reported
amounts of revenue and expenses during the reporting periods. Making estimates requires management to exercise significant judgment.
Certain of these estimates could be affected by external conditions, including those unique to the Company’s businesses, and
general economic conditions. These external conditions could have an effect on the Company’s estimates that could cause actual
results to differ materially from its estimates. Actual results could differ from those estimates. The Company re-evaluates all of
its accounting estimates at least quarterly based on these conditions and records adjustments when necessary. Significant estimates
relied upon in preparing these statements include revenue recognition, accounts receivable reserves, accrued expenses, share-based
compensation and the recoverability of the Company’s net deferred tax assets and any related valuation allowance.
Principles of Consolidation
The consolidated financial statements include
the accounts of the Company and its wholly-owned subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation.
Reclassification
Certain amounts in the prior consolidated financial
statements have been reclassified to conform to the presentation of the current period financial statements. These reclassifications had
no impact on the results of operations, changes in equity, or cash flows.
Cash and Cash Equivalents
Cash equivalents are short-term, highly liquid
investments that are readily convertible to cash with original maturities of three months or less at the date acquired. The Company had
$300 and $0 of investment securities that were deemed cash equivalents at May 31, 2022, and May 31, 2021, respectively.
Accounts Receivable
Included in accounts receivable on the balance
sheets are amounts primarily related to customers. The Company estimates losses on receivables based on known troubled accounts and historical
experience of losses incurred. Receivables are considered impaired and written off when it is probable that all contractual payments due
will not be collected in accordance with the terms of the related agreement. Based on experience and the judgment of management, there
was no allowance for doubtful accounts at May 31, 2022, and May 31, 2021.
Revenue Recognition
The Company follows the Financial Accounting Standards
Board’s (“FASB”) Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers
(Topic 606), as amended. This standard requires a company to recognize revenues when it transfers goods or services to customers in an
amount that reflects the consideration that it expects to receive for them.
Under ASU
No. 2014-09, the Company recognizes revenue when a customer obtains control of promised goods or services, or when they are shipped to
a customer, in an amount that reflects the consideration that it expects to receive in exchange for them. The Company recognizes revenues
following the five-step model prescribed under ASU No. 2014-09: (a) it identifies a contract with a customer; (b) it identifies the
performance obligations in the contract; (c) it determines the transaction price; (d) it allocates the transaction price to the performance
obligations in the contract; and (e) it recognizes revenues when (or as) it satisfies its performance obligation.
The Company generates revenue from multiple streams, namely, clinical trials, consulting fees, seminars and merchandise sales. Revenues from product
sales are recognized when a customer obtains control of the Company’s product, which occurs at a point in time or over time, typically
upon shipment to the customer or when services are fulfilled, and the customers receive benefit from such services. Revenue is deferred
and a liability is established to the extent the Company receives payments from customers in advance of goods being shipped or services
are rendered.
The Company
expenses incremental costs of obtaining a contract as and when incurred if the expected amortization period of the asset that it would
have been recognized is one year or less or the amount is immaterial.
A performance obligation is a contractual
promise to transfer a distinct product or service to a customer and is the unit of account in the new revenue standard. The contract
transaction price is allocated to each distinct performance obligation and recognized as revenue when, or as, the performance obligation
is satisfied. Each contract has a single performance obligation as the promise to transfer the individual goods or services is not separately
identifiable from other promises in the contracts and, therefore, not distinct. Revenue from contracts that satisfy the criteria for
overtime recognition is recognized as the work progresses. The majority of our revenue is derived from services provided to customers
and is executed typically over a period that is typically between 1 to 12 months, based on evaluation of when these services are rendered.
Our contracts will continue to be recognized over time because of the continuous transfer of control to the customer as services are
rendered to customers. Payments made by customers in advance of services being rendered are recorded as deferred revenue.
Our significant payment terms for our customer contracts vary based
on the revenue stream. Franchising business clients are required to advance a percentage of the franchise fee upon acceptance of the contract.
These advances when received are accounted for as contract liabilities on the consolidated balance sheet and are subsequently recognized
in revenue when they are earned. Contracts for our clinical trials typically provided for progress payments based on the number of patients
seen, with final payments generally due within 30 days upon completion of work or the termination of the contract. Revenue is recognized
when all performance obligations under the terms of a contract are satisfied. The Company requires advance payments from our consulting
customers and these payments are recorded as contract liabilities on the consolidated balance sheet until service is performed and revenue
is recognized. These advance payments are not treated as financing component based on the guidance in ASC 606-10-32-196-16 and -17, whereby
the timing of when services are provided are at the discretion of the customers or a substantial amount of the consideration promised
by the customer is variable and not in the control of the customer or the Company. There is no significant
financing component to any of our contracts.
Contracts
for educational services require nonrefundable payment in advance and are recorded as revenue when received.
There is
no significant financing component to any of our contracts.
Contract Modifications
Contracts
for the Company’s clinical trial business are subject to modification. These modifications may create new, or change existing, enforceable
rights and obligations of the parties thereto. Modifications are generally effected pursuant to an amendment or addendum to the original
contract. A contract modification is accounted for as a new contract if it reflects an increase in scope that is regarded as distinct
from the original contract and is priced in line with the standalone price for the related services. If a contract modification is not
considered a new contract, the modification is combined with the original contract and the impact on revenue recognition will depend on
whether the remaining services are distinct from the original contract. If they are distinct from those in the original contract, all
remaining performance obligations will be accounted for on a prospective basis with unrecognized consideration allocated to the remaining
performance obligations. If the remaining goods or services are not distinct, the modification will be treated as if it were a part of
the existing contract and the effect that the contract modification has on the transaction price and on the measure of progress toward
satisfaction of the performance obligations are recognized as an adjustment to revenue (either as an increase in or a reduction of revenue)
at the date of the contract modification on a cumulative catch-up basis.
Remaining Performance Obligation
The Company follows ASC 606, which requires the
allocation of the transaction price to the remaining performance obligations of a contract and applies a practical expedient allowing
it not to disclose the amount of the transaction price allocated to the remaining performance obligations for contracts with an original
expected duration of one year or less. As of February 28, 2023, and May 31, 2022, the Company had no remaining performance obligations.
Share-Based Payments
ASC 718,
“Compensation – Stock Compensation,” prescribes accounting and reporting standards for all share-based
payment transactions. In June 2018, FASB issued ASU No. 2018-07, Compensation – Stock Compensation (Topic 718): Improvements
to Nonemployee Share-Based Payment Accounting, which aligns accounting for share-based payments issued to non-employees to that of
employees under the existing guidance of Topic 718, with certain exceptions. This update supersedes previous guidance for share-based
payments to non-employees under Subtopic 505-50, Equity – Equity-Based Payments to Non-Employees. This guidance became effective
for the Company on January 1, 2019. Based on its completed analysis, the Company has determined that adopting this guidance will not have
a material impact on its financial statements. The Company follows FASB guidance related to equity-based payments, which requires that
equity-based compensation be accounted for using a fair value method and recognized as expense in the accompanying statements of operations.
Equity-based compensation expense will be recognized as compensation expense.
Leases
The Company has adopted ASU 2016-02, Leases
(Topic 842), along with related clarifications and improvements, under which lessees are required to recognize a lease liability,
which represents the discounted obligation to make future minimum lease payments and a corresponding right-of-use asset on the balance
sheet for most leases. The guidance retains the historical accounting for lessors and does not make significant changes to the recognition,
measurement, and presentation of expenses and cash flows by a lessee. Enhanced disclosures are also required to give financial statement
users the ability to assess the amount, timing and uncertainty of cash flows arising from leases.
Cash Flows
The Company follows ASU 2016-18, “Statement
of Cash Flows (Topic 230),” requiring that the statement of cash flows explain the change in the total cash, cash equivalents,
and amounts generally described as restricted cash or restricted cash equivalents. The provisions of this guidance are to be applied using
a retrospective approach, which requires application of the guidance for all periods presented.
Fair Value Measurements
The Company has adopted ASC Topic 820, Fair
Value Measurements, which defines fair value as used in numerous accounting pronouncements, establishes a framework for measuring
fair value and expands disclosure of fair-value measurements.
The estimated fair value of certain financial
instruments, including cash and cash equivalents, accounts receivable, accounts payable and accrued expenses, is carried at historical
cost basis, which approximates their fair values because of the short-term nature of these instruments. The carrying amounts of the Company’s
short- and long-term credit obligations approximate fair value because the effective yields on these obligations, which include contractual
interest rates taken together with other features, such as concurrent issuances of warrants and/or embedded conversion options, are comparable
to rates of returns for instruments of similar credit risk.
ASC Topic 820 defines fair value as the exchange
price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market
for the asset or liability in an orderly transaction between market participants on the measurement date. ASC Topic 820 also establishes
a fair-value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs
when measuring fair value. ASC Topic 820 describes three levels of inputs that may be used to measure fair value:
Level 1: Quoted prices in active markets for identical assets
or liabilities.
Level 2: Quoted prices for similar assets and liabilities
in active markets or inputs that are observable.
Level 3: Inputs that are unobservable (for example, cash
flow modeling inputs based on assumptions).
Income Taxes
The Company accounts for income taxes in accordance
with Accounting Standards Codification No. 740, “Income Taxes” (“ASC 740”). This codification prescribes
the use of the asset and liability method whereby deferred tax asset and liability account balances are determined based on differences
between financial reporting and tax bases of assets and liabilities and for carryforward tax losses. Deferred taxes are measured using
the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company provides a valuation allowance,
if necessary, to reduce deferred tax assets to their estimated realizable value if it is more likely than not that some portion or all
of the deferred tax asset will not be realized.
Deferred tax liabilities and assets are classified
as current or noncurrent based on the classification of the related asset or liability for financial reporting or according to the expected
reversal dates of the specific temporary differences, if not related to an asset or liability for financial reporting.
The Company accounts for uncertain tax positions
in accordance with the provisions of ASC 740, which provides guidance as to the determination of whether tax benefits claimed or expected
to be claimed on a tax return should be recorded in its unaudited financial statements, under which a company may recognize the tax benefit
from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing
authorities, based on the technical merits of the position.
The tax benefits recognized in unaudited financial
statements from such a position are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon
ultimate settlement. Accordingly, the Company would report a liability for unrecognized tax benefits resulting from uncertain tax positions
taken or expected to be taken in a tax return. The Company elects to recognize any interest and penalties, if any, related to unrecognized
tax benefits in tax expense.
Loss per Share
The Company computes basic earnings per share
amounts in accordance with Accounting Standards Codification Topic 260, “Earnings per Share.” Basic earnings per share
is calculated by dividing net income (loss) available to common stockholders by the weighted average number of common shares outstanding
during the reporting period. Diluted loss per share is computed by dividing net loss by the weighted average number of shares of common
stock, common stock equivalents and potentially dilutive securities outstanding during the period. At May 31, 2022, and May 31, 2021,
the Company had no dilutive securities.
Recently Issued Accounting Standards
The Company does not believe there are any other
recently issued, but not yet effective, accounting standards that would have a significant impact on the Company’s financial position
or results of operations.
Note 3 – Going Concern
The accompanying audited financial statements
have been prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”),
which contemplate the Company’s continuation as a going concern in accordance with ASC 240-40-50. The Company’s history of
recurring losses, negative working capital and negative cash flows from operating activities raises substantial doubt about its ability
to continue as a going concern. The Company has not generated any profits since inception and its current cash balances will not meet
its working capital needs. During the year ended May 31, 2022, the Company had a net loss from operations of $885,171, net cash used in
operations of $885,704, working capital deficit of $173,807 and an accumulated deficit of $3,650,156.
The ability of the Company to continue as a going
concern depends on the successful execution of its operating plan, which includes expanding its operations and raising either debt or
equity financing. There is no assurance that the Company will be able to expand its operations or obtain such financing on satisfactory
terms or at all. If the Company is unsuccessful in these endeavors, it may be required to curtail or cease its operations.
The accompanying financial statements do not include
any adjustments related to the recoverability or classification of asset carrying amounts or the amounts and classification of liabilities
that may result should the Company be unable to continue as a going concern.
Note 4 – Debt
PPP Loans
During 2021 and 2020, the Company received one
loan of $31,750, two loans of $20,833 each and three loans of $5,000 each under the Payroll Protection Program (“PPP”). The
PPP was established in 2020 as part of the Coronavirus Aid, Relief and Economic Security Act (“CARES Act”) to provide loans
to qualifying businesses for amounts up to 2.5 times their average monthly payroll expenses. At May 31, 2022, and May 31, 2021, the Company’s
outstanding PPP loans of $41,666 and $88,416, respectively, were recorded as current liabilities. On April 21, 2021, pursuant to the
CARES Act, the Company applied for and received forgiveness for four of these loans in the aggregate amount of $31,965. Loan forgiveness
was recorded as other income during 2022.
EIDL Loan
In May 2020, the Company received $143,100 from
the Small Business Administration as an Economic Injury Disaster Loan (“EIDL”) to help fund its operations during the Covid-19
pandemic. The loan bears interest at the rate of 3.75% per annum and is payable in monthly installments of $698 over a 30-year period,
with deferral of payments for the first 12 months. An additional $10,000 borrowed under EIDL, which was provided for payroll, was forgiven
and recorded as other income in 2020.
In June 2020, the Company received proceeds of
$106,200 from the Small Business Administration through a second EIDL loan to help fund its operations during the Covid-19 pandemic. The
loan bears interest at the rate of 3.75% per annum and is payable in monthly installments of $518 over a 30-year period. An additional
$4,000 under EIDL, which was provided for payroll was forgiven and recorded as Other Income during 2020.
The Company’s EIDL loans were recorded in
the balance sheet as follows:
| | |
May 31, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| SBA (EIDL) current portion | |
$ | 5,561 | | |
$ | 27,731 | |
| SBA (EIDL) noncurrent portion | |
| 243,739 | | |
| 221,569 | |
| | |
$ | 249,300 | | |
$ | 249,300 | |
Line of Credit
On November 16, 2020, the Company received proceeds
of $15,000 under a line of credit provided by an unrelated party with a limit of $15,000. Borrowings under the line of credit bear interest
at the rate of 4.17% per month. There we no balances outstanding at May 31, 2022, and May 31, 2021.
Short-Term Loans
The Company has entered into agreements under
which it sold receivables to third parties. In accordance with ASC 470, these transactions are treated as loans encumbering the receivables
of the Company in the event of default and are accounted for as a debt, such that payments are allocated to principal and interest expense
as they are made. These transactions are as follows:
| · | On December 10, 2020, the Company entered into a financing agreement with an unrelated party
for a loan of $45,000 at an annual interest rate of 42%, to be repaid at the weekly rate of $1,997. This loan was repaid in May 2021. |
| · | On January 14, 2021, the Company entered into a financing agreement with an unrelated party
for a loan of $22,500 at an annual interest rate of 46%, to be repaid at the rate of $1,027 per week for 32 weeks. The loan was repaid
in May 2021. |
| · | In May 2022, the Company entered into a financing agreement with an unrelated party
for a loan of $50,000 at an annual interest rate of 20.9%, to be repaid at the rate of $1,218 per week for one year. At May 31, 2022,
the outstanding balance, including interest, was $60,814. |
Note 5 – Right-of-Use Assets and Lease
Liabilities
The Company leases real property from unrelated
parties under leases that are classified as operating leases. The right-of-use assets for operating leases are included in right-of-use
assets on the balance sheets, with the corresponding lease liability in liabilities. Lease expense is recognized on a straight-line basis
over the lease term. Renewals and terminations are included in the calculation of right-of-use assets and lease liabilities when they
are considered reasonably certain to be exercised. When the implicit rate is unknown, the incremental borrowing rate, based on the commencement
date, is used in determining the present value of lease payments.
The following amounts related
to leases were recorded in the balance sheets:
| | |
May 31, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| Right-of-use asset | |
$ | 63,213 | | |
$ | 96,889 | |
| Less: Accumulated amortization | |
| (2,915 | ) | |
| (2,717 | ) |
| Right-of-use asset, net | |
$ | 60,298 | | |
$ | 94,172 | |
| | |
| | | |
| | |
| Lease liabilities – current | |
$ | 44,054 | | |
$ | 43,963 | |
| Lease liabilities – noncurrent | |
| 3,804 | | |
| 40,911 | |
| | |
$ | 47,858 | | |
$ | 84,874 | |
The Company reimburses for an office space operating
lease under a month-to-month arrangement, payable at the discretion of management.
The Company’s total operating lease expense
was $75,225 and $72,244 during the years ended May 31, 2022, and May 31, 2021, respectively. See Note 10 for additional lease information.
Note 6 -- Revenue
Most of the Company’s revenue is generated
by the performance of services to customers and recognized at a point in time based on the evaluation of when the customer obtains control
of the products. Revenue is recognized when all performance obligations under the terms of a contract are satisfied, net of certain taxes
and gain/loss resulting from changes in foreign currency. Revenue is recorded when customer acceptance is received and all performance
obligations have been satisfied. Sales of goods typically do not include multiple products and/or service elements.
The table below summarizes the Company’s
disaggregated revenue information:
| | |
Year Ended May 31, | |
| | |
2022 | | |
2021 | |
| Clinical trials | |
$ | 196,637 | | |
$ | 706,008 | |
| Consulting fees | |
| – | | |
| 17,289 | |
| Franchise fees | |
| – | | |
| – | |
| Seminar fees | |
| 13,985 | | |
| 38,440 | |
| Royalty | |
| 1,678 | | |
| – | |
| Merchandise | |
| 2,680 | | |
| – | |
| Total revenue | |
$ | 214,980 | | |
$ | 761,737 | |
Cost of Revenue
Cost of revenue consists of third-party costs
associated with the patient stipend, sleep study fees and audio/video fees. At May 31, 2022, and May 31, 2021, cost of revenue totaled
$46,763 and $108,311, respectively.
Note 7 – Stockholders’ Deficit
The Company is authorized to issue 20,010,000,000
of capital stock, of which 20,000,000,000 shares are common stock, without par value, and 10,000,000 are preferred stock.
Preferred Stock
The Company has designated 2,500,000 shares of
preferred stock as Series A Convertible Preferred Stock (the “Series A Stock”). Each share of Series A Stock entitles the
holder to receive dividends at the rate determined by the Board. In the event of liquidation, such holders are entitled to be paid out
of the assets of the Corporation available for distribution to its common stockholders, whether from capital, surplus or earnings, and
before any payment is made in respect of the shares of Common Stock, an amount equal to the greater of: (i) the then-current market price
of the Series A Stock, as detailed by OTC, or ten cents ($0.10) per share of Series A Stock, subject to adjustment for stock dividends,
combinations, splits, recapitalizations and the like with respect to the Series A Stock, plus all accrued but unpaid dividends. Each share
of Series A Stock is convertible, at the option of the holder, at any time one year after the date of issuance of such shares, into that
number of shares of Common Stock that is equal to the quotient obtained by dividing the Series A Preference Price then in effect for each
share of Series A Stock by the greater of: (i) ten cents ($0.10) per share, or (ii) seventy-five percent (75%) of the Market Price (as
defined) of the Common Stock on the conversion date, subject to adjustment in certain events. Series A Stock is not redeemable. The Series
A Stock possesses one-half of the voting power of the Company’s stockholders. At May 31, 2022, and May 31, 2021, there were 2,500,000
shares of Series A Stock issued and outstanding.
Common Stock
Issuances and Surrenders
On December 22, 2020, an officer surrendered to
the Company 279,532,795 shares of Common Stock that had been erroneously issued to him.
On December 23, 2020, an officer of the Company
sold 1,000,000,000 shares of Common Stock to the Company for $1,000, reducing ownership of the Company’s equity to 500,000,000 shares
of Common Stock and 500,000 shares of Series A Preferred.
During the year ended May 31, 2022, the Company
sold 798,760,199 shares of Common Stock for $825,290, during the year ended May 31, 2021, and during the year ended May 31, 2020, the
Company sold 117,797,617 shares of Common Stock for $183,868.
During the year ended May 31, 2022, the Company
issued 20,000,000 shares of Common Stock for services rendered; these shares had a market value of $12,000 on the date of their issuance.
At May 31, 2022, and May 31, 2021, there were
respectively 8,612,998,299 and 7,814,238,100 shares of Common Stock issued and outstanding.
Note 8 – Share-Based Compensation
During the years ended May 31, 2022, and May 31,
2021, the Company issued no shares of Common Stock to its employees as additional compensation.
Note 9 – Income Taxes
The Company provides for income taxes under ASC
740. Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recorded based on the differences between
the financial statement and tax basis of assets and liabilities and the tax rates in effect when these differences are expected to reverse.
A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize tax
assets through future operations.
On December 22, 2017, the 2017 Tax Cuts and Jobs
Act (the “Tax Act”) was enacted into law, making significant changes to the Code. These changes included a federal corporate
tax rate decrease from 35% to 21% for tax years beginning after December 31, 2017, the transition of U.S. international taxation
from a worldwide tax system to a territorial system and a one-time transition tax on the mandatory deemed repatriation of foreign earnings.
The Company is required to recognize the effect of the tax law changes in the period of enactment, such as re-measuring its U.S. deferred
tax assets and liabilities as well as reassessing the net realizability of our deferred tax assets and liabilities. The Tax Act did not
give rise to any material impact on the balance sheets and statements of operations due to the Company’s historical worldwide loss
position and the full valuation allowance on its net U.S. deferred tax assets. The reconciliation of taxes at the federal and state statutory
rate to the Company’s provision for income taxes for the years ended May 31, 2022, and May 31, 2021, was as follows:
| May 31, 2022 |
| Income tax expense (benefit) at the statutory rate | |
$ | 611,045 | |
| Valuation allowance | |
| (611,045 | ) |
| Income tax expense per books | |
$ | – | |
| | |
| | |
| May 31, 2021 | |
| Income tax expense (benefit) at the statutory rate | |
$ | (141,571 | ) |
| Valuation allowance | |
| 141,871 | |
| Income tax expense per books | |
$ | – | |
Due to changes in ownership provisions of the
income tax laws of the United States of America, net operating loss carryforwards of approximately $2,909,738 and $2,729,253 at May 31,
2022, and May 31, 2021, respectively, for federal income tax reporting purposes are subject to annual limitations. When a change in ownership
occurs, net operating loss carryforwards may be limited as to use in future years. They generally expire 20 years from when incurred.
Income taxes for 2017 to 2021 remain subject to
examination.
Note 10 – Commitments and Contingencies
The Company leases premises of approximately 4,500
square feet located at 6201 Bonhomme Road, Suites 460S and 466S, Houston, Texas. The lease currently provides for the base rent of $3,381.96
per month, increasing to (i) $3,529.00 per month on July 1, 2020, (ii) $3,676.04 per month on July 1, 2021, and (iii) $3,823.08
per month on July 1, 2022, subject to CPI increase. For information regarding the recording of the right-of-use asset and the lease liability
in the balance sheets in respect of this lease, see Note 5.
Two of the Company’s officers leased 1,400
square feet in Houston, Texas (the “Officers’ Leased Property”), under a lease, the term of which commenced on February
29, 2020, and expired on March 14, 2022, at a rent of $3,449 per month. These officers made a portion of these premises available to the
Company for use as office space on a month-to-month basis, for which the Company paid them $2,817 per month. On March 15, 2022, these
officers entered into a new lease for the same premises, which expires on September 14, 2022, at a rent of $3,008 per month, and these
officers continued to make a portion of these premises available to the Company for use as office space, for which the Company is paying
them $2,817 per month.
Note 11 – Related Party Transactions
See Note 7 – Issuance and Surrenders for
information respecting the Company’s purchase of Common Stock from one of its officers and Note 10 for information respecting the
lease of real property to the Company by two of its officers.
During the year ended May 31, 2021, the Company
advanced $15,000 to one of its stockholders, of which $12,000 remains outstanding.
The Company also has related party liabilities
outstanding to certain shareholders totaling $15,838 and $10,808 at May 31, 2022, and May 31, 2021, respectively.
Note 12 – Off-Balance-Sheet Arrangements
The Company has no off-balance sheet arrangements.
Note 13 – Concentration of Risk
The Company has revenue, net of taxes and foreign
currency gain/loss of $214,980 and $761,737 for the years ending May 31, 2022, and May 31, 2021, respectively.
The Company had three customers that provided
46% of gross revenue for the year ended May 31, 2022, and 11 customers that provided 70% of gross revenue for the year ended May 31, 2021.
Note 14 – Subsequent Events
During the years ended May 31, 2022, and May 31,
2021, the COVID-19 pandemic had a material adverse effect on the Company’s educational business because governmental measures that
we imposed to control it resulted in the closing of classrooms and other educational venues, and also hindered the Company’s franchising
and consulting activities. As the pandemic has abated, some of these restrictions have been removed and the Company is beginning to resume
normal operations. If the pandemic does not continue to abate, because of infections resulting from emerging virus variants or for other
reasons, restrictions could be reimposed or increased. The ultimate impact of the pandemic will depend on future developments, which are
highly uncertain and cannot be predicted.
On March 11, 2022, the Company issued 55,000,000
shares of Common Stock to an unrelated party. On that date, it also agreed to issue 11,250,000 shares of Common Stock to another unrelated
party upon completion of certain services; these shares were issued on September 30, 2022.
On June 22, 2022, pursuant to the CARES Act,
the Company applied for and received forgiveness for its outstanding PPP loan in the amount of $41,666. The forgiven amount will be recorded
as other income in the Company’s statements of operations for the quarter ending August 31, 2022.
On June 26, 2022, the Company issued 125,000,000
shares of Common Stock to an unrelated party for $75,000.
On June 29, 2022, the Company borrowed $12,500
from an unrelated party at an annual interest rate of 14%.
On July 20, 2022, the Company filed amended and
restated articles of incorporation with the Secretary of State of the State of Colorado. Among other things, the amended and restated
articles of incorporation:
| · | Amended the terms of the Company’s Series A Convertible Preferred Stock (i) to change the par value
of the shares of that series from $0.001 per share to no par value per share, (ii) to change the dividends to which such shares are entitled
to receive from an amount at the discretion of the Board to the dividend to be paid on the shares of Common Stock into which such shares
are convertible, (iii) to reduce the voting power of such shares from 50% of the Company’s voting power to the voting power of the
number of shares of Common Stock into which such shares are convertible, (iv) to eliminate redemption at the option of the holder and
provide for redemption at the option of the Company for a redemption price of the number of shares of Common Stock into which the redeemed
shares are convertible and (v) to provide that such shares are senior to the Common Stock and junior to the Series B Convertible Preferred
Stock described below. |
| | | |
| · | Designated a series of preferred stock, named Series B Preferred Convertible Preferred Stock, comprising
1,000 shares (“Series B Preferred”). The shares of this series have no par value, are not entitled to dividends, have no liquidation
rights, are not redeemable, are not convertible, have 60% of the Company’s voting power and rank senior to the Common Stock and
Series A Convertible Preferred Stock. |
| | | |
| · | Eliminated the personal liability of directors to the Company or its stockholders for monetary damages
for breach of their fiduciary duties as such to the full extent permitted by law. |
| | | |
| · | Provided that the Company indemnify, to the full extent permitted by law, any person who is or was a director
or officer of the Company and may indemnify any other person against any claim, liability or expense arising against or incurred by such
person made a party to a proceeding because he is or was a director, officer, agent, fiduciary or employee of the Company or because he
is or was serving another entity as a director, officer, partner, trustee, employee, fiduciary or agent at the Company’s request. |
Also, on July 20, 2022, the Company adopted its
2022 Equity Incentive Plan, which provides for the grant of incentive and non-statutory stock options, stock appreciation rights, restricted
stock, unrestricted stock, restricted stock units and performance awards to directors, officers, employees and consultants, as determined
by the Board, as plan administrator. The Company will recognize as share-based compensation expense all share-based payments to employees
over the requisite service period (generally the vesting period) in its statements of income based on the fair values of the awards that
are issued.
On August 3, 2022, the Company borrowed $15,000
from an unrelated party at an annual interest rate of 42.5%.
On August 8, 2022, the Company sold $61,155 of
its future receivables to an unrelated party for $45,000. The terms of this sale require the Company to deliver receivables at the rate
of $3,057 per week for 20 weeks.
On August 10, 2022, the Company issued 1,000 shares
of Series B Preferred to one of its officers in exchange for his surrender of 595,467,205 shares of Common Stock.
On September 7, 2022, the Company issued 62,500,000
shares of Common Stock and 16,888,889 shares of Common Stock to two unrelated parties in consideration of $50,000 and $12,667, respectively.
On September 15, 2022, the officers that leased
the Officers’ Leased Property entered into a new lease for the same premises, which expires on March 14, 2023, at a rent of $3,038
per month, and these officers continued to make a portion of these premises available to the Company for use as office space, for which
the Company is paying them $2,817 per month.
On October 17, 2022, the Company issued 625,000,000
shares of Common Stock to an unrelated party in consideration of $250,000.
Management has evaluated all other subsequent
events when these consolidated financial statements were issued and has determined that none of them requires disclosure herein.
PART II — INFORMATION NOT REQUIRED IN
THE PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the expenses expected
to be incurred by us in connection with the issuance and distribution of the securities being registered. No portion of such expenses
will be borne by the Selling Stockholders.
| SEC Registration | |
$ | 907 | |
| Legal Fees and Expenses* | |
$ | 75,000 | |
| Accounting Fees* | |
$ | 62,000 | |
| Miscellaneous* | |
$ | 12,093 | |
| Total | |
$ | 150,000 | |
Item 14. Indemnification of Directors and Officers.
Under Section 7-109-102 of the Colorado Business
Corporation Act (the “CBCA”), a corporation may indemnify a person made a party to a proceeding because he is or was a director
against liability incurred in the proceeding if (a) his conduct was in good faith and (b) he reasonably believed (i) in
the case of conduct in an official capacity with the corporation, that such conduct was in the corporation’s best interests; and
(ii) in all other cases, that such conduct was at least not opposed to the corporation’s best interests and (c) in the
case of any criminal proceeding, the person had no reasonable cause to believe that his conduct was unlawful. However, a corporation
may not indemnify a director under this section (a) in connection with a proceeding by or in the right of the corporation in which
he was adjudged liable to the corporation; or (b) in connection with any other proceeding charging that he derived an improper personal
benefit, whether or not involving action in an official capacity, in which proceeding he was adjudged liable on the basis that he derived
an improper personal benefit. The termination of a proceeding by judgment, order, settlement, conviction, or upon a plea of nolo contendere
or its equivalent is not, of itself, determinative that the director did not meet the requisite standard of conduct. Indemnification
permitted under this section in connection with a proceeding by or in the right of the corporation is limited to reasonable expenses
incurred in connection with the proceeding.
The CBCA further provides that, unless limited
by its articles of incorporation, a corporation shall indemnify a person who was wholly successful, on the merits or otherwise, in the
defense of any proceeding to which the person was a party because the person is or was a director or officer of the corporation, against
reasonable expenses incurred by the person in connection with the proceeding. The Registrant’s articles of incorporation contain
no such limitation. In addition, a director or officer, who is or was a party to a proceeding, may apply for indemnification to the court
conducting the proceeding or to another court of competent jurisdiction. The CBCA allows a corporation to indemnify and advance expenses
to an officer, employee, fiduciary or agent of the corporation to the same extent as a director.
Pursuant to the foregoing, the Registrant’s
amended and restated articles of incorporation require it to indemnify, to the full extent permitted by law, any person who is or was
a director or officer of the Registrant and may indemnify any other person against any claim, liability or expense arising against or
incurred by such person made a party to a proceeding because he is or was a director, officer, agent, fiduciary or employee of the Registrant
or because he is or was serving another entity as a director, officer, partner, trustee, employee, fiduciary or agent at the Company’s
request.
Under Section 7-108-402 of the CBCA, a corporation
may, in its articles of incorporation, eliminate or limit the personal liability of a director to the corporation or its shareholders
for monetary damages for breach of his fiduciary duty as a director, except that such provision may not eliminate or limit the liability
of a director to the corporation or its shareholders for monetary damages for any breach of his duty of loyalty to the corporation or
its shareholders, acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, unlawful
distributions or any transaction from which he directly or indirectly derived an improper personal benefit. No such provision may eliminate
or limit the liability of a director to the corporation or to its shareholders for monetary damages for any act or omission occurring
before the date when such provision became effective. As permitted by the CBCA, the Registrant’s amended and restated articles of
incorporation provide that the personal liability of the Company’s directors to the Company or its stockholders is limited to the
full extent permitted by the CBCA.
In addition, Section 7-108-402 provides that no director or officer
shall be personally liable for any injury to person or property arising out of a tort committed by an employee unless he was personally
involved in the situation giving rise to the litigation or unless he committed a criminal offense in connection with such situation,
without restricting other common-law protections and rights that he may have.
Section 7-109-108 of the CBCA provides that a
corporation may purchase and maintain insurance on behalf of a person who is or was a director, officer, employee, fiduciary or agent
of the corporation, or who, while a director, officer, employee, fiduciary or agent of the corporation, is or was serving at the request
of the corporation as a director, officer, partner, trustee, employee, fiduciary or agent of another entity or an employee benefit plan,
against liability asserted against or incurred by the person in that capacity or arising from the person’s status as a director,
officer, employee, fiduciary or agent, whether or not the corporation would have power to indemnify the person against the same liability
under the CBCA. The Registrant has not purchased such insurance.
Insofar as indemnification for liabilities
arising under the Securities Act may be permitted to directors, officers or persons controlling the Registrant pursuant to the foregoing
provisions, the Registrant has been informed that in the opinion of the Securities and Exchange Commission, such indemnification is against
public policy as expressed in the Act and is therefore unenforceable.
Item 15. Recent sales of unregistered securities.
On December 20, 2019, the Registrant issued 4,790,072,957
shares of Common Stock as merger consideration in respect of the merger of PUI with and into the Registrant to 23 persons pursuant to
the exemption from registration afforded by Section 4(a)(2) of the Securities Act. Of these shares, (i) 4,595,467,025 shares were
issued to the chief executive officer of PUI, who became the chief executive officer and a director of the Registrant pursuant to the
related merger agreement and (ii) the remainder were issued to 22 persons who had purchased them from PUI over a period of several
years prior to the merger.
On January 5, 2020, the Registrant issued 90,000,000
shares of Common Stock to two persons for $90,000 pursuant to the exemption from registration afforded by Section 4(a)(2) of the Securities
Act. Of these shares, 40,000,000 were issued to a director of the Registrant.
On January 5, 2020, the Registrant issued
47,650,000 shares of Common Stock to 13 persons in exchange for shares of PUI that they had received as employee benefits over a period
of several years prior to the merger pursuant to the exemption from registration afforded by Section 4(a)(2) of the Securities Act.
On January 5, 2020, the Registrant issued
10,250,000 shares of Common Stock to 13 persons who were not residents of the United States persons in exchange for shares of PUI that
they had received as employee benefits over a period of several years prior to the merger. By virtue of the foreign status of these
persons, these issuances were not subject to the registration provisions of the Securities Act.
In addition, the Company has issued unregistered
shares of Common Stock as follows:
| Date | |
No. of Shares | | |
Class of Securities | |
Value ($) | | |
Transaction Type | |
Exemption Claimed |
| 01/24/20 | |
| 1,000,000 | | |
Common Stock | |
| 1,000 | | |
Employee benefit | |
4(a)(2) of the Securities Act |
| 02/15/20 | |
| 2,000,000 | | |
Common Stock | |
| 2,000 | | |
Employee benefit | |
4(a)(2) of the Securities Act |
| 02/15/20 | |
| 150,000 | | |
Common Stock | |
| 150 | | |
Employee benefit | |
4(a)(2) of the Securities Act |
| 02/15/20 | |
| 250,000 | | |
Common Stock | |
| 250 | | |
Employee benefit | |
4(a)(2) of the Securities Act |
| 02/19/20 | |
| 500,000 | | |
Common Stock | |
| 500 | | |
Employee benefit | |
4(a)(2) of the Securities Act |
| 02/19/20 | |
| 5,000,000 | | |
Common Stock | |
| 5,000 | | |
Employee benefit | |
4(a)(2) of the Securities Act |
| 02/19/20 | |
| 500,000 | | |
Common Stock | |
| 500 | | |
Employee benefit | |
4(a)(2) of the Securities Act |
| 02/19/20 | |
| 1,000,000 | | |
Common Stock | |
| 1,000 | | |
Employee benefit | |
4(a)(2) of the Securities Act |
| 02/19/20 | |
| 250,000 | | |
Common Stock | |
| 250 | | |
Employee benefit | |
4(a)(2) of the Securities Act |
| 02/19/20 | |
| 1,000,000 | | |
Common Stock | |
| 1,000 | | |
Employee benefit | |
4(a)(2) of the Securities Act |
| 03/15/20 | |
| 7,000,000 | | |
Common Stock | |
| 9,800 | | |
Cash | |
4(a)(2) of the Securities Act |
| 03/15/20 | |
| 5,000,000 | | |
Common Stock | |
| 5,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 03/16/20 | |
| 2,143,000 | | |
Common Stock | |
| 3,000 | | |
Cash | |
4(a)(2) of the Securities Act; foreign |
| 03/16/20 | |
| 6,429,000 | | |
Common Stock | |
| 9,000 | | |
Cash | |
4(a)(2) of the Securities Act; foreign |
| 04/24/20 | |
| 7,142,857 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/24/20 | |
| 62,500,000 | | |
Common Stock | |
| 12,500 | | |
Cash | |
4(a)(2) of the Securities Act |
| 05/08/20 | |
| 500,000 | | |
Common Stock | |
| 500 | | |
Employee benefit | |
4(a)(2) of the Securities Act |
| 06/26/20 | |
| 7,000,000 | | |
Common Stock | |
| 9,800 | | |
Cash | |
4(a)(2) of the Securities Act |
| 06/26/20 | |
| 50,000,000 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 06/26/20 | |
| 3,571,428 | | |
Common Stock | |
| 5,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 03/15/21 | |
| 50,000,000 | | |
Common Stock | |
| 150,000 | | |
Settlement of litigation | |
4(a)(2) of the Securities Act; foreign |
| 03/15/21 | |
| 7,500,000 | | |
Common Stock | |
| 22,500 | | |
Cash | |
4(a)(2) of the Securities Act |
| 03/26/21 | |
| 13,392,857 | | |
Common Stock | |
| 3,750 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/09/21 | |
| 1,893,939 | | |
Common Stock | |
| 5,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/09/21 | |
| 8,928,571 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/09/21 | |
| 8,152,174 | | |
Common Stock | |
| 15,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/09/21 | |
| 10,080,645 | | |
Common Stock | |
| 25,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/21/21 | |
| 3,750,000 | | |
Common Stock | |
| 9,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/28/21 | |
| 10,714,286 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/29/21 | |
| 178,571,429 | | |
Common Stock | |
| 50,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 05/01/21 | |
| 6,944,444 | | |
Common Stock | |
| 15,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 05/08/21 | |
| 2,500,000 | | |
Common Stock | |
| 5,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 05/10/21 | |
| 36,764,706 | | |
Common Stock | |
| 50,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 05/18/21 | |
| 2,500,000 | | |
Common Stock | |
| 5,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 05/21/21 | |
| 12,500,000 | | |
Common Stock | |
| 2,500 | | |
Cash | |
4(a)(2) of the Securities Act |
| 05/24/21 | |
| 3,750,000 | | |
Common Stock | |
| 7,500 | | |
Cash | |
4(a)(2) of the Securities Act |
| 06/03/21 | |
| 8,928,857 | | |
Common Stock | |
| 9,800 | | |
Cash | |
4(a)(2) of the Securities Act |
| 06/11/21 | |
| 14,705,882 | | |
Common Stock | |
| 20,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 06/25/21 | |
| 6,250,000 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 06/26/21 | |
| 6,250,000 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 09/21/21 | |
| 10,000,000 | | |
Common Stock | |
| 40,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 11/30/21 | |
| 40,000,000 | | |
Common Stock | |
| 50,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 11/30/21 | |
| 1,893,939 | | |
Common Stock | |
| 2,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/04/22 | |
| 55,555,555 | | |
Common Stock | |
| 50,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/04/22 | |
| 27,777,778 | | |
Common Stock | |
| 25,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/04/22 | |
| 10,000,000 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/04/22 | |
| 200,000,000 | | |
Common Stock | |
| 200,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/07/22 | |
| 30,000,000 | | |
Common Stock | |
| 30,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/21/22 | |
| 20,000,000 | | |
Common Stock | |
| 20,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/24/22 | |
| 10,000,000 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/31/22 | |
| 10,000,000 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 03/02/22 | |
| 20,000,000 | | |
Common Stock | |
| 12,000 | | |
Services | |
4(a)(2) of the Securities Act |
| 03/03/22 | |
| 94,117,647 | | |
Common Stock | |
| 84,700 | | |
Cash | |
4(a)(2) of the Securities Act |
| 03/09/22 | |
| 11,111,111 | | |
Common Stock | |
| 1,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 03/11/22 | |
| 55,000,000 | | |
Common Stock | |
| 38,500 | | |
Cash | |
4(a)(2) of the Securities Act |
| 03/28/22 | |
| 70,588,234 | | |
Common Stock | |
| 70,600 | | |
Cash | |
4(a)(2) of the Securities Act |
| 03/28/22 | |
| 41,025,641 | | |
Common Stock | |
| 41,025 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/01/22 | |
| 55,555,555 | | |
Common Stock | |
| 50,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 06/26/22 | |
| 125,000,000 | | |
Common Stock | |
| 50,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 08/10/22 |
|
|
1,000 |
|
|
Series B Preferred |
|
|
-- |
|
|
1 |
|
4(a)(2) of the Securities Act |
| 09/07/22 |
|
|
62,500,000 |
|
|
Common Stock |
|
|
54,200 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 09/07/22 |
|
|
16,888,889 |
|
|
Common Stock |
|
|
12,667 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 09/30/22 |
|
|
11,250,000 |
|
|
Common Stock |
|
|
-- |
|
|
Services |
|
4(a)(2) of the Securities Act |
| 10/17/22 |
|
|
625,000,000 |
|
|
Common Stock |
|
|
250,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 11/01/22 |
|
|
70,000,000 |
|
|
Common Stock |
|
|
-- |
|
|
Services Agreements |
|
4(a)(2) of the Securities Act |
| 11/01/22 |
|
|
16,000,000 |
|
|
Common Stock |
|
|
-- |
|
|
Employee benefit |
|
4(a)(2) of the Securities Act |
| 12/01/22 |
|
|
4,000,000 |
|
|
Common Stock |
|
|
2,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 12/09/22 |
|
|
20,000,000 |
|
|
Common Stock |
|
|
10,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 12/12/22 |
|
|
22,222,222 |
|
|
Common Stock |
|
|
10,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 01/09/23 |
|
|
100,000,000 |
|
|
Common Stock |
|
|
25,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 01/09/23 |
|
|
16,000,000 |
|
|
Common Stock |
|
|
4,800 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 01/18/23 |
|
|
300,000,000 |
|
|
Common Stock |
|
|
150,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 02/23/23 |
|
|
14,285,714 |
|
|
Common Stock |
|
|
5,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 03/02/23 |
|
|
12,500,000 |
|
|
Common Stock |
|
|
5,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 03/09/23 |
|
|
150,000,000 |
|
|
Common Stock |
|
|
50,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 03/18/23 |
|
|
62,500,000 |
|
|
Common Stock |
|
|
25,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 04/15/23 |
|
|
150,000,000 |
|
|
Common Stock |
|
|
50,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 04/25/23 |
|
|
200,000,000 |
|
|
Common Stock |
|
|
50,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 05/31/23 |
|
|
25,000,000 |
|
|
Common Stock |
|
|
10,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 05/31/23 |
|
|
25,000,000 |
|
|
Common Stock |
|
|
5,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 06/20/23 |
|
|
25,000,000 |
|
|
Common Stock |
|
|
10,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 06/22/23 |
|
|
25,000,000 |
|
|
Common Stock |
|
|
10,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 06/29/23 |
|
|
10,000,000 |
|
|
Common Stock |
|
|
5,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 06/20/23 |
|
|
28,571,428 |
|
|
Common Stock |
|
|
10,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 07/01/23 |
|
|
12,500,000 |
|
|
Common Stock |
|
|
5,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 07/01/23 |
|
|
12,500,000 |
|
|
Common Stock |
|
|
5,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
______________
1 Issued in exchange for 595,467,205 shares of Common
Stock.
The proceeds of the securities issued for cash were used
for general corporate purposes.
Item 16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) Exhibits.
Exhibit
Number
| Description |
| 3.1 | Amended and Restated
Articles of Organization, filed with the Secretary of State of the State of Colorado on July 20,
2022. |
| 3.2 | Amendment to the Articles of Incorporation, filed with the Secretary of State of
the State of Colorado on December 6, 2022. |
| 3.3 | By-Laws. |
| 5 | Opinion of Barry J. Miller PLLC.* |
| 10.1 | 2022
Incentive Award Plan.+ |
| 10.2 | Lease, dated July 1, 2016, by and between 6201 Bonhomme,
L.P. as landlord and Precision Research Institute, L.L.C., as tenant (includes amendments). |
| 10.3 | Apartment Lease, dated March 2, 2023, by and between SPUSG HSTN North Tower, as Lessor, and Dante Picazo and Henry Levinski, as tenants.** |
| 10.4 | U.S. Small Business Note, dated April 16, 2021, made
by Elizabeth Hernandez and assumed by the Registrant. |
| 10.5 | Forward Purchase Agreement (Fixed ACH Delivery), dated May 13, 2022, by and between Kapitos LLC and the Registrant. |
| 10.6 | First Electronic Bank Revolving Credit Agreement, dated December 10, 2020, by and between Registrant and First Electronic Bank. |
| 10.7 | Business Line of Credit Agreement, dated October
8, 2019, by and between Headway Capital, LLC and Pharmacology University, Inc. |
| 10.8 | Future Receivables Sale and Purchase Agreement, dated as of August 8, 2022, by and between Park Avenue Funding and the Registrant |
| 10.9 | Agreement, dated as of November 1, 2022, by and between the Registrant and Henry Levinski.+ ** |
| 10.10 | Agreement, dated as of August 1, 2019, by and between Universidad de Bogata Jorge Tadeo Lozano and the Registrant.** |
| 10.11 | Clinical Trial Agreement, dated as of August 19, 2022, by and between Alpha Research Institute, LLC and Pharmaceutical Research Associates, Inc. |
| 10.12 | Master Research Services Agreement, dated as of June 9, 2021, by and between the Registrant and SeraTrials, LLC and amendments thereto |
| 10.13 | Third Amendment to Office Lease, by and between Precision Research Institute, LLC and 6201 Bonhomme, LP |
| 10.14 | Future Receipts Sale and Purchase Agreement, dated April 20,
2023, by and between Cloudfund LLC and the Registrant. |
| 10.15 | Future Receivables Sale and Purchase Agreement, dated
March 30, 2023, by and between Amerifund Group LLC and the Registrant. |
| 21 | Subsidiaries of the Registrant. |
| 23.1 | Consent of PWR CPA, LLP.** |
| 23.2 | Consent of Barry J. Miller PLLC. Included in Exhibit 5. |
| 24 | Power
of Attorney. Included on the signature page of the registration statement filed on August 24, 2022. |
| 107 | Filing Fee Table (Amended).* |
__________
* Filed herewith
** Filed previously
+ Management contract or Compensatory
Plan.
(b) Financial
Statement Schedules.
All schedules are omitted because the required
information is either not present, not present in material amounts or is presented within the consolidated financial statements included
in the Prospectus that is part of this registration statement.
Item 17. Undertakings.
The undersigned hereby undertakes:
(1) To
file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
| (i) | To include any prospectus required by Section 10(a)(3) of
the Securities Act of 1933; |
| (ii) | To reflect in the prospectus
any facts or events arising after the effective date of the registration statement (or the
most recent post-effective amendment thereof) which, individually or in the aggregate, represent
a fundamental change in the information set forth in the registration statement. Notwithstanding
the foregoing, any increase or decrease in volume of securities offered (if the total dollar
value of securities offered would not exceed that which was registered) and any deviation
from the low or high end of the estimated maximum offering range may be reflected in the
form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate,
the changes in volume and price represent no more than a 20 percent change in the maximum
aggregate offering price set forth in the “Calculation of Registration Fee” table
in the effective registration statement; and |
| (iii) | To include any material information
with respect to the plan of distribution not previously disclosed in the registration statement
or any material change to such information in the registration statement. |
(2) That,
for the purpose of determining liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a
new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed
to be the initial bona fide offering thereof.
(3) To
remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination
of the offering.
(4) That,
for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b)
as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses
filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used
after effectiveness; provided, however, that no statement made in a registration statement or prospectus that is part of the registration
statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is
part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify
any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such
document immediately prior to such date of first use.
(5) That,
for the purpose of determining liability of the Registrant under the Securities Act of 1933 to any purchaser in the initial distribution
of the securities, the undersigned Registrant undertakes that in a primary offering of securities of the undersigned Registrant pursuant
to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities
are offered or sold to such purchaser by means of any of the following communications, the undersigned Registrant will be a seller to
the purchaser and will be considered to offer or sell such securities to such purchaser:
| (i) | Any preliminary prospectus or prospectus
of the undersigned Registrant relating to the offering required to be filed pursuant to Rule
424; |
| (ii) | Any free writing prospectus relating
to the offering prepared by or on behalf of the undersigned Registrant or used or referred
to by the undersigned Registrant; |
| (iii) | The portion of any other free writing
prospectus relating to the offering containing material information about the undersigned
Registrant or its securities provided by or on behalf of the undersigned Registrant;
and |
| (iv) | Any other communication that is an offer in the offering made
by the undersigned Registrant to the purchaser. |
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1933, the Registrant has duly caused this amendment to the registration statement to be signed on its behalf by the undersigned,
thereunto duly authorized, in the City of Houston, Texas.
Date: July 10, 2023
| |
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS, INC. |
| |
|
| |
By: /s/ Dante Picazo |
| |
Dante Picazo |
| |
Chief Executive Officer |
Pursuant to the requirements of the Securities
Act, this registration statement has been signed below by the following persons in the capacities set forth opposite their names and
on the dates indicated.
| Person |
|
Title |
|
Date |
| |
|
|
|
|
| * |
|
Chief Executive Officer and Director |
|
July 10, 2023 |
| Dante Picazo |
|
(Principal Executive Officer and Principal Accounting Officer) |
|
|
| |
|
|
|
|
| /s/ Henry Levinski |
|
Director |
|
July 10, 2023 |
| Henry Levinski |
|
|
|
|
| |
|
|
|
|
| * |
|
Director |
|
July 10, 2023 |
| Jose Torres |
|
|
|
|
| *By: |
/s/
Henry Levinski |
| |
Henry Levinski
Attorney-in-Fact |
Exhibit 5
BARRY J. MILLER
7146 Pebble Park Drive
West Bloomfield, Michigan 48322
Telephone: (248) 232-8039 – Fax: (248) 246-9524
Email: bjmiller@bjmpllc.com
July 10, 2023
Board of Directors
Cannabis Bioscience International Holdings, Inc.
Re: Registration Statement on Form S-1 (Registration
No. 333-267039)
Ladies and Gentlemen:
I have acted as counsel for Cannabis Bioscience International
Holdings, Inc., a Colorado corporation (the “Company”), in connection with the registration statement on Form S-1 of the Company
(Registration No. 333-267039) filed with the United States Securities and Exchange Commission (the “ Commission” ) relating
to (i) the issuance and sale by the Company of 6,250,000,000 shares of the Company’s common stock, without par value (“ Common
Stock” ), and (ii) the sale by the Selling Stockholders (as that term is defined in the Registration Statement) of 4,033,405,599
shares of Common Stock. This opinion is being furnished to you at your request to enable you to comply with the requirements of Item 601(b)(5)
of Regulation S-K promulgated under the Securities Act of 1933 in connection with the Registration Statement.
In connection with this opinion, I have examined such
matters of fact and questions of law as I have deemed relevant. I have relied upon the foregoing and upon certificates and other assurances
of officers of the Company and others as to factual matters without having independently verified them.
In rendering this opinion, I have assumed: (i) information
contained in documents reviewed by me is true, complete and correct; (ii) the genuineness and authenticity of all signatures; (iii) the
authenticity of all documents submitted to me as originals; (iv) the conformity to authentic originals of all documents submitted to me
as copies; (v) the accuracy, completeness and authenticity of certificates of public officials; (vi) the due authorization, execution
and delivery of all documents by parties other than the Company; and (vii) the legal capacity of all natural persons.
Based upon the foregoing, I am of the opinion that
(i) the shares of Common Stock to be issued and sold by the Company have been duly and validly authorized and, when issued and delivered
by the Company, and paid for in accordance with the prospectus that is part of the Registration Statement, will be validly issued, fully
paid and nonassessable and (ii) the shares of Common Stock to be sold by the Selling Stockholders are duly authorized, validly issued,
fully paid and nonassessable.
This opinion is rendered only with respect to the
Colorado Business Corporation Act, as amended, the applicable provisions of the Colorado Constitution and any reported judicial decisions
interpreting them. I express no opinion (i) with respect to the applicability thereto, or the effect thereon, of the laws of any other
jurisdiction or as to any matters of municipal law or the laws of any local agencies within Colorado or (ii) as to the form or content
of the Registration Statement.
I hereby consent to the filing of this opinion with the Commission as Exhibit 5.1 to the Registration Statement and the reference to me
therein under the caption “Legal Matters.” In giving this consent, I do not thereby admit that I am included in the category
of persons whose consent is required under Section 7 of the Securities Act or the rules and regulations of the Commission.
Very truly yours,
/s/ Barry J. Miller
Exhibit 107
Calculation of Filing Fee
Tables
S-1
(Form Type)
Cannabis Bioscience International Holdings, Inc.
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered and Carry Forward Securities
| |
Security
Type |
Security
Class Title |
Fee
Calculation or Carry Forward Rule |
Amount
Registered |
Proposed
Maximum Offering Price Per Unit |
Maximum
Aggregate Offering Price |
Fee
Rate |
Amount
of Registration Fee |
Carry
Forward Form Type |
Carry
Forward File Number |
Carry
Forward Initial effective date |
Filing
Fee Previously Paid In Connection with Unsold Securities to be
Carried
Forward |
| Newly
Registered Securities |
| Fees
to be Paid |
Common
Stock |
Common
Stock |
457 |
10,283,940,599 |
0.0008 |
8,227,153 |
.0001102 |
906.63 |
|
|
|
|
| Fees
Previously Paid |
Common
Stock |
Common
Stock |
457 |
10,160,369,171 |
0.0008 |
8,128,295.34 |
|
895.74 |
|
|
|
|
| Previous
Registered Securities |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Total
Offering Amounts |
|
|
|
906.63 |
|
|
|
|
| |
Total
Fees Previously Paid |
|
|
|
895.74 |
|
|
|
|
| |
Total
Fee Offsets |
|
|
|
- |
|
|
|
|
| |
Net
Fee Due |
|
|
|
10.89 |
|
|
|
|
China Infrastructure Con... (PK) (USOTC:CHNC)
Historical Stock Chart
From Apr 2024 to May 2024
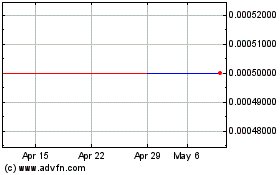
China Infrastructure Con... (PK) (USOTC:CHNC)
Historical Stock Chart
From May 2023 to May 2024