000071893700007189372024-01-082024-01-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): January 8, 2024
STAAR Surgical Company
(Exact Name of Registrant as Specified in Charter)
|
|
|
Delaware |
0-11634 |
95-3797439 |
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
|
|
25651 Atlantic Ocean Drive., Lake Forest, California |
|
92630 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: 626-303-7902
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
|
☐ |
Written communication pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ |
Pre-commencement communication pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ |
Pre-commencement communication pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
Common |
STAA |
NASDAQ |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1 933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On January 8, 2024, STAAR Surgical Company (the “Company”) published a press release reporting selected preliminary financial results for the quarter and fiscal year ended December 29, 2023, a copy of which is furnished as Exhibit 99.1 to this Report and is incorporated herein by this reference. The financial information is unaudited and subject to adjustment in the final audited financial statements to be filed with the Company’s Annual Report on Form 10-K. The Company expects to report its complete 2023 financial results on its fourth-quarter and full-year earnings call, which it will hold later in the first quarter of 2024.
Item 7.01 Regulation FD Disclosure.
Representatives of the Company will give presentations to investors commencing on January 8, 2024. A copy of the slide presentation they will share is furnished as Exhibit 99.2 to this Report and is incorporated herein by this reference.
The information furnished herewith pursuant to Items 2.02 and 7.01 of this Current Report, including Exhibits 99.1 and 99.2 shall not be deemed to be “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section. The information in Items 2.02 and 7.01 of this Current Report shall not be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date of this Current Report, regardless of any general incorporation language in the filing.
Item 9.01 Financial Statements and Exhibits
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
STAAR Surgical Company |
|
January 8, 2024 |
By: |
/s/ Tom Frinzi |
|
|
Thomas Frinzi |
|
|
President and Chief Executive Officer |
Exhibit 99.1

STAAR Surgical Announces Preliminary Results for Fourth Quarter and Fiscal 2023
Net Sales Up 19% for Fourth Quarter and 13% for Fiscal 20231
ICL Sales Up 22% for Fourth Quarter and 18% for Fiscal 2023
Provides Fiscal 2024 Sales Outlook of $335 Million to $340 Million
LAKE FOREST, CA, January 8, 2024 --- STAAR Surgical Company (NASDAQ: STAA), a leading developer, manufacturer and marketer of the EVO family of Implantable Collamer® Lenses (EVO ICL) for myopia, astigmatism and presbyopia, today announced preliminary results for the fourth quarter and fiscal year ended December 29, 2023. Preliminary net sales were approximately $76.5 million for the fourth quarter and approximately $322.5 million for fiscal 2023. Operating margin is expected to be approximately 5% for the fourth quarter and fiscal 2023.
“Fiscal 2023 was a year of positive market share gains, sales growth and continued profitability for STAAR. Our teams finished the year on a strong note with global ICL sales increasing 22% in the fourth quarter including 26% growth in APAC,” said Tom Frinzi, President and CEO of STAAR Surgical. “ICL unit growth also compares particularly favorably to recent industry trends, outpacing refractive industry growth by at least 25 points for the third year in a row.2 Global ICL sales increased 18% for fiscal 2023 driven by 21% growth in APAC, 7% growth in EMEA and 10% growth in the Americas.”
Mr. Frinzi continued, “2023 was also a year of transition and fast learning as we launched new market development strategies while continuing to invest in innovation. Global voice of customer surveys, the most recent completed in November, show willingness to recommend and satisfaction with product quality and clinical outcomes continue to exceed 95%. These data bolster our confidence that utilization of our ICL technology will continue to move down the diopter curve opening up a larger portion of the vast addressable market opportunity. We anticipate clinical journals will publish two new peer-reviewed papers in the first half of 2024 that should further enhance surgeon confidence in measurement and lens size selection across the wide range of diagnostic devices and tools surgeons use to measure the eye for our lenses. Additionally, we intend to continue our investment in innovation projects such as intermediate lens sizes and AI driven tools.”
“Our sales outlook for fiscal 2024 balances the recent industry trends and challenging economic environment for consumers against our conviction that ICL will continue to capture refractive market share globally. We therefore expect our net sales for fiscal 2024 will be approximately $335 million to $340 million which contemplates above-industry rates of growth of approximately 10% for ICL sales in both China and the U.S.3 We will continue to be disciplined in our investments on the path to realizing our market opportunity and anticipate our GAAP operating margin and earnings will be at least breakeven for fiscal 2024. We are confident our actions will increasingly resonate with a growing number of surgeons and patients in both large and emerging markets globally,” concluded Mr. Frinzi.
Cash, cash equivalents and investments available for sale was approximately $230 million as of December 29, 2023, an increase of approximately $28 million from $201.7 million at September 29, 2023. Accounts receivable was approximately $90 million as of December 29, 2023.
The Company expects to report complete fourth quarter and fiscal year results on or about February 21 and provided today’s information due to investor meetings taking place this week. The financial information in
this release is unaudited and subject to adjustment in the final audited financial statements to be filed with the Company’s Annual Report on Form 10-K.
1 Fiscal 2023 preliminary net sales of approximately $322.5 million includes approximately $3 million of low margin Other Products Cataract IOLs, a business the Company previously announced it would exit by the end of 2023. Fiscal 2022 reported net sales of $284.4 million included approximately $14 million of Other Products Cataract IOLs. For this reason, ICL sales may be a more insightful growth comparison on a go-forward basis.
2 Global ICL unit growth exceed refractive industry growth by 38 points (2021); 28 points (2022) and 26 points (2023). The Company estimates global refractive industry procedures increased 10% (2021); increased 5% (2022); and decreased 7% (2023) Y/Y based on data available as of January 2, 2024.
3 The Company estimates China and U.S. refractive procedures will be approximately flat Y/Y in 2024 as of January 2, 2024.
About STAAR Surgical
STAAR, which has been dedicated solely to ophthalmic surgery for over 40 years, designs, develops, manufactures and markets implantable lenses for the eye. These lenses are intended to provide visual freedom for patients, lessening or eliminating the reliance on glasses or contact lenses. All of these lenses are foldable, which permits the surgeon to insert them through a small incision. STAAR’s lens used in refractive surgery is called an Implantable Collamer® Lens or “ICL,” which includes the EVO ICL™ product line. More than 2,500,000 ICLs have been sold to date and STAAR markets these lenses in over 75 countries. To learn more about the ICL go to: EVOICL.com. Headquartered in Lake Forest, CA, the company operates manufacturing and packaging facilities in Aliso Viejo, CA, Monrovia, CA and Nidau, Switzerland. For more information, please visit the Company’s website at www.staar.com.
Safe Harbor
All statements that are not statements of historical fact are forward-looking statements, including statements about any of the following: any financial projections (including sales), plans, strategies, and objectives of management for 2024 and beyond or prospects for achieving such plans, expectations for sales, revenue, margin, expenses or earnings, and any statements of assumptions underlying any of the foregoing, including those relating to financial performance in the upcoming quarter, fiscal year 2024 and beyond. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include risks and uncertainties related to global economic conditions, as well as the factors set forth in the Company’s Annual Report on Form 10-K for the year ended December 30, 2022 under the caption “Risk Factors,” which is on file with the Securities and Exchange Commission and available in the “Investor Information” section of the company’s website under the heading “SEC Filings.” We disclaim any intention or obligation to update or revise any financial projections or forward-looking statement due to new information or events. These statements are based on expectations and assumptions as of the date of this press release and are subject to numerous risks and uncertainties, which could cause actual results to differ materially from those described in the forward-looking statements. The risks and uncertainties include the following: global economic conditions; the impact of COVID-19; the discretion of regulatory agencies to approve or reject existing, new or improved products, or to require additional actions before or after approval, or to take enforcement action; international trade disputes and substantial dependence on demand from Asia; and the willingness of surgeons and patients to adopt a new or improved product and procedure.
We intend to use our website as a means of disclosing material non-public information and for complying with our disclosure obligations under Regulation FD. Such disclosures will be included on our website in
the ‘Investor Relations’ sections. Accordingly, investors should monitor such portions of our website, in addition to following our press releases, SEC filings and public conference calls and webcasts.
CONTACT: Investors & Media
Brian Moore
Vice President, Investor Relations and Corporate Development
(626) 303-7902, Ext. 3023
bmoore@staar.com
NASDAQ: STAA Investor Presentation January 2024 Exhibit 99.2
All statements that are not statements of historical fact are forward-looking statements, including statements about any of the following: any financial projections (including sales), plans, strategies, and objectives of management for 2024 and beyond or prospects for achieving such plans, expectations for sales, revenue, margin, expenses or earnings, and any statements of assumptions underlying any of the foregoing, including those relating to financial performance in the upcoming quarter, fiscal year 2024 and beyond. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include risks and uncertainties related to global economic conditions, as well as the factors set forth in the Company’s Annual Report on Form 10-K for the year ended December 30, 2022 under the caption “Risk Factors,” which is on file with the Securities and Exchange Commission and available in the “Investor Information” section of the company’s website under the heading “SEC Filings.” We disclaim any intention or obligation to update or revise any financial projections or forward-looking statement due to new information or events. These statements are based on expectations and assumptions as of the date of this presentation and are subject to numerous risks and uncertainties, which could cause actual results to differ materially from those described in the forward-looking statements. The risks and uncertainties include the following: global economic conditions; the impact of COVID-19; the discretion of regulatory agencies to approve or reject existing, new or improved products, or to require additional actions before or after approval, or to take enforcement action; international trade disputes and substantial dependence on demand from Asia; and the willingness of surgeons and patients to adopt a new or improved product and procedure. We intend to use our website as a means of disclosing material non-public information and for complying with our disclosure obligations under Regulation FD. Such disclosures will be included on our website in the ‘Investor Relations’ sections. Accordingly, investors should monitor such portions of our website, in addition to following our press releases, SEC filings and public conference calls and webcasts. Forward Looking Statements 02

03 STAAR Surgical / Our Vision The first Choice for Doctors and Patients Seeking Visual Freedom

04 Preliminary Results PRELIMINARY RESULTS Please refer to Appendix Slide 27 for additional details by geography Preliminary sales of ~$76.5M for 4Q23 and ~$322.5M for fiscal 2023 sales, above outlook for low-end of $320M to $325M range provided on November 1, 2023 Net sales up 19% Y/Y for 4Q23 and up 13% Y/Y for fiscal 2023 reflecting global ICL growth, partially offset by previously announced and planned exit of lower margin cataract IOL business Cash, cash equivalents and investments available for sale was ~$230M as of December 29, 2023, an increase of ~$28M from $201.7M at September 29, 2023. Accounts receivable was ~$90M at December 29, 2023. ICL sales up 22% for 4Q23 and up 18% for fiscal 2023 Gross margin expected to be 79% to 80% for fiscal 2023 Operating margin expected to be approximately 5% for fiscal 2023 We delivered our sixth straight year of sales growth in fiscal 2023 and our profitability, strong balance sheet and free cash flow generation allows STAAR the flexibility to make a myriad of investment and capital allocation decisions designed to create shareholder value.

05 4Q23 and Fiscal 2023 COMMENTARY AND OUTLOOK FOR 2024 Fiscal 2023 Global ICL unit growth +19% Y/Y significantly outpaced refractive industry procedure growth which the Company estimates was down 7% Y/Y Expect 1Q24 sales of approximately $72M and that accounts receivable will continue to trend lower Raising ASPs remain an option, most likely in concert with new product introductions/ innovation given the Company has not taken a price increase since 2016 Balancing the recent industry trends and a challenging economic environment for consumers against our conviction that ICL will continue to capture refractive market share globally, we expect our net sales for fiscal 2024 will be approximately $335 million to $340 million which contemplates above-industry rates of growth of approximately 10% for ICL sales in both China and the U.S. Sales in the U.S. are expected to remain flattish sequentially through 1H24 accelerating in 2H24 to ~$5M+ per quarter. We will continue to be disciplined in our investments on the path to realizing our market opportunity and anticipate our GAAP operating margin and earnings will be at least breakeven for fiscal 2024. STAAR could choose to be much more profitable if we were not investing heavily to monetize the vast market opportunity and a business that we believe can become much larger over time
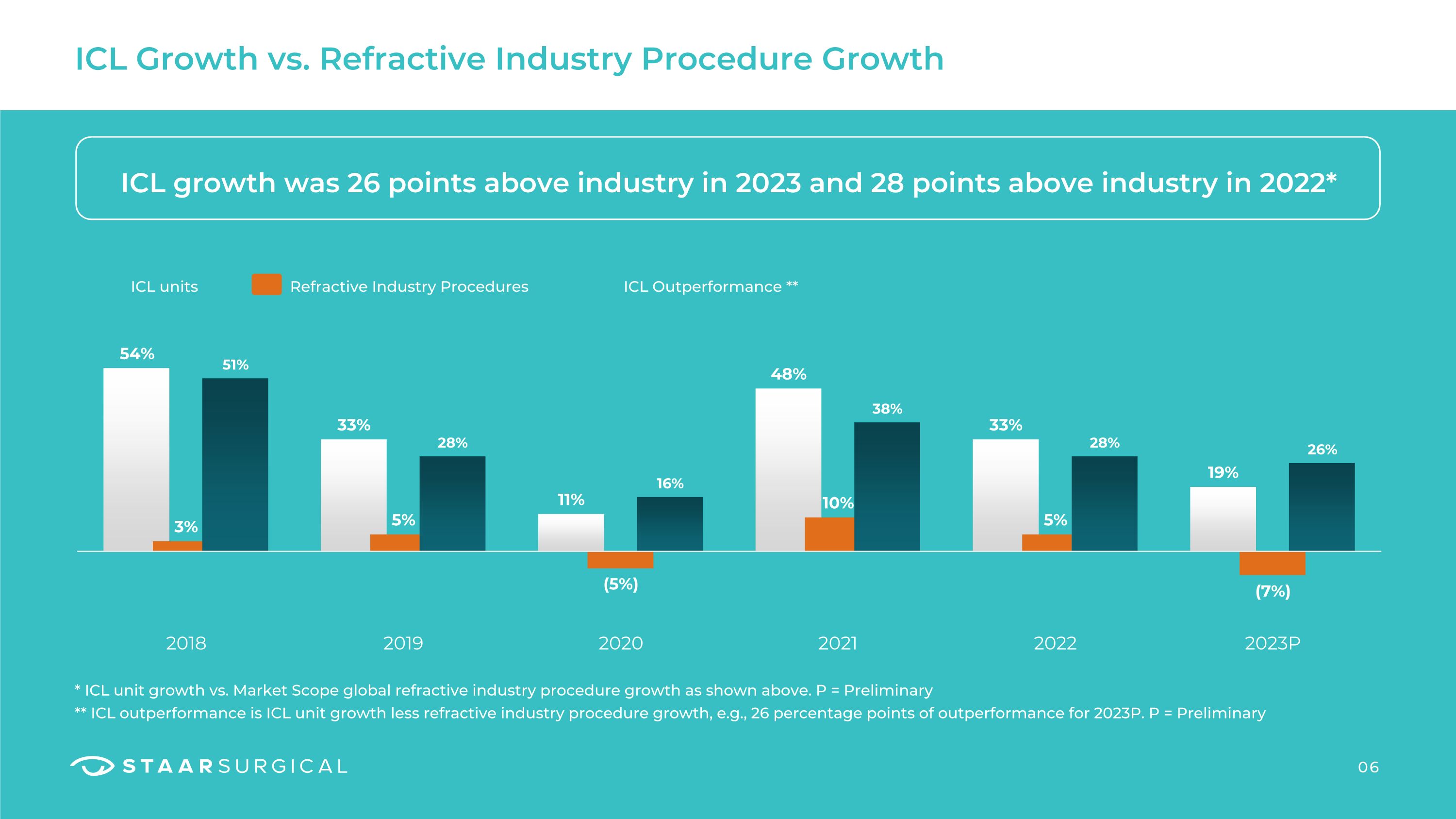
ICL Growth vs. Refractive Industry Procedure Growth ICL growth was 26 points above industry in 2023 and 28 points above industry in 2022* * ICL unit growth vs. Market Scope global refractive industry procedure growth as shown above. P = Preliminary
** ICL outperformance is ICL unit growth less refractive industry procedure growth, e.g., 26 percentage points of outperformance for 2023P. P = Preliminary ICL units Refractive Industry Procedures ICL Outperformance ** 06
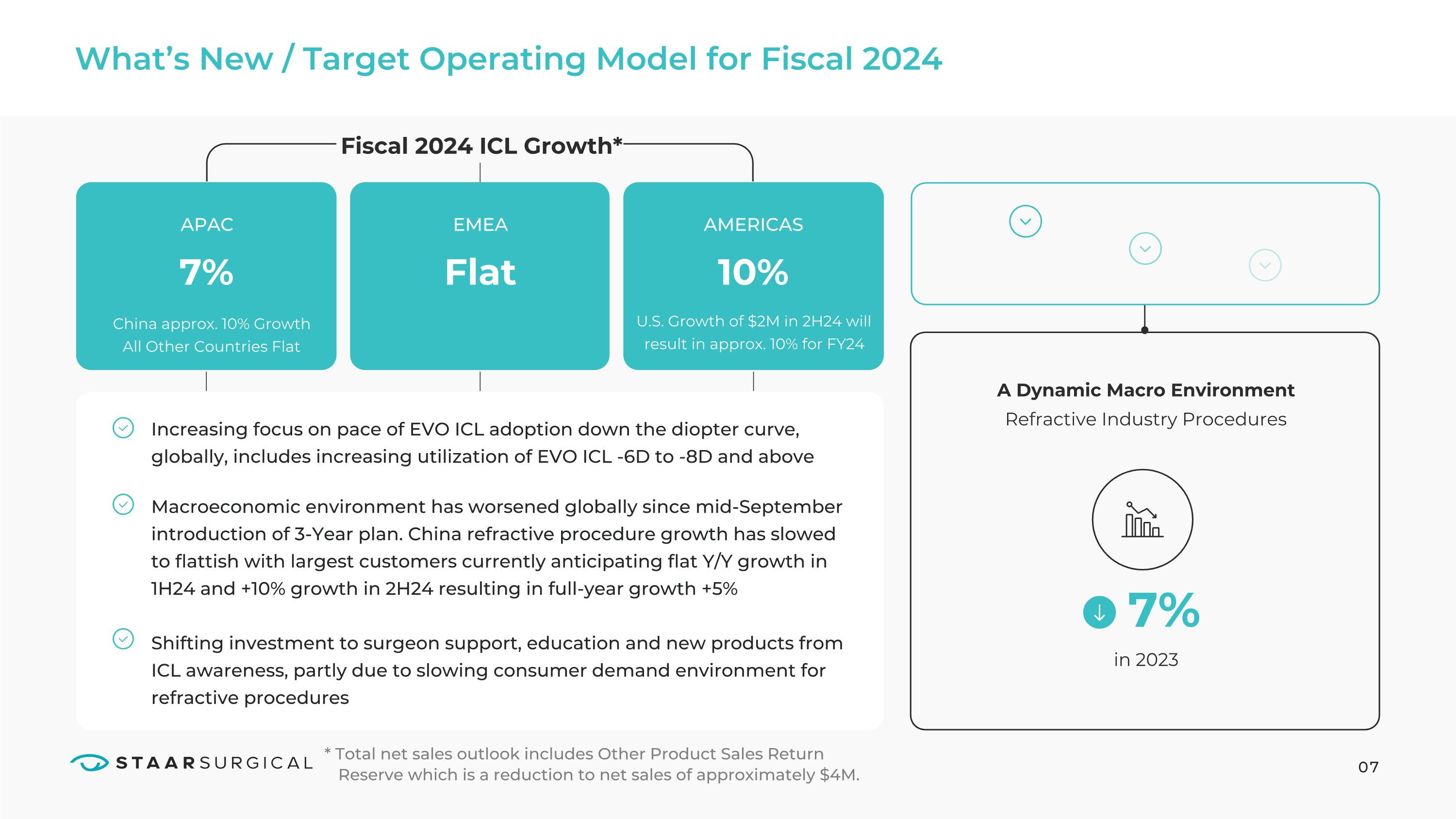
What’s New / Target Operating Model for Fiscal 2024 07 Increasing focus on pace of EVO ICL adoption down the diopter curve, globally, includes increasing utilization of EVO ICL -6D to -8D and above Shifting investment to surgeon support, education and new products from ICL awareness, partly due to slowing consumer demand environment for refractive procedures Macroeconomic environment has worsened globally since mid-September introduction of 3-Year plan. China refractive procedure growth has slowed to flattish with largest customers currently anticipating flat Y/Y growth in 1H24 and +10% growth in 2H24 resulting in full-year growth +5% APAC Fiscal 2024 ICL Growth* 7% EMEA AMERICAS A Dynamic Macro Environment Refractive Industry Procedures in 2023 U.S. Growth of $2M in 2H24 will result in approx. 10% for FY24 Flat 10% 7% * Total net sales outlook includes Other Product Sales Return Reserve which is a reduction to net sales of approximately $4M. China approx. 10% Growth All Other Countries Flat
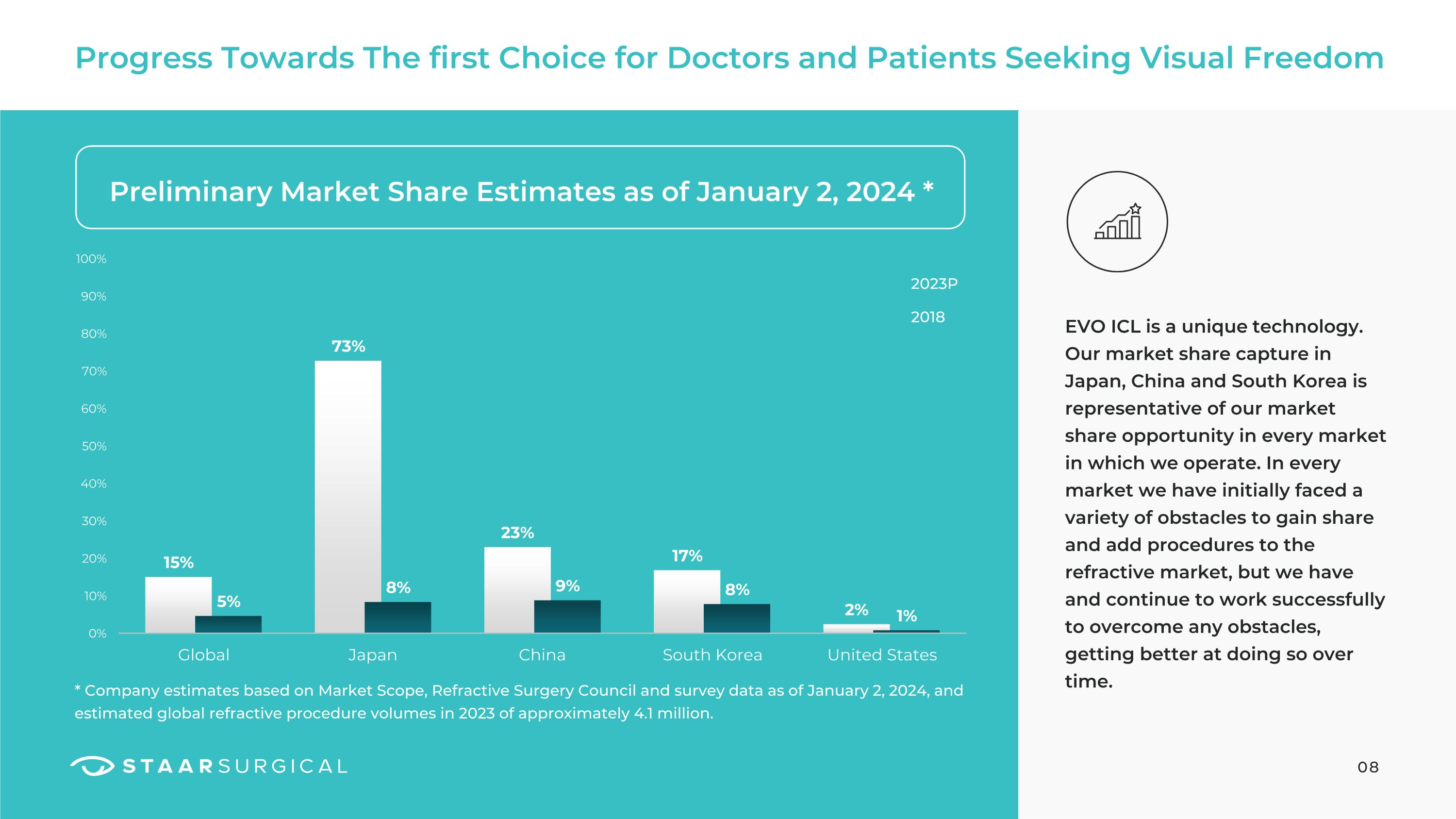
Progress Towards The first Choice for Doctors and Patients Seeking Visual Freedom Preliminary Market Share Estimates as of January 2, 2024 * * Company estimates based on Market Scope, Refractive Surgery Council and survey data as of January 2, 2024, and estimated global refractive procedure volumes in 2023 of approximately 4.1 million. 08 2023P 2018 EVO ICL is a unique technology. Our market share capture in Japan, China and South Korea is representative of our market share opportunity in every market in which we operate. In every market we have initially faced a variety of obstacles to gain share and add procedures to the refractive market, but we have and continue to work successfully to overcome any obstacles, getting better at doing so over time.
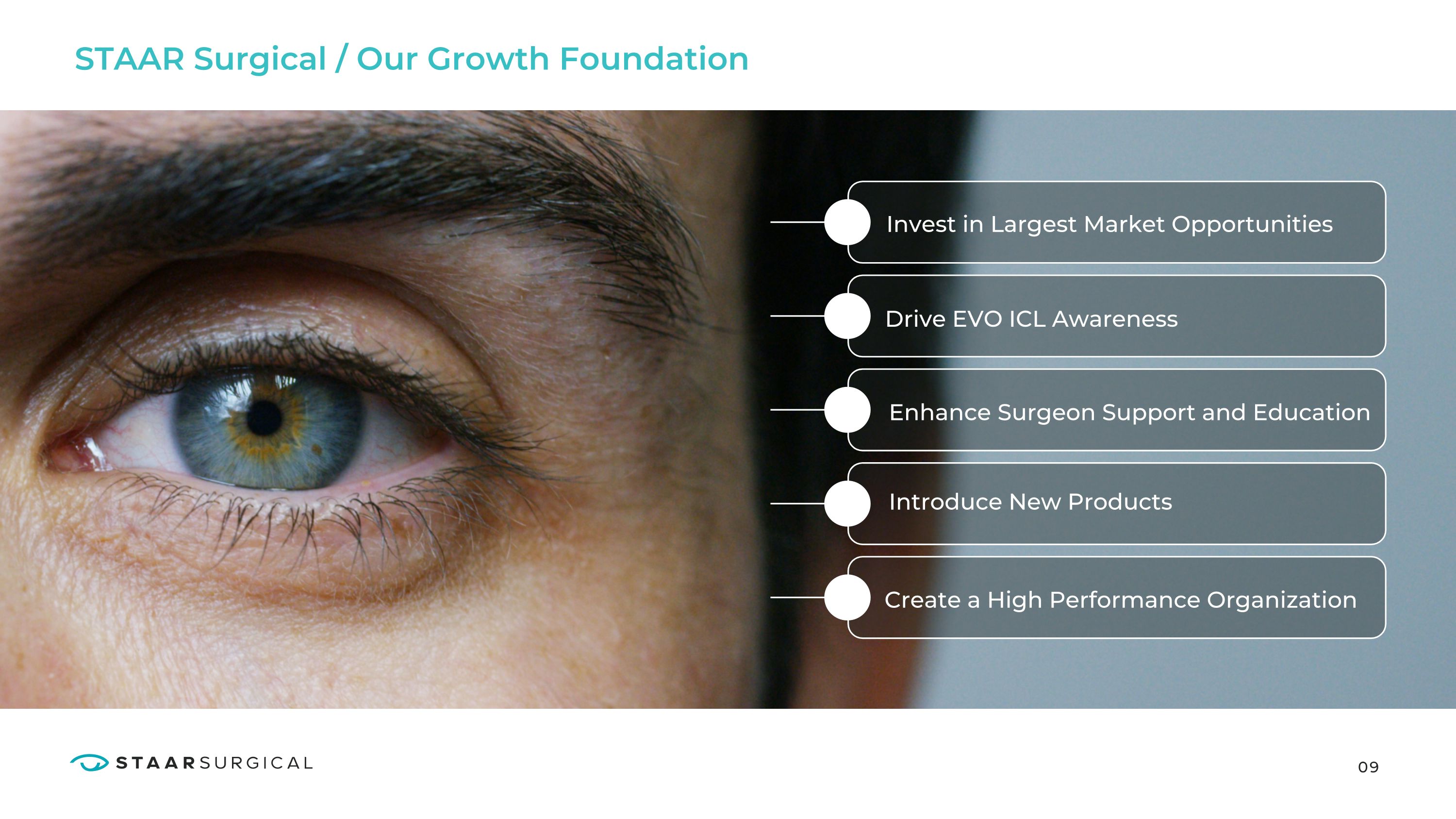
09 STAAR Surgical / Our Growth Foundation Invest in Largest Market Opportunities Drive EVO ICL Awareness Enhance Surgeon Support and Education Introduce New Products Create a High Performance Organization
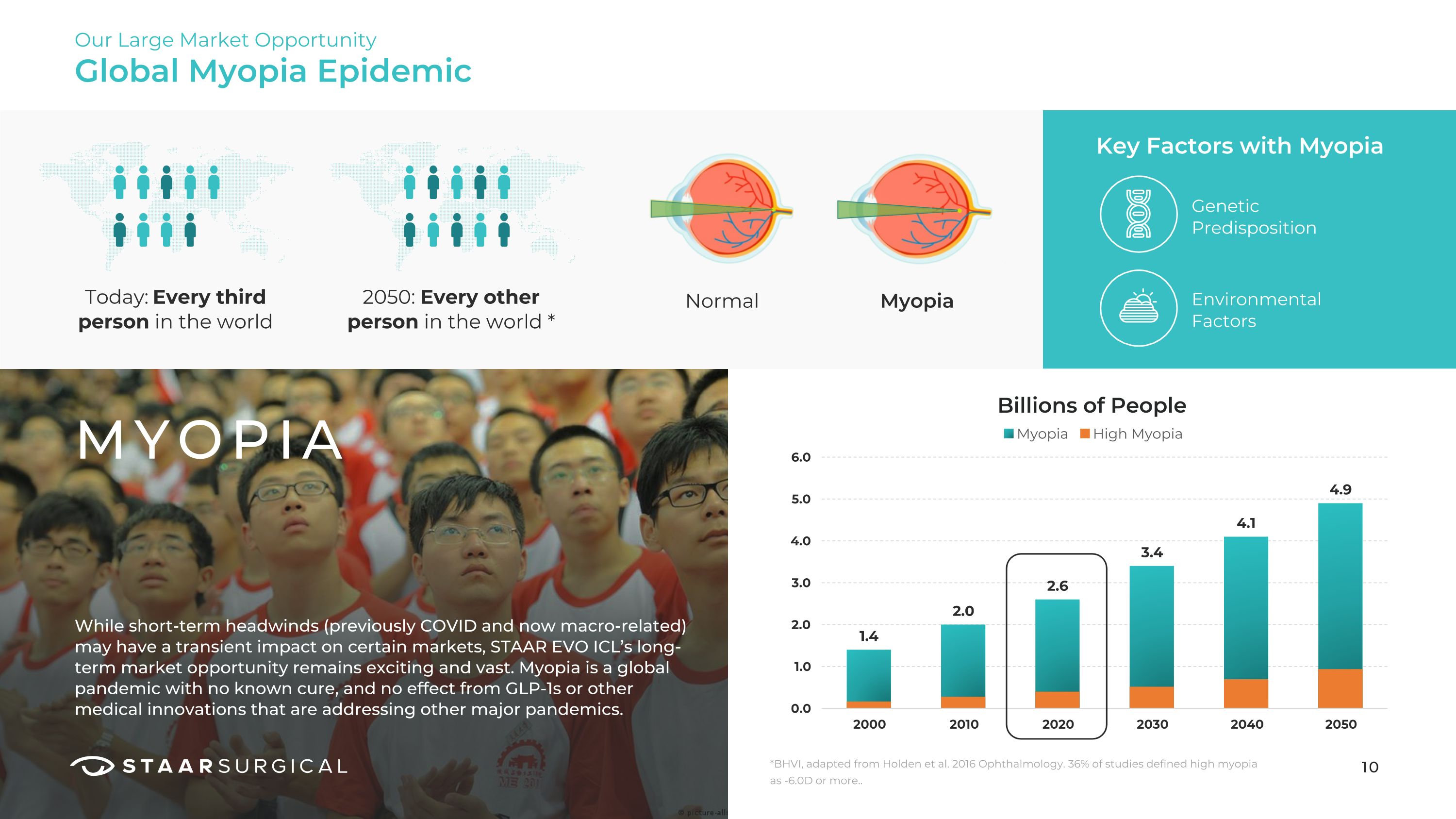
Global Myopia Epidemic Our Large Market Opportunity MYOPIA Today: Every third person in the world While short-term headwinds (previously COVID and now macro-related) may have a transient impact on certain markets, STAAR EVO ICL’s long-term market opportunity remains exciting and vast. Myopia is a global pandemic with no known cure, and no effect from GLP-1s or other medical innovations that are addressing other major pandemics. 2050: Every other person in the world * Normal Myopia Key Factors with Myopia Environmental Factors Genetic Predisposition 10 *BHVI, adapted from Holden et al. 2016 Ophthalmology. 36% of studies defined high myopia as -6.0D or more.. Billions of People
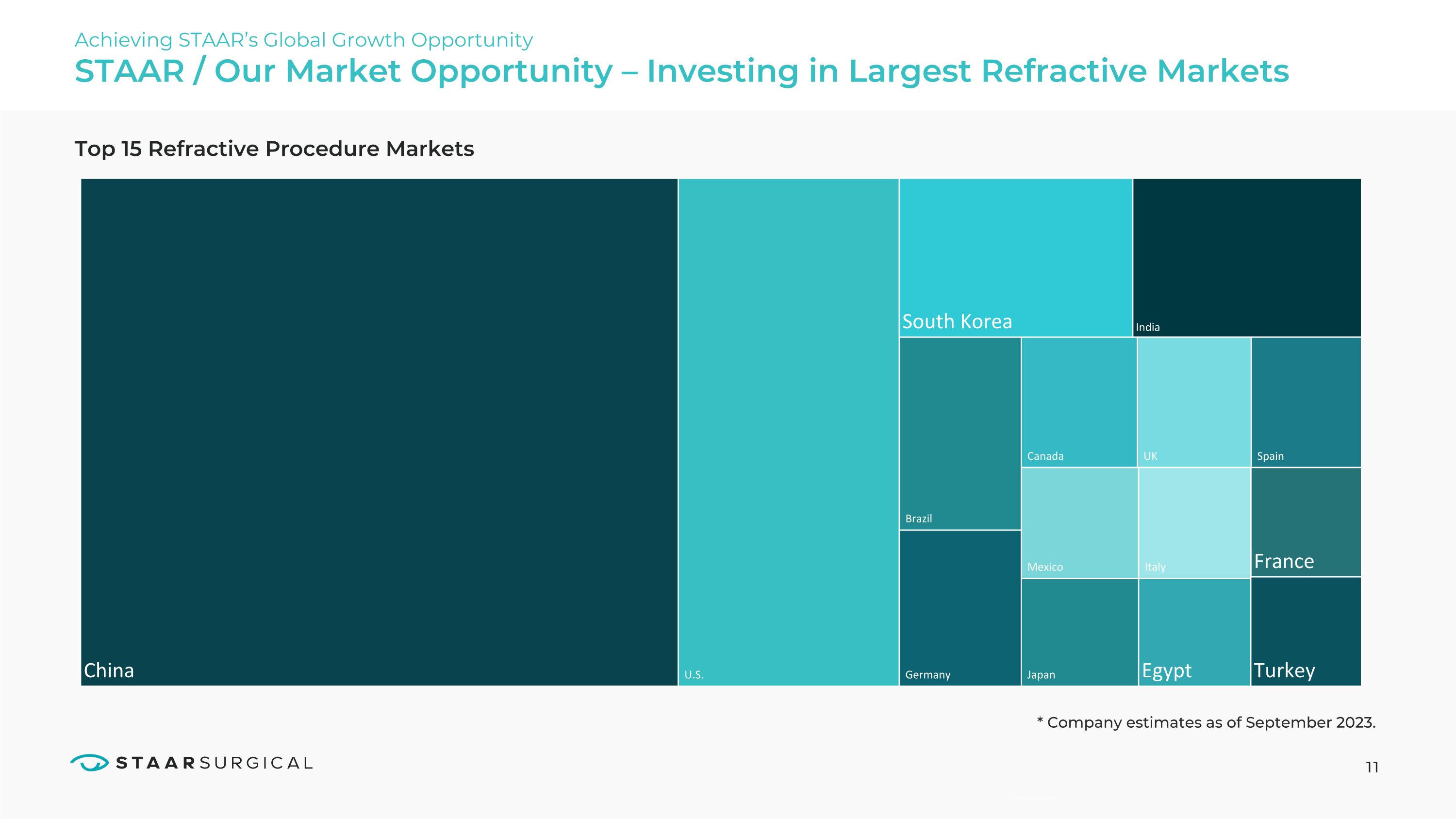
STAAR / Our Market Opportunity – Investing in Largest Refractive Markets Top 15 Refractive Procedure Markets Achieving STAAR’s Global Growth Opportunity 11 * Company estimates as of September 2023. U.S. South Korea Brazil Germany Canada Mexico Japan India UK Italy France Spain Egypt Turkey China
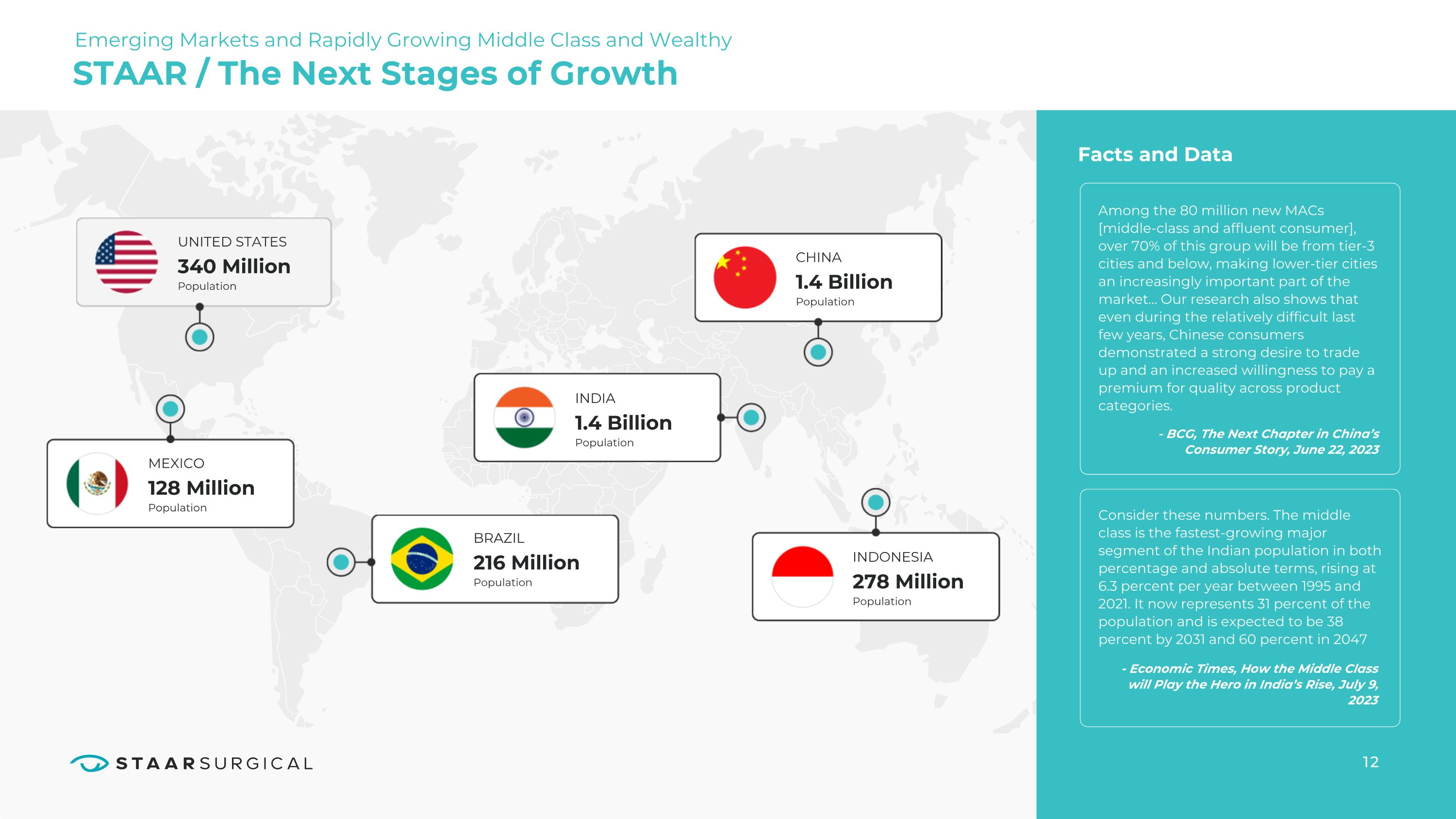
12 STAAR / The Next Stages of Growth UNITED STATES Population 340 Million MEXICO Population 128 Million INDONESIA Population 278 Million BRAZIL Population 216 Million Facts and Data Among the 80 million new MACs [middle-class and affluent consumer], over 70% of this group will be from tier-3 cities and below, making lower-tier cities an increasingly important part of the market… Our research also shows that even during the relatively difficult last few years, Chinese consumers demonstrated a strong desire to trade up and an increased willingness to pay a premium for quality across product categories. - BCG, The Next Chapter in China’s Consumer Story, June 22, 2023 Consider these numbers. The middle class is the fastest-growing major segment of the Indian population in both percentage and absolute terms, rising at 6.3 percent per year between 1995 and 2021. It now represents 31 percent of the population and is expected to be 38 percent by 2031 and 60 percent in 2047 - Economic Times, How the Middle Class will Play the Hero in India’s Rise, July 9, 2023 INDIA Population 1.4 Billion CHINA Population 1.4 Billion Emerging Markets and Rapidly Growing Middle Class and Wealthy
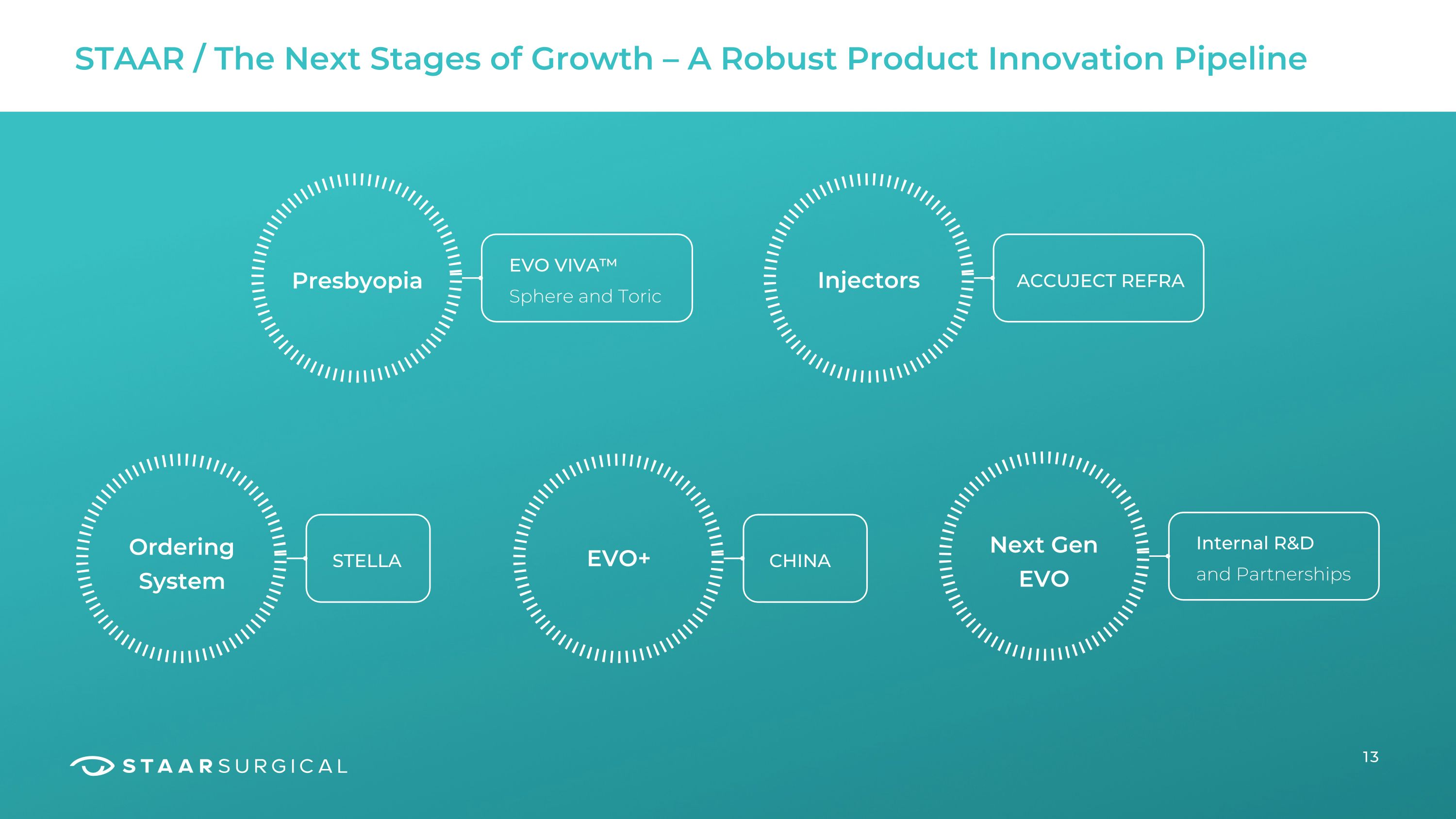
Presbyopia STAAR / The Next Stages of Growth – A Robust Product Innovation Pipeline EVO VIVA™ Sphere and Toric Injectors ACCUJECT REFRA Ordering System EVO+ Next Gen
EVO STELLA Internal R&D CHINA and Partnerships 13

How? STAAR / Vital Few Projects and Investments to Drive Growth 14 Evergreen and Refreshed as Data and Voice of Customer Dictates… Increase surgeon support and education Presbyopia (Viva™) New lens delivery devices globally (New Accuject/Global injector program) Simplify our ICL ordering process (New Stella ordering system) EVO+ in China (New lens family for China) US Growth (Targeting meaningful growth in 2H 2024) Faster production/Increased capacity to deliver our lenses to customers faster 2024 2023 2023 2024 2024 2024 2025 01 02 03 04 05 06 07
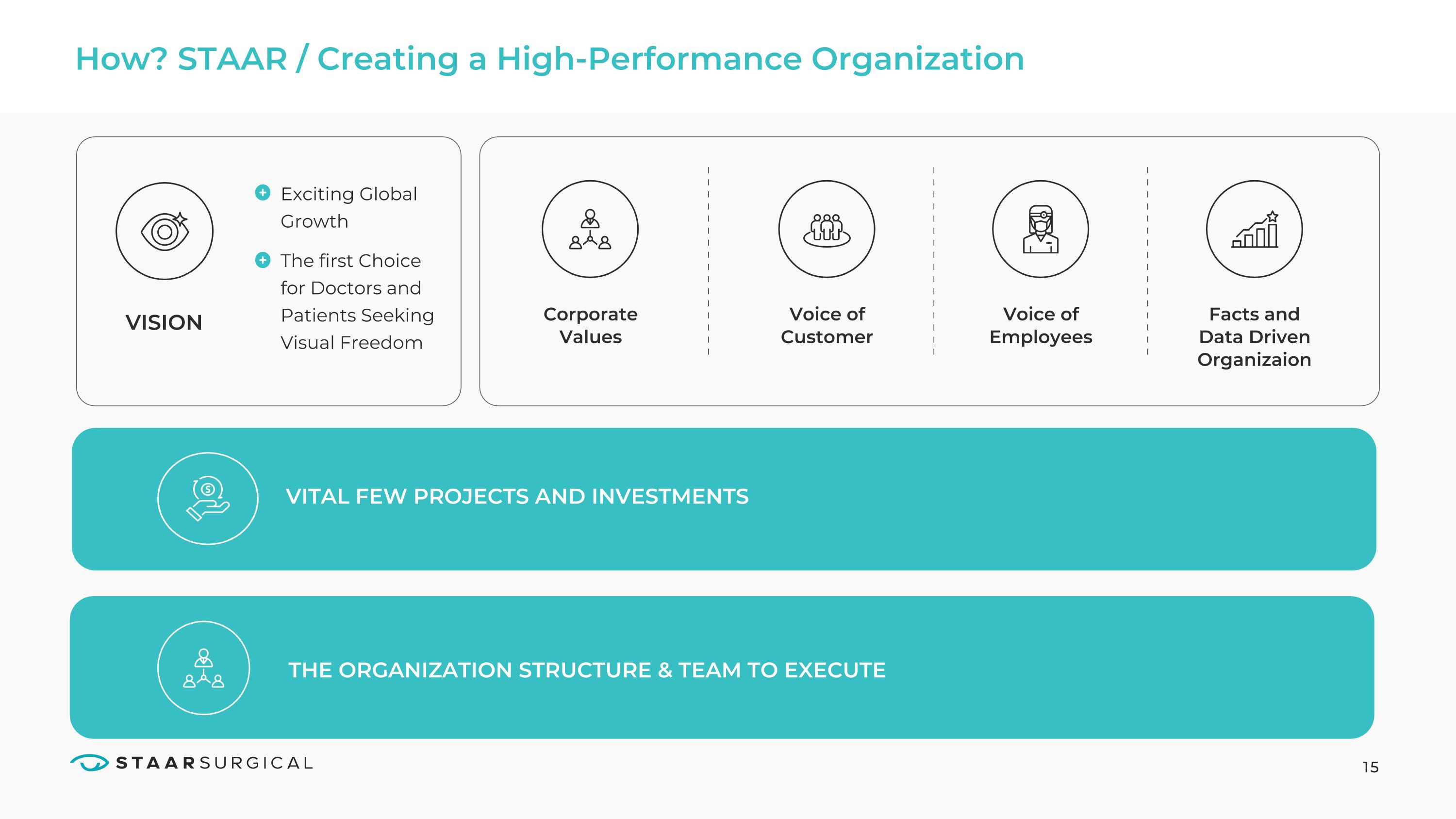
15 How? STAAR / Creating a High-Performance Organization VISION Voice of Customer Corporate Values Exciting Global Growth
The first Choice for Doctors and Patients Seeking Visual Freedom Voice of Employees Facts and Data Driven Organizaion VITAL FEW PROJECTS AND INVESTMENTS VITAL FEW PROJECTS AND INVESTMENTS THE ORGANIZATION STRUCTURE & TEAM TO EXECUTE

16 Why ICL? Milestone Sales Achievements STAAR expects to sell more ICLs in the next three years (2024-2026) than the first 25 years of ICL sales combined. TOTAL ICLs SOLD… ~ 20 YEARS ~ 3 YEARS < 2 YEARS VISION 2026 1999 - 2Q19 2Q22 1Q24E 4Q26E 1 M 2 M 3 M 6 M
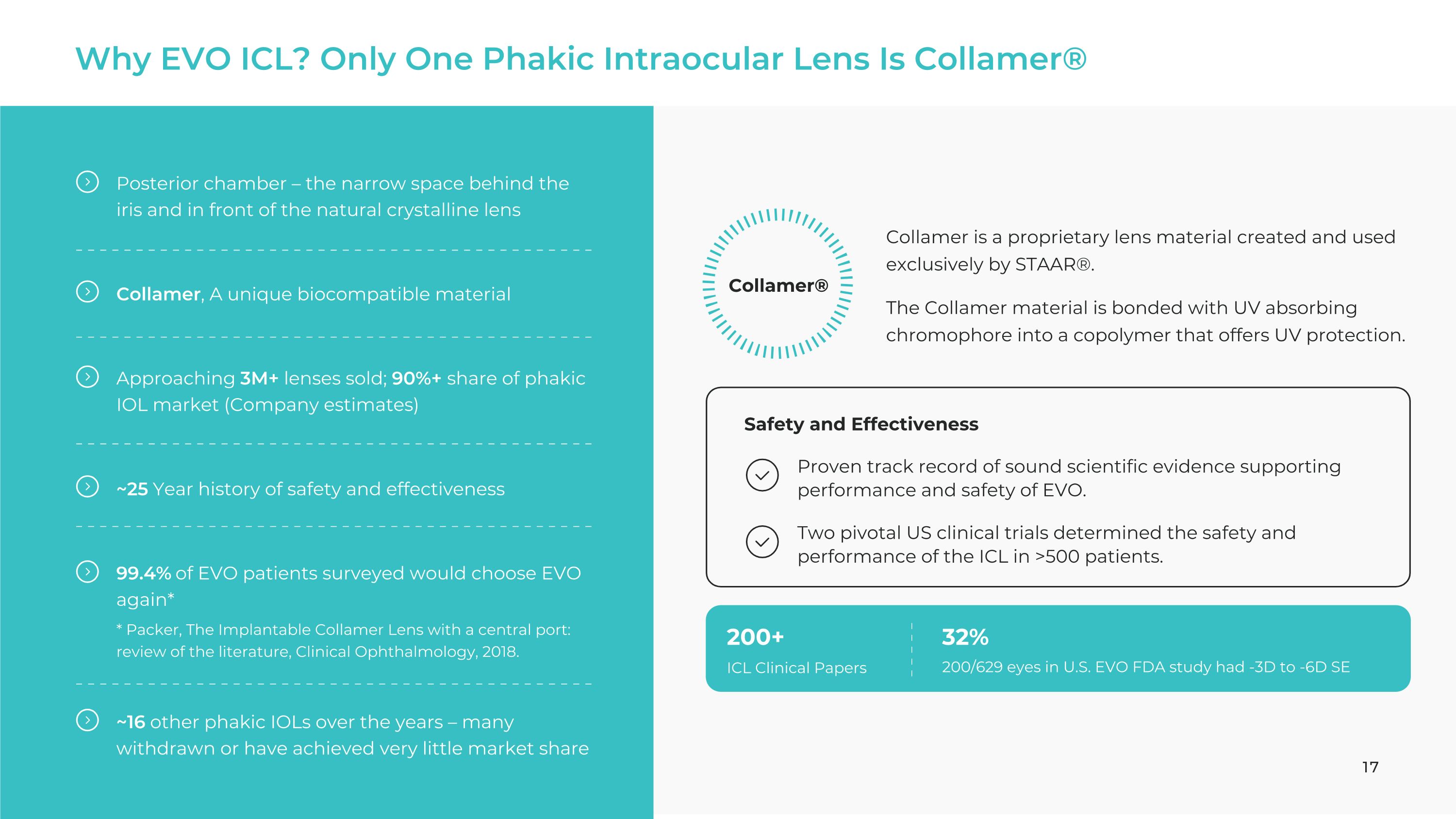
Why EVO ICL? Only One Phakic Intraocular Lens Is Collamer® Posterior chamber – the narrow space behind the iris and in front of the natural crystalline lens Collamer, A unique biocompatible material Approaching 3M+ lenses sold; 90%+ share of phakic IOL market (Company estimates) ~25 Year history of safety and effectiveness 99.4% of EVO patients surveyed would choose EVO again* * Packer, The Implantable Collamer Lens with a central port: review of the literature, Clinical Ophthalmology, 2018. ~16 other phakic IOLs over the years – many withdrawn or have achieved very little market share Collamer® Collamer is a proprietary lens material created and used exclusively by STAAR®.
The Collamer material is bonded with UV absorbing chromophore into a copolymer that offers UV protection. Proven track record of sound scientific evidence supporting performance and safety of EVO. Two pivotal US clinical trials determined the safety and performance of the ICL in >500 patients. 32% 200/629 eyes in U.S. EVO FDA study had -3D to -6D SE 200+ ICL Clinical Papers Safety and Effectiveness 17
Why EVO ICL? A Disruptive Technology 18 Sharp, clear vision No removal of corneal tissue Excellent vision at night Removable by a surgeon Does not cause dry eye syndrome Protection from UV rays Quick procedure and recovery Additive Bio-Compatible Quiet in the Eye
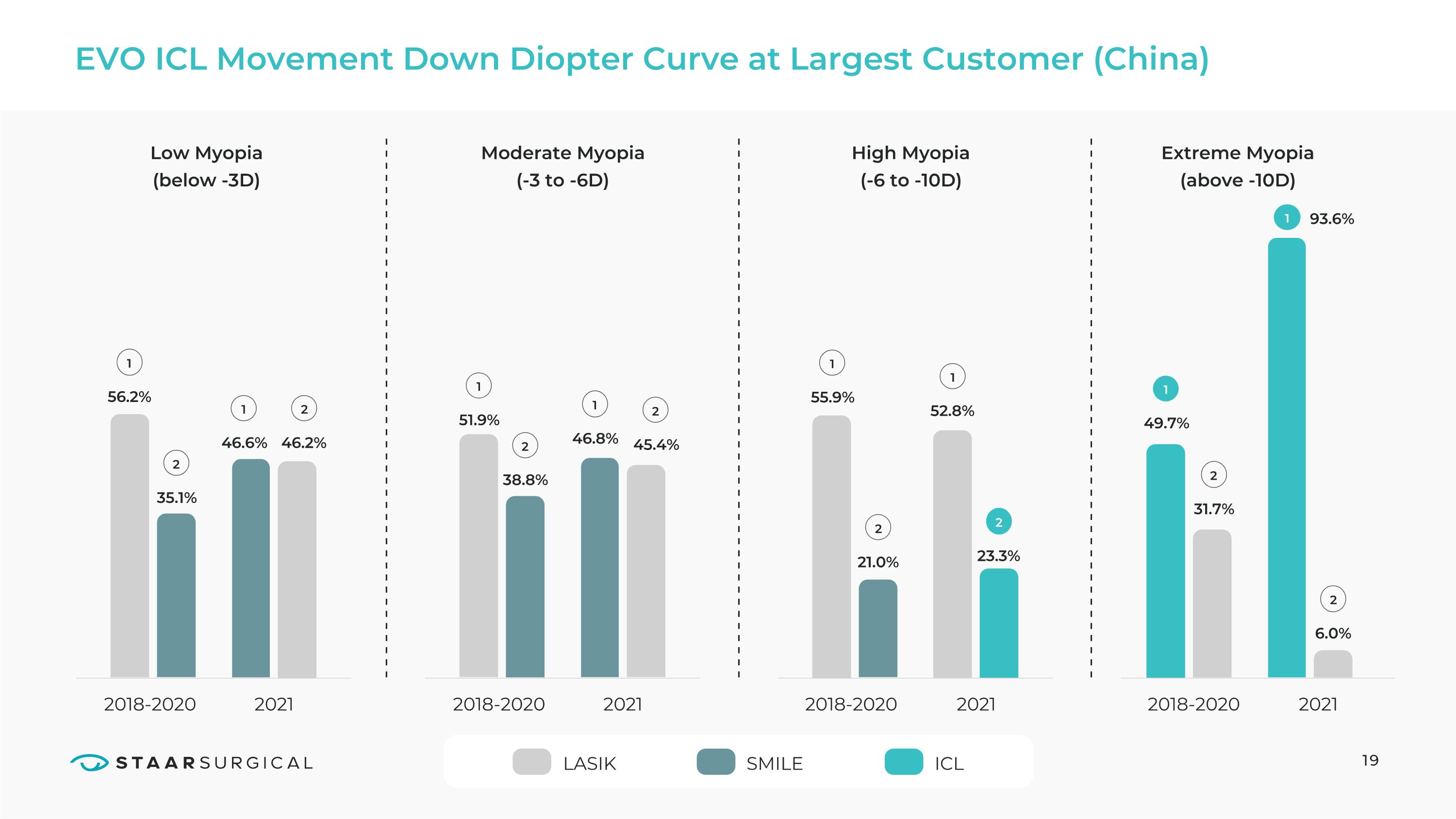
EVO ICL Movement Down Diopter Curve at Largest Customer (China) 2018-2020 Low Myopia
(below -3D) 46.6% 46.8% 55.9% 93.6% 52.8% 51.9% 56.2% 46.2% 35.1% 45.4% 21.0% 31.7% 23.3% 49.7% 6.0% 38.8% Moderate Myopia
(-3 to -6D) High Myopia
(-6 to -10D) Extreme Myopia
(above -10D) 2021 2018-2020 2021 2018-2020 2021 2018-2020 2021 1 1 1 1 1 SMILE 1 2 1 LASIK 2 2 2 2 1 2 2 ICL 2 19
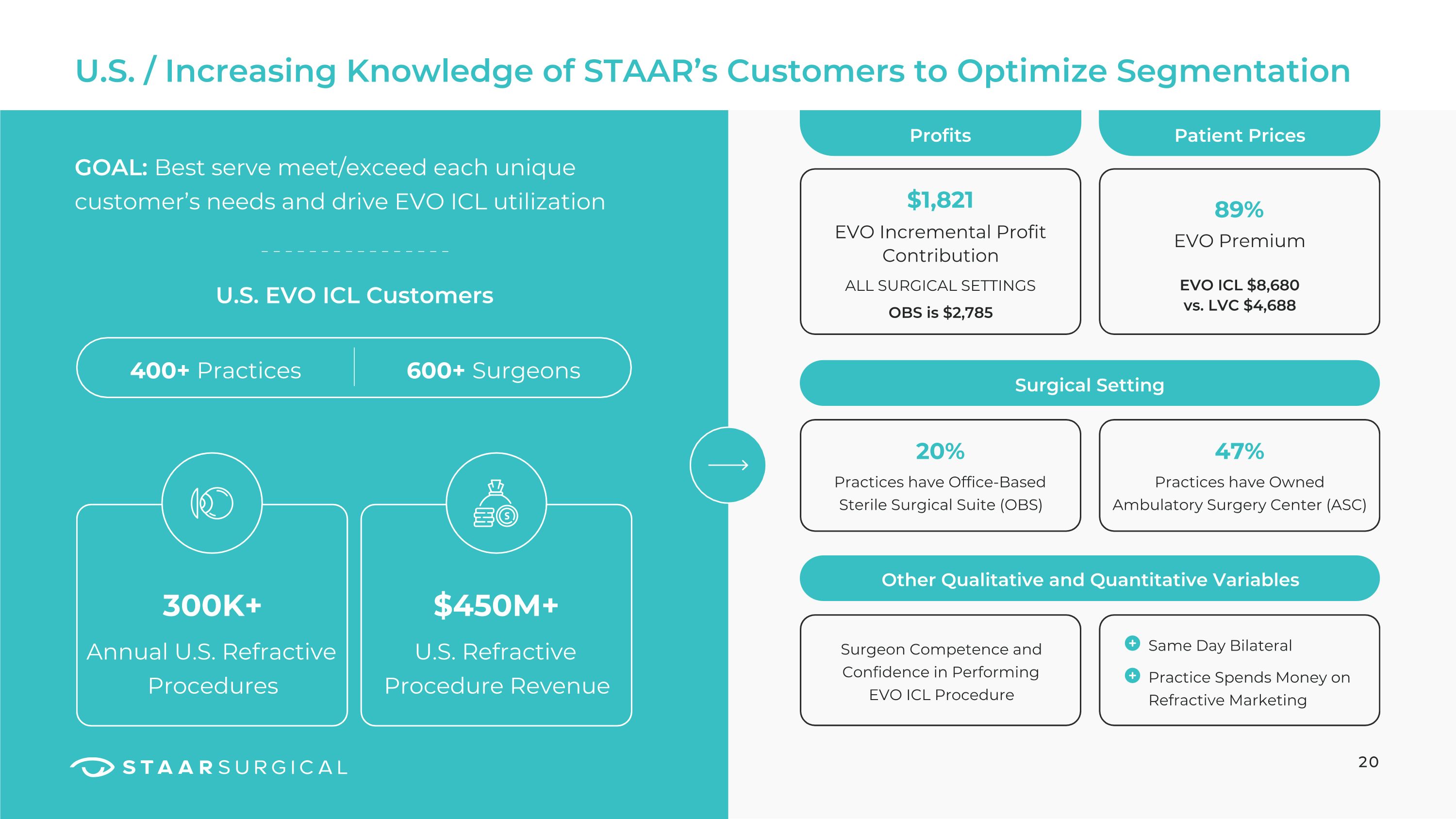
U.S. / Increasing Knowledge of STAAR’s Customers to Optimize Segmentation 20 GOAL: Best serve meet/exceed each unique customer’s needs and drive EVO ICL utilization 400+ Practices Annual U.S. Refractive Procedures U.S. Refractive Procedure Revenue 600+ Surgeons U.S. EVO ICL Customers 300K+ $450M+ Surgical Setting Profits Other Qualitative and Quantitative Variables $1,821 Patient Prices EVO Incremental Profit Contribution 47% Practices have Owned Ambulatory Surgery Center (ASC) ALL SURGICAL SETTINGS 20% Practices have Office-Based Sterile Surgical Suite (OBS) 89% EVO Premium EVO ICL $8,680 �vs. LVC $4,688 OBS is $2,785 Surgeon Competence and Confidence in Performing EVO ICL Procedure Same Day Bilateral
Practice Spends Money on Refractive Marketing
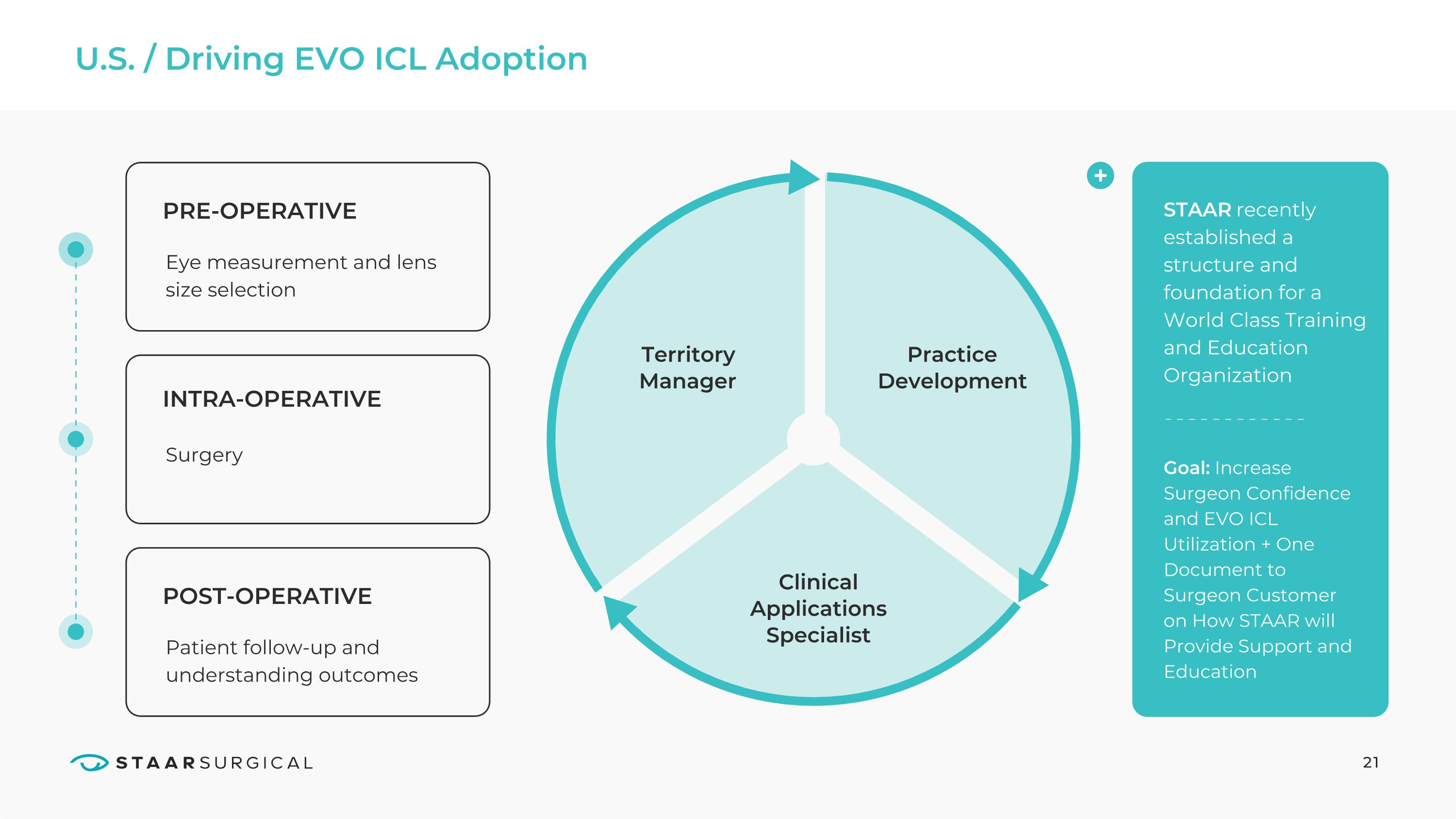
U.S. / Driving EVO ICL Adoption 21 PRE-OPERATIVE INTRA-OPERATIVE POST-OPERATIVE Eye measurement and lens size selection Surgery Patient follow-up and understanding outcomes Territory Manager Practice Development Clinical Applications Specialist STAAR recently established a structure and foundation for a World Class Training and Education Organization Goal: Increase Surgeon Confidence and EVO ICL Utilization + One Document to Surgeon Customer on How STAAR will Provide Support and Education
Joe Jonas
Campaign
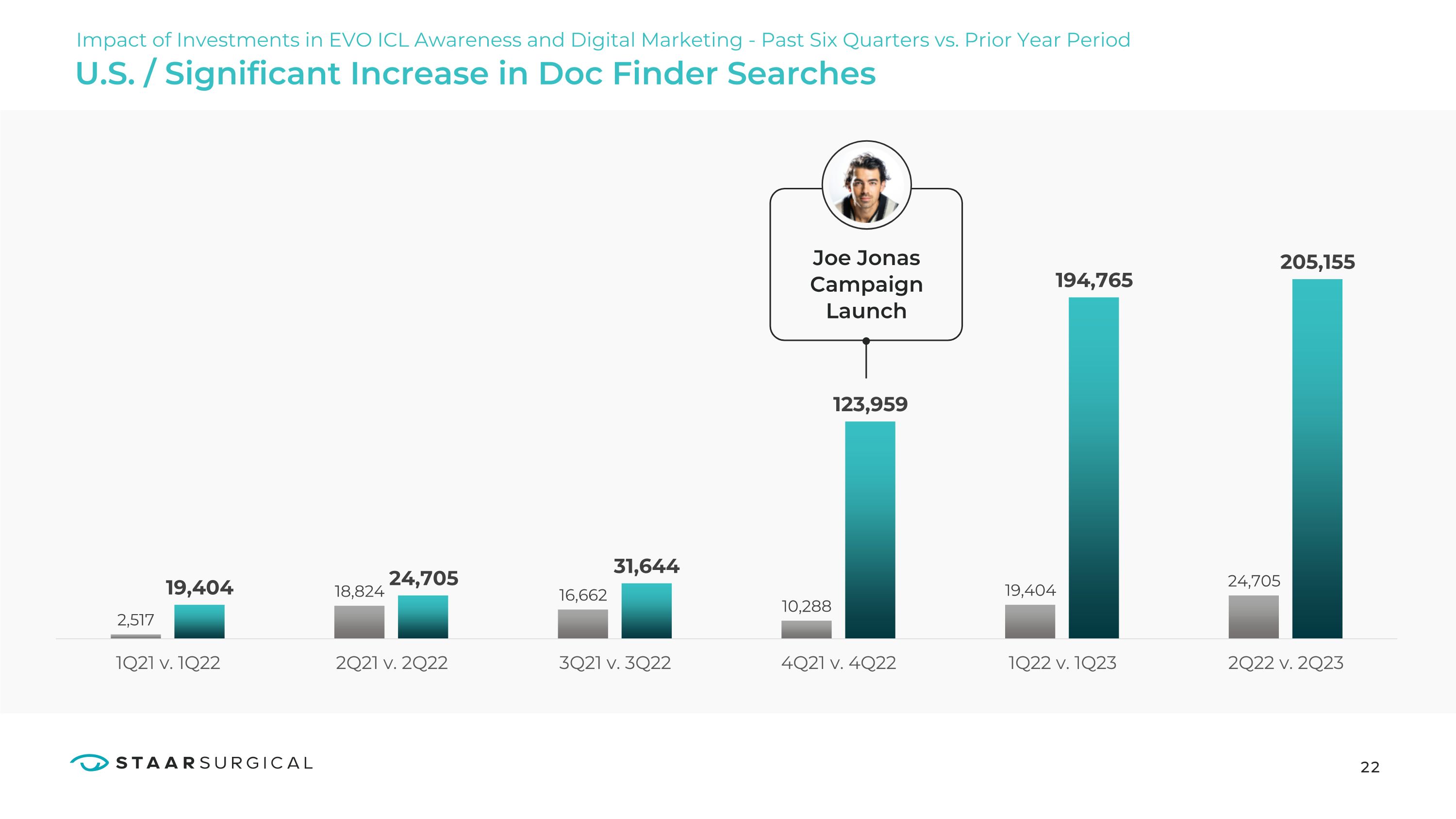
Launch U.S. / Significant Increase in Doc Finder Searches Impact of Investments in EVO ICL Awareness and Digital Marketing - Past Six Quarters vs. Prior Year Period 22 19,404 2.517 1Q21v.1Q22 18,824 24,705 2Q21v.2Q22 16,662 31,644 3Q21v.3Q22 10,288 123,959 4Q21v.4Q22 19,404 1Q22v.1Q23 194,765 24,705 2Q22v.2Q23 205,155

Our Goal / Happy EVO ICL Patients and Surgeons Globally! 23
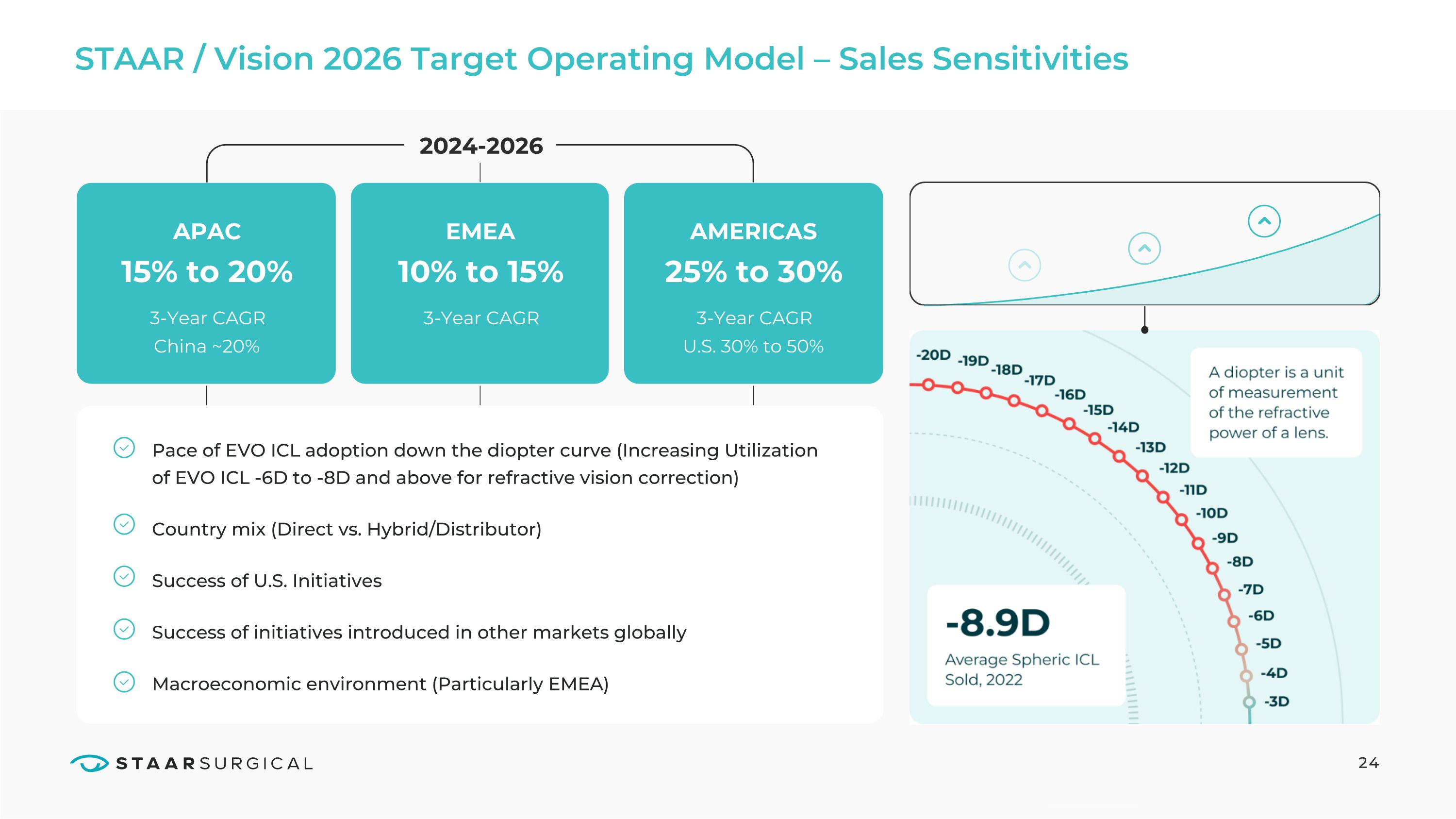
STAAR / Vision 2026 Target Operating Model – Sales Sensitivities 24 APAC 2024-2026 3-Year CAGR China ~20% 15% to 20% EMEA AMERICAS 3-Year CAGR 3-Year CAGR U.S. 30% to 50% 10% to 15% 25% to 30% Pace of EVO ICL adoption down the diopter curve (Increasing Utilization of EVO ICL -6D to -8D and above for refractive vision correction)
Country mix (Direct vs. Hybrid/Distributor)
Success of U.S. Initiatives
Success of initiatives introduced in other markets globally
Macroeconomic environment (Particularly EMEA) A diopter is a unit of measurement of the refractive power of a lens. -8.9D Average Spheric ICL Sold, 2022 -20D, -19D, -18D, -17D, -16D, -15D, -14D, -13D, -12D, -11D, -10D, -9D, -8D, -7D, -6D, -5D, -4D, -3D

SALES OPERATING MARGIN 15% 20% Approximately Approximately TO 12% 16% Approximately Approximately TO 3-Year Sales CAGR (2024-2026) GAAP OPERATING MARGIN (2024-2026) ANNUAL GROWTH RANGE Y/Y ANNUAL OPERATING MARGIN $500 TO $550 MILLION Approximately Fiscal 2026 Sales $60 TO $90 MILLION Approximately Fiscal 2026 Operating Income TARGET SALES AND OPERATING MODEL 25 STAAR / Vision 2026 Target Sales and Operating Model
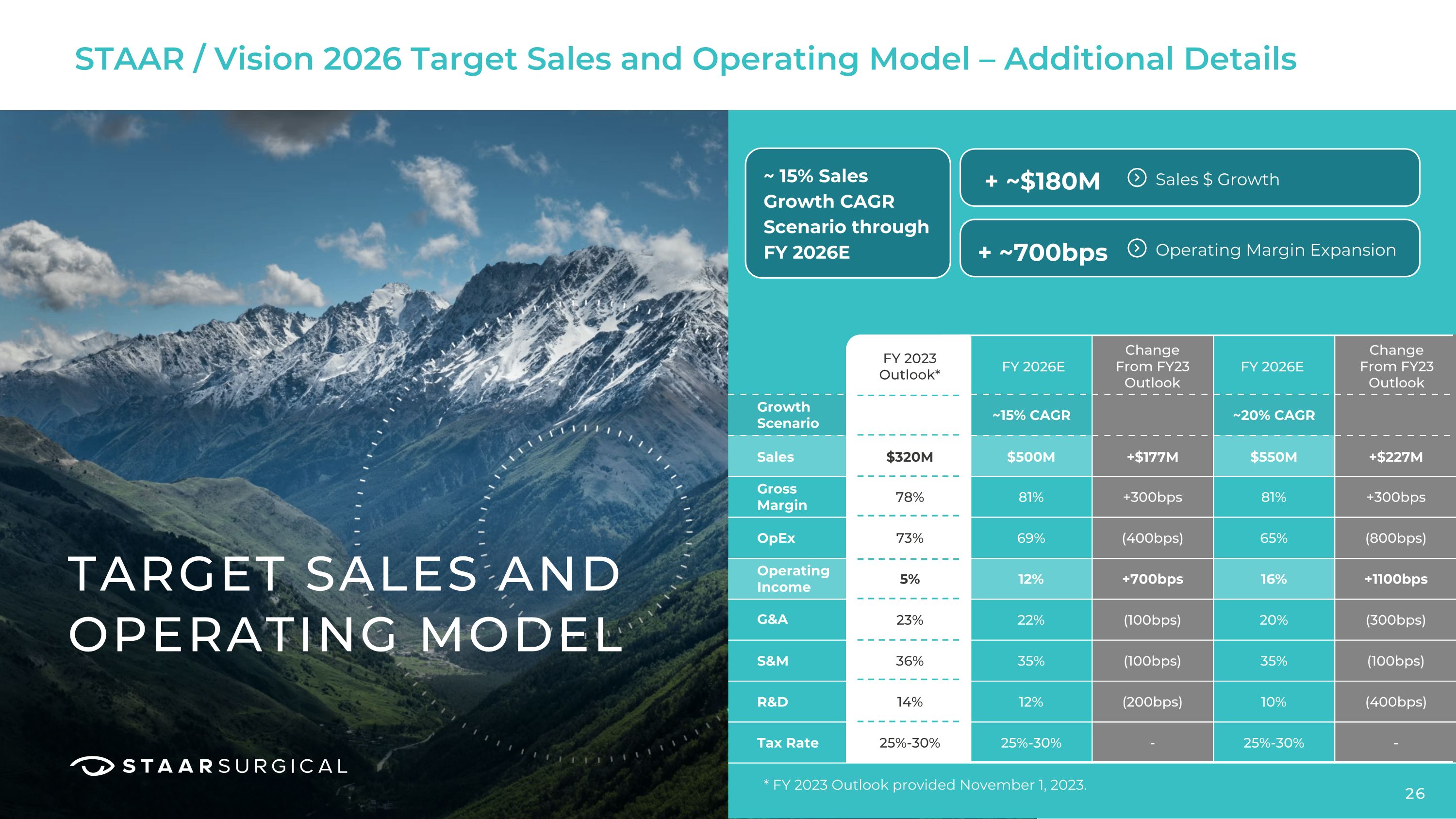
STAAR / Vision 2026 Target Sales and Operating Model – Additional Details TARGET SALES AND OPERATING MODEL FY 2023 Outlook* Growth Scenario ~15% CAGR ~20% CAGR Sales Gross Margin OpEx G&A S&M R&D Tax Rate Operating Income $320M $500M $550M +$177M +$227M 81% 81% 78% 69% 65% +300bps +300bps 12% 16% 73% 22% 20% (400bps) (800bps) 35% 35% 5% 12% 10% +700bps +1100bps 25%-30% 25%-30% 23% (100bps) (300bps) 36% (100bps) (100bps) 14% (200bps) (400bps) 25%-30% - - FY 2026E FY 2026E Change
From FY23 Outlook Change
From FY23 Outlook ~ 15% Sales Growth CAGR Scenario through FY 2026E + ~$180M + ~700bps Sales $ Growth Operating Margin Expansion 26 * FY 2023 Outlook provided November 1, 2023.
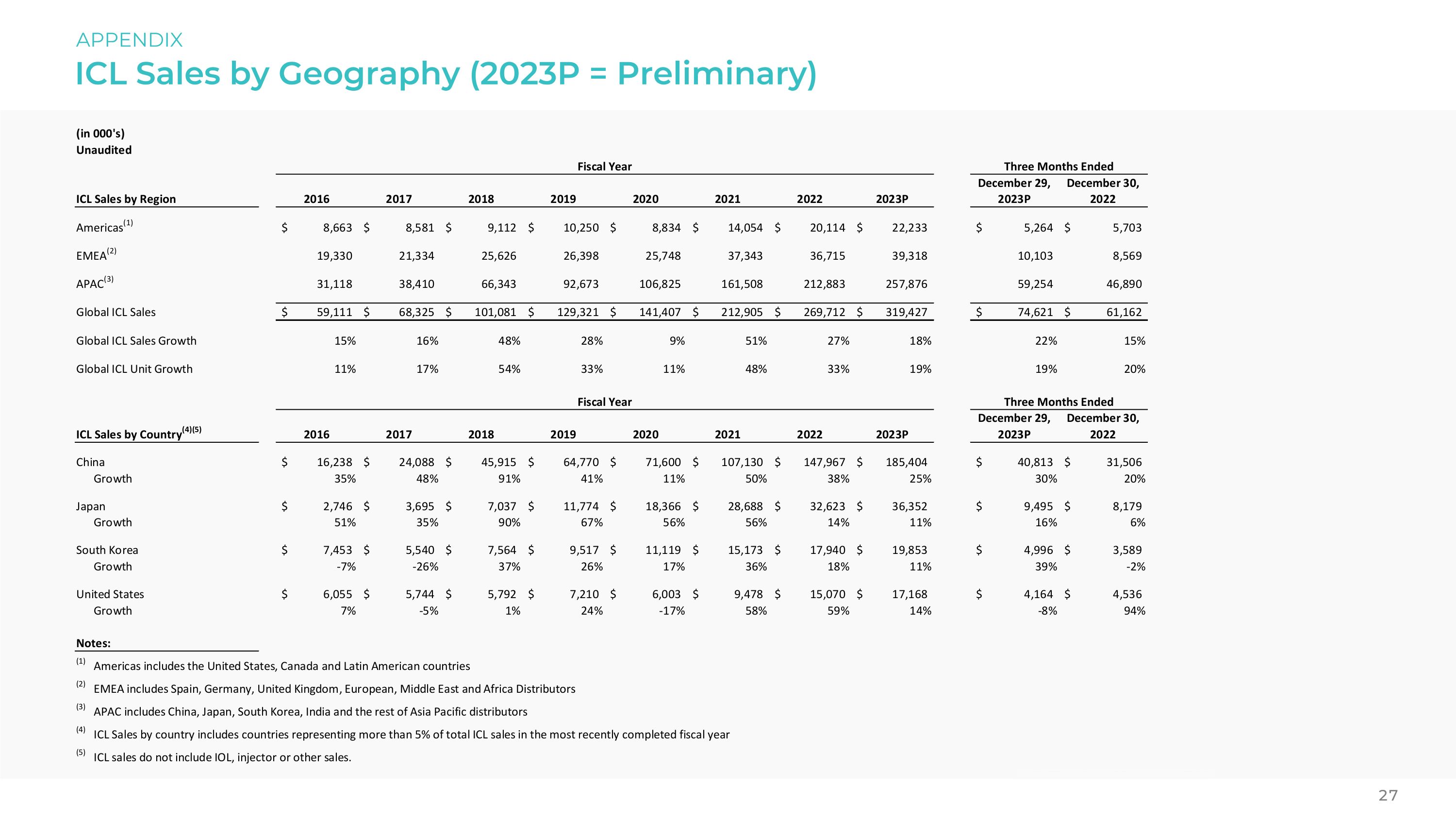
ICL Sales by Geography (2023P = Preliminary) APPENDIX 27 (in 000's) Unaudited Fiscal Year Three Months Ended ICL Sales by Region 2016 2017 2018 2019 2020 2021 2022 2023P December 29, 2023P December 29, 2022 Americas(1) $ 8,663 $ 8,581 $ 9,112 $ 10,250 $ 8,834 $ 14,054 $ 20,114 $ 22,233 $ 5,264 $ 5,703 EMEA(2) 19,330 21,334 25,626 26,398 25,748 37,343 36,715 39,318 10,103 8,569 APAC(3) 31,118 38,410 66,343 92,673 106,825 161,508 212,883 257,876 59,254 46,890 Global ICL Sales $ 59,111 $ 68,325 $ 101,081 $ 129,321 $ 141,407 $ 212,905 $ 269,712 $ 319,427 $ 74,621 $ 61,162 Global ICL Sales Growth 15% 16% 48% 28% 9% 51% 27% 18% 22% 15% Global ICL Unit Growth 11% 17% 54% 33% 11% 48% 33% 19% 19% 20% (in 000's) Unaudited Fiscal Year Three Months Ended ICL Sales by Country(4)(5) 2016 2017 2018 2019 2020 2021 2022 2023P December 29, 2023P December 29, 2022 China 16,238 24,088 45,915 64,770 71,600 107,130 147,967 185,404 40,813 31,506 Growth 35% 48% 91% 41% 11% 50% 38% 25% 30% 20% Japan 2,746 3,695 7,037 11,774 18,366 28,688 32,623 36,352 9,495 8,179 Growth 51% 35% 90% 67% 56% 56% 14% 11% 16% 6% South Lorea 7,453 5,540 7,564 9,517 11,119 15,173 17,940 19,853 4,996 3,589 Growth -7% -26% 37% 26% 17% 36% 18% 11% 39% -2% United States 6,055 5,744 5,792 7,210 6,003 9,478 15,070 17,168 4,164 4,536 Growth 7% -5% 1% 24% -17% 58% 59% 14% -8% 94% Notes: (1) Americas includes the United States, Canada and Latin American countries (2) EMEA includes Spain, Germany, United Kingdom, European, Middle East and Africa Distributors (3) APAC includes China, Japan, South Korea, India and the rest of Asia Pacific distributors (4) ICL Sales by country includes countries representing more than 5% of total ICL sales in the most recently completed fiscal year (5) ICL sales do not include IOL, injector or other sales.

IT’S OUR TIME… IT’S EVO’S TIME!
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
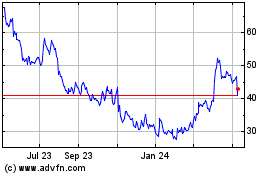
STAAR Surgical (NASDAQ:STAA)
Historical Stock Chart
From Mar 2024 to Apr 2024
STAAR Surgical (NASDAQ:STAA)
Historical Stock Chart
From Apr 2023 to Apr 2024