UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 13E-3
RULE 13e-3 TRANSACTION STATEMENT
PURSUANT TO SECTION 13(e)
OF THE SECURITIES EXCHANGE ACT OF 1934
(Amendment No. 1)
Pardes
Biosciences, Inc.
(Name of the Issuer)
Pardes
Biosciences, Inc.
(Name of Person(s) Filing Statement)
Common Stock, par value $0.0001 per share
(Title of Class of Securities)
69945Q 105
(CUSIP Number
of Common Stock)
Thomas G. Wiggans
Chief Executive Officer
Pardes Biosciences, Inc.
2173 Salk Avenue, Suite 250
PMB#052
Carlsbad,
California 92008
(415) 649-8758
(Name, Address and Telephone Number of Person Authorized to Receive Notices and Communications on Behalf of the Person(s) Filing Statement)
With copies to:
|
|
|
| Douglas N. Cogen, Esq.
Ethan A. Skerry, Esq. Ran
D. Ben-Tzur, Esq. Jennifer J. Hitchcock, Esq.
Michael S. Pilo, Esq.
Fenwick & West LLP
555 California Street, 12th Floor
San Francisco, California 94104
(415) 875-2300 |
|
Elizabeth H. Lacy
General Counsel and Corporate Secretary
Pardes Biosciences, Inc.
2173 Salk Avenue, Suite 250
PMB#052 Carlsbad,
California 92008 (415) 649-8758 |
This statement is filed in connection with (check the appropriate box):
|
|
|
|
|
| a. |
|
☐ |
|
The filing of solicitation materials or an information statement subject to Regulation 14A, Regulation 14C or Rule 13e-3(c) under the Securities Exchange Act of 1934. |
| b. |
|
☐ |
|
The filing of a registration statement under the Securities Act of 1933. |
| c. |
|
☒ |
|
A tender offer. |
| d. |
|
☐ |
|
None of the above. |
Check the following box if the soliciting materials or information statement referred to in checking box (a) are
preliminary copies: ☐
Check the following box if the filing is a final amendment reporting the results of the
transaction: ☐
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THIS
TRANSACTION, PASSED UPON THE MERITS OR FAIRNESS OF THIS TRANSACTION, OR PASSED UPON THE ADEQUACY OR ACCURACY OF THE DISCLOSURE IN THIS SCHEDULE 13E-3. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
INTRODUCTION
This Amendment No. 1 (“Amendment No. 1”), together with the attached exhibits (this
“Statement”) amends and restates the Rule 13e-3 Transaction Statement filed with the United States Securities and Exchange Commission (“SEC”) on
July 28, 2023 on Schedule 13E-3. This Statement is being filed by Pardes Biosciences, Inc. (“Pardes” or the “Company”) and relates to the offer
(the “Offer”) to purchase by MediPacific Sub, Inc., a Delaware corporation (“Purchaser”), and wholly owned subsidiary of MediPacific, Inc. (“Parent”), all of the issued and
outstanding shares of common stock, par value $0.0001 per share, of Pardes (the “Common Stock”) that is the subject of the Rule 13e-3 transaction described below (the
“Shares”) (other than (i) Shares held in the treasury of Pardes immediately prior to the effective time of the Merger (as defined below), (ii) Shares owned, directly or indirectly, by the Foresite Stockholders (as
defined in that certain Agreement and Plan of Merger, dated as of July 16, 2023, by and among Pardes, Purchaser and Parent (the “Merger Agreement”)), Parent, Purchaser or any other subsidiary of Parent at the
commencement of the Offer and that are owned by Parent, Purchaser or any other subsidiary of Parent immediately prior to the effective time of the Merger), for a price of (i) $2.02 per Share (the “Base Price Per Share”), (ii)
an additional amount of cash of up to $0.17 per Share (such amount as finally determined pursuant to Section 2.01(d) of the Merger Agreement, the “Additional Price Per Share,” and together with the Base Price Per
Share, the “Cash Amount”), and (iii) one non-transferable contingent value right per Share (each a “CVR”) (such amount, or any different amount per share
paid pursuant to the Offer (as defined below) to the extent permitted under the Merger Agreement, being the “CVR Amount”), and, together with the Cash Amount, the “Offer Price”), upon the terms and
subject to the conditions set forth in the Offer to Purchase dated July 28, 2023 (as amended and restated on August 17, 2023, and as may be further amended or supplemented from time to time, the “Offer to Purchase”),
which is annexed to and filed with this Statement as Exhibit (a)(1)(A) and in the related Letter of Transmittal, which is annexed to and filed with this Statement as Exhibit (a)(1)(B), which, together with any amendments or supplements thereto,
collectively constitute the “Offer.” The Offer is being made pursuant to the Merger Agreement. Pursuant to the terms of the Merger Agreement, the Company, Parent and Purchaser calculated the Additional Price Per Share on
August 17, 2023, based on the Company’s expected Closing Net Cash (as defined in the Merger Agreement) as of immediately prior to the Expiration Date of approximately $132.31 million and determined that the Additional Price Per Share will be
$0.11 Per Share. As a result, the total Cash Amount is $2.13 as determined in accordance with Section 2.01(d) of the Merger Agreement. The Merger Agreement provides, among other things, for the terms and conditions of the Offer and the
subsequent merger of Purchaser with and into Pardes (the “Merger”) in accordance with Section 251(h) of the Delaware General Corporation Law (the “DGCL”).
The information contained in the Tender Offer Statement filed under cover of Schedule TO by Parent with the SEC on July 28, 2023 (as amended and restated
on August 17, 2023, and as may be further amended or supplemented from time to time, the “Schedule TO”), including the Offer to Purchase, and the Solicitation/Recommendation Statement on Schedule 14D-9 filed by Pardes with the SEC on July 28, 2023 (as amended and restated on August 17, 2023, and as may be further amended or supplemented from time to time, the “Schedule 14D-9”), attached hereto as Exhibit (a)(1)(F) and is incorporated herein by reference and, except as described below, the responses to each item in this Statement are qualified in their entirety by the
information contained in the Schedule TO, the Offer to Purchase and the Schedule 14D-9. The cross references identified herein are being supplied pursuant to General Instruction G to Schedule 13E-3 and indicate the location in the Schedule TO and Schedule 14D-9 of the information required to be included in response to the respective Items of this
Statement.
Concurrently with the execution of the Merger Agreement, and as a condition and inducement to Pardes’ willingness to enter into the
Merger Agreement and the CVR Agreement (as defined below), Foresite Capital Opportunity Fund V, L.P., Foresite Capital Fund V, L.P. and FS Development Holdings II, LLC (collectively, the “Guarantors”), affiliates of Parent,
have duly executed and delivered to Pardes a limited guaranty (the “Limited Guaranty”), dated as of the date of the Merger Agreement, in favor of Pardes and the holders of CVRs, in respect of certain of Parent’s and
Purchaser’s obligations arising under, or in connection with, the Merger Agreement and the Contingent Value Rights Agreement (the “CVR Agreement”) that Pardes expects to enter into with a rights agent and a
representative, agent and attorney in-fact of the holders of the CVR. The Guarantors’
obligations under the Limited Guaranty are subject to a cap of $7.5 million with respect to obligations
to Pardes arising under or in connection with the Merger Agreement and $400,000 with respect to obligations to the holders of the CVRs arising under or in connection with the CVR Agreement.
Any information contained in the documents incorporated herein by reference shall be deemed modified or superseded for purposes of this Statement to the
extent that any information contained herein modifies or supersedes such information. All information contained in this Statement concerning Purchaser, Parent or their affiliates has been provided by such person and not by any other person.
| ITEM 1. |
SUMMARY TERM SHEET |
The information set forth in the section of the Offer to Purchase entitled “Summary Term Sheet” is incorporated herein by reference.
| ITEM 2. |
SUBJECT COMPANY INFORMATION |
The information set forth in the Schedule 14D-9 under the heading “Item 1. Subject Company
Information—Name and Address” is incorporated herein by reference.
The information set forth in the Schedule 14D-9 under the heading “Item 1. Subject Company
Information—Securities” is incorporated herein by reference.
| |
(c) |
Trading Market and Price |
The information set forth in the Offer to Purchase under the heading “Special Factors—Section 5. Price Range of Shares;
Dividends” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings “Special Factors—Section 5. Price Range of
Shares; Dividends” and “The Tender Offer—Section 10. Dividends and Distributions” is incorporated herein by reference.
| |
(e) |
Prior Public Offerings |
Not applicable.
| |
(f) |
Prior Stock Purchases |
The information set forth in the Schedule 14D-9 under the heading “Special Factors—Past Contacts,
Transactions, Negotiations and Agreements—Arrangements with Parent and Purchaser and Their Affiliates” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the heading “The Tender Offer—Section 6. Certain Information
Concerning Parent and Purchaser” is incorporated herein by reference.
| ITEM 3. |
IDENTITY AND BACKGROUND OF FILING PERSON |
The filing person and subject company is Pardes Biosciences, Inc. The business address of Pardes is 2173 Salk Avenue, Suite 250, PMB #052, Carlsbad, California
92008, and Pardes’ telephone number is (415)-649-8758.
The
information set forth in the Schedule 14D-9 under the headings “Special Factors—Purposes, Alternatives, Reasons and Effects—Identity and Background of Filing Person,” “Item
1. Subject Company Information” and “Annex 1. Business and Background of the Company’s Directors and Executive Officers” is incorporated herein by reference.
The information in the Offer to Purchase under the headings “The Tender Offer–Section 6. Certain Information Concerning Parent
and Purchaser” and “Schedule A–Information Concerning Members of the Boards of Directors and the Executive Officers of Purchaser, Parent and the Guarantors” is incorporated herein by reference.
| |
(b) |
Business and Background of Entities |
The information set forth in the Schedule 14D-9 under the headings “Special Factors–Purposes,
Alternatives, Reasons and Effects–Identity and Background of Filing Person–Business and Background of the Company’s Directors and Executive Officers,” “Item 1. Subject Company Information” and “Annex
1. Business and Background of the Company’s Directors and Executive Officers” is incorporated herein by reference.
The information set
forth in the Offer to Purchase under the headings “The Tender Offer–Section 6. Certain Information Concerning Parent and Purchaser” and “Schedule A–Information Concerning Members of the Boards of
Directors and the Executive Officers of Purchaser, Parent and the Guarantors” is incorporated herein by reference.
| |
(c) |
Business and Background of Natural Persons |
The information set forth in the Schedule 14D-9 under the headings “Special Factors–Purposes,
Alternatives, Reasons and Effects–Identity and Background of Filing Person–Business and Background of the Company’s Directors and Executive Officers,” “Item 1–Subject Company Information”
and “Annex 1. Business and Background of the Company’s Directors and Executive Officers” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings “The Tender Offer–Section 6. Certain Information
Concerning Parent and Purchaser” and “Schedule A–Information Concerning Members of the Boards of Directors and the Executive Officers of Purchaser, Parent and the Guarantors” is incorporated herein by reference.
| ITEM 4. |
TERMS OF THE TRANSACTION |
The information set forth in the Schedule 14D-9 under the headings “Special Factors–Purposes,
Alternatives, Reasons and Effects–Identity and Background of Filing Person–Tender Offer and Merger,” “Special Factors–Past Contacts, Transactions, Negotiations and Agreements,”
“Special Factors–The Solicitation or Recommendation,” “Special Factors–Purposes of the Transaction and Plans or Proposals,” “Special Factors–Additional Information–Named Executive
Officer Golden Parachute Compensation; Appraisal Rights” and “Item 1. Subject Company Information–Securities” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings “Summary Term Sheet,” “Special Factors—
Section 2. Purpose of the Offer and Plans for Pardes,” “Special Factors—Section 7. Certain U.S. Federal Income Tax Consequences of the Offer and the Merger,” “The
Tender Offer–Section 1. Terms of the Offer,” “The Tender Offer–Section 2. Acceptance for Payment and Payment for Shares,” “The Tender
Offer—Section 3. Procedures for Tendering Shares,” “The Tender Offer—Section 4. Withdrawal Rights,” and “The Tender Offer—Section 7. Summary of the Merger
Agreement and Certain Other Agreements” is incorporated herein by reference.
The information set forth in the Schedule 14D-9 under the headings “Special Factors—Purposes,
Alternatives, Reasons and Effects–Identity and Background of Filing Person–Tender Offer and Merger, “Special Factors—Past Contacts, Transactions, Negotiations and Agreements and “Special Factors—Additional
Information” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings “Summary Term
Sheet,” “Special Factors—Section 1. Background of the Offer; Contacts with Pardes,” “Special Factors—Section 2. Purpose of the Offer and Plans for Pardes,”
“The Tender Offer—Section 6. Certain Information Concerning Parent and Purchaser,” “The Tender Offer—Section 7. Summary of the Merger Agreement and Certain Other
Agreements” and “The Tender Offer–Section 13. Interests of Certain Pardes Directors and Executive Officers in the Offer and the Merger” is incorporated herein by reference.
The information set forth in the Schedule 14D-9 under the heading “Special Factors–Additional
Information—Appraisal Rights” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the heading
“The Tender Offer—Section 11. Certain Legal Matters; Regulatory Approvals—Takeover Laws; Appraisal Rights” is incorporated herein by reference.
| |
(e) |
Provisions for Unaffiliated Security Holders |
The filing person has not made any provision in connection with the transaction to grant unaffiliated security holders access to the corporate files of the
filing person or to obtain counsel or appraisal services at the expense of the filing person.
| |
(f) |
Eligibility for Listing or Trading |
Not applicable.
| ITEM 5. |
PAST CONTACTS, TRANSACTIONS, NEGOTIATIONS AND AGREEMENTS |
The information set forth in the Schedule 14D-9 under the headings “Special Factors—Purposes
Alternatives, Reasons and Effects–Identity and Background of Filing Person—Tender Offer and Merger” and “Special Factors—Past Contacts, Transactions, Negotiations and Agreements” is
incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings “Summary Term Sheet,”
“Special Factors—Section 1. Background of the Offer; Contacts with Pardes” “The Tender Offer—Section 6. Certain Information Concerning Parent and Purchaser,” and
“The Tender Offer—Section 13. Interests of Certain Pardes Directors and Executive Officers in the Offer and the Merger” is incorporated herein by reference.
| |
(b)-(c) |
Significant Corporate Events; Negotiations or Contacts |
The information set forth in the Schedule 14D-9 under the headings “Special Factors—Purposes,
Alternatives, Reasons and Effects–Identity and Background of Filing Person—Tender Offer and Merger,” “Special Factors— Past Contacts, Transactions, Negotiations and Agreements” and “Special
Factors—The Solicitation or Recommendation” is incorporated herein by reference.
The information set forth in the Offer to Purchase under
the headings “Summary Term Sheet,” “Special Factors—Section 1. Background of the Offer; Contacts with Pardes,” “Special Factors—Section 2. Purpose of the Offer
and Plans for Pardes,” “The Tender Offer—Section 6. Certain Information Concerning Parent and Purchaser,” “The Tender Offer—Section 7. Summary of the Merger
Agreement and Certain Other Agreements” and “The Tender Offer—Section 13. Interests of Certain Pardes Directors and Executive Officers in the Offer and the Merger” is incorporated herein by
reference.
| |
(e) |
Agreements Involving the Subject Company’s Securities |
The information set forth in the Schedule 14D-9 under the headings “Special Factors—Purposes,
Alternatives, Reasons and Effects—Identity and Background of Filing Person–Tender Offer and Merger,” “Special Factors— Past Contacts, Transactions, Negotiations and Agreements” and “Special
Factors—The Solicitation or Recommendation” is incorporated herein by reference.
The information set forth in the Offer to Purchase under
the headings “Summary Term Sheet,” “Special Factors— Section 1. Background of the Offer; Contacts with Pardes,” “Special Factors—Section 2. Purpose of the
Offer and Plans for Pardes,” “The Tender Offer— Section 6. Certain Information Concerning Parent and Purchaser” and “The Tender Offer—Section 7. Summary of the Merger
Agreement and Certain Other Agreements” is incorporated herein by reference.
| ITEM 6. |
PURPOSES OF THE TRANSACTION AND PLANS OR PROPOSALS |
| |
(b) |
Use of Securities Acquired |
The information set forth in the Offer to Purchase under the headings “Summary Term Sheet,” “Special
Factors–Section 6. Possible Effects of the Offer on the Market for the Shares; Nasdaq Listing; Exchange Act Registration and Margin Regulations,” “Special
Factors–Section 2. Purpose of the Offer and Plans for Pardes,” “The Tender Offer–Section 7. Summary of the Merger Agreement and Certain Other Agreements” and “The
Tender Offer–Section 11. Certain Legal Matters; Regulatory Matters–Appraisal Rights” is incorporated herein by reference.
The information set forth in the Schedule 14D-9 under the headings “Special
Factors—Purposes, Alternatives, Reasons and Effects—Identity and Background of Filing Person–Tender Offer and Merger,” “Special Factors—Past Contacts, Transactions, Negotiations and
Agreements,” “Special Factors—The Solicitation or Recommendation” and “Special Factors—Purposes of the Transaction and Plans or Proposals” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings “Summary Term Sheet,” “Special
Factors—Section 1. Background of the Offer; Contacts with Pardes,” “Special Factors—Section 2. Purpose of the Offer and Plans for Pardes,” “Special
Factors—Section 6. Possible Effects of the Offer on the Market for the Shares; Nasdaq Listing; Exchange Act Registration and Margin Regulations,” “The Tender Offer—Section 7. Summary
of the Merger Agreement and Certain Other Agreements,” “The Tender Offer—Section 8. Source and Amount of Funds” and “The Tender Offer—Section 13. Interests of Certain
Pardes Directors and Executive Officers in the Offer and the Merger” is incorporated herein by reference.
| ITEM 7. |
PURPOSES, ALTERNATIVES, REASONS AND EFFECTS |
The information set forth in the Schedule 14D-9 under the headings “Special Factors—Purposes,
Alternatives, Reasons and Effects—Identity and Background of Filing Person—Tender Offer and Merger,” “Special Factors— Past Contacts, Transactions, Negotiations and Agreements—Arrangements with Parent and
Purchaser and Their Affiliates,” “Special Factors—The Solicitation or Recommendation—Reasons for the Recommendation; Fairness of the Offer and Merger” and “Special Factors—Purposes of the Transaction
and Plans or Proposals” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings
“Summary Term Sheet,” “Special Factors—Section 2. Purpose of the Offer and Plans for Pardes,” “Special Factors—Section 6. Possible of Effects of the Offer on the Market for the
Shares; Nasdaq Listing; Exchange Act Registration and Margin Regulations,” “The Tender Offer–Section 6. Certain Information Concerning Parent and Purchaser” and “The Tender
Offer—Section 7. Summary of the Merger Agreement and Certain Other Agreements” is incorporated herein by reference.
The information set forth in the Schedule 14D-9 under the headings “Special Factors—The Solicitation or
Recommendation—Background of the Offer and the Merger; Reasons for the Recommendation; Fairness of the Offer and Merger” and “Special Factors—Purposes of the Transaction and Plans or Proposals” is incorporated
herein by reference.
The information set forth in the Offer to Purchase under the headings “Special Factors—Section 1.
Background of the Offer; Contacts with Pardes,” “Special Factors—Section 2. Purpose of the Offer and Plans for Pardes” and “The Tender Offer—Section 7. Summary
of the Merger Agreement and Certain Other Agreements” is incorporated herein by reference.
The information set forth in the Schedule 14D-9 under the heading “Special Factors—The Solicitation or
Recommendation—Background of the Offer and the Merger; Reasons for the Recommendation; Fairness of the Offer and Merger” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings “Summary Term Sheet,” “Special
Factors—Section 1. Background of the Offer; Contacts with Pardes,” “Special Factors—Section 2. Purpose of the Offer and Plans for Pardes,” “Special
Factors—Section 3. Position of Parent and Purchaser Regarding Fairness of the Offer and the Merger” and “The Tender Offer—Section 7. Summary of the Merger Agreement and Certain Other
Agreements” is incorporated herein by reference.
The information set forth in the Schedule 14D-9 under the headings “Special Factors—Purposes,
Alternatives, Reasons and Effects–Identity and Background of Filing Person–Tender Offer and Merger,” “Special Factors— Past Contacts, Transactions, Negotiations and Agreements,” “Special
Factors—The Solicitation or Recommendation,” “Special Factors— Persons/Assets Retained, Employed, Compensated or Used,” “Special Factors— Purposes of the Transaction and Plans or
Proposals” and “Special Factors—Additional Information” is incorporated herein by reference.
The information set forth in
the Offer to Purchase under the headings “Summary Term Sheet,” “Special Factors—Section 2. Purpose of the Offer and Plans for Pardes,” “Special
Factors—Section 6. Possible Effects of the Offer on the Market for the Shares; Nasdaq Listing; Exchange Act Registration and Margin Regulations,” “Special Factors–Section 7. Certain
U.S. Federal Income Tax Consequences of the Offer and the Merger,” “The Tender Offer— Section 1. Terms of the Offer,” “The Tender Offer—Section 7. Summary of
the Merger Agreement and Certain Other Agreements,” “The Tender Offer—Section 8. Source and Amount of Funds,” “The Tender Offer—Section 10. Dividends and
Distributions,” “The Tender Offer—Section 11. Certain Legal Matters; Regulatory Approvals—Appraisal Rights,” “The Tender Offer—Section 12. Fees and
Expenses,” and “The Tender Offer—Section 13. Interests of Certain Pardes Directors and Executive Officers in the Offer and the Merger” is incorporated herein by reference.
| ITEM 8. |
FAIRNESS OF THE TRANSACTION |
The information set forth in the Schedule 14D-9 under the headings “Special Factors—The Solicitation or
Recommendation,” “Special Factors–Persons/Assets Retained, Employed, Compensated or Used” and “Special Factors—Additional Information” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings “Special Factors—Section 1. Background of the Offer;
Contacts with Pardes,” “Special Factors—Section 2. Purpose of the Offer and Plans for Pardes,” “Special Factors—Section 3. Position of Parent and Purchaser Regarding
Fairness of the Offer and the Merger” and “The Tender Offer—Section 7. Summary of the Merger Agreement and Certain Other Agreements” is incorporated herein by reference.
| |
(b) |
Factors Considered in Determining Fairness |
The information set forth in the Schedule 14D-9 under the headings “Special Factors—The Solicitation or
Recommendation,” “Special Factors— Persons/Assets Retained, Employed, Compensated or Used,” “Special Factors—Additional Information” and “Annex 2: Opinion of Financial
Advisor” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings “Special
Factors—Section 1. Background of the Offer; Contacts with Pardes,” “Special Factors—2. Purpose of the Offer and Plans for Pardes,” “Special
Factors—Section 3. Position of Parent and Purchaser Regarding Fairness of the Offer and the Merger” and “The Tender
Offer—Section 7. Summary of the Merger Agreement and Certain Other Agreements” is incorporated herein by reference.
The
information set forth in Exhibits (c)(1)-(c)(9) is incorporated herein by reference.
| |
(c) |
Approval of Security Holders |
The information set forth in the Schedule 14D-9 under the headings “Special Factors—Purposes,
Alternatives, Reasons and Effects—Identity and Background of Filing Person–Tender Offer and Merger” and “Special Factors—Additional Information—Stockholder Approval of the Merger Not
Required” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings “Summary Term
Sheet,” “Special Factors—Section 2. Purpose of the Offer and Plans for Pardes” and “The Tender Offer—Section 7. Summary of the Merger Agreement and
Certain Other Agreements” is incorporated herein by reference.
| |
(d) |
Unaffiliated Representative |
The information set forth in the Schedule 14D-9 under the heading “Special Factors—The Solicitation or
Recommendation” is incorporated herein by reference.
No unaffiliated representative has been retained by a majority of directors who are not
employees of the subject company to act solely on behalf of unaffiliated security holders for purposes of negotiating the terms of this transaction and/or preparing a report concerning the fairness of the transaction.
| |
(e) |
Approval of Directors |
The information set forth in the Schedule 14D-9 under the heading “Special Factors—The Solicitation or
Recommendation” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings “Special
Factors—Section 2. Purpose of the Offer and Plans for Pardes,” and “The Tender Offer—Section 7. Summary of the Merger Agreement and Certain Other Agreements; Summary of the
Merger Agreement and Certain Other Agreements—Pardes Board Recommendation and Special Committee Recommendation” is incorporated herein by reference.
The Merger Agreement and Offer were approved by a majority of the directors of Pardes who are not employees of Pardes.
The information set forth in the Schedule 14D-9 under the heading “Special Factors—The Solicitation or
Recommendation—Background of the Offer and the Merger; Reasons for the Recommendation; Fairness of the Offer and Merger” is incorporated herein by reference.
| ITEM 9. |
REPORTS, OPINIONS, APPRAISALS AND NEGOTIATIONS |
| |
(a) - (b) |
Report, Opinion or Appraisal; Preparer and Summary of the Report, Opinion or Appraisal
|
The information set forth in the Schedule 14D-9 under the headings “Special
Factors—The Solicitation or Recommendation,” “Special Factors— Persons/Assets Retained, Employed, Compensated or Used” and “Annex 2— Opinion of Financial Advisor,” and the information set
forth as Exhibits (c)(1)-(c)(9) is incorporated herein by reference. Leerink Partners LLC has consented to the inclusion of its opinion and discussion materials in their entirety as Exhibits (c)(1)-(c)(9) to this Schedule 13E-3.
The information set forth in the Offer to Purchase under the headings “Special
Factors—Section 1. Background of the Offer; Contacts with Pardes” and “Special Factors—Section 4. Reports, Appraisals and Negotiations” and in Exhibits (c)(1) – (c)(9)
hereto is incorporated herein by reference.
| |
(c) |
Availability of Documents |
The reports, opinions or appraisals referenced in this Item 9 are available for inspection and copying at Foresite Capital Affiliates principal executive
offices located at 900 Larkspur Landing Circle, Suite 150, Larkspur, California 94939, during regular business hours, by any interested stockholder of Pardes or a representative of such interested stockholder who has been so designated in writing by
such interested stockholder.
| ITEM 10. |
SOURCE AND AMOUNTS OF FUNDS OR OTHER CONSIDERATION |
The information set forth in the Offer to Purchase under the headings “Summary Term Sheet” and “The Tender
Offer——Section 8. Source and Amount of Funds” is incorporated herein by reference.
Not applicable.
The information set forth in the Schedule 14D-9 under the heading “Special Factors—Persons/Assets
Retained, Employed, Compensated or Used” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the
heading “The Tender Offer—12. Fees and Expenses” is incorporated herein by reference.
Not applicable.
| ITEM 11. |
INTEREST IN SECURITIES OF THE SUBJECT COMPANY |
The information set forth in Schedule 14D-9 under the heading “Special Factors—Past Contacts,
Transactions, Negotiations, and Agreements” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the
heading “The Tender Offer—Section 6. Certain Information Concerning Parent and Purchaser” and “Schedule A—Information Concerning Members of the Boards of Directors and the Executive Officers of
Purchaser, Parent and the Guarantors—Security Ownership of Certain Beneficial Owners” is incorporated herein by reference.
| |
(b) |
Securities Transactions |
The information set forth in Schedule 14D-9 under the headings “Special Factors—Past Contacts,
Transactions, Negotiations and Agreements—Arrangements with Parent and Purchaser and Their Affiliates” and “Item 6. Interest in Securities of the Subject Company” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings “The Tender Offer—Section 6. Certain Information
Concerning Parent and Purchaser” and “Schedule A—Information Concerning Members of the Boards of Directors and the Executive Officers of Purchaser, Parent and the Guarantors—Security Ownership of Certain Beneficial
Owners” is incorporated herein by reference.
| ITEM 12. |
THE SOLICITATION OR RECOMMENDATION |
| |
(d) |
Intent to Tender or Vote in a Going-Private Transaction |
The information set forth in the Schedule 14D-9 under the heading “Special Factors—The Solicitation or
Recommendation—Intent to Tender” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings
“Summary Term Sheet,” “Special Factors—Section 2. Purpose of the Offer and Plans for Pardes,” “The Tender Offer—Section 7. Summary of the Merger Agreement and
Certain Other Agreements—Summary of the Merger Agreement and Certain Other Agreements–Pardes Board Recommendation and Special Committee Recommendation” is incorporated herein by reference.
| |
(e) |
Recommendations of Others |
The information set forth in the Schedule 14D-9 under the heading “Special Factors—The Solicitation or
Recommendation” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings “Summary Term
Sheet,” “Special Factors—Section 2. Purpose of the Offer and Plans for Pardes,” “Special Factors—Section 3. Position of Parent and Purchaser Regarding Fairness
of the Offer and Merger” and “The Tender Offer—Section 7. Summary of the Merger Agreement and Certain Other Agreements—Summary of the Merger Agreement and Certain Other Agreements—Pardes Board
Recommendation and Special Committee Recommendation” is incorporated herein by reference.
| ITEM 13. |
FINANCIAL STATEMENTS |
| |
(a) |
Financial Information |
The information set forth in the Offer to Purchase under the heading “The Tender Offer—Section 5. Certain Information
Concerning Pardes” is incorporated herein by reference.
The audited financial statements of Pardes as of and for the fiscal years ended
December 31, 2022 and December 31, 2021 are incorporated herein by reference to Part II, Item 8 of Pardes’ Annual Report on Form 10-K for the fiscal year ended December 31, 2022 filed with
the SEC on March 14, 2023. The unaudited condensed financial statements of Pardes for the three months ended March 31, 2023 are incorporated herein by reference to Item 1 of Pardes’ Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2023, filed with the SEC on May 5, 2023.
| |
(b) |
Pro Forma Information |
Not applicable.
The information set forth in the Schedule 14D-9 under the heading “Special
Factors—Additional Information–Summary Financial Information” is herein incorporated by reference.
| ITEM 14. |
PERSONS/ASSETS RETAINED, EMPLOYED, COMPENSATED OR USED |
| |
(a) |
Solicitations or Recommendations |
The information set forth in the Schedule 14D-9 under the heading “Special Factors–Persons/Assets
Retained, Employed, Compensated or Used” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the
heading “The Tender Offer—12. Fees and Expenses” is incorporated herein by reference.
| |
(b) |
Employees and Corporate Assets |
The information set forth in the Schedule 14D-9 under the heading “Special Factors—Persons/Assets
Retained, Employed, Compensated or Used” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the
heading “The Tender Offer—12. Fees and Expenses” is incorporated herein by reference.
| ITEM 15. |
ADDITIONAL INFORMATION |
| |
(b) |
Golden Parachute Compensation |
The information set forth in the Schedule 14D-9 under the heading “Special Factors—Past Contacts,
Transactions, Negotiations and Agreements—Arrangements Between Pardes and its Executive Officers, Directors and Affiliates—Golden Parachute Compensation” is incorporated herein by reference.
| |
(c) |
Other Material Information |
The information set forth in the Schedule 14D-9 under the heading “Special Factors—Additional
Information” is incorporated herein by reference.
The information set forth in the Offer to Purchase under the headings “The Tender
Offer—Section 8. Source and Amount of Funds” and “The Tender Offer–Section 14. Miscellaneous” is incorporated herein by reference.
The following exhibits are filed herewith:
|
|
|
| Exhibit No. |
|
Description |
|
|
| (a)(1)(A) |
|
Amended and Restated Offer to Purchase, dated August 17, 2023 (incorporated herein by reference to Exhibit (a)(1)(A) to the Schedule TO). |
|
|
| (a)(1)(B) |
|
Form of Letter of Transmittal (incorporated herein by reference to Exhibit (a)(1)(B) to the Schedule TO). |
|
|
| (a)(1)(C) |
|
Form of Letter to Brokers, Dealers, Commercial Banks, Trust Companies and Other Nominees (incorporated herein by reference to Exhibit (a)(1)(C) to the Schedule TO). |
|
|
| (a)(1)(D) |
|
Form of Letter to Clients for Use by Brokers, Dealers, Commercial Banks, Trust Companies and Other Nominees (incorporated herein by reference to Exhibit (a)(1)(D) to the Schedule TO). |
|
|
| (a)(1)(E) |
|
Form of Summary Advertisement as published in The New York Times on July 28, 2023 (incorporated by reference to Exhibit (a)(1)(E) to the Schedule TO). |
|
|
| (a)(1)(F) |
|
Solicitation/Recommendation Statement on Schedule 14D-9 (incorporated by reference to Schedule 14D-9 filed by Pardes with the SEC on July 28, 2023,
as amended and restated on August 17, 2023). |
|
|
| (a)(1)(G) |
|
Press Release issued by Pardes on July 17, 2023 (incorporated by reference to Exhibit 99.1 to the Form 8-K filed by Pardes with the SEC on July 17, 2023). |
|
|
| (a)(1)(H) |
|
Press Release issued by Purchaser on August 17, 2023 (incorporated herein by reference to Exhibit (a)(1)(G) to the Schedule TO). |
|
|
| (b) |
|
None. |
|
|
| (c)(1) |
|
Opinion of Leerink Partners LLC, dated July 16, 2023 (incorporated by reference to Annex 2 of the Schedule 14D-9). |
|
|
| (c)(2) |
|
Materials Prepared for the Board of Directors, dated July 16, 2023, from Leerink Partners LLC. |
|
|
| (c)(3)*+ |
|
Strategic Process Update, dated June 12, 2023, from Leerink Partners LLC. |
|
|
| (c)(4)*+ |
|
Discussion Materials, dated April 13, 2023, from Leerink Partners LLC. |
|
|
| (c)(5)*+ |
|
Discussion Materials, dated May 2023, from Leerink Partners LLC. |
|
|
| (c)(6)*+ |
|
Discussion Materials, dated May 24, 2023, from Leerink Partners LLC. |
|
|
| (c)(7)*+ |
|
Discussion Materials, dated May 25, 2023, from Leerink Partners LLC. |
|
|
| (c)(8)*+ |
|
Discussion Materials, dated June 10, 2023, from Leerink Partners LLC. |
|
|
| (c)(9)*+ |
|
Discussion Materials, dated June 11, 2023, from Leerink Partners LLC. |
|
|
| (d)(1) |
|
Agreement and Plan of Merger, dated as of July 16, 2023, among Pardes, Parent and Purchaser (incorporated by reference to Exhibit 2.1 to the Form 8-K, filed by Pardes with the SEC on
July 17, 2023). |
|
|
| (d)(2) |
|
Form of Contingent Value Rights Agreement, by and between Parent, Purchaser, the Rights Agent and the Representative (incorporated herein by reference to Exhibit C to Exhibit 2.1 to the
Form 8-K filed by Pardes with the SEC on July 17, 2023). |
|
|
| (d)(3) |
|
Mutual Confidentiality Agreement, dated June 13, 2023 between Pardes and Foresite Capital Management, LLC (incorporated by reference to Exhibit (d)(2) to the Schedule TO). |
|
|
| (d)(4) |
|
Limited Guaranty, dated July 16, 2023, by Pardes, FS Development Holdings II, LLC, Foresite Capital Fund V, L.P. and Foresite Capital Opportunity Fund V, L.P. (incorporated by reference to Exhibit 10.1 to the Company’s
Current Report on Form 8-K filed by Pardes with the SEC on July 17, 2023). |
|
|
|
| Exhibit No. |
|
Description |
|
|
| (d)(5) |
|
Registration Rights Agreement, dated December 23, 2021, by and among Pardes Biosciences, Inc. and the stockholders party thereto (incorporated by reference to Exhibit 10.1 on Form
8-A12B/A filed by Pardes with the SEC on December 23, 2021). |
|
|
| (d)(6) |
|
Voting Agreement, dated December 23, 2021, by and among Pardes Biosciences, Inc. and the other parties thereto (incorporated by reference to Exhibit 10.2 on Form 8-K filed by Pardes with
the SEC on December 30, 2021). |
|
|
| (d)(7) |
|
Lockup Agreement, dated December 23, 2021, by and among Pardes and the other parties thereto (incorporated by reference to Exhibit 10.3 on Form 8-K filed by Pardes with the SEC on
December 30, 2021). |
|
|
| (d)(8) |
|
Form of Indemnification Agreement for Directors of Pardes Biosciences, Inc. (incorporated by reference to Exhibit 10.6 to Form 8-K filed by Pardes with the SEC on December 30,
2021). |
|
|
| (d)(9) |
|
Form of Indemnification Agreement for Executive Officers of Pardes Biosciences, Inc. (incorporated by reference to Exhibit 10.7 to Form 8-K filed by Pardes with the SEC on December 30,
2021). |
|
|
| (d)(10) |
|
2021 Stock Option and Incentive Plan (incorporated by reference to Annex E to Pardes’ Proxy Statement/Prospectus on Form 424B3 filed by Pardes with the SEC on December 1, 2021). |
|
|
| (d)(11) |
|
Forms of Award Agreements under the 2021 Stock Option and Incentive Plan (incorporated by reference to Exhibit 10.5 on Form 8-K filed by Pardes with the SEC on December 30,
2021). |
|
|
| (d)(12) |
|
2022 Inducement Plan (incorporated by reference to Exhibit 99.3 to the Form S-8 filed by Pardes with the SEC on March 2, 2022). |
|
|
| (d)(13) |
|
Form of Award Agreements under the 2022 Inducement Plan (incorporated by reference to Exhibit 99.4 to the Form S-8 filed by Pardes with the SEC on March 2, 2022). |
|
|
| (d)(14) |
|
Amended and Restated Offer Letter, dated December 23, 2020, by and between Pardes Biosciences, Inc. and Uri A. Lopatin, M.D.
(incorporated by reference to Exhibit 10.9 on Form 8-K filed by Pardes with the SEC on December 30,
2021). |
|
|
| (d)(15) |
|
Offer Letter, dated January 20, 2021, by and between Pardes Biosciences, Inc. and Heidi Henson (incorporated by reference to Exhibit 10.10 to Form S-1 filed by Pardes with the SEC on
January 21, 2022). |
|
|
| (d)(16) |
|
Retention Bonus Agreement, dated June 2, 2023, by and between Pardes Biosciences, Inc. and Heidi Henson (incorporated by reference to Exhibit 10.1 to Form 8-K filed by Pardes with the SEC
on June 5, 2023). |
|
|
| (d)(17) |
|
Employment Agreement dated March 1, 2022, by and between Pardes Biosciences, Inc. and Thomas G. Wiggans (incorporated by reference to Exhibit 10.18 to Form 10-K filed by Pardes with the
SEC on March 29, 2022). |
|
|
| (d)(18) |
|
Letter Agreement dated July 16, 2023 with Thomas Wiggans (incorporated by reference to Exhibit 10.2 to Form 8-K filed by Pardes with the SEC on July 17, 2023). |
|
|
| (d)(19) |
|
Executive Severance Plan (incorporated by reference to Exhibit 10.13 on Form 8-K filed by Pardes with the SEC on December 30, 2021). |
|
|
| (d)(20) |
|
Senior Executive Cash Incentive Bonus Plan (incorporated by reference to Exhibit 10.13 to
Form S-1 filed by Pardes with the SEC on January 21, 2022). |
|
|
| (d)(21) |
|
Transition and Separation Agreement and General Release of Claims dated March 25, 2022, by and between Pardes Biosciences, Inc. and Uri A. Lopatin, M.D. (incorporated by reference to Exhibit 10.21 to Form 10-K filed by Pardes with the SEC on March 29, 2022). |
|
|
|
| Exhibit No. |
|
Description |
|
|
| (d)(22) |
|
Consulting Agreement dated March 25, 2022, by and between Pardes Biosciences, Inc. and Uri A. Lopatin, M.D. (incorporated by reference to Exhibit 10.22 to Form 10-K filed by Pardes with
the SEC on March 29, 2022). |
|
|
| (d)(23) |
|
Separation Agreement and General Release of Claims, dated May 15, 2023, by and between Pardes Biosciences, Inc. and Brian P. Kearney, PharmD. (incorporated by reference to Exhibit 10.1 to Form
8-K filed by Pardes with the SEC on May 18, 2023). |
|
|
| (d)(24) |
|
Consulting Agreement, executed May 15, 2023, by and between Pardes Biosciences, Inc. and Brian P. Kearney, PharmD. (incorporated by reference to Exhibit 10.2 to Form 8-K filed by Pardes
with the SEC on May 18, 2023). |
|
|
| (d)(25) |
|
Letter Agreement dated as of February 16, 2021, by and among FS Development Corp. II, FS Development Corp. II’s officers and directors, and FS Development Holdings II, LLC (incorporated by reference to Exhibit 10.4 to Form
8-K filed by Pardes with the SEC on February 19, 2021). |
|
|
| (d)(26) |
|
FS Development Corp. II Support Agreement, dated as of June 29, 2021, by and among FS Development Corp. II, Pardes Biosciences, Inc., FS Development Holdings II, LLC and certain supporting stockholders of FS Development Corp.
II (incorporated by reference to Exhibit 10.1 to Form 8-K filed by Pardes with the SEC on June 29, 2021). |
|
|
| (f)* |
|
Section 262 of the Delaware General Corporation Law. |
|
|
| (g) |
|
None. |
|
|
| (107)* |
|
Calculation of Filing Fee Tables. |
| + |
Certain portions of this exhibit have been redacted and separately filed with the Securities and Exchange
Commission pursuant to a request for confidential treatment. |
SIGNATURES
After due inquiry and to the best of my knowledge and belief, I certify that the information set forth in this Statement is true, complete and correct.
|
|
|
|
|
|
|
| Dated: August 17, 2023 |
|
|
|
PARDES BIOSCIENCES, INC. |
|
|
|
|
|
|
|
|
By: |
|
/s/ Thomas G. Wiggans |
|
|
|
|
|
|
Name: Thomas G. Wiggans |
|
|
|
|
|
|
Title: Chief Executive Officer and
Chair of the Board of Directors |
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT HAVE BEEN OMITTED AND REPLACED WITH “[ XYZ ]”. SUCH IDENTIFIED INFORMATION HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT (I) IS NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS
PRIVATE OR CONFIDENTIAL.
Exhibit (c)(3)

PROJECT PACIFIC STRATEGIC PROCESS UPDATE JUNE 12, 2023 SVb Securities Confidential

“[ XYZ
]” indicates information that has been omitted on the basis of a confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and
Exchange Commission.
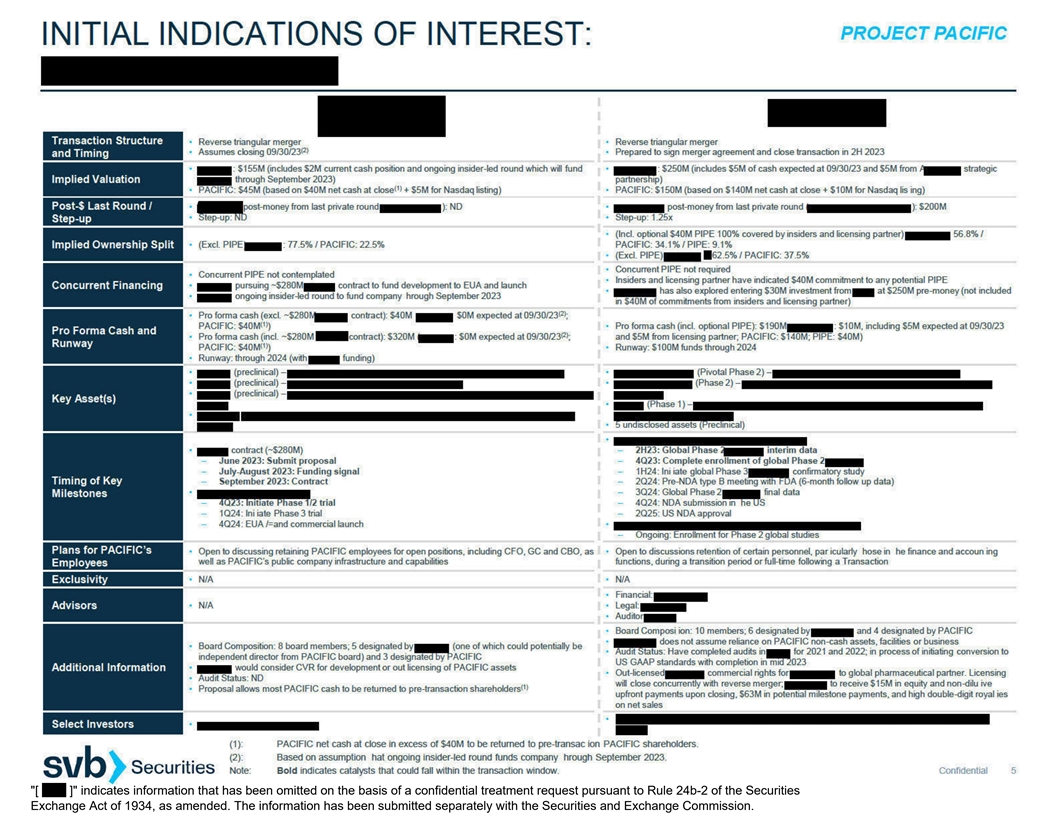
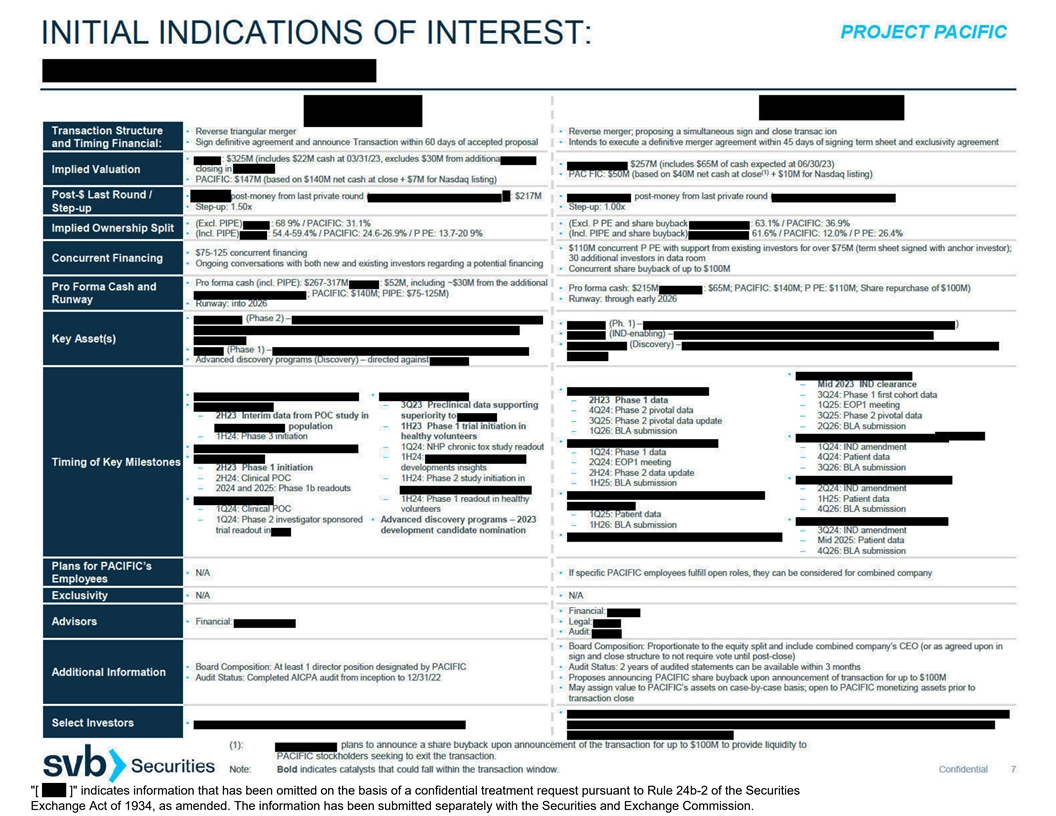
PROJECT PACIFIC (1):All private companies have withdrawn from the process. SUMMARY OF STRATEGIC ALTERNATIVES PROCESS AS OF JUNE 13,
2023 Following Pacific’s suspension of further clinical development of pomotrelvir, Pacific engaged SVB Securities to explore a range of strategic alternatives, including an acquisition, merger, business combination, or other transaction. SVB
Securities, management and the Pacific Special Committee considered an initial list of 133 companies and five parties with potential interest in acquiring Pacific via a cash tender offer (“financial buyers”). This list included inbound
interest from companies and financial buyers. Management and the Pacific Special Committee decided to include nine private companies and three potential financial buyers in the process, based on a determination of the potential merits of a
transaction with such party. During the initial outreach process, SVB Securities was directed to contact these parties to determine the level of interest in a potential strategic transaction with Pacific. Of the contacted private companies, eight
requested a process letter and eight submitted non-binding indications of interest (IOI). Two of the three contacted financial buyers submitted non-binding IOIs. Pacific
Special Committee selected three private companies on which to begin further detailed due diligence, including inviting them to conduct a confidential management presentation. After additional diligence and analysis, the three private companies were
provided feedback from the Pacific Special Committee that their proposals would need to include a return of capital to Pacific’s shareholders. Following additional feedback that the return of capital would need to be at least $100M, two of the
private companies withdrew from the process. One private company withdrew from the process to pursue an alternative strategic transaction. Following feedback from the Pacific Special Committee on their initial IOIs, the two financial buyers
submitted revised proposals between June 9 and June 10, 2023, and subsequently revised their proposals again on June 12, 2023. 9 / 3 8 / 2 CONSIDERED: CONTACTED: SUBMITTED IOI: 133 / 5 Companies / Financial Buyers Companies /
Financial Buyers Companies / Financial Buyers CONDUCTED MGMT. PRESENTATIONS(1)(N=3) SUBMITTED REVISED PROPOSALS (N=2)1 Confidential

“[ XYZ
]” indicates information that has been omitted on the basis of a confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and
Exchange Commission.
PROJECT PACIFIC XOMA CORPORATION’S OFFERS FOR PACIFICCURRENT 2ND OFFER 1ST OFFER Date of Offer June 12,
2023 June 9, 2023 May 30, 2023 Transaction Structure Cash tender offer “Back-end” merger to acquire any shares not tendered Requested Response Date N/A Timing 3Q23
expected close Support Agreements Certain of PACIFIC’s key shareholders Treatment of Stock Options Terminated as of closing of “back-end” merger In-the-money options cashed out for intrinsic value Exclusivity 30 days 10-day extension if initial period lapses and parties remain in negotiations unless party
provides 10-day written notice of non-renewal Cash Consideration as $ per Share $2.08 per share(1) $2.06 per share(1) $2.07 per share(2) Aggregate Consideration $128.5M
cash(3)(PACIFIC net cash minus $12.5M, assuming PACIFIC net cash at close is $141M) $127M cash(4)(PACIFIC net cash minus $14M, assuming PACIFIC net cash at close is $141M) $127M cash(5)(assumes PACIFIC net cash at close is $141M) CVR for 80% of net
proceeds from sale of PACIFIC’s assets Excess net cash at close greater than $139M will be returned to existing PACIFIC shareholders CVR for 80% of the net proceeds from the sale of PACIFIC’s legacy assets, such as
pomotrelvir, PBI-2158, the backup leads and the IP Cash Consideration as % of PACIFIC Net Cash 91%(6) 90%(6) 90%(7) Cash Consideration as a Premium to Closing Price as of 06/09/23(8) 11% 10%
11%

PROJECT PACIFIC FORESITE CAPITAL’SOFFERS FOR PACIFICCURRENT 2ND OFFER 1ST OFFER Date of
Offer June 12, 2023 June 10, 2023 June 5, 2023 Transaction Structure Cash tender offer for outstanding shares not currently owned by FORESITE Requested Response Date N/A June 12, 2023, 5pm ET (2 days from
offer date) June 8, 2023, 5pm ET (3 days from offer date) Timing N/A Support Agreements N/A Treatment of Stock Options N/A Exclusivity N/A Cash Consideration as $ per Share $2.09 per share $2.09 per share $1.93 per share Aggregate Consideration
$129M cash(1)(PACIFIC net cash minus $12M, assuming PACIFIC net cash at close is $141M) CVR for 80% of the net proceeds payable from any license or disposition involving Pomotrelvirwithin five years of closing $129M cash(1)(PACIFIC net cash minus
$12M, assuming PACIFIC net cash at close is $141M) CVR for 90% of the net proceeds payable from any license or disposition involving Pomotrelvirwithin one year of closing $119M cash(2)(assumes PACIFIC net cash at close is $141M) CVR for 80% of the
net proceeds payable from any license or disposition involving Pomotrelvirwithin one year of closing Cash Consideration as % of PACIFIC Net Cash(3) 91% 91% 84% Cash Consideration as a Premium to Closing Price as of 06/09/23(4) 12% 12% 3%

$1'5(3/$&(':,7+³>;<=@´68&+,'(17,),(',1)250$7,21
+$6%((1(;&/8'(')5207+,6(;+,%,7%(&$86(,7 , ,61270$7(5,$/$1' ,, ,67+(7<3(7+$77+(5(*,675$1775($76$635,9$7(25&21),'(17,$/ Exhibit (c)(4) &(57$,1&21),'(17,$/3257,2162)7+,6(;+,%,7+$9(%((120,77(' PROJECT PACIFIC STRATEGIC
ALTERNATIVES CONSIDERATIONS APRIL 13, 2023 Confidential

PROJECT PACIFIC SITUATION OVERVIEW • Pardes Biosciences is
evaluating its strategic options following the decision to suspend the clinical development of pomotrelvir, reduce headcount by 85% and conserve its current cash balance of ~$172 million. • Pardes’ attractive cash balance and the current
weakness in the capital markets provide Pardes with a range of strategic alternatives to optimize value for shareholders. – Merge with a private company – In-license or acquire clinical assets or new technologies – Merge with a
public company – Return capital to shareholders • Private company merger counterparties could have complementary assets and operating synergies or could be in different therapeutic areas that the board believes will create a compelling
story for new and existing investors. • In-licensing or acquiring assets should be explored but can be challenging given both the competitive intensity and funding requirements to acquire and develop high-quality, clinical-stage assets through
to value inflecting milestones. • Public-to-public mergers are rare for pre-commercial biopharma companies as public company targets with attractive assets often have financing options that are more efficient and feasible than a merger with a
cash-rich fallen angel. • The return of capital option may be more efficient than in the past given the emergence of parties willing to acquire cash-rich companies for up to 80-90% of their net cash balance plus CVRs via a tender offer that
can close in ~45 days vs the traditional dissolution process that can take up to three years to complete. • The typical private merger process can take approximately two to three months to announcement and can take up to another three to four
months to close in the event the transaction does not qualify for a sign-and-close. • Our experience as a leading strategic advisor, history of screening and sourcing select targets for a number of similarly situated clients, differentiated
knowledge resources and deep relationships with potential merger partners make us an ideal advisor for the board as it navigates options and executes potential strategic transactions. Confidential 1

PROJECT PACIFIC PUBLIC / PRIVATE MERGERS CAN WORK AS A GO PUBLIC
ALTERNATIVE FOR HIGH-QUALITY COMPANIES $ IN MILLIONS; SORTED BY ANNOUNCEMENT DATE (NEWEST TO OLDEST) RECENTLY Private Company Private Company Private Company Private Company Private Company ANNOUNCED Merger with Merger with Merger with Merger with
Merger with PUBLIC / PRIVATE MERGERS: March 2023 February 2023 December 2022 November 2022 November 2022 HIGH QUALITY COMPANIES CURRENTLY TRADING ON NASDAQ VIA A PUBLIC / PRIVATE MERGER: Private Company Private Company Private Company Private
Company Private Company Private Company Merger with Merger with Merger with Merger with Merger with Merger with Celtrix Pharmaceuticals May 2000 October 2020 October 2020 January 2018 November 2016 July 2016 • $98MM follow-on (Sep. 2021)
• $184MM follow-on (Nov. 2021) • $84MM follow-on (Jan. 2018) • $52MM follow-on (May 2017) • $144MM follow-on (Dec. 2017) • $403MM follow-on (Sep. 2017) • $450MM convertible debt • $270MM follow-on (Aug.2022)
• $121MM follow-on (May 2022) • $63MM follow-on (Nov. 2018) • $75MM follow-on (Jan. 2018) • $440MM follow-on (Jun. 2018) (Jan. 2018) • $91MM follow-on (Apr. 2019) • $46MM follow-on (Jan. 2020) • $159MM
proceeds under • $287MM follow-on (May 2019) $200M ATM (Dec. 2022) • $98MM follow-on (Dec. 2019) • $160MM follow-on (Sep. 2020) • $259MM follow-on (May 2020) • $288MM follow-on / $575MM • $299MM follow-on (Dec.
2020) • Acquisition by Ipsen (closed convertible debt (May 2021) March 2023) • Renovacor Acquisition • $275MM follow-on / $500MM (Dec. 2022) term loan/royalty (Oct. 2022) • $100MM follow-on (Oct. 2022) Acquired for Mkt. Cap:
$1.5B Mkt. Cap: $1.7B Mkt. Cap: $1.4B Mkt. Cap: $5.6B Mkt. Cap: $2.3B (1) $1.0B by Ipsen (1) Does not include $10/share CVRs. Note: Data as of 04/06/23. Recently announced transactions include private company mergers that have not yet closed.
Confidential 2 Source: FactSet, Dealogic, SEC filings and press releases.

PROJECT PACIFIC SELECTION METHODOLOGY FOR A PRIVATE COMPANY MERGER
TRANSACTION Leverage SVB Securities' relationships with high-quality private biopharma companies with stated interest in alternative go-public transactions MEDACorp knowledge resource enables diligence of potential opportunities Relationships with
100+ high quality private biopharma companies help expedite screening process Focused list of attractive target companies for Pardes to pursue Confidential 3

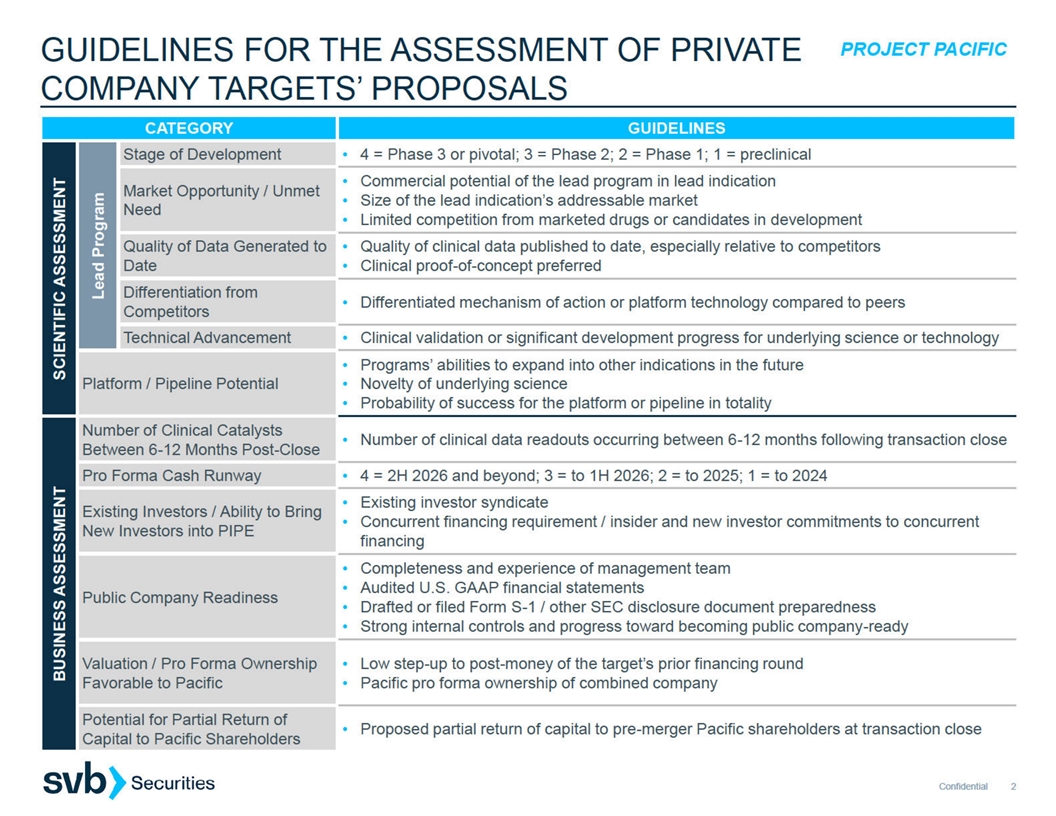
PROJECT PACIFIC CRITERIA FOR DETERMINING ATTRACTIVENESS OF A PRIVATE
COMPANY MERGER COUNTERPARTY ILLUSTRATIVE SCREENING CRITERIA USED BY PARDES BIOSCIENCES Attractiveness of target’s lead asset, pipeline, technology, and business case 1. 2. Near-term, value-driving catalysts within pro forma cash runway
Management, board and existing investor quality 3. 4. Readiness to be a U.S. publicly traded company Need for additional financing and commitment from private company investors to provide such 5. 6. Proposed valuation and ownership split including
premium assigned to private company over expected cash at close Fit and value assigned to public company’s programs 7. ILLUSTRATIVE SCREENING CRITERIA USED BY PRIVATE COMPANY 1. Sufficient cash on balance sheet to fund activities through key
value-driving catalysts 2. Quality of public company investor base – overlap with private company is often helpful Material litigation, liabilities or sustained operational commitments 3. Personnel with complementary domain expertise
(scientific, development, or commercial) 4. Confidential 4

PROJECT PACIFIC COMPONENTS OF VALUE IN A PRIVATE COMPANY MERGER PARDES
BIOSCIENCES PRIVATE TARGET Estimated net cash at close Base value is post-money valuation of last private financing • Primary value driver • Value may be adjusted based on market conditions • Ability to maximize cash preservation
via operational • Meaningful data generation or update since last streamlining financing could justify a value adjustment • Target valuation may be more negotiable in the absence of a high-quality institutional shareholder Valuation
supported by: Listing value Clinical programs / Intrinsic valuation Market based valuation other assets • Key analysis to inform • Crossover to IPO step- • Additional value driver • Potential for right inherent value of
future up, comparable partner to ascribe value cash flows • Dependent on equity companies analysis capital market • CVRs can be employed • Sensitive to changes in • Reference point to conditions to retain value for
assumptions inform value shareholders • Less reliable for earlier- • Dependent on market stage companies conditions Relative value, informed by the above metrics, will be determined via negotiation of the exchange ratio Confidential
5

CVRS CAN BE EMPLOYED TO RETAIN VALUE FOR PROJECT PACIFIC SHAREHOLDERS
Mechanism of Special dividend to Aduro shareholders of record prior Special dividend to OncoMed shareholders of record in to merger becoming effective March 2019 CVR Issuance Terminates at earlier of: • Payment by Mereo of each milestone
eligible to be 10 years Period attained • Expiration of period for each trigger event Non-tradable Non-tradable Tradability • Celgene exercises its option to license Oncomed’s etigilimab and pays associated $35mm milestone •
Disposition or license of Aduro’s non-renal assets before 12/31/19 Trigger • Revenue resulting from ownership of subsidiary Event(s) • Mereo enters partnership or other agreement established to hold any non-renal assets regarding
navicixizumab within 18 months of the merger closing • Additional Mereo ADRs based on exchange ratio calculated by dividing the milestone received by the • Any consideration paid to Aduro with respect to the Mereo’s 10-day VWAP
following announcement disposition or license of any non-renal assets that Celgene exercised its option (subject to issuance limitation of 40% of issued share capital) • Proceeds resulting from ownership in any Payment subsidiary established
by Aduro before the six- • 70% of net proceeds of milestone payments month anniversary of the closing date to hold any received by Mereo within 5 years of merger from non-renal assets future partnership or investment transactions in relation
to navicixizumab (subject to aggregate cap of $79.7mm) Confidential 6

PROJECT PACIFIC CASE STUDY: CONCENTRA’S ACQUISITION OF JOUNCE
POST REDX MERGER ANNOUNCEMENT TIMELINE • 02/23: Jounce announced an all-stock merger with Redx Pharma (37% / 63% split, respectively) • 03/14: Concentra offered to acquire Jounce for $1.80 cash per share + a CVR • 03/27: Jounce
announced acquisition by Concentra for $1.85 cash per share + CVRs TRANSACTION DETAILS • On 04/07, Concentra will commence a tender offer to acquire all outstanding shares of Jounce for $1.85 per share + CVRs ‒ Estimated / minimum Jounce
net cash at close: $115M / $110M ‒ Upfront cash of $1.85 per share represents 61% of current net cash per share, 85% of estimated net cash at close and 89% of minimum net cash ‒ Jounce’s liquidation analysis suggested distribution
would result in proceeds of 61% of current net cash and 89% of merger minimum cash • CVRs are for proceeds from (1) a transaction for Jounce’s programs and (2) certain specified cost savings (1) 80% of proceeds from a transaction for
Jounce’s programs within two years of merger close; 10-year tail on payments (2) 100% of the potential aggregate value of certain specified potential cost savings, including: o Any reduction in the amount of the expected lease obligation o Any
increase in net working capital • Concurrent with the merger, Jounce announced an 84% RIF (compared to a 57% RIF with the Redx merger) and restructuring costs of $6.5M • Jounce’s board also recommended not moving forward with the
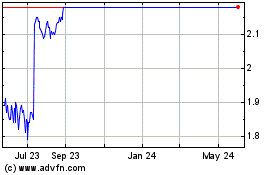
Redx merger, causing Redx shares to decline 11% TRADING PERFORMANCE PERFORMANCE RELATIVE TO CASH PER SHARE Share TANGCAPITALMANAGEMENT Price 03/27: Announced $3.00 acquisition by Concentra Merger (2/23) + Offer (3/14): Merger (3/27): (1) Current Net
Cash Per Share: $3.01 57% RIF $1.80 + CVR $1.85 + CVRs $2.50 T-0 +1 Day T-0 +1 Day T-0 +1 Day (2) 03/14: Announced Est. Net Cash at Close Per Share: $2.16 Concentra’s offer to acquire $2.00 Jounce Share Price $0.99 $1.10 $1.06 $1.49 $1.51
$1.83 (3) Merger Min. Net Cash Per Share: $2.07 Change 11% 41% 21% $1.50 Share Price as a % of Net Cash Share Price as % of: $1.00 (1) Latest Net Cash 33% 37% 35% 50% 50% 61% (2) Est. Net Cash at Close 46% 51% 49% 69% 70% 85% $0.50 02/23: Announced
merger 03/06: Tang Capital acquired (3) with Redx a 9.7% stake in Jounce Merger Min. Net Cash 48% 53% 51% 72% 73% 88% $0.00 1/3 1/10 1/17 1/24 1/31 2/7 2/14 2/21 2/28 3/7 3/14 3/21 (1): Net cash per share of $3.01, assuming $160M last reported net
cash and 53.1 fully diluted shares outstanding using treasury stock methodology. (2): Net cash per share of $2.16, assuming $115M net cash at close and 53.1 fully diluted shares outstanding using treasury stock methodology. Confidential 7 (3): Net
cash per share of $2.07, assuming $110M net cash at close and 53.1 fully diluted shares outstanding using treasury stock methodology.

6HFXULWLHVDQG([FKDQJH&RPPLVVLRQDPHQGHG7KHLQIRUPDWLRQKDVEHHQVXEPLWWHGVHSDUDWHOZLWKWKH
RIWKH6HFXULWLHV([FKDQJH$FWRIDV VLVRIDFRQILGHQWLDOWUHDWPHQWUHTXHVWSXUVXDQWWR5XOHE ³>;<=@´LQGLFDWHVLQIRUPDWLRQWKDWKDVEHHQRPLWWHGRQWKHED PROJECT PACIFIC ILLUSTRATIVE POTENTIAL MERGER CANDIDATES Oncology Autoimmune & Inflammation (1)
(1) (1) Vida Ventures (1) (1) (1) Portfolio Company (2) (1): Public company. (2): Highest stage of development not disclosed. Inbound Interest Source: Company websites. Confidential 8 Preclinical Phase 1 & 1/2 Phase 2 & 3

6HFXULWLHVDQG([FKDQJH&RPPLVVLRQDPHQGHG7KHLQIRUPDWLRQKDVEHHQVXEPLWWHGVHSDUDWHOZLWKWKH
RIWKH6HFXULWLHV([FKDQJH$FWRIDV VLVRIDFRQILGHQWLDOWUHDWPHQWUHTXHVWSXUVXDQWWR5XOHE ³>;<=@´LQGLFDWHVLQIRUPDWLRQWKDWKDVEHHQRPLWWHGRQWKHED PROJECT PACIFIC ILLUSTRATIVE POTENTIAL MERGER CANDIDATES (CONT.) Cardiology / X (1) (2) Cardio- CNS
Anti-Viral Genetic Medicines Rare Diseases Others metabolic (4) (3) (4) (4) Project Nuage (5) (1): Excludes oncology focused companies. (2): Other therapeutics areas includes endocrinology, gastrointestinal diseases, HCS therapies, hepatology,
ophthalmology, pulmonology, xenotransplantation, companies with multiple therapeutic areas as well as a healthcare information technology platform. (3): Commercial-stage. (4): Public company. (5): Highest stage of development not disclosed. Inbound
Interest Source: Company websites. Confidential 9 Preclinical Phase 1 & 1/2 Phase 2 & 3 or Later

PROJECT PACIFIC ILLUSTRATIVE PRIVATE COMPANY MERGER PROCESS TIMELINE
THROUGH ANNOUNCEMENT MONTH APRIL MAY JUNE JULY WEEK OF 10 17 24 1 8 15 22 29 5 12 19 26 3 10 17 24 Weekly reoccurring meeting to track process Private company target selection Formally initiate outreach to selected parties Send process letter and
receive initial proposals Evaluate proposals and advance parties to further diligence Due diligence Management presentations Receipt of final proposals Negotiate final terms and definitive documentation If Nasdaq agrees no shareholder approval is
required, the merger can close shortly after announcement. Confidential 10

PROJECT PACIFIC Disclosures This information (including, but not
limited to, prices, quotes and statistics) has been obtained from sources that we believe reliable, but we do not represent that it is accurate or complete and it should not be relied upon as such. All information is subject to change without
notice. The information is intended for Institutional Use Only and is not an offer to sell or a solicitation to buy any product to which this information relates. SVB Securities LLC (“Firm”), its officers, directors, employees,
proprietary accounts and affiliates may have a position, long or short, in the securities referred to in this report, and/or other related securities, and from time to time may increase or decrease the position or express a view that is contrary to
that contained in this report. The Firm's research analysts, salespeople, traders and other professionals may provide oral or written market commentary or trading strategies that are contrary to opinions expressed in this report. The Firm's asset
management group and proprietary accounts may make investment decisions that are inconsistent with the opinions expressed in this document. The past performance of securities does not guarantee or predict future performance. Transaction strategies
described herein may not be suitable for all investors. This document may not be reproduced or circulated without SVB Securities’ written authority. Additional information is available upon request by contacting the Editorial Department, SVB
Securities LLC, 53 State Street, 40th Floor, Boston, MA 02109. Like all Firm employees, research analysts receive compensation that is impacted by, among other factors, overall firm profitability, which includes revenues from, among other business
units, Institutional Equities, Research, and Investment Banking. Research analysts, however, are not compensated for a specific investment banking services transaction. To the extent SVB Securities' research reports are referenced in this material,
they are either attached hereto or information about these companies, including prices, rating, market making status, price charts, compensation disclosures, Analyst Certifications, etc. is available on
https://svbsecurities.bluematrix.com/bluematrix/Disclosure2. SVB MEDACorp LLC (MEDACorp), an affiliate of SVB Securities LLC, is a global network of independent healthcare professionals (Key Opinion Leaders and consultants) providing industry and
market insights to SVB Securities and its clients. © 2023 SVB Securities LLC. All Rights Reserved. Member FINRA/SIPC. SVB Securities LLC is a member of SVB Financial Group. Confidential

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT HAVE BEEN OMITTED AND
REPLACED WITH [ XYZ ] . SUCH IDENTIFIED INFORMATION HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT (I) IS NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL. Exhibit (c)(5)

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.


CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT HAVE BEEN OMITTED AND
REPLACED WITH [ XYZ ] . SUCH IDENTIFIED INFORMATION HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT (I) IS NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL. Exhibit (c)(6) PROJECT PACIFIC STRATEGIC PROCESS
UPDATE MAY 24, 2023

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.
[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.
[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.


APPENDIX

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT HAVE BEEN OMITTED AND
REPLACED WITH [ XYZ ] . SUCH IDENTIFIED INFORMATION HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT (I) IS NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL. Exhibit (c)(7) PROJECT PACIFIC SUMMARY OF INITIAL
INDICATIONS OF INTEREST MAY 25, 2023

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.


APPENDIX
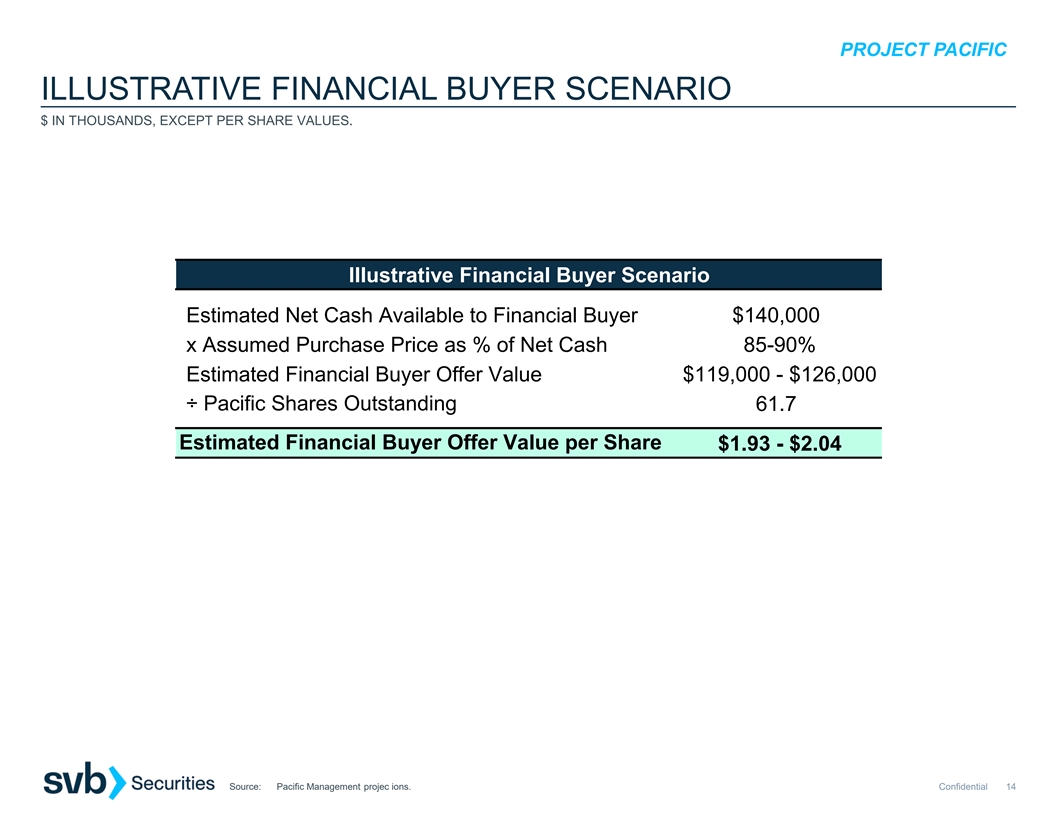
PROJECT PACIFIC ILLUSTRATIVE FINANCIAL BUYER SCENARIO $ IN THOUSANDS,
EXCEPT PER SHARE VALUES. Illustrative Financial Buyer Scenario Estimated Net Cash Available to Financial Buyer $140,000 x Assumed Purchase Price as % of Net Cash 85-90% Estimated Financial Buyer Offer Value $119,000 - $126,000 ÷ Pacific Shares
Outstanding 61.7 Estimated Financial Buyer Offer Value per Share $1.93 - $2.04 Source: Pacific Management projec ions. Confidential 14

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT HAVE BEEN OMITTED AND
REPLACED WITH [ XYZ ] . SUCH IDENTIFIED INFORMATION HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT (I) IS NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL. Exhibit (c)(8)

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

PROJECT PACIFIC HOW PRIORITIZED COUNTERPARTIES ARE VALUING PACIFIC AND
THEMSELVES $ IN USD MILLIONS. SORTED ALPHABETICALLY. PACIFIC VALUATION COUNTERPARTY PF OWNERSHIP PROPOSED TRANSACTION RETURN OF CASH ASSETS / PRO FORMA TARGET PIPE PARTY STRUCTURE CAPITAL DELIVERED LISTING TECHNOLOGY CVR TOTAL VALUATION STEP-UP CASH
RUNWAY (INSIDER COMMITMENT) PACIFIC PARTY PIPE ACTIVE (1) (1) Sign-and-close $100 $40 $10 - None $50 $257 1.00x Until early 2026 $110 (68%) 12.0% 61.6% 26.4% (2) (2) S-4 110 30 15 - None 45 257 1.00x Until early 2026 120 (63%) 10.7% 60.9% 28.4% (3)
S-4 - 140 15 - None 155 310 1.00x Through 2027 - 33.3% 66.7% - (4) (5) (3) S-4 60 80 15 - None 95 310 1.00x [Through 2027] - 23.5% 76.5% - PASSED FOLLOWING SPECIAL COMMITTEE FEEDBACK PROVIDED ON 06/07/23 S-4 - $140 - - None $140 402-434 1.50-1.62x
Into 2H 2026 - 24.4-25.8% 74.2-75.6% - (6) (5) S-4 $40 100 - - None 100 402-434 1.50-1.62x [Into 2H 2026] - 18.7-19.9% 80.1-81.3% - (1): plans to announce a share buyback upon announcement of the transaction for up to $100M to provide liquidity to
PACIFIC stockholders seeking to exit the transaction. (2): revised its proposed share buyback to return up to $110M to PACIFIC stockholders. (3): Ongoing discussions with investors to extend , which would increase post-money for and valuation for
proposed transac ion accordingly. would not require a concurrent financing for the transaction. (4): would be open to returning $60M of the $140M available estimated net cash at close to the shareholders of PACIFIC. (5): Pro forma runway per initial
indication of interest. The company has since proposed a return of capital mechanism and has not given guidance on how hat may impact the pro forma runway. (6): would consider a scenario that dividends any cash net of $100M from the PACIFIC balance
sheet back to PACIFIC shareholders. Note: Green shading denotes revised proposal communicated following further feedback from PACIFIC’s special committee. Blue shading denotes revised proposal communicated following initial feedback from
PACIFIC’s special committee. Confidential 2 [ ] indicates information that has been omitted on the basis of a confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been
submitted separately with the Securities and Exchange Commission.

PROJECT PACIFIC FINANCIAL BUYERS’ OFFERS FOR PACIFIC Date of
Offer • May 30, 2023 • June 9, 2023 • June 5, 2023 • Cash tender offer • Cash tender offer for outstanding shares not Transaction Structure • “Back-end” merger to acquire any shares not tendered
currently owned by FORESITE Requested Response Date • N/A • June 8, 2023, 5pm ET (3 days from offer date) Timing • 3Q23 expected close • N/A Support Agreements • Certain of PACIFIC’s key shareholders • N/A
• Terminated as of closing of “back-end” merger Treatment of Stock Options • N/A • In-the-money options cashed out for intrinsic value • 30 days Exclusivity • 10-day extension if initial period lapses and
parties remain in negotiations unless party provides • N/A 10-day written notice of non-renewal Cash Consideration as $ per (1) (2) • $2.07 per share • $2.02 per share • $1.93 per share Share (4) • $125M cash (PACIFIC
net cash minus $14M, (5) assuming PACIFIC net cash at close is $139M) • $119M cash (assumes PACIFIC net cash at (3) • $127M cash (assumes PACIFIC net cash • Excess net cash at close greater than $139M will close is $141M) at close
is $141M) Aggregate Consideration be returned to existing PACIFIC shareholders • CVR for 80% of the net proceeds payable from • CVR for 80% of net proceeds from sale of • CVR for 80% of the net proceeds from the sale of any license
or disposition involving Pomotrelvir PACIFIC’s assets PACIFIC’s legacy assets, such as pomotrelvir, within one year of closing PBI-2158, the backup leads and the IP Cash Consideration as % of (6) (7) (8) • 90% • 90% •
84% PACIFIC Net Cash Cash Consideration as a Premium to Closing Price as • 11% • 8% • 3% (9) of 06/09/23 (1): Calculated assuming aggregate cash considera ion of $127M, based on aggregate cash consideration equal to 90% of assumed
PACIFIC net cash at close per initial proposal received on 05/30/23, and ~61.7M PACIFIC common shares outstanding. Initial proposal of aggregate cash considera ion equal to $135M, based on assumed PACIFIC net cash at close of $150M, implied cash
consideration of $2.19 per share. (2): Calculated based on aggregate cash consideration of $139M assumed PACIFIC net cash at close minus $14M and ~61.7M PACIFIC common shares outstanding per proposal received on 06/09/23. (3): Assumed cash
consideration equal to 90% of assumed PACIFIC net cash at close based on initial proposal received on 05/30/23. Initial proposed cash consideration of $135M was based on assumed PACIFIC cash at close of $150M. (4): Cash consideration of $139M
assumed PACIFIC net cash at close minus $14M, representing approximately 90% of the assumed net cash, per proposal received on 06/09/23. (5): Based on offer price of $1.93 per share and ~61.7M common shares outstanding as of 05/01/23 per
PACIFIC’s latest 10-Q filing, including ~16.8M shares (~27.2% of common shares outstanding) owned by FORESITE and its affiliates at the offer date. (6): Based aggregate consideration of $135M and assumed PACIFIC net cash at close of $150M per
proposal received on 05/30/23. (7): Based aggregate consideration of $125M and assumed PACIFIC net cash at close of $139M per proposal received on 06/09/23. (8): Based on $141M assumed PACIFIC net cash at close per FORESITE proposal. Confidential 3
(9): Based on PACIFIC closing price of $1.87 per share as of 06/09/23. [ ] indicates information that has been omitted on the basis of a confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The
information has been submitted separately with the Securities and Exchange Commission.

PROJECT PACIFIC DISCOUNTS TO NET CASH IMPLIED BY RECENT FINANCIAL
BUYERS’ PROPOSALS ($ in millions, except per share values) Terms of Proposal Cash Consideration Non-Contingent Offer Target As a % of Premium to Ann. Financial Cash per Equity Total per Aggregate Contingent Net Cash Net Cash Unaffected Offer
(1) Date Company Buyer Share per Share Share Value Value Rights at Close at Close Price Accepted 80% of the net proceeds payable from any (2) 05/30/23 $8.00 - $8.00 $53 license or disposition of the MEI's clinical $76 70% 11% O assets 80% of the net
proceeds payable from any 05/22/23 5.75 - 5.75 480 570 84% 55% O license or disposition of Atea’s programs Echo Lake (3) 03/21/23 1.60 - 1.60 58 None 87 67% 90% O Capital 80% of the net proceeds for ten years post- closing from any license or
disposition of (4) 03/14/23 1.85 - 1.85 96 Jounce’s programs effected within two years 115 85% 75% P of closing and 100% of the potential aggregate value of certain potential cost savings Mean: 76% 58% Median: 77% 65% 25th Percentile: 69% 44%
75th Percentile: 84% 78% (1): Estimated net cash at close per offer filing unless otherwise noted. (2): Expected net cash of ~$93M at 06/30/23 per the S-4 filing, net of ~$17M of total minimum operating lease payments (carrying value of $13M due to
present value discounting) as of 03/31/23. (3): $90M cash and cash equivalents net of $3M total liabilities as of 03/31/23. (4): Represents final offer accepted on 03/27/23, increased from the 03/14/23 offer of $1.80 per share + CVR. Note: Includes
unsolicited offers from financial buyers for biopharma companies trading below net cash. Excludes unsolicited offers for companies which total liabilities exceeded cash and cash equivalents at he time of the offer. Source: Company press releases and
filings. Confidential 4

APPENDIX

A. CATALYSTS IN PRO FORMA CASH RUNWAY OF STRATEGICS THAT HAVE SUBMITTED
INDICATIONS OF INTEREST

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

B. INITIAL INDICATIONS OF INTEREST FROM STRATEGICS INVITED TO
MANAGEMENT PRESENTATIONS

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST FROM STRATEGICS
INVITED TO MANAGEMENT PRESENTATIONS: • Reverse merger; proposing a simultaneous sign and close transaction Transaction Structure • Reverse triangular merger • Intends to execute a definitive merger agreement within 45 days of
signing term sheet and exclusivity and Timing • Due diligence could be completed by both parties in 45 days agreement • $257M (includes $65M of cash expected at 06/30/23) • : $310M (includes $130M cash expected at 06/30/23) Implied
Valuation (1) • PACIFIC: $50M (based on $40M net cash at close + $10M for Nasdaq listing) • PACIFIC: $155M (based on $140M net cash at close + $15M for Nasdaq listing) Post-$ Last Round / • post-money from last private round ):
$257M • post-money from last private round ( ): $310M Step-up • Step-up: 1.00x • Step-up: 1.00x • (Excl. PIPE and share buyback) : 63.1% / PACIFIC: 36.9% Implied Ownership Split • (Excl. PIPE) 66.7% / PACIFIC: 33.3%
• (Incl. P PE and share buyback) : 61.6% / PACIFIC: 12.0% / PIPE: 26.4% • $110M concurrent PIPE with support from existing investors for over $75M (term sheet signed with • No planned financing concurrent with the transaction,
however, is in discussions to extend Concurrent Financing anchor investor); 30 additional investors in data room , which would increase post-money for and valua ion for proposed • Concurrent share buyback of up to $100M transaction accordingly
• Pro forma cash: $215M : $65M; PACIFIC: $140M; PIPE: $110M; Share repurchase of $100M) • Pro forma cash: $270M ( : $130M; PACIFIC: $140M) Pro Forma Cash and Runway • Runway: Until early 2026 • Runway: through 2027, not
including potential milestones payments from related to • (IND-enabling) – • (Ph. 1) – • (Phase 1 complete) – • (IND-enabling) – Key Asset(s) • Entered into a global license and development
agreement with in for • (Discovery) – • • • – Mid 2023 IND clearance – 2H23 Phase 1 data – 3Q24: Phase 1 first cohort data – 4Q24: Phase 2 pivotal data – 1Q25: EOP1 meeting – 3Q25:
Phase 2 pivotal data update – 3Q25: Phase 2 pivotal data • – 1Q26: BLA submission – 2Q26: BLA submission • – 1H23 IND filing • • – 2H23 Phase 2 POC initiation – 1H24: Phase 1 HV study
completion – 1Q24: Phase 1 data – 1Q24: IND amendment – 2Q25: Phase 2 POC study completion – 4Q24: Phase 1 PET study completion – 2Q24: EOP1 meeting – 4Q24: Patient data • – 2Q25: Chronic tox studies
Timing of Key Milestones – 2H24: Phase 2 data update – 3Q26: BLA submission – 3Q25: Biomarker studies completion – 1H25: BLA submission • – 2023 and 2024 initiate multiple Phase 2 – 3Q25: Phase 2a trial
initiation • – 2Q24: IND amendment POC studies • – 1H25: Patient data – 1Q25: Phase 2 POC studies completion – 4Q25: IND submission – 1Q25: Patient data – 4Q26: BLA submission – 1Q26: Phase 1
study initiation – 1H26: BLA submission • • – 3Q24: IND amendment – Mid 2025: Patient data – 4Q26: BLA submission Plans for PACIFIC’s • If specific PAC FIC employees fulfill open roles, they can be
considered for combined company • N/A Employees Exclusivity • N/A • 45 days • Financial: • Legal: Advisors • Audit: • Legal: • Auditor: • Board Composition: Proportionate to the equity split and
include combined company’s CEO (or as agreed upon in sign and close structure to not require vote until post-close) • Board Composition: 7-9 Board seats; up to 2 designated by PAC FIC • Audit Status: 2 years of audited statements
can be available within 3 months • Audit Status: 2019-2021 audits completed; 2022 audit to be completed by 06/30/23 Additional Information • Proposes announcing PAC FIC share buyback upon announcement of transaction for up to $100M
• Ongoing discussions with investors to extend , which would increase post-money • May assign value to PACIFIC’s assets on case-by-case basis; open to PACIFIC monetizing assets prior for and valuation for proposed transaction
accordingly to transaction close • • Select Investors (1): plans to announce a share buyback upon announcement of the transaction for up to $110M to provide liquidity to PAC FIC stockholders seeking to exit the transaction per the
Company’s revised proposal dated 06/09/23. The original proposal planned for a $100M share buyback. (2): would be open to returning $60M of the $140M available estimated net cash at close to the shareholders of PACIFIC. The revised transaction
structure was communicated following feedback on the initial proposal regarding returning PACIFIC cash to pre-merger PACIFIC shareholders via dividend. Note: Bold indicates catalysts that could fall within the transaction window. Confidential 11 [ ]
indicates information that has been omitted on the basis of a confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange
Commission.

C. INITIAL INDICATIONS OF INTEREST FROM DE-PRIORITIZED
STRATEGICS

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

D. INITIAL INDICATION OF INTEREST FROM STRATEGIC WHO HAS
PASSED

PROJECT PACIFIC INITIAL INDICATION OF INTEREST FROM STRATEGIC WHO HAS
PASSED: (1) Transaction Structure • Reverse triangular merger and Timing • Entry into a definitive merger agreement within 30-45 days of being selected to move forward • : $402-434M (includes $142M of cash expected at 06/30/23)
Implied Valuation • PACIFIC: $140M (based on $140M net cash at close + $0M for Nasdaq listing) Post-$ Last Round / • ’ post-money from last private round ( ): $268M Step-up • Step-up: 1.50x-1.62x Implied Ownership Split
• (Excl. PIPE) : 74.2-75.6% / PACIFIC: 24.4-25.8% Concurrent Financing • No planned financing concurrent with the transaction • Pro forma cash: $282M ( : $142M expected at 06/30/23; PACIFIC: $140M) Pro Forma Cash and Runway •
Runway: into 2H 2026 • (Phase 1/2) – • (IND-enabling) – Key Asset(s) • (Discovery) – • (Discovery) – • (IND-enabling) – • – Mid-2024: Phase 1/2 clinical POC data • •
– 1Q24: In vivo POC – 4Q23: IND filing – 2H24: Development candidate selection – 2Q24: Initiate Phase 1/2 trial – 4Q25: IND filing Timing of Key Milestones – 2H25: Phase 1/2 clinical POC data – 1H26:
Initiate Phase 1/2 trial • • – 4Q23: In vivo POC – 2H23: IDE filing – 1H24: Development candidate selection – 1Q24: CTA filing – 2Q25: IND filing – 3Q25: Initiate Phase 1/2 trial Plans for
PACIFIC’s Employees • N/A Exclusivity • N/A • Legal: Advisors • Auditor: • Board Composition: 10 members; 9 designated by and 1 designated by PACIFIC Additional Information • Audit Status: 5 years of audited
financials, including 2022 • Select Investors (1): would consider a scenario that dividends any cash net of $100M from the PACIFIC balance sheet back to PACIFIC shareholders. The revised transaction structure was communicated following
feedback on the initial proposal regarding returning PACIFIC cash to pre-merger PACIFIC shareholders via dividend. Note: Bold indicates catalysts that could fall within the transaction window. Confidential 17 [ ] indicates information that has been
omitted on the basis of a confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

E. ILLUSTRATIVE PROCESS TIMELINE
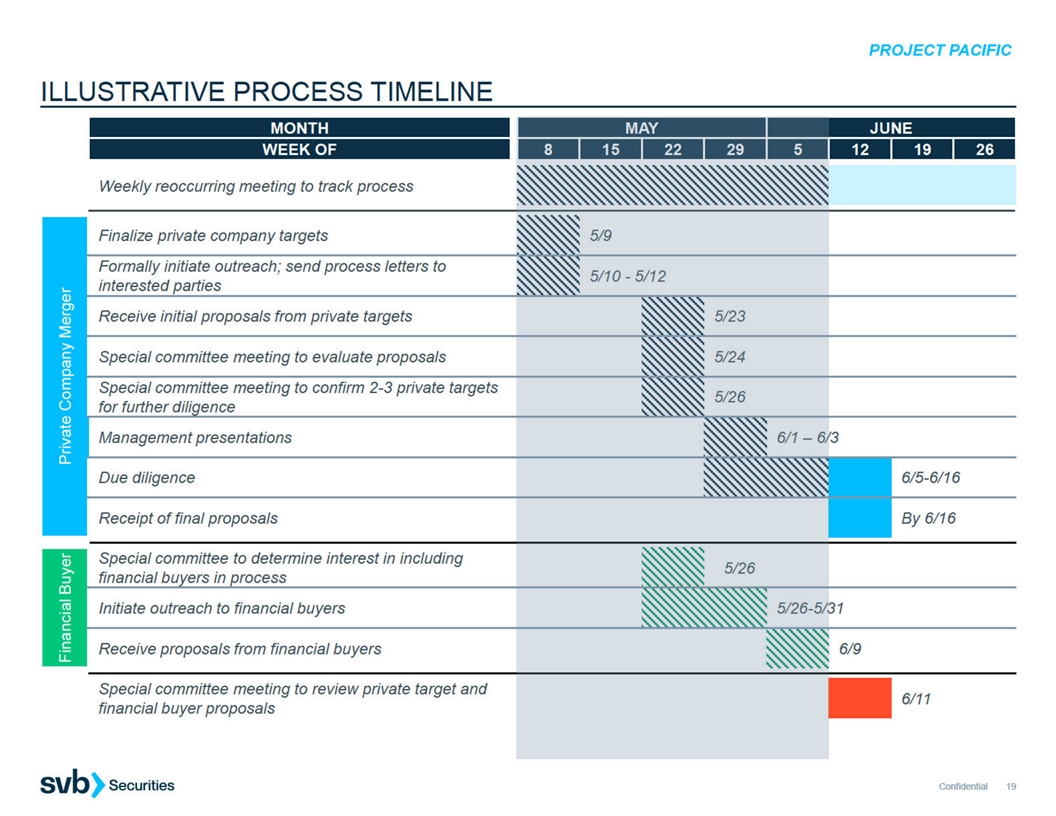

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT HAVE BEEN OMITTED AND
REPLACED WITH [ XYZ ] . SUCH IDENTIFIED INFORMATION HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT (I) IS NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL. Exhibit (c)(9)

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

PROJECT PACIFIC HOW PRIORITIZED COUNTERPARTIES ARE VALUING PACIFIC AND
THEMSELVES $ IN USD MILLIONS. SORTED ALPHABETICALLY. PACIFIC VALUATION COUNTERPARTY PF OWNERSHIP PROPOSED TRANSACTION RETURN OF CASH ASSETS / PRO FORMA TARGET PIPE PARTY STRUCTURE CAPITAL DELIVERED LISTING TECHNOLOGY CVR TOTAL VALUATION STEP-UP CASH
RUNWAY (INSIDER COMMITMENT) PACIFIC PARTY PIPE ACTIVE (1) S-4 - $140 $15 - None $155 $310 1.00x Through 2027 - 33.3% 66.7% - (2) (3) (1) S-4 $60 80 15 - None 95 310 1.00x [Through 2027] - 23.5% 76.5% - PASSED FOLLOWING SPECIAL COMMITTEE FEEDBACK S-4
- $140 - - None $140 $402-434 1.50-1.62x Into 2H 2026 - 24.4-25.8% 74.2-75.6% - (4) (4) S-4 $40 100 - - None 100 402-434 1.50-1.62x [Into 2H 2026] - 18.7-19.9% 80.1-81.3% - (5) (5) Sign-and-close 100 40 $10 - None 50 257 1.00x Until early 2026 $110
(68%) 12.0% 61.6% 26.4% (6) (6) S-4 110 30 15 - None 45 257 1.00x Until early 2026 120 (63%) 10.7% 60.9% 28.4% (1): Ongoing discussions with investors to extend by , which would increase post-money for and valuation for proposed transac ion
accordingly. would not require a concurrent financing for the transaction. (2): would be open to returning $60M of the $140M available estimated net cash at close to the shareholders of PACIFIC. (3): Pro forma runway per initial indication of
interest. The company has since proposed a return of capital mechanism and has not given guidance on how that may impact the pro forma runway. (4): would consider a scenario that dividends any cash net of $100M from the PACIFIC balance sheet back to
PACIFIC shareholders. (5): plans to announce a share buyback upon announcement of the transaction for up to $100M to provide liquidity to PACIFIC stockholders seeking to exit the transaction. (6): revised its proposed share buyback to return up to
$110M to PACIFIC stockholders. Note: Green shading denotes revised proposal communicated following further feedback from PACIFIC’s special committee. Blue shading denotes revised proposal communicated following initial feedback from
PACIFIC’s special committee. Confidential 2 [ ] indicates information that has been omitted on the basis of a confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been
submitted separately with the Securities and Exchange Commission.

PROJECT PACIFIC OFFERS FOR PACIFIC CURRENT PRIOR Date of Offer •
June 9, 2023 • May 30, 2023 • Cash tender offer Transaction Structure • “Back-end” merger to acquire any shares not tendered Requested Response Date • N/A Timing • 3Q23 expected close Support Agreements
• Certain of PACIFIC’s key shareholders • Terminated as of closing of “back-end” merger Treatment of Stock Options • In-the-money options cashed out for intrinsic value • 30 days Exclusivity • 10-day
extension if initial period lapses and parties remain in negotiations unless party provides 10-day written notice of non-renewal (1) (2) Cash Consideration as $ per Share • $2.06 per share • $2.07 per share (3) • $127M cash
(PACIFIC net cash minus $14M, assuming PACIFIC net cash at close is $141M) • Excess net cash at close greater than $139M will be (4) • $127M cash (assumes PACIFIC net cash at close is $141M) Aggregate Consideration returned to existing
PACIFIC shareholders • CVR for 80% of net proceeds from sale of PACIFIC’s assets • CVR for 80% of the net proceeds from the sale of PACIFIC’s legacy assets, such as pomotrelvir, PBI- 2158, the backup leads and the IP Cash
Consideration as % of PACIFIC (5) (6) • 90% • 90% Net Cash Cash Consideration as a Premium to • 10% • 11% (7) Closing Price as of 06/09/23 (1): Calculated based on aggregate consideration of $127M and ~61.7M common shares
outstanding per PACIFIC’s latest 10-Q filing. (2): Calculated assuming aggregate cash considera ion of $127M, based on aggregate cash consideration equal to 90% of assumed PACIFIC net cash at close per initial proposal received on 05/30/23,
and ~61.7M PACIFIC common shares outstanding. Initial proposal of aggregate cash considera ion equal to $135M, based on assumed PACIFIC net cash at close of $150M, implied cash consideration of $2.19 per share. (3): Cash consideration of $141M
assumed PACIFIC net cash at close per management guidance minus $14M per ’s proposal received on 06/09/23, representing approximately 90% of the assumed net cash. (4): Assumed cash consideration equal to 90% of assumed PACIFIC net cash at
close based on initial proposal received on 05/30/23. Initial proposed cash consideration of $135M was based on ’s assumed PACIFIC cash at close of $150M. (5): Based aggregate consideration of $127M and assumed PACIFIC net cash at close of
$141M per management guidance. (6): Based aggregate consideration of $135M and assumed PACIFIC net cash at close of $150M per ’s proposal received on 05/30/23. Confidential 3 (7): Based on PACIFIC closing price of $1.87 per share as of
06/09/23. [ ] indicates information that has been omitted on the basis of a confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities
and Exchange Commission.

PROJECT PACIFIC FORESITE CAPITAL’S OFFERS FOR PACIFIC CURRENT
PRIOR Date of Offer • June 10, 2023 • June 5, 2023 Transaction Structure • Cash tender offer for outstanding shares not currently owned by FORESITE • June 12, 2023, 5pm ET (2 days from offer date) • June 8, 2023, 5pm ET
(3 days from offer date) Requested Response Date Timing • N/A Support Agreements • N/A Treatment of Stock Options • N/A Exclusivity • N/A Cash Consideration as $ per Share • $2.09 per share • $1.93 per share (1)
• $129M cash (PACIFIC net cash minus $12M, (2) assuming PACIFIC net cash at close is $141M) • $119M cash (assumes PACIFIC net cash at close is $141M) Aggregate Consideration • CVR for 90% of the net proceeds payable from any
• CVR for 80% of the net proceeds payable from any license or license or disposition involving Pomotrelvir within one disposition involving Pomotrelvir within one year of closing year of closing Cash Consideration as % of PACIFIC • 91%
• 84% (3) Net Cash Cash Consideration as a Premium to • 12% • 3% (4) Closing Price as of 06/09/23 (1): Based on assumed $141M PACIFIC net cash at close less $12M per FORESITE’s proposal dated 06/10/23. Cash tender to be equal
to aggregate considera ion of $129M multiplied by the percentage of common shares not owned by FORESITE and its affiliates, ~16.8M shares (~27.2% of common shares outstanding). (2): Based on offer price of $1.93 per share and ~61.7M common shares
outstanding per PACIFIC’s latest 10-Q filing, including ~16.8M shares (~27.2% of common shares outstanding) owned by FORESITE and its affiliates at the offer date. (3): Based on $141M assumed PACIFIC net cash at close per FORESITE proposal.
Confidential 4 (4): Based on PACIFIC closing price of $1.87 per share as of 06/09/23.

PROJECT PACIFIC DISCOUNTS TO NET CASH IMPLIED BY RECENT FINANCIAL
BUYERS’ PROPOSALS ($ in millions, except per share values) Terms of Proposal Cash Consideration Non-Contingent Offer Target As a % of Premium to Ann. Financial Cash per Equity Total per Aggregate Contingent Net Cash Net Cash Unaffected Offer
(1) Date Company Buyer Share per Share Share Value Value Rights at Close at Close Price Accepted 80% of the net proceeds payable from any (2) 05/30/23 $8.00 - $8.00 $53 license or disposition of the MEI's clinical $76 70% 11% O assets 80% of the net
proceeds payable from any 05/22/23 5.75 - 5.75 480 570 84% 55% O license or disposition of Atea’s programs Echo Lake (3) 03/21/23 1.60 - 1.60 58 None 87 67% 90% O Capital 80% of the net proceeds for ten years post- closing from any license or
disposition of (4) 03/14/23 1.85 - 1.85 96 Jounce’s programs effected within two years 115 85% 75% P of closing and 100% of the potential aggregate value of certain potential cost savings Mean: 76% 58% Median: 77% 65% 25th Percentile: 69% 44%
75th Percentile: 84% 78% (1): Estimated net cash at close per offer filing unless otherwise noted. (2): Expected net cash of ~$93M at 06/30/23 per the S-4 filing, net of ~$17M of total minimum operating lease payments (carrying value of $13M due to
present value discounting) as of 03/31/23. (3): $90M cash and cash equivalents net of $3M total liabilities as of 03/31/23. (4): Represents final offer accepted on 03/27/23, increased from the 03/14/23 offer of $1.80 per share + CVR. Note: Includes
unsolicited offers from financial buyers for biopharma companies trading below net cash. Excludes unsolicited offers for companies which total liabilities exceeded cash and cash equivalents at he time of the offer. Source: Company press releases and
filings. Confidential 5

APPENDIX

A. CATALYSTS IN PRO FORMA CASH RUNWAY OF STRATEGICS THAT HAVE SUBMITTED
INDICATIONS OF INTEREST

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

B. INITIAL INDICATIONS OF INTEREST FROM STRATEGIC WHO HAS BEEN
PRIORITIZED
PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST FROM STRATEGIC WHO HAS
BEEN PRIORITIZED: Transaction Structure • Reverse triangular merger and Timing • Due diligence could be completed by both parties in 45 days • : $310M (includes $130M cash expected at 06/30/23) Implied Valuation • PACIFIC:
$155M (based on $140M net cash at close + $15M for Nasdaq listing) Post-$ Last Round / • ’s post-money from last private round $310M Step-up • Step-up: 1.00x Implied Ownership Split • (Excl. PIPE) : 66.7% / PACIFIC: 33.3%
• No planned financing concurrent with the transaction, however, is in discussions to extend , which would increase Concurrent Financing post-money for and valuation for proposed transaction accordingly • Pro forma cash: $270M ( $130M;
PACIFIC: $140M) Pro Forma Cash and Runway • Runway: through 2027, not including potential milestones payments from related to • (IND-enabling) – • (Phase 1 complete) – Key Asset(s) • Entered into a global license
and development agreement with in for • • – 1H23: IND filing • – 1H24: Phase 1 HV study completion – 2H23: Phase 2 POC initiation – 4Q24: Phase 1 PET study completion – 2Q25: Phase 2 POC study
completion – 2Q25: Chronic tox studies Timing of Key Milestones • – 3Q25: Biomarker studies completion – 2023 and 2024: initiate multiple Phase 2 POC studies – 3Q25: Phase 2a trial initiation – 1Q25: Phase 2 POC
studies completion • – 4Q25: IND submission – 1Q26: Phase 1 study initiation Plans for PACIFIC’s Employees • N/A Exclusivity • 45 days • Legal: Advisors • Auditor: • Board Composition: 7-9 Board
seats; up to 2 designated by PACIFIC • Audit Status: 2019-2021 audits completed; 2022 audit to be completed by 06/30/23 Additional Information • Ongoing discussions with investors to extend , which would increase post-money for and
valuation for proposed transaction accordingly • Select Investors (1): would be open to returning $60M of the $140M available estimated net cash at close to the shareholders of PACIFIC. The revised transaction structure was communicated
following feedback on the initial proposal regarding returning PACIFIC cash to pre-merger PACIFIC shareholders via dividend. Note: Bold indicates catalysts that could fall within the transaction window. Confidential 12 [ ] indicates information that
has been omitted on the basis of a confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

C. INITIAL INDICATIONS OF INTEREST FROM DE-PRIORITIZED
STRATEGICS
[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

D. INITIAL INDICATION OF INTEREST FROM STRATEGIC WHO HAS
PASSED

[ ] indicates information that has been omitted on the basis of a
confidential treatment request pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. The information has been submitted separately with the Securities and Exchange Commission.

E. ILLUSTRATIVE PROCESS TIMELINE

Exhibit (f)
Section 262 of the General Corporation Law of the State of Delaware
§ 262 Appraisal rights
(a) Any
stockholder of a corporation of this State who holds shares of stock on the date of the making of a demand pursuant to subsection (d) of this section with respect to such shares, who continuously holds such shares through the effective date of
the merger, consolidation, or conversion, who has otherwise complied with subsection (d) of this section and who has neither voted in favor of the merger, consolidation or conversion nor consented thereto in writing pursuant to §228 of
this title shall be entitled to an appraisal by the Court of Chancery of the fair value of the stockholder’s shares of stock under the circumstances described in subsections (b) and (c) of this section. As used in this section, the
word “stockholder” means a holder of record of stock in a corporation; the words “stock” and “share” mean and include what is ordinarily meant by those words; the words “depository receipt” mean a receipt or
other instrument issued by a depository representing an interest in 1 or more shares, or fractions thereof, solely of stock of a corporation, which stock is deposited with the depository; the words “beneficial owner” mean a person who is
the beneficial owner of shares of stock held either in voting trust or by a nominee on behalf of such person; and the word “person” means any individual, corporation, partnership, unincorporated association or other entity.
(b) Appraisal rights shall be available for the shares of any class or series of stock of a constituent or converting corporation in a merger,
consolidation or conversion to be effected pursuant to § 251 (other than a merger effected pursuant to § 251(g) of this title), § 252, § 254, § 255, § 256, § 257, § 258, § 263, § 264 or § 266 of
this title (other than, in each case and solely with respect to a domesticated corporation, a merger, consolidation or conversion authorized pursuant to and in accordance with the provisions of § 388 of this title):
(1) Provided, however, that no appraisal rights under this section shall be available for the shares of any class or series of stock, which
stock, or depository receipts in respect thereof, at the record date fixed to determine the stockholders entitled to receive notice of the meeting of stockholders, or at the record date fixed to determine the stockholders entitled to consent
pursuant to § 228 of this title, to act upon the agreement of merger or consolidation or the resolution providing for conversion (or, in the case of a merger pursuant to § 251(h) of this title, as of immediately prior to the execution of
the agreement of merger), were either: (i) listed on a national securities exchange or (ii) held of record by more than 2,000 holders; and further provided that no appraisal rights shall be available for any shares of stock of the
constituent corporation surviving a merger if the merger did not require for its approval the vote of the stockholders of the surviving corporation as provided in § 251(f) of this title.
(2) Notwithstanding paragraph (b)(1) of this section, appraisal rights under this section shall be available for the shares of any class or
series of stock of a constituent or converting corporation if the holders thereof are required by the terms of an agreement of merger or consolidation, or by the terms of a resolution providing for conversion, pursuant to §251, § 252,
§ 254, § 255, § 256, § 257, § 258, § 263, §264 or § 266 of this title to accept for such stock anything except:
a. Shares of stock of the corporation surviving or resulting from such merger or consolidation, or of the converted entity if such entity is a
corporation as a result of the conversion, or depository receipts in respect thereof;
b. Shares of stock of any other corporation, or
depository receipts in respect thereof, which shares of stock (or depository receipts in respect thereof) or depository receipts at the effective date of the merger, consolidation or conversion will be either listed on a national securities exchange
or held of record by more than 2,000 holders;
c. Cash in lieu of fractional shares or fractional depository receipts described in the
foregoing paragraphs (b)(2)a. and b. of this section; or
d. Any combination of the shares of stock, depository receipts and cash in lieu
of fractional shares or fractional depository receipts described in the foregoing paragraphs (b)(2)a., b. and c. of this section.
(3) In the event all of the stock of a subsidiary Delaware corporation party to a merger
effected under § 253 or § 267 of this title is not owned by the parent immediately prior to the merger, appraisal rights shall be available for the shares of the subsidiary Delaware corporation.
(4) [Repealed.]
(c) Any
corporation may provide in its certificate of incorporation that appraisal rights under this section shall be available for the shares of any class or series of its stock as a result of an amendment to its certificate of incorporation, any merger or
consolidation in which the corporation is a constituent corporation, the sale of all or substantially all of the assets of the corporation or a conversion effected pursuant to § 266 of this title. If the certificate of incorporation contains
such a provision, the provisions of this section, including those set forth in subsections (d), (e), and (g) of this section, shall apply as nearly as is practicable.
(d) Appraisal rights shall be perfected as follows:
(1) If a proposed merger, consolidation or conversion for which appraisal rights are provided under this section is to be submitted for
approval at a meeting of stockholders, the corporation, not less than 20 days prior to the meeting, shall notify each of its stockholders who was such on the record date for notice of such meeting (or such members who received notice in accordance
with §255(c) of this title) with respect to shares for which appraisal rights are available pursuant to subsection (b) or (c) of this section that appraisal rights are available for any or all of the shares of the constituent
corporations or the converting corporation, and shall include in such notice either a copy of this section (and, if 1 of the constituent corporations or the converting corporation is a nonstock corporation, a copy of §114 of this title) or
information directing the stockholders to a publicly available electronic resource at which this section (and, § 114 of this title, if applicable) may be accessed without subscription or cost. Each stockholder electing to demand the appraisal
of such stockholder’s shares shall deliver to the corporation, before the taking of the vote on the merger, consolidation or conversion, a written demand for appraisal of such stockholder’s shares; provided that a demand may be delivered
to the corporation by electronic transmission if directed to an information processing system (if any) expressly designated for that purpose in such notice. Such demand will be sufficient if it reasonably informs the corporation of the identity of
the stockholder and that the stockholder intends thereby to demand the appraisal of such stockholder’s shares. A proxy or vote against the merger, consolidation or conversion shall not constitute such a demand. A stockholder electing to take
such action must do so by a separate written demand as herein provided. Within 10 days after the effective date of such merger, consolidation or conversion, the surviving, resulting or converted entity shall notify each stockholder of each
constituent or converting corporation who has complied with this subsection and has not voted in favor of or consented to the merger, consolidation or conversion, and any beneficial owner who has demanded appraisal under paragraph (d)(3) of this
section, of the date that the merger, consolidation or conversion has become effective; or
(2) If the merger, consolidation or
conversion was approved pursuant to § 228, § 251(h), § 253, or § 267 of this title, then either a constituent or converting corporation before the effective date of the merger, consolidation or conversion, or the surviving,
resulting or converted entity within 10 days after such effective date, shall notify each stockholder of any class or series of stock of such constituent or converting corporation who is entitled to appraisal rights of the approval of the merger,
consolidation or conversion and that appraisal rights are available for any or all shares of such class or series of stock of such constituent or converting corporation, and shall include in such notice either a copy of this section (and, if 1 of
the constituent corporations or the converting corporation is a nonstock corporation, a copy of § 114 of this title) or information directing the stockholders to a publicly available electronic resource at which this section (and § 114 of
this title, if applicable) may be accessed without subscription or cost. Such notice may, and, if given on or after the effective date of the merger, consolidation or conversion, shall, also notify such stockholders of the effective date of the
merger, consolidation or conversion. Any stockholder entitled to appraisal rights may, within 20 days after the date of giving such notice or, in the case of a merger approved pursuant to § 251(h) of this title, within the later of the
consummation of the offer contemplated by § 251(h) of this title and 20 days after the date of giving such notice, demand in writing from the surviving or resulting entity the appraisal of such holder’s shares; provided that a demand may
be delivered to such entity by electronic transmission if directed to an information processing system (if any) expressly designated for that purpose in such notice. Such demand will be sufficient if it reasonably informs such entity of the identity
of the stockholder and that the stockholder intends thereby to demand the appraisal of such holder’s shares. If such notice did not notify stockholders of the effective date of the merger, consolidation or conversion, either (i) each such
2
constituent corporation or the converting corporation shall send a second notice before the effective date of the merger, consolidation or conversion notifying each of the holders of any class or
series of stock of such constituent or converting corporation that are entitled to appraisal rights of the effective date of the merger, consolidation or conversion or (ii) the surviving, resulting or converted entity shall send such a second
notice to all such holders on or within 10 days after such effective date; provided, however, that if such second notice is sent more than 20 days following the sending of the first notice or, in the case of a merger approved pursuant to §
251(h) of this title, later than the later of the consummation of the offer contemplated by § 251(h) of this title and 20 days following the sending of the first notice, such second notice need only be sent to each stockholder who is entitled
to appraisal rights and who has demanded appraisal of such holder’s shares in accordance with this subsection and any beneficial owner who has demanded appraisal under paragraph (d)(3) of this section. An affidavit of the secretary or assistant
secretary or of the transfer agent of the corporation or entity that is required to give either notice that such notice has been given shall, in the absence of fraud, be prima facie evidence of the facts stated therein. For purposes of determining
the stockholders entitled to receive either notice, each constituent corporation or the converting corporation may fix, in advance, a record date that shall be not more than 10 days prior to the date the notice is given, provided, that if the notice
is given on or after the effective date of the merger, consolidation or conversion, the record date shall be such effective date. If no record date is fixed and the notice is given prior to the effective date, the record date shall be the close of
business on the day next preceding the day on which the notice is given.
(3) Notwithstanding subsection (a) of this section (but
subject to this paragraph (d)(3)), a beneficial owner may, in such person’s name, demand in writing an appraisal of such beneficial owner’s shares in accordance with either paragraph (d)(1) or (2) of this section, as applicable;
provided that (i) such beneficial owner continuously owns such shares through the effective date of the merger, consolidation or conversion and otherwise satisfies the requirements applicable to a stockholder under the first sentence of
subsection (a) of this section and (ii) the demand made by such beneficial owner reasonably identifies the holder of record of the shares for which the demand is made, is accompanied by documentary evidence of such beneficial owner’s
beneficial ownership of stock and a statement that such documentary evidence is a true and correct copy of what it purports to be, and provides an address at which such beneficial owner consents to receive notices given by the surviving, resulting
or converted entity hereunder and to be set forth on the verified list required by subsection (f) of this section.
(e) Within 120
days after the effective date of the merger, consolidation or conversion, the surviving, resulting or converted entity, or any person who has complied with subsections (a) and (d) of this section hereof and who is otherwise entitled to
appraisal rights, may commence an appraisal proceeding by filing a petition in the Court of Chancery demanding a determination of the value of the stock of all such stockholders. Notwithstanding the foregoing, at any time within 60 days after the
effective date of the merger, consolidation or conversion, any person entitled to appraisal rights who has not commenced an appraisal proceeding or joined that proceeding as a named party shall have the right to withdraw such person’s demand
for appraisal and to accept the terms offered upon the merger, consolidation or conversion. Within 120 days after the effective date of the merger, consolidation or conversion, any person who has complied with the requirements of subsections
(a) and (d) of this section hereof, upon request given in writing (or by electronic transmission directed to an information processing system (if any) expressly designated for that purpose in the notice of appraisal), shall be entitled to
receive from the surviving, resulting or converted entity a statement setting forth the aggregate number of shares not voted in favor of the merger, consolidation or conversion (or, in the case of a merger approved pursuant to § 251(h) of this
title, the aggregate number of shares (other than any excluded stock (as defined in § 251(h)(6)d. of this title)) that were the subject of, and were not tendered into, and accepted for purchase or exchange in, the offer referred to in §
251(h)(2) of this title), and, in either case, with respect to which demands for appraisal have been received and the aggregate number of stockholders or beneficial owners holding or owning such shares (provided that, where a beneficial owner makes
a demand pursuant to paragraph (d)(3) of this section, the record holder of such shares shall not be considered a separate stockholder holding such shares for purposes of such aggregate number). Such statement shall be given to the person within 10
days after such person’s request for such a statement is received by the surviving, resulting or converted entity or within 10 days after expiration of the period for delivery of demands for appraisal under subsection (d) of this section
hereof, whichever is later.
(f) Upon the filing of any such petition by any person other than the surviving, resulting or converted
entity, service of a copy thereof shall be made upon such entity, which shall within 20 days after such service file in the office of the Register in Chancery in which the petition was filed a duly verified list containing the names and addresses of
all persons who have demanded appraisal for their shares and with whom agreements as to the value of
3
their shares have not been reached by such entity. If the petition shall be filed by the surviving, resulting or converted entity, the petition shall be accompanied by such a duly verified list.
The Register in Chancery, if so ordered by the Court, shall give notice of the time and place fixed for the hearing of such petition by registered or certified mail to the surviving, resulting or converted entity and to the persons shown on the list
at the addresses therein stated. The forms of the notices by mail and by publication shall be approved by the Court, and the costs thereof shall be borne by the surviving, resulting or converted entity.
(g) At the hearing on such petition, the Court shall determine the persons who have complied with this section and who have become entitled to
appraisal rights. The Court may require the persons who have demanded an appraisal for their shares and who hold stock represented by certificates to submit their certificates of stock to the Register in Chancery for notation thereon of the pendency
of the appraisal proceedings; and if any person fails to comply with such direction, the Court may dismiss the proceedings as to such person. If immediately before the merger, consolidation or conversion the shares of the class or series of stock of
the constituent or converting corporation as to which appraisal rights are available were listed on a national securities exchange, the Court shall dismiss the proceedings as to all holders of such shares who are otherwise entitled to appraisal
rights unless (1) the total number of shares entitled to appraisal exceeds 1% of the outstanding shares of the class or series eligible for appraisal, (2) the value of the consideration provided in the merger, consolidation or conversion
for such total number of shares exceeds $1 million, or (3) the merger was approved pursuant to § 253 or § 267 of this title.
(h) After the Court determines the persons entitled to an appraisal, the appraisal proceeding shall be conducted in accordance with the rules
of the Court of Chancery, including any rules specifically governing appraisal proceedings. Through such proceeding the Court shall determine the fair value of the shares exclusive of any element of value arising from the accomplishment or
expectation of the merger, consolidation or conversion, together with interest, if any, to be paid upon the amount determined to be the fair value. In determining such fair value, the Court shall take into account all relevant factors. Unless the
Court in its discretion determines otherwise for good cause shown, and except as provided in this subsection, interest from the effective date of the merger, consolidation or conversion through the date of payment of the judgment shall be compounded
quarterly and shall accrue at 5% over the Federal Reserve discount rate (including any surcharge) as established from time to time during the period between the effective date of the merger, consolidation or conversion and the date of payment of the
judgment. At any time before the entry of judgment in the proceedings, the surviving, resulting or converted entity may pay to each person entitled to appraisal an amount in cash, in which case interest shall accrue thereafter as provided herein
only upon the sum of (1) the difference, if any, between the amount so paid and the fair value of the shares as determined by the Court, and (2) interest theretofore accrued, unless paid at that time. Upon application by the surviving,
resulting or converted entity or by any person entitled to participate in the appraisal proceeding, the Court may, in its discretion, proceed to trial upon the appraisal prior to the final determination of the persons entitled to an appraisal. Any
person whose name appears on the list filed by the surviving, resulting or converted entity pursuant to subsection (f) of this section may participate fully in all proceedings until it is finally determined that such person is not entitled to
appraisal rights under this section.
(i) The Court shall direct the payment of the fair value of the shares, together with interest, if
any, by the surviving, resulting or converted entity to the persons entitled thereto. Payment shall be so made to each such person upon such terms and conditions as the Court may order. The Court’s decree may be enforced as other decrees in the
Court of Chancery may be enforced, whether such surviving, resulting or converted entity be an entity of this State or of any state.
(j)
The costs of the proceeding may be determined by the Court and taxed upon the parties as the Court deems equitable in the circumstances. Upon application of a person whose name appears on the list filed by the surviving, resulting or converted
entity pursuant to subsection (f) of this section who participated in the proceeding and incurred expenses in connection therewith, the Court may order all or a portion of such expenses, including, without limitation, reasonable attorney’s
fees and the fees and expenses of experts, to be charged pro rata against the value of all the shares entitled to an appraisal not dismissed pursuant to subsection (k) of this section or subject to such an award pursuant to a reservation of
jurisdiction under subsection (k) of this section.
4
(k) From and after the effective date of the merger, consolidation or conversion, no person
who has demanded appraisal rights with respect to some or all of such person’s shares as provided in subsection (d) of this section shall be entitled to vote such shares for any purpose or to receive payment of dividends or other
distributions on such shares (except dividends or other distributions payable to stockholders of record at a date which is prior to the effective date of the merger, consolidation or conversion); provided, however, that if no petition for an
appraisal is filed within the time provided in subsection (e) of this section, or if a person who has made a demand for an appraisal in accordance with this section shall deliver to the surviving, resulting or converted entity a written
withdrawal of such person’s demand for an appraisal in respect of some or all of such person’s shares in accordance with subsection (e) of this section, then the right of such person to an appraisal of the shares subject to the
withdrawal shall cease. Notwithstanding the foregoing, no appraisal proceeding in the Court of Chancery shall be dismissed as to any person without the approval of the Court, and such approval may be conditioned upon such terms as the Court deems
just, including without limitation, a reservation of jurisdiction for any application to the Court made under subsection (j) of this section; provided, however that this provision shall not affect the right of any person who has not commenced
an appraisal proceeding or joined that proceeding as a named party to withdraw such person’s demand for appraisal and to accept the terms offered upon the merger, consolidation or conversion within 60 days after the effective date of the
merger, consolidation or conversion, as set forth in subsection (e) of this section.
(l) The shares or other equity interests of the
surviving, resulting or converted entity to which the shares of stock subject to appraisal under this section would have otherwise converted but for an appraisal demand made in accordance with this section shall have the status of authorized but not
outstanding shares of stock or other equity interests of the surviving, resulting or converted entity, unless and until the person that has demanded appraisal is no longer entitled to appraisal pursuant to this section.
5
Exhibit 107
Calculation of Filing Fee Tables
Schedule 13E-3
(Form Type)
Pardes
Biosciences, Inc.
(Exact Name of Registrant and Name of Person Filing Statement)
Table 1 - Transaction Value
|
|
|
|
|
|
|
| |
|
|
|
| |
|
Transaction
valuation |
|
Fee
Rate |
|
Amount
of Filing Fee |
| |
|
|
|
| Fees to Be
Paid |
|
$132,350,817.00(1) |
|
0.0001102 |
|
$14,585.06 (2) |
| |
|
|
|
| Fees Previously
Paid |
|
$136,088,312.78 |
|
|
|
$14,996.93 |
| |
|
|
|
| Total Transaction
Valuation |
|
$132,350,817.00 |
|
|
|
|
| |
|
|
|
| Total Fees Due for
Filing |
|
|
|
|
|
$14,585.06 |
| |
|
|
|
| Total Fees
Previously Paid |
|
|
|
|
|
$14,996.93 |
| |
|
|
|
| Total Fee
Offsets |
|
|
|
|
|
$14,585.06 |
| |
|
|
|
|
Net Fee Due |
|
|
|
|
|
$0.00 |
Table 2—Fee Offset Claims and Sources
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
| |
|
Registrant
or Filer Name |
|
Form
or Filing
Type |
|
File
Number |
|
Initial
Filing
Date |
|
Filing
Date |
|
Fee
Offset
Claimed |
|
Fee
Paid with
Fee Offset
Source |
| |
|
|
|
|
|
|
|
| Fee Offset
Claims |
|
|
|
Schedule
TO |
|
231123926 |
|
July 28,
2023 |
|
|
|
$14,585.06 |
|
|
| |
|
|
|
|
|
|
|
|
Fee Offset Sources |
|
MediPacific Sub, Inc;
MediPacific, Inc.; FS
Development Holdings
II, LLC; Foresite Capital
Management V, LLC;
Foresite
Capital
Opportunity Fund V,
L.P.; Foresite Capital
Opportunity Management
V, LLC; Foresite Capital
Fund V, L.P.; James
Tananbaum |
|
Schedule
TO |
|
231123926 |
|
|
|
July 28,
2023 |
|
|
|
$14,585.06 |
| (1) |
Estimated solely for the purpose of calculating the filing fee. The transaction value was calculated by adding
(i) the product of (a) $2.13, which is the price per share of common stock of Pardes Biosciences, Inc. (the “Company”) (each, a “Share”), and (b) 62,054,756 Shares issued and outstanding (including restricted Shares) and
(ii) the product of (a) the difference between (1) $2.13 and (2) an exercise price of $2.04 (the weighted-average exercise price of the outstanding options) and (b) stock options representing the right to purchase an aggregate of
1,935,408. The calculation of the transaction value is based on information provided by the Company as of August 15, 2023. |
| (2) |
The amount of the filing fee was calculated by multiplying the transaction valuation by 0.0001102.
|
Pardes Biosciences (NASDAQ:PRDS)
Historical Stock Chart
From Apr 2024 to May 2024
Pardes Biosciences (NASDAQ:PRDS)
Historical Stock Chart
From May 2023 to May 2024