Filed Pursuant to Rule 424(b)(3)
Registration No. 333-261443
PROSPECTUS
37,500,000 Shares of Common Stock
This prospectus relates to the resale, from time to time, of up to 37,500,000 shares (the “Shares”) of NovaBay Pharmaceuticals, Inc.’s (“us”, “we”, “our”, “NovaBay”, or the “Company”) common stock, par value $0.01 per share (the “Common Stock”) upon the conversion of 15,000 shares of our Series B Non-Voting Convertible Preferred Stock sold to the selling stockholders (the “Selling Stockholders”) in a private placement offering that was consummated on November 2, 2021 (the “Private Placement”) pursuant to a Securities Purchase Agreement, dated October 29, 2021, by and between the Company and each of the Selling Stockholders (the “Securities Purchase Agreement”). We are registering the Shares held by the Selling Stockholders pursuant to the terms and conditions of the Registration Rights Agreement, dated as of October 29, 2021, which we entered into with the Selling Stockholders in connection with the Private Placement.
The Selling Stockholders may sell all or a portion of the Shares being offered pursuant to this prospectus at the prevailing market prices at the time of sale or at negotiated prices. We provide more information about the Selling Stockholders in the section entitled “Selling Stockholders” on page 48 of this prospectus.
We will not receive any proceeds from the sale of the Shares by the Selling Stockholders. The Selling Stockholders will each bear all commissions and discounts, if any, attributable to their respective sales of the Shares. For more information with respect to the sale of shares of Common Stock offered by this prospectus, see “Plan of Distribution.”
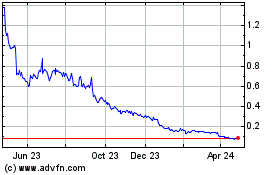
Our Common Stock is listed on the NYSE American under the symbol “NBY.” The last reported sale price of our Common Stock on November 30, 2021 was $0.50 per share.
Investing in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider carefully the risks that we have described under the caption “Risk Factors” on page 7.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is December 10, 2021.
TABLE OF CONTENTS
|
|
Page
|
|
About this Prospectus
|
2
|
|
Prospectus Summary
|
4
|
|
Risk Factors
|
7
|
|
Special Note Regarding Forward-Looking Statements
|
21
|
|
Description of the Private Placement
|
22
|
|
The Acquisition Purchase Agreement
|
26
|
|
Unaudited Pro Forma Condensed Combined Financial Information
|
31
|
|
Use of Proceeds
|
40
|
|
Market of Our Common Stock
|
40
|
|
Dividend Policy
|
40
|
|
Principal Stockholders
|
41
|
|
Description of Capital Stock
|
43
|
|
Selling Stockholders
|
48
|
|
Plan of Distribution
|
50
|
|
Legal Matters
|
52
|
|
Experts
|
52
|
|
Where You Can Find More Information
|
52
|
|
Incorporation of Certain Documents by Reference
|
53
|
About This Prospectus
This prospectus is part of a registration statement on Form S-1 filed with the U.S. Securities and Exchange Commission (the “SEC”), using a “shelf” registration process. By using a shelf registration statement, the Selling Stockholders may sell up to 37,500,000 shares of Common Stock from time to time in one or more offerings as described in this prospectus. We will not receive any proceeds from the sale of Common Stock by the Selling Stockholders.
We may also file a prospectus supplement or post-effective amendment to the registration statement of which this prospectus forms a part that may contain material information relating to this offering. The prospectus supplement or post-effective amendment may also add, update or change information contained in this prospectus with respect to the offering. If there is any inconsistency between the information in this prospectus and the applicable prospectus supplement or post-effective amendment, you should rely on the prospectus supplement or post-effective amendment, as applicable. As permitted by the rules and regulations of the SEC, the registration statement filed by us includes additional information that has been incorporated by reference, including reports we file with the SEC, that are not contained in this prospectus. Before purchasing any securities, you should carefully read this prospectus, any post-effective amendment, and any applicable prospectus supplement, together with the documents incorporated by reference and other additional information that we file with the SEC described in the “Where You Can Find More Information” and “Incorporation of Certain Documents by Reference” sections of this prospectus.
This prospectus, any post-effective amendment, and any applicable prospectus supplement, and the documents incorporated by reference in this prospectus, any post-effective amendment, and any applicable prospectus supplement, include important information about us and the securities being offered. You should rely only on this prospectus, any post-effective amendment, and any applicable prospectus supplement, and the information incorporated or deemed to be incorporated by reference in this prospectus. We have not, and the Selling Stockholders have not, authorized anyone to provide you with information that is in addition to, or different from, the information that is contained, or incorporated by reference, in this prospectus, any post-effective amendment, or any applicable prospectus supplement prepared by or on behalf of us or to which we have referred you. If anyone provides you with different or inconsistent information, you should not rely on it. We and the Selling Stockholders take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus does not constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction.
We further note that the representations, warranties and covenants made by us in the Securities Purchase Agreement, the Registration Rights Agreement and any other agreement that is filed as an exhibit to any document that is incorporated by reference in this prospectus were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
The information appearing in this prospectus, any post-effective amendment and any applicable prospectus supplement to this prospectus is accurate only as of the date on the front of the document and any information we have incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus or any sale of a security. Our business, financial condition, results of operations and prospects may have changed since those dates. This prospectus contains, and any post-effective amendment or any prospectus supplement may contain, market data and industry statistics and forecasts that are based on independent industry publications and other publicly available information. Although we believe these sources are reliable, we do not guarantee the accuracy or completeness of this information and we have not independently verified this information. In addition, the market and industry data and forecasts that may be included in this prospectus, any post-effective amendment or any prospectus supplement may involve estimates, assumptions and other risks and uncertainties and are subject to change based on various factors, including those discussed in the “Risk Factors” section of this prospectus, any post-effective amendment and the applicable prospectus supplement. Accordingly, investors should not place undue reliance on this information.
This prospectus, any post-effective amendment and any applicable prospectus supplement to this prospectus contains summaries of certain provisions contained in some of the documents described herein or therein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the section entitled “Where You Can Find More Information.”
This prospectus, any post-effective amendment and any applicable prospectus supplement to this prospectus and the information incorporated herein and therein by reference include trademarks, service marks and trade names owned by us, our subsidiary DERMAdoctor, LLC, or other companies. All trademarks, service marks and trade names included or incorporated by reference into this prospectus, any post-effective amendment and any applicable prospectus supplement to this prospectus are the property of their respective owners.
Unless the context indicates otherwise in this prospectus, any post-effective amendment and any applicable prospectus supplement to this prospectus, the terms “NovaBay,” the “Company,” the “Registrant,” “we,” “our” or “us” in this prospectus refer to NovaBay Pharmaceuticals, Inc.
PROSPECTUS SUMMARY
This summary highlights, and is qualified in its entirety by, the more detailed information and financial statements contained elsewhere in this prospectus or incorporated by reference in this prospectus. This summary does not contain all of the information that may be important to you or that you need to consider in making your investment decision. You should carefully read the entire prospectus, especially the “Risk Factors” section beginning on page 7 of this prospectus, and our financial statements and other information incorporated by reference in this prospectus before deciding to invest in our Common Stock. Each of the risk factors could adversely affect our business, operating results and financial condition, as well as adversely affect the value of an investment in our securities.
Our Company
We are a pharmaceutical company that develops and sells scientifically created and clinically proven consumer products for the eyecare and skincare markets. A majority of our revenue historically has come from a single product, Avenova®, a U.S. Food and Drug Administration (“FDA”) cleared product sold in the United States that has proven in laboratory testing to have broad antimicrobial properties as it removes foreign material including microorganisms and debris from skin around the eye, including the eyelid. Avenova is formulated with our proprietary, stable and pure form of hypochlorous acid. Avenova’s target market is the millions of Americans who suffer from minor irritation of the skin around the eye (commonly referred to as blepharitis), as well as anyone who suffers from dry eye (commonly described as a gritty sandy sensation while blinking).
Late in 2020, we launched a rebranded CelleRx® into the beauty industry as CelleRx® Clinical Reset™. As part of our growth strategy into the beauty industry, we recently completed the acquisition of DERMAdoctor, LLC (“DERMAdoctor”) on November 5, 2021 (the “DERMAdoctor Acquisition”). DERMAdoctor is an omni-channel skincare company that primarily focuses on the creation of products that are designed to target common skin concerns, ranging from aging and blemishes to dry skin, perspiration and keratosis pilaris. DERMAdoctor develops, markets, distributes and sells more than 30 dermatologist-developed skincare products, which are sold through well-known traditional retailers, digital beauty retailers and online, including through the DERMAdoctor branded website. In addition, the DERMAdoctor products are also sold in international markets through a network of international distributors. We expect that the DERMAdoctor product portfolio will complement our CelleRx Clinical Reset products, and will together strengthen our U.S. market presence in the beauty industry. The combination of DERMAdoctor’s business with our legacy company will result in significant growth in our revenue going forward.
Although we had recent record Avenova sales in 2020, launched CelleRx Clinical Reset into the beauty industry and completed the DERMAdoctor Acquisition, our company has historically incurred net losses. Although we expect that our recent DERMAdoctor acquisition will result in significant future growth opportunities to our company that will result in potentially achieving profitability, there is no assurance that these efforts will ultimately result in us achieving or maintaining sustained profitability, which is a strategic goal of our company. For more information, see “Risks Related to the DERMAdoctor Acquisition and DERMAdoctor’s Business” and “Risks Relating to Our Liquidity” in the section entitled “Risk Factors” on page 7 of this prospectus.
Additional Information
For additional information related to our business and operations, please refer to the reports incorporated herein by reference, including our most recent Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as described under the caption “Incorporation of Certain Information by Reference.”
Company Information
We were incorporated under the laws of the State of California on January 19, 2000, as NovaCal Pharmaceuticals, Inc. It had no operations until July 1, 2002, on which date it acquired all of the operating assets of NovaCal Pharmaceuticals, LLC, a California limited liability company. In February 2007, it changed its name from NovaCal Pharmaceuticals, Inc. to NovaBay Pharmaceuticals, Inc. In June 2010, we changed the state in which we were incorporated and are now incorporated under the laws of the State of Delaware.
Our corporate address is 2000 Powell Street, Suite 1150, Emeryville, California 94608, and our telephone number is (510) 899-8800. Our website address is www.novabay.com. Information found on, or accessible through, our website is not a part of, and is not incorporated into, this prospectus, and you should not consider it part of this prospectus. Our website address is included in this document as an inactive textual reference only.
Recent Developments
New Strategic Direction
After years of focusing on growing and marketing our Avenova brand, we have been focusing our strategic direction toward growing (i) organically by diversifying our business developing additional products and entering into additional business lines and markets, and/or (ii) pursuing a strategic transaction with a third party. Due to the amount of time and money that pharmaceutical research and development costs, our company has been exploring other business areas and industries for strategic growth. We recently executed upon this business strategy with the launch of our CelleRx Clinical Reset, and our acquisition of DERMAdoctor that was completed on November 5, 2021 (the “Acquisition Closing”). We believe that the combination of the DERMAdoctor Acquisition and CelleRx Clinical Reset executes upon both of these strategies.
About DERMAdoctor
DERMAdoctor initially began operations as an e-commerce company selling third-party beauty products and skincare products on the internet and providing skincare advice and information on its website. DERMAdoctor eventually discontinued sales of third-party products in 2011 to focus on sales of its own proprietary skincare line that is marketed and sold under the DERMAdoctor® name. DERMAdoctor has developed a comprehensive portfolio of dermatological solutions to address common skincare concerns including: keratosis pilaris, rosacea and eczema, anti-aging, SPF, hyperhidrosis, excessive hair, and acne. DERMAdoctor’s products are organized into six product families, which are: (i) Kakadu C®; (ii) KP Duty®; (iii) Antiperspirant; (iv) Wrinkle Revenge®; (v) Ain’t Misbehavin®; and (vi) a category of other products, which include Lucky Bamboo®, Calm, Cool & Corrected®, and Urban Veil®. DERMAdoctor’s Kakadu C® products, which includes eight Vitamin C related products, comprised 50.7% of DERMAdoctor’s gross sales in 2020 while KP Duty® products, Antiperspirant products, Wrinkle Revenge® products, Ain’t Misbehavin® and the other products comprised 19.3%, 12.2%, 4.1%, 4.1%, and 9.6%, respectively. DERMAdoctor has a development pipeline of 18 additional new product solutions to address a variety of skin conditions in various stages of development. We plan to launch two new Calm, Cool & Corrected products in the first quarter of 2022, which we expect to be followed by one new Kakadu C product and one new KP Duty product in the second quarter of 2022. The DERMAdoctor business relies upon third party suppliers and manufacturers to produce its products. See “Risk Factors—Risks Related to the DERMAdoctor Acquisition” beginning on page 7 of this prospectus. There are currently over 40 commercial relationships that supply the DERMAdoctor products. For additional information regarding DERMAdoctor, see the section titled “DERMAdoctor Business” in our Definitive Proxy Statement on Schedule 14A, filed with the SEC on November 12, 2021, which is incorporated by reference into this prospectus.
DERMAdoctor Acquisition
On September 27, 2021, we entered into a Membership Unit Purchase Agreement (the “Acquisition Purchase Agreement”) by and among (i) us, (ii) DERMAdoctor, (iii) Dr. Jeffrey Kunin and Dr. Audrey Kunin (the “Founders”); (iv) Papillon Partners, Inc., a Missouri corporation that is owned by the Founders (“Papillon”); and (v) Midwest Growth Partners, L.L.L.P., an Iowa limited liability limited partnership (“MGP” and together with Papillon, the “Sellers”). Pursuant to the Acquisition Purchase Agreement, the Company acquired 100% of the membership units of DERMAdoctor for an agreed upon purchase price of approximately $15.0 million (the “Purchase Price”). The Purchase Price was comprised of a payment of approximately $12.0 million in cash to the Sellers at the Acquisition Closing, which amount was (i) reduced to account for payments we made to satisfy DERMAdoctor’s indebtedness and its transaction expenses as of the Acquisition Closing, and (ii) increased by the DERMAdoctor cash and cash equivalents that remained as of the Acquisition Closing. The remaining amount of the Purchase Price is comprised of up to an aggregate of $3.0 million in earn out payments after the Acquisition Closing, which are contingent upon the legacy DERMAdoctor business achieving predetermined contribution margin financial targets for the 2022 and the 2023 calendar fiscal years (the “Earn Out Payments”). For additional information regarding the DERMAdoctor Acquisition and the Acquisition Purchase Agreement, see “The Acquisition Purchase Agreement” beginning on page 26 of this prospectus.
The Private Placement and Stockholder Action
On October 29, 2021, NovaBay entered into the Securities Purchase Agreement with the purchasers named therein (the “Purchasers”). Pursuant to the Securities Purchase Agreement, we agreed to sell in the Private Placement (i) an aggregate of 15,000 shares of our newly-created Preferred Stock convertible into an aggregate of 37,500,000 shares (the “Conversion Shares”) of Common Stock and (ii) Common Stock warrants (the “Warrants”) exercisable for 37,500,000 shares (the “Warrant Shares”) of Common Stock for an aggregate purchase price of $15,000,000. The Private Placement closed on November 2, 2021 (the “Private Placement Closing Date”). We used a portion of the net proceeds from the Private Placement to partially fund the Purchase Price to acquire DERMAdoctor, with the remaining amount to be used for working capital purposes.
Each share of the Preferred Stock that we issued in the Private Placement had a purchase price of $1,000 per share and is initially convertible at a conversion price of $0.40 into 2,500 Conversion Shares, or an aggregate of 37,500,000 Conversion Shares, subject to limitations, including receipt of stockholder approval (the “Share Conversion Approval”) in accordance with Section 713(a) and (b) of the NYSE American Company Guide (the “Company Guide”). Until the Share Conversion Approval is received from stockholders, the holders of the Preferred Stock are limited in converting their shares to an aggregate of 19.99% of the outstanding shares of Common Stock immediately prior to the Private Placement Closing Date, or 8,984,178 shares of Common Stock (the “Issuable Maximum”). The Preferred Stock does not have any preemptive rights or a preference upon any liquidation, dissolution or winding-up of NovaBay. The Preferred Stock does, however, have anti-dilution protection in the event that we sell or grant any Common Stock or any other securities of our Company, subject to certain limited exceptions, that would entitle the holder thereof to acquire Common Stock at an effective price per share that is lower than the then applicable conversion price of the Preferred Stock. As a result, the conversion price of the Preferred Stock will be reduced to such lower price; this protection afforded the holders of the Preferred Stock is referred to as a “full-ratchet” anti-dilution protection. The Preferred Stock has other customary anti-dilution protections. The powers, preferences, rights, qualifications, limitations and restrictions applicable to the Preferred Stock, including the anti-dilution protections, are set forth in the Certificate of Designation of Preferences, Rights and Limitations of the Series B Non-Voting Convertible Preferred Stock filed with the Secretary of State of the State of Delaware (the “Certificate of Designation”) on November 1, 2021. See “Description of Capital Stock” for additional information regarding the Preferred Stock and the Certificate of Designation.
Under the terms of the Securities Purchase Agreement, we agreed to reserve and maintain a sufficient number of shares of Common Stock upon the future conversion of the Preferred Stock and exercise of the Warrants in accordance with their terms. After reservation of the Conversion Shares, which has occurred, we did not have a sufficient number of authorized shares of Common Stock under our Amended and Restated Certificate of Incorporation, as amended (the “Certificate of Incorporation”), to reserve for issuance upon exercise of all of the Warrants. Accordingly, pursuant to the Securities Purchase Agreement, we are taking the necessary corporate action to seek approval of our stockholders to amend the Certificate of Incorporation to provide for an increase in the number of authorized but unissued shares of Common Stock to allow for the full exercise of the Warrants (the “Authorized Share Increase”). The Warrants will not be immediately exercisable, unless and until approvals for both the Authorized Share Increase and the Share Conversion Approval are received from our stockholders.
On the Private Placement Closing Date, in connection with the Securities Purchase Agreement, we entered into a registration rights agreement with the Purchasers (the “Registration Rights Agreement”) to register the Conversion Shares and the Warrant Shares. Pursuant to the Registration Rights Agreement, we are required to prepare and file with the SEC: (i) an initial registration statement on Form S-1 (the “Initial Registration Statement”) covering the resale of the Conversion Shares within 30 calendar days of the Placement Closing Date (the “Initial Filing Deadline”); and (ii) a second registration statement on Form S-1 (the “Second Registration Statement” and together with the Initial Registration Statement, the “Registration Statements”) covering the resale of the Warrant Shares, within 30 calendar days following stockholder approval for both the Authorized Share Increase and the Share Conversion Approval (the “Second Filing Deadline”). This registration statement has been filed to comply with the Company’s obligation to file an Initial Registration Statement. For additional information regarding the Registration Rights Agreement, see “The Private Placement—Registration Rights Agreement” on page 24 of this prospectus.
The Offering
This prospectus relates to the resale by the Selling Stockholders listed in this prospectus of up to 37,500,000 shares of our Common Stock, which shares are issuable upon the conversion of the Preferred Stock held by the Selling Stockholders. Accordingly, these shares will become eligible for sale by the Selling Stockholders under this prospectus only as the Preferred Stock is converted. Shares of Common Stock that may be offered in this offering, when issued upon conversion of the Preferred Stock, will be fully paid and non-assessable. Our Common Stock is currently listed on the NYSE American under the symbol “NBY.” All of the Shares will be sold by the Selling Stockholders. Such Selling Stockholders may sell their shares of our Common Stock from time to time at market prices prevailing at the time of sale, at prices related to the prevailing market price, or at negotiated prices. We will not receive any of the proceeds from sales by the Selling Stockholders of any Shares covered by this prospectus.
RISK FACTORS
Investing in our Common Stock involves a high degree of risk. You should consider carefully the risk factors described below, and all other information and documents contained in or incorporated by reference in this prospectus (as supplemented and amended), including the risks described under the caption “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2020, before deciding whether to buy our Common Stock. The risks described in this prospectus or incorporated by reference into this prospectus are not the only ones we face, but those that we consider to be material. Additional risks not presently known to us or that we currently believe are immaterial may also significantly impair our business operations and could result in a complete loss of your investment. There may be other unknown or unpredictable economic, business, competitive, regulatory or other factors that could have material adverse effects on our future results. Past financial performance may not be a reliable indicator of future performance, and historical trends should not be used to anticipate results or trends in future periods. If any of the following risks actually occur, our business, financial condition, results of operations or cash flow could be seriously harmed. This could cause the market price of our Common Stock to decline, and you could lose all or part of your investment. Please also read carefully the section below entitled “Special Note Regarding Forward-Looking Statements.”
Overview
As described in this prospectus, we recently completed two significant transactions for our Company, which are the Private Placement and the DERMAdoctor Acquisition. These transactions are both material and will be transformative to NovaBay’s business, operations and strategic direction, as well as to its overall capital structure on a go forward basis. Accordingly, as a result of these changes, there will be new and additional risks and uncertainties that we may encounter and have to face in the future, as well as those risks that we already had prior to completing these transactions.
Risks Related to the Private Placement
As a result of the Preferred Stock and the Warrants that we issued in the Private Placement, our stockholders will experience significant dilution as a result of the issuance of shares of our Common Stock upon future conversion of the Preferred Stock and the exercise of the Warrants.
The holders of the Preferred Stock that we issued in the Private Placement are entitled to convert a portion of their Preferred Stock into Common Stock at any time, provided that upon conversion, the aggregate number of shares of Preferred Stock converted by all of the holders does not exceed 19.99% of our issued and outstanding shares of Common Stock immediately prior to the closing of the Private Placement. If the holders of the Preferred Stock convert all of the shares of the Preferred Stock that they are entitled to convert, then it would result in 37,500,000 additional shares of Common Stock being issued and outstanding, which conversion would result in significant dilution of our existing stockholders. Additionally, if the Authorized Share Increase and the Share Conversion Approval are approved at our upcoming special meeting of stockholders, then there will not be the limitation upon the conversion of the remaining shares of Preferred Stock into Common Stock. Also, if both of these actions are approved by stockholders, then the Warrants will become exercisable and may be exercised thereafter into an aggregate of 37,500,000 shares of Common Stock. If all of the shares of Preferred Stock were converted based on the conversion price as of the date hereof, and all of the Warrants were exercised, it would result in a total of 75,000,000 additional shares of Common Stock becoming issued and outstanding, which represents 168% of the total number of shares of Common Stock currently outstanding. Accordingly, upon the conversion of some or all of the Preferred Stock and/or exercise of the Warrants, the percentage ownership and voting power held by our existing stockholders will be significantly reduced and our stockholders will experience significant dilution.
In addition, if stockholders approve the Authorized Shares Increase, we will more than double the number of authorized shares of Common Stock that NovaBay may issue in the future, which our Board will have discretion to issue in the future, including, without limitation, in connection with future capital raise transactions and financings, except to the extent prohibited or limited by the terms of the Private Placement. Stockholders will not have a right to approve any such issuances or transactions, unless required by our governing documents or applicable law, and any such issuance of our Common Stock in the future may be dilutive to stockholders.
If we offer Common Stock or other securities in the future and the price that we sell those securities for is less than the current conversion price of our Preferred Stock, then we will be required to issue additional shares of Common Stock to the holders of the Preferred Stock upon conversion, which will be dilutive to all of our other stockholders.
The Certificate of Designation for the Preferred Stock contains anti-dilution provisions, which provisions require the lowering of the conversion price, as then in effect, to the purchase price of equity or equity-linked securities issued by us in subsequent offerings, if lower than the current conversion price. A reduction in the conversion price of the Preferred Stock will result in a greater number of shares of Common Stock being issuable upon conversion of the Preferred Stock for no additional consideration, causing greater dilution to our stockholders. Furthermore, as there is no floor on the conversion price, we cannot determine the total number of shares issuable upon conversion. Accordingly, it is possible that we may not have a sufficient number of authorized and available shares to satisfy the conversion of the Preferred Stock if we enter into a future transaction that reduces the applicable conversion price.
We will incur significant transaction and acquisition-related costs in connection with the DERMAdoctor Acquisition and the Private Placement and such expenditures may create significant liquidity and cash flow risks for us.
We have incurred and expect to incur significant, nonrecurring costs associated with the DERMAdoctor Acquisition, including costs associated with the continued integration of the DERMAdoctor’s business. In addition, we have and expect to continue to incur additional significant, nonrecurring costs in connection with completing the Private Placement, including the solicitation of stockholders to approve the Authorized Share Increase and the Share Conversion Approval and holding a special meeting of stockholders to approve the same. While we have assumed that this level of expense will be incurred, there are factors beyond our control that could affect the total amount, including in the case of the DERMAdoctor Acquisition integration expenses. Moreover, many of the expenses that will be incurred are, by their nature, difficult to estimate accurately. To the extent these acquisition and integration expenses are higher than anticipated and we do not have sufficient cash, including the additional capital raised in the Private Placement, then we may experience liquidity or cash flow issues.
We do not have enough authorized shares of Common Stock to issue upon conversion of all of the Preferred Stock and the exercise of the Warrants and we require stockholder approval of the Authorized Share Increase in order to have a sufficient number of shares of Common Stock and there is no assurance that stockholder approval will be obtained which could materially and adversely impact us.
We do not have enough shares of Common Stock currently authorized under our Certificate of Incorporation to issue upon the conversion of all of the shares of Preferred Stock and the full exercise of all the Warrants that we issued in the Private Placement, and we are relying upon stockholders to approve the Authorized Share Increase at a special meeting of stockholders in order to have sufficient underlying shares. We currently have 100,000,000 shares of Common Stock authorized under our Certificate of Incorporation. Of those authorized shares that remain available for issuance, we have reserved shares of Common Stock upon conversion of the Preferred Stock; however, we do not have enough shares for full exercise of the Warrants. Accordingly, none of the Warrants may be exercised, unless and until the Authorized Share Increase and the Share Conversion Approval are approved by stockholders. If our stockholders do not approve these proposals, we are required under the Securities Purchase Agreement to continue to hold meetings of our stockholders to solicit approval every four months until these proposals are approved.
In addition, pursuant to the Purchase Agreement, we are restricted until 90 days after the later of (i) the date of the Share Conversion Approval and the Authorized Share Increase are approved by stockholders and effective and (ii) the Initial Registration Statement is declared effective by the SEC, from (x) issuing or entering into any agreement to issue or announce the issuance or proposed issuance of any shares of Common Stock or equivalents, (y) incur, enter into any agreement to incur or announce the incurrence or proposed incurrence of any indebtedness or (z) filing any registration statement or any amendment or supplement thereto, other than as contemplated by the Registration Rights Agreement. Accordingly, if we do not receive stockholder approval of the Authorized Share Increase and the Share Conversion Approval, then we will be restricted in our ability to raise capital using Common Stock and Common Stock equivalents or incur indebtedness until such approval is obtained, which could take an extended period of time. During this period of time, the restriction on our ability to raise capital through the issuance of our Common Stock and equivalents would have a material adverse effect on our ability to raise capital in the future using these securities, which has been our primary source of capital and liquidity over the years.
The market price of our Common Stock may decline as a result of the DERMAdoctor Acquisition, and the issuance of the Preferred Stock and the Warrants in connection with the Private Placement.
We are unable to predict the potential future effects that the closing of the DERMAdoctor Acquisition on November 5, 2021 and/or the issuance of the Preferred Stock and the Warrants in the Private Placement on November 2, 2021 will have on the near-term or future trading activity and market price of our Common Stock. In addition, we also cannot predict the future effects on the trading activity and market price of our Common Stock if stockholders approve the Authorized Share Increase and the Share Conversion Approval. If the Authorized Share Increase and the Share Conversion Approval are approved and the holders of the Preferred Stock are able to convert all of their shares into Common Stock and the Warrants are exercised into Common Stock, then there will be up to 75,000,000 additional shares of our Common Stock available for public trading. Even if the Authorized Share Increase and the Share Conversion Approval are not approved, the holders of the Preferred Stock will be able to convert a certain number of their shares of Preferred Stock into Common Stock up to an amount equal to 19.99% of our outstanding Common Stock immediately prior to the closing of the Private Placement. If, as a result of the Private Placement or the DERMAdoctor Acquisition, there are sales of a substantial number of shares of our Common Stock in the public market, or the perception that such sales might occur, it could have a material adverse effect on the price of our Common Stock.
Risks Related to the DERMAdoctor Acquisition and DERMAdoctor’s Business
In expanding our operations significantly with the DERMAdoctor Acquisition, we are further dependent on third parties to supply raw materials used in our products and to manufacture our products. Any interruption or failure by these suppliers or other disruptions to our supply chain may materially adversely affect our business, financial condition, results of operations and cash flows.
Historically, we have predominately relied on a single product, Avenova, for our primary revenue stream, which is comprised of our proprietary, stable and pure form of hypochlorous acid. In acquiring DERMAdoctor, we are greatly expanding our product offerings and operations, as DERMAdoctor has an extensive global platform, currently selling over 30 products in various countries, with over 40 commercial relationships that supply its products from around the globe. While product sales in the United States have historically driven DERMAdoctor’s revenue, it has strategically sought international opportunities for the sale and distribution of its products. DERMAdoctor’s products are currently offered internationally in China, the Middle East, Europe, Canada, and Central and South America.
With this larger operational business and range of product offerings around the globe, comes additional opportunity for us as well as corresponding risk in certain areas. A key risk area, which is emphasized further by the current pandemic environment, is that of supply chain risk. DERMAdoctor uses third party contract manufacturers and suppliers, some internationally, to obtain substantially all raw materials, components and packaging products and to manufacture finished products relating to the DERMAdoctor brand, encompassing its over 30 product offerings. There are many aspects of the supply chain process that are outside of our control, which range from third party manufacturing aspects, weather and natural disasters, terrorism, pandemics (such as the COVID-19 pandemic), strikes, government action, or other reasons that could impair our ability to manufacture, transport or deliver our products. We endeavor to take adequate steps to mitigate the likelihood or potential impact of a supply chain disruption that might occur. In some cases, DERMAdoctor has sufficient other suppliers across its broad range of products that it is able to mitigate its supply chain risk within a reasonable time frame. In other cases, DERMAdoctor may be more dependent upon a particular supplier, specifically for certain ingredient mixtures needed for its products; however, in the occasional circumstance where a supplier is unable to deliver a particular ingredient mixture and another supplier of such ingredient mixture is not readily available, DERMAdoctor may be able to make formulation adjustments to the various ingredient mixture supplies to achieve the same or similar product. A risk remains that DERMAdoctor may not be able to source a particular product, packaging or formula component in the quantities or timeframe that a customer desires. Particularly since a large portion of DERMAdoctor’s revenue is from wholesale customers that place large orders, if a customer order is missed or not appropriately delivered, it could materially adversely affect our business or financial results.
DERMAdoctor’s operating results are dependent on sales to a few significant retail partners and the loss of, or substantial decline in, sales to one of these retail partners could have a material adverse effect on its expected future revenues and profitability.
Retail partners of DERMAdoctor account for a substantial percentage of its net sales revenue, and the loss of all or a portion of the sales to any one of these customers could have a material adverse effect on the results of operations generated by the DERMAdoctor business. In particular, sales to retail partners accounted for approximately 50.8% of DERMAdoctor’s gross sales revenue in fiscal 2020. We expect that a small group of retail partners will continue to account for a significant portion of DERMAdoctor’s gross sales revenue for the foreseeable future. Although DERMAdoctor has long-standing relationships with its major retail partners, it generally does not, consistent with industry norms, have written agreements that require these retail partners to buy from DERMAdoctor or to purchase a minimum amount of its products. A substantial decrease in sales to any of DERMAdoctor’s major retail partners could have a material adverse effect on DERMAdoctor’s business and our financial condition and operating results, which would have an adverse impact on the expected benefits of the DERMAdoctor Acquisition.
Uncertainty about the DERMAdoctor Acquisition may adversely affect the relationships that we and DERMAdoctor have with our respective customers, service providers and employees.
Parties with whom we or DERMAdoctor do business may experience uncertainty associated with the recent DERMAdoctor Acquisition, including with respect to current or future business relationships with us or DERMAdoctor. These business relationships may be subject to disruption as end customers, suppliers, manufactures, distributors and others may attempt to (i) negotiate changes in existing business relationships, (ii) delay, defer or cease supplying or manufacturing for, or purchasing products from, us or DERMAdoctor or (iii) consider entering into business relationships with parties other than us or DERMAdoctor, including our competitors or those of DERMAdoctor. These disruptions, if they occur, could have a material adverse effect on the combined business and upon our operating results and financial condition.
Uncertainties associated with the DERMAdoctor Acquisition may cause a loss of management personnel and other key employees that could adversely affect our future business, operations and financial results.
With the DERMAdoctor Acquisition having been completed, the ongoing integration of the combined businesses could disrupt our business. We and DERMAdoctor are both dependent on the experience and industry knowledge of our respective senior management and other key employees to develop new products and execute our respective business plans. Our success will depend in part upon our ability to retain both key management personnel and key employees of DERMAdoctor. Although we entered into employment agreements with Dr. Audrey Kunin and Dr. Jeffrey Kunin, who are also incentivized to team with us over the next few years to achieve potential Earn Out Payments pursuant to the terms of the Purchase Agreement, and all of the other employees of DERMAdoctor continued as employees after the Acquisition Closing, there is no guarantee that current and prospective future employees we hire for the DERMAdoctor business will continue with us, which may have an adverse effect on our ability to effectively integrate DERMAdoctor into our business or for us to attract or retain key management and other key personnel. All of DERMAdoctor’s existing and new products in development were conceived by its product design team led by Dr. Audrey Kunin, who is a board-certified dermatologist. Dr. Kunin is continuing post-transaction in her leadership role of new product development, and the loss of Dr. Kunin’s service in this capacity could have a material and adverse effect on our ability to effectively develop and launch new products until such position could be filled by us.
The DERMAdoctor Acquisition involves risks associated with acquisitions and integrating acquired businesses and the intended benefits of the DERMAdoctor Acquisition may not be realized by us.
The DERMAdoctor Acquisition, which was completed on November 5, 2021, involves risks that are associated with acquisitions and integrating acquired businesses and their operations with existing operations, including:
|
|
●
|
our senior management’s attention may be diverted from the management of daily operations to the integration of DERMAdoctor’s products and business that we have acquired;
|
|
|
●
|
the challenges, including delays or any other unanticipated changed circumstances, and costs involved in integrating and/or developing the DERMAdoctor products and other assets that we have acquired;
|
|
|
●
|
failure of the products and other assets that we acquired in the DERMAdoctor Acquisition to generate anticipated revenues, to enhance the growth of our CelleRx Clinical Reset products and/or otherwise perform in accordance with our expectations; and
|
|
|
●
|
failure to achieve the anticipated efficiencies and cost savings or realize other expected benefits of the DERMAdoctor Acquisition within the expected time frame or at all.
|
If these risks or other unexpected costs and liabilities were to materialize, any desired benefits of the DERMAdoctor Acquisition may not be fully realized, if at all, and our future financial performance and results of operations could be negatively impacted. In addition, if the combined company does not perform as we or the market expects, then this could have an adverse effect on the price of our Common Stock.
The unaudited pro forma condensed combined financial information included in this prospectus are presented for illustrative purposes only and may not be an indication of our future financial condition or results of operations.
The unaudited pro forma condensed combined financial information included in this prospectus are presented for illustrative purposes only, are based on various adjustments and assumptions, many of which are preliminary, and may not be an indication of our financial condition or results of operations following the DERMAdoctor Acquisition and the Private Placement. Our actual and future financial condition and results of operations may not be consistent with, or evident from, these unaudited pro forma condensed combined financial information included in this prospectus. In addition, the assumptions used in preparing the unaudited pro forma financial data may not prove to be accurate, and other factors may affect our financial condition or results of operations.
Actual results may differ from any statements made by us concerning the anticipated impact of the DERMAdoctor Acquisition on the operating results of the combined company, and these differences could be material.
This prospectus contains a number of forward-looking statements. Although we believe that we have a reasonable basis for such forward-looking statements, these statements are based on our projections of future events that are subject to risks, uncertainties and other factors that may cause the combined company’s actual results, level of activity, performance or achievements expressed or implied by these forward-looking statements to differ in a material way. Additional risks and uncertainties that could cause actual results to differ materially from currently anticipated results include, but are not limited to, risks relating to our ability to successfully integrate DERMAdoctor; unanticipated increases in costs or expenses; our ability to realize expected cost synergies; our ability to reach profitability as a result of the DERMAdoctor Acquisition, and the other risks identified in this prospectus and the documents included herein that we urge you to read. Our actual financial condition and results of operations as a result of the DERMAdoctor Acquisition may not be consistent with, or evident from, the statements provided in this prospectus. Consequently, actual results or developments anticipated by us may not be realized or, even if substantially realized, may not have the expected consequences to, or effects on, us. In particular, while the DERMAdoctor Acquisition is expected to be accretive to NovaBay’s profitability in the first year after close, there can be no assurance with respect to the timing and scope of the accretive effect or whether it will be accretive at all. Any failure to meet expectations regarding the prospects for the combined company could have a material adverse effect on our business, financial condition, results of operation, as well as the trading price and/or volume of our Common Stock.
Charges to earnings resulting from the application of the purchase method of accounting following the Closing of the DERMAdoctor Acquisition may adversely affect the market value of our Common Stock.
The DERMAdoctor Acquisition will be accounted for using the purchase method of accounting, which will result in charges to earnings that could have an adverse impact on the market value of our Common Stock. Under the purchase method of accounting, the total estimated Purchase Price will be allocated to DERMAdoctor’s pro forma net tangible assets and identifiable intangible assets based on their respective fair values as of the Acquisition Closing. Any excess of the Purchase Price over those fair values will be recorded as goodwill. As a result of the consolidation of DERMAdoctor with our Company, we will incur additional amortization expense based on the identifiable amortizable intangible assets acquired pursuant to the Purchase Agreement and their relative useful lives. Additionally, to the extent the value of goodwill or identifiable intangible assets or other long-lived assets may become impaired, we may be required to incur charges relating to the impairment. These amortization and potential impairment charges could have a material impact on the combined company’s results of operations.
Risks Relating to Our Liquidity
We have a history of losses and we may never achieve or maintain sustained profitability.
We have historically incurred net losses, and we may never achieve or maintain sustained profitability. In addition, at this time, we expect to incur substantial acquisition related expenses as well as marketing and sales expenses as we integrate the DERMAdoctor business and its products with ours and we continue efforts to increase sales of our Avenova, CelleRx Clinical Reset and newly acquired DERMAdoctor products, and our results of operations may fluctuate significantly.
While we believe that increased revenues and operating expense efficiencies expected to be achieved as a result of the DERMAdoctor Acquisition will result in our Company achieving profitability, there is no assurance that this will occur. We will need to generate significant revenues to achieve and maintain profitability. Even with the combined sales of Avenova, CelleRx Clinical Reset and DERMAdoctor products, there is no assurance that we will be able to generate sufficient revenues to achieve or maintain profitability. Our failure to achieve and subsequently maintain profitability could have a material adverse impact on the market price of our Common Stock.
Risks Relating to Owning Our Common Stock
The price of our Common Stock may fluctuate substantially, which may result in losses to our stockholders.
The stock prices of many companies in the pharmaceutical and biotechnology industry have generally experienced wide fluctuations, which are often unrelated to the operating performance of those companies. The market price of our Common Stock is likely to be volatile and could fluctuate in response to, among other things:
|
|
●
|
the announcement of new products by us or our competitors;
|
|
|
●
|
the announcement of partnering arrangements by us or our competitors;
|
|
|
●
|
quarterly variations in our or our competitors’ results of operations;
|
|
|
●
|
changes in our earnings estimates, investors’ perceptions, recommendations by securities analysts or our failure to achieve analysts' earnings estimates;
|
|
|
●
|
developments in our industry;
|
|
|
●
|
the sale of a substantial number of shares of Common Stock by any large stockholder, especially within a short period of time; and
|
|
|
●
|
general, economic and market conditions, including volatility in the financial markets, a decrease in consumer confidence and other factors unrelated to our operating performance or the operating performance of our competitors.
|
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
Under Section 382 of the Internal Revenue Code of 1986, as amended (the “Code”), if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss (“NOL”) carryforwards and other pre-change tax attributes (such as research tax credits) to offset its post-change income may be limited. Since our formation, we have raised capital through the issuance of capital stock on many occasions which, combined with the purchasing stockholders’ subsequent disposition of those shares, may have resulted in one or more changes of control, as defined by Section 382 of the Code. We have not currently completed a study to assess whether any change of control has occurred, or whether there have been multiple changes of control since our formation, due to the significant complexity and cost associated with such study. If we have experienced a change of control at any time since our formation, our NOL carryforwards and tax credits may not be available, or their utilization could be subject to an annual limitation under Section 382. In addition, since we may need to raise additional funding to finance our operations, we may undergo further ownership changes in the future. If we earn net taxable income, our ability to use our pre-change NOL carryforwards to offset United States federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us.
We have not paid dividends or repurchased stock in the past and do not expect to pay dividends or repurchase stock in the future, and any return on investment may be limited to the value of our stock.
We have never paid cash dividends on, or repurchased shares of, our Common Stock and do not anticipate paying cash dividends or repurchasing shares of our Common Stock in the foreseeable future. In addition, we do not anticipate paying any dividends or repurchasing any shares of our Preferred Stock; however, if we pay dividends on our shares of Common Stock, we are required to pay dividends on our Preferred Stock on an as converted basis. The payment of dividends on, or the repurchase of shares of, our Common Stock or Preferred Stock will depend on our earnings, financial condition and other business and economic factors affecting us at such time as our Board of Directors may consider relevant. If we do not pay dividends or repurchase stock, holders of our Common Stock will experience a return on their investment in our shares only if our stock price appreciates.
China Pioneer Pharma Holdings Limited (“China Pioneer”) and/or China Kington Asset Management (“China Kington”) have influence over our corporate matters.
Pioneer Pharma (Hong Kong) Company, Ltd., a subsidiary of China Pioneer, currently beneficially owns approximately 11.5% of our outstanding Common Stock (with potential dilution down to at least 4.3% due to the Private Placement). Our director Mr. Xinzhou “Paul” Li is the chairman of China Pioneer. Mr. Mijia “Bob” Wu is a Non-Executive Director of China Pioneer as well as the Managing Director of China Kington (an affiliated party of NovaBay due to various historical transactions as disclosed in reports filed with the SEC). As a result, subject to the fiduciary duties Messrs. Li and Wu owe to NovaBay in their role as directors, China Pioneer and China Kington have input on all matters before our Board of Directors and may be able to exercise influence over all matters requiring board and stockholder approval.
If our stockholders' equity does not meet the minimum standards of the NYSE American or we are not able to comply with other continued listing requirements, we may be subject to delisting procedures.
Our Common Stock is currently listed on the NYSE American. If we are unable to comply with the continued listing requirements of the NYSE American, our Common Stock would be delisted from the NYSE American, which would limit investors’ ability to effect transactions in our Common Stock and subject us to additional trading restrictions. In order to maintain our listing, we must maintain certain share prices, financial and share distribution targets, including maintaining a minimum amount of stockholders’ equity and a minimum number of public stockholders. In addition to these objective standards, NYSE American may delist the securities of any issuer for other reasons involving the judgment of NYSE American. Historically, our stockholders’ equity has at times been below the minimum requirements of Section 1003(a) of the Company Guide though we have met all such minimum requirements since October 13, 2020. In accordance with Section 1009(h) of the Company Guide, if we are again determined to be below any of the continued listing standards in the future, the NYSE American will take the appropriate action which, depending on the circumstances, may include initiating its compliance procedures or initiating delisting proceedings. If our Common Stock is delisted, this could, among other things, substantially impair our ability to raise additional funds; result in a loss of institutional investor interest and fewer financing opportunities for us; and/or result in potential breaches of representations or covenants of our warrants, subscription agreements or other agreements pursuant to which we made representations or covenants relating to our compliance with applicable listing requirements. Claims related to any such breaches, with or without merit, could result in costly litigation, significant liabilities and diversion of our management's time and attention and could have a material adverse effect on our financial condition, business and results of operations.
We may issue additional shares of our Common Stock, other series or classes of preferred stock or other equity securities without your approval, which would dilute your ownership interests and may depress the market price of your shares.
We may issue additional shares of our Common Stock, other series or classes of preferred stock, in addition to the Preferred Stock we recently issued and sold in the Private Placement, or other equity securities of equal or senior rank in the future in connection with, among other things, future acquisitions, repayment of outstanding indebtedness or under our 2017 Omnibus Incentive Plan, without stockholder approval, in a number of circumstances.
Our issuance of additional shares of our Common Stock, preferred stock or other equity securities of equal or senior rank could have the following effects:
|
|
●
|
your proportionate ownership interest in NovaBay will decrease;
|
|
|
●
|
the relative voting strength of each previously outstanding share of Common Stock may be diminished; or
|
|
|
●
|
the market price of your shares of Common Stock may decline.
|
We may require additional capital funding, the receipt of which may impair the value of our Common Stock and Preferred Stock.
If we expand more rapidly than currently anticipated or if our working capital needs exceed our current expectations, we may need to raise additional capital through public or private equity offerings or debt financings. Our future capital requirements depend on many factors including our research, development, sales and marketing activities. We do not know whether additional financing will be available when needed, or will be available on terms favorable to us. If we cannot raise needed funds on acceptable terms, we may not be able to develop or enhance our products, take advantage of future opportunities or respond to competitive pressures or unanticipated requirements. To the extent we raise additional capital by issuing equity securities, our stockholders may experience substantial dilution and the new equity securities may have greater rights, preferences or privileges than our existing Common Stock. In addition, the new equity securities may be offered in the future at a price that is below the then in effect conversion price of the Preferred Stock, which would result in the lowering of the conversion price of the Preferred Stock and a greater number of shares of Common Stock being issuable upon conversion of the Preferred Stock for no additional consideration, causing even greater dilution to our stockholders.
Offers or availability for sale of a substantial number of shares of our Common Stock, including as a result of the conversion of the Preferred Stock and/or the exercise of the Warrants, may cause the price of our publicly traded securities to decline.
Sales of a significant number of shares of our Common Stock in the public market could harm the market price of our Common Stock and make it more difficult for us to raise funds through future offerings of Common Stock. The Preferred Stock that we issued in the Private Placement provides for conversion into an aggregate of 37,500,000 shares of Common Stock (based on the current conversion price), subject to limitations on the number of shares of Preferred Stock that may be converted until the Share Conversion Approval of the stockholders is obtained. Until the Share Conversion Approval is received, holders of Preferred Stock may still convert a pro rata portion of their Preferred Stock for up to 8,984,178 shares of Common Stock, representing 19.99% of our Common Stock immediately prior to the Private Placement. In addition, the Preferred Stock may become convertible into a greater number of shares of Common Stock that would be available for sale as a result of the full-ratchet anti-dilution price protection in the Certificate of Designation for the Preferred Stock, which would be triggered if we were to issue Common Stock in the future at an effective Common Stock purchase price that is less than the current conversion price for the Preferred Stock. In the Private Placement, we also issued Warrants that may be exercisable into 37,500,000 of Common Stock, provided that the stockholders approve the Authorized Share Increase and the Share Conversion Approval. If all of the Preferred Stock were converted based on the conversion price as of the date hereof, and all of the Warrants were exercised, it would result in a total of 75,000,000 additional shares of Common Stock becoming issued and outstanding, which represents 168% of the total number of shares of Common Stock currently outstanding. In addition, our stockholders and the holders of our stock options and other warrants may also sell amounts of our Common Stock in the public market. The sale of a significant portion of any of these shares of Common Stock in the public market at one time could create downward pressure on the market price of our Common Stock. In addition, the fact that our stockholders, including the holders of Preferred Stock, option holders and warrant holders, including the Warrants, could sell substantial amounts of our Common Stock in the public market, whether or not sales have occurred or are occurring, could make it more difficult for us to raise additional financing through the sale of equity or equity-related securities in the future at a time and/or at a price that we deem reasonable or appropriate, or at all.
Risks Relating to Our Business
Our business may be adversely affected by the continuing coronavirus outbreak.
In March 2020, the World Health Organization declared COVID-19 a global pandemic and the United States declared a national emergency with respect to COVID-19. In response to the COVID-19 outbreak, “shelter in place” orders and other public health measures were implemented across much of the United States, including the San Francisco Bay area counties where our headquarters is located. Due to “shelter in place” orders and other public health guidance measures, we implemented a temporary work-from-home policy for all staff members that has since been lifted. Overall, the impact of COVID-19 to date has been minimal on the sales of Avenova as an increase in online sales has made up for the decrease in prescription revenue. In addition, we recently acquired DERMAdoctor and have not yet integrated its business, operations or products with ours and the impact of COVID-19 on this integration and/or the future sales of the DERMAdoctor products is uncertain. Although we and DERMAdoctor have not experienced a material disruption in our supply chain to date due to COVID-19, as the pandemic continues, the availability of raw materials, goods and/or services from our suppliers could be disrupted and/or not provided in a timely manner or in the quantities that we require in order to operate our business in the ordinary course, which could materially and adversely affect our product sales, customer service levels and our overall business. In addition, any increases in the costs of goods and services for our business that could result from such disruptions in our supply chain or as a result of inflation in the overall costs of goods and services may adversely affect our profit margins if we are unable to pass along any higher costs in the form of price increases or otherwise achieve cost efficiencies in our operations.
The COVID-19 global pandemic continues to evolve. The extent to which the outbreak may continue to affect our business, financial condition and results of operations will depend on future developments, which are uncertain and cannot be predicted at this time, such as the duration of the outbreak, evolution of COVID-19 into novel strands of the disease, travel restrictions and actions to contain the outbreak or treat its impact, such as social distancing, quarantines or lock-downs in the United States and elsewhere, business closures or business disruptions and the effectiveness of actions taken in the United States and elsewhere to contain and treat the disease through vaccination. Future developments in these and other areas present material uncertainty and risk with respect to our business, financial condition and results of operations. Since the success of our business, including DERMAdoctor’s business, relies upon the strength of the United States and other retail economies, any sustained economic downturn in the United States or the other countries in which we conduct business could materially and adversely affect our business, operating results and financial condition.
Our future success is largely dependent on the successful commercialization of our products, particularly Avenova, and of the newly acquired DERMAdoctor products.
If we are unable to establish and maintain adequate sales, marketing and distribution capabilities or enter into or maintain agreements with third parties to do so, we may be unable to successfully commercialize our products, including the products that we recently acquired as a result of the DERMAdoctor Acquisition. While we believe we are creating an efficient commercial organization, we may not be able to judge correctly the size and experience of the sales and marketing force and the scale of distribution necessary to be successful. Establishing and maintaining sales, marketing, and distribution capabilities are expensive and time-consuming. Such expenses may be disproportionate compared to the revenues we may be able to generate on sales of Avenova, CelleRx Clinical Reset and/or our DERMAdoctor products, which could cause our commercialization efforts to be unprofitable or less profitable than expected.
Acceptance and use of Avenova, CelleRx Clinical Reset and/or the DERMAdoctor products by physicians, retail partners and other customers may depend on a number of factors including: (i) perceptions by members of the healthcare community, including physicians, about the safety and effectiveness of our products; (ii) published studies demonstrating the cost-effectiveness of our products relative to competing products; (iii) availability of reimbursement for our products from government or commercial payers as relates to Avenova; and (iv) effectiveness of marketing and distribution efforts by us and our licensees and distributors, if any. The failure of any of our products to find market acceptance would harm our business and could require us to seek additional financing to fund our operations.
We face substantial competition in the eye care and the skincare markets in which we operate.
Avenova faces intense competition in the eye care market, which is focused on cost-effectiveness, price, service, product effectiveness and quality, patient convenience and technological innovation. There is substantial competition in the eye care market from companies of all sizes in the United States and abroad, including, among others, large companies such as Allergan plc and Shire plc, and against products such as Restasis, Xiidra, eye wipes, baby shampoo and soap. There are also over-the-counter products that contain hypochlorous acid that compete with Avenova.
For our newly acquired DERMAdoctor products and CelleRx Clinical Reset that operate in the skincare and beauty industry, we also face vigorous competition form companies globally, including large multinational consumer products companies that have many skincare brands under ownership and standalone skincare brands, including those that may target the latest trends or specific distribution channels. The skincare and beauty industry is highly competitive and subject to rapid changes due to consumer preferences and industry trends. Competition in the skincare industry is generally based on the introduction of new products, pricing of products, quality of products and packaging, brand awareness, perceived value and quality, innovation, in-store presence and visibility, promotional activities, advertising, editorials, e-commerce and mobile-commerce initiatives and other activities. We must compete with a high volume of new product introductions and existing products by diverse companies across several different distribution channels. Our skincare and other beauty products face, and will continue to face, competition for consumer recognition and market share with products that have achieved significant national and international brand name recognition and consumer loyalty, such as those offered by global prestige beauty companies like Avon Products, Inc., Elizabeth Arden, Inc., The Estée Lauder Companies, Inc., Johnson & Johnson, Inc., L’Oréal Group, Shiseido, Coty, Mary Kay, Inc. and The Proctor & Gamble Company, each of which have launched skincare brands. In addition, we compete with brands including Dr. Dennis Gross, Kate Somerville, Murad, Perricone M.D., Dr. Brandt, Clarins, Clinique, Dermalogica, Exuviance, La Roche Posay and Vichey. We also compete with numerous other companies that market skincare products. Competition may increase further as existing competitors enhance their offerings or additional companies enter our markets or modify their existing products to compete directly with our products.
These companies that we compete against in the eye care, skincare and beauty industries may have substantially greater financial, technical and marketing resources, longer operating histories, greater brand recognition and larger customer bases than we do and may be able to respond more effectively to changing business and economic conditions than we can. If our competitors respond more quickly to new or emerging technologies and changes in customer requirements, our products may be rendered obsolete or non-competitive. In addition, if our competitors develop more effective or affordable products, or achieve earlier intellectual property protection or product commercialization than we do, our operating results will materially suffer. Competition may increase further as existing competitors enhance their offerings or additional companies enter our markets or modify their existing products to compete directly with our products. We may not be able to sustain growth as competitive pressures, including pricing pressure from competitors, increase. Our ability to compete depends on the continued strength of our brand and products, the success of our marketing, innovation and execution strategies, the continued diversity of our product offerings, the successful management of new product introductions and innovations, strong operational execution, including in order fulfillment, and our success in entering new markets and expanding our business in existing geographies. If we are unable to continue to compete effectively, it could have a material adverse effect on our business, results of operations and financial condition.
We are dependent on third parties to supply raw materials used in our products and to manufacture our products. Any interruption or failure by these suppliers or other disruptions to our supply chain may materially adversely affect our business, financial condition, results of operations and cash flows.
Our ability to make, move, and sell our products is critical to our success. Damage or disruption to our supply chain, including third-party manufacturing, assembly or transportation and distribution capabilities, due to weather, including any potential effects of climate change, natural disaster, fire or explosion, terrorism, pandemics (such as the COVID-19 pandemic), strikes, government action, or other reasons beyond our control or the control of our suppliers and business partners, could impair our ability to manufacture or sell our products. Failure to take adequate steps to mitigate the likelihood or potential impact of such events, or to effectively manage such events if they occur, particularly when a product is sourced from a single supplier or location, could adversely affect our business or financial results.
Further, we rely on third parties to supply raw materials, components and packaging products, to manufacture finished products, and distribute our products. Any interruption or failure by our suppliers, distributors and other partners to meet their obligations on schedule or in accordance with our expectations, misappropriation of our proprietary information, including trade secrets and know-how, or any termination by these third parties of their arrangements with us, which, in each case, could be the result of one or many factors outside of our control, could delay or prevent the manufacture or commercialization of our products, disrupt our operations or cause reputational harm to our company, particularly with wholesale customers, any or all of which could have a material adverse effect on our business, financial condition, results of operations and cash flows.
In particular, a large portion of DERMAdoctor’s revenue is from wholesale customers, which in accordance with standard industry practice, do not typically operate under written contracts or advance commitments for products. That being said, such wholesale customers generally place large orders shortly before such products are needed. Due to cost and product shelf life, DERMAdoctor is not able to keep a large inventory on hand though must have the ability to quickly provide products to its wholesale customers upon demand. Therefore, DERMAdoctor relies on its third party suppliers to be able to quickly respond to DERMAdoctor’s product needs, and if such supply chain is interrupted, could cause a material adverse effect on our business, reputation with wholesale customers and financial condition.
Significant disruptions of information technology systems or breaches of information security could adversely affect our businesses.
We rely upon information technology systems to operate our businesses. In the ordinary course of business, we collect, store and transmit large amounts of confidential information (including, but not limited to, personal information and intellectual property), and we deploy and operate an array of technical and procedural controls to maintain the confidentiality and integrity of such confidential information. We also have outsourced aspects of our operations to third parties, including significant elements of our information technology infrastructure and, as a result, we are managing independent vendor relationships with third parties who may or could have access to our confidential information. The size and complexity of our information technology and information security systems, and those of our third-party vendors with whom we contract, make such systems potentially vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by our employees or vendors, or from attacks by malicious third parties. Such attacks are of ever-increasing levels of sophistication and are made by groups and individuals with a wide range of motives and expertise, including organized criminal groups, “hacktivists,” nation states and others. While we have invested in the protection of data and information technology, there can be no assurance that our efforts will prevent service interruptions or security breaches. Any such interruption or breach of our systems could adversely affect our business operations and/or result in the loss of critical or sensitive confidential information or intellectual property, and could result in financial, legal, business and reputational harm to us.
Adverse U.S. or international economic conditions could negatively affect our business, financial condition and results of operations.
Consumer spending on skincare and beauty products, as well as eye care products, is influenced by general economic conditions and the availability of discretionary income. Adverse U.S. or international economic conditions or periods of inflation or high energy prices may contribute to higher unemployment levels, decreased consumer spending, reduced credit availability and declining consumer confidence and demand, each of which poses a risk to our business. A decrease in consumer spending or in retailer and consumer confidence and demand for our products could have a significant negative impact on our net sales and profitability, including our operating margins and return on invested capital. These economic conditions could cause some of our retail customers or suppliers to experience cash flow or credit problems and impair their financial condition, which could disrupt our business and adversely affect product orders, payment patterns and default rates and increase our bad debt expense. In addition, deterioration in global financial markets could make future financing difficult or more expensive, which could have a material adverse effect on our ability to finance the acquisition of inventory for sale to our customers.
Risk Related to Government Regulation
We expect continuous revenue from sales of Avenova, which is classified as a cleared medical device by the FDA, but we cannot guarantee that the FDA will continue to allow us to market and sell Avenova as a cleared medical device, which marketing inability would halt our sales and marketing of Avenova and cause us to lose revenue and materially and adversely affect our results of operations and the value of our business.
Our ability to continue to commercialize Avenova and generate revenue from Avenova depends upon, among other things:
|
|
●
|
the FDA allowing us to continue marketing Avenova as an FDA cleared medical device;
|
|
|
●
|
acceptance in the medical community;
|
|
|
●
|
the safety of Avenova’s predicate devices;
|
|
|
●
|
the number of patients who use Avenova;
|
|
|
●
|
coverage or reimbursement by third-party payors;
|
|
|
●
|
our ability to successfully market Avenova;
|
|
|
●
|
reopening and resurgence of patient visits to eyecare specialists after the nationwide shelter-in-place mandates cease; and
|
|
|
●
|
the amount and nature of competition from competing companies with similar products.
|
The sale of Avenova will be subject to, among other things, regulatory and commercial and market uncertainties that may be outside of our control. The clearance that we have received from the FDA for our Avenova, NeutroPhase, PhaseOne and other products is subject to strict limitations on the indicated uses for which the products may be marketed. The labeling, packaging, adverse event reporting, storage, advertising, promotion and record keeping for all of our products, including those that are not subject to FDA clearance, are subject to extensive regulatory requirements.
In addition, there can be no assurance that government regulations applicable to our products will not change and thereby prevent the marketing of some or all of our products for a period of time or permanently. The FDA’s policies may change and additional government regulations may be enacted that could modify, prevent or delay regulatory approval of our products. We cannot predict the likelihood, nature or extent of adverse government regulation that may arise from future legislation or administrative action, either in the U.S. or in other countries. We cannot guarantee that Avenova, our other cleared products, or products that may be approved or cleared for marketing in the future, will not be materially adversely impacted by a change in industry standards or regulations. If changes to industry standards, practices or regulations applicable to Avenova or our other cleared products that we may market and sell in the future cause a delay in continued commercialization or if we cannot make a change to satisfy the industry standards, practices or regulations, we may not be able to meet market demand which may have a materially adverse effect on our business, financial condition, results of operations, and prospects.
Additionally, the FDA may request that we submit another 510(k) premarket submission that compares to another predicate device. If we are unable to find an adequate predicate device that is substantially equivalent to Avenova for the treatment claims that we use to sell and market Avenova, we may not be able to obtain the necessary FDA clearance to continue to market and sell Avenova without performing comprehensive clinical trials. In such event, we would need to seek premarket approval from the FDA for the applicable product before we could continue to sell and market Avenova in the United States, which would be significantly more time consuming, expensive, and uncertain.
Our commercialized products such as Avenova, CelleRx Clinical Reset and our DERMAdoctor products are not approved by the FDA as a drug, and we rely solely on the 510(k) clearance for Avenova and certain of our other products as a medical device.
Our business and future growth depend on the development, use and sale of products that are subject to FDA regulation, clearance and approval. Under the U.S. Federal Food, Drug, and Cosmetic Act and other laws, we are prohibited from promoting our products for off-label uses. This means that we may not make claims about the safety or effectiveness of our products and may not proactively discuss or provide information on the use of our products, except as allowed by the FDA. As Avenova is a medical device, we may only make very limited claims that pertain to its cleared intended use. Without claims of efficacy, market acceptance of our products may be slow. The 510(k) status of Avenova also affects our ability to obtain formal insurance reimbursement by payors, and affects our ability to obtain Medicare coverage.
The FDA does not currently require pre-market approval for products intended to be sold as non-prescription skincare products, such as our CelleRx Clinical Reset and DERMAdoctor products, so long as they are not marketed for the treatment or prevention of a disease, or as affecting the structure or function of the human body. However, the FDA may in the future require pre-market approval, clearance or registration/notification of skincare products. Moreover, such products could also be regulated as both drugs and skincare simultaneously, as the categories are not mutually exclusive. If the FDA determines that any of our products intended to be sold as skincare should be classified and regulated as drug products, and we are unable to comply with applicable drug requirements, we may be unable to continue to market those products. Any inquiry into the regulatory status of our skincare products and any related interruption in the marketing and sale of these products could damage our reputation and image in the marketplace.
There is significant risk that the FDA or other federal or state law enforcement authorities may determine that the nature and scope of our sales and marketing activities constitutes the promotion of our products for non-FDA-approved uses in violation of applicable law and as the sale of unapproved drugs, which is prohibited under applicable law. We face the risk that the FDA may take enforcement action against us for the way that we promote and sell our products. This risk may grow with the increased visibility of Avenova online, as well as the FDA’s increased focus on antimicrobial products in the wake of the COVID-19 pandemic. We also face the risk that the FDA or other regulatory authorities might pursue enforcement actions based on past activities that we have discontinued or changed, including sales activities, arrangements with institutions and doctors, educational and training programs and other activities.
Government investigations concerning the promotion of unapproved drug products, off-label uses and related issues are typically expensive, disruptive and burdensome and generate negative publicity. If our promotional activities are found to be in violation of applicable law or if we agree to a settlement in connection with an enforcement action, we would likely face significant fines and penalties and be required to substantially limit and change our sales and promotion activities.
Developments after a product reaches the market may adversely affect sales of our products.
Even after obtaining regulatory clearances, certain developments may decrease demand for our products, including the re-review of products that are already marketed; new scientific information and evolution of scientific theories; the recall or loss of regulatory clearance of products that are already marketed; changing government standards or public expectations regarding safety, efficacy or labeling changes; and greater scrutiny in advertising and promotion. If previously unknown side effects are discovered or if there is an increase in negative publicity regarding known side effects of a product, it could significantly reduce demand for the product or require us to take actions that could negatively affect sales, including removing the product from the market, restricting its distribution or applying for labeling changes. In addition, some health authorities appear to have become more cautious when examining new products and are re-reviewing select products that are already marketed, adding further to the uncertainties in the regulatory processes.
There is also greater regulatory scrutiny, especially in the United States, on advertising (in particular, direct to consumer advertising), promotion and pricing of pharmaceutical products. Certain regulatory changes or decisions could make it more difficult for us to sell our products. If we are not able to maintain regulatory compliance, we may be subject to fines, suspension or withdrawal of regulatory clearance, product recalls, seizure of products, operating restrictions, injunctions, warning letters, criminal prosecution and other enforcement actions. Any of these events could prevent us from marketing our products and our business may not be able to continue past such concerns. If any of the above occurs to Avenova, CelleRx Clinical Reset or our DERMAdoctor products, our business, results of operations, financial condition and cash flows could be materially adversely affected.
We do not have our own manufacturing capacity, and we rely on partnering arrangements or third-party manufacturers for the manufacture of our products and potential products.
The FDA and other governmental authorities require that all of our products, including those of DERMAdoctor, be manufactured in strict compliance with federal Quality Systems Regulations and other applicable government regulations and corresponding foreign standards. We do not currently operate manufacturing facilities for production of our products. As a result, we have partnered with third parties to manufacture our products or rely on contract manufacturers to supply, store and distribute our products and help us meet legal requirements. As we have limited control over our commercial partners, any performance failure on their part (including failure to deliver compliant, quality components or finished goods on a timely basis or properly branded products) could affect the commercialization of our products, producing additional losses and reducing or delaying product revenues. If any of our commercial partners or manufacturers have violated or is alleged to have violated any laws or regulations during the performance of their obligations to us, it is possible that we could suffer significant financial, operational and reputational harm or other negative outcomes, including costly corrective actions, including suspending manufacturing operations, changing product formulations, suspending sales of nonconforming products, or initiating product recalls, change product labelling, packaging or advertising or take other corrective action and possible legal consequences.
Our products require precise, high-quality manufacturing. The failure to achieve and maintain high manufacturing standards could result in patient injury or death, product recalls or withdrawals, delays or failures in product testing or delivery, cost overruns or other problems that could seriously harm our business. Contract manufacturers and partners often encounter difficulties involving production yields, quality control and quality assurance, as well as shortages of qualified personnel. Accordingly, we and our third-party manufacturers are also subject to periodic unannounced inspections by the FDA to determine compliance with the FDA's requirements, including primarily current Good Manufacturing Practice (“cGMP”), the Quality Systems Regulations (“QSR”), medical device reporting regulations (where applicable for Avenova), proper and compliant labeling and other applicable government regulations and corresponding foreign standards, including ISO 13485.
The results of these inspections can include inspectional observations on FDA’s Form 483, untitled letters, warning letters, or other forms of enforcement. If the FDA were to conclude that we are not in compliance with applicable laws or regulations, or that any of our FDA-cleared products are ineffective, make additional therapeutic claims that are not commensurate to the accepted labeling claims, or pose an unreasonable health risk, the FDA could take a number of regulatory actions, including preventing us from manufacturing any or all of our products or performing laboratory testing on human specimens, which could materially adversely affect our business. In addition, a prolonged interruption in the manufacturing of one or more of our products as a result of non-compliance could decrease our supply of products available for sale, which could reduce our net sales, gross profits and market share, as well as harm our overall business, prospects, financial condition and results of operations.
Avenova's FDA-clearance and our other products that have been cleared by the FDA or products that we may obtain FDA-clearance in the future, if at all, are subject to limitations on the intended uses for which the product may be marketed, which can reduce our potential to successfully commercialize the product and generate revenue from the product. If the FDA determines that our promotional materials, labeling, training or other marketing or educational activities constitute promotion of an unapproved use, it could request that we cease or modify our training or promotional materials or subject us to regulatory enforcement actions. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our training or other promotional materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities. In addition, we may be required to conduct costly post-market testing and surveillance to monitor the safety or effectiveness of our products, and we must comply with medical device reporting requirements where applicable for Avenova, including the reporting of adverse events and malfunctions related to our products. Later discovery of previously unknown problems with our products, including unanticipated adverse events or adverse events of unanticipated severity or frequency, manufacturing problems, or failure to comply with regulatory requirements such as QSR, may result in changes to labeling, restrictions on such products or manufacturing processes, withdrawal of the products from the market, voluntary or mandatory recalls, a requirement to repair, replace or refund the cost of any medical device we manufacture or distribute, fines, suspension of regulatory clearance to one or all of our products that may be cleared in the future, product seizures, injunctions or the imposition of civil or criminal penalties which would adversely affect our business, operating results and prospects.
If we were to lose, or have restrictions imposed on, FDA clearances we may receive in the future, our business, operations, financial condition and results of operations would likely be materially adversely impacted.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents we have filed with the SEC that are incorporated by reference contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934 (the “Exchange Act”). These statements relate to future events or to our future operating or financial performance and involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking statements.
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential,” variations of these words, and similar expressions intended to identify forward-looking statements. Without limiting the generality of the forgoing, forward-looking statements contained in this prospectus include our expectations regarding our future growth, operational and financial performance and business prospects and opportunities. These statements reflect our current views with respect to future events and are based on assumptions and are subject to known and unknown risks and uncertainties and other factors. As a result of a number of known and unknown risks and uncertainties, our results or performance may be materially different from those expressed or implied by these forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements.
We discuss in greater detail many of these risks under the heading “Risk Factors” contained in this prospectus or otherwise described in our filings with the SEC, including our Annual Report on Form 10-K, in our subsequently filed Quarterly Reports on Form 10-Q, as well as any amendments thereto reflected in subsequent filings with the SEC, which are incorporated by reference into this prospectus in their entirety. Also, these forward-looking statements represent our estimates and assumptions only as of the date of the document containing the applicable statement. Unless required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information or future events or developments. Thus, you should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. You should read this prospectus together with the documents we have filed with the SEC that are incorporated by reference completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements in the foregoing documents by these cautionary statements.
DESCRIPTION OF THE PRIVATE PLACEMENT
The following is a summary of the material provisions of the Securities Purchase Agreement, the Certificate of Designation, the Warrant and the Registration Rights Agreement, but does not purport to describe all of the terms thereof and may not contain all of the information about the Securities Purchase Agreement, the Certificate of Designation, the Warrant, and the Registration Rights Agreement that is important to you. The following summary is qualified in its entirety by reference to the complete text of the Securities Purchase Agreement, the Certificate of Designation, the Warrant, and the Registration Rights Agreement, which were filed as Exhibits 1.1, 3.1, 4.1 and 10.1 to our Current Report on Form 8-K, filed with the SEC on November 1, 2021 and is incorporated into this prospectus by reference. You should refer to the full text of the Securities Purchase Agreement, the Certificate of Designation, the Warrant, the Registration Rights Agreement and the related documents for details about the transaction, and carefully read this entire prospectus, including other documents we have incorporated herein, and the other documents to which we have referred you.
Private Placement and Securities Purchase Agreement
On October 29, 2021, we entered into the Securities Purchase Agreement with the Purchasers whereby NovaBay sold an aggregate of 15,000 shares of the Preferred Stock convertible into 37,500,000 Conversion Shares and Warrants exercisable for 37,500,000 Warrant Shares for an aggregate purchase price of $15,000,000. NovaBay closed the Private Placement on November 2, 2021. We used a portion of the net proceeds from the Private Placement to fund the Purchase Price to acquire DERMAdoctor, which transaction was completed on November 5, 2021, and we anticipate using the remainder of the net proceeds for working capital purposes. For additional information about the DERMAdoctor Acquisition and the Acquisition Purchase Agreement, see “The Acquisition Purchase Agreement” in this prospectus.
The Preferred Stock is not fully convertible into the Conversion Shares until such time as the Share Conversion Approval is received from our stockholders in accordance with Section 713(a) and (b) of the Company Guide. Until the Share Conversion Approval is received from stockholders, the holders of Preferred Stock may convert their shares, pro rata, up to an aggregate of the Issuable Maximum. The powers, preferences, rights, qualifications, limitations and restrictions applicable to this newly created Preferred Stock, including the anti-dilution protections, are set forth in the Certificate of Designation filed with the Secretary of State of the State of Delaware on November 1, 2021. For additional information about the powers, preferences, rights, qualifications, limitations and restrictions with respect to the Preferred Stock, see “Description of Capital Stock” in this prospectus beginning on page 43.
The Warrants are not exercisable unless and until the Share Conversion Approval and the Authorized Share Increase are approved by stockholders. Upon stockholder approval, the Warrants will become exercisable at an exercise price of $0.53 per share, subject to adjustment, and will remain exercisable for six (6) years from the date they become exercisable.
The consummation of the Private Placement was subject to the satisfaction or waiver of, among other customary closing conditions, the accuracy of the representations and warranties in the Securities Purchase Agreement, the compliance by the parties with the covenants in the Securities Purchase Agreement, the absence of any legal order barring the Private Placement, no material adverse effect having occurred with respect to NovaBay, and no suspension in the trading of the Common Stock. The parties were also provided with customary termination rights, including the right of NovaBay or any Purchaser to terminate the Securities Purchase Agreement if the consummation of the Private Placement had not occurred within five days after the signing. These termination rights were not exercised by any party to the Securities Purchase Agreement.
Under the Securities Purchase Agreement, we made representations and warranties with respect to its business customary for transactions of a similar nature, including, with respect to organization and qualification, subsidiaries, authorization, capitalization, non-contravention, financial statements, reports and filings with the SEC, the non-occurrence of certain events from the date of the latest balance sheet of NovaBay, litigation, title to assets, labor matters, intellectual property, tax matters, insurance, the exemption under the federal securities laws with respect to the Private Placement and compliance with other laws.
In addition, under the Securities Purchase Agreement, each Purchaser made representations and warranties customary for transactions of a similar nature, including with respect to organization, authorization, non-contravention, investment intent, accredited investor status, certain trading activities, brokers and finders, and the independent evaluation of the decision to purchase the Preferred Stock and the Warrants. In addition, the Purchasers acknowledged there was limited information disclosed with respect to the DERMAdoctor Acquisition in our filings with the SEC, and determined that they were proceeding with their investment in the Private Placement as contemplated by this Agreement.
Certain representations of NovaBay and the Purchasers were qualified in whole or in part by a materiality standard for purposes of determining whether a breach of such representations and warranties has occurred. Moreover, certain representations and warranties in the Securities Purchase Agreement were used for the purpose of allocating risk among the parties, rather than establishing matters of fact. Accordingly, investors and securityholders should not rely on the representations and warranties in the Securities Purchase Agreement as characterizations of actual state of facts about the parties.
We agreed to certain covenants under the Securities Purchase Agreement after the Private Placement Closing Date, including among others, the following:
|
|
●
|
We would timely file a Form D with respect to the Preferred Stock and the Warrants sold pursuant to the Securities Purchase Agreement;
|
|
|
●
|
We would use the net proceeds of the Private Placement toward funding the DERMAdoctor Acquisition and for working capital purposes, and we would not use such proceeds for: (i) the satisfaction of any portion of our debt (other than payment of trade payables in the ordinary course of our business and prior practices), (ii) the redemption of any Common Stock or Common Stock equivalents, (iii) the settlement of any outstanding litigation or (iv) in violation of FCPA or OFAC regulations;
|
|
|
●
|
We would maintain a reserve equal to 37,500,000 shares of Common Stock from our duly authorized shares of Common Stock for all of the Common Stock that would be issuable upon conversion of the Preferred Stock up to the Issuable Maximum, and to maintain, after the Share Conversion Approval and the Authorized Share Increase were approved by stockholders, a reserve of a number of shares of Common Stock in order to satisfy the full conversion of the Preferred Stock and the full exercise of the Warrants;
|
|
|
●
|
We would not (i) issue or enter into any agreement to issue or announce the issuance or proposed issuance of any shares of Common Stock or Common Stock equivalents, (ii) incur, enter into any agreement to incur or announce the incurrence or proposed incurrence of any indebtedness, or (iii) file any registration statement or any amendment or supplement thereto, in each case other than as contemplated pursuant to the Registration Rights Agreement, until ninety (90) days after the later of (x) the date of the Share Conversion Approval and the Authorized Share Increase having been approved by stockholders and being effective or (y) the effective date of the Initial Registration Statement providing for the registration of the Conversion Shares;
|
|
|
●
|
We are required to hold a special meeting of stockholders for the purpose of obtaining stockholder approval of the Share Conversion Approval in accordance with the Company Guide and the Authorized Share Increase and to use our reasonable best efforts to (i) solicit stockholders’ approval of such proposals, (ii) have all management-appointed proxyholders vote their proxies in favor of such proposals and (iii) cause our Board of Directors to recommend to the stockholders that they recommend such proposals;
|
|
|
●
|
We will call a meeting of our stockholders every four (4) months to obtain approval of the Share Conversion Approval and the Authorized Share Increase, in the event that these proposals are not approved at our special stockholder meeting;
|
|
|
●
|
We agreed to pay partial liquidated damages to the Purchasers in certain circumstances, which include when we (i) are not in compliance with the public information requirements under Rule 144 of the Securities Act or (ii) have not removed restrictive legends within the timing provided for in the Securities Purchase Agreement; and
|
|
|
●
|
We would not, for a period of one (1) year, undertake a reverse or forward stock split or reclassification of our Common Stock without the prior written consent of the Purchasers holding a majority in interest of the shares of Preferred Stock.
|
The Purchasers made certain covenants under the Securities Purchase Agreement and the Certificate of Designation, including among others, the following:
|
|
●
|
The shares of Common Stock issued to the Purchasers upon the conversion of the Preferred Stock may be disposed of only pursuant to an effective registration statement under, and in compliance with, the requirements of the Securities Act, or pursuant to an available exemption from, or in a transaction not subject to, the registration requirements of the Securities Act, and in compliance with any applicable U.S. state and federal securities laws.
|
|
|
●
|
Each Purchaser agreed that it would not have the right to convert any portion of the Preferred Stock, to the extent that, after giving effect to the conversion, the Purchaser would, together with such Purchaser’s affiliates or other persons with whom they are acting as a group together, would beneficially own (i) in excess of the Beneficial Ownership Limitation and/or (ii) the Issuable Maximum. Each Purchaser may from time to time change the beneficial ownership limitation to any other percentage not in excess of 9.99%, subject to the requirements set forth in the Securities Purchase Agreement and the Certificate of Designation. The “Beneficial Ownership Limitation” shall be 4.99% (or, upon election by a Purchaser prior to the issuance of any shares, 9.99%) of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of shares of Common Stock issuable upon conversion of Preferred Stock and Warrants, if any, held by the Purchaser.
|
Ladenburg Thalmann & Co. Inc. served as the placement agent for the Private Placement and received a commission equal to eight percent (8%) of the gross proceeds of the securities sold to the Purchasers at the closing of the Private Placement.
Registration Rights Agreement
On the Private Placement Closing Date, pursuant to the Securities Purchase Agreement, we entered into the Registration Rights Agreement with the Purchasers. Pursuant to the Registration Rights Agreement, we are required to prepare and file with the SEC: (i) the Initial Registration Statement covering the resale of the Conversion Shares within 30 calendar days following the Private Placement Closing Date (the “Initial Filing Deadline”); and (ii) a Second Registration Statement on Form S-1 covering the resale of the Warrant Shares within 30 calendar days following approval by stockholders of the Share Conversion Approval and the Authorized Share Increase (the “Second Filing Deadline”). We will use commercially reasonable efforts to cause the Registration Statements to be declared effective by the SEC within 60 calendar days of the Initial Filing Deadline (or 90 days if full review by the SEC) in the case of the Initial Registration Statement and 60 days (or 90 days if full review by the SEC) in the case of the Second Registration Statement. We also agreed, among other things, to indemnify the Purchasers, their officers, directors, members, employees and agents, successors and assigns under the registration statement from certain liabilities and to pay all fees and expenses (excluding any legal fees of the Purchaser(s), and any underwriting discounts and selling commissions) incident to our obligations under the Registration Rights Agreement. The Private Placement was exempt from registration pursuant to Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder, as a transaction by an issuer not involving a public offering. The Purchasers have represented to us that they have acquired the securities for investment only and not with a view to, or for sale in connection with, any distribution thereof, and appropriate legends have been affixed to the securities issued in this transaction.
Use of Proceeds
Gross proceeds from the Private Placement were approximately $15.0 million, with net proceeds of approximately $13.4 million after deducting commissions and estimated offering costs. We used the net proceeds from the Private Placement to fund a portion of the Purchase Price of approximately $12.0 million to satisfy our financing condition to closing the DERMAdoctor Acquisition, and the balance is expected to be used for working capital purposes. For additional information about the DERMAdoctor Acquisition and the Acquisition Purchase Agreement, please see “The Acquisition Purchase Agreement” in this prospectus.
Description of the Preferred Stock
Pursuant to the Certificate of Designation, we authorized 15,000 shares of the Preferred Stock, of which all have been issued to the Purchasers. Except as otherwise required by law, the Preferred Stock does not have voting rights. However, we agreed that, as long as any shares of Preferred Stock are outstanding, we will not, without the affirmative vote of the holders of a majority of the then outstanding shares of the Preferred Stock, (i) alter or change adversely the powers, preferences or rights given to the Preferred Stock or alter or amend the Certificate of Designation, (ii) amend the Certificate of Incorporation or other charter documents in any manner that adversely affects any rights of the holders of the Preferred Stock, (iii) increase the number of authorized shares of Preferred Stock, or (iv) enter into any agreement with respect to any of the foregoing. The Preferred Stock does not have any preemptive rights or a preference upon any liquidation, dissolution or winding-up of NovaBay. We do not have any other class or series of preferred stock currently issued or outstanding.
Prior to stockholder approval of the Share Conversion Approval and the Authorized Share Increase, the holders of the Preferred Stock will be permitted to convert a limited number of shares of the Preferred Stock (which shall not exceed their pro rata portion of the Issuable Maximum) into shares of Common Stock. This limited conversion right provided to holders of the Preferred Stock does not permit conversion into more than an aggregate of 19.99% of outstanding shares of Common Stock immediately prior to the Private Placement Closing Date unless or until the Share Conversion Approval is received from our stockholders. Notwithstanding the approval of the Share Conversion Approval, the holders of Preferred Stock are also subject to the Beneficial Ownership Limitation.
The Preferred Stock does not have any preemptive rights or a preference upon any liquidation, dissolution or winding-up of NovaBay. The Preferred Stock, however, does have anti-dilution protections that could result in an increase in the number of shares of Common Stock to be issued upon conversion of the Preferred Stock in the future, in certain circumstances, including if we: (i) pay a dividend or otherwise make a distribution or distributions on shares of its Common Stock or other equity securities; (ii) subdivide outstanding shares of Common Stock into a larger number of shares or combine (including by way of reverse stock split) outstanding shares of Common Stock into a smaller number of shares; or (ii) issue by reclassification of shares of Common Stock any shares of capital stock of the Company. In addition, these anti-dilution protections also apply if we sell or grant any Common Stock or any other securities, subject to certain limited exceptions, which would entitle the holder thereof to acquire Common Stock at an effective price per share that is lower than the applicable conversion price of the Preferred Stock, and, as a result, the conversion price of the Preferred Stock will be reduced to such lower price, which is referred to as a “full-ratchet” anti-dilution protection. For additional information about the rights and preferences of the Preferred Stock, see “Description of Capital Stock—Preferred Stock” in this prospectus beginning on page 44 and the Certificate of Designation, which is incorporated by reference into this prospectus.
THE ACQUISITION PURCHASE AGREEMENT
The following summary describes the material provisions of the Acquisition Purchase Agreement that provides for the acquisition of DERMAdoctor, which closed on November 5, 2021. The following summary of the Acquisition Purchase Agreement is subject to, and qualified in its entirety by reference to, the Acquisition Purchase Agreement, which was filed as Exhibit 2.1 to our Current Reports on Form 8-K, filed with the SEC on September 28, 2021 and on November 12, 2021, and which are incorporated into this prospectus by reference. You are urged to read the Acquisition Purchase Agreement carefully and in its entirety, as it is the legal document governing the DERMAdoctor Acquisition, and carefully read this entire prospectus, including other documents we have incorporated herein, and the other documents to which we have referred you. Business and operational information regarding DERMAdoctor can be found in the section titled “DERMAdoctor Business” included in our Definitive Proxy Statement on Schedule 14A filed with the SEC on November 12, 2021, which is incorporated by reference into this prospectus, and with respect to us, in our filings with the SEC that are incorporated herein. See “Where You Can Find Additional Information” and “Incorporation of Certain Documents by Reference” in this prospectus.
The Acquisition Purchase Agreement and the following summary have been included to provide you with information regarding the terms of the Acquisition Purchase Agreement. The assertions embodied in the representations and warranties contained in the Acquisition Purchase Agreement of NovaBay, the Sellers and the Founders were made solely for purposes of the Acquisition Purchase Agreement and may be subject to important qualifications and limitations agreed to by the parties thereto in connection with negotiating its terms. In particular, the representations and warranties contained in the Acquisition Purchase Agreement were made solely for the benefit of the parties to the Acquisition Purchase Agreement and were negotiated for the purpose of allocating risk between the parties to the Acquisition Purchase Agreement rather than to establish matters of fact. The assertions embodied in those representations and warranties also are qualified by information in confidential disclosure schedules that the parties have exchanged in connection with signing the Acquisition Purchase Agreement. Although NovaBay, DERMAdoctor, the Sellers and the Founders do not believe that the confidential disclosure schedules contain information that the federal securities laws require to be publicly disclosed, the disclosure schedules do contain information that modifies, qualifies and creates exceptions to the representations and warranties set forth in the Acquisition Purchase Agreement. Accordingly, neither our stockholders nor investors in our Common Stock should rely on the representations and warranties as characterizations of the actual state of facts, since they were only made as of the date of the Acquisition Purchase Agreement and are modified in important part by the underlying disclosure schedules. Moreover, information concerning the subject matter of the representations and warranties may have changed since the date of the Acquisition Purchase Agreement, which subsequent information may or may not be fully reflected in our public disclosures, including those that we file with the SEC.
The DERMAdoctor Acquisition and the Closing
On September 27, 2021, we entered into the Acquisition Purchase Agreement with DERMAdoctor by and among (i) us, (ii) DERMAdoctor, (iii) the Founders; and (iv) the Sellers. The Acquisition Purchase Agreement provides for the acquisition of 100% of the issued and outstanding membership units of DERMAdoctor, of which 82.2% were owned by Papillon and 17.8% were owned by MGP. The Acquisition Purchase Agreement was approved and adopted by our Board of Directors and was also approved and adopted by the managers of DERMAdoctor and the Sellers. The DERMAdoctor Acquisition, since paid for in cash, was not subject to the approval of our stockholders.
On November 5, 2021, after receiving the proceeds from the Private Placement and the satisfaction or waiver of the other closing conditions, we closed the DERMAdoctor Acquisition. DERMAdoctor is now our wholly-owned subsidiary.
Consideration in the DERMAdoctor Acquisition
Pursuant to the terms of the Acquisition Purchase Agreement, we acquired 100% of the issued and outstanding membership units of DERMAdoctor, all of which were owned by the Sellers, for an agreed upon Purchase Price of approximately $15.0 million. The Purchase Price is comprised of a payment of approximately $12.0 million in cash to the Sellers that was made at the Acquisition Closing, which amount was (i) reduced to account for the amounts that we paid to satisfy DERMAdoctor’s indebtedness and its transaction expenses as of the Acquisition Closing, and (ii) increased by the DERMAdoctor cash and cash equivalents that remained as of the Acquisition Closing. In addition, $1.2 million of the Purchase Price paid at the Acquisition Closing was deposited into an escrow account, which amount is available to NovaBay to satisfy indemnification obligations of the Founders and the Sellers in accordance with the Acquisition Purchase Agreement. In addition, the Purchase Price includes up to an additional aggregate amount of $3.0 million in Earn Out Payments that will be contingent upon the legacy DERMAdoctor business achieving predetermined financial targets for the 2022 and the 2023 calendar years, subject to a maximum aggregate Earn Out Payment of $1.5 million for each Earn Out Period. Payment of an Earn Out Payment to the Sellers, if earned, will be conditioned upon both of the Founders remaining employees of DERMAdoctor throughout the entirety of the applicable Earn Out Period where an Earn Out Payment, if any, becomes payable; provided, however, this condition shall not apply if (x) we terminate either of the Founders “without cause”, as defined under their respective employment agreements, or (y) the death or disability of either Founder occurs. If earned, subject to certain parameters, the Sellers may elect for the Earn Out Payments to be paid in cash or unregistered shares of our Common Stock, with the number of shares determined by dividing the applicable Earn Out Payment by the average closing stock price of our Common Stock on the prior ten trading days before the Earn Out Payment is finally determined. Such shares of the Common Stock would be issued pursuant to a private placement exemption from registration provided by Section 4(a)(2) of the Securities Act and by Rule 506 of Regulation D.
Conditions to the Completion of the DERMAdoctor Acquisition
The obligation of NovaBay, on the one hand, and DERMAdoctor, the Sellers and the Founders, on the other hand, to consummate the DERMAdoctor Acquisition was subject to the satisfaction or waiver of agreed upon and other customary closing conditions, including the absence of injunction or the enactment of any law that would restrain, prohibit or otherwise prevent the consummation of the DERMAdoctor Acquisition.
Our obligation to consummate the DERMAdoctor Acquisition was subject to the satisfaction or waiver of certain conditions at or prior to the Acquisition Closing that included:
|
|
●
|
completing a financing(s) transaction for at least $7.2 million to fund the Purchase Price;
|
|
|
●
|
the accuracy of the representations and warranties of DERMAdoctor, the Sellers and the Founders contained in the Acquisition Purchase Agreement, subject to those representations and warranties with specified materiality and material adverse effect qualifications;
|
|
|
●
|
DERMAdoctor’s, the Sellers’ and/or the Founders’ performance of covenants and agreements required by the Acquisition Purchase Agreement, in all material respects;
|
|
|
●
|
the Sellers having delivered their membership units and any lien releases that may exist on such membership units; and
|
|
|
●
|
there shall not have occurred nor shall any event or casualty have occurred that, individually or in the aggregate, could reasonably be expected to result in a material adverse effect on DERMAdoctor and its business.
|
The Sellers’ and the Founders’ obligation to consummate the DERMAdoctor Acquisition were subject to the satisfaction or waiver of certain conditions at or prior to the Acquisition Closing that included:
|
|
●
|
NovaBay having delivered reasonable evidence of the successful completion of the financing for at least $7.2 million to fund the Purchase Price;
|
|
|
●
|
the accuracy of the representations and warranties of NovaBay contained in the Acquisition Purchase Agreement, subject to those representations and warranties with specified materiality and material adverse effect qualifications; and
|
|
|
●
|
NovaBay’s performance of covenants and agreements required by the Acquisition Purchase Agreement, in all material respects, including delivery of the Purchase Price.
|
The conditions to closing were satisfied or waived by the parties as of the Acquisition Closing.
Representations, Warranties and Covenants
Pursuant to the Acquisition Purchase Agreement, NovaBay, DERMAdoctor, the Sellers and the Founders made certain customary representations and warranties relating to their respective companies, businesses, security ownership and other matters related to the DERMAdoctor Acquisition. Such representations and warranties generally remained accurate through the completion of the DERMAdoctor Acquisition in all material respects, unless such representation was made as of a specific date. In addition, the Acquisition Purchase Agreement contained covenants with respect to DERMAdoctor conducting its businesses in the ordinary course during the period between the execution of the Acquisition Purchase Agreement and the Acquisition Closing. The representations, warranties and covenants were, in certain circumstances, qualified by materiality and knowledge as provided in the Acquisition Purchase Agreement, and did survive the Acquisition Closing for purposes of the indemnification obligations of the parties, which are further described immediately below under “—Indemnification and Escrow.”
Indemnification and Escrow
In connection with the DERMAdoctor Acquisition, the Sellers, severally, and Papillon and the Founders, jointly and severally, agreed to indemnify and hold harmless NovaBay, DERMAdoctor and their respective affiliates from and against losses and legal proceedings that may be sustained and that arise out of such matters as (i) inaccuracies or breaches by the Sellers, the Founders or DERMAdoctor of their respective representations and warranties in the Acquisition Purchase Agreement, (ii) breaches or non-fulfillment by the Sellers, the Founders and DERMAdoctor of any covenants and obligations in the Acquisition Purchase Agreement, (iii) pre-closing tax liabilities of DERMAdoctor, (iv) the inaccuracy of the DERMAdoctor indebtedness, transaction expenses or DERMAdoctor cash and cash equivalents as of the Acquisition Closing; and (v) claims or legal proceedings by any Seller, Founder or any other actual or purported beneficial owner of DERMAdoctor relating to any alleged action or failure to act on its behalf by DERMAdoctor. Also, we agreed to indemnify and hold harmless DERMAdoctor, the Sellers and the Founders and their respective affiliates from and against losses and legal proceedings that may be sustained and that arise out of (x) inaccuracies and breaches by us of our representations and warranties in the Acquisition Purchase Agreement; (y) breaches or non-fulfillment by us of our covenants in the Acquisition Purchase Agreement; and (z) liabilities of DERMAdoctor arising from actions or events taken by us after the Acquisition Closing.
The representations and warranties of each party to the Acquisition Purchase Agreement, other than specified fundamental representations of DERMAdoctor, the Sellers and the Founders, will survive for a period of 12 months after the Acquisition Closing. The fundamental representations of DERMAdoctor, the Sellers and the Founders include those representations in the Acquisition Purchase Agreement with respect to title of the DERMAdoctor membership units, DERMAdoctor indebtedness, DERMAdoctor capitalization, power and organization, no violation, due authorization and enforceability, and broker fees, which shall survive until the seven year anniversary of the Acquisition Closing. Indemnification obligations for breaches of pre-closing tax liabilities shall survive 60 days after the expiration of the statute of limitations, including any extensions. The covenants contained in the Acquisition Purchase Agreement shall survive the Acquisition Closing until performed, unless a shorter period of survival is specifically set forth in the Acquisition Purchase Agreement.
At the Acquisition Closing, $1.2 million of the Purchase Price was deposited into escrow and is being held for a period of 12 months after the Acquisition Closing, which amount is available to us to satisfy indemnification obligations of the Founders and the Sellers in accordance with the Acquisition Purchase Agreement. The amount held in escrow reflects the amount of the Sellers’ and the Founders’ liability for indemnification under the Acquisition Purchase Agreement, except in those circumstances that involve breaches or inaccuracies of fundamental representations, pre-closing tax liabilities, fraud, breaches or non-fulfillment of covenants and obligations, where the limitation of liability shall be the amount of the Cash Consideration.
Interests of DERMAdoctor’s Officers and its Managers in the DERMAdoctor Acquisition
Employment Agreements; Equity Grants and Board Appointment
In addition to the consideration that was received by the Founders in connection with the consummation of the DERMAdoctor Acquisition as the indirect owners of Papillon, NovaBay entered into employment agreements with each of Dr. Audrey Kunin and Dr. Jeffrey Kunin at the Acquisition Closing to serve as the Chief Product Officer and President of DERMAdoctor, respectively, having previously served as the Chief Creative Officer and Chief Executive Officer, respectively, of DERMAdoctor. The employment agreements provide for: (i) a term of two years; (ii) a minimum annual base salary of $150,000 and $200,000 for Dr. Jeffrey Kunin and Dr. Audrey Kunin, respectively; (iii) the opportunity for Dr. Jeffrey Kunin to earn an annual performance bonus in an amount up to 35% of his base salary, which will be dependent on the legacy DERMAdoctor business achieving specified financial margin targets each year; (iv) the opportunity for Dr. Audrey Kunin to earn an annual performance bonus of up to 100% of her base salary, of which 60% will be dependent on achievement of specific milestones, her performance and our financial progress as evaluated by our executive management team, and 40% of which is dependent upon the legacy DERMAdoctor business achieving specified financial margin targets each year; and (v) equity grants to Dr. Audrey Kunin of 300,000 performance restricted stock units and 150,000 stock options (to vest over a two year period) under our 2017 Omnibus Incentive Plan at the Acquisition Closing. Additionally, pursuant to the Acquisition Purchase Agreement, we entered into a separate letter agreement at the Closing with Dr. Audrey Kunin that provides for her appointment to our Board of Directors, including the related timing with respect to such appointment occurring prior to our 2022 annual stockholder meeting and the qualifications and conditions to be met in order to serve on our Board. For additional detailed information regarding these agreements, we have filed copies of the employment agreements with Drs. Jeffrey Kunin and Audrey Kunin, the letter agreement with Dr. Audrey Kunin and the related equity grants as part of our Current Report on Form 8-K filed with the SEC on November 12, 2021, which is incorporated by reference into this prospectus.
Non-Competition and Releases
Pursuant to the Acquisition Purchase Agreement and in connection with the Acquisition Closing, each Seller and Founder agreed that for a period of five (5) years after the Acquisition Closing, they would not do any of the following:
|
|
●
|
engage, directly or indirectly, in any competitive business activities without the prior written consent of NovaBay; provided, however, this restriction shall not prevent the Sellers or the Founders from owning for investment purposes up to 5% of the outstanding securities of a publicly-traded company engaged in a competitive business activity;
|
|
|
●
|
solicit, or assist any person or entity, other than NovaBay or DERMAdoctor, to solicit, any officer, director, executive or employee of NovaBay or DERMAdoctor to leave his or her employment; provided, however, this restriction shall not prohibit the Sellers or the Founders from solicitation through a general advertisement not targeted at officers, directors, executives or employees of NovaBay or DERMAdoctor and hiring such person responding to any such general advertisement; or
|
|
|
●
|
for themselves or for the benefit of any other person or entity, contact any person or entity that has manufactured, supplied, distributed, resold, licensed or purchased products or services from DERMAdoctor or otherwise provided services or materials to DERMAdoctor at any time within two years prior to the Closing Date or any person or entity that DERMAdoctor was actively soliciting as of the Closing Date for the purpose of selling any products or services or supplying materials, products or services that constitute a competitive business activity.
|
A “competitive business activity” for purposes of these restrictions shall mean any business activities that are competitive with the activities of the DERMAdoctor business, or any means of providing services to any person or entity that are the same or substantially similar to the DERMAdoctor business, whether as an employee, owner, independent contractor or consultant.
In connection with the consummation of the Acquisition, the Sellers and the Founders also agreed on their own behalf and on behalf of their respective owners, affiliates, heirs, successors, trustees, executors, administrators, assigns and any other person or entity to provide a release to DERMAdoctor from any claims, except for those claims that relate to enforcing their rights under the Acquisition Purchase Agreement or with respect to rights to unpaid employment compensation or expense reimbursement. For detailed information concerning the non-competition and non-solicitation covenants and the releases provided reference is made to the Acquisition Purchase Agreement, which is incorporated by reference into this prospectus.
Termination Fee
Pursuant to the Acquisition Purchase Agreement, if we validly terminated the Acquisition Purchase Agreement due to an uncured breach of representation, warranty, covenant or agreement of DERMAdoctor, the Sellers or the Founders, then the Sellers would have been required to pay us a termination fee equal to $120,000. In addition, if the Founders, on behalf of the Sellers, validly terminated the Acquisition Purchase Agreement due to an uncured breach of representation, warranty, covenant or agreement by NovaBay or NovaBay had not completed the financing transaction to fund the Purchase Price by November 17, 2021, then NovaBay would have been obligated to pay the Sellers an aggregate termination fee equal to $120,000. This termination fee is no longer payable as a result of the DERMAdoctor Acquisition having been consummated by the parties on November 5, 2021.
Expenses
Prior to the Acquisition Closing, DERMAdoctor, the Founders and the Sellers agreed to reasonably cooperate and assist us, at our cost and expense, in connection with securing the financing in the Private Placement to fund the Purchase Price, including in connection with the preparation and filing of a proxy statement or other filings necessary to obtain stockholder approval of the Share Conversion Approval. The other fees and expenses incurred in connection with the Acquisition Purchase Agreement and the transactions contemplated thereby, including fees and disbursements of counsel, financial advisors and accountants, were paid by the party incurring such fees and expenses, except the amount of the DERMAdoctor transaction expenses that were paid out of the Cash Consideration at the Acquisition Closing.
Amendments
The Acquisition Purchase Agreement may be terminated, amended, supplemented or modified in writing signed by the party against which the enforcement of the termination, amendment, supplement, or modification shall be sought.
Governing Law
The Acquisition Purchase Agreement is governed by and construed in accordance with the laws of the State of Delaware.
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
Introduction
The following unaudited pro forma condensed combined financial information is presented to illustrate the estimated effect of the DERMAdoctor Acquisition pursuant to the terms of the Acquisition Purchase Agreement for the Purchase Price, which was up to approximately $15.0 million. The DERMAdoctor Acquisition closed on November 5, 2021 (the “Acquisition Closing”). The Purchase Price was comprised of a payment to the Sellers of approximately $12.0 million in cash at the Acquisition Closing, which amount was (i) reduced by amounts paid to satisfy DERMAdoctor’s indebtedness and its transaction expenses as of the Acquisition Closing, and (ii) increased by the amount of remaining DERMAdoctor cash and cash equivalents as of the Acquisition Closing. In addition, $1.2 million of the Purchase Price paid at the Acquisition Closing was deposited into an escrow account, which amount is available to NovaBay to satisfy indemnification obligations of the Founders and the Sellers in accordance with the Acquisition Purchase Agreement. The remaining amount of the Purchase Price is comprised of up to an aggregate of $3.0 million in earn out payments, which are contingent upon the legacy DERMAdoctor business achieving predetermined financial targets for the 2022 and the 2023 calendar years (the “Earn Out Payments”).
For more information about the DERMAdoctor Acquisition, see the section entitled “The Acquisition Purchase Agreement” in this prospectus.
The unaudited pro forma condensed combined financial information also presents the pro forma effects of the Private Placement that was completed on November 2, 2021. Pursuant to the Securities Purchase Agreement, we sold 15,000 shares of Preferred Stock and the Warrants for gross proceeds of $15.0 million, which proceeds were partially used to fund the Purchase Price at the Acquisition Closing. For more information about the Private Placement, see the section titled “Description of the Private Placement” in this prospectus.
The unaudited pro forma condensed combined financial information presented has been prepared in accordance with Article 11 of Regulation S-X, Pro Forma Financial Information, as amended by the SEC’s final rule, Release No. 33-10786 “Amendments to Financial Disclosures about Acquired and Disposed Businesses,” and are being provided pursuant to Rule 3-05 of Regulation S-X as a result of the DERMAdoctor Acquisition having been completed.
Release No. 33-10786 replaces the existing pro forma adjustment criteria with simplified requirements to depict the accounting for the transaction (the “Transaction Accounting Adjustments”) and the option to present the reasonably estimable synergies and other transaction effects that have occurred or are reasonably expected to occur (“Management’s Adjustments”). NovaBay has elected not to present Management’s Adjustments and has only presented Transaction Accounting Adjustments in the following unaudited pro forma condensed combined financial information.
The unaudited pro forma condensed combined balance sheet as of September 30, 2021 combines the historical balance sheets of NovaBay and DERMAdoctor on a pro forma basis giving effect to the DERMAdoctor Acquisition and Private Placement as if they had been completed on September 30, 2021. The unaudited pro forma condensed combined statements of operations for the nine months ended September 30, 2021 and for the year ended December 31, 2020 combine the historical statements of operations of NovaBay and DERMAdoctor giving effect to the DERMAdoctor Acquisition and Private Placement as if they had been completed on January 1, 2020, the earliest period presented.
The pro forma adjustments and allocation of the Purchase Price are preliminary, are based on management’s current estimates of the fair value of the acquired assets and assumed liabilities, and are based on currently available information, including preliminary work performed by independent valuation specialists. In addition to the DERMAdoctor Acquisition, the unaudited pro forma condensed combined financial information provides for the anticipated effects of and the use of proceeds from the Private Placement.
As of the date of this prospectus, we have not completed the detailed valuation analysis and calculations necessary to arrive at final estimates of the fair market value of the assets of DERMAdoctor to be acquired and the liabilities to be assumed and the related allocations of the Purchase Price, nor have we identified all adjustments necessary to conform DERMAdoctor’s accounting policies to our accounting policies. Accordingly, certain of DERMAdoctor’s assets and liabilities are presented at their respective carrying amounts and should be treated as preliminary values.
Actual results will differ from the unaudited pro forma condensed combined financial information provided herein once we have completed the valuation analysis necessary to finalize the required Purchase Price allocations and identified any additional conforming accounting policy changes for DERMAdoctor. There can be no assurance that such finalization will not result in material changes to the unaudited pro forma condensed combined financial information presented.
Additionally, we have not yet completed a detailed analysis of the accounting impact of the Private Placement and therefore, the pro forma presentation of the Private Placement is also preliminary. Actual results will differ from the unaudited pro forma condensed combined financial information provided herein once we have completed a detailed analysis.
There can be no assurance that such finalization of the items above will not result in material changes to the unaudited pro forma condensed combined financial information presented.
Assumptions and estimates underlying the unaudited pro forma adjustments set forth in the unaudited pro forma condensed combined financial statements are described in the accompanying notes below. The unaudited pro forma adjustments represent management’s estimates based on information available as of the date of these unaudited pro forma condensed combined financial statements and are subject to change as additional information becomes available and analyses are performed.
The unaudited pro forma condensed combined financial information and accompanying notes are based on, and should be read in conjunction with, (i) the historical audited consolidated financial statements of NovaBay and accompanying notes for the year ended December 31, 2020, which are included in our Annual Report on Form 10-K filed with the SEC on March 25, 2021 and the historical unaudited consolidated financial statements of NovaBay and accompanying notes for the period ended September 30, 2021, which are included in our Quarterly Report on Form 10-Q filed with the SEC on November 12, 2021, which are both incorporated into this prospectus, (ii) the historical audited financial statements of DERMAdoctor and accompanying notes included for the year ended December 31, 2020 and the historical unaudited financial statements of DERMAdoctor and accompanying notes for the nine-month period ended September 30, 2021, each of which are included in our Current Report on Form 8-K/A filed with the SEC on December 1, 2021, which is incorporated into this prospectus.
The following unaudited pro forma condensed combined financial statements are provided for illustrative purposes only and are based on currently available information and assumptions that we believe are reasonable under the circumstances. They do not purport to represent what our actual consolidated results of operations or the consolidated financial position would have been had the DERMAdoctor Acquisition and Private Placement been completed on the dates indicated, or on any other date, nor are they necessarily indicative of our future consolidated results of operations or consolidated financial position after the DERMAdoctor Acquisition is completed. Accordingly, our actual financial position and results of operations will differ, perhaps significantly, from the pro forma amounts reflected herein due to a variety of factors, including changes in value not currently identified and changes in operating results of NovaBay and DERMAdoctor following the date of the unaudited pro forma condensed combined financial statements.
Unaudited Pro Forma Condensed Combined Balance Sheet
As of September 30, 2021
(in thousands)
|
|
|
NovaBay
Pharmaceuticals,
Inc.
|
|
|
DERMAdoctor, LLC
|
|
|
Transaction
Accounting
Adjustments
|
|
|
Other Transaction
Adjustments
|
|
|
Notes
|
|
|
Pro Forma
Combined
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
9,028
|
|
|
$
|
1
|
|
|
$
|
(12,000
|
)
|
|
$
|
13,375
|
|
|
(j)
|
|
|
$
|
10,404
|
|
|
Accounts receivable, net of allowance for doubtful accounts
|
|
|
843
|
|
|
|
1,093
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,936
|
|
|
Inventory, net of allowance
|
|
|
969
|
|
|
|
2,672
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3,641
|
|
|
Prepaid expenses and other current assets
|
|
|
657
|
|
|
|
17
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
674
|
|
|
Total current assets
|
|
|
11,497
|
|
|
|
3,783
|
|
|
|
(12,000
|
)
|
|
|
13,375
|
|
|
|
|
|
|
16,655
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating lease right-of-use assets
|
|
|
170
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
170
|
|
|
Intangible assets, net
|
|
|
-
|
|
|
|
66
|
|
|
|
2,960
|
|
|
|
|
|
|
(a) (i)
|
|
|
|
3,026
|
|
|
Goodwill
|
|
|
-
|
|
|
|
-
|
|
|
|
6,452
|
|
|
|
|
|
|
(b)
|
|
|
|
6,452
|
|
|
Property and equipment, net
|
|
|
96
|
|
|
|
57
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
153
|
|
|
Other assets
|
|
|
476
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
476
|
|
|
TOTAL ASSETS
|
|
$
|
12,239
|
|
|
$
|
3,906
|
|
|
$
|
(2,588
|
)
|
|
$
|
13,375
|
|
|
|
|
|
$
|
26,932
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
1,354
|
|
|
$
|
38
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
1,392
|
|
|
Related party accounts payable
|
|
|
-
|
|
|
|
45
|
|
|
$
|
(45
|
)
|
|
|
|
|
|
(d)
|
|
|
|
-
|
|
|
Accrued liabilities
|
|
|
1,325
|
|
|
|
900
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,225
|
|
|
Fair value of earn-out payments
|
|
|
-
|
|
|
|
-
|
|
|
|
750
|
|
|
|
|
|
|
(c)
|
|
|
|
750
|
|
|
Related party notes payable
|
|
|
-
|
|
|
|
1,645
|
|
|
|
(1,645
|
)
|
|
|
|
|
|
(d)
|
|
|
|
-
|
|
|
Operating lease liability
|
|
|
195
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
195
|
|
|
Total current liabilities
|
|
|
2,874
|
|
|
|
2,628
|
|
|
|
(940
|
)
|
|
|
|
|
|
|
|
|
|
4,562
|
|
|
Operating lease liability- non-current
|
|
|
1
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1
|
|
|
TOTAL LIABILITIES
|
|
$
|
2,875
|
|
|
$
|
2,628
|
|
|
$
|
(940
|
)
|
|
|
|
|
|
|
|
|
$
|
4,563
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible preferred stock
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
$
|
13,375
|
|
|
(j)
|
|
|
$
|
13,375
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders' equity (1)
|
|
$
|
9,364
|
|
|
$
|
1,278
|
|
|
$
|
(1,648
|
)
|
|
|
|
|
|
(e) (i)
|
|
|
$
|
8,994
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY
|
|
$
|
12,239
|
|
|
$
|
3,906
|
|
|
$
|
(2,588
|
)
|
|
$
|
13,375
|
|
|
|
|
|
$
|
26,932
|
|
(1) - Presented as “Total equity” in DERMAdoctor’s financial statements as of September 30, 2021
Unaudited Pro Forma Condensed Combined Statement of Operations
For the nine-months ended September 30, 2021
(in thousands)
|
|
|
NovaBay
Pharmaceuticals,
Inc.
|
|
|
DERMAdoctor,
LLC
|
|
|
Transaction
Accounting
Adjustments
|
|
|
Notes
|
|
|
Pro Forma
Combined
|
|
|
Sales:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product revenue, net
|
|
$
|
5,761
|
|
|
$
|
4,604
|
|
|
$
|
(449
|
)
|
|
(f)
|
|
|
$
|
9,916
|
|
|
Other revenue, net
|
|
|
19
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
19
|
|
|
Total sales, net
|
|
|
5,780
|
|
|
|
4,604
|
|
|
|
(449
|
)
|
|
|
|
|
|
9,935
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of goods sold
|
|
|
1,562
|
|
|
|
1,801
|
|
|
|
|
|
|
|
|
|
|
3,363
|
|
|
Gross Profit
|
|
|
4,218
|
|
|
|
2,803
|
|
|
|
(449
|
)
|
|
|
|
|
|
6,572
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
36
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
36
|
|
|
Sales and marketing
|
|
|
5,323
|
|
|
|
1,226
|
|
|
|
(449)
|
|
|
(f)
|
|
|
|
6,100
|
|
|
General and administrative expenses
|
|
|
4,527
|
|
|
|
1,076
|
|
|
|
(697
|
)
|
|
(h)
|
|
|
|
4,906
|
|
|
Amortization of intangibles
|
|
|
-
|
|
|
|
-
|
|
|
|
159
|
|
|
(i)
|
|
|
|
159
|
|
|
Total operating expenses
|
|
|
9,886
|
|
|
|
2,302
|
|
|
|
(987
|
)
|
|
|
|
|
|
11,201
|
|
|
Operating net income (loss)
|
|
|
(5,668
|
)
|
|
|
501
|
|
|
|
538
|
|
|
|
|
|
|
(4,629
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense), net
|
|
|
2
|
|
|
|
510
|
|
|
|
(316)
|
|
|
(g)
|
|
|
|
196
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) before provision for income taxes
|
|
|
(5,666
|
)
|
|
|
1,011
|
|
|
|
222
|
|
|
|
|
|
|
(4,433
|
)
|
|
Net Income (loss) and comprehensive net income (loss)
|
|
$
|
(5,666
|
)
|
|
$
|
1,011
|
|
|
$
|
222
|
|
|
|
|
|
$
|
(4,433
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per share (basic)
|
|
$
|
(0.13
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
(0.10
|
)
|
|
Net loss per share (diluted)
|
|
$
|
(0.13
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
(0.10
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average shares of common stock (basic)
|
|
|
43,100
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
43,100
|
|
|
Weighted average shares of common stock (diluted)
|
|
|
43,100
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
43,100
|
|
Unaudited Pro Forma Condensed Combined Statement of Operations
For the year ended December 31, 2020
(in thousands)
|
|
|
NovaBay
Pharmaceuticals,
Inc.
|
|
|
DERMAdoctor,
LLC
|
|
|
Transaction
Accounting
Adjustments
|
|
Notes
|
|
Pro Forma
Combined
|
|
|
Sales:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product revenue, net
|
|
$
|
9,916
|
|
|
$
|
8,387
|
|
|
$
|
(202
|
)
|
(f)
|
|
$
|
18,101
|
|
|
Other revenue, net
|
|
|
18
|
|
|
|
-
|
|
|
|
|
|
|
|
|
18
|
|
|
Total sales, net
|
|
|
9,934
|
|
|
|
8,387
|
|
|
|
(202
|
)
|
|
|
|
18,119
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of goods sold
|
|
|
3,970
|
|
|
|
4,133
|
|
|
|
|
|
|
|
|
8,103
|
|
|
Gross Profit
|
|
|
5,964
|
|
|
|
4,254
|
|
|
|
(202
|
)
|
|
|
|
10,016
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
285
|
|
|
|
-
|
|
|
|
|
|
|
|
|
285
|
|
|
Sales and marketing
|
|
|
6,173
|
|
|
|
1,983
|
|
|
|
(202
|
)
|
(f)
|
|
|
7,954
|
|
|
General and administrative expenses
|
|
|
5,932
|
|
|
|
1,499
|
|
|
|
|
|
|
|
|
7,431
|
|
|
Amortization of intangibles
|
|
|
-
|
|
|
|
-
|
|
|
|
211
|
|
(i)
|
|
|
211
|
|
|
Total operating expenses
|
|
|
12,390
|
|
|
|
3,482
|
|
|
|
9
|
|
|
|
|
15,881
|
|
|
Operating net income (loss)
|
|
|
(6,426
|
)
|
|
|
772
|
|
|
|
(211
|
)
|
|
|
|
(5,865
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-cash (loss) on changes in fair value of warrant liability
|
|
|
(5,216
|
)
|
|
|
-
|
|
|
|
|
|
|
|
|
(5,216
|
)
|
|
Non-cash gain on changes in fair value of embedded derivative liability on changes in fair value of warrant liability
|
|
|
3
|
|
|
|
-
|
|
|
|
|
|
|
|
|
3
|
|
|
Other income (expense), net
|
|
|
605
|
|
|
|
(133
|
)
|
|
|
316
|
|
(g)
|
|
|
788
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) before provision for income taxes
|
|
|
(11,034
|
)
|
|
|
639
|
|
|
|
105
|
|
|
|
|
(10,290
|
)
|
|
Provision for income taxes
|
|
|
(5
|
)
|
|
|
-
|
|
|
|
|
|
|
|
|
(5
|
)
|
|
Net Income (loss) and comprehensive net income (loss)
|
|
$
|
(11,039
|
)
|
|
$
|
639
|
|
|
$
|
105
|
|
|
|
$
|
(10,295
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per share (basic)
|
|
$
|
(0.31
|
)
|
|
|
|
|
|
|
|
|
|
|
$
|
(0.29
|
)
|
|
Net loss per share (diluted)
|
|
$
|
(0.31
|
)
|
|
|
|
|
|
|
|
|
|
|
$
|
(0.29
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average shares of common stock (basic)
|
|
|
35,076
|
|
|
|
|
|
|
|
|
|
|
|
|
35,076
|
|
|
Weighted average shares of common stock (diluted)
|
|
|
35,076
|
|
|
|
|
|
|
|
|
|
|
|
|
35,076
|
|
Note 1. Basis of Pro Forma Presentation
The unaudited pro forma condensed combined financial statements are derived from the historical consolidated financial statements of NovaBay and the historical financial statements of DERMAdoctor. The unaudited pro forma condensed combined financial statements are prepared as a business combination using the purchase accounting method.
The unaudited pro forma condensed combined balance sheet has been prepared to reflect the transaction as if the DERMAdoctor Acquisition had been completed on September 30, 2021. The unaudited pro forma condensed combined statements of operations for the nine months ended September 30, 2021 and for the year ended December 31, 2020 combine the historical statements of operations of NovaBay and DERMAdoctor giving effect to the DERMAdoctor Acquisition as if it had been completed on January 1, 2020, the earliest period presented.
The DERMAdoctor Acquisition will be accounted for under the purchase accounting method of accounting in accordance with FASB ASC 805, Business Combinations, using the fair value concepts defined in ASC 820, Fair Value Measurements and Disclosures. We are treated as the “acquirer” and DERMAdoctor is treated as the “acquired” company for financial reporting purposes. Accordingly, the purchase consideration allocated to the DERMAdoctor business’s assets and liabilities for preparation of the unaudited pro forma condensed combined sheet is based upon its estimated preliminary fair values assuming the DERMAdoctor Acquisition was completed as of September 30, 2021. The amount of the purchase consideration that was in excess of the estimated preliminary fair values of the DERMAdoctor business’s net assets and liabilities at September 30, 2021 is recorded as goodwill in the unaudited pro forma condensed combined balance sheet.
We have not yet performed the detailed valuation studies necessary to arrive at the final estimates of the fair value of DERMAdoctor’s acquired assets, assumed liabilities and the related allocations of the Purchase Price.
The unaudited pro forma condensed financial information includes pro forma adjustments that are (i) directly attributable to the DERMAdoctor Acquisition, (ii) factually supportable, and (iii) with respect to the unaudited condensed pro forma statements of operations, expected to have a continuing impact on the results of operations of the combined company.
Actual results may differ from these unaudited pro forma condensed combined financial statements once we have determined the final Purchase Price for DERMAdoctor and have completed the valuation studies necessary to finalize the required Purchase Price allocations and identified any additional conforming accounting policy changes for DERMAdoctor. There can be no assurance that such finalization will not result in material changes to the unaudited pro forma condensed combined financial information presented. The preliminary unaudited pro forma Purchase Price allocation has been made solely for preparing these unaudited pro forma condensed combined financial statements.
Additionally, we have not yet completed a detailed analysis of the accounting impact of the Private Placement and therefore, the pro forma presentation of the Private Placement is also preliminary. Actual results will differ from the unaudited pro forma condensed combined financial information provided herein once we have completed a detailed analysis. These unaudited pro forma condensed combined financial statements are presented for illustrative purposes only and do not give effect to any cost savings from operating efficiencies, revenue synergies, differences in stand-alone costs of the DERMAdoctor business or costs for the integration of DERMAdoctor’s business operations with NovaBay. These unaudited pro forma condensed combined financial statements do not purport to represent what the actual consolidated results of operations of NovaBay would have been had the DERMAdoctor Acquisition been completed on the dates assumed, nor are they necessarily indicative of future consolidated results of operations or consolidated financial position. Any transaction, separation or integration costs will be expensed in the appropriate accounting periods after completion of the DERMAdoctor Acquisition.
Note 2. Accounting Policies
The unaudited pro forma condensed combined financial statements may not reflect all reclassifications necessary to conform DERMAdoctor’s presentation to that of NovaBay’s due to limitations on the availability of information as of the date of this prospectus. As a result of the completion of the DERMAdoctor Acquisition, we will further review DERMAdoctor’s accounting policies. As a result of that review, we may identify differences between the accounting policies of the two companies that, when conformed, could have a material impact on the combined financial statements.
Note 3. Preliminary Purchase Consideration and Purchase Price Allocation
Under the purchase method of accounting, the identifiable assets acquired and liabilities assumed are recorded at the fair values. The Purchase Price allocation provided in these unaudited pro forma condensed combined financial statements is preliminary and based on estimates of the fair value as of September 30, 2021 and not the actual date of the Acquisition Closing.
We have engaged a third-party valuation company to assist us with the valuation of DERMAdoctor and its assets and liabilities. The detailed valuation necessary to estimate the fair value of the assets acquired and liabilities assumed has not yet been completed; accordingly, the adjustments to record the assets acquired and liabilities assumed at fair value reflect the best estimate of NovaBay based on the information currently available and are subject to change once additional analyses are completed.
There can be no assurance that such third-party valuation work will not result in material changes from the preliminary accounting treatment included in the accompanying unaudited pro forma condensed combined financial statements.
The Purchase Price as provided in the Acquisition Purchase Agreement provides for the Sellers to receive up to $15.0 million in connection with the DERMAdoctor Acquisition, of which $12.0 million in cash was paid at the Acquisition Closing and up to $3.0 million in Earn Out Payments may become payable to the Sellers in the future if the legacy DERMAdoctor business achieves predetermined financial targets for the 2022 and the 2023 calendar years. In addition, $1.2 million of the Purchase Price at the Acquisition Closing was deposited into an escrow account, which amount is available to NovaBay to satisfy indemnification obligations of the Founders and the Sellers in accordance with the Acquisition Purchase Agreement. As a result of the contingent nature of the Earn Out Payments and for purposes of the unaudited pro forma condensed combined financial statements, we have estimated the total amount for the DERMAdoctor Acquisition to be $12.75 million, comprised of a cash payment of $12.0 million and $750,000 for the preliminary estimated fair value of the Earn Out Payments.
|
|
|
Amount
|
|
|
Cash transferred
|
|
$
|
12,000,000
|
|
|
Estimated fair value of Earn Out payments
|
|
|
750,000
|
|
|
Estimated fair value of consideration transferred
|
|
$
|
12,750,000
|
|
The estimated fair value of the Earn Out Payments is preliminary and based upon available information and certain assumptions, known at the time of this report, which management believes are reasonable. This preliminary fair value estimate for the Earn Out Payments may change as additional information becomes available and such changes could be material. Upon final determination of the fair value of the Earn Out Payments, any differences in the actual Earn Out Payments will be recorded in operating income (expense) in the consolidated statements of operations.
The table below represents a preliminary allocation of the estimated total Purchase Price to the DERMAdoctor business’s assets and liabilities in the DERMAdoctor Acquisition based on our preliminary estimate of their respective fair values:
|
Description
|
|
Fair Value ( in thousands)
|
|
|
Assets acquired:
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
1
|
|
|
Accounts receivable, net of allowance for doubtful accounts
|
|
|
1,093
|
|
|
Inventory, net of allowance
|
|
|
2,672
|
|
|
Prepaid expenses and other current assets
|
|
|
17
|
|
|
Property and equipment, net
|
|
|
57
|
|
|
Intangible assets
|
|
|
3,396
|
|
|
Goodwill
|
|
|
6,452
|
|
|
Total assets acquired
|
|
$
|
13,688
|
|
|
|
|
|
|
|
|
Liabilities assumed:
|
|
|
|
|
|
Accounts payable
|
|
$
|
38
|
|
|
Accrued liabilities
|
|
|
900
|
|
|
Total liabilities acquired
|
|
|
938
|
|
|
Estimated fair value of net assets acquired
|
|
$
|
12,750
|
|
Goodwill represents the amount of the Purchase Price in excess of the estimated preliminary amounts assigned to the fair value of the DERMAdoctor assets acquired and the liabilities assumed. Since these amounts are estimates, the final amount of goodwill recorded may differ materially from the amount presented.
Our unaudited preliminary allocation of the estimated total Purchase Price includes certain identifiable intangible assets with an estimated fair value of approximately $3.3 million. Customer relationships and trade secrets / product formulation are expected to have a finite life and are expected to be amortized using the straight-line method over the respective lives of each asset, while trade name is not expected to be amortized. Trade name will be tested for impairment at least annually for events or circumstances that may indicate a possible impairment exists.
Goodwill is expected to be recorded for the excess of the consideration transferred over the preliminary fair value of the assets acquired and liabilities assumed. Goodwill will not be amortized, but instead will be tested for impairment at least annually for events or circumstances that may indicate a possible impairment exists. In the event management determines that the value of goodwill has been impaired, we will incur an impairment charge during the period in which the determination is made.
|
Intangible Asset
|
|
Fair Value (in thousands)
|
|
Useful Life
|
|
Amortization Method
|
|
|
Customer Relationships
|
|
$
|
160
|
|
6.5 Years
|
|
Straight line
|
|
|
Trade Secrets / Product Formulation
|
|
|
840
|
|
4.5 Years
|
|
Straight line
|
|
|
Trade Name
|
|
|
2,330
|
|
Indefinite
|
|
N/A
|
|
|
Goodwill
|
|
|
6,452
|
|
Indefinite
|
|
N/A
|
|
|
|
|
$
|
9,782
|
|
|
|
|
|
The unaudited pro forma condensed combined statements of operations for the nine months ended September 30, 2021 and for the year ended December 31, 2020 include pro forma adjustments related to the amortization of the intangible assets acquired. For pro forma purposes, the finite life intangible assets are amortized on a straight-line basis beginning on January 1, 2020, as if the DERMAdoctor Acquisition occurred on that date.
The preliminary fair value of the identifiable intangible assets acquired was estimated using a combination of asset-based and income-based valuation methodologies. The asset-based valuation methodology established a fair value estimate based on the cost of replacing the asset, less amortization from functional use and economic obsolescence, if present and measurable. The income-based valuation methodology utilizes a discounted cash flow technique where the expected future economic benefits of ownership of an asset are discounted back to present value. This valuation technique requires us to make certain assumptions about future operating performance and cash flow, and other such variables which are discounted to present value using a discount rate that reflects the risk factors associated with future cash flow, the characteristics of the assets acquired, and the experience of the acquired business. Such valuation methodologies and estimates are subject to change, possibly materially, as additional information becomes available and as additional analyses are performed.
The unaudited preliminary allocation of the estimated Purchase Price has been made solely for the purpose of preparing these unaudited pro forma condensed combined financial statements.
The final total consideration and amounts allocated to DERMAdoctor’s acquired assets and assumed liabilities could differ materially from the preliminary amounts presented in these unaudited pro forma condensed combined financial statements. A decrease in the fair value of the assets or an increase in the fair value of the liabilities from the preliminary valuations presented would result in a dollar-for-dollar corresponding increase in the amount of goodwill that will result from the DERMAdoctor Acquisition. In addition, if the value of the property and equipment and identifiable intangible assets is higher than the amounts included in these unaudited pro forma condensed combined financial statements, it may result in higher depreciation and amortization expense than is presented in the unaudited pro forma condensed combined statements of operations. Any such increases could be material and could result in our actual future financial condition and results of operations differing materially from those presented in the unaudited pro forma condensed combined financial statements.
Note 4. Adjustments to Unaudited Pro Forma Condensed Combined Financial Statements
The unaudited pro forma condensed combined financial information has been prepared to illustrate the effect of the DERMAdoctor Acquisition and has been prepared for informational purposes only. They do not purport to indicate the results of future operations or the financial position of either company.
The historical financial statements have been adjusted in the unaudited pro forma condensed combined financial information to give pro forma effect to events that are directly attributable to the DERMAdoctor Acquisition, factually supportable, and with respect to the statements of operations, expected to have a continuing impact on the results of NovaBay.
The following items are presented as reclassifications in the unaudited pro forma condensed combined financial statements for purposes of conforming DERMAdoctor’s classification of certain assets, liabilities, income, and expenses to our presentation for the combined presentation:
|
|
(a)
|
Adjustment includes preliminary estimated fair value of intangible assets acquired by NovaBay.
|
|
|
(b)
|
Adjustment reflects preliminary estimated goodwill.
|
|
|
(c)
|
Adjustment reflects the preliminary estimated fair value of the Earn Out Payments of $750 thousand. This contingent consideration is included in the preliminary estimated fair value of the consideration transferred in the DERMAdoctor Acquisition.
|
|
|
(d)
|
Adjustment offsets related party accounts payable and related party notes payable. These amounts were paid at the Acquisition Closing from the cash portion of the Purchase Price.
|
|
|
(e)
|
Adjustment includes the elimination of the historical Members’ Deficit of DERMAdoctor and the preliminary cumulative impact of the amortization of identifiable intangible assets.
|
|
|
(f)
|
These adjustments reclassify certain amounts set forth under the line item “Selling expenses” in DERMAdoctor’s audited and unaudited financial statements to “Product revenue, net” in the unaudited pro forma condensed combined statements of operations to conform to NovaBay’s presentation.
|
|
|
(g)
|
Adjustment includes pro forma accounting adjustment for DERMAdoctor’s Paycheck Protection Program Loan (PPP Loan) forgiveness amount to conform to NovaBay’s presentation. The PPP Loan was forgiven on September 3, 2021.
|
|
|
(h)
|
Represents pro forma adjustment to eliminate transaction expenses related to the DERMAdoctor Acquisition incurred by NovaBay and DERMAdoctor.
|
|
|
(i)
|
Includes the cumulative impact of preliminary amortization expense of approximately $370,000 for identifiable intangible assets acquired.
|
|
|
(j)
|
Represents gross proceeds of $15.0 million from the Private Placement in which the Company issued 15,000 shares of Preferred Stock, together with the Warrants at a price of $1,000 per share, less approximately $1,625,000 in preliminary estimated placement agent, attorney and other offering expenses paid in connection with the Private Placement.
|
USE OF PROCEEDS
We will not receive any of the proceeds from the sale of the Shares by the Selling Stockholders pursuant to this prospectus. The Shares covered by this prospectus are issuable upon conversion of the 15,000 shares of Preferred Stock into 37,500,000 shares of Common Stock as described in “Description of the Private Placement” beginning on page 22 of this prospectus. The Selling Stockholders will pay all incremental selling expenses relating to the sale of their Shares, including underwriters’ or agents’ commissions and discounts, brokerage fees, underwriter marketing costs and all reasonable fees and expenses of any legal counsel representing the Selling Stockholders. We will bear all other costs, fees and expenses incurred in effecting the registration of the Shares covered by this prospectus, including, without limitation, all registration and filing fees, printing and delivery fees, NYSE American listing fees and fees and expenses of our counsel and our accountants.
MARKET OF OUR COMMON STOCK
Market Information
Our Common Stock is listed on the NYSE American, under the symbol “NBY.”
Holders
As of November 26, 2021, we had 44,958,364 shares of Common Stock outstanding and there were approximately 131 holders of record of our Common Stock. We have 15,000 shares of Preferred Stock that have been issued in the Private Placement, and no other preferred stock outstanding. This figure does not reflect persons or entities that hold their stock in nominee or “street” name through various brokerage firms.
DIVIDEND POLICY
We have not paid cash dividends on our Common Stock since our inception. We currently expect to retain earnings primarily for use in the operation and expansion of our business; therefore, we do not anticipate paying any cash dividends in the foreseeable future. Any future determination to pay cash dividends will be at the discretion of our Board of Directors and will be dependent upon our financial condition, results of operations, capital requirements, restrictions under any existing indebtedness and other factors the Board of Directors deems relevant.
PRINCIPAL STOCKHOLDERS
The following table indicates information as of November 26, 2021 regarding the ownership of our Common Stock by:
|
|
●
|
each person who is known by us to own more than five percent (5%) of our shares of Common Stock;
|
|
|
●
|
our current executive officers who are each Named Executive Officers under the SEC’s proxy rules;
|
|
|
●
|
each of our directors; and
|
|
|
●
|
all of our directors and executive officers as a group.
|
The percentage of shares beneficially owned is based on 44,958,364 shares of our Common Stock outstanding as of November 26, 2021. Except as indicated in the footnotes to this table, and as affected by applicable community property laws, all persons listed have sole voting and investment power for all shares shown as beneficially owned by them and no shares are pledged.
|
Name and Address of Beneficial Owner(1)
|
|
Number of
Shares
Beneficially
Owned
|
|
|
Percent
of Class
|
|
|
Beneficial Owners Holding More Than 5%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pioneer Pharma (Hong Kong) Company Ltd. (“Pioneer Hong Kong”) (2)
|
|
|
5,188,421
|
|
|
|
11.5
|
%
|
|
682 Castle Peak Road
|
|
|
|
|
|
|
|
|
|
Lai Chi Kok, Kowloon, Hong Kong
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Jian Ping Fu (“Mr. Fu”) (3)
|
|
|
4,000,000
|
|
|
|
8.9
|
%
|
|
11 Williams Road
|
|
|
|
|
|
|
|
|
|
Mt. Eliza, Melbourne VIC 3930
|
|
|
|
|
|
|
|
|
|
Australia
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Executive Officers and Directors
|
|
|
|
|
|
|
|
|
|
Justin M. Hall, Esq.(4)
|
|
|
368,140
|
|
|
|
*
|
|
|
Andrew Jones (5)
|
|
|
202,319
|
|
|
|
*
|
|
|
Audrey Kunin (6)
|
|
|
91,133
|
|
|
|
*
|
|
|
Jeff Kunin (7)
|
|
|
91,133
|
|
|
|
*
|
|
|
Paul E. Freiman, Ph.D.(8)
|
|
|
124,409
|
|
|
|
*
|
|
|
Xinzhou (Paul) Li (2), (9)
|
|
|
65,248
|
|
|
|
*
|
|
|
Swan Sit (10)
|
|
|
20,000
|
|
|
|
*
|
|
|
Mijia (Bob) Wu, M.B.A. (11)
|
|
|
55,244
|
|
|
|
*
|
|
|
Yenyou (Jeff) Zheng, Ph.D. (12)
|
|
|
20,000
|
|
|
|
*
|
|
|
All directors and executive officers as a group (9 persons)
|
|
|
946,492
|
|
|
|
2.1
|
%
|
* Less than one percent (1%).
|
(1)
|
The address for each director and officer of NovaBay listed is c/o NovaBay Pharmaceuticals, Inc., 2000 Powell Street, Suite 1150, Emeryville, CA 94608. Number of shares beneficially owned and percent of class is calculated in accordance with SEC rules. A beneficial owner is deemed to beneficially own shares the beneficial owner has the right to acquire within 60 days of November 26, 2021. For purposes of calculating the percent of class held by a single beneficial owner, the shares that such beneficial owner has the right to acquire within 60 days of November 26, 2021 are also deemed to be outstanding; however, such shares are not deemed to be outstanding for purposes of calculating the percentage ownership of any other beneficial owner.
|
|
(2)
|
Director Xinzhou (Paul) Li is a Director of Pioneer Hong Kong and Chairman and Executive Director of China Pioneer Pharma Holdings Limited (“China Pioneer”), the parent company of Pioneer Hong Kong. Pioneer Hong Kong is a wholly-owned subsidiary of China Pioneer Pharma, and Mr. Li and his family own a majority interest in China Pioneer Pharma. Mr. Li disclaims beneficial ownership of the shares of the Company Common Stock held by Pioneer Hong Kong. Pioneer Hong Kong and China Pioneer Pharma (by virtue of China Pioneer Pharma’s indirect ownership of Pioneer Hong Kong), share voting power and share investment power over the 5,188,421 shares.
|
|
(3)
|
Based upon information contained in the Schedule 13D filed by Mr. Fu with the SEC on September 16, 2016, as amended by Amendment No. 1 filed with the SEC on April 16, 2019 and Amendment No. 2 filed with the SEC on August 24, 2020, Mr. Fu beneficially owned 4,000,000 shares of Common Stock as of August 1, 2020, with sole voting power over 4,000,000 shares, shared voting power over no shares, sole dispositive power over 4,000,000 shares and shared dispositive power over no shares.
|
|
(4)
|
Includes (i) 3,405 shares of Common Stock held directly by Mr. Hall (with sole voting power over 3,405 shares, shared voting power over no shares, sole investment power over 3,405 shares and shared investment power over no shares), and (ii) 364,735 shares issuable upon exercise of outstanding options which are exercisable as of November 26, 2021 or within 60 days after such date. Does not include 500,000 performance restricted stock units granted to Mr. Hall on May 4, 2021 that will vest based on the achievement of three performance goals at the end of a three-year performance period ending December 31, 2023.
|
|
(5)
|
Includes (i) 82,006 shares of Common Stock held directly by Mr. Jones (with sole voting power over 82,006 shares, shared voting power over no shares, sole investment power over 82,006 shares and shared investment power over no shares), and (ii) 120,313 shares issuable upon exercise of outstanding options which are exercisable as of November 26, 2021 or within 60 days after such date. Does not include 250,000 performance restricted stock units granted to Mr. Jones on May 4, 2021 that will vest based on the achievement of three performance goals at the end of a three-year performance period ending December 31, 2023.
|
|
(6)
|
Consists of 91,133 shares held by The Audrey G. Kunin Trust of which Mrs. Kunin serves as the trustee (with sole voting and investment power). Does not include 300,000 performance restricted stock units granted to Mrs. Kunin on November 8, 2021 that will vest based on the achievement of three performance goals at the end of a three-year performance period ending December 31, 2023.
|
|
(7)
|
Consists of 91,133 shares held by The Audrey G. Kunin Trust of which Mr. Kunin's spouse serves as the trustee (with sole voting and investment power).
|
|
(8)
|
Includes (i) 2,311 shares held by the Paul Freiman and Anna Mazzuchi Freiman Trust, of which Mr. Freiman and his spouse are trustees (with sole voting power over 625 shares, shared voting power over 1,061 shares, sole investment power over no shares and shared investment power over 1,686 shares), and (ii) 122,097 shares issuable upon exercise of outstanding options which are exercisable as of November 26, 2021, or within 60 days after such date.
|
|
(9)
|
Reflects 65,248 shares issuable upon exercise of outstanding options which are exercisable as of November 26, 2021, or within 60 days after such date.
|
|
(10)
|
Reflects 20,000 shares issuable upon exercise of outstanding options which are exercisable as of November 26, 2021, or within 60 days after such date.
|
|
(11)
|
Reflects 55,244 shares issuable upon exercise of outstanding options which are exercisable as of November 26, 2021, or within 60 days after such date. As Non-Executive Director of China Pioneer Pharma, Mr. Wu disclaims beneficial ownership of the shares of the Common Stock held by China Pioneer Pharma and Pioneer Hong Kong. See Note (2) above for shares of the Company owned by China Pioneer Pharma and Pioneer Hong Kong.
|
|
(12)
|
Reflects 20,000 shares issuable upon exercise of outstanding options which are exercisable as of November 26, 2021, or within 60 days after such date.
|
DESCRIPTION OF CAPITAL STOCK
Our authorized capital stock currently consists of 100,000,000 shares of Common Stock with a $0.01 par value per share, and 5,000,000 shares of preferred stock with a $0.01 par value per share. A description of material terms and provisions of the Certificate of Incorporation and the Bylaws affecting the rights of holders of the Company’s capital stock is set forth below. The description is intended as a summary, and is qualified in its entirety by reference to the Company’s Certificate of Incorporation and the Bylaws, which are available in our filings with the SEC. As of November 26, 2021, there were 44,958,364 shares of Common Stock outstanding, and 15,000 shares of the Preferred Stock outstanding.
The Company recently completed the Private Placement pursuant to the terms of the Securities Purchase Agreement, which provided for the issuance and sale to the Selling Stockholders of an aggregate of 15,000 shares of Preferred Stock convertible into 37,500,000 shares of Common Stock and Warrants exercisable for 37,500,000 shares of Common Stock for an aggregate purchase price of $15.0 million. As a result of the Private Placement and the issuance of the Preferred Stock and the Warrants, the number of shares of Common Stock that will be potentially issued upon conversion of the Preferred Stock and the exercise of the Warrants will be 75,000,000 shares, which number of shares exceeds our currently authorized shares of Common Stock under our Certificate of Incorporation. As a result, and pursuant to the Securities Purchase Agreement, we agreed with the Selling Stockholders to take the necessary corporate action to increase the number of authorized shares of Common Stock in sufficient amount to allow for the conversion of all of the shares of Preferred Stock and the full exercise of the Warrants. The Authorized Share Increase will be considered by stockholders at a special meeting to be held by us.
On November 1, 2021, the Board approved, subject to stockholder approval, an amendment to our Certificate of Incorporation to increase our authorized shares of Common Stock from 100,000,000 shares to 150,000,000 shares. An increase in the number of authorized shares of Common Stock to 150,000,000 shares will increase our total authorized capitalization to 155,000,000 shares of capital stock, which includes our previously authorized 5,000,000 shares of preferred stock. The additional Common Stock to be authorized by stockholder approval will have rights identical to the currently outstanding shares of our Common Stock. If our stockholders approve the Authorized Share Increase, then we expect to file a Certificate of Amendment to the Certificate of Incorporation with the Secretary of State of the State of Delaware, which will result in an increase in our authorized Common Stock.
Common Stock
Dividend rights. Subject to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of our Common Stock are entitled to receive dividends out of funds legally available if the Board, in its discretion, determines to issue dividends and then only at the times and in the amounts that the Board may determine.
Voting rights. Each holder of Common Stock is entitled to one vote for each share of Common Stock held on all matters submitted to a vote of stockholders. Our Certificate of Incorporation does not provide for the right of stockholders to cumulate votes for the election of directors. Our Certificate of Incorporation establishes a classified Board, divided into three classes with staggered three-year terms. Only one class of directors is elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms.
No preemptive or similar rights. Our Common Stock is not entitled to preemptive rights and is not subject to conversion, redemption or sinking fund provisions. The rights, preferences and privileges of the holders of our Common Stock are subject to, and may be adversely affected by, the rights of the holders of any series of our preferred stock that NovaBay may designate and issue in the future.
Right to receive liquidation distributions. Upon our dissolution, liquidation or winding-up, the assets legally available for distribution to holders of our Common Stock are distributable ratably among the holders of our Common Stock, subject to prior satisfaction of all outstanding debt and liabilities and the preferential or pari passu rights and payment of liquidation preferences, if any, on any outstanding shares of our preferred stock, including the Preferred Stock.
The rights of the holders of Common Stock are subject to, and may be adversely affected by, the rights of holders of shares of the Preferred Stock, as described below, and any other preferred stock that we may designate and issue in the future.
Preferred Stock
Under the terms of the Certificate of Incorporation, the Board is authorized to issue up to 5,000,000 shares of preferred stock in one or more series without stockholder approval. Other than the Preferred Stock, we do not currently have any shares of preferred stock issued and outstanding.
Our Certificate of Incorporation authorized the Board, subject to limitations prescribed by Delaware law, to issue preferred stock in one or more series, to establish from time to time the number of shares to be included in each series and to fix the designation, powers, preferences and rights of the shares of each series and any of its qualifications, limitations or restrictions. The Board can also increase or decrease the number of shares of any series, but not below the number of shares of that series then outstanding, without any further vote or action by our stockholders. The Board may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the Common Stock. The issuance of preferred stock, while providing flexibility in connection with financings, possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring, discouraging or preventing a change in control of the Company, may adversely affect the market price of our Common Stock and the voting and other rights of the holders of Common Stock, and may reduce the likelihood that stockholders will receive dividend payments and payments upon liquidation.
Series B Non-Voting Convertible Preferred Stock
On November 2, 2021, we issued 15,000 shares of the Preferred Stock, all of which may be converted into 37,500,000 shares of Common Stock at the election of the holders of the Preferred Stock, subject to (i) limitations upon the amount of shares that may be converted until stockholders approve the Share Conversion Approval and (ii) the beneficial ownership limitation described below. The following is a summary of the terms of the Preferred Stock, which is qualified in its entirety by the Certificate of Designation, which was filed as Exhibit 3.1 to our Current Report on Form 8-K filed with the SEC on November 1, 2021 and which is incorporated into this prospectus by reference.
Rank
The Preferred Stock ranks as to dividends or distributions of assets upon our liquidation, dissolution or winding up, whether voluntarily or involuntarily, as follows:
|
|
●
|
on par with our Common Stock;
|
|
|
●
|
senior to any class or series of our capital stock hereafter created specifically ranking by its terms junior to the Preferred Stock; and
|
|
|
●
|
junior to any class or series of our capital stock hereafter created specifically ranking by its terms senior to the Preferred Stock.
|
Conversion and Limitations
Pursuant to the terms of the Securities Purchase Agreement, each share of the Preferred Stock that we issued in the Private Placement is convertible at a current conversion price of $0.40 (subject to further adjustment in certain cases) into an aggregate of 37,500,000 shares of Common Stock, subject to limitations upon conversion, including receipt of stockholder approval of the Authorized Share Increase and the Share Conversion Approval. Until the Share Conversion Approval is received from stockholders, the holders of the Preferred Stock may only convert their pro rata share of Preferred Stock up to an aggregate of 19.99% of the outstanding shares of Common Stock immediately prior to the Private Placement Closing Date, or 8,984,178 shares of Common Stock. The Preferred Stock is subject to another limitation upon conversion of the Preferred Stock to the extent that, after giving effect to such conversion, the holder of such Preferred Stock (together with the holder’s affiliates, and any other persons acting as a group together with the holder or any of the holder’s affiliates), would beneficially own in excess of 4.99% or 9.99% of the outstanding Common Stock. Any holder may increase or decrease such percentage to any other percentage not in excess of 9.99% of the total number of shares of Common Stock then issued and outstanding provided that such increase in percentage shall not be effective until sixty-one days after notice to us.
Liquidation Preference
In the event of our liquidation, dissolution or winding up, holders of Preferred Stock are entitled to receive the same amount as a holder of Common Stock.
Voting Rights
Shares of Preferred Stock generally have no voting rights, except as required by law and except that the consent of the majority of holders of the outstanding Preferred Stock is required to: (i) alter or change adversely the powers, preferences or rights given to the Preferred Stock or alter or amend the Certificate of Designation, (ii) amend our Certificate of Incorporation or other charter documents in any manner that adversely affects any rights of the holders of Preferred Stock, (iii) increase the number of authorized shares of Preferred Stock, and (iv) enter into any agreement with respect to any of the foregoing.
Dividends
Holders of Preferred Stock are entitled to receive, and we are required to pay, dividends on shares of the Preferred Stock equal (on an as if converted to Common Stock basis) to and in the same form as dividends actually paid on shares of the Common Stock when, as and if such dividends are paid on shares of the Common Stock. The Preferred Stock is not entitled to any other dividends.
Redemption
We are not obligated to redeem or repurchase any shares of Preferred Stock. Shares of Preferred Stock are not otherwise entitled to any redemption rights, or mandatory sinking fund or analogous fund provisions.
Listing
There is no established public trading market for the Preferred Stock, and the Preferred Stock has not been listed on any national securities exchange or trading system.
Dilution Protection
In the event we, at any time after the first date of issue of the Preferred Stock and while at least one share of Preferred Stock is outstanding: (a) pays a dividend or otherwise make a distribution or distributions on shares of its Common Stock or any other equity or equity equivalent securities payable in shares of Common Stock (which, for avoidance of doubt, shall not include any shares of Common Stock issued by us upon conversion of the Preferred Stock or any debt securities), (b) subdivides outstanding shares of Common Stock into a larger number of shares, (c) combines (including by way of reverse stock split) outstanding shares of Common Stock into a smaller number of shares or (d) issues by reclassification of shares of Common Stock any shares of our capital stock, then, in each case, the conversion price of the Preferred Stock shall be adjusted as provided in the Certificate of Designation. Any adjustment made pursuant to the Certificate of Designation shall become effective immediately after the effective date of the applicable event described in subsections (a) through (d) above. In addition, in the event that we or any of our subsidiaries, as applicable, at any time while the Preferred Stock is outstanding sells or grants any option to purchase or sells or grants any right to reprice, or otherwise disposes of or issues (or announces any sale, grant or any option to purchase or other disposition), any Common Stock or any of our securities or any of our subsidiaries which would entitle the holder thereof to acquire at any time Common Stock at an effective price per share that is lower than the then conversion price of the Preferred Stock, then the conversion price of the Preferred Stock will be reduced to such lower price; this protection afforded the holder of the Preferred Stock is referred to as a “full-ratchet” anti-dilution protection. This full-ratchet anti-dilution protection is subject to termination as provided in the Certificate of Designation upon the earlier of: (i) our Common Stock achieving an average trading price during any 10 days during a 30-consecutive trading day period that is 250% of the conversion price of the Preferred Stock and the trading volume during such period exceeds $500,000 per trading day; provided that the Initial Registration Statement and the Second Registration Statement remain effective during this measurement period; or (ii) 75% of the Preferred Stock issued having been converted. The holders of Preferred Stock do not have any preemptive rights as a result of their ownership of Preferred Stock.
Fundamental Transactions
If, at any time that shares of Preferred Stock were outstanding, we effected a merger, a sale of substantially all assets or engage in another type of change of control transaction, as described in the Certificate of Designation and referred to as a “Fundamental Transaction”, then a holder of Preferred Stock would have the right to receive, upon any subsequent conversion of a share of Preferred Stock (in lieu of conversion shares) for each issuable conversion share, the same kind and amount of securities, cash or property as such holder would have been entitled to receive upon the occurrence of such fundamental transaction if such holder had been, immediately prior to such fundamental transaction, the holder of Common Stock. In connection with a Fundamental Transaction, the holders of Preferred Stock may instead receive in exchange for their shares of Preferred Stock a security of the successor entity evidenced by a written instrument substantially similar in form and substance to the Preferred Stock, which is convertible for a corresponding number of shares of capital stock of such successor entity equivalent to the shares of Common Stock upon conversion of the Preferred Stock and with a conversion price consistent with the conversion price of the Preferred Stock then currently in effect. If we are not the surviving entity in any such fundamental transaction, then it shall cause any successor entity to assume in writing all of the obligations of the Company under the Certificate of Designation, the Securities Purchase Agreement, Registration Rights Agreement and the Warrant in accordance with the provisions of the Certificate of Designation.
Registration Rights
In connection with the Private Placement, we entered into the Registration Rights Agreement with the Purchasers that provided for the resale of the Conversion Shares and the Warrant Shares. Pursuant to the terms of the Registration Rights Agreement, we are required to prepare and file: (i) an Initial Registration Statement on Form S-1 with the SEC within 30 calendar days following the Private Placement Closing Date covering the resale of Conversion Shares which have then been issued with the SEC within 30 calendar days following the Private Placement Closing Date; and (ii) the Second Registration Statement on Form S-1 covering the resale of any additional Conversion Shares and the Warrant Shares within 30 calendar days following approval by stockholders of the increase in the Common Stock and the issuance of the Conversion Shares and the Warrant Shares subject to NYSE listing rules. We will use our best efforts to cause the Initial Registration Statement and the Second Registration Statement to be declared effective by the SEC within the timing set forth in the Registration Rights Agreement. The Registration Rights Agreement provides for payment of liquidated damages to the Purchasers in the event we are not able to perform our obligations with respect to registering the Common Stock. In addition, pursuant to the Registration Rights Agreement, we also agreed, among other things, to indemnify the Purchasers, their officers, directors, members, employees and agents, successors and assigns under the registration statement from certain liabilities and pay all fees and expenses (excluding any legal fees of the Purchaser(s), and any underwriting discounts and selling commissions) incident to our obligations under the Registration Rights Agreement. For additional information regarding the Registration Rights Agreement, see “The Private Placement—Registration Rights Agreement” on page 22 of this prospectus and also a copy of the Registration Rights Agreement, which was filed as Exhibit 10.1 to our Current Report on Form 8-K filed with the SEC on November 1, 2021, and which is incorporated into this prospectus by reference.
Description of the Warrants in the Private Placement
The Warrants were issued together with the Preferred Stock in the Private Placement. The Warrants are not immediately exercisable and will not be exercisable unless and until the Share Conversion Approval and the Authorized Share Increase are approved by our stockholders. Upon receiving stockholder approval of these proposals, the Warrants will become exercisable by the holders for up to 37,500,000 shares of Common Stock at an exercise price of $0.53 per share, subject to customary anti-dilution adjustments as provided in the Warrant. The Warrants will remain exercisable for a period of six (6) years from the initial exercise date. The Warrants are subject to a provision prohibiting the exercise of such Warrants to the extent that, after giving effect to such exercise, the holder of such Warrant (together with the holder’s affiliates, and any other persons acting as a group together with the holder or any of the holder’s affiliates), would beneficially own in excess of 4.99% or 9.99% of the outstanding Common Stock. The Warrants do not have any preemptive rights or a preference upon any liquidation, dissolution or winding-up of NovaBay. For additional information about the terms of the Warrant, a copy of the Warrant we issued in the Private Placement is filed as Exhibit 4.1 to our Current Report on Form 8-K filed with the SEC on November 1, 2021, and which is incorporated into this prospectus by reference.
Outstanding Warrants
As of November 26, 2021, we had the Warrants and other common stock warrants outstanding to purchase an aggregate of 44,581,508 shares of Common Stock with a weighted-average exercise price of $0.71 per share. The Warrants to purchase an aggregate of 37,500,000 shares of Common Stock are not immediately exercisable, unless and until approvals for both the Authorized Share Increase and the Share Conversion Approval are received from our stockholders.
Anti-takeover effects of provisions of our Certificate of Incorporation and Bylaws and Delaware law
Our Certificate of Incorporation provides that the Board is divided into three classes with staggered three-year terms. Only one class of directors is elected at each annual meeting of stockholders, with the other classes continuing for the remainder of their respective three-year terms. Because holders of Common Stock do not have cumulative voting rights in the election of directors, stockholders holding a majority of the shares of Common Stock outstanding are able to elect all of our directors. The Board is able to elect a director to fill a vacancy created by the expansion of the Board or due to the resignation or departure of an existing board member. Our Certificate of Incorporation and Bylaws also provide that all stockholder actions must be effected at a duly called meeting of stockholders and not by written consent, and that only the Board pursuant to a resolution adopted by a majority of the total number of authorized directors may call a special meeting of stockholders. In addition, our Bylaws include a requirement for the advance notice of nominations for election to the Board or for proposing matters that can be acted upon at a stockholders’ meeting. Our Certificate of Incorporation provides for the ability of the Board to issue, without stockholder approval, up to 5,000,000 shares of preferred stock with terms set by the Board, which rights could be senior to those of our Common Stock. The Certificate of Incorporation and Bylaws also provide that approval of at least 66-2/3% of the shares entitled to vote at an election of directors will be required to adopt, amend or repeal the Bylaws, or repeal the provisions of our Certificate of Incorporation regarding the election of directors and the inability of stockholders to take action by written consent in lieu of a meeting.
The foregoing provisions make it difficult for holders of Common Stock to replace the Board. In addition, the authorization of undesignated preferred stock makes it possible for the Board to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control of NovaBay.
Section 203 of the Delaware General Corporation Law
We are subject to the provisions of Section 203 of the Delaware General Corporation Law regulating corporate takeovers. This section prevents some Delaware corporations from engaging, under some circumstances, in a business combination, which includes a merger or sale of at least 10% of the corporation’s assets with any interested stockholder, meaning a stockholder who, together with affiliates and associates, owns or, within three years prior to the determination of interested stockholder status, did own 15% or more of the corporation’s outstanding voting stock, unless:
|
|
●
|
the transaction is approved by the Board prior to the time that the interested stockholder became an interested stockholder;
|
|
|
●
|
upon consummation of the transaction which resulted in the stockholder’s becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced; or
|
|
|
●
|
at or subsequent to such time that the stockholder became an interested stockholder, the business combination is approved by the Board and authorized at an annual or special meeting of stockholders by at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder.
|
A Delaware corporation may “opt out” of these provisions with an express provision in its original certificate of incorporation or an express provision in its certificate of incorporation or bylaws resulting from a stockholders’ amendment approved by at least a majority of the outstanding voting shares. We do not plan to “opt out” of these provisions. The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts to acquire NovaBay.
Transfer Agent and Registrar
Computershare Shareholder Services, Inc., located in Providence, Rhode Island, Providence County, is the transfer agent and registrar for our Common Stock and preferred stock in the United States and Computershare Investor Services, Inc., located in Toronto, Ontario, Canada, is the co-transfer agent and registrar for our Common Stock in Canada.
Listing on the NYSE American
Our Common Stock is listed on the NYSE American under the symbol “NBY.”
SELLING STOCKHOLDERS
The shares of Common Stock being offered by the Selling Stockholders are those issuable to the Selling Stockholders upon the conversion of our Preferred Stock. For additional information regarding the issuance of the Preferred Stock, see “Description of the Private Placement” in this prospectus. We are registering the shares of Common Stock in order to permit the Selling Stockholders to offer the shares for resale from time to time. Except for the ownership of the Preferred Stock issued pursuant to the Securities Purchase Agreement and as disclosed below, the Selling Stockholders have not had any material relationship with us within the past three years.
The table below lists the Selling Stockholders and other information regarding the beneficial ownership (as determined under Section 13(d) of the Exchange Act and the rules and regulations thereunder) of the shares of Common Stock by each of the Selling Stockholders. These rules generally provide that a person is the beneficial owner of securities if such person has or shares the power to vote or direct the voting thereof, or to dispose or direct the disposition thereof, or has the right to acquire such powers within 60 days. The second column lists the number of shares of Common Stock beneficially owned by each Selling Stockholder, based on its ownership of the shares of common stock and warrants, as of November 26, 2021, assuming conversion of the Preferred Stock held by the Selling Stockholder on that date, without regard to any limitations on conversion. The third column lists the shares of Common Stock being offered by this prospectus by the Selling Stockholders.
In accordance with the terms of the Registration Rights Agreement with the Selling Stockholders, this prospectus generally covers the resale of the sum of the number of shares of Common Stock initially issuable upon conversion of the Preferred Stock issued to the Selling Stockholders in the Private Placement assuming the shares of Preferred Stock were converted in full, without regarding to any limitations on conversion contained in the Certificate of Designations for the Preferred Stock as of November 26, 2021. Because the conversion price of the Preferred Stock may be adjusted upon the occurrence of certain other events, the number of shares that will actually be issued may be more or less than the number of shares being offered by this prospectus. The fourth column assumes the sale of all of the shares offered by the Selling Stockholders pursuant to this prospectus.
Under the terms of the Preferred Stock, a Selling Stockholder may not convert or exercise, as applicable, shares of Preferred Stock to the extent such exercise would cause such Selling Stockholder, together with its affiliates and attribution parties, to beneficially own a number of shares of Common Stock, which would exceed 4.99% or 9.99%, as applicable, of our then outstanding Common Stock following such conversion, excluding for purposes of such determination shares of Common Stock issuable upon conversion of such Preferred Stock which have not been converted. In addition, the conversion of the Preferred Stock is subject to limitation up to the Issuable Maximum until the Share Conversion Approval is received from stockholders. The number of shares in the second and fourth columns do not reflect this limitation.
Percentage ownership is based on 44,958,364 shares of Common Stock outstanding as of November 26, 2021. The Selling Stockholders may sell all, some or none of their shares in this offering. See “Plan of Distribution.” This information in the table and the footnotes is based upon our review of public filings, our stockholder, optionholder and warrantholder registers and information furnished to us by the Selling Stockholders. Based on this information, we believe that none of the Selling Stockholders are broker-dealers or affiliates of broker-dealers.
|
Name of Selling Stockholder
|
|
|
Shares of
Common
Stock
Owned Prior
to Offering(1)
|
|
|
Shares of
Common
Stock Being
Offered(2)
|
|
|
Shares of
Common
Stock
Owned
After
Offering(1) (3)
|
|
|
Percent
Owned
After the
Offering(1) (3)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Altium Growth Fund, LP(4)
|
|
|
10,000,000
|
|
|
10,000,000
|
|
|
0
|
|
|
0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Alpha Capital Anstalt(5)
|
|
|
10,000,000
|
|
|
10,000,000
|
|
|
0
|
|
|
0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lincoln Park Capital Fund, LLC(6)
|
|
|
4,375,000
|
|
|
4,375,000
|
|
|
0
|
|
|
0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hudson Bay Master Fund Ltd.(7)
|
|
|
4,375,000
|
|
|
4,375,000
|
|
|
0
|
|
|
0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FGP Protective Opportunity Master Fund SP(8)
|
|
|
3,750,000
|
|
|
3,750,000
|
|
|
0
|
|
|
0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
District 2 Capital Fund LP(9)
|
|
|
2,500,000
|
|
|
2,500,000
|
|
|
0
|
|
|
0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bigger Capital Fund, LP(10)
|
|
|
2,500,000
|
|
|
2,500,000
|
|
|
0
|
|
|
0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Number of Shares:
|
|
|
37,500,000
|
|
|
37,500,000
|
|
|
|
|
|
|
|
*Less than 1%
|
(1)
|
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Includes 100% of the shares of Common Stock issuable upon conversion of the Preferred Stock at the current conversion price. The shares of Common Stock listed in the table do not include the shares of Common Stock issuable upon exercise of the Warrants owned by each of the Selling Stockholders, as the Warrants are not currently exercisable and will not be exercisable, unless and until approvals for both the Authorized Share Increase and the Share Conversion Approval are received from our stockholders.
|
|
|
|
|
(2)
|
Pursuant to the Certificate of Designation, a Selling Stockholder may not convert shares of Preferred Stock if as a result of such conversion such Selling Stockholder, its affiliates and any other person whose beneficial ownership of shares of Common Stock would be aggregated with the Selling Stockholder’s for purposes of Section 13(d) of the Exchange Act, would beneficially own more than 4.99% of our common stock (or 9.99% if such Selling Stockholder has previously provided not less than 61 days’ prior notice to us of such increase). In addition, the conversion of the Preferred Stock is subject to limitation up to the Issuable Maximum until the Share Conversion Approval is received from stockholders. For purposes of this Selling Stockholder table, we have disregarded these limitations.
|
|
|
|
|
(3)
|
Assumes for each Selling Stockholder the conversion in full of all shares of Preferred Stock held by such Selling Stockholder and the sale of all shares offered hereby.
|
|
(4)
|
Consists of 10,000,000 shares of Common Stock issuable upon conversion of the 4,000 shares of Preferred Stock owned by Altium Growth Fund, LP. Altium Capital Management, LP, the investment manager of Altium Growth Fund, LP, has voting and investment power over these securities. Jacob Gottlieb is the managing member of Altium Capital Growth GP, LLC, which is the general partner of Altium Growth Fund, LP. Each of Altium Growth Fund, LP and Jacob Gottlieb disclaims beneficial ownership over these shares.
|
|
(5)
|
Consists of 10,000,000 shares of Common Stock issuable upon conversion of the 4,000 shares of Preferred Stock owned by Alpha Capital Anstalt. Nicola Feuerstein, Director of Alpha Capital Anstalt, has sole voting and dispositive power over the NovaBay securities held by Alpha Capital Anstalt.
|
|
(6)
|
Consists of 4,375,000 shares of Common Stock issuable upon conversion of the 1,750 shares of Preferred Stock owned by Lincoln Park Capital Fund, LLC. Joshua Scheinfeld and Jonathan Cope are the principals of Lincoln Park Capital Fund, LLC and are deemed to be the beneficial owners of the NovaBay securities. Messrs. Scheinfeld and Cope have shared voting and disposition power with respect to the NovaBay securities owned by Lincoln Park Capital Fund, LLC.
|
|
(7)
|
Consists of 4,375,000 shares of Common Stock issuable upon conversion of the 1,750 shares of Preferred Stock owned by Hudson Bay Master Fund Ltd. Hudson Bay Capital Management LP, the investment manager of Hudson Bay Master Fund Ltd., has voting and investment power over these NovaBay securities. Sander Gerber is the managing member of Hudson Bay Capital GP LLC, which is the general partner of Hudson Bay Capital Management LP. Each of Hudson Bay Master Fund Ltd. and Sander Gerber disclaims beneficial ownership over these securities.
|
|
(8)
|
Consists of 3,750,000 shares of Common Stock issuable upon conversion of the 1,500 shares of Preferred Stock owned by FGP Protective Opportunity Master Fund SP, which is a segregated portfolio of FGP Protective Opportunity Master Fund SPC. The principal of FGP Protective Opportunity Master Fund SP is Gregory Pepin. Gregory Pepin has the voting and dispositive power with respect to the securities.
|
|
(9)
|
Consists of 2,500,000 shares of Common Stock issuable upon conversion of the 1,000 shares of Preferred Stock owned by District 2 Capital Fund LP (“District 2 CF”). Bigger Capital Fund GP, LLC (“Bigger GP”) is a general partner of Bigger Capital Fund, LP (“Bigger Capital”) and District 2 Capital LP (“District 2”) is the investment manager of District 2 CF. Michael Bigger is the managing member of Bigger GP and District and District 2 Holdings LLC (“District 2 Holdings”), which is the managing member of District 2 GP LLC (“District 2 GP”), the general partner of District 2 CF. Therefore, Mr. Bigger, District 2, District 2 Holdings and District 2 CF may be deemed to be the beneficial owner, and have the shared power to dispose of or direct the disposition, of the shares reported as beneficially owned by District 2 CF and Mr. Bigger and Bigger GP may be deemed to be the beneficial owner, and have the shared power to dispose of or direct the disposition, of the shares reported as beneficially owned by Bigger Capital and District 2 CF.
|
|
(10)
|
Consists of 2,500,000 shares of Common Stock issuable upon conversion of the 1,000 shares of Preferred Stock owned by Bigger Capital Fund, LP. Bigger Capital, LLC is the investment manager of Bigger Capital Fund, LP. Mr. Michael Bigger is a managing partner of Bigger Capital GP, LLC, which is the general partner of Bigger Capital Fund, LP, and has sole voting and investment power over the NovaBay securities. Bigger Capital GP, LLC and Mr. Bigger may deemed to beneficially own the shares beneficially held by Bigger Capital Fund, LP.
|
Relationships with the Selling Stockholders
None of the Selling Stockholders have had any position, office or other material relationship with NovaBay.
PLAN OF DISTRIBUTION
Each Selling Stockholder of the securities and any of their pledgees, assignees and successors-in-interest may, from time to time, sell, transfer or otherwise dispose of any or all of shares of Common Stock covered by this prospectus on the NYSE American or any other stock exchange, market or trading facility on which the securities are traded or in private transactions. These sales may be at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing market price, at varying prices determined at the time of sale or at negotiated prices. A Selling Stockholder may use any one or more of the following methods when selling securities:
|
|
●
|
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
|
●
|
block trades in which the broker-dealer will attempt to sell the Shares or Warrant Shares as agent, but may position and resell a portion of the block as principal to facilitate the transaction;
|
|
|
●
|
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
|
●
|
an exchange distribution in accordance with the rules of the applicable exchange;
|
|
|
●
|
privately negotiated transactions;
|
|
|
●
|
settlement of short sales, to the extent permitted by law;
|
|
|
●
|
through the writing or settlement of options or other hedging transactions, whether through an option exchange or otherwise;
|
|
|
●
|
in transactions through broker-dealers that agree with the Selling Stockholders to sell a specified number of such securities at a stipulated price per security;
|
|
|
●
|
a combination of any such methods of sale; or
|
|
|
●
|
any other method permitted pursuant to applicable law.
|
The Selling Stockholders may also sell securities under Rule 144 or any other exemption from registration under the Securities Act, if available, rather than under this prospectus.
Broker‑dealers engaged by the Selling Stockholders may arrange for other brokers‑dealers to participate in sales. Broker‑dealers may receive commissions or discounts from the Selling Stockholders (or, if any broker‑dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or markdown in compliance with FINRA Rule 2121.
In connection with the sale of the securities or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they assume. The Selling Stockholders may also sell securities short and deliver these securities to close out their short positions, or loan or pledge the securities to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Stockholders and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Each Selling Stockholder has informed the Company that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the securities.
The Company is required to pay certain fees and expenses incurred by the Company incident to the registration of the securities. The Company has agreed to indemnify the Selling Stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
We agreed to keep this prospectus effective until the earlier of (i) the date on which the securities may be resold by the Selling Stockholders without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for us to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of similar effect or (ii) all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in market making activities with respect to the Common Stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the Selling Stockholders will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the Common Stock by the Selling Stockholders or any other person. We will make copies of this prospectus available to the Selling Stockholders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
LEGAL MATTERS
Certain legal matters with respect to the validity of the issuance of the securities offered hereby will be passed upon by our counsel, Squire Patton Boggs (US) LLP, Washington, DC.
EXPERTS
The consolidated financial statements of NovaBay appearing in its Annual Report on Form 10-K for the year ended December 31, 2020 have been audited by OUM & Co. LLP, an independent registered public accounting firm, as set forth in their report thereon, included therein, and incorporated herein by reference. Such consolidated financial statements are incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The financial statements of DERMAdoctor referred to in this prospectus for the year ended December 31, 2020 have been audited by MarksNelson LLC, a certified public accountant and independent accounting firm, as set forth in their report thereon, and incorporated herein by reference. Such financial statements are incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s website at http://www.sec.gov. The SEC’s website contains reports, proxy and information statements and other information regarding issuers, such as us, that file electronically with the SEC. You may also read and copy any document we file with the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. You may also obtain copies of these documents at prescribed rates by writing to the SEC. Please call the SEC at 1-800-SEC-0330 for further information on the operation of its Public Reference Room.
We also maintain a website at http://www.novabay.com/investors/sec-filings, through which you can access our SEC filings. The information set forth on our website is not part of this prospectus.
We have filed with the SEC a registration statement under the Securities Act that registers the distribution of these securities. The registration statement, including the attached exhibits and schedules, contains additional relevant information about us and the securities. This prospectus does not contain all of the information set forth in the registration statement. You can get a copy of the registration statement, at prescribed rates, from the SEC at the address listed above. The registration statement and the documents referred to below under “Incorporation of Certain Documents by Reference” are also available on our Internet website, http://www.novabay.com, under “Investors—SEC Filings.” We have not incorporated by reference into this prospectus the information on our website, and you should not consider it to be a part of this prospectus.
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
You should consider the incorporated information as if we reproduced it in this prospectus. The SEC allows us to “incorporate by reference” the information we file with the SEC into this prospectus. This permits us to disclose important information to you by referring you to other documents that we filed separately with the SEC. Any information referred to in this way is considered part of this prospectus. The information incorporated by reference is an important part of this prospectus, and information that we file later with the SEC will automatically update and supersede this information. This prospectus omits certain information contained in the registration statement, as permitted by the SEC. You should refer to the registration statement, including the exhibits, for further information about us and the securities we may offer pursuant to this prospectus. Statements in this prospectus regarding the provisions of certain documents filed with, or incorporated by reference in, the registration statement are not necessarily complete and each statement is qualified in all respects by that reference. We incorporate by reference the following documents that have been filed with the SEC (other than information furnished under Item 2.02 or Item 7.01 of Form 8-K and all exhibits related to such items):
|
|
●
|
our Annual Report on Form 10-K for the year ended December 31, 2020, as filed with the SEC on March 25, 2021;
|
|
|
●
|
our Quarterly Reports on Form 10-Q for the quarterly periods ended March 31, 2021, June 30, 2021 and September 30, 2021, as filed with the SEC on May 6, 2021, August 12, 2021 and November 12, 2021, respectively;
|
|
|
●
|
our Current Reports on Form 8-K, as filed with the SEC on February 8, 2021, May 14, 2021, May 24, 2021, June 11, 2021, June 30 , 2021, July 21, 2021, September 28, 2021, November 1, 2021, November 2, 2021, November 3, 2021 and November 12, 2021, as amended December 1, 2021;
|
|
|
●
|
our Definitive Proxy Statement on Schedule 14A filed with the SEC on April 7, 2021 and our Definitive Proxy Statement on Schedule 14A filed with the SEC on November 12, 2021; and
|
|
|
●
|
the description of our Common Stock in our registration statement on Form 8-A, as filed with the SEC on August 29, 2007, and updated by our Current Report on Form 8-K filed with the SEC on June 29, 2010.
|
Any information in any of the foregoing documents will automatically be deemed to be modified or superseded to the extent that information in this prospectus modifies or replaces such information. We also incorporate by reference any future filings (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items) made with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act prior to the termination of the offering of the securities made by this prospectus, including those made after the date of the initial filing of the registration statement of which this prospectus is a part and prior to effectiveness of such registration statement. Information in such future filings updates and supplements the information provided in this prospectus. Any statements in any such future filings will automatically be deemed to modify and supersede any information in any document we previously filed with the SEC that is incorporated or deemed to be incorporated herein by reference to the extent that statements in the later filed document modify or replace such earlier statements.
We will provide, upon written or oral request, without charge to each person, including any beneficial owner, to whom a copy of this prospectus is delivered, a copy of any or all of the information incorporated herein by reference (exclusive of exhibits to such documents unless such exhibits are specifically incorporated by reference herein). You may request in writing or orally a copy of these filings, at no cost, by writing or telephoning us at the following address:
NovaBay Pharmaceuticals, Inc.
2000 Powell Street, Suite 1550
Emeryville, CA 94608
(510) 899-8800
Attn: Corporate Secretary
37,500,000 Shares of Common Stock
PROSPECTUS
We have not authorized any dealer, salesperson or other person to give any information or to make any representations not contained in this prospectus. You must not rely on any unauthorized information. This prospectus is not an offer to sell these securities in any jurisdiction where an offer or sale is not permitted. The information in this prospectus is current as of the date of this prospectus. You should not assume that this prospectus is accurate as of any other date.
December 10, 2021
NovaBay Pharmaceuticals (AMEX:NBY)
Historical Stock Chart
From Aug 2024 to Sep 2024
NovaBay Pharmaceuticals (AMEX:NBY)
Historical Stock Chart
From Sep 2023 to Sep 2024