FDA Gives Accelerated Approval to BeiGene's Brukinsa
November 14 2019 - 4:37PM
Dow Jones News
By Stephen Nakrosis
The U.S. Food and Drug Administration said Thursday it had
granted accelerated approval to BeiGene Ltd.'s (BGNE) Brukinsa, or
zanubrutinib, for the treatment of certain adult patients with
lymphoma.
The FDA said the approval was granted "for the treatment of
adult patients with mantle cell lymphoma who have received at least
one prior therapy."
According to the FDA, clinical trials showed 84% of patients saw
tumor shrinkage with BeiGene's therapy.
BeiGene's application was given a breakthrough therapy
designation, the FDA said, which "expedites the development and
review of drugs that are intended to treat a serious
condition."
--Write to Stephen Nakrosis at stephen.nakrosis@wsj.com
(END) Dow Jones Newswires
November 14, 2019 16:22 ET (21:22 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
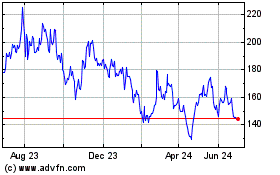
BeiGene (NASDAQ:BGNE)
Historical Stock Chart
From Mar 2024 to Apr 2024
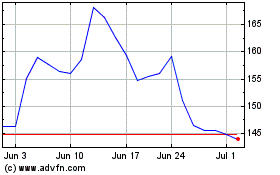
BeiGene (NASDAQ:BGNE)
Historical Stock Chart
From Apr 2023 to Apr 2024