GlaxoSmithKline Gets EU Approval for Jemperli Drug
April 23 2021 - 10:45AM
Dow Jones News
By Adriano Marchese
GlaxoSmithKline PLC said Friday that the European Commission has
granted conditional marketing authorization for Jemperli, a
treatment for endometrial cancer.
The British pharmaceutical giant said the approval makes
Jemperli, also known as dostarlimab, the first anti-PD-1 therapy
available for endometrial cancer in Europe.
The drug is a treatment for patients with mismatch repair
deficient recurrent or advanced endometrial cancer.
"Today's approval of dostarlimab means that for the first time
in Europe, these women will have access to a new, innovative and
much-needed therapy," Hal Barron, chief scientific officer and
president of research and development, said.
Write to Adriano Marchese at adriano.marchese@wsj.com
(END) Dow Jones Newswires
April 23, 2021 10:30 ET (14:30 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
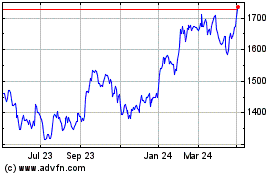
Gsk (LSE:GSK)
Historical Stock Chart
From Mar 2024 to Apr 2024
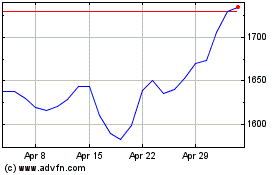
Gsk (LSE:GSK)
Historical Stock Chart
From Apr 2023 to Apr 2024