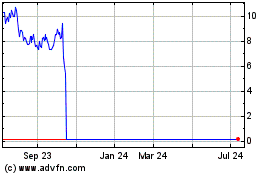
VBL Shares Drop 16% After FDA Withholds Clearance for New Cancer Treatment Batches
June 15 2021 - 1:40PM
Dow Jones News
By Chris Wack
VBL Therapeutics Ltd. shares were down 16% to $2.26 Tuesday
after the company said it was notified by the Food and Drug
Administration that clearance of new VB-111 batches for use in the
U.S. is currently pending the completion of a technical review
evaluating the comparability of VB-111 manufacturing between
different source sites.
The company's Phase 3 study is investigating ofranergene
obadenovec, or VB-111, for the treatment of platinum-resistant
ovarian cancer.
Until new batches are cleared, VBL expects a temporary shortage
of study drug supply for the U.S.
Accordingly, recruitment of new patients in the U.S. will be
temporarily paused, it said. Treatment will continue as usual for
all U.S. patients currently enrolled. To date, the study has
enrolled 75% of the planned 400 patients.
VBL recently amended the primary endpoint of the study based on
requested changes by the company that were reviewed by the FDA. The
study now includes a second, separate primary endpoint of
progression free survival, in addition to the original primary
endpoint of the trial, overall survival. Successfully meeting
either primary endpoint is expected to be sufficient to support BLA
submission.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
June 15, 2021 13:29 ET (17:29 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
Vascular Biogenics (NASDAQ:VBLT)
Historical Stock Chart
From Mar 2024 to Apr 2024
Vascular Biogenics (NASDAQ:VBLT)
Historical Stock Chart
From Apr 2023 to Apr 2024