Registration
No. 333-______
As
filed with the Securities and Exchange Commission on November 22, 2023
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
S-3
REGISTRATION
STATEMENT UNDER THE SECURITIES ACT OF 1933
SAFETY
SHOT, INC.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
2844 |
|
83-2455880 |
| (State
or jurisdiction of |
|
(Primary
Standard Industrial |
|
(I.R.S.
Employer |
| incorporation
or organization) |
|
Classification
Code Number) |
|
Identification
No.) |
1061
E. Indiantown Rd., Ste. 110
Jupiter,
FL 33477
(561)
244-7100
(Address,
including zip code, and telephone number, including area code of registrant’s principal executive offices)
Brian
S. John
Chief
Executive Officer
Safety
Shot, Inc.
1061
E. Indiantown Rd., Ste. 110
Jupiter,
FL 33477
(561)
244-7100
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies
to:
| Arthur
S. Marcus, Esq. |
| Sichenzia
Ross Ference Carmel LLP |
| 1185
Avenue of the Americas, 31 FL |
| New
York, NY 10036 |
| Telephone:
(212) 930-9700 |
| Facsimile:
(212) 930-9725 |
Approximate
date of commencement of proposed sale to the public: From time to time after the effective date of this registration statement.
If
the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check
the following box. ☐
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933 check the following box. ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”,
“smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large
accelerated filer |
☐ |
Accelerated
filer |
☐ |
| Non-accelerated
filer |
☒ |
Smaller
reporting company |
☒ |
| |
|
Emerging
growth company |
☒ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
The
registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the
registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective
in accordance with Section 8(a) of the Securities Act of 1933, or until this registration statement shall become effective on such date
as the Securities and Exchange Commission, acting pursuant to Section 8(a), may determine.
THE
INFORMATION IN THIS PROSPECTUS IS NOT COMPLETE AND MAY BE CHANGED. THE SELLING SHAREHOLDERS MAY NOT SELL THESE SECURITIES UNTIL THE REGISTRATION
STATEMENT FILED WITH THE SECURITIES AND EXCHANGE COMMISSION IS EFFECTIVE. THIS PROSPECTUS IS NOT AN OFFER TO SELL THESE SECURITIES AND
IS NOT SOLICITING AN OFFER TO BUY THESE SECURITIES IN ANY STATE WHERE THE OFFER OR SALE IS NOT PERMITTED.
Subject
to Completion, dated November 22, 2023
PROSPECTUS
SAFETY
SHOT, INC.
1,978,796
Shares of Common Stock
This
prospectus relates to the resale or other disposition from time to time in one or more offerings of up to 1,978,796 shares of
our common stock, par value $0.001, by the selling stockholder named herein. The shares that may be offered and sold from time to time
pursuant to this prospectus include (i) up to 550,000 previously issued shares of common stock; and (ii) up to 1,428,796
shares of common stock issuable upon the exercise of the common stock purchase warrant (the “Warrants”).
The
terms of the Warrants are described in greater detail under “Description of Capital Stock”, beginning on page 11.
We
will not receive any proceeds from the sale of shares of common stock by the selling stockholder pursuant to this prospectus. However,
we will receive proceeds from the exercise of the Warrants.
The
selling stockholder identified in this prospectus, or its permitted transferees or other successors-in-interest, may offer the shares
of our common stock from time to time through public or private transactions at prevailing market prices, at prices related to prevailing
market prices, or at privately negotiated prices. We provide additional information about how the selling stockholder may sell its shares
of common stock in the section entitled “Plan of Distribution” in this prospectus.
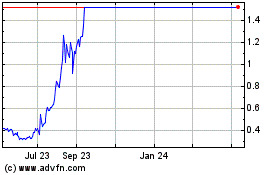
Our
common stock is listed on the NASDAQ Capital Market under the symbol “SHOT.” On November 21, 2023, the last reported
sale price of our common stock was $4.79 per share.
Investing
in our securities involves a high degree of risk. Before making any investment decision, you should carefully review and consider all
the information in this prospectus and the documents incorporated by reference herein, including the risks and uncertainties described
under “Risk Factors” beginning on page 9.
NEITHER
THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED
IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The
date of this prospectus is _______________, 2023.
SAFETY
SHOT, INC.
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
This
prospectus is a part of a registration statement that we have filed with the U.S. Securities and Exchange Commission (the “SEC”
or the “Commission”) pursuant to which the selling stockholder named herein may, from time to time, offer and sell or otherwise
dispose of the shares of our common stock covered by this prospectus.
This
prospectus and the documents incorporated by reference into this prospectus include important information about us, the securities being
offered and other information you should know before investing in our common stock. Before purchasing any common stock, you should carefully
read both this prospectus and any applicable prospectus supplement, together with the additional information described under the heading
“Where You Can Find More Information” and “Incorporation of Certain Information by Reference.”
Neither
we, nor the selling stockholder, have authorized anyone to provide you with any information or to make any representations other than
those contained in this prospectus or in any applicable prospectus supplement prepared by or on behalf of us or to which we have referred
you. We and the selling stockholder take no responsibility for, and can provide no assurance as to the reliability of, any other information
that others may give you. The selling stockholder will not make an offer to sell these securities in any jurisdiction where the offer
or sale is not permitted. You should assume that the information appearing in this prospectus and in any applicable prospectus supplement
to this prospectus is accurate only as of the date on its respective cover, that the information appearing in any applicable free writing
prospectus is accurate only as of the date of that free writing prospectus, and that any information incorporated by reference is accurate
only as of the date of the document incorporated by reference, unless we indicate otherwise. Our business, financial condition, results
of operations and prospects may have changed since those dates. This prospectus incorporates by reference, and any prospectus supplement
or free writing prospectus may contain and incorporate by reference, market data and industry statistics and forecasts that are based
on independent industry publications and other publicly available information. Although we believe these sources are reliable, we do
not guarantee the accuracy or completeness of this information and we have not independently verified this information. In addition,
the market and industry data and forecasts that may be included or incorporated by reference in this prospectus, any prospectus supplement
or any applicable free writing prospectus may involve estimates, assumptions and other risks and uncertainties and are subject to change
based on various factors, including those discussed under the heading “Risk Factors” contained in this prospectus, the applicable
prospectus supplement and any applicable free writing prospectus, and under similar headings in other documents that are incorporated
by reference into this prospectus. Accordingly, investors should not place undue reliance on this information.
All
references in this prospectus to the “Company”, “we”, “us”, or “our”, are to Safety Shot,
Inc., a Delaware corporation, and its consolidated subsidiaries unless the context dictates otherwise.
WHERE
YOU CAN FIND MORE INFORMATION
This
prospectus is part of the registration statement on Form S-3 filed with the SEC under the Securities Act of 1933, as amended, or the
Securities Act, and does not contain all the information set forth in the registration statement. Whenever a reference is made in this
prospectus to any of our contracts, agreements, or other documents, the reference may not be complete and you should refer to the exhibits
that are a part of the registration statement or the exhibits to the reports or other documents incorporated herein by reference for
a copy of such contract, agreement, or other document.
We
are currently subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and in accordance
therewith files periodic reports, proxy statements, and other information with the SEC. Our SEC filings are available to you on the SEC’s
website at www.sec.gov.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus includes statements and information that may constitute forward-looking statements within the meaning of Section 27A of the
Securities Act and Section 21E of the Exchange Act. Statements that are “forward-looking statements” include any projections
of earnings, revenue or other financial items, any statements of the plans, strategies or objectives of management for future operations,
any statements concerning proposed new projects or other developments, any statements regarding future economic conditions or performance,
any statements of management’s beliefs, goals, strategies, intentions and objectives, any statements concerning potential acquisitions,
and any statements of assumptions underlying any of the foregoing. Words such as “may,” “will,” “should,”
“could,” “would,” “predicts,” “potential,” “continue,” “expects,”
“anticipates,” “future,” “outlook,” “strategy,” “positioned,” “intends,”
“plans,” “believes,” “projects,” “estimates” and similar expressions, as well as statements
in the future tense, identify forward-looking statements.
These
statements are necessarily subjective and involve known and unknown risks, uncertainties and other important factors that could cause
our actual results, performance or achievements, or industry results, to differ materially from any future results, performance or achievements
described in or implied by such statements. Actual results may differ materially from expected results described in our forward-looking
statements, including with respect to correct measurement and identification of factors affecting our business or the extent of their
likely impact, the accuracy and completeness of the publicly available information with respect to the factors upon which our business
strategy is based or the success of our business. In addition, even if results are consistent with the forward-looking statements contained
in this prospectus, those results may not be indicative of results or developments in subsequent periods. Furthermore, industry forecasts
are likely to be inaccurate, especially over long periods of time and in industries particularly sensitive to market conditions, such
as the seafood industry.
Forward-looking
statements should not be read as a guarantee of future performance or results, and will not necessarily be accurate indications of whether,
or the times by which, our performance or results may be achieved. Forward-looking statements are based on information available at the
time those statements are made and management’s belief as of that time with respect to future events, and are subject to risks
and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward-looking
statements.
Should
one or more of the risks or uncertainties described above or elsewhere in this prospectus occur, or should underlying assumptions prove
incorrect, our actual results and plans could differ materially from those expressed in any forward-looking statements. Readers are cautioned
not to place undue reliance on forward-looking statements, which speak only as of the date they are made. Except as required by law,
we disclaim all responsibility to publicly update any information contained in a forward-looking statement or any forward-looking statement.
All
forward-looking statements attributable to us or to persons acting on our behalf, including any such forward-looking statements made
subsequent to the publication of this prospectus, are expressly qualified in their entirety by this cautionary statement.
PROSPECTUS
SUMMARY
This
summary highlights information contained elsewhere or incorporated by reference into this prospectus. Because it is a summary, it does
not contain all of the information that you should consider before investing in our common stock. You should read this entire prospectus
carefully, including the section entitled “Risk Factors,” any applicable prospectus supplement and the documents that we
incorporate by reference into this prospectus and any applicable prospectus supplement, before making an investment decision.
THE
COMPANY
Safety
Shot Inc. (NASDAQ: SHOT) was formerly known as Jupiter Wellness Inc. In August 2023, the Company acquired certain assets of GBB Drink
Lab Inc which included the blood alcohol detox drink Safety Shot, an over-the-counter drink that can lower blood alcohol content to allow
recovery from the effects of alcohol at a rate faster than would occur normally. Concurrently with the acquisition, the Company changed
its name to Safety Shot, Inc. and changed its NASDAQ trading symbol to SHOT. The Company plans on rolling out Safety Shot in 2024.
Safety
Shot has a well-established clinical development infrastructure and fits within the Company’s existing over-the-counter and prescription-grade
health and wellness products. The Company will continue its current product lines as an operating division and is committed to supporting
health and wellness by developing innovative solutions to a range of conditions. We take pride in our research and development of over-the-counter
(OTC) products and intellectual property, which aim to address some of the most prevalent health and wellness concerns today. Our product
pipeline includes a diverse range of products, such as hair loss treatments, eczema creams, vitiligo solutions, and sexual wellness products,
that cater to different health and wellness needs. We are dedicated to staying up-to-date with the latest scientific research and technology,
ensuring that our products are effective, safe, and meet the highest industry standards.
To
achieve our mission, we rely on a team of highly skilled and experienced professionals who are committed to advancing our vision of health
and wellness. Our team includes scientists, researchers, product developers, and business experts who collaborate to create new products
and enhance existing ones. We also partner with industry leaders and organizations to leverage the latest technologies and expand our
reach.
We
generate revenue through various channels, including the sales of our OTC and consumer products, as well as licensing royalties. Our
products are available through various retailers and e-commerce platforms, making them accessible to a broad customer base. Additionally,
we collaborate with other companies to license our intellectual property, creating additional revenue streams and expanding our global
presence.
We
signed agreements to license JW-700 to Taisho, a $2.6 billion revenue company and Japan’s leading seller of minoxidil products.
Taisho plans on launching the product commercially in 2024. In India, the Company signed an agreement with Cosmofix Technovation Pvt
Ltd and Sanpellegrino Cosmetics to license its JW-700 and Photocil products. Additional licensing opportunities for these products are
being pursued primarily in overseas markets.
Products
Roadmap
Safety
Shot plans to launch initially online and through Amazon in the near future and plans to launch in Big Box stores in 2024.
The
Company is advancing several formulations to address psoriasis and vitiligo (Photocil), increase the effectiveness of minoxidil to treat
hair loss (JW-700 “minoxidil booster”), women’s sexual wellness (JW-500), and jellyfish sting prevention sunscreen
(NoStingz), and atopic dermatitis/eczema (JW- 110).
Photocil
was launched commercially in India in Q3 2022 as a treatment for vitiligo and psoriasis. Photocil is a topical cream that works with
natural sunlight to provide patients with safe and effective phototherapy at home by blocking harmful radiation and permitting the passage
of therapeutic UV radiation from the sun.
NoStingz
provides an effective barrier against the stinging mechanism of jellyfish cnidocyte preventing the delivery of venom to the victim. Applied
like other topical sun screen products, the product is clinically proven to protect users from jellyfish, sea lice, and UVA/UVB rays.
JW-700,
currently being licensed abroad and developed for US launch, the product has been clinically shown to increase the enzymes needed for
minoxidil to work, sulfotransferase enzymes, by using the product topically in conjunction with topical minoxidil. Additional studies
and formulation work are ongoing.
JW-500
was born out of clinical trials designed to establish a topical treatment for the restoration of nipple sensitivity for breast augmentation
patients, in addition to patients who had undergone chemotherapy or lumpectomy surgery following a cancer diagnosis. During early studies,
women reported not only increased sensitivity but also increased libido. The Company plans to file for a pre-IND meeting with the US
FDA and seek Orphan Drug Designation. An expedited 505(b)(2) regulatory pathway for development is being considered as the current formulation
contains an already approved drug.
Research
and Development
Our
research and development team is continually looking to develop new therapeutic products, while continually improving and enhancing our
existing products and product candidates to address customer demands and emerging trends. Our team is currently working to further improve
the protection provided by NoStingz and develop more effective formulas for our JW-700 product .
We
have conducted extensive research and experimentation involving a substantial number of subjects under the influence of intoxicants.
Our findings indicate that the safety shot has proven to completely inhibit alcohol absorption. Furthermore, in diverse cases, it has
demonstrated a reduction in Blood Alcohol Content, as measured by the premier Breathalyzer in the market. The observable enhancements
in cognitive abilities among the test subjects have been meticulously documented.
Currently,
we are actively engaged in a double-blinded clinical trial, with anticipated completion by the first quarter of 2024. The outcomes of
this trial will be subjected to publication, providing comprehensive insights into the efficacy and potential of our safety shot.
Sales
and Marketing
We
primarily sell our products through third-party physical retail stores and partners who license and distribute them to other markets.
Currently, our products are licensed for distribution in over 31 countries. The majority of our sales occur via traditional physical
retailers, including their websites. We also sell via online retailers, such as Amazon and Walmart. To drive loyalty, word-of-mouth marketing,
and sustainable growth, we invest in customer experience and customer relationship management. Our marketing investments are directed
towards driving profitable growth through advertising, public relations, and brand promotion activities, including digital platforms,
sponsorships, collaborations, brand activations, and channel marketing. Additionally, we continue to invest in our marketing and brand
development efforts by investing capital expenditures on product displays to support our channel marketing via our retail partners.
Manufacturing,
Logistics and Fulfillment
We
outsource the manufacturing of our products to contract manufacturers, who produce them according to our formulation specifications.
Our products are manufactured by contract manufacturers in India and the US. The majority of our products will then be shipped to third-party
warehouses and to our corporate offices, which can either transport them to our distributors, retailers, or directly to our customers.
Our third-party warehouses are located in the US. We use a limited number of logistics providers to deliver our products to both distributors
and retailers, which allows us to lessen order fulfillment time, cut shipping costs, and improve inventory flexibility.
SRM
Entertainment
On
December 9, 2022, the Company entered into a stock exchange agreement (the “Exchange Agreement”) with SRM Entertainment,
Inc. (“SRM”) to govern the separation of SRM the Company. On May 26, 2023, we amended and restated the Exchange Agreement
(the “Amended and Restated Exchange Agreement”) to include additional information regarding the distribution and the separation
of SRM and the Company. The separation as set forth in the Amended and Restated Exchange Agreement with the Company closed August 14,
2023. Pursuant to the Amended and Restated Exchange Agreement, on May 31, 2023, SRM issued to the Company 6,500,000 shares of SRM Common
Stock (representing 79.3% of SRM’s outstanding shares of Common Stock) in exchange for 2 ordinary shares of SRM Ltd owned by the
Company (representing all of the issued and outstanding ordinary shares of SRM) (the “Share Exchange”). On August 14, 2023,
SRM consummated its Initial Public Offering (“IPO”), pursuant to which it sold 1,250,000 shares of its common stock at a
price of $5.00 per share. In connection with the Share Exchange and SRM’s IPO, the Company distributed 2,000,000 shares of SRM’s
common stock to the Company’s stockholders and certain warrant holders (out of the 6.5 million shares issued in May 2023) which
occurred on the effective date of the Registration Statement but prior to the closing of the IPO. Following such distribution, the Company
owns 4.5 million of the 9,450,000 shares of common stock outstanding and SRM is now a minority owned subsidiary of the Company.
Competitive
Strengths
We
are committed to driving continuous improvement through innovation. Since our inception, we have made significant investments in research
and development and have acquired a substantial portfolio of intellectual property, which continues to grow each year. Our commitment
to innovation has allowed us to create unique products that address unmet needs in the market, all backed by rigorous clinical research.
Our focus on research and development has enabled us to stay ahead of the curve and provide our customers with products that are not
only effective but also innovative. We take pride in our patent portfolio and the continuous growth we have achieved, as it showcases
our dedication to creating new and unique solutions for our customers. By staying committed to innovation, we are confident in our ability
to meet the ever-changing needs of the market and continue to be a leading player in the wellness industry.
Recent
Developments
On
January 19, 2023, the Company entered into a Securities Purchase Agreement (the “PIPE Agreement”) with certain purchasers,
for the issuance of 8,631,574 common stock warrants (the “PIPE Offering”) at a price of $0.125 per warrant, comprised of
two common stock warrants (the “Common Warrants,”), each to purchase up to one share of Common Stock per Common Warrant with
an exercise price of $1.00 per share, with (a) 4,315,787 Common Warrants being immediately exercisable for three years following 6 months
from the closing of the PIPE Offering, and (b) 4,315,787 Common Warrants being immediately exercisable for five years following 6 months
from the closing of the PIPE Offering. Concurrently to the PIPE Agreement, the Company entered into a Securities Purchase Agreement (the
“RD Agreement”) with certain purchasers, pursuant to which on January 23, 2023, 4,315,787 shares of common stock, par value
$0.001 (the “Common Stock”), at a price of $0.70 per share were issued to the purchasers (the “RD Offering”).
The Common Stock was issued pursuant to a Registration Statement on Form S-3 filed by the Company with the Securities and Exchange Commission
(the “Commission”) on September 28, 2022 (File No. 333-267644) and declared effective on November 9, 2022. The aggregate
gross proceeds to the Company from both the PIPE Offering and the RD Offering were approximately $4.1 million, with the purchase price
of one share, one 3- year warrant and one 5-year warrant as $0.95. The net proceeds were $3,450,675.
On
March 31, 2023 the Company entered into a Financial Advisory Agreement (“FSA”) with Greentree Financial Group, Inc. to render
certain professional services to the Company. In connection with the FSA, the Company issued 500,000 restricted shares of its common
stock to Greentree.
On
July 10, 2023, the Company entered into an asset purchase agreement (the “Agreement”) with GBB Labs, Inc., a Delaware corporation
(“Buyer”), GBB Drink Lab Inc., a Florida corporation (“Seller”), 2V Consulting LLC, a Florida limited liability
company, the Jarrett A Boon Revocable Trust Dated October 22, 2014, Gregory D. Blackman, an individual and Brothers Investment 7777.
Pursuant to the Agreement, the Buyer shall purchase certain assets relating to the Seller’s business for a consideration comprising
of: (a) the sum of Two Hundred Thousand U.S. Dollars (US $200,000) (the “Cash Purchase Price”); and (b) 5,000,000 Common
Shares (the “Consideration Shares” and together with the Cash Purchase Price, collectively, the “Purchase Price”).
The acquisition was closed on August 31, 2023.
Intellectual
Property
We
filed Provisional Patent (CBD Formulations and Uses Thereof: USAN: 62/884,995) on a combination of CBD and Aspartame on August 8, 2019.
The patent is to cover any products that contain a combination of CBD and Aspartame. This initially will cover the products under the
CaniDermRX Brand. The provisional patent application was converted into a full US patent application (No.: 16/987,941) and PCT application
(PCT/US2020/045408I) on August 9, 2020. If issued, the patent will give patent protection until 2040.
We
filed Provisional Patent (CBD Sunscreen Formulations and Uses Thereof: USAN: 63/005,854) on our CBD-infused sunscreen products on August
6, 2020. The patent is to cover any products under our CaniSun product line that contains CBD. The priority date starts at the time the
provisional is converted into a full patent application, which will occur on April 6, 2021. If issued, the patent will give patent protection
until 2041.
We
filed Provisional Patent (Oroanasal CBD formulations and uses thereof (No.: 63/042,458) on June 22, 2020. This covers the use of CBD
products for the treatment of respiratory viruses.
As
of the date, the Company owns two patents in relation to the over the counter drinks.
Government
Regulation
Since
1937, Cannabis sativa L. has been a federally regulated Schedule I drug under the Controlled Substances Act, 21 U.S.C. § 811 (the
“CSA”), regulated by the Drug Enforcement Agency (the “DEA”).
It
was not until 2014 when a distinction between the use of Cannabis sativa L. for medical, recreational, and industrial purposes was made
via Section 7606 of the Agricultural Act of 2014, which cleared a legal path for industrial hemp to be grown in three limited circumstances,
1) by researchers at an institute of higher education, 2) by state departments of agriculture, or 3) by farmers participating in a research
program permitted and overseen by a state department of agriculture.
In
2016, the DEA, U.S. Department of Agriculture, and the FDA issued a joint statement detailing the guidelines for growth of industrial
hemp as part of state-sanctioned research programs. Those guidelines state that hemp can only be sold in states with pilot programs,
plants and seeds can only cross state lines as part of permitted state research programs, and seeds can only be imported by individuals
registered with the DEA.
We
believe the recent passage of the Farm Bill will allow us to expand our marketplace opportunities. On December 20, 2018, President Donald
J. Trump signed into law the Agriculture Improvement Act of 2018, otherwise known as the “Farm Bill”. Prior to its passage,
hemp, a member of the cannabis family, and hemp-derived CBD were classified as a Schedule I controlled substances, and so were deemed
to be illegal under the CSA. With the passage of the Farm Bill, hemp cultivation is broadly permitted. The Farm Bill explicitly allows
the transfer of hemp-derived products across state lines for commercial or other purposes. It also puts no restrictions on the sale,
transport, or possession of hemp-derived products, so long as those items are produced in a manner consistent with the law.
Under
Section 10113 of the Farm Bill, hemp cannot contain more than 0.3 percent THC. THC refers to the chemical compound found in cannabis
that produces the psychoactive “high” associated with cannabis. Any cannabis plant that contains more than 0.3 percent THC
would be considered non-hemp cannabis—or marijuana—under federal law and would thus face no legal protection under this new
legislation and would be an illegal Schedule 1 drug under the CSA.
Additionally,
there will be significant, shared state-federal regulatory power over hemp cultivation and production. Under Section 10113 of the Farm
Bill, state departments of agriculture must consult with the state’s governor and chief law enforcement officer to devise a plan
that must be submitted to the Secretary of the United States Department of Agriculture or USDA. A state’s plan to license and regulate
hemp can only commence once the Secretary of USDA approves that state’s plan. In states opting not to devise a hemp regulatory
program, USDA will construct a regulatory program under which hemp cultivators in those states must apply for licenses and comply with
a federally run program. This system of shared regulatory programming is similar to options states had in other policy areas such as
health insurance marketplaces under the Affordable Care Act, or workplace safety plans under Occupational Health and Safety Act—both
of which had federally-run systems for states opting not to set up their own systems.
The
Farm Bill outlines actions that are considered violations of federal hemp law (including such activities as cultivating without a license
or producing cannabis with more than 0.3% THC). The Farm Bill details possible punishments for such violations, pathways for violators
to become compliant, and even which activities qualify as felonies under the law, such as repeated offenses.
One
of the goals of the Agricultural Act of 2014 was to generate and protect research into hemp. The Farm Bill continues this effort. Section
7605 re-extends the protections for hemp research and the conditions under which such research can and should be conducted. Further,
section 7501 of the Farm Bill extends hemp research by including hemp under the Critical Agricultural Materials Act. This provision recognizes
the importance, diversity, and opportunity of the plant and the products that can be derived from it, but also recognizes that there
is still a lot to learn about hemp and its products from commercial and market perspectives.
Overall,
we believe that our sunscreen products comply with the FDA Final Rule for sunscreen products under 21 CFR 352 Sunscreen products for
Over-the-Counter Human Use. Therefore, we believe that our sunscreen products fall within the FDA monograph and that FDA premarket approval
and testing is not required. Our products have been tested for SPF Evaluation (SPF rating), Critical Wave Length (Broad Spectrum claim)
and Water Resistance, each of which is defined within the monograph and labeled accordingly.
Our
products are tested each time they are manufactured. DCR Labs manufactures our products and is compliant with the FDA’s Current
Good Manufacturing Practice (“CGMP”) regulations in accordance with 21 CFR 210/211 (required for Over-the-Counter drug products).
DCR Labs has self-imposed health and safety standards to ensure compliance with the FDA’s CGMPs.
FDA
Regulation of Hemp Extracts
The
FDA is generally responsible for protecting the public health by ensuring the safety, efficacy, and security of (1) prescription and
over the counter drugs; (2) biologics including vaccines, blood & blood products, and cellular and gene therapies; (3) foodstuffs
including dietary supplements, bottled water, and baby formula; and, (4) medical devices including heart pacemakers, surgical implants,
prosthetics, and dental devices.
Regarding
its regulation of drugs, the FDA process requires a review that begins with the filing of an investigational new drug (IND) application,
with follow-on clinical studies and clinical trials that the FDA uses to determine whether a drug is safe and effective, and therefore
subject to approval for human use by the FDA.
Aside
from the FDA’s mandate to regulate drugs, the FDA also regulates dietary supplement products and dietary ingredients under the
Dietary Supplement Health and Education Act of 1994. This law prohibits manufacturers and distributors of dietary supplements and dietary
ingredients from marketing products that are adulterated or misbranded. This means that these firms are responsible for evaluating the
safety and labeling of their products before marketing to ensure that they meet all the requirements of the law and FDA regulations,
including, but not limited to the following labeling requirements: (1) identifying the supplement; (2) nutrition labeling; (3) ingredient
labeling; (4) claims; and, (5) daily use information.
The
FDA has not approved cannabis, marijuana, hemp or derivatives as a safe and effective drug for any indication. We intend to file an IND
with the FDA for our CaniDermRX products in the event the pending provisional patent on an Aspartame/CBD combination is approved. As
of the date hereof, our products containing CBD derived from industrial hemp are not marketed or sold using claims that their use is
a safe and effective treatment for any medical condition subject to the FDA’s jurisdiction.
The
FDA has concluded that products containing cannabis or industrial hemp derived CBD are excluded from the dietary supplement definition
under sections 201(ff)(3)(B)(i) and (ii) of the U.S. Food, Drug & Cosmetic Act, respectively. The FDA’s position is that products
containing cannabis, CBD or derivatives are Schedule 1 drugs under the Controlled Substances Act, and so are illegal. Our products containing
CBD derived from industrial hemp are not marketed or sold as dietary supplements. However, at some indeterminate future time, the FDA
may choose to generally change its position concerning products containing hemp derived CBD, and may choose to enact regulations that
are applicable to such products. In this event, our industrial hemp based products containing CBD may be subject to regulation.
Our
products contain controlled substances as defined in the Controlled Substances Act (CSA). Controlled substances that are pharmaceutical
products are subject to a high degree of regulation under the CSA, which establishes, among other things, certain registration, manufacturing
quotas, security, recordkeeping, reporting, import, export and other requirements administered by the DEA.
Despite
recent approvals by the FDA and DEA for a newly approved medication which contains cannabidiol (CBD), the scheduling of these substances,
many of which are beyond our control, could jeopardize our ability to obtain regulatory approval for and successfully market our products.
Any such setback in our pursuit of regulatory approval would have a material adverse effect on our business and prospects.
FDA
Regulation of CBD
On
June 25, 2018 the US Federal Drug Administration (FDA) approved Epidiolex. Epidiolex is the first and only FDA-approved prescription
cannabidiol (CBD). It is approved to treat seizures associated with Lennox-Gastaut syndrome (LGS), Dravet syndrome, or tuberous sclerosis
complex (TSC) in patients 1 year of age and older. Accordingly, the FDA has designated CBD as a drug and the need for all marketed products
to follow FDA guidelines for safety and efficacy. It is not yet clear how this will affect thousands of CBD products already on the market
given the multitude of state and local regulations that cover this field.
Employees
As
of this prospectus, we had eight full-time employees. We believe our relations with our employees to be good.
Properties
Currently,
we do not own any real property. We rent an office space at 1061 E. Indiantown Rd., Ste. 110, Jupiter, FL 33477 for $15,038 per month.
The Company entered into the office lease effective July 1, 2021, which has a primary term of the lease of five years with one renewal
option for an additional three years.
THE
OFFERING
| Common
stock outstanding |
|
41,422,339
shares. (1) |
| |
|
|
| Common
stock being offered |
|
550,000
shares of common stock and 1,428,796 shares issuable upon the exercise of outstanding Warrants. |
| |
|
|
| Use
of proceeds |
|
The
gross proceeds if all the warrant holders, as of the date of this prospectus, exercise their Warrants will be approximately $1,328,778;
however, we are unable to predict the timing or amount of potential warrant exercises. All of such proceeds will be used for
research and development studies and the patent and legal costs associated thereto, and for general working capital purposes. It
is possible that some of the Warrants may expire and never be exercised. |
| |
|
|
| Nasdaq
symbols |
|
Our
common stock are listed on the Nasdaq Capital Market under the symbols “SHOT.” |
| |
|
|
| Risk
factors |
|
You
should carefully consider the information set forth in this prospectus and, in particular, the specific factors set forth in the
“Risk Factors” section in the Form 10-K and Form S-1 incorporated herein by reference before deciding whether or not
to invest in common stock. |
| (1) |
As
of November 21, 2023, this number excludes the approximately 34,919,809 shares of common stock issuable upon exercise
of outstanding warrants and options. |
RISK
FACTORS
Investing
in our securities involves a high degree of risk. Before making an investment decision, you should carefully consider any risk factors
set forth in the applicable prospectus supplement and the documents incorporated by reference in this prospectus, including the factors
discussed under the heading “Risk Factors” in our (i) most recent Form 10-K for the year ended December 31, 2022, as filed
with the SEC on April 3, 2023; and (ii) the Registration Statement on Form S-1, filed with SEC on June 22, 2023, and declared effective
on July 3, 2023, as updated by our subsequent annual, quarterly and other reports and documents that are incorporated by reference into
this prospectus. See “Where You Can Find More Information” and “Information We Incorporate By Reference.” Each
of the risks described in these documents could materially and adversely affect our business, financial condition, results of operations
and prospects, and could result in a partial or complete loss of your investment. Additional risks and uncertainties not presently known
to us, or that we currently deem immaterial, may also adversely affect our business. In addition, past financial performance may not
be a reliable indicator of future performance and historical trends should not be used to anticipate results or trends in future periods.
USE
OF PROCEEDS
All
shares of our common stock offered by this prospectus are being registered for the account of the selling stockholder and we will not
receive any proceeds from the sale of shares of our common stock by the selling stockholder. However, we will receive proceeds from the
exercise of the Warrants and Options. Unless otherwise specified in the applicable prospectus supplement, we intend to use these proceeds,
if any, for general working capital purposes.
SELLING
STOCKHOLDER
This
prospectus relates to the possible resale by the selling stockholder from time to time of up to an aggregate of 1,978,796 shares
of our common stock. When we refer to the “selling stockholder” in this prospectus, we mean the stockholder listed in the
table below and the donees, pledgees, transferees, assignees or other successors-in-interest and others who later come to hold any of
the selling stockholder’s interest in shares of our common stock covered by this prospectus.
The
following table sets forth, as of the date of this prospectus, the name of the selling stockholder and the aggregate amount of shares
of common stock that the selling stockholder may offer pursuant to this prospectus. Information with respect to beneficial ownership is based on information
obtained from such selling stockholder and publicly available information. Information with respect to shares beneficially owned after
the offering assumes the sale of all of the shares offered and no other purchases or sales of common stock.
| Name
of Selling Stockholder |
|
Number
of shares of Common Stock Owned Prior to Offering (7) |
|
|
Maximum
Number of shares of Common Stock to be Sold Pursuant to this Prospectus |
|
|
Number
of shares of Common Stock Owned After Offering |
|
| District
2 Capital Fund LP(1) |
|
|
316,008 |
|
|
|
46,010 |
|
|
|
316,008 |
|
| Bigger
Capital fund, LP(2) |
|
|
1,706,050 |
|
|
|
46,010 |
|
|
|
1,706,050 |
|
| Intracoastal
Capital LLC(3) |
|
|
871,656 |
|
|
|
38,342 |
|
|
|
833,314 |
|
| Armistice
Capital, LLC(4) |
|
|
3,203,348 |
|
|
|
153,364 |
|
|
|
3,203,348 |
|
| Hudson
Bay Master Fund Ltd.(5) |
|
|
564,655 |
|
|
|
38,342 |
|
|
|
564,655 |
|
| Sabby
Volatility Warrant Master Fund, Ltd.(6) |
|
|
805,806 |
|
|
|
506,728 |
|
|
|
805,806 |
|
| Greentree
Financial Group, Inc.(8) |
|
|
1,889,167 |
|
|
|
862,500 |
(9) |
|
|
1,889,167 |
|
| L&H,
Inc.(10) |
|
|
416,135 |
|
|
|
287,500 |
(11) |
|
|
416,135 |
|
| |
(1) |
The
shares are beneficially owned by Michael Bigger as the Managing Member of District 2 Capital Fund LP |
| |
(2) |
The
shares are beneficially owned by Michael Bigger as the Managing Member of Bigger Capital fund, LP |
| |
(3) |
Mitchell
P. Kopin (“Mr. Kopin”) and Daniel B. Asher (“Mr. Asher”), each of whom are managers of Intracoastal Capital
LLC (“Intracoastal”), have shared voting control and investment discretion over the securities reported herein that are
held by Intracoastal. As a result, each of Mr. Kopin and Mr. Asher may be deemed to have beneficial ownership (as determined under
Section 13(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) of the securities reported herein
that are held by Intracoastal. |
| |
(4) |
The
securities are directly held by Armistice Capital Master Fund Ltd., a Cayman Islands exempted company (the “Master Fund”),
and may be deemed to be beneficially owned by: (i) Armistice Capital, LLC (“Armistice Capital”), as the investment manager
of the Master Fund; and (ii) Steven Boyd, as the Managing Member of Armistice Capital. The warrants are subject to a beneficial
ownership limitation of 4.99%, which such limitation restricts the Selling Stockholder from exercising that portion of the warrants
that would result in the Selling Stockholder and its affiliates owning, after exercise, a number of shares of Common Stock in excess
of the beneficial ownership limitation. The amounts and percentages in the table do not give effect to the beneficial ownership limitations.
The address of the Master Fund is c/o Armistice Capital, LLC, 510 Madison Ave, 7th Floor, New York, NY 10022 |
| |
(5) |
Hudson
Bay Capital Management LP, the investment manager of Hudson Bay Master Fund Ltd., has voting and investment power over these securities.
Sander Gerber is the managing member of Hudson Bay Capital GP LLC, which is the general partner of Hudson Bay Capital Management
LP. Each of Hudson Bay Master Fund Ltd. and Sander Gerber disclaims beneficial ownership over these securities |
| |
(6) |
Sabby
Management, LLC, in its capacity as the investment manager of Sabby Volatility Warrant Master Fund, Ltd., has the power to vote and
the power to direct the disposition of all securities held by Sabby Volatility Warrant Master Fund, Ltd. Hal Mintz is the Managing
Member of Sabby Management, LLC. Each of Sabby Volatility Warrant Master Fund, Ltd., Sabby Management, LLC and Mr. Mintz disclaim
beneficial ownership of these securities, except to the extent of any pecuniary interest therein. |
| |
(7) |
Includes
shares of common stock issuable upon the exercise of the outstanding warrants, as applicable. |
| |
(8) |
Robert C Cottone, vice president of Greentree Financial
Group, Inc. (“Greentree”), has voting control and investment discretion over the securities reported herein that are
held by Greentree. |
| |
(9) |
Includes 600,000 shares of common stock issuable upon
the exercise of the Warrants. |
| |
(10) |
Linwen Huang, president of L&H, Inc. (“L&H”),
has voting control and investment discretion over the securities reported herein that are held by L&H. |
| |
(11) |
Includes 200,000 shares of common stock issuable upon
the exercise of the Warrants. |
DESCRIPTION
OF CAPITAL STOCK
The
following description of the Company’s capital stock is a summary and does not purport to be complete. It is subject to and qualified
in its entirety by reference to the Company’s Amended and Restated Certificate of Incorporation and Amended and Restated By-laws,
copies of which are incorporated by reference as exhibits to the registration statement of which this prospectus is a part.
Authorized
Capital
Our
authorized capital stock consists of 100,000,000 shares of common stock, par value $0.001 per share, and 100,000 shares of preferred
stock, par value $0.001 per share.
Common
Stock
Common
stock outstanding
As
of November 21, 2023, there were 41,422,339 shares of our common stock outstanding.
Voting
rights
Subject
to the rights granted to holders of any preferred stock issued by us, each share of common stock entitles the holder to one vote, either
in person or by proxy, at meetings of stockholders. The holders are not permitted to vote their shares cumulatively.
Dividend
rights
Subject
to the rights granted to holders of any preferred stock issued by us, holders of common stock are entitled to receive ratably such dividends,
if any, as may be declared by the Board out of funds legally available.
Rights
upon liquidation
Subject
to the rights granted to holders of any preferred stock issued by us, upon our liquidation, dissolution or winding up, the holders of
our common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment
of all of our debts and other liabilities.
Other
rights
Holders
of our common stock do not have any pre-emptive rights or other subscription rights, conversion rights, redemption or sinking fund provisions.
Preferred
Stock
Under
the terms of our second amended and restated certificate of incorporation, our Board is authorized to issue shares of preferred stock
in one or more series without stockholder approval. Our Board has the discretion to determine the rights, preferences, privileges and
restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each
series of preferred stock.
The
purpose of authorizing our Board to issue preferred stock and determination its rights and preferences is to eliminate delays associated
with a stockholder vote on specific issuances. The issuance of preferred stock, while providing flexibility in connection with possible
acquisitions, future financings and other corporate purposes, could have the effect of making it more difficult for a third party to
acquire, or could discourage a third party from seeking to acquire, a majority of our outstanding voting stock.
Warrants
During
2020, the Company issued a total of 1,123,333 warrants, with each warrant to purchase one share of common stock, consisting of 1,073,333
warrants issued in connection with the Company’s initial public offering at an exercise price of $8.50 per share, expiring in October
2025, and 50,000 warrants issued in connection with the Endorsement Agreement with Tee-2-Green at an exercise price of $3.90, expiring
in November 2025.
During
2021, the Company issued 525,001 warrants in relation to loans amounting to $3,150,000 to the Company issued by the investors. As of
the date of this prospectus there are 1,648,334 warrants outstanding. The exercise price of these warrants was later adjusted to $0.93
per share. In addition, the Company issued 11,607,142 warrants to purchase common stock of the under public offering on July 21, 2021.These
are the warrants for which the underlying shares are being re-registered hereunder. The exercise price of these warrants was later adjusted
to $1.40 per share.
During
the year ended December 31, 2022, the Company issued a total of 2,260,000 warrants with an exercise price of between $1.00 and $2.79
and five year terms in connection with two convertible promissory notes, and during 2021 in connection with the issuance of three convertible
promissory notes, the Company issued 525,000 warrants with an exercise price of $6.00 and five-year term. The exercise price of these
warrants was later adjusted to $0.93 per share.
Options
During
2020, certain Directors and a consultant were granted stock options to purchase a total of 211,330 additional shares of the Company’s
common stock. The options have a three-year term with an exercise price between $0.25 and $4.49. On January 25, 2021, the Company issued
20,000 options with an exercise price of $5.59 (market price) and a three-year term to Nancy Kaufman, as a new director. On February
25, 2021 the Company issued 33,330 options, pursuant to Dr. Alila’s agreement, with an exercise price of $0.25 with a three-year
term. The relative fair value of the 2020 options using the Black-Scholes valuation model totals $75,645. As of March 31, 2021, the Company
had 355,990 options outstanding. Subsequent to March 31, 2021, the Company issued 251,526 options to its officers.
On
December 30, 2022, the Company, in connection with the 2022 Equity Incentive Plan, granted the directors and officers of the Company
options to purchase shares of common stock. The table below shows the options granted to each director and officers, and their respective
terms.
| Name | |
Options | | |
Exercise Price | | |
Term | |
| Brian S John | |
| 1,050,000 | | |
$ | 0.836 | | |
| Five years from the grant date | |
| Dr. Glynn Wilson | |
| 1,050,000 | | |
$ | 0.7600 | | |
| Five years from the grant date | |
| Doug McKinnon | |
| 500,000 | | |
$ | 0.7600 | | |
| Five years from the grant date | |
| Christopher Melton | |
| 50,000 | | |
$ | 0.7600 | | |
| Five years from the grant date | |
| Dr. Skander Fani | |
| 50,000 | | |
$ | 0.7600 | | |
| Five years from the grant date | |
| Nancy Torres Kauffman | |
| 50,000 | | |
$ | 0.7600 | | |
| Five years from the grant date | |
| Gary Hermann | |
| 50,000 | | |
$ | 0.7600 | | |
| Five years from the grant date | |
In
addition to the directors and officers, on December 30, 2022, the Company granted 100,000 options to purchase shares of common stock,
at an exercise price of $0.7600 and a five year term, to Mesers. Markita Russell, Paul Jones and Zachary Greave, each. The company also
granted 50,000 options to purchase shares of common stock, at an exercise price of $0.7600 and a five year term, to Mesers. Michelle
Basantes, George Hall, and Dr. Hector Alia.
During
the year ended December 31, 2022 the Company entered into an Investor Relations Consulting Agreement under the terms of which the Company
issued 300,000 two-year options, immediately vested, with an exercise price of $1.00. The Company recorded an expense of $142,169 in
connection with this issuance.
The
fair value of these warrants was measured using the Black-Scholes valuation model at the grant date. The table below sets forth the assumptions
for Black-Scholes valuation model on the respective reporting date.
| |
|
|
|
|
|
|
|
|
|
Market |
|
|
|
|
|
|
|
| |
|
Number |
|
|
|
|
|
|
|
Price |
|
|
|
|
|
|
|
| Reporting |
|
of |
|
|
Term |
|
Exercise |
|
|
on
Grant |
|
|
Volatility |
|
|
Fair |
|
| Date |
|
Options |
|
|
(Years) |
|
Price |
|
|
Date |
|
|
Percentage |
|
|
Value |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 1/01/21
– 6/30/21 |
|
|
306,730 |
|
|
3 |
|
$ |
0.25-5.59 |
|
|
$ |
3.78-5.59 |
|
|
|
148
209 |
% |
|
$ |
1,244,179 |
|
| 7/1/21-9/30/21 |
|
|
777,220 |
|
|
5 |
|
$ |
1.77 |
|
|
$ |
1.58 |
|
|
|
127 |
% |
|
$ |
816,158 |
|
| 10/01/21
– 12/31/21 |
|
|
3,300,000 |
|
|
3 |
|
$ |
1.30 |
|
|
$ |
1.30 |
|
|
|
129 |
% |
|
$ |
2,983,393 |
|
| 01/01/22 |
|
|
300,000 |
|
|
2 |
|
$ |
1.00 |
|
|
$ |
0.80 |
|
|
|
126 |
% |
|
$ |
142,169 |
|
| 12/30/2022 |
|
|
3,250,000 |
|
|
3 |
|
$ |
0.76 |
|
|
|
0.76 |
|
|
|
166 |
% |
|
$ |
2,026,122 |
|
During
the year ended December 31, 2022, the Company cancelled a total of 211,000 options to management and reallocated these to cover shares
of the Company’s stock to be issued under the Company’s Incentive Stock Plan.
During
the year ended December 31, 2022, the Company recognized $2,048,270 as compensation expense related to the option grants. At December
31, 2022 and 2021, the Company had 8,134,280 and 4,584,280 options outstanding, respectively.
2021
Private Placement Notes and Warrants
On
May 11, 2021, we entered into a loan agreement (the “May 11 Loan Agreement”), pursuant to which we sold approximately $2,500,000
of notes (the “May 11 Notes”) and 416,667 warrants at an exercise price of $6.00.
On
May 24, 2021, we entered into a loan agreement (the “May 24 Loan Agreement”), pursuant to which we sold approximately $150,000
of notes (the “May 24 Notes”) and 25,000 warrants at an exercise price of $6.00 per share.
On
May 28, 2021, we entered into a loan agreement (the “May 28 Loan Agreement, with the May 11 Loan Agreement and the May 24 Loan
Agreement, collectively as “2021 Loan Agreements”), pursuant to which we sold approximately $500,000 of notes (the “May
28 Notes,” collectively with May 11 Notes and May 24 Notes as the “2021 Notes”) and 83,334 warrants at an exercise
price of $6.00 per share.
The
2021 Notes have a six months term and are convertible into shares of Common Stock of the Company at $6.00 per share. Interest shall accrue
on the notes at 8% annually, payable on a quarterly basis. The 2021 Notes held by a particular holder will not be convertible to the
extent such conversion would result in such holder owning more than 4.99% of the number of Common Stock outstanding after giving effect
to the issuance of Common Stock issuable upon conversion of such note calculated in accordance with Section 13(d) of the Exchange Act.
Upon not less than sixty-one (61) days advance written notice, at any time or from time to time, the holder at its sole discretion, may
waive the 4.99% conversion limit. However, under any circumstance, the holder may not convert the 2021 Note if such conversion would
cause holder’s beneficial ownership (as defined by Section 13(d) of the Securities Exchange Act of 1934, as amended) of the Company
to exceed 9.99% of its total issued and outstanding common or voting shares. Any common shares converted under the 2021 Note need to
be delivered to the holder within three (3) business days of the receipt of conversion notice.
The
warrants are exercisable immediately for a period of five years for cash, at an exercise price of $6.00 per share of Common Stock. The
warrants held by a particular holder will not be exercisable to the extent such conversion would result in such holder owning more than
4.99% of the number of shares of Common Stock outstanding after giving effect to the issuance of Common Stock issuable upon exercise
of such warrants calculated in accordance with Section 13(d) of the Exchange Act. Upon not less than sixty-one (61) days advance written
notice, at any time or from time to time, the warrant holder at its sole discretion, may waive the 4.99% ownership limit. However, under
any circumstance, the warrant holder may not exercise the warrant if such exercise would cause such Warrant holder’s beneficial
ownership (as defined by Section 13(d) of the Securities Exchange Act of 1934, as amended) of the Common Stock of the Company to exceed
9.99% of its total issued and outstanding Common Stock or voting shares.
Pursuant
to the 2021 Loan Agreements, 2021 Notes and warrants we agreed to file the registration statement of which this prospectus forms a part
with the SEC and to cause such registration statement to become effective as promptly as practicable after filing, and are required to
cause such registration statement to remain effective until the Common Stock offered hereby have been sold or may be freely sold without
limitations or restrictions as to volume or manner of sale pursuant to Rule 144 under the Securities Act. The exercise price of the
warrants was reduced to $0.93 per share.
2022
Private Placement Notes and Warrants
On
April 20, 2022, we entered into the Greentree Loan, pursuant to which we sold approximately $1,500,000 of Greentree Notes and 1,100,000
Greentree Warrants at an exercise price of $2.79.
On
April 20, 2022, we entered into the L&H Loan, pursuant to which we sold approximately $500,000 of L&H Notes and 360,000 L&H
Warrants at an exercise price of $2.79.
The
Notes have an original issuance discount of five percent (5%), an interest rate of eight percent (8%), and a conversion price of $2.79
per share, subject to an adjustment downward if the Company is in default of the terms of the Notes. Provided, the Notes may be converted
at a default price of $1.00 per share in the event of default as stated therein. The Warrants have a five (5) year term, an exercise
price of $2.79 per share, have a cashless conversion feature until such time as the shares underlying the Warrants are included in an
effective registration and certain anti-dilution protection. In connection with the 2023 private placement described immediately below
the exercise price of the Warrants and the conversion price of the Notes was reduced to $0.93 per share.
Pursuant
to the Loan Agreements, Notes and warrants we agreed to file the registration statement of which this prospectus forms a part with the
SEC and to cause such registration statement to become effective as promptly as practicable after filing, and are required to cause such
registration statement to remain effective until the Common Stock offered hereby have been sold or may be freely sold without limitations
or restrictions as to volume or manner of sale pursuant to Rule 144 under the Securities Act.
2023
Private Placement of Warrants
On
January 19, 2023, the Company entered into the PIPE Agreement with certain purchasers, for the issuance of 8,631,574 common stock warrants
comprising of two common stock warrants, each to purchase up to one share of Common Stock per Common Warrant with an exercise price of
$1.00 per share, with (a) 4,315,787 warrants being immediately exercisable for two and one-half years following 6 months from the closing
of the PIPE Offering, and (b) 4,315,787 warrants being immediately exercisable for four and one-half years following 6 months from the
closing of the PIPE Offering. As a result of the spin off of SRM, these exercise price of these warrants adjusted to $0.93 per share
and the amount of warrants adjusted to an aggregate of 9,218,521 warrants. We are registering herein, 586,947 warrants issued as a result
of the spin off adjustment.
Pursuant
to the PIPE Agreements, registration rights agreement and the Warrants we agreed to file a registration statement and to cause such registration
statement to become effective as promptly as practicable after filing, and are required to cause such registration statement to remain
effective until the Common Stock offered hereby have been sold or may be freely sold without limitations or restrictions as to volume
or manner of sale pursuant to Rule 144 under the Securities Act. That registration statement became effective on July 3, 2023.
The
entire discussion regarding the securities PIPE Agreements, registration rights agreement and related agreements is qualified in its
entirety to the forms of such agreements which have been filed as exhibits to our Current Report on Form 8-K, filed with the SEC on January
25, 2023 which are incorporated by reference into the registration statement to which this prospectus forms a part.
Anti-Takeover
Effects
Our
second amended and restated certificate of incorporation and amended and restated bylaws will include a number of provisions that may
have the effect of delaying, deferring or preventing a party from acquiring control of us and encouraging persons considering unsolicited
tender offers or other unilateral takeover proposals to negotiate with our Board rather than pursue non-negotiated takeover attempts.
The provisions include the items described below.
Potential
Effects of Authorized but Unissued Stock
We
have shares of common stock available for future issuance without stockholder approval. We may utilize these additional shares for a
variety of corporate purposes, including future public offerings to raise additional capital, to facilitate corporate acquisitions or
payment as a dividend on the capital stock.
The
existence of unissued and unreserved common stock and preferred stock may enable our Board to issue shares to persons friendly to current
management or to issue preferred stock with terms that could render more difficult or discourage a third-party attempt to obtain control
of us by means of a merger, tender offer, proxy contest or otherwise, thereby protecting the continuity of our management. In addition,
our Board has the discretion to determine designations, rights, preferences, privileges and restrictions, including voting rights, dividend
rights, conversion rights, redemption privileges and liquidation preferences of each series of preferred stock, all to the fullest extent
permissible under the Delaware General Corporation Law and subject to any limitations set forth in our second amended and restated certificate
of incorporation. The purpose of authorizing the Board to issue preferred stock and to determine the rights and preferences applicable
to such preferred stock is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock,
while providing desirable flexibility in connection with possible financings, acquisitions and other corporate purposes, could have the
effect of making it more difficult for a third party to acquire, or could discourage a third party from acquiring, a majority of our
outstanding voting stock.
Limitations
of Director Liability and Indemnification of Directors, Officers and Employees
Our
second amended and restated certificate of incorporation limits the liability of directors to the maximum extent permitted by Delaware
law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary
duties as directors.
Our
amended and restated bylaws provide that we will indemnify our directors and officers to the fullest extent permitted by law, and may
indemnify employees and other agents. Our amended and restated bylaws also provide that we are obligated to advance expenses incurred
by a director or officer in advance of the final disposition of any action or proceeding.
We
currently do not have a policy of directors’ and officers’ liability insurance but intend to obtain such a policy in the
near future.
Our
amended and restated bylaws, subject to the provisions of Delaware Law, contain provisions which allow the corporation to indemnify any
person against liabilities and other expenses incurred as the result of defending or administering any pending or anticipated legal issue
in connection with service to us if it is determined that person acted in good faith and in a manner which he or she reasonably believed
was in the best interest of the corporation. Insofar as indemnification for liabilities arising under the Securities Act of 1933 as amended,
or the Securities Act, may be permitted to our directors, officers and controlling persons, we have been advised that in the opinion
of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act and is, therefore,
unenforceable.
The
limitation of liability and indemnification provisions in our amended and restated bylaws may discourage stockholders from bringing a
lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against
directors and officers, even though an action, if successful, might provide a benefit to us and our stockholders. Our results of operations
and financial condition may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant
to these indemnification provisions.
At
present, there is no pending litigation or proceeding involving any of our directors or officers as to which indemnification is required
or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
Requirements
for Advance Notification of Stockholder Nominations and Proposals
Our
amended and restated bylaws establish advance notice procedures with respect to stockholder proposals and nomination of candidates for
election as directors.
Limits
on Special Meetings
Special
meetings may be called for any purpose and at any time by the Chairman of the Board, the President (if there be one) or by any member
of the Board. Business transacted at each special meeting shall be confined to the purposes stated in the notice of such meeting.
Election
and Removal of Directors
Our
Board is elected annually by our stockholders. The number of directors that shall constitute the whole Board shall not be less than three
(3) nor more than seven (7) directors.
Directors
are elected by a plurality of the votes of shares of our capital stock present in person or represented by proxy at a meeting and entitled
to vote in the election of directors. Each director shall hold office until a successor is duly elected and qualified or until his or
her earlier death, resignation or removal.
Newly
created directorships resulting from any increase in the number of directors or any vacancies in the Board resulting from death, resignation,
retirement, disqualification, removal from office or any other cause may be filled, so long as there is at least one remaining director,
only by the Board, provided that a quorum is then in office and present, or by a majority of the directors then in office, if less than
a quorum is then in office, or by the sole remaining director. Directors elected to fill a newly created directorship or other vacancies
shall hold office until such director’s successor has been duly elected and qualified or until his or her earlier death, resignation
or removal as hereinafter provided.
Any
director may be removed from office at any time for cause, at a meeting called for that purpose, but only by the affirmative vote of
the holders of at least 66-2/3% of the voting power of all outstanding shares of our capital stock entitled to vote generally in the
election of directors, voting together as a single class.
Our
second amended and restated certificate of incorporation and amended and restated bylaws do not provide for cumulative voting in the
election of directors.
Amendments
to Our Governing Documents
The
affirmative vote of the holders of at least 66-2/3% of the voting power of all outstanding shares of our capital stock entitled to vote
generally in the election of directors, shall be required to adopt any provision inconsistent with, to amend or repeal any provision
of, or to adopt a bylaw inconsistent with, Articles Two, Seven, Eight and Nine of our Second Amended and Restated Certificate of Incorporation.
Our
amended and restated bylaws may be amended or repealed and new bylaws may be adopted by the stockholders and/or the Board. Any bylaws
adopted, amended or repealed by the Board may be amended or repealed by the stockholders.
Listing
Our
Common Stock and warrants are listed on Nasdaq under the symbols “SHOT” and “SHOTW”, respectively.
Transfer
Agent, Warrant Agent and Registrar
The
transfer agent and registrar for our Common Stock offered in this Offering is VSTOCK Transfer, LLC.
PLAN
OF DISTRIBUTION
The
selling stockholder may, from time to time, sell any or all of their securities covered hereby on Nasdaq or any other stock exchange,
market or trading facility on which the securities are traded or in private transactions. These sales may be at fixed or negotiated prices.
The selling stockholder may use any one or more of the following methods when selling securities:
| ● |
ordinary
brokerage transactions and transactions in which the broker dealer solicits purchasers; |
| |
|
| ● |
block
trades in which the broker dealer will attempt to sell the securities as agent but may position and resell a portion of the block
as principal to facilitate the transaction; |
| |
|
| ● |
purchases
by a broker dealer as principal and resale by the broker dealer for its account; |
| |
|
| ● |
an
exchange distribution in accordance with the rules of the applicable exchange; |
| |
|
| ● |
privately
negotiated transactions; |
| |
|
| ● |
settlement
of short sales; |
| |
|
| ● |
in
transactions through broker dealers that agree with the selling stockholder to sell a specified number of such securities at a stipulated
price per security; |
| |
|
| ● |
through
the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| |
|
| ● |
a
combination of any such methods of sale; or |
| |
|
| ● |
any
other method permitted pursuant to applicable law. |
The
selling stockholder may also sell securities under Rule 144 or any other exemption from registration under the Securities Act, if available,
rather than under this prospectus.
Broker
dealers engaged by the selling stockholder may arrange for other brokers dealers to participate in sales. Broker dealers may receive
commissions or discounts from the selling stockholder (or, if any broker dealer acts as agent for the purchaser of securities, from the
purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction
not in excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup
or markdown in compliance with FINRA Rule 2121.
In
connection with the sale of the securities or interests therein, the selling stockholder may enter into hedging transactions with broker-dealers
or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they
assume. The selling stockholder may also sell securities short and deliver these securities to close out their short positions, or loan
or pledge the securities to broker-dealers that in turn may sell these securities. The selling stockholder may also enter into option
or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the
delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer
or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The
selling stockholder and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters”
within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers
or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts
under the Securities Act. The selling stockholder has informed us that it does not have any written or oral agreement or understanding,
directly or indirectly, with any person to distribute the securities.
We
are required to pay certain fees and expenses incurred by us incident to the registration of the securities. We have agreed to indemnify
the selling stockholder against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
We
agreed to keep this prospectus effective until the earlier of (i) the date on which the securities may be resold by the selling stockholder
without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for
us to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of similar effect
or (ii) all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar
effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable state
securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered
or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is
complied with.
Under
applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously
engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M,
prior to the commencement of the distribution. In addition, the selling stockholder will be subject to applicable provisions of the Exchange
Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the common
stock by the selling stockholder or any other person. We will make copies of this prospectus available to the selling stockholder and
have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including
by compliance with Rule 172 under the Securities Act).
LEGAL
MATTERS
Certain
legal matters related to the securities offered by this prospectus will be passed upon on our behalf by The Sichenzia Ross Ference Carmel
LLP. If legal matters in connection with offerings made pursuant to this prospectus are passed upon by counsel for the underwriters,
dealers or agents, if any, such counsel will be named in the prospectus supplement relating to such offering.
EXPERTS
The
consolidated financial statements of the Company as of December 31, 2022 and 2021 incorporated in this prospectus by reference from the
Company’s Annual Report on Form 10-K for the year ended December 31, 2022 have been audited by M&K CPAS, PLLC, an independent
registered public accounting firm, as stated in their report thereon, and have been incorporated by reference in this prospectus and
registration statement in reliance upon such report and upon the authority of such firm as experts in accounting and auditing.
INCORPORATION
OF CERTAIN INFORMATION BY REFERENCE
The
SEC allows us to “incorporate by reference” information into this prospectus, which means that we can disclose important
information about us by referring you to another document filed separately with the SEC. The information incorporated by reference is
considered to be a part of this prospectus. This prospectus incorporates by reference the documents and reports listed below (other than
portions of these documents that are either (1) described in paragraph (e) of Item 201 of Regulation S-K or paragraphs (d)(1)-(3) and
(e)(5) of Item 407 of Regulation S-K promulgated by the SEC or (2) deemed to have been furnished and not filed in accordance with SEC
rules, including Current Reports on Form 8-K furnished under Item 2.02 or Item 7.01 (including any financial statements or exhibits relating
thereto furnished pursuant to Item 9.01), unless otherwise indicated therein:
| |
● |
Our
Annual Report on Form 10-K for the year ended December 31, 2022 (our “Annual Report”), filed with the SEC on April 3,
2023. |
| |
|
|
| |
● |
Our
Quarterly Report on Form 10-Q for the three and nine months ended September 30, 2023 (our “Quarterly Report”), filed
with the SEC on November 16, 2023. |
| |
|
|
| |
● |
Registration
Statement on Form S-1, filed with SEC on June 22, 2023, and declared effective on July 3, 2023. |
| |
|
|
| |
● |
Our
Current Reports on Form 8-K, filed with the SEC August 21, 2023, August 25, 2023, September 6, 2023, and September 15, 2023. |
| |
|
|
| |
● |
The
description of our Common Stock in our Registration Statement on Form S-1/A filed with the Commission on July 28, 2020, and amended
on October 26, 2020. |
We
also incorporate by reference the information contained in all other documents we file with the SEC pursuant to Sections 13(a), 13(c),
14 or 15(d) of the Exchange Act (other than portions of these documents that are either (1) described in paragraph (e) of Item 201 of
Regulation S-K or paragraphs (d)(1)-(3) and (e)(5) of Item 407 of Regulation S-K promulgated by the SEC or (2) deemed to have been furnished
and not filed in accordance with SEC rules, including Current Reports on Form 8-K furnished under Item 2.02 or Item 7.01 (including any
financial statements or exhibits relating thereto furnished pursuant to Item 9.01, unless otherwise indicated therein)) after the date
of this prospectus and prior to the completion of the offering of all securities covered by this prospectus and any applicable prospectus
supplement. The information contained in any such document will be considered part of this prospectus from the date the document is filed
with the SEC.
If
you make a request for such information in writing or by telephone, we will provide you, without charge, a copy of any or all of the
information incorporated by reference into this prospectus. Any such request should be directed to:
Safety
Shot, Inc.
1061
E. Indiantown Rd., Suite. 110
Jupiter,
FL 33477
(561)
244-7100
You
should rely only on the information contained in, or incorporated by reference into, this prospectus, in any applicable prospectus supplement
or in any free writing prospectus filed by us with the SEC. We have not authorized anyone to provide you with different or additional
information. The selling stockholder is not offering to sell or soliciting any offer to buy any securities in any jurisdiction where
the offer or sale is not permitted. You should not assume that the information in this prospectus or in any document incorporated by
reference is accurate as of any date other than the date on the front cover of the applicable document.
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
Item
14. Other Expenses of Issuance and Distribution
The
following table sets forth the costs and expenses payable by us in connection with the sale of common stock being registered. All amounts
are estimates except for the SEC registration fee.
| SEC registration fee | |
$ | 1,399 | |
| Legal fees and expenses | |
| 60,000 | |
| Accounting fees and expenses | |
| 10,000 | |
| Printing and Miscellaneous Expenses | |
| 8,601 | |
| Total | |
$ | 80,000 | |
Item
15. Indemnification of Directors and Officers
Safety
Shot, Inc. is incorporated under the laws of the State of Delaware. Reference is made to Section 102(b)(7) of the General Corporation
Law of the State of Delaware, as amended (the “DGCL”), which enables a corporation in its original certificate of
incorporation or an amendment thereto to eliminate or limit the personal liability of a director for violations of the director’s
fiduciary duty, except (1) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (2) for acts
or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (3) pursuant to Section 174 of
the DGCL, which provides for liability of directors for unlawful payments of dividends or unlawful stock purchase or redemptions or (4)
for any transaction from which the director derived an improper personal benefit.
Section
145(a) of the DGCL provides, in general, that a corporation may indemnify any person who was or is a party or is threatened to be made
a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative
(other than an action by or in the right of the corporation), because he or she is or was a director, officer, employee or agent of the
corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation,
partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts
paid in settlement actually and reasonably incurred by the person in connection with such action, suit or proceeding, if he or she acted
in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation and, with
respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
Section
145(b) of the DGCL provides, in general, that a corporation may indemnify any person who was or is a party or is threatened to be made
a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor
because the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation
as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses
(including attorneys’ fees) actually and reasonably incurred by the person in connection with the defense or settlement of such
action or suit if he or she acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests
of the corporation, except that no indemnification shall be made with respect to any claim, issue or matter as to which he or she shall
have been adjudged to be liable to the corporation unless and only to the extent that the adjudicating court determines that, despite
the adjudication of liability but in view of all of the circumstances of the case, he or she is fairly and reasonably entitled to indemnity
for such expenses which the adjudicating court shall deem proper.
Section
145(g) of the DGCL provides, in general, that a corporation may purchase and maintain insurance on behalf of any person who is or was
a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer,
employee or agent of another corporation, partnership, joint venture, trust or other enterprise against any liability asserted against
such person and incurred by such person in any such capacity, or arising out of his or her status as such, whether or not the corporation
would have the power to indemnify the person against such liability under Section 145 of the DGCL.
Our
bylaws, subject to the provisions of the DGCL, contain provisions which allow the corporation to indemnify any person against liabilities
and other expenses incurred as the result of defending or administering any pending or anticipated legal issue in connection with service
to us if it is determined that person acted in good faith and in a manner which he reasonably believed was in the best interest of the
corporation. Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers
and controlling persons, we have been advised that in the opinion of the Securities and Exchange Commission, such indemnification is
against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable.
As
permitted by the DGCL, the registrant has entered into separate indemnification agreements with each of the registrant’s directors
and certain of the registrant’s officers which require the registrant, among other things, to indemnify them against certain liabilities
which may arise by reason of their status as directors, officers or certain other employees.
The
registrant expects to obtain and maintain insurance policies under which its directors and officers are insured, within the limits and
subject to the limitations of those policies, against certain expenses in connection with the defense of, and certain liabilities which
might be imposed as a result of, actions, suits or proceedings to which they are parties by reason of being or having been directors
or officers. The coverage provided by these policies may apply whether or not the registrant would have the power to indemnify such person
against such liability under the provisions of the DGCL.
These
indemnification provisions and the indemnification agreements entered into between the registrant and the registrant’s officers
and directors may be sufficiently broad to permit indemnification of the registrant’s officers and directors for liabilities (including
reimbursement of expenses incurred) arising under the Securities Act of 1933.
Item
16. Exhibits
| Exhibit
No. |
|
Exhibit
Description |
| 3.1 |
|
Amended and Restated Certificate of Incorporation, incorporated herein by reference to Exhibit 2.1 to Jupiter Wellness, Inc.’s Form 1-A filed with the Securities and Exchange Commission on June 21, 2019. |
| 3.2 |
|
Bylaws, incorporated herein by reference to Exhibit 2.2 to Jupiter Wellness, Inc.’s Form 1-A filed with the Securities and Exchange Commission on June 21, 2019. |
| 3.3 |
|
Amended and Restated Bylaws, incorporated by reference to Exhibit 3.3 of the Company’s Registration Statement filed with the SEC on July 14, 2020. |
| 3.4 |
|
Certificate of Amendment of Certificate of Incorporation, incorporated by reference to Exhibit 3.4 of the Company’s Registration Statement filed with the SEC on June 17, 2020. |
| 3.5 |
|
Second Amended and Restated Certificate of Incorporation, incorporated by reference to Exhibit 3.5 of the Company’s Registration Statement filed with the SEC on June 17, 2020. |
| 4.1 |
|
Common Stock Purchase Warrant, incorporated by reference to Exhibit 4.1 of the Company’s Registration Statement filed with the SEC on July 14, 2020. |
| 4.2 |
|
Representative’s Warrant, incorporated by reference to Exhibit 4.2 of the Company’s Registration Statement filed with the SEC on June 17, 2020. |
| 4.3 |
|
Form of Warrant included in Unit, incorporated by reference to Exhibit 4.3 of the Company’s Registration Statement filed with the SEC on June 17, 2020. |
| 4.4 |
|
Form of Warrant Agent Agreement, incorporated by reference to Exhibit 4.4 of the Company’s Registration Statement filed with the SEC on June 17, 2020. |
| 4.5 |
|
Form of Common Stock Purchase Warrant, incorporated by reference to Exhibit 10.2 of the Company’s Current Report filed with the SEC on May 13, 2021. |
| 4.6 |
|
Form of Convertible Promissory Note, incorporated by reference to Exhibit 10.3 of the Company’s Current Report filed with the SEC on May 13, 2021. |
| 4.7 |
|
Form of Common Stock Purchase Warrant (3 years) dated January 23, 2023, incorporated by reference to the Exhibit 4.7 of the Registration Statement filed with SEC on June 22, 2023. |
| 4.8 |
|
Form of Common Stock Purchase Warrant (5 years) dated January 23, 2023, incorporated by reference to the Exhibit 4.8 of the Registration Statement filed with SEC on June 22, 2023. |
| 5.1 |
|
Opinion
of Sichenzia Ross Carmel Ference LLP* |
| 10.1
|
|
Common Stock and Warrant Subscription Agreement, incorporated by reference to Exhibit 10.1 of the Company’s Registration Statement filed with the SEC on July 14, 2020. |
| 10.2 |
|
Independent Director’s Contract between the Company and Dr. Hector Alila, dated February 25, 2019, incorporated by reference to Exhibit 10.2 of the Company’s Registration Statement filed with the SEC on July 14, 2020. |
| 10.3 |
|
Independent Director’s Contract between the Company and Timothy G. Glynn, dated March 13, 2019, incorporated by reference to Exhibit 10.3 of the Company’s Registration Statement filed with the SEC on July 14, 2020. |
| 10.4 |
|
Independent Director’s Contract between the Company and Christopher Melton, dated July 29, 2019, incorporated by reference to Exhibit 10.4 of the Company’s Registration Statement filed with the SEC on July 14, 2020. |
| 10.5 |
|
Employment Agreement with Douglas O. McKinnon, dated August 5, 2019, incorporated by reference to Exhibit 10.5 of the Company’s Registration Statement filed with the SEC on July 14, 2020. |
| 10.6 |
|
Form of Regulation A Subscription Agreement, incorporated herein by reference to Exhibit 4.1 to Jupiter Wellness, Inc.’s Form 1-A/A filed with the Securities and Exchange Commission on August 19, 2019. |
| 10.7 |
|
Employment Agreement with Dr. Glynn Wilson, dated October 15, 2019, incorporated by reference to Exhibit 10.7 of the Company’s Registration Statement filed with the SEC on July 14, 2020. |
| 10.8 |
|
Employment Agreement with Brian John, dated February 1, 2020, incorporated by reference to Exhibit 10.8 of the Company’s Registration Statement filed with the SEC on June 17, 2020. |
| 10.9 |
|
Employment Agreement with Richard Miller, dated February 1, 2020, incorporated by reference to Exhibit 10.9 of the Company’s Registration Statement filed with the SEC on June 17, 2020. |
| 10.10 |
|
2020 Equity Incentive Plan, incorporated by reference to Exhibit 10.10 of the Company’s Registration Statement filed with the SEC on June 17, 2020. |
| 10.11 |
|
Confidential Membership Interest Purchase Agreement dated February 20, 2020 by and between Jupiter Wellness, Inc., Magical Beasts LLC. and Krista Whitley, incorporated by reference to Exhibit 10.11 of the Company’s Registration Statement filed with the SEC on June 17, 2020. |
| 10.12 |
|
Sales Distribution Agreement dated February 20, 2020 between Jupiter Wellness Inc. and Ayako Holdings, Inc., incorporated by reference to Exhibit 10.12 of the Company’s Registration Statement filed with the SEC on June 17, 2020. |
| 10.13 |
|
Distribution Agreement, dated November 5, 2020, incorporated by reference to the Company’s Current Report on Form 8-K, filed with the SEC on November 9, 2020. |
| 10.14 |
|
Endorsement Agreement, dated November 10, 2020, incorporated by reference to the Company’s Current Report on Form 8-K, filed with the SEC on November 19, 2020. |
| 10.15 |
|
Share Exchange Agreement, dated November 30, 2020, incorporated by reference to the Company’s Current Report on Form 8-K, filed with the SEC on December 3, 2020. |
| 10.16 |
|
Independent Director’s Agreement, dated January 20, 2021, incorporated by reference to the Company’s Current Report on Form 8-K, filed with the SEC on January 26, 2021. |
| 10.17 |
|
Omnibus Amendment dated January 25, 2021, incorporated by reference to the Company’s Current Report on Form 8-K, filed with the SEC on January 29, 2021. |
| 10.18 |
|
First Amendment to Common Stock Option Agreement dated January 25, 2021, incorporated by reference to the Company’s Current Report on Form 8-K, filed with the SEC on January 29, 2021. |
| 10.19 |
|
Employment Agreement dated as of January 20, 2021, incorporated by reference to the Company’s Current Report on Form 8-K, filed with the SEC on February 3, 2021. |
| 10.20 |
|
Form of Loan Agreement, incorporated by reference to Exhibit 10.1 of the Company’s Current Report filed with the SEC on May 13, 2021. |
| 10.21 |
|
Form of the PIPE Securities Purchase Agreement, incorporated by reference to Exhibit 10.1 of the Company’s Current Report filed with the SEC on January 25, 2023 |
| 10.22 |
|
Form of the RD Securities Purchase Agreement, incorporated by reference to Exhibit 10.2 of the Company’s Current Report filed with the SEC on January 25, 2023 |
| 10.23 |
|
Form of the Registration Rights Agreement, incorporated by reference to Exhibit 10.3 of the Company’s Current Report filed with the SEC on January 25, 2023 |
| 14.1 |
|
Code of Ethics, incorporated by reference to Exhibit 14.1 of the Company’s Registration Statement filed with the SEC on July 14, 2020. |
| 14.2 |
|
Corporate Governance Guidelines, incorporated by reference to Exhibit 14.2 of the Company’s Registration Statement filed with the SEC on July 14, 2020. |
| 23.1 |
|
Consent of M&K CPAS PLLC, an independent registered public accounting firm * |
| 23.2 |
|
Consent
of Sichenzia Ross Ference Carmel LLP (included in exhibit 5.1)* |
| 24.1 |
|
Power of Attorney (included in signature page to this registration statement)* |
| 107 |
|
Filing Fee Table* |
*Filed
herewith
Item
17. Undertakings
The
Company hereby undertakes:
| (a)(1) |
To
file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| |
i. |
To
include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
| |
|
|
| |
ii. |
To
reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent
post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set
forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if
the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end
of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b)
if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set
forth in the “Calculation of Registration Fee” table in the effective registration statement. |
| |
|
|
| |
iii. |
To
include any material information with respect to the plan of distribution not previously
disclosed in the registration statement or any material change to such information in the
registration statement;
provided,
however, that paragraphs (1)(i), (1)(ii), and (1)(iii) above do not apply if the information required to be included in a post-effective
amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to Section
13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is
contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement. |
| (2)
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be
deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that
time shall be deemed to be the initial bona fide offering thereof. |
| |
| (3)
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at
the termination of the offering. |
| (4)
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser: |
| |
| (i)
Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of
the date the filed prospectus was deemed part of and included in the registration statement; and |
| |
| (ii)
Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance
on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information
required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement
as of the earlier of the date such form of prospectus is first used after effectiveness or date of the first sale of securities in
the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at
that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities
in the registration statement to which the prospectus relates, and the offering of such securities at that time shall be deemed to
be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus
that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration
statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior
to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part
of the registration statement or made in any such document immediately prior to such effective date. |
| |
| (5)
That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial
distribution of the securities, in a primary offering of securities of the undersigned registrant pursuant to this registration statement,
regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such
purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will
be considered to offer or sell such securities to such purchaser: |
| |
| (i)
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to
Rule 424; |
| |
| (ii)
Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to
by the undersigned registrant; |
| |
| (iii)
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant
or its securities provided by or on behalf of the undersigned registrant; and |
| |
| (iv)
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| |
| (6)
That, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report
pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit
plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in
the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the
offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| |
| (7)
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, and controlling
persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion
of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In
the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred
or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding)
is asserted against the registrant by such director, officer or controlling person in connection with the securities being registered,
the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court
of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities
Act and will be governed by the final adjudication of such issue. |
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf
by the undersigned, thereunto duly authorized, in the City of Miami, State of Florida on November 22, 2023.
| SAFETY
SHOT, INC. |
|
| |
|
|
| By: |
/s/
Brian S. John |
|
| |
Brian
S. John
Chief
Executive Officer and Executive Chairman (Principal Executive Officer) |
|
POWER
OF ATTORNEY
KNOW
ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Brian S. John, his or her true
and lawful attorney-in-fact and agent, each with full power of substitution and resubstituting, for him or her and in his or her name,
place, and stead, in any and all capacities, to (i) act on, sign and file with the Securities and Exchange Commission any and all amendments
(including post-effective amendments) to this registration statement together with all schedules and exhibits thereto and any subsequent
registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, together with all schedules and exhibits
thereto, (ii) act on, sign and file such certificates, instruments, agreements and other documents as may be necessary or appropriate
in connection therewith, (iii) act on and file any supplement to any prospectus included in this registration statement or any such amendment
or any subsequent registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and (iv) take any
and all actions which may be necessary or appropriate to be done, as fully for all intents and purposes as he or she might or could do
in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or his or her substitute or substitutes, may lawfully
do or cause to be done by virtue hereof.
Pursuant
to the requirements of the Securities Act, this registration statement has been signed by the following persons in the capacities and
on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/
Brian S. John |
|
Director
and Chief Executive Officer (principal executive officer) |
|
November
22, 2023 |
| Brian
S. John |
|
|
|
|
| |
|
|
|
|
| /s/
Markita L. Russell |
|
Chief
Financial Officer (principal financial and accounting officer) |
|
November
22, 2023 |
| Markita
L. Russell |
|
|
|
|
| |
|
|
|
|
| /s/
Glynn Wilson |
|
Chairman
and Chief Science Officer |
|
November
22, 2023 |
| Dr.
Glynn Wilson |
|
|
|
|
| |
|
|
|
|
| /s/
Dr. Hector Alila |
|
Director |
|
November
22, 2023 |
| Dr.
Hector Alila |
|
|
|
|
| |
|
|
|
|
| /s/
Christopher Marc Melton |
|
Director |
|
November
22, 2023 |
| Christopher
Marc Melton |
|
|
|
|
| |
|
|
|
|
| /s/
Nancy Torres Kaufman |
|
Director |
|
November
22, 2023 |
| Nancy
Torres Kaufman |
|
|
|
|
| |
|
|
|
|
| /s/
Jarrett Boon |
|
Director |
|
November
22, 2023 |
| Jarrett
Boon |
|
|
|
|
| |
|
|
|
|
| /s/
John Gulyas |
|
Director |
|
November
22, 2023 |
| John
Gulyas |
|
|
|
|
Exhibit
5.1
November
22, 2023
Safety
Shot, Inc.
1061
E. Indiantown Road, Suite 110
Jupiter,
FL - 33477
Re:
Registration Statement on Form S-3
Ladies
and Gentlemen:
We
have acted as counsel to Safety Shot, Inc., a Delaware corporation (the “Company”), in connection with the preparation of
a registration statement on Form S-3 (the “Registration Statement”), filed by the Company with the Securities and Exchange
Commission (the “Commission”) pursuant to the Securities Act of 1933, as amended (the “Securities Act”), relating
to the resale from time to time, as set forth in the Registration Statement, the form of prospectus contained therein (the “Prospectus”)
by the selling stockholders identified in the Registration Statement (the “Selling Stockholders”) of up to 1,978,796
(the “Shares”) shares of our common stock, par value $0.001 (the “Common Stock”), including (i) up to 550,000
shares of common stock; and (ii) up to 1,428,796 shares of common stock issuable upon the exercise of the common stock purchase
warrant (the “Warrants”)..
You
have requested our opinion as to the matters set forth below in connection with the Registration Statement. For purposes of rendering
the opinions set forth below, we have examined (i) the Registration Statement, including the exhibits filed therewith, (ii) the Prospectus,
(iii) the Company’s certificate of incorporation, as amended (the “Certificate of Incorporation”), (iv) the Company’s
bylaws, as amended (the “Bylaws”), (v) the corporate resolutions and other actions of the Company that authorize and provide
for the filing of the Registration Statement, and we have made such other investigation as we have deemed appropriate. We have not independently
established any of the facts so relied on.
For
purposes of this opinion letter, we have assumed the accuracy and completeness of each document submitted to us, the genuineness of all
signatures on original documents, the authenticity of all documents submitted to us as originals, the conformity to original documents
of all documents submitted to us as facsimile, electronic, certified, conformed or photostatic copies thereof, and the due execution
and delivery of all documents where due execution and delivery are prerequisites to the effectiveness thereof. We have further assumed
the legal capacity of natural persons, that persons identified to us as officers of the Company are actually serving in such capacity,
that the representations of officers and employees of the Company are correct as to questions of fact, that the board of directors will
have taken all action necessary to set the issuance price of the Warrants to be offered and sold and that each party to the documents
we have examined or relied on (other than the Company) has the power, corporate or other, to enter into and perform all obligations thereunder
and also have assumed the due authorization by all requisite action, corporate or other, the execution and delivery by such parties of
such documents, and the validity and binding effect thereof on such parties. We have not independently verified any of these assumptions.
The
opinions expressed in this opinion letter are limited to the Delaware General Corporation Law (the “DGCL”) and the applicable
statutory provisions of the Delaware Constitution and the reported judicial decisions interpreting such statute and provisions. We are
not opining on, and we assume no responsibility for, the applicability to or effect on any of the matters covered herein of (a) any other
laws; (b) the laws of any other jurisdiction; or (c) the laws of any county, municipality or other political subdivision or local governmental
agency or authority.
Based
on the foregoing and subject to the limitations, qualifications, and assumptions set forth herein, we are of the opinion that the Shares,
including when issued upon valid exercise of the Warrants in accordance with the terms of the Warrants, including without limitation
payment of the specified exercise price therefor, will be validly issued, fully paid, and non-assessable. The Warrants represent a binding
commitment of the Company.
The
opinion above is subject to the effects of (i) bankruptcy, insolvency, fraudulent conveyance, fraudulent transfer, reorganization, receivership,
moratorium and other similar laws relating to or affecting enforcement of creditors’ rights or remedies generally, (ii) general
principles of equity, whether such principles are considered in a proceeding of law or at equity, and (iii) an implied covenant of good
faith, reasonableness and fair dealing and standards of materiality.
This
opinion is limited to the DGCL, including the statutory provisions of the DGCL and all applicable provisions of the Delaware Constitution
and reported judicial decisions interpreting these laws. We hereby consent to the filing of this opinion as an exhibit to the Registration
Statement and to the use of our name under the caption “Legal Matters” in the Prospectus. In giving our consent, we do not
thereby admit that we are experts with respect to any part of the Registration Statement or the Prospectus within the meaning of the
term “expert,” as used in Section 11 of the Securities Act or the rules and regulations promulgated thereunder by the Commission,
nor do we admit that we are in the category of persons whose consent is required under Section 7 of the Securities Act or the rules and
regulations thereunder.
| |
Yours truly, |
| |
|
| |
/s/
Sichenzia Ross Ference Carmel LLP |
| |
Sichenzia Ross Ference
Carmel LLP |
Exhibit
23.1

CONSENT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We
hereby consent to the incorporation in this Registration Statement on Form S-3, of our report dated April 3, 2023, of Safety Shot, Inc.
(formerly Jupiter Wellness, Inc.) relating to the audit of the consolidated financial statements as of December 31, 2022 and 2021, and
for the periods then ended, and the reference to our firm under the caption “Experts” in the Registration Statement.
/s/
M&K CPA’s, PLLC
Houston,
TX
November
22, 2023
Exhibit
107
Calculation
of Filing Fee Table
Form
S-3
(Form
Type)
Safety
Shot, Inc.
(Exact
name of Registrant as Specified in its Charter)
| | |
Table 1 – Newly Registered Securities |
| |
Security Type | |
Security Class Title | |
Fee Calculation Rule | |
Amount Registered(1) | | |
Proposed Maximum Offering Price Per Unit(2) | | |
Maximum Aggregate Offering Price | | |
Fee Rate | | |
Amount of Registration Fee(2) | | |
Carry Forward Form Type | | |
Carry Forward File Number | | |
Carry Forward Initial Effective Date | | |
Filing Fee Previously Paid in Connection with Unsold Securities to be Carried Forward | |
| | |
| |
| |
| |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Fees to Be Paid | |
Equity | |
Common Stock, par value $0.001 per share | |
Rule 457(o) | |
| 550,000 | | |
$ | 4.79 | | |
$ | 2,634,500.00 | | |
| 0.0001476 | | |
$ | 388.85 | | |
| | | |
| | | |
| | | |
| | |
| Fees to Be Paid | |
Other | |
Warrants(3) | |
Rule 457(o) | |
| 1,428,796 | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Fees to Be Paid | |
Equity | |
Common Stock, par value $0.001 per share underlying Warrants(3) | |
Rule 457(o) | |
| 1,428,796 | | |
$ | 4.79 | | |
$ | 6,843,932.84 | | |
| 0.0001476 | | |
$ | 1,010.16 | | |
| | | |
| | | |
| | | |
| | |
| | |
Table 2 – Carry Forward Securities | |
| Carry Forward Securities | |
| |
| |
| |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
Total Offering Amounts | | | |
| | | |
| | | |
$ | 9,478,432.84 | | |
| | | |
| | | |
| | | |
| | |
| | |
Total Fees Previously Paid | | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
Total Fee Offsets | | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
Net Fee Due | | | |
| | | |
| | | |
$ | 1,399.02 | | |
| | | |
| | | |
| | | |
| | |
(1)
Pursuant to Rule 416 under the Securities Act of 1933, as amended (the “Securities Act”), the shares of common stock offered
hereby also include an indeterminate number of additional shares of common stock as may from time to time become issuable by reason of
stock splits, stock dividends, recapitalizations or other similar transactions.
(2)
With respect to the shares of common stock offered by the selling stockholders named herein, the offering price has been estimated at
the last reported sale price of our common stock was $4.79 per share on November 21, 2023, for the purpose of calculating
the registration fee in accordance with Rule 457(o) under the Securities Act.
(3)
In accordance with Rule 457(g) under the Securities Act, because the Ordinary Shares underlying the warrants are registered hereby, no
separate registration fee is required with respect to the warrants registered hereby.
Jupiter Wellness (NASDAQ:JUPW)
Historical Stock Chart
From Apr 2024 to May 2024
Jupiter Wellness (NASDAQ:JUPW)
Historical Stock Chart
From May 2023 to May 2024