FDA Lifts Partial Hold on Curis' Lymphoma Study
August 18 2022 - 8:52AM
Dow Jones News
By Dean Seal
Curis Inc. said Thursday the U.S. Food and Drug Administration
has lifted the partial clinical hold on its TakeAim Lymphoma study,
a Phase 1/2 trial of emavusertib in patients with B-cell
malignancies.
The cancer-focused biotechnology company announced the hold back
in April, saying at the time that it was unexpected. The
notification came from the FDA Division of Hematologic Malignancies
2, which regulates clinical studies in lymphoma.
The FDA lifted the hold after reviewing a comprehensive data
package that Curis submitted, according to the company.
Chief Executive James Dentzer said the company is working to
resume enrollment of new patients in the third quarter.
Shares soared 29.5% to $1.36 in premarket trading.
Write to Dean Seal at dean.seal@wsj.com
(END) Dow Jones Newswires
August 18, 2022 08:37 ET (12:37 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
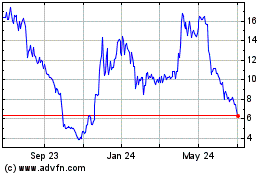
Curis (NASDAQ:CRIS)
Historical Stock Chart
From Mar 2024 to Apr 2024
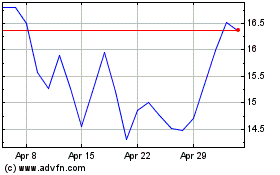
Curis (NASDAQ:CRIS)
Historical Stock Chart
From Apr 2023 to Apr 2024