LumiraDx Shares Rise 13% Premarket on EUA Submission to FDA
October 15 2021 - 9:46AM
Dow Jones News
By Chris Wack
LumiraDx Ltd. shares were up 13%, to $8.70, after the company
said it has submitted the LumiraDx SARS-CoV-2 & Flu A/B Test to
the U.S. Food and Drug Administration for Emergency Use
Authorization.
The company said the microfluidic immunofluorescence assay can
quickly verify infection for patients suspected of flu and/or
Covid-19 to aid diagnosis and clinical decision making.
The LumiraDx SARS-CoV-2 & Flu A/B Test is a rapid
microfluidic immunofluorescence assay intended for the simultaneous
detection of SARS-CoV-2, Influenza A, and Influenza B viral antigen
direct from self or clinician collected nasal swab specimens from
individuals suspected of viral infection consistent with Covid-19
by their healthcare provider within the first 12 days of the onset
of symptoms.
The company currently has five tests in the market globally, and
an additional 10 tests scheduled for regulatory submission or
clearance by the end of 2022, including tests for troponin and
congestive heart failure.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
October 15, 2021 09:31 ET (13:31 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
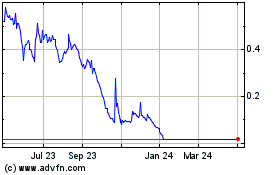
LumiraDx (NASDAQ:LMDX)
Historical Stock Chart
From Mar 2024 to Apr 2024
LumiraDx (NASDAQ:LMDX)
Historical Stock Chart
From Apr 2023 to Apr 2024