|
PROSPECTUS SUPPLEMENT
|
Filed pursuant to Rule 424(b)(5)
|
|
(To Prospectus dated June 16, 2017)
|
Registration Statement No. 333-218517
|

Ekso
Bionics Holdings, Inc.
Up to $25,000,000
Common Stock
Ekso Bionics Holdings, Inc. (“Ekso Bionics”) has
entered into a Controlled Equity Offering
SM
sales agreement with Cantor Fitzgerald & Co. (“Cantor Fitzgerald”)
relating to the sale of the shares of our common stock, par value $0.001 (the “sales agreement”), offered by this prospectus
supplement. In accordance with the terms of the sales agreement, we may offer and sell shares of our common stock having an aggregate
offering price of up to $25,000,000 under this prospectus supplement from time to time through Cantor Fitzgerald, acting as agent.
Sales of our common stock, if any, under this prospectus supplement
may be made in sales deemed to be an “at the market offering” as defined in Rule 415(a)(4) promulgated under the Securities
Act of 1933, as amended (the “Securities Act”). Cantor Fitzgerald will act as sales agent on a best efforts basis and
use commercially reasonable efforts consistent with its normal trading and sales practices to sell on our behalf all of the shares
of common stock requested to be sold by us, on mutually agreed terms between Cantor Fitzgerald and us. There is no arrangement
for funds to be received in any escrow, trust or similar arrangement.
Cantor Fitzgerald will be entitled to compensation at a fixed
commission rate of 3.0% of the gross sales price per share sold. In connection with the sale of our common stock on our behalf,
Cantor Fitzgerald will be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation
of Cantor Fitzgerald will be deemed to be underwriting commissions or discounts.
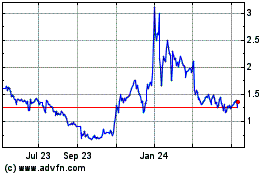
Our common stock trades on Nasdaq Capital Market (“Nasdaq”) under the symbol “EKSO.”
On August 17, 2018, the last reported sale price of the common stock on Nasdaq was $2.72 per share.
Investing in our securities involves
a high degree of risk. You should carefully read and consider the risk factors described in this prospectus supplement, the accompanying
base prospectus and in the documents incorporated by reference into this prospectus supplement and the base prospectus. See “Risk
Factors” beginning on page S-4.
Neither the Securities and Exchange Commission nor
any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement is
truthful or complete. Any representation to the contrary is a criminal offense.

The date of this prospectus supplement
is August 21, 2018
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying base prospectus
are part of a registration statement that we have filed with the Securities and Exchange Commission, or SEC, utilizing a “shelf”
registration process. Under this prospectus supplement, we may offer shares of our common stock having an aggregate offering price
of up to $25,000,000 from time to time at prices and on terms to be determined by market conditions at the time of offering.
We provide information to you about this offering of shares
of our common stock in two separate documents that are bound together: (1) this prospectus supplement, which describes the specific
details regarding this offering; and (2) the accompanying base prospectus, which provides general information, some of which may
not apply to this offering. Generally, when we refer to this “prospectus,” we are referring to both documents combined.
If information in this prospectus supplement is inconsistent with the accompanying base prospectus, you should rely on this prospectus
supplement. However, if any statement in one of these documents is inconsistent with a statement in another document having a later
date—for example, a document incorporated by reference in this prospectus—the statement in the document having the
later date modifies or supersedes the earlier statement as our business, financial condition, results of operations and prospects
may have changed since the earlier dates.
We further note that the representations, warranties and covenants
made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference in this prospectus were
made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among
the parties to such agreement, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations,
warranties or covenants were accurate only as of the date when made. Accordingly, such representations and warranties should not
be relied on as accurately representing the current state of our affairs or obligations.
You should rely only on the information contained in, or incorporated
by reference into, this prospectus and in any free writing prospectus that we may authorize for use in connection with this offering.
We have not, and Cantor Fitzgerald has not, authorized any other person to provide you with different information. If anyone provides
you with different or inconsistent information, you should not rely on it. We are not, and Cantor Fitzgerald is not, making an
offer to sell or soliciting an offer to buy our securities in any jurisdiction in which an offer or solicitation is not authorized
or in which the person making that offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make
an offer or solicitation. You should assume that the information appearing in this prospectus, the documents incorporated by reference
into this prospectus, and in any free writing prospectus that we may authorize for use in connection with this offering, is accurate
only as of the date of those respective documents. Our business, financial condition, results of operations and prospects may have
changed since those dates.
You should read this prospectus, the documents incorporated by reference into this prospectus, and
any free writing prospectus that we may authorize for use in connection with this offering, in their entirety before making an
investment decision. You should also read and consider the information in the documents to which we have referred you in the sections
of this prospectus entitled “Where You Can Find More Information” and “Incorporation of Certain Documents By
Reference.”
We are offering to sell, and seeking offers to buy, shares of
common stock only in jurisdictions where offers and sales are permitted. The distribution of this prospectus and the offering of
the common stock in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession
of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the common stock and
the distribution of this prospectus outside the United States. This prospectus does not constitute, and may not be used in connection
with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus by any person in any jurisdiction
in which it is unlawful for such person to make such an offer or solicitation.
For purposes of this prospectus, references to the terms “Ekso
Bionics,” “the Company,” “we,” “us” and “our” refer to Ekso Bionics Holdings,
Inc., unless the context otherwise requires.
This prospectus and the information incorporated by reference
herein and therein include trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks
and trade names included or incorporated by reference into this prospectus are the property of their respective owners.
PROSPECTUS SUMMARY
The following summary highlights certain information contained
elsewhere in this prospectus supplement, the accompanying base prospectus, any free writing prospectus that we have been authorized
to use and the documents incorporated by reference herein and in the accompanying base prospectus. This summary does not contain
all the information you will need in making your investment decision. You should carefully read this entire prospectus supplement
the accompanying base prospectus, any free writing prospectus that we have been authorized to use and the documents incorporated
by reference herein and in the accompanying base prospectus. You should pay special attention to the “Risk Factors”
section of this prospectus and the financial statements and other information incorporated by reference in this prospectus.
Company Overview
Ekso Bionics designs, develops and sells
exoskeletons that augment human strength, endurance, and mobility. Our exoskeleton technology serves multiple markets and can be
used both by able-bodied users as well as by persons with physical disabilities. We have sold, rented or leased devices that (a)
enable individuals with neurological conditions affecting gait (stroke and spinal cord injury) to rehabilitate and to walk again
and (b) allow industrial workers to perform heavy duty work for extended periods.
We believe the commercial opportunity for
exoskeleton technology adoption is accelerating as a result of recent advancements in material technologies, electronic and electrical
engineering, control technologies, and sensor and software development. Taken individually, many of these advancements have become
ubiquitous in peoples’ everyday lives. We believe we have learned how to integrate these existing technologies and wrap the
result around a human being efficiently, elegantly and safely, supported by an industry leading intellectual property portfolio.
We further believe we can do so across a broad spectrum of applications, from persons with lower limb paralysis to able-bodied
users.
Additional details of these programs and related strategic agreements
are contained in our annual report on Form 10-K for the year ended December 31, 2017.
Company Information
Our corporate headquarters are located in Richmond, California
94804. Our telephone number is (510) 984-1761, and our website address is www.eksobionics.com. Our official Twitter account is
@EksoBionics. The information on or accessible through our website or our Twitter account does not constitute part of this prospectus
supplement or the accompanying prospectus and should not be relied upon in connection with making any investment in our securities.
The common stock of Ekso Bionics is listed on the Nasdaq Capital
Market (“Nasdaq”) under the symbol “EKSO.”
THE OFFERING
|
|
|
|
|
Common stock to be offered by us
|
|
Shares of our common stock having an aggregate offering price of up to $25,000,000
|
|
|
|
|
Common shares to be outstanding after this offering
|
|
Up to 70,023,522 shares of common stock, assuming sales of 9,191,176 shares of our common stock
in this offering at an assumed offering price of $2.72 per share, which was the last reported sale price of our common stock
on Nasdaq on August 17, 2018. The actual number of shares issued will vary depending on the sales prices under this
offering.
|
|
|
|
|
Plan of Distribution
|
|
An “at the market offering” of shares of common stock that may be made from time to time
through our sales agent, Cantor Fitzgerald. See “Plan of Distribution” on page S-26.
|
|
|
|
|
Use of Proceeds
|
|
We intend to use the net proceeds primarily for general corporate purposes, which may include acquisitions,
research and development activities, capital expenditures, selling, general and administrative costs, facilities expansion and
to meet working capital needs. See “Use of Proceeds” on page S-24.
|
|
|
|
|
Risk Factors
|
|
Before investing in our common stock, you should carefully read and consider the “Risk Factors”
beginning on page S-4 of this prospectus, and any documents incorporated by reference, for certain considerations relevant to an
investment in our common stock.
|
|
|
|
|
Nasdaq Capital Market symbol
|
|
“EKSO.”
|
The number of shares of common stock to be outstanding after this offering is based on 60,832,346 shares
of common stock outstanding as of June 30, 2018 and a total offering of an aggregate of
9,191,176
shares of our common stock at a public offering price of $2.72 per share, which was the last reported sale price of our common
stock on Nasdaq on August 17, 2018, and excludes as of June 30, 2018:
|
|
·
|
2,916,453 shares of common stock issuable upon the exercise of stock
options outstanding at a weighted average exercise price of $4.60 per share, 78,514 restricted stock units which will, after vesting,
be settled in shares of our common stock, and 5,093,211 shares of our common stock reserved for issuance under our Amended and
Restated 2014 Equity Incentive Plan (“2014 Incentive Plan”);
|
|
|
·
|
500,000 shares of our common stock reserved for issuance under our
Employee Stock Purchase Plan (“ESPP”); and
|
|
|
·
|
3,395,532 shares of common stock issuable upon the exercise of warrants
outstanding at a weighed exercise price of $7.43 per share.
|
RISK FACTORS
Investing in our securities involves a high degree of risk.
You should carefully consider the specific risks described below, as well as the other information contained in this prospectus
and the other documents incorporated by reference, before making an investment decision. See the sections of this prospectus entitled
“Where You Can Find More Information” and “Incorporation of Certain Information By Reference.” Any of the
risks we describe below or in the information incorporated herein by reference in this prospectus could cause our business, financial
condition or operating results to suffer. The market price of our common stock could decline if one or more of these risks and
uncertainties develop into actual events. You could lose all or part of your investment.
Risks Related to our Business and the
Industry in Which We Operate
We have a limited operating history
upon which investors can evaluate our future prospects.
Although we were incorporated in 2005,
we did not sell our first Ekso medical device until 2012 and did not sell our first industrial unit until 2016. Therefore, we have
limited operating history upon which an evaluation of our business plan or performance and prospects can be made. Our business
and prospects must be considered in light of the potential problems, delays, uncertainties and complications encountered in connection
with a newly established business and creating a new industry. The risks include, but are not limited to, the possibility that
we will not be able to develop functional and scalable products and services, or that although functional and scalable, our products
and services will not be economical to market; that our competitors hold proprietary rights that preclude us from marketing such
products; that our competitors market a superior or equivalent product; that we are not able to upgrade and enhance our technologies
and products to accommodate new features and expanded service offerings; or that we fail to receive necessary regulatory clearances
for our products. To successfully introduce and market our products at a profit, we must establish brand name recognition and competitive
advantages for our products. There are no assurances that we can successfully address these challenges. If we are unsuccessful,
we and our business, financial condition and operating results could be materially and adversely affected.
Given the limited operating history, management
has little basis on which to forecast future demand for our products from our existing customer base, much less new customers.
Our current and future expense levels are based largely on estimates of planned operations and future revenues rather than experience.
It is difficult to accurately forecast future revenues because our business is new and our market has not been developed. If our
forecasts prove incorrect, our business, operating results and financial condition will be materially and adversely affected. Moreover,
we may be unable to adjust our spending in a timely manner to compensate for any unanticipated reduction in revenue. As a result,
any significant reduction in revenues would immediately and adversely affect our business, financial condition and operating results.
The industries in which we operate
are highly competitive and subject to rapid technological change. If our competitors are better able to develop and market products
that are safer, more effective, less costly, easier to use, or are otherwise more attractive, we may be unable to compete effectively
with other companies.
The medical technology and industrial robotics
industries are characterized by intense competition and rapid technological change, and we will face competition on the basis of
product features, clinical outcomes, price, services and other factors. Competitors may include large medical device and other
companies, some of which have significantly greater financial and marketing resources than we do, and firms that are more specialized
than we are with respect to particular markets. Our competition may respond more quickly to new or emerging technologies, undertake
more extensive marketing campaigns, have greater financial, marketing and other resources than we do or may be more successful
in attracting potential customers, employees and strategic partners.
Our competitive position will depend on
multiple, complex factors, including our ability to achieve market acceptance for our products, develop new products, implement
production and marketing plans, secure regulatory approvals for products under development and protect our intellectual property.
Competitors may offer, or may attempt to develop, more efficacious, safer, cheaper, or more convenient alternatives to our products,
including alternatives that could make the need for robotic exoskeletons obsolete. The development of new or improved products,
processes or technologies by other companies may render our products or proposed products obsolete or less competitive. The entry
into the market of manufacturers located in low-cost manufacturing locations may also create pricing pressure, particularly in
developing markets. Our future success depends, among other things, upon our ability to compete effectively against current technology,
as well as to respond effectively to technological advances, and upon our ability to successfully implement our marketing strategies
and execute our research and development plan.
Our products or exoskeletons generally
may not be accepted in the market.
We cannot be certain that our current products
or any other products we may develop or market will achieve or maintain market acceptance. Market acceptance of our products depends
on many factors, including our ability to convince key opinion leaders to provide recommendations regarding our products, convince
distributors and customers that our technology is an attractive alternative to other technologies, convince health insurers and
other third party payers to cover and provide adequate payments for any products that are used for medical or therapeutic purposes,
demonstrate that our products are reliable and supported by us in the field, supply and service sufficient quantities of products
directly or through marketing alliances, and price products competitively in light of the current macroeconomic environment, which,
particularly in the case of the medical device industry, is becoming increasingly price sensitive.
In addition, the market for medical and
industrial exoskeletons is new and unproven. We cannot be certain that the market for robotic exoskeletons will continue to develop,
or that robotic exoskeletons for medical or industrial use will achieve market acceptance. If the exoskeleton market fails to develop,
or develops more slowly than we anticipate, we and our business, financial condition and operating results could be materially
and adversely affected.
Protecting our patent and other proprietary
rights can be costly, and we may not be able to attain, defend or maintain such rights, which could harm our business.
Our long-term success largely depends on
our ability to market technologically competitive products. Failure to protect or to obtain, maintain or extend adequate patent
and other intellectual property rights could materially adversely impact our competitive advantage and impair our business. Our
issued patents may not be sufficient to protect our intellectual property and our patent applications may not result in issued
patents. Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful
protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors
may be able to circumvent our patents by developing similar or alternative technologies or products in a non-infringing manner
or may challenge the validity of our patents. Our attempts to prevent third parties from circumventing our intellectual property
and other rights ultimately may be unsuccessful. We may also fail to take the required actions or pay the necessary fees to maintain
any of our patents that issue.
Furthermore, we have not filed applications
for all of our patents internationally and may not be able to prevent third parties from using our proprietary technologies or
may lose access to technologies critical to our products in other countries. These include, in some cases, countries in which we
are currently selling products and countries in which we intend to sell products in the future.
Intellectual property litigation
and infringement claims could cause us to incur significant expenses or prevent us from selling certain of our products.
The industries in which we operate, including,
in particular, the medical device industry, are characterized by extensive intellectual property litigation and, from time to time,
we might be the subject of claims by third parties of potential infringement or misappropriation. Regardless of outcome, such claims
are expensive to defend and divert the time and effort of our management and operating personnel from other business issues. A
successful claim or claims of patent or other intellectual property infringement against us could result in our payment of significant
monetary damages and/or royalty payments or negatively impact our ability to sell current or future products in the affected category
and could have a material adverse effect on our business, cash flows, financial condition or results of operations.
Because competition in our industry is
intense, competitors may infringe or otherwise violate our issued patents, patents of our licensors or other intellectual property.
To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming.
Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that
we infringe their patents. In addition, in a patent infringement proceeding, a court may decide that a patent of ours is invalid
or unenforceable, in whole or in part, construe the patent’s claims narrowly, or refuse to stop the other party from using
the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation
proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly. We may also elect to enter
into license agreements in order to settle patent infringement claims or to resolve disputes prior to litigation, and any such
license agreements may require us to pay royalties and other fees that could be significant. Furthermore, because of the substantial
amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential
information could be compromised by disclosure.
Some of the patents and patent applications
in the intellectual property portfolio are not within our complete control, which could reduce the value of such patents.
Some of our U.S. patents (which have associated
international patents and applications) are co-owned by the Regents of the University of California Berkeley (“UC Berkeley”).
UC Berkeley has licensed its rights under many of these patents to us, but we do not have a license to UC Berkeley’s rights
under three of these patents.
UC Berkeley has licensed their U.S. rights
in two of these three co-owned patents to an unrelated third party.
The third patent is a continuation-in-part
of a patent that UC Berkeley did license to us. Under the terms of the relevant license agreement between us and UC Berkeley, we
have exclusive rights to any claims that are fully supported by the specification in the parent application. But, any claims that
are not based on the specification in the parent application are co-owned by UC Berkeley and us, and UC Berkeley’s rights
in respect of such claims are not exclusively licensed to us. There is no assurance that we will be able to obtain a license to
UC Berkeley’s rights in any such claims on commercially reasonable terms or at all, and UC Berkeley may choose to license
its rights to third parties instead of us.
In addition, in connection with our acquisition
of certain assets from Equipois, we assumed the rights and obligations of Equipois with respect to certain patents and patent applications
under an in-license of intellectual property from a third party and subject to an out-license of that intellectual property to
an unrelated third party for use in a particular field. We do not have complete control over the prosecution of these patent applications.
As well, the license of intellectual property rights under these patents to third parties could reduce the value of our patent
portfolio and limit any income or license fees that we might receive if we were to attempt to transfer or license our rights under
any of our co-owned or licensed patents.
If we fail to comply with our obligations
in the agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to
our business relationships with our licensors, we could lose intellectual property rights that are important to our business.
We
are a party to two exclusive license agreements and one amendment with UC Berkeley, covering ten patents exclusively licensed to
us. In addition, in connection with our acquisition of certain assets from Equipois, we assumed the rights and obligations of Equipois
with respect to certain patents and patent applications under an in-license of intellectual property from a third party and subject
to an out-license of that intellectual property to an unrelated third party for use in a particular field.. We may also need to
obtain additional licenses from others to advance our research and development activities or allow the commercialization of our
devices or any other devices we may identify and pursue. Our license agreements with UC Berkeley and the rights and obligations
that we assumed in connection with the Equipois acquisition impose various development, diligence, commercialization, and other
obligations on us, and we any future license agreements may impose similar or other obligations on us. For example, under our license
agreements with UC Berkeley we are required to submit a commercialization plan with performance milestones and progress report
to UC Berkeley, and must satisfy specified minimum annual royalty payment obligations. In spite of our efforts, our licensors might
conclude that we have materially breached our obligations under such license agreements and might therefore terminate the license
agreements, thereby removing or limiting our ability to develop and commercialize products and technology covered by these license
agreements. If our license agreements with UC Berkeley is terminated, or if our agreements granting us intellectual property rights
in connection with the Equipois acquisition or any future agreements granting us material intellectual property rights are terminated
or impeded in a material way, competitors or other third parties would have the freedom to seek regulatory approval of, and to
market, products that may be identical or functionally similar to our devices and we may be required to cease our development and
commercialization of such devices. Any of the foregoing could have a material adverse effect on our competitive position, business,
financial conditions, results of operations and prospects.
Moreover,
disputes may arise between us and our counterparties regarding intellectual property subject to a licensing agreement, including:
|
|
·
|
the
scope of rights granted under the license agreement and other interpretation-related issues;
|
|
|
·
|
the
extent to which our devices, technology and processes infringe on intellectual property of the licensor that is not subject to
the licensing agreement;
|
|
|
·
|
the
sublicensing of patent and other rights under our collaborative research and development relationships;
|
|
|
·
|
our
diligence obligations under the license agreement and what activities satisfy those diligence obligations;
|
|
|
·
|
the
ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us
and our partners; and
|
|
|
·
|
the
priority of invention of patented or patentable technology.
|
In
addition, certain provisions in our license agreement with UC Berkeley may be susceptible to multiple interpretations. The resolution
of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant
intellectual property or technology, or increase what we believe to be our financial or other obligations under the agreement,
either of which could have a material adverse effect on our business, financial condition, results of operations and prospects.
Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing
arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected devices,
which could have a material adverse effect on our business, financial conditions, results of operations and prospects.
Patent terms may be inadequate to
protect our competitive position on our devices for an adequate amount of time.
Patents have a
limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally
20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and
the protection it affords, is limited. Even if patents covering our devices are obtained, once the patent life has expired, we
may be open to competition from competitive products. Given the amount of time required for the development, testing and regulatory
review of new devices, patents protecting such devices might expire before or shortly after such devices are commercialized. As
a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing
products similar or identical to ours.
If we fail to obtain or maintain
necessary regulatory clearances or approvals for our medical device products, or if clearances or approvals for future products
or modifications to existing products are delayed or not issued, our commercial operations would be harmed.
Our EksoGT product is a medical device
that is subject to extensive regulation by the Food and Drug Administration (“FDA”), the European Union and other governmental
authorities both inside and outside of the United States. These agencies enforce laws and regulations that govern the development,
testing, manufacturing, labeling, advertising, marketing and distribution, and market surveillance of our medical products. Our
failure to comply with these complex laws and regulations could have a material adverse effect on our business, results of operations,
financial condition and cash flows.
In the United States, before we can market
a new medical device, or a new use of, new claim for or significant modification to an existing product, we must first receive
either clearance under Section 510(k) of the FDCA or approval of a premarket approval (“PMA”) application from the
FDA, unless an exemption applies. Both the PMA and the 510(k) clearance process can be expensive, lengthy and uncertain. The FDA’s
510(k) clearance process may take anywhere from several months to over a year. The process of obtaining a PMA is much more costly
and uncertain than the 510(k) clearance process and generally takes from one to three years, or even longer, from the time the
application is filed with the FDA. In addition, PMA generally requires the performance of one or more clinical trials.
The FDA also has substantial discretion
in the medical device review process. Despite the time, effort and cost, we cannot assure you that any particular device will
be approved or cleared by the FDA. Any delay or failure to obtain necessary regulatory approvals could harm our business. Failure
can occur at any stage, and we could encounter problems that cause us to repeat or perform additional development, standardized
testing, pre-clinical studies and clinical trials. Any delay or failure to obtain necessary regulatory approvals could harm our
business.
The FDA or other non-U.S. regulatory authorities
can delay, limit or deny clearance or approval of a medical device candidate for many reasons, including:
|
|
·
|
a medical device candidate may not be deemed to be substantially equivalent to a device lawfully marketed either as a grandfathered device or one that was cleared through the 510(k) premarket notification process;
|
|
|
·
|
a medical device candidate may not be deemed to be in conformance with applicable standards and regulations;
|
|
|
·
|
FDA or other regulatory officials may not find the data from pre-clinical studies and clinical trials or other product testing date to be sufficient;
|
|
|
·
|
other non-U.S. regulatory authorities may not approve our processes or facilities or those of any of our third-party manufacturers, thereby restricting export; or
|
|
|
·
|
the FDA or other non-U.S. regulatory authorities may change clearance or approval policies or adopt new regulations.
|
Even after regulatory clearance or approval
has been granted, a cleared or approved product and its manufacturer are subject to extensive regulatory requirements relating
to manufacturing, labeling, packaging, adverse event reporting, storage, advertising and promotion for the product. If we fail
to comply with the regulatory requirements of the FDA or other non-U.S. regulatory authorities, or if previously unknown problems
with our products or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions,
including:
|
|
·
|
restrictions on the products, manufacturers or manufacturing process;
|
|
|
·
|
adverse inspectional observations (Form 483), warning letters, non-warning letters incorporating inspectional observations;
|
|
|
·
|
civil or criminal penalties or fines;
|
|
|
·
|
injunctions;
|
|
|
·
|
product seizures, detentions or import bans;
|
|
|
·
|
voluntary or mandatory product recalls and publicity requirements;
|
|
|
·
|
suspension or withdrawal of regulatory clearances or approvals;
|
|
|
·
|
total or partial suspension of production;
|
|
|
·
|
imposition of restrictions on operations, including costly new manufacturing requirements;
|
|
|
·
|
refusal to clear or approve pending applications or premarket notifications; and
|
|
|
·
|
import and export restrictions.
|
If imposed on us, any of these sanctions
could have a material adverse effect on our reputation, business, results of operations and financial condition.
Modifications to our EksoGT and our
future products may require new 510(k) clearances or premarket approvals, or may require us to cease marketing or recall the modified
products until clearances are obtained
On April 4, 2016, we received clearance
from the FDA to market our EksoGT robotic exoskeleton for use in the treatment of individuals with hemiplegia due to stroke, individuals
with spinal cord injuries at levels T4 to L5, and individuals with spinal cord injuries at levels of T3 to C7 (ASIA D), in accordance
with the device’s labeling. On July 19, 2016, we received clearance from the FDA to expand/clarify the indications and labeling
to expressly include individuals with hemiplegia due to stroke who have upper extremity function of at least 4/5 in only one arm.
Our prior cleared indications for use statement required that individuals with hemiplegia due to stroke have upper extremity function
of at least 4/5 in both arms.
An element of our strategy is to continue
to upgrade the EksoGT to incorporate new software and hardware enhancements. Any modification to a 510(k)-cleared device, including
our EksoGT, that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended
use, design, or manufacture, requires a new 510(k) clearance or, possibly, a PMA. The FDA requires every manufacturer to make this
determination in the first instance based on the final guidance document issued by the FDA in October 2017 addressing when to submit
a new 510(k) application due to modifications to 510(k)-cleared devices and a separate guidance document on when to submit a new
510(k) application due to software changes to 510(k)-cleared devices. Although largely aligned with the FDA’s longstanding
guidance document issued in 1997, the 2017 guidance includes targeted changes intended to provide additional clarity on when a
new 510(k) application is needed. The FDA may review our determinations regarding whether new clearances or approvals are necessary,
and may not agree with our decisions. If the FDA disagrees with our determinations for any future changes, or prior changes to
previously marketed products, as the case may be, we may be required to cease marketing or to recall the modified products until
we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties.
The manufacture of our products is
subject to extensive post-market regulation by the FDA. Our failure to meet strict regulatory requirements could require us to
pay fines, incur other costs or even close our facilities.
We are required to comply with the FDA’s
Quality System Regulation (“QSR”) which is a complex regulatory scheme that covers the procedures and documentation
of the design, testing, production, process controls, quality assurance, labeling, packaging, handling, storage, distribution,
installation, servicing and shipping of our marketed products. These regulatory requirements may significantly increase our production
costs and may even prevent us from making our products in amounts sufficient to meet market demand. If we change our approved manufacturing
process, the FDA may need to review the process before it may be used. The FDA enforces the QSR through periodic announced and
unannounced inspections of manufacturing facilities. Failure to comply with regulatory requirements such as QSR may result in changes
to labeling, restrictions on such products or manufacturing processes, withdrawal of the products from the market, voluntary or
mandatory recalls, a requirement to repair, replace or refund the cost of any medical device we manufacture or distribute, fines,
suspension of regulatory approvals, product seizures, injunctions or the imposition of civil or criminal penalties which would
adversely affect our business, operating results and prospects.
Federal, state and non-U.S. regulations
regarding the manufacture and sale of medical devices are subject to future changes. The complexity, timeframes and costs associated
with obtaining marketing clearances are unknown. Although we cannot predict the impact, if any, these changes might have on our
business, the impact could be material.
We may be subject to fines, penalties
or injunctions if we are determined to be promoting the use of our products for unapproved or “off-label” uses.
Any cleared or approved product may be
promoted only for its indicated uses and our promotional materials must comply with FDA and other applicable laws and regulations.
We believe that the specific use for which our products are marketed fall within the scope of the indications for use that have
been cleared by the FDA. However, if the FDA determines that our promotional materials or training constitutes promotion of an
unapproved use, it could request that we modify our promotional materials or subject us to regulatory or enforcement actions, including
the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine and criminal penalties. It is also possible
that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials
to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities,
such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged and adoption of the products
would be impaired.
Failure to comply with anti-kickback
and fraud regulations could result in substantial penalties and changes in our business operations.
Although we do not provide healthcare services,
submit claims for third-party reimbursement, or receive payments directly from Medicare, Medicaid or other third-party payers for
our products, we are subject to healthcare fraud and abuse regulation and enforcement by federal, state and foreign governments,
which could significantly impact our business. These laws may constrain the business and financial arrangements and relationships
through which we conduct our operations, including how we research, market, sell and distribute any product for which we have obtained
regulatory approval, or for which we obtain regulatory approval in the future. The principal U.S. federal laws implicated include,
but are not limited to, those that prohibit, among other things, (i) filing, or causing to be filed, false or improper claims for
federal payment, known as the false claims laws, (ii) payment, solicitation or receipt of unlawful inducements, directly or indirectly,
for the referral of business reimbursable under federally-funded health care programs, known as the anti-kickback laws, and (iii)
health care service providers from seeking reimbursement for providing certain services to a patient who was referred by a physician
who has certain types of direct or indirect financial relationships with the service provider, known as the Stark law. Many states
have similar laws that apply to reimbursement by state Medicaid and other government funded programs as well as in some cases to
all payers. In addition, we may be subject to federal and state data privacy laws that govern the privacy and security of health
information in specified circumstances, many of which differ from each other in significant ways and may not have the same effect,
thus complicating compliance efforts.
Efforts to ensure that our business arrangements will comply with applicable healthcare laws and regulations
will involve substantial costs. We are subject to the risk that a person or government could allege we have engaged in fraud or
other misconduct, even if none occurred. It is possible that governmental and enforcement authorities will conclude that our business
practices do not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other
healthcare laws and regulations. If our operations are found to be in violation of any of the laws described above or any other
governmental regulations that apply to us now or in the future, we may be subject to penalties, including civil and criminal penalties,
damages, fines, disgorgement, exclusion from governmental health care programs, additional integrity oversight and reporting obligations,
contractual damages, reputational harm and the curtailment or restructuring of our operations, any of which could adversely affect
our ability to operate our business and our financial results.
Changes in law or regulation could
make it more difficult and costly for us to manufacture, market and distribute our products or obtain or maintain regulatory approval
of new or modified products.
From time to time, legislation is drafted and introduced in Congress that could significantly change the
statutory provisions governing the regulatory approval, manufacture and marketing of regulated devices. In addition, FDA regulations
and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products.
Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times
of future products. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency in ways that may
significantly affect our business and our products. Elections could result in significant changes in, and uncertainty with respect
to, legislation, regulation and government policy that could significantly impact our business and the health care industry. It
is impossible to predict whether legislative changes will be enacted or FDA regulations, guidance or interpretations changed, and
what the impact of such changes, if any, may be.
Any change in the laws or regulations that
govern the clearance and approval processes relating to our current and future products could make it more difficult and costly
to obtain clearance or approval for new products, or to produce, market, and distribute existing products. Significant delays in
receiving clearance or approval, or the failure to receive clearance or approval, for any new products would have an adverse effect
on our ability to expand our business.
Healthcare changes in the U.S. and
other countries, including recently enacted legislation reforming the U.S. healthcare system, could have a negative impact on our
future operating results.
In the United States and some foreign
jurisdictions, there have been a number of legislative and regulatory proposals to change the health care system in ways that
could affect our ability to sell our products profitably. For example, in 2010, the Patient Protection and Affordable Care Act
(“ACA”) was enacted into law. The legislation seeks to reform the United States healthcare system. It is far-reaching
and is intended to expand access to health insurance coverage, improve quality and reduce costs over time. We expect the law will
have a significant impact upon various aspects of our business operations. The ACA reduces Medicare and Medicaid payments to hospitals,
clinical laboratories and pharmaceutical companies, and could otherwise reduce the volume of medical procedures. These factors,
in turn, could result in reduced demand for our products and increased downward pricing pressure. It is also possible that the
ACA will result in lower reimbursements. While the ACA is intended to expand health insurance coverage to uninsured persons in
the United States, the impact of any overall increase in access to healthcare on sales of our products remains uncertain. The
new U.S. Presidential administration and the majority party in both Houses of the U.S. Congress have indicated their desire to
repeal the Affordable Care Act. It is unclear whether, when and how that repeal will be effectuated and what the effect on the
healthcare sector will be. However, in December 2017, the Tax Cuts and Jobs Act was enacted and signed into law, one part of which
repeals the “individual mandate” introduced by the ACA starting in 2019. The repeal of the “individual mandate”
may have an adverse effect on ACA insurance markets and lead to further legislative changes. In addition, the new law imposes
a 2.3 percent excise tax on medical devices that will apply to United States sales of our medical device product. Although a moratorium
was placed on the medical device excise tax in 2016, 2017 and 2018, absent further legislative action, the medical device excise
tax will apply to sales of our medical device product beginning on January 1, 2020. There have been other changes to the ACA since
the enactment of the Tax Cuts and Jobs Act, and Congress could still consider additional legislation to repeal or replace all
or certain elements of the ACA. In addition, other reform legislation has been passed subsequent to the enactment of the ACA,
including measures that reduced reimbursement for certain providers and entities under federal health care programs. The outlook
for the healthcare sector is unclear, and we are unable to predict the future course of federal or state healthcare legislation
and regulations. Changes in the law or regulatory framework that reduce our revenues or increase our costs could also harm our
business, financial condition and results of operations and cash flows.
If our medical products, or malfunction
of our medical products, cause or contribute to a death or a serious injury, we will be subject to medical device reporting regulations,
which can result in voluntary corrective actions or agency enforcement actions.
Under the FDA’s medical device reporting
(“MDR”) regulations, we are required to report to the FDA any incident in which our product may have caused or contributed
to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or
contribute to death or serious injury. For example, we have been informed of a limited number of events with respect to our EksoGT
devices that have been determined to be reportable pursuant to the MDR regulations. In each case, the required MDR report was
filed with the FDA.
In addition, all manufacturers bringing
medical devices to market in the European Economic Area are legally bound to report any incident that led or might have led to
the death or serious deterioration in the state of health of a patient, user or other person, and which the manufacturer’s
device is suspected to have caused, to the competent authority in whose jurisdiction the incident occurred. In such case, the manufacturer
must file an initial report with the relevant competent authority, which would be followed by further evaluation or investigation
of the incident and a final report indicating whether further action is required. The events described above that were reported
to the FDA were also reported to the relevant EU regulatory authorities.
We are also required to follow detailed
recordkeeping requirements for all Company-initiated medical device corrections and removals, and to report such corrective and
removal actions to the FDA if they are carried out in response to a risk to health and have not otherwise been reported under the
MDR regulations. Any adverse event involving our products could result in future voluntary corrective actions, such as recalls
or customer notifications, or agency action, such as inspection or enforcement action. Recalls of our products, or agency actions
relating to our failure to comply with our reporting or recordkeeping obligations, could harm our reputation and financial results.
Discovery of serious safety issues
with our products, or a recall of our products either voluntarily or at the direction of the FDA or another governmental authority,
could have a negative impact on us.
The FDA and similar foreign governmental
authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects
in design or manufacture or in the event that a product poses an unacceptable risk to health. In addition, manufacturers may, under
their own initiative, recall a product if any material deficiency in a device is found. A government-mandated or voluntary recall
by us could occur as a result of an unacceptable risk to health, component failures, manufacturing errors, design or labeling defects
or other deficiencies and issues. To date, we have initiated only one field action in which we voluntarily accelerated our maintenance
schedule based on field usage.
When a medical human exoskeleton is used
by a paralyzed individual to walk, the individual relies completely on the exoskeleton to hold them upright. There are many exoskeleton
components that, if they were to fail catastrophically, could cause a fall resulting in severe injury or death of the patient.
Certain of our competitors have reported injuries caused by the malfunction of human exoskeleton devices (in at least one case
to the FDA). Injuries caused by the malfunction or misuse of human exoskeleton devices, even where such malfunction or misuse occurs
with respect to one of our competitor’s products, could cause regulatory agencies to implement more conservative regulations
on the medical human exoskeleton industry, which could significantly increase our operating costs.
Similarly, when an industrial exoskeleton
is used by a healthy individual — for example to operate heavy machinery overhead — malfunction
of the device at an inopportune moment could result in severe injury or death of the person using the device. Such occurrences
could result in regulatory action on the part of OSHA or its foreign counterparts.
Any future recalls of any of our products
could divert managerial and financial resources, impair our ability to manufacture our products in a cost-effective and timely
manner, and have an adverse effect on our reputation, results of operations and financial condition. In some circumstances, such
adverse events could also cause delays in new product approvals. We may also be required to bear other costs or take other actions
that may have a negative impact on our future sales and our ability to generate profits.
In addition, personal injuries relating
to the use of our products could also result in product liability claims being brought against us. Any product liability claim
brought against us, with or without merit, could result in substantial damages, be costly and time-consuming to defend and could
increase our insurance rates or prevent us from securing insurance coverage in the future.
We could be exposed to significant
liability claims if we are unable to obtain insurance at adequate levels or otherwise protect ourselves against potential product
liability claims.
The testing, manufacture, marketing and
sale of medical devices and industrial products entail the inherent risk of liability claims or product recalls. Although we maintain
product liability insurance, the coverage is subject to deductibles and limitations, and may not be adequate to cover future claims.
A successful product liability claim or product recall could inhibit or prevent the successful commercialization of our products,
cause a significant financial burden on us, or both, which in either case could have a material adverse effect on our business
and financial condition.
Warranty claims or any other service
and repairs provided by the Company at its expense could have a material adverse effect on our business.
Sales of our EksoGT generally include a
one-year warranty for parts and services in the U.S. and a two-year warranty in Europe, the Middle East and Africa. We also generally
provide customers with an option to purchase an extended warranty for up to an additional three years. The costs associated with
such warranties, including any warranty-related legal proceedings, could have a material adverse effect on our results of operations,
cash flows and liquidity. As we enhance our product and in an effort to build our brand and drive adoption, the Company has elected
to incur increased service expenses related to an accelerated maintenance program, field corrections and the implementation of
technological improvements developed subsequent to many of our units being placed into service, sometimes outside of its warranty
and contractual obligations. Continuation of these activities could have a material adverse effect on our results of operations,
cash flows and liquidity.
If we are not able to both obtain
and maintain adequate levels of third-party reimbursement for our products, it would have a material adverse effect on our business.
Healthcare providers and related facilities
are generally reimbursed for their services through payment systems managed by various third-party payers, including governmental
agencies worldwide, private insurance companies, and managed care organizations. The manner and level of reimbursement in any
given case may depend on the site of care, the procedure(s) performed, the final patient diagnosis, the device(s) utilized, available
budget, or a combination of these factors, and coverage and payment levels are determined at each payer’s discretion. The
adoption of our product by our customers will depend on their ability to obtain adequate reimbursement for treatments provided
using our product from third-party payers. The coverage policies and reimbursement levels of these third-party payers may impact
the decisions of healthcare providers and facilities regarding which medical products they purchase and the prices they are willing
to pay for those products. Reimbursement rates can also affect the acceptance rates of new technologies.
We have no direct control over payer decision-making
with respect to coverage and payment levels for our medical device products. Additionally, we expect many payers to continue to
explore cost-containment strategies (e.g., comparative and cost-effectiveness analyses, so-called “pay-for-performance”
programs implemented by various public and private payers, and expansion of payment bundling schemes such as Accountable Care Organizations,
and other such methods that shift medical cost risk to providers) that may potentially impact coverage and/or payment levels for
our current products or products we develop.
In addition to the ACA, which is intended
to reduce the cost of healthcare over time, initiatives sponsored by government agencies, legislative bodies and the private sector
to limit the growth of healthcare costs, including price regulation and competitive pricing, are ongoing in markets where we do
business. Pricing pressure has also increased in these markets due to continued consolidation among health care providers, trends
toward managed care, the shift towards governments becoming the primary payers of health care expenses and laws and regulations
relating to reimbursement and pricing generally. Reductions in reimbursement levels or coverage or other cost-containment measures
could adversely affect customer demand or the price customers may be willing to pay for our products and could result in decreased
revenue.
Clinical studies regarding our products
may not provide sufficient data to either cause third-party payers to approve reimbursement or to make human exoskeletons a standard
of care.
Our business plan relies on broad adoption
of human exoskeletons to provide neuro-rehabilitation in the form of gait training to individuals who have suffered a neurological
injury or disorder. Although use of human exoskeletons in neuro-rehabilitation is new, use of robotic devices to provide gait training
has been going on for over a decade and the clinical studies relating to such devices have had both positive and negative outcomes.
Much of the rehabilitation community has rejected the use of such devices based on the data from some of these studies. Although
we believe that human exoskeletons will outperform such robotic equipment, this has not been proven. Furthermore, it may prove
impossible to prove an advantage in a timely manner, or at all, which could prevent broad adoption of our products.
Part of our business plan relies on broad
adoption of our robotic exoskeleton to provide “early mobilization” of individuals who have been immobilized by an
injury, disease, or other condition. Although the health benefits of other methods of “early mobilization” have been
demonstrated in clinical studies in fields such as stroke, those studies did not test early mobilization with human exoskeletons
directly. To date, our device has been the subject of several clinical trials, some of which have been partially sponsored by
us, but most of which are non-Ekso-sponsored independent studies conducted by rehabilitation institutions. Data from these studies
was provided to the FDA as part of our 510(k) application submissions. In addition, there are several ongoing independent studies
to investigate additional indications for use for our device, as well as to evaluate clinical and non-clinical outcomes of using
the Ekso device, and we are currently in the planning stage for several Company-led studies. Further, a Company-sponsored clinical
trial, entitled WISE (Walking Improvement for SCI with Exoskeletons), is being conducted to evaluate improvement in independent
gait speeds of SCI patients undergoing rehabilitation with the EksoGT and to compare it to both conventional therapy and a control
group.
If current and future clinical trials do
not provide sufficient data to support our belief that early mobilization through the use of exoskeletons improves health outcomes,
or such studies actually contradict that belief, market acceptance of the human exoskeletons could fail to increase or could decrease
and our business could be harmed.
Any studies that we initiate, whether
to drive market adoption and support commercialization, or to support additional product submissions or new claims, will be expensive
and time consuming, which could harm our financial results.
Initiating and completing clinical trials
necessary to drive market adoption and support commercialization, or to support additional product submissions or new claims, is
time consuming and expensive. Conducting successful clinical studies requires the enrollment of large numbers of patients, and
suitable patients may be difficult to identify and recruit. Delays in patient enrollment or failure of patients to continue to
participate in a clinical trial may cause an increase in costs and delays in future clearances or approvals of our products or
result in the failure of the clinical trial. Such increased costs and delays or failures could adversely affect our business, results
of operations and prospects.
In addition, all clinical trial activities
that we undertake are subject to extensive regulation and review by numerous governmental authorities both in the United States
and abroad. Clinical trials intended to support a 510(k) applications or PMA must be conducted in compliance with the FDA’s
Good Clinical Practice regulations and similar requirements in foreign jurisdictions. Sufficient and appropriate clinical protocols
to demonstrate safety and efficacy may be required and we may not adequately develop such protocols to support future clearances
and approvals. Compliance with these regulations is costly, and any failure to do so could delay or prevent us from using data
obtained from such activities to support our claims that a product is safe and effective.
The results of clinical trials may
not support new product submissions or claims or may result in the discovery of adverse side effects.
Despite considerable time and expense invested
in clinical trials, the FDA may not consider any data that we obtain adequate to demonstrate safety and efficacy for future submissions.
Even if our clinical trials are completed as planned, we cannot be certain that their results will support our intended claims
or demonstrate that our product candidates are safe and effective for the proposed indicated uses, which could cause us to abandon
a product candidate and may delay development of others. Moreover, the results of early clinical trials are not necessarily predictive
of future results, and any product we advance into clinical trials may not have favorable results in later clinical trials. Any
delay or termination of our clinical trials or studies could delay the filing of associated product submissions and, ultimately,
our ability to commercialize products requiring submission of clinical data or relying on clinical data for market acceptance.
It is also possible that patients enrolled
in a clinical trial will experience adverse side effects that are not currently part of the product candidate’s safety profile,
which could cause us to delay or abandon development of such product
Our business may suffer if we are
not able to attract and retain key employees.
Our success depends on our ability to identify,
hire, train and retain highly qualified managerial, technical and sales and marketing personnel. In addition, as we introduce new
products or services, we will need to hire additional personnel. Currently, competition for personnel with the required knowledge,
skill and experiences is intense, particularly in the San Francisco Bay area, where we are headquartered, and we may not be able
to attract, assimilate or retain such personnel. The inability to attract and retain the necessary managerial, technical and sales
and marketing personnel could have a material adverse effect on our business, results of operations and financial condition.
Changes in our management team may
adversely affect our operations.
Over the last several months, we have experienced
turnover in our senior management. Most recently, Maximilian Scheder-Bieschin, our Chief Financial Officer, ceased being an employee
of the Company as of August 1, 2018 and transitioned to being a consultant of the Company, where he will remain our Chief Financial
Officer and engaged in day-to-day operations until his replacement is hired and begins work with us. At that point, Mr. Scheder-Bieschin
will cease being our Chief Financial Officer, but will remain a consultant to us until December 31, 2018 to assist with the transition
of his role and responsibilities. As well, Gregory Davault, previously our Chief Marketing Officer, resigned effective as of May
15, 2018.
While we expect to engage in an orderly
transition process as we integrate newly appointed officers and managers, we face a variety of risks and uncertainties relating
to management transition, including diversion of management attention from business concerns, failure to retain other key personnel
or loss of institutional knowledge. These risks and uncertainties could result in operational and administrative inefficiencies
and added costs, which could adversely impact our results of operations, stock price and research and development of our products.
We will experience long and variable
sales cycles, which could have a negative impact on our results of operations for any given quarter and may result in volatility
in our stock price.
The EksoGT has a lengthy sale and purchase
order cycle because it is a major capital item and generally requires the approval of senior management at purchasing institutions,
which may contribute to substantial fluctuations in our quarterly operating results. Other factors that may cause our operating
results to fluctuate include:
|
|
·
|
general economic uncertainties and political concerns;
|
|
|
|
|
|
|
·
|
the introduction of new products or product lines;
|
|
|
|
|
|
|
·
|
product modifications;
|
|
|
|
|
|
|
·
|
the level of market acceptance of new products;
|
|
|
|
|
|
|
·
|
the availability of coverage and adequate reimbursement by third-party payers
of services provided using our products;
|
|
|
·
|
the timing and amount of research and development and other expenditures;
|
|
|
|
|
|
|
·
|
timing of the receipt of orders from, and product shipments to, distributors and customers;
|
|
|
|
|
|
|
·
|
changes in the distribution arrangements for our products;
|
|
|
|
|
|
|
·
|
manufacturing or supply delays;
|
|
|
|
|
|
|
·
|
the time needed to educate and train additional sales and manufacturing personnel; and
|
|
|
|
|
|
|
·
|
costs associated with defending our intellectual property.
|
In addition to these factors, expenditures
are based, in part, on expected future sales. If sales levels in a particular quarter do not meet expectations, we may be unable
to adjust operating expenses quickly enough to compensate for the shortfall of sales, and our results of operations may be adversely
affected.
International sales of our products
account for a portion of our revenues, which will expose us to certain operating risks. If we are unable to successfully manage
our international activities, our net sales, results of operations and financial condition could be adversely impacted.
Our business currently depends in part
on our activities in Europe and other foreign markets and we are actively looking to broaden our footprint in Asia. Our international
activities are subject to a number of risks inherent in selling and operating abroad. These include:
|
|
·
|
failure of local laws to provide the same degree of protection against infringement of our intellectual property rights;
|
|
|
|
|
|
|
·
|
protectionist laws and business practices that favor local competitors, which could slow our growth in international markets;
|
|
|
|
|
|
|
·
|
the expense of establishing facilities and operations in new foreign markets;
|
|
|
|
|
|
|
·
|
building an organization capable of supporting geographically dispersed operations;
|
|
|
|
|
|
|
·
|
challenges caused by distance, language and cultural differences;
|
|
|
|
|
|
|
·
|
challenges caused by differences in legal regulations, markets, and customer preferences, which may limit our ability to adapt our products or succeed in other regions;
|
|
|
·
|
multiple, conflicting, and changing laws and regulations, including complications due to unexpected changes in regulatory requirements, foreign laws, tax schemes, international import and export legislation, trading and investment policies, exchange controls and tariff and other trade barriers;
|
|
|
|
|
|
|
·
|
foreign tax consequences;
|
|
|
|
|
|
|
·
|
fluctuations in currency exchange rates and foreign currency translation adjustments;
|
|
|
|
|
|
|
·
|
foreign exchange controls that might prevent us from repatriating income earned outside the United States;
|
|
|
|
|
|
|
·
|
imposition of public sector controls;
|
|
|
|
|
|
|
·
|
differing payer reimbursement regimes, governmental payers or patient self-pay systems and
price controls;
|
|
|
|
|
|
|
·
|
political, economic and social instability; and
|
|
|
|
|
|
|
·
|
restrictions on the export or import of technology.
|
Some of the countries in which we operate
and seek to expand are in emerging markets where legal systems may be less developed or familiar to us. Other jurisdictions in
which we conduct business may establish legal and regulatory regimes that differ materially from United States laws and regulations.
Compliance with diverse legal requirements is costly and time-consuming and requires significant resources. Violations of one or
more of these regulations in the conduct of our business could result in significant fines or monetary damages, criminal sanctions
against us or our officers, prohibitions on doing business, unfavorable publicity and other reputational damage, restrictions on
our ability to process information and allegations by our clients that we have not performed our contractual obligations.
As we look to expand into China, we may
be exposed to the additional risks of doing business in China. Our success in the Chinese markets may be adversely affected by
China’s continuously evolving laws and regulations, including those relating to taxation, import and export tariffs, currency
controls, anti-corruption, environmental regulations, indigenous innovation, and intellectual property rights and enforcement of
those rights. Enforcement of existing laws or agreements may be inconsistent. In addition, changes in the political environment,
governmental policies or United States-China relations could result in revisions to laws or regulations or their interpretation
and enforcement, exposure of our proprietary intellectual property, increased taxation, restrictions on imports, import duties
or currency revaluations, which could have an adverse effect on our business plans and operating results.
If we are unable to meet and overcome these
challenges, then our international operations may not be successful, which could adversely affect our net sales, results of operations
and financial condition and limit our growth.
The disruption or loss of relationships
with vendors and suppliers for the components of our products could materially adversely affect our business.
Our ability to manufacture and market our
products successfully is dependent on relationships with both third party vendors and suppliers. Although most of the raw materials
that we use to manufacture our products are readily available from a number of suppliers, we generally procure raw materials and
components through purchase orders, with no guaranteed supply arrangements. Our inability to obtain sufficient quantities of various
components, if and as required in the future, may subject us to:
|
|
·
|
delays in delivery or shortages in components that could interrupt and delay manufacturing and result in cancellations of orders for our products;
|
|
|
·
|
increased component prices and supply delays as we establish alternative suppliers;
|
|
|
|
|
|
|
·
|
inability to develop alternative sources for product components;
|
|
|
|
|
|
|
·
|
required modifications of our products, which may cause delays in product shipments, increased manufacturing costs, and increased product prices; and
|
|
|
|
|
|
|
·
|
increased inventory costs as we hold more inventory than we otherwise might in order to avoid problems from shortages or discontinuance, which may result in write-offs if we are unable to use all such products in the future.
|
In addition, failure of any one supplier’s
components could result in a product recall, which could materially adversely affect our business, operations and cash flows.
We may be unable to manage our growth
and entry into new business areas.
If demand for our exoskeleton products
exceeds our capacity to provide services timely and efficiently, then we may need to expand our operations accordingly and swiftly.
Our management believes that establishing industry leadership will require us to:
|
|
·
|
test, introduce and develop new products and services including enhancements to our existing products;
|
|
|
|
|
|
|
·
|
develop and expand the breadth of products and services offered;
|
|
|
|
|
|
|
·
|
develop and expand our market presence through relationships with third parties; and
|
|
|
|
|
|
|
·
|
generate satisfactory revenues from such expanded products or services to fund the foregoing requirements while obtaining and maintaining satisfactory profit margins.
|
To be able to expand our operations in
a cost-effective or timely manner and increase the overall market acceptance of our products and services in this manner, we will
need additional capital and technical and managerial human resources. These additional resources may not be available to us. Our
failure to timely and efficiently expand our operations and successfully achieve the four requirements listed above could have
a material adverse effect on our business, results of operations and financial condition.
New product introductions may adversely
impact our financial results.
We may introduce new products with enhanced
features and extended capabilities from time to time. The products may be subject to various regulatory processes, and we may need
to obtain and maintain regulatory approvals in order to sell our new products. If a potential purchaser believes that we plan to
introduce a new product in the near future or if a potential purchaser is located in a country where a new product that we have
introduced has not yet received regulatory approval, planned purchases may be deferred or delayed. As a result, new product introductions
may adversely impact our financial results.
The acquisition of other companies,
businesses, or technologies could result in operating difficulties, dilution, and other harmful consequences.
We may selectively pursue strategic acquisitions,
any of which could be material to our business, operating results, and financial condition. Future acquisitions could divert management’s
time and focus from operating our business. In addition, integrating an acquired company, business or technology is risky and may
result in unforeseen operating difficulties and expenditures associated with integrating employees from the acquired company into
our organization and integrating each company’s accounting, management information, human resources and other administrative
systems to permit effective management. The anticipated benefits of future acquisitions may not materialize. Future acquisitions
or dispositions could result in potentially dilutive issuances of our equity securities, the incurrence of debt, contingent liabilities
or amortization expenses, or write-offs of goodwill, any of which could harm our financial condition. Future acquisitions may also
require us to obtain additional financing, which may not be available on favorable terms or at all.
We may incur losses associated with
currency fluctuations and may not be able to effectively hedge our exposure
Our operating results are subject to volatility
due to fluctuations in foreign currency exchange rates. Our primary exposure to fluctuations in foreign currency exchange rates
relates to revenue and operating expenses denominated in currencies other than the U.S. dollar. The weakening of foreign currencies
relative to the U.S. dollar adversely affects our foreign currency-denominated revenue. In the past, we have not hedged our exposures
to foreign currencies or entered into any other derivative instruments and we have no current plans to do so. See “Item 7A.
Quantitative and Qualitative Disclosures About Market Risk” for additional discussion on the impact of foreign exchange risk.
Natural or other disasters could
disrupt our business and result in loss of revenue or in higher expenses.
Natural disasters, terrorist activities,
military conflict and other business disruptions could seriously harm our revenue and financial condition and increase our costs
and expenses. Our corporate headquarters are located in California, a seismically active region. A natural disaster in any of our
major markets in North America or Europe could have a material adverse impact on our operations, operating results and financial
condition. Further, any unanticipated business disruption caused by Internet security threats, damage to global communication networks
or otherwise could have a material adverse impact on our operating results.
Risks Related to our Financial Condition
We have a history of losses and we
may not achieve or sustain profitability in the future.
We have incurred losses in each fiscal
year since our incorporation in 2005. Our net losses were $29.1 million, $23.5 million, and $19.6 million for the years ended December
31, 2017, 2016, and 2015, respectively. As of December 31, 2017 and June 30, 2018, we had an accumulated deficit of $144.2 million
and $160.0 million, respectively.
Our future profitability is dependent upon
our ability to successfully execute our business plan. We can provide no assurance regarding when, if ever, we will become profitable.
Even if we do become profitable, we may not be able to sustain or increase profitability on a quarterly or annual basis. Accordingly,
we may continue to generate losses for the foreseeable future and, in the extreme case, discontinue operations.
We may not be able to reduce the
cost to manufacture or service our products as planned.
Our business plan assumes that exoskeletons
can be manufactured more inexpensively than they are currently being manufactured. However, we have not yet found a way to significantly
reduce the manufacturing cost of our products and doing so may prove more difficult than expected or even impossible. For example,
if expectations for greater functionality of the products drive costs up as other factors drive costs down, the result may be that
the overall cost of manufacturing the product stays the same or even increases. Likewise, we currently provide service and support
of our products for our customers at a high standard (both in and out of warranty), and plan on continuing to do so. Our business
plan also assumes that as we continue to improve our product, we achieve improved levels of product reliability and decreased service
cost and frequency, which also may prove more difficult than expected.
We may not be able to leverage our
cost structure or achieve better margins.
Due to the early stage of our commercial
efforts, and particularly the early stage of customer adoption of our products, our current sales and marketing, research and development,
and general and administrative expenses are each a higher percentage of sales than they will need to be for us to reach profitability.
While we do expect these expenses to grow as our business grows, we also expect these expenses to decline as a percentage of revenues
over time. If we are unable to leverage these costs and grow revenues at a greater pace than these operating expenses as we expect,
we will not be able to achieve viable operating margins and profitability.
If we are unable to obtain additional
financing on acceptable terms, we may have to curtail our growth or cease our development plans and operations.
The operation of our business and our growth
efforts will require significant cash outlays and advance capital equipment expenditures and commitments.
We have been largely dependent on capital
raised through the sale of equity securities in various public and private offerings, and going forward will be largely dependent
on capital raised in any future offerings, to implement our business plan and support our operations.
Based upon our current cash resources,
the recent rate of using cash for operations and investment, and assuming modest increases in current revenue offset by incremental
increases in expenses related to increased sales and marketing and research and development, and a potential increase in rental
activity from our medical device business, we believe it has sufficient resources to meet its financial obligations into the first
quarter of 2019. We will require significant additional financing. We intend to pursue opportunities to obtain additional financing
in the future through public or private equity and/or debt financings, corporate collaborations, or warrant solicitations.
We anticipate for the foreseeable future
that cash on hand and cash generated from operations will not be sufficient to meet our cash requirements, and that we will need
to raise additional capital through investments to fund our operations and growth. We cannot assure you that we will be able to
raise additional working or growth capital as needed on terms acceptable to us, if at all. If we are unable to raise capital as
needed, we may be required to reduce the scope of our business development activities, which could harm our business plans, financial
condition and operating results, or cease our operations entirely.
Our reported financial results may
be adversely affected by changes in our accounting policies or in accounting principles generally accepted in the United States.
Generally accepted accounting principles
in the United States are subject to interpretation by the Financial Accounting Standards Board, the American Institute of Certified
Public Accountants, the SEC and various bodies formed to promulgate and interpret appropriate accounting principles. The accounting
principles and accompanying accounting pronouncements, implementation guidelines and interpretations for many aspects of our business,
including revenue recognition, are highly complex and involve subjective judgments. Some of these policies require the use of estimates
and assumptions that may affect the value of our assets and liabilities, and financial results. We may be required or determine
that it is appropriate to change our accounting policies or the manner in which they are implemented as circumstances change and
additional information becomes known. A change in applicable rules, their interpretation, or their application could have a significant
effect on our reported financial results, and could affect the reporting of transactions completed before the announcement of a
change.
Changes in tax laws or exposure to
additional income tax liabilities could have a material adverse impact on our financial condition and results of operations.
We are subject to income taxes as well
as non-income based taxes, in both the U.S. and various jurisdictions outside the U.S. On December 22, 2017, the Tax Cuts and
Jobs Act was enacted into law. This new law includes significant changes to the U.S. corporate income tax system, including a
permanent reduction in the corporate income tax rate from 35% to 21%, limitations on the deductibility of interest expense and
executive compensation and the transition of U.S. international taxation from a worldwide tax system to a territorial tax system.
The Company is currently assessing the impact of this legislation, but currently anticipates no major short-term impact.
In addition, we are subject to ongoing
tax audits in various jurisdictions. Tax authorities may disagree with certain positions we have taken and assess additional taxes
and penalties. We regularly assess the likely outcomes of these audits in order to determine the appropriateness of our tax provision.
However, there can be no assurance that we will accurately predict the outcomes of these audits, and the actual outcomes of these
audits could have a material impact on our consolidated earnings and financial condition.
Risks Related to our Common Stock
We may raise additional funds in the future through the
issuances of equity securities or debt, which funding may be dilutive to stockholders or impose operational restrictions on us.
We may need to raise additional capital through the sale of
equity securities or the issuance of short- and long-term debt. If we raise additional funds by issuing shares of our common stock,
our stockholders will experience dilution. If we raise additional funds by issuing securities exercisable or convertible into shares
of our common stock, our stockholders will experience dilution in the event the securities are exercised or converted, as the case
may be, into shares of our common stock. Further, prices at which new investors would be willing to purchase our securities may
be lower than the price at which existing stockholders purchased their shares, which may create downward pressure on the trading
price of the common stock. In addition, the terms of any new securities may include liquidation or other preferences that may adversely
affect the rights of our existing stockholders.
Debt financing may involve agreements containing covenants limiting
or restricting our ability to take specific actions, such as incurring additional debt, issuing equity securities, making capital
expenditures for certain purposes or above a certain amount, or declaring dividends. In addition, any equity securities or debt
that we issue may have rights, preferences and privileges senior to those of the securities held by our stockholders.
The ability of our Board of Directors to issue additional
stock may prevent or make more difficult certain transactions, including a sale or merger of the Company.
Our Board of Directors is authorized to issue up to 10,000,000
shares of preferred stock with powers, rights and preferences designated by it. Shares of voting or convertible preferred stock
could be issued, or rights to purchase such shares could be issued, to create voting impediments or to frustrate persons seeking
to effect a takeover or otherwise gain control of us. The ability of the Board of Directors to issue such additional shares of
preferred stock, with rights and preferences it deems advisable, could discourage an attempt by a party to acquire control of us
by tender offer or other means. Such issuances could therefore deprive stockholders of benefits that could result from such an
attempt, such as the realization of a premium over the market price for their shares in a tender offer or the temporary increase
in market price that such an attempt could cause. Moreover, the issuance of such additional shares of preferred stock to persons
friendly to the Board of Directors could make it more difficult to remove incumbent officers and directors from office even if
such change were to be favorable to stockholders generally.
Being a public company is expensive
and administratively burdensome.
As a public reporting company, we are subject
to the information and reporting requirements of the Securities Act, the Securities Exchange Act of 1934, as amended, and other
federal securities laws, rules and regulations related thereto, including compliance with the Sarbanes-Oxley Act. Complying with
these laws and regulations requires the time and attention of our Board of Directors and management, and increases our expenses.
Among other things, we are required to:
|
|
·
|
maintain and evaluate a system of internal controls over financial reporting in compliance with the requirements of Section 404 of the Sarbanes-Oxley Act and the related rules and regulations of the SEC and the Public Company Accounting Oversight Board;
|
|
|
|
|
|
|
·
|
maintain policies relating to disclosure controls and procedures;
|
|
|
|
|
|
|
·
|
prepare and distribute periodic reports in compliance with our obligations under federal securities laws;
|
|
|
|
|
|
|
·
|
institute a more comprehensive compliance function, including with respect to corporate governance; and
|
|
|
|
|
|
|
·
|
involve, to a greater degree, our outside legal counsel and accountants in the above activities.
|
The costs of preparing and filing annual
and quarterly reports, proxy statements and other information with the SEC and furnishing audited reports to stockholders is expensive
and much greater than that of a privately-held company, and compliance with these rules and regulations may require us to hire
additional financial reporting, internal controls and other finance personnel, and will involve a material increase in regulatory,
legal and accounting expenses and the attention of management. We anticipate that these costs and compliance initiatives will increase
as a result of the fact that we ceased to be an “emerging growth company,” as defined in the Jumpstart Our Business
Startups Act of 2012, as of December 31, 2017. In particular, we are now subject to certain disclosure requirements that are applicable
to other public companies that had not been applicable to us as an emerging growth company. These requirements include:
|
|
·
|
compliance with the auditor attestation
requirements in the assessment of our internal control over financial reporting;
|
|
|
·
|
compliance with any requirement that may
be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s
report providing additional information about the audit and the financial statements;
|
|
|
·
|
full disclosure and analysis obligations
regarding executive compensation; and
|
|
|
·
|
compliance with regulatory requirements
of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously
approved.
|
There can be no assurance that we will
be able to comply with the applicable regulations in a timely manner, if at all. In addition, being a public company makes it more
expensive for us to obtain director and officer liability insurance. In the future, we may be required to accept reduced coverage
or incur substantially higher costs to obtain this coverage. These factors could also make it more difficult for us to attract
and retain qualified executives and members of our Board of Directors, particularly directors willing to serve on our audit committee.
Any failure to maintain effective
internal control over our financial reporting could materially adversely affect us.
Section 404 of the Sarbanes-Oxley Act of
2002 requires us to include in our annual reports on Form 10-K and quarterly reports on Form 10-Q an assessment by management of
the effectiveness of our internal control over financial reporting. We previously reported a material weakness in our information
technology general controls as of December 31, 2016, and as a result, determined that our internal control over financial reporting
was not effective at December 31, 2016.
As a natural course of business, management
has, over the course of 2017, been working to further strengthen our internal controls. Specifically, we have increased segregation
of duties and implemented a more robust accounting and enterprise resource planning system (which became operational in October
2017). While we believe that the policies, processes and procedures we have put in place over the course of 2017 will be sufficient
to render our internal controls over financial reporting effective, our initiatives may not prove successful and management may
not be able to conclude that our internal control over financial reporting is effective. Furthermore, even if management were to
reach such a conclusion, if our independent registered public accounting firm is not satisfied with the adequacy of our internal
control over financial reporting, or if the independent auditors interpret the requirements, rules or regulations differently than
we do, then (if required in the future) they may decline to attest to management’s assessment or may issue a report that
is qualified. Any of these events could result in a loss of investor confidence in the reliability of our financial statements,
which in turn could negatively affect the price of our common stock
In particular, we must perform system and
process evaluation and testing of our internal control over financial reporting to allow management and our independent registered
public accounting firm to report on the effectiveness of our internal control over financial reporting, as required by Section
404. Our compliance with Section 404 may require that we incur substantial accounting expense and expend significant management
efforts.
We have never paid and do not intend to pay cash dividends.
Cash dividends have never been declared
or paid on our common stock, and we do not anticipate such a declaration or payment for the foreseeable future. We expect to use
future earnings, if any, to fund business growth. Therefore, stockholders will not receive any funds absent a sale of their shares
of common stock. If we do not pay dividends, our common stock may be less valuable because a return on investment will only occur
if our stock price appreciates.
The market price of our common stock has been, and may
continue to be, highly volatile, and such volatility could cause the market price of our common stock to decrease and could cause
you to lose some or all of your investment in our common stock.
During the period from our initial listing on Nasdaq in August
2016 through July 31, 2018, the market price of our common stock fluctuated from a high of $5.76 per share to a low of $1.07 per
share, and our stock price continues to fluctuate. The market price of our common stock may continue to fluctuate significantly
in response to numerous factors, some of which are beyond our control, such as:
|
|
·
|
our
ability to grow our revenue and customer base;
|
|
|
·
|
the
announcement of new products or product enhancements by us or our competitors;
|
|
|
·
|
developments
concerning regulatory oversight and approvals;
|
|
|
·
|
variations
in our and our competitors’ results of operations;
|
|
|
·
|
changes
in earnings estimates or recommendations by securities analysts, if our common stock is covered by analysts;
|
|
|
·
|
successes
or challenges in our collaborative arrangements or alternative funding sources;
|
|
|
·
|
developments
in the rehabilitation and industrial robotics markets;
|
|
|
·
|
the
results of product liability or intellectual property lawsuits;
|
|
|
·
|
future
issuances of common stock or other securities;
|
|
|
·
|
the
addition or departure of key personnel;
|
|
|
·
|
announcements
by us or our competitors of acquisitions, investments or strategic alliances; and
|
|
|
·
|
general
market conditions and other factors, including factors unrelated to our operating performance.
|
Trading of our common stock is limited, and trading restrictions
imposed on us by applicable regulations may further reduce trading in our common stock, making it difficult for our stockholders
to sell their shares; and future sales of common stock could reduce our stock price.
Trading of our common stock is currently conducted on Nasdaq.
The liquidity of our common stock is limited, not only in terms of the number of shares that can be bought and sold at a given
price, but also as it may be adversely affected by delays in the timing of transactions and low coverage by research analysts’
the media, if at all. These factors may result in different prices for our common stock than might otherwise be obtained in a more
liquid market and could also result in a larger spread between the bid and asked prices for our common stock. In addition, without
a large public float, our common stock is less liquid than the stock of companies with broader public ownership, and, as a result,
the trading prices of our common stock may be more volatile. In the absence of an active public trading market, an investor may
be unable to liquidate his or her investment in our common stock. Trading of a relatively small volume of our common stock may
have a greater impact on the trading price of our stock than would be the case if our public float were larger. We cannot predict
the prices at which our common stock will trade in the future, if at all.
Sales by stockholders of substantial amounts of our shares of
common stock, the issuance of new shares of common stock by us or the perception that these sales may occur in the future could
materially and adversely affect the market price of our common stock, and you may lose all or a portion of your investment in our
common stock.
Risks Related to this Offering
A substantial number of shares may be sold in the market
following this offering, which may depress the market price for our common stock.
Sales of a substantial number of shares of our common stock in the public market following this offering
could cause the market price of our common stock to decline. Although there can be no assurance that all $25,000,000 worth of shares
being offered under this prospectus will be sold or the price at which any such shares might be sold, assuming that an aggregate
of
9,191,176 shares of our common stock are sold during
the term of the sales agreement with Cantor Fitzgerald, in each case, for example, at a price of $2.72 per share, the last reported
sale price of our common stock on Nasdaq on August 17, 2018, upon completion of this offering, based on our shares outstanding
as of June 30, 2018, we will have outstanding an aggregate of 70,023,522 shares of common stock, assuming no exercise of outstanding
stock options, settlement of restricted stock units, exercises of warrants or other sales or issuances of stock. A substantial
majority of the outstanding shares of our common stock are, and all of the shares sold in this offering upon issuance will be,
freely tradable without restriction or further registration under the Securities Act of 1933, as amended, or the Securities Act,
unless these shares are owned or purchased by “affiliates” as that term is defined in Rule 144 under the Securities
Act. In addition, we have also registered all of the shares of common stock that we may issue pursuant to the exercise of outstanding
stock options and settlement of restricted stock units granted under our 2014 Incentive Plan and all of the shares of common stock
that we may issue under our ESPP, and as of June 30, 2018, 5,093,211 shares of our common stock were reserved for issuance of future
awards under our 2014 Incentive Plan and 500,000 shares of common stock were reserved for issuance under our ESPP. As
a result, these shares can be freely sold in the public market upon issuance, subject to restrictions related to affiliate sales
under the securities laws. Additionally, as of June 30, 2018, 3,395,532 shares of common stock were issuable upon the exercise
of outstanding warrants at a weighted exercise price of $7.43 per share. The issuance of 3,107,164 of the shares subject to these
warrants (with a weighted exercise price of $7.75 have been registered pursuant to an effective registration statement and prospectus,
and the remaining 288,368 shares subject to warrants (with a weighted exercise price of $4.00) will only be freely tradable after
issuance if registered in the future under the Securities Act or in reliance on any available exemptions from the registration
requirement of the Securities Act.
Management will have broad discretion as to the use of
proceeds from this offering and we may use the net proceeds in ways with which you may disagree.
We intend to use the net proceeds of this offering for general
corporate purposes, which may include acquisitions, research and development activities, capital expenditures, facilities expansion
and working capital needs. Our management will have broad discretion in the application of the net proceeds from this offering
and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. Accordingly,
you will be relying on the judgment of our management with regard to the use of net proceeds, and you will not have the opportunity,
as part of your investment decision, to assess whether the proceeds are being used appropriately. Our failure to apply these funds
effectively could have a material adverse effect on our business, delay the development of our products and cause the price of
our common stock to decline.
If you purchase the common stock sold in this offering,
you may experience immediate dilution as a result of this offering.
Because the price per share of our common stock being offered
may be higher than the net tangible book value per share of our common stock, you will experience dilution to the extent of the
difference between the offering price per share of common stock you pay in this offering and the net tangible book value per share
of our common stock immediately after this offering. Our net tangible book value as of June 30, 2018, was approximately $7.2 million,
or $0.12 per share of common stock. Net tangible book value per share is equal to our total tangible assets minus total liabilities,
all divided by the number of shares of common stock outstanding.
Because the sales of the shares offered hereby will be made
directly into the market or in negotiated transactions, the prices at which we sell these shares will vary and these variations
may be significant. Purchasers of the shares we sell, as well as our existing stockholders, will experience significant dilution
if we sell shares at prices significantly below the price at which they invested.
CAUTIONARY NOTE REGARDING FORWARD LOOKING
INFORMATION
This prospectus and the documents we incorporate by reference
in this prospectus contains forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E
of the Securities Exchange Act of 1934, as amended, or the Exchange Act. All statements, other than statements of historical facts,
included or incorporated in this prospectus regarding our strategy, future operations, financial position, future revenues, projected
costs, prospects, plans and objectives of management are forward-looking statements. We may, in some cases, use words such as “believe,”
“anticipate,” “should,” “intend,” “plan,” “will,” “estimate,”
“project,” “expect” and similar expressions, although not all forward-looking statements contain these
identifying words. These statements are based on assumptions and assessments made by our management in light of its experience
and its perception of historical trends, current conditions, expected future developments and other factors our management believes
to be appropriate. These forward-looking statements are subject to a number of risks and uncertainties, including those risks described
or incorporated by reference in this prospectus under “Risk Factors” above.
Forward-looking statements included or incorporated by reference
in this prospectus include, for example, statements about:
|
|
·
|
our ability to obtain adequate financing to fund operations and to develop or enhance our technology;
|
|
|
·
|
our ability to obtain or maintain regulatory approval to market the Company’s medical devices;
|
|
|
·
|
the anticipated timing, cost and progress of the development and commercialization of new products or services, and improvements to our existing products, and related impacts on our profitability and cash position;
|
|
|
·
|
our ability to effectively market and sell our products and expand our business, both in unit sales and product diversification;
|
|
|
·
|
our ability to achieve broad customer adoption of our products and services;
|
|
|
·
|
our ability to complete clinical trials on a timely basis and that completed clinical trials will be sufficient to support commercialization of our products;
|
|
|
·
|
existing or increased competition;
|
|
|
·
|
rapid changes in technological solutions available to our markets;
|
|
|
·
|
volatility with our business, including long and variable sales cycles, which could have a negative impact on our results of operations for any given quarter;
|
|
|
·
|
our ability to obtain or maintain patent protection for the Company’s intellectual property;
|
|
|
·
|
the scope, validity and enforceability of our and third party intellectual property rights;
|
|
|
·
|
significant government regulation of medical devices and the healthcare industry;
|
|
|
·
|
our customers’ ability to get third party reimbursement for our products and services associated with them;
|
|
|
·
|
our failure to implement our business plan or strategies;
|
|
|
·
|
our ability to retain or attract key employees;
|
|
|
·
|
stock volatility or illiquidity;
|
|
|
·
|
our ability to maintain adequate internal controls over financial reporting; and
|
|
|
·
|
overall economic and market conditions.
|
Any such forward-looking statements are not guarantees of future
performance and actual results and developments and business decisions may differ from those contemplated by such forward-looking
statements. We disclaim any duty to update any forward-looking statements. You should also carefully consider other information
set forth in reports or other documents that we file with the Securities and Exchange Commission.
USE OF PROCEEDS
The amount of proceeds from this offering will depend upon the
number of shares of our common stock sold and the market price at which they are sold. There can be no assurance that we will be
able to sell any shares under or fully utilize the sales agreement with Cantor Fitzgerald as a source of financing. We intend to
use the net proceeds, if any, from the sale of the shares of common stock under this prospectus for general corporate purposes,
which may include acquisitions, research and development activities, capital expenditures, selling, general and administrative
costs, facilities expansion, and to meet working capital needs. We expect from time to time to evaluate the acquisition of businesses,
products and technologies for which a portion of the net proceeds may be used, although we currently are not planning or negotiating
any such transactions
The amounts actually spent for each purpose may vary significantly
depending upon numerous factors, including the amount and timing of the proceeds from this offering and progress with the achievement
of our corporate goals. Expenditures will also depend upon the establishment and maintenance of manufacturing and supply chains,
the availability of additional financing and other factors. Investors will be relying on the judgment of our management regarding
the application of the proceeds of any sale of shares of our common stock.
As of the date of this prospectus, we cannot specify with certainty
all of the particular uses of the proceeds from this offering. Accordingly, we will retain broad discretion over the use of such
proceeds. Pending the use of the net proceeds from this offering as described above, we may invest the net proceeds in investment-grade,
interest-bearing securities.
DILUTION
Our net tangible book value as of June 30, 2018 was approximately
$7.2 million, or $0.12 per share. Net tangible book value per share is determined by dividing our total tangible assets, less total
liabilities, by the number of shares of our common stock outstanding as of June 30, 2018. Dilution with respect to net tangible
book value per share represents the difference between the amount per share paid by purchasers of shares of common stock in this
offering and the net tangible book value per share of our common stock immediately after this offering.
After giving effect to the assumed sale of
9,191,176
shares of our common stock in this offering at an assumed offering price of $2.72 per share, the last reported sale price of
our common stock on Nasdaq on August 17, 2018, and after deducting estimated offering commissions and estimated offering expenses
payable by us, our as adjusted net tangible book value as of June 30, 2018 would have been approximately $31.3 million, or $0.45
per share. This represents an immediate increase in net tangible book value of $0.33 per share to existing stockholders and immediate
dilution of $2.27 per share to investors purchasing our common stock in this offering at the public offering price. The following
table illustrates this dilution on a per share basis:
|
Assumed public offering price per share
|
|
|
|
|
|
$
|
2.72
|
|
|
Net tangible book value per share of as June 30, 2018
|
|
$
|
0.12
|
|
|
|
|
|
|
Increase in net tangible book value per share attributable to this offering
|
|
$
|
0.33
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As adjusted, net tangible book value per share as of June 30, 2018, after giving effect to this offering
|
|
|
|
|
|
$
|
0.45
|
|
|
|
|
|
|
|
|
|
|
|
|
Dilution per share to new investors purchasing our common stock in this offering
|
|
|
|
|
|
$
|
2.27
|
|
The shares subject to the sales agreement with Cantor Fitzgerald are being sold from time to time at various
prices. An increase of $1.00 per share in the price at which the shares are sold from the assumed offering price of $2.72 per
share shown in the table above, assuming all of our common stock in the aggregate amount of $25 million during the term of the
sales agreement with Cantor Fitzgerald are sold at that price, would increase our as adjusted net tangible book value per share
after the offering to $0.46 per share and would increase the dilution in net tangible book value per share to new investors in
this offering to $3.26 per share, after deducting commissions and estimated aggregate offering expenses payable by us. A decrease
of $1.00 per share in the price at which the shares are sold from the assumed offering price of $2.72 per share shown in the
table above, assuming all of our common stock in the aggregate amount of $25 million during the term of the sales agreement with
Cantor Fitzgerald is sold at that price, would decrease our adjusted net tangible book value per share after the offering to $0.42
per share and would decrease the dilution in net tangible book value per share to new investors in this offering to $1.30 per
share, after deducting commissions and estimated offering expenses payable by us. This information is supplied for illustrative
purposes only and may differ based on the actual offering price and the actual number of shares offered.
The above discussion and table are based on 60,832,346 shares
of our Common Stock outstanding as of June 30, 2018, and exclude as of that date:
|
|
·
|
2,916,453 shares of common stock issuable upon the exercise of stock
options outstanding at a weighted average exercise price of $4.60 per share, 78,514 restricted stock units which will, after vesting,
be settled in shares of our common stock, and 5,093,211 shares of our common stock reserved for issuance under our 2014 Incentive
Plan;
|
|
|
·
|
500,000 shares of our common stock reserved for issuance under our
ESPP; and
|
|
|
·
|
3,395,532 shares of common stock issuable upon the exercise of warrants
outstanding at a weighed exercise price of $7.43 per share.
|
To the extent that options or warrants outstanding as of June
30, 2018 have been or may be exercised or other shares issued, investors purchasing our common stock in this offering may experience
further dilution. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even
if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised
through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to
our stockholders.
PLAN OF DISTRIBUTION
We have entered into a sales agreement with Cantor Fitzgerald, under which we may issue and sell shares
of our common stock from time to time through Cantor Fitzgerald acting as a sales agent, including sales having an aggregate gross
sales price of up to $25 million pursuant to this prospectus supplement. The sales agreement has been filed as an exhibit to a
Current Report on Form 8-K which is incorporated by reference in this registration statement on Form S-3, of which this prospectus
forms a part. See “Incorporation of Certain Documents by Reference” beginning on page S-28.
Cantor Fitzgerald may sell the shares of common stock by any
method that is deemed to be an “at the market offering” as defined in Rule 415(a)(4) promulgated under the Securities
Act. Subject to the terms of the placement notice, Cantor Fitzgerald may also sell the shares of common stock by any other method
permitted by law, including in privately negotiated transactions.
Cantor Fitzgerald will offer the shares of our common stock
subject to the terms and conditions of the sales agreement on a daily basis or as otherwise agreed upon by us and Cantor Fitzgerald.
We will designate the maximum number of shares of common stock to be sold through Cantor Fitzgerald on a daily basis or otherwise
determine such maximum number together with Cantor Fitzgerald. Subject to the terms and conditions of the sales agreement, Cantor
Fitzgerald will use its commercially reasonable efforts to sell on our behalf all of the shares of common stock so designated or
determined. We may instruct Cantor Fitzgerald not to sell shares of common stock if the sales cannot be effected at or above the
price designated by us in any such instruction. We or Cantor Fitzgerald may suspend the offering of shares of common stock being
made through Cantor Fitzgerald under the sales agreement upon proper notice to the other party.
We will pay Cantor Fitzgerald commissions, in cash, for its
services in acting as agent in the sale of our common stock. Cantor Fitzgerald will be entitled to compensation at a fixed commission
rate of 3.0% of the aggregate gross proceeds from each sale of our common stock. Because there is no minimum offering amount required
as a condition to close this offering, the actual total public offering amount, commissions and proceeds to us, if any, are not
determinable at this time. We have also agreed to reimburse Cantor Fitzgerald for certain specified fees and documented expenses,
including the fees and documented expenses of its legal counsel in an amount not to exceed $50,000, as provided in the sales agreement.
We estimate that the total expenses for the offering, excluding compensation and reimbursements payable to Cantor Fitzgerald under
the terms of the sales agreement, will be approximately $120,000.
Settlement for sales of common stock will occur on the second
business day following the date on which any sales are made, or on some other date that is agreed upon by us and Cantor Fitzgerald
in connection with a particular transaction, in return for payment of the net proceeds to us. Sales of our common stock as contemplated
in this prospectus will be settled through the facilities of The Depository Trust Company or by such other means as we and Cantor
Fitzgerald may agree upon. There is no arrangement for funds to be received in an escrow, trust or similar arrangement.
Cantor Fitzgerald will use its commercially reasonable efforts
consistent with its normal trading and sales practices to sell on our behalf all of the shares of common stock requested to be
sold by us, subject to the conditions set forth in the sales agreement. In connection with the sale of the common stock on our
behalf, Cantor Fitzgerald will be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation
of Cantor Fitzgerald will be deemed to be underwriting commissions or discounts. We have agreed to provide indemnification and
contribution to Cantor Fitzgerald against certain civil liabilities, including liabilities under the Securities Act.
The offering of our common stock pursuant to the sales agreement
will terminate as permitted therein. We and Cantor Fitzgerald may each terminate the sales agreement at any time upon 10 days’
prior notice.
Cantor Fitzgerald and its affiliates may in the future provide
various investment banking, commercial banking and other financial services for us and our affiliates, for which services they
may in the future receive customary fees. To the extent required by Regulation M, Cantor Fitzgerald will not engage in any market
making activities involving our common stock while the offering is ongoing under this prospectus.
This prospectus in electronic format may be made available on
a website maintained by Cantor Fitzgerald and Cantor Fitzgerald may distribute this prospectus electronically.
LEGAL MATTERS
The validity of the securities being offered by this prospectus
will be passed upon by Snell & Wilmer L.L.P., Las Vegas, Nevada. Cantor Fitzgerald & Co. is being represented in connection
with this offering by Cooley LLP, New York, New York.
EXPERTS
The consolidated balance sheets of Ekso Bionics Holdings, Inc.
as of December 31, 2017 and 2016, and the related consolidated statements of operations and comprehensive loss, stockholders’
equity and cash flows for each of the years in the three-year period ended December 31, 2017, have been audited by OUM & Co.
LLP, independent registered public accounting firm, as stated in their report which is incorporated herein by reference. Such financial
statements have been incorporated herein by reference in reliance on the report of such firm given upon their authority as experts
in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form
S-3 under the Securities Act, of which this prospectus forms a part. The rules and regulations of the SEC allow us to omit from
this prospectus certain information included in the registration statement. For further information about us and the securities
we are offering under this prospectus, you should refer to the registration statement and the exhibits and schedules filed with
the registration statement. With respect to the statements contained in this prospectus regarding the contents of any agreement
or any other document, in each instance, the statement is qualified in all respects by the complete text of the agreement or document,
a copy of which has been filed as an exhibit to the registration statement or an item incorporated by reference in the registration
statement.
We file annual, quarterly and current reports, proxy statements
and other information with the SEC. Our SEC filings, including the registration statement and exhibits, are available to the public
at the SEC’s website at
http://www.sec.gov
. You may also read, without charge, and copy the documents we file, at
the SEC’s public reference rooms at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You can request copies of these
documents by writing to the SEC and paying a fee for the copying cost. Please call the SEC at 1-800-SEC-0330 for further information
on the public reference rooms.
We maintain an Internet site at www.eksobionics.com. Our official
Twitter account is @EksoBionics. We have not incorporated by reference into this prospectus the information on our website or Twitter
account, and you should not consider any of the information posted on or hyper-linked to our website or Twitter account to be a
part of this prospectus.
INCORPORATION OF CERTAIN DOCUMENTS BY
REFERENCE
The SEC allows us to “incorporate by reference”
the information we file with the SEC, which means we can disclose important information to you by referring you to those documents.
The information we incorporate by reference is an important part of this prospectus, and certain information that we will later
file with the SEC will automatically update and supersede this information. We incorporate by reference the documents listed below
as well as any future filings made with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of
this prospectus until we sell all of the securities under this prospectus, except that we do not incorporate any document or portion
of a document that is “furnished” to the SEC, but not deemed “filed.” The following documents filed with
the SEC are incorporated by reference in this prospectus:
|
|
·
|
our
Annual Report on Form 10-K for the year ended December 31, 2017 filed with the SEC on March 13, 2018;
|
|
|
·
|
our
Quarterly Report on Form 10-Q for the quarter ended March 31, 2018 filed with the SEC on May 7, 2018, and our Quarterly Report
on Form 10-Q for the quarter ended June 30, 2018 filed with the SEC on August 7, 2018;
|
|
|
·
|
our Current Reports on Form 8-K filed with
the SEC on January 26, 2018, March 13, 2018 (Item 5.02), March 28, 2018, May 15, 2018, June 12, 2018, July 10, 2018 and August
13, 2018; and
|
|
|
·
|
the description of our common
stock contained in our Registration Statement on Form 8-A filed on May 6, 2015 and August 8, 2016, including any amendment
or report filed for the purpose of updating such description.
|
We also incorporate by reference into this prospectus additional
documents that we may file with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, excluding, in each case,
information deemed furnished and not filed until we sell all of the securities we are offering. Any statements contained in a previously
filed document incorporated by reference into this prospectus is deemed to be modified or superseded for purposes of this prospectus
to the extent that a statement contained in this prospectus, or in a subsequently filed document also incorporated by reference
herein, modifies or supersedes that statement.
We will provide to you at no cost a copy of any and all of the
information incorporated by reference into the registration statement of which this prospectus is a part. You may make a request
for copies of this information in writing or by telephone. Requests should be directed to:
Ekso Bionics Holdings, Inc.
1414 Harbour Way South, Suite 1201
Richmond, California 94804
Attn: Chief Financial Officer
PROSPECTUS
Ekso Bionics Holdings, Inc.
$75,000,000
COMMON STOCK
PREFERRED STOCK
WARRANTS
RIGHTS
UNITS
Ekso Bionics Holdings, Inc., a Nevada corporation
(“Ekso Bionics”), may offer and sell from time to time, pursuant to this prospectus, in one or more series or issuances
and on terms that Ekso Bionics will determine at the time of the offering, any of the securities described in this prospectus,
and any combination of those securities, up to an aggregate amount of $75,000,000.
This prospectus describes the general terms
of these securities and the general manner in which these securities will be offered. We will provide you with the specific
terms of any offering in one or more supplements to this prospectus. The prospectus supplements will also describe the specific
manner in which these securities will be offered and may also supplement, update or amend information contained in this document.
You should read this prospectus and any prospectus supplement, as well as any documents incorporated by reference into this prospectus
or any prospectus supplement, carefully before you invest.
We may offer and sell these securities
directly to you, through agents designated from time to time, or to or through underwriters or dealers. For additional information
on the methods of sale, you should refer to the section entitled “Plan of Distribution” in this prospectus and in the
applicable prospectus supplement. If any underwriters or agents are involved in the sale of our securities with respect to
which this prospectus is being delivered, the names of such underwriters or agents and any applicable fees, commissions or discounts
and over-allotment options will be set forth in the applicable prospectus supplement. The price to the public of such securities
and the net proceeds that we expect to receive from such sale will also be set forth in a prospectus supplement.
Our common stock is listed on the Nasdaq
Capital Market under the symbol “EKSO.” On June 2, 2017, the last reported sale price of our common stock was $1.37
per share. The applicable prospectus supplement will contain information, where applicable, as to any other listing, if any, on
the Nasdaq Capital Market or any securities market or other securities exchange of the securities covered by the prospectus supplement.
Prospective purchasers of our securities are urged to obtain current information as to the market prices of our securities, where
applicable.
INVESTING IN OUR SECURITIES INVOLVES
A HIGH DEGREE OF RISK. BEFORE DECIDING WHETHER TO INVEST IN OUR SECURITIES, YOU SHOULD CONSIDER CAREFULLY THE RISKS THAT WE HAVE
DESCRIBED ON PAGE 4 OF THIS PROSPECTUS UNDER THE CAPTION “RISK FACTORS.” WE MAY INCLUDE SPECIFIC RISK FACTORS IN SUPPLEMENTS
TO THIS PROSPECTUS UNDER THE CAPTION “RISK FACTORS.” THIS PROSPECTUS MAY NOT BE USED TO SELL OUR SECURITIES UNLESS ACCOMPANIED
BY A PROSPECTUS SUPPLEMENT.
Neither the Securities and Exchange
Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus
is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is June 16,
2017.
TABLE OF CONTENTS
EXPLANATORY NOTE – STOCK SPLIT
Effective May 4, 2016, the Company effected
a one-for-seven reverse split of its common stock. As a result of the reverse stock split, every seven shares of issued and outstanding
common stock were converted into one share of issued and outstanding common stock. All references in this prospectus supplement
to shares and net tangible book value per share have been retroactively restated to reflect the reverse stock split.
ABOUT THIS PROSPECTUS
This prospectus is part of a registration
statement on Form S-3 that we filed with the Securities and Exchange Commission, or SEC, using a “shelf” registration
process. Under this shelf registration process, we may offer and sell, from time to time, shares of our common stock and
preferred stock, various series of warrants or rights to purchase any of such securities, either individually or in units, in one
or more offerings, with a total value of up to $75,000,000. This prospectus provides you with a general description of the
securities we may offer. Each time we offer a type or series of securities under this prospectus, we will provide a prospectus
supplement that will contain specific information about the terms of that offering.
This prospectus does not contain all of
the information included in the registration statement. For a more complete understanding of the offering of the securities,
you should refer to the registration statement, including its exhibits. The prospectus supplement may also add, update or
change information contained or incorporated by reference in this prospectus. However, no prospectus supplement will offer
a security that is not registered and described in this prospectus at the time of its effectiveness. This prospectus, together
with the applicable prospectus supplements and the documents incorporated by reference into this prospectus, includes all material
information relating to the offering of securities under this prospectus. You should carefully read this prospectus, the
applicable prospectus supplement, the information and documents incorporated herein by reference and the additional information
under the heading “Where You Can Find More Information” before making an investment decision.
You should rely only on the information
we have provided or incorporated by reference in this prospectus or any prospectus supplement. We have not authorized anyone
to provide you with information different from that contained or incorporated by reference in this prospectus. No dealer,
salesperson or other person is authorized to give any information or to represent anything not contained or incorporated by reference
in this prospectus. You must not rely on any unauthorized information or representation. This prospectus is an offer
to sell only the securities offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so.
You should assume that the information in this prospectus or any prospectus supplement is accurate only as of the date on the front
of the document and that any information we have incorporated herein by reference is accurate only as of the date of the document
incorporated by reference, regardless of the time of delivery of this prospectus or any sale of a security.
We further note that the representations,
warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference
in the accompanying prospectus were made solely for the benefit of the parties to such agreement, including, in some cases, for
the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty
or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made.
Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state
of our affairs.
This prospectus may not be used to consummate
sales of our securities, unless it is accompanied by a prospectus supplement. To the extent there are inconsistencies between
any prospectus supplement, this prospectus and any documents incorporated by reference, the document with the most recent date
will control.
Unless the context otherwise requires,
“Ekso Bionics,” “the Company,” “we,” “us,” “our” and similar terms refer to Ekso
Bionics Holdings, Inc. and our subsidiaries.
PROSPECTUS SUMMARY
The following is a summary of what we
believe to be the most important aspects of our business and the offering of our securities under this prospectus. We urge
you to read this entire prospectus, including the more detailed consolidated financial statements, notes to the consolidated financial
statements and other information incorporated by reference from our other filings with the SEC or included in any applicable prospectus
supplement. Investing in our securities involves risks. Therefore, you should carefully consider the risk factors set forth
in any prospectus supplements and in our most recent annual and quarterly filings with the SEC, as well as other information in
this prospectus and any prospectus supplements and the documents incorporated by reference herein or therein, before purchasing
our securities. Each of the risk factors could adversely affect our business, operating results and financial condition,
as well as adversely affect the value of an investment in our securities.
Overview
Ekso Bionics designs, develops and sells
exoskeletons that augment human strength, endurance, and mobility. Our exoskeleton technology serves multiple markets and can be
used both by able-bodied users as well as by persons with physical disabilities. We have sold, rented or leased devices that (a)
enable individuals with neurological conditions affecting gait (stroke and spinal cord injury) to rehabilitate and to walk again
and (b) allow industrial workers to perform heavy duty work for extended periods.
Additional Information
For additional information related to our
business and operations, please refer to the reports incorporated herein by reference, including our Annual Report on Form 10-K
for the year ended December 31, 2016, as described under the caption “Incorporation of Documents by Reference” on page
15 of this prospectus.
Corporate Information
We were incorporated under the laws of
the State of Nevada in January 2012 under the name PN Med Group, Inc. and changed our name to Ekso Bionics Holdings, Inc. in December
2013. Ekso Bionics, Inc. was incorporated under the laws of the State of Delaware in January 19, 2005 and in January 2014 completed
a reverse merger transaction with and became a wholly-owned subsidiary of Ekso Bionics Holdings, Inc. In connection with the reverse
merger transaction and pursuant to a split-off agreement and general release, we transferred our pre-merger assets and liabilities
to our pre-merger majority stockholders, in exchange for the surrender by them and cancellation of 2,497,586 shares of our common
stock. As a result of the merger and split-off, we discontinued our pre-merger business and acquired the business of Ekso Bionics,
and have continued the existing business operations of Ekso Bionics as a publicly-traded company under the name Ekso Bionics Holdings,
Inc.
Our principal executive offices are located
at 1414 Harbour Way South, Suite 1201, Richmond, California 94804 and our telephone number is (510) 984-1761.
We make available free of charge on or
through our website our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and all amendments
to those reports as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Our internet
address is
www.eksobionics.com
. This website address is intended to be an inactive, textual reference only; none of the
material on this website is part of this prospectus supplement. Copies of our annual reports on Form 10-K will be furnished without
charge to any person who submits a written request directed to the attention of our Secretary, at our offices located at 1414 Harbour
Way South, Suite 1201, Richmond, California, 94804. The SEC maintains an internet site (http://www.sec.gov) that contains reports,
proxy and information statements, and other information regarding issuers that file electronically with the SEC.
Implications of Being an Emerging Growth Company
As a company with less than $1.0 billion
in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business
Startups, or JOBS, Act enacted in April 2012. An emerging growth company may take advantage of reduced reporting requirements that
are otherwise applicable to public companies. These provisions include, but are not limited to:
|
|
·
|
not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes Oxley Act of 2002;
|
|
|
·
|
reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and
|
|
|
·
|
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
|
We may take advantage of these provisions
for up to five years after the first sale of our common equity securities pursuant to an effective registration statement under
the Securities Act of 1933, as amended (the “Securities Act”). However, if certain events occur prior to the end of such
five year period, including if we become a “large accelerated filer,” our annual gross revenues exceed $1 billion or
we issue more than $1 billion of non-convertible debt in any three year period, we would cease to be an emerging growth company
prior to the end of such five year period. We will cease being an emerging growth company as of December 31, 2017.
We may choose to take advantage of some
but not all of these reduced burdens. We have taken advantage of certain of the reduced disclosure obligations regarding executive
compensation in this registration statement and may elect to take advantage of other reduced burdens in future filings. As a result,
the information that we provide to our stockholders may be different than you might receive from other public reporting companies
in which you hold equity interests.
Under the JOBS Act, emerging growth companies
can delay adopting new or revised accounting standards until such time as those standards apply to private companies. However,
we have irrevocably elected not to avail ourselves of this extended transition period for complying with new or revised accounting
standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are
not emerging growth companies.
Offerings Under This Prospectus
Under this prospectus, we may offer shares
of our common stock and preferred stock, various series of warrants or rights to purchase any of such securities, either individually
or in units, with a total value of up to $75,000,000, from time to time at prices and on terms to be determined by market conditions
at the time of the offering. This prospectus provides you with a general description of the securities we may offer.
Each time we offer a type or series of securities under this prospectus, we will provide a prospectus supplement that will describe
the specific amounts, prices and other important terms of the securities.
The prospectus supplement also may add,
update or change information contained in this prospectus or in documents we have incorporated by reference into this prospectus.
However, no prospectus supplement will fundamentally change the terms that are set forth in this prospectus or offer a security
that is not registered and described in this prospectus at the time of its effectiveness.
We may sell the securities directly to
investors or to or through agents, underwriters or dealers. We, and our agents, underwriters or dealers, reserve the right
to accept or reject all or part of any proposed purchase of securities. If we offer securities through agents, underwriters
or dealers, we will include in the applicable prospectus supplement:
|
|
·
|
the names of those agents, underwriters or dealers;
|
|
|
·
|
applicable fees, discounts and commissions to be paid to them;
|
|
|
·
|
details regarding over-allotment options, if any; and
|
|
|
·
|
the net proceeds to us.
|
This prospectus may not be used
to consummate a sale of any securities unless it is accompanied by a prospectus supplement.
RISK FACTORS
Investing in our securities involves significant
risk. The prospectus supplement applicable to each offering of our securities will contain a discussion of the risks applicable
to an investment in Ekso Bionics. Prior to making a decision about investing in our securities, you should carefully consider
the specific factors discussed under the heading “Risk Factors” in the applicable prospectus supplement, together with
all of the other information contained or incorporated by reference in the prospectus supplement or appearing or incorporated by
reference in this prospectus. You should also consider the risks, uncertainties and assumptions discussed under the heading
“Risk Factors” included in our most recent annual report on Form 10-K, as revised or supplemented by our subsequent quarterly
reports on Form 10-Q or our current reports on Form 8-K on file with the SEC, all of which are incorporated herein by reference,
and which may be amended, supplemented or superseded from time to time by other reports we file with the SEC in the future.
The risks and uncertainties we have described are not the only ones we face. Additional risks and uncertainties not presently
known to us or that we currently deem immaterial may also affect our operations. The occurrence of any of these risks might
cause you to lose all or part of your investment in the offered securities.
SPECIAL NOTE REGARDING FORWARD-LOOKING
STATEMENTS
The SEC encourages companies to disclose
forward-looking information so that investors can better understand a company’s future prospects and make informed investment decisions.
This prospectus and the documents we have filed with the SEC that are incorporated herein by reference contain such “forward-looking
statements” within the meaning of the Private Securities Litigation Reform Act of 1995.
Such statements in connection with any
discussion of future operations or financial performance are identified by the use of words such as “may,” “anticipate,”
“estimate,” “expect,” “project,” “intend,” “plan,” “believe,” and other
words and terms of similar meaning. Forward-looking statements include, but are not limited to, statements regarding: (i)
the plans and objectives of management for future operations, including plans or objectives relating to the design, development
and commercialization of human exoskeletons, (ii) a projection of income (including income/loss), earnings (including earnings/loss)
per share, capital expenditures, dividends, capital structure or other financial items, (iii) our future financial performance,
including any such statement contained in a discussion and analysis of financial condition by management or in the results of operations
included pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”), (iv) our beliefs
regarding the potential for commercial opportunity for exoskeleton technology in general and our exoskeleton products in particular,
(v) our beliefs regarding potential clinical and other health benefits of our medical devices, and (vi) the assumptions underlying
or relating to any statement described in points (i), (ii), (iii), (iv) or (v) above.
We may not actually achieve the plans,
intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking
statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking
statements we make. We have included important cautionary statements in this Registration Statement, particularly in the “Risk
Factors” section, that we believe could cause actual results or events to differ materially from the forward-looking statements
that we make. For a summary of such factors, please refer to the section entitled “Risk Factors” in this prospectus,
as updated and supplemented by the discussion of risks and uncertainties under “Risk Factors” contained in any supplements
to this prospectus and in our most recent annual report on Form 10-K, as revised or supplemented by our subsequent quarterly reports
on Form 10-Q or our current reports on Form 8-K, as well as any amendments thereto, as filed with the SEC and which are incorporated
herein by reference. The information contained in this document is believed to be current as of the date of this document.
We do not intend to update any of the forward-looking statements after the date of this document to conform these statements to
actual results or to changes in our expectations, except as required by law.
In light of these assumptions, risks and
uncertainties, the results and events discussed in the forward-looking statements contained in this prospectus or in any document
incorporated herein by reference might not occur. Investors are cautioned not to place undue reliance on the forward-looking
statements, which speak only as of the date of this prospectus or the date of the document incorporated by reference in this prospectus.
We are not under any obligation, and we expressly disclaim any obligation, to update or alter any forward-looking statements, whether
as a result of new information, future events or otherwise. All subsequent forward-looking statements attributable to us
or to any person acting on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred
to in this section.
ABOUT EKSO BIONICS
Ekso Bionics designs, develops and sells
exoskeleton technology to augment human strength, endurance and mobility. Our exoskeleton technology serves multiple markets and
can be used both by able-bodied users as well as by persons with physical disabilities. We have sold, rented or leased devices
that (a) enable individuals with neurological conditions affecting gait (stroke and spinal cord injury) to rehabilitate and to
walk again and (b) allow industrial workers to perform heavy duty work for extended periods.
Today, our medical exoskeleton, Ekso GT,
is used as a rehabilitation tool to allow physicians and therapists to rehabilitate patients who have suffered a stroke or spinal
cord injury. With its unique features designed specifically for hospitals and its proprietary SmartAssist software, Ekso GT allows
for the early mobilization of patients, with high step count and high dosage treatments. The intent is to allow the patient’s central
nervous system to take advantage of a person’s neuroplasticity to maximize a patient’s recovery.
For able-bodied industrial workers, last
year we introduced a new product innovation for aerial work platforms (AWP) and scaffolding, the EksoZeroG, which is intended to
significantly improve workforce productivity while dramatically reducing workplace related injuries in order to keep workers healthy,
strong, and safe. EksoZeroG is a mobile arm mount that makes heavy tools feel weightless and enables workers to be more productive
and safer. In 2017, we are focusing on increasing sales of the EksoZeroG by pursuing alternative channels such as rental agreements
with construction equipment and heavy tool providers. In addition, we are exploring potential strategic alternatives to accelerate
product and market adoption of our industrial products, on our own and/or in collaboration with others.
Ekso Bionics believes the commercial opportunity
for exoskeleton technology adoption is accelerating as a result of recent advancements in material technologies, electronic and
electrical engineering, control technologies, and sensor and software development. Taken individually, many of these advancements
have become ubiquitous in peoples’ everyday lives. We believe we have learned how to integrate these existing technologies and
wrap the result around a human being efficiently, elegantly and safely, supported by an industry leading intellectual property
portfolio. We further believe that we can do so across a broad spectrum of applications, from persons with lower limb paralysis
to able-bodied users.
USE OF PROCEEDS
We cannot assure you that we will receive
any proceeds in connection with securities which may be offered pursuant to this prospectus. Unless otherwise indicated in
the applicable prospectus supplement, we intend to use any net proceeds from the sale of securities under this prospectus for our
operations and to increase our investments (i) in our clinical, sales and marketing initiatives to accelerate adoption of the Ekso
in the rehabilitation market, (ii) in our research, development and commercialization activities with respect to an Ekso robotic
exoskeleton for home use, and/or (iii) in the development and commercialization of able-bodied exoskeletons for industrial use,
and for other general corporate purposes, including, but not limited to, working capital, intellectual property protection and
enforcement, capital expenditures, investments, acquisitions and collaborations. We have not determined the amounts we plan
to spend on any of the areas listed above or the timing of these expenditures. As a result, our management will have broad
discretion to allocate the net proceeds, if any, we receive in connection with securities offered pursuant to this prospectus for
any purpose. Pending application of the net proceeds as described above, we may initially invest the net proceeds in short-term,
investment-grade, or interest-bearing securities.
PLAN OF DISTRIBUTION
General Plan of Distribution
We may offer securities under this prospectus
from time to time pursuant to underwritten public offerings, negotiated transactions, “at the market” sales, block trades
or a combination of these methods. We may sell the securities:
|
|
·
|
to or through underwriters or dealers;
|
|
|
·
|
through agents;
|
|
|
·
|
directly to one or more purchasers;
|
|
|
·
|
through a combination of such methods; or
|
|
|
·
|
through any other method described in a prospectus supplement.
|
The distribution of
securities may be effected, from time to time, in one or more transactions, including:
|
|
·
|
block transactions (which may involve crosses) and transactions on the Nasdaq Capital Market or any other organized market where the securities may be traded;
|
|
|
·
|
purchases by a dealer as principal and resale by the dealer for its own account pursuant to a prospectus supplement;
|
|
|
·
|
ordinary brokerage transactions and transactions in which a dealer solicits purchasers;
|
|
|
·
|
sales “at the market” to or through a market maker or into an existing trading market, on an exchange or otherwise; and
|
|
|
·
|
sales in other ways not involving market makers or established trading markets, including direct sales to purchasers.
|
We may distribute the securities from time
to time in one or more transactions at:
|
|
·
|
a fixed price or prices, which may be changed from time to time;
|
|
|
·
|
market prices prevailing at the time of sale;
|
|
|
·
|
prices related to the prevailing market prices; or
|
|
|
·
|
negotiated prices.
|
We may directly
solicit offers to purchase the securities being offered by this prospectus. We may also designate agents to solicit offers to purchase
the securities from time to time. The prospectus supplement relating to a series of the offered securities will name any underwriters,
dealers or agents involved in the offer or sale of the securities.
If we utilize
a dealer in the sale of the securities being offered by this prospectus, we will sell the securities to the dealer, as principal.
The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale.
If we utilize
an underwriter in the sale of the securities being offered by this prospectus, we will execute an underwriting agreement with the
underwriter at the time of sale, and we will provide the name of any underwriter in the prospectus supplement which the underwriter
will use to make resales of the securities to the public. In connection with the sale of the securities, we, or the purchasers
of the securities for whom the underwriter may act as agent, may compensate the underwriter in the form of underwriting discounts
or commissions. The underwriter may sell the securities to or through dealers, and the underwriter may compensate those dealers
in the form of discounts, concessions or commissions.
With respect to
underwritten public offerings, negotiated transactions and block trades, we will provide in the applicable prospectus supplement
information regarding any compensation we pay to underwriters, dealers or agents in connection with the offering of the securities,
and any discounts, concessions or commissions allowed by underwriters to participating dealers. Underwriters, dealers and agents
participating in the distribution of the securities may be deemed to be underwriters within the meaning of the Securities Act,
and any discounts and commissions received by them and any profit realized by them on resale of the securities may be deemed to
be underwriting discounts and commissions. We may enter into agreements to indemnify underwriters, dealers and agents against civil
liabilities, including liabilities under the Securities Act, or to contribute to payments they may be required to make in respect
thereof.
If so indicated in the applicable prospectus
supplement, we will authorize underwriters, dealers or other persons acting as our agents to solicit offers by certain institutions
to purchase securities from us pursuant to delayed delivery contracts providing for payment and delivery on the date stated in
each applicable prospectus supplement. Each contract will be for an amount not less than, and the aggregate amount of securities
sold pursuant to such contracts shall not be less nor more than, the respective amounts stated in each applicable prospectus supplement.
Institutions with whom the contracts, when authorized, may be made include commercial and savings banks, insurance companies, pension
funds, investment companies, educational and charitable institutions and other institutions, but shall in all cases be subject
to our approval. Delayed delivery contracts will not be subject to any conditions except that:
|
|
·
|
the purchase by an institution of the securities covered under that contract shall not at the time of delivery be prohibited under the laws of the jurisdiction to which that institution is subject; and
|
|
|
·
|
if the securities are also being sold to underwriters acting as principals for their own account, the underwriters shall have purchased such securities not sold for delayed delivery. The underwriters and other persons acting as our agents will not have any responsibility in respect of the validity or performance of delayed delivery contracts.
|
One or more firms, referred to as “remarketing
firms,” may also offer or sell the securities, if a prospectus supplement so indicates, in connection with a remarketing arrangement
upon their purchase. Remarketing firms will act as principals for their own accounts or as our agents. These remarketing firms
will offer or sell the securities in accordance with the terms of the securities. Each prospectus supplement will identify and
describe any remarketing firm and the terms of its agreement, if any, with us and will describe the remarketing firm’s compensation.
Remarketing firms may be deemed to be underwriters in connection with the securities they remarket. Remarketing firms may be entitled
under agreements that may be entered into with us to indemnification by us against certain civil liabilities, including liabilities
under the Securities Act, and may be customers of, engage in transactions with or perform services for us in the ordinary course
of business.
Certain underwriters may use this prospectus
and any accompanying prospectus supplement for offers and sales related to market-making transactions in the securities. These
underwriters may act as principal or agent in these transactions, and the sales will be made at prices related to prevailing market
prices at the time of sale. Any underwriters involved in the sale of the securities may qualify as “underwriters” within
the meaning of Section 2(a)(11) of the Securities Act. In addition, the underwriters’ commissions, discounts or concessions may
qualify as underwriters’ compensation under the Securities Act and the rules of the Financial Industry Regulatory Authority, Inc.,
or FINRA.
Shares of our common stock sold pursuant
to the registration statement of which this prospectus is a part will be listed on the Nasdaq Capital Market. The applicable
prospectus supplement will contain information, where applicable, as to any other listing, if any, on the Nasdaq Capital Market
or any securities market or other securities exchange of the securities covered by the prospectus supplement. Underwriters
may make a market in our common stock, but will not be obligated to do so and may discontinue any market making at any time without
notice. We can make no assurance as to the liquidity of or the existence, development or maintenance of trading markets for any
of the securities.
In order to facilitate the offering of
the securities, certain persons participating in the offering may engage in transactions that stabilize, maintain or otherwise
affect the price of the securities. This may include over-allotments or short sales of the securities, which involve the
sale by persons participating in the offering of more securities than we sold to them. In these circumstances, these persons
would cover such over-allotments or short positions by making purchases in the open market or by exercising their over-allotment
option. In addition, these persons may stabilize or maintain the price of the securities by bidding for or purchasing the
applicable security in the open market or by imposing penalty bids, whereby selling concessions allowed to dealers participating
in the offering may be reclaimed if the securities sold by them are repurchased in connection with stabilization transactions.
The effect of these transactions may be to stabilize or maintain the market price of the securities at a level above that which
might otherwise prevail in the open market. These transactions may be discontinued at any time.
The underwriters, dealers and agents may
engage in other transactions with us, or perform other services for us, in the ordinary course of their business.
DESCRIPTION OF COMMON STOCK
We are authorized to issue 71,428,571 shares
of common stock, par value $0.001 per share. On May 25, 2017, we had 25,723,854 shares of common stock outstanding and approximately
255 stockholders of record.
The following summary of certain provisions
of our common stock does not purport to be complete. You should refer to our articles of incorporation and our bylaws, both
of which are included as exhibits to the registration statement of which this prospectus is a part. The summary below is
also qualified by provisions of applicable law.
General
The holders of outstanding shares of common
stock are entitled to receive dividends out of assets or funds legally available for the payment of dividends of such times and
in such amounts as the board from time to time may determine. Holders of common stock are entitled to one vote for each share held
on all matters submitted to a vote of stockholders. There is no cumulative voting of the election of directors then standing for
election. The common stock is not entitled to pre-emptive rights and is not subject to conversion or redemption. Upon liquidation,
dissolution or winding up of our company, the assets legally available for distribution to stockholders are distributable ratably
among the holders of the common stock after payment of liquidation preferences, if any, on any outstanding payment of other claims
of creditors. Each outstanding share of common stock is duly and validly issued, fully paid and non-assessable.
Transfer Agent and Registrar
The transfer agent and registrar for our
common stock is VStock Transfer, LLC. The transfer agent’s address is 18 Lafayette Place, Woodmere, New York 11598 and its telephone
number is (212) 828-8436.
Listing
Our common stock is listed on the Nasdaq
Capital Market under the symbol “EKSO.”
DESCRIPTION OF PREFERRED STOCK
We are authorized to issue 10,000,000 shares
of preferred stock, par value $0.001 per share, of which 15,000 shares were designated as Series A Convertible Preferred Stock.
As of May 25, 2017, no shares of our preferred stock were outstanding. The following summary of certain provisions of our
preferred stock does not purport to be complete. You should refer to our articles of incorporation and our bylaws, both of
which are included as exhibits to the registration statement of which this prospectus is a part. The summary below is also
qualified by provisions of applicable law.
General
Our board of directors may, without further
action by our stockholders, from time to time, direct the issuance of shares of preferred stock in one or more series and may,
at the time of issuance, determine the rights, preferences and limitations of each series, including voting rights, dividend rights
and redemption and liquidation preferences. Satisfaction of any dividend preferences of outstanding shares of preferred stock
would reduce the amount of funds available for the payment of dividends on shares of our common stock. Holders of shares
of preferred stock may be entitled to receive a preference payment in the event of any liquidation, dissolution or winding-up of
our company before any payment is made to the holders of shares of our common stock. In some circumstances, the issuance
of shares of preferred stock may render more difficult or tend to discourage a merger, tender offer or proxy contest, the assumption
of control by a holder of a large block of our securities or the removal of incumbent management. Upon the affirmative vote
of our board of directors, without stockholder approval, we may issue shares of preferred stock with voting and conversion rights
which could adversely affect the holders of shares of our common stock.
If we offer a specific series of preferred
stock under this prospectus, we will describe the terms of the preferred stock in the prospectus supplement for such offering and
will file a copy of the certificate establishing the terms of the preferred stock with the SEC. To the extent required, this
description will include:
|
|
·
|
the title and stated value;
|
|
|
·
|
the number of shares offered, the liquidation preference, if any, per share and the purchase price;
|
|
|
·
|
the dividend rate(s), period(s) and/or payment date(s), or method(s) of calculation for such dividends;
|
|
|
·
|
whether dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate;
|
|
|
·
|
the procedures for any auction and remarketing, if any;
|
|
|
·
|
the provisions for a sinking fund, if any;
|
|
|
·
|
the provisions for redemption, if applicable;
|
|
|
·
|
any listing of the preferred stock on any securities exchange or market;
|
|
|
·
|
whether the preferred stock will be convertible into our common stock, and, if applicable, the conversion price (or how it will be calculated) and conversion period;
|
|
|
·
|
whether the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange price (or how it will be calculated) and exchange period;
|
|
|
·
|
voting rights, if any, of the preferred stock;
|
|
|
·
|
a discussion of any material and/or special U.S. federal income tax considerations applicable to the preferred stock;
|
|
|
·
|
the relative ranking and preferences of the preferred stock as to dividend rights and rights upon liquidation, dissolution or winding up of the affairs of the Company; and
|
|
|
·
|
any material limitations on issuance of any class or series of preferred stock ranking pari passu with or senior to the series of preferred stock as to dividend rights and rights upon liquidation, dissolution or winding up of the Company.
|
Series A Preferred Stock
In December 2014, our board of directors
created out of the authorized and unissued shares of our preferred stock a series of preferred stock designated as the Series A
Convertible Preferred Stock (the “Series A preferred stock”), comprising up to 15,000 shares of preferred stock. The
material terms of the Series A preferred stock are described below.
Conversion; Beneficial Ownership Limitation
.
Subject to certain ownership limitations as described below, a holder of shares of Series A preferred stock may convert the Series
A preferred stock into shares of our common stock at any time after the initial issuance date at a conversion ratio determined
by dividing the stated value of the Series A preferred stock (or $1,000) by the “Conversion Price.” The Conversion Price
initially was $1.01 per share, subject to adjustment as described below. On and after the close of business on the date of any
such conversion, the holder converting such Series A preferred stock would be deemed to be the holder of record of the common stock
issuable upon such conversion, such Series A preferred stock would cease to be outstanding, and all rights whatsoever with respect
to such shares (except the right to receive the common stock) would terminate.
Subject to limited exceptions, a holder
of shares of Series A preferred stock does not have the right to convert any portion of its Series A preferred stock if the holder,
together with its affiliates, would beneficially own in excess of 4.99% of the number of shares of our common stock outstanding
immediately after giving effect to its conversion (the “Beneficial Ownership Limitation”); provided, however, that upon
61 days’ prior notice to us, the holder may increase or decrease the Beneficial Ownership Limitation, provided that in no event
shall the Beneficial Ownership Limitation exceed 9.99%.
Adjustments to Conversion Price
.
The conversion price is subject to adjustment in the case of stock splits, stock dividends, combinations of shares and similar
recapitalization transactions. In addition, if we sell or grant any right to purchase or sell any common stock or common stock
equivalents entitling any person to acquire shares of common stock (subject to exceptions for certain exempt issuances, including
issuances pursuant to equity compensation plans, certain issuances to consultants, issuances in connection with exercise or exchange
of common stock equivalents already outstanding and issuances pursuant to strategic transactions or to strategic investors) at
an effective price per share that is lower than the then Conversion Price of the Series A preferred stock, then the Conversion
Price would be further reduced to equal the effective price per share applicable to such sale. As a result of the one-for-seven
reverse stock split completed on May 4, 2016 and the sale of common stock in an underwritten offering on August 9, 2016 at a price
per share less than the Conversion Price then in effect, the conversion price of the Series A preferred stock is $3.74 per share.
Voting Rights
. Except as required
by law, holders of the Series A preferred stock do not have rights to vote on any matters, questions or proceedings, including
the election of directors. However, if any shares of Series A preferred stock are outstanding, we cannot, without the affirmative
vote of the holders of 75% or more of the then outstanding shares of the Series A preferred stock, (1) alter or change adversely
the powers, preferences or rights given to the Series A preferred stock or alter or amend the certificate of designation, (2) amend
our articles of incorporation or other charter documents in any manner that adversely affects any rights of the holders of Series
A preferred stock, (3) increase the number of authorized shares of Series A preferred stock, or (4) enter into any agreement with
respect to any of the foregoing.
Dividends
. Shares of Series A preferred
stock are not be entitled to receive any dividends, unless and until specifically declared by our board of directors. The holders
of the Series A preferred stock participate, on an as-if-converted-to-common stock basis, in any dividends to the holders of common
stock.
Liquidation
. In the event of either
a voluntary or involuntary liquidation, dissolution or winding up of us, the assets of which constitute all or substantially all
of the assets of our business, in a single transaction or series of transactions, the holders of Series A preferred stock would
be entitled to participate, on an as-if-converted-to-common stock basis, in any distributions to the holders of common stock.
Exchange Listing
. We do not plan
on making an application to list the Series A preferred stock on any national securities exchange or other nationally recognized
trading system.
Transfer Agent and Registrar
The transfer agent and registrar for our
preferred stock will be set forth in the applicable prospectus supplement.
DESCRIPTION OF WARRANTS
General
As of May 25, 2017, warrants to purchase
an aggregate of 5,091,910 shares of our common stock are issued and outstanding, of which warrants to purchase 88,368 shares of
common stock have an exercise price of $9.66 per share and expire on May 20, 2020 (the “Pre-Merger Warrants”), warrants
to purchase 425,906 shares of common stock have an exercise price of $7.00 per share and expire on January 15, 2019, warrants to
purchase 1,077,816 shares of common stock have an exercise price of $14.00 per share and expire on January 15, 2019, warrants to
purchase 1,633,642 shares have an exercise price of $3.74 and expire on December 28, 2020, and warrants to purchase 1,866,178 shares
have an exercise price of $4.10 and expire on October 6, 2022. The Pre-Merger Warrants may, at the option of the holders, be exercised
on a cashless exercise basis, which means that in lieu of paying the aggregate exercise price for the shares being purchased upon
exercise of the warrants for cash, the holder will forfeit a number of shares underlying the warrants with a fair market value
(as defined in the warrant) equal to such aggregate exercise price. We will not receive additional proceeds to the extent these
warrants are exercised on a cashless exercise basis.
We may issue warrants to purchase shares
of our common stock and/or preferred stock in one or more series together with other securities or separately, as described in
the applicable prospectus supplement. Below is a description of certain general terms and provisions of the warrants that
we may offer. Particular terms of the warrants will be described in the warrant agreements and the prospectus supplement
relating to the warrants.
The applicable prospectus supplement will
contain, where applicable, the following terms of and other information relating to the warrants:
|
|
·
|
the specific designation and aggregate number of, and the price at which we will issue, the warrants;
|
|
|
·
|
the currency or currency units in which the offering price, if any, and the exercise price are payable;
|
|
|
·
|
the designation, amount and terms of the securities purchasable upon exercise of the warrants;
|
|
|
·
|
if applicable, the exercise price for shares of our common stock and the number of shares of common stock to be received upon exercise of the warrants;
|
|
|
·
|
if applicable, the exercise price for shares of our preferred stock, the number of shares of preferred stock to be received upon exercise, and a description of that series of our preferred stock;
|
|
|
·
|
the date on which the right to exercise the warrants will begin and the date on which that right will expire or, if you may not continuously exercise the warrants throughout that period, the specific date or dates on which you may exercise the warrants;
|
|
|
·
|
whether the warrants will be issued in fully registered form or bearer form, in definitive or global form or in any combination of these forms, although, in any case, the form of a warrant included in a unit will correspond to the form of the unit and of any security included in that unit;
|
|
|
·
|
any applicable material U.S. federal income tax consequences;
|
|
|
·
|
the identity of the warrant agent for the warrants and of any other depositaries, execution or paying agents, transfer agents, registrars or other agents;
|
|
|
·
|
the proposed listing, if any, of the warrants or any securities purchasable upon exercise of the warrants on any securities exchange;
|
|
|
·
|
if applicable, the date from and after which the warrants and the common stock and/or preferred stock will be separately transferable;
|
|
|
·
|
if applicable, the minimum or maximum amount of the warrants that may be exercised at any one time;
|
|
|
·
|
information with respect to book-entry procedures, if any;
|
|
|
·
|
the anti-dilution provisions of the warrants, if any;
|
|
|
·
|
any redemption or call provisions;
|
|
|
·
|
whether the warrants may be sold separately or with other securities as parts of units; and
|
|
|
·
|
any additional terms of the warrants, including terms, procedures and limitations relating to the exchange and exercise of the warrants.
|
Transfer Agent and Registrar
The transfer agent and registrar for any
warrants will be set forth in the applicable prospectus supplement.
DESCRIPTION OF RIGHTS
General
We may issue rights to our stockholders
to purchase shares of our common stock, preferred stock or the other securities described in this prospectus. We may offer
rights separately or together with one or more additional rights, preferred stock, common stock, or warrants, or any combination
of those securities in the form of units, as described in the applicable prospectus supplement. Each series of rights will
be issued under a separate rights agreement to be entered into between us and a bank or trust company, as rights agent. The
rights agent will act solely as our agent in connection with the certificates relating to the rights of the series of certificates
and will not assume any obligation or relationship of agency or trust for or with any holders of rights certificates or beneficial
owners of rights. The following description sets forth certain general terms and provisions of the rights to which any prospectus
supplement may relate. The particular terms of the rights to which any prospectus supplement may relate and the extent, if
any, to which the general provisions may apply to the rights so offered will be described in the applicable prospectus supplement.
To the extent that any particular terms of the rights, rights agreement or rights certificates described in a prospectus supplement
differ from any of the terms described below, then the terms described below will be deemed to have been superseded by that prospectus
supplement. We encourage you to read the applicable rights agreement and rights certificate for additional information before
you decide whether to purchase any of our rights.
We will provide in a prospectus supplement
the following terms of the rights being issued:
|
|
·
|
the date of determining the stockholders entitled to the rights distribution;
|
|
|
·
|
the aggregate number of shares of common stock, preferred stock or other securities purchasable upon exercise of the rights;
|
|
|
·
|
the exercise price;
|
|
|
·
|
the aggregate number of rights issued;
|
|
|
·
|
whether the rights are transferrable and the date, if any, on and after which the rights may be separately transferred;
|
|
|
·
|
the date on which the right to exercise the rights will commence, and the date on which the right to exercise the rights will expire;
|
|
|
·
|
the method by which holders of rights will be entitled to exercise;
|
|
|
·
|
the conditions to the completion of the offering, if any;
|
|
|
·
|
the withdrawal, termination and cancellation rights, if any;
|
|
|
·
|
whether there are any backstop or standby purchaser or purchasers and the terms of their commitment, if any;
|
|
|
·
|
whether stockholders are entitled to oversubscription rights, if any;
|
|
|
·
|
any applicable material U.S. federal income tax considerations; and
|
|
|
·
|
any other terms of the rights, including terms, procedures and limitations relating to the distribution, exchange and exercise of the rights, as applicable.
|
Each right will entitle the holder of rights
to purchase for cash the principal amount of shares of common stock, preferred stock or other securities at the exercise price
provided in the applicable prospectus supplement. Rights may be exercised at any time up to the close of business on the
expiration date for the rights provided in the applicable prospectus supplement.
Holders may exercise rights as described
in the applicable prospectus supplement. Upon receipt of payment and the rights certificate properly completed and duly executed
at the corporate trust office of the rights agent or any other office indicated in the prospectus supplement, we will, as soon
as practicable, forward the shares of common stock, preferred stock or other securities, as applicable, purchasable upon exercise
of the rights. If less than all of the rights issued in any rights offering are exercised, we may offer any unsubscribed
securities directly to persons other than stockholders, to or through agents, underwriters or dealers or through a combination
of such methods, including pursuant to standby arrangements, as described in the applicable prospectus supplement.
Rights Agent
The rights agent for any rights we offer
will be set forth in the applicable prospectus supplement.
DESCRIPTION OF UNITS
The following description, together with
the additional information that we include in any applicable prospectus supplements, summarizes the material terms and provisions
of the units that we may offer under this prospectus. While the terms we have summarized below will apply generally to any
units that we may offer under this prospectus, we will describe the particular terms of any series of units in more detail in the
applicable prospectus supplement. The terms of any units offered under a prospectus supplement may differ from the terms
described below.
General
We may issue units consisting of common
stock, preferred stock, one or more warrants or rights for the purchase of common stock or preferred stock in one or more series,
in any combination. Each unit will be issued so that the holder of the unit is also the holder of each security included
in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each security included in the unit.
The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred
separately, at any time or at any time before a specified date.
We will describe in the applicable prospectus
supplement the terms of the series of units being offered, including:
|
|
·
|
the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately;
|
|
|
·
|
any provisions of the governing unit agreement that differ from those described below; and
|
|
|
·
|
any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units.
|
The provisions described in this section,
as well as those set forth in any prospectus supplement or as described under “Description of Common Stock,” “Description
of Preferred Stock,” “Description of Warrants,” and “Description of Rights” will apply to each unit, as
applicable, and to any common stock, preferred stock, warrant, or right included in each unit, as applicable.
Unit Agent
The name and address of the unit agent
for any units we offer will be set forth in the applicable prospectus supplement.
Issuance in Series
We may issue units in such amounts and
in such numerous distinct series as we determine.
Enforceability of Rights by Holders of Units
Each unit agent will act solely as our
agent under the applicable unit agreement and will not assume any obligation or relationship of agency or trust with any holder
of any unit. A single bank or trust company may act as unit agent for more than one series of units. A unit agent will
have no duty or responsibility in case of any default by us under the applicable unit agreement or unit, including any duty or
responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a unit may,
without the consent of the related unit agent or the holder of any other unit, enforce by appropriate legal action its rights as
holder under any security included in the unit.
CERTAIN PROVISIONS OF NEVADA LAW AND
OF THE COMPANY’S ARTICLES OF INCORPORATION AND BYLAWS
Anti-Takeover Provisions
Nevada Law
We may in the future become subject to
Nevada’s control share laws. A corporation is subject to Nevada’s control share law if it has more than 200 stockholders of record,
at least 100 of whom are residents of Nevada, and if the corporation does business in Nevada, including through an affiliated corporation.
This control share law may have the effect of discouraging corporate takeovers. The Company currently has fewer than 100 stockholders
of record who are residents of Nevada.
The control share law focuses on the acquisition
of a “controlling interest,” which means the ownership of outstanding voting shares that would be sufficient, but for
the operation of the control share law, to enable the acquiring person to exercise the following proportions of the voting power
of the corporation in the election of directors: (1) one-fifth or more but less than one-third; (2) one-third or more but less
than a majority; or (3) a majority or more. The ability to exercise this voting power may be direct or indirect, as well as individual
or in association with others.
The effect of the control share law is
that an acquiring person, and those acting in association with that person, will obtain only such voting rights in the control
shares as are conferred by a resolution of the stockholders of the corporation, approved at a special or annual meeting of stockholders.
The control share law contemplates that voting rights will be considered only once by the other stockholders. Thus, there is no
authority to take away voting rights from the control shares of an acquiring person once those rights have been approved. If the
stockholders do not grant voting rights to the control shares acquired by an acquiring person, those shares do not become permanent
non-voting shares. The acquiring person is free to sell the shares to others. If the buyer or buyers of those shares themselves
do not acquire a controlling interest, the shares are not governed by the control share law any longer.
If control shares are accorded full voting
rights and the acquiring person has acquired control shares with a majority or more of the voting power, a stockholder of record,
other than the acquiring person, who did not vote in favor of approval of voting rights for the control shares, is entitled to
demand fair value for such stockholder’s shares.
In addition to the control share law, Nevada
has a business combination law, which prohibits certain business combinations between Nevada corporations and “interested
stockholders” for two years after the interested stockholder first becomes an interested stockholder, unless (a) the corporation’s
board of directors approves the combination in advance or (b) the corporation’s board of directors and at least 60% of the corporation’s
disinterested stockholders approve the combination at an annual or special meeting. For purposes of Nevada law, an interested stockholder
is any person who is: (a) the beneficial owner, directly or indirectly, of 10% or more of the voting power of the outstanding voting
shares of the corporation, or (b) an affiliate or associate of the corporation and at any time within the previous two years was
the beneficial owner, directly or indirectly, of 10% or more of the voting power of the then-outstanding shares of the corporation.
The definition of “business combination” contained in the statute is sufficiently broad to cover virtually any kind of
transaction that would allow a potential acquirer to use the corporation’s assets to finance the acquisition or otherwise to benefit
its own interests rather than the interests of the corporation and its other stockholders.
The effect of Nevada’s business combination
law is to potentially discourage a party interested in taking control of the Company from doing so if it cannot obtain the approval
of our board of directors.
Authorized but Unissued Shares
The
authorized but unissued shares of common stock and preferred stock are available for future issuance without stockholder approval,
subject to any limitations imposed by the listing standards of any exchange on which our shares are listed.
These
additional
shares may be used for a variety of corporate finance transactions, acquisitions and employee benefit plans. The existence of authorized
but unissued and unreserved common stock and preferred stock could make more difficult or discourage an attempt to obtain control
of us by means of a proxy contest, tender offer, merger or otherwise.
Special Meeting of Stockholders;
Advance Notice Requirements; Stockholder Action
Our by-laws provide
that, except as otherwise required by law or the articles of incorporation, special meetings of the stockholders can only be called
by our board of directors, by the chairman of our board of directors or by certain of our officers. In addition, our by-laws establish
an advance notice procedure for stockholder proposals to be brought before an annual meeting of stockholders, including proposed
nominations of candidates for election to our board of directors. Stockholders at an annual meeting may only consider proposals
or nominations specified in the notice of the meeting or brought before the meeting by or at the direction of our board of directors,
or by a stockholder of record on the record date for the meeting who is entitled to vote at the meeting and who has delivered timely
written notice in proper form to our secretary of the stockholder’s intention to bring such business before the meeting. These
provisions could have the effect of delaying until the next stockholder meeting stockholder actions that are favored by the holders
of a majority of our outstanding voting securities. In addition, our by-laws require that stockholder actions must be effected
at a duly called stockholders meeting and prohibit actions by our stockholders by written consent.
Limitation of Liability and Indemnification
Nevada Revised Statutes (NRS) Sections
78.7502 and 78.751 provide us with the power to indemnify any of our directors, officers, employees and agents. The person
entitled to indemnification must have conducted himself in good faith, and must reasonably believe that his conduct was in, or
not opposed to, our best interests. In a criminal action, the director, officer, employee or agent must not have had reasonable
cause to believe that his conduct was unlawful.
Under NRS Section 78.751, advances for
expenses may be made by agreement if the director or officer affirms in writing that he has met the standards for indemnification
and will personally repay the expenses if it is determined that such officer or director did not meet those standards.
Our by-laws state that we shall indemnify
every (i) present or former director, officer, employee or agent of us and (ii) any person who served at our request as a director,
officer, member, manager, partner, trustee, fiduciary, employee or agent of another corporation, limited liability company, partnership,
joint venture, trust, employee benefit plan or other enterprise (each an “Indemnitee”).
Our by-laws provide that we shall indemnify
an Indemnitee against expenses, including attorneys’ fees and disbursements, and costs (and in connection with a proceeding other
than a proceeding by or in the right of the Company, judgments, fines and amounts paid in settlement) actually and reasonably incurred
by such person in connection with any proceeding in which such Indemnitee was, is or is threatened to be named as defendant or
respondent, or in which he was or is a witness without being named a defendant or respondent, by reason, in whole or in part, of
his serving or having served, or having been nominated or designated to serve, if it is determined that the Indemnitee (a) conducted
himself in good faith and in a manner which such Indemnitee reasonably believed to be in or not opposed to our best interests,
or with respect to any criminal proceeding, had no reasonable cause to believe that his conduct was unlawful or (b) is not liable
pursuant to NRS Section 78.138; provided, however, that in the event that an Indemnitee is found liable to us, we will have no
obligation to indemnify such Indemnitee unless, and only to the extent that the court in which such action or suit was brought
or other court of competent jurisdiction determines that, in view of all the circumstances of the case, such person is fairly and
reasonably entitled to indemnity for such expenses and costs as a court of competent jurisdiction or such other court shall deem
proper.
The termination of any proceeding by judgment,
order, settlement or conviction, or on a plea of nolo contendere or its equivalent, is not of itself determinative that the Indemnitee
did not meet the requirements set forth in clauses (a) or (b) above. An Indemnitee shall be deemed to have been found liable in
respect of any claim, issue or matter only after the Indemnitee shall have been so adjudged by a court of competent jurisdiction
after exhaustion of all appeals therefrom.
In addition to our by-laws, have entered
into an Indemnification Agreement with each of our directors and executive officers pursuant to which we are required to indemnify,
and to advance expenses on behalf of, such persons to the fullest extent permitted by applicable law and our governing documents.
We believe that entering into these agreements helps us to attract and retain highly competent and qualified persons to serve the
Company.
Insofar as indemnification for liabilities
arising under the Securities Act may be permitted to our directors, officers and controlling persons, we have been advised that
in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is, therefore,
unenforceable.
LEGAL MATTERS
Nutter, McClennen and Fish, LLP, Boston,
Massachusetts, will pass upon the validity of the issuance of the securities to be offered by this prospectus.
EXPERTS
OUM & Co. LLP, independent registered
public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K for the year
ended December 31, 2016, as set forth in their report, which is incorporated by reference in this prospectus and elsewhere in the
registration statement. Our financial statements are incorporated by reference in reliance on OUM & Co. LLP’s report, given
on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We are subject to the reporting requirements
of the Securities Exchange Act of 1934, as amended, and file annual, quarterly and current reports, proxy statements and other
information with the SEC. You may read and copy these reports, proxy statements and other information at the SEC’s public
reference facilities at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You can request copies of these documents
by writing to the SEC and paying a fee for the copying cost. Please call the SEC at 1-800-SEC-0330 for more information about the
operation of the public reference facilities. SEC filings are also available at the SEC’s web site at http://www.sec.gov.
We
have filed with the SEC a registration statement under the Securities Act relating to the offering of these securities. The registration
statement, including the attached exhibits, contains additional relevant information about us and the securities. This prospectus
does not contain all of the information set forth in the registration statement. You can obtain a copy of the registration statement,
at prescribed rates, from the SEC at the address listed above.
The
registration statement and the documents referred to below under “Incorporation by Reference” are also available on our
Internet website www.eksobionics.com. We have not incorporated by reference into this prospectus the information on our website,
and you should not consider it to be a part of this prospectus
.
INCORPORATION OF DOCUMENTS BY REFERENCE
The
SEC allows us to incorporate by reference into this prospectus certain information we file with it, which means that we can disclose
important information by referring you to those documents. The information incorporated by reference is considered to be a part
of this prospectus, and information that we file later with the SEC will automatically update and supersede information contained
in this prospectus and any accompanying prospectus supplement. We incorporate by reference the documents listed below that we have
previously filed with the SEC (excluding any portions of any Form 8-K that are not deemed “filed” pursuant to the General
Instructions of Form 8-K):
|
|
·
|
our Annual Report on Form 10-K for the fiscal year ended December 31, 2016 filed on March 15, 2017;
|
|
|
·
|
the portions of our definitive proxy statement on Schedule 14A filed on April 28, 2017 that are deemed “filed” with the SEC under the Exchange Act;
|
|
|
·
|
our Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2017, filed on May 9, 2017;
|
|
|
·
|
our Current Reports on Form 8-K filed on January 6, 2017, March 23, 2017, April 5, 2017, April 26, 2017, and May 23, 2017; and
|
|
|
·
|
the description of our common stock contained in our Registration Statement on Form 8-A, filed on May 6, 2015 pursuant to Section 12(b) of the Exchange Act, which incorporates by reference the description of the shares of our common stock contained in our Registration Statement on Form S-1 (File No. 333-195783) filed on May 7, 2014 and declared effective by the SEC on June 20, 2014, and any amendment or report filed with the SEC for purposes of updating such description; and
|
|
|
·
|
all reports and other documents subsequently filed by us pursuant to Sections 13(a), 13(c), 14 and 15(d) of the Exchange Act after the date of this prospectus and prior to the termination or completion of the offering of securities under this prospectus shall be deemed to be incorporated by reference in this prospectus and to be a part hereof from the date of filing such reports and other documents.
|
Any statement contained in this prospectus
or in a document incorporated or deemed to be incorporated by reference into this prospectus will be deemed to be modified or superseded
for purposes of this prospectus to the extent that a statement contained in this prospectus or any other subsequently filed document
that is deemed to be incorporated by reference into this prospectus modifies or supersedes the statement. Any statement so modified
or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
We
will provide to each person, including any beneficial owner, to whom this prospectus is delivered, upon written or oral request,
at no cost to the requester, a copy of any or all of the information that is incorporated by reference in this prospectus. Requests
for such documents should be directed to:
Investor Relations, Ekso Bionics Holdings, Inc., 1414 Harbour Way South, Suite
1201, Richmond, California 94804, (510) 984-1761.
You
may also access the documents incorporated by reference in this prospectus through our website at www.eksobionics.com. Except for
the specific incorporated documents listed above, no information available on or through our website shall be deemed to be incorporated
in this prospectus or the registration statement of which it forms a part.
This
prospectus may contain information that updates, modifies or is contrary to information in one or more of the documents incorporated
by reference in this prospectus. You should rely only on the information incorporated by reference or provided in this prospectus.
We have not authorized anyone else to provide you with different information. You should not assume that the information in this
prospectus is accurate as of any date other than the date of this prospectus or the date of the documents incorporated by reference
in this prospectus.

Up to
$25,000,000
Common
Stock
PROSPECTUS SUPPLEMENT

August 21, 2018
Ekso Bionics (NASDAQ:EKSO)
Historical Stock Chart
From Mar 2024 to Apr 2024
Ekso Bionics (NASDAQ:EKSO)
Historical Stock Chart
From Apr 2023 to Apr 2024