UNITED STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM 6-K
REPORT OF
FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE
SECURITIES EXCHANGE ACT OF 1934
For the month of January 2019
Commission File Number: 001-31995
MEDICURE
INC.
(Translation of registrant's name into English)
2-1250 Waverley Street
Winnipeg, MB Canada R3T 6C6
(Address of principal executive offices)
Indicate by check mark whether the registrant
files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F x
Form 40-F o
Indicate by check mark if the registrant is
submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): o
Indicate by check mark if the registrant is
submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): o
Indicate by check mark whether the registrant
by furnishing the information contained in this Form is also thereby furnishing the information to the Commission pursuant to Rule
12g3-2(b) under the Securities Exchange Act of 1934.
Yes o
No x
If “Yes” is marked, indicate below
the file number assigned to the registrant in connection with Rule 12g3-2(b): 8a72____.
EXHIBIT
LIST
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
Medicure Inc. |
| |
(Registrant) |
| |
|
| |
|
|
| Date: January 28, 2019 |
By: |
/s/ Dr. Albert D. Friesen |
| |
Dr. Albert D. Friesen |
| |
Title: President & CEO |
Exhibit 99.1
Medicure Announces Agreement to Market
ReDS™ Device for Congestive Heart Failure Patients in the United States
INVESTS US$10.0 MILLION
IN SENSIBLE MEDICAL
WINNIPEG, Jan. 28, 2019 /CNW/ - Medicure Inc.
("Medicure" or the "Company") (TSXV:MPH, OTC:MCUJF), a cardiovascular pharmaceutical company,
is pleased to announce it has entered into an agreement with Sensible Medical Innovations Inc. ("Sensible") to become
the exclusive marketing partner for the ReDS™ point of care system ("ReDS") in the United States. ReDS is a non-invasive,
FDA-cleared medical device that provides an accurate, actionable and absolute measurement of lung fluid which is important in the
management of congestive heart failure. The lung fluid measurements are used in guiding treatment and monitoring a heart failure
patient's condition and may lead to a significant decrease in readmissions and hospital costs. Clinical studies have shown
an 87% reduction in heart failure readmission rates for patients using the ReDS system at home for three months post-discharge
versus those who were treated with usual care alone. ReDS is already marketed to U.S. hospitals by Sensible and Medicure expects
to begin marketing ReDS immediately using its existing commercial organization. Under the terms of the agreement, Medicure
will receive a percentage of total U.S. sales revenue of the device and must meet minimum annual sales quotas.
In addition, Medicure has invested US$10.0
million in Sensible for a 7.71% equity stake on a fully diluted basis. In connection with the investment, Medicure's President
and CEO, Dr. Albert D. Friesen, has been appointed to the Board of Directors of Sensible.
"Medicure is pleased to introduce the
ReDS device in an effort to improve the quality of life of heart failure patients. The device is being sold directly to hospitals
and fits well with Medicure's existing commercial operation and our mission of being a significant, value based, cardiovascular
company focused on the U.S. market." commented Dr. Friesen.
"These are exciting times for Sensible
Medical. The partnership with Medicure is a natural fit to fuel the company's vision to expand the reach of the ReDS device and
make it available to millions of heart failure patients that suffer from low quality of life and repeated hospital admissions that
are a major burden to the healthcare economy. With this partnership, we expect accelerated sales growth in the U.S."
commented Amir Ronen, Sensible Medical's CEO.
About ReDS Point of Care System
ReDS is intended for use by qualified health
care practitioners and by patients, under the direction of a physician, in hospitals, hospital-type facilities and home environment,
for the non-invasive monitoring and management of patients with fluid management problems in a variety of medically accepted clinical
applications. ReDS is indicated for patients: with fluid management problems; taking diuretic medication; living with heart failure;
or recovering from a coronary artery disease related event.
About Medicure
Medicure is a pharmaceutical company focused
on the development and commercialization of therapies for the U.S. cardiovascular market. The present focus of the Company is the
marketing and distribution of AGGRASTAT® (tirofiban hydrochloride) injection and ZYPITAMAGTM (pitavastatin)
tablets in the United States, where they are sold through the Company's U.S. subsidiary, Medicure Pharma, Inc. For more information
on Medicure please visit www.medicure.com.
About Sensible
Sensible is a market leader in medical radar
monitoring and imaging technology. ReDS™ was adapted for medical use from military 'see-through-wall' technology. The technology
is well-positioned to be a difference maker in a wide range of applications and to become the next-generation lung fluid monitoring
modality.
www.sensible-medical.com
To be added to Medicure's e-mail list, please
visit:
http://medicure.mediaroom.com/alerts
Neither the TSX Venture Exchange nor its
Regulation Services Provider (as that term is defined in policies of the TSX Venture Exchange) accepts responsibility for the adequacy
or accuracy of this release.
Forward Looking Information: Statements
contained in this press release that are not statements of historical fact, including, without limitation, statements containing
the words "believes", "may", "plans", "will", "estimates", "continues",
"anticipates", "intends", "expects" and similar expressions, may constitute "forward-looking
information" within the meaning of applicable Canadian and U.S. federal securities laws (such forward-looking information
and forward-looking statements are hereinafter collectively referred to as "forward-looking statements"). Forward-looking
statements, include the target launch date for new products, the estimated number of products the Company will be selling in the
future, the potential benefits of the ReDS™ system, estimates, analysis and opinions of management of
the Company made in light of its experience and its perception of trends, current conditions and expected developments, as well
as other factors which the Company believes to be relevant and reasonable in the circumstances. Inherent in forward-looking statements
are known and unknown risks, uncertainties and other factors beyond the Company's ability to predict or control that may cause
the actual results, events or developments to be materially different from any future results, events or developments expressed
or implied by such forward-looking statements, and as such, readers are cautioned not to place undue reliance on forward-looking
statements. Such risk factors include, among others, the Company's future product revenues, stage of development, additional capital
requirements, risks associated with the completion and timing of clinical trials and obtaining regulatory approval to market the
Company's products, the ability to protect its intellectual property, dependence upon collaborative partners, changes in government
regulation or regulatory approval processes, and rapid technological change in the industry. Such statements are based on a number
of assumptions which may prove to be incorrect, including, but not limited to, assumptions about: general business and economic
conditions; the impact of changes in Canadian-US dollar and other foreign exchange rates on the Company's revenues, costs and results;
the timing of the receipt of regulatory and governmental approvals for the Company's research and development projects; the availability
of financing for the Company's commercial operations and/or research and development projects, or the availability of financing
on reasonable terms; results of current and future clinical trials; the uncertainties associated with the acceptance and demand
for new products and market competition. The foregoing list of important factors and assumptions is not exhaustive. The Company
undertakes no obligation to update publicly or otherwise revise any forward-looking statements or the foregoing list of factors,
other than as may be required by applicable legislation. Additional discussion regarding the risks and uncertainties relating to
the Company and its business can be found in the Company's other filings with the applicable Canadian securities regulatory authorities
or the US Securities and Exchange Commission, and in the "Risk Factors" section of its Form 20F for the year ended December
31, 2017.
AGGRASTAT® (tirofiban hydrochloride)
is a registered trademark of Medicure International Inc.
 View
original content:http://www.prnewswire.com/news-releases/medicure-announces-agreement-to-market-reds-device-for-congestive-heart-failure-patients-in-the-united-states-300785383.html
View
original content:http://www.prnewswire.com/news-releases/medicure-announces-agreement-to-market-reds-device-for-congestive-heart-failure-patients-in-the-united-states-300785383.html
SOURCE Medicure Inc.
View original content: http://www.newswire.ca/en/releases/archive/January2019/28/c4072.html
%CIK: 0001133519
For further information: James Kinley, Chief Financial Officer,
Tel. 888-435-2220, Fax 204-488-9823, E-mail: info@medicure.com, www.medicure.com
CO: Medicure Inc.
CNW 17:00e 28-JAN-19
This regulatory filing also includes additional resources:
ex991.pdf
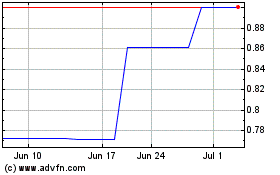
Medicure (PK) (USOTC:MCUJF)
Historical Stock Chart
From Mar 2024 to Apr 2024
Medicure (PK) (USOTC:MCUJF)
Historical Stock Chart
From Apr 2023 to Apr 2024