As
submitted to the Securities and Exchange Commission on August 24, 2020
Registration
No. 333-238883
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
PRE-EFFECTIVE AMENDMENT NO. 1 TO
FORM
S-1
REGISTRATION
STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
KRAIG
BIOCRAFT LABORATORIES, INC.
(Exact
name of registrant as specified in its charter)
|
Wyoming
|
|
7372
|
|
83-0459707
|
(State
or other jurisdiction of
incorporation or organization)
|
|
(Primary
Standard Industrial
Classification Code Number)
|
|
(I.R.S.
Employer
Identification Number)
|
2723
South State St. Suite 150
Ann
Arbor, Michigan 48104
Tel.
(734) 619-8066
(Address,
including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Kim
Thompson, CEO
Kraig
Biocraft Laboratories, Inc.
2723
South State St., Suite 150, Ann Arbor, Michigan 48104
(734)
619-8066
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
With
a Copy to:
Louis
Taubman, Esq.
Hunter Taubman Fischer & Li LLC
1450 Broadway, 26th Floor
New York, NY 10018
(917) 512-0827
|
|
Barry
I. Grossman, Esq.
Sarah
E. Williams, Esq.
Ellenoff
Grossman & Schole LLP
1345
Avenue of the Americas
New York, New York 10105
(212)
370-1300
|
Approximate
date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration
statement.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under
the Securities Act of 1933 check the following box: [X]
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please
check the following box and list the Securities Act registration statement number of the earlier effective registration statement
for the same offering. [ ]
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list
the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list
the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,”
“smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large
accelerated filer
|
[ ]
|
Accelerated
filer
|
[ ]
|
|
Non-accelerated
filer
|
[X]
|
Smaller
reporting company
|
[X]
|
|
|
|
Emerging
growth company
|
[ ]
|
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for
complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Exchange Act.
[ ]
CALCULATION
OF REGISTRATION FEE
|
Title
of Each Class of
Securities
to be Registered
|
|
Proposed
Maximum Aggregate Offering Price (1)
|
|
|
Amount
of Registration Fee(1)
|
|
|
Units consisting of shares
of Class A Common Stock, no par value, and warrants to purchase shares of Class A Common Stock, no par value
|
|
$
|
11,500,000
|
(3)
|
|
$
|
1,492.70
|
|
|
Class A Common Stock included as part
of the Units (2)
|
|
|
-
|
|
|
|
-
|
|
|
Warrants to purchase Class A Common
Stock included as part of the Units (2)(4)
|
|
|
-
|
|
|
|
-
|
|
|
Class A Common Stock underlying the
Warrants(2)
|
|
$
|
11,500,000
|
|
|
$
|
1,492.70
|
|
|
Representative’s Warrants(4)(5)
|
|
|
-
|
|
|
|
-
|
|
|
Class A Common Stock underlying Representative’s
Warrants (5)
|
|
$
|
1,012,000
|
|
|
|
131.36
|
|
|
Total
|
|
$
|
24,012,000
|
|
|
$
|
3,116.76
|
(6)
|
|
|
(1)
|
Estimated solely for the purpose of calculating
the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended,
based on an estimate of the proposed maximum aggregate offering price. Includes shares of Class A common stock and/or
warrants to purchase shares of Class A common stock that the underwriters have the option to purchase to cover
over-allotments, if any.
|
|
|
|
|
|
|
(2)
|
In
addition, pursuant to Rule 416 under the Securities Act of 1933, this Registration Statement includes an indeterminate number
of additional shares as may be issuable as a result of stock splits or stock dividends which occur during this continuous
offering.
|
|
|
|
|
|
|
(3)
|
Equal to $10,000,000 of the Units to be offered
by us plus the underwriter’s option to purchase up to an additional 15% of the total number of Units offered by us,
or up to an additional $1,500,000 of shares of Class A common stock and/or warrants to purchase shares of Class A common
stock at the public offering price, less underwriting discounts, to cover over-allotments, if any, within 45 days after
the date of this prospectus.
|
|
|
|
|
|
|
(4)
|
No
separate registration fee is required pursuant to Rule 457(g) under the Securities Act.
|
|
|
|
|
|
|
(5)
|
Upon the closing this offering, the Registrant will
issue to Maxim Group, LLC (“Maxim”) warrants to purchase a number of shares of Class A common stock equal to 8%
of the total number of securities sold in this offering (the “Representative’s Warrants”). The exercise
price of the Representative’s Warrants is equal to 110% of the offering price of the Class A common stock offered hereby.
The Representative’s Warrants are exercisable upon the 6-month anniversary of the date of effectiveness of this Registration
Statement and will terminate 5-years after the date of effectiveness of this Registration Statement. As estimated
solely for the purpose of calculating the registration fee pursuant to Rule 457(g) under the Securities Act,
the proposed maximum aggregate offering price of the Representative’s Warrants is $5.775, which is equal to 110% of
$800,000 (8% of $10,000,000).
|
|
|
|
|
|
|
(6)
|
$3,116.76 was previously paid.
|
The
Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until
the Registrant shall file a further amendment which specifically states that this registration statement shall thereafter become
effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall
become effective on such date as the Securities and Exchange Commission, acting pursuant to such Section 8(a), may determine.
The
information in this preliminary prospectus is not complete and may be changed. We may not sell or accept an offer to buy these
securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus
is not an offer to sell these securities and we are not soliciting any offer to buy these securities in any jurisdiction where
such offer or sale is not permitted.
SUBJECT
TO COMPLETION
PRELIMINARY
PROSPECTUS DATED August 24, 2020
KRAIG
BIOCRAFT LABORATORIES, INC.
1,904,762
Units1
Each
Unit Consisting of One Share of Class A Common Stock
One
Warrant to Purchase One Share of Class A Common
Stock
This is a firm commitment underwritten
public offering by Kraig Biocraft Laboratories, Inc., a Wyoming corporation (the “Company”) of 1,904,762
units (the “Units”), based on an assumed initial offering price of $5.25 per Unit, the midpoint
of the anticipated price range of the Units. We anticipate a public offering price between $4.25 and $6.25 per Unit.
Each Unit consists of 1 share of
our Class A common stock, no par value (the “Common Stock”) and one warrant (a “Purchase Warrant”)
to purchase one share of our Common Stock at an exercise price of $5.25 per share (100% of the price of each Unit sold
in this offering based on an assumed initial offering price of $5.25 per Unit, the midpoint of the anticipated price range). The
Units have no stand-alone rights and will not be certificated or issued as stand-alone securities. The shares of Common Stock
and Purchase Warrants comprising the Units are immediately separable and will be issued separately in this offering. Each Purchase
Warrant offered hereby is immediately exercisable on the date of issuance and will expire five years from the date of issuance.
The public offering price will be determined by us and Maxim Group LLC (“Maxim”), as representatives of the underwriters,
taking into consideration several factors as described in “Underwriting – Determination of Offering Price”,
and will not be based upon the price of our Common Stock on the OTCQB Marketplace (the “OTCQB”).
We are a
voluntary reporting company under Section 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
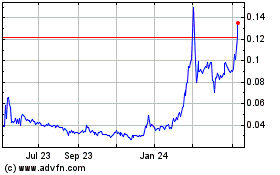
Our Common Stock is currently quoted on the OTCQB under the symbol “KBLB.” As of August 20, 2020, the last
reported sales price for our Common Stock as quoted on the OTCQB was $0.155 per share. There is no established trading
market for the Purchase Warrants. Quotes of stock trading prices on an over-the-counter marketplace may not be indicative of the
market price on a national securities exchange. There is a limited public trading market for our Common Stock. We have applied
to list our Common Stock and the Purchase Warrants on the Nasdaq Capital Market (“Nasdaq”) under the symbols “KBLB”
and “KBLBW,” respectively. We believe that upon the completion of the offering contemplated by this prospectus,
we will meet the standards for listing on the Nasdaq Capital Market or other national exchange. We cannot guarantee that we will
be successful in listing our common stock or our Purchase Warrants on Nasdaq or other national exchange, or, if successful, that
an active trading market for our Common Stock or Purchase Warrants will develop or be sustained. If we are unable to list our
Common Stock on Nasdaq or other national exchange, the Company will not consummate this offering.
The offering price of the Units will
be determined between the underwriters and us at the time of pricing, considering our historical performance and capital structure,
prevailing market conditions, and overall assessment of our business, and may be at a discount to the current market price. Therefore,
the assumed public offering price used throughout this prospectus may not be indicative of the actual public offering price for
our Common Stock and the Purchase Warrants.
__________________________
Investing
in our securities involves a high degree of risk. You should purchase Units only if you can afford a complete loss of your investment.
You should carefully consider the risk factors described under the heading “Risk Factors” beginning on page 14 of
this prospectus and under similar headings in any amendments or supplements before purchasing shares of our Common Stock.
|
|
|
Price
to Public(3)
|
|
|
Total
(No
Exercise Of Over- Allotment)
|
|
|
Total
(Full
Exercise of Over-Allotment)
|
|
|
Public Offering Price Per Unit
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
|
Underwriting Discounts (1)(2)
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
|
Proceeds to the Company (before expenses)
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
(1)
We refer you to the section of this prospectus entitled “Underwriting” for additional information regarding underwriter
compensation.
(2)
We estimate the total expenses payable by us, excluding the underwriting discount, will be approximately $[●].
(3) Based on an assumed price to the
public in this Offering of $5.25 per Unit, the midpoint of the price range set forth on the cover page of this prospectus.
We have
granted the underwriters a 45-day option to purchase up to an additional 285,715 shares of Common Stock and/or Purchase
Warrants to purchase 285,715 shares of Common Stock from us at the assumed public offering price, less the underwriting
discount, to cover over-allotments, if any, within forty-five (45) days from the date of this prospectus.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or
determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The
underwriters expect to deliver the shares of Common Stock and Purchase Warrants to purchasers on or about [●], 2020, subject
to customary closing conditions, including that, upon the closing of the offering, the Common Stock would qualify for listing
on the Nasdaq Capital Market or other national exchange.
Sole-Book
Running Manager
Maxim
Group LLC
The
date of this prospectus is August 24, 2020
1 Number of Units is based
on an assumed price to the public of $5.25 per Unit, the midpoint of the price range set forth on the cover page of this prospectus,
and no exercise of the underwriter’s option to cover over-allotments.
EXPLANATORY NOTE
We are filing this Amendment No. 1 to
our registration statement on Form S-1, initially filed on June 2, 2020 (File No. 333-238883) (the
“Registration Statement”) pursuant to a comment we received from the Securities and Exchange Commission. Pursuant
to such comment, we removed reference to a fixed price for the Units. Until the actual public offering price of the Units is determined
by the Company and the underwriters, we shall use a bona fide price range. As a result, we use the midpoint of such price range
to complete certain tables and provide certain information in this Registration Statement. In such instances, we may make a statement
such as, “Based on an assumed price to the public in this Offering of $5.25 per Unit, the midpoint of the price range set
forth on the cover page of this prospectus” or “at the assumed public offering price of $5.25, the midpoint of the
price range set forth on the cover page of this prospectus.” The use of the word “assume” in these instances
is solely to disclose to the public that to make such related calculations, we had to assume a specific price.
No additional securities are being registered under this
Amendment No. 1. All applicable registration fees were paid at the time of the original filing of the Registration Statement.
Table
of Contents
About
this Prospectus
We
and the underwriters have not authorized anyone to provide any information or to make any representations other than those contained
in this prospectus or in any free writing prospectuses prepared by us or on our behalf or to which we have referred you. We take
no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you.
This prospectus is an offer to sell only the Units offered hereby, but only under circumstances and in jurisdictions where it
is lawful to do so. We are not making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted
or where the person making the offer or sale is not qualified to do so or to any person to whom it is not permitted to make such
offer or sale. The information contained in this prospectus is current only as of the date on the front cover of the prospectus.
Our business, financial condition, results of operations and prospects may have changed since that date.
Persons
who come into possession of this prospectus and any applicable free writing prospectus in jurisdictions outside the United States
are required to inform themselves about and to observe any restrictions as to this offering and the distribution of this prospectus
and any such free writing prospectus applicable to that jurisdiction. See “Underwriting” for additional information
on these restrictions.
Industry
and Market Data
Unless
otherwise indicated, information in this prospectus concerning economic conditions, our industry, our markets and our competitive
position is based on a variety of sources, including information from third-party industry analysts and publications and our own
estimates and research. Some of the industry and market data contained in this prospectus are based on third-party industry publications.
This information involves a number of assumptions, estimates and limitations.
The
industry publications, surveys and forecasts and other public information generally indicate or suggest that their information
has been obtained from sources believed to be reliable. None of the third-party industry publications used in this prospectus
were prepared on our behalf. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety
of factors, including those described in “Risk Factors” in this prospectus. These and other factors could cause results
to differ materially from those expressed in these publications.
Trademarks
This
prospectus contains references to our trademarks and service marks and to those belonging to other entities. Solely for convenience,
trademarks and trade names referred to in this prospectus may appear without the ® or TM symbols, but such references
are not intended to indicate, in any way, that we will not assert, to the fullest extent possible under applicable law, our rights
or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’
trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by any other companies.
PROSPECTUS
SUMMARY
This
summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should
consider in making your investment decision. Before investing in our Units, you should carefully read this entire prospectus,
including our financial statements and the related notes and the information set forth under the headings “Risk Factors”
and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere
in this prospectus and our consolidated financial statements and the accompanying notes included in this prospectus. Except as
otherwise indicated herein or as the context otherwise requires, references in this prospectus to “Kraig.” “Kraig
Labs,” “the Company,” “we,” “us,” and “our” refer to Kraig Biocraft Laboratories,
Inc. together with its wholly-owned subsidiary Prodigy Textiles Co., Ltd., a Vietnamese corporation (“Prodigy Textiles”).
Company
Overview
Kraig
Biocraft Laboratories, Inc., a Wyoming corporation, is a corporation organized to develop high strength fibers using recombinant
DNA technology for commercial applications in technical textile. We use genetically engineered silkworms that produce spider silk
to create our recombinant spider silk. Applications include performance apparel, workwear, filtration, luxury fashion, flexible
composites, medical implants, and more. We believe that we have been a leader in the research and development of commercially
scalable and cost effective spider silk for technical textile. Our primary proprietary fiber technology includes natural and engineered
variants of spider silk produced in domesticated mulberry silkworms. Our business brings twenty-first century biotechnology to
the historical silk industry, permitting us to introduce materials with innovative properties and claims into an established commercial
ecosystem of silkworm rearing, silk spinning and weaving, and manufacture of garments and other products that can include our
specialty fibers and textiles. Specialty fibers are engineered for specific uses that require exceptional strength, flexibility,
heat resistance and/or chemical resistance. The specialty fiber market is exemplified by two synthetic fiber products that come
from petroleum derivatives: (1) aramid fibers; and (2) ultra-high molecular weight polyethylene fibers. The technical textile
industry involves products for both industrial and consumer products, such as filtration fabrics, medical textiles (e.g.,
sutures and artificial ligaments), safety and protective clothing and fabrics used in military and aerospace applications (e.g.,
high-strength composite materials).
We
are using genetic engineering technologies to develop fibers with greater strength, resiliency and flexibility for use in our
target markets, namely the specialty fiber and technical textile industries. We believe that the genetically engineered protein-based
fibers we seek to produce have properties that are in some ways superior to the materials currently available in the marketplace.
Production of our product in commercial quantities holds what we believe to be potential life-saving ballistic resistant material,
which we believe is lighter, thinner, more flexible, and tougher than steel. Other potential applications for spider silk based
recombinant fibers include use as structural material and for any application in which light weight and high strength are required.
We believe that fibers made with recombinant protein-based polymers will make significant inroads into the specialty fiber and
technical textile markets.
Through
our technologies, the introduction of the gene sequence based on those found in native spider silk, results in a germline transformation
and is therefore self-perpetuating. This technology is in essence a protein expression platform which has other potential applications
including diagnostics and pharmaceutical production. Moreover, our technologies are “green” inasmuch as our fibers
and textiles do not require petroleum inputs and our production processes are traditional and eco-friendly.
Our
management believes that access to financing has been the biggest hurdle to the Company’s ability to expand and create products
for commercialization. Due to our limited financing opportunities, we were unable to perform our operations in a manner that we
believe would have led to expedited growth and opportunities for our products. Although we have made progress in the commercialization
of our spider silk technology, it has not been at the rate it could have been with more financing. Management believes that the
proceeds from this offering will address our financing issues and pave the way for expedited growth.
Products
Our
products exploit the unique characteristics of spider silk, specifically dragline silk from Nephila clavipes (golden orb-web
spider) and variants thereof. Such fibers possess unique mechanical properties in terms of strength, resilience and flexibility.
Through the use of genetic engineering, we believe that we have produced a variety of unique transgenic silkworm strains that
produce recombinant spider silk. Our recombinant spider silk fiber blends the silk proteins found in spider silk with the native
silkworm silk proteins. This approach allows for the cost-effective and eco-responsible production of spider silk at
commercial production levels.
Monster
Silk®
Monster
Silk® was the first recombinant spider silk fiber product we developed. Monster Silk incorporates the natural elasticity of
spider silk to make a silk fiber which is more flexible that conventional silk fibers and textiles. We have produced sample products
using Monster Silk® including knit fabrics, gloves, and shirts in collaboration with textile mills. We expect that Monster
Silk® will have market applications across the traditional textile markets where its increased flexibility will provide increased
durability and comfort.
Dragon
SilkTM
Dragon
SilkTM is the next evolution in recombinant spider silk, combining the elasticity of Monster Silk® with additional
high strength elements of native spider silk. Some samples of Dragon SilkTM have demonstrated strength beyond that
of native spider silk. This combination of strength and elasticity results in a silk fiber which is soft and flexible, yet tougher
than leading synthetic fiber available on the market. Based on inquires we have received from end market leaders, we believe
that Dragon SilkTM- will have applications in performance apparel, durable workwear, luxury goods and apparel, and
composites.
Other
Products
We
are continuing to develop new recombinant silks and other protein-based fibers and materials using our genetic engineering capabilities.
We recently unveiled our first knock in knock out spider silk material using our silkworm based production platform. These new
spider silk fibers offer increased silk purity. Due to the biocompatible and biodegradable properties of silk, we expect that
the new materials developed using this higher purity process will create opportunities for products in the medical industry, including
sutures, grafts, and implants.
Intellectual
Property
The
Company’s intellectual property blends licensed technologies with in-house developments in spider silk polymers. As part
of our intellectual property portfolio, we have licensed the exclusive right to commercialize certain patented spider silk gene
sequences in silkworm. Through our in-house research operations, we have expanded on our intellectual property portfolio with
six additional provisional patent filings based on new discoveries, inventions, and methodologies for the production of spider
silk.
Under
a series of intellectual property and collaborative research agreements (the “Notre Dame Agreements”) with the University
of Notre Dame (“Notre Dame”), we were issued and exercised our right to exclusive commercial use for spider silk technologies
developed under that agreement. We have worked collaboratively with Notre Dame to develop fibers with the mechanical characteristics
of spider silk. We are applying this proprietary genetic engineering technology to domesticated silkworms, which to our knowledge,
is the only proven commercially scaled system for producing silk.
In
2017, we opened a research and development facility to expand on the work conducted at Notre Dame. Since opening this new facility,
we have expanded our intellectual property portfolio with six additional provisional patent filings based on new discoveries and
inventions and made numerous advancements that have decreased the development time for new technologies, none of which rely on
the patented materials produced with Notre Dame. We will continue to utilize this in-house research facility to expand and strengthen
our patent portfolio while also maintaining and growing our trade secret technologies approach to genetic engineering. We are
actively working to develop and patent new approaches to the development of genetically engineering silkworms, underlying construction
techniques, and fundamental genetic sequences for improved material performance.
The
Notre Dame Agreements will last for the duration of the patented materials that we developed with Notre Dame. The new technologies
that we are developing in our internal research labs does not rely on the Notre Dame patented materials and as a result will not
be impacted by an expiration of that agreement.
Strengths
We
have developed a method for the production of high-performance bio-degradable, bio-compatible, and ecologically friendly recombinant
spider silk to replace the existing global infrastructure for mundane silk manufacturing. This system of operations utilizes genetically
modified silkworms that have been engineered to produce silks based on the proteins and physical characteristics of native spider
silk, a material that has been prized for its physical and chemical properties. By adapting the common silkworm and the production
process for the manufacture of traditional silk, we are able to leverage a global production model which currently processes more
than 150,000 metric tons of silk per year.2 Our technology is a direct drop-in replacement for traditional silk manufacturing,
allowing any silk operation employing our silkworm technology to immediately be converted without any additional need for capital
investment. We are currently securing global patent protection for our technologies in silk producing and silk consuming countries.
2
https://inserco.org/en/statistics
On
April 16, 2020, we announced the successful development of a new technology platform, based on a non-CRISPR gene editing knock-in
knock-out technology. CRISPR is the most recent and efficient gene editing technology3; CRISPR stands for “Clustered
regularly interspaced short palindromic repeats.” This is our first knock-in knock-out technology of essentially pure spider
silk transgenic. This new system is built on our eco-friendly and cost-effective silkworm production system, which we believe
is significantly more advanced than any competing methods. Knock-in knock-out technology allows for the targeting of specific
locations and genetic traits for modification, addition, and removal. This new capability will accelerate new product developments,
which should allow us to bring products to market more quickly. This capability also allows for genetic trait modifications that
were previously impractical, creating opportunities for products outside of silk fibers and increased flexibility in production
location.
Strategies
Our
approach to disrupting the performance and technical textile market is to adapt our existing infrastructure and capacity to produce
our high-performance silk with minimal capital investment. When we were formed, this idea of utilizing the existing production
systems and capacity by simply modifying the input was fundamental. Our proprietary recombinant spider silkworms are a direct
drop-in replacement for any commercial silk producing operations. Our genetically engineered silks are produced using the same
equipment and processes that traditional silk uses. In designing our technologies in this manner, we have minimized our need for
expansion capital, limiting our direct investment and contracting with existing secondary fiber processors where the majority
of large scale equipment is needed. Through our subsidiary, Prodigy Textiles in Vietnam, we have established the relationships
necessary to secure these contracted secondary services.
We
are actively pursuing relationships within target end markets to secure product collaborations with key market channel leaders.
Due to the unique nature of our product, we received numerous unsolicited requests from leading businesses across a range of attractive
end markets requesting materials for applications development. This substantial demand for spider silk materials across the broad
spectrum of applications for high performance fibers and textiles, combined with the limited initial production capacity, has
provided the opportunity to be selective in choosing market channel partners best able to quickly bring our product to market
at scale. We are working under non-disclosure agreements to secure these collaborative development agreements and to establish
limited channel exclusivity for firms we believe mirror our culture of innovation. With recent advancements in our manufacturing
capacity, we expect to generate revenues from these relationships in 2020.
Additionally,
we are currently focused on the expansion of our production facilities in Vietnam. We are currently utilizing a mix of direct
staffing and contract labor to support its in-house production. We are exploring a production expansion model utilizing contract
production consistent with the distributed model used for mundane silk production. We will also consider technology licensing
models with companies, regions, or countries where its silkworms could be produced under exclusive license.
Recent
Developments
From
2016 through 2018, we were contracted directly by the U.S. Army for the development and delivery of next generation fibers. Due
to confidentiality agreements, we cannot publicly disclose the details of our ongoing efforts to establish additional market channel
partnerships, joint ventures, and material transfer agreements.
In
February, 2019, we signed a non-binding memorandum of understanding (“MOU”) with Polartec LLC for the development
of products for the protective textile markets.
On
April 16, 2020, the Company announced that it successfully developed a new technology platform, based on a non-CRISPR gene editing
knock-in knock-out technology. This is the first knock-in knock-out technology essentially pure spider silk transgenic in the
Company’s history. The new technology, which is the result of over ten years of effort, hits the target of one of the Company’s
primary technological goals and potentially opens the door for large scale U.S. production. Other than the silkworm’s remaining
specifically desired native silk protein elements, we are now able to produce nearly pure spider silk. Based on our internal studies,
the new technology has a purity rate that is about ten times greater than Dragon Silk, a fiber that the Company developed with
its previous tools. Dragon Silk has already demonstrated to be tougher than many fibers used in bullet proof vests and the Company
expects that the increased spider silk purity, created using this new approach, will yield materials beyond those capabilities.
This new system utilizes the Company’s eco-friendly and cost-effective silkworm production system, which we believe is significantly
more advanced than any of the competing methods. We have already begun the validation process for the first of these new transgenics
and anticipate that U.S. production will be possible as early as 2022 or 2023.
3
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5131771/
https://www.yourgenome.org/facts/what-is-genome-editing
https://ghr.nlm.nih.gov/primer/genomicresearch/genomeediting
This
does not affect our current work of overseeing our production facility to ramp up our production of Dragon SilkTM and
Monster Silk®, as these fibers are designed to address their own markets.
In
August 2019, we received authorization to begin rearing genetically enhanced silkworms at our production facility in Vietnam.
We received our investment registration certificate for the facility in April 2018. In October 2019, the Company delivered the
first batch of these silkworms and began operations. These silkworms will serve as the basis for the commercial expansion of our
proprietary silk technology. On November 4, 2019, we reported that we had successfully completed rearing the first batch of its
transgenic silkworms at the Quang Nam production factory. Seasonal challenges in late December 2019 slowed production operations,
however, we believe that we can resume expansion of the production of our specialized silk in 2020, as soon as travel and work
restrictions for COVID-19 are lifted, since the seasonal challenges will have also been alleviated. As of the date hereof, Vietnam
does not have any work restrictions impacting our facility. However, current U.S. travel restrictions may prevent us from shipping
the eggs we are now producing in the U.S. to Vietnam; as soon as the eggs are shipped, we can restart operations at the facility.
We believe that we will be able to target a capacity of 40 metric tons of our recombinant spider silk fiber per annum from this
factory once it reaches maximum utilization. This capacity will allow us to address initial demand for our products and materials
for various applications in the protective, performance, and luxury textile markets.
In
light of the significant uncertainty regarding the impact of the COVID-19 pandemic, on March 19, 2020, we furloughed non-essential
staff consistent with leading health official recommendations in order to help prevent the spread of COVID-19. This decision was
made in an abundance of caution and primarily impacted staff at our fully owned subsidiary, Prodigy Textiles, in Vietnam
and resulted in the temporary closing of silk rearing operations at that facility. We believe that this temporary suspension
of rearing operations will result in a delay of 4-6 months in the Company’s production expansion schedule; however,
due to the fact that U.S. travel restrictions could be extended, the delay could be longer. The Company supported its furloughed
staff and paid their salaries through June 30, 2020. During the duration of the furlough, the Company CEO did
not receive or accrue any pay. On July 1, 2020, furloughed staff returned to work preparing the factory in Vietnam to receive
the next shipment of silkworm eggs, which is out of our control. The global pandemic of COVID-19 continues to evolve rapidly,
and we will continue to monitor the situation closely, including its potential effect on our plans and timelines.
Reverse
Stock Split
On
or prior to the effective date of this registration statement of which this prospectus forms a part we will amend our articles
of incorporation to effect a reverse stock split of our Common Stock (the “Reverse Stock Split”).
At
our 2019 annual stockholders meeting, our stockholders approved a reverse stock split of our issued and outstanding Common Stock
by a ratio of not less than one-for-ten and not more than one-for-forty at any time prior to July 23, 2020, with the exact ratios
to be set at a whole number within this range, as determined by our board of directors (the “Board”) in its sole discretion
and approved and adopted the articles of amendment to our articles of incorporation to affect the same. Since we are still
in the uplisting process with Nasdaq, but past July 23, 2020, such stockholder approval expired. However, on [ ], 2020 we obtained
the written consent from a majority of our stockholders to effect the Reverse Stock Split.at a ratio of 1-for-[ ].
On August [ ], 2020, the Board
established a ratio for the Reverse Stock Split of the issued and outstanding shares of our Common Stock of 1-for-[ ] and
the Reverse Stock Split was effective at 12:01 a.m. on [______]. No fractional shares will be issued in the Reverse
Stock Split and any remaining share fractions will be rounded up to the next whole share.
In
connection with the Reverse Stock Split, the issued and outstanding shares of our Common Stock will be combined by the
1-for-[ ] ratio. Also, all shares of our Common Stock subject to outstanding equity awards and the exercise price of any
such award (if applicable) and the number of shares remaining available for issuance under the 2019 Employee Stock Option Plan,
and all shares underlying outstanding warrants, convertible notes, and other derivative securities of the Company, including exercise
prices and conversion prices (if applicable) will be proportionately adjusted for the Reverse Stock Split.
As
stated elsewhere in this registration statement, the lack of sufficient financing has been our biggest hurdle to achieving the
rate of growth management estimates is possible with our product. Without adequate funding, we are unable to expand operations
and accept opportunities for large scale production of our products, and our progress toward commercialization has slowed. The
funds from this offering will allow us to accomplish these goals, which management believes will increase shareholder value. As
part of our efforts to uplist our Common Stock, we are effecting the Reverse Stock Split because we believe it is our best option
to meet one of the criteria to obtain an initial listing. Although we cannot guarantee this outcome, a decrease in the number
of issued and outstanding shares of common stock often results in an increase in the per share market price of common stock. We
believe increasing the trading price of our Common Stock will assist in our capital-raising efforts by making our Common Stock
more attractive to a broader range of investors. We hope that the decrease in the number of shares of our issued and outstanding
Common Stock as a consequence of the Reverse Stock Split, and the anticipated increase in the price per share, will encourage
greater interest in our Common Stock by the financial community and the investing public, help us attract and retain employees
and other service providers, help us raise additional capital through the sale of stock in the future if needed, and possibly
promote greater liquidity for our stockholders with respect to those shares presently held by them. (See, Risk Factors –
“Our Reverse Stock Split may not result in a proportional increase in the per share price of our Common Stock”).
Decrease in Series A Preferred Stock
Voting Power
In line
with the Reverse Stock Split, on August [ ], 2020, the sole holder of our Series A Preferred Stock, Mr. Thompson, approved, via
written consent, to reduce the voting rights of the Series A preferred stock by a ratio of 1 for [ ], consistent with the Reverse
Stock Split (the “Reduced Voting Power”), to be effective as of the date of the Reverse Stock Split. Accordingly,
the Series A Preferred Stock now represents [ ] votes (31.89 %
of the voting power on all matters submitted to a stockholder vote). Our Board and a majority of our stockholders approved, via
written consent, to amend our articles of incorporation to reflect the Reduced Voting Power. As a result of the Reduced Voting
Power, we will no longer be considered a “Controlled Company,” which Nasdaq Stock Market Rules define as any company
of which more than 50% of the voting power for the election of directors is held by an individual, a group or another company.
Summary
of Risk Factors
Risks
Associated with Our Business
As
an OTCQB listed Company, we have limited resources with pressing capital requirements as we attempt to ramp up production. We
are mindful of the challenges involved in achieving growth without compromising our ability to manage our operating risks and
comply with rules and regulations. As we are commercializing a new technology, no one is in a position to know all of the risks,
obstacles, and hurdles that the Company will face as it works to commercialize its new technology. We are aware of numerous risks
associated with our business, including:
|
|
●
|
Our
ability to continue as a going concern;
|
|
|
|
|
|
|
●
|
Our
ability to generate significant revenues and to become profitable;
|
|
|
|
|
|
|
●
|
Our
ability to estimate future expenses;
|
|
|
|
|
|
|
●
|
Our
ability to maintain an effective system of internal controls;
|
|
|
|
|
|
|
●
|
Our
ability to protect our intellectual property rights and to secure additional rights domestically and internationally;
|
|
|
|
|
|
|
●
|
Our
ability to successfully manage our growth domestically and internationally;
|
|
|
|
|
|
|
●
|
Our
ability to attract and retain the services of key personnel;
|
|
|
|
|
|
|
●
|
Our
reliance on independent third-party collaborators to develop and deliver products to market;
|
|
|
|
|
|
|
●
|
Our
reliance on key management personnel and future need for highly skilled personnel;
|
|
|
|
|
|
|
●
|
Our
ability to successfully develop sales and marketing for our products;
|
|
|
|
|
|
|
●
|
Market
acceptance of pricing and performance for products we develop;
|
|
|
|
|
|
|
●
|
Our
ability to generate sustainable earnings and net operating profits;
|
|
|
|
|
|
|
●
|
Our
ability to adapt to regulatory and technology changes impacting our industry;
|
|
|
|
|
|
|
●
|
The
potential for product liability claims regarding our products and the use of genetically modified organisms (“GMO’s”)
in our production system;
|
|
|
|
|
|
|
●
|
Actions
taken or omitted to be taken by third parties including our suppliers and competitors, as well as legislative, regulatory,
judicial and other governmental authorities;
|
|
|
|
|
|
|
●
|
Competition
in our industry;
|
|
|
|
|
|
|
●
|
The
loss of or failure to obtain any license or permit necessary or desirable in the operation of our business;
|
|
|
|
|
|
|
●
|
The
availability of additional capital to support development;
|
|
|
|
|
|
|
●
|
An
active, liquid, and orderly market for our common stock or Purchase Warrants may not develop;
|
|
|
|
|
|
|
●
|
The
Purchase Warrants may not have any value;
|
|
|
●
|
Our
production system is based upon living transgenic organisms;
|
|
|
|
|
|
|
●
|
Our
business, operations and plans and timelines could be adversely affected by the effects of health epidemics, including the
recent COVID-19 pandemic;
|
|
|
|
|
|
|
●
|
We intend to effect the Reverse Stock Split of
our outstanding Common Stock immediately following the effective date but prior to the closing of the offering; however, the
Reverse Stock Split may not increase our stock price sufficiently and we may not be able to list our Common Stock on the Nasdaq
Capital Market or any other exchange in which case this offering may not be completed; and,
|
|
|
|
|
|
|
●
|
Certain
other risks and uncertainties set forth elsewhere in this prospectus under the section titled “Risk Factors”
|
Corporate
Information
Kraig Biocraft Laboratories, Inc. is a Wyoming
corporation. Our Common Stock is quoted on the OTCQB under the ticker symbol “KBLB”. On the effective date of the
registration statement of which this prospectus forms a part, we expect that trading on Nasdaq or other national
exchange will be under the same symbol. We also intend to seek a listing for the Purchase Warrants on Nasdaq or other national
exchange under the symbol “KBLBW.”
As of August
20, 2020, there are 854,410,001 shares of Common Stock issued and outstanding. Kim Thompson, our founder and Chief
Executive Officer, owns approximately 24.7% of our issued and outstanding Common Stock. As of August 20, 2020, there
are 2 shares of super voting Series A Preferred Stock issued and outstanding, all of which are owned by Kim Thompson, which
represent approximately 31.89% of all voting rights of our capital stock (See,
“Description of Securities” for additional information about our securities).
Our
principal executive office and mailing address is 2723 South State St., Suite 150, Ann Arbor, Michigan 48104. Our telephone number
is (734) 619-8066. Our corporate website is http://www.kraiglabs.com. The information contained on, or that can be accessed
through, our website is not part of, and is not incorporated by reference into, this prospectus and should not be relied upon
with respect to this offering.
The
Offering
The
following Summary contains general information about this offering. The Summary is not intended to be complete. You should read
the full text and more specific details contained elsewhere in this prospectus.
|
Securities
offered by us
|
|
1,904,762
Units2, with each Unit consisting of one share of our Common Stock
and one Purchase Warrant to purchase one share of our Common Stock
at an exercise price of $5.25 per share. The Units will not be certificated and
the shares of Common Stock and the Purchase Warrants are immediately separable and will
be issued separately in this offering.
|
|
|
|
|
|
Common
Stock offered by us
|
|
1,904,762
shares of Common Stock.
|
|
|
|
|
|
Purchase
Warrants offered by us
|
|
Purchase Warrants to purchase 1,904,762 shares
of Common Stock, each Purchase Warrant providing the right to purchase 1 share of our Common stock. Each
Purchase Warrant will have an exercise price per share of $5.25 per share, the midpoint of the price range set forth on
the cover page of this prospectus, will be exercisable on the original issuance date, and will expire on the fifth anniversary
of the original issuance date. Each holder of Purchase Warrants will be prohibited from exercising its Purchase Warrant for
shares of our Common Stock if, as a result of such exercise, the holder, together with its affiliates, would own more than
4.99% of the total number of shares of our Common Stock then issued and outstanding. However, any holder may increase such
percentage to any other percentage not in excess of 9.99%, provided that any increase in such percentage shall not be effective
until 61 days after such notice to us. This prospectus also relates to the offering of the shares of Common Stock issuable
upon exercise of the Purchase Warrants.
|
2 Based on an assumed
initial offering price of $5.25 per Unit, the midpoint of the price range set forth on the cover page of this
prospectus.
|
Price
per Unit
|
|
The purchase price will be between
$4.25 and $6.25 per Unit; the assumed public offering price of $5.25 per Unit that is disclosed in this document is the midpoint
of such range. The actual number of Units we will offer will be determined based on the actual public offering price.
|
|
|
|
|
|
Over-Allotment
option to purchase additional share of our Common Stock and/or Purchase Warrants
|
|
We have
granted to the underwriters an option within 45-days of the date of this prospectus to purchase up to 285,715
additional shares of Common Stock and/or Purchase Warrants to purchase 285,715 shares of Common Stock at the assumed
public offering price listed on the cover page of this prospectus, less the underwriting discount, to cover
over-allotments, if any. If the representative of the underwriters exercises the option in full, the total underwriting
discounts and commissions payable by us will be $[●] and the total proceeds to us, before expenses, will be
$[●].
|
|
|
|
|
|
Common
Stock outstanding prior to completion of this offering(1)
|
|
[ ].
|
|
|
|
|
|
Common
Stock outstanding immediately after this offering(2)
|
|
[ ] (or [ ]
if the underwriters’ option to purchase additional shares from us is exercised in full).
|
|
|
|
|
|
Proposed
Nasdaq Listing of our Common Stock
|
|
Our
Common Stock is currently quoted on the OTCQB. In connection with this offering, we have applied and expect to have our Common
Stock listed on the Nasdaq Capital Market under the symbol “KBLB”.
|
|
|
|
|
|
Proposed
Nasdaq listing for Purchase Warrants
|
|
There
is no established public trading market for the Purchase Warrants. We intend to seek a listing for the Purchase Warrants on
Nasdaq under the symbol “KBLBW,” however we cannot assure you that we will be successful listing the Purchase
Warrants on Nasdaq or, if successful, that an active trading market for the Purchase Warrants will develop or be sustained.
|
|
|
|
|
|
Use
of proceeds
|
|
We
estimate that the net proceeds from the sale of 1,904,762 Units in the offering
will be approximately $10,000,000, or approximately $11,500,000 if the
underwriters exercise their option to purchase additional shares of Common Stock and/or
Purchase Warrants in full, based on an assumed price to the public in this Offering
of $5.25 per Unit, the midpoint of the price range set forth on the cover page of this
prospectus, in each case, after deducting the underwriting discounts and estimated
offering expenses.
We
intend to use the net proceeds from the offering for (i) expansion of our production operations capacity in Vietnam, including
capital equipment, facilities improvements and staffing; and (ii) working capital and general business purposes.
The
amount and timing of our actual expenditures will depend on numerous factors, including the status of our acquisition and
development efforts, sales and marketing activities, and the amount of cash generated or used by our operations. We may find
it necessary or advisable to use portions of the proceeds for other purposes, and we will have broad discretion in the application
of the net proceeds.
|
|
|
|
|
|
Reverse
Stock Split
|
|
On
or prior to the effective date of the registration statement of which this prospectus
forms a part we will amend our articles of incorporation to effect the Reverse Stock
Split. At our 2019 annual stockholders meeting, our stockholders approved the Reverse
Stock Split of a ratio of not less than one-for-ten and not more than one-for-forty at
any time prior to July 23, 2020, with the exact ratios to be set at a whole number within
this range, as determined by our Board in its sole discretion and approved and adopted
the articles of amendment to our articles of incorporation to affect the same. Since
we are still in the uplisting process with Nasdaq, but past July 23, 2020, such stockholder
approval expired. However, on August [ ], 2020 we obtained the written consent from a
majority of our stockholders to effect the Reverse Stock Split.at a ratio of 1-for-[ ].
We
intend to effectuate the Reverse Stock Split immediately following the effective date but prior to the closing of the
offering.
|
|
|
|
In connection with the Reverse Stock
Split, the issued and outstanding shares of our Common Stock will be combined by the 1-for-[ ] ratio. Also,
all shares of our Common Stock subject to outstanding equity awards and the exercise price of any such award (if applicable)
and the number of shares remaining available for issuance under the 2019 Employee Stock Option Plan, and all shares underlying
outstanding warrants, convertible notes, and other derivative securities of the Company, including exercise prices and conversion
prices (if applicable) will be proportionately adjusted for the Reverse Stock Split.
|
|
|
|
|
|
Risk
factors
|
|
The
Units offered hereby involves a high degree of risk. You should read “Risk Factors,” and other information included
in this prospectus for a discussion of factors to consider before deciding to invest in our securities.
|
|
(1)
|
The number of Common Stock to be outstanding before
this offering is based on 854,410,001 shares of Common Stock outstanding as of August 20, 2020, and excludes:
|
|
|
●
|
7,440,000
shares of our Common Stock issuable upon the exercise of
stock options outstanding;
|
|
|
●
|
65,995,920
shares of our Common Stock underlying any outstanding warrants;
and
|
|
|
●
|
0
shares of our Common Stock issuable upon the conversion
of notes and other evidence of indebtedness.
|
|
(2)
|
The number of Common
Stock to be outstanding after this offering is based on 854,410,001 shares of Common Stock outstanding as of August 20, 2020,
and excludes:
|
|
|
●
|
7,440,000 shares of our Common Stock issuable upon the exercise
of stock options outstanding;
|
|
|
●
|
65,995,920 shares of our Common Stock underlying any outstanding
warrants; and
|
|
|
●
|
0 shares of our Common Stock issuable upon the conversion
of notes and other evidence of indebtedness.
|
Except as otherwise indicated, all
information in this prospectus assumes:
|
|
●
|
that the assumed
public offering price is $5.25 per Unit, which is the mid-point of the estimated offering price range described on the cover
of this prospectus;
|
|
|
●
|
no exercise of
65,995,920 outstanding warrants;
|
|
|
●
|
no exercise of
the Purchase Warrants included in the Units;
|
|
|
●
|
no exercise of
the Representative’s Warrants;
|
|
|
●
|
no exercise of
the Representative’s option to purchase additional shares of Common Stock and/or Purchase Warrants from us in this offering;
and
|
|
|
●
|
no exercise of
the 7,440,000 shares of our Common Stock issuable upon the exercise of stock options outstanding.
|
Summary
of Financial Data
The
summary of financial data set forth below should be read together with our financial statements and the related notes to those
statements, as well as the section of this prospectus titled “Management’s Discussion and Analysis of Financial Condition
and Results of Operations.” The statements of operations data for the years ended December 31, 2019 and 2018 have been derived
from our audited financial statements included elsewhere in this prospectus. We have derived the statement of operations data
for the six months ended June 30, 2020 and 2019 and the balance sheet data as of June 30, 2020 from our unaudited
financial statements appearing elsewhere in this prospectus. Our historical results are not necessarily indicative of the results
that should be expected in any future periods, and our results for any interim period are not necessarily indicative of results
that should be expected for any full year.
|
|
|
Six
Months ended
June 30, 2020
|
|
|
Six
Months Ended
June 30, 2019
|
|
|
Year Ended
December 31, 2019
|
|
|
Year Ended
December 31, 2018
|
|
|
|
|
US$
|
|
|
US$
|
|
|
US$
|
|
|
US$
|
|
|
|
|
(unaudited)
|
|
|
(unaudited)
|
|
|
(audited)
|
|
|
(audited)
|
|
|
Statement of operation data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
401,620
|
|
|
Gross profit
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
401,620
|
|
|
Operating expenses
|
|
$
|
3,434,993
|
|
|
$
|
747,253
|
|
|
$
|
2,621,867
|
|
|
$
|
1,361,567
|
|
|
Operating income (loss)
|
|
$
|
(3,434,993
|
)
|
|
$
|
(747,253
|
)
|
|
$
|
(2,621,867
|
)
|
|
$
|
(959,947
|
)
|
|
Other non-operating income (expense), net
|
|
$
|
(185,143
|
)
|
|
$
|
(132,990
|
)
|
|
$
|
(287,983
|
)
|
|
$
|
(209,030
|
)
|
|
Provision for income taxes
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Net income (loss)
|
|
$
|
(3,620,136
|
)
|
|
$
|
(880,243
|
)
|
|
$
|
(2,909,850
|
)
|
|
$
|
(1,168,977
|
)
|
|
Earnings (loss) per share, basic
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Earnings (loss) per share, diluted
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Weighted average shares outstanding, basic
|
|
|
844,468,378
|
|
|
|
828,912,974
|
|
|
|
835,587,422
|
|
|
|
816,874,442
|
|
|
Weighted average shares outstanding, diluted
|
|
|
844,468,378
|
|
|
|
828,912,974
|
|
|
|
835,587,422
|
|
|
|
816,874,442
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance sheet data
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current assets
|
|
$
|
63,182
|
|
|
$
|
674,228
|
|
|
$
|
156,769
|
|
|
$
|
20,555
|
|
|
Total assets
|
|
$
|
586,152
|
|
|
$
|
1,276,033
|
|
|
$
|
750,850
|
|
|
$
|
71,383
|
|
|
Current liabilities
|
|
$
|
6,436,073
|
|
|
$
|
4,696,189
|
|
|
$
|
5,584,383
|
|
|
$
|
4,530,606
|
|
|
Total liabilities
|
|
$
|
6,899,755
|
|
|
$
|
5,343,298
|
|
|
$
|
6,138,908
|
|
|
$
|
4,530,606
|
|
|
Total equity (deficit)
|
|
$
|
(6,313,603
|
)
|
|
$
|
(4,067,265
|
)
|
|
$
|
(5,388,058
|
)
|
|
$
|
(4,459,223
|
)
|
*
* Share amounts do not give effect to the Reverse Split.
RISK
FACTORS
An
investment in our Units involves a high degree of risk. You should carefully consider the following risk factors, together with
the other information contained in this prospectus, before you decide to buy any Units. Any of the following risks could cause
our business, results of operations and financial condition to suffer materially, causing the market price of our shares of Common
Stock to decline, in which event you may lose part or all of your investment. Additional risks and uncertainties not currently
known to us or that we currently do not deem material may also become important factors that may materially and adversely affect
our business.
Risk
Related to Our Company
The
report of the independent registered public accounting firm on our 2019 and 2018 financial statements contains a going concern
qualification.
The
report of the independent registered public accounting firm covering our financial statements for the years ended December 31,
2019 and December 31, 2018 stated that certain factors, including that we have a working capital and shareholder deficit, raised
substantial doubt as to our ability to continue as a going concern. Because we are not yet producing sufficient revenue to sustain
our operating costs, we are dependent upon raising capital to continue our business. If we are unable to raise capital, our ability
to continue could remain an ongoing concern.
We
may be unable to generate significant revenues and may never become profitable.
We
generated $401,620, $0 and $0 in revenue for the years ended December 31, 2018 and 2019 and the quarter ended June 30,
2020, respectively, and do not currently have any recurring sources of revenues, making it difficult to predict when we will
be profitable. We expect to incur significant research and development costs for the foreseeable future. We may not be able to
successfully market fiber products we produce in the future that will generate significant revenues. In addition, any revenues
that we may generate may be insufficient for us to become profitable.
As
a result of our limited operating history, we may not be able to correctly estimate our future revenues, operating expenses, need
for investment capital, or stability of operations, which could lead to cash shortfalls.
We
have a limited operating history from which to evaluate our business. Our failure to develop additional transgenic silkworms would
have a material adverse effect on our ability to continue operating. Accordingly, our prospects must be considered in light of
the risks, expenses, and difficulties frequently encountered by companies at our stage of development. We may not be successful
in addressing such risks, and the failure to do so could have a material adverse effect on our business, operating results and
financial condition.
Because
of this limited operating history and because of the emerging nature of our fiber product we are producing, our historical financial
data is of limited value in estimating future operating expenses. Our budgeted operating expense levels are based in part on our
expectations concerning future revenues. However, our ability to generate any revenues depends largely on the market acceptance
of the fibers we develop, which is difficult to forecast accurately. The failure of our target markets to adopt our products would
have a material adverse effect on our business.
Our
operating results may fluctuate as a result of a number of factors, many of which are outside of our control. For these reasons,
comparing our operating results on a period-to-period basis may not be meaningful, and you should not rely on our past results
as any indication of our future performance. Our quarterly and annual expenses are likely to increase substantially over the next
several years depending upon the level of fiber development activities. Our operating results in future quarters may fall below
expectations. Any of these events could adversely impact our business prospects and make it more difficult to raise additional
equity capital at an acceptable price per share.
We
derived all of our revenue from a single large customer.
Historically,
we have relied on one customer, the U.S. Army, for all of our revenue and accounts receivable. The U.S. Army comprised 100% of
our revenue from 2016 to 2018. When our contract with the U.S. Army expired, we did not have any other customers. As such, we
did not produce any revenue in 2019 and have been financing our operations with an equity investment from a shareholder. Failure
to secure additional customers will adversely affect our ability to sustain operations.
We
may be unable to maintain an effective system of internal controls and accurately report our financial results or prevent fraud,
which may cause our current and potential stockholders to lose confidence in our financial reporting and adversely impact our
business and our ability to raise additional funds in the future.
Effective
internal controls are necessary for us to provide reliable financial statements and effectively prevent fraud. We have no internal
audit function. As we noted in our annual report on Form 10-K for the year ended December 31, 2019, we reported that our internal
control over financial reporting was not effective for the purposes for which it is intended because we had material weaknesses,
as described below; we also noted in our quarterly report on Form 10-Q for the six months ended June 30, 2020
that (i) there were no changes in our internal control over financial reporting that has materially affected, or is reasonably
likely to materially affect, the our internal control over financial reporting, and (ii) our disclosure controls and procedures
were not effective to cause the information required to be disclosed by us in reports that we file or submit under the Exchange
Act to be recorded, processed, summarized and reported within the time periods prescribed by SEC, and that such information is
accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, as appropriate,
to allow timely decisions regarding required disclosure. Though we have taken some steps to address our material weaknesses in
our internal control over financial reporting, including education of management of disclosure requirements and financial reporting
controls, we still have not eliminated the material weakness in our internal controls over financial reporting. If we cannot provide
reliable financial statements or prevent fraud, our operating results and our reputation could be harmed as a result, causing
stockholders and/or prospective investors to lose confidence in management and making it more difficult for us to raise additional
capital in the future.
In
our “Management’s Annual Report on Internal Control Over Financial Reporting” that appeared in our annual report
on Form 10-K for the year ended December 31, 2019, we reported that our internal control over financial reporting was not effective
for the purposes for which it is intended based on a material weakness associated with our lack of qualified resources to perform
the internal audit functions properly, no segregation of duties that results in ineffective controls over financial reporting
and lack of control over related party transactions. As reported in our most recent we are taking some remediation steps to help
address our material weaknesses in our internal control over financial reporting, but we do not expect to remediate the weaknesses
in our internal controls over financial reporting until the time when we start to commercialize a recombinant fiber (and, therefore,
may have sufficient cash flow for hiring personnel to handle our accounting and reporting functions).
We
currently do not have patent rights to the products we are seeking to develop and we currently license some of the genetic sequences
and genetic engineering technology we need to develop our products. If any third party challenges our claim to intellectual property
rights in the fiber products we are seeking to develop or the intellectual property rights that we license, our business may be
materially harmed.
We
do not have utility or design patents to the fibers and products we are seeking to develop. It is possible that the fiber products
we are seeking to develop could be imitated or directly manufactured and sold by a competitor. In addition, some or all of our
research, development ideas and proposed products may be covered by patent rights held by some other entity. In that event, we
could incur substantial liability, we could be subject to litigation and claims, and our business would be materially adversely
affected.
In
addition to the Notre Dame Agreements, we also entered into intellectual property licensing agreements with the University of
Wyoming and Sigma-Aldrich. Pursuant to these licensing agreements, we have obtained certain exclusive rights to use intellectual
property and genetic sequences owned by these universities. However, we have no guarantee of the viability of these intellectual
property rights or the rights that we have licensed do not infringe on the legal rights of third parties. The intellectual property
rights that we have licensed could be challenged or voided or we could realize that the licensed intellectual property is worthless
and without utility. We may also need to license additional intellectual property from persons or entities in order to successfully
complete our research and development, and we cannot be certain that we will be able to enter into a license agreement with such
persons or entities. If we cannot enter into such license agreements, our operations will be adversely affected and our prospects
negatively affected.
We
have no assurance of the future grant of patents for technologies we hope to develop. If we are unable to secure intellectual
property protection rights for new technologies developed, our business would be materially harmed.
We
may not successfully manage any growth that we may experience.
Our
future success will depend upon not only product development, but also on the expansion of our operations and the effective management
of any such growth, which will place a significant strain on our management and on our administrative, operational, and financial
resources. To manage any such growth, we must expand our facilities, augment our operational, financial and management systems,
and hire and train additional qualified personnel. If we are unable to manage our growth effectively, our business would be harmed
as our growth could be adversely affected by such mismanagement.
Our
development of recombinant silk products and development programs depends upon third-parties.
As
we bring our product to market we will need to develop new relations and collaborations with wholesalers, retailers, silk spinners,
weavers, and freight handlers and end product developers. We expect to depend upon independent collaborations with textile producers,
to conduct development of applications for our transgenic silkworm and recombinant silk polymers, such as recombinant spider silk.
We expect that these collaborators would perform services under agreements with us. Such agreements are often standard-form agreements
typically not subject to extensive negotiation. These collaborators would not be our employees, and in general we would not control
the amount or timing of resources that they devoted to our product development programs. These future collaborators may not assign
as great a priority to our programs or pursue them as diligently as we would if we were undertaking such programs ourselves. If
outside collaborators fail to devote sufficient time and resources to product developments programs using our transgenic silkworm
technologies, or if their performance is substandard, our introduction of protein based fiber products will be delayed or may
not result at all. These future collaborators may also have relationships with other commercial entities, some of whom may compete
with us.
If
conflicts arise with our collaborators, they may act in their self-interests, which may be adverse to our interests.
Conflicts
may arise in collaborations we have entered into or may enter into due to one or more of the following:
|
●
|
disputes
with respect to payments that we believe are due under a collaboration agreement;
|
|
|
|
|
●
|
disagreements
with respect to ownership of intellectual property rights;
|
|
|
|
|
●
|
unwillingness
on the part of a collaborator to keep us informed regarding the progress of its development and commercialization activities,
or to permit public disclosure of these activities;
|
|
|
|
|
●
|
delay
of a collaborator’s development or commercialization efforts with respect to our product development; or
|
|
|
|
|
●
|
termination
or non-renewal of the collaboration.
|
In
addition, in our collaborations, we may be required to agree not to conduct independently, or with any third party, any research
or developments that are competitive with the research conducted under our collaborations. Our collaborations may have the effect
of limiting the areas of research or product commercialization that we may pursue, either alone or with others. Our collaborators,
however, may be able to develop, either alone or with others, products in related fields that are competitive with the products
or potential products that are the subject of these collaborations.
If
we lose the services of key management personnel, we may not be able to execute our business strategy effectively.
Our
future success depends in a large part upon the continued service of Kim Thompson, who is our founder, Chief Executive Officer,
Chief Financial Officer, President, and sole director. Mr. Thompson is critical to our overall management as well as the development
of our technology, our culture and our strategic direction. While Mr. Thompson does not possess formal training in the field of
scientific research and development, his core ideas and inventions serve as the basis for our technologies and products. We do
not maintain a key-person life insurance policy on Mr. Thompson. The loss of Mr. Thompson would materially harm our business.
As
our business grows, we will need to hire highly skilled personnel and, if we are unable to hire, retain, or motivate additional
qualified personnel, we may not be able to grow effectively.
Our
performance will be largely dependent on the talents and efforts of highly skilled individuals. Our future success depends on
our continuing ability to identify, hire, develop, motivate, and retain highly skilled personnel for all areas of our company.
Competition in our industry for qualified employees remains intense as the skills we require in our employees are highly specialized.
We compete with companies in the biotechnology and pharmaceutical industries that seek to retain scientists with genetic engineering
experience and expertise. We expect that over the longer term we will continue to face stiff competition and may not be able to
successfully recruit or retain such personnel. Attracting and retaining qualified personnel will be critical to our success.
If
we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market products
we may develop, we may not be able to generate product revenue.
We
do not currently have an organization for the sales, marketing and distribution of any fiber products that we expect to develop.
In order to market any products that may be developed, we must build our sales, marketing, managerial and other non-technical
capabilities or make arrangements with third parties to perform these services. In addition, we have no experience in developing,
training or managing a sales force and will incur substantial additional expenses in doing so. The cost of establishing and maintaining
a sales force may exceed its cost effectiveness. Furthermore, we will compete with many companies that currently have extensive
and well-funded marketing and sales operations. Our marketing and sales efforts may be unable to compete successfully against
these companies. If we are unable to establish adequate sales, marketing and distribution capabilities, whether independently
or with third parties, we may not be able to generate product revenue and may not become profitable.
We
may be unable to meet or sustain pricing or material performance requirements.
We
are not aware of any other company that has established a commercially viable and cost-effective production system for recombinant
spider silks. If we are unable to match or sustain the pricing requirement for our target markets or maintain material performance
expectations, it may have a material adverse impact on our business.
We
have limited intellectual property protection in foreign markets, which could affect our ability to grow our markets and increase
our revenue.
The
intellectual property that we licensed from Notre Dame, University of Wyoming and Sigma-Aldrich is covered by a series of U.S.
patents and U.S. patent applications with limited or no international patent protection. Foreign competitors could be using the
same technology that we have licensed, which would affect our ability to expand our markets beyond the United States. We are aware
that international laboratories and potential competitors are using the “piggyback” gene splicing technology for the
genetic modification of silkworm. Such limited foreign intellectual property could affect our ability to introduce fiber products
in international markets or effectively compete in such markets.
We
may fail to foresee challenges with international operations.
The
Company and its current management have no history or experience in establishing and developing international business units and
production facilities. Our future success will depend heavily upon on our ability to oversee production operations at facilities
and locations outside of the United States and management’s day-to-day oversight. Unforeseen challenges in sustaining efficient
operations, decreases in product quality, language and cultural difference, or theft of intellectual property from these satellite
production facilities, or other yet undiscovered challenges could have an adverse material effect on us.
The
patents underlying our license agreements could expire or be invalidated prior to our commercializing our specialty fibers, which
would result in the loss of our competitive edge and could negatively impact our revenues and results of operations.
The
patent rights that we license could expire or be invalidated before we are ready to market or commercialize any fiber product,
or while we are still in research and development phases of proposed products, in which event the patents would be worthless and
would not protect us from potential competitors who would then have low barriers to entry and who would be in a position to compete
more effectively with us.
Our
management has no previous experience in developing, producing, marketing, or selling recombinant fiber which may have a negative
effect on our ability to develop or sell our products.
Since,
to our knowledge, commercialization of spider silk on a large scale has yet to be accomplished, we are not aware of any candidates
with specific experience in this field. There may be numerous hurdles and obstacles that we are not currently able to foresee.
Our
current management has limited experience in developing, marketing and selling recombinant fiber and the other products that we
intend to develop and market. Additionally, our current management has no formal training in the business of scientific research
and development, which may be critical to our success. The inexperience of our management and lack of experienced workforce may
negatively affect our ability to succeed in developing, marketing and/or distributing our proposed products.
We
may be unprepared for technological changes in our industry, which could result in our products being obsolete or replaced by
better technology.
The
industry in which we participate is subject to rapid business and technological changes. The business, technology, marketing,
legal and regulatory changes that could occur may have a material adverse impact on us. New inventions and product innovations
may make our proposed products obsolete. Potential customers may prefer existing materials to our new technology. New materials
may come to market that outperform our technologies. Other researchers may develop and patent technologies which make our line
of research obsolete. We may not have the financial or technical ability to keep up with our competitors.
Our
business is based on scientific research which has not demonstrated commercial viability and makes our business highly risky.
We
are engaging in research and development of new recombinant silk fibers. Due to the speculative nature of this scientific research,
our chances of success are uncertain and we cannot guarantee that we will succeed in developing new fibers that deliver performance
results to meet customer requirements or obtain commercial acceptance. An investment in us, therefore, is highly speculative and
risky.
The
fibers we develop could expose us to product liability claims, which could have a negative impact on our results of operations.
The
fibers we are seeking to develop may subject us to product liability claims if widely used, including but not limited to design
defect, environmental hazards, quality control, and durability of product. This potential liability is increased by virtue of
the fact that our products in development may be used as protective and safety materials. The fibers and end products we are developing
are based on a GMO and are subject to public opinion, risks, and concerns regarding GMOs. There is tremendous potential liability
to any person who is injured by, or while using, one of our products. As a manufacturer, we may be strictly liable for any damage
caused by our products. This liability might not be covered by insurance, or may exceed any coverage that we may obtain.
If
we experience product recalls, we may incur significant and unexpected costs and damage to our reputation and, therefore, could
have a material adverse effect on our business, financial condition, or results of operations.
We
may be subject to product recalls, withdrawals, or seizures if any of our products are believed to cause injury. A recall, withdrawal,
or seizure of any of our products could materially and adversely affect consumer confidence in our brands and lead to decreased
demand for our products. In addition, a recall, withdrawal, or seizure of any of our products would require significant management
attention, would likely result in substantial and unexpected expenditures and could materially and adversely affect our business,
financial condition, or results of operations.
Ethical,
legal and social concerns about synthetic biologically engineered products and processes could limit or prevent the use of products
or processes using our technologies, limit consumer acceptance and limit our revenues.
Our
technologies involve the use of genetically engineered (“GE”) products or technologies. Public perception about the
safety and environmental hazards of, and ethical concerns over, GE products and processes could influence public acceptance of
our and our collaborators’ technologies, products and processes.
The
subject of GMOs has received negative publicity, which has aroused public debate. This adverse publicity has led to, and could
continue to lead to, greater regulation and trade restrictions on imports of genetically altered products. Further, there is a
risk that products produced using our technologies could cause adverse health effects or other adverse events, which could also
lead to negative publicity.
There
is also an active and vocal group of opponents to GMOs who wish to ban or restrict the technology and who, at a minimum, hope
to sway consumer perceptions and acceptance of this technology. Their efforts include regulatory legal challenges and labeling
campaigns for genetically modified products, as well as application of pressure to consumer retail outlets seeking a commitment
not to carry genetically modified products. Further, these groups have a history of bringing legal action against companies attempting
to bring new biotechnology products to market. We may be subject to future litigation brought by one or more of these organizations
in their attempt to block the development or sale of our products. In addition, animal rights groups and various other organizations
and individuals have attempted to stop genetic engineering activities by pressing for legislation and additional regulation in
these areas. We may not be able to overcome the negative consumer perceptions and potential legal hurdles that these organizations
seek to instill or assert against our products, and our business could be harmed.
If
we are not able to overcome the ethical, legal and social concerns relating to genetic engineering, products and processes using
our technologies may not be accepted. These concerns could result in increased expenses, regulatory scrutiny, delays or other
impediments to our programs or the public acceptance and commercialization of products and processes dependent on our technologies
or inventions. Our ability to develop and commercialize products, or processes using our technologies could be limited by public
attitudes and governmental regulation.
Inadvertent
releases or unintended consequences of releases of synthetic biology technologies by us or others could lead to adverse effects
on our business and results of operations.
The
genetically engineered technologies that we develop may have significantly enhanced strength and elasticity characteristics compared
to those found in native silkworms. While we produce these technologies only for use in a controlled industrial environment, the
release of such technologies into uncontrolled environments could have unintended consequences. Any adverse effect resulting from
such a release, by us or others, could have a material adverse effect on the public acceptance of our products and our business
and financial condition. Such a release could result in enhanced regulatory activity and we could have exposure to liability for
any resulting harm.
Potential
future regulations limiting our ability to sell or produce genetically engineered products could harm our business.
We
expect to develop biologic products using GMOs. Products derived from GMOs may in some instances be subject to bans or additional
regulation by federal, state, local and foreign government agencies. These agencies may not allow us or our collaborators and
licensees to produce and market products derived from GMOs in a timely manner or under technically or commercially feasible conditions.
Further,
we and our current and future collaborators and licensees are subject to regulations in the other countries in which we operate
outside of the U.S., which may have different rules and regulations depending on the jurisdiction. Different countries have different
rules regarding which products qualify as GMO. If any of these countries expand the definition of GMO and increase the regulatory
burden on GMO products, our business could be harmed.
Other
changes in regulatory requirements, laws and policies, or evolving interpretations of existing regulatory requirements, laws and
policies, may result in increased compliance costs, delays, capital expenditures and other financial obligations that could adversely
affect our business or financial results.
Our
operations would be negatively affected by any dispute with our collaborating universities or by labor unrest (such as disputes,
strikes or lockouts) between such universities and their academic staff.
We
have signed intellectual property, sponsored research and collaborative research agreements with one or more universities. The
continued cooperation of these universities, as well as the cooperation of other institutions and or universities is essential
for the success of the Company. In the event of a material dispute with one or more of the universities, such a dispute could
create a cessation of operations for a period of time that could be detrimental to our operations and survival. Additionally,
a material dispute between any such university and its employees could create a cessation of operations for a period of time that
could be detrimental to our product development.
Our
competitors are larger with greater financial resources than we have and we may face increased competition due to the low barriers
of entry to our industry.
We
compete directly with numerous other companies with similar product lines and/or distribution that have extensive capital, resources,
market share, and brand recognition. There are few barriers to entry on the industry in which we compete, namely the textile,
specialty fabric and technical textile industries. This creates the strong possibility of new competitors emerging, and of others
succeeding in developing the same or similar fibers that we are trying to develop. The effects of this increased competition may
be materially adverse to us and our stockholders.
We
may face various governmental regulations, which could increase our costs and lower our future profitability.
We
face various governmental regulations regarding import/export, taxes, transgenics and biological research. Transgenic product
manufacture and distribution, environmental regulation and packaging requirements may be adverse to our operations, research and
development, revenues, and potential profit. We are especially at risk from governmental restriction and regulations related to
the development of materials by use of transgenic organisms and GMOs. Federal and state regulations impose strict regulation on
the use, storage, and transportation of such transgenic organisms. Such rules impose severe penalties on us for any breach of
regulations, for any spill, release, or contamination caused while the substances are under our direct or indirect ownership or
control. We are not aware of any such breach of governmental regulation, or of any spill, release, or contamination, however,
if such a release, or other regulatory breach does occur in the future, the resulting clean-up costs, and/or fines and penalties,
would cause a material negative effect on the Company and our financial future.
We
face additional challenges associated with operating in overseas locations, which have additional governmental regulations and
restrictions. Those regulations are subject to change and interpretation relating to GMO’s. Such changes could limit our
ability to sell or produce GMO products. We cannot be assured that what is allowed today will be allowable in the future.
We
may face unforeseen challenges with the import and export of our products.
The
success of our operations depends on our ability to ship the silk fibers and yarns produced by GMO’s. There is no guarantee
that the existing authorizations and interpretations of rules will remain in effect. Increasing restrictions on the shipping of
products with GMO’s or importation of GMO’s may materially impact our business.
Our
international operations will be subject to the laws of the jurisdictions in which we operate.
A
significant portion of our business operations will occur in Vietnam. We will be generally subject to laws and regulations applicable
to foreign investment in Vietnam. The Vietnamese legal system is based, at least in part, on written statutes. However, since
these laws and regulations are relatively new and the Vietnamese legal system continues to rapidly evolve, the interpretations
of many laws, regulations and rules are not always uniform and enforcement of these laws, regulations and rules involves uncertainties.
We
cannot predict the effect of future developments in the legal systems of developing countries, including the promulgation of new
laws, changes to existing laws or the interpretation or enforcement thereof, the preemption of local regulations by national laws,
or the overturn of local government’s decisions by the superior government. These uncertainties may limit legal protections
available to us.
We
may be adversely affected by economic and political conditions in the countries where we operate.
We
operate in Vietnam. Economic and political changes in these countries, such as inflation rates, recession, foreign ownership restrictions,
restrictions on transfer of funds into or out of a country and similar factors may adversely affect results of operations.
While
it is our understanding that the economy in Vietnam has grown significantly in the past 20 years, the growth has been uneven,
both geographically and among various economic sectors. The government of Vietnam has implemented various measures to encourage
or control economic growth and guide the allocation of resources. Some of these measures benefit the overall Vietnamese economy,
but may also have a negative effect on us. For example, our financial condition and results of operations may be adversely affected
by government control over capital investments, land use or changes in tax regulations that are applicable to us.
The
Vietnamese economy has been transitioning from a planned economy to a more market-oriented economy4. Although in
recent years the Vietnamese government has implemented measures emphasizing the utilization of market forces for economic reform,
the reduction of state ownership of productive assets and the establishment of sound corporate governance in business enterprises,
a substantial portion of the productive assets in Vietnam are still owned by the Vietnamese government. The continued control
of these assets and other aspects of the national economy by Vietnam government could materially and adversely affect our business.
The Vietnamese government also exercises significant control over Vietnamese economic growth through the allocation of resources,
controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment
to particular industries or companies. Efforts by the Vietnamese government to slow the pace of growth of the Vietnamese economy
could negatively affect our business.
Our
business, operations and plans and timelines have been adversely affected by the effects of health epidemics, including the recent
COVID-19 pandemic, on the manufacturing, production and other business activities performed by us or by third parties with whom
we conduct business, including our suppliers, target end market potential collaboration partners, and others.
4 https://www.lowyinstitute.org/publications/missing-middle-political-economy-economic-restructuring-vietnam
Our
business could be further adversely affected by health epidemics wherever we have business operations. In addition, health epidemics
could cause significant disruption in the operations of third-party manufacturers, CROs and other third parties upon whom we rely.
For example, in December 2019, a novel strain of coronavirus, SARS-CoV-2, causing a disease referred to as COVID-19, was reported
to have surfaced in Wuhan, China. Since then, COVID-19 has spread to multiple countries worldwide, including the United States.
Our headquarters is located in Michigan and our manufacturers are located in Vietnam. This spread resulted in the Company furloughing
all non-essential personnel and pausing its production operations. In March 2020, the World Health Organization declared the COVID-19
outbreak a pandemic, and the U.S. government imposed travel restrictions on travel between the United States, Europe and certain
other countries. Further, the President of the United States declared the COVID-19 pandemic a national emergency and invoked powers
under the Stafford Act, the legislation that directs federal emergency disaster response, and under the Defense Production Act,
the legislation that facilitates the production of goods and services necessary for national security and for other purposes.
We have implemented work-from-home policies for all employees. The effects of the executive order and our work-from-home policies
may negatively impact productivity, disrupt our business and delay our timelines, the magnitude of which will depend, in part,
on the length and severity of the restrictions and other limitations on our ability to conduct our business in the ordinary course.
These and similar, and perhaps more severe, disruptions in our operations could negatively impact our business, operating results
and financial condition.
Quarantines,
shelter-in-place and similar government orders, or the expectation that such orders, shutdowns or other restrictions could occur,
whether related to COVID-19 or other infectious diseases, could impact personnel at third-party manufacturing facilities in the
United States and other countries, or the availability or cost of materials, which could disrupt our supply chain or end markets.
For example, we may face shortages in mulberry feed for our silkworms as a result shifting agricultural priorities, or a drop
in demand for our finished materials as a result of an economic downturn. In addition, closures of transportation carriers and
modal hubs could materially impact our development and any future commercialization timelines.
If
our relationships with our suppliers or other vendors are terminated or scaled back as a result of the COVID-19 pandemic or other
health epidemics, we may not be able to enter into arrangements with alternative suppliers or vendors or do so on commercially
reasonable terms or in a timely manner. Switching or adding additional suppliers or vendors involves substantial cost and requires
management time and focus. In addition, there is a natural transition period when a new supplier or vendor commences work. As
a result, delays occur, which could adversely impact our ability to meet our desired clinical development and any future commercialization
timelines. Although we carefully manage our relationships with our suppliers and vendors, there can be no assurance that we will
not encounter challenges or delays in the future or that these delays or challenges will not have an adverse impact on our business,
financial condition and prospects. See “—Risks Related to Our Dependence on Third Parties.”
The
spread of COVID-19, which has caused a broad impact globally, may materially affect us economically. While the potential economic
impact brought by, and the duration of, COVID-19 may be difficult to assess or predict, a widespread pandemic could result in
significant disruption of global financial markets, reducing our ability to access capital, which could in the future negatively
affect our liquidity. In addition, a recession or market correction resulting from the spread of COVID-19 could materially affect
our business and the value of our common stock.
We
expect the temporary closing of our facility in Vietnam to result in a 4-6 months delay in our production expansion schedule,
although given the uncertainty of the situation, it could be longer. As of the date hereof, Vietnam does not have any work restrictions
impacting our facility. However, current U.S. travel restrictions may prevent us from shipping the eggs we are now producing in
the U.S. to Vietnam; as soon as the eggs are shipped, we can restart operations at the facility. However, the global pandemic
of COVID-19 continues to evolve rapidly and therefore we cannot guarantee that we will be able to ship our eggs to Vietnam in
a timely manner, if at all or that we will not have to close the facility again in the future. The ultimate impact of the COVID-19
pandemic or a similar health epidemic is highly uncertain and subject to change. We do not yet know the full extent of potential
delays or impacts on our business, our clinical trials, healthcare systems or the global economy as a whole. However, these effects
could have a material impact on our operations, and we will continue to monitor the COVID-19 situation closely. See, “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Impact of COVID-19 Outbreak.”
Our
production system is based upon living transgenic organisms.
Our
production system is based on transgenic silkworms. Therefore, anything affecting the lifecycle of those silkworms, including
but not limited to disease, climate, or nutrition could have significant negative effects on our production or our products. Such
negative effects to our production could materially adversely affect our operations and ability to receive revenue in the
future.
Risks
Related to This Offering and Our Common Stock
Our
sole Director owns a significant percentage of our outstanding voting securities which could reduce the ability of minority stockholders
to effect certain corporate actions.
Collectively,
due to the voting rights features of the Series A Preferred Stock (which is owned solely by our chief executive officer),
our officers and directors own an aggregate of 222,768,160 shares of our capital stock, or approximately 49.65%
of our outstanding voting securities. As a result, currently, and after this offering, they will possess significant influence
and can elect a majority of our Board and authorize or prevent proposed significant corporate transactions without the votes of
any other stockholders. They are expected to have significant influence over a decision to enter into any corporate transaction
and have the ability to prevent any transaction that requires the approval of stockholders, regardless of whether or not our other
stockholders believe that such transaction is in our best interests. Such concentration of voting power could have the effect
of delaying, deterring, or preventing a change of control or other business combination, which could, in turn, have an adverse
effect on the market price of our Common Stock or prevent our shareholders from realizing a premium over the then-prevailing market
price for their Common Stock.
Our
management will have broad discretion as to the use of proceeds from this offering, and we may not use the proceeds effectively.
Our
management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in
ways that do not improve our results of operations or enhance the value of our Common Stock. You will not have the opportunity,
as part of your investment decision, to assess whether these proceeds are being used appropriately. Our failure to apply these
funds effectively could have a material adverse effect on our business and cause the price of our common stock to decline.
Our
Reverse Stock Split may not result in a proportional increase in the per share price of our Common Stock.
The
effect of the Reverse Stock Split on the market price for our Common Stock cannot be accurately predicted. In particular, we cannot
assure you that the prices for shares of the common stock after the Reverse Stock Split will increase proportionately to prices
for shares of our Common Stock immediately before the Reverse Stock Split. The market price of our common stock may also be affected
by other factors which may be unrelated to the Reverse Stock Split or the number of shares issued and outstanding.
Furthermore,
even if the market price of our Common Stock does rise following the Reverse Stock Split, we cannot assure you that the market
price of our Common Stock immediately after the proposed Reverse Stock Split will be maintained for any period of time. Moreover,
because some investors may view the Reverse Stock Split negatively, we cannot assure you that the Reverse Stock Split will not
adversely impact the market price of our common stock. There is also the possibility that liquidity may be adversely affected
by the reduced number of shares which would be issued and outstanding when the Reverse Stock Split is effected, particularly if
the price per share of our Common Stock begins a declining trend after the Reverse Stock Split is effected. Accordingly, our total
market capitalization after the Reverse Stock Split may be lower than the market capitalization before the Reverse Stock Split.
Investors
in this offering will experience immediate and substantial dilution in net tangible book value.
The public offering price will be substantially
higher than the net tangible book value per share of our outstanding shares of our Common Stock. As a result, investors in this
offering will incur immediate dilution of $[ ] per share, based on the assumed public offering price of $5.25
per Unit (the midpoint of the range indicated on the front cover of this prospectus). Investors in this offering will
pay a price per share that substantially exceeds the book value of our assets after subtracting our liabilities. See “Dilution”
for a more complete description of how the value of your investment will be diluted upon the completion of this offering.
We
may need to raise additional capital by sales of our securities, which may adversely affect the market price of our Common Stock
and your rights in us may be reduced.
We
expect to continue to incur product development and selling, general and administrative costs, and in order to satisfy our funding
requirements, we will need to continue to raise additional capital above and beyond the anticipated proceeds of this offering.
The sale or the proposed sale of substantial amounts of our Common Stock or other securities in the public markets may adversely
affect the market price of our Common Stock and our stock price may decline substantially. Our stockholders may experience substantial
dilution and a reduction in the price that they are able to obtain upon sale of their shares. Also, new equity securities issued
may have greater rights, preferences or privileges than our existing Common Stock. Furthermore, additional
capital may not be available in sufficient amounts or on reasonable terms, if at all, and our ability to raise additional capital
may be adversely impacted by potential worsening global economic conditions and the recent disruptions to and volatility in the
credit and financial markets in the United States and worldwide resulting from the ongoing COVID-19 pandemic.
We
do not intend to pay dividends.
We
have never declared or paid any cash dividends on our securities. We currently intend to retain our earnings for funding growth
and, therefore, do not expect to pay any dividends in the foreseeable future.
An
active, liquid, and orderly market for our common stock or Purchase Warrants may not develop.
Our Common Stock is expected to trade on Nasdaq
or other national exchange as of the effective date of the registration statement of which this prospectus forms
a part and we intend to seek a listing on Nasdaq or other national exchange for our Purchase Warrants. An active trading
market for our Common Stock or Purchase Warrants may never develop or be sustained. If an active market for our Common Stock or
Purchase Warrants does not continue to develop or is not sustained, it may be difficult for investors to sell shares of our Common
Stock or Purchase Warrants without depressing the market price and investors may not be able to sell the shares of our Common
Stock or Purchase Warrants at all. An inactive market may also impair our ability to raise capital by selling our Common Stock
or Purchase Warrants and may impair our ability to acquire other businesses, applications, or technologies using our Common Stock
or Purchase Warrants as consideration, which, in turn, could materially adversely affect our business.
While
we are seeking to list our Common Stock and Purchase Warrants on Nasdaq, there is no assurance that either of such securities
will be listed on Nasdaq or any stock exchange.
While
we are seeking to list our Common Stock and Purchase Warrants on Nasdaq, we cannot ensure that either of such securities will
be accepted for listing on Nasdaq or with regard to the Purchase Warrants, any exchange. Should our Purchase Warrants be rejected
for listing on Nasdaq, we will seek to have our Purchase Warrants quoted on the OTC Markets, in which event the trading price
of our Purchase Warrants could suffer, the trading market for our Purchase Warrants may be less liquid, and our Purchase Warrant
price may be subject to increased volatility. If we fail to have our Purchase Warrants quoted on the OTC Markets, there will be
no public market for our Purchase Warrants.
The
Purchase Warrants may not have any value.
Each
Purchase Warrant will have an exercise price equal to the per share public offering of our Units and will expire on the fifth
anniversary of the date they first become exercisable. In the event our Common Stock price does not exceed the exercise price
of the Purchase Warrants during the period when the warrants are exercisable, the Purchase Warrants may not have any value.
The
market price of our Common Stock may be volatile and may be affected by market conditions beyond our control, and you may not
be able to sell our Common Stock.
Publicly
traded companies generally have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate
to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our
securities, regardless of our actual operating performance.
The
market for our Common Stock is characterized by significant price volatility when compared to seasoned issuers, and we expect
that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. The volatility in our
share price is attributable to a number of factors. First, our shares of Common Stock are sporadically and thinly traded. As a
consequence of this lack of liquidity, the trading of relatively small quantities of shares by our stockholders may disproportionately
influence the price of those shares in either direction. The price for our shares Common Stock could, for example, decline precipitously
in the event that a large number of shares of our Common Stock are sold on the market without commensurate demand, as compared
to a seasoned issuer which could better absorb those sales without adverse impact on its share price. Second, we are a speculative
or “risky” investment due to our limited operating history and lack of revenue to date, and uncertainty of future
market acceptance for our potential products. As a consequence of this enhanced risk, more risk-averse investors may, under the
fear of losing all or most of their investment in the event of negative news or lack of progress, be more inclined to sell their
shares on the market more quickly and at greater discounts than would be the case with the stock of a seasoned issuer. Many of
these factors are beyond our control and may decrease the market price of our Common Stock, regardless of our operating performance.
We cannot make any predictions or projections as to what the prevailing market price for our Common Stock will be at any time,
including as to whether our Common Stock will sustain its current market price, or as to what effect the sale of shares or the
availability of Common Stock for sale at any time will have on the prevailing market price.
The
market price of our Common Stock is subject to significant fluctuations in response to, among other factors:
|
|
●
|
the
significant downward pressure on our Common Stock price caused by the sale of a significant number of shares could cause our
Common Stock price to decline, thus allowing short sellers of our Common Stock an opportunity to take advantage of any decrease
in the value of our Common Stock;
|
|
|
●
|
the
presence and action of short sellers in our Common Stock;
|
|
|
●
|
market
acceptance of our existing products, as well as products in development;
|
|
|
●
|
the
timing of regulatory approvals;
|
|
|
●
|
our
ability or the ability of third-party distributors to sell, market, and distribute our products;
|
|
|
●
|
our
ability or the ability of our contract manufacturers to manufacture our products efficiently;
|
|
|
●
|
changes
in our financial performance or a change in financial estimates or recommendations by securities analysts;
|
|
|
●
|
our
ability to raise additional funds to complete development of our pharmaceutical product candidates;
|
|
|
●
|
announcements
of innovations or new products or services by us or our competitors;
|
|
|
●
|
the
emergence of new competitors or success of our existing competitors;
|
|
|
●
|
operating
and market price performance of other companies that investors deem comparable;
|
|
|
●
|
sales
or purchases of our Common Stock by insiders;
|
|
|
●
|
commencement
of, or involvement in, litigation;
|
|
|
●
|
changes
in governmental regulations; and
|
|
|
●
|
general
economic conditions and slow or negative growth of related markets.
|
In
addition, if the market for stock in our industry, or the stock market in general, experiences a loss of investor confidence,
the market price of our common stock could decline for reasons unrelated to our business, financial condition or results of operations.
If
any of the foregoing occurs, it could cause the price of our Common Stock to fall and may expose us to lawsuits that, even if
unsuccessful, could be costly to defend and distract our Board and management.
We
are currently subject to penny stock regulations and restrictions and if we continue to be subject to such regulations and restrictions
you may have difficulty selling shares of our Common Stock.
The
Commission has adopted regulations which generally define so-called “penny stocks” as an equity security that has
a market price of less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exemptions.
Our Common Stock is a “penny stock”, and we are subject to Rule 15g-9 under the Exchange Act, or the Penny Stock Rule.
This rule imposes additional sales practice requirements on broker-dealers that sell such securities to persons other than established
customers and “accredited investors” (generally, individuals with a net worth in excess of $1,000,000 or annual income
exceeding $200,000, or $300,000 together with their spouses). For transactions covered by Rule 15g-9, a broker-dealer must make
a special suitability determination for the purchaser and receive the purchaser’s written consent to the transaction prior
to sale. As a result, this rule affects the ability of broker-dealers to sell our securities and affects the ability of purchasers
to sell any of our securities in the secondary market.
For
any transaction involving a penny stock, unless exempt, the rules require delivery, prior to any transaction in a penny stock,
of a disclosure schedule prepared by the Commission relating to the penny stock market. Disclosure is also required to be made
about sales commissions payable to both the broker-dealer and the registered representative and current quotations for the securities.
Finally, monthly statements are required to be sent disclosing recent price information for the penny stock held in the account
and information on the limited market in penny stock.
After
giving effect to this offering and the Reverse Stock Split and our listing on Nasdaq or other national exchange, our Common
Stock will not be a “penny stock”. There can be no assurance that our shares of Common Stock will continue to not
be a “penny stock” because of its price or qualification for exemption from the Penny Stock Rule. In any event, even
if our Common Stock were exempt from the Penny Stock Rule, we would remain subject to Section 15(b)(6) of the Exchange Act, which
gives the Commission the authority to restrict any person from participating in a distribution of penny stock if the Commission
finds that such a restriction would be in the public interest.
In
addition to the “penny stock” rules described above, the Financial Industry Regulatory Authority (“FINRA”)
has adopted similar rules that may also limit a stockholder’s ability to buy and sell our Common Stock. FINRA rules require
that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment
is suitable for such customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers
must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives
and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative
low priced securities will not be suitable for at least some customers. The FINRA requirements make it more difficult for broker-dealers
to recommend that their customers buy our Common Stock, which may limit your ability to buy and sell our stock and have an adverse
effect on the market for our shares.
Risk
Factors Related to Uplisting on the Nasdaq Capital Market or Another National Exchange
Our
ability to uplist our Common Stock to the Nasdaq Capital Market or another national exchange is contingent on our meeting applicable
initial listing criteria.
We
have applied for our Common Stock to be listed on the Nasdaq Capital Market or another national securities exchange. Each exchange
requires companies desiring to list their common stock to meet certain listing criteria including total number of stockholders;
minimum stock price, total value of public float, and in some cases total stockholders’ equity and market capitalization.
Our failure to meet such applicable listing criteria would prevent us from listing our Common Stock on the Nasdaq Capital Market
or another national exchange. In the event we are unable to uplist our Common Stock, our Common Stock will continue to be quoted
on the OTCQB, which is generally considered less liquid and more volatile than a national securities exchange. Our failure to
uplist our Common Stock could make it more difficult for you to trade our Common Stock, could prevent our common stock trading
on a frequent and liquid basis and could result in the value of our Common Stock being less than it would be if we were able to
uplist.
If
the Nasdaq Capital Market or another national exchange does not list our securities for quotation, it could limit investors’
ability to make transactions in our securities and subject us to additional trading restrictions.
We
have applied for our Common Stock to be listed on the Nasdaq Capital Market, or another national securities exchange. After giving
effect to this offering, we expect to meet, on a pro forma basis, the Nasdaq Capital Market’s minimum initial listing standards,
which generally only mandate that we meet certain requirements relating to stockholders’ equity, market capitalization,
aggregate market value of publicly held shares, bid price and distribution requirements. We cannot guarantee that we will be able
to meet those initial listing requirements. If the Nasdaq Capital Market or another national exchange does not list our common
stock for trading on its exchange, we could face significant material adverse consequences, including:
|
|
●
|
a
limited availability of market quotations for our securities;
|
|
|
●
|
reduced
liquidity with respect to our securities;
|
|
|
●
|
a
determination that our shares of or Common Stock are “penny stock,” which will require brokers desiring to recommend
our shares of Common Stock to their clients to adhere to more stringent rules, possibly resulting in a reduced level of trading
activity in the secondary trading market for our shares of common stock;
|
|
|
●
|
a
limited amount of news and analyst coverage for our Company; and
|
|
|
●
|
a
decreased ability to issue additional securities or obtain additional financing in the future.
|
The
National Securities Markets Improvement Act of 1996, which is a federal statute, prevents or preempts the states from regulating
the sale of certain securities, which are referred to as “covered securities.” If our Common Stock is listed on the
Nasdaq Capital Market or another national exchange, such securities will be covered securities. Although the states are preempted
from regulating the sale of our securities, the federal statute does allow the states to investigate companies if there is a suspicion
of fraud, and, if there is a finding of fraudulent activity, then the states can regulate or bar the sale of covered securities
in a particular case. Further, if we were no longer listed on the Nasdaq Capital Market or another national exchange, our securities
would not be covered securities and we would be subject to regulation in each state in which we offer our securities.
If
our application to uplist is approved, our failure to meet the continued listing requirements of the Nasdaq Capital Market or
another national exchange could result in a delisting of our Common Stock.
If
our application to list our Common Stock on the Nasdaq Capital Market or another national exchange is approved, and thereafter
we fail to satisfy the continued listing requirements of the Nasdaq Capital Market or another national exchange, such as the corporate
governance requirements or the minimum closing bid price requirement, the Nasdaq Capital Market or another national exchange may
take steps to delist our common stock. Such a delisting would likely have a negative effect on the price of our common stock and
would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a delisting, we anticipate
that we would take actions to restore our compliance with the Nasdaq Capital Market or another national exchange’s listing
requirements, but we can provide no assurance that any such action taken by us would allow our Common Stock to remain listed on
the Nasdaq Capital Market or another national exchange, stabilize our market price, improve the liquidity of our common stock,
prevent our common stock from dropping below the Nasdaq Capital Market or another national exchange ’s minimum bid price
requirement, or prevent future non-compliance with the Nasdaq Capital Market or another national exchange ’s listing requirements.
We
will continue to incur significant increased costs as a result of operating as a public company, and our management will be required
to devote substantial time to new compliance requirements as a result of our planned efforts to uplist our Common Stock to the
Nasdaq Capital Market or another national exchange.
We
will continue to incur significant increased costs as a result of operating as a public company, and our management will be required
to devote substantial time to new compliance requirements if our uplisting to the Nasdaq Capital Market or another national exchange
is successful. As a public company, we will continue to incur significant legal, accounting and other expenses. For example, if
our uplisting application to the Nasdaq Capital Market or another national exchange is successful, we will be subject to mandatory
reporting requirements of the Exchange Act, which require, among other things, that we continue to file with the SEC annual, quarterly
and current reports with respect to our business and financial condition, that we were not required to file as a voluntary reporting
company (though we did file such reports with the SEC on a voluntary basis). We have incurred and will continue to incur costs
associated with the preparation and filing of these SEC reports. Furthermore, if our planned uplisting to the Nasdaq Capital Market
or another national exchange is successful, we will be subject to mandatory new corporate governance and other compliance requirements.
In addition, the Sarbanes-Oxley Act, as well as rules subsequently implemented by the SEC, the Dodd-Frank Wall Street Reform and
Consumer Protection Act and the Nasdaq Capital Market or another national exchange have imposed various other requirements on
public companies. Stockholder activism, the current political environment and the current high level of government intervention
and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance
costs and impact (in ways we cannot currently anticipate) the way we operate our business. Our management and other personnel
will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations have and
will continue to increase our legal and financial compliance costs and will make some activities more time-consuming and costly.
In
addition, if and when we cease to be a smaller reporting company and become subject to Section 404(b) of the Sarbanes-Oxley Act,
we will be required to furnish an attestation report on internal control over financial reporting issued by our independent registered
public accounting firm. To achieve compliance with Section 404 within the prescribed time period, we will continue to be engaged
in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In
this regard, we will need to dedicate substantially greater internal resources, potentially engage outside consultants and adopt
a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve
control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous
reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that our
independent registered public accounting firm, when required, will not be able to conclude within the prescribed timeframe that
our internal control over financial reporting is effective as required by Section 404. This could result in an adverse reaction
in the financial markets due to a loss of confidence in the reliability of our financial statements.
Our
Common Stock is currently quoted on the OTCQB, which may not be indicative of the price at which our stock may trade upon listing
on the Nasdaq Capital Market or another national exchange.
Our
Common Stock is quoted on the OTCQB. The OTCQB is a significantly more limited market than the Nasdaq Capital Market or another
national exchange. Although we intend to list our Common Stock on the Nasdaq Capital Market or another national exchange in connection
with this offering, an adequate trading market for the securities may not develop or be sustained after this rights offering.
In addition, if our shares are approved for listing on the Nasdaq Capital Market or another national exchange, the per share trading
prices may differ significantly to trading prices previously quoted while on the OTCQB. There can be no assurance that the Company’s
share price will demonstrate the same trading characteristics of historical price and volume on the Nasdaq Capital Market that
were demonstrated while listed on the OTCQB. Accordingly, after listing, we could fail to satisfy the continued listing requirements
of the Nasdaq Capital Market or another national exchange, such as the minimum closing bid price requirement. The Nasdaq Capital
Market or another national exchange could take steps to delist our Common Stock. Such a delisting would likely have a negative
effect on the price of our Common Stock and would impair your ability to sell or purchase our Common Stock.
In
addition, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the
market prices of equity securities of many companies, including very recently in connection with the ongoing COVID-19 pandemic,
which has resulted in decreased stock prices for many companies notwithstanding the lack of a fundamental change in their underlying
business models or prospects. These fluctuations have often been unrelated or disproportionate to the operating performance of
those companies. Broad market and industry factors, including potentially worsening economic conditions and other adverse effects
or developments relating to the ongoing COVID-19 pandemic, political, regulatory and other market conditions, may negatively affect
the market price of shares of our common stock, regardless of our actual operating performance. The market price of shares of
our common stock may decline below the initial public offering price, and you may lose some or all of your investment.
DISCLOSURE
REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus contains forward-looking statements about our industry and us that involve substantial risks and uncertainties. Our
actual results could differ materially from those discussed in the forward-looking statements. All statements other than statements
of historical facts contained in this prospectus, including statements regarding our future results of operations and financial
condition, business strategy and plans, and objectives of management for future operations, are forward-looking statements. In
some cases, you can identify forward-looking statements by words such as “anticipate,” “believe,” “continue,”
“could,” “design,” “estimate,” “expect,” “intend,” “may,”
“plan,” “potentially,” “predict,” “project,” “should,” “will”
or the negative of these terms or other similar expressions. We have based these forward-looking statements largely on our current
expectations and projections about future events and financial trends that we believe may affect our financial condition, results
of operations, business strategy and financial needs. These forward-looking statements are subject to a number of known and unknown
risks, uncertainties and assumptions, including risks described in the section titled “Risk Factors” and elsewhere
in this prospectus and should not be relied upon.
The
forward-looking statements in this prospectus include statements about:
|
|
●
|
Our
ability to continue as a going concern;
|
|
|
|
|
|
|
●
|
Our
ability to generate significant revenues and to become profitable;
|
|
|
|
|
|
|
●
|
Our
ability to estimate future expenses;
|
|
|
|
|
|
|
●
|
The
effect of the ongoing COVID-19 pandemic;
|
|
|
|
|
|
|
●
|
Our
ability to maintain an effective system of internal controls;
|
|
|
|
|
|
|
●
|
Our
ability to protect our intellectual property rights and to secure additional rights domestically and internationally;
|
|
|
|
|
|
|
●
|
Our
ability to successfully manage our growth domestically and internationally;
|
|
|
|
|
|
|
●
|
Our
ability to retain the services of key personnel;
|
|
|
|
|
|
|
●
|
Our
reliance on independent third-party collaborators to develop and deliver products to market;
|
|
|
|
|
|
|
●
|
Our
reliance on key management personnel and future need for highly skilled personnel;
|
|
|
|
|
|
|
●
|
Our
ability to successfully develop sales and marketing for our products;
|
|
|
|
|
|
|
●
|
Market
acceptance of pricing and performance for products we develop;
|
|
|
|
|
|
|
●
|
Our
ability to generate sustainable earnings and net operating profits;
|
|
|
|
|
|
|
●
|
Our
ability to adapt to regulatory and technology changes impacting our industry;
|
|
|
|
|
|
|
●
|
The
potential for product liability claims regarding our products and the use of GMO’s in our production system;
|
|
|
|
|
|
|
●
|
Actions
taken or omitted to be taken by third parties including our suppliers and competitors, as well as legislative, regulatory,
judicial and other governmental authorities;
|
|
|
|
|
|
|
●
|
Competition
in our industry;
|
|
|
|
|
|
|
●
|
The
loss of or failure to obtain any license or permit necessary or desirable in the operation of our business;
|
|
|
|
|
|
|
●
|
The
availability of additional capital to support development;
|
|
|
|
|
|
|
●
|
Our
production system is based upon living transgenic organisms; and
|
|
|
|
|
|
|
●
|
Certain
other risks and uncertainties set forth elsewhere in this prospectus under the section titled “Risk Factors”.
|
These
risks are not exhaustive. Other sections of this prospectus may include additional factors that could harm our business and financial
performance. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to
time, and it is not possible for our management to predict all risk factors nor can we assess the impact of all factors on our
business or the extent to which any factor, or combination of factors, may cause actual results to differ from those contained
in, or implied by, any forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking
statements.
Although
we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results,
levels of activity, performance or achievements, which speak only as of their dates. Except as required by law, we undertake no
obligation to update publicly any forward-looking statements for any reason after the date of this prospectus or to conform these
statements to actual results or to changes in our expectations.
USE
OF PROCEEDS
We
estimate that the net proceeds from the sale of 1,904,762 Units at the assumed public offering price of $5.25
per Unit, the midpoint of the price range set forth on the cover page of this prospectus, will be approximately $10,000,000
or approximately $11,500,000 if the underwriters exercise their option to purchase additional shares of Common Stock
and/or Purchase Warrants in full, in each case after deducting the underwriting discounts and commissions and estimated offering
expenses payable by us.
We
intend to use the net proceeds from the offering for expansion of our production operations in (i) Vietnam including capital equipment,
facilities improvements, and staffing, and, (ii) working capital and general business purposes. The amounts and timing of these
expenditures may vary significantly depending on a number of factors, including the amount of cash generated by our operations,
competitive developments, and the rate of growth, if any, of our business. We may find it necessary or advisable to use portions
of the proceeds for other purposes, and we will have broad discretion in the application of the net proceeds. In addition, while
we have not entered into any agreements, commitments or understandings relating to any significant transaction as of the date
of this prospectus, we may use a portion of the net proceeds to pursue acquisitions, joint ventures, and other strategic transactions.
If we obtain additional financing through the issuance of debt or convertible debt securities, then we may use the net proceeds
of this offering to repay any such indebtedness.
|
Use of Proceeds
|
|
|
|
|
|
|
|
Production Operations Expansion
|
|
$
|
4,520,000
|
|
|
Research and Product Development
|
|
$
|
770,000
|
|
|
Working Capital
|
|
$
|
2,300,000
|
|
|
Corporate Operations
|
|
$
|
462,000
|
|
|
Accrued Expenses
|
|
$
|
204,600
|
|
|
Debt Repayment
|
|
$
|
726,000
|
|
|
Offering Expenses
|
|
$
|
1,000,000
|
|
Although
we believe that the net proceeds from this offering, together with our cash and cash equivalents, and anticipated cash flows from
operating activities will be sufficient to meet our anticipated working capital requirements and capital expenditures in the ordinary
course of business for the next 12 months, we cannot guarantee that this will be the case. We cannot specify with certainty all
of the particular uses for the net proceeds to be received upon the closing of this offering.
DIVIDEND
POLICY
We
have never declared or paid any cash dividends on our capital stock, and we do not currently intend to pay any cash dividends
on our capital stock in the foreseeable future. We currently intend to retain all available funds and any future earnings to support
operations and to finance the growth and development of our business. Any future determination to pay dividends will be made at
the discretion of our Board, subject to applicable laws and will depend upon, among other factors, our results of operations,
financial condition, contractual restrictions and capital requirements. Our future ability to pay cash dividends on our capital
stock may also be limited by the terms of any debt instruments or preferred securities issued in the future.
CAPITALIZATION
The
following table sets forth our capitalization as of June 30, 2020:
|
●
|
on
an actual basis (column 1);
|
|
|
|
|
●
|
on a pro forma basis, to give effect to the issuance
of two shares of Common Stock issuable upon conversion of the Series A preferred stock; (See “Certain Relationships
and Related Transactions”) (column 2);
|
|
●
|
on a pro forma as-adjusted basis to give effect
to the proposed Reverse Stock Split of the outstanding common stock at a 1-for-[__] ratio, the sale of 1,904,762
Units by us in this offering at the assumed public offering price of $5.25, the midpoint of the price range set forth
on the cover page of this prospectus, and after deducting the underwriting discounts and estimated offering expenses payable
by us (column 3).
|
You
should read this capitalization table in conjunction with “Use of Proceeds,” “Selected Consolidated Financial
and Operating Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations”
and the consolidated financial statements and the related notes appearing elsewhere in this prospectus.
As
of June 30, 2020
|
|
|
|
1
|
|
|
|
2
|
|
|
|
3
|
|
|
|
|
|
Actual
(unaudited)
|
|
|
|
Pro
Forma
(unaudited)
|
|
|
|
Pro
Forma
As
Adjusted
(unaudited)
|
|
|
|
|
|
US$
|
|
|
|
US$
|
|
|
|
US$
(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
|
54,710
|
|
|
|
|
|
|
|
|
|
|
Common Stock Class
A, No Par Value
|
|
|
16,757,079
|
|
|
|
|
|
|
|
|
|
|
Common Stock Class
B, No Par Value Stock
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
Preferred Stock Series
A, No Par Value Stock
|
|
|
5,217,800
|
|
|
|
|
|
|
|
|
|
|
Common Stock issuable
|
|
|
22,000
|
|
|
|
|
|
|
|
|
|
|
Additional
paid-in capital
|
|
|
5,107,560
|
|
|
|
|
|
|
|
|
|
|
Statutory reserves
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
Contributed capital
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
Retained earnings/(Losses)
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive
loss
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
Total Stockholders’
Deficit
|
|
|
(6,313,603
|
)
|
|
|
|
|
|
|
|
|
|
Total
capitalization
|
|
|
20,845,546
|
|
|
|
|
|
|
|
|
|
|
|
(1)
|
The as adjusted number of shares to be outstanding immediately after this offering as shown
above is based on shares outstanding as of August 20, 2020. The as adjusted information discussed above is illustrative
only and will be further adjusted based on the actual public offering price and other terms of this offering to be determined
by the Company and the underwriters.
|
DILUTION
If
you invest in our Common Stock, your interest will be diluted immediately to the extent of the difference between the public offering
price per share of common stock that is part of the Unit you will pay in this offering and the as adjusted net tangible
book value per share of our Common Stock immediately after this offering.
As of June
30, 2020, our as-adjusted net tangible book value was approximately $(6,313,603), or $(0.0074764) per share
of Common Stock. Our as-adjusted net tangible book value per share set forth below represents our total tangible assets, less
total liabilities, divided by 844,468,378 the number of shares of our Common Stock outstanding on June 30, 2020
after giving effect to the Reverse Stock Split.
After
giving effect to the Reverse Stock Split and the sale of 1,904,762 Units in this offering at the assumed public
offering price of $5.25 per Unit, the midpoint of the estimated initial public offering price range set forth on the
cover page of this prospectus, after deducting the underwriter discounts and estimated offering expenses payable by us, the
as adjusted net tangible book value as of June 30, 2020 would have been approximately $4,079,053, or $0.17722
per share. This represents an immediate increase in net tangible book value to existing stockholders of $0.456 per
share. The public offering price per share will significantly exceed the net tangible book value per share. Accordingly, new investors
who purchase shares of Common Stock in this offering will suffer an immediate dilution of their investment of $5.076 per
share. We determine dilution by subtracting the net tangible book value per share after the offering from the amount of cash that
a new investor paid for a share of Common Stock.
The
following table illustrates this per share dilution to the new investors, after giving effect to the Reverse Stock Split:
|
Assumed Public offering price
per Unit
|
|
$
|
5.25
|
|
|
Net tangible book value per share as of
June 30, 2020
|
|
|
0.0074764
|
|
|
Increase in net tangible book value per share attributable to the offering
|
|
|
[ ]
|
|
|
As-adjusted net tangible book value per share as of after giving effect to the offering
|
|
|
[ ]
|
|
|
Dilution per share to new investors
|
|
$
|
[ ]
|
|
Each $1.00 increase (decrease) in the
assumed public offering price of $5.25 per Unit (the midpoint of the estimated initial public offering price range set
forth on the cover page of this prospectus) per share would increase (decrease) our pro forma net tangible book value after
this offering by approximately $2 million, or approximately $[ ] per share, and increase (decrease) the
dilution per share to new investors by approximately $[ ] per share, after deducting underwriting discounts
and estimated offering fees and expenses payable by us, and assuming that the number of shares offered by us, as set forth on
the cover page of this prospectus, remains the same. We may also increase or decrease the number of shares we are offering. An
increase (decrease) of 100,000 shares in the number of shares offered by us would increase (decrease) our pro forma net tangible
book value after this offering by approximately $0.5 million, or $[ ] per share, and increase (decrease)
the dilution per share to new investors by approximately $[ ] per share, after deducting underwriting discounts
and estimated offering fees and expenses payable by us, and assuming that the public offering price remains the same. The pro
forma information discussed above is illustrative only and will be adjusted based on the actual public offering price and other
terms of this offering determined at pricing.
If the underwriters exercise their option
to purchase additional shares of Common Stock and/or Purchase Warrants in full, our pro forma net tangible book value per share
after this offering would be $[ ] per share. This amount represents an immediate increase in net tangible book
value of $[ ] per share to our existing stockholders and an immediate dilution in net tangible book value of
$[ ] per share to new investors purchasing shares of our common stock in this offering.
The
following charts illustrate our pro forma proportionate ownership, upon completion of this offering by present stockholders and
investors in this offering, compared to the relative amounts paid by each. The charts reflect payment by present stockholders
as of the date the consideration was received and by investors in this offering at the public offering price. The charts further
assume no changes in net tangible book value other than those resulting from the offering.
|
|
|
Shares
Purchased
|
|
|
Total
Consideration
|
|
|
Average
Price
|
|
|
|
|
Amount
(#)
|
|
|
Percent
(%)
|
|
|
Amount
($)
|
|
|
Percent
(%)
|
|
|
Per
Share ($)
|
|
|
Existing
stockholders
|
|
|
|
(1)
|
|
|
|
%
|
|
|
|
|
|
|
|
%
|
|
$
|
|
|
|
New
investors
|
|
|
1,904,762
|
|
|
|
|
%
|
|
|
|
|
|
|
|
%
|
|
$
|
5.25
|
|
|
Total
|
|
|
|
|
|
|
100.0
|
%
|
|
|
|
|
|
|
100.0
|
%
|
|
$
|
|
|
|
(1)
|
The number of Common
Stock to be outstanding after this offering is based on 854,410,001 shares of Common Stock outstanding as of August, 20, 2020,
and excludes:
|
|
|
●
|
7,440,000 shares
of our Common Stock issuable upon the exercise of stock options outstanding;
|
|
|
●
|
1,904,762 shares
of our Common Stock underlying the Purchase Warrants;
|
|
|
●
|
138,528 shares
of our Common Stock underlying the Representative’s Warrants;
|
|
|
●
|
65,995,920 shares
of our Common Stock underlying any outstanding warrants; and
|
|
|
●
|
0 shares of our
Common Stock issuable upon the conversion of notes and other evidence of indebtedness.
|
The table below
assumes the underwriters’ exercise their over-allotment option, solely into shares of Common Stock, in full:
|
|
|
Shares
Purchased
|
|
|
Total
Consideration
|
|
|
Average
Price
|
|
|
|
|
Amount
(#)
|
|
|
Percent
(%)
|
|
|
Amount
($)
|
|
|
Percent
(%)
|
|
|
Per
Share ($)
|
|
|
Existing
stockholders
|
|
|
[ ]
|
(1)
|
|
|
[ ]
|
%
|
|
|
[ ]
|
|
|
|
|
%
|
|
$
|
|
|
|
New
investors
|
|
|
2,190,476
|
|
|
|
[ ]
|
%
|
|
|
[ ]
|
|
|
|
[ ]
|
%
|
|
$
|
5.25
|
|
|
Total
|
|
|
[ ]
|
|
|
|
100.0
|
%
|
|
|
|
|
|
|
100.0
|
%
|
|
$
|
|
|
|
(1)
|
The number of Common
Stock to be outstanding after this offering is based on 854,410,001 shares of Common Stock outstanding as of August 20, 2020,
and excludes:
|
|
|
●
|
7,440,000 shares of our Common Stock issuable upon the exercise of stock options outstanding;
|
|
|
●
|
1,904,762 shares of our Common Stock
underlying the Purchase Warrants;
|
|
|
●
|
138,528 shares of our Common Stock underlying
the Representative’s Warrants;
|
|
|
●
|
65,995,920
shares of our Common Stock underlying any outstanding warrants; and
|
|
|
●
|
0 shares of our Common Stock issuable upon the conversion of notes and other evidence of indebtedness.
|
MARKET
PRICE AND DIVIDENDS ON OUR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market
Information
Our Common Stock is quoted under the symbol
“KBLB” on the OTCQB. You should be aware that over-the-counter market quotations may reflect inter-dealer prices,
without retail mark-up, mark-down or commissions and may not necessarily represent actual transactions. On the effective date
of the registration statement of which this prospectus forms a part, we expect that trading on Nasdaq or other national
exchange will be under the same symbol. The high and low bid quotations for our shares of our common stock for each full quarterly
period within the two most recent fiscal years and the first fiscal quarter of our current fiscal year are (prices set forth below
represent inter-dealer quotations, without retail markup, markdown or commission and may not be reflective of actual transactions):
The
prices set forth below are NOT adjusted for the effect of the Reverse Stock Split.
|
|
|
High
|
|
|
Low
|
|
|
Fiscal 2018
|
|
|
|
|
|
|
|
|
|
Quarter ended March 31, 2018
|
|
$
|
0.04
|
|
|
$
|
0.03
|
|
|
Quarter ended June 30, 2018
|
|
$
|
0.071
|
|
|
$
|
0.0292
|
|
|
Quarter ended September 30, 2018
|
|
$
|
0.07
|
|
|
$
|
0.05
|
|
|
Quarter ended December 31, 2018
|
|
$
|
0.0691
|
|
|
$
|
0.0428
|
|
|
Fiscal 2019
|
|
|
|
|
|
|
|
|
|
Quarter ended March 31, 2019
|
|
$
|
0.08
|
|
|
$
|
0.05
|
|
|
Quarter ended June 30, 2019
|
|
$
|
0.481
|
|
|
$
|
0.0629
|
|
|
Quarter ended September 30 2019
|
|
$
|
0.49
|
|
|
$
|
0.19
|
|
|
Quarter ended December 31, 2019
|
|
$
|
0.23
|
|
|
$
|
0.18
|
|
|
Fiscal 2020
|
|
|
|
|
|
|
|
|
|
Quarter ended March 31, 2020
|
|
$
|
0.299
|
|
|
$
|
0.1055
|
|
|
Quarter ended June 30, 2020
|
|
$
|
0.2999
|
|
|
$
|
0.12
|
|
|
July 1, 2020 to August 20, 2020
|
|
$
|
0.2031
|
|
|
$
|
0.126
|
|
As of August 20, 2020, the
last reported sale price of our Common Stock on the OTCQB was $0.155 per share.
Holders
As of August 20, 2020, we had 32
holders of record of our Common Stock and 1 holder of our Series A Preferred Stock. The holders of Common Stock are entitled to
one vote for each share held of record on all matters submitted to a vote of stockholders. Following his agreement to reduce
the voting power of the Series A Preferred Stock, which will not take effect until the Reverse Stock Split is effective, and on
a post-Reverse Stock Split basis, the holder of Series A Preferred Stock is entitled to [ ] votes for each
share held of record on all matters upon which the Company’s stockholders may vote. Holders of the Common Stock have no
preemptive rights and no right to convert their Common Stock into any other securities; each share of Series A Preferred Stock
is convertible into one share of Common Stock at the holder’s option. There are no redemption or sinking fund provisions
applicable to the Common Stock.
Dividends
We
have never declared or paid any cash dividend on our capital stock, and we do not currently intend to pay any cash dividends on
our capital stock in the foreseeable future. We currently anticipate that we will retain future earnings to support reinvestments
in our business and/or to repay outstanding debt from time to time. Any payment of cash dividends in the future will be at the
discretion of our Board and will depend upon, among other things, future operating earnings and cash flows, future capital requirements,
contractual restrictions (including those contained in the agreements and instruments governing our debt and the certificates
of designation of our convertible preferred stock) and general business conditions.
Securities
Authorized for Issuance under Equity Compensation Plans
The
following table discloses information as of the date of this prospectus, with respect to compensation plans (including individual
compensation arrangements) under which our equity securities are authorized for issuance, aggregated as follows:
Equity
Compensation Plan Information
|
Plan
category
|
|
Number
of securities to be issued upon exercise of outstanding options, warrants and rights
|
|
|
Weighted-average
exercise price of outstanding options, warrants and rights
|
|
|
Number
of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column
(a))
|
|
|
|
|
(a)
|
|
|
(b)
|
|
|
(c)
|
|
|
Equity compensation plans
approved by security holders
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
Equity compensation plans not approved
by security holders
|
|
|
27,440,000
|
|
|
|
0
|
|
|
|
69,449,360
|
|
|
Total
|
|
|
27,440,000
|
|
|
|
0
|
|
|
|
69,449,360
|
|
2019
Employee Stock Option Plan
Effective
December 9, 2019, we adopted the 2019 Employee Stock Option Plan (“Plan”), with 80,000,000 shares issuable pursuant
to the Plan. Beginning on January 1, 2020 and continuing on each January 1st that the Plan is in place, an additional
number of shares equal to the lesser of: (i) 2% of the number of shares of Common Stock outstanding (fully-diluted) on the immediately
preceding December 31 and (ii) such lower number of shares as may be determined by the Board or committee, shall be added to the
number of shares issuable under the Plan. As of the date of this prospectus, 27,440,000 options have been issued pursuant to the
Plan and 69,449,360 shares remain issuable pursuant to the Plan, based on the terms of the Plan as set forth above.
Eligibility. The
Plan provides for the grant of incentive stock options to our employees and any parent and subsidiary corporations’ employees
and for the grant of nonqualified share options, restricted shares, restricted share units, share appreciation rights, share bonuses
and performance awards to our employees, directors and consultants and our parent and subsidiary corporations employees and consultants.
Administration. The
Plan is administered by the Board or by a committee of not fewer than 2 members, each of whom is an outside Director and all of
whom are disinterested, designated by the Board to administer the Plan. The plan administrator determines the terms of all awards.
Types
of Awards. The Plan allows for the grant of nonqualified stock options, incentive stock options, restricted share options,
restricted stock units, stock appreciation rights, stock bonuses and performance awards.
Award
Agreements. All awards under the Plan are evidenced by an award agreement which shall set forth the number of shares
subject to the award and the terms and conditions of the award, which shall be consistent with the Plan.
Term
of Awards. The term of awards granted under the Plan is ten years.
Vesting
Schedule and Price. The plan administrator has the sole discretion in setting the vesting period and, if applicable,
exercise schedule of an award, determining that an award may not vest for a specified period after it is granted and accelerating
the vesting period of an award. The plan administrator determines the exercise or purchase price of each award, to the extent
applicable.
Transferability. Unless
the plan administrator provides otherwise, the Plan does not allow for the transfer of awards other than by will or the laws of
descent and distribution. Unless otherwise permitted by the plan administrator, options may be exercised during the lifetime of
the optionee only by the optionee or the optionee’s guardian or legal representative.
Adjustments. In
the event the Board or committee determines that any dividend or distribution, recapitalization, stock split, reorganization,
merger, consolidate, split-up, spin-off, or other similar corporate transact or event affects the shares subject to the Plan such
that an adjustment is determined by the Board or committee to be appropriate to prevent dilution or enlargement of the benefits
intended to be made under the Plan, appropriate adjustments will be made to the share maximums and exercise prices, as applicable.
Governing
Law and Compliance with Law. The Plan and awards granted under it are governed by and construed in accordance with the
laws of the Wyoming. Shares will not be issued under an award unless the issuance is permitted by applicable law.
Amendment
and Termination. The Plan terminates ten years from the date it was approved, unless it is terminated earlier by our
Board. The Board may amend, alter, suspend, discontinue, or terminate the plan, including, without limitation, any amendment,
alternation, suspension, discontinuation, or termination that would impart the rights of any participant, or any other holder
or beneficiary of any award thertofore granted, without the consent of any share owner, participant, other holder or beneficiary
of an award, or other person, unless required by applicable law.
BUSINESS
Overview
Kraig
Biocraft Laboratories, Inc. is a corporation that was incorporated under the laws of Wyoming on April 25, 2006. The inventor of
our technology concept, Kim Thompson, is the founder of Kraig Biocraft Laboratories, Inc. and serves as our Chief Executive Officer,
Chief Financial Officer and is currently our sole director. We develop high strength fibers using recombinant DNA technology for
commercial and defensive applications in both the specialty fiber and technical textile industries. We use genetically engineered
silkworms that produce spider silk to create our recombinant spider silk. Our proprietary fibers include natural and engineered
variants of spider silk produced in domesticated mulberry silkworms. Our business brings twenty-first century biotechnology to
the historical silk industry, permitting us to introduce materials with innovative properties and claims into an established commercial
ecosystem of silkworm rearing, silk spinning and weaving, and the manufacturing of garments and other products that can include
our specialty fibers and textiles.
We
believe our genetic engineering capabilities will allow us to develop specialized spider silk fibers with greater strength, resiliency
and flexibility than traditional silks. Target markets for these materials are expected to be in traditional textiles and technical
textile industries, including performance textiles and specialty fibers. Moreover, our technologies are “green” since
fibers and textiles do not require petroleum inputs and our production processes are traditional and eco-friendly.
One
key market for our products is the technical textile market for specialty fibers. Specialty fibers are engineered for specific
uses that require exceptional strength, flexibility, heat resistance and/or chemical resistance. The specialty fiber market is
currently dominated by two synthetic fiber products that come from petroleum derivatives: (i) aramid fibers, and (ii) ultra-high
molecular weight polyethylene fibers. The technical textile industry involves products for both industrial and consumer products,
such as filtration fabrics, medical textiles (e.g., sutures and artificial ligaments), safety and protective clothing and
fabrics used in military and aerospace applications (e.g., high-strength composite materials). Our recombinant spider silk
fibers offer a tougher material which is a biodegradable and sustainable alternative to those existing synthetic petroleum derivative
fibers.
The
Products
To
date, our focus has been on understanding and exploiting the unique characteristics of spider silk, specifically dragline silk
from Nephila clavipes (golden orb-web spider) and variants thereof. Such fibers possess unique mechanical properties in
terms of strength, resilience and flexibility. Natural spider silk and other high strength natural proteins are made of repeating
blocks of simple amino-acids. Through the process of genetic engineering we customize these proteins to generate materials with
tailored physical properties.
We
are currently in the process of scaling up production of our two leading products, Monster Silk® and Dragon SilkTM
at our production facility located in Vietnam.
Monster
Silk®
Monster
Silk® was the first recombinant spider silk fiber product that we developed. Monster Silk incorporates the natural elasticity
of spider silk to make a silk fiber which is more flexible that conventional silk fibers and textiles. We have produced sample
products using Monster Silk® including knit fabrics, gloves, and shirts in collaboration with textile mills. We expect that
Monster Silk® will have market applications across the traditional textile markets where its increased flexibility will provide
increased durability and comfort.
Dragon
SilkTM
Dragon
SilkTM is the next evolution in recombinant spider silk, combining the elasticity of Monster Silk® with additional
high strength elements of native spider silk. Some samples of Dragon SilkTM have demonstrated strength beyond that
of native spider silk. This combination of strength and elasticity result in a silk fiber which is soft and flexibly, yet tougher
than any synthetic fiber available on the market. Based on inquires we have received from end market leader, we believe that Dragon
SilkTM- will have applications in performance apparel, durable workwear, luxury goods and apparel, and composites.
We
are establishing relationships with key market leaders in several end markets and are working to establish exclusive
development and sales agreements. For example, we have signed a non-binding, memorandum of understanding with Polartec, LLC (“Polartec”),
a leader in high performance non-woven fabrics to develop applications for defensive and protective textiles. Polartec announced
a commitment to using 100% biodegradable and recycled materials throughout its product line, and we believe that our engineered
“green” silk can contribute to Polartec’s announced goal of fully recycled and biodegradable fleece, knits,
fills, and fabric. We are currently in discussions with other end market product manufactures to secure sales and development
agreements and hope to begin delivering product and generating revenue in 2020.
On
April 16, 2020, we announced that we successfully developed a new technology platform, based on a non-CRISPR gene editing knock-in
knock-out technology. This is the first knock-in knock-out technology essentially pure spider silk transgenic in our history.
The new technology, which is the result of over ten years of effort, hits the target of one of our primary technological goals
and what we believe opens the door for large scale U.S. production. Other than the silkworm’s remaining specifically desired
native silk protein elements, we are now able to produce nearly pure spider silk. Based on our internal studies, the new technology
has a purity rate that is about ten times greater than Dragon Silk, a fiber that we developed with our previous tools. Based on
our internal testing, Dragon Silk has demonstrated to be tougher than many fibers used in bullet proof vests and we expect that
the increased spider silk purity, created using this new approach, will yield materials beyond those capabilities. This new system
utilizes our eco-friendly and cost-effective silkworm production system, which we believe is more advanced than any of the competing
methods. We have already begun the validation process for the first of these new transgenics and anticipate that U.S. production
will be possible as early as 2022 or 2023.
Our
Technology
Our
technology builds upon the unique advantages of the domesticated silkworm. The silkworm is an efficient commercial and industrial
producer of protein based polymers, and forty percent (40%) of the caterpillars’ weight is devoted to the silk glands. The
silk glands produce large amounts of an insoluble protein called fibroin, which the silkworm spins into a composite protein thread
(silk).
We
use our genetic engineering technology to create proprietary recombinant silk polymers from the silkworms. On September 29, 2010,
we, along with our collaborators at Notre Dame created approximately twenty different strains of transgenic silkworm which produce
recombinant silk polymers. In April 2011, we entered into a licensing agreement with Sigma-Aldrich which provides us the use of
Sigma-Aldrich’s zinc finger technology to accelerate and enhance our product development. In October of 2017, with the support
of funding from the U.S. Army, we transitioned our research operations out of Notre Dame and into our own research and development
headquarters.
Our
transgenic silkworms are created by inserting the genes expressing spider silk with either natural or engineered amino acid sequences
into the embryos of the silkworm. The spider silk sequence is introduced to the embryo of the silkworm and incorporated into the
silkworm genome using state of the art molecular biology approaches. The spider sequence is created on a circular loop of DNA
called a plasmid. We developed a method to alter the plasmid DNA to more readily allow the mixing and matching of various spider
DNA genes. In this way, we can combine different spider genetic cassettes to create a fiber with the desired physical and mechanical
properties more rapidly than through conventional methods.
In
addition to this ability to easily mix and match DNA construction, we have also adapted new approaches to accelerate the rate
we generate new transgenic silkworms. Our initial approaches limited us to process silkworm eggs at a rate of roughly 50-200 a
day, however, we have developed a new approach which allows us to process thousands of eggs a day. Utilizing both visual and non-visual
genetic markers, we have successfully developed methods to speed up the screening of potentially transgenic silkworms, which allows
for rapid screening of transgenic eggs. The eggs expressing the new spider silk constructs are propagated while the eggs without
the visual marker are discarded, greatly increasing the efficiency of our screen for transgenic eggs. This new approach has been
highly effective in increasing the rate of development for new transgenics. We have employed this new procedure and have filed
provisional patent applications to protect its use.
We
utilize the latest advancements in molecular biology and genetic engineering to deliver targeted gene incorporations. The new
constructs are designed to integrate in the silkworm genome directly where the native silkworm silk is created. First made public
in April 2020, this new capability allows for the full knock out and knock in replacement of the native silkworm heavy chain silk
protein. We believe that this increased expression and incorporation of the spider protein into the silkworm cocoon leading to
increased performance and open the door for additional opportunities beyond fibers and textiles.
Comparison
of the Properties of Spider Silk and Steel
|
|
|
Material Toughness (1)
|
|
Tensile Strength (2)
|
|
Weight
(3)
|
|
|
Dragline spider silk
|
|
120,000-160,000
|
|
1,100-2,900
|
|
|
1.18-1.36
|
|
|
Aramid Fibers
|
|
30,000-50,000
|
|
2,600-4,100
|
|
|
1.44
|
|
|
Steel
|
|
2,000-6,000
|
|
300-2,000
|
|
|
7.84
|
|
|
1
|
Measured
by the energy required to break a continuous filament, expressed in joules per kilogram (J/kg). A .357 caliber bullet has
approximately 925 joules of kinetic energy at impact.
|
|
2
|
Tensile
strength refers to the greatest longitudinal stress the fiber can bear, measured by force over area in units of newtons per
square meter. The measurement here is in millions of pascals.
|
|
3
|
In
grams per cubic centimeter of material.
|
This
comparison table was the result of research performed by Randolph Lewis, Ph.D. at the University of Wyoming. Such work was summarized
in an article entitled “Spider Silk: Ancient Ideas for New Biomaterials” which was published in Chemicals Review,
volume 106, issue 9, pages 3672–3774. The measurements in joules in the table above are a conversion from Dr. Lewis’
measurements in newtons/meter squared.
We
believe that the genetically engineered protein-based fibers we currently produce have properties that are superior to the materials
currently available in the marketplace, including but not limited to, aramid fibers, ultra-high molecular weight polyethylene,
and steel. For example, as noted above, the ability of spider silk to absorb in excess of 100,000 joules of kinetic energy per
kilogram makes it a potentially ideal material for structural blast protection.
Production
of this material in commercial quantities holds the potential of a life-saving ballistic resistant material, which is lighter,
thinner, more flexible, and tougher than steel. However, the Company does not currently have any life-saving ballistic products
and could be some time before we are able to produce such a product from the material. Other applications for spider silk based
recombinant fibers include use as structural material and for any application in which light weight and high strength are required.
We believe that fibers made with recombinant protein-based polymers will make significant inroads into the specialty fiber and
technical textile markets. Our interactions with manufacturers of high performance textiles, including Polartec and other leaders
in performance textiles, convince us that there is an eager commercial market for our innovative, sustainable, and differentiated
technology and products.
Manufacturing
Our
spider silk technology is designed for easy plug and play incorporation into the existing silk production model. We manufacture
and plan to continue to manufacture our proprietary spider silk fibers using traditional silkworm production practices (sericulture).
Over
several years, we sought a license for an Enterprise Registration Certificate (“ERC”) in order to begin operations
in Quang Nam Province, Vietnam, an important region for sericulture. In May 2018, we announced that we were granted an ERC, and
thereafter formed a subsidiary, Prodigy Textiles, Co., Ltd. (“Prodigy Textiles”) to implement our Vietnam business.
In June 2018, we announced that we had entered collaborative agreements with several silk farming cooperatives in Quang Nam Province,
Vietnam, and that these cooperatives had begun planting mulberry, the key production input for our technology; in July 2018, we
celebrated the grand opening of Prodigy Textiles’ facility in Quang Nam province. In December 2018, we made the first shipment
of our specialized silkworm to Vietnam to conduct trial rearing and to demonstrate their safety. In October 2019, we delivered
the first batch of production silkworms and began operations. These silkworms will serve as the basis for the commercial expansion
of our proprietary silk technology. On November 4, 2019, we reported that we successfully completed rearing the first batch of
its transgenic silkworms at the Quang Nam production factory. Seasonal challenges in late December 2019 slowed production operations,
however, the Company expects to resume expansion of the production of its specialized silk through 2020, so long as Vietnam does
not impose any restrictions again due to Covid-19 or other reasons and so long as U.S. travel restrictions do not prevent us from
shipping eggs to Vietnam, which is out of our control.
We
contract local farmer and farming cooperatives to provide fresh mulberry for our operations. Prodigy Textiles has hired local
workers with experience in sericulture production to care for and raise our silkworms through the five instars, or stages, of
the silkworm life cycle, including the final instar when the mature caterpillars produce a cocoon comprised of pure silk. These
cocoons are then reeled to our specifications to form the final recombinant spider silk threads such as Dragon SilkTM
and Monster Silk®.
By
utilizing existing production methodology in traditional silk regions to produce our high performance materials, we leverage historical
knowledge, available labor and existing capital infrastructure for production, spinning, and weaving of our recombinant spider
silk materials. This approach reduces the risk to our manufacturing operations and decreases our need for upfront capital expenditure.
We
believe that we will be able to target a capacity of 40 metric tons of recombinant spider silk fiber per annum from this factory
once it reaches maximum utilization. This capacity will allow us to address our anticipated initial demand for applications in
the protective, performance, and luxury textile markets from Polartec and other companies with whom we have a relationship, but
which we cannot disclose due to non-disclosure agreements.
Our
long-term goal for Prodigy Textiles is to create a research center for development of our specialized silk, to contract with local
farming cooperatives to grow upwards of 2,500 hectares of mulberry (which would allow for production of up to 250 metric tons
of our high strength silk per year), and to serve as our principal manufacturing center.
In addition, in light of the significant
uncertainty regarding the impact of the COVID-19 pandemic, on March 19, 2020, we furloughed non-essential staff consistent with
leading health official recommendations in order to help prevent the spread of COVID-19. This decision was made in an abundance
of caution and will primarily impact staff at our fully owned subsidiary, Prodigy Textiles, in Vietnam and will result in the
temporary closing of silk rearing operations at that facility. On July 1, 2020 Prodigy Textiles staff returned to the factory
to clean, sterilize, and prepare the facility for restarting operations. Silk production operations will resume as soon as the
next shipment of silkworms eggs can be delivered from the Company’s U.S. headquarters, which is out of our control and we
cannot estimate when that will occur. The global pandemic of COVID-19 continues to evolve rapidly, and we will continue to
monitor the situation closely, including its potential effect on our plans and timelines. See, “Management’s Discussion
and Analysis of Financial Condition and Results of Operations – Impact of COVID-19 Outbreak.”
The
Market
We
are focusing our work on the creation of new fibers with innovative properties including fibers with potential high performance
and technical fiber applications for the technical textiles market, including protective textiles. The protective textiles market
is currently dominated by two classes of product: (i) aramid fibers, and (ii) ultra-high molecular weight polyethylene fibers.
These existing products serve the need for materials with high strength, resilience, but are unable to delivery flexibility. Because
these synthetic performance fibers are stronger and tougher than steel, they are used in a wide variety of military, industrial,
and consumer applications.
The
military and police are among the users of protective textiles for its ballistic protection. The materials are also used for industrial
applications requiring superior strength and toughness, e.g., critical cables and abrasion/impact resistant components. Performance
fibers are also employed in safety equipment, high strength composite materials for the aero-space industry and for ballistic
protection by the defense industry.
The
global market for technical textiles was estimated at greater than $234 billion in 2017 and projected to reach nearly $335 billion
by 2025.
These
are industrial materials which have become essential products for both industrial and consumer applications. The market for technical
textiles can be defined as consisting of:
|
●
|
Medical
textiles;
|
|
●
|
Geotextiles;
|
|
●
|
Textiles
used in Defense and Military;
|
|
●
|
Safe
and Protective Clothing;
|
|
●
|
Filtration
Textiles;
|
|
●
|
Textiles
used in Transportation;
|
|
●
|
Textiles
used in Buildings;
|
|
●
|
Composites
with Textile Structure; and,
|
|
●
|
Functional
and Sportive Textiles.
|
We
believe that the superior mechanical characteristics of the next generation of protein-based polymers (in other words, genetically
engineered silk fibers), will open up new applications for the technology. The materials which we are working to produce are many
times tougher and stronger than steel.
We
are actively pursuing relationships within target end markets to secure product collaborations with key market channel leaders.
Due to the unique nature of our product, we received numerous unsolicited requests from leading businesses across a range of attractive
end markets requesting materials for applications development. This substantial demand for spider silk materials across the broad
spectrum of applications for high performance fibers and textiles, combined with the limited initial production capacity, has
provided the opportunity to be selective in choosing market channel partners best able to quickly bring our product to market
at scale. We are working under non-disclosure agreements to secure these collaborative development agreements and to establish
limited channel exclusivity for firms we believe mirror our culture of innovation. With recent advancements in our manufacturing
capacity, we expect to generate revenues from these relationships in 2020.
Research
and Development
In
2007, we entered into the first series of the Notre Dame Agreements to develop new transgenic silkworms. On September 29, 2010,
we announced that we had achieved our longstanding goal of producing new silk fibers composed of recombinant proteins. In 2016,
we received a contract from the U.S. Army to deliver the first samples of its recombinant spider silk materials. In 2017, this
contract was expanded to include research into the development of stronger silk materials. As a result of that contract, the Company
brought its research operations in-house, opening its own research laboratories and expanding its scientific staff. This transition
to in-house operations has led to a series of new technical breakthroughs and is believed to have accelerated the pace of new
development. We intend to turn its technology to the development and production of high performance polymers.
During
the fiscal years ended December 31, 2019 and 2018, we have spent approximately 12,450 hours and 13,160 hours, respectively, on
research and development activities, which consisted primarily of laboratory research on genetic engineering by our in-house research
operations.
As
of the date of this Registration Statement, our research and development efforts remain focused on growing our internal capabilities,
but we may consider renewing funding of the collaborative research and development of high strength polymers with Notre Dame or
other joint development opportunities; we have not had any formal discussions regarding any such collaborations.
We
have initiated commercial scale production of our recombinant materials including Monster Silk® and Dragon SilkTM.
Additionally, we plan to accelerate both our microbiology and selective breeding programs, as well as providing more resources
for their material testing protocols in 2020.
Our
Intellectual Property Approach
Our
intellectual property strategy utilizes a blended approach of licensed technologies and in-house developments. As part of our
intellectual property portfolio, we have licensed the exclusive right to use certain patented spider silk gene sequences in silkworm.
Under the exclusive license agreement with the University of Wyoming (the “Exclusive Licensing Agreement”), we have
obtained certain exclusive rights to use numerous genetic sequences which are the subject of U.S. patents.
Under
the Notre Dame Agreements, we were issued and exercised our right to exclusive commercial use for spider silk technologies developed
under that agreement. We have worked collaboratively with the university to develop fibers with the mechanical characteristics
of spider silk. We are applying this proprietary genetic engineering technology to domesticated silkworms, which to our knowledge,
is the only proven commercially scaled system for producing silk.
In
2017, we opened a research and development facility to expand on the work conducted at Notre Dame. Since opening this new facility,
we have expanded our intellectual property portfolio with six additional provisional patent filings based on new discoveries and
inventions and made numerous advancements that have decreased the development time for new technologies, none of which rely on
the patented material from our collaboration with Notre Dame. We will continue to utilize this in-house research facility to expand
and strengthen its patent portfolio while also maintaining and growing its trade secret technologies approach to genetic advancement.
We are actively working to develop and patent new approaches to the development of genetically engineering silkworms, underlying
construction techniques, and fundamental genetic sequences for improved material performance.
The
Notre Dame Agreements will last for the duration of the patented materials that we developed with Notre Dame. The new technologies
that we are developing in our internal research labs does not rely on the Notre Dame patented materials and as a result will not
be impacted by an expiration of those agreements.
The
introduction of the gene sequence, in the manner employed by us, results in a germline transformation and is therefore self-perpetuating.
License
Agreements/Intellectual Property
We
have obtained certain rights to use a number of university created, and patented, spider silk proteins, gene sequences and methodologies.
As
part of the Notre Dame Agreements, we exercised our option for the exclusive commercial rights to technologies derived from a
family of patent applications filed in various jurisdictions worldwide. As of the date of this Registration Statement, two patents
have been issued, number 10-1926286 in South Korea and number 2011314072 in Australia. These locations are not currently silk
producing locations, however, we believe protecting our technologies in these locations will be beneficial to our future operations.
In
addition to the patents related to licensed technologies from Notre Dame listed above, we have filed six provisional patent applications
based on technologies developed and discovered as a result of its independent research operations.
Table
of Patent Applications and Status
|
Title
|
|
Country
|
|
Application
No.
|
|
Filing
Date
|
|
Patent
No.
|
|
Patent
Date
|
|
Status*
|
|
Chimeric
Spider Silk and Methods of Use Thereof
|
|
United
States of America
|
|
16/221267
|
|
14-Dec-2018
|
|
|
|
|
|
Published
|
|
Transgenic
Silkworms Capable of Producing Chimeric Spider Silk Polypeptides and Fibers
|
|
United
States of America
|
|
16/246318
|
|
11-Jan-2019
|
|
|
|
|
|
Published
|
|
Transgenic
Silkworms Capable of Producing Chimeric Spider Silk Polypeptides and Fibers
|
|
United
States of America
|
|
16/275159
|
|
13-Feb-2019
|
|
|
|
|
|
Published
|
|
A
chimeric spider silk polypeptide, composite fiber comprising the polypeptide and method of making a chimeric spider silk fiber
|
|
Vietnam
|
|
1-2013-01306
|
|
25-Apr-2013
|
|
|
|
|
|
Under
Exam
|
|
Chimeric
Spider Silk and Uses thereof
|
|
Australia
|
|
2011314072
|
|
26-Apr-2013
|
|
2011314072
|
|
13-Jul-2017
|
|
Granted
|
|
Chimeric
Spider Silk and Uses thereof
|
|
Australia
|
|
2019201497
|
|
05-Mar-2019
|
|
|
|
|
|
Pending
|
|
Chimeric
Spider Silk and Uses thereof
|
|
Brazil
|
|
BR112013007247-4
|
|
27-Mar-2013
|
|
|
|
|
|
Under
Exam
|
|
Chimeric
Spider Silk and Uses thereof
|
|
Canada
|
|
2812791
|
|
28-Sep-2011
|
|
|
|
|
|
Under
Exam
|
|
Chimeric
Spider Silk and Uses thereof
|
|
China
(People’s Republic)
|
|
201180057127.1
|
|
28-May-2013
|
|
|
|
|
|
Pending
|
|
Chimeric
Spider Silk and Uses thereof
|
|
China
(People’s Republic)
|
|
201710335250.4
|
|
12-May-2017
|
|
|
|
|
|
Published
|
|
Chimeric
Spider Silk and Uses thereof
|
|
China
(People’s Republic)
|
|
2018110261070.8
|
|
04-Sep-2018
|
|
|
|
|
|
Pending
|
|
Chimeric
Spider Silk and Uses thereof
|
|
European
Patent Convention
|
|
11833071.1
|
|
26-Apr-2013
|
|
|
|
|
|
Under
Exam
|
|
Chimeric
Spider Silk and Uses thereof
|
|
India
|
|
3574/DELNP/2013
|
|
22-Apr-2013
|
|
|
|
|
|
Under
Exam
|
|
Chimeric
Spider Silk and Uses thereof
|
|
Japan
|
|
2013-530432
|
|
26-Mar-2013
|
|
|
|
|
|
Pending
|
|
Chimeric
Spider Silk and Uses Thereof
|
|
Japan
|
|
2019-142869
|
|
02-Aug-2019
|
|
|
|
|
|
Pending
|
|
Chimeric
Spider Silk and Uses thereof
|
|
Korea,
Republic of
|
|
10-2017-7005086
|
|
22-Feb-2017
|
|
10-1926286
|
|
30-Nov-2018
|
|
Granted
|
|
Chimeric
Spider Silk and Uses thereof
|
|
Korea,
Republic of
|
|
10-2018-7034773
|
|
30-Nov-2018
|
|
|
|
|
|
Under
Exam
|
|
Method
of producing auto-assembling high molecular weight proteins
|
|
United
States of America
|
|
Provisional
|
|
23-May-19
|
|
|
|
|
|
Pending
|
|
Transgenic
Silkworm Capable of Sustaining Non-Mulberry Diet
|
|
United
States of America
|
|
Provisional
|
|
23-May-19
|
|
|
|
|
|
Pending
|
|
Non-invasive
genetic screening method for Bombyx Mori and other molting caterpillars
|
|
United
States of America
|
|
Provisional
|
|
23-May-19
|
|
|
|
|
|
Pending
|
|
Method
for creating high molecular weight proteins using auto-assembly in Bombyx Mori
|
|
United
States of America
|
|
Provisional
|
|
23-May-19
|
|
|
|
|
|
Pending
|
|
Method
of producing non-native proteins in Bombyx Mori
|
|
United
States of America
|
|
Provisional
|
|
23-May-19
|
|
|
|
|
|
Pending
|
|
Method
for the genetic removal and replacement of heavy chain fibroin of Bombyx Mori
|
|
United
States of America
|
|
Provisional
|
|
19-Feb-20
|
|
|
|
|
|
Pending
|
*
The terms in this column have the following meanings:
Published:
Pending patent applications that have been published by a corresponding state Patent Office (e.g., the U.S. Patent and Trademark
Office) or international patent authority (e.g., the World Intellectual Property Association).
Pending:
Patent applications that have been submitted to a corresponding state Patent Office for examination but that have not been issued
or abandoned.
Under
Exam: Pending patent applications currently being examined by a corresponding state Patent Office.
Granted:
Patent applications that have been allowed by a corresponding state Patent Office and that have passed through the registration
process; a granted patent application is synonymous with a “patent” and is conferred the associated patent rights
for the given jurisdiction.
In
addition to patent protection for intellectual property developed by the Company and through its collaborative research agreements,
the Company has developed specialized skills and knowledge in the field of selective breeding, performance selection, and husbandry.
This information is considered to be trade secrets and will play a critical role in the development of unique strains of new transgenic
with diverse mechanical properties. These operations and knowledge held as trade secrets provide an additional layer of security
and protection for the products and technologies we seek to develop.
In
2014, the following six trademarks were issued to the Company; the Company shall use these trademarks for product branding in
the future:
|
Marks
|
|
|
|
Monster
SilkTM
|
|
SpiderpillarTM
|
|
SpilkTM
|
|
Monster
WormTM
|
|
Spider
WormTM
|
|
Spider
MothTM
|
Notre
Dame Agreements
As
discussed above, in 2007 we entered into the first series of Notre Dame Agreements. We provide financial support to ongoing research
and development of transgenic silkworms and the creation of recombinant silk fibers. In exchange, we have an option to obtain
the exclusive global commercialization rights to the technology developed pursuant to the research effort.
Following
the first agreement, we entered into successive intellectual property and collaborative research agreements with Notre Dame to
provide different levels of financial support. The trend has been for an increase in financial support for the research and development
in nearly every successive agreement. In June 2012, we entered into an Intellectual Property / Collaborative Research Agreement
with Notre Dame (“2012 Notre Dame Research Agreement”). On March 4, 2015, we entered into a new Intellectual Property
/ Collaborative Research Agreement with Notre Dame extending the agreement through March 2016 (“2015 Notre Dame Research
Agreement”). Under the 2015 Notre Dame Research agreement, the Company provided approximately $534,000 in financial support.
On September 20, 2015, the 2015 Notre Dame Research Agreement was amended to increase the total funding by approximately $179,000;
in February 2016, the 2015 Notre Dame Research Agreement was extended to July 31, 2016 and in August 2016, the 2015 Notre Dame
Research Agreement was extended to December 31, 2016. In May 2017, the 2015 Notre Dame Research Agreement was amended to increase
the total funding by approximately $189,000 and the duration of the 2015 Notre Dame Research Agreement was extended to September
30, 2017. With the funding we received from the U.S. Army, we were able to conduct our research and development in-house, at less
cost, and therefore we did not extend the 2015 Notre Dame Research Agreement after September 30, 2017, but in the future we may
consider forming new collaborative research agreements.
In
2011, we exercised our option to obtain the global commercialization rights to the technology developed under the Notre Dame Agreements,
which resulted in a separate license agreement with Notre Dame (the “2011 Notre Dame Agreement”). Pursuant to the
2011 Notre Dame Agreement, Notre Dame filed an international patent application and numerous national patent applications on technology
relating to the creation and use of recombinant spider silks and we received exclusive and non-exclusive rights to certain spider
silk technologies including commercial rights with the right to sublicense such intellectual property. The 2011 Notre Dame Agreement
obligates us to reimburse Notre Dame for costs associated with the filing, prosecuting and maintaining of such patents and patent
applications. In exchange for the rights to commercialization, Notre Dame has received 2,200,000 shares of our Common Stock
and we have agreed to pay Notre Dame royalties equal to 2% of our gross sales of the licensed products and 10% of any sublicensing
fees received by the Company on licensed technology. We have also agreed to pay to Notre Dame $50,000 a year, which will be reduced
from the total amount of royalties paid in the same year. The $50,000 payment to Notre Dame is not owed for any year in which
the Company is sponsoring research within Notre Dame.
Exclusive
License Agreement with the University of Wyoming
In
May 2006, we entered into a license agreement with the University of Wyoming, pursuant to which we have licensed the right to
commercialize the production by silkworms of certain synthetic and natural spider silk proteins and the genetic sequencing for
such spider silk proteins. These spider silk proteins and genetic sequencing are covered by patents held by the University of
Wyoming. Our license allows us only to use silkworms to produce the licensed proteins and genetic sequencing. We have the right
to sublicense the intellectual property that we license from the University of Wyoming. Our license agreement with the University
of Wyoming required that we pay licensing and research fees to the university in exchange for an exclusive license in our field
of use for certain university-developed intellectual property including patented spider silk gene sequences. Pursuant to the agreement,
we issued 17,500,000 shares of our Common Stock to the University Foundation. Our license agreement with the University of Wyoming
was to continue until the later of (i) expiration of the last-to-expire patent we license from the University of Wyoming under
this license agreement in such country or (ii) ten years from the date of first commercial sale of a licensed product in such
country. There are no royalties payable to the University of Wyoming under the terms of our agreement with them. We anticipate
making arrangements with the University of Wyoming to address any accrued or unpaid fees which may exist.
Cooperative
Agreement in Vietnam
On
December 30, 2015, we entered into a cooperative agreement with a provincial government office in Vietnam for the research and
pilot production of hybrid silkworms. In April 2018, we received our investment registration certificate for our facility in Vietnam.
On May 1, 2018, we were issued our ERC so that we can begin our operations in Vietnam. We have established a subsidiary in Vietnam
where we will develop and produce hybrid silkworms. Management believes the ERC puts the Company on a path to scale at a much
greater level by harnessing existing silk production infrastructure with the capacity to match the existing demand for their spider
silk materials.
Other
Agreements
On October 15, 2013, we entered into an intellectual
property agreement with a scientific researcher relating to the development of new recombinant silk fibers. Under the terms of
that agreement, the scientific researcher transferred his rights of intellectual property, inventions and trade secrets which
the researcher develops relating to recombinant silk to us. Upon signing, the researcher received 8,000,000 common stock purchase
warrants from the Company, exercisable 24 months from the date of the agreement. As per the terms of the agreement, the researcher
received an additional 10,000,000 warrants after creating a new recombinant silk fiber for us that met specified performance
characteristics and another 8,000,000 warrants for performing the contract in good faith. The warrants described above all contain
a cashless exercise provision and are exercisable on the 24-month anniversary of the date on which they were issuable under the
agreement.
Governmental
Regulations
We
are subject to U.S. federal, state and local laws and regulations, as well as Vietnam central, provisional, and district laws
and regulations. These laws and regulations govern, among other things, labor relations, the labeling and safety of the products
we sell, the methods we use to sell these products and/or the production of the products we sell. We believe that we are in material
compliance with all such applicable laws and regulations, although no assurance can be provided that this will remain true in
the future.
Environment
We
seek to comply with all applicable statutory and administrative requirements concerning environmental quality. Expenditures for
compliance with federal state and local environmental laws have not had, and are not expected to have, a material effect on our
capital expenditures, results of operations or competitive position.
While
being environmentally conscious is the objective of all producers in this industry, the fermentation process used by our competitors
produces high levels of carbon dioxide. CO2 is a greenhouse gas and is argued to be the leading cause of global warming.
In stark contrast, Kraig Labs’ mulberry trees and the silk from silkworms have proven to be effective at sequestering carbon
dioxide and are renewable resources. Mulberry trees are also very low maintenance, while still providing essential global green-cover
and significantly help in reducing soil erosion in areas.
In
addition to climate impacts of the fermentation approach, solvents typically used to wet-spin fibers can have significant environmental
impacts. DMSO, a common wet-spinning solvent, can be absorbed directly through human skin, carrying with it potentially dangerous
side effects. This is another reason we pride ourselves on the use of silkworms, which do not require the use of DMSO, to produce
our products.
Competition
We
compete directly with numerous other companies which seek to develop similar product lines and/or distribution that have extensive
capital, resources, market share, and brand recognition.
There
are presently three primary competitors that we face in our industry, Bolt Threads, Inc., Spiber Inc. and AMSilk, all of which
have significantly greater financial resources than us. Additionally, there are few barriers to entry in our industry, which creates
the strong possibility of new competitors emerging, and of others succeeding in developing the same or similar fibers for application
that we are trying to develop. The effects of this increased competition may be materially adverse to us and our stockholders.
As this is an emergent industry there is no one yet producing at commercial scale or having captured a significant portion of
the market demand. We believe that our technology offers more cost-effective methods with lower environmental impact than technologies
used by our identified competitors, however, new technologies could be developed that remove this advantage.
Bolt
Threads, Inc., Spiber Inc. and AMSilk have raised and spent significant capital in pursuit of the same results that we have achieved,
but through different and more complex means. The Company believes that its competitors will continue to overspend while struggling
to deliver the results that we have been able to achieve utilizing the existing global infrastructure.
Based
on our research and internal assessments, the following chart illustrates why we believe we have a competitive advantage over
our three main, known competitors:

Property
Our
principal executive office is located at 2723 South State St., Suite 150, Ann Arbor, Michigan. We pay an annual rent of $2,508
for conference facilities, mail, fax, and reception services located at this location.
On
May 9, 2019, we signed a 5-year property lease for 4,560.57 square meters of space in the Socialist Republic of Vietnam at a current
rent of approximately $45,150 in each of year one and two and with a 5% increase per year for years three through five.
On
January 23, 2017, we signed an 8-year property lease with the Kim Thompson, our Chief Executive Officer, Chief Financial Officer,
President, sole director, and controlling shareholder, for land in Texas where the Company grows its mulberry. We pay a monthly
rent of $960.
On
September 5, 2019, we signed a new two-year lease for a 5,000 square foot property in Lansing, MI that commenced on October 1,
2019 and ends on September 30, 2021, for its research and development headquarters. We pay an annual rent of $42,000 for year
one of the lease and will pay $44,800 for year two of the lease.
Employees
We
currently employ 10 people at its U.S. facilities, 9 full-time and 1 part-time. We employ 5 full time personnel at our Vietnamese
subsidiary. We plan to hire more persons on as-needed basis.
Legal
Proceedings
There
is no material litigation, arbitration, governmental proceeding or any other legal proceeding currently pending or known to be
contemplated against us or any members of our management team in their capacity as such, and we and the members of our management
team have not been subject to any such proceeding in the 10 years preceding the date of this prospectus. We may however be involved,
from time to time, in claims and lawsuits incidental to the conduct of our business in the ordinary course. We carry insurance
coverage in such amounts as we believe to be reasonable under the circumstances and that may or may not cover any or all of our
liabilities in respect of these matters. We do not believe that the ultimate resolution of these matters will have a material
adverse impact on our consolidated financial position, cash flows or results of operations, but cannot guarantee same.
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF
FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
You
should read the following discussion and analysis of our financial condition and results of operations in conjunction with our
consolidated financial statements and the related notes included elsewhere in this prospectus. This discussion contains forward-looking
statements that involve risks and uncertainties. Our actual results and the timing of selected events could differ materially
from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Risk
Factors” and elsewhere in this prospectus. Readers are cautioned not to place undue reliance on forward-looking statements,
since such statements speak only as of the date they were made. Forward-looking statements involve risks and uncertainties that
could cause actual events or results to differ materially from the events or results described in the forward-looking statements,
including any forward-looking statements contained in this prospectus. The events described in forward-looking statements might
not occur or might occur to a different extent or at a different time than described in the forward-looking statements. We undertake
no obligation, except to the extent required by federal securities laws, to publicly update or revise any forward-looking statements
contained in this prospectus, whether as a result of new information, future events, or otherwise.
The following section reflects management’s
views on the financial condition as of December 31, 2019 and 2018, and the results of operations and cash flows for the fiscal
years ended December 31, 2019 and 2018 and the six months ended June 30, 2020 and 2019. This section is provided
as a supplement to, and should be read in conjunction with, the Company’s audited consolidated financial statements and
related notes to the consolidated financial statements contained elsewhere in this prospectus.
Overview
Kraig
Biocraft Laboratories, Inc. is a corporation organized under the laws of Wyoming on April 25, 2006. We were organized to develop
high strength fibers using recombinant DNA technology for commercial applications in technical textile. We use genetically engineered
silkworms that produce spider silk to create our recombinant spider silk. Applications include performance apparel, workwear,
filtration, luxury fashion, flexible composites, medical implants, and more. We believe that we have been a leader in the research
and development of commercially scalable and cost effective spider silk for technical textile. Our primary proprietary fiber technology
includes natural and engineered variants of spider silk produced in domesticated mulberry silkworms. Our business brings twenty-first
century biotechnology to the historical silk industry, permitting us to introduce materials with innovative properties and claims
into an established commercial ecosystem of silkworm rearing, silk spinning and weaving, and manufacture of garments and other
products that can include our specialty fibers and textiles. Specialty fibers are engineered for specific uses that require exceptional
strength, flexibility, heat resistance and/or chemical resistance. The specialty fiber market is exemplified by two synthetic
fiber products that come from petroleum derivatives: (1) aramid fibers; and (2) ultra-high molecular weight polyethylene fibers.
The technical textile industry involves products for both industrial and consumer products, such as filtration fabrics, medical
textiles (e.g., sutures and artificial ligaments), safety and protective clothing and fabrics used in military and aerospace
applications (e.g., high-strength composite materials).
We
are using genetic engineering technologies to develop fibers with greater strength, resiliency and flexibility for use in our
target markets, namely the specialty fiber and technical textile industries.
The
Report of Independent Registered Public Accounting Firm to our financial statements as of December 31, 2019 includes an explanatory
paragraph stating that our net loss from operations and net capital deficiency at
December 31, 2019 raise substantial doubt about our ability to continue as a going concern. The financial statements do not include
any adjustments that might result from the outcome of this uncertainty.
In August 2019, we received authorization
to begin rearing genetically enhanced silkworms at our production facility in Vietnam. We received our investment registration
certificate for the facility in April 2018. In October 2019, we delivered the first batch of these silkworms and began operations.
These silkworms will serve as the basis for the commercial expansion of our proprietary silk technology. On November 4, 2019,
we reported that we had successfully completed rearing the first batch of its transgenic silkworms at the Quang Nam production
factory. Seasonal challenges in late December 2019 slowed production operations, however, we believe that we can resume expansion
of the production of our specialized silk in 2020, as soon as travel and work restrictions for COVID-19 are lifted, since the
seasonal challenges will have also been alleviated. Travel and shipping restrictions may prevent us from shipping the eggs we
are now producing in the U.S. to Vietnam; as soon as the eggs are shipped, we can restart operations at the facility (See, “Impact
of COVID-19 Outbreak” below). We believe that we will be able to target a capacity of 40 metric tons of our recombinant
spider silk fiber per annum from this factory once it reaches maximum utilization. This capacity will allow us to address initial
demand for our products and materials for various applications in the protective, performance, and luxury textile markets.
On April 16, 2020, we announced that we
successfully developed a new technology platform, based on a non-CRISPR gene editing knock-in knock-out technology. CRISPR is
the most recent and efficient gene editing technology3
CRISPR stands for “Clustered regularly interspaced short palindromic repeats.” This is our first knock-in knock-out
technology of essentially pure spider silk transgenic. This new system is built on our eco-friendly and cost-effective silkworm
production system, which we believe is significantly more advanced than any competing methods. Knock-in knock-out technology allows
for the targeting of specific locations and genetic traits for modification, addition, and removal. This new capability will accelerate
new product developments, which should allow us to bring products to market more quickly. This capability also allows for genetic
trait modifications that were previously impractical, creating opportunities for products outside of silk fibers and increased
flexibility in production location. Based on our internal studies, the new technology has a purity rate that is significantly
greater than Dragon Silk, a fiber that we developed with our previous tools. Samples of Dragon Silk has already demonstrated to
be tougher than many fibers used in bullet proof vests and we expect that the increased spider silk purity, created using this
new approach, will yield materials beyond those capabilities. This new system utilizes our eco-friendly and cost-effective silkworm
production system, which we believe is significantly more advanced than any of the competing methods. We have already begun the
validation process for the first of these new transgenics and anticipate that U.S. production will be possible as early as 2022
or 2023.
This does not affect our current work of
overseeing our production facility to ramp up our production of Dragon SilkTM and Monster Silk®, as these
fibers are designed to address their own markets.
Plan
of Operations
During
the next twelve months, we expect to take the following steps in connection with the further development of our business and the
implementation of our plan of operations:
|
●
|
We
plan to accelerate both our microbiology and selective breeding programs as well as provide more resources for our material
testing protocols. We spent approximately $123,050 over the last 12 months on research and development of high strength polymers.
In 2019, we directed our research and development efforts on growing our internal capabilities; we plan to continue
to dedicate our efforts in 2020 to grow our internal research and development programs.
|
|
|
|
|
●
|
We
expect to spend approximately $13,700 on collaborative research and development of high strength polymers and spider silk
protein at the University of Wyoming over the next twelve months. This level of research spending at the university is also
a requirement of our licensing agreement with the university.
|
|
|
|
|
●
|
We
plan to continue the expansion of our production operations at our Quang Nam, Vietnam factory in accordance with our investment
and enterprise registration certificates, including the planting of additional mulberry fields in collaboration with local
farming cooperatives and the hiring of additional direct staff for our factory as needed.
|
|
●
|
We
will consider buying an established revenue producing company in a compatible business, in order to broaden our financial
base and facilitate the commercialization of our products; as of the date hereof, we have not had any formal discussion or
entered into any definitive agreements regarding any such purchase.
|
|
|
|
|
●
|
We
will also actively consider pursuing collaborative research opportunities with both private and university laboratories in
areas of research which overlap the company’s existing research and development. One such potential area for collaborative
research which the company is considering is protein expression platforms. If our financing allows, management will give strong
consideration to increasing the breadth of our research to include protein expression platform technologies.
|
|
|
|
|
●
|
We
plan to actively pursue collaborative research and product testing opportunities with companies in the biotechnology, materials,
textile and other industries.
|
|
|
|
|
●
|
We
plan to actively pursue collaborative commercialization, marketing and manufacturing opportunities with companies in the textile
and material sectors for the fibers we developed and for any new polymers that we create in the remainder of 2020 and going
forward.
|
|
|
|
|
●
|
We
plan to actively pursue the development of commercial scale production of our recombinant materials including Monster Silk®
and Dragon SilkTM.
|
|
|
|
|
●
|
As
soon as travel and work restrictions related to the COVID-19 pandemic are lifted, including those that restrict the shipping
of eggs from the U.S. to Vietnam, we plan resume production operations at our factory in Vietnam and expect to continue to
expand our monthly production volumes to address demand for materials.
|
|
|
|
|
●
|
We
have initiated and plan to accelerate our efforts for large scale U.S. production. This work will include the research and
production of a new transgenic tailored specifically domestic production.
|
Limited
Operating History
We
have not previously demonstrated that we will be able to expand our business through an increased investment in our research and
development efforts. We cannot guarantee that the research and development efforts described in this filing will be successful.
Our business is subject to risks inherent in growing an enterprise, including limited capital resources, risks inherent in the
research and development process and possible rejection of our products in development.
If
financing is not available on satisfactory terms, we may be unable to continue our research and development and other operations.
Equity financing will result in dilution to existing stockholders.
3
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5131771/
https://www.yourgenome.org/facts/what-is-genome-editing
https://ghr.nlm.nih.gov/primer/genomicresearch/genomeediting
Impact
of COVID-19 Outbreak
On
January 30, 2020, the World Health Organization declared the coronavirus outbreak a “Public Health Emergency of International
Concern” and on March 10, 2020, declared it to be a pandemic. Actions taken around the world to help mitigate the spread
of the coronavirus include restrictions on travel, and quarantines in certain areas, and forced closures for certain types of
public places and businesses. The coronavirus and actions taken to mitigate it have had and are expected to continue to have an
adverse impact on the economies and financial markets of many countries, including the geographical area in which the Company
operates. While the closures and limitations on movement, domestically and internationally, are expected to be temporary, if the
outbreak continues on its current trajectory the duration of the supply chain disruption could reduce the availability, or result
in delays, of materials or supplies to and from the Company, which in turn could materially interrupt the Company’s business
operations. Given the speed and frequency of the continuously evolving developments with respect to this pandemic, the Company
cannot reasonably estimate the magnitude of the impact to its consolidated results of operations. The Company’s manufacturing
facilities support business that have been deemed essential by their respective state governments and remain operational. We have
taken every precaution possible to ensure the safety of our employees.
On March 19, 2020,
we furloughed non-essential staff consistent with leading health official recommendations in order to help prevent the spread
of COVID-19. This decision was made in an abundance of caution and will primarily impact staff at our fully owned subsidiary,
Prodigy Textiles, in Vietnam and will result in the temporary closing of silk rearing operations at that facility. As of the date
hereof, Vietnam does not have any work restrictions impacting our facility. However, current U.S. travel restrictions may prevent
us from shipping the eggs we are now producing in the U.S. to Vietnam; as soon as the eggs are shipped, we can restart operations
at the facility. We believe that this temporary suspension of rearing operations will result in a delay of 4-6 months in
the Company’s production expansion schedule, however, due to the fact that U.S. travel restrictions could be extended, the
delay could be longer. The Company supported its furloughed staff through June 30, 2020. During the duration of
the furlough, Kim Thompson, our Chief Executive Officer, Chief Financial Officer, President, and sole director did not receive
or accrue any pay. On July 1, 2020, furloughed staff returned to work preparing the factory
in Vietnam to receive the next shipment of silkworm eggs. The global pandemic of COVID-19 continues to evolve rapidly, and we
will continue to monitor the situation closely, including its potential effect on our plans and timelines.
Additionally, it is reasonably possible that
estimates made in the financial statements have been, or will be, materially and adversely impacted in the near term as a result
of these conditions, including losses on inventory; impairment losses related to goodwill and other long-lived assets and current
obligations.
Results of Operations for the Six
months ended June 30, 2020 compared to the Six Months Ended June 30, 2019
Our revenue, operating expenses, and net loss
from operations for the six month period ended June 30, 2020 as compared to the six month period ended June
30, 2019, were as follows – some balances on the prior period’s combined financial statements have been reclassified
to conform to the current period presentation:
|
|
|
Six
Months Ended
June 30,
|
|
|
|
|
|
%
Change
Increase
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Change
|
|
|
(Decrease)
|
|
|
NET REVENUES
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
|
0.00
|
%
|
|
OPERATING EXPENSES:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and Administrative
|
|
|
3,141,059
|
|
|
|
240,153
|
|
|
|
2,900,906
|
|
|
|
1207.94
|
%
|
|
Professional Fees
|
|
|
50,474
|
|
|
|
188,228
|
|
|
|
(137,754
|
)
|
|
|
-73.18
|
%
|
|
Officer’s Salary
|
|
|
199,415
|
|
|
|
266,205
|
|
|
|
(66,790
|
)
|
|
|
-25.09
|
%
|
|
Rent - Related Party
|
|
|
6,263
|
|
|
|
9,426
|
|
|
|
(3,163
|
)
|
|
|
-33.56
|
%
|
|
Research and Development
|
|
|
37,782
|
|
|
|
43,241
|
|
|
|
(5,459
|
)
|
|
|
-12.62
|
%
|
|
Total operating expenses
|
|
|
3,434,993
|
|
|
|
747,253
|
|
|
|
2,687,740
|
|
|
|
359.68
|
%
|
|
Loss from operations
|
|
|
(3,434,993
|
)
|
|
|
(747,253
|
)
|
|
|
(2,687,740
|
)
|
|
|
359.68
|
%
|
|
Interest expense
|
|
|
(185,143
|
)
|
|
|
(137,615
|
)
|
|
|
(47,528
|
)
|
|
|
34.54
|
%
|
|
Interest income
|
|
|
-
|
|
|
|
4,625
|
|
|
|
(4,625
|
)
|
|
|
100.00
|
%
|
|
Net Loss
|
|
$
|
(3,620,136
|
)
|
|
$
|
(880,243
|
)
|
|
|
(2,739,893
|
)
|
|
|
311.27
|
%
|
Net Revenues:
During the six months ended June 30, 2020, we realized $0 of revenues from our business. During the
six months ended June 30, 2019, we realized $0 of revenues from our business. The change in revenues between the quarter
ended June 30, 2020 and 2019 was $0 or 0%.
Cost of Revenues:
Costs of revenues for the six months ended June 30, 2020 were $0, as compared to $0 for the six months ended
June 30, 2019, a change of $0 or 0%.
Gross Profit: During
the six months ended June 30, 2020, we realized a gross profit of $0, as compared to $0 for the six months
ended June 30, 2019, a change of $0 or 0%.
Research and development
expenses: During the six months ended June 30, 2020, we incurred $37,782 of research and development
expenses. During the six months ended June 30, 2019, we incurred $43,241 of research and development expenses,
a decrease of $5,459 or 12.62% compared with the same period in 2019. This decrease was due to a reduction in research
spend.
Professional Fees:
During the six months ended June 30, 2020, we incurred $50,474 of professional expenses, which decreased
by $137,754 or 73.18% from $188,228 for the six months ended June 30, 2019. This decrease was
primarily due to a decrease in professional fees associated with valuation services for the Company’s stock.
Officers Salary:
During the six months ended June 30, 2020, officers’ salary expenses decreased to $199,415 or 25.09% from
$266,205 for the six months ended June 30, 2019.
General and Administrative
Expense: General and administrative expenses increased by $2,900,906 or 1,207.94% to $3,141,059 for the
six months ended June 30, 2020 from $240,153 for the six months ended June 30, 2019. Our general
and administrative expenses for the six months ended June 30, 2020 consisted of other general and administrative
expenses (which includes expenses such as auto, business development, SEC filings, investor relations, general office,
warrants issued for services) of $3,023,867, travel of $14 and office salary of $117,178 for a total of $3,131,829.
Our general and administrative expenses for the six months ended June 30, 2019 consisted of consulting fees
of $5,787 and other general and administrative expenses (which includes expenses such as Auto, Business Development, SEC
Filing, Investor Relations, General Office) of $126,149, Travel of $14,765 and office salary of $93,452,
for a total of $240,153. The primary reason for this increase June 30, 2020 was primarily due to an increase
in the expense associated with the issuance of warrants for services.
Rent – Related
Party: During the six months ended June 30, 2020, rent- related party expense decreased to $6,263 or
33.56% from $9,426 for the six months ended June 30, 2019. The rent-related party expense was attributable
to the signing on January 23, 2017 the Company signed an eight year property lease with the Company’s President.
Interest Expense:
Interest expense increase by $47,528 to $185,143 for the six month period ended June 30, 2020 from
$137,615 for the six month period ended June 30, 2019. The increase was primarily due to interest on certain
Company loans.
Interest Income:
Interest income decreased by $4,625 to $0 for the six month period ended June 30, 2020 from $4,625
for the six month period ended June 30, 2019. The decrease was primarily due to interest on bank accounts.
Net Loss: Net loss
increased by $2,739,893, or 311.27 %, to a net loss of $3,620,136 for the six month period ended June
30, 2020 from a net loss of $880,243 for the six month period ended June 30, 2019. This increase in net loss
was primarily attributable to both increases in general and administrative expenses and a decrease in revenues.
Results
of Operations for the Years ended December 31, 2019 and 2018.
Our
revenue, operating expenses, and net loss from operations for the years ended December 31, 2019 as compared to the year ended
December 31, 2018, are included in the table below. Some balances on the prior period’s combined financial statements have
been reclassified to conform to the current period presentation:
|
|
|
Year Ended
|
|
|
Increase
|
|
|
|
|
|
|
|
December 31
|
|
|
(Decrease)
|
|
|
|
|
|
|
|
2019
|
|
|
2018
|
|
|
Change
|
|
|
% Change
|
|
|
NET REVENUES
|
|
|
-$
|
|
|
$
|
401,620
|
|
|
|
(401,620
|
)
|
|
|
-100.00
|
%
|
|
OPERATING EXPENSES:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and Administrative
|
|
|
1,413,982
|
|
|
|
515,875
|
|
|
|
898,107
|
|
|
|
174.09
|
%
|
|
Professional Fees
|
|
|
342,845
|
|
|
|
157,976
|
|
|
|
184,869
|
|
|
|
117.02
|
%
|
|
Officer’s Salary
|
|
|
627,197
|
|
|
|
528,127
|
|
|
|
99,070
|
|
|
|
18.76
|
%
|
|
Rent - Related Party
|
|
|
14,793
|
|
|
|
11,520
|
|
|
|
3,273
|
|
|
|
28.41
|
%
|
|
Research and Development
|
|
|
223,050
|
|
|
|
148,069
|
|
|
|
74,981
|
|
|
|
50.64
|
%
|
|
Total operating expenses
|
|
|
2,621,867
|
|
|
|
1,361,567
|
|
|
|
1,260,300
|
|
|
|
92.56
|
%
|
|
Loss from operations
|
|
|
(2,621,867
|
)
|
|
|
(959,947
|
)
|
|
|
(1,661,920
|
)
|
|
|
173.13
|
%
|
|
Gain on forgiveness of debt
|
|
|
-
|
|
|
|
19,924
|
|
|
|
(19,924
|
)
|
|
|
100.00
|
%
|
|
Interest expense
|
|
|
(294,352
|
)
|
|
|
(228,954
|
)
|
|
|
(65,398
|
)
|
|
|
28.56
|
%
|
|
Interest Income
|
|
|
6,369
|
|
|
|
-
|
|
|
|
6,369
|
|
|
|
100.00
|
%
|
|
Net Loss
|
|
$
|
(2,909,850
|
)
|
|
$
|
(1,168,977
|
)
|
|
|
(1,740,873
|
)
|
|
|
148.93
|
%
|
Net Revenues: During
the year ended December 31, 2019, we realized $0 of revenues from our business. During the year ended December 31, 2018, we realized
$401,620 of revenues from our business. The change in revenues between the years ended December 31, 2019 and 2018 was $401,620
or 100%. This decrease was related to loss of our only customer, the U.S. Army, whose contract concluded in 2018. In 2020, the
Company expects to have its commercial production facility operational, allowing for the production and sale of products to the
technical textile markets.
Research and development
expenses: During year ended December 31, 2019 we incurred $223,050 research and development expenses. During year ended December
31, 2018 we incurred $148,069 of research and development expenses, an increase of $74,981 or 50.64% compared with the same period
in 2018. The research and development expenses are attributable to the research and development with the Notre Dame University;
the increase was due to the timing of research related activity and costs by insources the Company’s research operations.
Professional Fees:
During year ended December 31, 2019, we incurred $342,845 professional expenses, which increased by $184,869 or 117.02% from $157,976
for the year ended December 31, 2018. The increase in professional fees expense was attributable to increased expenses related
to investor relations services during year ended December 31, 2019.
Officers Salary:
During year ended December 31, 2010, officers’ salary expenses increased to $627,197 or 18.76% compared to $528,127 for year
ended December 31, 2018. This increase was due to an increase in the salaries of our executive officers.
General and Administrative
Expense: General and administrative expenses increased by $898,107 or 174.09% to $1,413,982 for year ended December 31, 2019
from $515,875 for year ended December 31, 2018. Our general and administrative expenses for year ended December 31, 2019 consisted
of consulting fees of $5,787 and other general and administrative expenses (which includes expenses such as Auto, Business Development,
filings with the Commission, Investor Relations, General Office, warrant Compensation) of $1,123,787, Travel of $40,734, office
salary of $243,674 for a total of $1,413,982. Our general and administrative expenses for year ended December 31, 2018 consisted
of consulting fees of $26,538 and other general and administrative expenses (which includes expenses such as Auto, Business Development,
filings with the Commission, Investor Relations, General Office, warrant Compensation) of $325,897, Travel of $19,141, office salary
of $144,299 for a total of $515,875. The primary reason for the increase in comparing year ended December 31, 2019 to the corresponding
period for 2018 was mainly due to general business expenses and warrants issuances for services.
Rent – Related
Party: During the year ended December 31 2019, rent-related party expense increased to $14,793 or 28.41% compared to $11,520
for the year ended December 31, 2018. The increase in rent-related party expense was attributable to the additional month of rent
recorded in 2019 due to the implementation of ASC 842 and the amortization of rent expenses over the term of the lease.
Gain on Forgiveness
of Debt: Gain on forgiveness of debt decreased by $19,924 to $0 for the year ended December 31, 2019 compared to $19,924 for
the year ended December 31, 2018. The increase was primarily due to the issuance of stock as payment for consulting services.
Interest Expense:
Interest expense increased to $294,352, or 28.56% for the year ended December 31, 2019 compared to $228,954 for the year ended
December 31, 2018. The increase was primarily due to interest on the related party loans and accounts payable and accrued expenses
to the related parties.
Interest Income:
Interest income increased by $6,369 to $6,369 for the year ended December 31, 2019 from $0 for the year ended December 31, 2018.
The increase was primarily due to interest on bank accounts.
Net Loss: Net loss
increased by $1,740,873, or 148.92%, to a net loss of $2,909,850 for the year ended December 31, 2019 from a net loss of $1,168,977
for the year ended December 31, 2018. This increase in net loss was driven primarily by increased in research and development,
warrant compensation, and professional fees.
Capital Resources and Liquidity
Our financial statements
have been presented on the basis that we have a going concern, which contemplates the realization of assets and satisfaction of
liabilities in the normal course of business. As presented in the financial statements, we incurred a net loss of $2,909,850 during
the year ended December 31, 2019, and losses are expected to continue in the near term. Since our inception, the accumulated deficit
is $29,797,906 at December 31, 2019. Refer to Note 2 for our discussion of stockholder deficit. During the six months ended
June 30, 2020, and losses are expected to continue in the near term. The accumulated deficit is $33,418,042
at June 30, 2020. Refer to Note 2 of our unaudited financial statements for our discussion of stockholder deficit. We have
been funding our operations through private loans and the sale of common stock in private placement transactions. Refer to Note
5 and Note 7 in the financial statements for our discussion of notes payable and shares issued, respectively. Our cash resources
are insufficient to meet our planned business objectives without additional financing. These and other factors raise substantial
doubt about our ability to continue as a going concern. The accompanying financial statements do not include any adjustments to
reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities
that may result from the possible inability of our company to continue as a going concern.
Management anticipates
that significant additional expenditures will be necessary to develop and expand our business before significant positive operating
cash flows can be achieved. Our ability to continue as a going concern is dependent upon our ability to raise additional capital
and to ultimately achieve sustainable revenues and profitable operations. At December 31, 2019 and June 30, 2020, we had
$125,024 and $54,710, respectively of cash on hand. In addition to our cash on hand, we are regularly financed through
debt issuance to our Chief Executive Officer, Chief Financial Officer, director and our principal shareholder. With our current
cash on hand and such additional funding, the Company is able to sustain its operations for at least the next 90-120 days. These
funds are insufficient to complete our business plan and as a consequence, we will need to seek additional funds, primarily through
the issuance of debt or equity securities for cash to operate our business. No assurance can be given that any future financing
will be available or, if available, that it will be on terms that are satisfactory to us. Even if we are able to obtain additional
financing, it may contain undue restrictions on our operations, in the case of debt financing or cause substantial dilution for
our stockholders, in the case of equity financing.
Management has undertaken
steps as part of a plan to improve operations with the goal of sustaining our operations for the next twelve months and beyond.
These steps include (a) raising additional capital and/or obtaining financing; (b) controlling overhead and expenses; and (c) executing
material sales or research contracts. There can be no assurance that we can successfully accomplish these steps and it is uncertain
that the Company will achieve a profitable level of operations and obtain additional financing. There can be no assurance that
any additional financing will be available to the Company on satisfactory terms and conditions, if at all. As of the date of this
Report, we have not entered into any formal agreements regarding the above.
In the event we are unable
to continue as a going concern, we may elect or be required to seek protection from its creditors by filing a voluntary petition
in bankruptcy or may be subject to an involuntary petition in bankruptcy. To date, management has not considered this alternative,
nor does management view it as a likely occurrence.
Assets and Liabilities for December 31, 2019 and 2018 (Audited)
and June 30, 2020 (Unaudited)
|
|
|
June 30,
2020
|
|
|
December 31, 2019
|
|
|
December 31, 2018
|
|
|
Cash
|
|
$
|
54,710
|
|
|
$
|
125,024
|
|
|
$
|
13,697
|
|
|
Accounts receivable
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Prepaid expenses
|
|
$
|
8,472
|
|
|
$
|
31,745
|
|
|
$
|
6,858
|
|
|
Total current assets
|
|
$
|
63,182
|
|
|
$
|
156,769
|
|
|
$
|
20,555
|
|
|
Total assets
|
|
$
|
586,152
|
|
|
$
|
750,850
|
|
|
$
|
71,383
|
|
|
Total current liabilities
|
|
$
|
6,436,073
|
|
|
$
|
5,584,383
|
|
|
$
|
4,530,606
|
|
|
Total liabilities
|
|
|
6,899,755
|
|
|
$
|
6,138,908
|
|
|
$
|
4,530,606
|
|
At June 30, 2020, we had a working
capital deficit of $6,372,891 compared to a working capital deficit of $5,427,614 at December 31, 2019. Current liabilities
increased to $6,436,073 at June 30, 2020 from $5,584,383 at December 31, 2019, primarily as a result of accounts
payable, note payable and accrued compensation.
At December 31, 2019, we had a working capital
deficit of $5,427,614, compared to a working capital deficit of $4,510,051 at December 31, 2018. Current liabilities increased
to $5,584,383 at December 31, 2019 from $4,530,606 at December 31, 2018, primarily as a result of primarily as a result of accounts
payable and accrued compensation.
For the six months ended June 30,
2020, net cash used in operations of $697,909 was the result of a net loss of $3,620,136 offset by offset by
depreciation expense of $13,118, options issuance to related parties of $2,655,124, imputed interest on related
party loans of $21,972, decrease in prepaid expenses of $23,273 and a decrease in operating lease right of use of
$57,993, an increase of accrued expenses and other payables-related party of $277,050, decrease in accounts payable
of $72,448 and a decrease in operating lease liabilities of $53,855. For the six months ended June 30,
2019, net cash used in operations of $455,469 was the result of a net loss of $880,243 offset by offset by depreciation
expense of $13,403, warrants cancellation of $19,915, imputed interest on related party loans of $10,457, increase
in prepaid expenses of $28,387 and decrease in operating lease right of use of $30,433, an increase of accrued
expenses and other payables-related party of $373,469, an increase in accounts payable of $73,420 and a decrease
in operating lease liabilities of $28,106.
For the year ended December 31, 2019, net cash
used in operations of $1,087,881 was the result of a net loss of $2,909,850 offset by depreciation expense of $30,781, warrant
cancellation of $19,915, options issued to related parties of $696,934, imputed interest on related party loans of $22,337, increase
in prepaid expenses of $24,887, a decrease in operating lease right of use of $86,326, an increase of accrued expenses and other
payables-related party of $795,633, an increase in accounts payable of $314,369 and a decrease in operating lease liabilities of
$79,609. For the year ended December 31, 2018, net cash used in operations of $235,005 was the result of a net loss of $1,168,977
offset by depreciation expense of $26,632, gain on forgiveness of debt of $19,924, imputed interest on related party loans of $11,909,
warrants issued to consultants of $72,575, decrease in accounts receivable of $25,872, and an increase in other receivable of $2,394,
an increase of accrued expenses and other payables-related party of $682,976, an increase in accounts payable of $136,326.
Net cash used in our investing activities
were $0 and $0 for the six months ended June 30, 2020 and June 30, 2019, respectively. Our investing activities
for the six months ended June 30, 2019 are attributable to purchases of fixed assets.
Net cash used in our investing activities
were $100,792 and $11,448 for the year ended December 31, 2019 and December 31, 2018, respectively. Our investing activities for
the years ended December 31, 2019 and 2018 are attributable to purchases of fixed assets. Our financing activities resulted in
a cash inflow of $627,595 for the six months ended June 30, 2020, which is represented by $30,000
loan repayment, $550,000 proceeds from a stockholder note payable, $17,495 contributed capital from a stockholder
and $90,100 proceeds from the SBA Paycheck Protection Loan. Our financing activities resulted in a cash inflow of $1,116,000
for the six months ended June 30, 2019, which is represented by $1,000,000 proceeds from issuance of common stock,
$4,000 loan repayment and $120,000 proceeds from shareholder note payable.
Our financing activities resulted in a cash
inflow of $1,300,000 for the year ended December 31, 2019, which is represented by $1,000,000 proceeds from the issuance of common
stock, $20,000 loan repayment and $320,000 proceeds from a shareholder note payable. Our financing activities resulted in cash
inflow of $242,000 for the year ended December 31, 2018, which is represented by shareholder loan payable.
Critical Accounting Policies
Our financial statements and related public
financial information are based on the application of accounting principles generally accepted in the United States (“GAAP”).
GAAP requires the use of estimates, assumptions, judgments and subjective interpretations of accounting principles that have an
impact on the assets, liabilities, and revenue and expense amounts reported. These estimates can also affect supplemental information
contained in our external disclosures including information regarding contingencies, risk and financial condition. We believe
our use of estimates and underlying accounting assumptions adhere to GAAP and are consistently and conservatively applied. We
base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances.
Actual results may differ materially from these estimates under different assumptions or conditions. We continue to monitor significant
estimates made during the preparation of our financial statements.
Our significant accounting policies are summarized
in Note 1 of our financial statements. While all these significant accounting policies impact its financial condition and results
of operations, we view certain of these policies as critical. Policies determined to be critical are those policies that have the
most significant impact on our financial statements and require management to use a greater degree of judgment and estimates. Actual
results may differ from those estimates. Our management believes that given current facts and circumstances, it is unlikely that
applying any other reasonable judgments or estimate methodologies would cause a material effect on our results of operations, financial
position or liquidity for the periods presented in this Registration Statement.
Recent Accounting Pronouncements
In February 2016, the FASB issued ASU 2016-02,
“Leases” Topic 842, which amends the guidance in former ASC Topic 840, Leases. The new standard increases transparency
and comparability most significantly by requiring the recognition by lessees of right-of-use (“ROU”) assets and lease
liabilities on the balance sheet for all leases longer than 12 months. Under the standard, disclosures are required to meet the
objective of enabling users of financial statements to assess the amount, timing, and uncertainty of cash flows arising from leases.
For lessees, leases will be classified as finance or operating, with classification affecting the pattern and classification of
expense recognition in the income statement. We adopted the new lease guidance effective January 1, 2019 using the modified retrospective
transition approach, applying the new standard to all of its leases existing at the date of initial application which is the effective
date of adoption. Consequently, financial information will not be updated and the disclosures required under the new standard will
not be provided for dates and periods before January 1, 2019. We elected the package of practical expedients which permits us to
not reassess (1) whether any expired or existing contracts are or contain leases, (2) the lease classification for any expired
or existing leases, and (3) any initial direct costs for any existing leases as of the effective date. We did not elect the hindsight
practical expedient which permits entities to use hindsight in determining the lease term and assessing impairment. The adoption
of the lease standard did not change our previously reported consolidated statements of operations and did not result in a cumulative
catch-up adjustment to opening equity. As a result, the Company has recorded Right-to-use assets and corresponding Lease obligations
as more fully discussed in Note 4.
All other newly issued accounting pronouncements
but not yet effective have been deemed either immaterial or not applicable
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements,
financings, or other relationships with unconsolidated entities or other persons, also known as “special purpose entities”
(SPEs).
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS
ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE
GOVERNANCE
Below is a list of our executive officers and
sole director as of the date of this Registration Statement.
|
NAME
|
|
AGE
|
|
POSITION
|
|
DATE
APPOINTED
|
|
Kim Thompson
|
|
58
|
|
President, Chief Executive Officer, Chief Financial
Officer and Sole Director
|
|
April 25, 2006
|
|
Jonathan R. Rice
|
|
40
|
|
Chief Operating Officer
|
|
January 20, 2015
|
|
Anurag Gupta*
|
|
55
|
|
Director – Elect
|
|
To
be appointed prior to effectiveness
|
|
Julie R. Bishop*
|
|
40
|
|
Director – Elect
|
|
To
be appointed prior to effectiveness
|
|
Greg Scheessele*
|
|
59
|
|
Director – Elect
|
|
To
be appointed prior to effectiveness
|
|
Kenneth Le
|
|
55
|
|
President of Prodigy Textiles
|
|
July 2019
|
* These individuals have indicated his/her
consent to occupy such position upon closing of this offering and such persons are collectively referred to herein as the “Director
Nominees”
The following summarizes the occupation and business experience
during the past five years for our officers, current director and Director Nominees.
Kim Thompson. Mr. Kim Thompson was a
founder of the California law firm of Ching & Thompson which was founded in 1997 where he focused primarily on commercial litigation.
He has been a partner in the Illinois law firm of McJessy, Ching & Thompson since 2004 where he also emphasizes commercial
and civil rights litigation. Mr. Thompson received his bachelor’s degree in applied economics from James Madison College,
Michigan State University, and his Juris Doctorate from the University of Michigan. He is the named inventor or co-inventor on
a number of provisional patent applications including inventions relating to biotechnology and mechanics. Mr. Thompson is the inventor
of the technology concept that lead to the formation of the Company. We believe that Mr. Thompson is well suited to serve as our
director because of his knowledge of biotechnology, legal expertise, education and background in economics.
Jonanthan R. Rice. Mr. Jonathan R. Rice
worked at Ultra Electronics, Adaptive Materials Inc., a Michigan company (“UEA”) from 2002 through 2015. At the time
he left UEA, Mr. Rice worked as the Director of Advanced Technologies, where he was responsible for new products development and
commercialization. He was also the Corporate Facility Security Officer for UEA from2006 through 2015, where Mr. Rice ensured UEA’s
compliance with federal regulations under the National Industrial Security Program Operating Manual and completed its annual security
audit. During 2004 through 2007 while working as an Engineering Manager at UEA, Mr. Rice, among other things, led the design and
development of multiple fuel cell and power management systems, established a team to identify and eliminate production and performance
limitation, authored technical progress and final reports for customers and provided training to military personnel on use of fuel
cell systems. From 2002 through 2005, Mr. Rice also served as UEA’s Production Manager in charge of developing manufacturing
process and techniques and sourcing the production equipment for UEA’s products. Mr. Rice graduated from Michigan Technological
University in 2002 with a degree of Bachelors of Science Chemical Engineering. Mr. Rice received his Masters of Business Administration
at Michigan State University in 2016.
Anurag Gupta. Mr.
Anurag Gupta is a C-Suite executive with nearly 30 years of experience in US based global corporations. He has extensive expertise
in leading businesses internationally in both start-up and established business environments. Mr. Gupta currently serves on the
Board of Directors of Southern Graphics Systems (SGSCo), a PE backed global company providing packaging and marketing production
services to the CPG, retail and printer space. He is also an Executive Advisor to the company. Additionally, Mr. Gupta serves on
the Board of Directors of Roseburg Forest Products, Inc., a multi-billion dollar leading manufacturer and marketer of wood products
in USA, Canada and Japan, and is Chairman of its Strategy & Risk Committee. Mr. Gupta is also an investor and Director on the
Board of Drive My Way, Inc., a post-revenue start-up company in digital recruiting marketplace, powered by a proprietary, patent-protected
platform that is revolutionizing truck driver recruiting in USA. Mr. Gupta’s previous corporate roles include serving as
CEO of Global Data Services at TBG, AG from December 2016 to December 2017, a Private Equity firm where he helped Acquire DTN business
from Schneider Electric. As Executive Vice President of CMS Division at IHS Markit from April 2013 to December 2016 (NASDAQ listed:
INFO), an over $25 billion market capitalization company, Mr. Gupta led multiple global business lines along with corporate strategy,
M&A and product development for the company. During his tenure, the company acquired over 25 businesses across multiple industry
verticals. As President of Europe, Middle East and Africa (EMEA) region at BrightPoint, Inc from December 2009 to October 2012
(NASDAQ listed: CELL) and later at Ingram Micro from October 2012 to March 2013 (NYSE listed: IM), Mr. Gupta was responsible for
running a business of $2.7 billion in revenue across 30 countries in EMEA. He was also the head of Investor Relations at BrightPoint,
Inc. from April 2003 to November 2009 where he was the recipient of the Stevie Business Award for the Best Investor Relations Program.
As CEO of Teamcall Ltd., a Motorola Joint Venture Company which Mr. Gupta helped create in the mid-1990s, he pioneered the launch
of Mobile technology and business in India. Mr. Gupta has participated 3 times in ringing the opening bell at NASDAQ and once in
the closing bell at NYSE. Mr. Gupta graduated Magna Cum Laude and earned his Bachelors in Electrical Engineering in 1987 and his
Masters in Electrical Engineering in 1990 from the University of Toledo, Ohio, USA where his Master’s Thesis was funded by
NASA Lewis Research Center. He earned his Masters of Business Administration in 1994 from the Stuart School of Business, at Illinois
Institute of Technology, Chicago, USA. Mr. Gupta provides the board with significant expertise in business finance and management
of growing global operation as well as a strong history of board and committee service
Julie R. Bishop.
Ms. Julie R. Bishop is a licensed C.P.A. that brings extensive leadership experience and expertise in finance and accounting.
Ms. Bishop is currently the Vice President of Global Accounting and Reporting at Verizon Media, the media and technology business
unit of Verizon, a publicly traded telecom company. Prior to that role, Ms. Bishop was the Senior Manager and then Director of
Global Accounting and then Senior Director, Global Accounting at Yahoo Inc., a publicly traded media and technology company purchased
by Verizon in 2017. Ms. Bishop has been serving at Verizon Media (formerly Yahoo Inc.) since 2011. Ms. Bishop served as Accounting
Manager at HD Waterworks, a distribution company and business unit of HD Supply, from 2009 to 2011. In addition, Ms. Bishop served
as an auditor of publicly traded companies from 2002 to 2009 at Ernst & Young, LLP. Ms. Bishop also serves on the board of
a private entity. Ms. Bishop received Bachelors and Masters Degrees in Accounting from Southern Illinois University of Edwardsville
in 2001 and 2002, respectively. Ms. Bishop provides the board with significant expertise in accounting and finance, particularly
in global publicly traded companies, which she developed over her long career working in publicly traded companies and auditing
at a leading global audit firm. She also brings the board valuable management and leadership expertise, critical perspective on
strategic planning and risk management, and additional insights into the technology industry.
Greg Scheessele. Mr. Gregory (Greg)
Scheessele has thirty-seven years of global manufacturing business leadership experience. He is the CEO of his own advisory firm,
Gerette, LLC. Prior to launching Gerette, LLC in 2018, Mr. Sheessele led the NAFTA and South American business units of TMD Friction
Holdings GmbH as the Executive Vice President, TMD Americas (“TMD”). Prior to joining TMD in 2005, Mr. Scheessele was
the Group Vice President, Global Operations with Pall Corporation where he worked over twelve years in various global operations
executive positions at Pall Corporation and Gelman Sciences (Pall acquisition). Mr. Scheessele developed his engineering and manufacturing
management skills while working for General Motors – Powertrain Division for ten years. Mr. Scheessele has served as a Board
Director or Trustee for various automotive component, engineering, technology firms and non-profit organizations for the past twelve
years. Mr. Scheessele currently is a director or trustee for an educational non-profit in the Detroit Metro area and a privately
held manufacturing company in Wisconsin. Mr. Scheessele has more than 13 years of experience serving as a director for both non-profit
and for profit organizations. He has served in roles as an inside director and independent director and has more than 4 years of
experience on compensation and finance committees. Mr. Scheessele graduated from Purdue University with a Bachelor of Science –
Mechanical Engineering. Mr. Scheessele also earned a Master of Science – Industrial and Systems Engineering from the University
of Michigan. Mr. Scheessele provides the board with significant expertise in international manufacturing operations and management
oversight as well as a strong history of service on board committees.
Kenneth Le. Mr. Le was appointed as
our Director of government relations and the President of Prodigy Textiles in July 2019. In light of his position with our subsidiary
and the duties associated with such position, we believe Mr. Le meets the definition of “executive officer” as such
term is defined in the Exchange Act. Kenneth Le has over 25 years of successful international business experience specializing
in entrepreneurial enterprises. In January 2009, Mr. Le was appointed to Dot VN, Inc. Strategic Advisory Board and will assist
in strategic planning for future growth and development of key projects of the domain name registration and business e-commerce
solutions company in Vietnam. As current managing partner of Pacific Bay Ventures, Mr. Le is working on a joint venture developing
1,550 hectares as a mixed use residential industrial park in conjunction with Dat Quang Chu Lai Industrial Park, JSP in Tam An
city in Chu Lai province, Vietnam’s first international open economic trade zone. He is Managing Director of Minh Nhat Company
and developing Da Deh Lake, an eco-resort of over 500 hectares in a surrounded lake in the Lam Dong province, the third and the
largest plateau province on the Central Highlands three hours outside of Ho Chi Minh City in Vietnam. Mr. Le has extensive high-level
business contacts in Southeast Asia, many of which he has helped bring together acting as international liaison.
Term of Office
Our directors are appointed for a one-year
term to hold office until the next annual general meeting of our stockholders or until removed from office in accordance with our
bylaws. Our officers are appointed by our Board and hold office until removed by the Board. Mr. Thompson is employed as the Chief
Executive Officer and Chief Financial Officer of the Company pursuant to a five year employment contract.
Involvement in Certain Legal Proceedings
To the best of the Company’s knowledge,
none of the following events occurred during the past ten years that are material to an evaluation of the ability or integrity
of any of our executive officers, directors, Director Nominees or promoters:
(1) A petition under the
Federal bankruptcy laws or any state insolvency law was filed by or against, or a receiver, fiscal agent or similar officer was
appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within
two years before the time of such filing, or any corporation or business association of which he was an executive officer at or
within two years before the time of such filing;
(2) Convicted in a criminal
proceeding or is a named subject of a pending criminal proceeding (excluding traffic violations and other minor offenses);
(3) Subject of any order,
judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily
enjoining him from, or otherwise limiting, the following activities:
(i) Acting as a futures
commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction
merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing,
or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any
investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice
in connection with such activity;
(ii) Engaging in any type
of business practice; or
(iii) Engaging in any activity
in connection with the purchase or sale of any security or commodity or in connection with any violation of Federal or State securities
laws or Federal commodities laws;
(4) Subject of any order,
judgment or decree, not subsequently reversed, suspended or vacated, of any Federal or State authority barring, suspending or otherwise
limiting for more than 60 days the right of such person to engage in any activity described y such activity;
(5) Found by a court of
competent jurisdiction in a civil action or by the Commission to have violated any Federal or State securities law, and the judgment
in such civil action or finding by the Commission has not been subsequently reversed, suspended, or vacated;
(6) Found by a court of
competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any Federal commodities
law, and the judgment in such civil action or finding by the Commodity Futures Trading Commission has not been subsequently reversed,
suspended or vacated;
(7) Subject of, or a party
to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or
vacated, relating to an alleged violation of:
(i) Any Federal or State
securities or commodities law or regulation; or
(ii) Any law or regulation
respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order
of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition
order; or
(iii) Any law or regulation
prohibiting mail or wire fraud or fraud in connection with any business entity; or
(8) Subject of, or a party to, any sanction
or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26)
of the Exchange Act (15 U.S. C 78c(a)(26)), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act
(7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its
members or persons associated with a member.
Director Independence and Board Committees
We are not currently required under the Securities
and Exchange Act to maintain any committees of our Board.
Nasdaq listing standards require that a majority
of our Board be independent within one year of our initial public offering. An “independent director” is defined generally
as a person other than an officer or employee of the company or its subsidiaries or any other individual having a relationship
which in the opinion of the Company’s Board, would interfere with the director’s exercise of independent judgment in
carrying out the responsibilities of a director. Our Board has determined that Messrs. Gupta and Scheessele and Ms. Bishop are
“independent directors” as defined in the Nasdaq listing standard and applicable SEC rules.
Pursuant to Nasdaq listing rules we will establish
three standing committees - an audit committee in compliance with Section 3(a)(58)(A) of the Exchange Act, a compensation committee
and a nominating and governance committee, each comprised of independent directors. Under Nasdaq listing rule 5615(b)(1), a company
listing in connection with its initial public offering is permitted to phase in its compliance with the independent committee requirements.
We do not intend to rely on the phase-in schedules set forth in Nasdaq listing rule 5615(b)(3).
Audit Committee. Upon the effectiveness
of the registration statement of which this prospectus forms a part, we will establish an audit committee of the Board.
Under the Nasdaq listing standards and applicable SEC rules, we are required to have at least three members of the audit committee,
all of whom must be independent, subject to certain phase-in provisions. Messrs. Gupta and Scheessele and Ms. Bishop meet
the independent director standard under Nasdaq listing standards and under Rule 10-A-3(b)(1) of the Exchange Act. Ms. Bishop will
serve as chairman of our audit committee. Each member of the audit committee is financially literate and our Board has determined
that Ms. Bishop qualifies as an “audit committee financial expert” as defined in applicable SEC rules.
We will adopt an audit committee charter, which
will detail the purpose and principal functions of the audit committee, including:
|
|
●
|
appoint, compensate, and oversee the work of any registered public accounting firm employed by us;
|
|
|
|
|
|
|
●
|
resolve any disagreements between management and the auditor regarding financial reporting;
|
|
|
|
|
|
|
●
|
pre-approve all auditing and non-audit services;
|
|
|
|
|
|
|
●
|
retain independent counsel, accountants, or others to advise the audit committee or assist in the conduct of an investigation;
|
|
|
|
|
|
|
●
|
seek any information it requires from employees-all of whom are directed to cooperate with the audit committee’s requests-or external parties;
|
|
|
|
|
|
|
●
|
meet with our officers, external auditors, or outside counsel, as necessary; and
|
|
|
|
|
|
|
●
|
oversee that management has established and maintained processes to assure our compliance with all applicable laws, regulations and corporate policy.
|
Compensation Committee. Upon the effectiveness
of the registration statement of which this prospectus forms a part, we will establish a compensation committee of the Board.
Messrs. Gupta and Scheessele and Ms. Bishop will serve as members of our compensation
committee. Under the Nasdaq listing standards and applicable SEC rules, we are required to have at least two members of the compensation
committee, all of whom must be independent, subject to certain phase-in provisions. Messrs. Gupta and Scheessele and Ms.
Bishop meet the independent director standard under Nasdaq listing standards applicable to
members of the compensation committee
We will adopt a compensation committee charter,
which will detail the purpose and responsibility of the compensation committee, including:
|
|
●
|
discharge the responsibilities of the Board relating to compensation of the our directors, executive officers and key employees;
|
|
|
|
|
|
|
●
|
assist the Board in establishing appropriate incentive compensation and equity-based plans and to administer such plans;
|
|
|
|
|
|
|
●
|
oversee the annual process of evaluation of the performance of our management; and
|
|
|
|
|
|
|
●
|
perform such other duties and responsibilities as enumerated in and consistent with compensation committee’s charter.
|
The charter will permit the committee to retain
or receive advice from a compensation consultant and will outline certain requirements to ensure the consultants independence or
certain circumstances under which the consultant need not be independent. However, as of the date hereof, the Company has not retained
such a consultant.
Nominating and Governance Committee.
Upon the effectiveness of the registration statement of which this prospectus forms a part, we will establish a nominating and
governance committee of the Board that will be comprised of independent directors. Messrs. Gupta and Scheessele and Ms. Bishop
will serve as members of our nominating and governance.
We will adopt a nominating and governance committee charter, which will detail the purpose and responsibilities of the nominating
and governance committee, including:
|
|
●
|
assist the Board by identifying qualified candidates for director nominees, and to recommend to the board of directors the director nominees for the next annual meeting of stockholders;
|
|
|
|
|
|
|
●
|
lead the Board in its annual review of its performance;
|
|
|
|
|
|
|
●
|
recommend to the board director nominees for each committee of the Board; and
|
|
|
|
|
|
|
●
|
develop and recommend to the Board corporate governance guidelines applicable to us.
|
Meetings of the Board of Directors
During its fiscal year ended December 31, 2019,
the Board did not meet on any occasion, but rather transacted business by unanimous written consent seven times.
Family Relationships
There are no family relationships by between
or among the members of the Board or other executive officers of the Company.
Indemnification
Our amended and restated articles of incorporation
and amended and restated bylaws include provisions limiting the liability of directors and officers and indemnifying them under
certain circumstances. See “Indemnification of Directors and Officers” for further information. We intend to secure
directors’ and officers’ liability insurance following the completion of this offering.
Insofar as indemnification for liabilities
arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling the Company pursuant to
Wyoming law, we are informed that in the opinion of the Securities and Exchange Commission, such indemnification is against public
policy as expressed in the Securities Act and is therefore unenforceable.
EXECUTIVE COMPENSATION
The following summary compensation table sets forth all compensation
awarded to, earned by, or paid to the named executive officer during the years ended December 31, 2019 and 2018 in all capacities
for the accounts of our executive, including the Chief Executive Officer (CEO) and Chief Financial Officer (CFO).
On March 19, 2020, the Mr. Thompson agreed
to suspend payment or accrual of his salary until such time as the Company’s employees whom were furloughed due to the COVID-19
pandemic return to work.
SUMMARY COMPENSATION TABLE
|
Name and principal position
|
|
Year
|
|
|
Salary
($)
|
|
|
Bonus ($)
|
|
|
Stock Awards ($)
|
|
|
Option Awards ($)
|
|
|
Non-Equity Incentive Plan Compensation ($)
|
|
|
Nonqualified Deferred Compensation Earnings ($)
|
|
|
All Other Compensation ($)
|
|
|
Total ($)
|
|
Kim Thompson
President, CEO, CFO and Director
|
|
|
2019
|
|
|
$
|
354,791
|
(1)
|
|
$
|
70,958
|
(2)
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
52,798
|
(3)
|
|
$
|
478,547
|
|
|
|
|
|
2018
|
|
|
$
|
334,708
|
(4)
|
|
$
|
66,942
|
(5)
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
55,934
|
(6)
|
|
$
|
457,584
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Jonathan R. Rice
COO
|
|
|
2019
|
|
|
$
|
150,774
|
(7)
|
|
$
|
24,000
|
(8)
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
13,295
|
(9)
|
|
$
|
188,069
|
|
|
|
|
|
2018
|
|
|
$
|
125,398
|
(10)
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
15,324
|
(11)
|
|
$
|
142,285
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Kenneth Le President of Prodigy Textiles(12)
|
|
|
2019
|
|
|
$
|
27,692
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
237,748
|
(12)(13)
|
|
$
|
265,440
|
|
|
|
|
|
2018
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
(1)
|
This represents the annual salary payable to Mr. Thompson pursuant to the then current terms of his employment agreement. However, in light of the Company’s cash position, Mr. Thompson agreed to defer all such annual compensation until such time as our cash position improves. See the section, “Employment Agreements” below for additional information regarding the payback terms of the deferred compensation.
|
|
(2)
|
This represents the annual bonus payable to Mr. Thompson pursuant to the then current terms of his employment agreement. However, in light of the Company’s cash position, Mr. Thompson agreed to defer this bonus until such time as our cash position improves. See the section, “Employment Agreements” below for additional information regarding the payback terms of all of Mr. Thompson’s deferred compensation.
|
|
(3)
|
This amount includes: $49,833 in medical insurance and medical reimbursement we agreed to cover for Mr. Thompson pursuant to his employment agreement and $2,965 in reimbursement for office and travel related expenses. However, in light of the Company’s cash position, Mr. Thompson agreed to defer all such reimbursement until such time as our cash position improves. See the section, “Employment Agreements” below for additional information regarding the payback terms of these funds, which we deem “accounts payable – related party.”
|
|
(4)
|
This represents the annual salary payable to Mr. Thompson pursuant to the then current terms of his employment agreement. However, in light of the Company’s cash position, Mr. Thompson agreed to defer all such annual compensation until such time as our cash position improves. See the section, “Employment Agreements” below for additional information regarding the payback terms of the deferred compensation.
|
|
(5)
|
This represents the annual bonus payable to Mr. Thompson pursuant to the then current terms of his employment agreement. However, in light of the Company’s cash position, Mr. Thompson agreed to defer this bonus until such time as our cash position improves. See the section, “Employment Agreements” below for additional information regarding the payback terms of all of Mr. Thompson’s deferred compensation.
|
|
(6)
|
This amount includes: $48,565 in medical insurance and medical reimbursement we agreed to cover for Mr. Thompson pursuant to his employment agreement and $7,161 in reimbursement for office and travel related expenses. However, in light of the Company’s cash position, Mr. Thompson agreed to defer all such reimbursement until such time as our cash position improves. See the section, “Employment Agreements” below for additional information about regarding the payback terms of these funds, which we deem “accounts payable – related party.”
|
|
(7)
|
This represents the annual salary paid to Mr. Rice pursuant to the then current terms of his employment agreement. In 2019, Mr. Rice’s annual base salary was $150,774. However, in light of the Company’s cash position, the Company has deferred the payment of $23,076 of the $150,774. See the section, “Employment Agreements” below for additional information regarding the payback terms of all of Mr. Rice’s deferred compensation. In addition to his annual base salary Mr. Rice was reimbursed for $12,274 in medical insurance premiums and $1,020 in phone service expenses, pursuant to his employment agreement recorded and reported under “all other compensation”.
|
|
(8)
|
This represents the annual bonus payable to Mr. Rice pursuant to the then current terms of his employment agreement. However, in light of the Company’s cash position, the Company has deferred the payment of $20,000 of the $24,000. See the section, “Employment Agreements” below for additional information regarding the payback terms of all of Mr. Rice’s deferred compensation.
|
|
(9)
|
In 2019, Mr. Rice received $12,274 in medical insurance and medical reimbursement and $1,020 in phone service expenses and travel related, pursuant to his employment agreement.
|
|
(10)
|
This represents the annual salary that was paid to Mr. Rice pursuant to the then current terms of his employment agreement. In 2018, Mr. Rice’s annual base salary was $140,000, which includes $125,398 in wages and $15,324 in other expenses payable to Mr. Rice pursuant to the then current terms of his employment agreement, the latter of which are included in the “all other compensation” column.
|
|
(11)
|
This amount represents “all other compensation” payable to Mr. Rice pursuant to the then current terms of his employment agreement, including $14,284 in medical insurance and $1,040 in phone service expenses.
|
|
(12)
|
On July 3, 2019, the Board appointed Mr. Kenneth Le as the Company’s Director of government relations and President of Prodigy Textiles. Mr. Le’s employment agreement has a term of one year and can be terminated by either the Company or Mr. Le at any time. Under the employment agreement, Mr. Le is entitled to annual cash compensation of $60,000. In addition, Mr. Le was issued two three-year warrants to purchase 2,000,000 shares of Common Stock at an exercise price of $0.2299 per share, which are exercisable as of August 2021 and August 2022, respectively.
|
|
(13)
|
This amount represents the value of the warrants granted to Mr. Le in 2019.
|
Employment Agreements
Chief Executive Officer and Chief Financial
Officer
On November 10, 2010, the Company entered
into an employment agreement with Kim Thompson, its President, Chief Executive Officer, Chief Financial Officer and sole director,
effective January 1, 2011 through the December 31, 2015. The agreement was for a term of five years at an annual salary of $210,000
in 2011, with a 6% annual increase thereafter. For the year ended December 31, 2015, the annual salary was $281,027, but in light
of the Company’s cash position, Mr. Thompson deferred such compensation. On January 1, 2016, the agreement was renewed with
the same terms for another 5 years with an annual salary of $297,889 for the year ended December 31, 2016, but in light of the
Company’s cash position, Mr. Thompson deferred such compensation. On January 1, 2017, the agreement renewed with the same
terms for another 5 years, but with an annual salary of $315,764 for the year ended December 31, 2017, but in light of the Company’s
cash position, Mr. Thompson deferred such compensation. On January 1, 2018, the agreement renewed again with the same terms for
another 5 years, but with an annual salary of $334,708 for the year ended December 31, 2018, but in light of the Company’s
cash position, Mr. Thompson deferred such compensation. On January 1, 2019, the agreement renewed again with the same terms for
another 5 years, but with an annual salary of $354,791 for the year ended December 31, 2019. On January 1, 2020, the agreement
renewed again with the same terms for another 5 years, but with an annual salary of $376,078 for the year ended December 31, 2020.
As of June 30, 2020 and December 31, 2019, the accrued salary balance is $2,616,686 and $2,535,203, respectively. See,
“Certain Relationships And Related Transactions, And Director Independence - Accrued Salaries and Officer Loans –
Mr. Thompson, CEO/President.”
Base pay will be increased each January 1st,
for the subsequent twelve month periods by 6%. Mr. Thompson will also be entitled to life, disability, health and dental insurance
as well as an annual bonus in an amount equal to 20% of the base salary. In light of the Company cash position, Mr. Thompson declined
the life and disability insurance.
The agreement also calls for the retention
of the executive as a consultant following the termination of employment with compensation during such consultancy based upon the
Company reaching certain milestones:
Upon the expiration or termination of this
agreement for any reason, or by either party, Company agrees that it will employ Executive as a consultant for a period of four
(4) years and at a rate of $4,500 per month.
|
(a)
|
In the event that Company achieves gross sales of five million dollars ($5,000,000) or more, or one million dollars ($1,000,000) or more in net income, in any year during the term of this agreement, or upon the Company’s achieving an average market capitalization over a 240 consecutive calendar day period, in excess of $70,000,000 during the term of this agreement, then the consulting period will be for five (5) years and the consulting rate will be increased to $5,500 per month.
|
|
(b)
|
In the event that Company achieves gross sales of ten million dollars ($10,000,000) or more, or two million dollars ($2,000,000) or more in net income, in any year during the term of this agreement, or upon the Company’s achieving an average market capitalization over a 240 consecutive calendar day period, in excess of $90,000,000 during the term of this agreement, then the consulting period will be for six (6) years and the consulting rate will be increased to $7,500 per month.
|
Chief Operating Officer
On January 20, 2015, the Company entered into
an at-will employment agreement with Mr. Jonathan R. Rice, its Chief Operating Officer (the “2015 COO Employment Agreement”).
Although the 2015 COO Employment Agreement has been superseded (as described below), on January 23, 2015, Mr. Rice was issued
a three-year warrant to purchase 2,000,000 shares of common stock of the Company at an exercise price of $0.001 per share pursuant
to the COO Employment Agreement and on May 28, 2015, the Company issued a three-year warrant to purchase 3,000,000 shares of common
stock of the Company at an exercise price of $0.001 per share. The 3,000,000 share warrant fully vested on October 28,
2016. For the twelve months ended December 31, 2015, the Company recorded $121,448 for the warrants issued to Mr. Rice.
On January 14, 2016, the Company entered into
a new at-will employment agreement with Mr. Rice (the “2016 COO Employment Agreement”). The 2016 COO Employment Agreement
had a term of one year and can be terminated by either the Company or Mr. Rice at any time. Under the 2016 COO Employment Agreement,
Mr. Rice is entitled to an annual cash compensation of $140,000, which includes salary, health insurance, 401K retirement plan
contributions, and other benefits. In addition, on March 30, 2016, Mr. Rice was issued a three-year warrant to purchase 6,000,000
shares of common stock of the Company at an exercise price of $0.001 per share pursuant to the 2016 COO Employment Agreement. Additionally,
on August 4, 2016, the Company approved a performance retention bonus to Mr. Rice of $20,000 which was payable on March 31, 2018,
but which has not yet been paid. For the twelve months ended December 31, 2018, the Company recorded $0 for the warrants issued
to related party.
The Company extended the 2016 COO Employment
Agreement to a term ending on January 31, 2019. On March 15, 2019, the Company signed an extension of its at-will employment agreement
with its COO, extending the term to January 31, 2020. On May 19, 2020, the Company signed an extension of its at-will employment
agreement with its COO, extending the term to January 31, 2021. The COO Employment Agreement can be terminated by either
the Company or Mr. Rice at any time. For the twelve months ended December 31, 2018, the Company recorded $0 for the warrants issued
to related party.
On August 4, 2016, the Company issued an accommodation
to Mr. Rice awarding him a bonus of $20,000 payable on March, 31, 2018. In light of the Company’s cash position, this bonus
has been deferred.
On January 9, 2018, the Company extended the
expiration date of the January 2015 Warrant from January 19, 2018 to January 31, 2020. On January 10, 2020, the Company extended
the expiration date of the January 2015 Warrant from January 31, 2020 to January 10, 2025.
On April 26, 2019, the Company signed an agreement
to increase Mr. Rice’s base salary by $20,000 per year and issue a one-time $20,000 bonus. The salary increase and the bonus
is accrued and to be paid in full earlier by the direction of the Board or upon the earlier of:
|
|
●
|
The Company maintaining $6,000,000 or more in working capital;
|
|
|
●
|
Upon the transfer of ownership of more than 50% of the Corporation’s voting share or an assignment for the benefit of creditors or bankruptcy; or,
|
|
|
●
|
Upon the fifth year anniversary of the salary increase and the bonus issuance.
|
On October 21, 2019, the Company signed another
agreement to increase Mr. Rice’s base salary by another $20,000 per year (effective August 15, 2019). Such salary increase
is accrued and to be paid on the same terms as set forth above regarding the April 2019 increase.
Over the years, in light of our cash position,
certain amounts of Mr. Rice’s annual compensation have been deferred. As a result of these deferments, we accrued a total
of $82,902 and $64,352 in deferred compensation payable to Mr. Rice as of June 30, 2020 and December 31, 2019, respectively.
See, “Certain Relationships And Related Transactions, And Director Independence - Accrued Salaries and Officer Loans –
Mr. Rice, COO.”
Compensation of Directors
Directors are permitted to receive fixed fees
and other compensation for their services as directors. The Board has the authority to fix the compensation of directors. No amounts
have been paid to, or accrued to, directors in such capacity.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL
OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information
regarding the beneficial ownership of our Common Stock and Series A Preferred Stock as of the date of this Registration Statement
by (a) each stockholder who is known to us to own beneficially 5% or more of our outstanding Common Stock, (b) directors, (c) our
executive officers, and (d) all executive officers and directors as a group. Beneficial ownership is determined according to the
rules of the SEC, and generally means that person has beneficial ownership of a security if he or she possesses sole or shared
voting or investment power of that security and includes options, warrants and other securities convertible or exercisable into
shares of Common Stock, provided that such securities are currently exercisable or convertible or exercisable or convertible within
60 days of the date hereof. Each director or officer, as the case may be, has furnished us with information with respect to their
beneficial ownership. Except as otherwise indicated, all persons listed below have (i) sole voting power and investment power with
respect to their Common Stock, except to the extent that authority is shared by spouses under applicable law, and (ii) record and
beneficial ownership with respect to their Common Stock.
Title of
Class
|
|
Name and Address of
Beneficial Owner
|
|
Amount and
Nature of
Beneficial
|
|
|
Percent of
Class (1)
|
|
|
Percent of All
Voting Classes
|
|
|
Class A Common Stock
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Kim Thompson
|
|
|
209,468,162
|
(2)
|
|
|
24.52
|
%
|
|
|
16.70
|
%
|
|
|
|
2723 South State St Suite 150
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ann Arbor, MI 48104
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Jonathan R. Rice
|
|
|
11,000,000
|
(3)
|
|
|
1.29
|
%
|
|
|
0.88
|
%
|
|
|
|
2723 South State St Suite 150
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ann Arbor, MI 48104
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Anurag Gupta
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
2723 South State St Suite 150
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ann Arbor, MI 48104
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Julie R. Bishop
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
2723 South State St Suite 150
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ann Arbor, MI 48104
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Greg Scheessele
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
2723 South State St Suite 150
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ann Arbor, MI 48104
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Kenneth Le
|
|
|
2,300,000
|
(4)
|
|
|
0.27
|
%
|
|
|
0.18
|
%
|
|
|
|
2723 South State St Suite 150
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ann Arbor, MI 48104
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All executive officers and directors as a group (5 Persons)
|
|
|
222,768,160
|
|
|
|
26.07
|
%
|
|
|
17.76
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series A Preferred Stock
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Kim Thompson
|
|
|
2
|
|
|
|
100
|
%
|
|
|
31.89
|
%
|
|
|
|
2723 South State St Suite 150
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ann Arbor, MI 48104
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Jonathan R. Rice
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
2723 South State St Suite 150
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ann Arbor, MI 48104
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Anurag Gupta
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
2723 South State St Suite 150
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ann Arbor, MI 48104
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Julie R. Bishop
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
2723 South State St Suite 150
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ann Arbor, MI 48104
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Greg Scheessele
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
2723 South State St Suite 150
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ann Arbor, MI 48104
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Kenneth Le
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
2723 South State St Suite 150
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ann Arbor, MI 48104
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All executive officers and directors as a group (5 Persons)
|
|
|
2
|
|
|
|
100
|
%
|
|
|
31.89
|
%
|
(1) The percent of class is based on 854,410,001
shares of our Common Stock issued and outstanding as of the date hereof.
(2) Such shares include 209,468,160
shares of Common Stock that are owned by Mr. Thompson, and 2 shares of Common Stock that may be issued upon conversion of the
Series A Preferred Stock that are owned by Mr. Thompson. Mr. Thompson owns one warrant to purchase 20,000,000 shares of Common
Stock at an exercise price of $0.115, but he cannot receive such shares within the next 60 days because the earliest the warrants
are exercisable is February 2025.
(3) Mr. Rice owns 8 other warrants to purchase
a total of 12,000,000 shares of Common Stock at an exercise price ranging from $0.115 to $0.2299, but he cannot receive such shares
within the next 60 days because the earliest the warrants are exercisable is February 2021.
(4) Mr. Le owns two three-year warrants to
purchase a total of 2,000,000 shares of Common Stock at an exercise price of $0.2299 per share, but he cannot receive such shares
within the next 60 days because the earliest the warrants are exercisable is August 2021.
Change in Control
As of the date of this Registration Statement, there were no arrangements
which may result in a change in control of the Company.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Except as disclosed herein, no director, executive
officer, shareholder holding at least 5% of shares of our common stock, or any family member thereof, had any material interest,
direct or indirect, in any transaction, or proposed transaction since January 1, 2018, in which the amount involved in the transaction
exceeds the lesser of $120,000 or one percent of the average of our total assets at the year-end for the last two completed fiscal
years.
Related Party Transactions
Accrued Salaries and Officer Loans
Mr. Thompson, CEO, CFO, and President
Mr. Thompson agreed to
defer a significant portion of the compensation and other payments, as set forth below, owed to him. Mr. Thompson has also agreed
not to collect or accrue any salary while the Company has employees furloughed over concerns regarding the current global pandemic.
|
|
●
|
Annual Compensation: As of June 30, 2020, Kim
Thompson, our CEO accrued $2,616,686 of unpaid salary, which represents a portion of the annual compensation owed to him pursuant
to the terms of his employment agreements during such time period. As of June 30, 2020, there was $1,281,509
in accrued interest (collectively with the unpaid salary, the “Accrued CEO Salary”).
|
|
|
●
|
Company Loans: Mr. Thompson has loaned the Company an
aggregate of $1,552,000 and has been repaid $160,000, leaving a balance of $1,392,000 (including interest, the
CEO Loans”). As of June 30, 2020, there was $58,855 in loan interest.
|
|
|
●
|
Royalty Payments: Mr. Thompson was entitled to certain
royalties as compensation for the transfer of intellectual property he owned to the Company. As of June 30, 2020, there
was $65,292 in royalty payments payable to Mr. Thompson (“Royalty Payments”).
|
|
|
●
|
As of June 30, 2020, there was $319,005 included
in accounts payable and accrued expense payable to Mr. Thompson (“APAE,” together with Accrued CEO Salary,
CEO Loans and Royalty Payments, the “CEO Deferred Payments”).
|
Mr. Thompson also agreed to the following
repayment schedule of the CEO Deferred Payments:
|
|
·
|
$500,000
will be paid on the 3-month anniversary of the effective date of this registration statement;
|
|
|
·
|
50%
of the remaining balance will be paid on the 12-month anniversary of the effective date
of this registration statement; and,
|
|
|
·
|
The
remainder will be paid on the 18-month anniversary of the effective date of this registration
statement.
|
Mr. Rice, COO
As previously stated,
in light of our cash position, Mr. Rice agreed to defer a portion of the compensation owed to him. Between December 31, 2016 and
June 30, 2020, Mr. Rice accrued a total of $82,902 in unpaid salary (“Accrued COO Salary”).
In consideration of the
Company’s current and potential future cash position, the Company and Mr. Rice entered into an agreement in November 2019
regarding how the Company shall repay such salary. Pursuant to such agreement, the Company shall repay Mr. Rice in full either
by the direction of the Board or upon the earlier of:
|
|
●
|
The Company maintaining $6,000,000 or more in working capital;
|
|
|
●
|
Upon the transfer of ownership of more than 50% of the Corporation’s voting share or an assignment for the benefit of creditors or bankruptcy; or,
|
|
|
●
|
Upon the fifth-year anniversary of the salary increase and the bonus issuance.
|
Property Leases
On January 23, 2017, the Company signed an
8-year property lease with the Company’s CEO, CFO, President and controlling shareholder for land in Texas. The Company pays
$960 per month starting on February 1, 2017 and uses this facility to grow mulberry for its U.S. silk operations.
The Company is not a subsidiary of any company.
Related Party Policy
Our current Code of Ethics requires the CEO
and CFO to avoid, wherever possible, actual conflicts of interest in personal and professional
relationships; however, we have not yet adopted a formal policy for the review, approval or ratification of related party
transactions. Accordingly, the transactions discussed above were not reviewed, approved or ratified in accordance with any such
policy. A conflict of interest situation can arise when a person takes actions or has interests that may make it difficult to perform
his or her work objectively and effectively. Conflicts of interest may also arise if a person, or a member of his or her family,
receives improper personal benefits as a result of his or her position.
We also require each of our directors and executive
officers to annually complete a directors’ and officers’ questionnaire that elicits information about related party
transactions.
These procedures are intended to determine
whether any such related party transaction impairs the independence of a director or presents a conflict of interest on the part
of a director, employee or officer.
Prior to the consummation of this offering,
we will adopt a new code of ethics requiring us to avoid, wherever possible, all conflicts of interests, except under guidelines
or resolutions approved by our Board (or the appropriate committee of our board) or as disclosed in our public filings with the
SEC. Under our code of ethics, conflict of interest situations will include any financial transaction, arrangement or relationship
(including any indebtedness or guarantee of indebtedness) involving the company. A form of the code of ethics that we plan to adopt
prior to the consummation of this offering is filed as an exhibit to the registration statement of which this prospectus is a part.
In addition, our audit committee, pursuant
to a written charter that we will adopt prior to the consummation of this offering, will be responsible for reviewing and approving
related party transactions to the extent that we enter into such transactions. An affirmative vote of a majority of the members
of the audit committee present at a meeting at which a quorum is present will be required in order to approve a related party transaction.
A majority of the members of the entire audit committee will constitute a quorum. Without a meeting, the unanimous written consent
of all of the members of the audit committee will be required to approve a related party transaction. A form of the audit committee
charter that we plan to adopt prior to the consummation of this offering is filed as an exhibit to the registration statement of
which this prospectus is a part. We also require each of our directors and executive officers to complete a directors’ and
officers’ questionnaire that elicits information about related party transactions.
DESCRIPTION OF SECURITIES
General
Our original articles of incorporation authorized
60,000,000 shares of Class A common stock, 25,000,000 shares of Class B common stock with no par value per share and 10,000,000
shares of preferred stock with no par value per share on a pre-reverse split basis. On March 18, 2009, we amended our articles
of incorporation to provide for unlimited authorized shares, no par value, of Class A common stock and Class B common stock, and
preferred stock. In December 2013, we further amended our articles of incorporation to designate Series A of the Company’s
preferred stock, no par value; there are two shares of Series A preferred stock authorized. There are no provisions in our charter
or by-laws that would delay, defer or prevent a change in our control. As of the date hereof, we have 854,410,001 shares
of Class A common stock, 0 shares of Class B common stock and 2 shares of Series A Preferred Stock outstanding. The Class B common stock is not listed on the OTCQB or any other market and we are not seeking to have it listed
on Nasdaq or another national exchange.
On or prior to the effective date of the registration
statement of which this prospectus forms a part, we will amend our articles of incorporation to effect the Reverse Stock Split.
At our 2019 annual stockholder meeting, our stockholders approved a reverse stock split of the Company’s issued and
outstanding Common Stock by a ratio of not less than one-for-ten and not more than one-for-forty at any time prior to July 23,
2020, with the exact ratios to be set at a whole number within this range, as determined by our Board in its sole discretion and
approved and adopted the articles of amendment to our articles of incorporation to affect the same. As we continue the uplisting
process with Nasdaq, but are past July 23, 2020, such stockholder approval expired. However, on August [ ], 2020 we
obtained the written consent from a majority of our stockholders to effect the Reverse Stock Split.at a ratio of 1-for-[ ]
In line with
the Reverse Stock Split, on August [ ] 2020, the sole holder of our Series A Preferred Stock, Mr. Thompson, approved,
via written consent, to reduce the voting rights of the Series A preferred stock by a ratio of 1 for [ ], consistent
with the Reverse Stock Split (the “Reduced Voting Power”), to be effective as of the date of the Reverse Stock Split. Accordingly, the Series A Preferred Stock now represents
[ ] votes (31.89 % of the voting
power on all matters submitted to a stockholder vote). Our Board and a majority of our stockholders approved, via written consent,
amending our articles of incorporation to reflect the Reduced Voting Power. On or prior to the effective date of the registration
statement of which this prospectus forms a part, we will amend our articles of incorporation to effect the Reduced Voting Power.
The Reverse Stock Split will not impact
the number of authorized shares of Common Stock or Series A Preferred Stock.
Common Stock
As of August 20, 2020, 854,410,001
shares of Class A common stock were issued and outstanding and held by 32 stockholders of record, and we had no shares of
Class B common stock issued and outstanding. Holders of our Common Stock are entitled to one vote for each share on all matters
submitted to a stockholder vote; Class B common stock does not have any voting rights.
Holders of Common Stock do not have cumulative
voting rights.
Holders of a majority of the shares of Common
Stock voting for the election of directors can elect all of the directors. Holders of our common stock representing a majority
of the voting power of our capital stock issued and outstanding and entitled to vote, represented in person or by proxy, are necessary
to constitute a quorum at any meeting of our stockholders. A vote by the holders of a majority of our outstanding shares is required
to effectuate certain fundamental corporate changes such as liquidation, merger or an amendment to our Articles of Incorporation.
Although there are no provisions in our charter
or by-laws that may delay, defer or prevent a change in control, we are authorized, without stockholder approval, to issue shares
of preferred stock that may contain rights or restrictions that could have this effect.
Holders of both classes of common stock are
entitled to share in all dividends that the Board, in its discretion, declares from legally available funds. In the event of liquidation,
dissolution or winding up, each outstanding share entitles its holder to participate pro rata in all assets that remain after payment
of liabilities and after providing for each class of stock, if any, having preference over the common stock. Holders of both classes
of our common stock have no pre-emptive rights, no conversion rights and there are no redemption provisions applicable to our common
stock.
Preferred Stock
Our Board has the authority, without further
action by the stockholders, to issue from time to time the preferred stock in one or more series for such consideration and with
such relative rights, privileges, preferences and restrictions that the Board may determine. The preferences, powers, rights and
restrictions of different series of preferred stock may differ with respect to dividend rates, amounts payable on liquidation,
voting rights, conversion rights, redemption provisions, sinking fund provisions and purchase funds and other matters. The issuance
of preferred stock could adversely affect the voting power or other rights of the holders of common stock.
Effective December 17, 2013, the Company amended
its Articles of Incorporation to designate Series A of the Company’s preferred stock, no par value. Under the amendment,
there are two shares of Series A preferred stock authorized. The holders of Series A preferred stock are entitled to vote together
with the holders of the Company’s common stock on all matters upon which the Company’s stockholders may vote.
Each share of
Series A preferred stock is entitled to 200,000,000 votes on all such matters on a pre-reverse basis. Each share of Series
A preferred stock is convertible into one share of the Company’s common stock at the holder’s option. On December
19, 2013, the Company issued two shares of Series A preferred stock to Kim Thompson, the Company’s founder, CEO, CFO, President,
and sole director.
The shares of Series A preferred stock were
issued to Mr. Thompson in exchange for an agreement to extend to October, 30, 2014 the date on which the Company would pay certain
debts owed to Mr. Thompson. As part of the transaction, Mr. Thompson also agreed to forgive $30,000 which the Company owed to him
as compensation. In connection with the transaction, the Company incurred a loss on settlement of debt of $5,187,800.
Dividends
Since inception we have not paid any cash dividends
on our capital stock. We currently do not anticipate paying any cash dividends in the foreseeable future on our common stock, when
issued pursuant to this offering. Although we intend to retain our earnings, if any, to finance the exploration and growth of our
business, our Board will have the discretion to declare and pay dividends in the future. Payment of dividends in the future will
depend upon our earnings, capital requirements, and other factors, which our Board may deem relevant.
Certain Anti-Takeover Effects
Certain provisions of Wyoming law may have
an anti-takeover effect and may delay or prevent a tender offer or other acquisition transaction that a shareholder might consider
to be in his or her best interest. The summary of the provisions of Wyoming law set forth below does not purport to be complete
and is qualified in its entirety by reference to Wyoming law.
The issuance of shares of preferred stock,
the issuance of rights to purchase such shares, and the imposition of certain other adverse effects on any party contemplating
a takeover could be used to discourage an unsolicited acquisition proposal. For instance, the issuance of the preferred stock,
if the option to acquire such shares is exercised, would impede a business combination by the voting rights that would enable a
holder to block such a transaction. In addition, under certain circumstances, the issuance of other preferred stock could adversely
affect the voting power of holders of our common stock.
Under Wyoming law, a director, in determining
what he reasonably believes to be in or not opposed to the best interests of the corporation, does not need to consider only the
interests of the corporation’s stockholders in any takeover matter but may also, in his discretion, may consider any of the
following:
|
|
(i)
|
The interests of the corporation’s employees, suppliers, creditors and customers;
|
|
|
|
|
|
|
(ii)
|
The economy of the state and nation;
|
|
|
|
|
|
|
(iii)
|
The impact of any action upon the communities in or near which the corporation’s facilities or operations are located;
|
|
|
|
|
|
|
(iv)
|
The long-term interests of the corporation and its stockholders, including the possibility that those interests may be best served by the continued independence of the corporation; and
|
|
|
|
|
|
|
(v)
|
Any other factors relevant to promoting or preserving public or community interests.
|
The outstanding Series A Preferred Stock can
deter a takeover.
Because our Board is not required to make
any determination on matters affecting potential takeovers solely based on its judgment as to the best interests of our stockholders,
our board of directors could act in a manner that would discourage an acquisition attempt or other transaction that some, or a
majority, of our stockholders might believe to be in their best interests or in which such stockholders might receive a premium
for their stock over the then market price of such stock. Our Board of directors presently does not intend to seek stockholder
approval prior to the issuance of currently authorized stock, unless otherwise required by law or applicable stock exchange
rules.
Transfer Agent
The transfer agent for our Common Stock is
Olde Monmouth Stock Transfer Co., Inc.
Listing
Our Common stock is quoted on the OTCQB under
the trading symbol KBLB”. We have applied to have our Common Stock listed on the Nasdaq Capital Market under the symbol “KBLB”
and our Purchase Warrants listed on the Nasdaq Capital Market under the symbol “KBLBW”.
Limitation on Liability and Indemnification
Matters
See the section of this prospectus entitled
“Management — Indemnification”.
Penny Stock Regulation
The SEC has adopted regulations which generally
define “penny stock” to be any equity security that has a market price of less than Five Dollars ($5.00) per
share or an exercise price of less than Five Dollars ($5.00) per share. Such securities are subject to rules that impose additional
sales practice requirements on broker-dealers who sell them. For transactions covered by these rules, the broker-dealer must make
a special suitability determination for the purchaser of such securities and have received the purchaser’s written consent
to the transaction prior to the purchase. Additionally, for any transaction involving a penny stock, unless exempt, the rules require
the delivery, prior to the transaction, of a disclosure schedule prepared by the SEC relating to the penny stock market. The broker-dealer
also must disclose the commissions payable to both the broker-dealer and the registered representative, current quotations for
the securities and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer’s
presumed control over the market. Finally, among other requirements, monthly statements must be sent disclosing recent price information
for the penny stock held in the account and information on the limited market in penny stocks. As our Common Stock immediately
following this Offering may be subject to such penny stock rules, purchasers in this Offering will in all likelihood find it more
difficult to sell their Common Stock shares in the secondary market.
DESCRIPTION OF SECURITIES THAT WE ARE OFFERING
We are offering 1,904,762 Units in
this offering at an assumed initial offering price of $5.25 per Unit. Each Unit consisting of one share of our Common
Stock together with one Purchase Warrants to purchase one share of our Common Stock. The shares of Common Stock
accompanying Purchase Warrants will be issued separately. We are also registering the shares of Common Stock issuable from time
to time upon exercise of the Purchase Warrants offered hereby.
Common Stock
The material terms and provisions of our Common
Stock and each other class of our securities that qualifies or limits our Common Stock are described in the section entitled “Description
of Securities” in this prospectus.
Purchase Warrants to be Issued in this Offering
The
following summary of certain terms and provisions of the Purchase Warrants offered hereby is not complete and is subject to, and
qualified in its entirety by, the provisions of the warrant agent agreement between us and Olde Monmouth, as warrant agent,
and the form of Purchase Warrant, both of which are filed as exhibits to the registration statement of which this prospectus is
a part. Prospective investors should carefully review the terms and provisions set forth in the warrant agent agreement, including
the annexes thereto, and form of Purchase Warrant.
Form. Pursuant
to a warrant agent agreement between us and Olde Monmouth, as warrant agent, the Purchase Warrants will be issued in book-entry
form and shall initially be represented only by one or more global warrants deposited with the warrant agent, as custodian on
behalf of The Depository Trust Company, or DTC, and registered in the name of Cede & Co., a nominee of DTC, or as otherwise
directed by DTC.
Exercisability. The Purchase Warrants
are exercisable at any time after their original issuance and at any time up to the date that is five years after their original
issuance. The Purchase Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly
executed exercise notice and, at any time a registration statement registering the issuance of the shares of Common Stock underlying
the Purchase Warrants under the Securities Act is effective and available for the issuance of such shares, or an exemption from
registration under the Securities Act is available for the issuance of such shares, by payment in full in immediately available
funds for the number of shares of Common Stock purchased upon such exercise. If a registration statement registering the issuance
of the shares of Common Stock underlying the Purchase Warrants under the Securities Act is not effective or available and an exemption
from registration under the Securities Act is not available for the issuance of such shares, the holder may, in its sole discretion,
elect to exercise the Purchase Warrant through a cashless exercise, in which case the holder would receive upon such exercise the
net number of shares of Common Stock determined according to the formula set forth in the Purchase Warrant. No fractional shares
of Common Stock will be issued in connection with the exercise of a Purchase Warrant. In lieu of fractional shares, we will pay
the holder an amount in cash equal to the fractional amount multiplied by the exercise price.
Exercise Limitation. A holder will not
have the right to exercise any portion of the Purchase Warrant if the holder (together with its affiliates) would beneficially
own in excess of 4.99% (or, upon election of the holder, 9.99%) of the number of shares of our Common Stock outstanding immediately
after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Purchase Warrants.
However, any holder may increase or decrease such percentage, provided that any increase will not be effective until the 61st
day after such election.
Exercise Price. The Purchase Warrants
will have an exercise price of $5.25 per share, which is 100% of the assumed public offering price and which is the
midpoint of the price range set forth on the cover page of this prospectus. The exercise price is subject to appropriate adjustment
in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events
affecting our Common Stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Transferability. Subject to applicable
laws, the Purchase Warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing. There is no established
trading market for the Purchase Warrants. We intend to seek a listing for the Purchase Warrants on Nasdaq or other national
exchange under the symbol “KBLBW,” however we cannot assure you that we will be successful listing the Purchase
Warrants on Nasdaq or other national exchange or, if successful, that an active trading market for the Purchase Warrants
will develop or be sustained.
Fundamental Transactions. If a fundamental
transaction as defined in the Purchase Warrant occurs, then the successor entity will succeed to, and be substituted for us, and
may exercise every right and power that we may exercise and will assume all of our obligations under the Purchase Warrants with
the same effect as if such successor entity had been named in the Purchase Warrant itself. If holders of our Common Stock are given
a choice as to the securities, cash or property to be received in a fundamental transaction, then the holder shall be given the
same choice as to the consideration it receives upon any exercise of the Purchase Warrant following such fundamental transaction.
Rights as a Stockholder. Except as otherwise
provided in the Purchase Warrants or by virtue of such holder’s ownership of shares of our Common Stock, the holder of a
Purchase Warrant does not have the rights or privileges of a holder of our Common Stock, including any voting rights, until the
holder exercises the Purchase Warrant.
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, only a limited public
market for our Common Stock existed on the OTCQB. Future sales of substantial amounts of our Common Stock in the public market,
including shares issued upon exercise of outstanding warrants, or the anticipation of such sales, could adversely affect prevailing
market prices of our Common Stock from time to time and could impair our ability to raise equity capital in the future.
Upon the closing of this offering, we will
have [ ] shares of our Common Stock outstanding (assuming no exercise of the underwriter’s option to purchase additional
shares of Common Stock and/or Purchase Warrants). All of the shares sold in this offering will be freely tradable unless purchased
by our “affiliates,” as that term is defined in Rule 144 under the Securities Act of 1933, as amended, or the Securities
Act.
Lock-Up
For further details on the lock-up agreements,
see the section entitled “Underwriting – Lock Up Agreements.”
Rule 144
In general, under Rule 144 of the Securities
Act, as in effect on the date of this prospectus, any person who is not our affiliate at any time during the preceding three months,
and who has beneficially owned their shares for at least six months, including the holding period of any prior owner other than
one of our affiliates, would be entitled to sell an unlimited number of shares of our Common Stock provided current public information
about us is available, and, after owning such shares for at least one year, including the holding period of any prior owner other
than one of our affiliates, would be entitled to sell an unlimited number of shares of our Common Stock without restriction.
A person who is our affiliate or who was our
affiliate at any time during the preceding three months, and who has beneficially owned restricted securities for at least
six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell within any three-month
period a number of shares that does not exceed the greater of:
|
|
●
|
1% of the number
of shares of our Common Stock then outstanding, which will equal approximately 8,544,100 shares, or
|
|
|
●
|
the average weekly
trading volume of our Common Stock during the four calendar weeks preceding the filing of a Notice of Proposed Sale of Securities
pursuant to Rule 144 with respect to the sale.
|
Sales
under Rule 144 by our affiliates are also subject to manner of sale provisions and notice requirements and to the availability
of current public information about us.
Rule
701
In
general, under Rule of the Securities Act, any of our employees, directors, consultants or advisors who purchased shares from
us in connection with a qualified compensatory stock or option plan or other written agreement and in compliance with Rule 701,
is eligible to resell those shares 90 days after the effective date of the registration statement of which this prospectus
forms a part in reliance on Rule 144, but without compliance with the various restrictions, including the holding period,
contained in Rule 144.
CERTAIN U.S. FEDERAL INCOME TAX CONSIDERATIONS
This section summarizes certain U.S. federal
income tax considerations relating to the purchase, ownership and disposition of our Common Stock. This summary does not provide
a complete analysis of all potential tax considerations. The information provided below is based upon provisions of the Internal
Revenue Code of 1986, as amended (the “Code”), Treasury regulations promulgated thereunder and administrative rulings
and judicial decisions, all as currently in effect. These authorities may change at any time, possibly on a retroactive basis,
or the U.S. Internal Revenue Service (the “IRS”), might interpret the existing authorities differently. In either case,
the tax considerations of purchasing, owning or disposing of Common Stock could differ from those described below.
This discussion is addressed only to U.S. holders
(defined below) which hold our shares of Common Stock as a “capital asset” within the meaning of Section 1221 of the
Code (generally, property held for investment). This discussion does not address all of the U.S. federal income tax considerations
that might be relevant to a beneficial owner in light of such beneficial owner’s particular circumstances or to beneficial
owners subject to special treatment under the U.S. federal income tax laws, including:
|
|
●
|
a broker, dealer or trader in securities, currencies, commodities, or notional principal contracts;
|
|
|
●
|
a bank, financial institution or insurance company;
|
|
|
●
|
a regulated investment company, a real estate investment trust or grantor trust;
|
|
|
●
|
a tax-exempt entity or organization, including an individual retirement account or Roth IRA as defined in Section 408 or 408A of the Code, respectively;
|
|
|
●
|
a person holding the Common Stock as part of a hedging, integrated, or conversion transaction or a straddle, or a person deemed to sell Common Stock under the constructive sale provisions of the Code;
|
|
|
●
|
a trader in securities that has elected the mark-to-market method of tax accounting for securities;
|
|
|
●
|
an entity that is treated as a partnership or other pass-through entity for U.S. federal income tax purposes;
|
|
|
●
|
a person who is a partner or investor in a partnership or other pass-through entity that holds the Common Stock;
|
|
|
●
|
a U.S. person whose “functional currency” is not the U.S. dollar;
|
|
|
●
|
a controlled foreign corporation or passive foreign investment company;
|
|
|
●
|
a qualified foreign pension fund or an entity that is wholly owned by one or more qualified foreign pension funds; or
|
|
|
●
|
a U.S. expatriate.
|
For purposes of this discussion, a “U.S.
holder” is a beneficial owner of a share of Common Stock that is, for U.S. federal income tax purposes:
|
|
●
|
an individual who is a citizen or resident of the United States;
|
|
|
●
|
a corporation (or any other entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the District of Columbia;
|
|
|
●
|
an estate the income of which is subject to U.S. federal income taxation regardless of its source; or
|
|
|
●
|
a trust if (1) it is subject to the primary supervision of a court within the United States and one or more U.S. persons have the authority to control all substantial decisions of the trust or (2) it has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
|
For purposes of this discussion, a “non-U.S.
holder” is a beneficial owner of a share of Common Stock that is (i) a foreign corporation, (ii) a nonresident alien individual,
or (iii) a foreign estate or trust that in each case is not subject to U.S. federal income tax on a net-income basis on income
or gain from a share of Common Stock.
If a partnership holds shares of Common Stock,
the tax treatment of a partner will generally depend upon the status of the partner and the activities of the partnership. A partnership
holding shares of Common Stock or a partner therein should consult its own tax advisors as to the tax consequences of holding and
disposing of shares of Common Stock.
You are urged to consult your tax advisor
with respect to the application of the U.S. federal income tax laws to your particular situation, as well as any tax consequences
of the purchase, ownership and disposition of our Common Stock arising under the U.S. federal estate or gift tax rules or under
the laws of any U.S. state or local or any non-U.S. or other taxing jurisdiction or under any applicable tax treaty.
Certain U.S. Federal Income Tax Considerations
for U.S. Holders of Common Stock
Dividends on our Common Stock
We do not expect to declare or pay any distributions
on our Common Stock in the foreseeable future. If we do make any distributions on shares of our Common Stock, however, such distributions
will be includible in the gross income of a U.S. holder as ordinary dividend income to the extent paid out of current or accumulated
earnings and profits, as determined for U.S. federal income tax purposes. Any portion of a distribution in excess of current or
accumulated earnings and profits would be treated as a return of the holder’s tax basis in its Common Stock and then as gain
from the sale or exchange of the Common Stock. Under current law, if certain requirements are met, a preferential U.S. federal
income tax rate will apply to any dividends paid to a holder of Common Stock who is a U.S. individual.
Distributions to U.S. holders that are corporate
stockholders, constituting dividends for U.S. federal income tax purposes, may qualify for the dividends received deduction, or
DRD, which is generally available to corporate stockholders. No assurance can be given that we will have sufficient earnings and
profits (as determined for U.S. federal income tax purposes) to cause any distributions to be eligible for a DRD. In addition,
a DRD is available only if certain holding periods and other taxable income requirements are satisfied.
Sale of Common Stock
A U.S. holder of Common Stock will generally
recognize gain or loss on the taxable sale, exchange, or other taxable disposition of such stock in an amount equal to the difference
between such U.S. holder’s amount realized on the sale and its adjusted tax basis in the Common Stock sold. A U.S. holder’s
amount realized should equal the amount of cash and the fair market value of any property received in consideration of its stock.
The gain or loss should be capital gain or loss and should be long-term capital gain or loss if the Common Stock is held for more
than one year at the time of disposition. The deductibility of capital losses for U.S. federal income tax purposes is subject to
limitations under the Code. Under current law, long-term capital gain recognized by an individual U.S. holder is generally eligible
for a preferential U.S. federal income tax rate.
Information Reporting and Backup Withholding
Information reporting requirements generally
will apply to payments of dividends on shares of Common Stock and to the proceeds of a sale of Common Stock unless a U.S. holder
is an exempt recipient, such as a corporation. Backup withholding will apply to those payments if a U.S. holder fails to provide
its correct taxpayer identification number and certification of exempt status, or fails to report in full dividend income. Any
amounts withheld under the backup withholding rules will be allowed as a refund or a credit against U.S. federal income tax liability,
provided the required information is timely furnished to the IRS.
UNDERWRITING
Maxim Group LLC is acting as the sole book-running
manager and as representative of the underwriters of this offering (the “Representative”). Subject to the terms and
conditions of an underwriting agreement between us and the Representative, we have agreed to sell to each underwriter named below,
and each underwriter named below has severally agreed to purchase, at the public offering price less the underwriting discounts
set forth on the cover page of this prospectus, the number of shares of our Common Stock and corresponding Purchase Warrants listed
next to its name in the following table on a firm commitment basis:
|
Underwriter
|
|
Number of Shares of Common Stock
|
|
|
Number of Purchase Warrants
|
|
|
Maxim Group LLC
|
|
|
1,904,476
|
|
|
|
1,904,476
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
|
|
The underwriters are committed to purchase
all the shares of our Common Stock and corresponding Purchase Warrants offered by this prospectus if they purchase any shares of
our Common Stock and corresponding Purchase Warrants. The underwriting agreement also provides that if an underwriter defaults,
the purchase commitments of non-defaulting underwriters may be increased or the offering may be terminated. The underwriters are
not obligated to purchase the shares of our Common Stock and/or Purchase Warrants covered by the underwriters’ over-allotment
option described below. The underwriters are offering the shares of our Common Stock and corresponding Purchase Warrants, subject
to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, and other
conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer’s certificates and
legal opinions. The underwriters reserve the right to withdraw, cancel, or modify offers to the public and to reject orders in
whole or in part.
Over-Allotment Option
We have granted
to the underwriters an option, exercisable no later than 45 calendar days after the date of this prospectus, to purchase up to
an additional 285,715 shares of our Common Stock and/or Purchase Warrants to purchase 285,715 shares of our Common
Stock at the assumed public offering price
listed on the cover page of this prospectus, less underwriting discounts. The underwriters may exercise this option only to cover
over-allotments, if any, made in connection with this offering and may exercise this option to purchase additional shares of Common
Stock and/or Purchase Warrants. To the extent the option is exercised and the conditions of the underwriting agreement are satisfied,
we will be obligated to sell to the underwriters, and the underwriters will be obligated to purchase, these additional shares
of our Common Stock and/or Purchase Warrants.
Discounts and Commissions
We have agreed to provide the underwriters
with an underwriting discount equal to eight percent (8.0%) of the public offering price for all securities sold in this offering.
The Representative has advised us that the
underwriters propose to offer the shares of Common Stock and corresponding Purchase Warrants directly to the public at the public
offering price set forth on the cover of this prospectus. In addition, the Representative may offer some of the shares of Common
Stock and corresponding Purchase Warrants to other securities dealers at such price less a concession of up to $[●] per share
of Common Stock and corresponding Purchase Warrant. After the offering to the public, the offering price and other selling terms
may be changed by the Representative without changing the Company’s proceeds from the underwriters’ purchase of the
shares of Common Stock and corresponding Purchase Warrants.
The following table summarizes the public offering
price, underwriting discounts, and proceeds before estimated offering fees and expenses to us assuming both no exercise and full
exercise of the underwriters’ option to purchase additional shares of our Common Stock and/or Purchase Warrants. The underwriting
discounts are equal to the public offering price per share and related Purchase Warrants less the amount per share the underwriters
pay us for the shares of our common stock and Purchase Warrants.
|
|
|
|
|
|
Total
|
|
|
|
Per
Share
|
|
|
No
Exercise
|
|
|
Full
Exercise
|
|
Public
offering price
|
|
$
|
|
|
$
|
|
|
$
|
|
Underwriting
discount (1)
|
|
$
|
|
|
$
|
|
|
$
|
|
Proceeds
to us, before fees and expenses (2)
|
|
$
|
|
|
$
|
|
|
$
|
|
|
(1)
|
Assumes and underwriting discount of 8.0% of the public offering price for all securities sold in this offering.
|
|
|
(2)
|
We estimate that the total expenses of this offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, will be approximately $[●]. This figure includes expense reimbursements we have agreed to pay Maxim for reimbursement of its expenses related to the offering up to a maximum aggregate expense allowance of $125,000 (including any advance toward the maximum expense allowance). In accordance with FINRA Rule 5110, the reimbursement fee described in the preceding sentence is deemed underwriting compensation for this offering.
|
We have also agreed to indemnify the underwriters
against certain liabilities, including civil liabilities under the Securities Act or to contribute to payments that the underwriters
may be required to make in respect of those liabilities.
Except as disclosed in this prospectus, the
underwriters have not received, and will not receive, from us any other item of compensation or expense in connection with this
offering considered by FINRA to be underwriting compensation under its rule of fair price. The underwriting discount was determined
through an arms’ length negotiation between us and the underwriter.
Representative’s Warrants
We have agreed to issue to the
Representative (or its designed affiliates) share purchase warrants (the “Representative’s Warrants”) to
purchase up to a total of 8% of the shares of Common Stock sold in this offering. The Representative’s Warrants will be
non-exercisable for six (6) months after the effective date of the registration statement of which this prospectus forms a
part and will expire five (5) years after such effective date. The Representative’s Warrants will be exercisable at a
price equal to 110.0% of the public offering price in connection with this offering. The Representative’s Warrants
shall not be redeemable. The Company will register the shares of Common Stock underlying the Representative’s Warrants
under the Securities Act and will file all necessary undertakings in connection therewith. The Representative’s
Warrants may not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale,
derivative, put, or call transaction that would result in the effective economic disposition of the securities by any person
for a period of 180 days following the effective date of the registration statement of which this prospectus forms a part,
except that they may be assigned, in whole or in part, to any officer or partner of the Representative and to members of the
underwriting syndicate or selling group and officers and partners thereof. The Representative’s Warrants may be
exercised as to all or a lesser number of shares of Common Stock for a period of five (5) years after the effective date of
the registration statement of which this prospectus forms a part, will provide for cashless exercise and will contain
provisions for one demand registration of the sale of the underlying shares of common stock at the Company’s expense,
an additional demand registration at the warrant holders’ expense, and unlimited “piggyback” registration
rights at the Company’s expense for a period of five (5) and seven (7) years, respectively, following the
effective date of the registration statement of which this prospectus forms a part. The Representative’s Warrants shall
further provide for anti-dilution protection (adjustment in the number and price of such warrants and the shares underlying
such warrants) resulting from corporate events (which would include dividends, reorganizations, mergers, etc.) when the
public stockholders have been proportionally affected and otherwise in compliance with FINRA Rule 5110(f)(2)(G)(vi).
Right of First Refusal
For a period of twelve (12) months
from the commencement of sales of the Offering, the Representative is granted the right of first refusal to act as lead
managing underwriter and book running manager or minimally as co-lead manager and co-book runner and/or co-lead placement agent
with at least 100% of the economics for any and all of our future public and private equity, equity-linked, convertible or debt
(excluding commercial bank debt) offerings during such twelve (12) month period of the Company, or any successor to or any subsidiary
of the Company.
Determination
of Offering Price
Prior to this offering, there has only been
limited public market for our Common Stock. The public offering price of the shares of Common Stock was determined by negotiation
between us and the Representative. The principal factors considered in determining the public offering price of the shares of
Common Stock, including the exercise price of the Warrants included:
|
●
|
the information in this prospectus and otherwise available to the underwriters, including our financial information;
|
|
|
|
|
●
|
the history and the prospects for the industry in which we compete;
|
|
|
|
|
●
|
the ability of our management;
|
|
|
|
|
●
|
the prospects for our future earnings;
|
|
|
|
|
●
|
the present state of our development and our current financial condition;
|
|
|
|
|
●
|
the general condition of the economy and the securities markets in the United States at the time of this offering;
|
|
|
|
|
●
|
the recent market prices of, and the demand for, publicly-traded securities of generally comparable companies; and
|
|
|
|
|
●
|
other factors as were deemed relevant.
|
We cannot be sure that the public offering
price will correspond to the price at which the shares will trade in the public market following this offering or that an active
trading market for the shares will develop or continue after this offering.
Lock-Up Agreements
We and each
of our officers, and directors have
agreed not to offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any
Common Stock or other securities convertible into or exercisable or exchangeable for Common Stock for a period of six months
from the date this offering is completed without the prior written consent of the Representative.
The Representative may in its sole discretion
and at any time without notice release some or all of the shares subject to lock-up agreements prior to the expiration of the lock-up
period. When determining whether or not to release shares from the lock-up agreements, the underwriters will consider, among other
factors, the security holder’s reasons for requesting the release, the number of shares for which the release is being requested
and market conditions at the time.
Price Stabilization, Short Positions and
Penalty Bids
In connection with this offering, the underwriters
may engage in transactions that stabilize, maintain or otherwise affect the price of our common stock. Specifically, the underwriters
may over-allot in connection with this offering by selling more Units than are set forth on the cover page of this prospectus.
This creates a short position in our Common Stock and/or Purchase Warrants for its own account. The short position may
be either a covered short position or a naked short position. In a covered short position, the number of shares Common Stock and/or
Purchase Warrants over-allotted by the underwriters is not greater than the number of shares of our Common Stock and/or
Purchase Warrants that they may purchase in the over-allotment option. In a naked short position, the number of shares of
our Common Stock and/or Purchase Warrants involved is greater than the number of shares Common Stock and/or Purchase
Warrants in the over-allotment option. To close out a short position, the underwriters may elect to exercise all or part of
the over-allotment option. The underwriters may also elect to stabilize the price of our Common Stock and/or Purchase Warrants
or reduce any short position by bidding for, and purchasing, Common Stock in the open market.
The underwriters may also impose a penalty
bid. This occurs when a particular underwriter or dealer repays selling concessions allowed to it for distributing a security in
this offering because the underwriter repurchases that security in stabilizing or short covering transactions.
Finally, the underwriters may bid for, and
purchase, shares of our Common Stock in market making transactions, including “passive” market making transactions
as described below.
These activities may stabilize or maintain
the market price of our Common Stock at a price that is higher than the price that might otherwise exist in the absence of these
activities. The underwriters are not required to engage in these activities, and may discontinue any of these activities at any
time without notice.
In connection with this offering, the underwriters
and selling group members, if any, or their affiliates may engage in passive market making transactions in our Common Stock immediately
prior to the commencement of sales in this offering, in accordance with Rule 103 of Regulation M under the Exchange Act. Rule 103
generally provides that:
●
a passive market maker may not effect transactions or display bids for our Common Stock in excess of the highest independent bid
price by persons who are not passive market makers;
●
net purchases by a passive market maker on each day are generally limited to 30% of the passive market maker’s average daily
trading volume in our common stock during a specified two-month prior period or 200 shares, whichever is greater, and must be
discontinued when that limit is reached; and
●
passive market making bids must be identified as such.
Electronic
Distribution
A
prospectus in electronic format may be made available on a website maintained by the representatives of the underwriters and may
also be made available on a website maintained by other underwriters. The underwriters may agree to allocate a number of shares
to underwriters for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives
of the underwriters to underwriters that may make Internet distributions on the same basis as other allocations. In connection
with the offering, the underwriters or syndicate members may distribute prospectuses electronically. No forms of electronic prospectus
other than prospectuses that are printable as Adobe® PDF will be used in connection with this offering.
The
underwriters have informed us that they do not expect to confirm sales of shares offered by this prospectus to accounts over which
they exercise discretionary authority.
Other
than the prospectus in electronic format, the information on any underwriter’s website and any information contained in
any other website maintained by an underwriter is not part of the prospectus or the registration statement of which this prospectus
forms a part, has not been approved and/or endorsed by us or any underwriter in its capacity as underwriter and should not be
relied upon by investors.
Affiliations
Certain
of the underwriters and their affiliates may provide, from time to time, investment banking and financial advisory services to
us in the ordinary course of business, for which they may receive customary fees and commissions.
Foreign
Regulatory Restrictions on Purchase of our Shares
We
have not taken any action to permit a public offering of our shares outside the United States or to permit the possession or distribution
of this prospectus outside the United States. People outside the United States who come into possession of this prospectus must
inform themselves about and observe any restrictions relating to this offering of our shares and the distribution of this prospectus
outside the United States.
Indemnification
We
have agreed to indemnify the underwriters against liabilities relating to the offering arising under the Securities Act and the
Exchange Act and to contribute to payments that the underwriters may be required to make for these liabilities.
Selling
Restrictions
Canada.
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited
investors, as defined in National Instrument 45 106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario),
and are permitted clients, as defined in National Instrument 31 103 Registration Requirements, Exemptions and Ongoing Registrant
Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject
to, the prospectus requirements of applicable securities laws.
Securities
legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this
prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages
are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province
or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province
or territory for particulars of these rights or consult with a legal advisor.
Pursuant
to section 3A.3 of National Instrument 33 105 Underwriting Conflicts (NI 33 105), the underwriters are not required to
comply with the disclosure requirements of NI 33 105 regarding underwriter conflicts of interest in connection with this offering.
DISCLOSURE
OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons
controlling the registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the SEC
such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
LEGAL
MATTERS
The
validity of the shares of our Common Stock offered hereby and other legal matters concerning this Offering relating to Wyoming
law will be passed upon for us by Hathaway & Kunz, LLP, Cheyenne, Wyoming. Certain legal matters in connection with this Offering
with respect to the United States federal securities law and New York law will be passed upon for us by Hunter Taubman Fischer
& Li LLC, New York, New York. Ellenoff Grossman & Schole LLP, is acting as counsel to the underwriters in connection with
the securities offered hereby.
EXPERTS
The
consolidated financial statements as of December 31, 2019 and 2018, included in this prospectus have been audited and so included
in reliance on the report of M&K CPAS, PLLC, an independent registered public accounting firm, given on the authority of said
firm as experts in auditing and accounting.
WHERE
YOU CAN FIND ADDITIONAL INFORMATION
We
have filed with the SEC a registration statement on Form S-1, including relevant exhibits and schedules under the Securities Act,
covering the Common Stocks offered by this prospectus. You should refer to our registration statements and their exhibits and
schedules if you would like to find out more about us and about the Common Stocks. This prospectus summarizes material provisions
of contracts and other documents that we refer you to. Since the prospectus may not contain all the information that you may find
important, you should review the full text of these documents.
The
registration statements, reports and other information so filed can be inspected and copied at the public reference facilities
maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. You can request copies of these documents upon payment of
a duplicating fee, by writing to the SEC. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the
public reference rooms. The SEC also maintains a website that contains reports, proxy statements and other information about issuers,
such as us, who file electronically with the SEC. The address of that website is http://www.sec.gov. The information on
that website is not a part of this prospectus.
No
dealers, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus.
You must not rely on any unauthorized information or representations. This prospectus is an offer to sell only the securities
offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this
prospectus is current only as of its date.
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
Item
13. Other Expenses of Issuance and Distribution
The
following table sets forth the various expenses, all of which will be borne by the registrant, in connection with the sale and
distribution of the securities being registered, other than the underwriting discounts and commissions. All amounts shown are
estimates except for the SEC registration fee and the FINRA filing fee.
|
SEC registration fee
|
|
$
|
3,116.76
|
|
|
FINRA filing fee
|
|
$
|
4,102
|
|
|
Nasdaq initial and listing fees
|
|
$
|
80,000
|
|
|
Accounting fees and expenses
|
|
$
|
*
|
|
|
Legal fees and expenses
|
|
$
|
*
|
|
|
Printing and Engraving
|
|
$
|
*
|
|
|
Transfer agent and registrar fees
|
|
$
|
*
|
|
|
Miscellaneous
|
|
$
|
*
|
|
|
Total
|
|
$
|
*
|
|
*
To be provided by amendment.
Item
14. Indemnification of Directors and Officers.
Pursuant
to the Registrant’s Articles of Incorporation, as amended and Bylaws, the Registrant may indemnify any person (including
his estate) made or threatened to be made a party to any suit or proceeding, whether civil or criminal, by reason of the fact
that he was a director or officer of the Registrant or served at the Registrant’s request as a director or officer of a
subsidiary of the Registrant, against judgments, fines, amounts paid in settlement and reasonable expenses, including attorney
fees actually and necessarily incurred as a result of such threat, suit or proceeding, or any appeal therein, to the fullest extent
permitted by the General Corporation Law of the State of Wyoming.
Item
15. Recent Sales of Unregistered Securities.
During the last three years, the Registrant
has not issued unregistered securities to any person, except as described below. None of these transactions involved any underwriters,
underwriting discounts or commissions, except as specified below, or any public offering, and, unless otherwise indicated below,
the Registrant believes that each transaction was exempt from the registration requirements of the Securities Act by virtue of
Section 4(a)(2) thereof and/or Rule 506 of Regulation D promulgated thereunder, and/or Regulation S promulgated thereunder regarding
offshore offers and sales. All recipients had adequate access, though their relationships with the Registrant, to information about
the Registrant.
On
January 25, 2017, the Company issued 750,000 shares of common stock to a consultant for services rendered.
On
February 6, 2017 the Company issued a warrant for 750,000 share of common stock to a consultant for services rendered.
On February 9, 2018, the Company issued 3-year
warrant to purchase 3,000,000 shares of common stock at an exercise price of $0.056 per share to a consultant for services rendered.
The warrants had a fair value of $52,660, based upon the Black-Scholes option-pricing model on the date of grant and are fully
vested on the date granted. Warrants will be exercisable on August 9, 2019, and for a period of 2 years expiring on August 9,
2021. During the year ended December 31, 2018, the Company recorded $52,660 as an expense for warrants issued.
On March 20, 2018, the Company issued 4-year
warrant to purchase 600,000 shares of common stock at an exercise price of $0.001 per share to a consultant for services rendered.
The warrants had a fair value of $19,915, based upon the Black-Scholes option-pricing model on the date of grant and fully vested
on March 20, 2018. Warrants will be exercisable on March 20, 2019, and for a period of 3 years expiring on March 20, 22. During
the year ended December 31, 2018, the Company recorded $19,915 as an expense for warrants issued. The warrants were canceled
during the fiscal year ended December 31, 2019 in exchange for a cash payment of $6,000.
On
April 6, 2018, the Company issued 36,000 shares with a fair value of $1,076 ($0.0299/share) to a consultant as consideration for
consulting fees owed from October 1, 2014 through December 31, 2018 of $21,000.
On
March 9, 2019, the Company entered into a purchase agreement with one investor (the “Purchase Agreement”). Pursuant
to the Purchase Agreement, the Company issued the investor 14,797,278 Units at a purchase price of $0.06758 per Unit, for total
gross proceeds to the Company of $1,000,000. The Units consist of 14,797,278 shares of the Company’s common stock and two
warrants (the “Warrants”): (i) one warrant entitles the investor to purchase up to 14,797,278 shares of common stock
at an exercise price of $0.06 per share (the “6 Cent Warrants”) and (ii) one warrant entitles the investor to purchase
up to 7,398,639 shares of common stock at an exercise price of $0.08 per share (the “8 Cent Warrant”). The securities
sold in the private placement were issued in reliance on an exemption from registration under Regulation S of the Securities Act
of 1933, as amended (“Regulation S”). The bases for the availability of this exemption include the facts that the
sales of the securities were made to a non-U.S. person (as defined under Rule 902 section (k)(2)(i) of Regulation S), pursuant
to an offshore transaction, and no directed selling efforts were made in the United States by the issuer, a distributor, any of
their respective affiliates, or any person acting on behalf of any of the foregoing.
On
March 20, 2019, the Company issued 4,052,652 shares of its Common Stock as payment for $243,159 of certain debt owed to Notre
Dame.
On
August 8, 2019, the Company issued a set of four (4) warrants totaling 500,000 shares to a related party, with an exercise price
of $0.2299 per share.
On
August 8, 2019, the Company issued a set of two (2) warrants totaling 2,000,000 shares to a related party, with an exercise price
of $0.2299 per share.
On
August 8, 2019, the Company issued a set of three (3) warrant totaling 6,000,000 shares to a related party, with an exercise price
of $0.2299 per share.
On
August 14, 2019, the Company issued 7,967,871 shares in connection with the cashless exercise of the 8,000,000 warrants.
On
September 26, 2019, the Company issued 766,667 shares is connection with the cashless exercise of 1,000,000 warrants.
On July 30, 2020, the Company issued
9,941,623 shares of common stock in connection with the cashless exercise of 10,000,000 warrants.
Item
16. Exhibits and Financial Statement Schedules.
(a)
The following exhibits are filed as part of this Registration Statement:
EXHIBIT
INDEX
|
EXHIBIT
NUMBER
|
|
DESCRIPTION
|
|
|
|
|
|
1.1
|
|
Form
of Underwriting Agreement**
|
|
|
|
|
|
3.1
|
|
Articles of Incorporation (1)
|
|
|
|
|
|
3.2
|
|
Articles of Amendment (3)
|
|
|
|
|
|
3.3
|
|
Articles of Amendment, filed with the Wyoming Secretary of State on November 15, 2013 (6)
|
|
|
|
|
|
3.4
|
|
Articles of Amendment, filed with the Wyoming Secretary of State on December 17, 2013 (7)
|
|
|
|
|
|
3.5
|
|
By-Laws (1)
|
|
|
|
|
|
4.1
|
|
Form of Warrant issued Mr. Jonathan R. Rice (14)
|
|
|
|
|
|
4.2
|
|
Form
of Purchase Warrant**
|
|
|
|
|
|
4.3
|
|
Form
of Representative’s Warrant**
|
|
|
|
|
|
4.4
|
|
Form of Warrant Agent Agreement**
|
|
|
|
|
|
4.5
|
|
Form of Lock-Up Agreement**
|
|
|
|
|
|
5.1
|
|
Opinion
of Wyoming counsel, as to the validity of the Common Stock **
|
|
|
|
|
|
5.2
|
|
Opinion
of US counsel, as to the validity of the Common Stock **
|
|
|
|
|
|
5.3
|
|
Opinion
of [ ], as to U.S. federal tax matters **
|
|
|
|
|
|
10.1
|
|
Employment Agreement, dated November 10, 2010, by and between Kraig Biocraft Laboratories, Inc. and Kim Thompson (8)
|
|
|
|
|
|
10.6
|
|
Addendum to the Founder’s Stock Purchase and Intellectual Property Transfer Agreement, dated December 26, 2006, and the Founder’s Stock Purchase and Intellectual Property Transfer Agreement dated April 26, 2006 (3)
|
|
|
|
|
|
10.7
|
|
Intellectual Property/Collaborative Research Agreement, dated March 20, 2010, by and between Kraig Biocraft Laboratories and The University of Notre Dame du Lac. (2)
|
|
|
|
|
|
10.11
|
|
License Agreement, dated October 28, 2011, between the Company and University of Notre Dame du Lac. (12)
|
|
|
|
|
|
10.12
|
|
Intellectual Property / Collaborative Research Agreement, dated June 6, 2012, between the Company and University of Notre Dame du Lac. (12)
|
|
|
|
|
|
10.14
|
|
Employment Agreement, dated January 19, 2015, between the Company and Mr. Jonathan R. Rice (11)
|
|
|
|
|
|
10.15
|
|
Intellectual Property and Collaborative Research Agreements, dated March 4, 2015, between the Company and University of Notre Dame du Lac. (15)
|
|
|
|
|
|
10.16
|
|
2019 Employee Stock Option Plan (16)
|
|
|
|
|
|
14.1
|
|
Code of Business Conduct and Ethics (13)
|
|
|
|
|
|
14.2
|
|
Code of Ethics (17)
|
|
101.INS
|
|
XBRL Instance Document *
|
|
101.CAL
|
|
XBRL Taxonomy Extension Calculation Linkbase Document *
|
|
101.SCH
|
|
XBRL Taxonomy Extension Schema Document *
|
|
101.DEF
|
|
XBRL Taxonomy Extension Definition Linkbase Document *
|
|
101.LAB
|
|
XBRL Taxonomy Extension Labels Linkbase Document *
|
|
101.PRE
|
|
XBRL Taxonomy Extension Presentation Linkbase Document *
|
*Filed
herewith
**
To be filed by amendment
(1)
Incorporated by reference to our Registration Statement on Form SB-2 (Reg. No. 333-146316) filed with the SEC on September 26,
2007.
(2)
Incorporated by reference to our annual report on Form 10-K for the year ended December 31, 2009 filed with the SEC on April 15,
2010.
(3)
Incorporated by reference to our Registration Statement on Form S-1 (Reg. No. 333-162316) filed with the SEC on October 2, 2009.
(4)
Incorporated by reference to our Current Report on Form 8-K filed with the SEC on June 29, 2011.
(5)
Incorporated by reference to our Quarterly Report on Form 10-Q filed with the SEC on May 15, 2013.
(6)
Incorporated by reference to our Current Report on Form 8-K filed with the SEC on November 22, 2013.
(7)
Incorporated by reference to our Current Report on Form 8-K filed with the SEC on December 19, 2013.
(8)
Incorporated by reference to our Registration Statement on Form S-1 (Reg. No. 333-175936) filed with the SEC on August 1, 2011.
(9)
Incorporated by reference to our Registration Statement on Form S-1 (Reg. No. 333-199820) filed with the SEC on November 3, 2014.
(10)
Incorporated by reference to our Amendment No. 1 to Registration Statement on Form S-1/A (Reg. No. 333-199820) filed with the
SEC on January 7, 2015.
(11)
Incorporated by reference to our Current Report on Form 8-K filed with the SEC on January 21, 2015.
(12)
Incorporated by reference to our Amendment No. 2 to Registration Statement on Form S-1/A (Reg. No. 333-199820) filed with the
SEC on January 30, 2015.
(13)
Incorporated by reference to Exhibit 14.1 to our Annual Report on Form 10-KSB for the year ended December 31, 2007 filed with
the SEC on March 26, 2008.
(14)
Incorporated by reference to Exhibit 4.1 to the Annual Report on Form 10-K filed on March 31, 2015.
(15)
Incorporated by reference to Exhibit 10.15 to the Annual Report on Form 10-K filed on March 31, 2015.
(16)
Incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2019 filed with the SEC on March 27,
2020.
(17) Incorporated by reference to our Registration
Statement on Form S-1 (Reg. No. 333-238883) filed with the SEC on June 2, 2020.
Item
17. Undertakings.
The
undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement,
certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each
purchaser.
Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling
persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion
of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and
is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by
the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense
of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities
being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent,
submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed
in the Securities Act and will be governed by the final adjudication of such issue.
The
undersigned registrant hereby undertakes:
(1)
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i)
To include any prospectus required by section 10(a)(3) of the Securities Act of 1933, as amended;
(ii)
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent
post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set
forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if
the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high
end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule
424(b) (§230.424(b) of this chapter) if, in the aggregate, the changes in volume and price represent no more than 20% change
in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective
registration statement.
(iii)
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement
or any material change to such information in the registration statement;
(2)
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall
be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at
that time shall be deemed to be the initial bona fide offering thereof.
(3)
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold
at the termination of the offering.
The
undersigned registrant hereby undertakes that:
(1)
For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus
filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant
pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement
as of the time it was declared effective.
(2)
For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form
of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering
of such securities at that time shall be deemed to be the initial bona fide offering thereof.
The
undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each
filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934
(and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities
Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration
statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the
initial bona fide offering thereof.
SIGNATURES
Pursuant to the requirements
of the Securities Act of 1933, the Registrant has duly caused this Pre-Effective Amendment 1 to the registration statement
to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of New York, NY, on August 24, 2020.
|
|
KRAIG
BIOCRAFT LABORATORIES, INC.
|
|
|
|
|
|
|
By:
|
/s/
Kim Thompson
|
|
|
|
President,
Chief Executive Officer and Chief Financial Officer
|
|
|
|
(Principal
Executive Officer and Principal Financial and Accounting Officer)
|
SIGNATURES
Pursuant to the requirements of the Securities
Act of 1933, this Pre-Effective Amendment 1 to the registration statement has been signed by the following persons in the
capacities and on the dates indicated.
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/
Kim Thompson
|
|
President,
Chief Executive Officer, Chief
|
|
August
24, 2020
|
|
Kim
Thompson
|
|
Financial Officer and Sole Director
|
|
|
KRAIG
BIOCRAFT LABORATORIES, INC.
INDEX
TO FINANCIAL STATEMENTS
Unaudited
Interim Financial Statements for the Six Months Ended June 30, 2020
Audited
Consolidated Financial Statements for the Fiscal Years Ended December 31, 2019 and 2018
Kraig Biocraft Laboratories, Inc. and Subsidiary
Kraig
Biocraft Laboratories, Inc. and Subsidiary
Condensed
Consolidated Balance Sheets
|
|
|
June 30,
2020
|
|
|
December
31, 2019
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Assets
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
54,710
|
|
|
$
|
125,024
|
|
|
Prepaid expenses
|
|
|
8,472
|
|
|
|
31,745
|
|
|
Total Current Assets
|
|
|
63,182
|
|
|
|
156,769
|
|
|
|
|
|
|
|
|
|
|
|
|
Property and Equipment, net
|
|
|
104,203
|
|
|
|
117,321
|
|
|
Operating lease right-of-use asset, net
|
|
|
415,249
|
|
|
|
473,242
|
|
|
Security deposit
|
|
|
3,518
|
|
|
|
3,518
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Assets
|
|
$
|
586,152
|
|
|
$
|
750,850
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’
DEFICIT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Liabilities
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued expenses
|
|
$
|
488,500
|
|
|
$
|
560,948
|
|
|
Note payable - related party
|
|
|
1,192,000
|
|
|
|
642,000
|
|
|
Note payable
|
|
|
|
|
|
|
|
|
|
Royalty agreement payable - related party
|
|
|
65,292
|
|
|
|
65,292
|
|
|
Accounts payable and accrued expenses - related party
|
|
|
4,422,515
|
|
|
|
4,145,465
|
|
|
Operating lease liability, current
|
|
|
117,666
|
|
|
|
110,678
|
|
|
Loan payable
|
|
|
60,000
|
|
|
|
60,000
|
|
|
SBA Paycheck Protection Loan
|
|
|
90,100
|
|
|
|
-
|
|
|
Total Current Liabilities
|
|
|
6,436,073
|
|
|
|
5,584,383
|
|
|
|
|
|
|
|
|
|
|
|
|
Long Term Liabilities
|
|
|
|
|
|
|
|
|
|
Loan payable, net of current
|
|
|
155,244
|
|
|
|
185,244
|
|
|
Operating lease liability, net
of current
|
|
|
308,438
|
|
|
|
369,281
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Liabilities
|
|
|
6,899,755
|
|
|
|
6,138,908
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and Contingencies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Deficit
|
|
|
|
|
|
|
|
|
|
Preferred stock, no par value; unlimited shares authorized,
none, issued and outstanding
|
|
|
-
|
|
|
|
-
|
|
|
Preferred stock Series A, no par value; 2 and 2 shares
issued and outstanding, respectively
|
|
|
5,217,800
|
|
|
|
5,217,800
|
|
|
Common stock Class A, no par value; unlimited shares
authorized, 844,468,378 and 844,468,378 shares issued and outstanding, respectively
|
|
|
16,757,079
|
|
|
|
16,757,079
|
|
|
Common Stock Issuable, 1,122,311 and 1,122,311 shares,
respectively
|
|
|
22,000
|
|
|
|
22,000
|
|
|
Additional paid-in capital
|
|
|
5,107,560
|
|
|
|
2,412,969
|
|
|
Accumulated Deficit
|
|
|
(33,418,042
|
)
|
|
|
(29,797,906
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total Stockholders’ Deficit
|
|
|
(6,313,603
|
)
|
|
|
(5,388,058
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total Liabilities and Stockholders’
Deficit
|
|
$
|
586,152
|
|
|
$
|
750,850
|
|
Kraig
Biocraft Laboratories, Inc. and Subsidiary
Condensed
Consolidated Statements of Operations
(Unaudited)
|
|
|
For the Three Months Ended
|
|
|
For the Six Months Ended
|
|
|
|
|
June 30,
2020
|
|
|
June 30,
2019
|
|
|
June 30,
2020
|
|
|
June 30,
2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and Administrative
|
|
|
348,278
|
|
|
|
122,186
|
|
|
|
3,141,059
|
|
|
|
240,153
|
|
|
Professional Fees
|
|
|
31,100
|
|
|
|
37,917
|
|
|
|
50,474
|
|
|
|
188,228
|
|
|
Officer’s Salary
|
|
|
54,853
|
|
|
|
148,050
|
|
|
|
199,415
|
|
|
|
266,205
|
|
|
Rent - Related Party
|
|
|
3,128
|
|
|
|
6,153
|
|
|
|
6,263
|
|
|
|
9,426
|
|
|
Research and Development
|
|
|
32,970
|
|
|
|
20,937
|
|
|
|
37,782
|
|
|
|
43,241
|
|
|
Total Operating Expenses
|
|
|
470,329
|
|
|
|
335,243
|
|
|
|
3,434,993
|
|
|
|
747,253
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from Operations
|
|
|
(470,329
|
)
|
|
|
(335,243
|
)
|
|
|
(3,434,993
|
)
|
|
|
(747,253
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other Income/(Expenses)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
(98,468
|
)
|
|
|
(70,695
|
)
|
|
|
(185,143
|
)
|
|
|
(137,615
|
)
|
|
Interest income
|
|
|
-
|
|
|
|
3,441
|
|
|
|
-
|
|
|
|
4,625
|
|
|
Total Other Income/(Expenses)
|
|
|
(98,468
|
)
|
|
|
(67,254
|
)
|
|
|
(185,143
|
)
|
|
|
(132,990
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (Loss) before Provision for Income Taxes
|
|
|
(568,797
|
)
|
|
|
(402,497
|
)
|
|
|
(3,620,136
|
)
|
|
|
(880,243
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provision for Income Taxes
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (Loss)
|
|
$
|
(568,797
|
)
|
|
$
|
(402,497
|
)
|
|
$
|
(3,620,136
|
)
|
|
$
|
(880,243
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Income (Loss) Per Share - Basic and Diluted
|
|
$
|
(0.00
|
)
|
|
$
|
(0.00
|
)
|
|
$
|
(0.00
|
)
|
|
$
|
(0.00
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares outstanding during the period - Basic and Diluted
|
|
|
844,468,378
|
|
|
|
835,733,840
|
|
|
|
844,468,378
|
|
|
|
828,912,974
|
|
Kraig
Biocraft Laboratories, Inc. and Subsidiary
Condensed
Consolidated Statement of Changes in Stockholders Deficit
For
the six months ended June 30, 2020
(Unaudited)
|
|
|
|
Preferred
Stock - Series A
|
|
|
|
Common
Stock - Class A
|
|
|
|
Common
Stock - Class B
|
|
|
|
Common
Stock -
Class A Shares
To be issued
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
|
Shares
|
|
|
|
Par
|
|
|
|
Shares
|
|
|
|
Par
|
|
|
|
Shares
|
|
|
|
Par
|
|
|
|
Shares
|
|
|
|
Par
|
|
|
|
APIC
|
|
|
|
Deficit
|
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2019
|
|
|
2
|
|
|
$
|
5,217,800
|
|
|
|
844,468,378
|
|
|
$
|
16,757,079
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
1,122,311
|
|
|
$
|
22,000
|
|
|
$
|
2,412,969
|
|
|
$
|
(29,797,906
|
)
|
|
$
|
(5,388,058
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued for services - related parties
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
2,612,411
|
|
|
|
-
|
|
|
|
2,612,411
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued for services
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
42,713
|
|
|
|
-
|
|
|
|
42,713
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contributed capital - related party
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
17,495
|
|
|
|
-
|
|
|
|
17,495
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Imputed interest - related party
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
21,972
|
|
|
|
-
|
|
|
|
21,972
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for the six months ended June 30, 2020
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(3,620,136
|
)
|
|
|
(3,620,136
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, June 30, 2020
|
|
|
2
|
|
|
$
|
5,217,800
|
|
|
|
844,468,378
|
|
|
$
|
16,757,079
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
1,122,311
|
|
|
$
|
22,000
|
|
|
$
|
5,107,560
|
|
|
$
|
(33,418,042
|
)
|
|
$
|
(6,313,603
|
)
|
Kraig
Biocraft Laboratories, Inc. and Subsidiary
Condensed
Consolidated Statement of Changes in Stockholders Deficit
For
the six months ended June 30, 2019
(Unaudited)
|
|
|
Preferred
Stock - Series A
|
|
|
Common
Stock - Class A
|
|
|
Common
Stock - Class B
|
|
|
Common
Stock Class A Shares To be issued
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
Shares
|
|
|
Par
|
|
|
Shares
|
|
|
Par
|
|
|
Shares
|
|
|
Par
|
|
|
Shares
|
|
|
Par
|
|
|
APIC
|
|
|
Deficit
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2018
|
|
|
2
|
|
|
$
|
5,217,800
|
|
|
|
816,883,910
|
|
|
$
|
15,145,798
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
1,122,311
|
|
|
$
|
22,000
|
|
|
$
|
2,043,235
|
|
|
$
|
(26,888,056
|
)
|
|
$
|
(4,459,223
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Units issued for cash
|
|
|
-
|
|
|
|
-
|
|
|
|
14,797,278
|
|
|
|
1,000,000
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,000,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares issued in exchange for accounts payable
|
|
|
-
|
|
|
|
-
|
|
|
|
4,052,652
|
|
|
|
281,659
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
281,659
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cancellation of warrants issued for services
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(19,915
|
)
|
|
|
-
|
|
|
|
(19,915
|
)
|
|
Imputed interest - related party
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
10,457
|
|
|
|
-
|
|
|
|
10,457
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for the six months ended June 30, 2019
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(880,243
|
)
|
|
|
(880,243
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, June 30, 2019
|
|
|
2
|
|
|
$
|
5,217,800
|
|
|
|
835,733,840
|
|
|
$
|
16,427,457
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
1,122,311
|
|
|
$
|
22,000
|
|
|
$
|
2,033,777
|
|
|
$
|
(27,768,299
|
)
|
|
$
|
(4,067,265
|
)
|
Kraig
Biocraft Laboratories, Inc. and Subsidiary
Condensed
Consolidated Statements of Cash Flows
(Unaudited)
|
|
|
For the
six months ended
June 30,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Cash Flows From Operating Activities:
|
|
|
|
|
|
|
|
|
|
Net Loss
|
|
$
|
(3,620,136
|
)
|
|
$
|
(880,243
|
)
|
|
Adjustments to reconcile net loss to net cash used in
operations
|
|
|
|
|
|
|
|
|
|
Depreciation expense
|
|
|
13,118
|
|
|
|
13,403
|
|
|
Imputed interest - related party
|
|
|
21,972
|
|
|
|
10,457
|
|
|
Fair value of options issued for services
|
|
|
2,655,124
|
|
|
|
-
|
|
|
Warrants issued/(cancelled) to consultants
|
|
|
-
|
|
|
|
(19,915
|
)
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Decrease (Increase) in prepaid expenses
|
|
|
23,273
|
|
|
|
(28,387
|
)
|
|
Operating lease right-of-use, net
|
|
|
57,993
|
|
|
|
30,433
|
|
|
Increase in accrued expenses and other payables - related
party
|
|
|
277,050
|
|
|
|
373,469
|
|
|
(Decrease) Increase in accounts payable
|
|
|
(72,448
|
)
|
|
|
73,420
|
|
|
Operating lease liabilities, current
|
|
|
(53,855
|
)
|
|
|
(28,106
|
)
|
|
Net Cash Used In Operating Activities
|
|
|
(697,909
|
)
|
|
|
(455,469
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Investing Activities:
|
|
|
|
|
|
|
|
|
|
Purchase of Fixed Assets and Leasehold Improvements
|
|
|
-
|
|
|
|
-
|
|
|
Net Cash Used In Investing Activities
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Financing Activities:
|
|
|
|
|
|
|
|
|
|
Proceeds from Notes Payable - related party
|
|
|
550,000
|
|
|
|
120,000
|
|
|
Principal payments on debt
|
|
|
(30,000
|
)
|
|
|
(4,000
|
)
|
|
Contributed capital - related party
|
|
|
17,495
|
|
|
|
-
|
|
|
Proceeds from SBA Paycheck Protection Loan
|
|
|
90,100
|
|
|
|
-
|
|
|
Proceeds from issuance of common stock
|
|
|
-
|
|
|
|
1,000,000
|
|
|
Net Cash Provided by Financing
Activities
|
|
|
627,595
|
|
|
|
1,116,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Increase in Cash
|
|
|
(70,314
|
)
|
|
|
660,531
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash at Beginning of Period
|
|
|
125,024
|
|
|
|
13,697
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash at End of Period
|
|
$
|
54,710
|
|
|
$
|
674,228
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of
cash flow information:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid for interest
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Cash paid for taxes
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of
non-cash investing and financing activities:
|
|
|
|
|
|
|
|
|
|
Shares issued in connection with
cashless warrants exercise
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Settlement of accounts payable
with note payable
|
|
$
|
-
|
|
|
$
|
265,244
|
|
|
Settlement of accounts payable
with stock issuance
|
|
$
|
-
|
|
|
$
|
281,659
|
|
|
Adoption of lease standard ASC 842
|
|
$
|
-
|
|
|
$
|
559,568
|
|
Kraig
Biocraft Laboratories, Inc.
Notes
to Condensed Consolidated Financial Statements as of June 30, 2020 and 2019
NOTE
1 SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES AND ORGANIZATION
(A)
Basis of Presentation
The
accompanying unaudited condensed financial statements have been prepared in accordance with accounting principles generally accepted
in The United States of America and the rules and regulations of the Securities and Exchange Commission for interim financial
information. Accordingly, they do not include all the information necessary for a comprehensive presentation of financial position
and results of operations.
It
is management’s opinion, however that all material adjustments (consisting of normal recurring adjustments) have been made
which are necessary for a fair financial statements presentation. The results for the interim period are not necessarily indicative
of the results to be expected for the year.
Kraig
Biocraft Laboratories, Inc. (the “Company”) was incorporated under the laws of the State of Wyoming on April 25, 2006.
The Company was organized to develop high strength, protein based fiber, using recombinant DNA technology, for commercial applications
in the textile and specialty fiber industries.
(B)
Foreign Currency
The
assets and liabilities of Prodigy Textiles, Co., Ltd. (the Company’s Vietnamese subsidiary) whose functional currency is
the Vietnamese Dong, are translated into US dollars at period-end exchange rates prior to consolidation. Income and expense items
are translated at the average rates of exchange prevailing during the period. The adjustments resulting from translating the Company’s
financial statements are reflected as a component of other comprehensive (loss) income. Foreign currency transaction gains and
losses are recognized in net earnings based on differences between foreign exchange rates on the transaction date and settlement
date.
(C)
Use of Estimates
In
preparing financial statements in conformity with generally accepted accounting principles, management is required to make estimates
and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities
at the date of the financial statements and revenues and expenses during the reported period. Actual results could differ from
those estimates.
(D)
Cash
For
the purposes of the cash flow statements, the Company considers all highly liquid investments with original maturities of three
months or less at the time of purchase to be cash equivalents. There were no cash equivalents as of June 30, 2020 or December
31, 2019.
(E)
Loss Per Share
Basic
and diluted net loss per common share is computed based upon the weighted average common shares outstanding as defined by the
Financial Accounting Standards Board (“FASB” Accounting Standards Codification (“ASC”) No. 260, “Earnings
per Share.” For June 30, 2020 and June 30, 2019, warrants were not included in the computation of income/ (loss) per share
because their inclusion is anti-dilutive.
The
computation of basic and diluted loss per share for June 30, 2020 and June 30, 2019 excludes the common stock equivalents of the
following potentially dilutive securities because their inclusion would be anti-dilutive:
|
|
|
June 30, 2020
|
|
|
June 30, 2019
|
|
|
Stock Warrants (Exercise price - $0.001/share)
|
|
|
55,995,917
|
|
|
|
57,995,917
|
|
|
Stock Options (Exercise price - $0.1150/Share)
|
|
|
27,340,000
|
|
|
|
-
|
|
|
Convertible Preferred Stock
|
|
|
2
|
|
|
|
2
|
|
|
Total
|
|
|
83,335,919
|
|
|
|
57,995,919
|
|
(F)
Research and Development Costs
The
Company expenses all research and development costs as incurred for which there is no alternative future use. These costs also
include the expensing of employee compensation and employee stock based compensation.
(G)
Income Taxes
The
Company accounts for income taxes under FASB Codification Topic 740-10-25 (“ASC 740-10-25”). Under ASC No. 740-10-25,
deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial
statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities
are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are
expected to be recovered or settled. Under ASC No. 740-10-25, the effect on deferred tax assets and liabilities of a change in
tax rates is recognized in income in the period that includes the enactment date.
On
December 22, 2017, the 2017 Tax Cuts and Jobs Act (the “Tax Act”) was enacted into law and the new legislation contains
several key tax provisions that affected us, including a one-time mandatory transition tax on accumulated foreign earnings and
a reduction of the corporate income tax rate to 21% effective January 1, 2018, among others. We are required to recognize the
effect of the tax law changes in the period of enactment, such as determining the transition tax, remeasuring our U.S. deferred
tax assets and liabilities as well as reassessing the net realizability of our deferred tax assets and liabilities. In December
2017, the SEC staff issued Staff Accounting Bulletin No. 118, Income Tax Accounting Implications of the Tax Cuts and Jobs Act
(SAB 118), which allows us to record provisional amounts during a measurement period not to extend beyond one year of the
enactment date. Since the Tax Act was passed late in the fourth quarter of 2017, and ongoing guidance and accounting interpretation
are expected over the next 12 months, we consider the accounting of the transition tax, deferred tax re-measurements, and other
items to be incomplete due to the forthcoming guidance and our ongoing analysis of final year-end data and tax positions. We expect
to complete our analysis within the measurement period in accordance with SAB 118.
Effective
January 1, 2009, the Company adopted guidance regarding accounting for uncertainty in income taxes. This guidance clarifies the
accounting for income taxes by prescribing the minimum recognition threshold an income tax position is required to meet before
being recognized in the financial statements and applies to all federal or state income tax positions. Each income tax position
is assessed using a two-step process. A determination is first made as to whether it is more likely than not that the income tax
position will be sustained, based upon technical merits, upon examination by the taxing authorities. If the income tax position
is expected to meet the more likely than not criteria, the benefit recorded in the financial statements equals the largest amount
that is greater than 50% likely to be realized upon its ultimate settlement. As of June 30, 2020 and December 31, 2019 there were
no amounts that had been accrued in respect to uncertain tax positions.
Fair
value accounting requires bifurcation of embedded derivative instruments such as conversion features in convertible debt or equity
instruments, and measurement of their fair value for accounting purposes. In determining the appropriate fair value, the Company
uses the Black-Scholes option-pricing model. In assessing the convertible debt instruments, management determines if the convertible
debt host instrument is conventional convertible debt and further if there is a beneficial conversion feature requiring measurement.
If the instrument is not considered conventional convertible debt, the Company will continue its evaluation process of these instruments
as derivative financial instruments.
Once
determined, derivative liabilities are adjusted to reflect fair value at each reporting period end, with any increase or decrease
in the fair value being recorded in results of operations as an adjustment to fair value of derivatives. In addition, the fair
value of freestanding derivative instruments such as warrants, are also valued using the Black-Scholes option-pricing model.
(H)
Stock-Based Compensation
The
Company accounts for stock-based compensation for employees and directors in accordance with ASC 718, Compensation (“ASC
718”). ASC 718 requires all share-based payments to employees, including grants of employee stock options, to be recognized
in the statement of operations based on their fair values. Under the provisions of ASC 718, stock-based compensation costs are
measured at the grant date, based on the fair value of the award, and are recognized as expense over the employee’s requisite
service period (generally the vesting period of the equity grant). The fair value of the Company’s common stock options
are estimated using the Black Scholes option-pricing model with the following assumptions: expected volatility, dividend rate,
risk free interest rate and the expected life. The Company expenses stock-based compensation by using the straight-line method.
In accordance with ASC 718 and, excess tax benefits realized from the exercise of stock-based awards are classified as cash flows
from operating activities. All excess tax benefits and tax deficiencies (including tax benefits of dividends on share-based payment
awards) are recognized as income tax expense or benefit in the condensed consolidated statements of operations.
The
Company accounts for stock-based compensation awards issued to non-employees for services, as prescribed by ASC 718-10, at either
the fair value of the services rendered or the instruments issued in exchange for such services, whichever is more readily determinable,
using the measurement date guidelines enumerated in ASU 2018-07.
(I)
Recent Accounting Pronouncements
In
June 2018, the FASB issued Accounting Standards Update (“ASU”) No. 2018-07, “Compensation — Stock Compensation
(Topic 718),” (“ASU 2018-07”). ASU 2018-07 is intended to reduce cost and complexity and to improve financial
reporting for nonemployee share-based payments. Currently, the accounting requirements for nonemployee and employee share-based
payment transactions are significantly different. ASU 2018-07 expands the scope of Topic 718, Compensation — Stock Compensation
(which currently only includes share-based payments to employees) to include share-based payments issued to nonemployees for goods
or services. Consequently, the accounting for share-based payments to nonemployees and employees will be substantially aligned.
This ASU supersedes Subtopic 505-50, Equity — Equity-Based Payments to Nonemployees. The amendments in this ASU are effective
for fiscal years beginning after December 15, 2018, and interim periods within that fiscal year. Early adoption is permitted,
but no earlier than a company’s adoption date of Topic 606, Revenue from Contracts with Customers. The Company early adopted
ASU 2018-07 effective April 1, 2018. The adoption of this ASU did not have a material impact on the Company’s condensed
consolidated financial statements.
In
February 2016, the FASB issued ASU 2016-02, “Leases” Topic 842, which amends the guidance in former ASC Topic 840,
Leases. The new standard increases transparency and comparability most significantly by requiring the recognition by lessees
of right-of-use (“ROU”) assets and lease liabilities on the balance sheet for all leases longer than 12 months. Under
the standard, disclosures are required to meet the objective of enabling users of financial statements to assess the amount, timing,
and uncertainty of cash flows arising from leases. For lessees, leases will be classified as finance or operating, with classification
affecting the pattern and classification of expense recognition in the income statement. The Company adopted the new lease guidance
effective January 1, 2019 using the modified retrospective transition approach, applying the new standard to all of its leases
existing at the date of initial application which is the effective date of adoption. Consequently, financial information will
not be updated and the disclosures required under the new standard will not be provided for dates and periods before January 1,
2019. We elected the package of practical expedients which permits us to not reassess (1) whether any expired or existing contracts
are or contain leases, (2) the lease classification for any expired or existing leases, and (3) any initial direct costs for any
existing leases as of the effective date. We did not elect the hindsight practical expedient which permits entities to use hindsight
in determining the lease term and assessing impairment. The adoption of the lease standard did not change our previously reported
consolidated statements of operations and did not result in a cumulative catch-up adjustment to opening equity. As a result, the
Company has recorded Right-to-use assets and corresponding Lease obligations as more fully discussed in Note 4.
All
other newly issued accounting pronouncements but not yet effective have been deemed either immaterial or not applicable.
(J)
Equipment
The
Company values property and equipment at cost and depreciates these assets using the straight-line method over their expected
useful life. The Company uses a five year life for automobiles.
In
accordance with FASB ASC No. 360, Property, Plant and Equipment, the Company carries long-lived assets at the lower of
the carrying amount or fair value. Impairment is evaluated by estimating future undiscounted cash flows expected to result from
the use of the asset and its eventual disposition. If the sum of the expected undiscounted future cash flow is less than the carrying
amount of the assets, an impairment loss is recognized. Fair value, for purposes of calculating impairment, is measured based
on estimated future cash flows, discounted at a market rate of interest.
There
were no impairment losses recorded for the six months ended June 30, 2020 and 2019.
(K)
Fair Value of Financial Instruments
We
hold certain financial assets, which are required to be measured at fair value on a recurring basis in accordance with the Statement
of Financial Accounting Standard No. 157, “Fair Value Measurements” (“ASC Topic 820-10”). ASC Topic
820-10 establishes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The
hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level
1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). ASC Topic 820-10 defines fair value as
the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants
on the measurement date. Level 1 instruments include cash, account receivable, prepaid expenses, inventory and account payable
and accrued liabilities. The carrying values are assumed to approximate the fair value due to the short term nature of the instrument.
The
three levels of the fair value hierarchy under ASC Topic 820-10 are described below:
|
|
●
|
Level
1 - Valuations based on quoted prices in active markets for identical assets or liabilities that an entity has the ability
to access. We believe our carrying value of level 1 instruments approximate their fair value at June 30, 2020 and December
31, 2019.
|
|
|
|
|
|
|
●
|
Level
2 - Valuations based on quoted prices for similar assets or liabilities, quoted prices for identical assets or liabilities
in markets that are not active, or other inputs that are observable or can be corroborated by observable data for substantially
the full term of the assets or liabilities.
|
|
|
|
|
|
|
●
|
Level
3 - Valuations based on inputs that are supported by little or no market activity and that are significant to the fair value
of the assets or liabilities. We consider depleting assets, asset retirement obligations and net profit interest liability
to be Level 3. We determine the fair value of Level 3 assets and liabilities utilizing various inputs, including NYMEX price
quotations and contract terms.
|
|
|
|
|
June
30,
2020
|
|
|
|
December
31,
2019
|
|
|
Level 1
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Level 2
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Level 3
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Total
|
|
$
|
-
|
|
|
$
|
-
|
|
(L)
Revenue Recognition
During
the year ended December 31, 2019, the Company’s revenues were generated primarily from a contract with the U.S. Government.
The Company performs work under this cost-plus-fixed-fee contract. Under the base phase of that contract the Company produced
recombinant spider silk woven into ballistic shootpack panels. Those shootpack panels were delivered to the U.S. Government customer.
Under an option period award starting in July 2017, to that original contract, the Company has worked to develop new recombinant
silks.
Effective
January 1, 2018, the Company adopted ASC No. 606 — Revenue from Contracts with Customers. Under ASC No. 606, the Company
recognizes revenue from the commercial sales of products, licensing agreements and contracts by applying the following steps:
(1) identify the contract with a customer; (2) identify the performance obligations in the contract; (3) determine the transaction
price; (4) allocate the transaction price to each performance obligation in the contract; and (5) recognize revenue when each
performance obligation is satisfied.
For
the three and six months ended June 30, 2020 and 2019, the Company recognized $0 and $0 respectively in revenue from the Government
contract. These revenues were generated for work performed in the development and production of the Company’s recombinant
silks under the base and option period phases of our ongoing contract with the US Army.
On
July 24, 2017, the Company signed a contract option extension with the US Army to research and deliver recombinant spider silk
fibers and threads. This contract option increased the total contract award by an additional $921,130 to a total of $1,021,092
and added 12 months to the contract duration. This effort was scheduled to end on September 24, 2018, but the Company requested
an extension of this contract option period through April 2019 to complete the work. The Company has been in communication with
the contracting office and is working with them as they determine the best path forward; Management believes there is a possibility
of securing a follow-up contract to complete the delivery of all materials for the contract. The Company is also continuing to
pursue additional contract opportunities with the Department of Defense, Department of Energy and other governmental agencies.
(M)
Concentration of Credit Risk
The
Company at times has cash in banks in excess of FDIC insurance limits. At June 30, 2020 and December 31, 2019, the Company had
approximately $0 and $0, respectively in excess of FDIC insurance limits.
For
the three and six months ended June 30, 2020 and 2019, the Company booked $0 and $0 for doubtful accounts.
NOTE
2 GOING CONCERN
As
reflected in the accompanying financial statements, the Company has a working capital deficiency of $6,372,891 and stockholders’
deficiency of $6,313,603 and used $697,909 of cash in operations for six months ended June 30, 2020. This raises substantial doubt
about its ability to continue as a going concern. The ability of the Company to continue as a going concern is dependent on the
Company’s ability to raise additional capital and implement its business plan. The financial statements do not include any
adjustments that might be necessary if the Company is unable to continue as a going concern.
Management
believes that actions presently being taken to obtain additional funding and implement its strategic plans provide the opportunity
for the Company to continue as a going concern.
NOTE
3 EQUIPMENT
At
June 30, 2020 and December 31, 2019, property and equipment, net, is as follows:
|
|
|
As of June 30,
2020
|
|
|
December 31,
2019
|
|
|
Automobile
|
|
$
|
41,805
|
|
|
$
|
41,805
|
|
|
Laboratory Equipment
|
|
|
96,536
|
|
|
|
96,536
|
|
|
Office Equipment
|
|
|
7,260
|
|
|
|
7,260
|
|
|
Leasehold Improvements
|
|
|
85,389
|
|
|
|
85,389
|
|
|
Less: Accumulated Depreciation
|
|
|
(126,787
|
)
|
|
|
(113,669
|
)
|
|
Total Property and Equipment, net
|
|
$
|
104,203
|
|
|
$
|
117,321
|
|
Depreciation
expense for the six months ended June 30, 2020 and 2019, was $13,118 and $13,403, respectively
Depreciation
expense for the three months ended June 30, 2020 and 2019, was $5,740 and $6,738, respectively.
NOTE
4 - RIGHT TO USE ASSETS AND LEASE LIABILITY
Since
September of 2015, we rent office space at 2723 South State Street, Suite 150, Ann Arbor, Michigan 48104, which is our principal
place of business. We pay an annual rent of $2,508 for conference facilities, mail, fax, and reception services located at our
principal place of business.
On
January 23, 2017 the Company signed an 8 year property lease with the Company’s President for land in Texas where the Company
grows its mulberry. The Company pays a monthly rent of $960. Rent expense – related party for the six months ended June
30, 2020 and 2019, was $6,263 and $6,153, respectively (See Note 9).
On
September 13, 2017, the Company signed a new two year lease commencing on October 1, 2017 and ending on September 30, 2019. The
Company pays an annual rent of $39,200 for the year one of lease and $42,000 for the year two of lease for office and manufacturing
space.
On
May 9, 2019 the Company signed a 5 year property lease with the Socialist Republic of Vietnam which consists of 4,560.57 square
meters of space, which it leases at a current rent of approximately $45,150 per year one and two and with the 5% increase per
year for years three through five.
In
February 2016, the FASB issued ASU 2016-02, “Leases” Topic 842, which amends the guidance in former ASC Topic 840,
Leases. The new standard increases transparency and comparability most significantly by requiring the recognition by lessees
of right-of-use (“ROU”) assets and lease liabilities on the balance sheet for all leases longer than 12 months. Under
the standard, disclosures are required to meet the objective of enabling users of financial statements to assess the amount, timing,
and uncertainty of cash flows arising from leases. For lessees, leases will be classified as finance or operating, with classification
affecting the pattern and classification of expense recognition in the income statement.
The
Company adopted the new lease guidance effective January 1, 2019 using the modified retrospective transition approach, applying
the new standard to all of its leases existing at the date of initial application which is the effective date of adoption. Consequently,
financial information will not be updated and the disclosures required under the new standard will not be provided for dates and
periods before January 1, 2019. We elected the package of practical expedients which permits us to not reassess (1) whether any
expired or existing contracts are or contain leases, (2) the lease classification for any expired or existing leases, and (3)
any initial direct costs for any existing leases as of the effective date. We did not elect the hindsight practical expedient
which permits entities to use hindsight in determining the lease term and assessing impairment. The adoption of the lease standard
did not change our previously reported consolidated statements of operations and did not result in a cumulative catch-up adjustment
to opening equity. The adoption of the new guidance resulted in the recognition of ROU assets of $529,135 and lease liabilities
of $531,462.
The
interest rate implicit in lease contracts is typically not readily determinable. As such, the Company utilizes its incremental
borrowing rate, which is the rate incurred to borrow on a collateralized basis over a similar term an amount equal to the lease
payments in a similar economic environment. In calculating the present value of the lease payments, the Company elected to utilize
its incremental borrowing rate based on the remaining lease terms as of the January 1, 2019 adoption date. This rate was determined
to be 8% and the Company determined the initial present value, at inception, of $559,568.
Operating
lease ROU assets and operating lease liabilities are recognized based on the present value of the future minimum lease payments
over the lease term at the commencement date. The operating lease ROU asset also includes any lease payments made and excludes
lease incentives and initial direct costs incurred, if any.
The
Company has elected the practical expedient to combine lease and non-lease components as a single component. The lease expense
is recognized over the expected term on a straight-line basis. Operating leases are recognized on the balance sheet as right-of-use
assets, current operating lease liabilities and non-current operating lease liabilities.
The
new standard also provides practical expedients and certain exemptions for an entity’s ongoing accounting. We have elected
the short-term lease recognition exemption for all leases that qualify. This means, for those leases where the initial lease term
is one year or less or for which the ROU asset at inception is deemed immaterial, we will not recognize ROU assets or lease liabilities.
Those leases are expensed on a straight line basis over the term of the lease
Right
to use assets is summarized below:
|
|
|
June 30, 2020
|
|
|
Right to use assets, net – related party
|
|
$
|
50,905
|
|
|
Right to use assets, net
|
|
|
49,404
|
|
|
Right to use assets, net
|
|
|
314,940
|
|
|
Total
|
|
$
|
415,249
|
|
During
the six months ended June 30, 2020, the Company recorded $54,963 as lease expense to current period operations.
During
the six months ended June 30, 2020, the Company recorded $6,263 as lease expense – related party to current period operations.
Lease
liability is summarized below:
|
|
|
June 30, 2020
|
|
|
Right to use liability, net – related party
|
|
|
52,432
|
|
|
Right to use liability, net
|
|
|
51,474
|
|
|
Right to use liability, net
|
|
|
322,198
|
|
|
Total
|
|
|
426,104
|
|
|
Less: short term portion
|
|
$
|
(117,666
|
)
|
|
Long term position
|
|
$
|
308,438
|
|
Lease
expense for the six months ended June 30, 2020 was comprised of the following:
|
Operating lease expense
|
|
$
|
32,164
|
|
|
Operating lease expense
|
|
$
|
22,799
|
|
|
Operating lease expense – related party
|
|
$
|
6,263
|
|
NOTE
5 ACCRUED INTEREST – RELATED PARTY
On
June 6, 2016, the Company received a $50,000 loan from our principal stockholder. Subsequently on December 1, 2017, the Company
received an additional $30,000 loan from the same stockholder. On January 8, 2018 and March 31, 2018 the Company received an additional
loan of $100,000 and $15,000, respectively. The Company received additional loan funds from the same stockholder as follows: $20,000
on April 26, 2018; $15,000 on June 21, 2018; $15,000 on June 29, 2018; $20,000 on July 5, 2018; $26,000 on October 1, 2018; $11,000
on October 12, 2018; $20,000 on December 21, 2018; $3,000 on January 4, 2019; $30,000 on January 17, 2019; $30,000 on February
1, 2019; $20,000 on February 15, 2019; $20,000 on March 1, 2019; $17,000 on January 4, 2019, $100,000 on November 20, 2019, $100,000
on December 18, 2019, $100,000 on January 24, 2020, $100,000 on February 19, 2020 $100,000 on March 9, 2020, $100,000 on April
8, 2020 and $150,000 on June 3, 2020. Pursuant to the terms of the loan, the advance bears an interest at 3%, is unsecured, and
due on demand. Total loan payable to principal stockholder for as of December 31, 2019 is $642,000. Total loan payable to this
principal stockholder as of June 30, 2020 is $1,192,000. During the six months ended June 30, 2020, the Company recorded $21,972
as an in-kind contribution of interest related to the loan and recorded accrued interest payable of $14,161. During the three
months ended June 30, 2019, the Company recorded $10,457 as an in-kind contribution of interest related to the loan and recorded
accrued interest payable of $7,033.
NOTE
6 NOTE PAYABLE
On
March 1, 2019, the Company entered into an unsecured promissory note with Notre Dame - an unrelated party in the amount of $265,244
in exchange for outstanding account payable due to the debtor. Pursuant to the terms of the note, the note bears 10% interest
per year from the date of default until the date the loan is paid in full. The term of the loan is twenty four months. The loan
repayment commenced immediately over a twenty-four month period according to the following table. During the six months ended
June 30, 2020, the Company paid $30,000 of the loan balance (See Note 8 (A)):
1.
$1,000 per month for the first six months;
2.
$2,000 per month for the months seven and eight;
3.
$5,000 per month for months nine through twenty three; and,
4.
Final payment of all remaining balance, in the amount of $180,224 in month 24.
On
April 16, 2020, the Company, was granted a loan (the “Loan”) from The Huntington National Bank, in the aggregate amount
of $90,100, pursuant to the Paycheck Protection Program (the “PPP”) under Division A, Title I of the CARES Act, which
was enacted March 27, 2020.
The
Loan, which was in the form of a Note dated on or about April 16, 2020 issued by the Borrower, matures on or about April 16, 2022
and bears interest at an approximate rate of 1% per annum. The Note may be prepaid by the Borrower at any time prior to maturity
with no prepayment penalties. Funds from the Loan may only be used for payroll costs, costs used to continue group health care
benefits, mortgage payments, rent, utilities, and interest on other debt obligations incurred before February 15, 2020. The Company
intends to use the entire Loan amount for qualifying expenses. Under the terms of the PPP, certain amounts of the Loan may be
forgiven if they are used for qualifying expenses as described in the CARES Act.
NOTE
7 STOCKHOLDERS’ DEFICIT
(A)
Common Stock Issued for Cash
On
March 9, 2019, the Company entered into a purchase agreement with one investor (the “Purchase Agreement”). Pursuant
to the Purchase Agreement, the Company issued the investor 14,797,278 Units at a purchase price of $0.06758 per Unit, for total
gross proceeds to the Company of $1,000,000. The Units consist of 14,797,278 shares of the Company’s Class A Common Stock
(the “Common Stock”) and two warrants (the “Warrants”): (i) one warrant entitles the investor to purchase
up to 14,797,278 shares of Common Stock at an exercise price of $0.06 per share (the “6 Cent Warrants”) and (ii) one
warrant entitles the investor to purchase up to 7,398,639 shares of Common Stock at an exercise price of $0.08 per share (the
“8 Cent Warrant”). The Warrants shall be exercisable at any time from the issuance date until the following expiration
dates:
●
½ of all $0.06 Warrants shall expire on March 8, 2021;
●
½ of all $0.06 Warrants shall expire on March 8, 2022;
●
½ of all $0.08 Warrants shall expire on March 8, 2022;
●
½ of all $0.08 Warrants shall expire on March 8, 2023.
(B)
Common Stock Issued for Services
Shares
issued for services as mentioned below were valued at the closing price of the stock on the date of grant.
On
March 20, 2019, the Company issued 4,052,652 shares of its class A common stock with a fair value of $281,659 ($0.0695/share)
on the date of settlement. The Company settled $243,159 of accounts payable to the University of Notre Dame. The Company recorded
an additional amount of $38,500 based on the fair value of the shares on the date of settlement. See Note 8 (A).
(C)
Common Stock Warrants and Options
On
February 19, 2020 the Company issued a 20-year option to purchase 20,000,000 shares of common stock at an exercise price of $0.0115
per share to a related party for services rendered. The options had a fair value of $2,198,411, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on the date granted. Options will be exercisable on February 19, 2025, and for
a period of 15 years expiring on February 19, 2040. During the six months ended June 30, 2020, the Company recorded $2,198,411
as an expense for options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
125.19
|
%
|
|
Expected term
|
|
|
3
years
|
|
|
Risk free interest rate
|
|
|
1.56
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
February 19, 2020 the Company issued a 10-year option to purchase 6,000,000 shares of common stock at an exercise price of $.00115
per share to a related party for services rendered. The options had a fair value of $626,047, based upon the Black-Scholes option-pricing
model on the date of grant and 2,000,000 options are fully vested on the date granted and 1,000,000 options vest at the end of
each successive year for four years. Options will be exercisable on February 19, 2021, and for a period of 10 years expiring on
February 19, 2030. During the six months ended June 30, 2020, the Company recorded $208,682 as an expense for options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
125.19
|
%
|
|
Expected term
|
|
|
3
years
|
|
|
Risk free interest rate
|
|
|
1.50
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
February 19, 2020 the Company issued a 7-year option to purchase 1,340,000 shares of common stock at an exercise price of $0.0115
per share to employees for services rendered. The options had a fair value of $133,063, based upon the Black-Scholes option-pricing
model on the date of grant and 268,000 options are fully vested on the date granted and the remaining option vest equally over
the remaining 4 years at the end of each successive year. Options will be exercisable on February 19, 2021, and for a period of
6 years expiring on February 19, 2027. During the six months ended June 30, 2020, the Company recorded $26,613 as an expense for
options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
125.19
|
%
|
|
Expected term
|
|
|
6
years
|
|
|
Risk free interest rate
|
|
|
1.46
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
September 26, 2019, the Company issued 766,667 shares in connection with the cashless exercise of the 1,000,000 warrants. On August
14, 2019, the Company issued 7,967,871 shares in connection with the cashless exercise of the 8,000,000 warrants.
On
August 8, 2019, the Company issued a 2-year option to purchase 2,000,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $267,574, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on the date granted. Options will be exercisable on August 8, 2020, and for a
period of 3 years expiring on August 8, 2024. During the year ended December 31, 2019, the Company recorded $267,574 as an expense
for options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
2
years
|
|
|
Risk free interest rate
|
|
|
1.62
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 2-year option to purchase 2,000,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $267,574, based upon the Black-Scholes option-pricing
model on the date of grant and is fully vested on August 8, 2020. Options will be exercisable on August 8, 2022, and for a period
of 3 years expiring on August 8, 2025. During the six months ended June 30, 2020, the Company recorded $133,056 as an expense
for options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
2
years
|
|
|
Risk free interest rate
|
|
|
1.62
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 3-year option to purchase 2,000,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $291,842, based upon the Black-Scholes option-pricing
model on the date of grant and is fully vested on August 8, 2021. Options will be exercisable on August 8, 2023, and for a period
of 3 years expiring on August 8, 2026. During the six months ended June 30, 2020, the Company recorded $72,262 as an expense for
options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
3
years
|
|
|
Risk free interest rate
|
|
|
1.54
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 2-year option to purchase 1,000,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $118,874, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on the date granted. Options will be exercisable on August 8, 2020, and for a
period of 3 years expiring on August 8, 2023. During the year ended December 31, 2019, the Company recorded $118,874 as an expense
for options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
2
years
|
|
|
Risk free interest rate
|
|
|
1.62
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 2-year option to purchase 1,000,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $118,874, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on the date granted. Options will be exercisable on August 8, 2021, and for a
period of 3 years expiring on August 8, 2024. During the year ended December 31, 2019, the Company recorded $118,874 as an expense
for options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
2
years
|
|
|
Risk free interest rate
|
|
|
1.62
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 2-year option to purchase 125,000 shares of common stock at an exercise price of $0.2299
per share to an employee for services rendered. The options had a fair value of $14,859, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on the date granted. Options will be exercisable on August 8, 2020, and for a
period of 3 years expiring on August 8, 2023. During the year ended December 31, 2019, the Company recorded $14,859, as an expense
for options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
2
years
|
|
|
Risk free interest rate
|
|
|
1.62
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 2-year option to purchase 125,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $16,723, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on August 8, 2020. Options will be exercisable on August 8, 2022, and for a period
of 3 years expiring on August 8, 2025. During the six months ended June 30, 2020, the Company recorded $8,316, as an expense for
options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
2
years
|
|
|
Risk free interest rate
|
|
|
1.62
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 2-year options to purchase 125,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $18,240, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on August 8, 2021. Options will be exercisable on August 8, 2023, and for a period
of 3 years expiring on August 8, 2026. During the six months ended June 30, 2020, the Company recorded $4,542, as an expense for
options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
3
years
|
|
|
Risk free interest rate
|
|
|
1.54
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 2-year options to purchase 125,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $19,525, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on August 8, 2022. Options will be exercisable on August 8, 2024, and for a period
of 3 years expiring on August 8, 2027. During the six months ended June 30, 2020, the Company recorded $3,242, as an expense for
options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
3
years
|
|
|
Risk free interest rate
|
|
|
1.54
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
March 20, 2018, the Company issued a 4-year warrant to purchase 600,000 shares of common stock at an exercise price of $0.001
per share to a consultant for services rendered. The warrants had a fair value of $19,915, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on March 20, 2018. Warrants will be exercisable on March 20, 2019, and for a period
of 3 years expiring on March 20, 2022. During the year ended December 31, 2019, the Company recorded $19,915 as an expense for
warrants issued. On April 5, 2019, the Company cancelled 600,000 warrant issued to a consultant on February 20, 2018 in exchange
for $6,000 cash payment. In addition the Company also recorded a $19,915 reduction to warrant expense related to the warrant cancellation.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
97.56
|
%
|
|
Expected term
|
|
|
4
years
|
|
|
Risk free interest rate
|
|
|
2.65
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
September 26, 2019, the Company issued 766,667 shares in connection with the cashless exercise of the 1,000,000 warrants.
On
August 14, 2019, the Company issued 7,967,871 shares in connection with the cashless exercise of the 8,000,000 warrants.
|
|
|
|
Number
of
Warrants
|
|
|
Weighted
Average
Exercise
Price
|
|
Weighted
Average
Remaining
Contractual Life
(in
Years)
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
December 31, 2019
|
|
|
|
55,395,917
|
|
|
|
|
|
2.77
|
|
Granted
|
|
|
|
-
|
|
|
|
-
|
|
|
|
Exercised
|
|
|
|
-
|
|
|
|
-
|
|
|
|
Cancelled/Forfeited
|
|
|
|
-
|
|
|
|
-
|
|
|
|
Balance, June 30,
2020
|
|
|
|
55,395,917
|
|
|
|
|
|
2.27
|
|
Intrinsic
Value
|
|
|
$
|
9,799,286
|
|
|
|
|
|
|
For
the six months ended June 30, 2020, the following warrants were outstanding:
Exercise Price
Warrants
Outstanding
|
|
|
Warrants
Exercisable
|
|
|
Weighted Average
Remaining
Contractual Life
|
|
|
Aggregate
Intrinsic Value
|
|
|
$
|
0.001
|
|
|
|
21,000,000
|
|
|
|
1.15
|
|
|
$
|
4,326,000
|
|
|
$
|
0.056
|
|
|
|
3,000,000
|
|
|
|
1.1
|
|
|
$
|
618,000
|
|
|
$
|
0.04
|
|
|
|
2,300,000
|
|
|
|
1.20
|
|
|
$
|
473,800
|
|
|
$
|
0.06
|
|
|
|
7,398,639
|
|
|
|
0.69
|
|
|
$
|
1,524,120
|
|
|
$
|
0.06
|
|
|
|
7,398,639
|
|
|
|
1.69
|
|
|
$
|
1,524,120
|
|
|
$
|
0.08
|
|
|
|
3,699,320
|
|
|
|
1.69
|
|
|
$
|
762,060
|
|
|
$
|
0.08
|
|
|
|
3,699,320
|
|
|
|
2.69
|
|
|
$
|
762,060
|
|
|
$
|
0.2299
|
|
|
|
8,500,000
|
|
|
|
4.89
|
|
|
$
|
1,751,000
|
|
For
the year ended December 31, 2019, the following warrants were outstanding:
Exercise Price
Warrants
Outstanding
|
|
|
Warrants
Exercisable
|
|
|
Weighted Average
Remaining
Contractual Life
|
|
|
Aggregate
Intrinsic Value
|
|
|
$
|
0.001
|
|
|
|
21,000,000
|
|
|
|
1.65
|
|
|
$
|
4,069,800
|
|
|
$
|
0.056
|
|
|
|
3,000,000
|
|
|
|
1.61
|
|
|
$
|
387,600
|
|
|
$
|
0.04
|
|
|
|
2,300,000
|
|
|
|
1.70
|
|
|
$
|
445,740
|
|
|
$
|
0.06
|
|
|
|
7,398,639
|
|
|
|
1.19
|
|
|
$
|
1,433,856
|
|
|
$
|
0.06
|
|
|
|
7,398,639
|
|
|
|
2.19
|
|
|
$
|
1,433,856
|
|
|
$
|
0.08
|
|
|
|
3,699,320
|
|
|
|
2.19
|
|
|
$
|
716,928
|
|
|
$
|
0.08
|
|
|
|
3,699,320
|
|
|
|
3.19
|
|
|
$
|
719,928
|
|
|
$
|
0.2299
|
|
|
|
8,500,000
|
|
|
|
5.39
|
|
|
$
|
1,647,300
|
|
For
the six months ended June 30, 2020, the following options were outstanding:
|
|
|
|
|
|
|
|
|
|
Weighted Average
|
|
|
Exercise
|
|
|
Options
|
|
|
Options
|
|
|
Remaining
|
|
|
Price
|
|
|
Outstanding
|
|
|
Exercisable
|
|
|
Contractual Life
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
0.115
|
|
|
|
-
|
|
|
|
22,267,800
|
|
|
|
23.09
|
|
(D)
Amendment to Articles of Incorporation
On
February 16, 2009, the Company amended its articles of incorporation to amend the number and class of shares the Company is authorized
to issue as follows:
|
●
|
Common
stock Class A, unlimited number of shares authorized, no par value
|
|
●
|
Common
stock Class B, unlimited number of shares authorized, no par value
|
|
●
|
Preferred
stock, unlimited number of shares authorized, no par value
|
Effective
December 17, 2013, the Company amended its articles of incorporation to designate a Series A no par value preferred stock. Two
shares of Series A Preferred stock have been authorized.
(E)
Common Stock Issued for Debt
None
(F)
Capital contribution – Related Party
For
the six months ended June 30, 2020, the Company recorded $17,495 as contribution of capital by Chief Financial Officer.
NOTE
8 COMMITMENTS AND CONTINGENCIES
On
November 10, 2010, the Company entered into an employment agreement with its CEO, effective January 1, 2011 through the December
31, 2015. The term of the agreement is a five year period at an annual salary of $210,000. There is a 6% annual increase. For
the year ending December 31, 2015, the annual salary was $281,027. The employee is also to receive a 20% bonus based on the annual
based salary. Any stock, stock options bonuses have to be approved by the board of directors. On January 1, 2016 the agreement
was renewed with the same terms for another 5 years with an annual salary of $297,889 for the year ended December 31, 2016. On
January 1, 2017 the agreement renewed with the same terms for another 5 years, but with an annual salary of $315,764 for the year
ended December 31, 2017. On January 1, 2019 the agreement renewed again with the same terms for another 5 years, but with an annual
salary of $354,791 for the year ended December 31, 2019. On January 1, 2020 the agreement renewed again with the same terms for
another 5 years, but with an annual salary of $376,078 for the year ended December 31, 2020. As of June 30, 2020 and December
31, 2019, the accrued salary balance is $2,616,686 and $2,535,203, respectively. (See Note 9).
On
January 20, 2015, the board of directors appointed Mr. Jonathan R. Rice as our Chief Operating Officer. Mr. Rice’s employment
agreement has a term of one year and can be terminated by either the Company or Mr. Rice at any time. Under the employment agreement,
Mr. Rice is entitled to an annual cash compensation of $120,000, which includes salary, health insurance, 401K retirement plan
contributions, etc. The Company also agreed to reimburse Mr. Rice for his past educational expenses of approximately $11,000.
In addition, Mr. Rice was issued a three-year warrant to purchase 2,000,000 shares of common stock of the Company at an exercise
price of $0.001 per share (the “January 2015 Warrant”) pursuant to the employment agreement. Additionally, on May
28, 2015, the Company issued a three-year warrant to purchase 3,000,000 shares of common stock of the Company at an exercise price
of $0.001 per share (the “May 20165 Warrant”) to Mr. Rice. The 2,000,000 share warrant fully vested on October 28,
2016. For the year ended December 31, 2015, the Company recorded $121,448 for the warrants issued to Mr. Rice. On January 14,
2016, the Company signed a new employment agreement with Mr. Rice. The employment agreement has a term of one year and can be
terminated by either the Company or Mr. Rice at any time. Under the employment agreement, Mr. Rice is entitled to annual cash
compensation of $140,000, which includes salary, health insurance, 401K retirement plan contributions, etc. In addition, Mr. Rice
was issued a three-year warrant to purchase 6,000,000 shares of common stock of the Company at an exercise price of $0.001 per
share pursuant to the employment agreement. On January 9, 2018, the Company extended the expiration date of the January 2015 warrant
from January 19, 2018 to January 31, 2020, and on January 10, 2020 the Company extended the expiration date of the warrant to
January 23, 2015 and on March 15, 2018, the Company signed an extension of its at-will employment agreement with its COO, extending
the term to January 31, 2019. On March 25, 2019, the Company signed an extension of its at-will employment agreement with its
COO, extending the term to January 1, 2020. On April 26, 2019, the Company signed an agreement to increase Mr. Rice’s base
salary by $20,000 per year and issue a one-time $20,000 bonus. Additionally, on August 15, 2019, the Company signed an agreement
to increase Mr. Rice’s base salary by an additional $20,000 per year. The salary increase and the bonus is accrued and to
be paid in full earlier by the direction of the Board or upon the earlier of:
●The
Company maintaining $6,000,000 or more in working capital,
●Upon
the transfer of ownership of more than 50% of the Corporation’s voting share or an assignment for the benefit of creditors
or bankruptcy, or
●Upon
the fifth year anniversary of the salary increase and the bonus issuance.
As
of June 30, 2020 and December 31, 2019 the Company owes $82,902 and $64,352, respectively, to Mr. Rice for payroll payable.
On
October 21, 2019, the Company signed an agreement to increase Mr. Rice’s base salary by $20,000 per year (effective August
15, 2019). The salary increase is accrued and to be paid in full earlier by the direction of the Board or upon the earlier of:
●The
Company maintaining $6,000,000 or more in working capital,
●Upon
the transfer of ownership of more than 50% of the Corporation’s voting share or an assignment for the benefit of creditors
or bankruptcy, or
●Upon
the fifth year anniversary of the salary increase and the bonus issuance.
On
July 3, 2019, the board of directors appointed Mr. Kenneth Le as the Company’s Director of Government relations and President
of Prodigy Textiles. Mr. Le’s employment agreement has a term of one year and can be terminated by either the Company or
Mr. Rice at any time. Under the employment agreement, Mr. Le is entitled to annual cash compensation of $60,000. In addition,
Mr. Le was issued two three-year warrants to purchase 2,000,000 shares of common stock of the Company at an exercise price of
$0.2299 per share. As of June 30, 2020 and December 31, 2019, the accrued salary balance is $533 and $1,154, respectively.
(A)
License Agreement
On
May 8, 2006, the Company entered into a license agreement. Pursuant to the terms of the agreement, the Company paid a non- refundable
license fee of $10,000. The Company will pay a license maintenance fee of $10,000 on the one year anniversary of this agreement
and each year thereafter. The Company will pay an annual research fee of $13,700 with first payment due January 2007, then on
each subsequent anniversary of the effective date commencing May 4, 2007. The annual research fees are accrued by the Company
for future payment. Pursuant to the terms of the agreement the Company may be required to pay additional fees aggregating up to
a maximum of $10,000 a year for patent maintenance and prosecution relating to the licensed intellectual property.
On
October 28, 2011, the Company entered into a license agreement with the University of Notre Dame. Under the agreement, the Company
received exclusive and non-exclusive rights to certain spider silk technologies including commercial rights with the right to
sublicense such intellectual property. In consideration of the licenses granted under the agreement, the Company agreed to issue
to the University of Notre Dame 2,200,000 shares of its common stock and to pay a royalty of 2% of net sales. The license agreement
has a term of 20 years which can be extended on an annual basis after that. It can be terminated by the University of Notre Dame
if the Company defaults on its obligations under the agreement and fails to cure such default within 90 days of a written notice
by the university. The Company can terminate the agreement upon a 90 day written notice subject to payment of a termination fee
of $5,000 if the termination takes place within 2 years after its effectiveness, $10,000 if the termination takes place within
4 years after its effectiveness and $20,000 if the Agreement is terminated after 4 years. On May 5, 2017, the Company signed an
addendum to that agreement relating to tangible property and project intellectual property. On March 1, 2019, the Company singed
an addendum to that agreement. The Company entered into a separate loan agreement and promissory noted dated March 1, 2019 as
a payment for expenses paid by the University prior to January 31, 2019 totaling $265,244 and issued 4,025,652 shares of Class
A common stock with a fair value of $281,659 as payment of certain debt. In the event of default the license agreement will be
terminated. During the six months ended June 30, 2020, the Company paid $30,000 of the balance (See Notes 6).
(B)
Royalty and Research Agreements
On
May 1, 2008 the Company entered into a five year consulting agreement for research and development. Pursuant to the terms of the
agreement, the Company will be required to pay $1,000 per month, or at the Company’s option, the consulting fee may be paid
in the form of Company common stock based upon the greater of $0.05 per share or the average of the closing price of the Company’s
shares over the five days preceding such stock issuance. On April 6, 2018, the Company issued 36,000 shares with a fair value
of $1,076 ($0.0299/share) to a consultant as consideration for consulting fees owed from October 1, 2014 through December 31,
2019 of $21,000. The issuance of shares resulted in gain on settlement of accounts payable of $19,924. On April 1, 2018, the Company
ended the consulting agreement and no additional compensation will be issued. (See Note 7 (B)).
On
December 26, 2006, the Company entered into an addendum to the intellectual property transfer agreement with Mr. Thompson, its
CEO. In accordance with FASB ASC No 480, Distinguishing Liabilities from Equity, the Company determined that the present
value of the payment of $120,000 that was due on December 26, 2007. As of June 30, 2020 and December 31, 2019, the outstanding
balance is $65,292. As of December 31, 2019, the Company recorded interest expense and related accrued interest payable of $2,623.
In 2020 the Company recorded $980 in interest expensed and related accrued interest payable. As of June 30, 2020 the Company recorded
interest expense and related accrued interest payable of $7,523.
On
December 30, 2015, the Company entered into a cooperative agreement for the research and pilot production of hybrid silkworms
in Vietnam. Under this agreement, the Company will establish a subsidiary in Vietnam where it will develop and produce hybrid
silkworms. On April 24, 2018, the Company announced that it had received its investment registration certificate for its new Vietnamese
subsidiary Prodigy Textiles Co., Ltd. On May 1, 2018, the Company announced that it had received its enterprise registration certificate
for its new Vietnamese subsidiary Prodigy Textiles Co., Ltd.
(C)
Consulting Agreement
On
February 20, 2018, the Company signed an agreement with a consultant to provide services. Under this agreement the consultant
will receive a warrant for 600,000 shares of common stock and may be awarded additional warrants for up to 3,000,000 shares of
common stock if performance metrics are achieved. On March 20, 2018, the Company issued a 4-year warrant to purchase 600,000 shares
of common stock at an exercise price of $0.001 per share to a consultant for services rendered. The warrants had a fair value
of $19,915, based upon the Black-Scholes option-pricing model on the date of grant and are fully vested on March 20, 2018. Warrants
will be exercisable on March 20, 2019, and for a period of 3 years expiring on March 20, 2022. During the year ended December
31, 2018, the Company recorded $19,915 as an expense for warrants issued (See Note 7 (C)). On April 5, 2019, the Company cancelled
600,000 warrant issued to a consultant on February 20, 2018 in exchange for $6,000 cash payment.
(D)
Operating Lease Agreements
Since
September of 2015, we rent office space at 2723 South State Street, Suite 150, Ann Arbor, Michigan 48104, which is our principal
place of business. We pay an annual rent of $2,508 for conference facilities, mail, fax, and reception services located at our
principal place of business.
On
May 9, 2019 the Company signed an 5 year property lease Socialist Republic of Vietnam which consists of 4,560.57 square meters
of space, which it leases at a current rent of approximately $45,150 per year one and two and with the 5% increase per year for
years three through five.
On
January 23, 2017 the Company signed an 8 year property lease with the Company’s President for land in Texas where the Company
grows its mulberry. The Company pays a monthly rent of $960. Rent expense – related party for the six months ended June
30, 2020 and 2019, was $6,263 and $6,153, respectively (See Note 9).
On
September 13, 2017, the Company signed a new two year lease commencing on October 1, 2017 and ending on March 31, 2020. The Company
pays an annual rent of $39,200 for the year one of lease and $42,000 for the year two of lease for office and manufacturing space.
On September 5, 2019, the Company signed a new two-year lease for this 5,000 square foot property in Lansing, MI that commenced
on October 1, 2019 and ends on September 30, 2021, for its research and development headquarters. The Company pays an annual rent
of $42,000 for year one of the lease and $44,800 for year two of the lease.
NOTE
9 RELATED PARTY TRANSACTIONS
On
December 26, 2006, the Company entered into an addendum to the intellectual property transfer agreement with Mr. Thompson, its
CEO. Pursuant to the addendum, the Company agreed to issue either 200,000 preferred shares with the following preferences; no
dividends and voting rights equal to 100 common shares per share of preferred stock or the payment of $120,000, the officer agreed
to terminate the royalty payments due under the agreement and give title to the exclusive license for the non-protective apparel
use of the intellectual property to the Company. On the date of the agreement, the Company did not have any preferred stock authorized
with the required preferences. In accordance with FASB ASC No. 480, Distinguishing Liabilities from Equity, the Company
determined that the present value of the payment of $120,000 that was due on December 26, 2007, one year anniversary of the addendum,
should be recorded as an accrued expense until such time as the Company has the ability to assert that it has preferred shares
authorized. As of June 30, 2020 the outstanding balance is $65,292. Additionally, the accrued expenses are accruing 7% interest
per year. As of June30, 2020, the Company recorded interest expense and related accrued interest payable of $7,523.
On
November 10, 2010, the Company entered into an employment agreement, with its CEO, effective January 1, 2011 through the December
31, 2015. Subsequently, on January 1, 2018 the agreement renewed with the same terms for another 5 years with an annual salary
of $334,708 for the year ended December 31, 2019. As of June 30, 2020 and December 31, 2019, the accrued salary balance is $2,616,686
and $2,535,203, respectively.
On
January 14, 2016 the Company signed a new employment agreement with Mr. Rice, the Company’s COO. The employment agreement
has a term of one year and can be terminated by either the Company or Mr. Rice at any time. Under the employment agreement, Mr.
Rice is entitled to annual cash compensation of $140,000, which includes salary, health insurance, 401K retirement plan contributions,
etc. In addition, Mr. Rice was issued a three-year warrant to purchase 6,000,000 shares of common stock of the Company at an exercise
price of $0.001 per share pursuant to the employment agreement. On January 9, 2018, the Company extended the expiration date of
a warrant for 2,000,000 shares of common stock from January 19, 2018 to January 31, 2020 and on January 10, 2020, the Company
extended the expiration date of the warrant to January 23, 2015 for Mr. Rice. Additionally, on March 15, 2018, the Company signed
an extension of its at-will employment agreement with its COO. On April 26, 2019, the Company signed an agreement to increase
Mr. Rice’s base salary by $20,000 per year and issue a one-time $20,000 bonus. Additional, on August 15, 2019, the Company
signed an agreement to increase Mr. Rice’s base salary by an additional $20,000 per year. The salary increase and the bonus
is accrued and to be paid in full earlier by the direction of the Board or upon the earlier of:
|
|
●
|
The
Company maintaining $6,000,000 or more in working capital,
|
|
|
|
|
|
|
●
|
Upon
the transfer of ownership of more than 50% of the Corporation’s voting share or an assignment for the benefit of creditors
or bankruptcy, or
|
|
|
|
|
|
|
●
|
Upon
the fifth year anniversary of the salary increase and the bonus issuance.
|
As
of June 30, 2020 and December 31, 2019, the Company owes $82,902 and $64,351, respectively, to Mr. Rice for payroll payable.
On
July 3, 2019, the board of directors appointed Mr. Kenneth Le as the Company’s Director of Government relations and President
of Prodigy Textiles. Mr. Le’s employment agreement has a term of one year and can be terminated by either the Company or
Mr. Rice at any time. Under the employment agreement, Mr. Le is entitled to an annual cash compensation of $60,000. In addition,
Mr. Le was issued two three-year warrants to purchase 2,000,000 shares of common stock of the Company at an exercise price of
$0.2299 per share. As of June 30, 2020 and December 31, 2019, the accrued salary balance is $533 and $1,154, respectively.
On
June 6, 2016, the Company received a $50,000 loan from our principal stockholder. Subsequently on December 1, 2017, the Company
received an additional $30,000 loan from the same stockholder. On January 8, 2018 and March 31, 2018 the Company received an additional
loan of $100,000 and $15,000, respectively. The Company received additional loan funds from the same stockholder as follows: $20,000
on April 26, 2018; $15,000 on June 21, 2018; $15,000 on June 29, 2018; $20,000 on July 5, 2018; $26,000 on October 1, 2018; $11,000
on October 12, 2018; $20,000 on December 21, 2018; $3,000 on January 4, 2019; $30,000 on January 17, 2019; $30,000 on February
1, 2019; $20,000 on February 15, 2019; $20,000 on March 1, 2019; $17,000 on January 4, 2019, $100,000 on November 20, 2019, $100,000
on December 18, 2019, $100,000 on January 24, 2020, $100,000 on February 19, 2020, $100,000 on April 8, 2020 and $150,000 on June
3, 2020. Pursuant to the terms of the loan, the advance bears an interest at 3%, is unsecured, and due on demand. Total loan payable
to principal stockholder for as of December 31, 2019 is $642,000. Total loan payable to this principal stockholder as of June
30, 2020 is $1,192,000. During the six months ended June 30, 2020, the Company recorded $21,972 as an in-kind contribution of
interest related to the loan and recorded accrued interest payable of $14,161. During the six months ended June 30, 2019, the
Company recorded $10,457 as an in-kind contribution of interest related to the loan and recorded accrued interest payable of $7,033.
On
January 23, 2017, the Company signed an 8 year property lease with the Company’s President for land in Texas. The Company
pays $960 per month starting on February 1, 2017 and uses this facility to grow mulberry for its U.S. silk operations. Rent expense
– related party for six months ended June 30, 2020 and 2019 was $6,263 and $6,153, respectively.
As
of June 30, 2020 and December 31, 2019, there was $319,005 and $304,539, respectively, included in accounts payable and accrued
expenses - related party, which is owed to the Company’s Chief Executive Officer and Chief Operations Officer.
As
of June 30, 2020, there was $1,344,534 of accrued interest- related party and $58,855 in shareholder loan interest – related
party included in accounts payable and accrued expenses – related party, which is owed to the Company’s Chief Executive
officer.
As
of December 31, 2019, there was $1,196,503 of accrued interest- related party and $43,715 in shareholder loan interest –
related party included in accounts payable and accrued expenses – related party, which is owed to the Company’s Chief
Executive officer.
As
of June 30, 2020, the Company owes $2,616,686 in accrued salary to principal stockholder, $82,902 to the Company’s COO,
$533 to Director of Prodigy Textiles and $11,274 to its office employees.
As
of December 31, 2019, the Company owes $2,535,203 in accrued salary to principal stockholder, $64,351 to the Company’s COO,
$1,153 to Director of Prodigy Textiles and $4,477 to its office employees.
The
Company owes $65,292 in royalty payable to related party as of June 30, 2020 and December 31, 2019.
NOTE
10 SUBSEQUENT EVENTS
The
Company has analyzed its operations subsequent to July 31, 2020 through the date these financial statements were issued, and has
determined that, other than disclosed below, it does not have any material subsequent events to disclose.
On
July 17, 2020, the Company received $100,000 from a principal stockholder. Pursuant to the terms of the loan, the advances bear
an interest at 3%, is unsecured and due on demand.
On July 30, 2020, the Company issued 9,941,623
shares of Common stock in connection with the cashless exercise of 10,000,000 warrants.

REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
the Board of Directors and
Stockholders of Kraig Biocraft Laboratories, Inc.
Opinion
on the Financial Statements
We
have audited the accompanying consolidated balance sheets of Kraig Biocraft Laboratories, Inc. (the Company) as of December 31,
2019 and 2018, and the related statements of operations, stockholders’ deficit, and cash flows for each of the years in
the two-year period ended December 31, 2019, and the related notes and schedules (collectively referred to as the financial statements).
In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of
December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the years in the two-year period
ended December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
Basis
for Opinion
These
financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on
the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company
Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance
with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the
PCAOB.
We
conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit
to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error
or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial
reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but
not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting.
Accordingly, we express no such opinion.
Our
audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to
error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence
regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles
used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements.
We believe that our audits provide a reasonable basis for our opinion.
The
accompanying financial statements have been prepared assuming that the Company will continues as a going concern. As discussed
in Note 2 to the financial statements, the Company has suffered a net loss from operations and has a net capital deficiency, which
raises substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are
discussed in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
M&K
CPAS, PLLC.
We
have served as the Company’s auditor since 2013.
Houston,
TX
March
27, 2020
Kraig
Biocraft Laboratories, Inc. and Subsidiary
Consolidated
Balance Sheets
|
|
|
December
31, 2019
|
|
|
December
31, 2018
|
|
|
ASSETS
|
|
|
|
|
|
|
|
Current Assets
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
125,024
|
|
|
$
|
13,697
|
|
|
Prepaid
expenses
|
|
|
31,745
|
|
|
|
6,858
|
|
|
Total
Current Assets
|
|
|
156,769
|
|
|
|
20,555
|
|
|
|
|
|
|
|
|
|
|
|
|
Property and Equipment, net
|
|
|
117,321
|
|
|
|
47,310
|
|
|
Operating lease right-of-use asset,
net
|
|
|
473,242
|
|
|
|
-
|
|
|
Security deposit
|
|
|
3,518
|
|
|
|
3,518
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
Assets
|
|
$
|
750,850
|
|
|
$
|
71,383
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES
AND STOCKHOLDERS’ DEFICIT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Liabilities
|
|
|
|
|
|
|
|
|
|
Accounts payable
and accrued expenses
|
|
$
|
560,948
|
|
|
$
|
793,482
|
|
|
Note payable - related
party
|
|
|
642,000
|
|
|
|
322,000
|
|
|
Royalty agreement
payable - related party
|
|
|
65,292
|
|
|
|
65,292
|
|
|
Accounts payable
and accrued expenses - related party
|
|
|
4,145,465
|
|
|
|
3,349,832
|
|
|
Operating lease
liability, current
|
|
|
110,678
|
|
|
|
-
|
|
|
Loan
payable
|
|
|
60,000
|
|
|
|
-
|
|
|
Total
Current Liabilities
|
|
|
5,584,383
|
|
|
|
4,530,606
|
|
|
|
|
|
|
|
|
|
|
|
|
Long Term Liabilities
|
|
|
|
|
|
|
|
|
|
Loan payable, net
of current
|
|
|
185,244
|
|
|
|
-
|
|
|
Operating
lease liability, net of current
|
|
|
369,281
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
Liabilities
|
|
|
6,138,908
|
|
|
|
4,530,606
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and Contingencies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’
Deficit
|
|
|
|
|
|
|
|
|
|
Preferred stock Series
A, no par value; 2 and 2 shares issued and outstanding, respectively
|
|
|
5,217,800
|
|
|
|
5,217,800
|
|
|
Common stock Class
A, no par value; unlimited shares authorized, 844,468,378 and 816,883,910 shares issued and outstanding, respectively
|
|
|
16,757,079
|
|
|
|
15,145,798
|
|
|
Common stock Class
B, no par value; unlimited shares authorized, no shares issued and outstanding
|
|
|
-
|
|
|
|
-
|
|
|
Common Stock Issuable,
1,122,311 and 1,122,311 shares, respectively
|
|
|
22,000
|
|
|
|
22,000
|
|
|
Additional paid-in
capital
|
|
|
2,412,969
|
|
|
|
2,043,235
|
|
|
Accumulated
Deficit
|
|
|
(29,797,906
|
)
|
|
|
(26,888,056
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total
Stockholders’ Deficit
|
|
|
(5,388,058
|
)
|
|
|
(4,459,223
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total
Liabilities and Stockholders’ Deficit
|
|
$
|
750,850
|
|
|
$
|
71,383
|
|
Kraig
Biocraft Laboratories, Inc. and Subsidiary
Consolidated
Statements of Operations
|
|
|
For
the Years Ended
|
|
|
|
|
December
31, 2019
|
|
|
December
31, 2018
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
$
|
-
|
|
|
$
|
401,620
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating Expenses
|
|
|
|
|
|
|
|
|
|
General and Administrative
|
|
|
1,413,982
|
|
|
|
515,875
|
|
|
Professional Fees
|
|
|
342,845
|
|
|
|
157,976
|
|
|
Officer’s Salary
|
|
|
627,197
|
|
|
|
528,127
|
|
|
Rent - Related Party
|
|
|
14,793
|
|
|
|
11,520
|
|
|
Research and
Development
|
|
|
223,050
|
|
|
|
148,069
|
|
|
Total
Operating Expenses
|
|
|
2,621,867
|
|
|
|
1,361,567
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from Operations
|
|
|
(2,621,867
|
)
|
|
|
(959,947
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Other Income/(Expenses)
|
|
|
|
|
|
|
|
|
|
Gain on forgiveness of debt
|
|
|
-
|
|
|
|
19,924
|
|
|
Interest expense
|
|
|
(294,352
|
)
|
|
|
(228,954
|
)
|
|
Interest income
|
|
|
6,369
|
|
|
|
-
|
|
|
Total
Other Income/(Expenses)
|
|
|
(287,983
|
)
|
|
|
(209,030
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net
(Loss) before Provision for Income Taxes
|
|
|
(2,909,850
|
)
|
|
|
(1,168,977
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Provision
for Income Taxes
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
(Loss)
|
|
$
|
(2,909,850
|
)
|
|
$
|
(1,168,977
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net
Income (Loss) Per Share - Basic and Diluted
|
|
$
|
(0.00
|
)
|
|
$
|
(0.00
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
average number of shares outstanding during the period - Basic and Diluted
|
|
|
835,587,422
|
|
|
|
816,874,442
|
|
Kraig
Biocraft Laboratories, Inc. and Subsidiary
Consolidated
Statements of Cash Flows
|
|
|
For
the years ended December 31,
|
|
|
|
|
2019
|
|
|
2018
|
|
|
Cash Flows From Operating
Activities:
|
|
|
|
|
|
|
|
|
|
Net Loss
|
|
$
|
(2,909,850
|
)
|
|
$
|
(1,168,977
|
)
|
|
Adjustments to reconcile
net loss to net cash used in operations
|
|
|
|
|
|
|
|
|
|
Depreciation expense
|
|
|
30,781
|
|
|
|
26,632
|
|
|
Gain on forgiveness
of debt
|
|
|
-
|
|
|
|
(19,924
|
)
|
|
Imputed interest
- related party
|
|
|
22,337
|
|
|
|
11,909
|
|
|
Fair value of options
issued for services
|
|
|
696,934
|
|
|
|
-
|
|
|
Warrants issued/(cancelled)
to consultants
|
|
|
(19,915
|
)
|
|
|
72,575
|
|
|
Changes in operating
assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Increase in prepaid
expenses
|
|
|
(24,887
|
)
|
|
|
(2,394
|
)
|
|
(Increase) in accounts
receivables, net
|
|
|
-
|
|
|
|
25,872
|
|
|
Operating lease
right-of-use, net
|
|
|
86,326
|
|
|
|
-
|
|
|
Increase in accrued
expenses and other payables - related party
|
|
|
795,633
|
|
|
|
682,976
|
|
|
Increase (Decrease)
in accounts payable
|
|
|
314,369
|
|
|
|
136,326
|
|
|
Operating
lease liabilities, current
|
|
|
(79,609
|
)
|
|
|
-
|
|
|
Net
Cash Used In Operating Activities
|
|
|
(1,087,881
|
)
|
|
|
(235,005
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Investing
Activities:
|
|
|
|
|
|
|
|
|
|
Purchase of Fixed
Assets and Domain Name
|
|
|
(100,792
|
)
|
|
|
(11,448
|
)
|
|
Net
Cash Used In Investing Activities
|
|
|
(100,792
|
)
|
|
|
(11,448
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Financing
Activities:
|
|
|
|
|
|
|
|
|
|
Proceeds from Notes Payable - related
party
|
|
|
320,000
|
|
|
|
242,000
|
|
|
Principal payments on debt
|
|
|
(20,000
|
)
|
|
|
-
|
|
|
Proceeds from
issuance of common stock
|
|
|
1,000,000
|
|
|
|
-
|
|
|
Net
Cash Provided by Financing Activities
|
|
|
1,300,000
|
|
|
|
242,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Increase in Cash
|
|
|
111,327
|
|
|
|
(4,453
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash at Beginning
of Year
|
|
|
13,697
|
|
|
|
18,150
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
at End of Year
|
|
$
|
125,024
|
|
|
$
|
13,697
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental
disclosure of cash flow information:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid for
interest
|
|
$
|
-
|
|
|
$
|
10
|
|
|
Cash paid for
taxes
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental
disclosure of non-cash investing and financing activities:
|
|
|
|
|
|
|
|
|
|
Shares
issued in connection with cashless warrants exercise
|
|
$
|
329,622
|
|
|
$
|
-
|
|
|
Settlement
of accounts payable with note payable
|
|
$
|
265,244
|
|
|
$
|
-
|
|
|
Settlement
of accounts payable with stock issuance
|
|
$
|
281,659
|
|
|
$
|
1,076
|
|
|
Adoption
of lease standard ASC 842
|
|
$
|
559,568
|
|
|
$
|
-
|
|
Kraig
Biocraft Laboratories, Inc. and Subsidiary
Consolidated
Statement of Changes in Stockholders Deficit
For
the years ended December 31, 2019 and 2018
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
Stock -
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred
Stock -
|
|
|
Common
Stock -
|
|
|
Common
Stock -
|
|
|
Class
A Shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series
A
|
|
|
Class
A
|
|
|
Class
B
|
|
|
To
be issued
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
Shares
|
|
|
Par
|
|
|
Shares
|
|
|
Par
|
|
|
Shares
|
|
|
Par
|
|
|
Shares
|
|
|
Par
|
|
|
APIC
|
|
|
Deficit
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
December 31, 2017
|
|
|
2
|
|
|
$
|
5,217,800
|
|
|
|
816,847,910
|
|
|
$
|
15,144,722
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
1,122,311
|
|
|
$
|
22,000
|
|
|
$
|
1,958,751
|
|
|
$
|
(25,719,079
|
)
|
|
$
|
(3,375,806
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued for
services
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
72,575
|
|
|
$
|
-
|
|
|
$
|
72,575
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock issued for services
($0.0299/Sh)
|
|
|
-
|
|
|
$
|
-
|
|
|
|
36,000
|
|
|
$
|
1,076
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
1,076
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Imputed interest - related
party
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
11,909
|
|
|
$
|
-
|
|
|
$
|
11,909
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss for the year ended December 31, 2018
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
(1,168,977
|
)
|
|
$
|
(1,168,977
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
December 31, 2018
|
|
|
2
|
|
|
$
|
5,217,800
|
|
|
|
816,883,910
|
|
|
$
|
15,145,798
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
1,122,311
|
|
|
$
|
22,000
|
|
|
$
|
2,043,235
|
|
|
$
|
(26,888,056
|
)
|
|
$
|
(4,459,223
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Units issued for cash
|
|
|
-
|
|
|
$
|
-
|
|
|
|
14,797,278
|
|
|
$
|
1,000,000
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
1,000,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares issued in exchange
for accounts payable
|
|
|
-
|
|
|
$
|
-
|
|
|
|
4,052,652
|
|
|
$
|
281,659
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
281,659
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options issued for services
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
696,934
|
|
|
$
|
-
|
|
|
$
|
696,934
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of 9,000,000
warrants in exchange for stock
|
|
|
-
|
|
|
$
|
-
|
|
|
|
8,734,538
|
|
|
$
|
329,622
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
(329,622
|
)
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cancellation of warrants
issued for services
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
(19,915
|
)
|
|
$
|
-
|
|
|
$
|
(19,915
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Imputed interest - related
party
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
22,337
|
|
|
$
|
-
|
|
|
$
|
22,337
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss for the year ended December 31, 2019
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
(2,909,850
|
)
|
|
$
|
(2,909,850
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
December 31, 2019
|
|
|
2
|
|
|
$
|
5,217,800
|
|
|
|
844,468,378
|
|
|
$
|
16,757,079
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
1,122,311
|
|
|
$
|
22,000
|
|
|
$
|
2,412,969
|
|
|
$
|
(29,797,906
|
)
|
|
$
|
(5,388,058
|
)
|
NOTE
1 SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES AND ORGANIZATION
(A)
Organization
Kraig
Biocraft Laboratories, Inc. (the “Company”) was incorporated under the laws of the State of Wyoming on April 25, 2006.
The Company was organized to develop high strength, protein based fiber, using recombinant DNA technology, for commercial applications
in the textile and specialty fiber industries.
On
March 5, 2018, the Company issued a board resolution authorizing investment in a Vietnamese subsidiary and appointing a representative
for the subsidiary.
On
April 24, 2018, the Company announced that it had received its investment registration certificate for its new Vietnamese subsidiary
Prodigy Textiles Co., Ltd.
On
May 1, 2018, the Company announced that it had received its enterprise registration certificate for its new Vietnamese subsidiary
Prodigy Textiles Co., Ltd.
On
October 8, 2019, the Company delivered the first batch of its transgenic silkworm eggs to its production factory in Quang Nam,
Vietnam for the purpose of commencing production at that facility.
On
November 4, 2019, the Company reported that it had successfully completed rearing the first batch of its transgenic silkworms
at its production factory in Quang Nam, Vietnam. The Company expects to continue expanding production of its specialized silk.
In
January 2020, the Company reported challenges related to climate and access to high quality mulberry during the winter months at
its subsidiary in Vietnam. The Company announced that it had already begun planting additional mulberry fields to ensure no future
shortages.
(B)
Foreign Currency
The
assets and liabilities of Prodigy Textiles, Co., Ltd. (the Company’s Vietnamese subsidiary) whose functional currency is
the Vietnamese Dong, are translated into US dollars at period-end exchange rates prior to consolidation. Income and expense items
are translated at the average rates of exchange prevailing during the period. The adjustments resulting from translating the Company’s
financial statements are reflected as a component of other comprehensive (loss) income. Foreign currency transaction gains and
losses are recognized in net earnings based on differences between foreign exchange rates on the transaction date and settlement
date.
(C)
Use of Estimates
In
preparing financial statements in conformity with generally accepted accounting principles, management is required to make estimates
and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities
at the date of the financial statements and revenues and expenses during the reported period. Actual results could differ from
those estimates.
(D)
Cash
For
the purposes of the cash flow statements, the Company considers all highly liquid investments with original maturities of three
months or less at the time of purchase to be cash equivalents. There were no cash equivalents as of December 31, 2019 or December
31, 2018.
(E)
Loss Per Share
Basic
and diluted net loss per common share is computed based upon the weighted average common shares outstanding as defined by the
Financial Accounting Standards Board (“FASB” Accounting Standards Codification (“ASC”) No. 260, “Earnings
per Share.” For December 31, 2019 and December 31, 2018, warrants were not included in the computation of income/ (loss)
per share because their inclusion is anti-dilutive.
The
computation of basic and diluted loss per share for December 31, 2019 and December 31, 2018 excludes the common stock equivalents
of the following potentially dilutive securities because their inclusion would be anti-dilutive:
|
|
|
December
31, 2019
|
|
|
December
31, 2018
|
|
|
Stock Warrants (Exercise price - $0.001-$0.2299/share)
|
|
|
55,995,917
|
|
|
|
36,400,000
|
|
|
Convertible Preferred Stock
|
|
|
2
|
|
|
|
2
|
|
|
Total
|
|
|
55,995,919
|
|
|
|
36,400,002
|
|
(F)
Research and Development Costs
The
Company expenses all research and development costs as incurred for which there is no alternative future use. These costs also
include the expensing of employee compensation and employee stock based compensation.
(G)
Income Taxes
The
Company accounts for income taxes under FASB Codification Topic 740-10-25 (“ASC 740-10-25”). Under ASC No. 740-10-25,
deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial
statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities
are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are
expected to be recovered or settled. Under ASC No. 740-10-25, the effect on deferred tax assets and liabilities of a change in
tax rates is recognized in income in the period that includes the enactment date.
The
net deferred tax liability in the accompanying balance sheets includes the following amounts of deferred tax assets and liabilities:
|
|
|
2019
|
|
|
2018
|
|
|
Expected income tax recovery
(expense) at the statutory rate of 21%
|
|
$
|
(610,845
|
)
|
|
$
|
(1,412,328
|
)
|
|
Tax effect of expenses that are not
deductible for income tax purposes (net of other amounts deductible for tax purposes)
|
|
|
142,167
|
|
|
|
11,057
|
|
|
Change in valuation allowance
|
|
|
(468,678
|
)
|
|
|
(1,423,384
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Provision for income taxes
|
|
$
|
-
|
|
|
$
|
-
|
|
The
components of deferred income taxes are as follows:
|
|
|
Years
Ended December,
|
|
|
|
|
2019
|
|
|
2018
|
|
|
Deferred tax liability:
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Deferred tax asset
|
|
|
|
|
|
|
|
|
|
Net
Operating Loss Carryforward
|
|
|
3,379,542
|
|
|
|
2,910,863
|
|
|
Valuation allowance
|
|
|
(3,379,542
|
)
|
|
|
(2,910,863
|
)
|
|
Net deferred tax
asset
|
|
|
-
|
|
|
|
-
|
|
|
Net
deferred tax liability
|
|
$
|
-
|
|
|
$
|
-
|
|
The
valuation allowance was established to reduce the deferred tax asset to the amount that will more likely than not be realized.
This is necessary due to the Company’s continued operating losses and the uncertainty of the Company’s ability to
utilize all of the net operating loss carryforwards before they will expire through the year 2039.
The
net change in the valuation allowance for the year ended December 31, 2019 and 2018 was an increase of $610,845 and a decrease
of $1,412,328, respectively.
Fair
value accounting requires bifurcation of embedded derivative instruments such as conversion features in convertible debt or equity
instruments, and measurement of their fair value for accounting purposes. In determining the appropriate fair value, the Company
uses the Black-Scholes option-pricing model. In assessing the convertible debt instruments, management determines if the convertible
debt host instrument is conventional convertible debt and further if there is a beneficial conversion feature requiring measurement.
If the instrument is not considered conventional convertible debt, the Company will continue its evaluation process of these instruments
as derivative financial instruments.
Once
determined, derivative liabilities are adjusted to reflect fair value at each reporting period end, with any increase or decrease
in the fair value being recorded in results of operations as an adjustment to fair value of derivatives. In addition, the fair
value of freestanding derivative instruments such as warrants, are also valued using the Black-Scholes option-pricing model.
(H)
Stock-Based Compensation
In
December 2004, the FASB issued FASB ASC No. 718, Compensation – Stock Compensation. Under FASB ASC No. 718, companies
are required to measure the compensation costs of share-based compensation arrangements based on the grant- date fair value and
recognize the costs in the financial statements over the period during which employees are required to provide services. Share-based
compensation arrangements include stock options, restricted share plans, performance-based awards, share appreciation rights and
employee share purchase plans. As such, compensation cost is measured on the date of grant at their fair value. Such compensation
amounts, if any, are amortized over the respective vesting periods of the option grant. The Company applies this statement prospectively.
Equity
instruments (“instruments”) issued to other than employees are recorded on the basis of the fair value of the instruments,
as required by FASB ASC No. 718. FASB ASC No. 505, Equity Based Payments to Non-Employees defines the measurement date
and recognition period for such instruments. In general, the measurement date is when either a (a) performance commitment, as
defined, is reached or (b) the earlier of (i) the non-employee performance is complete or (ii) the instruments are vested. The
measured value related to the instruments is recognized over a period based on the facts and circumstances of each particular
grant as defined in the FASB ASC.
(I)
Recent Accounting Pronouncements
In
February 2016, the FASB issued ASU 2016-02, “Leases” Topic 842, which amends the guidance in former ASC Topic 840,
Leases. The new standard increases transparency and comparability most significantly by requiring the recognition by lessees
of right-of-use (“ROU”) assets and lease liabilities on the balance sheet for all leases longer than 12 months. Under
the standard, disclosures are required to meet the objective of enabling users of financial statements to assess the amount, timing,
and uncertainty of cash flows arising from leases. For lessees, leases will be classified as finance or operating, with classification
affecting the pattern and classification of expense recognition in the income statement. The Company adopted the new lease guidance
effective January 1, 2019 using the modified retrospective transition approach, applying the new standard to all of its leases
existing at the date of initial application which is the effective date of adoption. Consequently, financial information will
not be updated and the disclosures required under the new standard will not be provided for dates and periods before January 1,
2019. We elected the package of practical expedients which permits us to not reassess (1) whether any expired or existing contracts
are or contain leases, (2) the lease classification for any expired or existing leases, and (3) any initial direct costs for any
existing leases as of the effective date. We did not elect the hindsight practical expedient which permits entities to use hindsight
in determining the lease term and assessing impairment. The adoption of the lease standard did not change our previously reported
consolidated statements of operations and did not result in a cumulative catch-up adjustment to opening equity. As a result, the
Company has recorded Right-to-use assets and corresponding Lease obligations as more fully discussed in Note 4.
Those
amendments do not have an accounting effect. For public business entities, the amendments in Part I of this update are effective
for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. Early adoption is permitted
for all entities, including adoption in an interim period. If an entity early adopts the amendments in an interim period, any
adjustments should be reflected as of the beginning of the fiscal year that includes that interim period. The Company is currently
reviewing the impact of adoption of ASU 2017-11 on its financial statements.
All
other newly issued accounting pronouncements but not yet effective have been deemed either immaterial or not applicable. The 2018
financial statements have been reclassified to conform to the 2019 presentation.
(J)
Equipment
The
Company values property and equipment at cost and depreciates these assets using the straight-line method over their expected
useful life. The Company uses a five year life for automobiles.
In
accordance with FASB ASC No. 360, Property, Plant and Equipment, the Company carries long-lived assets at the lower of
the carrying amount or fair value. Impairment is evaluated by estimating future undiscounted cash flows expected to result from
the use of the asset and its eventual disposition. If the sum of the expected undiscounted future cash flow is less than the carrying
amount of the assets, an impairment loss is recognized. Fair value, for purposes of calculating impairment, is measured based
on estimated future cash flows, discounted at a market rate of interest.
There
were no impairment losses recorded for the years ended December 31, 2019 and 2018.
(K)
Fair Value of Financial Instruments
We
hold certain financial assets, which are required to be measured at fair value on a recurring basis in accordance with the Statement
of Financial Accounting Standard No. 157, “Fair Value Measurements” (“ASC Topic 820-10”). ASC Topic
820-10 establishes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The
hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level
1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). ASC Topic 820-10 defines fair value as
the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants
on the measurement date. Level 1 instruments include cash, account receivable, prepaid expenses, inventory and account payable
and accrued liabilities. The carrying values are assumed to approximate the fair value due to the short term nature of the instrument.
The
three levels of the fair value hierarchy under ASC Topic 820-10 are described below:
|
|
●
|
Level
1 - Valuations based on quoted prices in active markets for identical assets or liabilities that an entity has the ability
to access. We believe our carrying value of level 1 instruments approximate their fair value at December 31, 2019 and December
31, 2018.
|
|
|
|
|
|
|
●
|
Level
2 - Valuations based on quoted prices for similar assets or liabilities, quoted prices for identical assets or liabilities
in markets that are not active, or other inputs that are observable or can be corroborated by observable data for substantially
the full term of the assets or liabilities.
|
|
|
|
|
|
|
●
|
Level
3 - Valuations based on inputs that are supported by little or no market activity and that are significant to the fair value
of the assets or liabilities. We consider depleting assets, asset retirement obligations and net profit interest liability
to be Level 3. We determine the fair value of Level 3 assets and liabilities utilizing various inputs, including NYMEX price
quotations and contract terms.
|
|
|
|
December
31, 2019
|
|
|
December
31, 2018
|
|
|
Level 1
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Level 2
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Level 3
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Total
|
|
$
|
-
|
|
|
$
|
-
|
|
(L)
Revenue Recognition
During
the year ended December 31, 2018, the Company’s revenues were generated primarily from a contract with the U.S. Government.
The Company performs work under this cost-plus-fixed-fee contract. Under the base phase of that contract the Company produced
recombinant spider silk woven into ballistic shootpack panels. Those shootpack panels were delivered to the U.S. Government customer.
Under an option period award starting in July 2017, to that original contract, the Company has worked to develop new recombinant
silks. This contract ended in September of 2018.
Effective
January 1, 2018, the Company adopted ASC No. 606 — Revenue from Contracts with Customers. Under ASC No. 606, the Company
recognizes revenue from the commercial sales of products, licensing agreements and contracts by applying the following steps:
(1) identify the contract with a customer; (2) identify the performance obligations in the contract; (3) determine the transaction
price; (4) allocate the transaction price to each performance obligation in the contract; and (5) recognize revenue when each
performance obligation is satisfied. For the comparative periods, revenue has not been adjusted and continues to be reported under
ASC No. 605 — Revenue Recognition. Under ASC No. 605, revenue is recognized when the following criteria are met: (1) persuasive
evidence of an arrangement exists;(2) the performance of service has been rendered to a customer or delivery has occurred; (3)
the amount of fee to be paid by a customer is fixed and determinable; and (4) the collectability of the fee is reasonably assured.
For
the years ended December 31, 2019 and 2018, the Company recognized $0 and $401,620 respectively in revenue from the Government
contract. These revenues were generated for work performed in the development and production of the Company’s recombinant
silks under the base and option period phases of our ongoing contract with the US Army.
On
July 24, 2017, the Company signed a contract option extension with the US Army to research and deliver recombinant spider silk
fibers and threads. This contract option increased the total contract award by an additional $921,130 to a total of $1,021,092
and added 12 months to the contract duration. This effort was scheduled to end on September 24, 2018, but the Company requested
an extension of this contract option period through April 2019 to complete the work. The Company has been in communication with
the contracting office and is working with them as they determine the best path forward; Management believes there is a possibility
of securing a follow-up contract to complete the delivery of all materials for the contract. The Company is also continuing to
pursue additional contract opportunities with the Department of Defense, Department of Energy and other governmental agencies.
(M)
Concentration of Credit Risk
The
Company at times has cash in banks in excess of FDIC insurance limits. At December 31, 2019 and December 31, 2018, the Company
had approximately $0 and $0, respectively in excess of FDIC insurance limits.
For
the years ended December 31, 2019 and 2018, the Company had a concentration of sales of:
|
|
|
December
31, 2019
|
|
|
December
31, 2018
|
|
|
Customer
|
|
|
|
|
|
|
|
Customer A
|
|
|
-
|
|
|
|
100
|
%
|
|
Customer A
|
|
$
|
-
|
|
|
$
|
401,620
|
|
For
the years ended December 31, 2019 and 2018, the Company booked $0 and $0 for doubtful accounts.
NOTE
2 GOING CONCERN
As reflected in the accompanying
financial statements, the Company has a working capital deficiency of $5,427,614 and stockholders’ deficiency of
$5,388,058 and used $1,087,881 of cash in operations for year ended December 31, 2019. This raises substantial doubt about
its ability to continue as a going concern. The ability of the Company to continue as a going concern is dependent on the
Company’s ability to raise additional capital and implement its business plan. The financial statements do not include
any adjustments that might be necessary if the Company is unable to continue as a going concern.
Management
believes that actions presently being taken to obtain additional funding and implement its strategic plans provide the opportunity
for the Company to continue as a going concern.
NOTE
3 EQUIPMENT
At
December 31, 2019 and December 31, 2018, property and equipment, net, is as follows:
|
|
|
December
31, 2019
|
|
|
December
31, 2018
|
|
|
Automobile
|
|
$
|
41,805
|
|
|
$
|
41,805
|
|
|
Laboratory Equipment
|
|
|
96,536
|
|
|
|
73,194
|
|
|
Office Equipment
|
|
|
7,260
|
|
|
|
7,260
|
|
|
Leasehold Improvements
|
|
|
85,388
|
|
|
|
7,938
|
|
|
Less: Accumulated Depreciation
|
|
|
(113,668
|
)
|
|
|
(82,887
|
)
|
|
Total Property and Equipment, net
|
|
$
|
117,321
|
|
|
$
|
47,310
|
|
Depreciation
expense for the years ended December 31, 2019 and 2018, was $30,781 and $26,632, respectively.
NOTE
4 - RIGHT TO USE ASSETS AND LEASE LIABILITY
Since
September of 2015, we rent office space at 2723 South State Street, Suite 150, Ann Arbor, Michigan 48104, which is our principal
place of business. We pay an annual rent of $2,508 for conference facilities, mail, fax, and reception services located at our
principal place of business.
On
January 23, 2017, the Company signed an 8 year property lease with the Company’s President for land in Texas where the Company
grows its mulberry. The Company pays a monthly rent of $960. Rent expense – related party for the years ended December 31,
2019 and 2018, was $11,913 and $5,760, respectively (See Note 9).
On
September 13, 2017, the Company signed a new two year lease commencing on October 1, 2017 and ending on December 31, 2019. The
Company pays an annual rent of $39,200 for the year one of lease and $42,000 for the year two of lease for office and manufacturing
space. On September 5, 2019, the Company signed a new two-year lease for this 5,000 square foot property in Lansing, MI that commenced
on October 1, 2019 and ends on September 30, 2021, for its research and development headquarters. The Company pays an annual rent
of $42,000 for year one of the lease and $44,800 for year two of the lease.
On
May 9, 2019, the Company signed a 5 year property lease with the Socialist Republic of Vietnam which consists of 4,560.57 square
meters of space, which it leases at a current rent of approximately $45,150 per year one and two and with the 5% increase per
year for years three through five.
In
February 2016, the FASB issued ASU 2016-02, “Leases” Topic 842, which amends the guidance in former ASC Topic 840,
Leases. The new standard increases transparency and comparability most significantly by requiring the recognition by lessees
of right-of-use (“ROU”) assets and lease liabilities on the balance sheet for all leases longer than 12 months. Under
the standard, disclosures are required to meet the objective of enabling users of financial statements to assess the amount, timing,
and uncertainty of cash flows arising from leases. For lessees, leases will be classified as finance or operating, with classification
affecting the pattern and classification of expense recognition in the income statement.
The
Company adopted the new lease guidance effective January 1, 2019 using the modified retrospective transition approach, applying
the new standard to all of its leases existing at the date of initial application which is the effective date of adoption. Consequently,
financial information will not be updated and the disclosures required under the new standard will not be provided for dates and
periods before January 1, 2019. We elected the package of practical expedients which permits us to not reassess (1) whether any
expired or existing contracts are or contain leases, (2) the lease classification for any expired or existing leases, and (3)
any initial direct costs for any existing leases as of the effective date. We did not elect the hindsight practical expedient
which permits entities to use hindsight in determining the lease term and assessing impairment. The adoption of the lease standard
did not change our previously reported consolidated statements of operations and did not result in a cumulative catch-up adjustment
to opening equity. The adoption of the new guidance resulted in the recognition of ROU assets of $559,568 and lease liabilities
of $559,568.
The
interest rate implicit in lease contracts is typically not readily determinable. As such, the Company utilizes its incremental
borrowing rate, which is the rate incurred to borrow on a collateralized basis over a similar term an amount equal to the lease
payments in a similar economic environment. In calculating the present value of the lease payments, the Company elected to utilize
its incremental borrowing rate based on the remaining lease terms as of the January 1, 2019 adoption date. This rate was determined
to be 8% and the Company determined the initial present value, at inception, of $559,568.
Operating
lease ROU assets and operating lease liabilities are recognized based on the present value of the future minimum lease payments
over the lease term at the commencement date. The operating lease ROU asset also includes any lease payments made and excludes
lease incentives and initial direct costs incurred, if any.
The
Company has elected the practical expedient to combine lease and non-lease components as a single component. The lease expense
is recognized over the expected term on a straight-line basis. Operating leases are recognized on the balance sheet as right-of-use
assets, current operating lease liabilities and non-current operating lease liabilities.
The
new standard also provides practical expedients and certain exemptions for an entity’s ongoing accounting. We have elected
the short- term lease recognition exemption for all leases that qualify. This means, for those leases where the initial lease
term is one year or less or for which the ROU asset at inception is deemed immaterial, we will not recognize ROU assets or lease
liabilities. Those leases are expensed on a straight line basis over the term of the lease
Right
to use assets is summarized below:
|
|
|
December
31,
|
|
|
Right to use assets, net
– related party
|
|
$
|
69,884
|
|
|
Right to use assets, net
|
|
|
53,799
|
|
|
Right to use assets, net
|
|
|
349,559
|
|
|
Total
|
|
$
|
473,242
|
|
During
the year ended December 31, 2019, the Company recorded $75,575 as lease expense to current period operations.
During
the year ended December 31, 2019, the Company recorded $11,913 as lease expense – related party to current period operations.
Lease
liability is summarized below:
|
|
|
December
31,
|
|
|
Right to use liability,
net – related party
|
|
|
55,372
|
|
|
Right to use liability, net
|
|
|
70,901
|
|
|
Right to use liability, net
|
|
|
353,686
|
|
|
Total
|
|
|
479,959
|
|
|
Less: short term portion
|
|
$
|
(110,678
|
)
|
|
Long term position
|
|
$
|
369,281
|
|
Lease
expense for the year ended December 31, 2019 was comprised of the following:
|
Operating lease expense
|
|
$
|
43,666
|
|
|
Operating lease expense
|
|
$
|
64,327
|
|
|
Operating lease expense – related
party
|
|
$
|
14,793
|
|
NOTE
5 ACCRUED INTEREST – RELATED PARTY
On
June 6, 2016, the Company received a $50,000 loan from our principal stockholder. Subsequently on December 1, 2017, the Company
received an additional $30,000 loan from the same stockholder. On January 8, 2018 and March 31, 2018 the Company received an additional
loan of $100,000 and $15,000, respectively. The Company received additional loan funds from the same stockholder as follows: $20,000
on April 26, 2018; $15,000 on June 21, 2018; $15,000 on June 29, 2018; $20,000 on July 5, 2018; $26,000 on October 1, 2018; $11,000
on October 12, 2018; $20,000 on December 21, 2018; $3,000 on January 4, 2019; $30,000 on January 17, 2019; $30,000 on February
1, 2019; $20,000 on February 15, 2019; $20,000 on March 1, 2019; $17,000 on January 4, 2019, $100,000 on November 20, 2019 and
$100,000 on December 18, 2019. Pursuant to the terms of the loan, the advance bears an interest at 3%, is unsecured, and due on
demand. Total loan payable to principal stockholder for as of December 31, 2018 is $322,000. Total loan payable to this principal
stockholder as of December 31, 2019 is $642,000. During the year ended December 31, 2019, the Company recorded $22,337 as an in-kind
contribution of interest related to the loan and recorded accrued interest payable of $15,581. During the year ended December
31, 2018, the Company recorded $11,909 as an in-kind contribution of interest related to the loan and recorded accrued interest
payable of $7,071.
NOTE
6 NOTE PAYABLE
On
March 1, 2019, the Company entered into an unsecured promissory note with Notre Dame - an unrelated party in the amount of $265,244
in exchange for outstanding account payable due to the debtor. Pursuant to the terms of the note, the note bears 10% interest
per year from the date of default until the date the loan is paid in full. The term of the loan is twenty four months. The loan
repayment commenced immediately over a twenty-four month period according to the following table. During the year ended December
31, 2019, the Company paid $20,000 of the loan balance (See Note 8 (A)):
1.
$1,000 per month for the first six months;
2.
$2,000 per month for the months seven and eight;
3.
$5,000 per month for months nine through twenty three; and,
4.
Final payment of all remaining balance, in the amount of $180,224 in month 24.
NOTE
7 STOCKHOLDERS’ DEFICIT
(A)
Common Stock Issued for Cash
On
March 9, 2019, the Company entered into a purchase agreement with one investor (the “Purchase Agreement”). Pursuant
to the Purchase Agreement, the Company issued the investor 14,797,278 Units at a purchase price of $0.06758 per Unit, for total
gross proceeds to the Company of $1,000,000. The Units consist of 14,797,278 shares of the Company’s Class A Common Stock
(the “Common Stock”) and two warrants (the “Warrants”): (i) one warrant entitles the investor to purchase
up to 14,797,278 shares of Common Stock at an exercise price of $0.06 per share (the “6 Cent Warrants”) and (ii) one
warrant entitles the investor to purchase up to 7,398,639 shares of Common Stock at an exercise price of $0.08 per share (the
“8 Cent Warrant”). The Warrants shall be exercisable at any time from the issuance date until the following expiration
dates:
●½
of all $0.06 Warrants shall expire on March 8, 2021;
●½
of all $0.06 Warrants shall expire on March 8, 2022;
●½
of all $0.08 Warrants shall expire on March 8, 2022;
●½
of all $0.08 Warrants shall expire on March 8, 2023.
(B)
Common Stock Issued for Services
Shares
issued for services as mentioned below were valued at the closing price of the stock on the date of grant.
On
March 20, 2019, the Company issued 4,052,652 shares of its class A common stock with a fair value of $281,659 ($0.0695/share)
on the date of settlement. The Company settled $243,159 of accounts payable to the University of Notre Dame. The Company recorded
an additional amount of $38,500 based on the fair value of the shares on the date of settlement. See Note 8 (A).
On
April 6, 2018, the Company issued 36,000 shares with a fair value of $1,076 ($0.0299/share) to a consultant as consideration for
consulting fees owed from October 1, 2014 through December 31, 2018 of $21,000. The issuance of shares resulted in gain on settlement
of accounts payable of $19,924. See Note 8(B).
On
March 20, 2018, the Company issued 4-year warrant to purchase 600,000 shares of common stock at an exercise price of $0.001 per
share to a consultant for services rendered. The warrants had a fair value of $19,915, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on March 20, 2018. This warrant was cancelled on April 4, 2019 for a cash payment
of $6,000.
(C)
Common Stock Warrants and Options
On
September 26, 2019, the Company issued 766,667 shares in connection with the cashless exercise of the 1,000,000 warrants. On August
14, 2019, the Company issued 7,967,871 shares in connection with the cashless exercise of the 8,000,000 warrants.
On
August 8, 2019, the Company issued a 2-year option to purchase 2,000,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $267,574, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on the date granted. Options will be exercisable on August 8, 2020, and for a
period of 3 years expiring on August 8, 2024. During the year ended December 31, 2019, the Company recorded $267,574 as an expense
for options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
2
Years
|
|
|
Risk free interest rate
|
|
|
1.62
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 2-year option to purchase 2,000,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $267,574, based upon the Black-Scholes option-pricing
model on the date of grant and is fully vested on August 8, 2020. Options will be exercisable on August 8, 2022, and for a period
of 3 years expiring on August 8, 2025. During the year ended December 31, 2019, the Company recorded $106,006 as an expense for
options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
2
Years
|
|
|
Risk free interest rate
|
|
|
1.62
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 3-year option to purchase 2,000,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $291,842, based upon the Black-Scholes option-pricing
model on the date of grant and is fully vested on August 8, 2021. Options will be exercisable on August 8, 2023, and for a period
of 3 years expiring on August 8, 2026. During the year ended December 31, 2019, the Company recorded $57,889 as an expense for
options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
3
Years
|
|
|
Risk free interest rate
|
|
|
1.54
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 2-year option to purchase 1,000,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $118,874, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on the date granted. Options will be exercisable on August 8, 2020, and for a
period of 3 years expiring on August 8, 2023. During the year ended December 31, 2019, the Company recorded $118,874 as an expense
for options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
2
Years
|
|
|
Risk free interest rate
|
|
|
1.62
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 2-year option to purchase 1,000,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $118,874, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on the date granted. Options will be exercisable on August 8, 2021, and for a
period of 3 years expiring on August 8, 2024. During the year ended December 31, 2019, the Company recorded $118,874 as an expense
for options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
2
Years
|
|
|
Risk free interest rate
|
|
|
1.62
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 2-year option to purchase 125,000 shares of common stock at an exercise price of $0.2299
per share to an employee for services rendered. The options had a fair value of $14,859, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on the date granted. Options will be exercisable on August 8, 2020, and for a
period of 3 years expiring on August 8, 2023. During the year ended December 31, 2019, the Company recorded $14,859 as an expense
for options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
2
Years
|
|
|
Risk free interest rate
|
|
|
1.62
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 2-year option to purchase 125,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $16,723, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on August 8, 2020. Options will be exercisable on August 8, 2022, and for a period
of 3 years expiring on August 8, 2025. During the year ended December 31, 2019, the Company recorded $6,625, as an expense for
options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
2
Years
|
|
|
Risk free interest rate
|
|
|
1.62
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 2-year options to purchase 125,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $18,240, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on August 8, 2021. Options will be exercisable on August 8, 2023, and for a period
of 3 years expiring on August 8, 2026. During the year ended December 31, 2019, the Company recorded $3,618, as an expense for
options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
3
Years
|
|
|
Risk free interest rate
|
|
|
1.54
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
August 8, 2019, the Company issued a 2-year options to purchase 125,000 shares of common stock at an exercise price of $0.2299
per share to a related party for services rendered. The options had a fair value of $19,525, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on August 8, 2022. Options will be exercisable on August 8, 2024, and for a period
of 3 years expiring on August 8, 2027. During the year ended December 31, 2019, the Company recorded $2,615, as an expense
for options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
105.73
|
%
|
|
Expected term
|
|
|
3
Years
|
|
|
Risk free interest rate
|
|
|
1.54
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
March 20, 2018, the Company issued a 4-year warrant to purchase 600,000 shares of common stock at an exercise price of $0.001
per share to a consultant for services rendered. The warrants had a fair value of $19,915, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on March 20, 2018. Warrants will be exercisable on March 20, 2019, and for a period
of 3 years expiring on March 20, 2022. During the year ended December 31, 2018, the Company recorded $19,915 as an expense for
warrants issued. On April 5, 2019, the Company cancelled 600,000 warrant issued to a consultant on February 20, 2018 in exchange
for $6,000 cash payment. In addition the Company also recorded a $19,915 reduction to warrant expense related to the warrant cancellation.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
97.56
|
%
|
|
Expected term
|
|
|
4
Years
|
|
|
Risk free interest rate
|
|
|
2.65
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
On
February 9, 2018, the Company issued a 3-year o to purchase 3,000,000 shares of common stock at an exercise price of $0.056 per
share to a consultant for services rendered. The options had a fair value of $52,660, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on the date granted. Options will be exercisable on August 9, 2019, and for a
period of 2 years expiring on August 9, 2021. During the year ended December 31, 2018, the Company recorded $52,660 as
an expense for options issued.
|
Expected dividends
|
|
|
0
|
%
|
|
Expected volatility
|
|
|
96.95
|
%
|
|
Expected term
|
|
|
3
Years
|
|
|
Risk free interest rate
|
|
|
2.26
|
%
|
|
Expected forfeitures
|
|
|
0
|
%
|
|
|
|
Number
of Warrants
|
|
|
Weighted
Average Exercise Price
|
|
|
Weighted
Average Remaining Contractual Life (In Years)
|
|
|
Balance as of December 31, 2018
|
|
|
34,300,000
|
|
|
|
|
|
|
|
3.0
|
|
|
Granted
|
|
|
30,695,917
|
|
|
|
|
|
|
|
2.94
|
|
|
Exercised
|
|
|
(9,000,000
|
)
|
|
|
|
|
|
|
|
|
|
Cancelled/Forfeited
|
|
|
(600,000
|
)
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2019
|
|
|
55,395,917
|
|
|
|
|
|
|
|
2.77
|
|
|
Intrinsic Value
|
|
$
|
10,852,009
|
|
|
|
|
|
|
|
|
|
For
the year ended December 31, 2019, the following warrants were outstanding:
|
Exercise
Price Warrants Outstanding
|
|
|
Warrants
Exercisable
|
|
|
Weighted
Average Remaining Contractual Life
|
|
|
Aggregate
Intrinsic Value
|
|
|
$
|
0.001
|
|
|
|
21,000,000
|
|
|
|
1.65
|
|
|
$
|
4,069,800
|
|
|
$
|
0.056
|
|
|
|
3,000,000
|
|
|
|
1.61
|
|
|
$
|
387,600
|
|
|
$
|
0.04
|
|
|
|
2,300,000
|
|
|
|
1.70
|
|
|
$
|
445,740
|
|
|
$
|
0.06
|
|
|
|
7,398,639
|
|
|
|
1.19
|
|
|
$
|
1,433,856
|
|
|
$
|
0.06
|
|
|
|
7,398,639
|
|
|
|
2.19
|
|
|
$
|
1,433,856
|
|
|
$
|
0.08
|
|
|
|
3,699,320
|
|
|
|
2.19
|
|
|
$
|
716,928
|
|
|
$
|
0.08
|
|
|
|
3,699,320
|
|
|
|
3.19
|
|
|
$
|
719,928
|
|
|
$
|
0.2299
|
|
|
|
8,500,000
|
|
|
|
5.39
|
|
|
$
|
1,647,300
|
|
For
the year ended December 31, 2018, the following warrants were outstanding:
|
Exercise
Price Warrants Outstanding
|
|
|
Warrants
Exercisable
|
|
|
Weighted
Average Remaining Contractual Life
|
|
|
Aggregate
Intrinsic Value
|
|
|
$
|
0.001
|
|
|
|
29,600,000
|
|
|
|
2.9
|
|
|
$
|
1,523,900
|
|
|
$
|
0.056
|
|
|
|
3,000,000
|
|
|
|
2.6
|
|
|
$
|
147,000
|
|
|
$
|
0.04
|
|
|
|
2,300,000
|
|
|
|
2.7
|
|
|
$
|
112,700
|
|
(D)
Amendment to Articles of Incorporation
On
February 16, 2009, the Company amended its articles of incorporation to amend the number and class of shares the Company is authorized
to issue as follows:
●Common
stock Class A, unlimited number of shares authorized, no par value
●Common
stock Class B, unlimited number of shares authorized, no par value
●Preferred
stock, unlimited number of shares authorized, no par value
Effective
December 17, 2013, the Company amended its articles of incorporation to designate a Series A no par value preferred stock. Two
shares of Series A Preferred stock have been authorized and were issued
(E)
Common Stock Issued for Debt
None
NOTE
8 COMMITMENTS AND CONTINGENCIES
On
November 10, 2010, the Company entered into an employment agreement with its CEO, effective January 1, 2011 through the December
31, 2015. The term of the agreement is a five year period at an annual salary of $210,000. There is a 6% annual increase. For
the year ending December 31, 2015, the annual salary was $281,027. The employee is also to receive a 20% bonus based on the annual
based salary. Any stock, stock options bonuses have to be approved by the board of directors. On January 1, 2016 the agreement
was renewed with the same terms for another 5 years with an annual salary of $297,889 for the year ended December 31, 2016. On
January 1, 2017 the agreement renewed with the same terms for another 5 years, but with an annual salary of $315,764 for the year
ended December 31, 2017. On January 1, 2019 the agreement renewed again with the same terms for another 5 years, but with an annual
salary of $354,791 for the year ended December 31, 2018. As of December 31, 2019 and December 31, 2018, the accrued salary balance
is $2,535,203 and $2,109,454, respectively. (See Note 9).
On
January 20, 2015, the board of directors appointed Mr. Jonathan R. Rice as our Chief Operating Officer. Mr. Rice’s employment
agreement has a term of one year and can be terminated by either the Company or Mr. Rice at any time. Under the employment agreement,
Mr. Rice is entitled to an annual cash compensation of $120,000, which includes salary, health insurance, 401K retirement plan
contributions, etc. The Company also agreed to reimburse Mr. Rice for his past educational expenses of approximately $11,000.
In addition, Mr. Rice was issued a three-year warrant to purchase 2,000,000 shares of common stock of the Company at an exercise
price of $0.001 per share (the “January 2015 Warrant”) pursuant to the employment agreement. Additionally, on May
28, 2015, the Company issued a three-year warrant to purchase 3,000,000 shares of common stock of the Company at an exercise price
of $0.001 per share (the “May 20165 Warrant”) to Mr. Rice. The 2,000,000 share warrant fully vested on October 28,
2016. For the year ended December 31, 2015, the Company recorded $121,448 for the warrants issued to Mr. Rice. On January 14,
2016, the Company signed a new employment agreement with Mr. Rice. The employment agreement has a term of one year and can be
terminated by either the Company or Mr. Rice at any time. Under the employment agreement, Mr. Rice is entitled to annual cash
compensation of $140,000, which includes salary, health insurance, 401K retirement plan contributions, etc. In addition, Mr. Rice
was issued a three-year warrant to purchase 6,000,000 shares of common stock of the Company at an exercise price of $0.001 per
share pursuant to the employment agreement. For the year ended December 31, 2016, the Company recorded $193,652 for the warrants
issued to Mr. Rice in 2016. For the year ended December 31, 2017, the Company recorded $17,473 for the warrants issued to Mr.
Rice in2016. On January 9, 2018, the Company extended the expiration date of the January 2015 Warrant from January 19, 2018 to
January 31, 2020 and on March 15, 2018, the Company signed an extension of its at-will employment agreement with its COO, extending
the term to January 31, 2019. On March 25, 2019, the Company signed an extension of its at-will employment agreement with its
COO, extending the term to January 1, 2020. On April 26, 2019, the Company signed an agreement to increase Mr. Rice’s base
salary by $20,000 per year and issue a one-time $20,000 bonus. Additionally, on August 15, 2019, the Company signed an agreement
to increase Mr. Rice’s base salary by an additional $20,000 per year. The salary increase and the bonus is accrued and to
be paid in full earlier by the direction of the Board or upon the earlier of
●The
Company maintaining $6,000,000 or more in working capital,
●Upon
the transfer of ownership of more than 50% of the Corporation’s voting share or an assignment for the benefit of creditors
or bankruptcy, or
●Upon
the fifth year anniversary of the salary increase and the bonus issuance.
As
of December 31, 2019 and December 31, 2018 the Company owes $64,352 and $24,433, respectively, to Mr. Rice for payroll payable.
On
October 21, 2019, the Company signed an agreement to increase Mr. Rice’s base salary by $20,000 per year (effective August
15, 2019). The salary increase is accrued and to be paid in full earlier by the direction of the Board or upon the earlier of:
●The
Company maintaining $6,000,000 or more in working capital,
●Upon
the transfer of ownership of more than 50% of the Corporation’s voting share or an assignment for the benefit of creditors
or bankruptcy, or
●Upon
the fifth year anniversary of the salary increase and the bonus issuance.
On
July 3, 2019, the board of directors appointed Mr. Kenneth Le as the Company’s Director of Government relations and President
of Prodigy Textiles. Mr. Le’s employment agreement has a term of one year and can be terminated by either the Company or
Mr. Rice at any time. Under the employment agreement, Mr. Le is entitled to annual cash compensation of $60,000. In addition,
Mr. Le was issued two three-year warrants to purchase 2,000,000 shares of common stock of the Company at an exercise price of
$0.2299 per share. As of December 31, 2019, the accrued salary balance is $1,154.
(A)
License Agreement
On
May 8, 2006, the Company entered into a license agreement. Pursuant to the terms of the agreement, the Company paid a non- refundable
license fee of $10,000. The Company will pay a license maintenance fee of $10,000 on the one year anniversary of this agreement
and each year thereafter. The Company will pay an annual research fee of $13,700 with first payment due January 2007, then on
each subsequent anniversary of the effective date commencing May 4, 2007. The annual research fees are accrued by the Company
for future payment. Pursuant to the terms of the agreement the Company may be required to pay additional fees aggregating up to
a maximum of $10,000 a year for patent maintenance and prosecution relating to the licensed intellectual property.
On
October 28, 2011, the Company entered into a license agreement with the University of Notre Dame. Under the agreement, the Company
received exclusive and non-exclusive rights to certain spider silk technologies including commercial rights with the right to
sublicense such intellectual property. In consideration of the licenses granted under the agreement, the Company agreed to issue
to the University of Notre Dame 2,200,000 shares of its common stock and to pay a royalty of 2% of net sales. The license agreement
has a term of 20 years which can be extended on an annual basis after that. It can be terminated by the University of Notre Dame
if the Company defaults on its obligations under the agreement and fails to cure such default within 90 days of a written notice
by the university. The Company can terminate the agreement upon a 90 day written notice subject to payment of a termination fee
of $5,000 if the termination takes place within 2 years after its effectiveness, $10,000 if the termination takes place within
4 years after its effectiveness and $20,000 if the Agreement is terminated after 4 years. On May 5, 2017, the Company signed an
addendum to that agreement relating to tangible property and project intellectual property. On March 1, 2019, the Company singed
an addendum to that agreement. The Company entered into a separate loan agreement and promissory noted dated March 1, 2019 as
a payment for expenses paid by the University prior to January 31, 2019 totaling $265,244 and issued 4,025,652 shares of Class
A common stock with a fair value of $281,659 as payment of certain debt. In the event of default the license agreement will be
terminated. During the year ended December 31, 2019, the Company paid $20,000 of the balance (See Notes 6).
(B)
Royalty and Research Agreements
On
May 1, 2008 the Company entered into a five year consulting agreement for research and development. Pursuant to the terms of the
agreement, the Company will be required to pay $1,000 per month, or at the Company’s option, the consulting fee may be paid
in the form of Company common stock based upon the greater of $0.05 per share or the average of the closing price of the Company’s
shares over the five days preceding such stock issuance. On April 6, 2018, the Company issued 36,000 shares with a fair value
of $1,076 ($0.0299/share) to a consultant as consideration for consulting fees owed from October 1, 2014 through December 31,
2018 of $21,000. The issuance of shares resulted in gain on settlement of accounts payable of $19,924. On April 1, 2018, the Company
ended the consulting agreement and no additional compensation will be issued. (See Note 7 (B)).
On
December 26, 2006, the Company entered into an addendum to the intellectual property transfer agreement with Mr. Thompson, its
CEO. In accordance with FASB ASC No 480, Distinguishing Liabilities from Equity, the Company determined that the present
value of the payment of $120,000 that was due on December 26, 2007. As of December 31, 2018 and December 31, 2017, the outstanding
balance is $65,292. In 2019 the Company recorded $1,959 in interest expensed and related accrued interest payable. As of December
31, 2019, the Company recorded interest expense and related accrued interest payable of $6,543.
On
December 30, 2015, the Company entered into a cooperative agreement for the research and pilot production of hybrid silkworms
in Vietnam. Under this agreement, the Company will establish a subsidiary in Vietnam where it will develop and produce hybrid
silkworms. On April 24, 2018, the Company announced that it had received its investment registration certificate for its new Vietnamese
subsidiary Prodigy Textiles Co., Ltd. On May 1, 2018, the Company announced that it had received its enterprise registration certificate
for its new Vietnamese subsidiary Prodigy Textiles Co., Ltd.
(C)
Consulting Agreement
On
February 9, 2018, the Company issued a 3-year warrant to purchase 3,000,000 shares of common stock at an exercise price of $0.056
per share to a consultant for services rendered. The warrants had a fair value of $52,660, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on the date granted. Warrants will be exercisable on August 9, 2019, and for a
period of 2 years expiring on August 9, 2021. During the year ended December 31, 2018, the Company recorded $52,660 as
an expense for warrants issued (See Note 7 (C)).
On
February 20, 2018, the Company signed an agreement with a consultant to provide services. Under this agreement the consultant
will receive a warrant for 600,000 shares of common stock and may be awarded additional warrants for up to 3,000,000 shares of
common stock if performance metrics are achieved. On March 20, 2018, the Company issued a 4-year warrant to purchase 600,000 shares
of common stock at an exercise price of $0.001 per share to a consultant for services rendered. The warrants had a fair value
of $19,915, based upon the Black-Scholes option-pricing model on the date of grant and are fully vested on March 20, 2018. Warrants
will be exercisable on March 20, 2019, and for a period of 3 years expiring on March 20, 2022. During the year ended December
31, 2018, the Company recorded $19,915 as an expense for warrants issued (See Note 7 (C)). On April 5, 2019, the Company cancelled
600,000 warrant issued to a consultant on February 20, 2018 in exchange for $6,000 cash payment.
(D)
Operating Lease Agreements
Since
September of 2015, we rent office space at 2723 South State Street, Suite 150, Ann Arbor, Michigan 48104, which is our principal
place of business. We pay an annual rent of $2,508 for conference facilities, mail, fax, and reception services located at our
principal place of business.
On
May 9, 2019, the Company signed a 5 year property lease Socialist Republic of Vietnam which consists of 4,560.57 square meters
of space, which it leases at a current rent of approximately $45,150 per year one and two and with the 5% increase per year for
years three through five.
On
January 23, 2017, the Company signed an 8 year property lease with the Company’s President for land in Texas where the Company
grows its mulberry. The Company pays a monthly rent of $960. Rent expense – related party for the years ended December 31,
2019 and 2018, was $14,793 and $11,520, respectively (See Note 9).
On
September 13, 2017, the Company signed a new two year lease commencing on October 1, 2017 and ending on December 31, 2019. The
Company pays an annual rent of $39,200 for the year one of lease and $42,000 for the year two of lease for office and manufacturing
space. On September 5, 2019, the Company signed a new two-year lease for this 5,000 square foot property in Lansing, MI that commenced
on October 1, 2019 and ends on September 30, 2021, for its research and development headquarters. The Company pays an annual rent
of $42,000 for year one of the lease and $44,800 for year two of the lease.
NOTE
9 RELATED PARTY TRANSACTIONS
On
December 26, 2006, the Company entered into an addendum to the intellectual property transfer agreement with Mr. Thompson, its
CEO. Pursuant to the addendum, the Company agreed to issue either 200,000 preferred shares with the following preferences; no
dividends and voting rights equal to 100 common shares per share of preferred stock or the payment of $120,000, the officer agreed
to terminate the royalty payments due under the agreement and give title to the exclusive license for the non-protective apparel
use of the intellectual property to the Company. On the date of the agreement, the Company did not have any preferred stock authorized
with the required preferences. In accordance with FASB ASC No. 480, Distinguishing Liabilities from Equity, the Company
determined that the present value of the payment of $120,000 that was due on December 26, 2007, one year anniversary of the addendum,
should be recorded as an accrued expense until such time as the Company has the ability to assert that it has preferred shares
authorized. As of December 31, 2019 the outstanding balance is $65,292. Additionally, the accrued expenses are accruing 7% interest
per year. As of December 31, 2019, the Company recorded interest expense and related accrued interest payable of $6,543.
On
November 10, 2010, the Company entered into an employment agreement, with its CEO, effective January 1, 2011 through the December
31, 2015. Subsequently, on January 1, 2018 the agreement renewed with the same terms for another 5 years with an annual salary
of $334,708 for the year ended December 31, 2018. As of December 31, 2019 and December 31, 2018, the accrued salary balance is
$2,464,244 and $2,109,454, respectively.
On
January 14, 2016 the Company signed a new employment agreement with Mr. Rice, the Company’s COO. The employment agreement
has a term of one year and can be terminated by either the Company or Mr. Rice at any time. Under the employment agreement, Mr.
Rice is entitled to annual cash compensation of $140,000, which includes salary, health insurance, 401K retirement plan contributions,
etc. In addition, Mr. Rice was issued a three-year warrant to purchase 6,000,000 shares of common stock of the Company at an exercise
price of $0.001 per share pursuant to the employment agreement. For the year ended December 31, 2016, the Company recorded $193,654
for the warrants issued to Mr. Rice. For the year ended December 31, 2017 the Company recorded $17,473 for the warrants
issued to Mr. Rice in 2016. On January 9, 2018, the Company extended the expiration date of a warrant for 2,000,000 shares of
common stock from January 19, 2018 to January 31, 2020 for Mr. Rice. On January 10, 2020, the Company extended the expiration
date of a warrant for 2,000,000 shares of common stock from January 31, 2020 to January 10, 2025 for Mr. Rice. Additionally,
on March 15, 2018, the Company signed an extension of its at-will employment agreement with its COO. On April 26, 2019, the Company
signed an agreement to increase Mr. Rice’s base salary by $20,000 per year and issue a one-time $20,000 bonus. On August
8, 2019 Mr. Rice was issued a set of three five-year warrant to purchase a total of 6,000,000 shares of common stock of the Company
at an exercise price of $0.2299 per share pursuant to the employment agreement. Additionally, on August 15, 2019, the Company
signed an agreement to increase Mr. Rice’s base salary by an additional $20,000 per year. The salary increase and the bonus
is accrued and to be paid in full earlier by the direction of the Board or upon the earlier of:
●The
Company maintaining $6,000,000 or more in working capital,
●Upon
the transfer of ownership of more than 50% of the Corporation’s voting share or
an assignment for the benefit of creditors or bankruptcy, or
●Upon
the fifth year anniversary of the salary increase and the bonus issuance.
On
October 21, 2019, the Company signed an agreement to increase Mr. Rice’s base salary by $20,000 per year (effective August
15, 2019). The salary increase is accrued and to be paid in full earlier by the direction of the Board or upon the earlier of:
●The
Company maintaining $6,000,000 or more in working capital,
●Upon
the transfer of ownership of more than 50% of the Corporation’s voting share or an assignment for the benefit of creditors
or bankruptcy, or
●Upon
the fifth year anniversary of the salary increase and the bonus issuance.
As
of December 31, 2019 and December 31, 2018, the Company owes $64,351 and $24,433, respectively, to Mr. Rice for payroll payable.
On
July 3, 2019, the board of directors appointed Mr. Kenneth Le as the Company’s Director of Government relations and President
of Prodigy Textiles. Mr. Le’s employment agreement has a term of one year and can be terminated by either the Company or
Mr. Le at any time. Under the employment agreement, Mr. Le is entitled to an annual cash compensation of $60,000. In addition,
Mr. Le was issued two three-year warrants to purchase 2,000,000 shares of common stock of the Company at an exercise price of
$0.2299 per share. As of December 31, 2019, the accrued salary balance is $1,154.
On
June 6, 2016, the Company received a $50,000 loan from our principal stockholder. Subsequently on December 1, 2017, the Company
received an additional $30,000 loan from the same stockholder. On January 8, 2018 and March 31, 2018 the Company received an additional
loan of $100,000 and $15,000, respectively. The Company received additional loan funds from the same stockholder as follows: $20,000
on April 26, 2018; $15,000 on June 21, 2018; $15,000 on June 29, 2018; $20,000 on July 5, 2018; $26,000 on October 1, 2018; $11,000
on October 12, 2018; $20,000 on December 21, 2018; $3,000 on January 4, 2019; $30,000 on January 17, 2019; $30,000 on February
1, 2019; $20,000 on February 15, 2019; $20,000 on March 1, 2019; $17,000 on January 4, 2019, $100,000 on November 20, 2019, and
$100,000 on December 18, 2019. Pursuant to the terms of the loan, the advance bears an interest at 3%, is unsecured, and due on
demand. Total loan payable to principal stockholder for as of December 31, 2018 is $322,000. Total loan payable to this principal
stockholder as of December 31, 2019 is $642,000. During the year ended December 31, 2019, the Company recorded $22,337 as an in-kind
contribution of interest related to the loan and recorded accrued interest payable of $15,581. During the year ended December
31, 2018, the Company recorded $11,909 as an in-kind contribution of interest related to the loan and recorded accrued interest
payable of $7,071.
On
January 23, 2017, the Company signed an 8 year property lease with the Company’s President for land in Texas. The Company
pays $960 per month starting on February 1, 2017 and uses this facility to grow mulberry for its U.S. silk operations. Rent expense
– related party for years ended December 31, 2019 and 2018 was $14,793 and $11,520, respectively.
As
of December 31, 2019 and December 31, 2018, there was $304,539 and $247,652, respectively, included in accounts payable and accrued
expenses - related party, which is owed to the Company’s Chief Executive Officer and Chief Operations Officer.
As
of December 31, 2019, there was $1,196,503 of accrued interest- related party and $43,715 in shareholder loan interest –
related party included in accounts payable and accrued expenses – related party, which is owed to the Company’s Chief
Executive officer.
As
of December 31, 2018, there was $940,158 of accrued interest- related party and $28,135 in shareholder loan interest – related
party included in accounts payable and accrued expenses – related party, which is owed to the Company’s Chief Executive
officer.
As
of December 31, 2019, the Company owes $2,535,203 in accrued salary to principal stockholder, $64,351 to the Company’s
COO, $1,153 to Director of Prodigy Textiles and $4,477 to its office employees.
As
of December 31, 2018, the Company owes $2,109,454 in accrued salary to principal stockholder, $24,433 to the Company’s COO,
and $7,640 to its office employees.
The
Company owes $65,292 in royalty payable to related party as of December 31, 2019 and December 31, 2018.
NOTE
10 SUBSEQUENT EVENTS
The
Company has analyzed its operations subsequent to December 31, 2019 through the date these financial statements were issued, and
has determined that, other than disclosed below, it does not have any material subsequent events to disclose.
On January 24, 2020, the Company received
$100,000 from a principal stockholder. Pursuant to the terms of the loan, the advances bear an interest at 3%, is unsecured and
due on demand.
On
February 19, 2020, the Board of Directors issued a total of 27,440,000 share options under the 2019 Employee Stock Option
Plan.
On
February 19, 2020, the Company received $100,000 from a principal stockholder. Pursuant to the terms of the loan, the advances
bear an interest at 3%, is unsecured and due on demand.
On
March 9, 2020, the Company received $100,000 from a principal stockholder. Pursuant to the terms of the loan, the advances bear
an interest at 3%, is unsecured and due on demand.
On
March 19, 2020, the Company furloughed non-essential staff consistent with leading health official recommendations in order
to help prevent the spread of COVID-19. This decision was made in an abundance of caution and will primarily impact staff at
our fully owned subsidiary, Prodigy Textiles, in Vietnam and will result in the temporary closing of silk rearing operations
at that facility. During the duration of the furlough, the Company CEO, CFO and President will not receive or accrue any pay.
Kraig Biocraft Laborator... (QB) (USOTC:KBLB)
Historical Stock Chart
From Apr 2024 to May 2024
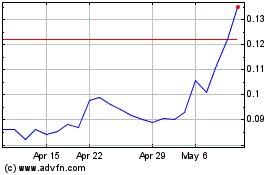
Kraig Biocraft Laborator... (QB) (USOTC:KBLB)
Historical Stock Chart
From May 2023 to May 2024