false 0001609809 0001609809 2024-09-12 2024-09-12
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): September 12, 2024
SERES THERAPEUTICS, INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-37465 |
|
27-4326290 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
| 101 Cambridgepark Drive Cambridge, MA |
|
02140 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (617) 945-9626
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
| Common stock, par value $0.001 per share |
|
MCRB |
|
The Nasdaq Stock Market LLC (Nasdaq Global Select Market) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01. |
Regulation FD Disclosure. |
On September 12, 2024, Seres Therapeutics, Inc. (the “Company”) posted a slide presentation on the SER-155 Phase 1b Cohort 2 Study results in the “Investors and News” portion of its website at www.serestherapeutics.com. The Company also issued a press release in connection with the foregoing. A copy of the slide presentation and press release are attached as Exhibit 99.1 and Exhibit 99.2, respectively, to this Current Report on Form 8-K (this “Current Report”) and incorporated herein by reference.
On September 12, 2024, the Company posted an updated corporate presentation in the “Investors and News” portion of its website at www.serestherapeutics.com. A copy of the slide presentation is attached as Exhibit 99.3 to this Current Report and incorporated herein by reference.
The information in Item 7.01 of this Current Report, including Exhibit 99.1, Exhibit 99.2 and Exhibit 99.3 attached hereto, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1, Exhibit 99.2 and Exhibit 99.3.
On September 12, 2024, the Company announced topline clinical data from Cohort 2 of its SER-155 Phase 1b placebo-controlled study in patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT). Study results demonstrate that SER-155 was associated with a significant reduction in both bloodstream infections (BSIs) and systemic antibiotic exposure, as well as a lower incidence of febrile neutropenia, as compared to placebo through day 100 post HSCT.
The Company believes that the SER-155 Phase 1 study results support the Company’s corporate strategy to develop its platform, comprised of a pipeline of designed live biotherapeutics, in multiple medically vulnerable patient populations at high risk of life-threatening bacterial infections and associated negative clinical outcomes. The Company intends to seek Breakthrough Therapy designation, given the high unmet medical need associated with BSIs, and discuss advancing development of SER-155 for allo-HSCT with the U.S. Food and Drug Administration (FDA). The Company also intends to evaluate SER-155 in additional patient populations that have a high risk of serious bacterial infections.
SER-155 Phase 1b Study Design
The SER-155 Phase 1b study (NCT04995653) included two cohorts. Cohort 1 was designed to assess safety and drug pharmacology, specifically the drug strain engraftment in the gastrointestinal tract. Cohort 1 included 13 subjects who received any dosing of the SER-155 regimen, with 11 subjects subsequently receiving an allo-HSCT. Results from this cohort, announced in May 2023, showed SER-155 was generally well tolerated and resulted in successful drug strain engraftment and a reduction in pathogen domination in the GI microbiome relative to a historical control cohort.
Study Cohort 2 utilized a randomized, double-blinded 1:1 placebo-controlled design to further evaluate safety and drug strain engraftment, as well as key secondary and exploratory endpoints such as the incidence of bacterial bloodstream infections and related medical consequences such as febrile neutropenia and antibiotic use. Cohort 2 included 45 patients in the intention-to-treat (ITT) population. Of the ITT population, 20 received SER-155 and 14 received placebo, each of whom subsequently received an allo-HSCT, with data available for clinical evaluation through day 100, the study’s prespecified primary observation point. Exploratory hypothesis testing was conducted at the two-sided α=0.05 level. Ninety-five percent (95%) 2-sided confidence intervals (CIs) were determined, where specified. No adjustment for multiplicity was done. A subset of patient samples was available for drug pharmacology analysis.
The median age in Cohort 2 was 63, and most subjects had acute myeloid leukemia, acute lymphocytic leukemia, myelodysplastic syndrome or myeloproliferative neoplasia as their primary disease and received reduced-intensity conditioning pre-transplant. Most patients received peripheral blood stem cells from a matched unrelated donor. A majority received post-transplant cyclophosphamide as part of their graft-versus-host disease (GvHD) prophylaxis.
Summary of Cohort 2 Study Results
Consistent with the observations from the Phase 1b study Cohort 1, SER-155 was generally well-tolerated, and no treatment-emergent serious adverse events related to drug were observed. SER-155 bacterial strains engrafted into the gastrointestinal tract of patients following the administration of SER-155.
The incidence of BSIs was significantly lower in the SER-155 arm compared with the placebo arm (2/20 (10%) vs. 6/14 (42.9%), respectively; [Odds Ratio: 0.15; 95% CI: 0.01, 1.13, p=0.0423]). In addition, while antibiotic starts were similar in each arm, patients administered SER-155 were treated with antibiotics for a significantly shorter duration compared to patients in the placebo arm (9.2 days vs. 21.1 days, respectively, with a mean difference of -11.9 days [95% CI: -23.85, -0.04; p=0.0494]). The incidence of febrile neutropenia was lower in patients administered SER-155 compared to placebo (65% vs. 78.6%, respectively; [Odds Ratio: 0.51; 95% CI: 0.07, 2.99; p=0.4674]). Six cases of gastrointestinal infections (C. difficile infections) were observed in the study, with four cases (20%) in the SER-155 arm and two cases (14.3%) in the placebo arm.
Recent changes in the allo-HSCT standard of care and the increasing use of post-transplant cyclophosphamide as part of prophylactic therapy for GvHD have reduced rates of GvHD overall in this patient population. The rates of GvHD in the study were low, with two cases of grade 2 GvHD observed in each arm, and no cases of grade 3 or 4 GvHD were observed.
In Cohort 2, the ability to detect pathogen domination (i.e., relative abundance in the GI ≥30%) in the placebo arm, and differences between the study arms, was constrained due to the limited number of placebo stool samples and an imbalance in the number of available stool samples between the arms. Observed pathogen domination events were low in the placebo and SER-155 arms with no significant differences identified. In a comparison of the prevalence of pathogen domination versus a larger allo-HSCT historical control cohort, pathogen domination in SER-155 subjects was substantially lower, providing further evidence of SER-155 activity.
The overall safety results in the first 100 days showed that SER-155 was generally well-tolerated, with no observed treatment-related serious adverse events. In Cohort 2, all but one subject in the placebo arm experienced at least one treatment-emergent adverse event (“TEAE”). The most common TEAEs were diarrhea and nausea. Four subjects experienced TEAEs that led to discontinuation. Nineteen subjects, or 48% of subjects, experienced serious adverse events, none of which were considered related to SER-155. Three deaths were reported in the study, none of which were considered related to SER-155. Fourteen subjects, or 35% of the subjects, experienced adverse events of special interest, including bloodstream infections, GI infection and invasive infection.
Seres fully owns worldwide rights for the commercialization of SER-155.
| Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits.
The following Exhibits 99.1 through 99.3 relate to Item 7.01 and shall be deemed to be furnished, and not filed:
Important Additional Information About the Transaction and Where to Find It
In connection with the proposed transaction involving Seres Therapeutics, Inc. (“Seres”) and Société des Produits Nestlé S.A. (“SPN”), Seres filed a definitive proxy statement with the Securities and Exchange Commission (the “SEC”). Seres may also file other relevant material with the SEC regarding the proposed transaction. Beginning on August 26, 2024, Seres mailed the definitive proxy statement to its stockholders. INVESTORS AND STOCKHOLDERS OF SERES ARE URGED TO READ THE DEFINITIVE PROXY STATEMENT AND OTHER RELEVANT MATERIALS CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION ABOUT SERES AND THE PROPOSED TRANSACTION. Investors may obtain a free copy of these materials (when they are available) and other documents filed by Seres with the SEC at the SEC’s website at www.sec.gov or from Seres at its website at ir.serestherapeutics.com.
Participants in the Solicitation
Seres and certain of its directors, executive officers and other members of management and employees may be deemed to be participants in soliciting proxies from its stockholders in connection with the proposed transaction. Information regarding the persons who may, under the rules of the SEC, be considered to be participants in the solicitation of Seres’ stockholders in connection with the proposed transaction is set forth in Seres’ definitive proxy statement for its stockholder meeting, which was filed with the SEC on August 26, 2024, at which the proposed transaction will be submitted for approval by Seres’ stockholders. You may also find additional information about Seres’ directors and executive officers in Seres’ Annual Report on Form 10-K for the fiscal year ended December 31, 2023, which was filed with the SEC on March 5, 2024, Seres’ Definitive Proxy Statement for its 2024 annual meeting of stockholders, which was filed with the SEC on March 5, 2024, and in subsequently filed Current Reports on Form 8-K and Quarterly Reports on Form 10-Q.
Forward-Looking Statements
This communication contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this communication that do not relate to matters of historical fact should be considered forward-looking statements, including statements about the potential benefits of any of our products or product candidates; the ultimate safety and efficacy data of SER-155; study results; plans to seek FDA feedback; clinical data and clinical trials; our intentions related to the development of SER-155; our intention to seek Breakthrough Therapy Designation; the ability of live biotherapeutics to prevent or reduce infections; or the timing of any of the foregoing; the financial terms, timing and completion of the sale of VOWST assets to SPN; the receipt of future payments and the use of proceeds of the transaction; the timing and results of our clinical studies and data readouts; future product candidates, development plans and commercial opportunities; operating plans and our future cash runway; our ability to generate additional capital; our planned strategic focus; anticipated timing of any of the foregoing and other statements which are not historical fact.
These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: (1) we have incurred significant losses, are not currently profitable and may never become profitable; (2) our need for additional funding; (3) our history of operating losses; (4) the restrictions in our debt agreement; (5) our novel approach to therapeutic intervention; (6) our reliance on third parties to conduct our clinical trials and manufacture our product candidates; (7) the competition we will face; (8) risks associated with our clinical trials; (9) whether the FDA grants Breakthrough Therapy Designation; (10) our ability to protect our intellectual property; (11) our ability to retain key personnel and to manage our growth; and (12) risks related to the proposed transaction under the Purchase Agreement with SPN for the sale of the VOWST business to SPN. These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q filed with the SEC, on August 13, 2024, and our other reports filed with the SEC could cause actual results to differ materially from those
indicated by the forward-looking statements made in this communication. Any such forward-looking statements represent management’s estimates as of the date of this communication. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this communication.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
| Date: September 12, 2024 |
|
SERES THERAPEUTICS, INC. |
|
|
|
|
|
|
|
|
By: |
|
/s/ Thomas J. DesRosier |
|
|
|
|
Name: |
|
Thomas J. DesRosier |
|
|
|
|
Title: |
|
Chief Legal Officer and Executive Vice President |

Exhibit 99.1 SER-155 Phase 1b Readout September 12, 2024

Disclaimers Forward Looking Statements This communication contains
forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this communication that do not relate to matters of historical fact should be considered forward-looking statements,
including statements about the potential benefits of any of our products or product candidates; the ultimate safety and efficacy data of SER-155; study results; plans to seek FDA feedback; clinical data and clinical trials; our intentions related to
the development of SER-155; our intention to seek Breakthrough Therapy Designation; the ability of live biotherapeutics to prevent or reduce infections; or the timing of any of the foregoing and other statements which are not historical fact. These
forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results,
performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: (1) we have incurred significant
losses, are not currently profitable and may never become profitable; (2) our need for additional funding; (3) our history of operating losses; (4) the restrictions in our debt agreement; (5) our novel approach to therapeutic intervention; (6) our
reliance on third parties to conduct our clinical trials and manufacture our product candidates; (7) the competition we will face; (8) risks associated with our clinical trials; (9) whether the FDA grants Breakthrough Therapy Designation; (10) our
ability to protect our intellectual property; (11) our ability to retain key personnel and to manage our growth; and (12) risks related to the proposed transaction under the Purchase Agreement with Société des Produits Nestlé S.A for
the sale of the VOWST business to SPN. These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q filed with the SEC, on August 13, 2024, and our other reports filed with the SEC
could cause actual results to differ materially from those indicated by the forward-looking statements made in this communication. Any such forward-looking statements represent management’s estimates as of the date of this communication. While
we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing
our views as of any date subsequent to the date of this communication. Seres Therapeutics, Inc. © 2024 2 2

Disclaimers Important Information About the Transaction and Where to
Find it In connection with the proposed transaction involving Seres Therapeutics, Inc. (“Seres”) and Société des Produits Nestlé S.A. (“SPN”), Seres filed a definitive proxy statement with the Securities and
Exchange Commission (the “SEC”). Seres may also file other relevant material with the SEC regarding the proposed transaction. Beginning on August 26, 2024, Seres mailed the definitive proxy statement to its stockholders. INVESTORS AND
STOCKHOLDERS OF SERES ARE URGED TO READ THE DEFINITIVE PROXY STATEMENT AND OTHER RELEVANT MATERIALS CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION ABOUT SERES AND THE PROPOSED
TRANSACTION. Investors may obtain a free copy of these materials (when they are available) and other documents filed by Seres with the SEC at the SEC’s website at www.sec.gov or from Seres at its website at ir.serestherapeutics.com.
Participants in the Solicitation Seres and certain of its directors, executive officers and other members of management and employees may be deemed to be participants in soliciting proxies from its stockholders in connection with the proposed
transaction. Information regarding the persons who may, under the rules of the SEC, be considered to be participants in the solicitation of Seres’ stockholders in connection with the proposed transaction is set forth in Seres’ definitive
proxy statement for its stockholder meeting, which was filed with the SEC on August 26, 2024, at which the proposed transaction will be submitted for approval by Seres’ stockholders. You may also find additional information about Seres’
directors and executive officers in Seres’ Annual Report on Form 10-K for the fiscal year ended December 31, 2023, which was filed with the SEC on March 5, 2024, Seres’ Definitive Proxy Statement for its 2024 annual meeting of
stockholders, which was filed with the SEC on March 5, 2024, and in subsequently filed Current Reports on Form 8-K and Quarterly Reports on Form 10-Q. Seres Therapeutics, Inc. © 2024 3 3
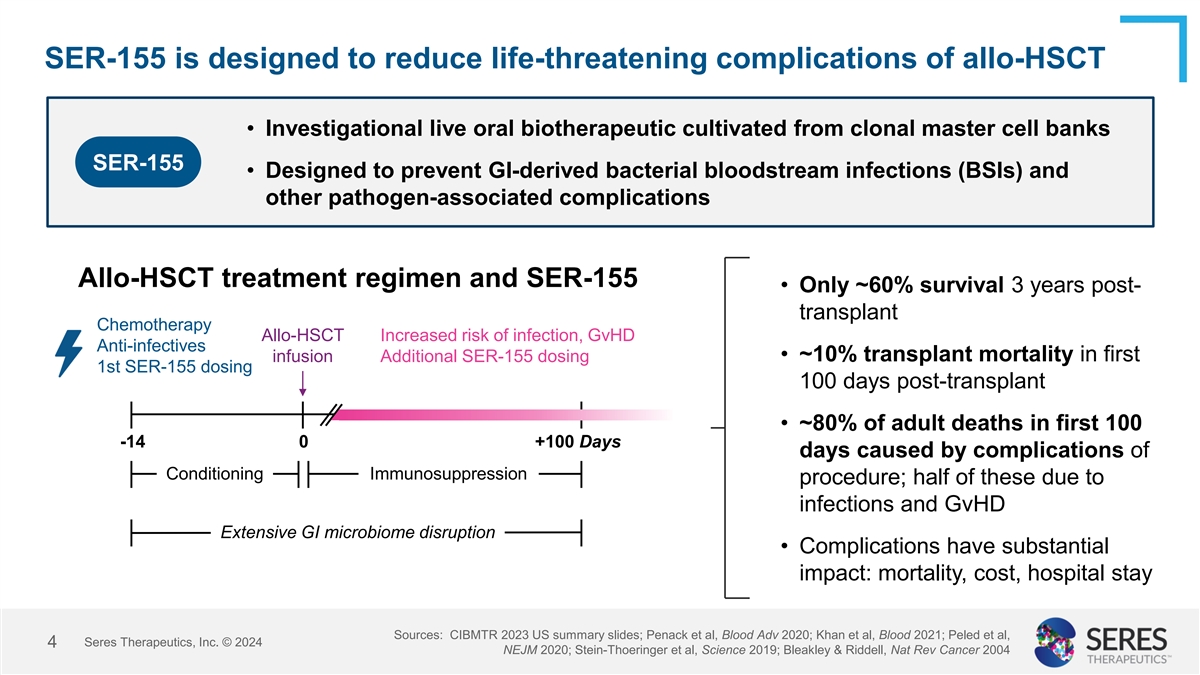
SER-155 is designed to reduce life-threatening complications of
allo-HSCT • Investigational live oral biotherapeutic cultivated from clonal master cell banks SER-155 • Designed to prevent GI-derived bacterial bloodstream infections (BSIs) and other pathogen-associated complications Allo-HSCT
treatment regimen and SER-155 • Only ~60% survival 3 years post- transplant Chemotherapy Allo-HSCT Increased risk of infection, GvHD Anti-infectives • ~10% transplant mortality in first infusion Additional SER-155 dosing 1st SER-155
dosing 100 days post-transplant • ~80% of adult deaths in first 100 -14 0 +100 Days days caused by complications of Conditioning Immunosuppression procedure; half of these due to infections and GvHD Extensive GI microbiome disruption •
Complications have substantial impact: mortality, cost, hospital stay Sources: CIBMTR 2023 US summary slides; Penack et al, Blood Adv 2020; Khan et al, Blood 2021; Peled et al, Seres Therapeutics, Inc. © 2024 4 NEJM 2020; Stein-Thoeringer et
al, Science 2019; Bleakley & Riddell, Nat Rev Cancer 2004
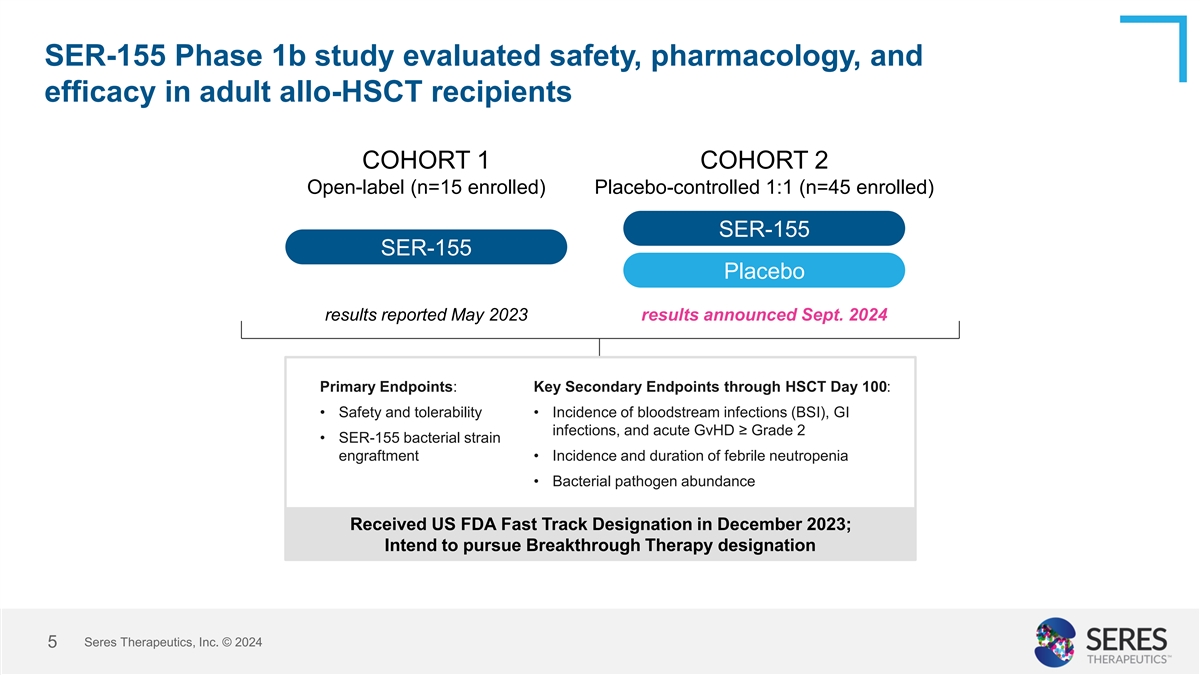
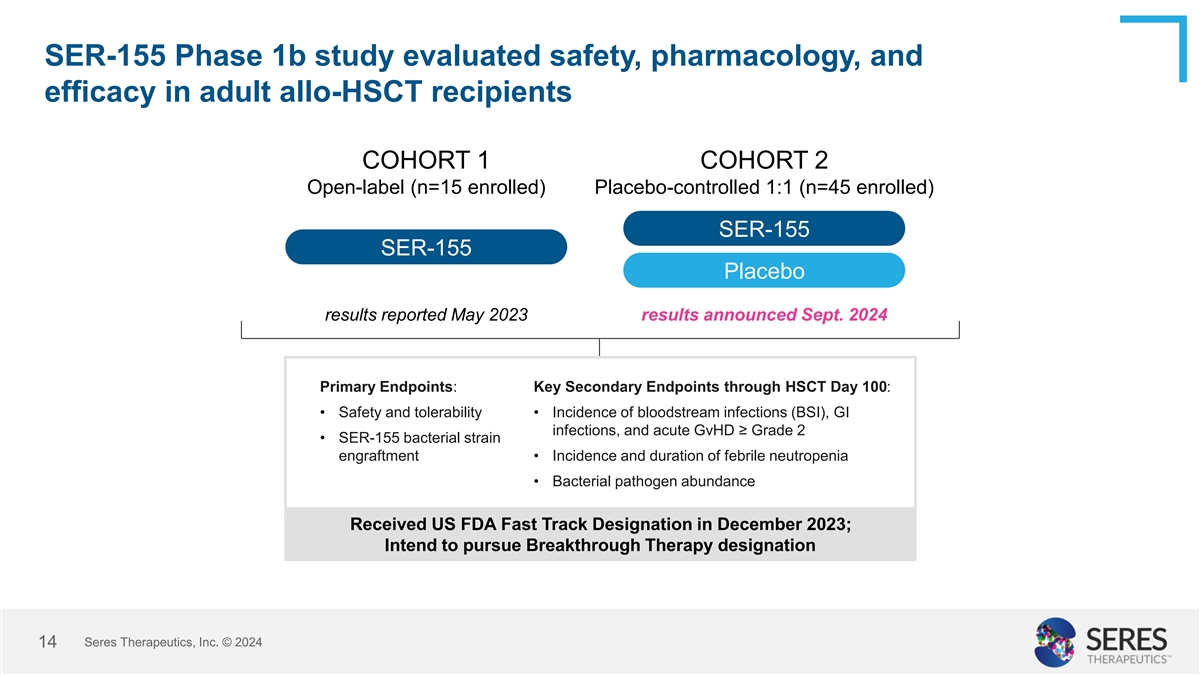
SER-155 Phase 1b study evaluated safety, pharmacology, and efficacy in
adult allo-HSCT recipients COHORT 1 COHORT 2 Open-label (n=15 enrolled) Placebo-controlled 1:1 (n=45 enrolled) SER-155 SER-155 Placebo results reported May 2023 results announced Sept. 2024 Primary Endpoints: Key Secondary Endpoints through HSCT Day
100: • Safety and tolerability • Incidence of bloodstream infections (BSI), GI infections, and acute GvHD ≥ Grade 2 • SER-155 bacterial strain engraftment • Incidence and duration of febrile neutropenia •
Bacterial pathogen abundance Received US FDA Fast Track Designation in December 2023; Intend to pursue Breakthrough Therapy designation Seres Therapeutics, Inc. © 2024 5
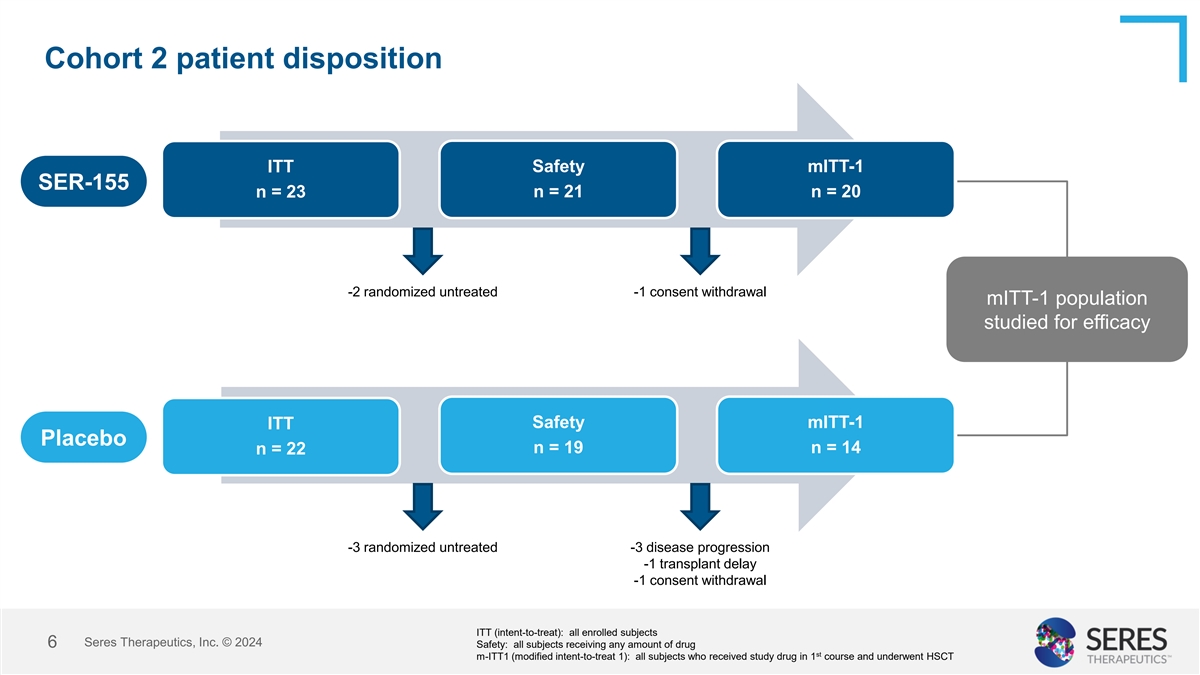
Cohort 2 patient disposition ITT Safety mITT-1 SER-155 n = 23 n = 21 n =
20 -2 randomized untreated -1 consent withdrawal mITT-1 population studied for efficacy Safety mITT-1 ITT Placebo n = 19 n = 14 n = 22 -3 randomized untreated -3 disease progression -1 transplant delay -1 consent withdrawal ITT (intent-to-treat):
all enrolled subjects Seres Therapeutics, Inc. © 2024 6 Safety: all subjects receiving any amount of drug st m-ITT1 (modified intent-to-treat 1): all subjects who received study drug in 1 course and underwent HSCT
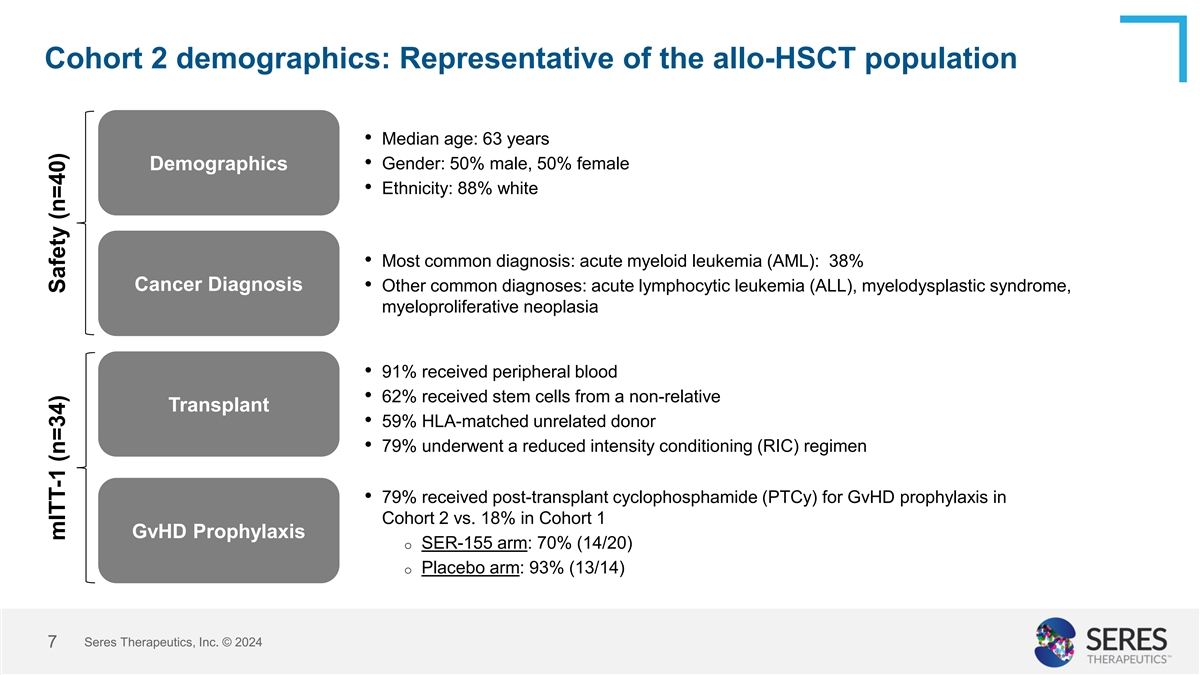
Cohort 2 demographics: Representative of the allo-HSCT population
• Median age: 63 years Demographics • Gender: 50% male, 50% female • Ethnicity: 88% white • Most common diagnosis: acute myeloid leukemia (AML): 38% Cancer Diagnosis • Other common diagnoses: acute lymphocytic leukemia
(ALL), myelodysplastic syndrome, myeloproliferative neoplasia • 91% received peripheral blood • 62% received stem cells from a non-relative Transplant • 59% HLA-matched unrelated donor • 79% underwent a reduced intensity
conditioning (RIC) regimen • 79% received post-transplant cyclophosphamide (PTCy) for GvHD prophylaxis in Cohort 2 vs. 18% in Cohort 1 GvHD Prophylaxis o SER-155 arm: 70% (14/20) o Placebo arm: 93% (13/14) Seres Therapeutics, Inc. © 2024
7 mITT-1 (n=34) Safety (n=40)
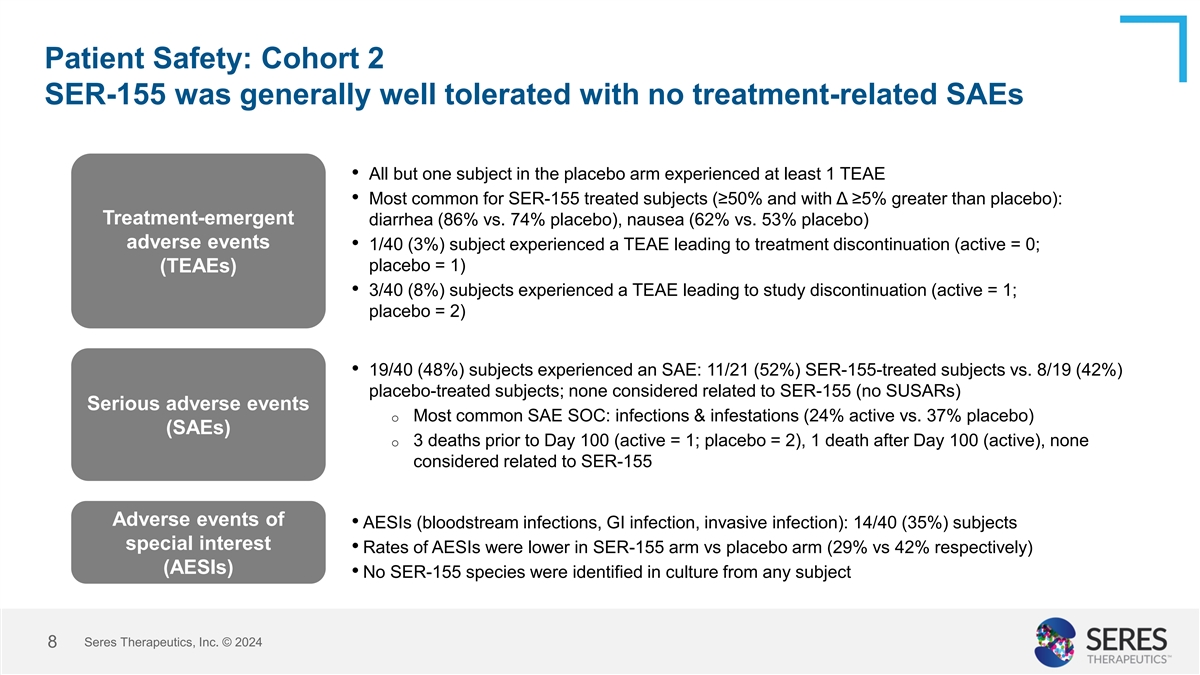
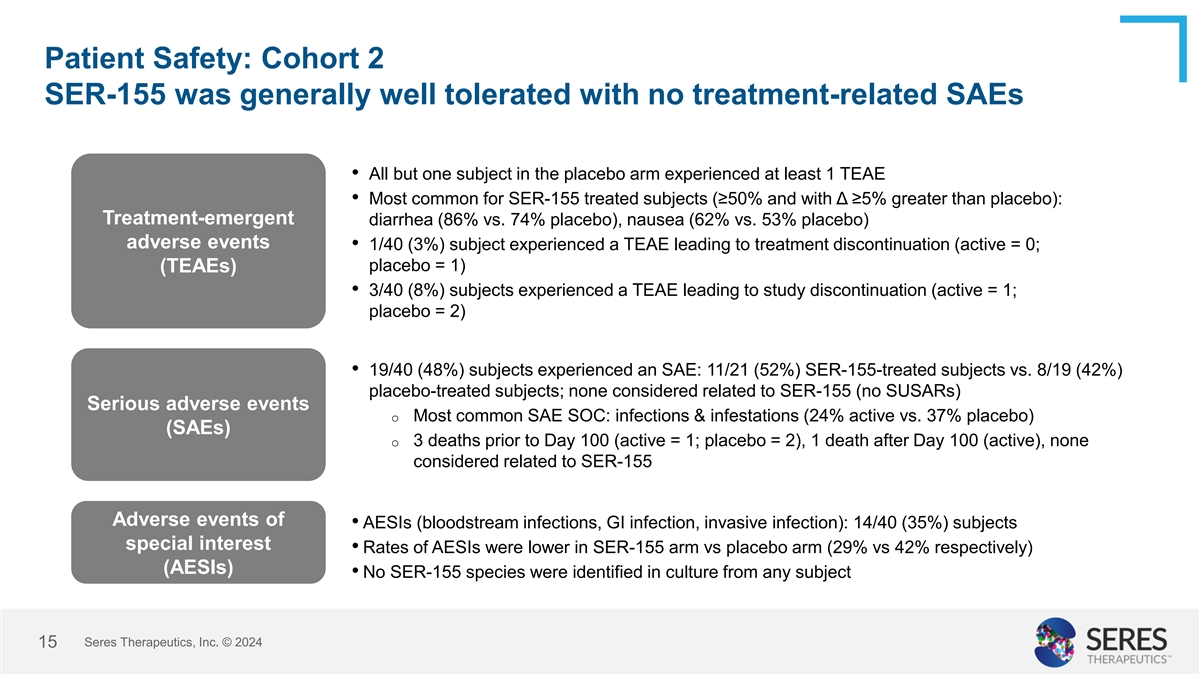
Patient Safety: Cohort 2 SER-155 was generally well tolerated with no
treatment-related SAEs • All but one subject in the placebo arm experienced at least 1 TEAE • Most common for SER-155 treated subjects (≥50% and with Δ ≥5% greater than placebo): Treatment-emergent diarrhea (86% vs. 74%
placebo), nausea (62% vs. 53% placebo) adverse events • 1/40 (3%) subject experienced a TEAE leading to treatment discontinuation (active = 0; placebo = 1) (TEAEs) • 3/40 (8%) subjects experienced a TEAE leading to study discontinuation
(active = 1; placebo = 2) • 19/40 (48%) subjects experienced an SAE: 11/21 (52%) SER-155-treated subjects vs. 8/19 (42%) placebo-treated subjects; none considered related to SER-155 (no SUSARs) Serious adverse events o Most common SAE SOC:
infections & infestations (24% active vs. 37% placebo) (SAEs) o 3 deaths prior to Day 100 (active = 1; placebo = 2), 1 death after Day 100 (active), none considered related to SER-155 Adverse events of • AESIs (bloodstream infections, GI
infection, invasive infection): 14/40 (35%) subjects special interest • Rates of AESIs were lower in SER-155 arm vs placebo arm (29% vs 42% respectively) (AESIs) • No SER-155 species were identified in culture from any subject Seres
Therapeutics, Inc. © 2024 8
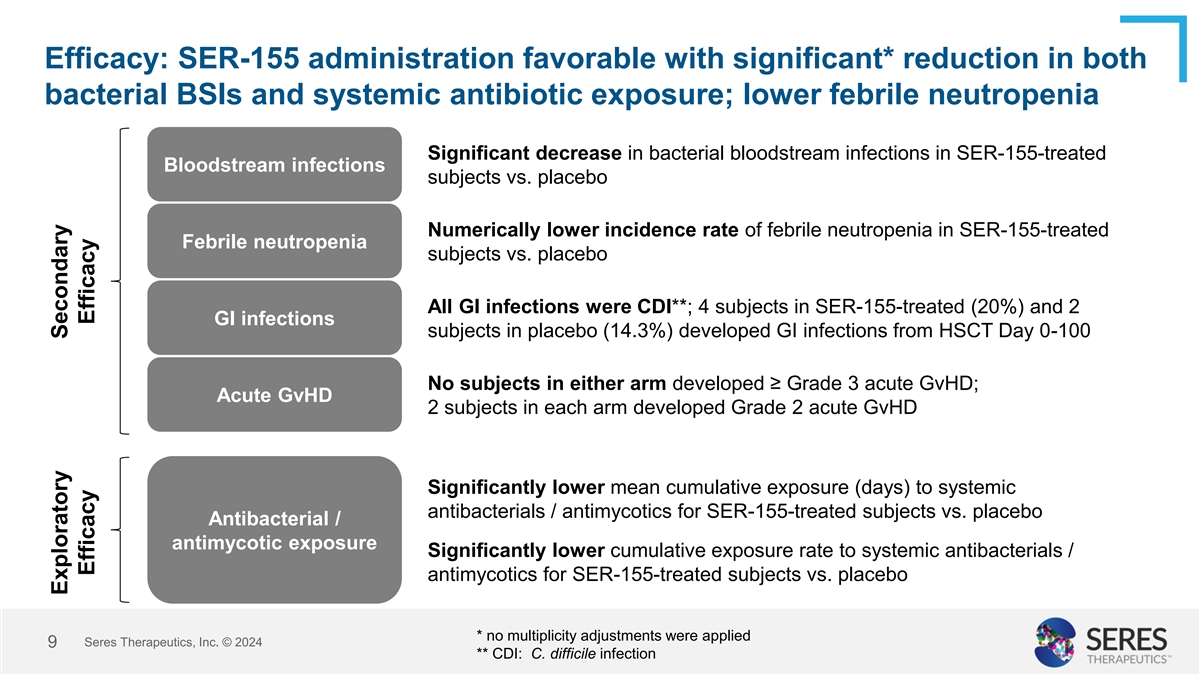
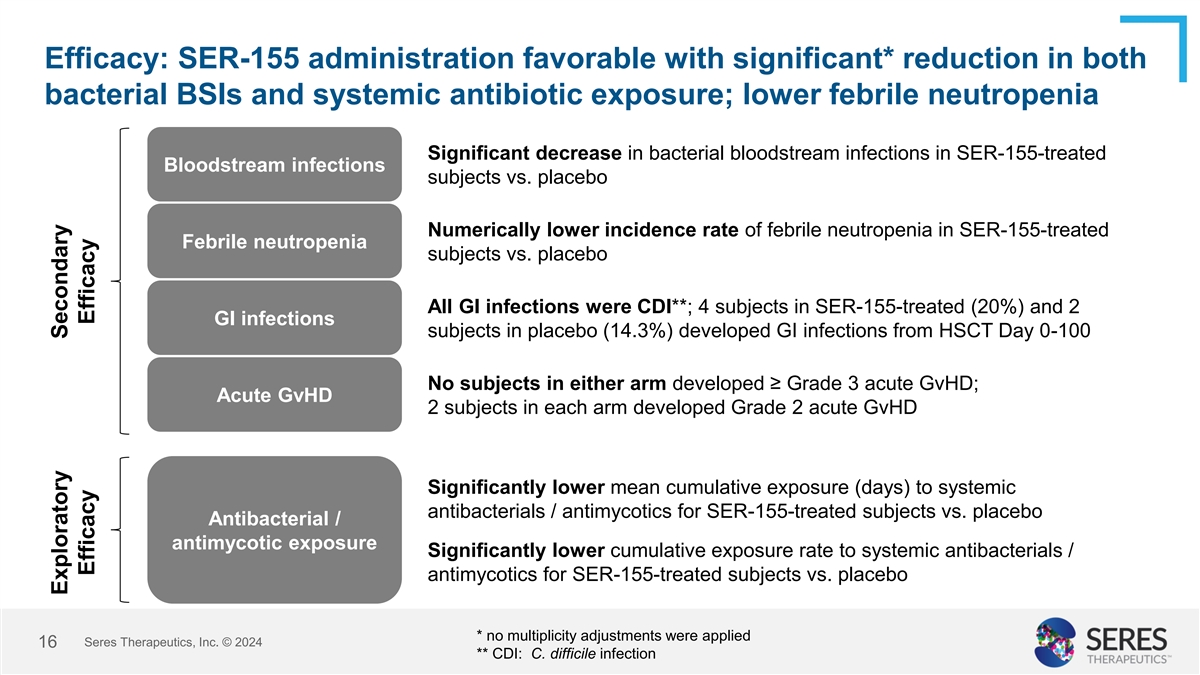
Efficacy: SER-155 administration favorable with significant* reduction
in both bacterial BSIs and systemic antibiotic exposure; lower febrile neutropenia Significant decrease in bacterial bloodstream infections in SER-155-treated Bloodstream infections subjects vs. placebo Numerically lower incidence rate of febrile
neutropenia in SER-155-treated Febrile neutropenia subjects vs. placebo All GI infections were CDI**; 4 subjects in SER-155-treated (20%) and 2 GI infections subjects in placebo (14.3%) developed GI infections from HSCT Day 0-100 No subjects in
either arm developed ≥ Grade 3 acute GvHD; Acute GvHD 2 subjects in each arm developed Grade 2 acute GvHD Significantly lower mean cumulative exposure (days) to systemic antibacterials / antimycotics for SER-155-treated subjects vs. placebo
Antibacterial / antimycotic exposure Significantly lower cumulative exposure rate to systemic antibacterials / antimycotics for SER-155-treated subjects vs. placebo * no multiplicity adjustments were applied Seres Therapeutics, Inc. © 2024 9 **
CDI: C. difficile infection Exploratory Secondary Efficacy Efficacy
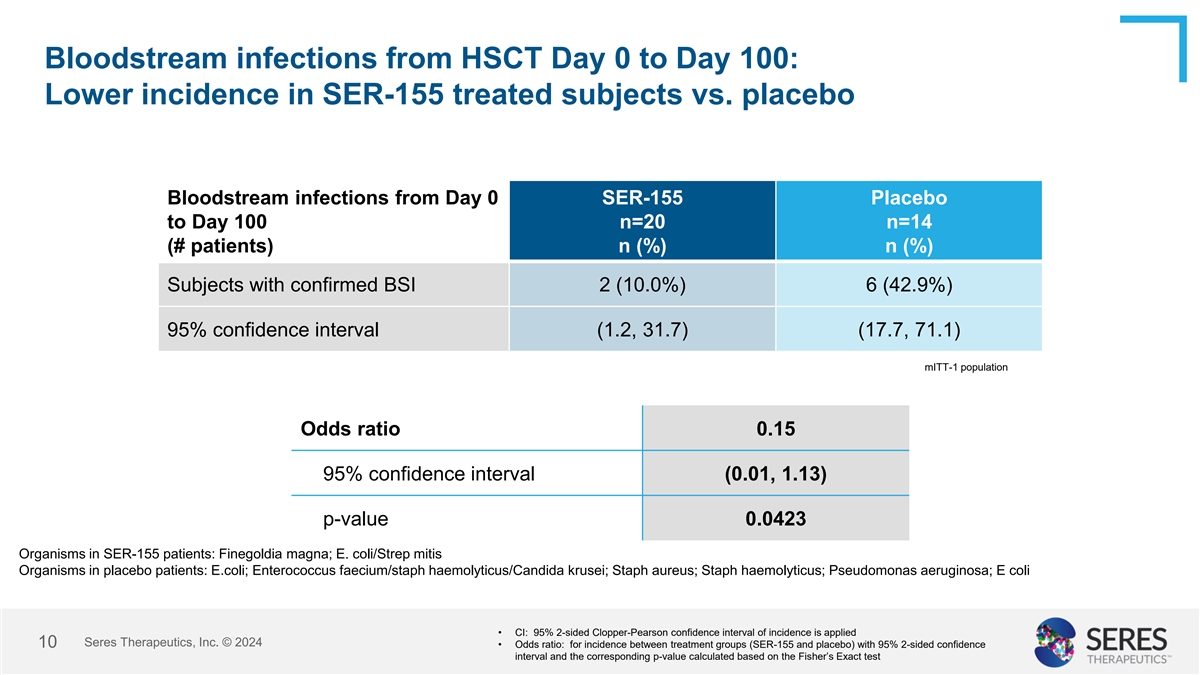
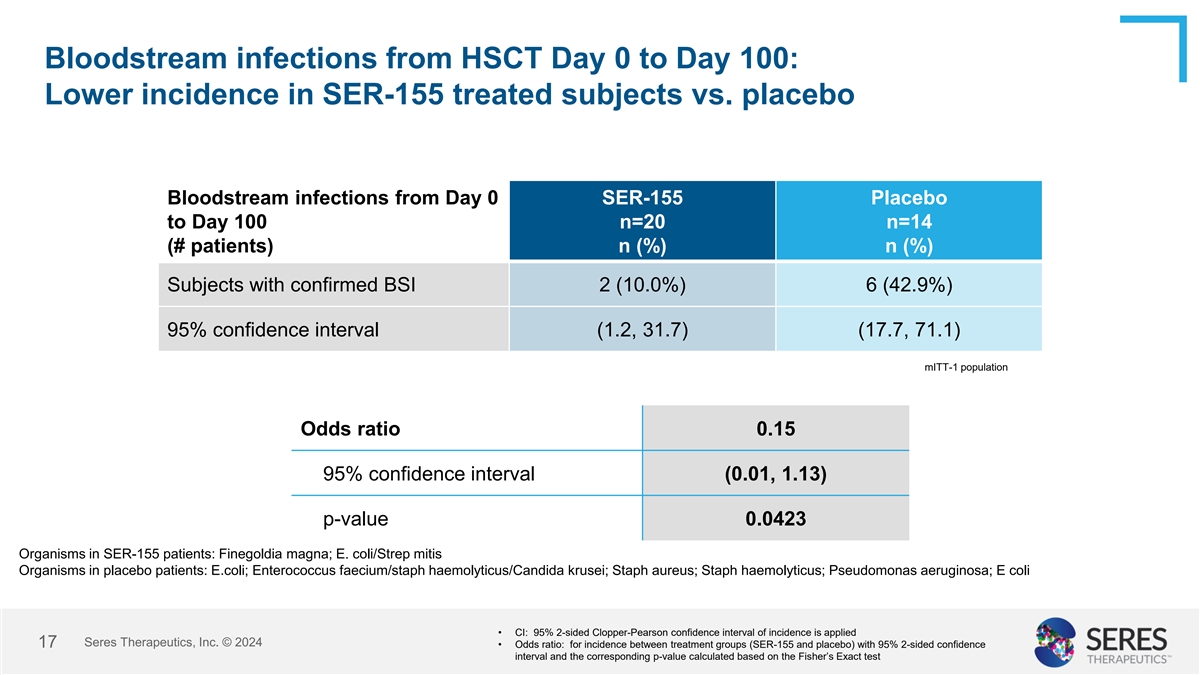
Bloodstream infections from HSCT Day 0 to Day 100: Lower incidence in
SER-155 treated subjects vs. placebo Bloodstream infections from Day 0 SER-155 Placebo to Day 100 n=20 n=14 (# patients) n (%) n (%) Subjects with confirmed BSI 2 (10.0%) 6 (42.9%) 95% confidence interval (1.2, 31.7) (17.7, 71.1) mITT-1 population
Odds ratio 0.15 95% confidence interval (0.01, 1.13) p-value 0.0423 Organisms in SER-155 patients: Finegoldia magna; E. coli/Strep mitis Organisms in placebo patients: E.coli; Enterococcus faecium/staph haemolyticus/Candida krusei; Staph aureus;
Staph haemolyticus; Pseudomonas aeruginosa; E coli • CI: 95% 2-sided Clopper-Pearson confidence interval of incidence is applied Seres Therapeutics, Inc. © 2024 10 • Odds ratio: for incidence between treatment groups (SER-155 and
placebo) with 95% 2-sided confidence interval and the corresponding p-value calculated based on the Fisher’s Exact test
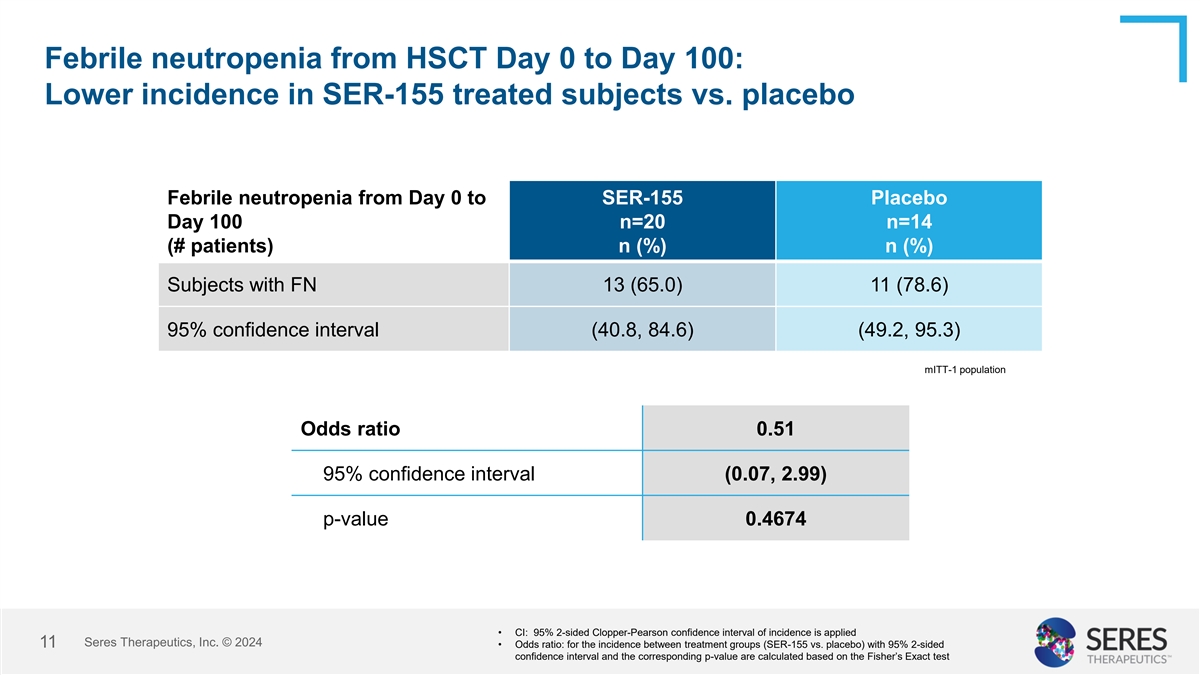
Febrile neutropenia from HSCT Day 0 to Day 100: Lower incidence in
SER-155 treated subjects vs. placebo Febrile neutropenia from Day 0 to SER-155 Placebo Day 100 n=20 n=14 (# patients) n (%) n (%) Subjects with FN 13 (65.0) 11 (78.6) 95% confidence interval (40.8, 84.6) (49.2, 95.3) mITT-1 population Odds ratio
0.51 95% confidence interval (0.07, 2.99) p-value 0.4674 • CI: 95% 2-sided Clopper-Pearson confidence interval of incidence is applied Seres Therapeutics, Inc. © 2024 11 • Odds ratio: for the incidence between treatment groups
(SER-155 vs. placebo) with 95% 2-sided confidence interval and the corresponding p-value are calculated based on the Fisher’s Exact test
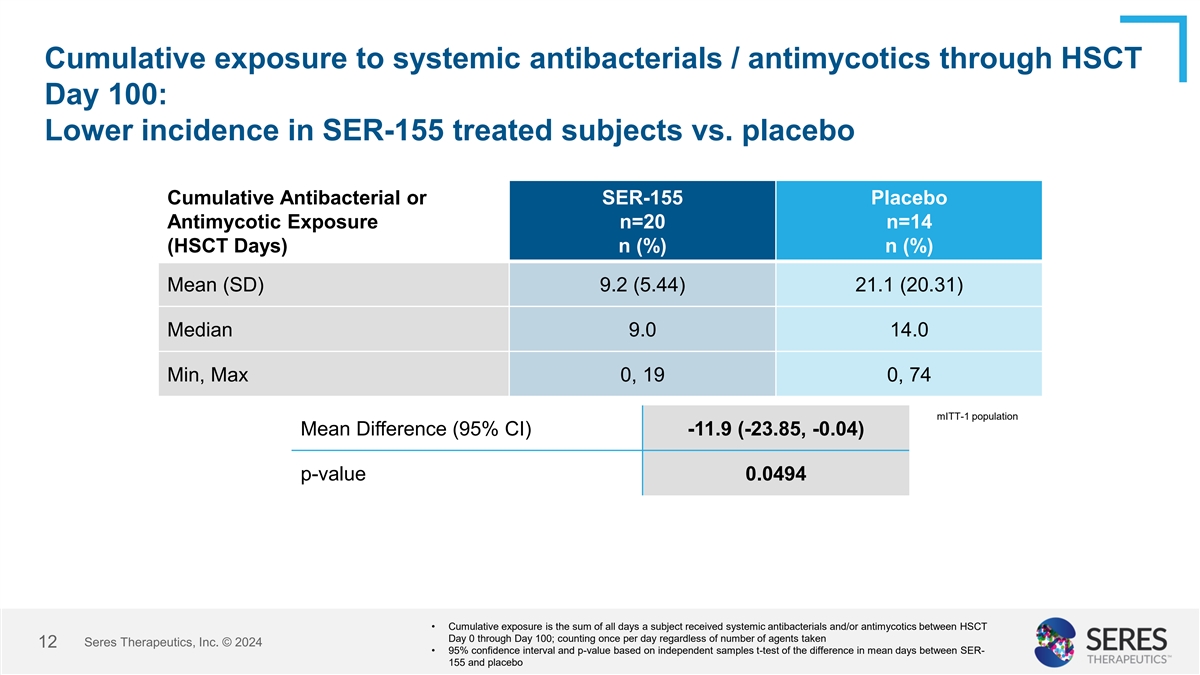
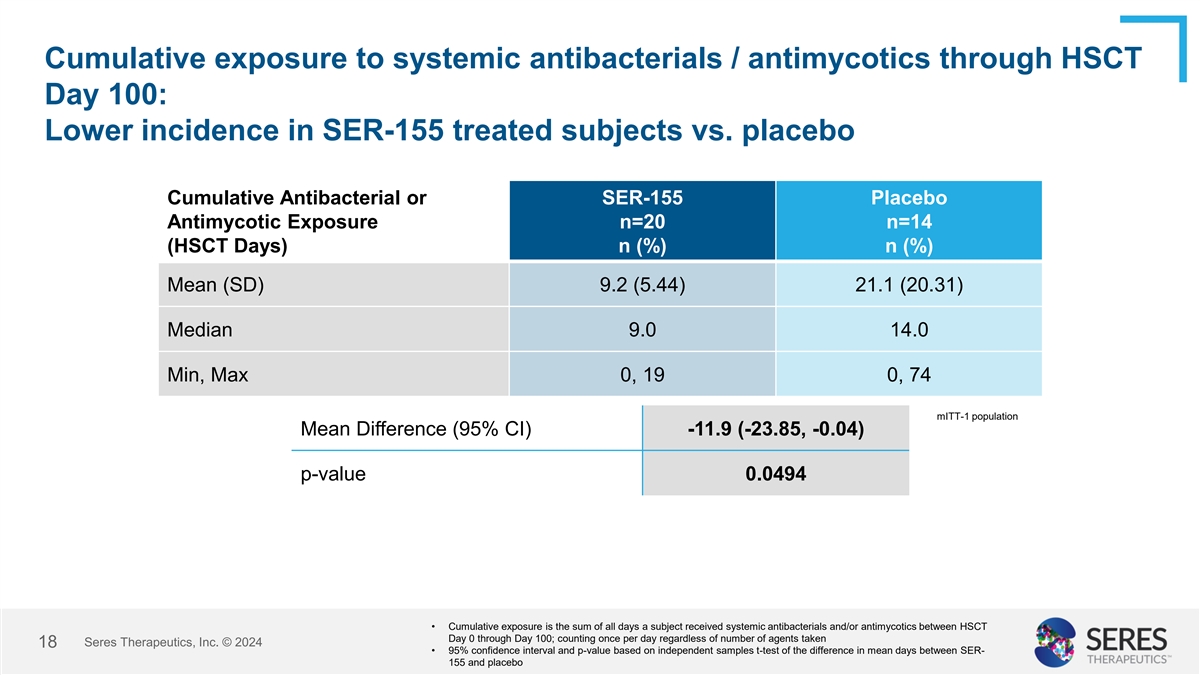
Cumulative exposure to systemic antibacterials / antimycotics through
HSCT Day 100: Lower incidence in SER-155 treated subjects vs. placebo Cumulative Antibacterial or SER-155 Placebo Antimycotic Exposure n=20 n=14 (HSCT Days) n (%) n (%) Mean (SD) 9.2 (5.44) 21.1 (20.31) Median 9.0 14.0 Min, Max 0, 19 0, 74 mITT-1
population Mean Difference (95% CI) -11.9 (-23.85, -0.04) p-value 0.0494 • Cumulative exposure is the sum of all days a subject received systemic antibacterials and/or antimycotics between HSCT Day 0 through Day 100; counting once per day
regardless of number of agents taken Seres Therapeutics, Inc. © 2024 12 • 95% confidence interval and p-value based on independent samples t-test of the difference in mean days between SER- 155 and placebo
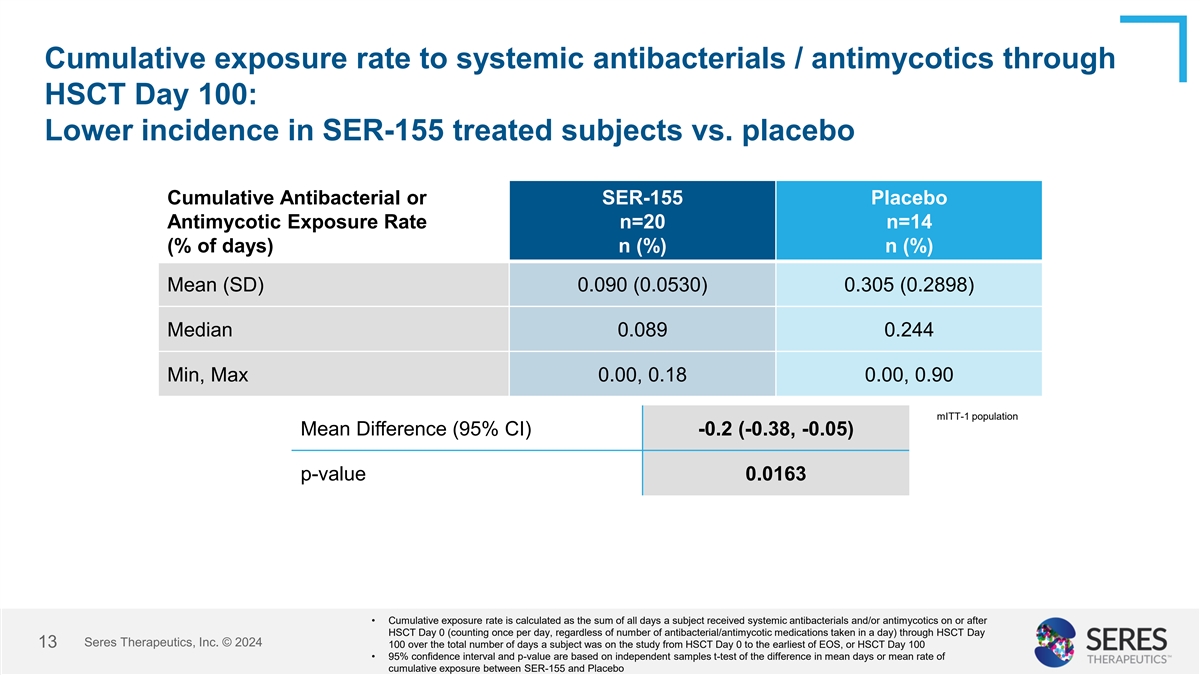
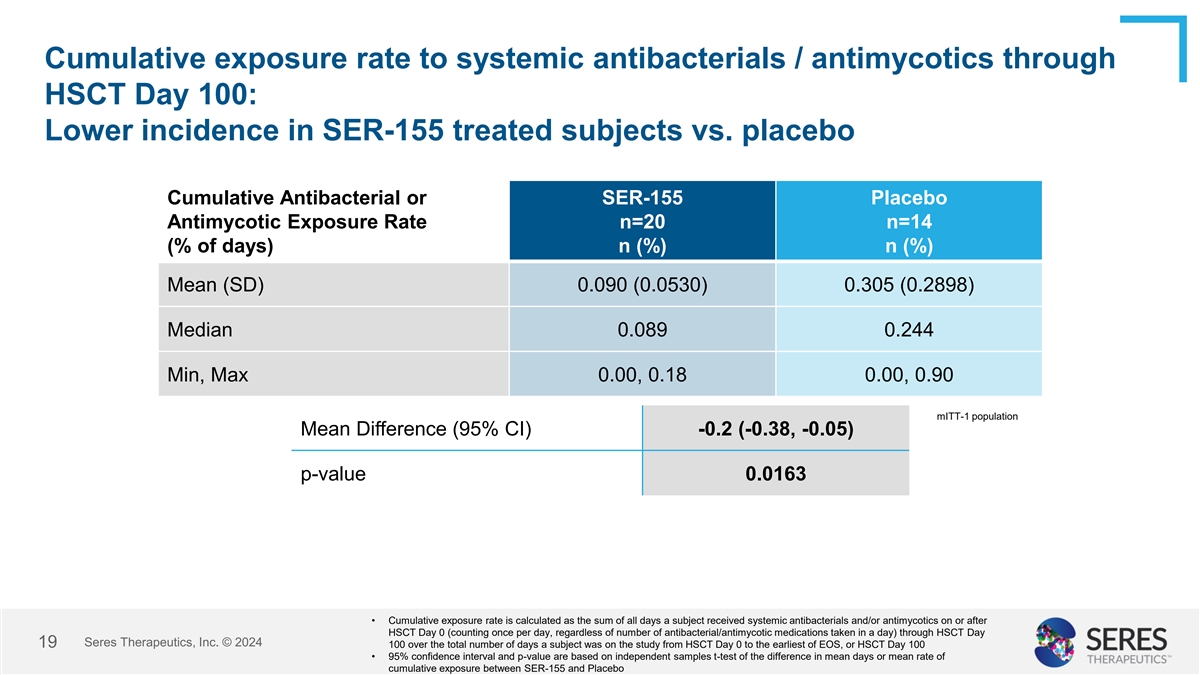
Cumulative exposure rate to systemic antibacterials / antimycotics
through HSCT Day 100: Lower incidence in SER-155 treated subjects vs. placebo Cumulative Antibacterial or SER-155 Placebo Antimycotic Exposure Rate n=20 n=14 (% of days) n (%) n (%) Mean (SD) 0.090 (0.0530) 0.305 (0.2898) Median 0.089 0.244 Min, Max
0.00, 0.18 0.00, 0.90 mITT-1 population Mean Difference (95% CI) -0.2 (-0.38, -0.05) p-value 0.0163 • Cumulative exposure rate is calculated as the sum of all days a subject received systemic antibacterials and/or antimycotics on or after HSCT
Day 0 (counting once per day, regardless of number of antibacterial/antimycotic medications taken in a day) through HSCT Day Seres Therapeutics, Inc. © 2024 13 100 over the total number of days a subject was on the study from HSCT Day 0 to the
earliest of EOS, or HSCT Day 100 • 95% confidence interval and p-value are based on independent samples t-test of the difference in mean days or mean rate of cumulative exposure between SER-155 and Placebo
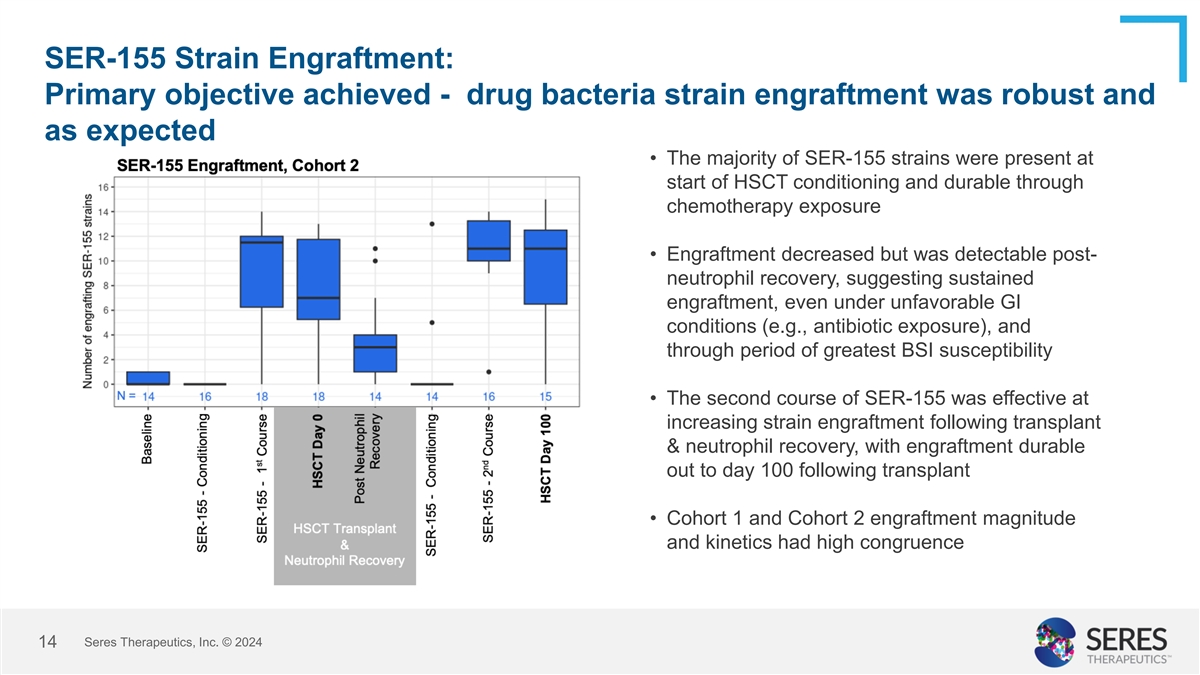
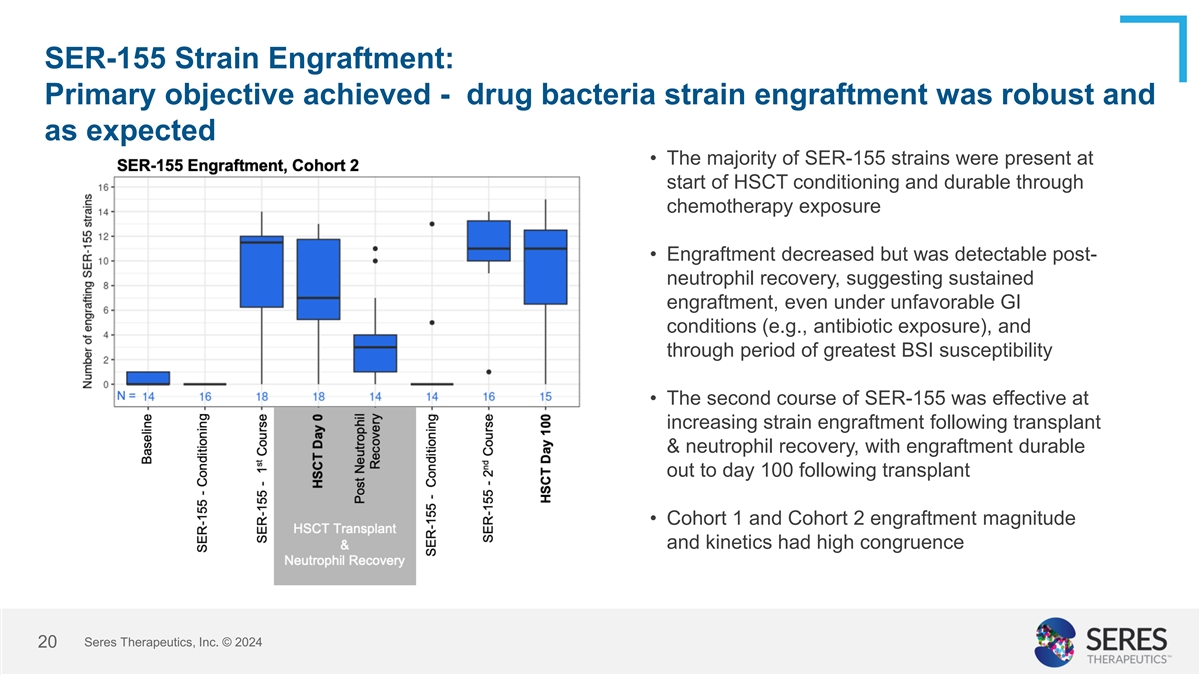
SER-155 Strain Engraftment: Primary objective achieved - drug bacteria
strain engraftment was robust and as expected • The majority of SER-155 strains were present at start of HSCT conditioning and durable through chemotherapy exposure • Engraftment decreased but was detectable post- neutrophil recovery,
suggesting sustained engraftment, even under unfavorable GI conditions (e.g., antibiotic exposure), and through period of greatest BSI susceptibility • The second course of SER-155 was effective at increasing strain engraftment following
transplant & neutrophil recovery, with engraftment durable out to day 100 following transplant • Cohort 1 and Cohort 2 engraftment magnitude and kinetics had high congruence Seres Therapeutics, Inc. © 2024 14
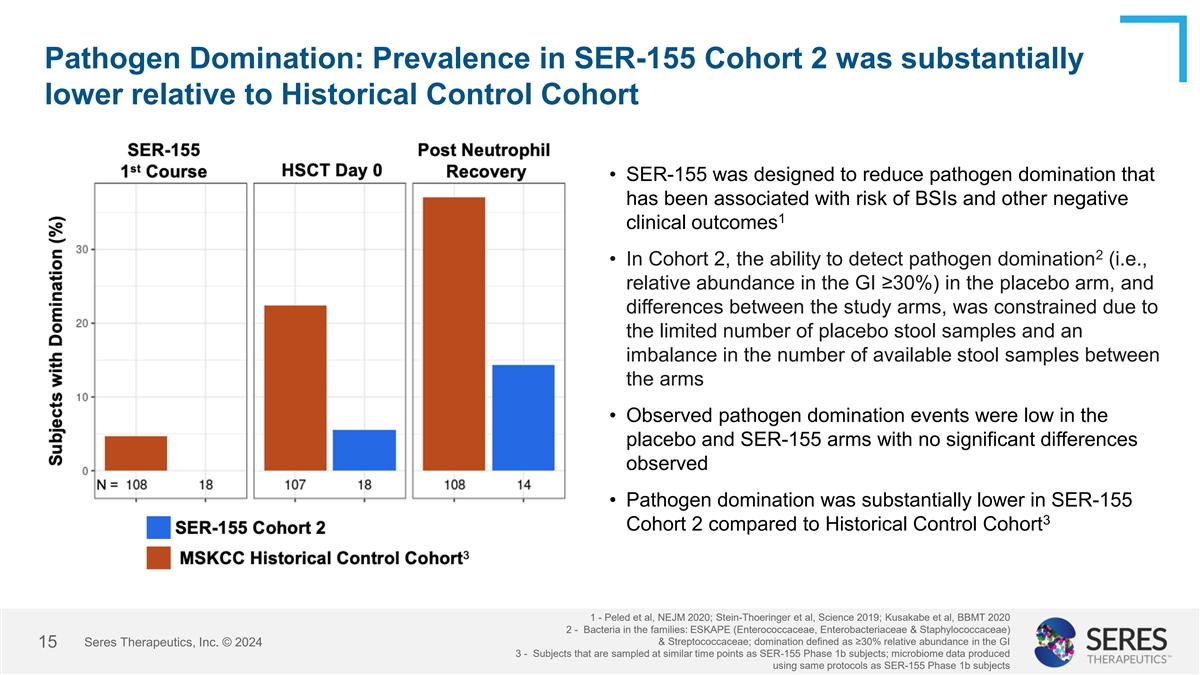
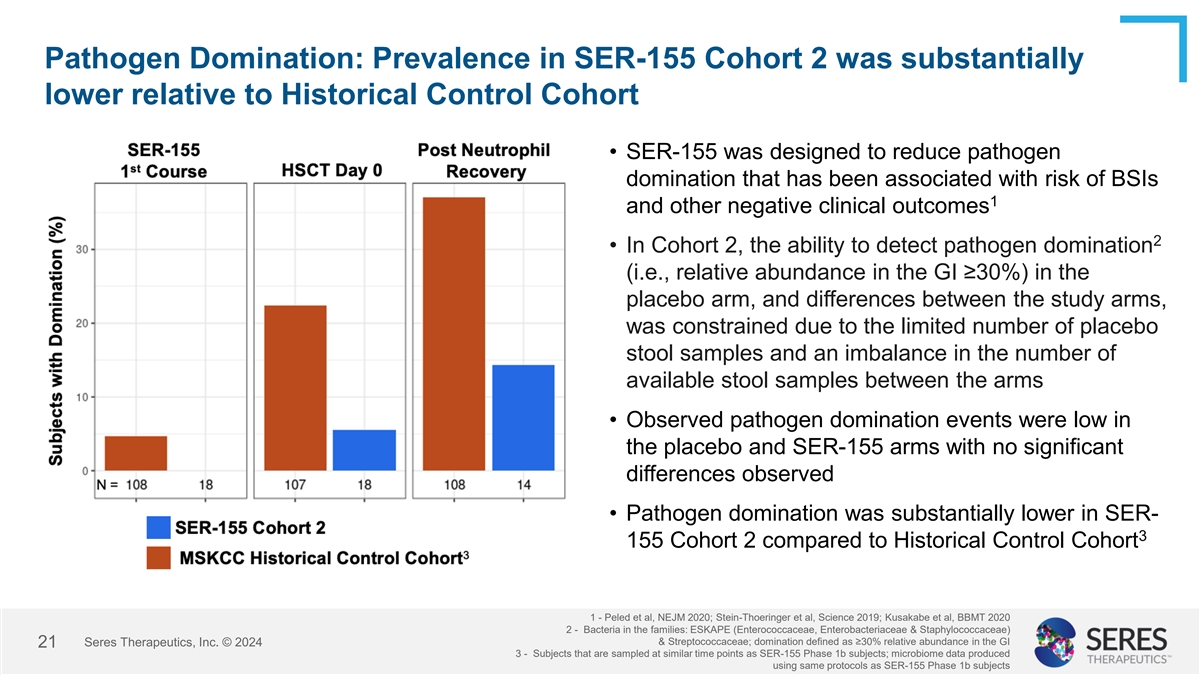
Pathogen Domination: Prevalence in SER-155 Cohort 2 was substantially
lower relative to Historical Control Cohort • SER-155 was designed to reduce pathogen domination that has been associated with risk of BSIs and other negative 1 clinical outcomes 2 • In Cohort 2, the ability to detect pathogen domination
(i.e., relative abundance in the GI ≥30%) in the placebo arm, and differences between the study arms, was constrained due to the limited number of placebo stool samples and an imbalance in the number of available stool samples between the arms
• Observed pathogen domination events were low in the placebo and SER-155 arms with no significant differences observed • Pathogen domination was substantially lower in SER-155 3 Cohort 2 compared to Historical Control Cohort 1 - Peled
et al, NEJM 2020; Stein-Thoeringer et al, Science 2019; Kusakabe et al, BBMT 2020 2 - Bacteria in the families: ESKAPE (Enterococcaceae, Enterobacteriaceae & Staphylococcaceae) & Streptococcaceae; domination defined as ≥30%
relative abundance in the GI Seres Therapeutics, Inc. © 2024 15 3 - Subjects that are sampled at similar time points as SER-155 Phase 1b subjects; microbiome data produced using same protocols as SER-155 Phase 1b subjects
Summary and Next Steps Seres to engage with FDA on advancement Phase 1
results support Seres’ strategy to of SER-155 allo-HSCT program pursue SER-155 and other live biotherapeutics for prevention of serious bacterial infections • Intend to evaluate SER-155 in additional patient • Seek Breakthrough
Therapy designation given populations with high risk of serious bacterial high unmet medical need associated with infections bloodstream infections • Opportunities to address multiple medically • Pursue additional designations (Orphan
Drug vulnerable patient groups with SER-155 and designation, Qualified Infectious Disease Product) additional pipeline programs Seres Therapeutics, Inc. © 2024 16
Exhibit 99.2
Seres Therapeutics Reports SER-155 Phase 1b Placebo-Controlled Cohort 2 Study Safety and Clinical
Results in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplant (allo-HSCT)
SER-155 administration was associated with a significant reduction in both bacterial bloodstream
infections (BSIs) and systemic antibiotic exposure, as well as lower incidence of febrile neutropenia, as compared to placebo through day 100 post HSCT
Demonstrated generally well tolerated safety profile and confirmed drug bacteria strain engraftment; no treatment-related serious adverse
events
Company to seek Breakthrough Therapy designation from the FDA, given the high unmet medical need associated with BSIs, and
discuss plans to advance development of SER-155 in allo-HSCT
Results support Seres’
strategy to pursue SER-155 and other live biotherapeutics for prevention of a broader range of serious bacterial infections in multiple medically vulnerable patient populations
Conference call at 8:30 a.m. ET today
CAMBRIDGE, Mass.—September 12, 2024— Seres Therapeutics, Inc. (Nasdaq: MCRB), a leading live biotherapeutics company, today reports topline clinical
data from Cohort 2 of its SER-155 Phase 1b placebo-controlled study in patients undergoing allo-HSCT. In this patient population, infections are frequent, severe and often life-threatening. BSIs are one of the
three leading causes of death in allo-HSCT patients. Additionally, transplant related complications, such as infections, increase the recovery burden for patients as well as increase treatment costs due to readmissions, prolonged hospital stays, and
increased time in intensive care units. Seres believes that current options to address infections are not sufficient and that SER-155 has the potential to bring significant value to patients, healthcare
providers, and the healthcare system.
SER-155 is an investigational live oral biotherapeutic cultivated from
clonal master cell banks designed to prevent GI-derived bacterial bloodstream infections and other pathogen-associated complications. Study results demonstrate that
SER-155 was associated with a significant reduction in both bloodstream infections and systemic antibiotic exposure as well as a lower incidence of febrile neutropenia, as compared to placebo through day 100
post HSCT. SER-155 was generally well tolerated, with no observed treatment-related serious adverse events.
The
Company believes that the SER-155 Phase 1 study results support Seres’ corporate strategy to develop its platform, comprised of a pipeline of designed live biotherapeutics, in multiple medically
vulnerable patient populations at high risk of life-threatening bacterial infections and associated negative
clinical outcomes. Seres intends to seek Breakthrough Therapy designation, given the high unmet medical need associated with BSIs, and discuss advancing development of SER-155 for allo-HSCT with the U.S. Food and Drug Administration (FDA). The Company also intends to evaluate SER-155 in additional patient populations that have a high risk of
serious bacterial infections.
“The placebo-controlled Phase 1b study Cohort 2 results provide further evidence supporting the potential of SER-155 to reduce the risk of bacterial bloodstream infections, a leading cause of mortality and morbidity in patients undergoing allo-HSCT,” said Lisa von Moltke, M.D., Chief Medical Officer of Seres
Therapeutics. “Given our encouraging clinical results and the severe consequences of bacterial infections, we will pursue Breakthrough Therapy designation with the FDA. We also look forward to discussing our plans to further develop SER-155 with the Agency.”
“Bacterial infections such as bacteremia (bacteria in blood) are a frequent and
often life-threatening complication faced by patients undergoing HSCT as well as other patients with cancer.” said infectious diseases physician David Fredricks, M.D., Professor, Vaccine and Infectious Disease Division, and Professor, Clinical
Research Division at the Fred Hutchinson Cancer Center in Seattle. “Many of these infections arise from bacteria in the gastrointestinal tract. Investigational live oral biotherapeutics such as SER-155
hold promise as a novel approach that could protect patients against these serious bacterial infections, resulting in improved patient outcomes, together with reduced use of antibiotics. The data from the
SER-155 Phase 1b study, including results showing lower infection rates, less systemic antibiotic exposure, and reduced incidence of febrile neutropenia events, support continued development in
allo-HSCT.”
SER-155 Phase 1b Study Design
The SER-155 Phase 1b study (NCT04995653) included two cohorts. Cohort 1 was designed to assess safety and drug
pharmacology, specifically the drug strain engraftment in the gastrointestinal tract. Cohort 1 included 13 subjects who received any dosing of the SER-155 regimen, with 11 subjects subsequently receiving an
allo-HSCT. Results from this cohort, announced in May 2023, showed SER-155 was generally well tolerated and resulted in successful drug strain engraftment and a reduction in pathogen domination in the GI
microbiome relative to a historical control cohort.
Study Cohort 2 utilized a randomized, double-blinded 1:1 placebo-controlled design to further
evaluate safety and drug strain engraftment, as well as key secondary and exploratory endpoints such as the incidence of bacterial bloodstream infections and related medical consequences such as febrile neutropenia and antibiotic use. Cohort 2
included 45 patients in the intention-to-treat (ITT) population. Of the ITT population, 20 received SER-155 and 14 received
placebo, each of whom subsequently received an allo-HSCT, with data available for clinical evaluation through day 100, the study’s prespecified primary observation point. Exploratory hypothesis testing was conducted at the two-sided α=0.05 level. Ninety-five percent (95%) 2-sided confidence intervals (CIs) were determined, where specified. No adjustment for multiplicity was done. A subset
of patient samples was available for drug pharmacology analysis.
The median age in Cohort 2 was 63, and most subjects had acute myeloid leukemia, acute lymphocytic leukemia,
myelodysplastic syndrome or myeloproliferative neoplasia as their primary disease and received reduced-intensity conditioning pre-transplant. Most patients received peripheral blood stem cells from a matched
unrelated donor. A majority received post-transplant cyclophosphamide as part of their graft-versus-host disease (GvHD) prophylaxis.
Summary of Cohort
2 Study Results
Consistent with the observations from the Phase 1b study Cohort 1, SER-155 was generally well
tolerated, and no treatment-emergent serious adverse events related to drug were observed. SER-155 bacterial strains engrafted into the gastrointestinal tract of patients following the administration of SER-155.
The incidence of BSIs was significantly lower in the SER-155 arm
compared with the placebo arm (2/20 (10%) vs. 6/14 (42.9%), respectively; [Odds Ratio: 0.15; 95% CI: 0.01, 1.13, p=0.0423]). In addition, while antibiotic starts were similar in each arm, patients administered
SER-155 were treated with antibiotics for a significantly shorter duration compared to patients in the placebo arm (9.2 days vs. 21.1 days, respectively, with a mean difference of -11.9 days [95% CI: -23.85, -0.04; p=0.0494]). The incidence of febrile neutropenia was lower in patients administered SER-155 compared to placebo (65% vs. 78.6%, respectively; [Odds Ratio: 0.51; 95% CI: 0.07, 2.99; p=0.4674]). Six cases of gastrointestinal infections (C. difficile infections) were observed in the study, with
four cases (20%) in the SER-155 arm and two cases (14.3%) in the placebo arm.
Recent changes in the allo-HSCT
standard of care and the increasing use of post-transplant cyclophosphamide as part of prophylactic therapy for GvHD have reduced rates of GvHD overall in this patient population. The rates of GvHD in the study were low, with two cases of grade 2
GvHD observed in each arm, and no cases of grade 3 or 4 GvHD were observed.
In Cohort 2, the ability to detect pathogen domination (i.e., relative
abundance in the GI ≥30%) in the placebo arm, and differences between the study arms, was constrained due to the limited number of placebo stool samples and an imbalance in the number of available stool samples between the arms. Observed
pathogen domination events were low in the placebo and SER-155 arms with no significant differences identified. In a comparison of the prevalence of pathogen domination versus a larger allo-HSCT historical
control cohort, pathogen domination in SER-155 subjects was substantially lower, providing further evidence of SER-155 activity.
“The SER-155 Phase 1b results generate further evidence to support Seres’ strategy as SER-155 is our second live biotherapeutic, after VOWST, designed to prevent serious bacterial infections and associated negative clinical outcomes in medically vulnerable populations. An estimated 40,000 patients
worldwide undergo allogeneic stem cell transplantation each year. Adding autologous stem cell transplants (auto-HSCT), a natural adjacent patient population, approximately doubles this figure. The potential to contract a bloodstream infection during
the allogeneic transplant process is significant, with incidence reports in the literature reaching up to 45%. Allogeneic transplant-related complications, including infections, increase already significant treatment costs by approximately $180,000
per patient. Given this high unmet need, we believe SER-155 could provide meaningful value for patients and the healthcare system,” said Eric Shaff, Chief Executive Officer of Seres Therapeutics.
Mr. Shaff continued, “We are particularly encouraged by the consistency of related efficacy
outcomes in this study, especially the significantly lower rates of bloodstream infections and systemic antibiotic exposure as well as fewer instances of febrile neutropenia, as compared to placebo. Supported by these data and our well-established
clinical, pharmacological, CMC, and regulatory capabilities, we plan to engage with the FDA to seek Breakthrough Therapy designation and discuss advancing development of SER-155 in allo-HSCT. With SER-155 and additional pipeline programs, we believe we may have the opportunity to address multiple patient groups, including allo-HSCT, auto-HSCT, CAR-T, chronic liver
disease, cancer neutropenia, and solid organ transplants, thereby potentially creating significant commercial opportunities.”
Seres fully owns
worldwide rights for the commercialization of SER-155.
Conference Call Information
Seres’ management will host a conference call today, September 12, 2024, at 8:30 a.m. ET. The conference call may be accessed by calling 1-800-715-9871 (international callers dial 1-646-307-1963) and referencing the conference ID number 622932. To join the live webcast, please visit the “Investors and News” section of the Seres website at www.serestherapeutics.com. A
webcast replay will be available on the Seres website beginning approximately two hours after the event and will be archived for at least 21 days.
About SER-155
SER-155 is an investigational live biotherapeutic designed to prevent
GI-derived bloodstream infections, enhance epithelial barrier integrity to reduce the likelihood of bacterial translocation from the gut to the bloodstream, and induce immune tolerance responses to reduce the
incidence of GvHD. SER-155 contains 16 bacterial strains selected using Seres’ reverse translation discovery and development platform technologies to optimize
SER-155’s functional profile. The design incorporates biomarker data from human clinical data and screening data from nonclinical human cell-based assays and in vivo disease models. SER-155 has been evaluated in a Phase 1b placebo-controlled study in patients undergoing allo-HSCT. SER-155 has received FDA Fast Track designation for reducing the risk of
infection and GvHD in patients undergoing HSCT. The early development of the program was supported by Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X), a global non-profit partnership accelerating antibacterial products to address drug-resistant bacteria.
About Seres Therapeutics
Seres
Therapeutics, Inc. (Nasdaq: MCRB) is a commercial-stage company focused on improving patient outcomes in medically vulnerable populations through novel live biotherapeutics. Seres led the successful development and approval of VOWST™, the first FDA-approved orally administered microbiome therapeutic. The Company is developing SER-155, designed to
prevent gastrointestinal-derived bloodstream infections, enhance epithelial barrier integrity, and induce immune tolerance responses to reduce the incidence of graft-versus-host-disease (GvHD). The Company is also advancing additional cultivated
oral live biotherapeutics for medically vulnerable populations, including those with chronic liver disease, cancer neutropenia, and solid organ transplants. For more information, please visit www.serestherapeutics.com.
Important Additional Information About the Transaction and Where to Find It
In connection with the proposed transaction involving Seres Therapeutics, Inc. (“Seres”) and Société des Produits Nestlé
S.A. (“SPN”), Seres filed a definitive proxy statement with the Securities and Exchange Commission (the “SEC”). Seres may also file other relevant material with the SEC regarding the proposed transaction. Beginning on
August 26, 2024, Seres mailed the definitive proxy statement to its stockholders. INVESTORS AND STOCKHOLDERS OF SERES ARE URGED TO READ THE DEFINITIVE PROXY STATEMENT AND OTHER RELEVANT MATERIALS CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME
AVAILABLE BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION ABOUT SERES AND THE PROPOSED TRANSACTION. Investors may obtain a free copy of these materials (when they are available) and other documents filed by Seres with the SEC at the
SEC’s website at www.sec.gov or from Seres at its website at ir.serestherapeutics.com.
Participants in the Solicitation
Seres and certain of its directors, executive officers and other members of management and employees may be deemed to be participants in soliciting proxies
from its stockholders in connection with the proposed transaction. Information regarding the persons who may, under the rules of the SEC, be considered to be participants in the solicitation of Seres’ stockholders in connection with the
proposed transaction is set forth in Seres’ definitive proxy statement for its stockholder meeting, which was filed with the SEC on August 26, 2024, at which the proposed transaction will be submitted for approval by Seres’
stockholders. You may also find additional information about Seres’ directors and executive officers in Seres’ Annual Report on Form 10-K for the fiscal year ended December 31, 2023, which was
filed with the SEC on March 5, 2024, Seres’ Definitive Proxy Statement for its 2024 annual meeting of stockholders, which was filed with the SEC on March 5, 2024, and in subsequently filed Current Reports on Form 8-K and Quarterly Reports on Form 10-Q.
Forward-Looking Statements
This communication contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements
contained in this communication that do not relate to matters of historical fact should be considered forward-looking statements, including statements about the potential benefits of any of our products or product candidates; the ultimate safety and
efficacy data of SER-155; study results; plans to seek FDA feedback; clinical data and clinical trials; our intentions related to the development of SER-155; our
intention to seek Breakthrough Therapy designation; the ability of live biotherapeutics to prevent or reduce infections; or the timing of any of the foregoing and other statements which are not historical fact.
These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known
and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking
statements, including, but not limited to, the following: (1) we have incurred significant losses, are not currently profitable and may never become profitable; (2) our need for additional funding; (3) our history of operating losses;
(4) the restrictions in our debt agreement; (5) our novel approach to therapeutic intervention; (6) our reliance on third parties to conduct our clinical trials and manufacture our product candidates; (7) the competition we will
face; (8) risks associated with our clinical trials; (9) whether the FDA grants Breakthrough Therapy designation; (10) our ability to protect our intellectual
property; (11) our ability to retain key personnel and to manage our growth; and (12) risks related to the proposed transaction under the Purchase Agreement with Société
des Produits Nestlé S.A. for the sale of the VOWST business to SPN. These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q filed with
the SEC, on August 13, 2024, and our other reports filed with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this communication. Any such forward-looking statements
represent management’s estimates as of the date of this communication. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to
change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this communication.
Investor and Media Contacts:
IR@serestherapeutics.com
Carlo Tanzi, Ph.D.
Kendall Investor Relations
ctanzi@kendallir.com

Exhibit 99.3 Seres Therapeutics Investor Presentation September 12,
2024

Disclaimers Forward Looking Statements This communication contains
forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this communication that do not relate to matters of historical fact should be considered forward-looking statements,
including statements about the potential benefits of any of our products or product candidates; the ultimate safety and efficacy data of SER-155; study results; plans to seek FDA feedback; clinical data and clinical trials; our intentions related to
the development of SER-155; our intention to seek Breakthrough Therapy Designation; the ability of live biotherapeutics to prevent or reduce infections; or the timing of any of the foregoing; the financial terms, timing and completion of the sale of
VOWST assets to SPN; the receipt of future payments and the use of proceeds of the transaction; the timing and results of our clinical studies and data readouts; future product candidates, development plans and commercial opportunities; operating
plans and our future cash runway; our ability to generate additional capital; our planned strategic focus; anticipated timing of any of the foregoing and other statements which are not historical fact. These forward-looking statements are based on
management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be
materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: (1) we have incurred significant losses, are not currently profitable and
may never become profitable; (2) our need for additional funding; (3) our history of operating losses; (4) the restrictions in our debt agreement; (5) our novel approach to therapeutic intervention; (6) our reliance on third parties to conduct our
clinical trials and manufacture our product candidates; (7) the competition we will face; (8) risks associated with our clinical trials; (9) whether the FDA grants Breakthrough Therapy Designation; (10) our ability to protect our intellectual
property; (11) our ability to retain key personnel and to manage our growth; and (12) risks related to the proposed transaction under the Purchase Agreement with SPN for the sale of the VOWST business to SPN. These and other important factors
discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q filed with the SEC, on August 13, 2024, and our other reports filed with the SEC could cause actual results to differ materially from those indicated by the
forward-looking statements made in this communication. Any such forward-looking statements represent management’s estimates as of the date of this communication. While we may elect to update such forward-looking statements at some point in the
future, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this communication. Seres
Therapeutics, Inc. © 2024 2 2

Disclaimers Important Information About the Transaction and Where to
Find it In connection with the proposed transaction involving Seres Therapeutics, Inc. (“Seres”) and Société des Produits Nestlé S.A. (“SPN”), Seres filed a definitive proxy statement with the Securities and
Exchange Commission (the “SEC”). Seres may also file other relevant material with the SEC regarding the proposed transaction. Beginning on August 26, 2024, Seres mailed the definitive proxy statement to its stockholders. INVESTORS AND
STOCKHOLDERS OF SERES ARE URGED TO READ THE DEFINITIVE PROXY STATEMENT AND OTHER RELEVANT MATERIALS CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION ABOUT SERES AND THE PROPOSED
TRANSACTION. Investors may obtain a free copy of these materials (when they are available) and other documents filed by Seres with the SEC at the SEC’s website at www.sec.gov or from Seres at its website at ir.serestherapeutics.com.
Participants in the Solicitation Seres and certain of its directors, executive officers and other members of management and employees may be deemed to be participants in soliciting proxies from its stockholders in connection with the proposed
transaction. Information regarding the persons who may, under the rules of the SEC, be considered to be participants in the solicitation of Seres’ stockholders in connection with the proposed transaction is set forth in Seres’ definitive
proxy statement for its stockholder meeting, which was filed with the SEC on August 26, 2024, at which the proposed transaction will be submitted for approval by Seres’ stockholders. You may also find additional information about Seres’
directors and executive officers in Seres’ Annual Report on Form 10-K for the fiscal year ended December 31, 2023, which was filed with the SEC on March 5, 2024, Seres’ Definitive Proxy Statement for its 2024 annual meeting of
stockholders, which was filed with the SEC on March 5, 2024, and in subsequently filed Current Reports on Form 8-K and Quarterly Reports on Form 10-Q. Seres Therapeutics, Inc. © 2024 3 3
September 2024: SER-155 Phase 1b placebo-controlled Cohort 2 study
results in allo-HSCT • Phase 1b study in adults undergoing allogeneic hematopoietic stem cell transplant (allo-HSCT) to assess SER-155 safety and pharmacology, with additional endpoints such as incidence of bacterial bloodstream infections and
related medical consequences such as antibiotic use and febrile neutropenia • Demonstrated generally well tolerated safety profile and confirmed drug bacteria strain engraftment • SER-155 administration associated with following outcomes
compared to placebo at day 100 post-HSCT: Significant reduction Significant reduction Lower incidence of in bacterial in systemic antibiotic febrile neutropenia bloodstream infections exposure • Company to seek Breakthrough Therapy designation
from the FDA given the high unmet medical need associated with bloodstream infections Results support Seres’ strategy to develop SER-155 and other live biotherapeutics to prevent serious bacterial infections in medically vulnerable patient
populations Seres Therapeutics, Inc. © 2024 4
Transforming patient outcomes using proprietary consortia of live
biotherapeutics Strong foundation Favorable Phase 1b Blockbuster opportunity Expansive potential clinical data in SER- • Validated platform with • Accelerate SER-155 • SER-147 designed to ® 155 allo-HSCT VOWST clinical and
development in allo-HSCT prevent infections in chronic regulatory success liver disease; anticipate IND • Well tolerated safety • Potential to initiate multiple readiness in H2 '25 profile; no treatment- • Asset sale strengthens
clinical studies in the next 12- related SAEs balance sheet, expected 18 months • Current focus of preventing to extend runway into Q4 life-threatening infections • Drug bacteria • Potential to evaluate SER- ‘25 engraftment
as expected 155 in additional patient • Potential to treat immune- • Wholly-owned cultivated populations at high risk of related diseases (including • Significant reduction in pipeline: SER-155, SER- serious bacterial infections
IBD) bloodstream infections 147, beyond (e.g., autologous HSCT, and systemic CAR-T, blood cancers, solid antibacterial exposure organ transplant) • Lower incidence of febrile neutropenia Seres Therapeutics, Inc. © 2024 5
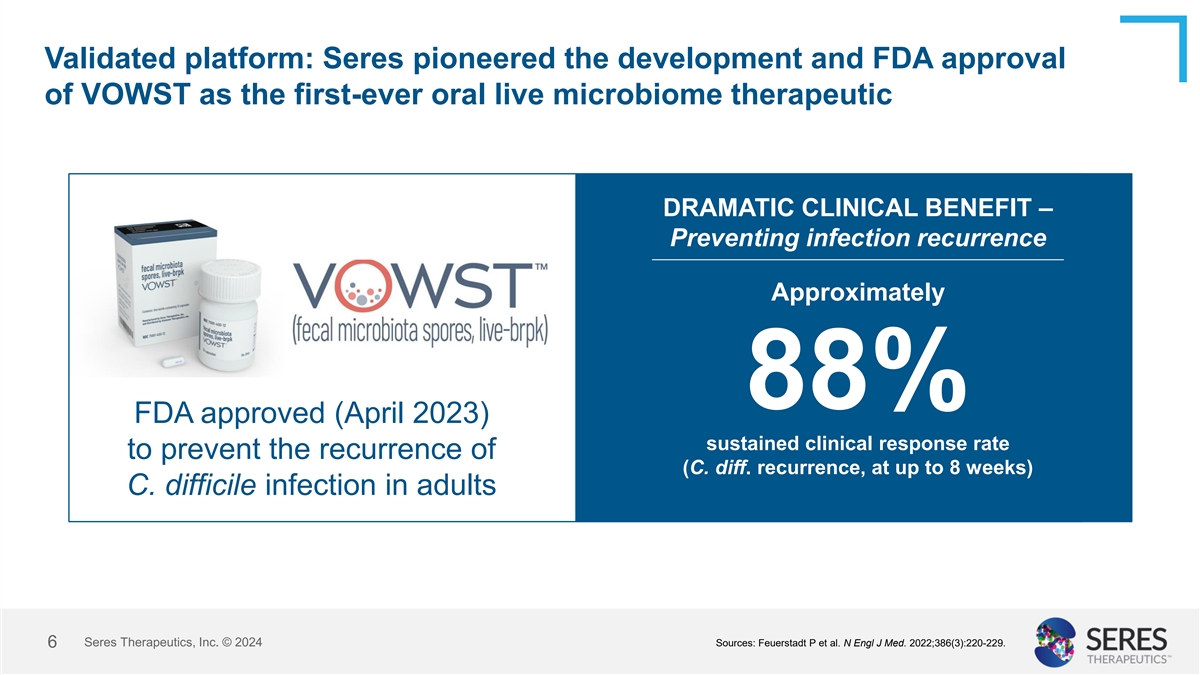
Validated platform: Seres pioneered the development and FDA approval of
VOWST as the first-ever oral live microbiome therapeutic DRAMATIC CLINICAL BENEFIT – Preventing infection recurrence Approximately 88% FDA approved (April 2023) sustained clinical response rate to prevent the recurrence of (C. diff.
recurrence, at up to 8 weeks) C. difficile infection in adults Seres Therapeutics, Inc. © 2024 Sources: Feuerstadt P et al. N Engl J Med. 2022;386(3):220-229. 6
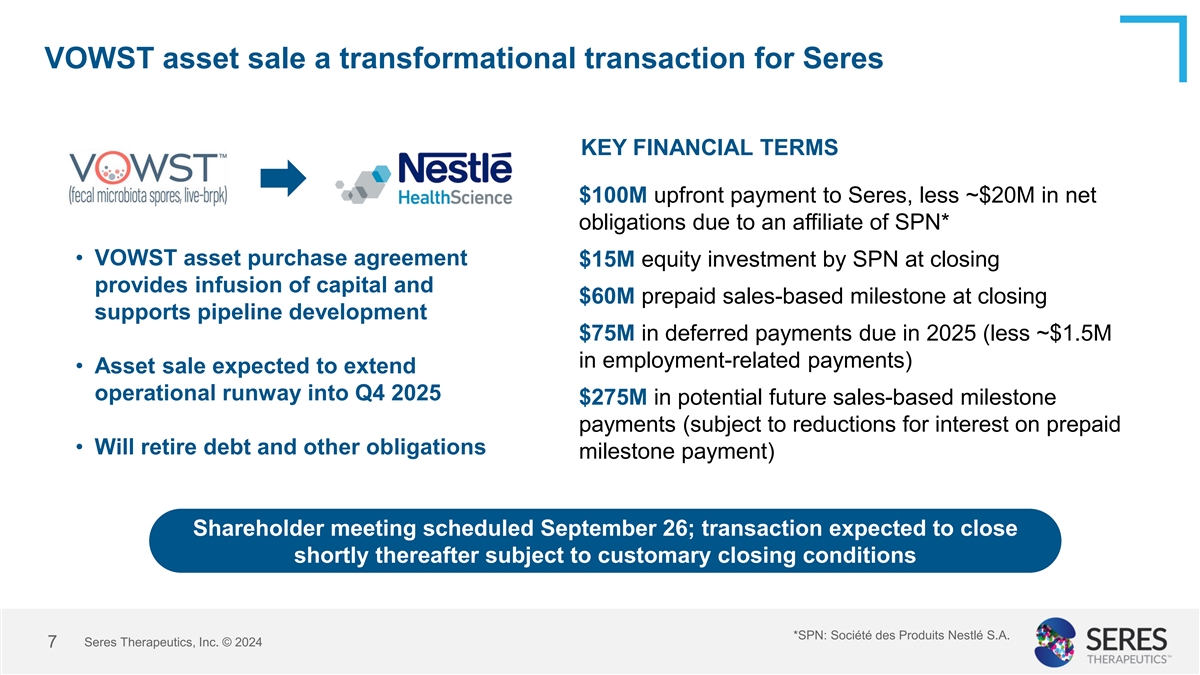
VOWST asset sale a transformational transaction for Seres KEY FINANCIAL
TERMS $100M upfront payment to Seres, less ~$20M in net obligations due to an affiliate of SPN* • VOWST asset purchase agreement $15M equity investment by SPN at closing provides infusion of capital and $60M prepaid sales-based milestone at
closing supports pipeline development $75M in deferred payments due in 2025 (less ~$1.5M in employment-related payments) • Asset sale expected to extend operational runway into Q4 2025 $275M in potential future sales-based milestone payments
(subject to reductions for interest on prepaid • Will retire debt and other obligations milestone payment) Shareholder meeting scheduled September 26; transaction expected to close shortly thereafter subject to customary closing conditions
*SPN: Société des Produits Nestlé S.A. Seres Therapeutics, Inc. © 2024 7
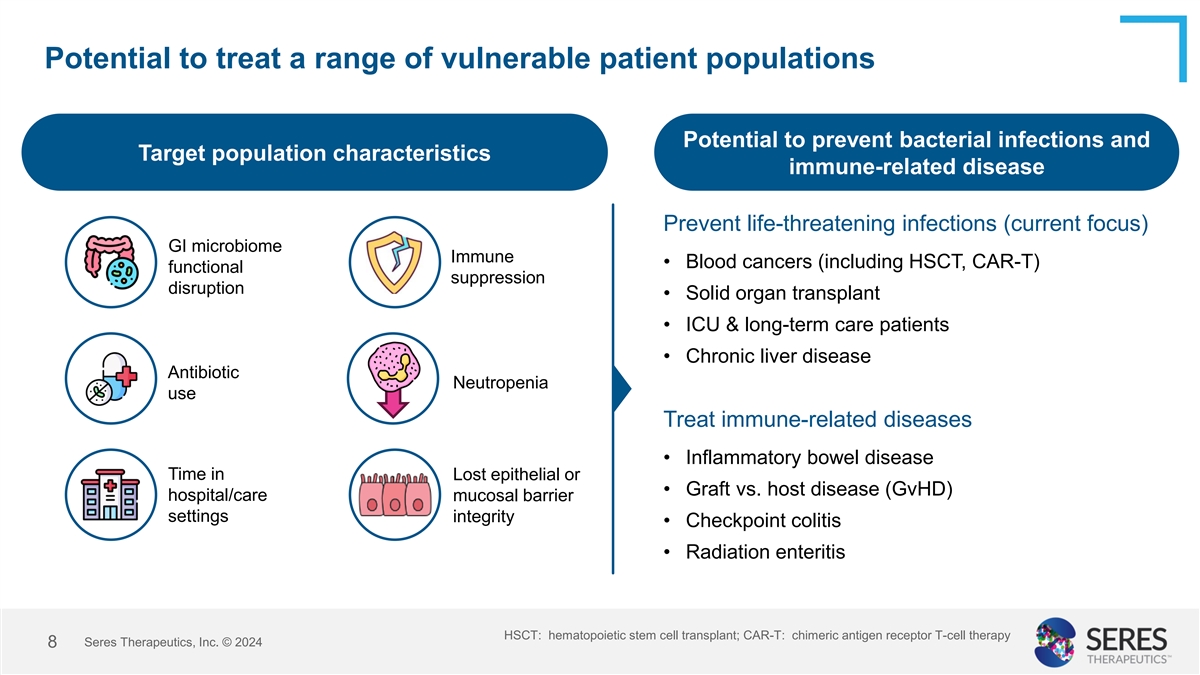
Potential to treat a range of vulnerable patient populations Potential
to prevent bacterial infections and Target population characteristics immune-related disease Prevent life-threatening infections (current focus) GI microbiome Immune • Blood cancers (including HSCT, CAR-T) functional suppression disruption
• Solid organ transplant • ICU & long-term care patients • Chronic liver disease Antibiotic Neutropenia use Treat immune-related diseases • Inflammatory bowel disease Time in Lost epithelial or • Graft vs. host
disease (GvHD) hospital/care mucosal barrier settings integrity • Checkpoint colitis • Radiation enteritis HSCT: hematopoietic stem cell transplant; CAR-T: chimeric antigen receptor T-cell therapy Seres Therapeutics, Inc. © 2024
8
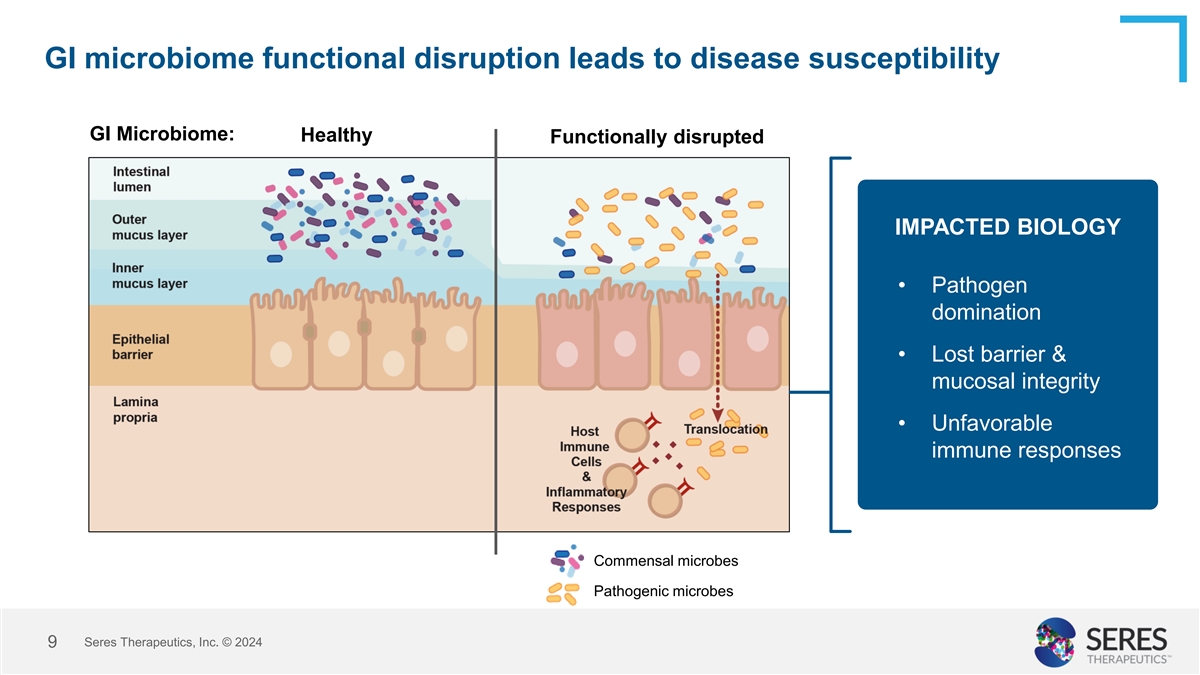
GI microbiome functional disruption leads to disease susceptibility GI
Microbiome: Healthy Functionally disrupted IMPACTED BIOLOGY • Pathogen domination • Lost barrier & mucosal integrity • Unfavorable immune responses Commensal microbes Pathogenic microbes Seres Therapeutics, Inc. © 2024
9
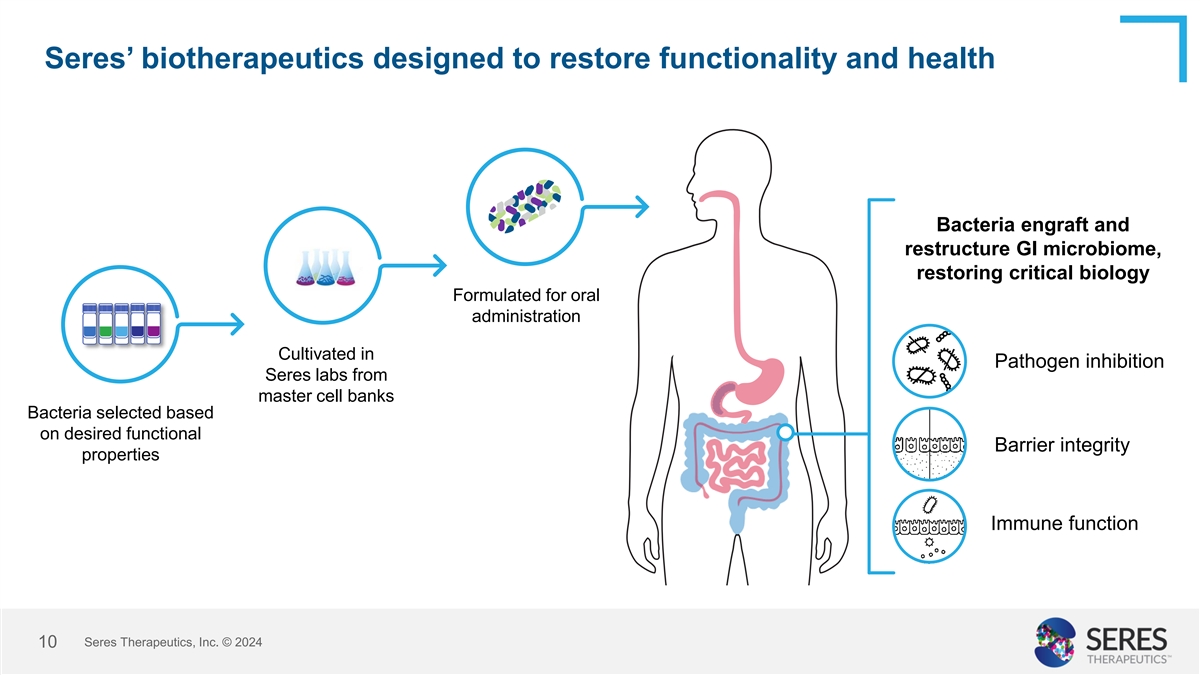
Seres’ biotherapeutics designed to restore functionality and
health Bacteria engraft and restructure GI microbiome, restoring critical biology Formulated for oral administration Cultivated in Pathogen inhibition Seres labs from master cell banks Bacteria selected based on desired functional Barrier integrity
properties Immune function Seres Therapeutics, Inc. © 2024 10
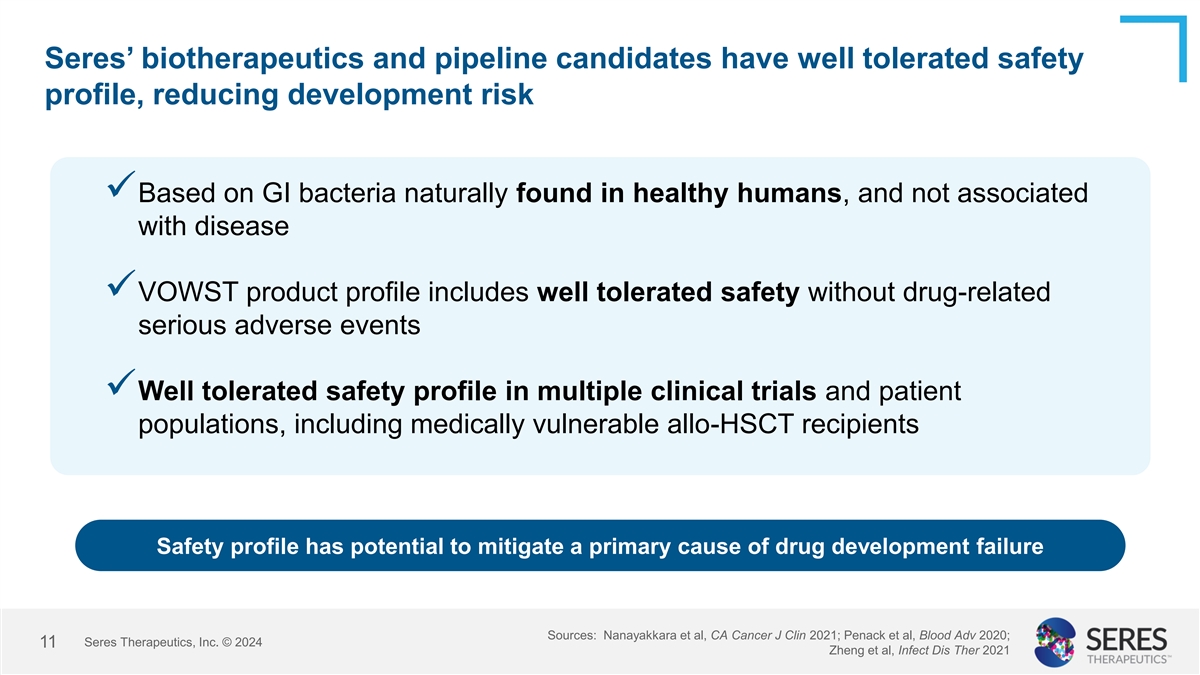
Seres’ biotherapeutics and pipeline candidates have well
tolerated safety profile, reducing development risk ✓Based on GI bacteria naturally found in healthy humans, and not associated with disease ✓VOWST product profile includes well tolerated safety without drug-related serious adverse
events ✓Well tolerated safety profile in multiple clinical trials and patient populations, including medically vulnerable allo-HSCT recipients Safety profile has potential to mitigate a primary cause of drug development failure Sources:
Nanayakkara et al, CA Cancer J Clin 2021; Penack et al, Blood Adv 2020; Seres Therapeutics, Inc. © 2024 11 Zheng et al, Infect Dis Ther 2021
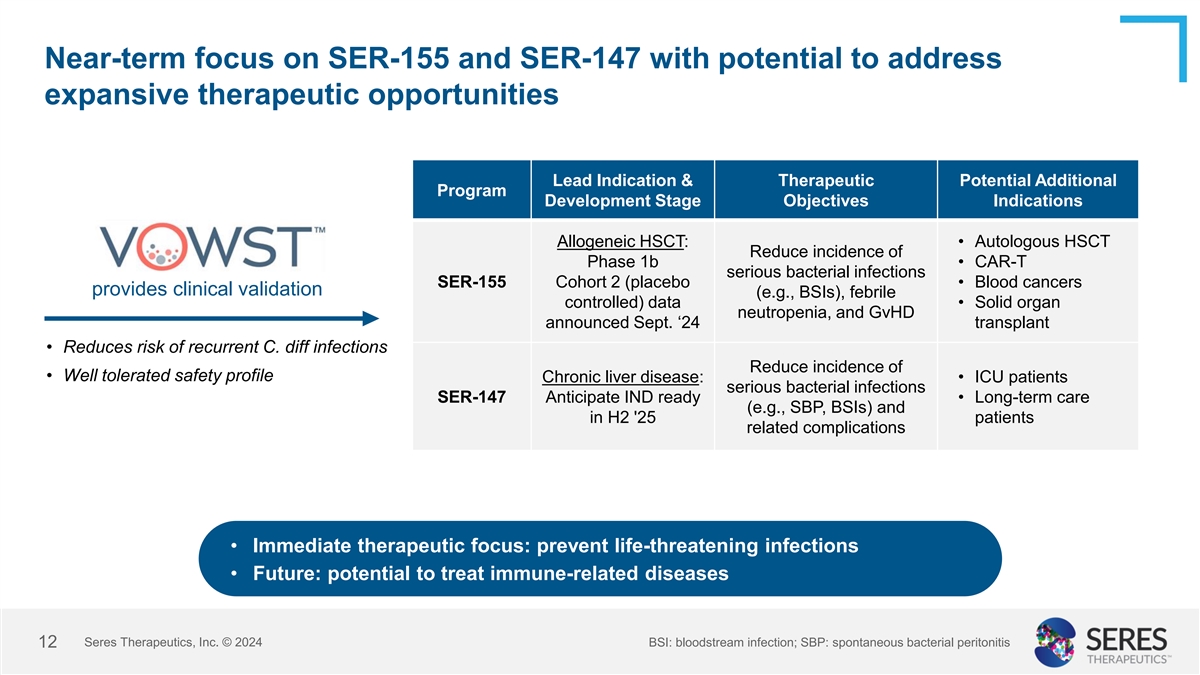
Near-term focus on SER-155 and SER-147 with potential to address
expansive therapeutic opportunities Lead Indication & Therapeutic Potential Additional Program Development Stage Objectives Indications Allogeneic HSCT: • Autologous HSCT Reduce incidence of Phase 1b • CAR-T serious bacterial
infections SER-155 Cohort 2 (placebo • Blood cancers provides clinical validation (e.g., BSIs), febrile controlled) data • Solid organ neutropenia, and GvHD announced Sept. ‘24 transplant • Reduces risk of recurrent C. diff
infections Reduce incidence of • Well tolerated safety profile Chronic liver disease: • ICU patients serious bacterial infections SER-147 Anticipate IND ready • Long-term care (e.g., SBP, BSIs) and in H2 '25 patients related
complications • Immediate therapeutic focus: prevent life-threatening infections • Future: potential to treat immune-related diseases Seres Therapeutics, Inc. © 2024 BSI: bloodstream infection; SBP: spontaneous bacterial peritonitis
12
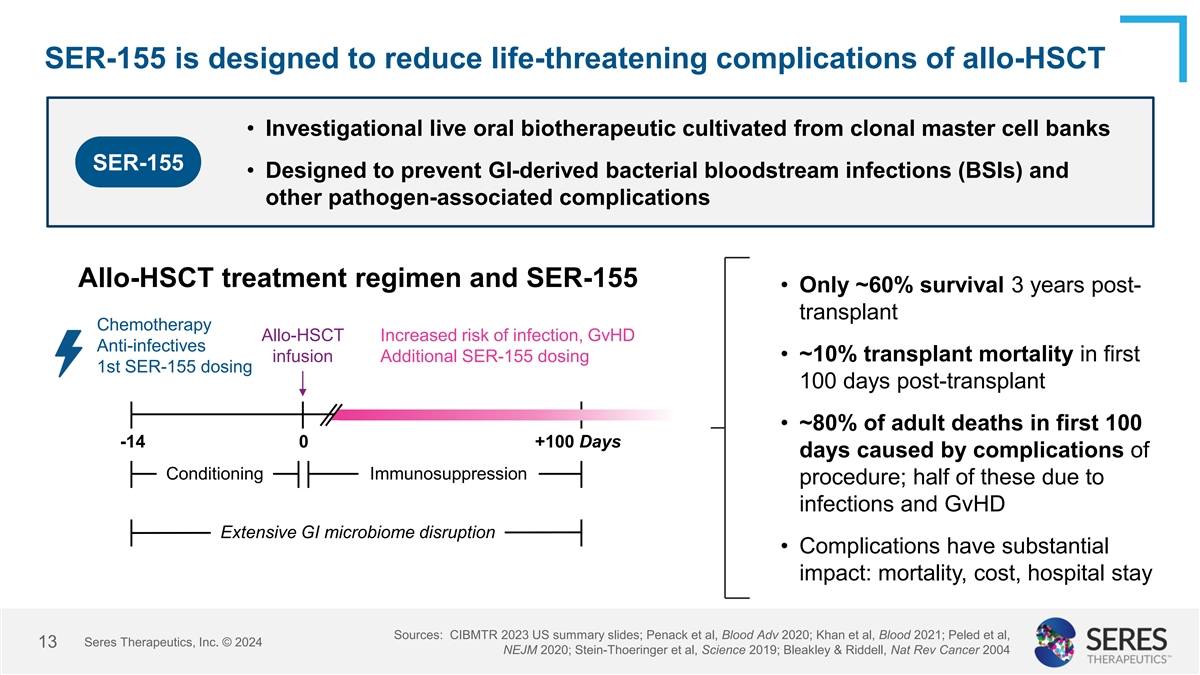
SER-155 is designed to reduce life-threatening complications of
allo-HSCT • Investigational live oral biotherapeutic cultivated from clonal master cell banks SER-155 • Designed to prevent GI-derived bacterial bloodstream infections (BSIs) and other pathogen-associated complications Allo-HSCT
treatment regimen and SER-155 • Only ~60% survival 3 years post- transplant Chemotherapy Allo-HSCT Increased risk of infection, GvHD Anti-infectives • ~10% transplant mortality in first infusion Additional SER-155 dosing 1st SER-155
dosing 100 days post-transplant • ~80% of adult deaths in first 100 -14 0 +100 Days days caused by complications of Conditioning Immunosuppression procedure; half of these due to infections and GvHD Extensive GI microbiome disruption •
Complications have substantial impact: mortality, cost, hospital stay Sources: CIBMTR 2023 US summary slides; Penack et al, Blood Adv 2020; Khan et al, Blood 2021; Peled et al, Seres Therapeutics, Inc. © 2024 13 NEJM 2020; Stein-Thoeringer et
al, Science 2019; Bleakley & Riddell, Nat Rev Cancer 2004
SER-155 Phase 1b study evaluated safety, pharmacology, and efficacy in
adult allo-HSCT recipients COHORT 1 COHORT 2 Open-label (n=15 enrolled) Placebo-controlled 1:1 (n=45 enrolled) SER-155 SER-155 Placebo results reported May 2023 results announced Sept. 2024 Primary Endpoints: Key Secondary Endpoints through HSCT Day
100: • Safety and tolerability • Incidence of bloodstream infections (BSI), GI infections, and acute GvHD ≥ Grade 2 • SER-155 bacterial strain engraftment • Incidence and duration of febrile neutropenia •
Bacterial pathogen abundance Received US FDA Fast Track Designation in December 2023; Intend to pursue Breakthrough Therapy designation Seres Therapeutics, Inc. © 2024 14
Patient Safety: Cohort 2 SER-155 was generally well tolerated with no
treatment-related SAEs • All but one subject in the placebo arm experienced at least 1 TEAE • Most common for SER-155 treated subjects (≥50% and with Δ ≥5% greater than placebo): Treatment-emergent diarrhea (86% vs. 74%
placebo), nausea (62% vs. 53% placebo) adverse events • 1/40 (3%) subject experienced a TEAE leading to treatment discontinuation (active = 0; placebo = 1) (TEAEs) • 3/40 (8%) subjects experienced a TEAE leading to study discontinuation
(active = 1; placebo = 2) • 19/40 (48%) subjects experienced an SAE: 11/21 (52%) SER-155-treated subjects vs. 8/19 (42%) placebo-treated subjects; none considered related to SER-155 (no SUSARs) Serious adverse events o Most common SAE SOC:
infections & infestations (24% active vs. 37% placebo) (SAEs) o 3 deaths prior to Day 100 (active = 1; placebo = 2), 1 death after Day 100 (active), none considered related to SER-155 Adverse events of • AESIs (bloodstream infections, GI
infection, invasive infection): 14/40 (35%) subjects special interest • Rates of AESIs were lower in SER-155 arm vs placebo arm (29% vs 42% respectively) (AESIs) • No SER-155 species were identified in culture from any subject Seres
Therapeutics, Inc. © 2024 15
Efficacy: SER-155 administration favorable with significant* reduction
in both bacterial BSIs and systemic antibiotic exposure; lower febrile neutropenia Significant decrease in bacterial bloodstream infections in SER-155-treated Bloodstream infections subjects vs. placebo Numerically lower incidence rate of febrile
neutropenia in SER-155-treated Febrile neutropenia subjects vs. placebo All GI infections were CDI**; 4 subjects in SER-155-treated (20%) and 2 GI infections subjects in placebo (14.3%) developed GI infections from HSCT Day 0-100 No subjects in
either arm developed ≥ Grade 3 acute GvHD; Acute GvHD 2 subjects in each arm developed Grade 2 acute GvHD Significantly lower mean cumulative exposure (days) to systemic antibacterials / antimycotics for SER-155-treated subjects vs. placebo
Antibacterial / antimycotic exposure Significantly lower cumulative exposure rate to systemic antibacterials / antimycotics for SER-155-treated subjects vs. placebo * no multiplicity adjustments were applied Seres Therapeutics, Inc. © 2024 16
** CDI: C. difficile infection Exploratory Secondary Efficacy Efficacy
Bloodstream infections from HSCT Day 0 to Day 100: Lower incidence in
SER-155 treated subjects vs. placebo Bloodstream infections from Day 0 SER-155 Placebo to Day 100 n=20 n=14 (# patients) n (%) n (%) Subjects with confirmed BSI 2 (10.0%) 6 (42.9%) 95% confidence interval (1.2, 31.7) (17.7, 71.1) mITT-1 population
Odds ratio 0.15 95% confidence interval (0.01, 1.13) p-value 0.0423 Organisms in SER-155 patients: Finegoldia magna; E. coli/Strep mitis Organisms in placebo patients: E.coli; Enterococcus faecium/staph haemolyticus/Candida krusei; Staph aureus;
Staph haemolyticus; Pseudomonas aeruginosa; E coli • CI: 95% 2-sided Clopper-Pearson confidence interval of incidence is applied Seres Therapeutics, Inc. © 2024 17 • Odds ratio: for incidence between treatment groups (SER-155 and
placebo) with 95% 2-sided confidence interval and the corresponding p-value calculated based on the Fisher’s Exact test
Cumulative exposure to systemic antibacterials / antimycotics through
HSCT Day 100: Lower incidence in SER-155 treated subjects vs. placebo Cumulative Antibacterial or SER-155 Placebo Antimycotic Exposure n=20 n=14 (HSCT Days) n (%) n (%) Mean (SD) 9.2 (5.44) 21.1 (20.31) Median 9.0 14.0 Min, Max 0, 19 0, 74 mITT-1
population Mean Difference (95% CI) -11.9 (-23.85, -0.04) p-value 0.0494 • Cumulative exposure is the sum of all days a subject received systemic antibacterials and/or antimycotics between HSCT Day 0 through Day 100; counting once per day
regardless of number of agents taken Seres Therapeutics, Inc. © 2024 18 • 95% confidence interval and p-value based on independent samples t-test of the difference in mean days between SER- 155 and placebo
Cumulative exposure rate to systemic antibacterials / antimycotics
through HSCT Day 100: Lower incidence in SER-155 treated subjects vs. placebo Cumulative Antibacterial or SER-155 Placebo Antimycotic Exposure Rate n=20 n=14 (% of days) n (%) n (%) Mean (SD) 0.090 (0.0530) 0.305 (0.2898) Median 0.089 0.244 Min, Max
0.00, 0.18 0.00, 0.90 mITT-1 population Mean Difference (95% CI) -0.2 (-0.38, -0.05) p-value 0.0163 • Cumulative exposure rate is calculated as the sum of all days a subject received systemic antibacterials and/or antimycotics on or after HSCT
Day 0 (counting once per day, regardless of number of antibacterial/antimycotic medications taken in a day) through HSCT Day Seres Therapeutics, Inc. © 2024 19 100 over the total number of days a subject was on the study from HSCT Day 0 to the
earliest of EOS, or HSCT Day 100 • 95% confidence interval and p-value are based on independent samples t-test of the difference in mean days or mean rate of cumulative exposure between SER-155 and Placebo
SER-155 Strain Engraftment: Primary objective achieved - drug bacteria
strain engraftment was robust and as expected • The majority of SER-155 strains were present at start of HSCT conditioning and durable through chemotherapy exposure • Engraftment decreased but was detectable post- neutrophil recovery,
suggesting sustained engraftment, even under unfavorable GI conditions (e.g., antibiotic exposure), and through period of greatest BSI susceptibility • The second course of SER-155 was effective at increasing strain engraftment following
transplant & neutrophil recovery, with engraftment durable out to day 100 following transplant • Cohort 1 and Cohort 2 engraftment magnitude and kinetics had high congruence Seres Therapeutics, Inc. © 2024 20
Pathogen Domination: Prevalence in SER-155 Cohort 2 was substantially
lower relative to Historical Control Cohort • SER-155 was designed to reduce pathogen domination that has been associated with risk of BSIs 1 and other negative clinical outcomes 2 • In Cohort 2, the ability to detect pathogen domination
(i.e., relative abundance in the GI ≥30%) in the placebo arm, and differences between the study arms, was constrained due to the limited number of placebo stool samples and an imbalance in the number of available stool samples between the arms
• Observed pathogen domination events were low in the placebo and SER-155 arms with no significant differences observed • Pathogen domination was substantially lower in SER- 3 155 Cohort 2 compared to Historical Control Cohort 1 - Peled
et al, NEJM 2020; Stein-Thoeringer et al, Science 2019; Kusakabe et al, BBMT 2020 2 - Bacteria in the families: ESKAPE (Enterococcaceae, Enterobacteriaceae & Staphylococcaceae) & Streptococcaceae; domination defined as ≥30%
relative abundance in the GI Seres Therapeutics, Inc. © 2024 21 3 - Subjects that are sampled at similar time points as SER-155 Phase 1b subjects; microbiome data produced using same protocols as SER-155 Phase 1b subjects
Viral prophylaxis provides precedent in medically vulnerable patients
Prevymis - increasingly used for viral infection prophylaxis (e.g., allo-HSCT and • Overall cost of allo-HSCT is high solid organ transplant populations) (~$400K US year 1 allo-HSCT costs) • Transplant-related complications (e.g., $605M
‘23 infections) raise cost by ~$180K WW sales • Infections result in longer hospital stays, readmissions, increased ICU utilization • Reduces CMV infection in allo-HSCT recipients • Lowers mortality rate Sources: CMS.gov;
Broder et al, Am Drug Health Benefits 2017; Perales et al, Biol Seres Therapeutics, Inc. © 2024 22 Blood Marrow Transplant 2017; Merck SEC filings

SER-155 allo-HSCT commercial opportunity is meaningful Serious
bacterial infections are frequent, creating a strong medical ✓ rationale for prophylaxis to prevent infections and complications ~40K transplants per year worldwide ✓ Very high overall cost of allo-HSCT and related cost of ✓
complications supports financial rationale to treat Well-defined treatment centers could rapidly adopt as a new ✓ standard of care Sources: CIBMTR; HRSA; Passweg et al, BMT 2021; IQVIA; Broder et al, Am Drug Health Benefits Seres
Therapeutics, Inc. © 2024 23 2017; Perales et al, Biol Blood Marrow Transplant 2017
Accelerating SER-155 clinical development with positive Ph1b outcomes
Aim to accelerate SER-155 development in allo-HSCT • Potential to follow successful precedent from VOWST development Potential global SER-155 Engage with FDA on advancement of SER-155 allo- development and HSCT program commercialization in
• Seek Breakthrough Therapy designation broad range of • Pursue additional designations (Orphan Drug, Qualified Infectious Disease Product) indications in medically vulnerable populations Evaluate SER-155 in additional patient
populations with high risk of serious bacterial infections Seres Therapeutics, Inc. © 2024 24
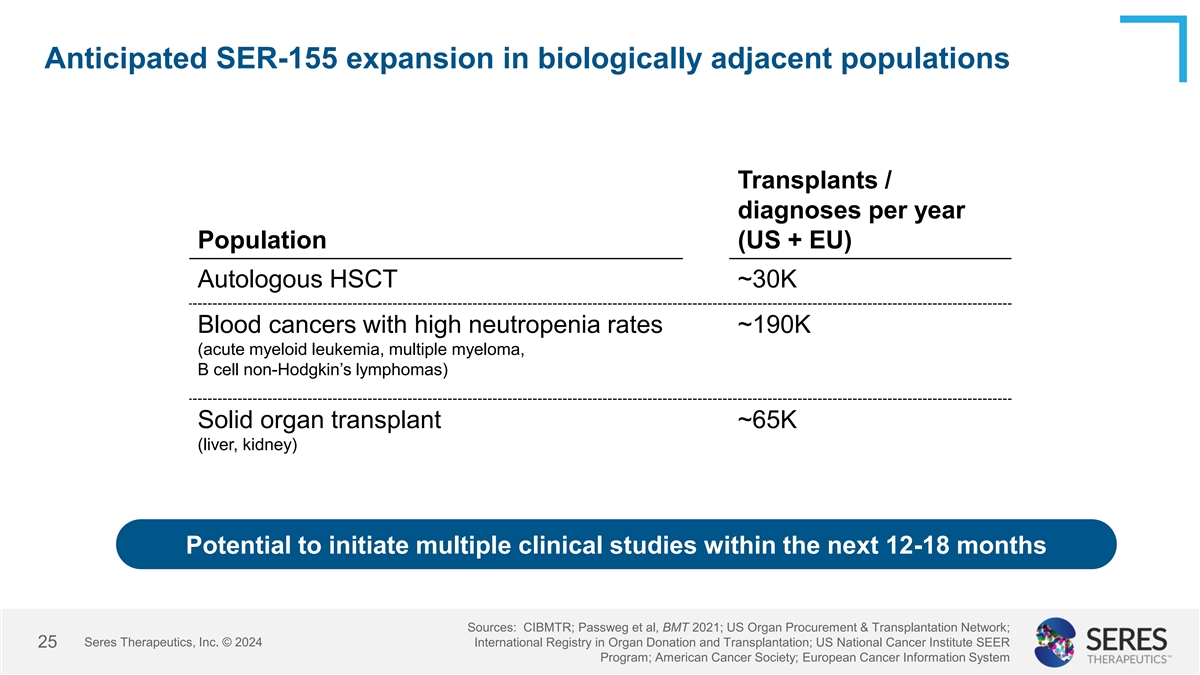
Anticipated SER-155 expansion in biologically adjacent populations
Transplants / diagnoses per year Population (US + EU) Autologous HSCT ~30K Blood cancers with high neutropenia rates ~190K (acute myeloid leukemia, multiple myeloma, B cell non-Hodgkin’s lymphomas) Solid organ transplant ~65K (liver, kidney)
Potential to initiate multiple clinical studies within the next 12-18 months Sources: CIBMTR; Passweg et al, BMT 2021; US Organ Procurement & Transplantation Network; Seres Therapeutics, Inc. © 2024 International Registry in Organ Donation
and Transplantation; US National Cancer Institute SEER 25 Program; American Cancer Society; European Cancer Information System
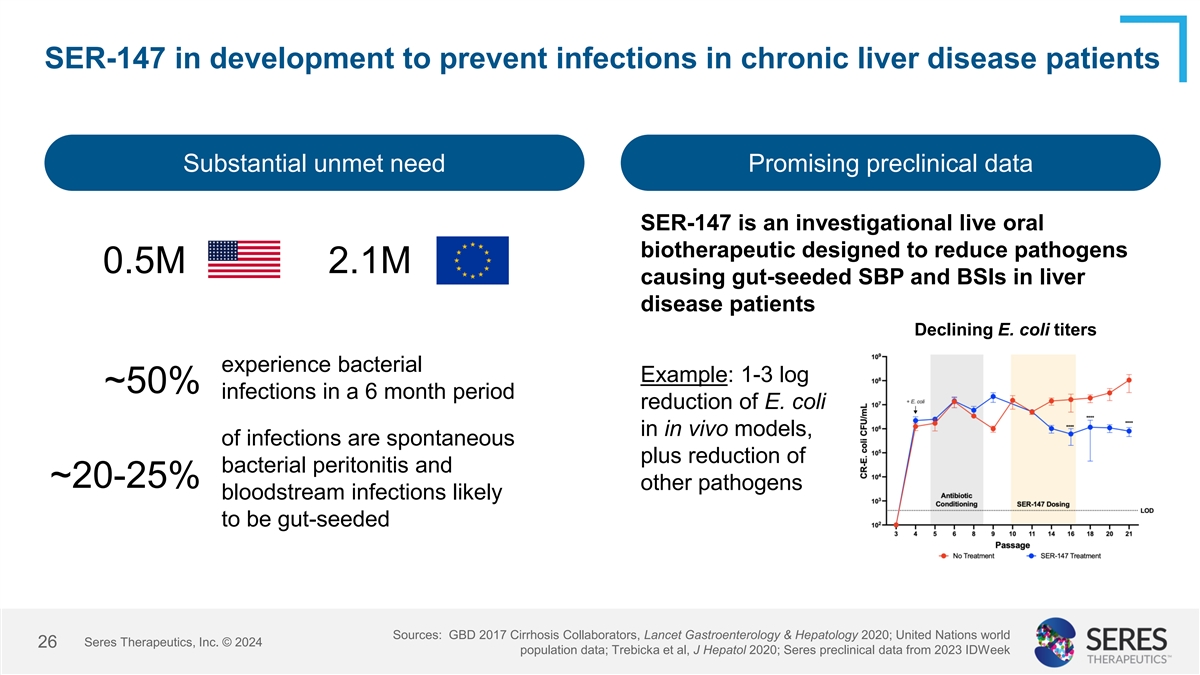
SER-147 in development to prevent infections in chronic liver disease
patients Substantial unmet need Promising preclinical data SER-147 is an investigational live oral biotherapeutic designed to reduce pathogens 0.5M 2.1M causing gut-seeded SBP and BSIs in liver disease patients Declining E. coli titers experience
bacterial Example: 1-3 log ~50% infections in a 6 month period reduction of E. coli in in vivo models, of infections are spontaneous plus reduction of bacterial peritonitis and ~20-25% other pathogens bloodstream infections likely to be gut-seeded
Sources: GBD 2017 Cirrhosis Collaborators, Lancet Gastroenterology & Hepatology 2020; United Nations world Seres Therapeutics, Inc. © 2024 26 population data; Trebicka et al, J Hepatol 2020; Seres preclinical data from 2023
IDWeek
End-to-end capabilities & expertise for discovery and development
of bacterial live biotherapeutics GI microbiome biomarker & drug Clinical translation target identification & patient subpopulation insights Proven discovery, Lead candidate design, Proprietary know-how on clinical screening, optimization,
& drug pharmacology, and trial design and execution pharmacology clinical capabilities Novel biotherapeutic GMP Regulatory expertise pioneering a manufacturing & quality novel biotherapeutic class Seres Therapeutics, Inc. © 2024
27
Manufacturing platform delivers defined consortia in oral formulation
using cost-effective production Strain isolation and characterization pipeline to rapidly identify cGMP- suitable medium components Highly intensive strain bioprocessing leveraging flexible, single-use manufacturing technology for cost-effective
production Novel formulations enabling consistent drug product composition, drug stability for distribution, and targeted drug delivery Quality systems to ensure product quality and stability, extending prior regulatory successes, including
developing product release specifications with the FDA Seres Therapeutics, Inc. © 2024 28
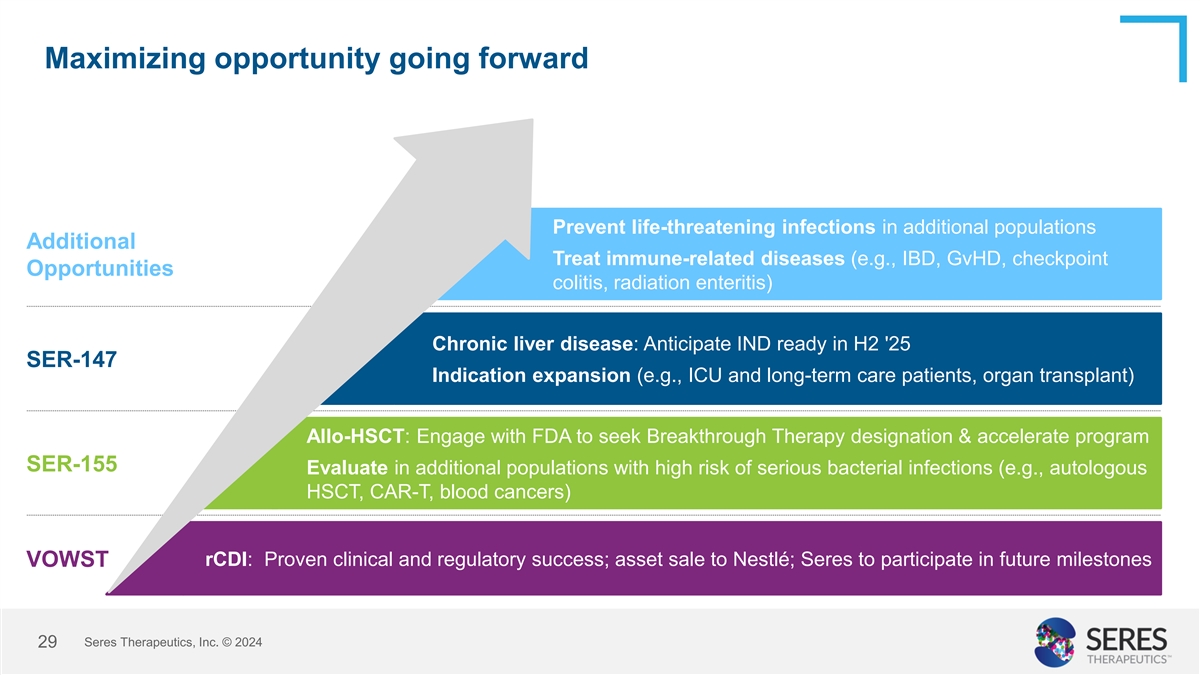
Maximizing opportunity going forward Prevent life-threatening
infections in additional populations Additional Treat immune-related diseases (e.g., IBD, GvHD, checkpoint Opportunities colitis, radiation enteritis) Chronic liver disease: Anticipate IND ready in H2 '25 SER-147 Indication expansion (e.g., ICU and
long-term care patients, organ transplant) Allo-HSCT: Engage with FDA to seek Breakthrough Therapy designation & accelerate program SER-155 Evaluate in additional populations with high risk of serious bacterial infections (e.g., autologous HSCT,
CAR-T, blood cancers) rCDI: Proven clinical and regulatory success; asset sale to Nestlé; Seres to participate in future milestones VOWST Seres Therapeutics, Inc. © 2024 29
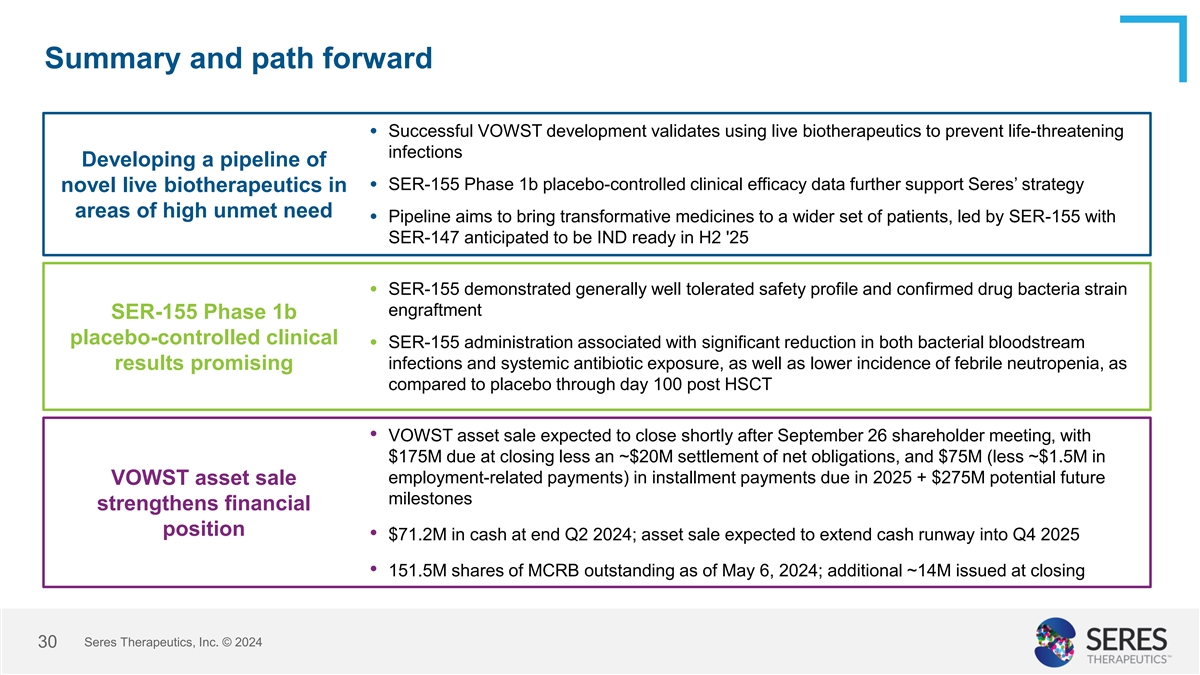
Summary and path forward Successful VOWST development validates using
live biotherapeutics to prevent life-threatening • infections Developing a pipeline of SER-155 Phase 1b placebo-controlled clinical efficacy data further support Seres’ strategy novel live biotherapeutics in • areas of high unmet
need Pipeline aims to bring transformative medicines to a wider set of patients, led by SER-155 with • SER-147 anticipated to be IND ready in H2 '25 SER-155 demonstrated generally well tolerated safety profile and confirmed drug bacteria
strain • engraftment SER-155 Phase 1b placebo-controlled clinical SER-155 administration associated with significant reduction in both bacterial bloodstream • infections and systemic antibiotic exposure, as well as lower incidence of
febrile neutropenia, as results promising compared to placebo through day 100 post HSCT • VOWST asset sale expected to close shortly after September 26 shareholder meeting, with $175M due at closing less an ~$20M settlement of net obligations,
and $75M (less ~$1.5M in employment-related payments) in installment payments due in 2025 + $275M potential future VOWST asset sale milestones strengthens financial position • $71.2M in cash at end Q2 2024; asset sale expected to extend cash
runway into Q4 2025 • 151.5M shares of MCRB outstanding as of May 6, 2024; additional ~14M issued at closing Seres Therapeutics, Inc. © 2024 30
v3.24.2.u1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Seres Therapeutics (NASDAQ:MCRB)
Historical Stock Chart
From Feb 2025 to Mar 2025
Seres Therapeutics (NASDAQ:MCRB)
Historical Stock Chart
From Mar 2024 to Mar 2025