false000102914200010291422024-01-082024-01-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): January 08, 2024 |
Dynavax Technologies Corporation
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-34207 |
33-0728374 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
2100 Powell Street, Suite 720 |
|
Emeryville, California |
|
94608 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: 510 848-5100 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, $0.001 par value |
|
DVAX |
|
Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On January 8, 2024, Dynavax Technologies Corporation ("Dynavax") issued a press release announcing its preliminary unaudited fourth quarter and full year 2023 financial highlights. A copy of the press release is attached as Exhibit 99.1 to this Current Report on Form 8-K, and is incorporated herein by reference.
The preliminary selected financial results included in the press release are based upon estimates and information available as of the date of the press release. Accordingly, undue reliance should not be placed on these preliminary estimates. In addition, the Company has not yet completed its financial close process for the quarter and year ended December 31, 2023, therefore the estimates included in the press release regarding net product revenue and cash and cash equivalents, and marketable securities are preliminary, unaudited and are subject to change upon completion of the Company’s financial statement closing procedures and the audit of the Company’s consolidated financial statements.
Item 7.01 Regulation FD Disclosure.
The Company has posted a presentation (the “Presentation”) to its website at www.dynavax.com, in the “Events & Presentations” subsection of the “News & Events” tab. A copy of the Presentation is attached as Exhibit 99.2 to this current report and is incorporated herein by reference.
All of the information furnished in this Form 8-K, including the accompanying Exhibits 99.1 and 99.2, shall not be deemed "filed" for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this current report and in the accompanying exhibit shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by Dynavax, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits. The following exhibit is furnished herewith:
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
Dynavax Technologies Corporation |
|
|
|
|
Date: |
January 8, 2024 |
By: |
/s/ Kelly MacDonald |
|
|
|
Kelly MacDonald
Senior Vice President, CFO |

Dynavax Announces Preliminary Unaudited Fourth Quarter and Full Year 2023 Financial Highlights
•Preliminary full year 2023 HEPLISAV-B® vaccine net product revenue of approximately $213 million, a 69% year-over-year increase
•Significant gains in HEPLISAV-B market share in key market segments, with total U.S. market share increasing to approximately 44% compared to approximately 35% at the end of 2022
•Strengthened financial position with cash, cash equivalents and marketable securities at year end increasing to approximately $742 million; expects to be cash flow positive for full year 2024
EMERYVILLE, CA – January 8, 2024 – Dynavax Technologies Corporation (Nasdaq: DVAX), a commercial-stage biopharmaceutical company developing and commercializing innovative vaccines, today announced preliminary, unaudited financial highlights for the fourth quarter and full year ended December 31, 2023.
“In 2023, we delivered a record year of revenue for HEPLISAV-B, driven by the expansion of the adult hepatitis B vaccine market in the U.S., and our team’s progress toward establishing HEPLISAV-B as the market leading vaccine. We are extremely pleased with our market share growth in the fourth quarter, which enabled us to achieve our increased product revenue guidance for the year despite the impact of expected seasonality due to increased focus on respiratory disease vaccines during the fall and winter seasons. We believe the seasonal market decline for adult hepatitis B vaccines will be limited to the fourth quarter in line with the administration of the vast majority of influenza and COVID-19 vaccines,” said Ryan Spencer, Chief Executive Officer of Dynavax. “Turning to this year, we believe HEPLISAV-B is well-positioned entering 2024, supported by significant market share gains in the total market and in key market segments. We remain extremely confident in the long-term growth of the hepatitis B market, with HEPLISAV-B expected to achieve a majority market share in the U.S. In addition to HEPLISAV-B, we continue to advance our pipeline of innovative vaccine candidates and continue to pursue strategic opportunities to accelerate our growth.”
Preliminary Fourth Quarter and Full Year 2023 Financial and Commercial Highlights
•Preliminary HEPLISAV-B vaccine net product revenue for the fourth quarter and full year 2023 were approximately $51 million and $213 million, respectively, representing year-over-year growth of approximately 46% and 69% compared to the fourth quarter and full year 2022.
•HEPLISAV-B total market share in the U.S. increased to approximately 44%, compared to approximately 35% at the end of 2022.
•HEPLISAV-B market share in the retail pharmacy segment increased to approximately 60%, compared to approximately 42% at the end of 2022. HEPLISAV-B market share in the Integrated Delivery Networks (IDNs) and Large Clinics segment increased to approximately 58%, compared to approximately 47% at the end of 2022.
•Cash, cash equivalents and marketable securities were approximately $742 million as of December 31, 2023.
The preliminary selected financial results contained herein are unaudited, subject to adjustment, and provided as an estimate in advance of the Company’s announcement of complete financial results, for the three and twelve months ended December 31, 2023. Market share data are preliminary and are as of the latest market data available on December 22, 2023.
Expected Commercial and Pipeline Milestones
HEPLISAV-B® [Hepatitis B Vaccine (Recombinant), Adjuvanted]
HEPLISAV-B vaccine is the first and only adult hepatitis B vaccine approved in the U.S., the European Union and Great Britain that enables series completion with only two doses in one month. Hepatitis B vaccination is universally recommended for adults aged 19-59 in the U.S.

•Driven by the Centers for Disease Control and Prevention's Advisory Committee of Immunization Practices (ACIP) universal recommendation for adult hepatitis B vaccination, Dynavax continues to expect the adult hepatitis B vaccine market in the U.S. to expand at an annual growth rate of approximately 10 - 15% over the next several years to a total market of approximately $800 million by 2027, one of the largest adult vaccine markets in the U.S., with HEPLISAV-B well-positioned to achieve a majority market share.
•A supplemental Biologic License Application (sBLA) for HEPLISAV-B vaccination of adults on hemodialysis is currently under priority review by the U.S. Food and Drug Administration (FDA) with a Prescription Drug User Fee Act (PDUFA) action date planned for May 13, 2024.
Clinical Pipeline
Dynavax is advancing a pipeline of differentiated product candidates that leverage its CpG 1018® adjuvant, which has demonstrated its ability to enhance the immune response with a favorable tolerability profile in a wide range of clinical trials and real-world commercial use.
Shingles vaccine program:
Z-1018 is an investigational vaccine candidate being developed for the prevention of shingles in adults aged 50 and older.
•Dynavax expects to submit an Investigational New Drug Application (IND) to the FDA to support initiation of a Phase 1/2 trial of Z-1018 in the first half of 2024.
Tdap vaccine program:
Tdap-1018 is an investigational vaccine candidate intended for active booster immunization against tetanus, diphtheria, and pertussis (Tdap).
•Dynavax plans to submit an IND to the FDA to support the initiation of a Phase 2 human challenge study of Tdap-1018 in the second half of 2024, upon completion of the independent study conducted by the Canadian Center for Virology to establish the human challenge dose.
Plague vaccine program:
Dynavax is developing a plague (rF1V) vaccine candidate adjuvanted with CpG 1018® currently in a Phase 2 clinical trial in collaboration with, and fully funded by, the U.S. Department of Defense.
•Dynavax anticipates top line data for the randomized, active-controlled Phase 2 clinical trial evaluating immunogenicity, safety, and tolerability of the plague vaccine candidate in 2024.
J.P. Morgan Healthcare Conference Presentation Webcast Details
Dynavax will present at the 42nd Annual J.P. Morgan Healthcare Conference on Thursday, January 11 at 11:15 a.m. PT.
The presentation will be webcast and may be accessed through the “Events & Presentations” page on the “Investors” section of the Company’s website at https://investors.dynavax.com/events-presentations.
Important U.S. Product Information
HEPLISAV-B is indicated for the prevention of infection caused by all known subtypes of hepatitis B virus in adults aged 18 years and older.
For full U.S. Prescribing Information for HEPLISAV-B, please visit the following website at https://www.heplisavbhcp.com, and click the “Prescribing Information” link in the “Important Safety Information” section.
Important U.S. Safety Information (ISI)
Do not administer HEPLISAV-B to individuals with a history of a severe allergic reaction (e.g., anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of HEPLISAV-B, including yeast.

Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of HEPLISAV-B.
Immunocompromised persons, including individuals receiving immunosuppressant therapy, may have a diminished immune response to HEPLISAV-B.
Hepatitis B has a long incubation period. HEPLISAV-B may not prevent hepatitis B infection in individuals who have an unrecognized hepatitis B infection at the time of vaccine administration.
The most common patient-reported adverse reactions reported within 7 days of vaccination were injection site pain (23% to 39%), fatigue (11% to 17%), and headache (8% to 17%).
About Dynavax
Dynavax is a commercial-stage biopharmaceutical company developing and commercializing innovative vaccines to help protect the world against infectious diseases. The Company has two commercial products, HEPLISAV-B® vaccine [Hepatitis B Vaccine (Recombinant), Adjuvanted], which is approved in the U.S., the European Union and Great Britain for the prevention of infection caused by all known subtypes of hepatitis B virus in adults 18 years of age and older, and CpG 1018® adjuvant, currently used in multiple adjuvanted COVID-19 vaccines. Dynavax is advancing CpG 1018 adjuvant as a premier vaccine adjuvant with adjuvanted vaccine clinical programs for shingles and Tdap, and through global collaborations, currently focused on adjuvanted vaccines for COVID-19, plague, seasonal influenza and universal influenza. For more information about our marketed products and development pipeline, visit www.dynavax.com.
Forward-Looking Statements
This press release contains "forward-looking" statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which are subject to a number of risks and uncertainties. All statements that are not historical facts are forward-looking statements. Forward-looking statements can generally be identified by the use of words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “intend,” “will,” “may,” “plan,” “project,” “potential,” “seek,” “should,” “think,” “toward,” “will,” “would” and similar expressions, or the negatives thereof, or they may use future dates. Forward-looking statements made in this document include statements regarding our expected financial results and market share as of and for the year and quarter ended December 31, 2023, expectations regarding future growth and market share, and the timing of IND filings, initiation and completion of clinical studies, the publication of results, and interaction with regulators. Actual results may differ materially from those set forth in this press release due to the risks and uncertainties inherent in our business, including, the risk that actual demand for our products may differ from our expectations, risks relating to our ability to commercialize and supply HEPLISAV-B, risks related to the timing of completion and results of current clinical studies, risks related to the development and pre-clinical and clinical testing of vaccines containing CpG 1018 adjuvant, as well as other risks detailed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2023 and periodic filings made thereafter, as well as discussions of potential risks, uncertainties and other important factors in our other filings with the U.S. Securities and Exchange Commission. These forward-looking statements are made as of the date hereof, are qualified in their entirety by this cautionary statement and we undertake no obligation to revise or update information herein to reflect events or circumstances in the future, even if new information becomes available. Information on Dynavax's website at www.dynavax.com is not incorporated by reference in our current periodic reports with the SEC.
For Investors/Media:
Paul Cox
pcox@dynavax.com
510-665-0499
Nicole Arndt
narndt@dynavax.com
510-665-7264

Developing and �Commercializing �Innovative Vaccines 42nd Annual J.P. Morgan Healthcare Conference January 2024 Nasdaq: DVAX

Forward-Looking Statements Statements contained in this presentation regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including statements about Dynavax's expected financial results and market share as of and for the year and quarter ended December 31, 2023, expectations regarding future growth and market shares, expectations for vaccine markets, the company's strategic priorities, and expectations regarding the timing of IND filings, initiation and completion of clinical studies, publication of results and interaction with regulators. These forward-looking statements are based upon management’s current expectations, are subject to known and unknown risks and uncertainties, and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, including, without limitation; risks related to Dynavax’s ability to successfully commercialize and supply HEPLISAV-B and grow market share, which among other things will require Dynavax to successfully negotiate and enter into contracts with wholesalers, distributors, group purchasing organizations, and other parties, and maintain those contractual relationships, maintain and build its commercial infrastructure, and access prescribers and other key health care providers to discuss HEPLISAV-B; risks related to market adoption and competing products; risks related to whether payors will cover and provide timely and adequate reimbursement for HEPLISAV-B; risks related to the completion, timing of completion and results of our clinical studies; and risks associated with the development, pre-clinical and clinical testing, and commercialization of vaccines in the U.S. and outside the U.S., including vaccines for COVID-19, shingles, plague and pertussis. These and other risks and uncertainties are described in Dynavax’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, or any subsequent periodic filing made by us, under the heading “Risk Factors”. Dynavax undertakes no obligation to revise or update information herein to reflect events or circumstances in the future, even if new information becomes available. © Copyright DYNAVAX 2024

Versatile proprietary adjuvant technology Commercial vaccine with continued growth potential and significant addressable market Differentiated vaccine development pipeline targeting large indications with unmet need Fully-integrated infrastructure supporting U.S. commercialization & global manufacturing Strong financial profile Dynavax �at a Glance A commercial-stage biopharmaceutical company committed to developing and commercializing novel vaccines to help protect the world against infectious diseases by utilizing proven, innovative adjuvant technology. © Copyright DYNAVAX 2024
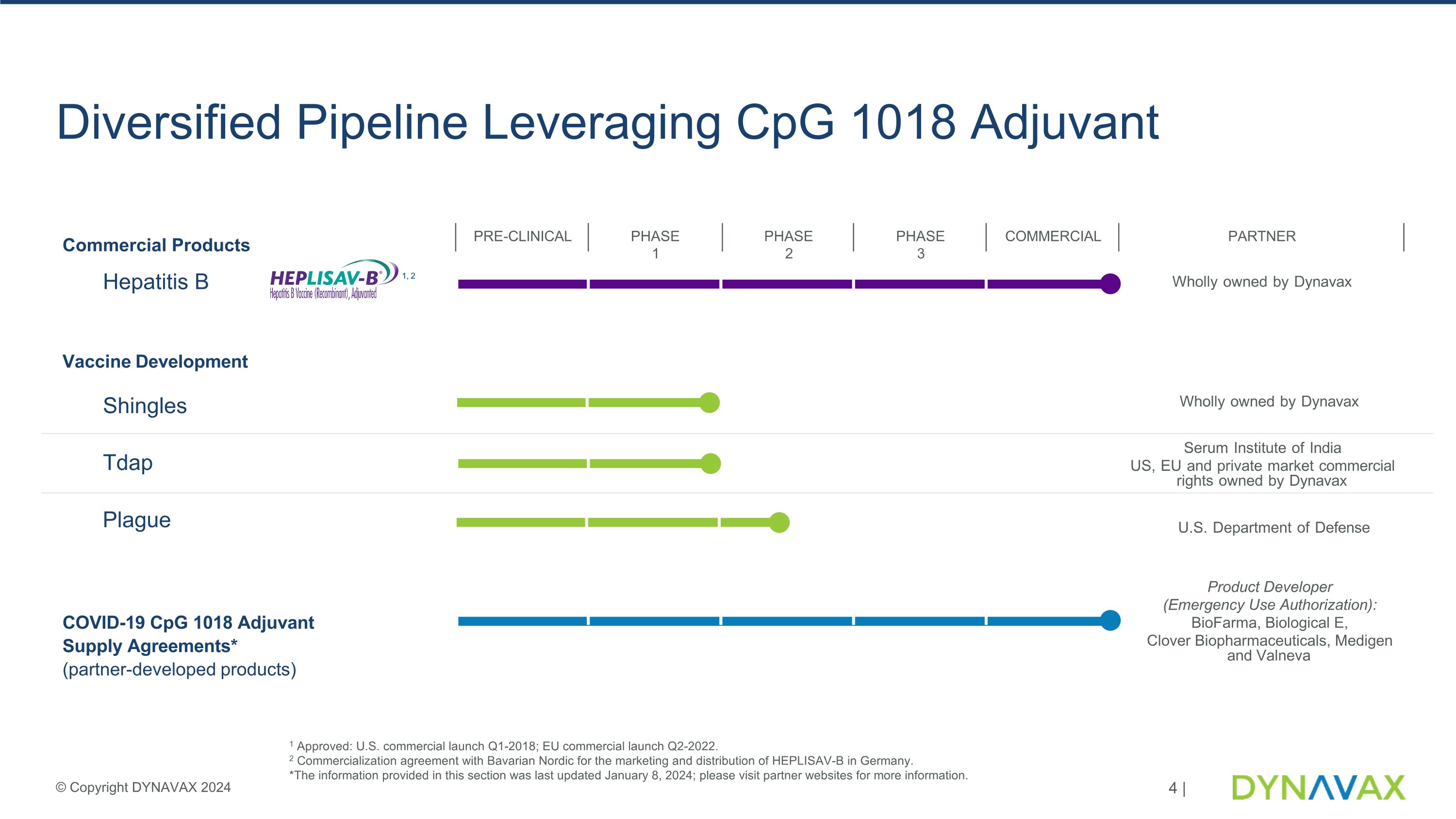
Diversified Pipeline Leveraging CpG 1018 Adjuvant U.S. Department of Defense Wholly owned by Dynavax Serum Institute of India US, EU and private market commercial rights owned by Dynavax Wholly owned by Dynavax Commercial Products Hepatitis B PRE-CLINICAL PHASE 1 PHASE 2 PHASE 3 COMMERCIAL PARTNER 1, 2 Vaccine Development Shingles COVID-19 CpG 1018 Adjuvant Supply Agreements* (partner-developed products) Plague Tdap 1 Approved: U.S. commercial launch Q1-2018; EU commercial launch Q2-2022.�2 Commercialization agreement with Bavarian Nordic for the marketing and distribution of HEPLISAV-B in Germany.�*The information provided in this section was last updated January 8, 2024; please visit partner websites for more information. Product Developer (Emergency Use Authorization): BioFarma, Biological E, Clover Biopharmaceuticals, Medigen and Valneva © Copyright DYNAVAX 2024

Dynavax Core Strategic Priorities Advance Differentiated Vaccine Pipeline Identify Strategic Opportunities to Accelerate Growth Increase market share to become the market leader by 2027 Maximize total addressable market based on the ACIP Universal Recommendation Leverage foundational commercial asset to support company growth and pipeline development Deliver on our innovative and diversified pipeline leveraging CpG 1018® adjuvant with proven antigens Build adult vaccine portfolio of �best-in-class products Advance innovative pre-clinical and discovery efforts leveraging collaborations Continue disciplined allocation of capital aligned with corporate strategy to deliver long-term value through internal and external innovation Prioritize external opportunities with high synergy assets in vaccines, or other modalities in infectious diseases, to further leverage our expertise and capabilities © Copyright DYNAVAX 2024 Drive Growth in

Executing on Our Strategy: Q4 and FY 2023 Preliminary Unaudited Financial & Business Highlights Preliminary Q4 and FY 2023 Financial Results HEPLISAV-B sBLA in Hemodialysis: sBLA under review by FDA with PDUFA action date expected in May 2024. Shingles Program: Received Type B meeting feedback from the U.S. FDA on clinical development plan and expect to submit IND to support the initiation of a Phase 1/2 trial. Tdap Program: Plan to submit an IND to the U.S. FDA to support initiation of a Phase 2 human challenge study. Plague Program: Executed contract modification to support advancement into a nonhuman primate challenge study, which was initiated in August. Pipeline Advancement HEPLISAV-B®: Continued Net Revenue Growth ~$51 M in Q4 ’23 net product revenue Increased ~46% year-over-year ~$213 M in FY 23 net product revenue Increased ~69% year-over-year HEPLISAV-B: Significant Market Share Capture ~44% in total market share compared to ~35% at end of Q4 ‘22 ~60% in retail segment share compared to ~42% at end of Q4 ‘22 ~58% in IDN/Large Clinics segment share compared to ~47% at end of Q4 ‘22 Strengthened Financial Profile ~$742 M in cash, cash equivalents and marketable securities as of December 31, 2023 Compared to $624 M at end of FY 2022 © Copyright DYNAVAX 2024 *Preliminary selected financial results contained herein are unaudited, subject to adjustment, and provided as an estimate in advance of the Company’s announcement of complete financial results for the three and twelve months ended December 31, 2023. These estimates represent management's expectations as of January 8, 2024.

Commercial Product © Copyright DYNAVAX 2024
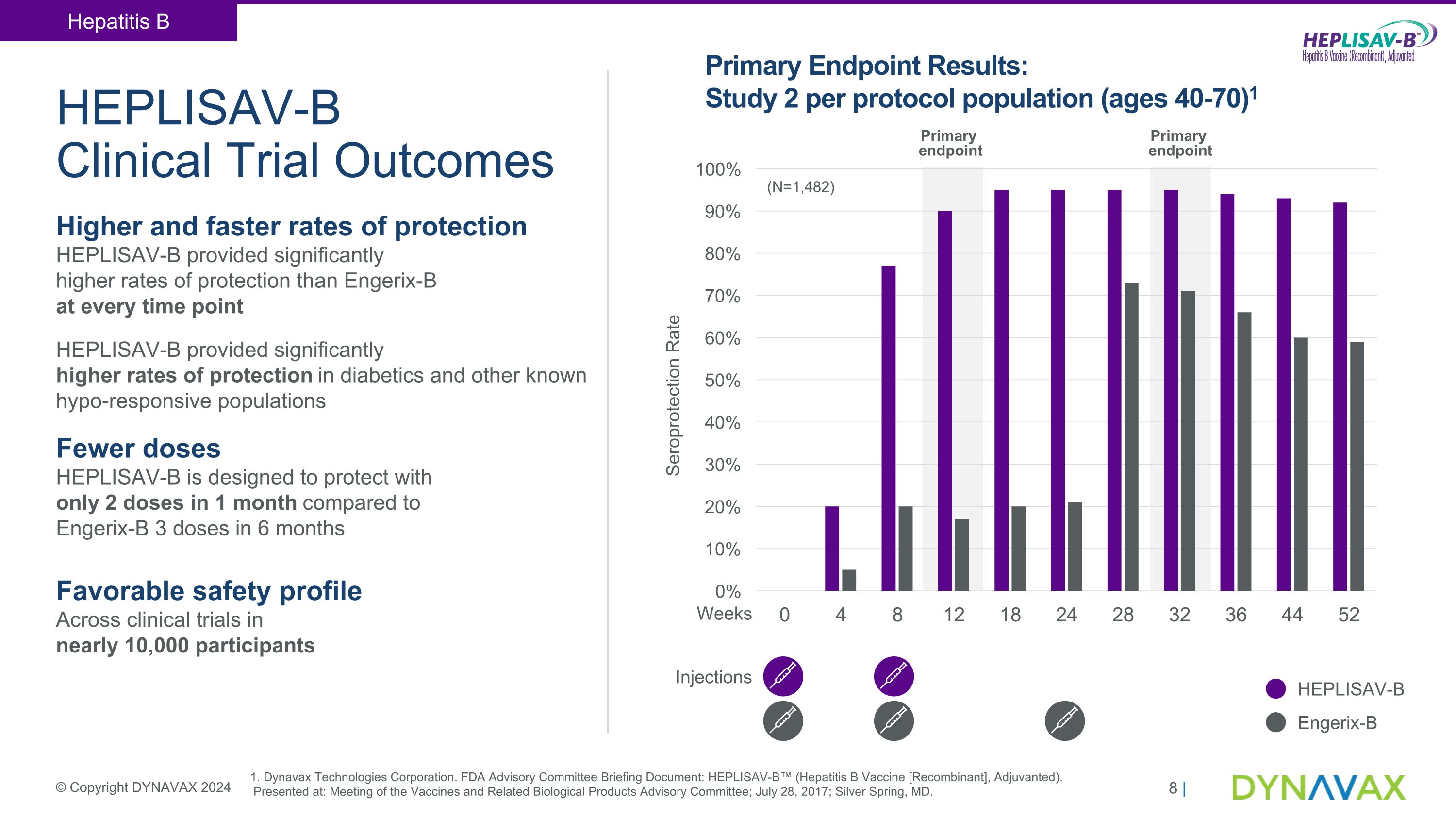
HEPLISAV-B �Clinical Trial Outcomes Higher and faster rates of protection HEPLISAV-B provided significantly �higher rates of protection than Engerix-B �at every time point HEPLISAV-B provided significantly �higher rates of protection in diabetics and other known hypo-responsive populations Fewer doses �HEPLISAV-B is designed to protect with �only 2 doses in 1 month compared to �Engerix-B 3 doses in 6 months Favorable safety profile �Across clinical trials in �nearly 10,000 participants Weeks Primary Endpoint Results: Study 2 per protocol population (ages 40-70)1 Injections Primary �endpoint Primary �endpoint (N=1,482) HEPLISAV-B Engerix-B 1. Dynavax Technologies Corporation. FDA Advisory Committee Briefing Document: HEPLISAV-B™ (Hepatitis B Vaccine [Recombinant], Adjuvanted).� Presented at: Meeting of the Vaccines and Related Biological Products Advisory Committee; July 28, 2017; Silver Spring, MD. Hepatitis B © Copyright DYNAVAX 2024 Seroprotection Rate

There is No Cure �for Hepatitis B - �Prevention is Essential Hepatitis B is an incurable liver infection caused by the hepatitis B virus transmitted by bodily fluids. When the virus attacks the liver, the resulting health complications can be lifelong or even deadly. 1 out of 3 people have been infected with hepatitis B (2 billion people) ~1.5 million people become newly infected each year ~300 million more infectious than HIV ~80% of people are unaware of their infection, increasing risk of unknowingly spreading it to others 30-59 years age range where new infections are highest 7 days virus can survive outside the body on surfaces people living with hepatitis B Globally1 100x Hepatitis B is Hepatitis B 1. Source: https://www.hepb.org/what-is-hepatitis-b/what-is-hepb/facts-and-figures/, https://doi.org/10.1007%2Fs13337-015-0247-y © Copyright DYNAVAX 2024
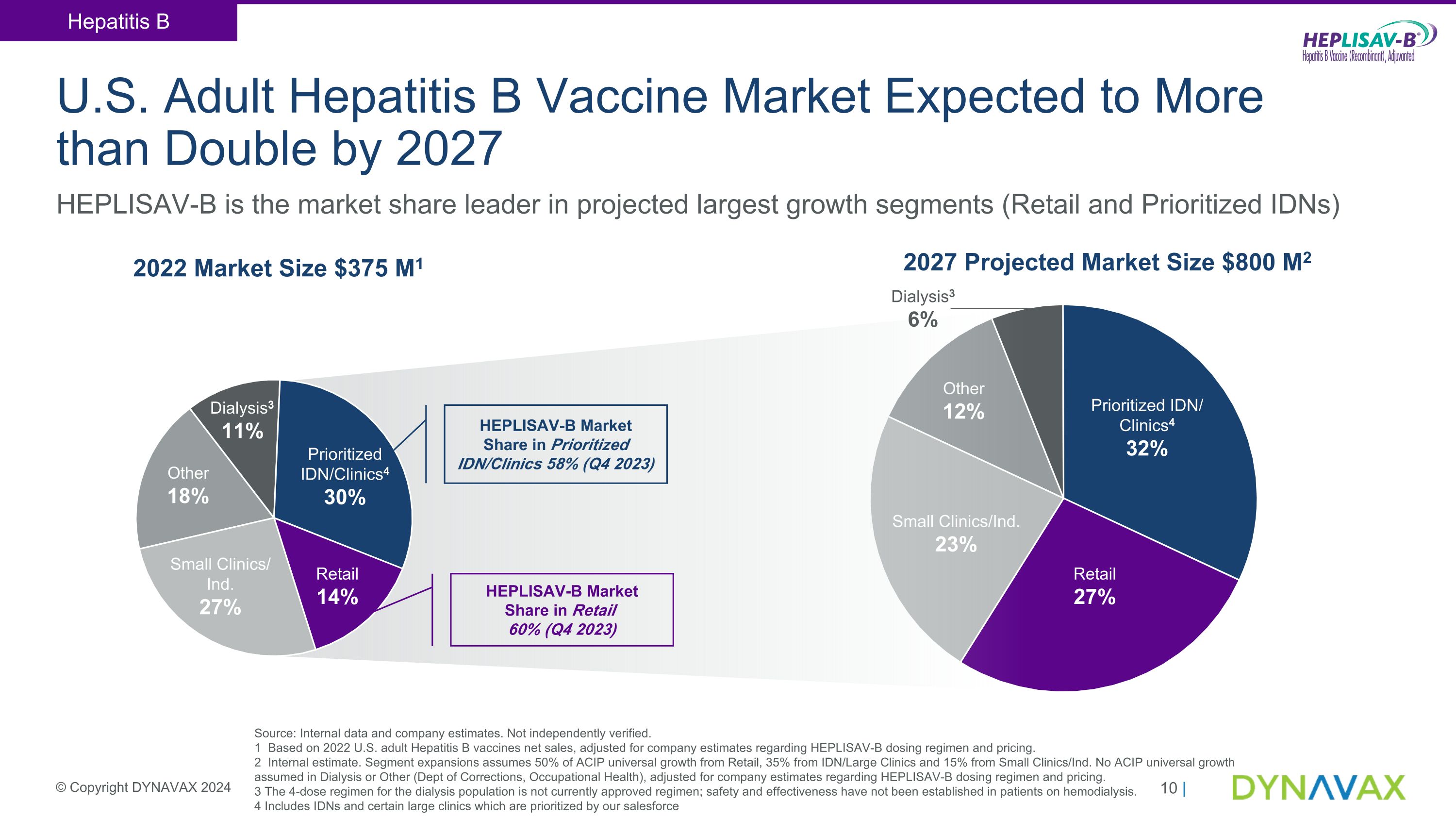
U.S. Adult Hepatitis B Vaccine Market Expected to More than Double by 2027 © Copyright DYNAVAX 2024 HEPLISAV-B is the market share leader in projected largest growth segments (Retail and Prioritized IDNs) 2027 Projected Market Size $800 M2 Prioritized IDN/Clinics4 30% Small Clinics/�Ind. 27% Prioritized IDN/ �Clinics4 32% Retail 14% Retail 27% Other 18% Dialysis3 11% 2022 Market Size $375 M1 Small Clinics/Ind. 23% Other 12% Dialysis3 6% Hepatitis B Source: Internal data and company estimates. Not independently verified. 1 Based on 2022 U.S. adult Hepatitis B vaccines net sales, adjusted for company estimates regarding HEPLISAV-B dosing regimen and pricing. 2 Internal estimate. Segment expansions assumes 50% of ACIP universal growth from Retail, 35% from IDN/Large Clinics and 15% from Small Clinics/Ind. No ACIP universal growth assumed in Dialysis or Other (Dept of Corrections, Occupational Health), adjusted for company estimates regarding HEPLISAV-B dosing regimen and pricing. 3 The 4-dose regimen for the dialysis population is not currently approved regimen; safety and effectiveness have not been established in patients on hemodialysis. 4 Includes IDNs and certain large clinics which are prioritized by our salesforce HEPLISAV-B Market Share in Prioritized IDN/Clinics 58% (Q4 2023) HEPLISAV-B Market Share in Retail 60% (Q4 2023)
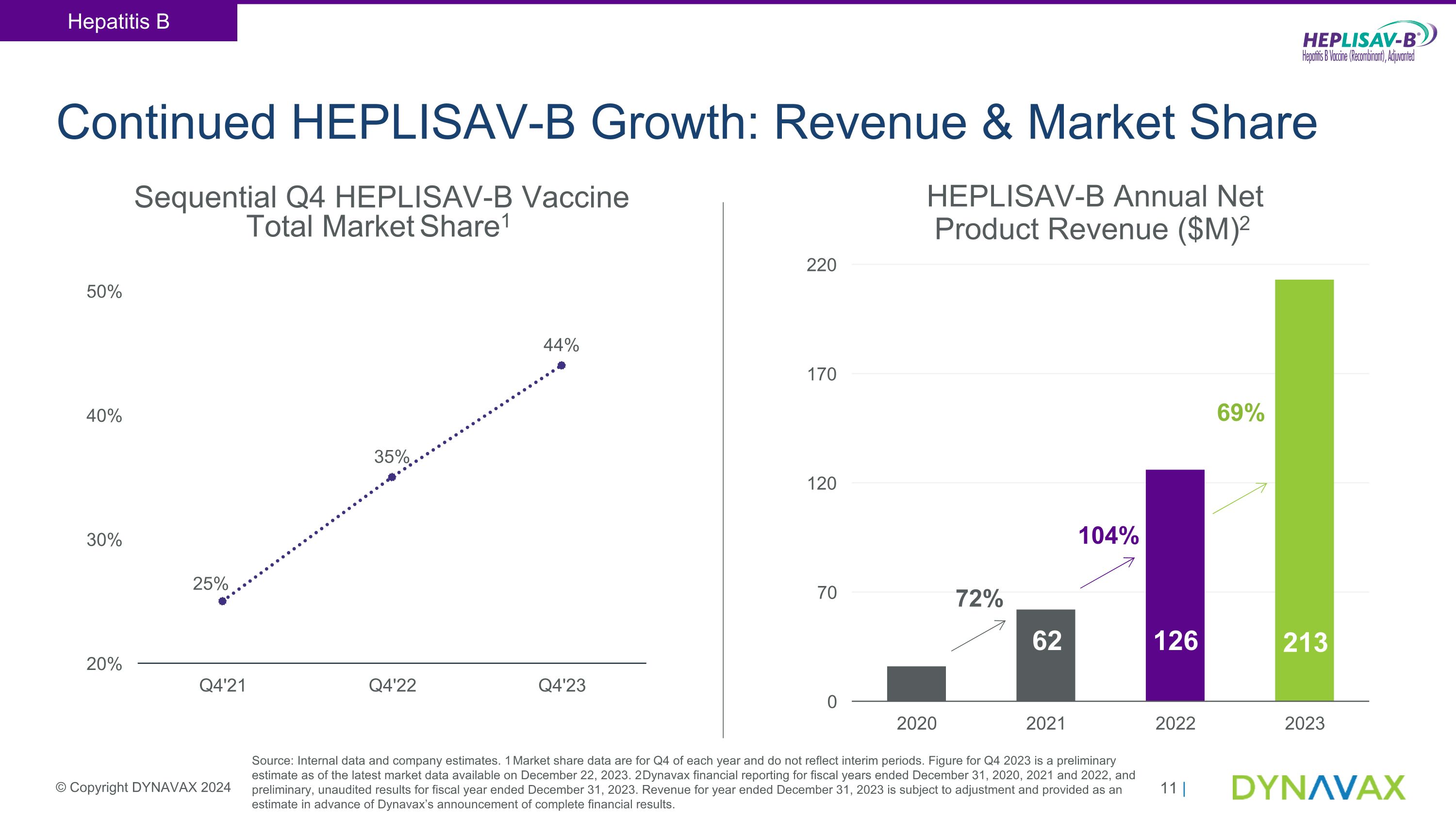
Continued HEPLISAV-B Growth: Revenue & Market Share Sequential Q4 HEPLISAV-B Vaccine Total Market Share1 Source: Internal data and company estimates. 1 Market share data are for Q4 of each year and do not reflect interim periods. Figure for Q4 2023 is a preliminary estimate as of the latest market data available on December 22, 2023. 2 Dynavax financial reporting for fiscal years ended December 31, 2020, 2021 and 2022, and preliminary, unaudited results for fiscal year ended December 31, 2023. Revenue for year ended December 31, 2023 is subject to adjustment and provided as an estimate in advance of Dynavax’s announcement of complete financial results. Hepatitis B © Copyright DYNAVAX 2024 HEPLISAV-B Annual Net Product Revenue ($M)2 36 62 126 104% 72% 69% 213

Vaccine Development Herpes Zoster (Shingles) | Tetanus, Diphtheria, and Pertussis (Tdap) | Plague © Copyright DYNAVAX 2024

Shingles Program: New Options Needed �Current Market-Leading Vaccine Associated with Adverse Events1 Herpes Zoster (shingles) is an extremely painful consequence of the reactivation of a latent varicella-zoster virus (VZV), the same virus that causes varicella (chickenpox). Shingles 1 Package Insert - SHINGRIX (fda.gov) 2 Based on annual Shingrix sales Global market size: ~$3.5B in 20222 Program Status: Recent Updates: Phase 1 study results presented at the 2023 ACVR meeting in June 2023. Dynavax recently received Type B meeting feedback from the FDA on the Z-1018 clinical development plan. Upcoming Milestones: Plans to submit an IND to the U.S. FDA to support the initiation of a Phase 1/2 trial of Z-1018 in the first half of 2024. In the U.S.: Herpes zoster rates are increasing among adults in the U.S., especially among younger adults. Opportunity: Utilizing CpG 1018 adjuvant in a shingles vaccine may improve vaccine tolerability while maintaining comparable efficacy due to its ability to generate high levels of CD4+ T cell responses, which is key in controlling reactivation of the zoster virus and preventing shingles © Copyright DYNAVAX 2024
Tdap Vaccine Program (tetanus, diphtheria, and pertussis)�Intended for booster immunization against Tdap Since 1991, when acellular pertussis vaccines replaced whole-cell vaccines, whooping cough cases have increased by 85% due to: Tdap Global market size: ~$1.2B in 20224 Program Status: Recent Updates: Pertussis challenge study in nonhuman primates (NHP) demonstrated protection from disease and robust Type 1 T helper (Th1) cell responses upon challenge in NHPs vaccinated with Tdap-1018. Dynavax recently received Type B meeting feedback from the FDA on the Tdap-1018 clinical development plan. Upcoming Milestones: Plans to submit an IND to the U.S. FDA to support initiation of a Phase 2 human challenge study in 2G 2024, upon completion of the independent study conducted by the Canadian Center for Virology to establish the human challenge dose. In the U.S.: Tetanus and diphtheria are rare, but pertussis continues to spread.3 Opportunity: Utilizing CpG 1018 adjuvant is expected to improve the durability of protection against pertussis by redirecting T cell responses and enhancing protective antibody responses in a booster vaccine. Sources: 1 Updated as of January 2023 (data through 2019), Centers for Disease Control and Prevention (https://www.cdc.gov/pertussis/surv-reporting/cases-by-year.html) 2 https://www.cdc.gov/vaccines/vd/dtap-tdap-td/public/index.html 3 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4482312 4 Based on global 2022 sales of Boostrix and Adacel Waning efficacy: Effectiveness decreases 40-60% four years post vaccination1 Asymptomatic transmission: current acellular vaccines do not prevent asymptomatic infection or transmission2 © Copyright DYNAVAX 2024

Program Status: Recent Updates: Contract modification with U.S. DoD to support advancement into NHP challenge study, agreement now totaling $33.7 million through 2025. NHP challenge study was initiated in August 2023. Upcoming Milestones: Dosing has been completed in Part 2 of the Phase 2, with top line data expected in 2024. Plague Plague Vaccine Program�Phase 2 program conducted in collaboration with, and funded by, the U.S. DoD Government agencies research and stockpile medical countermeasures – biologics, drugs, devices – which may be used in the event of a potential public health emergency stemming from a biological attack or a naturally occurring emerging disease. Opportunity: We believe incorporating CpG 1018 adjuvant with rF1V plague vaccine will improve the durability of protection with fewer doses administered over a shorter time period. In the U.S.: There is no approved vaccine in the U.S. © Copyright DYNAVAX 2024

Delivering on Dynavax’s Value Proposition © Copyright DYNAVAX 2024 Building on Key �Recent Accomplishments 2024 Expectations HEPLISAV-B: net product revenue of $213 M in 2023 (69% Y/Y growth) Shingles and Tdap programs: data and regulatory feedback support continued development Plague program: expanded contract with U.S. Department of Defense Strong capital position of ~$742 M in cash, cash equivalents and marketable securities at Q4’23 end HEPLISAV-B continued revenue growth, and expansion of U.S. hepatitis B vaccine market share Advance innovative vaccine pipeline, including regulatory and clinical activities across pipeline programs Expects positive cash flow for FY 2024 Identify and pursue strategic opportunities to accelerate growth *Preliminary selected financial results contained herein are unaudited, subject to adjustment, and provided as an estimate in advance of the Company’s announcement of complete financial results as of December 31, 2023. This estimate represents management's expectations as of January 8, 2024.
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Dynavax Technologies (NASDAQ:DVAX)
Historical Stock Chart
From Mar 2024 to Apr 2024
Dynavax Technologies (NASDAQ:DVAX)
Historical Stock Chart
From Apr 2023 to Apr 2024