ADMA Biologics Gets FDA Approval for Aseptic Fill-Finish Machine
September 08 2021 - 7:50AM
Dow Jones News
By Chris Wack
ADMA Biologics Inc. said the U.S. Food and Drug Administration
has granted approval for its in-house aseptic fill-finish machine,
the VanRx SA25.
The biopharmaceutical company said that with the VanRx
operational, it is anticipating meaningfully improved gross
margins, enhanced patient-supply consistency, accelerated
inventory-production-cycle times, and increased control and
visibility of commercial-product-lot releases, creating more
predictable near-term revenue results.
The VanRx fill-finish machine uses a closed isolator design,
allowing for the removal of human interventions and providing safe
drug products for patients. The VanRx machine has the capability of
rapidly switching between different container and closure formats,
enabling aseptic filling in a variety of different fill volumes and
presentation sizes.
ADMA said it will continue to work with its third-party contract
manufacturing organization fill-finish partner who will continue to
fill a portion of ADMA's production at their site. The CMO's site
would remain in ADMA's FDA-approved product Biologics License
Applications to provide the company with alternatives to ensure
continued supply-chain robustness.
ADMA Biologics shares were up 20%, to $1.52, in premarket
trading.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
September 08, 2021 07:35 ET (11:35 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
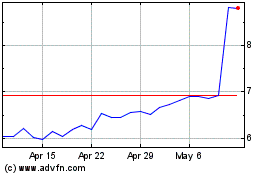
Adma Biologics (NASDAQ:ADMA)
Historical Stock Chart
From Mar 2024 to Apr 2024
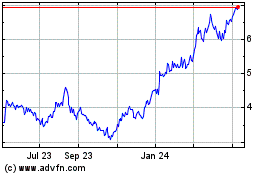
Adma Biologics (NASDAQ:ADMA)
Historical Stock Chart
From Apr 2023 to Apr 2024