Filed Pursuant to Rule 424(b)(3)
Registration No. 333-208330
PROSPECTUS

GALENA BIOPHARMA, INC.
$100,000,000
Common
Stock
Preferred Stock
Warrants
Rights
Units
We may, from
time to time, offer and sell shares of common stock, shares of preferred stock, warrants or rights, either separately or in units, in one or more offerings. The preferred stock and warrants may be convertible into or exercisable or exchangeable for
common stock or preferred stock. The rights may be exercisable for common stock or preferred stock. We will specify in the accompanying prospectus supplement more specific information about any such offering. The aggregate initial offering price of
all securities sold under this prospectus will not exceed $100,000,000, including the U.S. dollar equivalent if the public offering of any such securities is denominated in one or more foreign currencies, foreign currency units or composite
currencies.
We may offer these securities for sale directly to investors or through underwriters, dealers or agents. We will set forth
the names of any underwriters, dealers or agents and their compensation in the accompanying prospectus supplement.
This prospectus may
not be used to sell any of these securities unless accompanied by a prospectus supplement.
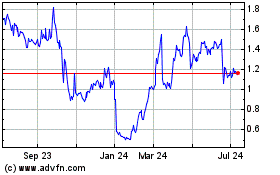
Our common stock is traded on The NASDAQ
Capital Market under the symbol “GALE.” On December 3, 2015, the last reported sale price of our common stock on The NASDAQ Capital Market was $1.45 per share.
Investing in our securities involves risks. See the section entitled “Risk Factors” in the
accompanying prospectus supplement and in the documents we incorporate by reference in this prospectus.
NEITHER THE SECURITIES AND
EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ADEQUACY OR ACCURACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is December 22, 2015
Prospectus No. 1
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission, or “SEC,” using a
“shelf” registration, or continuous offering, process. Under this shelf registration process, we may, from time to time, offer and sell shares of common stock, shares of preferred stock, warrants or rights, either separately or in units,
in one or more offerings with a maximum aggregate offering price of $100,000,000.
This prospectus provides you with a general description
of the securities we may offer. Each time we sell securities, we will provide a prospectus supplement that will contain specific information about the terms of that offering and the offered securities. Any prospectus supplement may also add, update
or change information contained in this prospectus. Any statement that we make in this prospectus will be modified or superseded by any inconsistent statement made by us in a prospectus supplement. The registration statement we filed with the SEC
includes exhibits that provide more detail of the matters discussed in this prospectus. You should read this prospectus and the related exhibits filed with the SEC and any prospectus supplement, together with additional information described under
the heading “Where You Can Find More Information,” before making your investment decision.
Unless otherwise indicated,
information contained or incorporated by reference in this prospectus concerning our industry, including our general expectations and market opportunity, is based on information from our own management estimates and research, as well as from
industry and general publications and research, surveys and studies conducted by third parties. Management estimates are derived from publicly available information, our knowledge of our industry and assumptions based on such information and
knowledge, which we believe to be reasonable. In addition, assumptions and estimates of our and our industry’s future performance are necessarily uncertain due to a variety of factors, including those referred to in “Risk Factors”
below in this prospectus. These and other factors could cause our future performance to differ materially from our assumptions and estimates.
NeuVax™ is our trademark used in this prospectus. This prospectus also includes the
Zuplenz® trademark and Abstral® and other trademarks, trade names and service marks that are the property of other organizations but in
certain instances have been licensed to us. Solely for convenience, trademarks and trade names referred to in this prospectus sometimes appear without the ® and ™ symbols, but those
references are not intended to indicate that we will not assert, to the fullest extent under applicable law, our rights, or that the applicable owner will not assert its rights, to these trademarks and trade names.
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the other documents we have filed with the SEC that are incorporated herein by reference contain forward-looking
statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve risks and uncertainties, as well as assumptions that, if they never materialize or prove incorrect, could cause our results to differ materially from
those expressed or implied by such forward-looking statements. All statements other than statements of historical fact are statements that could be deemed forward-looking statements, including any projections of financing needs, revenue, expenses,
earnings or losses from operations, or other financial items, any statements of the plans, strategies and objectives of management for future operations, any statements concerning product research, development and commercialization plans and
timelines, any statements regarding safety and efficacy of product candidates, any statements of expectation or belief and any statements of assumptions underlying any of the foregoing. In addition, forward-looking statements may contain the words
“believe,” “anticipate,” “expect,” “estimate,” “intend,” “plan,” “project,” “will be,” “will continue,” “will result,” “seek,”
“could,” “may,” “might,” or any variations of such words or other words with similar meanings. All forward-looking statements attributable to us or to persons acting on our behalf are expressly qualified in their
entirety by the cautionary statements and risk factors set forth under “Risk Factors” and elsewhere in this prospectus and set forth in our Form 10-K for the year ended December 31, 2014 and subsequent Quarterly Reports on Form 10-Q
filed with the SEC. See “Where You Can Find More Information” and “Incorporation of Certain Documents by Reference” in this prospectus for information on how to access or obtain our reports filed with the SEC.
Given their inherent uncertainty, you should not place undue reliance on these forward-looking statements. You should read this prospectus and
the documents that we reference in this prospectus with the understanding that our actual future results may be materially different from what we expect. Except as required by law, we do not undertake any obligation to update or revise any
forward-looking statements contained in this prospectus, whether as a result of new information, future events or otherwise.
RISK FACTORS
Investing in our securities involves significant risks. The prospectus supplement
relating to a particular offering will contain a discussion of risks applicable to an investment in the securities offered. Prior to making a decision about investing in our securities, you should carefully consider the specific factors discussed
under the heading “Risk Factors” in the applicable prospectus supplement together with all of the other information contained in the prospectus supplement or appearing or incorporated by reference in this prospectus.
1
Prospectus No. 1
ABOUT GALENA
Overview
Galena Biopharma, Inc.
(“we,” “us,” “our,” “Galena” or the “company”) is a biopharmaceutical company committed to the development and commercialization of targeted oncology therapeutics that address major unmet medical
needs. Galena’s development portfolio is focused primarily on addressing the rapidly growing patient populations of cancer survivors by harnessing the power of the immune system to prevent cancer recurrence. The Company’s pipeline consists
of multiple mid- to late-stage clinical assets, including novel cancer immunotherapy programs led by NeuVax™ (nelipepimut-S) and GALE-301. NeuVax is currently in a pivotal, Phase 3 clinical trial with several concurrent Phase 2 trials ongoing
both as a single agent and in combination with other therapies. GALE-301 is in a Phase 2a clinical trial in ovarian and endometrial cancers and in a Phase 1b given sequentially with GALE-302.
We are seeking to build value for shareholders through pursuit of the following objectives:
| |
• |
|
Develop novel cancer immunotherapies to address unmet medical needs through the use of peptide-based vaccines targeting well-established tumor antigens. One of our key strategies is to target the adjuvant, minimum
residual disease setting, in high risk patients who are more likely to benefit from treatment via immunotherapy. Our immunotherapy programs are currently targeting two key areas: secondary prevention to seek to significantly decrease the risk of
disease recurrence in breast cancer, gastric cancer, endometrial and ovarian cancers; and a planned trial positioning earlier in the breast cancer treatment spectrum via primary prevention. |
| |
• |
|
Expand our development pipeline by enhancing the clinical and geographic footprint of our technologies. We can accomplish this through the initiation of new clinical trials as well as through acquisition of additional
development stage products in related oncology indications. |
| |
• |
|
Leverage valuable partnerships and collaborations, as well as investigator-sponsored trial arrangements, to maximize the scope of potential clinical opportunities in a cost effective and efficient manner.
|
| |
• |
|
Focus our resources on our valuable and expanding clinical development programs. On November 19, 2015, we sold our Abstral® (fentanyl) Sublingual Tablets
product and related assets and we have determined to sell or otherwise dispose of our Zuplenz® (ondansetron) Oral Soluble Film product and related assets and to cease our commercial operations
to seek to maximize value to our shareholders. |
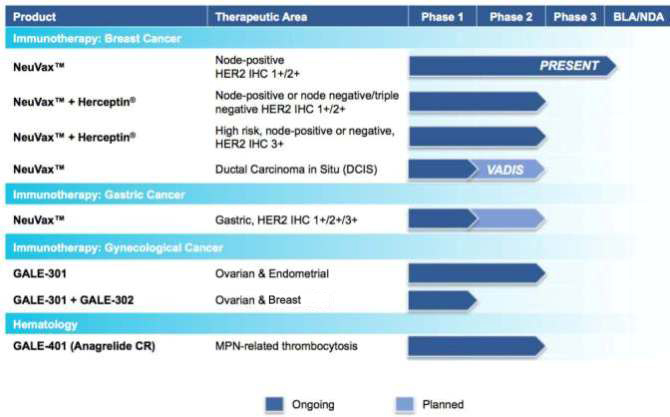
The chart below summarizes the current status of our clinical development
pipeline:
2
Prospectus No. 1
Novel Cancer Immunotherapies
Our targeted cancer immunotherapy approach is currently based upon two key areas: preventing secondary recurrence of cancer, which is becoming
increasingly important as the number of cancer survivors continues to grow; and, positioning earlier in the breast cancer treatment spectrum via primary prevention. Once a patient’s tumor becomes metastatic, the outcome is most often fatal,
making the prevention of recurrence a potentially critical component of overall patient care. Our programs primarily target patients in the adjuvant (after-surgery) setting who have relatively healthy immune systems, but may still have minimal
residual disease. Minimal residual disease, or single cancer cells (occult cancer cells) or micrometastasis, are undetectable by current radiographic scanning technologies, but we know can result in disease recurrence.
Our therapies utilize an immunodominant peptide combined with the immune adjuvant, recombinant human granulocyte macrophage-colony stimulating
factor (rhGM-CSF), and work by harnessing the patient’s own immune system to seek out and attack any residual cancer cells. Using peptide immunogens has many potential clinical advantages, including a favorable safety profile, since these drugs
may lack the toxicities typical of most cancer therapies. They also have the potential to evoke long-lasting protection through activation of the immune system and a convenient, intradermal mode of delivery. We are currently engaged in multiple
clinical trials with NeuVax™ (nelipepimut-S), GALE-301, and GALE-302, targeting the prevention of recurrence in breast, gastric, ovarian and endometrial cancers.
NeuVax™ (nelipepimut-S)
NeuVax™ (nelipepimut-S), our lead product candidate, is a cancer immunotherapy targeting human epidermal growth factor receptor (HER2)
expressing cancers. NeuVax is the immunodominant nonapeptide derived from the extracellular domain of the HER2 protein, a well-established and validated target for therapeutic intervention in breast and gastric carcinomas. The NeuVax vaccine is
combined with GM-CSF for injection under the skin, or intradermal administration. Data has shown that an increased presence of circulating tumor cells (CTCs) may predict Disease Free Survival (DFS) and Overall Survival (OS)—suggesting a
dormancy of isolated micrometastases, which, over time, may lead to recurrence. After binding to the specific HLA molecules on antigen presenting cells, the nelipepimut-S sequence stimulates specific cytotoxic T lymphocyte (CTLs). These activated
CTLs recognize, neutralize and destroy, through cell lysis, HER2 expressing cancer cells, including occult cancer cells and micrometastatic foci. The nelipepimut immune response can also generate CTLs to other immunogenic peptides through inter- and
intra-antigenic epitope spreading.
Breast Cancer: According to the National Cancer Institute, over 230,000 women in the U.S. are
diagnosed with breast cancer annually. While improved diagnostics and targeted therapies have decreased breast cancer mortality in the U.S., metastatic breast cancer remains incurable. Approximately 75% of breast cancer patients have tissue test
positive for some increased amount of the HER2 receptor, which is associated with disease progression and decreased survival. Only approximately 20% to 30% of all breast cancer patients—those with HER2 immunohistochemistry (IHC) 3+ disease, or
IHC 2+ and fluorescence in situ hybridization (FISH) positive—have a HER2 directed, approved treatment option available. This leaves the majority of breast cancer patients with low-to-intermediate HER2 IHC 1+/2+ ineligible for therapy and
without an effective targeted treatment option to prevent cancer recurrence.
We have multiple trials currently ongoing for NeuVax. For
our pivotal, Phase 3 PRESENT (Prevention of Recurrence in Early-Stage, Node- Positive Breast Cancer with Low to Intermediate HER2 Expression with NeuVax Treatment) trial, NeuVax is targeting the
30,000-40,000 of the 230,000 female breast cancer patients annually diagnosed in the U.S. who are at a higher risk of their breast cancer recurring, which we refer to as “disease recurrence,” after achieving “no evidence of
disease” (NED) status, (or becoming a “survivor”) with standard-of-care therapy (surgery, chemotherapy, radiation). These high-risk patients have a particular molecular signature and disease status: HER2 IHC 1+/2+ (oncoprotein
associated with aggressive tumor growth), node positive (disease present in the axillary lymph nodes prior to surgery), and HLA A2/A3 (human leukocyte antigen from A2/A3 patients who have the same loci of genes which represents approximately 65% of
the population). Up to 25% of resectable, node-positive breast cancer patients, having no radiographic evidence of disease following surgery and adjuvant chemo/radiation therapy, are expected to relapse within three years following diagnosis. The
prognosis upon recurrence is very poor. These cancer patients presumably still had isolated, undetected tumor CTCs that led to a recurrence of cancer in the breast (local recurrence) or in another location (metastatic disease). In addition to our
Phase 3 trial, we currently have two additional Phase 2 breast cancer trials ongoing with NeuVax in combination with trastuzumab (Herceptin®; Genentech/Roche) targeting the prevention of
recurrence in expanded indications.
We also recently announced our intent to initiate a Phase 2 trial with NeuVax as a single agent in
patients with ductal carcinoma in situ (DCIS) in collaboration with the National Cancer Institute (NCI), potentially positioning NeuVax earlier in the treatment cycle towards primary prevention. The trial will have an immunological endpoint
evaluating NeuVax peptide-specific cytotoxic T lymphocyte (CTL; CD8+ T cell) response in vaccinated patients. DCIS, is defined by the NCI as a noninvasive condition in which abnormal cells are found in the lining of a breast duct, and is the most
common type of breast cancer. The abnormal cells have not spread outside the duct to other tissues in the breast. In some cases, DCIS may become invasive cancer and spread to other tissues, and at this time, there is no way to know which lesions
could become invasive. Current treatment options for DCIS include breast- conserving surgery and radiation therapy with or without tamoxifen, breast-conserving surgery without radiation therapy, or total mastectomy with or without tamoxifen.
According to the American Cancer Society, in 2014 there were an estimated 51,933 diagnoses of DCIS.
3
Prospectus No. 1
Gastric Cancer: Gastric cancer (also known as stomach cancer) is a disease in which the
cells forming the inner lining of the stomach become abnormal and start to divide uncontrollably, forming a cancerous tumor mass. Cancer can develop in any of the five sections of the stomach. Symptoms and outcomes of the disease will vary depending
on the location of the cancer. Stomach cancer is one of the leading causes of cancer deaths in several areas of the world, most notably in Asia. Annually, almost one million people will be diagnosed worldwide with stomach cancer and over 700,000
will die from the disease. More than 90% of stomach cancers are caused by adenocarcinomas, malignant cancers that originate in glandular tissues. Overexpression of the HER2 receptor occurs in approximately 20% of gastric and gastro-esophageal
junction adenocarcinomas, predominantly those of the intestinal type. Overall, without regard to the stage of cancer, only approximately 28% of patients with stomach cancer live at least five years following diagnosis and new adjuvant treatments are
needed to prevent disease recurrence.
We currently have a number of ongoing or planned clinical trials designed to expand the clinical
and geographical footprint of NeuVax:
| |
• |
|
Phase 3 Ongoing: Our Phase 3 PRESENT (Prevention of Recurrence in Early- Stage, Node-Positive Breast Cancer with Low to Intermediate HER2 Expression with NeuVax Treatment) study
targeted enrollment of 700 HER2 1+/2+ patients under a Special Protocol Assessment (SPA) granted by the U.S. Food and Drug Administration (FDA). The multinational, multicenter, randomized, double-blinded PRESENT trial is ongoing in North
America, Western and Eastern Europe, and Israel. The trial is fully enrolled with 758 patients. |
| |
• |
|
Phase 2b Ongoing: A randomized, multicenter, investigator-sponsored, 300 patient Phase 2b clinical trial is enrolling HER2 1+/2+ node-positive and high-risk node-negative breast cancer patients who are HLA A2+, A3+,
A24+ or A26+ to study NeuVax in combination with trastuzumab in the adjuvant setting. This trial is co-funded by Genentech/Roche (providing both trastuzumab and monetary support) and Galena (providing NeuVax and monetary support). |
| |
• |
|
Phase 2 Ongoing: An investigator-sponsored trial is ongoing to study NeuVax in combination with trastuzumab. The study will enroll 100 node positive and negative HER2 IHC 3+ patients or HER2 gene-amplified breast cancer
patients who are HLA A2+ or HLA A3+ and are determined to be at high-risk for recurrence. Partial funding for this trial comes from the Department of Defense (DoD) through the Congressionally Directed Medical Research Program via legislation known
as the Defense Appropriations Act. The grant was awarded under a Breast Cancer Research Program with the Breakthrough Award given to the lead investigator for the trial. |
| |
• |
|
Phase 2 Planned: A clinical trial, entitled, VADIS: Phase 2 trial of the Nelipepimut-S Peptide VAccine in Women with DCIS of the Breast is planned to initiate by the end of 2015/early 2016. The
Phase 2 trial will be a single-blind, double arm, randomized, controlled trial in pre- or post-menopausal patients with DCIS and are HLA-A2 positive. VADIS will be co-funded and run in collaboration with the National Cancer Institute (NCI).
|
| |
• |
|
Phase 2 Planned: A Phase 2 clinical trial in patients with gastric cancer is expected to initiate in 2016. The trial will be run in India by our partner, Dr. Reddy’s Laboratories, Ltd., as part of our NeuVax
commercialization agreement in that region with Dr. Reddy’s. |
GALE-301 and GALE-302
Our second immunotherapy franchise targets folate binding protein receptor-alpha, a well-validated therapeutic target, which is highly
over-expressed (20-80 fold) in ovarian, endometrial and breast cancers. Both GALE-301 (E39) and GALE-302 (E39’) are immunogenic peptides that can stimulate CTLs to recognize and destroy FBP-expressing cancer cells. GALE-301 consists of the FBP
peptide E39 combined with GM-CSF, and is currently in a Phase 2a clinical trial for the prevention of recurrence in patients with ovarian and endometrial cancers. GALE-302 is an attenuated version of the E39 peptide and is currently in a Phase 1b
randomized, single-center trial investigating a novel vaccination series using GALE-301 and GALE-302 to evaluate the immune response and monitor long-term immunity. Current treatments after surgery for these diseases are principally with platinum
based chemotherapeutic agents and patients suffer a high recurrence rate; and, most patients relapse with an extremely poor prognosis. Although not powered for efficacy, promising preliminary results from the Phase 2a clinical trial of GALE-301 were
presented in September 2015 at the European Cancer Congress and demonstrated statistically significant data with the estimate for disease free survival at two years at 85.7% (1000 mcg dose group) vs. 33.6% for the control group (p < .02), and
that GALE-301 was well-tolerated with primarily Grade 1 and 2 toxicities and elicited a strong in vivo immune response.
In November 2015, we presented preliminary data at the Society for Immunotherapy of Cancer Conference on the primary vaccine series (PVS) from
a randomized Phase lb trial with GALE-301 and GALE-302 demonstrating that the in vivo immune response is enhanced with the use of the attenuated E39’ (GALE-302) after E39 (GALE-301). Both agents were shown to be immunogenic and well tolerated
with no differences in toxicities between primary vaccine sequences.
Ovarian and Endometrial Cancer: According to the NCI
Surveillance, Epidemiology, and End Results (SEER) Program, new cases of ovarian cancer occur at an annual rate of 12.1 per 100,000 women in the U.S., with an estimated 21,290 cases for 2015. Although ovarian cancer represents about 1.3% of all
cancers, it represents about 2.4% of all cancer deaths, or an estimated 14,180 deaths in 2015. Approximately 1.3% of women will be diagnosed with ovarian cancer at
4
Prospectus No. 1
some point during their lifetime (2010 – 2012 data). The prevalence of ovarian cancer in the U.S. is about 192,000 women, and the five-year survivorship for women with ovarian cancer is
45.6%. Due to the lack of specific symptoms, the majority of ovarian cancer patients are diagnosed at later stages of the disease, with an estimated 75% of women presenting with advanced-stage (III or IV) disease. These patients have their
tumors routinely surgically debulked to minimal residual disease, and then are treated with platinum- and/or taxane-based chemotherapy. While many patients respond to this treatment regimen and become clinically free-of-disease, the majority of
these patients will relapse. Depending upon their level of residual disease, the risk for recurrence after completion of primary therapy ranges from 60% to 85%. Unfortunately for these women, once the disease recurs, treatment options are limited
and the disease remains incurable.
According to the NCI SEER Program, new cases of endometrial cancer occur at an annual rate of
25.1 per 100,000 women in the U.S., with an estimated 54,870 cases for 2015. Although endometrial cancer represents about 3.3% of all cancers, it represents about 1.7% of all cancer deaths, or an estimated 10,170 deaths in 2015. Approximately
2.8% of women will be diagnosed with endometrial cancer at some point during their lifetime (2010 – 2012 data). The prevalence of endometrial cancer in the U.S. is about 620,000 women, and the five-year survivorship for women with endometrial
cancer is 81.7%.
Hematology
GALE-401 (anagrelide controlled release (CR))
In January 2014, we announced the acquisition of the worldwide rights to anagrelide controlled release (CR), which we renamed GALE-401, through
our acquisition of Mills Pharmaceuticals, LLC. GALE-401 contains the active ingredient anagrelide, an FDA-approved product, for the treatment of patients with myeloproliferative neoplasms (MPNs) to lower abnormally elevated platelet levels. The
currently available immediate release (IR) version of anagrelide causes adverse events that are believed to be dose and plasma concentration dependent. Therefore, reducing the maximum concentration (Cmax) is hypothesized to reduce the side effects,
but preserve efficacy.
Multiple Phase 1 studies in 98 healthy subjects have shown GALE-401 reduces the Cmax of anagrelide following oral
administration, appears to be well-tolerated at the doses administered, and to be capable of reducing platelet levels. The Phase 1 program provided the desired PK/PD (pharmacokinetic/pharmacodynamic) profile to enable the initiation of the ongoing
Phase 2 proof-of-concept trial. The Phase 2, open label, single arm, proof-of-concept trial enrolled 18 patients in the United States for the treatment of thrombocytosis, or elevated platelet counts in patients with MPNs. Phase 2 top-line safety and
efficacy data was presented in June 2015 at the European Hematology Association 20th Congress, and we expect to present a more mature data set at the 57th American Society of Hematology Annual
Meeting in December 2015. Based on a regulatory meeting with the FDA, Galena believes a 505(b)(2) regulatory filing is an acceptable pathway for development and potential approval of GALE-401.
Myeloproliferative neoplasms: MPNs are a closely related group of hematological malignancies in which the bone marrow cells that produce the
body’s blood cells develop and function abnormally. The main MPNs are Polycythemia Vera (PV), Essential Thrombocythemia (ET), Primary Myelofibrosis (PMF), and Chronic Myelogenous Leukemia (CML), all of which are associated with high platelet
counts. The MPNs are progressive blood cancers that can strike anyone at any age, and for which there is no known cure.
Strategic Review of
Commercial Operations
During the quarter ended September 30, 2015, we completed a strategic review of the commercial business
and operations. As a result of the review, we recently sold our Abstral® (fentanyl) Sublingual Tablets product and related assets, and we are pursuing the sale or other disposition of our
Zuplenz® (odansetron) Oral Soluble Film product and related assets described below.
Abstral® (fentanyl) Sublingual Tablets
On November 19, 2015, we and Sentynl Therapeutics Inc., or Sentynl, entered into an asset purchase agreement pursuant to which we sold to
Sentynl our Abstral® (fentanyl) Sublingual Tablets product and related assets, including our rights in the asset purchase agreement and the license agreement between us and Orexo AB dated
March 15, 2013, and March 18, 2013, respectively, and the settlement and license agreement among Orexo AB, Actavis Laboratories FL, Inc, and us dated October 23, 2015. Sentynl has assumed our future obligations under the Orexo agreements,
except that we will continue to be responsible for certain product channel liabilities. We also will continue to be responsible for any pre-closing liabilities and obligations related to Abstral.
Under the purchase agreement we received from Sentynl an $8 million upfront cash payment and are entitled to up to an aggregate of
$4 million in future cash payments based on Sentynl’s achievement of specified Abstral net sales milestones. The purchase agreement also includes customary representations, warranties, covenants and indemnities by Sentynl and us.
Zuplenz® (ondansetron) Oral Soluble Film
Zuplenz® (ondansetron) Oral Soluble Film, is approved by the FDA in adult patients for
the prevention of highly and moderately emetogenic chemotherapy-induced nausea and vomiting (CINV), radiotherapy-induced nausea and vomiting (RINV), and post-operative nausea and vomiting (PONV). Zuplenz is also approved in pediatric patients
treated with moderately emetogenic
5
Prospectus No. 1
CINV. Nausea and vomiting are two of the most common side-effects experienced by post-surgery patients and patients receiving chemotherapy or radiation. Zuplenz utilizes MonoSol’s
proprietary PharmFilm® technology, an oral soluble film that dissolves on the tongue in less than 30 seconds, therefore eliminating the burden of swallowing pills during periods of emesis. The
active pharmaceutical ingredient in Zuplenz, ondansetron, belongs to a class of medications called serotonin 5-HT3 receptor antagonists and works by blocking the action of serotonin, a natural substance that may cause nausea and vomiting.
Alliance Partners in Therapeutic Areas
We are actively looking to leverage our technology platforms by seeking to work with pharmaceutical and biotechnology partners in a number of
therapeutic areas in oncology. Our team has experience targeting products in multiple indications, and based on this experience, we believe we can expand the clinical utility of our current development candidates as well as discover more drug
candidates by working with partners than we can develop with our own resources. We are seeking to work with partners in the discovery and development of drugs in a number of therapeutic areas and technology platforms.
Intellectual Property
Patents and other
intellectual property rights are crucial to our success. It is our policy to protect our intellectual property rights through available means, including filing and prosecuting patent applications in the U.S. and other countries, protecting trade
secrets, and utilizing regulatory protections such as data exclusivity. We also include restrictions regarding use and disclosure of our proprietary information in our contracts with third parties, and utilize customary confidentiality agreements
with our employees, consultants, clinical investigators and scientific advisors to protect our confidential information and know-how. Together with our licensors, we also rely on trade secrets to protect our combined technology especially where we
do not believe patent protection is appropriate or obtainable. It is our policy to operate without infringing on, or misappropriating, the proprietary rights of others. The following chart summarizes our intellectual property rights:
6
Prospectus No. 1
|
|
|
|
|
|
|
|
|
| Drug Candidate |
|
Indication |
|
Scope |
|
Strategic Partner |
|
Estimated
Exclusivity
Period |
| NeuVax™ (nelipepimut-S) |
|
Breast cancer recurrence |
|
Filed and pending or issued
worldwide |
|
University of
Texas/MDACC/
Henry M. Jackson
Foundation |
|
2028 |
|
|
|
|
|
| NeuVax™ (nelipepimut-S) |
|
Gastric |
|
Filed and pending or issued
worldwide |
|
Dr. Reddy’s
Laboratories |
|
2028 |
|
|
|
|
|
| NeuVax™ (nelipepimut-S) |
|
DCIS |
|
Filed and pending or issued
worldwide |
|
National Cancer
Institute/
University of
Texas, MD
Anderson Cancer
Center |
|
2028 |
|
|
|
|
|
| NeuVax™ in combination with Herceptin® |
|
Breast cancer |
|
Filed and pending or issued
worldwide |
|
Henry M. Jackson
Foundation,
Genentech/Roche |
|
2026 |
|
|
|
|
|
| NeuVax™ in combination with other compounds |
|
Breast cancer |
|
Filed and pending or issued
worldwide |
|
Pending |
|
2037 |
|
|
|
|
|
| GALE-301 & GALE-302 |
|
Breast, ovarian and endometrial cancer |
|
Filed and pending or issued
worldwide |
|
Henry M. Jackson
Foundation |
|
2035 |
|
|
|
|
|
| GALE-401 (Anagrelide Controlled Release) |
|
Platelet Lowering |
|
Filed and pending or issued
worldwide |
|
BioVascular, Inc. |
|
2029 |
Out-License Agreements
Teva Pharmaceuticals
Effective December 3, 2012, we entered into a license and supply agreement with ABIC Marketing Limited, a subsidiary of Teva
Pharmaceuticals (“ABIC”). Under the agreement, we granted ABIC exclusive rights to seek marketing approval in Israel for our NeuVax product candidate for the treatment of breast cancer following its approval by the FDA or the European
Medicines Agency, and to market, sell and distribute NeuVax in Israel assuming such approval is obtained. ABIC’s rights also include a right of first refusal in Israel for all future indications for which NeuVax may be approved.
Under the license and supply agreement, ABIC will assume responsibility for regulatory registration of NeuVax in Israel, provide financial
support for local development, and commercialize the product in the region in exchange for making royalty payments to us based on future sales of NeuVax. ABIC also agrees in the license and supply agreement to purchase all supplies of NeuVax from us
at a price determined according to a specified formula.
Dr. Reddy’s Laboratories Ltd.
Effective January 14, 2014, we entered into a strategic development and commercialization partnership with Dr. Reddy’s
Laboratories Ltd. (“Dr. Reddy’s”), under which we licensed commercial rights in India to Dr. Reddy’s for NeuVax in breast and gastric cancers. Under the agreement, Dr. Reddy’s will lead the Phase 2 development of
NeuVax in India in gastric cancer, significantly expanding the potential patient population addressable with NeuVax.
Kwangdong
Pharmaceutical Co., Ltd.
Effective April 30, 2009, we entered into a license agreement with Kwangdong Pharmaceutical Co, Ltd
(Kwangdong). Under the agreement, we granted Kwangdong exclusive rights to seek marketing approval in The Republic of Korea (South Korea) for our NeuVax product candidate for the treatment of breast cancer following its approval by the FDA or the
European Medicines Agency, and to market, sell and distribute NeuVax in South Korea assuming such approval is obtained.
Recent Developments (in
reverse chronological order)
Litigation Settlement
On December 4, 2015, we reported that we had reached an agreement in principle to settle the consolidated shareholder derivative
action, captioned In re Galena Biopharma, Inc. Derivative Litigation, Civil Action No. 3:14-cv-00382-SI, currently pending in the United States District Court for the District of Oregon against the Company and/or certain of its
current and former officers and directors and the consolidated securities putative class action lawsuits, captioned In re Galena Biopharma, Inc. Securities Litigation, Civil Action No. 3:14-cv-00367-SI, pending against the
Company, certain of its current and former officers and directors, and other defendants in the United States District Court for the District of Oregon.
Strategic Review of Commercial Operations – We have completed a strategic review of our commercial operations.
During the three months ended September 30, 2015, we completed a strategic review of the commercial business and operations. As a result
of the review, we recently sold our Abstral® (fentanyl) Sublingual Tablets product and related assets, and we have determined to sell or otherwise dispose of our Zuplenz® (odansetron) Oral Soluble Film product and related assets and to cease our commercial operations.
GALE-302 Immunological Data Optimizing GALE-301 Presented - We presented preliminary immunologic data for our GALE-301 and
GALE-302 Phase 1b clinical trial at the Society for Immunotherapy of Cancer (SITC) 30th Anniversary Annual Meeting.
On November 7,
2015 we presented the poster, entitled, “Preliminary report of a clinical trial supporting the sequential use of an attenuated E39 peptide (E39’) to optimize the immunologic response to the FBP (E39+GM-CSF) vaccine,” that compared
three primary vaccine series (PVS) sequences of GALE-301 (E39) and GALE-302 (E39’) in ovarian and breast cancer patients to optimize the ex vivo immune responses, local reactions (LR), and delayed type hypersensitivity (DTH) reactions. The data
demonstrated that the in vivo immune response is enhanced with the use of the attenuated E39’ (GALE-302) after E39 (GALE-301). The optimal vaccination sequence utilizing three inoculations of GALE-301 followed by three inoculations of GALE-302
produced the most prominent and statistically significant LR and DTH responses.
Patent Infringement Litigation – We
have settled the patent infringement litigation involving Abstral.
On October 27, 2015, we reported that on October 23,
2015 we, Orexo AB and Actavis Laboratories Fl, Inc. (“Actavis”) had entered into a settlement and license agreement, which will permit Actavis to enter the market with a generic or authorized generic version of Abstral in June 2018 or
earlier under certain circumstances. The litigation, Orexo AB v. Actavis Laboratories FL, Inc., CA
7
Prospectus No. 1
No. 3:15-cv-00826, was dismissed with prejudice by the United States District Court for the District of New Jersey before and the agreement will be filed with the Federal Trade Commission
and the Department of Justice under applicable law.
Collaboration with the National Cancer Institute – We announced a
Phase 2 clinical trial with NeuVax in Ductal Carcinoma in Situ Patients.
On September 30, 2015, we announced a collaboration
with the National Cancer Institute (NCI) to initiate a Phase 2 clinical trial with NeuVax in patients diagnosed with Ductal Carcinoma in Situ (DCIS). The trial will be entitled VADIS: Phase 2 trial of the Nelipepimut-S Peptide Vaccine in Women with
DCIS of the Breast. The University of Texas M.D. Anderson Cancer Center (MDACC) Phase I and II Chemoprevention Consortium will be the lead clinical trial site for this multi-center trial with Elizabeth Mittendorf, M.D., Ph.D. serving as the
study Principal Investigator. The Consortium is funded through the Division of Cancer Prevention at NCI, which will provide financial and administrative support for the trial. We will provide NeuVax, as well as additional financial and
administrative support. The single-blind, double arm, randomized, controlled trial is expected to initiate in the fourth quarter of 2015/ first quarter of 2016.
GALE-301 Phase 2a Clinical Trial Data Presented – We announced positive data for our GALE-301 Phase 2a clinical trial
On September 28, 2015 we announced the poster presentation of positive data from the GALE-301 Phase 2a portion of the Phase
1/2a clinical trial at the European Cancer Congress 2015, providing updated data for all ovarian and endometrial cancer patients who have received at least twelve months of treatment on the study. The poster was entitled, entitled
“Preliminary results of the phase I/IIa dose finding trial of a folate binding protein vaccine GALE-301 (E39) + GM-CSF in ovarian and endometrial cancer patients to prevent recurrence,” and as presented, the clinical recurrence rate based
on all treatment cohorts was 41% in the Vaccine Group (VG) (n=29) versus 55% in the Control Group (CG) (n=22), p=0.41. However, in the 1000 mcg VG cohort (n=15), there have only been two clinical recurrences (13.3% versus 55% CG, p=0.02), and
the two-year Disease Free Survival (DFS) estimate is 85.7% (1000 mcg patients) versus 33.6% (CG), p < 0.02, as compared by Kaplan-Meir and Log rank tests.
IDMC Provides Recommendation to Reduce Cardiac Monitoring in the PRESENT Trial – We announced that the Independent Data
Monitoring Committee (IDMC) for the NeuVax Phase 3 clinical trial recommended to the Company that it can reduce the cardiac toxicity monitoring for patients in our PRESENT study
On August 24, 2015 we announced that the Independent Data Monitoring Committee (IDMC) for the NeuVax Phase 3 clinical trial recommended
to the Company that we can reduce the cardiac toxicity monitoring for patients in our PRESENT study. Following its most recent meeting in June 2015, the IDMC recommended routine cardiac monitoring could be reduced in the PRESENT trial and that such
a reduction is justified and consistent with the pre-specified Cardiac Toxicity Monitoring Stopping Rules defined in the study protocol. The IDMC concluded that cardiac toxicity monitoring by echocardiogram (ECHO) or multiple-gated acquisition
(MUGA) scans could be reduced. The IDMC had no other suggestions and recommended the trial continue as planned.
GALE 401 Phase 2
Clinical Trial Data Presented - We presented data from our Phase 2 clinical trial of GALE-401 at the European Hematology Association 20th Congress
On June 15, 2015, we announced our poster presentation entitled “Phase 2 Study of a Novel Controlled-Release Formulation of
Anagrelide (GALE-401) in Subjects with Myeloproliferative Neoplasm (MPN)-Related Thrombocytosis,” which was presented on June 13, 2015. The Phase 2 study demonstrated that GALE-401 was well tolerated with primarily Grade 1 and 2
toxicities in 16 of the 18 subjects enrolled. The efficacy shown in the trial compares favorably to historical anagrelide immediate release (IR) response rates with the following platelet response: overall response rate (ORR) of 78% (14/18);
complete response (CR) of 39% (7/18); partial response (PR) of 39% (7/18). The median time to response was 5 weeks (range, 3-10), and the median duration of response has not yet been reached.
Published Abstracts related to GALE-301 and Leica Biosystem’s Companion Diagnostic - We presented two abstract publications
at the American Society of Clinical Oncology (ASCO) 2015 Annual Meeting.
On May 27, 2015, we announced two abstract
publications at the ASCO 2015 Annual Meeting related to our cancer immunotherapy programs:
We presented data related to GALE-301 in
abstract #e14031, entitled, “Preliminary Results of the Phase I/IIa Dose Finding Trial of a Folate Binding Protein Vaccine (E39+GM-CSF) in Ovarian and Endometrial Cancer Patients to Prevent Recurrence.” The more mature data set from this
trial was presented in September 2015 as mentioned above.
8
Prospectus No. 1
We presented data related to our NeuVax Phase 3 PRESENT trial companion diagnostic in abstract
#e11609, entitled, “Analytical Validation of BOND Oracle HER2 IHC System for Identifying Low to Intermediate HER2 Expressing Breast Cancer in NeuVax PRESENT Phase 3 Clinical Trial.” This data demonstrated a direct correlation between cell
line receptor load, quantitative measure of HER2 protein, and IHC score. The ability to discriminate HER2 protein expression at the low and intermediate levels in breast cancer tumors will identify patients for new treatments in development such as
NeuVax. Specifically, the validation of the Bond Oracle HER2 IHC System to distinguish lower levels of HER2+ expressions supports its use as a companion diagnostic.
NeuVax Phase 3 PRESENT Over-Enrollment Completed - We announced the completion of enrollment in our NeuVax Phase 3 PRESENT
clinical trial.
On April 14, 2015, we announced the completion of enrollment in the NeuVax™ Phase 3 PRESENT clinical
trial. As anticipated, we over-enrolled the trial by 7.7% with a total of 758 patients now in the intent-to-treat (ITT) population. The protocol for the PRESENT trial called for 700 patients; and we expect this higher number of ITT patients will
increase the confidence in both the timing and quality of the statistics and the final outcome of the trial. The primary endpoint is currently expected to be reached in 2018, after the last patient dosed reaches her 36th month of treatment, or a
total of 141 events (recurrence or death) occur, whichever comes later.
Expanded Patient Population in NeuVax Combination
Trial - We announced the expansion of the patient population in the NeuVax and trastuzumab combination trial in HER2 1+/2+ patients to include HLA A24+ or HLA-A26+ patients.
On March 26, 2015, we announced that human leukocyte antigen (HLA) - A24+ or HLA-A26+ women are now eligible for enrollment into the
ongoing Phase 2b clinical trial with NeuVax in combination with trastuzumab. The trial evaluates node positive and triple negative, node negative breast cancer patients with immunohistochemistry (IHC) HER2 1+/2+ expressing tumors who are
disease-free after standard of care therapy.
Enrolled 700th Patient in NeuVax Phase 3 PRESENT Clinical Trial —We
announced enrollment of the 700th patient in our Phase 3 PRESENT clinical trial.
On February 9, 2015, we announced the
enrollment in the NeuVax Phase 3 PRESENT clinical trial of the 700th patient, which is the patient enrollment target as defined by the PRESENT Phase 3 clinical trial protocol.
Financial Condition
We had cash and cash
equivalents of approximately $34.8 million as of September 30, 2015. We believe that our existing cash and cash equivalents together with funding available under our purchase agreement with Lincoln Park Capital Fund, LLC and sales agreements
with MLV & Co. and Maxim Group LLC should be sufficient to fund our operations for at least one year. This projection is based on our current planned operations and revenue expectations and is subject to changes in our plans and
uncertainties inherent in our business, and we may need to seek to replenish our existing cash and cash equivalents sooner than we project. There is no guarantee that any additional funding will be available to us on acceptable terms, or at all. If
we fail to obtain additional funding when needed, we would be forced to scale back or terminate our operations, or to seek to merge with or to be acquired by another company.
Purchase Agreement with Lincoln Park Capital Fund, LLC
On November 18, 2014, we entered into a purchase agreement with Lincoln Park Capital Fund, LLC, or Lincoln Park. The purchase agreement
provides that in our discretion, upon the terms and subject to the conditions and limitations set forth therein, Lincoln Park currently is irrevocably committed to purchase from us an aggregate of up to $42,194,000 of additional shares of our common
stock over the remaining term of the purchase agreement.
There is no upper limit on the price per share that Lincoln Park must pay for
our common stock under the purchase agreement, but in no event will shares be sold to Lincoln Park on a day our closing price is less than $1.00 per share. The purchase price will be equitably adjusted for any reorganization, recapitalization,
non-cash dividend, stock split or other similar transaction occurring during the business days used to compute the purchase price.
There
are no trading volume requirements or restrictions under the purchase agreement, but there are limitations on the number of shares we can sell to Lincoln Park as described below. We will control the timing and amount of any sales of our common stock
to Lincoln Park, and we may terminate the purchase agreement at any time without fee, penalty or cost, upon one business day notice to Lincoln Park.
The purchase agreement limits our sales of shares of common stock to Lincoln Park to a maximum of 19.99% of our total outstanding shares of
common stock as of November 18, 2014, or approximately 42,270,277 shares, including 7,932,121 shares sold
9
Prospectus No. 1
to Lincoln Park prior to the date of this prospectus, unless we first obtain stockholder approval under the rules of The NASDAQ Capital Market or unless the average price of all shares of our
common stock sold to Lincoln Park exceeds $1.889, which represents the closing bid price of our common stock on November 18, 2014 as reported on The NASDAQ Capital Market plus $0.0491, such that such sales to Lincoln Park are considered to be
at least “at market” under the applicable NASDAQ rules.
The purchase agreement also prohibits us from selling Lincoln Park any
shares of common stock if those shares, when aggregated with all other shares of our common stock then beneficially owned by Lincoln Park and its affiliates, would result in Lincoln Park and its affiliates having beneficial ownership of more than
9.99% of the then total outstanding shares of our common stock (approximately 16,174,000 shares as of September 30, 2015), as calculated pursuant to Section 13(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and
Rule 13d-3 thereunder.
Sales Agreements with MLV & Co. and Maxim Group LLC
We entered into sales agreements with MLV & Co. LLC, or MLV, and Maxim Group LLC, or Maxim, on May 24, 2013, under which we may
currently issue and sell our common stock having a cumulative aggregate offering price of up to $20,000,000.
The sales, if any, of shares
made under the sales agreements will be made on The NASDAQ Capital Market by means of ordinary brokers’ transactions at market prices, in block transactions or as otherwise agreed by MLV or Maxim and us. We may instruct MLV and Maxim not to
sell common stock if the sales cannot be effected at or above the price designated by us from time to time. We or MLV or Maxim may suspend the offering of common stock upon notice and subject to other conditions. As agents, MLV and Maxim will not
engage in any transactions that stabilize the price of our common stock.
We will pay each of MLV and Maxim commissions for its services
in acting as agent in the sale of common stock. MLV and Maxim will be entitled to compensation at a fixed commission rate of 3.0% of the gross sales price per share sold.
Corporate Information
Our principal
executive offices are located at 2000 Crow Canyon Place, Suite 380, San Ramon, California 94583, and our phone number is (855) 855-4253. Our website address is www.galenabiopharma.com. We do not incorporate the information on our website into
this prospectus, and you should not consider such information part of this prospectus.
We were incorporated as Argonaut Pharmaceuticals,
Inc. in Delaware on April 3, 2006 and changed our name to RXi Pharmaceuticals Corporation on November 28, 2006. On September 26, 2011, we changed our company name from RXi Pharmaceuticals Corporation to Galena Biopharma, Inc.
USE OF PROCEEDS
Unless we state otherwise in the accompanying prospectus supplement, we currently intend to use the net proceeds from this offering to augment
our working capital and for general corporate purposes, including our Phase 3 PRESENT study and other clinical trials of our product candidates. General corporate purposes also may include, but are not limited to, payments relating to the
ongoing defense or settlement of pending stockholder lawsuits or the possible sale or other disposition of our marketed products and discontinuation of our commercial operations, repayment of indebtedness, financing of capital expenditures and
potential future acquisitions and strategic investments. Pending application of the net proceeds, we expect to invest the net proceeds in investment-grade, interest-bearing securities.
10
Prospectus No. 1
FINANCIAL RATIOS
We present below the ratio of our earnings to combined fixed charges and preference dividends, if any. Earnings consist of our net loss plus
fixed charges. Fixed charges consist of interest expense and amortization of debt issuance costs. We recorded no preference dividends in any of the periods presented.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Year Ended December 31, |
|
|
Nine
Months
Ended
September
30, |
|
| |
|
2010 |
|
|
2011 |
|
|
2012 |
|
|
2013 |
|
|
2014 |
|
|
2015 |
|
| Ratio of earnings (loss) to combined fixed charges and preference dividends(1) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
| Deficiency in earnings (loss) to cover fixed charges |
|
|
N/A |
|
|
|
N/A |
|
|
|
N/A |
|
|
|
N/A |
|
|
|
N/A |
|
|
|
N/A |
|
| (1) |
We did not record earnings in any of the periods presented. Accordingly, our earnings were insufficient to cover fixed charges in such periods, and we are unable to disclose a ratio of earnings to combined fixed charges
and preference dividends for such periods. |
THE SECURITIES THAT WE MAY OFFER
We, directly or through agents, dealers or underwriters designated from time to time, may offer, issue and sell, together or separately, up to
$100,000,000 in the aggregate of:
| |
• |
|
shares of our common stock, par value $0.0001 per share; |
| |
• |
|
shares of our preferred stock, par value $0.0001 per share; |
| |
• |
|
warrants to purchase our common stock or preferred stock; |
| |
• |
|
rights to purchase our common stock or preferred stock; and |
| |
• |
|
any combination of the securities listed above, separately or as units, each on terms to be determined at the time of sale. |
The common stock, preferred stock, warrants, rights and units collectively are referred to in this prospectus as the “securities.”
We have summarized below the material terms of the various types of securities that we may offer. We will describe in the applicable
prospectus supplement the detailed terms of the securities offered by that supplement. If indicated in the prospectus supplement, the terms of the offered securities may differ from the terms summarized below.
DESCRIPTION OF CAPITAL STOCK
General
Our authorized capital stock
consists of 280,000,000 shares, all which includes:
| |
• |
|
275,000,000 shares of common stock, par value $0.0001 per share, and |
| |
• |
|
5,000,000 shares of preferred stock, par value $0.0001 per share. |
As of September 30,
2015, there were 161,900,446 shares of common stock outstanding held by approximately 544 stockholders of record, and no shares of preferred stock outstanding. The number of holders of record does not include shares held in “street name”
through brokers.
11
Prospectus No. 1
Common Stock
Each holder of our common stock is entitled to one vote for each share of common stock held on all matters submitted to a vote of stockholders.
Common stockholders are not entitled to cumulative voting in the election of directors by our certificate of incorporation. This means that the holders of a majority of the shares voted will be able to elect all of the directors then standing for
election. Subject to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of our common stock are entitled to receive dividends out of assets legally available at the times and in the
amounts that our board of directors may determine from time to time.
Upon our liquidation, dissolution or winding-up, the holders of our
common stock will be entitled to share ratably in all assets remaining after payment of all liabilities and the liquidation preferences of any series of capital stock ranking senior to the common stock upon liquidation. Holders of common stock have
no preemptive or conversion rights or other subscription rights. There will be no redemption or sinking fund provisions applicable to the common stock. All outstanding shares of common stock are fully paid and nonassessable, and the shares of common
stock to be issued under this prospectus, when they are paid for, will be fully paid and nonassessable.
Preferred Stock
Our board of directors is authorized, subject to any limitations prescribed by law, without further vote or action by the stockholders, to
issue from time to time any of the authorized shares of preferred stock in one or more series without stockholder approval. Each such series of preferred stock shall have such number of shares, designations, preferences, voting powers,
qualifications, and special or relative rights or privileges as shall be determined by our board of directors, which may include, among others, dividend rights, voting rights, liquidation preferences, conversion rights and preemptive rights.
Our board of directors will fix the rights, preferences, privileges, qualifications and restrictions of the preferred stock of each series
that we sell under this prospectus in the certificate of designation relating to each such series. We will incorporate by reference as an exhibit to the registration statement of which this prospectus is a part or as an exhibit to one or more
current reports on Form 8-K, the form of any certificate of designation that describes the terms of the series of preferred stock we are offering before the issuance of the related series of preferred
stock. This description will include:
| |
• |
|
the title and stated value; |
| |
• |
|
the number of shares we are offering; |
| |
• |
|
the liquidation preference per share; |
| |
• |
|
the purchase price per share; |
| |
• |
|
the dividend rate per share, dividend period, payment date or dates and method of calculation of dividends; |
| |
• |
|
whether dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate; |
| |
• |
|
our right, if any, to defer payment of dividends and the maximum length of any such deferral period; |
| |
• |
|
the procedures for any auction and remarketing, if any; |
| |
• |
|
the provisions for a sinking fund, if any; |
| |
• |
|
the provisions for redemption or repurchase, if applicable, and any restrictions on our ability to exercise those redemption and repurchase rights; |
| |
• |
|
any listing of the preferred stock on any securities exchange or market; |
| |
• |
|
whether the preferred stock will be convertible into our common stock or other securities of ours, including warrants, and, if applicable, the conversion price, or how it will be calculated, and under what circumstances
and the mechanism by which it may be adjusted, and the conversion period; |
| |
• |
|
whether the preferred stock will be exchangeable into debt securities or other securities of ours, and, if applicable, the exchange price, or how it will be calculated, and under what circumstances it may be adjusted,
and the exchange period; |
| |
• |
|
preemptive rights, if any; |
12
Prospectus No. 1
| |
• |
|
restrictions on transfer, sale or other assignment, if any; |
| |
• |
|
a discussion of any material United States federal income tax considerations applicable to the preferred stock; |
| |
• |
|
the relative ranking and preferences of the preferred stock as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; |
| |
• |
|
any limitations on issuances of any class or series of preferred stock ranking senior or on a parity with the series of preferred stock being issued as to dividend rights and rights if we liquidate, dissolve or wind up
our affairs; and |
| |
• |
|
any other specific terms, rights, preferences, privileges, qualifications or limitations of, or restrictions on, the preferred stock. |
If we issue and sell shares of preferred stock pursuant to this prospectus, the shares will be fully paid and nonassessable and will not have,
or be subject to, any preemptive or similar rights.
The laws of the State of Delaware, the state of our incorporation, provide that the
holders of preferred stock will have the right to vote separately as a class on any proposal involving fundamental changes in the rights of holders of such preferred stock. This right is in addition to any voting rights that may be provided for in
the applicable certificate of designation.
We believe the power to issue preferred stock will provide our board of directors with
flexibility in connection with certain possible corporate transactions. The issuance of preferred stock, however, could adversely affect the voting power of holders of our common stock, restrict their rights to receive payment upon liquidation, and
have the effect of delaying, deferring, or preventing a change in control which may be beneficial to our stockholders.
Anti-Takeover Effects of
Delaware Law and Our Restated Certificate of Incorporation and Bylaws
Certain provisions of Delaware law, our amended and restated
certificate of incorporation and our amended and restated bylaws contain provisions that could have the effect of delaying, deferring or discouraging another party from acquiring control of us. These provisions, which are summarized below, are
expected to discourage certain types of coercive takeover practices and inadequate takeover bids. These provisions are also designed, in part, to encourage persons seeking to acquire control of us to first negotiate with our board of directors. We
believe that the benefits of increased protection of our potential ability to negotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging such proposals, including proposals that are priced above the then-current
market value of our common stock, because, among other reasons, the negotiation of such proposals could result in an improvement of their terms.
Certificate of Incorporation and Bylaws
Our amended and restated certificate of incorporation and amended and restated bylaws include provisions that:
| |
• |
|
authorize our board of directors to issue, without further action by the stockholders, up to 5,000,000 shares of undesignated preferred stock; |
| |
• |
|
require that any action to be taken by our stockholders be effected at a duly called annual or special meeting and not by written consent; |
| |
• |
|
specify that special meetings of our stockholders can be called only by our board of directors, the chairman of the board or the chief executive officer; |
| |
• |
|
provide that our board of directors will be classified, with directors serving staggered three-year terms; |
| |
• |
|
provide that directors may be removed only for cause and may only be removed for cause only by the holders of 75% of our outstanding capital stock entitled to vote generally in the election of directors;
|
| |
• |
|
provide that vacancies on our board of directors may be filled only by a majority of directors then in office, even though less than a quorum; |
| |
• |
|
provide for a 75% vote of stockholders to amend our amended and restated bylaws, unless the amendment has been approved by a majority of our directors who are not affiliated or associated with any person or entity
holding 10% or more of the voting power of our outstanding capital stock; and |
13
Prospectus No. 1
| |
• |
|
establish an advance notice procedure for stockholder proposals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to our board of directors.
|
Delaware Anti-Takeover Statute
We are subject to the provisions of Section 203 of the Delaware General Corporation Law regulating corporate takeovers. In general,
Section 203 prohibits a publicly-held Delaware corporation from engaging, under certain circumstances, in a business combination with an interested stockholder for a period of three years following the date the person became an interested
stockholder unless:
| |
• |
|
prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder;
|
| |
• |
|
upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the
transaction commenced, excluding for purposes of determining the voting stock outstanding, but not the outstanding voting stock owned by the interested stockholder, (1) shares owned by persons who are directors and also officers and
(2) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| |
• |
|
at or subsequent to the date of the transaction, the business combination is approved by the board of directors of the corporation and authorized at an annual or special meeting of stockholders, and not by written
consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock which is not owned by the interested stockholder. |
Generally, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to
the “interested stockholder.” Generally, an “interested stockholder” is a person who, together with affiliates and associates, owns or, within three years prior to the determination of interested stockholder status, did own 15%
or more of a corporation’s outstanding voting stock. We expect the existence of this provision to have an anti-takeover effect with respect to transactions our board of directors does not approve in advance. We also anticipate that
Section 203 may discourage business combinations or other attempts that might result in a premium over the market price for the shares of common stock held by our stockholders.
The provisions of Delaware law, our restated certificate of incorporation and our amended and restated bylaws could have the effect of
discouraging others from attempting hostile takeovers and, as a consequence, they may also inhibit temporary fluctuations in the market price of our common stock that often result from actual or rumored hostile takeover attempts. These provisions
may also have the effect of preventing changes in our management. It is possible that these provisions could make it more difficult to accomplish transactions that stockholders may otherwise deem to be in their best interests.
Listing
Our common stock is listed on
The NASDAQ Capital Market under the symbol “GALE.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Computershare Trust Company, N.A.
DESCRIPTION OF WARRANTS
We may issue warrants for the purchase of preferred stock or common stock, or a combination thereof. We may issue warrants independently or
together with any other securities offered by any prospectus and may be attached to or separate from the other offered securities. Each series of warrants will be issued under a separate warrant agreement to be entered into by us with a warrant
agent. The warrant agent will act solely as our agent in connection with the warrants and will not assume any obligation or relationship of agency or trust for or with any holders or beneficial owners of warrants. Further terms of the warrants and
the applicable warrant agreements will be set forth in the applicable prospectus supplement.
The prospectus supplement relating to any
particular issue of warrants will describe the terms of the warrants, including, as applicable, the following:
| |
• |
|
the title of the warrants; |
14
Prospectus No. 1
| |
• |
|
the aggregate number of the warrants; |
| |
• |
|
the price or prices at which the warrants will be issued; |
| |
• |
|
the designation, terms and number of shares of debt securities, preferred stock or common stock purchasable upon exercise of the warrants; |
| |
• |
|
the designation and terms of the offered securities, if any, with which the warrants are issued and the number of the warrants issued with each offered security; |
| |
• |
|
the date, if any, on and after which the warrants and the related debt securities, preferred stock or common stock will be separately transferable; |
| |
• |
|
the price at which each share of debt securities, preferred stock or common stock purchasable upon exercise of the warrants may be purchased; |
| |
• |
|
the date on which the right to exercise the warrants shall commence and the date on which that right shall expire; |
| |
• |
|
the minimum or maximum amount of the warrants which may be exercised at any one time; |
| |
• |
|
information with respect to book-entry procedures, if any; |
| |
• |
|
a discussion of certain federal income tax considerations; and |
| |
• |
|
any other terms of the warrants, including terms, procedures and limitations relating to the exchange and exercise of the warrants. |
We and the warrant agent may amend or supplement the warrant agreement for a series of warrants without the consent of the holders of the
warrants issued thereunder to effect changes that are not inconsistent with the provisions of the warrants and that do not materially and adversely affect the interests of the holders of the warrants.
DESCRIPTION OF RIGHTS
We may issue rights to purchase common stock or preferred stock. The accompanying prospectus supplement will contain the material terms and
conditions for each right.
We will describe in the applicable prospectus supplement the terms and conditions of the issue of rights being
offered, the rights agreement relating to the rights and the rights certificates representing the rights, including, as applicable:
| |
• |
|
the title of the rights; |
| |
• |
|
the date of determining the stockholders entitled to the rights distribution; |
| |
• |
|
the title, aggregate number of shares of common stock or preferred stock purchasable upon exercise of the rights; |
| |
• |
|
the aggregate number of rights issued; |
| |
• |
|
the date, if any, on and after which the rights will be separately transferable; |
| |
• |
|
the date on which the right to exercise the rights will commence and the date on which the right will expire; and |
| |
• |
|
any other terms of the rights, including terms, procedures and limitations relating to the distribution, exchange and exercise of the rights. |
Each right will entitle the holder of rights to purchase for cash the principal amount of shares of common stock or preferred stock at the
exercise price provided in the applicable prospectus supplement. Rights may be exercised at any time up to the close of business on the expiration date for the rights provided in the applicable prospectus supplement. After the close of business on
the expiration date, all unexercised rights will be void.
15
Prospectus No. 1
Holders may exercise rights as described in the applicable prospectus supplement. Upon receipt of
payment and the rights certificate properly completed and duly executed at the corporate trust office of the rights agent or any other office indicated in the prospectus supplement, we will, as soon as practicable, forward the shares of common stock
or preferred stock purchasable upon exercise of the rights. If less than all of the rights issued in any rights offering are exercised, we may offer any unsubscribed securities directly to persons other than stockholders, to or through agents,
underwriters or dealers or through a combination of such methods, including pursuant to standby underwriting arrangements, as described in the applicable prospectus supplement.
DESCRIPTION OF UNITS
We may offer and issue units that consist of shares of our common stock or preferred stock and warrants to purchase additional shares of our
common stock or preferred stock. For example, we may elect to issue units for a specified price per unit, with each unit consisting of one share of our common stock or preferred stock and one warrant to purchase an additional share of our common
stock or preferred stock at a specified price. The holder of a unit will also hold each of the securities that is included in the unit.
We have provided in the preceding sections of this prospectus a general description of our common stock, preferred stock, and warrants that we
may offer. If we elect to offer units, we will describe the specific terms of the units in a supplement to this prospectus. Among other things, the prospectus supplement will describe, to the extent applicable:
| |
• |
|
the price of each unit; |
| |
• |
|
the securities comprising each unit; |
| |
• |
|
the exercise price of the warrants comprising part of the units; |
| |
• |
|
the aggregate number of units offered; |
| |
• |
|
the number of shares of common stock or preferred stock that may be purchased upon the exercise of each warrant comprising part of a unit; |
| |
• |
|
the terms of any right by us to redeem any of the securities comprising the units; |
| |
• |
|
the date on which the right to exercise the warrants forming part of the units will commence and the date on which this right will expire; |
| |
• |
|
any transfer restrictions on the units, including whether the securities comprising the units may be transferred separately; |
| |
• |
|
the terms on which the units or warrants forming part of the units may be amended; |
| |
• |
|
with respect to preferred stock forming part of the units, the other matters listed above under “Description of Capital Stock—Preferred Stock”; |
| |
• |
|
with respect to warrants forming part of the units, the other matters listed above under “Description of Warrants”; and |
| |
• |
|
the material United States federal income tax consequences applicable to the units. |
PLAN OF DISTRIBUTION
We may sell the securities offered by this prospectus to one or more underwriters or dealers for public
offering and sale by them or to investors directly or through agents. The accompanying prospectus supplement will set forth the terms of the offering and the method of distribution and will identify any firms acting as underwriters, dealers or
agents in connection with the offering, including:
| |
• |
|
the name or names of any underwriters, dealers or agents; |
| |
• |
|
the purchase price of the securities and the proceeds to us or to any selling stockholder from the sale; |
| |
• |
|
any underwriting discounts and other items constituting compensation to underwriters, dealers or agents; |
| |
• |
|
any public offering price; |
16
Prospectus No. 1
| |
• |
|
any discounts or concessions allowed or reallowed or paid to dealers; and |
| |
• |
|
any securities exchange or market on which the securities offered in the prospectus supplement may be listed. |
Only those underwriters identified in such prospectus supplement are deemed to be underwriters in connection with the securities offered in
the prospectus supplement.
The distribution of the securities may be effected from time to time in one or more transactions at a fixed
price or prices, which may be changed, or at prices determined as the applicable prospectus supplement specifies. The securities may be sold through a rights offering, forward contracts or similar arrangements. In connection with the sale of the
securities, underwriters, dealers or agents may be deemed to have received compensation from us or selling stockholders in the form of underwriting discounts or commissions and also may receive commissions from securities purchasers for whom they
may act as agent. Underwriters may sell the securities to or through dealers, and the dealers may receive compensation in the form of discounts, concessions or commissions from the underwriters or commissions from the purchasers for whom they may
act as agent. Some of the underwriters, dealers or agents who participate in the securities distribution may engage in other transactions with, and perform other services for, us in the ordinary course of business.
We will provide in the applicable prospectus supplement information regarding any underwriting discounts or other compensation that we pay to
underwriters or agents in connection with the securities offering, and any discounts, concessions or commissions which underwriters allow to dealers. Underwriters, dealers and agents participating in the securities distribution may be deemed to be
underwriters, and any discounts and commissions they receive and any profit they realize on the resale of the securities may be deemed to be underwriting discounts and commissions under the Securities Act of 1933. Underwriters and their controlling
persons, dealers and agents may be entitled, under agreements entered into with us, to indemnification against and contribution toward specific civil liabilities, including liabilities under the Securities Act.
The securities may or may not be listed on a national securities exchange. In connection with an offering, the underwriters may purchase and
sell securities in the open market. These transactions may include short sales, stabilizing transactions and purchases to cover positions created by short sales. Short sales involve the sale by the underwriters of a greater number of securities than
they are required to purchase in an offering. Stabilizing transactions consist of bids or purchases made for the purpose of preventing or retarding a decline in the market price of the securities while an offering is in progress. The underwriters
also may impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the underwriters have repurchased securities sold by or for the account of that
underwriter in stabilizing or short-covering transactions. These activities by the underwriters may stabilize, maintain or otherwise affect the market price of the securities. As a result, the price of the securities may be higher than the price
that otherwise might exist in the open market. If these activities are commenced, they may be discontinued by the underwriters at any time.
Any underwriter who is qualified market maker on The Nasdaq Capital Market may engage in passive market making transactions in the securities
on The Nasdaq Capital Market in accordance with Rule 103 of Regulation M, during the business day prior to the pricing of the offering, before the commencement of offers or sales of the securities. Passive market makers must comply with applicable
volume and price limitations and must be identified as passive market makers. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for such security; if all independent bids are lowered
below the passive market maker’s bid, however, the passive market maker’s bid must then be lowered when certain purchase limits are exceeded.
Certain underwriters, dealers or agents and their associates may engage in transactions with and perform services for us in the ordinary
course of our business.
LEGAL MATTERS
TroyGould PC, Los Angeles, California, has rendered an opinion with respect to the securities offered by this prospectus. Sanford J.
Hillsberg, the Chairman of our company, is an attorney with TroyGould PC. TroyGould PC owned 63,941 shares of our common stock as of September 30, 2015.
EXPERTS
The consolidated financial statements of the Company as of December 31, 2014 and 2013, and for the years then ended, and the effectiveness of
internal control over financial reporting of the Company as of December 31, 2014, have been incorporated by reference herein in reliance upon the reports of Moss Adams LLP, independent registered public accounting firm, incorporated by reference
herein, and upon the authority of said firm as experts in accounting and auditing.
17
Prospectus No. 1
The consolidated financial statements of Galena Biopharma, Inc. for the year ended
December 31, 2012 incorporated by reference in this prospectus have been so incorporated in reliance on the report of BDO USA, LLP, an independent registered public accounting firm, incorporated herein by reference, given on the authority of
said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-3 with the SEC under the Securities Act of 1933. This prospectus is part of the registration
statement but the registration statement includes and incorporates by reference additional information and exhibits. We file annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy the
registration statement and any other document we file with the SEC at the public reference room maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. You may obtain information on the operation of the public reference room by calling
the SEC at 1-800-SEC-0330. The SEC also maintains a web site that contains reports, proxy and information statements and other information regarding companies, such as ours, that file documents electronically with the SEC. The address of that site
on the world wide web is http://www.sec.gov. The information on the SEC’s web site is not part of this prospectus, and any references to this web site or any other web site are inactive textual references only.
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
The SEC allows us to “incorporate by reference” information from other documents that we file with it, which means that we can
disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we
filed with the SEC prior to the date of this prospectus, while information that we file later with the SEC will automatically update and supersede the information in this prospectus. We incorporate by reference into this prospectus and the
registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC:
| |
• |
|
our Annual Report on Form 10-K for the year ended December 31, 2014, which was filed on March 5, 2015, and amended on March 10, 2015 (Items 6, 7, and 8 from said Annual Report have been recast for discontinued
operations as reflected in the Current Report on Form 8-K filed on December 4, 2015); |
| |
• |
|
our Quarterly Report on Form 10-Q for the three months ended March 31, 2015 filed with the SEC on May 7, 2015; |
| |
• |
|
our Quarterly Report on Form 10-Q for the three months ended June 30, 2015 filed with the SEC on August 6, 2015; |
| |
• |
|
our Quarterly Report on Form 10-Q for the three months ended September 30, 2015 filed with the SEC on November 9, 2015; |
| |
• |
|
our Current Reports on Form 8-K filed with the SEC on January 14, 2015, February 9, 2015, March 5, 2015, March 16, 2015, April 9, 2015, May 7, 2015, June 24, 2015, August 6, 2015, October 27, 2015, October 30, 2015,
November 20, 2015, and December 4, 2015, respectively; and |
| |
• |
|
the description of our common stock contained in our registration statement on Form 8-A (File No. 001-33958) filed pursuant to Section 12 of the Exchange Act, as amended from time to time. |
We also incorporate by reference into this prospectus all documents filed by us with the SEC pursuant to Sections 13(a), 13(c), 14
or 15(d) of the Securities Exchange Act of 1934 prior to the termination of this offering, including all such documents that we may file with the SEC after the date of the initial registration statement and prior to the effectiveness of the
registration statement, except that we are not incorporating any information furnished under Item 2.02 or Item 7.01 of any Current Report on Form 8-K we may subsequently file.
You may request a copy of the documents incorporated by reference into this prospectus, at no cost, by writing or telephoning us at the
following address:
Galena Biopharma, Inc.
2000 Crow Canyon Place, Suite 380
San Ramon, California 94583
Attention: Investor Relations
Phone: (855) 855-4253
Copies of these documents are also available, without charge, through the “Investor Relations” section of our website
(www.galenabiopharma.com) as soon as reasonably practicable after it are filed with the SEC. The information contained on our website is not a part of this prospectus.
18
Prospectus No. 1
PROSPECTUS

$100,000,000
Common Stock
Preferred
Stock
Warrants
Rights
Units
The date of this prospectus is December 22, 2015
SELLAS Life Sciences (NASDAQ:SLS)
Historical Stock Chart
From Mar 2024 to Apr 2024
SELLAS Life Sciences (NASDAQ:SLS)
Historical Stock Chart
From Apr 2023 to Apr 2024