Table of Contents
Filed pursuant to Rule 424(b)(4)
Registration Number 333-215730
PROSPECTUS
36,090,857 shares of common stock
This prospectus relates to the sale of up to 36,090,857 shares of our common stock by persons who have purchased shares in a series of private placements. The aforementioned persons are sometimes referred to in this prospectus as the selling stockholders. The shares offered under this prospectus by the selling stockholders may be sold on the public market, in negotiated transactions with a broker-dealer or market maker as principal or agent, or in privately negotiated transactions not involving a broker dealer. The prices at which the selling stockholder may sell the shares may be determined by the prevailing market price of the shares at the time of sale, may be different than such prevailing market prices or may be determined through negotiated transactions with third parties. We will not receive proceeds from the sale of our shares by the selling stockholders.
Each selling stockholder may be considered an “underwriter” within the meaning of the Securities Act of 1933, as amended.
Since January 23, 2008, our common stock has been quoted on the OTC Markets “OTCQB” marketplace (formerly known as the “OTC Bulletin Board”) under the trading symbol “BLGO.” The selling stockholders will sell up the shares at prices established on the OTC Bulletin Board during the term of this offering, at prices different than prevailing market prices or at privately negotiated prices. On September 12, 2019, the last reported sale price of our common stock on the OTC Markets was $0.235.
The securities offered in this prospectus involve a high degree of risk. You should consider the risk factors beginning on page 3 before purchasing our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is September 12, 2019
TABLE OF CONTENTS
Unless otherwise specified, the information in this prospectus is set forth as of September 12, 2019, and we anticipate that changes in our affairs will occur after such date. We have not authorized any person to give any information or to make any representations, other than as contained in this prospectus, in connection with the offer contained in this prospectus. If any person gives you any information or makes representations in connection with this offer, do not rely on it as information we have authorized. This prospectus is not an offer to sell our common stock in any state or other jurisdiction to any person to whom it is unlawful to make such offer.
PROSPECTUS SUMMARY
The following summary highlights selected information from this prospectus and may not contain all the information that is important to you. You should read this entire prospectus, including the section titled “Risk Factors,” and our financial statements and the notes included in the Annual Report on Form 10-K for year ended December 31, 2018 and Quarterly Report on Form 10-Q for the three- and six-month periods ended June 30, 2019, incorporated herein by reference, before deciding to invest in our Common Stock. When we refer in this prospectus to “BioLargo,” the “company,” “our company,” “we,” “us” and “our,” we mean BioLargo, Inc., a Delaware corporation, and its wholly owned subsidiaries, BioLargo Life Technologies, Inc., a California corporation, Odor-No-More, Inc., a California corporation, BioLargo Water Investment Group, Inc., a California corporation (and its subsidiary, BioLargo Water, Inc., a Canadian corporation), BioLargo Development Corp., a California corporation, BioLargo Engineering, Science & Technologies, LLC, Tennessee limited liability company, and our partially owned subsidiary Clyra Medical Technologies, Inc., a California corporation. This prospectus contains forward-looking statements and information relating to BioLargo. See “Cautionary Note Regarding Forward Looking Statements” on page 15.
Our Company
BioLargo, Inc. is a Delaware corporation.
Our principal executive offices are located at 14921 Chestnut St., Westminster, California 92683. Our telephone number is (888) 400-2863.
The Registration Statement
This prospectus covers 36,090,857 shares of stock, all of which are offered for sale by the selling stockholders.
ABOUT THIS REGISTRATION
|
|
|
|
Securities Being Registered
|
This Prospectus covers the following shares, all of which are being sold by the selling stockholders: 20,159,062 shares of common stock of BioLargo issuable upon the exercise of outstanding warrants to purchase common stock, and 15,931,795 outstanding shares.
|
|
|
|
|
Initial Offering Price
|
The selling stockholders will sell up to 36,090,857 shares at prices established on the OTC Electronic Bulletin Board during the term of this offering, at prices different than prevailing market prices or at privately negotiated prices. We may receive up to approximately $9.5 million in the event the selling stockholders exercise warrants to purchase 20,159,062 shares.
|
|
|
|
|
Termination of the Offering
|
The offering will conclude when all the 36,090,857 shares of common stock registered hereby have been sold by the selling stockholders.
|
|
|
|
|
Risk Factors
|
An investment in our common stock is highly speculative and involves a high degree of risk. See “Risk Factors” beginning on page 3.
|
RISK FACTORS
An investment in our common stock is highly speculative, involves a high degree of risk and should be made only by investors who can afford a complete loss. You should carefully consider the following risk factors, together with the other information in this prospectus, including our financial statements and the related notes, before you decide to buy our common stock. If any of the following risks actually occurs, then our business, financial condition or results of operations could be materially adversely affected, the trading of our common stock could decline, and you may lose all or part of your investment therein.
Risks Relating to our Business
Our limited operating history makes evaluation of our business difficult.
We have limited and only nominal historical financial data upon which to base planned operating expenses or forecast accurately our future operating results. Because our operations are not yet sufficient to fund our operational expenses, we rely on investor capital to fund operations. Our limited operational history make it difficult to forecast the need for future financing activities. Further, our limited operating history will make it difficult for investors and securities analysts to evaluate our business and prospects. Our failure to address these risks and difficulties successfully could seriously harm us.
We have never generated any significant revenues, have a history of losses, and cannot assure you that we will ever become or remain profitable.
We have not yet generated any significant revenue from operations, and, accordingly, we have incurred net losses every year since our inception. To date, we have dedicated most of our financial resources to research and development, general and administrative expenses, and initial sales and marketing activities. We have funded the majority of our activities through the issuance of convertible debt or equity securities. Although sale of our CupriDyne Clean products are increasing, and we are devoting more energy and money to our sales and marketing activities, we continue to anticipate net losses and negative cash flow for the foreseeable future. Our ability to reach positive cash flow depends on many factors, including our ability to fund sales and marketing activities, and the rate of client adoption. There can be no assurance that our revenues will be sufficient for us to become profitable in 2019 or future years, or thereafter maintain profitability. We may also face unforeseen problems, difficulties, expenses or delays in implementing our business plan, including generally the need for odor control products in solid waste handling operations, which we may not fully understand or be able to predict.
Our cash requirements are significant. We will require additional financing to sustain our operations and without it we may not be able to continue operations.
Our cash requirements and expenses will continue to be significant. Our net cash used in continuing operations for the year ended December 31, 2018 was almost $4,000,000, over $300,000 per month, and this trend has continued in 2019. During calendar year 2018, we generated only $1,364,000 in total gross revenues, and in the first six months of 2019, only $790,000. In order to become profitable, we must significantly increase our revenues and reduce our expenses. Although our revenues are increasing through sales of our products and from our engineering division, we expect to continue to use cash in 2019 as it becomes available.
At December 31, 2018 and June 30, 2019, we had working capital deficits of approximately $1,536,000 and $3,473,000. Our auditor’s report for the year ended December 31, 2018 includes an explanatory paragraph to their audit opinion stating that our recurring losses from operations and working capital deficiency raise substantial doubt about our ability to continue as a going concern. We do not currently have sufficient financial resources to fund our operations or those of our subsidiaries. Therefore, we need additional financing to continue these operations.
During 2019, we have raised over $4.0 million through the issuance of promissory notes and stock purchase warrants. These funds have been used to refinance existing debt (which was approximately $3.5 million as of December 31, 2018), and for working capital.
We have one long-term financing instrument in place. In August 2017, we entered into a three-year purchase agreement with Lincoln Park Capital Fund LLC (“Lincoln Park”) through which we may direct Lincoln Park to purchase shares of our common stock at prices that depend on the market price of our stock (the “LPC Agreement”). Over time, and subject to multiple limitations, we may direct Lincoln Park to purchase up to $10,000,000 of our common stock. Since inception of the LPC Agreement, through December 31, 2018, we directed Lincoln Park to purchase 4,025,733 shares of our common stock, and received $1,349,969 in proceeds. During the year ended December 31, 2018, we directed Lincoln Park to purchase 2,850,733 shares of our common stock, and received $838,884 in proceeds. As of the date of this prospectus, we have not used this financing instrument in 2019. The extent to which we rely on Lincoln Park as a source of funding in 2019 will depend on a number of factors, including the prevailing market price of our common stock, and the extent to which we are able to secure working capital from other sources. If obtaining sufficient funding from Lincoln Park were to prove unavailable or prohibitively dilutive, we will need to secure another source of funding in order to satisfy our working capital needs. Even if we were receive the full maximum commitment of $10,000,000 in aggregate gross proceeds from sales of our common stock to Lincoln Park during the three year term of the LPC Agreement, we may still need additional capital to fully implement our business, operating and development plans. Should the financing we require to sustain our working capital needs be unavailable or prohibitively expensive when we require it, the consequences could be a material adverse effect on our business, operating results, financial condition and prospects.
From time to time, we issue stock, instead of cash, to pay some of our operating expenses. These issuances are dilutive to our existing stockholders.
We are party to agreements that provide for the payment of, or permit us to pay at our option, securities rather than cash in consideration for services provided to us. We include these provisions in agreements to allow us to preserve cash. When we pay employees, vendors and consultants in stock or stock options, we do so at a premium. We anticipate that we will continue to do so in the future. All such issuances are dilutive to our stockholders because they increase (and will increase in the future) the total number of shares of our common stock issued and outstanding, even though such arrangements assist us with managing our cash flow. These issuances also increase the expense amount recorded.
Our stockholders face further potential dilution in any new financing.
Our private securities offerings typically provide for convertible securities, including notes and warrants. Any additional capital that we raise would dilute the interest of the current stockholders and any persons who may become stockholders before such financing. Given the low price of our common stock, such dilution in any financing of a significant amount could be substantial.
Our stockholders face further potential adverse effects from the terms of any preferred stock that may be issued in the future.
Our certificate of incorporation authorizes 50 million shares of preferred stock. None are outstanding as of the date of this prospectus. In order to raise capital to meet expenses or to acquire a business, our board of directors may issue additional stock, including preferred stock. Any preferred stock that we may issue may have voting rights, liquidation preferences, redemption rights and other rights, preferences and privileges. The rights of the holders of our common stock will be subject to, and in many respects subordinate to, the rights of the holders of any such preferred stock. Furthermore, such preferred stock may have other rights, including economic rights, senior to our common stock that could have a material adverse effect on the value of our common stock. Preferred stock, while providing desirable flexibility in connection with possible acquisitions and other corporate purposes, can also have the effect of making it more difficult for a third party to acquire a majority of our outstanding voting stock, thereby delaying, deferring or preventing a change in control of our company.
There are several specific business opportunities we are considering in further development of our business. None of these opportunities is yet the subject of a definitive agreement, and most or all of these opportunities will require additional funding obligations on our part, for which funding is not currently in place.
In furtherance of our business plan, we are presently considering a number of opportunities to promote our business, to further develop and broaden, and to license, our technology with third parties. While discussions are underway with respect to such opportunities, there are no definitive agreements in place with respect to any of such opportunities at this time. There can be no assurance that any of such opportunities being discussed will result in definitive agreements or, if definitive agreements are entered into, that they will be on terms that are favorable to us.
Moreover, should any of these opportunities result in definitive agreements being executed or consummated, we may be required to expend additional monies above and beyond our current operating budget to promote such endeavors. No such financing is in place at this time for such endeavors, and we cannot assure you that any such financing will be available, or if it is available, whether it will be on terms that are favorable to our company.
We expect to incur future losses and may not be able to achieve profitability.
Although we are generating limited revenue from the sale of our products, and we expect to generate revenue from new products we are introducing, and eventually from other license or supply agreements, we anticipate net losses and negative cash flow to continue for the foreseeable future until our products are expanded in the marketplace and they gain broader acceptance by resellers and customers. Our current level of sales is not sufficient to support the financial needs of our business. We cannot predict when or if sales volumes will be sufficiently large to cover our operating expenses. We intend to expand our marketing efforts of our products as financial resources are available, and we intend to continue to expand our research and development efforts. Consequently, we will need to generate significant additional revenue or seek additional financings to fund our operations. This has put a proportionate corresponding demand on capital. Our ability to achieve profitability is dependent upon our efforts to deliver a viable product and our ability to successfully bring it to market, which we are currently pursuing. Although our management is optimistic that we will succeed in licensing our technology, we cannot be certain as to timing or whether we will generate sufficient revenue to be able to operate profitably. If we cannot achieve or sustain profitability, then we may not be able to fund our expected cash needs or continue our operations. If we are not able to devote adequate resources to promote commercialization of our technology, then our business plans will suffer and may fail.
Because we have limited resources to devote to sales, marketing and licensing efforts with respect to our technology, any delay in such efforts may jeopardize future research and development of technologies and commercialization of our technology. Although our management believes that it can finance commercialization efforts through sales of our securities and possibly other capital sources, if we do not successfully bring our technology to market, our ability to generate revenues will be adversely affected.
Our internal controls are not effective.
We have determined that our disclosure controls and procedures and our internal control over financial reporting are currently not effective. The lack of effective internal controls could materially adversely affect our financial condition and ability to carry out our business plan.
Our management team for financial reporting, under the supervision and with the participation of our chief executive officer and our chief financial officer, conducted an evaluation of the effectiveness of the design and operation of our internal controls. Recognizing the dynamic nature and growth of the Company’s business in the past two years, including the growth of the core operations and the increase in the number of employees, management has recognized the strain on the overall internal control environment. As a result, management has concluded that its internal controls over financial reporting are not effective. Management identified a material weakness with respect to deficiencies in its financial closing and reporting procedures. Management believes this is due to a lack of resources. Management intends to add accounting personnel and operating staff and more sophisticated systems in order to improve its reporting procedures and internal controls, subject to available capital. Until we have adequate resources to address these issues, any material weaknesses may materially adversely affect our ability to report accurately our financial condition and results of operations in the future in a timely and reliable manner. In addition, although we continually review and evaluate internal control systems to allow management to report on the sufficiency of our internal controls, we cannot assure you that we will not discover additional weaknesses in our internal control over financial reporting. Any such additional weakness or failure to remediate the existing weakness could materially adversely affect our financial condition or ability to comply with applicable financial reporting requirements and the requirements of the Company’s various financing agreements.
If we are not able to manage our anticipated growth effectively, we may not become profitable.
We anticipate that expansion will continue to be required to address potential market opportunities for our technology and our products. Our existing infrastructure is limited. While we believe our current manufacturing processes as well as our office and warehousing provide the basic resources to expand as we grow sales of CupriDyne Clean to more than $2 million per month, our infrastructure will need more staffing to support manufacturing, customer service, administration as well as sales/account executive functions. There can be no assurance that we will have the financial resources to create new infrastructure, or that any such infrastructure will be sufficiently scalable to manage future growth, if any. There also can be no assurance that, if we invest in additional infrastructure, we will be effective in expanding our operations or that our systems, procedures or controls will be adequate to support such expansion. In addition, we will need to provide additional sales and support services to our partners if we achieve our anticipated growth with respect to the sale of our technology for various applications. Failure to properly manage an increase in customer demands could result in a material adverse effect on customer satisfaction, our ability to meet our contractual obligations, and our operating results.
Some of the products incorporating our technology will require regulatory approval.
The products in which our technology may be incorporated have both regulated and non-regulated applications. The regulatory approvals for certain applications may be difficult, impossible, time consuming and/or expensive to obtain. While our management believes such approvals can be obtained for the applications contemplated, until those approvals from the FDA or the EPA or other regulatory bodies, at the federal and state levels, as may be required are obtained, we may not be able to generate commercial revenues for regulated products. Certain specific regulated applications and their use require highly technical analysis and additional third-party validation and will require regulatory approvals from organizations like the FDA. Certain applications may also be subject to additional state and local agency regulations, increasing the cost and time associated with commercial strategies. Additionally, most products incorporating our technology that may be sold in the European Union (“EU”) will require EU and possibly also individual country regulatory approval. All such approvals, including additional testing, are time-consuming, expensive and do not have assured outcomes of ultimate regulatory approval.
We need to outsource and rely on third parties for the manufacture of the chemicals, material components or delivery apparatus used in our technology, and part of our future success will be dependent on the timeliness and effectiveness of the efforts of these third parties.
We do not have the required financial and human resources or capability to manufacture the chemicals necessary to make our odor control products. Our business model calls for the outsourcing of the manufacture of these chemicals in order to reduce our capital and infrastructure costs as a means of potentially improving our financial position and the profitability of our business. Accordingly, we must enter agreements with other companies that can assist us and provide certain capabilities, including sourcing and manufacturing, which we do not possess. We may not be successful in entering into such alliances on favorable terms or at all. Even if we do succeed in securing such agreements, we may not be able to maintain them. Furthermore, any delay in entering into agreements could delay the development and commercialization of our technology or reduce its competitiveness even if it reaches the market. Any such delay related to such future agreements could adversely affect our business.
If any party to which we have outsourced certain functions fails to perform its obligations under agreements with us, the commercialization of our technology could be delayed or curtailed.
To the extent that we rely on other companies to manufacture the chemicals used in our technology, or sell or market products incorporating our technology, we will be dependent on the timeliness and effectiveness of their efforts. If any of these parties does not perform its obligations in a timely and effective manner, the commercialization of our technology could be delayed or curtailed because we may not have sufficient financial resources or capabilities to continue such efforts on our own.
We rely on a small number of key supply ingredients in order to manufacture our products.
All of the supply ingredients used to manufacture our products are readily available from multiple suppliers. However, commodity prices for these ingredients can vary significantly, and the margins that we are able to generate could decline if prices rise. If our manufacturing costs rise significantly, we may be forced to raise the prices for our products, which may reduce their acceptance in the marketplace.
If our technology or products incorporating our technology do not gain market acceptance, it is unlikely that we will become profitable.
The potential markets for products into which our technology can be incorporated are rapidly evolving, and we have many successful competitors including some of the largest and most well-established companies in the world. (see, herein: “Description At this time, our technology is unproven in all but one industry – waste management – and the use of our technology by others, and the sales of our products, is relatively nominal. The commercial success of products incorporating our technology will depend on the adoption of our technology by commercial and consumer end users in various fields.
Market acceptance may depend on many factors, including:
|
|
●
|
the willingness and ability of consumers and industry partners to adopt new technologies from a company with little or no history in the industry;
|
|
|
●
|
our ability to convince potential industry partners and consumers that our technology is an attractive alternative to other competing technologies;
|
|
|
●
|
our ability to license our technology in a commercially effective manner;
|
|
|
●
|
our ability to continue to fund operations while our products move through the process of gaining acceptance, before the time in which we are able to scale up production to obtain economies of scale; and
|
|
|
●
|
our ability to overcome brand loyalties.
|
If products incorporating our technology do not achieve a significant level of market acceptance, then demand for our technology itself may not develop as expected, and, in such event, it is unlikely that we will become profitable.
Any revenues that we may earn in the future are unpredictable, and our operating results are likely to fluctuate from quarter to quarter.
We believe that our future operating results will fluctuate due to a variety of factors, including:
|
|
●
|
delays in product development by us or third parties;
|
|
|
●
|
market acceptance of products incorporating our technology;
|
|
|
●
|
changes in the demand for, and pricing of, products incorporating our technology;
|
|
|
●
|
competition and pricing pressure from competitive products; and
|
|
|
●
|
expenses related to, and the results of, proceedings relating to our intellectual property.
|
We expect our operating expenses will continue to fluctuate significantly in 2019 and beyond, as we continue our research and development and increase our marketing and licensing activities. Although we expect to generate revenues from licensing our technology in the future, revenues may decline or not grow as anticipated, and our operating results could be substantially harmed for a particular fiscal period. Moreover, our operating results in some quarters may not meet the expectations of stock market analysts and investors. In that case, our stock price most likely would decline.
Some of our revenue is dependent on the award of new contracts from the U.S. government, which we do not directly control.
A substantial portion of our revenue and is generated from sales to the U.S. defense logistics agency through a bid process in response to request for bids. The timing and size of requests for bids is unpredictable and outside of our control. The number of other companies competing for these bids is also unpredictable and outside of our control. In the event of more competition for these awards, we may have to reduce our margins. These variables make it difficult to predict when or if we will sell more products to the US government, which in turns makes it difficult to stock inventory and purchase raw materials.
We have limited product distribution experience, and we rely in part on third parties who may not successfully sell our products.
We have limited product distribution experience and rely in part on product distribution arrangements with third parties. In our future product offerings, we may rely solely on third parties for product sales and distribution. We also plan to license our technology to certain third parties for commercialization of certain applications. We expect to enter into additional distribution agreements and licensing agreements in the future, and we may not be able to enter into these additional agreements on terms that are favorable to us, if at all. In addition, we may have limited or no control over the distribution activities of these third parties. These third parties could sell competing products and may devote insufficient sales efforts to our products. As a result, our future revenues from sales of our products, if any, will depend on the success of the efforts of these third parties.
We may not be able to attract or retain qualified senior personnel.
We believe we are currently able to manage our current business with our existing management team. However, as we expand the scope of our operations, we will need to obtain the full-time services of additional senior management and other personnel. Competition for highly-skilled personnel is intense, and there can be no assurance that we will be able to attract or retain qualified senior personnel. Our failure to do so could have an adverse effect on our ability to implement our business plan. As we add full-time senior personnel, our overhead expenses for salaries and related items will increase from current levels and, depending upon the number of personnel we hire and their compensation packages, these increases could be substantial.
If we lose our key personnel or are unable to attract and retain additional personnel, we may be unable to achieve profitability.
Our future success is substantially dependent on the efforts of our senior management, particularly Dennis P. Calvert, our president and chief executive officer. The loss of the services of Mr. Calvert or other members of our senior management may significantly delay or prevent the achievement of product development and other business objectives. Because of the scientific nature of our business, we depend substantially on our ability to attract and retain qualified marketing, scientific and technical personnel. There is intense competition among specialized and technologically-oriented companies for qualified personnel in the areas of our activities. If we lose the services of, or do not successfully recruit, key marketing, scientific and technical personnel, then the growth of our business could be substantially impaired. At present, we do not maintain key man insurance for any of our senior management, although management is evaluating the potential of securing this type of insurance in the future as may be available.
Nondisclosure agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information.
In order to protect our proprietary technology and processes, we rely in part on nondisclosure agreements with our employees, potential licensing partners, potential manufacturing partners, testing facilities, universities, consultants, agents and other organizations to which we disclose our proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover trade secrets and proprietary information, and in such cases we could not assert any trade secret rights against such parties. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position. Since we rely on trade secrets and nondisclosure agreements, in addition to patents, to protect some of our intellectual property, there is a risk that third parties may obtain and improperly utilize our proprietary information to our competitive disadvantage. We may not be able to detect unauthorized use or take appropriate and timely steps to enforce our intellectual property rights.
We may become subject to product liability claims.
As a business that manufactures and markets products for use by consumers and institutions, we may become liable for any damage caused by our products, whether used in the manner intended or not. Any such claim of liability, whether meritorious or not, could be time-consuming and/or result in costly litigation. Although we maintain general liability insurance, our insurance may not cover potential claims of the types described above and may not be adequate to indemnify for all liabilities that may be imposed. Any imposition of liability that is not covered by insurance or is in excess of insurance coverage could harm our business and operating results, and you may lose some or all of any investment you have made, or may make, in our company.
Litigation or the actions of regulatory authorities may harm our business or otherwise distract our management.
Substantial, complex or extended litigation could cause us to incur major expenditures and distract our management. For example, lawsuits by employees, former employees, investors, stockholders, partners, customers or others, or actions taken by regulatory authorities, could be very costly and substantially disrupt our business. As a result of our financing activities over time, and by virtue of the number of people that have invested in our company, we face increased risk of lawsuits from investors. Such lawsuits or actions could from time to time be filed against our company and/or our executive officers and directors. Such lawsuits and actions are not uncommon, and we cannot assure you that we will always be able to resolve such disputes or actions on terms favorable to our company.
If we suffer negative publicity concerning the safety or efficacy of our products, our sales may be harmed.
If concerns should arise about the safety or efficacy of any of our products that are marketed, regardless of whether or not such concerns have a basis in generally accepted science or peer-reviewed scientific research, such concerns could adversely affect the market for those products. Similarly, negative publicity could result in an increased number of product liability claims, whether or not those claims are supported by applicable law.
The licensing of our technology or the manufacture, use or sale of products incorporating our technology may infringe on the patent rights of others, and we may be forced to litigate if an intellectual property dispute arises.
If we infringe or are alleged to have infringed another party’s patent rights, we may be required to seek a license, defend an infringement action or challenge the validity of the patents in court. Patent litigation is costly and time consuming. We may not have sufficient resources to bring these actions to a successful conclusion. In addition, if we do not obtain a license, do not successfully defend an infringement action or are unable to have infringed patents declared invalid, we may:
|
|
●
|
incur substantial monetary damages;
|
|
|
●
|
encounter significant delays in marketing our current and proposed product candidates;
|
|
|
●
|
be unable to conduct or participate in the manufacture, use or sale of product candidates or methods of treatment requiring licenses;
|
|
|
●
|
lose patent protection for our inventions and products; or
|
|
|
●
|
find our patents are unenforceable, invalid or have a reduced scope of protection
|
Parties making such claims may be able to obtain injunctive relief that could effectively block our company’s ability to further develop or commercialize our current and proposed product candidates in the United States and abroad and could result in the award of substantial damages. Defense of any lawsuit or failure to obtain any such license could substantially harm our company. Litigation, regardless of outcome, could result in substantial cost to, and a diversion of efforts by, our company.
Our patents are expensive to maintain, our patent applications are expensive to prosecute, and thus we are unable to file for patent protection in many countries.
Our ability to compete effectively will depend in part on our ability to develop and maintain proprietary aspects of our technology and either to operate without infringing the proprietary rights of others or to obtain rights to technology owned by third parties. Pending patent applications relating to our technology may not result in the issuance of any patents or any issued patents that will offer protection against competitors with similar technology. We must employ patent attorneys to prosecute our patent applications both in the United States and internationally. International patent protection requires the retention of patent counsel and the payment of patent application fees in each foreign country in which we desire patent protection, on or before filing deadlines set forth by the International Patent Cooperation Treaty (“PCT”). We therefore choose to file patent applications only in foreign countries where we believe the commercial opportunities require it, considering our available financial resources and the needs for our technology. This has resulted, and will continue to result, in the irrevocable loss of patent rights in all but a few foreign jurisdictions.
Patents we receive may be challenged, invalidated or circumvented in the future, or the rights created by those patents may not provide a competitive advantage. We also rely on trade secrets, technical know-how and continuing invention to develop and maintain our competitive position. Others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets.
We are subject to risks related to future business outside of the United States.
Over time, we may develop business relationships outside of North America, and as those efforts are pursued, we will face risks related to those relationships such as:
|
|
●
|
foreign currency fluctuations;
|
|
|
●
|
unstable political, economic, financial and market conditions;
|
|
|
●
|
import and export license requirements;
|
|
|
●
|
increases in tariffs and taxes;
|
|
|
●
|
high levels of inflation;
|
|
|
●
|
restrictions on repatriating foreign profits back to the United States;
|
|
|
●
|
greater difficulty collecting accounts receivable and longer payment cycles;
|
|
|
●
|
less favorable intellectual property laws, and the lack of intellectual property legal protection;
|
|
|
●
|
regulatory requirements;
|
|
|
●
|
unfamiliarity with foreign laws and regulations; and
|
|
|
●
|
changes in labor conditions and difficulties in staffing and managing international operations.
|
The volatility of certain raw material costs may adversely affect operations and competitive price advantages for products that incorporate our technology.
Most of the chemicals and other key materials that we use in our business, such as minerals, fiber materials and packaging materials, are neither generally scarce nor price sensitive, but prices for such chemicals and materials can be cyclical. Super Absorbent Polymer (SAP) beads, which are a petrochemical derivative, have been subject to periodic scarcity and price volatility from time to time during recent years, although prices are relatively stable at present. Should the volume of our sales increase dramatically, we may have difficulty obtaining SAP beads or other raw materials at a favorable price. Supply and demand factors, which are beyond our control, generally affect the price of our raw materials. We try to minimize the effect of price increases through production efficiency and the use of alternative suppliers, but these efforts are limited by the size of our operations. If we are unable to minimize the effects of increased raw material costs, our business, financial condition, results of operations and cash flows may be materially adversely affected.
Certain of our products sales historically have been highly impacted by fluctuations in seasons and weather.
Industrial odor control products have proven highly effective in controlling volatile organic compounds that are released as vapors produced by decomposing waste material. Such vapors are produced with the highest degree of intensity in temperatures between 40 degrees Fahrenheit (5 degrees Celsius) and 140 degrees Fahrenheit (60 degrees Celsius). When weather patterns are cold or in times of precipitation, our clients are less prone to use our odor control products, presumably because such vapors are less noticeable or, in the case of precipitation, can be washed away or altered. This leads to unpredictability in use and sales patterns for, especially, our CupriDyne Clean product line which accounts for over one-half our total sales.
The cost of maintaining our public company reporting obligations is high.
We are obligated to maintain our periodic public filings and public reporting requirements, on a timely basis, under the rules and regulations of the SEC. In order to meet these obligations, we will need to continue to raise capital. If adequate funds are not available, we will be unable to comply with those requirements and could cease to be qualified to have our stock traded in the public market. As a public company, we incur significant legal, accounting and other expenses. In addition, the Sarbanes-Oxley Act of 2002, as well as related rules adopted by the SEC, has imposed substantial requirements on public companies, including certain corporate governance practices and requirements relating to internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act.
Risks Relating to our Common Stock
The sale or issuance of our common stock to Lincoln Park may cause dilution, and the sale of the shares of common stock acquired by Lincoln Park, or the perception that such sales may occur, could cause the price of our common stock to fall.
On August 25, 2017, we entered into the LPC Agreement with Lincoln Park, pursuant to which Lincoln Park has committed to purchase up to $10,000,000 of our common stock, noted above in our Risks Related to our Business. We generally have the right to control the timing and amount of any sales of our shares to Lincoln Park. Sales of our common stock, if any, to Lincoln Park will depend on market conditions and other factors to be determined by us. We may ultimately decide to sell to Lincoln Park all, some or none of the shares of our common stock that may be available for us to sell pursuant to the LPC Agreement. If and when we do sell shares to Lincoln Park, after Lincoln Park has acquired the shares, Lincoln Park may resell all, some or none of those shares at any time or from time to time in its discretion. Therefore, sales to Lincoln Park by us could result in substantial dilution to the interests of other holders of our common stock, as well as sales of our stock by Lincoln Park into the open market causing reductions in the price of our common stock. Additionally, the sale of a substantial number of shares of our common stock to Lincoln Park, or the anticipation of such sales, could make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise desire to effect sales.
Our common stock is thinly traded and largely illiquid.
Our stock is currently quoted on the OTC Markets (OTCQB). Being quoted on the OTCQB has made it more difficult to buy or sell our stock and from time to time has led to a significant decline in the frequency of trades and trading volume. Continued trading on the OTCQB will also likely adversely affect our ability to obtain financing in the future due to the decreased liquidity of our shares and other restrictions that certain investors have for investing in OTCQB traded securities. While we intend to seek listing on the Nasdaq Stock Market (“Nasdaq”) or another national stock exchange when our company is eligible, there can be no assurance when or if our common stock will be listed on Nasdaq or another national stock exchange.
The market price of our stock is subject to volatility.
Because our stock is thinly traded, its price can change dramatically over short periods, even in a single day. An investment in our stock is subject to such volatility and, consequently, is subject to significant risk. The market price of our common stock could fluctuate widely in response to many factors, including:
|
|
●
|
developments with respect to patents or proprietary rights;
|
|
|
●
|
announcements of technological innovations by us or our competitors;
|
|
|
●
|
announcements of new products or new contracts by us or our competitors;
|
|
|
●
|
actual or anticipated variations in our operating results due to the level of development expenses and other factors;
|
|
|
●
|
changes in financial estimates by securities analysts and whether any future earnings of ours meet or exceed such estimates;
|
|
|
●
|
conditions and trends in our industry;
|
|
|
●
|
new accounting standards;
|
|
|
●
|
general economic, political and market conditions and other factors; and
|
|
|
●
|
the occurrence of any of the risks described herein.
|
You may have difficulty selling our shares because they are deemed “penny stocks”.
Because our common stock is not quoted or listed on a national securities exchange, if the trading price of our common stock remains below $5.00 per share, which we expect for the foreseeable future, trading in our common stock will be subject to the requirements of certain rules promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which require additional disclosure by broker-dealers in connection with any trades involving a stock defined as a penny stock (generally, any non-Nasdaq equity security that has a market price of less than $5.00 per share, subject to certain exceptions). Such rules require the delivery, before any penny stock transaction, of a disclosure schedule explaining the penny stock market and the risks associated therewith and impose various sales practice requirements on broker-dealers who sell penny stocks to persons other than established customers and accredited investors (generally defined as an investor with a net worth in excess of $1,000,000 or annual income exceeding $200,000 individually or $300,000 together with a spouse). For these types of transactions, the broker-dealer must make a special suitability determination for the purchaser and have received the purchaser’s written consent to the transaction before the sale. The broker-dealer also must disclose the commissions payable to the broker-dealer and current bid and offer quotations for the penny stock and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market. Such information must be provided to the customer orally or in writing before or with the written confirmation of trade sent to the customer. Monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks. The additional burdens imposed on broker-dealers by such requirements could discourage broker-dealers from effecting transactions in our common stock, which could severely limit the market liquidity of our common stock and the ability of holders of our common stock to sell their shares.
Because our shares are deemed “penny stocks,” new rules make it more difficult to remove restrictive legends.
Rules put in place by the Financial Industry Regulatory Authority (FINRA) require broker-dealers to perform due diligence before depositing unrestricted common shares of penny stocks, and as such, some broker-dealers, including many national firms (such as eTrade and Charles Schwab), are refusing to deposit previously restricted common shares of penny stocks. As such, it may be more difficult for purchases of shares in our private securities offerings to deposit the shares with broker-dealers and sell those shares on the open market.
Because we will not pay dividends in the foreseeable future, stockholders will only benefit from owning common stock if it appreciates.
We have never declared or paid a cash dividend to stockholders. We intend to retain any earnings that may be generated in the future to finance operations. Accordingly, any potential investor who anticipates the need for current dividends from his investment should not purchase our common stock.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
All statements, other than statements of historical fact, included in this prospectus regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects and plans and objectives of management are forward-looking statements. The words “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
We have based these forward-looking statements on our current expectations and projections about future events. Although we believe that the expectations underlying our forward-looking statements are reasonable, these expectations may prove to be incorrect, and all of these statements are subject to risks and uncertainties. Therefore, you should not place undue reliance on our forward-looking statements. We have included important risks and uncertainties in the cautionary statements included in this prospectus, particularly the section titled “Risk Factors” incorporated by reference herein. We believe these risks and uncertainties could cause actual results or events to differ materially from the forward-looking statements that we make. Should one or more of these risks and uncertainties materialize, or should underlying assumptions, projections or expectations prove incorrect, actual results, performance or financial condition may vary materially and adversely from those anticipated, estimated or expected. Our forward-looking statements do not reflect the potential impact of future acquisitions, mergers, dispositions, joint ventures or investments that we may make. We do not assume any obligation to update any of the forward-looking statements contained herein, whether as a result of new information, future events or otherwise, except as required by law. In the light of these risks and uncertainties, the forward-looking events and circumstances discussed in this prospectus may not occur, and actual results could differ materially from those anticipated or implied in the forward-looking statements. Any forward-looking statement made by us in this prospectus is based only on information currently available to us and speaks only as of the date on which it is made.
USE OF PROCEEDS
This prospectus relates to shares of our common stock that may be offered and sold from time to time by the selling stockholders upon exercise of outstanding warrants to purchase common stock. We will receive no proceeds from the sale of shares of common stock by the selling stockholders in this offering. We may receive up to $9.5 million in aggregate gross proceeds upon exercise of the underlying warrants. See “Plan of Distribution” elsewhere in this prospectus for more information.
We expect to use any proceeds that we receive under the exercise of the warrants to help fund general working capital for our corporate operations and repayment of debt
DIVIDEND POLICY
We have never declared or paid a cash dividend to stockholders. We intend to retain any earnings that may be generated in the future to finance operations.
CAPITALIZATION
The following table sets forth our actual cash and cash equivalents and our capitalization as of December 31, 2018 and June 30, 2019, and as adjusted to give effect to the exercise of the warrants underlying the shares of common stock offered hereby.
You should read this information in conjunction with “Managements’ Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes appearing in our Annual Report on Form 10-K for the periods ended December 31, 2018 and our unaudited financial statements appearing in the Quarterly Report on Form 10-Q for the three and six months ended June 30, 2019.
|
|
|
December 31, 2018
(in thousands)
|
|
|
June 30, 2019
(in thousands)
|
|
|
|
|
Audited
|
|
|
Unaudited
|
|
|
As Adjusted(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
655
|
|
|
$
|
706
|
|
|
$
|
10,181
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
$
|
4,308
|
|
|
$
|
5,502
|
|
|
$
|
5,502
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ DEFICIT:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible Preferred Series A, $.00067 Par Value, 50,000,000 Shares Authorized, -0- Shares Issued and Outstanding, at June 30, 2019 and as adjusted.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock, $.00067 Par Value, 400,000,000 Shares Authorized, 141,466,071 and 152,054,904 Shares Issued at December 31, 2018 and June 30, 2019, respectively, and 172,213,966 as adjusted
|
|
|
95
|
|
|
|
102
|
|
|
|
116
|
|
|
Additional paid-in capital
|
|
|
110,222
|
|
|
|
114,745
|
|
|
|
124,206
|
|
|
Accumulated other comprehensive loss
|
|
|
(90
|
)
|
|
|
(98
|
)
|
|
|
(98
|
)
|
|
Accumulated deficit
|
|
|
(111,723
|
)
|
|
|
(116,876
|
)
|
|
|
(116,876
|
)
|
|
Total BioLargo stockholders’ deficit
|
|
|
(1,496
|
)
|
|
|
(2,127
|
)
|
|
|
7,348
|
|
|
Non-controlling interest
|
|
|
373
|
|
|
|
208
|
|
|
|
208
|
|
|
Total stockholders’ deficit
|
|
|
(1,123
|
)
|
|
|
(1,919
|
)
|
|
|
7,556
|
|
|
Total liabilities and stockholders’ deficit
|
|
$
|
3,185
|
|
|
$
|
3,583
|
|
|
$
|
13,058
|
|
|
(1)
|
Assumes the selling stockholders exercise all 20,159,062 shares available for purchase under the stock purchase warrants, at an aggregate average exercise price $0.47 per share. Given our stock currently trades at less than $0.50 per share, we do not expect the selling stockholders will exercise all warrants, and thus do not expect to receive $9,475,000 cash as reflected in the “as adjusted” column in the above table.
|
DILUTION
The net tangible book value of our company as of June 30, 2019 was negative $3,812,000 or approximately $(0.025) per share of common stock. Net tangible book value per share is determined by dividing the net tangible book value of our company (total tangible assets less total liabilities) by the number of outstanding shares of our common stock.
Assuming net proceeds of $9,475,000 from the sale of shares to the selling stockholders pursuant to the stock purchase warrants (see Note 1 in the Capitalization section immediately above), our adjusted net tangible book value as of June 30, 2019 would have been $5,663,000 or $0.033 per share. This represents an immediate increase in net tangible book value of approximately $0.058 per share to existing stockholders.
MARKET PRICE OF AND DIVIDENDS ON COMMON EQUITY
AND RELATED STOCKHOLDER MATTERS
Market Information
Since January 23, 2008, our common stock has been quoted on the OTC Markets “OTCQB” marketplace (formerly known as the “OTC Bulletin Board”) under the trading symbol “BLGO”.
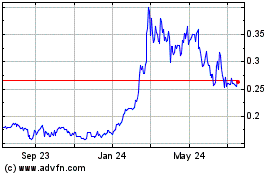
The table below represents the quarterly high and low closing prices of our common stock for the last three fiscal years as reported by www.otcmarkets.com.
|
|
|
2017
|
|
|
2018
|
|
|
2019
|
|
|
|
|
High
|
|
|
Low
|
|
|
High
|
|
|
Low
|
|
|
High
|
|
|
Low
|
|
|
First Quarter
|
|
$
|
0.83
|
|
|
$
|
0.47
|
|
|
$
|
0.41
|
|
|
$
|
0.21
|
|
|
$
|
0.27
|
|
|
$
|
0.16
|
|
|
Second Quarter
|
|
$
|
0.53
|
|
|
$
|
0.39
|
|
|
$
|
0.45
|
|
|
$
|
0.23
|
|
|
$
|
0.31
|
|
|
$
|
0.16
|
|
|
Third Quarter
|
|
$
|
0.66
|
|
|
$
|
0.42
|
|
|
$
|
0.45
|
|
|
$
|
0.22
|
|
|
|
N/A
|
|
|
|
N/A
|
|
|
Fourth Quarter
|
|
$
|
0.52
|
|
|
$
|
0.39
|
|
|
$
|
0.30
|
|
|
$
|
0.18
|
|
|
|
N/A
|
|
|
|
N/A
|
|
The closing price for our common stock on September 13, 2017, was $0.51 per share. The closing price for our common stock on September 12, 2019, was $0.235 per share.
Holders of our Common Stock
As of September 12, 2019, 157,604,022 shares of our common stock were outstanding and held of record by approximately 530 stockholders of record, and approximately 2,600 beneficial owners.
Dividends
We have never declared or paid a cash dividend to stockholders. We intend to retain any earnings that may be generated in the future to finance operations.
Securities Authorized for Issuance Under Equity Compensation Plans
On March 7, 2018, our board of directors adopted BioLargo, Inc. 2018 Equity Incentive Plan (“2018 Equity Plan”) as a means of providing our directors, key employees, and consultants additional incentive to provide services. This plan was approved by our stockholders at our annual meeting on May 23, 2018. The Compensation Committee administers this plan, except for awards made to non-employee directors. The plan allows for the grant of stock options, restricted stock awards, stock bonus awards, stock appreciation rights, restricted stock units and performance awards in any combination, separately or in tandem. Subject to the terms of the 2018 Equity Plan, the Compensation Committee will determine the terms and conditions of awards, including the times when awards vest or become payable and the effect of certain events such as termination of employment. Under the 2018 Equity Plan, 40,000,000 shares of our common stock are reserved for issuance under awards. Each January 1, through January 1, 2028, the number of shares available for grant and issuance will be increased by the lesser of 2,000,000 and such number of shares set by the Board. As of December 31, 2018, and June 30, 2019, we had issued options under the plan to purchase 1,318,517 and 5,046,883 shares, respectively.
On August 7, 2007, our board of directors adopted the BioLargo, Inc. 2007 Equity Incentive Plan (“2007 Equity Plan”) as a means of providing our directors, key employees, and consultants additional incentive to provide services. This plan expired on September 6, 2017. The Compensation Committee administers this plan. The plan allowed for grants of common shares or options to purchase common shares. As plan administrator, the Compensation Committee has sole discretion to set the price of the options. The Compensation Committee may at any time amend the plan.
Under the 2007 Equity Plan, as amended in 2011, 12,000,000 shares of our common stock are reserved for issuance under awards. Only shares actually issued under the 2007 Equity Plan will reduce the share reserve. If we acquire another entity through a merger or similar transaction and issue replacement awards under the 2007 Equity Plan to employees, officers and directors of the acquired entity, those awards, to the extent permitted under applicable laws and securities exchange rules, will not reduce the number of shares reserved for the 2007 Equity Plan.
In addition to the 2007 Equity Plan, our board of directors has approved a plan for employees, consultants and vendors by which outstanding amounts owed to them by our company may be converted to common stock or options to purchase common stock. The conversion and exercise price is based on the closing price of our common stock on the date of agreement. If an option is issued, the number of shares purchasable by the option is calculated by dividing the amount owed by the exercise price, times one and one-half.
Equity Compensation Plan Information as of June 30, 2019
|
|
|
Number of
|
|
|
|
|
|
|
|
|
|
|
securities
|
|
|
|
|
|
|
|
|
|
|
to be issued upon
|
|
|
Weighted average
|
|
|
|
|
|
|
|
exercise of
|
|
|
exercise price of
|
|
|
Number of
|
|
|
|
|
outstanding
|
|
|
outstanding
|
|
|
securities
|
|
|
|
|
options,
|
|
|
options,
|
|
|
remaining
|
|
|
|
|
warrants and
|
|
|
warrants and
|
|
|
available
|
|
|
Plan category
|
|
rights
|
|
|
rights
|
|
|
for future issuance
|
|
|
|
|
(a)
|
|
|
(b)
|
|
|
(c)
|
|
|
Equity compensation plans approved by security holders (1)
|
|
|
13,896,334
|
|
|
$
|
0.37
|
|
|
|
36,953,167
|
|
|
Equity compensation plans not approved by security holders (2)
|
|
|
19,597,901
|
|
|
|
0.42
|
|
|
|
n/a
|
|
|
Total
|
|
|
33,494,235
|
|
|
$
|
|
|
|
|
---
|
|
|
(1)
|
Includes 8,849,451 shares issuable under the 2007 Equity Plan, which expired September 6, 2017, and 5,046,883 shares issued under the 2018 Equity Incentive Plan adopted by the Board on March 7, 2018 and subsequently approved by stockholders on May 23, 2018.
|
|
|
|
|
(2)
|
This includes various issuances to specific individuals either as a conversion of un-paid obligations pursuant to a plan adopted by our board of directors, or as part of their agreement for services.
|
DESCRIPTION OF BUSINESS
BioLargo, Inc. is a corporation organized under the laws of the state of Delaware. Since January 23, 2008, our common stock has been quoted on the OTC Bulletin Board (now called the OTCQB – the OTC Markets “Venture Marketplace”) under the trading symbol “BLGO”.
As used in this report, “we” and “Company” refers to (i) BioLargo, Inc., a Delaware corporation; (ii) its wholly-owned subsidiaries BioLargo Life Technologies, Inc., a California corporation; Odor-No-More, Inc., a California corporation; BioLargo Development Corp., a California corporation; BioLargo Engineering, Science & Technologies, LLC, a Tennessee limited liability company; and BioLargo Water Investment Group, Inc., a California corporation and sole shareholder of Canadian subsidiary BioLargo Water, Inc.; and (iii) Clyra Medical Technologies, Inc. (“Clyra”), a partially owned subsidiary.
The following discussion and analysis should be read in conjunction with our unaudited consolidated financial statements and the related notes to the consolidated financial statements included elsewhere in this report.
Our Business- A Sustainable Products, Technology and Solutions Provider
BioLargo, Inc. is an innovative technology developer and environmental engineering company driven by a mission to “make life better” by delivering robust, sustainable solutions for a broad range of industries and applications, with a focus on clean water, clean air, and advanced wound care. We develop and commercialize disruptive technologies by providing the capital, support, and expertise to expedite them from “cradle” to “maturity”. Our business strategy is straightforward: we invent or acquire technologies that we believe have the potential to be disruptive in large commercial markets; we incubate and develop these technologies to advance them and promote their commercial success as we leverage our considerable scientific, engineering, and entrepreneurial talent; we then monetize these technical assets through a variety of business structures that may include licensure, joint venture, sale, spin off, or by deploying direct to market strategies. We seek to unlock the value of our portfolio of underlying technologies to both advance our purposeful mission while we create value for our stockholders.
Our first significant commercial success is currently unfolding in our subsidiary, Odor-No-More, Inc., which is focused on odor and volatile organic compound (“VOC”) control products sold under the brands CupriDyne Clean and Nature’s Best Science. We are gearing up for rapid growth as our products are experiencing more widespread market adoption in the waste handling industry through national purchasing agreements with four of the largest industry members. To this end, we have recently begun to offer a menu of services to our clients including engineering design, construction, and installation of misting systems and related equipment used to deliver our liquid chemistry products, as well as ongoing maintenance services for installed systems. We have also begun expanding with early adopters into new vertical segments such as wastewater treatment, the cannabis industry and various industrial facilities like steel manufacturing and livestock processing operations. In 2019 we executed a five-year white-label distribution agreement with Cannabusters, Inc. a company organized and owned by Mabre Corporation to feature our odor and VOC control technology to the cannabis industry in combination with their air handling and air quality systems. We believe this to be an important opportunity for BioLargo’s odor and VOC control products, as the cannabis and hemp industries are predicted to grow significantly in the US in the coming years and are known to contend with significant odor and VOC challenges (read more under Emerging High-Growth Opportunity in Cannabis / Hemp Industry).
Our second commercial operation, BioLargo Engineering, Science & Technologies, LLC (“BLEST”), provides professional engineering and consulting services to third party clients on a fee-for-service basis, and also serves as our in-house engineering team to advance the development of our proprietary technologies and complement service offerings of our other business segments.
In addition to our two operating subsidiaries, we have technologies and products in the development pipeline progressing towards commercialization, including our water treatment system for decontamination and disinfection (our “Advanced Oxidation System”, or “AOS” – see Pilot Projects discussion below), and our medical products focused on healing chronic wounds, including our recently acquired stem cell therapy called the SkinDiscTM, which is focused on regenerative tissue management and is licensed to our subsidiary Clyra Medical Technologies, Inc. (“Clyra Medical”).
We believe our current success with our industrial odor and VOC control products serves to validate our overall business strategy which is focused on technology-based products and services capable of disrupting the status quo in their applicable industry market segment. We believe that the future of our medical and clean water technologies has similar and also very large market opportunities ahead as they are introduced commercially.
Odor-No-More Industrial Odor and VOC Solutions
Our CupriDyne Clean industrial products reduce and eliminate tough odors and VOC’s in various industrial settings, delivered through misting systems, sprayers, water trucks and similar water delivery systems. We believe the product is the number one performing odor-control product in the market, and offers substantial savings to our customers compared with competing products.
Waste Handling
Our customer base for our odor and VOC business is expanding. We are now selling product to four of the largest solid waste handling companies in the country, and also have secured multiple flagship clients in the wastewater treatment industry, which we expect to become a priority market. We are also expanding with early adopters into new industrial markets, including steel manufacturing, paper production, construction, building and facilities management, livestock production, and the cannabis industry. Opportunities for our products are available internationally. We have in the past and plan to continue marketing these products through industry associations like the “Technology Approval Group” program offered by Isle Utilities that serves the wastewater treatment industry. We also have a number of potential partners actively engaged in commercial trials around the globe and we are actively in discussion with a number of groups to leverage our commercial focus through distribution partnerships.
Many of our customers have adopted CupriDyne Clean as a replacement for non-performing competitive products, some of which have been in use by customers for decades. Upon using CupriDyne Clean, our customers consistently express a very high degree of satisfaction with its performance compared to prior solutions. Because of this, we are realizing systematic adoption by our very large corporate customers and expect to serve these customers for years to come. Our experience has helped refine our value proposition and assemble a comprehensive menu of products and services. Our success in this market has validated the market opportunity for our products and services and encourages us to continue investing in infrastructure and sales and marketing to increase revenues. We estimate there are approximately 2,000 active landfills1, 8,000 transfer stations2, and 15,000 wastewater treatment agencies3 in the United States. While all may not have ongoing odor problems or neighbor complaints, we believe many of the facilities have need for a disruptive odor solution like CupriDyne Clean.
The total addressable market for the waste handling and wastewater treatment industries is greater than $1.3 billion. While we are still assessing the size of the cannabis, agriculture and steel manufacturing industries, we believe they could readily double the market opportunities for our product CupriDyne Clean.
Turn-key Full-service Solutions
At the request of our clients, we have begun offering a menu of services to landfills, transfer stations, and wastewater treatment facilities. These services include ongoing maintenance and on-site support services to assist our clients in the design and continued use of the various systems that deliver our liquid products in the field (such as misting systems). We have recently expanded these serves to engineering design, construction and installation. Our engineering team at BLEST has been instrumental in supporting these operations. Our system design, build and install business continues to grow. We have completed multiple installs during the last quarter and have several bids outstanding for CupriDyne Clean delivery systems.
Regional Adoption
Sales of our CupriDyne Clean products and related services were initially made at the local level, on a per-location/facility basis. We would demonstrate our product to the manager of operations at a transfer station or landfill, and he or she ultimately would decide whether to use our products. If owned by a national company, in some instances before the operations manager could buy our products, we were required to obtain official “vendor” status with the company and sign a “national purchasing agreement” (“NPA”). Doing so required a tremendous amount of effort and time. These agreements typically include the addition of our line of products which will be offered through an online purchasing portal to the members around the nation. The process of integrating the data is often delayed by months from the start date of our agreements given their very technical nature. As an example, we just completed work to finish this portion of the startup process with our fourth national agreement account. These processes establish an easy and familiar selling and purchasing process for the ongoing and long-term relationships we seek to develop. We now have NPAs with four of the largest solid waste handling companies in the United States. Some of these accounts are now introducing us to regional managers around the country who have the ability to direct the facilities in their region to use our product. Because of our continued success with our existing clients, our national accounts are expanding their support for, and expanding resources to encourage increased awareness and broad adoption of our products and services. It is also important to note that we are often replacing companies that have served these customers for 20 to 30 years giving support for our claim of ‘disruption’ to an industry.
1 “Municipal Solid Waste Landfills - Economic Impact Analysis for the Proposed New Subpart to the New Source Performance Standards” (2014), by U.S. Environmental Protection Agency Office of Air and Radiation and Office of Air Quality Planning and Standards.
2 The top 5 Waste Management companies in the US, as of 2011, operated 624 transfer stations, and 565 landfills. “Municipal Solid Waste Landfills - Economic Impact Analysis for the Proposed New Subpart to the New Source Performance Standards” (2014), by U.S. Environmental Protection Agency Office of Air and Radiation and Office of Air Quality Planning and Standards. This is a ratio of 1:4 (landfill to transfer stations). The estimated number of transfer stations is this ratio multiplied by the approximate 1,900 total landfills, and then rounded.
3 1“Failure to Act, The Economic Impact of Current Investment Trends in Water and Wastewater Treatment Infrastructure” (2011), by American Society of Civil Engineers and Economic Development Research Group. Figure includes treatment facilities owned and operated by municipalities, as well as those owned and/or operated by private entities contracting with municipalities.
We believe that “regional adoption” is a scalable approach for the larger solid waste handling companies that, with sufficient resources, we can implement nationwide. Our current national accounts represent the opportunity to serve more than 3,000 local operations around North America. Because of our success serving the transfer stations, material transfer facilities, and landfills, these very large companies are also evaluating the use of CupriDyne Clean in various transportation segments as well.
We now have a body of evidence that has been developed through direct work with our large national accounts that supports our product claims, namely superior performance, cost savings and service excellence. As a result, we are receiving support from the leadership of our national accounts to help expand our services within their organizations. This support has and will continue to demand that we increase our activity to deliver RFPs (requests for proposals), follow up with and make site visits as a result of introductions to local operators by regional and corporate leaders, follow up on referrals from local operators to other local operators and provide high level customer service and responsiveness to regional office requests for site visits, and offer our products and services to multiple locations with these regional operations. This activity is increasing and as a result we are focused on adding qualified staff to our team and believe that sales will continue to increase as a function of increased staffing. Our experience has shown that the cycle from identifying a new customer that wants to use our products to installing delivery systems and related equipment (if needed), to deploying our products can take from 60 to 180 days. The work is demanding but we know the up-front investment by our team will be rewarded with expanded adoption and recurring revenues. We are continually reminded that in many instances we are replacing companies that have been serving these customers for decades.
We believe that our products will become known as the odor and VOC elimination product that will become selected as a “best practices” tool for the waste handling industry. As we continue to achieve that level of recognition, we believe our large national accounts will want to modify their stance to encourage their local operators around the country to choose our product as the top performer and highest value provider.
Expanding our Brand
CupriDyne Clean is gaining a reputation as “the one that actually works” to control odors and VOCs. We are constantly reminded that decision-makers in many industries, including the waste handling industry, have been conditioned to believe that “nothing actually works” to address industrial nuisance odors. We are working to help change the industry mindset to being proactive, investing to avoid problems rather than to rush to fix problems that have escalated to an emergency intervention status. One of our most important branding goals is to educate decision-makers that the “cost of doing nothing” can be the most expensive choice by a customer. The alternative is to use the best-performing odor mitigation product – CupriDyne Clean – to save them money and reduce or eliminate their risk and costs associated with managing odors and VOCs. Our company is committed to raise the bar of awareness with consistent performance, brand awareness and marketing to help industry see the value and make the correct choice to use and deploy CupriDyne Clean. In the past few months, we have received opening orders from more than 14 new customers, from both national accounts and new independent customers as a result of our marketing and branding awareness. We expect that trend to continue. New Product Expansion with Existing Customers
In line with our mandate as an innovator and full services solution provider, Odor-No-More was recently asked by one of its national customers to expand the use of its CupriDyne based products to include a wash out and odor control product for transportation devices, compactors and containers. While this work is still early, our first trials demonstrate that the new product saves our customers money and labor costs. Although sales for this new product have just begun, we believe the opportunity for this product is significant.
Additionally, we have been approached by two of our large customers to develop a series of educational and training tools to assist in their continuing focus to refine ‘best practices’ operating procedures for odor management at waste processing facilities. While this work is early, and our scope of services and role is still being defined, we believe this is another important validation of how we are being adopted as a reliable and high value total solutions provider.
Emerging High-Growth Opportunity in Cannabis / Hemp Industry
Odor-No-More recently entered into a 5-year “white-label” distribution agreement with Cannabusters, Inc., a sister company to Mabre Air Systems, to sell its CupriDyne Clean odor and VOC control products to Cannabis and Hemp grow and production facilities, which represent a target market that management’s research indicates is in sore need of new odor control products and services. Cannabusters has decades of experience with air quality management through their sister company Mabre Air Systems, a leader in air quality control systems in Italy. Cannabusters has committed to a comprehensive marketing program that includes more than 25 trade show events over the next two years to quickly introduce the Cannabusters product to the cannabis and hemp industries.
The cannabis industry is facing increased scrutiny by regulators to better control of hazardous air pollutants called terpenes that are a natural part of production and processing. These gases can also cause malodors that demand attention and can be problematic as these companies seek to maintain good community relations and avoid legal entanglements or lawsuits over nuisance odors. Odor abatement operating procedures are part and parcel to the permitting processes for companies involved in the industry and have typically included traditional carbon filters. With the growth and concentration of cannabis related operators, the industry has come to recognize that the volume of terpenes and air flow in a typical operation are often more than the traditional carbon filter-based systems can manage effectively. Odor complaints persist. We have been able to successfully demonstrate that our products are effective as eliminating these VOC’s and related odors, just as we have done in the waste handling industry. As a result, we have had a number of experts in the cannabis industry tell us that our products could become part of the ‘best practices’ operating procedures for this industry and are working toward that goal.
The global legal cannabis market is expected to grow to $146.4B in 2025 at an astounding 34.6% annual growth rate. Some call cannabis the 21st century’s gold rush. With an estimated 15,000 companies operating in our California alone, we believe the opportunity for our product is significant. A number of recent examples have surfaced with leading companies in this industry that highlight the nuisance odor issue and their inability to adequately manage the volume of terpenes escaping the operations. To that end, we are organizing a series of strategic relationships within the Cannabis industry to capture the opportunity quickly. We are working to finalize agreements with equipment manufacturers, regulatory consultants, key opinion leaders, and marketing partners. Our value proposition is unmatched for odor and VOC control and this is another great example how our platform continues to expand in high value markets.
Wastewater Treatment
We have begun selling products and services to wastewater treatment facilities in our local markets. Our clients are prominent municipal agencies and have indicated a desire to expand the use of our products and services to additional locations in their service areas. As a result of our success in the field, a client featured our product as an example of ‘Best Practices’ for the wastewater treatment industry at a national water quality conference hosted by the Water Environment Federation. We anticipate overall longer selling cycles given the technical sophistication of the customers in this market, and believe that channel partnerships with leading companies that already sell and service this highly technical market will be required for our ultimate success. We are encouraged and are evaluating various strategies to maximize our marketing and selling proposition into this mature and well-established market. We are actively engaged in discussions with potential distribution partners and leading engineering firms with well established relationships to the clients in order to service this very large market.
Infrastructure and Capital Needs for Odor-No-More
We recognize the scope of the opportunity for CupriDyne Clean and related services, and understand the task of building the personnel and infrastructure to become a disruptive company in the waste handling industry. In the United States, we currently operate out of two locations – Southern California and Tennessee. As of now, our manufacturing facilities are located in California. However, we expect to expand our manufacturing and staffing in our Tennessee operation as we achieve critical mass in that region. We are also contemplating the opportunity to establish a manufacturing facility in Canada to serve the Canadian odor and VOC control market. In the meantime, as a result of the rapid adoption we are experiencing in our local Southern California market, we want to focus on adding staff and infrastructure to meet the obvious need for our products and services. We believe that we need to invest in qualified sales and support personnel to properly focus our energies on capturing the client opportunities already under contract with our national accounts and expand revenues accordingly. As of August 1, 2019, we added waste-industry veteran Mitch Noto to our team as Director of Business Development.
We believe that a significant number of personnel will be required to fully service the solid waste handling and wastewater treatment industries. We plan to expand as adequate capital to fund these needs becomes available.
Full Service Environmental Engineering
In September 2017, we formed a subsidiary (BioLargo Engineering, Science & Technologies, LLC, or “BLEST”), for the purpose of offering full service environmental engineering to third parties, and to provide engineering support services to our internal teams to accelerate the commercialization of our AOS technologies. Its website is found at www.BioLargoEngineering.com.
BLEST focuses its efforts in four areas:
|
|
●
|
Providing engineering services to third-party clients;
|
|
|
●
|
Supporting the AOS development efforts by working with our Canadian subsidiary, BioLargo Water;
|
|
|
●
|
Supporting our team at Odor-No-More to provide engineering and design of the CupriDyne Clean delivery systems to the waste handling industry; and
|
|
|
●
|
Developing new products or engineered solutions for high value targets like:
|
|
|
o
|
our work on behalf of the US EPA as funded through a SBIR Phase I grants to develop potential solutions for managing PFAS contaminants in water;
|
|
|
o
|
our work to refine and validate the CupriDyne Clean’s efficacy and delivery systems for managing terpenes from cannabis production;
|
|
|
o
|
our work to provide initial proof of claim for CupriDyne Clean’s efficacy in high volume industrial settings for VOC and air contaminant mitigation; and
|
|
|
o
|
Legionella prevention and monitoring systems
|
The subsidiary is based in Oak Ridge (a suburb of Knoxville), Tennessee, and employs seven scientists and engineers who collectively have over two hundred years of experience in diverse engineering fields. The team is led by Randall Moore, who served as Manager of Operations for Consulting and Engineering for the Knoxville office of CB&I Environmental & Infrastructure and was formerly a leader at The Shaw Group, Inc., a Fortune 500 global engineering firm. The other team members are also former employees of CB&I and Shaw. The team is highly experienced across multiple industries and they are considered experts in their respective fields, including chemical engineering, wastewater treatment (including design, operations, data gathering and data evaluation), process safety, energy efficiency, air pollution, design and control, technology evaluation, technology integration, air quality management & testing, engineering management, permitting, industrial hygiene, applied research and development, air testing, environmental permitting, HAZOP review, chemical processing, thermal design, computational fluid dynamics, mechanical engineering, mechanical design, NEPDES permitting, RCRA/TSCA compliance and permitting, project management, storm water design & permitting, computer assisted design (CAD), bench chemistry, continuous emission monitoring system operator, data handling and evaluation and decommissioning and decontamination of radiological and chemical contaminated facilities.
Business Development at BLEST
BLEST has had success in several noteworthy areas in the past months. The company is increasing its customer base and executing more and larger projects than in its first year. Additionally, BLEST has made strides toward creating lucrative new opportunities through development of new processes, which BioLargo intend to seek new IP for where possible.
BLEST was recently awarded three subcontracts to do work on U.S. Air Force bases in Texas, Kansas, Illinois and Arizona. Primary contractor Bhate Environmental Associates, Inc. has bid multiple projects with BioLargo to conduct “Fence-to-Fence (F2F) environmental compliance”. The total value of the contracts awarded (split between the prime contractor Bhate and its subcontractors, including BLEST) is in excess of $15 million over five years (with one year guaranteed). BLEST is responsible for one of the three major components of the services: air quality compliance.
BLEST was recently awarded an SBIR Phase I Competitive Grant by the Environmental Protection Agency in the amount of $100,000 to investigate solutions for the removal of per- and polyfluoroalkyl substances (PFAS) from water. PFAS have been linked to cancer, fertility problems, asthma, and more, and are present in a vast range of manufactured goods including food, common household products (e.g., cleaning products, cookware), and electronics. PFAS also pose widespread and serious water safety problems around the world, with governments and industry actively seeking new technologies and processes to eliminate PFAS from groundwater and drinking water. BLEST will compete for a Phase II grant for $1,000,000 in funding to finish the product design and start a go to market campaign.
BLEST recently began a feasibility and placement study for 1.1 million tons of magnesium rich production tailings in Northern California for a new client.
Notably, BLEST recently begun work to develop a new process by which to manage and mitigate Legionella contamination in the water distribution systems of large buildings including hospitals, office buildings, condos, and more. BioLargo recently filed a patent for this new process, which is referenced below in the Intellectual Property section. BLEST intends to leverage this patented process to offer Legionella mitigation services to customers.
BLEST has recently been notified that as a result of its recent audit work on assisting a leading healthcare products company in transitioning to the 2015 revision of the ISO 14001 standard for environmental management systems (EMS) it is being awarded another small project from the client. The new time and materials project involved preparing a detailed GAP analysis, and subsequently updating the client’s EMS procedures to reflect the significant changes to the new EMS standard which places new emphasis on upper management involvement, the life cycle of products and services, emergency preparedness and response, and sustainability. There is also a new focus on evaluating risks and opportunities and integrating this assessment into the EMS program.
The formation of BLEST was predicated on the concept that 60% of the revenue would be provided by external clients and the remaining 40% would be provided by internal clients (i.e. BioLargo Water or Odor-No-More). By reaching this goal, BLEST will provide direct positive cash flow to the BioLargo, Inc. while fulfilling its mission to provide professional engineering services to the internal client base. For calendar 2018, the ratio was approximately 40% of revenue provided by external clients and 60% provided by internal sources. In the second quarter of 2019, BLEST has now reached and exceeded its target threshold of 60% of revenues coming from external clients and less than 40% coming from internal sources, meaning BLEST has achieved its goal of generating the majority of its revenues from external contracts. . This occurred and is occurring principally because of an increasing number of perpetual contracts including the U.S. Air Force contracts, Citizens Gas Utility District, HAVCO, Powell Valley Utilities, and APTIM/Picatinny Arsenal. These perpetual contracts, which are anticipated to be renewed annually, will provide a steady base load of outside client revenue that is reasonably predictable and secure.
In addition to continued organic growth in the external client base, BLEST is developing new technologies and services for water pollution control, the microbrewery sector, legionella prevention in public buildings and hospitals. They are evaluating similar approaches for the cannabis industry as well. These markets are expanding in areas across the United States and represent significant opportunities for BLEST.
BLEST management believes the company can expect growth in several additional areas. For one, BLEST is under contract to design, build, and install wastewater treatment equipment and “treatment trains” for clients in collaboration with BioLargo’s water technology subsidiary BioLargo Water. Not only does this represent important synergy between two BioLargo business units, but it offers BLEST the opportunity to become a total water treatment solutions provider for customers in the widely under-served small industrial wastewater treatment sector. Another area of predicted growth is the conduct of environmental engineering and permitting work for large industrial facilities such as fuel conversion plants, an area in which BLEST has experienced an increasing number of contracts in the past quarter.
BioLargo Water and the Advanced Oxidation System - AOS
BioLargo Water is our wholly owned subsidiary located on campus at the University of Alberta, Canada, that has been primarily engaged in the research and development of our Advanced Oxidation System (AOS). The AOS is our patented water treatment device that generates a series of highly oxidative species of iodine and other molecules that, because of its proprietary configuration and inner constituents, allow it to eliminate pathogenic organisms and organic contaminants as water passes through the device and it performs with extreme efficacy while consuming very little electricity. Its key application is rapid and efficient decontamination and disinfection of various wastewaters. The AOS recently began its first pre-commercial pilot project, wherein an AOS and treatment train has been installed on-site at Sunworks Farm, a poultry farm in Alberta. This pilot project is discussed in more detail in the Pre-commercial Pilot Projects section below.
The key value proposition of the AOS is its ability to eliminate a wide variety of contaminants with high performance while consuming extremely low levels of both input electricity and chemistry – a trait made possible by the complex set of highly oxidative iodine compounds generated within the AOS reactor. Our proof-of-concept studies and case studies have generated results that project the AOS will be more cost- and energy-efficient than commonly used advanced water treatment technologies such as UV, electro-chlorination, and ozonation. This value proposition sets the AOS technology above other water treatment options, as we believe the AOS may allow safe and reliable water treatment for significantly lower cost compared to its competitors and may even enable advanced water treatment in applications where it otherwise would have been prohibitively costly.
The AOS has the potential to allow reliable and cost-effective water treatment in numerous industries and applications where high-level disinfection or elimination of hard-to-treat organic contaminants is required. We believe the total serviceable market for our AOS is $10.75 billion for the poultry processing, food & beverage, and storm water segments with a target beachhead market for poultry processing in North America at an estimated $240 million.
Our AOS was the result of breakthroughs in both advanced iodine electrochemistry and advances in materials engineering, and its invention led to BioLargo’s co-founding of a multi-year industrial research chair whose goal was to solve the contaminated water issues associated with the Canadian Oil Sands at the University of Alberta Department of Engineering in conjunction with the top five oil companies in Canada, the regional water district, and various environmental agencies of the Canadian government. Based on recovering oil prices and our ongoing work in Canada, in 2018 re reinitiated discussions with stakeholders in the oil sands industry to support the completion of AOS development for oil and gas water treatment and to discuss the initiation of pre-commercial and commercial pilots for our AOS to help treat and remediate oil sands process-affected water (“OSPW”) found in tailings ponds in the Canadian oil sands, an application that currently has no good economically viable solution. We have been unsuccessful in raising grant or private funds for this project, and, therefore we will continue to focus on energies on other markets until such time as proper resources are available.
Our AOS is an award-winning invention that is supported by science and engineering financial support and highly competitive grants (66 and counting) from various federal and provincial funding agencies in Canada such as NSERC, NRC- IRAP, and Alberta Innovates and in the United States by the Metropolitan Water District of Southern California.
Our immediate goals for the development and commercialization of the AOS are: 1) to secure direct investment into the BioLargo Water subsidiary to empower its staff to complete its development cycle, 2) complete the ongoing pre-commercial field pilot studies which are necessary to generate the techno-economic data required to secure commercial trials, entice future customers, and commence traversal of regulatory pathways, 3) conduct the first commercial trials with the AOS, and 4) secure first sale of the AOS. It is our belief that once pre-commercial pilots have concluded with the AOS, we will be able to entice major water industry players to partner with BioLargo Water to accelerate market adoption of the AOS..
Recent AOS Milestones
The most important advances in AOS development in recent months have been 1) recent validation of the AOS as an effective and transformative water treatment technology able to eliminate hard-to-treat “micropollutants” from wastewater; 2) design and engineering advances and changes to the AOS in preparation for piloting and scale-up for industrial flow-rates and conditions; and 3) the planning and design of pre-commercial field pilot projects.
One recent and important AOS milestone was the demonstration that it eliminated or reduced the toxicity of certain high-concern pharmaceutical byproducts (micropollutants) common in some municipal wastewater (“MWW”) streams. Currently, there are no economically viable solutions to remove these compounds from MWW, and incumbent technologies fall short. We believe that the value proposition for our AOS for use as a new technology solution for the municipal water treatment industry to efficiently remove micropollutants could increase our total serviceable market to 5% or more of the total industry which is recognized at + $700 billion globally or approximately $35 billion.
Several advances and improvements to the AOS have also been made in recent months with the purpose of preparing the technology for pre-commercial piloting, commercial piloting, and subsequent mass production, as well as to prepare it for scale-up to allow industrial flow rates. These advancements have largely been proprietary physical improvements to the AOS, including the transitioning of the AOS to using inner substrates more amenable to mass-production and greater flow rates and pressures. Management believes it will continue to advance the scale-up to higher volume throughputs of water flow and enhances the AOS ability to be more compact and longer lasting in the field. This work is not complete, but management believes it does represent a significant step forward to achieving high throughput quality results. Importantly, we have also designed and begun assembling our own proprietary water treatment train that will be used in pilots for the AOS and that will pave the way for complete wastewater treatment in industrial settings.
Pre-commercial Pilot Projects for AOS
We are now underway on multiple pre-commercial field pilot projects.
The first project involves treating poultry wastewater on-site at a facility in Alberta Canada, with support from the Poultry Growers Association. In this pilot, the AOS is being assessed for its ability to eliminate bacteria and other contaminants from poultry processing wastewater effectively and cost-efficiently and to establish operating costs (OPEX) and capital costs (CAPEX) in a field setting. BioLargo Water built and installed a complete “treatment train” with equipment to address all aspects of the client’s water treatment needs, including organic contaminants, suspended solids, and biological organisms, in addition to the connected AOS unit. Therefore, this pilot also represents BioLargo’s first assessment as a “total solutions provider”, which could open the door for a wider array of future water treatment market opportunities. Funded in part by Canadian government grants, this system is operating successfully. We hope to report data from the project before the end of the year.
In another pilot project, the AOS is being used on-site at a Californian micro-brewery as a polishing (final) step in a wastewater “treatment train” whose goal is to reduce wastewater contaminant load to levels that would allow the microbrewery to reduce its wastewater discharge fines and enable water reuse. The treatment train includes several pieces of wastewater treatment equipment including a proprietary technology developed and manufactured by our project partner Aquacycl, an emerging wastewater treatment technology company based in the San Diego area that was introduced to our company by The Maritime Alliance, a trade organization in San Diego committed to fostering maritime business and technology innovation. This pilot will help establish the efficacy of the AOS in a field setting, the OPEX and CAPEX of the system, and the AOS’ ability to “plug and play” in the context of diverse supporting equipment and logistics.
In addition, we recently commenced a pre-commercial demonstration pilot that will utilize the company’s Advanced Oxidation System (AOS) to treat captured stormwater in Southern California at BioLargo’s Westminster, California facility. The pilot’s goal is to demonstrate the technical and economic feasibility of deploying the AOS to enable stormwater treatment and reuse, an important and emerging water management application in the US and Canada. The pilot project is supported in part by research and development funding of to up to $189,000 from the National Research Council of Canada Industrial Research Assistance Program (NRC IRAP). BioLargo Water is collaborating on the project with Richard Watson & Associates, Inc. and Carollo Engineers, Inc. Richard Watson has been active in stormwater quality management since 1990 and currently consults to three watershed management groups in Los Angeles County. Carollo Engineers, a leading environmental engineering firm providing cost-effective, innovative, and reliable water treatment solutions, will provide engineering and water treatment validation for the project. The goals of this demonstration pilot will focus on the efficacy of the AOS to treat captured stormwater to water reuse standards. The pilot will also help establish the capital and operating costs of the AOS in this application, a crucial step before potential commercial pilot clients and paying customers would consider the technology in this industrial setting.
All of these pilot projects represent an important step for our AOS technology, as well as for our company. We are confident in our disruptive water treatment technology and have proven its treatment capabilities in the lab. However, pilot projects for the AOS, as with any technology, are crucial to prove its reliability to industry stakeholders as well the capital cost and operating costs of our technology at-scale. These data will be critical to pave the way for future market adoption. As a reminder, we have many other pilots in evaluation to support this same cause.
We believe that our current designs for the AOS are cost-effective, commercially viable and should be ready for their first commercial launch in late 2019 or early 2020. We secured a patent on the AOS in 2018, and another in March 2019. We intend to continue refining and improving the AOS continually to accomplish a series of goals: expanded patent coverage, extended useful life, lower capital costs, lower energy costs, optimized performance, precise configurations for specific industry challenges, portability, and identifying its performance limits. Our current and most pressing goal for the AOS, as evidenced by the pilot projects described above, is to demonstrate its efficacy in field settings, which is a crucial and necessary step for the commercialization of any water treatment system.
Advanced Wound Care - Clyra Medical
We initially formed Clyra Medical to commercialize our technology in the medical products industry, which we believe can be disruptive to many competing product lines. Our initial product designs focus in the “advanced wound care” field, which includes traumatic injury, diabetic ulcers, and chronic hard-to-heal wounds. We also have designs for products focused on preventing or controlling infections. In late 2018, we also acquired our second technology, a stem cell therapy technology, SkinDisc, that is both complementary to our antimicrobial product designs and it also presents a high value proposition to offer stand-alone products to the advanced wound care industry to assist in regenerating tissue. With the addition of highly skilled team members with extensive experience and proven track record of success in the medical industry and, the addition of the SkinDisc, we have expanded our plans to focus and build out a complete line of products to deliver state of the art solutions to assist in healing wounds. Therefore, we are also presently evaluating a number of additional licensing opportunities to add complementary technologies and products to our medical products portfolio with the goal of offering a complete menu of proprietary and patent protected products to better serve the advanced wound care patient population with state-of-the-art medical products. We are presently seeking pre-market clearance for our first advanced wound care product (application in process), from the U.S. Food & Drug Administration (“FDA”) under Section 510(k) of the Food, Drug, and Cosmetic Act.
We believe the total addressable market for Clyra Medical’s existing product designs in the advanced wound care market, dental, orthopedics and regenerative tissue markets will exceed $2.5 billion by 2022.
Our first and original advanced wound care product combines the broad-spectrum antimicrobial capabilities of iodine in a platform complex that promotes and facilitates wound healing. Our products are highly differentiated from existing antimicrobials in multiple ways - by the gentle nature in which they perform, extremely low dosing of active ingredients, reduced product costs, extended antimicrobial activity, and biofilm efficacy. In addition, iodine has no known acquired microbial resistance, unlike many competing products. We believe the future markets for some of our product designs may also include infection control and wound therapy in orthopedics, dental and veterinary markets. We also intend to pursue and study the use of our technology as a complimentary and synergistic platform for use with regenerative tissue therapy.
We have three patent applications pending for medical products, and are preparing additional applications. While these patent applications are pending, we intend to continue expanding patent coverage as we refine and expand our medical products.
We are in the process of obtaining regulatory approval (pre-market clearance) from the FDA for our first advanced wound care product. These efforts are ongoing as of the date of this report. Although the process has taken considerable time and money, and we have faced a number of delays as a result of the FDA’s requirements of us, we remain highly encouraged by our current interactions with the FDA staff and our current position. The process has confirmed that our product design falls in the scope of the 510(k) process and the pathway to clearance has now been better defined by senior staff at FDA. We are preparing to report to the FDA results of a 30-day animal study that confirmed the Clyra product has no adverse effects on wound healing. This animal study is the last material item asked of us by FDA staff, and we believe we can submit this new data and have a response back from the FDA as soon as possible, with expectations of delivery within weeks and a timely response from the FDA promptly thereafter. While we remain confident that we will ultimately receive premarket clearance for this product, and we continue to invest substantial recourses in anticipation of our ultimate success, we are continually reminded by legal counsel that we can make no assurance or prediction as to success of these efforts, or whether additional information will be requested after this animal study, and must wait patiently for the process with the FDA to conclude. Notwithstanding these disclosures, having spent a significant amount of time and money responding to the various technical questions by the staff, including two trips to Washington D.C., we are confident we will see a successful conclusion.
We believe this product’s future role in the advanced wound care industry will be disruptive to many incumbent competing products like silver, hypochlorous acid and even other iodine-based products and therefore our extraordinary investment of time and money will have significant opportunity to generate a considerable return on investment as the products find their way through the FDA process for clearance and then to market adoption. Simply stated, we believe it is worth it and that we will succeed.
Our second technology and its related products center around the SkinDisc technology which we acquired in late 2018 from Scion Solutions, LLC (“Scion”). Scion is led by Spencer Brown, a medical device industry veteran with more than 35 years’ experience in sales, account management, and distribution in the medical device industry. The SkinDisc product was developed by Dr. Brock Liden, a renowned medical podiatrist and expert in wound care and diabetic limb salvage. The SkinDisc is a therapy product that uses a patient’s own bone marrow and plasma in a unique mixture to generate a cell-rich bio gel for use with chronic wounds. It has been tested in over 250 patient cases with no adverse effects, and has successfully aided in the salvage of limbs that otherwise would have been amputated. The regenerative tissue therapy technique has been shown to assist in successful wound closure in time frames as short at 4 to 7 weeks with one or two applications and is patent pending.
Clyra Medical also continues to actively work on the development of new products.
Clyra is currently successfully recruiting Key Opinion Leaders from the medial field to join Clyra’s Medical Advisory Board and is actively evaluating a number of technologies and products to add to its product portfolio in anticipation of its near-term plans to launch its commercial sales efforts.
We are committed to see these advanced wound care products go to market and we believe they will make a positive impact for a greater good around the world and generate meaningful financial results for our stockholders.
Scion Solutions Acquisition – SkinDisc™
On September 26, 2018, we and Clyra Medical agreed to a transaction whereby we would acquire the intangible assets of Scion Solutions, LLC (“Scion”), and in particular its stem cell-based technology, the SkinDisc, and the know-how of key team members to support further research as well as the sale and distribution of Clyra Medical’s products based on our BioLargo technologies.
The parties entered into a Stock Purchase Agreement and Plan of Reorganization (“Purchase Agreement”) whereby Clyra Medical acquired (and then sold to BioLargo) the Scion intangible assets, including the SkinDisc. The consideration provided to Scion is subject to an escrow agreement and earn out provisions and includes: (i) 21,000 shares of the Clyra Medical common stock; (ii) 10,000 shares of Clyra Medical common stock redeemable for BioLargo common shares (detailed below); and (iii) a promissory note in the principal amount of $1,250,000 to be paid through new capital investments and revenue, as detailed below. The Clyra Medical common stock was initially held in escrow subject to the new entity raising $1,000,000 “base capital” to fund its business operations, which was raised effective December 17, 2018 (see below). One-half of the common stock was released to scion, and the second half remains subject to the following performance metrics, each vesting one-fifth of the remaining shares of common stock: (a) notification of FDA premarket clearance of certain orthopedics products, or recognition by Clyra Medical of $100,000 gross revenue; (b) the recognition by Clyra Medical of $100,000 in aggregate gross revenue; (c) the granting of all or any part of the patent application for the SkinDisc product, or recognition by Clyra Medical of $500,000 in gross revenue; (d) recognition by Clyra Medical of $1,000,000 in aggregate gross revenue; and (e) recognition by Clyra Medical of $2,000,000 in gross revenue. In addition, Clyra and Scion entered into the $1,250,000 promissory note called for by the Purchase Agreement. The promissory note accrues interest at the rate of 5%. Principal and interest due under the note are to be paid periodically at a rate of 25% of investment proceeds received. If the note is not paid off within 18 months after the date of issuance, it is automatically extended for additional 12-month periods until the note is repaid in full. Payments after the initial 18-month maturity date are required to be made as investment proceeds are received, at a rate of 25% of such proceeds, and 5% of Clyra Medical’s gross revenues.
Immediately following Clyra Medical’s purchase of Scion’s assets, Clyra Medical sold to BioLargo the assets, along with 12,755 Clyra Medical common shares. In exchange, BioLargo issued Clyra Medical 7,142,858 shares of BioLargo common stock. Concurrently, BioLargo licensed back to Clyra Medical the Scion assets. Scion may exchange its 10,000 Clyra Medical common shares for the 7,142,858 shares of BioLargo common stock issued to Clyra Medical, subject to the escrow and earn-out provisions described above. As of December 31, 2018, per the Closing Agreement, one-half of these shares have been earned and thus may be redeemed, and one-half remain subject to the earn-out provisions.
On December 17, 2018, we entered into a closing agreement (“Closing Agreement”) reflecting the satisfaction of the obligation to raise $1,000,000 “base capital” established under the Purchase Agreement. With the satisfaction of the obligation to raise $1,000,000 in base capital, Clyra Medical agreed to release to Scion one-half of the shares of Clyra common stock exchanged for the Scion assets. The remaining Clyra Medical common shares remain subject to the Escrow Agreement dated September 26, 2018, subject to the metrics identified above. We were initially introduced to the SkinDisc product and Scion Solutions through Dr. Liden and Tanya Rhodes’s consulting work with Clyra Medical (both Dr. Liden and Ms. Rhodes have ownership interest in Scion). Prior to the execution of the above-described agreements, BioLargo did not have any material relationship with Scion’s founder Spencer Brown.
Intellectual Property
We have 20 patents issued, including 18 in the United States, and multiple pending. We believe these patents provide a foundation from which to continue building our patent portfolio, and we believe that our technology is sufficiently useful and novel that we have a reasonable basis upon which to rely on our patent protections. We also rely on trade secrets and technical know-how to establish and maintain additional protection of our intellectual property. As our capital resources permit, we expect to expand our patent protection as we continue to refine our inventions as well as make new discoveries. See the detailed discussion below of our patent portfolio.
We regard our intellectual property as critical to our ultimate success. Our goal is to obtain, maintain and enforce patent protection for our products and technologies in geographic areas of commercial interest and to protect our trade secrets and proprietary information through laws and contractual arrangements.
Our Chief Science Officer, Mr. Kenneth R. Code, has been involved in the research and development of the technology since 1997. He has participated in the Canadian Federal Scientific Research and Experimental Development program, and he was instrumental in the discovery, preparation and filing of the first technology patents. He has worked with manufacturers, distributors and suppliers in a wide variety of industries to gain a full appreciation of the potential applications and the methodologies applicable to our technology for their manufacture and performance. He continues to research methods and applications to continue to expand the potential uses of our technology as well as work to uncover new discoveries that may provide additional commercial applications to help solve real world problems in the field of disinfection.
We incurred approximately $1,700,000 in expense related to our research and development activities in 2018, an increase of approximately $100,000 over the prior year. We have shifted the focused in our Canadian research facility to focus on commercializing our AOS technology and thus expect these expenses to decrease in 2019.
We believe that our suite of intellectual property covers the presently targeted major areas of focus for our licensing strategy. The description of our intellectual property, at present, is as follows:
|
|
●
|
U.S. Patent 10,238,990, issued on March 26, 2019, and 10,051,866, issued on August 21, 2018, which protect our AOS system.
|
|
|
●
|
U.S. Patent 10,046,078 issued on August 15, 2018, which encompasses our CupriDyne Clean misting systems used at transfer stations and landfills.
|
|
|
●
|
U.S. Patent 9,883,653 issued on February 8, 2018, which encompasses a litter composition used in the absorption of animal wastes.
|
|
|
●
|
U.S. Patent 9,414,601 issued on August 16, 2016, relating to the use of an article for application to a surface to provide antimicrobial and/or anti-odor activity. At least one of the reagents is coated with a water-soluble, water dispersible or water-penetrable covering that prevents ambient conditions of 50% relative humidity at 25ºC from causing more than 10% of the total reagents exposed to the ambient conditions from reacting in a twenty-four-hour period.
|
|
|
●
|
U.S. Patent 8,846,067, issued on September 30, 2014, which encompasses a method of treating a wound or burn on tissue to reduce microbe growth about a wound comprising applying an antimicrobial composition to the wound or burn on tissue using a proprietary stable iodine gel or liquid. This patent covers our technology as used in products being developed by our subsidiary, Clyra Medical Technologies.
|
|
|
●
|
U.S. Patent 8,757,253, issued on June 24, 2014, relating to the moderation of oil extraction waste environments.
|
|
|
●
|
U.S. Patent 8,734,559, issued on May 27, 2014, relating to the moderation of animal waste environments.
|
|
|
●
|
U.S. Patent 8,679,515 issued on March 25, 2014, titled “Activated Carbon Associated with Alkaline or Alkali Iodide,” which provides protection for our BioLargo® AOS filter.
|
|
|
●
|
U.S. Patent 8,642,057, issued on February 14, 2014, titled “Antimicrobial and Antiodor Solutions and Delivery Systems,” relating to our liquid antimicrobial solutions, including our gels, sprays and liquids imbedded into wipes and other substrates.
|
|
|
●
|
U.S. Patent 8,574,610, issued on November 5, 2013, relating to flowable powder compositions, including our cat litter additive.
|
|
|
●
|
U.S. Patent 8,257,749, issued on September 4, 2012, relating to the use of our technology as protection of against antimicrobial activity in environments that need to be protected or cleansed of microbial or chemical material. These environments include closed and open environments and absorbent sheet materials that exhibit stability until activated by aqueous environments. The field also includes novel particle technology, coating technology or micro-encapsulation technology to control the stability of chemicals that may be used to kill or inhibit the growth of microbes to water vapor or humidity for such applications.
|
|
|
●
|
U.S. Patent 8,226,964, issued on July 24, 2012, relating to use of our technology as a treatment of residue, deposits or coatings within large liquid carrying structures such as pipes, drains, ducts, conduits, run-offs, tunnels and the like, using iodine, delivered in a variety of physical forms and methods, including using its action to physically disrupt coatings. The iodine’s disruptive activity may be combined with other physical removal systems such as pigging, scraping, tunneling, etching or grooving systems or the like.
|
|
|
●
|
U.S. Patent 8,021,610, issued on September 20, 2011, titled “System providing antimicrobial activity to an environment,” relating to the reduction of microbial content in a land mass. Related to this patent are patents held in Canada and the European Union.
|
|
|
●
|
U.S. Patent 7,943,158, issued on May 17, 2011, titled “Absorbent systems providing antimicrobial activity,” relating to the reduction of microbial content by providing molecular iodine to stabilized reagents.
|
|
|
●
|
U.S. Patent 7,867,510, issued on January 11, 2011, titled “Material having antimicrobial activity when wet,” relating to articles for delivering stable iodine-generating compositions.
|
|
|
●
|
U.S. Patent 6,328,929, issued on December 11, 2001, titled “Method of delivering disinfectant in an absorbent substrate,” relating to method of delivering disinfectant in an absorbent substrate.
|
|
|
●
|
U.S. Patent 6,146,725, issued on November 14, 2000, titled “absorbent composition,” relating to an absorbent composition to be used in the transport of specimens of bodily fluids.
|
Pending Patent Applications
Most recently, we filed two patent applications in the United States for our advanced wound care formulas. The inventions in these applications form the basis for the work at Clyra Medical and the products for which that subsidiary intends to seek FDA approval. In addition to these applications, we have filed patent applications in multiple foreign countries, including the European Union, pursuant to the PCT, and other provisional applications.
Subject to adequate financing, we intend to continue to expand and enhance our suite of intellectual property through ongoing focus on product development, new intellectual property development and patent applications, and further third-party testing and validations for specific areas of focus for commercial exploitation. We currently anticipate that additional patent applications will be filed during the next 12 months with the USPTO and the PCT, although we are uncertain of the cost of such patent filings, which will depend on the number of such applications prepared and filed. The expense associated with seeking patent rights in multiple foreign countries is expensive and will require substantial ongoing capital resources. However, we cannot give any assurance that adequate capital will be available. Without adequate capital resources, we will be forced to abandon patent applications and irrevocably lose rights to our technologies.
Competition
We believe that our products contain unique characteristics that distinguish them from competing products. In spite of these unique characteristics, our products face competition from products with similar prices and similar claims. We face stiff competition from companies in all of our market segments, and many of our competitors are larger and better-capitalized.
For example, we would compete with the following leading companies in our respective markets:
|
|
●
|
Disinfecting/Sanitizing: Johnson & Johnson, BASF Corporation, Dow Chemical Co., E.I. DuPont De Nemours & Co., Chemical and Mining Company of Chile, Inc., Proctor and Gamble Co., Diversey, Inc., EcoLab, Inc., Steris Corp., Clorox, and Reckitt Benckiser.
|
|
|
●
|
Water Treatment: GE Water, Trojan UV, Ecolab, Pentair, Xylem and Siemens AG.
|
|
|
●
|
Medical Markets: Smith & Nephew, 3M, ConvaTec and Derma Sciences.
|
|
|
●
|
Pet Market: Arm & Hammer and United Pet Group (owner of Nature’s Miracle branded products).
|
|
|
●
|
Industrial Odor Control: NCM Odor Control and OMI Industries.
|
Each of these named companies and many other competitors are significantly more capitalized than we are and have many more years of experience in producing and distributing products.
Additionally, our technology and products incorporating our technology must compete with many other applications and long embedded technologies currently on the market (such as, for example, chlorine for disinfection).
In addition to the competition we face for our existing products, we are aware of other companies engaged in research and development of other novel approaches to applications in some or all the markets identified by us as potential fields of application for our products and technologies. Many of our present and potential competitors have substantially greater financial and other resources and larger research and development staffs than we have. Many of these companies also have extensive experience in testing and applying for regulatory approvals.
Finally, colleges, universities, government agencies, and public and private research organizations conduct research and are becoming more active in seeking patent protection and licensing arrangements to collect royalties for the use of technology that they have developed, some of which may be directly competitive with our applications.
Governmental Regulation
We will have products (each a ‘‘Medical Device”) that will be subject to the Federal Food, Drug, and Cosmetic Act, as amended (including the rules and regulations promulgated thereunder, the “FDCA”), or similar Laws (including Council Directive 93/42/EEC concerning medical devices and its implementing rules and guidance documents) in any foreign jurisdiction (the FDCA and such similar Laws, collectively, the “Regulatory Laws”) that are developed, manufactured, tested, distributed or marketed by our company or its subsidiary Clyra. Each such Medical Device will need to be developed, manufactured, tested, distributed, and marketed in compliance with all applicable requirements under the Regulatory Laws, including those relating to investigational use, premarket clearance or marketing approval to market a medical device, good manufacturing practices, labeling, advertising, record keeping, filing of reports and security, and in compliance with the Advanced Medical Technology Association Code of Ethics on Interactions with Healthcare Professionals.
We believe that no article or part of any Medical Device intended to be manufactured or distributed by our company or any of our subsidiaries will be classified as (i) adulterated within the meaning of Sec. 501 of the FDCA (21 U.S.C. § 351) (or other Regulatory Laws), (ii) misbranded within the meaning of Sec. 502 of the FDCA (21 U.S.C. § 352) (or other Regulatory Laws) or (iii) a product that is in violation of Sec 510 of the FDCA (21 U.S.C. § 360) or Sec. 515 of the FDCA (21 U.S.C. § 360e) (or other Regulatory Laws).
Neither our company nor any of its subsidiaries, nor, to the knowledge of our company, any officer, employee or agent of our company or any of its subsidiaries, has been convicted of any crime or engaged in any conduct for which such Person or entity could be excluded from participating in the federal health care programs under Section 1128 of the Social Security Act of 1935, as amended (the “Social Security Act”), or any similar Law in any foreign jurisdiction.
Neither our company nor any of its subsidiaries has received any written notice that the FDA or any other Governmental Authority has commenced, or threatened to initiate, any action to enjoin research, development, or production of any Medical Device.
Employees
As of the date of this prospectus, we employ 25 persons. We also engage consultants on an as needed basis who provide certain specified services to us.
Description of Property
Our company owns no real property. We are party to three commercial property leases for our corporate offices and manufacturing facility in California, our research and development facility in Canada, and our engineering division in Tennessee.
We currently lease approximately 9,000 square feet of office and industrial space at 14921 Chestnut St., Westminster, California 92683. The current lease term is from September 1, 2016 to August 31, 2020, at a monthly base rent of $8,379 throughout the term. In addition to serving as our principal offices, it is also a manufacturing facility where we manufacture our products, including our CupriDyne Clean Industrial Odor, and Specimen Transport Solidifiers.
We also lease approximately 1,500 square feet of office and lab space from the University of Alberta. The current lease term expires on January 31, 2020, at monthly fee of $5,266 Canadian dollars. These offices serve as our primary research and development facilities.
We also lease approximately 13,000 square feet of office and warehouse space at 105 Fordham Road, Oak Ridge, Tennessee, 37830, for our professional engineering division. The lease term is from September 1, 2017 through August 31, 2020, at a monthly base rent of $5,400 throughout the term.
Our telephone number is (888) 400-2863.
Legal Proceedings
Our company is not a party to any legal proceeding.
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This discussion contains forward-looking statements about our business and operations. Our actual results may differ materially from those we currently anticipate as a result of many factors, including those we described under “Risk Factors” and elsewhere in this prospectus. Certain statements contained in this discussion, including, without limitation, statements containing the words “believes,” “anticipates,” “expects” and the like, constitute “forward-looking statements” within the meaning of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). However, as we will issue “penny stock,” as such term is defined in Rule 3a51-1 promulgated under the Exchange Act, we are ineligible to rely on these safe harbor provisions. Such forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any of the future results, performance or achievements expressed or implied by such forward-looking statements. Given these uncertainties, readers are cautioned not to place undue reliance on such forward-looking statements. We disclaim any obligation to update any of such factors or to announce publicly the results of revision of any of the forward-looking statements contained herein to reflect future events or developments. For information regarding risk factors that could have a material adverse effect on our business, refer to the “Risk Factors” section of this prospectus beginning on page 3.
Results of Operations—Comparison of the three and six months ended June 30, 2018 and 2019
We operate our business in distinct business segments:
|
|
●
|
Odor-No-More, which manufactures and sells our odor and VOC control products and services, including our flagship product, CupriDyne Clean;
|
|
|
●
|
BLEST, our professional engineering services division supporting our internal business units and serving outside clients on a fee for service basis;
|
|
|
●
|
BioLargo Water, our Canadian division that has been historically pure research and development, and is now transitioning to focus on commercializing our AOS system;
|
|
|
●
|
Clyra Medical, our partially owned subsidiary focused on the Advanced Wound Care industry; and
|
|
|
●
|
Our corporate operations, which support the operating segments with legal, accounting, human resources, and other services.
|
We invest cash into each of these segments on a regular basis, as none of the segments yet generates enough cash to fund their operations. However, both Odor-No-More and BLEST are trending towards cash-flow positive, and we expect each of those two segments to begin to generate positive cash for BioLargo in 2019. Additionally, Clyra Medical raises capital directly, rather than relying on BioLargo for cash to operate.
Revenue for the three and six months ended June 30, 2019 was $426,000 and $790,000, respectively. This is a 30% and 34% increase over the same periods in 2018. We generated revenue from two of our operating divisions – Odor-No-More and BLEST. Our business segments obtain cash to support operations in different ways. Odor-No-More and BLEST generate revenues from third parties, and receive funding as needed from their parent corporation, BioLargo. Our Canadian team, BioLargo Water, receives funds from government research grants (reported on our financial statements as “Other income – Grant income”), and receives funding as needed from BioLargo. Clyra Medical, however, relies on direct investment from third parties for 100% of its operating costs and is not supported with capital from BioLargo’s corporate budget or fundraising.
Odor-No-More
Our wholly owned subsidiary Odor-No-More generates revenues through sales of our flagship product CupriDyne Clean, by providing design, installation, and maintenance services on the systems that deliver CupriDyne Clean at its clients’ facilities, and through sales of odor absorption products to the U.S. Government.
Revenue (Odor-No-More)
Odor-No-More’s revenues for the six months ended June 30, 2019, increased $76,000 (34%) from the same period in 2018. Its revenue for the three months ended June 30, 2019, was equal to that of the same period in 2018. The fluctuation in our revenues is due to timing of orders, weather at customer facilities (such as rain and snow), and shipping schedules. Approximately three-quarters of our revenue is generated from sales of CupriDyne Clean products and related services, and the remaining mostly from sales to the U.S. military.
Sales of our CupriDyne Clean products increased 31% and 24% in the three and six months ended June 30, 2019, as compared to same periods in 2018, due to the acquisition of more clients and client locations, and the sale and delivery of more products. Of our CupriDyne Clean sales, approximately one-half were made pursuant to “national purchasing agreements” (“NPA”) with the four largest waste handling companies in the United States. With the addition of an industry veteran as Director of Business Development, and increased capital resources, we expect sales to our NPA clients as well as new independent customers will increase in the remainder of 2019.
Sales to the U.S. military are primarily our Specimen Transport Solidifier pouches, and are made to the U.S. Defense Logistics Agency through our distributor Downeast Logistics. These sales decreased by 65% and 59% in the three and six months ended June 30, 2019 as compared with the same periods in 2018. The vast majority of these sales are made through a bid process in response to a request for bids to which any qualified government vendor can respond, and our decreased revenue in 2019 is due to a reduced number of opportunities from the government for our products, and to the cyclical nature and timing of the government procurement process. We cannot know in advance the frequency or size of such requests from the US Government, or whether our bids will be successful, and as such we are uncertain as to our future revenues through this system. We believe that the sales of CupriDyne Clean will continue to grow and help offset this segment of our business which we do not view as a high growth opportunity.
Cost of Goods Sold (Odor-No-More)
Odor-No-More’s cost of goods sold includes costs of raw materials, contract manufacturing, and portions of salaries and expenses related to the manufacturing of our products. For installation and other services, it includes labor and materials. As a percentage of gross sales, Odor-No-More’s costs of goods was 43% and 45% in the three and six months ended June 30, 2019 versus 61% in the same periods in 2018. In mid-2018, because of higher volumes, Odor-No-More was able to decrease its costs by purchasing raw materials directly from manufacturers at more favorable prices, resulting in the year-to-year cost of goods decrease.
Selling, General and Administrative Expense (Odor-No-More)
Odor-No-More’s Selling, General and Administrative (“SG&A”) expenses are remaining consistent between the three and six months ended June 30, 2019 and 2018. They are averaging $235,000 per quarter in 2019 compared to an average of $223,000 in 2018. These expenses have increased alongside Odor-No-More’s efforts to increase revenues by hiring additional sales and support staff. We expect its SG&A expenses to increase in 2019 as it continues to add sales and support personnel as its number of customers and revenues increase.
Operating Loss (Odor-No-More)
Odor-No-More had a net operating loss of $51,000 and $141,000 for the three and six months ended June 30, 2019. This was a 65% and 45% improvement over the same periods in 2018. Odor-No-More is continuing to increase sales to work toward profitability. Its gross margin from product sales is at 55%, and its loss from operations is trending downward. We believe these trends will continue. The loss from operations is trending downward for two reasons. First, Odor-No-More was able to reduce its product costs as a result of its increased volume (purchasing power). Second, increased sales resulted in increased gross margin contributing to the company’s operational costs.
We expect that Odor-No-More’s sales will continue to increase. By the end of 2019, assuming the company is properly capitalized with a marketing budget and additional salespeople, we expect that Odor-No-More will no longer require a cash subsidy to operate.
BLEST (engineering division)
Revenue (BLEST)
BLEST generated $241,000 and $424,000 of revenue for the three and six months ended June 30, 2019. Included in that total is intersegment revenue of $130,000 and $250,000 for the three and six months ended June 30, 2019. Intersegment revenue is eliminated in consolidation.
BLEST generated $111,000 and $174,000 of revenues from third party clients in the three and six months ended June 30, 2019, compared to $11,000 and $50,000 in revenue in same periods in 2018. The increase is due to an increase in the number of client contracts being serviced. The impact of its recently signed subcontracts to service the United States Air Force will begin generating revenues in the third quarter of 2019.
Cost of Goods (Services) Sold (BLEST)
BLEST’s cost of services includes employee labor as well as subcontracted labor costs. In the three and six months ended June 30, 2019, its cost of services were 84% and 83% of its revenues, versus 72% and 62% in the three and six months ended June 30, 2018. Costs were higher in the six-month comparison as we utilized sub-contractors with lower margins and we had fixed fee contracts that were not profitable. Those trends are declining as we add new, profitable contracts.
Selling, General and Administrative Expense (BLEST)
BLEST selling, general and administrative expenses during the three and six months ended June 30, 2019 totaled $107,000 and $198,000, which is comparable to the same periods in 2018. BLEST primarily delivers services to its clients, most of its labor costs are included in its cost of services (for third party clients), and research and development for its work on BioLargo technologies.
Operating Loss (BLEST)
BLEST had a net operating loss of $108,000 and $137,000 during the three and six months ended June 30, 2019, compared to $69,000 and $117,000 for the same periods in 2018. Because the subsidiary had an operating loss, we invested cash during the year to allow it to maintain operations. BLEST’s need for a cash subsidy to support its operations has decreased over time. We expect this trend to continue, and expect that in the remainder of 2019 its revenues will continue to increase. We expect that this subsidiary will become profitable and contribute cash to our corporate operations.
Other Income
Our wholly owned Canadian subsidiary has been awarded more than 67 research grants over the years from various Canadian public and private agencies, including the Canadian National Research Institute – Industrial Research Assistance Program (NRC-IRAP), the National Science and Engineering Research Council of Canada (NSERC), and the Metropolitan Water District of Southern California’s Innovative Conservation Program “ICP”. The research grants received are considered reimbursement grants related to costs we incur and therefore are included as Other Income. We continued to win grants and it is important to note that amounts paid directly to third parties are not included as income in our financial statements. Our grant income increased $9,000 and $86,000 in the three and six months ended June 30, 2019, compared with the same periods in 2018. This increase is due to additional and higher value grants awarded in 2019.
Although we are continuing to apply for government and industry grants, and indications from the various grant agencies is highly encouraging, we cannot be certain of continuing those successes in the future.
Selling, General and Administrative Expense – company-wide consolidated results
Our SG&A expenses include both cash expenses (for example, salaries to employees) and non-cash expenses (for example, stock option compensation expense). Our SG&A expenses decreased by 1% ($14,000) during the three months ended June 30, 2019 compared to the three months ended June 30, 2018, and increased by 8% ($210,000) for the six-month periods. The largest components of our SG&A expenses included (in thousands):
|
|
|
Three months
|
|
|
Six months
|
|
|
|
|
June 30, 2018
|
|
|
June 30, 2019
|
|
|
June 30, 2018
|
|
|
June 30, 2019
|
|
|
Salaries and payroll related
|
|
$
|
521
|
|
|
$
|
476
|
|
|
$
|
972
|
|
|
$
|
969
|
|
|
Professional fees
|
|
|
187
|
|
|
|
164
|
|
|
|
379
|
|
|
|
360
|
|
|
Consulting
|
|
|
192
|
|
|
|
299
|
|
|
|
353
|
|
|
|
590
|
|
|
Office expense
|
|
|
258
|
|
|
|
224
|
|
|
|
468
|
|
|
|
464
|
|
|
Sales and marketing
|
|
|
63
|
|
|
|
34
|
|
|
|
117
|
|
|
|
93
|
|
|
Investor relations
|
|
|
28
|
|
|
|
37
|
|
|
|
61
|
|
|
|
82
|
|
|
Board of director expense
|
|
|
68
|
|
|
|
68
|
|
|
|
135
|
|
|
|
135
|
|
Consulting expense increased in 2019 due to increased cash at Clyra and resulting increased research and development activities, and our hiring firms related to business development and brand exposure. . We have also increased our investor relations expense to continue to develop and spread the word about our company.
Research and Development
Our company-wide research and development expenses decreased by 14% and 17% compared to the three and six months ended June 30, 2018. In some areas, such as in our medical subsidiary, these expenses increased as the company was better financed and ramping up activities in anticipation of an FDA decision regarding their first wound care product. In Canada, we have transitioned from pure research and development towards a focus on commercializing the AOS system, decreasing R&D.
Interest expense
Our interest expense for the three and six months ended June 30, 2019 decreased by $1,231,000 (71%) and $1,078,000 (42%) compared with the three and six months ended June 30, 2018. Of our total interest expense, $40,000 was paid in cash, and the remaining is non-cash expenses related to financing transactions. Our interest expense decreased in 2019 primarily because (i) over $5 million in debt matured in the first six months of 2018, and (ii) we accepted cash from some convertible noteholders to reduce their conversion prices. We expect our interest expense to increase in the second half of 2019 due to the recent issuance of more than $2 million in Twelve Month OID Notes. Each investor also received a stock purchase warrant and we record the relative fair value of the warrants and the intrinsic value of the beneficial conversion feature sold with the convertible notes which typically results in a full discount on the proceeds from the convertible notes. This discount is then amortized as interest expense over the term of the convertible notes. Ultimately, it is management’s objective to secure equity and discontinue the use of convertible interest-bearing debt to finance its ongoing growth. In the six months ended June 30, 2019, we recorded non-cash expenses of $1,254,000 related to amortization of the fair value of warrants issued in connection with our debt, and $228,000 related to debt extension.
Net Loss
Net loss for the three and six months ended June 30, 2019 was $1,987,000 ($0.01 per share) and $4,736,000 ($0.03 per share). Net loss for the three and six months ended June 30, 2018 was $3,600,000 ($0.03 per share) and $6,029,000 ($0.05 per share). Our net loss in 2019 has decreased primarily due to lower interest expense and an increase in revenue. Of our total net loss, approximately $2.9 million (60%) was non-cash expense, and the remaining $1.85 million (40%) was cash used in operating activities.
The net loss per business segment is as follows (in thousands):
|
|
|
Three months
|
|
|
Six months
|
|
|
|
|
June 30, 2018
|
|
|
June 30, 2019
|
|
|
June 30, 2018
|
|
|
June 30, 2019
|
|
|
BioLargo corporate
|
|
|
(3,056
|
)
|
|
|
(1,486
|
)
|
|
|
(4,980
|
)
|
|
|
(3,591
|
)
|
|
Odor-no-more
|
|
|
(145
|
)
|
|
|
(51
|
)
|
|
|
(254
|
)
|
|
|
(141
|
)
|
|
Clyra
|
|
|
(177
|
)
|
|
|
(332
|
)
|
|
|
(376
|
)
|
|
|
(631
|
)
|
|
BLEST
|
|
|
(71
|
)
|
|
|
(26
|
)
|
|
|
(117
|
)
|
|
|
(137
|
)
|
|
BioLargo Water
|
|
|
(151
|
)
|
|
|
(92
|
)
|
|
|
(302
|
)
|
|
|
(237
|
)
|
|
Net loss
|
|
|
(3,600
|
)
|
|
|
(1,987
|
)
|
|
|
(6,029
|
)
|
|
|
(4,736
|
)
|
Liquidity and Capital Resources
For the six months ended June 30, 2019, we had a net loss of $4,736,000, used $1,851,000 cash in operations, and at June 30, 2019, had a working capital deficit of $3,473,000 and current assets of $1,001,000. We do not have sufficient working capital and do not believe gross profits will be sufficient to fund our current level of operations or pay our debt due prior to December 31, 2019, and will have to obtain further investment capital to continue to fund operations and seek to refinance our existing debt. We have been, and anticipate that we will continue to be, limited in terms of our capital resources. During the year ended December 31, 2018, and the six months ended June 30, 2019, we generated revenues of $1,364,000 and $790,000 through our business segments (Odor-No-More and BLEST – see Note 10, “Business Segment Information”). Neither generated enough revenues to fund their operations. We have $2,119,000 in debt obligations due in the next 12 months (see Notes 4 and 12): (i) $1,724,000in notes that are convertible at the option of the holder, (ii) a $145,000 note due September 6, 2019, and (iii) a line of credit in the amount of $250,000 due on 30-day demand beginning September 1, 2019. We intend to either refinance or renegotiate these obligations, as our cash position is insufficient to maintain our current level of operations and pay these liabilities. Thus, we will be required to raise additional capital. We continue to raise money through private securities offerings, and continue to negotiate for more substantial financings from private and institutional investors. During the six months ended June 30, 2019, we received $1,924,000 net cash provided by financing activities, and at June 30, 2019 had cash of $706,000. Subsequent to June 30, 2019, we received $2,360,000 from new financing activities. No assurance can be made of our success at raising money through private or public offerings.
Clyra Medical is unique in that it funds its operations through third party investments, as it has done since 2016. We do not currently intend and are under no obligation to subsidize its operations in the future.
Results of Operations—Comparison of the years ended December 31, 2017 and 2018
We operate our business in distinct business segments:
|
|
●
|
Odor-No-More, which manufactures and sells our odor and VOC control products and services, including our flagship product, CupriDyne Clean;
|
|
|
●
|
BLEST, our professional engineering services division supporting our internal business units and serving outside clients on a fee for service basis;
|
|
|
●
|
BioLargo Water, our Canadian division that has been historically pure research and development, and is now transitioning to focus on commercializing our AOS system;
|
|
|
●
|
Clyra Medical, our partially owned subsidiary focused on the Advanced Wound Care industry; and
|
|
|
●
|
Our corporate operations, which support the operating segments with legal, accounting, human resources, and other services.
|
We invest cash into each of these segments on a regular basis, as none of the segments yet generates enough cash to fund their operations. However, both Odor-No-More and BLEST are trending towards cash-flow positive, and we expect each of those two segments to begin to generate positive cash for BioLargo in 2019.
Annual revenue for the year ended December 31, 2018 was $1,364,000, more than double the revenue of $516,000 in 2017. We generated revenue from two of our operating divisions – Odor-No-More and BLEST. Our business segments obtain cash to support operations in different ways. Odor-No-More and BLEST generate revenues from third parties, and receive funding as needed from their parent corporation, BioLargo. Our Canadian team, BioLargo Water, receives funds from government research grants (reported on our financial statements as “Other income – Grant income”), and receives funding as needed from BioLargo. Clyra Medical, however, relies direct investment from third parties for 100% of its operating costs and is not supported with capital from BioLargo’s corporate budget or fundraising.
Odor-No-More
Our wholly owned subsidiary Odor-No-More generates revenues through sales of our flagship product CupriDyne Clean, by providing design, installation, and maintenance services on the systems that deliver CupriDyne Clean at its clients’ facilities, and through sales of odor absorption products to the U.S. Government. Although Odor-No-More did not generate a net profit in 2018, its revenues continued to increase throughout the year, and in the fourth quarter of 2018 its net loss was only $68,000 (for the year, its net loss was $433,000).
Revenue (Odor-No-More)
Odor-No-More’s revenues more than doubled in 2018, to $1,123,000, comprised of $1,016,000 in product sales, and $107,000 in design, installation and maintenance services (including related parts). Of product sales, approximately 50% was generated from sales of CupriDyne Clean products, and approximately one-third from sales to the U.S. military.
Sales of our CupriDyne Clean products increased 68% from the prior year, due to the acquisition of more clients and client locations, and the sale and delivery of more products than in years past. Of our CupriDyne Clean sales, approximately two-thirds were made pursuant to “national purchasing agreements” (“NPA”) with the four largest waste handling companies in the United States. We expect our sales to NPA clients to continue to increase in 2019 as we expect to continue to add new service locations for those customers. And, for one such company, we have only recently become fully authorized in their corporate system, opening up potential sales to their more than 1,000 U.S. locations.
Sales to the U.S. military are primarily our Specimen Transport Solidifier pouches, and are made to the U.S. Defense Logistics Agency through our distributor Downeast Logistics. These sales increased by almost three-fold in 2018 as compared with 2017. The vast majority of these sales are made through a bid process in response to a request for bids to which any qualified government vendor can respond, and our increased revenue in 2018 is due to an increased volume of sales from the bidding process. We cannot know in advance the frequency or size of such requests from the US Government, or whether our bids will be successful, and as such we are uncertain as to our future revenues through this system.
Cost of Goods Sold (Odor-No-More)
Odor-No-More’s cost of goods sold includes costs of raw materials, contract manufacturing, and portions of salaries and expenses related to the manufacturing of our products. As a percentage of gross sales, Odor-No-More’s costs of goods was 51% in 2018 versus 64% in 2017. In mid-2018, because of higher volumes, Odor-No-More was able to decrease its costs by purchasing raw materials directly from manufacturers at more favorable prices, resulting in the year-to-year cost of goods decrease.
Selling, General and Administrative Expense (Odor-No-More)
Odor-No-More’s Selling, General and Administrative (“SG&A”) expenses include both cash and non-cash expense related to its operations. Odor-No-More’s SG&A expenses increased to $969,000 in 2018, as compared with $661,000 in 2017, an increase of 47%. These expenses have increased alongside Odor-No-More’s efforts to increase revenues by hiring additional sales and support staff. We expect its SG&A expenses to increase in 2019 as it continues to add sales and support personnel as its number of customers and revenues increase.
Net Loss (Odor-No-More)
Odor-No-More generated $1,123,000 in revenue, a gross margin of $552,000, and had total costs and expenses of $985,000, resulting in a net loss of $433,000. Odor-No-More is trending toward profitability. Its gross margin from product sales has increased significantly since 2017, and its loss from operations is trending downward:
We believe these trends will continue. The loss from operations is trending downward for two reasons. First, Odor-No-More was able to reduce its product costs as a result of its increased volume (purchasing power). Second, increased sales resulted in increased gross margin contributing to the company’s operational costs.
Because the subsidiary had a net loss, we invested cash into it during the year to allow it to fund its operations. However, this need for cash decreased as 2018 progressed, and in the fourth quarter of 2018, it needed only $51,000 cash (as compared with over $400,000 for the year). We expect that Odor-No-More’s sales will continue to increase, and thus its gross margin will continue to increase. By the end of 2019, we expect that Odor-No-More will no longer require a cash subsidy to operate, but will be contributing cash to our corporate operations.
BLEST (engineering division)
Revenue (BLEST)
Our engineering segment (BLEST) generated $241,000 of revenues from third party clients in its first full year of operation, versus only $12,000 in revenue in its first three months of operation in 2017. BLEST’s revenues increased in the latter part of the year, with approximately one-half of its revenues generated during the fourth quarter 2018. Its revenues do not include over $600,000 of work performed on internal BioLargo projects, such as its further engineering and development of the AOS water filtration system. Our engineers are performing a critical role in the AOS pilot projects, some of which are supported by third-party research grants and has been instrumental in developing and supporting a professional engineered design service for misting systems being sold by our Odor-No-More operating unit.
Cost of Goods (Services) Sold (BLEST)
BLEST’s cost of services includes employee labor as well as subcontracted labor costs. In 2018, its cost of services were 71% of its revenues, versus 66% in 2017. We expect the cost of services to remain stable in 2019.
Selling, General and Administrative Expense (BLEST)
BLEST’S SG&A expenses include both cash and non-cash expense related to its operations, although because it primarily delivers services to its clients, most of its labor costs are included in its cost of services (for third party clients), and research and development for its work on BioLargo technologies. Because BLEST began operations in the fourth quarter of 2017, and thus its SG&A expenses of $409,000 in 2018 does not have a comparable period in 2017. We expect these expenses to increase only slightly in 2019, as the staff required to increase service to its clients and revenues will be included in cost of services.
Net Loss (BLEST)
BLEST generated $241,000 in revenue, a gross margin of $69,000, and had total costs and expenses of $991,000, resulting in a net loss of $750,000.
While we are unable to record revenues generated from intracompany services by the engineering group to other operating divisions, it is important to note that the net loss would be eliminated if BLEST were an outside contract for hire services company selling services to our water company or our industrial odor and VOC control operating unit.
Because the subsidiary had a net loss, we invested cash during the year to allow it to maintain operations. BLEST’s need for a cash subsidy to support its operations decreased considerably towards the end of calendar year 2018. We expect this trend to continue, and expect that in 2019 its sales will continue to increase, and thus its gross profit will continue to increase. By the end of 2019, we expect that it will no longer require a cash subsidy to operate, but will be contributing cash to our corporate operations.
Other Income
Our wholly owned Canadian subsidiary has been awarded more than 65 research grants over the years from various Canadian public and private agencies, including the Canadian National Research Institute – Industrial Research Assistance Program (NRC-IRAP), the National Science and Engineering Research Council of Canada (NSERC), and the Metropolitan Water District of Southern California’s Innovative Conservation Program “ICP”. The research grants received are considered reimbursement grants related to costs we incur and therefore are included as Other Income. The amount of grant income remained consistent between 2017 and 2018. We continued to win grants and it is important to note that amounts paid directly to third parties are not included as income in our financial statements.
Our Canadian subsidiary applied for and received a refund on our income taxes pursuant to the “Scientific Research and Experimental Development (SR&ED) Program”, a Canadian federal tax incentive program designed to encourage Canadian businesses to conduct research and development in Canada. For the year ended December 31, 2017 and 2018, we received $71,000 and $73,000.
Although we are continuing to apply for government and industry grants, and indications from the various grant agencies is highly encouraging, we cannot be certain of continuing those successes in the future.
Selling, General and Administrative Expense – company wide
Our SG&A expenses include both cash expenses (for example, salaries to employees) and non-cash expenses (for example, stock option compensation expense). Our SG&A expenses increased by 19% ($834,000) in 2018 to $5,264,000.4 Our non-cash expenses (through the issuance of stock and stock options) increased in 2018 compared with 2017 ($2,242,000 compared to $1,564,000) because our employees, vendors and consultants chose to receive a greater number of stock and stock options in lieu of cash owed. The largest components of our SG&A expenses included (in thousands):
|
|
|
Year ended
December 31, 2017
|
|
|
Year ended
December 31, 2018
|
|
|
Salaries and payroll related
|
|
$
|
1,610
|
|
|
$
|
1,973
|
|
|
Professional fees
|
|
|
651
|
|
|
|
800
|
|
|
Consulting
|
|
|
810
|
|
|
|
839
|
|
|
Office expense
|
|
|
627
|
|
|
|
987
|
|
|
Board of director expense
|
|
|
306
|
|
|
|
280
|
|
|
Sales and marketing
|
|
|
224
|
|
|
|
246
|
|
|
Investor relations
|
|
|
201
|
|
|
|
139
|
|
Our salaries and payroll-related and office-related expenses increased in 2018 due to the addition of our engineering subsidiary for the full year of 2018 compared to only three months of 2017. Our professional fees increased in 2018 due to increased needs for legal and accounting as a result of the registration statements filed during 2018, the special stockholder meeting held in September 2018 and other work related to our efforts to list our common stock on a national exchange, and the purchase of the intellectual property of Scion Solutions (see Part I, Item I, “Advanced Wound Care – Clyra Medical,” above). Office expense increased due to the addition our engineering segment in Tennessee. Our investor relations fees decreased in 2018 compared with 2017 due to a reduction in the use of outside investor relation firms during that period. The Company has maintained investor relations support with internal personnel.
4 This includes all of our operational segments (including Odor-No-More and BLEST discussed above).
Research and Development
In the year ended December 31, 2018, we spent approximately $1,700,000 in the research and development of our technologies and products. This was a slight increase of 5% ($86,000) over 2017. This number does not include over $300,000 in internal billings from our engineering division’s work on the AOS system.
As we transition our Canadian operations from pure research and development towards a focus on commercializing the AOS system, we expect their contribution to our total research and development expenses to decrease in 2019. We expect this to be offset by increased research and development at Clyra Medical, which we expect to be funded entirely from its own resources.
Interest expense
Our interest expense for the year ended December 31, 2018 was $3,494,000, a decrease of $366,000 compared with 2017, and of which $54,000 was paid in cash, and the remainder, $3,440,000, is non-cash expense. Our non-cash interest related expenses were comprised primarily as follows: (i) $2,766,000 as one-time, non-cash debt discounts related to warrants issued in conjunction with debt instruments being amortized over the life of the debt instrument (in 2017, it was $3,058,000), and (ii) $524,000 related to interest paid in stock on debt instruments. While we cannot predict our interest expense in 2019, our outstanding debt as of December 31, 2018 was substantially less than as of December 31, 2019, and thus we expect our interest expense in 2019 to decline.
We record the relative fair value of the warrants and the intrinsic value of the beneficial conversion feature sold with the convertible notes payable which typically results in a full discount on the proceeds from the convertible notes. This discount is being amortized as interest expense over the term of the convertible notes. We expect our interest expense to decrease in 2019 because the total amount we amortize (the line item on our balance sheet “Discount on convertible notes payable and line of credit, net of amortization”) decreased by $1,784,000 in 2018 – from $2,107,000 at December 31, 2017, to $323,000 at December 31, 2018. However, any decrease would be offset if we issue new debt instruments in 2019 that are combined with warrants, or if we issue new warrants as consideration to extend maturity dates on existing debt instruments.
Net Loss
Net loss for the year ended December 31, 2018 was $10,696,000 a loss of $0.09 per share, compared to a net loss for the year ended December 31, 2017 of $9,547,000 a loss of $0.10 per share. Our net loss this year was somewhat offset by an increase in revenue; nevertheless, the net loss increased mainly due to the increase in financing costs, non-cash interest expense to obtain capital, and increased payroll and related office expenses which are primarily associated with the start-up expenses related to our engineering operating unit. The decrease in net loss per share for the year ended December 31, 2018 is primarily attributable to the increase in the number of shares outstanding from 2017 to 2018.
The net loss per business segment is as follows (in thousands):
|
Net loss
|
|
Year ended
December 31, 2017
|
|
Year ended
December 31, 2018
|
|
Odor-No-More
|
|
$
|
(500)
|
|
$
|
(433)
|
|
BLEST
|
|
|
(90)
|
|
|
(750)
|
|
Clyra Medical
|
|
|
(915)
|
|
|
(883)
|
|
BioLargo Water
|
|
|
(741)
|
|
|
(571)
|
|
Corporate
|
|
|
(7,301)
|
|
|
(8,059)
|
|
Consolidated net loss
|
|
$
|
(9,547)
|
|
$
|
(10,696)
|
It is important to note that of the corporate net loss of $8,059,000, interest expense was $3,494,000, of which $3,440,000 was a non-cash expense. R & D was $1,700,000 primarily attributed to the accelerated development of the AOS technology. These two items alone account for $5.2 million in losses of the consolidated loss of $10,696,000 in total losses. With expanding sales, we believe that Odor-No-More and BLEST (engineering) can achieve positive cash flow from operations. However, with the continued development costs associated with Clyra Medical (even though it is financed directly through the sale of stock in Clyra), and with the addition of any ongoing development costs associated with BioLargo Water to be incurred through pre-commercial piloting, we expect to continue to incur a net loss for the foreseeable future.
We have made considerable investments in our water and medical technologies as well as supporting the start-up expenses for our engineering team. We believe those investment will pay off as we now are narrowly focused on commercial sales.
Liquidity and Capital Resources
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of our business. For the year ended December 31, 2018, we had a net loss of $10,696,000, used $3,891,000 cash in operations, and at December 31, 2018, we had a working capital deficit of $1,536,000, and current assets of $955,000. At December 31, 2018, we had convertible debt, promissory notes, and line of credit obligations outstanding with an aggregate principal balance of $3,487,000, an accumulated deficit of $111,723,000, and net stockholders’ deficit of $1,496,000. We do not believe gross profits will be sufficient to fund our current level of operations or pay our debt due prior to December 31, 2019, and thus we believe we will have to raise additional investment capital to both fund our operations and refinance our existing debt.
We operate our business in five distinct business segments. Each of these segments obtains cash to fund operations in unique ways. Odor-No-More and BLEST generate cash by selling products and services. Clyra Medical obtains cash from third party investments of sales of its common stock. BioLargo Water generates cash through government research grants and tax credits. Our corporate operations generate cash through private offerings of stock, debt instruments, and warrants. In 2018, cash was generated as follows (in thousands):
|
|
|
2017 (year)
|
|
|
2018 (Year)
|
|
|
SOURCES OF INCOME AND CASH
|
|
|
|
|
|
|
|
|
|
Revenue from operations
|
|
$
|
516
|
|
|
$
|
1,364
|
|
|
Grant income
|
|
|
140
|
|
|
|
158
|
|
|
Tax credit income
|
|
|
71
|
|
|
|
73
|
|
|
Cash investments (to BioLargo)
|
|
|
3,373
|
|
|
|
2,637
|
|
|
Cash investments (to Clyra)
|
|
|
750
|
|
|
|
1,005
|
|
|
Total:
|
|
$
|
4,850
|
|
|
$
|
5,237
|
|
Only two segments (Odor-No-More and BLEST) generated revenues in the year ended December 31, 2018. As such, we provided a cash subsidy to each business segment to allow it to fund its operations. For our two revenue generating divisions, cash needs have decreased as their revenues have increased. In the fourth quarter of 2018, Odor-No-More’s gross margin and cash receipts from clients were such that it needed only $51,000 extra cash from corporate to meet its operational expenses. BLEST similarly increased its revenues such that it needed little cash from corporate during the fourth quarter to maintain it operations. We expect these trends to continue, and expect that at some point in the calendar year 2019 both Odor-No-More and BLEST will be generating profits and contributing cash to corporate operations.
In the first quarter of 2019, we shifted focus at our Canadian subsidiary (BioLargo Water) from pure research and development to commercializing the AOS system. In doing so, we reduced our research staff and thus reduced its monthly cash needs by $15,000.
Clyra Medical is unique in that it funds its operations through third party investments. We do not intend to subsidize its operations in the future.
We used almost four million dollars cash in our total operations in 2018. At December 31, 2018, we had current assets of just less than one million dollars. Thus, to maintain the same level of operations in 2019, and notwithstanding the increasing revenues at Odor-No-More and BLEST, we expect to continue to need to raise investment capital. In 2018, we conducted private securities offerings and received $3,642,000 net proceeds. Since first acquiring the BioLargo technology in the spring of 2007, we have received investment capital of approximately $22,000,000 which we have invested in development and commercialization efforts. We intend to continue to raise money through private securities offerings for the foreseeable future. Although we engaged an investment banking firm and filed a registration statement to raise $7,500,000 in conjunction with an application for listing our common stock on the Nasdaq Capital Markets, no assurance can be made that we will move forward in the near future with that offering or our listing application. We may reconsider and postpone these efforts as management believes our current market capitalization does not reflect the true value of the Company or recognize the significant business opportunities that lie ahead. Our board intends to evaluate these and other factors, including the anticipated dilution to our stockholders of an offering of the size required to meet the initial and continued listing requirements. No assurance can be made of our success at raising money through private or public offerings, or of our intended listing on a national exchange.
Critical Accounting Policies
Our discussion and analysis of our results of operations and liquidity and capital resources are based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates and judgments, including those related to revenue recognition, valuation of offerings of debt with equity or derivative features which include the valuation of the warrant component, any beneficial conversion feature and potential derivative treatment, and share-based payments. We base our estimates on anticipated results and trends and on various other assumptions that we believe are reasonable under the circumstances, including assumptions as to future events. These estimates form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. By their nature, estimates are subject to an inherent degree of uncertainty. Actual results that differ from our estimates could have a significant adverse effect on our operating results and financial position. We believe that the following significant accounting policies and assumptions may involve a higher degree of judgment and complexity than others.
The methods, estimates and judgments the Company uses in applying these most critical accounting policies have a significant impact on the results of the Company reports in its financial statements.
Revenue Recognition
We adopted ASU 2014-09, “Revenue from Contracts with Customers”, Topic 606, on January 1, 2018. The guidance focuses on the core principle for revenue recognition.
The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, an entity should apply the following steps:
Step 1: Identify the contract(s) with a customer.
Step 2: Identify the performance obligations in the contract.
Step 3: Determine the transaction price.
Step 4: Allocate the transaction price to the performance obligations in the contract.
Step 5: Recognize revenue when (or as) the entity satisfies a performance obligation.
We have revenue from two subsidiaries, Odor-No-More and BLEST. Odor-No-More identifies its contract with the customer through a written purchase order , in which the details of the contract are defined including the transaction price and method of shipment. The only performance obligation is to create and ship the product and each product has separate pricing. Odor-No-More recognizes revenue at a point in time when the order for its goods are shipped if its agreement with the customer is FOB Odor-No-More’s warehouse facility, and when goods are delivered to its customer if its agreement with the customer is FOB destination. Revenue is recognized with a reduction for sales discounts, as appropriate and negotiated in the customer’s purchase order.
BLEST identifies services to be performed in a written contract, which specifies the performance obligations and the rate at which the services will be billed. Each service is separately negotiated and priced. Revenue is recognized as services are performed and completed. BLEST’s contracts typically call for invoicing for time and materials incurred for that contract. To date, there have been no discounts or other financing terms for the contracts.
In the future, we may generate revenues from royalties or license fees from our intellectual property. In the event we do so, we anticipate a licensee would pay a license fee in one or more installments and ongoing royalties based on their sales of products incorporating or using our licensed intellectual property. Upon entering into a licensing agreement, we will determine the appropriate method of recognizing the royalty and license fees.
Warrants and Conversion Features
Warrants issued with our convertible and non-convertible debt instruments are accounted for under the fair value and relative fair value method.
The warrant is first analyzed per its terms as to whether it has derivative features or not. If the warrant is determined to be a derivative and not qualify for equity treatment, then it is measured at fair value using the Black Scholes option model, and recorded as a liability on the balance sheet. The warrant is re-measured at its then current fair value at each subsequent reporting date (it is “marked-to-market”).
If the warrant is determined to not have derivative features, it is recorded into equity at its fair value using the Black Scholes option model, however, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the convertible note.
Convertible debt instruments are recorded at fair value, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the warrant. Further, the convertible debt instrument is examined for any intrinsic beneficial conversion feature (“BCF”) of which the conversion price is less than the closing common stock price on date of issuance. If the relative fair value method is used to value the convertible debt instrument and there is an intrinsic BCF, a further analysis is undertaken of the BCF using an effective conversion price which assumes the conversion price is the relative fair value divided by the number of shares the convertible debt is converted into by its terms. The BCF value is accounted for as equity.
The warrant and BCF relative fair values are also recorded as a discount to the convertible promissory notes. As present, these equity features of the convertible promissory notes have recorded a discount to the convertible notes that is substantially equal to the proceeds received.
Share-based Payments
It is the Company’s policy to expense share-based payments as of the date of grant or over the term of the vesting period in accordance with Auditing Standards Codification Topic 718 “Share-Based Payment.” Application of this pronouncement requires significant judgment regarding the assumptions used in the selected option pricing model, including stock price volatility and employee exercise behavior. Most of these inputs are either highly dependent on the current economic environment at the date of grant or forward-looking expectations projected over the expected term of the award.
Fair Value Measurement
Generally accepted accounting principles establishes a hierarchy to prioritize the inputs of valuation techniques used to measure fair value. The hierarchy gives the highest ranking to the fair values determined by using unadjusted quoted prices in active markets for identical assets (Level 1) and the lowest ranking to fair values determined using methodologies and models with unobservable inputs (Level 3). Observable inputs are those that market participants would use in pricing the assets based on market data obtained from sources independent of the Company. Unobservable inputs reflect the Company’s assumptions about inputs market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. The Company has determined the appropriate level of the hierarchy and applied it to its financial assets and liabilities.
Management believes the carrying amounts of the Company’s financial instruments as of December 31, 2018 and June 30, 2019 approximate their respective fair values because of the short-term nature of these instruments. Such instruments consist of cash, accounts receivable, prepaid assets, accounts payable, convertible notes, and other assets and liabilities.
Recent Accounting Pronouncements
See Note 2 to the Consolidated Financial Statements, “Summary of Significant Accounting Policies – Recent Accounting Pronouncements”, for the applicable accounting pronouncements affecting the Company.
MANAGEMENT
Executive Officers and Directors
The following table sets forth information about our executive officers and directors as of the date of this prospectus:
|
Name
|
|
Age
|
|
Position
|
|
|
Dennis P. Calvert
|
|
56
|
|
President, CEO, Chairman, Director
|
|
|
Charles K. Dargan II
|
|
64
|
|
CFO
|
|
|
Kenneth R. Code
|
|
72
|
|
Chief Science Officer, Director
|
|
|
Joseph L. Provenzano
|
|
50
|
|
Vice President of Operations, Corporate Secretary, Director
|
|
|
Dennis E. Marshall(2)(3)(5)
|
|
76
|
|
Director
|
|
|
Kent C. Roberts III(3)(5)
|
|
59
|
|
Director
|
|
|
John S. Runyan(1)(4)(6)
|
|
80
|
|
Director
|
|
|
Jack B. Strommen
|
|
49
|
|
Director
|
______________
|
(1)
|
Member of Audit Committee
|
|
(2)
|
Member of Compensation Committee
|
|
(3)
|
Chairman of Audit Committee
|
|
(4)
|
Chairman of Compensation Committee
|
|
(5)
|
Member of Nominating Committee
|
|
(6)
|
Chairman of Nominating Committee
|
Dennis P. Calvert is our President, Chief Executive Officer and Chairman of the Board. He also serves in the same positions for BioLargo Life Technologies, Inc. and BioLargo Water U.S.A., Inc., both wholly owned subsidiaries, and chairman of the board of directors of our subsidiaries Odor-No-More, Inc., Clyra Medical Technologies, Inc. and BioLargo Water, Inc. (Canada). Mr. Calvert was appointed a director in June 2002 and has served as President and Chief Executive Officer since June 2002, Corporate Secretary from September 2002 until March 2003 and Chief Financial Officer from March 2003 through January 2008. Mr. Calvert holds a B.A. degree in Economics from Wake Forest University, where he was a varsity basketball player. Mr. Calvert also studied at Columbia University and Harding University. He also serves on the board of directors at The Maximum Impact Foundation, a 501(c)(3), committed to bridging the gap for lifesaving work around the globe for the good of man and in the name of Christ. He serves as a Director of Sustain SoCal (formerly known as Sustain OC) in and serves on their “Technology Breakthrough” committee. Sustain SoCal is a trade association that seeks to promote economic growth in the Orange County clean technology industry. Most recently, he joined the Board of Directors at The Maritime Alliance of San Diego and also serves on the Board of Directors of Tilly’s Life Center, a nonprofit charitable foundation aimed at empowering teens with a positive mindset and enabling them to effectively cope with crisis, adversity and tough decisions. He is also an Eagle Scout. He is married and has two children. He has been an active coach in youth sports organizations and ministry activity in his home community. Mr. Calvert has an extensive entrepreneurial background as an operator, investor and consultant. Prior to his work with BioLargo, he had participated in more than 300 consulting projects and more than 50 acquisitions as well as various financing transactions and companies that ranged from industrial chemicals, healthcare management, finance, telecommunications and consumer products.
Charles K. Dargan II is our Chief Financial Officer and has served as such since February 2008. Since January 2003, Mr. Dargan has served as founder and principal of CFO 911, an organization of senior executives that provides accounting, finance and operational expertise to both public and private companies who are at strategic inflection points of their development and helps them effectively transition from one business stage to another. From March 2000 to January 2003, Mr. Dargan was the Chief Financial Officer of Semotus Solutions, Inc., an American Stock Exchange-listed wireless mobility software company. Mr. Dargan also serves as a director of Hiplink Software, Inc. and CPSM, Inc. Further, Mr. Dargan began his finance career in investment banking with Drexel Burnham Lambert and later became Managing Director of two regional firms, including Houlihan Lokey Howard & Zukin, where he was responsible for the management of the private placement activities of the firm. Mr. Dargan received his B.A. degree in Government from Dartmouth College, and his M.B.A. degree and M.S.B.A. degree in Finance from the University of Southern California. Mr. Dargan is a CPA (inactive).
Kenneth R. Code is our Chief Science Officer. He has been a director since April 2007. Mr. Code is our single largest stockholder. He is the founder of IOWC, which is engaged in the research and development of advanced disinfection technology, and from which our company acquired its core iodine technology in April 2007. Mr. Code has authored several publications and holds several patents, with additional patents pending, concerning advanced iodine disinfection. Mr. Code graduated from the University of Calgary, Alberta, Canada.
Joseph L. Provenzano is our Vice President of Operations, Corporate Secretary. He has been a director since June 2002, assumed the role of Corporate Secretary in March 2003, was appointed Executive Vice President of Operations in January 2008 and was elected President of our subsidiary, Odor-No-More, Inc., upon the commencement of its operations in January 2010. He is a co-inventor on several of our company’s patents and proprietary manufacturing processes, and he has developed over 30 products from our CupriDyne® technology. Mr. Provenzano began his corporate career in 1988 in the marketing field. In 2001 he began work with an investment holding company to manage their mergers and acquisitions department, participating in more than 50 corporate mergers and acquisitions.
Dennis E. Marshall has been a director since April 2006. Mr. Marshall has over 46 years of experience in real estate, asset management, management level finance and operations-oriented management. Since 1981, Mr. Marshall has been a real estate investment broker in Orange County, California, representing buyers and sellers in investment acquisitions and dispositions. From March 1977 to January 1981, Mr. Marshall was a real estate syndicator at McCombs Corporation as well as the assistant to the Chairman of the Board. While at McCombs Corporation, Mr. Marshall became the Vice President of Finance, where he financially monitored numerous public real estate syndications. From June 1973 to September 1976, Mr. Marshall served as an equity controller for the Don Koll Company, an investment builder and general contractor firm, at which Mr. Marshall worked closely with institutional equity partners and lenders. Before he began his career in real estate, Mr. Marshall worked at Arthur Young & Co. (now Ernst & Young) from June 1969 to June 1973, where he served as Supervising Senior Auditor and was responsible for numerous independent audits of publicly held corporations. During this period, he obtained Certified Public Accountant certification. Mr. Marshall earned a degree in Accounting from the University of Texas, Austin in 1966 and earned a Master of Science Business Administration from the University of California, Los Angeles in 1969. Mr. Marshall serves as Chairman of the Audit Committee.
Kent C. Roberts III has been a director since August 2011. Mr. Roberts is an analyst and portfolio manager for Vulcan Capital is Seattle Washington. He joined Vulcan Capital in April 2017. Mr. Roberts has had a long and successful career in the asset management business as a north American practice leader or at the senior partner level. His investment experience spans 25 years where he served in senior positions in business management, trading, currency risk management, business development and marketing strategy, as well as governance and oversight roles. He has worked for both large firms as well as boutiques that bring unique investment expertise to investors around the world. Those firms include: Global Evolution USA, First Quadrant and Bankers Trust Company. He has presented at numerous industry conferences and as a guest speaker at numerous industry conferences and events. Before entering the financial services industry Mr. Roberts worked in the oil and gas exploration industry. Mr. Roberts received a MBA in Finance from the University of Notre Dame and a BS in Agriculture and Watershed Hydrology from the University of Arizona. Mr. Roberts holds a series 3 securities license.
John S. Runyan has been a director since October 2011. He has spent his career in the food industry. He began as a stock clerk at age 12, and ultimately served the Fleming Companies for 38 years, his last 10 years as a Senior Executive Officer in its corporate headquarters where he was Group President of Price Impact Retail Stores with annual sales of over $3 billion. He retired from Fleming Companies in 2001, and then established JSR&R Company Executive Advising, with a primary emphasis in the United States and international food business. His clients have included Coca Cola, Food 4 Less Price Impact Stores, IGA, Inc., Golden State Foods, Bozzuto Companies Foodstuffs New Zealand, Metcash Australia and McLane International. In 2005, he joined Associated Grocers in Seattle, Washington as President and CEO, overseeing its purchase in 2007 by Unified Grocers, at which time he became Executive Advisor to its CEO and to its President. Mr. Runyan currently serves on the board of directors of Western Association of Food Chains and Retailer Owned Food Distributors of America. Additionally, Mr. Runyan served eight years as a board member of the City of Hope’s Northern California Food Industry Circle, which included two terms as President, and was recognized with the City of Hope “Spirit of Life” award. He was the first wholesale executive to be voted “Man of the Year” by Food People Publication. He is a graduate of Washburn University, which recognized his business accomplishments in 2007 as the honoree from the School of Business “Alumni Fellow Award.” Mr. Runyan serves as Chairman of the Compensation and Nominating/Corporate Governance Committees.
Jack B. Strommen has been a director since June 2017, and also is a member of the board of directors of our subsidiary, Clyra Medical Technologies, as the representative of Sanatio Capital LLC. Mr. Strommen is the CEO of PD Instore, a leader in the design, production and installation of retail environments and displays for many Fortune 500 companies including Target, Adidas, Verizon, Disney and Sony. He also is the Chairman of Our House Films, an angel investor in several private companies ranging from bio-tech to med-tech to real estate, and serves on the board of directors of several private and public companies. A relentless force of growth, Mr. Strommen has taken his company, PD Instore, to new and ever increasing levels of success. Mr. Strommen purchased the family owned, local based printing firm, from his grandfather in 1999. With his vision and leadership, it went from a local company with $25M in revenues to a global company with $180M in global sales. Mr. Strommen led the company in a private sale in 2015, remaining as CEO.
CORPORATE GOVERNANCE
Our corporate website, www.biolargo.com, contains the charters for our Audit and Compensation Committees and certain other corporate governance documents and policies, including our Code of Ethics. Any changes to these documents and any waivers granted with respect to our Code of Ethics will be posted at www.biolargo.com. In addition, we will provide a copy of any of these documents without charge to any stockholder upon written request made to Corporate Secretary, BioLargo, Inc., 14921 Chestnut St., Westminster, California 92683. The information at www.biolargo.com is not, and shall not be deemed to be, a part of this prospectus.
Director Independence
Our board of directors has determined that each of Messrs. Marshall, Roberts, Strommen and Runyan is independent as defined under applicable Nasdaq Stock Market, LLC (“Nasdaq”) listing standards. Our board of directors has determined that neither Mr. Calvert, Mr. Provenzano, nor Mr. Code is independent as defined under applicable Nasdaq listing standards. Neither Mr. Calvert, Mr. Provenzano, nor Mr. Code serve on any committee of our board of directors.
Meetings of our Board of Directors
Our board of directors held five meetings during 2017, and acted via unanimous written consent four times. Each of the incumbent directors attended all the meetings of our board of directors and committees on which the director served, except for two absences at the annual board meeting in June 2017, and one absence at a meeting in August 2017. Each of our directors is encouraged to attend our Annual Meeting of Stockholders, when these are held, and to be available to answer any questions posed by stockholders to such director.
Communications with our Board of Directors
The following procedures have been established by our board of directors to facilitate communications between our stockholders and our board of directors:
|
|
•
|
|
Stockholders may send correspondence, which should indicate that the sender is a Stockholder, to our board of directors or to any individual director, by mail to Corporate Secretary, BioLargo, Inc., 14921 Chestnut St., Westminster, California 92683.
|
|
|
•
|
|
Our Corporate Secretary will be responsible for the first review and logging of this correspondence and will forward the communication to the director or directors to whom it is addressed unless it is a type of correspondence which our board of directors has identified as correspondence which may be retained in our files and not sent to directors. Our board of directors has authorized the Corporate Secretary to retain and not send to directors communications that: (a) are advertising or promotional in nature (offering goods or services), (b) solely relate to complaints by clients with respect to ordinary course of business customer service and satisfaction issues or (c) clearly are unrelated to our business, industry, management or Board or committee matters. These types of communications will be logged and filed but not circulated to directors. Except as set forth in the preceding sentence, the Corporate Secretary will not screen communications sent to directors.
|
|
|
•
|
|
The log of stockholder correspondence will be available to members of our board of directors for inspection. At least once each year, the Corporate Secretary will provide to our board of directors a summary of the communications received from stockholders, including the communications not sent to directors in accordance with the procedures set forth above.
|
Our stockholders also may communicate directly with the non-management directors as a group, by mail addressed to Dennis E. Marshall, c/o Corporate Secretary, BioLargo, Inc., 14921 Chestnut St., Westminster, California 92683.
Our Audit Committee has established procedures for the receipt, retention and treatment of complaints regarding questionable accounting, internal controls and financial improprieties or auditing matters. Any of our employees may confidentially communicate concerns about any of these matters by mail addressed to Audit Committee, c/o Corporate Secretary, BioLargo, Inc., 14921 Chestnut St., Westminster, California 92683.
All the reporting mechanisms also are posted on our corporate website, www.biolargo.com. Upon receipt of a complaint or concern, a determination will be made whether it pertains to accounting, internal controls or auditing matters and, if it does, it will be handled in accordance with the procedures established by the Audit Committee.
Committees of our Board of Directors
Our board of directors has established an Audit Committee, a Compensation Committee, and a Nominating and Corporate Governance Committee.
The Audit Committee meets with management and our independent registered public accounting firm to review the adequacy of internal controls and other financial reporting matters. Dennis E. Marshall served as Chairman of the Audit Committee during 2015 and continues to serve in that capacity. John S. Runyan also serves on the Audit Committee. Our board of directors has determined that Mr. Marshall qualifies as an “audit committee financial expert” as defined in Item 401(h) of Regulation S-K of the Securities Exchange Act of 1934, as amended. The Audit Committee met four times in 2018.
The Compensation Committee reviews the compensation for all our officers and directors and affiliates. The Committee also administers our equity incentive option plan. Mr. Runyan served as Chairman of the Compensation Committee during 2018. Mr. Marshall also serves on the Compensation Committee. The Compensation Committee met once and acted by consent three times during 2018.
Our board of directors did not modify any action or recommendation made by the Compensation Committee with respect to executive compensation for the 2017 or 2018 fiscal years. It is the opinion of the Compensation Committee that the executive compensation policies and plans provide the necessary total remuneration program to properly align their performance and the interests of our stockholders using competitive and equitable executive compensation in a balanced and reasonable manner, for both the short and long term.
The Nominating and Corporate Governance Committee was established in November 2018. Its responsibilities include to identify and screen individuals qualified to become members of the Board, to make recommendations to the Board regarding to the Board regarding the selection and approval of the nominees for director to be submitted to a stockholder vote at the annual meeting of stockholders, subject to approval by the Board, to development corporate governance guidelines and oversee corporate governance practices, to develop a process for an annual evaluation of the Board and its committees, to review all director compensation and benefits, to review, approve and oversee and related party transaction, to develop and recommend director independent standards, and to develop and recommend a company code of conduct, to investigate any alleged breach and enforce the provisions of the code. This committee has not yet held any meetings or taken any formal action.
Our board of directors follows a written code of ethics that applies to its principal executive officers, principal financial officer, principal accounting officer or controller, or persons performing similar functions.
Leadership Structure of our Board of Directors
Mr. Calvert serves as both principal executive officer and Chairman of the Board. Our company does not have a lead independent director. Messrs. Marshall, Roberts, Strommen and Runyan serve as independent directors who provide active and effective oversight of our strategic decisions. As of the date of this prospectus, our company has determined that the leadership structure of our board of directors has permitted our board of directors to fulfill its duties effectively and efficiently and is appropriate given the size and scope of our company and its financial condition.
Our Board of Directors’ Role in Risk Oversight
As a smaller company, our executive management team, consisting of Messrs. Calvert, Code and Provenzano, are also members of our board of directors. Our board of directors, including our executive management members and independent directors, is responsible for overseeing our executive management team in the execution of its responsibilities and for assessing our company’s approach to risk management. Our board of directors exercises these responsibilities on an ongoing basis as part of its meetings and through its committees. Each member of the management team has direct access to the other Board members, and our committees of our board of directors, to ensure that all risk issues are frequently and openly communicated. Our board of directors closely monitors the information it receives from management and provides oversight and guidance to our executive management team regarding the assessment and management of risk. For example, our board of directors regularly reviews our company’s critical strategic, operational, legal and financial risks with management to set the tone and direction for ensuring appropriate risk taking within the business.
Family Relationships
There are no family relationships among the directors and executive officers of our company.
EXECUTIVE COMPENSATION
The following table sets forth all compensation earned for services rendered to our company in all capacities for the fiscal years ended December 31, 2017 and 2018, by our principal executive officer and our three most highly compensated executive officers other than our principal executive officer, collectively referred to as the “Named Executive Officers.”
Summary Compensation Table
|
Name and
Principal
Positions
|
Year
|
|
Salary
|
|
|
Stock
Awards(1)
|
|
|
Option
Awards(1)
|
|
|
All other
Compensation
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dennis P. Calvert,
|
2017
|
|
$
|
288,603
|
(2)
|
|
$
|
—
|
(3)
|
|
$
|
195,894
|
(4)
|
|
$
|
49,600
|
(5)
|
|
$
|
534,097
|
|
|
Chairman, Chief Executive Officer and President
|
2018
|
|
$
|
288,603
|
(2)
|
|
$
|
—
|
(3)
|
|
$
|
335,820
|
(4)
|
|
$
|
31,325
|
(5)
|
|
$
|
655,748
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Kenneth R. Code,
|
2017
|
|
$
|
288,603
|
(6)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
72,600
|
(5)
|
|
$
|
361,203
|
|
|
Chief Science Officer
|
2018
|
|
$
|
288,603
|
(6)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
12,600
|
(5)
|
|
$
|
301,203
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Charles K. Dargan
|
2017
|
|
$
|
—
|
|
|
|
|
|
|
$
|
236,250
|
(7)
|
|
$
|
—
|
|
|
$
|
236,250
|
|
|
Chief Financial Officer
|
2018
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
87,750
|
(7)
|
|
$
|
—
|
|
|
$
|
87,750
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Joseph Provenzano,
|
2017
|
|
$
|
169,772
|
(8)
|
|
$
|
—
|
|
|
$
|
47,000
|
(9)
|
|
$
|
12,900
|
(10)
|
|
$
|
229,672
|
|
|
Corporate Secretary; President Odor-No-More, Inc
|
2018
|
|
$
|
169,772
|
(8)
|
|
$
|
—
|
|
|
$
|
37,600
|
(9)
|
|
$
|
16,565
|
(5)
|
|
$
|
224,937
|
|
________________
|
(1)
|
Our company recognizes compensation expense for stock option awards on a straight-line basis over the applicable service period of the award, which is the vesting period. Share-based compensation expense is based on the grant date fair value estimated using the Black-Scholes method. The amounts in the “Stock Awards” and “Option Awards” columns reflect the aggregate fair value of awards of stock or options calculated as of the grant date. These amounts do not represent cash paid to or realized by any of the recipients during the years indicated.
|
|
(2)
|
In 2017 and 2018 the employment agreement for Mr. Calvert provided for a base salary of $288,603 and other compensation for health insurance and an automobile allowance. During the year ended December 31, 2017, Mr. Calvert agreed to forego $27,796 of cash compensation due to him and accept 71,273 shares of our common stock in lieu thereof, at $0.39 per share. During the year ended December 31, 2018, Mr. Calvert agreed forego $151,149 of cash compensation due to him and accept 534,619 shares of our common stock in lieu thereof, at prices ranging between $0.24 - $0.43 per share. The common stock issued to Mr. Calvert is subject to a “lock up agreement” that prohibits Mr. Calvert from selling the shares until the earlier of (i) the sale of the Company; (ii) the successful commercialization of BioLargo’s products or technologies as demonstrated by its receipt of at least $3,000,000 in cash, or the recognition of $3,000,000 in revenue, over a 12-month period from the sale of products and/or the license of technology; and (iii) the Company’s breach of the employment agreement between the Company and Calvert dated May 2, 2017 and resulting in Calvert’s termination. (See “Employment Agreements—Dennis P. Calvert” and “Outstanding Equity Awards at Fiscal Year-End” below for more details).
|
|
(3)
|
On May 2, 2017, pursuant to his employment agreement, we granted to our president, Dennis P. Calvert, 1,500,000 shares of common stock, subject to a “lock-up agreement” whereby the shares remain unvested until the occurrence of certain events. As no such events occurred during 2017, and thus no shares vested, the value of the award in 2017 was recorded as zero. (See “Employment Agreements—Dennis P. Calvert” and “Outstanding Equity Awards at Fiscal Year-End” below for more details.)
|
|
(4)
|
On May 2, 2017, pursuant to his employment agreement, we granted to our president, Dennis P. Calvert, an option to purchase 3,731,322 shares of the Company’s common stock. The option is a non-qualified stock option, exercisable at $0.45 per share, the closing price of our common stock on the grant date, exercisable for ten years from the date of grant, and vesting in equal increments on the anniversary of the option agreement for five years. Any portion of the option which has not yet vested shall immediately vest in the event of, and prior to, a change of control, as defined in the employment agreement. The option cliff vests in 4 equal amounts on each anniversary of the option agreement. The option agreement contains the other terms standard in option agreements issued by the Company, including provisions for a cashless exercise. The fair value of this option totaled $1,679,095 and will be amortized monthly through May 2, 2022. During the year ended December 31, 2017 and 2018, we recorded $195,894 and $335,820, respectively, of selling, general and administrative expense related to the option.
|
|
(5)
|
Includes health insurance premiums, automobile allowance, and bonus.
|
|
(6)
|
In 2017 and 2018 the employment agreement for Mr. Code provided for a base salary of $288,603 and other compensation of $12,600. During the year ended December 31, 2017, Mr. Code agreed to forego $30,198 of cash compensation due to him and accept 77,432 shares of our common stock in lieu thereof, at $0.39 per share. During the year ended December 31, 2018, Mr. Calvert agreed forego $167,535 of cash compensation due to him and accept 596,417 shares of our common stock in lieu thereof, at prices ranging between $0.24 - $0.43 per share. See “Employment Agreements—Kenneth R. Code” and “Outstanding Equity Awards at Fiscal Year-End” below for more details.
|
|
(7)
|
Our Chief Financial Officer, Charles K. Dargan II, did not receive any cash compensation during the years ended December 31, 2017 and 2018. His only compensation is the issuance, each year, of an option to purchase 300,000 shares of our common stock, with 25,000 shares vesting each month. The value set forth in the table reflects the fair value of the options issued that vested during the 12 months of the years indicated. See “Employment Agreements—Charles K. Dargan II” and “Outstanding Equity Awards at Fiscal Year-End” below for more details.
|
|
(8)
|
In 2017 and 2018, the employment agreement for Mr. Provenzano provided for a base salary of $169,772, and other compensation for health insurance and automobile allowance. See “Employment Agreements – Joseph Provenzano” and “Outstanding Equity Awards at Fiscal Year-End” below for more details.
|
|
(9)
|
On October 23, 2017, we issued to Mr. Provenzano an option to purchase 100,000 shares of our common stock at $0.47 per share, which expires October 23, 2027, and vests monthly in 10,000 share increments beginning November 23, 2017. The remaining fair value of $37,600 vested during 2018.
|
|
(10)
|
Includes a $7,500 cash bonus and $5,400 in automobile expense.
|
Employment Agreements
Dennis P. Calvert
On May 2, 2017, we and our President and Chief Executive Officer Dennis P. Calvert entered into an employment agreement (the “Calvert Employment Agreement”), replacing in its entirety the previous employment agreement with Mr. Calvert dated April 30, 2007.
The Calvert Employment Agreement provides that Mr. Calvert will continue to serve as the President and Chief Executive Officer of the Company and receive base compensation equal to his current rate of pay of $288,603 annually. In addition to this base compensation, the agreement provides that he is eligible to participate in incentive plans, stock option plans, and similar arrangements as determined by the Company’s Board of Directors, health insurance premium payments for himself and his immediate family, a car allowance of $800 per month, paid vacation of four weeks per year, and bonuses in such amount as the Compensation Committee may determine from time to time.
The Calvert Employment Agreement provides that Mr. Calvert will be granted an option (the “Option”) to purchase 3,731,322 shares of the Company’s common stock. The Option shall be a non-qualified stock option, exercisable at $0.45 per share, which represents the market price of the Company’s common stock as of the date of the agreement, exercisable for ten years from the date of grant and vesting in equal increments over five years. Notwithstanding the foregoing, any portion of the Option which has not yet vested shall be immediately vested in the event of, and prior to, a change of control, as defined in the Calvert Employment Agreement. The agreement also provides for a grant of 1,500,000 shares of common stock, subject to the execution of a “lock-up agreement” whereby the shares remain unvested unless and until the earlier of (i) a sale of the Company, (ii) the successful commercialization of the Company’s products or technologies as demonstrated by its receipt of at least $3,000,000 in cash, or the recognition of $3,000,000 in revenue, over a 12-month period from the sale of products and/or the license of technology, and (iii) the Company’s breach of the employment agreement resulting in his termination. The Option contains the other terms standard in option agreements issued by the Company, including provisions for a cashless exercise.
The Calvert Employment Agreement has a term of five years, unless earlier terminated in accordance with its terms. The Calvert Employment Agreement provides that Mr. Calvert’s employment may be terminated by the Company due to his death or disability, for cause, or upon a merger, acquisition, bankruptcy or dissolution of the Company. “Disability” as used in the Calvert Employment Agreement means physical or mental incapacity or illness rendering Mr. Calvert unable to perform his duties on a long-term basis (i) as evidenced by his failure or inability to perform his duties for a total of 120 days in any 360-day period, or (ii) as determined by an independent and licensed physician whom the Company selects, or (iii) as determined without recourse by the Company’s disability insurance carrier. “Cause” means that Mr. Calvert has (i) engaged in willful misconduct in connection with the Company’s business; or (ii) been convicted of, or plead guilty or nolo contendre in connection with, fraud or any crime that constitutes a felony or that involves moral turpitude or theft. If Mr. Calvert’s employment is terminated due to merger or acquisition, then he will be eligible to receive the greater of (i) one year’s compensation plus an additional one half year for each year of service since the effective date of the employment agreement or (ii) one year’s compensation plus an additional one half year for each year remaining in the term of the agreement. Otherwise, he is only entitled to receive compensation due through the date of termination.
The Calvert Employment Agreement requires Mr. Calvert to keep certain information confidential, not to solicit customers or employees of the Company or interfere with any business relationship of the Company, and to assign all inventions made or created during the term of the Calvert Employment Agreement as “work made for hire”.
Kenneth R. Code
We entered into an employment agreement dated as of April 29, 2007 with Mr. Code, our Chief Science Officer (the “Code Employment Agreement”), which we amended on December 28, 2012 such that his salary will remain at $288,603, the level paid in April 2012, with no further automatic increases. The Code Employment Agreement can automatically renew for one year periods on April 29th of each year but may be terminated “without cause” at any time upon 120 days’ notice, and upon such termination, Mr. Code would not receive the severance originally provided for. All other terms in the 2007 agreement remain the same in the Code Employment Agreement.
In addition, Mr. Code will be eligible to participate in incentive plans, stock option plans, and similar arrangements as determined by our board of directors. When such benefits are made available to our senior employees, Mr. Code is also eligible to receive health insurance premium payments for himself and his immediate family, a car allowance of $800 per month, paid vacation of four weeks per year plus an additional two weeks per year for each full year of service during the term of the agreement up to a maximum of 10 weeks per year, life insurance equal to three times his base salary and disability insurance.
The Code Employment Agreement further requires Mr. Code to keep certain information confidential, not to solicit customers or employees of our company or interfere with any business relationship of our company, and to assign all inventions made or created during the term of the Code Employment Agreement as “work made for hire”.
Charles K. Dargan II
Charles K. Dargan, II has served as our Chief Financial Officer since February 2008 pursuant to an engagement agreement with his company, CFO 911, that has been renewed each year. For the renewal effective February 1, 2015, Mr. Dargan was compensated through the issuance of an option to purchase an additional 300,000 shares of our common stock, at an exercise price of $0.57 per share, to expire September 30, 2025, and vest over the term of the engagement with 120,000 shares vested as of September 30, 2015, and the remaining shares to vest 15,000 monthly, provided that the Engagement Extension Agreement with Mr. Dargan has not been terminated prior to each vesting date. Mr. Dargan receives no cash compensation from our company and continues to serve as our Chief Financial Officer.
On February 10, 2017, we and Mr. Dargan further extended his engagement agreement. The extension provides for an additional term to expire September 30, 2017 (the “Extended Term”), and is retroactively effective to the termination of the prior extension on October 1, 2016. This more recent extension again compensates Mr. Dargan through the issuance of an option to purchase 300,000 shares of the Company’s common stock. The strike price of the option is $0.69 per share, which is equal to the closing price of the Company’s common stock on February 10, 2017, expires February 10, 2027, and vests over the term of the engagement with 125,000 shares having vested as of February 10, 2017, and the remaining shares to vest 25,000 shares monthly beginning March 1, 2017, and each month thereafter, so long as his agreement is in full force and effect.
On December 31, 2017, we and Mr. Dargan further extended his engagement agreement. The extension provides for an additional term to expire September 30, 2018 (the “Extended Term”), and is retroactively effective to the termination of the prior extension on October 1, 2017. This more recent extension again compensates Mr. Dargan through the issuance of an option to purchase 300,000 shares of the Company’s common stock. The strike price of the option is $0.39 per share, which is equal to the closing price of the Company’s common stock on December 29, 2017, expires December 31, 2027, and vests over the term of the engagement with 75,000 shares having vested as of December 31, 2017, and the remaining shares to vest 25,000 shares monthly beginning January 31, 2018, and each month thereafter, so long as his agreement is in full force and effect.
On January 16, 2019, we and Mr. Dargan formally agreed to extend his engagement agreement. The extension provides for an additional term to expire September 30, 2019, and is retroactively effective to the termination of the prior extension on September 30, 2018. Mr. Dargan has been serving as the Company’s Chief Financial Officer since such termination pursuant to the terms of the December 31, 2017 extension. This extension again compensates Mr. Dargan through the issuance of an option to purchase 300,000 shares of the Company’s common stock, at a strike price equal to the closing price of the Company’s common stock on January 16, 2019 of $0.223, to expire January 16, 2029, and to vest over the term of the engagement with 75,000 shares having vested as of December 31, 2018, and the remaining shares to vest 25,000 shares monthly beginning January 31, 2019, and each month thereafter, so long as the engagement agreement is in full force and effect. The Option was issued pursuant to the Company’s 2018 Equity Incentive Plan. The issuance of the Option is Mr. Dargan’s sole source of compensation for the extended term. As was the case in all prior terms of his engagement, there is no cash component of his compensation for the Extended Term. Mr. Dargan is eligible to be reimbursed for business expenses he incurs in connection with the performance of his services as the Company’s Chief Financial Officer (although he has made no such requests for reimbursement in the past). All other provisions of the Engagement Agreement not expressly amended pursuant to the Engagement Extension Agreement remain the same, including provisions regarding indemnification and arbitration of disputes.
Joseph Provenzano
Mr. Provenzano has served as Vice President of Operations since January 1, 2008, in addition to continuing to serve as our Corporate Secretary. On June 18, 2019, we and Mr. Provenzano entered into an employment agreement (the “Provenzano Employment Agreement”), replacing in its entirety the previous employment agreement with Mr. Provenzano dated January 1, 2008.
The Provenzano Employment Agreement provides that Mr. Provenzano will serve as our Executive Vice President of Operations, as well as the President and Chief Executive Officer of our wholly owned subsidiary Odor-No-More. Mr. Provenzano’s base compensation will remain at his current rate of $169,772 annually. In addition to this base compensation, the agreement provides that he is eligible to participate in incentive plans, stock option plans, and similar arrangements as determined by the our Board of Directors, health insurance premium payments for himself and his immediate family, a car allowance covering the expenses of his personal commercial grade truck which the company uses in company operations on a continual basis, paid vacation of four weeks per year, and bonuses in such amount as the Compensation Committee may determine from time to time.
In conjunction with this agreement, our Compensation Committee awarded Mr. Provenzano an option to purchase common stock and restricted stock units under our 2018 Equity Incentive Plan (see Note 5).
The Provenzano Employment Agreement has a term of five years, unless earlier terminated in accordance with its terms. The Provenzano Employment Agreement provides that Mr. Provenzano’s employment may be terminated by the Company due to his death or disability, for cause, or upon a merger, acquisition, bankruptcy or dissolution of the Company. “Disability” as used in the Provenzano Employment Agreement means physical or mental incapacity or illness rendering Mr. Provenzano unable to perform his duties on a long-term basis (i) as evidenced by his failure or inability to perform his duties for a total of 120 days in any 360-day period, or (ii) as determined by an independent and licensed physician whom the Company selects, or (iii) as determined without recourse by the Company’s disability insurance carrier. “Cause” means that Mr. Provenzano has (i) engaged in willful misconduct in connection with the Company’s business; or (ii) been convicted of, or plead guilty or nolo contendre in connection with, fraud or any crime that constitutes a felony or that involves moral turpitude or theft. If Mr. Provenzano’s employment is terminated due to merger or acquisition, then he will be eligible to receive the greater of (i) one year’s compensation plus an additional one half year for each year of service since the effective date of the employment agreement or (ii) one year’s compensation plus an additional one half year for each year remaining in the term of the agreement. Otherwise, he is only entitled to receive compensation due through the date of termination.
The Provenzano Employment Agreement requires Mr. Provenzano to keep certain information confidential, not to solicit customers or employees of the Company or interfere with any business relationship of the Company, and to assign all inventions made or created during the term of the Provenzano Employment Agreement as “work made for hire”.
Director Compensation
Each director who is not an officer or employee of our company receives an annual retainer of $60,000, paid in cash or shares of our common stock, or options to purchase our common stock (pursuant to a plan put in place by our board of directors), in our sole discretion. Historically, all but one director has received the entirety of his fees in the form of options to purchase stock, rather than cash. In addition, Mr. Marshall and Mr. Runyan each receive an additional $15,000 for their services as the chairman of the Audit Committee and chairman of the Compensation Committee, respectively. The following table sets forth information for the fiscal years ended December 31, 2018 regarding compensation of our non-employee directors. Our employee directors do not receive any additional compensation for serving as a director.
|
Name
|
|
Fees Earned or Fees Paid in Cash
|
|
|
Option
Awards
|
|
|
Non-Equity Incentive Plan Compensation
|
|
|
All Other Compensation
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dennis E. Marshall
|
|
$
|
75,000
|
(1)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
75,000
|
|
|
Jack B. Strommen
|
|
$
|
60,000
|
(2)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
60,000
|
|
|
Kent C. Roberts III
|
|
$
|
60,000
|
(3)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
60,000
|
|
|
John S. Runyan
|
|
$
|
75,000
|
(4)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
75,000
|
|
_________________
|
|
(1)
|
In 2018, Mr. Marshall earned director fees of $75,000, which included compensation for serving as Chairman of the Audit Committee of our board of directors. None of these fees was paid in cash. During 2018, Mr. Marshall received options in lieu of cash consisting of (i) on March 31, 2018, an issuance of an option to purchase 72,394 shares of our common stock at $0.26 per share, (ii) on June 30, 2018, an issuance of an option to purchase 43,605 shares of our common stock at $0.43 per share, (iii) on September 30, 2018, an issuance of an option to purchase 69,444 shares of our common stock at $0.27 per share, and (iv) on December 31, 2018, an issuance of an option to purchase 78,125 shares of our common stock at $0.24 per share.
|
|
|
(2)
|
In 2018 Mr. Strommen earned director fees of $60,000. During 2018, Mr. Strommen received options in lieu of cash consisting of (i) on March 31, 2018, an issuance of an option to purchase 57,916 shares of our common stock at $0.43 per share, (ii) on June 30, 2018, an issuance of an option to purchase 34,884 shares of our common stock at $0.43 per share, (iii) on September 30, 2018, an option to purchase 55,556 shares of our common stock at $0.27 per share, and (iv) on December 31, 2018, an option to purchase 62,500 shares of our common stock at $0.34 per share.
|
|
|
(3)
|
In 2018 Mr. Roberts earned director fees of $60,000. During 2018, Mr. Roberts received options in lieu of cash consisting of (i) on March 31, 2018, an issuance of an option to purchase 57,916 shares of our common stock at $0.43 per share, (ii) on June 30, 2018, an issuance of an option to purchase 34,884 shares of our common stock at $0.43 per share, (iii) on September 30, 2018, an option to purchase 55,556 shares of our common stock at $0.27 per share, and (iv) on December 31, 2018, an option to purchase 62,500 shares of our common stock at $0.34 per share.
|
|
|
(4)
|
In 2018, Mr. Runyan earned director fees of $75,000. None of these fees was paid in cash. During 2018, Mr. Runyan received options in lieu of cash consisting of (i) on March 31, 2018, an issuance of an option to purchase 72,394 shares of our common stock at $0.26 per share, (ii) on June 30, 2018, an issuance of an option to purchase 43,605 shares of our common stock at $0.43 per share, (iii) on September 30, 2018, an issuance of an option to purchase 69,444 shares of our common stock at $0.27 per share, and (iv) on December 31, 2018, an issuance of an option to purchase 78,125 shares of our common stock at $0.24 per share.
|
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth information regarding unexercised stock options and equity incentive plan awards for each of the Named Executive Officers outstanding as of December 31, 2018. All stock or options that were granted to the Named Executive Officers during the fiscal year ended December 31, 2018 have fully vested, except as indicated.
|
Name
|
|
Number of
Securities
Underlying
Unexercised
Options (#)
Exercisable
|
|
Number of
Securities
Underlying
Unexercised
Options (#)
Unexercisable
|
|
Equity
Incentive
Plan
Awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options
|
|
|
Option
Exercise
Price
|
|
|
Share
Price on
Grant Date
|
|
Option
Expiration
Date
|
|
Dennis P. Calvert
|
|
|
3,731,322
|
|
|
|
|
--
|
|
|
$
|
0.45
|
|
|
$
|
0.45
|
|
May 2, 2027
|
|
|
|
|
60,000
|
|
|
|
|
--
|
|
|
$
|
0.55
|
|
|
$
|
0.37
|
|
April 27, 2019
|
|
|
|
|
691,974
|
|
|
|
|
--
|
|
|
$
|
0.55
|
|
|
$
|
0.37
|
|
April 27, 2019
|
|
|
|
|
200,000
|
|
|
|
|
--
|
|
|
$
|
0.575
|
|
|
$
|
0.50
|
|
February 1, 2020
|
|
Charles K. Dargan II
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.38
|
|
|
$
|
0.38
|
|
January 31, 2019
|
|
|
|
|
50,000
|
|
|
|
|
--
|
|
|
$
|
0.28
|
|
|
$
|
0.28
|
|
February 23, 2019
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.30
|
|
|
$
|
0.30
|
|
April 29, 2019
|
|
|
|
|
36,000
|
|
|
|
|
--
|
|
|
$
|
0.50
|
|
|
$
|
0.30
|
|
April 29, 2019
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.45
|
|
|
$
|
0.45
|
|
May 31, 2019
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.45
|
|
|
$
|
0.45
|
|
June 30, 2019
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.50
|
|
|
$
|
0.50
|
|
July 31, 2019
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.43
|
|
|
$
|
0.43
|
|
August 31, 2019
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.40
|
|
|
$
|
0.40
|
|
September 30, 2019
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.45
|
|
|
$
|
0.45
|
|
October 31, 2019
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.57
|
|
|
$
|
0.57
|
|
November 30, 2019
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.70
|
|
|
$
|
0.70
|
|
December 31, 2019
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.50
|
|
|
$
|
0.50
|
|
January 31, 2020
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.45
|
|
|
$
|
0.45
|
|
February 28, 2020
|
|
|
|
|
60,000
|
|
|
|
|
--
|
|
|
$
|
0.575
|
|
|
$
|
0.50
|
|
February 1, 2020
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.50
|
|
|
$
|
0.50
|
|
March 31, 2020
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.39
|
|
|
$
|
0.39
|
|
April 29, 2020
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.31
|
|
|
$
|
0.31
|
|
May 31, 2020
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.25
|
|
|
$
|
0.25
|
|
June 30, 2020
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.24
|
|
|
$
|
0.24
|
|
July 31, 2020
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.23
|
|
|
$
|
0.23
|
|
August 30, 2020
|
|
|
|
|
200,000
|
|
|
|
|
--
|
|
|
$
|
0.30
|
|
|
$
|
0.30
|
|
August 4, 2020
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.35
|
|
|
$
|
0.35
|
|
September 30, 2020
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.42
|
|
|
$
|
0.42
|
|
October 31, 2020
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.40
|
|
|
$
|
0.40
|
|
November 30, 2020
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.50
|
|
|
$
|
0.50
|
|
December 31, 2020
|
|
|
|
|
10,000
|
|
|
|
|
--
|
|
|
$
|
0.42
|
|
|
$
|
0.42
|
|
January 31, 2021
|
|
|
|
|
120,000
|
|
|
|
|
--
|
|
|
$
|
0.41
|
|
|
$
|
0.41
|
|
February 28, 2021
|
|
|
|
|
300,000
|
|
|
|
|
--
|
|
|
$
|
0.35
|
|
|
$
|
0.35
|
|
April 10, 2022
|
|
|
|
|
410,000
|
|
|
|
|
--
|
|
|
$
|
0.30
|
|
|
$
|
0.30
|
|
December 28, 2022
|
|
|
|
|
300,000
|
|
|
|
|
--
|
|
|
$
|
0.30
|
|
|
$
|
0.30
|
|
July 17, 2023
|
|
|
|
|
300,000
|
|
|
|
|
--
|
|
|
$
|
0.30
|
|
|
$
|
0.30
|
|
June 23, 2024
|
|
|
|
|
300,000
|
|
|
|
|
--
|
|
|
$
|
0.57
|
|
|
$
|
0.57
|
|
September 30, 2025
|
|
|
|
|
300,000
|
|
|
|
|
--
|
|
|
$
|
0.69
|
|
|
$
|
0.69
|
|
February 10, 2027
|
|
|
|
|
300,000
|
|
|
|
|
--
|
|
|
$
|
0.39
|
|
|
$
|
0.39
|
|
December 31, 2027
|
|
Kenneth R. Code
|
|
|
200,000
|
|
|
|
|
--
|
|
|
$
|
1.03
|
|
|
$
|
0.94
|
|
July 17, 2023
|
|
|
|
|
200,000
|
|
|
|
|
--
|
|
|
$
|
0.575
|
|
|
$
|
0.50
|
|
February 1, 2020
|
|
Joseph L. Provenzano
|
|
|
30,000
|
|
|
|
|
|
|
|
$
|
0.50
|
|
|
$
|
0.37
|
|
April 27, 2019
|
|
|
|
|
200,000
|
|
|
|
|
|
|
|
$
|
0.575
|
|
|
$
|
0.50
|
|
February 1, 2020
|
|
|
|
|
296,203
|
|
|
|
|
|
|
|
$
|
0.30
|
|
|
$
|
0.30
|
|
August 4, 2020
|
|
|
|
|
200,000
|
|
|
|
|
|
|
|
$
|
0.41
|
|
|
$
|
0.41
|
|
March 21 2021
|
|
|
|
|
100,000
|
|
|
|
|
|
|
|
$
|
0.45
|
|
|
$
|
0.45
|
|
October 23 2027
|
Limitation of Liability and Indemnification Matters
As permitted by the Delaware general corporation law, we have included a provision in our certificate of incorporation to eliminate the personal liability of our directors for monetary damages for breach of their fiduciary duties as directors, except for liability (i) for any breach of the director’s duty of loyalty to our company, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) under section 174 of the Delaware general corporation law or (iv) for any transaction from which the director derived an improper personal benefit. Our certificate of incorporation also provides that our company shall, to the full extent permitted by section 145 of the Delaware general corporation law, as amended from time to time, indemnify all persons whom it may indemnify pursuant thereto.
In addition, our Bylaws provide that we are required to indemnify our officers and directors even when indemnification would otherwise be discretionary, and we are required to advance expenses to our officers and directors as incurred in connection with proceedings against them for which they may be indemnified.
We may enter into indemnification agreements with our officers and directors containing provisions that are in some respects broader than the specific indemnification provisions contained in the Delaware general corporation law. The indemnification agreements would require us to indemnify our officers and directors against liabilities that may arise by reason of their status or service as officers and directors other than for liabilities arising from willful misconduct of a culpable nature, to advance their expenses incurred as a result of any proceeding against them as to which they could be indemnified, and to obtain our directors’ and officers’ insurance if available on reasonable terms. As of the date of this prospectus, our company has not entered into any indemnification agreement with any of its directors or officers, except for Mr. Strommen.
We have obtained directors’ and officers’ liability insurance in amounts comparable to other companies of our size and in our industry.
No pending litigation or proceeding involving a director, officer, employee or other agent of our company currently exists as to which indemnification is being sought. We are not aware of any threatened litigation that may result in claims for indemnification by any director, officer, employee or other agent of our company.
See “Disclosure of SEC Position on Indemnification for Securities Act Liabilities.”
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information regarding the beneficial ownership of shares of our Common stock as of September 12, 2019, including rights to acquire beneficial ownership of shares of our Common stock within 60 days of September 12, 2019, by (a) all stockholders known to the Company to be beneficial owners of more than 5% of the outstanding Common stock; (b) each director, (c) each Named Executive Officer, and (d) all directors and executive officers of the Company as a group:
|
Name and Address of Beneficial Owner(1)
|
Amount of Beneficial
Ownership
|
Percent of
Class(2)
|
|
Kenneth R. Code(4)
|
23,266,703
|
14.6%
|
|
Dennis P. Calvert(5)
|
9,636,555
|
6.0%
|
|
Jack B. Strommen(6)
|
8,603,094
|
5.4%
|
|
Charles K. Dargan II(7)
|
3,396,244
|
2.1%
|
|
Dennis E. Marshall(8)
|
2,842,881
|
1.8%
|
|
Joseph L. Provenzano(9)
|
2,096,946
|
1.3%
|
|
Kent C. Roberts II(10)
|
2,004,778
|
1.3%
|
|
John S. Runyan(11)
|
1,851,716
|
1.2%
|
|
All directors and officers as a group (8 persons)
|
53,548,917
|
33.7%
|
__________________
|
(1)
|
Except as noted in any footnotes below, each person has sole voting power and sole dispositive power as to all of the shares shown as beneficially owned by them. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities.
|
|
(2)
|
The address for all directors and the Named Executive Officers is: c/o BioLargo, Inc., 14921 Chestnut St., Westminster, CA 92683, except for: Kent C. Roberts II’s address is 1146 Oxford Road, San Marino, CA 91108; Charles K. Dargan II’s address is 18841 NE 29th Avenue, Suite 700, Aventura FL 33180; and John S. Runyan’s address is 30001 Hillside Terrace, San Juan Capistrano, CA 92675.
|
|
(3)
|
Our company has only one class of stock outstanding. The sum of 157,604,022 shares of common stock outstanding on September 12, 2019. For purposes of the above table, an additional 13,539,521 shares of common stock subject to options currently exercisable or exercisable within 60 days by the directors and officers are deemed outstanding for determining the number of shares beneficially owned by the directors and officers, and the directors and officers as a group, and for computing the percentage ownership of the person holding such options, but are not deemed outstanding for computing the percentage ownership of any other person.
|
|
(4)
|
Includes 22,139,012 shares owned indirectly by Mr. Code issued on April 29, 2007 to IOWC Technologies, Inc. in connection with the acquisition by our company of certain intellectual property and other assets on that date. Includes 400,000 shares issuable to Mr. Code upon exercise of options.
|
|
(5)
|
Includes 1,528,695 shares, and an option to purchase 691,974 shares, of common stock held by New Millennium Capital Partners, LLC, which is wholly owned and controlled by Mr. Calvert. Includes 200,000 shares issuable to Mr. Calvert upon exercise of other options granted from time to time by our company.
|
|
(6)
|
Includes 369,823 shares issuable to Mr. Strommen upon exercise of options; includes 3,257,143 shares issuable to Mr. Strommen upon the exercise of warrants. Includes 400,000 shares issuable to Mr. Strommen upon conversion of a convertible promissory note.
|
|
(7)
|
Includes 2,985,000 shares issuable to Mr. Dargan upon exercise of options.
|
|
(8)
|
Includes 2,504,371 shares issuable to Mr. Marshall upon exercise of options.
|
|
(9)
|
Includes 796,203 shares issuable to Mr. Provenzano upon exercise of options.
|
|
(10)
|
Includes 1,400,912 shares issuable to Mr. Roberts upon exercise of options.
|
|
(11)
|
Includes 1,586,069 shares issuable to Mr. Runyan upon exercise of options.
|
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Our company has adopted a policy that all transactions between our company and its executive officers, directors and other affiliates must be approved by a majority of the members of our board of directors and by a majority of the disinterested members of our board of directors, and must be on terms no less favorable to our company than could be obtained from unaffiliated third parties.
From time to time, our company is unable to pay in full amounts due to its officers for salary and business expenses, and those amounts are recorded as liabilities in our financial statements. These amounts are then paid in the future as our company’s cash position allows, or through the issuance of our common stock, or an option to purchase common stock, pursuant to a plan adopted by our board for the payment of outstanding payables.
On June 30, 2019, we issued options to purchase 293,478 shares of our common stock at an exercise price of $0.23 per share to four members of our board of directors, in lieu of $67,500 in fees, as follows: 81,522 to Mr. Marshall in exchange for $18,750 in fees due; 65,217 to Mr. Strommen in exchange for $15,000 in fees due; 65,217to Mr. Roberts in exchange for $15,000 in fees due; and 81,522 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On March 31, 2019, we issued options to purchase 421,876 shares of our common stock at an exercise price of $0.16 per share to four members of our board of directors, in lieu of $67,500 in fees, as follows: 117,188 to Mr. Marshall in exchange for $18,750 in fees due; 93,750 to Mr. Strommen in exchange for $15,000 in fees due; 93,750 to Mr. Roberts in exchange for $15,000 in fees due; and 117,188 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On March 31, 2019, we issued an aggregate 579,996 shares of our common stock to two executive officers in exchange for a reduction of $92,799 of salary and unreimbursed business expenses owed to the officers.
On December 31, 2018, we issued options to purchase 281,250 shares of our common stock at an exercise price of $0.22 per share to four members of our board of directors, in lieu of $67,500 in fees, as follows: 78,125 to Mr. Marshall in exchange for $18,750 in fees due; 62,500 to Mr. Strommen in exchange for $15,000 in fees due; 62,500to Mr. Roberts in exchange for $15,000 in fees due; and 78,125 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On December 31, 2018, we issued an aggregate 381,801 shares of our common stock to two executive officers in exchange for a reduction of $91,632 of salary and unreimbursed business expenses owed to the officers.
On September 30, 2018, we issued options to purchase 250,000 shares of our common stock at an exercise price of $0.27 per share to four members of our board of directors, in lieu of $67,500 in fees, as follows: 69,444 to Mr. Marshall in exchange for $18,750 in fees due; 55,556 to Mr. Strommen in exchange for $15,000 in fees due; 55,556 to Mr. Roberts in exchange for $15,000 in fees due; and 69,444 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On September 30, 2018, we issued an aggregate 249,258 shares of our common stock to two executive officers in exchange for a reduction of $67,300 of salary owed to the officers.
On June 29, 2018, we issued options to purchase 156,978 shares of our common stock at an exercise price of $0.31 per share to four members of our board of directors, in lieu of $62,500 in fees, as follows: 43,605 to Mr. Marshall in exchange for $18,750 in fees due; 34,884 to Mr. Strommen in exchange for $15,000 in fees due; 34,884 to Mr. Roberts in exchange for $15,000 in fees due; and 43,605 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On June 29, 2018, we issued an aggregate 176,950 shares of our common stock to two executive officers in exchange for a reduction of $76,087 of salary owed to the officers.
On March 31, 2018, we issued options to purchase 260,620 shares of our common stock at an exercise price of $0.295 per share to four members of our board of directors, in lieu of $62,500 in fees, as follows: 72,394 to Mr. Marshall in exchange for $18,750 in fees due; 57,916 to Mr. Strommen in exchange for $15,000 in fees due; 57,916 to Mr. Roberts in exchange for $15,000 in fees due; and 72,394 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On March 31, 2018, we issued an aggregate 323,030 shares of our common stock to two executive officers in exchange for a reduction of $83,664 of salary owed to the officers.
On March 31, 2017, we issued options to purchase an aggregate 130,000 shares of our common stock at an exercise price of $0.50 per share to four members of our board of directors, in lieu of $65,000 in fees, as follows: 37,500 to Mr. Marshall in exchange for $18,750 in fees due; 25,000 to Mr. Cox in exchange for $12,500 in fees due; 30,000 to Mr. Roberts in exchange for $15,000 in fees due; 37,500 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On June 30, 2017, we issued options to purchase 145,350 shares of our common stock at an exercise price of $0.43 per share to four members of our board of directors, in lieu of $62,500 in fees, as follows: 43,605 to Mr. Marshall in exchange for $18,750 in fees due; 18,992 to Mr. Cox in exchange for $8,167 in fees due; 34,884 to Mr. Roberts in exchange for $15,000 in fees due; 43,605 to Mr. Runyan in exchange for $18,750 in fees due; and 4,264 to Mr. Strommen in exchange for $1,833 in fees due. The options expire 10 years from the date of grant.
On September 30, 2017, we issued options to purchase 132,354 shares of our common stock at an exercise price of $0.51 per share to four members of our board of directors, in lieu of $62,500 in fees, as follows: 36,765 to Mr. Marshall in exchange for $18,750 in fees due; 29,412 to Mr. Roberts in exchange for $15,000 in fees due; 36,765 to Mr. Runyan in exchange for $18,750 in fees due; and 29,412 to Mr. Strommen in exchange for $15,000 in fees due. The options expire 10 years from the date of grant.
On December 31, 2017, we issued options to purchase 173,078 shares of our common stock at an exercise price of $0.39 per share to four members of our board of directors, in lieu of $62,500 in fees, as follows: 48,077 to Mr. Marshall in exchange for $18,750 in fees due; 38,462 to Mr. Strommen in exchange for $15,000 in fees due; 38,462 to Mr. Roberts in exchange for $15,000 in fees due; and 48,077 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On December 31, 2017, we issued an aggregate 148,705 shares of our common stock to two executive officers in exchange for a reduction of $57,994 of salary owed to the officers.
During 2016, we issued options to purchase 170,377 shares of our common stock to a member of our Board of Directors, Mr. Marshall, in exchange for the payment of $86,250 of board of director fees due. Pursuant to a plan adopted by our Board of Directors for the reduction of outstanding accounts payable, the options were issued at a strike price equal to the closing price of our common stock on the date of the agreement, and the number of shares purchasable was calculated by dividing the total amount of fees due by the exercise price and multiplying that number by one point five. As a result, the aggregate value of the options issued to Mr. Marshall was equal to $86,250.
During 2016, we issued options to purchase 121,115 shares of our common stock to a member of our Board of Directors, Mr. Runyan, in exchange for the payment of $63,750 of board of director fees due. Pursuant to a plan adopted by our Board of Directors for the reduction of outstanding accounts payable, the options were issued at a strike price equal to the closing price of our common stock on the date of the agreement, and the number of shares purchasable was calculated by dividing the total amount of fees due by the exercise price and multiplying that number by one point five. As a result, the aggregate value of the options issued to Mr. Runyan was equal to $63,750.
On March 31, 2017, we issued options to purchase an aggregate 130,000 shares of our common stock at an exercise price of $0.50 per share to four members of our board of directors, in lieu of $65,000 in fees, as follows: 37,500 to Mr. Marshall in exchange for $18,750 in fees due; 25,000 to Mr. Cox in exchange for $12,500 in fees due; 30,000 to Mr. Roberts in exchange for $15,000 in fees due; 37,500 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On June 30, 2017, we issued options to purchase 145,349 shares of our common stock at an exercise price of $0.43 per share to four members of our board of directors, in lieu of $62,500 in fees, as follows: 43,605 to Mr. Marshall in exchange for $18,750 in fees due; 18,992 to Mr. Cox in exchange for $8,167 in fees due; 34,884 to Mr. Roberts in exchange for $15,000 in fees due; 43,605 to Mr. Runyan in exchange for $18,750 in fees due; and 4,264 to Mr. Roberts in exchange for $1,833 in fees due. The options expire 10 years from the date of grant.
Mr. Strommen was first elected to our board of directors on June 20, 2017. Prior to joining our board, Mr. Strommen invested in the Company’s 2015 Unit Offering, receiving a promissory note and stock purchase warrant. Pursuant to the terms of the notes issued to investors in the 2015 Unit Offering, the Company has elected to pay interest due by issuing common stock. On June 26, 2017, and September 20, 2017, Mr. Strommen was issued 71,423 and 61,792 shares of our common stock, respectively, in payment of interest. All other investors in the 2015 Unit Offering were also issued shares on those days. Prior to those dates, and prior to joining the board, Mr. Strommen had been issued 339,868 shares of our common stock in payment of interest.
On March 28, 2018, Mr. Strommen invested $100,000 in the Company’s private securities offering, receiving a promissory note in the face amount of $100,000, bearing annual interest at the rate of 12%, which is convertible into the Company’s common stock by Mr. Strommen at any time, or the Company at the April 30, 2021 maturity, at the rate of $0.30 per share. Investors in the offering also receive a stock purchase warrant to purchase the number of shares calculated by dividing the investment amount by the note conversion price. Mr. Strommen received a warrant to purchase 333,334 shares of common stock at $0.48 per share, which expires April 20, 2023.
DESCRIPTION OF CAPITAL STOCK
As reflected in the Certificate of Incorporation as amended May 25, 2018, our authorized capital stock consists of 400,000,000 shares of common stock, par value $0.00067 per share, and 50,000,000 shares of preferred stock, par value $0.00067 per share.
|
Authorized and Issued Stock
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of shares at September 12, 2019
|
|
|
Title of Class
|
|
Authorized
|
|
|
Outstanding
|
|
|
Reserved (1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock, par value $0.00067 per share
|
|
|
400,000,000
|
|
|
|
157,604,022
|
|
|
|
75,111,575
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock, $0.00067 par value per share
|
|
|
50,000,000
|
|
|
|
-0-
|
|
|
|
|
|
|
|
(1)
|
The 75,111,575 shares reserved for future issuances includes 17,397,258 shares issuable to Lincoln Park pursuant to the Purchase Agreement.
|
Common Stock
Dividends. Each share of our common stock is entitled to receive an equal dividend, if one is declared. We cannot provide any assurance that we will declare or pay cash dividends on our common stock in the future. Any future determination to declare cash dividends will be made at the discretion of our board of directors, subject to applicable laws, and will depend on our financial condition, results of operations, capital requirements, general business conditions and other factors that our board of directors may deem relevant. Our board of directors may determine it to be necessary to retain future earnings (if any) to finance operations. See “Risk Factors” and “Dividend Policy.”
Liquidation. If our company is liquidated, then assets that remain (if any) after the creditors are paid and the owners of preferred stock receive liquidation preferences (as applicable) will be distributed to the owners of our common stock pro rata.
Voting Rights. Each share of our common stock entitles the owner to one vote. There is no cumulative voting. A simple majority can elect all of the directors at a given meeting, and the minority would not be able to elect any director at that meeting.
Preemptive Rights. Owners of our common stock have no preemptive rights. We may sell shares of our common stock to third parties without first offering such shares to current stockholders.
Redemption Rights. We do not have the right to buy back shares of our common stock except in extraordinary transactions, such as mergers and court approved bankruptcy reorganizations. Owners of our common stock do not ordinarily have the right to require us to buy their common stock. We do not have a sinking fund to provide assets for any buy back.
Conversion Rights. Shares of our common stock cannot be converted into any other kind of stock except in extraordinary transactions, such as mergers and court approved bankruptcy reorganizations.
Nonassessability. All outstanding shares of our common stock are fully paid and nonassessable.
Preferred Stock
Our certificate of incorporation authorizes our board of directors to issue “blank check” preferred stock. Our board of directors may divide this preferred stock into series and establish the rights, preferences and privileges thereof. Our board of directors may, without prior stockholder approval, issue any or all of the shares of this preferred stock with dividend, liquidation, conversion, voting or other rights that could adversely affect the relative voting power or other rights of our common stock. Preferred stock could be used as a method of discouraging, delaying or preventing a takeover or other change in control of our company. Issuances of preferred stock in the future could have a dilutive effect on our common stock.
As of the date of this prospectus, there are no shares of our preferred stock outstanding.
DESCRIPTION OF THE OFFERING
This is a registration of shares that were previously sold by the Company in a series of private placements. This prospectus relates to the sale of up to 36,090,857 shares of our common stock by selling stockholders.
SELLING STOCKHOLDERS
The following table presents information regarding the selling stockholders and the shares of our common stock that may be sold by them pursuant to this prospectus.
Each of the selling shareholders acquired their shares from the company for cash (or as payment of accrued interest) in a private placement transaction which was exempt from registration pursuant Regulation D promulgated under the Securities Act of 1933, except Freedom Investors Corp., Sexton Equities LLC, and Randall A. Heller, each of whom received their warrants to purchase shares as broker-dealer commissions.
Except for Jack Strommen, who is a shareholder of Clyra and may be a consultant to Clyra, none of the selling stockholders has had within the past three years any position, office or other material relationship with our company or any of its predecessors or affiliates. Other than Freedom Investors Corp., Sexton Equities LLC, and Randall A. Heller, no selling stockholder is a broker-dealer or an affiliate of a broker-dealer. Freedom Investors Corp. is a registered broker-dealer and purchased the shares being offered under this prospectus in the ordinary course of its business. Sexton Equities LLC, and Randall A. Heller are affiliated persons of Freedom Investor Corp. At the time of purchase, Freedom Investors Corp., Sexton Equities LLC, and Randall A. Heller did not have any agreement or understanding, directly or indirectly, with any person to distribute the shares. Freedom Investor Corp. is deemed to be an underwriter with respect to the shares of stock it offers for sale under this prospectus. Shares beneficially owned prior to the offering includes shares that may be issued to the selling stockholders pursuant to the exercise of stock purchase warrants and conversion of convertible promissory notes.
Our company issued the shares being offered for resale pursuant to this prospectus to the selling stockholders in private placements that we effected in 2013, 2015, and 2016 in exchange for consideration that we received from the selling stockholders.
|
Name
|
Number of
Shares of
Common stock
Beneficially
Owned Prior to
Offering(1)
|
Number of
Shares of
Common
Stock Being
Offered
|
Shares of
Common
Stock
Beneficially
Owned After
the Offering
|
Percentage
Beneficially
Owned
After
Registration
|
|
Andrea Kochensparger
|
986,992
|
986,992
|
–
|
*
|
|
Anthony J. Jacobson
|
876,596
|
585,559
|
291,037
|
*
|
|
Austin N. Heberger
|
149,883
|
149,883
|
–
|
*
|
|
Best Home Choices, LLC(2)
|
179,858
|
179,858
|
–
|
*
|
|
Black Mountain Equities, Inc.(3)
|
640,000
|
640,000
|
–
|
*
|
|
BMS Endeavor, LLP(4)
|
612,117
|
212,117
|
400,000
|
*
|
|
Brandan Adams and Dr. Shelley Thompson
|
202,771
|
202,771
|
–
|
*
|
|
Brandan M Adams
|
191,429
|
191,429
|
–
|
*
|
|
Brian Griffith
|
201,502
|
201,502
|
–
|
*
|
|
Brian Griffith and Lorelei Griffith
|
107,135
|
107,135
|
–
|
*
|
|
Bruce Evans
|
500,000
|
500,000
|
–
|
*
|
|
Bruce Kelber
|
92,857
|
40,000
|
52,857
|
*
|
|
C1P Solutions Inc.(5)
|
431,221
|
345,507
|
85,714
|
*
|
|
California Clock Co.(6)
|
210,568
|
210,568
|
–
|
*
|
|
Carl Soderlund
|
161,224
|
161,224
|
–
|
*
|
|
Chris T. Washburn
|
71,429
|
71,429
|
–
|
*
|
|
Christopher A. Herr
|
691,050
|
691,050
|
–
|
*
|
|
Coastal Real Estate Investments Inc.(7)
|
273,817
|
273,817
|
–
|
*
|
|
Daniel J. Conger
|
485,233
|
485,233
|
–
|
*
|
|
David Azari
|
1,054,335
|
1,054,335
|
–
|
*
|
|
Demosthenes Dionis
|
147,819
|
147,819
|
–
|
*
|
|
Dennis DeSmith
|
124,000
|
60,000
|
64,000
|
*
|
|
Don and Bryn Oates
|
40,000
|
40,000
|
–
|
*
|
|
Douglas Goularte
|
207,440
|
207,440
|
–
|
*
|
|
Douglas J. Morgan
|
333,981
|
333,981
|
–
|
*
|
|
Duane Fitzgerald
|
209,471
|
209,471
|
–
|
*
|
|
Ermelinda Arriola
|
745,283
|
745,283
|
–
|
*
|
|
Eva Wald
|
150,117
|
150,117
|
–
|
*
|
|
Freedom Investor Corp.
|
128,800
|
128,800
|
–
|
*
|
|
G. Scott McComb
|
133,652
|
133,652
|
–
|
*
|
|
Gemini Master Fund, Ltd.(8)
|
480,000
|
480,000
|
–
|
*
|
|
Golfbully Venture Capital, LLC(9)
|
211,592
|
211,592
|
–
|
*
|
|
Harvey Bibicoff
|
502,095
|
502,095
|
–
|
*
|
|
Irving Cantor
|
150,140
|
150,140
|
–
|
*
|
|
Jack Strommen
|
6,854,154
|
6,854,154
|
–
|
*
|
|
James C. Hilbert
|
798,255
|
798,255
|
–
|
*
|
|
Jeanne M. Stratta
|
290,473
|
290,473
|
–
|
*
|
|
Jeffrey Jackson
|
200,000
|
200,000
|
–
|
*
|
|
Jeffrey Jeremiah McCarty
|
186,802
|
186,802
|
–
|
–*
|
|
Jennifer Blake
|
207,769
|
207,769
|
–
|
*
|
|
John J. Dombroski
|
50,000
|
50,000
|
–
|
*
|
|
John L. Martino
|
484,777
|
484,777
|
–
|
*
|
|
Johnathan Rubic
|
180,601
|
180,601
|
–
|
*
|
|
Jonathan Phillips
|
50,000
|
50,000
|
–
|
*
|
|
Joseph A. Martino
|
765,248
|
765,248
|
–
|
*
|
|
Julius Argumedo
|
102,307
|
102,307
|
–
|
*
|
|
Kent Shuster
|
465,514
|
465,514
|
–
|
*
|
|
Larry Backus
|
267,688
|
80,000
|
187,688
|
*
|
|
Larry Levine
|
1,259,931
|
1,259,931
|
–
|
*
|
|
Mark Sherman
|
397,517
|
397,517
|
–
|
*
|
|
Matthew B. Madden
|
134,272
|
134,272
|
–
|
*
|
|
Michael A. Krever
|
127,964
|
127,964
|
–
|
*
|
|
Michael B. Greenberg
|
206,541
|
206,541
|
–
|
*
|
|
Michael Rivkind
|
108,364
|
108,364
|
–
|
*
|
|
Moshe and Gabriel Azari
|
1,644,219
|
1,644,219
|
–
|
*
|
|
Neta Phillips
|
50,000
|
50,000
|
–
|
*
|
|
Nicholas H. Nguyen
|
466,543
|
466,543
|
–
|
*
|
|
Nicholas Steele
|
93,350
|
93,350
|
–
|
*
|
|
Partner Ship Inc.
|
697,157
|
622,157
|
75,000
|
*
|
|
Patricia Jonikaitis
|
212,301
|
212,301
|
–
|
*
|
|
Paul McDermott
|
74,666
|
74,666
|
–
|
*
|
|
Pedro Arriola
|
278,099
|
102,672
|
175,427
|
*
|
|
Peter Jonikaitis
|
212,406
|
212,406
|
–
|
*
|
|
Peter K Nitz
|
257,762
|
257,762
|
–
|
*
|
|
Phillip Harris
|
100,000
|
100,000
|
–
|
*
|
|
R. Jonathan Robinson
|
499,114
|
499,114
|
–
|
*
|
|
Ralph C. Jenney and Joanne M. Jenney
|
241,919
|
241,919
|
–
|
*
|
|
Randall A. Heller
|
40,000
|
40,000
|
–
|
*
|
|
Raymond A. Pronto
|
1,307,901
|
1,307,901
|
–
|
*
|
|
Robert A. Commandeur
|
504,094
|
504,094
|
–
|
*
|
|
Robert G Szewc
|
273,852
|
273,852
|
–
|
*
|
|
Rona K. Krenik and Mark Krenik
|
148,601
|
148,601
|
–
|
*
|
|
Scott Garver
|
147,661
|
147,661
|
–
|
*
|
|
Sean M. Tabor
|
249,357
|
249,357
|
–
|
*
|
|
Sexton Equities LLC(10)
|
12,000
|
12,000
|
–
|
*
|
|
Stephen E. Harmon
|
293,046
|
293,046
|
–
|
*
|
|
Stephen W. Prough and Kathleen R. Prough
|
210,489
|
210,489
|
–
|
*
|
|
Susan Carlisle
|
93,276
|
93,276
|
–
|
*
|
|
Teri Nowe Myers and Scott Garver
|
295,446
|
295,446
|
–
|
*
|
|
Terry Hartshorn and Sharon Hartshorn
|
631,598
|
631,598
|
–
|
*
|
|
Tim C. Wachter
|
169,930
|
169,930
|
–
|
*
|
|
Timothy C. Larsen
|
220,629
|
46,000
|
174,629
|
*
|
|
Timothy J. Ertmer and Jodi L. Ertmer
|
416,912
|
416,912
|
–
|
*
|
|
Timothy Romanow
|
250,000
|
150,000
|
100,000
|
*
|
|
Tom and Yolanda Talbot
|
548,004
|
488,004
|
60,000
|
*
|
|
Vince J. Severino
|
1,831,364
|
1,831,364
|
–
|
*
|
|
Wesley Larsen
|
280,996
|
250,996
|
30,000
|
*
|
|
William Waligora
|
211,933
|
211,933
|
–
|
*
|
|
TOTAL
|
37,787,209
|
36,090,857
|
1,696,352
|
0.9%
|
|
|
(1)
|
Except as noted in any footnotes below, each person has sole voting power and sole dispositive power as to all of the shares shown as beneficially owned by them. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Our company has only one class of stock outstanding. The sum of 99,040,328 shares of common stock outstanding on June 5, 2017, and 58,547,926 shares of common stock subject to options, warrants and convertible notes currently exercisable or convertible, or exercisable or convertible within 60 days, are deemed outstanding for determining the number of shares beneficially owned by the stockholders.
|
|
|
(2)
|
Julius Argumedo is the manager of the limited liability company and therefore has dispositive power but disclaims any direct beneficial ownership.
|
|
|
(3)
|
Black mountain Equities, Inc. is managed by Adam Baker who has dispositive power but disclaims beneficial ownership.
|
|
|
(4)
|
Dispositive power held by Mark Stangret who disclaims beneficial ownership.
|
|
|
(5)
|
Dispositive power held by Julius Argumedo, Gerarld Argumedo, and Nicole Argumedo, each of whom disclaims beneficial ownership.
|
|
|
(6)
|
Dispositive power held by Woodrow Young who disclaims beneficial ownership.
|
|
|
(7)
|
Dispositive power held by Robert Capetz who disclaims beneficial ownership.
|
|
|
(8)
|
Gemini Strategies Inc. is the investment manager for Gemini Master Fund, Ltd. Gemini Strategies Inc. is managed by Steven Winters who has dispositive power but disclaims beneficial ownership.
|
|
|
(9)
|
Dispositive power held by Kyle Berger who disclaims beneficial ownership.
|
|
|
(10)
|
Dispositive power held by AJ Sexton who disclaims beneficial ownership.
|
* Less than one percent (1%)
PLAN OF DISTRIBUTION
This prospectus covers the sale of up to 36,090,857 shares of our common stock by the selling stockholders. See “Description of Offering.”
The Selling Stockholders are “underwriters,” and may be deemed to be an “underwriter,” within the meaning of the Securities Act. The Selling Stockholders and any of their respective pledgees, donees, assignees and successors-in-interest may, from time to time, sell any or all of their shares of common stock being offered under this prospectus on any stock exchange, market or trading facility on which shares of our common stock are traded or in private transactions. These sales may be at fixed or negotiated prices. The Selling Stockholders may use any one or more of the following methods when disposing of shares:
|
|
☐
|
ordinary brokerage transactions and transactions in which the broker-dealer solicits investors;
|
|
|
☐
|
block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
|
|
|
☐
|
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
|
☐
|
an exchange distribution in accordance with the rules of the applicable exchange;
|
|
|
☐
|
privately negotiated transactions;
|
|
|
☐
|
to cover short sales made after the date that this Registration Statement is declared effective by the Commission;
|
|
|
☐
|
broker-dealers may agree with the Selling Stockholder to sell a specified number of such shares at a stipulated price per share;
|
|
|
☐
|
a combination of any such methods of sale; and
|
|
|
☐
|
any other method permitted pursuant to applicable law; provided, however, that Selling Stockholders who are executive officers or directors of the Company have agreed to sell the shares they hold personally through their individual broker (not a broker that may be engaged on behalf of the Company, if applicable) and not as principal acting for their own accounts.
|
The Selling Stockholder may also sell shares under an exemption from the registration requirements under the Securities Act, if available, rather than under this prospectus.
Broker-dealers engaged by the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Stockholder (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated.
The Selling Stockholders may from time to time pledge or grant a security interest in some or all of the Shares owned by it and, if it defaults in the performance of their secured obligations, the pledgees or secured parties may offer and sell shares of common stock from time to time under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act of 1933 amending the list of Selling Stockholders to include the pledgee, transferee or other successors in interest as Selling Stockholders under this prospectus.
The Selling Stockholders also may transfer the shares of common stock in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
Any broker-dealers or agents that are involved in selling the shares offered under this prospectus may be deemed to be "underwriters," within the meaning of the Securities Act in connection with these sales. Commissions received by these broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Any broker-dealers or agents that are deemed to be underwriters may not sell shares offered under this prospectus unless and until we set forth the names of the underwriters and the material details of their underwriting arrangements in a supplement to this prospectus or, if required, in a replacement prospectus included in a post-effective amendment to the registration statement of which this prospectus is a part.
The Company has advised the Selling Stockholders that it may not use shares registered on this Registration Statement to cover short sales of common stock made prior to the date on which this Registration Statement shall have been declared effective by the Commission. If the Selling Stockholder uses this prospectus for any sale of the common stock, it will be subject to the prospectus delivery requirements of the Securities Act. The Selling Stockholder will be responsible to comply with the applicable provisions of the Securities Act and Exchange Act, and the rules and regulations thereunder promulgated, including, without limitation, Regulation M, as applicable to such Selling Stockholder in connection with resales of their respective shares under this Registration Statement. The Equity Purchaser has agreed not to engage in any direct or indirect short selling of our common stock during the term of the Purchase Agreement.
The company is required to pay all fees and expenses incident to the registration of the shares, but the company will not receive any proceeds from the sale of the common stock by Selling Stockholders.
Blue Sky Restrictions on Resale
If the selling stockholder desires to sell shares of our common stock under this prospectus in the United States, then the selling stockholder will also need to comply with state securities laws, also known as “Blue Sky laws,” with regard to secondary sales. All states offer a variety of exemptions from registration for secondary sales. Many states, for example, have an exemption for secondary trading of securities registered under Section 12(g) of the Exchange Act or for securities of issuers that publish continuous disclosure of financial and non-financial information in a recognized securities manual, such as Standard & Poor’s.
Any person who purchases shares of our common stock from the selling stockholder under this prospectus who then desires to sell such shares also will have to comply with Blue Sky laws regarding secondary sales.
DISCLOSURE OF SEC POSITION ON
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
As permitted by the Delaware general corporation law, we have included a provision in our certificate of incorporation to eliminate the personal liability of our directors for monetary damages for breach of their fiduciary duties as directors, except for liability (i) for any breach of the director’s duty of loyalty to our company, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) under section 174 of the Delaware general corporation law or (iv) for any transaction from which the director derived an improper personal benefit. Our certificate of incorporation also provides that our company shall, to the full extent permitted by section 145 of the Delaware general corporation law, as amended from time to time, indemnify all persons whom it may indemnify pursuant thereto.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to the directors, officers or persons controlling the registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
In the event that a claim for indemnification against such liabilities (other than the payment by our company of expenses incurred or paid by such director, officer or controlling person of our company in the successful defense of any action, suit or proceeding) is asserted by any director, officer or controlling person of our company in connection with the securities being registered in the registration statement of which this prospectus is a part, the registrant will, unless in the opinion of counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by our company is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
LEGAL OPINION
The validity of the shares covered by the registration statement of which this prospectus is a part has been passed upon for us by Wilson, Bradshaw & Cao, LLP (formerly Wilson & Oksam, LLP).
EXPERTS
The consolidated financial statements included in this prospectus for the years ended December 31, 2017 and 2018 have been audited by Haskell & White LLP, an independent registered public accounting firm, to the extent and for the periods set forth in their report appearing elsewhere herein (which expressed an unqualified opinion and includes an explanatory paragraph referring to conditions that raise substantial doubt about BioLargo, Inc. and subsidiaries’ ability to continue as a going concern) and are included in reliance upon such report given upon the authority of said firm as experts in auditing and accounting.
ADDITIONAL INFORMATION
We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, and file reports, proxy statements and other information with the SEC. These reports, proxy statements and other information may be inspected and copied at the public reference facilities maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549 and at the SEC’s regional offices located at the Northwestern Atrium Center, 500 West Madison Street, Suite 1400, Chicago, Illinois 60661 and 233 Broadway, New York, New York 10279. You can obtain copies of these materials from the Public Reference Section of the SEC upon payment of fees prescribed by the SEC. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC’s website (www.SEC.gov) contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC.
We have filed a registration statement on Form S-1 with the SEC under the Securities Act of 1933, as amended, with respect to the securities offered in this prospectus. This prospectus, which is filed as part of a registration statement, does not contain all of the information set forth in the registration statement, some portions of which have been omitted in accordance with the SEC’s rules and regulations. Statements made in this prospectus as to the contents of any contract, agreement or other document referred to in this prospectus are not necessarily complete and are qualified in their entirety by reference to each such contract, agreement or other document that is filed as an exhibit to the registration statement. The registration statement may be inspected without charge at the public reference facilities maintained by the SEC, and copies of such materials can be obtained from the Public Reference Section of the SEC at prescribed rates. You may obtain additional information regarding our company on our website, located at www.BioLargo.com.
BIOLARGO, INC.
INDEX TO FINANCIAL STATEMENTS
|
Index to Consolidated Financial Statements of BioLargo, Inc. as of December 31, 2018 and for the three and six months ended June 30, 2018 and 2019
|
|
|
|
|
|
|
|
Consolidated Balance Sheets as of December 31, 2018 and June 30, 2019 (unaudited)
|
F-2
|
|
|
Consolidated Statements of Operations and Comprehensive Loss for the Three and Six Months Ended June 30, 2018 and 2019 (unaudited)
|
F-3
|
|
|
Consolidated Statement of Stockholders’ Deficit for the Three and Six Months Ended June 30, 2018 and 2019 (unaudited)
|
F-4
|
|
|
Consolidated Statements of Cash Flows for the Three and Six Months Ended June 30, 2018 and 2019 (unaudited)
|
F-5
|
|
|
Notes to Consolidated Financial Statements
|
F-6
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Index to Audited Consolidated Financial Statements of BioLargo, Inc. as of December 31, 2017 and 2018
|
|
|
|
|
|
|
|
Report of Independent Registered Public Accounting Firm
|
F-28
|
|
|
Consolidated Balance Sheets as of December 31, 2017 and December 31, 2018
|
F-29
|
|
|
Consolidated Statements of Operations for the years ended December 31, 2017 and 2018
|
F-30
|
|
|
Consolidated Statements of Stockholders’ Equity (Deficit) for the years ended December 31, 2017 and 2018
|
F-31
|
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2017 and 2018
|
F-32
|
|
|
Notes to Consolidated Financial Statements
|
F-33
|
PART I – FINANCIAL INFORMATION
Item 1. Financial Statements
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2018 AND JUNE 30, 2019
(in thousands, except for per share data)
|
|
|
DECEMBER 31,
2018
|
|
|
JUNE 30, 2019
(unaudited)
|
|
|
Assets
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
655
|
|
|
$
|
706
|
|
|
Accounts receivable
|
|
|
257
|
|
|
|
232
|
|
|
Inventories, net of allowance
|
|
|
26
|
|
|
|
38
|
|
|
Prepaid expenses and other current assets
|
|
|
17
|
|
|
|
25
|
|
|
Total current assets
|
|
|
955
|
|
|
|
1,001
|
|
|
In-process research and development (Note 8)
|
|
|
1,893
|
|
|
|
1,893
|
|
|
Equipment, net of depreciation
|
|
|
126
|
|
|
|
108
|
|
|
Other non-current assets
|
|
|
35
|
|
|
|
35
|
|
|
Right-of-use, operating lease, net of amortization
|
|
|
—
|
|
|
|
370
|
|
|
Deferred offering cost
|
|
|
176
|
|
|
|
176
|
|
|
Total assets
|
|
$
|
3,185
|
|
|
$
|
3,583
|
|
|
Liabilities and stockholders’ equity (deficit)
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued expenses
|
|
$
|
501
|
|
|
$
|
676
|
|
|
Clyra Medical note payable (Note 8)
|
|
|
—
|
|
|
|
1,007
|
|
|
Notes payable
|
|
|
400
|
|
|
|
534
|
|
|
Line of credit
|
|
|
430
|
|
|
|
430
|
|
|
Convertible notes payable
|
|
|
1,365
|
|
|
|
2,863
|
|
|
Discount on convertible notes payable, and line of credit, net of amortization
|
|
|
(205
|
)
|
|
|
(1,180
|
)
|
|
Lease liability
|
|
|
—
|
|
|
|
116
|
|
|
Customer deposit
|
|
|
—
|
|
|
|
28
|
|
|
Total current liabilities
|
|
|
2,491
|
|
|
|
4,474
|
|
|
Long-term liabilities:
|
|
|
|
|
|
|
|
|
|
Convertible notes and note payable
|
|
|
285
|
|
|
|
210
|
|
|
Clyra Medical note payable (Note 8)
|
|
|
1,007
|
|
|
|
—
|
|
|
Liability to Clyra Medical shareholder (Note 8)
|
|
|
643
|
|
|
|
643
|
|
|
Discount on convertible notes payable, net of amortization
|
|
|
(118
|
)
|
|
|
(79
|
)
|
|
Lease liability
|
|
|
—
|
|
|
|
254
|
|
|
Total long-term liabilities
|
|
|
1,817
|
|
|
|
1,028
|
|
|
Total liabilities
|
|
|
4,308
|
|
|
|
5,502
|
|
|
COMMITMENTS, CONTINGENCIES (Note 11)
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY (DEFICIT):
|
|
|
|
|
|
|
|
|
|
Preferred Series A, $.00067 Par Value, 50,000,000 Shares Authorized, -0- Shares Issued and Outstanding, at December 31, 2018 and June 30, 2019, respectively.
|
|
|
—
|
|
|
|
—
|
|
|
Common stock, $.00067 Par Value, 400,000,000 Shares Authorized, 141,466,071 and 152,054,904 Shares Issued, at December 31, 2018 and June 30, 2019, respectively.
|
|
|
95
|
|
|
|
102
|
|
|
Additional paid-in capital
|
|
|
110,222
|
|
|
|
114,745
|
|
|
Accumulated other comprehensive loss
|
|
|
(90
|
)
|
|
|
(98
|
)
|
|
Accumulated deficit
|
|
|
(111,723
|
)
|
|
|
(116,876
|
)
|
|
Total BioLargo Inc. and Subsidiaries stockholders’ equity (deficit)
|
|
|
(1,496
|
)
|
|
|
(2,127
|
)
|
|
Non-controlling interest (Note 8)
|
|
|
373
|
|
|
|
208
|
|
|
Total stockholders’ equity (deficit)
|
|
|
(1,123
|
)
|
|
|
(1,919
|
)
|
|
Total liabilities and stockholders’ equity (deficit)
|
|
$
|
3,185
|
|
|
$
|
3,583
|
|
The accompanying notes are an integral part of these unaudited consolidated financial statements.
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
FOR THE THREE AND SIX MONTHS ENDED JUNE 30, 2018 AND 2019
(in thousands, except for share and per share data)
(unaudited)
|
|
|
THREE MONTHS
|
|
|
SIX MONTHS
|
|
|
|
|
JUNE 30,
2018
|
|
|
JUNE 30,
2019
|
|
|
JUNE 30,
2018
|
|
|
JUNE 30,
2019
|
|
|
Revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product revenue
|
|
$
|
316
|
|
|
$
|
316
|
|
|
$
|
540
|
|
|
$
|
617
|
|
|
Service revenue
|
|
|
11
|
|
|
|
110
|
|
|
|
50
|
|
|
|
173
|
|
|
Total revenue
|
|
|
327
|
|
|
|
426
|
|
|
|
590
|
|
|
|
790
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of goods sold
|
|
|
(194
|
)
|
|
|
(136
|
)
|
|
|
(328
|
)
|
|
|
(276
|
)
|
|
Cost of service
|
|
|
(7
|
)
|
|
|
(92
|
)
|
|
|
(36
|
)
|
|
|
(143
|
)
|
|
Gross profit
|
|
|
126
|
|
|
|
198
|
|
|
|
226
|
|
|
|
371
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative expenses
|
|
|
1,316
|
|
|
|
1,302
|
|
|
|
2,486
|
|
|
|
2,696
|
|
|
Research and development
|
|
|
425
|
|
|
|
367
|
|
|
|
947
|
|
|
|
793
|
|
|
Depreciation
|
|
|
13
|
|
|
|
16
|
|
|
|
23
|
|
|
|
31
|
|
|
Operating loss:
|
|
|
(1,628
|
)
|
|
|
(1,487
|
)
|
|
|
(3,230
|
)
|
|
|
(3,149
|
)
|
|
Other (expense) income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
(1,729
|
)
|
|
|
(498
|
)
|
|
|
(2,561
|
)
|
|
|
(1,483
|
)
|
|
Debt conversion expense
|
|
|
(276
|
)
|
|
|
—
|
|
|
|
(276
|
)
|
|
|
—
|
|
|
Loss on debt extinguishment
|
|
|
—
|
|
|
|
(44
|
)
|
|
|
—
|
|
|
|
(228
|
)
|
|
Grant income
|
|
|
33
|
|
|
|
42
|
|
|
|
38
|
|
|
|
124
|
|
|
Total other expense:
|
|
|
(1,972
|
)
|
|
|
(500
|
)
|
|
|
(2,799
|
)
|
|
|
(1,587
|
)
|
|
Net loss
|
|
|
(3,600
|
)
|
|
|
(1,987
|
)
|
|
|
(6,029
|
)
|
|
|
(4,736
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to noncontrolling interest
|
|
|
(95
|
)
|
|
|
(192
|
)
|
|
|
(202
|
)
|
|
|
(365
|
)
|
|
Net loss attributable to common shareholders
|
|
$
|
(3,505
|
)
|
|
$
|
(1,795
|
)
|
|
$
|
(5,827
|
)
|
|
$
|
(4,371
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per share attributable to common shareholders:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss per share attributable to shareholders – basic and diluted
|
|
$
|
(0.03
|
)
|
|
$
|
(0.01
|
)
|
|
$
|
(0.05
|
)
|
|
$
|
(0.03
|
)
|
|
Weighted average number of common shares outstanding:
|
|
|
118,748,451
|
|
|
|
145,700,515
|
|
|
|
111,760,954
|
|
|
|
143,983,182
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(3,600
|
)
|
|
$
|
(1,987
|
)
|
|
$
|
(6,029
|
)
|
|
$
|
(4,736
|
)
|
|
Foreign currency translation
|
|
|
13
|
|
|
|
(4
|
)
|
|
|
1
|
|
|
|
(8
|
)
|
|
Comprehensive loss
|
|
|
(3,587
|
)
|
|
|
(1,991
|
)
|
|
|
(6,028
|
)
|
|
|
(4,744
|
)
|
|
Comprehensive loss attributable to noncontrolling interest
|
|
|
(95
|
)
|
|
|
(192
|
)
|
|
|
(202
|
)
|
|
|
(365
|
)
|
|
Comprehensive loss attributable to common stockholders
|
|
$
|
(3,492
|
)
|
|
$
|
(1,799
|
)
|
|
$
|
(5,826
|
)
|
|
$
|
(4,379
|
)
|
The accompanying notes are an integral part of these unaudited consolidated financial statements.
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE THREE AND SIX MONTHS ENDED JUNE 30, 2018 AND 2019
(in thousands, except for share data)
(unaudited)
|
|
|
Common stock
|
|
|
Additional
paid-in
|
|
|
Accumulated
|
|
|
Accumulated
other
comprehensive
|
|
|
Non-
controlling
|
|
|
|
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
capital
|
|
|
deficit
|
|
|
loss
|
|
|
interest
|
|
|
Total
|
|
|
Balance, December 31, 2017
|
|
|
104,164,465
|
|
|
$
|
70
|
|
|
$
|
97,093
|
|
|
$
|
(101,205
|
)
|
|
$
|
(62
|
)
|
|
$
|
695
|
|
|
$
|
(3,409
|
)
|
|
Issuance of common stock for services
|
|
|
714,436
|
|
|
|
—
|
|
|
|
196
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
196
|
|
|
Issuance of common stock for interest
|
|
|
617,072
|
|
|
|
—
|
|
|
|
165
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
165
|
|
|
Financing fee in stock
|
|
|
252,385
|
|
|
|
—
|
|
|
|
85
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
85
|
|
|
Sale of stock for cash
|
|
|
658,226
|
|
|
|
—
|
|
|
|
168
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
168
|
|
|
Stock option compensation expense
|
|
|
—
|
|
|
|
—
|
|
|
|
320
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
320
|
|
|
Warrants and beneficial conversion feature issued as discount on convertible notes payable, note payable and line of credit
|
|
|
—
|
|
|
|
—
|
|
|
|
282
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
282
|
|
|
Deemed dividend
|
|
|
—
|
|
|
|
—
|
|
|
|
297
|
|
|
|
(297
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(2,323
|
)
|
|
|
—
|
|
|
|
(107
|
)
|
|
|
(2,430
|
)
|
|
Foreign currency translation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
8
|
|
|
|
—
|
|
|
|
8
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, March 31, 2018
|
|
|
106,406,584
|
|
|
$
|
70
|
|
|
$
|
98,606
|
|
|
$
|
(103,825
|
)
|
|
$
|
(54
|
)
|
|
$
|
588
|
|
|
$
|
(4,615
|
)
|
|
Conversion of notes
|
|
|
19,298,723
|
|
|
|
13
|
|
|
|
6,215
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
6,228
|
|
|
Issuance of common stock for services
|
|
|
733,821
|
|
|
|
—
|
|
|
|
250
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
250
|
|
|
Issuance of common stock for interest
|
|
|
1,302,734
|
|
|
|
1
|
|
|
|
327
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
329
|
|
|
Sale of stock for cash
|
|
|
617,145
|
|
|
|
—
|
|
|
|
212
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
212
|
|
|
Warrant exercise price reduction for cash
|
|
|
—
|
|
|
|
—
|
|
|
|
149
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
149
|
|
|
Stock option compensation expense
|
|
|
—
|
|
|
|
—
|
|
|
|
376
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
376
|
|
|
Warrants and beneficial conversion feature issued as discount on convertible notes payable, note payable and line of credit
|
|
|
—
|
|
|
|
—
|
|
|
|
32
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
33
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(3,505
|
)
|
|
|
—
|
|
|
|
(95
|
)
|
|
|
(3,600
|
)
|
|
Foreign currency translation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(7
|
)
|
|
|
—
|
|
|
|
(7
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, June 30, 2018
|
|
|
128,359,007
|
|
|
$
|
84
|
|
|
$
|
106,167
|
|
|
$
|
(107,330
|
)
|
|
$
|
(61
|
)
|
|
$
|
493
|
|
|
$
|
(645
|
)
|
|
|
|
Common stock
|
|
|
Additional
paid-in
|
|
|
Accumulated
|
|
|
Accumulated
other
comprehensive
|
|
|
Non-
controlling
|
|
|
Total stockholders’
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
capital
|
|
|
deficit
|
|
|
Loss
|
|
|
interest
|
|
|
equity (deficit)
|
|
|
Balance, December 31, 2018
|
|
|
141,466,071
|
|
|
$
|
95
|
|
|
$
|
110,222
|
|
|
$
|
(111,723
|
)
|
|
$
|
(90
|
)
|
|
$
|
373
|
|
|
$
|
(1,123
|
)
|
|
Conversion of notes
|
|
|
1,638,479
|
|
|
|
1
|
|
|
|
218
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
219
|
|
|
Issuance of common stock for service
|
|
|
1,229,541
|
|
|
|
1
|
|
|
|
205
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
206
|
|
|
Issuance of common stock for interest
|
|
|
139,362
|
|
|
|
—
|
|
|
|
25
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
25
|
|
|
Stock option compensation expense
|
|
|
—
|
|
|
|
—
|
|
|
|
352
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
352
|
|
|
Warrants and conversion feature issued as discount on convertible notes payable and line of credit
|
|
|
—
|
|
|
|
—
|
|
|
|
1,115
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,115
|
|
|
Issuance of Clyra Medical common stock
|
|
|
—
|
|
|
|
—
|
|
|
|
21
|
|
|
|
—
|
|
|
|
—
|
|
|
|
89
|
|
|
|
110
|
|
|
Fair value of warrants for extension of debt
|
|
|
—
|
|
|
|
—
|
|
|
|
56
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
56
|
|
|
Deemed dividend for the change in accounting for derivative liability
|
|
|
—
|
|
|
|
—
|
|
|
|
342
|
|
|
|
(342
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(2,576
|
)
|
|
|
—
|
|
|
|
(173
|
)
|
|
|
(2,749
|
)
|
|
Foreign currency translation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(4
|
)
|
|
|
—
|
|
|
|
(4
|
)
|
|
Balance, March 31, 2019
|
|
|
144,473,453
|
|
|
|
97
|
|
|
|
112,556
|
|
|
|
(114,641
|
)
|
|
|
(94
|
)
|
|
|
289
|
|
|
|
(1,793
|
)
|
|
Conversion of notes
|
|
|
2,767,833
|
|
|
|
2
|
|
|
|
294
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
296
|
|
|
Issuance of common stock for service
|
|
|
981,684
|
|
|
|
—
|
|
|
|
213
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
213
|
|
|
Issuance of common stock for interest
|
|
|
87,748
|
|
|
|
—
|
|
|
|
15
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
15
|
|
|
Warrant exercise
|
|
|
3,744,456
|
|
|
|
3
|
|
|
|
101
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
104
|
|
|
Stock issuance to officer (see note 7)
|
|
|
500,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Stock option compensation expense
|
|
|
—
|
|
|
|
—
|
|
|
|
296
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
296
|
|
|
Warrants and conversion feature issued as discount on convertible notes payable and line of credit
|
|
|
—
|
|
|
|
—
|
|
|
|
756
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
756
|
|
|
Issuance of Clyra Medical common stock
|
|
|
—
|
|
|
|
—
|
|
|
|
74
|
|
|
|
—
|
|
|
|
—
|
|
|
|
111
|
|
|
|
185
|
|
|
Deemed dividend for the change in accounting for derivative liability
|
|
|
—
|
|
|
|
—
|
|
|
|
440
|
|
|
|
(440
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,795
|
)
|
|
|
—
|
|
|
|
(192
|
)
|
|
|
(1,987
|
)
|
|
Foreign currency translation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(4
|
)
|
|
|
—
|
|
|
|
(4
|
)
|
|
Balance, June 30, 2019
|
|
|
152,555,174
|
|
|
$
|
102
|
|
|
$
|
114,745
|
|
|
$
|
(116,876
|
)
|
|
$
|
(98
|
)
|
|
$
|
208
|
|
|
$
|
(1,919
|
)
|
The accompanying notes are an integral part of these unaudited consolidated financial statements.
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE SIX MONTHS ENDED JUNE 30, 2018 AND 2019
(in thousands, except for per share data)
(unaudited)
|
|
|
JUNE 30,
2018
|
|
|
JUNE 30,
2019
|
|
|
Cash flows from operating activities
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(6,029
|
)
|
|
$
|
(4,736
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Stock option compensation expense
|
|
|
696
|
|
|
|
648
|
|
|
Common stock issued in lieu of salary to officers and fees for services from vendors
|
|
|
446
|
|
|
|
419
|
|
|
Common stock issued for interest
|
|
|
484
|
|
|
|
40
|
|
|
Interest expense related to amortization of the discount on convertible notes payable and line of credit and deferred financing costs
|
|
|
2,019
|
|
|
|
1,254
|
|
|
Interest expense related to the fair value of warrants issued as consent for variable debt
|
|
|
—
|
|
|
|
54
|
|
|
Debt conversion expense
|
|
|
276
|
|
|
|
—
|
|
|
Loss on extinguishment of debt
|
|
|
—
|
|
|
|
229
|
|
|
Bad debt expense
|
|
|
1
|
|
|
|
—
|
|
|
Deferred offering expense
|
|
|
8
|
|
|
|
—
|
|
|
Depreciation expense
|
|
|
23
|
|
|
|
31
|
|
|
Changes in assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
3
|
|
|
|
24
|
|
|
Inventories
|
|
|
28
|
|
|
|
(12
|
)
|
|
Accounts payable and accrued expenses
|
|
|
31
|
|
|
|
178
|
|
|
Prepaid expenses and other current assets
|
|
|
(39
|
)
|
|
|
(8
|
)
|
|
Customer deposits
|
|
|
—
|
|
|
|
28
|
|
|
Net cash used in operating activities
|
|
|
(2,053
|
)
|
|
|
(1,851
|
)
|
|
Cash flows from investing activities
|
|
|
|
|
|
|
|
|
|
Leasehold improvements
|
|
|
(26
|
)
|
|
|
(14
|
)
|
|
Net cash used in investing activities
|
|
|
(26
|
)
|
|
|
(14
|
)
|
|
Cash flows from financing activities
|
|
|
|
|
|
|
|
|
|
Proceeds from convertible notes payable
|
|
|
463
|
|
|
|
1,825
|
|
|
Proceeds from conversion inducement
|
|
|
357
|
|
|
|
—
|
|
|
Proceeds from warrant exercise-price reduction
|
|
|
148
|
|
|
|
—
|
|
|
Proceeds from warrant exercise
|
|
|
—
|
|
|
|
104
|
|
|
Proceeds from the sale of stock in Clyra Medical
|
|
|
—
|
|
|
|
295
|
|
|
Repayment of note payable
|
|
|
—
|
|
|
|
(300
|
)
|
|
Proceeds from sale of stock to Lincoln Park Capital
|
|
|
381
|
|
|
|
—
|
|
|
Proceeds from line of credit
|
|
|
390
|
|
|
|
—
|
|
|
Net cash provided by financing activities
|
|
|
1,739
|
|
|
|
1,924
|
|
|
Net effect of foreign currency translation
|
|
|
1
|
|
|
|
(8
|
)
|
|
Net change in cash
|
|
|
(339
|
)
|
|
|
51
|
|
|
Cash at beginning of year
|
|
|
990
|
|
|
|
655
|
|
|
Cash at end of period
|
|
$
|
651
|
|
|
$
|
706
|
|
|
Supplemental disclosures of cash flow information
|
|
|
|
|
|
|
|
|
|
Cash paid during the year for:
|
|
|
|
|
|
|
|
|
|
Interest
|
|
$
|
5
|
|
|
$
|
40
|
|
|
Income taxes
|
|
$
|
5
|
|
|
$
|
3
|
|
|
Non-cash investing and financing activities
|
|
|
|
|
|
|
|
|
|
Fair value of warrants issued with convertible notes
|
|
$
|
225
|
|
|
$
|
1,817
|
|
|
Conversion of convertible notes payable into common stock
|
|
$
|
530
|
|
|
$
|
515
|
|
|
Convertible Notes issued with Original Issue Discount
|
|
$
|
—
|
|
|
$
|
373
|
|
|
Exercise of stock options
|
|
$
|
2
|
|
|
$
|
—
|
|
|
Fair value of stock issued for financing fees
|
|
$
|
85
|
|
|
$
|
—
|
|
|
Right of use, operating lease and liability
|
|
$
|
—
|
|
|
$
|
370
|
|
|
Deemed dividend
|
|
$
|
297
|
|
|
$
|
782
|
|
The accompanying notes are an integral part of these unaudited consolidated financial statements.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
Note 1. Business and Organization
Description of Business
BioLargo, Inc. delivers innovative and sustainable technology-based products and services, as well as environmental engineering expertise, across a broad range of industries with an overriding mission to “make life better” with a focus on clean water, clean air, and advanced wound care. Our business strategy is straightforward: we invent or acquire technologies that we believe have the potential to be disruptive in large commercial markets; we develop and validate these technologies to advance and promote their commercial success as we leverage our considerable scientific, engineering, and entrepreneurial talent; we then monetize these technical assets through a variety of business structures that may include licensure, joint venture, sale, spin off, or by deploying direct to market strategies.
Liquidity / Going concern
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of our business. For the six months ended June 30, 2019, we had a net loss of $4,736,000, used $1,851,000 cash in operations, and at June 30, 2019, had a working capital deficit of $3,473,000 and current assets of $1,001,000. We do not have sufficient working capital and do not believe gross profits will be sufficient to fund our current level of operations or pay our debt due prior to December 31, 2019, and will have to obtain further investment capital to continue to fund operations and seek to refinance our existing debt. We have been, and anticipate that we will continue to be, limited in terms of our capital resources. During the year ended December 31, 2018, and the six months ended June 30, 2019, we generated revenues of $1,364,000 and $790,000 through our business segments (Odor-No-More and BLEST – see Note 10, “Business Segment Information”). Neither generated enough revenues to fund their operations. We have $2,119,000 in debt obligations due in the next 12 months (see Notes 4 and 12): (i) $1,724,000in notes that are convertible at the option of the holder, (ii) a $145,000 note due September 6, 2019, and (iii) a line of credit in the amount of $250,000 due on 30-day demand beginning September 1, 2019. We intend to either refinance or renegotiate these obligations, as our cash position is insufficient to maintain our current level of operations and pay these liabilities. Thus, we will be required to raise additional capital. We continue to raise money through private securities offerings, and continue to negotiate for more substantial financings from private and institutional investors. During the six months ended June 30, 2019, we received $1,924,000 net cash provided by financing activities, and at June 30, 2019 had cash of $706,000. Subsequent to June 30, 2019, we received $2,540,000 from new financing activities. No assurance can be made of our success at raising money through private or public offerings.
The foregoing factors raise substantial doubt about our ability to continue as a going concern. Ultimately, our ability to continue as a going concern is dependent upon our ability to attract significant new sources of capital, attain a reasonable threshold of operating efficiencies and achieve profitable operations by licensing or otherwise commercializing products incorporating our technologies. The consolidated financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern.
Organization
We are a Delaware corporation formed in 1991. We have five wholly-owned subsidiaries: BioLargo Life Technologies, Inc., organized under the laws of the State of California in 2006; Odor-No-More, Inc., organized under the laws of the State of California in 2009; BioLargo Water Investment Group Inc., which wholly owns BioLargo Water, Inc., organized under the laws of Canada in 2014; BioLargo Development Corp., organized under the laws of the State of California in 2016; and BioLargo Engineering Science and Technologies, LLC, organized under the laws of the State of Tennessee in 2017 (“BLEST”). Additionally, we own 41.4% of Clyra Medical Technologies, Inc. (“Clyra Medical”), organized under the laws of the State of California in 2012, and consolidate their financial statements (see Notes 2 and 8).
The unaudited consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America for interim financial information and pursuant to Rule 8-03 of Regulation S-X under the Securities Act of 1933, as amended. Accordingly, they do not include all of the information and notes required by generally accepted accounting principles for annual financial statements. In the opinion of management, all adjustments (consisting only of normal recurring adjustments) considered necessary for a fair presentation have been included. For some of our activities, we are still operating in the early stages of the sales and distribution process, and therefore our operating results for the six months ended June 30, 2019 are not necessarily indicative of the results that may be expected for the year ending December 31, 2019, or for any other period. These unaudited consolidated financial statements and notes should be read in conjunction with the Company’s audited consolidated financial statements and accompanying notes included in the Annual Report on Form 10-K for the year ended December 31, 2018 filed with the Securities and Exchange Commission (the “SEC”) on March 29, 2019, as amended.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
Note 2. Summary of Significant Accounting Policies
In the opinion of management, the accompanying balance sheet and related statements of operations, cash flows, and stockholders’ deficit include all adjustments, consisting only of normal recurring items, necessary for their fair presentation in conformity with accounting principles generally accepted in the United States of America.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company, its wholly owned subsidiaries, and Clyra Medical. Management believes Clyra Medical’s financial statements are appropriately consolidated with that of the Company after reviewing the guidance of ASC Topic 810, “Consolidation”, and concluding that BioLargo controls Clyra Medical. While BioLargo does not have voting interest control through a majority stock ownership of Clyra Medical (it owns 41% of the outstanding voting stock), it does exercise control under the “Variable Interest Model”: there is substantial board overlap, BioLargo is the primary beneficiary since it has the power to direct Clyra Medical’s activities that most significantly impact Clyra Medical’s performance, and it has the obligation to absorb losses or receive benefits (through royalties and licensing) that could be potentially significant to Clyra Medical. BioLargo has consolidated Clyra Medical’s operations for all periods presented. All intercompany accounts and transactions have been eliminated (see Note 8).
Foreign Currency
The Company has designated the functional currency of BioLargo Water, Inc., our Canadian subsidiary, to be the Canadian dollar. Therefore, translation gains and losses resulting from differences in exchange rates are recorded in accumulated other comprehensive income.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less when acquired to be cash equivalents. Substantially all cash equivalents are held in short-term money market accounts at one of the largest financial institutions in the United States. From time to time, our cash account balances are greater than the Federal Deposit Insurance Corporation insurance limit of $250,000 per owner per bank, and during such times, we are exposed to credit loss for amounts in excess of insured limits in the event of non-performance by the financial institution. We do not anticipate non-performance by our financial institution.
As of December 31, 2018 and June 30, 2019, our cash balances were made up of the following (in thousands):
|
|
|
December 31,
2018
|
|
|
June 30,
2019
|
|
|
BioLargo, Inc. and wholly owned subsidiaries
|
|
$
|
193
|
|
|
$
|
553
|
|
|
Clyra Medical Technologies, Inc.
|
|
|
462
|
|
|
|
153
|
|
|
Total
|
|
$
|
655
|
|
|
$
|
706
|
|
Accounts Receivable
Trade accounts receivable are recorded net of allowances for doubtful accounts. Estimates for allowances for doubtful accounts are determined based on payment history and individual customer circumstances. The allowance for doubtful accounts as of December 31, 2018 and June 30, 2019 was zero.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
Credit Concentration
We have a limited number of customers that account for significant portions of our revenue. During the six months ended June 30, 2018 and 2019, we had two customers that each accounted for more than 10% of consolidated revenues in the respective periods, as follows:
|
|
|
June 30,
2018
|
|
|
June 30,
2019
|
|
|
Customer A
|
|
|
43
|
%
|
|
|
18
|
%
|
|
Customer B
|
|
|
15
|
%
|
|
|
10
|
%
|
We had four customers that accounted for more than 10% of consolidated accounts receivable at December 31, 2018 and at June 30, 2019 as follows:
|
|
|
December 31,
2018
|
|
|
June 30,
2019
|
|
|
|
|
|
|
|
|
|
|
|
|
Customer W
|
|
|
12
|
%
|
|
|
11
|
%
|
|
Customer X
|
|
|
31
|
%
|
|
<10
|
%
|
|
Customer Y
|
|
<10
|
%
|
|
|
10
|
%
|
|
Customer Z
|
|
<10
|
%
|
|
|
10
|
%
|
Inventory
Inventories are stated at the lower of cost or net realizable value using the average cost method. The allowance for obsolete inventory as of December 31, 2018 and June 30, 2019 was $3,000. As of December 31, 2018 and June 30, 2019, inventories consisted of (in thousands):
|
|
|
December 31,
2018
|
|
|
June 30,
2019
|
|
|
|
|
|
|
|
|
|
|
|
|
Raw material
|
|
$
|
14
|
|
|
$
|
26
|
|
|
Finished goods
|
|
|
12
|
|
|
|
12
|
|
|
Total
|
|
$
|
26
|
|
|
$
|
38
|
|
Other Assets
Other assets consisted of security deposits of $35,000 related to our real estate leases.
Impairment
Long-lived and definite lived intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If the sum of the expected future undiscounted cash flows from the use of the asset and its eventual disposition is less than the carrying amount of the asset, then an impairment loss is recognized. The impairment loss is measured based on the fair value of the asset. Any resulting impairment is recorded as a reduction in the carrying value of the related asset in excess of fair value and a charge to operating results. As of December 31, 2018 and June 30, 2019, management determined that there was no impairment of its long-lived assets.
Earnings (Loss) Per Share
We report basic and diluted earnings (loss) per share (“EPS”) for common and common share equivalents. Basic EPS is computed by dividing the loss attributable to common shareholders by the weighted average shares outstanding. Diluted EPS is computed by adding to the weighted average shares the dilutive effect if stock options and warrants were exercised into common stock. For the three and six months ended June 30, 2018 and 2019, the denominator in the diluted EPS computation is the same as the denominator for basic EPS due to the anti-dilutive effect of the warrants and stock options on the Company’s net loss.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and revenues and expenses during the period reported. Actual results could differ from those estimates. Estimates are used when accounting for stock-based transactions, debt transactions, derivative liabilities, allowance for bad debt, asset depreciation and amortization, among others.
The methods, estimates and judgments we use in applying these most critical accounting policies have a significant impact on the results of our financial statements.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
Share-Based Compensation Expense
We recognize compensation expense for stock option awards on a straight-line basis for employees over the applicable service period of the award, which is the vesting period. We recognize compensation expense for stock option awards for non-employees at the fair value on the grant date. Generally, the options issued to non-employees have been earned upon issuance. For the instances that options are issued to non-employees with a vesting schedule, the fair value is recorded on each vesting date. Share-based compensation expense is based on the grant date fair value estimated using the Black-Scholes Option Pricing Model.
For stock and stock options issued to consultants and other non-employees for services, the Company measures and records an expense as of the earlier of the date at which either: a commitment for performance by the non-employee has been reached or the non-employee’s performance is complete. The equity instruments are measured at the current fair value, and for stock options, the instruments are measured at fair value using the Black Scholes option model.
The following methodology and assumptions were used to calculate share-based compensation for the six months ended June 30, 2018 and 2019:
|
|
|
2018
|
|
|
2019
|
|
|
|
|
Non Plan
|
|
2018 Plan
|
|
|
Non Plan
|
|
2018 Plan
|
|
|
Risk free interest rate
|
|
2.43
|
-
|
2.91%
|
|
|
2.91
|
%
|
|
2.00
|
-
|
2.65%
|
|
2.00
|
-
|
2.65%
|
|
|
Expected volatility
|
|
548
|
-
|
563%
|
|
|
548
|
%
|
|
147
|
-
|
152%
|
|
147
|
-
|
152%
|
|
|
Expected dividend yield
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
Forfeiture rate
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
Life in years
|
|
|
7
|
|
|
|
7
|
|
|
|
7
|
|
|
|
7
|
|
|
Expected price volatility is the measure by which our stock price is expected to fluctuate during the expected term of an option. Expected volatility is derived from the historical daily change in the market price of our common stock, as we believe that historical volatility is the best indicator of future volatility.
The risk-free interest rate used in the Black-Scholes calculation is based on the prevailing U.S. Treasury yield as determined by the U.S. Federal Reserve. We have never paid any cash dividends on our common stock and do not anticipate paying cash dividends on our common stock in the foreseeable future.
Historically, we have not had significant forfeitures of unvested stock options granted to employees and Directors. A significant number of our stock option grants are fully vested at issuance or have short vesting provisions. Therefore, we have estimated the forfeiture rate of our outstanding stock options as zero.
Warrants
Warrants issued with our convertible promissory notes, note payables, line of credit are accounted for under the fair value and relative fair value method.
The warrant is first analyzed per its terms as to whether it has derivative features or not. If the warrant is determined to be a derivative and not qualify for equity treatment, then it is measured at fair value using the Black Scholes option model, and recorded as a liability on the balance sheet. The warrant is re-measured at its then current fair value at each subsequent reporting date (it is “marked-to-market”).
If the warrant is determined to not have derivative features, it is recorded into equity at its fair value using the Black Scholes option model, however, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the convertible note.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
The convertible note issued with the warrant is recorded at its fair value, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the warrant. Further, the convertible promissory note is examined for any intrinsic beneficial conversion feature (“BCF”) of which the convertible price of the note is less than the closing common stock price on date of issuance. If the relative fair value method is used to value the convertible promissory note and there is an intrinsic BCF, a further analysis is undertaken of the BCF using an effective conversion price which assumes the conversion price is the relative fair value divided by the number of shares the convertible debt is converted into by its terms. The BCF value is accounted for as equity.
The warrant and BCF relative fair values are also recorded as a discount to the convertible promissory notes. As presented, these equity features of the convertible promissory notes have recorded a discount to the convertible notes that is substantially equal to the proceeds received.
Non-Cash Transactions
We have established a policy relative to the methodology to determine the value assigned to each intangible we acquire, and/or services or products received for non-cash consideration of our common stock. The value is based on the market price of our common stock issued as consideration, at the date of the agreement of each transaction or when the service is rendered or product is received.
Revenue Recognition
We adopted ASU 2014-09, “Revenue from Contracts with Customers”, Topic 606, on January 1, 2018. The guidance focuses on the core principle for revenue recognition.
The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, an entity should apply the following steps:
Step 1: Identify the contract(s) with a customer.
Step 2: Identify the performance obligations in the contract.
Step 3: Determine the transaction price.
Step 4: Allocate the transaction price to the performance obligations in the contract.
Step 5: Recognize revenue when (or as) the entity satisfies a performance obligation.
For product revenue, we identify the contract with the customer through a written purchase order (which may be part of a national purchasing agreement), in which the details of the contract are defined including the transaction price and method of shipment. The only performance obligation is to create and ship the product and each product has separate pricing. We recognize revenue at a point in time when the order for its goods are shipped if the agreement with our customer is FOB our warehouse facility, and when goods are delivered to its customer if the agreement with our customer is FOB destination. Revenue is recognized with a reduction for sales discounts, as appropriate and negotiated in the customer’s purchase order.
For service revenue, we identify services to be performed in a written contract, which specifies the performance obligations and the rate at which the services will be billed. Each service is separately negotiated and priced. Revenue is recognized as services are performed and completed. Service contracts typically call for invoicing for time and materials incurred for that contract. To date, there have been no discounts or other financing terms for the contracts.
In the future, we may generate revenues from royalties or license fees from our intellectual property. In the event we do so, we anticipate a licensee would pay a license fee in one or more installments and ongoing royalties based on their sales of products incorporating or using our licensed intellectual property. Upon entering into a licensing agreement, we will determine the appropriate method of recognizing the royalty and license fees.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
Government Grants
We have been awarded multiple research grants from governmental and quasi-governmental institutions. The grants received are considered “other income” and are included in our Consolidated Statements of Operations and Comprehensive Loss. We received our first grant in 2015 and have been awarded over 60 grants totaling over $3.6 million. Some of the funds from these grants are given directly to third parties (such as the University of Alberta or a third-party research scientist) to support research on our technology. The grants have terms generally ranging between six and eighteen months and support a majority, but not all, of the related research budget costs. This cooperative research allows us to utilize (i) a depth of resources and talent to accomplish highly skilled work, (ii) financial aid to support research and development costs, (iii) independent and credible validation of our technical claims.
The grants typically provide for (i) recurring monthly amounts, (ii) reimbursement of costs for research talent for which we invoice to request payment, and (iii) ancillary cost reimbursement for research talent travel related costs. All awarded grants have specific requirements on how the money is spent, typically to employ researchers. None of the funds may be used for general administrative expenses or overhead in the United States. These grants have substantially increased our level of research and development activities in Canada. We continue to apply for Canadian government and agency grants to fund research and development activities. Not all of our grant applications have been awarded, and no assurance can be made that any pending grant application, or any future grant applications, will be awarded.
Income Taxes
The asset and liability approach is used to recognize deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of asset and liabilities. Deferred tax assets and liabilities are determined based on the differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The effect on deferred tax asset and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
We account for uncertainties in income tax law under a comprehensive model for the financial statement recognition, measurement, presentation and disclosure of uncertain tax positions taken or expected to be taken in income tax returns as prescribed by generally accepted accounting principles (“GAAP”). Under GAAP, the tax effects of a position are recognized only if it is “more-likely-than-not” to be sustained by the taxing authority as of the reporting date. If the tax position is not considered “more-likely-than-not” to be sustained, then no benefits of the position are recognized.
Fair Value of Financial Instruments
Management believes the carrying amounts of the Company’s financial instruments (excluding debt and equity instruments) as of December 31, 2018 and June 30, 2019 approximate their respective fair values because of the short-term nature of these instruments. Such instruments consist of cash, accounts receivable, prepaid assets, accounts payable, lines of credit, and other assets and liabilities.
Tax Credits
Our research and development activities in Canada may entitle our Canadian subsidiary to claim benefits under the “Scientific Research and Experimental Development Program”, a Canadian federal tax incentive program designed to encourage Canadian businesses of all sizes and in all sectors to conduct research and development in Canada. Benefits under the program include credits to taxable income. If our Canadian subsidiary does not have taxable income in a reporting period, we instead receive a tax refund from the Canadian Revenue Authority. Those refunds are classified in Other Income on our Consolidated Statement of Operations and Comprehensive Loss.
Recent Accounting Pronouncements
In August 2018, the FASB issued Accounting Standards Update No. 2018-13, “Fair Value Measurement (Topic 820), Disclosure Framework—Changes to the Disclosure Requirements for Fair Value Measurement.” The amendments in this update modify the disclosure requirements on fair value measurements in Topic 820, Fair Value Measurement. The amendments in this update are effective for public business entities for fiscal years beginning after December 15, 2019, including interim periods within that fiscal year. Management has not concluded its evaluation of the guidance. Its initial analysis is that it does not believe the new guidance will substantially impact the Company’s financial statements.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
In June 2018, The FASB issued Accounting Standards Update No. 2018-07, “Compensation – Stock Compensation (topic 718): Improvements to Nonemployee Share-Based Payment Accounting”. The amendments in this update expand the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. An entity should apply the requirements of Topic 718 to nonemployee awards except for specific guidance on inputs to an option pricing model and the attribution of cost (that is, the period of time over which share-based payment awards vest and the pattern of cost recognition over that period). The amendments specify that Topic 718 applies to all share-based payment transactions in which a grantor acquires goods or services to be used or consumed in a grantor’s own operations by issuing share-based payment awards. The amendments also clarify that Topic 718 does not apply to share-based payments used to effectively provide (1) financing to the issuer or (2) awards granted in conjunction with selling goods or services to customers as part of a contract accounted for under Topic 606, Revenue from Contracts and Customers. The amendments in this update are effective for public business entities for fiscal years beginning after December 15, 2018, including interim periods within that fiscal year. This new guidance did not materially impact our stock compensation expense.
In February 2016, the FASB issued ASU Update No. 2016-02, “Leases,” which will require lessees to recognize most leases on their balance sheets as a right-of-use asset with a corresponding lease liability, and lessors to recognize a net lease investment. Additional qualitative and quantitative disclosures will also be required. We adopted this standard effective January 1, 2019 using the modified retrospective transition method approved by the FASB in July 2018, which resulted in a $399,000 gross up of assets and liabilities; this balance may fluctuate over time as we enter into new leases, extend or terminate current leases. As of June 30, 2019, the gross up of our balance sheet related to our operating leases totals $370,000.
Note 3. Lincoln Park Financing
On August 25, 2017, we entered into a stock purchase agreement (“LPC Purchase Agreement”) with Lincoln Park Capital Fund, LLC (“Lincoln Park”), pursuant to which Lincoln Park agreed to purchase from us at our request up to an aggregate of $10 million of our common stock (subject to certain limitations) from time to time over a period of three years. The LPC Purchase Agreement allows us, from time to time and at our sole discretion, to direct Lincoln Park to purchase shares of our common stock, subject to limitations in both volume and dollar amount. The purchase price of the shares that may be sold to Lincoln Park under the Purchase Agreement is the lower of (i) the lowest sale price on the date of purchase, or (ii) the average of the three lowest closing prices in the prior 12 business days. There are no restrictions on future financings, rights of first refusal, participation rights, penalties or liquidated damages in the LPC Purchase Agreement or LPC RRA other than a prohibition on entering into a “Variable Rate Transaction,” as defined in the Purchase Agreement. Lincoln Park may not assign or transfer its rights and obligations under the Purchase Agreement.
We did not sell any shares to Lincoln Park during the six months ended June 30, 2019. During the six months ended June 30, 2018, we elected to sell to Lincoln Park 1,256,751 shares of our common stock for which we received $381,000. Additionally, we issued Lincoln Park 18,260 “additional commitment” shares.
We record stock sales in our equity statement and the additional commitment shares issued reduce the deferred offering costs on our balance sheet.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
Note 4. Debt Obligations
The following table summarizes our debt obligations outstanding as of December 31, 2018 and as of June 30, 2019 (in thousands). The Company raised $2,360,000 new financing after June 30, 2019, and refinanced some of the obligations in the table (see Note 12).
|
|
|
December 31,
2018
|
|
|
June 30,
2019
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Notes payable and line of credit
|
|
|
|
|
|
|
|
|
|
Notes payable, mature September 6, 2019
|
|
$
|
400
|
|
|
$
|
484
|
|
|
Note payable, due on demand 60 days’ notice (or March 8, 2023)
|
|
|
—
|
|
|
|
50
|
|
|
Line of credit, due on demand 30 days’ notice after September 1, 2019
|
|
|
430
|
|
|
|
430
|
|
|
Note payable issued by Clyra Medical to Scion, matures June 17, 2020 (Note 8)
|
|
|
—
|
|
|
|
1,007
|
|
|
Total notes payable and line of credit
|
|
$
|
830
|
|
|
$
|
1,971
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible notes payable:
|
|
|
|
|
|
|
|
|
|
Convertible note, matured January 11, 2019
|
|
|
300
|
|
|
|
—
|
|
|
Convertible notes, mature December 31, 2019(1)
|
|
|
75
|
|
|
|
75
|
|
|
Convertible note, matures July 15, 2019
|
|
|
550
|
|
|
|
125
|
|
|
Convertible note, matures July 20, 2019(1)
|
|
|
440
|
|
|
|
440
|
|
|
Convertible note, matures October 7, 2019
|
|
|
—
|
|
|
|
370
|
|
|
Convertible notes, mature November 5, 2019 and December 7, 2019
|
|
|
—
|
|
|
|
554
|
|
|
Convertible nine-month OID notes, mature beginning October 2019
|
|
|
—
|
|
|
|
213
|
|
|
Convertible note, matures April 18, 2020
|
|
|
—
|
|
|
|
220
|
|
|
Convertible notes, mature February 14 and March 17, 2020
|
|
|
—
|
|
|
|
200
|
|
|
Convertible note, matures March 4, 2020
|
|
|
—
|
|
|
|
110
|
|
|
Convertible 12-month OID notes, mature beginning June 2020
|
|
|
—
|
|
|
|
531
|
|
|
Convertible notes payable, mature June 20, 2020(1)
|
|
|
—
|
|
|
|
25
|
|
|
Total convertible notes payable
|
|
$
|
1,365
|
|
|
$
|
2,863
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
$
|
2,195
|
|
|
$
|
4,834
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-term liabilities:
|
|
|
|
|
|
|
|
|
|
Note payable issued by Clyra Medical to Scion, matures June 17, 2020 (See Note 8)
|
|
|
1,007
|
|
|
|
—
|
|
|
Convertible notes payable, mature June 20, 2020(1)
|
|
|
25
|
|
|
|
—
|
|
|
Convertible notes payable, mature April 20, 2021(1)
|
|
|
100
|
|
|
|
100
|
|
|
Convertible notes, mature June 15, 2021(1)
|
|
|
110
|
|
|
|
110
|
|
|
Note payable, matures March 8, 2023 (or on demand 60 days’ notice)
|
|
|
50
|
|
|
|
—
|
|
|
Total long-term liabilities
|
|
$
|
1,292
|
|
|
$
|
210
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
3,487
|
|
|
$
|
5,044
|
|
(1) These notes are convertible at our option at maturity.
The following discussion includes debt instruments to which amendments were made during the three months ended June 30, 2019, and includes other activity that management deemed appropriate to disclose. Each of the debt instruments contained in the above table are disclosed more fully in the financial statements contained in the Company’s Annual Report filed March 29, 2019.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
Notes payable, mature September 6, 2019
On June 4, 2019, we exercised our right to extend the maturity dates of two promissory notes due June 5, 2019 which were originally issued September 19, 2018 to Vernal Bay Investments, LLC (“Vernal”) and Chappy Bean, LLC (“Chappy Bean”). Our election to extend the maturity dates increased the principal amount of each note by 10%, such that the aggregate principal balance of the two notes increased to $484,000 as of June 4, 2019. Subsequent to June 30, 2019, we refinanced one of the two notes (see Note 12).
Convertible Note, matures July 15, 2019 (Vista Capital)
On January 7, 2019, we and Vista Capital agreed to amend the convertible promissory note originally issued December 14, 2017 (“Vista 2017 Note”) and extend its maturity date to April 15, 2019. The principal amount of the note was increased to $605,100. The note will continue to earn interest at the rate of five percent per annum. The amendment re-defined the conversion price to equal 80% of the lowest closing bid price of the Company’s common stock during the 25 consecutive trading days immediately preceding the conversion date. The amendment also reduced the prepayment penalty from 20% to 15%, such that a prepayment requires the payment of an additional 15% of the then outstanding balance, and reduced the penalty for a default from 30% to 25% of the outstanding balance. The intrinsic value of the beneficial conversion feature resulted in a fair value totaling $487,000, all of which was recorded as interest expense during the six months ended June 30, 2019.
On March 28, 2019, we and Vista agreed to further extend the maturity date of the Vista 2017 Note, to July 15, 2019. In consideration for the extension, we agreed to increase the principal balance of the note by 10 percent, to $420,000. The increase in principal totaling $38,000 was recorded as a loss on debt extinguishment on our statement of operations. On July 16, 2019, we and Vista further agreed to extend the maturity date to August 31, 2019. No additional consideration was given for the extension (see Note 12).
During the six months ended June 30, 2019, Vista Capital elected to convert $515,000 of the outstanding principal of the Vista 2017 Note, and we issued 4,406,312 shares of our common stock to Vista pursuant to the conversions. As of June 30, 2019, the outstanding balance on the Vista Note totaled $125,000. Subsequent to June 30, 2019, we and Vista agreed to extend the maturity date of the Note to February 28, 2020 (see Note 12).
Convertible Note, matures October 7, 2019 (Vista Capital)
On January 7, 2019, Vista Capital invested $300,000 and we issued a convertible promissory note (the “Vista 2019 Note”) in the principal amount of $330,000, maturing nine months from the date of issuance (October 7, 2019). The Vista 2019 Note earned a one-time interest charge of 12%, recorded as a discount on convertible notes and will be amortized over the term of the note. The Vista 2019 Note allows Vista Capital to convert the note to our common stock at any time at a price equal to 65% of the lowest closing bid price of the Company’s common stock during the 25 consecutive trading days immediately preceding the conversion date. The Vista 2019 Note contains standard provisions of default, and precludes the issuance of shares to the extent that Vista Capital would beneficially own more than 4.99% of our common stock. The Vista 2019 Note also includes a term that allows Vista Capital to adopt any term of a future financing more favorable than what is provided in the note. For example, these provisions could include a more favorable interest rate, conversion price, or original issue discount. The Vista 2019 Note also requires that we include the shares underlying conversion of the note on the next registration statement we file with the SEC (but not the registration statement filed November 6, 2018). The intrinsic value of the beneficial conversion feature resulted in a fair value totaling $300,000, and is recorded as a discount on convertible notes on our balance sheet. This discount will be amortized over the term of the note as interest expense, all of which will be recorded in 2019. Subsequent to June 30, 2019, we and Vista agreed to extend the maturity date of the Note to April 7, 2020 (see Note 12).
Convertible Notes, mature November 5, 2019 and December 7, 2019 (Tangiers)
On January 31, 2019, we issued a 12% Convertible Promissory Note to Tangiers Global, LLC (“Tangiers”) in the aggregate principal amount of up to $495,000 (the “Tangiers Note”). The note allows for two payments, each due in nine months after receipt, and incurs a guaranteed interest of 12% at inception. The initial payment of $300,000 was received on February 5, 2019, representing a $330,000 principal amount and 10% original issue discount. It is due November 5, 2019. We received the second payment, in the amount of $150,000, on March 7, 2019, increasing the principal amount due under the note to $495,000. This second amount, plus guaranteed interest, is due December 7, 2019. In the aggregate, the principal amount of the note, plus guaranteed interest, totals $554,000.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
The Tangiers Note is convertible at the option of Tangiers at a conversion price equal to 75% of the lowest closing bid price of the Company’s common stock during the 25 consecutive trading days prior to the conversion date. The intrinsic value of the beneficial conversion feature resulted in a fair value totaling $185,000, and is recorded as a discount on convertible notes on our balance sheet. This discount will be amortized over the term of the note as interest expense, all of which will be recorded in 2019.
We may prepay the Tangiers Note up to 180 days after the effective date. If a prepayment is made within 90 days, we must pay a prepayment penalty of 25%; from 91 to 180 days, we must pay a prepayment penalty of 30%. We may pay such prepayment penalties, if we so choose, by issuing common stock at the conversion price. If such shares are not eligible for removal of restrictions pursuant to a registration statement or Rule 144 within 10 trading days following the six-month anniversary of the effective date, Tangiers may rescind the stock issuance and force the Company to pay the prepayment penalty in cash. Upon the occurrence of an event of default, as such term is defined under the Tangiers Note, additional interest will accrue from the date of the event of default at a rate equal to the lower of 22% per annum or the highest rate permitted by law, and an additional 25% shall be added to the principal amount of the note.
In connection with the Tangiers Note, the Company caused its transfer agent to reserve 3,000,000 shares of the Company’s common stock, in the event that the Tangiers Note is converted.
On July 29, 2019, Tangiers elected to convert $369,600 into equity, and subsequently agreed to invest an additional $350,000 (see Note 12).
Convertible Nine-Month OID Notes
During the three months ended March 31, 2019, we issued convertible promissory notes (each, an “OID Note”) in the aggregate principal amount of $213,000, with a 25% original issue discount. These notes were initially convertible into shares of the Company’s common stock at a conversion price of $0.25 per share, and mature nine months from the date of issuance. Our agreement with the investors provided that the initial conversion price may be adjusted downward in the event the Company subsequently issues a convertible promissory note at a lower conversion rate (with this lower conversion rate becoming the adjusted conversion rate under the OID Note), or conducts an equity offering at a per-share price less than $0.25. Each investor also received a stock purchase warrant equal to 75% of the principal amount, divided by the conversion price of $0.25 (see Note 6).
On June 7, 2019, we began issuing twelve-month OID notes at a lower conversion price ($0.17; see “Convertible Twelve-month OID notes”, below). As such, we reduced conversion prices of these notes to $0.17, resulting in an increase of 300,000 shares available for purchase under the warrants.
Convertible Note, matures April 18, 2020
On April 18, 2019, we received $188,000 and issued a convertible note to Bellridge Capital, LP (“Bellridge”) in the principal amount of $220,000 (the “Bellridge Note”), representing a 10% original issue discount, and a deduction of $10,000 for legal fees paid to the investor. The note is due April 18, 2020 and earns interest at 10% per annum. We and Bellridge concurrently entered into a Securities Purchase Agreement through which, upon our mutual consent, Bellridge may invest up to an additional $400,000 (in two tranches) that would be reflected in two additional notes, each of which would mature one year from the date of issuance.
The Bellridge Note is convertible at the option of Bellridge at a conversion price equal to 70% of the lowest closing bid price of the Company’s common stock during the 25 trading days prior to the conversion date. We may prepay the Bellridge Note at any time. If we do so up to 90 days after the effective date, the amount due is equal to 125% of the unpaid principal amount of the note along with any accrued interest, and thereafter, the amount due is 130% of the unpaid principal amount of the note along with any accrued interest. Upon the occurrence of an event of default, as such term is defined under the Bellridge Note, additional interest will accrue from the date of the event of default at a rate equal to the lesser of 24% per annum or the highest rate permitted by law. The intrinsic value of the beneficial conversion feature resulted in a fair value totaling $120,000, and is recorded as a discount on convertible notes on our balance sheet. This discount will be amortized over the twelve-month term of the note as interest expense.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
Convertible notes, mature February 14 and March 17, 2020
On May 14, 2019, we received $95,000 and issued a convertible note to Crossover Capital Fund I, LP (“Crossover Capital”) in the principal amount of $110,000 , representing a 10% original issue discount, and a deduction of $5,000 for legal fees and due diligence. The note is due nine months from the date of issuance, on February 14, 2020.
On June 17, 2019, we received a second investment from Crossover Capital in the amount of $77,000 and issued a convertible note in the principal amount of $90,000, representing a 10% original issue discount, and a deduction of $5,000 for legal fees and due diligence. The note is due nine months from the date of issuance, March 17, 2020.
Concurrently with these two investments, we and Crossover Capital entered into Securities Purchase Agreements. The notes are convertible at the option of Crossover Capital at a conversion price equal to 70% of the lowest closing bid price of our common stock during the 25 trading days prior to the conversion date. We may prepay the notes up to 180 days after issuance, by paying a prepayment penalty that increases from 5% within the first 30 days, to 30% during the last 30. Upon the occurrence of an event of default, as such term is defined under the note, additional interest will accrue from the date of the event of default at a rate equal to the lesser of 24% per annum or the highest rate permitted by law. The intrinsic value of the beneficial conversion features resulted in an aggregate fair value of $134,000, and is recorded as a discount on convertible notes on our balance sheet. This discount will be amortized over the nine-month terms of the notes as interest expense.
Convertible note, matures March 4, 2020
On June 4, 2019, we received $95,000 and issued a convertible note to EMA Financial, LLC (“EMA”) in the principal amount of $110,000 (the “EMA Note”), representing a 10% original issue discount, and a deduction of $5,000 for legal and diligence fees. The note is due nine-months from the date of issuance, on March 4, 2020, and earns interest at a rate of 10% per annum.
The EMA Note is convertible at the option of EMA at a conversion price equal to 70% of the lowest closing bid price of the Company’s common stock during the 25 trading days prior to the conversion date. We may prepay the EMA Note at any time. If we do so up to 90 days after the effective date, the amount due is equal to 125% of the unpaid principal amount of the note along with any accrued interest, and thereafter, the amount due is 130% of the unpaid principal amount of the note along with any accrued interest. Upon the occurrence of an event of default, as such term is defined under the EMA Note, additional interest will accrue from the date of the event of default at a rate equal to the lesser of 24% per annum or the highest rate permitted by law. The intrinsic value of the beneficial conversion feature resulted in a fair value totaling $77,000, and is recorded as a discount on convertible notes on our balance sheet. This discount will be amortized over the nine-month term of the note as interest expense.
Convertible Twelve-month OID notes
During the three months ended June 30, 2019, we received $425,000 and issued convertible promissory notes (each, a “12-Month OID Note”) in the aggregate principal amount of $531,000, with a 25% original issue discount, to four accredited investors. The original issuance discount totaled $106,000 and is recorded as a discount on convertible notes payable on our balance sheet. The intrinsic value of the beneficial conversion features resulted in an aggregate fair value of $425,000, and is recorded as a discount on convertible notes on our balance sheet. The discounts will be amortized and recorded to interest expense over the term of the notes. These notes mature twelve months from the date of issuance. The earliest maturity date is June 7, 2020.
Each Twelve-month OID Note is convertible by the investor at any time at $0.17 per share. This initial conversion price shall be adjusted downward in the event the Company subsequently issues a convertible promissory note at a lower conversion rate (with this lower conversion rate becoming the adjusted conversion rate under the note), or conducts an equity offering at a per-share price less than $0.17. The notes earn interest at a rate of five percent (5%) per annum, due at maturity. The Company may prepay the notes only upon 10 days’ notice to the investor, during which time the investor may exercise his/her right to convert the note to stock.
We must prepay the OID Notes upon the conclusion of a “qualifying offering” (an offering raising $3.5 million or more); in the event a qualified offering is not concluded prior to the maturity date, or the Note is otherwise not paid in full, the Company shall redeem the notes by issuing the number of shares of common stock equal to the outstanding balance divided by the lower of (i) the current conversion price and (ii) seventy percent (70%) of the lowest daily volume weighted average price (“VWAP”) during the 25 trading days immediately preceding the conversion.
In addition to the note, each OID investor will receive a warrant to purchase common stock exercisable at $0.25 per share (see Note 6).
Subsequent to June 30, 2019, we issued additional 12-month OID notes and closed the offering (see Note 12).
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
Note 5. Share-Based Compensation
Issuance of Common Stock in exchange for payment of payables
Payment of Officer Salaries
On March 29, 2019, we issued 579,996 shares of our common stock in lieu of $93,000 of accrued salary and unreimbursed business expenses owed to two of our officers. The price-per-share of $0.16 was based on the closing price of our common stock on the last business day of the month. These shares were issued pursuant to our 2018 Equity Incentive Plan.
On June 28, 2019, we issued 465,875 shares of our common stock in lieu of $107,000 of accrued salary and unreimbursed business expenses owed to two of our officers. The price-per-share of $0.23 was based on the closing price of our common stock on the last business day of the month. These shares were issued pursuant to our 2018 Equity Incentive Plan.
On March 31, 2018, we issued 323,030 shares of our common stock in lieu of $84,000 of accrued salary and unreimbursed business expenses owed to two of our officers. The price-per-share of $0.26 was based on the closing price of our common stock on the last business day of the month.
On June 29, 2018, we issued 176,947 shares of our common stock in lieu of $76,000 of accrued salary and unreimbursed business expenses owed to two of our officers. The price-per-share of $0.43 was based on the closing price of our common stock on the last business day of the month.
Payment of Consultant Fees
During the three months ended June 30, 2019, we issued 515,809 shares of our common stock at a range of $0.16 – $0.23 per share in lieu of $107,000 accrued and unpaid obligations to consultants.
During the three months ended June 30, 2018, we issued 556,874 shares of our common stock, at prices ranging between $0.17 - $0.23 per share, in lieu of $174,000 of accrued and unpaid obligations to consultants.
Payment of Interest on Notes
During the three months ended June 30, 2019, we issued 87,478 shares of our common stock, at prices ranging between $0.23 - $0.43 per share, in lieu of $15,000 of accrued interest due on promissory notes.
During the three months ended June 30, 2018, we issued 1,302,734 shares of our common stock, at prices ranging between $0.23 - $0.45 per share, in lieu of $329,000 of accrued interest due on promissory notes.
Restricted Stock Units
On May 28, 2019, our Compensation Committee, in conjunction with the approval of a new employment agreement for our Vice President of Operations and President of our subsidiary Odor-No-More, granted Joseph L. Provenzano a restricted stock unit of 500,000 shares of common stock, subject to the execution of a “lock-up agreement” whereby the shares remain unvested unless and until the earlier of (i) a sale of the Company, (ii) the successful commercialization of the Company’s products or technologies as demonstrated by its receipt of at least $3,000,000 in cash, or the recognition of $3,000,000 in revenue, over a 12-month period from the sale of products and/or the license of technology, and (iii) the Company’s breach of the employment agreement resulting in his termination.
Stock Option Expense
During the six months ended June 30, 2018 and 2019, we recorded an aggregate $696,000 and $648,000, respectively, in selling general and administrative expense related to the issuance and vesting of stock options. We issued options through our 2018 Equity Incentive Plan, and outside of this plan.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
2018 Equity Incentive Plan
On June 22, 2018, our stockholders adopted the BioLargo 2018 Equity Incentive Plan (“2018 Plan”) as a means of providing our directors, key employees and consultants additional incentive to provide services. Both stock options and stock grants may be made under this plan for a period of 10 years. Our Board of Director’s Compensation Committee administers this plan. As plan administrator, the Compensation Committee has sole discretion to set the price of the options. The plan authorizes the following types of awards: (i) incentive and non-qualified stock options, (ii) restricted stock awards, (iii) stock bonus awards, (iv) stock appreciation rights, (v) restricted stock units, and (vi) performance awards. The total number of shares reserved and available for awards pursuant to this Plan as of the date of adoption of this 2018 Plan by the Board was 40 million shares. The number of shares available to be issued under the 2018 Plan increases automatically each January 1st by the lesser of (a) 2 million shares, or (b) such number of shares determined by our Board.
Activity for our stock options under the 2018 Plan from inception through June 30, 2018 and from December 31, 2018 through the six months ended June 30, 2019, is as follows:
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Aggregate
|
|
|
|
|
Options
|
|
|
Exercise
|
|
|
Price per
|
|
|
intrinsic
|
|
|
As of June 30, 2018:
|
|
Outstanding
|
|
|
Price per share
|
|
|
share
|
|
|
Value(1)
|
|
|
Inception, June 22, 2018
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
Granted
|
|
|
296,976
|
|
|
|
0.43
|
|
|
|
0. 43
|
|
|
|
|
|
|
Balance, June 30, 2018
|
|
|
296,976
|
|
|
$
|
0.43
|
|
|
$
|
0.43
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Aggregate
|
|
|
|
|
Options
|
|
|
Exercise
|
|
|
Price per
|
|
|
intrinsic
|
|
|
As of June 30, 2019:
|
|
Outstanding
|
|
|
Price per share
|
|
|
share
|
|
|
Value(1)
|
|
|
Balance, December 31, 2018
|
|
|
1,318,517
|
|
|
$0.22
|
–
|
0.43
|
|
|
$
|
0.30
|
|
|
|
|
|
|
Granted
|
|
|
3,728,366
|
|
|
0.16
|
–
|
0.22
|
|
|
|
0.18
|
|
|
|
|
|
|
Expired
|
|
|
—
|
|
|
|
—
|
|
|
|
|
—
|
|
|
|
|
|
|
Balance, June 30, 2019
|
|
|
5,046,833
|
|
|
$0.16
|
–
|
0.43
|
|
|
$
|
0.21
|
|
|
$
|
109,000
|
|
(1) – Aggregate intrinsic value based on closing common stock price of $0.23 at June 30, 2019.
The options to purchase 3,528,366 shares granted during the six months ended June 30, 2019 are comprised of options issued to employees, consultants, officers, and directors. We issued options to purchase 513,012 shares of our common stock to employees and consultants in lieu of salary and fees due at an exercise price on the respective grant dates ranging between $0.16 - $0.25 per share. The fair value of these options totaled $93,000 and is recorded as selling, general and administrative expense. We issued options to purchase 715,354 shares of our common stock to members of our board of directors for services performed, in lieu of cash, at an exercise price on the respective grant date of $0.16 and $0.23 per share. We issued options to purchase 300,000 shares of our common stock at an exercise price on the respective grant date of $0.22 per share to our Chief Financial Officer as described immediately below. We issued options to purchase 1,000,000 shares of our common stock at an exercise price on the respective grant date of $0.17 per share to our Vice President of Operations as described below. We issued options to purchase 1,200,000 shares of our common stock at an exercise price on the respective grant date of $0.17 per share to our Vice President of Sales as described below. The fair value of the 2018 Plan options issued during the six months ended June 30, 2019, totaled $137,000 and is recorded as selling, general and administrative expenses.
Chief Financial Officer Contract Extension
On January 16, 2019, we agreed to extend the engagement agreement dated February 1, 2008 (the “Engagement Agreement”, which had been previously extended multiple times) with our Chief Financial Officer, Charles K. Dargan, II. The Engagement Extension Agreement dated as of January 16, 2019 (the “Engagement Extension Agreement”) provides for an additional term to expire September 30, 2019 (the “Extended Term”), and is retroactively effective to the termination of the prior extension on September 30, 2018. Mr. Dargan has been serving as the Company’s Chief Financial Officer since such termination pursuant to the terms of the December 31, 2018 extension.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
For the Extended Term, Mr. Dargan was issued an option (“Option”) to purchase 300,000 shares of the Company’s common stock, at a strike price equal to the closing price of the Company’s common stock on January 16, 2019 of $0.22, to expire January 16, 2029, and to vest over the term of the engagement with 75,000 shares having vested as of June 30, 2019, and the remaining shares to vest 25,000 shares monthly beginning January 31, 2019, and each month thereafter, so long as the Engagement Agreement is in full force and effect. The Option was issued pursuant to the Company’s 2018 Equity Incentive Plan. The fair value of the option totaled $67,000, of which $50,000 was recorded as selling, general and administrative expense during the six months ended June 30, 2019.
The issuance of the Option is Mr. Dargan’s sole source of compensation for the Extended Term. As was the case in all prior terms of his engagement, there is no cash component of his compensation for this term. Mr. Dargan is eligible to be reimbursed for business expenses he incurs in connection with the performance of his services as the Company’s Chief Financial Officer (although he has made no such requests for reimbursement in the past). All other provisions of the Engagement Agreement not expressly amended pursuant to the Engagement Extension Agreement remain the same, including provisions regarding indemnification and arbitration of disputes.
Vice President of Operations Contract Extension
On May 28, 2019, the Compensation Committee of the Board of Directors approved the terms of an employment agreement for our Vice President of Sales and issued him options to purchase an aggregate 1,200,000 shares of the Company’s common stock pursuant to the terms of our 2018 Plan. The exercise price of the first option to purchase 200,000 shares is equal to the closing price of our common stock on the May 28 grant date, at $0.17 per share. One-third of the option vests upon grant, the next third at the first anniversary of the grant, and the final third upon the second anniversary of the grant. Fair value of $34,000 was recorded as selling, general and administrative expenses at issuance. The remaining options to purchase an aggregate 1,000,000 shares are unvested at grant date, and contingent upon certain performance metrics based on sales of our Odor-No-More subsidiary, none of which have been met. As such, no additional fair value was recorded and we are unable to estimate at this time if these metrics will be met. Upon execution of his employment agreement on July 5, 2019, an additional option to purchase 300,000 shares was granted, with an exercise price as of July 5 ($0.25), vesting 100,000 shares on the first, second and third anniversary of the agreement.
Vice President of Sales
On May 28, 2019, the Compensation Committee of the Board of Directors approved the terms of an employment agreement for our Vice President of Sales and issued him options to purchase an aggregate 1,200,000 shares of the Company’s common stock pursuant to the terms of our 2018 Plan. The exercise price of the first option to purchase 200,000 shares is equal to the closing price of our common stock on the May 28 grant date, at $0.17 per share. One-third of the option vests upon grant, the next third at the first anniversary of the grant, and the final third upon the second anniversary of the grant. Fair value of $34,000 was recorded as selling, general and administrative expenses at issuance. The remaining options to purchase an aggregate 1,000,000 shares are unvested at grant date, and contingent upon certain performance metrics based on sales of our Odor-No-More subsidiary, none of which have been met. As such, no additional fair value was recorded and we are unable to estimate at this time if these metrics will be met. Upon execution of his employment agreement on July 5, 2019, an additional option to purchase 300,000 shares was granted, with an exercise price as of July 5 ($0.25), vesting 100,000 shares on the first, second and third anniversary of the agreement.
2007 Equity Incentive Plan
On September 7, 2007, and as amended April 29, 2011, the BioLargo, Inc. 2007 Equity Incentive Plan (“2007 Plan”) was adopted as a means of providing our directors, key employees and consultants additional incentive to provide services. Both stock options and stock grants may be made under this plan for a period of 10 years, which expired on September 7, 2017. The Board’s Compensation Committee administers this plan. As plan administrator, the Compensation Committee has sole discretion to set the price of the options. As of September 2017, the Plan was closed to further stock option grants.
Activity for our stock options under the 2007 Plan for the six months ended June 30, 2018 and 2019 is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Aggregate
|
|
|
|
|
Options
|
|
|
|
Exercise
|
|
|
Price per
|
|
|
intrinsic
|
|
|
As of June 30, 2018:
|
|
Outstanding
|
|
|
|
price per share
|
|
|
share
|
|
|
Value(1)
|
|
|
Balance, December 31, 2017
|
|
|
9,831,586
|
|
|
|
$0.23
|
–
|
1.89
|
|
|
$
|
0.44
|
|
|
|
|
|
|
Expired
|
|
|
(70,000
|
)
|
|
|
1.45
|
–
|
1.89
|
|
|
|
1.79
|
|
|
|
|
|
|
Balance, June 30, 2018
|
|
|
9,761,586
|
|
|
|
$0.23
|
–
|
1.65
|
|
|
$
|
0.43
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of June 30, 2019:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2018
|
|
|
9,691,586
|
|
|
|
$0.23
|
–
|
0.94
|
|
|
$
|
0.43
|
|
|
|
|
|
|
Expired
|
|
|
(842,136
|
)
|
|
|
0.28
|
–
|
0.70
|
|
|
|
0.49
|
|
|
|
|
|
|
Balance, June 30, 2019
|
|
|
8,849,451
|
|
|
|
$0.23
|
–
|
1.65
|
|
|
$
|
0.46
|
|
|
$
|
—
|
|
(1) – Aggregate intrinsic value based on closing common stock price of $0.23 at June 30, 2019.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
Non-Plan Options issued
During the six months ended June 30, 2019, we issued options to purchase 970,380 shares of our common stock at exercise prices ranging between $0.16 – $0.25 per share to vendors for fees for service resulting in a fair value totaling $194,000. The fair value of the options issued and vested during the six months ended June 30, 2019 totaled $367,000, is recorded in our selling, general and administrative expense.
During the six months ended June 30, 2018, we issued options to purchase 1,008,268 shares of our common stock at exercise prices ranging between $0.23 – $0.43 per share to vendors and to members of our board of directors in exchange for unpaid obligations for their services. The fair value of the options totaled $261,000 and is recorded as selling, general and administrative expenses.
Activity of our non-plan stock options issued for the six months ended June 30, 2018 and 2019 is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
Non-plan
|
|
|
|
|
|
|
|
|
average
|
|
|
Aggregate
|
|
|
|
|
Options
|
|
|
|
Exercise
|
|
|
price per
|
|
|
intrinsic
|
|
|
As of June 30, 2018:
|
|
outstanding
|
|
|
|
price per share
|
|
|
share
|
|
|
value(1)
|
|
|
Balance, December 31, 2017
|
|
|
20,018,408
|
|
|
|
$0.25
|
–
|
1.00
|
|
|
$
|
0.51
|
|
|
|
|
|
|
Granted
|
|
|
1,008,268
|
|
|
|
0.23
|
–
|
0.43
|
|
|
|
0.26
|
|
|
|
|
|
|
Expired
|
|
|
(2,400,000
|
)
|
|
|
|
0.99
|
|
|
|
|
0.99
|
|
|
|
|
|
|
Balance, June 30, 2018
|
|
|
18,626,676
|
|
|
|
$0.25
|
–
|
1.00
|
|
|
$
|
0.45
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of June 30, 2019:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2018
|
|
|
19,319,496
|
|
|
|
$0.23
|
–
|
1.00
|
|
|
$
|
0.43
|
|
|
|
|
|
|
Granted
|
|
|
970,380
|
|
|
|
0.16
|
–
|
0.25
|
|
|
|
0.19
|
|
|
|
|
|
|
Expired
|
|
|
(691,975
|
)
|
|
|
|
0.55
|
|
|
|
|
0.55
|
|
|
|
|
|
|
Balance, June 30, 2019
|
|
|
19,597,901
|
|
|
|
$0.16
|
–
|
1.00
|
|
|
$
|
0.42
|
|
|
$
|
34,000
|
|
(1) – Aggregate intrinsic value based on closing common stock price of $0.23 at June 30, 2019.
Note 6. Warrants
We have certain warrants outstanding to purchase our common stock, at various prices, as described in the following table:
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
average
|
|
|
Aggregate
|
|
|
|
|
Warrants
|
|
|
|
Exercise
|
|
|
price per
|
|
|
intrinsic
|
|
|
As of June 30, 2018:
|
|
outstanding
|
|
|
|
price per share
|
|
|
share
|
|
|
value(1)
|
|
|
Balance, December 31, 2017
|
|
|
22,104,817
|
|
|
|
$0.125
|
–
|
1.00
|
|
|
$
|
0.45
|
|
|
|
|
|
|
Issued
|
|
|
2,611,513
|
|
|
|
0.25
|
–
|
0.48
|
|
|
|
0.35
|
|
|
|
|
|
|
Expired
|
|
|
(2,683,400
|
)
|
|
|
|
0.40
|
|
|
|
|
0.40
|
|
|
|
|
|
|
Balance, June 30, 2018
|
|
|
22,032,930
|
|
|
|
$0.125
|
–
|
1.00
|
|
|
$
|
0.44
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of June 30, 2019:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2018
|
|
|
26,872,430
|
|
|
|
$0.25
|
–
|
1.00
|
|
|
$
|
0.42
|
|
|
|
|
|
|
Issued
|
|
|
9,031,871
|
|
|
|
0.10
|
–
|
0.25
|
|
|
|
0.15
|
|
|
|
|
|
|
Exercised
|
|
|
(5,205,746
|
)
|
|
|
0.10
|
–
|
0.12
|
|
|
|
0.11
|
|
|
|
|
|
|
Balance, June 30, 2019
|
|
|
30,698,555
|
|
|
|
$0.10
|
–
|
1.00
|
|
|
$
|
0.39
|
|
|
$
|
547,000
|
|
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
Warrants issued as part of debt extension
On March 5, 2019, we executed amendments extending the maturity dates of notes issued to Vernal Bay and Chappy Bean (see Note 4, subsection titled “Notes payable, mature September 6, 2019”). As consideration for this extension, we agreed to reduce the exercise price, and increase the number of shares purchasable, by the warrants held by Vernal Bay and Chappy Bean. Vernal Bay had been issued a warrant to purchase 1,387,500 shares at $0.25 per share, expiring September 19, 2023. We agreed to lower the exercise price to $0.20 per share, and proportionately increase the number of shares in the warrant to 1,734,375. By doing so, the maximum investment amount under the warrant of $346,875 remained the same. Chappy Bean’s warrant to purchase 600,000 shares was similarly modified, such that it now allows for the purchase of 750,000 shares at $0.20 per share. The reduction in warrant exercise price resulted in a fair value of $56,000 recorded as loss on debt extinguishment in the three months ended March 31, 2019. In the aggregate, the number of shares purchasable under these two warrants increased by 496,875.
Warrants issued as consent for variable rate debt
On January 7 and January 31, 2019, Lincoln Park Capital Fund, LLC agreed to waive the provisions of the Purchase Agreement dated August 25, 2017, prohibiting variable rate transactions. As consideration for the waivers, we issued to Lincoln Park a warrant to purchase 300,000 shares of our common stock at $0.25 per share, expiring five years from the date of grant. In the event the shares underlying the warrant are not registered, the warrant allows the holder to do a “cashless” exercise. The fair value of these warrants totaled $54,000 and was recorded as a discount on note payable on our consolidated balance sheet and will amortize to interest expense in 2019 over the term of the notes. (See Note 4).
Warrants Issued concurrently with the Nine-month OID notes
In conjunction with the issuance of our nine-month OID notes (see Note 4), we issued each investor a warrant to purchase common stock for $0.25 per share, expiring 5 years from the date of issuance. During the three months ended March 31, 2019, we issued warrants to purchase 637,500 shares of our common stock to the three investors. The fair value of these warrants totaled $89,000 and was recorded as a discount on note payable on our consolidated balance sheet and will amortize to interest expense over the term of the notes. During the three months ended June 30, 2019, we reduced the conversion prices of the notes from $0.25 to $0.17, and this resulted in an increase in the number of warrants purchasable by the investors by 300,000 to 937,500, which resulted our recording the fair value of $85,000, which is recorded as a deemed dividend.
Warrants Issued concurrently with Twelve-month OID notes
During the three-months ended June 30, 2019, we issued warrants to purchase 2,619,485 shares of our common stock to purchasers of our Twelve-month OID Notes (see Note 4). The warrants allow the holder to purchase common stock for $0.25 per share, expiring 5 years from the date of issuance. The number of shares purchasable under each warrant was equal to the 75% of the principal balance of the investor’s note, divided by $0.17 (thus, for example, a $300,000 investment would yield a note with principal balance of $375,000, and a warrant allowing for the purchase of up to 1,654,412 shares). The warrant will allow for cashless exercise after 18 months so long as the shares underlying the warrant are not registered. The Company does not have the obligation to register the shares underlying the warrant, but intends to dos o The fair value of these warrants totaled $252,000 and was recorded as a discount on note payable on our consolidated balance sheet and will amortize to interest expense over the term of the notes.
Warrants exercised
During the three months ended June 30, 2019, we received $104,000 from the exercise of a warrant to purchase 866,666 shares.
During the three months ended June 30, 2019, Vista Capital exercised its stock purchase warrant issued September 12, 2018, electing to utilize the cashless exercise features in the warrant. As a result, we issued Vista Capital 2,877,790 shares of common stock. A previous adjustment to the number of shares available for purchase under the warrant resulted in a fair value totaling $355,000, recorded as a deemed dividend in our statement of stockholders’ equity.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
Fair Value
To determine interest expense related to our outstanding warrants issued in conjunction with debt offerings, the fair value of each award grant is estimated on the date of grant using the Black-Scholes option pricing model and the relative fair values are amortized over the life of the warrant. For the determination of expense of warrants issued for services, extinguishment of debt and settlement management also uses the option-pricing model. The principal assumptions we used in applying this model were as follows:
|
|
|
June 30,
2018
|
|
|
June 30,
2019
|
|
|
Risk free interest rate
|
|
|
2.54%
|
|
|
|
1.70
|
–
|
2.62%
|
|
|
Expected volatility
|
|
|
252%
|
|
|
|
86
|
–
|
110%
|
|
|
Expected dividend yield
|
|
|
—
|
|
|
|
|
—
|
|
|
|
Forfeiture rate
|
|
|
—
|
|
|
|
|
—
|
|
|
|
Expected life in years
|
|
5
|
–
|
10
|
|
|
2
|
–
|
5
|
|
The risk-free interest rate is based on U.S. Treasury yields in effect at the time of grant. Expected volatilities are based on historical volatility of our common stock. The expected life in years is based on the contract term of the warrant.
Note 7. Accounts Payable and Accrued Expenses
Accounts payable and accrued expenses included the following (in thousands):
|
|
|
December 31,
2018
|
|
|
June 30,
2019
|
|
|
Accounts payable and accrued expense
|
|
$
|
302
|
|
|
$
|
328
|
|
|
Accrued interest
|
|
|
122
|
|
|
|
209
|
|
|
Accrued payroll
|
|
|
77
|
|
|
|
139
|
|
|
Total accounts payable and accrued expenses
|
|
$
|
501
|
|
|
$
|
676
|
|
Note 8. Noncontrolling Interest – Clyra Medical
We consolidate the operations of our partially owned subsidiary Clyra Medical (see Note 2).
Acquisition of In-process Research and Development
On September 26, 2018, Clyra Medical entered into a transaction with Scion Solutions, LLC, for the purchase of its intellectual property, including its SkinDisc. The consideration provided to Scion is subject to an escrow agreement (“Escrow Agreement”) and earn out provisions and includes: (i) 21,000 shares of the Clyra Medical common stock; (ii) 10,000 shares of Clyra Medical common stock redeemable for 7,142,858 BioLargo common shares (detailed below); and (iii) a promissory note in the principal amount of $1,250,000 to be paid through new capital investments and revenue, as detailed below. This consideration was initially held in escrow pending Clyra Medical raising $1 million “base capital” to fund its business operations.
On December 17, 2018, the parties entered into a closing agreement (“Closing Agreement”) reflecting the satisfaction of the obligation to raise $1 million “base capital”; at that time, one-half of the shares of Clyra Medical common stock exchanged for the Scion assets were released to Scion. The remaining Clyra Medical common shares (a total of 15,500 shares) remain subject to the Escrow Agreement’s performance metrics, each vesting one-fifth of the remaining shares of common stock: (a) notification of FDA premarket clearance of certain orthopedics products, or recognition by Clyra Medical of $100,000 gross revenue; (b) the recognition by Clyra Medical of $100,000 in aggregate gross revenue; (c) the granting of all or any part of the patent application for the SkinDisc product, or recognition by Clyra Medical of $500,000 in gross revenue; (d) recognition by Clyra Medical of $1 million in aggregate gross revenue; and (e) recognition by Clyra Medical of $2 million in gross revenue.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
Scion Solutions – Note Payable and Clyra Liability
The promissory note in the principal amount of $1,250,000 issued by Clyra Medical to Scion on September 26, 2018 (“Clyra-Scion Note”) accrues interest at the rate of 5%. Principal and interest due under the note are to be paid periodically at a rate of 25% of investment proceeds received by Clyra Medical. If the note is not paid off within 18 months after the date of issuance, it is automatically extended for additional 12-month periods until the note is repaid in full. Payments after the initial 18-month maturity date are required to be made in annual installments in an amount equal to the greater of (i) 25% of investment proceeds received during the 12-month period, and (ii) 5% of Clyra Medical’s gross revenues. At June 30, 2019, the balance due on the Clyra-Scion Note equaled $1,007,000.
Non-Controlling Interest
During the six months ended June 30, 2019, Clyra sold $295,000 of its common stock at a price of $200 per share. The shares of BioLargo common stock held by Clyra for the benefit of Scion (the redemption shares) are recorded on our balance sheet as a liability to “Clyra Medical Shareholder”.
As of June 30, 2019, Clyra Medical had the following common and preferred shares outstanding:
|
Shareholder
|
|
Shares
|
|
|
Percent
|
|
|
BioLargo, Inc.
|
|
28,053
|
|
|
41.4%
|
|
|
Sanatio Capital(1)
|
|
11,520
|
|
|
17.0%
|
|
|
Scion Solutions(2)
|
|
15,500
|
|
|
22.9%
|
|
|
Other
|
|
12,697
|
|
|
18.7%
|
|
|
Total
|
|
67,770
|
|
|
|
|
Notes:
(1) Includes 9,830 Series A Preferred shares (see below), and 1,690 common shares.
(2) Does not include an additional 15,500 shares held in escrow subject to performance metrics.
Sanatio Capital purchased Series A Preferred shares in 2015. Sanatio Capital is owned by Jack B. Strommen, who subsequently joined BioLargo’s board of directors. Preferred Shares accrue an annual dividend of 8% for a period of five years. Although the dividends began to accrue immediately, Clyra Medical has no obligation to declare a dividend until a product of the company has received a premarket approval by the United States Federal Drug Administration (“FDA”), or for which a premarket notification pursuant to form 510(k) has been submitted and for which the FDA has given written clearance to market the product in the United States (either, “FDA Approval”). After FDA Approval, annually on December 20, and unless prohibited by California law governing distributions to shareholders, Clyra Medical is required to declare and pay any accruing dividends to holders of Preferred Shares then accrued but unpaid. As the declaration and payment of such dividends is contingent on an uncertain future event, no liability has been recorded for the dividends. The accumulated and undeclared dividend balance as of June 30, 2019 is $215,000.
Holders of Preferred Shares are entitled to preferential payments in the event of a liquidation, dissolution or winding up of the company, in an amount equal to any accrued and unpaid dividends. After such preference, any remaining assets are distributed pro-rata between holders of Clyra Medical common stock and Preferred Shares as if the Preferred Shares had converted to Clyra Medical common stock. Holders of Preferred Shares may convert the shares to Clyra Medical common stock initially on a one-to-one basis. The conversion formula is subject to change in the event Clyra Medical sells stock at a lower price than the price paid by Sanatio.
Preferred shares may be converted to common shares on a one-to-one basis, and have voting rights equal to common shares on a one-to-one basis.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
Note 9. BioLargo Engineering, Science and Technologies, LLC
In September 2017, we commenced a full-service environmental engineering firm and formed a Tennessee entity named BioLargo Engineering, Science & Technologies, LLC (“BLEST”). In conjunction with the start of this subsidiary, we entered into a three-year office lease in the Knoxville, Tennessee area, and entered into employment agreements with seven scientists and engineers. (See Note 10 “Business Segment Information”.) The company was capitalized with two classes of membership units: Class A, 100% owned by BioLargo, and Class B, held by management of BLEST, and which initially have no “profit interest,” as that term is defined in Tennessee law. However, over the succeeding five years, the Class B members can earn up to a 30% profit interest. They also have been granted options to purchase up to an aggregate 2 million shares of BioLargo, Inc. common stock. The profit interest and option shares are subject to a five year vesting schedule tied to the performance of the subsidiary, including gross revenue targets that increase over time, obtaining positive cash flow by March 31, 2018 (which was not met), collecting 90% of its account receivables, obtaining a profit of 10% in its first year (and increasing in subsequent years), making progress in the scale-up and commercialization of our AOS system, and using BioLargo research scientists (such as our Canadian team) for billable work on client projects. These criteria are to be evaluated annually by BLEST’s compensation committee (which includes BioLargo’s president, CFO, and BLEST’s president). Given the significant performance criteria, the Class B units and the stock options will only be recognized in compensation expense if or when the criteria are satisfied. No such units have been issued.
Note 10. Business Segment Information
BioLargo currently has four operating business segments, plus its corporate entity which is responsible for general corporate operations, including administrative functions, finance, human resources, marketing, legal, etc. The four operational business segments are:
1. Odor-No-More (“ONM”) -- which is selling odor and volatile organic control products and services (located in Westminster, California);
2. Clyra Medical (“Clyra”) -- which is engaged in developing medical products and preparing launch into commercial activity with approval of its FDA 510 (K) application in process;
3. BLEST -- which provides professional engineering services on a time and materials basis for outside clients and supports our internal operations as needed (located in Oak Ridge, Tennessee);
4. BioLargo Water (“Water”) -- which has now shifted its focus from R&D/product development to commercializing the AOS technology (located in Edmonton, Alberta Canada).
Historically, none of our operating business units operated at a profit and therefore each required additional cash to meet its monthly expenses. The additional sources of the cash to fund the shortfall from operations of Odor-No-More, BLEST and BioLargo Water have been provided by BioLargo’s sales of debt or equity, research grants, and tax credits. Clyra Medical has been funded by third party investors who invest directly in Clyra Medical in exchange for equity ownership in that entity. For example, during the year ended December 31, 2018, we provided Odor-No-More with approximately $417,000 in cash to supplement its operations. As this subsidiary’s sales have increased (from approximately $500,000 in calendar year 2017 to over $1 million in calendar year 2018), and its gross margins have improved, it has generated more cash for its operations and relied less on corporate to supplement its cash to pay its bills. For the six months ended June 30, 2019, we provided it with approximately $54,000 in cash to supplement its operations.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
The segment information for the three and six months ended June 30, 2018 and 2019, is as follows (in thousands):
|
|
|
Three months ended June 30,
|
|
|
Six months ended June 30,
|
|
|
|
|
2018
|
|
|
2019
|
|
|
2018
|
|
|
2019
|
|
|
Revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Odor-No-More
|
|
$
|
316
|
|
|
$
|
315
|
|
|
$
|
540
|
|
|
$
|
616
|
|
|
BLEST
|
|
|
161
|
|
|
|
241
|
|
|
|
350
|
|
|
|
424
|
|
|
BLEST - Intercompany revenue
|
|
|
(150
|
)
|
|
|
(130
|
)
|
|
|
(300
|
)
|
|
|
(250
|
)
|
|
Total
|
|
$
|
327
|
|
|
$
|
426
|
|
|
$
|
590
|
|
|
$
|
790
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BioLargo corporate
|
|
$
|
(1,052
|
)
|
|
$
|
(956
|
)
|
|
$
|
(2,145
|
)
|
|
$
|
(1,904
|
)
|
|
Odor-No-More
|
|
|
(145
|
)
|
|
|
(51
|
)
|
|
|
(254
|
)
|
|
|
(141
|
)
|
|
Clyra
|
|
|
(177
|
)
|
|
|
(319
|
)
|
|
|
(376
|
)
|
|
|
(606
|
)
|
|
BLEST
|
|
|
(70
|
)
|
|
|
(27
|
)
|
|
|
(115
|
)
|
|
|
(137
|
)
|
|
Water
|
|
|
(184
|
)
|
|
|
(134
|
)
|
|
|
(340
|
)
|
|
|
(361
|
)
|
|
Total
|
|
$
|
(1,628
|
)
|
|
$
|
(1,487
|
)
|
|
$
|
(3,230
|
)
|
|
$
|
(3,149
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BioLargo Corporate
|
|
$
|
(1,729
|
)
|
|
$
|
(485
|
)
|
|
$
|
(2,559
|
)
|
|
$
|
(1,458
|
)
|
|
Clyra
|
|
|
—
|
|
|
|
(13
|
)
|
|
|
(2
|
)
|
|
|
(25
|
)
|
|
Total
|
|
$
|
(1,729
|
)
|
|
$
|
(498
|
)
|
|
$
|
(2,561
|
)
|
|
$
|
(1,483
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development expense
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BioLargo Corporate
|
|
$
|
(281
|
)
|
|
$
|
(210
|
)
|
|
$
|
(624
|
)
|
|
$
|
(382
|
)
|
|
Odor-No-More
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Clyra
|
|
|
(57
|
)
|
|
|
(106
|
)
|
|
|
(144
|
)
|
|
|
(155
|
)
|
|
BLEST
|
|
|
(105
|
)
|
|
|
(73
|
)
|
|
|
(205
|
)
|
|
|
(195
|
)
|
|
Water
|
|
|
(132
|
)
|
|
|
(108
|
)
|
|
|
(274
|
)
|
|
|
(317
|
)
|
|
Intersegement BioLargo corporate
|
|
|
150
|
|
|
|
130
|
|
|
|
300
|
|
|
|
256
|
|
|
Total
|
|
$
|
(425
|
)
|
|
$
|
(367
|
)
|
|
$
|
(947
|
)
|
|
$
|
(793
|
)
|
|
As of June 30, 2019
|
|
BioLargo
|
|
|
ONM
|
|
|
Clyra
|
|
|
BLEST
|
|
|
Water
|
|
|
Elimination
|
|
|
Total
|
|
|
Tangible assets
|
|
$
|
1,000
|
|
|
$
|
198
|
|
|
$
|
302
|
|
|
$
|
163
|
|
|
$
|
33
|
|
|
$
|
(6
|
)
|
|
$
|
1,690
|
|
|
Intangible assets
|
|
|
1,893
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,893
|
|
|
As of December 31, 2018
|
|
BioLargo
|
|
|
ONM
|
|
|
Clyra
|
|
|
BLEST
|
|
|
Water
|
|
|
Elimination
|
|
|
Total
|
|
|
Tangible assets
|
|
$
|
353
|
|
|
$
|
220
|
|
|
$
|
462
|
|
|
$
|
230
|
|
|
$
|
33
|
|
|
$
|
(6
|
)
|
|
$
|
1,292
|
|
|
Intangible assets
|
|
|
1,893
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,893
|
|
Note 11. Commitments and Contingencies
Provenzano Employment Agreement
On June 18, 2019, we and the head of our Odor-No-More subsidiary, Joseph L. Provenzano, entered into an employment agreement (the “Provenzano Employment Agreement”), replacing in its entirety the previous employment agreement with Mr. Provenzano dated January 1, 2008.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
The Provenzano Employment Agreement provides that Mr. Provenzano will serve as our Executive Vice President of Operations, as well as the President and Chief Executive Officer of our wholly owned subsidiary Odor-No-More. Mr. Provenzano’s base compensation will remain at his current rate of $169,772 annually. In addition to this base compensation, the agreement provides that he is eligible to participate in incentive plans, stock option plans, and similar arrangements as determined by the our Board of Directors, health insurance premium payments for himself and his immediate family, a car allowance covering the expenses of his personal commercial grade truck which the company uses in company operations on a continual basis, paid vacation of four weeks per year, and bonuses in such amount as the Compensation Committee may determine from time to time.
In conjunction with this agreement, our Compensation Committee awarded Mr. Provenzano an option to purchase common stock and restricted stock units under our 2018 Equity Incentive Plan (see Note 5).
The Provenzano Employment Agreement has a term of five years, unless earlier terminated in accordance with its terms. The Provenzano Employment Agreement provides that Mr. Provenzano’s employment may be terminated by the Company due to his death or disability, for cause, or upon a merger, acquisition, bankruptcy or dissolution of the Company. “Disability” as used in the Provenzano Employment Agreement means physical or mental incapacity or illness rendering Mr. Provenzano unable to perform his duties on a long-term basis (i) as evidenced by his failure or inability to perform his duties for a total of 120 days in any 360-day period, or (ii) as determined by an independent and licensed physician whom Company selects, or (iii) as determined without recourse by the Company’s disability insurance carrier. “Cause” means that Mr. Provenzano has (i) engaged in willful misconduct in connection with the Company’s business; or (ii) been convicted of, or plead guilty or nolo contendre in connection with, fraud or any crime that constitutes a felony or that involves moral turpitude or theft. If Mr. Provenzano’s employment is terminated due to merger or acquisition, then he will be eligible to receive the greater of (i) one year’s compensation plus an additional one half year for each year of service since the effective date of the employment agreement or (ii) one year’s compensation plus an additional one half year for each year remaining in the term of the agreement. Otherwise, he is only entitled to receive compensation due through the date of termination.
The Provenzano Employment Agreement requires Mr. Provenzano to keep certain information confidential, not to solicit customers or employees of the Company or interfere with any business relationship of the Company, and to assign all inventions made or created during the term of the Provenzano Employment Agreement as “work made for hire”.
Office Leases
We have long-term operating leases for office, industrial and laboratory space in Westminster, California, Oak Ridge, Tennessee, and Alberta, Canada. Payments made under operating leases are charged to the Consolidated Statement of Operations and Comprehensive Loss on a straight-line basis over the term of the operating lease agreement. For the six months ended June 30, 2018 and 2019, total rental expense was $108,000 and $104,000, respectively. On January 1, 2019, we adopted ASC 842 which resulted in a right-of-use asset and lease liability, the adoption resulted in an immaterial cumulative effect of an accounting change that was not recorded. Our right-of-use asset and lease liability operating leases included our office space BioLargo/ONM and BLEST. Our BioLargo/ONM lease has a 4-year extension and we included this extension in the net present value of our lease payments, which used the incremental borrowing cost to BioLargo of 18%. Total remaining operating lease payments is $699,000. BioLargo Water operations lease is considered short-term and not included.
The leases have no additional payment terms such as common area maintenance payments, tax sharing payments or other allocable expenses. Likewise, the leases do not contain other terms and conditions of use, such as variable lease payments, residual value guaranties or other restrictive financial terms.
Clyra Medical Consulting Agreement
Our partially owned subsidiary Clyra Medical (see Note 8) entered into a consulting agreement with Beach House Consulting, LLC, through which Jack B. Strommen will be providing consulting services to Clyra Medical related to its sales and marketing activities once it has received FDA Approval (as defined in Note 8 and the associated agreement) on a product, at which point the agreement provides that Mr. Strommen is to receive $23,000 per month for a period of four years. This agreement has not started, and the total cash obligation related to the agreement would be $1.1 million.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
Note 12. Subsequent Events.
Management has evaluated subsequent events through the date of the filing of this report and management noted the following for disclosure.
Financing activities
Subsequent to June 30, 2019, we accepted subscriptions for an aggregate $2,360,000 in new investments, received proceeds of $180,000 from the exercise of stock purchase warrants, and refinanced and/or converted to equity an aggregate $1,313,800 in current liabilities. These transactions are detailed in the following paragraphs.
Subsequent to June 30, 2019, we accepted subscriptions for an aggregate $1,410,000 to purchase our Twelve-Month OID Notes and stock purchase warrants to accredited investors (See Note 4, subheading “Convertible Twelve-month OID notes”). Once these subscriptions are fully processed, we will have issued promissory notes in the aggregate principal amount of $1,762,500, and warrants to purchase 7,775,735 shares at $0.25 per share (see Note 6). This offering of 12-month OID notes and stock purchase warrants was closed on July 29, 2019.
On August 9, 2019, we received $600,000 from one accredited investor and issued a promissory note in the principal amount of $600,000, maturing in two years, accruing interest at 15% to be paid in cash monthly, and which converts to common stock at the holder’s option at $0.30 per share. This investor also received a warrant to purchase 1,200,000 shares of our common stock at $0.30 per share, expiring five years from the grant date.
Subsequent to June 30, 2019, the holder of stock purchase warrants elected to purchase an aggregate 1.5 million shares for $180,000.
On July 29, 2019, Tangiers Global, LLC, elected to convert $330,000 principal amount and $39,600 accrued interest due on its promissory note issued January 31, 2019, into 2,640,000 shares of common stock. Additionally, Tangiers subscribed to invest $350,000 into a Twelve-Month OID Note (see Note 4) in the principal amount of $437,500, and a stock purchase with the same terms and conditions as those issued to the Twelve-Month OID Note investors, allowing for the purchase of 1,930,147 shares.
Three holders of a secured line of credit issued in 2018 elected to convert $205,000 of principal owed into Twelve Month OID Notes in the principal amount of $256,250, and stock purchase warrants allowing for the purchase of 1,130,515 shares.
On the July 20, 2019 maturity date, the holder of a note in the principal amount of $440,000 elected to convert the principal amount due on the note into 2,000 shares of Clyra Medical common stock held by BioLargo, and $105,600 in accrued and unpaid interest into 384,980 shares of BioLargo common stock at $0.2743 per share (the 20-day closing average). This reduced BioLargo’s ownership in Clyra Medical by 3% to 38.9%.
A holder of a note in the principal amount of $338,800, for which we would have been required to satisfy in cash on September 6, 2019, agreed to satisfy the note through the issuance an amended and restated convertible promissory note due in 12 months with a 25% original issue discount. (Vernal; see Note 4, subheading “Notes payable, mature September 6, 2019”.) The entire note balance, including $41,200 in accrued and unpaid interest, was satisfied through the issuance of an amended and restated note in the principal amount of $475,000, and a warrant to purchase 2,095,588 shares of our common stock. The amended and restated note is convertible by the holder at $0.17 per share. The interest rate was reduced from 18% to 5% per annum. The warrant expires in five years and may be exercised at $0.25 per share. After 18 months, it allows the holder to do a “cashless” exercise unless the shares underlying the warrant are registered with the SEC.
On August 13, 2019, we and Vista Capital agreed to extend by six months the maturity dates of its two promissory notes, the first in the principal amount of $125,000, now due on February 28, 2020, and the second in the principal amount of $330,000, now due on April 7, 2020.
In summary, since June 30, 2019, we added $2,540,000 cash to our balance sheet, and refinanced and/or converted an aggregate $1,313,800 in current liabilities. These new investments and refinancing activities added $2,931,250 in current liabilities and $600,000 in long term liabilities to our balance sheet.
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders
BioLargo, Inc. and Subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of BioLargo, Inc. and Subsidiaries (the “Company”) as of December 31, 2017 and 2018, and the related consolidated statements of operations, stockholders’ equity (deficit), and cash flows for each of the two years in the period ended December 31, 2018, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the consolidated financial position of the Company as of December 31, 2017 and 2018, and the consolidated results of its operations and its cash flows for each of the two years in the period ended December 31, 2018, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has experienced recurring losses, negative cash flows from operations, has limited capital resources, a net stockholders’ deficit, and significant debt obligations coming due in the near term. These matters raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures include examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ HASKELL & WHITE LLP
We have served as the Company’s auditor since 2011.
Irvine, California
March 29, 2019
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2017 AND DECEMBER 31, 2018
(in thousands, except for per share data)
|
|
|
DECEMBER 31,
2017
|
|
|
DECEMBER
31, 2018
|
|
|
Assets
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
990
|
|
|
$
|
655
|
|
|
Accounts receivable, net of allowance
|
|
|
94
|
|
|
|
257
|
|
|
Inventories, net of allowance
|
|
|
54
|
|
|
|
26
|
|
|
Prepaid expenses and other current assets
|
|
|
20
|
|
|
|
17
|
|
|
Total current assets
|
|
|
1,158
|
|
|
|
955
|
|
|
|
|
|
|
|
|
|
|
|
|
In-process research and development (Note 3)
|
|
|
—
|
|
|
|
1,893
|
|
|
Equipment, net of depreciation
|
|
|
109
|
|
|
|
126
|
|
|
Other non-current assets, net of amortization
|
|
|
34
|
|
|
|
35
|
|
|
Deferred offering cost
|
|
|
195
|
|
|
|
176
|
|
|
Total assets
|
|
$
|
1,496
|
|
|
$
|
3,185
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and stockholders’ equity (deficit)
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued expenses
|
|
$
|
224
|
|
|
$
|
501
|
|
|
Notes payable
|
|
|
—
|
|
|
|
400
|
|
|
Line of credit
|
|
|
—
|
|
|
|
430
|
|
|
Convertible notes payable
|
|
|
5,249
|
|
|
|
1,365
|
|
|
Discount on convertible notes payable, and line of credit, net of amortization
|
|
|
(1,257
|
)
|
|
|
(205
|
)
|
|
Total current liabilities
|
|
|
4,216
|
|
|
|
2,491
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-term liabilities:
|
|
|
|
|
|
|
|
|
|
Convertible notes and note payable
|
|
|
1,539
|
|
|
|
285
|
|
|
Clyra Medical note payable (Notes 3 and 10)
|
|
|
—
|
|
|
|
1,007
|
|
|
Liability to Clyra Medical shareholder (Notes 3 and 10)
|
|
|
—
|
|
|
|
643
|
|
|
Discount on convertible notes payable, net of amortization
|
|
|
(850
|
)
|
|
|
(118
|
)
|
|
Total liabilities
|
|
|
4,905
|
|
|
|
4,308
|
|
|
|
|
|
|
|
|
|
|
|
|
COMMITMENTS, CONTINGENCIES (Note 13)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY (DEFICIT):
|
|
|
|
|
|
|
|
|
|
Preferred Series A, $.00067 Par Value, 50,000,000 Shares Authorized, -0- Shares Issued and Outstanding, at December 31, 2017 and December 31, 2018, respectively.
|
|
|
—
|
|
|
|
—
|
|
|
Common stock, $.00067 Par Value, 200,000,000 Shares Authorized, 104,164,465 and 141,466,071 Shares Issued, at December 31, 2017 and December 31, 2018, respectively.
|
|
|
70
|
|
|
|
95
|
|
|
Additional paid-in capital
|
|
|
97,093
|
|
|
|
110,222
|
|
|
Accumulated other comprehensive loss
|
|
|
(62
|
)
|
|
|
(90
|
)
|
|
Accumulated deficit
|
|
|
(101,205
|
)
|
|
|
(111,723
|
)
|
|
Total Biolargo Inc. and Subsidiaries stockholders’ equity (deficit)
|
|
|
(4,104
|
)
|
|
|
(1,496
|
)
|
|
Non-controlling interest (Note 10)
|
|
|
695
|
|
|
|
373
|
|
|
Total stockholders’ equity (deficit)
|
|
|
(3,409
|
)
|
|
|
(1,123
|
)
|
|
Total liabilities and stockholders’ equity (deficit)
|
|
$
|
1,496
|
|
|
$
|
3,185
|
|
See accompanying notes to consolidated financial statements and report of Independent Registered Public Accounting Firm.
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
FOR THE YEARS ENDED DECEMBER 31, 2017 AND 2018
(in thousands, except for per share data)
|
|
|
DECEMBER
31, 2017
|
|
|
DECEMBER
31, 2018
|
|
|
Revenue
|
|
|
|
|
|
|
|
|
|
Product revenue
|
|
$
|
504
|
|
|
$
|
1,123
|
|
|
Service revenue
|
|
|
12
|
|
|
|
241
|
|
|
Total revenue
|
|
|
516
|
|
|
|
1,364
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of revenue
|
|
|
|
|
|
|
|
|
|
Cost of goods sold
|
|
|
(315
|
)
|
|
|
(571
|
)
|
|
Cost of service
|
|
|
(8
|
)
|
|
|
(172
|
)
|
|
Total cost of revenue
|
|
|
(323
|
)
|
|
|
(743
|
)
|
|
Gross profit
|
|
|
193
|
|
|
|
621
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative expenses
|
|
|
4,429
|
|
|
|
5,264
|
|
|
Research and development
|
|
|
1,630
|
|
|
|
1,719
|
|
|
Depreciation and amortization
|
|
|
30
|
|
|
|
50
|
|
|
Total operating expenses
|
|
|
6,089
|
|
|
|
7,033
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
(5,896
|
)
|
|
|
(6,412
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense):
|
|
|
|
|
|
|
|
|
|
Grant income
|
|
|
140
|
|
|
|
158
|
|
|
Tax credit income
|
|
|
71
|
|
|
|
73
|
|
|
Interest expense
|
|
|
(3,862
|
)
|
|
|
(3,494
|
)
|
|
Debt conversion expense
|
|
|
—
|
|
|
|
(276
|
)
|
|
Loss on extinguishment of debt
|
|
|
—
|
|
|
|
(745
|
)
|
|
Total other (expense) income
|
|
|
(3,651
|
)
|
|
|
(4,284
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
(9,547
|
)
|
|
|
(10,696
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to noncontrolling interest
|
|
|
(429
|
)
|
|
|
(475
|
)
|
|
Net loss attributable to common shareholders
|
|
$
|
(9,118
|
)
|
|
$
|
(10,221
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per share attributable to common stockholders:
|
|
|
|
|
|
|
|
|
|
Loss per share attributable to shareholders – basic and diluted
|
|
$
|
(0.10
|
)
|
|
$
|
(0.09
|
)
|
|
Weighted average number of common shares outstanding:
|
|
|
98,941,169
|
|
|
|
122,000,940
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss attributable to common shareholders
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(9,547
|
)
|
|
$
|
(10,696
|
)
|
|
Foreign translation adjustment
|
|
|
20
|
|
|
|
(28
|
)
|
|
Comprehensive loss
|
|
|
(9,527
|
)
|
|
|
(10,724
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss attributable to noncontrolling interest
|
|
|
(429
|
)
|
|
|
(475
|
)
|
|
Comprehensive loss attributable to shareholders
|
|
$
|
(9,098
|
)
|
|
$
|
(10,249
|
)
|
See accompanying notes to consolidated financial statements and report of Independent Registered Public Accounting Firm.
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE YEARS ENDED DECEMBER 31, 2017 AND 2018
(in thousands, except for share data)
|
|
|
Common stock
|
|
|
Additional
paid-in
|
|
|
Accumulated
|
|
|
Accumulated
other
comprehensive
|
|
|
Non-
controlling
|
|
|
Total
stockholders’
equity
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
capital
|
|
|
deficit
|
|
|
Loss
|
|
|
interest
|
|
|
(deficit)
|
|
|
Balance, December 31, 2016
|
|
|
92,975,970
|
|
|
$
|
62
|
|
|
$
|
90,610
|
|
|
$
|
(91,915
|
)
|
|
$
|
(82
|
)
|
|
$
|
555
|
|
|
$
|
(770
|
)
|
|
Issuance of common stock for service
|
|
|
984,070
|
|
|
|
1
|
|
|
|
461
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
462
|
|
|
Issuance of common stock for interest
|
|
|
1,436,751
|
|
|
|
1
|
|
|
|
673
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
674
|
|
|
Stock to CEO
|
|
|
1,500,000
|
|
|
|
1
|
|
|
|
(1
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Conversion of notes
|
|
|
2,316,748
|
|
|
|
2
|
|
|
|
890
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
892
|
|
|
Exercise of warrants
|
|
|
510,000
|
|
|
|
—
|
|
|
|
153
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
153
|
|
|
Exercise of stock options
|
|
|
2,501,937
|
|
|
|
2
|
|
|
|
(2
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Financing fee in stock
|
|
|
738,998
|
|
|
|
—
|
|
|
|
304
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
304
|
|
|
Sale of stock for cash
|
|
|
1,199,991
|
|
|
|
1
|
|
|
|
510
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
511
|
|
|
Stock option compensation expense
|
|
|
—
|
|
|
|
—
|
|
|
|
1,103
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,103
|
|
|
Warrants and conversion feature issued as discount on convertible notes payable and line of credit
|
|
|
—
|
|
|
|
—
|
|
|
|
1,145
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,145
|
|
|
Purchase of Clyra Medical common stock
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(40
|
)
|
|
|
(40
|
)
|
|
Issuance of Clyra Medical common stock
|
|
|
—
|
|
|
|
—
|
|
|
|
411
|
|
|
|
—
|
|
|
|
—
|
|
|
|
609
|
|
|
|
1,020
|
|
|
Deemed dividend for the change in accounting for derivative liability
|
|
|
—
|
|
|
|
—
|
|
|
|
344
|
|
|
|
(344
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Cumulative effect of change in accounting for derivative liability
|
|
|
—
|
|
|
|
—
|
|
|
|
492
|
|
|
|
172
|
|
|
|
—
|
|
|
|
—
|
|
|
|
664
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(9,118
|
)
|
|
|
—
|
|
|
|
(429
|
)
|
|
|
(9,547
|
)
|
|
Foreign currency translation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
20
|
|
|
|
—
|
|
|
|
20
|
|
|
Balance, December 31, 2017
|
|
|
104,164,465
|
|
|
$
|
70
|
|
|
$
|
97,093
|
|
|
$
|
(101,205
|
)
|
|
$
|
(62
|
)
|
|
$
|
695
|
|
|
$
|
(3,409
|
)
|
|
Conversion of notes
|
|
|
18,859,100
|
|
|
|
13
|
|
|
|
6,177
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
6,190
|
|
|
Inducement to convert notes
|
|
|
2,749,197
|
|
|
|
2
|
|
|
|
630
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
632
|
|
|
Issuance of common stock for service
|
|
|
3,214,121
|
|
|
|
2
|
|
|
|
906
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
908
|
|
|
Issuance of common stock for interest
|
|
|
2,042,196
|
|
|
|
1
|
|
|
|
523
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
524
|
|
|
Financing fee in common stock
|
|
|
402,385
|
|
|
|
—
|
|
|
|
127
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
127
|
|
|
Issuance of common stock for the acquisition of In-process research and development
|
|
|
7,142,858
|
|
|
|
5
|
|
|
|
(5
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Sale of stock for cash
|
|
|
2,891,749
|
|
|
|
2
|
|
|
|
837
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
839
|
|
|
Warrant exercise price reduction for cash
|
|
|
—
|
|
|
|
—
|
|
|
|
149
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
149
|
|
|
Stock option compensation expense
|
|
|
—
|
|
|
|
—
|
|
|
|
1,335
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,335
|
|
|
Warrants and conversion feature issued as discount on convertible notes payable and line of credit
|
|
|
—
|
|
|
|
—
|
|
|
|
795
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
795
|
|
|
Issuance of Clyra Medical common stock
|
|
|
—
|
|
|
|
—
|
|
|
|
852
|
|
|
|
—
|
|
|
|
—
|
|
|
|
153
|
|
|
|
1,005
|
|
|
Fair value of warrants for extension of debt
|
|
|
—
|
|
|
|
—
|
|
|
|
506
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
506
|
|
|
Deemed dividend for the change in accounting for derivative liability
|
|
|
—
|
|
|
|
—
|
|
|
|
297
|
|
|
|
(297
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(10,221
|
)
|
|
|
—
|
|
|
|
(475
|
)
|
|
|
(10,696
|
)
|
|
Foreign currency translation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(28
|
)
|
|
|
—
|
|
|
|
(28
|
)
|
|
Balance, December 31, 2018
|
|
|
141,466,071
|
|
|
$
|
95
|
|
|
$
|
110,222
|
|
|
$
|
(111,723
|
)
|
|
$
|
(90
|
)
|
|
$
|
373
|
|
|
$
|
(1,123
|
)
|
See accompanying notes to consolidated financial statements and report of Independent Registered Public Accounting Firm.
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2017 AND 2018
(in thousands, except for per share data)
|
|
|
DECEMBER
31, 2017
|
|
|
DECEMBER
31, 2018
|
|
|
Cash flows from operating activities
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(9,547
|
)
|
|
$
|
(10,696
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Stock option compensation expense
|
|
|
1,103
|
|
|
|
1,335
|
|
|
Common stock issued for in lieu of salary to officers and fees for services from vendors
|
|
|
421
|
|
|
|
898
|
|
|
Common stock issued for interest
|
|
|
674
|
|
|
|
524
|
|
|
Interest expense related to amortization of the discount on convertible notes payable and line of credit and deferred financing costs
|
|
|
3,058
|
|
|
|
2,766
|
|
|
Loss on extinguishment of debt
|
|
|
—
|
|
|
|
745
|
|
|
Debt conversion expense
|
|
|
—
|
|
|
|
276
|
|
|
Deferred offering expense
|
|
|
11
|
|
|
|
19
|
|
|
Financing fee paid in stock
|
|
|
—
|
|
|
|
42
|
|
|
Amortization and depreciation expense
|
|
|
30
|
|
|
|
50
|
|
|
Bad debt expense
|
|
|
3
|
|
|
|
—
|
|
|
Changes in assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
(29
|
)
|
|
|
(163
|
)
|
|
Inventories
|
|
|
(20
|
)
|
|
|
28
|
|
|
Accounts payable and accrued expenses
|
|
|
114
|
|
|
|
284
|
|
|
Accrued officer bonus
|
|
|
(80
|
)
|
|
|
—
|
|
|
Prepaid expenses and other assets
|
|
|
(22
|
)
|
|
|
1
|
|
|
Net cash used in operating activities
|
|
|
(4,284
|
)
|
|
|
(3,891
|
)
|
|
Cash flows from investing activities
|
|
|
|
|
|
|
|
|
|
Equipment purchases
|
|
|
(29
|
)
|
|
|
(58
|
)
|
|
Net cash used in investing activities
|
|
|
(29
|
)
|
|
|
(58
|
)
|
|
Cash flows from financing activities
|
|
|
|
|
|
|
|
|
|
Proceeds from convertible notes payable
|
|
|
1,799
|
|
|
|
705
|
|
|
Proceeds from the sale of stock in Clyra Medical
|
|
|
750
|
|
|
|
1,005
|
|
|
Repayment of Clyra Medical note payable
|
|
|
—
|
|
|
|
(243
|
)
|
|
Proceeds from sale of stock to Lincoln Park Capital
|
|
|
511
|
|
|
|
839
|
|
|
Proceeds from notes payable
|
|
|
—
|
|
|
|
400
|
|
|
Proceeds from line of credit
|
|
|
250
|
|
|
|
430
|
|
|
Proceeds from conversion inducement
|
|
|
—
|
|
|
|
357
|
|
|
Proceeds from warrant buy down
|
|
|
—
|
|
|
|
149
|
|
|
Proceeds from warrant exercise
|
|
|
153
|
|
|
|
—
|
|
|
Repurchase of Clyra Medical shares
|
|
|
(40
|
)
|
|
|
—
|
|
|
Repayment of letter of credit
|
|
|
(50
|
)
|
|
|
—
|
|
|
Net cash provided by financing activities
|
|
|
3,373
|
|
|
|
3,642
|
|
|
Net effect of foreign currency translation
|
|
|
20
|
|
|
|
(28
|
)
|
|
Net change in cash
|
|
|
(920
|
)
|
|
|
(335
|
)
|
|
Cash at beginning of year
|
|
|
1,910
|
|
|
|
990
|
|
|
Cash at end of year
|
|
$
|
990
|
|
|
$
|
655
|
|
|
Supplemental disclosures of cash flow information
|
|
|
|
|
|
|
|
|
|
Cash paid during the year for:
|
|
|
|
|
|
|
|
|
|
Interest
|
|
$
|
9
|
|
|
$
|
54
|
|
|
Income taxes
|
|
$
|
5
|
|
|
$
|
13
|
|
|
Non-cash investing and financing activities
|
|
|
|
|
|
|
|
|
|
Fair value of warrants issued with convertible notes and letter of credit
|
|
$
|
3,006
|
|
|
$
|
795
|
|
|
Conversion of lines of credit into convertible notes payable
|
|
$
|
250
|
|
|
$
|
—
|
|
|
Conversion of convertible notes payable into common stock
|
|
$
|
891
|
|
|
$
|
6,190
|
|
|
Convertible Notes issued with Original Issue Discount
|
|
$
|
70
|
|
|
$
|
85
|
|
|
Note payable issued for intellectual property
|
|
$
|
—
|
|
|
$
|
1,250
|
|
|
Liability to Scion Solutions, LLC
|
|
|
|
|
|
$
|
643
|
|
|
Fair value of stock issued for equipment
|
|
$
|
40
|
|
|
$
|
10
|
|
|
Fair value of stock issued for financing fees
|
|
$
|
304
|
|
|
$
|
85
|
|
|
Fair value of stock issued for conversion of Clyra Medical line of credit
|
|
$
|
250
|
|
|
$
|
—
|
|
|
Stock grant to CEO
|
|
$
|
1
|
|
|
$
|
—
|
|
|
Exercise of stock options
|
|
$
|
12
|
|
|
$
|
—
|
|
|
Deemed dividend
|
|
$
|
344
|
|
|
$
|
297
|
|
|
Cumulative effect of change in account for derivative liability
|
|
$
|
664
|
|
|
$
|
—
|
|
See accompanying notes to consolidated financial statements and report of Independent Registered Public Accounting Firm
Note 1. Business and Organization
Description of Business
BioLargo, Inc. delivers innovative and sustainable technology-based products and services, as well as environmental engineering expertise, across a broad range of industries with an overriding mission to “make life better” with a focus on clean water, clean air, and advanced wound care. Our business strategy is straightforward: we invent or acquire technologies that we believe have the potential to be disruptive in large commercial markets; we develop and validate these technologies to advance and promote their commercial success as we leverage our considerable scientific, engineering, and entrepreneurial talent; we then monetize these technical assets through a variety of business structures that may include licensure, joint venture, sale, spin off, or by deploying direct to market strategies.
Liquidity / Going concern
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of our business. For the year ended December 31, 2018, we had a net loss of $10,696,000, used $3,891,000 cash in operations, and at December 31, 2018, we had a working capital deficit of $1,536,000, and current assets of $955,000. We do not believe gross profits will be sufficient to fund our current level of operations or pay our debt due prior to December 31, 2019, and will have to obtain further investment capital to continue to fund operations and seek to refinance our existing debt. We have been, and anticipate that we will continue to be, limited in terms of our capital resources. During the year ended December 31, 2018, we generated revenues of $1,364,000 through two subsidiaries (Odor-No-More and BLEST – see Note 2, “Business Segment Information”). Neither generated enough revenues to fund their operations, and thus in order for those two, and our other, business segments to continue to operate throughout 2018, we conducted private securities offerings. During the year ended December 31, 2018, we received $3,642,000 net proceeds from various private securities offerings, and ended the year with total cash and cash equivalents of $655,000. Our cash position as of date hereof is insufficient to pay our debt obligations due in April 2019, and thus we must either refinance or renegotiate these obligations. Our cash position is insufficient to maintain our current level of operations and research/development, and thus we will be required to raise substantial additional capital to continue to fund our operations in 2019, as well as our future business plans. We continue to raise money through private securities offerings (see Note 14), and continue to negotiate for more substantial financings from private and institutional investors. Although we engaged an investment banking firm and filed a registration statement to raise $7,500,000 in conjunction with an application for listing our common stock on the Nasdaq Capital Markets, no assurance can be made that we will move forward in the near future with that offering or our listing application. We may reconsider and postpone these efforts as management believes our current market capitalization does not reflect the true value of the Company or recognize the significant business opportunities that lie ahead. Our board intends to evaluate these and other factors, including the anticipated dilution to our stockholders of an offering of the size required to meet the initial and continued listing requirements. No assurance can be made of our success at raising money through private or public offerings, or of our intended listing on a national exchange. No assurance can be made of our success at raising money through private or public offerings, or of our intended listing on a national exchange.
The foregoing factors raise substantial doubt about our ability to continue as a going concern. Ultimately, our ability to continue as a going concern is dependent upon our ability to attract significant new sources of capital, attain a reasonable threshold of operating efficiencies and achieve profitable operations by licensing or otherwise commercializing products incorporating our technologies. The consolidated financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern.
Organization
We are a Delaware corporation formed in 1991. We have five wholly-owned subsidiaries: BioLargo Life Technologies, Inc., organized under the laws of the State of California in 2006; Odor-No-More, Inc., organized under the laws of the State of California in 2009; BioLargo Water, Inc., organized under the laws of Canada in 2014; BioLargo Development Corp., organized under the laws of the State of California in 2016; and BioLargo Engineering Science and Technologies, LLC, organized under the laws of the State of Tennessee in 2017 (“BLEST”). Additionally, we own 42.3% of Clyra Medical Technologies, Inc. (“Clyra Medical”), organized under the laws of the State of California in 2012, and consolidate their financial statements (see Note 10).
Note 2. Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of the Company, its wholly owned subsidiaries, and Clyra Medical. Management believes Clyra Medical’s financial statements are appropriately consolidated with that of the Company after reviewing the guidance of ASC Topic 810, “Consolidation”, and concluding that BioLargo controls Clyra Medical. While BioLargo does not have voting interest control through a majority stock ownership of Clyra Medical (it owns 42.3% of the outstanding voting stock), it does exercise control under the “Variable Interest Model.” There is substantial board overlap, BioLargo is the primary beneficiary since it has the power to direct Clyra Medical’s activities that most significantly impact Clyra Medical’s performance, and it has the obligation to absorb losses or receive benefits (through royalties and licensing) that could be potentially significant to Clyra Medical. Biolargo has consolidated Clyra Medical’s operations for all periods presented. (See Note 10).
All intercompany accounts and transactions have been eliminated.
Foreign Currency
The Company has designated the functional currency of Biolargo Water, Inc., our Canadian subsidiary, to be the Canadian dollar. Therefore, translation gains and losses resulting from differences in exchange rates are recorded in accumulated other comprehensive income.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less when acquired to be cash equivalents. Substantially all cash equivalents are held in short-term money market accounts at one of the largest financial institutions in the United States. From time to time, our cash account balances are greater than the Federal Deposit Insurance Corporation insurance limit of $250,000 per owner per bank, and during such times, we are exposed to credit loss for amounts in excess of insured limits in the event of non-performance by the financial institution. We do not anticipate non-performance by our financial institution.
As of December 31, 2017 and 2018, our cash balances were made up of the following (in thousands):
|
|
|
2017
|
|
|
2018
|
|
|
Biolargo, Inc. and wholly owned subsidiaries
|
|
$
|
462
|
|
|
$
|
193
|
|
|
Clyra Medical Technologies, Inc.
|
|
|
528
|
|
|
|
462
|
|
|
Total
|
|
$
|
990
|
|
|
$
|
655
|
|
Accounts Receivable
Trade accounts receivable are recorded net of allowances for doubtful accounts. Estimates for allowances for doubtful accounts are determined based on payment history and individual customer circumstances. The allowance for doubtful accounts as of December 31, 2017 was $3,000. As of December 31, 2018, although our accounts receivable balance had increased, such increase was due to increased sales, and based on our history of collections, we decreased the allowance for doubtful accounts to zero.
Credit Concentration
We have a limited number of customers that account for significant portions of our revenue. During the years ended December 31, 2017 and 2018, we had three and one customer that each accounted for more than 10% of consolidated revenues in the respective periods, as follows:
|
|
|
2017
|
|
|
2018
|
|
|
Customer A
|
|
|
27
|
%
|
|
<10
|
%
|
|
Customer B
|
|
|
24
|
%
|
|
|
33
|
%
|
|
Customer C
|
|
|
11
|
%
|
|
<10
|
%
|
We had five customers that each accounted for more than 10% of consolidated accounts receivable at December 31, 2017 and two customers at December 31, 2018 as follows:
|
|
|
2017
|
|
|
2018
|
|
|
Customer X
|
|
|
19
|
%
|
|
|
12
|
%
|
|
Customer Y
|
|
|
10
|
%
|
|
|
31
|
%
|
|
Customer Z
|
|
|
12
|
%
|
|
<10
|
%
|
|
Customer AA
|
|
|
12
|
%
|
|
<10
|
%
|
|
Customer BB
|
|
|
10
|
%
|
|
<10
|
%
|
Inventory
Inventories are stated at the lower of cost or net realizable value using the average cost method. The allowance for obsolete inventory as of December 31, 2017 and 2018 was $3,000. As of December 31, 2017 and 2018, inventories consisted of (in thousands):
|
|
|
2017
|
|
|
2018
|
|
|
Raw material
|
|
$
|
34
|
|
|
$
|
14
|
|
|
Finished goods
|
|
|
20
|
|
|
|
12
|
|
|
Total
|
|
$
|
54
|
|
|
$
|
26
|
|
Other Assets
Other Assets consisted of security deposits of $35,000 related to our business offices.
Impairment
Long-lived and definite lived intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If the sum of the expected future undiscounted cash flows from the use of the asset and its eventual disposition is less than the carrying amount of the asset, then an impairment loss is recognized. The impairment loss is measured based on the fair value of the asset. Any resulting impairment is recorded as a reduction in the carrying value of the related asset in excess of fair value and a charge to operating results. For the years ended December 31, 2017 and 2018, management determined that there was no impairment of its long-lived assets.
Earnings (Loss) Per Share
We report basic and diluted earnings (loss) per share (“EPS”) for common and common share equivalents. Basic EPS is computed by dividing reported earnings by the weighted average shares outstanding. Diluted EPS is computed by adding to the weighted average shares the dilutive effect if stock options and warrants were exercised into common stock. For the years ended December 31, 2017 and 2018, the denominator in the diluted EPS computation is the same as the denominator for basic EPS due to the anti-dilutive effect of the warrants and stock options on the Company’s net loss.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and revenues and expenses during the period reported. Actual results could differ from those estimates. Estimates are used when accounting for stock-based transactions, debt transactions, derivative liabilities, allowance for bad debt, asset depreciation and amortization, among others.
The methods, estimates and judgments we use in applying these most critical accounting policies have a significant impact on the results of our financial statements.
Share-Based Compensation Expense
We recognize compensation expense for stock option awards on a straight-line basis for employees over the applicable service period of the award, which is the vesting period. We recognize compensation expense for stock option awards for non-employees at the fair value on the grant date. Generally, the options issued to non-employees have been earned upon issuance. For the instances that options are issued to non-employees with a vesting schedule, the fair value is recorded on each vesting date. Share-based compensation expense is based on the grant date fair value estimated using the Black-Scholes Option Pricing Model.
For stock and stock options issued to consultants and other non-employees for services, the Company measures and records an expense as of the earlier of the date at which either: a commitment for performance by the non-employee has been reached or the non-employee’s performance is complete. The equity instruments are measured at the current fair value, and for stock options, the instruments are measured at fair value using the Black Scholes option model.
For equity instruments issued and outstanding where performance is not complete, but the instrument has been recorded, those instruments are measured again at their then current fair market values at each of the reporting dates (they are “marked-to market”) until the performance and the contract are complete.
The following methodology and assumptions were used to calculate share-based compensation for the years ended December 31, 2017 and 2018:
|
|
|
2017
|
|
|
2018
|
|
|
|
|
Non Plan
|
|
|
2007 Plan
|
|
|
Non Plan
|
|
|
2018 Plan
|
|
|
Risk free interest rate
|
|
2.29
|
–
|
2.43
|
%
|
|
2.31
|
–
|
2.40
|
%
|
|
2.43
|
–
|
2.91
|
%
|
|
2.89
|
–
|
2.91
|
%
|
|
Expected volatility
|
|
563
|
–
|
601
|
%
|
|
578
|
–
|
601
|
%
|
|
538
|
–
|
563
|
%
|
|
489
|
–
|
548
|
%
|
|
Expected dividend yield
|
|
|
—
|
|
|
|
|
—
|
|
|
|
|
—
|
|
|
|
|
—
|
|
|
|
Forfeiture rate
|
|
|
—
|
|
|
|
|
—
|
|
|
|
|
—
|
|
|
|
|
—
|
|
|
|
Life in years
|
|
|
7
|
|
|
|
|
5
|
|
|
|
|
7
|
|
|
|
|
7
|
|
|
Expected price volatility is the measure by which our stock price is expected to fluctuate during the expected term of an option. Expected volatility is derived from the historical daily change in the market price of our common stock, as we believe that historical volatility is the best indicator of future volatility.
The risk-free interest rate used in the Black-Scholes calculation is based on the prevailing U.S. Treasury yield as determined by the U.S. Federal Reserve. We have never paid any cash dividends on our common stock and do not anticipate paying cash dividends on our common stock in the foreseeable future.
Historically, we have not had significant forfeitures of unvested stock options granted to employees and Directors. A significant number of our stock option grants are fully vested at issuance or have short vesting provisions. Therefore, we have estimated the forfeiture rate of our outstanding stock options as zero.
Warrants
Warrants issued with our convertible and non-convertible debt instruments are accounted for under the fair value and relative fair value method.
The warrant is first analyzed per its terms as to whether it has derivative features or not. If the warrant is determined to be a derivative and not qualify for equity treatment, then it is measured at fair value using the Black Scholes option model, and recorded as a liability on the balance sheet. The warrant is re-measured at its then current fair value at each subsequent reporting date (it is “marked-to-market”).
If the warrant is determined to not have derivative features, it is recorded into equity at its fair value using the Black Scholes option model, however, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the convertible note.
Convertible debt instruments are recorded at fair value, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the warrant. Further, the convertible debt instrument is examined for any intrinsic beneficial conversion feature (“BCF”) of which the conversion price is less than the closing common stock price on date of issuance. If the relative fair value method is used to value the convertible debt instrument and there is an intrinsic BCF, a further analysis is undertaken of the BCF using an effective conversion price which assumes the conversion price is the relative fair value divided by the number of shares the convertible debt is converted into by its terms. The BCF value is accounted for as equity.
The warrant and BCF relative fair values are also recorded as a discount to the convertible promissory notes. As present, these equity features of the convertible promissory notes have recorded a discount to the convertible notes that is substantially equal to the proceeds received.
Non-Cash Transactions
We have established a policy relative to the methodology to determine the value assigned to each intangible we acquire, and/or services or products received for non-cash consideration of our common stock. The value is based on the market price of our common stock issued as consideration, at the date of the agreement of each transaction or when the service is rendered or product is received.
Revenue Recognition
We adopted ASU 2014-09, “Revenue from Contracts with Customers”, Topic 606, on January 1, 2018. The guidance focuses on the core principle for revenue recognition.
The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, an entity should apply the following steps:
Step 1: Identify the contract(s) with a customer.
Step 2: Identify the performance obligations in the contract.
Step 3: Determine the transaction price.
Step 4: Allocate the transaction price to the performance obligations in the contract.
Step 5: Recognize revenue when (or as) the entity satisfies a performance obligation.
We have revenue from two subsidiaries, Odor-No-More and BLEST. Odor-No-More identifies its contract with the customer through a written purchase order , in which the details of the contract are defined including the transaction price and method of shipment. The only performance obligation is to create and ship the product and each product has separate pricing. Odor-No-More recognizes revenue at a point in time when the order for its goods are shipped if its agreement with the customer is FOB Odor-No-More’s warehouse facility, and when goods are delivered to its customer if its agreement with the customer is FOB destination. Revenue is recognized with a reduction for sales discounts, as appropriate and negotiated in the customer’s purchase order.
BLEST identifies services to be performed in a written contract, which specifies the performance obligations and the rate at which the services will be billed. Each service is separately negotiated and priced. Revenue is recognized as services are performed and completed. BLEST’s contracts typically call for invoicing for time and materials incurred for that contract. To date, there have been no discounts or other financing terms for the contracts.
In the future, we may generate revenues from royalties or license fees from our intellectual property. In the event we do so, we anticipate a licensee would pay a license fee in one or more installments and ongoing royalties based on their sales of products incorporating or using our licensed intellectual property. Upon entering into a licensing agreement, we will determine the appropriate method of recognizing the royalty and license fees.
Government Grants
We have been awarded multiple research grants from the Canadian National Research Institute – Industrial Research Assistance Program (NRC-IRAP) and the National Science and Engineering Research Council of Canada (NSERC). The grants received are considered other income and are included in our consolidated statements of operations. We received our first grant in 2015 and have been awarded over 65 grants totaling over $3.6 million. Some of the funds from these grants are given directly to third parties (such as the University of Alberta or a third-party research scientist) to support research on our technology. The grants have terms generally ranging between six and eighteen months and support a majority, but not all, of the related research budget costs. This cooperative research allows us to utilize (i) a depth of resources and talent to accomplish highly skilled work, (ii) financial aid to support research and development costs, (iii) independent and credible validation of our technical claims.
The grants typically provide for (i) recurring monthly amounts, (ii) reimbursement of costs for research talent for which we invoice to request payment, and (iii) ancillary cost reimbursement for research talent travel related costs. All awarded grants have specific requirements on how the money is spent, typically to employ researchers. None of the funds may be used for general administrative expenses or overhead in the United States. These grants have substantially increased our level of research and development activities in Canada. We continue to apply for Canadian government and agency grants to fund research and development activities. Not all of our grant applications have been awarded, and no assurance can be made that any pending grant application, or any future grant applications, will be awarded.
Income Taxes
The asset and liability approach is used to recognize deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of asset and liabilities. Deferred tax assets and liabilities are determined based on the differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The effect on deferred tax asset and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
We account for uncertainties in income tax law under a comprehensive model for the financial statement recognition, measurement, presentation and disclosure of uncertain tax positions taken or expected to be taken in income tax returns as prescribed by generally accepted accounting principles (“GAAP”). Under GAAP, the tax effects of a position are recognized only if it is “more-likely-than-not” to be sustained by the taxing authority as of the reporting date. If the tax position is not considered “more-likely-than-not” to be sustained, then no benefits of the position are recognized.
Fair Value of Financial Instruments
Management believes the carrying amounts of the Company’s financial instruments (excluding debt and equity instruments) as of December 31, 2017 and 2018 approximate their respective fair values because of the short-term nature of these instruments. Such instruments consist of cash, accounts receivable, prepaid assets, accounts payable, lines of credit, and other assets and liabilities.
Tax Credits
Our research and development activities in Canada may entitle our Canadian subsidiary to claim benefits under the “Scientific Research and Experimental Development Program”, a Canadian federal tax incentive program designed to encourage Canadian businesses of all sizes and in all sectors to conduct research and development in Canada. Benefits under the program include credits to taxable income. If our Canadian subsidiary does not have taxable income in a reporting period, we instead receive a tax refund from the Canadian Revenue Authority. Those refunds are classified in Other Income on our Consolidated Statement of Operations and Comprehensive Loss.
Recent Accounting Pronouncements
In August 2018, the FASB issued Accounting Standards Update No. 2018-13, “Fair Value Measurement (Topic 820), Disclosure Framework—Changes to the Disclosure Requirements for Fair Value Measurement.” The amendments in this update modify the disclosure requirements on fair value measurements in Topic 820, Fair Value Measurement.
The amendments in this update are effective for public business entities for fiscal years beginning after December 15, 2019, including interim periods within that fiscal year. Management has not concluded its evaluation of the guidance. Its initial analysis is that it does not believe the new guidance will substantially impact the Company’s financial statements.
In June 2018, The FASB issued Accounting Standards Update No. 2018-07, “Compensation – Stock Compensation (topic 718): Improvements to Nonemployee Share-Based Payment Accounting”. The amendments in this update expand the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. An entity should apply the requirements of Topic 718 to nonemployee awards except for specific guidance on inputs to an option pricing model and the attribution of cost (that is, the period of time over which share-based payment awards vest and the pattern of cost recognition over that period). The amendments specify that Topic 718 applies to all share-based payment transactions in which a grantor acquires goods or services to be used or consumed in a grantor’s own operations by issuing share-based payment awards. The amendments also clarify that Topic 718 does not apply to share-based payments used to effectively provide (1) financing to the issuer or (2) awards granted in conjunction with selling goods or services to customers as part of a contract accounted for under Topic 606, Revenue from Contracts and Customers. The amendments in this update are effective for public business entities for fiscal years beginning after December 15, 2018, including interim periods within that fiscal year. Management has not concluded its evaluation of the guidance. Its initial analysis is that the new guidelines would likely decrease the stock compensation expense, but not so as to substantially impact the Company’s financial statements.
In February 2016, the FASB issued ASU Update No. 2016-02, “Leases,” which will require lessees to recognize most leases on their balance sheets as a right-of-use asset with a corresponding lease liability, and lessors to recognize a net lease investment. Additional qualitative and quantitative disclosures will also be required. We will adopt this standard effective January 1, 2019 using the modified retrospective transition method approved by the FASB in July 2018. Management believes the adoption of the new standard will gross up assets and liabilities; however, we do not believe there will be a material impact on our states of operations.
Note 3. Acquisition of In-process Research and Development
On September 26, 2018, BioLargo and Clyra Medical entered into a transaction whereby BioLargo would acquire the intangible assets of Scion Solutions, LLC (“Scion”), and in particular its in-process research and development of the “SkinDisc,” a method for treating advanced hard-to-treat wounds including diabetic ulcers. In addition to a pending patent application, the assets include the technical know-how and data developed by the Scion team.
The consideration provided to Scion is subject to an escrow agreement dated September 26, 2018 (“Escrow Agreement”) and earn out provisions and includes: (i) 21,000 shares of the Clyra Medical common stock; (ii) 10,000 shares of Clyra Medical common stock redeemable for 7,142,858 BioLargo common shares (detailed below); and (iii) a promissory note in the principal amount of $1,250,000 to be paid through new capital investments and revenue, as detailed below. This consideration was initially held in escrow pending Clyra Medical raising $1 million “base capital” to fund its business operations.
On December 17, 2018, the parties entered into a closing agreement (“Closing Agreement”) reflecting the satisfaction of the obligation to raise $1 million “base capital”; at that time, one-half of the shares of Clyra Medical common stock exchanged for the Scion assets were released to Scion. The remaining Clyra Medical common shares (a total of 15,500 shares) remain subject to the Escrow Agreement’s performance metrics, each vesting one-fifth of the remaining shares of common stock: (a) notification of FDA premarket clearance of certain orthopedics products, or recognition by Clyra Medical of $100,000 gross revenue; (b) the recognition by Clyra Medical of $100,000 in aggregate gross revenue; (c) the granting of all or any part of the patent application for the SkinDisc product, or recognition by Clyra Medical of $500,000 in gross revenue; (d) recognition by Clyra Medical of $1 million in aggregate gross revenue; and (e) recognition by Clyra Medical of $2 million in gross revenue.
The promissory note in the principal amount of $1,250,000 issued by Clyra Medical to Scion on September 26, 2018 accrues interest at the rate of 5%. Principal and interest due under the note are to be paid periodically at a rate of 25% of investment proceeds received by Clyra Medical. If the note is not paid off within 18 months after the date of issuance, it is automatically extended for additional 12-month periods until the note is repaid in full. Payments after the initial 18-month maturity date are required to be made in annual installments in an amount equal to the greater of (i) 25% of investment proceeds received during the 12-month period, and (ii) 5% of Clyra Medical’s gross revenues.
Immediately following Clyra Medical’s purchase of Scion’s intangible assets, Clyra Medical sold to BioLargo the assets, along with 12,755 Clyra Medical common shares. In exchange, BioLargo issued Clyra Medical 7,142,858 shares of BioLargo common stock. Concurrently, BioLargo licensed back to Clyra Medical the Scion assets. Scion may exchange its 10,000 Clyra Medical common shares for the 7,142,858 shares of BioLargo common stock issued to Clyra Medical, subject to the escrow and earn-out provisions described above. As of December 31, 2018, per the Closing Agreement, one-half of these shares have been earned and thus may be redeemed, and one-half remain subject to the earn-out provisions. The fair value of the 7,142,858 BioLargo shares is $1,286,000, and one-half of this value is included on our December 31, 2018 balance sheet as (i) “In-process research and development” asset, and (ii) a “Clyra Medical shareholder” liability.
Note 4. Lincoln Park Financing
On August 25, 2017, we entered into a stock purchase agreement (“LPC Purchase Agreement”) with Lincoln Park Capital Fund, LLC (“Lincoln Park”), pursuant to which Lincoln Park agreed to purchase from us at our request up to an aggregate of $10 million of our common stock (subject to certain limitations) from time to time over a period of three years. Concurrently, we entered into a registration rights agreement with Lincoln Park (“LPC RRA”), pursuant to which we were required to file with the Securities and Exchange Commission (“SEC”) a registration statement on Form S-1 to register for resale under the Securities Act of 1933, as amended, the shares of common stock that have been or may be issued to Lincoln Park under the LPC Purchase Agreement. The registration statement was filed, and on September 22, 2017, it was deemed effective by the SEC. The LPC Purchase Agreement allows us, from time to time and at our sole discretion, to direct Lincoln Park to purchase shares of our common stock, subject to limitations in both volume and dollar amount. The volume of shares is limited to a maximum of 50,000 shares if our stock closes at less than $0.50 per share, 75,000 if it closes from $0.50 to $0.74 per share, 100,000 if it closes from $0.75 to $1.24 per share, and 200,000 if it closes at or above $1.25 per share. The maximum dollar amount for any single purchase is $500,000. There are no trading volume requirements under the LPC Purchase Agreement, and we alone control the timing and amount of any sales of our common stock to Lincoln Park. The purchase price of the shares that may be sold to Lincoln Park under the Purchase Agreement is the lower of (i) the lowest sale price on the date of purchase, or (ii) the average of the three lowest closing prices in the prior 12 business days. The purchase price per share will be equitably adjusted for any reorganization, recapitalization, non-cash dividend, stock split, or other similar transaction occurring during the business days used to compute such price. We may at any time in our sole discretion terminate the LPC Purchase Agreement without fee, penalty or cost upon one business day notice. There are no restrictions on future financings, rights of first refusal, participation rights, penalties or liquidated damages in the LPC Purchase Agreement or LPC RRA other than a prohibition on entering into a “Variable Rate Transaction,” as defined in the Purchase Agreement. Lincoln Park may not assign or transfer its rights and obligations under the Purchase Agreement.
In consideration for entering into the LPC Purchase Agreement, on August 25, 2017, we issued to Lincoln Park 488,998 shares of common stock as an “initial commitment fee.” For no additional consideration, when and if Lincoln Park purchases (at the Company’s discretion) any portion of the $10 million aggregate commitment, we are required to issue up to 488,998 shares, pro-rata, as “additional commitment shares”. For example, if we elect, at our sole discretion, to require Lincoln Park to purchase $25,000 of our stock, then we would issue 1,222 additional commitment shares, which is the product of $25,000 (the amount we have elected to sell) divided by $10 million (total amount we can sell Lincoln Park pursuant to the LPC Purchase Agreement) multiplied by 488,998 (the total number of additional commitment shares). The additional commitment shares will only be issued pursuant to this formula as and when we elect at our discretion to sell stock to Lincoln Park.
During the years ended December 31, 2017 and 2018, our transactions pursuant to the Purchase Agreement with Lincoln Park totaled:
|
|
|
Year ended
December 31, 2017
|
|
|
Year ended
December 31, 2018
|
|
|
Shares sold to Lincoln Park
|
|
|
1,199,991
|
|
|
|
2,891,749
|
|
|
Additional Commitment Shares issued to Lincoln Park
|
|
|
24,991
|
|
|
|
41,016
|
|
|
Total shares issued to Lincoln Park:
|
|
|
1,224,982
|
|
|
|
2,932,765
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross proceeds to BioLargo:
|
|
$
|
511,000
|
|
|
$
|
839,000
|
|
We recorded the stock sales in our equity statement and the additional shares issued reduce the deferred offering costs.
Note 5. Debt Obligations
The following table summarizes our debt obligations outstanding as of December 31, 2017 and 2018 (in thousands).
|
|
|
2017
|
|
|
2018
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Notes payable, mature January 5, 2019(1)
|
|
$
|
—
|
|
|
$
|
400
|
|
|
Line of credit, matures September 1, 2019 or later (on 30 day demand)
|
|
|
—
|
|
|
|
430
|
|
|
Convertible notes payable:
|
|
|
|
|
|
|
|
|
|
Convertible notes, mature June 1, 2018
|
|
|
4,469
|
|
|
|
—
|
|
|
Convertible notes, mature July 18, 2018
|
|
|
280
|
|
|
|
—
|
|
|
Convertible notes, mature December 31, 2019(2)
|
|
|
—
|
|
|
|
75
|
|
|
Convertible notes, mature January 11, 2019(1)
|
|
|
—
|
|
|
|
300
|
|
|
Convertible note, matures April 15, 2019(1)
|
|
|
500
|
|
|
|
550
|
|
|
Convertible note, matures July 20, 2019(2)
|
|
|
—
|
|
|
|
440
|
|
|
Total convertible notes payable
|
|
$
|
5,249
|
|
|
$
|
1,365
|
|
|
Total current liabilities
|
|
$
|
5,249
|
|
|
$
|
2,195
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-term liabilities:
|
|
|
|
|
|
|
|
|
|
Note payable issued by Clyra Medical to Scion, matures June 17, 2020 (See Notes 3 and 10)
|
|
|
—
|
|
|
|
1,007
|
|
|
Convertible notes payable, mature September 17, 2019
|
|
|
284
|
|
|
|
—
|
|
|
Convertible notes payable, mature December 31, 2019(1)
|
|
|
292
|
|
|
|
—
|
|
|
Convertible notes payable, mature June 20, 2020(1)
|
|
|
523
|
|
|
|
25
|
|
|
Convertible notes payable, mature July 20, 2019
|
|
|
440
|
|
|
|
—
|
|
|
Convertible notes payable, mature April 20, 2021(1)
|
|
|
—
|
|
|
|
100
|
|
|
Convertible notes, mature June 15, 2021(1)
|
|
|
—
|
|
|
|
110
|
|
|
Note payable, matures March 8, 2023 (or on demand 60 days’ notice)
|
|
|
—
|
|
|
|
50
|
|
|
Total long-term liabilities
|
|
$
|
1,539
|
|
|
$
|
1,292
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
6,788
|
|
|
$
|
3,487
|
|
(1) See Note 14 “Subsequent Events”
(2) These notes are convertible at our option at maturity.
For the years ended December 31, 2017 and 2018 we recorded $3,862,000 and $3,494,000 of interest expense related to the amortization of discounts on convertible notes payable and coupon interest from our convertible notes and line of credit.
Conversion of Debt Obligations
Of the $6,788,000 in debt obligations outstanding as of December 31, 2017, during the year ended December 31, 2018, $6,190,000 were converted into shares of our common stock.
Early Conversion of Unit Notes
In May 2018, prior to their maturity dates, we issued 17,255,811 shares of our common stock in satisfaction of $4,626,000 of convertible promissory notes issued in our “unit” offerings at varying conversion prices, maturing on the following dates (in thousands):
|
|
|
2018
|
|
|
Convertible notes payable, mature June 1, 2018
|
|
$
|
3,647
|
|
|
Convertible notes payable, mature September 17, 2019
|
|
|
284
|
|
|
Convertible notes payable, mature December 31, 2019
|
|
|
217
|
|
|
Convertible notes payable, mature June 20, 2020
|
|
|
478
|
|
|
Total debt converted into shares, May 2018
|
|
$
|
4,626
|
|
These conversions were voluntary on the part of the noteholders and prior to the various maturity dates on notes that were issued in prior “unit” offerings conducted by the Company (2015 Unit Offering, Winter 2016 Unit Offering, and Summer 2017 Unit Offering). We offered these noteholders incentives to convert their notes early. Noteholders with conversion prices of $0.25 and $0.30 were offered incentive shares equal to one and one-half times the number of shares issuable for the payment of interest that would accrue from the last interest payment date of March 20, 2018, through the maturity of the note, at a fixed price of $0.25 per share (for example, a note that would have yielded $1,000 in interest, would receive 1,000 times 1.5 divided by 0.25 equals 6,000 incentive shares). We offered holders of notes with conversion prices higher than $0.30 the ability to reduce their conversion price to $0.30 by paying additional funds equal to six percent or twenty percent of their original investment (6% for notes with original conversion prices of $0.35, and 20% for notes with original conversion prices of $0.55 and $0.57). The additional funds did not increase the amount of the note payable, nor did the reduced conversion price affect the number of shares purchasable under the warrant issued with their “unit” investment. Holders of 40 notes elected to pay an aggregate $357,000 to reduce the conversion prices of their notes to $0.30. As a result of the reduction in conversion prices, an additional 2,749,197 shares were issuable pursuant to the notes upon conversion. The fair value of these additional shares was $632,000. Additional interest expense of $276,000 was recorded as part of the debt conversion and is the amount by which the fair value of the additional shares exceeded the cash received by the Company. Holders of 41 notes with original conversion prices of $0.30 and $0.25 elected to convert early and received 966,318 additional “incentive shares” for their agreement to do so.
Conversion of 2015 Unit Offering Notes at Maturity
On June 1, 2018, we elected to convert the $822,000 outstanding promissory notes remaining in our 2015 Unit Offering on their June 1, 2018 maturity date into 2,488,819 shares of our common stock. Of the shares issued, 2,411,004 were issued in satisfaction of principal amounts due on notes with conversion prices of $0.25, $0.35, and $0.55, and 77,815 shares were issued in satisfaction of $20,000 of accrued and unpaid interest.
Conversion of one-year convertible notes, mature July 18, 2018
On July 2, 2018, the holders of two one-year notes in the aggregate principal amount of $280,000 which were due to mature on July 18, 2018, tendered an offer to the Company to convert 100% of the balance due on the outstanding notes into shares of our common stock in lieu of receiving cash. We accepted the offer and agreed to convert the principal balance of $280,000 and $9,000 in outstanding interest into an aggregate 1,153,600 shares of our common stock, at $0.25 per share.
Conversion of convertible note, matures October 16, 2018 (FirstFire)
On January 16, 2018, we entered into a securities purchase agreement (the “FirstFire Purchase Agreement”) and a registration rights agreement (the “FirstFire RRA”) with FirstFire Global Opportunity Fund, LLC (“FirstFire”), and issued a nine-month promissory note (the “FirstFire Note”) in the principal amount of $150,000 at 5% annual interest convertible into shares of common stock of the Company at $0.394 per share, subject to the terms, and certain limitations and conditions set forth in the FirstFire Purchase Agreement and FirstFire Note.
Pursuant to the FirstFire Purchase Agreement, the Company issued 75,000 shares of common stock to FirstFire as a commitment fee (the “FirstFire Commitment Shares”) at $0.39 per share and $29,000 is recorded as a discount on convertible notes and will amortize to interest expense over the term of the note. Pursuant to the FirstFire RRA, because our common stock traded lower as of the date the FirstFire Commitment Shares were registered ($0.3147 on February 8, 2018), we issued 36,536 additional commitment shares of our common stock and $11,000 is recorded as additional discount on convertible notes and will amortize to interest expense over the term of the note.
The FirstFire Note contains a price protection provision such that if we issue a security with any term more favorable to the holder of such security that was not similarly provided in the FirstFire Note, then the Company shall notify FirstFire of such additional or more favorable term and such term, at its option, shall become a part of the FirstFire Note. As a result of our sale of common stock at $0.25, the conversion price of the FirstFire Note was reduced from $0.394 to $0.25.
In June 2018, FirstFire elected to convert $96,000 of the outstanding principal balance of the FirstFire Note and we issued 383,047 shares, plus 11,902 shares for outstanding interest. On July 15, 2018, FirstFire elected to convert the remaining outstanding principal amount of $54,000, plus interest, and we issued 217,960 shares at $0.25 per share.
Notes payable, mature January 5, 2019
On September 19, 2018, we received $400,000 and issued promissory notes originally due January 5, 2019 and incurring interest at an annual rate of 12%, and stock purchase warrants (see Note 7), to two investors. We exercised our option to extend the maturity date of these notes by 60 days by giving written notice on January 3, 2019, and as a result the principal amount of the notes increased by 10%, effective as of the date of the notice, and amended the notes to further extend the maturity date (see Note 14 “Subsequent Events”).
Convertible Note, matures January 11, 2019 (Triton)
On October 16, 2018, we entered into a Securities Purchase Agreement (“Triton Purchase Agreement”) with Triton Fund, LP (“Triton”) for a $225,000 bridge loan, and issued a promissory note in the principal amount of $300,000 (the “Triton Note”). The Triton Note incurs interest at an annual rate of 5%, and was scheduled to mature January 11, 2019. We agreed to repay the note through any financing we close in excess of $3 million. The $75,000 original issue discount is recorded as a discount on our convertible note and will be amortized to interest expense over the term of the note.
In addition to the note, we issued a stock purchase warrant to Triton (the “Triton Warrant”) allowing Triton to purchase up to an aggregate 1 million shares of our common stock for $0.25 per share, until October 12, 2023 (see Note 7).
In addition to the foregoing, we donated 150,000 shares of our common stock to the student-run Triton Fund, LLC. The closing price of our common stock on the date of grant was $0.28 and we recorded $42,000 of interest expense.
On January 8, 2019, we paid the Triton Note in full (see Note 14 “Subsequent Events”).
Convertible Note, matures April 15, 2019 (Vista Capital)
On December 18, 2017, we received $500,000 pursuant to a securities purchase agreement (the “Vista Purchase Agreement”) and a registration rights agreement (the “Vista RRA”) with Vista Capital Investments, LLC (“Vista Capital”), and issued a convertible promissory note (the “Vista 2017 Note”) in the aggregate principal amount of $500,000 at 5% annual interest, which was originally convertible into shares of common stock of the Company at $0.394 per share, subject to the terms, and certain limitations and conditions, set forth in the Vista Purchase Agreement and Vista Note. The Vista 2017 Note was originally scheduled to mature on September 18, 2018.
Pursuant to the Vista RRA, we filed a registration statement to register the shares of common stock into which the Vista 2017 Note is convertible, and the 250,000 shares issued as a commitment fee, which was recorded as a discount on convertible notes in the amount of $99,000 and was amortized to interest expense over the term of the note. The registration statement was deemed effective by the SEC on February 8, 2018. Because the closing price of our common stock was lower on the date the registration of these shares was deemed effective, we were required to issue an additional 140,849 shares of our common stock as additional commitment shares. The beneficial conversion feature resulted in a $20,000 relative fair value recorded as an additional discount. The discount was amortized monthly to interest expense through September 18, 2018.
The Vista 2017 Note contains a price protection provision such that if we issue a security with any term more favorable to the holder of such security that was not similarly provided in the Vista 2017 Note, then we shall notify Vista Capital of such additional or more favorable term and such term, at its option, shall become a part of the Vista 2017 Note. As a result of our sale of common stock at $0.25, the conversion price of the Vista 2017 Note was reduced from $0.394 to $0.25.
In June 2018, Vista Capital elected to convert $52,000 of the outstanding principal and interest balance of the Vista Note and we issued 208,100 shares of our common stock.
On September 12, 2018, Vista Capital agreed to extend the maturity date of the Vista 2017 Note to December 18, 2018. In return, we increased the principal outstanding balance by 20% or $92,000. In addition, we issued the note holder a warrant to purchase 1,812,000 shares of our common stock at $0.25 per share, which was fair valued using the Black Scholes option model at $488,000 (see Note 6). We accounted for this as a modification of the note. Per the guidance of ASC 470-50, Debt Modifications and Extinguishments, modified terms are considered substantially different if the present value of the cash flows after modification differ by at least 10% prior to the modification. The major change in the instrument is the increase in OID of $92,000, and with a discount rate of 5% (equal to the interest rate) and a one-month extension, the present value is $165,975, which is over the 10% test. Therefore, the substantial modification is an extinguishment of the debt. Biolargo accounted for the $166,667 as a loss on extinguishment. This was recorded as a loss on debt extinguishment of debt on our Consolidated Statement of Operations and Comprehensive Loss.
On December 18, 2018, Vista Capital elected to convert $166,667 of the outstanding principal and interest of the Vista 2017 Note in conjunction with our agreement that the principal amount of the note had increased by $166,667 as a result of the OID provisions in the Triton Note (above), and we issued 666,668 shares of our common stock. As of December 31, 2018, the outstanding balance on the Vista Note totaled $550,000.
Vista Capital agreed to further extend the December 15, 2018 maturity date of this note (see Note 14, “Subsequent Events”).
Per the guidance of ASC 470-50, Debt Modifications and Extinguishments, modified terms are considered substantially different, if the present value of the cash flows after modification differ by at least 10% prior to the modification. With the increase in principal, the Vista Note met the 10% cash flow test and therefore the Company accounted for the transaction as an extinguishment of debt. The increased principal, and the warrant fair value treated as a fee for the extension, produced a $578,942 loss on extinguishment of the convertible debt. The new 5% Convertible Note is recorded at principal value with a 90-day maturity.
Two-Year Convertible Note, matures July 20, 2019
On July 20, 2017, the Company accepted $400,000 from one accredited investor, and issued a promissory note with a 10% original issue discount in the principal amount of $440,000 due in two years, which accrues interest at 12%. The note originally provided that interest was to be paid quarterly beginning October 1, 2017, in either cash, common stock, or an option to purchase common stock, in the holder’s discretion. On January 25, 2018, the interest provisions in the note were modified such that the 12% annual simple interest is due at maturity.
At maturity, the note automatically converts, at the holder’s option, into either BioLargo common shares at $0.42 per share, 2,000 shares of Clyra Medical common stock held by BioLargo, or any combination thereof. The fair value of the beneficial conversion feature resulted in a $171,000 discount recorded on our balance sheet as a discount on convertible notes payable, net of current portion. The discount will be amortized monthly as interest expense through July 20, 2019.
Lines of credit, mature September 1, 2019
On March 1, 2018, we received $390,000, and on September 1, 2018, we received $40,000, pursuant to a line of credit accruing interest at a rate of 18% per annum, for which we have pledged our inventory and accounts receivable as collateral. Interest is paid quarterly; the holder may choose to receive interest payments in (i) cash, (ii) our common stock, calculated based on the 20-day average closing price, or (iii) options to purchase our common stock, priced at the 20-day average closing price, the number of shares doubled, and expiring 10 years from the date of grant. The holder of the line of credit has the right to call due the outstanding principal amount on 30-days’ notice at any time after September 1, 2019.
Each creditor, for no additional consideration, received a warrant to purchase our common stock. The warrant allows for the purchase of the number of common shares equal to the investment amount (e.g., one warrant share for each dollar invested), at a price of $0.35 and expires March 1, 2023.
Convertible Notes, mature December 31, 2019 (Winter 2016 Unit Offering)
Of the $292,000 of promissory notes issued in our Winter 2016 Unit Offering, all but $75,000 were converted in May 2018 (see table above). This note, held by one investor, is due December 31, 2019. At maturity, we have the option to convert this note into common stock at $0.57 per share.
Convertible Notes, mature June 20, 2020 (Summer 2017 Unit Offering)
On May 24, 2017, we commenced a private securities offering (titled the “Summer 2017 Unit Offering”) which offered the sale of $1.5 million of “Units,” each Unit consisting of a convertible promissory note and stock purchase warrant. Concurrently, we issued Pricing Supplement No. 1 setting the initial unit/conversion price at $0.42 per share, and the initial warrant exercise price at $0.65 per share. The promissory notes issued to investors mature June 20, 2020, and bear interest at the rate of 12% per annum on the amount invested. Any interest due will be paid quarterly in arrears in cash or shares of common stock. If paid by the issuance of common stock, interest is paid at a conversion price equal to the average closing price of the Company’s common stock over the 20 trading days prior to the interest payment due date. The principal amount of the note may be paid by the issuance of shares of common stock, or cash, upon maturity at the Company’s election. Promissory notes may be converted at any time by the investor, at maturity by the Company, or by the Company prior to maturity, so long as the following conditions are met: (i) the Shares issued as payment are registered with the SEC; and (ii) the Company’s common stock closes for ten consecutive trading days at or above three times the Unit price.
In addition to the convertible promissory note, each investor received a warrant allowing for the purchase of the number of shares of BioLargo common stock equal to the investment amount divided by the unit/conversion price (e.g., one warrant share for each share of common stock which the investor is eligible to receive through conversion of the note). (See Note 7.) The warrants expire on June 20, 2022. The Company may “call” the warrants, requiring the investor to exercise their warrants within 30 days or forever lose the rights to do so, only if the following conditions have been met: (i) the underlying Shares are registered with the SEC and (ii) the Company’s common stock closes for 10 consecutive trading days at or above two times the exercise price.
We received a total of $604,000 of investments in this offering, from ten accredited investors. Of that amount, $524,000 were received in 2017, and $80,000 were received in 2018. The offering documents assured the investors that in the event a subsequent pricing supplement offered a lower conversion or exercise price, prior investors would be given those favorable terms. On December 29, 2017, we issued a pricing supplement lowering the unit price to $0.394. On February 12, 2018, we issued a third pricing supplement lowering the unit price to $0.30, and the warrant exercise price to $0.48 per share. As a result of these reductions, we notified each investor of the decrease in conversion price, and increased the number of warrant shares available to each investor.
In May 2018, investors holding notes in the principal amount of $478,000 elected to convert their notes to common stock (reflected in the table above). As a result of these conversions, we issued an aggregate 2,372,817 shares of our common stock (1,595,670 for principal, and 777,146 for interest). On November 11, 2018, a holder elected to convert a note in the principal amount of $100,000 and we issued 333,334 shares of common stock. As of December 31, 2018, one note in the principal amount of $25,000 remained outstanding on this offering.
Convertible Note, matures April 20, 2021 (Spring 2018 Unit Offering)
On March 26, 2018, we commenced a private securities offering (titled the “Spring 2018 Unit Offering”) which offered the sale of $1.5 million of “Units,” each Unit consisting of a convertible promissory note and stock purchase warrant. We set the initial unit/conversion price at $0.30 per share, and the initial warrant exercise price at $0.48 per share. The promissory notes issued to investors mature April 20, 2021, and incur interest at the rate of 12% per annum. Interest due will be paid quarterly in arrears in cash or shares of common stock, at our option. If paid by the issuance of common stock, interest is paid at a conversion price equal to the average closing price of the Company’s common stock over the 20 trading days prior to the interest payment due date. The principal amount of the note may be paid by the issuance of shares of common stock, or cash, upon maturity at the Company’s election. The notes may be converted at any time by the investor, at maturity by the Company, or by the Company prior to maturity, so long as the following conditions are met: (i) the shares issued as payment are registered with the SEC; and (ii) the Company’s common stock closes for ten consecutive trading days at or above three times the Unit price.
In addition to the convertible promissory note, each investor will receive a warrant allowing for the purchase of the number of shares of BioLargo common stock equal to the investment amount divided by the unit/conversion price (e.g., one warrant share for each share of common stock which the investor is eligible to receive through conversion of the note).
We received one investment in this offering, in March 2018, for $100,000, and issued a warrant to purchase 333,333 shares (see Note 7). This investment was received from an entity owned/controlled by a member of our board of directors. In light of the decreasing price of our common stock, in September 2018, we issued a pricing supplement reducing the unit price to $0.25 per share and reducing the warrant exercise price to $0.40 per share. As a result of the issuance of this pricing supplement, the unit and warrant price of the prior investor were changed to reflect these new prices. We received no further investments in this offering.
Convertible Notes, mature June 15, 2021 (OID Note)
On June 15, 2018, we received $75,000 and issued a convertible promissory note (titled the “OID Note”) in the principal amount of $82,500. On August 7, 2018, we received $25,000 and issued an OID Note in the principal amount of $32,500. These notes are convertible into shares of the Company’s common stock at a conversion price of $0.30 per share. The original issuance discount totaled $10,000, recorded as a discount on convertible notes payable on our balance sheet. The discount will be amortized and recorded to interest expense over the term of the note. The OID Notes mature June 15, 2021, and incurs interest at the rate of 15% per annum. Interest due will be paid quarterly in arrears in shares of common stock, paid at a conversion price equal to the average closing price of the Company’s common stock over the 20 trading days prior to the interest payment due date. The OID Notes are convertible by the investors at any time, and convertible by the Company (i) at maturity, (ii) in the event the Company’s stock price closes at two times the conversion price for 20 consecutive days, provided that either the shares underlying the convertible note are registered with the SEC, or more than six months has elapsed since the date of the investment.
Note payable, matures March 8, 2023 (or on demand)
On March 8, 2018, we received $50,000 and entered into a note payable. The note is due on upon demand from the noteholder, with sixty days’ notice. In the absence of the demand, the maturity date is March 8, 2023. In lieu of interest, we issued the note holder a warrant (see Note 7).
Note 6. Share-Based Compensation
Common Stock
On May 2, 2017, pursuant to an employment agreement with the Company’s president, Dennis P. Calvert (see Note 13), we issued Mr. Calvert 1.5 million shares of common stock, subject to a “lock-up agreement” whereby the shares remain unvested unless and until certain conditions are met (see Note 13). None of these conditions have been met. The Company will expense the fair value of the stock if and when it is probable that any of the conditions above are met.
Issuance of Common Stock in exchange for payment of payables
Payment of Officer Salaries
During 2018, we issued 1,131,036 shares of our common stock at range of $0.24 - $0.43 per share in lieu of accrued and unpaid salary totaling $319,000. The shares issued to Mr. Calvert are unvested at the date of grant and subject to a lock-up agreement restricting vesting and sale until the earlier of (i) the consummation of a sale (in a single transaction or in a series of related transactions) of BioLargo by means of a sale of (a) a majority of the then outstanding common stock of BioLargo (whether by merger, consolidation, sale or transfer of common stock, reorganization, recapitalization or otherwise) or (b) all or substantially all of the assets of BioLargo; and (ii) the successful commercialization of BioLargo’s products or technologies as demonstrated by its receipt of at least $3,000,000 in cash, or the recognition of $3,000,000 in revenue, over a 12-month period from the sale of products and/or the license of technology; and (iii) the Company’s breach of the employment agreement between the Company and Calvert and resulting in Calvert’s termination.
During 2017, we issued 148,705 shares of our common stock at $0.39 per share in lieu of $58,000 of accrued and unpaid obligations to our officers.
Payment of Consultant Fees and Accrued Interest
During 2018, we issued 4,125,281 shares of our common stock at a range of $0.23 – $0.42 per share in lieu of $1,012,000 of accrued interest and accrued and unpaid obligations to consultants.
During 2017, we issued 2,272,116 shares of our common stock at a range of $0.39 – $0.70 per share in lieu of $1,078,000 of accrued interest and accrued and unpaid obligations to consultants.
All of these offerings and sales were made in reliance on the exemption from registration contained in Section 4(2) of the Securities Exchange Act and/or Regulation D promulgated thereunder as not involving a public offering of securities.
Stock Option Expense
During the years ended December 31, 2017 and 2018, we recorded an aggregate $1,103,000 and $1,335,000, respectively, in selling general and administrative expense related to the issuance of stock options. We issued options through our 2018 Equity Incentive Plan, our (now expired) 2007 Equity Incentive Plan, and outside of these plans.
2018 Equity Incentive Plan
On June 22, 2018, our stockholders adopted the BioLargo 2018 Equity Incentive Plan (“2018 Plan”) as a means of providing our directors, key employees and consultants additional incentive to provide services. Both stock options and stock grants may be made under this plan for a period of 10 years. It is set to expire on its terms on June 22, 2028. Our Board of Director’s Compensation Committee administers this plan. As plan administrator, the Compensation Committee has sole discretion to set the price of the options. The plan authorizes the following types of awards: (i) incentive and non-qualified stock options, (ii) restricted stock awards, (iii) stock bonus awards, (iv) stock appreciation rights, (v) restricted stock units, and (vi) performance awards. The total number of shares reserved and available for awards pursuant to this Plan as of the date of adoption of this 2018 Plan by the Board is 40 million shares. The number of shares available to be issued under the 2018 Plan increases automatically each January 1st by the lesser of (a) 2 million shares, or (b) such number of shares determined by our Board.
Activity for our stock options under the 2018 Plan from June 22, 2018, inception date through the year ended December 31, 2018, is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Aggregate
|
|
|
As of December 31, 2018:
|
|
Options
|
|
|
Exercise
|
|
|
Price per
|
|
|
intrinsic
|
|
|
|
|
Outstanding
|
|
|
Price per share
|
|
|
share
|
|
|
Value(1)
|
|
|
Inception, June 22, 2018
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Granted
|
|
|
1,318,517
|
|
$
|
0.22
|
–
|
0.43
|
|
|
$
|
0.34
|
|
|
|
|
|
|
Expired
|
|
|
—
|
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2018
|
|
|
1,318,517
|
|
$
|
0.22
|
–
|
0.43
|
|
|
$
|
0.34
|
|
|
$
|
1,000
|
|
(1) – Aggregate intrinsic value based on closing common stock price of $0.24 at December 31, 2018.
The options to purchase 1,318,517 shares issued during the year ended December 31, 2018 are comprised of options issued to employees, consultants, officers, and directors: (i) we issued options to purchase 630,289 shares of our common stock at an exercise price on the respective grant date ranging from $0.22 to $0.43 per share to employees and consultants in lieu of salary and amounts owed; the fair value of these options totaled $187,000 and is recorded as selling, general and administrative expenses; and (ii) we issued options to purchase 688,228 shares of our common stock at an exercise price on the respective grant dates ranging from $0.24 to $0.43 per share to members of our board of directors for services performed, in lieu of cash. The fair value of these options totaled $203,000 and is recorded as selling, general and administrative expenses.
2007 Equity Incentive Plan
On September 7, 2007, and as amended April 29, 2011, the BioLargo, Inc. 2007 Equity Incentive Plan (“2007 Plan”) was adopted as a means of providing our directors, key employees and consultants additional incentive to provide services. Both stock options and stock grants may be made under this plan for a period of 10 years, which expired on September 7, 2017. The Board’s Compensation Committee administers this plan. As plan administrator, the Compensation Committee has sole discretion to set the price of the options. As of September 2017, the Plan was closed to further stock option grants.
On June 19, 2017, the date of our annual stockholders’ meeting, we recorded the issuance of options to purchase an aggregate 40,000 shares of our common stock to the non-employee members of our Board of Directors, pursuant to the terms of the 2007 Plan which calls for an annual automatic issuance. The exercise price of $0.43 equals the price of our common stock on the grant date. The fair value of these options totaled $16,000 and was recorded as selling, general and administrative expense.
On February 10, 2017, we extended our engagement agreement with our Chief Financial Officer (and, see Note 14). The sole consideration for the one-year extension was the issuance of an option to purchase 300,000 shares of our common stock, at an exercise price of $0.69 per share which was equal to the closing price of our common stock on the date of grant. The option expires February 10, 2027, and vests over the term of the engagement with 125,000 shares having vested as of February 10, 2017, and the remaining shares to vest 25,000 shares monthly beginning March 1, 2017, and each month thereafter, so long as his agreement is in full force and effect. The fair value of the option totaled $207,000 and is recorded in selling, general and administrative expense. The option has fully vested.
Activity for our stock options under the 2007 Plan for the years ended December 31, 2017 and 2018 is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Aggregate
|
|
|
|
|
Options
|
|
|
Exercise
|
|
|
Price per
|
|
|
intrinsic
|
|
|
|
|
Outstanding
|
|
|
price per share
|
|
|
share
|
|
|
Value(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2016
|
|
|
9,916,586
|
|
$
|
0.22
|
–
|
1.89
|
|
|
$
|
0.44
|
|
|
|
|
|
Granted
|
|
|
340,000
|
|
|
0.39
|
–
|
0.69
|
|
|
|
0.65
|
|
|
|
|
|
Expired
|
|
|
(425,000
|
)
|
|
0.40
|
–
|
0.94
|
|
|
|
0.91
|
|
|
|
|
|
Not issued, 2007 Plan closed September 2017
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2017
|
|
|
9,831,586
|
|
|
0.22
|
–
|
1.89
|
|
|
|
0.44
|
|
|
|
|
|
Expired
|
|
|
(140,000
|
)
|
|
0.35
|
–
|
1.89
|
|
|
|
1.41
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2018
|
|
|
9,691,586
|
|
$
|
0.23
|
–
|
0.94
|
|
|
$
|
0.43
|
|
$
|
<1,000
|
|
(1) – Aggregate intrinsic value based on closing common stock price of $0.24 at December 31, 2018.
Non-Plan Options issued
During the year ended December 31, 2018, we issued options to purchase 1,701,088 shares of our common stock at exercise prices ranging between $0.39 – $0.67 per share to members of our board of directors and vendors for fees for services. The fair value of the options issued totaled $434,000, of which $414,000 is recorded in our selling, general and administrative expense. The remaining $20,000 of fair value will vest during 2019.
During the year ended December 31, 2017, we issued options to purchase 1,433,999 shares of our common stock at exercise prices ranging between $0.39 – $0.67 per share to members of our board of directors and vendors for fees for services totaling $716,000.
On December 29, 2017, we extended our engagement agreement with our Chief Financial Officer. The sole consideration for the one-year extension was the issuance of an option to purchase 300,000 shares of our common stock, at an exercise price of $0.39 per share which was equal to the closing price of our common stock on the date of grant. The option expires December 19, 2027, and vests over the term of the engagement with 75,000 shares having vested as of December 19, 2017 and the remaining shares to vest 25,000 shares monthly through September 30, 2018, so long as his agreement is in full force and effect. The fair value of the option totaled $117,000, and during the year ended December 31, 2017, we recorded $29,000 of selling, general and administrative expense. During the year ended December 31, 2018, we recorded the remaining $88,000 to selling general and administrative expense.
On October 23, 2017, we issued to our corporate Secretary an option to purchase 100,000 shares of our common stock at $0.45 per share, which expires October 23, 2027, and vests monthly in 10,000 share increments beginning November 23, 2017. The fair value of this option totaled $45,000, of which $9,000 was recorded as selling, general and administrative expense during 2017. The remaining $34,000 of fair value was recorded as selling general and administrative expense on our December 31, 2018.
October 17, 2017, we issued an option to two employees to each purchase 100,000 shares of our common stock at $0.47 per share, which expires October 17, 2027, and vests monthly in 10,000 share increments beginning November 23, 2017. The fair value of these options totaled $94,000, of which $19,000 was recorded as selling, general and administrative expense during 2017. The remaining $75,000 of fair value was recorded in 2018 as selling general and administrative expense on our December 31, 2018.
On September 5, 2017, we issued options to purchase 2 million shares of our common stock to the employees of our newly created engineering subsidiary (see Note 11). The options are non-qualified stock options, exercisable at $0.45 per share, the closing price of our common stock as of the grant date, exercisable for ten years from the date of grant and subject to vesting in five equal increments on the anniversary of the agreement for five years based on certain performance milestones related to the operations of the subsidiary. (See Note 11 for details of the performance milestones.) The options contain other terms standard in option agreements issued by the Company, including provisions for a cashless exercise. No compensation expense has yet been recognized for these options.
On May 2, 2017, pursuant to his employment agreement (see Note 13), we granted to our president, Dennis P. Calvert, an option to purchase 3,731,322 shares of the Company’s common stock. The option is a non-qualified stock option, exercisable at $0.45 per share, the closing price of our common stock on the grant date, exercisable for ten years from the date of grant, and vesting in equal increments on the anniversary of the agreement for five years. Any portion of the option which has not yet vested shall immediate vest in the event of, and prior to, a change of control, as defined in the employment agreement. The option contains the other terms standard in option agreements issued by the Company, including provisions for a cashless exercise. The fair value of this option totaled $1,679,000 and will be expensed monthly through May 2, 2022. During the years ended December 31, 2017, and 2018 we recorded $196,000 and $336,000 of selling, general and administrative expense.
Activity of our non-plan stock options issued for the years ended December 31, 2017 and 2018 is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
Non-plan
|
|
|
|
|
|
|
|
|
average
|
|
|
Aggregate
|
|
|
|
|
Options
|
|
|
Exercise
|
|
|
price per
|
|
|
intrinsic
|
|
|
|
|
outstanding
|
|
|
price per share
|
|
|
share
|
|
|
value(1)
|
|
|
Balance, December 31, 2016
|
|
|
20,148,766
|
|
|
$
|
0.18
|
–
|
1.00
|
|
|
$
|
0.40
|
|
|
|
|
|
|
Granted
|
|
|
7,765,401
|
|
|
|
0.39
|
–
|
0.69
|
|
|
|
0.46
|
|
|
|
|
|
|
Exercised
|
|
|
(3,866,630
|
)
|
|
|
|
|
0.18
|
|
|
|
0.18
|
|
|
|
|
|
|
Expired
|
|
|
(4,029,129
|
)
|
|
|
|
|
0.18
|
|
|
|
0.18
|
|
|
|
|
|
|
Balance, December 31, 2017
|
|
|
20,018,408
|
|
|
|
0.25
|
–
|
1.00
|
|
|
|
0.51
|
|
|
|
|
|
|
Granted
|
|
|
1,701,088
|
|
|
|
0.23
|
–
|
0.43
|
|
|
|
0.26
|
|
|
|
|
|
|
Expired
|
|
|
(2,400,000
|
)
|
|
|
|
|
0.99
|
|
|
|
0.99
|
|
|
|
|
|
|
Balance, December 31, 2018
|
|
|
19,319,496
|
|
|
$
|
0.23
|
–
|
1.00
|
|
|
$
|
0.43
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, December 31, 2018
|
|
|
19,319,496
|
|
|
$
|
0.23
|
–
|
1.00
|
|
|
$
|
0.43
|
|
|
|
|
|
|
Unvested
|
|
|
(90,000
|
)
|
|
|
0.26
|
–
|
0.28
|
|
|
|
0.27
|
|
|
|
|
|
|
Vested and outstanding, December 31, 2018
|
|
|
19,229,496
|
|
|
$
|
0.23
|
–
|
1.00
|
|
|
$
|
0.43
|
|
|
$
|
1,000
|
|
(1) – Aggregate intrinsic value based on closing common stock price of $0.24 at December 31, 2018.
Exercise of Stock Option
On April 30, 2017, our president, Dennis P. Calvert, delivered a notice of exercise of 3,866,630 shares pursuant to his stock option agreement dated April 30, 2007. The exercise price was $0.18 per share, and the Company issued to Mr. Calvert 2,501,937 shares, calculated by multiplying the difference between the market price of $0.51 and the exercise price of $0.18 with the number of shares exercised, and dividing that amount by the market price. No cash consideration was tendered with respect to the exercise. The remaining 3,866,629 shares available for purchase under the option agreement expired unexercised. Pursuant to a “lock-up agreement” dated April 30, 2017, Mr. Calvert agreed to restrict the sales of the shares received until the earlier of (i) the consummation of a sale (in a single transaction or in a series of related transactions) of the Company by means of a sale of (a) a majority of the then outstanding common stock (whether by merger, consolidation, sale or transfer of common stock, reorganization, recapitalization or otherwise) or (b) all or substantially all of its assets; and (ii) the successful commercialization of the Company’s products or technologies as demonstrated by its receipt of at least $3 million in cash, or the recognition of $3 million in revenue, over a 12-month period from the sale of products and/or the license of technology; and (iii) the Company’s breach of the employment agreement between the Company and Calvert dated May 2, 2017 and resulting in Calvert’s termination.
Note 7. Warrants
We have certain warrants outstanding to purchase our common stock, at various prices, as described in the following table:
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
average
|
|
|
Aggregate
|
|
|
|
|
Warrants
|
|
|
Exercise
|
|
|
price per
|
|
|
intrinsic
|
|
|
|
|
outstanding
|
|
|
price per share
|
|
|
share
|
|
|
value(1)
|
|
|
Balance, December 31, 2016
|
|
|
20,035,114
|
|
|
$
|
0.25
|
–
|
1.00
|
|
|
$
|
0.45
|
|
|
|
|
|
|
Granted
|
|
|
2,289,703
|
|
|
|
0.42
|
–
|
0.70
|
|
|
|
0.41
|
|
|
|
|
|
|
Exercised
|
|
|
(510,000
|
)
|
|
|
|
|
0.30
|
|
|
|
0.30
|
|
|
|
|
|
|
Expired
|
|
|
(250,000
|
)
|
|
|
|
|
0.40
|
|
|
|
0.40
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2017
|
|
|
22,104,817
|
|
|
|
0.25
|
–
|
1.00
|
|
|
|
0.45
|
|
|
|
|
|
|
Granted
|
|
|
7,451,013
|
|
|
|
0.25
|
–
|
0.48
|
|
|
|
0.29
|
|
|
|
|
|
|
Expired
|
|
|
(2,683,400
|
)
|
|
|
|
|
0.40
|
|
|
|
0.40
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2018
|
|
|
26,872,430
|
|
|
$
|
0.25
|
–
|
1.00
|
|
|
$
|
0.43
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, December 31, 2018
|
|
|
26,872,430
|
|
|
$
|
0.25
|
–
|
1.00
|
|
|
$
|
0.42
|
|
|
|
|
|
|
Unvested
|
|
|
(87,500
|
)
|
|
|
|
|
0.35
|
|
|
|
0.35
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vested and outstanding, December 31, 2018
|
|
|
26,784,930
|
|
|
$
|
0.25
|
–
|
1.00
|
|
|
$
|
0.42
|
|
|
$
|
—
|
|
Warrants issued concurrently with promissory notes
In conjunction with a $225,000 investment and note issued in the principal amount of $300,000 to Triton (see Note 5, “Convertible note payable, matures January 11, 2019 (Triton)”), we issued a stock purchase warrant to Triton allowing Triton to purchase up to an aggregate 1,000,000 shares of our common stock for $0.25 per share, expiring October 12, 2023. The relative fair value of this warrant totaled $225,000 and was recorded as a discount on our convertible notes and will be amortized to interest expense through the January 11, 2019 maturity of the note.
We may “call” the warrant if the closing price of our common stock equals or exceeds $0.50 for 10 consecutive trading days and the shares underlying the warrant are subject to an effective registration statement with the Securities and Exchange Commission. If we call the warrant, Triton would have 30 days to exercise its rights to purchase shares under the warrant or forever forfeit such rights. If the shares underlying the warrant are not registered, Triton may exercise the warrant pursuant to a formula (a “cashless” exercise).
On September 19, 2018, pursuant to the terms of the convertible notes payable due January 5, 2019 (see Note 5, “Convertible Notes, mature January 5, 2019”), we issued warrants to purchase up to an aggregate 1,387,500 shares of our common stock at an exercise price of $0.25 per share. These warrants expire September 19, 2023. We may “call” the warrants if the closing price of our common stock equals or exceeds $2.50 for 10 consecutive trading days and the shares underlying the warrant are subject to an effective registration statement with the Securities and Exchange Commission. If we call the warrants, each investor would have 30 days to exercise its rights to purchase shares under the warrant or forever forfeit such rights.
The relative fair value of these warrants resulted in $217,000 recorded as a discount on our consolidated balance sheet in the period issued. The discount will amortize to interest expense through the maturity date of the convertible notes.
On September 12, 2018, Vista Capital agreed to extend the maturity date of its note dated December 18, 2017 (See Note 5, “Convertible Note, matures April 15, 2019 (Vista Capital)”). Pursuant to our amendment of the Note extending the maturity date, we issued Vista Capital a warrant to purchase 1,812,000 shares of our common stock at $0.25 per share. This warrant expires September 12, 2023. The fair value of this warrant resulted in $488,000 of loss on extinguishment of debt in 2018.
On March 8, 2018, we issued a warrant to purchase up to 150,000 shares of our common stock (subject to vesting) at an exercise price of $0.35 per share to the holder of a note of the same date in the principal amount of $50,000 (see Note 5, “Note payable, matures March 8, 2023 (or on demand)”). The warrant expires February 28, 2023. At the end of each month, 6,250 shares vest as long as the note payable is outstanding. At December 31, 2018, 56,250 shares had vested. The fair value the warrant totaled $7,000 and was recorded as interest expense.
Reduction of Warrant Exercise Price
In May 2018, certain holders of outstanding warrants to purchase common stock received in prior unit offerings paid us cash in exchange for a reduction of the exercise price in their warrant(s). In the aggregate, we received $149,000 from holders of 37 warrants which allow for the purchase of an aggregate 4,326,358 shares of our common stock. Exercise prices of these warrants were reduced to $0.30. Management determined that the appropriate accounting treatment for the reduction in the exercise price of the warrants was a capital transaction rather than a contract modification treatment analogous to changes in stock option contracts. As such, the fair value was equal to the cash received totaling $149,000.
Warrants Issued Concurrently with Spring 2018 Unit Offering
During 2018, pursuant to the terms of our Spring 2018 Unit Offering (see Note 5, “Convertible Note, matures April 20, 2021 (Spring 2018 Unit Offering)”), we issued a warrant to purchase up to 333,333 shares of our common stock at an exercise price of $0.48 per share to the investor in the Spring 2018 Offering. The warrant expires April 20, 2023. The relative fair value of the warrant resulted in $49,000 recorded as a discount on our convertible notes on our consolidated balance sheet in the period issued. Subsequent to the issuance of this warrant, the unit price for this offering was reduced, and as a result, the Company was obligated to increase the number of shares available for purchase under the warrant from 333,333 to 400,000. The exercise price of the warrant was concurrently reduced. The fair value of this warrant resulted in $17,000 recorded as interest expense during the year ended December 31, 2018.
The Company may “call” the warrants issued in the Spring 2018 Offering, requiring the holder to exercise their warrant within 30 days or forever lose the rights to do so, if the following conditions have been met: (i) the shares of common stock underlying the warrants are registered with the SEC and (ii) the Company’s common stock closes for 10 consecutive trading days at or above two times the exercise price.
Warrants Issued Concurrently with Line of Credit Offering
During 2018, pursuant to the terms of our Line of Credit (see Note 5, “Line of Credit, matures September 1, 2019”), we issued warrants to purchase up to an aggregate of 430,000 shares of our common stock. Of this amount 390,000 shares of our common stock are at an exercise price of $0.35 per share and 40,000 shares are at an exercise price of $0.25 per share. These warrants expire March 1, 2023. The relative fair value of these warrants resulted in $98,000 recorded as a discount on our convertible notes payable and line of credit on our consolidated balance sheet in the period issued.
The Company may “call” these warrants, requiring the holder to exercise their warrants within 30 days or forever lose the rights to do so, if the following conditions have been met: (i) the shares of common stock underlying the warrants are registered with the SEC and (ii) the Company’s common stock closes for 10 consecutive trading days at or above two times the exercise price.
Warrants Issued to Summer 2017 Unit Offering Investors
Pursuant to the terms of our Summer 2017 Unit Offering (see Note 5), we issued warrants to purchase an aggregate 1,246,906 shares of our common stock, at an exercise price of $0.65 per share. These warrants expire June 20, 2022. The relative fair value of these warrants resulted in $524,000 recorded as a long-term discount on our convertible notes.
The offering documents assured the investors that in the event a subsequent pricing supplement offered a lower conversion or exercise price, prior investors would be given those favorable terms. On December 29, 2017, we issued a second pricing supplement, lowering the conversion price to $0.394. As a result of this reduction, we notified each investor of the decrease in conversion price, and increased the number of warrant shares available to each investor. In the aggregate, the number of warrant shares increased by 82,283, such that the warrants, in the aggregate, allow for the purchase of 1,329,189 shares. The relative fair value of these additional warrants resulted in $32,000 recorded as a long-term discount on our convertible notes.
On February 12, 2018, we issued a third pricing supplement, lowering the unit price to $0.30. As a result of this reduction, the number of shares purchasable pursuant to warrants issued to prior investors increased by an aggregate 416,478 shares. Additionally, during the three months ended March 31, 2018, we accepted two final investments in the aggregate amount of $80,000, pursuant to the third pricing supplement, and issued these investors warrants to purchase an aggregate 266,667 shares. The relative fair value of these warrants, including the increase in purchasable shares, resulted in $103,000 recorded as a discount on our consolidated balance sheet in the period issued.
Warrants Issued to One-Year Noteholders
In conjunction with three separate investments of one-year convertible notes, we issued three sets of warrants to purchase an aggregate 400,000 shares to two investors. These warrants were issued July 8, 2016 (400,000 shares at $0.65 exercise price), December 30, 2016 (400,000 shares at $0.75 exercise price), and July 18, 2017 (400,000 shares at $0.65 exercise price). The fair value of these warrants resulted in a $280,000 discount recorded on our balance sheet as of December 31, 2017 as a discount on convertible note payable and will be amortized monthly as interest expense in 2017.
Each of these warrants contained provisions that required a reduction to the exercise price and increase to the number of warrant shares in the event that we sold our common stock at a lower price than the exercise price (subject to some exceptions). During the year ended December 31, 2017, we adjusted downward the warrant exercise price three times to $0.394, resulting in a fair value totaling $344,000, recorded as a deemed dividend in our statement of stockholders’ equity. During the year ended December 31, 2018, we adjusted downward the warrant exercise price to $0.25, resulting in a fair value totaling $297,000, recorded as a deemed dividend in our statement of stockholders’ equity.
Exercise of Warrants
During the year ended December 31, 2017, we issued 510,000 shares of our common stock from the exercise of outstanding stock purchase warrants and in exchange we received proceeds totaling $153,000.
Fair Value – Interest Expense
To determine interest expense related to our outstanding warrants issued in conjunction with debt offerings, the fair value of each award grant is estimated on the date of grant using the Black-Scholes option pricing model and the relative fair values are amortized over the life of the warrant. For the determination of expense of warrants issued for services, extinguishment of debt and settlement management also uses the option-pricing model. The principal assumptions we used in applying this model were as follows:
|
|
|
2017
|
|
|
2018
|
|
|
Risk free interest rate
|
|
1.71
|
–
|
2.10%
|
|
|
2.54
|
–
|
3.00%
|
|
|
Expected volatility
|
|
221
|
–
|
297%
|
|
|
105
|
–
|
127%
|
|
|
Expected dividend yield
|
|
|
—
|
|
|
|
|
—
|
|
|
|
Forfeiture rate
|
|
|
—
|
|
|
|
|
—
|
|
|
|
Expected life in years
|
|
3
|
–
|
5
|
|
|
3
|
–
|
5
|
|
The risk-free interest rate is based on U.S. Treasury yields in effect at the time of grant. Expected volatilities are based on historical volatility of our common stock. The expected life in years is based on the contract term of the warrant.
Note 8. Accounts Payable and Accrued Expenses
Accounts payable and accrued expenses included the following (in thousands):
|
|
|
December
|
|
|
December
|
|
|
|
|
|
31, 2017
|
|
|
|
31, 2018
|
|
|
Accounts payable and accrued expense
|
|
$
|
88
|
|
|
$
|
302
|
|
|
Accrued interest
|
|
|
51
|
|
|
|
122
|
|
|
Accrued payroll
|
|
|
85
|
|
|
|
77
|
|
|
Total accounts payable and accrued expenses
|
|
$
|
224
|
|
|
$
|
501
|
|
Note 9. Provision for Income Taxes
Given our historical losses from operations, income taxes have been limited to the minimum franchise tax assessed by the State of California. Our subsidiary BLEST is a Tennessee limited liability company and as such, is not consolidated in our corporate tax return. As a pass through entity, it does not pay federal taxes. However, the state of Tennessee charges franchise and excise taxes for limited liability companies, and thus BLEST will incur a nominal franchise tax and will not pay an excise tax unless and until it is profitable.
At December 31, 2018, we had federal and California tax net operating loss carry-forwards (“NOLs”) of approximately $58.5 million. Due to changes in our ownership through common stock issuances throughout the year, the utilization of NOLs may be subject to annual limitations and discounts under provisions of the Internal Revenue Code. We have not conducted a complete analysis to determine the extent of these limitations or any future limitation. Such limitations could result in the permanent loss of a significant portion of the NOLs. Given the impact of the Tax Cuts and Jobs Act (“TCJA”) signed into law on December 22, 2017, the future expected corporate tax rate was reduced to 21%. Accordingly, the Company measured its deferred tax asset for these NOLs and estimated a deferred tax asset of approximately $11.1 million for federal, and $4.6 million for California. Under the TCJA, NOLs may be carried forward indefinitely; however, the NOLs are limited to the lesser of (1) the aggregate of the NOL carryovers to such year, plus the NOL carry-backs to such year, or (2) 80% of taxable income (determined without regard to the deduction) (Sec. 172(a)). Generally, NOLs can no longer be carried back but are allowed to be carried forward indefinitely (Sec. 172(b)(1)(A)). Nevertheless, for California purposes, the additional taxable income limitations on NOL carryforwards as well as the indefinite time to use the NOLs have not been adopted. Therefore for California, NOLs expire after 20 years. As such, ours will begin to expire in for the tax period ending December 31, 2021. Realization of our deferred tax assets, which relate to operating loss carry-forwards and timing differences, is dependent on future earnings. The timing and amount of future earnings are uncertain and therefore we have established a 100% valuation allowance.
Note 10. Noncontrolling Interest – Clyra Medical
We consolidate the operations of our partially owned subsidiary Clyra Medical (see Note 2, “Principles of Consolidation”).
On March 31, 2017, Clyra Medical received $250,000 from an existing Clyra Medical shareholder (Sanatio Capital LLC), and issued a line of credit note accruing interest at a rate of 10% per annum and a 5% original issue discount.
In April 2017, BioLargo purchased 500 shares of Clyra Medical common stock from a former member of Clyra Medical’s management for $40,000.
In August 2017, Clyra Medical commenced a private securities offering of its common shares at a price of $160 per share, and accepted $1 million in subscriptions from two investors. Of that amount, BioLargo invested $250,000 and was issued 1,562.5 shares. Concurrently, Sanatio Capital converted the outstanding amount due on its line of credit at $160 per share into 1,690 Clyra Medical common shares.
On September 26, 2018, Clyra Medical entered into a transaction with Scion Solutions, LLC, for the purchase of its intellectual property, including its SkinDisc (see Note 3). Shortly thereafter, it commenced a private securities offering to raise the funds necessary to meet the closing obligations in the Scion transaction. As of December 31, 2018, it had raised $1,005,000 at a price of $200 per share. On December 17, 2018, it announced it had met the closing obligations for the Scion transaction (see Note 3).
As of December 31, 2018, Clyra Medical had the following common and preferred shares outstanding:
|
Shareholder
|
|
Shares
|
|
|
Percent
|
|
|
BioLargo, Inc.
|
|
|
28,053
|
|
|
|
42.3
|
%
|
|
Sanatio Capital(1)
|
|
|
11,520
|
|
|
|
17.4
|
%
|
|
Scion Solutions(2)
|
|
|
15,500
|
|
|
|
23.4
|
%
|
|
Other
|
|
|
11,222
|
|
|
|
16.9
|
%
|
|
Total
|
|
|
66,295
|
|
|
|
|
|
Notes:
(1) Includes 9,830 Series A Preferred shares (see below), and 1,690 common shares.
(2) Does not include an additional 15,500 shares held in escrow subject to performance metrics (see Note 3).
Sanatio Capital purchased Series A Preferred shares in 2015. Sanatio Capital is owned by Jack B. Strommen, who subsequently joined BioLargo’s board of directors. Preferred Shares accrue an annual dividend of 8% for a period of five years. Although the dividends began to accrue immediately, Clyra Medical has no obligation to declare a dividend until a product of the company has received a premarket approval by the United States Federal Drug Administration (“FDA”), or for which a premarket notification pursuant to form 510(k) has been submitted and for which the FDA has given written clearance to market the product in the United States (either, “FDA Approval”). After FDA Approval, annually on December 20, and unless prohibited by California law governing distributions to shareholders, Clyra Medical is required to declare and pay any accruing dividends to holders of Preferred Shares then accrued but unpaid. As the declaration and payment of such dividends is contingent on an uncertain future event, no liability has been recorded for the dividends. The accumulated and undeclared dividend balance as of December 31, 2018 is $185,000.
Holders of Preferred Shares are entitled to preferential payments in the event of a liquidation, dissolution or winding up of the company, in an amount equal to any accrued and unpaid dividends. After such preference, any remaining assets are distributed pro-rata between holders of Clyra Medical common stock and Preferred Shares as if the Preferred Shares had converted to Clyra Medical common stock. Holders of Preferred Shares may convert the shares to Clyra Medical common stock initially on a one-to-one basis. The conversion formula is subject to change in the event Clyra Medical sells stock at a lower price than the price paid by Sanatio.
Preferred shares may be converted to common shares on a one-to-one basis, and have voting rights equal to common shares on a one-to-one basis.
Note 11. Biolargo Engineering, Science and Technologies, LLC
In September 2017, we commenced a full-service environmental engineering firm and formed a Tennessee entity named BioLargo Engineering, Science & Technologies, LLC (“BLEST”). In conjunction with the start of this subsidiary, we entered into a three-year office lease in the Knoxville, Tennessee area, and entered into employment agreements with seven scientists and engineers. (See Note 12 “Business Segment Information”.) The company was capitalized with two classes of membership units: Class A, 100% owned by Biolargo, and Class B, held by management of BLEST, and which initially have no “profit interest,” as that term is defined in Tennessee law. However, over the succeeding five years, the Class B members can earn up to a 30% profit interest. They also have been granted options to purchase up to an aggregate 2 million shares of BioLargo, Inc. common stock. The profit interest and option shares are subject to a five year vesting schedule tied to the performance of the subsidiary, including gross revenue targets that increase over time, obtaining positive cash flow by March 31, 2018 (which was not met), collecting 90% of its account receivables, obtaining a profit of 10% in its first year (and increasing in subsequent years), making progress in the scale-up and commercialization of our AOS system, and using BioLargo research scientists (such as our Canadian team) for billable work on client projects. These criteria are to be evaluated annually by BLEST’s compensation committee (which includes BioLargo’s president, CFO, and BLEST’s president), beginning September 2018. The details of these transactions were reported on a Form 8-K filed with the SEC on September 8, 2017. Given the significant performance criteria, the Class B units and the stock options will only be recognized in compensation expense if or when the criteria are satisfied.
The Compensation Committee met on September 26, 2018 and reviewed the operating performance of the engineering subsidiary and determined that the performance metrics were not met and as a result, did not award any Class B units or stock options. The Committee decided to roll forward one additional year to the time allowed for the performance metrics to be met and for the Class B units and stock options to be awarded.
Note 12. Business Segment Information
BioLargo currently has four operating business segments, plus its corporate entity which is responsible for general corporate operations, including administrative functions, finance, human resources, marketing, legal, etc. The four operational business segments are:
|
|
1.
|
Odor-No-More -- which is selling odor and volatile organic control products and services (located in Westminster, California);
|
|
|
2.
|
BLEST -- which provides professional engineering services on a time and materials basis for outside clients and supports our internal operations as needed (located in Oak Ridge, Tennessee);
|
|
|
3.
|
BioLargo Water -- which historically focused entirely on R&D, and has now shifted its focus to commercializing the AOS technology (located in Edmonton, Alberta Canada); and
|
|
|
4.
|
Clyra Medical -- which is engaged in developing medical products and preparing launch into commercial activity with approval of its FDA 510 (K) application in process (located in Florida).
|
Historically, none of our operating business units operated at a profit and therefore each required additional cash to meet its monthly expenses. The additional sources of the cash to fund the shortfall from operations of Odor-No-More, BLEST and BioLargo Water have been provided by BioLargo’s sales of debt or equity, research grants, and tax credits. Clyra Medical has been funded by third party investors who invest directly in Clyra Medical in exchange for equity ownership in that entity. For example, during the year ended December 31, 2018, we provided Odor-No-More with approximately $417,000 in cash to supplement its operations. As this subsidiary’s sales have increased (from approximately $500,000 in calendar year 2017 to over $1 million in calendar year 2018), and its gross margins have improved, it has generated more cash for its operations and relied less on corporate to supplement its cash to pay its bills.
The segment information for the years December 31, 2017 and 2018, is as follows (in thousands):
|
|
|
2017
|
|
|
2018
|
|
|
Revenues
|
|
|
|
|
|
|
|
|
|
Odor-No-More
|
|
$
|
504
|
|
|
$
|
1,123
|
|
|
BLEST
|
|
|
12
|
|
|
|
241
|
|
|
Consolidated revenue
|
|
$
|
516
|
|
|
$
|
1,364
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of goods/services
|
|
|
|
|
|
|
|
|
|
Odor-No-More
|
|
$
|
(315
|
)
|
|
$
|
(571
|
)
|
|
BLEST
|
|
|
(8
|
)
|
|
|
(172
|
)
|
|
Consolidated costs of goods/services
|
|
$
|
(323
|
)
|
|
$
|
(743
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
|
|
|
|
|
|
|
Odor-No-More
|
|
$
|
(500
|
)
|
|
$
|
(433
|
)
|
|
BLEST
|
|
|
(90
|
)
|
|
|
(750
|
)
|
|
Clyra Medical
|
|
|
(915
|
)
|
|
|
(883
|
)
|
|
BioLargo Water
|
|
|
(741
|
)
|
|
|
(571
|
)
|
|
Corporate
|
|
|
(7,301
|
)
|
|
|
(8,059
|
)
|
|
Consolidated net loss
|
|
$
|
(9,547
|
)
|
|
$
|
(10,696
|
)
|
|
|
|
2017
|
|
|
2018
|
|
|
Assets, net
|
|
|
|
|
|
|
|
|
|
Odor-No-More
|
|
$
|
211
|
|
|
$
|
219
|
|
|
BLEST
|
|
|
—
|
|
|
|
250
|
|
|
Clyra Medical
|
|
|
529
|
|
|
|
462
|
|
|
BioLargo Water
|
|
|
64
|
|
|
|
34
|
|
|
Corporate
|
|
|
692
|
|
|
|
2,220
|
|
|
Consolidated assets, net
|
|
$
|
1,496
|
|
|
$
|
3,185
|
|
Note 13. Commitments and Contingencies
Calvert Employment Agreement
On May 2, 2017, the Company entered into an employment agreement with its President and Chief Executive Officer Dennis P. Calvert (the “Calvert Employment Agreement”), replacing in its entirety the previous employment agreement with Mr. Calvert dated April 30, 2007.
The Calvert Employment Agreement provides that Mr. Calvert will continue to serve as our President and Chief Executive Officer and receive base compensation equal to his current rate of pay of $289,000 annually. During the year ended December 31, 2018, Mr. Calvert took only one-half ($147,000) in cash – the balance was paid in shares of our common stock with significant restrictions on resale (see Note 6). In addition to this base compensation, the agreement provides that he is eligible to participate in incentive plans, stock option plans, and similar arrangements as determined by the Company’s Board of Directors, health insurance premium payments for himself and his immediate family, a car allowance, paid vacation of four weeks per year, and bonuses in such amount as the Compensation Committee may determine from time to time.
Pursuant to the Calvert Employment Agreement, we granted Mr. Calvert a non-qualified stock option (the “Option”) to purchase 3,731,322 shares of our common stock, exercisable at $0.45 per share, which represented the market price of the Company’s common stock as of the date of the agreement, exercisable for ten years from the date of grant and vesting in equal increments over five years (see Note 7). The Option provides that any portion of the Option which has not yet vested shall be immediately vested in the event of, and prior to, a change of control, as defined in the Calvert Employment Agreement. The Calvert Employment Agreement also provides for a grant of 1,500,000 shares of common stock, subject to the execution of a “lock-up agreement” whereby the shares remain unvested unless and until the earlier of (i) a sale of the Company, (ii) the successful commercialization of the Company’s products or technologies as demonstrated by its receipt of at least $3 million in cash, or the recognition of $3 million in revenue, over a 12-month period from the sale of products and/or the license of technology, and (iii) the Company’s breach of the employment agreement resulting in his termination. The Option contains the other terms standard in option agreements issued by the Company, including provisions for a cashless exercise.
The Calvert Employment Agreement has a term of five years, unless earlier terminated in accordance with its terms. The Calvert Employment Agreement provides that Mr. Calvert’s employment may be terminated by the Company due to his death or disability, for cause, or upon a merger, acquisition, bankruptcy or dissolution of the Company. “Disability” as used in the Calvert Employment Agreement means physical or mental incapacity or illness rendering Mr. Calvert unable to perform his duties on a long-term basis (i) as evidenced by his failure or inability to perform his duties for a total of 120 days in any 360-day period, or (ii) as determined by an independent and licensed physician whom Company selects, or (iii) as determined without recourse by the Company’s disability insurance carrier. “Cause” means that Mr. Calvert has (i) engaged in willful misconduct in connection with the Company’s business; or (ii) been convicted of, or plead guilty or nolo contendre in connection with, fraud or any crime that constitutes a felony or that involves moral turpitude or theft. If Mr. Calvert’s employment is terminated due to merger or acquisition, then he will be eligible to receive the greater of (i) one year’s compensation plus an additional one-half year for each year of service since the effective date of the employment agreement or (ii) one year’s compensation plus an additional one-half year for each year remaining in the term of the agreement. Otherwise, he is only entitled to receive compensation due through the date of termination.
The Calvert Employment Agreement requires Mr. Calvert to keep certain information confidential, not to solicit customers or employees of the Company or interfere with any business relationship of the Company, and to assign all inventions made or created during the term of the Calvert Employment Agreement as “work made for hire”.
Office Leases
We have long-term operating leases for office, industrial and laboratory space in Westminster, California, Oak Ridge, Tennessee, and Alberta, Canada. Payments made under operating leases are charged to the Consolidated Statement of Operations and Comprehensive Loss on a straight-line basis over the term of the operating lease agreement. For the years ended December 31, 2017 and 2018, total rental expense was $183,000 and $213,000, respectively.
Future minimum lease payments as of December 31, 2018 are as follows (in thousands):
|
|
|
Total
|
|
|
|
|
|
|
|
|
2019
|
|
$
|
229
|
|
|
2020
|
|
|
231
|
|
|
Total future minimum lease payments
|
|
$
|
460
|
|
Clyra Medical Consulting Agreement
Our partially owned subsidiary Clyra Medical (see Note 10) entered into a consulting agreement with Beach House Consulting, LLC, through which Jack B. Strommen will be providing consulting services to Clyra Medical related to its sales and marketing activities once it has received FDA Approval (as defined in Note 10 and the associated agreement) on a product, at which point the agreement provides that Mr. Strommen is to receive $23,000 per month for a period of four years. This agreement has not started, and the total cash obligation related to the agreement would be $1.1 million.
Note 14. Subsequent Events.
Management has evaluated subsequent events through the date of the filing of this Annual Report and management noted the following for disclosure.
Debt obligations – Extension of notes due January 5, 2019
By letter dated January 3, 2019, we notified the holders of two promissory notes in the aggregate principal amount of $400,000 (the first held by Vernal Bay Investments, LLC (“Vernal”) in the original principal amount of $280,000, and the second held by Chappy Bean, LLC (“Chappy Bean”) in the original principal amount of $120,000) of our election to extend by 60 days, to March 6, 2019, the maturity dates of the notes (see Note 5). As provided in the notes, our election to extend increased the principal amount of each note by 10%, such that the aggregate principal balance of the two notes increased to $440,000 as of January 3, 2019.
On March 5, 2019, we executed amendments to these two notes that extended the maturity dates initially to June 6, 2019, and provide that we may further extend the maturity dates to September 6, 2019 by giving written notice of such extension and increasing the principal due on the notes at that time by 10%. As consideration of the extension of the maturity dates reflected in the March 5, 2019 amendments, we (i) increased the annual percentage rate of interest from 12% to 18%, effective as of March 7, 2019, and (ii) lowered the exercise price, and increased the number of shares available, on warrants that had been previously issued to the two investors (at the time of their original investment). With respect to the warrants, Vernal Bay had been issued a warrant to purchase 1,387,500 shares at $0.25 per share, expiring September 19, 2023. We agreed to lower the exercise price to $0.20 per share, and proportionately increase the number of shares in the warrant to 1,734,375. By doing so, the maximum investment amount under the warrant of $346,875 remained the same. Chappy Bean’s warrant to purchase 600,000 shares was similarly modified, such that it now allows for the purchase of 750,000 shares at $0.20 per share.
Debt Obligations –Convertible Note, matures April 15, 2019 (Vista Capital)
On January 7, 2019, we and Vista Capital agreed to amend the convertible promissory note originally issued December 14, 2017 (“Vista 2017 Note”; see Note 5) and extend its maturity date to April 15, 2019. The principal amount of the note was increased to $605,100. The note will continue to earn interest at the rate of five percent per annum. The amendment re-defined the conversion price to equal 80% of the lowest closing bid price of the Company’s common stock during the 25 consecutive trading days immediately preceding the conversion date. The amendment also reduced the prepayment penalty from 20% to 15%, such that a prepayment requires the payment of an additional 15% of the then outstanding balance, and reduced the penalty for a default from 30% to 25% of the outstanding balance.
Per the guidance of ASC 470-50, “Debt Modifications and Extinguishments,” modified terms are considered substantially different, if the present value of the cash flows after modification differ by at least 10% prior to the modification. With the increase in principal, the Vista 2017 Note met the 10% cash flow test and therefore the Company accounted for the transaction as an extinguishment of debt. The increased principal, and the warrant fair value treated as a fee for the extension, produced a $746,000 loss on extinguishment of the convertible debt. The new 5% Convertible Note is recorded at principal value with a 90-day maturity.
Subsequent to the January 7, 2019 amendment, Vista Capital has chosen to convert $225,000 of the Vista 2017 Note (credited to interest and then principal), and received an aggregate 1,679,248 shares of our common stock. We and Vista Capital have agreed to further extend the maturity date from April 15, 2019 to July 15, 2019, and as consideration have increased the principal balance of the note by 10%. Accounting for these conversions and the extension, the principal amount due on the note is $420,452
Concurrently with the January 7, 2019 extension of the Vista 2017 Note, Vista Capital invested an additional $300,000 and we issued a convertible promissory note (the “Vista 2019 Note”) in the principal amount of $330,000, maturing nine months from the date of issuance (October 7, 2019). The Vista 2019 Note earned a one-time interest charge of 12%. The Vista 2019 Note allows Vista Capital to convert the note to our common stock at any time at a price equal to 65% of the lowest closing bid price of the Company’s common stock during the 25 consecutive trading days immediately preceding the conversion date. The Vista 2019 Note contains standard provisions of default, and precludes the issuance of shares to the extent that Vista Capital would beneficially own more than 4.99% of our common stock. We may pre-pay the Vista 2019 Note within 90 days of the issuance date by giving 10 business day notice of the intent to pre-pay, and then tendering 120% of the outstanding balance of the note. Vista Capital has the option to convert the note to common stock during the 10-day period. The Vista 2019 Note also includes a term that allows Vista Capital to adopt any term of a future financing more favorable than what is provided in the note. For example, these provisions could include a more favorable interest rate, conversion price, or original issue discount. The Vista 2019 Note also requires that we include the shares underlying conversion of the note on the next registration statement we file with the SEC (but not the registration statement filed November 6, 2018).
With respect to the above transactions with Vista Capital, Lincoln Park Capital Fund, LLC agreed to waive the provisions of the Purchase Agreement dated August 25, 2017, prohibiting variable rate transactions. As consideration for this waiver, we issued to Lincoln Park a warrant to purchase 250,000 shares of our common stock at $0.25 per share, expiring five years from the date of grant. In the event the shares underlying the warrant are not registered, the warrant allows the holder to do a “cashless” exercise.
Debt Obligations – Payment of Note due January 11, 2019 (Triton)
On January 9, 2019, we tendered payment of the outstanding principal amount and interest due on the promissory note issued to Triton Funds, LP dated October 12, 2018 (see Notes 5 and 7). Other than the outstanding warrants, we have no ongoing business dealings with Triton.
Debt Obligations – Note due November 5, 2019 (Tangiers Global)
On January 31, 2019, we issued a 12% Convertible Promissory Note to Tangiers Global, LLC (“Tangiers”) in the aggregate principal amount of up to $495,000 (the “Tangiers Note”). The initial principal amount of the Tangiers Note is $330,000, for which Tangiers paid a purchase price of $300,000 on February 5, 2019, representing a 10% original issue discount, due November 5, 2019. As originally contemplated, we received an additional $150,000 from Tangiers pursuant to an amendment to the note dated March 7, 2019, increasing the principal amount due under the note to $495,000.
The Tangiers Note is convertible at the option of Tangiers at a conversion price equal to 75% of the lowest closing bid price of the Company’s common stock during the 25 consecutive trading days prior to the conversion date. We may prepay the Tangiers Note up to 180 days after the effective date. If a prepayment is made within 90 days, we must pay a prepayment penalty of 25%; from 91 to 180 days, we must pay a prepayment penalty of 30%. We may pay such prepayment penalties, if we so choose, by issuing common stock at the conversion price. If such shares are not eligible for removal of restrictions pursuant to a registration statement or Rule 144 within 10 trading days following the six-month anniversary of the effective date, Tangiers may rescind the stock issuance and force the Company to pay the prepayment penalty in cash. Upon the occurrence of an event of default, as such term is defined under the Tangiers Note, additional interest will accrue from the date of the event of default at a rate equal to the lower of 22% per annum or the highest rate permitted by law, and an additional 25% shall be added to the principal amount of the note.
In connection with the Tangiers Note, the Company caused its transfer agent to reserve 3,000,000 shares of the Company’s common stock, in the event that the Tangiers Note is converted.
With respect to the above transaction with Tangiers, Lincoln Park consented to waive the provisions of the Purchase Agreement dated August 25, 2017 prohibiting variable rate transactions. As consideration for the consent, we agreed to issue Lincoln Park a stock purchase warrant allowing for the purchase of 50,000 shares of our common stock at $0.25 per share, expiring five years from the date of grant. In the event the shares underlying the warrant are not registered, the warrant allows the holder to do a “cashless” exercise.
Chief Financial Officer Contract Extension
On January 16, 2019, we agreed to extend the engagement agreement dated February 1, 2008 (the “Engagement Agreement”, which had been previously extended multiple times) with our Chief Financial Officer, Charles K. Dargan, II. The Engagement Extension Agreement dated as of January 16, 2019 (the “Engagement Extension Agreement”) provides for an additional term to expire September 30, 2019 (the “Extended Term”), and is retroactively effective to the termination of the prior extension on September 30, 2018. Mr. Dargan has been serving as the Company’s Chief Financial Officer since such termination pursuant to the terms of the December 31, 2017 extension.
For the Extended Term, Mr. Dargan was issued an option (“Option”) to purchase 300,000 shares of the Company’s common stock, at a strike price equal to the closing price of the Company’s common stock on January 16, 2019 of $0.223, to expire January 16, 2029, and to vest over the term of the engagement with 75,000 shares having vested as of December 31, 2018, and the remaining shares to vest 25,000 shares monthly beginning January 31, 2019, and each month thereafter, so long as the Engagement Agreement is in full force and effect. The Option was issued pursuant to the Company’s 2018 Equity Incentive Plan.
The issuance of the Option is Mr. Dargan’s sole source of compensation for the Extended Term. As was the case in all prior terms of his engagement, there is no cash component of his compensation for this term. Mr. Dargan is eligible to be reimbursed for business expenses he incurs in connection with the performance of his services as the Company’s Chief Financial Officer (although he has made no such requests for reimbursement in the past). All other provisions of the Engagement Agreement not expressly amended pursuant to the Engagement Extension Agreement remain the same, including provisions regarding indemnification and arbitration of disputes.
F-61
BioLargo (QB) (USOTC:BLGO)
Historical Stock Chart
From Mar 2024 to Apr 2024
BioLargo (QB) (USOTC:BLGO)
Historical Stock Chart
From Apr 2023 to Apr 2024