PMDA Adds Licenses of GastroPlus®
May 21 2019 - 8:30AM
Business Wire
Japanese government organization to train
reviewers to efficiently analyze PBPK model submissions
Simulations Plus, Inc. (Nasdaq: SLP), the leading provider of
modeling and simulation solutions for the pharmaceutical,
biotechnology, chemicals, and consumer goods industries, today
announced that it has received an order from the Pharmaceuticals
and Medical Devices Agency (PMDA) in Japan to add licenses to its
GastroPlus® software suite.
John DiBella, Lancaster division president for Simulations Plus,
said: “The adoption of physiologically based pharmacokinetic (PBPK)
modeling to support various applications during the drug
development process has increased over the years, partly driven by
encouragement from global regulatory agencies. The U.S. Food and
Drug Administration (FDA) and European Medicines Agency (EMA) have
led these efforts, and it is exciting to see PMDA invest in the
GastroPlus platform in anticipation of more submissions coming to
them for review. This news is most welcomed by the >30, and
growing, domestic Japanese pharmaceutical companies that have been
utilizing our technologies for years, as they will hopefully engage
more frequently with PMDA to identify how the simulation results
can potentially be applied to reduce time to market and get
medicines to patients more cost effectively.”
Views expressed in this press release do not necessarily reflect
the official policies of the Pharmaceuticals and Medical Devices
Agency; nor does any mention of trade names, commercial practices,
or organization imply endorsement by the Japanese Government.
About Simulations Plus, Inc.
Simulations Plus, Inc., is a premier developer of drug discovery
and development software as well as a leading provider of both
preclinical and clinical pharmacometric consulting services for
regulatory submissions and quantitative systems pharmacology models
for drug-induced liver injury, drug-induced kidney injury, and
nonalcoholic fatty liver disease. Our software is licensed to and
used in the conduct of drug research by major pharmaceutical,
biotechnology, chemical, and consumer goods companies and
regulatory agencies worldwide. Our innovations in integrating new
and existing science in medicinal chemistry, computational
chemistry, pharmaceutical science, biology, and physiology into our
software have made us the leading software provider for
physiologically based pharmacokinetic modeling and simulation. For
more information, visit our website at
www.simulations-plus.com.
Safe Harbor Statement Under the Private Securities Litigation
Reform Act of 1995 – With the exception of historical
information, the matters discussed in this press release are
forward-looking statements that involve a number of risks and
uncertainties. Words like “believe,” “expect” and “anticipate” mean
that these are our best estimates as of this writing, but that
there can be no assurances that expected or anticipated results or
events will actually take place, so our actual future results could
differ significantly from those statements. Factors that could
cause or contribute to such differences include, but are not
limited to: our ability to maintain our competitive advantages,
acceptance of new software and improved versions of our existing
software by our customers, the general economics of the
pharmaceutical industry, our ability to finance growth, our ability
to continue to attract and retain highly qualified technical staff,
our ability to identify and close acquisitions on terms favorable
to the Company, and a sustainable market. Further information on
our risk factors is contained in our quarterly and annual reports
and filed with the U.S. Securities and Exchange Commission.
Follow us on Twitter | LinkedIn
View source
version on businesswire.com: https://www.businesswire.com/news/home/20190521005222/en/
Simulations Plus Investor
RelationsMs. Renee
Bouche661-723-7723renee@simulations-plus.com
Hayden IRMr. Cameron
Donahue651-653-1854cameron@haydenir.com
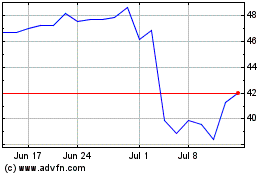
Simulations Plus (NASDAQ:SLP)
Historical Stock Chart
From Mar 2024 to Apr 2024
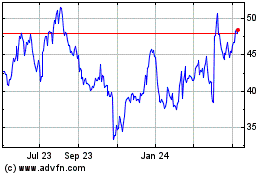
Simulations Plus (NASDAQ:SLP)
Historical Stock Chart
From Apr 2023 to Apr 2024